- Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy
Liver cancer is a leading cause of cancer-related deaths worldwide, highlighting the need for innovative approaches to understand its complex biology and develop effective treatments. While traditional in vivo animal models have played a vital role in liver cancer research, ethical concerns and the demand for more human-relevant systems have driven the development of advanced in vitro models. Spheroids and organoids have emerged as powerful tools due to their ability to replicate tumor microenvironment and facilitate preclinical drug development. Spheroids are simpler 3D culture models that partially recreate tumor structure and cell interactions. They can be used for drug penetration studies and high-throughput screening. Organoids derived from stem cells or patient tissues that accurately emulate the complexity and functionality of liver tissue. They can be generated from pluripotent and adult stem cells, as well as from liver tumor specimens, providing personalized models for studying tumor behavior and drug responses. Liver organoids retain the genetic variability of the original tumor and offer a robust platform for high-throughput drug screening and personalized treatment strategies. However, both organoids and spheroids have limitations, such as the absence of functional vasculature and immune components, which are essential for tumor growth and therapeutic responses. The field of preclinical modeling is evolving, with ongoing efforts to develop more predictive and personalized models that reflect the complexities of human liver cancer. By integrating these advanced in vitro tools, researchers can gain deeper insights into liver cancer biology and accelerate the development of novel treatments.
Introduction
Primary liver cancers, notably cholangiocarcinoma (CCA) and hepatocellular carcinoma (HCC) encompass a heterogeneous group of malignancies that present significant clinical challenges. These cancers are often characterized by a lack of specific biomarkers and frequently manifest as asymptomatic in their early stages, resulting in delays in diagnosis and adverse prognoses (Valle et al., 2021; Tsung et al., 2024; Banales et al., 2020). CCA is further categorized based on its anatomical location within the biliary tree, which includes intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA) forms (Ilyas et al., 2018). Currently, surgical resection is the only curative treatment available for these primary liver cancers, underscoring the urgent need for effective pharmacological interventions (Orcutt and Anaya, 2018).
In this landscape, advanced in vitro three-dimensional (3D) culture models have emerged as crucial tools for enhancing our understanding of liver cancer biology. These sophisticated models allow for a more accurate representation of the tumor microenvironment, facilitating the exploration of molecular mechanisms, identification of new therapeutic targets, and expedited yet reliable screening of potential novel drugs (Nuciforo and Heim, 2021; Blidisel et al., 2021). Additionally, patient-derived organoids (PDOs) are gaining prominence as innovative platforms for developing personalized treatment strategies.
This review highlights recent studies using 3D models to assess drug responses and advance treatment options for liver cancer. By focusing on their integration into preclinical research, it underscores their significance in discovering and developing effective therapies.
3D in vitro models in primary liver cancer
Spheroids in hepatocellular carcinoma: insights into cancer biology and therapeutic strategies
Since the 1970s, tumor spheroids have been employed to simulate tumor biology, forming 3D multicellular aggregates primarily derived from two-dimensional (2D) cancer cell cultures and occasionally including stromal components such as endothelial cells and fibroblasts (Inch et al., 1970; Shoval et al., 2017; Österholm et al., 2012). These spheroids self-assemble using anchoring-independent culture methods or scaffold systems like Matrigel droplets (Calvisi et al., 2023; Jubelin et al., 2022). A significant advantage of spheroids over 2D cultures is their ability to maximize cell-to-cell interactions and replicate the gradients of oxygen and drug transport found within tumors (Habanjar et al., 2021).
In HCC, the existence of a subpopulation of cancer cells with stem-like characteristics is well documented (Yamashita et al., 2009). Tumor spheroids are particularly useful for investigating potential stemness markers in HCC that may serve as targets for anti-cancer stem cell therapies (Wang YY. et al., 2024; Roy et al., 2024).
Sorafenib, a multi-tyrosine kinase inhibitor, is the first targeted therapy approved for HCC. Although it exhibits significant anticancer and anti-angiogenic effects, some patients develop resistance (Kong et al., 2021). Recent studies have created spheroids from sorafenib-resistant HuH7 cell lines to evaluate alternative treatments in a fibrotic microenvironment (Sariyar and Karagonlar, 2023; Sariyar et al., 2023). In their work, Sarıyar et al. noted a significant reduction in the CD133-positive stem cell population and an increase in CD24 and EpCAM-positive cells in sorafenib-resistant spheroids, suggesting that these markers may contribute to drug resistance. They tested new drugs, Gefitinib (an EGFR inhibitor) and PP2 (a Src-family kinase inhibitor), finding that their combination was more effective in inducing cell death in resistant spheroids compared to single treatments (Sariyar et al., 2023).
To assess the toxicity of anti-HCC therapies while preserving healthy liver tissue, Royo et al. developed 3D spheroids from both HCC cells (HEPG2 and HuH7) and healthy liver cells. Treatments with standard anti-HCC drugs (Dacarbazine, Methotrexate, Sorafenib) revealed a marked decrease in tumor cells, with Sorafenib showing the strongest impact. The study also tracked liver-derived extracellular vesicles as indicators of hepatocyte damage, revealing that spheroid treatments increased vesicle release, thereby providing a dual approach to evaluating drug efficacy and toxicity (Royo et al., 2024).
Anti-PD-1 immune checkpoint inhibitors (ICIs) are approved systemic therapies for HCC (Sankar et al., 2024). However, patients with mutated β-catenin often have poor outcomes (Akasu et al., 2021). A recent study utilizing HCC-derived spheroids explored the role of β-catenin in immune evasion, showing that silencing β-catenin enhanced the infiltration of peripheral blood mononuclear cells (PBMCs) into spheroids (Dantzer et al., 2024). Conversely, treatment with CHIR-99021, a GSK3β inhibitor, reduced immune infiltration, indicating a possible mechanism by which β-catenin aids tumor escape from immune surveillance.
In efforts to enhance NK cell-mediated tumor responses, a recent study introduced antibody-based therapies known as NK cell engagers (NKCEs), specifically targeting Glypican-3 (GPC3) in HCC (Arulanandam et al., 2023). The addition of CYT-303, an NKCE that binds both NK cells and GPC3, significantly augmented the cytotoxic effects of peripheral blood-derived NK cells on Hep3B spheroids in a dose-dependent manner, offering a promising avenue for immunotherapy in HCC.
Investigating the effects of CHIR-99021 on stromal cells, one study developed spheroids composed of HCC cells and hepatic stellate cells (HSCs) (Song et al., 2024). Given the connection between liver fibrosis and HCC, these mixed spheroids demonstrated increased expression of epithelial-mesenchymal transition (EMT) markers. Treatment with CHIR-99021 reduced these markers and highlighted the potential for antifibrotic strategies in HCC therapy. Table 1 summarizes the drugs tested and their targets identified through HCC spheroid research.
Spheroids derived from HCC cells exhibit complex architectures that enhance the reliability of 3D models, especially when incorporating stromal components. Co-culturing with immune cells like T and NK lymphocytes offers efficient platforms for evaluating novel therapies aimed at boosting anti-tumor immunity. These developments underscore the importance of 3D models in advancing our understanding and treatment of liver cancers.
3D spheroid models for cholangiocarcinoma research
Similar to HCC, spheroids have gained prominence in CCA, where they effectively mimic the low oxygen levels present in tumor environments (Vanichapol et al., 2015). Metabolomic studies have demonstrated that iCCA spheroids exhibit altered metabolic profiles, including heightened glucose consumption and lactate excretion, indicative of a glycolytic shift (Ciufolini et al., 2024). Furthermore, analyses of various iCCA cell lines confirmed that spheroids display diminished antioxidant capacity and increased oxidative stress (Phukhum et al., 2023). These metabolic alterations enhance the relevance of iCCA spheroid cultures as models for studying anaerobic metabolism and tumor stress.
