- 1Institute of Experimental Genetics, Helmholtz Munich GmbH, German Research Center for Environmental Health, Neuherberg, Germany
- 2DZD – German Center for Diabetes Research, Neuherberg, Germany
Sexual dimorphism involves distinct anatomical, physiological, behavioral, and developmental differences between males and females of the same species, influenced by factors prior to conception and during early development. These sex-specific traits contribute to varied phenotypes and individual disease risks within and across generations and understanding them is essential in mammalian studies. Hormones, sex chromosomes, and imprinted genes drive this dimorphism, with over half of quantitative traits in wildtype mice showing sex-based variation. This review focuses on the impact of paternal non-genetic factors on sexual dimorphism. We synthesize current research on how paternal health before conception affects offspring phenotypes in a sex-specific manner, examining mechanisms such as DNA methylation, paternally imprinted genes, sperm RNA, and seminal plasma. Additionally, we explore how paternal influences indirectly shape offspring through maternal behavior, uterine environment, and placental changes, affecting males and females differently. We propose mechanisms modulating sexual dimorphism during development, underscoring the need for sex-specific documentation in animal studies.
Introduction
Sexual dimorphism (SDM) refers to the phenotypic variations between males and females within the same species. The global prevalence of SDM across sexually reproducing species has been documented in a wide range of studies. Sex differences have significantly been evident in phenotypic traits, gene expression, and diseases. A broad spectrum of qualitative and quantitative phenotypic traits, ranging from behavioral to health-related, have been observed to display significant sex differences in many mammalian species, most notably in humans (Choleris et al., 2018; Karp et al., 2017; Weiss et al., 2006; Rawlik et al., 2016). In terms of gene expression, advanced technologies such as qRT-PCR, microarray as well as bulk and single-cell RNA-seq analyses, have identified a few hundred to several thousands of sexually dimorphic genes in a variety of animal and human tissues such as liver, muscles, adipose tissue, brain, etc. These genes are involved in a wide range of biological processes, mainly related to metabolism, endocrine and immune response among many other cell signaling pathways (Yang et al., 2006; Seo et al., 2016; Oliva et al., 2020; Khodursky et al., 2022). In the context of diseases, the influence of SDM can be apparent in various ways, including disease course, onset, expressivity, associated symptoms, prognosis, and prevalence. Since identifying and understanding sex disparities in diseases is therapeutically relevant, many diseases have been investigated in a sex-specific manner. Specifically, cardiovascular diseases (Miller, 2012), renal diseases (Neugarten and Golestaneh, 2019), neurological diseases (Zalewska et al., 2022), autoimmune diseases (McCombe et al., 2009), respiratory diseases (Reddy and Oliver, 2023), infectious diseases (Gay et al., 2021), and several types of cancers (Ma et al., 2016) in both human and animal models exhibit clear sexual dimorphism.
Given the prevalence of SDM across diverse biological and physiological aspects, a mechanistic understanding of its ontogeny is crucial to dissecting and comprehending the sex differences in complex traits and diseases. While still not entirely understood, the main underlying mechanisms include sex chromosomes and sex hormones, but also involve epigenetic modifications and sex specificities during developmental programming. Extensive studies have unequivocally shown that gonadal sex hormones (estrogens, progestins, and androgens) are primary mediators in a wide array of phenotypes displaying SDM. The sexually dimorphic effects of sex hormones can be either organizational (permanent) or activational (reversible). However, the emergence of sex differences before the development of gonads (Lowe et al., 2015; Werner et al., 2017) suggests that genetic and epigenetic factors are involved in SDM. Sex chromosome effects refer to the differential action of genes present on the sex chromosomes in female (XX) and male (YX) cells. Sex chromosome effects can be mainly attributed to the presence of the Y chromosome and the role of X chromosome dosage (Snell and Turner, 2018). Using sex-chromosome-modified mouse models such as XY* and Four Core Genotypes (FCG) has effectively dissected whether a phenotypic sex difference is influenced by sex chromosomes, gonadal sex hormones, or interactions of the two (Burgoyne and Arnold, 2016; Blencowe et al., 2022).
The role of epigenetics in SDM has been manifested in some key processes such as X chromosome inactivation (XCI) and genomic imprinting - Figure 1A. XCI refers to the process of silencing one of the two X chromosomes through DNA methylation, histone modifications and non-coding RNAs to achieve dosage compensation and balanced gene expression (Avner and Heard, 2001). Nevertheless, some X-linked genes (both in humans and mice) escape silencing by XCI which leads to different gene expression levels between female and male cells due to the bi-allelic expression. As a result, this provides a source of sex differences in phenotypic traits and disease susceptibility. For instance, DDX3X (Dead Box Helicase 3, X-Linked), KDM6A/UTX (Lysine Demethylase 6A/UTX), MAGEC3 (Melanoma Antigen Gene Family, Member C3), CNKSR2 (Connector Enhancer of Kinase Suppressor of Ras 2), KDM5C (Lysine Demethylase 5C), ATRX (Alpha Thalassemia/Intellectual Disability Syndrome X-Linked) are tumor suppressor X-linked genes located in the non-pseudoautosomal region (PAR) which has been reported to escape silencing by XCI and thus contribute to cancer sex bias (Dunford et al., 2017). Another example that shows the implication of XCI in SDM is the abnormal expression of the lncRNA Xist, an X-inactive specific transcript. In particular, the inhibition of Xist gene expression can suppress cell proliferation, indicating that high expression of the lncRNA Xist might account for the sex differences in the proliferative potential of pulmonary arterial endothelial cells in women and consequently boost their susceptibility to pulmonary arterial hypertension (Qin et al., 2021).

Figure 1. Graphical illustration of how epigenetics may impact sexual dimorphism and how paternal inheritance mechanisms can affect offspring phenotypes in a sex-specific manner. (A) highlights the main epigenetic mechanisms that potentially affect sexual dimorphism (SDM), including X-chromosome inactivation (XCI), genomic imprinting, and X-linked miRNAs. Male and female offspring can be potentially affected differently by fathers in both direct and indirect ways as shown in (B). For instance, direct mechanisms involve sperm-borne factors such as DNA methylation and imprinting, chromatin modifications, small non-coding RNAs, and other factors in the seminal plasma. Indirect ways include paternal influences on maternal behavior, the uterine environment, and placental alterations. Although human figures (male, female, and infant) are used to illustrate sex-specific variance of paternal epigenetic inheritance mechanisms, these mechanisms are primarily inferred from animal models, which still require confirmation in human studies. Abbreviations: H3K27me3 – Histone H3 lysine 27 trimethylation, H3K9me3 – Histone H3 lysine 9 trimethylation, H3K9ac–Histone H3 lysine 9 acetylation, H2AK11pUb–Histone H2A lysine 11 polyubiquitination, Xist–X-inactive specific transcript, Tsix–Xist antisense transcript, Jpx–Jpx RNA, RepA–Repeat-associated non-coding RNA, Firre–Functional Intergenic Repeat RNA, Igf2r–Insulin-like growth factor 2 receptor, H19 – H19 imprinted maternally expressed transcript, Pw1 – Paternally Expressed Gene 3, Igf2 – Insulin-like growth factor 2 (Chen and Zhang, 2020; Fukuda et al., 2014; Fang et al., 2019; Żylicz et al., 2019; Sharp et al., 2011; Tinker and Brown, 1998; Panning et al., 1997; Lee et al., 1999; Froberg et al., 2013; Yang et al., 2015).
