- 1Department of Periodontology, Anam Hospital, Korea University, Seoul, South Korea
- 2Department of Oral Microbiology and Immunology, School of Dentistry and Dental Research Institute, Seoul National University, Seoul, South Korea
Periodontitis is a chronic inflammation of the periodontium caused by persistent bacterial infection that leads to the breakdown of connective tissue and bone. Because the ability to reconstruct the periodontium is limited after alveolar bone loss, early diagnosis and intervention should be the primary goals of periodontal treatment. However, periodontitis often progresses without noticeable symptoms, and many patients do not seek professional dental care until the periodontal destruction progresses to the point of no return. Furthermore, the current diagnosis of periodontitis depends on time-consuming clinical measurements. Therefore, there is an unmet need for near-patient testing to diagnose periodontitis. Saliva is an optimal biological fluid to serve as a near-patient diagnostic tool for periodontitis. Recent developments in point-of-care (POC) testing indicate that a diagnostic test for periodontitis using saliva is now technically feasible. A number of promising salivary biomarkers associated with periodontitis have been reported. A panel of optimal biomarkers must be carefully selected based on the pathogenesis of periodontitis. The biggest hurdle for the POC diagnosis of periodontitis using saliva may be the process of validation in a large, diverse patient population. Therefore, we propose the organization of an International Consortium for Biomarkers of Periodontitis, which will gather efforts to identify, select, and validate salivary biomarkers for the diagnosis of periodontitis.
Usefulness of Salivary Diagnostics for Periodontitis
Periodontitis is a chronic inflammation of the periodontium caused by persistent bacterial infection that leads to the breakdown of connective tissue and bone (Ji et al., 2014). Due to its chronic nature, periodontitis progresses without causing severe discomfort in the oral cavity, and patients often seek professional care only after the periodontium is considerably destroyed. Thus, there is a need to diagnose periodontitis in its initial stages using an easy, safe, and easily accessible method. Periodontitis is currently diagnosed using radiography and clinical measurements of probing pocket depth (PD), bleeding on probing (BOP), and clinical attachment level (CAL) (Salvi et al., 2008). However, these traditional clinical measurements are time-consuming and yield limited information because they are indicators of previous periodontal disease rather than present disease activity. Moreover, they are inadequate for predicting susceptible individuals who might be at risk of periodontitis in the future. The best predictor of gingival inflammation to date is BOP, but there are too many false positives associated with this method (Lang et al., 1990). There is an unmet need for near-patient testing to diagnose periodontitis.
Saliva is an optimal biological fluid to serve as the diagnostic tool for periodontitis. The collection of saliva is safe, non-invasive, and simple, and saliva can be collected repeatedly with minimum discomfort to the patient. A number of promising biomarkers have been already identified in saliva that correlate with the clinical parameters of periodontitis (Miller et al., 2010; AlMoharib et al., 2014; Taylor, 2014). Saliva contains locally produced proteins, genetic/genomic biomarkers such as DNA and mRNA, and various metabolites that originate from the host and the bacteria (Cuevas-Córdoba and Santiago-García, 2014). However, the diagnosis of periodontitis using saliva has a limitation in detecting disease activity at each individual tooth site; traditional clinical measurements are required in order to accomplish this. In this respect, the diagnosis of periodontitis using saliva must be realized as a point-of-care (POC) testing. POC testing is defined as medical testing conducted outside of a laboratory at or near the site of patient care, including the patient's bedside, the doctor's office, and the patient's home (Song et al., 2014). If periodontitis is diagnosed by a POC device using saliva, patients could easily diagnose their periodontitis at home and visit dental clinics at a suitable time. In dental clinics, current disease activity and responses to treatment can be easily monitored at a chair-side. A POC device for diagnosing periodontitis would also assist medical doctors in assessing the periodontal status of their patients because periodontitis is associated with many systemic diseases, such as atherosclerosis, coronary heart disease, diabetes mellitus, and rheumatoid arthritis (Scannapieco, 2005; Kobayashi and Yoshie, 2015). When medical doctors prescribe bisphosphonate or other medicines associated with medication-related osteonecrosis of the jaw (MRONJ), they can consider the periodontal status of their patients in advance to prevent the development of MRONJ, a common complication of medication combined with tooth extraction (Katsarelis et al., 2015). Recent developments in POC testing indicate that the diagnosis of periodontitis using saliva is now technically feasible.
POC Technologies for Molecular Diagnostics
Technologies for detecting biomarker signals in biofluids have advanced significantly. In particular, the combination of microfluidic and lab-on-a-chip technologies allows for real-time monitoring of biomarkers in a small volume of a bodily fluid at POC sites (Sackmann et al., 2014). Lab-on-a-chip approaches integrate processing steps such as sampling, sample preparation, detection, and data analysis into one small device (Su et al., 2015). Microfluidics-based devices can analyze diverse clinical samples, including blood, saliva, nasal aspirate, and urine (Su et al., 2015).
Diagnostic targets detected by POC technologies include nucleic acids, proteins, metabolites and other small molecules (Song et al., 2014; Su et al., 2015). For example, nucleic acid can be amplified by on-chip PCR (non-isothermal) or on-chip isothermal amplification techniques (Su et al., 2015). Many PCR-based POC devices for the detection of pathogens such as influenza, RSV, HIV, Methicillin-resistant Staphylococcus aureus, Clostridium difficile, and malaria are already commercially available (Su et al., 2015). POC DNA tests have also been developed to detect genetic mutations associated with various cancers (Yang et al., 2014). The microfluidic detection of protein biomarkers generally relies on antibody-based immunoassays. Aptamers, DNA, or RNA oligonucleotides designed to bind to various biomolecules with high specificity and sensitivity are an alternative to antibodies (Toh et al., 2015). The simple lateral flow assay is rapid and specific but not sensitive or quantitative. Diverse new technologies have been developed to improve sensitivities and to allow for quantitative measurements of multiplex protein biomarkers (Gaster et al., 2009; Warsinke, 2009; Rissin et al., 2010; de la Rica and Stevens, 2012). Glucose is the best-known metabolite targeted by POC testing, with a long history of use (Wilkins and Atanasov, 1996). Now a much wider range of analytes can be quantified using POC technology (Sia and Chin, 2011). For example, the i-STAT POC device, millions of which are sold annually, electrochemically measures blood gas (pH, PCO2, PO2, TCO2, HCO3, base excess, and sO2), electrolyte (sodium, potassium, chloride, TCO2, anion gap, ionized calcium, glucose, urea nitrogen, creatinine, and lactate), and hematology (hematocrit and hemoglobin) parameters (Lauks, 1998). A microfluidic device that measures nitric oxide has also been developed (Halpin and Spence, 2010).
Various approaches have been developed to detect the target molecules, but optical detection and electrochemical detection are the ones most commonly adopted. Optical detection methods implemented in POC devices include absorbance colorimetry, chemiluminescence, fluorescence, surface-enhanced Raman scattering spectroscopy, and surface plasmon resonance (Gubala et al., 2012; Su et al., 2015). Electrochemical detection methods include amperometric, potentiometric, and impedimetric measurements (Su et al., 2015).
