
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol., 03 June 2024
Sec. Molecular and Cellular Reproduction
Volume 12 - 2024 | https://doi.org/10.3389/fcell.2024.1335028
Introduction: Epidemiological evidence over the last few decades has consistently shown that exposure to endocrine-disrupting chemicals (EDCs) is associated with adverse reproductive health outcomes, including male and female infertility, poor-pregnancy outcomes, and increased risk of diseases in childhood and beyond. To investigate the effects of EDCs and lifestyle on all aspects of reproduction (including early oocyte development, fertilization, embryo development, embryo implantation, abortion, and preterm birth).
Methods: We performed this cohort study on patients receiving in vitro fertilization (IVF) treatment. Biological samples including urine, serum, follicular fluid, semen, fetal tissue, decidua, and placenta, were obtained.
Results: By studying the correlations between reproductive outcomes and environmental pollutant exposure and lifestyle, we determined the toxicological mechanisms and health effects of EDCs on female reproductive health. We found that higher concentrations of per- and polyfluoroalkyl substances were correlated with polycystic ovary syndrome (PCOS). Using specific biomarkers, we also detected the concentrations of organophosphorus flame retardants (OPFRs) in urine and found that OPFRs may disrupt hormone homeostasis.
Discussion: All of these results reveal EDCs may disrupt female reproduction.
According to the World Health Organization, about 15% of women of childbearing age worldwide suffer from infertility (Huang et al., 2024), and by the end of the 21st century, infertility will become the third major class of disease in the world following cancer and cardiovascular disease (Attina et al., 2016). The prevalence of infertility among women of childbearing age in China is 15%–20% (Gao et al., 2022). Epidemiological evidence over the last few decades has consistently shown that endocrine-disrupting chemicals (EDCs) are associated with adverse reproductive health outcomes, including male and female infertility, poor pregnancy outcomes, and increased risk of diseases in childhood and beyond (Harley et al., 2010; Bergman et al., 2013; Messerlian et al., 2016). The Environment and Reproductive Health (EARTH) study enrolled couples undergoing in vitro fertilization (IVF)/embryo transfer (ET) treatment to explore the effects of environmental chemicals and lifestyle factors (such as diet and smoking) (Messerlian et al., 2018). In the 21st century, Chinese scholars focus on the influence of environmental factors and social, psychological, and biological factors on the reproductive outcomes of parents and offspring during pregnancy. However, current studies have several limitations, such as small samples and inconsistent results. To resolve these problems, we designed a cohort study with a larger sample in 2018. This cohort study enrolled couples receiving in vitro fertilization–embryo transfer (IVF-ET) treatment to explore all aspects of human reproduction (including sperm quality, oocyte quality, fertilization, embryo development, embryo implantation, abortion, and preterm birth). Biological samples including urine, serum, follicular fluid, semen, fetal tissue, decidua, and placenta were obtained. Follicular fluid, as the fluid human oocytes are directly exposed to in vivo, was shown to be an important research medium for studying the effects of EDCs on the quality and development of oocytes (Basuino and Silveira, 2016). Therefore, it is better to study the concentrations of EDCs in follicular fluid than in blood to determine their effects on oocytes and female reproductive outcomes overall.
In 2018, we established the cohort study Peking University Environmental Reproductive Health Cohort (PKU-ERC), an ongoing perspective cohort of couples seeking care at the Reproductive Medical Center, Department of Obstetrics and Gynecology, Peking University Peoples’ Hospital, Beijing, China. Clinical physicians from the Reproductive Medical Center identified potentially eligible patients in clinical practice. Women aged 18–40 years who came to the reproductive center for their first IVF/ICSI treatment were recruited for the cohort study. Patients who received donor egg transplantation and hormone drug treatment 3 months before their first visit were excluded. Informed consent was provided by all participants who agreed to participate in the study. This study was approved by the Ethics Committee of Peking University People’s Hospital.
The study started in January 2018 and has recruited 1,883 couples through December 2022.
The basic personal information and samples of all enrolled patients at different stages were collected according to the established follow-up timeline (Figures 1, 2). After the patients were enrolled, the basic personal information about the enrolled couples was collected through questionnaires. First, patients provided personal information, including age, height, weight, occupation, education background, daily medication, personal and family member health, physical labor and exercise, and female reproductive history. The living environment and workplace environment, mainly including the time of exposure to decorations and their materials, ventilation time, and indoor exposure time, were also included in the questionnaire. In addition, the lifestyle questionnaires cover all aspects of lifestyle according to the Kadoorie Study of Chronic Disease in China (KSCDC) (Chen et al., 2005), including drinking, smoking, and coffee consumption. We surveyed the habits of personal and family skin products, cleaning agents, and other chemicals, such as lotions, soaps, house cleaning chemicals, plastics, pesticides, and weight-loss products. Patient depression and anxiety were also assessed using a psychiatric questionnaire.
