
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 23 June 2023
Sec. Cancer Cell Biology
Volume 11 - 2023 | https://doi.org/10.3389/fcell.2023.1131199
This article is part of the Research TopicRole of non-coding RNAs in Development and Metastasis of Solid TumorsView all 5 articles
The long non-coding RNA (lncRNA) cancer susceptibility 11 (CASC11) is a newly identified lncRNA located on chromosome 8q24.21. The expression of lncRNA CASC11 has been found to be elevated in different cancer types and the prognosis of the tumor is inversely correlated with the high CASC11 expression. Moreover, lncRNA CASC11 has an oncogenic function in cancers. The biological characteristics of the tumors, such as proliferation, migration, invasion, autophagy, and apoptosis can be controlled by this lncRNA. In addition to interacting with miRNAs, proteins, transcription factors, and other molecules, the lncRNA CASC11 modulates signaling pathways including Wnt/β-catenin and epithelial-mesenchymal transition. In this review, we have summarized studies on the role of lncRNA CASC11 in the carcinogenesis from cell lines, in vivo, and clinical perspectives.
According to the ENCODE project, although more than 80% of the human genome is transcribed, about 98% of these transcripts do not encode proteins (Harrow et al., 2012). A particular type of RNAs, called long non-coding RNAs (lncRNAs) lacks the ability to code for proteins but are involved in important cellular processes (Bridges et al., 2021). LncRNAs appear to play a variety of roles in the regulation of epigenetic modifications, transcription, post-transcriptional modifications, and translation, according to numerous studies that have been conducted up to now (Bhat et al., 2016; Peng et al., 2017). They can interact with proteins while still being linked to their transcriptional site or they can interact with chromatin-modifying complexes to regulate transcription of target genes in cis or trans, respectively (Rinn et al., 2007; Wang et al., 2008). In addition, the possibility of lncRNAs interacting with microRNAs (miRNAs) to carry out their biological functions has long been known (Jalali et al., 2013). Undeniably, lncRNAs are involved in the pathogenesis of many diseases, including various cancers (Chen F. et al., 2019).
Different functions of lncRNAs depend on their localization and their specific interfaces with DNA, RNA and proteins. Through these interactions, lncRNAs regulate chromatin function and modulate the establishment and function of membraneless nuclear bodies. Most notably, lncRNAs can change the stability and translation of mRNAs in the cytoplasm. Similar to protein coding genes, lncRNAs interfere with signaling pathways (Statello et al., 2021).
The coding gene of lncRNA cancer susceptibility 11 (CASC11) is an lncRNA encoded by a gene on chromosome 8q24.21 and has two transcript variants (https://www.ncbi.nlm.nih.gov/gene/100270680) (Figure 1). There are other CASC genes in the human genome such as CASC1 (chr 12p12.1), CASC2 (chr 10q26.11) and CAS3 (17q21.1). Notably, CASC8 is also affiliated with the lncRNA class.
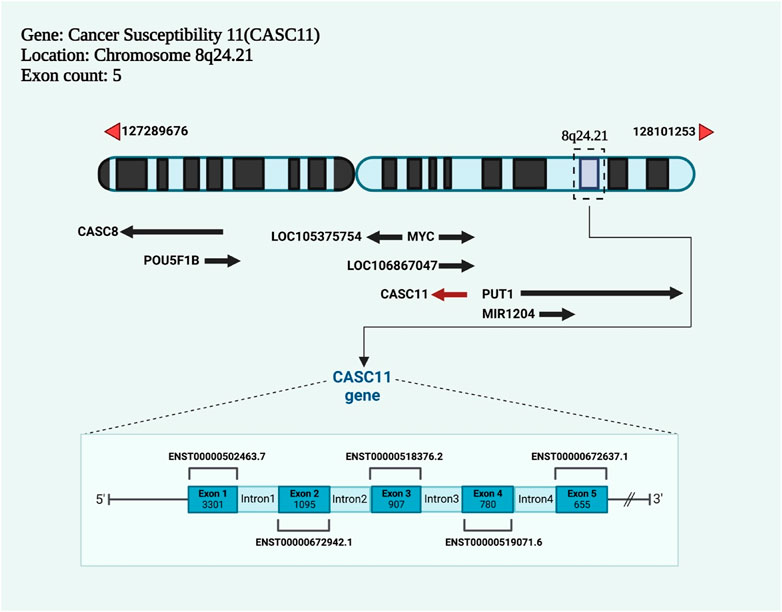
FIGURE 1. The newly discovered lncRNA cancer susceptibility 11 (CASC11) has a coding gene with five exons and is located on chromosome 8q24.21.
