- Department of Pathology, General Hospital of Northern Theater Command, Shenyang, China
Osteoporosis is characterized by a high incidence rate, with significant effects on people’s lives. The underlying mechanisms are complex, with no treatments for the condition. Recent studies have indicated that melatonin can be used to treat osteoporosis by promoting osteoblast proliferation and differentiation, and inhibiting osteoclast differentiation. Specifically, in vivo mechanisms are initiated by stabilizing biological rhythms in bone tissue. In healthy organisms, these biological rhythms are present in bone tissue, and are characterized by bone formation during the day, and bone resorption at night. When this rhythm is disrupted, osteoporosis occurs. Thus, taking appropriate medication at different times of the day could produce different effects on osteoporosis rhythms. In this review, we characterized these processes, and provided treatments and management strategies for individuals with osteoporosis.
1 Osteoporosis
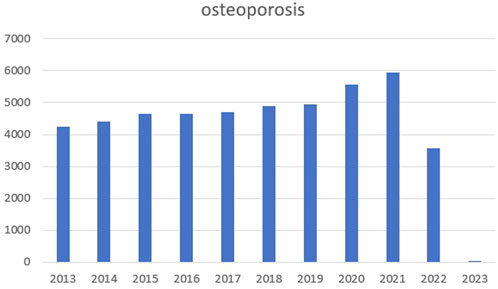
Osteoporosis is a complex pathogenic metabolic disease (Loffler et al., 2020; Zhang et al., 2022) caused by imbalanced bone formation and resorption (Johnston & Slemenda, 1995; Gu et al., 2020). By 2050, the number of hip fracture patients in Asia will more than double, accounting for 50% of the total hip fracture in the world (Wu et al., 2021). We searched PubMed for relevant articles in the past 10 years with osteoporosis as the keyword and made statistics. We found that the number of articles in the past two or 3 years has increased significantly, as shown in the figure below (Figure 1). The Wnt signaling pathway is believed to play an important role in bone homeostasis and bone related diseases, hence new osteoporosis therapies targeting Wnt signaling are emerging (Manolagas, 2014; Zhang et al., 2022). Similarly, the protein kinase B (Akt) signaling pathway is closely associated with osteoporosis treatments (Liu et al., 2018; Jiang et al., 2022). In ovariectomized rats, the Mongolian medicine, echinops has been shown to be effective for osteoporosis by Akt (Liu et al., 2018). In an osteoporosis rat model, mitogen activated protein kinase (MAPK) is involved in osteoblast proliferation and differentiation (Pan et al., 2018). While in RAW264.7 cells, bisphosphonate zoledronate inhibits osteoclast migration, differentiation and bone resorption via the AMP-activated protein kinase (AMPK) pathway, suggesting this route could become a new target for disease treatment (Dong et al., 2018). Although Wnt/Akt/MAPK/AMPK pathways exert important functions in bone homeostasis and bone related diseases, and new therapies are constantly emerging, the current therapeutic environment for osteoporosis is limited, therefore new side-effect free drugs and therapeutic targets are required (Gera et al., 2022; Kobayakawa et al., 2022).
2 Melatonin and diabetic osteoporosis
Melatonin is a hormone secreted by the pineal gland, and its chemical essence is a methoxyindole (Amaral & Cipolla-Neto, 2018). Melatonin secretion generates a typical circadian rhythm, which helps regulating the normal circadian rhythms of the body (Amaral & Cipolla-Neto, 2018). Recent studies have shown the hormone acts on bone tissue to exert its biological effects. Low melatonin concentrations have been used to treat osteoporosis by promoting bone growth (Xiao et al., 2020), while high concentrations treat scoliosis by inhibiting this growth (Qiu et al., 2020). Melatonin improved bone trabecular microstructure and reduced autophagy levels (50 mg/kg was more efficacious than 100 mg/kg) in 4-month-old male SPF SD rats (Zhang et al., 2016). In osteoblasts cultured in high glucose, melatonin promoted osteogenic activities, and appeared to inhibit autophagy levels by inhibiting the extracellular-regulated protein kinase (ERK) signaling pathway (10 μM was better than 1 mM), and delayed diabetic osteoporosis (Zhang et al., 2016). In 6-week-old adult female SD rats, when compared with the diabetic alone group, body weight, serum superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and Ca2+ levels, lumbar spine and left and right femur BMD of the diabetic + melatonin group were significantly increased), while blood glucose, serum malondialdehyde (MDA), and parathyroid hormone (PTH) levels were significantly decreased and no significant differences in serum phosphorus levels (Jing & Wang, 2017). Aerobic exercise and melatonin improve diabetic osteoporosis; both are more effective than exercise alone (Jing & Wang, 2017). Thus, melatonin may effectively reduce blood Ca2+ and PTH levels, augment BMD by improving antioxidant stress, reducing the effects of high glucose oxidative stress on bone metabolism, and regulating glucose metabolism (Jing & Wang, 2017).
3 Melatonin and osteoclast differentiation
3.1 Signaling pathways
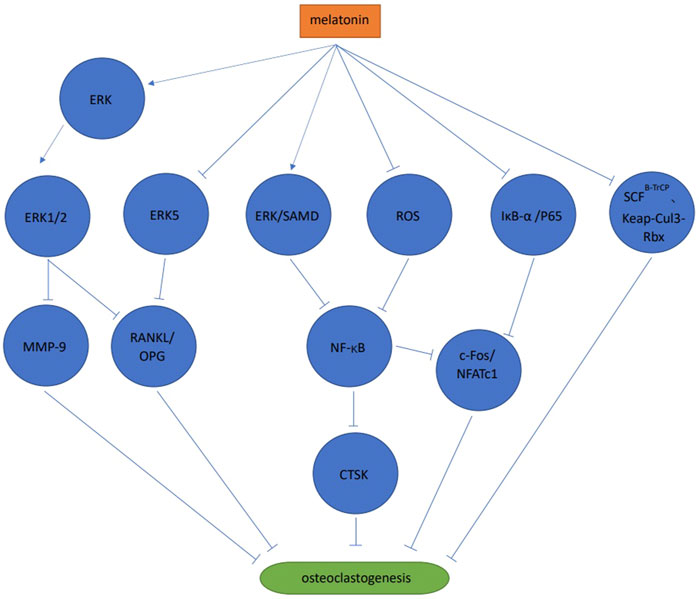
Melatonin activated the ERK1/2 signaling pathway by phosphorylating ERK in RAW264.7 cells, thereby inhibiting osteoclast differentiation via the melatonin receptor (Satue et al., 2015). When compared with the control group, melatonin significantly inhibited bone resorption, but no significant differences were observed in tartrate-resistant acid phosphatase (TRAP) positive cell numbers (Satue et al., 2015). Melatonin also significantly reduced matrix metallopeptidase nine expression, but no significant effects on cathepsin K expression were observed (Satue et al., 2015). Melatonin inhibited osteoclast differentiation and cathepsin K expression via ERK and NF-κB pathways in RAW264.7 cells (Wang et al., 2019). Also in RAW264.7 cells, melatonin significantly inhibited NFATc1 and c-Fos expression by blocking the phosphorylation of IjB-A and p65 rather than IKKa, to inhibit receptor activator of nuclear factor-kappa B ligand (RANKL) induced osteoclast differentiation, F-actin loop formation and bone resorption, in a melatonin concentration dependent manner. The study also reported that melatonin exerted no effects on mitogen-activated protein kinases (MAPK) and phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling pathways (Ping et al., 2017b). When co-culturing human mesenchymal stem cell (MSC) and peripheral blood mononuclear cell (PBMCs), MSDK (melatonin, strontium (citrate), vitamin D3 and vitamin K2) inhibited osteoclast differentiation by increasing osteoprotegerin (OPG) and reducing RANKL levels (Maria et al., 2017). These authors observed that melatonin increased OPG/RANKL levels via MEK1/2 and MEK5 signaling pathways to inhibit osteoclast differentiation. This process was mediated by the melatonin receptor type 1 B (MT2) (Maria et al., 2018). During osteoclast differentiation of mouse bone marrow mononuclear cells (BMMS) induced by RANKL, melatonin significantly inhibited osteoclast differentiation at pharmacological concentrations (10−4 mol/L, 10−5 mol/L, 10−6 mol/L) but not physiological concentrations (10−8 mol/L, 10−9 mol/L, 10−10 mol/L, 10−11 mol/L). The results showed that the process was melatonin concentration dependent. Melatonin inhibited osteoclast differentiation and bone resorption by inhibiting the NF-κB signaling pathway, mediated by reactive oxygen species but not SIRT1 (Zhou et al., 2017). In mouse BMMSs melatonin inhibited osteoclast differentiation by down-regulating the NF-κB pathway, thereby reducing NFATc1 expression. However, these anti-osteoclast differentiation effects were not associated with the melatonin receptor type 1A (MT1) and MT2, and no effects were observed towards the MAPKs (ERK, JNK, and p38) (Kim et al., 2017). Melatonin inhibited osteoclast differentiation via ubiquitination, which is a process mediated by ubiquitin ligase SCFB−TrCP and Kelch-like ECH-associated protein 1-cullin 3-RING-box 1 (Keap-Cul3-Rbx), or the proteasome (Vriend & Reiter, 2016). Melatonin effectively inhibited osteoclast differentiation and bone resorption in mouse bone marrow cells (including osteoclast and osteoblast precursors) at pharmacological concentrations (1 × 10−4 mol/L–5 × 10−4 mol/L). This process may be mediated by other cells (e.g., bone marrow stem cells) rather than melatonin acting directly on osteoclasts, suggesting interactions between different osteocytes could regulate the bone resorption/formation balance (Koyama et al., 2002) (Figure 2).
