
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol., 20 June 2022
Sec. Cell Growth and Division
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.931115
This article is part of the Research TopicEditors' Showcase 2021: Insights in Cell Growth and DivisionView all 11 articles
Ubiquitin Binding Protein 2-like (UBAP2L, also known as NICE-4) is a ubiquitin- and RNA-binding protein, highly conserved in metazoans. Despite its abundance, its functions have only recently started to be characterized. Several studies have demonstrated the crucial involvement of UBAP2L in various cellular processes such as cell cycle regulation, stem cell activity and stress-response signaling. In addition, UBAP2L has recently emerged as a master regulator of growth and proliferation in several human cancers, where it is suggested to display oncogenic properties. Given that this versatile protein is involved in the regulation of multiple and distinct cellular pathways, actively contributing to the maintenance of cell homeostasis and survival, UBAP2L might represent a good candidate for future therapeutic studies. In this review, we discuss the current knowledge and latest advances on elucidating UBAP2L cellular functions, with an aim to highlight the importance of targeting UBAP2L for future therapies.
Ubiquitin Associated Protein 2-Like (UBAP2L) or NICE-4 is a highly conserved protein in vertebrates (Chang et al., 2018). Encoded by the KIAA0144 gene located on the chromosomal region 1q21, NICE-4 was originally identified by Marenholz and colleagues in an effort to discover new Human Epidermal Differentiation Complex (EDC)-encoded genes (Marenholz et al., 2001). Five different isoforms produced by alternative splicing have been reported for UBAP2L, that are broadly expressed in nearly all tissues. Despite its abundant expression, UBAP2L has only recently attracted attention of broad scientific community which led to the discovery of its highly versatile roles. Interestingly, UBAP2L orthologs have been identified in metazoans such as Prion-like (Q/N-rich)-domain-bearing protein (PQN-59) in Caenorhabditis elegans and lingerer in Drosophila melanogaster (Uhlén et al., 2015).
UBAP2L is a 1,087 amino-acid (aa)-long protein, structurally composed of a N-terminal Ubiquitin-Associated Domain (UBA; aa 49-89), an Arginine-Glycine-Glycine (RGG; aa 131-190) domain and three predicted RNA-Binding regions (aa 239-257, aa 282-290 and aa 850-864) (Castello et al., 2016) (Figure 1). SILAC analysis demonstrated that UBAP2L cofractionates with ubiquitin in aggregates following proteasomal inhibition, emphasizing the functionality of its UBA domain (Wilde et al., 2011). Moreover, ribosome profiling studies demonstrated that UBAP2L promotes translation of target mRNAs suggesting that it can act as a ribosome-binding protein essential for protein synthesis (Luo et al., 2020). In addition, UBAP2L harbors a Domain of Unknown Function (DUF; aa 495-526). Prediction tools have unraveled several disordered regions prone to undergo Liquid-Liquid Phase Separation (LLPS) as well as several Nuclear Localization Signals (NLS) and Nuclear Export Signals (NES), suggesting that UBAP2L is shuttling between the cytoplasm and the nucleus. Such atypical domain organization classifies UBAP2L in both Ubiquitin-binding and RNA-binding proteins superfamilies, highlighting its potential involvement in a plethora of cellular processes.
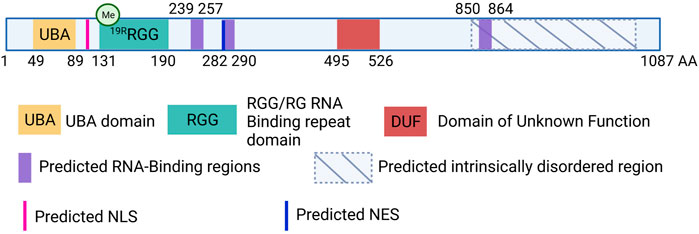
FIGURE 1. Schematic view of the human UBAP2L protein and its domain organization. UBAP2L (1087 AA) is composed of a Ubiquitin-associated domain (UBA, yellow), an Arginine-Glycine-Glycine (RGG, green) domain and a Domain of Unknow Function (DUF, red). Additional RNA-binding regions have been predicted and are painted in purple. Moreover, UBAP2L is predicted to contain several Nuclear Localization Signals (NLS) and Nuclear Export Signals (NES) (pink and dark blue respectively). Several UBAP2L regions have been proposed to be intrinsically disordered (IDR), and prone to liquid-liquid phase-separation. The most conserved ones are shown with hatched lines. It is important to note that for simplicity we chose to depict only some of the predicted NLS, NES and IDR regions of UBAP2L in the scheme, and that this does not exclude the existence of other similar motifs or regions. Similarly, documented methylation modification on 19 different arginines (19R) present within the RGG domain has been indicated schematically.
