
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 16 November 2023
Sec. Stem Cell Research
Volume 11 - 2023 | https://doi.org/10.3389/fcell.2023.1278278
This article is part of the Research TopicCoordinated Regulation of the Balance Between Stem Cell Self-renewal and DifferentiationView all 5 articles
Hair follicle (HF) homeostasis is regulated by various signaling pathways. Disruption of such homeostasis leads to HF disorders, such as alopecia, pigment loss, and hair aging, which is causing severe health problems and aesthetic concerns. Among these disorders, hair aging is characterized by hair graying, hair loss, hair follicle miniaturization (HFM), and structural changes to the hair shaft. Hair aging occurs under physiological conditions, while premature hair aging is often associated with certain pathological conditions. Numerous investigations have been made to determine the mechanisms and explore treatments to prevent hair aging. The most well-known hypotheses about hair aging include oxidative stress, hormonal disorders, inflammation, as well as DNA damage and repair defects. Ultimately, these factors pose threats to HF cells, especially stem cells such as hair follicle stem cells, melanocyte stem cells, and mesenchymal stem cells, which hamper hair regeneration and pigmentation. Here, we summarize previous studies investigating the above mechanisms and the existing therapeutic methods for hair aging. We also provide insights into hair aging research and discuss the limitations and outlook.
Hair follicles (HFs) are constituted by different cell types, including hair follicle stem cells (HFSCs), non-HFSC epithelial cells, immune cells, neurons, mesenchymal cells, adipocytes, and melanocytes. Other structures, such as sebaceous glands (SGs), blood vasculature, and arrector pili muscle (APM), are also important HF components (Lei et al., 2021) (Figure 1). Generally, HF status depends on the hair cycle, which can be roughly divided into three stages, including anagen (the growing phase), catagen (the transition phase), and telogen (the resting phase). These phases are modulated by genes, age, microenvironment, diet, and psychological factors (Alonso and Fuchs, 2006). HF homeostasis is disrupted due to aging, gene mutations, nutritional imbalance, hormonal dysregulation, the inflammatory microenvironment, etc., which will lead to various HF disorders such as hair aging (Palmer et al., 2020). Although hair-related diseases are not life-threatening, they can significantly influence people’s social activities and psychological wellbeing (Mercke et al., 2000; Cotsarelis and Millar, 2001). Among these disorders, hair aging is manifested by hair graying, hair loss, hair thinning, hair follicle miniaturization (HFM), structural changes, lipid composition change, and curvature in the hair fiber (Trüeb, 2006). There are multiple causes of hair aging, including genetic defects, systemic diseases, ultraviolet (UV) radiation, nutritional imbalance, environmental pollution, and physical damage (D’Orazio et al., 2013).
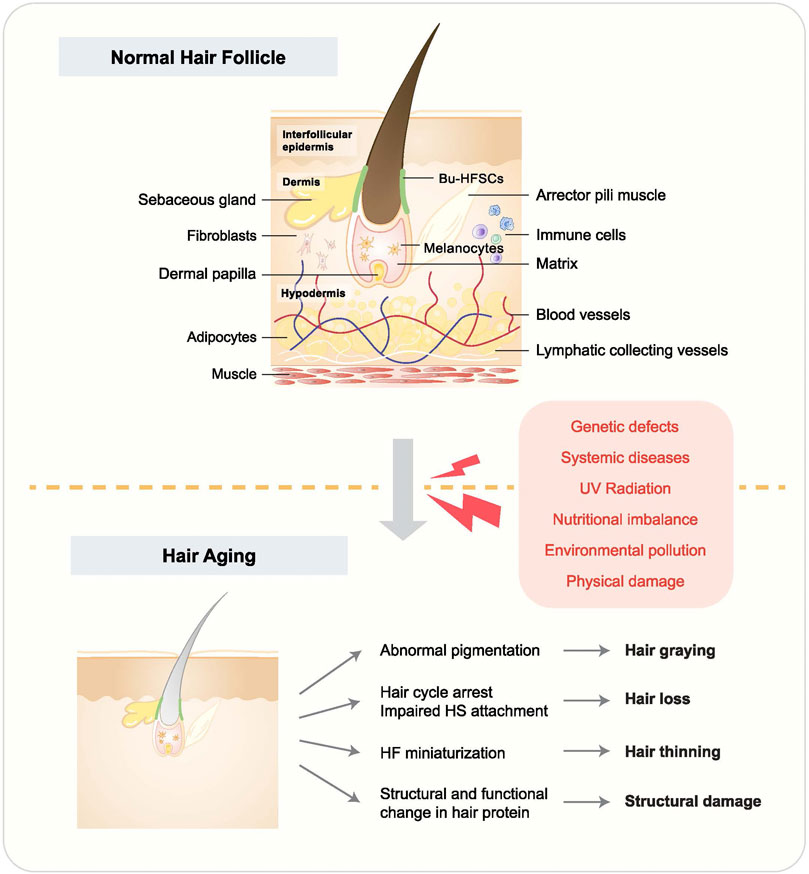
FIGURE 1. Hair follicle structure and hair aging phenotypes. HF accommodates multiple tissues and cells, and their malfunction leads to hair aging. Causes of hair aging are listed in the red box. Hair aging is largely manifested by hair graying, hair loss, hair thinning, and hair shaft structural change.
Hair aging is often accompanied by hair graying, hair loss, and hair thinning (Figure 1). The hair pigmentation process starts with melanocyte stem cells (McSCs), which differentiate into melanocytes to produce pigmentation units. During anagen, melanocytes go through mitosis and are activated, manifested by increasing dendricity. Through the dendrites, they can transfer melanosomes, which contain melanin (Van Neste and Tobin, 2004; Tobin, 2008). Hair graying happens when the pigmentation process is disrupted (Nishimura et al., 2005). For example, it was recently reported that McSCs could switch between transit-amplifying status and quiescence status and reside in a dynamic niche, indicating a potential role of McSC mobility in regulating cell stemness and hair graying (Sun et al., 2023). Hair loss, however, is mostly related to HFSC dysfunction and depletion. Physiologically, HFSCs are activated at anagen and stay quiescent at telogen. Whereas, in alopecia, HFSCs are depleted or remain in a quiescent status, leading to irreversible or reversible hair loss, respectively (Hu X.-M. et al., 2021). HFSCs are regulated by intrinsic and extrinsic cues, such as Wnt and bone morphogenetic protein (BMP) signaling, as well as skin wounding (Kandyba et al., 2013; Lee et al., 2021). Hair thinning can be a transitional status before hair loss, frequently occurring with HFM, which is manifested by the reduction of the diameter of HFs and hair shaft (Anastassakis, 2022).
Numerous theories exist about the primary mechanism underlying hair aging. The most well-known one is the thesis of oxidative stress, which accounts for multiple kinds of cell dysfunction such as mitochondrial damage and upregulated inflammatory signaling (Williams et al., 2020). Additionally, extensive research is being done on other possibilities, including hormone-induced premature hair aging, inflammation-predominant hair aging, and DNA damage-driven hair aging (Figure 2). The following sections will give detailed depictions of these concepts. In this review, we try to outline and update the signaling pathway underlying these hair aging hypotheses and provide insights into the current progress and limitations of hair aging research.
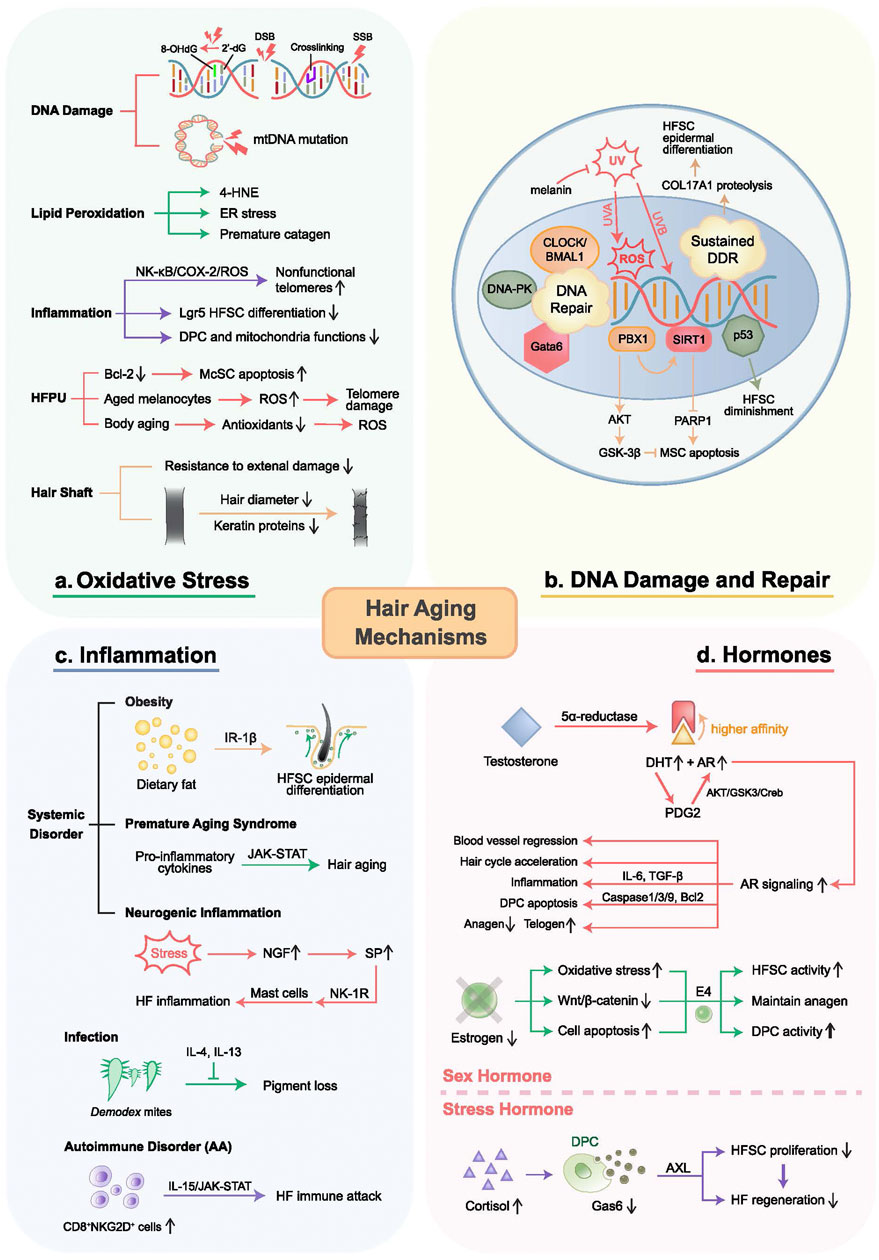
FIGURE 2. Mechanisms of hair aging. Proposed mechanisms of hair aging include oxidative stress, inflammation, DNA damage and repair, as well as hormone disorders. (A) Oxidative stress causes DNA damage, protein modifications, lipid oxidation, chronic inflammation, and HFPU damage. (B) DNA damage can be caused by UV and genetic defects such as p53 and p63, leading to HF-residing stem cell dysfunctions through the PBX1/AKT/GSK-3β and PBX1/SIRT1/PARP1 axes. Sustained DNA damage-induced DDR results in HFSC epidermal differentiation. Cell resistance to DNA damage is determined by DNA repair capacity, regulated by CLOCK/BMAL1, DNA-PK, and Gata6. (C) Inflammation results from systemic disorders, infections, and autoimmune disorders, mediated by inflammatory signaling and immune cell attack. (D) Androgens activate AR signaling, upregulating genes related to hair growth inhibition, apoptosis, vascular regression, DPC aging, and hair aging, exacerbated by PGD2. Conversely, estrogens maintain the hair cycle, protect against oxidative stress, and promote follicle health. E4 induces DP fibroblasts, increasing anagen follicles and CD34+ cells in the outer root sheath to delay HF aging. Chronic stress accelerates hair loss and follicle aging through the cortisol-Gas6-AXL axis. DSB, double-strand break; SSB, single-strand break.