Genomic imprinting is another potential key player in SDM. It represents the process of suppressing a subset of genes in one parent using DNA methylation, resulting in monoallelic parental-specific expression. Since some of the imprinted genes (IGs) are involved in growth, metabolism, and brain functions (Tucci et al., 2019), it is plausible that these imprinted genes play a part in the sex differences observed in these physiological processes. To provide evidence for this speculation, the imprinted-X liability threshold model suggests that a certain imprinted X-linked gene(s) (only active when inherited paternally) is protective in nature against phenotypic expression of many autism-related traits and therefore raises the threshold for females to develop autism (Skuse, 2000). In 2010, Gregg et al. revealed through their in-depth analysis that the imprinted expression of interleukin-18, a cytokine that regulates neuroinflammation, is expressed in female brains but not in male brains (Gregg et al., 2010). Another study has also shown a link between interleukin-18 gene expression and multiple sclerosis, a highly sex-specific disease with a noticeable prevalence in females (Herrera et al., 2008).
Another epigenetic mechanism that could account for sex differences is the differential expression of X-linked microRNAs, which are known to regulate the post-transcriptional expression of numerous genes involved in immune response, cytokine production, apoptosis regulation, and cell lineage determination (Pinheiro et al., 2011; Hewagama, 2013). This notion is supported by the fact that the X chromosome carries a higher number of miRNA-encoding genes when compared to the autosomes and Y chromosome and several miRNA-encoding genes on the X-chromosome have been found to escape XCI (Migliore et al., 2021). In multiple studies, differential expression of X-linked miRNAs between the sexes has been reported as a possible mechanism behind sex differences in several diseases, including multiple sclerosis, cerebral ischemia, autoimmune diseases, and various malignancies (Migliore et al., 2021).
A growing body of evidence has shown that the epigenome can be affected and reprogrammed by environmental factors, resulting in changes to the expression of many phenotypic characteristics. Emerging studies have also demonstrated that these environmentally induced traits can be passed on from parents to offspring in a sex-specific manner. Traditionally, parentally induced epigenetic effects in offspring have been mainly attributed to mothers because there were no established mechanisms for transferring epigenetic factors between fathers and offspring. However, sperm- and ejaculate-mediated mechanisms of transfer have revealed that epigenetic effects via the paternal line can be highly significant (Lassi et al., 2021; Tomar et al., 2024; Comas-Armangue et al., 2022). Since paternal contributions are pleiotropic and have a sex-specific impact, understanding the mechanisms underlying paternal epigenetic inheritance could provide a beneficial strategy for understanding SDM in phenotypic traits and diseases.
Of particular interest for the scope of this review, SDM can emerge early in development due to epigenetically programmed differences, often influenced by the parental health status at conception. More recently, paternal health at conception has gained substantial relevance for its impact on offspring health and recent studies have highlighted that factors such as metabolic and circadian states may affect gene expression profiles in male and female embryos differently, contributing to observable sex-specific phenotypic differences in offspring, including susceptibility to metabolic and neuropsychiatric disorders (Comas-Armangue et al., 2022; Billah et al., 2022; Costa et al., 2018; Cambiasso et al., 2022; Solomon and Zerach, 2020; Sun et al., 2023) In this review, we will specifically focus on paternal programming of sexually dimorphic phenotypes and discuss molecular mechanisms and physiological relevance.
Evidence of sexual dimorphism in paternal inheritance
Various studies have documented that environmental perturbations on parents result in sexually dimorphic impact on offspring. These studies range over multiple species with most documenting only maternal (Risal et al., 2021), whereas some comparing the impact of maternal and paternal effects (Hedegger et al., 2020). These comparisons also give a unique insight into a possible additive nature of the perturbation. Paternal inheritance is mediated directly via sperm borne factors like DNA methylation and imprinting, chromatin modifications as well as small non-coding RNAs, along with its nutritious soup, the seminal plasma- Figure 1B.
Gasterosteus aculeatus, commonly known as the three-spine stickleback offers a great model system to study evolutionary adaptations and epigenetic mechanisms (Reid et al., 2021). A study using the three-spine sticklebacks in freshwater has shown comparison of maternal and paternal stress using predation risk with sexually dimorphic impact on offspring brain gene expression (Hellmann et al., 2020a). The same study further reported risk-prone male offspring born from stressed fathers and anxious offspring (sex-independent) from stressed mothers (Hellmann et al., 2020a), while a second study expanded on the impact of paternal stress on F2 and found sex specific differences in offspring based on maternal/paternal lineage in F1 (Hellmann et al., 2020b). Drosophila melanogaster, another widely-used model to study epigenetics, has been used to show the sex-specific intergenerational impact of paternal stress, using protein restriction during larval development (Zeender et al., 2023). In particular, daughters of affected fathers showed enhanced fecundity when their diet aligned with that of the fathers compared to controls. Whereas the sons showed the same abundance of offspring but faster mating (Zeender et al., 2023).
Studies in rats show promising insight into understanding the sex-dependent variance of behavioral phenotypes. A study showed the impact of increasing doses of Morphine in male rats (during adolescence) on its offspring, and observed a sexually dimorphic impact on anxiety-like behavior in offspring (Azadi et al., 2021). Paternal self-administration of morphine also induces sexually dimorphic results in offspring, with object recognition memory deficit present in females but not male offspring (Ellis et al., 2020). A study with paternal preconception exposure to Cannabis showed male and female offspring having inverse methylation-expression relationship with their respective sex controls (Schrott et al., 2022). The study also reported cardiomegaly in offspring along with significant differences in both offspring sexes in response to addition of a washout period, but only in female offspring in the absence of a washout period (Schrott et al., 2022). Paternal preconception exposure to Nicotine has also shown sexually dimorphic outcomes, with male offspring exhibiting locomotor hyperactivity, exclusively during adolescence, and female offspring exhibiting reduced response latency (Hawkey et al., 2019).
Metabolic health of offspring is also affected by paternal impact in Rats as shown with high-protein diet that induced increased insulin sensitivity in male offspring (Gong et al., 2021). Beta cell plasticity of these paternally exposed male offspring was enhanced as well in response to high fat diet metabolic challenge (Gong et al., 2021). Studying offspring of obese fathers also showed higher susceptibility to impairment in the male offspring compared to the female offspring, when subjected to high fat diet (Sanchez-Garrido et al., 2018).
A study with rats subjected to predatory stress showed that the maternal and paternal effect was not additive, in agreement with Hellmann et al.‘s findings in three-spined sticklebacks (Hellmann et al., 2020a). The authors also made comparisons with onset of acute stress of the offspring and observed that female non-acute stressed offspring were more impacted with paternal stress in comparison to combined, whereas acutely stressed pups were more impacted with combined stress than maternal stress present alone (Azizi et al., 2019). These phenotypes offer insight into the complex nature of inheritance of SDM.
While studies with rats give more phenotypic insight into behavior, studies with mice evidently give a deeper understanding of mechanistic differences during development, and aid in hypothesizing possible mechanisms of SDM. Studies with fathers exposed to early life unexpected stress exposure with maternal separation showed that only male offspring exhibit behavior of social anxiety, although the phenotype was absent in fathers (Franklin et al., 2011). Here, depressive-like behavior of fathers was inherited to female, but not male offspring, and it could be reversed with anti-depressants. Interestingly, the second effect was inherited by grandsons through the male lineage, thus, phenotype skipping a generation. Chronic stress exposure to fathers in adulthood through social defeat has also been shown to affect the offspring, with males showing a more robust phenotype as well as exclusive increase in plasma corticosterone and decrease in Vascular endothelial growth factor (VEGF) (Dietz et al., 2011). The same study also reported that IVF did not mimic the phenotype, indicating involvement of factors other than sperm, by itself (Dietz et al., 2011). Paternal chronic stress with restraint stressor/forced swim test also shows sex specific impact on offspring anxiety-like and depression-like behavior, with males exhibiting reduction and females-an increase (Mashoodh et al., 2023). Interestingly, the changes in males have been correlated to differences in maternal investment in pregnancy. Despite these studies showing sex-specific behavioral differences, chronic paternal stress with varied stressors has been found to translate in reduced HPA axis responsiveness in the offspring, in a non-sex-specific manner, further depicting the complexities of the subject (Rodgers et al., 2013).
