
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cell Dev. Biol. , 16 February 2023
Sec. Cancer Cell Biology
Volume 11 - 2023 | https://doi.org/10.3389/fcell.2023.1057484
This article is part of the Research Topic Signaling by Extracellular Vesicles in Immune Response, Tissue Regeneration and Cancer: New Insights and Common Trends View all 4 articles
 Polina V. Shnaider1,2,3
Polina V. Shnaider1,2,3 Irina Yu. Petrushanko4
Irina Yu. Petrushanko4 Olga I. Aleshikova5
Olga I. Aleshikova5 Nataliya A. Babaeva5
Nataliya A. Babaeva5 Lev A. Ashrafyan5
Lev A. Ashrafyan5 Ekaterina I. Borovkova6
Ekaterina I. Borovkova6 Julia E. Dobrokhotova6
Julia E. Dobrokhotova6 Ivan M. Borovkov7
Ivan M. Borovkov7 Victoria O. Shender2,8
Victoria O. Shender2,8 Elena Khomyakova9*
Elena Khomyakova9*Ovarian cancer is known to be the most lethal malignancy among all gynecological cancers affecting a large number of women worldwide. The treatment of ovarian cancer is challenging due to the high recurrence rate of the disease and is further complicated by acquired chemoresistance. Most ovarian cancer deaths are the result of the metastatic spread of drug-resistant cells. The theory of cancer stem cells (CSC) suggests that both tumor initiation and progression are driven by a population of undifferentiated capable of self-renewal, tumor initiation and development of chemoresistance. The CD117 mast/stem cell growth factor receptor (KIT) is the most commonly used marker for ovarian CSCs. Here, we analyze the correlation between CD117 expression and histological tumor type in ovarian cancer cell lines (SK-OV-3 and MES-OV) and in small/medium extracellular vesicles (EVs) isolated from the urine of ovarian cancer patients. We have demonstrated that the abundance of CD117 on cells and EVs is correlated with tumor grade and therapy resistance status. Moreover, using small EVs isolated from ovarian cancer ascites, it was shown that recurrent disease is characterized by a much higher abundance of CD117 on EVs than primary tumor.
Ovarian cancer (OC) is the most lethal gynecologic malignancy. Even after surgical cytoreduction in combination with platinum-based chemotherapy, the 5-year survival rate for ovarian cancer patients remains 30%–40% and about 80% of patients will have a recurrence (van Zyl et al., 2018; Berek et al., 2021). The main reason for the low survival rate of patients with ovarian cancer is the late cancer diagnosis as well as acquisition of therapy resistance by relapsed tumors (Lheureux et al., 2019). It has been recently shown that chemotherapy can provoke the spread of therapy-resistant clones or activation of compensatory signaling pathways, which allows the tumor cells to cope with damage and initiate tumor repopulation (Izar et al., 2020; Shnaider et al., 2020; Kan et al., 2022; Zhang et al., 2022). Thus, there is a need to develop new methods for prediction of tumor aggressiveness and reliable early detection of cancer progression along with new chemotherapy regimens. The Cancer Stem Cell hypothesis posits that tumor initiation and progression are driven by a rare subpopulation of non-differentiated cells. These cells carry mesenchymal markers, which can be used as biomarkers of tumor aggressiveness, prediction of treatment success and prognosis (Blassl et al., 2016; Harris et al., 2021).
It has been repeatedly shown that CD117 (a product of the c-KIT gene) is expressed in many aggressive cancers (including ovarian cancers) as well as in recurrent and resistant tumors and predicts poor survival of patients (Chau et al., 2013; Conic et al., 2015; Stemberger-Papić et al., 2015; Yang et al., 2017; Foster et al., 2018; Fang et al., 2020). Along with other tyrosine kinases, CD117 participates in important processes of cancer progression: proliferation, metabolism, cell growth, regulation of cell migration, differentiation and apoptosis (Foster et al., 2018).
Another common cancer marker is Epithelial Cell Adhesion Molecule (EpCAM). However, the role of EpCam in tumor initiation, progression, and therapy resistance in not well established. Despite the role of EpCam as mediator of epithelial cell-cell adhesion, some works mention it as a marker of tumor initiating cells (Gires et al., 2009). However, during chemotherapy, epithelial tumor cells often undergo an epithelial-mesenchymal transition. Such cells acquire a more mesenchymal phenotype, which leads to the loss of the EpCAM epithelial marker (Hyun et al., 2016). Thus, EpCam does not reflect the phenotype of cancer-initiating cells and further studies of the role of this glycoprotein in tumor initiation and progression, as well as the possibility of its application in diagnostics, is required.
The standard approach for tumor profiling is tissue biopsy where molecular markers are analyzed in the tumor punction (Mari et al., 2019). However, in the case of ovarian cancer, tissue biopsy has severe limitations since it requires serious surgical interventions that is not well tolerated by patients and cannot be performed several times during the course of the therapy. The liquid biopsy is the most suitable method for biomaterial sampling (Mari et al., 2019). Initially, only circulating tumor cells and circulating tumor DNA were studied in the context of liquid biopsies. Extracellular vesicles (EVs) have been attracting significant research interest because they constitute a promising tool for important medical applications including liquid biopsy tests (Poulet et al., 2019; Zhou et al., 2020; Yu et al., 2022). EVs are found in body fluids and in the cultured media of different cell lines. They are enriched with proteins, lipids and nucleic acids (Zhang and Yu, 2019; Yokoi and Ochiya, 2021) which reflect the composition of the cell of origin. EVs are involved in various cellular processes including intercellular communication and immune regulation (Zhang and Yu, 2019; Yokoi and Ochiya, 2021). EV-based liquid biopsies would allow non-invasive profiling of protein, RNA and DNA tumor markers, monitoring the modifications in tumor phenotype during therapy, prediction of tumor response to therapy and post-treatment follow-up of the patients.