The expression of lncRNA CASC11 has been found to be elevated in different cancer types and the prognosis of the tumor is inversely correlated with the high CASC11 expression. As a result, lncRNA CASC11 has an oncogenic function in cancers. The biological characteristics of malignant cells, such as proliferation, migration, invasion, autophagy, and apoptosis can be controlled by this lncRNA. In addition to interacting with miRNAs, proteins, transcription factors, and other molecules, the lncRNA CASC11 modulates signaling pathways including Wnt/β-catenin and epithelial-mesenchymal transition (EMT) to carry out these regulatory functions (Zheng et al., 2021; Wang et al., 2022).
In this review, we have summarized studies on the role of lncRNA CASC11 in the carcinogenesis from cell lines, in vivo, and clinical perspectives. The data summarized in this manuscript highlights the importance of CASC11 in the carcinogenesis and suggests this lncRNA as a putative target for anti-cancer therapies.
The role of CASC11 in the carcinogenesis has been evaluated in several cancer cell lines. In bladder cancer cell lines, upregulation of CASC11 has led to suppression of miR-150 expression. However, miR-150 overexpression could not affect expression of CASC11. Over-expression of CASC11 promotes, while miR-150 overexpression inhibits cancer cell proliferation. In addition, miR-150 could attenuate the increasing effect of CASC11 upregulation on proliferation of cancer cells. Conversely, upregulation of CASC11 could not affect migration and invasion of bladder cancer cells. Cumulatively, CASC11 has a role in regulation of proliferation of bladder cancer cells through modulation of miR-150 levels (Wang et al., 2019).
Similarly, CASC11 has an oncogenic role in cervical cancer. In these cells, CASC11 silencing has inhibited proliferation, migratory potential and invasiveness and induced their apoptosis. Upregulation of CASC11 could facilitate cancer cell proliferation, migration and invasive abilities and suppress their apoptosis. Mechanistically, CASC11 promotes migration and invasion of cervical cancer cells through inducing activity of Wnt/β-catenin signaling (Hsu et al., 2019).
Similar to bladder cancer, CASC11 has been shown to sponge certain miRNAs in colorectal cancer cell. Experiments in colorectal cancer cells have shown the ability of CASC11 to bind with miR-646 and miR-381-3p in the cytoplasm. Besides, miR-646 and miR-381-3p inhibitors could reverse the inhibitory effects of CASC11 knock out on proliferation of colorectal cancer cells. Notably, RAB11FIP2 has been found to be a common target of miR-646 and miR-381-3p. Mechanistically, CASC11 regulates PI3K/AKT pathway through regulation of miR-646 and miR-381-3p/RAB11FIP2 axis (Zhang et al., 2021). CASC11 can also enhance proliferation of colorectal cancer cells through targeting hnRNP-K and activating WNT/β-catenin signaling (Figure 2). Moreover, c-Myc has been shown to directly bind to the promoter of CASC11 and increase histone acetylation to induce expression of CASC11 (Zhang et al., 2016). CASC11 knockdown in esophageal cancer cells has led to enhancement of cell apoptosis. Moreover, its silencing has resulted in upregulation of expression of KLF6 protein. Based on the results of recovery experiments, CASC11 and KLF6 have been shown to be mutually regulated (Chen SG. et al., 2019). Another study in gastric cancer cells has shown that expression of CASC11 is induced by overexpression of LINC01116. Similarly, CASC11 overexpression has resulted in up-regulation of LINC01116. Both lncRNAs have important roles in induction of invasion and migration of gastric cancer cells (Su et al., 2019). CASC11 can also promote malignant features in gastric cancer through regulation of cell cycle pathway (Zhang et al., 2018).
