
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 10 March 2025
Sec. Signaling
Volume 13 - 2025 | https://doi.org/10.3389/fcell.2025.1522294
The mammalian p38 MAPK pathway plays a vital role in transducing extracellular environmental stresses into numerous intracellular biological processes. The p38 MAPK have been linked to a variety of cellular processes including inflammation, cell cycle, apoptosis, development and tumorigenesis in specific cell types. The p38 MAPK pathway has been implicated in the development of many human diseases and become a target for treatment of cancer. Although MAPK p38 pathway has been extensively studied, many questions still await clarification. More comprehensive understanding of the MAPK p38 pathway will provide new possibilities for the treatment of human diseases. Hog1 in S. cerevisiae is the conserved homolog of p38 in mammalian cells and the HOG MAPK signaling pathway in S. cerevisiae has been extensively studied. The deep understanding of HOG MAPK signaling pathway will help provide clues for clarifying the p38 signaling pathway, thereby furthering our understanding of the relationship between p38 and disease. In this review, we elaborate the functions of p38 and the relationship between p38 and human disease. while also analyzing how Hog1 regulates cellular processes in response to environmental stresses. 1, p38 in response to various stresses in mammalian cells.2, The functions of mammalian p38 in human health.3, Hog1 as conserved homolog of p38 in response to environmental stresses in Saccharomyces cerevisiae. 1, p38 in response to various stresses in mammalian cells. 2, The functions of mammalian p38 in human health. 3, Hog1 as conserved homolog of p38 in response to environmental stresses in S. cerevisiae.
Cells have evolved sophisticated sensory mechanisms and information transduction systems to respond to environmental challenges and ensure survival (Nadal and Posas, 2015). Eukaryotic cells, ranging from yeast to mammals, the multiple mitogen-activated protein kinase (MAPK) cascades play a crucial role in regulating various cellular processes (de Nadal et al., 2002; Westfall et al., 2004).
In mammals, p38 MAPK is one of the most significant signaling pathways in the MAPK cascade (Obata et al., 2000; Chen et al., 2001; Kyriakis and Avruch, 2001; Pearson et al., 2001; Johnson and Lapadat, 2002; Goldsmith et al., 2004). p38 MAPK is involved in multiple essential functions including apoptosis, cytokine production, transcriptional regulation, and cytoskeletal reorganization (Figure 1). This pathway also regulates the activity and expression of key inflammatory mediators, including cytokines and proteases, which are critical for cancer progression (Sheikh-Hamad and Gustin, 2004; Obata et al., 2000) as well as diseases related to inflammatory responses (Wang et al., 2024), including colitis, arthritis, atherosclerosis, lung diseases, human immunodeficiency virus infection, Alzheimer’s disease (AD) (Hugon and Paquet, 2021), and cell carcinoma (Martínez-Limón et al., 2020), tumors (Bulavin and Fornace, 2004) among other diseases (Figure 1). Givern its critical role in these processes, p38 MAPK has been extensively studied as a therapeutic target, with p38 inhibitors explored for clinical treaments (Han et al., 2020; Asih et al., 2020).
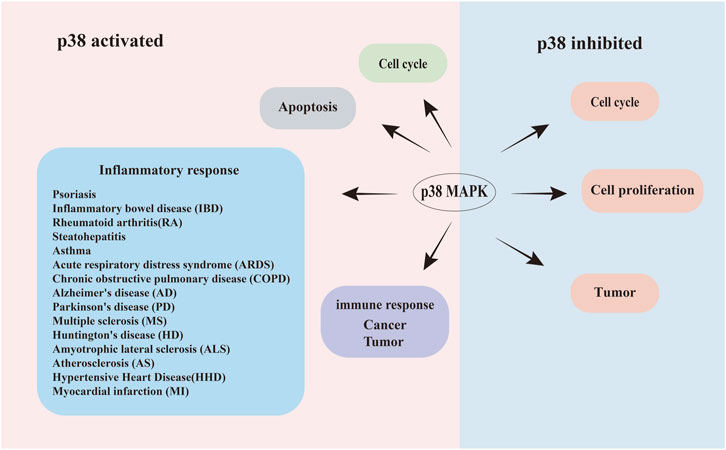
Figure 1. Schematic illustration of the bidirectional roles of p38 signaling in human diseases. Dual regulatory roles of p38 MAPK activation and inhibition in disease pathogenesis. Left panel (Activation): phosphorylated p38 (Red area) drives pathological processes, including: apoptosis dysregulation, acceleration or retardation of the cell cycle, Inflammatory disorders (Psoriasis, inflammatory bowel disease (IBD), rheumatoid arthritis (RA), steatohepatitis, asthma, acute respiratory distress syndrome (ARDS), and chronic obstructive pulmonary disease (COPD). Neuroinflammatory/neurodegenerative diseases: Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), atherosclerosis (AS), hypertensive heart disease (HHD), and myocardial infarction (MI)), tumor progression, and cancer cell proliferation. Right panel (Inhibition): pharmacological suppression of p38 (blue area) attenuates disease progression through: enhanced cellular survival, slowed tumor growth. Arrows indicate directional signaling flow.
Interestingly, the p38 MAPK pathway has a homologous counterpart in Saccharomyces cerevisiae, where the high osmolarity glycerol (HOG) pathway performs a similar function (Galcheva-Gargova et al., 1994; Cooper, 1994; Han et al., 1994; de Nadal et al., 2011). The HOG pathway, a yeast-specific MAPK signaling cascade, is crucial for the cell’s response to environmental stressors such as osmotic stress (de Nadal et al., 2011). The key protein in this pathway, Hog1, is a conserved homologue of p38 (Han et al., 1994). The similarities between the mammalian p38 MAPK pathway and the yeast HOG pathway provide valuable insights into the conserved nature of MAPK signaling across species, and understanding these pathways in yeast may offer new directions for studying p38 MAPK in human diseases. This discussion will first explore the p38 pathway and its relationship to human diseases, while also examining how the HOG MAPK pathway responds to various external stimuli, providing new insights and directions for studies on p38. How MAP kinase p38 affects human health.
p38 was originally identified as a protein with a molecular weight of 38 kDa, characterized by the rapid phosphorylation of its tyrosine residues in response to various environmental stimuli (Han et al., 1994). These stimuli include heat shock, changes in osmotic pressure, oxidative stress, genotoxic agents and DNA-damaging agents such as cisplatin, adriamycin, ultraviolet light, and γ-radiation (Kyriakis and Avruch, 2012). Additionally, p38 is activated by inflammatory cytokines, pathogen-associated molecular patterns (PAMPs), danger-associated molecular patterns (DAMPs) (Martínez-Limón et al., 2020) and lipopolysaccharide (LPS) stimulation (Han et al., 1994). p38 MAPK represents a group of highly conserved protein kinases, and phosphorylated p38 MAPK can activate a diverse range of substrates, including transcription factors, protein kinases, and various cytoplasmic and nuclear proteins (Obata et al., 2000; Coulthard et al., 2009).
In mammalian cells, four homologous p38 MAPK proteins are encoded by different genes: p38α. (MAPK14), p38β (MAPK11), p38γ (MAPK12), and p38δ (MAPK13) (Cuenda and Rousseau, 2007) (Figure 2). These proteins are broadly expressed, yet their expression patterns differ across tissues. p38α is universally expressed in all cell types, while p38β is predominantly found in the brain, thymus, and spleen. p38γ is abundant in skeletal muscle, and p38δ levels are higher in the pancreas, intestines, adrenal glands, kidney and heart (Mertens et al., 1996; Goedert et al., 1997; Jiang et al., 1997; Beardmore et al., 2005; Cuenda and Rousseau, 2007; Cuenda and Sanz-Ezquerro, 2017). The external environment activates MAP3K, including TAK1 (Moriguchi et al., 1996), ASK1 (Ichijo et al., 1997), DLK (Hirai et al., 1997), MLK3 and ZAK1 (Doza et al., 1995; Brancho et al., 2003). This activation leads to the stimulation activation of upstream MAP2K, specifically MKK3 and MKK6, which in turn activates the downstream p38 kinase, resulting in their phosphorylation (Figure 2). All four p38 kinases possess conserved Thr-Gly-Tyr (TGY) biphosphorylated motifs (Han et al., 2020). Notably, among of these four types of p38 MAPK, p38β is exclusively phosphorylated by MKK6, while only p38α is specifically activated by MKK4 (Enslen et al., 1998; Alonso et al., 2000; Cuadrado and Nebreda, 2010) (Figure 2).
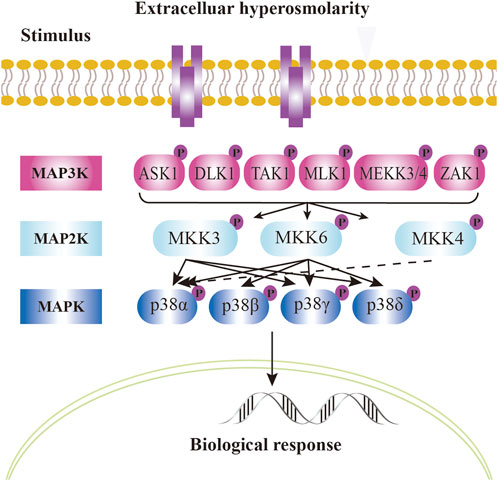
Figure 2. p38 signal pathway in mammalian cells. Mammalian p38 kinases consist of four proline - directed serine/threonine kinases, namely, p38α, p38β, p38γ, and p38δ, which are encoded by four genes. The classical activation of p38 follows a three - tiered mechanism. The stimuli for p38 activation are cellular stressors, such as oxidative stress, inflammatory stimuli/cytokines, ultraviolet radiation, and cell membrane osmotic pressure. Upstream stimuli activate MAP kinases (MAPKKK), such as kinases like ASK1, TAK1, MLK, etc. These kinases, in turn, phosphorylate and activate MAPKK, such as MKK3, MKK4, or MKK6. MKK, in turn, phosphorylates and activates p38 kinases on threonine and tyrosine residues in the activation loop. In the case of p38α, it is activated by MKK4 (dashed arrow). The dual phosphorylation of p38 can be detected by phosphorylation - specific antibodies and serves as a marker of p38 activation. Dual - phosphorylated p38 is fully active and targets downstream phosphorylation substrates, altering their structure, activity, function, localization, or interaction with other biomolecules, thus regulating cellular responses. The phosphorylated state is represented by “P”. Solid arrows and dashed arrows indicate the directionality of the signal.
Activated p38 rapidly accumulates and translocates to the nucleus, where it phosphorylates various transcriptional regulators that coordinate specific gene expression programs (Ono and Han, 2000; Cuenda and Rousseau, 2007). Additionally, it may interact with other signaling pathways, binding to non-p38-regulated transcription factors to trigger diverse responses, including inflammation, cell cycle arrest, apoptosis, senescence, cytokine production and RNA splicing regulation (Coulthard et al., 2009; Sanz-Ezquerro and Cuenda, 2021) (Figure 1). Notably, strong and sustained p38 activation is linked to apoptosis, senescence and terminal cell differentiation (Puri et al., 2000; Haq et al., 2002). In contrast, low-level p38 activation supports cell survival (Puri et al., 2000; Haq et al., 2002; Macé et al., 2005).
When cells are exposed to stress, defects in cell growth occur as cell cycle checkpoint systems and protective responses are activated. Energy is redirected from other cell functions to support the stress response. In mammalian cells, the p38 MAPK pathway plays a pivotal role in regulating cell cycle progression under various stress conditions, such as osmotic stress, reactive oxygen species, DNA damage and aging. These stresses lead to defects in cell cycle delay, impairing cell viability. (Duch et al., 2012; Barnum and O'Connell, 2014; Martínez- Limón et al., 2020). p38 MAPK is particularly important for regulating cell proliferation during the G1/S and G2/M phases of the cell cycle. (Barnum and O'Connell, 2014; Martínez- Limón et al., 2020). For instance, p38 MAPK enhances cell survival by downregulating the expression of the retinoblastoma (RB) tumor suppressor gene, which is a key regulator of G1 phase restriction point in metastasis (Ambrosino and Nebreda, 2001; Gubern et al., 2016).
In the G1/S transition, p38 interacts with several key regulatory proteins, such as Cyclin D1, Cdc25A, and p53, to modulate cell cycle progression. (Bulavin and Fornace, 2004). Cyclin D1, encoded by the human CCND1 gene, is essential for the G1/S transition (Tchakarska and Sola, 2020). Inhibition of p38 MAPK during this transition lead to a downregulation of cyclin D1 levels, slowing the conversion from G1 to S phase, thereby impeding cancer cell cycle progression and reducing cancer incidence (Lavoie et al., 1996). Additionally, p38 MAPK regulates the stability of Cdc25A, its activation can inhibit Cdc25A activity, further slowing cell cycle proliferation (Galaktionov et al., 1995; Mailand et al., 2000; Bartek and Lukas, 2001; Zhao et al., 2002; Goloudina et al., 2003). Notably, Cdc25A is often overexpressed in primary human breast cancer, where it could serve as a potential therapeutic target (Cangi et al., 2000). Meanwhile, p53 functions as a tumor suppressor at the G1/S checkpoint by upregulating proteins such as p21Cip1/WAF1, GADD45, and 14-3-3σ, which prevents progression to S phase and induce G1 phase stagnation (Takekawa et al., 2000; Ho and Benchimol, 2003; Stramucci et al., 2018).
Furthermore, the G2/M checkpoints are crucial for halting mitotic progression in response to chromatin damaged or incomplete replication (Bulavin and Fornace, 2004). Studies indicate that p38α is involved in G2/M checkpoints, promoting cell cycle arrest and facilitating DNA repair (Wagner and Nebreda, 2009). Experiments with transgenic mice expressing an active mkk6Δ mutant in immature thymus cells have demonstrated sustained activation of the p38 MAPK pathway inhibits both cell cycle progression and differentiation (Diehl et al., 2000; Ambrosino and Nebreda, 2001). Despite these findings, the precise molecular mechanisms through which p38 MAPK integrates with other key cell cycle checkpoints, including the DNA damage response and tumor suppressor signaling pathways, remain incompletely understood. Further research is needed to elucidate these interactions and their implications for cell cycle regulation under stress conditions.
Apoptosis is a genetically controlled, multistep process of cell death that eliminates damaged cells while preventing inflammatory response (Obata et al., 2000). In many biological systems, the activation of p38 MAPK activity is associated with apoptosis, whereas its inhibition can reduce apoptosis events (Ziegler-Heitbrock et al., 1992; Cardone et al., 1997; Obata et al., 2000). The role of p38 MAPK in apoptosis may depend on the mode and duration of its activation (Obata et al., 2000). For instance, brief activation of p38 MAPK promotes erythroid differentiation of SKT6 cells, while prolonged activation leads to apoptosis (Nagata and Todokoro, 1999; Macé et al., 2005). Similarly, early activation of p38 MAPK can prevent apoptosis in neutrophils treated with tumor necrosis factor (TNF)-α, whereas later activation appears to facilitate apoptosis in these cells (Roulston et al., 1998).
Some studies suggest that p38 MAPK influences apoptosis both upstream and downstream of cysteine protease (Cardone et al., 1997; Ziegler-Heitbrock et al., 1992), which are central to the apoptosis pathway and exist as inactive zymogens (Fernandes-Alnemri et al., 1996; Cahill et al., 1996). Moreover, activation of MEKK can stimulate the p38 signaling pathway, promoting apoptosis in T cells and fibroblasts (Huang et al., 1997). Interestingly, heat stress has been shown to inhibit LPS-induced apoptosis by blocking the calpain/p38 MPAK pathway (Liu et al., 2016). Overall, the p38 MAPK pathway plays a crucial role in regulating cell fate, yet the precise mechanism underlying this process remains to be fully elucidated.
The p38 MAPK pathway is recognized for its complex role in the inflammatory response, where it is involved not only in promoting inflammation but also in mediating anti-inflammatory processes. Activation of p38 MAPK can stimulate the expression of transcription factors such as AP-1 (Garcia et al., 1998; Yang et al., 2014) through pro-inflammatory mediators, including interleukin-1 (IL-1), IL-6, TNF. This stimulation further enhances the production of pro-inflammatory cytokines, thereby intensifying the inflammatory response (Guzman-Martinez et al., 2019; Chan et al., 2020; Liao et al., 2021). Concurrently, p38 MAPK also plays a role in regulating anti-inflammatory mediators such as IL-10 and transforming growth factor-β (TGF-β), which may inhibit inflammation under certain conditions (Guzman-Martinez et al., 2019; Chan et al., 2020; Liao et al., 2021). Due to this dual functionality, p38 MAPK is considered a key regulator of the inflammatory response (Garcia et al., 1998; Yang et al., 2014). The NLRP3 inflammasome is a multiple protein complex that detects pathogens and danger signals, promoting the maturation and release of inflammatory factors such as IL-1β (Ising et al., 2019). Abnormal activation of the NLRP3 inflammasome has been linked to various inflammatory diseases, including AD (Ising et al., 2019). The p38 MAPK signaling pathway exhibits a dual role in the activation and expression of the NLRP3 inflammasome (Wang et al., 2024). Specifically, phosphorylation of p38 MAPK enhances the function of the NLRP3 inflammasome, leading to a heightened inflammatory response (Wang et al., 2024). Conversely, the inhibition or absence of p38 MAPK may result in excessive activation of the NLRP3 inflammasome during its activation phase, which can exert an anti-inflammatory effect through the regulation of mitochondrial Ca2+ uptake (Chanjitwiriya et al., 2020).