One of the features of advanced stages of ovarian cancer is the formation of ascitic fluid in the abdominal cavity. Ascites contain proteins, lipids, nucleic acids and metabolites as well as extracellular vesicles secreted by both tumor cells and associated stromal and immune cells (Ahmed and Stenvers, 2013; Shender et al., 2014; Rickard et al., 2021). Another body fluid of great value for liquid biopsies is urine since it contains large quantities of extracellular vesicles that allow non-invasive profiling of urogenital tumors. Moreover, urine-based diagnostics allows the profiling of early-stage tumors prior to the formation of ascitic fluid.
In the present work, we performed an analysis of the expression of mesenchymal marker CD117 and cell adhesion glycoprotein EpCam in tumor cells and the abundance of these markers on the surface of tumor-derived extracellular vesicles. We analyzed two ovarian cancer cell lines MES-OV and SK-OV-3 and clinical samples from patients with ovarian cancer with different histological types and grades to study the correlation between CD117/EpCam abundance and aggressiveness of the tumors.
Human ovarian cancer cell line SK-OV-3 (ATCC® HTB-77™) and human cystadenocarcinoma cell line MES-OV (ATCC® CRL-3272™) were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Gibco) and Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12; Gibco), accordingly, supplemented with 10% fetal bovine serum (FBS; Gibco), 2 mM glutamine (GlutaMAX; Gibco), and penicillin/streptomycin (1%) (Gibco), at 37°C in a humidified atmosphere containing 5% CO2.
SK-OV-3 and MES-OV cells were collected and fixed with 4% PFA, blocked with 2% FBS solution and incubated overnight with primary antibodies: CD117-PE (BD Pharmigen), EpCam (Abcam), CD9-FITC (BD Pharmigen). Next, cells were washed three times with PBS and incubated with secondary antibodies if needed for 1 h (Goat anti-Rabbit IgG, Alexa 647 (Invitrogen) for EpCam). Stained samples were analyzed with NovoCyte Flow Cytometer (ACEA Biosciences). The data were analysed with Kaluza software (Beckman Coulter, United States).
At 90% confluence SK-OV-3 and MES-OV cells were washed 3 times with PBS and maintained in 30 mL FBS-free, phenol-red-free DMEM (Gibco). After 24 h cell culture supernatants (28 mL per 175 cm2 flasks) were harvested and centrifuged at 500 g for 5 min at 14°C (Eppendorf Centrifuge 5804 R, A-4-44 swinging-bucket rotor) to pellet dead cells followed by centrifugation at 4,000 g for 20 min at 4°C in the same rotor to eliminate debris and large vesicles. Small and medium extracellular vesicles were collected by ultrafiltration of supernatants with 100-kDa Amicon Ultra-15 Centrifugal Filter Units (Millipore) according to manufacturer protocol.
Measurements of particle size distribution (PSD) and concentration were made with Nanosight NS300 instrument (Malvern) based on Nanoparticle Tracking Analysis (NTA). Measurements were performed with 405 nm, 65 mW laser and high sensitivity sCMOS camera. Samples were diluted with particle-free PBS (pH = 7.4) to reach the optimal concentration for NTA according to ASTM E2834–12. All measurements were made under the same camera settings (Shutter: 1,206-1,300, Gain: 366-512, camera level: 16, time: 60 s) and processing conditions (NTA 3.3 build 3.3.203, Detection Threshold: 5). Measurements were done in several repeats (3–5) to collect at least 4,000 particles in total. To obtain the joint histogram of PSD for multiple measurements, single particle diameters and track lengths from each measurement were collected into a global table and binned with weights proportional to track lengths. A decrease of the total vesicle concentration during the storage (at 4°C) was taken into account.
Ascitic fluids from ovarian cancer patient were obtained from the Russian Scientific Center of Roentgen Radiology (Moscow, Russia), Ministry of Healthcare of the Russian Federation. The study was approved by the Ethics Committee of the Russian Scientific Center of Roentgen Radiology (agreement and protocol no. 30-2018/E from 13 November 2018), and written informed consent was obtained from all the patients who participated. The ascites of primary tumor (ovarian papillary cystadenocarcinoma) was collected before chemotherapy. The relapsed ascites were collected after six courses of neoadjuvant paclitaxel/carboplatin chemotherapy. Fresh ascitic fluids were centrifuged at 300 g for 15 min to pellet cells and cell debris (Eppendorf Centrifuge 5804 R, A-4-44 swinging-bucket rotor). The supernatants were collected and stored at −80°C until further processing.
For EVs isolation from ovarian cancer ascites samples, 500 µL of the ascitic fluids were centrifuged at 500 g for 15 min, then at 10,000 g for 30 min in an F-45–24-11 rotor (Eppendorf) at 4°C. Then, the upper-phase containing largest lipoproteins was removed, 12 mL of PBS buffer was added to supernatant followed by concentration with 100-kDa Amicon Ultra-15 Centrifugal Filter Units (Millipore) and washing with PBS buffer using the same filter unit.
Urine samples from ovarian cancer patients and individuals without cancer were obtained from Department of Obstetrics and Gynecology, Faculty of Medicine, Pirogov Russian National Research Medical University (Figure 4A). Written informed consent was obtained from all the patients who participated. The urine of cancer patients was collected before treatment. To isolate extracellular vesicles, urine was centrifuged at 4,000 g for 20 min at 4°C in the A-4-44 swinging-bucket rotor, concentrated with 100-kDa Amicon Ultra-15 Centrifugal Filter Units (Millipore) and washed twice with PBS buffer.