Salivary Biomarkers of Periodontitis
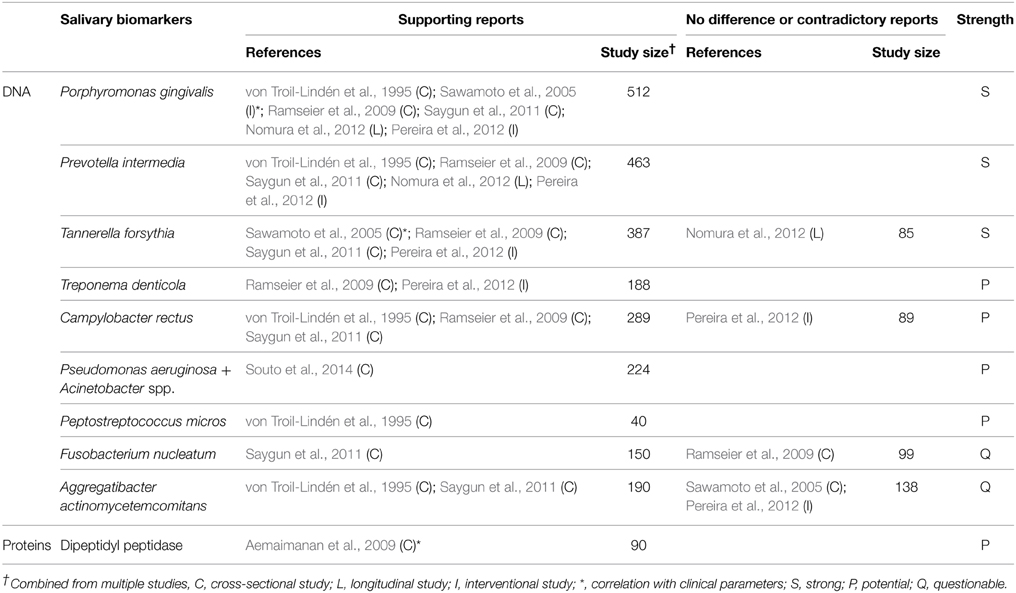
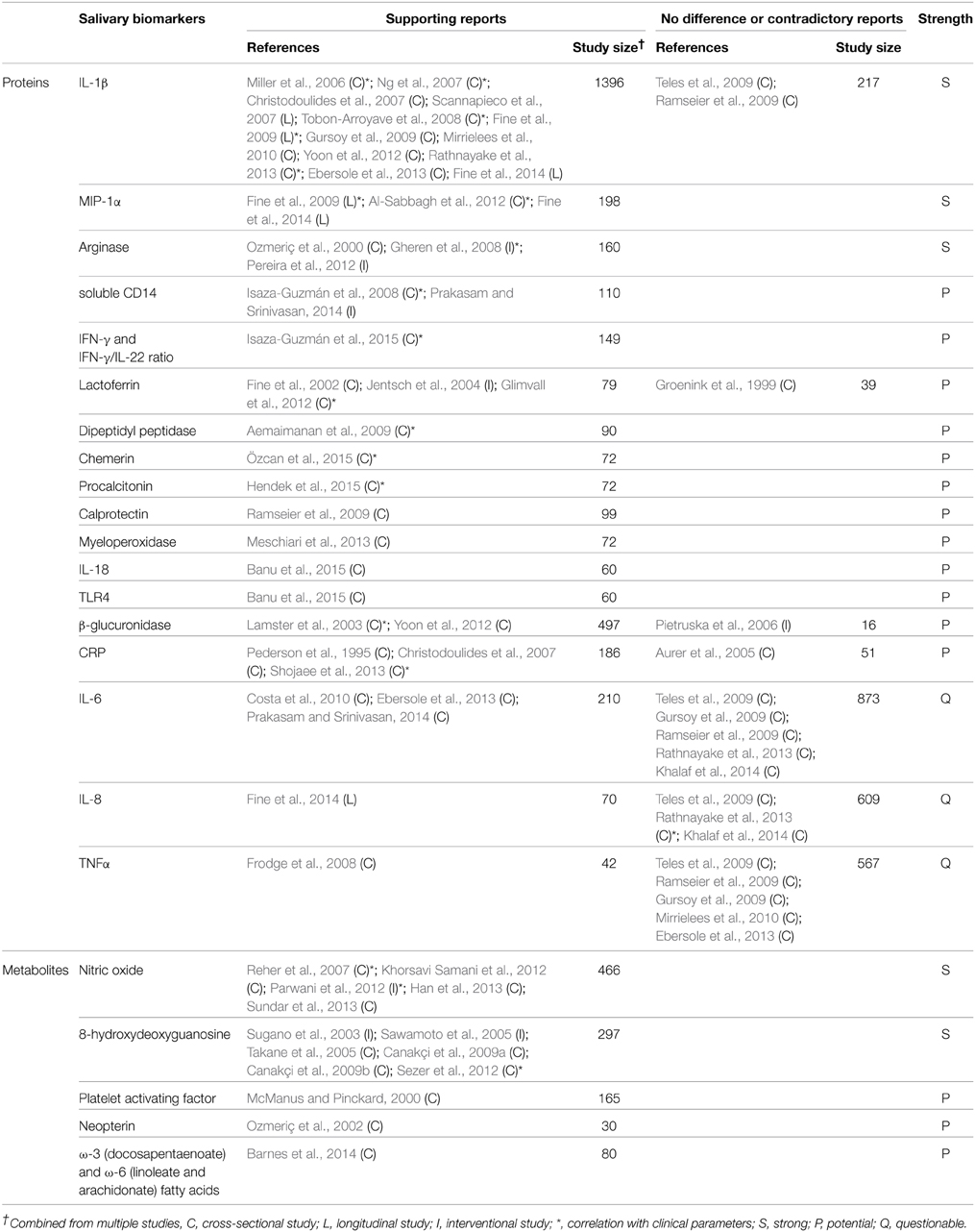
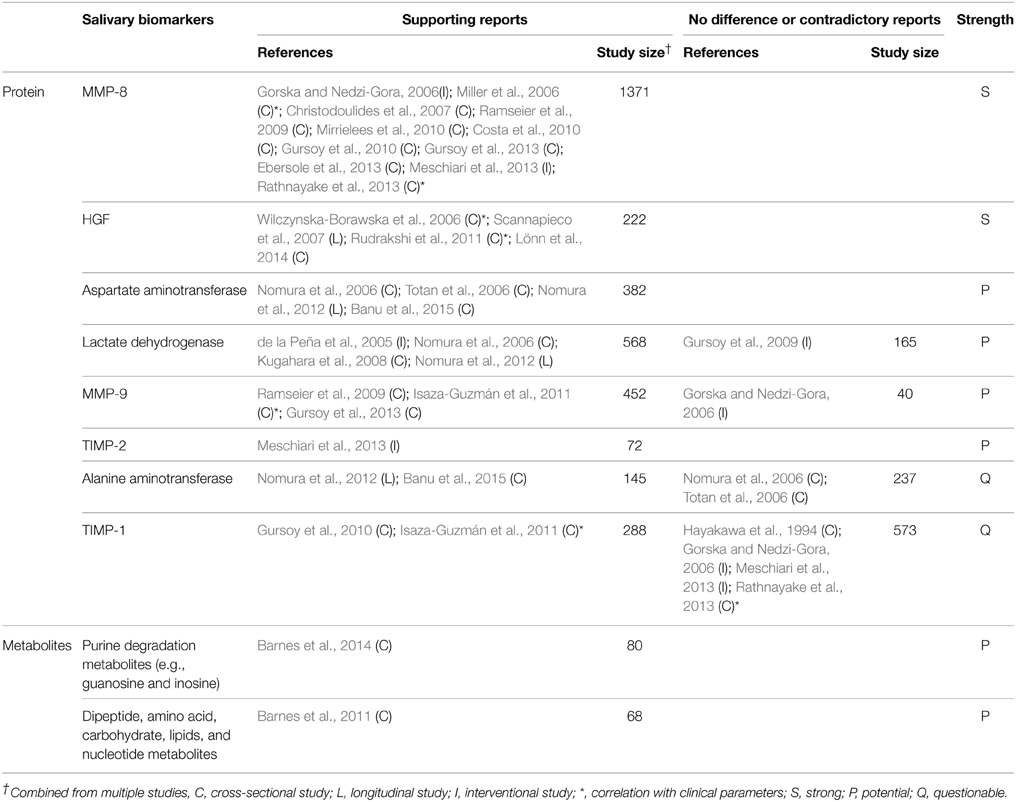
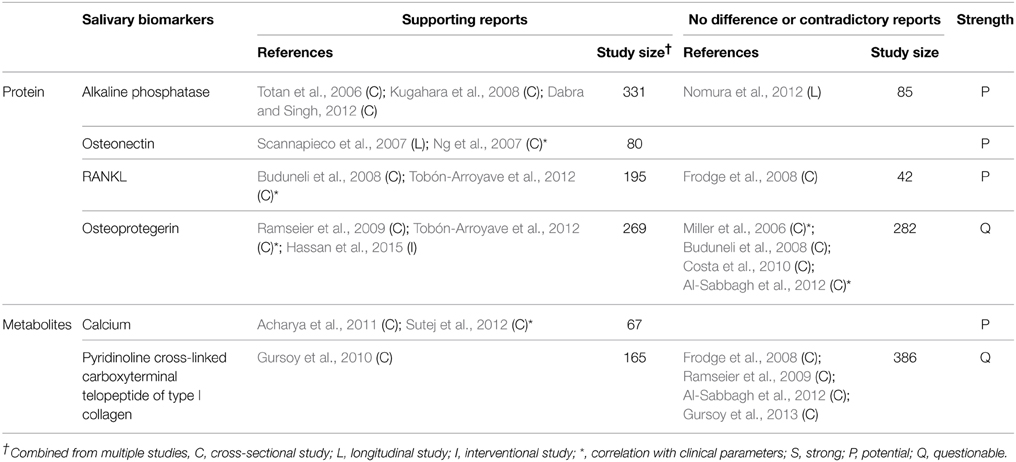
Ideal biomarkers of periodontitis must be able to (1) diagnose the presence of periodontal disease, (2) reflect the severity of the disease (3) monitor the response of the disease to treatment, and (4) predict the prognosis/progress of the disease. A number of biomarkers that satisfy at least one of the four requirements have been identified in saliva (Tables 1–4). Salivary biomarkers of periodontal disease can originate from both bacteria and the host. As periodontitis progresses, gingival inflammation, soft tissue destruction, and bone destruction occur sequentially and release associated proteins or metabolites into the saliva. Therefore, host-derived biomarkers are categorized according to whether they reflect inflammation, soft tissue destruction, or bone destruction. The biomarkers that satisfy three of the four requirements in at least three separate studies are classified as strong (S) biomarkers. When the number of studies that reported no difference or contradictory results is equal or greater than those with supporting results, the biomarkers are classified as questionable (Q). The remaining biomarkers are classified as potential (P).
Bacteria-derived Biomarkers
Bacteria-derived biomarkers include DNA and proteins. The levels of well-known pathogenic bacteria, such as Aggregatibacter actinomycetemcomitans, the three red complex species, and several species of the orange complex in saliva were determined by targeting a specific area of the 16S rRNA gene (Table 1). Among them, only Porphyromonas gingivalis, Prevotella intermedia, and Tannerella forsythia have been proved by multiple studies as strong biomarkers of periodontitis. Recent studies using high-throughput sequencing of the 16S rRNA gene have identified new species/phylotypes that are associated with periodontitis (Griffen et al., 2012; Göhler et al., 2014). Given the complexity of dental biofilm, the potential of the newly identified species/phylotypes to serve as salivary biomarkers of periodontitis needs to be