Additionally, the p38 MAPK signaling pathway plays a crucial role in the functional activation, proliferation and migration of macrophages, as well as in regulating their phagocytic capabilities, which are essential for inflammatory responses (Senokuchi et al., 2005; Hou et al., 2022; Wang et al., 2024). Increased phosphorylation levels of p38 MAPK enhance both the phagocytic ability of macrophages and their production IL-10. Specifically, p38α is vital in mediating inflammatory responses, notably in conditions such as psoriasis, while p38β has also been implicated in various inflammatory diseases, (Johansen et al., 2005), including inflammatory bowel disease (IBD), rheumatoid arthritis (RA), steatohepatitis (Zhang and Reynolds, 2019; Otsuka et al., 2010; Liang et al., 2013), asthma, acute respiratory distress syndrome (ARDS) and chronic obstructive pulmonary disease (COPD). Furthermore, studies have highlighted the complex pro-inflammatory and anti-inflammatory roles of p38γ and p38δ in cytokine production during innate immune responses (Escós et al., 2016), particularly in collagen-induced arthritis (CIA) (Han et al., 2020). These isoforms regulate the expression of cytokines, chemokines, and inducible nitric oxide synthases, which are crucial for the innate inflammatory response against infections by controlling the expression of multiple protein-coding genes involved in the activation, recruitment of immune cells, and the elimination of pathogens in bone marrow derived macrophages (Risco et al., 2012; Alsina-Beauchamp et al., 2018).
Inhibiting p38 MAPK can help prevent inflammation and the death of muscle fiber, thus providing a potential treatment for various forms of muscular dystrophy forms of muscular dystrophy (Brennan et al., 2021). Microglia, the innate immune effector cells of the central nervous system, undergo phenotypic changes and release inflammatory mediators, which are pivotal in neuroinflammation associated with conditions such as stroke (Park et al., 2015). The p38 MAPK pathway is integral to the functioning of microglia and other cell types (Park et al., 2015; Kheiri et al., 2018; Yan and Zhao et al., 2020; Yang et al., 2020; Fan et al., 2021; Gaikwad et al., 2021; Li et al., 2020; Lo et al., 2022). Neuroinflammation significantly contributes to neurodegenerative diseases, including AD (Peel et al., 2004), Parkinson’s disease (PD) (Thomas et al., 2008), and multiple sclerosis (MS), with chronic oxidative stress further exacerbating neurodegenerative changes (Solleiro-Villavicencio et al., 2018). Aditionally, p38 MAPK signaling is implicated in the pathogenesis of other neurodegenerative diseases, such as Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS) (Perregaux et al., 1995; Johnson and Bailey, 2003; Hollenbach et al., 2004; Culbert et al., 2006; Van Eldik et al., 2007; Thomas et al., 2008; Feng et al., 2019), as well as various cardiovascular diseases, including atherosclerosis (AS), obesity-related cardiac hypertrophy (ORCH) (Wang et al., 2016), myocardial infarction (MI) and cerebrovascular disease (Bassi et al., 2008; Muslin, 2008). Moreover, p38 MAPK is particularly important in the context of chronic pain diseases (Coulthard et al., 2009).
The importance of the p38 signaling pathway in regulating the immune response has garnered significant attention in the context of carcinogenesis (Martínez-Limón et al., 2020). Immune cells can profoundly influence tumor progression through the secretion of cytokines and chemokines (Martínez-Limón et al., 2020).
The effects of p38 on tumor are complex and multifaceted.
First, the activation of p38 can promote tumorigenesis. For instance, p38α regulates the induction of the pro-inflammatory mediator Cyclooxygenase-2 (COX-2), which may contribute to cancer progression in various cancers, including non-melanoma skin cancer, breast cancer, and glioma (Bachelor and Bowden, 2004; Timoshenko et al., 2006; Xu and Shu, 2007). Additionally, p38α can inhibit inflammation-related intestinal epithelial damage and tumorigenesis, while also promoting the proliferation and survival of colon cancer cells (Bachstetter et al., 2011). Furthermore, p38γ expression is crucial for proliferation of colon cancer (Chen et al., 2000) and liver tumors (Tang et al., 2005). Inhibition of p38 has been shown to decrease the expression of TGF-β-dependent MMP- 9, thereby reducing bone metastasis of breast cancer in mouse models (Suarez-Cuervo et al., 2004), and preventing bone metastasis of prostate cancer cells (Arechederra et al., 2015). Chronic inflammatory diseases, particularly those affecting the gastrointestinal tract, are associated with an increased risk of cancer development (Grivennikov and Karin, 2011). The p38 pathway regulates the production of key cytokines such as TNF, IL-6, IL-1, COX-2, IL-17 and other cytokines, which play significant roles in tumor growth, survival, and tumorigenesis (Martínez-Limón et al., 2020).
Second, the activation of p38 can inhibit tumor occurrence. The p38α and p38β subtypes (Ambrosino and Nebreda, 2001) inhibit G0, G1/S and G2/M cell cycle checkpoint control, leading to growth arrest and induction of apoptosis (Bulavin and Fornace, 2004; Kummer et al., 1997; She et al., 2001) or senescence (Wang et al., 2002; Haq et al., 2002; Bulavin et al., 2002). p38 downregulates the expression of cyclin through phosphorylation, thereby inhibiting cell proliferation across various cancer cell lines (Gubern et al., 2016). Moreover, p38α can limit the proliferation of hematopoietic stem cells (Tamura et al., 2000), cardiomyocytes (Engel et al., 2005) and pancreatic islets (Wong et al., 2009). Additionally, constitutive activation of the p38 MAPK pathway, through MKK3 or MKK6, can induce senescence in several cell types (Wang et al., 2002; Haq et al., 2002) and inhibit tumor formation.
Thus, p38 not only has the capacity to inhibit tumor cell proliferation, but also act as a tumor promoter (Martínez-Limón et al., 2020). Experimental evidence suggests that low p38 activity in the early stages of cancer may facilitate tumor formation and growth, while increased activation of this pathway in advanced tumor stages may be beneficial (Igea and Nebreda, 2015).
Due to its crucial role in regulating cellular functions, p38 is currently being extensively studied as a drug target, and various inhibitors (Genovese, 2009) are being investigated for the treatment of diseases such as pain, asthma, cognitive impairment, RA, PD (Coulthard et al., 2009), cancer, myelodysplastic syndrome and depression (Johansen et al., 2005). In 2011, the European Commission approved Esbriet (pirfenidone), identified as a p38γ inhibitor, for the treatment of idiopathic pulmonary fibrosis (Moran, 2011). Another notable example is Ralimetinib (or LY2228820), a potent and selective inhibitor of p38α and p38β, utilized as either a single agent or in combination therapy for ovarian cancer, glioblastoma and metastatic breast cancer (Vergote et al., 2020). Furthermore, p38α inhibitors may be beneficial in treating tumors reliant on the progression of p38 MAPK activity, potentially enhancing the efficacy of DNA-damaging chemotherapy by inhibiting p38α-mediated cell cycle arrest and affecting DNA repair mechanisms (Wagner and Nebreda, 2009).
Despite these advancements, our understanding of the p38 signaling pathway remains limited. Nonetheless, novel drug targets for p38 kinase or its downstream components continue to be promising candidates for the development of new therapies addressing a wide range of human diseases.
Many signaling pathways present in yeast have equivalent systems in mammalian cells, exhibiting extensive functional conservation (Han et al., 1994; Sheikh-Hamad and Gustin, 2004; Jiménez et al., 2020). Mammalian p38 MAPK is both structurally and functionally homologous of yeast HOG MAPK (Galcheva-Gargova et al., 1994; Han et al., 1994; Nadal and Posas, 2015). Notably, it has been reported that p38 can complement the normal functions of HOG MAPK in mutant yeast strains (Han et al., 1994). Consequently, in depth studies of the HOG signaling pathway in yeast, which serves as the conserved homologue of p38 in mammalian cells, may provide novel insights for disease treatment targeting p38.
In S. cerevisiae, the HOG signaling pathway has been extensively investigated. It coordinates multiple cellular functions, regulates cell survival and growth, and plays a crucial role in osmotic signaling (Nadal and Posas, 2022). Initially, the HOG signaling pathway in S. cerevisiae was thought to be activated solely by osmotic stress. However, recent studies have revealed that Hog1 is also activated by various other environmental stresses, including heat (Winkler et al., 2002; Yamamoto et al., 2008; Dunayevich et al., 2018), cold (Hayashi and Maeda, 2006; Panadero et al., 2006), oxidative (Singh, 2000; Haghnazari and Heyer, 2004; Lee et al., 2017), acid (citric acid (Lawrence et al., 2004), acetic acid (Mollapour and Piper, 2006)), heavy metals [e.g., copper (Ren et al., 2022), cadmium (Jiang et al., 2014; Zhao et al., 2021), iron (Martins et al., 2018)], metalloid (arsenic (Sotelo and Rodríguez-Gabriel, 2006; García et al., 2004; Lee and Levin, 2018), antimony (Thorsen et al., 2006)), LPS (Marques et al., 2006), curcumin (CUR) (Azad et al., 2014), hypoxia (Hickman et al., 2011) and caffeine (Elhasi and Blomberg, 2023), methylglyoxal (MG) (Aguilera et al., 2005), KP1019 (Singh et al., 2014) and carbon stress (Vallejo and Mayinger, 2015), cesium chloride (Del Vescovo et al., 2008). In this section, we primarily describe the HOG signaling pathway and its activation by various environmental stresses.
Saccharomyces cerevisiae is frequently exposed to adverse environments, necessitating the evolution of regulatory mechanisms that enable stress adaptation survival (Udom et al., 2019; Yao et al., 2020; Lucena et al., 2020; Nadal and Posas, 2022). The HOG MAPK pathway in yeast, a structural and functional homologue of the mammalian p38 MAPK (Nadal and Posas, 2015), plays a crucial role in mediating cellular adaptation to stress, particularly in high osmotic pressure environments. Under osmotic stress, HOG activation occurs through two independent mechanisms, each involving a sensing mechanism and a tertiary cascade of MAPKKK (Ssk2, Ssk22, and Ste11), MAPKK (Pbs2), and MAPK (Hog1) (Duch et al., 2012). Ultimately, this cascade activates downstream substrate (Brewster et al., 1993; Maeda et al., 1994; Maeda et al., 1995; Posas and Saito, 1997; O'Rourke et al., 2002; Hohmann, 2002; O'Rourke and Herskowitz, 2004; Tatebayashi et al., 2007; Macia et al., 2009; de Nadal et al., 2011; Saito and Posas, 2012; Westfall et al., 2004) (Figure 3). One branch of this pathway involves the osmotic stress receptor Sln1, a complex variant of the well known bacterial two-component system (Maeda et al., 1994; Hohmann, 2002). In this pathway, the histidine kinase activity of Sln1 is inhibited, leading to the dephosphorylation of its downstream target, Ssk1, which activates downstream MAPKKK (Ssk2, Ssk22) (Posas et al., 1996; Posas et al., 1998; West & Stock, 2001; Horie et al., 2008) (Figure 3). The other branch features the membrane protein Sho1, which interacts with Ste20 and Ste50 to activate MAPKKK (Ste11) (Maeda et al., 1995; Posas and Saito, 1997; Drogen et al., 2000; Raitt et al., 2000; Reiser et al., 2000; Macia et al., 2009) (Figure 3). In yeast, the mucin-like transmembrane proteins Hkr1 and Msb2 are potential osmosensors and share redundant functions with Sho1 (O'Rourke and Herskowitz, 2002; de Nadal et al., 2007; Tatebayashi et al., 2007; Tanaka et al., 2014) (Figure 3). In addition, another element, Opy2, which is a transmembrane protein, acts as the membrane anchor for Ste11/Ste50 (Wu et al., 2006). The MAPKKK from both branches (Ssk2, Ssk22 and Ste11) subsequently activate the common MAPKK (Pbs2), which acts as both a scaffolding protein and a kinase, inducing the phosphorylation of Thr174 and Tyr176 to activate Hog1 (MAPK) (Brewster et al., 1993) (Figure 3). Activation of the Hog1 pathway is similar to that of the p38 MAPK pathway in mammals, and is achieved through the MAPK cascade reaction. Phosphorylated Hog1 translocates to the nucleus, where it accumulates (Ferrigno et al., 1998; Reiser et al., 1999) and recruits RNA polymerase and transcription factors [Msn2 (Schmitt and McEntee, 1996), Msn4, and Hot1 (Rep et al., 1999), Msn1 (Estruch and Carlson, 1990)] to the promoters of hyperosmolarity-associated genes [CTT1, GPD1, GPD2, GPP1, GPP2, STL1 and HSP12 (O'Rourke et al., 2002; Hohmann, 2002; O'Rourke and Herskowitz, 2004)], thus regulating intranuclear osmotic pressure through specific chromatin remodeling factors to ensure normal transcription and expression of relevant genes under hypertonic conditions (Posas et al., 2000; Capaldi et al., 2008; Babazadeh et al., 2014; Hohmann, 2015; Udom et al., 2019). Furthermore, pbs2Δ mutant or hog1Δ mutant, which encode MAPK kinase (MAPKK) and MAPK, respectively, lead to increased osmosensitivity and decreased glycerol levels (Brewster et al., 1993). Hog1 research reveals how transcription factors mediate gene expression in response to stress, and these mechanisms are equally applicable in mammals.
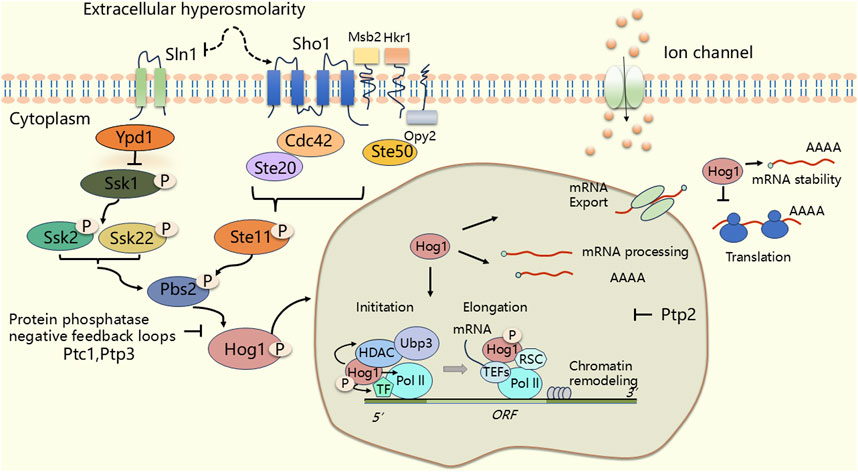
Figure 3. The HOG (High Osmolarity Glycerol) signal pathway in Saccharomyces cerevisiae. Under osmotic stress, Pbs2 integrates signals from two major independent upstream osmotic sensing pathways: the Sln1 and Sho1 branches. Upon activation, Pbs2 triggers the activation of Hog1. The activated Hog1 portion enters the nucleus and regulates transcription (RNA polymerase initiates transcription at the promoter, synthesizes the RNA chain, and continues to extend forward.). The other part of Hog1 remains in the cytoplasm and directly regulates post-translational processes such as translation. Thereby initiating a series of osmotic adaptive responses. The phosphorylation state is represented by “P”. An arrow indicates an activation state, while the T-bar symbol represents an inhibitory state.
However, sustained activation of Hog1 can be detrimental to cell growth, making negative feedback regulation of HOG-MAPK signaling essential (Sacristán-Reviriego et al., 2015; Vázquez-Ibarra et al., 2020). In yeast cells, the protein phosphatases that inactivate the HOG signaling pathway are divided into two classes. The first class consists of protein tyrosine phosphatases (PTPs) specifically Ptp2 and Ptp3. Ptp2 is predominantly located in the nucleus (Mattison et al., 1999), where it binds and dephosphorylates Hog1, playing a crucial role in the negative feedback regulation of the HOG-MAPK signaling pathway (Maeda et al., 1994; Wurgler-Murphy et al., 1997; Jacoby et al., 1997; Mattison and Ota, 2000; Saito and Tatebayashi, 2004) (Figure 3). The second class comprises type 2C Ser/Thr phosphatases (PTCs) Ptc1, Ptc2 and Ptc3, which specifically dephosphorylates.
Thr174 of Hog1, thereby preventing overactivation of Hog1 phosphorylation (Warmka et al., 2001; Young et al., 2002; Sacristán-Reviriego et al., 2015). The coordinated action of activation and negative feedback regulation of the HOG signaling pathway ensures that organisms can effectively adapt to environmental changes.