The 3D structure and metabolic changes observed in iCCA spheroids contribute to an enriched stem gene expression profile, significantly enhancing their tumorigenic properties compared to 2D cultures (Raggi et al., 2017). Consequently, spheroids are frequently used to evaluate novel treatments for CCA (Marin et al., 2019).
Recent advancements include the development of novel 3D heterospheroids composed of human cancer-associated fibroblasts (CAFs) and iCCA cells (Mancarella et al., 2024). CAFs are known to facilitate CCA progression through extracellular matrix deposition and interaction with malignant cells (Carloni et al., 2022). Studies have shown that these heterospheroids enhance iCCA cell proliferation and invasion. Notably, treatment with Crenigacestat, a γ-secretase inhibitor, reduced the viability and invasion of hCAF-iCCA heterospheroids (Mancarella et al., 2024), highlighting the potential for targeting stromal interactions in therapeutic strategies.
Mesenchymal stromal cells (MSCs) are emerging as potential components in the tumor microenvironment of iCCA, as they can contribute to liver fibrosis and differentiate into CAFs (Haga et al., 2015; Gan et al., 2021; Russo et al., 2006). The concept of MSCs pertains to a subset of non-hematopoietic cells found in the stromal bone marrow that are multipotent and possess the ability to self-renew. Recently, this definition has broadened to include cells originating from any connective tissue that can produce various types of stromal cells. MSCs also circulate in the bloodstream and can migrate to sites of inflammation (Dominici et al., 2006; Bianco et al., 2013; Ridge et al., 2017). Recent research demonstrated that adding MSCs to spheroids derived from patient-derived xenograft (PDX) models could restore lost signaling pathways, indicating their dual role in either promoting or inhibiting tumor growth. This emphasizes the importance of stromal elements in CCA modeling (Sueca-Comes et al., 2024).
The past decade has seen an increased exploration of ICIs in cancer, including anti-PD-L1 therapies approved for various cancers and CCA (Fiste et al., 2021). Recent studies utilized iCCA spheroids to test RNA-based anti-PD-L1 therapies, revealing that multicellular spheroids better mimic the tumor microenvironment and can effectively assess immunomodulatory responses (Gondaliya et al., 2024). Table 1 summarizes the drugs tested and their targets identified through CCA spheroid research.
3D in vitro models, particularly tumor spheroids, offer enhanced insights into CCA biology and the tumor microenvironment, proving essential for developing new therapeutic strategies. Their ability to incorporate stromal components and accurately reflect metabolic and immune interactions makes them invaluable for preclinical cancer research.
Liver patient-derived organoids
The concept of organoids emerged in 2009, originating with the development of 3D cultures that mimic the structure and function of human organs, initially focusing on intestinal organoids. This foundational research paved the way for liver organoids, providing insights into liver tissue regeneration and early-stage diseases, and eventually extending to liver cancer models (Sato et al., 2009; Huch et al., 2013; Takebe et al., 2013). PDOs are 3D cultures derived from tumor tissues that maintain the architecture and heterogeneity of the original tumors. They are typically sourced from surgically resected tissues or needle biopsies, allowing for minimal tissue use and timely sample collection (Nuciforo and Heim, 2021; Nuciforo et al., 2018; Thorel et al., 2024).
PDOs are cultured in specialized matrices, such as Matrigel, with nutrient-rich media, preserving the histological and genetic characteristics of the parent tumor (Broutier et al., 2017). A recent biobank of liver cancer PDOs includes 44 HCC, 5 hepatoblastoma (HB), 12 iCCA, and 4 mixed HCC-CCA PDOs (Ji et al., 2023). Comprehensive genomic, epigenomic, transcriptomic, and proteomic analyses identified four molecular subtypes of liver cancer PDOs: L-LM (best prognosis), L-PL (poor prognosis, high proliferative signals), L-ICC (RAS signaling), and L-DM (altered drug metabolism) with distinct drug responses.
High-throughput screening revealed general sensitivity to TOP2 inhibitors, HDAC inhibitors, and BET PROTAC inhibitors while uncovering subtype-specific responses, L-PL showed high sensitivity to PI3K pathway inhibitors, while L-DM exhibited sensitivity to FGFR inhibitors. Studies indicated a relationship between Lenvatinib resistance and EGFR expression, and predictive models based on PDO proteogenomic data identified potentially effective drug combinations, such as Lenvatinib plus Temsirolimus (a mTOR inhibitor).
A recent report established long-term PDO cultures from 66 liver cancer patients, achieving a 40.9% success rate. This involved a two-step digestion method to minimize fibrotic tissue and utilized different media conditions for initiation and passaging. Drug screening from these PDOs yielded a successful treatment regimen for a diagnosed iCCA patient, highlighting the predictive potential of PDOs (Rao et al., 2024).
Studies employing pharmacogenomic profiling of liver cancer PDOs revealed significant intra-tumor heterogeneity, complicating treatment responses. Screening over 100 patients provided insights into drug sensitivities, revealing a cumulative sensitivity of 73% to seven targeted therapies, yet only 37.1% of patients benefited from monotherapy. Transcriptomic analysis identified 254 genes associated with Lenvatinib sensitivity, and a machine-learning approach yielded a panel of predictive biomarkers (Yang H. et al., 2024).
Additional research using PDOs and xenografts assessed a panel of 80 drugs to identify alternatives for Lenvatinib resistance. Key candidates included Romidepsin (an HDAC inhibitor), which displayed consistent effectiveness and enhanced immune responses when combined with anti-PD1 therapy (Sun L. et al., 2024). In a study focusing on the Chinese population, 64 organoid lines were evaluated for genomic and transcriptomic profiles, identifying variable genes and enrichment in pathways related to proliferation, resistance mechanisms, and immune evasion; this research emphasized the role of PDOs in predicting drug efficacy (Zhu et al., 2024).
As interest in PDOs for drug testing grows, numerous recent studies have aimed to evaluate the effectiveness of new therapeutic agents using these models. Table 1 summarizes the drugs tested and their targets identified through PDO research.
Hepatocellular carcinoma patient-derived organoids (HCC-PDOs): challenges and advances
The establishment of HCC-PDOs has been particularly challenging due to factors such as low success rates, difficulties in developing them from well-differentiated specimens, larger necrotic areas, the predominance of healthy cells over malignant ones, and the heterogeneous nature of HCC tumors (Broutier et al., 2017; Sun L. et al., 2024; Li K. et al., 2024; Airola et al., 2024; Zhang et al., 2023). Despite these challenges, successful cultivation of HCC-PDOs has demonstrated their ability to accurately recapitulate tumor biology, thus representing a substantial advancement in disease modeling and providing valuable tools for identifying therapeutic targets and biomarkers.
PDOs may help maximize the application of drugs that have shown promise in preclinical studies but failed in clinical settings. For instance, a study by Lim et al. screened 268 drugs in PDOs derived from HCC-PDX and identified three proteasome inhibitors (Bortezomib, Carfilzomib, Ixazomib) and one CDK inhibitor (Dinaciclib) as having significant antitumor effects. Their combination was found to have the highest cytotoxicity with minimal effects on non-malignant cells, confirming stronger tumor inhibition than sorafenib (Lim JJ. et al., 2022).
The potential of HCC-PDOs in studying liver regeneration was recently reported, using PDOs generated from poorly differentiated HCC specimens injected into the right superior lobe of immunodeficient mice. The findings indicated an enhanced regenerative potential compared to animals that were not subjected to resection, thereby providing a model with greater physiological relevance than traditional models (Haak et al., 2024).