Exposure to various compounds has also been documented to be paternally inherited in mice. Paternal valproic acid exposure has been shown to affect behavior of both sexes similarly, but deficits in the sensorimotor gating were only observed in females (Ibi et al., 2019). Prenatal paternal exposure to alcohol has been reported to cause greater intrauterine growth restriction in males than females (Chang et al., 2019). The same study also reported metabolic dysregulation in the offspring with males having decreased glucose and insulin and females having increased insulin (Chang et al., 2019). Paternal exposure to glucocorticoids is shown to affect male offspring more than female, in the context of pro-anxiety behaviors (Short et al., 2016), whereas the same evidently affected memory retention in females, not males (Yeshurun et al., 2017). In the context of glucocorticoids, our group has recently shown disrupted levels of it in the seminal plasma as a result of circadian disruption have affect metabolic health of male offspring, and the impact on female offspring is minimal and different (e.g., Lower weight) (Lassi et al., 2021). Differences in metabolic phenotypes were also observed on paternal high fat diet treatment leading to the F2 generation, with males showing obese hyperglycemic phenotype with upregulated glycolysis and females showing lean hyperglycemic phenotype with upregulated gluconeogenesis and lipolysis (Park et al., 2018). It is likely from these observations that a different set of response cascade in males and females is in place and is dependent on the type of stress.
Finally, case studies in humans aid towards increasing the clinical relevance of all animal models. Studies with paternal exposure to organic pollutants have reported effects on sex ratio. In Michigan fish eaters, exposure to polychlorinated biphenyls have been documented to affect secondary sex ratio with increased male offspring (Karmaus et al., 2002), whereas in a Faroe island males, they found the levels of persistent organic compounds to negatively correlate with Y:X sex ratio in sperm (Kvist et al., 2014). Paternal opioid use, starting after childbirth, has been associated with significantly increased odds ratio of obesity in sons but not in daughters (Jalali et al., 2021). Paternal smoking exposure has been found to be associated with greater body mass index (BMI) in 9 year old sons (Pembrey et al., 2006). Furthermore, paternal smoking exposure is also significantly associated with childhood overweight/obesity in sons, not daughters, and the phenotype is evident with pre-conception and post-conception exposure, but not post-natal exposure (You et al., 2022). Smoking is also established to cause alterations in DNA methylation (Jenkins et al., 2017) and sperm chromatin condensation (Laqqan and Yassin, 2022), indicating an epigenetic link. Well established cohorts have also demonstrated that grandfather’s food supply is associated exclusively with grandson’s increased mortality risk ratio (Pembrey et al., 2006; Vagero et al., 2018). Various studies have also been documented for metabolic phenotype and obesity (Comas-Armangue et al., 2022), but they unfortunately don’t yet provide a clear mechanistic insight.
Mechanisms involved in paternally transmitted signal for SDM
While numerous studies report sexual dimorphism (SDM) in phenotypes, the mechanisms governing SDM remain poorly understood. Key questions persist, such as: (a) which signals communicate parental stress versus which are passive observations, and (b) what the boundaries of programming are in terms of developmental stages or offspring age. Additionally, it remains uncertain whether these mechanisms respond uniformly to various stress types. A central debate, often referred to as the “chicken and egg problem,” questions whether SDM is developmentally programmed due to parental influence alongside genomic and epigenomic contributions, or if it emerges in adulthood through hormonal pathways and gonadal activity. Understanding how inheritance mechanisms may intersect with SDM pathways to drive significant phenotypic differences remains an intriguing area of inquiry. Currently, there are competing hypotheses suggesting both early developmental programming and postnatal activation as potential drivers of SDM.
The mechanisms of paternal inheritance mediated by both germ cell and non-germ cell components of the male reproductive tract and including DNA methylation, chromatin organization and the activity of small non-coding RNAs have been extensively reviewed (Daxinger and Whitelaw, 2012; Chen et al., 2016; Lempradl, 2020) and also summarized here in Figure 1. Global studies have identified differences such as sperm hypomethylation associated with low-protein diets (Watkins et al., 2018) and gestational arsenic exposure (Nohara et al., 2020) associated to SDM in offspring phenotypes. However, due to current methodological limitations, single-sperm chromatin and DNA methylation analyses are not yet feasible. This makes it unclear whether these differences are already encoded within sperm carrying either the X or Y chromosome. There is, however, much more evidence in support of RNA mediated inheritance.
Long term restraint stress has been shown to cause differential DNA methylation at regions, that are further inherited paternally (Zheng et al., 2021) and this has been proposed to be mediated by small non-coding RNA (sncRNA), which further highlights the interactive nature of epigenetic mechanisms. Increased abundance of several miRNAs in sperm has also been reported in the case of paternal obesity, that interestingly resulted in more pronounced transcriptomic changes in male blastocysts, compared to female (Hedegger et al., 2020). Reduced levels of certain miRNA family members in sperm from fathers with chronic social instability have been associated with elevated anxiety and defective sociability in female offspring (Champroux et al., 2024). Interestingly, restoring the miRNA member in pre-implantation embryo has shown to reduce the phenotype, which strengthens the case of RNA mediated inheritance, or at least highlights its importance for early development and late-onset phenotypes.
Spermatogenesis is a complex process and in recent years, there has been more attention towards contribution from the epididymis, either directly via epididymosomes (Chen et al., 2016), or through the environment provided for maturation. We have recently shown that the epidydimal spermatozoa are sensitive to environmental stimuli and transfer the signal through mitochondrial tRNAs (mt-tRNA) and their fragments (Tomar et al., 2024). Paternal high fat diet treatment caused a spike in these mt-tRNAs, and they were inherited at fertilization. Interestingly, despite being inherited and found in both male and female early 2-cell embryos, only male embryos were transcriptionally reprogrammed and only male offspring showed impaired metabolic homeostasis (Tomar et al., 2024). These findings support the idea that a vast proportion of sexually dimorphic phenotypes are established early in development before any possible hormonal contribution. In keeping with this, the currently available data from two-cell to pre-implantation stages has also been repurposed to study sex differences and uncovered early differences forming in two waves, both long before hormonal signaling, further supporting the theory of developmental programming (Richardson et al., 2023). Finally, we cannot rule out maternal reaction to paternal stress while defining pre-implantation programming. As mentioned before, maternal investment in the pregnancy is documented to be altered, specifically for male offspring in terms of prenatal weight gain and nursing (Mashoodh et al., 2023). Apart from behavioral reaction, mechanistic differences can also be programmed during fetal development (Watkins et al., 2020). The seminal plasma from paternal low protein diet has been shown to blunt maternal immunological responses (Watkins et al., 2018), and it has also been documented to have a sexually dimorphic impact on their vascular function, with females displaying significantly greater acetylcholine-mediated vasodilation responses to nitric oxide synthesis inhibitor, and males displaying a significant reduction (Morgan et al., 2020).
