- 1Department of Bioengineering, Los Angeles, CA, United States
- 2Department of Urology, Los Angeles, CA, United States
- 3UCLA Mattel Children's Hospital, Los Angeles, CA, United States
In recent decades, reproductive science has revolutionized the options for biological parenthood for the 20–50% of infertility cases affected by male factors. However, current solutions exclude those who are infertile due to absent testicular tissue. This includes anorchic 46, XY individuals due to trauma or congenital factors and transgender men with a 46, XX genotype. There is a clinical need for methods to restore testicular function independent of pre-existing testicular tissue. This mini-review analyzes studies that have applied non-testicular cell lines to generate germline and non-germline testicular parenchymal components. While only 46, XY cell lines have been evaluated in this context to date, the potential for future application of cell lines from 46, XX individuals is also included. Additionally, the role of varied culture methods, media supplementation, and biologic and synthetic scaffolds to further support testicular parenchyma generation are critiqued. De novo testicular tissue generation in this manner will require a focus on both cellular and environmental aspects of tissue engineering. Put together, these studies highlight the future potential for expanded clinical, reproductive, and endocrine management options for individuals who are currently excluded from aspects of biologic reproduction most consistent with their gender identity and reproductive preferences.
Introduction
Infertility impacts nearly 50 million couples globally. Male factor infertility is identified as the primary cause in 20–30% and as a contributing factor in 50% of total heterosexual infertile couples (Agarwal et al., 2015). Assisted reproductive techniques (ART) have revolutionized infertility care, but there are limitations. This includes methods that require the presence of germ cells from testicular tissue. Therefore, lack of functional testicular tissue in anorchic patients and transgender males excludes these individuals from reproductive techniques yielding biological children consistent with male gender identity. To address this limitation, the objective of this mini-review is to highlight areas of progress and opportunity surrounding the current state of testicular parenchymal generation using non-testicular cell lines and varied biologic or hybrid microenvironments.
History of testicular tissue engineering
Researchers have been incrementally developing the capacity for in vitro spermatogenesis for more than a century. One of the earliest described methods (Champy, 1920) yielded early meiotic spermatocytes by culturing adult rabbit testis fragments in plasma. For decades, researchers preserved cell composition, microenvironment, and spatial arrangement of the testis by utilizing tissue fragments. Later in the 20th century, studies using cells from dissociated testes were published, still preserving the cell composition of testes, but aiming to replicate the microenvironment without testicular tissue. Since the 1990s, further co-culture has been evaluated by combining isolated germline cells and/or spermatogonial stem cell (SSC) with somatic cell lines. More recently, the seeding of either isolated germline cells or co-culture methods on biocompatible scaffolds has been explored to achieve in vitro spermatogenesis (Bhaskar et al., 2022), including further focus on the importance of not only the scaffold structure but also the culture methods and media conditions comprising the cellular microenvironment. All methods described above applied testis-derived cells to a wide array of culture conditions and have successfully yielded haploid cells. This review explores studies that in the past decade have taken these historic methods of testicular tissue engineering a step further by aiming to replicate the cell composition and/or microenvironment of the testis without the requirement of native testicular tissue or autologous testicular cell lines.
De novo generation of testicular parenchymal cells
Introduction to primary testicular cell lines
The testis serves two primary roles: reproductive and endocrine. In the reproductive context, spermatogenesis occurs in the seminiferous tubules and represents a continuum from SSC through spermatocytes, spermatids, and spermatozoa. Sertoli cells provide structural and metabolic support to the differentiating spermatogenic cells and are responsive to follicle stimulating hormone (FSH) secreted by the pituitary gland. In the endocrine context, the hypothalamic-pituitary-testicular axis includes Leydig cells and operates via a feedback loop comprising luteinizing hormone (LH) and testosterone to maintain androgenic homeostasis.