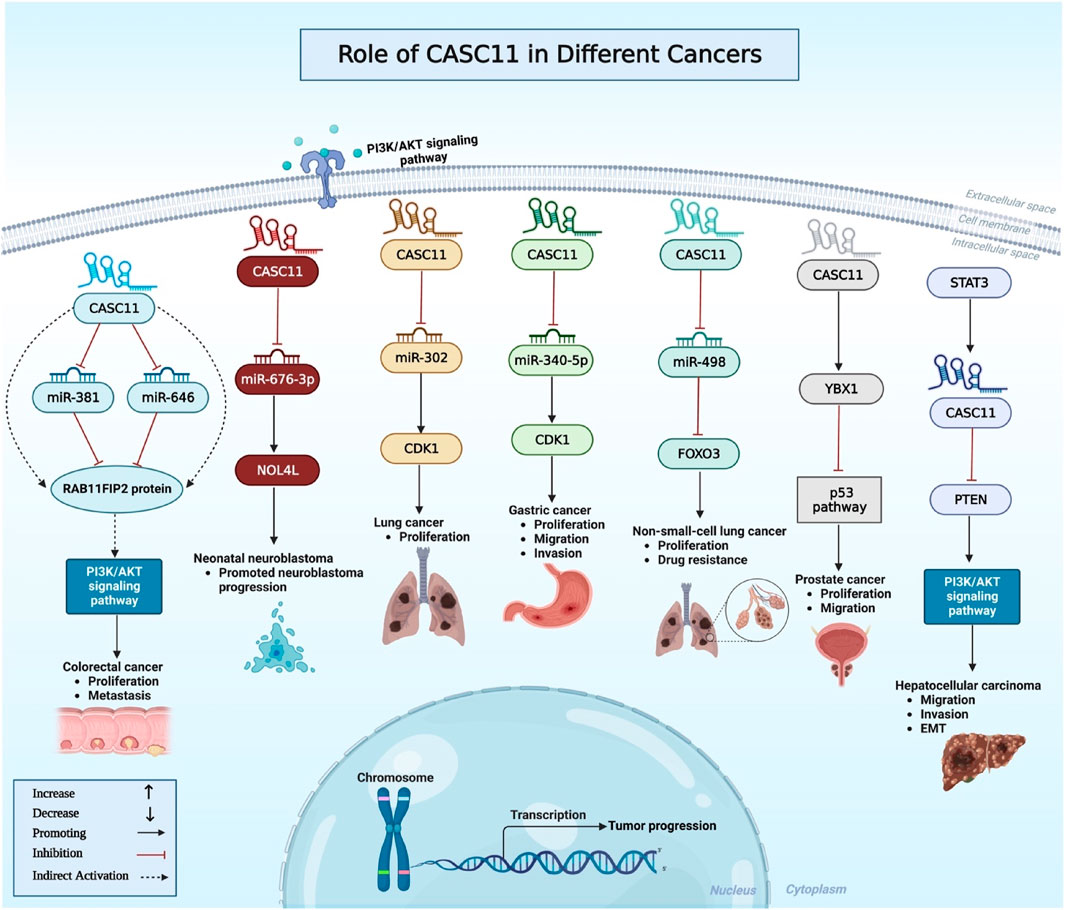
FIGURE 2. This diagram depicts the association between CASC11 and oncogenic signaling pathways in a variety of malignancies. CASC11 promotes tumor cell proliferation, invasion, migration, and survival by targeting specific genes like PTEN and YBX1 and sponging certain miRNAs. Some examples of these miRNAs are miR-381, miR-646, miR-676-3p, miR-340-5p, and miR-498.
In the glioma cells, CASC11 has been demonstrated to sponge miR-498 and increase expression of FOXK1 (Jin et al., 2019). Table 1 shows the results of cell line assays to determine function of CASC11 in various cancer types.
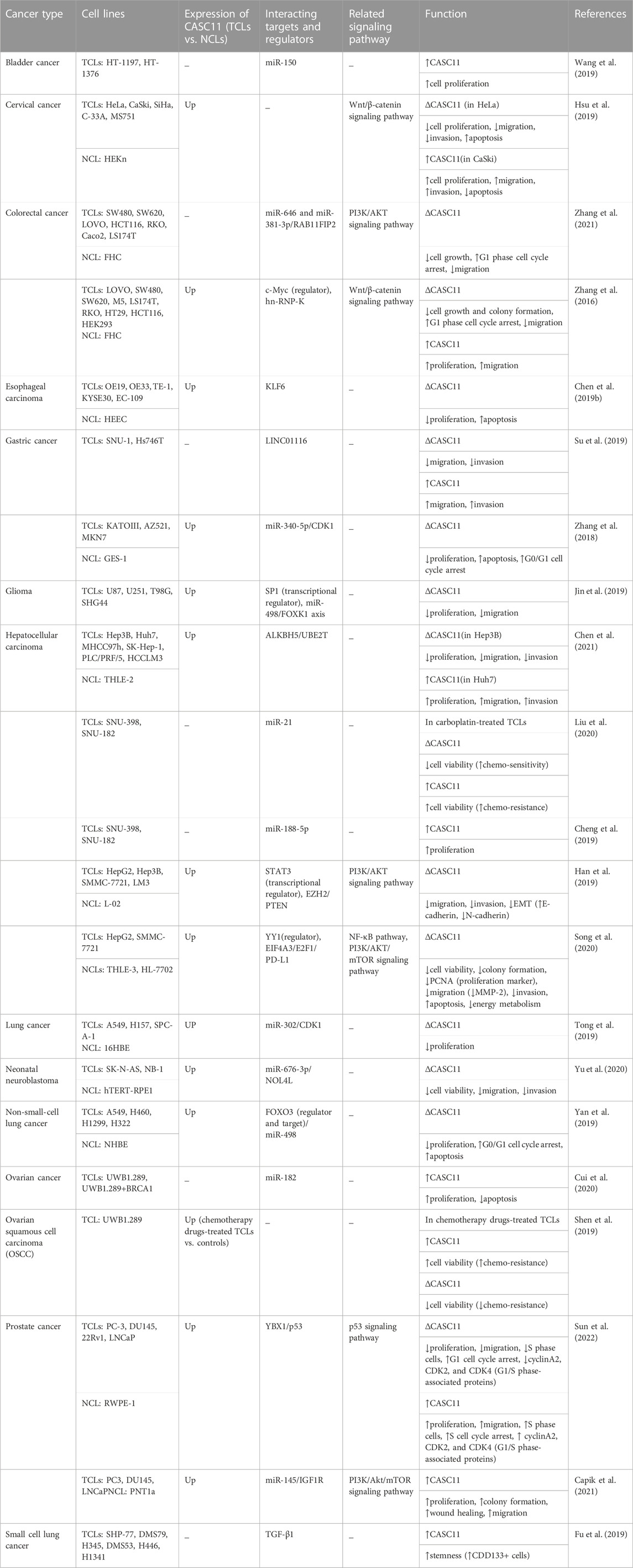
TABLE 1. Cell line assays to determine function of CASC11 in various cancer types (TCLs: tumor cell lines, NCL: normal cell line, ∆: knockdown or deletion, EMT: epithelial-mesenchymal transition).
Obtained from https://app.biorender.com/biorender-templates. BioRender was used in accordance with the terms of the Academic License.
Consistent with in vitro studies, animal studies have affirmed the oncogenic role of CASC11. In animal models of cervical cancer, CASC11 silencing has led to reduction of tumor volume and weight and downregulation of β-catenin (Hsu et al., 2019). Similarly, experiments in animal models of colorectal cancer have shown the role of CASC11 in enhancement of tumor growth. Moreover, miR-646 and miR-381-3p inhibitors have been shown to reverse the inhibitory effects of CASC11 silencing on tumor growth and metastasis (Zhang et al., 2021). Besides, CASC11 silencing has reduced Ki-67 expression and suppressed metastases of colorectal cancer to lung and liver (Zhang et al., 2016). Other studies in animal models of glioma, hepatocellular carcinoma, lung cancer and prostate cancer support oncogenic role of CASC11 (Table 2).