Post-implantation, placenta plays a crucial role in providing the necessary nourishment to the fetus. Fetal growth restriction has been reported as a consequence of paternal stress (Lassi et al., 2021). It is also well established that male and female placentas are different in their gene expression, miRNA profiles, as well as histopathology based on the requirement of the fetus (Gabory et al., 2013; Christians, 2022) thus being a key player in conveying stress signals in a sexually dimorphic manner. Paternal preconception stress is shown to have a divergent effect on the transcriptional profiles of placentae at E12.5, with female featuring increase in carbohydrate, lipid and amino acid metabolism, whereas males show reduction of immune-regulatory genes (Cisse et al., 2020). Another study showed that paternal high fat diet caused low placental weights for males but not females, whereas a combination with exercise indicated decrease in expression of pro-inflammatory molecule mRNAs, exclusively in female placentae (Claycombe-Larson et al., 2020). In females, this is also exclusively linked to reduced levels of sperm miRNA 193b, which indicates a sex-specific communication between father-placenta-daughter.
Parentally imprinted gene Pw1 (Paternally Expressed Gene 1) has been shown to be involved in SDM that arises postnatally, using Pw1 deficient mice that showed reduced masculinization of body composition in males as well as reduced testosterone at puberty and reduced growth hormones levels at early postnatal developmental points (Tanaka et al., 2022). Interestingly, this was also one of the imprinted genes found to be affected in male offspring of fathers with early life stress (Thivisol et al., 2023). Igf2 (Insulin-like Growth Factor 2), another imprinted gene was found to be increased in offspring of fathers with glucocorticoid exposure (Short et al., 2016), as well as maternal separation induced early life stress (Thivisol et al., 2023). On the other hand, it has also been reportedly lowly expressed upon paternal stress, in adult male rat offspring hippocampus and neocortex, but not in neonatal offspring (Ordyan et al., 2022) IGF2 treatment (human recombinant) in rats during late pregnancy showed male fetuses to be more stimulated and affected, further documenting its role in SDM (White et al., 2018). These cases support the theory that SDM phenotypes are established postnatally.
Hormones also significantly influence SDM in various physiological systems including metabolism (Santos-Marcos et al., 2023; Sandovici et al., 2022) and immunity (Shepherd et al., 2020). Sex-dimorphic differences appearing early post-natal are owed to growth and sex-specific hormone related pathways and have been linked, for example, to the pulsatile nature of growth hormone release in males and continuous in females (Gabory et al., 2009). Paternal obesity is reported to reduce luteinizing hormone (LH) levels in male offspring, but not female (Sanchez-Garrido et al., 2018). It has also been recently established using paternal exposure to inorganic compounds that the metabolic phenotype in female offspring is induced by estrogen which was further confirmed with absence of phenotype using hepatic knockout of estrogen receptor α/ß (Xue et al., 2023). This sheds light on possibilities of long-term programming of stress signals in a sex-dependent manner, but doesn’t rule out an initial trigger based on epigenetic developmental programming. The above mechanisms are summarized in Table 1.
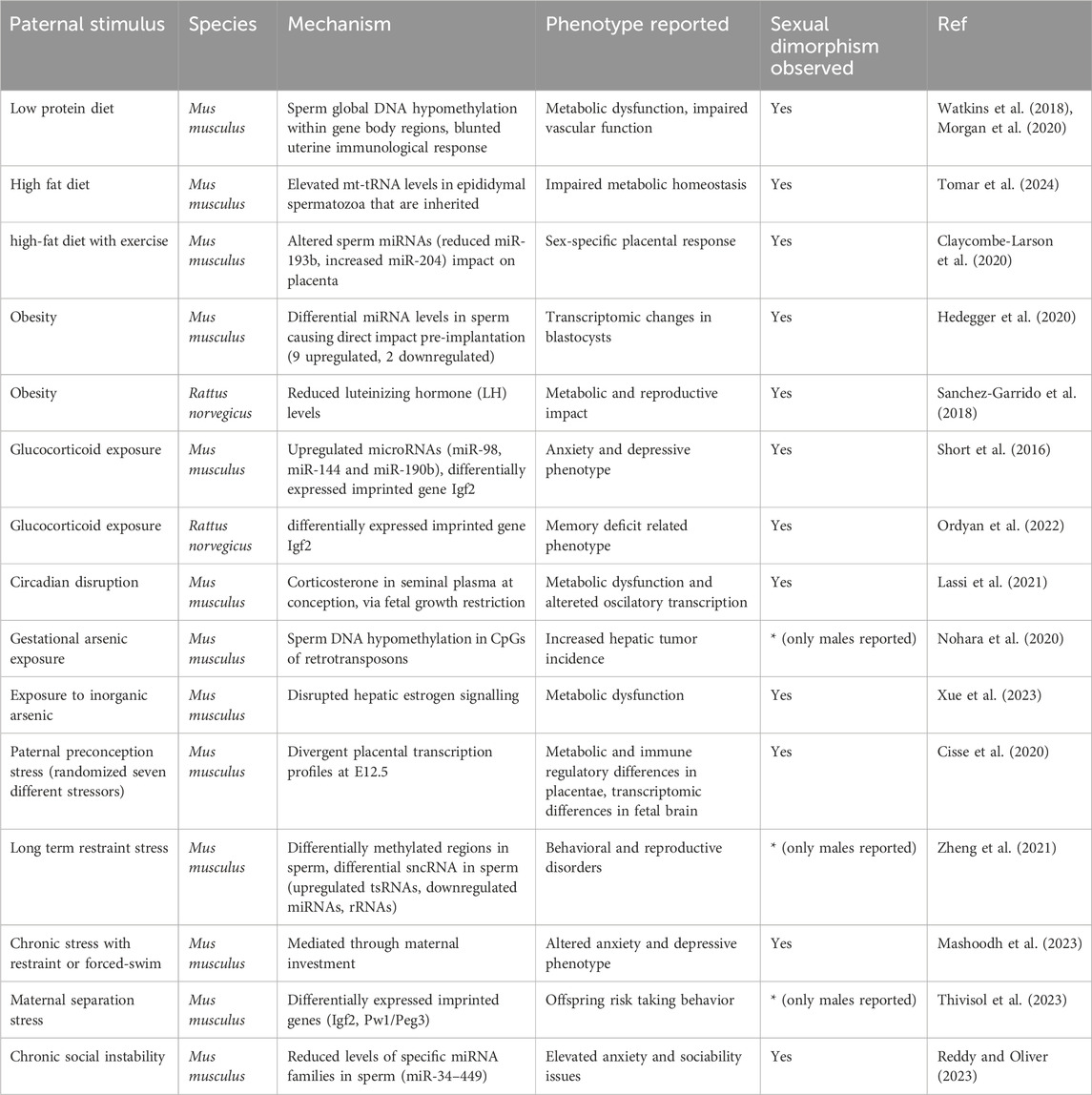
Table 1. Models and modes of paternal influence on sexual dimorphism The table summarizes documented models of paternal stimuli with their modes/mechanisms of paternal influence. We also summarize in brief, the phenotypes and observed instances of sexual dimorphism. Abbreviations: Igf2- Insulin-like Growth Factor 2, sncRNA-small non-coding RNA, tsRNA-tRNA-derived small RNAs, Pw1/Peg3- Paternally Expressed Gene 3.
Discussion
In summary, we show evidence of the sexual dimorphic nature of paternal inheritance in various species and the current understanding of the mechanistic underpinnings behind it. However, systematically compiling the sexually dimorphic contributions of fathers towards various offspring phenotypes and complex traits has a handful of limitations underlined in the mechanistic complexities of paternal epigenetic inheritance, research design constraints, and the persistent knowledge gaps. For instance, several mechanistic routes and machineries by which paternal signals are passed on to the offspring remain enigmatic, especially throughout the early stages of development when cell signaling pathways are dynamic as well as not yet clearly defined. Moreover, male germ cell heterogeneity poses a considerable challenge for our understanding of which sperm-borne factors or signals are specifically implicated in paternal inheritance and SDM. Furthermore, investigating and studying both offspring sexes incredibly aids in unraveling the sexually dimorphic effects and ensures a clear thorough understanding of paternal impact across generations.