The HOG pathway plays a crucial role in various cellular processes. First, it determines the short-term translation response following hyperosmotic shock, regulating protein synthesis to help the cell adapt to osmotic stress (Warringer et al., 2010; de Nadal et al., 2011). Second, the p38/HOG stress-activated protein kinase network is involved in coordinating growth and division in Candida albicans, although its exact role remains under debate (Sellam et al., 2019). Third, Hog1 has a dual role in the HOG pathway, acting both as a direct kinase and as a coordinator of secondary signaling mediated by effector kinases such as Rck2 (Romanov et al., 2017). In addition, under hypertonic stress, Hog1 directly binds to the n-terminal regulatory domain of Fps1, and in this case phosphorylates Rgc2 at multiple sites, shutting down the saccharomyces cerevisians glycerol channel Fps1, thereby regulating cellular osmotic balance (Tamás et al., 1999; Beese et al., 2009; Lee et al., 2013). Finally, in S. cerevisiae, Hog1 MAPK prevents crosstalk between the HOG pathway and the pheromone response MAPK pathway, ensuring specific signal transduction under osmotic stress conditions (O'Rourke and Herskowitz, 1998; Vázquez-Ibarra et al., 2020).
In addition to its classical role under hyperosmotic stress in S. cerevisiae, the HOG signaling pathway has been shown to be activated by various environmental stresses. Therefore, in the section, we will primarily discuss the activation of the HOG signaling pathway in response to other environmental stressors. The Hog1 study revealed that cells adapt to environmental stress, which has similarities to the adaptation of the mammalian p38 MAPK pathway to cell physiological functions in response to environmental stress or inflammation. The aim is to provide new perspectives for the study of p38 MAPK in mammalian cells.
Cells of living organisms are constantly exposed to environmental changes that can be detrimental, including rising temperatures (30°C–37°C) (Parsell and Lindquist, 1993; Piper, 1993; Dunayevich et al., 2018). These temperature increases can damage vital cell structures and impair essential biological functions (Richter et al., 2010; de Nadal et al., 2011). In response to specific stresses, cells regulate intracellular effectors and intracellular signaling pathways (de Nadal et al., 2011).
In yeast cells, there are two main classes of signaling pathways that detect and respond to sudden changes in external temperature. One pathway involves the accumulation of denatured protein 26, which is conserved in the heat-induced responses, leading to the activation of Heat Stress Factor (HSF) and the transient expression of Heat Stress Proteins (HSPs) (Franzmann et al., 2008; de Nadal et al., 2011). The other pathway responds directly to temperature changes through key heat-senstive structures, such as DNA, RNA, proteins and lipids, either by participating in or activating signal transduction pathways (Franzmann et al., 2008; de Nadal et al., 2011).
Winkler A et al. demonstrated that heat stress (treated at 37°C) promotes Hog1 phosphorylation and Hog1-dependent gene expression via the Sho1 phosphorylation branch (Figure 4) (Winkler et al., 2002). Some researchers also suggest that Hog1 is involved in heat shock responses due to the transient increase in pressure (de Nadal et al., 2011). Transcription of genes such as HSP12, CTT1 or ALD3 is induced within 1–3 min of stimulation (Gasch et al., 2000; de Nadal et al., 2011). Additionally, studies have shown that the Ptp2 and Ptp3 can inactivate Hog1 to prevent excessive activation of HOG MAPK, thereby avoiding cell death (Winkler et al., 2002).
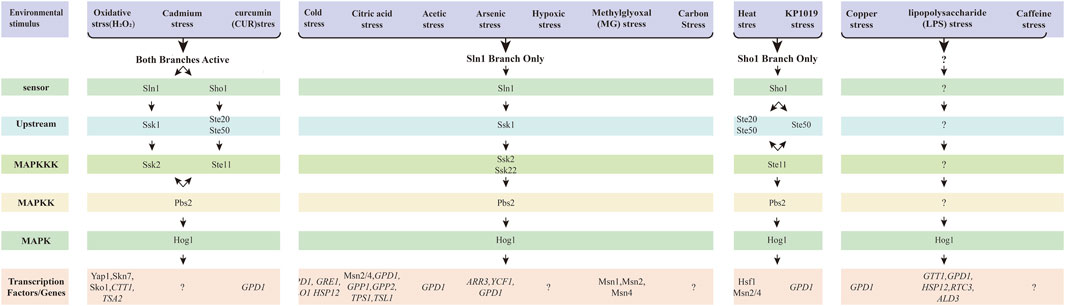
Figure 4. The HOG signal pathway in response to the other enviromental stresses. The HOG pathway has two branches, and different environmental stimuli can activate distinct branches, thereby regulating different transcription factors. These stresses pass through the Sln1-dependent branch, the Sho1-dependent branch, or involve both branches simultaneously. Pathway-specific protein complexes are common and necessary for signaling. Under different stress conditions, the transcription factors or genes corresponding to the bottom are different. Arrows represent the flow of information, while question marks indicate uncertainty.
Following the activation of Hog1, the heat shock transcription factor Hsf1 and the general stress transcription factors Msn2 and Msn4 (collectively referred to as Msn2/4) are activated as master regulators of the heat shock response in S. cerevisiae (Figure 4) (Yamamoto et al., 2008). Hsf1 and Msn2/4 induce the expression of proteins that protect cellular components from thermal inactivation (Amorós and Estruch, 2001; Grably et al., 2002). Hsf1 is rapidly and transiently activated to counteract the detrimental effects of misfolded proteins and restore proteins homeostasis by inducing the expression of chaperones and other protective proteins under heat shock conditions (Hightower, 1990; Akerfelt et al., 2010; Rhodius and Mutalik, 2010). Additionally, Hsf1 promotes the transcription of many HSP family target genes during the recovery period following severe heat shock, including HSP104, HSP82 and HSP70 (Lindquist and Kim, 1996; Sanchez et al., 1993).
Simultaneously the transcription factors Msn2/4 were also activated to regulate the target genes such as HSP12, CTT1 and ALD3 in response to heat shock (Gasch et al., 2000; de Nadal et al., 2011). However, the sensor system and detailed mechanism of the HOG signaling pathway remain largely unexplored.
Low temperatures (0°C–13°C) (Schade et al., 2004) can lead to decreased membrane fluidity (Henry and Keith, 1971; Alonso et al., 1997; Swan and Watson, 1997; Horváth et al., 1998; Hayashi and Maeda, 2006; Panadero et al., 2006), reduced enzyme activity, impaired protein translation efficiency, changes in lipid composition, disruptions in the synthesis of damaged proteins, and decreased secondary stability of DNA and RNA structures in various organisms, including yeast (Panadero et al., 2006; López-Malo et al., 2013). When yeast is exposed to low temperatures, it triggers a rapid and dynamic stress response known as the cold shock response (Aguilera et al., 2007). This response has been extensively studied in bacteria and plants, but less so in fungi. Yeast cells, in particular, HOG signal pathway has been shown to play a crucial role in adapting to cold stress, providing valuable insights into cellular mechanisms that protect against frostbite induced by low temperatures (Hayashi and Maeda, 2006; Panadero et al., 2006; Aguilera et al., 2007).
Using Western blot analysis, Hayashiet al. demonstrated that the HOG signaling pathway is activated only in a Sln1branched dependency manner when cells are exposed to cold stress (at 0 °C) (Figure 4) (Hayashi and Maeda, 2006). The transcription factors Msn2 and Msn4 bind to Stress Response Element (STRE) and are involved in the coordinated regulation of low temperature response genes (Kandror et al., 2004; Schade et al., 2004). However, there is currently no direct evidence that Hog1 induces Msn2/Msn4 expression under cold stress. Panadero et al. (Panadero et al., 2006) analyzed mRNA samples from wild-type and hog1Δ mutant cells using Northern blot and examined the transcription profiles of several genes. They found that the cold-induced expression of GPD1, GRE1, GLO1 and HSP12 was nearly completely inhibited in the hog1Δ mutant (Figure 4). This suggests that Hog1 may be the primary kinase regulating the expression of these genes.
Additionally, studies have shown that when cells are transferred to 12°C or 4°C, wild-type cells accumulate significant amounts of glycerol, resulting in improved freezing tolerance. In contrast, hog1Δ and gpd1Δ mutants exhibit lower survival rates after freezing. This suggests that an increased glycerol content following a temperature drop may confer frost resistance (Panadero et al., 2006). However, cells with mutations in the HOG signaling pathway grow more slowly under cold stress but are not fatal indicating that other, potentially more critical signaling pathways may also be involved in response to cold stress.
Oxygen can cause forms of cellular damage through intermediates with differing reactivity and distribution (Bilsland et al., 2004). For instance, endogenous metabolites produced by cells, such as hydrogen peroxide (H2O2), reactive free radicals, and other oxidants, can lead to oxidative damage to proteins, lipids, and DNA (Davies, 1995; Wallace, 1998; Singh, 2000). To protect against oxidative damage, organisms constantly monitor their environment and respond to oxidative stress through signal transduction mechanisms (Lee et al., 2017). Oxidants like hydrogen peroxide, menadione, ultraviolet light and у-radiation can induce oxidative stress (Demple, 1998; Jamieson, 1992; Hidalgo et al., 1997; Kielbassa et al., 1997; Wallace, 1998; Zheng et al., 1998). In S. cerevisiae, the most typical HOG signal in the MAPK cascade is associated with oxidative stress (Singh, 2000; Haghnazari and Heyer, 2004).
Singh (Singh, 2000) found that sln1Δ ssk1Δ double mutants and sho1Δ ssk2Δ double mutants exhibited sensitivity only to H2O2 and diamine, but not to the other oxidants like menadione, ultraviolet light, and у-radiation. The Sho1 branch and Sln1branch were shown to protect cells only from oxidative damage caused by H2O2 and diamine, rather than other oxidants (Figure 4).
Surprisingly, a hallmark of the osmotic stress response of S. cerevisiae is that upstream kinases, such as MAPKK phosphorylate Hog1 kinase at tyrosine residues (Maeda et al., 1994; Schüller et al., 1994; Gustin et al., 1998). However, when yeast cells were exposed to H2O2, Hog 1 tyrosine phosphorylation did not increase. Similarly, no significant increase in tyrosine phosphorylation was observed after exposure to other oxidants, consistent with the findings of Schüller et al. (1994). These results suggest that direct phosphorylation of Hog1 is not involved in H2O2- induced stress signaling. Interestingly, the hog1Δ mutant was sensitive to H2O2, while the hog1Δ mutant expressing mouse p38 kinase demonstrated resistance to H2O2. Thus, the expression of p38 kinase compensates for the loss of Hog1 and protects cells from H2O2-induced damage (Singh, 2000).
Lee et al. (2017) found that Hog1 MAPK could be activated by oxidative stress (specifically H2O2) through spot dilution assay and Western blot analysis. However, the activation mechanism was shown to only through Ssk1-Ssk2 in Sln1 branch, rather than Ssk1-Ssk22. While only a few cells exhibited nucleus localization of Hog1 upon H2O2 exposure, most were remained in the cytoplasm. Additionally, Lee YM et al. detected that both Hog1 and Rck2 were activated and phosphorylated upon H2O2 stress. Interestingly, the number of cells with Hog1 in the nucleus was significantly higher in rckΔ mutant cells compared to wild-type cells (Bilsland et al., 2004).
Several important transcription factors, including Yap1, Skn7 and Sko1, which are regulated by Hog1, play crucial roles in the oxidative stress response. These factors are necessary for the regulation of genes involved in protection against oxidative damage (Figure 4) (Rep et al., 2001). Furthermore, the genes of CTT1 and TSA2, which encode antioxidants, are induced in respond to oxidative stress by these transcriptions factors (Schüller et al., 1994; Wong et al., 2003). Massive genes associated with the CRE and STRE elements, regulated by Hog1, are induced under oxidative conditions (Lee et al., 2017; Yaakoub et al., 2022). Currently, it remains controversy regarding the signaling pathway involved in the oxidative stress response. Much work and further investigation are needed in this research area to address existing challenges.
Acids can be categorized as weak or strong, and as organic or inorganic. Regardless of the type, the growth of organisms requires adaptation to external hydrogen ion concentrations (Lawrence et al., 2004). Acid stress is influenced not only by the toxicity of high concentrations of hydrogen ions but also depends on the chemical nature of the acid to which the organism is exposed (Lawrence et al., 2004). In fact, different properties of acids can produce different inhibitory effects; for instance, different weak organic acids can exert significantly different inhibitory effects on microorganisms, even at the same pH (Salmond et al., 1984). In S. cerevisiae, the HOG MAPK pathway has been showed to regulate resistance to citric acid as well as acetic acid during acid stress (Lawrence et al., 2004; Mollapour and Piper, 2006).
Citric acid, an intermediate metabolite of the tricarboxylic acid (TCA) cycle, is a key component of normal respiratory metabolism in S. cerevisiae and is commonly used as a preservative to prevent microbial growth. Lawrence et al. (Lawrence et al., 2004) were the first to discover that the HOG MAPK pathway is vital for the regulation of citric acid stress adaptation, utilizing screening S. cerevisiae cleavage, transcriptional analysis, and determination of protein expression changes. By screening the S. cerevisiae mutant cells, including HOG1, SSK1, MSN2, PBS2, PTC2, PTP2 and PTP3, it was confirmed that the HOG MAPK pathway was activated by the Sln1 branch upon citric acid stress (Figure 4). The genes regulated by the HOG pathway, which are involved in glycerol and trehalose metabolism (e.g., GPD1, GPP1, GPP2, TPS1, and TSL1) (Figure 4), general stress response (e.g., CTT1, HSP42, and DDR48), and cell wall integrity (e.g., SPI1 and CWP1), were upregulated in wild type yeast cells under citric acid stress. Additionally, genes such as CTT1, ALD3, PNC1, DDR48 and YDL204w, which serve as marker for the transcription factor Msn2/4, were also upregulated, indicating the activation of Msn2/4 in response to citric acid stress. Moreover, Hog1 was found to negatively regulate the expression of Bmh1p, Pdb1p, Ura1p, Fba1, Ydr533cp, Gnd1p and Car1p during citric acid exposure. The study clearly demonstrated that the HOG MAPK pathway was activated by the Sln1 branch to regulate gene expression to alter biological processes and cause glycerol accumulation in response to citric acid stress (Lawrence et al., 2004). Furthermore, the HOG MAPK pathway was similarly activated by the Sln1 branch in response to acetate stress (Mollapour and Piper, 2006). The GPD1 gene and glycerol content were induced only in an acetate culture with a pH of 6.8, but not in culture with a pH of 4.5 (Mollapour and Piper, 2006; Ludovico et al., 2001; Ludovico et al., 2003). In summary, the HOG pathway by Sln1 branch is activated in response to both citric acid and acetic acid stress. However, the mechanism governing gene expression following the activation of the HOG signaling pathway remains unclear.
The widespread use of heavy metals and their improper disposal pose a serious threat to the environment and human health. While transition metals, heavy metals and quasi-metals can be toxic, some transition metals are essential trace elements necessary for biological function. All cells have mechanisms for metal ion homeostasis. typically involving a balance between uptake and efflux systems (Tamás and Wysocki, 2001; Rosen, 2002). Heavy metals are cofactors for several microbial enzymes and are present at low concentrations required for normal biological function in yeast (Kirchman and Botta, 2007). However, when the concentration exceeds permissible thresholds, heavy metals can impair cellular function and viability (Dong et al., 2013; Jiang et al., 2014; Gerwien et al., 2018; Ren et al., 2022).
In S. cerevisiae, regulatory mechanisms for developing tolerance to various metals have been identified, including heavy metals such as copper (Ren et al., 2022), cadmium (Jiang et al., 2014; Zhao et al., 2021), iron (Martins et al., 2018)), as well as metalloids like arsenic (Sotelo and Rodríguez-Gabriel, 2006; García et al., 2004; Lee and Levin, 2018), antimony (Thorsen et al., 2006). These mechanisms are crucial for the survival and adaptation of yeast in environments contaminated with toxic metals.
Copper is essential for life, yet it can become toxic when its concentration exceeds physiological limits. In cellular contexts, copper ions can exist in various valence states (Ren et al., 2022). The toxicity of copper primarily arises from its REDOX properties, enabling it to participate in Fenton-like reactions that generate harmful reactive oxygen species (ROS) (Ren et al., 2022), This leads to cellular damage, including protein oxidation, DNA and RNA cleavage, and lipid peroxidation that compromises membrane integrity (Dong et al., 2013; Gerwien et al., 2018; Castro et al., 2021).
Ren M et al. demonstrated for the first time that copper exposure induce oxidative stress, including increased levels of ROS and malondialdehyde (MDA), the enzymes activity of GSH and SOD, and upregulated expression of related genes to protect cells defend against oxidative toxicity (Ren et al., 2022). In addition, trace amounts of Hog1 were activated under copper stress, leading to the upregulation of gene expression for CTT1, GPD1, HSP12, RTC3, and ALD3, as confirmed through phenotypic assays, Western blot assays and RT-PCR (Ren et al., 2022) (Figure 4). However, the specific branch regulating Hog1 activation in response to copper remains unidentified. Furthermore, copper exposure resulted in significant cell cycle arrest in G1 phase, while Hog1 was partially involved in regulating cell cycle progression (Ren et al., 2022). Despite these findings, the HOG pathway response to copper exposure is under-researched, and the precise mechanisms involved are still unclear.
