
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Cell Dev. Biol. , 28 March 2025
Sec. Molecular and Cellular Pathology
Volume 13 - 2025 | https://doi.org/10.3389/fcell.2025.1562346
This article is part of the Research Topic 7th International Symposium on Peripheral Nerve Regeneration: Peripheral Nerve Regeneration - Advances and New Directions View all 11 articles
The skin’s integrity and functionality are repaired through a series of processes that are conducted in a systematic and timely manner during wound healing (WH). Hemostasis, inflammation, proliferation, and remodeling are the four phases of WH that overlap (Fernández-Guarino et al., 2023a). The process of wound regeneration following an injury is significantly influenced by neurogenic stimuli; this is illustrated by the discovery that delayed wound repair occurs in animal models after the surgical removal of cutaneous nerves (Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023b). In addition, recent studies suggest that glial support cells may be key players in wound repair, and skin wounding triggers glial dedifferentiation and proliferation. In confirmation of this hypothesis, ablation of injury-activated glia leads to reduced tumor growth factor (TGF) β signaling and impaired wound repair (Peña and Martin, 2024). Studies in mice show that cutaneous nerve endings and Schwann cells surround a population of Lgr6+ epidermal stem cells in hair follicles, and denervation leads to the shifting of these cells toward a differentiated state, impairing wound re-epithelialization. Sensory nerves in the skin are also a source of Sonic Hedgehog (Shh), which induces Gli1 expression in cells of the hair follicle that contribute to WH by becoming epidermal stem cells (Peña and Martin, 2024). Skin wounding may damage the cutaneous vasculature and innervation, with axotomy studies in zebrafish enabling live imaging of the clearance of axon debris by macrophages and epidermal cells. The relationship between innervation and repair is reciprocal, with wounding triggering increased cutaneous nerve sprouting in some contexts (Peña and Martin, 2024).
The activation of critical processes during this phase of WH has been demonstrated by the release of numerous neuropeptides from cutaneous innervation. Substance P (SP) appears to be a significant factor in the inflammatory phase; however, other neuropeptides have also been identified and are reviewed. SP induces microvascular permeability and vasodilation by increasing the release of nitric oxide and exerting direct effects on endothelial cells. The expression of adhesion molecules on endothelial cells, monocyte chemotaxis, and inflammatory cell activity is upregulated by SP. SP also regulates the synthesis and release of pro-inflammatory cytokines, including interleukins, TGFα, and tumor necrosis factor (TNF)α, which are essential components during the inflammatory phase of wound healing. Neutral endopeptidase (NEP) is a zinc metalloprotease that inhibits the actions of SP through enzymatic degradation and competes with neurokinin-1 receptor 1(NK-1R). Inflammatory signaling in wound repair is significantly influenced by the interactions between SP and NEP (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023a; Ivanov et al., 2023; Noble et al., 2024).
Neurokinin A (NKA) is a bioactive tachykinin released into the skin following an injury. It activates cutaneous target cells, including keratinocytes and dermal endothelial cells, preferentially through the neurokinin-2 receptor (NK-2R), thereby contributing to the regulation of skin inflammation during WH (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023b; Ivanov et al., 2023; Noble et al., 2024).
Sensory cutaneous nerves contain corticotropin-releasing hormone (CRH), as evidenced by immunohistochemistry investigations. CRH induces skin mast cell (MC) degranulation and operates as a pro-inflammatory mediator, thereby increasing vascular permeability and the release of pro-inflammatory cytokines. CRH has also been demonstrated to promote angiogenesis in the epidermis (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023a; Ivanov et al., 2023; Noble et al., 2024).
Calcitonin gene-related peptide (CGRP) is a vasodilator that can stimulate angiogenesis and improve plasma extravasation. CGRP has been demonstrated to enhance the inflammatory response of other mediators, including SP. Activin, a member of the TGF-β superfamily, has been demonstrated to upregulate CGRP expression in innervating sensory neurons and to increase after wounding, underscoring its regulatory function in WH (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023b; Ivanov et al., 2023; Noble et al., 2024).
The role of nerve growth factor (NGF) as a modulator of the inflammatory phase of WH is underscored by its ability to increase the release of CGRP from peripheral nerve terminals into peripheral tissue (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023a; Ivanov et al., 2023; Noble et al., 2024).
