
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 15 February 2023
Sec. Cancer Cell Biology
Volume 11 - 2023 | https://doi.org/10.3389/fcell.2023.1124615
This article is part of the Research TopiclncRNAs: Application in Immunotherapy, Radiotherapy, and ChemotherapyView all 9 articles
 Soudeh Ghafouri-Fard1
Soudeh Ghafouri-Fard1 Atefeh Harsij2
Atefeh Harsij2 Bashdar Mahmud Hussen3
Bashdar Mahmud Hussen3 Snur Rasool Abdullah4
Snur Rasool Abdullah4 Aria Baniahmad5
Aria Baniahmad5 Mohammad Taheri6,7*
Mohammad Taheri6,7* Guive Sharifi7*
Guive Sharifi7*LncRNA prostate androgen-regulated transcript 1 (PART1) is an important lncRNA in the carcinogenesis whose role has been firstly unraveled in prostate cancer. Expression of this lncRNA is activated by androgen in prostate cancer cells. In addition, this lncRNA has a role in the pathogenesis intervertebral disc degeneration, myocardial ischemia-reperfusion injury, osteoarthritis, osteoporosis and Parkinson’s disease. Diagnostic role of PART1 has been assessed in some types of cancers. Moreover, dysregulation of PART1 expression is regarded as a prognostic factor in a variety of cancers. The current review provides a concise but comprehensive summary of the role of PART1 in different cancers and non-malignant disorders.
Long non-coding RNAs (lncRNAs) have diverse roles in the carcinogenesis through modulation of gene expression. They can be localized in the nucleus or cytoplasm, thus regulating expression of genes through epigenetic, transcriptional and post-transcriptional mechanisms (Zhang et al., 2019a; Hussen et al., 2022). These effects are mediated through interactions with mRNAs, DNA molecules, proteins, and miRNAs (Zhang et al., 2019a; Ghafouri-Fard et al., 2022). The majority of identified lncRNAs are transcribed by RNA polymerase II; thus, they share several structural features with mRNAs, particularly in terms of having cap structure and poly A tail (Marchese et al., 2017). Yet, most lncRNAs lack coding capacity. The ENCODE project has annotated approximately 16,000 lncRNA genes in humans. These genes can produce more than 28,000 distinctive transcripts (Derrien et al., 2012).
LncRNAs have been shown to be involved in the carcinogenesis through modulation of expression of several tumor suppressor genes and oncogenes. Their altered expression in malignant cells have been associated with diverse abnormalities in the cell cycle regulation, cell proliferation, differentiation and apoptosis (Jiang et al., 2019). During the carcinogenesis process, lncRNAs regulate cell migration, invasion and stemness, thus they have prominent roles in the metastasis (Jiang et al., 2019).
LncRNA prostate androgen-regulated transcript 1 (PART1) is an important lncRNA in the carcinogenesis whose role has been firstly unraveled in prostate cancer. Expression of this lncRNA is activated by androgen in prostate cancer cells (Lin et al., 2000). Being encoded by a gene on gene to chromosome 5q12, PART1 has multiple alternatively transcripts none of them encoding a protein product (Figure 1). Expression assays have revealed biased expression of PART1 in brain, prostate, salivary gland, placenta and bladder (https://www.ncbi.nlm.nih.gov/gene/25859).
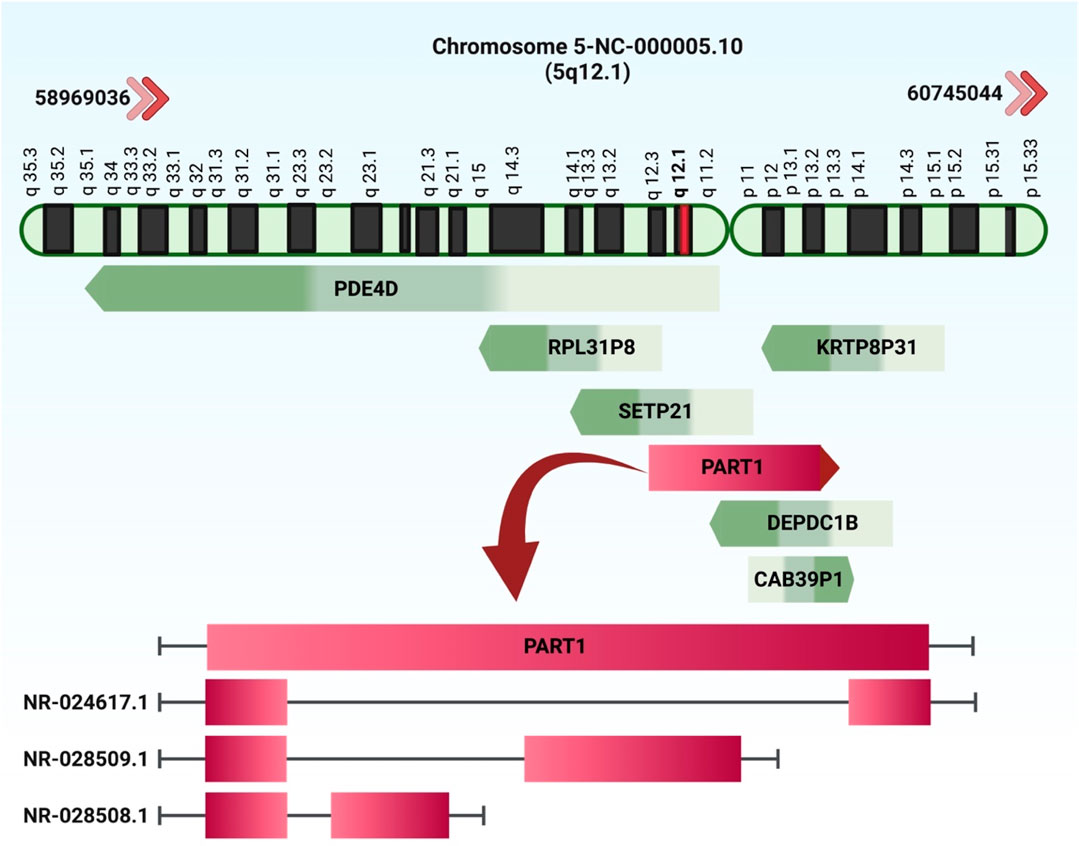
FIGURE 1. The chromosomal location of the prostate androgen-regulated transcript 1 (PART1) was initially identified in PCa. The NCBI database reveals the existence of three transcripts: NR 024617.1 (2.5 kb), NR 028509.1 (5.9 kb), and NR 028508.1 (2.1 kb).
This lncRNA has dual roles in human tissues, being regarded as an oncogene in some tissues but tumor suppressor gene in others (Ran et al., 2022). The current review provides a concise but comprehensive summary of the role of PART1 in different cancers and non-malignant disorders.
Functional studies in a variety of cancer-derived cell lines have assessed the consequences of up-regulation or silencing of PART1. Moreover, these studies have revealed a number of PART1 counterparts. In bladder cancer cells, enhanced expression of PART1 has promoted cell proliferation and invasiveness and suppressed cell apoptosis. On the other hand, PART1 silencing has suppressed cell proliferation and invasion and promoted apoptosis (Hu et al., 2019). In breast cancer cells, knockdown of PART1 has led to decreased proliferation, invasion and migration. Besides, miR-4516 has been found to be a direct counterpart of PART1. Suppression of miR-4516 has been found to rescue the effects of PART1 knockdown on breast cancer cells. Therefore, PART1 binding with miR-4516 promotes development of this type of cancer (Wang and Xu, 2020). Another study in breast cancer cells has shown that PART1 silencing improves the sensitivity of these cells to cisplatin, promotes cell apoptosis, and decreases expression proteins contributing in drug resistance (Lou et al., 2020). PART1 has also been found to be is enriched in triple negative breast cancer cells and in Aldefluorhigh cancer stem cells. PART1 silencing in these cell lines has reduced cell proliferation, migration, and mammosphere forming ability. This lncRNA has been able to affect expression of several genes, including myosin-Va, MYO5A, zinc fingers and homeoboxes protein 2 and ZHX2. In addition, expression of miR-190a-3p, miR-937-5p, miR-22-5p, miR-30b-3p, and miR-6870-5p has been shown to be affected by PART1. PART1 has a direct interaction with miR-937-5p (Cruickshank et al., 2021).
PART1 has also been among lncRNAs being targeted by the tumor suppressor protein ΔNp63α in cervical cancer cells (Liu et al., 2020).
