- 1Department of Health Sciences and Technology, GAIHST, Gachon University, Seongnam, South Korea
- 2Department of Molecular Medicine, School of Medicine, Gachon University, Seongnam, South Korea
Stem cell-based therapeutics have gained tremendous attention in recent years due to their wide range of applications in various degenerative diseases, injuries, and other health-related conditions. Therapeutically effective bone marrow stem cells, cord blood- or adipose tissue-derived mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), and more recently, induced pluripotent stem cells (iPSCs) have been widely reported in many preclinical and clinical studies with some promising results. However, these stem cell-only transplantation strategies are hindered by the harsh microenvironment, limited cell viability, and poor retention of transplanted cells at the sites of injury. In fact, a number of studies have reported that less than 5% of the transplanted cells are retained at the site of injury on the first day after transplantation, suggesting extremely low (<1%) viability of transplanted cells. In this context, 3D porous or fibrous national polymers (collagen, fibrin, hyaluronic acid, and chitosan)-based scaffold with appropriate mechanical features and biocompatibility can be used to overcome various limitations of stem cell-only transplantation by supporting their adhesion, survival, proliferation, and differentiation as well as providing elegant 3-dimensional (3D) tissue microenvironment. Therefore, stem cell-based tissue engineering using natural or synthetic biomimetics provides novel clinical and therapeutic opportunities for a number of degenerative diseases or tissue injury. Here, we summarized recent studies involving various types of stem cell-based tissue-engineering strategies for different degenerative diseases. We also reviewed recent studies for preclinical and clinical use of stem cell-based scaffolds and various optimization strategies.
Introduction
Previous studies demonstrates that stem cell transplantation ameliorates tissue damage and robustly regenerates diseased or injured organs via various repair mechanisms (Moeinabadi-Bidgoli et al., 2021). Currently, various types of stem cells, such as induced pluripotent stem cells (iPSCs) (Netsrithong and Wattanapanitch, 2021), embryonic stem cells (ESCs) (Lan et al., 2020), and mesenchymal stem cells (MSCs) (Hernandez et al., 2020) have been shown to enhance tissue repair/regeneration by modulating immune reactions and/or direct differentiation into target cells. Although stem cell-based therapeutic approaches have emerged as a promising alternative for the treatment of various degenerative diseases, their full-scale clinical application is limited by the relatively low cell viability at the sites of injury of the transplanted cells (Zhang S. et al., 2020) and their poor multilineage differentiation into target tissues (Park JS. et al., 2021). For example, neural stem cell transplantation is widely used to treat ischemic brain injury or neurodegenerative disease (Zhao L. et al., 2021); however, their differentiation into fully functional cells of neural lineage and stable engraftment, followed by reconnection with host neural cells at the injured site are still obstacles to overcome (Kwon et al., 2018; Katoh et al., 2019).
To overcome their current limitations, biomaterial-based tissue engineering using many different types of biocompatible cell-seeded scaffolds has been tried to enhance the cell viability, growth potential, and multilineage differentiation of transplanted stem cells (Xu et al., 2020; Belludi et al., 2021; Gogele et al., 2022; Zheng et al., 2022). A number of naturally derived and synthetic biomaterials have been currently developed to restore the function and structure of damaged tissues (Park S. R. et al., 2021; Mazzoni et al., 2021). Indeed, various biodegradable protein-based natural polymers, including collagen, fibrin, gelatin, hyaluronic acid, and poly (lactic-co-glycolic) acid have been widely used to construct scaffolds for the regeneration of multiple tissues such as bone, cartilage, ligament, neural tissues, skin, skeletal muscle, and blood vessels (Rice et al., 2013; Wang et al., 2020; Bonferoni et al., 2021).
The biomaterial scaffold-based approaches are primarily designed to incorporate living therapeutic cells within a porous 3D scaffold and signaling molecules or growth factors by providing a tissue architecture for cell infiltration and proliferation to promote the repair and regeneration of damaged tissue (Salerno and Netti, 2021). For example, the combination of several biodegradable 3D scaffolds and transplanted stem cells is an attractive therapeutic strategy for the regenerations of injured tissues by facilitating cell survival and retention (Abbott et al., 2012; Dash et al., 2018; Nagano et al., 2021). Therefore, in this article, we will critically discuss recent advances and limitations of various stem cell-based tissue-engineering therapies and their preclinical or clinical application in multiple types of disease for tissue regeneration.
Bioengineering Techniques Accelerate Stem Cell-Based Therapeutic Effects
Due to their relatively high self-renewal capacity, the ability to differentiate into multiple cellular lineages, and immunomodulatory properties, various types of stem cells, especially MSCs have been extensively investigated over the past decade as a promising cell resource in a number of preclinical and clinical studies to treat or ameliorate various incurable diseases, such as cirrhosis, diabetes, inflammatory disease, liver failure, and neurodegenerative disorders (Chen J. et al., 2021; Chen X. et al., 2021). Despite substantial progress in stem cell therapies, many challenges remain to be overcome prior to their therapeutic application. Regardless of the source, one of the major bottlenecks of their clinical application is the large-scale in vitro expansion prior to transplantation to obtain adequate numbers of stem cells, normally millions to hundreds of millions of stem cells required per patient (Wei et al., 2014; Garcia-Bernal et al., 2021). Therefore, 3D porous or fibrous biomaterials-based scaffold can be used to overcome these limitations by supporting their adhesion, survival, proliferation, and differentiation as well as providing elegant 3-dimensional (3D) tissue microenvironment (Summarized in Figure 1).
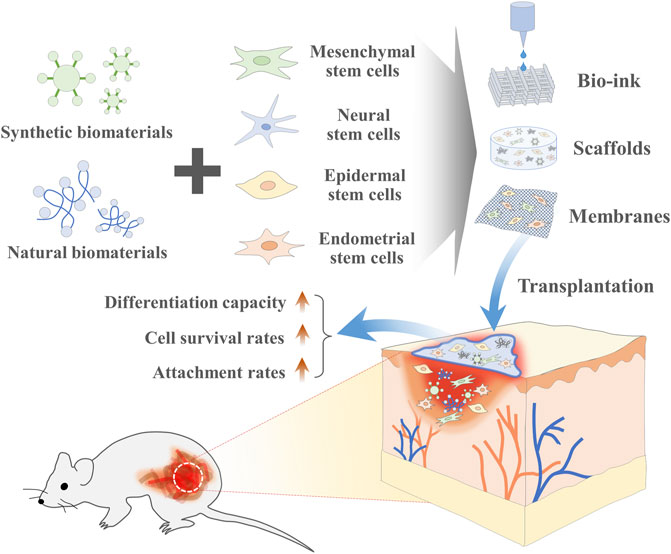
FIGURE 1. The combination of stem cells and biomaterial-based tissue engineering can promote regenerative capacity of stem cells. Various synthetic or natural biomaterials-based 3-dimensional (3D) scaffolds can be used to overcome various current limitations of stem cells-based therapies by supporting their adhesion, survival, proliferation, and differentiation as well as providing elegant 3D tissue microenvironment at the sites of injury.
Scaffolds based on synthetic or natural biomaterials provide various chemical cues and mechanical supports for the transplanted stem cells at specific sites of injured tissues. Typically, Gilbert et al. (2010) loaded muscle stem cells to the tunable polyethylene glycol (PEG) hydrogel platform to properly regenerate damaged skeletal muscle by increasing their survival rates and self-renewability in the sites of injury. In addition, synthetic peptide-acrylate surfaces support self-renewal of human ESCs with similar morphology and phenotypic marker expression compared to cells cultured on Matrigel (Melkoumian et al., 2010). Kim et al. (2010) also developed synthetic peptide substrates that recognize cell surface glycosaminoglycan for long-term in vitro expansion of human pluripotent stem cells. In addition, the maintenance of stem cells in their undifferentiated state during in vitro cell expansion, efficient control of stem cell differentiation into target tissues both pre- and post-administration, and adequate protection of the stem cells during and after transplantation to patients are challenges (Neofytou et al., 2015; Liu et al., 2020). Various natural or synthetic biomaterial-based tissue engineering techniques may offer novel solutions to overcome these obstacles.
A specific microenvironment that provides signaling factors and elements plays a significant role in regulating cell survival and differentiation (Zhang et al., 2019). In this context, to effectively mimic the in vivo microenvironment, combining various biomaterials with stem cells is a powerful tool for controlling stem cell viability and fate by facilitating cell-matrix/cell-cell interactions (Gelmi and Schutt, 2021). In contrast to traditional 2D cell culture conditions, the 3D culture platform using biomaterial-based scaffolds provides a more in vivo physiologic architecture for implanted stem cells by supporting physical interactions with extracellular matrix (ECM) components and releasing signaling molecules (Li et al., 2017). Therefore, chemical, electrical, mechanical, surface topography, and structural characteristics need to be comprehensively evaluated when manufacturing a biomaterials-based scaffold (Martino et al., 2012). In particular, efficient ex vivo expansion and differentiation of implanted stem cells into target tissues on synthetic or natural microcarriers largely depends on their potential to regulate cell shape (spreading or round) and cell organization (aggregate size) (Sart et al., 2013). Microcarrier techniques are often used in bioreactors to promote stem cell expansion in vitro (Bardy et al., 2013; Li et al., 2014). For example, Bardy et al. (2013) achieved approximately 7-fold increased expansion of human iPSCs with significantly elevated differentiation into neural progenitor cells using a matrigel-based microcarrier (MC) culture system.
In addition, the highly hydrated and microporous hydrogels, a class of biomaterial scaffolds, provide ideal 3D microenvironments to stably encapsulate stem cells together with signaling molecules, peptides, or growth factors (Aguado et al., 2012; Yan et al., 2012). Hydrogels, made of various natural polymers such as natural polymers like collagen, alginates, gelatin, and hyaluronic acid, serve as immunological barriers to protect the implanted stem cells from host immune attack while maintaining permeability to therapeutic molecules or growth factors (Nafea et al., 2011). In addition, Gaffey et al (2015) reported that endothelial progenitor cells, which are seeded into hyaluronic acid (HA) shear-thinning hydrogel and then transplanted into the ischemic rat hearts, resulted in enhanced engraft efficiency and reduced myocardial fibrosis compared with stem cells treated with normal saline. Injectable hydrogels based on peptide amphiphiles also significantly enhanced the transplantation of muscle stem cells in injured muscles by ensuring growth factor retention and thus increasing proliferation of encapsulated cells (Sleep et al., 2017).
The Correlation Between the Physical Properties of Biomaterials and Their Target Tissue
Because different tissues or cells have their own chemical compositions and physical characteristics, the designed biomaterials used in tissue engineering should have a strong functional and structural affinity for targeted tissue and cell types to properly stimulate tissue regeneration (Abbas et al., 2020; Peressotti et al., 2021). The structural or mechanical properties of the biomaterial surface such as charges, chemical compositions, and hydrophobicity may play key roles in regulating their diverse biological functions (Echeverria Molina et al., 2021; Pearce and O’reilly, 2021). The therapeutic effects are largely dependent on the reciprocal interactions between the transplanted biomaterials with specific mechanical characteristics and 3D microenvironment of targeted tissues (Barthes et al., 2014; Nicolas et al., 2020; Antmen et al., 2021). For example, relatively strong mechanical properties may be required to regenerate hard tissues such as bones or teeth which may be subjected to weight-loading or strain (Mastrogiacomo et al., 2019), whereas porous, soft, and highly viscose biomaterials are needed to restore the functions of the soft tissues such as skin and internal organs (Bonferoni et al., 2021).
In addition, the use of biomaterials also largely depends on the predicted application types (open implantation (attachment) strategies vs internal injection or less invasive treatments) (Kohane and Langer, 2008; Qi et al., 2015; Raucci et al., 2020). Generally, biomaterial-based tissue mimetics are fully fabricated outside the body (in vitro) and then should be transplanted surgically. Therefore, for such applications, the whole tissue mimetics need to have relatively low viscosity with certain level of mechanical strength that can be implanted. Conversely, biomaterials fabricated for internal injection have relatively high viscosity and soft mechanical strength with more cohesive and gel-like characteristics. Currently, a number of studies have investigated the effects of structural (mechanical) and chemical characteristics of biomaterials on various cellular functions such as self-renewability, migratory capacity, and metabolic activity (Ma et al., 2019; Zhao X. et al., 2021; Peressotti et al., 2021).
Regulation of Stem Cell Microenvironment With Biomaterial Scaffolds
Tissue resident stem cells are located in particular 3D microenvironment, defined as niches to interact with neighboring cells, matrix components, bound or secreted biomolecules, and growth factors, which are essential for their sufficient therapeutic effects after administration (He et al., 2014). Interestingly, various synthetic or natural biomaterial-based scaffolds can provide a longer and more efficient cellular microenvironment for administrated stem cells retention and survival rates in injured tissues and subsequently enhance their therapeutic potential after administration. Indeed, insulin like growth factor-1 (IGF-I) conjugated fibrin micro-beads significantly increased bulk stability and muscle regeneration capacity of human smooth muscle cells (Vardar et al., 2019).
Kim et al. (2021) also observed that adipose-derived stem cells, which were self-assembled with the cartilage-dECM-decorated nanofibrils, enhanced their survival rates and differentiation capacity and subsequently enhanced therapeutic efficiency in osteochondral defect mice by mimicking structural and biochemical cartilage-specific microenvironment. In addition, the combination of chitosan-coated silicone tube and neurosphere cells induced from human adipose-derived MSCs increased their myelinated axons density and myelin thickness and thus exhibited substantial improvements in nerve regeneration after 6 weeks of surgery (Hsueh et al., 2014).
Similarly, hydrogel comprised of thiol-functionalized hyaluronic acid and hyperbranched poly (ethylene glycol) diacrylate (HB-PEGDA) polymer significantly enhances cell growth, survival, and paracrine activity of adipose-derived MSCs and subsequently accelerated wound closure and reduced the scar formation in burn skin wound model (Dong et al., 2020). Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides can bind strongly to extracellular vesicles (EVs) derived from MSCs. This EV-RGD hydrogels significantly attenuated histopathological damage, decreased tubular injury and in turn increased therapeutic efficacy in renal repair in early phases of acute kidney injury (Zhang C. et al., 2020).
Various types of growth factors (cytokines) can increase stem cell proliferation, migratory capacity, and differentiation potential towards a specific lineage (Meng et al., 2008). In this context, Ferguson et al. conjugated epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) with dextrin to support growth and differentiation of mouse neural stem cells via controllable growth factor release (Ferguson et al., 2018). Similarly, Zhao et al. synthesized a chitosan hydrogel scaffolds by conjugating C domain peptide of IGF-1 onto chitosan and co-transplanted with human placenta-derived MSCs into a murine hindlimb ischemia model.
Transplanted scaffolds substantially reduced fibrosis and collagen deposition, along with increased angiogenesis by protecting H2O2-induced apoptosis and decreasing inflammation (Zhao J et al., 2019). In addition, nitric oxide (NO), a short-lived and highly reactive free radical, is an essential intra- and extracellular messenger with critical signaling roles in regulating diverse cellular functions associated with tissue regeneration (Midgley et al., 2020). However, the short biological half-life of NO (5–10 s) and diffusion distance severely limited its therapeutic application in stem cell-based regenerative medicine (Hughes et al., 2009). Thus, Nie et al. (2017) developed a controllable chitosan NO-releasing hydrogel (CS-NO) to promote direct endothelial differentiation of mouse ESCs by activating PI3K/Akt signaling pathway without adding growth factors. Similarly, Zhang et al. developed NO-releasing chitosan hydrogel to increase proangiogenic capacities of human placenta-derived MSCs and thus enhance their therapeutic efficiency in hindlimb ischemia model (Zhang K. et al., 2020).
Regeneration and Repair of Skin Wounds
The skin is the largest organ in the human body and is made up of three basic layers: dermis, epidermis, and hypodermis or subcutaneous tissue. It supports and protects the underlying skeletal muscles, bones, ligaments, internal organs, connective tissues (Bhardwaj et al., 2017). It contains many specialized cells and complex multi-layered structures with different mechanical behaviors (Biggs et al., 2020). Skin provides the first line of defense against any pathogenic infection as an immune-protective organ (Di Meglio et al., 2011). Thus, skin is the most vulnerable to infection and requires rapid wound healing. Application of stem cell technologies in wound healing and skin regeneration has increased considerably in recent decade (Kucharzewski et al., 2019; Nourian Dehkordi et al., 2019). Several distinct stem cell populations have been identified and characterized in the epidermis with distinct locations and functions (Rognoni and Watt, 2018), and strong skin repair and regenerative abilities (Yang et al., 2020). Stimulating the local stem cell populations and promoting specific microenvironment (termed stem cell niche) that enhances their self-renewal capacity and differentiation potential may accelerate the regeneration and repair of injured skin (Wong et al., 2012; Fuchs and Blau, 2020).
Scaffolds based on synthetic or natural biomaterials provide various chemical cues and mechanical supports for the transplanted stem cells at specific sites of injured skin (Riha et al., 2021; Weng et al., 2021). For example, although G-protein coupled receptor 6-positive (LGR6+) epithelial stem cell populations were isolated from the follicular bulge and substantial wound-healing effects were achieved when transplanted into full-thickness cutaneous wounds (Lough et al., 2014), it resulted in poor therapeutic efficacy with limited epithelialization, hair growth, and angiogenesis. Therefore, to address these limitations, Denver et al. developed LGR6+ epithelial stem cell-seeded scaffold to provide deliverable, immediate and viable soft tissue mimetics that significantly improved the rates of healing, epithelialization, angiogenesis or vasculogenesis, and hair growth in full-thickness wounds (Lough et al., 2016).
In addition to LGR6+ epidermal stem cells, MSCs derived from various tissues including bone marrow, umbilical cord blood, and adipose tissue (Laverdet et al., 2014) are also extensively used to regenerate injured skin. Indeed, MSC-transplanted wounds exhibited significantly accelerated cutaneous healing with increased re-epithelialization, vascularization, and differentiation into epithelial cells and keratinocytes (Wu et al., 2007; Sasaki et al., 2008). Bone marrow (BM)-derived MSC-seeded collagen-chitosan scaffolds having mechanical properties similar to that of the soft tissues significantly improved the degree of collagen deposition and wound healing rates (Abolgheit et al., 2021). Formigli et al. (2015) also demonstrated that BM-MSCs seeded on synthetic polysiloxane polymer-based scaffold largely ameliorated cutaneous wounds, enhanced reepithelization and collagen deposition, and promoted vascularization and hair growth at the implantation sites by releasing various paracrine factors and/or recruiting endogenous progenitor cells.
Adipose tissue derived (AD)-MSCs or secretome significantly enhanced the healing process in early phase by increasing the migration and proliferation of dermal fibroblasts and inhibiting the inflammatory response (Ma et al., 2021). These intracutaneously injected AD-MSCs at the dermal and subcutaneous layers can survive in vivo for up to 1 year in immunodeficient mice and differentiate into adipocytes to provide architectural support and microenvironment for re-epithelialization and subsequent dermal repair (Koellensperger et al., 2014). Furthermore, AD-MSCs implanted into the curcumin-loaded collagen scaffold markedly accelerated ulcer healing process (Mardani et al., 2020). Similarly, Lotfi et al. (2019) demonstrated that the combination of AD-MSCs and keratinocytes on gelatin/chitosan/β-glycerol phosphate nanoscaffold can be used in cutaneous wound healing by reducing wound size as well as increasing angiogenesis of the dermis and dermal thickness.