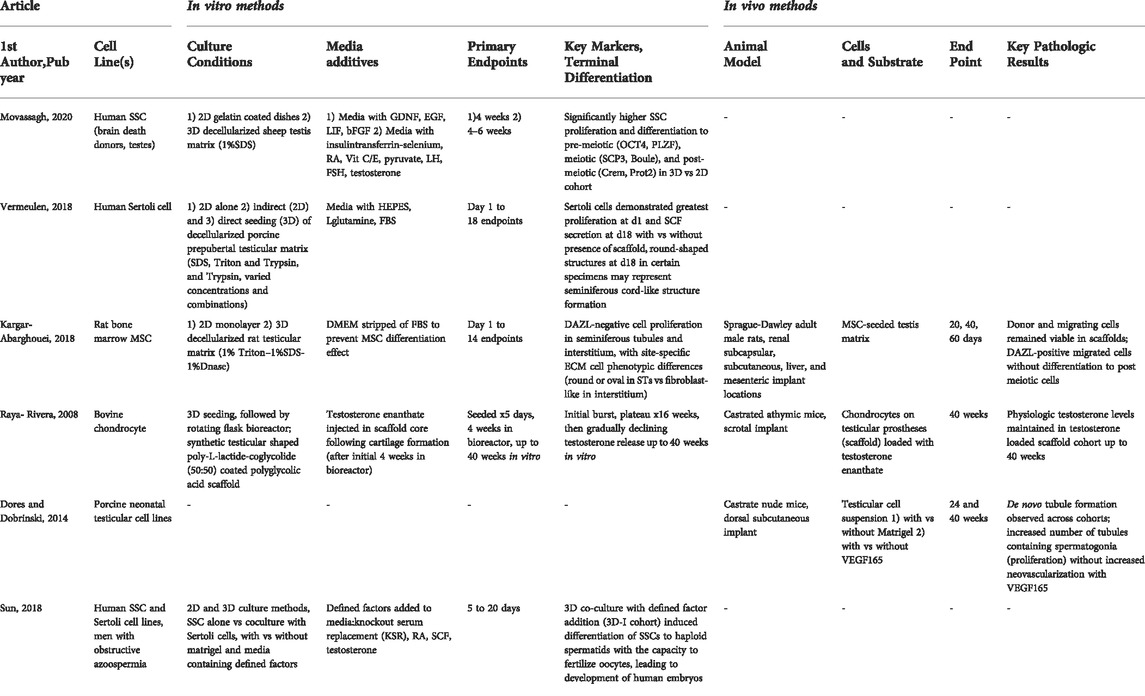
In this section, the most successful studies in the derivation of testicular cell lines from non-testicular progenitors and the varied microenvironments and culture conditions applied to accomplish this task are described. Study details are included in Table 1.
Induced pluripotent and embryonic stem cells (iPSC, ESC)
iPSC cell lines are derived from adult tissues that, after genetic manipulation with a core set of genes, can be expanded and differentiated into organ-specific cell lines (Doss and Sachinidis, 2019). Given their versatility, iPSCs are one of the most common cell lines applied to date for testicular parenchymal tissue generation from progenitor cell lines.
Culturing iPSCs has demonstrated the importance of culture method and conditions to generate both germ cell and non-germ cell lines. In 2-dimensional (2D) co-culture conditions, human iPSC and ESC cell lines were cultured in SSC conditions with GDNF (Glial cell line-derived Neurotrophic Factor). Differentiation was demonstrated by presence of germ cell markers followed by a subset differentiated to round spermatids (Easley IV et al., 2012). Small animal model cell lines have likewise demonstrated iPSC differentiation potential. In another study, cell lines were extracted from embryoid bodies cultured by hanging drop method and derived from iPSCs and mouse ESCs. Subsequently, an iPSC-matrigel suspension was dorsally injected in immunodeficient mice. The resultant grafts displayed ectopic seminiferous tubule formation and differentiation to post-meiotic germ cells (Cai et al., 2013). In a similar study initiating culture via embryoid bodies followed by transplantation of cells with testicular tissue into host mice, seminiferous tubule-like structures were likewise observed (Yang et al., 2012).
Two more recent iPSC applications have advanced further toward the ultimate objective of deriving an effective testicular environment. In one of these studies, various testicular cell types including spermatogonia, Leydig, peritubular myoid, and endothelial cells were derived from human iPSCs using cell-line specific 2D culture media conditions. Resulting cells were then co-cultured as organoids, leading to formation of tubular structures, mature somatic cell, and post-meiotic gametes (Robinson et al., 2021). Ultimately the promise of restored fertility has also occurred with the application of iPSCs in an animal model. In this study, mouse PGCLCs (Primordial Germ Cell-like Cells) were differentiated, expanded, and co-cultured with reconstituted testes (single cell layer of neonatal somatic testicular cells), followed by transplantation of derived Gonadal Stem Cell-like Cells (GSCLCs) into the testes of male mice lacking endogenous spermatogenesis. This subsequently resulted in haploid round spermatids and led to viable offspring using intracytoplasmic sperm injection (ICSI) (Ishikura et al., 2021).