The function of neuropeptide Y (NPY) and CGRP in WH has been demonstrated in mouse models and is associated with its pro- and anti-inflammatory features. This role involves macrophage-derived NPY within the adipose tissue, a powerful modulator of inflammation, and fosters chemotaxis and angiogenesis (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023b; Ivanov et al., 2023; Noble et al., 2024).
In recent years, the list of mediators involved in the process of WH has been expanded to include nitric oxide (NO), an extracellular molecular messenger. Nitric oxide synthase (NOs), an enzyme complex, is responsible for the production of NO, which is distinguished by an overregulation of the inducible isoform in response to stress. In reality, the enzyme’s production is elevated in the presence of inflammatory mediators, apoptotic corpses, or bacterial antigens. Consequently, it has been postulated that iNOs is involved in the inflammatory phase of wound repair, a phase during which it increases vasodilation and antibacterial activity. As a result, it has been demonstrated that iNOS plays a function in the process of wound repair (Nardini et al., 2024) (Figure 1) (See Table 1).
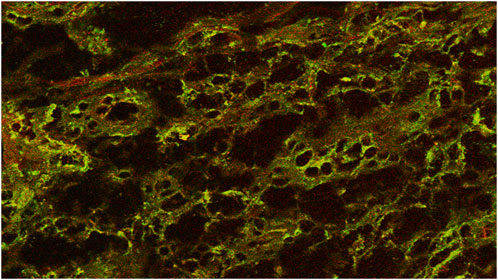
Figure 1. Coexpression of iNos in enolase-labeled unipolar neurons in a chronic wound. Confocal microscope 20×.
SP stimulates DNA synthesis, which results in potent proliferative effects on fibroblasts, keratinocytes, and endothelial cells. It also promotes angiogenesis through the potential mediation of NO. SP is essential for the remodeling of granulation tissue by promoting the proliferation and migration of dermal fibroblasts and by enhancing the expression of epidermal growth factor and its associated receptor (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023a; Ivanov et al., 2023; Noble et al., 2024).
Polypeptide neurotrophins, such as NGF, are present in the central and peripheral nervous systems, as well as in a variety of cells, such as fibroblasts, epithelial cells, keratinocytes, and immune cells. NGF plays a critical role in the survival, function, and differentiation of sensory and autonomic nerves. Additionally, NGF exhibits anti-inflammatory properties. In particular, NGF has been hypothesized to facilitate the proliferation of local immature cells in lesions, the formation of blood vessels, and neurite overgrowth. In animal studies and a human case study, NGF has been demonstrated to facilitate epithelial healing and angiogenesis. The secretion of NGF in the epidermis is induced by neurokinin A (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023b; Ivanov et al., 2023; Noble et al., 2024).
Additional neuropeptides that have been identified as contributing to the proliferative phase include gastrin-releasing peptide (GRP), CGRP, galanin, vasoactive intestinal peptide (VIP), and pituitary adenylate cyclase-activating peptide (PACAP).
CGRP is extensively distributed throughout the central and peripheral nervous system. While its potential to facilitate WH is uncertain, certain studies have demonstrated that it may facilitate angiogenesis, proliferation of keratinocytes, and migration. Keratinocyte proliferation and migration are facilitated by CGRP (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023a; Ivanov et al., 2023; Noble et al., 2024).
Galanin is a peptide that is released from afferent nerves and has anti-proliferative effects in tissue. It signals through G-protein coupled receptors. In contrast, an in vitro study demonstrated that galanin stimulated the upregulation of NGF (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023b; Ivanov et al., 2023; Noble et al., 2024).
Vasoactive intestinal peptide (VIP) has been demonstrated to function as a growth factor for the proliferation of keratinocytes and as a modulator of their migration. Additionally, VIP induces the release of histamine by MC, which results in vasodilation. VIP may be involved in the re-innervation of traumatized tissue as it has been demonstrated to accelerate the regeneration of the sciatic nerve in rats following transection. SP, CGRP, and VIP, as demonstrated by Cheret et al. (2013), modulate matrix metalloproteinase (MMP) activities and influence collagen-1 and collagen-3 production during cutaneous WH (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023a; Ivanov et al., 2023; Noble et al., 2024).