Cadmium is a highly toxic trace elements that accumulates in organisms, (Genchi et al., 2020; Farooq et al., 2020; El-Esawi et al., 2020; Ozturk et al., 2021), triggering a cascade of death and adaptive signals in eukaryotic cells, including the formation of oxidants as well as the prevention of DNA damage and DNA repair (Zhang and Reynolds, 2019). It is classified as a carcinogen affecting various tissues (Bertin and Averbeck, 2006; Bishak et al., 2015; Larsson et al., 2015). The HOG pathway identified as crucial for yeast cells in resisting cadmium-induced toxicity (Ozturk et al., 2021; Zhao et al., 2021).
In Jiang et al. employed phenotypic screening of mutants lacking components of the HOG signaling pathway alongside Western blot analysis, revealing that the upstream branches Sln1 and Sho1 can activate the HOG signaling pathway in response to cadmium stress (Figure 4) (Jiang et al., 2014). Notably, the MAPKKK involved in cadmium signaling within the Sln1 branch was identified as Ssk2, rather than Ssk22 (Jiang et al., 2014) (Figure 4). CYS3, CYS4, and GSH1, which are involved in cysteine and glutathione biosynthesis, were identified by transcriptomic, proteomic, and degenomic methods, suggesting that cysteine and glutathione are essential for cadmium tolerance in yeast cells (Jiang et al., 2014). It was observed that ROS levels and cell death levels were induced under the influence of Cd in mutant cells such as hog1∆ mutant and pbs2∆ mutant (Zhao et al., 2021). Furthermore, cadmium-induced Hog1 phosphorylation was shown to require the Unfolded protein-response (UPR) pathway (Zhao et al., 2021). The loss of HAC1 and IRE1 was found to enhance the nuclear accumulation of Hog1 and increase Slt2 localization in the cytoplasm and bud neck, suggesting that both Hog1 and Slt2 are crucial for regulating cellular processes in the absence of the UPR signaling pathway (Zhao et al., 2021). However, the mechanisms by which the HOG signaling pathway regulates downstream transcription factors and gene expression remain unclear.
Arsenic is a toxic metalloid that is widely present in the environment, and exposure has been linked to various diseases, including liver, kidney, and lung cancers (Evens et al., 2004; Thorsen et al., 2006). Despite their toxicity, arsenic-containing drugs have become part of modern therapies (Thorsen et al., 2006). Studies involving arsenite have shown that activation of the HOG pathway is crucial for tolerance in S. cerevisiae.
Sotelo and Rodríguez-Gabriel (2006) first demonstrated that Hog1 is rapidly phosphorylated in response to arsenic stress and triggers a transcriptional response via the Sln1 branch of the HOG pathway (Figure 4). The abundance of several mRNAs in response to sodium arsenic in the wild type and the hog1Δ mutant was examined by quantitative reverse transcript PCR (García et al., 2004). Among the monitored mRNAs, the transcription factor Arr1, which is critical for upregulating several genes involved in the sodium arsenic response (Bouganim et al., 2001; Menezes et al., 2004), including the plasma membrane transporter ARR3; the vacuolar transporter YCF1; and Glycerol-3-phosphate dehydrogenase GPD1, which plays a crucial role in hypertonic stress responses and is regulated by Hog1 activity (Albertyn et al., 1994), it was observed that ARR3 mRNA was strongly induced under arsenic treatment, whereas its induction was significantly diminished in hog1Δ mutant cells (Sotelo and Rodríguez-Gabriel, 2006) (Figure 4). The defective induction of ARR3 and YCF1 expression is consistent with the high sensitivity of the hog1Δ mutant to sodium arsenic (Sotelo and Rodríguez-Gabriel, 2006). Furthermore, analysis of strains lacking the transcription factors Sko1, Msn2/4, Hot1, and Smp1, all of which are regulated by Hog1, it was found that Hog1 regulated the arsenic response independently of these factors (Sotelo and Rodríguez-Gabriel, 2006). Thorsen M et al. confirmed the above results and showed that the increase in arsenite influx was dependent on the aqueous triglyceride protein Fps1 (Thorsen et al., 2006).
Lee and Levin (2018) investigated the two main forms of inorganic arsenic: trivalent arsenate [As (III)] and pentavalent arsenate [As (V)]. Their findings revealed that Hog1 is activated only when As (III) is converted to Mas (III), a metabolite that activates Hog1 by inhibiting its tyrosine-specific phosphatases Ptp2 and Ptp3. These phosphatases typically maintain Hog1 in a state of low activity, representing a negative feedback mechanism within the HOG pathway. Hog1 is activated through arsenate [As (V)] through a MAPK cascade (Lee and Levin, 2018; Lee et al., 2019), a mechanism that differs from the activation by As (III). Both As (III) and As (V) stimulate the expression of arsenic-protected genes, including ACR2 and ACR3 genes, through the ap-1 like transcription factor ACR1 (Wysocki et al., 2004). Notably, the induction of ACR3 by As (III) was found to be partially dependent on Hog1, while its induction by As (V) occurred independently of Hog1. Despite these insights, the underlying mechanisms remain unclear.
A typical activator of the immune and inflammatory systems is lipopolysaccharide (LPS), a component of the outer leaflets of the cell wall of Gram-negative bacteria, commonly referred to as bacterial endotoxin. LPS triggers systemic changes associated with infectious shock (Han et al., 1994; Marques et al., 2006).
Since p38 mediates the action of LPS in mammalian cells, Marques et al. (Marques et al., 2006) investigated the adaptive response of the HOG pathway to LPS in S. cerevisiae. They found that exposure to LPS induced Hog1 phosphorylation and translocation to the nucleus, where it is in a catalytically active state and upregulates GPD1 mRNA levels (Figure 4). However, further exploration is needed to elucidate the upstream branch of the HOG signaling pathway and their regulatory effects on gene expression.
CUR, an active polyphenol derived from the spice turmeric, has garnered significant attention for its diverse therapeutic and preventative applications. CUR has been used as a dietary supplement for many years and is widely used in Ayurvedic medicines (Aggarwal et al., 2007). CUR has good therapeutic potential and may play an important role in the prevention of neurodegenerative disorders, many forms of cancer including colon and pancreatic cancer cancers, intestinal disorders, and other disorders (Gupta et al., 2013; Shehzad et al., 2013; Monroy et al., 2013; Vera-Ramirez et al., 2013). However, the exact mechanism underlying these effects remain under investigation (Azad et al., 2014). New aspects of the CUR-induced stress response in S. cerevisiae have provided additional understanding of the treatment of CUR. Azad G. K et al. first discovered that CUR exposure causes Hog1 phosphorylation in S. cerevisiae across both Sln1 and Sho1 branches and the Ssk2 in the Sln1 branch is required for CUR-induced Hog1 to achieve optimal phosphorylation by Western blotting and mutant cell assays to achieve (Figure 4) (Azad et al., 2014). The expression of GPD1 regulated by Hog1 was significantly increased by RT-PCR. Meanwhile, immunoblotting indicated a notable reduction in phosphorylated Hog1 levels following the addition of iron, suggesting that CUR-induced iron deficiency contributes to Hog1 phosphorylation, which can be restored by iron supplementation (Azad et al., 2014). However, the specific transcription factors and gene expressions regulated by Hog1 remain clear.
Oxygen is a critical electron acceptor in aerobic respiration and is essential for the biosynthesis of important cellular components such as steroids, unsaturated fatty acids (UFAs), and hemoglobin (Rosenfeld and Beauvoit, 2003; Hickman et al., 2011). However, oxygen also has a downside side, during metabolism, it produces reactive oxygen species, which can damage cellular components (Jamieson, 1998; Hickman et al., 2011). To adapt to changes in oxygen levels in the environment, most organisms respond to changes in oxygen levels through different mechanisms. In S. cerevisiae, the response to hypoxia is regulated via the HOG signaling pathway.
Hickman et al. studied hypoxia induction in S. cerevisiae by analyzing the hypoxic-induced seripauperin (PAU) gene and showed for the first time that Hog1 is phosphorylated and involved in the hypoxic growth response by phosphorylation-specific antiserum analysis and Western blot analysis (Hickman et al., 2011). Additionally, the ssk1Δ ste11Δ double mutants exhibited the same defect as the ssk1Δ single mutant, suggesting that Ste11 does no play a role in the hypoxic activation of Hog1. This indicates that the upstream Sln1 branch of the HOG pathway is involved in hypoxia stress (Figure 4). Under hypoxia conditions, the mRNA levels of three hypoxia genes (DAN1, INO1 and TDH1) were also increased in the hog1Δ mutant. This suggests that HOG signaling pathway is not the primary signaling pathway for hypoxia stress, and other pathways may play a more central role in this adaptive response.
Caffeine, a purine analogue of methylxanthines, occurs naturally in many plants as a secondary metabolite. In plants, caffeine serves a protective role and acts as an insecticide, paralyzing and killing herbivorous insects (Elhasi and Blomberg, 2023). While caffeine has both positive and negative effects on human health, its ability to modulate various neurotransmitter systems can significantly affect physiological functions. S. cerevisiae is highly responsive to caffeine, which influences cell growth, morphology, DNA repair, intracellular calcium homeostasis, and cell cycle progression (Kuranda et al., 2006; Elhasi and Blomberg, 2023). Elhasi and Blomberg found that caffeine treatment induces rapid, intense, and transient phosphorylation of Hog1 (Elhasi and Blomberg, 2023). Phosphorylated Hog1 is immediately accumulated in the nucleus under caffeine stress with HOG1-GFP cells (Figure 4) (Elhasi and Blomberg, 2023). However, the specific pathway through which caffeine affects Hog1 phosphorylation remains unclear, and the mechanism of cellular transcription following activation require further investigation.
MG is a glycolytic by product formed during the dephosphorylation of dihydroxyacetone phosphate (DHAP), which is an intermediate in the interconversion of DHAP and glyceraldehyde phosphate (GA3P) (Phillips and Thornalley, 1993). The production of MG in S. cerevisiae occurs as a non-enzymatic, spontaneous process (Martins et al., 2001). In addition, MG is known to have significant toxic effects, influencing DNA and proteins (Vander Jagt et al., 1992). To prevent the overaccumulation of MG, yeast cells initiate a genetic response when internal concentrations reach a certain threshold.
Aguilera J et al. showed that the HOG signaling pathway regulates the genetic response of yeast to MG (Aguilera et al., 2005). Western blot analyses revealed that hog1Δ mutant and upstream sln1Δ branch mutant exhibited sensitivity and impairment under MG treatment, while sho1Δ ssk1Δ double mutants displayed an attenuated response similar to that of ssk1Δ single mutant. This indicates that MG response primarily depends on the Sln1 branch (Figure 4). The absence of Hog1 protein led to reduced MG-dependent mRNA accumulation of the three reporter genes GPD1, GLO1 and GRE3 (Aguilera et al., 2005). Furthermore, the combined deletion of the transcription factors Msn1, Msn2 and Msn4 virtually eliminated the accumulation of GLO1, GRE3, and GPD1 mRNAs (Figure 4) (Aguilera et al., 2005).
Interestingly, MG does not induce hyperphosphorylation of Hog1 or its nuclear translocation in the parental strains (Aguilera et al., 2005). Although the phosphorylated form of Hog1 is crucial for transcriptional activity, dual phosphorylation of Hog1 is essential for triggering transcriptional responses (Aguilera et al., 2005). The activity of the HOG pathway enhance the expression of MG response genes under both non-induced and inducible conditions, thereby protecting cells from this toxic glycolytic byproduct (Aguilera et al., 2005). However, studies on MG stress are limited, the specific mechanism involved remain unclear.
Ruthenium is a non-essential transition metal known for its diverse coordination chemistry, making it an attractive candidate for development of pharmacologically active compounds. Among these, KP1019 has emerged as a promising ruthenium-containing drug candidates for cancer therapy. Research indicates that KP1019 induces DNA damage in S. cerevisiae, resulting in delayed cell cycle progression and ultimately leading to cell death (Stevens et al., 2013). Singh et al. were the first to investigate the tolerance of the HOG pathway to KP1019. Their phenotype and Western blotting analyses demonstrated that KP1019 induces the Hog1 phosphorylation (Singh et al., 2014). Additionally, it was found that GPD1 mRNA level was upregulated within 30 min after KP1019 treatment by RT-PCR (Singh et al., 2014). Experiments involving mutant deletion strains revealed that Hog1 activation is primarily regulated by the Sho1 branch (Figure 4) (Singh et al., 2014). Therefore, the activation of HOG pathway by KP1019 is mediated by the Sho1 branch. Despite the potential of KP1019 as a novel anticancer agent, little is known about the specific biological pathways and molecules it targets (Singh et al., 2014).
Yeast cells detect external nutrient levels through signaling pathways that regulate metabolism and transcription. They preferentially utilize glucose and fructose as carbon sources and favors fermentation over oxidative phosphorylation to harness energy and precursor molecules for biosynthesis. Consequently, yeast cells can rapidly and extensively modify their transcriptional programs in response to fluctuations in glucose levels (Vallejo and Mayinger, 2015). Piao H et al. reported that glucose starvation induced Hog1 phosphorylation (Piao et al., 2012). During glucose starvation, Hog1 phosphorylation is slower and completely dependent on Ssk1,but not on Sho1 (Vallejo and Mayinger, 2015). However, the specific transcription factors involved and the resulting changes in mRNA levels remain unclear.
From single cells to mammals, organisms respond to a variety of environmental stimuli through the MAPK signaling pathway to maximize survival. In mammals, the p38 signaling pathway is a crucial MAPK signaling pathway that significantly impacts human health and serves as an important area of research for various diseases. p38 plays a role not only in cellular regulation but also as a therapeutic target for conditions such as immune response, neurodegenerative diseases, inflammation, cancer, and viral infections. Although p38-related drugs have been extensively studied in clinic settings, their successes have been limited, and practical applications remain scarce. Thus, accurate and in-depth studies on p38 are still ongoing.
The main role of Hog1 is to regulate the response of cells to changes in the external environment under stress conditions such as high osmotic pressure. In mammals, the p38 MAPK pathway has a similar structure and function, especially in cellular stress response. Therefore, the study of Hog1 activation mechanism provides a framework for understanding the activation of p38 in mammals.
In the Hog1 signaling pathway, several key proteins mediate signaling processes, including kinases, splices, and transcription factors. For example, Ypd1 and Ssk1 regulate Hog1 activation upstream. Similarly, in mammals, p38 activates downstream signaling through the regulation of its upstream kinases, such as MKK3/MKK6. The Hog1 study provides a better understanding of how these transcription factors respond to environmental stress, as well as how p38 regulates stress responses through similar signaling mechanisms, and advances research into the role of these factors in mammalian systems. p38 can activate or inhibit the Hog1 signaling pathway through certain conditions, and vice versa. An understanding of this interplay can help develop more nuanced intervention strategies. The mammalian p38 MAPK pathway and the yeast HOG pathway are highly homologous in structure and function. S. cerevisiae as a well-studied model eukaryote, provides insights into the conserved of the HOG pathway across eukaryotes. We hypothesize that by examining the changes in S. cerevisiae in response to environmental stimuli, in conjunction with studies on the p38 signaling pathway, we can gain valuable insight into the role of p38 in human disease and health. Research on the stress response of S. cerevisiae can enhance our understanding of stress response in microorganisms and mammals, ultimately aiding the development of therapeutic strategies for various diseases and contributing to improved human health. Future studies should focus on the HOG pathway of resistance mechanisms under various adverse conditions, refining existing models. Future studies can start from the aspects of targeting specific transcription factors, kinase interactions, signaling pathway feedback mechanisms, etc., to provide new targets and strategies for the treatment of various diseases (such as cancer, inflammatory diseases, neurodegenerative diseases, etc.). Cross-species comparative studies, especially the analogy between Hog1 and p38, will provide theoretical basis and practical guidance for the development of more accurate and effective targeted therapies. and exploring new access mechanisms. Further exploration of the relationship between the HOG and p38 MAPK pathways in response to stress is necessary, including identifying specific transcription factors or promoters involved. Another layer of the relationship between HOG pathway and p38 MAPK pathway in response to adversity, whether there are homologues, is not clear, whether kinases such as Hog1 and their dependent factors can help p38 in disease management and treatment. Given the significance of the Hog1/p38 signaling pathway in both yeast and mammalian cells, further investment in understanding this mechanism is warranted for the advancement of human health.
GD: Funding acquisition, Supervision, Writing–original draft, Writing–review and editing. KZ: Writing–original draft, Writing–review and editing. CS: Writing–original draft. MS: Writing–original draft. JP: Writing–review and editing. DM: Writing–original draft, Writing–review and editing. WG: Funding acquisition, Supervision, Writing–review and editing. HZ: Funding acquisition, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (31471835).