investigated. The activity of dipeptidyl peptidase IV in saliva has been shown to be associated with periodontitis and the presence of P. gingivalis (Aemaimanan et al., 2009). Dipeptidyl peptidase IV is a serine protease that cleaves X-Pro dipeptide from the N-terminus of polypeptide chains, thus contributing to collagen degradation (Banbula et al., 2000). DPP4 in saliva may originate from both the host and bacteria, including P. gingivalis (Aemaimanan et al., 2009).
Host-derived Inflammatory Biomarkers
Periodontitis begins with inflammation of the gingival tissue in response to dental biofilm. As inflammatory biomarkers in saliva, diverse enzymes (arginase, dipeptidyl peptidase IV, β-glucuronidase, and myeloperoxidase), anti-microbial proteins (lactoferrin and calprotectin), inflammatory cytokines (IL-1β, IL-6, IL-18, IFN-γ, and MIP-1α), and proteins that mediate inflammation (chemerin, CRP, TLR4, soluble CD14, and procalcitonin) have been studied (Table 2). Particularly, IL-1β, MIP-1α, and arginase are strong biomarkers that correlate with inflammatory parameters of periodontitis, such as the gingival index or BOP (Miller et al., 2006; Gheren et al., 2008; Al-Sabbagh et al., 2012; Rathnayake et al., 2013). In addition to protein biomarkers, nitric oxide, 8-hydroxydeoxyguanosine, platelet activating factor and fatty acid metabolites (neopterin, docosapentaenoate, linoleate, and arachidonate) have been identified as inflammation-associated biomarkers in saliva (Table 2).
Host-derived Biomarkers Associated with Soft Tissue Destruction
As periodontitis progresses, soft tissues are destroyed, releasing several enzymes and proteins that are involved in tissue destruction into the saliva. Among them, MMP-8, MMP-9, HGF, lactate dehydrogenase, aspartate aminotransferase, and TIMP-2 are strong or potential biomarkers of periodontitis (Table 3). In addition, a recent metabolomic profiling of saliva revealed increased amounts of metabolites originated from macromolecular degradation, including dipeptides (proteins), oligo/mono-saccharides (polysaccharides), lysolipids, fatty acids, and monoacylglycerol (glycerophospholipid and triacylglycerol), and uridine (DNA/RNA) in periodontitis (Table 3).