Plasma levels of CASC11 has been found to be up-regulated, while levels of miR-150 has been down-regulated in early stages bladder cancer compared with their levels in healthy controls. Notably, altered expressions of these two transcripts could separate patients with bladder cancer from healthy subjects. Moreover, CASC11 expression has been inversely correlated with miR-150 expression in patients with bladder cancer but not in cancer-free subjects (Wang et al., 2019). In patients with cervical cancer, CASC11 expression has been positively associated with tumor size and FIGO staging and negatively correlated to the survival of patients (Hsu et al., 2019). CASC11 has also been found to be up-regulated in colorectal cancer tissues in association with tumor dimension, serosal invasion, metastasis to lymph node, and TNM stage (Zhang et al., 2016). Besides, expression of CASC11 in the esophageal carcinoma tissues has been remarkably higher than its expression in adjacent normal tissues. Up-regulation of CASC11 has been associated with higher pathological stage and lower overall survival rate in this cancer (Chen SG. et al., 2019). In gastric cancer tissues, expression of CASC11 has been found to be increased parallel with up-regulation of another lncRNA, namely, LINC01116. Expression levels of both lncRNAs have been higher in tissue samples with higher clinical stages (Su et al., 2019). Other studies that reported up-regulation of CASC11 in tumor tissues are shown in Table 3.