The authors especially thank the help of Prof. Francesc Posas from the Universitat Pompeu Fabra.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aggarwal, B. B., Sundaram, C., Malani, N., and Ichikawa, H. (2007). Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 595, 1–75. doi:10.1007/978-0-387-46401-5_1
Aguilera, J., Randez-Gil, F., and Prieto, J. A. (2007). Cold response in Saccharomyces cerevisiae: new functions for old mechanisms. FEMS Microbiol. Rev. 31 (3), 327–341. doi:10.1111/j.1574-6976.2007.00066.x
Aguilera, J., Rodríguez-Vargas, S., and Prieto, J. A. (2005). The HOG MAP kinase pathway is required for the induction of methylglyoxal-responsive genes and determines methylglyoxal resistance in Saccharomyces cerevisiae. Mol. Microbiol. 56 (1), 228–239. doi:10.1111/j.1365-2958.2005.04533.x
Akerfelt, M., Morimoto, R. I., and Sistonen, L. (2010). Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. cell Biol. 11 (8), 545–555. doi:10.1038/nrm2938
Albertyn, J., Hohmann, S., Thevelein, J. M., and Prior, B. A. (1994). GPD1, which encodes glycerol- 3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 14 (6), 4135–4144. doi:10.1128/mcb.14.6.4135
Alonso, A., Queiroz, C. S., and Magalhães, A. C. (1997). Chilling stress leads to increased cell membrane rigidity in roots of coffee (Coffea arabica L.) seedlings. Biochimica biophysica acta 1323 (1), 75–84. doi:10.1016/s0005-2736(96)00177-0
Alonso, G., Ambrosino, C., Jones, M., and Nebreda, A. R. (2000). Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J. Biol. Chem. 275 (51), 40641–40648. doi:10.1074/jbc.M007835200
Alsina-Beauchamp, D., Escós, A., Fajardo, P., González-Romero, D., Díaz-Mora, E., Risco, A., et al. (2018). Myeloid cell deficiency of p38γ/p38δ protects against candidiasis and regulates antifungal immunity. EMBO Mol. Med., 10(5), e8485. doi:10.15252/emmm.201708485
Ambrosino, C., and Nebreda, A. R. (2001). Cell cycle regulation by p38 MAP kinases. Biol. cell 93 (1-2), 47–51. doi:10.1016/s0248-4900(01)01124-8
Amorós, M., and Estruch, F. (2001). Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Mol. Microbiol. 39 (6), 1523–1532. doi:10.1046/j.1365-2958.2001.02339.x
Arechederra, M., Priego, N., Vázquez-Carballo, A., Sequera, C., Gutiérrez-Uzquiza, Á., Cerezo- Guisado, M. I., et al. (2015). p38 MAPK down-regulates fibulin 3 expression through methylation of gene regulatory sequences: role in migration and invasion. J. Biol. Chem. 290 (7), 4383–4397. doi:10.1074/jbc.M114.582239
Asih, P. R., Prikas, E., Stefanoska, K., Tan, A. R. P., Ahel, H. I., and Ittner, A. (2020). Functions of p38 MAP kinases in the central nervous system. Front. Mol. Neurosci. 13, 570586. doi:10.3389/fnmol.2020.570586
Azad, G. K., Singh, V., Thakare, M. J., Baranwal, S., and Tomar, R. S. (2014). Mitogen-activated protein kinase Hog1 is activated in response to curcumin exposure in the budding yeast Saccharomyces cerevisiae. BMC Microbiol. 14, 317. doi:10.1186/s12866-014-0317-0
Babazadeh, R., Furukawa, T., Hohmann, S., and Furukawa, K. (2014). Rewiring yeast osmostress signalling through the MAPK network reveals essential and non-essential roles of Hog1 in osmoadaptation. Sci. Rep. 4, 4697. doi:10.1038/srep04697
Bachelor, M. A., and Bowden, G. T. (2004). UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Seminars cancer Biol. 14 (2), 131–138. doi:10.1016/j.semcancer.2003.09.017
Bachstetter, A. D., Xing, B., de Almeida, L., Dimayuga, E. R., Watterson, D. M., and Van Eldik, L. J. (2011). Microglial p38α MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ). J. neuroinflammation 8, 79. doi:10.1186/1742-2094-8-79
Barnum, K. J., and O'Connell, M. J. (2014). Cell cycle regulation by checkpoints. Methods Mol. Biol. Clift. N.J. 1170, 29–40. doi:10.1007/978-1-4939-0888-2_2
Bartek, J., and Lukas, J. (2001). Mammalian G1-and S-phase checkpoints in response to DNA damage. Curr. Opin. cell Biol. 13 (6), 738–747. doi:10.1016/s0955-0674(00)00280-5
Bassi, R., Heads, R., Marber, M. S., and Clark, J. E. (2008). Targeting p38-MAPK in the ischaemic heart: kill or cure? Curr. Opin. Pharmacol. 8 (2), 141–146. doi:10.1016/j.coph.2008.01.002
Beardmore, V. A., Hinton, H. J., Eftychi, C., Apostolaki, M., Armaka, M., Darragh, J., et al. (2005). Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol. Cell. Biol. 25 (23), 10454–10464. doi:10.1128/MCB.25.23.10454-10464.2005
Beese, S. E., Negishi, T., and Levin, D. E. (2009). Identification of positive regulators of the yeast fps1 glycerol channel. PLoS Genet. 5 (11), e1000738. doi:10.1371/journal.pgen.1000738
Bertin, G., and Averbeck, D. (2006). Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88 (11), 1549–1559. doi:10.1016/j.biochi.2006.10.001
Bilsland, E., Molin, C., Swaminathan, S., Ramne, A., and Sunnerhagen, P. (2004). Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol. Microbiol. 53 (6), 1743–1756. doi:10.1111/j.1365-2958.2004.04238.x
Bishak, Y. K., Payahoo, L., Osatdrahimi, A., and Nourazarian, A. (2015). Mechanisms of cadmium carcinogenicity in the gastrointestinal tract. Asian Pac. J. cancer Prev. APJCP 16 (1), 9–21. doi:10.7314/apjcp.2015.16.1.9
Bouganim, N., David, J., Wysocki, R., and Ramotar, D. (2001). Yap1 overproduction restores arsenite resistance to the ABC transporter deficient mutant ycf1 by activating ACR3 expression. Biochem. cell Biol. = Biochimie Biol. Cell. 79 (4), 441–448. doi:10.1139/o01-033
Brancho, D., Tanaka, N., Jaeschke, A., Ventura, J. J., Kelkar, N., Tanaka, Y., et al. (2003). Mechanism of p38 MAP kinase activation in vivo. Genes and Dev. 17 (16), 1969–1978. doi:10.1101/gad.1107303
Brennan, C. M., Emerson, C. P., Owens, J., and Christoforou, N. (2021). p38 MAPKs roles in skeletal muscle physiology, disease mechanisms, and as potential therapeutic targets. JCI insight 6 (12), e149915. doi:10.1172/jci.insight.149915
Brewster, J. L., de Valoir, T., Dwyer, N. D., Winter, E., and Gustin, M. C. (1993). An osmosensing signal transduction pathway in yeast signal transduction pathway in yeast. Sci. (New York, N.Y.), 259(5102), 1760–1763. doi:10.1126/science.7681220
Bulavin, D. V., Demidov, O. N., Saito, S., Kauraniemi, P., Phillips, C., Amundson, S. A., et al. (2002). Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 31 (2), 210–215. doi:10.1038/ng894
Bulavin, D. V., and Fornace, A. J. (2004). p38 MAP kinase's emerging role as a tumor suppressor. Adv. cancer Res. 92, 95–118. doi:10.1016/S0065-230X(04)92005-2
Cahill, M. A., Peter, M. E., Kischkel, F. C., Chinnaiyan, A. M., Dixit, V. M., Krammer, P. H., et al. (1996). CD95 (APO-1/Fas) induces activation of SAP kinases downstream of ICE-like proteases. Oncogene 13 (10), 2087–2096.
PubMed Abstract PubMed Abstract PubMed Abstract | Google Scholar
Cangi, M. G., Cukor, B., Soung, P., Signoretti, S., Moreira, G., Ranashinge, M., et al. (2000). Role of the Cdc25A phosphatase in human breast cancer. J. Clin. investigation 106 (6), 753–761. doi:10.1172/JCI9174
Capaldi, A. P., Kaplan, T., Liu, Y., Habib, N., Regev, A., Friedman, N., et al. (2008). Structure and function of a transcriptional network activated by the MAPK Hog1. Nat. Genet. 40 (11), 1300–1306. doi:10.1038/ng.235
Cardone, M. H., Salvesen, G. S., Widmann, C., Johnson, G., and Frisch, S. M. (1997). The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell 90 (2), 315–323. doi:10.1016/s0092-8674(00)80339-6
Castro, C., Carvalho, A., Gaivão, I., and Lima-Brito, J. (2021). Evaluation of copper-induced DNA damage in Vitis vinifera L. using Comet-FISH. Environ. Sci. Pollut. Res. Int. 28 (6), 6600–6610. doi:10.1007/s11356-020-10995-7
Chan, C. C., Damen, M. S. M. A., Moreno-Fernandez, M. E., Stankiewicz, T. E., Cappelletti, M., Alarcon, P. C., et al. (2020). Type I interferon sensing unlocks dormant adipocyte inflammatory potential. Nat. Commun. 11 (1), 2745. doi:10.1038/s41467-020-16571-4
Chanjitwiriya, K., Roytrakul, S., and Kunthalert, D. (2020). Quercetin negatively regulates IL-1β production in Pseudomonas aeruginosa-infected human macrophages through the inhibition of MAPK/NLRP3 inflammasome pathways. PloS one 15 (8), e0237752. doi:10.1371/journal.pone.0237752
Chen, G., Hitomi, M., Han, J., and Stacey, D. W. (2000). The p38 pathway provides negative feedback for Ras proliferative signaling. J. Biol. Chem. 275 (50), 38973–38980. doi:10.1074/jbc.M002856200
Chen, Z., Gibson, T. B., Robinson, F., Silvestro, L., Pearson, G., Xu, B., et al. (2001). MAP kinases. Chem. Rev. 101 (8), 2449–2476. doi:10.1021/cr000241p
Cooper, J. A. (1994). MAP kinase pathways. Straight and narrow or tortuous and intersecting? Curr. Biol. CB 4 (12), 1118–1121. doi:10.1016/s0960-9822(00)00251-7
Coulthard, L. R., White, D. E., Jones, D. L., McDermott, M. F., and Burchill, S. A. (2009). p38 (MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 15 (8), 369–379. doi:10.1016/j.molmed.2009.06.005
Cuadrado, A., and Nebreda, A. R. (2010). Mechanisms and functions of p38 MAPK signalling. Biochem. J. 429 (3), 403–417. doi:10.1042/BJ20100323
Cuenda, A., and Rousseau, S. (2007). p38 MAP-kinases pathway regulation, function and role in human diseases. Biochimica biophysica acta 1773 (8), 1358–1375. doi:10.1016/j.bbamcr.2007.03.010
Cuenda, A., and Sanz-Ezquerro, J. J. (2017). p38γ and p38δ: from spectators to key physiological players. Trends Biochem. Sci. 42 (6), 431–442. doi:10.1016/j.tibs.2017.02.008
Culbert, A. A., Skaper, S. D., Howlett, D. R., Evans, N. A., Facci, L., Soden, P. E., et al. (2006). MAPK-activated protein kinase 2 deficiency in microglia inhibits pro-inflammatory mediator release and resultant neurotoxicity. Relevance to neuroinflammation in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 281 (33), 23658–23667. doi:10.1074/jbc.M513646200
Davies, K. J. (1995). Oxidative stress: the paradox of aerobic life. Biochem. Soc. Symp. 61, 1–31. doi:10.1042/bss0610001
Del Vescovo, V., Casagrande, V., Bianchi, M. M., Piccinni, E., Frontali, L., Militti, C., et al. (2008). Role of Hog1 and Yaf9 in the transcriptional response of Saccharomyces cerevisiae to cesium chloride. Physiol. genomics 33 (1), 110–120. doi:10.1152/physiolgenomics.00251.2007
Demple, B. (1998). A bridge to control. Sci. (New York, N.Y.) 279 (5357), 1655–1656. doi:10.1126/science.279.5357.1655
de Nadal, E., Alepuz, P. M., and Posas, F. (2002). Dealing with osmostress through MAP kinase activation. EMBO Rep. 3 (8), 735–740. doi:10.1093/embo-reports/kvf158
de Nadal, E., Ammerer, G., and Posas, F. (2011). Controlling gene expression in response to stress. Nat. Rev. Genet. 12 (12), 833–845. doi:10.1038/nrg3055
de Nadal, E., Real, F. X., and Posas, F. (2007). Mucins, osmosensors in eukaryotic cells? Trends cell Biol. 17 (12), 571–574. doi:10.1016/j.tcb.2007.10.001
Diehl, N. L., Enslen, H., Fortner, K. A., Merritt, C., Stetson, N., Charland, C., et al. (2000). Activation of the p38 mitogen-activated protein kinase pathway arrests cell cycle progression and differentiation of immature thymocytes in vivo. J. Exp. Med. 191 (2), 321–334. doi:10.1084/jem.191.2.321
Dong, K., Addinall, S. G., Lydall, D., and Rutherford, J. C. (2013). The yeast copper response is regulated by DNA damage. Mol. Cell. Biol. 33 (20), 4041–4050. doi:10.1128/MCB.00116-13
Doza, Y. N., Cuenda, A., Thomas, G. M., Cohen, P., and Nebreda, A. R. (1995). Activation of the MAP kinase homologue RK requires the phosphorylation of Thr-180 and Tyr-182 and both residues are phosphorylated in chemically stressed KB cells. FEBS Lett. 364 (2), 223–228. doi:10.1016/0014-5793(95)00346-b
Drogen, F., O'Rourke, S. M., Stucke, V. M., Jaquenoud, M., Neiman, A. M., and Peter, M. (2000). Phosphorylation of the MEKK Ste11p by the PAK-like kinase Ste20p is required for MAP kinase signaling in vivo. Curr. Biol. CB 10 (11), 630–639. doi:10.1016/s0960-9822(00)00511-x
Duch, A., de Nadal, E., and Posas, F. (2012). The p38 and Hog1 SAPKs control cell cycle progression in response to environmental stresses. FEBS Lett. 586 (18), 2925–2931. doi:10.1016/j.febslet.2012.07.034
Dunayevich, P., Baltanás, R., Clemente, J. A., Couto, A., Sapochnik, D., Vasen, G., et al. (2018). Heat-stress triggers MAPK crosstalk to turn on the hyperosmotic response pathway. Sci. Rep. 8 (1), 15168. doi:10.1038/s41598-018-33203-6
El-Esawi, M. A., Elkelish, A., Soliman, M., Elansary, H. O., Zaid, A., and Wani, S. H. (2020). Serratia marcescens BM1 enhances cadmium stress tolerance and phytoremediation potential of soybean through modulation of osmolytes, leaf gas exchange, antioxidant machinery, and stress-responsive genes expression. Antioxidants Basel, Switz. 9 (1), 43. doi:10.3390/antiox9010043
Elhasi, T., and Blomberg, A. (2023). Caffeine activates HOG-signalling and inhibits pseudohyphal growth in Saccharomyces cerevisiae. BMC Res. notes 16 (1), 52. doi:10.1186/s13104-023-06312-3
Engel, F. B., Schebesta, M., Duong, M. T., Lu, G., Ren, S., Madwed, J. B., et al. (2005). p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes and Dev. 19 (10), 1175–1187. doi:10.1101/gad.1306705
Enslen, H., Raingeaud, J., and Davis, R. J. (1998). Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 273 (3), 1741–1748. doi:10.1074/jbc.273.3.1741
Escós, A., Risco, A., Alsina-Beauchamp, D., and Cuenda, A. (2016). p38γ and p38δ mitogen activated protein kinases (MAPKs), new stars in the MAPK galaxy. Front. cell Dev. Biol. 4, 31. doi:10.3389/fcell.2016.00031
Estruch, F., and Carlson, M. (1990). Increased dosage of the MSN1 gene restores invertase expression in yeast mutants defective in the Snf1 protein kinase. Nucleic acids Res. 18 (23), 6959–6964. doi:10.1093/nar/18.23.6959
Evens, A. M., Tallman, M. S., and Gartenhaus, R. B. (2004). The potential of arsenic trioxide in the treatment of malignant disease: past, present, and future. Leukemia Res. 28 (9), 891–900. doi:10.1016/j.leukres.2004.01.011
Fan, K., Yang, J., Gong, W. Y., Pan, Y. C., Zheng, P., and Yue, X. F. (2021). NLRP3 inflammasome activation mediates sleep deprivation-induced pyroptosis in mice. PeerJ 9, e11609. doi:10.7717/peerj.11609
Farooq, M., Ullah, A., Usman, M., and Siddique, K. H. M. (2020). Application of zinc and biochar help to mitigate cadmium stress in bread wheat raised from seeds with high intrinsic zinc. Chemosphere 260, 127652. doi:10.1016/j.chemosphere.2020.127652
Feng, Y., Fang, Z., Liu, B., and Zheng, X. (2019). p38 MAPK plays a pivotal role in the development of acute respiratory distress syndrome. Clin. Sao Paulo, Braz. 74, e509. doi:10.6061/clinics/2019/e509
Fernandes-Alnemri, T., Armstrong, R. C., Krebs, J., Srinivasula, S. M., Wang, L., Bullrich, F., et al. (1996). In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc. Natl. Acad. Sci. United States of America 93 (15), 7464–7469. doi:10.1073/pnas.93.15.7464
Ferrigno, P., Posas, F., Koepp, D., Saito, H., and Silver, P. A. (1998). Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. EMBO J. 17 (19), 5606–5614. doi:10.1093/emboj/17.19.5606
Franzmann, T. M., Menhorn, P., Walter, S., and Buchner, J. (2008). Activation of the chaperone Hsp26 is controlled by the rearrangement of its thermosensor domain. Mol. cell 29 (2), 207–216. doi:10.1016/j.molcel.2007.11.025