Host-derived Biomarkers Associated with Bone Destruction
Salivary biomarkers of bone remodeling can be used as indicators of bone destruction in periodontitis. These include alkaline phosphatase, osteonectin, RANKL, and calcium (Table 4). A positive correlation between the levels of salivary calcium and CAL has been reported (Sutej et al., 2012).
Further Considerations
Among the various salivary biomarkers listed, P. gingivalis has been shown to satisfy all four requirements of ideal biomarkers for periodontitis in at least one study for each requirement. However, single biomarker detection may not be effective enough for accurate diagnoses without false-positive or false-negative results. Periodontitis is a disease that involves complex interactions between bacteria and the host immune system. The combination of the host-derived biomarkers, which reflect inflammation, soft tissue destruction, and bone destruction together with bacteria-derived biomarkers, may be useful to diagnose not only the presence of periodontitis but also the degree of progression and the response to therapy. A number of host-derived biomarkers have already shown strong associations with periodontitis.
The fact that the concentration of biomarkers can be affected by the saliva flow rate, circadian rhythm, age, the physiological status of the patients, and other factors raises concern over the accuracy and reproducibility of diagnoses using salivary biomarkers (Nový, 2014). Although within-subject correlations between unstimulated and stimulated samples and over time have been reported for some salivary proteins (Rudney et al., 1985), such studies have not been done for all salivary proteomes or metabolomes. Nevertheless, many biomarkers, in numerous studies, have shown consistent associations with periodontitis. For example, significantly higher levels of salivary MMP-8 in periodontitis than in healthy controls were observed in six studies that used the non-stimulatory whole saliva samples (Miller et al., 2006; Christodoulides et al., 2007; Ramseier et al., 2009; Costa et al., 2010; Mirrielees et al., 2010; Ebersole et al., 2013) and in five studies that used stimulated whole saliva samples (Gorska and Nedzi-Gora, 2006; Gursoy et al., 2010, 2013; Meschiari et al., 2013; Rathnayake et al., 2013). These findings suggest that within-subject variations in the concentration of salivary biomarkers can be overcome for diagnostic purposes if a biomarker with substantial inter-group differences is chosen.
POC Devices in Periodontology
A few POC devices have been developed for the salivary diagnosis of periodontitis. A device called the Integrated Microfluidic Platform for Oral Diagnostics (IMPOD) was able to detect salivary proteins with a low sample volume requirements (10 μL) and considerable sensitivity (nM-pM) by integrating sample pretreatment (filtering, enrichment, mixing) with electrophoretic immunoassays and a laser-induced fluorescence detection system. Using this device, rapid (< 10 min) measurements of MMP-8, TNF-α, IL-6, and CRP in saliva were performed (Herr et al., 2007a,b). However, validation in the clinical setting has not yet been reported.
A group at the University of Texas at Austin developed a lab-on-a-chip (LOC) system that integrates microfluidics and a fluorescence-based optical system in which sandwich immunoassays are performed on chemically sensitized beads. They reported the application of the LOC system for the multiplex measurement of three salivary biomarkers, C-reactive protein, MMP-8, and IL-1β, which are related to the clinical expression of periodontitis. The LOC approach yielded a limit of detection five orders of magnitude lower than that of a standard ELISA, and the results obtained by the LOC approach were consistent with ELISA results (Christodoulides et al., 2007). Whether the POC device using this LOC approach can accurately measure levels of the salivary biomarker MMP-8 and thus indicate if a patient has periodontal health, gingivitis or periodontal disease is currently being studied in a clinical trial (NCT02403297 at ClinicalTrials.gov).
Suggestions for Organizing the International Consortium for Salivary Biomarkers of Periodontitis
For the success of POC diagnostics of periodontitis using saliva, it is important to validate the candidate biomarkers with large populations which suitably account for diversity such as those related to race, region, gender, and age. In periodontal research, full-mouth or selected teeth have been examined to diagnose periodontitis, and different criteria have been also used to classify the severity of periodontitis. Such variations in the classifications of patients make it difficult to integrate the results of different studies. Furthermore, variations in saliva sampling methods (e.g., resting vs. stimulatory, whole vs. individual gland sampling), target biomarkers, and detection methods of salivary biomarkers prevent direct comparisons of the data obtained from different studies or centers. We propose the organization of an International Consortium for Salivary Biomarkers of Periodontitis (ICSBP). The ICSBP can put forth collaborative efforts to create standardized protocols for clinical research, including a uniform method for clinical diagnoses of periodontitis. In addition, the ICSBP can accelerate the validation of biomarkers and the implementation of salivary diagnostics by sharing clinical samples and experience.
Funding
This work was supported by the National Research Foundation of Korea (Daejun, Korea) Grant 2014050477 funded by the Korean Government through the Oromaxillofacial Dysfunction Research Center for the Elderly at Seoul National University and by Basic Science Research Program through the National Research Foundation grants 2013R1A1A3005669 and 2014R1A1A1005507.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Acharya, A., Kharadi, M. D., Dhavale, R., Deshmukh, V. L., and Sontakke, A. N. (2011). High salivary calcium level associated with periodontal disease in Indian subjects–a pilot study. Oral Health Prev. Dent. 9, 195–200.
Aemaimanan, P., Sattayasai, N., Wara-aswapati, N., Pitiphat, W., Suwannarong, W., Prajaneh, S., et al. (2009). Alanine aminopeptidase and dipeptidyl peptidase IV in saliva of chronic periodontitis patients. J. Periodontol. 80, 1809–1814. doi: 10.1902/jop.2009.090233
AlMoharib, H. S., AlMubarak, A., AlRowis, R., Geevarghese, A., Preethanath, R. S., and Anil, S. (2014). Oral fluid based biomarkers in periodontal disease: part 1. Saliva. J. Int. Oral Health 6, 95–103.
Al-Sabbagh, M., Alladah, A., Lin, Y., Kryscio, R. J., Thomas, M. V., Ebersole, J. L., et al. (2012). Bone remodeling-associated salivary biomarker MIP-1α distinguishes periodontal disease from health. J. Periodontal Res. 47, 389–395. doi: 10.1111/j.1600-0765.2011.01445.x
Aurer, A., Jorgic-Srdjak, K., Plancak, D., Stavljenic-Rukavina, A., and Aurer-Kozelj, J. (2005). Proinflammatory factors in saliva as possible markers for periodontal disease. Coll. Antropol. 29, 435–439.