Although UBAP2L was initially described as an interactor of the Human Zona Pellucida Sperm-binding protein 3 (ZP3) (Naz and Dhandapani, 2010), during the last decade additional studies have demonstrated its direct involvement in cell growth, mitotic progression, stem cell activity, apoptosis and stress response signaling (Bordeleau et al., 2014; Li and Huang, 2014; Chai et al., 2016; Maeda et al., 2016; Youn et al., 2018; Huang et al., 2020). Moreover, UBAP2L is overexpressed in different types of cancer, displaying oncogenic potential and often correlating with poor prognosis (Li and Huang, 2014; Zhao et al., 2015; Bai et al., 2016; Chai et al., 2016; Aucagne et al., 2017; He et al., 2018; Yoshida et al., 2020; Guan et al., 2021). Of note, UBAP2L KO mice die before birth or within minutes after surgical delivery from acute respiratory failure, demonstrating that UBAP2L holds housekeeping functions, essential for living organisms (Aucagne et al., 2017). This review discusses the current knowledge and the latest advances on elucidating NICE-4 cellular functions, with an aim to highlight the importance of targeting NICE4 for future therapies.
As mentioned above, UBAP2L KO mice die prematurely, pointing to a potential role for UBAP2L during development. Interestingly, in C. elegans, PQN-59 has been shown to modulate gene expression thus playing a key role in cell fate specification during development (Carlston et al., 2021). In an embryo, undifferentiated cells, called stem cells, give rise to one or several types of differentiated cells which later form mature tissues and organs. UBAP2L was proposed to be modified by O-Linked N-Acetylglucosamine (O-Glc-NAc) in mouse MC3T3E1 differentiating osteoblasts (Nagel et al., 2013). Interestingly, UBAP2L is found enriched in osteoblasts and as such it is used as an osteoblast marker (Guan et al., 2021). More globally, UBAP2L expression is increased in other types of undifferentiated cells such as mouse and human hematopoietic and leukemic stem cells. In the above study, Bordeleau and colleagues propose a model in which UBAP2L forms a complex with the Polycomb group (PcG) proteins BMI1 and Ring Finger Protein 2 (RNF2), thereby regulating long-term repopulating hematopoietic stem cells (LT-HSCs) independently of Ink4a/Arf locus repression, a popular target of BMI1. The authors suggest that at least two Polycomb-repressive complexes can assemble in order to regulate HSC function, which are distinguishable by the presence or the absence of UBAP2L (Bordeleau et al., 2014). Further investigations are needed in order to elucidate UBAP2L’s precise role as part of the Polycomb complex since the exact mechanism has not been fully understood yet. A partial answer has been provided by Lin et al. who used rat bone marrow mesenchymal stem cells (BMSCs) overexpressing UBAP2L to transplant it to rats suffering from semi-sectioned spinal cord injury (SPI) and to monitor the recovery of the injured tissue (Lin et al., 2018). UBAP2L overexpressing cells exhibited stronger neuronal differentiation potential, which led to faster spinal cord function recovery. Mechanistically, UBAP2L overexpression results in increased expression of the cell cycle related protein cyclin D1 and of p38 MAPK, and more importantly to decreased expression of Caspase 3, a key apoptotic factor responsible for the majority of post-SCI neuronal death (Yu and Fehlings, 2011). Overall, the authors propose that UBAP2L overexpression in BMSCs promotes neuronal proliferation and survival, limits contingent damage like post-SCI inflammation and eventually leads to SCI repair (Lin et al., 2018). Given that the UBAP2L locus has been associated with other neuronal disorders such as bipolar or anorexia nervosa disorders (eQTLGen Consortium et al., 2019; Iranzo-Tatay et al., 2022), it would be of great interest to further investigate its potential role in the development of other neurological and aging-related neurodegenerative diseases.
In eukaryotes, mitosis is a crucial process which needs to be tightly regulated in time and space to allow for faithful division of a mother cell into two identical daughter cells (McIntosh, 2016). UBAP2L has been proposed to regulate cell division. Its depletion impairs chromosome alignment during metaphase and potentiates Spindle Assembly Checkpoint (SAC) response. Chromosome misalignment phenotypes upon UBAP2L depletion occur due to the disruption of stable k-fibers, suggesting defects in proper microtubule-kinetochore (MT-KT) attachment, which in turn hinders proper chromosome segregation and mitosis completion (Maeda et al., 2016). Maeda and colleagues further showed that UBAP2L RGG/RG domain is responsible for the multi- and micronucleation phenotypes observed in UBAP2L downregulated HeLa cells and more importantly that this function is mediated by the methylation of the arginines within the RGG/RG domain by the methyl-transferase PRMT1. Although the construct lacking this post-translational modification is properly localized at the spindle, it cannot rescue chromosome misalignment during metaphase observed in UBAP2L depleted cells suggesting that UBAP2L RGG/RG domain methylation is essential for proper MT-KT attachments, accurate chromosome distribution and proper mitotic progression. Consistently, UBAP2L depletion leads to an enrichment of G2/Mitotic (G2/M) population in HeLa cells (Maeda et al., 2016), in ZR-75-30 and in T-47D breast cancer cells (He et al., 2018) and in DU145 prostate cancer cells (Li and Huang, 2014) pointing to an important role of UBAP2L as a cell cycle regulator.