The above-mentioned studies affirm the differentiation potential of iPSCs to generate haploid gametes as well as somatic cells. Future studies that confirm the genetic stability, safety of implantation, and efficacy of gametes in producing progeny are needed to improve understanding of iPSC translational potential.
Very small embryonic-like stem cells (VSEL)
Another potential cell line for clinical translation are VSELs, a relatively rare cell population in the gonads. VSELs are regarded as a pluripotent and quiescent (during steady state) subpopulation among SSCs, comprising ∼0.03% of testicular cells and sharing numerous PGCLC markers (Ratajczak et al., 2019). In addition to the testes, VSELs can be found in bone marrow and other adult tissues, entering the cell cycle during times of stress (Kassmer and Krause, 2013).
After induction of azoospermia in mice via injection of the alkylating agent busulphan, the VSEL population persists within the testes. However, VSELs did not spontaneously differentiate until allogeneic Sertoli cells were transplanted, suggesting that paracrine signaling between Sertoli cells and the VSEL population may be key for resumption of spermatogenesis. These findings emphasize that supporting cell lines are critical components of a microenvironment in which VSELs can differentiate into germ cell lines capable of in vitro fertilization (IVF) (Anand et al., 2014). VSELs are a potentially valuable autologous source of progenitor cells for spermatogenesis resumption requiring further evaluation.
Mesenchymal stem/progenitor cells (MSC)
MSCs derived from multiple sources have generated germline and non-germline testicular cells, supporting reproductive and hormonal function while providing microenvironment mediation. Several studies have demonstrated the potential for targeted MSC differentiation to germ cell lines; like iPSCs the importance of the culture method and conditions cannot be over-emphasized. However, unlike iPSCs most studies noted the use of 2D culture methods. Umbilical cord derived MSCs have been differentiated using varied 2D culture methods. In vitro, cell differentiation to small round cell morphology expressing a range of pre, meitoic, and post-meiotic markers has been observed. When injected into the rete testis of azoospermic mice, transplanted cells migrated to intratubular spaces and differentiated to germline cells. (Shlush et al., 2017; Dissanayake et al., 2018). Adipose derived MSCs have also demonstrated germline differentiation when injected into the rete testis of rats with azoospermia. Particularly promising for future translation, restoration of male rat fertility was demonstrated by production of live offspring (Cakici et al., 2013).
Additional progress has been made in deriving non-germline cell types from MSCs. In a study using umbilical cord derived MSCs to produce male gametes, cells expressing Sertoli-specific markers were observed during in vitro differentiation (Shlush et al., 2017). Additionally, in vitro culture of bone marrow derived MSCs in a cell-specific media resulted in significant expression of 3β-hydroxysteroid, a Leydig cell specific antigen, in differentiated cells (Hou et al., 2016).
Taken together, these studies establish the feasibility of differentiating MSCs into a range of testicular cell types, with the potential to develop both reproductive and hormonal function in infertile animal models. However, only a single study restored fertility using these methods. Recapitulating the testicular microenvironment is critical in establishing the reproductive potential of MSCs for individuals lacking testicular tissue.
Generation of testicular cell lines from 46, XX individuals
In the process of fetal development, primordial gonads were classically described as bi-potential (Adams and McLaren, 2002). In the determination process of gonadal differentiation, the SRY (Sex Determining Region Y) gene plays a major role in testicular development. In fact, manipulating genes within the SRY pathway can result in 46, XX primordial gonads that develop a testis-like structure (Ottolenghi et al., 2007). A more recent study examined whether testicular tissue development and spermatogenesis could be induced in mice without the presence of a Y chromosome. Transgenic modifications of SRY, SOX9 (SRY-Box Transcription Factor), and Eif2s3x (eukaryotic initiation factor), homologs for SRY on the X chromosome resulted in mice with evidence of spermatogenesis despite absence of the Y chromosome. One of the modifications yielded spermatids that enabled production of live offspring (Yamauchi et al., 2016), indicating the potential of genetic modifications to change the fate of PGCs from 46, XX individuals.
While there have been no such studies using human tissues, investigation of genetic mechanisms in individuals with conditions resulting in differences in sexual development likewise indicate the importance of SRY and downstream effector genes on testicular parenchyma development (Wein, et al., 2012). SOX10 overexpression (Polanco et al., 2010) and R-spondin (Rspo1) frameshift mutation (Parma et al., 2006) are examples of such genes observed as drivers of male phenotype in 46, XX individuals.