3.2 Animal studies
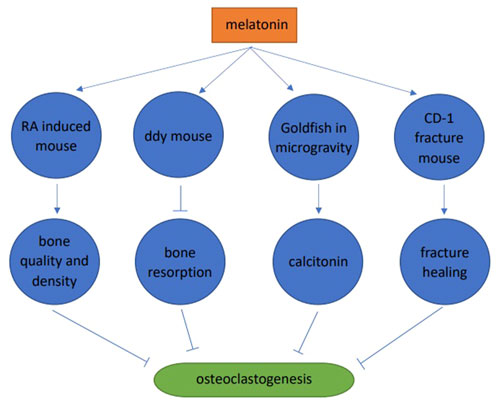
Melatonin significantly improved the microstructure of bone trabeculae, increased bone trabeculae in the retinoic acid-induced osteoporosis mouse model, improved femur and vertebrae microstructures, and improved bone quality and density (Wang et al., 2019). Melatonin (pharmacological dose) inhibited bone resorption and increased bone mass in 4-week-old male ddY mice. Melatonin significantly increased BMD (36%), bone mass (49%), and trabecular thickness (19%). These skeletal improvements were believed to be due to melatonin inhibiting RANKL expression and secretion in osteoblasts, and further inhibiting osteoclast differentiation (Koyama et al., 2002). Melatonin inhibited osteoclast differentiation in goldfish scales by promoting calcitonin secretion in microgravity environments in space (Ikegame et al., 2019). In a CD-1 fracture mouse model, melatonin promoted fracture healing by inhibiting RANKL induced osteoclast differentiation, thereby reducing bone resorption (Histing et al., 2012) (Figure 3).
3.3 Peri-prosthetic osteolysis
From radiological and histomorphological studies, melatonin inhibited bone resorption and promoted bone formation in a pericranial osteolysis mouse model, induced by titanium particles. When compared with untreated mice, numbers of osteoclasts in mice treated with low and high melatonin concentrations decreased significantly, with melatonin appearing to regulate RANKL/OPG levels, mediated by the Wnt/β-catenin signaling pathway (Ping et al., 2017a).
3.4 Human studies
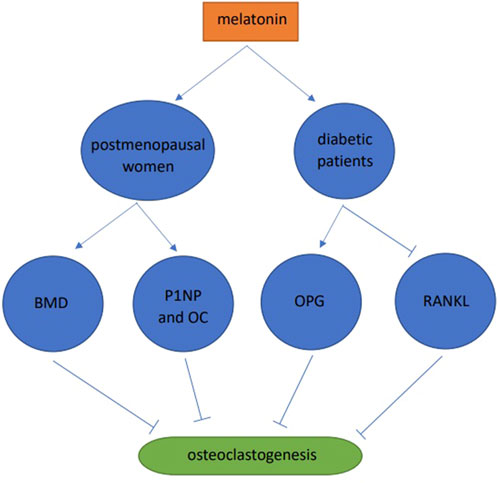
A one-year double-blind clinical trial in postmenopausal women was conducted and when compared with the placebo group, the BMD of patients in the MSDK group increased by 4.3% and 2.2% in the lumbar spine and left hip, respectively. In addition, melatonin increased serum levels of the bone formation markers, one procollagen n-propyl peptide (P1NP) and osteocalcin (OC). At the same time, there are significant improvements of mood and sleep quality in MSDK group (Maria et al., 2017). 30 healthy and 30 diabetic subjects were studied, and when compared with the healthy group, RANKL levels in diabetic saliva were increased, OPG was decreased, and blood melatonin was decreased. After taking melatonin, the periodontal markers, gingival index and pocket depth were significantly increased, RANKL in saliva was significantly decreased, and OPG increased (Cutando et al., 2014). Combined, these data showed that melatonin slowed down osteoclast formation by improving alveolar bone quality and preventing periodontal disease (Figure 4).
4 Circadian rhythm genes
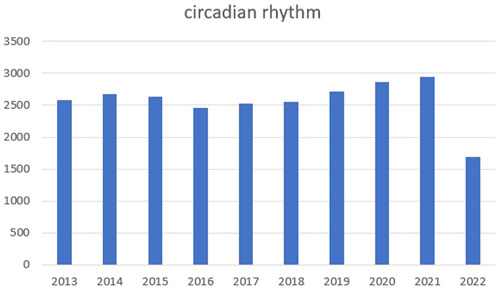
Circadian rhythm reflects alternations in daily biological behaviors and physiological activities, such as sleeping, waking, eating, and fasting (Welz et al., 2019; Stothard et al., 2020). We searched PubMed for relevant articles in the past 10 years with circadian rhythm as the keyword and made statistics. We found that the number of articles in the past two or 3 years has increased significantly, as shown in the figure below (Figure 5). In vivo, circadian rhythms are regulated and maintained by endogenous clock genes (Oishi et al., 2005; Liu et al., 2022), which are involved in several physiological activities, including metabolism (Peek et al., 2017), cell cycle (Han et al., 2016; Dong et al., 2019), sleep wake cycle (Ikegami et al., 2019), bone formation (Fu et al., 2005; Sato et al., 2007; Hirata et al., 2022; Zhao et al., 2022), and heart rate and blood pressure (Wang et al., 2008). However, when clock gene expression is aberrant, the body experience abnormalities and possible disease, including sleep disorders (Patke et al., 2017), diabetes (Marcheva et al., 2010), obesity (Paschos et al., 2012; Chaix et al., 2019), osteoporosis (Tang et al., 2020), and tumors (Altman et al., 2015). Thus, for disease diagnostics and treatments, the significance of body rhythms is vital. For example, when doing blood test, we recommend at the same time of the day. What is more, taking medicine at different times of the day can achieve different effects.
In mammals, the suprachiasmatic nucleus of the hypothalamus regulates circadian rhythms, with light driving the process through the retinal hypothalamic tract (Crosio et al., 2000). A series of complex protein networks produce stable 24-h circadian rhythms (Edery et al., 1994). Of these, heterodimeric CLOCK-BMAL1 acts on promoter or intronic sequences of PER and CRY genes to activate transcription and translation via the E-box enhancer, thus exerting positive regulatory roles (Hamaguchi et al., 2004; Kavakli et al., 2022). The PER and CRY complex enter the nucleus from the cytoplasm and inhibits the transcription and translation of CLOCK-BMAL1, thus exerting a negative regulatory role (Hirayama et al., 2003; Rasmussen et al., 2022). After CLOCK-BMAL1 levels are reduced, PER and CRY transcription and translation decreases, and CLOCK-BMAL1 inhibition decreases, thus forming a feedback loop (Liu et al., 2008).
ROR and REV-ERB are important regulators of this feedback loop (Liu et al., 2008; Gustafson & Partch, 2015; Mansingh & Handschin, 2022). The transcription and translation of REV-ERB is activated by the CLOCK-BMAL1 heterodimer and inhibited by CRY/PER, resulting in circadian rhythm changes in REV-ERBα (Liu et al., 2008; Gustafson & Partch, 2015). Conversely, REV-ERBα inhibits the transcription and translation of BMAL1 and CLOCK. RORα promotes the transcription and translation of BMAL1 and CLOCK by binding to the DNA binding element rore in the BMAL1 promoter competitively with REV-ERBα(Guillaumond et al., 2005). The REV-ERBα/RORα feedback loop and the positive and negative limbs of the circadian clock gene loop interact to maintain circadian rhythm stability.
5 Biorhythms and bone metabolism during the normal physiological state
5.1 In vitro bone remodeling markers are rhythmic in nature
The Per1 luciferase transgene was used as a bioluminescent indicator in the calvaria of neonatal rats in vitro, and the Per1 rhythmic expression cycle was 25 ± 4 h (McElderry et al., 2013). The tibial cortical bone of 8-week-old rats was resected, cultured in vitro, and bone collagen formation exhibited circadian rhythm fluctuations. When compared with daytime light, bone collagen accumulation and secretion was less in dark periods (Russell et al., 1985) (Table 1).