An interesting feature of UBAP2L protein is its ability to aggregate and to regulate protein synthesis as indicated above (Wilde et al., 2011; Luo et al., 2020). mRNA turnover and protection under stress conditions have been associated with the formation of Stress Granules (SG) (Parker and Sheth, 2007). In an attempt to identify new components and/or regulators of cytosolic RNA granules, Youn and colleagues performed proximity-based proteomics and identified UBAP2L as a critical factor for efficient SG assembly following stress induced by the arsenite treatment. Importantly, the DUF domain of UBAP2L containing an phenylalanine-glycine phenylalanine-glycine (FG-FG) motif is critical for G3BP1 (Ras GTPase-activating protein-binding protein 1) recognition and binding in flies (Baumgartner et al., 2013) and is responsible for G3BP1 assembly in HeLa cells. In contrast, UBA and RGG domains of UBAP2L seem to be dispensable for SG formation (Youn et al., 2018). Subsequent studies by another group demonstrated the crucial role of the RGG domain of UBAP2L for SG competence under stress-null and stress conditions (Huang et al., 2020). More precisely, under stress conditions, UBAP2L methylation by PRMT1 is decreased, enabling UBAP2L’s interaction with SG components and subsequently promoting SG assembly. The authors show that UBAP2L’s DUF domain is still very important for G3BP1/2 NTF2-like domain binding and localization. In fact, depletion of the DUF domain promotes UBAP2L shuttling from the cytoplasm to the nucleus, impeding its interaction with G3BP1/2 and consecutively abolishes SG formation (Huang et al., 2020). Further work from Gotta group, propose that UBAP2L forms SG cores to which G3BP1 is subsequently recruited to allow for SG maturation, suggesting that UBAP2L acts upstream of G3BP1 in SG nucleation (Cirillo et al., 2020). Intriguingly, this phenomenon seems to be specific to human cells as a recent study from the same group established that PQN-59 and GTBP-1 (the human UBAP2L and G3BP1/2 orthologs respectively) are not essential for SG assembly in C. elegans (Abbatemarco et al., 2021). Interestingly, additional types of subcellular complexes can be assembled under stress conditions. Among them, the nuclear “twins” of SG are called paraspeckles (PS). These ribonucleoproteins (RNP) granules assemble around the long noncoding RNA (lncRNA) NEAT1 (Fox et al., 2018). Upon stress induction, SGs regulate PSs assembly via the sequestration of important negative regulators of PS formation such as UBAP2L (An et al., 2019). For the moment, we still lack sufficient knowledge to explain the molecular mechanism behind this regulation and it would be important to understand if and how UBAP2L acts as a global regulator of stress-induced complex assemblies, in addition to its well-established role in SGs.
Recent work has demonstrated that UBAP2L is overexpressed in a variety of cancers and as such it has gained significant attention of researchers over the past years. Although its aberrant expression is a common feature of very different types of tumors, the way UBAP2L acts to promote carcinogenesis appears to be highly variable (Figure 2), highlighting UBAP2L’s versatile functions not only in healthy tissues but also under pathological conditions. As mentioned above, UBAP2L is broadly expressed in almost all tissues. Likewise, this abundance is also found and exacerbated in distinct tumor types such as prostate, breast, uterine, cervical, non-small cell lung and gastric cancers, glioma, colorectal and hepatocellular carcinoma (HCC) and lung adenocarcinoma (Li and Huang, 2014; Zhao et al., 2015; Bai et al., 2016; Chai et al., 2016; Aucagne et al., 2017; Wang et al., 2017; Ye et al., 2017; He et al., 2018; Li et al., 2018; Lin et al., 2018; Pan et al., 2020; Yoshida et al., 2020; Guan et al., 2021; Yang et al., 2021; Li et al., 2022). In nearly all cited cancer studies, UBAP2L is suggested to act as an oncogene promoting cancer cell proliferation and growth in vitro and in vivo, thus providing an explanation to the existing negative correlation between UBAP2L expression and patients’ prognosis.
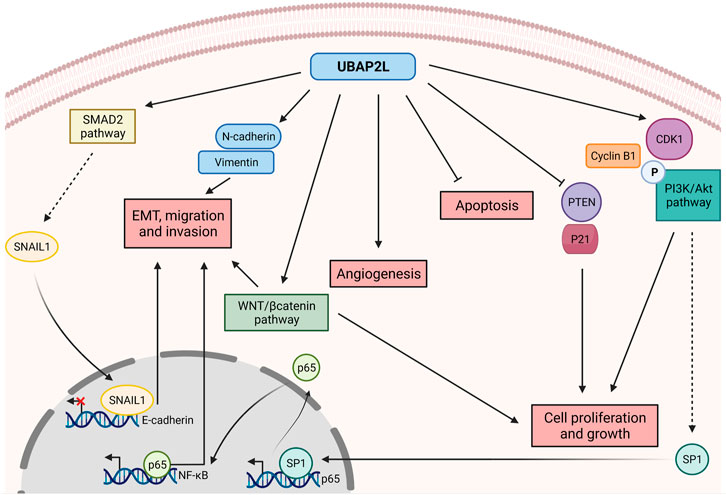
FIGURE 2. Versatile roles of UBAP2L in promoting cancer disease. UBAP2L upregulates key cell cycle regulators such as CyclinB1, CDK1 and the PI3K/Akt pathway, while it inhibits the expression of tumor suppressors such as PTEN and P21, thereby promoting cell proliferation and growth. PI3K/Akt activation enhances SP1 levels which in turn activates P65 expression, thereby activating NF-κB pathway and favoring epithelial-mesenchymal transition (EMT), migration and invasion. The metastatic potential of UBAP2L-overexpressing cells is also sustained by the activation of the SMAD2 pathway, triggering the transcriptional repressor SNAIL1 to the E-cadherin promoter, shutting down its expression. Cancer cells overexpressing UBAP2L are characterized by hyperactivation of the WNT/βcatenin pathway and by upregulation of mesenchymal factors such as N-cadherin and Vimentin, resulting in increased invasion and proliferation. Finally, UBAP2L favors tumor vascularization while inhibiting cancer cells apoptosis. Overall, UBAP2L promotes cancer progression by regulating various axes of tumorigenesis known as the hallmarks of cancer.