Profiling of CD81, CD117 and EpCam markers on EVs was performed with ExoSence FACS kit beta (Exosome Analytics, France). Pre-purified EVs (the EVs quantity per reaction is shown in Table 1) were incubated with antiCD9 coated magnetic beads for 18 h followed by incubation of EVs-bead complexes with CD81-PE, CD117-PE or EpCam-PE antibodies according to the manufacturer’s protocol and analysed either with BD LSRFortessa™ Cell Analyzer or CytoFLEX cytometer (Beckman Coulter). The data were analysed with Kaluza software (Beckman Coulter, United States).
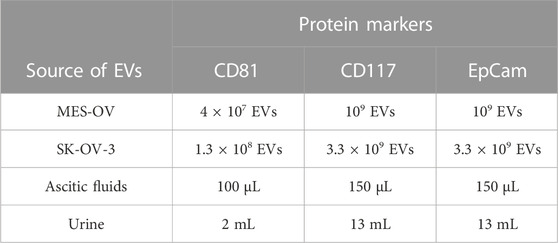
TABLE 1. The quantity of EVs (for cell lines) and volume of body fluids (for clinical samples) taken for each reaction.
Elevated levels of receptor tyrosine kinase CD117 expression have been shown to be associated with chemoresistance of ovarian cancer cells to platinum-based drugs (Tomao et al., 2013). We assessed the expression level of CD117 in two ovarian cancer cell lines: SK-OV-3 and MES-OV. Both cell lines were established from ascitic fluids of ovarian cancer patients (ATCC HTB-77™ and ATCC CRL-3272™). SK-OV-3 cells are a well-known ovarian cancer model with an aggressive phenotype. Despite the absence of available information concerning the tumor grade, SK-OV-3 cells demonstrate resistance to platinum-based drugs, have a high invasive potential and tumorigenicity among the other ovarian cancer cell lines (Hallas-Potts et al., 2019). MES-OV cells have been little studied. They represent moderately differentiated (grade 2) ovarian tumor but have traits of a mesenchymal phenotype. Figure 1A represents flow histograms for CD117, EpCam and CD9 staining of MES-OV and SK-OV-3 cells. As follows from the results, EpCam expression is observed in more than 90% of the cells. However, it is important to note that EpCam median specific fluorescent signal is 10 times lower for SK-OV-3 cells as compared to MES-OV cells (2 × 105 a. u. for MES-OV cells vs. 2 × 104 a. u. for SK-OV-3 cells; Figures 1A, B). An explication of this discrepancy could be the fact that the quantity of EpCam antigens is essentially lower in SK-OV-3 cells than in MES-OV cells. The opposite is observed for CD117 expression. As shown in Figure 1, about half (47%) of SK-OV-3 cells are CD117-positive, compared with only 12% of MES-OV cells. Thus, taking into consideration EpCam and CD117 expression pattern, one can conclude that SK-OV-3 cells have a more pronounced mesenchymal-like phenotype than MES-OV cells, which corresponds well to highly aggressive properties of SK-OV-3 cells.
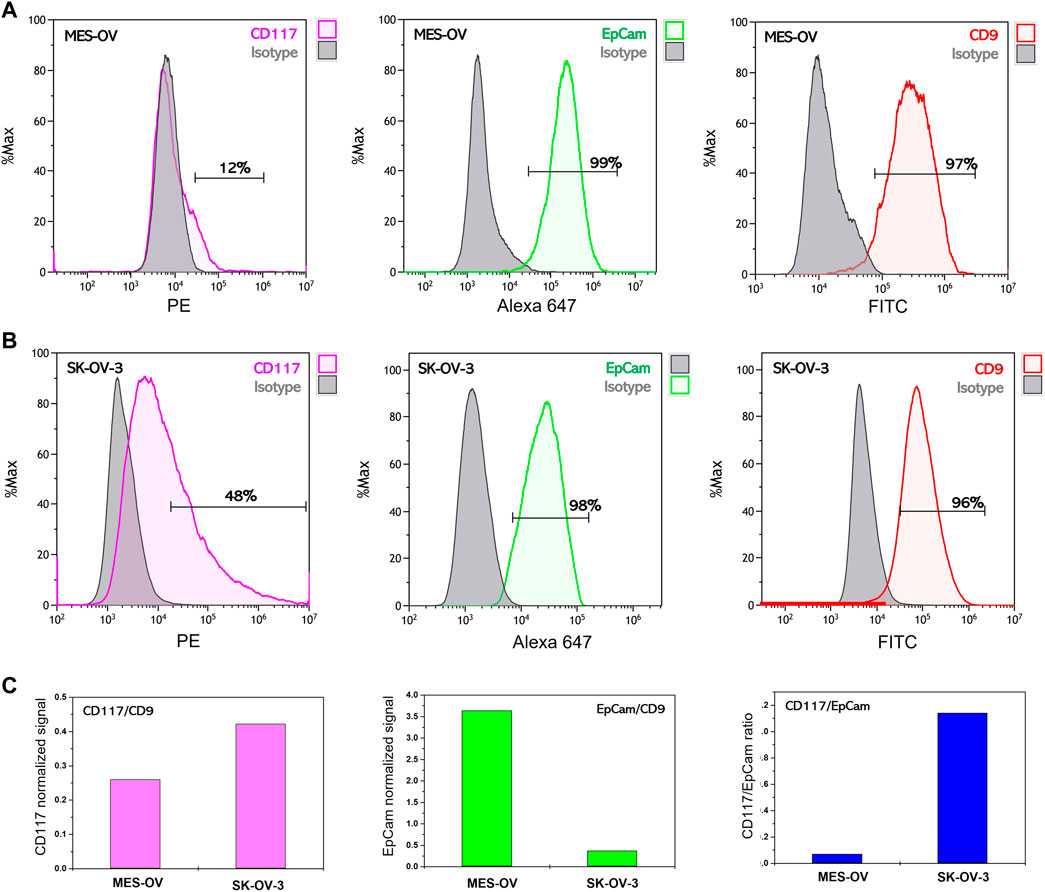
FIGURE 1. Flow cytometry analysis of membrane surface protein expression on MES-OV and SK-OV-3 cells. Flow histograms of CD117-PE, EpCam-Alexa 647 and CD9-PE staining of MES-OV (A) and SK-OV-3 cells (B). (C) Ratios of median fluorescent signals.