Recreating the testicular microenvironment: Biologic and structural factors
Introduction to the use of scaffolds in testicular tissue engineering using non-testicular cell lines
A potential regenerative option for those lacking functional native spermatogenesis is to populate a 3-dimensional (3D) construct consisting of either a biologic or synthetic scaffold with select cell lines. In the first section the outcomes of recellularization of biologic scaffolds with stem cells will be discussed; the subsequent section will discuss advancements in synthetic scaffold engineering. Study details are included in Table 2.
The role of the extracellular matrix (ECM) as a culture substrate
As in other areas of tissue engineering, one strategy to create an optimal microenvironment has been to use testicular ECM with or without additional scaffolding, thereby maintaining its critical growth factors and additional protein components present (Siu and Cheng, 2004). When applied in vitro, human and porcine SSCs cultured on decellularized human ECM indeed provided a microenvironment that successfully maintained human germline progenitor cells (Murdock et al., 2019).
Not only does the ECM affect maintenance of spermatogonial cell lines; it also influences cellular differentiation and maturation. In a study comparing the culture of human SSCs on a decellularized sheep testis membrane versus 2D culture media, cells cultured on the biologic scaffold expressed higher levels of pre-meiotic, meiotic, and post-meiotic differentiation markers than cell lines maintained in culture media alone (Movassagh et al., 2020). In addition to growth and differentiation of SSCs, researchers have studied attachment and proliferation of Sertoli cells using decellularized testicular ECM. In this setting, Sertoli cells maintained viability on decellularized porcine testicular ECM and demonstrated attachment, proliferation, and orthotopic organization on the biologic scaffold. A decellularized animal model such as this one may be an attractive option for translational use, as it does not require human donor organs (Vermeulen et al., 2018). Further studies are needed to optimize ECM decellularization and to improve understanding of the organization and cellular behavior of each component of the healthy testis in an in vitro environment.
To demonstrate biocompatibility and feasibility of biologic scaffolds for in vivo implantation, decellularized rat testicular ECM was cultured with bone marrow derived MSCs followed by transplantation to several different anatomic locations within the rat (renal subcapsular, subcutaneous tissue, liver, and mesentery). Constructs implanted into liver and mesentery remained intact with demonstrated biocompatibility. Additionally, vascularized constructs contained multiple classes of non-germline testicular cells. This supports the potential to use in vivo models as functional bioreactors to achieve cellular differentiation of non-germline testicular cells (Kargar-Abarghouei et al., 2018).
De novo synthetic scaffolds in generation of testicular parenchymal components
While decellularized tissue matrices can provide a microenvironment conducive to differentiation and propagation of testicular germline and non-germline cells, future clinical implementation of decellularized tissue is limited by the challenge of scaling this technology for high volume tissue production. An alternative approach is to develop and apply de novo synthetic, polymer-based biomaterial scaffolds to support select cell lines. The inherent reproducibility and scalability make synthetic scaffold technology an attractive alternative.
An example of a hybrid biomaterial synthesized scaffold developed to support testosterone delivery was created by seeding a poly-l-lactic acid (PLLA) coated polyglycolic acid (PGA) polymer with chondrocytes to support cartilage development. Following an initial culture period, the scaffold was loaded with testosterone enanthate. The scaffold sustained hormonal elution in vitro and in vivo, thereby demonstrating the potential to apply scaffolds to retain physiologic levels of intratesticular testosterone. Such hormonal-structural microenvironments will be critical to increase functionality and efficiency of spermatogenesis. (Raya-Rivera et al., 2008). Another study aimed to investigate the role of a growth factor during testicular tissue formation and spermatogenesis in a synthetic scaffold. Varied concentrations of germline cells were seeded onto a Matrigel scaffold with and without added vascular endothelial growth factor (VEGF-165), followed by subsequent implantation into castrate immunocompromised mice. Due to increased tubules with spermatogonia in growth factor containing constructs, this experiment led the authors to conclude that VEGF may have a protective role against transient hypoxia during testicular tissue formation and spermatogenesis (Dores and Dobrinski, 2014).
Further studies using Matrigel-based scaffolds indicated that these findings are not isolated to animal cell lines. Human SSCs differentiated into spermatids when cultured on a 3D scaffold. These spermatids then fertilized isolated mouse oocytes via ICSI (Sun et al., 2018). Taken together, synthetic scaffolds can significantly enhance the function of hybrid constructs and may play a role in future restoration of both endocrine and reproductive function for individuals lacking native functional testicular tissue.
