- 1Garvan Institute of Medical Research, Sydney, NSW, Australia
- 2St Vincent’s Clinical School, University of NSW, Sydney, NSW, Australia
CDK4/6 inhibitors have become game-changers in the treatment of estrogen receptor-positive (ER+) breast cancer, and in combination with endocrine therapy are the standard of care first-line treatment for ER+/HER2-negative advanced breast cancer. Although CDK4/6 inhibitors prolong survival for these patients, resistance is inevitable and there is currently no clear standard next-line treatment. There is an urgent unmet need to dissect the mechanisms which drive intrinsic and acquired resistance to CDK4/6 inhibitors and endocrine therapy to guide the subsequent therapeutic decisions. We will review the insights gained from preclinical studies and clinical cohorts into the diverse mechanisms of CDK4/6 inhibitor action and resistance, and highlight potential therapeutic strategies in the context of CDK4/6 inhibitor resistance.
1 Introduction
Over 70% of breast cancers are estrogen-receptor positive (ER+) and human epidermal growth factor 2 negative (HER2-) (Howlader et al., 2014). Endocrine therapy (ET) forms the backbone of systemic treatment for this breast cancer subtype. An estimated 20% of patients have innate resistance to ET, and others acquire resistance to ET over a period of time (Anurag et al., 2018). The addition of CDK4/6 inhibitors to ET is efficacious in suppressing cell proliferation, delaying cancer progression and improving survival, and represents the current standard of care first-line therapy for advanced ER+/HER2-breast cancer.
The CDK4/6 inhibitors palbociclib, ribociclib, and abemaciclib are each indicated in the first-line for recurrent unresectable or metastatic ER+/HER2-breast cancer in combination with an aromatase inhibitor (AI), with concurrent ovarian function suppression for pre-menopausal women (Gradishar et al., 2022). There is also data to support the use of combination of a CDK4/6 inhibitor with the selective estrogen receptor degrader (SERD) fulvestrant, particularly in patients with progression on or early relapse after adjuvant AI (Gradishar et al., 2022). Abemaciclib is also FDA approved as monotherapy in refractory advanced disease, having shown single-agent anti-tumor activity in prospective trials (Dickler et al., 2017; Hamilton E. et al., 2022; Hamilton E. P. et al., 2022). More recently, the addition of abemaciclib to adjuvant ET has demonstrated invabsive disease-free survival (IDFS) benefit in high-risk early-stage ER + cancer (Harbeck et al., 2021). The overall survival data is still immature in this abemaciclib trial, as is data from a similarly large phase III study of ribociclib added to AIs in high-risk early-stage ER+ cancer (ClinicalTrials.gov Identifier: NCT03701334).
Palbociclib, ribociclib and abemaciclib differ in biochemical kinase activity assays, their effects on cell proliferation, and on senescence in ER + breast cancer (Fry et al., 2004; Gelbert et al., 2014; Chen et al., 2016; Hafner et al., 2019). All three inhibit the enzymatic activity of CDK4/6 at nanomolar levels and have greater selectivity for CDK4 than CDK6 (Klein et al., 2018; Hafner et al., 2019). Abemaciclib also has the broadest CDK inhibition activity, with additional inhibitory activity against other CDKs including CDK1, 2 and 9 (Hafner et al., 2019). At higher concentrations and with prolonged treatment, abemaciclib has been observed to cause apoptosis (Torres-Guzman et al., 2017), which is in contrast to the cytostatic effect of palbociclib and ribociclib (Jost et al., 2021).
There are also different dosing schedules and toxicity profiles associated with the three agents. They all result in myelosuppression, particularly neutropenia. The incidence of febrile neutropenia however is very low (Braal et al., 2021). Abemaciclib has the least effect on bone marrow suppression and is administered continuously, while patients treated with palbociclib or ribociclib require a 7-day break in the 28-day treatment cycle to allow bone marrow recovery (Chen et al., 2016; Patnaik et al., 2016; Kwapisz, 2017; Klein et al., 2018; Hafner et al., 2019). In contrast, abemaciclib has a greater frequency of gastrointestinal side effects compared to palbociclib and ribociclib (Chen et al., 2016; Kwapisz, 2017; Braal et al., 2021), which may be mediated through CDK9 inhibition (Braal et al., 2021). Prolongation of the QTc interval has been observed with ribociclib, and QTc monitoring after starting treatment is required (Braal et al., 2021).
Other newer CDK4/6 inhibitor agents include dalpiciclib, which has demonstrated a positive progression free survival (PFS) readout in combination with fulvestrant in the second- or third-line setting in the phase III DAWNA-1 trial (Xu et al., 2021), and in combination with an AI in the first-line setting in the phase III DAWNA-2 trial (Xu et al., 2022). Lerociclib had favorable signals of efficacy and tolerability in a dose escalation and expansion trial in combination with fulvestrant (Bulat et al., 2020).
Patients with metastatic disease will eventually progress on the combination therapy due to intrinsic or acquired resistance to the ET, the CDK4/6 inhibitor or both therapies (O'Leary et al., 2018). These scenarios are not differentiated clinically, and currently there is no standard next-line treatment. A better understanding of the mechanisms of CDK4/6 inhibitor and ET resistance and early detection of predictors for resistance may best inform how to sequence or combine currently available and emerging therapies, and potentially identify new drug targets. There is unlikely to be a one size fits all therapeutic approach, and the identification of predictive biomarkers is key to tailoring an individualized next-line treatment plan. Herein we will provide preclinical and clinical perspectives on the management of advanced ER+/HER2-breast cancer following progression on combination ET and CDK4/6 inhibitors.
2 Mechanisms of CDK4/6 inhibitor action
2.1 CDK4/6 inhibitors induce cell cycle arrest
The best characterized mechanism by which CDK4/6 inhibitors act is the inhibition of retinoblastoma protein (Rb) phosphorylation, leading to G1 cell cycle arrest in tumor cells (O'Brien et al., 2018). Palbociclib inhibits growth of both ER+ and ER-negative breast cancer tumors, but only in the context of Rb expression (Fry et al., 2004). Palbociclib inhibition of CDK4 activity blocks the disassembly of the Rb related protein p130 within the DREAM (DP, Rb-like, E2F And MuvB) complex during cell cycle entry (Schade et al., 2019) (Figure 1A), and double Rb and p130 knockout primary fibroblast cells are significantly more resistant to CDK4/6 inhibition compared to Rb knockout cells. This demonstrates that CDK4/6 inhibitors act through Rb and p130, and that a functional Rb axis is required for CDK4/6 inhibitor activity. The loss of functional Rb is a relatively uncommon mechanism of intrinsic resistance (O'Leary et al., 2016; Kumarasamy et al., 2022). While alterations in expression of DREAM complex members are documented in many cancers and correlate with cancer prognosis (Duan et al., 2022; Wang and Liu, 2022), its mechanistic role in CDK4/6 inhibitor resistance is not defined. CDK4/6 inhibition also induces G1 cytostatic arrest by generating DNA replication stress (Crozier et al., 2022).
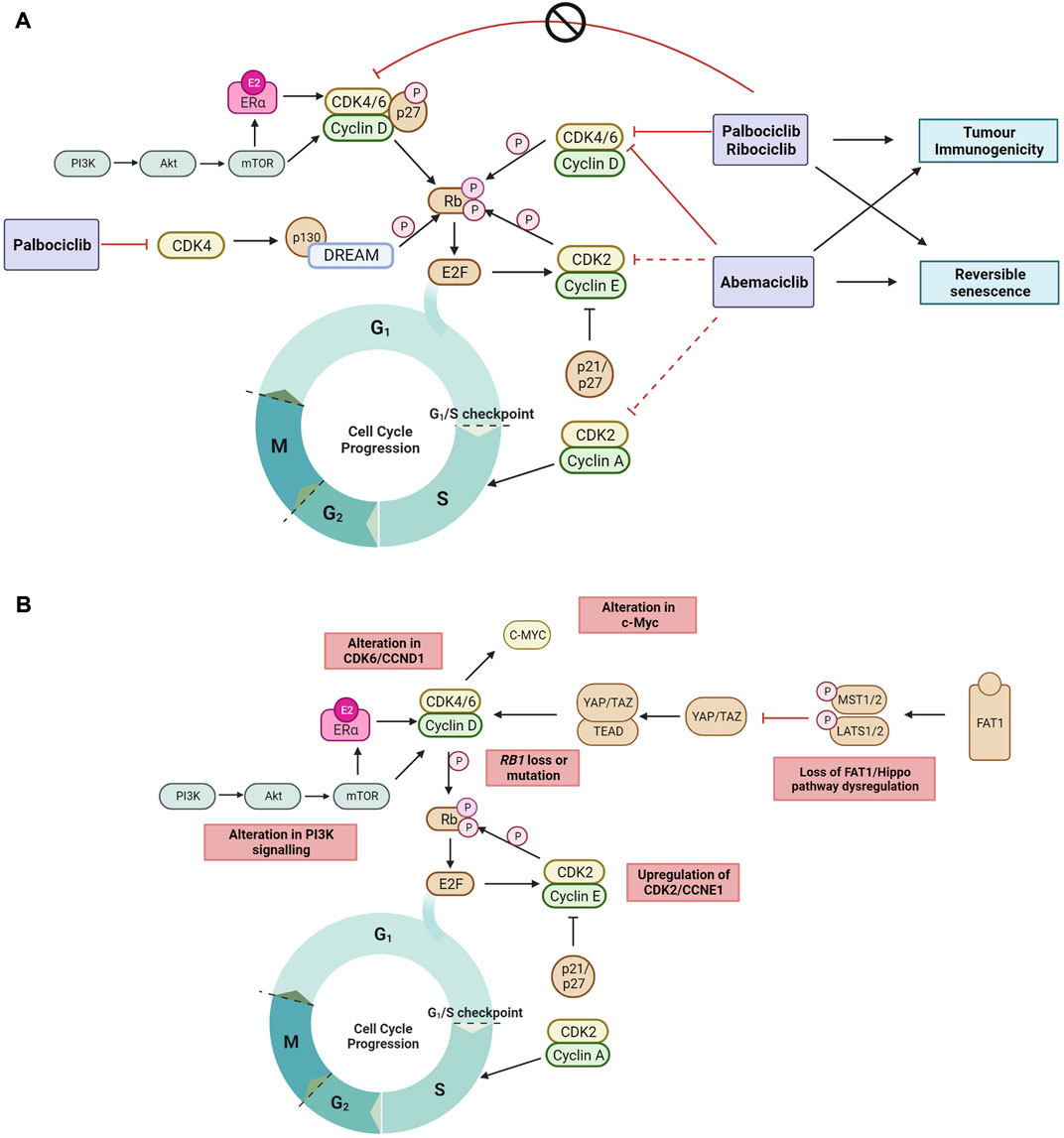
FIGURE 1. (A) The recently proposed mechanisms by which clinical CDK4/6 inhibitors act to block the cell cycle progression to DNA synthesis S phase and G2 phase but cannot inhibit tyrosine kinase phosphorylated p27-CDK4/6-CycD complexes in ER + breast cancer (Guiley et al., 2019; Hafner et al., 2019; Schade et al., 2019; Pack et al., 2021). Non-cell cycle effects of CDK4/6 inhibition include reversible senescence (Thangavel et al., 2011; Torres-Guzman et al., 2017; Vijayaraghavan et al., 2017; Marinelli et al., 2020; Maskey et al., 2021; Mayayo-Peralta et al., 2021) and enhanced tumor immunogenicity (Goel et al., 2017; Peuker et al., 2022) (B) The mechanisms of ET and CDK4/6 inhibitor resistance currently reported in literature are highlighted. Black arrow shows pathway induction; red line shows pathway inhibition; dotted line indicates lower relative inhibition activity; P, phosphorylation. Images created with BioRender.com.
