- Fujian Key Laboratory of Pathogenic Fungi and Mycotoxins, Key Laboratory of Biopesticide and Chemical Biology of Education Ministry, and School of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, China
Aspergillus flavus is one of the most important opportunistic pathogens of crops and animals. The carcinogenic mycotoxin, aflatoxins produced by this pathogen cause a health problem to human and animals. Since cyclic AMP signaling controls a range of physiological processes, like fungal development and infection when responding to extracellular stimuli in fungal pathogens, in this study, we investigated the function of adenylate cyclase, a core component of cAMP signaling, in aflatoxins biosynthesis and virulence on plant seeds in A. flavus. A gene replacement strategy was used to generate the deletion mutant of acyA that encodes the adenylate cyclase. Severe defects in fungal growth, sporulation and sclerotia formation were observed in the acyA deletion mutant. The defect in radical growth could be partially rescued by exogenous cAMP analog. The acyA mutant was also significantly reduced in aflatoxins production and virulence. Similar to the former studies in other fungi, The acyA mutant showed enhancing tolerance to oxidative stress, but more sensitive to heat stress. Overall, the pleiotropic defects of the acyA deletion mutant indicates that the cAMP-PKA pathway is involved in fungal development, aflatoxins biosynthesis and plant seed invasion in A. flavus.
Introduction
Aspergillus flavus is a saprophytic soilborne fungus that contaminates food-stuffs and a broad range of important agricultural crops, including maize, peanut, and cottonseed, with the most carcinogenic metabolite, aflatoxins (AFs) (Amaike and Keller, 2011; Yang et al., 2015). This fungus is also one of the most opportunistic pathogen of human and animals causing aspergillosis diseases or liver cancer either through consumption of contaminated food or through invasive growth (Hedayati et al., 2007; Amaike and Keller, 2011; Yang et al., 2015). Because of this, A. flavus causes food shortages, significant economic losses and health problems all over the world especially in warm and moist fields (Amaike and Keller, 2011; Bai et al., 2015). Effective strategies of combating this pathogen are required to alleviate its potential deleterious effects.
The cAMP/PKA signaling pathway, which utilizes cyclic AMP (cAMP) as a second messenger, controls a range of physiological processes in eukaryotic cell (Oliver et al., 2002; Xue et al., 2008). In fungi, cAMP regulates both metabolism and morphogenesis (Thevelein and de Winde, 1999; Lengeler et al., 2000; Fillinger et al., 2002). As study in S. cerevisiae, cAMP regulates carbon metabolism, cell cycle progression, and pseudohyphal growth (Ward et al., 1995). In filamentous fungi, such as N. crassa, cAMP signaling is also required for hyphal tip growth, conidiation, and carbon metabolism (Kays et al., 2000). In many plant-pathogenic fungi, cAMP signaling is also involved in toxin production and virulence (D'Souza and Heitman, 2001; Lee et al., 2003; Shimizu et al., 2003; Choi and Xu, 2010; Hu et al., 2014). For example, in F. graminearum, cAMP signaling has been shown to be important for the production of DON, which is one of the best characterized virulence factors (Hu et al., 2014; Jiang et al., 2016). In A. nidulans, cAMP/PKA pathway is also known to negatively regulate sterigmatocystin biosynthesis (Shimizu et al., 2003).
In the cAMP network, the intracellular cAMP level is synthesized by the core component, adenylyl cyclase, when responding to extracellular stimuli. Adenylate cyclase has been studied for decade, which is shown to have pleiotropic effects on growth, conidiation, sexual development, and virulence in phytopathogenic fungi (Terenzi et al., 1979; Adachi and Hamer, 1998; Choi and Xu, 2010). Although adenylate cyclase plays various roles in a wide range of fungi, the function of adenylate cyclase in secondary metabolism and infection in Aspergillus has yet been investigated. In this study, we are interested in revealing the roles of adenylate cyclase in modulating the cAMP levels and signaling during different stages of development and AF biosynthesis in A. flavus. Disruption of cAMP signaling by deletion of the A. flavus adenylyl cyclase gene has pleiotropic effects on fungal development, conidiation and stress responses. We also provide data on the impact of cAMP-signaling on aflatoxin biosynthesis and virulence in A. flavus.
Materials and Methods
Strain and Culture Conditions
Aspergillus flavus wild-type (WT) strain and all the transformants in this study (Table 1) were cultured on potato dextrose agar (PDA) for mycelia growth assays. For determining sensitivities to various stresses, fungal growth was assayed after incubation at 37°C for 4 d on PDA plates with 200 μg/mL Congo red, 100 μg/mL Calcofluor white (CFW), 100 μg/mL sodium dodecyl sulfate (SDS), 20 mM/L hydrogen peroxide (H2O2), 2.0 mM/L tert-butyl hydroperoxide (tBooH), 1M NaCl and 1M KCl. To analyse conidia production, 1 × 103 A. flavus conidia was pointed onto PDA agar media, then grown for 5 d at 37°C under dark conditions. Three 1.5 cm diameter cores were harvested from the center of each plate and homogenized in 3 mL of distilled water, and the spore number was counted haemocytometrically. For sclerotial production analysis, sclerotial inducing WKM medium was used (Chang et al., 2012). Cultures were grown at 37°C for 7 d under dark condition, and the plates were then sprayed with 70% ethanol to kill and wash away conidia to aid in enumeration of sclerotial. The experiment was conducted with technical triplicates for each strain, and was repeated thrice.
Targeted Gene Deletion and Complementation
To generate the acyA deletion strain (ΔacyA) and the ΔacyA complementary strain (ΔacyA-C) of A. flavus, a previously described approach was used (Yang et al., 2016). The primers used in this study to amplify the fragment for gene knockout were listed in Table 2. The fusion PCR approach was used to generate gene replacement constructs for the acyA gene, and the fusion PCR product was transformed into protoplasts of the A. flavus PTS strain. For complementation, acyA ORF with its native promoter was amplified using primer pairs CM-acyA/F and CM-acyA/R (Table 2), and then cloned into the pPTR I vector (Takara). The recombinant plasmid, harboring acyA ORF with its native promoter and a pyrithiamine-resistance marker, was transformed into protoplasts of the ΔacyA mutant. Preparation of protoplasts and fungal transformation were performed as previously described (Cary et al., 2006; Abdel-Hadi et al., 2011; Yang et al., 2016).
Seed Infections
The ability of the wild-type and all the mutant strains to infect crop seeds was assayed as described previously (Tsitsigiannis and Keller, 2006; Kale et al., 2008; Yang et al., 2016). The peanut cotyledons treated with A. flavus conidia were incubated at 29°C for 5 d at dark conditions, and the filter paper was moistened daily. After incubation for 5 d, the peanut and maize seeds were harvested in 50 mL Falcon tubes, weighed, then with vortex for 2 min to release the spores in 15 mL of sterile water supplemented with 0.05% Tween 80. Conidiation was counted haemocytometrically. There were three replicated plates per strain and the experiment was repeated thrice.
Aflatoxins Analysis
To analyse aflatoxin (AF) production, 10 mL aliquot of a 106 spore/mL suspension of A. flavus conidia was incubated into Glucose Minimal Media(GMM) (Shimizu and Keller, 2001) medium in the dark at 29°C for 5 d. AF extraction was followed as previously described (Yang et al., 2016). Thin Layer Chromatography (TLC) was used to analyse AF production, performed as previously described (Yang et al., 2016). The experiment was conducted with technical triplicates for each strain, and was repeated thrice.