Discussion
In vitro spermatogenesis has been a topic of growing interest in the field of stem cell biology. As in many areas of tissue engineering, success in early studies of induced spermatogenesis have been defined by their recapitulation of key components of the structure and biology of the testicular microenvironment. However, re-creating the in vivo milieu using synthetic approaches is a considerable challenge. The research studies evaluated in this mini-review focused on progress toward establishment of the testicular niche, resulting in differentiated germline and non-germline testicular cell lines derived from non-testicular cells. The ultimate goal of this work is to provide a new option for individuals who desire fertility consistent with a male gender identity without the requirement of testicular tissue.
The studies presented vary in key components that can inform future directions in testicular tissue engineering. One is their use of 46, XY progenitor cell line selection. iPSCs have been extensively studied and demonstrate particular promise, and this is anticipated regardless of 46, XX versus 46, XY source. Varied sources may however have divergent differentiation potential and this does require further evaluation. VSELs have high differentiation potential but are limited by relative scarcity and uncertainty regarding their reproductive potential. MSCs are an attractive option due to their ease of isolation and ubiquitous presence in adults. However, further evaluation of relative cell line source does require evaluation. Although future studies are needed, MSCs may be a feasible and optimal translational candidate, particularly as genetic alterations required by iPSCs may not be required in these cell lines. Future studies are needed to evaluate the use of 46, XX cell lines and the genetic alterations that may be required to generate each component of testicular parenchyma in an autologous manner using these sources.
Another critical aspect by which these studies varied was in cell and tissue culture practices, including their structural and biologic environments. Key areas of variation included the use of 2D or 3D culture, culture methods (e.g air-liquid interface, drop culture, organoids), media selection and steps in its use, and the role that cell-cell interactions and the secretome of each cell in culture may have had in co-culture studies. Additionally, the epigenome of cells impacts the yield of their differentiation to higher levels and using pharmaco-epigenetic agents, the differentiation yield can be significantly influenced (Ishikura et al., 2021). Finally, it is important that the substrate and/or scaffold on which cells are differentiated recapitulates key components and structure of ECM, with multiple aspects requiring future research into their independent and combined effects (Smith and Ma, 2004).
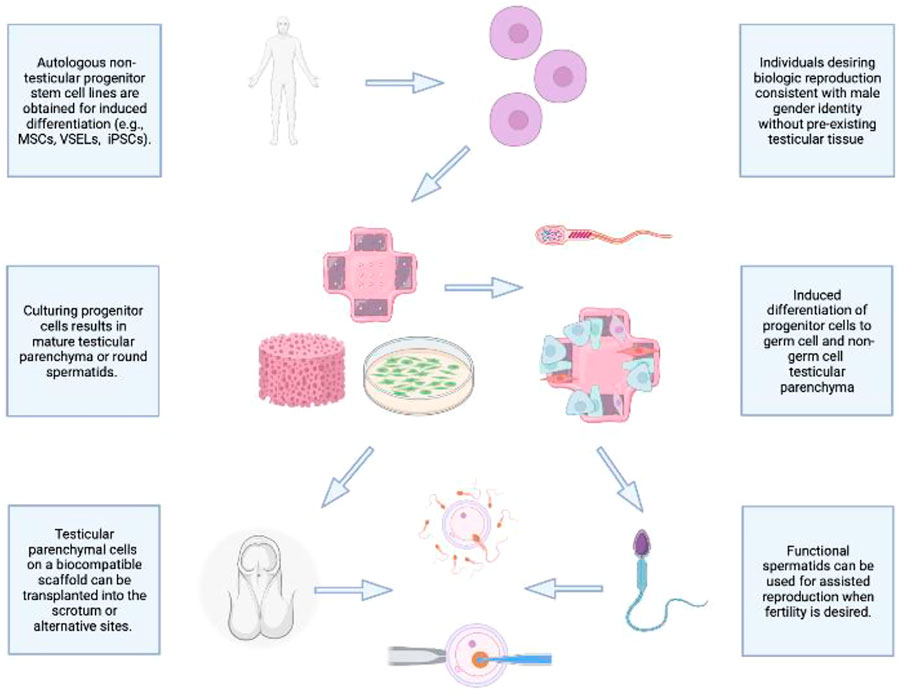
As demonstrated in Figure 1, an ideal future prospect would be to combine the best performing cell line and scaffold-based culture in a manner that supports prolonged biologic function following cellular differentiation. The priority will be to achieve reproducible, efficient in vitro generation of fully differentiated haploid gametes from a non-testicular progenitor cell line derived from 46, XX or 46, XY individuals. To achieve this, seeding in a 3D environment using a scaffold that emulates key components of the healthy testis microenvironment and structure will be invaluable, including promotion of cell-cell interactions. The resulting structure can be adapted for auto-transplantation, thereby supporting gametogenesis and endocrine functions of a healthy testis in an autologous, biocompatible manner. While this prospect is currently out of reach, several key independent functions of the ultimate structure have been demonstrated, including maintenance of testosterone physiological concentration, cell differentiation of progenitor cells into both germline and non-germline cells using a biological scaffold, and testis-like structural support generation for progenitor cells transplanted into host animals. As this work proceeds, it will be key to evaluate each step for its potential short- and long-term effects on epigenetic and genetic stability and transmission for effective and safe biologic reproduction for all individuals, regardless of gonadal presence or gender identity.