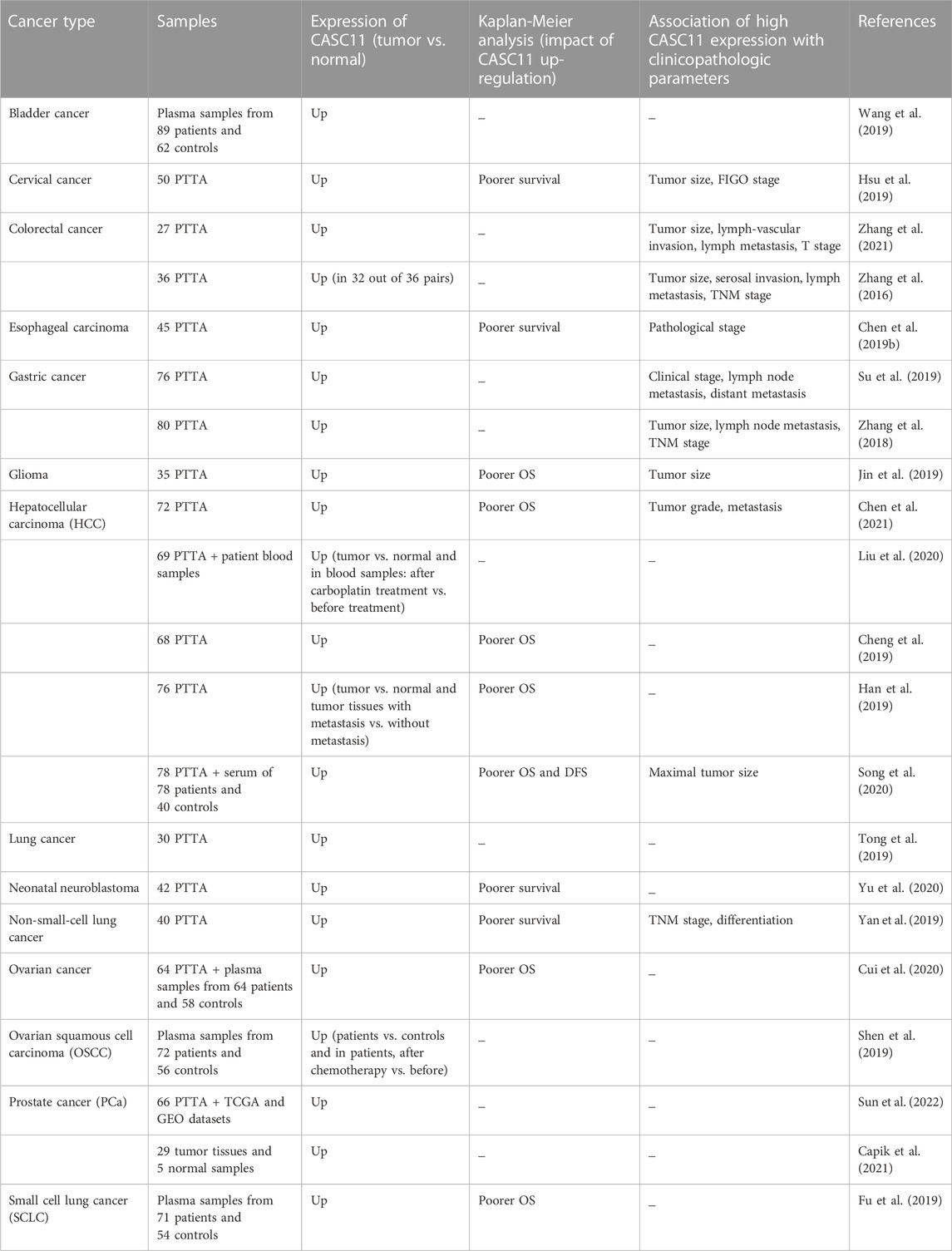
TABLE 3. CASC11 expression in clinical samples of cancer (PTTA: pairs of tumor tissues and adjacent normal tissues, TNM: tumor-node-metastasis, T stage: tumor stage, OS: overall survival, DFS: disease-free survival, FIGO: international federation of gynecology and obstetrics, TCGA: the cancer genome atlas, GEO: gene expression omnibus).
CASC11 is an lncRNA participating in the pathoetiology of diverse cancers as well as atherosclerosis, coronary artery disease and postmenopausal osteoporosis. It is universally up-regulated in malignant tissues and cancer cell lines compared with controls. Therefore, CASC11 can be regarded as an oncogenic lncRNA. This observation has also been affirmed in xenograft models of different cancers. Mechanistical studies have shown the sponging effect of CASC11 on miR-150, miR-646, miR-381-3p, miR-340-5p, miR-498, miR-21, miR-188-5p, miR-302, miR-676-3p, miR-498, miR-182, and miR-145. Moreover, expression of CASC11 has been shown to be regulated by c-Myc, STAT3, YY1, and FOXO3. Therefore, a complex network exists between cancer-related transcription factors, CASC11 and miRNAs. Identification of further molecules being involved in this network would facilitate design of novel therapeutic options for cancer.