The Free Radical Hypothesis was first posited in the 1950s and is widely used to elucidate the mechanisms underlying the aging process (Arck et al., 2006). It is stated that aging is caused by the built-up redox damage to the biomolecules due to the imbalance in the oxidation-reduction processes (Arck et al., 2006). As an evolved version of the theory, mitochondrial dysfunction, has been highlighted as the major contributor of oxidative stress. It is stated that oxidative stress may lead to mitochondrial DNA (mtDNA) mutations and deletions (Ziada et al., 2020). Oxidative damage is mainly created by excessive reactive oxygen species (ROS), primarily produced during metabolic process (Liu et al., 2022).
ROS comprises a group of highly reactive oxygen molecules or free radicals, mostly generated in mitochondria during oxidative phosphorylation, such as superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH) (Balaban et al., 2005). External factors such as UV radiation, ionizing radiation, and environmental toxins may also increase levels of ROS (Tirichen et al., 2021). Although most cells have certain capabilities to clear up ROS, oxidative damage can still occur when the antioxidant systems are overwhelmed by the adverse impacts of elevated ROS levels, leading to premature aging.
Current research has demonstrated that mechanisms underlying oxidative damage in HFs mainly involve ROS-induced DNA damage, impaired antioxidant enzyme activity, oxidative modification of hair proteins, induction of chronic inflammation, and damage to hair follicle pigmentary unit (HFPU) (Trüeb, 2021).
ROS can damage DNA through various mechanisms. ROS, particularly hydroxyl radicals (•OH), can directly react with the DNA molecule’s bases, causing base oxidation, deoxyribose damage, and DNA strand breaks. For instance, ROS can cause base oxidation including 8-oxoguanine and 8-hydroxy-2′-deoxyguanosine (8-OHdG), as being pivotal markers for measuring the effect of endogenous oxidative damage to DNA. These modifications disrupt normal base pairing, interfering with DNA replication and transcription (Tarng et al., 2000). Moreover, recent research has indicated that ROS might reduce the fidelity of mitochondrial polymerase, potentially leading to an increase in somatic transition mutations (Anderson et al., 2020). In addition to that, another role of ROS is to serve as signaling molecules and affect the generation of mitochondria, further contributing to the spread of mtDNA mutations (Napoli et al., 2013; Martins et al., 2021).
Moreover, ROS can oxidize unsaturated fatty acids, thus establishing structural and functional changes in the cell membrane, involving loss of selective permeability, unusual opening of ion channels, etc. For example, 4-hydroxynonenal (4-HNE) is a reactive aldehyde generated from the reaction between ROS and polyunsaturated fatty acids in lipids (Barrera, 2012; Juan et al., 2021). 4-HNE forms adducts with protein amino acid residues (mainly cysteine, histidine, lysine) through Michael addition and Schiff base formation, leading to protein crosslinking and aggregation (Su et al., 2019). Additionally, 4-HNE can further activate the Keap1/Nrf2/ARE pathway, consequently promoting oxidative stress. In addition, lipid peroxidation can cause endoplasmic reticulum (ER) stress, leading to protein misfolding and aggregation (Milkovic et al., 2023). Specifically, Naito et al. found that topical application of linolein hydroperoxides, a type of lipid peroxide, induced premature catagen phase onset in murine hair cycles (Csala et al., 2015). Furthermore, with age, the activities of superoxide dismutase (SOD) and catalase (CAT) are diminished, and their ability to clear the ROS is impaired, thereby increasing susceptibility to oxidative damage (Naito et al., 2008).
Oxidative stress can also trigger oxidative modifications in hair proteins, such as keratin (Rybka et al., 2013). The concomitant structural and functional changes in the proteins can result in hair shaft aging. Studies have found that aged hair has a reduced diameter and decreased keratin-associated proteins (KAPs) (Trüeb, 2009). Additionally, oxidative stress can lead to lipid peroxidation in the hair shaft, particularly peroxidation of cholesterol, thus abolishing its abilities to protect the hair shaft from chemical damage, to prevent hair softening, to reduce diameter shrinkage, and to preserve hair shine (Giesen et al., 2011).
Recent studies have demonstrated that ROS can upregulate the transcription of various proinflammatory mediators and impair tissue regeneration (Brosche et al., 2001; Shafiq et al., 2021). For example, the diminished tissue regenerative potential and accelerated aging in nfkb1−/− mice were shown to be associated with chronic activation of NF-κB/COX-2/ROS axis by inflammation, which resulted in the accumulation of nonfunctional telomeres (Jurk et al., 2014; Mittal et al., 2014). Additionally, Yang, et al. revealed that wound-induced IL-1α facilitates the differentiation of leucine-rich repeat G protein-coupled receptor 5 (Lgr5) HFSCs by decreasing inflammatory cell infiltration and ROS levels, thereby promoting the neogenesis of aged hair follicles (Zhang et al., 2016). Furthermore, it was documented that Argan press cake can protect DPCs from H2O2-induced oxidative stress by attenuating inflammation and downregulating ROS-associated gene expression, thus restoring cellular viability and mitochondrial function. However, there is no direct evidence that ROS-induced inflammation accelerates hair aging (Yang et al., 2023). While studies have demonstrated that restraining ROS and inflammation can facilitate HF regeneration, the precise mechanisms by which ROS and inflammation directly impact HF aging or impede its regeneration remain to be fully elucidated.
Furthermore, hair aging is commonly manifested by pigment loss due to dysfunction of HFPU, which is highly sensitive to ROS. Bcl-2, an anti-apoptotic protein, maintains the redox balance within the follicular stem cell niche. It is reported that depletion of Bcl-2 exacerbates oxidative stress, inducing McSCs selective apoptosis and consequently hampering the repopulation of melanocytes in anagen follicles (Bejaoui et al., 2021). Research also indicates that with age, the clearance capacity of ROS declines, resulting in ROS accumulation that damages HFPU, leading to hair graying (Seiberg, 2013). Consistent with Harman’s original free radical aging thesis, another hypothesis suggests that melanin can act as an antioxidant, but the process of melanin synthesis itself generates a substantial amount of ROS (Arck et al., 2006). This underscores the significance of maintaining a harmonious redox balance in young and healthy HF, signifying the importance of properly-working ROS clearance machinery. Additionally, similar to the skin aging induced by senescent epidermal melanocytes, it is believed that HF melanocytes are likely to serve as cellular stress sensors, and paracrine signals from aging melanocytes in the HFPU increase ROS production and induce telomere damage, accelerating HFPU aging (O’Sullivan et al., 2021).
Androgens, such as testosterone and DHT, are primarily secreted by the adrenal glands, the testicles in the male reproductive system, and the ovaries in the female reproductive system (Stone et al., 2021). Testosterone and DHT influence HFs by binding to androgen receptors (AR) within the follicular cells. Testosterone can be converted into DHT by 5α-reductase, with a higher affinity of approximately 5–10 folds for ARs. In the balding scalp of male pattern hair loss (MPHL) patients, 5α-reductase and DHT were found to increase abnormally, thus overactivating the AR signaling (Handelsman and Feingold, 2000). Consequently, AR signaling upregulated the expression of dikkopf-related protein 1 (DKK-1), an inhibitor of WNT signaling, IL-6, and transforming growth factor β (TGF-β). As a result, hair growth and the telogen-to-anagen transition were inhibited (Ustuner, 2013). Besides, microvascular vessel aggregation around DP is crucial for anagen initiation (Cerut et al., 2018). Recently, Deng et al. reported that AR-induced blood vessel regression around DP in the initial stage of HFM might be one of the causes of pattern hair loss (PHL). Specifically, when ARs in the dermal papilla cells (DPCs) sensed the increase of androgen, they facilitated DKK-1 and TGF-β expression as well as antagonized Wnt/β-catenin pathways to suppress hair shaft growth (Stenn and Paus, 2001). TGF-β further shrank the blood vessels, thus synergistically inhibiting hair growth and promoting the process of HFM (Deng et al., 2022). Interestingly, Jung et al. reported that DHT could promote the accumulation of mitochondrial ROS (mtROS) in DPCs by activating membrane androgen receptor (mAR) signaling. The mtROS accumulation led to the formation of mitochondria-associated ER membranes (MAM) and thus induced mitochondrial Ca2+ accumulation through the interactions between VDAC on mitochondria and IP3R1 on ER, ultimately resulting in increased DPC aging. Also, they found that DPC aging could be mitigated by Cyanidin 3-O-arabinoside through its antioxidative effects and the inhibition of the p38 signaling pathways in an androgenetic alopecia (AGA) mouse model (Rushton et al., 2022).
Moreover, the research conducted by Garza et al. showed that testosterone production was associated with prostaglandin D (PGD) (J et al., 2022). PGD synthase was found to be overexpressed in the alopecia area, and evidence supports that surplus PGD2 can induce precocious catagen as well as shortened anagen (J et al., 2022). Jeong et al. also reported the specific mechanism of PGD2 interacting with AR signaling (Garza et al., 2012). Particularly, PGD2 interacted with the DP2 receptor to upregulate AR expression, thus increasing its downstream effectors such as TGF-β1 and LEF1. PGD2 also induces the expression of cell apoptosis genes such as caspase-3 and caspase-9. Additionally, PGD2 could activate the AKT/(glycogen synthase kinase-3β (GSK-3β)/Creb axis to further enhance AR signaling within hDPCs (Garza et al., 2012).