Endogenous CDK inhibitors, including p16, p18, p21 and p27, regulate the cell cycle in healthy cells in response to DNA damage, metabolic changes, and cellular stress (Abukhdeir and Park, 2008; Witkiewicz et al., 2011). However, endogenous CDK inhibitors can hinder the action of synthetic CDK4/6 inhibitors. In ER + breast cancer, overexpression of p16 has been associated with CDK4/6 inhibitor resistance and poor clinical outcome (Palafox et al., 2022). In addition, p18 binds to the CDK6-CycD complex blocking the therapeutic action of CDK4/6 inhibitors (palbociclib or abemaciclib), and conversely CDK4/6 inhibitor sensitivity is restored by suppressing the binding of p18 to CDK6 (Li et al., 2022). The endogenous CDK4/6 inhibitory proteins p21 and p27 prevent phosphorylation by the CDK4-Cyclin D complex, but also facilitate its stabilization and promote its activation. When phosphorylated by tyrosine kinases, p27 allosterically activates the CDK4-Cyclin D complex (Guiley et al., 2019) (Figure 1A). Interestingly, palbociclib has no inhibitory effect on the activity of tyrosine phosphorylated p27-CDK4/6-cycD trimer. Endogenous CDK4/6 inhibitors may also impact the cell cycle indirectly by interfering with CDK4/6 folding which prevents the formation of stable complexes comprising cyclin D, CDK4/6 and p21/p27, thereby releasing p21 to inhibit CDK2 activity and reduce cell proliferation (Guiley et al., 2019; Pack et al., 2021).
2.2 Non-cell cycle effects of CDK4/6 inhibitors
When cell proliferation stops in response to contact inhibition, mitogen withdrawal or cytostatic drugs such as CDK4/6 inhibitors, cells exit the cell cycle and enter a quiescent or senescent state. CDK4/6 inhibitors have been shown to induce morphological changes and increase senescence associated (SA)-β-galactosidase activity (Thangavel et al., 2011; Torres-Guzman et al., 2017; Vijayaraghavan et al., 2017; Marinelli et al., 2020; Mayayo-Peralta et al., 2021). This is reversible upon removal of CDK4/6 inhibition, as cells re-enter the cell cycle and resume cell proliferation (Vijayaraghavan et al., 2017), suggesting that CDK4/6 inhibitors do not induce irreversible senescence (Figure 1A). Inhibition of the mTOR signaling complex, mTORC1, during palbociclib exposure has been shown to prevent the induction of permanent senescence, while genetic depletion of TSC2, a negative regulator of mTORC1 during palbociclib exposure resulted in irreversible senescence (Maskey et al., 2021). In addition, abemaciclib and palbociclib treatment have been shown to downregulate mTOR signaling in small cell lung and breast cancer (Naz et al., 2018; Maskey et al., 2021), providing support that CDK4/6 inhibitors may induce reversible senescence through downregulating mTOR signaling.
Enhanced tumor immunogenicity and antitumor immune responses have also been observed with CDK4/6 inhibition (Goel et al., 2017; Deng et al., 2018; Schaer et al., 2018) (Figure 1A). Transcriptomic analysis of serial biopsies from the neoadjuvant NeoPalAna trial (Clinicaltrials.gov identifier NCT01723774) (Ma et al., 2017) showed that palbociclib induced tumor cell expression of endogenous retroviral elements. This results in increased intracellular levels of double-stranded RNA, increased production of type III interferons, and greater tumor antigen presentation. Palbociclib and abemaciclib also suppressed the proliferation of regulatory T cells and promoted cytotoxic T-cell-mediated clearance of tumor cells (Goel et al., 2017). Recently, it was also demonstrated in the RIBECCA trial (Clinicaltrials.gov identifier NCT03096847) that ribociclib treatment resulted in activation of an already existing immune response rather than a de novo immune induction in patients with ER + breast cancer (Peuker et al., 2022). Combination therapy of CDK4/6 inhibitors with immune checkpoint blockade has been shown to increase anti-tumor efficacy in preclinical models (Goel et al., 2017; Deng et al., 2018) and provides the rationale to evaluate this combination clinically.
3 Pivotal CDK4/6 inhibitor clinical trials in ER + breast cancer
Palbociclib, ribociclib and abemaciclib have demonstrated statistically significant and clinically meaningful PFS benefit when added to ET in both the first and second-line advanced ER+/HER2-breast cancer settings (Cristofanilli et al., 2016; Finn et al., 2016; Sledge et al., 2017; Hortobagyi et al., 2018; Slamon et al., 2018; Johnston et al., 2019). This has translated to overall survival (OS) benefit in pivotal trials with a fulvestrant backbone (Sledge et al., 2020; Slamon et al., 2021; Cristofanilli et al., 2022). In contrast, only ribociclib in combination with an AI have reported an improvement in OS (Hortobagyi et al., 2022; Lu et al., 2022). OS benefit was not seen for first-line palbociclib with letrozole (Finn et al., 2022), and OS results have yet to be reported for first-line abemaciclib with AI (Goetz et al., 2022b).
With the success of CDK4/6 inhibitors in the metastatic setting, they have subsequently been evaluated in the adjuvant setting for early-stage disease. The phase III monarchE trial reported that the addition of 2 years of abemaciclib to adjuvant ET improved invasive disease free (IDFS) survival in high-risk early-stage ER+/HER2-breast cancer (O'Shaughnessy et al., 2021). In contrast, the Penelope-B and PALLAS trials failed to show an IDFS benefit with the addition of 2 years and 1 year of palbociclib respectively to adjuvant ET (Loibl et al., 2021; Mayer et al., 2021). Potential contributing factors to the differing results including differences in study population, duration of CDK4/6 inhibitors, and pharmacological differences between the agents (Loibl et al., 2021; Mayer et al., 2021). The results of the NATALEE trial (Clinicaltrials.gov identifier NCT03701334) assessing the addition of 3 years of ribociclib to adjuvant ET are pending.
4 Mechanisms of resistance to combination CDK4/6 inhibitor and endocrine therapy
Other than ER, there are no other routinely used clinical biomarkers used to select patients for combination CDK4/6 inhibitor and ET. ER loss occurs in a minority of ER + breast cancer through the course of therapy, is associated with ET resistance, and remains an important predictor of CDK4/6 inhibitor and ET efficacy (Finn et al., 2020; Griffiths et al., 2021).
Mechanisms of CDK4/6 inhibitor resistance that have been identified are varied and affect cell cycle targets (e.g., Rb1, cyclin E, CDK2, CDK6, c-Myc and AURKA) and/or activated signaling targets (e.g., AKT1, FGFR, HER2, EGFR and RAS) (Wander et al., 2020; Asghar et al., 2022). Only a small percentage of patients are found to harbor each of the individual genomic aberrations that drive resistance at baseline, and a clearer picture of acquired genomic drivers of resistance is emerging with biomarker analysis, in particular circulating tumor DNA (ctDNA) based studies of pivotal CDK4/6 inhibitor trials (Asghar et al., 2022). In some instances, it is also possible that CDK4/6 inhibition delays the onset of endocrine resistance, and that resistance to combination is driven primarily by resistance to the ET backbone (O'Leary et al., 2018). In support of this, ctDNA biomarker analysis of MONARCH-3 trial found a lower incidence of ESR1 mutations in the abemaciclib plus AI arm compared to the AI alone arm (17% vs. 31% respectively) (Goetz et al., 2020). Here, we list the most well-characterized resistance mechanisms and acquired genomic aberrations identified to date (Figure 1B).
4.1 Loss of Rb1
As CDK4/6 inhibitors act to prevent Rb protein inactivation, mutations resulting in a biallelic loss of function in the RB1 gene have been identified as drivers of resistance to CDK4/6 inhibitors (Herrera-Abreu et al., 2016; Bertucci et al., 2019). Cells harboring a non-functional Rb protein have a dysfunctional proliferative capacity, continuing through the cell cycle unchecked even in the presence of CDK4/6 inhibitors. Combined analyses of ctDNA across three randomized trials of ET plus ribociclib therapy in patients with advanced breast cancer reported a low baseline incidence of RB1 mutation in 1.7% of patients (Condorelli et al., 2018; O'Leary et al., 2018; Kumarasamy et al., 2022). Importantly, the addition of ribociclib to ET showed poor PFS in patients with tumors harboring RB1 mutations compared to wildtype (Kumarasamy et al., 2022). The low occurrence of RB1 mutation prior to use of CDK4/6 inhibitors compares with an incidence of 2%–9% in patients at the time of progression on CDK4/6 inhibitor therapy, suggesting that it is more commonly an acquired mechanism of resistance (Asghar et al., 2022). Loss of heterozygosity (LOH) of RB1 is also significantly associated with intrinsic CDK4/6 inhibitor resistance, and RB1 mutation is frequently acquired in CDK4/6 inhibitor treated patients and pre-clinical models with pre-existing RB1 LOH (Palafox et al., 2022; Safonov et al., 2022).