Banbula, A., Bugno, M., Goldstein, J., Yen, J., Nelson, D., Travis, J., et al. (2000). Emerging family of proline-specific peptidases of Porphyromonas gingivalis: purification and characterization of serine dipeptidyl peptidase, a structural and functional homologue of mammalian prolyl dipeptidyl peptidase IV. Infect. Immun. 68, 1176–1182. doi: 10.1128/IAI.68.3.1176-1182.2000
Banu, S., Jabir, N. R., Mohan, R., Manjunath, N. C., Kamal, M. A., Kumar, K. R., et al. (2015). Correlation of Toll-like receptor 4, interleukin-18, transaminases, and uric acid in patients with chronic periodontitis and healthy adults. J. Periodontol. 86, 431–439. doi: 10.1902/jop.2014.140414
Barnes, V. M., Ciancio, S. G., Shibly, O., Xu, T., Devizio, W., Trivedi, H. M., et al. (2011). Metabolomics reveals elevated macromolecular degradation in periodontal disease. J. Dent. Res. 90, 1293–1297. doi: 10.1177/0022034511416240
Barnes, V. M., Kennedy, A. D., Panagakos, F., Devizio, W., Trivedi, H. M., Jönsson, T., et al. (2014). Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS ONE 18:e105181. doi: 10.1371/journal.pone.0105181
Buduneli, N., Biyikoglu, B., Sherrabeh, S., and Lappin, D. F. (2008). Saliva concentrations of RANKL and osteoprotegerin in smoker versus non-smoker chronic periodontitis patients. J. Clin. Periodontol. 35, 846–852. doi: 10.1111/j.1600-051X.2008.01310.x
Canakçi, C. F., Canakçi, V., Tatar, A., Eltas, A., Sezer, U., Ciçek, Y., et al. (2009a). Increased salivary level of 8-hydroxydeoxyguanosine is a marker of premature oxidative mitochondrial DNA damage in gingival tissue of patients with periodontitis. Arch. Immunol. Ther. Exp. 57, 205–211. doi: 10.1007/s00005-009-0026-9
Canakçi, C. F., Cicek, Y., Yildirim, A., Sezer, U., and Canakci, V. (2009b). Increased levels of 8-hydroxydeoxyguanosine and malondialdehyde and its relationship with antioxidant enzymes in saliva of periodontitis patients. Eur. J. Dent. 3, 100–106.
Christodoulides, N., Floriano, P. N., Miller, C. S., Ebersole, J. L., Mohanty, S., Dharshan, P., et al. (2007). Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann. N. Y. Acad. Sci. 1098, 411–428. doi: 10.1196/annals.1384.035
Costa, P. P., Trevisan, G. L., Macedo, G. O., Palioto, D. B., Souza, S. L., Grisi, M. F., et al. (2010). Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. J. Periodontol. 81, 384–391. doi: 10.1902/jop.2009.090510
Cuevas-Córdoba, B., and Santiago-García, J. (2014). Saliva: a fluid of study for OMICS. OMICS 18, 87–97. doi: 10.1089/omi.2013.0064
Dabra, S., and Singh, P. (2012). Evaluating the levels of salivary alkaline and acid phosphatase activities as biochemical markers for periodontal disease: a case series. Dent. Res. J. 9, 41–45. doi: 10.4103/1735-3327.92942
de la Peña, V. A., Dios, P. D., Rodríguez-Nuñez, I., and Rodríguez-Segade, S. (2005). Effect of ultrasonic scaling on salivary lactate dehydrogenase. Am. J. Dent. 18, 113–115.
de la Rica, R., and Stevens, M. M. (2012). Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 7, 821–824. doi: 10.1038/nnano.2012
Ebersole, J. L., Schuster, J. L., Stevens, J., Dawson, D. III. Kryscio, R. J., Lin, Y., et al. (2013). Patterns of salivary analytes provide diagnostic capacity for distinguishing chronic adult periodontitis from health. J. Clin. Immunol. 33, 271–279. doi: 10.1007/s10875-012-9771-3
Fine, D. H., Furgang, D., and Beydouin, F. (2002). Lactoferrin iron levels are reduced in saliva of patients with localized aggressive periodontitis. J. Periodontol. 73, 624–630. doi: 10.1902/jop.2002.73.6.624
Fine, D. H., Markowitz, K., Fairlie, K., Tischio-Bereski, D., Ferrandiz, J., Godboley, D., et al. (2014). Macrophage inflammatory protein-1α shows predictive value as a risk marker for subjects and sites vulnerable to bone loss in a longitudinal model of aggressive periodontitis. PLoS ONE 5:e98541. doi: 10.1371/journal.pone.0098541
Fine, D. H., Markowitz, K., Furgang, D., Fairlie, K., Ferrandiz, J., Nasri, C., et al. (2009). Macrophage inflammatory protein-1alpha: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J. Periodontol. 80, 106–113. doi: 10.1902/jop.2009.080296
Frodge, B. D., Ebersole, J. L., Kryscio, R. J., Thomas, M. V., and Miller, C. S. (2008). Bone remodeling biomarkers of periodontal disease in saliva. J. Periodontol. 79, 1913–1919. doi: 10.1902/jop.2008.080070
Gaster, R. S., Hall, D. A., Nielsen, C. H., Osterfeld, S. J., Yu, H., Mach, K. E., et al. (2009). Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat. Med. 15, 1327–1332. doi: 10.1038/nm.2032
Gheren, L. W., Cortelli, J. R., Rodrigues, E., Holzhausen, M., and Saad, W. A. (2008). Periodontal therapy reduces arginase activity in saliva of patients with chronic periodontitis. Clin. Oral Investig. 12, 67–72. doi: 10.1007/s00784-007-0146-8
Glimvall, P., Wickström, C., and Jansson, H. (2012). Elevated levels of salivary lactoferrin, a marker for chronic periodontitis? J. Periodontal Res. 47, 655–660. doi: 10.1111/j.1600-0765.2012.01479.x
Göhler, A., Hetzer, A., Holtfreter, B., Geisel, M. H., Schmidt, C. O., Steinmetz, I., et al. (2014). Quantitative molecular detection of putative periodontal pathogens in clinically healthy and periodontally diseased subjects. PLoS ONE 9:e99244. doi: 10.1371/journal.pone.0099244
Górska, R., and Nedzi-Góra, M. (2006). The effects of the initial treatment phase and of adjunctive low-dose doxycycline therapy on clinical parameters and MMP-8, MMP-9, and TIMP-1 levels in the saliva and peripheral blood of patients with chronic periodontitis. Arch. Immunol. Ther. Exp. 54, 419–426. doi: 10.1007/s00005-006-0047-6