Expression of the CD9 antigen, which is a common EV marker, was also analyzed in MES-OV and SK-OV-3 cells. At least 97% of MES-OV cells and 96% of SK-OV-3 cells are CD9-positive, and the median value of antiCD9-PE fluorescence is rather close for both cell lines (Figure 1A). To move towards the application of EVs in liquid biopsy tests for the profiling of tumor markers, it is necessary to prove the correspondence between the abundance of marker proteins in EVs and the expression pattern of these markers in the donor cells.
EVs were isolated from cultured media of MES-OV and SK-OV-3 cells by differential centrifugation followed by ultrafiltration (as described in Material and Methods). Centrifugation conditions were chosen to obtain a mixed population of small and medium-sized EVs [cut-off size 200 nm according to (Livshits et al., 2015)]. Size distribution and concentration of the MES-OV and SK-OV-3 EVs were analyzed by nanoparticle tracking analysis (NTA) The EVs size distribution corresponds well to the theoretical prediction for both cell lines (Supplementary Figure S1). The measured EVs’ mean size is 101±3.5 nm and 126.6±4.4 nm for MES-OV and SK-OV-3 vesicles, respectively.
To prove the correspondence of the CD117 and EpCam abundance on EVs to the profiles of CD117 and EpCam expression in the cells of origin, we analyzed the expression of these markers on SK-OV-3- and MES-OV-derived EVs. To approach real liquid biopsy test conditions where vesicle concentration varies between the clinical samples and is a priori unknown, in our experiments the concentrations of SK-OV-3- and MES-OV-derived EVs were also different (Materials and Methods section, Table 1). The abundance of CD81, CD117, and EpCam antigens were analyzed by flow analysis of EVs immunoprecipitated to antiCD9 coated magnetic beads and stained with the antibody of interest. AntiCD9 beads were chosen for EV capture since the CD9 antigen is widely represented in many ovarian cancer tumors including MES-OV and SK-OV-3 cell lines (Hwang et al., 2012; Lorico et al., 2021; Figure 1). Figure 2A shows flow histograms of CD81-PE, CD117-PE and EpCam-PE stained MES-OV and SK-OV-3-derived extracellular vesicles.
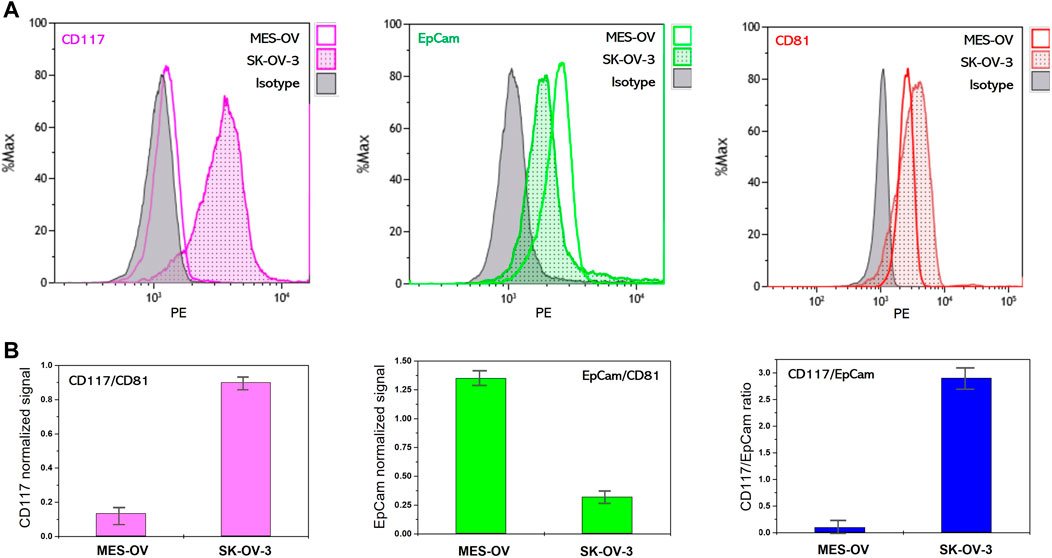
FIGURE 2. Flow cytometry analysis of membrane protein abundance on MES-OV and SK-OV-3-derived EVs. (A) Flow histograms of CD117-PE, EpCam-PE and CD81-PE staining of MES-OV and SK-OV-3-derived EVs immunoprecipitated on antiCD9 magnetic beads. (B) Ratios of median fluorescent signals. Error bars correspond to the standard deviation of median PE fluorescence calculated from the two measurements.