5.2 Animal bone remodeling markers are rhythmic in nature
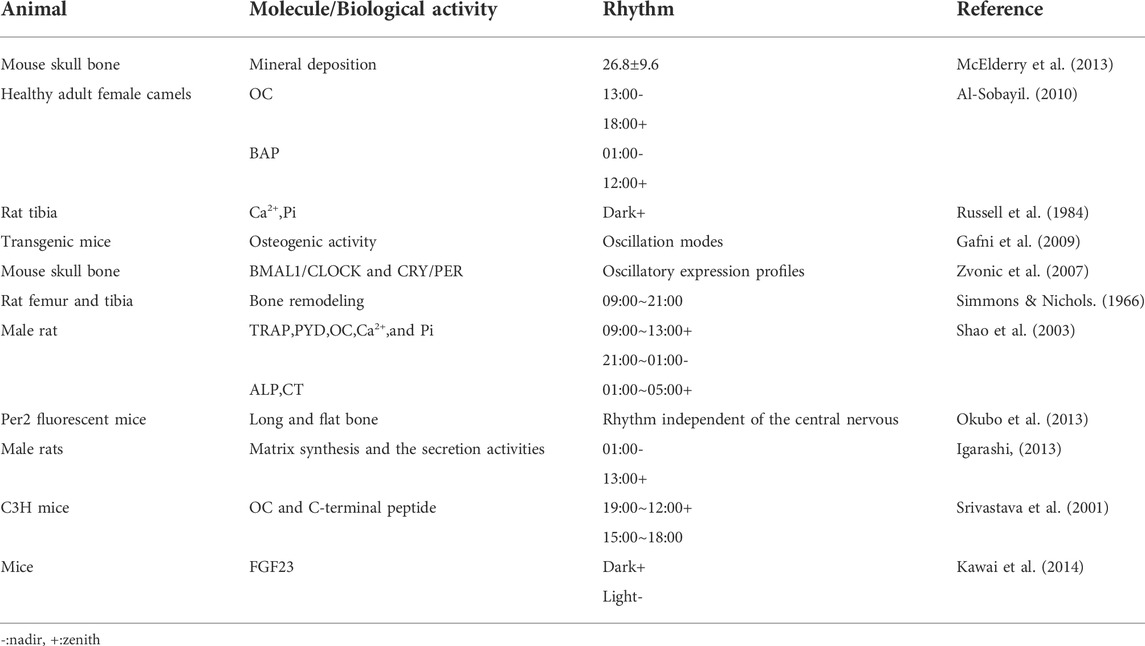
Several studies have shown that in vivo bone tissue metabolism generates a typical circadian rhythm, i.e., bone formation is dominant during the day, while bone absorption is dominant at night. Specifically, bone remodeling markers show a circadian rhythm. A non-invasive Raman microscope was used to track mineral accumulation in mouse skull bone tissue, and the rhythmic cycle of mineral deposition was 26.8 ± 9.6 h, and the earliest mineralization events occurred 6 hours after peak expression levels of Per1 (McElderry et al., 2013). Healthy adult female camels were studied and serum osteocalcin (OC) had a circadian rhythm over 24 h/day, with the lowest level at 13:00, and the highest at 18:00. Bone alkaline phosphatase (BAP) levels fluctuated slightly, reaching the lowest and highest levels at 1:00 and 12:00 noon, respectively (Al-Sobayil, 2010). In a biopsy study, bone tissue mineralization in the metaphysis of 4-week-old rats followed a circadian rhythm, indicating that calcium and inorganic phosphorus levels peaked during dark periods (Russell et al., 1984). Luminous signal intensity of osteoblasts (the human OC promoter was tagged with a luciferase reporter) in transgenic mice was analyzed, and the mouse maxillary complex, skull, tail, carpal bone, and tarsal bone all displayed oscillation modes of osteogenic activity (Gafni et al., 2009). In their transcriptome analysis, 26% of genes displayed circadian rhythms after a 12 h light/12 h dark cycle treatment. Two circadian rhythm transcription complexes (BMAL1/CLOCK and CRY/PER) and their mediators (DBP and REV-ERBα) showed oscillatory expression profiles (Zvonic et al., 2007). The strongest bone remodeling occurred in the photoperiod (9:00–21:00) (Simmons & Nichols, 1966). 5-Week-old male rats were subjected to a 12 h light/12 h dark, and serum TRAP, pyridinol (PYD), OC, Ca2+, and Pi levels displayed significant circadian rhythms, with peak levels between 9:00 and 13:00, and lowest levels between 21:00 and 1:00. In addition, alkaline phosphatase (ALP) and CT serum levels showed peak levels between 1:00 and 5:00. However, the authors observed no circadian rhythm changes in PTH serum levels (Shao et al., 2003). Per2 fluorescent mice was used to culture long (proximal femur and radius) and flat bone (skull and scapula) tissue in vitro for 6 months. Articular and epiphyseal cartilage in the growth plates of young mice underwent circadian rhythm, indicating peripheral bone tissue underwent circadian rhythm, independent of the central nervous system (Okubo et al., 2013). 4-week-old male rats were studied and matrix synthesis and secretion activities of chondrocytes and osteoblasts at different osteogenic sites, experienced the same circadian rhythms, i.e., peak levels appeared in the13:00 photoperiod, and the lowest levels appeared in the 1:00 dark period (Igarashi et al., 2013). In 6-week-old C3H/HEJ (C3H) mice, OC and C-terminal peptide levels underwent diurnal changes, with peak levels between 9:00 and 12:00, and the lowest levels between 15:00 and 18:00; the peak level was 26%–66% higher than the 24-h average level. However, serum ALP levels were less affected by circadian rhythms (Srivastava et al., 2001). Fibroblast growth factor 23 (FGF23) regulates phosphorus levels in osteoblasts. FGF23 expression levels displayed circadian rhythms in mice, i.e., serum levels were low in the daytime, but increased at night (Kawai et al., 2014). In the UMR-106 mouse osteoblast cell line, sympathetic nerves activated the expression levels of FGF23, and CRY1 inhibited this process by inhibiting ISO induced cAMP response element-binding protein (CREB) phosphorylation. Also, in BMAL1 knockout mice, the expression level of CRY1 was increased, and above process was also inhibited. These data indicated that FGF23 regulation by the sympathetic nerve was mediated by rhythmic proteins (Kawai et al., 2014) (Table 2).
5.3 Human bone remodeling markers are rhythmic in nature
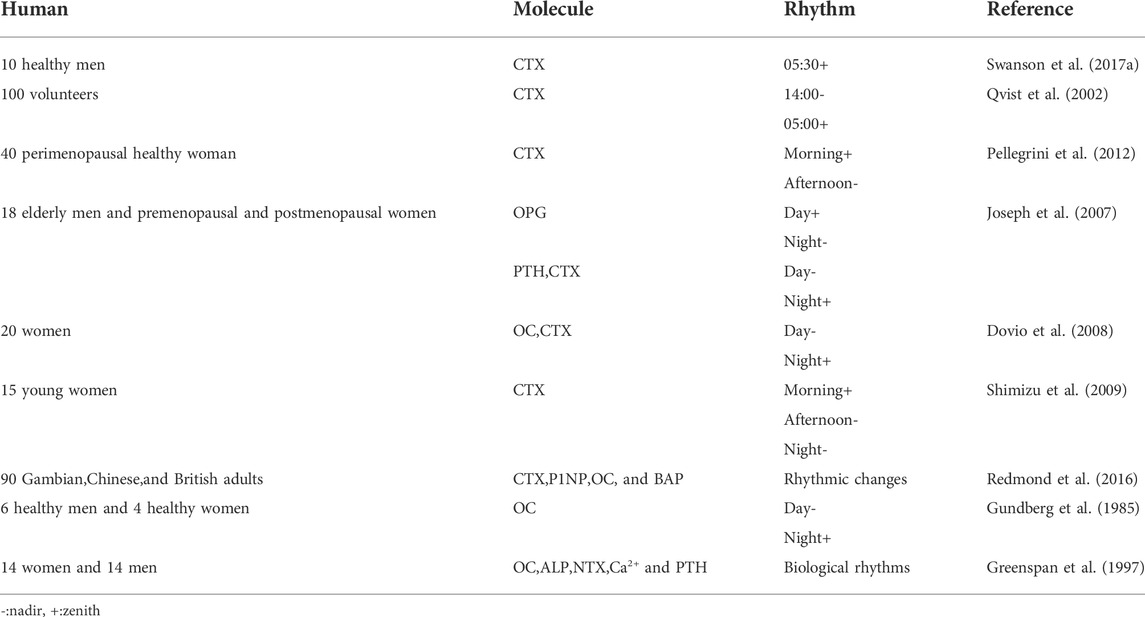
Serum collagen type 1 C-telopeptide (CTX), FGF23, sclerostin (SOST), and P1NP levels in healthy men (20–65 years old) were measured, and the bone resorption marker, CTX, exhibited peak circadian rhythms at 5:30 (range 1:30–07:30), FGF23 exhibited uncertain peak rhythms (range 2:30–11:30), while SOST and PINP exhibited none (Swanson C. et al., 2017). 100 volunteers were studied and serum CTX levels exhibited rhythmic changes over 24 h, with the highest levels at around 5:00 in the morning, and the lowest levels around 14:00 in the afternoon. Except to fasting, this rhythmic change was not affected by gender, age, menstrual status, bed rest, and other factors (Qvist et al., 2002). Saliva, serum, and urine from perimenopausal healthy women were analyzed at 9:30, 13:30 and, 17:30 and CTX levels peaked in the morning and dipped in the afternoon (Pellegrini et al., 2012). Peripheral venous blood (within 24 h) was collected from elderly men and premenopausal and postmenopausal women every hour, and PTH, OPG, and CTX levels displayed circadian rhythms. The highest OPG levels appeared in the daytime and the lowest appeared at night, while PTH and CTX levels had opposite patterns (Joseph et al., 2007). 20 women (25–65 years old) were studied and serum OC and CTX had the lowest levels during the day, and the highest at night. However, similar rhythms for OPG and soluble RANKL (sRANKL) were not detected in circulating blood (Dovio et al., 2008). The blood from 15 young women was analyzed at 8:00, 14:00, and 20:00, and CTX levels were increased in the morning, but decreased in the afternoon and night. However, no similar rhythms for ALP, OPG, and sRANKL were detected in circulating blood (Shimizu et al., 2009). Blood from volunteers aged 60–75 years old, comprising Gambian, Chinese, and British adults was studied. While there were ethnic differences in plasma levels of bone metabolism and PTH markers, their circadian rhythms were similar, i.e., plasma levels of CTX, P1NP, OC, and BAP showed rhythmic changes (Redmond et al., 2016). In a study involving six healthy men and four healthy women (20–30 years old), serum OC levels were decreased in the morning, but gradually increased in the afternoon and evening, and peaked at night (Gundberg et al., 1985). 14 women (74 ± 6 years old) and 14 men (80 ± 5 years old) were studied, and serum OC, ALP, urinary N-terminal peptide cross-linked collagen type 1, serum calcium, and PTH all exhibited biological rhythms (Greenspan et al., 1997) (Table 3).
