- 1Laboratorio de Inmunoquímica y Biotecnología, Departamento SAMP, Fac. Cs. Veterinarias, Universidad Nacional del Centro de la Provincia de Buenos Aires, Tandil, Buenos Aires, Argentina
- 2CONICET, Buenos Aires, Argentina
- 3Instituto de Patobiología, CNIA, INTA Castelar, Buenos Aires, Argentina
Shiga toxin-producing Escherichia coli (STEC) are characterized by the production of Shiga toxins (Stx) encoded by temperate bacteriophages. Stx production is linked to the induction of the phage lytic cycle. Several stx variants have been described and differentially associated with the risk of developing severe illness. The variant named stx2g was first identified in a STEC strain isolated from the faeces of healthy cattle. Analysis of stx2g-positive strains isolated from humans, animals, and environmental sources have shown that they have a close relationship. In this study, stx2g-positive STEC isolated from cattle were analyzed for phage and Stx production, with the aim to relate the results to differences observed in cytotoxicity. The presence of inducible phages was assessed by analyzing the bacterial growth/lysis curves and also by plaque assay. Bacterial growth curves in the absence of induction were similar for all isolates, however, notably differed among induced cultures. The two strains that clearly evidenced bacteriolysis under this condition also showed higher phage titers in plaque assays. However, only the phage plaques produced by one of these strains (FB 62) hybridized with a stx2-probe. Furthermore, the production of Stx was evaluated by enzyme immunoassay (EIA) and Western immunoblotting in overnight supernatants. By EIA, we detected Stx only in supernatants of FB 62, with a higher signal for induced than uninduced cultures. By immunoblotting, Stx2 could be detected after induction in all stx2g-positive isolates, but with lower amounts of Stx2B subunit in those supernatants where phages could not be detected. Taking into account all the results, several differences could be found among stx2g-positive strains. The strain with the highest cytotoxic titer showed higher levels of stx2-phages and toxin production by EIA, and the opposite was observed for strains that previously showed low cytotoxic titers, confirming that in stx2g-positive strains Stx production is phage-regulated.
Introduction
Shiga toxin-producing Escherichia coli (STEC) are important pathogens that can cause severe human diseases, including hemorrhagic colitis and hemolytic uremic syndrome (Karmali et al., 1985). STEC comprise a diverse group of E. coli strains characterized by the production of Shiga toxins (Stx1 and/or Stx2), which are regarded as their main virulence factors.
The genes encoding Stx are usually carried by bacteriophages. In general, stx genes are situated among genes controlled by the phage late promoter suggesting that Stx production is linked to the induction or progression of the phage lytic cycle (Neely and Friedman, 1998; O'Loughlin and Robins-Browne, 2001). Several variants of stx genes have been described, and have been differentially associated with the risk of developing severe illness (Friedrich et al., 2002; Beutin et al., 2004; Persson et al., 2007).
A probably emergent variant named Stx2g was identified by Leung et al. (2003) in STEC isolated from faeces of healthy cattle. These authors found that this stx2g variant had high similarity with stx2 genes associated with human disease, and besides, Stx2g cytotoxicity for HeLa and Vero cells was comparable to that of Stx2EDL933.
Other studies have also described strains carrying stx2g isolated from cattle, wastewater, aquatic environments, and humans (Garcia-Aljaro et al., 2005; Beutin et al., 2006; García-Aljaro et al., 2006; Beutin et al., 2007; Krüger et al., 2007; Persson et al., 2007; Garcia-Aljaro et al., 2009; Nguyen et al., 2011; Prager et al., 2011). Differences have been detected in regard to toxin production, cytotoxic activity, and stx-phage release among stx2g-positive strains (Beutin et al., 2006; García-Aljaro et al., 2006; Krüger et al., 2011; Prager et al., 2011). Interestingly, Prager et al. (2011) demonstrated that stx2g-positive strains isolated from humans, animals, and environmental sources have a close phylogenetic relationship, reinforcing the idea of human infections as a potential zoonotic disease. At present, the role of stx2g in human pathogenicity has not been evaluated.
In this study, stx2g-positive STEC isolated from cattle were analyzed for phage and Stx production, with the aim to relate the results to differences observed in cytotoxicity.