Artificial dermal substitutes, such as atelocollagen matrix, seeded with AD-MSCs were accurately incorporated into the regenerating capillaries, dermis, and epidermis at the site of wound healing in diabetic db/db mice (Nambu et al., 2011). In addition, silk fibroin scaffolds cellularized with human Wharton’s jelly derived-MSCs enhanced myofibroblast proliferation and re-epithelialization as well as reduced scar tissue formation and inflammatory infiltration in models of skin wound healing (Millan-Rivero et al., 2019). Human umbilical cord blood (UCB)-derived MSCs seeded on collagen-fibrin double-layered scaffolds exhibiting a highly porous and interconnected structure also significantly accelerated wound healing in mouse models of full-thickness skin wounds (Nan et al., 2015).
In addition, epidermolysis bullosa (EB) is a severe skin disease associated with skin fragility, which is caused by different genetic mutations of some genes involved in regulating adhesion of basal epidermal cells to the underlying basement membrane (Jackson et al., 2017; Alharthi et al., 2021). Therefore, gene therapeutic strategies linked to EB can be applied to induce tissue regeneration of EB skin lesions or prevent EB-indued wound healing abnormalities (Marinkovich and Tang, 2019; Gurevich et al., 2022). In this context, eenetically modified skin-derived stem cells have great potential as successful cell-based gene delivery systems locally or systemically. Indeed, several clinical trial observed that local implantation of transgenic epidermal stem cells can regenerate a functional epidermal layer, leading to permanent closure of a large chronic wound of EB skin lesions (Mavilio et al., 2006; De Rosa et al., 2014; Bauer et al., 2017). Similarly, Hirsch et al. (2017) also regeneration of the entire human epidermal layer by transplantation of transgenic stem cells into 7-year-old child suffering from life-threatening form of EB. Although these genetically modified skin stem cells could achieve a certain degree of therapeutic effects, the development of an effective gene transfer technology that can make a sufficient therapeutic effects remains a challenge to be overcome. In this context, development of novel biomaterials is being tried to effectively deliver therapeutic genes to target cells that constantly are involved in the regeneration of the dynamic epidermal tissue. For example, several positively charged polycationic molecules, such as polybrene, which has been widely used in many clinical studies, have applied to increase in vivo retroviral gene delivery efficiency. Recently, Barbier et al. (2022) developed a system for the efficient therapeutic gene transduction to keratinocytes and dermal fibroblasts in vivo using EF-c, that forms amyloid-like peptide nanofibrils. Similarly, Dakiw Piaceski et al. (2018) also combined therapeutic gene (COL7A1 gene, encoding type VII collagen) and tissue-engineered skin substitute for treating dystrophic EB.
Bone Regeneration and Repair
Previous studies have shown the biocompatibility, degradability, mechanical performance, and porosity of tissue-engineered 3D scaffolds for accelerated bone regeneration in various bone defect models (Zhang H.-X. et al., 2016; Lai et al., 2018; Liu et al., 2019). In addition, the use of biomaterial-based scaffolds for the local delivery of stem cells into bone defect sites decreases the potential risk of ectopic bone formation. The 3D structure of the scaffolds mimics the natural bone microenvironment and thus facilitates cell adhesion, growth, differentiation (Baker and Chen, 2012; Ahmad et al., 2018). Bone tissue bioengineering based on natural or synthetic biomaterial 3D scaffolds requires a promising stem cell source to successfully accelerate bone regeneration.
Various types of stem cells with osteogenic differentiation capacity in vivo, such as MSCs, ESCs, and iPSCs, have been used in 3D scaffold-based bone tissue engineering (Mizuno, 2009; Grassel and Lorenz, 2014). In particular, various tissue-derived MSCs are attractive candidates due to their high self-renewal and osteogenic differentiation potential (Rao and Stegemann, 2013). Indeed, MSCs play a critical role in bone regeneration and repair by differentiating into bone-forming osteoblasts to produce bone matrix (Florencio-Silva et al., 2015) and chondrocytes that subsequently undergo mineralization and resorption upon implantation (Knight and Hankenson, 2013) and thus appear to be a better cell source for 3D scaffold-based bone bioengineering than iPSCs and ESCs (Battafarano et al., 2021). Indeed, Cidonio et al. (2020) developed nanocomposite-based 3D scaffolds seeded with human BM-MSCs and umbilical vein endothelial cells (HUVECs) to closely recapitulate the architecture and function of bone tissue and ultimately promote skeletal differentiation. This BM-MSCs-laden nanoclay-based 3D printed scaffolds were found to significantly improve vascular ingrowth and bone tissue formation in vivo compared with acellular scaffolds (Cidonio et al., 2020). Redondo et al. (2018) also performed a pilot clinical trial using a cross-linked serum scaffold (BioMax) seeded with autologous BM-MSCs for treatment of maxillary cysts in nine patients. No inflammatory response or other serious adverse effects were found, and the density of cyst interior remarkably increased after the transplantation of this scaffold (Redondo et al., 2018).
Various bioengineering techniques have been used to integrate stem cells on the scaffold. For instance, Sartika et al. (2020) directly seeded AD-MSCs onto previously fabricated 3D silk fibroin (SF) scaffold with relatively high cell viability and enhanced proliferation. Another approach involved incorporating stem cells simultaneously with nanoclay-based bioinks during the fabrication of scaffold by Cidonio et al. (2020). Similarly, Qiao et al. (2021) fabricated a tri-layered stratified scaffold in which BM-MSCs were simultaneously integrated with gelatin methacrylamide (GelMA) hydrogel using a UV-light assisted, stepwise infiltration and crosslinking method.
In addition, a significant improvement in bone regeneration in the presence of stem cells has recently been demonstrated with various types of composites, ceramics, metals, and polymeric materials (Cidonio et al., 2020; Lozano et al., 2020; Volkov et al., 2020). Indeed, it has recently been shown that Zn2+−releasing β-tricalcium phosphate/poly (l-lactic acid) (TCP/PLLA) ceramics with periosteum-derived progenitor cells represent ideal scaffolds for bone regeneration and repair given their immunoregulatory capacity and unique osteoinductive activity (Huang et al., 2021). Similarly, Marcacci et al. (2007) transplanted a porous hydroxyapatite (HA) ceramic scaffold seeded with patient-derived BM-MSCs into four patients with large bone diaphysis defects, resulting in effective and long-term bone regeneration. Peng et al. (2011) also fabricated biphasic calcium phosphate (BCP) ceramic scaffolds using a 3D gel-lamination technique to seed autologous BM-MSCs in vitro. They implanted the BCP scaffold into canine models with bone defects, resulting in significantly greater osteointegration and new bone formation than in the absence of BMSC seeding scaffold.
Regarding the use of polymers associated with muscle stem cells, Peter et al. fabricated a polyglycolic acid mesh seeded with multipotent muscle stem cells, which were isolated from adult skeletal muscle, and found significantly enhanced therapeutic effects after transplantation into a calvarial defect rat model (Taub et al., 2009). Harada et al. (2014) also fabricated a copolymer poly (D, L-lactic-co-glycolic acid) (PLGA) scaffold seeded with cartilage-forming chondrocytes pre-differentiated in vitro from rat BM-MSCs and evaluated bone formation efficiency. Volkov et al. (2020) reported therapeutic effects of biocompatible and biodegradable polymer scaffolds, poly (3-hydroxybutyrate) with hydroxyapatite filled with alginate hydrogel, containing BM-MSCs in a rat model of parietal bone defect. Although the in vitro and in vivo toxic effects on human cells are currently under investigation (Sherry et al., 1989; Zhang XF. et al., 2016; Escarcega-Gonzalez et al., 2018), Zhang et al. (2015) demonstrated that silver nanoparticles (AgNps) encapsulated with collagen accelerated bone fracture healing by facilitating growth and osteogenic differentiation of MSCs and facilitating chemo-attraction of MSCs and fibroblasts into the site of injury.
Spinal Cord or Peripheral Nerve Injury Regeneration
Neural stem cell administration without a supporting ECM results in poor cell viability, low differentiation potential into neural lineage cells, and low transplantation rates at sites of spinal cord injury (Cao et al., 2001; Zhu et al., 2018). To overcome the limitations of neural stem cell therapy in spinal cord or peripheral nerve injury regeneration, tissue bioengineering-based regeneration and repair strategies received increased attention during the past decades. Based on this bioengineering approach, various natural and synthetic biomaterial-based scaffolds combined with different types of stem cells have been developed for direct transplantation into injured regions to regenerate and restore spinal cord or peripheral nerve function.
Chitosan, a chitin-deacetylated non-toxic product, been used as the tube biomaterial to facilitate axonal regeneration and to promote neural stem differentiation into neurons after spinal cord injury (Rao et al., 2018; Liu H. et al., 2021). For example, Li et al. (2009) developed chitosan carriers combined with well-known neurotrophic factor neurotrophin-3 (NT-3), which enhanced the viability and differentiation of neural stem cells into neurons. The chitosan carriers exhibited good biocompatibility with the neural stem cells and significantly promoted the differentiation of neural stem cells into neurons including cholinergic and GABAergic neurons. Similarly, Nomura et al. (2008) implanted chitosan channels seeded with neural stem cells between the cord stumps after complete spinal cord transection. They reported tissue bridge formation and increased astrocytic and oligodendrocytic differentiation in the chitosan channels in the spinal cord at 14 weeks after transplantation (Nomura et al., 2008).
Collagen is the most abundant protein constituting approximately 30–40% of the total protein in human body and a substantial portion of the ECM (Leon-Lopez et al., 2019). It is found in the proliferative regions during neurogenesis and suppresses proliferation and glial cell differentiation while increasing neuronal differentiation of neural progenitor cells (Ali et al., 1998). Kourgiantaki et al. (2020) demonstrated that porous collagen-based scaffolds (PCS) seeded with embryonic neural stem cells can be used to effectively transplant and protect implanted cells at sites of spinal cord injury for enhanced neuronal differentiation, leading to significantly improved therapeutic effects in mouse model. Zou et al. (2020) also fabricated a collagen sponge scaffold with human spinal cord-derived neural stem cells, and the transplantation of this scaffold effectively increased long-term cell survival and neural differentiation and improved the microenvironment at the site of spinal cord injury by decreasing inflammatory response and glial scar formation. A multi-channel collagen scaffold with axially aligned luminal conduits, which is loaded with neural stem cells, promotes neural stem cell activity and enhances cell proliferation without changing cell differentiation potential, thereby enhancing spinal cord repair following injury (Liu S. et al., 2021).
In addition, hyaluronic acid is a non-immunogenic non-sulfated anionic natural polysaccharide with highly conserved structure and biocompatibility that is present throughout the body including cartilage, soft connective tissues, neural tissues, skin, synovial fluids, and umbilical cords (Deepa et al., 2006; Burdick and Prestwich, 2011). It is a major component of the extracellular matrix and plays an important role in regulating angiogenesis, cell differentiation, cell signaling, matrix organizations, and tissue regeneration (Allison and Grande-Allen, 2006; Li et al., 2012), and therefore represents a key biomaterial in regenerative medicine (Shahi et al., 2020).
Three-dimensional (3D) biodegradable porous scaffolds with hyaluronic acid are central elements in bioengineering-based tissue regeneration (Zhai et al., 2020). Zarei-Kheirabadi et al. (2020) transplanted embryonic neural stem cells with commercially available hyaluronic acid-based hydrogel into the spinal cord injury rat to induce differentiation of these cells into astrocytes, neurons, and oligodendrocytes leading to increased neuronal myelination. Similarly, Arulmoli et al. (2016) found that scaffolds containing a combination of fibrin with interpenetrating networks of hyaluronic acid and laminin significantly promoted the growth and differentiation of human neural stem/progenitor cells while attenuating cell-mediated degradation. The scaffolds also enhanced vessel formation and complexity of human cord blood-derived endothelial cells when co-cultured with neural stem cells (Arulmoli et al., 2016). By incorporating single-walled carbon nanotubes and polypyrrole, Shin et al. (2017) developed porous catechol-functionalized hyaluronic acid hydrogels that significantly improved neuronal differentiation of human neural stem/progenitor cells with increased electrophysiological functionality. Calcium channel expression, intracellular, and calcium influx depolarization of loaded neural stem cells were markedly increased in 3D electroconductive hydrogels.
Vascularized Tissue Regeneration and Repair
Cardiovascular diseases are one of the leading causes of morbidity and mortality in developing countries and are rapidly increasing globally. As extensively reviewed and discussed by Zaragoza et al. (2011), both environmental and genetic factors play an important role in the pathogenesis of cardiovascular disease and its related disorders, which are complex and multifactorial and extremely difficult to treat.
Various biomaterial-based tissue engineering techniques have been suggested as alternative therapeutic strategies to overcome the limitations of current treatment in cardiovascular disease by directly transplanting vascularized cardiac tissue mimetics, which are fabricated according to the specific tissue microenvironment at the sites of injury (Nugent and Edelman, 2003). Selecting the appropriate cell source reflecting tissue-specific features is also important for the development of functional cardiac or vascular tissue mimetics, to ensure structural stability and accurate incorporation into the damaged sites (Vunjak-Novakovic et al., 2010).
Other major challenges associated with the application of vascular cells in cardiovascular diseases are the poor quality and quantity of proliferating cells, which deteriorates with the donor’s age and also depends on the selected cell type and isolation method (Janzen et al., 2006; Mimeault and Batra, 2009). In this context, various types of stem/progenitor cells are being used as sources of endothelial cells, smooth muscle cells, and vascular cells to facilitate vascular regeneration and incorporation into the engineered vasculature (Tsifaki et al., 2018; Vila Cuenca et al., 2021). In particular, MSCs from bone marrow or adipose tissues are an attractive source of vascular cells for tissue bioengineering (Pittenger et al., 2019).
Several previous studies reported the increased differentiation potential of AD-MSCs into functional contractile smooth muscle cells (Rodriguez et al., 2006; Heydarkhan-Hagvall et al., 2008; Harris et al., 2011), which were encapsulated with biomaterial-based hydrogels and proliferated on decellularized blood vessel scaffolds, suggesting their capacity for regenerating the vascular structures and functions (Harris et al., 2011). Li et al. (2020) implanted AD-MSCs on atelocollagen scaffolds, induced differentiation into cardiocyte-like cells using 5-azacytidine, and subsequently transplanted into mice with ischemic myocardium. Krawiec et al. (2017) also fabricated biodegradable, elastomeric, porous vascular scaffolds using non-toxic, cytocompatible poly (ester urethane) urea. This tissue-engineered vascular graft was seeded with AD-MSCs and transplanted into the infrarenal abdominal region of rat. Finally, a novel vascular-like tissue in vivo was detected by analyzing the ECM collagen and elastin within implanted vascular scaffolds (Krawiec et al., 2017). Decellularized aorta elastin scaffold with biodegradability and cytocompatibility in combination with human AD-MSCs was also developed by Kazemi et al. (2021).
BM-MSCs are recognized as a promising cell source to restore damaged myocardium after myocardial infarction due to their potential for differentiation into functional cardiomyocytes (Khan et al., 2017) and secretion of various paracrine factors (Song et al., 2017). Indeed, seeding of BM-MSCs porous scaffolds using collagen, which is a major constituent of the myocardial ECM, improves myocardial function by increasing trophic factor secretion and regulating immune modulatory function (Rashedi et al., 2017). Gong et al. successfully fabricated a small-diameter biodegradable vessel scaffold using polyglycolic acid (PGA) and BM-MSC-derived endothelial and smooth muscle cells (Gong and Niklason, 2008). Similarly, BM-MSCs seeded on polycaprolactone 3D nanofiber scaffolds induced cardiac lineage differentiation when transplanted into a rat myocardial infarction model in combination with Wnt/β-catenin signaling regulator IWP-2 and the native cardiac protein thymosin β4 (Marks and Kumar, 2015). Liu et al. (2007) embedded BM-MSCs in fibrin hydrogels, which were polymerized on approximately 4-mm diameter cylindrical scaffold to mimic the structure of blood vessels and transplanted into the jugular veins of lambs. The grafted scaffold exhibited a confluent endothelial layer and the embedded BM-MSCs secreted significant amounts of collagen (Liu et al., 2007).
Among human MSCs, umbilical cord (UCB)-derived MSCs are characterized by relatively low immunogenicity and high proliferative potential for in vitro expansion prior to clinical application (Li et al., 2015). A clinical trial revealed that UCB-MSCs are safe and effective cell source for patients with myocardial infarction (Gao et al., 2015). UCB-MSCs exhibited their capacity for self-organization into matrigel-mediated cell networks and activated angiogenic myeloid cells (Roura et al., 2012). Ultimately, UCB-MSC-seeded matrigel induced the formation of new functional microvasculature that is connected with the host vascular system in animal models of acute myocardial infarction (Roura et al., 2012). Pushp et al. (2020) seeded UCB-MSC-derived beating cardiomyocytes expressing various cardiac-specific genes on aligned polycaprolactone (PCL) nanofibrous scaffolds. The aligned PCL scaffolds stimulated parallel orientation of the seeded cells with the fibers, thus effectively regulating anisotropic conditions in vitro (Pushp et al., 2020).
Previous studies indicated that endothelial progenitor cells (EPCs), characterized by increased differentiation into functional endothelial cells, represent an appropriate source of endothelial regeneration via angiogenesis and re-endothelialization (Walter et al., 2002; Werner et al., 2002; Urbich and Dimmeler, 2004). In this context, Kaushal et al. (2001) isolated EPCs from ovine peripheral blood and seeded them onto decellularized 4 mm blood vessels obtained from porcine iliac arteries. The tissue-engineered small blood vessels showed contractility and nitric oxide-induced vascular contraction/relaxation similar to natural cardiac arteries (Kaushal et al., 2001). Lv et al. (2020) also developed cardiac ECM-chitosan-gelatin seeded with CD34-positive EPCs. It is a highly porous, biodegradable, non-toxic, and biocompatible 3D scaffold, which supported reendothelialization of seeded EPCs (Lv et al., 2020).
Tracheal Tissue Regeneration via Stem Cell-Based Tissue Engineering
The native trachea is a hollow tube composed of multiple cartilage rings interspersed with well-vascularized fibrous connective tissue (Udelsman et al., 2018). It is lined by pseudostratified ciliated columnar epithelium and plays an essential role in respiration and indirectly swallowing (Udelsman et al., 2018). Tracheal epithelium also plays a significant role in host defense by eliminating various environmental insults, such as pathogenic bacteria, dust particles, and mold (Nomoto et al., 2006). Narrowing or constriction of the trachea can lead to life-threatening respiratory emergencies because airway stenosis can prevent sufficient air supply to the lungs. Therefore, innovative interventions and treatments for tracheal regeneration have been developed by surgeons using biomaterial and tissue bioengineering technologies.