Since this lncRNA can be tracked in plasma, it is a possible novel biomarker for detection of cancer recurrence after accomplishment of appropriate therapies.
Moreover, up-regulation of CASC11 in tumor tissues has been related with poor prognosis and adverse clinicopathological characteristics such as metastasis, lymph node involvement, higher grades and advanced stages. Thus, CASC11 is a putative prognostic marker for diverse cancers.
Taken together, CASC11 is an oncogenic lncRNA with possible application as diagnostic and prognostic marker in cancer. Yet, three are several unsolved questions about the underlying mechanism of CASC11 up-regulation in cancers, possible impact of genetic polymorphisms on its function and activity, the role of epigenetic factors in its regulation and the interactions between CASC11 and other regulatory biomolecules. Finding the answers to these questions might facilitate design of novel therapeutic modalities for cancers.
SG-F wrote the draft and revised it. MT designed and supervised the study. AH, BH, and GS collected the data and designed the figures and tables. All authors contributed to the article and approved the submitted version.
The authors would like to thank the clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support, cooperation and assistance throughout the period of study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bhat, S. A., Ahmad, S. M., Mumtaz, P. T., Malik, A. A., Dar, M. A., Urwat, U., et al. (2016). Long non-coding RNAs: Mechanism of action and functional utility. Noncoding RNA Res. 1 (1), 43–50. PubMed PMID: 30159410. Pubmed Central PMCID: PMC6096411. Epub 2016/11/12. eng. doi:10.1016/j.ncrna.2016.11.002
Bridges, M. C., Daulagala, A. C., and Kourtidis, A. (2021). LNCcation: lncRNA localization and function. J. Cell Biol. 220 (2), e202009045. PubMed PMID: 33464299. Pubmed Central PMCID: PMC7816648. Epub 2021/01/20. eng. doi:10.1083/jcb.202009045
Capik, O., Sanli, F., Kurt, A., Ceylan, O., Suer, I., Kaya, M., et al. (2021). CASC11 promotes aggressiveness of prostate cancer cells through miR-145/IGF1R axis. Prostate Cancer Prostatic Dis. 24 (3), 891–902. PubMed PMID: 33753875. Epub 2021/03/24. eng. doi:10.1038/s41391-021-00353-0
Chen, F., Li, M., and Wang, L. (2021). LncRNA CASC11 promotes hepatocellular carcinoma progression via upregulation of UBE2T in a m(6)a-dependent manner. Front. Oncol. 11, 772671. PubMed PMID: 34900723. Pubmed Central PMCID: PMC8652064. Epub 2021/12/14. eng. doi:10.3389/fonc.2021.772671
Chen, F., Li, Z., Deng, C., and Yan, H. (2019a). Integration analysis for novel lncRNA markers predicting tumor recurrence in human colon adenocarcinoma. J. Transl. Med. 17 (1), 299. PubMed PMID: 31470869. Pubmed Central PMCID: PMC6717325. Epub 2019/09/01. eng. doi:10.1186/s12967-019-2049-2
Chen, S. G., Wang, C. H., He, R. Q., Xu, R. Y., and Ji, C. B. (2019b). LncRNA CASC11 promotes the development of esophageal carcinoma by regulating KLF6. Eur. Rev. Med. Pharmacol. Sci. 23 (20), 8878–8887. PubMed PMID: 31696474. Epub 2019/11/07. eng. doi:10.26355/eurrev_201910_19283
Cheng, N., Wu, J., Yin, M., Xu, J., Wang, Y., Chen, X., et al. (2019). LncRNA CASC11 promotes cancer cell proliferation in hepatocellular carcinoma by inhibiting miRNA-188-5p. Biosci. Rep. 39 (4). PubMed PMID: 30910841. Pubmed Central PMCID: PMC6488862. Epub 2019/03/27. eng. doi:10.1042/BSR20190251
Cui, Y., Shen, G., Zhou, D., and Wu, F. (2020). CASC11 overexpression predicts poor prognosis and regulates cell proliferation and apoptosis in ovarian carcinoma. Cancer Manag. Res. 12, 523–529. PubMed PMID: 32158258. Pubmed Central PMCID: PMC6985985. Epub 2020/03/12. eng. doi:10.2147/CMAR.S226801