Quantitative RT-PCR
For qRT-PCR, the mycelia of wild-type and all the mutant strains were harvested at growth stages (48 h and 72 h incubated on PDA). Total RNA was isolated with TRIzol reagent (Biomarker Technologies, Beijing, China), and the first-strand cDNA was synthesized with All-in-One First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). QRT-PCR was performed with the Thermo Fisher Scientific Real-time PCR System (Finland) using TransStart Top Green qPCR SuperMix (TransGen Biotech, Beijing, China). In the quantitative real-time PCR, AF structural gene aflD and regulator gene aflR were amplified by the primer pairs shown in Table 3, and actin gene was used as the endogenous reference gene. The relative quantification of each transcript was calculated following the 2−ΔΔCT method (Livak and Schmittgen, 2001). All qRT-PCR assays were conducted with technical triplicates for each sample, and the experiment was repeated three times.
Intracellular cAMP Measurement
Cultures of 2-day-old liquid mycelial were harvested, frozen in liquid nitrogen and lyophilized more than 6 h. Intracellular cAMP extraction was followed as previously described (Liu et al., 2007). The cAMP levels were quantified according to the Direct cAMP colorimetric (EIA) kit (Enzo Life Sciences, Exeter, UK). The experiment was conducted with technical triplicates for each strain, and was repeated thrice.
Tagging of AcyA with eGFP under the Native Promoter
To localize AcyA, a eGFP-pyrG fragment was amplified from pKNTG vector using primer pairs GFP-pyrG-F/ eGFP-pyrG-R (Table 2). A same approach was used to construct the AcyA-GFP fusion cassette as described previously (Zheng et al., 2012; Yang et al., 2016). In brief, a 1.0 kb fragment immediately upstream of the acyA stop codon and a 1.0 kb fragment immediately downstream of the acyA stop codon were amplified from wild-type strain using primer pairs acyA-eGFP/P1/acyA-eGFP/P3 and acyA/P4/acyA/P6, respectively. The AcyA-eGFP fusion PCR cassettes (using primer pairs acyA-eGFP/P2 and acyA-eGFP/P5) were transformed into A. flavus PTS protoplast, and the transformants embedding homologous integration were verified by PCR.
Microscopic Analysis
Bright field and epifluorescence microscopy was the Olympus BX51 microscope (Olympus, Japan) equipped with a ×20 0.5 NA (numerical aperture), ×40 1.3 NA, or ×100 1.40 NA Olympus oil immersion objective lens. Alternatively, confocal microscopy was used for time-lapse or live cell fluorescence imaging using a Nikon A1R laser scanning confocal microscope system (Nikon, Japan). GFP excitation was performed with 488 nm light (Em. 525/40 nm).
Statistical Analysis
All data were presented as means± standard deviation of at least three biological replicates samples. Statistical and significance analysis were performed using the GraphPad Prism 6 and regarded significant if p-values were < 0.05. Student's t-test was used when comparing two means for differences. For multiple comparisons, Tukey's multiple comparison test was used for significance analysis.
Results
Identification and Analysis of Adenylate Cyclase in A. flavus
cAMP/PKA pathway is one of the most important signaling pathways in eukaryotic organisms. To identify ortholog of the yeast adenylate cyclase in A. flavus, the protein sequences of yeast CYR1 (AAA34549.1) was used as queries for BlastP analyses in the NCBI using the Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). AFLA_128130 in A. flavus was predicted to encode a 2144 aa protein, showing 35% overall identity to yeast CYR1, which we designated herein as AcyA. A phylogenetic analysis of the evolutionary relationship of the AcyA in Aspergillus, Magnaporche. oryzae, Neurospora. crassa, Candida albicans, Saccharomvces cerevisiae, Pseudomonas sp., Mus sp., Homo sapiens, Rattus norvegicus, Saccharum hybrid cultivar, Arabidopsis thaliana, Pseudomonas sp., Haemophilus, and Escherichia coli (Figure 1A) revealed that AcyA were conserved among Aspergillus. The amino acid sequence alignment of adenylate cyclase in different fungi shows that AcyA contains several highly conserved domains (Figure S1), from the N- to C-terminus, including an Adenylate cyclase G-alpha binding domain, Ras-associating (RA) domain, Leucine-rich repeat domain, PPM-type phosphatase domain and Nucleotide cyclase, however, in bacteria or animals the adenylate cyclase only have two domains, the N-terminal domain and Nucleotide cyclase(Figure 1B).
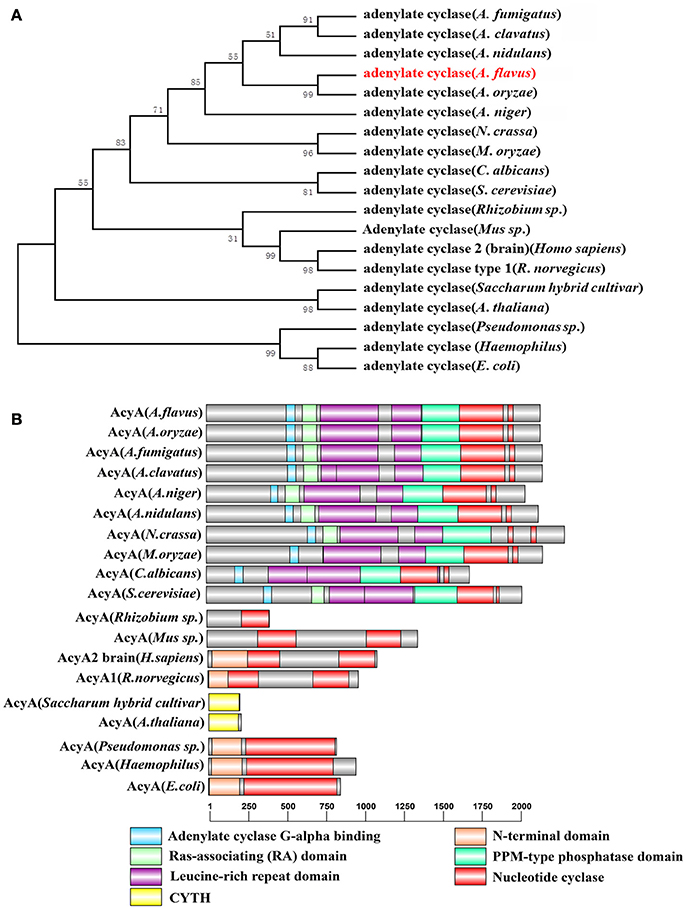
Figure 1. Identification of adenylate cyclase in A. flavus. (A) Phylogenetic analysis of adenylate cyclase. The phylogenetic tree based on all the available adenylate cyclase amino acid sequences from different organisms was constructed by MEGA5.0 software using the Neighbour-joining method. Bootstrap analysis was performed with 1000 replicates. (B) Sequence structure of AcyA was identified by SMART (http://smart.embl-heidelberg.de/smart/set_mode.cgi?NORMAL=1), and the domain architectures were visualized using software DOG 2.0.
Location of AcyA
In order to track the subcellular localization of AcyA, we generated eGFP tag at the C-terminus of AcyA protein under the control of theri native promoter. The AcyA::eGFP transformants showed a WT phenotype (data not shown), indicating that the AcyA-GFP fusion is fully functional. The strategy for their construction is illustrated in Figure 2A. As shown in Figure 2B, in the vegetative growth period, it showed a strong fluorescence signal in the cytoplasm and nucleus of the hyphae.