In colorectal cancer cells, three independent studies have shown possible mechanisms for contribution of PART1 in the carcinogenesis. First, PART1 has been shown to regulate this process through targeting miR-150-5p/miR-520h/CTNNB1 axis and inducing activity of Wnt/β-catenin pathway (Zhou et al., 2020a). Moreover, PART1 can function as a molecular sponge for miR-143 in these cells (Hu et al., 2017). Finally, through sponging miR-150-5p, PART1 can increase expression of LRG1 in colorectal cancer cells (Lou et al., 2020).
In esophageal squamous cell carcinoma cells, PART1 has been shown to acts as a tumor suppressor lncRNA in a single study (Zhao et al., 2021). FOXP2 has been shown to bind to the promoter region of PART1 in these cells to regulate its expression. Up-regulation of PART1 could suppress cell proliferation and invasion, while its downregulation promotes cell proliferation and invasion in esophageal squamous cell carcinoma (Figure 2). From a mechanistical point of view, PART1 functions as a molecular sponge for miR-18a-5p, leading to over-expression of SOX6 and inactivation of the β-catenin/c-myc axis (Zhao et al., 2021). On the other hand, another study has shown that exosome-mediated transport of PART1 leads to induction of gefitinib resistance in esophageal squamous cell carcinoma cells through sponging miR-129 (Kang et al., 2018).
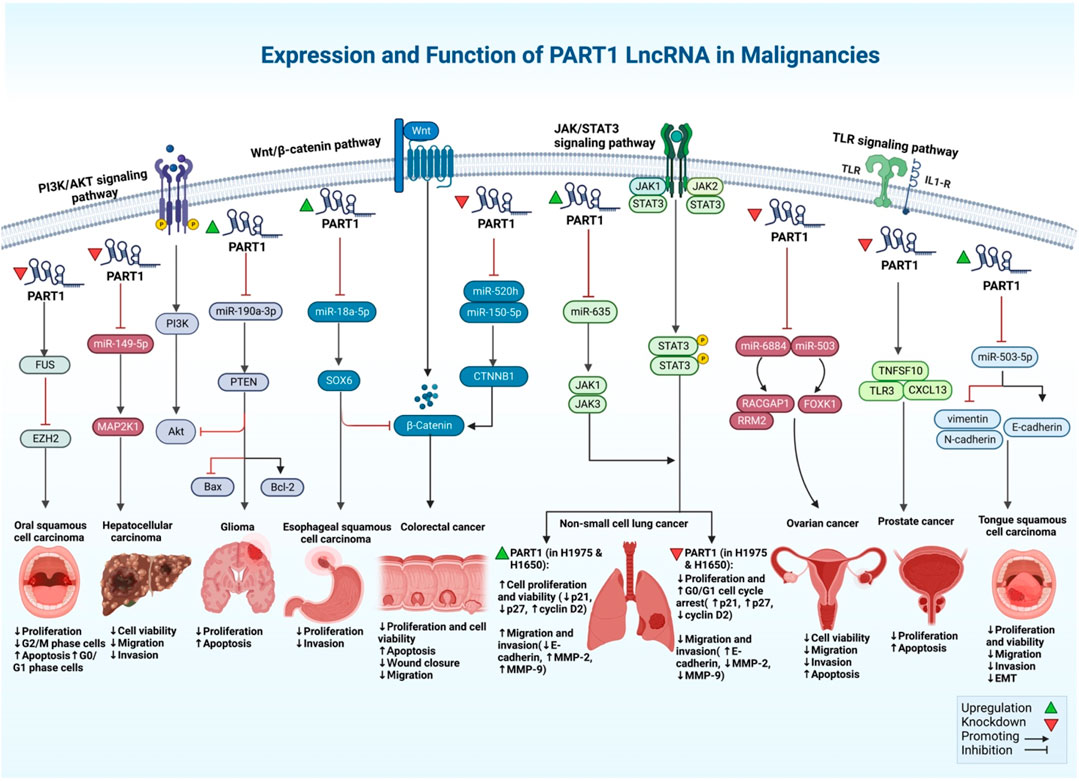
FIGURE 2. An illustration shows the different signaling pathways of PART1 lncRNA with its expression and function in different types of cancer.
PART1 has been shown to restrain aggressive gastric cancer via decreasing expression of PDGFB through PLZF-mediated recruitment of EZH2 (Han et al., 2020). Similarly, PART1 has a tumor suppressor role in glioma through sponging miR-190a-3p and inactivating PTEN/AKT signals (Jin et al., 2020). Moreover, it can block carcinogenic process in glioma through modulation of miR-374b/SALL1 axis (Deng et al., 2022). Tables 1, 2 summarize the results of in vitro studies that reported up-regulation and down-regulation of PART1, respectively.
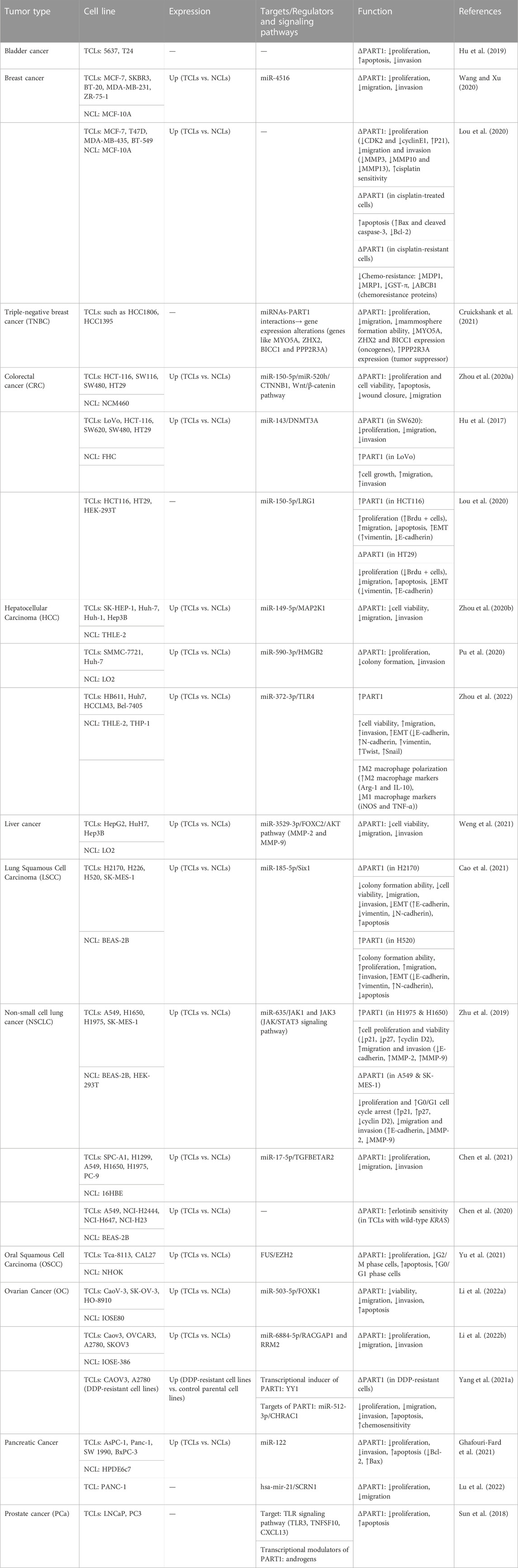
TABLE 1. In vitro experiments to examine expression and function of PART1 in malignancies in which PART1 has been up-regulated (TCLs: tumor cell lines, NCL: normal cell line, ∆: knockdown or deletion, EMT: epithelial-mesenchymal transition, Brdu: Bromodeoxyuridine, DDP: cisplatin, ↑: increase, ↓: decrease).

TABLE 2. In vitro experiments to examine expression and function of PART1 in malignancies in which PART1 has been down-regulated (↑: increase, ↓: decrease).
Different study groups have evaluated functional consequences of PART1 up-regulation or silencing on tumor formation in xenoraft models (Figure 3) (Table 3). Similar to in vitro studies, both tumor suppressor role and oncogenic role have been reported for PART1. Examples of the former type of function have been seen in animal models of cervical squamous cell carcinoma (Liu et al., 2020), gastric cancer (Han et al., 2020) and glioma (Deng et al., 2022) where up-regulation of PART1 has resulted in reduction of tumor growth. Animal models of colorectal carcinoma (Zhou et al., 2020a), hepatocellular carcinoma (Pu et al., 2020), lung cancer (Zhu et al., 2019), oral squamous cell carcinoma (Yu et al., 2021), ovarian cancer (Li et al., 2022b) and triple negative breast cancer (Cruickshank et al., 2021).