In prostate, breast cancers and HCC, UBAP2L depletion leads to an accumulation of G2/M cell population (Li and Huang, 2014; He et al., 2018; Li et al., 2018), whereas it was shown to increase the G0/G1 cells rate in Glioma and colorectal carcinoma, suggesting that UBAP2L may act during several cell cycle stages (Zhao et al., 2015; Chai et al., 2016). Additionally, UBAP2L is responsible for the multifaceted regulation of tumors’ cellular and molecular properties in order to promote cellular survival as well as migration. Compelling evidence suggests that oncogenic pathways rely on the establishment of a suitable micro-environment that provides nutrients and supports tumor development and survival as elegantly summarized in 2011 (Hanahan and Weinberg, 2011). Intriguingly, UBAP2L seems to be involved in the regulation of several hallmarks of cancer.
Firstly, as mentioned above, UBAP2L sustains cell proliferation potentially via the regulation of cell cycle signaling pathways. For instance, it has been observed that knockdown of UBAP2L increases p21 and decreases CDK1 and CyclinB1 expression in breast cancer cells (He et al., 2018). This observation was further confirmed in HCC in a study showing a gene enrichment analysis after UBAP2L depletion. As previously demonstrated, the authors found PTEN and p21 among the most upregulated genes, while CDK1, CyclinB1, p-PI3K and p-AKT were among the most downregulated genes following UBAP2L silencing (Li et al., 2018). The signaling pathways downstream of PTEN, TP53 and PI3K/Akt are commonly dysregulated and hijacked in cancerous cells in order to promote their growth as extensively reviewed in the past years (Hollander et al., 2011; Khemlina et al., 2017; Levine, 2020). Of particular interest, the PI3K/Akt pathway is implicated in a broad range of cellular processes including cell proliferation but also apoptosis, angiogenesis, replicative immortality, invasion and metastasis, pointing out to UBAP2L oncogene as a golden target for future anti-cancer therapies (Lien et al., 2017). The molecular mechanism of how UBAP2L might regulate the PI3K/Akt pathway can be partially explained by a study suggesting that UBAP2L activates the PI3K/Akt pathway by promoting a phosphorylation cascade which in turn triggers SP1 binding to P65 promoter, inducing its expression. UBAP2L enables P65 translocation into the nucleus and possibly activates NF-KB (Li et al., 2022), a pathway strongly associated to cancer progression (Zinatizadeh et al., 2021). However, further efforts are required in order to dissect how UBAP2L precisely regulates signaling pathways to enable cancer progression.
An additional common feature of cancer cells is the ability to undergo epithelial-mesenchymal transition (EMT) as a means to promote effective invasion and metastasis (Hanahan and Weinberg, 2011). Interestingly, wound-healing assays of HCC cells lacking UBAP2L, revealed defects in migration and invasion. Consistently, cells lacking UBAP2L harbor increased epithelial (E-cadherin, CK-18) and decreased mesenchymal markers (N-cadherin, vimentin) (Ye et al., 2017), highlighting UBAP2L’s crucial role in regulating the metastatic potential of cancer cells. In addition to HCC, the promotion of EMT by UBAP2L has also been reported in prostate, lung and gastric cancers (Li and Huang, 2014; Aucagne et al., 2017; Lin et al., 2021). Complementary studies verified these conclusions in vivo where inhibition of UBAP2L led to defective cancer invasion in xenografts (Guan et al., 2021). In addition, mice injected with Ubap2l−/− A549 cells show less nodules in their lungs, lighter lungs and increased survival 3 weeks after injection in contrast to mice injected with Ubap2l+/+ A549 cells (Aucagne et al., 2017), while the opposite result is observed in gastric cancer when UBAP2L is overexpressed (Li et al., 2022). Finally, it was recently suggested that UBAP2L positively regulates the expression of the transcriptional repressor SNAIL1 via the SMAD2 signaling pathway which subsequently binds to and inhibits the promoter of E-cadherin, hindering the expression of this epithelial marker in favor of mesenchymal ones, ultimately leading to EMT, invasion and metastasis (Ye et al., 2017).
