
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 14 April 2022
Sec. Stem Cell Research
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.880544
This article is part of the Research TopicUnderstanding the Causal Link Between Inflammation and Neurodevelopmental DisordersView all 8 articles
The gut microbiome has a tremendous influence on human physiology, including the nervous system. During fetal development, the initial colonization of the microbiome coincides with the development of the nervous system in a timely, coordinated manner. Emerging studies suggest an active involvement of the microbiome and its metabolic by-products in regulating early brain development. However, any disruption during this early developmental process can negatively impact brain functionality, leading to a range of neurodevelopment and neuropsychiatric disorders (NPD). In this review, we summarize recent evidence as to how the gut microbiome can influence the process of early human brain development and its association with major neurodevelopmental psychiatric disorders such as autism spectrum disorders, attention-deficit hyperactivity disorder, and schizophrenia. Further, we discuss how gut microbiome alterations can also play a role in inducing drug resistance in the affected individuals. We propose a model that establishes a direct link of microbiome dysbiosis with the exacerbated inflammatory state, leading to functional brain deficits associated with NPD. Based on the existing research, we discuss a framework whereby early diet intervention can boost mental wellness in the affected subjects and call for further research for a better understanding of mechanisms that govern the gut-brain axis may lead to novel approaches to the study of the pathophysiology and treatment of neuropsychiatric disorders.
The gut microbiota with trillions of microbial cells and thousands of species profoundly influences human physiology, including various diseases and disorders. The human gut microbiota starts colonizing during the pregnancy period (Collado et al., 2016). Collectively the genome of all these microorganisms represents the microbiome. The process of development and colonization of gut microbes co-occurs with brain development during pregnancy in a coordinated way until the first few years after birth (Jena et al., 2020; Acuña et al., 2021). An imbalance in the gut microbiome during the critical developmental phase can influence the overall developmental process, primarily neuronal plus glial development and maturation (Borre et al., 2014; Marín, 2016). The microbiota composition exhibits the highest intra- and inter-individual variability during the first 12 months of post-natal development until it reaches a stable adult-like organization at the age of ∼ 3 years and can influence the process associated with brain development and in shaping the immune profile of the individual. (Koenig et al., 2011; Bäckhed et al., 2015; Gensollen et al., 2016). Early-life colonization of the host’s mucosal surfaces is crucial for the development and maturation of the host’s immune system in a healthy individual (Gensollen et al., 2016).
However, exposure to factors such as maternal immune activation (MIA), poor diet, disease/infections and antibiotic overdose can lead to early life gut dysbiosis (Vangay et al., 2015; Neuman et al., 2018; Li et al., 2021). The altered gut microbiome can cause dysregulated immune activation, igniting systemic inflammation resulting in atypical brain development leading to symptoms associated with neurodevelopmental psychiatric disorders (NPD) (Figure 1) (Garay and McAllister, 2010; Han et al., 2021; J.; Lu and Claud, 2019; Munawar et al., 2021). Hence, the presence of a balanced microbiome is needed for the proper functioning of the immune system, which in turn regulates the neurodevelopmental trajectories (Garay and McAllister, 2010; Gensollen et al., 2016; Sotgiu et al., 2020).
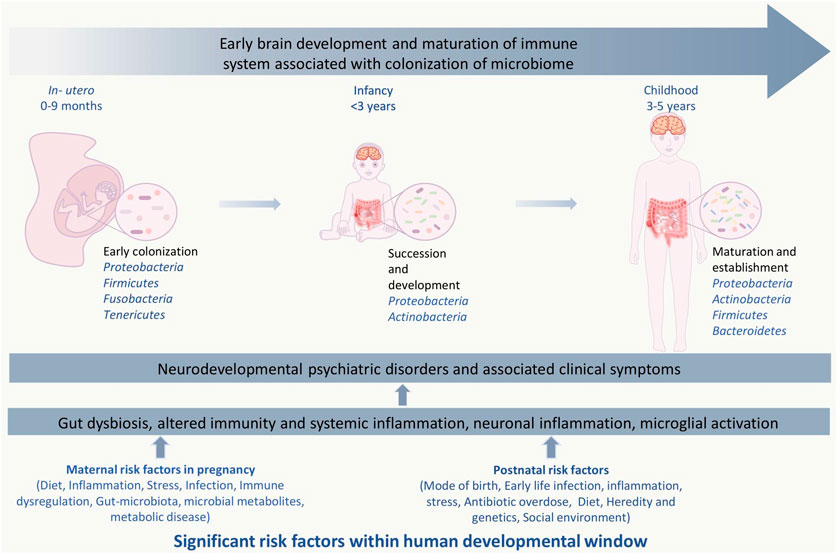
FIGURE 1. Significant risk factors affecting events with in human developmental window could bring on neurodevelopmental psychiatric disorders. The critical developmental window spans from the fetal stage until childhood, which consists of early colonization and development of the microbiome, development of the brain and nervous system, and development and maturation of the immune system. Within this period, the composition and diversity of the gut microbiome, genetics, maternal, and other reverent factors can alter the overall developmental homeostasis by causing disturbances in the immune system. Gut dysbiosis, immune alteration, and other factors induce microglial activation via inflammatory response, which leads to systemic and neuroinflammation. This negatively affects the brain development process and leads to abnormal brain development and functionality, including anxiety, depression, intellectual disability, and behavioral abnormality that can be seen in neurodevelopmental and psychotic disorders.