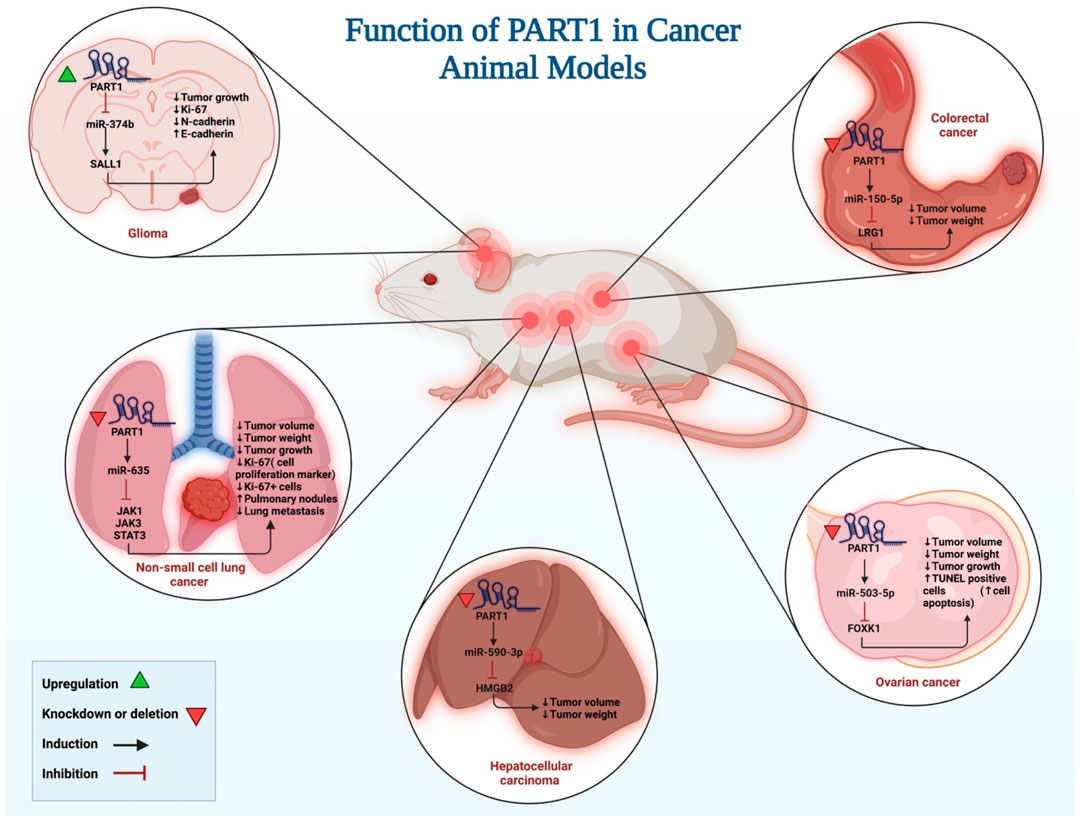
FIGURE 3. An illustration depicts the roles of PART1 activation and silencing in tumor formation in xenograft models, as well as the signaling pathways involved.
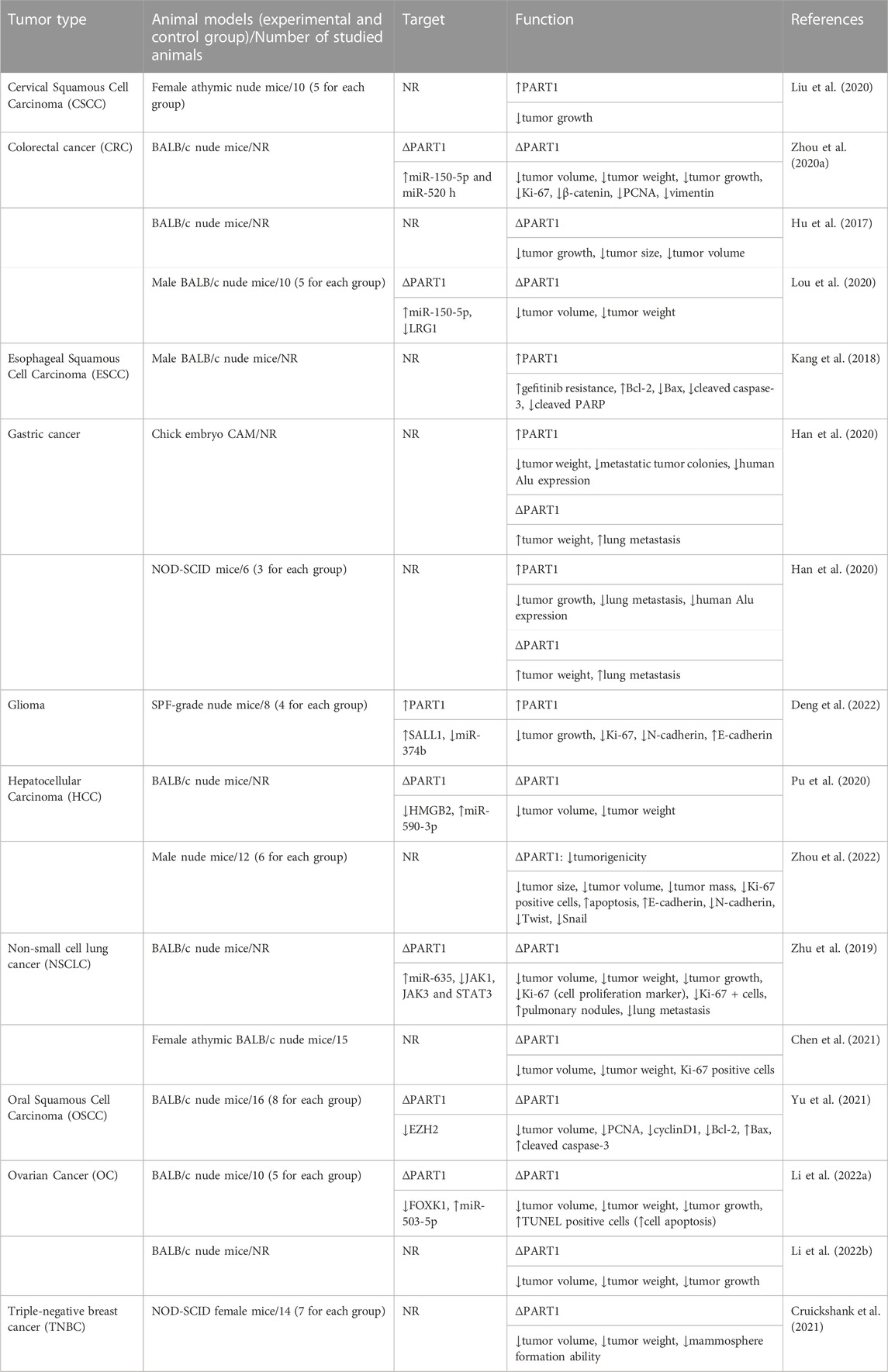
TABLE 3. Effects of PART1 in animal models for cancer (∆: knockdown or deletion, NR: not reported, CAM: chorioallantoic membrane, NOD-SCID mice: non-obese diabetic-severe combined immunodeficiency mice, TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling, SPF: specific-pathogen-free, ↑: increase, ↓: decrease).
Studies in clinical samples have shown up-regulation of PART1 in a variety of cancer tissues including bladder, breast and colorectal cancers (Tables 4, 5). However, there are a number of other cancerous tissues in which PART1 has been found to be down-regulated. For instance, expression of PART1 has been shown to be decreased in esophageal squamous cell carcinoma tissues parallel with down-regulation of SOX6. Notably, low expression of these two genes has been associated with TNM stage, lymph node metastasis and poor prognosis in these patients. Moreover, expression of FOXP2 has been reduced in these tissues in correlation with PART1 expression levels (Zhao et al., 2021). However, another study in this type of cancer has revealed up-regulation of PART1 in the sera samples of gefitinib non-responders versus responders (Kang et al., 2018). Moreover, PART1 is down-regulated in cervical squamous cell carcinoma tissues (Liu et al., 2020). In addition, dysregulation of PART1 has been associated with TNM stage, metastasis, tumor grade and diameter as well as histological type in a variety of cancers (Tables 4, 5).