As previously discussed, cancer cells must use many diverse strategies to escape the cellular surveillance mechanisms in order to survive and migrate. To this end, most of the signaling pathways exploited by normal cells have to be hijacked, to favor cancer progression. For example, components of the Wnt/β-catenin signaling which is a highly conserved pathway regulating fundamental developmental processes, has been frequently observed to be mutated in cancer (Nusse and Clevers, 2017). Not surprisingly, UBAP2L has been proposed to activate the Wnt/β-catenin signaling cascade in gastric cancer cells, leading to the expression of downstream pathway targets, known to be implicated in tumorigenesis and metastasis (Yook et al., 2006; Liu et al., 2010; Damsky et al., 2011; Lin et al., 2021). However, the precise molecular mechanisms driving UBAP2L’s oncogenic potential are not yet defined. UBAP2L has been reported as a BMI1 interactor as cited before (Bordeleau et al., 2014). Although BMI1 is essential for the activity of hematopoietic stem cells, it has also been suggested as a Wnt signaling activator by regulating the Wnt antagonist IDAX (Yu et al., 2018). Therefore, one hypothesis that could be further explored, might be that Wnt/β-catenin hyperactivity in UBAP2L-overexpressing tumors could be attributed to UBAP2L/BMI1 interaction.
Cancer cells must acquire resistance to cellular death to ensure their survival and expansion (Hanahan and Weinberg, 2011). In this context, UBAP2L is suggested to act as an anti-apoptotic factor possibly by regulating, through yet unknown mechanisms, the expression of crucial apoptotic factors such as Bad/Bax and the cleavage of PARP and caspase 3 (Li and Huang, 2014; Chai et al., 2016). Bypassing all checkpoints employed by the cellular machinery is a challenge for cancer cells. Nevertheless, tumor microenvironment is crucial for proper cancer dissemination across tissues. For instance, cancerous cells require a certain amount of nutrients and oxygen to function properly and these components are efficiently brought to the cells only if the tumor is properly vascularized. Interestingly, samples from HCC patients revealed a positive correlation between UBAP2L and VEGF expression, a crucial protein for angiogenesis. Consistently, micro vessel density was also found to be increased in UBAP2L overexpressing tumors (Wang et al., 2017) and a complementary study from another laboratory reported that UBAP2L downregulation decreases the average vascular length and number of vascular branches (Li et al., 2018), once more pointing to a potential role for UBAP2L in favoring angiogenesis.
Incremental studies were conducted on microRNAs (miRNAs), small nucleotides duplexes which post-transcriptionally regulate gene expression of their targets, being involved in general biological processes such as cell proliferation, apoptosis or brain development among others (Ambros, 2004). Intriguingly, UBAP2L was demonstrated to be targeted by different miRNA. First, in non-small cell lung cancer (NSCLC), miR-19a-3p directly inhibits UBAP2L, resulting in similar phenotypes as those observed upon UBAP2L downregulation, mainly inhibition of cell proliferation, migration and invasion (Pan et al., 2020). Similarly, UBAP2L was silenced by miR-148b-3p in gastric cancer cells leading to the same phenotypes as in NSCLC (Lin et al., 2021). Interestingly, the UBAP2L ortholog PQN-59 stabilizes several miRNAs involved in various cellular functions and interacts with RNA metabolism, transcription and translation cellular components similarly to UBAP2L, highlighting the importance of this protein in RNA regulation (Carlston et al., 2021). Supporting this hypothesis, UBAP2L localizes to stress granules and P-bodies under certain conditions, two structures highly linked to RNA turnover, miRNA or gene expression regulation (Leung et al., 2006).
Conclusively, although UBAP2L has been identified more than 20 years ago, its extremely versatile roles in various signaling pathways have been elucidated only recently. It would therefore be fascinating that future studies address the underlying precise molecular mechanisms that govern and direct UBAP2L’s functions towards such distinct signaling nodes to ensure cellular homeostasis. Our review aimed at highlighting the growing evidence on the oncogenic potential of UBAP2L that may identify UBAP2L as a promising target and stimulate research on UBAP2L-based future cancer therapies.
Conceptualization, Writing, Review and Editing: LG, EP, and IS.
LG was supported by Labex international PhD fellowship from IGBMC and IMC-Bio graduate school. EP was supported by postdoctoral fellowships from the ANR-10-LABX-0030-INRT. Research in IS laboratory was supported by the grant ANR-10-LABX-0030-INRT, a French State fund managed by the Agence Nationale de la Recherche under the frame program Investissements d’Avenir ANR-10-IDEX-0002-02, IGBMC, CNRS, Fondation ARC pour la recherche sur le cancer, Institut National du Cancer (INCa), Agence Nationale de la Recherche (ANR), Ligue Nationale contre le Cancer, Sanofi iAward Europe and Programme Fédérateur Aviesan, Plan Cancer, National collaborative project: “NANOTUMOR”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all members of the Sumara lab for helpful discussions on the manuscript. Figures were created with BioRender.com.