Clinical applicability for personalized therapy using HCC-PDOs is an emerging goal. For example, in the case of a 74-year-old patient with a rare neuroendocrine-differentiated HCC, PDOs were established post-surgery to guide treatment. Despite initial drug screenings, the patient’s condition deteriorated rapidly (Meier et al., 2022). Conversely, another case showed successful application of PDOs for pharmacological screening in a 55-year-old patient, leading to a significant reduction in tumor markers and size, ultimately facilitating surgical resection (He et al., 2024a).
Murine HCC organoids have also been established, particularly in transgenic mice with specific gene deletions in hepatic progenitor cells, leading to the development of aggressive HCC tumors with high metastatic potential (Zhang et al., 2023; Li et al., 2018).
Collectively, these developments underscore the importance of HCC-PDOs in precision medicine and the need for further studies to validate their clinical relevance.
Cholangiocarcinoma patient-derived organoids (CCA-PDOs): advances in disease modeling and treatment
Research on CCA-PDOs is expanding due to their potential in disease modeling, drug testing, and personalized medicine. Given the complex nature of CCA and the lack of effective treatments, PDOs provide valuable insights. Significant studies have established protocols for generating CCA-PDOs from bile duct tissues, successfully reproducing the tumor’s histological and genetic features (Saito et al., 2019; Maier et al., 2021).
Recent analyses of PDOs identified two major iCCA subtypes—small-duct and large-duct—with distinct genetic and histological characteristics. Integrative genomic profiling revealed differences in key signaling pathways (KRAS, TGFβ, and ERBB2) enriched in large-duct tumors, underscoring the potential of organoids for personalized therapeutic strategies (Lee et al., 2023).
A case report demonstrated the utility of PDOs in guiding conversion therapy for a 59-year-old woman with advanced pCCA. After initial therapies failed, PDOs were created from a biopsy to assess drug sensitivity. Results indicated responsiveness to Gemcitabine and Cisplatin, leading to an adjusted treatment regimen that resulted in significant tumor shrinkage, making surgical resection possible. Following surgery, the patient remained disease-free at the 12-month follow-up, highlighting the effectiveness of PDOs in personalized treatment planning (He et al., 2024b).
Innovative technologies are enhancing drug screening in CCA organoids. Kinome profiling across different organoid models revealed distinct kinase activity patterns that correlated with tumor responses to specific inhibitors, suggesting a promising approach to personalized treatment strategies targeting pathways like EGFR, PDGFRβ, and MAPK (Lieshout et al., 2022).
Label-free brightfield microscopy, in conjunction with an organoid-specific image analysis pipeline, demonstrated the selective growth inhibition of iCCA-PDOs by Sorafenib, particularly in tumor cells, and identified potential applications for low-dose Sorafenib in patients with KRAS mutations (Koch et al., 2022).
Another study developed a protocol for inducing branching morphogenesis in cholangiocyte and cholangiocarcinoma organoids, providing a model for studying biliary function and pathology (Ober et al., 2023).
Co-culture models of patient-derived organoids in liver cancer research
Despite significant advances in liver PDOs, challenges remain in replicating the complex interactions between tumors and their stroma and accurately reflecting intratumor heterogeneity.
A recent study examined the role of CAFs in HCC tumor initiation. Mice treated with diethylnitrosamine (DEN) had LGR5+ knock-in cells to model HCC. Co-culturing organoids with primary CAFs enhanced the proliferation of LGR5+ PDOs and increased tumor growth and metastasis in vivo (Zhang et al., 2023).
Another study developed a co-culture model of human HCC spheroids or PDX-derived organoids and endothelial cells in macroporous hydrogels. Direct co-cultures showed increased angiogenesis-related proteins and induced an inflammatory phenotype, suggesting a pro-angiogenic environment in HCC (Lim JTC. et al., 2022).
Zhou et al. (2022) established a co-culture system integrating CCA-PDOs with immune cells to evaluate immune-mediated cytotoxicity. The experiments demonstrated that T cells were the primary mediators of organoid cytotoxicity, producing effector cytokines like interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) upon interaction with organoids. Their findings revealed patient-specific cytotoxic effects, emphasizing the importance of soluble factors in immune responses (Zhou et al., 2022).
To study tumor-stroma interactions and chemotherapy resistance, a co-culture system was created using eCCA organoids and tumor-associated macrophages (TAMs). The findings indicated that eCCA organoids co-cultured with TAMs were more resistant to chemotherapy agents, underscoring TAMs’ role in supporting tumor growth and drug resistance. This model could serve as a robust platform for personalized drug testing and understanding TAMs’ contributions to treatment mechanisms (Guo et al., 2024).
Future developments may incorporate additional tumor microenvironment (TME) cells and optimized culture conditions to enhance preclinical models.
Organoid-on-a-chip technology in liver cancer research
The organoid-on-a-chip technology offers a promising approach to studying liver tumors and advancing drug development. This innovative technology integrates organoids with microfluidic devices, creating an environment that closely mimics in vivo conditions. Microfluidic devices facilitate precise control of factors like nutrient gradients, oxygen levels, and shear stress, simulating the dynamic environment of human tissues, thus offering a more physiologically relevant system than static (both 2D and 3D) cultures (Telles-Silva et al., 2022).
Recent developments have led to the creation of a microfluidic platform featuring hepatic spheroids and organoids, designed to sustain liver-specific functions through efficient nutrient and oxygen exchange. This vascular-like network enables continuous flow, closely simulating liver blood vessels. Cultured within this system, hepatic spheroids and organoids demonstrated sustained viability, preserved morphology, and liver-specific protein expression, highlighting a stable microenvironment. The organoids exhibited active liver enzymes, including critical CYP450 isoforms for drug metabolism. The platform successfully mirrored in vivo toxicity profiles in response to hepatotoxic drugs like acetaminophen, indicating its potential for accurate preclinical testing (Bonanini et al., 2022).
Zou et al. (2023) introduced a micro-engineered organoid-on-a-chip platform for predicting immunotherapy responses in HCC patients. This model integrates MSCs with HCC organoids to replicate key aspects of TME. Co-culturing PDOs with MSCs significantly enhanced organoid growth and the expression of tumor markers like alpha-fetoprotein and Ki67. The study assessed the platform’s utility in predicting immunotherapy responses by treating the organoid model with anti-PD-L1 antibody. The results revealed varying responses to immunotherapy, reflecting the heterogeneity observed in clinical settings. MSCs influence the immune microenvironment, promoting macrophage differentiation toward an M2 phenotype while enhancing immune cell recruitment and exhibiting immune suppression through cytokine secretion. These findings suggest that MSCs in the TME play a significant role in mediating resistance to immunotherapy, potentially explaining the variable patient responses (Zou et al., 2023).
Discussion
Applying spheroids and organoids in studying HCC and CCA represents a significant advancement in cancer research. These 3D models provide a more accurate representation of tumor architecture, cellular interactions, and heterogeneity than traditional 2D cell cultures. They are essential for investigating cancer biology, drug response, and resistance mechanisms. Figure 1 provides a schematic representation of the design of some recent models, along with their corresponding applications.