6 Biological rhythm disorders in bone tissue could lead to osteoporosis
The 24-h circadian rhythm is based on light/dark alternations, and facilitates sleep behaviors at night and awake activities during the day. Jet lag, fasting, eating, etc., disturb these rhythms, resulting in osteoporosis.
6.1 Cell/gene knockout models
Cry and Per genes in mouse osteoblasts were knocked out and the bone mass of vertebrae and long bones increased significantly (Fu et al., 2005). RANKL expression and osteoclasts increased in osteoblast specific BMAL1 knockout mice, while in BMAL1 overexpression mice, both RANKL and osteoclast levels decreased. These observations indicated that bone resorption and bone mass were regulated by the clock gene, BMAL1, in osteoblasts (Takarada et al., 2017). After a BMAL1 specific knockout mouse, osteoclast differentiation decreased and bone mass increased, suggesting BMAL1 up-regulated NFATc1 expression by binding to the E-box element of the NFATc1 promoter (Xu et al., 2016). Mouse per2 and cry2 genes were knocked out, and there are different pathways to regulate bone remodeling between per2 and cry2. Cry2 mainly affected the biological process of osteoclasts, and the content of TRAP in serum is significantly reduced after knockout. Per2 acts on the biological process related to osteoblasts, and the bone formation rate (BFR) is significantly increased after knockout (Maronde et al., 2010). In BMAL1 gene knockout mice, when compared with wild-type animals, the bone bridge between metaphysis and epiphysis in knockout mice had shortened long bones, thinned bone cortex, thinned bone trabecula, reduced bone density, accelerated aging, and decreased bone marrow MSC differentiation into osteoblasts (Samsa et al., 2016). BMD and volume of bone were decreased in BMAL1 knockout mice when compared with wild-type mice (Kondratov et al., 2006). BMAL1 restored osteogenic abilities by inhibiting the NF-κB signaling pathway during inhibition of MSC differentiation, induced by type 2 diabetes, and regulated the balance between osteogenesis and osteoclast differentiation (Li et al., 2018). MSCs were cultured in osteogenic induction medium, and REV-ERBα expression decreased during bone MSC osteogenesis, while REV-ERBα overexpression inhibited bone MSC proliferation and osteogenic ability (He et al., 2015) (Table 4).
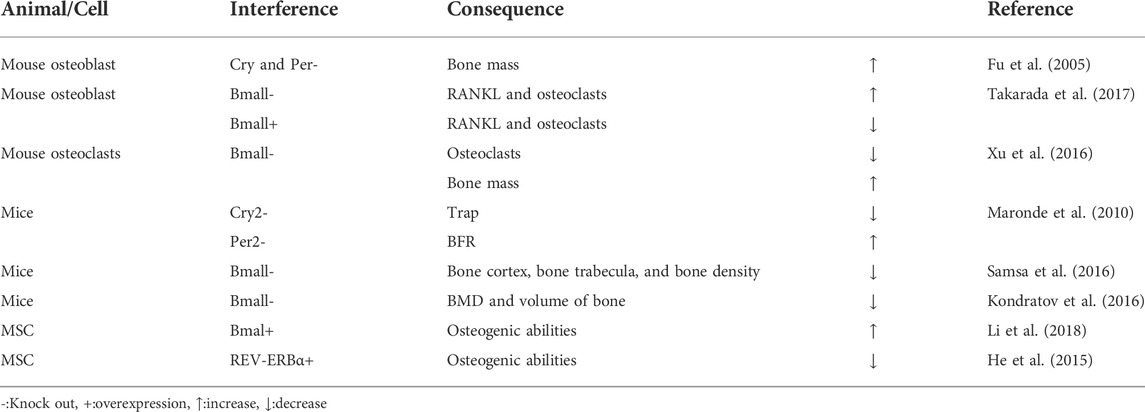
TABLE 4. Biological rhythm disorders in bone tissue lead to osteoporosis in cell/gene knockout models.
6.2 Animal studies
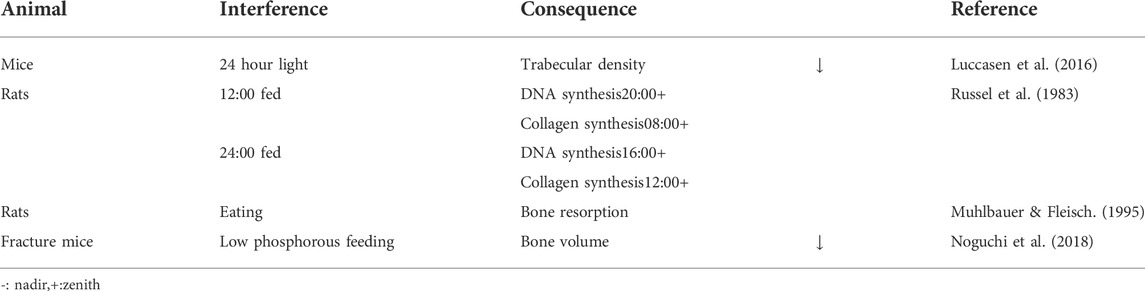
Wild-type mice were exposed to a 24 h light for 24 weeks. Their data showed that the central circadian rhythm pacing rate of suprachiasmatic nucleus (SCN) neurons decreased by 70%, with trabecular density also decreased. When mice returned to standard light/dark cycles, SCN neurons quickly returned to normal circadian rhythms, and osteoporosis gradually recovered (Lucassen et al., 2016). 4-week-old rats were exposed to a 12 h light (8:00–20:00) and 12 h dark (20:00–8:00) photoperiods for 4 weeks. One group was fed 4 hours after the beginning of light, and the other group fed 4 hours after the beginning of dark. The authors observed that different eating times disturbed the normal rhythms of DNA and collagen synthesis in rat tibias (Russell et al., 1983). The relationship between food intake and bone mass in 2-month-old rats was investigated, and a peak in bone resorption was observed after eating, however, dividing food into smaller pieces weakened this effect. However, the effect was not weakened in parathyroidectomy rats, suggesting calcitonin inhibited bone resorption, as mediated by osteoclasts. (Muhlbauer and Fleisch, 1995). Phosphate was an important metabolite in regulating circadian rhythm functions in bone tissue and peripheral non-bone tissue, via low phosphorus feeding in male 8–10 weeks fracture old mice (Noguchi et al., 2018) (Table 5).