Abbatemarco, S., Bondaz, A., Schwager, F., Wang, J., Hammell, C. M., and Gotta, M. (2021). PQN-59 and GTBP-1 Contribute to Stress Granule Formation but Are Not Essential for Their Assembly in C. elegans Embryos. J. Cell Sci. 134, jcs258834. doi:10.1242/jcs.258834
An, H., Tan, J. T., and Shelkovnikova, T. A. (2019). Stress Granules Regulate Stress-Induced Paraspeckle Assembly. J. Cell Biol. 218, 4127–4140. doi:10.1083/jcb.201904098
Aucagne, R., Girard, S., Mayotte, N., Lehnertz, B., Lopes‐Paciencia, S., Gendron, P., et al. (2017). UBAP2L Is Amplified in a Large Subset of Human Lung Adenocarcinoma and Is Critical for Epithelial Lung Cell Identity and Tumor Metastasis. FASEB J. 31, 5012–5018. doi:10.1096/fj.201601219RRR
Bai, D.-S., Wu, C., Yang, L.-X., Zhang, C., Zhang, P.-F., He, Y.-Z., et al. (2016). UBAP2 Negatively Regulates the Invasion of Hepatocellular Carcinoma Cell by Ubiquitinating and Degradating Annexin A2. Oncotarget 7, 32946–32955. doi:10.18632/oncotarget.8783
Baumgartner, R., Stocker, H., and Hafen, E. (2013). The RNA-Binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in Drosophila melanogaster. PLoS Genet. 9, e1003598. doi:10.1371/journal.pgen.1003598
Bordeleau, M.-E., Aucagne, R., Chagraoui, J., Girard, S., Mayotte, N., Bonneil, É., et al. (2014). UBAP2L Is a Novel BMI1-Interacting Protein Essential for Hematopoietic Stem Cell Activity. Blood 124, 2362–2369. doi:10.1182/blood-2014-01-548651
Carlston, C., Weinmann, R., Stec, N., Abbatemarco, S., Schwager, F., Wang, J., et al. (2021). PQN-59 Antagonizes microRNA-Mediated Repression during Post-embryonic Temporal Patterning and Modulates Translation and Stress Granule Formation in C. elegans. PLoS Genet. 17, e1009599. doi:10.1371/journal.pgen.1009599
Castello, A., Fischer, B., Frese, C. K., Horos, R., Alleaume, A.-M., Foehr, S., et al. (2016). Comprehensive Identification of RNA-Binding Domains in Human Cells. Mol. Cell 63, 696–710. doi:10.1016/j.molcel.2016.06.029
Chai, R., Yu, X., Tu, S., and Zheng, B. A. (2016). Depletion of UBA Protein 2-like Protein Inhibits Growth and Induces Apoptosis of Human Colorectal Carcinoma Cells. Tumor Biol. 37, 13225–13235. doi:10.1007/s13277-016-5159-y
Chang, K. T., Guo, J., di Ronza, A., and Sardiello, M. (2018). Aminode: Identification of Evolutionary Constraints in the Human Proteome. Sci. Rep. 8, 1357. doi:10.1038/s41598-018-19744-w
Cirillo, L., Cieren, A., Barbieri, S., Khong, A., Schwager, F., Parker, R., et al. (2020). UBAP2L Forms Distinct Cores that Act in Nucleating Stress Granules Upstream of G3BP1. Curr. Biol. 30, 698–707. e6. doi:10.1016/j.cub.2019.12.020
Damsky, W. E., Curley, D. P., Santhanakrishnan, M., Rosenbaum, L. E., Platt, J. T., Gould Rothberg, B. E., et al. (2011). β-Catenin Signaling Controls Metastasis in Braf-Activated Pten-Deficient Melanomas. Cancer Cell 20, 741–754. doi:10.1016/j.ccr.2011.10.030
Fox, A. H., Nakagawa, S., Hirose, T., and Bond, C. S. (2018). Paraspeckles: Where Long Noncoding RNA Meets Phase Separation. Trends Biochem. Sci. 43, 124–135. doi:10.1016/j.tibs.2017.12.001
Guan, W., Yang, N., Zuo, X., Wang, X., Cao, P., Chu, Y., et al. (2021). Heritable Variants in the Chromosome 1q22 Locus Increase Gastric Cancer Risk via Altered Chromatin Looping and Increased UBAP2L Expression. Mol. Cancer Res. 19, 1992–2002. doi:10.1158/1541-7786.MCR-21-0001
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of Cancer: The Next Generation. Cell 144, 646–674. doi:10.1016/j.cell.2011.02.013
He, J., Chen, Y., Cai, L., Li, Z., and Guo, X. (2018). UBAP2L Silencing Inhibits Cell Proliferation and G2/M Phase Transition in Breast Cancer. Breast Cancer 25, 224–232. doi:10.1007/s12282-017-0820-x
Hollander, M. C., Blumenthal, G. M., and Dennis, P. A. (2011). PTEN Loss in the Continuum of Common Cancers, Rare Syndromes and Mouse Models. Nat. Rev. Cancer 11, 289–301. doi:10.1038/nrc3037
Huang, C., Chen, Y., Dai, H., Zhang, H., Xie, M., Zhang, H., et al. (2020). UBAP2L Arginine Methylation by PRMT1 Modulates Stress Granule Assembly. Cell Death Differ. 27, 227–241. doi:10.1038/s41418-019-0350-5