4.2 Aberrant cyclin E and CDK2 activity
The circumvention of CDK4/6 inhibition via the alternate phosphorylation of Rb through an upregulated CDK2-cyclin E axis represents another mechanism of CDK4/6 inhibitor resistance (Wang et al., 2007). Amplification of cyclin E1 and E2 results in an increase in CDK2 activity, and reduced expression of p27 (Taylor-Harding et al., 2015; Herrera-Abreu et al., 2016; Yang et al., 2017). There are however discordant results clinically. Higher levels of CCNE1 mRNA were detected in resistant compared to sensitive tumors in the abemaciclib ABC-POP (Palafox et al., 2022) and palbociclib PALOMA-3 trials (Turner et al., 2019), whereas altered CCNE1 expression was not significantly associated with an altered response to palbociclib plus letrozole in the PALOMA-2 trial (Finn et al., 2020).
CDK2 mRNA expression has also been shown to be upregulated in palbociclib resistant ER + cell lines, and palbociclib sensitivity may be restored by CDK2 knockdown with siRNA, resulting in tumor suppression, increased apoptosis, and senescence (Pandey et al., 2020). However, CDK2 amplification has not been reported in clinical samples (Tadesse et al., 2020).
Aberrant CDK2 activation may also occur independently of CDK4/6 through mesenchymal-epithelial transition factor (c-MET) family receptor tyrosine kinase signaling and its downstream effector focal adhesion kinases (FAK) (Zhang et al., 2019). The incidence of acquired genomic aberrations in MET was approximately 8% in the ctDNA analysis of an abemaciclib monotherapy clinical trial (Goetz et al., 2020). Upregulation of the PI3K/AKT/mTOR pathway has also been shown to trigger non-canonical activity of CDK2 by binding to cyclin D, resulting in cell cycle progression and resistance to CDK4/6 inhibitors (Herrera-Abreu et al., 2016). These findings have led to interest in the clinical development of CDK2 inhibitors which will be described later.
4.3 Upregulation of CDK6 activity
Preclinical studies have demonstrated that CDK6 amplification may occur in response to prolonged CDK4/6 inhibitor exposure, and sensitivity can be restored following CDK6 knockdown (Alves et al., 2016; Yang et al., 2017). This finding was supported by the analysis of CDK6 protein expression in patients treated with combined CDK4/6 inhibitor and ET, whereby there was a significant inverse correlation with PFS (Al-Qasem et al., 2022). In the same study, the authors noted that a combined score of CDK6, p-CDK2, and cyclin E1 proteins also predicted a poorer outcome in this patient subgroup as well as in patients treated with ET alone.
Another mechanism resulting in increased CDK6 activity is through loss of FAT1, a member of the cadherin superfamily. Loss of function mutations in FAT1 are present in about 2% of breast tumors (Li et al., 2018). Inactivation or deletion of FAT1 results in activation of the Hippo signaling pathway which regulates cell growth and apoptosis (Steinhardt et al., 2008). Loss of FAT1 function results in an accumulation of YAP/TAZ transcription factors, which in turn promote overexpression of CDK6 and increase the growth inhibitory concentration of CDK4/6 inhibitors. This effect is most profound in biallelic FAT1 inactivation, where the PFS on CDK4/6 inhibitors have been reported at 2.4 months. In contrast, patients with missense mutations had only a slightly shorter PFS when compared to wildtype (10.1 vs. 11.3 months) (Li et al., 2018).
Exosomal miRNA-432-5p, a predicator target of SMAD4 and TGFBR3 can drive acquired CDK4/6 inhibitor resistance, independent of inherent genetic mutations, by increasing CDK6 expression, downregulation of SMAD4 and subsequent reduction in G1/S cell cycle arrest (Cornell et al., 2019). A “drug holiday” (removing palbociclib for a prolonged period) resulted in downregulation of CDK6, CCND1, and miRNA-432-5p while upregulating Rb. Consequently, the resistance of ER + breast cancer cells to palbociclib was reversed in in vitro and in vivo preclinical models (Cornell et al., 2019). This may explain why patients who have progressed on one CDK4/6 inhibitor may later respond to a different CDK4/6 inhibitor.
4.4 c-Myc alteration
C-Myc is activated by CDK2, 4 and 6, and has been shown to be upregulated in CDK4/6 inhibitor resistance in preclinical models (Pandey et al., 2020; Freeman-Cook et al., 2021). In support of this, ctDNA biomarkers analysis of the MONARCH-3 study demonstrated an enrichment of acquired MYC genomic alterations in the abemaciclib treatment arm (Goetz et al., 2020). c-Myc is also activated downstream of kinases such as S6K1. Elevated S6K1 have been reported in 9%–14% of breast cancer patients and studies have demonstrated that elevated S6K1 drives palbociclib resistance via activation of c-Myc signaling pathways in preclinical models and clinical breast cancer samples (Mo et al., 2022).
4.5 Activation of upstream signaling pathways
A study which compared whole exome sequencing data of CDK4/6 inhibitor resistant and sensitive breast tumors identified an enrichment of mutations and amplifications in AKT1, KRAS, HRAS, NRAS, FGFR2 and HER2 in the resistant samples (Wander et al., 2020), and similar findings were found in ctDNA biomarker analysis of the MONARCH-3 trial, whereby acquired mutations in EGFR and FGFR1 were observed in the abemaciclib arm (Goetz et al., 2020). Interestingly, PIK3CA mutations were not more frequently found in the abemaciclib arm. Furthermore, high phospho-AKT levels in metastases have been shown to be a biomarker for poor prognosis and correlated with a shorter PFS in patients who have received CDK4/6 inhibitors and ET (Alves et al., 2021).
5 Therapeutic strategies following progression on CDK4/6 inhibitors
Candidate therapeutic strategies following progression on combination CDK4/6 inhibitor and ET can broadly be divided into 1) changing to an alternative ET and/or CDK4/6 inhibitor, 2) adding a therapy which inhibits a resistance mechanism of CDK4/6 inhibitors, such as inhibitors of CDK2 and PI3K/AKT/mTOR pathways, and 3) non-endocrine therapy approaches including chemotherapies and antibody drug conjugates (ADCs). Below, we summarize the clinical data to date for these strategies. Supplementary Table S1 and Supplementary Table S2 provide summaries of completed and ongoing clinical trials respectively in the post CDK4/6 inhibitor ER+/HER2-population.
5.1 Continuation of CDK4/6 inhibitors beyond progression
It is currently unclear if there is benefit from continued CDK4/6 inhibition post-progression on combination ET and CDK4/6 inhibitor. The phase II MAINTAIN trial enrolled patients with advanced ER+/HER2-breast cancer who progressed on ET and a CDK4/6 inhibitor, of whom 84% received prior palbociclib. Patients were randomized to receive a change in their ET backbone (exemestane or fulvestrant) plus ribociclib, versus ET (exemestane or fulvestrant) plus placebo. The median PFS was superior for the ET plus ribociclib arm compared to ET with placebo, supporting a switch in both ET and CDK4/6 inhibitor at progression (5.3 vs. 2.8 months; HR 0.56, p = 0.004) (Kalinsky et al., 2020; Kalinsky et al., 2022).
The phase II PACE trial similarly enrolled patients with advanced ER+/HER2-breast cancer who received prior AI and CDK4/6 inhibitor, of which 91% received palbociclib (Mayer et al., 2022). Patients were randomized to fulvestrant alone, fulvestrant plus palbociclib, or fulvestrant plus palbociclib plus avelumab, a PD-L1 inhibitor. In contrast to the MAINTAIN study, there was no significant difference in PFS for the fulvestrant plus palbociclib arm compared to the fulvestrant alone arm (4.6 vs. 4.8 months respectively), suggesting that a switch in both ET and CDK4/6 inhibitor may be required to derive additional benefit. There interestingly was a longer PFS noted in patients receiving avelumab (8.1 months) which may warrant further investigation (Mayer et al., 2022). The triplet combination of atezolizumab, abemaciclib and fulvestrant is one of the treatment regimens being assessed in the ongoing MORPHEUS HR + BC (Clinicaltrials.gov identifier NCT03280563) platform trial.
The phase II BioPER single-arm study enrolled 33 patients with advanced ER+/HER2-breast cancer who had prior clinical benefit to but subsequently progressed on palbociclib plus ET (Albanell et al., 2023). Patients continued palbociclib but had switch of their ET. Clinical benefit rate was 34% and median PFS was 2.6 months (Albanell et al., 2023). A composite biomarker signature incorporating low Rb score (defined as <1% tumor cells with positive nuclear staining on immunohistochemistry), high cyclin E1 (defined as ≥10% tumor cells with positive nuclear staining on immunohistochemistry), and ESR1 mutation was associated with poorer PFS (Albanell et al., 2023). Other trials evaluating continued CDK4/6 inhibition are in progress and included in Supplementary Table S2.
Additional insights can be gained from a retrospective review of 87 patients who received abemaciclib with or without ET, following progression on palbociclib with ET, where the median PFS and OS were 5.3 and 17.2 months respectively (Wander et al., 2021). These results were remarkably similar to the MONARCH 1 study, where patients with ER+/HER2-breast cancer received abemaciclib following prior ET and chemotherapy but were CDK4/6 inhibitor naïve (Dickler et al., 2017). Interestingly, PFS was longer for patients who received sequential CDK4/6 inhibitor, compared to patients who had intervening non-CDK4/6 inhibitor therapy, even after adjusting for number of prior lines of therapy (Wander et al., 2021).
