
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 03 August 2023
Sec. Molecular and Cellular Pathology
Volume 11 - 2023 | https://doi.org/10.3389/fcell.2023.1215698
This article is part of the Research Topic Intervertebral Disc Degeneration: Mechanisms and Therapeutics View all 6 articles
Intervertebral disc degeneration is thought to be a major contributor to low back pain, the etiology of which is complex and not yet fully understood. To compensate for the lack of drug and surgical treatment, mesenchymal stem cells have been proposed for regenerative treatment of intervertebral discs in recent years, and encouraging results have been achieved in related trials. Mesenchymal stem cells can be derived from different parts of the body, among which mesenchymal stem cells isolated from the fetal umbilical cord have excellent performance in terms of difficulty of acquisition, differentiation potential, immunogenicity and ethical risk. This makes it possible for umbilical cord derived mesenchymal stem cells to replace the most widely used bone marrow-derived and adipose tissue derived mesenchymal stem cells as the first choice for regenerating intervertebral discs. However, the survival of umbilical cord mesenchymal stem cells within the intervertebral disc is a major factor affecting their regenerative capacity. In recent years biomaterial scaffolds in tissue engineering have aided the survival of umbilical cord mesenchymal stem cells by mimicking the natural extracellular matrix. This seems to provide a new idea for the application of umbilical cord mesenchymal stem cells. This article reviews the structure of the intervertebral disc, disc degeneration, and the strengths and weaknesses of common treatment methods. We focus on the cell source, cell characteristics, mechanism of action and related experiments to summarize the umbilical cord mesenchymal stem cells and explore the feasibility of tissue engineering technology of umbilical cord mesenchymal stem cells. Hoping to provide new ideas for the treatment of disc degeneration.
With the growth of the global population and accelerated aging, low back pain (LBP) has become common public health problem worldwide (Cieza et al., 2021). Currently, the prevalence of LBP is 9.4% worldwide and is the leading cause of disability and loss of productivity (Geurts et al., 2018; Wu et al., 2020; Knezevic et al., 2021). Annually, the direct cost of treating LBP is approximately US$ 30 billion and the indirect socio-economic losses account for approximately US$ 100 billion in the United States (Binch et al., 2021). Although the causative factors of LBP are complex and varied, intervertebral disc degeneration (IDD) is considered to be the most common cause of LBP(Maher et al., 2017). IDD can be caused by cellular senescence, genetics, mechanical loading, obesity and even smoking (Adams and Roughley, 2006; Xin et al., 2022).
At present, the treatment of IDD mainly includes conservative treatment and surgical treatment. Although these treatments can provide good relief to patients, they do not slow or reverse the reduction of extracellular matrix (ECM) and nucleus pulposus cells (NPCs), do not repair the patient’s disc tissue, and have unsatisfactory long-term outcomes. In contrast, regenerative therapies based on restoring the physiological structure and biomechanical function of the intervertebral disc (IVD) have gained widespread interest in recent years. Among them, the current research related to mesenchymal stem cells (MSCs) for IDD is the most extensive. MSCs are pluripotent stem cells with the ability to differentiate into a tri-spectrum of osteoblasts, chondrocytes and adipocytes (Pittenger et al., 1999). Positivity for surface markers CD73, CD105 and CD90 is a distinctive feature (Dominici et al., 2006). MSCs are used as the first choice for regenerating IVD because of their abundant source and easy availability, extremely low immunogenicity, strong ability to induce differentiation, and to proliferate in a low-oxygen, low-sugar environment (Miao et al., 2006).
In the past, bone marrow mesenchymal stem cells (BMMSCs) were considered to be the gold standard of regenerative therapy. However, recent studies have found that umbilical cord mesenchymal stem cells (UCMSCs) are superior to BMMSCs in terms of cell source and differentiation potential. The IVD is the largest avascular tissue in the body (Molladavoodi et al., 2020). The survival of UCMSCs injected into the intervertebral disc is limited by the complex anatomy and harsh microenvironment within the disc (Sun et al., 2020). In recent years, with the continuous development of biomaterials with good biocompatibility as well as mechanical properties, tissue engineering techniques by implanting pretreated MSCs into biomaterial scaffolds have compensated for the shortcomings of MSCs for the treatment of IDD. However, there is still controversy regarding the selection of the most suitable cell type as well as the biological material. In this paper, we explored the effectiveness and feasibility of UCMSCs and their tissue engineering for regenerating IVD, and we hope that this study will help researchers to explore more systematically the prospects of UCMSCs in regenerating IVD.
IVD is the largest avascular tissue in the body and consists of fibrocartilage tissue, which is one of the most important structures of the spine. Anatomically, IVD consists of three main components: the central highly hydrated gelatinous nucleus pulposus (NP), fibrous rings (AF) consisting of thin sheets of collagen fibers around the NP, and cartilage end plate (CEP) is a transparent cartilage structure that connects adjacent vertebrae (Smith et al., 2011; Molladavoodi et al., 2020). The above structure distributes the axial load from the spinal cone and increases the mobility of the spine. NP is rich in proteoglycans and type II collagen, and it is highly hydrated, such that physiologic osmotic pressures readily dissipate any mechanical forces transmitted through the spine (Frith et al., 2013). AF is a laminar structure consisting mainly of type I collagen in a highly oriented manner, with approximately 15–25 layers. The closer the AF is to NP, the higher content of type II collagen and water (Torre et al., 2019; Kamali et al., 2021). CEP consists of hyaline cartilage that lies between the soft tissue of the IVD and the bony structures of the vertebral body. CEP is essential to maintain the mechanical integrity of the IVD and the exchange of nutrients (Zhang X. B. et al., 2021). Essential substances such as glucose and oxygen permeate into the NP mainly through the CEP to maintain IVD activity (Frost et al., 2019). ECM is present in the extracellular environment of all tissues. The main components of the ECM within the IVD include: collagens, proteoglycan, and non-collagenous proteins. The intensity of normal IVD is primarily affected by the components of ECM (Roughley et al., 2006; Liang et al., 2022).
The specific mechanism leading to IDD is still unclear, and the prevailing view is that cell senescence, inadequate nutritional supply, repeated mechanical stress, obesity, trauma, genetics, and even smoking can lead to the development of IDD (Adams and Roughley, 2006; Gübitz et al., 2018; Okada et al., 2019; Zhang et al., 2019). Among them, genetic factors are the main cause of IDD, approximately 50%–70% of the variability in disc degeneration is caused by an individual’s genetic inheritance (Battié et al., 1995; Buckwalter, 1995; Battié et al., 2008). In the early stages of IDD, there is an altered NPCs phenotype and a decrease in cell numbers, as well as an upregulation of the expression of enzymes such as MMP that mediate the degradation of ECM. This results in the inhibition of proteoglycans, glycosaminoglycans, aggrecan and type II collagen production, and an increase in type I collagen production. Ultimately, this leads to a weakening of IVD hydration and a reduction in height. The axial pressure from the spine is dispersed through the NP to the adjacent AF, which leads to altered biomechanical function of the AF as well as structural damage (Freemont, 2009; Morris et al., 2021; Ohnishi et al., 2022). The rupture of AF provides a suitable microenvironment for the growth of sensory neurons and blood vessels, prompting their growth into the IVD and accelerating the development of pain (Yee et al., 2016). As IDD progresses further, high levels of inflammatory cytokines are produced by AF cells, NPCs, and immune cells. These inflammatory factors, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α, further aggravate IDD by exacerbating the inflammatory response, inhibiting IVD cell proliferation and differentiation, accelerating cellular senescence and apoptosis, and promoting ECM degradation (Rogers et al., 2017; Song et al., 2017; Wang et al., 2020). Calcification of CEP exacerbates metabolic disturbances within the IVD by affecting the exchange of substances (Wang et al., 2018). In addition, the above-mentioned series of pathological changes promote the production of reactive oxygen species (ROS) within the IVD and form a positive feedback loop, accelerating apoptosis within the disc (Feng et al., 2017).