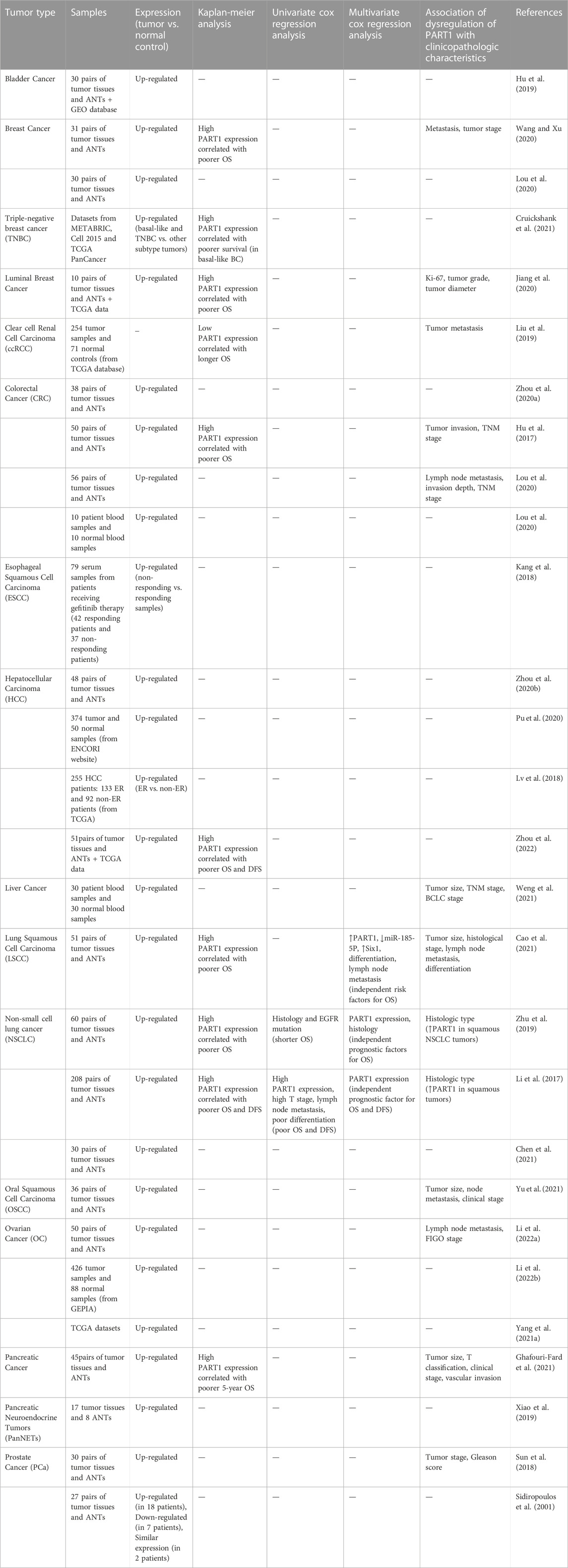
TABLE 4. Function of PART1 up-regulation in the development of malignancy on the basis of studies in clinical samples (ANTs: adjacent normal tissues, TCGA: the cancer genome atlas, METABRIC: molecular taxonomy of breast cancer international consortium, GEPIA: gene expression profiling interactive analysis, GEO: gene expression omnibus, GTEx: genotype–tissue expression, ENCORI: encyclopedia of RNA interaction, GBM: high-grade glioma, LGG: low-grade glioma, ER: early recurrence, BCLC: Barcelona clinic liver cancer, OS: overall survival, DFS: disease-free survival, FIGO: international federation of gynecology and obstetrics, TNM: tumor-node-metastasis, T stage: tumor stage, T classification: tumor classification).
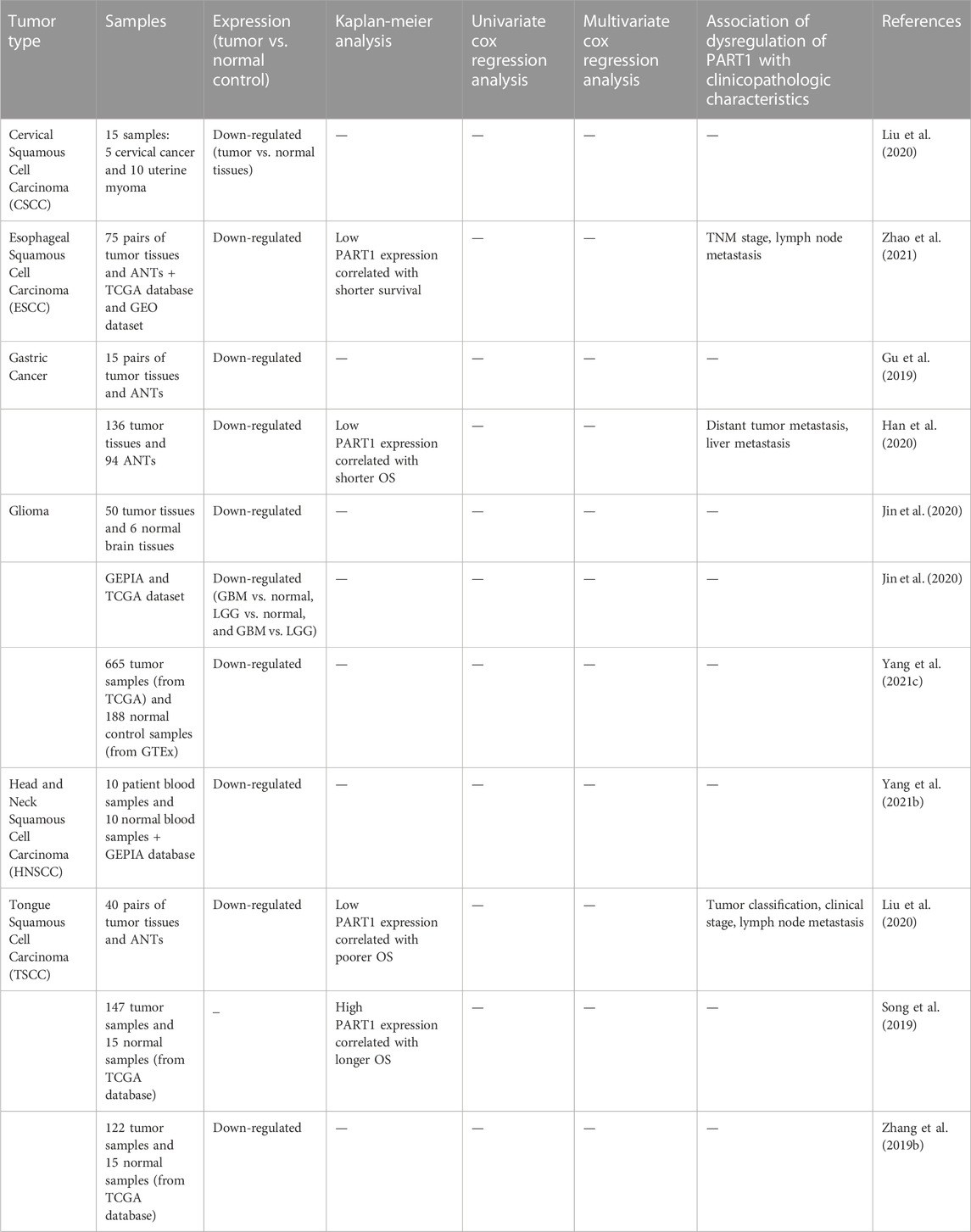
TABLE 5. Function of PART1 down-regulation in the development of malignancy on the basis of studies in clinical samples.
Diagnostic value of PART1 has been evaluated in the context of esophageal squamous cell carcinoma (Kang et al., 2018) and lung squamous cell carcinoma (Cao et al., 2021) (Table 6). In the former type of cancer, PART1 levels could differentiate between gefitinib responders and non-responders with AUC value of 0.839 (Kang et al., 2018). In the latter type of cancer, this lncRNA could separate cancerous and non-cancerous tissues with AUC value of 0.7857 (Cao et al., 2021).
PART1 is among lncRNAs that are dysregulated in SARS-CoV-2 infected cells as revealed by an in silico analysis of GSE147507 dataset. Expression of PART1 has been found to reduced in at least two independent SARS-CoV-2-infected cell lines. Dysregulated lncRNAs have been shown to interact with a variety of genes/proteins and miRNAs which have been linked with signaling pathways regulating viral infection, inflammatory responses and immune function (Laha et al., 2021). PART1 is alos involved in the pathogenesis of intervertebral disc degeneration via regulation of the miR-93/MMP2 axis (Gao et al., 2020) as well as miR-190a-3p expression (Zhang et al., 2021a). Tables 7, 8 summarize the role of PART1 in other non-malignant disorders based on cell line studies that reported up-regulation and down-regulation of PART1, respectively.