Materials and Methods
Bacterial Strains
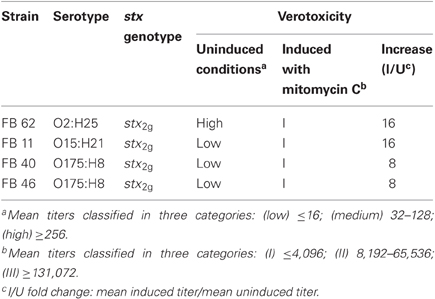
The stx2g-positive isolates analyzed in this study (Table 1) have been previously described regarding the serotype and other virulence factors (Padola et al., 2004; Krüger et al., 2007; Granobles Velandia et al., 2011). Cytotoxic activity was evaluated in a previous study showing differences among these isolates (Krüger et al., 2011). One of the strains, belonging to O2:H25 serotype had a high basal titer comparable to those obtained from strains carrying the stx2EDL933 subtype, but the others showed low basal cytotoxicity. All these stx2g-positive strains showed a low response to mitomycin C induction.
As a positive control of phage lysis the strain E. coli EDL933 (stx1 EDL933/stx2 EDL933, O157:H7) was used. This strain was kindly provided by Dr. J. Blanco (Laboratorio de Referencia de E. coli, Spain). The strain E. coli DH5α was used as host strain for phage detection.
Bacterial Growth/Lysis Curves
Bacteria were grown overnight in Luria Bertani (LB) medium at 37°C with shaking at 100 rpm. An aliquot was inoculated into fresh LB medium and incubated at 37°C and 180 rpm up to an optical density at 600 nm (OD600) ≈ 0.2−0.3. In that moment (named 0 h), each culture was subdivided into two flasks and mitomycin C was added to one of them to a final concentration of 0.5 μg/ml. The cultures were incubated overnight and monitored spectrophotometrically every hour for the first 5 h, and when necessary, dilutions of the samples were performed. Bacterial enumeration was also conducted by plating appropriate dilutions in duplicate by using LB agar plates. The assays were done at least three times.
Evaluation of Phage Production
To evaluate phage production, we followed the methodology described by Muniesa et al. (2004), with some modifications. At 3 h after mitomycin C induction, an aliquot of each culture was centrifuged for 10 min at 10,000 × g. The supernatants were filtered through low-protein-binding 0.22 μm membrane filters (Millex-GV, Millipore) and tenfold serially diluted. One hundred μl of each dilution were then mixed with 500 μl of an exponential phase culture of E. coli DH5α (OD600 ≈ 0.6−0.8) and incubated for 30 min at 37°C with shaking (180 rpm). The suspension was then mixed with 3 ml of LB soft agar supplemented with 3.2 mM CaCl2 and 0.5-1 μg/ml ampicillin (Muniesa et al., 2004; Santos et al., 2009), and poured onto LB agar plates. The plates were examined for the presence of lysis plaques following incubation for 18 h at 37°C. The assays were done at least three times.
Plaque Hybridization
Plaques were transferred onto nylon membranes positively charged (Roche Diagnostics GmbH) according to a standard procedure (Sambrook and Russell, 2001) and hybridized at 68°C with a stx2 specific probe. The probe was synthesized by PCR using stx2 generic primers (Paton and Paton, 1998), and labeled by incorporating digoxigenin 11-deoxyuridine triphosphate (Roche Diagnostics, Germany).
Evaluation of Extracellular Shiga Toxin Production
Stx production was evaluated in the supernatants of stx2g-positive strains after overnight incubation with or without mitomycin C, by using an enzyme immunoassay (EIA, Ridascreen® Verotoxin, R-Biopharm, Germany). The results were analyzed spectrophotometrically at 450 nm. The supernatant of the E. coli DH5α culture was included as negative control besides the negative control of the kit. Test results were recorded as weak positive (1+) if the extinction was >0.1–0.5 above the negative control, moderate (2+) (extinction > 0.5–1.0 above negative control) and strongly positive 3+ (>1.0–2.0) to 4+ (>2.0). The assays were done at least three times.
The supernatants of stx2g-positive strains after overnight incubation with mitomycin C were also evaluated by Western immunoblotting. Briefly, 12 μl of supernatants were separated by 12.5% SDS-PAGE (under reducing conditions) and transferred onto a nitrocellulose membrane (Hybond ECL, Amersham Pharmacia Biotech). The membrane was blocked overnight at 4°C with 5% skimmed milk in PBS-Tween 0.1%, and incubated with a 1:500 dilution of anti-Stx2B rabbit IgG in PBS-Tween 0.1% for 1 h at 37°C (Parma et al., 2011). After washing, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000) for 1 h at 37°C. Finally, membranes were revealed using DAB/H2O2 system (Pierce). As positive controls, recombinant Stx2B protein and the supernatant of an overnight culture of a stx2EDL933-positive E. coli strain were used.
