
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cell Death , 20 January 2025
Sec. Non-Apoptotic Regulated Cell Death
Volume 3 - 2024 | https://doi.org/10.3389/fceld.2024.1507960
 Sasiprapa Sonkaew1,2†
Sasiprapa Sonkaew1,2† Ruwaida Rajna1†
Ruwaida Rajna1† Yeon-Ji Park1
Yeon-Ji Park1 Jiong Yan1
Jiong Yan1 Zhaoshan Liu1
Zhaoshan Liu1 Siriporn Jitkaew3
Siriporn Jitkaew3 Zheng-Gang Liu1
Zheng-Gang Liu1 Swati Choksi1*
Swati Choksi1*Glucose deprivation (GD), a common metabolic stress condition, has been recognized as a potent inducer of necroptotic cell death. Our previous findings suggested that the mitochondrial protein, Noxa, may be involved in mediating the release of mitochondrial DNA during GD-induced ZBP1-dependent necroptotic pathway. However, the functional significance of Noxa in necroptosis under GD treatment remains unclear. Here, we investigated the role of Noxa in GD-induced necroptosis and the underlying molecular mechanisms governing its expression. We revealed that Noxa is required for the induction of necroptosis under GD. We also demonstrated that the upregulation of Noxa induced by GD is mediated by ATF4, a key transcription factor. These results provide insights into the regulatory mechanisms underlying Noxa dynamics during GD treatment and highlights its potential as a therapeutic target in cancer therapy and necroptosis-related diseases.
Necroptosis, a programmed form of necrotic cell death, has emerged as a critical mechanism in diverse pathological conditions, including inflammatory diseases, neurodegenerative disorders, and cancer (Dhuriya and Sharma, 2018; Galluzzi et al., 2018; Yan et al., 2022; Ye et al., 2023). Necroptosis can be triggered by various extracellular stimuli, including death receptor engagement, pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and cellular stressors such as hypoxia, oxidative stress, and metabolic alterations (Choi et al., 2019; Yuan and Ofengein, 2023). Death receptors, such as tumor necrosis factor receptor 1 (TNFR1), Fas receptor (Fas), and tumor necrosis factor-related apoptosis-inducing ligand receptor (TRAIL-R), can initiate necroptotic signaling through the recruitment and activation of receptor-interacting protein kinases (RIPKs), particularly RIPK1 and RIPK3 in a process known as the necrosome assembly (Newton et al., 2024; Yuan and Ofengein, 2023). Additionally, intracellular sensors, including Z-DNA binding protein 1 (ZBP1), can activate RIPK3 independently of death receptor signaling in response to viral infection or cellular stress, leading to necroptosis cell death (Chen et al., 2022; Jiao et al., 2020; Sridharan et al., 2017). Activation of RIPK1 or ZBP1 by upstream signals, through binding of death ligands to their receptors or the recognition of viral RNA, respectively, can lead to activation of RIPK3. Subsequently, phosphorylated RIPK3 (pRIPK3) phosphorylates MLKL (pMLKL), leading to its oligomerization, translocation to the plasma membrane, and disruption of membrane integrity, resulting in necroptotic cell death (Cai et al., 2013; Chen et al., 2013; Wang et al., 2014).
Metabolic stress conditions, such as glucose deprivation (GD), have been recognized as potent inducers of necroptotic cell death (León-Annicchiarico et al., 2015). GD disrupts cellular energy metabolism, leading to ATP depletion and metabolic imbalances, which can activate necroptosis. Our previous findings demonstrated that GD could trigger ZBP1-dependent necroptosis. Moreover, we found that Noxa, a mitochondrial protein, facilitated GD-induced necroptosis in cancer cells (Baik et al., 2021).
Noxa is a member of the BH3-only pro-apoptotic protein family where initially, it was identified that its apoptotic activity is primarily reliant on the tumor suppressor gene, p53 (Shibue et al., 2003). Noxa functions by binding and neutralizing anti-apoptotic proteins, particularly Mcl-1, thereby promoting apoptosis (Seo et al., 2003; Shibue et al., 2003; Oda et al., 2000). However, recent studies have suggested a broader role for Noxa beyond apoptosis, including its involvement in the regulation of cellular responses to various stressors, such as DNA damage, hypoxia, and endoplasmic reticulum stress (Ploner et al., 2008). Our previous study demonstrated a role for Noxa in mediating the release of mitochondrial DNA during GD-induced necroptosis, indicating a potential role in metabolic stress (Baik et al., 2021). However, the functional significance of Noxa in GD treatment remains incompletely understood.