Current therapeutic approaches for tracheal repair and regeneration include cell-free 3D tissue mimetics (Omori et al., 2005; Tatekawa et al., 2010), autologous tissues (Fabre et al., 2013; Ch’ng et al., 2014), and decellularized autologous tissues, which retain the native organ ultrastructure and are re-cellularized by the recipient-derived cells (Jungebluth et al., 2012a; Elliott et al., 2012; Berg et al., 2014; Haykal et al., 2014). Despite the wide variety of therapeutic strategies, these approaches are limited by low availability, insufficient mechanical strength, and morphological changes during or after transplantation, which can cause a narrowing or blockage of airway (Ch’ng et al., 2014).
In addition, allogeneic grafts increase the risk of disease transmission (microbiological contamination) and immune reactions against donor MHC, suggesting the need for immunosuppressive therapies to appropriately block the immune response against donor antigens in xenogeneic tissues. Due to these limitations, implantation of various fully differentiated allogeneic airway cells into the bioengineered tracheal grafts may be difficult clinically. In this context, stem cells have received substantial attention as an alternative cell source, because not only do they support tissue regeneration but also exhibit significant immunosuppressive function to prevent undesired immune response.
Currently, decellularized trachea is one of the optimal scaffolds, due to its increased pro-angiogenic activities, excellent biocompatibility, and original tubular structure (Jungebluth et al., 2012b). For example, Yao et al. (2021) developed decellularized tracheal scaffolds with vascularized BM-MSC sheet showing strong angiogenic potential. Similarly, Elliott et al. (2017) seeded autologous BM-MSCs onto decellularized tissue-engineered tracheal scaffold and transplanted into a patient with a long-segment congenital tracheal stenosis after conventional reconstructive techniques failed. Xu et al. (2019) also developed decellularized tracheal alternatives to repair long-segment tracheal defects using the laser micropore technique with BM-MSCs. The tracheal scaffold significantly increased the efficiency of cell attachment in addition to ensuring homogenous cell distribution throughout the scaffold. Additionally, the tissue-engineered trachea promoted chondrogenesis of the BM-MSCs and the formation of homogeneous neocartilage in vivo (Xu et al., 2019). Choi et al. (2021) reported that hyaluronic acid-coated polycaprolactone scaffolds resulted in better MSC adhesion rates and greater mucosal regeneration compared with non-coated scaffolds. Dikina et al. (2015) developed high-cell density, scaffold-free BM-MSC-derived cartilaginous rings and tubes, in which TGF β1-delivering gelatin microspheres were incorporated to enhance the chondrogenic differentiation efficiency of MSCs. Xu et al. (2021) developed chondroitin-sulfate-incorporated type-II atelocollagen (COL II/CS) biomimetic tracheas with significant chondrogenic potential using BM-MSCs to facilitate the formation of vascularized fibrous tissues and subsequently mimic the tracheal structure and function. The biomimetic tracheas exhibited successful tracheal reconstruction and showed satisfactory therapeutic outcomes in vivo (Xu et al., 2021).
Furthermore, Taniguchi et al. (2018) fabricated a new scaffold-free artificial trachea using bio-3D printing technology with spheroids consisting of various autologous cell types, such as chondrocytes, endothelial cells, and MSCs. This bio-3D-printed artificial tracheas had sufficient strength to transplant and support chondrogenesis and vasculogenesis in vivo (Taniguchi et al., 2018). In addition, the two-layered artificial trachea was fabricated with 3D-printed polycaprolactone (PCL) microfibers (outer layer) and electrospun PCL nanofibers (inner layer), and then seeded with iPSCs-derived chondrocytes, iPSCs-derived mesenchymal stem cells, and bronchial epithelial cells (Kim et al., 2020). The artificial trachea enhanced the regeneration of tracheal cartilage and tracheal mucosa in a rabbit model of defective tracheal segment (Kim et al., 2020). However, tissue-engineered tracheal transplantation is mainly limited by tracheal collapse associated with chondromalacia of transplantation cartilage (Kobayashi et al., 2010). Chondromalacia of tissue-engineered trachea might be mostly attributed to poor chondrogenic differentiation of seeded stem cells, insufficient cell retention or survival, and inadequate vascularization of transplanted trachea. To address the chondromalacia of transplantation cartilage, Yan et al. designed a new cell sheet scaffold using poly (trimethylene carbonate) (PTMC) and poly (lactic-co-glycolic acid) (PLGA) to fabricate a porous membrane structure for implanting BM-MSCs. Four weeks after transplantation of tissue-engineered trachea, the number of differentiated chondrocytes, the amount of cartilage matrix, and vascularization were significantly increased (Yan et al., 2017).
Reproductive Tract Tissue Regeneration
Naturally thin endometrial thickness or mechanical damage to the endometrial basal layer during artificial abortion may lead to implantation failure and subsequent female infertility (Revel, 2012). Although several pharmacological strategies, including steroid hormones (Sharma et al., 2018) and chemokines/growth factors (Yi et al., 2018) have been used to regenerate the uterine tissue, these therapeutic strategies failed to sufficiently increase endometrial receptivity and subsequent pregnancy outcomes. Therefore, the development of new alternative therapeutic approaches to increase uterine receptivity is imperative. In this context, substantial efforts have been devoted to restore thin or injured endometrium via transplantation of various types of stem cells (Nagori et al., 2011; Singh et al., 2014; Santamaria et al., 2016; Zhao N et al., 2019). Despite positive preliminary results, regenerating the damaged endometrium by administrating stem cells is a huge challenge, due to the lack of an appropriate niche specific for the transplanted stem cells within the tissues, leading to poor therapeutic outcomes.
One of the most promising strategies includes development of artificial microenvironments for endometrium via tissue engineering technologies using endometrial scaffolds. In this context, Xin et al. (2019) developed a collagen scaffold loaded with human UCB-MSCs, which promoted endometrial regeneration and restored fertility by promoting collagen remodeling, intrinsic endometrial cell growth, and the expressions of estrogen and progesterone receptors. Arezoo et al. (2021) developed decellularized uterine scaffolds loaded with menstrual blood stem cells, which differentiated into various endometrial cell types. Miyazaki et al. developed a rat decellularized uterine matrix via aortic perfusion with detergents. The decellularized endometrial tissue was then reseeded with adult and neonatal endometrial cells and MSCs (Miyazaki and Maruyama, 2014). The recellularized uterine matrix was transplanted into a partially excised uterine tissue, leading to successful endometrial regeneration and subsequent pregnancy outcomes almost comparable to normal endometrium (Miyazaki and Maruyama, 2014). Similarly, Campo et al. (2017) decellularized and subsequently recellularized porcine uterine extracellular matrix disks using a human stem cells. Tiemann et al. (2020) also decellularised sheep uterus while maintaining a high integrity of the extracellular components and recellularised with heterogeneous sheep fetal bone marrow stem cells.
In addition, Abbas et al. (2020) generated porous collagen scaffolds loaded with multi-cellular endometrial organoids containing both endometrial stromal and epithelial cells. These seeded cells developed a luminal epithelial layer on the surface of the porous scaffold (Abbas et al., 2020). Recently, Park et al. developed a multiple endometrial stem cell-laden artificial endometrium using two biodegradable natural polymers such as hyaluronic acid and collagen to recapitulate the multicellular and complex structure of endometrial tissue (Park S. R. et al., 2021). Severe tissue injuries were successfully resolved via implantation of stem cell-laden artificial endometrium into a mouse model of endometrial damage (Park S. R. et al., 2021).
The ovary is a highly complex and unique female reproductive organ producing an optimal number of mature oocytes with developmental competence via folliculogenesis and ovulation (Ding et al., 2017). Currently, approximately 1% of women develop premature ovarian failure (POF), also called primary ovarian insufficiency (POI) characterized by hypergonadotropic amenorrhea and subsequent depletion (dysfunction) of ovarian follicles before the age of 40 years (Welt, 2008). Various types of stem cells have been used in an attempt to restore ovarian function in animal models of premature ovarian insufficiency (Sun et al., 2013). For example, endometrial stem cells improve ovarian function, such as oocyte production and serum anti-Müllerian hormone in a rodent model of chemotherapy-induced POF (Reig et al., 2019). However, the stem cell-based therapeutic approaches are restricted by insufficient survival and attachment of administered stem cells within the target sites. Administered therapeutic cells diffused passively to other organs or surrounding tissues (Suuronen et al., 2006; Sun et al., 2013).
Therefore, to overcome these limitations, Su et al. (2016) developed a porous and biodegradable collagen-based matrix for migration and function of transplanted adipose-derived stem cells. Transplanted stem cells with collagen improved ovarian function impaired by chemotherapy by increasing their survival and attachment rates within the ovary, and thereby alleviated premature ovarian insufficiency in rodent models. In addition, the ECM also acts as a reservoir for various bioactive molecules, such as cytokines and growth factors, which stimulate follicular development, growth, and maturation (Sadr et al., 2018; Tomaszewski et al., 2021). Thus, decellularized ovarian ECM-based scaffold provides an optimal and native 3D microenvironment to enhance the therapeutic effects of administered stem cells. Indeed, Pennarossa et al. (2021) produced a decellularized ovarian bioscaffold, which mimics the microarchitecture and biological signals in natural ovarian tissue, and the differentiated mature ovarian cells derived from female germline stem cells were repopulated on decellularized bioscaffolds. Similarly, Hassanpour et al. (2018) developed a human decellularized ovarian scaffold seeded with Wharton jelly-derived MSCs. This ovary-specific scaffold served as a native niche to stem cells and successfully improved ovarian function by increasing serum estradiol and progesterone levels in an ovariectomized animal model (Hassanpour et al., 2018). Similarly, Yang et al. (2019) reported that collagen scaffold loaded with human UCB-MSCs improved ovarian function in mice with premature ovarian failure by enhancing the secretion of estrogen and anti-Mullerian hormone (AMH).
Stem Cell-Based Engineering Combined With Nanotechnology
Stem cell therapies have extraordinary potential for promoting regenerative medicine. However, their clinical application is largely restricted by the lack of effective methods for long-term in vivo monitoring of transplanted cells and insufficient therapeutic effects in vivo (Dong et al., 2021). The integration of stem cell biology with nanotechnology will potentially improve differentiation capacity, survival rates, and attachment, which in turn will significantly enhance the therapeutic outcomes of stem cell therapies in various incurable diseases (Wang et al., 2009).
Effective isolation of undifferentiated cell populations is considered crucial for successful stem cell-based therapeutics. Lui et al. (2013) developed a new method to effectively isolate stem/progenitor cells from the brain using CD133 antibodies conjugated to magnetic nanoparticles (Ab-MNPs), which develop into neurospheres and differentiate into various cell types. Similarly, Jing et al. (2007) selectively isolated CD34+ blood progenitor cells from circulation by CD34 antibody-conjugated magnetic nanoparticle with a purity of 60–96% using the continuous quadrupole magnetic flow sorter (QMS).
Highly advanced nanostructure-based scaffolds or hydrogels for optimal regulation of stem cell behavior have recently received considerable attention in regenerative medicine (Abdal Dayem et al., 2018; Zhang et al., 2021). Nanomaterial-based therapeutic approaches have been designed using various biodegradable and biocompatible nano-carriers or nano-fibers such as self-assembling peptides (Marchini et al., 2020), carbon nanotubes (Lalwani et al., 2017; Naskar et al., 2017), collagen nanoparticles (Bagher et al., 2016), graphene-oxide nanofibers (Zhou et al., 2019), polycaprolactone (PCL) (Lee et al., 2013), tricalcium silicate (C3S) (Jung et al., 2020), and tricalcium phosphate (TCP) (Nuschke et al., 2016) to promote stem cell differentiation and therapeutic outcomes. For example, Chuang et al. (2021) reported that titanium dioxide (TiO2) nanoparticles combined with polybutadiene substrates have a synergistic effect in stimulating proliferation and differentiation of dental pulp stem cells into bone or tooth cells.
Li et al. (2016) also developed a series of bovine serum albumin (BSA)-coated gold (Au) nanospheres with diameters of 40, 70 and 110 nm. The gold nanospheres showed good cytocompatibility without influencing the proliferation of human MSCs and promoted osteogenic differentiation (Li et al., 2016). Similarly, Ko et al. (2015) also designed gold nanoparticles with varying size (15, 30, 50, 75 and 100 nm) and similar surface chemistry and investigated their role in cytotoxicity, proliferation, and osteogenic differentiation of AD-MSCs.
Various carbon nanotube-based scaffolds have been designed to promote stem cell attachment, expansion, and differentiation into specific lineages (Nayak et al., 2010; Baik et al., 2011; Lee et al., 2014). For example, Nayak et al. (2010) developed thin films of pegylated multiwalled carbon nanotubes as porous scaffolds for human MSCs. The functionalized carbon nanotube-based scaffolds did not show cytotoxicity and significantly accelerated osteogenic differentiation of human MSCs (Nayak et al., 2010). Lee et al. (2014) also fabricated carbon nanotube-collagen hydrogels to mimic 3D microenvironment and promote neural differentiation of BM-MSCs and secretion of neurotrophic factors. In addition, carbon nanotubes have recently been utilized in other biomedical applications, such as bioimaging, biosensing, and drug delivery involving various bioactive agents (Harrison and Atala, 2007; Saito et al., 2009). These nanoparticles have been used to identify and track transplanted cells in vivo, and to improve the efficiency of stem cell-based therapy. Well-known nanoparticles for long-term in vivo tracking of transplanted stem cells include quantum dots (Kundrotas et al., 2019), magnetite nanoparticles (Guldris et al., 2017), and gold nanorods (Yu et al., 2021). Carboxylated quantum dots localize mainly to the perinuclear region of human BM-MSCs without adversely affecting their viability, and thus can be used as nonspecific and effective dyes for staining of BM-MSCs (Kundrotas et al., 2019).
Gold nanoparticles have also been widely used in computed tomography (CT) imaging and tracking of transplanted human MSCs because of their excellent biocompatibility and strong photoelectric absorption coefficient (Yu et al., 2021). Interestingly, stem cell-conjugated nanoparticles, which are loaded with therapeutic agents, can also be used as an effective photodynamic platform against tumor cells. For instance, the two-cycle photoactivations of BM-MSCs loaded with positively charged poly-methyl methacrylate core-shell fluorescent nanoparticles (FNPs) significantly induced apoptosis of osteosarcoma cells in both 2D and 3D conditions (Lenna et al., 2020). Recently, nanoparticles were used as non-viral gene delivery systems for stem cells.
Genetic modifications of stem cells have been extensively used to improve their paracrine secretion of certain growth factors, survival rates, and lineage-specific differentiation in vivo, which subsequently enhanced the therapeutic effects of administrated stem cells (Hodgkinson et al., 2010). Traditional viral vector systems have been widely utilized to stimulate delivery and stable expression of specific therapeutic genes with high efficiency in host cells. However, their clinical application is still restricted by possible oncogenic potential, strong immunogenicity, and limited gene-loading capacity (Lehrman, 1999; Walther and Stein, 2000). For example, Green et al. (2008) developed positively charged (∼10 mV) and small (∼200 nm), biodegradable polymeric nanoparticles to enhance nonviral gene delivery efficiency up to 4-fold higher than that of commercially available transfection agents in human ESCs. Xu et al. (2018) also developed iron oxide nanoparticles (IONPs) as an optimal gene delivery platform, which significantly improves the self-renewal capacity and the multi-lineage differentiation potential of human mesenchymal stem cells.
3D Bioprinting With Stem Cell Technology
3D bioprinting is a tissue-engineering technology that allows precise layer by layer deposition of biocompatible materials, live cells, and supporting components (referred to as bioinks) via a computer-controlled process to mimic complex 3D structural architecture and subsequently generate functional tissues or organs (Groll et al., 2016; Moroni et al., 2018). The development of tissue-specific bioinks provides an optimized 3D microenvironment with precise positioning of living cells layer by layer and thus mimics biological and physical properties of native tissues, which in turn facilitates repair of tissue defects and restoration of tissue structure and function (Murphy and Atala, 2014; Mandrycky et al., 2016; Lee et al., 2018). In this context, 3D bioprinting techniques combined with various biomaterials and growth factors overcome the low therapeutic efficacy of stem cell-based therapies for various human diseases by supporting their specific niche and micro-architecture, which influences the cell fates and survival rate (Skeldon et al., 2018).
Currently, multipotent MSCs are one of the most popular stem cell types used in 3D bioprinting, probably due to the relative ease to culture in vitro and high self-renewability, multipotency, and high safety (low immunogenicity and tumorigenicity) compared with other types of stem cells (Skeldon et al., 2018). Zhu et al. (2022) developed 3D-bioprinted bone-like tissue scaffolds using BM-MSCs and multifunctional nanocomposite bioink consisting of alginate dialdehyde-gelatin and mesoporous bioactive glass nanoparticles. Similarly, Costantini et al. (2016) fabricated hydrogel cartilage scaffolds loaded with 3D-bioprinted BM-MSCs using a bioink consisting of chondroitin sulfate amino ethyl methacrylate, gelatin methacrylamide, and hyaluronic acid methacrylate. They reported significantly enhanced viability and chondrogenic differentiation of loaded BM-MSCs within the fabricated hydrogel cartilage scaffolds (Costantini et al., 2016). Restan Perez et al. (2021) also designed neural tissues loaded with patient-derived AD-MSCs using fibrin-based bioink and microfluidic RX1 3D bioprinter, and analyzed the expression of various neural markers, dopamine release, and electrophysiological activity. Daly et al. (2016) fabricated hypertrophic cartilage templates loaded with BM-MSCs with vascularization and mineralization using gamma-irradiated alginate bioink incorporating Arg-Gly-Asp adhesion peptides (). Furthermore, the mechanical properties of this soft cartilage tissue can be reinforced with a network of 3D-printed polycaprolactone fibers. Du et al. (2015) bioprinted BM-MSC-laden methacrylamide gelatin scaffolds carrying collagen-binding domain (CBD)-BMP2-collagen microfibers at the micrometer scale. The CBD-BMP2-collagen microfibers effectively induced differentiation of BM-MSCs into osteogenic lineage within 14 days (Du et al., 2015).
Current 3D bioprinting technology facilitates printing of iPSC-derived differentiated cells or undifferentiated iPSCs mixed with bioinks derived from various biomaterial (Maiullari et al., 2018; Yeung et al., 2019). Importantly, 3D bioprinting of autologous iPSC-derived cells or tissues may not cause immune rejection or infection with organ transplants. Therefore, Kawai et al. (2021) recently developed scaffold-free tubular heart tissues using 3D bioprinting techniques with iPSCs-derived cardiomyocytes, human umbilical vein endothelial cells, and human fibroblasts. The beating of these 3D-bioprinted heart tissues was observed in mice with clear striations of the myocardium and vascularization after 1 month post-transplantation (Kawai et al., 2021). Zhang et al. used 3D-bioprinted endothelial cells within microfibrous hydrogel scaffolds to form a layer of confluent endothelium (Zhang Y. S. et al., 2016). The endothelialized 3D scaffolds were then seeded with human iPSC-derived cardiomyocytes to establish the endothelialized-myocardium organ-on-a-chip for cardiovascular toxicity evaluation (Zhang Y. S. et al., 2016). Ma et al. (2016) 3D bioprinted hydrogel-based microscale hepatic constructs loaded with human iPSCs-derived hepatic progenitor cells, AD-MSCs, and human umbilical vein endothelial cells. This 3D-bioprinted hepatic construct improved the expression of various liver-specific genes, metabolic product secretion, and cytochrome P450 expression (Ma et al., 2016).