Our understanding of the mechanisms in humans is deeply lacking, partly owing to the ethical complications. Animal models help in driving the science in the right direction, but diseases often tend to present differently in humans compared to other animal models and it is very important to confirm these findings in humans to ascertain clinical relevance. Finally, the necessity of performing systemic phenotyping and sex-stratified analyses in upcoming studies is highly pressing since paternal signals inherited by offspring could impact a broad range of traits, other than those intended to be examined or studied. A more extensive approach that embraces various traits might uncover earlier neglected or subtle effects, reinforcing a profound understanding of the sex-specific variances of paternal epigenetic inheritance.
Author contributions
SA: Visualization, Writing–original draft, Writing–review and editing, Conceptualization. SP: Conceptualization, Writing–original draft, Writing–review and editing, Visualization. RT: Conceptualization, Funding acquisition, Supervision, Writing–review and editing, Project administration, Resources, Writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors thank the Helmholtz Munich and the German Center for Diabetes Research for funding the Environmental Epigenetics Group, the DAAD (German Academic Exchange Service) and the EpiCrossBorders for funding the position of SA(funding program Nr. 57597951) and SP, respectively. The authors also thank the Fritz-Thyssen Foundation for funding RT’s work on epigenetic inheritance (Grant Nr. 10.19.2.027 MN - Epigenetic inheritance of diabetes in mammals).
Acknowledgments
The authors thank the Helmholtz Munich and the German Center for Diabetes Research for funding the Environmental Epigenetics Group, the DAAD (German Academic Exchange Service) and the EpiCrossBorders for funding the position of Shefa’ M. Aljabali (funding program Nr. 57597951) and SP, respectively. The authors also thank the Fritz-Thyssen Foundation for funding RT’s work on epigenetic inheritance (Grant Nr. 10.19.2.027 MN - Epigenetic inheritance of diabetes in mammals).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Avner, P., and Heard, E. (2001). X-chromosome inactivation: counting, choice and initiation. Nat. Rev. Genet. 2 (1), 59–67. doi:10.1038/35047580
Azadi, M., Zare, M., Pachenari, N., Shojaei, A., Semnanian, S., and Azizi, H. (2021). Sex-specific transgenerational effects of adolescent morphine exposure on short-term memory and anxiety behavior: male linage. Neurosci. Lett. 761, 136111. doi:10.1016/j.neulet.2021.136111
Azizi, N., Roshan-Milani, S., MahmoodKhani, M., Saboory, E., Gholinejad, Z., Abdollahzadeh, N., et al. (2019). Parental pre-conception stress status and risk for anxiety in rat offspring: specific and sex-dependent maternal and paternal effects. Stress 22 (5), 619–631. doi:10.1080/10253890.2019.1619075
Billah, M. M., Khatiwada, S., Morris, M. J., and Maloney, C. A. (2022). Effects of paternal overnutrition and interventions on future generations. Int. J. Obes. (Lond) 46 (5), 901–917. doi:10.1038/s41366-021-01042-7
Blencowe, M., Chen, X., Zhao, Y., Itoh, Y., McQuillen, C. N., Han, Y., et al. (2022). Relative contributions of sex hormones, sex chromosomes, and gonads to sex differences in tissue gene regulation. Genome Res. 32 (5), 807–824. doi:10.1101/gr.275965.121
Burgoyne, P. S., and Arnold, A. P. (2016). A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol. Sex. Differ. 7, 68. doi:10.1186/s13293-016-0115-5
Cambiasso, M. Y., Gotfryd, L., Stinson, M. G., Birolo, S., Salamone, G., Romanato, M., et al. (2022). Paternal alcohol consumption has intergenerational consequences in male offspring. J. Assist. Reprod. Genet. 39 (2), 441–459. doi:10.1007/s10815-021-02373-0
Champroux, A., Tang, Y., Dickson, D. A., Meng, A., Harrington, A., Liaw, L., et al. (2024). Transmission of reduced levels of miR-34/449 from sperm to preimplantation embryos is a key step in the transgenerational epigenetic inheritance of the effects of paternal chronic social instability stress. Epigenetics 19 (1), 2346694. doi:10.1080/15592294.2024.2346694
Chang, R. C., Wang, H., Bedi, Y., and Golding, M. C. (2019). Preconception paternal alcohol exposure exerts sex-specific effects on offspring growth and long-term metabolic programming. Epigenetics Chromatin 12 (1), 9. doi:10.1186/s13072-019-0254-0
Chen, Q., Yan, W., and Duan, E. (2016). Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 17 (12), 733–743. doi:10.1038/nrg.2016.106
Chen, Z., and Zhang, Y. (2020). Maternal H3K27me3-dependent autosomal and X chromosome imprinting. Nat. Rev. Genet. 21 (9), 555–571. doi:10.1038/s41576-020-0245-9
Choleris, E., Galea, L. A. M., Sohrabji, F., and Frick, K. M. (2018). Sex differences in the brain: implications for behavioral and biomedical research. Neurosci. Biobehav Rev. 85, 126–145. doi:10.1016/j.neubiorev.2017.07.005
Christians, J. K. (2022). The placenta's role in sexually dimorphic fetal growth strategies. Reprod. Sci. 29 (6), 1895–1907. doi:10.1007/s43032-021-00780-3
Cisse, Y. M., Chan, J. C., Nugent, B. M., Banducci, C., and Bale, T. L. (2020). Brain and placental transcriptional responses as a readout of maternal and paternal preconception stress are fetal sex specific. Placenta 100, 164–170. doi:10.1016/j.placenta.2020.06.019
Claycombe-Larson, K. G., Bundy, A. N., and Roemmich, J. N. (2020). Paternal high-fat diet and exercise regulate sperm miRNA and histone methylation to modify placental inflammation, nutrient transporter mRNA expression and fetal weight in a sex-dependent manner. J. Nutr. Biochem. 81, 108373. doi:10.1016/j.jnutbio.2020.108373
Comas-Armangue, G., Makharadze, L., Gomez-Velazquez, M., and Teperino, R. (2022). The legacy of parental obesity: mechanisms of non-genetic transmission and reversibility. Biomedicines 10 (10), 2461. doi:10.3390/biomedicines10102461
Costa, D. L., Yetter, N., and DeSomer, H. (2018). Intergenerational transmission of paternal trauma among US Civil War ex-POWs. Proc. Natl. Acad. Sci. U. S. A. 115 (44), 11215–11220. doi:10.1073/pnas.1803630115
Daxinger, L., and Whitelaw, E. (2012). Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 13 (3), 153–162. doi:10.1038/nrg3188
Dietz, D. M., Laplant, Q., Watts, E. L., Hodes, G. E., Russo, S. J., Feng, J., et al. (2011). Paternal transmission of stress-induced pathologies. Biol. Psychiatry 70 (5), 408–414. doi:10.1016/j.biopsych.2011.05.005