PACAP is located in sensory cutaneous nerves. It is a potent vasodilator and a member of the VIP peptide family. In response to neuronal activation, it is hypothesized that C-fibers release PACAP, which in turn results in extravasation and vasodilation. PACAP is involved in cutaneous inflammation by promoting the proliferation of human keratinocytes and producing histamine from MC (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023b; Ivanov et al., 2023; Noble et al., 2024) (See Table 1).
The function of neuropeptides or cutaneous innervation during the remodeling phase is poorly understood. In response to neuropeptides, sensory nerve fibers regenerate within the epidermis and dermis that have been repaired (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023a; Ivanov et al., 2023; Noble et al., 2024).
SP induces the production of NGF by human dermal microvascular endothelial cells in vitro, a process that is necessary for the regeneration of nerve fibers following cutaneous injury. NGF has also been proposed to expedite tissue remodeling. Sensory and sympathetic nerves express neurotrophin-3 (NT3), a neurotrophic growth factor that is crucial for the growth, proliferation, and maintenance of nerves (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023b; Ivanov et al., 2023; Noble et al., 2024).
Similarly, the postnatal survival or functional maturation of sensory neurons necessitates brain-derived neurotrophic factor (BDNF). Keratinocytes, fibroblasts, and myofibroblasts express BDNF and its receptors, which facilitate their proliferation and differentiation (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023a; Ivanov et al., 2023; Noble et al., 2024).
SP has been demonstrated to affect the degradation of wound collagen by increasing the activity of MMP-2 in fibroblasts. As demonstrated by Fujiwara et al. in vitro, direct neuronal contact accelerates the differentiation of fibroblasts into myofibroblasts, which subsequently secrete collagen fibers and elicit wound contraction. In addition, MMP activities and collagen-1 and collagen-3 productions are influenced by SP, CGRP, and VIP during cutaneous WH (Roosterman et al., 2006; Cheret et al., 2013; Laverdet et al., 2015; Martin and Nunan, 2015; Ashrafi et al., 2016; Kiya and Kubo, 2019; Oleszycka et al., 2022; Fernández-Guarino et al., 2023b; Ivanov et al., 2023; Noble et al., 2024) (See Table 1).
Chronic cutaneous lesions persist for 6 weeks to 8 weeks and exhibit an inflammatory response that maintains a balance between productive and degenerative processes. However, they do not adhere to the conventional, orderly, and timely repair process and progress through these phases without regaining the anatomical and functional integrity of the tissue. The process is impeded by numerous factors, with 140 pathologies implicated. Approximately 6.85% of individuals over 65 have at least one chronic illness, and 30% have three or more chronic conditions (Falanga et al., 2022; Fernández-Guarino et al., 2023a).
Skin ulcers are caused by various clinical symptoms and syndromes, but this investigation does not address the treatment of these conditions. Protease activity increases during the protracted inflammatory phase of ulcers, resulting in the disintegration of growth factors and other molecular signals that facilitate the reparative phase. The overproduction of pro-inflammatory cytokines and hydrolytic enzymes in chronic ulcers impedes the dominance of reparative processes over destructive ones (Martin and Nunan, 2015; Raziyeva et al., 2021; Grandi et al., 2022).
The body must maintain a balance between the synthesis of new tissue and the breakdown of old tissue in order to recuperate. Fibrinolytic systems and matrix metalloproteinases (MMPs) collaborate to eliminate fibrin and damaged extracellular matrix (ECM) from acute lesions, but lower tissue inhibitors of metalloproteinase (TIMP) concentrations and elevated MMP levels have been observed in chronic cutaneous lesions. This results in the matrix undergoing a subsequent reorganization, which exacerbates its degradation (Martin and Nunan, 2015; Raziyeva et al., 2021; Grandi et al., 2022).
Chronic WH mechanisms are similar to acute WH, but the dysregulation of MMP production is significantly associated with chronic wounds (CW), prolonging the inflammatory phase. Neutrophils are dispersed throughout the wound and emit a significant quantity of MMP, impairing the connective tissue matrix and elastase and deactivating vital proteins essential to the healing process (Martin and Nunan, 2015; Raziyeva et al., 2021; Grandi et al., 2022).
The immune system’s interactions with the nervous system are noteworthy in their ability to regulate WH processes (Chiu et al., 2012; Grandi et al., 2022). Recent research indicates that the transmission of neurotransmitters such as SP, protein gene product 9.5 (PGP 9.5), NO, NGF, NKA, NPY, CGRP, and VIP are crucial in chronic wounds, and such transmission is facilitated by interactions between MC and nerve cells, resulting in the release of ECM by fibroblasts, the elevation of TGFβ levels, and the reaction of infiltrating cells (Grandi et al., 2021).