6.3 Human studies
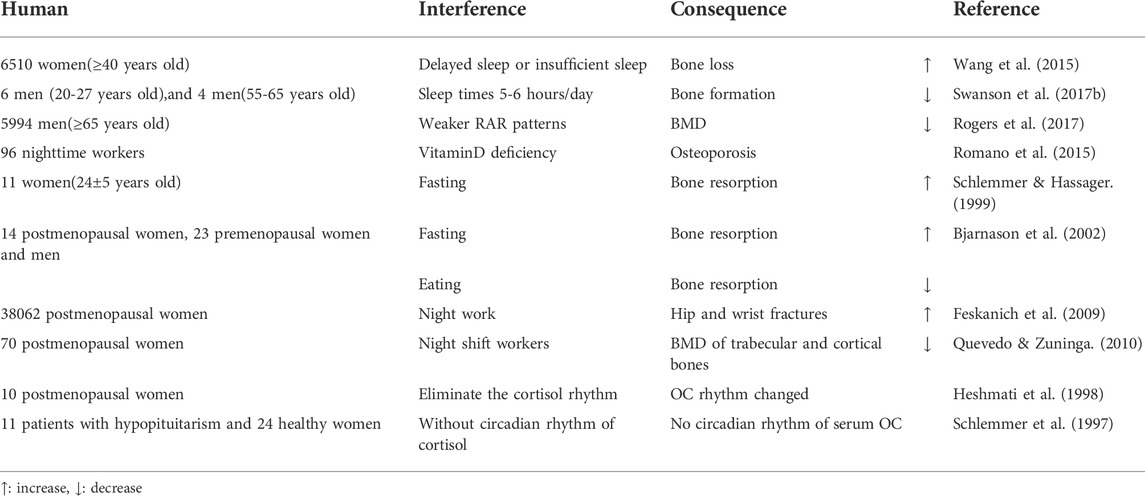
6,510 women (≥40 years old) were studied and when total sleep time is fixed, delayed sleep or insufficient sleep at night, led to increased bone loss in postmenopausal, but not in premenopausal women (Wang et al., 2015). Circadian rhythm interference studies were performed on six healthy 20–27 year old men, and four healthy 55–65 year old men for 3 weeks (sleep times were set at 5–6 h/day). After interference, serum P1NP levels decreased, while CTX levels remained unchanged, suggesting an imbalance in bone turnover, i.e., bone formation was reduced but bone resorption was unchanged. Thus, circadian rhythm disorder and sleep deprivation may be influential factors for bone damage (Swanson C. M. et al., 2017). The relationship between resting activity circadian rhythm patterns and BMD were monitored by following-up 5,994 elderly men (≥65 years old). Using a generalized linear model, the circadian activity ratio was related to hip joint and femoral neck BMD, but it was not an independent influential factors (Rogers et al., 2017). 96 nighttime and 100 daytime workers were studied, and night work caused vitamin D deficiency, potentially indicative for osteoporosis (Romano et al., 2015). A randomized crossover study on 11 healthy premenopausal women (24 ± 5 years old) was performed to assess circadian rhythm changes on bone turnover markers in subjects consisting of two periods: either 33 h of fasting (fasting) followed 1 week later by a 33-h period with regular meals eaten at 08:00–08:30,11:30–12:30 and 18:00–19:00 (control) or vice versa. Food intake had little effect on the diurnal changes of bone resorption, and was independent of PTH levels. Decreased iPTH (intact PTH) during fasting may be secondary to increased bone resorption caused by fasting (Schlemmer & Hassager, 1999). Serum CTX, a marker of bone resorption after fasting, oral glucose tolerance tests (OGTT) and normal eating were detected in postmenopausal women, premenopausal women and men. The circadian rhythm of bone resorption was mediated by eating and fasting, and independent with gender and menstrual status. The circadian rhythm of bone resorption suggests it decreases when eating during the day, and increases when fasting at night (Bjarnason et al., 2002). An 8-years follow-up study was conducted on 38,062 postmenopausal women and when compared with women who never worked a night shift, those with 20 years night work experience had an increased risk of hip and wrist fractures (Feskanich et al., 2009). 70 postmenopausal women (39 on night and 31 on day shift) were studied, and the BMD of trabecular and cortical bones of night shift workers was lower, suggesting night shift work altered circadian rhythms, and was a risk factor for osteoporosis (Quevedo & Zuniga, 2010). Cortisol secretion from 10 healthy postmenopausal women was intervened to study circadian rhythm etiology in bone. Subjects were divided into two groups. Firstly, endogenous cortisol secretion of both groups was blocked by metoprolone. Then, one group had different hydrocortisone doses according to the circadian rhythm at each time point, while the other group had the same hydrocortisone dose at each time point to eliminate the circadian rhythm. Serum OC levels changed in line with the circadian rhythm of serum cortisol, but procollagen type I carboxyl peptide (PICP) and bone resorption markers did not. This study showed that the circadian rhythm of bone resorption was not mediated by cortisol (Heshmati et al., 1998). A similar conclusion by studying 35 women (11 patients with hypopituitarism and 24 healthy women) was reached. In patients without circadian rhythm of cortisol, there were normal circadian rhythm changes of pyridine cross-linking compounds and PICP in urine, but no circadian rhythm changes of serum OC. These observations suggested that circadian changes in serum OC may have been mediated by endogenous circadian changes in serum cortisol, but serum cortisol could not control circadian changes in serum PICP, nor the rhythmic changes of pyridine cross-linking compounds in urine (Schlemmer et al., 1997) (Table 6).
7 The application of bone tissue rhythms
81 postmenopausal women were studied and the oral administration of 0.8 mg salmon calcitonin at three different time points (8:00, 17:00, and 22:00) reduced CTX serum levels, i.e., bone resorption was inhibited. In addition, different administration times had different effects on bone resorption i.e., taking the supplement before dinner exerted the best effects, with a bone resorption inhibition rate of 25% (Karsdal et al., 2008). 50 postmenopausal women with osteoporosis were investigated, after 12 months of treatment with tripatide (TPTD), lumbar BMD in the morning treatment group was higher than in the night treatment group. This showed that TPTD administration time had a significant impact on osteoporosis (Michalska et al., 2012). Pulsed electromagnetic fields (PEMF) can prevent osteoporosis, and was applied to 3-month-old ovariectomized female rats at different time points, and observed that the therapeutic effects from 9:00–15:00 were significantly improved when compared to the 0:00–6:00 period (Jing et al., 2010).
8 Future perspectives
Melatonin, as a therapy for osteoporosis, has been investigated by several pivotal studies, and valued by researchers and clinicians. Melatonin appears to adjust and restore circadian rhythms, deepen sleep, improve both sleep quality and the functional state of the body. There are biorhythms related proteins in bone tissue. Therefore, melatonin promotes bone formation and inhibits bone resorption. This latter mechanism is facilitated by the differentiation and maturation of osteoclasts, which is partly mediated by rhythmic proteins, therefore, melatonin as an osteoporosis therapeutic is feasible.
By consulting the literature and databases, we found no preclinical reports on melatonin or rhythmic protein as therapeutic targets for osteoporosis. Therefore, in the future, we must focus on how melatonin maintains normal bone biological rhythms via rhythm proteins.
By applying new technologies and performing more comprehensive human and animal studies, new biological rhythm mechanisms will be identified. These approaches will provide greater insights on biological rhythms, and contribute to our knowledge on osteoporosis. We hope in the future, melatonin will be incorporated into clinical practice as an osteoporosis therapy, and provide hope and relief for patients with the condition.
Author contributions
YT contributed to the conception and design. JM performed language editing, tabulation and drawing. All authors read and approved the final version of the manuscript.