Fu, Y., Zhang, P., Nan, H., Lu, Y., Zhao, J., Yang, M., et al. (2019). LncRNA CASC11 promotes TGF-β1, increases cancer cell stemness and predicts postoperative survival in small cell lung cancer. Gene 704, 91–96. PubMed PMID: 30965130. Epub 2019/04/10. eng. doi:10.1016/j.gene.2019.04.019
Han, Y., Chen, M., Wang, A., and Fan, X. (2019). STAT3-induced upregulation of lncRNA CASC11 promotes the cell migration, invasion and epithelial-mesenchymal transition in hepatocellular carcinoma by epigenetically silencing PTEN and activating PI3K/AKT signaling pathway. Biochem. Biophys. Res. Commun. 508 (2), 472–479. PubMed PMID: 30503497. Epub 2018/12/07. eng. doi:10.1016/j.bbrc.2018.11.092
Harrow, J., Frankish, A., Gonzalez, J. M., Tapanari, E., Diekhans, M., Kokocinski, F., et al. (2012). Gencode: The reference human genome annotation for the ENCODE project. Genome Res. 22 (9), 1760–1774. PubMed PMID: 22955987. Pubmed Central PMCID: PMC3431492. Epub 2012/09/08. eng. doi:10.1101/gr.135350.111
Hsu, W., Liu, L., Chen, X., Zhang, Y., and Zhu, W. (2019). LncRNA CASC11 promotes the cervical cancer progression by activating Wnt/beta-catenin signaling pathway. Biol. Res. 52 (1), 33. PubMed PMID: 31255182. Pubmed Central PMCID: PMC6599525. Epub 2019/07/01. eng. doi:10.1186/s40659-019-0240-9
Jalali, S., Bhartiya, D., Lalwani, M. K., Sivasubbu, S., and Scaria, V. (2013). Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One 8 (2), e53823. PubMed PMID: 23405074. Pubmed Central PMCID: PMC3566149. Epub 2013/02/14. eng. doi:10.1371/journal.pone.0053823
Jin, J., Zhang, S., Hu, Y., Zhang, Y., Guo, C., and Feng, F. (2019). SP1 induced lncRNA CASC11 accelerates the glioma tumorigenesis through targeting FOXK1 via sponging miR-498. Biomed. Pharmacother. 116, 108968. PubMed PMID: 31121483. Epub 2019/05/24. eng. doi:10.1016/j.biopha.2019.108968
Liu, H., Liu, T., Zhou, Y., Song, X., and Wei, R. (2020). Overexpression of long non-coding RNA cancer susceptibility 11 is involved in the development of chemoresistance to carboplatin in hepatocellular carcinoma. Oncol. Lett. 19 (3), 1993–1998. PubMed PMID: 32194694. Pubmed Central PMCID: PMC7039114. Epub 2020/03/21. eng. doi:10.3892/ol.2020.11265
Peng, W. X., Koirala, P., and Mo, Y. Y. (2017). LncRNA-mediated regulation of cell signaling in cancer. Oncogene 36 (41), 5661–5667. PubMed PMID: 28604750. Pubmed Central PMCID: PMC6450570. Epub 2017/06/13. eng. doi:10.1038/onc.2017.184
Rinn, J. L., Kertesz, M., Wang, J. K., Squazzo, S. L., Xu, X., Brugmann, S. A., et al. (2007). Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129 (7), 1311–1323. PubMed PMID: 17604720. Pubmed Central PMCID: PMC2084369. Epub 2007/07/03. eng. doi:10.1016/j.cell.2007.05.022
Shen, F., Feng, L., Zhou, J., Zhang, H., Xu, Y., Jiang, R., et al. (2019). Overexpression of CASC11 in ovarian squamous cell carcinoma mediates the development of cancer cell resistance to chemotherapy. Gene 710, 363–366. PubMed PMID: 31181314. Epub 2019/06/11. eng. doi:10.1016/j.gene.2019.06.011
Song, H., Liu, Y., Li, X., Chen, S., Xie, R., Chen, D., et al. (2020). Long noncoding RNA CASC11 promotes hepatocarcinogenesis and HCC progression through EIF4A3-mediated E2F1 activation. Clin. Transl. Med. 10 (7), e220. PubMed PMID: 33252856. Pubmed Central PMCID: PMC7643871. Epub 2020/12/01. eng. doi:10.1002/ctm2.220
Statello, L., Guo, C-J., Chen, L-L., and Huarte, M. (2021). Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22 (2), 96–118. doi:10.1038/s41580-020-00315-9
Su, X., Zhang, J., Luo, X., Yang, W., Liu, Y., Liu, Y., et al. (2019). LncRNA LINC01116 promotes cancer cell proliferation, migration and invasion in gastric cancer by positively interacting with lncRNA CASC11. Onco Targets Ther. 12, 8117–8123. PubMed PMID: 31632064. Pubmed Central PMCID: PMC6781852. Epub 2019/10/22. eng. doi:10.2147/OTT.S208133
