
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 13 March 2025
Sec. Cancer Cell Biology
Volume 13 - 2025 | https://doi.org/10.3389/fcell.2025.1563184
This article is part of the Research Topic Microbiota Homeostasis and Metabolic Reprogramming in Cancer Development and Digestive Diseases View all articles
Colorectal liver metastasis (CRLM) represents a major therapeutic challenge in colorectal cancer (CRC), with complex interactions between the gut microbiota and the liver tumor microenvironment (TME) playing a crucial role in disease progression via the gut-liver axis. The gut barrier serves as a gatekeeper, regulating microbial translocation, which influences liver colonization and metastasis. Through the gut-liver axis, the microbiota actively shapes the TME, where specific microbial species and their metabolites exert dual roles in immune modulation. The immunologically “cold” nature of the liver, combined with the influence of the gut microbiota on liver immunity, complicates effective immunotherapy. However, microbiota-targeted interventions present promising strategies to enhance immunotherapy outcomes by modulating the gut-liver axis. Overall, this review highlights the emerging evidence on the role of the gut microbiota in CRLM and provides insights into the molecular mechanisms driving the dynamic interactions within the gut-liver axis.
Colorectal cancer (CRC) is the third most prevalent malignancy globally, accounting for approximately 10% of all cancer cases, and remains the second leading cause of cancer-related mortality worldwide (Siegel et al., 2024). Among metastatic patterns in CRC, colorectal liver metastasis (CRLM) is the most common and represents the primary cause of death in CRC patients (Ciraci et al., 2024). Approximately 30%–50% of CRC patients develop CRLM during the disease course, underscoring its clinical significance (Siegel et al., 2023). Notably, the incidence of metastatic liver cancer surpasses that of primary liver cancer by 18–40 times, with CRC being the most frequent primary source of liver metastases (Tsilimigras et al., 2021).
The tendency of CRC to preferentially metastasize to the liver can be explained by the liver’s unique blood supply, with 75% of blood flow through the portal vein from the colon and rectum, provides a direct route for CRC cells to reach the liver (Chow and Chok, 2019; Vidal-Vanaclocha, 2008). The hepatic sinusoids, where blood flow is slow and the vascular permeability is high, also create a favorable environment for tumor cell retention and colonization (Mielgo and Schmid, 2020; Poisson et al., 2017). However, it is important to recognize that metastasis is a systemic process, and the portal vein also carries a wide range of substances besides tumor cells.
The gut, where CRC originates, is home to a highly complex and dynamic microbiota, which plays a pivotal role in the development and progression of CRC. Dysbiosis, characterized by an imbalance in microbial composition, is a hallmark of CRC, fostering chronic inflammation and driving tumor progression. Specific microbial species, such as Fusobacterium nucleatum (Zepeda-Rivera et al., 2024; Wang and Fang, 2023; Yu et al., 2017), Bacteroides fragilis (Purcell et al., 2017; Chung et al., 2018a; Boleij et al., 2015), and Escherichia coli (Swidsinski et al., 1998; Bonnet et al., 2014; Prorok-Hamon et al., 2014), have been linked to enhanced tumor invasiveness through direct interactions with CRC cells and by modulating the immune microenvironment in a tumor-promoting direction (Tilg et al., 2018; Gao et al., 2022; Sears and Garrett, 2014). Moreover, microbial metabolites, such as short-chain fatty acids (SCFAs) and bile acids, can have dual roles, either protecting against or exacerbating tumorigenesis, depending on the broader environmental context (Cai et al., 2022; Cong et al., 2024; Ohigashi et al., 2013; Ocvirk and O'Keefe, 2021). These microbial products, along with other byproducts, travel to the liver via the portal vein, establishing a dynamic gut-liver connection, known as the gut-liver axis (Tilg et al., 2022).
Under normal conditions, the liver processes these molecules and maintains systemic homeostasis through bile acid secretion and immune responses (Pabst et al., 2023). However, CRC and dysbiosis can overwhelm these regulatory mechanisms, leading to a disrupted gut-liver axis (Albillos et al., 2020). This disruption facilitates the establishment of a pro-metastatic niche in the liver, marked by immune suppression and inflammation, creating a favorable environment for CRC cells to colonize and thrive. Therefore, this review explores the impact of the gut microbiota on CRLM through the gut-liver axis, focusing on the complex interplay between gut barrier, dysbiosis, microbial metabolites, and inflammation within the liver tumor microenvironment (TME). In addition, we discuss potential microbiota-targeted therapies, such as probiotics and prebiotics, which may offer new strategies to improve therapeutic outcomes in CRLM. Our goal is to provide a comprehensive perspective on CRLM and the gut-liver axis in the hope that bench discoveries can be effectively translated into clinical applications.
The gut barrier is a complex, multi-layered structure that extends from the mucus layer to epithelial tight junctions (TJs), host-microbe interactions, and the gut-vascular barrier (GVB) (Leshem et al., 2020; Rao et al., 2021). It serves as a protective defense, shielding the body from harmful substances such as bacteria and toxins. The GVB, as the key component, consisting of endothelial cells, pericytes, and enteric glia, specifically prevents bacteria and metabolites from translocating from the gut to the liver (Grander et al., 2020). While the intestinal barrier regulates gut permeability, the GVB offers an additional layer of defense at the gut-liver interface, underscoring the essential role of the gut barrier in maintaining gut-liver homeostasis (Spadoni et al., 2015). Disruption of the gut barrier can lead to liver diseases (Mouries et al., 2019).
Increased intestinal permeability, commonly referred to as “leaky gut,” is a key factor in the development of liver diseases, especially through the gut-liver axis (Aburto and Cryan, 2024). Disruption of the GVB is closely linked to dysbiosis of the intestinal microbiome (Grander et al., 2018; Depommier et al., 2019). Changes in the composition of mucin and thickness of this layer, shaped by the microbiota, have been observed in pre-epithelial mucus layer in the colon of rats (Szentkuti et al., 1990). This barrier also can be compromised in patients undergoing treatment for CRC, where factors such as operative trauma, post-surgical complications, chemotherapy cytotoxicity, and antibiotics, can severely damage gut integrity (Yang et al., 2024; Schietroma et al., 2016; Gibiino et al., 2022). For instance, dysbiosis induced by a high-fat diet has been shown to result in intestinal barrier disruption, facilitating the entry of microbial components and metabolites into the bloodstream. This disruption is considered a precursor for the development of liver disorders including non-alcoholic fatty liver disease, steatohepatitis, and hepatocellular carcinoma (HCC) (Brescia and Rescigno, 2021). Furthermore, gut vascular impairment is linked to the formation of a “pre-metastatic niche” in the liver, where molecular and cellular changes at distant sites attract circulating tumor cells and support future metastatic growth (Peinado et al., 2017; Bertocchi et al., 2021) (Figure 1). These observations emphasize the crucial role of maintaining the physical integrity of the gut barrier in the gut-liver axis to prevent disease progression.

Figure 1. Impaired gut barrier enables CRLM The impaired gut barrier is a pivotal mediator of gut-liver axis dysfunction, facilitating CRLM. Detrimental factors such as operative trauma, chemotherapy, dysbiosis, antibiotics, and high-fat diets compromise gut epithelial integrity, resulting in increased permeability. Leaky epithelium and GVB allow microbial products, including bacteria and their metabolites, to translocate via the portal vein to the liver. This process triggers pre-metastatic niche formation in the liver, characterized by extracellular matrix (ECM) remodeling and chemoattractant production.
Disruption of the GVB plays a pivotal role in the formation of a pre-metastatic niche in liver metastasis of CRC (Murota and Jobin, 2021). Under normal conditions, the GVB acts as a critical protective layer that prevents bacteria and other large molecules from translocating across the intestinal epithelium into the bloodstream. However, when the GVB is compromised—such as during CRC or intestinal infection—bacteria can migrate from the gut into the liver (Ray, 2021). Notably, E. coli, particularly those expressing the Virf1 virulence factor, have been found to breach the GVB, triggering a cascade of immune and extracellular matrix changes in the liver that foster a pre-metastatic environment. This disruption not only facilitates bacterial dissemination to the liver but also creates favorable conditions for the seeding and growth of metastatic cancer cells. Elevated levels of PV-1, a marker for GVB disruption, in primary tumors have been linked to increased bacterial presence in liver metastases, further emphasizing the connection between GVB breakdown and CRC liver metastasis (Bertocchi et al., 2021). These findings highlight the crucial role of GVB integrity in microbial translocation via the gut-liver axis and emphasize its potential as a therapeutic target for preventing the formation of pre-metastatic niches in CRC liver metastasis.
While the GVB plays a critical role in maintaining microbial integrity and preventing the translocation of harmful bacteria, any disruption in this barrier can lead to severe complications. One such complication is an anastomotic leak, which occurs when the integrity of a surgical connection between two bowel segments is compromised (Figure 1). Similar to the breakdown of the GVB, an anastomotic leak allows bacterial dissemination and can lead to systemic infections, increasing the risk of CRLM (Mirnezami et al., 2011; Goto et al., 2017). Recent studies in mouse models revealed that anastomotic leak was associated with microbiota mediated systemic inflammation and development of CRLM (Hajjar et al., 2024; Hajjar et al., 2023). Specifically, the microbiota in patients with anastomotic leak showed diminished capacity to activate PPAR-γ, a receptor that plays a critical role in antineoplastic defense in the gut. Dietary interventions, such as inulin and 5-aminosalicylate (5-ASA), which activate PPAR-γ, were found to enhance gut barrier integrity, reduce the formation of anastomotic tumors, and prevent metastatic spread to the liver in mice (Hajjar et al., 2024). These findings underscore the importance of preventing anastomotic leak to improve oncological outcomes after CRC surgery and suggest that dietary modulation of the gut microbiota offers a novel strategy for promoting anastomotic healing and reducing CRLM.
The gut microbiota significantly influences the inflammatory environment in CRC, contributing to tumor initiation and progression. Dysbiosis, or an imbalance in the gut microbiome, has been linked to the promotion of colonic inflammation, a key factor in CRC initiation and progression. Various pathobionts, such as F. nucleatum, B. fragilis, and Enterococcus faecalis, have been implicated in this process. These microorganisms activate inflammatory pathways, including IL-17, NF-κB, and pattern recognition receptors, which not only exacerbate inflammation but also impair the gut barrier function (Kostic et al., 2013; Wu et al., 2009; Ruiz et al., 2005). In particular, B. fragilis has been shown to initiate an inflammatory cascade by disrupting the gut barrier, leading to the activation of TH17 cells and further propagation of the inflammatory response (Chung et al., 2018b). This ongoing inflammation is closely linked to the development of CRC, as it creates a tumor-promoting environment by altering immune TME.