Author contributions
HH, CD, and RS contributed to conception and design of the study. All authors wrote and edited sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMP4, Bone Morphogenic Protein 4; c-MYC, c-Myelocytomatosis Oncogene; CREM, cAMP Responsive Element Modulate; DAZL, Deleted in Azoospermia-Like; DMEM, Dulbecco’s Modified Eagle Medium; EB, Embryoid Body; ECM, Extracellular Matrix; EGF, Epidermal Growth Factor; EGFP, E-green Fluorescent Protein; Eif, Eukaryotic Initiation Factor; ESC, Embryonic Stem Cell; FBS, Fetal Bovine Serum; FGF, Fibroblast Growth Factor; FSH, Follicle Stimulating Hormone; FTM, First Trimester; GDNF, Glial-Derived Neurotrophic Factor; GFP, Green Fluorescent Protein; GSC, Gonadal Stem Cell; GSCLC, Gonadal Stem Cell-like Cells; HCG, Human Chorionic Gonadotropin; HEPES, 4-(2-Hydroxyethyl)-1- Piperazineethanesulfonic Acid; HFF, Human Foreskin Fibroblasts; HMG, Human Menopausal Gonadotropin; HSD, Hydroxysteroid Dehydrogenase; ICR, Institute of Cancer Research; ICSI, Intracytoplasmic Sperm Injection; iPSC, Induced Pluripotent Stem Cells; IVF, In vitro fertilization; KLF, Kruppel-like Factor; LH, Luteinizing Hormone; LIF, Leukemia Inhibitory Factor; MEF, Mouse Embryonic Fibroblasts; MSC, Mesenchymal Stem/Progenitor Cells, MVH: Mouse VASA Homologue; OCT-4, Octamer Binding Protein 4; PDGF, Platelet Derived Growth Factor; PGA, Polyglycolic Acid; PGC, Primordial Germ Cell; PGCLC, Primordial Germ Cell-like Cells; PLLA, Poly-l-lactic Acid; PLZF, Promyelocytic Leukemia Zinc Finger; PRM1, Protamine 1; PSC, Pluripotent Stem Cells; PVC, Perivascular Cells; RA, Retinoic Acid; Rspo1, R-spondin; SCF, Stem Cell Factor; SCP3, Single Cell Protein 3; SDS, Sodium Dodecil Sulfate; SFM, Serum Free Media; SOX, Sry-related HMG box; SRY, Sex Determining Region Y; SSC, Spermatogonial Stem Cells; VEGF, Vascular Endothelial Growth Factor; VSEL, Very Small Embryonic-Like Stem Cells.