Results and Discussion
In this study, stx2g-positive STEC isolates belonging to serotypes O2:H25, O15:H21 and O175:H8, which have previously shown differences in cytotoxicity titers, were analyzed for phage and Stx production, under inducing and non-inducing conditions.
The presence of inducible phages was assessed by analyzing the bacterial growth/lysis curves constructed for each strain and also by plaque assay using E. coli DH5α as host strain. The bacterial growth curves in the absence of mitomycin C were similar for all stx2g-positive isolates and also similar to that of E. coli EDL933. However, the bacterial growth/lysis curves notably differed when cultures were exposed to mitomycin C (Figure 1). Only two of the isolates (FB 62 and FB 11) clearly evidenced bacteriolysis under this condition. The strain FB 62 (serotype O2:H25), which had the highest cytotoxicity titer among stx2g-positive isolates (Krüger et al., 2011), showed an OD600 pattern with a maximum of 2.5 at 2 h after mitomycin C induction, followed by a significant decrease typical of host cell lysis, which reached the baseline OD600 at 5 h of culture. The FB 11 strain also showed a bacteriolytic pattern, but the maximum OD600 value, which occured 2 h after mitomycin C induction, was lower than 2.0. On the contrary, the other stx2g-positive isolates (FB 40 and FB 46) did not show a marked bacteriolytic pattern and their growth/lysis curves were similar to that of the stx2-negative strain E. coli DH5α. These two STEC isolates reached a maximum OD600 earlier (1 h after mitomycin C induction) with a lower value (1.0), and along the following 4 h of culture the OD600 decreased gradually.
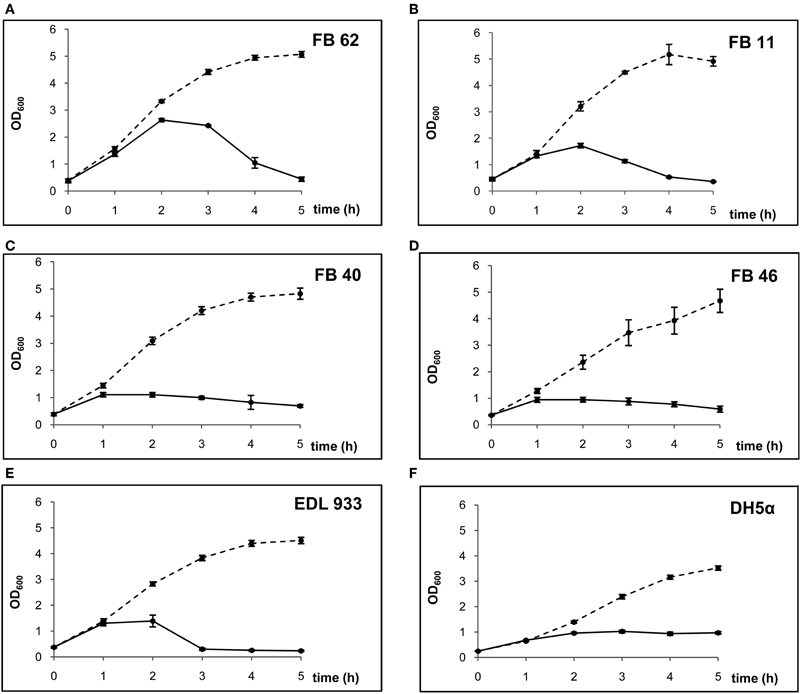
Figure 1. Growth/lysis curves of the isolates studied in presence and absence of mitomycin C (solid and dashed lines, respectively).
The different patterns were related to differences in the viable bacterial counts. In the FB 62 and FB 11 cultures, the bacterial counts remained stable comparing 0–1 h after mitomycin C induction, and then a drop was observed between 1 and 2 h (a 2 log for FB 62 and a 1.5 log for FB 11). In contrast, bacterial counts diminished earlier in FB 40 and FB 46, reaching a 2 log decrease in the first hour after the addition of mitomycin C.