5.2 Novel endocrine therapies
There are several emerging novel classes of ET, of which the oral SERDs are the largest group and are reviewed in detail elsewhere (Downton et al., 2022). To date there have been four completed randomized trials of oral SERDs in advanced ER+/HER2-breast cancer, after progression on prior ET with or without a CDK4/6 inhibitor, and results have been mixed. In both the phase III EMERALD trial of elacestrant versus physician’s choice ET (Bidard et al., 2022) and the phase II SERENA-2 trial of camizestrant versus fulvestrant (Oliveira et al., 2022), oral SERD treatment was associated with a statistically significant PFS benefit. In contrast, the phase II AMEERA-3 (Tolaney et al., 2022) and the phase II acelERA trial (Martin Jimenez et al., 2022) of amcenestrant and giredestrant respectively were both negative trials with no significant difference in PFS observed between the oral SERDs and physician’s choice ET. Of note, these four trials were of oral SERD monotherapy, EMERALD was the only trial that mandated prior CDK4/6 inhibitor, and there was heterogeneity across trials in the proportion of patients with ESR1 mutant disease. The ESR1 mutant subgroup derived a greater benefit compared to the ESR1 wildtype subgroups across these trials, supporting its use as a biomarker to identify ongoing ER dependence in this pretreated population of patients. Elacestrant is the first oral SERD to be FDA approved following progression on first line ET and CDK4/6 inhibitors in January 2023.
Other novel ET agents in development include the proteolysis targeting chimera (PROTAC) ARV-471 (Hurvitz et al., 2022), the complete ER antagonist (CERAN) OP-1250 (Patel et al., 2022), the selective ER covalent antagonists (SERCA) H3B 6545 (Johnston et al., 2022), the third-generation selective ER modulator (SERM) lasofoxifene (Goetz et al., 2022a; Damodaran et al., 2022), and the ShERPA (selective human ER partial agonists) TTC-352 (Dudek et al., 2020). These agents have demonstrated encouraging anti-tumor activity in early phase trials which have included patients treated previously with a CDK4/6 inhibitor (Supplementary Table S1).
Finally, there is preclinical data to support AR activation as a therapeutic strategy for ER + breast cancer, including ET and CDK4/6 inhibitor resistant ER + breast cancer models (Hickey et al., 2021). AR activation results in an altered distribution of ER chromatin binding and its interaction with essential co-activators (p300, SRC-3), resulting in the repression of ER-regulated cell cycle genes (Panet-Raymond et al., 2000). This has prompted clinical trials evaluating the selective androgen receptor modulator (SARM) enobosarm as monotherapy (Clinicaltrials.gov identifier NCT04869943) and in combination with abemaciclib (Clinicaltrials.gov identifier NCT05065411) in patients who have progressed on ET and a CDK4/6 inhibitor.
5.3 CDK2 inhibitor combinations
In light of the evidence for the role of CDK2 and cyclin E in CDK4/6 inhibitor resistance, another therapeutic approach would be to target CDK2 either concurrently with or following the development of CDK4/6 inhibitor resistance (Tadesse et al., 2020). Preclinical studies have demonstrated increased efficacy using the non-selective CDK2 inhibitor dinaciclib concurrently with palbociclib and letrozole, compared with palbociclib and letrozole alone (Al-Qasem et al., 2022). More specific CDK2 inhibitors are currently under evaluation in early phase trials enrolling patients with solid tumors including ER+/HER2-breast cancer. These include PF-07104091 (Clinicaltrials.gov identifiers NCT04553133 and NCT05262400), BLU-222 (Clinicaltrials.gov identifier NCT05252416), and the CDK2/4/6 inhibitor PF-06873600 (Clinicaltrials.gov identifier NCT03519178).
5.4 Combination therapies that target the PI3K/AKT/mTOR pathway
Similar to CDK4/6 inhibitors, small molecule inhibitors of the PI3K/AKT/mTOR pathway are also FDA approved for use in endocrine resistant ER+/HER2-breast cancer in combination with ET. Upregulation of this pathway is associated with ET and CDK4/6 inhibitor resistance as described earlier. Given the potential benefit of continued CDK4/6 inhibition and known crosstalk between the CDK4/6 and PI3K/AKT/mTOR pathways, a number of combinations that include inhibitors of PI3K/AKT/mTOR are under evaluation in patients who have progressed on CDK4/6 inhibitors. In addition to therapeutic efficacy, key considerations include toxicities and the costs of these multidrug regimens.
The seminal phase III SOLAR-1 trial compared alpelisib plus fulvestrant versus placebo plus fulvestrant in patients with advanced ER+/HER2-breast cancer who had relapsed after or progressed on prior AI. OS was extended by 7.9 months in patients with PIK3CA mutated cancer, establishing PIK3CA mutation as a predictive biomarker for alpelisib benefit (Andre et al., 2021). Notably, only 6% of participants in SOLAR-1 received a prior CDK4/6 inhibitor (Andre et al., 2019). In contrast, the phase II BYLieve trial assessed alpelisib and fulvestrant following progression on CDK4/6 inhibitors in patients with a detectable PIK3CA mutation. The median PFS and OS was lower than those observed in the PIK3CA mutated cohort of SOLAR-1, suggesting that the benefit of alpelisib may be attenuated in the setting of CDK4/6 inhibitor resistance (Rugo et al., 2021). Other studies of alpelisib and fulvestrant following progression on CDK4/6 inhibitors are ongoing and include CAPTURE (Australian Trials identifier ACTRN12619001117101), EPIK-B5 (Clinicaltrials.gov identifier NCT05038735), and SEQUEL-Breast (NCT05392608).
In vitro studies and analysis of patient samples demonstrate that activating AKT aberrations are enriched following treatment with CDK4/6 inhibitors and are associated with CDK4/6 inhibitor resistance, which contrasts with PI3K alterations (Wander et al., 2020). The combination of fulvestrant, palbociclib and capiversertib (an inhibitor of AKT1, AKT2 and AKT3) has been shown to be effective in suppressing tumor growth in preclinical models that were dually resistant to ET and CDK4/6 inhibitors (Alves et al., 2021). In the phase II FAKTION trial, patients with endocrine-resistant advanced ER+/HER2-breast cancer were randomized to receive fulvestrant and capiversertib versus fulvestrant and placebo (Jones et al., 2020). This trial included only CDK4/6 inhibitor naïve patients. PFS and OS were superior in the capiversertib arm, and the benefits were more pronounced in patients with evidence of PI3K/AKT/PTEN pathway alterations (Howell et al., 2022). Extending on this study, the phase III CAPItello-291 trial evaluated the same treatments and included 69% patients who had received prior CDK4/6 inhibitor therapy, and reported a significant improvement in PFS in the arm containing capiversertib compared with placebo (7.2 vs. 3.6 months; HR 0.60, p < 0.001) (Turner et al., 2022). The FINER trial (Clinicaltrials.gov identifier NCT04650581) is currently in progress assessing the combination of another AKT inhibitor ipatasertib with fulvestrant, after progression on ET and CDK4/6 inhibitors.
Data for everolimus (mTOR inhibitor) plus exemestane after progression on CDK4/6 inhibitors is limited to two small real-world studies. In these studies, median time on treatment or PFS was limited to a few months (Lupichuk et al., 2019; Cook et al., 2021). The phase I/II TRINITI trial evaluated the triplet combination of exemestane, ribociclib, and everolimus, of whom 92% had received prior CDK4/6 inhibitors. The clinical benefit rate (CBR) at week 24 was 41%, and median PFS was 5.7 months, an impressive result considering the heavily pretreated group of patients (Bardia et al., 2021). The phase Ib B2151009 trial assessed the pan-PI3K/mTOR inhibitor gedatolisib in combination with palbociclib and ET. Patients had received 0–3 prior lines of therapy for metastatic ER+/HER2-breast cancer, and 12-month PFS in this trial was 53% in patients who had received prior CDK4/6 inhibitor therapy (Wesolowski et al., 2022). CAPItello-292 (Clinicaltrials.gov identifier NCT04862663) and VIKTORIA-1 (Clinicaltrials.gov identifier NCT05501886) are other ongoing trials of triplet endocrine, CDK4/6 inhibitor and PI3K/AKT/mTOR pathway inhibitor therapy.
5.5 Non-endocrine therapy approaches
Chemotherapy remains an option for patients who have progressed on ËT alone and in combination with CDK4/6 inhibitors. It is generally held in reserve for rapidly progressive or endocrine-refractory disease. The development of new classes of ET is aimed at delaying the switch to chemotherapy, but such a strategy is only effective in patients whose tumors retain some dependence on ER signaling.
An emerging class of therapies used in ER + breast cancer is antibody drug conjugates (ADCs). These are complex molecules consisting of tumor-antigen targeting antibodies linked to a potent chemotherapy payload and have immune-mediated and cytotoxic properties. In addition to direct cytotoxic effects, ADCs with a cleavable linker can also exert bystander killing effects on neighboring cells which may or may not express the target antigen (Staudacher and Brown, 2017).
Trastuzumab deruxtecan is a HER2-directed ADC, consisting of trastuzumab, a humanized anti-HER2 antibody linked to a topoisomerase I inhibitor payload, approved for use in advanced HER2-positive breast cancer (Cortes et al., 2022). It was more recently evaluated in and demonstrated benefit also against tumors with HER2-low expression (defined as immunohistochemistry 1 + or 2+ with a negative in situ hybridization result) (Modi et al., 2020). In the pivotal phase III DESTINY-Breast04 trial, patients were randomized to receive trastuzumab deruxtecan or physician’s choice chemotherapy. 89% of patients had ER + disease, and of which, 70% had received prior CDK4/6 inhibitor therapy. In the ER + subgroup, the trastuzumab deruxtecan arm had an improved PFS (10.1 vs. 5.4 months; HR 0.51, p < 0.001) and OS (23.9 vs. 17.5 months; HR 0.64, p = 0.003) compared to the treatment of physician’s choice arm, and the benefit was similar for those who had received and not received prior CDK4/6 inhibitors (Modi et al., 2022).
Sacituzumab govitecan is an ADC, which is directed against trophoblast cell-surface antigen 2 (Trop-2) and is linked to a topoisomerase I inhibitor SN-38, cytotoxic payload (Syed, 2020). Trop-2 is a transmembrane glycoprotein which is overexpressed in many epithelial tumors, including >90% of ER+/HER2-breast cancers (Vidula et al., 2022). In the phase III TROPiCS-02 trial, patients with heavily pretreated (median of three prior systemic therapies) ER+/HER2-breast cancer and who received prior CDK4/6 inhibitors were randomized to sacituzumab govitecan or physician’s choice chemotherapy. The median PFS was 5.5 months for sacituzumab govitecan versus 4.0 months for chemotherapy (HR 0.66; p = 0.0003), and benefit was independent of Trop-2 tumor status (Rugo H. et al., 2022; Rugo et al., 2022b; Rugo et al., 2022c). The outcomes of DESTINY-Breast04 and TROPiCS-02 have established a clinical role for ADCs following progression on CDK4/6 inhibitors.