The modulation of the gut immune microenvironment by microbiota also plays a significant role in promoting CRC local invasion and distant metastasis. Microbial metabolites, such as formate produced by F. nucleatum, have been shown to drive cancer cell invasion and metastatic spread by activating key signaling pathways, including the aryl hydrocarbon receptor, which enhances cancer stemness (Ternes et al., 2022). Furthermore, diet-induced dysbiosis, particularly from a high-fat diet, can exacerbate gut inflammation and barrier dysfunction, creating a favorable environment for both CRC initiation and metastasis. For instance, increased abundance of Desulfovibrio in high-fat diet models contributes to inflammation in both the colorectum and liver, highlighting the link between gut microbiota changes and CRC metastasis (Yu et al., 2022). Together, these observations highlight that the gut microenvironment plays a foundational role in the gut-liver axis, setting the stage for metastasis.
The integrity of the gut barrier and the composition of the gut microbiota are intricately linked within the framework of the gut-liver axis, forming a dynamic system that regulates intestinal homeostasis and liver microenvironment. On the one hand, alterations in the gut microbiota—often manifesting as dysbiosis—can impair gut barrier function, exacerbating its permeability and fostering a cycle of microbial translocation. On the other hand, disruption of the gut barrier, through factors such as inflammation or infection, can create pathways for the translocation of gut microbiota into the liver, potentially enhancing metastatic dissemination and contributing to the formation of pre-metastatic niches in colorectal cancer.
The identity of microorganisms in primary and metastatic CRC suggests their translocation through the gut-liver axis. Fusobacterium nucleatum has been detected in 8%–13% of primary CRC in patient specimens (Nosho et al., 2016; Mima et al., 2015), with its abundance notably higher in CRC tissues from patients with metastasis (Chen et al., 2020). Notable, this bacterium is not only present in primary tumors but also in matched liver metastases, highlighting the stability of the microbiome between primary and metastatic sites (Bullman et al., 2017). Additionally, other associated species such as Bacteroides, Selenomonas, and Prevotella also remain consistent across both primary and metastatic tumors (Bullman et al., 2017) (Figure 2). These observations of microbiome stability from human samples provide direct evidence for the translocation of microbiome composition via the gut-liver axis, raising questions about if/how the gut microorganisms can directly influence its microenvironment.
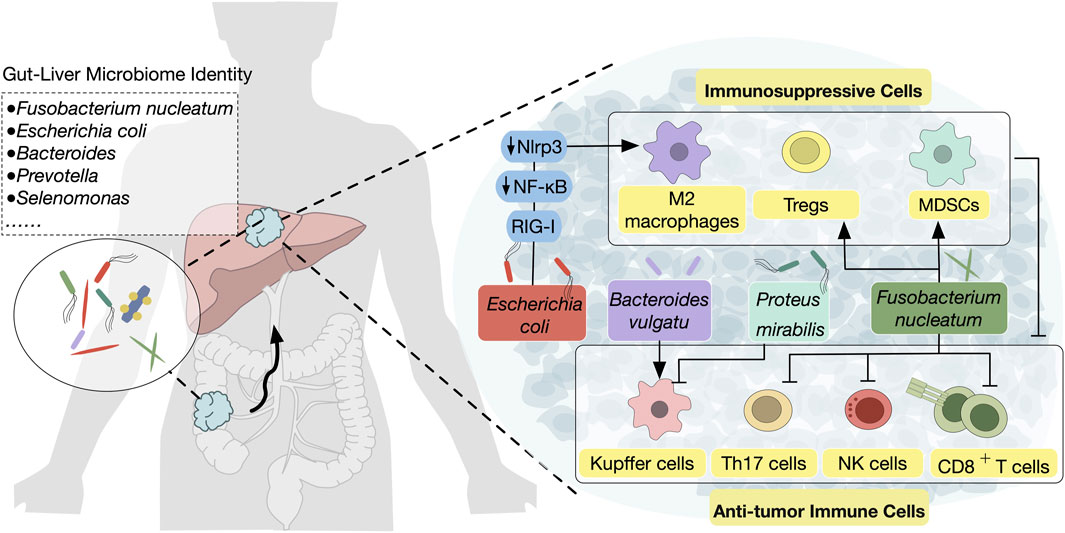
Figure 2. Microbiota translocation from the gut to the liver modulates the hepatic TME The complex interplay between gut microbiota and CRLM through the gut-liver axis are shown. Pathogenic bacteria such as Fusobacterium nucleatum, Escherichia coli, and Proteus mirabilis are translocated from the gut to the liver, where they contribute to tumor progression by inducing an immunosuppressive TME. Mechanisms include promoting the activation of MDSCs, Tregs, and M2 macrophages, which suppress anti-tumor immune populations such as NK cells, CD8+ T cells, and Kupffer cells. Conversely, certain bacterial species, such as Bacteroides vulgatus, can enhance anti-tumor immunity by increasing Kupffer cell activation.
The complex interplay between gut microbiota and the liver TME has garnered increasing attention, particularly regarding its role in modulating inflammatory responses and immune functions (Figure 2). For instance, a high-fat diet-induced microbiota imbalance, particularly with the abundance of Desulfovibrio, has been shown to promote gut barrier dysfunction and gut inflammation, creating a pro-inflammatory environment in the liver that favors CRC metastasis (Yu et al., 2022). Further investigation has revealed the involvement of specific bacteria in modulating immune responses. The balance of bacterial populations influences liver-resident Kupffer cells. Specifically, Bacteroides vulgatus has been linked to an increase in the proliferation of Kupffer cells, which subsequently reduces the incidence of CRLM. Conversely, bacteria such as Proteus mirabilis can lead to a reduction in Kupffer cells, promoting a more permissive environment for disseminating CRC cells (Yuan et al., 2022).
Fusobacterium nucleatum is a Gram-negative anaerobic bacterium and a known tumor-promoting bacterium that is often abundant in CRC tissues (Tilg et al., 2018). It also plays an important role in the progression of CRLMs by driving immune suppression in the liver microenvironment.
Recent evidence from patient tissue revealed that the presence of F. nucleatum in CRLM is associated with a reduced density of CD8+ T cells and an increased density of myeloid-derived suppressor cells (MDSCs), suggesting that F. nucleatum may contribute to immune suppression within the liver (Sakamoto et al., 2021). In response to F. nucleatum exposure, mice exhibit elevated plasma levels of pro-inflammatory cytokines such as IL-6 and TNF-α, along with a marked reduction in the hepatic infiltration of natural killer (NK) and T-helper 17 (Th17) cells—immune populations crucial for combating metastasis. Concurrently, an accumulation of regulatory T cells (Tregs) in the liver further fosters an immunosuppressive environment, providing a niche that supports CRC cell survival and growth (Yin et al., 2022).
Beyond direct bacterial colonization and immune modulation, F. nucleatum also influences the CRLM through a variety of secreted factors, one of the most intriguing being exosomes (Teng et al., 2018). These nanoscale vesicles facilitate the transfer of proteins, RNA, and lipids, which can significantly impact immune responses and tumor progression (Zhang et al., 2023). A recent study has highlighted the novel role of microbiota-associated exosomes in CRLM (Guo et al., 2020). Exosomes derived from F. nucleatum-infected CRC cells were found to contain miRNAs (such as miR-1246, miR-92b-3p, and miR-27a-3p) and proteins (including CXCL16, RhoA, and IL-8), which promote tumor cell migration and modulate systemic inflammation and immune responses. These exosomal markers were closely associated with F. nucleatum abundance and tumor stage in CRC patients, indicating their potential as biomarkers for CRLM. However, the precise mechanisms by which these exosomes modulate the liver TME remain to be fully explored.
Thus, these studies underscore F. nucleatum as a crucial driver of the immunosuppressive TME in CRLM, operating through both immune modulation and exosome secretion. The interplay between these mechanisms illustrates the complexity of F. nucleatum–host interactions, with microbial-driven metabolic and immune alterations playing a key role in CRLM progression. Disrupting these microbial influences, through targeted microbiota therapies or strategies to restore immune cell function, could enhance current treatments for metastatic CRC.
Escherichia coli is a ubiquitous bacterium that can be found in both the human gut and liver, where it has been implicated in various cancer-related processes (Arthur et al., 2012). While its role in primary CRC is largely attributed to its ability to promote tumorigenesis through the pks + genotype (Arthur et al., 2014), the pro-tumorigenic effect of E. coli in the liver, especially in CRLM, is distinctly driven by its inflammatory potential. In the liver, E. coli exerts a significant impact on immune modulation, fostering an environment conducive to metastasis.
One of the mechanisms through which E. coli contributes to this process is by influencing tumor metabolism (Gu et al., 2024; Wong and Yu, 2023). Lactate, as a byproduct of tumor metabolic reprogramming, has emerged as a potent factor influencing immune cell function (Xia et al., 2021). Specifically, lactate has been shown to drive M2 macrophage polarization and enhance the suppressive capabilities of regulatory T cells (Tregs), both of which contribute to an immunosuppressive TME. Furthermore, E. coli appears to play a pivotal role in this process, not only by altering metabolic pathways but also by influencing signaling cascades, such as the RIG-I-MAVS-NF-κB pathway, that regulate macrophage and Treg activity (Gu et al., 2024). The connection between E. coli-induced lactate production and immune phenotype changes challenges the traditional understanding of the microbiota as a passive factor in cancer progression. Instead, E. coli acts as a metabolic regulator that reprograms immune cells, creating an environment conducive to tumor growth and metastasis. The lactate-driven alteration of the immune microenvironment suggests that targeting this metabolic pathway could reprogram the immunosuppressive tumor milieu, potentially enhancing the effectiveness of current therapies. This highlights the need to consider microbiota-induced metabolic changes—specifically lactate production—as a critical factor in the development of new therapeutic strategies that target both tumor metabolism and immune modulation.