Building upon our previous findings, we aimed to elucidate the dynamic role of Noxa in GD-induced necroptosis and explore the regulatory pathways governing Noxa expression during GD treatment. In this study, we revealed that increased Noxa expression upon GD treatment positively correlated with the expression of pMLKL, the marker of necroptosis. Depletion of Noxa resulted in blocking necroptosis. Furthermore, we found that the upregulation of Noxa after GD treatment was dependent on Activating Transcription factor 4 (ATF4). These results provide evidence for the first time of the involvement of Noxa in necroptosis induced by GD.
The antibodies against NOXA, pMLKL, MLKL, and pRIPK3 were purchased from Abcam (ab13654, ab187091, ab184718, and ab209384). The following antibodies against ATF4 and RIPK3 from Cell Signaling (11815S and 13526S), β-actin (A2066, Sigma-Aldrich), p53 (554147, Pharmingen), and ZBP1 (703166, Invitrogen) were purchased.
All cell lines HT-29 (colorectal cancer cell line) and CFPAC-1 (pancreatic cancer cell line) were obtained from the American Type Culture Collection (ATCC). HT-29 cells were cultured in DMEM, while CFPAC-1 cells were cultured in RPMI 1640 media. All culture media were supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO, United States), and 1% Penicillin-Streptomycin (Gibco, Life Technologies, Gaithersburg, MD, United States), and 1% L-Glutamine (Corning, Mediatech, Inc., Manassas, VA, United States). For GD treatment, cells were cultured in glucose-depleted DMEM and RPMI media supplemented with 10% FBS, 1% Penicillin-Streptomycin, 1% L-Glutamine, and 0.5 mM glucose for HT-29 and CFPAC-1 cells. To upregulate ZBP1expression, cells were also pretreated with 20 ng/mL IFN-β (PeproTech) 16 h prior to GD treatment. These cell lines were cultured at 37°C in a humidified 5% CO2 incubator. Additionally, all cell lines were confirmed to be mycoplasma-free.
Cells underwent RNA extraction using the Qiagen RNeasy® Kit. RNA concentrations were determined using an ND-1000 spectrophotometer (NanoDrop, Thermo Fisher Scientific, Madison, WI, United States). Subsequently, cDNA synthesis was performed using random primers and a master mix of MultiScribe™ reverse transcriptase (Applied Biosystems, Thermo Fisher Scientific, Foster City, CA, United States). Quantitative PCR was conducted using SensiFAST Probe Hi-ROX Mix (Bioline) and the QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific). 2^−ΔΔCT method was employed to quantify the fold change of Noxa mRNA expression. Primer sequences for Noxa (PMAIP1) and GADPH are provided in Supplementary Table 1.
Cells were cultured and treated with GD in 6 cm dishes in a time-dependent experiment. Cells were then lysed in M2 buffer supplemented with phosphatase inhibitors A, B and protease inhibitor cocktail (Selleck Chemicals). A total of 40 µg of protein from lysed cells were separated by 4%–20% SDS-polyacrylamide gel electrophoresis and then transferred to a PVDF membrane (Immobilon, Millipore). The blots were blocked with 5% BSA and subsequently probed with anti-MLKL, anti-pMLKL, anti-pRIPK3, anti-RIPK3, anti-Noxa, anti-ATF4, anti-ZBP1, anti-p53, or anti-β-Actin antibodies. After overnight incubation, residual primary antibodies were washed away with TBST three times for 10 min each prior to incubation with secondary antibodies conjugated to Horseradish peroxidase (HRP). The blots were washed again with TBST three times for 10 min each before developing with enhanced chemiluminescence (Clarity™ Western ECL Substrate or ThermoScientific Super Signal™ West Femto) and visualized by ChemiDoc Touch Imaging System (Bio Rad, Hercules, CA, United States).