Gaikwad, S., Puangmalai, N., Bittar, A., Montalbano, M., Garcia, S., McAllen, S., et al. (2021). Tau oligomer induced HMGB1 release contributes to cellular senescence and neuropathology linked to Alzheimer's disease and frontotemporal dementia. Cell Rep., 36(3), 109419. doi:10.1016/j.celrep.2021.109419
Galaktionov, K., Lee, A. K., Eckstein, J., Draetta, G., Meckler, J., Loda, M., et al. (1995). CDC25 phosphatases as potential human oncogenes. Sci. (New York, N.Y.) 269 (5230), 1575–1577. doi:10.1126/science.7667636
Galcheva-Gargova, Z., Dérijard, B., Wu, I. H., and Davis, R. J. (1994). An osmosensing signal transduction pathway in mammalian cells. Sci. (New York, N.Y.), 265(5173), 806–808. doi:10.1126/science.8047888
Garcia, J., Lemercier, B., Roman-Roman, S., and Rawadi, G. (1998). A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-kappaB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J. Biol. Chem. 273 (51), 34391–34398. doi:10.1074/jbc.273.51.34391
García, R., Bermejo, C., Grau, C., Pérez, R., Rodríguez-Peña, J. M., Francois, J., et al. (2004). The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J. Biol. Chem. 279 (15), 15183–15195. doi:10.1074/jbc.M312954200
Gasch, A. P., Spellman, P. T., Kao, C. M., Carmel-Harel, O., Eisen, M. B., Storz, G., et al. (2000). Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. cell 11 (12), 4241–4257. doi:10.1091/mbc.11.12.4241
Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A., and Catalano, A. (2020). The effects of cadmium toxicity. Int. J. Environ. Res. public health 17 (11), 3782. doi:10.3390/ijerph17113782
Genovese, M. C. (2009). Inhibition of p38: has the fat lady sung? Arthritis rheumatism 60 (2), 317–320. doi:10.1002/art.24264
Gerwien, F., Skrahina, V., Kasper, L., Hube, B., and Brunke, S. (2018). Metals in fungal virulence. FEMS Microbiol. Rev. 42 (1), fux050. doi:10.1093/femsre/fux050
Goedert, M., Cuenda, A., Craxton, M., Jakes, R., and Cohen, P. (1997). Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 16 (12), 3563–3571. doi:10.1093/emboj/16.12.3563
Goldsmith, E. J., Cobb, M. H., and Chang, C. I. (2004). Structure of MAPKs. Methods Mol. Biol. Clift. N.J. 250, 127–144. doi:10.1385/1-59259-671-1:127
Goloudina, A., Yamaguchi, H., Chervyakova, D. B., Appella, E., Fornace, A. J., and Bulavin, D. V. (2003). Regulation of human Cdc25A stability by serine 75 phosphorylation is not sufficient to activate a S phase checkpoint. Cell cycleGeorget. Tex. 2 (5), 471–476. doi:10.4161/cc.2.5.482
Grably, M. R., Stanhill, A., Tell, O., and Engelberg, D. (2002). HSF and Msn2/4p can exclusively or cooperatively activate the yeast HSP104 gene cooperatively activate the yeast HSP104 gene. Mol. Microbiol., 44(1), 21–35. doi:10.1046/j.1365-2958.2002.02860.x
Grivennikov, S. I., and Karin, M. (2011). Inflammatory cytokines in cancer: tumor necrosis factor and interleukin 6 take the stage. Ann. rheumatic Dis. 70 (Suppl. 1), i104–i108. doi:10.1136/ard.2010.140145
Gubern, A., Joaquin, M., Marquès, M., Maseres, P., Garcia-Garcia, J., Amat, R., et al. (2016). The N-terminal phosphorylation of RB by p38 bypasses its inactivation by CDKs and prevents proliferation in cancer cells. Mol. cell 64 (1), 25–36. doi:10.1016/j.molcel.2016.08.015
Gupta, S. C., Kismali, G., and Aggarwal, B. B. (2013). Curcumin, a component of turmeric: from farm to pharmacy. BioFactors Oxf. Engl. 39 (1), 2–13. doi:10.1002/biof.1079
Gustin, M. C., Albertyn, J., Alexander, M., and Davenport, K. (1998). MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. MMBR 62 (4), 1264–1300. doi:10.1128/MMBR.62.4.1264-1300.1998
Guzman-Martinez, L., Maccioni, R. B., Andrade, V., Navarrete, L. P., Pastor, M. G., and Ramos- Escobar, N. (2019). Neuroinflammation as a common feature of neurodegenerative disorders. Frontiers in pharmacology. Front. Pharmacol. 10, 1008. doi:10.3389/fphar.2019.01008
Haghnazari, E., and Heyer, W. D. (2004). The Hog1 MAP kinase pathway and the Mec1 DNA damage checkpoint pathway independently control the cellular responses to hydrogen peroxide. DNA repair 3 (7), 769–776. doi:10.1016/j.dnarep.2004.03.043
Han, J., Lee, J. D., Bibbs, L., and Ulevitch, R. J. (1994). A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Sci. (New York, N.Y.) 265 (5173), 808–811. doi:10.1126/science.7914033
Han, J., Wu, J., and Silke, J. (2020). An overview of mammalian p38 mitogen-activated protein kinases, central regulators of cell stress and receptor signaling. F1000Research 9, 653. F1000 Faculty Rev-653. doi:10.12688/f1000research.22092.1
Haq, R., Brenton, J. D., Takahashi, M., Finan, D., Finkielsztein, A., Damaraju, S., et al. (2002). Constitutive p38 HOG mitogen-activated protein kinase activation induces permanent cell cycle arrest and senescence. Cancer Res. 62 (17), 5076–5082.
PubMed Abstract PubMed Abstract PubMed Abstract | Google Scholar
Hayashi, M., and Maeda, T. (2006). Activation of the HOG pathway upon cold stress in Saccharomyces cerevisiae Saccharomyces cerevisiae. J. Biochem., 139(4), 797–803. doi:10.1093/jb/mvj089
Henry, S. A., and Keith, A. D. (1971). Membrane properties of saturated fatty acid mutants of yeast revealed by spin labels. Chem. Phys. lipids 7 (4), 245–265. doi:10.1016/0009-3084(71)90004-1
Hickman, M. J., Spatt, D., and Winston, F. (2011). The Hog1 mitogen-activated protein kinase mediates a hypoxic response in Saccharomyces cerevisiae. Genetics 188 (2), 325–338. doi:10.1534/genetics.111.128322
Hidalgo, E., Ding, H., and Demple, B. (1997). Redox signal transduction: mutations shifting [2Fe-2S] centers of the SoxR sensor-regulator to the oxidized form. Cell 88 (1), 121–129. doi:10.1016/s0092-8674(00)81864-4
Hightower, L. E. (1990). “Stress Proteins In Biology and Medicine. Richard I. Morimoto, Alfred Tissieres, and Costa Georgopoulos, Eds. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 1990. x, 450 pp., illus. $97. Cold Spring Harbor Monograph Series 19,”Sci. (New York, N.Y.). Editors R. I. Morimoto, A. Tissieres, and C. Georgopoulos 249, 572–573. doi:10.1126/science.249.4968.572-a
Hirai, S. i., Katoh, M., Terada, M., Kyriakis, J. M., Zon, L. I., Rana, A., et al. (1997). MST/MLK2, a member of the mixed lineage kinase family, directly phosphorylates and activates SEK1, an activator of c-Jun N-terminal kinase/stress-activated protein kinase. J. Biol. Chem. 272 (24), 15167–15173. doi:10.1074/jbc.272.24.15167
Ho, J., and Benchimol, S. (2003). Transcriptional repression mediated by the p53 tumour suppressor. Cell death Differ. 10 (4), 404–408. doi:10.1038/sj.cdd.4401191
Hohmann, S. (2002). Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. MMBR 66 (2), 300–372. doi:10.1128/MMBR.66.2.300-372.2002
Hohmann, S. (2015). An integrated view on a eukaryotic osmoregulation system. Curr. Genet. 61 (3), 373–382. doi:10.1007/s00294-015-0475-0
Hollenbach, E., Neumann, M., Vieth, M., Roessner, A., Malfertheiner, P., and Naumann, M. (2004). Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. official Publ. Fed. Am. Soc. Exp. Biol. 18 (13), 1550–1552. doi:10.1096/fj.04-1642fje
Horie, T., Tatebayashi, K., Yamada, R., and Saito, H. (2008). Phosphorylated Ssk1 prevents unphosphorylated Ssk1 from activating the Ssk2 mitogen-activated protein kinase kinase kinase in the yeast high osmolarity glycerol osmoregulatory pathway. Mol. Cell Biol. 28 (17), 5172–5183. doi:10.1128/MCB.00589-08
Horváth, I., Glatz, A., Varvasovszki, V., Török, Z., Páli, T., Balogh, G., et al. (1998). Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a fluidity gene. Proc. Natl. Acad. Sci. U. S. A. 95 (7), 3513–3518. doi:10.1073/pnas.95.7.3513
Hou, X., Liu, Q., Gao, Y., Yong, L., Xie, H., Li, W., et al. (2022). Mesencephalic astrocyte-derived neurotrophic factor reprograms macrophages to ameliorate acetaminophen-induced acute liver injury via p38 MAPK pathway. Cell death and Dis. 13 (2), 100. doi:10.1038/s41419-022-04555-9
Huang, S., Jiang, Y., Li, Z., Nishida, E., Mathias, P., Lin, S., et al. (1997). Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity 6 (6), 739–749. doi:10.1016/s1074-7613(00)80449-5
Hugon, J., and Paquet, C. (2021). The PKR/p38/RIPK1 signaling pathway as a therapeutic target in Alzheimer's disease. Int. J. Mol. Sci. 22 (6), 3136. doi:10.3390/ijms22063136
Ichijo, H., Nishida, E., Irie, K., ten Dijke, P., Saitoh, M., Moriguchi, T., et al. (1997). Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Sci. (New York, N.Y.) 275 (5296), 90–94. doi:10.1126/science.275.5296.90
Igea, A., and Nebreda, A. R. (2015). The Stress Kinase p38α as a Target for Cancer Therapy. Cancer Res. 75 (19), 3997–4002. doi:10.1158/0008-5472.CAN-15-0173
Ising, C., Venegas, C., Zhang, S., Scheiblich, H., Schmidt, S. V., Vieira-Saecker, A., et al. (2019). NLRP3 inflammasome activation drives tau pathology. Nature, 575(7784), 669–673. doi:10.1038/s41586-019-1769-z
Jacoby, T., Flanagan, H., Faykin, A., Seto, A. G., Mattison, C., and Ota, I. (1997). Two protein-tyrosine phosphatases inactivate the osmotic stress response pathway in yeast by targeting the mitogen-activated protein kinase, Hog1. J. Biol. Chem. 272 (28), 17749–17755. doi:10.1074/jbc.272.28.17749
Jamieson, D. J. (1992). Saccharomyces cerevisiae has distinct adaptive responses to both hydrogen peroxide and menadione. J. Bacteriol. 174 (20), 6678–6681. doi:10.1128/jb.174.20.6678-6681.1992
Jamieson, D. J. (1998). Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast Chichester, Engl. 14 (16), 1511–1527. doi:10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S
Jiang, L., Cao, C., Zhang, L., Lin, W., Xia, J., Xu, H., et al. (2014). Cadmium-induced activation of high osmolarity glycerol pathway through its Sln1 branch is dependent on the MAP kinase kinase kinase Ssk2, but not its paralog Ssk22, in budding yeast. FEMS yeast Res. 14 (8), 1263–1272. doi:10.1111/1567-1364.12220
Jiang, Y., Gram, H., Zhao, M., New, L., Gu, J., Feng, L., et al. (1997). Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J. Biol. Chem. 272 (48), 30122–30128. doi:10.1074/jbc.272.48.30122
Jiménez, J., Queralt, E., Posas, F., and de Nadal, E. (2020). The regulation of Net1/Cdc14 by the Hog1 MAPK upon osmostress unravels a new mechanism regulating mitosis. Cell cycleGeorget. Tex. 19 (17), 2105–2118. doi:10.1080/15384101.2020.1804222
Johansen, C., Kragballe, K., Westergaard, M., Henningsen, J., Kristiansen, K., and Iversen, L. (2005). The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br. J. dermatology 152 (1), 37–42. doi:10.1111/j.1365-2133.2004.06304.x
Johnson, G. L., and Lapadat, R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Sci. (New York, N.Y.) 298 (5600), 1911–1912. doi:10.1126/science.1072682
Johnson, G. V., and Bailey, C. D. (2003). The p38 MAP kinase signaling pathway in Alzheimer's disease disease. Exp. Neurol., 183(2), 263–268. doi:10.1016/s0014-4886(03)00268-1
Kandror, O., Bretschneider, N., Kreydin, E., Cavalieri, D., and Goldberg, A. L. (2004). Yeast adapt to near-freezing temperatures by STRE/Msn2,4-dependent induction of trehalose synthesis and certain molecular chaperones. Mol. cell 13 (6), 771–781. doi:10.1016/s1097-2765(04)00148-0
Kheiri, G., Dolatshahi, M., Rahmani, F., and Rezaei, N. (2018). Role of p38/MAPKs in Alzheimer's disease: implications for amyloid beta toxicity targeted therapy. Rev. Neurosci. 30 (1), 9–30. doi:10.1515/revneuro-2018-0008
Kielbassa, C., Roza, L., and Epe, B. (1997). Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 18 (4), 811–816. doi:10.1093/carcin/18.4.811
Kirchman, P. A., and Botta, G. (2007). Copper supplementation increases yeast life span under conditions requiring respiratory metabolism. Mech. ageing Dev. 128 (2), 187–195. doi:10.1016/j.mad.2006.10.003
Kummer, J. L., Rao, P. K., and Heidenreich, K. A. (1997). Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J. Biol. Chem. 272 (33), 20490–20494. doi:10.1074/jbc.272.33.20490
Kuranda, K., Leberre, V., Sokol, S., Palamarczyk, G., and François, J. (2006). Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol. Microbiol. 61 (5), 1147–1166. doi:10.1111/j.1365-2958.2006.05300.x
Kyriakis, J. M., and Avruch, J. (2001). Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81 (2), 807–869. doi:10.1152/physrev.2001.81.2.807
Kyriakis, J. M., and Avruch, J. (2012). Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 92 (2), 689–737. doi:10.1152/physrev.00028.2011
Larsson, S. C., Orsini, N., and Wolk, A. (2015). Urinary cadmium concentration and risk of breast cancer: a systematic review and dose-response meta-analysis. Am. J. Epidemiol. 182 (5), 375–380. doi:10.1093/aje/kwv085
Lavoie, J. N., L'Allemain, G., Brunet, A., Müller, R., and Pouysségur, J. (1996). Cyclin D1 expression is regulated positively by the p42/p44 MAPK and negatively by the p38/HOG MAPK pathway. J. Biol. Chem. 271 (34), 20608–20616. doi:10.1074/jbc.271.34.20608
Lawrence, C. L., Botting, C. H., Antrobus, R., and Coote, P. J. (2004). Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: regulating adaptation to citric acid stress. Mol. Cell. Biol. 24 (8), 3307–3323. doi:10.1128/MCB.24.8.3307-3323.2004
Lee, J., and Levin, D. E. (2018). Intracellular mechanism by which arsenite activates the yeast stress MAPK Hog1. Mol. Biol. cell 29 (15), 1904–1915. doi:10.1091/mbc.E18-03-0185
Lee, J., Liu, L., and Levin, D. E. (2019). Stressing out or stressing in: intracellular pathways for SAPK activation. Curr. Genet. 65 (2), 417–421. doi:10.1007/s00294-018-0898-5
Lee, J., Reiter, W., Dohnal, I., Gregori, C., Beese-Sims, S., Kuchler, K., et al. (2013). MAPK Hog1 closes the S. cerevisiae glycerol channel Fps1 by phosphorylating and displacing its positive regulators. Genes and Dev. 27 (23), 2590–2601. doi:10.1101/gad.229310.113
Lee, Y. M., Kim, E., An, J., Lee, Y., Choi, E., Choi, W., et al. (2017). Dissection of the HOG pathway activated by hydrogen peroxide in Saccharomyces cerevisiae. Environ. Microbiol. 19 (2), 584–597. doi:10.1111/1462-2920.13499
Li, Y., Chen, N., Wu, C., Lu, Y., Gao, G., Duan, C., et al. (2020). Galectin-1 attenuates neurodegeneration in Parkinson's disease model by modulating microglial MAPK/IκB/NFκB axis through its carbohydrate-recognition domain. Brain, Behav. Immun. 83, 214–225. doi:10.1016/j.bbi.2019.10.015
Liang, L., Li, F., Bao, A., Zhang, M., Chung, K. F., and Zhou, X. (2013). Activation of p38 mitogen-activated protein kinase in ovalbumin and ozone-induced mouse model of asthma. Respirol. Carlt. Vic. 18 (Suppl. 3), 20–29. doi:10.1111/resp.12189
Liao, X., Zhang, W., Dai, H., Jing, R., Ye, M., Ge, W., et al. (2021). Neutrophil-Derived IL-17 Promotes Ventilator-Induced Lung Injury via p38 MAPK/MCP-1 Pathway Activation. Front. Immunol. 12, 768813. doi:10.3389/fimmu.2021.768813
Lindquist, S., and Kim, G. (1996). Heat-shock protein 104 expression is sufficient for thermotolerance in yeast. Proc. Natl. Acad. Sci. U. S. A. 93 (11), 5301–5306. doi:10.1073/pnas.93.11.5301
Liu, Z. F., Zheng, D., Fan, G. C., Peng, T., and Su, L. (2016). Heat stress prevents lipopolysaccharide-induced apoptosis in pulmonary microvascular endothelial cells by blocking calpain/p38 MAPK signalling. Apoptosis Int. J. Program. cell death 21 (8), 896–904. doi:10.1007/s10495-016-1263-0