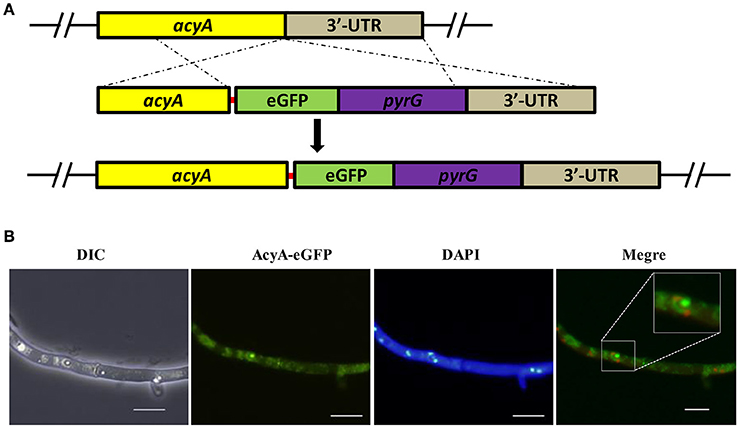
Figure 2. Subcellular distribution of AcyA-eGFP fusion protein. (A) Diagrammatic representation of the gene replacement strategy for construction of acyA(p)-acyA-egfp strain. The red line represents a 5 Gly–Ala linker between AcyA and eGFP to make sure these two proteins can function independently. (B) Localization of AcyA-eGFP in the vegetative growth period, mycelia were collected from YES media after grown at 37°C for 24 h.
AcyA Is Essential for Hyphal Growth and Conidiation
To gain insight into the function of adenylate cyclase during growth and morphogenesis in A. flavus, we generated gene-deletion mutants of acyA (ΔacyA) in the PTS wild-type strain. PEG-mediated gene targeting was used to replace the entire ORF of the acyA gene with the pyrG selective marker (Figure S2A). The selected transformants were confirmed to be knockouts by PCR, and the failure of gene expression in ΔacyA was also verified by RT-PCR (Figure S2B). Furthermore, we constructed a complementation strain (ΔacyA-C) by reintroducing the genomic DNA sequence encompassing acyA ORF and 1 kb promoter region. When grown on YES agar medium for 4 d at 37°C, the ΔacyA mutant was significantly reduced in growth rate compared to the wild-type and ΔacyA-C strain (Figure 3A). Colonies formed by the ΔacyA mutant also had limited aerial hyphal growth. Further microscopic examination showed that ΔacyA mutant tended to produce substantial hyperbranching (Figures 3B,C, Figure S3). These results indicated that the AcyA plays an important role in the vegetative growth and hyphal branching of A. flavus.
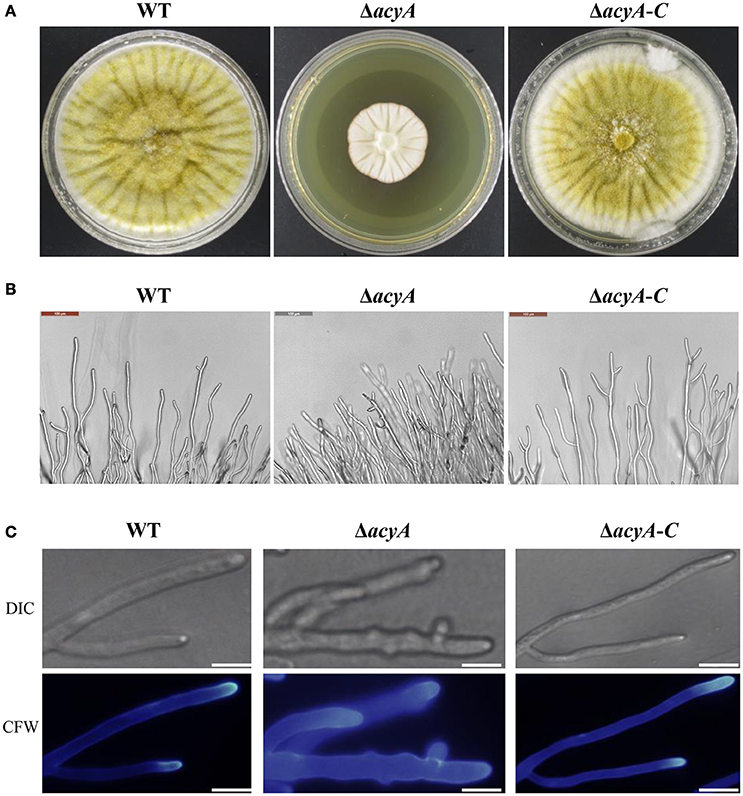
Figure 3. The ΔacyA mutant was defective in colony morphology. (A) Colonies formed by the wild type, ΔacyA and ΔacyA-C complemented strain on YES agar plates. (B) Microscopic examination revealed the difference mycelia tips of WT, ΔacyA and ΔacyA-C complemented strain, bars = 100 μm. (C) mycelia of WT, ΔacyA and ΔacyA-C complemented strain were stained with Calcofluor white (CFW) and examined by DIC or fluorescence microscopy, bars = 200 μm.
In addition to fungal growth, the ΔacyA mutant was found reduced severely in conidiation when compared with the wild-type or ΔacyA-C strain (Figure 4A). For further analysis of the defect in conidiation, we examined the conidiophore formation, and the result showed that the ΔacyA mutant failed to form normal conidiophore, and the wild-type phenotype could be basically recovered in the ΔacyA-C strain (Figure 4B). The transcript levels of the conidia-related genes brlA and abaA were also detected by qRT-PCR, and the result showed that the expression levels of these two genes were decreased significantly in the ΔacyA mutant compared to the wild-type and ΔacyA-C strain (Figure 4C). Taken together, these results reveal that AcyA is essential for hyphal growth and conidiation in A. flavus.
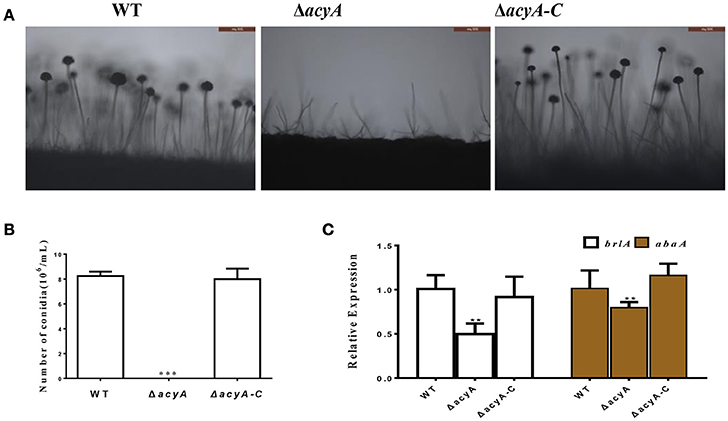
Figure 4. Requirement of acyA for conidiation. (A) Conidia formation was observed under a light microscope at 12 h after induction with illumination. (B) The number of conidia in wild-type and mutant strains were measured after grown on YES agar for 4 d at 37°C. (C) qRT-PCR revealed that the expression levels of conidia related gene brlA and abaA were decreased in the ΔacyA mutant. Values in (B,C) are the means plus standard errors (error bars) for three replicates. The asterisks ** and ***above the bars represent significantly different (p ≤ 0.01 or p ≤ 0.001).
AcyA Is Required for Sclerotial Reproduction in A. flavus
In order to adapt the stress condition, A. flavus is able to reproduce a survival structure sclerotia, which is considered to be a vestige of the cleistothecia. To determine if AcyA was involved in the formation of sclerotia, all the strains were grown on the sclerotia-inducing Wickerham medium at 37°C for 7 d. The results showed that sclerotia formation in ΔacyA mutant was severely blocked compared to that in wild type and ΔacyA-C strain (Figures 5A,B), indicating that AcyA is required for the formation of sclerotia in A. flavus. To further confirm this finding, we performed qRT-PCR to detect the transcript levels of the sclerotia-related genes, nsdC and nsdD, and the results showed that the expression levels of nsdC and nsdD were both significantly decreased in the ΔacyA mutant compared to that of WT and ΔacyA-C strains (Figure 5C), demonstrating that AcyA is necessary for the normal maturation of sclerotia.