In short, excessive androgens can induce the HF transition from the anagen to the catagen and telogen, effectively shortening the duration of the anagen. This premature entry into the resting phases leads to hair shedding, thereby contributing to HF aging (Jeong et al., 2018).
Estrogens are also important in hair cycle regulation. Studies have demonstrated that postmenopausal women are more likely to suffer from female pattern hair loss (FPHL) because of reduced ovary-derived estrogen. Conversely, elevated estrogen levels occur with increased anagen HFs, particularly during pregnancy (Ho et al., 2022). Estrogens are also important in maintaining normal HF functions (Hasan et al., 2022). Estrogens can activate the Wnt/β-catenin signaling to maintain HFSC proliferation and differentiation, promoting healthy hair growth (Chen et al., 2020). Estrogens also protect HFs from oxidative damage by regulating the expression of antioxidant enzymes, which slows down HF aging (Choi, 2020). Additionally, estrogens can inhibit the apoptosis pathway within HF cells by affecting the expression and activity of Bcl-2 family proteins and regulating the levels of apoptotic factors (Serruya and Maor, 2021). Furthermore, recent research has revealed that treatment with estetrol (E4), an endogenous estrogen produced by the fetal liver, could significantly increase the number of anagen HFs with enhanced hair matrix keratinocyte proliferation. Moreover, E4 treatment also stimulated DP fibroblast inductivity, as evidenced by an upregulation of versican expression and alkaline phosphatase activity in the DP, particularly in anagen HFs. E4 also promoted the generation of stem cell progeny by increasing the percentage of CD34+ cells (a marker for diverse progenitors) in the outer root sheath (ORS) (Hu et al., 2012). Thus, E4 has high potential for hair loss treatment.
Additionally, chronic stress can accelerate HF aging and hair loss by negatively impacting the activity and proliferation of HFSCs. Corticosterone (equal to cortisol in rodents), a major stress hormone, prevents HFSCs from entering the anagen, mainly through the corticosterone/Gas6/AXL axis. Mechanistically, cortisol binds to glucocorticoid receptors (GR), resulting in Gas6 suppression. Thereby, the AXL receptors cannot be sufficiently stimulated, which aborts HFSC activation and contributes to accelerated HF aging (Gerard et al., 2023).
Inflammation is another factor that causes hair to age. Inflammation is associated with cell death and compromised cell functions. It is reported that inflammation-induced ROS could impair mitochondrial functions, melanosome transfer, and melanocyte migration, leading to hair graying (Choi et al., 2021). In addition, HFSC aging is also largely regulated by inflammatory signals (Ji et al., 2017; Jo et al., 2018), which are closely correlated to hair thinning and hair loss. Generally, HF inflammation occurs in the context of systemic disorders, autoimmune disorders, and infections.
Hair aging happens with systemic disorders such as obesity, progeria diseases, and psychological stress. Morinaga et al. investigated the role of HFSCs in obesity-induced hair loss (Castellana et al., 2014). They discovered that high-fat diet (HFD) led to HFSC epidermal differentiation, mimicking aging HFs, which could be attributable to IL-1 inflammatory signaling. While local IL-1β administration substantially increased Cox2 expression in HFSCs from old mice, Cox2 was not upregulated in HFSCs from young mice, suggesting an age-dependent decrease in resistance to acute inflammatory signals. These results show that obesity-induced inflammatory signals account for premature hair loss, and the effects are age-dependent. Besides, Bedja et al. also observed hair graying and hair loss in atherosclerosis (ApoE−/−) mice supplied with HFD with high cholesterol, together with neutrophil infiltration and increased tumor necrosis factor-stimulated gene-6 (TSG-6) within the skin (Morinaga et al., 2021). A survey conducted by Shin et al. further demonstrated a strong association between obesity and premature hair graying (Bedja et al., 2018).
Systemic progeria diseases are manifested by the senescence of hair, including Hutchinson-Gilford progeria syndrome (HGPS) and Werner syndrome, and they often occur with augmented inflammation (Shin et al., 2015). For example, pro-inflammatory cytokines were found to mediate JAK-STAT signaling overactivation in HGPS (Trüeb, 2005). Other research further confirms the role of JAK-STAT in hair loss. Wang et al. found that JAK-STAT5 activation could induce HFSC quiescence as a downstream target of Oncostatin M (OSM), which was secreted by TREM2+ macrophages (Arnold and Djabali, 2019). Consistently, administration of baricitinib, a JAK inhibitor, was sufficient to blunt cell senescence by downregulating pro-inflammatory signals including IL-6, CXCL8, IL-4, and TNF-ɑ. Notably, baricitinib has recently been approved by the FDA as a treatment for alopecia areata (AA), an autoimmune disorder with phenotypes of hair loss with preserved HFs (Wang et al., 2019). AA is caused by immune privilege breakdown with the aggregation of NK cells, CD4+ T cells, and CD8+ T cells around HFs (Freitas et al., 2023). Among these immune cells, CD8+NKG2D+ cells play a central role in pathogenesis, whose survival depends on the IL-15/JAK-STAT pathway. Consistently, baricitinib, as a JAK inhibitor, was shown to be potent enough to alleviate AA and normalize AA signatures, including IFN-γ pathway as well as cytotoxic T lymphocyte (CTL) (Pratt et al., 2017). Interestingly, several studies have shown that hair aging is also associated with other autoimmune disorders, such as pernicious anemia and autoimmune thyroid disease (Trüeb, 2006).
Premature hair loss and graying can also result from systemic stress and the following neurogenic inflammation, which is generated by nerve activation and can lead to neuropeptide release, connecting the brain and the skin (Jabbari et al., 2015). In contrast to the systemic stress response commonly observed in other organs, neuropeptides can be produced locally in HFs to trigger immune cell infiltration, which causes cytokine-mediated inflammation in HFs (Matsuda et al., 2019). For example, it is indicated that stress-induced nerve growth factor (NGF) promoted substance P (SP) synthesis and release from the nerve ends in HFs. NGF and SP further stimulated neurogenic inflammation, primarily via mast cells (O’Sullivan et al., 2022). SP causes inflammation in HFs mainly through interaction with the neurokinin-1 receptor (NK-1R). Siebenhaar et al. illustrated that the SP/NK-1R could cause mast cell degranulation as well as activate CD8+ T cells in HFs of AA mouse models, indicating profound neurogenic inflammation (Paus et al., 2014). These results suggest that the SP/NK-1R/mast cell pathway plays an important part in psychoemotional stress (Siebenhaar et al., 2007).
Hair aging also happens as a result of infections. Research has shown an age-related microbiome change in scalp skin. Utilizing bacterial 16S rRNA sequencing, they found that aged skin accommodated a greater variety of microbiomes than young skin, and the microbiome exhibited significant proportional change (Liu et al., 2013). The microbiome discrepancy indicates its potential role in hair aging. It has already been demonstrated that HF-residing bacteria are associated with several scalp diseases, including folliculitis decalvans, AA, and AGA (Tirichen et al., 2021). However, how these microbiomes correlate to the hair aging process is still under investigation. Besides bacteria and fungi, microorganisms such as Demodex mites also contribute to hair aging. In common situations, Demodex remains at a low level, with increasing infestation in aging skin (Shibagaki et al., 2017). Upon Demodex infection, Ricardo-Gonzalez et al. observed substantial pigment loss and revealed a significant role of type 2 immunity in defense against Demodex, which was mediated by IL-4 and IL-13 (Lacey et al., 2011). Administration of IL-4 and IL-13 further inhibited HFSC proliferation as well as hair growth, suggesting that Demodex-induced hair aging is mediated through inflammatory signaling.
Another major triggering factor for cell dysfunction and tissue aging is DNA damage. DNA damage induces aging not only by changing the genetic profiles but also by altering the chromatin structure, the length of telomeres, mitochondrial functions, and proteostasis (Vermeij et al., 2014; Ricardo-Gonzalez et al., 2022). Ultimately, DNA damage accumulation is associated with cell transformation, senescence, and apoptosis (Best, 2009; Schumach et al., 2021). This also applies to the hair aging process, with a strong impact on HF-residing stem cells, including HFSCs, McSCs, and mesenchymal stem cells (MSCs). Despite their resistance to DNA damage under physiological conditions, their DNA repair capacity decreases with age (Maynard et al., 2015). Therefore, the influence on hair aging depends on the extent of DNA damage as well as the ability to repair. Here, we introduce multiple causes for DNA damage and how they lead to stem cell dysfunction in HF. We also highlight the role of the DNA repair system in preventing hair aging.
DNA damage in HF cells can be triggered by several factors, such as ultraviolet (UV) radiation, genetic defects, and chemotherapies (Schuler and Rübe, 2013). Photoaging happens through direct or indirect effects, mediated by UVB and UVA, respectively. UVB promotes direct DNA damage by rearranging nucleotides and generating photoproducts such as cyclobutane dimers, while UVA produces ROS to damage mitochondria and DNA (D’Orazio et al., 2013). Zhai et al. indicated that UVA radiation reduced the number of HFSCs and melanocytes in HFs, attributable to HFM and hair graying noted in mice. UVB, on the other hand, was sufficient to make histological alterations to HFs, with thickened epidermis and hyperplastic SGs (Yousefzadeh et al., 2021). It is believed that UV-induced DNA damage can be prevented by melanin, which absorbs UV penetrating through, emphasizing the role of McSCs and melanocytes in photoaging prevention (Zhai et al., 2021).
Genetic dysfunction accounts for hair aging as well, such as p53 and p63 mutations. The p53 transcription factor inhibits cell proliferation and induces cell death to accelerate the aging process, and it is commonly mutated in human cancers (García-Cao et al., 2002; Panich et al., 2016; Gritsenko et al., 2017). The introduction of T21D and S23D mutations in p53 mimics its phosphorylated state, inducing an alteration in Mdm2 binding region, a negative regulator of p53, thus magnifying p53 downstream effects (Rufini et al., 2013; Kim et al., 2014). p53 mutants displayed hair cycle arrest as well as declined SG activity and reduced thickness of subcutaneous adipose, which might contribute to the hair aging process (Rufini et al., 2013). In another study, p53 signaling was overactivated by Mdm2 deletion, resulting in significant hair loss and HFSC diminishment in aged mice but not in young mice. Concomitantly, wound healing capacity was impaired and hair regrowth was delayed, especially in old mice (Liu et al., 2010). Despite the accelerated aging induced by p53, its homolog p63 is able to prevent aging (Gannon et al., 2011). Su et al. demonstrated that the deletion of TAp63, an isoform of p63, triggered premature hair aging and HF loss with enhanced SA-β-gal signals, a cellular senescent marker. Interestingly, massive DNA damage was detected in TAp63−/− mice, suggesting that TAp63 might delay aging by maintaining genome stability (Craig et al., 2010).