Griffen, A. L., Beall, C. J., Campbell, J. H., Firestone, N. D., Kumar, P. S., Yang, Z. K., et al. (2012). Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 6, 1176–1185. doi: 10.1038/ismej.2011.191
Groenink, J., Walgreen-Weterings, E., Nazmi, K., Bolscher, J. G., Veerman, E. C., van Winkelhoff, A. J., et al. (1999). Salivary lactoferrin and low-Mr mucin MG2 in Actinobacillus actinomycetemcomitans-associated periodontitis. J. Clin. Periodontol. 26, 269–275. doi: 10.1034/j.1600-051X.1999.260501.x
Gubala, V., Harris, L. F., Ricco, A. J., Tan, M. X., and Williams, D. E. (2012). Point of care diagnostics: status and future. anal chem. point of care diagnostics: status and future. Anal. Chem. 84, 487–515. doi: 10.1021/ac2030199
Gursoy, U. K., Könönen, E., Huumonen, S., Tervahartiala, T., Pussinen, P. J., Suominen, A. L., et al. (2013). Salivary type I collagen degradation end-products and related matrix metalloproteinases in periodontitis. J. Clin. Periodontol. 40, 18–25. doi: 10.1111/jcpe.12020
Gursoy, U. K., Könönen, E., Pradhan-Palikhe, P., Tervahartiala, T., Pussinen, P. J., Suominen-Taipale, L., et al. (2010). Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J. Clin. Periodontol. 37, 487–493. doi: 10.1111/j.1600-051X.2010.01563.x
Gursoy, U. K., Könönen, E., Uitto, V. J., Pussinen, P. J., Hyvärinen, K., Suominen-Taipale, L., et al. (2009). Salivary interleukin-1beta concentration and the presence of multiple pathogens in periodontitis. J. Clin. Periodontol. 36, 922–927. doi: 10.1111/j.1600-051X.2009.01480.x
Halpin, S. T., and Spence, D. M. (2010). Direct plate-reader measurement of nitric oxide released from hypoxic erythrocytes flowing through a microfluidic device. Anal. Chem. 82, 7492–7497. doi: 10.1021/ac101130s
Han, D. H., Kim, M. S., Shin, H. S., Park, K. P., and Kim, H. D. (2013). Association between periodontitis and salivary nitric oxide metabolites among community elderly Koreans. J. Periodontol. 84, 776–784. doi: 10.1902/jop.2012.120237
Hassan, S. H., El-Refai, M. I., Ghallab, N. A., Kasem, R. F., and Shaker, O. G. (2015). Effect of periodontal surgery on osteoprotegerin levels in gingival crevicular fluid, saliva, and gingival tissues of chronic periodontitis patients. Dis. Markers 2015:341259. doi: 10.1155/2015/341259
Hayakawa, H., Yamashita, K., Ohwaki, K., Sawa, M., Noguchi, T., Iwata, K., et al. (1994). Collagenase activity and tissue inhibitor of metalloproteinases-1 (TIMP-1) content in human whole saliva from clinically healthy and periodontally diseased subjects. J. Periodontal Res. 29, 305–308. doi: 10.1111/j.1600-0765.1994.tb01226.x
Hendek, M. K., Erdemir, E. O., and Kisa, U. (2015). Evaluation of salivary procalcitonin levels in different periodontal diseases. J. Periodontol. 86, 820–826. doi: 10.1902/jop.2015.130751
Herr, A. E., Hatch, A. V., Giannobile, W. V., Throckmorton, D. J., Tran, H. M., Brennan, J. S., et al. (2007a). Integrated microfluidic platform for oral diagnostics. Ann. N.Y. Acad. Sci. 1098, 362–374. doi: 10.1196/annals.1384.004
Herr, A. E., Hatch, A. V., Throckmorton, D. J., Tran, H. M., Brennan, J. S., Giannobile, W. V., et al. (2007b). Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc. Natl. Acad. Sci. U.S.A. 27, 5268–5273. doi: 10.1073/pnas.0607254104
Isaza-Guzmán, D. M., Arias-Osorio, C., Martínez-Pabón, M. C., and Tobón-Arroyave, S. I. (2011). Salivary levels of matrix metalloproteinase (MMP)-9 and tissue inhibitor of matrix metalloproteinase (TIMP)-1: a pilot study about the relationship with periodontal status and MMP-9(- 1562C/T) gene promoter polymorphism. Arch. Oral Biol. 56, 401–411. doi: 10.1016/j.archoralbio.2010.10.021
Isaza-Guzmán, D. M., Aristizábal-Cardona, D., Martínez-Pabón, M. C., Velásquez-Echeverri, H., and Tobón-Arroyave, S. I. (2008). Estimation of sCD14 levels in saliva obtained from patients with various periodontal conditions. Oral Dis. 14, 450–456. doi: 10.1111/j.1601-0825.2007.01400.x
Isaza-Guzmán, D. M., Cardona-Vélez, N., Gaviria-Correa, D. E., Martínez-Pabón, M. C., Castaño-Granada, M. C., and Tobón-Arroyave, S. I. (2015). Association study between salivary levels of interferon (IFN)-gamma, interleukin (IL)-17, IL-21, and IL-22 with chronic periodontitis. Arch. Oral Biol. 60, 91–99. doi: 10.1016/j.archoralbio.2014.09.002
Jentsch, H., Sievert, Y., and Göcke, R. (2004). Lactoferrin and other markers from gingival crevicular fluid and saliva before and after periodontal treatment. J. Clin. Periodontol. 31, 511–514. doi: 10.1111/j.1600-051X.2004.00512.x
Ji, S., Choi, Y. S., and Choi, Y. (2014). Bacterial invasion and persistence: critical events in the pathogenesis of periodontitis? J. Periodontal Res. doi: 10.1111/jre.12248. [Epub ahead of print].
Katsarelis, H., Shah, N. P., Dhariwal, D. K., and Pazianas, M. (2015). Infection and medication-related osteonecrosis of the jaw. J. Dent. Res. 94, 534–539. doi: 10.1177/0022034515572021
Khalaf, H., Lönn, J., and Bengtsson, T. (2014). Cytokines and chemokines are differentially expressed in patients with periodontitis: possible role for TGF-β1 as a marker for disease progression. Cytokine 67, 29–35. doi: 10.1016/j.cyto.2014.02.007
Khorsavi Samani, M., Poorsattar Bejeh Mir, A., Kashiri, M., and Gujeq, D. (2012). Introducing cut-points for salivary nitric oxide to distinguish periodontitis from the normal periodontium. Minerva Stomatol. 61, 443–448.