We could only observe lysis plaques with the supernatants of FB 62 and FB 11 cultures, and the phage titers were higher from induced than from uninduced cultures (pfu increased from 1.0 × 102 to 3.0 × 103 for FB 62 and from 5.0 × 103 to 2.3 × 104 for FB 11). However, only the phages produced by FB 62 strain were stx2g-phages (as these phage plaques hybridized with a stx2-probe). The production of extracellular Stx was evaluated by EIA and Western immunoblotting in overnight supernatants. By EIA, we detected the toxin only in supernatants of FB 62 (with values of 3+ and 4+ for uninduced and induced cultures, respectively). By Western immunoblotting (using anti-Stx2B subunit antibodies), toxin production after mitomycin C induction was detected in all stx2g-positive isolates (Figure 2). Despite the same volume of supernatant form each culture was loaded onto the gel, a faint band was observed in strains FB 40 and FB 46 comparing to strains FB 11 and FB 62, evidencing the presence of lower amounts of toxin (B subunit) in those supernatants.

Figure 2. Stx2B detection by Western immunoblotting. Anti-Stx2B IgG was used as first antibody. Lanes 1–5: supernatants of isolates FB 11, FB 40, FB 46, FB 62, and positive control (O26:H11 STEC strain, harboring stx2EDL933 subtype), respectively. Lane 6: recombinant Stx2B protein.
Taking all the results into account, several differences could be found among the four stx2g-positive strains. The strain with the highest cytotoxic titer (FB 62) presented a bacteriolytic pattern when the growth curve under mitomycin C treatment was analyzed. As we expected, this strain also had high levels of Stx and stx2-phage production, and both were higher under inducing conditions. Therefore, it can be concluded that FB 62 strain has an inducible stx2-phage, and produces high amounts of Stx2, biologically active on Vero cells. Noticeably, this strain belongs to the same serotype (O2:H25) as the strain 7v isolated by Leung et al. (2003) from cattle, which is the reference strain for stx2g.
Regarding FB 11 strain, we observed that it carries one or more inducible phages because of both the presence of infective particles in the supernatants and the bacteriolytic pattern observed by monitoring the OD600 of the culture. These phages do not seem to encode stx2g, as no signal was obtained when the plaque hybridization assay was performed. Possible explanations could be that stx2g either is not phage encoded in this strain or is encoded in a defective stx-phage, or that lytic cycle of the stx2g-phage is repressed by other phage/s. Indeed, there are studies demonstrating that not all stx2 genes are associated with inducible prophages as well as studies that suggest the existence of regulatory mechanisms when two stx2-phages are present in a same strain (Teel et al., 2002; Muniesa et al., 2003; Zhang et al., 2005; Karama and Gyles, 2008).
The apparent absence of lytic cycle induction of stx2g-phages in FB 11 strain correlates with the low cytotoxic titer under inducing conditions. It seems it produces a low amount of toxin, which is undetectable by EIA but detectable by Western immunoblotting (Stx2B subunit). The epitopes recognized in the EIA are probably different from the ones detected by the anti-Stx2B antibodies used in the immunoblotting. Besides, limits caused by sensitivity of EIA-Ridascreen to detect low Stx production, such as the case of some stx2g-positive strains, have been reported by Beutin et al. (2006).
The FB 40 and FB 46 isolates, both with low cytotoxic titers on Vero cells and a low increase under inducing conditions, showed a particular behavior in the present study since both strains did not have OD600 curves typical of lytic cycle induction. Instead, they seemed to have a bacteriostatic pattern when incubated with mitomycin C, similarly to E. coli DH5α strain. Moreover, they showed an earlier decrease in viable bacterial counts than FB 11 and FB 62. Analyzing these isolates, neither phage plaques were obtained nor Stx production was detected by EIA, and the Stx2B subunit was detected by Western immunoblotting with low intensity. In this regard, Johansen et al. (2001) observed that the level of Stx production in bacteria that carry apparently defective phages is lower than in bacteria from which phages can be induced.
Interestingly, Prager et al. (2011), assessing Stx production by EIA-Ridascreen and by Vero cell cytotoxicity assays, detected some stx2g-positive strains that did not produce Stx2, some of which contained stx2g pseudogenes but others presented intact stx2g genes. Other authors have reported strains PCR-positive for stx2g with lack of Stx expression (García-Aljaro et al., 2006; Beutin et al., 2007; Miko et al., 2009).
In accordance with the present work, García-Aljaro et al. (2006) found that only those stx2g-positive strains that carried inducible stx2g-phages showed Stx production, and noticeably, these strains also belonged to O2:H25 serotype as FB 62 strain.
Our results highlight the variability among stx2g-positive strains and show that phage regulation can affect Stx2g production as differences in verocytotoxicity correlated both with differences in lytic cycle induction, and with phage and Stx production.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank M. R. Ortiz for her technical assistance. This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Fondo para la Investigación Científica y Tecnológica (FONCYT), and Secretaría de Ciencia, Arte y Tecnología-Universidad Nacional del Centro de la Provincia de Buenos Aires (SECAT-UNICEN). Claudia V. Granobles Velandia and Yanil R. Parma are holders of fellowships from CONICET. Alejandra Krüger and Paula M. A. Lucchesi are members of the Research Career of CONICET.