Other causes of DNA damage in HFs include irradiation, medical treatment, and environmental pollutants. Kim et al. uncovered the mechanisms for alkylating chemotherapy-induced permanent hair loss (Su et al., 2009). They revealed that HFSCs went through rapid proliferation after treatment with busulfan followed by cyclophosphamide, which impaired HFSCs’ resistance to DNA damage and depleted the HFSC pool. In addition to that, Titova et al. explained that DNA damage in the skin could also be caused by terahertz (THz) radiation, widely used in diagnostic imaging and security screening (Kim et al., 2019). Toxins such as patulin and ochratoxin A were found to impact genome stability in the skin as well (Saxena et al., 2009; Titova et al., 2013).
Mentioned above are the causes of DNA damage in HF cells, which often result in stem cell dysfunction to induce hair aging. For example, hair thinning and hair loss in aged mice occur with sustained DNA damage response (DDR) in aging stem cells. Particularly, DDR in aging HFSCs caused type XVII collagen (COL17A1) proteolysis, promoting epidermal differentiation of HFSCs and diminishing their stemness (Kumar et al., 2012). In addition to HFSCs, hair follicle mesenchymal stem cells (HF-MSCs) are also an important player in HF regeneration and wound healing, and their senescence might lead to hair aging. Wang et al. indicated that DNA damage-induced HF-MSC apoptosis could be rescued by PBX homeobox 1 (PBX1) overexpression (Matsumura et al., 2016). Further studies demonstrated that PBX1 alleviated HF-MSC apoptosis through a PBX1/AKT/GSK axis (Wang et al., 2021). In another study, PBX1 upregulation was accompanied by increased SIRT1 as well as decreased PARP1 expression, and DNA damage was also alleviated. These results suggest a PBX1/SIRT/PARP1 axis in DNA damage reduction and HF-MSC maintenance (Jiang et al., 2019). Additional study has revealed that shortened telomeres broadly affected stem cells within the epidermis, which impaired the epidermis as well as HF formation (Wang et al., 2023). Telomere shortening can happen from DNA damage and is proposed as one of the major aging mechanisms, restricting cell division and changing gene expression (Vermeij et al., 2014). Mechanically, telomere dysfunction reduces the number of epidermal stem cells and impedes their specification and differentiation through a BMP/pSmad/P63 axis, contributing to skin atrophy and hair loss.
Aside from DNA damage, defects in DNA repair also account for hair aging. Gata6 is a transcriptional factor expressed in HF progenitors. Ablation of Gata6 in mice exhibited cell cycle arrest in keratinocytes and impaired progenitor differentiation, accompanied by significant DNA damage marked by γ-H2AX and increased apoptosis marked by caspase3 (Liu et al., 2019). These results suggest the anti-aging role of Gata6 in HFs. Additionally, circadian clock proteins are involved in DNA repair, which can be transcriptionally activated by the CLOCK/BMAL1 heterodimer complex. Bmal1 deficiency facilitated ROS accumulation and thus DNA damage to HF-residing stem cells in aged mice, promoting hair aging (Wang et al., 2017). Furthermore, Sotiropoulou et al. found that DNA-PK could accelerate non-homologous end-joining repair of double-strand DNA breaks, increasing bulge-SCs’ resistance to DNA damage-induced apoptosis (Geyfman and Andersen, 2010). Collectively, these studies highlight the role of DNA repair in preventing hair aging.
With the increasing social emphasis on hair research, extensive investigations into hair loss treatment have been conducted over several decades. However, in the context of hair aging, the existing treatment methods remain significantly limited. Currently, topically applied minoxidil and orally administered finasteride are the two most commonly used drugs approved by the FDA for the treatment of hair loss (Sotiropoulou et al., 2010; Wang et al., 2019).
Initially, minoxidil acts as an oral antihypertensive medication as an activator of potassium (K+) ion channels (Devjani et al., 2023). Although the exact manner in which topical minoxidil promotes hair growth remains unclear, current studies indicate that it most likely works through vasodilation, anti-inflammatory action, induction of Wnt/β-catenin signaling, resistance to androgens, and modulation of the hair cycle (Campese, 1981). The most common formulations include 2% solution, 5% solution, foam, and spray in clinical treatment (Gupta et al., 2022). Many patients experienced adverse effects such as skin itching or allergies that were associated with PEG in the solution formulations (Kovacevic et al., 2020). Additionally, only about 60% of the male AGA patients respond to topical minoxidil treatment in practical use (Friedman et al., 2002). Clinically, patients’ suitability for minoxidil can be determined by the sulfotransferase assay (FSA) (Goren et al., 2014). However, the specific role of FSA in minoxidil treatment warrants further investigation due to the high variability of sulfotransferase expression.
Another FDA-approved drug for AGA treatment is finasteride. It selectively inhibits types II and III 5α-reductase isoenzymes and consequently hampers the conversion of testosterone into DHT, which has a stronger affinity for AR, thereby downregulating AR signaling (McCoy et al., 2019). Nonetheless, finasteride cannot sufficiently block type I 5α-reductase isoenzyme, DHT conversion does not stop completely, and when medication is stopped, DHT levels are restored in the second week (Rittmaster, 1994). Moreover, within 12 m following the discontinuation of treatment, the hair regrowth will be reversed (Rittmaster, 1994). Furthermore, clinical observations suggest that finasteride exhibits systemic effects, which potentially increase the risks of prostate cancer and sexual disorders in male patients (Lee et al., 2018), highlighting the need to develop a topical treatment approach (Mysore, 2012).
The constraints of these two drugs have prompted people to explore new therapies. For example, platelet-rich plasma (PRP) and low-level light therapy (LLLT) have emerged as alternative approaches for hair regeneration.
PRP is separated from patients’ whole blood and contains high levels of platelets, approximately 5–6 times more platelets than normal (Gupta and Talukder, 2022). Many growth factors and cytokines related to hair growth can be emitted by platelets in PRP, including PDGF, VEGF, EGF, and TGF-β (Dashore et al., 2021). Additionally, PRP can induce DPC proliferation by activating the ERK1/2, PI3K/AKT, and Wnt/β-catenin signaling pathways (Straum, 2020). Also, due to its few side effects as an autologous plasma product and promising treatment efficacy, PRP serves as an effective therapy alone or in combination with other treatments and hair transplantation (Xiao et al., 2019). Many studies have proven the effectiveness of PRP in different forms of alopecia, such as AGA, FPHL, and AA. Whereas, its mechanisms still need further clarification (Garg and Manchanda, 2017; Everts et al., 2020; Abdin et al., 2022).
LLLT utilizes lasers (600–1,100 nm) to promote hair growth and increase the proportion of anagen HFs, without an invasive procedure (Stevens and Khetarpal, 2018). Until 2023, more than 47 LLLT devices have been designed and approved by the FDA, and there have been multiple clinical studies demonstrating the efficacy of LLLT, which is even more potent than the FDA-approved drugs in some cases (Farivar et al., 2014; Pillai and Mysore, 2021). The convenience, high patient compliance, good efficacy, and minimal side effects have made it a highly anticipated therapeutic approach (Lueangarun et al., 2021). Current studies suggest that LLLT may promote hair growth by increasing adenosine triphosphate (ATP) production, regulating ROS, and reducing inflammatory PGE2 through photobiomodulation (Farivar et al., 2014; Stevens and Khetarpal, 2018; Taylor et al., 2020).
Nonetheless, there are numerous unresolved issues with this therapy. The exact mechanisms for promoting hair growth remain unclear, and there is a significant disparity in its efficacy between humans and animals. The challenge also lies in the standardization of treatment protocols, which should be based on individual differences such as hair lengths, colors, and skin tones (Sakurai et al., 2000).
Much effort has been made to reverse hair aging by boosting stem cell activity. Stem cell transplantation, hair follicle organoids, and stem cell-derived exosomes have gained attention as potential therapies to rejuvenate the HFs (Anastassakis and Anastassakis, 2023).
Stem cells possess intrinsic features such as self-renewal, mobility, anti-inflammatory properties, and immunity that promote repair and regeneration in aging tissues. Stem cell therapies for hair aging mainly include MSC treatment due to its pluripotent regenerative potential (Egger et al., 2020). Depending on the origin, these MSCs can be categorized into adipose tissue-derived MSCs (AD-MSCs), bone marrow-derived MSCs (BM-MSCs), and perinatal MSCs, etc. (Egger et al., 2020). Some studies have indicated that growth factors secreted by AD-MSCs can activate epidermal stem cells and DPCs. Furthermore, AD-MSCs display anti-inflammatory effects through cell-cell interactions as well as modulation of PGE2 and leukemia inhibitory factors. Clinically, MSC transplantation in AGA patients has achieved favorable therapeutic outcomes. However, the limits on the acquisition and cell quantity of AD-MSCs and BM-MSCs remain a bottleneck of this therapeutic approach (Shimizu et al., 2022).
Derived from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), hair follicle organoids are shown to promote hair regeneration (Lee et al., 2022; Salhab et al., 2022). Within hair follicle organoids, there are stratified layers of epidermis and dermis, as well as appendages such as neurons, SG, and DP (Lee et al., 2022). Also, hair follicle organoids exhibit higher hair production capacity compared to directly cultured HFs (Lee et al., 2020). Additionally, iPSC-derived hair follicle organoids significantly mitigate ethical concerns related to ESC treatment (Sun et al., 2021). However, current hair follicle organoid cultivation protocols are not able to induce sweat glands, immune cells, blood vessels and APM. Moreover, the HFs induced by current methods resemble vellus hair rather than thick terminal hair (Salhab et al., 2022). This issue might be tackled by optimizing the organoid culture protocol. According to the current method, the DP structure is derived from cranial neural crest (CNC) cells, while DP within the human scalp HF originates from mesodermal cells (Lee et al., 2022). Since DP is critical in controlling the duration of anagen, which distinguish the terminal hair and the vellus hair (O’Sullivan et al., 2021), establishing a method to culture ectoderm and mesoderm-derived hair follicle organoid might help break the bottleneck. Furthermore, preventing post-transplantation carcinogenesis is a critical concern in stem cell transplantation (Vatanashevanopakorn and Sartyoungkul, 2023).