A third ADC datopotamab deruxtecan, which also acts against Trop-2 and uses the same payload as trastuzumab deruxtecan has had encouraging results in a phase I trial that included heavily pretreated ER+/HER2-breast cancer (Meric-Bernstam et al., 2022), and is under further assessment in the phase III TROPION-Breast-01 trial (Clinicaltrials.gov identifier NCT05104866).
6 Conclusion
The combination of ET with a CDK4/6 inhibitor is the standard of care first line treatment for advanced ER+/HER2-breast cancer, and while practice changing and improving survival outcomes, patients eventually develop progressive disease due to intrinsic or acquired drug resistance. There are various systemic treatment options post-progression, but it is not currently clear how these are best sequenced. This represents a current clinical knowledge gap, and defining a biomarker led approach in this setting is critical. Patients whose tumors retain dependence of ER signaling can benefit from changing the endocrine therapy backbone, and where the mechanisms of resistance to combination ET and CDK4/6 inhibitor can be identified and targeted, this may represent a rationale strategy to delay the start of chemotherapy.
Current targeted therapy options include PI3K and PARP inhibitors in the setting of PIK3CA and BRCA1/2 mutations respectively (Burstein et al., 2021), but their use is limited by lack of universal access to genomic testing and treatment side effect profiles. There is an unmet need for efficacious and better tolerated therapies and this review has discussed the preclinical backdrop and status of continued CDK4/6 inhibition, addition of CDK2 inhibition, newer ET backbones, PI3K/AKT/mTOR inhibitors, AR agonists, and ADCs as strategies in the post-CDK4/6 inhibitor ER+/HER2-treatment landscape. The novel approach delivering chemotherapy to tumors through ADCs represent an exciting strategy which has been shown to be effective. The novel approach of delivering chemotherapy to tumors through ADCs represent an exciting strategy which has been shown to be effective.
There is intra- and inter-patient heterogeneity in breast cancer tumor biology, mechanisms of drug resistance, and tumor genomic profiles. Identification of biomarkers relevant to both intrinsic and acquired resistance may inform how to personalize the treatment approach for ER+/HER2-breast cancer patients. The major challenge is to accrue patients of each unique resistance subset for future clinical trials to test new combinations in the post-combination ET plus CDK4/6 inhibitor setting.
Author contributions
FZ, TD, AF, JH, CC, and EL contributed to conception, design and writing of the review. AF and FZ created Figure 1. TD and JH created Supplementary Tables S1, S2. All authors contributed to manuscript revision and approved the submitted version.
Funding
EL is supported by the National Breast Cancer Foundation Endowed Chair (EC17-02) and Love Your Sister Foundation. CC is supported by a Cancer Institute NSW Fellowship (2020/CDF1071). AF is supported by the Australian Commonwealth Government Research Training Program.
Acknowledgments
Images created with BioRender.com under the license agreement KQ252HN37R.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2023.1148792/full#supplementary-material
References
Abukhdeir, A. M., and Park, B. H. (2008). P21 and p27: Roles in carcinogenesis and drug resistance. Expert Rev. Mol. Med. 10, e19. doi:10.1017/S1462399408000744
Al-Qasem, A. J., Alves, C. L., Ehmsen, S., Tuttolomondo, M., Terp, M. G., Johansen, L. E., et al. (2022). Co-targeting CDK2 and CDK4/6 overcomes resistance to aromatase and CDK4/6 inhibitors in ER+ breast cancer. NPJ Precis. Oncol. 6 (1), 68. doi:10.1038/s41698-022-00311-6
Albanell, J., Perez-Garcia, J. M., Gil-Gil, M., Curigliano, G., Ruiz-Borrego, M., Comerma, L., et al. (2023). Palbociclib rechallenge for hormone receptor-positive/HER-negative advanced breast cancer: Findings from the phase II BioPER trial. Clin. Cancer Res. 29 (1), 67–80. doi:10.1158/1078-0432.CCR-22-1281
Alves, C. L., Ehmsen, S., Terp, M. G., Portman, N., Tuttolomondo, M., Gammelgaard, O. L., et al. (2021). Co-targeting CDK4/6 and AKT with endocrine therapy prevents progression in CDK4/6 inhibitor and endocrine therapy-resistant breast cancer. Nat. Commun. 12 (1), 5112. doi:10.1038/s41467-021-25422-9
Alves, C. L., Elias, D., Lyng, M., Bak, M., Kirkegaard, T., Lykkesfeldt, A. E., et al. (2016). High CDK6 protects cells from fulvestrant-mediated apoptosis and is a predictor of resistance to fulvestrant in estrogen receptor-positive metastatic breast cancer. Clin. Cancer Res. 22 (22), 5514–5526. doi:10.1158/1078-0432.CCR-15-1984
Andre, F., Ciruelos, E. M., Juric, D., Loibl, S., Campone, M., Mayer, I. A., et al. (2021). Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 32 (2), 208–217. doi:10.1016/j.annonc.2020.11.011
Andre, F., Ciruelos, E., Rubovszky, G., Campone, M., Loibl, S., Rugo, H. S., et al. (2019). Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380 (20), 1929–1940. doi:10.1056/NEJMoa1813904
Anurag, M., Ellis, M. J., and Haricharan, S. (2018). DNA damage repair defects as a new class of endocrine treatment resistance driver. Oncotarget 9 (91), 36252–36253. doi:10.18632/oncotarget.26363
Asghar, U. S., Kanani, R., Roylance, R., and Mittnacht, S. (2022). Systematic review of molecular biomarkers predictive of resistance to CDK4/6 inhibition in metastatic breast cancer. JCO Precis. Oncol. 6, e2100002. doi:10.1200/PO.21.00002
Bardia, A., Hurvitz, S. A., DeMichele, A., Clark, A. S., Zelnak, A., Yardley, D. A., et al. (2021). Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR(+)/HER2(-) advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin. Cancer Res. 27 (15), 4177–4185. doi:10.1158/1078-0432.CCR-20-2114
Bertucci, F., Ng, C. K. Y., Patsouris, A., Droin, N., Piscuoglio, S., Carbuccia, N., et al. (2019). Genomic characterization of metastatic breast cancers. Nature 569 (7757), 560–564. doi:10.1038/s41586-019-1056-z
Bidard, F. C., Kaklamani, V. G., Neven, P., Streich, G., Montero, A. J., Forget, F., et al. (2022). Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Results from the randomized phase III EMERALD trial. J. Clin. Oncol. 40, 3246–3256. doi:10.1200/JCO.22.00338
Braal, C. L., Jongbloed, E. M., Wilting, S. M., Mathijssen, R. H. J., Koolen, S. L. W., and Jager, A. (2021). Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: Similarities and differences. Drugs 81 (3), 317–331. doi:10.1007/s40265-020-01461-2
Bulat, I., Maglakelidze, M., Krastev, B., Arkenau, H. T., Murias, C., Baird, R. D., et al. (2020). 334P Lerociclib (G1T38), a continuously dosed oral CDK4/6 inhibitor, with fulvestrant in HR+/HER2-advanced breast cancer patients: Updated phase II results and dose selection. Ann. Oncol. 31, S380. doi:10.1016/j.annonc.2020.08.436
Burstein, H. J., Somerfield, M. R., Barton, D. L., Dorris, A., Fallowfield, L. J., Jain, D., et al. (2021). Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J. Clin. Oncol. 39 (35), 3959–3977. doi:10.1200/JCO.21.01392
Chen, P., Lee, N. V., Hu, W., Xu, M., Ferre, R. A., Lam, H., et al. (2016). Spectrum and degree of CDK drug interactions predicts clinical performance. Mol. Cancer Ther. 15 (10), 2273–2281. doi:10.1158/1535-7163.MCT-16-0300
Condorelli, R., Spring, L., O'Shaughnessy, J., Lacroix, L., Bailleux, C., Scott, V., et al. (2018). Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 29 (3), 640–645. doi:10.1093/annonc/mdx784
Cornell, L., Wander, S. A., Visal, T., Wagle, N., and Shapiro, G. I. (2019). MicroRNA-mediated suppression of the TGF-beta pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell Rep. 26 (10), 2667–2680.e7. doi:10.1016/j.celrep.2019.02.023
Cortes, J., Kim, S. B., Chung, W. P., Im, S. A., Park, Y. H., Hegg, R., et al. (2022). Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386 (12), 1143–1154. doi:10.1056/NEJMoa2115022
Cristofanilli, M., Rugo, H. S., Im, S. A., Slamon, D. J., Harbeck, N., Bondarenko, I., et al. (2022). Overall survival with palbociclib and fulvestrant in women with HR+/HER2- ABC: Updated exploratory analyses of PALOMA-3, a double-blind, phase III randomized study. Clin. Cancer Res. 28 (16), 3433–3442. doi:10.1158/1078-0432.CCR-22-0305