In addition to metabolic reprogramming, E. coli also promotes the recruitment and activation of innate immune cells, such as macrophages and inflammatory monocytes, which are key components of the hepatic pre-metastatic niche population. Translocation of E. coli into the liver results in preferential infiltration of these immune cells, further maintaining an inflammatory and pro-metastatic microenvironment (Bertocchi et al., 2021). In contrast, the introduction of beneficial bacteria such as Lactobacillus paracasei has been shown to reduce the infiltration of hepatic innate immune cells (Bertocchi et al., 2021), suggesting a protective effect against E. coli-induced immune reprogramming. This observation highlights the potential of microbial modulation as a therapeutic strategy to counteract the immune-dysregulatory effects of pathogenic bacteria in liver metastasis.
Microbiota-derived metabolites represent another powerful mechanism through which the gut microbiome influences liver function and pathology via gut-liver axis (Figure 3). The key metabolites—SCFAs and bile acids—are produced during microbial fermentation and metabolism within the gut (Koh et al., 2016; Lee et al., 2022). As signaling molecules, they not only regulate various physiological processes locally in the gut, but also crosstalk with the liver once released into the bloodstream. SCFAs, such as acetate, propionate, and butyrate, are produced by gut bacteria from dietary components, influencing host metabolism, epithelial barrier function, immune responses, and also liver processes like lipogenesis and gluconeogenesis (Martin-Gallausiaux et al., 2021). Bile acids, synthesized in the liver from cholesterol, are released into the small intestine as primary bile acids, where they aid in digestion and fat absorption. These primary bile acids are then modified by gut microbiota into secondary bile acids, reabsorbed in the terminal ileum and returned to the liver through the enterohepatic circulation, maintaining a balanced pool of bile acids for continuous use (Thakare et al., 2018). The gut microbiota significantly influences this bile acid metabolism, impacting not only digestive functions but also metabolic and immune regulation, thereby establishing a key link in the gut-liver axis (Guo et al., 2016; Hang et al., 2019; Campbell et al., 2020).

Figure 3. Gut microbiota-derived signals driving liver TME The influence of gut microbiota-derived metabolites on liver metastasis through the gut-liver axis is illustrated. Key interactions include microbial metabolites, such as LPS, engaging the TLR4/MD2 receptor complex, which activates the PI3K/AKT signaling pathway, facilitating CRC cell adhesion to liver sinusoidal endothelial cells. Concurrently, altered bile acid metabolism modulates NKT cell recruitment and T cell activity in the hepatic microenvironment, either promoting or suppressing metastatic progression. SCFAs, produced by commensal bacteria, can influence Th17 cell differentiation and enhance immune responses.
The pivotal role of gut microbiota in modulating liver tumor immunity through bile acid metabolism has been highlighted in a non-colorectal mouse model of liver metastases (Ma et al., 2018). This research demonstrated that Clostridium species can influence the growth of liver metastases by regulating the recruitment of NKT cells, a key immune cell subset involved in tumor surveillance. Specifically, the gut microbiota’s effect on bile acid metabolism has been found to play a central role in this process. Primary bile acids stimulate the expression of the chemokine CXCL16 in liver sinusoidal endothelial cells, which in turn recruits NKT cells to the liver. In contrast, secondary bile acids, produced by gut bacteria, suppress this effect. Notably, similar bile acid-mediated immune modulation patterns have been observed in human liver tissues, suggesting that this mechanism is relevant to CRLM and may open new therapeutic avenues for manipulating the gut microbiome in CRLM treatment (Dart, 2018).
In addition to NKT cells, bile acids have also been shown to affect tumor-specific CD8+ T cells in the liver, as evidenced in a recent study on mouse models of HCC (Varanasi et al., 2025). Elevated levels of the bile acid–conjugating enzyme BAAT (bile acid-CoA:amino acid N-acyltransferase) were found in HCC patients, with its deletion in mice leading to enhanced tumor-specific T cell responses and reduced tumor growth. Further mechanistic investigations revealed that primary bile acids induce oxidative stress in T cells, while certain secondary bile acids, such as lithocholic acid, impair T cell function through endoplasmic reticulum stress. Interestingly, ursodeoxycholic acid, a specific secondary bile acid, showed the opposite effect of lithocholic acid. Dietary supplementation with ursodeoxycholic acid has been found to mitigate this effect, restoring T cell function and enhancing the effectiveness of immunotherapy. Thus, bile acids exhibit a context-dependent and somewhat ambiguous role in regulating immune responses. While some bile acids, such as primary bile acids, impair T cell function, others, like ursodeoxycholic acid, demonstrate protective effects. This highlights the need for further research to better understand how bile acids modulate immune responses under different conditions, and how these effects can be harnessed to improve the efficacy of cancer immunotherapy.
Taken together, these findings highlight the complex dual role of bile acid metabolism in regulating liver immunity. Although current findings were primarily derived from HCC models, these insights are also highly relevant to CRLM, where similar immune suppression mechanisms in the liver TME could impair the effectiveness of immunotherapy. The modulation of bile acid metabolism could, therefore, serve as a promising therapeutic approach for improving immune responses and treatment outcomes in CRLM.
Conversely, SCFAs are closely linked to anti-tumor effects in CRC and CRLM (Wang et al., 2019). Dysbiosis, often observed in conditions like colitis and colitis-associated CRC, leads to a reduced abundance of SCFA-producing bacteria and a subsequent decrease in SCFA levels. This imbalance impairs the metabolic and immune responses of the host (Ibrahim et al., 2019). Notably, dietary interventions, such as high-fiber diets, have been shown to shift the microbiota composition towards SCFA-producing bacteria, improving gut health and potentially enhancing anti-tumor immunity (Bishehsari et al., 2018). In mouse models of CRLM, SCFA exposure has been demonstrated to increase the abundance of beneficial probiotic bacteria, improve anti-tumor responses, and reduce liver metastasis (Ma et al., 2020). Furthermore, the role of SCFAs in the gut-liver axis has been elucidated in HCC models (Li et al., 2016). Shotgun metagenome sequencing revealed crosstalk between gut microbial metabolites, T-cell differentiation, the secretion of inflammatory cytokines, and HCC tumorigenesis. Probiotic treatment, through the production of anti-inflammatory metabolites like SCFAs, was found to reduce the migration of Th17 cells from the intestine and peripheral blood to the liver and their polarization. This modulation of immune cell trafficking helps regulate the proinflammatory immune cell population, thereby slowing tumor progression and highlighting the potential of SCFAs and probiotics in cancer therapy (Li et al., 2016).
Lipopolysaccharide (LPS), a component of the outer membrane of Gram-negative bacteria, has been implicated in promoting tumorigenesis, with toll-like receptor 4 (TLR4) serving as its primary receptor on tumor cells (Park et al., 2009). Several studies in mouse models have demonstrated that LPS plays a critical role in promoting CRLM. A mechanistic example of this process is that LPS could enhance the metastatic capacity of CRC cells by facilitating their adhesion to the hepatic microvascular endothelial cells through the TLR4/MD2 complex, activating downstream signaling pathways such as PI3K/AKT (Hsu et al., 2011). In addition to directly influencing tumor cell behavior, LPS also shapes the immune microenvironment, contributing to an immunosuppressive milieu that hampers anti-tumor responses. Specifically, LPS has been associated with reduced efficacy of anti-PD-L1 immunotherapy in CRC, where blocking LPS or its receptor, TLR4, enhances T-cell infiltration into CRC, improves immune responses, and reduces liver metastasis (Song et al., 2018). Moreover, elevated LPS result from gut microbiota imbalance has been implicated in the secretion of cathepsin K (CTSK), which further promotes CRC metastasis by stimulating M2 polarization of tumor-associated macrophages (TAMs) through the TLR4/mTOR pathway (Li R. et al., 2019). Thus, blocking LPS within the gut-liver axis could provide a novel strategy for reducing CRC metastasis and enhancing immunotherapy outcomes.
Microbial metabolism generates various indole derivatives from tryptophan, which play critical roles in regulating host health and may influence cancer progression (Yu et al., 2024). These indole compounds, including I3C, IAld, and ILA, have been shown to inhibit the growth of CRC cells by reducing cell viability, promoting apoptosis (Megna et al., 2016; Sugimura et al., 2021). In addition, indole derivatives strengthen the intestinal barrier by modulating tight junctions and decreasing epithelial permeability, potentially preventing the metastatic spread of CRC to the liver via the gut-liver axis (Shin et al., 2020; Scott et al., 2020; Li et al., 2021; Jing et al., 2021). Furthermore, indoles exhibit anti-inflammatory effects by influencing immune responses, suggesting their potential to reduce chronic inflammation and inhibit CRLM (Cervantes-Barragan et al., 2017; Lei et al., 2020; Alexeev et al., 2021). However, current research remains limited, and direct evidence linking indole derivatives to CRLM is lacking. Future studies should focus on investigating the impact of indoles on CRC metastasis, particularly in the context of the gut-liver axis, to further assess their therapeutic potential in preventing or treating CRLM.
Among the mechanisms discussed, the influence of microbiota on immune response modulation stands out as a particularly promising strategy to overcome resistance and optimize immunotherapy. This underscores a critical opportunity for advancing the treatment of CRLM. The following section explores how the gut-liver axis can be leveraged to modulate the immune TME and enhance immunotherapy efficacy.
Immunotherapy, particularly immune checkpoint inhibitors (ICIs), has revolutionized cancer treatment by harnessing the immune system to combat malignancies (Chen and Mellman, 2017). The approval of ICIs, including anti-CTLA-4, anti-PD-1, and anti-PD-L1 antibodies, has led to durable clinical responses and long-term remissions in various cancers, including CRC (Topalian et al., 2012). These therapies function by blocking immune checkpoints, thereby reactivating the immune system’s ability to recognize and destroy tumor cells. However, the efficacy of ICB therapy in CRC remains limited, as only a subset of patients with microsatellite instability (MSI) or mismatch repair deficiency (dMMR) respond favorably to these treatments (Overman et al., 2018; Casak et al., 2021). The majority of CRC patients, who have microsatellite stability (MSS), do not benefit from ICB therapy, highlighting the challenge of identifying potential responders and the need to enhance the effectiveness of ICB therapy in this cohort.