Currently, the traditional treatment modalities for IDD include conservative treatment and surgery, and it is critical to choose the appropriate treatment for patients with different degrees of degeneration and clinical manifestations. For patients with early IDD, pharmacotherapy has a clear role in controlling pain and improving patient function and quality of life (van Middelkoop et al., 2011). However, the long-term use of drugs may lead to serious side effects as well as potential drug addiction and dependence, which makes the clinical use of drug therapy controversial (Hale et al., 2005; Wong et al., 2017). In addition, non-pharmacological treatments such as bed rest, brace immobilization, acupuncture, massage, electromagnetic or electrothermal therapy are also very effective in relieving acute attacks of LBP, and they are often combined with pharmacological treatments and surgery (Wegner et al., 2013; Glazov et al., 2014).
For patients with advanced IDD and those who have failed conservative treatment, surgery becomes the best option. Common surgical procedures include spinal decompression, spinal fusion and total disc replacement. Currently, spinal fusion is the most widely used in clinical practice and is considered the gold standard for the surgical treatment of IDD (Lee et al., 2016). Spinal fusion improves the stability of the spine and reduces patient pain by joining and fusing adjacent vertebrae (Phillips et al., 2013). It has been shown that when the vertebrae are fused, intervertebral motion between adjacent vertebrae is reduced, increasing the load on the surrounding tissues and adjacent IVD, which may further contribute to the development of IDD in adjacent segments (Kumar et al., 2001). For total disc replacement, prolonged wear of IVD prostheses prepared from metal and polymeric materials can lead to an immune inflammatory response and bone loss and are usually used only in the presence of single-segment IDD and in the absence of small joint disease. (Fritzell et al., 2001; Veruva et al., 2017; Werner et al., 2018). Surgical treatment can provide good relief to patients, but the high incidence of postoperative IVD stenosis and recurrence has become a major problem for physicians and patients (Scoville and Corkill, 1973; Hashimoto et al., 2019). Consequently, further explorations about more effective IDD treatment approaches are of great significance.
In recent years, researchers have begun to investigate regenerative therapies aimed at regulating anabolic and catabolic metabolism within the IVD and restoring ECM and NPCs. These methods mainly include direct injection of growth factors, cell transplantation, gene therapy and tissue engineering.
In cell therapy, researchers transplant cells with differentiation potential into the IVD to help restore the number of cells in the disc, promote ECM synthesis, and immune regulation. For discs with different degrees of development and degeneration, the selection of the appropriate cell type and source is critical for successful cell transplantation and disc regeneration. Common cell types include MSCs, intervertebral disc-derived stem cells (IVDSCs), and pluripotent stem cells (PSCs) (Hwang et al., 2009). These cells are related in terms of embryonic origin and genealogy. Among them, MSCs lack low expression of the major histocompatibility complex II and co-stimulatory factors CD80, CD86 and CD40, which leads to their low immunogenicity (Fouillard et al., 2007; Jacobs et al., 2013). In addition, MSCs have the advantages of a wide source, high proliferative capacity, multispectral differentiation potential and low tumorigenicity (Tsaryk et al., 2017).This seems to offer a promising alternative to regenerative therapy. However, the microenvironment of IVD is characterized by hypoxia, nutrient deficiency, acidity, hyperosmolarity, and mechanical loading. Its internal microenvironment further deteriorates during IVD denaturation with mechanical overload and accumulation of inflammatory cytokines and proteases (Lyu et al., 2019; Ryu et al., 2020; Vasanthan et al., 2020; Guerrero et al., 2021). This causes the survival of cells injected into the disc to be a primary issue. Some studies have reported that leakage during MSCs injection therapy can further aggravate IDD (Vadalà et al., 2012). In addition, the potential ethical issues of cell therapy, how to obtain sufficient numbers of MSCs for clinical treatment and the associated rejection reactions are still issues that we need to address urgently.
Direct injection of growth factors into the IVD has also been used as a new treatment modality. Growth factors commonly used in injectable therapy include bone morphogenetic protein (BMP)-7, BMP-2, transforming growth factor-β (TGF-β), insulin like growth factor (IGF), epidermal growth factor (EGF) and differentiation factor (GDF)-5 etc., (Knezevic et al., 2017; Cecerska-Heryć et al., 2022). However, growth factors have a short half-life and often require multiple injections for treatment, which makes the risk of injection-related injury as well as infection significantly higher. Growth factors are peptides that target cells. However, for patients with advanced IDD, only a small number of cells are present within the IVD, which makes growth factor therapy for IDD difficult to implement clinically. Gene therapy mainly involves the transfer of target genes into IVD cells via viral or non-viral vectors, which are amplified in vitro and then subsequently injected into the IVD. These IVD cells with the target gene will be retained in the IVD for a long time, promoting IVD regeneration and improving the patient’s symptoms. (Sampara et al., 2018). However, this gene therapy is difficult to mitigate the complications associated with viruses used for gene transfection and non-viral vectors, and the clinical application of this method is still limited to medically life-threatening diseases (Han, 2020; Takeoka et al., 2020). Moreover, most of the relevant studies at this stage are still in the experimental stage and still need a lot of investment in research.
MSCs were first discovered and isolated in the bone marrow of rats (Friedenstein et al., 1966), and more and more organs and tissues have been reported to provide a stable source of MSCs, including bone marrow, adipose tissue, umbilical cord, pulp, placenta, skin, tonsils etc., (Zuk et al., 2001; Lai et al., 2014; Ryu et al., 2014; Yan et al., 2014; Miceli et al., 2021). MSCs from different sources have certain similarities in morphology, differentiation potential and immunophenotype, but there are still differences in population numbers, growth rates, colony frequencies, success rates of isolation, immunosuppressive ability and gene expression profiles (Thirumala et al., 2013).
Among them, BMMSCs and ADMSCs isolated from adult tissues are most frequently used for therapeutic purposes (Wei et al., 2014). However, as patients age, the number of bone marrow tissue and adipose tissue MSCs decreases, the difficulty of obtaining them and the associated risks of invasive operations are increased, and the ability to proliferate and differentiate decreases. In recent years, UCMSCs derived from allogeneic embryos have been used as an ideal alternative. Compared to MSCs derived from adult tissues, UCMSCs have the following advantages. In terms of access, UCMSCs derived from fetal discarded umbilical cords does not generate ethical controversy and without invasive manipulation, which allows for a large and stable source of UCMSCs(Lu et al., 2006; Fong et al., 2012). In terms of biological properties, UCMSCs exhibit higher proliferative and differentiation potential, and UCMSCs are three times more likely than BMMSCs to induce differentiation into chondrocytes and to produce collagen (Hsieh et al., 2010; Zhang et al., 2011). In terms of application prospects, the rate and stability of in vitro expansion of UCMSCs are also significantly higher than that of BMMSCs (Sarugaser et al., 2005; Vellasamy et al., 2012; Vawda and Fehlings, 2013), which allows UCMSCs to meet the large number of stem cells required for clinical use. Finally, in terms of safety, UCMSCs also have lower immunogenicity and cause fewer teratomas. However, at present, how to preserve cells for a long time, improve the stability of in vitro expansion, and whether the umbilical cord donor is healthy is an urgent problem for us to solve. The advantages and disadvantages of UCMSCs, BMMSCs, and ADMSCs are shown in Table 1.
The human umbilical cord, the structure that connects the placenta to the developing fetus and thus provides the source of fetal nutrition, consists of two arteries and one vein surrounded by mucus connective tissue called the Wharton’s Jelly (WJ), with the outermost layer wrapped around the amniotic epithelium (Arutyunyan et al., 2016). The WJ makes up the majority of the cord and is composed of collagen fibrils, proteoglycans and stromal cells, which is the main source of UCMSCs. Among them, WJ-MSCs have significant advantages over MSCs from other parts of the umbilical cord in terms of quantity, ease of isolation, proliferation ability and viability.
At present, UCMSCs mainly achieves the purpose of treating IDD through the following main ways. On the one hand, UCMSCs have the potential to proliferate and multidirectionally differentiate, replenish NPCs by differentiating to NPCs, and promote the synthesis of ECM by NPCs (Steck et al., 2009; Fan et al., 2011). UCMSCs, on the other hand, have paracrine abilities. It can not only secrete growth factors and cytokines to regenerate IVD, but also secrete certain anti-inflammatory cytokines to regulate the inflammatory response of degenerative IVD, reduce pain and delay the process of IDD (Kuchroo et al., 2015; Shen et al., 2015; Beeravolu et al., 2018).