Cristofanilli, M., Turner, N. C., Bondarenko, I., Ro, J., Im, S.-A., Masuda, N., et al. (2016). Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 17 (4), 425–439. doi:10.1016/S1470-2045(15)00613-0
Crozier, L., Foy, R., Mouery, B. L., Whitaker, R. H., Corno, A., Spanos, C., et al. (2022). CDK4/6 inhibitors induce replication stress to cause long-term cell cycle withdrawal. EMBO J. 41 (6), e108599. doi:10.15252/embj.2021108599
Damodaran, S., Plourde, P. V., Moore, H. C. F., Anderson, I. C., and Portman, D. J. (2022). Open-label, phase 2, multicenter study of lasofoxifene (LAS) combined with abemaciclib (Abema) for treating pre- and postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer and an ESR1 mutation after progression on prior therapies. J. Clin. Oncol. 40 (16), 1022. doi:10.1200/JCO.2022.40.16_suppl.1022
Deng, J., Wang, E. S., Jenkins, R. W., Li, S., Dries, R., Yates, K., et al. (2018). CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 8 (2), 216–233. doi:10.1158/2159-8290.CD-17-0915
Dickler, M. N., Tolaney, S. M., Rugo, H. S., Cortés, J., Diéras, V., Patt, D., et al. (2017). MONARCH 1, A phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin. Cancer Res. 23 (17), 5218–5224. doi:10.1158/1078-0432.Ccr-17-0754
Downton, T., Zhou, F., Segara, D., Jeselsohn, R., and Lim, E. (2022). Oral selective estrogen receptor degraders (SERDs) in breast cancer: Advances, challenges, and current status. Drug Des. Devel Ther. 16, 2933–2948. doi:10.2147/DDDT.S380925
Duan, L., Perez, R. E., Calhoun, S., and Maki, C. G. (2022). RBL2/DREAM-mediated repression of the Aurora kinase A/B pathway determines therapy responsiveness and outcome in p53 WT NSCLC. Sci. Rep. 12 (1), 1049. doi:10.1038/s41598-022-05013-4
Dudek, A. Z., Liu, L. C., Fischer, J. H., Wiley, E. L., Sachdev, J. C., Bleeker, J., et al. (2020). Phase 1 study of TTC-352 in patients with metastatic breast cancer progressing on endocrine and CDK4/6 inhibitor therapy. Breast Cancer Res. Treat. 183 (3), 617–627. doi:10.1007/s10549-020-05787-z
Finn, R. S., Liu, Y., Zhu, Z., Martin, M., Rugo, H. S., Dieras, V., et al. (2020). Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naive metastatic breast cancer. Clin. Cancer Res. 26 (1), 110–121. doi:10.1158/1078-0432.CCR-19-0751
Finn, R. S., Martin, M., Rugo, H. S., Jones, S., Im, S.-A., Gelmon, K., et al. (2016). Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375 (20), 1925–1936. doi:10.1056/NEJMoa1607303
Finn, R. S., Rugo, H. S., Dieras, V. C., Harbeck, N., Im, S.-A., Gelmon, K. A., et al. (2022). Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J. Clin. Oncol. 40 (17), LBA1003. doi:10.1200/JCO.2022.40.17_suppl.LBA1003
Freeman-Cook, K., Hoffman, R. L., Miller, N., Almaden, J., Chionis, J., Zhang, Q., et al. (2021). Expanding control of the tumor cell cycle with a CDK2/4/6 inhibitor. Cancer Cell 39 (10), 1404–1421. doi:10.1016/j.ccell.2021.08.009
Fry, D. W., Harvey, P. J., Keller, P. R., Elliott, W. L., Meade, M., Trachet, E., et al. (2004). Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 3 (11), 1427–1438. doi:10.1158/1535-7163.1427.3.11
Gelbert, L. M., Cai, S., Lin, X., Sanchez-Martinez, C., Del Prado, M., Lallena, M. J., et al. (2014). Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 32 (5), 825–837. doi:10.1007/s10637-014-0120-7
Goel, S., DeCristo, M. J., Watt, A. C., BrinJones, H., Sceneay, J., Li, B. B., et al. (2017). CDK4/6 inhibition triggers anti-tumour immunity. Nature 548 (7668), 471–475. doi:10.1038/nature23465
Goetz, M., Hamilton, E., Campone, M., Hurvitz, S., Cortes, J., and Martin, M. (2020). “Acquired genomic alterations in circulating tumor DNA from patients receiving abemaciclib alone or in combination with endocrine therapy,” in ASCO Annual Meeting I. Journal of Clinical Oncology.
Goetz, M. P., Plourde, P., Stover, D. G., Bagegni, N., Vidal, G. A., Brufsky, A., et al. (2022a). LBA20 Open-label, randomized study of lasofoxifene (LAS) vs fulvestrant (Fulv) for women with locally advanced/metastatic ER+/HER2-breast cancer (mBC), an estrogen receptor 1 (ESR1) mutation, and disease progression on aromatase (AI) and cyclin-dependent kinase 4/6 (CDK4/6i) inhibitors. Ann. Oncol. 33, S1387–S1388. doi:10.1016/j.annonc.2022.08.015
Goetz, M. P., Toi, M., Huober, J., Sohn, J., Tredan, O., Park, I. H., et al. (2022b). LBA15 MONARCH 3: Interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2-advanced breast cancer (ABC). Ann. Oncol. 33, S1384. doi:10.1016/j.annonc.2022.08.009
Gradishar, W. J., Moran, M. S., Abraham, J., Aft, R., Agnese, D., Allison, K. H., et al. (2022). Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 20 (6), 691–722. doi:10.6004/jnccn.2022.0030
Griffiths, J. I., Chen, J., Cosgrove, P. A., O'Dea, A., Sharma, P., Ma, C., et al. (2021). Serial single-cell genomics reveals convergent subclonal evolution of resistance as early-stage breast cancer patients progress on endocrine plus CDK4/6 therapy. Nat. Cancer 2 (6), 658–671. doi:10.1038/s43018-021-00215-7
Guiley, K. Z., Stevenson, J. W., Lou, K., Barkovich, K. J., Kumarasamy, V., Wijeratne, T. U., et al. (2019). p27 allosterically activates cyclin-dependent kinase 4 and antagonizes palbociclib inhibition. Science 366 (6471), eaaw2106. doi:10.1126/science.aaw2106
Hafner, M., Mills, C. E., Subramanian, K., Chen, C., Chung, M., Boswell, S. A., et al. (2019). Multiomics profiling establishes the polypharmacology of FDA-approved CDK4/6 inhibitors and the potential for differential clinical activity. Cell Chem. Biol. 26 (8), 1067–1080. doi:10.1016/j.chembiol.2019.05.005
Hamilton, E., Cortes, J., Ozyilkan, O., Chen, S. C., Petrakova, K., Manikhas, A., et al. (2022a). nextMONARCH Phase 2 randomized clinical trial: overall survival analysis of abemaciclib monotherapy or in combination with tamoxifen in patients with endocrine-refractory HR +, HER2-metastatic breast cancer. Breast Cancer Res. Treat. 195 (1), 55–64. doi:10.1007/s10549-022-06662-9
Hamilton, E. P., Wang, J. S., Pluard, T., Morikawa, A., Dees, E. C., Jones, R. H., et al. (2022b). Abstract P1-17-10: H3B-6545, a novel selective estrogen receptor covalent antagonist (SERCA), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer - a phase II study. Cancer Res. 82 (4), P1–17–10. doi:10.1158/1538-7445.SABCS21-P1-17-10
Harbeck, N., Rastogi, P., Martin, M., Tolaney, S. M., Shao, Z. M., Fasching, P. A., et al. (2021). Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and ki-67 analysis from the monarchE study. Ann. Oncol. 32 (12), 1571–1581. doi:10.1016/j.annonc.2021.09.015
Herrera-Abreu, M. T., Palafox, M., Asghar, U., Rivas, M. A., Cutts, R. J., Garcia-Murillas, I., et al. (2016). Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 76 (8), 2301–2313. doi:10.1158/0008-5472.CAN-15-0728
Hickey, T. E., Selth, L. A., Chia, K. M., Laven-Law, G., Milioli, H. H., Roden, D., et al. (2021). The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat. Med. 27 (2), 310–320. doi:10.1038/s41591-020-01168-7
Hortobagyi, G. N., Stemmer, S. M., Burris, H. A., Yap, Y. S., Sonke, G. S., Hart, L., et al. (2022). Overall survival with ribociclib plus letrozole in advanced breast cancer. N. Engl. J. Med. 386 (10), 942–950. doi:10.1056/NEJMoa2114663
Hortobagyi, G. N., Stemmer, S. M., Burris, H. A., Yap, Y. S., Sonke, G. S., Paluch-Shimon, S., et al. (2018). Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 29 (7), 1541–1547. doi:10.1093/annonc/mdy155
Howell, S. J., Casbard, A., Carucci, M., Ingarfield, K., Butler, R., Morgan, S., et al. (2022). Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): Overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol. 23 (7), 851–864. doi:10.1016/S1470-2045(22)00284-4
Howlader, N., Altekruse, S. F., Li, C. I., Chen, V. W., Clarke, C. A., Ries, L. A., et al. (2014). US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 106 (5), dju055. doi:10.1093/jnci/dju055
Hurvitz, S. A., Schott, A. F., Ma, C., Hamilton, E. P., Nanda, R., Zahrah, G., et al. (2022). “GS3-03 ARV-471, a PROTAC estrogen receptor (ER) degrader in advanced ER-positive/human epidermal growth factor receptor (HER2)-negative breast cancer: Phase 2 expansion (VERITAC) of a phase 1/2 study,” in San Antonio Breast Cancer Symposium, San Antonio, Texas.