Liver metastasis plays a significant role in the resistance to immunotherapy in mCRC. The liver, as an immune-tolerant organ, creates a unique microenvironment that impedes the effectiveness of ICIs. Recent clinical trials have shown that liver metastases are associated with poorer clinical outcomes in CRC patients receiving immunotherapy, including reduced survival and lower response rates (Cohen et al., 2024; Chen et al., 2023). The immune tolerance mechanisms of the liver, which include T cell anergy and suppression by myeloid-derived cells such as Kupffer cells and myeloid-derived suppressor cells, contribute to this resistance (Crispe, 2014). Moreover, liver metastasis not only disrupts local immune responses but also exerts a systemic impact on the immune system. This is evidenced by reduced T cell diversity and functionality in both patients and preclinical models with liver metastasis (Yu et al., 2021; Lindblad and Lujambio, 2021). The underlying mechanism involves the depletion of antigen-specific CD8+ T cells from the peripheral circulation, coupled with the induction of T cell apoptosis via Fas-FasL signaling in interaction with hepatic macrophages. This process results in the establishment of a systemic immune desert, further compromising the overall immune response and highlighting liver metastasis as a pivotal factor in immunotherapy failure in mCRC (Yu et al., 2021).
The gut microbiota, through its influence on the gut-liver axis, plays a pivotal role in modulating liver antitumor immunity. Recent studies suggest that interventions aimed at modulating the gut microbiome, such as the use of probiotics, prebiotics, or fecal microbiota transplantation (FMT), may enhance the sensitivity of CRLM to immunotherapy. These interventions can restore the balance of gut microbiota, enhance T cell priming, and improve systemic immune responses, thus mitigating the immune suppression in the liver microenvironment and promoting better outcomes in CRLM patients undergoing ICIs. Given the interplay between gut microbiota and liver immunity, targeting the gut-liver axis represents a promising strategy for overcoming the resistance to immunotherapy in mCRC and improving patient responses to treatment.
FMT has gained attention as a strategy to modulate the gut microbiome for improving cancer immunotherapy. By transferring microbiota from ICI-responsive donors to germ-free animals, FMT can mimic the human immune response to immunotherapy (Matson et al., 2018; Shaikh et al., 2022; Huang et al., 2022; Goc et al., 2021). In melanoma, clinical trials have shown that FMT from ICI-responsive patients can overcome resistance to anti-PD-1 therapy, highlighting its potential to enhance immunotherapy efficacy (Baruch et al., 2021; Davar et al., 2021).
Recently, pre-clinical research has demonstrated the potential of FMT as a strategy to enhance the effectiveness of immunotherapy in CRLM (Jiang et al., 2023). In patients with CRLM who did not respond to anti-PD-1 therapy, a higher abundance of F. nucleatum and elevated succinic acid levels were linked to immunotherapy resistance. Remarkably, fecal microbiota transfer from patients who responded well to immunotherapy—characterized by low F. nucleatum levels—was able to restore sensitivity to anti-PD-1 monoclonal antibody therapy in mice. This finding highlights FMT as a promising approach for reprogramming the gut microbiota and potentially overcoming resistance to immunotherapy.
Antibiotics have long been thought to impair the efficacy of ICIs, largely due to their disruptive effects on the gut microbiota. The depletion of beneficial microbial species through antibiotic treatment has been shown to diminish immune system function, resulting in impaired responses to immunotherapy (Vetizou et al., 2015; Routy et al., 2018; Gopalakrishnan et al., 2018; Xu et al., 2020). Clinical evidence supports this notion, with studies linking antibiotic use in cancer patients to poorer outcomes when receiving ICIs (Matson et al., 2018; Gopalakrishnan et al., 2018). The disruption of the microbiome seems to hinder the ability of the immune system to effectively target and destroy tumor cells, underlining the importance of a balanced gut microbiota in optimizing immunotherapy efficacy.
However, contrasting findings suggest that antibiotics might not always hinder, but at times could even enhance, the effectiveness of cancer immunotherapy, particularly by modifying the tumor microenvironment. For example, in models of CRLM, broad-spectrum antibiotics (antibiotic cocktail including vancomycin hydrochloride, neomycin sulfate and ampicillin sodium) have been observed to reduce bacterial load both in the gut and liver, leading to decreased immune suppression in the liver metastatic niche. By alleviating the immunosuppressive effects of gut-derived E. coli, antibiotic treatment may improve the ability of immune system to combat tumor growth, thereby enhancing the response to ICIs (Bertocchi et al., 2021).
In this context, a Phase II clinical trial investigating the combination of tadalafil, nivolumab, and oral vancomycin in HCC and CRLM patients further complicates the narrative (D'Annibale et al., 2024). Building on the observation that secondary bile acids produced by gut bacteria can suppress the recruitment of NKT cells, the trial aimed to modulate the gut microbiome in order to enhance immune responses through bile acid metabolism and improved NKT cell recruitment (Dart, 2018). However, the treatment failed to produce meaningful clinical responses. While changes in bile acid levels and immune cell activity were observed, NKT cell activity was not enhanced, likely due to the accumulation of MDSCs in the tumor microenvironment (D'Annibale et al., 2024).
Alternatively, bacteriophages provide a more targeted approach compared to antibiotics, as they can selectively eliminate specific bacterial species or strains (Shkoporov et al., 2022). This precision could help preserve beneficial microbes while addressing harmful bacteria, offering a promising alternative for enhancing cancer immunotherapy outcomes.
Probiotics and engineered bacterial products have shown promising potential in enhancing the efficacy of ICIs in CRC, renal cell carcinoma, and melanoma. Specific strains, such as Akkermansia muciniphila, B. fragilis, and Bifidobacterium, as well as probiotic cocktails like Probio-M9 and VB800, have demonstrated the ability to potentiate ICI activity in preclinical models (Matson et al., 2018; Vetizou et al., 2015; Routy et al., 2018; Si et al., 2022; Li Y. et al., 2019; Zhang et al., 2022; Gao et al., 2021), with VB800 currently undergoing clinical trials in combination with nivolumab for MSS CRC patients (Tanoue et al., 2019). Furthermore, bioengineered bacterial therapies are offering new solutions by directly influencing the tumor environment. For example, in CRC mouse models, genetically modified E. coli that enhances l-arginine availability within tumors has been shown to strengthen T-cell activity, while engineered Lactobacillus lactis expressing specific immune-stimulating SagA proteins could enhance the response to ICIs (Griffin et al., 2021; Canale et al., 2021).
Engineered probiotics offer a novel strategy to address the immunologically suppressed microenvironment of MSS CRLM by leveraging their natural ability to selectively colonize tumor tissue (Danino et al., 2015). For example, E. coli Nissle 1917 has been developed as a delivery system for ICIs targeting PD-L1 and CTLA-4 (Gurbatri et al., 2020). Using a stabilized lysing release mechanism, this engineered probiotic can enable precise intratumoral delivery of nanobodies, enhancing local immune activation while reducing systemic toxicity. In preclinical models, the system outperformed traditional antibody therapies, inducing tumor regression, enhancing T-cell activation, and promoting systemic effects such as increased T-cell memory and an abscopal response. Combining this platform with cytokines like GM-CSF further amplified therapeutic efficacy, particularly in poorly immunogenic tumor models (Gurbatri et al., 2020). This innovative approach integrates synthetic biology with immunotherapy to refine checkpoint blockade delivery and improve outcomes in MSS CRLM.
Similarly, an innovative LPS-trap nanoparticle system has shown substantial potential in enhancing immunotherapy for CRLM. By selectively targeting and neutralizing LPS within the tumor microenvironment, this system could mitigate LPS-driven immunosuppression, restore T-cell infiltration, and significantly improve anti-PD-L1 therapy outcomes. In mouse models of MSS CRC, the LPS-trap system not only boosted immunotherapy efficacy but also effectively reduced liver metastases, highlighting its transformative role in modulating the gut-liver axis to enhance therapeutic outcomes (Song et al., 2018).
Recently, F. nucleatum, traditionally regarded as a pathogenic bacterium in CRC, has been revealed to play a paradoxical role in enhancing immunotherapy efficacy in MSS CRC (Wang et al., 2024). Specifically, research indicated that intratumoral F. nucleatum can produce butyric acid, which modulated CD8+ T cells by inhibiting histone deacetylase activity. This inhibition was shown to reduce PD-1 expression and reactivate T cell function, alleviating immune exhaustion and improving therapeutic responses. Both clinical and preclinical data further demonstrated that elevated levels of F. nucleatum within tumors were associated with improved outcomes in MSS CRC patients treated with anti-PD-1 therapy. These findings suggest a dual role for F. nucleatum—as both a pathogen and a potential enhancer of immunotherapy—emphasizing the intricate and multifaceted relationship between tumor-associated microbiota and immune modulation.
Given the contradictory findings and the complexity of underlying mechanisms, combining microbiota-based approaches with ICI therapy requires careful consideration. Future research should focus on the design and validation of targeted microbiota therapies to ensure both safety and efficacy, with their potential thoroughly evaluated in well-designed clinical trials.
As discussed above, microbiota-based interventions, including FMT, probiotics, prebiotics, and engineered bacteriophages, hold significant potential in CRC treatment by enhancing chemotherapy, improving immunotherapy efficacy, and mitigating treatment-associated side effects (Qu et al., 2023; Wang et al., 2023; White and Sears, 2024; Lang et al., 2023). While some of these strategies remain in preclinical stages, others have advanced to clinical trials, highlighting their translational relevance. A detailed summary of key clinical trials exploring microbiota applications in CRC is presented in (Table 1).
Microbiota-enhanced immunotherapy has shown promising results in clinical trials involving renal cell carcinoma (RCC) and melanoma (Baruch et al., 2021; Davar et al., 2021; Dizman et al., 2022). In CRC, preclinical evidence, particularly from murine studies, further supports the potential of microbiota-modulated immunotherapy. For example, a study assessing the combined effect of FMT and anti-PD-1 therapy in colorectal tumor-bearing mice demonstrated significantly improved survival and tumor control when compared to either treatment alone. Metagenomic analyses revealed notable shifts in the gut microbiota composition, with specific genera, including Bacteroides, identified as crucial contributors to the enhanced efficacy of anti-PD-1 therapy (Huang et al., 2022). Furthermore, metabolomic analyses identified potential metabolites, such as punicic acid and aspirin, which may exert immunomodulatory effects that further support the therapeutic impact of immunotherapy (Xu et al., 2020). These findings have deepened our understanding of how microbiota interventions can improve the clinical effectiveness of PD-1 inhibitors in CRC.