The female specimens included blood, urine, follicular fluid, villi from miscarriages, umbilical cord blood, and placenta. Male specimens included blood, urine, and semen. Women provided two fasting blood samples (on day 2 of the menstruation within 3 months before ovulation induction treatment and 3–9 days after ovulation induction). Women also provided fasting urine in the non-menstrual period within 3 months before ovulation induction therapy. For men, a fasting blood sample and fasting urine were collected before IVF/ICSI treatment. The urine, seminal fluid, and follicular fluid samples were labeled and stored at −80°C. If a woman was diagnosed with miscarriage during the fertility treatment, villi and decidua samples were collected. Placental and umbilical cord blood were collected during delivery. The umbilical cord blood was labeled and stored at −80°C. The villi, decidua, and placental tissue were labeled and stored in liquid nitrogen.
In the IVF-ET treatment, the enrolled population was followed up continuously to the preset time points (Figures 1, 2). Information on the number of oocytes, the quality of the oocytes obtained, the fertilization rate, the number of high-quality embryos, embryonic development, and the number of available embryos was recorded after oocyte retrieval. After the embryo transfer, we checked the pregnancy status, which included a routine β-hCG blood test on day 14 after embryo transfer.
Biochemical pregnancy was defined by a positive pregnancy test (β-hCG). Clinical pregnancy was defined as a pregnancy confirmed by ultrasound visualization of the gestational sac 4 weeks after embryo transfer. Miscarriage was defined as pregnancy loss within 28 weeks of gestation. Live birth was defined as a baby born after 28 weeks of gestation. The neonatal weight, length, mode of delivery, and complications were recorded at delivery.
For the quantification of per- and polyfluoroalkyl substances, we used LC-MS/MS (Thermo Fisher Scientific, San Jose, CA, U.S.A.) as described by Lindh et al. (Lindh et al., 2012). For the quantification of organophosphorus flame retardants, urine sample extraction and analysis of 4 target metabolites (4-hydroxyphenyl diphenyl phosphate, 2-ethyl-5-hydroxyhexyl diphenyl phosphate, phenyl diptolyl phosphate, and 2-ethylhexyl diphenyl phosphate) were performed following the methods developed in our previous study (Zhao et al., 2019). All ultrasound scans were performed by experienced senior physician using a Voluson E8 (GE Medical Systems, USA). Serum hormone levels were measured by automatic chemiluminescence method using ADVIA Centaur XP immunoassay system of Siemens, Germany.
The geometric means (GMs) and distribution percentiles for the urinary concentrations were calculated for all analytes. The correlations between the urinary concentrations of the polyfluoroalkyl substance (PFASs) or aryl-organophosphate ester (OPE) metabolites were calculated as Pearson’s correlation (r). The change in each reproductive hormone concentration was calculated as the difference in the marginal mean from the lowest quartile (Q1) to the highest quartile (Q4) divided by the marginal mean from Q1. The data analysis was performed using SPSS, version 26.0 (IBM Corporation). The statistical significance level of trends was p < 0.05 (Gao et al., 2022).
The height, weight, waist circumference, and blood pressure from the electronic records of each participant were recorded by trained study staff. According to the patient medical history, clinicians diagnosed the etiology of the disease. The ovarian reserve function and specific treatment plan of the patient were recorded in the electronic medical records.
The anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), testosterone (T), and progesterone (P) levels were measured on day 2 of the treatment. The sperm quality and routine hematuria test results were as follows: free thyroxine T4, free thyroxine T3, thyroid-stimulating hormone, thyroxine peroxidase, and urine creatinine; and glomerular filtration rate. Data extracted from the electronic medical records were also recorded in the cohort file. During each fertility treatment cycle, we extracted clinical information from the electronic medical records, including the mode of conception, cycle cancellation, oocyte parameters, early embryo development, implantation, biochemical pregnancy (with β-hCG measurements), clinical pregnancy (with ultrasound assessment), and infertility diagnosis assigned by a physician. We continued to follow up those achieving pregnancy, including their pregnancy complications and pathology, glucose tolerance tests during pregnancy, and delivery outcomes (e.g., livebirths, stillbirths, birthweight, gestational age, infant sex, complications, and pathologies).