Although the number of reports on MSCs for the treatment of IDD is relatively large, most of these studies have focused on BMMSCs and ADMSCs. The existing ex vivo studies on UCMSCs mainly focus on the effects of UCMSCs on IVD, promoting the proliferation and differentiation of UCMSCs and improving their survival rate, while the related clinical studies need to be further explored. Zhang et al. (Zhang et al., 2015) injected UCMSCs isolated from human umbilical cords into canine IVD after labeling with EGFP, and found that UCMSCs survived in the IVD for a long time, delaying the rate of disc height decline as well as upregulating the expression of disc matrix genes, aggrecan, type II collagen (COL2), and SOX-9 [SRY (sex determining region Y)-box 9]. Wharton’s jelly cells (WJCs) still detectable in the IVD at 24 weeks. Perez-Cruet et al. (Perez-Cruet et al., 2019) found that by transplanting human UCMSCs into the IDD model of the rabbit, NPCs derivatives of MSCs expressed known NP-specific genes, SOX9, ACAN, COL2, FOXF1, and KRT19. Transplanted cells survived, dispersed, and integrated into the degenerated IVD. IVD augmented with NPCs showed significant improvement in the histology, cellularity, sulfated glycosaminoglycan and water contents of the NP. Ekram et al. (Ekram et al., 2021) showed that human UCMSCs can be differentiated into cartilage progenitor cells by using cartilage induction medium. Both chondroprogenitor cells and human UCMSCs in animal intervertebral discs express SOX9, transforming growth factor (TGF)-β1, ACAN, BMP2 and GDF5 genes as well as regulate inflammatory responses. Transplanted chondroprogenitors showed better survival, homing, and distribution in IVD as compared to normal MSCs. However, Wu et al. (Wu et al., 2017) found that NP progenitor cells isolated from degenerated IVDs exhibited lower proliferative and differentiation capacity compared to UCMSCs. This might account for the distinct NP microenvironment and the poor capacity for disc regeneration. Although UCMSCs can persist in the IVD for a long time and promote regeneration, microenvironmental and other factors may have some negative effects on their regenerative capacity.
To further aid in the differentiation of UCMSCs, Lee et al. (Chon et al., 2013) cultured UCMSCs in a hypoxic environment with three differentiation conditions: NP differentiation media (containing 2.5% Matrigel™ solution to provide for a pseudo-three-dimensional laminin culture system) with no serum, or the same media supplemented with either IGF-1 or TGF-β1. The study proved that a pseudo-three-dimensional culture condition (laminin-1 rich) promoted HUCMSC differentiation under no serum conditions. Neither growth factor treatment generated distinct differences in NP-like phenotype for HUCMSC as compared with no-serum conditions. Khalid et al. (Khalid et al., 2022) found that Sox-9 and Six-1 transcription factor-transfected UCMSCs greatly enhanced gene expression of TGF β-1, BMP, Sox-9, Six-1 and Aggrecan and accelerated differentiation to chondrocytes.
In addition to the ability of UCMSCs to differentiate directly into NPCs, there is also an effect on NPCs in degenerating IVD. Han et al. (Han et al., 2018), Ruan et al. (Ruan et al., 2012) and Zeng et al. (Zeng et al., 2020) cultured human WJCs with NPCs with and without direct cell-cell contact, respectively. The results showed that co-culture induced NP-like cell differentiation in WJCs, with significantly increased expression of NP-maker, OCT4, Nanog and Tie2 genes, and direct cell-to-cell contact, which could produce more favorable gene expression. Yuan et al. (Yuan et al., 2021) found that, human UCMSCs exosomes were found to effectively improve the viability of NP cells and protect them from pyroptosis through targeting METTL14.
Clinical trials of human UCMSCs make very few, and it is essential to investigate their effectiveness and safety. yang et al. (Pang et al., 2014) treated 2 patients with chronic discogenic low back pain with HUC-MSC transplantation. During the 2-year follow-up period, VAS and ODI scores decreased significantly. However, the reliability of this trial was poor due to the limited number of patients. In addition, some scholars have explored the direct injection of umbilical cord tissue into the affected area to relieve spinal disorders, and found significant pain relief in most patients through long-term follow-up, but there were individual cases with no significant pain relief, and no adverse events were reported (Buck, 2019; Ross et al., 2022).
Although UCMSCs show strong potential for the treatment of IDD, Matta et al. (Matta et al., 2021) found in their experiments that treatment with NTG-101 resulted in the suppression of inflammation induced p38 and NFκB, leading to the suppression of catabolic genes, but Smad-2/3, Erk-1/2 and Akt-dependent signaling activation, inducing anabolic genes for IVD-NP. A single injection of NTG-101 into the degenerating disc showed superior benefits compared to transplantation of human UCMSCs. A summary of trials related to the use of UCMSCs for regenerative treatment of IDD is shown in Table 2. These experiments demonstrated that UCMSCs can significantly delay or reverse IDD by promoting ECM synthesis, supplementing NPCs and affecting IVD endocytosis, etc. Compared with other stem cells, UCMSCs exhibit high proliferation and differentiation potential. We also demonstrated the feasibility of using UCMSCs in clinical practice.
Tissue engineering techniques have received increasingly widespread attention in order to eliminate the effects of the harsh microenvironment and mechanical loading within the IVD on the regenerative capacity of MSCs. Tissue engineering of the IVD consists of three key components: 1) seed cells that can regenerate IVD; 2) a biocompatible scaffold made of bioactive materials that can mimic ECM; 3) bioactive factors that promote cell proliferation and differentiation and migration (Gkantsinikoudis et al., 2022). A schematic diagram of the tissue engineering of UCMSCs is shown in Figure 1.
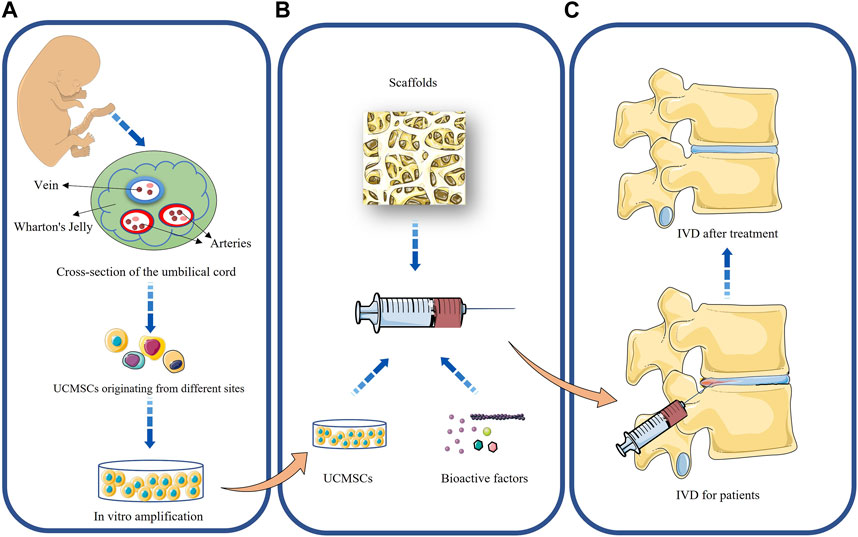
FIGURE 1. Application of UCMSCs to the treatment of intervertebral disc degeneration. (A): Acquisition and in vitro amplification of UCMSCs; (B): Tissue engineering of UCMSCs; (C): Repair of intervertebral disc by UCMSCs.
To help the survival of MSCs in the IVD, researchers have successively proposed culturing the cells in vitro under hypoxic conditions, heat treatment and the use of exogenous bioactive molecules (Kakudo et al., 2015; Baer et al., 2018; Chen et al., 2018). However, normal intra-IVD tissues consist of cells and ECM, and pretreatment for MSCs alone is not sufficient. With the development of biomaterials science in recent years, a variety of biomaterials have been used as scaffolds for cells to mimic the function of normal ECM. These biomaterials should be biocompatible and help restore cells and ECM within the IVD, reduce inflammatory response and inhibit pathological fibrosis (Huang et al., 2018). It is also indispensable to be able to stabilize the IVD under internal pressure load and to improve the stability of the spine. Biomaterial scaffolds are one of the three key elements in IVD tissue engineering, mostly hydrogel or solid scaffolds, which form the backbone of tissue regeneration. Differences in mechanical strength, void filling, cytotoxicity, immunogenicity, degradability and manufacturing cost exist among different materials, and it is particularly important to select the most appropriate type of scaffold material. According to the main source of materials, they can be classified as natural materials, synthetic materials and hybrid materials.