To generate Noxa and ATF4 knockouts in HT-29 cells (HT-29-sgNoxa and HT-29-sgATF4), we used the system previously described (Ran et al., 2013). Briefly, the lentiviral sgRNA vectors targeting Noxa and ATF4 were constructed by ligation of hybridized oligos into LentiCRISPR V2 (pXPR_001, GeCKO) vector. Noxa and ATF4 sgRNA sequences used to construct the knockouts are shown in Supplementary Table 1. 293T cells were transfected with sgRNA vector, psPAX2 (Addgene) and pCMV-VSV-g (Addgene) for 24 h. HT-29 cells were then infected with lentivirus containing supernatants with polybrene (Millipore) for 24 h. After 3 days of selection with 1–2 μg/mL puromycin (Sigma-Aldrich), the Noxa KO cells were placed into 96 well plate to undergo clonal selection. Single clones with complete knockout of Noxa were verified by DNA sequencing and Western blot analysis. HT-29-sgATF4 cells were used without clonal selection.
HT-29-sgControl, HT-29-sgNoxa and HT-29-sgATF4 cells were cultured in 96-well plates and treated with GD in a time-dependent experiment. To examine cell viability, we followed the manufacture described protocol (Chondrex). Briefly, after GD incubation of cells, MTT solution (0.5 mg/mL; Calbiochem®) was added to the cells and incubated for 2–4 h followed by 100 μL of dimethyl sulfoxide (DMSO). We measured the absorbance at 540 nm using the Tecan Spark 10M Multimode Plate Reader.
Paired t-Test was used to determine the statistical significance of differences between groups. Differences with p values < 0.05 were considered significant.
To examine the role of Noxa in GD-induced necroptosis, we investigated the expression of Noxa at both the mRNA and protein levels in GD-treated cancer cells. Upon exposure to GD, HT-29 cells exhibited a gradual but significant time-dependent increase in the expression of Noxa. Interestingly, the increase in Noxa levels coincided with elevated pMLKL levels (Figure 1A) and pRIPK3 (Supplementary Figure 1). Similar upregulation of Noxa and pMLKL after GD treatment was also observed in CFPAC-1 cells (Figure 1A). With increased necroptotic activity, we showed that the mRNA expression of Noxa was also upregulated (Figure 1B; Supplementary Table 1). Additionally, we examined cell viability of HT-29-sgControl and sgNoxa cells after time-dependent GD treatment and found that the loss of Noxa protected the cells from GD-induced death (Supplementary Figure 2). These findings suggested that GD triggers the upregulation of Noxa and that Noxa may contribute to GD-induced necroptosis in cancer cells.
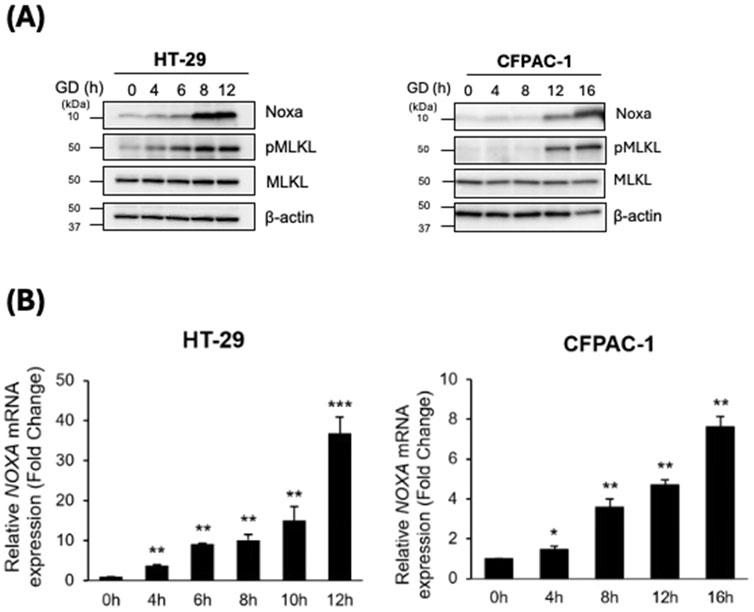
Figure 1. GD treatment upregulated Noxa expression. HT-29 and CFPAC-1 cells were treated with GD-depleted media for the indicated timepoints. (A) Cell lysates were examined by Western blot analysis with the indicated antibodies. (B) RNA was extracted from cells at the indicated timepoints and mRNA expression of Noxa was determined by RT-qPCR. Paired t-Test of each time point to the untreated control for both cell lines: *p < 0.05, **p < 0.01, ***p < 0.001.