Acknowledgments
Thanks for the people who helped me.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Sobayil, F. A. (2010). Circadian rhythm of bone formation biomarkers in serum of dromedary camels. Res. Vet. Sci. 89, 455–459. doi:10.1016/j.rvsc.2010.03.024
Altman, B., Hsieh, A., Sengupta, A., Krishnanaiah, S., Stine, Z., Walton, Z., et al. (2015). MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 22, 1009–1019. doi:10.1016/j.cmet.2015.09.003
Amaral, F., and Cipolla-Neto, J. (2018). A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 62, 472–479. doi:10.20945/2359-3997000000066
Bjarnason, N., Henriksen, E., Alexandersen, P., Christgau, S., Henriksen, D., and Christiansen, C. (2002). Mechanism of circadian variation in bone resorption. Bone 30, 307–313. doi:10.1016/s8756-3282(01)00662-7
Chaix, A., Lin, T., Le, H., Chang, M., and Panda, S. (2019). Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 29, 303–319. doi:10.1016/j.cmet.2018.08.004
Crosio, C., Cermakian, N., Allis, C., and Sassone-Corsi, P. (2000). Light induces chromatin modification in cells of the mammalian circadian clock. Nat. Neurosci. 3, 1241–1247. doi:10.1038/81767
Cutando, A., Lopez-Valverde, A., de Diego, R. G., de Vicente, J., Reiter, R., Fernandez, M. H., et al. (2014). Effect of topical application of melatonin to the gingiva on salivary osteoprotegerin, RANKL and melatonin levels in patients with diabetes and periodontal disease. Odontology 102, 290–296. doi:10.1007/s10266-013-0122-5
Dong, W., Qi, M., Wang, Y., Feng, X., and Liu, H. (2018). Zoledronate and high glucose levels influence osteoclast differentiation and bone absorption via the AMPK pathway. Biochem. Biophys. Res. Commun. 505, 1195–1202. doi:10.1016/j.bbrc.2018.10.059
Dong, Z., Zhang, G., Qu, M., Gimple, R., Wu, Q., Qiu, Z., et al. (2019). Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. 9, 1556–1573. doi:10.1158/2159-8290.CD-19-0215
Dovio, A., Generali, D., Tampellini, M., Berruti, A., Tedoldi, S., Torta, M., et al. (2008). Variations along the 24-hour cycle of circulating osteoprotegerin and soluble RANKL: A rhythmometric analysis. Osteoporos. Int. 19, 113–117. doi:10.1007/s00198-007-0423-z
Edery, I., Rutila, J., and Rosbash, M. (1994). Phase shifting of the circadian clock by induction of the Drosophila period protein. Sci. (New York, N.Y.) 263, 237–240. doi:10.1126/science.8284676
Feskanich, D., Hankinson, S. E., and Schernhammer, E. S. (2009). Nightshift work and fracture risk: The nurses' health study. Osteoporos. Int. 20, 537–542. doi:10.1007/s00198-008-0729-5
Fu, L., Patel, M., Bradley, A., Wagner, E., and Karsenty, G. (2005). The molecular clock mediates leptin-regulated bone formation. Cell 122, 803–815. doi:10.1016/j.cell.2005.06.028
Gafni, Y., Ptitsyn, A. A., Zilberman, Y., Pelled, G., Gimble, J. M., and Gazit, D. (2009). Circadian rhythm of osteocalcin in the maxillomandibular complex. J. Dent. Res. 88, 45–50. doi:10.1177/0022034508328012
Gera, S., Sampathi, S., Maddukuri, S., Dodoala, S., Junnuthula, V., and Dyawanapelly, S. (2022). Therapeutic potential of naringenin nanosuspension: In vitro and in vivo anti-osteoporotic studies. Pharmaceutics 14, 1449. doi:10.3390/pharmaceutics14071449
Greenspan, S. L., Dresner-Pollak, R., Parker, R. A., London, D., and Ferguson, L. (1997). Diurnal variation of bone mineral turnover in elderly men and women. Calcif. Tissue Int. 60, 419–423. doi:10.1007/s002239900256
Gu, H., Huang, Z., Chen, G., Zhou, K., Zhang, Y., Chen, J., et al. (2020). Network and pathway-based analyses of genes associated with osteoporosis. Med. Baltim. 99, e19120. doi:10.1097/MD.0000000000019120
Guillaumond, F., Dardente, H., Giguère, V., and Cermakian, N. (2005). Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J. Biol. Rhythms 20, 391–403. doi:10.1177/0748730405277232
Gundberg, C. M., Markowitz, M. E., Mizruchi, M., and Rosen, J. F. (1985). Osteocalcin in human serum: A circadian rhythm. J. Clin. Endocrinol. Metab. 60, 736–739. doi:10.1210/jcem-60-4-736
Gustafson, C. L., and Partch, C. L. (2015). Emerging models for the molecular basis of mammalian circadian timing. Biochemistry 54, 134–149. doi:10.1021/bi500731f
Hamaguchi, H., Fujimoto, K., Kawamoto, T., Noshiro, M., Maemura, K., Takeda, N., et al. (2004). Expression of the gene for Dec2, a basic helix-loop-helix transcription factor, is regulated by a molecular clock system. Biochem. J. 382, 43–50. doi:10.1042/BJ20031760
Han, Y., Meng, F., Venter, J., Wu, N., Wan, Y., Standeford, H., et al. (2016). miR-34a-dependent overexpression of Per1 decreases cholangiocarcinoma growth. J. Hepatol. 64, 1295–1304. doi:10.1016/j.jhep.2016.02.024
He, Y., Lin, F., Chen, Y., Tan, Z., Bai, D., and Zhao, Q. (2015). Overexpression of the circadian clock gene rev-erbα affects murine bone mesenchymal stem cell proliferation and osteogenesis. Stem Cells Dev. 24, 1194–1204. doi:10.1089/scd.2014.0437
Heshmati, H., Riggs, B., Burritt, M., McAlister, C., Wollan, P., and Khosla, S. (1998). Effects of the circadian variation in serum cortisol on markers of bone turnover and calcium homeostasis in normal postmenopausal women. J. Clin. Endocrinol. Metab. 83, 751–756. doi:10.1210/jcem.83.3.4627
Hirata, H., Kamohara, A., Murayama, M., Nishioka, K., Honda, H., Urano, Y., et al. (2022). A novel role of helix-loop-helix transcriptional factor Bhlhe40 in osteoclast activation. J. Cell. Physiol. doi:10.1002/jcp.30844
Hirayama, J., Fukuda, I., Ishikawa, T., Kobayashi, Y., and Todo, T. (2003). New role of zCRY and zPER2 as regulators of sub-cellular distributions of zCLOCK and zBMAL proteins. Nucleic Acids Res. 31, 935–943. doi:10.1093/nar/gkg174
Histing, T., Anton, C., Scheuer, C., Garcia, P., Holstein, J. H., Klein, M., et al. (2012). Melatonin impairs fracture healing by suppressing RANKL-mediated bone remodeling. J. Surg. Res. 173, 83–90. doi:10.1016/j.jss.2010.08.036
Igarashi, K., Saeki, S., and Shinoda, H. b. (2013). Diurnal rhythms in the incorporation and secretion of 3H-proline and 3H-galactose by cartilage cells and osteoblasts in various bone-forming sites in growing rats. Orthod. Waves 72, 11–15. doi:10.1016/j.odw.2012.09.001
Ikegame, M., Hattori, A., Tabata, M. J., Kitamura, K. I., Tabuchi, Y., Furusawa, Y., et al. (2019). Melatonin is a potential drug for the prevention of bone loss during space flight. J. Pineal Res. 67, e12594. doi:10.1111/jpi.12594
Ikegami, K., Refetoff, S., Van Cauter, E., and Yoshimura, T. (2019). Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 15, 590–600. doi:10.1038/s41574-019-0237-z
Jiang, C., Wang, Y., Zhang, M., and Xu, J. (2022). Cholesterol inhibits autophagy in RANKL-induced osteoclast differentiation through activating the PI3K/AKT/mTOR signaling pathway. Mol. Biol. Rep. doi:10.1007/s11033-022-07747-w
Jing, D., Shen, G., Huang, J., Xie, K., Cai, J., Xu, Q., et al. (2010). Circadian rhythm affects the preventive role of pulsed electromagnetic fields on ovariectomy-induced osteoporosis in rats. Bone 46, 487–495. doi:10.1016/j.bone.2009.09.021
Jing, H. F., and Wang, X. M. (2017). Effects of aerobic exercise combined with melatonin on osteoporosis of type II diabetic rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 33, 252–256. doi:10.12047/j.cjap.5395.2017.062
Johnston, C. C., and Slemenda, C. W. (1995). Pathogenesis of osteoporosis. Bone 17, 19S–22S. doi:10.1016/8756-3282(95)00202-o
Joseph, F., Chan, B. Y., Durham, B. H., Ahmad, A. M., Vinjamuri, S., Gallagher, J. A., et al. (2007). The circadian rhythm of osteoprotegerin and its association with parathyroid hormone secretion. J. Clin. Endocrinol. Metab. 92, 3230–3238. doi:10.1210/jc.2006-1832
Karsdal, M. A., Byrjalsen, I., Riis, B. J., and Christiansen, C. (2008). Investigation of the diurnal variation in bone resorption for optimal drug delivery and efficacy in osteoporosis with oral calcitonin. BMC Clin. Pharmacol. 8, 12. doi:10.1186/1472-6904-8-12