Clinical trials investigating microbiota-based interventions in conjunction with immunotherapy have also demonstrated encouraging results. A recent phase Ib/II trial exploring the combination of regorafenib and toripalimab in metastatic CRC reported an objective response rate (ORR) of 15.2% and a disease control rate (DCR) of 36.4%. The study found that Fusobacterium abundance in baseline fecal samples was associated with poor progression-free survival (PFS), suggesting that gut microbiota composition may influence treatment outcomes (Wang et al., 2021). Furthermore, a phase II trial evaluating the combination of FMT, tislelizumab, and fruquintinib in refractory MSS metastatic CRC revealed improved survival, with a median PFS of 9.6 months and an ORR of 20%. Notably, patients with high abundance of Proteobacteria and Lachnospiraceae showed better treatment response (Zhao et al., 2023). In a separate study (NCT04208958), the combination of VE800 with nivolumab in metastatic CRC demonstrated a median overall survival of 7.6 months and a PFS of 1.8 months. Although these results were limited, they highlight the potential of microbiota interventions to enhance immune checkpoint blockade. Together, these studies underscore the growing potential of microbiota modulation as a complementary strategy to enhance the efficacy of immunotherapy in CRC, providing promising therapeutic options for patients with refractory disease.
The process of CRLM follows a multi-step cascade, where microbiota and their metabolites influence each stage, from local invasion of CRC cells to their colonization and growth in the liver. This cascade is critically regulated through the gut-liver axis, a bidirectional communication pathway between the gut microbiota and the liver. The role of microbiota in each step of this cascade is complex and, in many cases, contradictory. While some microbes and microbial products promote cancer progression, others can enhance immune responses or mitigate tumor growth. As such, understanding the role of microbiota in the CRLM cascade is crucial for uncovering therapeutic strategies that can manipulate the gut-liver axis to improve immunotherapy outcomes in liver metastasis.
The influence of microbiota begins in the primary tumor, where dysbiosis plays a pivotal role in CRC progression and invasion. Certain bacterial species, such as F. nucleatum, E. coli, and B. fragilis contribute to tumorigenesis by creating a pro-inflammatory microenvironment and altering immune cell activity (Tilg et al., 2018; Gao et al., 2022; Sears and Garrett, 2014). These microorganisms have been shown to modulate the immune response in ways that promote tumor invasion and progression. However, the role of theses microbiota is not purely negative. In fact, some of them have been identified to enhance the efficacy of immunotherapy under certain conditions (Yuan et al., 2022; Wang et al., 2024), complicating the overall understanding of microbiota as both tumorigenic and immune-modulating agents.
Similarly, antibiotics, which are commonly used in the treatment of cancer patients to prevent infections, have a complex and often contradictory effect on immunotherapy outcomes. On the one hand, antibiotics can impair the gut microbiota, reducing diversity and disrupting the balance of beneficial microbes that support immune function. This disruption is often associated with reduced efficacy of ICB therapies, as the microbiota is crucial for the optimal activation of immune responses (Vetizou et al., 2015; Routy et al., 2018; Gopalakrishnan et al., 2018; Xu et al., 2020). On the other hand, the use of antibiotics may also reduce the abundance of certain pathogenic microbes that actively suppress the immune system. Thus, while antibiotics can inadvertently reduce the immune-enhancing potential of the microbiota, they may also provide benefits by alleviating microbial-induced immunosuppression (Bertocchi et al., 2021; Dart, 2018). The challenge lies in identifying the specific microbial species and understanding the precise mechanisms by which antibiotics influence immunotherapy efficacy, as the effects can vary depending on the type of bacteria being targeted.
Bile acids, a class of metabolites produced by the liver and influenced by gut microbiota, further exemplify the dual role of microbiota in immune modulation. Primary bile acids, synthesized in the liver, play a key role in regulating immune responses in the liver, particularly through the recruitment of NKT cells, which are essential for anti-tumor immunity (Dart, 2018). However, certain secondary bile acids, such as lithocholic acid, impair the function of tumor-specific T cells by inducing endoplasmic reticulum stress (Varanasi et al., 2025). To add to the complexity, ursodeoxycholic acid, another secondary bile acid was shown to enhance T cell function and improve the response to immunotherapy (Varanasi et al., 2025). This highlights the complex and often contradictory effects of bile acids in immune modulation and suggests that manipulating bile acid metabolism could offer a promising therapeutic strategy to enhance immunotherapy efficacy in CRLM.
To resolve the contradictions in the dual roles of microbiota and optimize its therapeutic potential, future research should focus on developing targeted microbiota-based interventions that selectively enhance beneficial microbes while suppressing harmful ones. Personalized microbiome modulation tailored to an individual’s microbial composition, could maximize immune-boosting effects and minimize tumor-promoting influences (Ratiner et al., 2024). Advances in metagenomic sequencing and microbiome analysis will be key to identifying specific microbial populations and their metabolites that influence CRC progression and immunotherapy response (Gu et al., 2019). Additionally, a deeper understanding of the gut-liver axis, particularly how microbiota and metabolites such as bile acids affect liver immunity, will provide new targets for improving CRLM treatment. The use of bacteriophages, capable of selectively eliminating pathogenic bacteria, offers a promising approach for achieving high specificity in microbiota modulation without disturbing beneficial microbes (Shkoporov et al., 2022). Combining these insights with current cancer therapies may lead to more effective, personalized treatment strategies for CRC and CRLM patients.
Future research on the gut microbiome in cancer immunotherapy should focus on larger clinical trials to better understand its role in treatment outcomes. Current studies with limited sample sizes need to be expanded to facilitate more robust subgroup analyses, accounting for patient health status, cancer types, and immunotherapy regimens (Sharafi et al., 2022). This approach will help clarify how the microbiome impacts responses to treatments, as seen in the varying relevance of PD-L1 expression in different cancer subtypes. Furthermore, precise classification of patient responses using criteria like response evaluation criteria in solid tumors (RECIST) will provide more detailed insights into the microbiome’s influence on treatment efficacy.
In addition, combination immunotherapies are becoming more common, but their impact on immune-related adverse events (irAEs) and treatment outcomes, particularly in relation to the gut microbiome, remains underexplored (Baxi et al., 2018). Exploring biomarkers for irAEs in these settings is crucial. Advances in metagenomics and multi-omics approaches offer promising tools to uncover the microbiome’s functional role, which could inform more targeted therapies. New technologies such as microbiome imaging and artificial intelligence-based models could enhance predictive capabilities, while standardizing methods across studies for immunotherapy trials will ensure more reliable results.
Q-LL: Conceptualization, Investigation, Writing–original draft. HZ: Conceptualization, Investigation, Writing–original draft. ZW: Supervision, Writing–review and editing. YC: Conceptualization, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (82003113).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aburto, M. R., and Cryan, J. F. (2024). Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota-gut-brain axis. Nat. Rev. Gastroenterol. Hepatol. 21 (4), 222–247. doi:10.1038/s41575-023-00890-0
Albillos, A., de Gottardi, A., and Rescigno, M. (2020). The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72 (3), 558–577. doi:10.1016/j.jhep.2019.10.003
Alexeev, E. E., Dowdell, A. S., Henen, M. A., Lanis, J. M., Lee, J. S., Cartwright, I. M., et al. (2021). Microbial-derived indoles inhibit neutrophil myeloperoxidase to diminish bystander tissue damage. FASEB J. 35 (5), e21552. doi:10.1096/fj.202100027r
Arthur, J. C., Gharaibeh, R. Z., Muhlbauer, M., Perez-Chanona, E., Uronis, J. M., McCafferty, J., et al. (2014). Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat. Commun. 5, 4724. doi:10.1038/ncomms5724
Arthur, J. C., Perez-Chanona, E., Muhlbauer, M., Tomkovich, S., Uronis, J. M., Fan, T. J., et al. (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338 (6103), 120–123. doi:10.1126/science.1224820
Baruch, E. N., Youngster, I., Ben-Betzalel, G., Ortenberg, R., Lahat, A., Katz, L., et al. (2021). Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 371 (6529), 602–609. doi:10.1126/science.abb5920
Baxi, S., Yang, A., Gennarelli, R. L., Khan, N., Wang, Z., Boyce, L., et al. (2018). Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 360, k793. doi:10.1136/bmj.k793