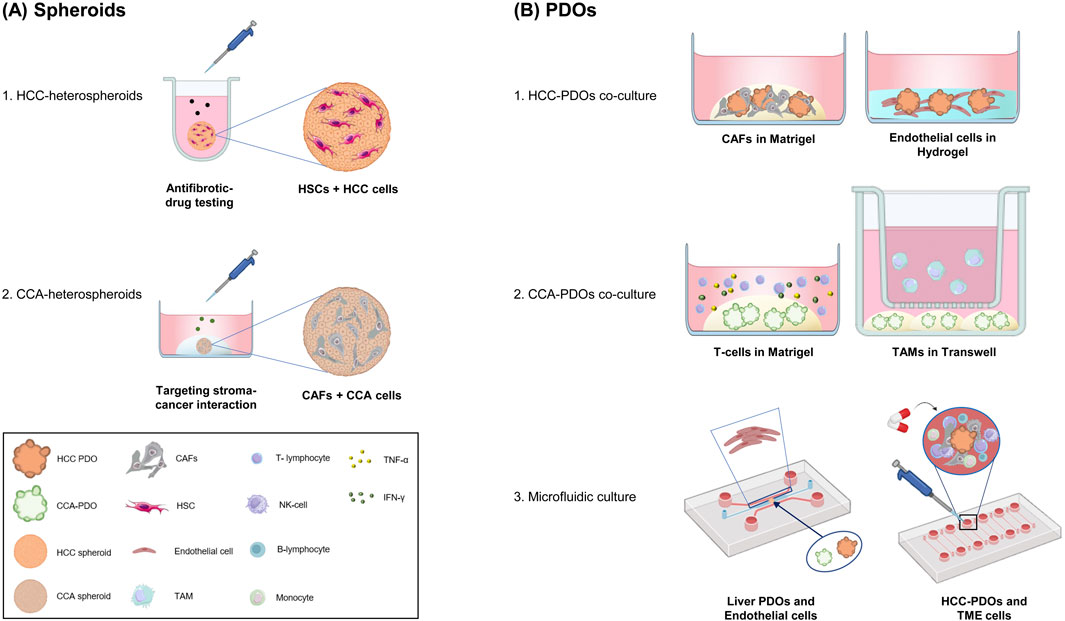
Figure 1. Advances in Spheroids and Organoids for Liver Cancer Research. A schematic overview of advances in the applications of spheroids and PDOs in liver cancer research. (A) Heterospheroids combining mesenchymal cells and cancer cells to better mimic the tumor microenvironment. The 3D cultures are used to investigate tumor-stroma interactions and test novel treatments. (B) PDOs: Co-cultures and microfluidic devices with PDOs and stroma components. 1) HCC-PDOs with CAFs or endothelial cells mimic the interaction between cancer cells and the TME. 2) CCA-PDOs with T-cells enable the study of tumor-immune cell interactions and checkpoint inhibitor responses; CCA-PDOs with TAMs highlighting the role of macrophages in tumor growth. 3) Organoids-on-Chip integrate organoids in a dynamic microfluidic culture, that incorporates endothelial cells and PBMCs. These systems mimic vascularization and immune surveillance, which are crucial for proving therapy efficacy. Cancer-Associated Fibroblasts (CAFs); cholangiocarcinoma (CCA); Hepatic Stellate Cells (HSCs); Hepatocellular Carcinoma (HCC); Patient-Derived Organoids (PDOs); Interferon-Gamma (IFN-γ); peripheral blood mononuclear cells (PBMCs), Tumor Necrosis Factor-alpha (TNF-α), Tumor-Associated Macrophages (TAMs); Tumor Microenvironment (TME). Figures were created with BioRender.com (free version), Microsoft Paint 3D and with Bioicons.com.
Despite these advancements, challenges persist, including variability in organoid cultures, the necessity for enhanced standardization, and difficulties in fully recapitulating the tumor microenvironment. Nevertheless, ongoing refinements of these models are expected to improve their clinical relevance, facilitating drug development and enhancing our understanding of cancer progression.
The development and use of spheroids and organoids in research represent a shift in biomedical sciences, especially when compared to traditional animal models. These advanced 3D cell culture systems offer significant advantages in terms of biological relevance by providing human-relevant alternatives to animal testing. They align closely with the principles of the 3Rs—Replacing, Reducing, and Refining—by substituting animal models with human-derived systems, diminishing reliance on animal studies, and refining experimental methodologies (Tosca et al., 2023). This approach allows for high-resolution insights into cellular dynamics and molecular mechanisms without the invasive techniques necessary in animal research.
These models present a promising avenue for personalizing cancer treatment, reducing reliance on animal models, and improving predictions of human-specific drug toxicity and efficacy, thus progressing liver cancer research and therapeutic innovation.
General conclusion
Research on both spheroids and organoids has revolutionized the field of liver cancer, offering in vitro models that faithfully replicate the characteristics of original tumors. These models serve as powerful tools for identifying therapeutic targets, biomarkers, and effective treatments, marking a significant advance toward the realization of personalized medicine.
Author contributions
MP: Writing–original draft, Writing–review and editing. AG: Writing–original draft, Writing–review and editing. ES-L: Writing–original draft, Writing–review and editing. FM: Writing–original draft, Writing–review and editing. CR: Conceptualization, Funding acquisition, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this work was provided by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG23117) to CR. CR is a member of the European Network for the Study of Cholangiocarcinoma (ENSCCA) and participates in the initiative COST Action EURO-CHOLANGIO-NET and Precision-BTC-Network granted by the COST Association (CA18122, CA22125)
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CCA, Cholangiocarcinoma; HCC, hepatocellular carcinoma; MSCs, Mesenchymal stromal cells; PDOs, patient-derived organoids; CAFs, cancer-associated fibroblasts; PDX, patient-derived xenograft; ICIs, immune checkpoint inhibitors; PBMCs, peripheral blood mononuclear cells; NKCEs, NK cell engagers; GPC3, glypican-3; HSCs, hepatic stellate cells; TAMs, tumor-associated macrophages; TME, tumor microenvironment.
References
Airola, C., Pallozzi, M., Cesari, E., Cerrito, L., Stella, L., Sette, C., et al. (2024). Hepatocellular-carcinoma-derived organoids: innovation in cancer research. Cells 13 (20), 1726. doi:10.3390/cells13201726
Akasu, M., Shimada, S., Kabashima, A., Akiyama, Y., Shimokawa, M., Akahoshi, K., et al. (2021). Intrinsic activation of β-catenin signaling by CRISPR/Cas9-mediated exon skipping contributes to immune evasion in hepatocellular carcinoma. Sci. Rep. 11 (1), 16732. doi:10.1038/s41598-021-96167-0
Arulanandam, A., Lin, L., Chang, H. M., Cerutti, M., Choblet, S., Gao, P., et al. (2023). Derivation and preclinical characterization of CYT-303, a novel NKp46-NK cell engager targeting GPC3. Cells 12 (7), 996. doi:10.3390/cells12070996
Banales, J. M., Marin, J. J. G., Lamarca, A., Rodrigues, P. M., Khan, S. A., Roberts, L. R., et al. (2020). Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterology and Hepatology 17 (9), 557–588. doi:10.1038/s41575-020-0310-z
Bian, S., Ni, W., Zhou, L., Tong, Y., Dai, C., Zhao, X., et al. (2024). Ubiquitin-specific protease 1 facilitates hepatocellular carcinoma progression by modulating mitochondrial fission and metabolic reprogramming via cyclin-dependent kinase 5 stabilization. Cell Death Differ. 31 (9), 1202–1218. doi:10.1038/s41418-024-01342-1
Bianco, P., Cao, X., Frenette, P. S., Mao, J. J., Robey, P. G., Simmons, P. J., et al. (2013). The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat. Med. 19 (1), 35–42. doi:10.1038/nm.3028
Blidisel, A., Marcovici, I., Coricovac, D., Hut, F., Dehelean, C. A., and Cretu, O. M. (2021). Experimental models of hepatocellular carcinoma-A preclinical perspective. Cancers 13 (15), 3651. doi:10.3390/cancers13153651
Blukacz, L., Nuciforo, S., Fucile, G., Trulsson, F., Duthaler, U., Wieland, S., et al. (2024). Inhibition of the transmembrane transporter ABCB1 overcomes resistance to doxorubicin in patient-derived organoid models of HCC. Hepatol. Commun. 8 (5), e0437. doi:10.1097/HC9.0000000000000437
Bonanini, F., Kurek, D., Previdi, S., Nicolas, A., Hendriks, D., de, R. S., et al. (2022). In vitro grafting of hepatic spheroids and organoids on a microfluidic vascular bed. Angiogenesis 25 (4), 455–470. doi:10.1007/s10456-022-09842-9