Kavakli, I. H., Ozturk, N., and Baris, I. (2022). Protein interaction networks of the mammalian core clock proteins. Adv. Protein Chem. Struct. Biol. 131, 207–233. doi:10.1016/bs.apcsb.2022.04.001
Kawai, M., Kinoshita, S., Shimba, S., Ozono, K., and Michigami, T. (2014). Sympathetic activation induces skeletal Fgf23 expression in a circadian rhythm-dependent manner. J. Biol. Chem. 289, 1457–1466. doi:10.1074/jbc.M113.500850
Kim, H. J., Kim, H. J., Bae, M. K., and Kim, Y. D. (2017). Suppression of osteoclastogenesis by melatonin: A melatonin receptor-independent action. Int. J. Mol. Sci. 18, E1142. doi:10.3390/ijms18061142
Kobayakawa, T., Miyazaki, A., Takahashi, J., and Nakamura, Y. (2022). Verification of efficacy and safety of ibandronate or denosumab for postmenopausal osteoporosis after 12-month treatment with romosozumab as sequential therapy: The prospective VICTOR study. Bone 162, 116480. doi:10.1016/j.bone.2022.116480
Kondratov, R., Kondratova, A., Gorbacheva, V., Vykhovanets, O., and Antoch, M. (2006). Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 20, 1868–1873. doi:10.1101/gad.1432206
Koyama, H., Nakade, O., Takada, Y., Kaku, T., and Lau, K. H. (2002). Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J. Bone Min. Res. 17, 1219–1229. doi:10.1359/jbmr.2002.17.7.1219
Li, X., Liu, N., Gu, B., Hu, W., Li, Y., Guo, B., et al. (2018). BMAL1 regulates balance of osteogenic-osteoclastic function of bone marrow mesenchymal stem cells in type 2 diabetes mellitus through the NF-κB pathway. Mol. Biol. Rep. 45, 1691–1704. doi:10.1007/s11033-018-4312-7
Liu, A. C., Tran, H. G., Zhang, E. E., Priest, A. A., Welsh, D. K., and Kay, S. A. (2008). Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 4, e1000023. doi:10.1371/journal.pgen.1000023
Liu, C., Li, N., Lu, Z., Sun, Q., Pang, X., Xiang, X., et al. (2022). CG and CHG methylation contribute to the transcriptional control of OsPRR37-output genes in rice. Front. Plant Sci. 13, 839457. doi:10.3389/fpls.2022.839457
Liu, Y., Wang, X., Chang, H., Gao, X., Dong, C., Li, Z., et al. (2018). Mongolian Medicine echinops prevented postmenopausal osteoporosis and induced ER/AKT/ERK pathway in BMSCs. Biosci. Trends 12, 275–281. doi:10.5582/bst.2018.01046
Loffler, M. T., Sollmann, N., Mei, K., Valentinitsch, A., Noel, P. B., Kirschke, J. S., et al. (2020). X-ray-based quantitative osteoporosis imaging at the spine. Osteoporos. Int. 31, 233–250. doi:10.1007/s00198-019-05212-2
Lucassen, E., Coomans, C., van Putten, M., de Kreij, S., van Genugten, J., Sutorius, R., et al. (2016). Environmental 24-hr cycles are essential for health. Curr. Biol. 26, 1843–1853. doi:10.1016/j.cub.2016.05.038
Manolagas, S. C. (2014). Wnt signaling and osteoporosis. Maturitas 78, 233–237. doi:10.1016/j.maturitas.2014.04.013
Mansingh, S., and Handschin, C. (2022). Time to train: The involvement of the molecular clock in exercise adaptation of skeletal muscle. Front. Physiol. 13, 902031. doi:10.3389/fphys.2022.902031
Marcheva, B., Ramsey, K., Buhr, E., Kobayashi, Y., Su, H., Ko, C., et al. (2010). Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631. doi:10.1038/nature09253
Maria, S., Samsonraj, R. M., Munmun, F., Glas, J., Silvestros, M., Kotlarczyk, M. P., et al. (2018). Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 64, e12465. doi:10.1111/jpi.12465
Maria, S., Swanson, M. H., Enderby, L. T., D'Amico, F., Enderby, B., Samsonraj, R. M., et al. (2017). Melatonin-micronutrients osteopenia treatment study (MOTS): A translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging 9, 256–285. doi:10.18632/aging.101158
Maronde, E., Schilling, A. F., Seitz, S., Schinke, T., Schmutz, I., van der Horst, G., et al. (2010). The clock genes Period 2 and Cryptochrome 2 differentially balance bone formation. PLoS One 5, e11527. doi:10.1371/journal.pone.0011527
McElderry, J. D., Zhao, G., Khmaladze, A., Wilson, C. G., Franceschi, R. T., and Morris, M. D. (2013). Tracking circadian rhythms of bone mineral deposition in murine calvarial organ cultures. J. Bone Min. Res. 28, 1846–1854. doi:10.1002/jbmr.1924
Michalska, D., Luchavova, M., Zikan, V., Raska, I., Kubena, A. A., and Stepan, J. J. (2012). Effects of morning vs. evening teriparatide injection on bone mineral density and bone turnover markers in postmenopausal osteoporosis. Osteoporos. Int. 23, 2885–2891. doi:10.1007/s00198-012-1955-4
Muhlbauer, R. C., and Fleisch, H. (1995). The diurnal rhythm of bone resorption in the rat. Effect of feeding habits and pharmacological inhibitors. J. Clin. Invest. 95, 1933–1940. doi:10.1172/JCI117875
Noguchi, T., Hussein, A. I., Horowitz, N., Carroll, D., Gower, A. C., Demissie, S., et al. (2018). Hypophosphatemia regulates molecular mechanisms of circadian rhythm. Sci. Rep. 8, 13756. doi:10.1038/s41598-018-31830-7
Oishi, K., Shirai, H., and Ishida, N. (2005). CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem. J. 386, 575–581. doi:10.1042/BJ20041150
Okubo, N., Minami, Y., Fujiwara, H., Umemura, Y., Tsuchiya, Y., Shirai, T., et al. (2013). Prolonged bioluminescence monitoring in mouse ex vivo bone culture revealed persistent circadian rhythms in articular cartilages and growth plates. PLoS One 8, e78306. doi:10.1371/journal.pone.0078306
Pan, B. L., Tong, Z. W., Li, S. D., Wu, L., Liao, J. L., Yang, Y. X., et al. (2018). Decreased microRNA-182-5p helps alendronate promote osteoblast proliferation and differentiation in osteoporosis via the Rap1/MAPK pathway. Biosci. Rep. 38, BSR20180696. doi:10.1042/BSR20180696
Paschos, G., Ibrahim, S., Song, W., Kunieda, T., Grant, G., Reyes, T., et al. (2012). Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 18, 1768–1777. doi:10.1038/nm.2979
Patke, A., Murphy, P., Onat, O., Krieger, A., Özçelik, T., Campbell, S., et al. (2017). Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell 169, 203–215. doi:10.1016/j.cell.2017.03.027
Peek, C., Levine, D., Cedernaes, J., Taguchi, A., Kobayashi, Y., Tsai, S., et al. (2017). Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 25, 86–92. doi:10.1016/j.cmet.2016.09.010
Pellegrini, G. G., Gonzales Chaves, M. M., Fajardo, M. A., Ponce, G. M., Toyos, G. I., Lifshitz, F., et al. (2012). Salivary bone turnover markers in healthy pre- and postmenopausal women: Daily and seasonal rhythm. Clin. Oral Investig. 16, 651–657. doi:10.1007/s00784-011-0538-7
Ping, Z., Hu, X., Wang, L., Shi, J., Tao, Y., Wu, X., et al. (2017a). Melatonin attenuates titanium particle-induced osteolysis via activation of Wnt/β-catenin signaling pathway. Acta Biomater. 51, 513–525. doi:10.1016/j.actbio.2017.01.034
Ping, Z., Wang, Z., Shi, J., Wang, L., Guo, X., Zhou, W., et al. (2017b). Inhibitory effects of melatonin on titanium particle-induced inflammatory bone resorption and osteoclastogenesis via suppression of NF-κB signaling. Acta Biomater. 62, 362–371. doi:10.1016/j.actbio.2017.08.046
Qiu, S., Tao, Z. B., Tao, L., and Zhu, Y. (2020). Melatonin induces mitochondrial apoptosis in osteoblasts by regulating the STIM1/cytosolic calcium elevation/ERK pathway. Life Sci. 248, 117455. doi:10.1016/j.lfs.2020.117455
Quevedo, I., and Zuniga, A. M. (2010). Low bone mineral density in rotating-shift workers. J. Clin. Densitom. 13, 467–469. doi:10.1016/j.jocd.2010.07.004
Qvist, P., Christgau, S., Pedersen, B. J., Schlemmer, A., and Christiansen, C. (2002). Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): Effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone 31, 57–61. doi:10.1016/s8756-3282(02)00791-3
Rasmussen, E. S., Takahashi, J. S., and Green, C. B. (2022). Time to target the circadian clock for drug discovery. Trends biochem. Sci. 47, 745–758. doi:10.1016/j.tibs.2022.04.009
Redmond, J., Fulford, A. J., Jarjou, L., Zhou, B., Prentice, A., and Schoenmakers, I. (2016). Diurnal rhythms of bone turnover markers in three ethnic groups. J. Clin. Endocrinol. Metab. 101, 3222–3230. doi:10.1210/jc.2016-1183