All patients were subjected to transvaginal ultrasound examination after 2–4 days of menstruation (except for amenorrhea) using a color Doppler ultrasound diagnostic instrument with a cavity volume probe frequency of 5–9 MHz (both two-dimensional and three-dimensional scanning functions). Before the examination, the patients were asked to empty their bladder, lie flat, and flex their legs. A probe with a sterile condom was placed into the vagina, and two-dimensional ultrasound was routinely performed to observe the structure of bilateral ovaries and determine the number of antral follicle counts (AFCs) that had a diameter of 2–9 mm in both ovaries (Hao et al., 2023).
We detected concentrations of per- and polyfluoroalkyl substances in follicular fluid to explore their adverse effects on the reproductive health of women. We tested the concentrations of 27 perfluorinated compounds in follicular fluid. Detailed information on the demographic and clinical characteristics of the participants is presented in Table 1. The levels of AMH and LH significantly increased with increasing concentrations of per- and polyfluoroalkyl substances (Table 2). AFCs increased with the concentrations of per- and polyfluoroalkyl substances. Regression analyses found a concentration-dependent positive correlation between their concentrations and the incidence of PCOS.
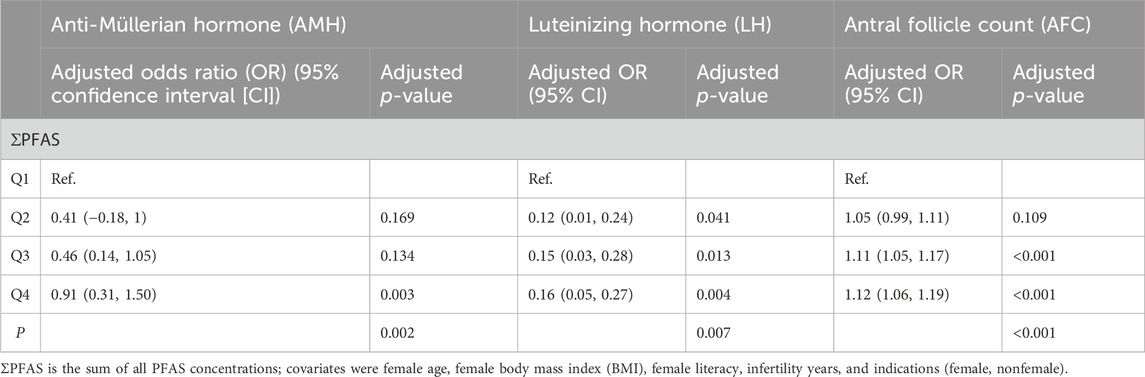
Table 2. Reproductive hormone levels and antral follicle counts (AFCs) by quartile of polyfluoroalkyl substance (PFAS) concentrations among 880 women.
We measured organophosphorus flame retardants (OPFRs) in the urine of 913 of 1,286 eligible participants. Except three hydroxylated metabolites of 2-ethylhexyl diphenyl phosphate (EHDPP), all aryl-OPE–triphenyl phosphate (TPhP), tricresyl phosphate (TCrP), and the diester compound diphenyl phosphate (DPhP) were detected in the urine samples. The target metabolites 5-OH-EHDPP, 4-OH-MDTP, DPhP, and 4-OH-TPhP were detected in the urine samples at frequencies of 94.6% (864/913), 93.3% (852/913), 92.1% (841/913), and 84.2% (769/913), respectively. The reproductive hormone levels were reported as quartiles of urinary aryl-organophosphate ester metabolite concentrations according to previous studies for relevant results (Gao et al., 2022). The results showed that the quartile of 4-hydroxyphenyl diphenyl phosphate (4-OH-TPhP) was positively associated with the progesterone (P) level (p trend = 0.008), and the P level in the highest quartile of 2-ethyl-5-hydroxyhexyl diphenyl phosphate (5-OH-EHDPP) was 7.2% (95% CI, 5.7%–8.7%) greater than that in the lowest quartile (Gao et al., 2022). The 17β-estradiol levels in the highest quartiles of 4-OH-TPhP and 5-OH-EHDPP were 15.0% (95% CI, 13.7%–16.1%) and 5.9% (95% CI, 15.7%–16.1%) lower than those in the lowest quartile, respectively. The anti-Müllerian hormone level linearly increased across the phenyl diptolyl-phosphate (4-OH-MDTP) quartiles (p trend = 0.036), and the follicle-stimulating hormone level exhibited the opposite trend (p trend = 0.0047). These results indicate that aryl-OPEs may disrupt hormone homeostasis, according to their specific biomarkers, and may negatively affect female reproduction.