Infections are a significant impediment to the healing process, as they prolong the inflammatory phase and lead to elevated MMP levels. Bacteria form polysaccharide biofilms, which are shielded from antibiotics by reduced penetration through the biofilm matrix, and genetic mutations modify their susceptibility to antibiotics. Biofilms periodically release single bacterial cells that can colonize new surfaces or degrade the collagen matrix in healed ulcers, a process known as “re-ulceration.” The cells that remain in the colony, die, or depart can be influenced by variations in the conditions within the biofilm.
Clinical lesions are generally more subdued when infected due to the release of endotoxins and proteases, disrupting the extracellular matrix and releasing mediators that exacerbate the local inflammatory response. This is a potential correlation between infection and an increase in exudate, which is inhibited by elevated levels of macromolecules, such as albumin and fibrin, as well as pro-inflammatory cytokines and MMP.
Photodynamic treatment (PDT) therapy for CW has been shown to increase the density of neuronal populations in the dermis, which are a component of the autonomous nervous system and contain the typical nerve mediators already defined for chronic wounds. The proportion of mast cells capable of secreting and containing NGF and VIP and iNOs compounds increases as a result of a single irradiation, consistent with the previously identified increase in mast cell degranulation index (Grandi et al., 2021; Grandi et al., 2022; Nardini et al., 2024).
Neuromodulation in WH is a concept of extreme interest. In fact, it is important to recognize that all the phases that characterize this important process have not yet been fully clarified, and it would not be surprising if, in the future, other mediators could be discovered. On the other hand, WH is itself a complex process, and it is no coincidence that the term neuromodulation is often replaced by the term neuroimmunomodulation, underlining how the cells of the immune system are involved in the various phases. An example is given by MCs, which are mainly located near nerves and play a role in WH. When an article proposed how the release of histamine stimulated that of acetylcholine, numerous publications followed the idea (Fantozzi et al., 1978).
The concept of neuroimmunomodulation becomes more complicated in chronic wounds treated with photodynamic therapy because, in this case, the nervous mediators are able to stimulate the secretion of the extracellular matrix by fibroblasts and that of the cellular infiltrate. It is precisely in this location that the mast cell expresses NGF, and this means that the increased content of this mediator in these cell types is fundamental for the possible healing of the wound (Siiskonen and Harvima, 2019; Grandi et al., 2021; Nardini et al., 2024). We are, therefore, faced with an incredible flow of ideas that must be placed in the right relationships to begin to understand the complexity of this phenomenon.
Advanced imaging techniques and high-throughput screening could be beneficial in studying neuroimmunomodulation in chronic wound healing.
An investigation into the molecular interactions that occur between neuropeptides, immune cells, and signaling pathways may allow for the discovery of new treatment options.
Additional clinical trials are required to determine whether PDT is effective in promoting wound healing through neuroimmunomodulation, its efficacy, and its probable mechanisms. This might provide a more detailed roadmap for further research studies and medicinal uses.