Reduced hydration within degenerated IVD is one of the main reasons for their development, and it is crucial to address the water content when selecting biomaterials for regenerating discs. Hydrophilic hydrogels are widely used in tissue engineering for IVD because they can be stored for longer periods of time and have a similar structure and function to ECM. Some scholars have verified that hydrogel scaffolds can help MSCs perform greater regenerative functions by mixing various materials into hydrogels and injecting them into animal IDD models in combination with BMMSCs (Smith et al., 2014; Zhang C. et al., 2021). However, it has also been reported that MSCs bound to fibrin carriers had little effect on porcine IVD regeneration and highly did not significantly improve (Acosta et al., 2011; Omlor et al., 2018).
Studies related to the use of UCMSCs alone for the treatment of IDD have yielded fairly promising results, but research in tissue engineering combining UCMSCs with biomaterial scaffolds still needs to receive more attention. To investigate the effects of tissue engineering of different types of biomaterials on UCMSCs and IVD, the investigators conducted relevant experiments.
Natural materials mainly include hydrogels, such as alginate, agarose, fibrin, hyaluronic, collagen, chitosan, and carboxymethylcellulose (Vaudreuil et al., 2019). These materials have excellent biocompatibility, better mimic natural ECM, and biodegrade to carbon dioxide and water in vivo. However, they have insufficient mechanical strength, rapid degradation rate, unstable biological properties and limited production capacity (Tang et al., 2021). Therefore, a single natural biomaterial scaffold is difficult to be used for tissue engineering of IVD. To investigate whether hydrogels prepared from natural materials are more useful for regenerating IVD in UCMSCs. Leckie et al. (Leckie et al., 2013) established a rabbit annulotomy model for IDD and subsequently randomized them into three groups, injected with UCMSCs alone, hydrogels alone and UCMSCs combined with fibrin hydrogels. Outcome analysis was performed by serial MRI observation as well as IVD histological staining at 4 weeks. The results showed that all three treatment groups exhibited a lower degree of degeneration than the control group in terms of total NP area and MRI index, with the UCMSCs combined with the hydrogel scaffold having the strongest regenerative effect on the IVD. And it showed significant fibrosis within the NP in the group injected with UCMSCs alone, and a significant decrease in the number of cells in the NP in the group injected with hydrogel alone, but maintained the highest number of ECM.
Choi et al. (Choi et al., 2020) found that T cells injected via needle resulted in cell damage and reduced viability and that cell viability was significantly increased when hyaluronan-methylcellulose (HAMC) was co-cultured with WJ-MSCs in vitro. This made the tissue engineering technique of combining HAMC with WJ-MSCs very attractive, so they randomly divided the rats into four groups to receive the following single intradiscal injections after establishing injury-induced IVD degeneration: 1) phosphate-buffered saline (PBS) vehicle, 2) HAMC, 3) WJ-MSCs, 4) WJ-MSCs/HAMC. The results showed that WJ-MSCs/HAMC had the strongest ability to regenerate IVD. Combined injection of WJ-MSCs and HAMC enhanced IVD regeneration through increase in cell survival, attenuation of the activation of iNOS, MMP-13, ADAMTS4 and COX-2, and significant upregulation of ECM, such as aggrecan and collagen type II. Ahn et al. (Ahn et al., 2015) found that the expression levels of TβRI/ALK5 and TβRII in WJ-MSCs from different donors differed between donors, which may affect the regenerative function of WJ-MSCs. They defined MSC-high TR as expression levels of TβRI/ALK5 and TβRII exceeding 5.0% and 20.0%, while MSC-low TR was defined at levels of approximately 1.0% and 7.0%. To this end, they injected cross-linked hyaluronic acid (XHA) scaffolds loaded with WJ-MSCs into a rabbit IDD model. The results showed that significant restoration of the disc water content in rabbits treated with MSC-highTR-loaded XHA scaffold in comparison to rabbits treated with the scaffold alone or MSC-lowTR-loaded XHA scaffold. In addition, morphological and histological analyses revealed that IVD regeneration was highest in rabbits transplanted with MSC-highTR-loaded XHA scaffold. The expression levels of TβRI/ALK5 and TβRII in WJ-MSCs could influence their secretion of cytokines such as GDF-15, MMP-1, and CCL-5 and their response to autocrine TGFβ ligands and that WJ-MSCs could improve IVD degeneration by releasing paracrine factors. Reppel et al. (Reppel et al., 2015) WJ-MSCs were embedded in alginate/hyaluronic acid hydrogels and then placed in vitro for culture. After 28 days of scaffold culture, results showed strong upregulation of cartilage-specific transcript expression. WJ-MSCs exhibited greater type II collagen synthesis than BMMSCs at both transcript and protein levels.
On the contrary, synthetic materials are relatively better than natural materials in terms of mechanical properties, while they are less biocompatible, hydrophilic and cell adhesion. In addition, degradation products of synthetic materials can induce inflammatory responses and reduce the rate of cell proliferation (Danhier et al., 2012). Synthetic materials mainly include poly (D, L-lactide) (PLA) and its derivatives, polyethylene glycol (PEG), polycarbonate urethane (PU), and poly (ε-caprolactone) (PCL). Considering the above reasons, composite materials combining natural and synthetic materials are widely used nowadays. It combines the advantages of both and reduces the impact of their respective disadvantages on regenerative IVD. Composite materials are a combination of two or more materials with different morphology or composition at the micro-/nanoscale (Tang et al., 2021). Li et al. (Li et al., 2017) established a novel biomimetic porous chitosan/poly (l-lactic acid) scaffold with human UCMSCs was applied in lumbar fusion. This approach demonstrated greater IVD regeneration than blank control and autologous bone in a rabbit model of IDD.
In addition, the decellularized matrix obtained by removing cells and antigens from autologous tissues through a series of methods also provides a new idea for tissue engineering of IVD (Xu et al., 2019; Qian et al., 2023). This type of scaffold is similar to ECM within IVD and can better assist cell adhesion, migration and proliferation. However, for humans, the stable access to sufficient amounts of decellularized matrix is the primary issue that limits its use for therapeutic purposes. Currently, WJ from the human umbilical cord is considered to be an ideal source of decellularized matrix scaffolds. These trials suggest that tissue engineering techniques have a stronger effect in restoring ECM and promoting cell proliferation and differentiation than UCMSCs injected alone for IDD. A summary of trials related to tissue engineering of biomaterial scaffolds combined with UCMSCs for regenerative treatment of IDD is shown in Table 3.
Currently, conventional treatment modalities for IDD provide good relief of symptoms in IDD patients, but their long-term outcomes are hardly satisfactory. A series of regenerative therapies have received much attention for their effectiveness in delaying or even reversing IDD. The higher proliferative differentiation potential, higher in vitro expansion rate and stability, lower immunogenicity and risk of infection make UCMSCs promising as an ideal choice for the treatment of IDD. In vitro and in vivo experiments have demonstrated that UCMSCs can differentiate into NPCs, promote ECM synthesis and regulate inflammatory responses within the IVD. However, the regenerative capacity of UCMSCs has reached opposite conclusions in some trials, and relevant clinical trials are scarce.The survival of UCMSCs under the harsh IVD microenvironment and pressure load is the main factor limiting their regenerative capacity. As the field of biomaterials continues to evolve, a new way of thinking is provided by the fact that UCMSCs have been shown to better perform their regenerative role by being attached to a variety of biomaterial scaffolds with excellent properties.
UCMSCs, as stem cells that have only recently entered the public eye, have been little studied, and existing experiments are similarly deficient. The main reason for this situation is the immaturity of UCMSCs in terms of extraction, storage and in vitro amplification. In recent years, cell banks for UCMSCs have been established in several countries with the aim of achieving access to large numbers of UCMSCs and helping heir clinical application. However, the number of established cell banks is currently much lower than expected. With the development and widespread use of 3D printing technology in the field of tissue engineering, 3D printed scaffolds may become a major trend. These scaffolds can be artificially adjusted to better meet the needs of UCMSCs survival and better repair the IVD. In addition, exosomes with lipid bilayers obtained by paracrine action of MSCs have been shown to promote tissue repair and regeneration. Because of their unique cell-free properties, they are significantly superior to MSCs in overcoming the microenvironment within IVD, immune rejection risk, tumorigenicity and ethical risk, which provides new ideas for further studies of UCMSCs. In the future, there are still many issues that need to be addressed for UCMSCs in regenerative treatment of IDD. More trials are needed to validate their effectiveness and safety in order to reduce the burden of IDD patients.