The difference in CD81 fluorescence values reflects the difference in input of MES-OV and SK-OV-3 vesicles. A CD117 signal is reliably detected on MES-OV extracellular vesicles, however the value of the signal is rather low. Contrary, a strong CD117 signal is observed for SK-OV-3-derived EVs. The opposite pattern was observed for EpCam expression. EpCam signal corresponding to MES-OV-derived vesicles is significantly higher than for SK-OV-3 EVs (Figure 2A).
However, since MES-OV and SK-OV-3 EVs input was different, direct comparison of the CD117 and EpCam signals between MES-OV- and SK-OV-3-derived vesicles is not possible and signals normalization is required. One of the possible approaches is normalization to EVs concentration measured by NTA. However, even though such an approach is common is laboratory practice, it is much less applicable in real liquid biopsy tests as NTA measurements strongly complicate the diagnostic procedure. In our study, we normalize CD117 and EpCam signal value obtained for MES-OV and SK-OV-3 vesicles to the value of the CD81 signal. Since tetraspanins CD9 and CD81 are believed to be common EVs markers, such a ratio reflects the portion of CD117-positive vesicles in total EVs population. As shown in Figure 2B, CD117 and EpCam normalized values inversely correlate in MES-OV- and SK-OV-3-derived EVs that fully corresponds to the profiles of original cells. This observation confirms correspondence of CD117 and EpCam expression profiles on cells and extracellular vesicles that allows the application of EVs in liquid biopsy tests for profiling of CD117 and EpCam markers on tumors.
We analyzed the evolution of the abundance of CD117 and EpCam markers on EVs in the course of the development of post-treatment relapsed ovarian tumor. In our pilot experiment, we performed comparative analysis of CD117, EpCam and CD81 abundance on EVs isolated from ascitic fluids of the patient with primary tumor (ovarian papillary cystadenocarcinoma) and of the same patient with post-treatment tumor relapse.
Figure 3A shows flow histograms corresponding to antiCD81-PE, antiCD117-PE and antiEpCam-PE staining of EVs immunocaptured with antiCD9 magnetic beads. As follows from the data, very high CD81 signal was observed for EVs isolated from just 100 µL of ascites of both primary and relapsed tumors. Slightly higher level of CD81 signal corresponding to EVs isolated from recurrent ascites reveals their higher concentration in the ascitic fluid of relapsed tumor. CD117 signal was undetectable on primary tumor EVs, however the CD117 signal is reliably detectable on relapsed tumor EVs. The opposite is observed for the expression of EpCam. EVs from primary tumor ascites showed a higher EpCam signal than EVs isolated from relapsed tumor ascites. Thus, as shown in the profiling data, the pattern of CD117 and EpCam abundance on primary tumor EVs corresponds to the profile of MES-OV cells and extracellular vesicles. On the other hand, recurrent tumor acquires an expression signature characterized by high CD117 and low EpCam expression as was observed for highly aggressive SK-OV-3 cells. These observations are in good agreement with the statement that relapsed ovarian tumors acquire mesenchymal properties and become more aggressive than primary tumors (Pastushenko et al., 2018).
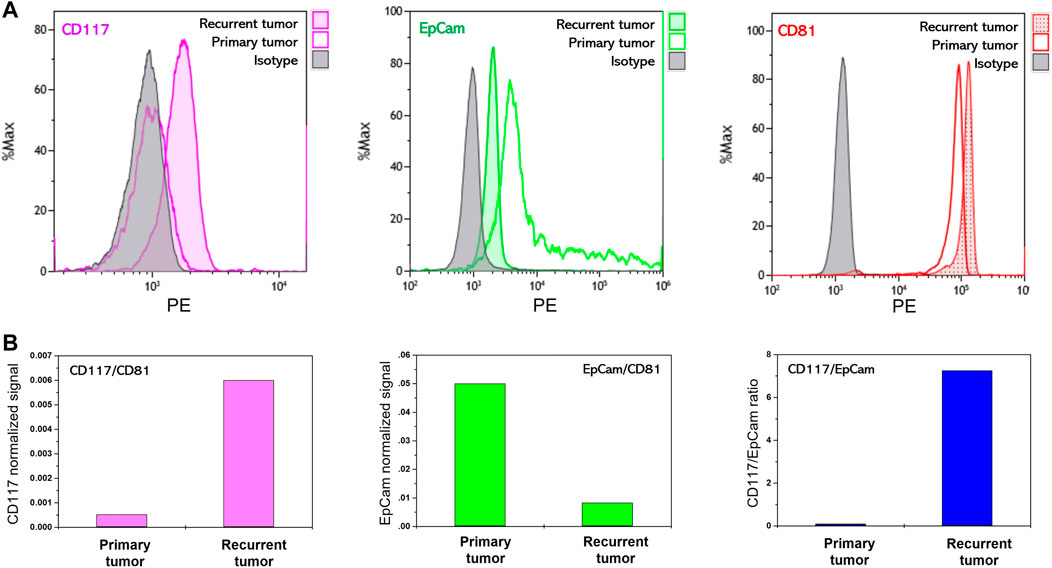
FIGURE 3. Flow cytometry analysis of membrane protein abundance on EVs isolated from ascites of ovarian cancer patient. (A) Flow histograms of CD117-PE, EpCam-PE and CD81-PE staining EVs immunoprecipitated on antiCD9 magnetic beads. (B) Ratios of median fluorescent signals.
CD117 abundance was analyzed on EVs isolated from the urine of patients with histologically characterized ovarian tumors and healthy donors (4 malignant and 2 benign tumors, Figure 4A). To estimate the proportion of CD117-positive EVs, normalization to the value of CD81 signal was applied (Figure 4B). As follows from the data, remarkable CD117 expression is detected only in two clinical samples. These samples correspond to patients with high grade serous ovarian carcinoma and papillary serous cystadenocarcinoma. No CD117 expression signal is observed either for healthy donors, or for patients with benign fibroma tumors and endometrioid adenocarconoma.