To investigate whether Noxa contributes to GD-induced necroptosis, we employed the CRISPR-Cas9 system to knockout Noxa expression in HT-29 cells (HT-29-sgNoxa) (Supplementary Table 1). Strikingly, HT-29-sgNoxa cells exhibited a decrease in pMLKL expression upon exposure to GD treatment when compared to HT-29-sgControl cells (Figure 2A). Loss of Noxa expression leading to decreased pMLKL expression highlights the role of Noxa in mediating GD-induced necroptosis in cancer cells (Figure 2A). Previous studies have shown that GD can activate p53-dependent Noxa upregulation in apoptotic cell death (Alves et al., 2006; Okoshi et al., 2007; Shibue et al., 2003). To investigate whether the upregulation of Noxa by p53 also occurs in necroptosis we induced GD stress in HT-29 cells. Interestingly, we observed that as Noxa expression was upregulated, p53 expression gradually decreased during GD exposure in HT-29 (Figure 2B). Noxa upregulation under GD treatment in and CFPAC1 cells, that do not express p53, is unaffected (Figure 2B). This suggests that the upregulation of Noxa is independent of p53 under GD induced necroptosis.
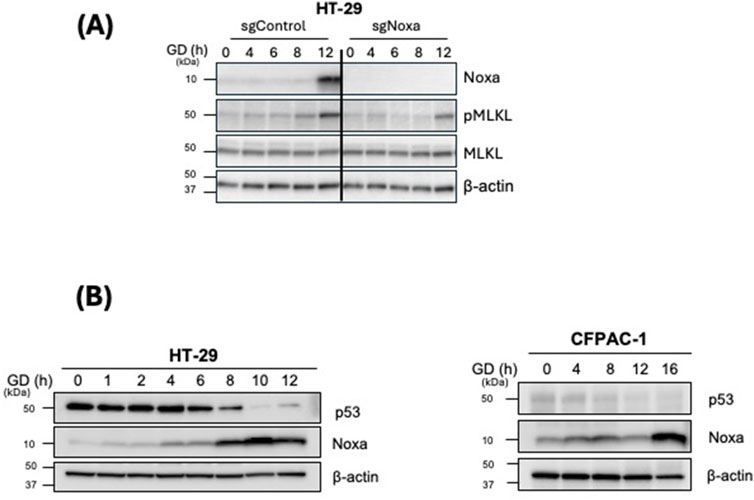
Figure 2. Noxa is required for GD-induced necroptosis and is p53-independent. (A) HT-29 sgControl and sgNoxa cells were treated with GD-depleted media for the indicated timepoints. Cell lysates were examined by Western blot analysis with the indicated antibodies. (B) HT-29 and CFPAC-1 cells were treated with GD for the indicated timepoints. Cell lysates were examined by Western blot analysis with the indicated antibodies.
It has been previously reported that Noxa expression could be regulated by a stress-induced transcription factor, ATF4 (León-Annicchiarico et al., 2015; Sharma et al., 2018). Therefore, we investigated ATF4 expression upon GD treatment in our cell models. Both HT-29 and CFPAC-1 cells, when treated with GD exhibited increased expression of ATF4 in a time-dependent manner (Figure 3A). Moreover, the increased ATF4 expression was positively correlated with increased Noxa level after GD treatment suggesting a ATF4-dependent Noxa expression in GD-induced necroptosis (Figure 3A). To further investigate whether the upregulation of Noxa is dependent on ATF4, we generated ATF4 knockout cells (HT-29-sgATF4, pool). The lower ATF4 expression levels led to almost no expression of Noxa after GD-treatment (Figure 3B; Supplementary Table 1). It is known that IFN-β treatment upregulates ZBP1 expression. Therefore, we first examined whether IFN-β induced upregulation of ZBP1 expression in HT-29 cells (Supplementary Figure 3). We found that the upregulation of ZBP1 prior to GD treatment does not affect the Noxa and ATF4 expression. However, IFN-β -induced upregulation of ZBP1 did lead to a more robust pMLKL upregulation as well as increased expression of Noxa response to GD treatment. When HT-29-sgATF4 cells are pretreated with IFN-β (to upregulate ZBP1 expression) prior to GD treatment, we observed lower pMLKL levels and lower Noxa expression compared to those in HT-29-sgControl cells (Figure 3C). Additionally, we examined cell viability of HT-29-sgControl and sgNoxa cells after time-dependent GD treatment and found that the loss of ATF4 protected the cells from GD-induced death (Supplementary Figure 4). This data suggests that ATF4-dependent upregulation of Noxa expression under GD treatment, is downstream of ZBP1.