To explore the influence of environmental factors on infertility, this study collected biological specimens (blood, urine, follicle fluid, serum, etc.); case reports; environmental exposure factor reports; lifestyle, diet, exercise, and mental health questionnaires from the enrolled patients; and follow-up data on ovulation induction, pregnancy outcomes, and delivery outcomes. By studying the correlations between the reproductive outcomes and exposure to environmental pollutants and lifestyle, the reproductive toxicology mechanism and health effects of environmental endocrine disruptors were revealed, laying a theoretical and technical basis for the systematic evaluation of the effects of endocrine-disrupting chemicals on female reproductive health.
Per- and polyfluoroalkyl substances and organophosphorus flame retardants are endocrine-disrupting chemicals that can disturb normal reproductive outcomes and cause adverse pregnancy outcomes. Per- and polyfluoroalkyl substances refer to hydrogen atoms on one or more carbon chains that are partially or completely depleted by fluorine atoms. PFASs are a class of substituted compounds with strong thermal stability, chemical stability, and excellent surface activity (Ding et al., 2020). Therefore, they are widely used in industrial production, packaging materials, fire foam, metal plating, and pesticides (Ding et al., 2020). PCOS is a heterogeneous disorder characterized by unclear etiopathogenesis that likely involves genetic and environmental components that synergistically contribute to its phenotypic expression (Xu et al., 2024). Its main manifestations include reproductive dysfunction characterized by abnormal follicular development and hyperactivity of steroid hormone synthesis and glucose metabolism disorders characterized by insulin resistance or associated obesity and hyperinsulinemia (Xu et al., 2024). For women of childbearing age, the main manifestation is being affected by continuous anovulation or nonovulation. Evidence from animal experiments shows that PFOS exposure inhibits the maturation and ovulation of mouse oocytes (Feng et al., 2015; Wang et al., 2018). A case–control study in China (n = 367) found that the serum concentration of perfluorododecanoic acid (PFDoDA) was significantly associated with PCOS, while the serum concentration of PFOS, PFOA, and other per- and polyfluoroalkyl substances was not associated with PCOS (Wang et al., 2019). Polycystic ovary syndrome has been associated with polymorphisms caused by peroxisome proliferator-activated receptor γ (PPARγ) (Y and Valsala Gopalakrishnan, 2020). The PPARγ agonist valproic acid (an antiepileptic drug) can cause symptoms associated with polycystic ovary syndrome (Cutia and Christian-Hinman, 2023). PFHxS, PFOS, PFOA, PFNA, and PFDA are agonists of PPARγ (Li et al., 2020). Human exposure to PFAS may affect female ovarian development and lead to female infertility. PPARγ activation downregulates aromatase, the key enzyme for E2 synthesis in human granulosa cells, and upregulates the expression of 3β-HSD I mRNA (the key enzyme for progesterone biosynthesis) in theca and granulosa cells to promote progesterone secretion (Hiromori et al., 2016). Our other study found that the E2 concentration is positively correlated with increasing concentrations of 4-OH-TPhP and 5-OH-EHDPP. In vitro studies have found that TPhP and EHDPP can also activate PPARγ, and the PPARγ agonistic activity may explain the association with E2 levels for TPhP and EHDPP. Moreover, EHDPP elicited stronger PPARγ agonistic activity (2.04 μM) than TPhP did (2.78 μM) (Hu et al., 2017; Yao et al., 2023). PPARγ also plays an essential role in hormone synthesis in granulosa cells and the placenta (Yoon et al., 2020).
This study has several strengths. First, it had a relatively large sample and data on endpoints. Second, follicular fluid are human oocytes directly exposed, were collected. The concentration of the target organ is more likely to represent the actual level of PFAS entering the female reproductive system. This study had a detailed design, included registered couples with complete information, and controlled for potential confounders. Its main limitation is that it was conducted in women undergoing IVF. Whether the results can be generalized to all women needs to be carefully evaluated.
The data of this study are not openly available and are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to HS, cm1pdmZAc2luYS5jb20=.