Broutier, L., Mastrogiovanni, G., Verstegen, M. M. A., Francies, H. E., Gavarró, L. M., Bradshaw, C. R., et al. (2017). Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23 (12), 1424–1435. doi:10.1038/nm.4438
Calvisi, D. F., Boulter, L., Vaquero, J., Saborowski, A., Fabris, L., Rodrigues, P. M., et al. (2023). Criteria for preclinical models of cholangiocarcinoma: scientific and medical relevance. Nat. Rev. Gastroenterology and Hepatology 20 (7), 462–480. doi:10.1038/s41575-022-00739-y
Carloni, R., Rizzo, A., Ricci, A. D., Di Federico, A., De Luca, R., Guven, D. C., et al. (2022). Targeting tumor microenvironment for cholangiocarcinoma: opportunities for precision medicine. Transl. Oncol. 25, 101514. doi:10.1016/j.tranon.2022.101514
Cigliano, A., Gigante, I., Serra, M., Vidili, G., Simile, M. M., Steinmann, S., et al. (2024). HSF1 is a prognostic determinant and therapeutic target in intrahepatic cholangiocarcinoma. J. Exp. Clin. Cancer Res. 43 (1), 253. doi:10.1186/s13046-024-03177-7
Ciufolini, G., Zampieri, S., Cesaroni, S., Pasquale, V., Bonanomi, M., Gaglio, D., et al. (2024). 3D modeling: insights into the metabolic reprogramming of cholangiocarcinoma cells. Cells 13 (18), 1536. doi:10.3390/cells13181536
Conboy, C. B., Yonkus, J. A., Buckarma, E. H., Mun, D. G., Werneburg, N. W., Watkins, R. D., et al. (2023). LCK inhibition downregulates YAP activity and is therapeutic in patient-derived models of cholangiocarcinoma. J. Hepatol. 78 (1), 142–152. doi:10.1016/j.jhep.2022.09.014
Conti, N. S., De Siervi, S., Luchinat, E., Magri, A., Messina, A., Brocca, L., et al. (2024). VDAC1-interacting molecules promote cell death in cancer organoids through mitochondrial-dependent metabolic interference. iScience 27 (6), 109853. doi:10.1016/j.isci.2024.109853
Dantzer, C., Vache, J., Brunel, A., Mahouche, I., Raymond, A. A., Dupuy, J. W., et al. (2024). Emerging role of oncogenic ss-catenin in exosome biogenesis as a driver of immune escape in hepatocellular carcinoma. Elife 13. doi:10.7554/eLife.95191
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8 (4), 315–317. doi:10.1080/14653240600855905
Dong, R., Wang, T., Dong, W., Zhu, H., Liu, Q., Liang, H., et al. (2024). Inhibition of PTPRE suppresses tumor progression and improves sorafenib response in hepatocellular carcinoma. Biomed. Pharmacother. 173, 116366. doi:10.1016/j.biopha.2024.116366
Elurbide, J., Colyn, L., Latasa, M. U., Uriarte, I., Mariani, S., Lopez-Pascual, A., et al. (2024). Identification of PRMT5 as a therapeutic target in cholangiocarcinoma. Gut 74, 116–127. doi:10.1136/gutjnl-2024-332998
Fan, L., Tian, C., Yang, W., Liu, X., Dhungana, Y., Yang, W., et al. (2024). HKDC1 promotes liver cancer stemness under hypoxia through stabilizing beta-catenin. Hepatology. doi:10.1097/HEP.0000000000001085
Fiste, O., Ntanasis-Stathopoulos, I., Gavriatopoulou, M., Liontos, M., Koutsoukos, K., Dimopoulos, M. A., et al. (2021). The emerging role of immunotherapy in intrahepatic cholangiocarcinoma. Vaccines. 9 (5), 422. doi:10.3390/vaccines9050422
Fu, W., Lin, Y., Bai, M., Yao, J., Huang, C., Gao, L., et al. (2024). Beyond ribosomal function: RPS6 deficiency suppresses cholangiocarcinoma cell growth by disrupting alternative splicing. Acta Pharm. Sin. B 14 (9), 3931–3948. doi:10.1016/j.apsb.2024.06.028
Gan, L. H., Shen, H., Li, X. D., Han, Z. P., Jing, Y. Y., Yang, X., et al. (2021). Mesenchymal stem cells promote chemoresistance by activating autophagy in intrahepatic cholangiocarcinoma. Oncol. Rep. 45 (1), 107–118. doi:10.3892/or.2020.7838
Gondaliya, P., Sayyed, A. A., Yan, I. K., Driscoll, J., Ziemer, A., and Patel, T. (2024). Targeting PD-L1 in cholangiocarcinoma using nanovesicle-based immunotherapy. Mol. Ther. 32 (8), 2762–2777. doi:10.1016/j.ymthe.2024.06.006
Guo, Y., Li, Q., Ye, Q., Jin, Y., Yu, Y., Zhang, X., et al. (2024). Construction and drug screening of Co-culture system using extrahepatic cholangiocarcinoma organoids and tumor-associated macrophages. Heliyon 10 (17), e36377. doi:10.1016/j.heliyon.2024.e36377
Haak, F., Hess, G. F., Sedlaczek, P., Soysal, S. D., Vosbeck, J., Piscuoglio, S., et al. (2024). A hepatocellular cancer patient-derived organoid xenograft model to investigate impact of liver regeneration on tumor growth. J. Vis. Exp. 204. doi:10.3791/66245
Habanjar, O., Diab-Assaf, M., Caldefie-Chezet, F., and Delort, L. (2021). 3D cell culture systems: tumor application, advantages, and disadvantages. Int. J. Mol. Sci. 22 (22), 12200. doi:10.3390/ijms222212200
Haga, H., Yan, I. K., Takahashi, K., Wood, J., Zubair, A., and Patel, T. (2015). Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J. Extracell. Vesicles 4, 24900. doi:10.3402/jev.v4.24900
He, Y. G., Wang, Z., Li, J., Xi, W., Zhao, C. Y., Huang, X. B., et al. (2024a). Pathologic complete response to conversion therapy in hepatocellular carcinoma using patient-derived organoids: a case report. World J. Gastrointest. Oncol. 16 (11), 4506–4513. doi:10.4251/wjgo.v16.i11.4506
He, Y. G., Zhang, L. Y., Li, J., Wang, Z., Zhao, C. Y., Zheng, L., et al. (2024b). Conversion therapy in advanced perihilar cholangiocarcinoma based on patient-derived organoids: a case report. World J. Gastrointest. Oncol. 16 (10), 4274–4280. doi:10.4251/wjgo.v16.i10.4274
Huang, T., Cao, H., Liu, C., Sun, X., Dai, S., Liu, L., et al. (2024b). MAL2 reprograms lipid metabolism in intrahepatic cholangiocarcinoma via EGFR/SREBP-1 pathway based on single-cell RNA sequencing. Cell Death Dis. 15 (6), 411. doi:10.1038/s41419-024-06775-7
Huang, Y. P., Wang, Y. X., Zhou, H., Liu, Z. T., Zhang, Z. J., Xiong, L., et al. (2024a). Surufatinib combined with photodynamic therapy induces ferroptosis to inhibit cholangiocarcinoma in vitro and in tumor models. Front. Pharmacol. 15, 1288255. doi:10.3389/fphar.2024.1288255
Huch, M., Dorrell, C., Boj, S. F., van Es, J. H., Li, V. S. W., van de Wetering, M., et al. (2013). In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494 (7436), 247–250. doi:10.1038/nature11826
Ilyas, S. I., Khan, S. A., Hallemeier, C. L., Kelley, R. K., and Gores, G. J. (2018). Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 15 (2), 95–111. doi:10.1038/nrclinonc.2017.157
Inch, W. R., McCredie, J. A., and Sutherland, R. M. (1970). Growth of nodular carcinomas in rodents compared with multi-cell spheroids in tissue culture. Growth 34 (3), 271–282.