HH wrote the manuscript. XL, JW, MS, and WZ revised the images. JZ and TS revised the tables. ZL reviewed and revised the full text. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Innovation Foundation of Dalian (2022JJ12SN045), and the Natural Science Foundation of Liaoning Province (2022-MS-322). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank the funding agencies for supporting our study. We would also like to thank all present and former lab members for helpful discussions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acosta, F. L., Metz, L., Adkisson, H. D., Liu, J., Carruthers-Liebenberg, E., Milliman, C., et al. (2011). Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng. Part A 17 (23-24), 3045–3055. doi:10.1089/ten.tea.2011.0229
Adams, M. A., and Roughley, P. J. (2006). What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 31 (18), 2151–2161. doi:10.1097/01.brs.0000231761.73859.2c
Ahn, J., Park, E. M., Kim, B. J., Kim, J. S., Choi, B., Lee, S. H., et al. (2015). Transplantation of human Wharton's jelly-derived mesenchymal stem cells highly expressing TGFβ receptors in a rabbit model of disc degeneration. Stem Cell Res. Ther. 6, 190. doi:10.1186/s13287-015-0183-1
Arutyunyan, I., Elchaninov, A., Makarov, A., and Fatkhudinov, T. (2016). Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016, 6901286. doi:10.1155/2016/6901286
Baer, P. C., Overath, J. M., Urbschat, A., Schubert, R., Koch, B., Bohn, A. A., et al. (2018). Effect of different preconditioning regimens on the expression profile of murine adipose-derived stromal/stem cells. Int. J. Mol. Sci. 19 (6), 1719. doi:10.3390/ijms19061719
Battié, M. C., Videman, T., Gibbons, L. E., Fisher, L. D., Manninen, H., and Gill, K. (1995). Determinants of lumbar disc degeneration: A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine (Phila Pa 1976) 20(24), 2601–2612. doi:10.1097/00007632-199512150-00001
Battié, M. C., Videman, T., Levälahti, E., Gill, K., and Kaprio, J. (2008). Genetic and environmental effects on disc degeneration by phenotype and spinal level: A multivariate twin study. Spine (Phila Pa 1976) 33 (25), 2801–2808. doi:10.1097/BRS.0b013e31818043b7
Beeravolu, N., Brougham, J., Khan, I., McKee, C., Perez-Cruet, M., and Chaudhry, G. R. (2018). Human umbilical cord derivatives regenerate intervertebral disc. J. Tissue Eng. Regen. Med. 12 (1), e579–e591. doi:10.1002/term.2330
Binch, A. L. A., Fitzgerald, J. C., Growney, E. A., and Barry, F. (2021). Cell-based strategies for IVD repair: Clinical progress and translational obstacles. Nat. Rev. Rheumatol. 17 (3), 158–175. doi:10.1038/s41584-020-00568-w
Buck, D. (2019). Amniotic umbilical cord particulate for discogenic pain. J. Am. Osteopath Assoc. 119 (12), 814–819. doi:10.7556/jaoa.2019.138
Buckwalter, J. A. (1995). Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 20 (11), 1307–1314. doi:10.1097/00007632-199506000-00022
Cecerska-Heryć, E., Goszka, M., Serwin, N., Roszak, M., Grygorcewicz, B., Heryć, R., et al. (2022). Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 64, 84–94. doi:10.1016/j.cytogfr.2021.11.003
Chen, Z., Chen, L., Zeng, C., and Wang, W. E. (2018). Functionally improved mesenchymal stem cells to better treat myocardial infarction. Stem Cells Int. 2018, 7045245. doi:10.1155/2018/7045245
Choi, U. Y., Joshi, H. P., Payne, S., Kim, K. T., Kyung, J. W., Choi, H., et al. (2020). An injectable hyaluronan-methylcellulose (HAMC) hydrogel combined with Wharton's jelly-derived mesenchymal stromal cells (WJ-MSCs) promotes degenerative disc repair. Int. J. Mol. Sci. 21 (19), 7391. doi:10.3390/ijms21197391
Chon, B. H., Lee, E. J., Jing, L., Setton, L. A., and Chen, J. (2013). Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin-rich pseudo-three-dimensional culture system. Stem Cell Res. Ther. 4 (5), 120. doi:10.1186/scrt331
Cieza, A., Causey, K., Kamenov, K., Hanson, S. W., Chatterji, S., and Vos, T. (2021). Global estimates of the need for rehabilitation based on the global burden of disease study 2019: A systematic analysis for the global burden of disease study 2019. Lancet 396 (10267), 2006–2017. doi:10.1016/s0140-6736(20)32340-0
Danhier, F., Ansorena, E., Silva, J. M., Coco, R., Le Breton, A., and Préat, V. (2012). PLGA-Based nanoparticles: An overview of biomedical applications. J. Control Release 161 (2), 505–522. doi:10.1016/j.jconrel.2012.01.043
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8 (4), 315–317. doi:10.1080/14653240600855905
Ekram, S., Khalid, S., Bashir, I., Salim, A., and Khan, I. (2021). Human umbilical cord-derived mesenchymal stem cells and their chondroprogenitor derivatives reduced pain and inflammation signaling and promote regeneration in a rat intervertebral disc degeneration model. Mol. Cell Biochem. 476 (8), 3191–3205. doi:10.1007/s11010-021-04155-9
Fan, C. G., Zhang, Q. J., and Zhou, J. R. (2011). Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. Rep. 7 (1), 195–207. doi:10.1007/s12015-010-9168-8
Feng, C., Yang, M., Lan, M., Liu, C., Zhang, Y., Huang, B., et al. (2017). Ros: Crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxid. Med. Cell Longev. 2017, 5601593. doi:10.1155/2017/5601593
Fong, C. Y., Gauthaman, K., Cheyyatraivendran, S., Lin, H. D., Biswas, A., and Bongso, A. (2012). Human umbilical cord Wharton's jelly stem cells and its conditioned medium support hematopoietic stem cell expansion ex vivo. J. Cell Biochem. 113 (2), 658–668. doi:10.1002/jcb.23395
Fouillard, L., Chapel, A., Bories, D., Bouchet, S., Costa, J. M., Rouard, H., et al. (2007). Infusion of allogeneic-related HLA mismatched mesenchymal stem cells for the treatment of incomplete engraftment following autologous haematopoietic stem cell transplantation. Leukemia 21 (3), 568–570. doi:10.1038/sj.leu.2404550
Freemont, A. J. (2009). The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatol. Oxf. 48 (1), 5–10. doi:10.1093/rheumatology/ken396
Friedenstein, A. J., Piatetzky, S., and Petrakova, K. V. (1966). Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 16 (3), 381–390. doi:10.1242/dev.16.3.381
Frith, J. E., Cameron, A. R., Menzies, D. J., Ghosh, P., Whitehead, D. L., Gronthos, S., et al. (2013). An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials 34 (37), 9430–9440. doi:10.1016/j.biomaterials.2013.08.072