In addition, biomimetic tissue-like constructs for neural engineering were developed using 3D bioprinting technologies for neurodegenerative diseases (Thomas and Willerth, 2017). In this context, Sharma et al. (2020) fabricated small spherical neural particles using microfluidics-based RX1 bioprinter with fibrin-based bioink and iPSC-derived neural progenitor cells. These 3D-bioprinted neural tissues expressed various neuronal markers (TUJ1, FOXA2, NURR1, TH, and PAX6) and glial markers (GFAP and O4) (Sharma et al., 2020). Jury et al. (2022) also developed 3D-bioprinted neural structures with hyaluronan and poly (ethylene glycol)-based hydrogels. The neuroepithelial stem cells within hydrogels undergo spontaneous differentiation into neural cells with significantly enhanced proliferation and viability. Huang et al. (2017) developed a graphene–polyurethane composite hydrogel as a potential bioink for enhanced survival and differentiation of neural stem cells within 3D-bioprinted neural tissue structures. The graphene-based hydrogel significantly increased the oxygen metabolism and the neural lineage differentiation of loaded neural stem cells (Huang et al., 2017). Liu et al. 3D-bioprinted neural stem cell-laden scaffolds, which maintain high cell viability (about 95%) and facilitate neural lineage differentiation for optimized neural networks (Liu X. et al., 2021). The 3D-bioprinted neural tissue structure significantly enhanced axon regeneration and reduced glial scar deposition in vivo (Liu X. et al., 2021).
Clinical Trials of Stem Cell-Based Bioengineering Therapeutics
Currently, various stem cell-based tissue engineering products provides novel clinical therapeutic opportunities for a number of degenerative diseases or tissue injury. For example, collagen sponge scaffolds (Condress®, Istituto Gentili, Milano, Italy) seeded with autologous dental pulp stem cells (DPSCs) were transplanted into the chronic periodontitis patients with deep intrabony defect. Various radiographic and clinical parameters were assessed at baseline, 6 and 12 months after surgery. The transplanted sites exhibited significantly more probing depth reduction, bone defect fill, clinical attachment, and periodontal regeneration than control groups (Ferrarotti et al., 2018).
Gjerde et al. (2018) also implanted biphasic calcium phosphate scaffolds loaded with bone marrow-derived MSCs into the resorbed alveolar ridge in the patients with severe mandibular ridge resorption. After 4–6 months of healing, new bone formation was assessed radiographically and clinically. The loaded MSCs could expand on the scaffolds and successfully induce significant formation of new bone, without adverse events.
In addition, Ding et al. (2018) observed that umbilical cord-derived MSCs on a collagen-based scaffold can restore the functions of primordial follicles through FOXO1 and FOXO3a signaling pathways in vitro. Transplantation of this collagen scaffold loaded with MSCs to the dormant ovaries of premature ovarian failure (POF) patients reactivated various ovarian functions, such as estrogen secretion, follicular growth, and ovulation. Successful clinical pregnancy was achieved in patients with POF after implantation of this collagen scaffold loaded with MSCs.
Recently, Meamar et al. seeded human placenta-derived MSCs onto the electrospun gelatin nanofibrous scaffolds (GNS) and cultured with platelet-rich plasma (PRP) for 7 days. This scaffold then implanted to the patients with diabetic foot ulcers (DFUs) and then various clinical parameters were assessed after 12-week. Indeed, wound healing, new capillary formation, and pain-free walking distance were significantly increased by the implantation of placenta-derived MSCs seeded GNS (Meamar et al., 2021).
Discussion
Currently, a number of biomaterials and multiple types of stem cells are available to mimic the structure, architecture, and function of specific tissues. Several studies continue to increase our understanding of transplanted tissue mimetics and their development in vivo to identify the stem cell type for clinical application. Further studies are needed to provide comprehensive insight into the growth and integration of stem cell-loaded tissue mimetics with host tissues. Indeed, various synthetic or natural biomaterial-based scaffolds can provide a longer and more efficient cellular microenvironment for administrated stem cells retention and survival rates in injured tissues and subsequently enhance their therapeutic potential after administration. For example, insulin like growth factor-1 (IGF-I) conjugated fibrin micro-beads significantly increased bulk stability and muscle regeneration capacity of human smooth muscle cells (Vardar et al., 2019).
Although stem cell-based tissue engineering therapeutic strategies have demonstrated successful outcomes in preclinical and clinical trials, several challenges still remain before their clinical application can be envisaged. Indeed, various stem cell-based tissue engineering products provides novel clinical therapeutic opportunities for a number of degenerative diseases or tissue injury. For example, collagen sponge scaffolds seeded with autologous dental pulp stem cells (DPSCs) were transplanted into the chronic periodontitis patients with deep intrabony defect (Ferrarotti et al., 2018). Gjerde et al also implanted biphasic calcium phosphate scaffolds loaded with bone marrow-derived MSCs into the resorbed alveolar ridge in the patients with severe mandibular ridge resorption (Gjerde et al., 2018). However, further studies are needed to address the immunological and related issues, challenges associated with appropriate integration of stem cell-loaded engineered mimetics into host tissues and their high variation in therapeutic efficiency, effects on the surrounding tissues, disease progression, age, or transplantation route. Thus, interdisciplinary research is absolutely necessary before full clinical applications of stem cell-based tissue engineering can be utilized n tissue repair and regeneration.
In addition, the interactions of transplanted stem cells and biomaterial-based scaffolds within the injured tissues are complex and multi-factorial, in addition to the need for an optimal platform to promote the functional restoration following injury. Focused investigations are needed to evaluate the types and combinations of national or synthetic biomaterials required to increase survival rate and multilineage differentiation of transplanted stem cells within 3D porous scaffolds and their interaction with host cells and microenvironment at the site of injury. Interestingly, various synthetic or natural biomaterial-based scaffolds can provide a longer and more efficient cellular microenvironment for administrated stem cells retention and survival rates in injured tissues and subsequently enhance their therapeutic potential after administration. Indeed, IGF-I conjugated fibrin micro-beads significantly increased bulk stability and muscle regeneration capacity of human smooth muscle cells (Vardar et al., 2019). In addition, the combination of chitosan-coated silicone tube and neurosphere cells induced from human adipose-derived MSCs exhibited substantial improvements in nerve regeneration (Hsueh et al., 2014).
Chemical groups, surface structure, physical properties or technique of biomaterial preparation need to be intensively analyzed to regulate the behavior and fate of transplanted stem cells. Because different tissues or cells have their own chemical compositions and physical characteristics, the designed biomaterials used in tissue engineering should have a strong functional and structural affinity for targeted tissue and cell types to properly stimulate tissue regeneration (Abbas et al., 2020; Peressotti et al., 2021). The structural or mechanical properties of the biomaterial surface such as charges, chemical compositions, and hydrophobicity may play key roles in regulating their diverse biological functions (Echeverria Molina et al., 2021; Pearce and O’reilly, 2021).
Further, the characteristics and tissue origin of transplanted stem cells should be determined before their application in the synthesis of biomaterial-based 3D porous scaffolds (Redondo et al., 2017). For example, stem cells derived from tissues with high turnover rates such as bone marrow, intestinal tract, and skin undergo rapid proliferation and regeneration, whereas stem cells isolated from the muscles and liver facilitate tissue regeneration (Redondo et al., 2017). By contrast, stem cells isolated from inactive regenerating tissues such as heart and brain exhibit low proliferation and poor regenerative potential (Redondo et al., 2017).
Author Contributions
I-SH performed the design, writing, and proofreading of this manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2021R1A2C2008424). This work was also supported by the Gachon University research fund of 2021 (GCU-202103460001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, Y., Brunel, L. G., Hollinshead, M. S., Fernando, R. C., Gardner, L., Duncan, I., et al. (2020). Generation of a Three-Dimensional Collagen Scaffold-Based Model of the Human Endometrium. Interface Focus. 10, 20190079. doi:10.1098/rsfs.2019.0079
Abbott, R. D., Howe, A. K., Langevin, H. M., and Iatridis, J. C. (2012). Live free or die: stretch-induced apoptosis occurs when adaptive reorientation of annulus fibrosus cells is restricted. Biochem. Biophysical Res. Commun. 421, 361–366. doi:10.1016/j.bbrc.2012.04.018
Abdal Dayem, A., Lee, S. B., and Cho, S. G. (2018). The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomater. (Basel) 8, 761. doi:10.3390/nano8100761
Abolgheit, S., Abdelkader, S., Aboushelib, M., Omar, E., and Mehanna, R. (2021). Bone Marrow-Derived Mesenchymal Stem Cells and Extracellular Vesicles Enriched Collagen Chitosan Scaffold in Skin Wound Healing (A Rat Model). J. Biomater. Appl. 36, 128–139. doi:10.1177/0885328220963920
Aguado, B. A., Mulyasasmita, W., Su, J., Lampe, K. J., and Heilshorn, S. C. (2012). Improving Viability of Stem Cells during Syringe Needle Flow through the Design of Hydrogel Cell Carriers. Tissue Eng. Part A 18, 806–815. doi:10.1089/ten.tea.2011.0391
Ahmad, T., Shin, H. J., Lee, J., Shin, Y. M., Perikamana, S. K. M., Park, S. Y., et al. (2018). Fabrication of In Vitro 3D Mineralized Tissue by Fusion of Composite Spheroids Incorporating Biomineral-Coated Nanofibers and Human Adipose-Derived Stem Cells. Acta Biomater. 74, 464–477. doi:10.1016/j.actbio.2018.05.035
Alharthi, R., Alnahdi, M. A., Alharthi, A., Almutairi, S., Al-Khenaizan, S., and Albalwi, M. A. (2021). Genetic Profile of Epidermolysis Bullosa Cases in King Abdulaziz Medical City, Riyadh, Saudi Arabia. Front. Genet. 12, 753229. doi:10.3389/fgene.2021.753229
Ali, S. A., Pappas, I. S., and Parnavelas, J. G. (1998). Collagen Type IV Promotes the Differentiation of Neuronal Progenitors and Inhibits Astroglial Differentiation in Cortical Cell Cultures. Dev. Brain Res. 110, 31–38. doi:10.1016/s0165-3806(98)00091-1
Allison, D. D., and Grande-Allen, K. J. (2006). Review. Hyaluronan: a Powerful Tissue Engineering Tool. Tissue Eng. 12, 2131–2140. doi:10.1089/ten.2006.12.2131
Antmen, E., Vrana, N. E., and Hasirci, V. (2021). The Role of Biomaterials and Scaffolds in Immune Responses in Regenerative Medicine: Macrophage Phenotype Modulation by Biomaterial Properties and Scaffold Architectures. Biomater. Sci. 9, 8090–8110. doi:10.1039/d1bm00840d
Arezoo, N., Mohammad, H., and Malihezaman, M. (2021). Tissue Engineering of Mouse Uterus Using Menstrual Blood Stem Cells (MenSCs) and Decellularized Uterine Scaffold. Stem Cell Res. Ther. 12, 475. doi:10.1186/s13287-021-02543-y
Arulmoli, J., Wright, H. J., Phan, D. T. T., Sheth, U., Que, R. A., Botten, G. A., et al. (2016). Combination Scaffolds of Salmon Fibrin, Hyaluronic Acid, and Laminin for Human Neural Stem Cell and Vascular Tissue Engineering. Acta Biomater. 43, 122–138. doi:10.1016/j.actbio.2016.07.043
Bagher, Z., Azami, M., Ebrahimi-Barough, S., Mirzadeh, H., Solouk, A., Soleimani, M., et al. (2016). Differentiation of Wharton's Jelly-Derived Mesenchymal Stem Cells into Motor Neuron-like Cells on Three-Dimensional Collagen-Grafted Nanofibers. Mol. Neurobiol. 53, 2397–2408. doi:10.1007/s12035-015-9199-x
Baik, K. Y., Park, S. Y., Heo, K., Lee, K.-B., and Hong, S. (2011). Carbon Nanotube Monolayer Cues for Osteogenesis of Mesenchymal Stem Cells. Small 7, 741–745. doi:10.1002/smll.201001930
Baker, B. M., and Chen, C. S. (2012). Deconstructing the Third Dimension: How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci. 125, 3015–3024. doi:10.1242/jcs.079509
Barbier, M. A., Piaceski, A. D., Larouche, D., Villeneuve, S. H., Ghani, K., Pope, E., et al. (2022). Efficient Gamma-Retroviral Transduction of Primary Human Skin Cells Using the EF-C Peptide as a Transduction Enhancer. Curr. Protoc. 2, e353. doi:10.1002/cpz1.353
Bardy, J. a., Chen, A. K., Lim, Y. M., Wu, S., Wei, S., Weiping, H., et al. (2013). Microcarrier Suspension Cultures for High-Density Expansion and Differentiation of Human Pluripotent Stem Cells to Neural Progenitor Cells. Tissue Eng. Part C. Methods 19, 166–180. doi:10.1089/ten.tec.2012.0146
Barthes, J., Özçelik, H., Hindié, M., Ndreu-Halili, A., Hasan, A., and Vrana, N. E. (2014). Cell Microenvironment Engineering and Monitoring for Tissue Engineering and Regenerative Medicine: the Recent Advances. Biomed. Res. Int. 2014, 921905. doi:10.1155/2014/921905
Battafarano, G., Rossi, M., De Martino, V., Marampon, F., Borro, L., Secinaro, A., et al. (2021). Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 22, 1128. doi:10.3390/ijms22031128
Bauer, J. W., Koller, J., Murauer, E. M., De Rosa, L., Enzo, E., Carulli, S., et al. (2017). Closure of a Large Chronic Wound through Transplantation of Gene-Corrected Epidermal Stem Cells. J. Investigative Dermatology 137, 778–781. doi:10.1016/j.jid.2016.10.038
Belludi, S. A., Singhal, L., and Gubbala, M. (2021). Peripheral Blood Mesenchymal Stem Cells and Platelet Rich Fibrin Matrix in the Management of Class II Gingival Recession: A Case Report. J. Dent. (Shiraz) 22, 67–70. doi:10.30476/DENTJODS.2020.81784.0
Berg, M., Ejnell, H., Kovács, A., Nayakawde, N., Patil, P. B., Joshi, M., et al. (2014). RETRACTED: Replacement of a Tracheal Stenosis with a Tissue-Engineered Human Trachea Using Autologous Stem Cells: A Case Report. Tissue Eng. Part A 20, 389–397. doi:10.1089/ten.tea.2012.0514
Bhardwaj, N., Chouhan, D., and Mandal, B. B. (2017). Tissue Engineered Skin and Wound Healing: Current Strategies and Future Directions. Curr. Pharm. Des. 23, 3455–3482. doi:10.2174/1381612823666170526094606
Biggs, L. C., Kim, C. S., Miroshnikova, Y. A., and Wickström, S. A. (2020). Mechanical Forces in the Skin: Roles in Tissue Architecture, Stability, and Function. J. Investigative Dermatology 140, 284–290. doi:10.1016/j.jid.2019.06.137
Bonferoni, M. C., Caramella, C., Catenacci, L., Conti, B., Dorati, R., Ferrari, F., et al. (2021). Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field. Pharmaceutics 13, 1341. doi:10.3390/pharmaceutics13091341
Burdick, J. A., and Prestwich, G. D. (2011). Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mat. 23, H41–H56. doi:10.1002/adma.201003963
Campo, H., Baptista, P. M., López-Pérez, N., Faus, A., Cervelló, I., and Simón, C. (2017). De- and Recellularization of the Pig Uterus: a Bioengineering Pilot Study. Biol. Reprod. 96, 34–45. doi:10.1095/biolre/bio143396
Cao, Q.-l., Zhang, Y. P., Howard, R. M., Walters, W. M., Tsoulfas, P., and Whittemore, S. R. (2001). Pluripotent Stem Cells Engrafted into the Normal or Lesioned Adult Rat Spinal Cord Are Restricted to a Glial Lineage. Exp. Neurol. 167, 48–58. doi:10.1006/exnr.2000.7536
Ch'ng, S., Wong, G. L., and Clark, J. R. (2014). Reconstruction of the Trachea. J. Reconstr. Microsurg 30, 153–162. doi:10.1055/s-0033-1358786
Chen, J., Luo, L., Tian, R., and Yu, C. (2021a). A Review and Update for Registered Clinical Studies of Stem Cells for Non-tumorous and Non-hematological Diseases. Regen. Ther. 18, 355–362. doi:10.1016/j.reth.2021.09.001
Chen, X., Wang, F., Huang, Z., Wu, Y., Geng, J., and Wang, Y. (2021b). Clinical Applications of Mesenchymal Stromal Cell-Based Therapies for Pulmonary Diseases: An Update and Concise Review. Int. J. Med. Sci. 18, 2849–2870. doi:10.7150/ijms.59218
Choi, J. S., Lee, M. S., Kim, J., Eom, M. R., Jeong, E. J., Lee, M., et al. (2021). Hyaluronic Acid Coating on Hydrophobic Tracheal Scaffold Enhances Mesenchymal Stem Cell Adhesion and Tracheal Regeneration. Tissue Eng. Regen. Med. 18, 225–233. doi:10.1007/s13770-021-00335-2