Ji, S. Y., Feng, L., Fu, Z. L., Wu, G. H., Wu, Y. C., Lin, Y. P., et al. (2023). Pharmaco-proteogenomic characterization of liver cancer organoids for precision oncology. Sci. Transl. Med. 15 (706), eadg3358. doi:10.1126/scitranslmed.adg3358
Ji, X., Yang, Z., Li, C., Zhu, S., Zhang, Y., Xue, F., et al. (2024). Mitochondrial ribosomal protein L12 potentiates hepatocellular carcinoma by regulating mitochondrial biogenesis and metabolic reprogramming. Metabolism 152, 155761. doi:10.1016/j.metabol.2023.155761
Jiang, L., Qi, X., Lai, M., Zhou, J., Yuan, M., You, J., et al. (2024). WDR20 prevents hepatocellular carcinoma senescence by orchestrating the simultaneous USP12/46-mediated deubiquitination of c-Myc. Proc. Natl. Acad. Sci. U. S. A. 121 (44), e2407904121. doi:10.1073/pnas.2407904121
Jubelin, C., Muñoz-Garcia, J., Griscom, L., Cochonneau, D., Ollivier, E., Heymann, M. F., et al. (2022). Three-dimensional in vitro culture models in oncology research. Cell Biosci. 12 (1), 155. doi:10.1186/s13578-022-00887-3
Kang, F. P., Chen, Z. W., Liao, C. Y., Wu, Y. D., Li, G., Xie, C. K., et al. (2024). Escherichia coli-induced cGLIS3-mediated stress granules activate the NF-κB pathway to promote intrahepatic cholangiocarcinoma progression. Adv. Sci. (Weinh) 11 (16), e2306174. doi:10.1002/advs.202306174
Kipcak, A., Sezan, S., Karpat, O., Kaya, E., Baylan, S., Sariyar, E., et al. (2024). Suppression of CTC1 inhibits hepatocellular carcinoma cell growth and enhances RHPS4 cytotoxicity. Mol. Biol. Rep. 51 (1), 799. doi:10.1007/s11033-024-09756-3
Koch, M., Nickel, S., Lieshout, R., Lissek, S. M., Leskova, M., van der Laan, L. J. W., et al. (2022). Label-free imaging analysis of patient-derived cholangiocarcinoma organoids after sorafenib treatment. Cells 11 (22), 3613. doi:10.3390/cells11223613
Kong, F. H., Ye, Q. F., Miao, X. Y., Liu, X., Huang, S. Q., Xiong, L., et al. (2021). Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics 11 (11), 5464–5490. doi:10.7150/thno.54822
Kopsida, M., Clavero, A. L., Khaled, J., Balgoma, D., Luna-Marco, C., Chowdhury, A., et al. (2024). Inhibiting the endoplasmic reticulum stress response enhances the effect of doxorubicin by altering the lipid metabolism of liver cancer cells. Mol. Metab. 79, 101846. doi:10.1016/j.molmet.2023.101846
Lau, H. C., Zhang, X., Ji, F., Lin, Y., Liang, W., Li, Q., et al. (2024). Lactobacillus acidophilus suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma through producing valeric acid. EBioMedicine 100, 104952. doi:10.1016/j.ebiom.2023.104952
Lee, H. S., Han, D. H., Cho, K., Park, S. B., Kim, C., Leem, G., et al. (2023). Integrative analysis of multiple genomic data from intrahepatic cholangiocarcinoma organoids enables tumor subtyping. Nat. Commun. 14 (1), 237. doi:10.1038/s41467-023-35896-4
Li, K., Ren, K., Du, S., Gao, X., and Yu, J. (2024a). Development of liver cancer organoids: reproducing tumor microenvironment and advancing research for liver cancer treatment. Technol. Cancer Res. Treat. 23, 15330338241285097. doi:10.1177/15330338241285097
Li, L. Y., Qian, M. X., Chen, I. H., Finkelstein, D., Onar-Thomas, A., Johnson, M., et al. (2018). Acquisition of cholangiocarcinoma traits during advanced hepatocellular carcinoma development in mice. Am. J. Pathology 188 (3), 656–671. doi:10.1016/j.ajpath.2017.11.013
Li, Y., Guo, M., Qiu, Y., Li, M., Wu, Y., Shen, M., et al. (2024b). Autophagy activation is required for N6-methyladenosine modification to regulate ferroptosis in hepatocellular carcinoma. Redox Biol. 69, 102971. doi:10.1016/j.redox.2023.102971
Lieshout, R., Faria, A. V. S., Peppelenbosch, M. P., van der Laan, L. J. W., Verstegen, M. M. A., and Fuhler, G. M. (2022). Kinome profiling of cholangiocarcinoma organoids reveals potential druggable targets that hold promise for treatment stratification. Mol. Med. 28 (1), 74. doi:10.1186/s10020-022-00498-1
Lim, J. J., Hooi, L., Dan, Y. Y., Bonney, G. K., Zhou, L., Chow, P. K. H., et al. (2022a). Rational drug combination design in patient-derived avatars reveals effective inhibition of hepatocellular carcinoma with proteasome and CDK inhibitors. J. Exp. and Clin. Cancer Res. 41 (1), 249. doi:10.1186/s13046-022-02436-9
Lim, J. T. C., Kwang, L. G., Ho, N. C. W., Toh, C. C. M., Too, N. S. H., Hooi, L., et al. (2022b). Hepatocellular carcinoma organoid co-cultures mimic angiocrine crosstalk to generate inflammatory tumor microenvironment. Biomaterials 284, 121527. doi:10.1016/j.biomaterials.2022.121527
Luk, I. S., Bridgwater, C. M., Yu, A., Boila, L. D., Yanez-Bartolome, M., Lampano, A. E., et al. (2024). SRC inhibition enables formation of a growth suppressive MAGI1-PP2A complex in isocitrate dehydrogenase-mutant cholangiocarcinoma. Sci. Transl. Med. 16 (747), eadj7685. doi:10.1126/scitranslmed.adj7685
Maier, C. F., Zhu, L., Nanduri, L. K., Kühn, D., Kochall, S., Thepkaysone, M. L., et al. (2021). Patient-derived organoids of cholangiocarcinoma. Int. J. Mol. Sci. 22 (16), 8675. doi:10.3390/ijms22168675
Majumdar, S., Chakraborty, A., Das, S., Gorain, M., Chatterjee, S., Dey, I., et al. (2024). Sponging of five tumour suppressor miRNAs by lncRNA-KCNQ1OT1 activates BMPR1A/BMPR1B-ACVR2A/ACVR2B signalling and promotes chemoresistance in hepatocellular carcinoma. Cell Death Discov. 10 (1), 274. doi:10.1038/s41420-024-02016-0
Mancarella, S., Gigante, I., Pizzuto, E., Serino, G., Terzi, A., Dituri, F., et al. (2024). Targeting cancer-associated fibroblasts/tumor cells cross-talk inhibits intrahepatic cholangiocarcinoma progression via cell-cycle arrest. J. Exp. Clin. Cancer Res. 43 (1), 286. doi:10.1186/s13046-024-03210-9
Marin, J. J. G., Herraez, E., Lozano, E., Macias, R. I. R., and Briz, O. (2019). Models for understanding resistance to chemotherapy in liver cancer. Cancers 11 (11), 1677. doi:10.3390/cancers11111677
Meier, M. A., Nuciforo, S., Coto-Llerena, M., Gallon, J., Matter, M. S., Ercan, C., et al. (2022). Patient-derived tumor organoids for personalized medicine in a patient with rare hepatocellular carcinoma with neuroendocrine differentiation: a case report. Commun. Med. (Lond). 2, 80. doi:10.1038/s43856-022-00150-3