Johnston, S., Martin, M., Di Leo, A., Im, S. A., Awada, A., Forrester, T., et al. (2019). MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5, 5. doi:10.1038/s41523-018-0097-z
Johnston, S., Pluard, T. J., Wang, J. S., Hamilton, E. P., Juric, D., Scholz, C. R., et al. (2022). Abstract P1-17-03: H3B-6545 in combination with palbociclib in women with metastatic estrogen receptor-positive (ER+), human epidermal growth factor receptor 2 (HER2)-negative breast cancer, phase 1b study. Cancer Res. 82 (4), P1-17. doi:10.1158/1538-7445.Sabcs21-p1-17-03
Jones, R. H., Casbard, A., Carucci, M., Cox, C., Butler, R., Alchami, F., et al. (2020). Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 21 (3), 345–357. doi:10.1016/S1470-2045(19)30817-4
Jost, T., Heinzerling, L., Fietkau, R., Hecht, M., and Distel, L. V. (2021). Palbociclib induces senescence in melanoma and breast cancer cells and leads to additive growth arrest in combination with irradiation. Front. Oncol. 11, 740002. doi:10.3389/fonc.2021.740002
Kalinsky, K., Accordino, M. K., Chiuzan, C., Mundi, P. S., Trivedi, M. S., Novik, Y., et al. (2022). A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. J. Clin. Oncol. 40 (17), LBA1004. doi:10.1200/JCO.2022.40.17_suppl.LBA1004
Kalinsky, K., Diamond, J. R., Vahdat, L. T., Tolaney, S. M., Juric, D., O'Shaughnessy, J., et al. (2020). Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: Final results from a phase I/II, single-arm, basket trial. Ann. Oncol. 31 (12), 1709–1718. doi:10.1016/j.annonc.2020.09.004
Klein, M. E., Kovatcheva, M., Davis, L. E., Tap, W. D., and Koff, A. (2018). CDK4/6 inhibitors: The mechanism of action may not Be as simple as once thought. Cancer Cell 34 (1), 9–20. doi:10.1016/j.ccell.2018.03.023
Kumarasamy, V., Nambiar, R., Wang, J., Rosenheck, H., Witkiewicz, A. K., and Knudsen, E. S. (2022). RB loss determines selective resistance and novel vulnerabilities in ER-positive breast cancer models. Oncogene 41 (27), 3524–3538. doi:10.1038/s41388-022-02362-2
Kwapisz, D. (2017). Cyclin-dependent kinase 4/6 inhibitors in breast cancer: Palbociclib, ribociclib, and abemaciclib. Breast Cancer Res. Treat. 166 (1), 41–54. doi:10.1007/s10549-017-4385-3
Li, Q., Jiang, B., Guo, J., Shao, H., Del Priore, I. S., Chang, Q., et al. (2022). INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discov. 12 (2), 356–371. doi:10.1158/2159-8290.CD-20-1726
Li, Z., Razavi, P., Li, Q., Toy, W., Liu, B., Ping, C., et al. (2018). Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell 34 (6), 893–905. doi:10.1016/j.ccell.2018.11.006
Loibl, S., Marmé, F., Martin, M., Untch, M., Bonnefoi, H., Kim, S.-B., et al. (2021). Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—the Penelope-B trial. J. Clin. Oncol. 39 (14), 1518–1530. doi:10.1200/jco.20.03639
Lu, Y. S., Im, S. A., Colleoni, M., Franke, F., Bardia, A., Cardoso, F., et al. (2022). Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with hr+/HER2- advanced breast cancer in MONALEESA-7: A phase III randomized clinical trial. Clin. Cancer Res. 28 (5), 851–859. doi:10.1158/1078-0432.CCR-21-3032
Ma, C. X., Gao, F., Luo, J., Northfelt, D. W., Goetz, M., Forero, A., et al. (2017). NeoPalAna: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin. Cancer Res. 23 (15), 4055–4065. doi:10.1158/1078-0432.Ccr-16-3206
Marinelli, O., Romagnoli, E., Maggi, F., Nabissi, M., Amantini, C., Morelli, M. B., et al. (2020). Exploring treatment with Ribociclib alone or in sequence/combination with Everolimus in ER(+)HER2(-)Rb wild-type and knock-down in breast cancer cell lines. BMC Cancer 20 (1), 1119. doi:10.1186/s12885-020-07619-1
Martin Jimenez, M., Lim, E., Chavez Mac Gregor, M., Bardia, A., Wu, J., Zhang, Q., et al. (2022). 211MO Giredestrant (GDC-9545) vs physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2-locally advanced/metastatic breast cancer (LA/mBC): Primary analysis of the phase II, randomised, open-label acelERA BC study. Ann. Oncol. 33, S633–S634. doi:10.1016/j.annonc.2022.07.250
Maskey, R. S., Wang, F., Lehman, E., Wang, Y., Emmanuel, N., Zhong, W., et al. (2021). Sustained mTORC1 activity during palbociclib-induced growth arrest triggers senescence in ER+ breast cancer cells. Cell Cycle 20 (1), 65–80. doi:10.1080/15384101.2020.1859195
Mayayo-Peralta, I., Faggion, B., Hoekman, L., Morris, B., Lieftink, C., Goldsbrough, I., et al. (2021). Ribociclib induces broad chemotherapy resistance and EGFR dependency in ESR1 wildtype and mutant breast cancer. Cancers (Basel) 13 (24), 6314. doi:10.3390/cancers13246314
Mayer, E. L., Dueck, A. C., Martin, M., Rubovszky, G., Burstein, H. J., Bellet-Ezquerra, M., et al. (2021). Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 22 (2), 212–222. doi:10.1016/S1470-2045(20)30642-2
Mayer, E. L., Ren, Y., Wagle, N., Mahtani, R., Ma, C., DeMichele, A., et al. (2022). “GS3-06 palbociclib after CDK4/6i and endocrine therapy (PACE): A randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated er+/HER2- metastatic breast cancer,” in San Antonio Breast Cancer Symposium, San Antonio, Texas.
Meric-Bernstam, F., Krop, I., Juric, D., Kogawa, T., Hamilton, E., Spira, A. I., et al. (2022). “PD13-08 phase 1 TROPION-PanTumor01 study evaluating datopotamab deruxtecan (Dato-DXd) in unresectable or metastatic hormone receptor-positive/HER2-negative breast cancer (BC),” in San Antonio Breast Cancer Symposium, San Antonio, Texas.
Mo, H., Liu, X., Xue, Y., Chen, H., Guo, S., Li, Z., et al. (2022). S6K1 amplification confers innate resistance to CDK4/6 inhibitors through activating c-Myc pathway in patients with estrogen receptor-positive breast cancer. Mol. Cancer 21 (1), 171. doi:10.1186/s12943-022-01642-5
Modi, S., Jacot, W., Yamashita, T., Sohn, J., Vidal, M., Tokunaga, E., et al. (2022). Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387 (1), 9–20. doi:10.1056/NEJMoa2203690
Modi, S., Park, H., Murthy, R. K., Iwata, H., Tamura, K., Tsurutani, J., et al. (2020). Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: Results from a phase Ib study. J. Clin. Oncol. 38 (17), 1887–1896. doi:10.1200/JCO.19.02318
Naz, S., Sowers, A., Choudhuri, R., Wissler, M., Gamson, J., Mathias, A., et al. (2018). Abemaciclib, a selective CDK4/6 inhibitor, enhances the radiosensitivity of non-small cell lung cancer in vitro and in vivo. Clin. Cancer Res. 24 (16), 3994–4005. doi:10.1158/1078-0432.CCR-17-3575
O'Brien, N., Conklin, D., Beckmann, R., Luo, T., Chau, K., Thomas, J., et al. (2018). Preclinical activity of abemaciclib alone or in combination with antimitotic and targeted therapies in breast cancer. Mol. Cancer Ther. 17 (5), 897–907. doi:10.1158/1535-7163.MCT-17-0290
O'Leary, B., Cutts, R. J., Liu, Y., Hrebien, S., Huang, X., Fenwick, K., et al. (2018). The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 8 (11), 1390–1403. doi:10.1158/2159-8290.CD-18-0264
O'Leary, B., Finn, R. S., and Turner, N. C. (2016). Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 13 (7), 417–430. doi:10.1038/nrclinonc.2016.26
O'Shaughnessy, J., Rastogi, P., Harbeck, N., Toi, M., Hegg, R., Sohn, J., et al. (2021). VP8-2021: Adjuvant abemaciclib combined with endocrine therapy (ET): Updated results from monarchE. Ann. Oncol. 32 (12), 1646–1649. doi:10.1016/j.annonc.2021.09.012
Oliveira, M., Pominchuck, D., Nowecki, Z., Hamilton, E., Kulyaba, Y., Andabekov, T., et al. (2022). “GS3-02 Camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: Results of the randomized, multi-dose Phase 2 SERENA-2 trial,” in San Antonio Breast Cancer Symposium, San Antonio, Texas.
Pack, L. R., Daigh, L. H., Chung, M., and Meyer, T. (2021). Clinical CDK4/6 inhibitors induce selective and immediate dissociation of p21 from cyclin D-CDK4 to inhibit CDK2. Nat. Commun. 12 (1), 3356. doi:10.1038/s41467-021-23612-z
Palafox, M., Monserrat, L., Bellet, M., Villacampa, G., Gonzalez-Perez, A., Oliveira, M., et al. (2022). High p16 expression and heterozygous RB1 loss are biomarkers for CDK4/6 inhibitor resistance in ER(+) breast cancer. Nat. Commun. 13 (1), 5258. doi:10.1038/s41467-022-32828-6
Pandey, K., Park, N., Park, K. S., Hur, J., Cho, Y. B., Kang, M., et al. (2020). Combined CDK2 and CDK4/6 inhibition overcomes palbociclib resistance in breast cancer by enhancing senescence. Cancers (Basel) 12 (12), 3566. doi:10.3390/cancers12123566
Panet-Raymond, V., Gottlieb, B., Beitel, L. K., Pinsky, L., and Trifiro, M. A. (2000). Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol. Cell Endocrinol. 167 (1-2), 139–150. doi:10.1016/s0303-7207(00)00279-3
Patel, M., Alemany, C., Mitri, Z., Makower, D., Borges, V., Sparano, J., et al. (2022). Abstract P1-17-12: Preliminary data from a phase I/II, multicenter, dose escalation study of OP-1250, an oral CERAN/SERD, in subjects with advanced and/or metastatic estrogen receptor (ER)-positive, HER2-negative breast cancer. Cancer Res. 82 (4), P1 17-12-P1-17-12. doi:10.1158/1538-7445.Sabcs21-p1-17-12
Patnaik, A., Rosen, L. S., Tolaney, S. M., Tolcher, A. W., Goldman, J. W., Gandhi, L., et al. (2016). Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 6 (7), 740–753. doi:10.1158/2159-8290.CD-16-0095
Peuker, C. A., Yaghobramzi, S., Grunert, C., Keilholz, L., Gjerga, E., Hennig, S., et al. (2022). Treatment with ribociclib shows favourable immunomodulatory effects in patients with hormone receptor-positive breast cancer-findings from the RIBECCA trial. Eur. J. Cancer 162, 45–55. doi:10.1016/j.ejca.2021.11.025
Rugo, H., Bardia, A., Marme, F., Cortes, J., Schmid, P., Loirat, D., et al. (2022a). “GS1-11 sacitizumab govitecan (SG) vs treatment of physician’s choice (TPC): Efficacy by trop-2 expression in the TROPiCS-02 study of patients (pts) with HR+/HER2- metastatic breast cancer (mBC),” in San Antonio Breast Cancer Symposium, San Antonio, Texas.