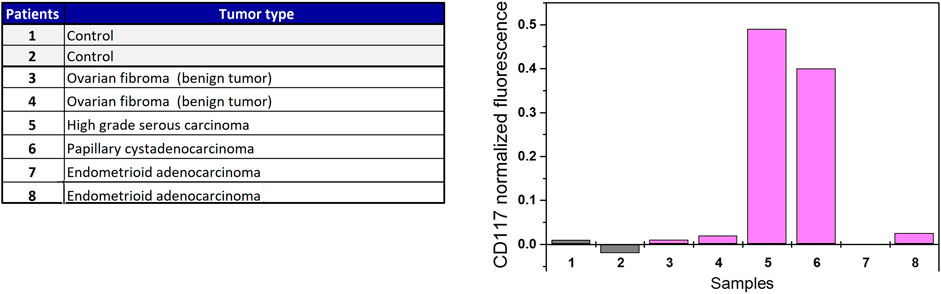
FIGURE 4. CD117 profiling of EVs isolated from urine of ovarian cancer patients and individuals without cancer.
The present work aimed at analyzing the abundance of mesenchymal marker CD117 and epithelial cell adhesion molecule (EpCam) on the surface of extracellular vesicles derived from ovarian tumors. Using ovarian cancer cell line models, we demonstrated the increased expression of CD117 in highly aggressive SK-OV-3 cells. On the contrary, the MES-OV cells, which corresponds to a middle-grade ovarian tumor, contains only a minor population of CD117-positive cells. These results are in good agreement with the statement that CD117 expression is associated with tumor aggressiveness. Interestingly, although all cell populations in MES-OV and SK-OV-3 cell lines stained positive for EpCam, the median value of the EpCam signal was significantly lower in SK-OV-3 cells, which can be explained by a lower quantity of EpCam antigens being present on the membrane of SK-OV-3 cells. The same effect was observed in the MDA-MB-231 cell line originated from an aggressive triple negative breast tumor (Bragina et al., 2022). Thus, at least in some cancers, the value of EpCam expression does not correlate with aggressiveness of the tumor and its role in cancer biology needs to be further studied.
To answer the question whether EVs could be used in liquid biopsy tests for non-invasive tumor profiling, it is necessary to prove that the expression profile of cancer markers on tumor cells matches the abundance of these markers on tumor-derived EVs. Using highly sensitive FACS kit, we succeeded in detecting both CD117 and EpCam antigens on the surface of SK-OV-3- and MES-OV-derived extracellular vesicles. Moreover, we demonstrated that the profiles of CD117 and EpCam expression in donor cells and their EVs are identical when the fluorescence signals were normalized to vesicle concentration.
We applied a similar approach for the profiling of EVs isolated from physiological fluids. We analyzed the abundance of CD117 on EVs isolated from the urine of patients with different histological types of ovarian tumors and healthy women. No CD117 signal was detected in control samples. Among the patients with benign and malignant tumors, the EVs from serous ovarian carcinoma and papillary serous cystadenocarcinoma gave a strongly positive CD117 signal. The abundance of CD117 on EVs isolated from urine of patients with highly invasive serous ovarian carcinoma is not surprising since these tumors are known to be highly invasive with a documented mesenchymal signature, particularly with high CD117 expression (Foster et al., 2018). On the other hand, the papillary serous cystadenocarcinoma is a less common and much less studied disease than the serous ovarian carcinoma. Particularly, to our knowledge, the CD117 expression is not characteristic of either papillary serous cystadenocarcinoma tumor samples or established papillary serous cystadenocarcinoma cell lines. Generally, papillary serous cystadenocarcinoma is considered a low-grade tumor, however, some works demonstrate its increased invasive potential and resistance to treatment [early post-treatment tumor relapse is described in (Kaku et al., 2004)]. Thus, the screening of papillary serous cystadenocarcinoma for CD117 expression may contribute to a better understanding of the behavior of this type of tumor.
We also analyzed the EVs isolated from ascitic fluids of the same patient with a primary tumor (ovarian papillary cystadenocarcinoma, before treatment) and then after early recurrence (after six courses of chemotherapy). On the EVs from the primary tumor, no CD117 signal was detected. However, the EVs from the recurrent tumor carry essential quantity of CD117 antigen to be reliably detected. The described clinical case supports the hypothesis that a very small population of tumor-initiating cells, including CD117-positive cells, can survive during the treatment and give rise to an aggressive recurrent tumor therapy resistant phenotype (which led to the death of the patient). Thus, a novel treatment approach targeting cancer-initiating cells and particularly CD117-positive tumor cells could improve outcomes. Moreover, the screening of EVs for CD117 abundance could be potentially used for diagnostics of tumor relapse during post-treatment follow-up of the patients.
In this study we performed normalization of CD117 and EpCam signals to the value of the CD81 signal. However, the most effective approach in diagnostics is when the “positive” marker is normalized to the “negative” marker (Erdbrügger et al., 2021). That means if a diagnostic test is aimed at distinguishing between mesenchymal and epithelial tumors, the ratio between mesenchymal and epithelial markers must be considered. If we consider EpCam as an exclusively epithelial marker and calculate the signal ratio CD117/EpCam, the difference between SK-OV-3 and MES-OV cell lines, as well as between primary and relapsed ovarian tumors, is more pronounced. However, extended studies of the role of EpCam in cancer progression is required before its expression could be attributed to aggressive or non-aggressive tumors.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The present study was approved by Ethics Committees of the Russian Scientific Center of Roentgen Radiology and Pirogov Russian National Research Medical University. Clinical samples were collected from patients after written informed consent was obtained. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: EK, VS, and PS; Methodology: EK, PS, VS, and IP; Investigation: EK, VS, and PS; Biological samples: OA, NB, LA, EB, JD, and IB; Resources: EK, VS, and PS; Writing Original Draft: EK, VS, and PS; Writing—Reviewing & Editing: EK, VS, and PS; Visualization: EK and VS; Supervision: EK and VS; Funding Acquisition: PS; Software: EK and PS. All authors contributed to the article and approved the submitted version.