Fritzell, P., Hägg, O., Wessberg, P., and Nordwall, A. (2001). 2001 volvo award winner in clinical studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: A multicenter randomized controlled trial from the Swedish lumbar spine study group. Spine (Phila Pa 1976) 26(23), 2521–2532. doi:10.1097/00007632-200112010-00002
Frost, B. A., Camarero-Espinosa, S., and Foster, E. J. (2019). Materials for the spine: Anatomy, problems, and solutions. Mater. (Basel) 12 (2), 253. doi:10.3390/ma12020253
Geurts, J. W., Willems, P. C., Kallewaard, J. W., van Kleef, M., and Dirksen, C. (2018). The impact of chronic discogenic low back pain: Costs and patients' burden. Pain Res. Manag. 2018, 4696180. doi:10.1155/2018/4696180
Gkantsinikoudis, N., Kapetanakis, S., Magras, I., Tsiridis, E., and Kritis, A. (2022). Tissue engineering of human intervertebral disc: A concise review. Tissue Eng. Part B Rev. 28 (4), 848–860. doi:10.1089/ten.TEB.2021.0090
Glazov, G., Yelland, M., and Emery, J. (2014). Low-dose laser acupuncture for non-specific chronic low back pain: A double-blind randomised controlled trial. Acupunct. Med. 32 (2), 116–123. doi:10.1136/acupmed-2013-010456
Gübitz, R., Lange, T., Gosheger, G., Heindel, W., Allkemper, T., Stehling, C., et al. (2018). Influence of age, BMI, gender and lumbar level on T1ρ magnetic resonance imaging of lumbar discs in healthy asymptomatic adults. Rofo 190 (2), 144–151. doi:10.1055/s-0043-115898
Guerrero, J., Häckel, S., Croft, A. S., Hoppe, S., Albers, C. E., and Gantenbein, B. (2021). The nucleus pulposus microenvironment in the intervertebral disc: The fountain of youth? Eur. Cell Mater 41, 707–738. doi:10.22203/eCM.v041a46
Hale, M. E., Dvergsten, C., and Gimbel, J. (2005). Efficacy and safety of oxymorphone extended release in chronic low back pain: Results of a randomized, double-blind, placebo- and active-controlled phase III study. J. Pain 6 (1), 21–28. doi:10.1016/j.jpain.2004.09.005
Han, I. B. (2020). Moving forward: Gene therapy for intervertebral disc degeneration. Neurospine 17 (1), 17–18. doi:10.14245/ns.2040108.054
Han, Z., Zhang, Y., Gao, L., Jiang, S., and Ruan, D. (2018). Human Wharton's jelly cells activate degenerative nucleus pulposus cells in vitro. Tissue Eng. Part A 24 (13-14), 1035–1043. doi:10.1089/ten.TEA.2017.0340
Hashimoto, K., Aizawa, T., Kanno, H., and Itoi, E. (2019). Adjacent segment degeneration after fusion spinal surgery-a systematic review. Int. Orthop. 43 (4), 987–993. doi:10.1007/s00264-018-4241-z
Hsieh, J. Y., Fu, Y. S., Chang, S. J., Tsuang, Y. H., and Wang, H. W. (2010). Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton's jelly of umbilical cord. Stem Cells Dev. 19 (12), 1895–1910. doi:10.1089/scd.2009.0485
Huang, Y. C., Hu, Y., Li, Z., and Luk, K. D. K. (2018). Biomaterials for intervertebral disc regeneration: Current status and looming challenges. J. Tissue Eng. Regen. Med. 12 (11), 2188–2202. doi:10.1002/term.2750
Hwang, N. S., Zhang, C., Hwang, Y. S., and Varghese, S. (2009). Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 1 (1), 97–106. doi:10.1002/wsbm.26
Jacobs, S. A., Pinxteren, J., Roobrouck, V. D., Luyckx, A., van't Hof, W., Deans, R., et al. (2013). Human multipotent adult progenitor cells are nonimmunogenic and exert potent immunomodulatory effects on alloreactive T-cell responses. Cell Transpl. 22 (10), 1915–1928. doi:10.3727/096368912x657369
Kakudo, N., Morimoto, N., Ogawa, T., Taketani, S., and Kusumoto, K. (2015). Hypoxia enhances proliferation of human adipose-derived stem cells via HIF-1ɑ activation. PLoS One 10 (10), e0139890. doi:10.1371/journal.pone.0139890
Kamali, A., Ziadlou, R., Lang, G., Pfannkuche, J., Cui, S., Li, Z., et al. (2021). Small molecule-based treatment approaches for intervertebral disc degeneration: Current options and future directions. Theranostics 11 (1), 27–47. doi:10.7150/thno.48987
Khalid, S., Ekram, S., Salim, A., Chaudhry, G. R., and Khan, I. (2022). Transcription regulators differentiate mesenchymal stem cells into chondroprogenitors, and their in vivo implantation regenerated the intervertebral disc degeneration. World J. Stem Cells 14 (2), 163–182. doi:10.4252/wjsc.v14.i2.163
Knezevic, N. N., Candido, K. D., Vlaeyen, J. W. S., Van Zundert, J., and Cohen, S. P. (2021). Low back pain. Lancet 398 (10294), 78–92. doi:10.1016/s0140-6736(21)00733-9
Knezevic, N. N., Mandalia, S., Raasch, J., Knezevic, I., and Candido, K. D. (2017). Treatment of chronic low back pain - new approaches on the horizon. J. Pain Res. 10, 1111–1123. doi:10.2147/jpr.S132769
Kuchroo, P., Dave, V., Vijayan, A., Viswanathan, C., and Ghosh, D. (2015). Paracrine factors secreted by umbilical cord-derived mesenchymal stem cells induce angiogenesis in vitro by a VEGF-independent pathway. Stem Cells Dev. 24 (4), 437–450. doi:10.1089/scd.2014.0184
Kumar, M. N., Jacquot, F., and Hall, H. (2001). Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur. Spine J. 10 (4), 309–313. doi:10.1007/s005860000207
Lai, D., Wang, F., Dong, Z., and Zhang, Q. (2014). Skin-derived mesenchymal stem cells help restore function to ovaries in a premature ovarian failure mouse model. PLoS One 9 (5), e98749. doi:10.1371/journal.pone.0098749
Leckie, S. K., Sowa, G. A., Bechara, B. P., Hartman, R. A., Coelho, J. P., Witt, W. T., et al. (2013). Injection of human umbilical tissue-derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 13 (3), 263–272. doi:10.1016/j.spinee.2012.12.004
Lee, Y. C., Zotti, M. G., and Osti, O. L. (2016). Operative management of lumbar degenerative disc disease. Asian Spine J. 10 (4), 801–819. doi:10.4184/asj.2016.10.4.801
Li, N., Li, Z., Li, R., Tian, J., Sun, G., Li, L., et al. (2017). A novel biomimetic scaffold with hUCMSCs for lumbar fusion. J. Mater Chem. B 5 (30), 5996–6007. doi:10.1039/c6tb02640k
Liang, H., Luo, R., Li, G., Zhang, W., Song, Y., and Yang, C. (2022). The proteolysis of ECM in intervertebral disc degeneration. Int. J. Mol. Sci. 23 (3), 1715. doi:10.3390/ijms23031715
Lu, L. L., Liu, Y. J., Yang, S. G., Zhao, Q. J., Wang, X., Gong, W., et al. (2006). Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 91 (8), 1017–1026.