References
Adams, I. R., and McLaren, A. (2002). Sexually dimorphic development of mouse primordial germ cells: Switching from oogenesis to spermatogenesis. Development 129 (5), 1155–1164. doi:10.1242/dev.129.5.1155
Agarwal, A., Mulgund, A., Hamada, A., and Chyatte, M. R. (2015). A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 13 (1), 37–39. doi:10.1186/s12958-015-0032-1
Anand, S., Bhartiya, D., Sriraman, K., Patel, H., and Manjramkar, D. D. (2014). Very small embryonic-like stem cells survive and restore spermatogenesis after busulphan treatment in mouse testis. J. Stem Cell Res. Ther. 4, 216. doi:10.4172/2157-7633.1000216
Bhaskar, R., Gupta, M. K., and Han, S. S. (2022). Tissue engineering approaches for the in vitro production of spermatids to treat male infertility: A review. Eur. Polym. J. 174, 111318. doi:10.1016/j.eurpolymj.2022.111318
Cai, H., Xia, X., Wang, L., Liu, Y., He, Z., Guo, Q., et al. (2013). In vitro and in vivo differentiation of induced pluripotent stem cells into male germ cells. Biochem. Biophys. Res. Commun. 433 (3), 286–291. doi:10.1016/j.bbrc.2013.02.107
Cakici, C., Buyrukcu, B., Duruksu, G., Haliloglu, A. H., Aksoy, A., Isık, A., et al. (2013). Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: The sperm generation. Biomed. Res. Int. 2013, 529589. doi:10.1155/2013/529589
Champy, C. H. (1920). De la méthode de culture des tissus. VI. Le testicule. Arch. Zool. Exptl Gen. 60, 461–500.
Dissanayake, D. M. A. B., Patel, H., and Wijesinghe, P. S. (2018). Differentiation of human male germ cells from Wharton's jelly-derived mesenchymal stem cells. Clin. Exp. Reprod. Med. 45 (2), 75–81. doi:10.5653/cerm.2018.45.2.75
Dores, C., and Dobrinski, I. (2014). De novo morphogenesis of testis tissue: An improved bioassay to investigate the role of VEGF165 during testis formation. Reproduction 148 (1), 109–117. doi:10.1530/REP-13-0303
Doss, M. X., and Sachinidis, A. (2019). Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 8 (5), 403. doi:10.3390/cells8050403
Easley IV, C. A., Phillips, B. T., McGuire, M. M., Barringer, J. M., Valli, H., Hermann, B. P., et al. (2012). Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2 (3), 440–446. doi:10.1016/j.celrep.2012.07.015
Hou, L., Dong, Q., Wu, Y. J., Sun, Y. X., Guo, Y. Y., and Huo, Y. H. (2016). Gonadotropins facilitate potential differentiation of human bone marrow mesenchymal stem cells into Leydig cells in vitro. Kaohsiung J. Med. Sci. 32 (1), 1–9. doi:10.1016/j.kjms.2015.10.008
Ishikura, Y., Ohta, H., Sato, T., Murase, Y., Yabuta, Y., Kojima, Y., et al. (2021). In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell 28 (12), 2167–2179.e9. doi:10.1016/j.stem.2021.08.005
Ishikura, Y., Yabuta, Y., Ohta, H., Hayashi, K., Nakamura, T., Okamoto, I., et al. (2016). In vitro derivation and propagation of spermatogonial stem cell activity from mouse pluripotent stem cells. Cell Rep. 17 (10), 2789–2804. doi:10.1016/j.celrep.2016.11.026
Kapałczyńska, M., Kolenda, T., Przybyła, W., Zajączkowska, M., Teresiak, A., Filas, V., et al. (2018). 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Arch. Med. Sci. 14 (4), 910–919. doi:10.5114/aoms.2016.63743
Kargar-Abarghouei, E., Vojdani, Z., Hassanpour, A., Alaee, S., and Talaei-Khozani, T. (2018). Characterization, recellularization, and transplantation of rat decellularized testis scaffold with bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 9 (1), 1–16. doi:10.1186/s13287-018-1062-3
Kassmer, S. H., and Krause, D. S. (2013). Very small embryonic-like cells: Biology and function of these potential endogenous pluripotent stem cells in adult tissues. Mol. Reprod. Dev. 80 (8), 677–690. doi:10.1002/mrd.22168
Movassagh, S. A., Movassagh, S. A., Dehkordi, M. B., Pourmand, G., Gholami, K., Talebi, A., et al. (2020). Isolation, identification and differentiation of human spermatogonial cells on three-dimensional decellularized sheep testis. Acta Histochem. 122 (8), 151623. doi:10.1016/j.acthis.2020.151623