This work was supported by grant 075-15-2019-1669 from the Ministry of Science and Higher Education of the Russian Federation (PS).
We thank Mr. Florian Boyer Chammard for critical reading and editing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2023.1057484/full#supplementary-material
Ahmed, N., and Stenvers, K. L. (2013). Getting to know ovarian cancer ascites: Opportunities for targeted therapy-based translational research. Front. Oncol. 3, 256. doi:10.3389/fonc.2013.00256
Berek, J. S., Renz, M., Kehoe, S., Kumar, L., and Friedlander, M. (2021). Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynaecol. Obstet. 155 (1), 61–85. doi:10.1002/ijgo.13878
Blassl, C., Kuhlmann, J. D., Webers, A., Wimberger, P., Fehm, T., and Neubauer, H. (2016). Gene expression profiling of single circulating tumor cells in ovarian cancer - establishment of a multi-marker gene panel. Mol. Oncol. 10, 1030–1042. doi:10.1016/j.molonc.2016.04.002
Bragina, V. A., Khomyakova, E., Orlov, A. V., Znoyko, S. L., Mochalova, E. N., Paniushkina, L., et al. (2022). Highly sensitive nanomagnetic quantification of extracellular vesicles by immunochromatographic strips: A tool for liquid biopsy. Nanomater. (Basel) 12, 1579. doi:10.3390/nano12091579
Chau, W. K., Ip, C. K., Mak, A. S. C., Lai, H.-C., and Wong, A. S. T. (2013). c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene 32, 2767–2781. doi:10.1038/onc.2012.290
Conic, I., Stanojevic, Z., Jankovic Velickovic, L., Stojnev, S., Ristic Petrovic, A., Krstic, M., et al. (2015). Epithelial ovarian cancer with CD117 phenotype is highly aggressive and resistant to chemotherapy. J. Obstet. Gynaecol. Res. 41, 1630–1637. doi:10.1111/jog.12758
Erdbrügger, U., Blijdorp, C. J., Bijnsdorp, I. V., Borràs, F. E., Burger, D., Bussolati, B., et al. (2021). Urinary extracellular vesicles: A position paper by the urine task force of the international society for extracellular vesicles. J. Extracell. Vesicles 10, e12093. doi:10.1002/jev2.12093
Fang, C.-H., Lin, Y.-T., Liang, C.-M., and Liang, S.-M. (2020). A novel c-Kit/phospho-prohibitin axis enhances ovarian cancer stemness and chemoresistance via Notch3-PBX1 and β-catenin-ABCG2 signaling. J. Biomed. Sci. 27, 42. doi:10.1186/s12929-020-00638-x
Foster, B. M., Zaidi, D., Young, T. R., Mobley, M. E., and Kerr, B. A. (2018). CD117/c-kit in cancer stem cell-mediated progression and therapeutic resistance. Biomedicines 6, 31. doi:10.3390/biomedicines6010031
Gires, O., Klein, C. A., and Baeuerle, P. A. (2009). On the abundance of EpCAM on cancer stem cells. Nat. Rev. Cancer 9, 143; author reply 143. doi:10.1038/nrc2499-c1
Hallas-Potts, A., Dawson, J. C., and Herrington, C. S. (2019). Ovarian cancer cell lines derived from non-serous carcinomas migrate and invade more aggressively than those derived from high-grade serous carcinomas. Sci. Rep. 9, 5515. doi:10.1038/s41598-019-41941-4
Harris, K. S., Shi, L., Foster, B. M., Mobley, M. E., Elliott, P. L., Song, C. J., et al. (2021). CD117/c-kit defines a prostate CSC-like subpopulation driving progression and TKI resistance. Sci. Rep. 11, 1465. doi:10.1038/s41598-021-81126-6
Hwang, J. R., Jo, K., Lee, Y., Sung, B.-J., Park, Y. W., and Lee, J.-H. (2012). Upregulation of CD9 in ovarian cancer is related to the induction of TNF-α gene expression and constitutive NF-κB activation. Carcinogenesis 33, 77–83. doi:10.1093/carcin/bgr257
Hyun, K.-A., Koo, G.-B., Han, H., Sohn, J., Choi, W., Kim, S.-I., et al. (2016). Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 7, 24677–24687. doi:10.18632/oncotarget.8250
Izar, B., Tirosh, I., Stover, E. H., Wakiro, I., Cuoco, M. S., Alter, I., et al. (2020). A single-cell landscape of high-grade serous ovarian cancer. Nat. Med. 26, 1271–1279. doi:10.1038/s41591-020-0926-0
Kaku, M., Ohara, N., Seima, Y., Imanishi, K., Tomura, N., Kobayashi, A., et al. (2004). A primary retroperitoneal serous cystadenocarcinoma with clinically aggressive behavior. Arch. Gynecol. Obstet. 270, 302–306. doi:10.1007/s00404-003-0550-5
Kan, T., Zhang, S., Zhou, S., Zhang, Y., Zhao, Y., Gao, Y., et al. (2022). Single-cell RNA-seq recognized the initiator of epithelial ovarian cancer recurrence. Oncogene 41, 895–906. doi:10.1038/s41388-021-02139-z
Lheureux, S., Braunstein, M., and Oza, A. M. (2019). Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 69, 280–304. doi:10.3322/caac.21559