Bertocchi, A., Carloni, S., Ravenda, P. S., Bertalot, G., Spadoni, I., Lo Cascio, A., et al. (2021). Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 39 (5), 708–724.e11. doi:10.1016/j.ccell.2021.03.004
Bishehsari, F., Engen, P. A., Preite, N. Z., Tuncil, Y. E., Naqib, A., Shaikh, M., et al. (2018). Dietary fiber treatment corrects the composition of gut microbiota, promotes SCFA production, and suppresses colon carcinogenesis. Genes (Basel) 9 (2), 102. doi:10.3390/genes9020102
Boleij, A., Hechenbleikner, E. M., Goodwin, A. C., Badani, R., Stein, E. M., Lazarev, M. G., et al. (2015). The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 60 (2), 208–215. doi:10.1093/cid/ciu787
Bonnet, M., Buc, E., Sauvanet, P., Darcha, C., Dubois, D., Pereira, B., et al. (2014). Colonization of the human gut by E. coli and colorectal cancer risk. Clin. Cancer Res. 20 (4), 859–867. doi:10.1158/1078-0432.CCR-13-1343
Brescia, P., and Rescigno, M. (2021). The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol. Med. 27 (9), 844–855. doi:10.1016/j.molmed.2021.06.007
Bullman, S., Pedamallu, C. S., Sicinska, E., Clancy, T. E., Zhang, X., Cai, D., et al. (2017). Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358 (6369), 1443–1448. doi:10.1126/science.aal5240
Cai, J., Sun, L., and Gonzalez, F. J. (2022). Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30 (3), 289–300. doi:10.1016/j.chom.2022.02.004
Campbell, C., McKenney, P. T., Konstantinovsky, D., Isaeva, O. I., Schizas, M., Verter, J., et al. (2020). Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581 (7809), 475–479. doi:10.1038/s41586-020-2193-0
Canale, F. P., Basso, C., Antonini, G., Perotti, M., Li, N., Sokolovska, A., et al. (2021). Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598 (7882), 662–666. doi:10.1038/s41586-021-04003-2
Casak, S. J., Marcus, L., Fashoyin-Aje, L., Mushti, S. L., Cheng, J., Shen, Y. L., et al. (2021). FDA approval summary: pembrolizumab for the first-line treatment of patients with MSI-H/dMMR advanced unresectable or metastatic colorectal carcinoma. Clin. Cancer Res. 27 (17), 4680–4684. doi:10.1158/1078-0432.ccr-21-0557
Cervantes-Barragan, L., Chai, J. N., Tianero, M. D., Di Luccia, B., Ahern, P. P., Merriman, J., et al. (2017). Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 357 (6353), 806–810. doi:10.1126/science.aah5825
Chen, D. S., and Mellman, I. (2017). Elements of cancer immunity and the cancer-immune set point. Nature 541 (7637), 321–330. doi:10.1038/nature21349
Chen, E. X., Loree, J. M., Titmuss, E., Jonker, D. J., Kennecke, H. F., Berry, S., et al. (2023). Liver metastases and immune checkpoint inhibitor efficacy in patients with refractory metastatic colorectal cancer: a secondary analysis of a randomized clinical trial. JAMA Netw. Open 6 (12), e2346094. doi:10.1001/jamanetworkopen.2023.46094
Chen, Y., Chen, Y., Zhang, J., Cao, P., Su, W., Deng, Y., et al. (2020). Fusobacterium nucleatum promotes metastasis in colorectal cancer by activating autophagy signaling via the upregulation of CARD3 expression. Theranostics 10 (1), 323–339. doi:10.7150/thno.38870
Chow, F. C., and Chok, K. S. (2019). Colorectal liver metastases: an update on multidisciplinary approach. World J. Hepatol. 11 (2), 150–172. doi:10.4254/wjh.v11.i2.150
Chung, L., Orberg, E. T., Geis, A. L., Chan, J. L., Fu, K., DeStefano Shields, C. E., et al. (2018a). Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host Microbe 23 (3), 421. doi:10.1016/j.chom.2018.02.004
Chung, L., Thiele Orberg, E., Geis, A. L., Chan, J. L., Fu, K., DeStefano Shields, C. E., et al. (2018b). Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host Microbe 23 (2), 203–214. doi:10.1016/j.chom.2018.01.007
Ciraci, P., Studiale, V., Taravella, A., Antoniotti, C., and Cremolini, C. (2024). Late-line options for patients with metastatic colorectal cancer: a review and evidence-based algorithm. Nat. Rev. Clin. Oncol. 22, 28–45. doi:10.1038/s41571-024-00965-0
Cohen, R., Raeisi, M., Chibaudel, B., Shi, Q., Yoshino, T., Zalcberg, J. R., et al. (2024). Prognostic value of liver metastases in colorectal cancer treated by systemic therapy: an ARCAD pooled analysis. Eur. J. Cancer 207, 114160. doi:10.1016/j.ejca.2024.114160
Cong, J., Liu, P., Han, Z., Ying, W., Li, C., Yang, Y., et al. (2024). Bile acids modified by the intestinal microbiota promote colorectal cancer growth by suppressing CD8(+) T cell effector functions. Immunity 57 (4), 876–889.e11. doi:10.1016/j.immuni.2024.02.014
Crispe, I. N. (2014). Immune tolerance in liver disease. Hepatology 60 (6), 2109–2117. doi:10.1002/hep.27254
Danino, T., Prindle, A., Kwong, G. A., Skalak, M., Li, H., Allen, K., et al. (2015). Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 7 (289), 289ra84. doi:10.1126/scitranslmed.aaa3519
D'Annibale, K. C., Ma, C., B, M. C. M., Xie, C., Hrones, D. M., Awosika, J., et al. (2024). Altering the gut microbiome and TME in advanced liver cancers: a phase II study of nivolumab, tadalafil, and oral vancomycin in refractory HCC or liver-dominant metastatic CRC or PDAC. J. Clin. Oncol. 42 (16_Suppl. l), e14642–e. doi:10.1200/jco.2024.42.16_suppl.e14642
Dart, A. (2018). Gut microbiota bile acid metabolism controls cancer immunosurveillance. Nat. Rev. Microbiol. 16 (8), 453. doi:10.1038/s41579-018-0053-9
Davar, D., Dzutsev, A. K., McCulloch, J. A., Rodrigues, R. R., Chauvin, J. M., Morrison, R. M., et al. (2021). Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371 (6529), 595–602. doi:10.1126/science.abf3363
Depommier, C., Everard, A., Druart, C., Plovier, H., Van Hul, M., Vieira-Silva, S., et al. (2019). Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25 (7), 1096–1103. doi:10.1038/s41591-019-0495-2
Dizman, N., Meza, L., Bergerot, P., Alcantara, M., Dorff, T., Lyou, Y., et al. (2022). Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat. Med. 28 (4), 704–712. doi:10.1038/s41591-022-01694-6
Gao, G., Ma, T., Zhang, T., Jin, H., Li, Y., Kwok, L. Y., et al. (2021). Adjunctive probiotic Lactobacillus rhamnosus probio-M9 administration enhances the effect of anti-PD-1 antitumor therapy via restoring antibiotic-disrupted gut microbiota. Front. Immunol. 12, 772532. doi:10.3389/fimmu.2021.772532
Gao, R., Wu, C., Zhu, Y., Kong, C., Zhu, Y., Gao, Y., et al. (2022). Integrated analysis of colorectal cancer reveals cross-cohort gut microbial signatures and associated serum metabolites. Gastroenterology 163 (4), 1024–1037.e9. doi:10.1053/j.gastro.2022.06.069
Gibiino, G., Binda, C., Cristofaro, L., Sbrancia, M., Coluccio, C., Petraroli, C., et al. (2022). Dysbiosis and gastrointestinal surgery: current insights and future research. Biomedicines 10 (10), 2532. doi:10.3390/biomedicines10102532
Goc, J., Lv, M., Bessman, N. J., Flamar, A. L., Sahota, S., Suzuki, H., et al. (2021). Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 184 (19), 5015–5030.e16. doi:10.1016/j.cell.2021.07.029
Gopalakrishnan, V., Spencer, C. N., Nezi, L., Reuben, A., Andrews, M. C., Karpinets, T. V., et al. (2018). Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359 (6371), 97–103. doi:10.1126/science.aan4236
Goto, S., Hasegawa, S., Hida, K., Uozumi, R., Kanemitsu, Y., Watanabe, T., et al. (2017). Multicenter analysis of impact of anastomotic leakage on long-term oncologic outcomes after curative resection of colon cancer. Surgery 162 (2), 317–324. doi:10.1016/j.surg.2017.03.005
Grander, C., Adolph, T. E., Wieser, V., Lowe, P., Wrzosek, L., Gyongyosi, B., et al. (2018). Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67 (5), 891–901. doi:10.1136/gutjnl-2016-313432
Grander, C., Grabherr, F., Spadoni, I., Enrich, B., Oberhuber, G., Rescigno, M., et al. (2020). The role of gut vascular barrier in experimental alcoholic liver disease and A. muciniphila supplementation. Gut Microbes 12 (1), 1851986. doi:10.1080/19490976.2020.1851986
Griffin, M. E., Espinosa, J., Becker, J. L., Luo, J. D., Carroll, T. S., Jha, J. K., et al. (2021). Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science 373 (6558), 1040–1046. doi:10.1126/science.abc9113
Gu, J., Xu, X., Li, X., Yue, L., Zhu, X., Chen, Q., et al. (2024). Tumor-resident microbiota contributes to colorectal cancer liver metastasis by lactation and immune modulation. Oncogene 43 (31), 2389–2404. doi:10.1038/s41388-024-03080-7
Gu, W., Miller, S., and Chiu, C. Y. (2019). Clinical metagenomic next-generation sequencing for pathogen detection. Annu. Rev. Pathol. 14, 319–338. doi:10.1146/annurev-pathmechdis-012418-012751
Guo, C., Xie, S., Chi, Z., Zhang, J., Liu, Y., Zhang, L., et al. (2016). Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity 45 (4), 802–816. doi:10.1016/j.immuni.2016.09.008
Guo, S., Chen, J., Chen, F., Zeng, Q., Liu, W. L., and Zhang, G. (2020). Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut 70, 1507–1519. doi:10.1136/gutjnl-2020-321187
Gurbatri, C. R., Lia, I., Vincent, R., Coker, C., Castro, S., Treuting, P. M., et al. (2020). Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 12 (530). doi:10.1126/scitranslmed.aax0876
Hajjar, R., Gonzalez, E., Fragoso, G., Oliero, M., Alaoui, A. A., Calve, A., et al. (2023). Gut microbiota influence anastomotic healing in colorectal cancer surgery through modulation of mucosal proinflammatory cytokines. Gut 72 (6), 1143–1154. doi:10.1136/gutjnl-2022-328389
Hajjar, R., Oliero, M., Fragoso, G., Ajayi, A. S., Alaoui, A. A., Vennin, R. H., et al. (2024). Modulating gut microbiota prevents anastomotic leak to reduce local implantation and dissemination of colorectal cancer cells after surgery. Clin. Cancer Res. 30 (3), 616–628. doi:10.1158/1078-0432.ccr-23-1601
Hang, S., Paik, D., Yao, L., Kim, E., Trinath, J., Lu, J., et al. (2019). Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 576 (7785), 143–148. doi:10.1038/s41586-019-1785-z