PN: Methodology, writing–original draft. SB: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Writing–original draft, and Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
I take this opportunity to thank the following colleagues for their contributions over the years: Nicola Pimpinelli, Alessandro Corsi, Vieri Grandi, and all the students who have contributed to the achievement of the various results obtained.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ashrafi, M., Baguneid, M., and Bayat, A. (2016). The role of neuromediators and innervation in cutaneous wound healing. Acta Derm. Venereol. 96, 587–594. doi:10.2340/00015555-2321
Cheret, J., Lebonvallett, N., Carré, J. L., Misery, L., and Le Gall-Ianotto, C. (2013). Role of neuropeptides, neurotrophins, and neurohormones in skin wound healing. Wound Repair Regen. 21, 772–788. doi:10.1111/wrr.12101
Chiu, I. M., von Hehn, C. A., and Woolf, C. J. (2012). Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15, 1063–1067. doi:10.1038/nn.3144
Falanga, V., Isseroff, R. R., Soulika, A. M., Romanelli, M., Margolis, D., Kapp, S., et al. (2022). Chronic wounds. Nat. Rev. Dis. Prim. 8, 50. doi:10.1038/s41572-022-00377-3
Fantozzi, R., Masini, E., Blandina, P., Mannaioni, P. F., and Bani-Sacchi, T. (1978). Release of histamine from rat mast cells by acetylcholine. Nature 273, 473–474. doi:10.1038/273473a0
Fernández-Guarino, M., Bacci, S., Pérez González, L. A., Bermejo-Martínez, M., Cecilia-Matilla, A., and Hernández-Bule, M. L. (2023a). The role of physical therapies in wound healing and assisted scarring. Int. J. Mol. Sci. 19, 7487. doi:10.3390/ijms24087487
Fernández-Guarino, M., Hernández-Bule, M. L., and Bacci, S. (2023b). Cellular and molecular processes in wound healing. Biomedicines 11, 2526. doi:10.3390/biomedicines11092526
Grandi, V., Corsi, A., Pimpinelli, N., and Bacci, S. (2022). Cellular mechanisms in acute and chronic wounds after PDT therapy: an update. Biomedicines 10, 1624. doi:10.3390/biomedicines10071624
Grandi, V., Paroli, G., Puliti, E., Bacci, S., and Pimpinelli, N. (2021). Single ALA-PDT irradiation induces increase in mast cells degranulation and neuropeptide acute response in chronic venous ulcers: a pilot study. Photodiagnosis Photodyn. Ther. 34, 102222. doi:10.1016/j.pdpdt.2021.102222
Ivanov, E., Akhmetshina, M., Erdiakov, A., and Gavrilova, S. (2023). Sympathetic system in wound healing: multistage control in normal and diabetic skin. Int. J. Mol. Sci. 24, 2045. doi:10.3390/ijms24032045
Kiya, K., and Kubo, T. (2019). Neurovascular interactions in skin wound healing. Neurochem. Int. 125, 144–150. doi:10.1016/j.neuint.2019.02.014
Laverdet, B., Danigo, A., Girard, B., Magy, L., Demiot, C., and Desmoulliere, A. (2015). Skin innervation: important roles during normal and pathological cutaneous repair. Histol. Histopathol. 30, 875–892. doi:10.14670/HH-11-610
Martin, P., and Nunan, R. (2015). Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 173, 370–378. doi:10.1111/bjd.13954
Nardini, P., Notari, L., Magazzini, M., Mariani, B., Rossi, F., Rossi, S., et al. (2024). Neuroimmunomodulatory effect of Nitric Oxide on chronic wound healing after photodynamic therapy. Photodiagnosis Photodyn. Ther. 47, 104078. doi:10.1016/j.pdpdt.2024.104078
Noble, A., Qubrosi, R., Cariba, S., Favaro, K., and Payne, S. L. (2024). Neural dependency in wound healing and regeneration. Dev. Dynam. 253, 181–203. doi:10.1002/dvdy.650
Oleszycka, E., Kwiecien, K., Kwiecinska, P., Morytko, A., Pocalun, N., Camacho, M., et al. (2022). Soluble mediators in the function of the epidermal-immune-neuro unit in the skin. Front. Immunol. 13, 1003970. doi:10.3389/fimmu.2022.1003970
Peña, O. A., and Martin, P. (2024). Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 25, 599–616. doi:10.1038/s41580-024-00715-1
Raziyeva, K., Kim, Y., Zharkinbekov, Z., Kassymbek, K., Jimi, S., and Saparov, A. (2021). Immunology of acute and chronic wound healing. Biomolecules 11, 700. doi:10.3390/biom11050700
Roosterman, D., Goerge, T., Schneider, S. W., Bunnett, N. W., and Steinhoff, M. (2006). Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol. Rev. 80, 1309–1379. doi:10.1152/physrev.00026.2005
Keywords: acute wounds, cellular infiltrate, chronic wounds, wound healing, neuromodulation
Citation: Nardini P and Bacci S (2025) Neuroimmunomodulation in chronic wounds: an opinion. Front. Cell Dev. Biol. 13:1562346. doi: 10.3389/fcell.2025.1562346
Received: 20 January 2025; Accepted: 28 February 2025;
Published: 28 March 2025.
Edited by:
Rui Alvites, University of Oporto, PortugalReviewed by:
Xiaolei Ding, Shanghai University, ChinaCopyright © 2025 Nardini and Bacci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Bacci, c3RlZmFuby5iYWNjaUB1bmlmaS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.