Livshits, M. A., Khomyakova, E., Evtushenko, E. G., Lazarev, V. N., Kulemin, N. A., Semina, S. E., et al. (2015). Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 5, 17319. doi:10.1038/srep17319
Lorico, A., Lorico-Rappa, M., Karbanová, J., Corbeil, D., and Pizzorno, G. (2021). CD9, a tetraspanin target for cancer therapy? Exp. Biol. Med. 246, 1121–1138. doi:10.1177/1535370220981855
Mari, R., Mamessier, E., Lambaudie, E., Provansal, M., Birnbaum, D., Bertucci, F., et al. (2019). Liquid biopsies for ovarian carcinoma: How blood tests may improve the clinical management of a deadly disease. Cancers 11, 774. doi:10.3390/cancers11060774
Pastushenko, I., Brisebarre, A., Sifrim, A., Fioramonti, M., Revenco, T., Boumahdi, S., et al. (2018). Identification of the tumour transition states occurring during EMT. Nature 556, 463–468. doi:10.1038/s41586-018-0040-3
Poulet, G., Massias, J., and Taly, V. (2019). Liquid biopsy: General concepts. Acta Cytol. 63, 449–455. doi:10.1159/000499337
Rickard, B. P., Conrad, C., Sorrin, A. J., Ruhi, M. K., Reader, J. C., Huang, S. A., et al. (2021). Malignant ascites in ovarian cancer: Cellular, acellular, and biophysical determinants of molecular characteristics and therapy response. Cancers 13, 4318. doi:10.3390/cancers13174318
Shender, V. O., Pavlyukov, M. S., Ziganshin, R. H., Arapidi, G. P., Kovalchuk, S. I., Anikanov, N. A., et al. (2014). Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol. Cell. Proteomics 13, 3558–3571. doi:10.1074/mcp.M114.041194
Shnaider, P. V., Ivanova, O. M., Malyants, I. K., Anufrieva, K. S., Semenov, I. A., Pavlyukov, M. S., et al. (2020). New insights into therapy-induced progression of cancer. Int. J. Mol. Sci. 21, 7872. doi:10.3390/ijms21217872
Stemberger-Papić, S., Vrdoljak-Mozetic, D., Ostojić, D. V., Rubesa-Mihaljević, R., Krigtofić, I., Brncić-Fisher, A., et al. (2015). Expression of CD133 and CD117 in 64 serous ovarian cancer cases. Coll. Antropol. 39, 745–753.
Tomao, F., Papa, A., Rossi, L., Strudel, M., Vici, P., Lo Russo, G., et al. (2013). Emerging role of cancer stem cells in the biology and treatment of ovarian cancer: Basic knowledge and therapeutic possibilities for an innovative approach. J. Exp. Clin. Cancer Res. 32, 48. doi:10.1186/1756-9966-32-48
van Zyl, B., Tang, D., and Bowden, N. A. (2018). Biomarkers of platinum resistance in ovarian cancer: What can we use to improve treatment. Endocr. Relat. Cancer 25, R303–R318. doi:10.1530/ERC-17-0336
Yang, B., Yan, X., Liu, L., Jiang, C., and Hou, S. (2017). Overexpression of the cancer stem cell marker CD117 predicts poor prognosis in epithelial ovarian cancer patients: Evidence from meta-analysis. Onco. Targets. Ther. 10, 2951–2961. doi:10.2147/OTT.S136549
Yokoi, A., and Ochiya, T. (2021). Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 74, 79–91. doi:10.1016/j.semcancer.2021.03.032
Yu, D., Li, Y., Wang, M., Gu, J., Xu, W., Cai, H., et al. (2022). Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 21, 56. doi:10.1186/s12943-022-01509-9
Zhang, K., Erkan, E. P., Jamalzadeh, S., Dai, J., Andersson, N., Kaipio, K., et al. (2022). Longitudinal single-cell RNA-seq analysis reveals stress-promoted chemoresistance in metastatic ovarian cancer. Sci. Adv. 8, eabm1831. doi:10.1126/sciadv.abm1831
Zhang, L., and Yu, D. (2019). Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 1871, 455–468. doi:10.1016/j.bbcan.2019.04.004
Keywords: extracellular vesicles, EVS, ovarian cancer, chemoresistance, prognositic biomarkers, liquid biopsy, CD117, EpCAM
Citation: Shnaider PV, Petrushanko IY, Aleshikova OI, Babaeva NA, Ashrafyan LA, Borovkova EI, Dobrokhotova JE, Borovkov IM, Shender VO and Khomyakova E (2023) Expression level of CD117 (KIT) on ovarian cancer extracellular vesicles correlates with tumor aggressiveness. Front. Cell Dev. Biol. 11:1057484. doi: 10.3389/fcell.2023.1057484
Received: 29 September 2022; Accepted: 19 January 2023;
Published: 16 February 2023.
Edited by:
George Vladimirovich Sharonov, Pirogov Russian National Research Medical University, RussiaReviewed by:
Alessandro Sarcinella, University of Turin, ItalyCopyright © 2023 Shnaider, Petrushanko, Aleshikova, Babaeva, Ashrafyan, Borovkova, Dobrokhotova, Borovkov, Shender and Khomyakova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Khomyakova, ZWxlbmEua2hvbXlha292YUBleG9zb21lLWFuYWx5dGljcy5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.