Dunford, A., Weinstock, D. M., Savova, V., Schumacher, S. E., Cleary, J. P., Yoda, A., et al. (2017). Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat. Genet. 49 (1), 10–16. doi:10.1038/ng.3726
Ellis, A. S., Toussaint, A. B., Knouse, M. C., Thomas, A. S., Bongiovanni, A. R., Mayberry, H. L., et al. (2020). Paternal morphine self-administration produces object recognition memory deficits in female, but not male offspring. Psychopharmacol. Berl. 237 (4), 1209–1221. doi:10.1007/s00213-019-05450-6
Fang, H., Disteche, C. M., and Berletch, J. B. (2019). X inactivation and escape: epigenetic and structural features. Front. Cell Dev. Biol. 7, 219. doi:10.3389/fcell.2019.00219
Franklin, T. B., Linder, N., Russig, H., Thöny, B., and Mansuy, I. M. (2011). Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One 6 (7), e21842. doi:10.1371/journal.pone.0021842
Froberg, J. E., Yang, L., and Lee, J. T. (2013). Guided by RNAs: X-inactivation as a model for lncRNA function. J. Mol. Biol. 425 (19), 3698–3706. doi:10.1016/j.jmb.2013.06.031
Fukuda, A., Tomikawa, J., Miura, T., Hata, K., Nakabayashi, K., Eggan, K., et al. (2014). The role of maternal-specific H3K9me3 modification in establishing imprinted X-chromosome inactivation and embryogenesis in mice. Nat. Commun. 5 (1), 5464. doi:10.1038/ncomms6464
Gabory, A., Attig, L., and Junien, C. (2009). Sexual dimorphism in environmental epigenetic programming. Mol. Cell Endocrinol. 304 (1-2), 8–18. doi:10.1016/j.mce.2009.02.015
Gabory, A., Roseboom, T. J., Moore, T., Moore, L. G., and Junien, C. (2013). Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol. Sex. Differ. 4 (1), 5. doi:10.1186/2042-6410-4-5
Gay, L., Melenotte, C., Lakbar, I., Mezouar, S., Devaux, C., Raoult, D., et al. (2021). Sexual dimorphism and gender in infectious diseases. Front. Immunol. 12, 698121. doi:10.3389/fimmu.2021.698121
Gong, P., Bailbé, D., Bianchi, L., Pommier, G., Liu, J., Tolu, S., et al. (2021). Paternal high-protein diet programs offspring insulin sensitivity in a sex-specific manner. Biomolecules 11 (5), 751. doi:10.3390/biom11050751
Gregg, C., Zhang, J., Butler, J. E., Haig, D., and Dulac, C. (2010). Sex-specific parent-of-origin allelic expression in the mouse brain. Science 329 (5992), 682–685. doi:10.1126/science.1190831
Hawkey, A. B., White, H., Pippen, E., Greengrove, E., Rezvani, A. H., Murphy, S. K., et al. (2019). Paternal nicotine exposure in rats produces long-lasting neurobehavioral effects in the offspring. Neurotoxicol Teratol. 74, 106808. doi:10.1016/j.ntt.2019.05.001
Hedegger, K., Philippou-Massier, J., Krebs, S., Blum, H., Kunzelmann, S., Förstemann, K., et al. (2020). Sex-specific programming effects of parental obesity in pre-implantation embryonic development. Int. J. Obes. (Lond) 44 (5), 1185–1190. doi:10.1038/s41366-019-0494-x
Hellmann, J. K., Bukhari, S. A., Deno, J., and Bell, A. M. (2020a). Sex-specific plasticity across generations I: maternal and paternal effects on sons and daughters. J. Anim. Ecol. 89 (12), 2788–2799. doi:10.1111/1365-2656.13364
Hellmann, J. K., Carlson, E. R., and Bell, A. M. (2020b). Sex-specific plasticity across generations II: grandpaternal effects are lineage specific and sex specific. J. Anim. Ecol. 89 (12), 2800–2812. doi:10.1111/1365-2656.13365
Herrera, B. M., Ramagopalan, S. V., Lincoln, M. R., Orton, S. M., Chao, M. J., Sadovnick, A. D., et al. (2008). Parent-of-origin effects in MS: observations from avuncular pairs. Neurology 71 (11), 799–803. doi:10.1212/01.wnl.0000312377.50395.00
Hewagama, A. (2013). Role of X-chromosome encoded miRNAs in autoimmunity: suppressing the suppressor and female predisposition. Rheumatol. Curr. Res. 3 (1), 118. doi:10.4172/2161-1149.1000118
Ibi, D., Fujiki, Y., Koide, N., Nakasai, G., Takaba, R., and Hiramatsu, M. (2019). Paternal valproic acid exposure in mice triggers behavioral alterations in offspring. Neurotoxicol Teratol. 76, 106837. doi:10.1016/j.ntt.2019.106837
Jalali, Z., Bahrampour, S., Khalili, P., Khademalhosseini, M., and Esmaeili Nadimi, A. (2021). Cohort-based analysis of paternal opioid use in relation to offspring's BMI and plasma lipid profile. Sci. Rep. 11 (1), 9462. doi:10.1038/s41598-021-88781-9
Jenkins, T. G., James, E. R., Alonso, D. F., Hoidal, J. R., Murphy, P. J., Hotaling, J. M., et al. (2017). Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology 5 (6), 1089–1099. doi:10.1111/andr.12416
Karmaus, W., Huang, S., and Cameron, L. (2002). Parental concentration of dichlorodiphenyl dichloroethene and polychlorinated biphenyls in Michigan fish eaters and sex ratio in offspring. J. Occup. Environ. Med. 44 (1), 8–13. doi:10.1097/00043764-200201000-00003
Karp, N. A., Mason, J., Beaudet, A. L., Benjamini, Y., Bower, L., Braun, R. E., et al. (2017). Prevalence of sexual dimorphism in mammalian phenotypic traits. Nat. Commun. 8, 15475. doi:10.1038/ncomms15475
Khodursky, S., Jiang, C. S., Zheng, E. B., Vaughan, R., Schrider, D. R., and Zhao, L. (2022). Sex differences in interindividual gene expression variability across human tissues. PNAS Nexus 1 (5), pgac243. doi:10.1093/pnasnexus/pgac243
Kvist, L., Giwercman, A., Weihe, P., Kold Jensen, T., Grandjean, P., Halling, J., et al. (2014). Exposure to persistent organic pollutants and sperm sex chromosome ratio in men from the Faroe Islands. Environ. Int. 73, 359–364. doi:10.1016/j.envint.2014.09.001
Laqqan, M. M., and Yassin, M. M. (2022). Cigarette heavy smoking alters DNA methylation patterns and gene transcription levels in humans spermatozoa. Environ. Sci. Pollut. Res. Int. 29 (18), 26835–26849. doi:10.1007/s11356-021-17786-8
Lassi, M., Tomar, A., Comas-Armangué, G., Vogtmann, R., Dijkstra, D. J., Corujo, D., et al. (2021). Disruption of paternal circadian rhythm affects metabolic health in male offspring via nongerm cell factors. Sci. Adv. 7 (22), eabg6424. doi:10.1126/sciadv.abg6424
Lee, J., Davidow, L. S., and Warshawsky, D. (1999). Tsix, a gene antisense to Xist at the X-inactivation centre. Nat. Genet. 21 (4), 400–404. doi:10.1038/7734
Lempradl, A. (2020). Germ cell-mediated mechanisms of epigenetic inheritance. Semin. Cell Dev. Biol. 97, 116–122. doi:10.1016/j.semcdb.2019.07.012