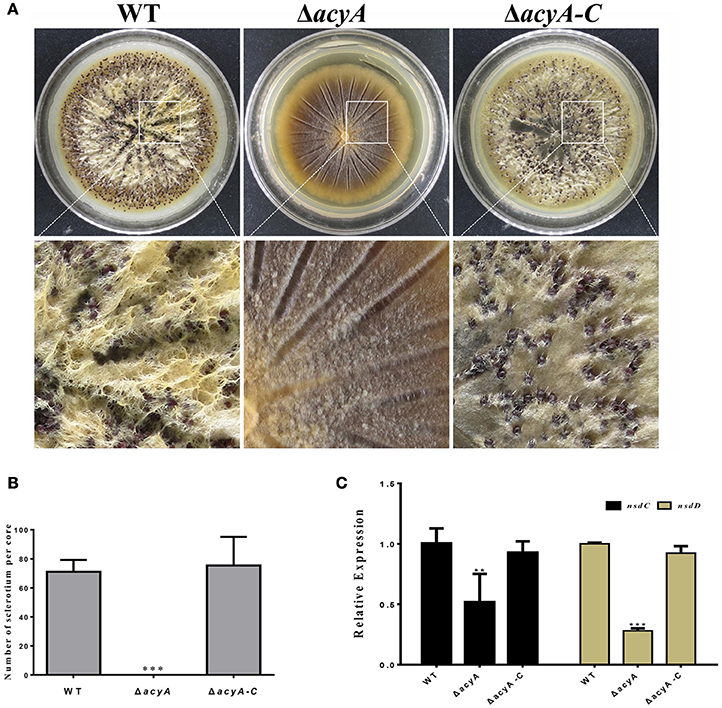
Figure 5. Deletion of acyA blocked sclerotia reproduction in A. flavus. (A) Sclerotia production among the wild type, acyA deletion mutant and ΔacyA-C strain were observed after grown on WKM agar for 7 d in dark condition. The plates were sprayed with 70% ethanol to allow visualization of sclerotia. (B) Sclerotial production was counted from three replicates of WKM plates in (A). (C) qRT-PCR revealed that the sclerotial related gene nsdC and nsdD were decreased in ΔacyA mutant. Values in (B,C) are the means plus standard errors (error bars) for three replicates. The asterisks ** and ***above the bars represent significantly different (p ≤ 0.01 or p ≤ 0.001).
AcyA Is Involved in Nutrient Sensing
In fungi, adenylyl cyclases are large proteins providing multiple points for signal sensing, including response to glucose and amino acids. To determine the function of acyA in sensing nutrient, we assay vegetative growth of acyA deletion mutant on different medium. As shown in Figure 6A, ΔacyA showed reduced aerial hyphae on all assayed media. When grown on nutrient-rich media PDA, ΔacyA, although defective in colony morphology, showed a largest colony diameter compared to other medium (p < 0.01; Figures 6B,C). When the cultures were replaced by the media which used the sucrose or dextrose as carbon source, ΔacyA was reduced 20~40% in colony diameter compare to the PDA media (Figure 6C), indicating that the acyA mutant was likely defective in nutrient sensing.
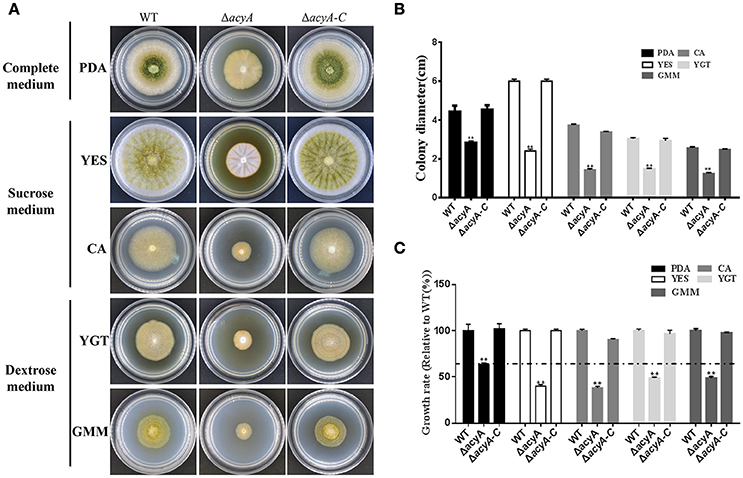
Figure 6. Defects of the acyA mutant in phenotype and growth rate when grown on different medium. (A) Phenotype of Wild-type (WT), ΔacyA and ΔacyA-C stains were grown on different medium for 4 d. (B) colony diameter of WT, ΔacyA and ΔacyA-C complemented strain on different cultures were assayed. (C) Growth rate of ΔacyA and ΔacyA-C strain relative to WT on PDA, YES, CA, YGT, and GMM media were measured, respectively. Values in (B,C) are the means plus standard errors (error bars) for three replicates. The asterisks **above the bars represents significantly different (p ≤ 0.01).
Altered Heat Shock Responses in the acyA Mutant
Because cAMP signaling is known to be involved in heat tolerance, we assayed hyphal growth at different temperature. As we can see in Figure 7, A. flavus hyphal grew fast at 37°C on PDA cultures (Figure 7A). When grown in the condition that is suitable for aflatoxin biosynthesis (29°C), the acyA mutant was reduced approximately 21.2% in growth rate, whereas the growth rate of WT and ΔacyA-C strains decreased slightly (Figure 7B). When the cultures were kept growing at 42°C for 2 d, the colony growth of ΔacyA was reduced almost 55%, while 42% for WT, and 44% for ΔacyA-C strain. These results indicated that the acyA mutant was much more sensitive to temperature stress than the wild-type for hyphal growth.
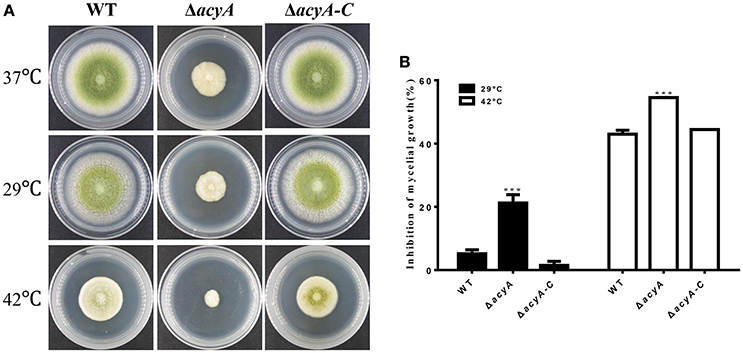
Figure 7. The acyA mutant decreased tolerance to changing temperatures. (A) Phenotype of Wild-type (WT), ΔacyA and acyA-C stains were grown on PDA medium at different temperature. Cultures were grown on PDA medium at 37°C for 2 d, then kept growing at 37°, 29°, or 42°C for 2 d. (B) Growth rate of hyphal growth at different temperature relative to that of 37°C were measured. Values in (B) are the means plus standard errors (error bars) for three replicates. The asterisks ***above the bars represent significantly different (p ≤ 0.001).