The studies involving humans were approved by the Scientific Research Development Fund of Peking University People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SZ: data curation, investigation, and writing–original draft. FG: data curation, funding acquisition, investigation, and writing–original draft. MF: investigation, resources, and writing–original draft. QZ: data curation, investigation, and writing–original draft. JG: funding acquisition, resources, and writing–original draft. HS: funding acquisition, supervision, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Scientific Research Development Fund of Peking University People’s Hospital (RD2020-27), the project Surgical Technique for Preserving Fertility Function in Tubal Pregnancy Under Laparoscope (BHTPP2022009), and the National Natural Science Foundation of China (21737001).
The authors thank the nurses at the Reproductive Center of Peking University People’s Hospital for their help in sample collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Attina, T. M., Hauser, R., Sathyanarayana, S., Hunt, P. A., Bourguignon, J.-P., Myers, J. P., et al. (2016). Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes and Endocrinol. 4 (12), 996–1003. doi:10.1016/S2213-8587(16)30275-3
Basuino, L., and Silveira, C. F. (2016). Human follicular fluid and effects on reproduction. JBRA Assist. Reprod. 20 (1), 38–40. doi:10.5935/1518-0557.20160009
Bergman, A., Heindel, J. J., Kasten, T., Kidd, K. A., Jobling, S., Neira, M., et al. (2013). The impact of endocrine disruption: a consensus statement on the state of the science. Environ. Health Perspect. 121 (4), A104–A106. doi:10.1289/ehp.1205448
Chen, Z., Lee, L., Chen, J., Collins, R., Wu, F., Guo, Y., et al. (2005). Cohort profile: the Kadoorie study of Chronic disease in China (KSCDC). Int. J. Epidemiol. 34 (6), 1243–1249. doi:10.1093/ije/dyi174
Cutia, C. A., and Christian-Hinman, C. A. (2023). Mechanisms linking neurological disorders with reproductive endocrine dysfunction: Insights from epilepsy research. Front. Neuroendocrinol. 71, 101084. doi:10.1016/j.yfrne.2023.101084
Ding, N., Harlow, S. D., Randolph, J. F., Loch-Caruso, R., and Park, S. K. (2020). Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 26 (5), 724–752. doi:10.1093/humupd/dmaa018
Feng, X., Wang, X., Cao, X., Xia, Y., Zhou, R., and Chen, L. (2015). Chronic exposure of female Mice to an environmental level of Perfluorooctane Sulfonate Suppresses Estrogen synthesis through Reduced Histone H3K14 Acetylation of the StAR promoter leading to Deficits in follicular development and ovulation. Toxicol. Sci. Official J. Soc. Toxicol. 148 (2), 368–379. doi:10.1093/toxsci/kfv197
Gao, F., Zhang, X., Shen, X., Zhao, F., Shen, H., and Hu, J. (2022). Exposure assessment of aryl-organophosphate esters based on specific urinary biomarkers and their associations with reproductive hormone homeostasis disruption in women of childbearing age. Environ. Int. 169, 107503. doi:10.1016/j.envint.2022.107503
Hao, Y., Wang, Y., Yan, L., Xu, X., Chen, D., Zhao, Y., et al. (2023). Synthetic Phenolic Antioxidants and their metabolites in follicular fluid and association with Diminished ovarian reserve: a case-control study. Environ. Health Perspect. 131 (6), 67005. doi:10.1289/EHP11309
Harley, K. G., Marks, A. R., Chevrier, J., Bradman, A., Sjodin, A., and Eskenazi, B. (2010). PBDE concentrations in women's serum and fecundability. Environ. Health Perspect. 118 (5), 699–704. doi:10.1289/ehp.0901450
Hiromori, Y., Yui, H., Nishikawa, J.-i., Nagase, H., and Nakanishi, T. (2016). Organotin compounds cause structure-dependent induction of progesterone in human choriocarcinoma Jar cells. J Steroid Biochem Mol Biol. 155 (Pt B), 190–198. doi:10.1016/j.jsbmb.2014.10.010