Lowe, R., Gemma, C., Rakyan, V. K., and Holland, M. L. (2015). Sexually dimorphic gene expression emerges with embryonic genome activation and is dynamic throughout development. BMC Genomics 16 (1), 295. doi:10.1186/s12864-015-1506-4
Ma, J., Malladi, S., and Beck, A. H. (2016). Systematic analysis of sex-linked molecular alterations and therapies in cancer. Sci. Rep. 6, 19119. doi:10.1038/srep19119
Mashoodh, R., Habrylo, I. B., Gudsnuk, K., and Champagne, F. A. (2023). Sex-specific effects of chronic paternal stress on offspring development are partially mediated via mothers. Horm. Behav. 152, 105357. doi:10.1016/j.yhbeh.2023.105357
McCombe, P. A., Greer, J. M., and Mackay, I. R. (2009). Sexual dimorphism in autoimmune disease. Curr. Mol. Med. 9 (9), 1058–1079. doi:10.2174/156652409789839116
Migliore, L., Nicoli, V., and Stoccoro, A. (2021). Gender specific differences in disease susceptibility: the role of epigenetics. Biomedicines 9 (6), 652. doi:10.3390/biomedicines9060652
Miller, V. M. (2012). Family matters: sexual dimorphism in cardiovascular disease. Lancet 379 (9819), 873–875. doi:10.1016/S0140-6736(12)60200-1
Morgan, H. L., Paganopoulou, P., Akhtar, S., Urquhart, N., Philomin, R., Dickinson, Y., et al. (2020). Paternal diet impairs F1 and F2 offspring vascular function through sperm and seminal plasma specific mechanisms in mice. J. Physiol. 598 (4), 699–715. doi:10.1113/JP278270
Neugarten, J., and Golestaneh, L. (2019). Influence of sex on the progression of chronic kidney disease. Mayo Clin. Proc. 94 (7), 1339–1356. doi:10.1016/j.mayocp.2018.12.024
Nohara, K., Suzuki, T., and Okamura, K. (2020). Gestational arsenic exposure and paternal intergenerational epigenetic inheritance. Toxicol. Appl. Pharmacol. 409, 115319. doi:10.1016/j.taap.2020.115319
Oliva, M., Muñoz-Aguirre, M., Kim-Hellmuth, S., Wucher, V., Gewirtz, A. D. H., Cotter, D. J., et al. (2020). The impact of sex on gene expression across human tissues. Science 369 (6509), eaba3066. doi:10.1126/science.aba3066
Ordyan, N., Malysheva, O. V., Holova, G. I., Akulova, V. K., and Pivina, S. G. (2022). Sex-dependent effects of stress in male rats on memory and expression of the insulin-like growth factor 2 receptor gene in the brains of offspring. Neurosci. Behav. Physiology 52 (2), 242–250. doi:10.1007/s11055-022-01232-4
Panning, B., Dausman, J., and Jaenisch, R. (1997). X chromosome inactivation is mediated by Xist RNA stabilization. Cell 90 (5), 907–916. doi:10.1016/s0092-8674(00)80355-4
Park, J. H., Yoo, Y., Cho, M., Lim, J., Lindroth, A. M., and Park, Y. J. (2018). Diet-induced obesity leads to metabolic dysregulation in offspring via endoplasmic reticulum stress in a sex-specific manner. Int. J. Obes. (Lond) 42 (2), 244–251. doi:10.1038/ijo.2017.203
Pembrey, M. E., Bygren, L. O., Kaati, G., Edvinsson, S., Northstone, K., Sjöström, M., et al. (2006). Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 14 (2), 159–166. doi:10.1038/sj.ejhg.5201538
Pinheiro, I., Dejager, L., and Libert, C. (2011). X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays 33 (11), 791–802. doi:10.1002/bies.201100047
Qin, S., Predescu, D., Carman, B., Patel, P., Chen, J., Kim, M., et al. (2021). Up-regulation of the long noncoding RNA X-inactive-specific transcript and the sex bias in pulmonary arterial hypertension. Am. J. Pathol. 191 (6), 1135–1150. doi:10.1016/j.ajpath.2021.03.009
Rawlik, K., Canela-Xandri, O., and Tenesa, A. (2016). Evidence for sex-specific genetic architectures across a spectrum of human complex traits. Genome Biol. 17 (1), 166. doi:10.1186/s13059-016-1025-x
Reddy, K. D., and Oliver, B. G. G. (2023). Sexual dimorphism in chronic respiratory diseases. Cell Biosci. 13 (1), 47. doi:10.1186/s13578-023-00998-5
Reid, K., Bell, M. A., and Veeramah, K. R. (2021). Threespine stickleback: a model system for evolutionary genomics. Annu. Rev. Genomics Hum. Genet. 22, 357–383. doi:10.1146/annurev-genom-111720-081402
Richardson, V., Engel, N., and Kulathinal, R. J. (2023). Comparative developmental genomics of sex-biased gene expression in early embryogenesis across mammals. Biol. Sex. Differ. 14 (1), 30. doi:10.1186/s13293-023-00520-z
Risal, S., Manti, M., Lu, H., Fornes, R., Larsson, H., Benrick, A., et al. (2021). Prenatal androgen exposure causes a sexually dimorphic transgenerational increase in offspring susceptibility to anxiety disorders. Transl. Psychiatry 11 (1), 45. doi:10.1038/s41398-020-01183-9
Rodgers, A. B., Morgan, C. P., Bronson, S. L., Revello, S., and Bale, T. L. (2013). Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 33 (21), 9003–9012. doi:10.1523/JNEUROSCI.0914-13.2013
Sanchez-Garrido, M. A., Ruiz-Pino, F., Velasco, I., Barroso, A., Fernandois, D., Heras, V., et al. (2018). Intergenerational influence of paternal obesity on metabolic and reproductive health parameters of the offspring: male-preferential impact and involvement of kiss1-mediated pathways. Endocrinology 159 (2), 1005–1018. doi:10.1210/en.2017-00705
Sandovici, I., Fernandez-Twinn, D. S., Hufnagel, A., Constância, M., and Ozanne, S. E. (2022). Sex differences in the intergenerational inheritance of metabolic traits. Nat. Metab. 4 (5), 507–523. doi:10.1038/s42255-022-00570-4
Santos-Marcos, J. A., Mora-Ortiz, M., Tena-Sempere, M., Lopez-Miranda, J., and Camargo, A. (2023). Interaction between gut microbiota and sex hormones and their relation to sexual dimorphism in metabolic diseases. Biol. Sex. Differ. 14 (1), 4. doi:10.1186/s13293-023-00490-2
Schrott, R., Modliszewski, J. L., Hawkey, A. B., Grenier, C., Holloway, Z., Evans, J., et al. (2022). Sperm DNA methylation alterations from cannabis extract exposure are evident in offspring. Epigenetics Chromatin 15 (1), 33. doi:10.1186/s13072-022-00466-3
Seo, M., Caetano-Anolles, K., Rodriguez-Zas, S., Ka, S., Jeong, J. Y., Park, S., et al. (2016). Comprehensive identification of sexually dimorphic genes in diverse cattle tissues using RNA-seq. BMC Genomics 17, 81. doi:10.1186/s12864-016-2400-4
Sharp, A. J., Stathaki, E., Migliavacca, E., Brahmachary, M., Montgomery, S. B., Dupre, Y., et al. (2011). DNA methylation profiles of human active and inactive X chromosomes. Genome Res. 21 (10), 1592–1600. doi:10.1101/gr.112680.110
Shepherd, R., Cheung, A. S., Pang, K., Saffery, R., and Novakovic, B. (2020). Sexual dimorphism in innate immunity: the role of sex hormones and epigenetics. Front. Immunol. 11, 604000. doi:10.3389/fimmu.2020.604000