AcyA Is Important for Response to Hyperosmotic Stresses and Oxidative Stresses
cAMP signaling plays a major role in regulating cellular responses to environmental signals. To test the ability of acyA in response to various environmental stress, we assayed the sensitivity of WT, ΔacyA and ΔacyA-C strains in response to osmotic stress and oxidative stress. As shown in Figures 8A,B, the growth of ΔacyA mutants was severely blocked after the addition of 1M NaCl or 1M KCl in PDA media compared to the WT and ΔacyA-C strain, indicating that AcyA is involved in responses to osmotic stresses in A. flavus. Since ΔacyA mutant exhibited increased sensitivity to osmotic stresses, we were also interested in determining the role of AcyA played in responses to other stresses. As we can see in Figures 8C,D, the wild-type and ΔacyA-C strain, but not ΔacyA, were much more sensitive to oxidative agent hydrogen peroxide. The growth of the wild-type and ΔacyA-C strains were severely blocked by the addition of tert-butyl hydroperoxide compared to the ΔacyA mutant, demonstrating that AcyA is likely to play a negative role in responses to oxidative stresses in A. flavus.
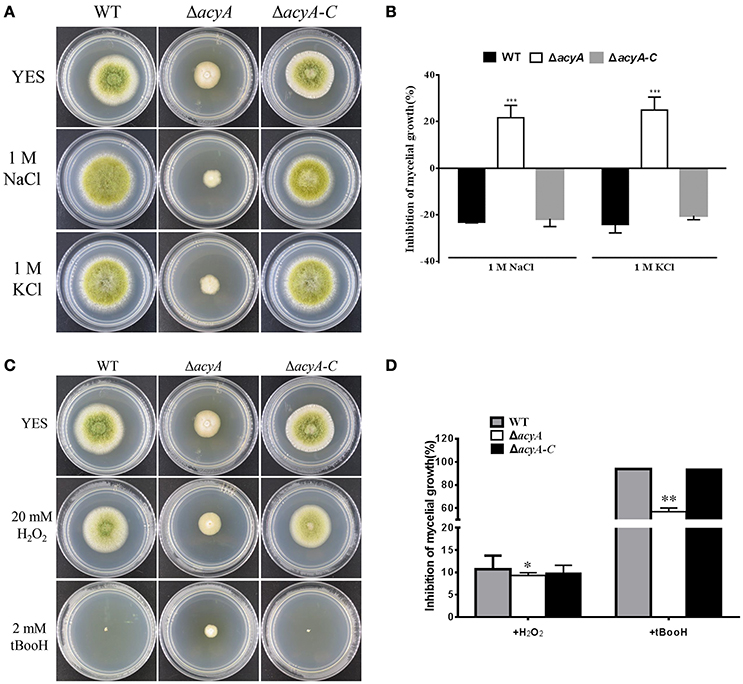
Figure 8. Sensitivity of the WT, acyA mutant and ΔacyA-C strain to hyperosmotic stresses and oxidative stresses. (A) Phenotype of WT, ΔacyA and acyA complementation stains were grown on PDA medium supplemented with or without 1M NaCl and 1M KCl for 3 d. (B) The inhibition rate of the mycelial radial growth was quantified after incubation on media with 1M NaCl and 1M KCl for 3 d. (C) Colonies of WT, ΔacyA and acyA complementation stains were grown on PDA medium supplemented with or without 20 mmol/L hydrogen peroxide (H2O2) or 2 mmol/L tBooH (tert-butyl hydroperoxide) for 3 d. (D) The inhibition rate of the mycelial radial growth was quantified after incubation on media with H2O2 and tBooH for 3 d. Values in (B,D) are the means plus standard errors (error bars) for three replicates. The asterisks *, **, and ***above the bars represent significantly different (p ≤ 0.05, p ≤ 0.01, or p ≤ 0.001, respectively).
AcyA Is Essential for Aflatoxin Biosynthesis
Aspergillus flavus is a well-known aflatoxins (AFs)-producing fungus, which are found to be one of the most toxic and carcinogenic natural contaminants. To examine the effect of AcyA on AF biosynthesis, AF production was tested from the WT, ΔacyA and ΔacyA-C strains by thin layer chromatography (TLC) at 5 d. The result indicated that the ΔacyA mutant failed to produce aflatoxin compared to WT and ΔacyA-C strains (Figures 9A,B). The qRT-PCR was also performed to detect the transcript levels of laeA, encoding a global regulator of many secondary metabolisms, the aflatoxin globally regulated gene aflR, and the structure genes, aflD (nor-1) and aflO (omtB), and the resluts showed that the expression levels of laeA, aflR and aflO were significantly decreased in the ΔacyA mutant compared to that in WT and ΔacyA-C (Figure 9C), however, the aflD transcript showed no difference among these strains (Figure 9C). All these results indicated that the AcyA might play an important role in regulating aflatoxins biosynthesis by reducing the AF regulator genes' expression in A. flavus.
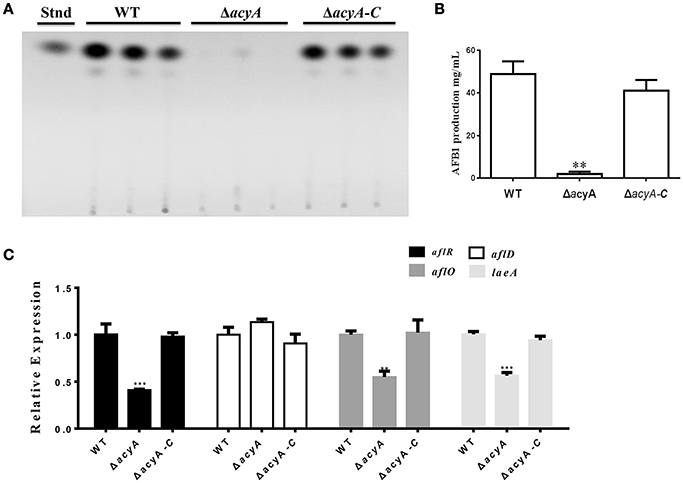
Figure 9. Aflatoxin production of the wild type, acyA deletion mutant and ΔacyA-C strain. (A) Aflatoxin production was detected by TLC after cultured in GMM liquid media for 5 d at 29°C in the dark. (B) Quantification of AFB1 production as in (A). (C) qRT-PCR results of the expression level of laeA, and the aflatoxin-related genes, aflR, aflD, and aflO from WT, ΔacyA and ΔacyA-C strains. Gene expression levels were normalized (ΔΔCT analysis) to actin. Line bars in each column denote standard errors of three experiments. The asterisks ** and *** represent significantly different (p ≤ 0.01 and p ≤ 0.01, respectively).
AcyA Contributes to the Full Virulence to Crop Seeds
To determine the function of AcyA in pathogenicity, peanut seed was inoculated with spore suspension from the wild-type, ΔacyA and ΔacyA-C strains. The ΔacyA grew less vigorously than wild-type and ΔacyA-C strains on crop seeds (Figure 10A). Conidial production was also measured in these strains on seed, and the acyA deletion mutant was found fail to produced conidia compared to WT andΔacyA-C strains (Figure 10B). We also detected the AF production from the infected peanut seed, which showed that the ΔacyA produced no detectable AF production compared to the WT and ΔacyA-C strain (Figure 10C). All these results indicated that AcyA contributed to the full virulence to crop seeds.