References
Beutin, L., Krause, G., Zimmermann, S., Kaulfuss, S., and Gleier, K. (2004). Characterization of Shiga-toxin producing Escherichia coli strains isolated from humans patients in Germany over a 3-year period. J. Clin. Microbiol. 42, 1099–1108.
Beutin, L., Miko, A., Krause, G., Pries, K., Haby, S., Steege, K., and Albrecht, N. (2007). Identification of human-pathogenic strains of Shiga toxin-producing Escherichia coli from food by combination of serotyping and molecular typing of Shiga toxin genes. Appl. Environ. Microbiol. 73, 4769–4775.
Beutin, L., Steinrück, H., Krause, G., Steege, K., Haby, S., Hultsch, G., and Appel, B. (2006). Comparative evaluation of RidascreenVerotoxin enzyme immunoassay for detection of Shiga-toxin producing strains of Escherichia coli (STEC) from food and other sources. J. Appl. Microbiol. 102, 630–639.
Friedrich, A. W., Bielaszewska, M., Zhang, W. L., Pulz, M., Kuczius, T., Ammon, A., and Karch, H. (2002). Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with symptoms. J. Infect. Dis. 185, 74–84.
Garcia-Aljaro, C., Muniesa, M., Blanco, J. E., Blanco, M., Blanco, J., Jofre, J., and Blanch, A. R. (2005). Characterization of Shiga toxin producing Escherichia coli isolated from aquatic environments. FEMS Microbiol. Lett. 246, 55–65.
García-Aljaro, C., Muniesa, M., Jofre, J., and Blanch, A. R. (2006). Newly identified bacteriophages carrying the stx2g gene isolated from Escherichia coli strains in polluted waters. FEMS Microbiol. Lett. 258, 127–135.
Garcia-Aljaro, C., Muniesa, M., Jofre, J., and Blanch, A. R. (2009). Genotypic and phenotypic diversity among induced stx2-carrying bacteriophages from environmental Escherichia coli strains. Appl. Environ. Microbiol. 75, 329–366.
Granobles Velandia, C. V., Sanso, A. M., Krüger, A., Suárez, L. V., Lucchesi, P. M. A., and Parma, A. E. (2011). Occurrence of subtilase cytotoxin and relation with other virulence factors in verotoxigenic Escherichia coli isolated from food and cattle in Argentina. Braz. J. Microbiol. 42, 711–715.
Johansen, B. K., Wasteson, Y., Granum, P. E., and Brynestad, S. (2001). Mosaic structure of Shiga-toxin-2-encoding phages isolated from Escherichia coli O157:H7 indicates frequent gene exchange between lambdoid phage genomes. Microbiology 147, 1929–1936.
Karama, M., and Gyles, C. L. (2008). Characterization of verotoxin-encoding phages from Escherichia coli O103:H2 strains of bovine and human origins. Appl. Environ. Microbiol. 74, 5153–5158.
Karmali, M. A., Petric, M., Lim, C., Fleming, P. C., Arbus, G. S., and Lior, H. (1985). The association between idiopathic hemolytic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151, 775–782. In: J. Infect. Dis. 189, 552–563.
Krüger, A., Lucchesi, P. M. A., and Parma, A. E. (2007). Evaluation of vt2-subtyping methods for identifying vt2g in verotoxigenic Escherichia coli. J. Med. Microbiol. 56, 1474–1478.
Krüger, A., Lucchesi, P. M. A., and Parma, A. E. (2011). Verotoxins in bovine and meat verotoxin-producing Escherichia coli isolates: type, number of variants, and relationship to cytotoxicity. Appl. Environ. Microbiol. 77, 73–79.
Leung, P. H. M., Peiris, J. S. M., Ng, W. W. S., Robins-Browne, R. M., Bettelheim, K. A., and Yam, W. C. (2003). A newly discovered verotoxin variant, VT2g, produced by bovine verocytotoxigenic Escherichia coli. Appl. Environ. Microbiol. 69, 7549–7553.
Miko, A., Pries, K., Haby, S., Steege, K., Albrecht, N., Krause, G., and Beutin, L. (2009). Assessment of Shigatoxin-producing Escherichia coli isolates from wildlife meat as potential pathogens for humans. Appl. Environ. Microbiol. 75, 6462–6470.