Chuang, Y.-C., Chang, C.-C., Yang, F., Simon, M., and Rafailovich, M. (2021). TiO2 Nanoparticles Synergize with Substrate Mechanics to Improve Dental Pulp Stem Cells Proliferation and Differentiation. Mater. Sci. Eng. C 118, 111366. doi:10.1016/j.msec.2020.111366
Cidonio, G., Glinka, M., Kim, Y.-H., Kanczler, J. M., Lanham, S. A., Ahlfeld, T., et al. (2020). Nanoclay-based 3D Printed Scaffolds Promote Vascular Ingrowth Ex Vivo and Generate Bone Mineral Tissue In Vitro and In Vivo. Biofabrication 12, 035010. doi:10.1088/1758-5090/ab8753
Costantini, M., Idaszek, J., Szöke, K., Jaroszewicz, J., Dentini, M., Barbetta, A., et al. (2016). 3D Bioprinting of BM-MSCs-Loaded ECM Biomimetic Hydrogels for In Vitro Neocartilage Formation. Biofabrication 8, 035002. doi:10.1088/1758-5090/8/3/035002
Dakiw Piaceski, A., Larouche, D., Larouche, D., Ghani, K., Bisson, F., Cortez Ghio, S., et al. (2018). Translating the Combination of Gene Therapy and Tissue Engineering for Treating Recessive Dystrophic Epidermolysis Bullosa. eCM 35, 73–86. doi:10.22203/ecm.v035a06
Daly, A. C., Cunniffe, G. M., Sathy, B. N., Jeon, O., Alsberg, E., and Kelly, D. J. (2016). 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering. Adv. Healthc. Mat. 5, 2353–2362. doi:10.1002/adhm.201600182
Dash, B. C., Xu, Z., Lin, L., Koo, A., Ndon, S., Berthiaume, F., et al. (2018). Stem Cells and Engineered Scaffolds for Regenerative Wound Healing. Bioeng. (Basel) 5, 23. doi:10.3390/bioengineering5010023
De Rosa, L., Carulli, S., Cocchiarella, F., Quaglino, D., Enzo, E., Franchini, E., et al. (2014). Long-term Stability and Safety of Transgenic Cultured Epidermal Stem Cells in Gene Therapy of Junctional Epidermolysis Bullosa. Stem Cell Rep. 2, 1–8. doi:10.1016/j.stemcr.2013.11.001
Deepa, S. S., Carulli, D., Galtrey, C., Rhodes, K., Fukuda, J., Mikami, T., et al. (2006). Composition of Perineuronal Net Extracellular Matrix in Rat Brain. J. Biol. Chem. 281, 17789–17800. doi:10.1074/jbc.m600544200
Di Meglio, P., Perera, G. K., and Nestle, F. O. (2011). The Multitasking Organ: Recent Insights into Skin Immune Function. Immunity 35, 857–869. doi:10.1016/j.immuni.2011.12.003
Dikina, A. D., Strobel, H. A., Lai, B. P., Rolle, M. W., and Alsberg, E. (2015). Engineered Cartilaginous Tubes for Tracheal Tissue Replacement via Self-Assembly and Fusion of Human Mesenchymal Stem Cell Constructs. Biomaterials 52, 452–462. doi:10.1016/j.biomaterials.2015.01.073
Ding, C., Li, H., Wang, Y., Wang, F., Wu, H., Chen, R., et al. (2017). Different Therapeutic Effects of Cells Derived from Human Amniotic Membrane on Premature Ovarian Aging Depend on Distinct Cellular Biological Characteristics. Stem Cell Res. Ther. 8, 173. doi:10.1186/s13287-017-0613-3
Ding, L., Yan, G., Wang, B., Xu, L., Gu, Y., Ru, T., et al. (2018). Transplantation of UC-MSCs on Collagen Scaffold Activates Follicles in Dormant Ovaries of POF Patients with Long History of Infertility. Sci. China Life Sci. 61, 1554–1565. doi:10.1007/s11427-017-9272-2
Dong, Y., Cui, M., Qu, J., Wang, X., Kwon, S. H., Barrera, J., et al. (2020). Conformable Hyaluronic Acid Hydrogel Delivers Adipose-Derived Stem Cells and Promotes Regeneration of Burn Injury. Acta Biomater. 108, 56–66. doi:10.1016/j.actbio.2020.03.040
Dong, Y., Wu, X., Chen, X., Zhou, P., Xu, F., and Liang, W. (2021). Nanotechnology Shaping Stem Cell Therapy: Recent Advances, Application, Challenges, and Future Outlook. Biomed. Pharmacother. 137, 111236. doi:10.1016/j.biopha.2021.111236
Du, M., Chen, B., Meng, Q., Liu, S., Zheng, X., Zhang, C., et al. (2015). 3D Bioprinting of BMSC-Laden Methacrylamide Gelatin Scaffolds with CBD-BMP2-Collagen Microfibers. Biofabrication 7, 044104. doi:10.1088/1758-5090/7/4/044104
Echeverria Molina, M. I., Malollari, K. G., and Komvopoulos, K. (2021). Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 9, 617141. doi:10.3389/fbioe.2021.617141
Elliott, M. J., Butler, C. R., Varanou-Jenkins, A., Partington, L., Carvalho, C., Samuel, E., et al. (2017). Tracheal Replacement Therapy with a Stem Cell-Seeded Graft: Lessons from Compassionate Use Application of a GMP-Compliant Tissue-Engineered Medicine. Stem Cells Transl. Med. 6, 1458–1464. doi:10.1002/sctm.16-0443
Elliott, M. J., De Coppi, P., Speggiorin, S., Roebuck, D., Butler, C. R., Samuel, E., et al. (2012). Stem-cell-based, Tissue Engineered Tracheal Replacement in a Child: a 2-year Follow-Up Study. Lancet 380, 994–1000. doi:10.1016/s0140-6736(12)60737-5
Escárcega-González, C. E., Garza-Cervantes, J. A., Vazquez-Rodríguez, A., Montelongo-Peralta, L. Z., Treviño-Gonzalez, M. T., Díaz Barriga Castro, E., et al. (2018). In Vivo antimicrobial Activity of Silver Nanoparticles Produced via a Green Chemistry Synthesis Using Acacia Rigidula as a Reducing and Capping Agent. Ijn 13, 2349–2363. doi:10.2147/ijn.s160605
Fabre, D., Kolb, F., Fadel, E., Mercier, O., Mussot, S., Le Chevalier, T., et al. (2013). Successful Tracheal Replacement in Humans Using Autologous Tissues: an 8-year Experience. Ann. Thorac. Surg. 96, 1146–1155. doi:10.1016/j.athoracsur.2013.05.073
Ferguson, E. L., Naseer, S., Powell, L. C., Hardwicke, J., Young, F. I., Zhu, B., et al. (2018). Controlled Release of Dextrin-Conjugated Growth Factors to Support Growth and Differentiation of Neural Stem Cells. Stem Cell Res. 33, 69–78. doi:10.1016/j.scr.2018.10.008
Ferrarotti, F., Romano, F., Gamba, M. N., Quirico, A., Giraudi, M., Audagna, M., et al. (2018). Human Intrabony Defect Regeneration with Micrografts Containing Dental Pulp Stem Cells: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 45, 841–850. doi:10.1111/jcpe.12931
Florencio-Silva, R., Sasso, G. R., Sasso-Cerri, E., Simões, M. J., and Cerri, P. S. (2015). Biology of Bone Tissue: Structure, Function, and Factors that Influence Bone Cells. Biomed. Res. Int. 2015, 421746. doi:10.1155/2015/421746
Formigli, L., Paternostro, F., Tani, A., Mirabella, C., Quattrini Li, A., Nosi, D., et al. (2015). MSCs Seeded on Bioengineered Scaffolds Improve Skin Wound Healing in Rats. Wound Repair Regen. 23, 115–123. doi:10.1111/wrr.12251
Fuchs, E., and Blau, H. M. (2020). Tissue Stem Cells: Architects of Their Niches. Cell Stem Cell 27, 532–556. doi:10.1016/j.stem.2020.09.011
Gaffey, A. C., Chen, M. H., Venkataraman, C. M., Trubelja, A., Rodell, C. B., Dinh, P. V., et al. (2015). Injectable Shear-Thinning Hydrogels Used to Deliver Endothelial Progenitor Cells, Enhance Cell Engraftment, and Improve Ischemic Myocardium. J. Thorac. Cardiovasc. Surg. 150, 1268–1277. doi:10.1016/j.jtcvs.2015.07.035
Gao, L. R., Chen, Y., Zhang, N. K., Yang, X. L., Liu, H. L., Wang, Z. G., et al. (2015). Intracoronary Infusion of Wharton's Jelly-Derived Mesenchymal Stem Cells in Acute Myocardial Infarction: Double-Blind, Randomized Controlled Trial. BMC Med. 13, 162. doi:10.1186/s12916-015-0399-z
García-Bernal, D., García-Arranz, M., Yáñez, R. M., Hervás-Salcedo, R., Cortés, A., Fernández-García, M., et al. (2021). The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy. Front. Cell Dev. Biol. 9, 650664. doi:10.3389/fcell.2021.650664
Gelmi, A., and Schutt, C. E. (2021). Stimuli-Responsive Biomaterials: Scaffolds for Stem Cell Control. Adv. Healthc. Mater 10, e2001125. doi:10.1002/adhm.202001125
Gilbert, P. M., Havenstrite, K. L., Magnusson, K. E. G., Sacco, A., Leonardi, N. A., Kraft, P., et al. (2010). Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science 329, 1078–1081. doi:10.1126/science.1191035
Gjerde, C., Mustafa, K., Hellem, S., Rojewski, M., Gjengedal, H., Yassin, M. A., et al. (2018). Cell Therapy Induced Regeneration of Severely Atrophied Mandibular Bone in a Clinical Trial. Stem Cell Res. Ther. 9, 213. doi:10.1186/s13287-018-0951-9
Gogele, C., Muller, S., Belov, S., Pradel, A., Wiltzsch, S., Lenhart, A., et al. (2022). Biodegradable Poly(D-L-Lactide-Co-Glycolide) (PLGA)-Infiltrated Bioactive Glass (CAR12N) Scaffolds Maintain Mesenchymal Stem Cell Chondrogenesis for Cartilage Tissue Engineering. Cells 11, 1577. doi:10.3390/cells11091577
Gong, Z., and Niklason, L. E. (2008). Small‐diameter Human Vessel Wall Engineered from Bone Marrow‐derived Mesenchymal Stem Cells (hMSCs). FASEB J. 22, 1635–1648. doi:10.1096/fj.07-087924
Grässel, S., and Lorenz, J. (2014). Tissue-engineering Strategies to Repair Chondral and Osteochondral Tissue in Osteoarthritis: Use of Mesenchymal Stem Cells. Curr. Rheumatol. Rep. 16, 452. doi:10.1007/s11926-014-0452-5
Green, J. J., Zhou, B. Y., Mitalipova, M. M., Beard, C., Langer, R., Jaenisch, R., et al. (2008). Nanoparticles for Gene Transfer to Human Embryonic Stem Cell Colonies. Nano Lett. 8, 3126–3130. doi:10.1021/nl8012665
Groll, J., Boland, T., Blunk, T., Burdick, J. A., Cho, D.-W., Dalton, P. D., et al. (2016). Biofabrication: Reappraising the Definition of an Evolving Field. Biofabrication 8, 013001. doi:10.1088/1758-5090/8/1/013001
Guldris, N., Argibay, B., Gallo, J., Iglesias-Rey, R., Carbó-Argibay, E., Kolen’ko, Y. V., et al. (2017). Magnetite Nanoparticles for Stem Cell Labeling with High Efficiency and Long-Term In Vivo Tracking. Bioconjugate Chem. 28, 362–370. doi:10.1021/acs.bioconjchem.6b00522
Gurevich, I., Agarwal, P., Zhang, P., Dolorito, J. A., Oliver, S., Liu, H., et al. (2022). In Vivo topical Gene Therapy for Recessive Dystrophic Epidermolysis Bullosa: a Phase 1 and 2 Trial. Nat. Med. 28, 780–788. doi:10.1038/s41591-022-01737-y
Harada, N., Watanabe, Y., Sato, K., Abe, S., Yamanaka, K., Sakai, Y., et al. (2014). Bone Regeneration in a Massive Rat Femur Defect through Endochondral Ossification Achieved with Chondrogenically Differentiated MSCs in a Degradable Scaffold. Biomaterials 35, 7800–7810. doi:10.1016/j.biomaterials.2014.05.052
Harris, L. J., Abdollahi, H., Zhang, P., Mcilhenny, S., Tulenko, T. N., and Dimuzio, P. J. (2011). Differentiation of Adult Stem Cells into Smooth Muscle for Vascular Tissue Engineering. J. Surg. Res. 168, 306–314. doi:10.1016/j.jss.2009.08.001
Harrison, B. S., and Atala, A. (2007). Carbon Nanotube Applications for Tissue Engineering. Biomaterials 28, 344–353. doi:10.1016/j.biomaterials.2006.07.044
Hassanpour, A., Talaei-Khozani, T., Kargar-Abarghouei, E., Razban, V., and Vojdani, Z. (2018). Decellularized Human Ovarian Scaffold Based on a Sodium Lauryl Ester Sulfate (SLES)-treated Protocol, as a Natural Three-Dimensional Scaffold for Construction of Bioengineered Ovaries. Stem Cell Res. Ther. 9, 252. doi:10.1186/s13287-018-0971-5
Haykal, S., Salna, M., Zhou, Y., Marcus, P., Fatehi, M., Frost, G., et al. (2014). Double-chamber Rotating Bioreactor for Dynamic Perfusion Cell Seeding of Large-Segment Tracheal Allografts: Comparison to Conventional Static Methods. Tissue Eng. Part C. Methods 20, 681–692. doi:10.1089/ten.tec.2013.0627
He, N., Zhang, L., Cui, J., and Li, Z. (2014). Bone Marrow Vascular Niche: Home for Hematopoietic Stem Cells. Bone Marrow Res. 2014, 128436. doi:10.1155/2014/128436
Hernández, R., Jiménez-Luna, C., Perales-Adán, J., Perazzoli, G., Melguizo, C., and Prados, J. (2020). Differentiation of Human Mesenchymal Stem Cells towards Neuronal Lineage: Clinical Trials in Nervous System Disorders. Biomol. Ther. 28, 34–44. doi:10.4062/biomolther.2019.065
Heydarkhan-Hagvall, S., Schenke-Layland, K., Yang, J. Q., Heydarkhan, S., Xu, Y., Zuk, P. A., et al. (2008). Human Adipose Stem Cells: a Potential Cell Source for Cardiovascular Tissue Engineering. Cells Tissues Organs 187, 263–274. doi:10.1159/000113407
Hirsch, T., Rothoeft, T., Teig, N., Bauer, J. W., Pellegrini, G., De Rosa, L., et al. (2017). Regeneration of the Entire Human Epidermis Using Transgenic Stem Cells. Nature 551, 327–332. doi:10.1038/nature24487
Hodgkinson, C. P., Gomez, J. A., Mirotsou, M., and Dzau, V. J. (2010). Genetic Engineering of Mesenchymal Stem Cells and its Application in Human Disease Therapy. Hum. Gene Ther. 21, 1513–1526. doi:10.1089/hum.2010.165
Hsueh, Y.-Y., Chang, Y.-J., Huang, T.-C., Fan, S.-C., Wang, D.-H., Jason Chen, J.-J., et al. (2014). Functional Recoveries of Sciatic Nerve Regeneration by Combining Chitosan-Coated Conduit and Neurosphere Cells Induced from Adipose-Derived Stem Cells. Biomaterials 35, 2234–2244. doi:10.1016/j.biomaterials.2013.11.081
Huang, C.-T., Kumar Shrestha, L., Ariga, K., and Hsu, S.-h. (2017). A Graphene-Polyurethane Composite Hydrogel as a Potential Bioink for 3D Bioprinting and Differentiation of Neural Stem Cells. J. Mat. Chem. B 5, 8854–8864. doi:10.1039/c7tb01594a
Huang, X., Huang, D., Zhu, T., Yu, X., Xu, K., Li, H., et al. (2021). Sustained Zinc Release in Cooperation with CaP Scaffold Promoted Bone Regeneration via Directing Stem Cell Fate and Triggering a Pro-healing Immune Stimuli. J. Nanobiotechnol 19, 207. doi:10.1186/s12951-021-00956-8
Hughes, K. J., Chambers, K. T., Meares, G. P., and Corbett, J. A. (2009). Nitric Oxides Mediates a Shift from Early Necrosis to Late Apoptosis in Cytokine-Treated β-cells that Is Associated with Irreversible DNA Damage. Am. J. Physiology-Endocrinology Metabolism 297, E1187–E1196. doi:10.1152/ajpendo.00214.2009
Jackson, C. J., Tønseth, K. A., and Utheim, T. P. (2017). Cultured Epidermal Stem Cells in Regenerative Medicine. Stem Cell Res. Ther. 8, 155. doi:10.1186/s13287-017-0587-1
Janzen, V., Forkert, R., Fleming, H. E., Saito, Y., Waring, M. T., Dombkowski, D. M., et al. (2006). Stem-cell Ageing Modified by the Cyclin-dependent Kinase Inhibitor p16INK4a. Nature 443, 421–426. doi:10.1038/nature05159
Jing, Y., Moore, L. R., Williams, P. S., Chalmers, J. J., Farag, S. S., Bolwell, B., et al. (2007). Blood Progenitor Cell Separation from Clinical Leukapheresis Product by Magnetic Nanoparticle Binding and Magnetophoresis. Biotechnol. Bioeng. 96, 1139–1154. doi:10.1002/bit.21202
Jung, Y., Yoon, J. Y., Dev Patel, K., Ma, L., Lee, H. H., Kim, J., et al. (2020). Biological Effects of Tricalcium Silicate Nanoparticle-Containing Cement on Stem Cells from Human Exfoliated Deciduous Teeth. Nanomater. (Basel) 10, 1373. doi:10.3390/nano10071373
Jungebluth, P., Bader, A., Baiguera, S., Möller, S., Jaus, M., Lim, M. L., et al. (2012a). The Concept of In Vivo Airway Tissue Engineering. Biomaterials 33, 4319–4326. doi:10.1016/j.biomaterials.2012.03.016
Jungebluth, P., Moll, G., Baiguera, S., and Macchiarini, P. (2012b). Tissue-engineered Airway: a Regenerative Solution. Clin. Pharmacol. Ther. 91, 81–93. doi:10.1038/clpt.2011.270
Jury, M., Matthiesen, I., Boroojeni, F. R., Ludwig, S. L., Civitelli, L., Winkler, T. E., et al. (2022). Bioorthogonally Cross-Linked Hyaluronan-Laminin Hydrogels for 3D Neuronal Cell Culture and Biofabrication. Adv. Healthc. Mater 11, e2102097. doi:10.1002/adhm.202102097
Katoh, H., Yokota, K., and Fehlings, M. G. (2019). Regeneration of Spinal Cord Connectivity through Stem Cell Transplantation and Biomaterial Scaffolds. Front. Cell. Neurosci. 13, 248. doi:10.3389/fncel.2019.00248
Kaushal, S., Amiel, G. E., Guleserian, K. J., Shapira, O. M., Perry, T., Sutherland, F. W., et al. (2001). Functional Small-Diameter Neovessels Created Using Endothelial Progenitor Cells Expanded Ex Vivo. Nat. Med. 7, 1035–1040. doi:10.1038/nm0901-1035
Kawai, Y., Tohyama, S., Arai, K., Tamura, T., Soma, Y., Fukuda, K., et al. (2021). Scaffold-Free Tubular Engineered Heart Tissue from Human Induced Pluripotent Stem Cells Using Bio-3D Printing Technology In Vivo. Front. Cardiovasc Med. 8, 806215. doi:10.3389/fcvm.2021.806215
Kazemi, T., Mohammadpour, A. A., Matin, M. M., Mahdavi-Shahri, N., Dehghani, H., and Kazemi Riabi, S. H. (2021). Decellularized Bovine Aorta as a Promising 3D Elastin Scaffold for Vascular Tissue Engineering Applications. Regen. Med. 16, 1037–1050. doi:10.2217/rme-2021-0062
Khan, I., Ali, A., Akhter, M. A., Naeem, N., Chotani, M. A., Iqbal, H., et al. (2017). Epac-Rap1-activated Mesenchymal Stem Cells Improve Cardiac Function in Rat Model of Myocardial Infarction. Cardiovasc Ther. 35, 1. doi:10.1111/1755-5922.12248
Kim, H. S., Mandakhbayar, N., Kim, H.-W., Leong, K. W., and Yoo, H. S. (2021). Protein-reactive Nanofibrils Decorated with Cartilage-Derived Decellularized Extracellular Matrix for Osteochondral Defects. Biomaterials 269, 120214. doi:10.1016/j.biomaterials.2020.120214
Kim, I. G., Park, S. A., Lee, S.-H., Choi, J. S., Cho, H., Lee, S. J., et al. (2020). Transplantation of a 3D-Printed Tracheal Graft Combined with iPS Cell-Derived MSCs and Chondrocytes. Sci. Rep. 10, 4326. doi:10.1038/s41598-020-61405-4
Klim, J. R., Li, L., Wrighton, P. J., Piekarczyk, M. S., and Kiessling, L. L. (2010). A Defined Glycosaminoglycan-Binding Substratum for Human Pluripotent Stem Cells. Nat. Methods 7, 989–994. doi:10.1038/nmeth.1532
Knight, M. N., and Hankenson, K. D. (2013). Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2, 306–316. doi:10.1089/wound.2012.0420