Murdock, M. H., David, S., Swinehart, I. T., Reing, J. E., Tran, K., Gassei, K., et al. (2019). Human testis extracellular matrix enhances human spermatogonial stem cell survival in vitro. Tissue Eng. Part A 25 (7-8), 663–676. doi:10.1089/ten.TEA.2018.0147
Ottolenghi, C., Pelosi, E., Tran, J., Colombino, M., Douglass, E., Nedorezov, T., et al. (2007). Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum. Mol. Genet. 16 (23), 2795–2804. doi:10.1093/hmg/ddm235
Parma, P., Radi, O., Vidal, V., Chaboissier, M. C., Dellambra, E., Valentini, S., et al. (2006). R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 38 (11), 1304–1309. doi:10.1038/ng1907
Polanco, J. C., Wilhelm, D., Davidson, T. L., Knight, D., and Koopman, P. (2010). Sox10 gain-of-function causes XX sex reversal in mice: Implications for human 22q-linked disorders of sex development. Hum. Mol. Genet. 19 (3), 506–516. doi:10.1093/hmg/ddp520
Ratajczak, M. Z., Ratajczak, J., and Kucia, M. (2019). Very small embryonic-like stem cells (VSELs). Circ. Res. 124 (2), 208–210. doi:10.1161/CIRCRESAHA.118.314287
Raya-Rivera, A. M., Baez, C., Atala, A., and Yoo, J. J. (2008). Tissue engineered testicular prostheses with prolonged testosterone release. World J. Urol. 26 (4), 351–358. doi:10.1007/s00345-008-0267-y
Robinson, M., Witherspoon, L., Willerth, S., and Flannigan, R., 2021. A novel organoid model of in vitro spermatogenesis using human induced pluripotent stem cells. Preprint bioRxiv. doi:10.1101/2021.06.04.447122
Shlush, E., Maghen, L., Swanson, S., Kenigsberg, S., Moskovtsev, S., Barretto, T., et al. (2017). In vitro generation of Sertoli-like and haploid spermatid-like cells from human umbilical cord perivascular cells. Stem Cell Res. Ther. 8 (1), 37–16. doi:10.1186/s13287-017-0491-8
Siu, M. K., and Cheng, C. Y. (2004). Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays 26 (9), 978–992. doi:10.1002/bies.20099
Smith, L. A., and Ma, P. X. (2004). Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B 39 (3), 125–131.
Sun, M., Yuan, Q., Niu, M., Wang, H., Wen, L., Yao, C., et al. (2018). Efficient generation of functional haploid spermatids from human germline stem cells by three-dimensional-induced system. Cell Death Differ. 25 (4), 749–766. doi:10.1038/s41418-017-0015-1
Vermeulen, M., Del Vento, F., De Michele, F., Poels, J., and Wyns, C. (2018). Development of a cytocompatible scaffold from pig immature testicular tissue allowing human sertoli cell attachment, proliferation and functionality. Int. J. Mol. Sci. 19 (1), 227. doi:10.3390/ijms19010227
Wein, A. J., Kavoussi, L. R., Campbell, M. F., and Walsh, P. C. (2012). “Disorders of sexual development: Etiology, evaluation and medical management,” in Campbell-walsh urology. 10th edn. (Philadelphia, PA: Elsevier Saunders), 3471.
Yamauchi, Y., Riel, J. M., Ruthig, V. A., Ortega, E. A., Mitchell, M. J., and Ward, M. A. (2016). Two genes substitute for the mouse Y chromosome for spermatogenesis and reproduction. Science 351 (6272), 514–516. doi:10.1126/science.aad1795
Keywords: male infertility, fertilty, tissue engineering and regenerative medicine, tissue engineering scaffold materials, disorders in sex differentiation, gender affirmation treatment, germline cells, somatic stem cells (SSCs)
Citation: Hosseini H, DeBenedetto C, Eleswarapu SV, Ng G and Sturm RM (2022) De novo testicular tissue generation from non-testicular cell lines, biologic and synthetic scaffolds: Current findings and future translational applications. Front. Cell Dev. Biol. 10:954196. doi: 10.3389/fcell.2022.954196
Received: 27 May 2022; Accepted: 26 September 2022;
Published: 02 November 2022.
Edited by:
Ruttachuk Rungsiwiwut, Srinakharinwirot University, ThailandReviewed by:
Rakesh Bhaskar, Yeungnam University, South KoreaBo Zheng, Nanjing Medical University, China
Copyright © 2022 Hosseini, DeBenedetto, Eleswarapu, Ng and Sturm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renea M. Sturm, cnN0dXJtQG1lZG5ldC51Y2xhLmVkdQ==
 Helia Hosseini
Helia Hosseini Christina DeBenedetto1
Christina DeBenedetto1 Sriram V. Eleswarapu
Sriram V. Eleswarapu Gladys Ng
Gladys Ng Renea M. Sturm
Renea M. Sturm