Muniesa, M., Blanco, J. E., Simon, M., Serra-Moreno, R., Blanch, A. R., and Jofre, J. (2004). Diversity of stx2 converting bacteriophages induced from Shiga-toxin- producing Escherichia coli strains isolated from cattle. Microbiology 150, 2959–2971.
Muniesa, M., Simon, M., Prats, G., Ferrer, D., Pañela, H., and Jofre, J. (2003). Shiga toxin 2-converting bacteriophages assoaciated with clonal variability in Escherichia coli O157:H7 strains of human origin isolated from a single outbreak. Infect. Inmun. 71, 4554–4562.
Neely, M. N., and Friedman, D. I. (1998). Functional and genetic analysis of regulatory regions of coliphage H-19B: location of shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol. Microbiol. 28, 1255–1267.
Nguyen, T. D., Vo, T. T., and Vu-Khac, H. (2011). Virulence factors in Escherichia coli isolated from calves with diarrhea in Vietnam. J. Vet. Sci. 12, 159–164.
O'Loughlin, E. V., and Robins-Browne, R. M. (2001). Effect of Shiga toxin and Shiga-like toxins on eukaryotic cells. Microbes Infect. 3, 493–507.
Padola, N. L., Sanz, M. E., Blanco, J. E., Blanco, M., Blanco, J., Etcheverría, A. I., Arroyo, G. H., Usera, M. A., and Parma, A. E. (2004). Serotypes and virulence genes of bovine Shigatoxigenic Escherichia coli (STEC) isolated from a feedlot in Argentina. Vet. Microbiol. 100, 3–9.
Parma, Y. R., Chacana, P. A., Rogé, A., Kahl, A., Cangelosi, A., Geoghegan, P., Lucchesi, P. M. A., and Fernández-Miyakawa, M. E. (2011). Antibodies anti-Shiga toxin 2 B subunit from chicken egg yolk: isolation, purification and neutralization efficacy. Toxicon 58, 380–388.
Paton, A. W., and Paton, J. C. (1998). Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36, 598–602.
Persson, S., Olsen, K. E. P., Ethelberg, S., and Scheutz, F. (2007). Subtyping method for Escherichia coli Shiga toxin (verocytotoxin) 2 variants and correlations to clinical manifestations. J. Clin. Microbiol. 45, 2020–2024.
Prager, R., Fruth, A., Busch, U., and Tietze, E. (2011). Comparative analysis of virulence genes, genetic diversity, and phylogeny of Shiga toxin 2g and heat-stable enterotoxin STIa encoding Escherichia coli isolates from human, animals and environmental sources. Int. J. Med. Microbiol. 301, 181–191.
Sambrook, J., and Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual, 3rd Edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Santos, S. B., Carvalho, C. M., Sillankorva, S., Nicolau, A., Ferreira, E., and Azeredo, J. (2009). The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 9, 148–157.
Teel, L. D., Melton-Celsa, A. R., Schmitt, C. K., and O'Brien, A. D. (2002). One of two copies of the gene for the activatable Shiga toxin type 2d in Escherichia coli O91:H21 strain B2F1 is associated with an inducible bacteriophage. Infect. Immun. 70, 4282–4291.
Keywords: cytotoxicity, Stx2g, phage induction, toxin production
Citation: Granobles Velandia CV, Krüger A, Parma YR, Parma AE and Lucchesi PMA (2012) Differences in Shiga toxin and phage production among stx2g-positive STEC strains. Front. Cell. Inf. Microbio. 2:82. doi: 10.3389/fcimb.2012.00082
Received: 29 March 2012; Paper pending published: 16 April 2012;
Accepted: 24 May 2012; Published online: 15 June 2012.
Edited by:
Nora L. Padola, Universidad Nacional del Centro de la Provincia de Buenos Aires, ArgentinaReviewed by:
V. K. Viswanathan, University of Arizona, USAMaite Muniesa, University of Barcelona, Spain
Copyright: © 2012 Granobles Velandia, Krüger, Parma, Parma and Lucchesi. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Alejandra Krüger, Laboratorio de Inmunoquímica y Biotecnología, Departamento SAMP, Universidad Nacional del Centro de la Provincia de Buenos Aires, Pinto 399, Tandil, Buenos Aires B7000, Argentina. e-mail:YWtydWdlckB2ZXQudW5pY2VuLmVkdS5hcg==


 Yanil R. Parma2,3
Yanil R. Parma2,3