Exosomes are tiny membrane-bound vehicles, about 40–160 nm in diameter, released from cells via the endosomal pathway. They serve as crucial messengers for the transfer of proteins, lipids, and RNA, facilitating communication between cells (Azar et al., 2021). SCD-Exos refer to exosomes carrying specific proteins and nucleic acids associated with stem cell functions. Unlike stem cells, exosomes cannot replicate, eliminating concerns about potential tumorigenesis after transplantation. In HF, exosomes are sourced from various cells, including DPCs and MSCs (Azar et al., 2021).
DPCs-Exos can regulate key signaling pathways like Wnt/β-catenin and BMP, promoting HFSC proliferation, regeneration, and HF formation. MSC-derived exosomes activate Akt signaling and increase the anti-apoptotic protein Bcl-2 in DPCs, transitioning HFs from a dormant to an active phase. While animal studies have been encouraging with minimal side effects, further clinical research is needed to understand their specific effects, mechanisms, and potential risks in the human body (Salhab et al., 2022).
Overall, this part provides a narrative overview of currently utilized pharmaceutical agents and selected prospective therapeutic approaches for addressing hair aging. It is evident that the existing therapeutic strategies for hair loss treatment have failed to produce satisfactory outcomes with significant adverse effects. The mechanisms are also poorly understood. The etiology of hair loss, substantial inter-individual variability, and disparities between animal models and humans in manifestation and therapeutic efficacy have resulted in inconsistent conclusions. Methodological flaws have further hampered the reliability and comparability of the data. Nevertheless, multiple new pharmacological targets have been employed in drug discovery, including JAK inhibitors and PGD inhibitors. Among which, baricitinib has recently been approved for the treatment of AA. It is hoped that as the understanding of these mechanisms becomes more comprehensive, more potent treatments will be developed to tackle the hair aging problem.
In our review, we explored many complex factors responsible for HF aging. The discussion highlighted mechanisms associated with the aging process.
Firstly, we emphasized the role of oxidative stress, which is mainly mediated by ROS. The oxidative stress might cause DNA damage, lipid peroxidation, and long-term inflammation, all of which contribute to hair aging. Furthermore, the mechanisms regarding how HFPU responds to oxidative stress and leads to hair graying have also been clarified.
Additionally, discussions about hormonal influences on hair aging focus largely on androgen and estrogen. Androgens, particularly DHT, have a substantial impact on HFs, contributing to diseases such as MPHL. Estrogens, on the other hand, play a crucial role in promoting HF growth as well as protecting HFs from antioxidant damage. Chronic stress-induced hormones such as cortisol also account for hair aging.
Besides, we conclude that hair aging can be triggered by inflammatory signaling. Among these immune disorders, the activation of JAK-STAT signaling is observed in multiple premature hair aging diseases such as AA, AGA, and cicatricial alopecia. Previously, JAK-STAT was known to be involved in immune response, tissue regeneration, and apoptosis (Hu X. et al., 2021). Given the critical role of JAK-STAT signaling in maintaining immune functions, it has great potential for inflammation-driven alopecia treatment. For example, JAK inhibitors are reported to alleviate the AA symptoms, probably by reducing cytotoxic T cells and IFN-γ signature in HFs (Harel et al., 2015). Moreover, stress-induced neurogenic inflammation is drawing people’s attention. Nowadays, more and more people are suffering from psychological diseases such as anxiety and depression, which often occur with premature hair graying and hair loss. Several studies have uncovered the correlation between perceived stress and HF disorders (Liu et al., 2013; Zhang et al., 2020; O’Sullivan et al., 2022). Therefore, stress-associated neurotransmitters such as norepinephrine might also participate in the aging process, which is still largely under-explored. In addition to that, a recent study has shown a microbiome discrepancy in aged and young scalp skin. Among these microbiomes, Acinetobacter was significantly increased in aged scalp skin, and further research is required to confirm its role in hair aging (Shibagaki et al., 2017). How other bacteria relate to hair aging, such as Malassezia, Staphylococcus spp., and Alternaria spp., is also worth investigating due to their potential ability to cause scalp diseases and HF disorders (Polak-Witka et al., 2020). Moreover, the interaction between these microbiomes and the innate immune system remains unclear, which is under extensive research (Sakamoto et al., 2021).
HF homeostasis is regulated by intrinsic and extrinsic signaling, emphasizing the role of the HF milieu in hair aging. For instance, recent research has demonstrated that lymphatic vessels are important for skin renewal (Gur-Cohen et al., 2019; Peña-Jimenez et al., 2019). The size of SGs also changes over time during aging, along with altered sebum composition (Hou et al., 2022). Additionally, dermal white adipose tissue (dWAT) became thicker with age, accompanied by aberrant inflammatory signaling (Chen et al., 2021). Sympathetic nerves within the HF regulate HFSC activation while depleting McSCs in HFs (Shwar et al., 2020; Zhang et al., 2020). These findings demonstrate that the HF milieu might be important for the hair aging process.
In conclusion, hair aging is a complex process influenced by numerous factors. In our review, we examine the features of hair aging, including hair graying, baldness, and HFM. These processes can be attributable to oxidative stress, hormonal imbalance, inflammation, and DNA damage. Understanding the complex mechanisms of hair aging and emerging trends in treatments helps us develop effective therapeutic strategies. With more and more in-depth research in this field, we anticipate further breakthroughs in hair aging mechanisms and treatments.
AL: Visualization, Writing–original draft, Writing–review and editing. YF: Writing–original draft, Writing–review and editing. LY: Writing–original draft, Writing–review and editing. JM: Writing–original draft, Writing–review and editing. XW: Funding acquisition, Supervision, Writing–review and editing. JC: Writing–review and editing. XX: Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Shenzhen Technology and Innovation Commission (JCYJ20200109142444449 and JCYJ20210324120007021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdin, R., Zhang, Y., and Jimenez, J. J. (2022). Treatment of androgenetic alopecia using PRP to target dysregulated mechanisms and pathways. Front. Med. 9, 843127. doi:10.3389/fmed.2022.843127
Anastassakis, K. (2022). “Female pattern hair loss,” in Androgenetic alopecia from A to Z 181–203 (Berlin, Germany: Springer International Publishing). doi:10.1007/978-3-030-76111-0_12
Anastassakis, K. (2023). “Low-level laser therapy (LLLT) and AGA,” in Androgenetic alopecia from A to Z: vol.3 hair restoration surgery, alternative treatments, and hair care. Editor K. Anastassakis (Berlin, Germany: Springer International Publishing), 597–624. doi:10.1007/978-3-031-10613-2_38
Anderson, A. P., Luo, X., Russell, W., and Yin, Y. W. (2020). Oxidative damage diminishes mitochondrial DNA polymerase replication fidelity. Nucleic acids Res. 48, 817–829. doi:10.1093/nar/gkz1018
Arck, P. C., Overall, R., Spatz, K., Liezman, C., Handjiski, B., Klapp, B. F., et al. (2006). Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 20, 1567–1569. doi:10.1096/fj.05-4039fje
Arnold, H., and Djabali, K. (2019). Inhibition of JAK-STAT signaling with baricitinib reduces inflammation and improves cellular homeostasis in progeria cells. Cells 8, 1276. doi:10.3390/cells8101276
Azar, J., Bahmad, H. F., Daher, D., Moubarak, M. M., Hadadeh, O., Monzer, A., et al. (2021). The use of stem cell-derived organoids in disease modeling: an update. Int. J. Mol. Sci. 22, 7667. doi:10.3390/ijms22147667
Balaban, R. S., Nemoto, S., and Finkel, T. (2005). Mitochondria, oxidants, and aging. Cell 120, 483–495. doi:10.1016/j.cell.2005.02.001
Barrera, G. (2012). Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 137289. doi:10.5402/2012/137289
Bedja, D., Yan, W., Lad, V., Iocco, D., Sivakumar, N., Bandaru, V. V. R., et al. (2018). Inhibition of glycosphingolipid synthesis reverses skin inflammation and hair loss in ApoE−/− mice fed western diet. Sci. Rep. 8, 11463. doi:10.1038/s41598-018-28663-9
Bejaoui, M., Taarji, N., Saito, M., Nakajima, M., and Isoda, H. (2021). Argan (Argania Spinosa) press cake extract enhances cell proliferation and prevents oxidative stress and inflammation of human dermal papilla cells. J. Dermatological Sci. 103, 33–40. doi:10.1016/j.jdermsci.2021.06.003
Best, B. P. (2009). Nuclear DNA damage as a direct cause of aging. Rejuvenation Res. 12, 199–208. doi:10.1089/rej.2009.0847
Brosche, T., Dressler, S., and Platt, D. (2001). Age-associated changes in integral cholesterol and cholesterol sulfate concentrations in human scalp hair and finger nail clippings. Aging Clin. Exp. Res. 13, 131–138. doi:10.1007/BF03351535
Campese, V. M. (1981). Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs 22, 257–278. doi:10.2165/00003495-198122040-00001
Castellana, D., Paus, R., and Perez-Moreno, M. (2014). Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 12, e1002002. doi:10.1371/journal.pbio.1002002
Ceruti, J. M., Leirós, G. J., and Balañá, M. E. (2018). Androgens and androgen receptor action in skin and hair follicles. Mol. Cell Endocrinol. 465, 122–133. doi:10.1016/j.mce.2017.09.009
Chen, J., Fan, Z. X., Zhu, D. C., Guo, Y. L., Ye, K., Dai, D., et al. (2021). Emerging role of dermal white adipose tissue in modulating hair follicle development during aging. Front. Cell Dev. Biol. 9, 728188. doi:10.3389/fcell.2021.728188
Chen, X., Liu, B., Li, Y., Han, L., Tang, X., Deng, W., et al. (2020). Dihydrotestosterone regulates hair growth through the wnt/β-catenin pathway in C57bl/6 mice and in vitro organ culture. Front. Pharmacol. 10, 1528. doi:10.3389/fphar.2019.01528
Choi, B. Y. (2020). Targeting wnt/β-catenin pathway for developing therapies for hair loss. Int. J. Mol. Sci. 21, 4915. doi:10.3390/ijms21144915
Choi, S., Zhang, B., Ma, S., Gonzalez-Celeiro, M., Stein, D., Jin, X., et al. (2021). Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 592, 428–432. doi:10.1038/s41586-021-03417-2
Cotsarelis, G., and Millar, S. E. (2001). Towards a molecular understanding of hair loss and its treatment. Trends Mol. Med. 7, 293–301. doi:10.1016/s1471-4914(01)02027-5
Craig, A. L., Holcakova, J., Finlan, L. E., Nekulova, M., Hrstka, R., Gueven, N., et al. (2010). DeltaNp63 transcriptionally regulates ATM to control p53 Serine-15 phosphorylation. Mol. Cancer. 9, 195. doi:10.1186/1476-4598-9-195
Csala, M., Kardon, T., Legeza, B., Lizák, B., Mandl, J., Margittai, É., et al. (2015). On the role of 4-hydroxynonenal in health and disease. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1852, 826–838. doi:10.1016/j.bbadis.2015.01.015
Dashore, S., Chouhan, K., Nanda, S., and Sharma, A. (2021). Preparation of platelet-rich plasma: national IADVL PRP taskforce recommendations. Indian Dermatol Online J. 12, S12–S23. doi:10.4103/idoj.idoj_269_21
Deng, Z., Chen, M., Liu, F., Wang, Y., Xu, S., Sha, K., et al. (2022). Androgen receptor-mediated paracrine signaling induces regression of blood vessels in the dermal papilla in androgenetic alopecia. J. Invest. Dermatol 142, 2088–2099.e9. doi:10.1016/j.jid.2022.01.003
Devjani, S., Ezemma, O., Kelley, K. J., Stratton, E., and Senna, M. (2023). Androgenetic alopecia: therapy update. Drugs 83, 701–715. doi:10.1007/s40265-023-01880-x
D’Orazio, J., Jarrett, S., Amaro-Ortiz, A., and Scott, T. (2013). UV radiation and the skin. IJMS 14, 12222–12248. doi:10.3390/ijms140612222
Egger, A., Tomic-Canic, M., and Tosti, A. (2020). Advances in stem cell-based therapy for hair loss. CellR4 Repair Replace. Regen. Reprogr. 8, e2894.