Myint, K. Z., Balasubramanian, B., Venkatraman, S., Phimsen, S., Sripramote, S., Jantra, J., et al. (2024). Therapeutic implications of ceritinib in cholangiocarcinoma beyond ALK expression and mutation. Pharm. (Basel) 17 (2), 197. doi:10.3390/ph17020197
Nuciforo, S., Fofana, I., Matter, M. S., Blumer, T., Calabrese, D., Boldanova, T., et al. (2018). Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 24 (5), 1363–1376. doi:10.1016/j.celrep.2018.07.001
Nuciforo, S., and Heim, M. H. (2021). Organoids to model liver disease. Jhep Rep. 3 (1), 100198. doi:10.1016/j.jhepr.2020.100198
Ober, K., Roos, F. J. M., van Tienderen, G. S., Köten, K., Klaassen, A., Mi, W., et al. (2023). Protocol for inducing branching morphogenesis in human cholangiocyte and cholangiocarcinoma organoids. Star. Protoc. 4 (3), 102431. doi:10.1016/j.xpro.2023.102431
Orcutt, S. T., and Anaya, D. A. (2018). Liver resection and surgical strategies for management of primary liver cancer. Cancer control. 25 (1), 1073274817744621. doi:10.1177/1073274817744621
Österholm, C., Lu, N., Lidén, Å., Karlsen, T. V., Gullberg, D., Reed, R. K., et al. (2012). Fibroblast EXT1-levels influence tumor cell proliferation and migration in composite spheroids. Plos One 7 (7), e41334. doi:10.1371/journal.pone.0041334
Pan, M., Luo, M., Liu, L., Chen, Y., Cheng, Z., Wang, K., et al. (2024a). EGR1 suppresses HCC growth and aerobic glycolysis by transcriptionally downregulating PFKL. J. Exp. Clin. Cancer Res. 43 (1), 35. doi:10.1186/s13046-024-02957-5
Pan, Y., Zhou, Y., Shen, Y., Xu, L., Liu, H., Zhang, N., et al. (2024b). Hypoxia stimulates PYGB enzymatic activity to promote glycogen metabolism and cholangiocarcinoma progression. Cancer Res. 84 (22), 3803–3817. doi:10.1158/0008-5472.CAN-24-0088
Phukhum, P., Phetcharaburanin, J., Chaleekarn, K., Kittirat, Y., Kulthawatsiri, T., Namwat, N., et al. (2023). The impact of hypoxia and oxidative stress on proteo-metabolomic alterations of 3D cholangiocarcinoma models. Sci. Rep. 13 (1), 3072. doi:10.1038/s41598-023-30204-y
Raggi, C., Correnti, M., Sica, A., Andersen, J. B., Cardinale, V., Alvaro, D., et al. (2017). Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J. Hepatology 66 (1), 102–115. doi:10.1016/j.jhep.2016.08.012
Rao, J., Song, C., Hao, Y., Chen, Z., Feng, S., Xu, S., et al. (2024). Leveraging patient-derived organoids for personalized liver cancer treatment. Int. J. Biol. Sci. 20 (13), 5363–5374. doi:10.7150/ijbs.96317
Ridge, S. M., Sullivan, F. J., and Glynn, S. A. (2017). Mesenchymal stem cells: key players in cancer progression. Mol. Cancer 16 (1), 31. doi:10.1186/s12943-017-0597-8
Roy, S. K., Srivastava, S., McCance, C., Shrivastava, A., Morvant, J., Shankar, S., et al. (2024). Clinical significance of PNO1 as a novel biomarker and therapeutic target of hepatocellular carcinoma. J. Cell Mol. Med. 28 (9), e18295. doi:10.1111/jcmm.18295
Royo, F., Garcia-Vallicrosa, C., Azparren-Angulo, M., Bordanaba-Florit, G., Lopez-Sarrio, S., and Falcon-Perez, J. M. (2024). Three-dimensional hepatocyte spheroids: model for assessing chemotherapy in hepatocellular carcinoma. Biomedicines 12 (6), 1200. doi:10.3390/biomedicines12061200
Russo, F. P., Alison, M. R., Bigger, B. W., Amofah, E., Florou, A., Amin, F., et al. (2006). The bone marrow functionally contributes to liver fibrosis. Gastroenterology 130 (6), 1807–1821. doi:10.1053/j.gastro.2006.01.036
Saito, Y., Muramatsu, T., Kanai, Y., Ojima, H., Sukeda, A., Hiraoka, N., et al. (2019). Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 27 (4), 1265–1276. doi:10.1016/j.celrep.2019.03.088
Sandech, N., Yang, M. C., Juntranggoor, P., Rukthong, P., Gorelkin, P., Savin, N., et al. (2024). Benja-ummarit induces ferroptosis with cell ballooning feature through ROS and iron-dependent pathway in hepatocellular carcinoma. J. Ethnopharmacol. 335, 118672. doi:10.1016/j.jep.2024.118672
Sankar, K., Gong, J., Osipov, A., Miles, S. A., Kosari, K., Nissen, N. N., et al. (2024). Recent advances in the management of hepatocellular carcinoma. Clin. Mol. Hepatology 30 (1), 1–15. doi:10.3350/cmh.2023.0125
Sariyar, E., and Karagonlar, Z. F. (2023). Modelling the sorafenib-resistant liver cancer microenvironment by using 3-D spheroids. Atla-Alternatives Laboratory Animals 51 (5), 301–312. doi:10.1177/02611929231193421
Sariyar, E., Karpat, O., Sezan, S., Baylan, S. M., Kipcak, A., Guven, K., et al. (2023). EGFR and Lyn inhibition augments regorafenib induced cell death in sorafenib resistant 3D tumor spheroid model. Cell Signal 105, 110608. doi:10.1016/j.cellsig.2023.110608
Sato, T., Vries, R. G., Snippert, H. J., van de Wetering, M., Barker, N., Stange, D. E., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459 (7244), 262–265. doi:10.1038/nature07935
Shoval, H., Karsch-Bluman, A., Brill-Karniely, Y., Stern, T., Zamir, G., Hubert, A., et al. (2017). Tumor cells and their crosstalk with endothelial cells in 3D spheroids. Sci. Rep. 7, 10428. doi:10.1038/s41598-017-10699-y
Song, Y., Kim, N., Heo, J., Shum, D., Heo, T., and Seo, H. R. (2024). Inhibition of DNMT3B expression in activated hepatic stellate cells overcomes chemoresistance in the tumor microenvironment of hepatocellular carcinoma. Sci. Rep. 14 (1), 115. doi:10.1038/s41598-023-50680-6
Sueca-Comes, M., Rusu, E. C., Ashworth, J. C., Collier, P., Probert, C., Ritchie, A., et al. (2024). The role of mesenchymal cells in cholangiocarcinoma. Dis. Model Mech. 4, 050716. doi:10.1242/dmm.050716
Sun, B., Ding, P., Song, Y., Zhou, J., Chen, X., Peng, C., et al. (2024b). FDX1 downregulation activates mitophagy and the PI3K/AKT signaling pathway to promote hepatocellular carcinoma progression by inducing ROS production. Redox Biol. 75, 103302. doi:10.1016/j.redox.2024.103302
Sun, L., Wan, A. H., Yan, S. J., Liu, R. N., Li, J. R., Zhou, Z. L., et al. (2024a). A multidimensional platform of patient-derived tumors identifies drug susceptibilities for clinical lenvatinib resistance. Acta Pharm. Sin. B 14 (1), 223–240. doi:10.1016/j.apsb.2023.09.015