Hu, W., Gao, F., Zhang, H., Hiromori, Y., Arakawa, S., Nagase, H., et al. (2017). Activation of peroxisome proliferator-activated receptor Gamma and disruption of progesterone synthesis of 2-ethylhexyl diphenyl phosphate in human placental choriocarcinoma cells: Comparison with triphenyl phosphate. Environ. Sci. Technol. 51 (7), 4061–4068. doi:10.1021/acs.est.7b00872
Huang, R., Yu, J. Y., He, W. C., and Liu, R. H. (2024). Feasibility analysis of China's medical insurance coverage of assisted reproductive technology. Sci. Rep. 14 (1), 7998. doi:10.1038/s41598-024-58640-4
Li, C.-H., Shi, Y.-L., Li, M., Guo, L.-H., and Cai, Y.-Q. (2020). Receptor-bound Perfluoroalkyl Carboxylic acids Dictate their activity on human and mouse peroxisome proliferator-activated receptor γ. Environ. Sci. Technol. 54 (15), 9529–9536. doi:10.1021/acs.est.0c02386
Lindh, C. H., Rylander, L., Toft, G., Axmon, A., Rignell-Hydbom, A., Giwercman, A., et al. (2012). Blood serum concentrations of perfluorinated compounds in men from Greenlandic Inuit and European populations. Chemosphere 88 (11), 1269–1275. doi:10.1016/j.chemosphere.2012.03.049
Messerlian, C., Williams, P. L., Ford, J. B., Chavarro, J. E., Minguez-Alarcon, L., Dadd, R., et al. (2018). The environment and reproductive health (EARTH) study: a Prospective Preconception cohort. Hum. Reprod. Open 2018 (2), hoy001. doi:10.1093/hropen/hoy001
Messerlian, C., Wylie, B. J., Minguez-Alarcon, L., Williams, P. L., Ford, J. B., Souter, I. C., et al. (2016). Urinary concentrations of Phthalate metabolites and pregnancy loss among women Conceiving with medically assisted reproduction. Epidemiology 27 (6), 879–888. doi:10.1097/EDE.0000000000000525
Wang, W., Zhou, W., Wu, S., Liang, F., Li, Y., Zhang, J., et al. (2019). Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ. Pollut. Barking, Essex 1987 247, 824–831. doi:10.1016/j.envpol.2019.01.039
Wang, X., Bai, Y., Tang, C., Cao, X., Chang, F., and Chen, L. (2018). Impact of Perfluorooctane Sulfonate on reproductive Ability of female Mice through Suppression of Estrogen receptor α-activated Kisspeptin Neurons. Toxicol. Sci. Official J. Soc. Toxicol. 165 (2), 475–486. doi:10.1093/toxsci/kfy167
Xu, X., Zhang, X., Chen, J., Du, X., Sun, Y., Zhan, L., et al. (2024). Exploring the molecular mechanisms by which per- and polyfluoroalkyl substances induce polycystic ovary syndrome through in silico toxicogenomic data mining. Ecotoxicol. Environ. Saf. 275, 116251. doi:10.1016/j.ecoenv.2024.116251
Y, D. P., and Valsala Gopalakrishnan, A. (2020). γ-Linolenic acid ameliorates DHEA induced pro-inflammatory response in polycystic ovary syndrome via PPAR-γ signaling in rats. Reprod. Biol. 20 (3), 348–356. doi:10.1016/j.repbio.2020.05.004
Yao, W., Liu, C., Qin, D. Y., Yuan, X. Q., Yao, Q. Y., Li, N. J., et al. (2023). Associations between Phthalate metabolite concentrations in follicular fluid and reproductive outcomes among women undergoing in vitro fertilization/Intracytoplasmic sperm Injection treatment. Environ. Health Perspect. 131 (12), 127019. doi:10.1289/EHP11998
Yoon, S. Y., Kim, R., Jang, H., Shin, D. H., Lee, J. I., Seol, D., et al. (2020). Peroxisome proliferator-activated receptor Gamma Modulator promotes neonatal mouse Primordial follicle activation in vitro. Int. J. Mol. Sci. 21 (9), 3120. doi:10.3390/ijms21093120
Keywords: environmental disrupting chemicals, reproductive health, cohort study, per- and polyfluoroalkyl substances, organophosphorus flame retardants
Citation: Zhang S, Gao F, Fu M, Zhang Q, Guan J and Shen H (2024) Reproductive toxicology of environmental endocrine-disrupting chemicals in women: a cohort study protocol. Front. Cell Dev. Biol. 12:1335028. doi: 10.3389/fcell.2024.1335028
Received: 08 November 2023; Accepted: 30 April 2024;
Published: 03 June 2024.
Edited by:
Asok K. Dasmahapatra, University of Mississippi, United StatesReviewed by:
Anitha Myla, Mississippi State Department of Health, United StatesCopyright © 2024 Zhang, Gao, Fu, Zhang, Guan and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Shen, cm1pdmZAc2luYS5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.