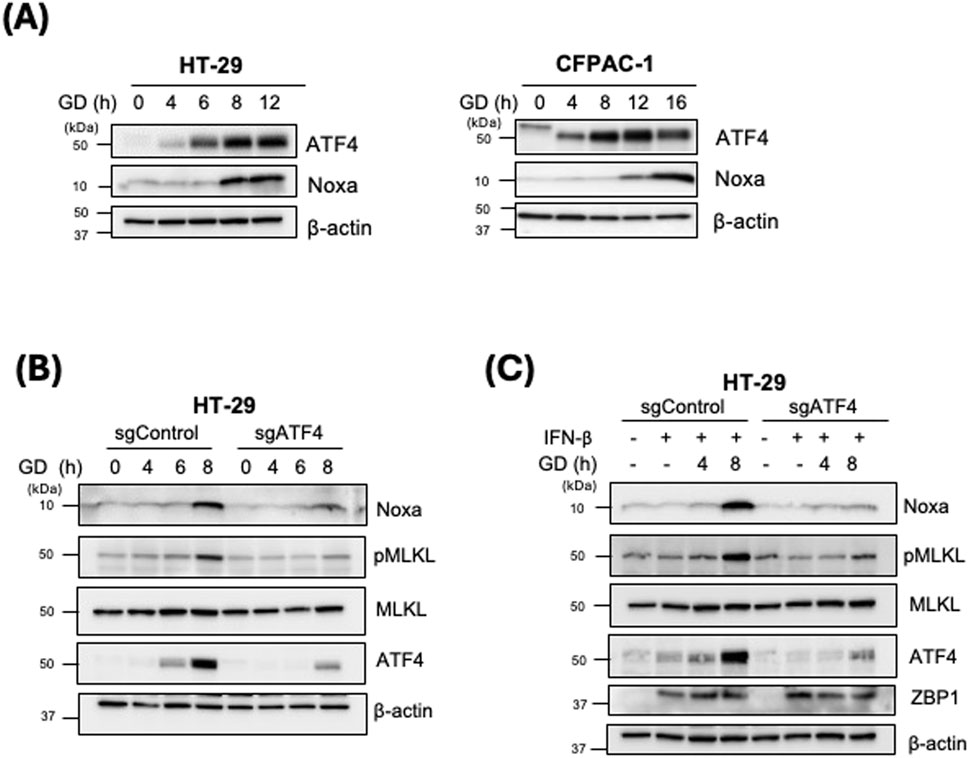
Figure 3. Increased Noxa expression induced by glucose deprivation is ATF4-dependent. (A) HT-29 and CFPAC-1 were treated with GD-depleted media for the indicated timepoints. Cell lysates were examined by Western blot analysis with the indicated antibodies. (B) HT-29 sgControl and sgATF4 cells were treated with GD-depleted media for the indicated timepoints. Cell lysates were examined by Western blot analysis with the indicated antibodies. (C) HT-29 sgControl and sgATF4 cells were treated with IFN-β for 16 h followed by GD treatment for the indicated timepoints. Cell lysates were examined by Western blot analysis with the indicated antibodies.
Noxa, a member of a subgroup of the Bcl-2 family has become an important target of study as a p53 tumor suppressor dependent, pro-apoptotic gene (Oda et al., 2000). During DNA damage as cells undergo apoptosis, Noxa mRNA expression is shown to be significantly upregulated. But this transcriptional upregulation is substantially downregulated in the absence of p53, which suggests that Noxa is critical for p53 activated apoptotic survival pathways (Shibue et al., 2003). Studies have parallelly shown that Noxa can be induced independent of p53 by the ATF4 transcription factor in cisplatin-induced apoptosis (Sharma et al., 2018). Interestingly, during metabolic and nutritional stress, ATF4 has been identified as promoting cell survival under such stress (León-Annicchiarico et al., 2015).