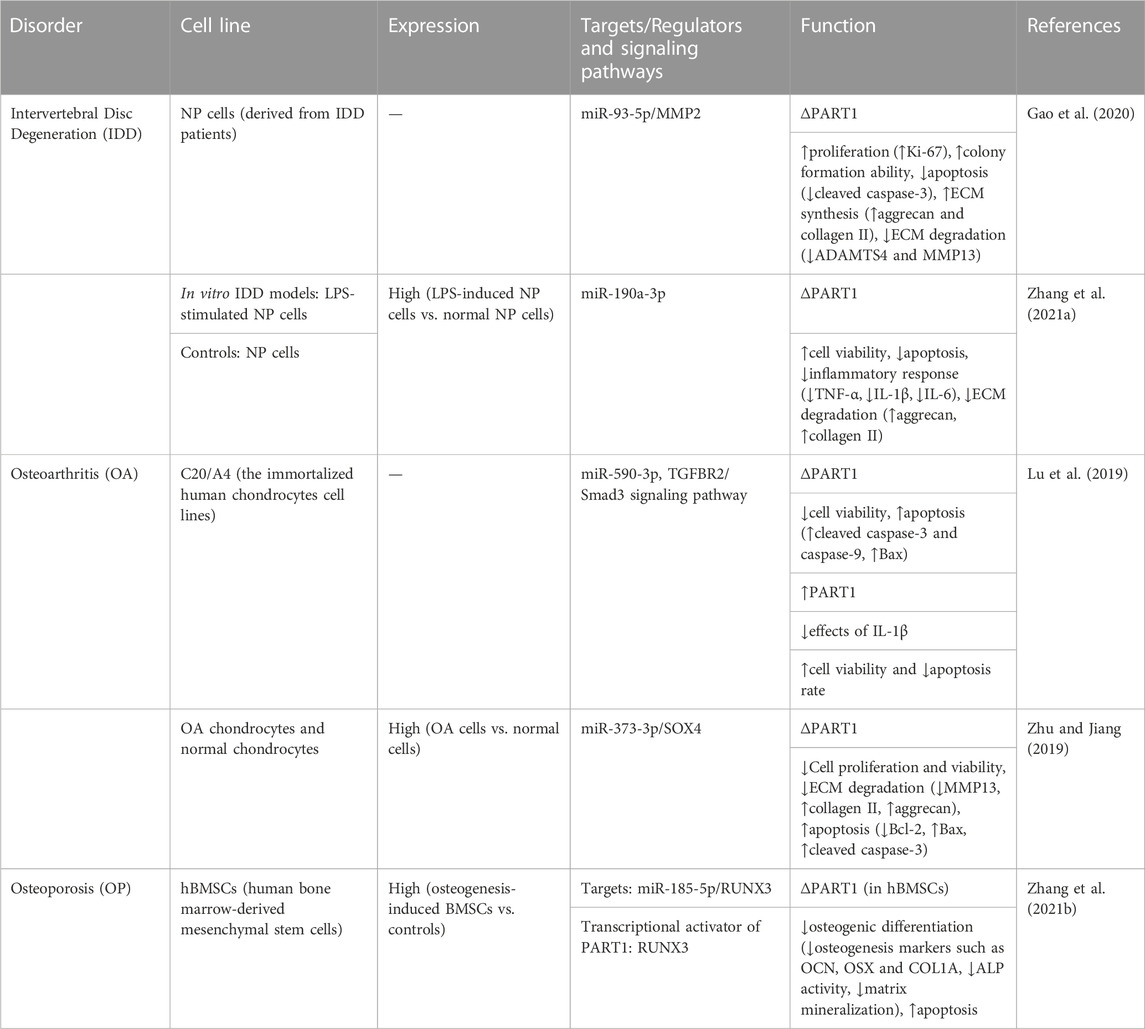
TABLE 7. Cell line studies on PART1 function in non-malignant illnesses in which PART1 has been up-regulated (∆: knockdown or deletion, NP cells: nucleus pulposus cells, ECM: extracellular matrix, LPS: lipopolysaccharide, MPP+: methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine, H/R: hypoxia/reoxygenation, ROS: reactive oxygen species, MMP13: matrix metallopeptidase13, MMP: mitochondrial membrane potential, ↑: increase, ↓: decrease).
Two different studies in animal models have shown the importance of PART1 in myocardial ischemia-reperfusion injury (Guo et al., 2021) and Parkinson’s disease (Shen et al., 2021) (Table 9). In animal models of myocardial ischemia-reperfusion injury, up-regulation of PART1 has resulted in the alleviation of tissue injury, enhancement of cardiac function and reduction of infarction size (Guo et al., 2021).
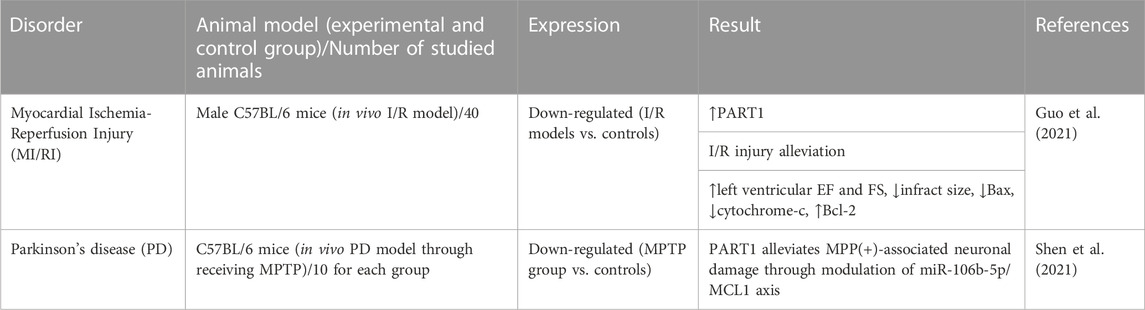
TABLE 9. Animal studies on the involvement of PART1 in non-malignant disorders (MPTP: methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine hydrochloride, I/R: Ischemia-Reperfusion, EF: ejection fraction, FS: fraction shortening, ↑: increase, ↓: decrease).
Experiments in clinical samples have shown down-regulation of PART1 in biological samples obtained from patients with Alzheimer’s disease (Huaying et al., 2020), Parkinson’s disease (Chi et al., 2019) and preeclampsia (Peñailillo et al., 2022). On the other hand, PART1 has been found to be up-regulated in nucleus pulposus samples of patients with intervertebral disc degeneration (Gao et al., 2020). Table 10 shows the results of studies on humans samples to ascertain how PART1 is expressed in non-cancerous disorders.
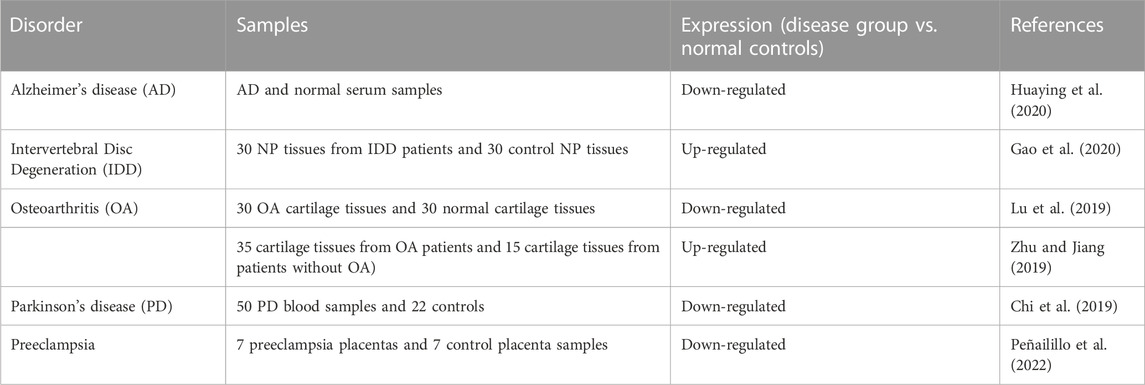
TABLE 10. Studies on humans samples to ascertain how PART1 is expressed in non-cancerous disorders (NP: nucleus pulposus).