Hsu, R. Y., Chan, C. H., Spicer, J. D., Rousseau, M. C., Giannias, B., Rousseau, S., et al. (2011). LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 71 (5), 1989–1998. doi:10.1158/0008-5472.CAN-10-2833
Huang, J., Zheng, X., Kang, W., Hao, H., Mao, Y., Zhang, H., et al. (2022). Metagenomic and metabolomic analyses reveal synergistic effects of fecal microbiota transplantation and anti-PD-1 therapy on treating colorectal cancer. Front. Immunol. 13, 874922. doi:10.3389/fimmu.2022.874922
Ibrahim, A., Hugerth, L. W., Hases, L., Saxena, A., Seifert, M., Thomas, Q., et al. (2019). Colitis-induced colorectal cancer and intestinal epithelial estrogen receptor beta impact gut microbiota diversity. Int. J. Cancer 144 (12), 3086–3098. doi:10.1002/ijc.32037
Jiang, S. S., Xie, Y. L., Xiao, X. Y., Kang, Z. R., Lin, X. L., Zhang, L., et al. (2023). Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe 31 (5), 781–797.e9. doi:10.1016/j.chom.2023.04.010
Jing, W., Dong, S., Luo, X., Liu, J., Wei, B., Du, W., et al. (2021). Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites. Pharmacol. Res. 164, 105358. doi:10.1016/j.phrs.2020.105358
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Backhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165 (6), 1332–1345. doi:10.1016/j.cell.2016.05.041
Kostic, A. D., Chun, E., Robertson, L., Glickman, J. N., Gallini, C. A., Michaud, M., et al. (2013). Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14 (2), 207–215. doi:10.1016/j.chom.2013.07.007
Lang, T., Zhu, R., Zhu, X., Yan, W., Li, Y., Zhai, Y., et al. (2023). Combining gut microbiota modulation and chemotherapy by capecitabine-loaded prebiotic nanoparticle improves colorectal cancer therapy. Nat. Commun. 14 (1), 4746. doi:10.1038/s41467-023-40439-y
Lee, J. Y., Tsolis, R. M., and Baumler, A. J. (2022). The microbiome and gut homeostasis. Science 377 (6601), eabp9960. doi:10.1126/science.abp9960
Lei, C., Mu, J., Teng, Y., He, L., Xu, F., Zhang, X., et al. (2020). Lemon exosome-like nanoparticles-manipulated probiotics protect mice from C. d iff infection. iScience 23 (10), 101571. doi:10.1016/j.isci.2020.101571
Leshem, A., Liwinski, T., and Elinav, E. (2020). Immune-microbiota interplay and colonization resistance in infection. Mol. Cell 78 (4), 597–613. doi:10.1016/j.molcel.2020.03.001
Li, J., Sung, C. Y., Lee, N., Ni, Y., Pihlajamaki, J., Panagiotou, G., et al. (2016). Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. U. S. A. 113 (9), E1306–E1315. doi:10.1073/pnas.1518189113
Li, J., Zhang, L., Wu, T., Li, Y., Zhou, X., and Ruan, Z. (2021). Indole-3-propionic acid improved the intestinal barrier by enhancing epithelial barrier and mucus barrier. J. Agric. Food Chem. 69 (5), 1487–1495. doi:10.1021/acs.jafc.0c05205
Li, R., Zhou, R., Wang, H., Li, W., Pan, M., Yao, X., et al. (2019a). Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 26 (11), 2447–2463. doi:10.1038/s41418-019-0312-y
Li, Y., Tinoco, R., Elmen, L., Segota, I., Xian, Y., Fujita, Y., et al. (2019b). Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5(-/-) mice. Nat. Commun. 10 (1), 1492. doi:10.1038/s41467-019-09525-y
Lindblad, K. E., and Lujambio, A. (2021). Liver metastases inhibit immunotherapy efficacy. Nat. Med. 27 (1), 25–27. doi:10.1038/s41591-020-01190-9
Ma, C., Han, M., Heinrich, B., Fu, Q., Zhang, Q., Sandhu, M., et al. (2018). Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360 (6391). doi:10.1126/science.aan5931
Ma, X., Zhou, Z., Zhang, X., Fan, M., Hong, Y., Feng, Y., et al. (2020). Sodium butyrate modulates gut microbiota and immune response in colorectal cancer liver metastatic mice. Cell Biol. Toxicol. 36 (5), 509–515. doi:10.1007/s10565-020-09518-4
Martin-Gallausiaux, C., Marinelli, L., Blottiere, H. M., Larraufie, P., and Lapaque, N. (2021). SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80 (1), 37–49. doi:10.1017/s0029665120006916
Matson, V., Fessler, J., Bao, R., Chongsuwat, T., Zha, Y., Alegre, M. L., et al. (2018). The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359 (6371), 104–108. doi:10.1126/science.aao3290
Megna, B. W., Carney, P. R., Nukaya, M., Geiger, P., and Kennedy, G. D. (2016). Indole-3-carbinol induces tumor cell death: function follows form. J. Surg. Res. 204 (1), 47–54. doi:10.1016/j.jss.2016.04.021
Mielgo, A., and Schmid, M. C. (2020). Liver tropism in cancer: the hepatic metastatic niche. Cold Spring Harb. Perspect. Med. 10 (3), a037259. doi:10.1101/cshperspect.a037259
Mima, K., Sukawa, Y., Nishihara, R., Qian, Z. R., Yamauchi, M., Inamura, K., et al. (2015). Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 1 (5), 653–661. doi:10.1001/jamaoncol.2015.1377
Mirnezami, A., Mirnezami, R., Chandrakumaran, K., Sasapu, K., Sagar, P., and Finan, P. (2011). Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann. Surg. 253 (5), 890–899. doi:10.1097/SLA.0b013e3182128929
Mouries, J., Brescia, P., Silvestri, A., Spadoni, I., Sorribas, M., Wiest, R., et al. (2019). Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 71 (6), 1216–1228. doi:10.1016/j.jhep.2019.08.005
Murota, Y., and Jobin, C. (2021). Bacteria break barrier to promote metastasis. Cancer Cell 39 (5), 598–600. doi:10.1016/j.ccell.2021.03.009
Nosho, K., Sukawa, Y., Adachi, Y., Ito, M., Mitsuhashi, K., Kurihara, H., et al. (2016). Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J. Gastroenterol. 22 (2), 557–566. doi:10.3748/wjg.v22.i2.557
Ocvirk, S., and O'Keefe, S. J. D. (2021). Dietary fat, bile acid metabolism and colorectal cancer. Semin. Cancer Biol. 73, 347–355. doi:10.1016/j.semcancer.2020.10.003
Ohigashi, S., Sudo, K., Kobayashi, D., Takahashi, T., Nomoto, K., and Onodera, H. (2013). Significant changes in the intestinal environment after surgery in patients with colorectal cancer. J. Gastrointest. Surg. 17 (9), 1657–1664. doi:10.1007/s11605-013-2270-x
Overman, M. J., Lonardi, S., Wong, K. Y. M., Lenz, H. J., Gelsomino, F., Aglietta, M., et al. (2018). Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 36 (8), 773–779. doi:10.1200/JCO.2017.76.9901
Pabst, O., Hornef, M. W., Schaap, F. G., Cerovic, V., Clavel, T., and Bruns, T. (2023). Gut-liver axis: barriers and functional circuits. Nat. Rev. Gastroenterol. Hepatol. 20 (7), 447–461. doi:10.1038/s41575-023-00771-6
Park, B. S., Song, D. H., Kim, H. M., Choi, B. S., Lee, H., and Lee, J. O. (2009). The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458 (7242), 1191–1195. doi:10.1038/nature07830
Peinado, H., Zhang, H., Matei, I. R., Costa-Silva, B., Hoshino, A., Rodrigues, G., et al. (2017). Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17 (5), 302–317. doi:10.1038/nrc.2017.6
Poisson, J., Lemoinne, S., Boulanger, C., Durand, F., Moreau, R., Valla, D., et al. (2017). Liver sinusoidal endothelial cells: physiology and role in liver diseases. J. Hepatol. 66 (1), 212–227. doi:10.1016/j.jhep.2016.07.009
Prorok-Hamon, M., Friswell, M. K., Alswied, A., Roberts, C. L., Song, F., Flanagan, P. K., et al. (2014). Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 63 (5), 761–770. doi:10.1136/gutjnl-2013-304739
Purcell, R. V., Pearson, J., Aitchison, A., Dixon, L., Frizelle, F. A., and Keenan, J. I. (2017). Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS One 12 (2), e0171602. doi:10.1371/journal.pone.0171602
Qu, R., Zhang, Y., Ma, Y., Zhou, X., Sun, L., Jiang, C., et al. (2023). Role of the gut microbiota and its metabolites in tumorigenesis or development of colorectal cancer. Adv. Sci. (Weinh) 10 (23), e2205563. doi:10.1002/advs.202205563
Rao, C., Coyte, K. Z., Bainter, W., Geha, R. S., Martin, C. R., and Rakoff-Nahoum, S. (2021). Multi-kingdom ecological drivers of microbiota assembly in preterm infants. Nature 591 (7851), 633–638. doi:10.1038/s41586-021-03241-8
Ratiner, K., Ciocan, D., Abdeen, S. K., and Elinav, E. (2024). Utilization of the microbiome in personalized medicine. Nat. Rev. Microbiol. 22 (5), 291–308. doi:10.1038/s41579-023-00998-9
Ray, K. (2021). Linking gut vascular barrier to CRC liver metastases. Nat. Rev. Gastroenterol. Hepatol. 18 (6), 368. doi:10.1038/s41575-021-00458-w
Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P. M., Alou, M. T., Daillere, R., et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359 (6371), 91–97. doi:10.1126/science.aan3706
Ruiz, P. A., Shkoda, A., Kim, S. C., Sartor, R. B., and Haller, D. (2005). IL-10 gene-deficient mice lack TGF-beta/Smad signaling and fail to inhibit proinflammatory gene expression in intestinal epithelial cells after the colonization with colitogenic Enterococcus faecalis. J. Immunol. 174 (5), 2990–2999. doi:10.4049/jimmunol.174.5.2990
Sakamoto, Y., Mima, K., Ishimoto, T., Ogata, Y., Imai, K., Miyamoto, Y., et al. (2021). Relationship between Fusobacterium nucleatum and antitumor immunity in colorectal cancer liver metastasis. Cancer Sci. 112 (11), 4470–4477. doi:10.1111/cas.15126
Schietroma, M., Pessia, B., Carlei, F., Cecilia, E. M., and Amicucci, G. (2016). Gut barrier function and systemic endotoxemia after laparotomy or laparoscopic resection for colon cancer: a prospective randomized study. J. Minim. Access Surg. 12 (3), 254–259. doi:10.4103/0972-9941.169982
Scott, S. A., Fu, J., and Chang, P. V. (2020). Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U. S. A. 117 (32), 19376–19387. doi:10.1073/pnas.2000047117
Sears, C. L., and Garrett, W. S. (2014). Microbes, microbiota, and colon cancer. Cell Host Microbe 15 (3), 317–328. doi:10.1016/j.chom.2014.02.007