Everts, P., Onishi, K., Jayaram, P., Lana, J. F., and Mautner, K. (2020). Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int. J. Mol. Sci. 21, 7794. doi:10.3390/ijms21207794
Farivar, S., Malekshahabi, T., and Shiari, R. (2014). Biological effects of low level laser therapy. J. Lasers Med. Sci. 5, 58–62.
Freitas, E., Guttman-Yassky, E., and Torres, T. (2023). Baricitinib for the treatment of alopecia areata. Drugs 83, 761–770. doi:10.1007/s40265-023-01873-w
Friedman, E. S., Friedman, P. M., Cohen, D. E., and Washenik, K. (2002). Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J. Am. Acad. Dermatology 46, 309–312. doi:10.1067/mjd.2002.119104
Gannon, H. S., Donehower, L. A., Lyle, S., and Jones, S. N. (2011). Mdm2–p53 signaling regulates epidermal stem cell senescence and premature aging phenotypes in mouse skin. Dev. Biol. 353, 1–9. doi:10.1016/j.ydbio.2011.02.007
García-Cao, I., García-Cao, M., Martín-Caballero, J., Criado, L. M., Klatt, P., Flores, J. M., et al. (2002). ‘Super p53’ mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 21, 6225–6235. doi:10.1093/emboj/cdf595
Garg, S., and Manchanda, S. (2017). Platelet-rich plasma—an ‘Elixir’ for treatment of alopecia: personal experience on 117 patients with review of literature. Stem Cell Investig. 4, 64. doi:10.21037/sci.2017.06.07
Garza, L. A., Liu, Y., Yang, Z., Alagesan, B., Lawson, J. A., Norberg, S. M., et al. (2012). Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 4, 126ra34. doi:10.1126/scitranslmed.3003122
Gerard, C., La Riche, A., Altendorf, S., Dion, V., Epping, L., Bíro, T., et al. (2023). FRI378 estetrol prolongs anagen in healthy female scalp hair follicles by positively modulating dermal papilla functions and generation of progenitor stem cells ex vivo. J. Endocr. Soc. 7, bvad114.1574. doi:10.1210/jendso/bvad114.1574
Geyfman, M., and Andersen, B. (2010). Clock genes, hair growth and aging. Aging 2, 122–128. doi:10.18632/aging.100130
Giesen, M., Gruedl, S., Holtkoetter, O., Fuhrmann, G., Koerner, A., and Petersohn, D. (2011). Ageing processes influence keratin and KAP expression in human hair follicles. Exp. Dermatol. 20, 759–761. doi:10.1111/j.1600-0625.2011.01301.x
Goren, A., Castano, J. A., McCoy, J., Bermudez, F., and Lotti, T. (2014). Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatol Ther. 27, 171–173. doi:10.1111/dth.12111
Gritsenko, D. A., Orlova, O. A., Linkova, N. S., and Khavinson, V.Kh. (2017). Transcription factor p53 and skin aging. Adv. Gerontol. 7, 114–119. doi:10.1134/s2079057017020072
Gupta, A. K., and Talukder, M. (2022). Topical finasteride for male and female pattern hair loss: is it a safe and effective alternative? J. Cosmet. Dermatol 21, 1841–1848. doi:10.1111/jocd.14895
Gupta, A. K., Talukder, M., Venkataraman, M., and Bamimore, M. A. (2022). Minoxidil: a comprehensive review. J. Dermatological Treat. 33, 1896–1906. doi:10.1080/09546634.2021.1945527
Gur-Cohen, S., Yang, H., Baksh, S. C., Miao, Y., Levorse, J., Kataru, R. P., et al. (2019). Stem cell–driven lymphatic remodeling coordinates tissue regeneration. Science 366, 1218–1225. doi:10.1126/science.aay4509
Handelsman, D. J. (2000). “Androgen physiology, pharmacology, use and misuse,” in Endotext. Editor K. R. Feingold (South Dartmouth: MDText.com, Inc.).
Harel, S., Higgins, C. A., Cerise, J. E., Dai, Z., Chen, J. C., Clynes, R., et al. (2015). Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci. Adv. 1, e1500973. doi:10.1126/sciadv.1500973
Hasan, R., Juma, H., Eid, F. A., Alaswad, H. A., Ali, W. M., and Aladraj, F. J. (2022). Effects of hormones and endocrine disorders on hair growth. Cureus 14, e32726. doi:10.7759/cureus.32726
Ho, C. H., Sood, T., and Zito, P. M. (2022). “Androgenetic alopecia,” in StatPearls (United States: StatPearls Publishing).
Hou, X., Wei, Z., Zouboulis, C. C., and Ju, Q. (2022). Aging in the sebaceous gland. Front. Cell Dev. Biol. 10, 909694. doi:10.3389/fcell.2022.909694
Hu, H., Zhang, S. b., Lei, X. h., Deng, Z. l., Guo, W. x., Qiu, Z. f., et al. (2012). Estrogen leads to reversible hair cycle retardation through inducing premature catagen and maintaining telogen. PLoS One 7, e40124. doi:10.1371/journal.pone.0040124
Hu, X., Li, J., Fu, M., Zhao, X., and Wang, W. (2021b). The JAK/STAT signaling pathway: from bench to clinic. Sig Transduct. Target Ther. 6, 402. doi:10.1038/s41392-021-00791-1
Hu, X.-M., Li, Z. X., Zhang, D. Y., Yang, Y. C., Fu, S. A., Zhang, Z. Q., et al. (2021a). A systematic summary of survival and death signalling during the life of hair follicle stem cells. Stem Cell Res. Ther. 12, 453. doi:10.1186/s13287-021-02527-y
Jabbari, A., Dai, Z., Xing, L., Cerise, J. E., Ramot, Y., Berkun, Y., et al. (2015). Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine 2, 351–355. doi:10.1016/j.ebiom.2015.02.015
Jeong, K. H., Jung, J. H., Kim, J. E., and Kang, H. (2018). Prostaglandin D2-mediated DP2 and AKT signal regulate the activation of androgen receptors in human dermal papilla cells. Int. J. Mol. Sci. 19, 556. doi:10.3390/ijms19020556
Ji, J., Ho, B. S. Y., Qian, G., Xie, X. M., Bigliardi, P. L., and Bigliardi-Qi, M. (2017). Aging in hair follicle stem cells and niche microenvironment. J. Dermatology 44, 1097–1104. doi:10.1111/1346-8138.13897
Jiang, Y., Liu, F., Zou, F., Zhang, Y., Wang, B., Zhang, Y., et al. (2019). PBX homeobox 1 enhances hair follicle mesenchymal stem cell proliferation and reprogramming through activation of the AKT/glycogen synthase kinase signaling pathway and suppression of apoptosis. Stem Cell Res. Ther. 10, 268. doi:10.1186/s13287-019-1382-y
Jo, S. K., Lee, J. Y., Lee, Y., Kim, C. D., Lee, J. H., and Lee, Y. H. (2018). Three streams for the mechanism of hair graying. Ann. Dermatol 30, 397–401. doi:10.5021/ad.2018.30.4.397
Juan, C. A., Pérez de la Lastra, J. M., Plou, F. J., and Pérez-Lebeña, E. (2021). The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22, 4642. doi:10.3390/ijms22094642
Jung, Y. H., Chae, C. W., Choi, G. E., Shin, H. C., Lim, J. R., Chang, H. S., et al. (2022). Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts. J. Biomed. Sci. 29, 17. doi:10.1186/s12929-022-00800-7
Jurk, D., Wilson, C., Passos, J. F., Oakley, F., Correia-Melo, C., Greaves, L., et al. (2014). Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 5, 4172. doi:10.1038/ncomms5172
Kandyba, E., Leung, Y., Chen, Y. B., Widelitz, R., Chuong, C. M., and Kobielak, K. (2013). Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc. Natl. Acad. Sci. U.S.A. 110, 1351–1356. doi:10.1073/pnas.1121312110
Kim, J., Nakasaki, M., Todorova, D., Lake, B., Yuan, C. Y., Jamora, C., et al. (2014). p53 induces skin aging by depleting Blimp1+ sebaceous gland cells. Cell Death Dis. 5, e1141. doi:10.1038/cddis.2014.87
Kim, J. Y., Ohn, J., Yoon, J. S., Kang, B. M., Park, M., Kim, S., et al. (2019). Priming mobilization of hair follicle stem cells triggers permanent loss of regeneration after alkylating chemotherapy. Nat. Commun. 10, 3694. doi:10.1038/s41467-019-11665-0
Kovacevic, M., McCoy, J., Shapiro, J., Sinclair, R., Vaño-Galvan, S., Goldust, M., et al. (2020). Novel “After Minoxidil” spray improves topical minoxidil compliance and hair style manageability. J. Cosmet. Dermatology 19, 2647–2649. doi:10.1111/jocd.13630
Kumar, R., Ansari, K. M., Chaudhari, B. P., Dhawan, A., Dwivedi, P. D., Jain, S. K., et al. (2012). Topical application of ochratoxin A causes DNA damage and tumor initiation in mouse skin. PLoS ONE 7, e47280. doi:10.1371/journal.pone.0047280
Lacey, N., Ní Raghallaigh, S., and Powell, F. C. (2011). Demodex mites – commensals, parasites or mutualistic organisms? Dermatology 222, 128–130. doi:10.1159/000323009
Lee, J., Rabbani, C. C., Gao, H., Steinhart, M. R., Woodruff, B. M., Pflum, Z. E., et al. (2020). Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582, 399–404. doi:10.1038/s41586-020-2352-3
Lee, J., van der Valk, W. H., Serdy, S. A., Deakin, C., Kim, J., Le, A. P., et al. (2022). Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells. Nat. Protoc. 17, 1266–1305. doi:10.1038/s41596-022-00681-y
Lee, S. A., Li, K. N., and Tumbar, T. (2021). Stem cell-intrinsic mechanisms regulating adult hair follicle homeostasis. Exp. Dermatol 30, 430–447. doi:10.1111/exd.14251
Lee, S. W., Juhasz, M., Mobasher, P., Ekelem, C., and Mesinkovska, N. A. (2018). A systematic review of topical finasteride in the treatment of androgenetic alopecia in men and women. J. Drugs Dermatol 17, 457–463.