Ko, W.-K., Heo, D. N., Moon, H.-J., Lee, S. J., Bae, M. S., Lee, J. B., et al. (2015). The Effect of Gold Nanoparticle Size on Osteogenic Differentiation of Adipose-Derived Stem Cells. J. Colloid Interface Sci. 438, 68–76. doi:10.1016/j.jcis.2014.08.058
Kobayashi, K., Suzuki, T., Nomoto, Y., Tada, Y., Miyake, M., Hazama, A., et al. (2010). A Tissue-Engineered Trachea Derived from a Framed Collagen Scaffold, Gingival Fibroblasts and Adipose-Derived Stem Cells. Biomaterials 31, 4855–4863. doi:10.1016/j.biomaterials.2010.02.027
Koellensperger, E., Lampe, K., Beierfuss, A., Gramley, F., Germann, G., and Leimer, U. (2014). Intracutaneously Injected Human Adipose Tissue-Derived Stem Cells in a Mouse Model Stay at the Site of Injection. J. Plastic, Reconstr. Aesthetic Surg. 67, 844–850. doi:10.1016/j.bjps.2014.02.021
Kohane, D. S., and Langer, R. (2008). Polymeric Biomaterials in Tissue Engineering. Pediatr. Res. 63, 487–491. doi:10.1203/01.pdr.0000305937.26105.e7
Kourgiantaki, A., Tzeranis, D. S., Karali, K., Georgelou, K., Bampoula, E., Psilodimitrakopoulos, S., et al. (2020). Neural Stem Cell Delivery via Porous Collagen Scaffolds Promotes Neuronal Differentiation and Locomotion Recovery in Spinal Cord Injury. NPJ Regen. Med. 5, 12. doi:10.1038/s41536-020-0097-0
Krawiec, J. T., Liao, H.-T., Kwan, L., D'amore, A., Weinbaum, J. S., Rubin, J. P., et al. (2017). Evaluation of the Stromal Vascular Fraction of Adipose Tissue as the Basis for a Stem Cell-Based Tissue-Engineered Vascular Graft. J. Vasc. Surg. 66, 883–890. e881. doi:10.1016/j.jvs.2016.09.034
Kucharzewski, M., Rojczyk, E., Wilemska-Kucharzewska, K., Wilk, R., Hudecki, J., and Los, M. J. (2019). Novel Trends in Application of Stem Cells in Skin Wound Healing. Eur. J. Pharmacol. 843, 307–315. doi:10.1016/j.ejphar.2018.12.012
Kundrotas, G., Karabanovas, V., Pleckaitis, M., Juraleviciute, M., Steponkiene, S., Gudleviciene, Z., et al. (2019). Uptake and Distribution of Carboxylated Quantum Dots in Human Mesenchymal Stem Cells: Cell Growing Density Matters. J. Nanobiotechnol 17, 39. doi:10.1186/s12951-019-0470-6
Kwon, S. G., Kwon, Y. W., Lee, T. W., Park, G. T., and Kim, J. H. (2018). Recent Advances in Stem Cell Therapeutics and Tissue Engineering Strategies. Biomater. Res. 22, 36. doi:10.1186/s40824-018-0148-4
Lai, Y., Cao, H., Wang, X., Chen, S., Zhang, M., Wang, N., et al. (2018). Porous Composite Scaffold Incorporating Osteogenic Phytomolecule Icariin for Promoting Skeletal Regeneration in Challenging Osteonecrotic Bone in Rabbits. Biomaterials 153, 1–13. doi:10.1016/j.biomaterials.2017.10.025
Lalwani, G., D'agati, M., Gopalan, A., Patel, S. C., Talukdar, Y., and Sitharaman, B. (2017). Three-dimensional Carbon Nanotube Scaffolds for Long-Term Maintenance and Expansion of Human Mesenchymal Stem Cells. J. Biomed. Mat. Res. 105, 1927–1939. doi:10.1002/jbm.a.36062
Lan, Y., Lu, C., Yang, Y., Liu, X., Guo, X., Xi, J., et al. (2020). Linc1557 Is Critical for the Initiation of Embryonic Stem Cell Differentiation by Directly Targeting the LIF/STAT3 Signaling Pathway. Stem Cells 38, 340–351. doi:10.1002/stem.3130
Laverdet, B., Micallef, L., Lebreton, C., Mollard, J., Lataillade, J.-J., Coulomb, B., et al. (2014). Use of Mesenchymal Stem Cells for Cutaneous Repair and Skin Substitute Elaboration. Pathol. Biol. 62, 108–117. doi:10.1016/j.patbio.2014.01.002
Lee, J. H., Lee, J.-Y., Yang, S. H., Lee, E.-J., and Kim, H.-W. (2014). Carbon Nanotube-Collagen Three-Dimensional Culture of Mesenchymal Stem Cells Promotes Expression of Neural Phenotypes and Secretion of Neurotrophic Factors. Acta Biomater. 10, 4425–4436. doi:10.1016/j.actbio.2014.06.023
Lee, J. H., Park, J.-H., Yun, Y.-R., Jang, J.-H., Lee, E.-J., Chrzanowski, W., et al. (2013). Tethering Bi-functional Protein onto Mineralized Polymer Scaffolds to Regulate Mesenchymal Stem Cell Behaviors for Bone Regeneration. J. Mat. Chem. B 1, 2731–2741. doi:10.1039/c3tb00043e
Lee, J. M., Sing, S. L., Zhou, M., and Yeong, W. Y. (2018). 3D Bioprinting Processes: A Perspective on Classification and Terminology. Int. J. Bioprint 4, 151. doi:10.18063/ijb.v4i2.151
Lehrman, S. (1999). Virus Treatment Questioned after Gene Therapy Death. Nature 401, 517–518. doi:10.1038/43977
Lenna, S., Bellotti, C., Duchi, S., Martella, E., Columbaro, M., Dozza, B., et al. (2020). Mesenchymal Stromal Cells Mediated Delivery of Photoactive Nanoparticles Inhibits Osteosarcoma Growth In Vitro and in a Murine In Vivo Ectopic Model. J. Exp. Clin. Cancer Res. 39, 40. doi:10.1186/s13046-020-01548-4
Leon-Lopez, A., Morales-Penaloza, A., Martinez-Juarez, V. M., Vargas-Torres, A., Zeugolis, D. I., and Aguirre-Alvarez, G. (2019). Hydrolyzed Collagen-Sources and Applications. Molecules 24, 4031. doi:10.3390/molecules24224031
Li, J., Li, J. E. J., Zhang, J., Wang, X., Kawazoe, N., and Chen, G. (2016). Gold Nanoparticle Size and Shape Influence on Osteogenesis of Mesenchymal Stem Cells. Nanoscale 8, 7992–8007. doi:10.1039/c5nr08808a
Li, Q., Li, M., Li, M., Zhang, Z., Ma, H., Zhao, L., et al. (2020). Adipose-derived Mesenchymal Stem Cell Seeded Atelocollagen Scaffolds for Cardiac Tissue Engineering. J. Mater Sci. Mater Med. 31, 83. doi:10.1007/s10856-020-06425-2
Li, T., Xia, M., Gao, Y., Chen, Y., and Xu, Y. (2015). Human Umbilical Cord Mesenchymal Stem Cells: an Overview of Their Potential in Cell-Based Therapy. Expert Opin. Biol. Ther. 15, 1293–1306. doi:10.1517/14712598.2015.1051528
Li, X., Yang, Z., and Zhang, A. (2009). The Effect of Neurotrophin-3/chitosan Carriers on the Proliferation and Differentiation of Neural Stem Cells. Biomaterials 30, 4978–4985. doi:10.1016/j.biomaterials.2009.05.047
Li, Y., He, L., Pan, S., Zhang, L., Zhang, W., Yi, H., et al. (2017). Three-dimensional Simulated Microgravity Culture Improves the Proliferation and Odontogenic Differentiation of Dental Pulp Stem Cell in PLGA Scaffolds Implanted in Mice. Mol. Med. Rep. 15, 873–878. doi:10.3892/mmr.2016.6042
Li, Y., Liu, M., and Yang, S. T. (2014). Dendritic Cells Derived from Pluripotent Stem Cells: Potential of Large Scale Production. Wjsc 6, 1–10. doi:10.4252/wjsc.v6.i1.1
Li, Y., Rodrigues, J., and Tomás, H. (2012). Injectable and Biodegradable Hydrogels: Gelation, Biodegradation and Biomedical Applications. Chem. Soc. Rev. 41, 2193–2221. doi:10.1039/c1cs15203c
Liu, B., Yang, F., Wei, X., Zhang, X., Zhang, Y., Wang, B., et al. (2019). An Exploratory Study of Articular Cartilage and Subchondral Bone Reconstruction with Bone Marrow Mesenchymal Stem Cells Combined with Porous tantalum/Bio-Gide Collagen Membrane in Osteonecrosis of the Femoral Head. Mater. Sci. Eng. C 99, 1123–1132. doi:10.1016/j.msec.2019.02.072
Liu, G., David, B. T., Trawczynski, M., and Fessler, R. G. (2020). Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev Rep 16, 3–32. doi:10.1007/s12015-019-09935-x
Liu, H., Zhao, Y., Tong, J., Shi, X., Chen, Y., and Du, Y. (2021a). Electrofabrication of Flexible and Mechanically Strong Tubular Chitosan Implants for Peripheral Nerve Regeneration. J. Mat. Chem. B 9, 5537–5546. doi:10.1039/d1tb00247c
Liu, J., Swartz, D., Peng, H., Gugino, S., Russell, J., and Andreadis, S. (2007). Functional Tissue-Engineered Blood Vessels from Bone Marrow Progenitor Cells. Cardiovasc. Res. 75, 618–628. doi:10.1016/j.cardiores.2007.04.018
Liu, S., Xie, Y. Y., Wang, L. D., Tai, C. X., Chen, D., Mu, D., et al. (2021b). A Multi-Channel Collagen Scaffold Loaded with Neural Stem Cells for the Repair of Spinal Cord Injury. Neural Regen. Res. 16, 2284–2292. doi:10.4103/1673-5374.310698
Liu, X., Hao, M., Chen, Z., Zhang, T., Huang, J., Dai, J., et al. (2021c). 3D Bioprinted Neural Tissue Constructs for Spinal Cord Injury Repair. Biomaterials 272, 120771. doi:10.1016/j.biomaterials.2021.120771
Lotfi, M., Naderi-Meshkin, H., Mahdipour, E., Mafinezhad, A., Bagherzadeh, R., Sadeghnia, H. R., et al. (2019). Adipose Tissue-Derived Mesenchymal Stem Cells and Keratinocytes Co-culture on Gelatin/chitosan/beta-Glycerol Phosphate Nanoscaffold in Skin Regeneration. Cell Biol. Int. 1, 1. doi:10.1002/cbin.11119
Lough, D. M., Wetter, N., Madsen, C., Reichensperger, J., Cosenza, N., Cox, L., et al. (2016). Transplantation of an LGR6+ Epithelial Stem Cell-Enriched Scaffold for Repair of Full-Thickness Soft-Tissue Defects. Plastic Reconstr. Surg. 137, 495–507. doi:10.1097/01.prs.0000475761.09451.00
Lough, D. M., Yang, M., Blum, A., Reichensperger, J. D., Cosenza, N. M., Wetter, N., et al. (2014). Transplantation of the LGR6+ Epithelial Stem Cell into Full-Thickness Cutaneous Wounds Results in Enhanced Healing, Nascent Hair Follicle Development, and Augmentation of Angiogenic Analytes. Plastic Reconstr. Surg. 133, 579–590. doi:10.1097/prs.0000000000000075
Lozano, D., Gil-Albarova, J., Heras, C., Sánchez-Salcedo, S., Gómez-Palacio, V. E., Gómez-Blasco, A., et al. (2020). ZnO-mesoporous Glass Scaffolds Loaded with Osteostatin and Mesenchymal Cells Improve Bone Healing in a Rabbit Bone Defect. J. Mater Sci. Mater Med. 31, 100. doi:10.1007/s10856-020-06439-w
Lui, C. N. P., Tsui, Y. P., Ho, A. S. L., Shum, D. K. Y., Chan, Y. S., Wu, C. T., et al. (2013). Neural Stem Cells Harvested from Live Brains by Antibody-Conjugated Magnetic Nanoparticles. Angew. Chem. Int. Ed. 52, 12298–12302. doi:10.1002/anie.201305482
Lv, J., Liu, W., Shi, G., Zhu, F., He, X., Zhu, Z., et al. (2020). Human Cardiac Extracellular Matrix-Chitosan-Gelatin Composite Scaffold and its Endothelialization. Exp. Ther. Med. 19, 1225–1234. doi:10.3892/etm.2019.8349
Ma, C., Kuzma, M. L., Bai, X., and Yang, J. (2019). Biomaterial‐Based Metabolic Regulation in Regenerative Engineering. Adv. Sci. 6, 1900819. doi:10.1002/advs.201900819
Ma, H., Lam, P. K., Siu, W. S., Tong, C. S. W., Lo, K. K. Y., Koon, C. M., et al. (2021). Adipose Tissue-Derived Mesenchymal Stem Cells (ADMSCs) and ADMSC-Derived Secretome Expedited Wound Healing in a Rodent Model - A Preliminary Study. Clin. Cosmet. Investig. Dermatol. 14, 753–764. doi:10.2147/ccid.s298105
Ma, X., Qu, X., Zhu, W., Li, Y.-S., Yuan, S., Zhang, H., et al. (2016). Deterministically Patterned Biomimetic Human iPSC-Derived Hepatic Model via Rapid 3D Bioprinting. Proc. Natl. Acad. Sci. U.S.A. 113, 2206–2211. doi:10.1073/pnas.1524510113
Maiullari, F., Costantini, M., Milan, M., Pace, V., Chirivì, M., Maiullari, S., et al. (2018). A Multi-Cellular 3D Bioprinting Approach for Vascularized Heart Tissue Engineering Based on HUVECs and iPSC-Derived Cardiomyocytes. Sci. Rep. 8, 13532. doi:10.1038/s41598-018-31848-x
Mandrycky, C., Wang, Z., Kim, K., and Kim, D.-H. (2016). 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 34, 422–434. doi:10.1016/j.biotechadv.2015.12.011
Marcacci, M., Kon, E., Moukhachev, V., Lavroukov, A., Kutepov, S., Quarto, R., et al. (2007). Stem Cells Associated with Macroporous Bioceramics for Long Bone Repair: 6- to 7-year Outcome of a Pilot Clinical Study. Tissue Eng. 13, 947–955. doi:10.1089/ten.2006.0271
Marchini, A., Favoino, C., and Gelain, F. (2020). Multi-Functionalized Self-Assembling Peptides as Reproducible 3D Cell Culture Systems Enabling Differentiation and Survival of Various Human Neural Stem Cell Lines. Front. Neurosci. 14, 413. doi:10.3389/fnins.2020.00413
Mardani, M., Sadeghzadeh, A., Tanideh, N., Andisheh-Tadbir, A., Lavaee, F., Zarei, M., et al. (2020). The Effects of Adipose Tissue-Derived Stem Cells Seeded onto the Curcumin-Loaded Collagen Scaffold in Healing of Experimentally- Induced Oral Mucosal Ulcers in Rat. Iran. J. Basic Med. Sci. 23, 1618–1627. doi:10.22038/ijbms.2020.48698.11171
Marinkovich, M. P., and Tang, J. Y. (2019). Gene Therapy for Epidermolysis Bullosa. J. Investigative Dermatology 139, 1221–1226. doi:10.1016/j.jid.2018.11.036
Marks, E. D., and Kumar, A. (2015). Bone Marrow Stem Cell Derived Cardiomyocyte Precursors Differentiated on Nanofiber Scaffolds Attenuate Scar Formation in a Rat Model of Myocardial Infarction. J. Cardiac Fail. 21, S92. doi:10.1016/j.cardfail.2015.06.276
Martino, S., D'angelo, F., Armentano, I., Kenny, J. M., and Orlacchio, A. (2012). Stem Cell-Biomaterial Interactions for Regenerative Medicine. Biotechnol. Adv. 30, 338–351. doi:10.1016/j.biotechadv.2011.06.015
Mastrogiacomo, S., Dou, W., Jansen, J. A., and Walboomers, X. F. (2019). Magnetic Resonance Imaging of Hard Tissues and Hard Tissue Engineered Bio-Substitutes. Mol. Imaging Biol. 21, 1003–1019. doi:10.1007/s11307-019-01345-2
Mavilio, F., Pellegrini, G., Ferrari, S., Di Nunzio, F., Di Iorio, E., Recchia, A., et al. (2006). Correction of Junctional Epidermolysis Bullosa by Transplantation of Genetically Modified Epidermal Stem Cells. Nat. Med. 12, 1397–1402. doi:10.1038/nm1504
Mazzoni, E., Iaquinta, M. R., Lanzillotti, C., Mazziotta, C., Maritati, M., Montesi, M., et al. (2021). Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 9, 613787. doi:10.3389/fbioe.2021.613787
Meamar, R., Ghasemi-Mobarakeh, L., Norouzi, M.-R., Siavash, M., Hamblin, M. R., and Fesharaki, M. (2021). Improved Wound Healing of Diabetic Foot Ulcers Using Human Placenta-Derived Mesenchymal Stem Cells in Gelatin Electrospun Nanofibrous Scaffolds Plus a Platelet-Rich Plasma Gel: A Randomized Clinical Trial. Int. Immunopharmacol. 101, 108282. doi:10.1016/j.intimp.2021.108282
Melkoumian, Z., Weber, J. L., Weber, D. M., Fadeev, A. G., Zhou, Y., Dolley-Sonneville, P., et al. (2010). Synthetic Peptide-Acrylate Surfaces for Long-Term Self-Renewal and Cardiomyocyte Differentiation of Human Embryonic Stem Cells. Nat. Biotechnol. 28, 606–610. doi:10.1038/nbt.1629
Meng, X., Li, C., Dong, Z., Liu, J., Li, W., Liu, Y., et al. (2008). Co-transplantation of bFGF-Expressing Amniotic Epithelial Cells and Neural Stem Cells Promotes Functional Recovery in Spinal Cord-Injured Rats. Cell Biol. Int. 32, 1546–1558. doi:10.1016/j.cellbi.2008.09.001
Midgley, A. C., Wei, Y., Li, Z., Kong, D., and Zhao, Q. (2020). Nitric-Oxide-Releasing Biomaterial Regulation of the Stem Cell Microenvironment in Regenerative Medicine. Adv. Mater 32, e1805818. doi:10.1002/adma.201805818
Millán-Rivero, J. E., Martínez, C. M., Romecín, P. A., Aznar-Cervantes, S. D., Carpes-Ruiz, M., Cenis, J. L., et al. (2019). Silk Fibroin Scaffolds Seeded with Wharton's Jelly Mesenchymal Stem Cells Enhance Re-epithelialization and Reduce Formation of Scar Tissue after Cutaneous Wound Healing. Stem Cell Res. Ther. 10, 126. doi:10.1186/s13287-019-1229-6
Mimeault, M., and Batra, S. K. (2009). Recent Insights into the Molecular Mechanisms Involved in Aging and the Malignant Transformation of Adult Stem/progenitor Cells and Their Therapeutic Implications. Ageing Res. Rev. 8, 94–112. doi:10.1016/j.arr.2008.12.001
Miyazaki, K., and Maruyama, T. (2014). Partial Regeneration and Reconstruction of the Rat Uterus through Recellularization of a Decellularized Uterine Matrix. Biomaterials 35, 8791–8800. doi:10.1016/j.biomaterials.2014.06.052
Mizuno, H. (2009). Adipose-derived Stem Cells for Tissue Repair and Regeneration: Ten Years of Research and a Literature Review. J. Nippon. Med. Sch. 76, 56–66. doi:10.1272/jnms.76.56
Moeinabadi-Bidgoli, K., Babajani, A., Yazdanpanah, G., Farhadihosseinabadi, B., Jamshidi, E., Bahrami, S., et al. (2021). Translational Insights into Stem Cell Preconditioning: From Molecular Mechanisms to Preclinical Applications. Biomed. Pharmacother. 142, 112026. doi:10.1016/j.biopha.2021.112026
Moroni, L., Boland, T., Burdick, J. A., De Maria, C., Derby, B., Forgacs, G., et al. (2018). Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 36, 384–402. doi:10.1016/j.tibtech.2017.10.015
Murphy, S. V., and Atala, A. (2014). 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 32, 773–785. doi:10.1038/nbt.2958
Nafea, E. H., Poole-Warren, A. M. L. A., Martens, P. J., and Martens, P. J. (2011). Immunoisolating Semi-permeable Membranes for Cell Encapsulation: Focus on Hydrogels. J. Control. Release 154, 110–122. doi:10.1016/j.jconrel.2011.04.022
Nagano, H., Suematsu, Y., Takuma, M., Aoki, S., Satoh, A., Takayama, E., et al. (2021). Enhanced Cellular Engraftment of Adipose-Derived Mesenchymal Stem Cell Spheroids by Using Nanosheets as Scaffolds. Sci. Rep. 11, 14500. doi:10.1038/s41598-021-93642-6
Nagori, C. B., Panchal, S. Y., and Patel, H. (2011). Endometrial Regeneration Using Autologous Adult Stem Cells Followed by Conception by In Vitro Fertilization in a Patient of Severe Asherman's Syndrome. J. Hum. Reprod. Sci. 4, 43–48. doi:10.4103/0974-1208.82360
Nambu, M., Ishihara, M., Kishimoto, S., Yanagibayashi, S., Yamamoto, N., Azuma, R., et al. (2011). Stimulatory Effect of Autologous Adipose Tissue-Derived Stromal Cells in an Atelocollagen Matrix on Wound Healing in Diabetic Db/db Mice. J. Tissue Eng. 2011, 158105. doi:10.4061/2011/158105
Nan, W., Liu, R., Chen, H., Xu, Z., Chen, J., Wang, M., et al. (2015). Umbilical Cord Mesenchymal Stem Cells Combined with a Collagenfibrin Double-Layered Membrane Accelerates Wound Healing. Wounds 27, 134–140.