Lo, J., Liu, C. C., Li, Y. S., Lee, P. Y., Liu, P. L., Wu, P. C., et al. (2022). Punicalagin attenuates LPS-induced inflammation and ROS production in microglia by inhibiting the MAPK/NF-κB signaling pathway and NLRP3 inflammasome activation. J. Inflamm. Res. 15, 5347–5359. doi:10.2147/JIR.S372773
López-Malo, M., Chiva, R., Rozes, N., and Guillamon, J. M. (2013). Phenotypic analysis of mutant and overexpressing strains of lipid metabolism genes in Saccharomyces cerevisiae: implication in growth at low temperatures. Int. J. food Microbiol. 162 (1), 26–36. doi:10.1016/j.ijfoodmicro.2012.12.020
Lucena, R. M., Dolz-Edo, L., Brul, S., de Morais, M. A., and Smits, G. (2020). Extreme Low Cytosolic pH Is a Signal for Cell Survival in Acid Stressed Yeast. Genes 11 (6), 656. doi:10.3390/genes11060656
Ludovico, P., Sansonetty, F., Silva, M. T., and Côrte-Real, M. (2003). Acetic acid induces a programmed cell death process in the food spoilage yeast Zygosaccharomyces bailii. FEMS yeast Res. 3 (1), 91–96. doi:10.1016/s1567-1356(02)00166-6
Ludovico, P., Sousa, M. J., Silva, M. T., Leão, C. L., and Côrte-Real, M. (2001). Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiol. Read. Engl. 147 (Pt 9), 2409–2415. doi:10.1099/00221287-147-9-2409
Macé, G., Miaczynska, M., Zerial, M., and Nebreda, A. R. (2005). Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid receptor endocytosis. EMBO J. 24 (18), 3235–3246. doi:10.1038/sj.emboj.7600799
Macia, J., Regot, S., Peeters, T., Conde, N., Solé, R., and Posas, F. (2009). Dynamic signaling in the Hog1 MAPK pathway relies on high basal signal transduction. Sci. Signal. 2 (63), ra13. doi:10.1126/scisignal.2000056
Maeda, T., Takekawa, M., and Saito, H. (1995). Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Sci. (New York, N.Y.), 269(5223), 554–558. doi:10.1126/science.7624781
Maeda, T., Wurgler-Murphy, S. M., and Saito, H. (1994). A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369 (6477), 242–245. doi:10.1038/369242a0
Mailand, N., Falck, J., Lukas, C., Syljuâsen, R. G., Welcker, M., Bartek, J., et al. (2000). Rapid destruction of human Cdc25A in response to DNA damage. Sci. (New York, N.Y.) 288 (5470), 1425–1429. doi:10.1126/science.288.5470.1425
Marques, J. M., Rodrigues, R. J., de Magalhães-Sant'ana, A. C., and Gonçalves, T. (2006). Saccharomyces cerevisiae Hog1 protein phosphorylation upon exposure to bacterial endotoxin. J. Biol. Chem. 281 (34), 24687–24694. doi:10.1074/jbc.M603753200
Martínez-Limón, A., Joaquin, M., Caballero, M., Posas, F., and de Nadal, E. (2020). The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 21 (6), 1913. doi:10.3390/ijms21061913
Martins, A. M., Cordeiro, C. A., and Ponces Freire, A. M. (2001). In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Lett. 499 (1-2), 41–44. doi:10.1016/s0014-5793(01)02519-4
Martins, T. S., Pereira, C., Canadell, D., Vilaça, R., Teixeira, V., Moradas-Ferreira, P., et al. (2018). The Hog1p kinase regulates Aft1p transcription factor to control iron accumulation. Biochimica biophysica acta. Mol. cell Biol. lipids 1863 (1), 61–70. doi:10.1016/j.bbalip.2017.10.001
Mattison, C. P., and Ota, I. M. (2000). Two protein tyrosine phosphatases, Ptp2 and Ptp3, modulate the subcellular localization of the Hog1 MAP kinase in yeast. Genes and Dev. 14 (10), 1229–1235. doi:10.1101/gad.14.10.1229
Mattison, C. P., Spencer, S. S., Kresge, K. A., Lee, J., and Ota, I. M. (1999). Differential regulation of the cell wall integrity mitogen–activated protein kinase pathway in budding yeast by the protein tyrosine phosphatases Ptp2 and Ptp3. Mol. Cell. Biol. 19, 7651–7660. doi:10.1128/MCB.19.11.7651
Menezes, R. A., Amaral, C., Delaunay, A., Toledano, M., and Rodrigues-Pousada, C. (2004). Yap8p activation in Saccharomyces cerevisiae under arsenic conditions. FEBS Lett. 566 (1-3), 141–146. doi:10.1016/j.febslet.2004.04.019
Mertens, S., Craxton, M., and Goedert, M. (1996). SAP kinase-3, a new member of the family of mammalian stress-activated protein kinases. FEBS Lett. 383 (3), 273–276. doi:10.1016/0014-5793(96)00255-4
Mollapour, M., and Piper, P. W. (2006). Hog1p mitogen-activated protein kinase determines acetic acid resistance in Saccharomyces cerevisiae. FEMS yeast Res. 6 (8), 1274–1280. doi:10.1111/j.1567-1364.2006.00118.x
Monroy, A., Lithgow, G. J., and Alavez, S. (2013). Curcumin and neurodegenerative diseases. BioFactors Oxf. Engl. 39 (1), 122–132. doi:10.1002/biof.1063
Moran, N. (2011). p38 kinase inhibitor approved for idiopathic pulmonary fibrosis. Nat. Biotechnol. 29 (4), 301. doi:10.1038/nbt0411-301
Moriguchi, T., Kuroyanagi, N., Yamaguchi, K., Gotoh, Y., Irie, K., Kano, T., et al. (1996). A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J. Biol. Chem. 271 (23), 13675–13679. doi:10.1074/jbc.271.23.13675
Muslin, A. J. (2008). MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin. Sci. Lond. Engl. 1979 115 (7), 203–218. doi:10.1042/CS20070430
Nadal, E., and Posas, F. (2015). Osmostress-induced gene expression--a model to understand how stress-activated protein kinases (SAPKs) regulate transcription. FEBS J. 282 (17), 3275–3285. doi:10.1111/febs.13323
Nadal, E., and Posas, F. (2022). The HOG pathway and the regulation of osmoadaptive responses in yeast. FEMS yeast Res. 22 (1), foac013. doi:10.1093/femsyr/foac013
Nagata, Y., and Todokoro, K. (1999). Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood 94 (3), 853–863. doi:10.1182/blood.v94.3.853.415a12_853_863
Obata, T., Brown, G. E., and Yaffe, M. B. (2000). MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit. care Med. 28 (4 Suppl. l), N67–N77. doi:10.1097/00003246-200004001-00008
Ono, K., and Han, J. (2000). The p38 signal transduction pathway: activation and function. Cell Signal 12, 1–13. doi:10.1016/s0898-6568(99)00071-6
O'Rourke, S. M., and Herskowitz, I. (1998). The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes and Dev. 12 (18), 2874–2886. doi:10.1101/gad.12.18.2874
O'Rourke, S. M., and Herskowitz, I. (2002). A third osmosensing branch in Saccharomyces cerevisiae requires the Msb2 protein and functions in parallel with the Sho1 branch. Mol. Cell. Biol. 22 (13), 4739–4749. doi:10.1128/MCB.22.13.4739-4749.2002
O'Rourke, S. M., and Herskowitz, I. (2004). Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol. Biol. Cell 15 (2), 532–542. doi:10.1091/mbc.e03-07-0521
O'Rourke, S. M., Herskowitz, I., and O'Shea, E. K. (2002). Yeast go the whole HOG for the hyperosmotic response. Trends Genet. TIG 18 (8), 405–412. doi:10.1016/s0168-9525(02)02723-3
Otsuka, M., Kang, Y. J., Ren, J., Jiang, H., Wang, Y., Omata, M., et al. (2010). Distinct effects of p38alpha deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology 138 (4), 1255–1265. doi:10.1053/j.gastro.2010.01.005
Ozturk, M., Metin, M., Altay, V., De Filippis, L., Ünal, B. T., Khursheed, A., et al. (2021). Molecular Biology of Cadmium Toxicity in Saccharomyces cerevisiae. Biol. trace Elem. Res. 199 (12), 4832–4846. doi:10.1007/s12011-021-02584-7
Panadero, J., Pallotti, C., Rodríguez-Vargas, S., Randez-Gil, F., and Prieto, J. A. (2006). A downshift in temperature activates the high osmolarity glycerol (HOG) pathway, which determines freeze tolerance in Saccharomyces cerevisiae. J. Biol. Chem. 281 (8), 4638–4645. doi:10.1074/jbc.M512736200
Park, J., Min, J. S., Kim, B., Chae, U. B., Yun, J. W., Choi, M. S., et al. (2015). Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 584, 191–196. doi:10.1016/j.neulet.2014.10.016
Parsell, D. A., and Lindquist, S. (1993). The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 27, 437–496. doi:10.1146/annurev.ge.27.120193.002253
Pearson, G., Robinson, F., Beers Gibson, T., Xu, B. E., Karandikar, M., Berman, K., et al. (2001). Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22 (2), 153–183. doi:10.1210/edrv.22.2.0428
Peel, A. L., Sorscher, N., Kim, J. Y., Galvan, V., Chen, S., and Bredesen, D. E. (2004). Tau phosphorylation in Alzheimer's disease: potential involvement of an APP-MAP kinase complex. Neuromolecular Med. 5 (3), 205–218. doi:10.1385/NMM:5:3:205
Perregaux, D. G., Dean, D., Cronan, M., Connelly, P., and Gabel, C. A. (1995). Inhibition of interleukin-1 beta production by SKF86002: evidence of two sites of in vitro activity and of a time and system dependence. Mol. Pharmacol. 48 (3), 433–442. doi:10.1016/s0026-895x(25)10491-4
Phillips, S. A., and Thornalley, P. J. (1993). The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 212 (1), 101–105. doi:10.1111/j.1432-1033.1993.tb17638.x
Piao, H., MacLean Freed, J., and Mayinger, P. (2012). Metabolic activation of the HOG MAP kinase pathway by Snf1/AMPK regulates lipid signaling at the Golgi. Traffic Copenhagen, Den. 13 (11), 1522–1531. doi:10.1111/j.1600-0854.2012.01406.x
Piper, P. W. (1993). Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 11 (4), 339–355. doi:10.1111/j.1574-6976.1993.tb00005.x
Posas, F., Chambers, J. R., Heyman, J. A., Hoeffler, J. P., de Nadal, E., and Ariño, J. (2000). The transcriptional response of yeast to saline stress. J. Biol. Chem. 275 (23), 17249–17255. doi:10.1074/jbc.M910016199
Posas, F., and Saito, H. (1997). Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Sci. (New York, N.Y.) 276 (5319), 1702–1705. doi:10.1126/science.276.5319.1702
Posas, F., Witten, E. A., and Saito, H. (1998). Requirement of STE50 for osmostress-induced activation of the STE11 mitogen-activated protein kinase kinase kinase in the high osmolarity glycerol response pathway. Mol. Cell. Biol. 18, 5788–5796. doi:10.1128/MCB.18.10.5788
Posas, F., Wurgler-Murphy, S. M., Maeda, T., Witten, E. A., Thai, T. C., and Saito, H. (1996). Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86 (6), 865–875. doi:10.1016/s0092-8674(00)80162-2
Puri, P. L., Wu, Z., Zhang, P., Wood, L. D., Bhakta, K. S., Han, J., et al. (2000). Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes and Dev. 14 (5), 574–584. doi:10.1101/gad.14.5.574
Raitt, D. C., Posas, F., and Saito, H. (2000). Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 19, 4623–4631. doi:10.1093/emboj/19.17.4623
Reiser, V., Ruis, H., and Ammerer, G. (1999). Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol. Biol. cell 10 (4), 1147–1161. doi:10.1091/mbc.10.4.1147
Reiser, V., Salah, S. M., and Ammerer, G. (2000). Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1 and Cdc42. Nat. Cell Biol. 2, 620–627. doi:10.1038/35023568
Ren, M., Li, R., Han, B., You, Y., Huang, W., Du, G., et al. (2022). Involvement of the high-osmolarity glycerol pathway of Saccharomyces cerevisiae in protection against copper Toxicity. Antioxidants Basel, Switz. 11 (2), 200. doi:10.3390/antiox11020200
Rep, M., Proft, M., Remize, F., Tamás, M., Serrano, R., Thevelein, J. M., et al. (2001). The Saccharomyces cerevisiae Sko1p transcription factor mediates HOG pathway-dependent osmotic regulation of a set of genes encoding enzymes implicated in protection from oxidative damage. Mol. Microbiol. 40 (5), 1067–1083. doi:10.1046/j.1365-2958.2001.02384.x
Rep, M., Reiser, V., Gartner, U., Thevelein, J. M., Hohmann, S., Ammerer, G., et al. (1999). Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell. Biol. 19 (8), 5474–5485. doi:10.1128/MCB.19.8.5474
Rhodius, V. A., and Mutalik, V. K. (2010). Predicting strength and function for promoters of the Escherichia coli alternative sigma factor, sigmaE. Proc. Natl. Acad. Sci. U. S. A. 107 (7), 2854–2859. doi:10.1073/pnas.0915066107
Richter, K., Haslbeck, M., and Buchner, J. (2010). The heat shock response: life on the verge of death. Mol. cell 40 (2), 253–266. doi:10.1016/j.molcel.2010.10.006
Risco, A., del Fresno, C., Mambol, A., Alsina-Beauchamp, D., MacKenzie, K. F., Yang, H. T., et al. (2012). p38γ and p38δ kinases regulate the Toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Proc. Natl. Acad. Sci. U. S. A. 109 (28), 11200–11205. doi:10.1073/pnas.1207290109
Romanov, N., Hollenstein, D. M., Janschitz, M., Ammerer, G., Anrather, D., and Reiter, W. (2017). Identifying protein kinase-specific effectors of the osmostress response in yeast. Sci. Signal. 10 (469), eaag2435. doi:10.1126/scisignal.aag2435
Rosen, B. P. (2002). Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Mol. and Integr. physiology 133 (3), 689–693. doi:10.1016/s1095-6433(02)00201-5
Rosenfeld, E., and Beauvoit, B. (2003). Role of the non-respiratory pathways in the utilization of molecular oxygen by Saccharomyces cerevisiae. Yeast Chichester, Engl. 20 (13), 1115–1144. doi:10.1002/yea.1026
Roulston, A., Reinhard, C., Amiri, P., and Williams, L. T. (1998). Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J. Biol. Chem. 273 (17), 10232–10239. doi:10.1074/jbc.273.17.10232
Sacristán-Reviriego, A., Martín, H., and Molina, M. (2015). Identification of putative negative regulators of yeast signaling through a screening for protein phosphatases acting on cell wall integrity and mating MAPK pathways. Fungal Genet. Biol. FG and B 77, 1–11. doi:10.1016/j.fgb.2015.02.011
Saito, H., and Posas, F. (2012). Response to hyperosmotic stress. Genetics 192 (2), 289–318. doi:10.1534/genetics.112.140863
Saito, H., and Tatebayashi, K. (2004). Regulation of the osmoregulatory HOG MAPK cascade in yeast. J. Biochem. 136 (3), 267–272. doi:10.1093/jb/mvh135
Salmond, C. V., Kroll, R. G., and Booth, I. R. (1984). The effect of food preservatives on pH homeostasis in Escherichia coli. J. general Microbiol. 130 (11), 2845–2850. doi:10.1099/00221287-130-11-2845
Sanchez, Y., Parsell, D. A., Taulien, J., Vogel, J. L., Craig, E. A., and Lindquist, S. (1993). Genetic evidence for a functional relationship between Hsp104 and Hsp70. J. Bacteriol. 175 (20), 6484–6491. doi:10.1128/jb.175.20.6484-6491.1993
Sanz-Ezquerro, J. J., and Cuenda, A. (2021). p38 Signalling Pathway. Int. J. Mol. Sci. 22 (3), 1003. doi:10.3390/ijms22031003
Schade, B., Jansen, G., Whiteway, M., Entian, K. D., and Thomas, D. Y. (2004). Cold adaptation in budding yeast. Mol. Biol. cell 15 (12), 5492–5502. doi:10.1091/mbc.e04-03-0167
Schmitt, A. P., and McEntee, K. (1996). Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 93 (12), 5777–5782. doi:10.1073/pnas.93.12.5777
Schüller, C., Brewster, J. L., Alexander, M. R., Gustin, M. C., and Ruis, H. (1994). The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene Saccharomyces cerevisiae CTT1 gene. EMBO J., 13(18), 4382–4389. doi:10.1002/j.1460-2075.1994.tb06758.x
Sellam, A., Chaillot, J., Mallick, J., Tebbji, F., Richard Albert, J., Cook, M. A., et al. (2019). The p38/HOG stress-activated protein kinase network couples growth to division in Candida albicans. PLoS Genet. 15 (3), e1008052. doi:10.1371/journal.pgen.1008052
Senokuchi, T., Matsumura, T., Sakai, M., Yano, M., Taguchi, T., Matsuo, T., et al. (2005). Statins suppress oxidized low density lipoprotein-induced macrophage proliferation by inactivation of the small G protein-p38 MAPK pathway. J. Biol. Chem. 280 (8), 6627–6633. doi:10.1074/jbc.M412531200
She, Q. B., Bode, A. M., Ma, W. Y., Chen, N. Y., and Dong, Z. (2001). Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 61 (4), 1604–1610.