Shaikh, F. Y., Gills, J. J., Mohammad, F., White, J. R., Stevens, C. M., Ding, H., et al. (2022). Murine fecal microbiota transfer models selectively colonize human microbes and reveal transcriptional programs associated with response to neoadjuvant checkpoint inhibitors. Cancer Immunol. Immunother. 71 (10), 2405–2420. doi:10.1007/s00262-022-03169-6
Sharafi, F., Hasani, S. A., Alesaeidi, S., Kahrizi, M. S., Adili, A., Ghoreishizadeh, S., et al. (2022). A comprehensive review about the utilization of immune checkpoint inhibitors and combination therapy in hepatocellular carcinoma: an updated review. Cancer Cell Int. 22 (1), 269. doi:10.1186/s12935-022-02682-z
Shin, J. H., Lee, Y. K., Shon, W. J., Kim, B., Jeon, C. O., Cho, J. Y., et al. (2020). Gut microorganisms and their metabolites modulate the severity of acute colitis in a tryptophan metabolism-dependent manner. Eur. J. Nutr. 59 (8), 3591–3601. doi:10.1007/s00394-020-02194-4
Shkoporov, A. N., Turkington, C. J., and Hill, C. (2022). Mutualistic interplay between bacteriophages and bacteria in the human gut. Nat. Rev. Microbiol. 20 (12), 737–749. doi:10.1038/s41579-022-00755-4
Si, W., Liang, H., Bugno, J., Xu, Q., Ding, X., Yang, K., et al. (2022). Lactobacillus rhamnosus GG induces cGAS/STING-dependent type I interferon and improves response to immune checkpoint blockade. Gut 71 (3), 521–533. doi:10.1136/gutjnl-2020-323426
Siegel, R. L., Giaquinto, A. N., and Jemal, A. (2024). Cancer statistics, 2024. CA Cancer J. Clin. 74 (1), 12–49. doi:10.3322/caac.21820
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A., and Jemal, A. (2023). Colorectal cancer statistics, 2023. CA Cancer J. Clin. 73 (3), 233–254. doi:10.3322/caac.21772
Song, W., Tiruthani, K., Wang, Y., Shen, L., Hu, M., Dorosheva, O., et al. (2018). Trapping of lipopolysaccharide to promote immunotherapy against colorectal cancer and attenuate liver metastasis. Adv. Mater 30 (52), e1805007. doi:10.1002/adma.201805007
Spadoni, I., Zagato, E., Bertocchi, A., Paolinelli, R., Hot, E., Di Sabatino, A., et al. (2015). A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350 (6262), 830–834. doi:10.1126/science.aad0135
Sugimura, N., Li, Q., Chu, E. S. H., Lau, H. C. H., Fong, W., Liu, W., et al. (2021). Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut 71 (10), 2011–2021. doi:10.1136/gutjnl-2020-323951
Swidsinski, A., Khilkin, M., Kerjaschki, D., Schreiber, S., Ortner, M., Weber, J., et al. (1998). Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology 115 (2), 281–286. doi:10.1016/s0016-5085(98)70194-5
Szentkuti, L., Riedesel, H., Enss, M. L., Gaertner, K., and Von Engelhardt, W. (1990). Pre-epithelial mucus layer in the colon of conventional and germ-free rats. Histochem J. 22 (9), 491–497. doi:10.1007/BF01007234
Tanoue, T., Morita, S., Plichta, D. R., Skelly, A. N., Suda, W., Sugiura, Y., et al. (2019). A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565 (7741), 600–605. doi:10.1038/s41586-019-0878-z
Teng, Y., Ren, Y., Sayed, M., Hu, X., Lei, C., Kumar, A., et al. (2018). Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe 24 (5), 637–652. doi:10.1016/j.chom.2018.10.001
Ternes, D., Tsenkova, M., Pozdeev, V. I., Meyers, M., Koncina, E., Atatri, S., et al. (2022). The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat. Metab. 4 (4), 458–475. doi:10.1038/s42255-022-00558-0
Thakare, R., Alamoudi, J. A., Gautam, N., Rodrigues, A. D., and Alnouti, Y. (2018). Species differences in bile acids I. Plasma and urine bile acid composition. J. Appl. Toxicol. 38 (10), 1323–1335. doi:10.1002/jat.3644
Tilg, H., Adolph, T. E., Gerner, R. R., and Moschen, A. R. (2018). The intestinal microbiota in colorectal cancer. Cancer Cell 33 (6), 954–964. doi:10.1016/j.ccell.2018.03.004
Tilg, H., Adolph, T. E., and Trauner, M. (2022). Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. 34 (11), 1700–1718. doi:10.1016/j.cmet.2022.09.017
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F., et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366 (26), 2443–2454. doi:10.1056/NEJMoa1200690
Tsilimigras, D. I., Brodt, P., Clavien, P. A., Muschel, R. J., D'Angelica, M. I., Endo, I., et al. (2021). Liver metastases. Nat. Rev. Dis. Prim. 7 (1), 27. doi:10.1038/s41572-021-00261-6
Varanasi, S. K., Chen, D., Liu, Y., Johnson, M. A., Miller, C. M., Ganguly, S., et al. (2025). Bile acid synthesis impedes tumor-specific T cell responses during liver cancer. Science. 387 (6730), 192–201. doi:10.1126/science.adl4100
Vetizou, M., Pitt, J. M., Daillere, R., Lepage, P., Waldschmitt, N., Flament, C., et al. (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350 (6264), 1079–1084. doi:10.1126/science.aad1329
Vidal-Vanaclocha, F. (2008). The prometastatic microenvironment of the liver. Cancer Microenviron. 1 (1), 113–129. doi:10.1007/s12307-008-0011-6
Wang, F., He, M. M., Yao, Y. C., Zhao, X., Wang, Z. Q., Jin, Y., et al. (2021). Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep. Med. 2 (9), 100383. doi:10.1016/j.xcrm.2021.100383
Wang, G., Yu, Y., Wang, Y. Z., Wang, J. J., Guan, R., Sun, Y., et al. (2019). Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J. Cell Physiol. 234 (10), 17023–17049. doi:10.1002/jcp.28436
Wang, N., and Fang, J. Y. (2023). Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 31 (2), 159–172. doi:10.1016/j.tim.2022.08.010
Wang, X., Fang, Y., Liang, W., Wong, C. C., Qin, H., Gao, Y., et al. (2024). Fusobacterium nucleatum facilitates anti-PD-1 therapy in microsatellite stable colorectal cancer. Cancer Cell 42 (10), 1729–1746.e8. doi:10.1016/j.ccell.2024.08.019
Wang, Z., Dan, W., Zhang, N., Fang, J., and Yang, Y. (2023). Colorectal cancer and gut microbiota studies in China. Gut Microbes 15 (1), 2236364. doi:10.1080/19490976.2023.2236364
White, M. T., and Sears, C. L. (2024). The microbial landscape of colorectal cancer. Nat. Rev. Microbiol. 22 (4), 240–254. doi:10.1038/s41579-023-00973-4
Wong, C. C., and Yu, J. (2023). Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 20 (7), 429–452. doi:10.1038/s41571-023-00766-x
Wu, S., Rhee, K. J., Albesiano, E., Rabizadeh, S., Wu, X., Yen, H. R., et al. (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15 (9), 1016–1022. doi:10.1038/nm.2015
Xia, L., Oyang, L., Lin, J., Tan, S., Han, Y., Wu, N., et al. (2021). The cancer metabolic reprogramming and immune response. Mol. Cancer 20 (1), 28. doi:10.1186/s12943-021-01316-8
Xu, X., Lv, J., Guo, F., Li, J., Jia, Y., Jiang, D., et al. (2020). Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal cancer via metabolic pathway. Front. Microbiol. 11, 814. doi:10.3389/fmicb.2020.00814
Yang, S., Hao, S., Ye, H., and Zhang, X. (2024). Crosstalk between gut microbiota and cancer chemotherapy: current status and trends. Discov. Oncol. 15 (1), 833. doi:10.1007/s12672-024-01704-8
Yin, H., Miao, Z., Wang, L., Su, B., Liu, C., Jin, Y., et al. (2022). Fusobacterium nucleatum promotes liver metastasis in colorectal cancer by regulating the hepatic immune niche and altering gut microbiota. Aging (Albany NY) 14 (4), 1941–1958. doi:10.18632/aging.203914
Yu, J., Green, M. D., Li, S., Sun, Y., Journey, S. N., Choi, J. E., et al. (2021). Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 27 (1), 152–164. doi:10.1038/s41591-020-1131-x
Yu, L., Lu, J., and Du, W. (2024). Tryptophan metabolism in digestive system tumors: unraveling the pathways and implications. Cell Commun. Signal 22 (1), 174. doi:10.1186/s12964-024-01552-7
Yu, T., Guo, F., Yu, Y., Sun, T., Ma, D., Han, J., et al. (2017). Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170 (3), 548–563. doi:10.1016/j.cell.2017.07.008
Yu, Y., Cai, Y., Yang, B., Xie, S., Shen, W., Wu, Y., et al. (2022). High-fat diet enhances the liver metastasis potential of colorectal cancer through microbiota dysbiosis. Cancers (Basel) 14 (11), 2573. doi:10.3390/cancers14112573
Yuan, N., Li, X., Wang, M., Zhang, Z., Qiao, L., Gao, Y., et al. (2022). Gut microbiota alteration influences colorectal cancer metastasis to the liver by remodeling the liver immune microenvironment. Gut Liver 16 (4), 575–588. doi:10.5009/gnl210177
Zepeda-Rivera, M., Minot, S. S., Bouzek, H., Wu, H., Blanco-Miguez, A., Manghi, P., et al. (2024). A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 628 (8007), 424–432. doi:10.1038/s41586-024-07182-w
Zhang, C., Liu, H., Sun, L., Wang, Y., Chen, X., Du, J., et al. (2023). An overview of host-derived molecules that interact with gut microbiota. Imeta 2 (2), e88. doi:10.1002/imt2.88
Zhang, S. L., Han, B., Mao, Y. Q., Zhang, Z. Y., Li, Z. M., Kong, C. Y., et al. (2022). Lacticaseibacillus paracasei sh2020 induced antitumor immunity and synergized with anti-programmed cell death 1 to reduce tumor burden in mice. Gut Microbes 14 (1), 2046246. doi:10.1080/19490976.2022.2046246
Zhao, W., Lei, J., Ke, S., Chen, Y., Xiao, J., Tang, Z., et al. (2023). Fecal microbiota transplantation plus tislelizumab and fruquintinib in refractory microsatellite stable metastatic colorectal cancer: an open-label, single-arm, phase II trial (RENMIN-215). EClinicalMedicine 66, 102315. doi:10.1016/j.eclinm.2023.102315
Keywords: colorectal cancer liver metastasis, gut microbiota, metabolites, gut-liver axis, tumor immune microenvironment
Citation: Liu Q-L, Zhou H, Wang Z and Chen Y (2025) Exploring the role of gut microbiota in colorectal liver metastasis through the gut-liver axis. Front. Cell Dev. Biol. 13:1563184. doi: 10.3389/fcell.2025.1563184
Received: 19 January 2025; Accepted: 26 February 2025;
Published: 13 March 2025.
Edited by:
Dongdong Wang, McMaster University, CanadaReviewed by:
Sweta Ghosh, University of Louisville, United StatesCopyright © 2025 Liu, Zhou, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Chen, eWFuY2hlbjA1MjRAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.