Lei, M., Lin, S.-J., and Chuong, C.-M. (2021). Editorial: hair follicle stem cell regeneration in aging. Front. Cell Dev. Biol. 9, 799268. doi:10.3389/fcell.2021.799268
Liu, D., Ou, L., Clemenson, G. D., Chao, C., Lutske, M. E., Zambetti, G. P., et al. (2010). Puma is required for p53-induced depletion of adult stem cells. Nat. Cell Biol. 12, 993–998. doi:10.1038/ncb2100
Liu, M., Liu, X., Wang, Y., Sui, Y., Liu, F., Liu, Z., et al. (2022). Intrinsic ROS drive hair follicle cycle progression by modulating DNA damage and repair and subsequently hair follicle apoptosis and macrophage polarization. Oxid. Med. Cell Longev. 2022, 8279269. doi:10.1155/2022/8279269
Liu, N., Wang, L. H., Guo, L. L., Wang, G. Q., Zhou, X. P., Jiang, Y., et al. (2013). Chronic restraint stress inhibits hair growth via substance P mediated by reactive oxygen species in mice. PLoS ONE 8, e61574. doi:10.1371/journal.pone.0061574
Liu, N., Yin, Y., Wang, H., Zhou, Z., Sheng, X., Fu, H., et al. (2019). Telomere dysfunction impairs epidermal stem cell specification and differentiation by disrupting BMP/pSmad/P63 signaling. PLoS Genet. 15, e1008368. doi:10.1371/journal.pgen.1008368
Lueangarun, S., Visutjindaporn, P., Parcharoen, Y., Jamparuang, P., and Tempark, T. (2021). A systematic review and meta-analysis of randomized controlled trials of United States food and drug administration-approved, home-use, low-level light/laser therapy devices for pattern hair loss: device design and Technology. J. Clin. Aesthet. Dermatol 14, E64–E75.
Martins, S. G., Zilhão, R., Thorsteinsdóttir, S., and Carlos, A. R. (2021). Linking oxidative stress and DNA damage to changes in the expression of extracellular matrix components. Front. Genet. 12, 673002. doi:10.3389/fgene.2021.673002
Matsuda, M., Huh, Y., and Ji, R.-R. (2019). Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 33, 131–139. doi:10.1007/s00540-018-2579-4
Matsumura, H., Mohri, Y., Binh, N. T., Morinaga, H., Fukuda, M., Ito, M., et al. (2016). Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 351, aad4395. doi:10.1126/science.aad4395
Maynard, S., Fang, E. F., Scheibye-Knudsen, M., Croteau, D. L., and Bohr, V. A. (2015). DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb. Perspect. Med. 5, a025130. doi:10.1101/cshperspect.a025130
McCoy, J., Goren, A., Naccarato, T., Kovacevic, M., Situm, M., Skudar, V. L., et al. (2019). Identification of the sulfotransferase iso-enzyme primarily responsible for the bio-activation of topical minoxidil. J. Biol. Regul. Homeost. Agents 33, 817–819.
Mercke, Y., Sheng, H., Khan, T., and Lippmann, S. (2000). Hair loss in psychopharmacology. Ann. Clin. Psychiatry 12, 35–42. doi:10.1023/a:1009074926921
Milkovic, L., Zarkovic, N., Marusic, Z., Zarkovic, K., and Jaganjac, M. (2023). The 4-hydroxynonenal–protein adducts and their biological relevance: are some proteins preferred targets? Antioxidants (Basel) 12, 856. doi:10.3390/antiox12040856
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., and Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 20, 1126–1167. doi:10.1089/ars.2012.5149
Morinaga, H., Mohri, Y., Grachtchouk, M., Asakawa, K., Matsumura, H., Oshima, M., et al. (2021). Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature 595, 266–271. doi:10.1038/s41586-021-03624-x
Mysore, V. (2012). Finasteride and sexual side effects. Indian Dermatol Online J. 3, 62–65. doi:10.4103/2229-5178.93496
Naito, A., Midorikawa, T., Yoshino, T., and Ohdera, M. (2008). Lipid peroxides induce early onset of catagen phase in murine hair cycles. Int. J. Mol. Med. 22, 725–729.
Napoli, E., Wong, S., and Giulivi, C. (2013). Evidence of reactive oxygen species-mediated damage to mitochondrial DNA in children with typical autism. Mol. Autism 4, 2. doi:10.1186/2040-2392-4-2
Nishimura, E. K., Cranter, S. R., and Fisher, D. E. (2005). Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Sci. New Ser. 307, 720–724. doi:10.1126/science.1099593
O’Sullivan, J. D. B., Peters, E. M. J., Amer, Y., Atuluru, P., Chéret, J., Rosenberg, A. M., et al. (2022). The impact of perceived stress on the hair follicle: towards solving a psychoneuroendocrine and neuroimmunological puzzle. Front. Neuroendocrinol. 66, 101008. doi:10.1016/j.yfrne.2022.101008
O’Sullivan, J. D. B., Nicu, C., Picard, M., Chéret, J., Bedogni, B., Tobin, D. J., et al. (2021). The biology of human hair greying. Biol. Rev. Camb Philos. Soc. 96, 107–128. doi:10.1111/brv.12648
Palmer, M. A., Blakeborough, L., Harries, M., and Haslam, I. S. (2020). Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp. Dermatol. 29, 299–311. doi:10.1111/exd.13993
Panich, U., Sittithumcharee, G., Rathviboon, N., and Jirawatnotai, S. (2016). Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 7370642–7370714. doi:10.1155/2016/7370642
Paus, R., Langan, E. A., Vidali, S., Ramot, Y., and Andersen, B. (2014). Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol. Med. 20, 559–570. doi:10.1016/j.molmed.2014.06.002
Peña-Jimenez, D., Fontenete, S., Megias, D., Fustero-Torre, C., Graña-Castro, O., Castellana, D., et al. (2019). Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J. 38, e101688. doi:10.15252/embj.2019101688
Pillai, J. K., and Mysore, V. (2021). Role of low-level light therapy (LLLT) in androgenetic alopecia. J. Cutan. Aesthet. Surg. 14, 385–391. doi:10.4103/JCAS.JCAS_218_20
Polak-Witka, K., Rudnicka, L., Blume-Peytavi, U., and Vogt, A. (2020). The role of the microbiome in scalp hair follicle biology and disease. Exp. Dermatol. 29, 286–294. doi:10.1111/exd.13935
Pratt, C. H., King, L. E., Messenger, A. G., Christiano, A. M., and Sundberg, J. P. (2017). Alopecia areata. Nat. Rev. Dis. Prim. 3, 17011. doi:10.1038/nrdp.2017.11
Ricardo-Gonzalez, R. R., Kotas, M. E., O'Leary, C. E., Singh, K., Damsky, W., Liao, C., et al. (2022). Innate type 2 immunity controls hair follicle commensalism by Demodex mites. Immunity 55, 1891–1908.e12. doi:10.1016/j.immuni.2022.08.001
Rittmaster, R. S. (1994). Finasteride. N. Engl. J. Med. 330, 120–125. doi:10.1056/NEJM199401133300208
Rufini, A., Tucci, P., Celardo, I., and Melino, G. (2013). Senescence and aging: the critical roles of p53. Oncogene 32, 5129–5143. doi:10.1038/onc.2012.640
Rushton, D. H., Westgate, G. E., and Van Neste, D. J. (2022). Following historical ‘tracks’ of hair follicle miniaturisation in patterned hair loss: are elastin bodies the forgotten aetiology? Exp. Dermatol 31, 102–109. doi:10.1111/exd.14393
Rybka, J., Kupczyk, D., Kędziora-Kornatowska, K., Pawluk, H., Czuczejko, J., Szewczyk-Golec, K., et al. (2013). Age-related changes in an antioxidant defense system in elderly patients with essential hypertension compared with healthy controls. Redox Rep. 16, 71–77. doi:10.1179/174329211X13002357050897
Sakamoto, K., Jin, S. P., Goel, S., Jo, J. H., Voisin, B., Kim, D., et al. (2021). Disruption of the endopeptidase ADAM10-Notch signaling axis leads to skin dysbiosis and innate lymphoid cell-mediated hair follicle destruction. Immunity 54, 2321–2337.e10. doi:10.1016/j.immuni.2021.09.001
Sakurai, Y., Yamaguchi, M., and Abiko, Y. (2000). Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblasts. Eur. J. Oral Sci. 108, 29–34. doi:10.1034/j.1600-0722.2000.00783.x
Salhab, O., Khayat, L., and Alaaeddine, N. (2022). Stem cell secretome as a mechanism for restoring hair loss due to stress, particularly alopecia areata: narrative review. J. Biomed. Sci. 29, 77. doi:10.1186/s12929-022-00863-6
Saxena, N., Ansari, K. M., Kumar, R., Dhawan, A., Dwivedi, P. D., and Das, M. (2009). Patulin causes DNA damage leading to cell cycle arrest and apoptosis through modulation of Bax, p53 and p21/WAF1 proteins in skin of mice. Toxicol. Appl. Pharmacol. 234, 192–201. doi:10.1016/j.taap.2008.09.033
Schuler, N., and Rübe, C. E. (2013). Accumulation of DNA damage-induced chromatin alterations in tissue-specific stem cells: the driving force of aging? PLoS ONE 8, e63932. doi:10.1371/journal.pone.0063932
Schumacher, B., Pothof, J., Vijg, J., and Hoeijmakers, J. H. J. (2021). The central role of DNA damage in the ageing process. Nature 592, 695–703. doi:10.1038/s41586-021-03307-7
Seiberg, M. (2013). Age-induced hair greying – the multiple effects of oxidative stress. Int. J. Cosmet. Sci. 35, 532–538. doi:10.1111/ics.12090
Serruya, R., and Maor, Y. (2021). Hair growth-promotion effects at the cellular level and antioxidant activity of the plant-based extract PhyllotexTM. Heliyon 7, e07888. doi:10.1016/j.heliyon.2021.e07888