Kobayashi, T., and Yoshie, H. (2015). Host responses in the link between periodontitis and rheumatoid arthritis. Curr. Oral Health Rep. 2, 1–8. doi: 10.1007/s40496-014-0039-2
Kugahara, T., Shosenji, Y., and Ohashi, K. (2008). Screening for periodontitis in pregnant women with salivary enzymes. J. Obstet. Gynaecol. Res. 34, 40–46. doi: 10.1111/j.1447-0756.2007.00681.x
Lamster, I. B., Kaufman, E., Grbic, J. T., Winston, L. J., and Singer, R. E. (2003). β-glucuronidase activity in saliva: relationship to clinical periodontal parameters. J. Periodontol. 74, 353–359. doi: 10.1902/jop.2003.74.3.353
Lang, N. P., Adler, R., Joss, A., and Nyman, S. (1990). Absence of bleeding on probing. An indicator of periodontal stability. J. Clin. Periodontol. 17, 714–721. doi: 10.1111/j.1600-051X.1990.tb01059.x
Lauks, I. R. (1998). Microfabricated biosensors and microanalytical systems for blood analysis. Acc. Chem. Res. 31, 317–324. doi: 10.1021/ar9700670
Lönn, J., Johansson, C. S., Nakka, S., Palm, E., Bengtsson, T., Nayeri, F., et al. (2014). High concentration but low activity of hepatocyte growth factor in periodontitis. J. Periodontol. 85, 113–122. doi: 10.1902/jop.2013.130003
McManus, L. M., and Pinckard, R. N. (2000). PAF, a putative mediator of oral inflammation. Crit. Rev. Oral Biol. Med. 11, 240–258. doi: 10.1177/10454411000110020701
Meschiari, C. A., Marcaccini, A. M., Santos Moura, B. C., Zuardi, L. R., Tanus-Santos, J. E., and Gerlach, R. F. (2013). Salivary MMPs, TIMPs, and MPO levels in periodontal disease patients and controls. Clin. Chim. Acta. 5, 140–146. doi: 10.1016/j.cca.2013.03.008
Miller, C. S., Foley, J. D., Bailey, A. L., Campell, C. L., Humphries, R. L., Christodoulides, N., et al. (2010). Current developments in salivary diagnostics. Biomark. Med. 4, 171–189. doi: 10.2217/bmm.09.68
Miller, C. S., King, C. P. Jr. Langub, M. C., Kryscio, R. J., and Thomas, M. V. (2006). Salivary biomarkers of existing periodontal disease: a cross-sectional study. J. Am. Dent. Assoc. 137, 322–329. doi: 10.14219/jada.archive.2006.0181
Mirrielees, J., Crofford, L. J., Lin, Y., Kryscio, R. J., Dawson, D. R. III, Ebersole, J. L., et al. (2010). Rheumatoid arthritis and salivary biomarkers of periodontal disease. J. Clin. Periodontol. 37, 1068–1074. doi: 10.1111/j.1600-051X.2010.01625.x
Ng, P. Y., Donley, M., Hausmann, E., Hutson, A. D., Rossomando, E. F., and Scannapieco, F. A. (2007). Candidate salivary biomarkers associated with alveolar bone loss: cross-sectional and in vitro studies. FEMS Immunol. Med. Microbiol. 49, 252–260. doi: 10.1111/j.1574-695X.2006.00187.x
Nomura, Y., Shimada, Y., Hanada, N., Numabe, Y., Kamoi, K., Sato, T., et al. (2012). Salivary biomarkers for predicting the progression of chronic periodontitis. Arch. Oral Biol. 57, 413–420. doi: 10.1016/j.archoralbio.2011.09.011
Nomura, Y., Tamaki, Y., Tanaka, T., Arakawa, H., Tsurumoto, A., Kirimura, K., et al. (2006). Screening of periodontitis with salivary enzyme tests. J. Oral Sci. 48, 177–183. doi: 10.2334/josnusd.48.177
Nový, B. B. (2014). Saliva and biofilm-based diagnostics: a critical review of the literature concerning sialochemistry. J. Evid. Based Dent. Pract. 14(Suppl.), 27–32. doi: 10.1016/j.jebdp.2014.04.004
Özcan, E., Saygun, N. I., Serdar, M. A., and Kurt, N. (2015). Evaluation of the salivary levels of visfatin, chemerin, and progranulin in periodontal inflammation. Clin. Oral Investig. 19, 921–928. doi: 10.1007/s00784-014-1308-0
Ozmeriç, N., Elgün, S., and Uraz, A. (2000). Salivary arginase in patients with adult periodontitis. Clin. Oral Investig. 4, 21–24. doi: 10.1007/s007840050108
Ozmeriç, N., Baydar, T., Bodur, A., Engin, A. B., Uraz, A., Eren, K., et al. (2002). Level of neopterin, a marker of immune cell activation in gingival crevicular fluid, saliva, and urine in patients with aggressive periodontitis. J. Periodontol. 73, 720–725. doi: 10.1902/jop.2002.73.7.720
Parwani, S. R., Chitnis, P. J., and Parwani, R. N. (2012). Salivary nitric oxide levels in inflammatory periodontal disease - a case-control and interventional study. Int. J. Dent. Hyg. 10, 67–73. doi: 10.1111/j.1601-5037.2011.00508.x
Pederson, E. D., Stanke, S. R., Whitener, S. J., Sebastiani, P. T., Lamberts, B. L., and Turner, D. W. (1995). Salivary levels of alpha 2-macroglobulin, alpha 1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Arch. Oral Biol. 40, 1151–1155. doi: 10.1016/0003-9969(95)00089-5
Pereira, A. L., Cortelli, S. C., Aquino, D. R., Franco, G. C., Cogo, K., Rodrigues, E., et al. (2012). Reduction of salivary arginine catabolic activity through periodontal therapy. Quintessence Int. 43, 777–787.
Pietruska, M., Bernaczyk, A., Knas, M., Pietruski, J., and Zwierz, K. (2006). Assessment of salivary levels of the chosen exoglycosidases in patients with aggressive periodontitis after treatment with doxycycline. Adv. Med. Sci. 51, 158–161.
Prakasam, S., and Srinivasan, M. (2014). Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral Dis. 20, 171–177. doi: 10.1111/odi.12085
Ramseier, C. A., Kinney, J. S., Herr, A. E., Braun, T., Sugai, J. V., Shelburne, C. A., et al. (2009). Identification of pathogen and host-response markers correlated with periodontal disease. J. Periodontol. 80, 436–446. doi: 10.1902/jop.2009.080480
Rathnayake, N., Akerman, S., Klinge, B., Lundegren, N., Jansson, H., Tryselius, Y., et al. (2013). Salivary biomarkers of oral health: a cross-sectional study. J. Clin. Periodontol. 40, 140–147. doi: 10.1111/jcpe.12038
Reher, V. G., Zenóbio, E. G., Costa, F. O., Reher, P., and Soares, R. V. (2007). Nitric oxide levels in saliva increase with severity of chronic periodontitis. J. Oral Sci. 49, 271–276. doi: 10.2334/josnusd.49.271
Rissin, D. M., Kan, C. W., Campbell, T. G., Howes, S. C., Fournier, D. R., Song, L., et al. (2010). Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599. doi: 10.1038/nbt.1641
Rudney, J. D., Kajander, K. C., and Smith, Q. T. (1985). Correlations between human salivary levels of lysozyme, lactoferrin, salivary peroxidase and secretory immunoglobulin A with different stimulatory states and over time. Arch. Oral Biol. 30, 765–771. doi: 10.1016/0003-9969(85)90129-3
Rudrakshi, C., Srinivas, N., and Mehta, D. S. (2011). A comparative evaluation of hepatocyte growth factor levels in gingival crevicular fluid and saliva and its correlation with clinical parameters in patients with and without chronic periodontitis: a clinico-biochemical study. J. Indian Soc. Periodontol. 15, 147–151. doi: 10.4103/0972-124X.84384
Sackmann, E. K., Fulton, A. L., and Beebe, D. J. (2014). The present and future role of microfluidics in biomedical research. Nature 13, 181–189. doi: 10.1038/nature13118
Salvi, G. E., Lindhe, J., and Lang, N. P. (2008). “Examination of patients with periodontal diseases,” in Clinical Periodontology and Implant Dentistry, eds N. P. Lang and J. Lindhe (Oxford: Munksgaard), 573–586.