Iranzo-Tatay, C., Hervas-Marin, D., Rojo-Bofill, L. M., Garcia, D., Vaz-Leal, F. J., Calabria, I., et al. (2022). Genome-wide DNA Methylation Profiling in Anorexia Nervosa Discordant Identical Twins. Transl. Psychiatry 12, 15. doi:10.1038/s41398-021-01776-y
Khemlina, G., Ikeda, S., and Kurzrock, R. (2017). The Biology of Hepatocellular Carcinoma: Implications for Genomic and Immune Therapies. Mol. Cancer 16, 149. doi:10.1186/s12943-017-0712-x
Leung, A. K. L., Calabrese, J. M., and Sharp, P. A. (2006). Quantitative Analysis of Argonaute Protein Reveals microRNA-dependent Localization to Stress Granules. Proc. Natl. Acad. Sci. U.S.A. 103, 18125–18130. doi:10.1073/pnas.0608845103
Levine, A. J. (2020). p53: 800 Million Years of Evolution and 40 Years of Discovery. Nat. Rev. Cancer 20, 471–480. doi:10.1038/s41568-020-0262-1
Li, D., and Huang, Y. (2014). Knockdown of Ubiquitin Associated Protein 2-like Inhibits the Growth and Migration of Prostate Cancer Cells. Oncol. Rep. 32, 1578–1584. doi:10.3892/or.2014.3360
Li, O., Zhao, C., Zhang, J., Li, F.-N., Yang, Z.-Y., Liu, S.-L., et al. (2022). UBAP2L Promotes Gastric Cancer Metastasis by Activating NF-Κb through PI3K/AKT Pathway. Cell Death Discov. 8, 123. doi:10.1038/s41420-022-00916-7
Li, Q., Wang, W., Hu, Y.-C., Yin, T.-T., and He, J. (2018). Knockdown of Ubiquitin Associated Protein 2-Like (UBAP2L) Inhibits Growth and Metastasis of Hepatocellular Carcinoma. Med. Sci. Monit. 24, 7109–7118. doi:10.12659/MSM.912861
Lien, E. C., Dibble, C. C., and Toker, A. (2017). PI3K Signaling in Cancer: beyond AKT. Curr. Opin. Cell Biol. 45, 62–71. doi:10.1016/j.ceb.2017.02.007
Lin, G.-l., Wang, H., Dai, J., Li, X., Guan, M., Ding, Q., et al. (2018). Upregulation of UBAP2L in Bone Marrow Mesenchymal Stem Cells Promotes Functional Recovery in Rats with Spinal Cord Injury. Curr. Med. Sci. 38, 1081–1089. doi:10.1007/s11596-018-1987-x
Lin, S., Yan, Z., Tang, Q., and Zhang, S. (2021). Ubiquitin-associated Protein 2 like (UBAP2L) Enhances Growth and Metastasis of Gastric Cancer Cells. Bioengineered 12, 10232–10245. doi:10.1080/21655979.2021.1982308
Liu, L., Zhu, X.-D., Wang, W.-Q., Shen, Y., Qin, Y., Ren, Z.-G., et al. (2010). Activation of β-Catenin by Hypoxia in Hepatocellular Carcinoma Contributes to Enhanced Metastatic Potential and Poor Prognosis. Clin. Cancer Res. 16, 2740–2750. doi:10.1158/1078-0432.CCR-09-2610
Luo, E.-C., Nathanson, J. L., Tan, F. E., Schwartz, J. L., Schmok, J. C., Shankar, A., et al. (2020). Large-scale Tethered Function Assays Identify Factors that Regulate mRNA Stability and Translation. Nat. Struct. Mol. Biol. 27, 989–1000. doi:10.1038/s41594-020-0477-6
Maeda, M., Hasegawa, H., Sugiyama, M., Hyodo, T., Ito, S., Chen, D., et al. (2016). Arginine Methylation of Ubiquitin‐associated Protein 2‐like Is Required for the Accurate Distribution of Chromosomes. FASEB J. 30, 312–323. doi:10.1096/fj.14-268987
Marenholz, I., Zirra, M., Fischer, D. F., Backendorf, C., Ziegler, A., and Mischke, D. (2001). Identification of Human Epidermal Differentiation Complex (EDC)-Encoded Genes by Subtractive Hybridization of Entire YACs to a Gridded Keratinocyte cDNA Library. Genome Res. 11, 341–355. doi:10.1101/gr.114801
McIntosh, J. R. (2016). Mitosis. Cold Spring Harb. Perspect. Biol. 8, a023218. doi:10.1101/cshperspect.a023218
Nagel, A. K., Schilling, M., Comte-Walters, S., Berkaw, M. N., and Ball, L. E. (2013). Identification of O-Linked N-Acetylglucosamine (O-GlcNAc)-Modified Osteoblast Proteins by Electron Transfer Dissociation Tandem Mass Spectrometry Reveals Proteins Critical for Bone Formation. Mol. Cell. Proteomics 12, 945–955. doi:10.1074/mcp.M112.026633
Naz, R. K., and Dhandapani, L. (2010). Identification of Human Sperm Proteins that Interact with Human Zona Pellucida3 (ZP3) Using Yeast Two-Hybrid System. J. Reproductive Immunol. 84, 24–31. doi:10.1016/j.jri.2009.10.006