Sun, X., Xin, S., Zhang, Y., Jin, L., Liu, X., Zhang, J., et al. (2022). Long non-coding RNA CASC11 interacts with YBX1 to promote prostate cancer progression by suppressing the p53 pathway. Int. J. Oncol. 61 (3), 110. PubMed PMID: 35904175. Pubmed Central PMCID: PMC9374466. Epub 2022/07/30. eng. doi:10.3892/ijo.2022.5400
Tong, W., Han, T. C., Wang, W., and Zhao, J. (2019). LncRNA CASC11 promotes the development of lung cancer through targeting microRNA-302/CDK1 axis. Eur. Rev. Med. Pharmacol. Sci. 23 (15), 6539–6547. PubMed PMID: 31378894. Epub 2019/08/06. eng. doi:10.26355/eurrev_201908_18539
Wang, B., Xu, W., Hu, C., Liu, K., Chen, J., Guo, C., et al. (2022). Critical roles of the lncRNA CASC11 in tumor progression and cancer metastasis: The biomarker and therapeutic target potential. Genes Dis. 9 (2), 325–333. PubMed PMID: 35224149. Pubmed Central PMCID: PMC8843879. Epub 2020/12/02. eng. doi:10.1016/j.gendis.2020.11.016
Wang, X., Arai, S., Song, X., Reichart, D., Du, K., Pascual, G., et al. (2008). Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 454 (7200), 126–130. PubMed PMID: 18509338. Pubmed Central PMCID: PMC2823488. Epub 2008/05/30. eng. doi:10.1038/nature06992
Wang, Y., Luo, X., Liu, Y., Han, G., and Sun, D. (2019). Long noncoding RNA RMRP promotes proliferation and invasion via targeting miR-1-3p in non–small-cell lung cancer. J. Cell. Biochem. 120 (9), 15170–15181. doi:10.1002/jcb.28779
Yan, R., Jiang, Y., Lai, B., Lin, Y., and Wen, J. (2019). The positive feedback loop FOXO3/CASC11/miR-498 promotes the tumorigenesis of non-small cell lung cancer. Biochem. Biophys. Res. Commun. 519 (3), 518–524. PubMed PMID: 31537383. Epub 2019/09/21. eng. doi:10.1016/j.bbrc.2019.08.136
Yu, Z., Zhang, J., and Han, J. (2020). Silencing CASC11 curbs neonatal neuroblastoma progression through modulating microRNA-676-3p/nucleolar protein 4 like (NOL4L) axis. Pediatr. Res. 87 (4), 662–668. PubMed PMID: 31645055. Epub 2019/10/24. eng. doi:10.1038/s41390-019-0625-z
Zhang, L., Kang, W., Lu, X., Ma, S., Dong, L., and Zou, B. (2018). LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle 17 (15), 1886–1900. PubMed PMID: 30200804. Pubmed Central PMCID: PMC6152531. Epub 2018/09/12. eng. doi:10.1080/15384101.2018.1502574
Zhang, W., Li, X., Zhang, W., Lu, Y., Lin, W., Yang, L., et al. (2021). The LncRNA CASC11 promotes colorectal cancer cell proliferation and migration by adsorbing miR-646 and miR-381-3p to upregulate their target RAB11FIP2. Front. Oncol. 11, 657650. PubMed PMID: 33937069. Pubmed Central PMCID: PMC8084185. Epub 2021/05/04. eng. doi:10.3389/fonc.2021.657650
Zhang, Z., Zhou, C., Chang, Y., Zhang, Z., Hu, Y., Zhang, F., et al. (2016). Long non-coding RNA CASC11 interacts with hnRNP-K and activates the WNT/β-catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Lett. 376 (1), 62–73. PubMed PMID: 27012187. Epub 2016/03/26. eng. doi:10.1016/j.canlet.2016.03.022
Keywords: CASC11, lncRNA, cancer, expression, biomarker
Citation: Ghafouri-Fard S, Harsij A, Hussen BM, Taheri M and Sharifi G (2023) A review on the role of CASC11 in cancers. Front. Cell Dev. Biol. 11:1131199. doi: 10.3389/fcell.2023.1131199
Received: 24 December 2022; Accepted: 16 June 2023;
Published: 23 June 2023.
Edited by:
Chandrama Mukherjee, Presidency University, IndiaReviewed by:
Md Afjalus Siraj, Yale University, United StatesCopyright © 2023 Ghafouri-Fard, Harsij, Hussen, Taheri and Sharifi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, TW9oYW1tYWQudGFoZXJpQHVuaS1qZW5hLmRl; Guive Sharifi, Z2libm93QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.