Several studies have shown that Noxa is regulated by a variety of signaling pathways through ATF3/ATF4 (Rozita et al., 2015; Qing et al., 2012; Wang et al., 2010; Yan et al., 2014). The upregulation of ATF4 can lead to the transcriptional upregulation of many stress-related genes containing the C/EBP-ATF response element (CRE) in their promoters. Specifically, Noxa is involved in the cytotoxic effects of several chemotherapeutic, such as genotoxic stressors (irradiation, etoposide), proteasome inhibitors (bortezomib), or ER stress inducers (thapsigargin, tunicamycin) (Núñez-Vázquez et al., 2020). Indeed, knockdown of NOXA strongly protects from ER-induced cell death triggered by numerous drugs such as bortezomib or thapsigargin. Studies have shown that ATF3 and ATF4 form a binding complex in NOXA promoter to induce apoptosis upon treatment with chemotherapy drugs such as cisplatin (Núñez-Vázquez et al., 2020). Although the induction of Noxa in apoptotic conditions has been widely studied, there continues to be a gap in our knowledge regarding the activation of Noxa under necroptotic conditions. In our previous study, we have demonstrated that ZBP1-induced necroptosis can be triggered under GD stress (Baik et al., 2021). Here, we demonstrate for the first time, that Noxa plays a role in necroptosis and the induction of Noxa expression is mediated by ATF4, a key transcription factor involved in the integrated stress response, not p53.
We have shown that GD stress alone can upregulate the expression of Noxa and pMLKL, a known necroptosis marker. Here, we find that in the absence of Noxa, the phosphorylation of MLKL is downregulated and resulting in less necroptosis. Therefore, Noxa is required for GD induced necroptosis. Finally, we demonstrated that blocking the upregulation of the transcription factor, ATF4, prevents the subsequent upregulation of Noxa and results in the loss of necroptosis, marked by lower pMLKL levels. This data suggests that ATF4-mediated expression of NOXA is critical for necroptosis. It has been shown that GD results in HIF-1α activation and HIF-1α directly interacts with the NOXA promoter (Lum et al., 2007; Kim et al., 2003). This could explain why we observed some Noxa expression at later time points in GD treated ATF4 KO cells. However, at the time points examined here, it is likely that the incomplete knock out of ATF4 in the cell pool causes the minor upregulation of Noxa expression. We also showed that p53 is not involved in the induction of Noxa under GD induced necroptosis.
Noxa can be marked as a potential cancer therapeutic target as it increasingly suggests that the upregulation of this BH3-only protein can increase apoptotic mechanisms in tumor and DNA damage (Ploner et al., 2008; Shibue et al., 2003). But the proapoptotic potential of Noxa is considered limited which potentially indicates that the nature of the induction of cell death by Noxa is variable and muti-faceted (Ploner et al., 2008).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
SS: Data curation, Writing–original draft. RR: Data curation, Writing–original draft. Y-JP: Data curation, Writing–review and editing. JY: Data curation, Writing–review and editing. ZL: Data curation, Writing–review and editing. SJ: Investigation, Writing–review and editing. Z-GL: Conceptualization, Writing–review and editing. SC: Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
S.S. would like to thank the scholarship from the Royal Golden Jubilee Ph.D. Program Scholarship from National Research Council of Thailand (NRCT) (NRCT5-RGJ63001-023) and Second Century Fund (C2F) from Chulalongkorn University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fceld.2024.1507960/full#supplementary-material
Alves, N. L., Derks, I. A. M., Berk, E., Spijker, R., van Lier, R. A. W., and Eldering, E. (2006). The noxa/mcl-1 Axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity 24 (6), 703–716. doi:10.1016/j.immuni.2006.03.018
Baik, J. Y., Liu, Z., Jiao, D., Kwon, H.-J., Yan, J., Kadigamuwa, C., et al. (2021). ZBP1 not RIPK1 mediates tumor necroptosis in breast cancer. Nat. Commun. 12 (1), 2666. doi:10.1038/s41467-021-23004-3
Cai, Z., Jitkaew, S., Zhao, J., Chiang, H.-C., Choksi, S., Liu, J., et al. (2013). Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 16 (1), 55–65. doi:10.1038/ncb2883
Chen, X., Dai, Y., Wan, X., Hu, X.-M., Zhao, W., Ban, X.-X., et al. (2022). ZBP1-Mediated necroptosis: mechanisms and therapeutic implications. Molecules 28 (1), 52. doi:10.3390/molecules28010052
Chen, X., Li, W., Ren, J., Huang, D., He, W., Song, Y., et al. (2013). Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 24 (1), 105–121. doi:10.1038/cr.2013.171
Choi, M. E., Price, D. R., Ryter, S. W., and Choi, A. M. K. (2019). Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight 4 (15), e128834. doi:10.1172/jci.insight.128834
Chondrex Inc (2023). MTT cell proliferation and viability assay. Chondrex.com. Available at: https://www.chondrex.com/products/mtt-cell-proliferation-and-viability-assay.