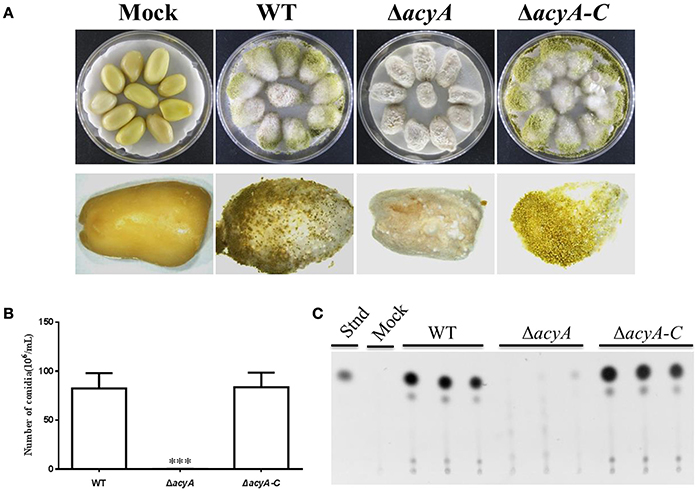
Figure 10. Host colonization of the wild type, acyA deletion mutant and ΔacyA-C strain. (A) The strains were grown on peanut seed in the dark at 29°C for 7 d. (B) Conidia production was assessed from infected peanut seed. (C) Aflatoxin production were detected by TLC, which were extracted from infected peanut seed. Values in panel B are the means plus standard errors (error bars) for three replicates. The asterisks ***above the bars represent significantly different (p ≤ 0.001).
AcyA Synthesizes Intracellular cAMP in A. flauvs
In order to assess whether the phenotypic defects in acyA deletion mutant was due to decreased levels of cAMP, we quantified and compared the steady-state levels of cAMP from the wild-type, ΔacyA and ΔacyA-C strains. cAMP levels were measured after 2 d incubation in the dark, and the result showed that levels of intracellular cAMP in the ΔacyA mutant was reduced more than 10-fold compared to than that in WT and ΔacyA-C strains (Figure 11A). These results indicate that AcyA is responsible for synthesizing intracellular cAMP in A. flavus. Here, we also assayed whether exogenous cAMP or 8-Bromo-cAMP would recover the phenotype defects in ΔacyA mutant. As shown in Figures 11B,C, the hyphal growth of the ΔacyA mutant showed a significant increase in the presence of 5 mM cAMP or 8-Bromo-cAMP, but showing no difference in the wild-type or ΔacyA-C strains. However, neither the exogenous cAMP nor 8-Bromo-cAMP could fully recover the defects in ΔacyA mutant, indicating additional roles for AcyA, which is a multi-domain protein, each of which might be important for its full function, not just synthesizing cAMP in A. flavus.
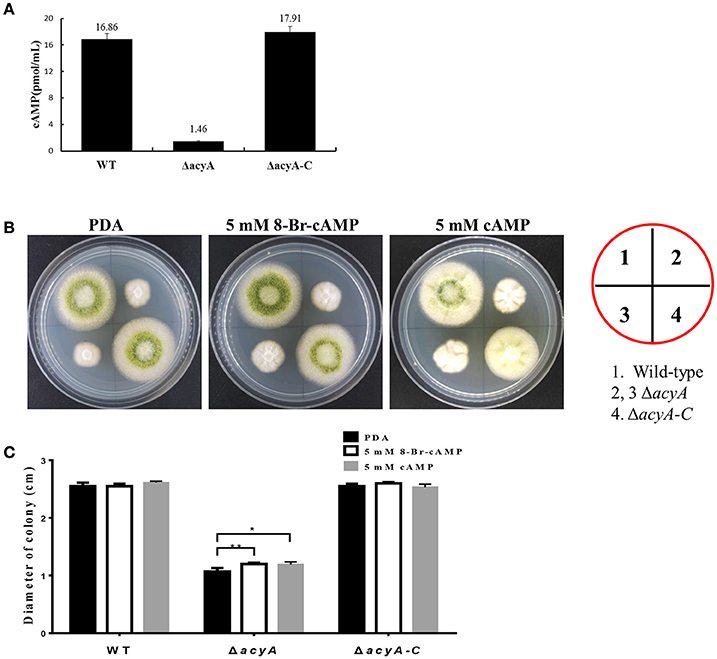
Figure 11. AcyA is responsible for intracellular cAMP levels in A. flavus. (A) Inactivation of acyA leads to decreased accumulation of cAMP levels. (B) Hyphal growth of the wild type, acyA deletion mutant and ΔacyA-C strain on PDA media supplemented without or with 5 mmol/L cAMP or 5 mmol/L 8-Bromo-cAMP. (C) colony diameter of WT, ΔacyA and ΔacyA-C complemented strain on PDA media supplemented without or with 5 mmol/L cAMP or 5 mmol/L 8-Bromo-cAMP were assayed. Values in panels A and C are the means plus standard errors (error bars) for three replicates. The asterisks * and **above the bars represent significantly different (p ≤ 0.05 or p ≤ 0.01, respectively).
Discussion
cAMP signaling pathway has been shown to be very important for nutrient sensing, asexual development and secondary metabolism in fungi (Shimizu et al., 2003; Grosse et al., 2008; Zhang et al., 2011; Hu et al., 2014). In this study, we functionally characterized acyA gene in A. flavus, and found that the acyA deletion mutant wasseverely reduced in aerial hyphal and radial growth. In A. nidulans and F. verticillioides, a reduction in growth of the adenylate cyclase gene deletion mutants was found, which was similar to that of ΔacyA in A. flavus(Fillinger et al., 2002; Choi and Xu, 2010). In N. crassa, inactivation of cr-1 was also severely blocked in vegetative development (Terenzi et al., 1979). However, in M. oryzae, the aerial hyphal of MAC1 deficient mutant was found significantly reduced, but showing no difference in radial growth (Choi and Dean, 1997; Adachi and Hamer, 1998). Therefore, although the role of adenylate cyclase is conserved and important in fungal growth, but remains different in various fungi.
In Aspergillus, The cAMP/PKA pathway has been shown to have a major role in stimulating vegetative growth and repressing conidiation (Shimizu and Keller, 2001; Krijgsheld et al., 2013). However, our study, suggests that AcyA is required for conidiation and sclerotia formation. Here, we found that inactivation of acyA in A. flavus failed to produce conidiation, and concurrently decreasing in expression of two conidia-specific genes brlA and abaA. A defect in sporogenous structure was also found in the ΔacyA mutant, which might result in abnormal sporulation in the mutant. Although the negative role of cAMP/PKA pathway acts in asexual development in Aspergillus, defect in sporulation is a common event found in the adenylate cyclase deficient mutants of previously known filamentous fungi. As we know, inactivation of AcyA, leading to a low level of intracellular cAMP, causes a severe defect in fungal growth, which might also affect conidia formation concurrently, which has to gone through a period of vegetative growth that is necessary for cells to acquire the ability to respond to development signals (Lee et al., 2016). Former studies had also shown that conidial and sclerotial production were likely to remain balanced (Amaike and Keller, 2011). Here, we found that inactivation of acyA in A. flavus failed to form sclerotia. We also found that deleting the acyA gene led to lower transcript levels of sclerotia formation related genes nsdC and nsdD, which might lead to abnormal sclerotia formation. In S. rolfsii and R. solani, exogenous cAMP were shown to alter their sclerotial production (Rollins and Dickman, 1998). In R. solani, cAMP has been shown to enhance sclerotial development even in non-sclerotium-forming strains (Rollins and Dickman, 1998). Aberrant sclerotia were also found in adenylate cyclase sac1 gene deletion mutant in S. sclerotiorum (Jurick and Rollins, 2007). These data indicate that cAMP signaling is likely to play an important role in regulating conidiation and sclerotial formation.