Nusse, R., and Clevers, H. (2017). Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 169, 985–999. doi:10.1016/j.cell.2017.05.016
Pan, Y., Jin, K., Xie, X., Wang, K., and Zhang, H. (2020). MicroRNA-19a-3p Inhibits the Cellular Proliferation and Invasion of Non-small Cell Lung Cancer by Downregulating UBAP2L. Exp. Ther. Med. 20 (3), 2252–2261. doi:10.3892/etm.2020.8926
Parker, R., and Sheth, U. (2007). P Bodies and the Control of mRNA Translation and Degradation. Mol. Cell 25, 635–646. doi:10.1016/j.molcel.2007.02.011
eQTLGen ConsortiumBIOS Consortiumthe Bipolar Disorder Working Group of the Psychiatric Genomics Consortium Stahl, E. A., Breen, G., Forstner, A. J., McQuillin, A., Ripke, S., Trubetskoy, V., et al. (2019). Genome-wide Association Study Identifies 30 Loci Associated with Bipolar Disorder. Nat. Genet. 51, 793–803. doi:10.1038/s41588-019-0397-8
Uhlén, M., Fagerberg, L., Hallström, B. M., Lindskog, C., Oksvold, P., Mardinoglu, A., et al. (2015). Tissue-based Map of the Human Proteome. Science 347, 1260419. doi:10.1126/science.1260419
Wang, W., Zhang, M., Peng, Y., and He, J. (2017). Ubiquitin Associated Protein 2-Like (UBAP2L) Overexpression in Patients with Hepatocellular Carcinoma and its Clinical Significance. Med. Sci. Monit. 23, 4779–4788. doi:10.12659/MSM.907071
Wilde, I. B., Brack, M., Winget, J. M., and Mayor, T. (2011). Proteomic Characterization of Aggregating Proteins after the Inhibition of the Ubiquitin Proteasome System. J. Proteome Res. 10, 1062–1072. doi:10.1021/pr1008543
Yang, Z., Li, G., Zhao, Y., Zhang, L., Yuan, X., Meng, L., et al. (2021). Molecular Insights into the Recruiting between UCP2 and DDX5/UBAP2L in the Metabolic Plasticity of Non-small-cell Lung Cancer. J. Chem. Inf. Model. 61, 3978–3987. doi:10.1021/acs.jcim.1c00138
Ye, T., Xu, J., Du, L., Mo, W., Liang, Y., and Xia, J. (2017). Downregulation of UBAP2L Inhibits the Epithelial-Mesenchymal Transition via SNAIL1 Regulation in Hepatocellular Carcinoma Cells. Cell Physiol. Biochem. 41, 1584–1595. doi:10.1159/000470824
Yook, J. I., Li, X.-Y., Ota, I., Hu, C., Kim, H. S., Kim, N. H., et al. (2006). A Wnt-Axin2-Gsk3β Cascade Regulates Snail1 Activity in Breast Cancer Cells. Nat. Cell Biol. 8, 1398–1406. doi:10.1038/ncb1508
Yoshida, K., Kajiyama, H., Inami, E., Tamauchi, S., Ikeda, Y., Yoshikawa, N., et al. (2020). Clinical Significance of Ubiquitin-Associated Protein 2-like in Patients with Uterine Cervical Cancer. Vivo 34, 109–116. doi:10.21873/invivo.11751
Youn, J.-Y., Dunham, W. H., Hong, S. J., Knight, J. D. R., Bashkurov, M., Chen, G. I., et al. (2018). High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies. Mol. Cell 69, 517–532. e11. doi:10.1016/j.molcel.2017.12.020
Yu, F., Zhou, C., Zeng, H., Liu, Y., and Li, S. (2018). BMI1 Activates WNT Signaling in Colon Cancer by Negatively Regulating the WNT Antagonist IDAX. Biochem. Biophysical Res. Commun. 496, 468–474. doi:10.1016/j.bbrc.2018.01.063
Yu, W. R., and Fehlings, M. G. (2011). Fas/FasL-mediated Apoptosis and Inflammation Are Key Features of Acute Human Spinal Cord Injury: Implications for Translational, Clinical Application. Acta Neuropathol. 122, 747–761. doi:10.1007/s00401-011-0882-3
Zhao, B., Zong, G., Xie, Y., Li, J., Wang, H., and Bian, E. (2015). Downregulation of Ubiquitin-Associated Protein 2-like with a Short Hairpin RNA Inhibits Human Glioma Cell Growth In Vitro. Int. J. Mol. Med. 36, 1012–1018. doi:10.3892/ijmm.2015.2323
Keywords: UBAP2L, mitosis, cancer, ubiquitin, stress signaling
Citation: Guerber L, Pangou E and Sumara I (2022) Ubiquitin Binding Protein 2-Like (UBAP2L): is it so NICE After All?. Front. Cell Dev. Biol. 10:931115. doi: 10.3389/fcell.2022.931115
Received: 28 April 2022; Accepted: 01 June 2022;
Published: 20 June 2022.
Edited by:
Philipp Kaldis, Lund University, SwedenReviewed by:
Permeen Yusoff, Singapore General Hospital, SingaporeCopyright © 2022 Guerber, Pangou and Sumara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Izabela Sumara, c3VtYXJhQGlnYm1jLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.