Sawamoto, Y., Sugano, N., Tanaka, H., and Ito, K. (2005). Detection of periodontopathic bacteria and an oxidative stress marker in saliva from periodontitis patients. Oral Microbiol. Immunol. 20, 216–220. doi: 10.1111/j.1399-302X.2005.00215.x
Saygun, I., Nizam, N., Keskiner, I., Bal, V., Kubar, A., Açikel, C., et al. (2011). Salivary infectious agents and periodontal disease status. J. Periodontal Res. 46, 235–239. doi: 10.1111/j.1600-0765.2010.01335.x
Scannapieco, F. A. (2005). Systemic effects of periodontal diseases. Dent. Clin. North Am. 49, 533–550. doi: 10.1016/j.cden.2005.03.002
Scannapieco, F. A., Ng, P., Hovey, K., Hausmann, E., Hutson, A., and Wactawski-Wende, J. (2007). Salivary biomarkers associated with alveolar bone loss. Ann. N.Y. Acad. Sci. 1098, 496–497. doi: 10.1196/annals.1384.034
Sezer, U., Ciçek, Y., and Canakçi, C. F. (2012). Increased salivary levels of 8-hydroxydeoxyguanosine may be a marker for disease activity for periodontitis. Dis. Markers 32, 165–172. doi: 10.3233/DMA-2011-0876
Shojaee, M., Fereydooni Golpasha, M., Maliji, G., Bijani, A., Aghajanpour Mir, S. M., and Mousavi Kani, S. N. (2013). C – reactive protein levels in patients with periodontal disease and normal subjects. Int. J. Mol. Cell. Med. 2, 151–155.
Sia, S. K., and Chin, C. D. (2011). Analytical chemistry: sweet solution to sensing. Nature Chem. 3, 659–660. doi: 10.1038/nchem.1119
Song, Y., Huang, Y. Y., Liu, X., Zhang, X., Ferrari, M., and Qin, L. (2014). Point-of-care technologies for molecular diagnostics using a drop of blood. Trends. Biotechnol. 32, 132–139. doi: 10.1016/j.tibtech.2014.01.003
Souto, R., Silva-Boghossian, C. M., and Colombo, A. P. (2014). Prevalence of Pseudomonas aeruginosa and Acinetobacter spp. in subgingival biofilm and saliva of subjects with chronic periodontal infection. Braz. J. Microbiol. 45, 495–501. doi: 10.1590/S1517-83822014000200017
Su, W., Gao, X., Jiang, L., and Qin, J. (2015). Microfluidic platform towards point-of-care diagnostics in infectious diseases. J. Chromatogr. A 16, 13–26. doi: 10.1016/j.chroma.2014.12.041
Sugano, N., Yokoyama, K., Oshikawa, M., Kumagai, K., Takane, M., Tanaka, H., et al. (2003). Detection of Streptococcus anginosus and 8-hydroxydeoxyguanosine in saliva. J. Oral Sci. 45, 181–184. doi: 10.2334/josnusd.45.181
Sundar, N. M., Krishnan, V., Krishnaraj, S., Hemalatha, V. T., and Alam, M. N. (2013). Comparison of the salivary and the serum nitric oxide levels in chronic and aggressive periodontitis: a biochemical study. J. Clin. Diagn. Res. 7, 1223–1227. doi: 10.7860/JCDR/2013/5386.3068
Sutej, I., Peros, K., Benutic, A., Capak, K., Basic, K., and Rosin-Grget, K. (2012). Salivary calcium concentration and periodontal health of young adults in relation to tobacco smoking. Oral Health Prev. Dent. 10, 397–403.
Takane, M., Sugano, N., Ezawa, T., Uchiyama, T., and Ito, K. (2005). A marker of oxidative stress in saliva: association with periodontally-involved teeth of a hopeless prognosis. J. Oral Sci. 47, 53–57. doi: 10.2334/josnusd.47.53
Taylor, J. J. (2014). Protein biomarkers of periodontitis in saliva. ISRN Inflamm. 22, 593151. doi: 10.1155/2014/593151
Teles, R. P., Likhari, V., Socransky, S. S., and Haffajee, A. D. (2009). Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J. Periodontal Res. 44, 411–417. doi: 10.1111/j.1600-0765.2008.01119.x
Tobón-Arroyave, S. I., Isaza-Guzmán, D. M., Restrepo-Cadavid, E. M., Zapata-Molina, S. M., and Martínez-Pabón, M. C. (2012). Association of salivary levels of the bone remodelling regulators sRANKL and OPG with periodontal clinical status. J. Clin. Periodontol. 39, 1132–1140. doi: 10.1111/jcpe.12012
Tobon-Arroyave, S. I., Jaramillo-Gonzalez, P. E., and Isaza-Guzman, D. M. (2008). Correlation between salivary IL-1β levels and periodontal clinical status. Arch. Oral Biol. 53, 346–352. doi: 10.1016/j.archoralbio.2007.11.005
Toh, S. Y., Citartan, M., Gopinath, S. C., and Tang, T. H. (2015). Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 64, 392–403. doi: 10.1016/j.bios.2014.09.026
Totan, A., Greabu, M., Totan, C., and Spinu, T. (2006). Salivary aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase: possible markers in periodontal diseases? Clin. Chem. Lab. Med. 44, 612–615. doi: 10.1515/cclm.2006.096
von Troil-Lindén, B., Torkko, H., Alaluusua, S., Jousimies-Somer, H., and Asikainen, S. (1995). Salivary levels of suspected periodontal pathogens in relation to periodontal status and treatment. J. Dent. Res. 74, 1789–1795. doi: 10.1177/00220345950740111201
Warsinke, A. (2009). Point-of-care testing of proteins. Anal. Bioanal. Chem. 393, 1393–1405. doi: 10.1007/s00216-008-2572-0
Wilczynska-Borawska, M., Borawski, J., Kovalchuk, O., Chyczewski, L., and Stokowska, W. (2006). Hepatocyte growth factor in saliva is a potential marker of symptomatic periodontal disease. J. Oral Sci. 48, 47–50. doi: 10.2334/josnusd.48.47
Wilkins, E., and Atanasov, P. (1996). Glucose monitoring: state of the art and future possibilities. Med. Eng. Phys. 18, 273–288. doi: 10.1016/1350-4533(95)00046-1
Yang, J., Wei, F., Schafer, C., and Wong, D. T. (2014). Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS ONE 14:e110641. doi: 10.1371/journal.pone.0110641
Yoon, A. J., Cheng, B., Philipone, E., Turner, R., and Lamster, I. B. (2012). Inflammatory biomarkers in saliva: assessing the strength of association of diabetes mellitus and periodontal status with the oral inflammatory burden. J. Clin. Periodontol. 39, 434–440. doi: 10.1111/j.1600-051X.2012.01866.x
Keywords: periodontitis, point-of care testing, saliva, bacteria-derived biomarkers, host-derived biomarkers
Citation: Ji S and Choi Y (2015) Point-of-care diagnosis of periodontitis using saliva: technically feasible but still a challenge. Front. Cell. Infect. Microbiol. 5:65. doi: 10.3389/fcimb.2015.00065
Received: 15 June 2015; Accepted: 21 August 2015;
Published: 03 September 2015.
Edited by:
Ulvi Kahraman Gürsoy, University of Turku, FinlandReviewed by:
Nick Stephen Jakubovics, Newcastle University, UKSinem Esra Sahingur, Virginia Commonwealth University, USA
Copyright © 2015 Ji and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youngnim Choi, Laboratory of Infection and Immunity, Department of Oral Microbiology and Immunology, School of Dentistry, Seoul National University, 101 Daehak-ro, Jongno-gu, Seoul 110-744, South Korea,eW91bmduaW1Ac251LmFjLmty
 Suk Ji
Suk Ji Youngnim Choi
Youngnim Choi