Takebe, T., Sekine, K., Enomura, M., Koike, H., Kimura, M., Ogaeri, T., et al. (2013). Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499 (7459), 481–484. doi:10.1038/nature12271
Telles-Silva, K. A., Pacheco, L., Komatsu, S., Chianca, F., Caires-Junior, L. C., Araujo, B. H. S., et al. (2022). Applied hepatic bioengineering: modeling the human liver using organoid and liver-on-a-chip technologies. Front. Bioeng. Biotechnol. 10, 845360. doi:10.3389/fbioe.2022.845360
Thorel, L., Perréard, M., Florent, R., Divoux, J., Coffy, S., Vincent, A., et al. (2024). Patient-derived tumor organoids: a new avenue for preclinical research and precision medicine in oncology. Exp. Mol. Med. 56 (7), 1531–1551. doi:10.1038/s12276-024-01272-5
Tosca, E. M., Ronchi, D., Facciolo, D., and Magni, P. (2023). Replacement, reduction, and refinement of animal experiments in anticancer drug development: the contribution of 3D in vitro cancer models in the drug efficacy assessment. Biomedicines 11 (4), 1058. doi:10.3390/biomedicines11041058
Tsung, C., Quinn, P. L., and Ejaz, A. (2024). Management of intrahepatic cholangiocarcinoma: a narrative review. Cancers 16 (4), 739. doi:10.3390/cancers16040739
Valle, J. W., Kelley, R. K., Nervi, B., Oh, D. Y., and Zhu, A. X. (2021). Biliary tract cancer. Lancet. 397 (10272), 428–444. doi:10.1016/S0140-6736(21)00153-7
Vanichapol, T., Leelawat, K., and Hongeng, S. (2015). Hypoxia enhances cholangiocarcinoma invasion through activation of hepatocyte growth factor receptor and the extracellular signal-regulated kinase signaling pathway. Mol. Med. Rep. 12 (3), 3265–3272. doi:10.3892/mmr.2015.3865
Wang, J., Xiu, M., Wang, J., Gao, Y., and Li, Y. (2024b). METTL16-SENP3-LTF axis confers ferroptosis resistance and facilitates tumorigenesis in hepatocellular carcinoma. J. Hematol. Oncol. 17 (1), 78. doi:10.1186/s13045-024-01599-6
Wang, Y. Y., Shen, M. M., and Gao, J. (2024a). Metadherin promotes stem cell phenotypes and correlated with immune infiltration in hepatocellular carcinoma. World J. Gastroenterology 30 (8), 901–918. doi:10.3748/wjg.v30.i8.901
Xiang, D., Liu, J., Wang, Y., Hu, D., Zhang, C., Zeng, T., et al. (2024). Oncofetal MCB1 is a functional biomarker for HCC personalized therapy. Adv. Sci. (Weinh) 11, e2401228. doi:10.1002/advs.202401228
Yamashita, T., Ji, J. F., Budhu, A., Forgues, M., Yang, W., Wang, H. Y., et al. (2009). EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 136 (3), 1012–1024. doi:10.1053/j.gastro.2008.12.004
Yan, S., Chen, L., Zhuang, H., Yang, H., Yang, Y., Zhang, N., et al. (2024). HDAC inhibition sensitize hepatocellular carcinoma to lenvatinib via suppressing AKT activation. Int. J. Biol. Sci. 20 (8), 3046–3060. doi:10.7150/ijbs.93375
Yang, H., Cheng, J. H., Zhuang, H., Xu, H. C., Wang, Y. N., Zhang, T. T., et al. (2024a). Pharmacogenomic profiling of intra-tumor heterogeneity using a large organoid biobank of liver cancer. Cancer Cell 42 (4), 535–551.e8. doi:10.1016/j.ccell.2024.03.004
Yang, S., Zheng, L., Li, L., Zhang, J., Wang, J., Zhao, H., et al. (2024b). Integrative multiomics analysis identifies molecular subtypes and potential targets of hepatocellular carcinoma. Clin. Transl. Med. 14 (6), e1727. doi:10.1002/ctm2.1727
Ye, P., Luo, S., Huang, J., Fu, X., Chi, X., Cha, J. H., et al. (2024). TESC associated with poor prognosis enhances cancer stemness and migratory properties in liver cancer. Clin. Exp. Med. 24 (1), 206. doi:10.1007/s10238-024-01469-y
Zhang, M. N., Fang, Y. Q., Fu, X., Liu, J. Y., Liu, Y., Zhu, Z. A., et al. (2023). Cancer-associated fibroblasts nurture LGR5 marked liver tumor-initiating cells and promote their tumor formation, growth, and metastasis. Cancer Med. 12 (17), 18032–18049. doi:10.1002/cam4.6408
Zhang, Q., Wei, T., Jin, W., Yan, L., Shi, L., Zhu, S., et al. (2024d). Deficiency in SLC25A15, a hypoxia-responsive gene, promotes hepatocellular carcinoma by reprogramming glutamine metabolism. J. Hepatol. 80 (2), 293–308. doi:10.1016/j.jhep.2023.10.024
Zhang, S., Jia, X., Dai, H., Zhu, X., Song, W., Bian, S., et al. (2024b). SERPINE2 promotes liver cancer metastasis by inhibiting c-Cbl-mediated EGFR ubiquitination and degradation. Cancer Commun. (Lond). 44 (3), 384–407. doi:10.1002/cac2.12527
Zhang, X., Rameika, N., Zhong, L., Rendo, V., Veanes, M., Kundu, S., et al. (2024a). Loss of heterozygosity of CYP2D6 enhances the sensitivity of hepatocellular carcinomas to talazoparib. EBioMedicine 109, 105368. doi:10.1016/j.ebiom.2024.105368
Zhang, X., Su, T., Wu, Y., Cai, Y., Wang, L., Liang, C., et al. (2024c). N6-Methyladenosine reader YTHDF1 promotes stemness and therapeutic resistance in hepatocellular carcinoma by enhancing NOTCH1 expression. Cancer Res. 84 (6), 827–840. doi:10.1158/0008-5472.CAN-23-1916
Zhou, G. Y., Lieshout, R., van Tienderen, G. S., de Ruiter, V., van Royen, M. E., Boor, P. P. C., et al. (2022). Modelling immune cytotoxicity for cholangiocarcinoma with tumour-derived organoids and effector T cells. Br. J. Cancer 127 (4), 649–660. doi:10.1038/s41416-022-01839-x
Zhu, Y. J., Tang, S. J., Yuan, Q. Y., Fu, J., He, J., Liu, Z., et al. (2024). Integrated characterization of hepatobiliary tumor organoids provides a potential landscape of pharmacogenomic interactions. Cell Rep. Med. 5 (2), 101375. doi:10.1016/j.xcrm.2023.101375
Keywords: 3D culture, organoids, spheroids, liver cancer, drug-screening
Citation: Pastore M, Giachi A, Spínola-Lasso E, Marra F and Raggi C (2025) Organoids and spheroids: advanced in vitro models for liver cancer research. Front. Cell Dev. Biol. 12:1536854. doi: 10.3389/fcell.2024.1536854
Received: 29 November 2024; Accepted: 20 December 2024;
Published: 09 January 2025.
Edited by:
Daniela Gabbia, University of Padova, ItalyReviewed by:
Alessio Romaldini, University of Modena and Reggio Emilia, ItalyCopyright © 2025 Pastore, Giachi, Spínola-Lasso, Marra and Raggi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Raggi, Y2hpYXJhLnJhZ2dpQHVuaWZpLml0
 Mirella Pastore
Mirella Pastore Alessia Giachi
Alessia Giachi Elena Spínola-Lasso
Elena Spínola-Lasso Fabio Marra
Fabio Marra Chiara Raggi
Chiara Raggi