PubMed Abstract PubMed Abstract PubMed Abstract | Google Scholar
Shehzad, A., Lee, J., and Lee, Y. S. (2013). Curcumin in various cancers. BioFactors Oxf. Engl. 39 (1), 56–68. doi:10.1002/biof.1068
Sheikh-Hamad, D., and Gustin, M. C. (2004). MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am. J. physiology. Ren. physiology 287 (6), F1102–F1110. doi:10.1152/ajprenal.00225.2004
Singh, K. K. (2000). The Saccharomyces cerevisiae Sln1p-Ssk1p two-component system mediates response to oxidative stress and in an oxidant-specific fashion. Free Radic. Biol. and Med. 29 (10), 1043–1050. doi:10.1016/s0891-5849(00)00432-9
Singh, V., Azad, G. K., Reddy M, A., Baranwal, S., and Tomar, R. S. (2014). Anti-cancer drug KP1019 induces Hog1 phosphorylation and protein ubiquitylation in Saccharomyces cerevisiae. Eur. J. Pharmacol. 736, 77–85. doi:10.1016/j.ejphar.2014.04.032
Solleiro-Villavicencio, H., and Rivas-Arancibia, S. (2018). Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. cell. neurosci. 12, 114. doi:10.3389/fncel.2018.00114
Sotelo, J., and Rodríguez-Gabriel, M. A. (2006). Mitogen-activated protein kinase Hog1 is essential for the response to arsenite in Saccharomyces cerevisiae. Eukaryot. cell 5 (10), 1826–1830. doi:10.1128/EC.00225-06
Stevens, S. K., Strehle, A. P., Miller, R. L., Gammons, S. H., Hoffman, K. J., McCarty, J. T., et al. (2013). The anticancer ruthenium complex KP1019 induces DNA damage, leading to cell cycle delay and cell death in Saccharomyces cerevisiae. Mol. Pharmacol. 83 (1), 225–234. doi:10.1124/mol.112.079657
Stramucci, L., Pranteda, A., and Bossi, G. (2018). Insights of crosstalk between p53 protein and the MKK3/MKK6/p38 MAPK signaling pathway in cancer. Cancers 10 (5), 131. doi:10.3390/cancers10050131
Suarez-Cuervo, C., Merrell, M. A., Watson, L., Harris, K. W., Rosenthal, E. L., Väänänen, H. K., et al. (2004). Breast cancer cells with inhibition of p38alpha have decreased MMP-9 activity and exhibit decreased bone metastasis in mice. Clin. and Exp. metastasis 21 (6), 525–533. doi:10.1007/s10585-004-3503-x
Swan, T. M., and Watson, K. (1997). Membrane fatty acid composition and membrane fluidity as parameters of stress tolerance in yeast. Can. J. Microbiol. 43 (1), 70–77. doi:10.1139/m97-010
Takekawa, M., Adachi, M., Nakahata, A., Nakayama, I., Itoh, F., Tsukuda, H., et al. (2000). p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK- p53 signaling in response to UV radiation. EMBO J. 19 (23), 6517–6526. doi:10.1093/emboj/19.23.6517
Tamás, M. J., Luyten, K., Sutherland, F. C., Hernandez, A., Albertyn, J., Valadi, H., et al. (1999). Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 31 (4), 1087–1104. doi:10.1046/j.1365-2958.1999.01248.x
Tamás, M. J., and Wysocki, R. (2001). Mechanisms involved in metalloid transport and tolerance acquisition. Curr. Genet. 40 (1), 2–12. doi:10.1007/s002940100234
Tamura, K., Sudo, T., Senftleben, U., Dadak, A. M., Johnson, R., and Karin, M. (2000). Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102 (2), 221–231. doi:10.1016/s0092-8674(00)00027-1
Tanaka, K., Tatebayashi, K., Nishimura, A., Yamamoto, K., Yang, H. Y., and Saito, H. (2014). Yeast osmosensors Hkr1 and Msb2 activate the Hog1 MAPK cascade by different mechanisms. Sci. Signal. 7 (314), ra21. doi:10.1126/scisignal.2004780
Tang, J., Qi, X., Mercola, D., Han, J., and Chen, G. (2005). Essential role of p38gamma in K-Ras transformation independent of phosphorylation. J. Biol. Chem. 280 (25), 23910–23917. doi:10.1074/jbc.M500699200
Tatebayashi, K., Tanaka, K., Yang, H. Y., Yamamoto, K., Matsushita, Y., Tomida, T., et al. (2007). Transmembrane mucins Hkr1 and Msb2 are putative osmosensors in the SHO1 branch of yeast HOG pathway. EMBO J. 26, 3521–3533. doi:10.1038/sj.emboj.7601796
Tchakarska, G., and Sola, B. (2020). The double dealing of cyclin D1. Cell cycleGeorget. Tex. 19 (2), 163–178. doi:10.1080/15384101.2019.1706903
Thomas, T., Timmer, M., Cesnulevicius, K., Hitti, E., Kotlyarov, A., and Gaestel, M. (2008). MAPKAP kinase 2-deficiency prevents neurons from cell death by reducing neuroinflammation--relevance in a mouse model of Parkinson's disease relevance in a mouse model of Parkinson's disease. J. Neurochem., 105(5), 2039– 2052. doi:10.1111/j.1471-4159.2008.05310.x
Thorsen, M., Di, Y., Tängemo, C., Morillas, M., Ahmadpour, D., Van der Does, C., et al. (2006). The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Mol. Biol. cell 17 (10), 4400–4410. doi:10.1091/mbc.e06-04-0315
Timoshenko, A. V., Chakraborty, C., Wagner, G. F., and Lala, P. K. (2006). COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br. J. cancer 94 (8), 1154–1163. doi:10.1038/sj.bjc.6603067
Udom, N., Chansongkrow, P., Charoensawan, V., and Auesukaree, C. (2019). Coordination of the cell wall integrity and high-osmolarity glycerol pathways in response to ethanol stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 85 (15), 005511-19–e619. doi:10.1128/AEM.00551-19
Vallejo, M. C., and Mayinger, P. (2015). Delayed turnover of unphosphorylated Ssk1 during carbon stress activates the yeast Hog1 Map kinase pathway. PloS one 10 (9), e0137199. doi:10.1371/journal.pone.0137199
Vander Jagt, D. L., Robinson, B., Taylor, K. K., and Hunsaker, L. A. (1992). Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. J. Biol. Chem. 267 (7), 4364–4369. doi:10.1016/s0021-9258(18)42844-x
Van Eldik, L. J., Thompson, W. L., Ralay Ranaivo, H., Behanna, H. A., and Martin Watterson, D. (2007). Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int. Rev. Neurobiol. 82, 277–296. doi:10.1016/S0074-7742(07)82015-0
Vázquez-Ibarra, A., Rodríguez-Martínez, G., Guerrero-Serrano, G., Kawasaki, L., Ongay-Larios, L., and Coria, R. (2020). Negative feedback-loop mechanisms regulating HOG- and pheromone- MAPK signaling in yeast. Curr. Genet. 66 (5), 867–880. doi:10.1007/s00294-020-01089-5
Vera-Ramirez, L., Pérez-Lopez, P., Varela-Lopez, A., Ramirez-Tortosa, M., Battino, M., and Quiles, J. L. (2013). Curcumin and liver disease. BioFactors Oxf. Engl. 39 (1), 88–100. doi:10.1002/biof.1057
Vergote, I., Heitz, F., Buderath, P., Powell, M., Sehouli, J., Lee, C. M., et al. (2020). A randomized, double-blind, placebo-controlled phase 1b/2 study of ralimetinib, a p38 MAPK inhibitor, plus gemcitabine and carboplatin versus gemcitabine and carboplatin for women with recurrent platinum-sensitive ovarian cancer. Gynecol. Oncol. 156 (1), 23–31. doi:10.1016/j.ygyno.2019.11.006
Wagner, E. F., and Nebreda, A. R. (2009). Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9 (8), 537–549. doi:10.1038/nrc2694
Wallace, S. S. (1998). Enzymatic processing of radiation-induced free radical damage in DNA. Radiat. Res. 150 (5 Suppl. l), S60–S79. doi:10.2307/3579809
Wang, J., Liu, Y., Guo, Y., Liu, C., Yang, Y., Fan, X., et al. (2024). Function and inhibition of p38 MAP kinase signaling: Targeting multiple inflammation diseases. Biochem. Pharmacol. 220, 115973. doi:10.1016/j.bcp.2023.115973
Wang, S., Luo, M., Zhang, Z., Gu, J., Chen, J., Payne, K. M., et al. (2016). Zinc deficiency exacerbates while zinc supplement attenuates cardiac hypertrophy in high-fat diet-induced obese mice through modulating p38 MAPK-dependent signaling. Toxicol. Lett. 258, 134–146. doi:10.1016/j.toxlet.2016.06.020
Wang, W., Chen, J. X., Liao, R., Deng, Q., Zhou, J. J., Huang, S., et al. (2002). Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol. Cell. Biol. 22 (10), 3389–3403. doi:10.1128/MCB.22.10.3389-3403.2002
Warmka, J., Hanneman, J., Lee, J., Amin, D., and Ota, I. (2001). Ptc1, a type 2C Ser/Thr phosphatase, inactivates the HOG pathway by dephosphorylating the mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 21 (1), 51–60. doi:10.1128/MCB.21.1.51-60.2001
Warringer, J., Hult, M., Regot, S., Posas, F., and Sunnerhagen, P. (2010). The HOG pathway dictates the short-term translational response after hyperosmotic shock. Mol. Biol. cell 21 (17), 3080–3092. doi:10.1091/mbc.E10-01-0006
West, A. H., and Stock, A. M. (2001). Histidine kinases and response regulator proteins in two-component signaling systems. Trends biochem. Sci. 26, 369–376. doi:10.1016/s0968-0004(01)01852-7
Westfall, P. J., Ballon, D. R., and Thorner, J. (2004). When the stress of your environment makes you go HOG wild. Sci. (New York, N.Y.) 306 (5701), 1511–1512. doi:10.1126/science.1104879
Winkler, A., Arkind, C., Mattison, C. P., Burkholder, A., Knoche, K., and Ota, I. (2002). Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot. cell 1 (2), 163–173. doi:10.1128/EC.1.2.163-173.2002
Wong, C. M., Ching, Y. P., Zhou, Y., Kung, H. F., and Jin, D. Y. (2003). Transcriptional regulation of yeast peroxiredoxin gene TSA2 through Hap1p, Rox1p, and Hap2/3/5p. Free Radic. Biol. and Med. 34 (5), 585–597. doi:10.1016/s0891-5849(02)01354-0
Wong, E. S., Le Guezennec, X., Demidov, O. N., Marshall, N. T., Wang, S. T., Krishnamurthy, J., et al. (2009). p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev. cell 17 (1), 142–149. doi:10.1016/j.devcel.2009.05.009
Wu, C., Jansen, G., Zhang, J., Thomas, D. Y., and Whiteway, M. (2006). Adaptor protein Ste50p links the Ste11p MEKK to the HOG pathway through plasma membrane association. Genes and Dev. 20 (6), 734–746. doi:10.1101/gad.1375706
Wurgler-Murphy, S. M., Maeda, T., Witten, E. A., and Saito, H. (1997). Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol. Cell. Biol. 17 (3), 1289–1297. doi:10.1128/MCB.17.3.1289
Wysocki, R., Fortier, P. K., Maciaszczyk, E., Thorsen, M., Leduc, A., Odhagen, A., et al. (2004). Transcriptional activation of metalloid tolerance genes in Saccharomyces cerevisiae requires the AP-1-like proteins Yap1p and Yap8p. Mol. Biol. cell 15 (5), 2049–2060. doi:10.1091/mbc.e03-04-0236
Xu, K., and Shu, H. K. (2007). EGFR activation results in enhanced cyclooxygenase-2 expression through p38 mitogen-activated protein kinase-dependent activation of the Sp1/Sp3 transcription factors in human gliomas. Cancer Res. 67 (13), 6121–6129. doi:10.1158/0008-5472.CAN-07-0141
Yaakoub, H., Mina, S., Calenda, A., Bouchara, J. P., and Papon, N. (2022). Oxidative stress response pathways in fungi. Cell. Mol. life Sci. CMLS 79 (6), 333. doi:10.1007/s00018-022-04353-8
Yamamoto, N., Maeda, Y., Ikeda, A., and Sakurai, H. (2008). Regulation of thermotolerance by stress-induced transcription factors in Saccharomyces cerevisiae. Eukaryot. cell 7 (5), 783–790. doi:10.1128/EC.00029-08
Yan, T., and Zhao, Y. (2020). Acetaldehyde induces phosphorylation of dynamin-related protein 1 and mitochondrial dysfunction via elevating intracellular ROS and Ca2+ levels. Redox Biol. 28, 101381. doi:10.1016/j.redox.2019.101381
Yang, L., Zhou, R., Tong, Y., Chen, P., Shen, Y., Miao, S., et al. (2020). Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 140, 104814. doi:10.1016/j.nbd.2020.104814
Yang, Y., Kim, S. C., Yu, T., Yi, Y. S., Rhee, M. H., Sung, G. H., et al. (2014). Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 352371. doi:10.1155/2014/352371
Yao, R., Zhou, P., Wu, C., Liu, L., and Wu, J. (2020). Sml1 Inhibits the DNA Repair Activity of Rev1 in Saccharomyces cerevisiae during Oxidative Stress. Appl. Environ. Microbiol. 86 (7), 028388-19–e2919. doi:10.1128/AEM.02838-19
Young, C., Mapes, J., Hanneman, J., Al-Zarban, S., and Ota, I. (2002). Role of Ptc2 type 2C Ser/Thr phosphatase in yeast high-osmolarity glycerol pathway inactivation. Eukaryot. cell 1 (6), 1032–1040. doi:10.1128/EC.1.6.1032-1040.2002
Zhang, H., and Reynolds, M. (2019). Cadmium exposure in living organisms: A short review. Sci. total Environ. 678, 761–767. doi:10.1016/j.scitotenv.2019.04.395
Zhao, H., Watkins, J. L., and Piwnica-Worms, H. (2002). Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl. Acad. Sci. U. S. A. 99 (23), 14795–14800. doi:10.1073/pnas.182557299
Zhao, Y., Li, S., Wang, J., Liu, Y., and Deng, Y. (2021). Roles of high osmolarity glycerol and cell wall integrity pathways in cadmium toxicity in Saccharomyces cerevisiae. Int. J. Mol. Sci. 22 (12), 6169. doi:10.3390/ijms22126169
Zheng, M., Aslund, F., and Storz, G. (1998). Activation of the OxyR transcription factor by reversible disulfide bond formation. Sci. (New York, N.Y.) 279 (5357), 1718–1721. doi:10.1126/science.279.5357.1718
Ziegler-Heitbrock, H. W., Blumenstein, M., Käfferlein, E., Kieper, D., Petersmann, I., Endres, S., et al. (1992). In vitro desensitization to lipopolysaccharide suppresses tumour necrosis factor, interleukin-1 and interleukin-6 gene expression in a similar fashion lipopolysaccharide suppresses tumour necrosis factor, interleukin-1 and interleukin-6 gene expression in a similar fashion. Immunology, 75(2), 264–268.
PubMed Abstract PubMed Abstract PubMed Abstract | Google Scholar
Keywords: HOG pathway, human health, osmotic stress, MAPK, p38
Citation: Du G, Zheng K, Sun C, Sun M, Pan J, Meng D, Guan W and Zhao H (2025) The relationship mammalian p38 with human health and its homolog Hog1 in response to environmental stresses in Saccharomyces cerevisiae. Front. Cell Dev. Biol. 13:1522294. doi: 10.3389/fcell.2025.1522294
Received: 09 November 2024; Accepted: 13 February 2025;
Published: 10 March 2025.
Edited by:
Sobia Tabassum, International Islamic University, Islamabad, PakistanReviewed by:
Sahezeel Awadia, University of Michigan, United StatesCopyright © 2025 Du, Zheng, Sun, Sun, Pan, Meng, Guan and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Du, ZHVnYW5nQHRqY3UuZWR1LmNu; Wenqiang Guan, Z3VhbndlbnFpYW5nQHRqY3UuZWR1LmNu; Hui Zhao, emhhb2h1aUB0amN1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.