Former studies have indicated that cAMP/PKA pathway is likely to negatively regulate AF biosynthesis in A. flavus. However, the roles of adenylate cyclase in AF or other secondary metabolism are yet characterized in Aspergillus. In this study, we found that inactivation of acyA results in a severe reduction in intracellular cAMP levels and concurrent decrease in AF production. It is interesting to wonder why a block of cAMP signaling by deleting acyA from A. flavus causes reduction in AF biosynthesis and its related genes' expression. There are two main reasons for the decreasing AF production in acyA deficient mutant. One for this, we think, is that inactivation of acyA led to severe defects in A. flavus development, which might affect AF biosynthesis. In the other hand, we hold the idea that deletion of acyA might alter the activated state of cAMP dependent PKA in the cAMP signaling pathway, which might affect the transcriptional level of aflR. In A. nidulans, activating the G-protein α-subunit, FadA, or PkaA (PKA catalytic subunit) inhibits aflR expression and subsequent stc expression. Previous studies also showed that exogenous cAMP and IBMX promoted AF production in A. parasiticus, while the treated cultures were found decreased in PKA activities (Roze et al., 2004a,b). Therefore, we hypothesis that, a block in AF production in the ΔacyA mutant might be caused by the changing activities of PKA, leading to the changing expression levels of AF related genes in A. flavus.
In many phytopathogenic and animal pathogenic fungi, like M. oryzae, C. neoformans and F. verticillioides, the cAMP-PKA pathway is known to function in virulence (Alspaugh et al., 2002; Choi and Xu, 2010). To address the effect of AcyA on A. flavus pathogenicity, we detected host colonization in the ΔacyA mutant. The ΔacyA mutant shows significantly reduction in pathogenicity, which might be caused by a number of factors. Firstly, deletion of acyA results in a defect of vegetative growth and conidiation, leading to the mutant grow less vigorously on the crop seeds compared to the wild-type. In A. flavus, the virulence is thought to be multifactorial and is mainly connected with development, sporulation, secondary metabolism, and other conditions(Amaike and Keller, 2011). In this study, we found that the the ΔacyA mutant fail to produce the secondary metabolism, aflatoxins, which would impair the pathogenicity of A. flavus on crop seeds. The roles of adenylate cyclase seem to be conserved in many other plant pathogenic fungi, just like M. oryzae, S. sclerotiorum and F. verticillioides, for which the adenylate cyclase gene is required for their pathogenesis. Given these properties, targeting cAMP/PKA pathway could be a good strategy to control A. flavus contaminating preharvest and postharvest seed crops.
Conclusions
In summary, our results provide evidence that adenylate cyclase AcyA is responsible for cAMP synthesis in A. flavus, and showing that AcyA has pleiotropic effects on growth, conidiation, virulence and AF biosynthesis. Our results also provide valuable information that could advance our understanding of the cAMP signaling in AF biosynthesis and would provide strategies to block AF production or A. flavus invasion in preharvest and postharvest agriculture crops.
Author Contributions
KY, QQ, and SW designed the experiments and wrote the manuscript. KY, QQ, YL, and LL performed all the experiments. CC and FZ performed a few experiments and data analysis. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding was provided for this research from (No. 2013CB127802), the grants of the National Natural Science Foundation of China (No. 31172297, No.31400100, No. 31000961), and the Scientific Research Foundation of Fujian Agriculture and Forestry University (YB2014007). We especially thanked Dr Perng Kuang Chang (Southern Regional Research Center, United States Department of Agriculture, New Orleans, USA), Prof. Yang Liu (Institute of Food Science and Technology CAAS), Prof. Kong Qing for their kindness to provide the strains. We thanked Dr Yanyun Li (FAFU, Life Science College) for her help in taking the confocal images. We thanked Center for Molecular Cell and Systems Biology, FAFU, Life Science College for providing facilities and support.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fcimb.2016.00190/full#supplementary-material
References
Abdel-Hadi, A. M., Caley, D. P., Carter, D. R., and Magan, N. (2011). Control of aflatoxin production of Aspergillus flavus and Aspergillus parasiticus using RNA silencing technology by targeting aflD (nor-1) gene. Toxins (Basel). 3, 647–659. doi: 10.3390/toxins3060647
Adachi, K., and Hamer, J. E. (1998). Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell 10, 1361–1374. doi: 10.1105/tpc.10.8.1361
Alspaugh, J. A., Pukkila-Worley, R., Harashima, T., Cavallo, L. M., Funnell, D., Cox, G. M., et al. (2002). Adenylyl cyclase functions downstream of the Gα a protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryotic Cell 1, 75–84. doi: 10.1128/EC.1.1.75-84.2002
Amaike, S., and Keller, N. P. (2011). Aspergillus flavus. Annu. Rev. Phytopathol. 49, 107–133. doi: 10.1146/annurev-phyto-072910-095221
Bai, Y., Wang, S., Zhong, H., Yang, Q., Zhang, F., Zhuang, Z., et al. (2015). Integrative analyses reveal transcriptome-proteome correlation in biological pathways and secondary metabolism clusters in A. flavus in response to temperature. Sci. Rep. 5:14582. doi: 10.1038/srep14582
Cary, J. W., Ehrlich, K. C., Bland, J. M., and Montalbano, B. G. (2006). The aflatoxin biosynthesis cluster gene, aflX, encodes an oxidoreductase involved in conversion of versicolorin A to demethylsterigmatocystin. Appl. Environ. Microbiol. 72, 1096–1101. doi: 10.1128/AEM.72.2.1096-1101.2006
Chang, P. K., Scharfenstein, L. L., Mack, B., and Ehrlich, K. C. (2012). Deletion of the Aspergillus flavus orthologue of A. nidulans fluG reduces conidiation and promotes production of sclerotia but does not abolish aflatoxin biosynthesis. Appl. Environ. Microbiol. 78, 7557–7563. doi: 10.1128/AEM.01241-12
Chang, P. K., Scharfenstein, L. L., Wei, Q., and Bhatnagar, D. (2010). Development and refinement of a high-efficiency gene-targeting system for Aspergillus flavus. J. Microbiol. Methods 81, 240–246. doi: 10.1016/j.mimet.2010.03.010
Choi, W., and Dean, R. A. (1997). The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 9, 1973–1983. doi: 10.1105/tpc.9.11.1973
Choi, Y. E., and Xu, J. R. (2010). The cAMP signaling pathway in Fusarium verticillioides is important for conidiation, plant infection, and stress responses but not fumonisin production. Mol. Plant Microbe Interact. 23, 522–533. doi: 10.1094/MPMI-23-4-0522
D'souza, C. A., and Heitman, J. (2001). Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25, 349–364. doi: 10.1111/j.1574-6976.2001.tb00582.x