PART1 is an lncRNA with diverse functions in the carcinogenesis (Lin et al., 2000). It can affect maintenance of cancer stem cells (Cruickshank et al., 2021) and epithelial to mesenchymal transition (Lou et al., 2020) in a variety of tissues. Moreover, it has a role in modulation of response of cancer cells to cisplatin, erlotinib and gefitinib. Mechanistically, PART1 can act as molecular sponge for a variety of miRNAs such as miR-4516, miR-150-5p, miR-143, miR-18a-5p, miR-129, miR-190a-3p, miR-374b, miR-149-5p, miR-590-3p, miR-372-3p, miR-3529-3p, miR-185-5p, miR-17-5p, miR-503-5p, miR-6884-5p, miR-512-3p, miR-122 and miR-503-5p. It can regulate activity of some cancer-related signaling pathways such as Wnt/β-catenin, PI3K/AKT, PTEN and JAK/STAT3 (Zhu et al., 2019).
Transcription of PART1 can be regulated by a number of transcription factors such as androgens, ∆Np63α, FOXP2, STAT1 and YY1. However, the importance of methylation marks in its promotor on PART1 expression has not been elucidated.
An important feature of PART1 participation in the carcinogenesis is its diverse roles and possibly its tissue-dependent functions in this process. Future studies should identify the mechanism of such tissue-dependent functions and determinants its oncogenic versus tumor suppressor roles.
Since dysregulation of PART1 in tumor tissues has been associated with aggressive behavior of cancer cells, PART1 can be regarded as a prognostic factor in different types of cancers. However, data regarding the application of PART1 as a diagnostic tool in cancer is not sufficient. Since abnormal expression of PART1 has been reported in a variety of cancers, it is possible that expression levels of PART1 can differentiate cancerous tissues from normal counterparts with appropriate diagnostic power.
Taken together, PART1 participates in the pathogenesis of cancer and a variety of non-cancerous conditions including neurodegenerative disorders. Diagnostic value of PART1 has been assessed in few types of cancers, including esophageal (Kang et al., 2018) and lung (Cao et al., 2021) cancers revealing promising results. Moreover, modulation of expression of PART1 in cancer cell lines or animal models of cancers have been associated with therapeutic benefits. However, this filed lacks sufficient data from clinical models. Future functional studies can provide important information about the underlying mechanisms and consequences of PART1 dysregulation in these disorders. The results of such studies can help in design of novel therapeutic modalities based on this lncRNA, particularly in cancers.
SG-F wrote the draft and revised it. MT and AB designed and supervised the study. BH, GS, AH, and SA collected the data and designed the figures and tables. All the authors read the submitted version and approved it.
The authors would like to thank the Clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support, cooperation and assistance throughout the period of study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Cao, Y., Zhang, R., Luo, X., and Yang, Y. (2021). LncRNA PART1 promotes lung squamous cell carcinoma progression via miR-185-5p/Six1 axis. Hum. Exp. Toxicol. 40 (6), 960–976. PubMed PMID: 33300377. Epub 2020/12/11. eng. doi:10.1177/0960327120979032
Chen, S. C., Diao, Y. Z., Zhao, Z. H., and Li, X. L. (2020). Inhibition of lncRNA PART1 chemosensitizes wild type but not KRAS mutant NSCLC cells. Cancer Manag. Res. 12, 4453–4460. PubMed PMID: 32606939. Pubmed Central PMCID: PMC7293907. Epub 2020/07/02. eng. doi:10.2147/CMAR.S245257
Chen, Y., Zhou, X., Huang, C., Li, L., Qin, Y., Tian, Z., et al. (2021). LncRNA PART1 promotes cell proliferation and progression in non-small-cell lung cancer cells via sponging miR-17-5p. J. Cell. Biochem. 122 (3-4), 315–325. PubMed PMID: 33368623. Epub 2020/12/29. eng. doi:10.1002/jcb.29714
Chi, L. M., Wang, L. P., and Jiao, D. (2019). Identification of differentially expressed genes and long noncoding RNAs associated with Parkinson's disease. Park. Dis. 2019, 6078251. PubMed PMID: 30867898. Pubmed Central PMCID: PMC6379850. Epub 2019/03/15. eng. doi:10.1155/2019/6078251
Cruickshank, B. M., Wasson, M. D., Brown, J. M., Fernando, W., Venkatesh, J., Walker, O. L., et al. (2021). LncRNA PART1 promotes proliferation and migration, is associated with cancer stem cells, and alters the miRNA landscape in triple-negative breast cancer. Cancers (Basel) 13 (11), 2644. PubMed PMID: 34072264. Pubmed Central PMCID: PMC8198907. Epub 2021/06/03. eng. doi:10.3390/cancers13112644
Deng, Y. W., Shu, Y. G., and Sun, S. L. (2022). LncRNA PART1 inhibits glioma proliferation and migration via miR-374b/SALL1 axis. Neurochem. Int. 157, 105347. PubMed PMID: 35490895. Epub 2022/05/02. eng. doi:10.1016/j.neuint.2022.105347
Derrien, T., Johnson, R., Bussotti, G., Tanzer, A., Djebali, S., Tilgner, H., et al. (2012). The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 22 (9), 1775–1789. doi:10.1101/gr.132159.111
Gao, D., Hao, L., and Zhao, Z. (2020). Long non-coding RNA PART1 promotes intervertebral disc degeneration through regulating the miR-93/MMP2 pathway in nucleus pulposus cells. Int. J. Mol. Med. 46 (1), 289–299. PubMed PMID: 32319551. Pubmed Central PMCID: PMC7255469. Epub 2020/04/23. eng. doi:10.3892/ijmm.2020.4580
Ghafouri-Fard, S., Hussen, B. M., Gharebaghi, A., Eghtedarian, R., and Taheri, M. (2021). LncRNA signature in colorectal cancer. Pathology-Research Pract. 222, 153432. doi:10.1016/j.prp.2021.153432
Ghafouri-Fard, S., Sohrabi, B., Hussen, B. M., Mehravaran, E., Jamali, E., Arsang-Jang, S., et al. (2022). Down-regulation of MEG3, PANDA and CASC2 as p53-related lncRNAs in breast cancer. Breast Dis. 41 (1), 137–143. PubMed PMID: 35034894. eng. doi:10.3233/BD-210069
Gu, W., Ren, J. H., Zheng, X., Hu, X. Y., and Hu, M. J. (2019). Comprehensive analysis of expression profiles of long non-coding RNAs with associated ceRNA network involved in gastric cancer progression. Mol. Med. Rep. 20 (3), 2209–2218. PubMed PMID: 31322220. Pubmed Central PMCID: PMC6691204. Epub 2019/07/20. eng. doi:10.3892/mmr.2019.10478
Guo, Z., Zhao, M., Jia, G., Ma, R., and Li, M. (2021). LncRNA PART1 alleviated myocardial ischemia/reperfusion injury via suppressing miR-503-5p/BIRC5 mediated mitochondrial apoptosis. Int. J. Cardiol. 338, 176–184. PubMed PMID: 34082009. Epub 2021/06/04. eng. doi:10.1016/j.ijcard.2021.05.044
Han, H., Wang, S., Meng, J., Lyu, G., Ding, G., Hu, Y., et al. (2020). Long noncoding RNA PART1 restrains aggressive gastric cancer through the epigenetic silencing of PDGFB via the PLZF-mediated recruitment of EZH2. Oncogene 39 (42), 6513–6528. PubMed PMID: 32901105. Epub 2020/09/10. eng. doi:10.1038/s41388-020-01442-5
Hu, Y., Ma, Z., He, Y., Liu, W., Su, Y., and Tang, Z. (2017). PART-1 functions as a competitive endogenous RNA for promoting tumor progression by sponging miR-143 in colorectal cancer. Biochem. Biophys. Res. Commun. 490 (2), 317–323. PubMed PMID: 28619512. Epub 2017/06/18. eng. doi:10.1016/j.bbrc.2017.06.042
Hu, X., Feng, H., Huang, H., Gu, W., Fang, Q., Xie, Y., et al. (2019). Downregulated long noncoding RNA PART1 inhibits proliferation and promotes apoptosis in bladder cancer. Technol. Cancer Res. Treat. 18, 1533033819846638. PubMed PMID: 31311442. Pubmed Central PMCID: PMC6636221. Epub 2019/07/18. eng. doi:10.1177/1533033819846638
Huaying, C., Xing, J., Luya, J., Linhui, N., Di, S., and Xianjun, D. (2020). A signature of five long non-coding RNAs for predicting the prognosis of Alzheimer's disease based on competing endogenous RNA networks. Front. Aging Neurosci. 12, 598606. PubMed PMID: 33584243. Pubmed Central PMCID: PMC7876075. Epub 2021/02/16. eng. doi:10.3389/fnagi.2020.598606
Hussen, B. M., Kheder, R. K., Abdullah, S. T., Hidayat, H. J., Rahman, H. S., Salihi, A., et al. (20222022). Functional interplay between long non-coding RNAs and Breast CSCs. Cancer Cell. Int. 22 (1), 233. doi:10.1186/s12935-022-02653-4
Jiang, M. C., Ni, J. J., Cui, W. Y., Wang, B. Y., and Zhuo, W. (2019). Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 9 (7), 1354–1366. PubMed PMID: 31392074. Pubmed Central PMCID: PMC6682721. Epub 2019/08/09. eng.