Rugo, H. S., Bardia, A., Marmé, F., Cortes, J., Schmid, P., Loirat, D., et al. (2022b). Sacituzumab govitecan in hormone receptor–positive/human epidermal growth factor receptor 2–negative metastatic breast cancer. J. Clin. Oncol. 22, 3365–3376. doi:10.1200/jco.22.01002
Rugo, H. S., Bardia, A., Marmé, F., Cortés, J., Schmid, P., Loirat, D., et al. (2022c). LBA76 Overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs treatment of physician's choice (TPC) in patients (pts) with HR+/HER2-metastatic breast cancer (mBC). Ann. Oncol. 33, S1386. doi:10.1016/j.annonc.2022.08.012
Rugo, H. S., Lerebours, F., Ciruelos, E., Drullinsky, P., Ruiz-Borrego, M., Neven, P., et al. (2021). Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 22 (4), 489–498. doi:10.1016/S1470-2045(21)00034-6
Safonov, A. M., Bandlamudi, C., Selenica, P., Marra, A., Ferraro, E., Mandelker, D., et al. (2022). “Allelic dosage of RB1 drives CDK4/6 inhibitor treatment resistance in metastatic breast cancer,” in ASCO Annual Meeting I. Journal of Clinical Oncology.
Savas, P., Lo, L. L., Luen, S. J., Blackley, E. F., Callahan, J., Moodie, K., et al. (2022). Alpelisib monotherapy for PI3K-altered, pretreated advanced breast cancer: A phase II study. Cancer Discov. 12 (9), 2058–2073. doi:10.1158/2159-8290.CD-21-1696
Schade, A. E., Oser, M. G., Nicholson, H. E., and DeCaprio, J. A. (2019). Cyclin D-CDK4 relieves cooperative repression of proliferation and cell cycle gene expression by DREAM and RB. Oncogene 38 (25), 4962–4976. doi:10.1038/s41388-019-0767-9
Schaer, D. A., Beckmann, R. P., Dempsey, J. A., Huber, L., Forest, A., Amaladas, N., et al. (2018). The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 22 (11), 2978–2994. doi:10.1016/j.celrep.2018.02.053
Slamon, D. J., Neven, P., Chia, S., Fasching, P. A., De Laurentiis, M., Im, S. A., et al. (2018). Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 36 (24), 2465–2472. doi:10.1200/JCO.2018.78.9909
Slamon, D. J., Neven, P., Chia, S., Jerusalem, G., De Laurentiis, M., Im, S., et al. (2021). Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. 32 (8), 1015–1024. doi:10.1016/j.annonc.2021.05.353
Sledge, G. W., Toi, M., Neven, P., Sohn, J., Inoue, K., Pivot, X., et al. (2017). Monarch 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35 (25), 2875–2884. doi:10.1200/jco.2017.73.7585
Sledge, G. W., Toi, M., Neven, P., Sohn, J., Inoue, K., Pivot, X., et al. (2020). The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial. JAMA Oncol. 6 (1), 116–124. doi:10.1001/jamaoncol.2019.4782
Staudacher, A. H., and Brown, M. P. (2017). Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 117 (12), 1736–1742. doi:10.1038/bjc.2017.367
Steinhardt, A. A., Gayyed, M. F., Klein, A. P., Dong, J., Maitra, A., Pan, D., et al. (2008). Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 39 (11), 1582–1589. doi:10.1016/j.humpath.2008.04.012
Syed, Y. Y. (2020). Sacituzumab govitecan: First approval. Drugs 80 (10), 1019–1025. doi:10.1007/s40265-020-01337-5
Tadesse, S., Anshabo, A. T., Portman, N., Lim, E., Tilley, W., Caldon, C. E., et al. (2020). Targeting CDK2 in cancer: Challenges and opportunities for therapy. Drug Discov. Today 25 (2), 406–413. doi:10.1016/j.drudis.2019.12.001
Taylor-Harding, B., Aspuria, P. J., Agadjanian, H., Cheon, D. J., Mizuno, T., Greenberg, D., et al. (2015). Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget 6 (2), 696–714. doi:10.18632/oncotarget.2673
Thangavel, C., Dean, J. L., Ertel, A., Knudsen, K. E., Aldaz, C. M., Witkiewicz, A. K., et al. (2011). Therapeutically activating RB: Reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr. Relat. Cancer 18 (3), 333–345. doi:10.1530/ERC-10-0262
Tolaney, S. M., Chan, A., Petrakova, K., Delaloge, S., Campone, M., Iwata, H., et al. (2022). 212MO AMEERA-3, a phase II study of amcenestrant (AMC) versus endocrine treatment of physician’s choice (TPC) in patients (pts) with endocrine-resistant ER+/HER2− advanced breast cancer (aBC). Ann. Oncol. 33, S634–S635. doi:10.1016/j.annonc.2022.07.251
Torres-Guzman, R., Calsina, B., Hermoso, A., Baquero, C., Alvarez, B., Amat, J., et al. (2017). Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget 8 (41), 69493–69507. doi:10.18632/oncotarget.17778
Turner, N. C., Liu, Y., Zhu, Z., Loi, S., Colleoni, M., Loibl, S., et al. (2019). Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 37 (14), 1169–1178. doi:10.1200/JCO.18.00925
Turner, N., Oliveira, M., Howell, S. J., Dalenc, F., Cortes, J., Gomez, H., et al. (2022). “GS3-04 capivasertib and fulvestrant for patients with aromatase inhibitor-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Results from the phase III CAPItello-291 trial,” in San Antonio Breast Cancer Symposium, San Antonio, Texas.
Vidula, N., Yau, C., and Rugo, H. (2022). Trophoblast Cell Surface Antigen 2 gene (TACSTD2) expression in primary breast cancer. Breast Cancer Res. Treat. 194 (3), 569–575. doi:10.1007/s10549-022-06660-x
Vijayaraghavan, S., Karakas, C., Doostan, I., Chen, X., Bui, T., Yi, M., et al. (2017). CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nat. Commun. 8, 15916. doi:10.1038/ncomms15916
Wander, S. A., Cohen, O., Gong, X., Johnson, G. N., Buendia-Buendia, J. E., Lloyd, M. R., et al. (2020). The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 10 (8), 1174–1193. doi:10.1158/2159-8290.CD-19-1390
Wander, S. A., Han, H. S., Zangardi, M. L., Niemierko, A., Mariotti, V., Kim, L. S. L., et al. (2021). Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: A multicenter experience. J. Natl. Compr. Canc Netw., 1–8. doi:10.6004/jnccn.2020.7662
Wander, S. A., Keenan, J. C., Niemierko, A., Juric, D., Spring, L. M., Supko, J., et al. (2022). “PD13-07 combination therapy with the AKT inhibitor, ipatasertib, endocrine therapy, and a CDK4/6 inhibitor for hormone receptor positive (HR+)/HER2 negative metastatic breast cancer (MBC): Results from the phase I TAKTIC trial,” in San Antonio Breast Cancer Symposium, San Antonio, Texas.
Wang, L., and Liu, X. (2022). Comprehensive analysis of the expression and prognosis for the DREAM complex in human cancers. Front. Genet. 13, 814725. doi:10.3389/fgene.2022.814725
Wang, L., Wang, J., Blaser, B. W., Duchemin, A. M., Kusewitt, D. F., Liu, T., et al. (2007). Pharmacologic inhibition of CDK4/6: Mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia. Blood 110 (6), 2075–2083. doi:10.1182/blood-2007-02-071266
Wesolowski, R., Rugo, H., Stringer-Reasor, E., Han, H. S., Specht, J. M., Dees, E. C., et al. (2022). “PD13-05 Updated results of a Phase Ib study of gedatolisib plus palbociclib and endocrine therapy in women with hormone receptor positive advanced breast cancer: Subgroup analysis by PIK3CA mutation status,” in San Antonio Breast Cancer Symposium, San Antonio, Texas.
Witkiewicz, A. K., Knudsen, K. E., Dicker, A. P., and Knudsen, E. S. (2011). The meaning of p16(ink4a) expression in tumors: Functional significance, clinical associations and future developments. Cell Cycle 10 (15), 2497–2503. doi:10.4161/cc.10.15.16776
Xu, B., Zhang, Q. Y., Zhang, P., Tong, Z., Sun, T., Li, W., et al. (2022). LBA16 dalpiciclib plus letrozole or anastrozole as first-line treatment for hr+/HER2-advanced breast cancer (DAWNA-2): A phase III trial. Ann. Oncol. 33, S1384–S1385. doi:10.1016/j.annonc.2022.08.010
Xu, B., Zhang, Q., Zhang, P., Hu, X., Li, W., Tong, Z., et al. (2021). Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: A randomized, phase 3 trial. Nat. Med. 27 (11), 1904–1909. doi:10.1038/s41591-021-01562-9
Yang, C., Li, Z., Bhatt, T., Dickler, M., Giri, D., Scaltriti, M., et al. (2017). Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36 (16), 2255–2264. doi:10.1038/onc.2016.379
Keywords: estrogen receptor, breast cancer, CDK4/6 inhibitor, endocrine therapy, resistance
Citation: Zhou FH, Downton T, Freelander A, Hurwitz J, Caldon CE and Lim E (2023) CDK4/6 inhibitor resistance in estrogen receptor positive breast cancer, a 2023 perspective. Front. Cell Dev. Biol. 11:1148792. doi: 10.3389/fcell.2023.1148792
Received: 20 January 2023; Accepted: 13 March 2023;
Published: 22 March 2023.
Edited by:
Andrew David Redfern, University of Western Australia, AustraliaReviewed by:
Muriel Le Romancer, INSERM U1052 Centre de Recherche en Cancerologie de Lyon, FranceShengchun Liu, First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2023 Zhou, Downton, Freelander, Hurwitz, Caldon and Lim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elgene Lim, ZS5saW1AZ2FydmFuLm9yZy5hdQ==
†These authors have contributed equally to this work
 Fiona H. Zhou
Fiona H. Zhou Teesha Downton1,2†
Teesha Downton1,2† Allegra Freelander
Allegra Freelander C. Elizabeth Caldon
C. Elizabeth Caldon Elgene Lim
Elgene Lim