Fillinger, S., Chaveroche, M. K., Shimizu, K., Keller, N., and D'Enfert, C. (2002). cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 44, 1001–1016. doi: 10.1046/j.1365-2958.2002.02933.x
Grosse, C., Heinekamp, T., Kniemeyer, O., Gehrke, A., and Brakhage, A. A. (2008). Protein kinase A regulates growth, sporulation, and pigment formation in Aspergillus fumigatus. Appl. Environ. Microbiol. 74, 4923–4933. doi: 10.1128/AEM.00470-08
Hedayati, M. T., Pasqualotto, A. C., Warn, P. A., Bowyer, P., and Denning, D. W. (2007). Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153, 1677–1692. doi: 10.1099/mic.0.2007/007641-0
Hu, S., Zhou, X., Gu, X., Cao, S., Wang, C., and Xu, J. R. (2014). The cAMP-PKA pathway regulates growth, sexual and asexual differentiation, and pathogenesis in Fusarium graminearum. Mol. Plant Microbe Interact. 27, 557–566. doi: 10.1094/MPMI-10-13-0306-R
Jiang, C., Zhang, C., Wu, C., Sun, P., Hou, R., Liu, H., et al. (2016). TRI6 and TRI10 play different roles in the regulation of deoxynivalenol (DON) production by cAMP signalling in Fusarium graminearum. Environ. Microbiol. 18, 3689–3701. doi: 10.1111/1462-2920.13279
Jurick, W. M. I.I., and Rollins, J. A. (2007). Deletion of the adenylate cyclase (sac1) gene affects multiple developmental pathways and pathogenicity in Sclerotinia sclerotiorum. Fungal Genet. Biol. 44, 521–530. doi: 10.1016/j.fgb.2006.11.005
Kale, S. P., Milde, L., Trapp, M. K., Frisvad, J. C., Keller, N. P., and Bok, J. W. (2008). Requirement of LaeA for secondary metabolism and sclerotial production in Aspergillus flavus. Fungal Genet. Biol. 45, 1422–1429. doi: 10.1016/j.fgb.2008.06.009
Kays, A. M., Rowley, P. S., Baasiri, R. A., and Borkovich, K. A. (2000). Regulation of conidiation and adenylyl cyclase levels by the Gα protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 20, 7693–7705. doi: 10.1128/MCB.20.20.7693-7705.2000
Krijgsheld, P., Bleichrodt, R., Van Veluw, G. J., Wang, F., Muller, W. H., Dijksterhuis, J., et al. (2013). Development in Aspergillus. Stud. Mycol. 74, 1–29. doi: 10.3114/sim0006
Lee, M. K., Kwon, N. J., Lee, I. S., Jung, S., Kim, S. C., and Yu, J. H. (2016). Negative regulation and developmental competence in Aspergillus. Sci. Rep. 6:28874. doi: 10.1038/srep28874
Lee, N., D'Souza, C. A., and Kronstad, J. W. (2003). Of smuts, blasts, mildews, and blights: cAMP signaling in phytopathogenic fungi. Annu. Rev. Phytopathol. 41, 399–427. doi: 10.1146/annurev.phyto.41.052002.095728
Lengeler, K. B., Davidson, R. C., D'souza, C., Harashima, T., Shen, W. C., Wang, P., et al. (2000). Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64, 746–785. doi: 10.1128/MMBR.64.4.746-785.2000
Liu, H., Suresh, A., Willard, F. S., Siderovski, D. P., Lu, S., and Naqvi, N. I. (2007). Rgs1 regulates multiple Gα subunits in Magnaporthe pathogenesis, asexual growth and thigmotropism. EMBO J. 26, 690–700. doi: 10.1038/sj.emboj.7601536
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Oliver, B. G., Panepinto, J. C., Askew, D. S., and Rhodes, J. C. (2002). cAMP alteration of growth rate of Aspergillus fumigatus and Aspergillus niger is carbon-source dependent. Microbiology 148, 2627–2633. doi: 10.1099/00221287-148-8-2627
Rollins, J. A., and Dickman, M. B. (1998). Increase in endogenous and exogenous cyclic AMP levels inhibits sclerotial development in sclerotinia sclerotiorum. Appl. Environ. Microbiol. 64, 2539–2544.
Roze, L. V., Beaudry, R. M., Keller, N. P., and Linz, J. E. (2004a). Regulation of aflatoxin synthesis by FadA/cAMP/protein kinase A signaling in Aspergillus parasiticus. Mycopathologia 158, 219–232. doi: 10.1023/B:MYCO.0000041841.71648.6e
Roze, L. V., Miller, M. J., Rarick, M., Mahanti, N., and Linz, J. E. (2004b). A novel cAMP-response element, CRE1, modulates expression of nor-1 in Aspergillus parasiticus. J. Biol. Chem. 279, 27428–27439. doi: 10.1074/jbc.M400075200
Shimizu, K., Hicks, J. K., Huang, T. P., and Keller, N. P. (2003). Pka, Ras and RGS protein interactions regulate activity of AflR, a Zn(II)2Cys6 transcription factor in Aspergillus nidulans. Genetics 165, 1095–1104.
Shimizu, K., and Keller, N. P. (2001). Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157, 591–600.
Terenzi, H. F., Jorge, J. A., Roselino, J. E., and Migliorini, R. H. (1979). Adenylyl cyclase deficient cr-1 (Crisp) mutant of Neurospora crassa: cyclic AMP-dependent nutritional deficiencies. Arch. Microbiol. 123, 251–258. doi: 10.1007/BF00406658
Thevelein, J. M., and de Winde, J. H. (1999). Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33, 904–918. doi: 10.1046/j.1365-2958.1999.01538.x
Tsitsigiannis, D. I., and Keller, N. P. (2006). Oxylipins act as determinants of natural product biosynthesis and seed colonization in Aspergillus nidulans. Mol. Microbiol. 59, 882–892. doi: 10.1111/j.1365-2958.2005.05000.x
Ward, M. P., Gimeno, C. J., Fink, G. R., and Garrett, S. (1995). SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol. Cell. Biol. 15, 6854–6863. doi: 10.1128/MCB.15.12.6854
Xue, C., Hsueh, Y. P., Chen, L., and Heitman, J. (2008). The RGS protein Crg2 regulates both pheromone and cAMP signalling in Cryptococcus neoformans. Mol. Microbiol. 70, 379–395. doi: 10.1111/j.1365-2958.2008.06417.x
Yang, K., Liang, L., Ran, F., Liu, Y., Li, Z., Lan, H., et al. (2016). The DmtA methyltransferase contributes to Aspergillus flavus conidiation, sclerotial production, aflatoxin biosynthesis and virulence. Sci. Rep. 6:23259. doi: 10.1038/srep23259
Yang, K., Zhuang, Z., Zhang, F., Song, F., Zhong, H., Ran, F., et al. (2015). Inhibition of aflatoxin metabolism and growth of Aspergillus flavus in liquid culture by a DNA methylation inhibitor. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 32, 554–563. doi: 10.1080/19440049.2014.972992
Zhang, H., Liu, K., Zhang, X., Tang, W., Wang, J., Guo, M., et al. (2011). Two phosphodiesterase genes, PDEL and PDEH, regulate development and pathogenicity by modulating intracellular cyclic AMP levels in Magnaporthe oryzae. PLoS ONE 6:e17241. doi: 10.1371/journal.pone.0017241
Keywords: adenylate cyclase, sclerotia, cAMP, Aspergillus flavus, aflatoxin
Citation: Yang K, Qin Q, Liu Y, Zhang L, Liang L, Lan H, Chen C, You Y, Zhang F and Wang S (2016) Adenylate Cyclase AcyA Regulates Development, Aflatoxin Biosynthesis and Fungal Virulence in Aspergillus flavus. Front. Cell. Infect. Microbiol. 6:190. doi: 10.3389/fcimb.2016.00190
Received: 28 September 2016; Accepted: 05 December 2016;
Published: 21 December 2016.
Edited by:
Brian Wickes, University of Texas Health Science Center at San Antonio, USACopyright © 2016 Yang, Qin, Liu, Zhang, Liang, Lan, Chen, You, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihua Wang, d3NoeXlsQHNpbmEuY29t
†These authors have contributed equally to this work.
 Kunlong Yang
Kunlong Yang Qiuping Qin
Qiuping Qin Yinghang Liu
Yinghang Liu Huahui Lan
Huahui Lan Shihua Wang
Shihua Wang