Naskar, D., Ghosh, A. K., Mandal, M., Das, P., Nandi, S. K., and Kundu, S. C. (2017). Dual Growth Factor Loaded Nonmulberry Silk Fibroin/carbon Nanofiber Composite 3D Scaffolds for In Vitro and In Vivo Bone Regeneration. Biomaterials 136, 67–85. doi:10.1016/j.biomaterials.2017.05.014
Nayak, T. R., Jian, L., Phua, L. C., Ho, H. K., Ren, Y., and Pastorin, G. (2010). Thin Films of Functionalized Multiwalled Carbon Nanotubes as Suitable Scaffold Materials for Stem Cells Proliferation and Bone Formation. ACS Nano 4, 7717–7725. doi:10.1021/nn102738c
Neofytou, E., O’Brien, C. G., Couture, L. A., and Wu, J. C. (2015). Hurdles to Clinical Translation of Human Induced Pluripotent Stem Cells. J. Clin. Invest. 125, 2551–2557. doi:10.1172/jci80575
Netsrithong, R., and Wattanapanitch, M. (2021). Advances in Adoptive Cell Therapy Using Induced Pluripotent Stem Cell-Derived T Cells. Front. Immunol. 12, 759558. doi:10.3389/fimmu.2021.759558
Nicolas, J., Magli, S., Rabbachin, L., Sampaolesi, S., Nicotra, F., and Russo, L. (2020). 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 21, 1968–1994. doi:10.1021/acs.biomac.0c00045
Nie, Y., Zhang, K., Zhang, S., Wang, D., Han, Z., Che, Y., et al. (2017). Nitric Oxide Releasing Hydrogel Promotes Endothelial Differentiation of Mouse Embryonic Stem Cells. Acta Biomater. 63, 190–199. doi:10.1016/j.actbio.2017.08.037
Nomoto, Y., Suzuki, T., Tada, Y., Kobayashi, K., Miyake, M., Hazama, A., et al. (2006). Tissue Engineering for Regeneration of the Tracheal Epithelium. Ann. Otol. Rhinol. Laryngol. 115, 501–506. doi:10.1177/000348940611500704
Nomura, H., Zahir, T., Kim, H., Katayama, Y., Kulbatski, I., Morshead, C. M., et al. (2008). Extramedullary Chitosan Channels Promote Survival of Transplanted Neural Stem and Progenitor Cells and Create a Tissue Bridge after Complete Spinal Cord Transection. Tissue Eng. Part A 14, 649–665. doi:10.1089/tea.2007.0180
Nourian Dehkordi, A., Mirahmadi Babaheydari, F., Chehelgerdi, M., and Raeisi Dehkordi, S. (2019). Skin Tissue Engineering: Wound Healing Based on Stem-Cell-Based Therapeutic Strategies. Stem Cell Res. Ther. 10, 111. doi:10.1186/s13287-019-1212-2
Nugent, H. M., and Edelman, E. R. (2003). Tissue Engineering Therapy for Cardiovascular Disease. Circulation Res. 92, 1068–1078. doi:10.1161/01.res.0000073844.41372.38
Nuschke, A., Rodrigues, M., Rivera, J., Yates, C., Whaley, D., Stolz, D., et al. (2016). Epidermal Growth Factor Tethered to β-Tricalcium Phosphate Bone Scaffolds via a High-Affinity Binding Peptide Enhances Survival of Human Mesenchymal Stem Cells/Multipotent Stromal Cells in an Immune-Competent Parafascial Implantation Assay in Mice. Stem Cells Transl. Med. 5, 1580–1586. doi:10.5966/sctm.2015-0326
Omori, K., Nakamura, T., Kanemaru, S., Asato, R., Yamashita, M., Tanaka, S., et al. (2005). Regenerative Medicine of the Trachea: the First Human Case. Ann. Otol. Rhinol. Laryngol. 114, 429–433. doi:10.1177/000348940511400603
Park, J. S., Park, G., and Hong, H. S. (2021a). Age Affects the Paracrine Activity and Differentiation Potential of Human Adipose-derived S-tem C-ells. Mol. Med. Rep. 23, 160. doi:10.3892/mmr.2020.11799
Park, S. R., Kim, S. R., Im, J. B., Park, C. H., Lee, H. Y., and Hong, I. S. (2021b). 3D Stem Cell-Laden Artificial Endometrium: Successful Endometrial Regeneration and Pregnancy. Biofabrication 13, 1. doi:10.1088/1758-5090/ac165a
Pearce, A. K., and O’Reilly, R. K. (2021). Polymers for Biomedical Applications: The Importance of Hydrophobicity in Directing Biological Interactions and Application Efficacy. Biomacromolecules 22, 4459–4469. doi:10.1021/acs.biomac.1c00434
Peng, J., Wen, C., Wang, A., Wang, Y., Xu, W., Zhao, B., et al. (2011). Micro-CT-based Bone Ceramic Scaffolding and its Performance after Seeding with Mesenchymal Stem Cells for Repair of Load-Bearing Bone Defect in Canine Femoral Head. J. Biomed. Mat. Res. 96B, 316–325. doi:10.1002/jbm.b.31770
Pennarossa, G., Ghiringhelli, M., Gandolfi, F., and Brevini, T. A. L. (2021). Creation of a Bioengineered Ovary: Isolation of Female Germline Stem Cells for the Repopulation of a Decellularized Ovarian Bioscaffold. Methods Mol. Biol. 2273, 139–149. doi:10.1007/978-1-0716-1246-0_9
Peressotti, S., Koehl, G. E., Goding, J. A., and Green, R. A. (2021). Self-Assembling Hydrogel Structures for Neural Tissue Repair. ACS Biomater. Sci. Eng. 7, 4136–4163. doi:10.1021/acsbiomaterials.1c00030
Pittenger, M. F., Discher, D. E., Péault, B. M., Phinney, D. G., Hare, J. M., and Caplan, A. I. (2019). Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 4, 22. doi:10.1038/s41536-019-0083-6
Pushp, P., Sahoo, B., Ferreira, F. C., Sampaio Cabral, J. M., Fernandes‐Platzgummer, A., and Gupta, M. K. (2020). Functional Comparison of Beating Cardiomyocytes Differentiated from Umbilical Cord‐derived Mesenchymal/stromal Stem Cells and Human Foreskin‐derived Induced Pluripotent Stem Cells. J. Biomed. Mater Res. 108, 496–514. doi:10.1002/jbm.a.36831
Qi, C., Yan, X., Huang, C., Melerzanov, A., and Du, Y. (2015). Biomaterials as Carrier, Barrier and Reactor for Cell-Based Regenerative Medicine. Protein Cell 6, 638–653. doi:10.1007/s13238-015-0179-8
Qiao, Z., Lian, M., Han, Y., Sun, B., Zhang, X., Jiang, W., et al. (2021). Bioinspired Stratified Electrowritten Fiber-Reinforced Hydrogel Constructs with Layer-specific Induction Capacity for Functional Osteochondral Regeneration. Biomaterials 266, 120385. doi:10.1016/j.biomaterials.2020.120385
Rao, J. S., Zhao, C., Zhang, A., Duan, H., Hao, P., Wei, R. H., et al. (2018). NT3-chitosan Enables De Novo Regeneration and Functional Recovery in Monkeys after Spinal Cord Injury. Proc. Natl. Acad. Sci. U. S. A. 115, E5595–E5604. doi:10.1073/pnas.1804735115
Rao, R. R., and Stegemann, J. P. (2013). Cell-based Approaches to the Engineering of Vascularized Bone Tissue. Cytotherapy 15, 1309–1322. doi:10.1016/j.jcyt.2013.06.005
Rashedi, I., Talele, N., Wang, X.-H., Hinz, B., Radisic, M., and Keating, A. (2017). Collagen Scaffold Enhances the Regenerative Properties of Mesenchymal Stromal Cells. PLoS One 12, e0187348. doi:10.1371/journal.pone.0187348
Raucci, M. G., D'amora, U., Ronca, A., and Ambrosio, L. (2020). Injectable Functional Biomaterials for Minimally Invasive Surgery. Adv. Healthc. Mater 9, e2000349. doi:10.1002/adhm.202000349
Redondo, L. M., García, V., Peral, B., Verrier, A., Becerra, J., Sánchez, A., et al. (2018). Repair of Maxillary Cystic Bone Defects with Mesenchymal Stem Cells Seeded on a Cross-Linked Serum Scaffold. J. Cranio-Maxillofacial Surg. 46, 222–229. doi:10.1016/j.jcms.2017.11.004
Redondo, P. A., Pavlou, M., Loizidou, M., and Cheema, U. (2017). Elements of the Niche for Adult Stem Cell Expansion. J. Tissue Eng. 8, 1. doi:10.1177/2041731417725464
Reig, A., Mamillapalli, R., Coolidge, A., Johnson, J., and Taylor, H. S. (2019). Uterine Cells Improved Ovarian Function in a Murine Model of Ovarian Insufficiency. Reprod. Sci. 26, 1633–1639. doi:10.1177/1933719119875818
Restan Perez, M., Sharma, R., Masri, N. Z., and Willerth, S. M. (2021). 3D Bioprinting Mesenchymal Stem Cell-Derived Neural Tissues Using a Fibrin-Based Bioink. Biomolecules 11, 1250. doi:10.3390/biom11081250
Revel, A. (2012). Defective Endometrial Receptivity. Fertil. Steril. 97, 1028–1032. doi:10.1016/j.fertnstert.2012.03.039
Rice, J. J., Martino, M. M., De Laporte, L., Tortelli, F., Briquez, P. S., and Hubbell, J. A. (2013). Engineering the Regenerative Microenvironment with Biomaterials. Adv. Healthc. Mater. 2, 57–71. doi:10.1002/adhm.201200197
Riha, S. M., Maarof, M., and Fauzi, M. B. (2021). Synergistic Effect of Biomaterial and Stem Cell for Skin Tissue Engineering in Cutaneous Wound Healing: A Concise Review. Polym. (Basel) 13, 1546. doi:10.3390/polym13101546
Rodríguez, L. V., Alfonso, Z., Zhang, R., Leung, J., Wu, B., and Ignarro, L. J. (2006). Clonogenic Multipotent Stem Cells in Human Adipose Tissue Differentiate into Functional Smooth Muscle Cells. Proc. Natl. Acad. Sci. U.S.A. 103, 12167–12172. doi:10.1073/pnas.0604850103
Rognoni, E., and Watt, F. M. (2018). Skin Cell Heterogeneity in Development, Wound Healing, and Cancer. Trends Cell Biol. 28, 709–722. doi:10.1016/j.tcb.2018.05.002
Roura, S., Bagó, J. R., Soler-Botija, C., Pujal, J. M., Gálvez-Montón, C., Prat-Vidal, C., et al. (2012). Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Promote Vascular Growth In Vivo. PLoS One 7, e49447. doi:10.1371/journal.pone.0049447
Sadr, S. Z., Fatehi, R., Maroufizadeh, S., Amorim, C. A., and Ebrahimi, B. (2018). Utilizing Fibrin-Alginate and Matrigel-Alginate for Mouse Follicle Development in Three-Dimensional Culture Systems. Biopreservation Biobanking 16, 120–127. doi:10.1089/bio.2017.0087
Saito, N., Usui, Y., Aoki, K., Narita, N., Shimizu, M., Hara, K., et al. (2009). Carbon Nanotubes: Biomaterial Applications. Chem. Soc. Rev. 38, 1897–1903. doi:10.1039/b804822n
Salerno, A., and Netti, P. A. (2021). Review on Computer-Aided Design and Manufacturing of Drug Delivery Scaffolds for Cell Guidance and Tissue Regeneration. Front. Bioeng. Biotechnol. 9, 682133. doi:10.3389/fbioe.2021.682133
Santamaria, X., Cabanillas, S., Cervelló, I., Arbona, C., Raga, F., Ferro, J., et al. (2016). Autologous Cell Therapy with CD133+ Bone Marrow-Derived Stem Cells for Refractory Asherman's Syndrome and Endometrial Atrophy: a Pilot Cohort Study. Hum. Reprod. 31, 1087–1096. doi:10.1093/humrep/dew042
Sart, S., Agathos, S. N., and Li, Y. (2013). Engineering Stem Cell Fate with Biochemical and Biomechanical Properties of Microcarriers. Biotechnol. Prog. 29, 1354–1366. doi:10.1002/btpr.1825
Sartika, D., Wang, C. H., Wang, D. H., Cherng, J. H., Chang, S. J., Fan, G. Y., et al. (2020). Human Adipose-Derived Mesenchymal Stem Cells-Incorporated Silk Fibroin as a Potential Bio-Scaffold in Guiding Bone Regeneration. Polym. (Basel) 12, 853. doi:10.3390/polym12040853
Sasaki, M., Abe, R., Fujita, Y., Ando, S., Inokuma, D., and Shimizu, H. (2008). Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 180, 2581–2587. doi:10.4049/jimmunol.180.4.2581
Shahi, M., Mohammadnejad, D., Karimipour, M., Rasta, S. H., Rahbarghazi, R., and Elahi, A. A. (2020). Hyaluronic Acid and Regenerative Medicine: New Insights into the Stroke Therapy. Curr. Mol. Med. 20, 675–691. doi:10.2174/1566524020666200326095837
Sharma, R., Smits, I. P. M., De La Vega, L., Lee, C., and Willerth, S. M. (2020). 3D Bioprinting Pluripotent Stem Cell Derived Neural Tissues Using a Novel Fibrin Bioink Containing Drug Releasing Microspheres. Front. Bioeng. Biotechnol. 8, 57. doi:10.3389/fbioe.2020.00057
Sharma, S., Rani, G., Bose, G., Saha, I., Bathwal, S., and Chakravarty, B. (2018). Tamoxifen Is Better Than Low-Dose Clomiphene or Gonadotropins in Women with Thin Endometrium (<7 Mm) after Clomiphene in Intrauterine Insemination Cycles: A Prospective Study. J. Hum. Reprod. Sci. 11, 34–39. doi:10.4103/jhrs.jhrs_9_17
Sherry, B., Smith, A. L., and Kronmal, R. A. (1989). Anemia during Haemophilus Influenzae Type B Meningitis: Lack of an Effect of Chloramphenicol. Dev. Pharmacol. Ther. 12, 188–199. doi:10.1159/000480946
Shin, J., Choi, E. J., Cho, J. H., Cho, A.-N., Jin, Y., Yang, K., et al. (2017). Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 18, 3060–3072. doi:10.1021/acs.biomac.7b00568
Singh, N., Mohanty, S., Seth, T., Shankar, M., Dharmendra, S., and Bhaskaran, S. (2014). Autologous Stem Cell Transplantation in Refractory Asherman′s Syndrome: A Novel Cell Based Therapy. J. Hum. Reprod. Sci. 7, 93–98. doi:10.4103/0974-1208.138864
Skeldon, G., Lucendo-Villarin, B., and Shu, W. (2018). Three-dimensional Bioprinting of Stem-Cell Derived Tissues for Human Regenerative Medicine. Philos. Trans. R. Soc. Lond B Biol. Sci. 373, 1. doi:10.1098/rstb.2017.0224
Sleep, E., Cosgrove, B. D., Mcclendon, M. T., Preslar, A. T., Chen, C. H., Sangji, M. H., et al. (2017). Injectable Biomimetic Liquid Crystalline Scaffolds Enhance Muscle Stem Cell Transplantation. Proc. Natl. Acad. Sci. U. S. A. 114, E7919–E7928. doi:10.1073/pnas.1708142114
Song, Y.-S., Joo, H.-W., Park, I.-H., Shen, G.-Y., Lee, Y., Shin, J. H., et al. (2017). Bone Marrow Mesenchymal Stem Cell-Derived Vascular Endothelial Growth Factor Attenuates Cardiac Apoptosis via Regulation of Cardiac miRNA-23a and miRNA-92a in a Rat Model of Myocardial Infarction. PLoS One 12, e0179972. doi:10.1371/journal.pone.0179972
Su, J., Ding, L., Cheng, J., Yang, J., Li, X. a., Yan, G., et al. (2016). Transplantation of Adipose-Derived Stem Cells Combined with Collagen Scaffolds Restores Ovarian Function in a Rat Model of Premature Ovarian Insufficiency. Hum. Reprod. 31, 1075–1086. doi:10.1093/humrep/dew041
Sun, M., Wang, S., Li, Y., Yu, L., Gu, F., Wang, C., et al. (2013). Adipose-derived Stem Cells Improved Mouse Ovary Function after Chemotherapy-Induced Ovary Failure. Stem Cell Res. Ther. 4, 80. doi:10.1186/scrt231
Suuronen, E. J., Veinot, J. P., Wong, S., Kapila, V., Price, J., Griffith, M., et al. (2006). Tissue-engineered Injectable Collagen-Based Matrices for Improved Cell Delivery and Vascularization of Ischemic Tissue Using CD133+ Progenitors Expanded from the Peripheral Blood. Circulation 114, I138–I144. doi:10.1161/CIRCULATIONAHA.105.001081
Taniguchi, D., Matsumoto, K., Tsuchiya, T., Machino, R., Takeoka, Y., Elgalad, A., et al. (2018). Scaffold-free Trachea Regeneration by Tissue Engineering with bio-3D Printing†. Interact. Cardiovasc Thorac. Surg. 26, 745–752. doi:10.1093/icvts/ivx444