Jiang, Z., Cheng, P., Luo, B., and Huang, J. (2020). Construction and analysis of a long non-coding RNA-associated competing endogenous RNA network identified potential prognostic biomarkers in luminal breast cancer. Onco Targets Ther. 13, 4271–4282. PubMed PMID: 32547061. Pubmed Central PMCID: PMC7244246. Epub 2020/06/18. eng. doi:10.2147/OTT.S240973
Jin, Z., Piao, L., Sun, G., Lv, C., Jing, Y., and Jin, R. (2020). Long non-coding RNA PART1 exerts tumor suppressive functions in glioma via sponging miR-190a-3p and inactivation of PTEN/AKT pathway. Onco Targets Ther. 13, 1073–1086. PubMed PMID: 32099409. Pubmed Central PMCID: PMC7007780. Epub 2020/02/27. eng. doi:10.2147/OTT.S232848
Kang, M., Ren, M., Li, Y., Fu, Y., Deng, M., and Li, C. (2018). Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J. Exp. Clin. Cancer Res. 37 (1), 171. PubMed PMID: 30049286. Pubmed Central PMCID: PMC6063009. Epub 2018/07/28. eng. doi:10.1186/s13046-018-0845-9
Laha, S., Saha, C., Dutta, S., Basu, M., Chatterjee, R., Ghosh, S., et al. (2021). In silico analysis of altered expression of long non-coding RNA in SARS-CoV-2 infected cells and their possible regulation by STAT1, STAT3 and interferon regulatory factors. Heliyon 7 (3), e06395. PubMed PMID: 33688586. Pubmed Central PMCID: PMC7914022. Epub 2021/03/11. eng. doi:10.1016/j.heliyon.2021.e06395
Li, M., Zhang, W., Zhang, S., Wang, C., and Lin, Y. (2017). PART1 expression is associated with poor prognosis and tumor recurrence in stage I-III non-small cell lung cancer. J. Cancer 8 (10), 1795–1800. PubMed PMID: 28819376. Pubmed Central PMCID: PMC5556642. Epub 2017/08/19. eng. doi:10.7150/jca.18848
Li, B., Lou, G., Zhang, J., Cao, N., and Yu, X. (2022). Repression of lncRNA PART1 attenuates ovarian cancer cell viability, migration and invasion through the miR-503-5p/FOXK1 axis. BMC Cancer 22 (1), 124. PubMed PMID: 35100978. Pubmed Central PMCID: PMC8802513. Epub 2022/02/02. eng. doi:10.1186/s12885-021-09005-x
Li, H., Lei, Y., Li, S., Li, F., and Lei, J. (2022). LncRNA PART1 stimulates the development of ovarian cancer by up-regulating RACGAP1 and RRM2. Reprod. Sci. 29 (8), 2224–2235. PubMed PMID: 35553409. Epub 2022/05/14. eng. doi:10.1007/s43032-022-00905-2
Lin, B., White, J. T., Ferguson, C., Bumgarner, R., Friedman, C., Trask, B., et al. (2000). PART-1: A novel human prostate-specific, androgen-regulated gene that maps to chromosome 5q12. Cancer Res. 60 (4), 858–863. PubMed PMID: 10706094. Epub 2000/03/08. eng.
Liu, B., Ma, T., Li, Q., Wang, S., Sun, W., Li, W., et al. (2019). Identification of a lncRNA-associated competing endogenous RNA-regulated network in clear cell renal cell carcinoma. Mol. Med. Rep. 20 (1), 485–494. PubMed PMID: 31180525. Pubmed Central PMCID: PMC6580006. Epub 2019/06/11. eng. doi:10.3892/mmr.2019.10290
Liu, H., Zhu, C., Xu, Z., Wang, J., Qian, L., Zhou, Q., et al. (2020). lncRNA PART1 and MIR17HG as ΔNp63α direct targets regulate tumor progression of cervical squamous cell carcinoma. Cancer Sci. 111 (11), 4129–4141. PubMed PMID: 32920922. Pubmed Central PMCID: PMC7648017. Epub 2020/09/14. eng. doi:10.1111/cas.14649
Lou, T., Ke, K., Zhang, L., Miao, C., and Liu, Y. (2020). LncRNA PART1 facilitates the malignant progression of colorectal cancer via miR-150-5p/LRG1 axis. J. Cell. Biochem. 121 (10), 4271–4281. PubMed PMID: 31898365. Epub 2020/01/04. eng. doi:10.1002/jcb.29635
Lu, C., Li, Z., Hu, S., Cai, Y., and Peng, K. (2019). LncRNA PART-1 targets TGFBR2/Smad3 to regulate cell viability and apoptosis of chondrocytes via acting as miR-590-3p sponge in osteoarthritis. J. Cell. Mol. Med. 23 (12), 8196–8205. PubMed PMID: 31571401. Pubmed Central PMCID: PMC6850963. Epub 2019/10/02. eng. doi:10.1111/jcmm.14690
Lu, S. Y., Hua, J., Liu, J., Wei, M. Y., Liang, C., Meng, Q. C., et al. (2022). Construction of a paclitaxel-related competitive endogenous RNA network and identification of a potential regulatory axis in pancreatic cancer. Transl. Oncol. 20, 101419. PubMed PMID: 35413498. Pubmed Central PMCID: PMC9018166. Epub 2022/04/13. eng. doi:10.1016/j.tranon.2022.101419
Lv, Y., Wei, W., Huang, Z., Chen, Z., Fang, Y., Pan, L., et al. (2018). Long non-coding RNA expression profile can predict early recurrence in hepatocellular carcinoma after curative resection. Hepatol. Res. 48 (13), 1140–1148. PubMed PMID: 29924905. Epub 2018/06/21. eng. doi:10.1111/hepr.13220
Marchese, F. P., Raimondi, I., and Huarte, M. (2017). The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 18 (1), 206–213. doi:10.1186/s13059-017-1348-2
Peñailillo, R., Monteiro, L. J., Acuña-Gallardo, S., García, F., Velásquez, V., Correa, P., et al. (2022). Identification of LOC101927355 as a novel biomarker for preeclampsia. Biomedicines 10 (6), 1253. PubMed PMID: 35740273. Pubmed Central PMCID: PMC9219905. Epub 2022/06/25. eng. doi:10.3390/biomedicines10061253
Pu, J., Tan, C., Shao, Z., Wu, X., Zhang, Y., Xu, Z., et al. (2020). Long noncoding RNA PART1 promotes hepatocellular carcinoma progression via targeting miR-590-3p/HMGB2 Axis. Onco Targets Ther. 13, 9203–9211. PubMed PMID: 32982307. Pubmed Central PMCID: PMC7502387. Epub 2020/09/29. eng. doi:10.2147/OTT.S259962
Ran, R., Gong, C. Y., Wang, Z. Q., Zhou, W. M., Zhang, S. B., Shi, Y. Q., et al. (2022). Long non-coding RNA PART1: Dual role in cancer. Hum. Cell. 35 (5), 1364–1374. PubMed PMID: 35864416. Epub 2022/07/22. eng. doi:10.1007/s13577-022-00752-y
Shen, Y., Cui, X., Xu, N., Hu, Y., and Zhang, Z. (2021). lncRNA PART1 mitigates MPP(+)-induced neuronal injury in SH-SY5Y cells via micRNA-106b-5p/MCL1 axis. Am. J. Transl. Res. 13 (8), 8897–8908. PubMed PMID: 34540003. Pubmed Central PMCID: PMC8430160. Epub 2021/09/21. eng.