Lyu, F. J., Cheung, K. M., Zheng, Z., Wang, H., Sakai, D., and Leung, V. Y. (2019). IVD progenitor cells: A new horizon for understanding disc homeostasis and repair. Nat. Rev. Rheumatol. 15 (2), 102–112. doi:10.1038/s41584-018-0154-x
Maher, C., Underwood, M., and Buchbinder, R. (2017). Non-specific low back pain. Lancet 389 (10070), 736–747. doi:10.1016/s0140-6736(16)30970-9
Matta, A., Karim, M. Z., Gerami, H., Benigno, B., and Erwin, W. M. (2021). A comparative study of mesenchymal stem cell transplantation and NTG-101 molecular therapy to treat degenerative disc disease. Sci. Rep. 11 (1), 14804. doi:10.1038/s41598-021-94173-w
Miao, Z., Jin, J., Chen, L., Zhu, J., Huang, W., Zhao, J., et al. (2006). Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 30 (9), 681–687. doi:10.1016/j.cellbi.2006.03.009
Miceli, V., Bulati, M., Iannolo, G., Zito, G., Gallo, A., and Conaldi, P. G. (2021). Therapeutic properties of mesenchymal stromal/stem cells: The need of cell priming for cell-free therapies in regenerative medicine. Int. J. Mol. Sci. 22 (2), 763. doi:10.3390/ijms22020763
Molladavoodi, S., McMorran, J., and Gregory, D. (2020). Mechanobiology of annulus fibrosus and nucleus pulposus cells in intervertebral discs. Cell Tissue Res. 379 (3), 429–444. doi:10.1007/s00441-019-03136-1
Morris, H., Gonçalves, C. F., Dudek, M., Hoyland, J., and Meng, Q. J. (2021). Tissue physiology revolving around the clock: Circadian rhythms as exemplified by the intervertebral disc. Ann. Rheum. Dis. 80 (7), 828–839. doi:10.1136/annrheumdis-2020-219515
Ohnishi, T., Iwasaki, N., and Sudo, H. (2022). Causes of and molecular targets for the treatment of intervertebral disc degeneration: A review. Cells 11 (3), 394. doi:10.3390/cells11030394
Okada, E., Daimon, K., Fujiwara, H., Nishiwaki, Y., Nojiri, K., Watanabe, M., et al. (2019). Ten-year longitudinal follow-up MRI study of age-related changes in thoracic intervertebral discs in asymptomatic subjects. Spine (Phila Pa 1976) 44 (22), E1317–e1324. doi:10.1097/brs.0000000000003145
Omlor, G. W., Lorenz, S., Nerlich, A. G., Guehring, T., and Richter, W. (2018). Disc cell therapy with bone-marrow-derived autologous mesenchymal stromal cells in a large porcine disc degeneration model. Eur. Spine J. 27 (10), 2639–2649. doi:10.1007/s00586-018-5728-4
Pang, X., Yang, H., and Peng, B. (2014). Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician 17 (4), E525–E530. doi:10.36076/ppj.2014/17/e525
Perez-Cruet, M., Beeravolu, N., McKee, C., Brougham, J., Khan, I., Bakshi, S., et al. (2019). Potential of human nucleus pulposus-like cells derived from umbilical cord to treat degenerative disc disease. Neurosurgery 84 (1), 272–283. doi:10.1093/neuros/nyy012
Phillips, F. M., Slosar, P. J., Youssef, J. A., Andersson, G., and Papatheofanis, F. (2013). Lumbar spine fusion for chronic low back pain due to degenerative disc disease: A systematic review. Spine (Phila Pa 1976) 38 (7), E409–E422. doi:10.1097/BRS.0b013e3182877f11
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284 (5411), 143–147. doi:10.1126/science.284.5411.143
Qian, H., He, L., Ye, Z., Wei, Z., and Ao, J. (2023). Decellularized matrix for repairing intervertebral disc degeneration: Fabrication methods, applications and animal models. Mater Today Bio 18, 100523. doi:10.1016/j.mtbio.2022.100523
Reppel, L., Schiavi, J., Charif, N., Leger, L., Yu, H., Pinzano, A., et al. (2015). Chondrogenic induction of mesenchymal stromal/stem cells from Wharton's jelly embedded in alginate hydrogel and without added growth factor: An alternative stem cell source for cartilage tissue engineering. Stem Cell Res. Ther. 6, 260. doi:10.1186/s13287-015-0263-2
Rogers, C., Fernandes-Alnemri, T., Mayes, L., Alnemri, D., Cingolani, G., and Alnemri, E. S. (2017). Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 8, 14128. doi:10.1038/ncomms14128
Ross, A., Gambrill, V., and Main, C. (2022). Clinical outcomes of amniotic membrane/umbilical cord particulate in spinal disorders: A retrospective study. J. Pain Res. 15, 3971–3979. doi:10.2147/jpr.S375201
Roughley, P. J., Melching, L. I., Heathfield, T. F., Pearce, R. H., and Mort, J. S. (2006). The structure and degradation of aggrecan in human intervertebral disc. Eur. Spine J. 15 (Suppl. 3), S326–S332. doi:10.1007/s00586-006-0127-7
Ruan, D., Zhang, Y., Wang, D., Zhang, C., Wu, J., Wang, C., et al. (2012). Differentiation of human Wharton's jelly cells toward nucleus pulposus-like cells after coculture with nucleus pulposus cells in vitro. Tissue Eng. Part A 18 (1-2), 167–175. doi:10.1089/ten.TEA.2011.0186
Ryu, J. S., Jeong, E. J., Kim, J. Y., Park, S. J., Ju, W. S., Kim, C. H., et al. (2020). Application of mesenchymal stem cells in inflammatory and fibrotic diseases. Int. J. Mol. Sci. 21 (21), 8366. doi:10.3390/ijms21218366
Ryu, K. H., Kim, S. Y., Kim, Y. R., Woo, S. Y., Sung, S. H., Kim, H. S., et al. (2014). Tonsil-derived mesenchymal stem cells alleviate concanavalin A-induced acute liver injury. Exp. Cell Res. 326 (1), 143–154. doi:10.1016/j.yexcr.2014.06.007
Sampara, P., Banala, R. R., Vemuri, S. K., Av, G. R., and Gpv, S. (2018). Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: A review. Gene Ther. 25 (2), 67–82. doi:10.1038/s41434-018-0004-0
Sarugaser, R., Lickorish, D., Baksh, D., Hosseini, M. M., and Davies, J. E. (2005). Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells 23 (2), 220–229. doi:10.1634/stemcells.2004-0166
Scoville, W. B., and Corkill, G. (1973). Lumbar disc surgery: Technique of radical removal and early mobilization. Technical note. J. Neurosurg. 39 (2), 265–269. doi:10.3171/jns.1973.39.2.0265
Shen, C., Lie, P., Miao, T., Yu, M., Lu, Q., Feng, T., et al. (2015). Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol. Med. Rep. 12 (1), 20–30. doi:10.3892/mmr.2015.3409
Smith, L. J., Gorth, D. J., Showalter, B. L., Chiaro, J. A., Beattie, E. E., Elliott, D. M., et al. (2014). In vitro characterization of a stem-cell-seeded triple-interpenetrating-network hydrogel for functional regeneration of the nucleus pulposus. Tissue Eng. Part A 20 (13-14), 1841–1849. doi:10.1089/ten.TEA.2013.0516
Smith, L. J., Nerurkar, N. L., Choi, K. S., Harfe, B. D., and Elliott, D. M. (2011). Degeneration and regeneration of the intervertebral disc: Lessons from development. Dis. Model Mech. 4 (1), 31–41. doi:10.1242/dmm.006403
Song, Y., Wang, Y., Zhang, Y., Geng, W., Liu, W., Gao, Y., et al. (2017). Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J. Cell Mol. Med. 21 (7), 1373–1387. doi:10.1111/jcmm.13067
Steck, E., Fischer, J., Lorenz, H., Gotterbarm, T., Jung, M., and Richter, W. (2009). Mesenchymal stem cell differentiation in an experimental cartilage defect: Restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 18 (7), 969–978. doi:10.1089/scd.2008.0213
Sun, Z., Liu, B., and Luo, Z. J. (2020). The immune privilege of the intervertebral disc: Implications for intervertebral disc degeneration treatment. Int. J. Med. Sci. 17 (5), 685–692. doi:10.7150/ijms.42238
Takeoka, Y., Yurube, T., and Nishida, K. (2020). Gene therapy approach for intervertebral disc degeneration: An update. Neurospine 17 (1), 3–14. doi:10.14245/ns.2040042.021
Tang, G., Liu, Z., Liu, Y., Yu, J., Wang, X., Tan, Z., et al. (2021). Recent trends in the development of bone regenerative biomaterials. Front. Cell Dev. Biol. 9, 665813. doi:10.3389/fcell.2021.665813
Thirumala, S., Goebel, W. S., and Woods, E. J. (2013). Manufacturing and banking of mesenchymal stem cells. Expert Opin. Biol. Ther. 13 (5), 673–691. doi:10.1517/14712598.2013.763925