Deer, E. L., González-Hernández, J., Coursen, J. D., Shea, J. E., Ngatia, J., Scaife, C. L., et al. (2010). Phenotype and genotype of pancreatic cancer cell lines. Pancreas 39 (4), 425–435. doi:10.1097/mpa.0b013e3181c15963
Dhuriya, Y. K., and Sharma, D. (2018). Necroptosis: a regulated inflammatory mode of cell death. J. Neuroinflammation 15 (1), 199. doi:10.1186/s12974-018-1235-0
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Agostinis, P., et al. (2018). Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death and Differ. 25 (3), 486–541. doi:10.1038/s41418-017-0012-4
Jiao, H., Wachsmuth, L., Kumari, S., Schwarzer, R., Lin, J., Eren, R. O., et al. (2020). Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 580 (7803), 391–395. doi:10.1038/s41586-020-2129-8
Kim, J.-Y., Ahn, H.-J., Jong, H. R., Suk, K., and Park, J.-H. (2003). BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1α. J. Exp. Med. 199 (1), 113–124. doi:10.1084/jem.20030613
León-Annicchiarico, C. L., Ramírez-Peinado, S., Domínguez-Villanueva, D., Gonsberg, A., Lampidis, T. J., and Muñoz-Pinedo, C. (2015). ATF4 mediates necrosis induced by glucose deprivation and apoptosis induced by 2-deoxyglucose in the same cells. FEBS J. 282 (18), 3647–3658. doi:10.1111/febs.13369
Lum, J. J., Bui, T., Gruber, M., Gordan, J. D., DeBerardinis, R. J., Covello, K. L., et al. (2007). The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes and Dev. 21 (9), 1037–1049. doi:10.1101/gad.1529107
Newton, K., Strasser, A., Nobuhiko, K., and Dixit, V. M. (2024). Cell death. Cell 187 (2), 235–256. doi:10.1016/j.cell.2023.11.044
Núñez-Vázquez, S., Sánchez-Vera, I., Saura-Esteller, J., Cosialls, A. M., Noisier, A. F. M., Albericio, F., et al. (2020). NOXA upregulation by the prohibitin-binding compound fluorizoline is transcriptionally regulated by integrated stress response-induced ATF3 and ATF4. FEBS J. 288 (4), 1271–1285. doi:10.1111/febs.15480
Oda, E., Ohki, R., Murasawa, H., Nemoto, J., Shibue, T., Yamashita, T., et al. (2000). Noxa, a BH3-only member of the bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288 (5468), 1053–1058. doi:10.1126/science.288.5468.1053
Okoshi, R., Ozaki, T., Yamamoto, H., Ando, K., Koida, N., Ono, S., et al. (2007). Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J. Biol. Chem. 283 (7), 3979–3987. doi:10.1074/jbc.m705232200
Ploner, C., Kofler, R., and Villunger, A. (2008). Noxa: at the tip of the balance between life and death. Oncogene 27 (Suppl. 1), S84–S92. doi:10.1038/onc.2009.46
Qing, G., Li, B., Vu, A., Skuli, N., Walton, Z. E., Liu, X., et al. (2012). ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 22 (5), 631–644. doi:10.1016/j.ccr.2012.09.021
Ran, F. A., Hsu, P. D., Wright, J., Agarwala, V., Scott, D. A., and Zhang, F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8 (11), 2281–2308. doi:10.1038/nprot.2013.143
Rödicker, F., and Pützer, B. M. (2021). p73 is effective in p53-null pancreatic cancer cells resistant to wild-type TP53 gene replacement. Cancer Res. 63 (11), 2737–2741. Available at: https://pubmed.ncbi.nlm.nih.gov/12782576/.