Shafiq, M., Chen, Y., Hashim, R., He, C., Mo, X., and Zhou, X. (2021). Reactive oxygen species-based biomaterials for regenerative medicine and tissue engineering applications. Front. Bioeng. Biotechnol. 9, 821288. doi:10.3389/fbioe.2021.821288
Shibagaki, N., Suda, W., Clavaud, C., Bastien, P., Takayasu, L., Iioka, E., et al. (2017). Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci. Rep. 7, 10567. doi:10.1038/s41598-017-10834-9
Shimizu, Y., Ntege, E. H., Sunami, H., and Inoue, Y. (2022). Regenerative medicine strategies for hair growth and regeneration: a narrative review of literature. Regen. Ther. 21, 527–539. doi:10.1016/j.reth.2022.10.005
Shin, H., Ryu, H. H., Yoon, J., Jo, S., Jang, S., Choi, M., et al. (2015). Association of premature hair graying with family history, smoking, and obesity: a cross-sectional study. J. Am. Acad. Dermatology 72, 321–327. doi:10.1016/j.jaad.2014.11.008
Shwartz, Y., Gonzalez-Celeiro, M., Chen, C. L., Pasolli, H. A., Sheu, S. H., Fan, S. M. Y., et al. (2020). Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182, 578–593. doi:10.1016/j.cell.2020.06.031
Siebenhaar, F., Sharov, A. A., Peters, E. M., Sharova, T. Y., Syska, W., Mardaryev, A. N., et al. (2007). Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata). J. Investigative Dermatology 127, 1489–1497. doi:10.1038/sj.jid.5700704
Sotiropoulou, P. A., Candi, A., Mascré, G., De Clercq, S., Youssef, K. K., Lapouge, G., et al. (2010). Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat. Cell Biol. 12, 572–582. doi:10.1038/ncb2059
Stenn, K. S., and Paus, R. (2001). Controls of hair follicle cycling. Physiol. Rev. 81, 449–494. doi:10.1152/physrev.2001.81.1.449
Stevens, J., and Khetarpal, S. (2018). Platelet-rich plasma for androgenetic alopecia: a review of the literature and proposed treatment protocol. Int. J. Womens Dermatol 5, 46–51. doi:10.1016/j.ijwd.2018.08.004
Stone, R. C., Aviv, A., and Paus, R. (2021). Telomere dynamics and telomerase in the biology of hair follicles and their stem cells as a model for aging research. J. Investigative Dermatology 141, 1031–1040. doi:10.1016/j.jid.2020.12.006
Straum, O. K. (2020). The optimal platelet concentration in platelet-rich plasma for proliferation of human cells in vitro—diversity, biases, and possible basic experimental principles for further research in the field: a review. PeerJ 8, e10303. doi:10.7717/peerj.10303
Su, L.-J., Zhang, J. H., Gomez, H., Murugan, R., Hong, X., Xu, D., et al. (2019). Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 5080843. doi:10.1155/2019/5080843
Su, X., Paris, M., Gi, Y. J., Tsai, K. Y., Cho, M. S., Lin, Y. L., et al. (2009). TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell 5, 64–75. doi:10.1016/j.stem.2009.04.003
Sun, H., Zhang, Y.-X., and Li, Y.-M. (2021). Generation of skin organoids: potential opportunities and challenges. Front. Cell Dev. Biol. 9, 709824. doi:10.3389/fcell.2021.709824
Sun, Q., Lee, W., Hu, H., Ogawa, T., De Leon, S., Katehis, I., et al. (2023). Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature 616, 774–782. doi:10.1038/s41586-023-05960-6
Tarng, D.-C., Huang, T. P., Wei, Y. H., Liu, T. Y., Chen, H. W., Wen Chen, T., et al. (2000). 8-hydroxy-2′-deoxyguanosine of leukocyte DNA as a marker of oxidative stress in chronic hemodialysis patients. Am. J. Kidney Dis. 36, 934–944. doi:10.1053/ajkd.2000.19086
Taylor, D. N., Winfield, T., and Wynd, S. (2020). Low-level laser light therapy dosage variables vs treatment efficacy of neuromusculoskeletal conditions: a scoping review. J. Chiropr. Med. 19, 119–127. doi:10.1016/j.jcm.2020.06.002
Tirichen, H., Yaigoub, H., Xu, W., Wu, C., Li, R., and Li, Y. (2021). Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress. Front. Physiology 12, 627837. doi:10.3389/fphys.2021.627837
Titova, L. V., Ayesheshim, A. K., Golubov, A., Fogen, D., Rodriguez-Juarez, R., Hegmann, F. A., et al. (2013). Intense THz pulses cause H2AX phosphorylation and activate DNA damage response in human skin tissue. Biomed. Opt. Express 4, 559–568. doi:10.1364/BOE.4.000559
Tobin, D. J. (2008). Human hair pigmentation biological aspects. Int. J. Cosmet. Sci. 30, 233–257. doi:10.1111/j.1468-2494.2008.00456.x
Trüeb, R. M. (2005). Aging of hair. J Cosmet. Dermatology 4, 60–72. doi:10.1111/j.1473-2165.2005.40203.x
Trüeb, R. M. (2006). Pharmacologic interventions in aging hair. Clin. Interventions Aging 1, 121–129. doi:10.2147/ciia.2006.1.2.121
Trüeb, R. M. (2009). Oxidative stress in ageing of hair. Int. J. Trichology 1, 6–14. doi:10.4103/0974-7753.51923
Trüeb, R. M. (2021). Oxidative stress and its impact on skin, scalp and hair. Int. J. Cosmet. Sci. 43, S9–S13. doi:10.1111/ics.12736
Ustuner, E. T. (2013). Cause of androgenic alopecia: crux of the matter. Plast. Reconstr. Surg. Glob. Open 1, e64. doi:10.1097/GOX.0000000000000005
Van Neste, D., and Tobin, D. J. (2004). Hair cycle and hair pigmentation: dynamic interactions and changes associated with aging. Micron 35, 193–200. doi:10.1016/j.micron.2003.11.006
Vatanashevanopakorn, C., and Sartyoungkul, T. (2023). iPSC-based approach for human hair follicle regeneration. Front. Cell Dev. Biol. 11, 1149050. doi:10.3389/fcell.2023.1149050
Vermeij, W. P., Hoeijmakers, J. H., and Pothof, J. (2014). Aging: not all DNA damage is equal. Curr. Opin. Genet. Dev. 26, 124–130. doi:10.1016/j.gde.2014.06.006
Wang, A. B., Zhang, Y. V., and Tumbar, T. (2017). Gata6 promotes hair follicle progenitor cell renewal by genome maintenance during proliferation. EMBO J. 36, 61–78. doi:10.15252/embj.201694572
Wang, E. C. E., Dai, Z., Ferrante, A. W., Drake, C. G., and Christiano, A. M. (2019). A subset of TREM2+ dermal macrophages secretes Oncostatin M to maintain hair follicle stem cell quiescence and inhibit hair growth. Cell Stem Cell 24, 654–669. doi:10.1016/j.stem.2019.01.011
Wang, Y., Sui, Y., Lian, A., Han, X., Liu, F., Zuo, K., et al. (2021). PBX1 attenuates hair follicle-derived mesenchymal stem cell senescence and apoptosis by alleviating reactive oxygen species-mediated DNA damage instead of enhancing DNA damage repair. Front. Cell Dev. Biol. 9, 739868. doi:10.3389/fcell.2021.739868
Wang, Y., Sui, Y., Niu, Y., Liu, D., Xu, Q., Liu, F., et al. (2023). PBX1-SIRT1 positive feedback loop attenuates ROS-mediated HF-MSC senescence and apoptosis. Stem Cell Rev Rep 19, 443–454. doi:10.1007/s12015-022-10425-w
Williams, R., Pawlus, A. D., and Thornton, M. J. (2020). Getting under the skin of hair aging: the impact of the hair follicle environment. Exp. Dermatol. 29, 588–597. doi:10.1111/exd.14109
Xiao, S., Wang, J., Chen, Q., Miao, Y., and Hu, Z. (2019). The mechanism of activated platelet-rich plasma supernatant promotion of hair growth by cultured dermal papilla cells. J. Cosmet. Dermatology 18, 1711–1716. doi:10.1111/jocd.12919
Yang, G., Chen, H., Chen, Q., Qiu, J., Qahar, M., Fan, Z., et al. (2023). Injury-induced interleukin-1 alpha promotes Lgr5 hair follicle stem cells de novo regeneration and proliferation via regulating regenerative microenvironment in mice. Inflamm. Regen. 43, 14. doi:10.1186/s41232-023-00265-7
Yousefzadeh, M., Henpita, C., Vyas, R., Soto-Palma, C., Robbins, P., and Niedernhofer, L. (2021). DNA damage—how and why we age? eLife 10, e62852. doi:10.7554/eLife.62852
Zhai, X., Gong, M., Peng, Y., and Yang, D. (2021). Effects of UV induced-photoaging on the hair follicle cycle of C57BL6/J mice. CCID 14, 527–539. doi:10.2147/CCID.S310487
Zhang, B., Ma, S., Rachmin, I., He, M., Baral, P., Choi, S., et al. (2020). Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 577, 676–681. doi:10.1038/s41586-020-1935-3
Zhang, J., Wang, X., Vikash, V., Ye, Q., Wu, D., Liu, Y., et al. (2016). ROS and ROS-mediated cellular signaling. Oxid. Med. Cell Longev. 2016, 4350965. doi:10.1155/2016/4350965
Keywords: hair aging, oxidative stress, hormonal disorders, inflammation, DNA damage, hair graying, hair loss
Citation: Liang A, Fang Y, Ye L, Meng J, Wang X, Chen J and Xu X (2023) Signaling pathways in hair aging. Front. Cell Dev. Biol. 11:1278278. doi: 10.3389/fcell.2023.1278278
Received: 16 August 2023; Accepted: 06 November 2023;
Published: 16 November 2023.
Edited by:
Marco Tatullo, University of Bari Medical School, ItalyReviewed by:
Elisabetta Palazzo, University of Modena and Reggio Emilia, ItalyCopyright © 2023 Liang, Fang, Ye, Meng, Wang, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xusheng Wang, d2FuZ3hzaDI3QG91dGxvb2suY29t; Jinsong Chen, Y2pzZnN5eXlAMTI2LmNvbQ==; Xuejuan Xu, c25vd2NhcmVzc3lvdUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.