Sidiropoulos, M., Chang, A., Jung, K., and Diamandis, E. P. (2001). Expression and regulation of prostate androgen regulated transcript-1 (PART-1) and identification of differential expression in prostatic cancer. Br. J. Cancer 85 (3), 393–397. PubMed PMID: 11487271. Pubmed Central PMCID: PMC2364080. Epub 2001/08/07. eng. doi:10.1054/bjoc.2001.1883
Song, Y., Pan, Y., and Liu, J. (2019). Functional analysis of lncRNAs based on competitive endogenous RNA in tongue squamous cell carcinoma. PeerJ 7, e6991. PubMed PMID: 31179185. Pubmed Central PMCID: PMC6544013. Epub 2019/06/11. eng. doi:10.7717/peerj.6991
Sun, M., Geng, D., Li, S., Chen, Z., and Zhao, W. (2018). LncRNA PART1 modulates toll-like receptor pathways to influence cell proliferation and apoptosis in prostate cancer cells. Biol. Chem. 399 (4), 387–395. PubMed PMID: 29261512. Epub 2017/12/21. eng. doi:10.1515/hsz-2017-0255
Wang, Z., and Xu, R. (2020). lncRNA PART1 promotes breast cancer cell progression by directly targeting miR-4516. Cancer Manag. Res. 12, 7753–7760. PubMed PMID: 32922076. Pubmed Central PMCID: PMC7457826. Epub 2020/09/15. eng. doi:10.2147/CMAR.S249296
Weng, Z., Peng, J., Wu, W., Zhang, C., Zhao, J., and Gao, H. (2021). Downregulation of PART1 inhibits proliferation and differentiation of Hep3B cells by targeting hsa-miR-3529-3p/FOXC2 Axis. J. Oncol. 2021, 7792223. PubMed PMID: 34484336. Pubmed Central PMCID: PMC8410447. Epub 2021/09/07. eng. doi:10.1155/2021/7792223
Xiao, Y., Yang, Y., Wang, Y., Li, X., and Wang, W. (2019). Five novel genes related to the pathogenesis and progression of pancreatic neuroendocrine tumors by bioinformatics analysis with RT-qPCR verification. Front. Neurosci. 13, 937. PubMed PMID: 31607839. Pubmed Central PMCID: PMC6771308. Epub 2019/10/15. eng. doi:10.3389/fnins.2019.00937
Yang, H., Zhang, X., Zhu, L., Yang, Y., and Yin, X. (2021). YY1-Induced lncRNA PART1 enhanced resistance of ovarian cancer cells to cisplatin by regulating miR-512-3p/CHRAC1 Axis. DNA Cell. Biol. 40 (6), 821–832. PubMed PMID: 34030482. Epub 2021/05/26. eng. doi:10.1089/dna.2021.0059
Yang, L., Lu, P., Yang, X., Li, K., Chen, X., and Qu, S. (2021). Excavating novel diagnostic and prognostic long non-coding RNAs (lncRNAs) for head and neck squamous cell carcinoma: An integrated bioinformatics analysis of competing endogenous RNAs (ceRNAs) and gene co-expression networks. Bioengineered 12 (2), 12821–12838. PubMed PMID: 34898376. Pubmed Central PMCID: PMC8810019. Epub 2021/12/14. eng. doi:10.1080/21655979.2021.2003925
Yang, Z., Gong, W., Zhang, T., and Gao, H. (2021). Molecular features of glioma determined and validated using combined TCGA and GTEx data analyses. Front. Oncol. 11, 729137. PubMed PMID: 34660294. Pubmed Central PMCID: PMC8516354. Epub 2021/10/19. eng. doi:10.3389/fonc.2021.729137
Yu, Q., Du, Y., Wang, S., and Zheng, X. (2021). LncRNA PART1 promotes cell proliferation and inhibits apoptosis of oral squamous cell carcinoma by blocking EZH2 degradation. J. Biochem. 169 (6), 721–730. PubMed PMID: 33725092. Epub 2021/03/17. eng. doi:10.1093/jb/mvab026
Zhang, X., Wang, W., Zhu, W., Dong, J., Cheng, Y., Yin, Z., et al. (2019). Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int. J. Mol. Sci. 20 (22), 5573. PubMed PMID: 31717266. Pubmed Central PMCID: PMC6888083. Epub 2019/11/14. eng. doi:10.3390/ijms20225573
Zhang, S., Cao, R., Li, Q., Yao, M., Chen, Y., and Zhou, H. (2019). Comprehensive analysis of lncRNA-associated competing endogenous RNA network in tongue squamous cell carcinoma. PeerJ 7, e6397. PubMed PMID: 30755833. Pubmed Central PMCID: PMC6368841. Epub 2019/02/14. eng. doi:10.7717/peerj.6397
Zhang, Z., Huo, Y., Zhou, Z., Zhang, P., and Hu, J. (2021). Role of lncRNA PART1 in intervertebral disc degeneration and associated underlying mechanism. Exp. Ther. Med. 21 (2), 131. PubMed PMID: 33376513. Pubmed Central PMCID: PMC7751492. Epub 2020/12/31. eng. doi:10.3892/etm.2020.9563
Zhang, J., Xu, N., Yu, C., Miao, K., and Wang, Q. (2021). LncRNA PART1/miR-185-5p/RUNX3 feedback loop modulates osteogenic differentiation of bone marrow mesenchymal stem cells. Autoimmunity 54 (7), 422–429. PubMed PMID: 34431433. Epub 2021/08/26. eng. doi:10.1080/08916934.2021.1966771
Zhao, Y., Zhang, Q., Liu, H., Wang, N., Zhang, X., and Yang, S. (2021). lncRNA PART1, manipulated by transcriptional factor FOXP2, suppresses proliferation and invasion in ESCC by regulating the miR-18a-5p/SOX6 signaling axis. Oncol. Rep. 45 (3), 1118–1132. PubMed PMID: 33432363. Pubmed Central PMCID: PMC7859983. Epub 2021/01/13. eng. doi:10.3892/or.2021.7931
Zhou, T., Wu, L., Ma, N., Tang, F., Zong, Z., and Chen, S. (2020). LncRNA PART1 regulates colorectal cancer via targeting miR-150-5p/miR-520h/CTNNB1 and activating Wnt/β-catenin pathway. Int. J. Biochem. Cell. Biol. 118, 105637. PubMed PMID: 31669140. Epub 2019/11/02. eng. doi:10.1016/j.biocel.2019.105637
Zhou, C., Wang, P., Tu, M., Huang, Y., Xiong, F., and Wu, Y. (2020). Long non-coding RNA PART1 promotes proliferation, migration and invasion of hepatocellular carcinoma cells via miR-149-5p/MAP2K1 Axis. Cancer Manag. Res. 12, 3771–3782. PubMed PMID: 32547213. Pubmed Central PMCID: PMC7248804. Epub 2020/06/18. eng. doi:10.2147/CMAR.S246311
Zhou, J., Che, J., Xu, L., Yang, W., Zhou, W., and Zhou, C. (2022). Tumor-derived extracellular vesicles containing long noncoding RNA PART1 exert oncogenic effect in hepatocellular carcinoma by polarizing macrophages into M2. Dig. Liver Dis. 54 (4), 543–553. PubMed PMID: 34497040. Epub 2021/09/10. eng. doi:10.1016/j.dld.2021.07.005
Zhu, D., Yu, Y., Wang, W., Wu, K., Liu, D., Yang, Y., et al. (2019). Long noncoding RNA PART1 promotes progression of non-small cell lung cancer cells via JAK-STAT signaling pathway. Cancer Med. 8 (13), 6064–6081. PubMed PMID: 31436388. Pubmed Central PMCID: PMC6792487. Epub 2019/08/23. eng. doi:10.1002/cam4.2494
Zhu, Y. J., and Jiang, D. M. (2019). LncRNA PART1 modulates chondrocyte proliferation, apoptosis, and extracellular matrix degradation in osteoarthritis via regulating miR-373-3p/SOX4 axis. Eur. Rev. Med. Pharmacol. Sci. 23 (19), 8175–8185. PubMed PMID: 31646607. Epub 2019/10/28. eng. doi:10.26355/eurrev_201910_19124
Keywords: lncRNA, PART1, cancer, biomarker, diagnsotic marker
Citation: Ghafouri-Fard S, Harsij A, Hussen BM, Abdullah SR, Baniahmad A, Taheri M and Sharifi G (2023) A review on the role of long non-coding RNA prostate androgen-regulated transcript 1 (PART1) in the etiology of different disorders. Front. Cell Dev. Biol. 11:1124615. doi: 10.3389/fcell.2023.1124615
Received: 15 December 2022; Accepted: 06 February 2023;
Published: 15 February 2023.
Edited by:
Wenjie Shi, Otto von Guericke University Magdeburg, GermanyReviewed by:
Reyhane Eghtedarian, University of Helsinki, FinlandCopyright © 2023 Ghafouri-Fard, Harsij, Hussen, Abdullah, Baniahmad, Taheri and Sharifi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, TW9oYW1tYWQudGFoZXJpQHVuaS1qZW5hLmRl; Guive Sharifi, Z2libm93QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.