Short, A. K., Fennell, K. A., Perreau, V. M., Fox, A., O'Bryan, M. K., Kim, J. H., et al. (2016). Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl. Psychiatry 6 (6), e837. doi:10.1038/tp.2016.109
Skuse, D. H. (2000). Imprinting, the X-chromosome, and the male brain: explaining sex differences in the liability to autism. Pediatr. Res. 47 (1), 9–16. doi:10.1203/00006450-200001000-00006
Snell, D. M., and Turner, J. M. A. (2018). Sex chromosome effects on male-female differences in mammals. Curr. Biol. 28 (22), R1313–R1324. doi:10.1016/j.cub.2018.09.018
Solomon, Z., and Zerach, G. (2020). The Intergenerational transmission of trauma: when children bear their father's traumatic past. Neuropsychiatrie de l'Enfance de l'Adolescence 68 (2), 65–75. doi:10.1016/j.neurenf.2020.01.004
Sun, J., Teng, M., Wu, F., Zhao, X., Li, Y., Zhao, L., et al. (2023). Adverse health effects and stresses on offspring due to paternal exposure to harmful substances. Crit. Rev. Environ. Sci. Technol. 53 (10), 1059–1084. doi:10.1080/10643389.2022.2129941
Tanaka, K., Besson, V., Rivagorda, M., Oury, F., Marazzi, G., and Sassoon, D. A. (2022). Paternally expressed gene 3 (Pw1/Peg3) promotes sexual dimorphism in metabolism and behavior. PLoS Genet. 18 (1), e1010003. doi:10.1371/journal.pgen.1010003
Thivisol, U., Ho, P., Li, B., Trompke, M., Hoffmann, L. B., Hannan, A. J., et al. (2023). Paternal early life stress exerts intergenerational effects on male C57Bl/6J offspring risk-taking behaviors and predator scent-induced c-Fos expression. Neuronal Signal 7 (2), NS20220097. doi:10.1042/NS20220097
Tinker, A. V., and Brown, C. J. (1998). Induction of XIST expression from the human active X chromosome in mouse/human somatic cell hybrids by DNA demethylation. Nucleic acids Res. 26 (12), 2935–2940. doi:10.1093/nar/26.12.2935
Tomar, A., Gomez-Velazquez, M., Gerlini, R., Comas-Armangué, G., Makharadze, L., Kolbe, T., et al. (2024). Epigenetic inheritance of diet-induced and sperm-borne mitochondrial RNAs. Nature 630 (8017), 720–727. doi:10.1038/s41586-024-07472-3
Tucci, V., Isles, A. R., Kelsey, G., and Ferguson-Smith, A. C.Erice Imprinting Group (2019). Genomic imprinting and physiological processes in mammals. Cell 176 (5), 952–965. doi:10.1016/j.cell.2019.01.043
Vagero, D., Pinger, P. R., Aronsson, V., and van den Berg, G. J. (2018). Paternal grandfather's access to food predicts all-cause and cancer mortality in grandsons. Nat. Commun. 9 (1), 5124. doi:10.1038/s41467-018-07617-9
Watkins, A. J., Dias, I., Tsuro, H., Allen, D., Emes, R. D., Moreton, J., et al. (2018). Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc. Natl. Acad. Sci. U. S. A. 115 (40), 10064–10069. doi:10.1073/pnas.1806333115
Watkins, A. J., Rubini, E., Hosier, E. D., and Morgan, H. L. (2020). Paternal programming of offspring health. Early Hum. Dev. 150, 105185. doi:10.1016/j.earlhumdev.2020.105185
Weiss, L. A., Pan, L., Abney, M., and Ober, C. (2006). The sex-specific genetic architecture of quantitative traits in humans. Nat. Genet. 38 (2), 218–222. doi:10.1038/ng1726
Werner, R. J., Schultz, B. M., Huhn, J. M., Jelinek, J., Madzo, J., and Engel, N. (2017). Sex chromosomes drive gene expression and regulatory dimorphisms in mouse embryonic stem cells. Biol. Sex. Differ. 8 (1), 28. doi:10.1186/s13293-017-0150-x
White, V., Jawerbaum, A., Mazzucco, M. B., Gauster, M., Desoye, G., and Hiden, U. (2018). IGF2 stimulates fetal growth in a sex- and organ-dependent manner. Pediatr. Res. 83 (1-1), 183–189. doi:10.1038/pr.2017.221
Xue, Y., Gong, Y., Li, X., Peng, F., Ding, G., Zhang, Z., et al. (2023). Sex differences in paternal arsenic-induced intergenerational metabolic effects are mediated by estrogen. Cell Biosci. 13 (1), 165. doi:10.1186/s13578-023-01121-4
Yang, F., Deng, X., Ma, W., Berletch, J. B., Rabaia, N., Wei, G., et al. (2015). The lncRNA Firre anchors the inactive X chromosome to the nucleolus by binding CTCF and maintains H3K27me3 methylation. Genome Biol. 16, 52–17. doi:10.1186/s13059-015-0618-0
Yang, X., Schadt, E. E., Wang, S., Wang, H., Arnold, A. P., Ingram-Drake, L., et al. (2006). Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16 (8), 995–1004. doi:10.1101/gr.5217506
Yeshurun, S., Rogers, J., Short, A. K., Renoir, T., Pang, T. Y., and Hannan, A. J. (2017). Elevated paternal glucocorticoid exposure modifies memory retention in female offspring. Psychoneuroendocrinology 83, 9–18. doi:10.1016/j.psyneuen.2017.05.014
You, Y., Liu, R., Zhou, H., Wu, R., Lin, R., Li, B., et al. (2022). Effect of exposure to paternal smoking on overweight and obesity in children: findings from the children lifeway cohort in shenzhen, southern China. Obes. Facts 15 (4), 609–620. doi:10.1159/000525544
Zalewska, T., Pawelec, P., Ziabska, K., and Ziemka-Nalecz, M. (2022). Sexual dimorphism in neurodegenerative diseases and in brain ischemia. Biomolecules 13 (1), 26. doi:10.3390/biom13010026
Zeender, V., Sbilordo, S. H., Roy, J., and Lüpold, S. (2023). Paternal condition affects offspring reproduction and life history in a sex-specific manner in Drosophila melanogaster. Evolution 77 (2), 467–481. doi:10.1093/evolut/qpac051
Zheng, X., Li, Z., Wang, G., Wang, H., Zhou, Y., Zhao, X., et al. (2021). Sperm epigenetic alterations contribute to inter- and transgenerational effects of paternal exposure to long-term psychological stress via evading offspring embryonic reprogramming. Cell Discov. 7 (1), 101. doi:10.1038/s41421-021-00343-5
Keywords: sexual dimoprhism, developmental programing, paternal inheritance, epigenetics, sperm RNAs, seminal plasma, imprinting
Citation: Aljabali SM, Pai S and Teperino R (2024) Paternal impact on the developmental programming of sexual dimorphism. Front. Cell Dev. Biol. 12:1520783. doi: 10.3389/fcell.2024.1520783
Received: 31 October 2024; Accepted: 25 November 2024;
Published: 06 December 2024.
Edited by:
Marta Lombó, University of León, SpainReviewed by:
Veronica Porreca, Sapienza University of Rome, ItalyAntonio Suglia, INSERM U1085 Institut de Recherche en Santé, Environnement et Travail, France
Copyright © 2024 Aljabali, Pai and Teperino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffaele Teperino, cmFmZmFlbGUudGVwZXJpbm9AaGVsbWhvbHR6LW11bmljaC5kZQ==
†These authors have contributed equally to this work and share first authorship
 Shefa’ M. Aljabali
Shefa’ M. Aljabali Shruta Pai
Shruta Pai Raffaele Teperino
Raffaele Teperino