Torre, O. M., Mroz, V., Bartelstein, M. K., Huang, A. H., and Iatridis, J. C. (2019). Annulus fibrosus cell phenotypes in homeostasis and injury: Implications for regenerative strategies. Ann. N. Y. Acad. Sci. 1442 (1), 61–78. doi:10.1111/nyas.13964
Tsaryk, R., Silva-Correia, J., Oliveira, J. M., Unger, R. E., Landes, C., Brochhausen, C., et al. (2017). Biological performance of cell-encapsulated methacrylated gellan gum-based hydrogels for nucleus pulposus regeneration. J. Tissue Eng. Regen. Med. 11 (3), 637–648. doi:10.1002/term.1959
Vadalà, G., Sowa, G., Hubert, M., Gilbertson, L. G., Denaro, V., and Kang, J. D. (2012). Mesenchymal stem cells injection in degenerated intervertebral disc: Cell leakage may induce osteophyte formation. J. Tissue Eng. Regen. Med. 6 (5), 348–355. doi:10.1002/term.433
van Middelkoop, M., Rubinstein, S. M., Kuijpers, T., Verhagen, A. P., Ostelo, R., Koes, B. W., et al. (2011). A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur. Spine J. 20 (1), 19–39. doi:10.1007/s00586-010-1518-3
Vasanthan, J., Gurusamy, N., Rajasingh, S., Sigamani, V., Kirankumar, S., Thomas, E. L., et al. (2020). Role of human mesenchymal stem cells in regenerative therapy. Cells 10 (1), 54. doi:10.3390/cells10010054
Vaudreuil, N., Henrikson, K., Pohl, P., Lee, A., Lin, H., Olsen, A., et al. (2019). Photopolymerizable biogel scaffold seeded with mesenchymal stem cells: Safety and efficacy evaluation of novel treatment for intervertebral disc degeneration. J. Orthop. Res. 37 (6), 1451–1459. doi:10.1002/jor.24208
Vawda, R., and Fehlings, M. G. (2013). Mesenchymal cells in the treatment of spinal cord injury: Current & future perspectives. Curr. Stem Cell Res. Ther. 8 (1), 25–38. doi:10.2174/1574888x11308010005
Vellasamy, S., Sandrasaigaran, P., Vidyadaran, S., George, E., and Ramasamy, R. (2012). Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J. Stem Cells 4 (6), 53–61. doi:10.4252/wjsc.v4.i6.53
Veruva, S. Y., Lanman, T. H., Isaza, J. E., Freeman, T. A., Kurtz, S. M., and Steinbeck, M. J. (2017). Periprosthetic UHMWPE wear debris induces inflammation, vascularization, and innervation after total disc replacement in the lumbar spine. Clin. Orthop. Relat. Res. 475 (5), 1369–1381. doi:10.1007/s11999-016-4996-8
Wang, J., Nisar, M., Huang, C., Pan, X., Lin, D., Zheng, G., et al. (2018). Small molecule natural compound agonist of SIRT3 as a therapeutic target for the treatment of intervertebral disc degeneration. Exp. Mol. Med. 50 (11), 1–14. doi:10.1038/s12276-018-0173-3
Wang, Y., Che, M., Xin, J., Zheng, Z., Li, J., and Zhang, S. (2020). The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 131, 110660. doi:10.1016/j.biopha.2020.110660
Wegner, I., Widyahening, I. S., van Tulder, M. W., Blomberg, S. E., de Vet, H. C., Brønfort, G., et al. (2013). Traction for low-back pain with or without sciatica. Cochrane Database Syst. Rev. 2013 (8), Cd003010. doi:10.1002/14651858.CD003010.pub5
Wei, C. C., Lin, A. B., and Hung, S. C. (2014). Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: Bench, bedside, and industry. Cell Transpl. 23 (4-5), 505–512. doi:10.3727/096368914x678328
Werner, J. H., Rosenberg, J. H., Keeley, K. L., and Agrawal, D. K. (2018). Immunobiology of periprosthetic inflammation and pain following ultra-high-molecular-weight-polyethylene wear debris in the lumbar spine. Expert Rev. Clin. Immunol. 14 (8), 695–706. doi:10.1080/1744666x.2018.1511428
Wong, J. J., Côté, P., Sutton, D. A., Randhawa, K., Yu, H., Varatharajan, S., et al. (2017). Clinical practice guidelines for the noninvasive management of low back pain: A systematic review by the ontario protocol for traffic injury management (OPTIMa) collaboration. Eur. J. Pain 21 (2), 201–216. doi:10.1002/ejp.931
Wu, A., March, L., Zheng, X., Huang, J., Wang, X., Zhao, J., et al. (2020). Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the global burden of disease study 2017. Ann. Transl. Med. 8 (6), 299. doi:10.21037/atm.2020.02.175
Wu, H., Zeng, X., Yu, J., Shang, Y., Tu, M., Cheang, L. H., et al. (2017). Comparison of nucleus pulposus stem/progenitor cells isolated from degenerated intervertebral discs with umbilical cord derived mesenchymal stem cells. Exp. Cell Res. 361 (2), 324–332. doi:10.1016/j.yexcr.2017.10.034
Xin, J., Wang, Y., Zheng, Z., Wang, S., Na, S., and Zhang, S. (2022). Treatment of intervertebral disc degeneration. Orthop. Surg. 14 (7), 1271–1280. doi:10.1111/os.13254
Xu, J., Liu, S., Wang, S., Qiu, P., Chen, P., Lin, X., et al. (2019). Decellularised nucleus pulposus as a potential biologic scaffold for disc tissue engineering. Mater Sci. Eng. C Mater Biol. Appl. 99, 1213–1225. doi:10.1016/j.msec.2019.02.045
Yan, K., Zhang, R., Chen, L., Chen, F., Liu, Y., Peng, L., et al. (2014). Nitric oxide-mediated immunosuppressive effect of human amniotic membrane-derived mesenchymal stem cells on the viability and migration of microglia. Brain Res. 1590, 1–9. doi:10.1016/j.brainres.2014.05.041
Yee, A., Lam, M. P., Tam, V., Chan, W. C., Chu, I. K., Cheah, K. S., et al. (2016). Fibrotic-like changes in degenerate human intervertebral discs revealed by quantitative proteomic analysis. Osteoarthr. Cartil. 24 (3), 503–513. doi:10.1016/j.joca.2015.09.020
Yuan, X., Li, T., Shi, L., Miao, J., Guo, Y., and Chen, Y. (2021). Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Mol. Med. 27 (1), 91. doi:10.1186/s10020-021-00355-7
Zeng, X., Lin, J., Wu, H., Yu, J., Tu, M., Cheang, L. H., et al. (2020). Effect of conditioned medium from human umbilical cord-derived mesenchymal stromal cells on rejuvenation of nucleus pulposus derived stem/progenitor cells from degenerated intervertebral disc. Int. J. Stem Cells 13 (2), 257–267. doi:10.15283/ijsc20027
Zhang, C., Gullbrand, S. E., Schaer, T. P., Boorman, S., Elliott, D. M., Chen, W., et al. (2021a). Combined hydrogel and mesenchymal stem cell therapy for moderate-severity disc degeneration in goats. Tissue Eng. Part A 27 (1-2), 117–128. doi:10.1089/ten.TEA.2020.0103
Zhang, X. B., Hu, Y. C., Cheng, P., Zhou, H. Y., Chen, X. Y., Wu, D., et al. (2021b). Targeted therapy for intervertebral disc degeneration: Inhibiting apoptosis is a promising treatment strategy. Int. J. Med. Sci. 18 (13), 2799–2813. doi:10.7150/ijms.59171
Zhang, X., Chen, J., Huang, B., Wang, J., Shan, Z., Liu, J., et al. (2019). Obesity mediates apoptosis and extracellular matrix metabolic imbalances via MAPK pathway activation in intervertebral disk degeneration. Front. Physiol. 10, 1284. doi:10.3389/fphys.2019.01284
Zhang, X., Hirai, M., Cantero, S., Ciubotariu, R., Dobrila, L., Hirsh, A., et al. (2011). Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J. Cell Biochem. 112 (4), 1206–1218. doi:10.1002/jcb.23042
Zhang, Y., Tao, H., Gu, T., Zhou, M., Jia, Z., Jiang, G., et al. (2015). The effects of human Wharton's jelly cell transplantation on the intervertebral disc in a canine disc degeneration model. Stem Cell Res. Ther. 6 (1), 154. doi:10.1186/s13287-015-0132-z
Keywords: intervertebral disc, intervertebral disc degeneration, mesenchymal stem cells, umbilical cord, tissue engineering
Citation: Huang H, Liu X, Wang J, Suo M, Zhang J, Sun T, Zhang W and Li Z (2023) Umbilical cord mesenchymal stem cells for regenerative treatment of intervertebral disc degeneration. Front. Cell Dev. Biol. 11:1215698. doi: 10.3389/fcell.2023.1215698
Received: 02 May 2023; Accepted: 27 July 2023;
Published: 03 August 2023.
Edited by:
Hongfei Xiang, The Affiliated Hospital of Qingdao University, ChinaReviewed by:
Saijilafu, Zhejiang University City College, ChinaCopyright © 2023 Huang, Liu, Wang, Suo, Zhang, Sun, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhonghai Li, bGl6aG9uZ2hhaXNwaW5lQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.