Rogers, T. S., Harrison, S., Swanson, C., Cauley, J. A., Barrett-Connor, E., Orwoll, E., et al. (2017). Fractures in Men Study Research, 2017: Rest-activity circadian rhythms and bone mineral density in elderly men. Bone Rep. 7, 156–163. doi:10.1016/j.bonr.2017.11.001
Romano, A., Vigna, L., Belluigi, V., Conti, D. M., Barberi, C. E., Tomaino, L., et al. (2015). Shift work and serum 25-OH vitamin D status among factory workers in Northern Italy: Cross-sectional study. Chronobiol. Int. 32, 842–847. doi:10.3109/07420528.2015.1048867
Russell, J. E., Grazman, B., and Simmons, D. J. (1984). Mineralization in rat metaphyseal bone exhibits a circadian stage dependency. Proc. Soc. Exp. Biol. Med. 176, 342–345. doi:10.3181/00379727-176-41880
Russell, J. E., Simmons, D. J., Huber, B., and Roos, B. A. (1983). Meal timing as a Zeitgeber for skeletal deoxyribonucleic acid and collagen synthesis rhythms. Endocrinology 113, 2035–2042. doi:10.1210/endo-113-6-2035
Russell, J. E., Walker, W. V., Fenster, R. J., and Simmons, D. J. (1985). In vitro evaluation of circadian patterns of bone collagen formation. Proc. Soc. Exp. Biol. Med. 180, 375–381. doi:10.3181/00379727-180-42192
Samsa, W. E., Vasanji, A., Midura, R. J., and Kondratov, R. V. (2016). Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone 84, 194–203. doi:10.1016/j.bone.2016.01.006
Sato, S., Hanada, R., Kimura, A., Abe, T., Matsumoto, T., Iwasaki, M., et al. (2007). Central control of bone remodeling by neuromedin U. Nat. Med. 13, 1234–1240. doi:10.1038/nm1640
Satue, M., Ramis, J. M., del Mar Arriero, M., and Monjo, M. (2015). A new role for 5-methoxytryptophol on bone cells function in vitro. J. Cell. Biochem. 116, 551–558. doi:10.1002/jcb.25005
Schlemmer, A., and Hassager, C. (1999). Acute fasting diminishes the circadian rhythm of biochemical markers of bone resorption. Eur. J. Endocrinol. 140, 332–337. doi:10.1530/eje.0.1400332
Schlemmer, A., Hassager, C., Alexandersen, P., Fledelius, C., Pedersen, B., Kristensen, I., et al. (1997). Circadian variation in bone resorption is not related to serum cortisol. Bone 21, 83–88. doi:10.1016/s8756-3282(97)00039-2
Shao, P., Ohtsuka-Isoya, M., and Shinoda, H. (2003). Circadian rhythms in serum bone markers and their relation to the effect of etidronate in rats. Chronobiol. Int. 20, 325–336. doi:10.1081/cbi-120019343
Shimizu, M., Onoe, Y., Mikumo, M., Miyabara, Y., Kuroda, T., Yoshikata, R., et al. (2009). Variations in circulating osteoprotegerin and soluble RANKL during diurnal and menstrual cycles in young women. Horm. Res. 71, 285–289. doi:10.1159/000208802
Simmons, D. J., and Nichols, G. (1966). Diurnal periodicity in the metabolic activity of bone tissue. Am. J. Physiol. 210, 411–418. doi:10.1152/ajplegacy.1966.210.2.411
Srivastava, A. K., Bhattacharyya, S., Li, X., Mohan, S., and Baylink, D. J. (2001). Circadian and longitudinal variation of serum C-telopeptide, osteocalcin, and skeletal alkaline phosphatase in C3H/HeJ mice. Bone 29, 361–367. doi:10.1016/s8756-3282(01)00581-6
Stothard, E. R., Ritchie, H. K., Birks, B. R., Eckel, R. H., Higgins, J., Melanson, E. L., et al. (2020). Early morning food intake as a risk factor for metabolic dysregulation. Nutrients 12, E756. doi:10.3390/nu12030756
Swanson, C. M., Shea, S. A., Wolfe, P., Cain, S. W., Munch, M., Vujovic, N., et al. (2017b). Bone turnover markers after sleep restriction and circadian disruption: A mechanism for sleep-related bone loss in humans. J. Clin. Endocrinol. Metab. 102, 3722–3730. doi:10.1210/jc.2017-01147
Swanson, C., Shea, S. A., Wolfe, P., Markwardt, S., Cain, S. W., Munch, M., et al. (2017a). 24-hour profile of serum sclerostin and its association with bone biomarkers in men. Osteoporos. Int. 28, 3205–3213. doi:10.1007/s00198-017-4162-5
Takarada, T., Xu, C., Ochi, H., Nakazato, R., Yamada, D., Nakamura, S., et al. (2017). Bone resorption is regulated by circadian clock in osteoblasts. J. Bone Min. Res. 32, 872–881. doi:10.1002/jbmr.3053
Tang, Z., Xu, T., Li, Y., Fei, W., Yang, G., and Hong, Y. (2020). Inhibition of CRY2 by STAT3/miRNA-7-5p promotes osteoblast differentiation through upregulation of CLOCK/BMAL1/P300 expression. Mol. Ther. Nucleic Acids 19, 865–876. doi:10.1016/j.omtn.2019.12.020
Vriend, J., and Reiter, R. J. (2016). Melatonin, bone regulation and the ubiquitin-proteasome connection: A review. Life Sci. 145, 152–160. doi:10.1016/j.lfs.2015.12.031
Wang, K., Wu, Y., Yang, Y., Chen, J., Zhang, D., Hu, Y., et al. (2015). The associations of bedtime, nocturnal, and daytime sleep duration with bone mineral density in pre- and post-menopausal women. Endocrine 49, 538–548. doi:10.1007/s12020-014-0493-6
Wang, N., Yang, G., Jia, Z., Zhang, H., Aoyagi, T., Soodvilai, S., et al. (2008). Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 8, 482–491. doi:10.1016/j.cmet.2008.10.009
Wang, X., Liang, T., Zhu, Y., Qiu, J., Qiu, X., Lian, C., et al. (2019). Melatonin prevents bone destruction in mice with retinoic acid-induced osteoporosis. Mol. Med. 25, 43. doi:10.1186/s10020-019-0107-0
Welz, P., Zinna, V., Symeonidi, A., Koronowski, K., Kinouchi, K., Smith, J., et al. (2019). BMAL1-Driven tissue clocks respond independently to light to maintain homeostasis. Cell 177, 1436–1447. doi:10.1016/j.cell.2019.05.009
Wu, C. H., Chang, Y. F., Chen, C. H., Lewiecki, E. M., Wuster, C., Reid, I., et al. (2021). Consensus statement on the use of bone turnover markers for short-term monitoring of osteoporosis treatment in the asia-pacific region. J. Clin. Densitom. 24, 3–13. doi:10.1016/j.jocd.2019.03.004
Xiao, L., Lin, J., Chen, R., Huang, Y., Liu, Y., Bai, J., et al. (2020). Sustained release of melatonin from GelMA liposomes reduced osteoblast apoptosis and improved implant osseointegration in osteoporosis. Oxid. Med. Cell. Longev. 2020, 6797154. doi:10.1155/2020/6797154
Xu, C., Ochi, H., Fukuda, T., Sato, S., Sunamura, S., Takarada, T., et al. (2016). Circadian clock regulates bone resorption in mice. J. Bone Min. Res. 31, 1344–1355. doi:10.1002/jbmr.2803
Zhang, M., Gao, Y., Li, Q., Cao, H., Yang, J., Cai, X., et al. (2022). Downregulation of DNA methyltransferase-3a ameliorates the osteogenic differentiation ability of adipose-derived stem cells in diabetic osteoporosis via Wnt/β-catenin signaling pathway. Stem Cell Res. Ther. 13, 397. doi:10.1186/s13287-022-03088-4
Zhang, W. L., Meng, H. Z., Yang, R. F., Yang, M. W., Sun, G. H., Liu, J. H., et al. (2016). Melatonin suppresses autophagy in type 2 diabetic osteoporosis. Oncotarget 7, 52179–52194. doi:10.18632/oncotarget.10538
Zhao, Y., Shao, G., Liu, X., and Li, Z. (2022). Assessment of the therapeutic potential of melatonin for the treatment of osteoporosis through a narrative review of its signaling and preclinical and clinical studies. Front. Pharmacol. 13, 866625. doi:10.3389/fphar.2022.866625
Zhou, L., Chen, X., Yan, J., Li, M., Liu, T., Zhu, C., et al. (2017). Melatonin at pharmacological concentrations suppresses osteoclastogenesis via the attenuation of intracellular ROS. Osteoporos. Int. 28, 3325–3337. doi:10.1007/s00198-017-4127-8
Keywords: circadian rhythm, melatonin, osteoclast differentiation, circadian rhythm genes, osteoporosis
Citation: Tian Y and Ming J (2022) The role of circadian rhythm in osteoporosis; a review. Front. Cell Dev. Biol. 10:960456. doi: 10.3389/fcell.2022.960456
Received: 03 June 2022; Accepted: 15 September 2022;
Published: 27 September 2022.
Edited by:
Roland Wohlgemuth, Lodz University of Technology, PolandReviewed by:
Chuandong Wang, Shanghai Jiao Tong University, ChinaIvana Skrlec, Josip Juraj Strossmayer University of Osijek, Croatia
Copyright © 2022 Tian and Ming. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Ming, bWpqZ2M3ODc4QDE2My5jb20=
 Yihao Tian
Yihao Tian Jian Ming*
Jian Ming*