Rozita, B.--Y., Sinha, K. M., Gururaj, A. E., Ahmed, Z., Rizvi, Y. Q., Huang, S., et al. (2015). A novel dual kinase function of the RET proto-oncogene negatively regulates activating transcription factor 4-mediated apoptosis. J. Biol. Chem. 290 (18), 11749–11761. doi:10.1074/jbc.m114.619833
Seo, Y.-W., Shin, J. N., Ko, K. H., Cha, J. H., Park, J. Y., Lee, B. R., et al. (2003). The molecular mechanism of noxa-induced mitochondrial dysfunction in p53-mediated cell death. J. Biol. Chem. 278 (48), 48292–48299. doi:10.1074/jbc.M308785200
Sharma, K., Vu, T.-T., Cook, W., Naseri, M., Zhan, K., Nakajima, W., et al. (2018). p53-independent Noxa induction by cisplatin is regulated by ATF3/ATF4 in head and neck squamous cell carcinoma cells. Mol. Oncol. 12 (6), 788–798. doi:10.1002/1878-0261.12172
Shibue, T., Takeda, K., Oda, E., Tanaka, H., Murasawa, H., Takaoka, A., et al. (2003). Integral role of Noxa in p53-mediated apoptotic response. Genes and Dev. 17 (18), 2233–2238. doi:10.1101/gad.1103603
Sridharan, H., Ragan, K. B., Guo, H., Gilley, R. P., Landsteiner, V. J., Kaiser, W. J., et al. (2017). Murine cytomegalovirus IE3-dependent transcription is required for DAI/ZBP1-mediated necroptosis. EMBO Rep. 18 (8), 1429–1441. doi:10.15252/embr.201743947
Wang, H., Sun, L., Su, L., Rizo, J., Liu, L., Wang, L.-F., et al. (2014). Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54 (1), 133–146. doi:10.1016/j.molcel.2014.03.003
Wang, Q., Shinkre, B. A., Lee, J., Weniger, M. A., Liu, Y., Chen, W., et al. (2010). The ERAD inhibitor eeyarestatin I is a bifunctional compound with a membrane-binding domain and a p97/VCP inhibitory group. PLoS ONE 5 (11), e15479. doi:10.1371/journal.pone.0015479
Yan, J., Wan, P., Choksi, S., and Liu, Z.-G. (2022). Necroptosis and tumor progression. Trends Cancer 8 (1), 21–27. doi:10.1016/j.trecan.2021.09.003
Yan, J., Zhong, N., Liu, G., Chen, K., Liu, X., Su, L., et al. (2014). Usp9x- and Noxa-mediated Mcl-1 downregulation contributes to pemetrexed-induced apoptosis in human non-small-cell lung cancer cells. Cell Death and Dis. 5 (7), e1316. doi:10.1038/cddis.2014.281
Ye, K., Chen, Z., and Xu, Y. (2023). The double-edged functions of necroptosis. Cell Death and Dis. 14 (2), 163. doi:10.1038/s41419-023-05691-6
Keywords: glucose deprivation, Noxa, ATF4, necroptosis, cell death
Citation: Sonkaew S, Rajna R, Park Y-J, Yan J, Liu Z, Jitkaew S, Liu Z-G and Choksi S (2025) ATF4-mediated expression of NOXA is critical for necroptosis driven by glucose deprivation. Front. Cell. Death 3:1507960. doi: 10.3389/fceld.2024.1507960
Received: 08 October 2024; Accepted: 17 December 2024;
Published: 20 January 2025.
Edited by:
Michael Joseph Morgan, Northeastern State University, United StatesCopyright © 2025 Sonkaew, Rajna, Park, Yan, Liu, Jitkaew, Liu and Choksi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Swati Choksi, Y2hva3Npc0BtYWlsLm5paC5nb3Y=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.