Tatekawa, Y., Kawazoe, N., Chen, G., Shirasaki, Y., Komuro, H., and Kaneko, M. (2010). Tracheal Defect Repair Using a PLGA-Collagen Hybrid Scaffold Reinforced by a Copolymer Stent with bFGF-Impregnated Gelatin Hydrogel. Pediatr. Surg. Int. 26, 575–580. doi:10.1007/s00383-010-2609-2
Taub, P. J., Yau, J., Spangler, M., Mason, J. M., and Lucas, P. A. (2009). Bioengineering of Calvaria with Adult Stem Cells. Plastic Reconstr. Surg. 123, 1178–1185. doi:10.1097/prs.0b013e31819f2949
Thomas, M., and Willerth, S. M. (2017). 3-D Bioprinting of Neural Tissue for Applications in Cell Therapy and Drug Screening. Front. Bioeng. Biotechnol. 5, 69. doi:10.3389/fbioe.2017.00069
Tiemann, T. T., Padma, A. M., Sehic, E., Bäckdahl, H., Oltean, M., Song, M. J., et al. (2020). Towards Uterus Tissue Engineering: a Comparative Study of Sheep Uterus Decellularisation. Mol. Hum. Reprod. 26, 167–178. doi:10.1093/molehr/gaaa009
Tomaszewski, C. E., Dilillo, K. M., Baker, B. M., Arnold, K. B., and Shikanov, A. (2021). Sequestered Cell-Secreted Extracellular Matrix Proteins Improve Murine Folliculogenesis and Oocyte Maturation for Fertility Preservation. Acta Biomater. 132, 313–324. doi:10.1016/j.actbio.2021.03.041
Tsifaki, M., Kelaini, S., Caines, R., Yang, C., and Margariti, A. (2018). Regenerating the Cardiovascular System through Cell Reprogramming; Current Approaches and a Look into the Future. Front. Cardiovasc. Med. 5, 109. doi:10.3389/fcvm.2018.00109
Udelsman, B., Mathisen, D. J., and Ott, H. C. (2018). A Reassessment of Tracheal Substitutes-A Systematic Review. Ann. Cardiothorac. Surg. 7, 175–182. doi:10.21037/acs.2018.01.17
Urbich, C., and Dimmeler, S. (2004). Endothelial Progenitor Cells. Circulation Res. 95, 343–353. doi:10.1161/01.res.0000137877.89448.78
Vardar, E., Vythilingam, G., Pinnagoda, K., Engelhardt, E. M., Zambelli, P. Y., Hubbell, J. A., et al. (2019). A Bioactive Injectable Bulking Material; a Potential Therapeutic Approach for Stress Urinary Incontinence. Biomaterials 206, 41–48. doi:10.1016/j.biomaterials.2019.03.030
Vila Cuenca, M., Cochrane, A., Van Den Hil, F. E., De Vries, A. A. F., Lesnik Oberstein, S. A. J., Mummery, C. L., et al. (2021). Engineered 3D Vessel-On-Chip Using hiPSC-Derived Endothelial- and Vascular Smooth Muscle Cells. Stem Cell Rep. 16, 2159–2168. doi:10.1016/j.stemcr.2021.08.003
Volkov, A. V., Muraev, A. A., Zharkova, I. I., Voinova, V. V., Akoulina, E. A., Zhuikov, V. A., et al. (2020). Poly(3-hydroxybutyrate)/hydroxyapatite/alginate Scaffolds Seeded with Mesenchymal Stem Cells Enhance the Regeneration of Critical-Sized Bone Defect. Mater. Sci. Eng. C 114, 110991. doi:10.1016/j.msec.2020.110991
Vunjak-Novakovic, G., Tandon, N., Godier, A., Maidhof, R., Marsano, A., Martens, T. P., et al. (2010). Challenges in Cardiac Tissue Engineering. Tissue Eng. Part B Rev. 16, 169–187. doi:10.1089/ten.teb.2009.0352
Walter, D. H., Rittig, K., Bahlmann, F. H., Kirchmair, R., Silver, M., Murayama, T., et al. (2002). Statin Therapy Accelerates Reendothelialization. Circulation 105, 3017–3024. doi:10.1161/01.cir.0000018166.84319.55
Walther, W., and Stein, U. (2000). Viral Vectors for Gene Transfer. Drugs 60, 249–271. doi:10.2165/00003495-200060020-00002
Wang, C. Y., Hong, P. D., Wang, D. H., Cherng, J. H., Chang, S. J., Liu, C. C., et al. (2020). Polymeric Gelatin Scaffolds Affect Mesenchymal Stem Cell Differentiation and its Diverse Applications in Tissue Engineering. Int. J. Mol. Sci. 21, 8632. doi:10.3390/ijms21228632
Wang, Z., Ruan, J., and Cui, D. (2009). Advances and Prospect of Nanotechnology in Stem Cells. Nanoscale Res. Lett. 4, 593–605. doi:10.1007/s11671-009-9292-z
Wei, A., Shen, B., Williams, L., and Diwan, A. (2014). Mesenchymal Stem Cells: Potential Application in Intervertebral Disc Regeneration. Transl. Pediatr. 3, 71–90. doi:10.3978/j.issn.2224-4336.2014.03.05
Welt, C. K. (2008). Primary Ovarian Insufficiency: a More Accurate Term for Premature Ovarian Failure. Clin. Endocrinol. 68, 499–509. doi:10.1111/j.1365-2265.2007.03073.x
Weng, T., Zhang, W., Xia, Y., Wu, P., Yang, M., Jin, R., et al. (2021). 3D Bioprinting for Skin Tissue Engineering: Current Status and Perspectives. J. Tissue Eng. 12, 1. doi:10.1177/20417314211028574
Werner, N., Priller, J., Laufs, U., Endres, M., Böhm, M., Dirnagl, U., et al. (2002). Bone Marrow-Derived Progenitor Cells Modulate Vascular Reendothelialization and Neointimal Formation. Atvb 22, 1567–1572. doi:10.1161/01.atv.0000036417.43987.d8
Wong, V. W., Levi, B., Rajadas, J., Longaker, M. T., and Gurtner, G. C. (2012). Stem Cell Niches for Skin Regeneration. Int. J. Biomater. 2012, 926059. doi:10.1155/2012/926059
Wu, Y., Chen, L., Scott, P. G., and Tredget, E. E. (2007). Mesenchymal Stem Cells Enhance Wound Healing through Differentiation and Angiogenesis. Stem Cells 25, 2648–2659. doi:10.1634/stemcells.2007-0226
Xin, L., Lin, X., Pan, Y., Zheng, X., Shi, L., Zhang, Y., et al. (2019). A Collagen Scaffold Loaded with Human Umbilical Cord-Derived Mesenchymal Stem Cells Facilitates Endometrial Regeneration and Restores Fertility. Acta Biomater. 92, 160–171. doi:10.1016/j.actbio.2019.05.012
Xu, B., Ye, J., Yuan, F.-Z., Zhang, J.-Y., Chen, Y.-R., Fan, B.-S., et al. (2020). Advances of Stem Cell-Laden Hydrogels with Biomimetic Microenvironment for Osteochondral Repair. Front. Bioeng. Biotechnol. 8, 247. doi:10.3389/fbioe.2020.00247
Xu, Q., Zhang, T., Wang, Q., Jiang, X., Li, A., Li, Y., et al. (2018). Uniformly Sized Iron Oxide Nanoparticles for Efficient Gene Delivery to Mesenchymal Stem Cells. Int. J. Pharm. 552, 443–452. doi:10.1016/j.ijpharm.2018.10.023
Xu, Y., Li, Y., Liu, Y., Li, H., Jia, Z., Tang, Y., et al. (2019). Surface Modification of Decellularized Trachea Matrix with Collagen and Laser Micropore Technique to Promote Cartilage Regeneration. Am. J. Transl. Res. 11, 5390–5403.
Xu, Y., Dai, J., Zhu, X., Cao, R., Song, N., Liu, M., et al. (2021). Biomimetic Trachea Engineering via a Modular Ring Strategy Based on Bone-Marrow Stem Cells and Atelocollagen for Use in Extensive Tracheal Reconstruction. Adv. Mater 34, e2106755. doi:10.1002/adma.202106755
Yan, B., Zhang, Z., Wang, X., Ni, Y., Liu, Y., Liu, T., et al. (2017). PLGA-PTMC-Cultured Bone Mesenchymal Stem Cell Scaffold Enhances Cartilage Regeneration in Tissue-Engineered Tracheal Transplantation. Artif. Organs 41, 461–469. doi:10.1111/aor.12805
Yan, C., Mackay, M. E., Czymmek, K., Nagarkar, R. P., Schneider, J. P., and Pochan, D. J. (2012). Injectable Solid Peptide Hydrogel as a Cell Carrier: Effects of Shear Flow on Hydrogels and Cell Payload. Langmuir 28, 6076–6087. doi:10.1021/la2041746
Yang, R., Wang, J., Chen, X., Shi, Y., and Xie, J. (2020). Epidermal Stem Cells in Wound Healing and Regeneration. Stem Cells Int. 2020, 9148310. doi:10.1155/2020/9148310
Yang, Y., Lei, L., Wang, S., Sheng, X., Yan, G., Xu, L., et al. (2019). Transplantation of Umbilical Cord-Derived Mesenchymal Stem Cells on a Collagen Scaffold Improves Ovarian Function in a Premature Ovarian Failure Model of Mice. Vitro Cell.Dev.Biol.-Animal 55, 302–311. doi:10.1007/s11626-019-00337-4
Yao, Z. Y., Feng, B. W., Liu, C. S., Liu, Y. M., Zhou, H. Y., Zhang, X. H., et al. (2021). The Application of a Bone Marrow Mesenchymal Stem Cell Membrane in the Vascularization of a Decellularized Tracheal Scaffold. Stem Cells Int. 2021, 6624265. doi:10.1155/2021/6624265
Yeung, E., Fukunishi, T., Bai, Y., Bedja, D., Pitaktong, I., Mattson, G., et al. (2019). Cardiac Regeneration Using Human‐induced Pluripotent Stem Cell‐derived Biomaterial‐free 3D‐bioprinted Cardiac Patch In Vivo. J. Tissue Eng. Regen. Med. 13, 2031–2039. doi:10.1002/term.2954
Yi, K. W., Mamillapalli, R., Sahin, C., Song, J., Tal, R., and Taylor, H. S. (2018). Bone Marrow-Derived Cells or C-X-C Motif Chemokine 12 (CXCL12) Treatment Improve Thin Endometrium in a Mouse Model. Biol. Reprod. 100 (1), 61–70. doi:10.1093/biolre/ioy175
Yu, C., Bao, H., Chen, Z., Li, X., Liu, X., Wang, W., et al. (2021). Enhanced and Long-Term CT Imaging Tracking of Transplanted Stem Cells Labeled with Temperature-Responsive Gold Nanoparticles. J. Mat. Chem. B 9, 2854–2865. doi:10.1039/d0tb02997a
Zaragoza, C., Gomez-Guerrero, C., Martin-Ventura, J. L., Blanco-Colio, L., Lavin, B., Mallavia, B., et al. (2011). Animal Models of Cardiovascular Diseases. J. Biomed. Biotechnol. 2011, 497841. doi:10.1155/2011/497841
Zarei-Kheirabadi, M., Sadrosadat, H., Mohammadshirazi, A., Jaberi, R., Sorouri, F., Khayyatan, F., et al. (2020). Human Embryonic Stem Cell-Derived Neural Stem Cells Encapsulated in Hyaluronic Acid Promotes Regeneration in a Contusion Spinal Cord Injured Rat. Int. J. Biol. Macromol. 148, 1118–1129. doi:10.1016/j.ijbiomac.2020.01.219
Zhai, P., Peng, X., Li, B., Liu, Y., Sun, H., and Li, X. (2020). The Application of Hyaluronic Acid in Bone Regeneration. Int. J. Biol. Macromol. 151, 1224–1239. doi:10.1016/j.ijbiomac.2019.10.169
Zhang, C., Shang, Y., Chen, X., Midgley, A. C., Wang, Z., Zhu, D., et al. (2020a). Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano 14, 12133–12147. doi:10.1021/acsnano.0c05681
Zhang, H.-X., Zhang, X.-P., Xiao, G.-Y., Hou, Y., Cheng, L., Si, M., et al. (2016a). In Vitro and In Vivo Evaluation of Calcium Phosphate Composite Scaffolds Containing BMP-VEGF Loaded PLGA Microspheres for the Treatment of Avascular Necrosis of the Femoral Head. Mater. Sci. Eng. C 60, 298–307. doi:10.1016/j.msec.2015.11.055
Zhang, K., Chen, X., Li, H., Feng, G., Nie, Y., Wei, Y., et al. (2020b). A Nitric Oxide-Releasing Hydrogel for Enhancing the Therapeutic Effects of Mesenchymal Stem Cell Therapy for Hindlimb Ischemia. Acta Biomater. 113, 289–304. doi:10.1016/j.actbio.2020.07.011
Zhang, P., Zhang, C., Li, J., Han, J., Liu, X., and Yang, H. (2019). The Physical Microenvironment of Hematopoietic Stem Cells and its Emerging Roles in Engineering Applications. Stem Cell Res. Ther. 10, 327. doi:10.1186/s13287-019-1422-7
Zhang, R., Lee, P., Lui, V. C. H., Chen, Y., Liu, X., Lok, C. N., et al. (2015). Silver Nanoparticles Promote Osteogenesis of Mesenchymal Stem Cells and Improve Bone Fracture Healing in Osteogenesis Mechanism Mouse Model. Nanomedicine Nanotechnol. Biol. Med. 11, 1949–1959. doi:10.1016/j.nano.2015.07.016
Zhang, S., Lachance, B. B., Moiz, B., and Jia, X. (2020c). Optimizing Stem Cell Therapy after Ischemic Brain Injury. J. Stroke 22, 286–305. doi:10.5853/jos.2019.03048
Zhang, X. F., Shen, W., and Gurunathan, S. (2016b). Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An In Vitro Model. Int. J. Mol. Sci. 17, 1603. doi:10.3390/ijms17101603
Zhang, Y. S., Arneri, A., Bersini, S., Shin, S.-R., Zhu, K., Goli-Malekabadi, Z., et al. (2016c). Bioprinting 3D Microfibrous Scaffolds for Engineering Endothelialized Myocardium and Heart-On-A-Chip. Biomaterials 110, 45–59. doi:10.1016/j.biomaterials.2016.09.003
Zhang, Y., Wang, P., Wang, Y., Li, J., Qiao, D., Chen, R., et al. (2021). Gold Nanoparticles Promote the Bone Regeneration of Periodontal Ligament Stem Cell Sheets through Activation of Autophagy. Ijn 16, 61–73. doi:10.2147/ijn.s282246
Zhao, J., Zhang, Q., Wang, Y., and Li, Y. (2019). Uterine Infusion with Bone Marrow Mesenchymal Stem Cells Improves Endometrium Thickness in a Rat Model of Thin Endometrium. Reprod. Sci. 22 (2), 181–188. doi:10.1177/1933719114537715
Zhao, L., Liu, J.-W., Shi, H.-Y., and Ma, Y.-M. (2021a). Neural Stem Cell Therapy for Brain Disease. Wjsc 13, 1278–1292. doi:10.4252/wjsc.v13.i9.1278
Zhao, N., Yue, Z., Cui, J., Yao, Y., Song, X., Cui, B., et al. (2019). IGF-1C Domain-Modified Hydrogel Enhances Therapeutic Potential of Mesenchymal Stem Cells for Hindlimb Ischemia. Stem Cell Res. Ther. 10, 129. doi:10.1186/s13287-019-1230-0
Zhao, X., Li, Q., Guo, Z., and Li, Z. (2021b). Constructing a Cell Microenvironment with Biomaterial Scaffolds for Stem Cell Therapy. Stem Cell Res. Ther. 12, 583. doi:10.1186/s13287-021-02650-w
Zheng, Z., Guo, Z., Zhong, F., Wang, B., Liu, L., Ma, W., et al. (2022). A Dual Crosslinked Hydrogel-Mediated Integrated Peptides and BMSC Therapy for Myocardial Regeneration. J. Control. Release 347, 127–142. doi:10.1016/j.jconrel.2022.04.010
Zhou, M., Lozano, N., Wychowaniec, J. K., Hodgkinson, T., Richardson, S. M., Kostarelos, K., et al. (2019). Graphene Oxide: A Growth Factor Delivery Carrier to Enhance Chondrogenic Differentiation of Human Mesenchymal Stem Cells in 3D Hydrogels. Acta Biomater. 96, 271–280. doi:10.1016/j.actbio.2019.07.027
Zhu, H., Monavari, M., Zheng, K., Distler, T., Ouyang, L., Heid, S., et al. (2022). 3D Bioprinting of Multifunctional Dynamic Nanocomposite Bioinks Incorporating Cu-Doped Mesoporous Bioactive Glass Nanoparticles for Bone Tissue Engineering. Small 18, e2104996. doi:10.1002/smll.202104996
Zhu, Y., Uezono, N., Yasui, T., and Nakashima, K. (2018). Neural Stem Cell Therapy Aiming at Better Functional Recovery after Spinal Cord Injury. Dev. Dyn. 247, 75–84. doi:10.1002/dvdy.24558
Keywords: stem cells, biomaterials, scaffolds, microenvironment, therapeutic effects
Citation: Hong I-S (2022) Enhancing Stem Cell-Based Therapeutic Potential by Combining Various Bioengineering Technologies. Front. Cell Dev. Biol. 10:901661. doi: 10.3389/fcell.2022.901661
Received: 22 March 2022; Accepted: 17 June 2022;
Published: 05 July 2022.
Edited by:
Carmine Gentile, University of Technology Sydney, AustraliaReviewed by:
Leonora Buzanska, Mossakowski Medical Research Centre (PAN), PolandNathaniel Huebsch, Washington University in St. Louis, United States
Copyright © 2022 Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: In-Sun Hong, aG9uZ3N0ZW1AZ2FjaG9uLmFjLmty
 In-Sun Hong
In-Sun Hong