NPDs are a spectrum of disorders arising from atypical brain development resulting in cognitive, emotional, and motor deficits (Kapoor, 2002; Villagomez et al., 2019; Munawar et al., 2021). These include attention deficit hyperactivity disorders (ADHD), autism spectrum disorders (ASD), and schizophrenia (SCZ) which affect children in their daily lives through the early adolescence and later periods of life. These NPDs are also associated with psychotic symptoms and behavioral abnormalities characterized by positive symptoms such as psychotic hallucinations, the eccentric overflow of thoughts, and negative symptoms like insensibility, emotional and interactive withdrawal, lethargy, etc. (Cowen et al., 2012). These behavioral abnormalities often change or maturate and become more severe as a child grows older; some disabilities and behavioral abnormalities remain permanent (Klimkeit et al., 2016).
An increasing number of preclinical and clinical studies have been focusing on how the interaction between the complex gut-microbial ecosystem and central nervous system can regulate brain development and its association with NPDs (Figure 2) (Yap and Martin, 2015; Ernesto Martínez-González and Andreo-Martínez, 2019; Bull-Larsen and Hasan, 2019; Yuan et al., 2019; Checa-Ros et al., 2021; Kelly et al., 2021). However, most of these studies have explored the relationship on core pathology associated with one clinical subtype of NPDs. It is evident that there is a need for a greater understanding of the complex coordinated underlying pathways that’s span major NPDs. In this timely review, we discuss the recent evidence establishing the direct role of commensal gut microbes and associated metabolites on human brain development and how alterations in gut microbiome brain axis mediated communications play a role in the pathology of ASD, ADHD, and SCZ, which stems from aberrant brain development. Secondly, we propose a hypothetical framework illustrating an association between gut microbiota and inflammation affecting brain development and preventive measures in association with diet and probiotic therapy at crucial time points to mitigate the initiation and proper management of existing neurodevelopmental psychiatric disorders.
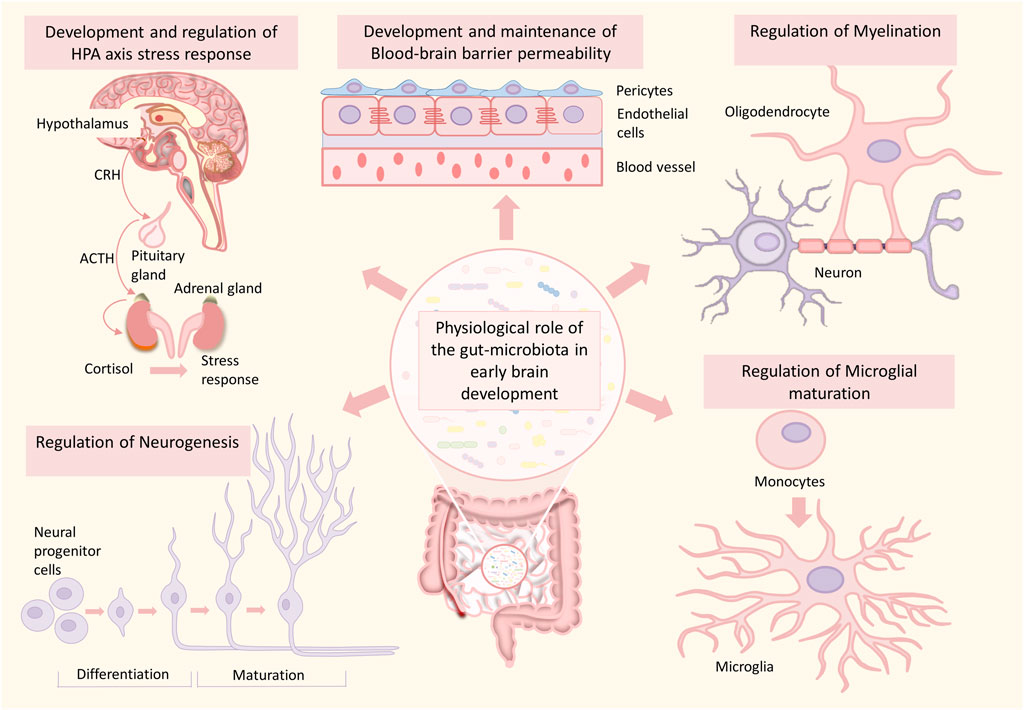
FIGURE 2. Role of the gut microbiome in healthy brain development. The gut microbiome plays an essential role in various processes of brain development such as neurogenesis, myelination, microglial maturation, development and maintenance of blood-brain barrier integrity, development of HPA-axis, and HPA-axis stress response. Any alterations in this developmental process can significantly increase the risk for neurodevelopmental disorders.
Following birth, the colonization of microbes initiates and is very much influenced by the mode of delivery (normal delivery or C-section) (Biasucci et al., 2010; Wampach et al., 2017). Some studies have shown that microbial colonization begins in-utero with the discrete microbial community present in the umbilical cord blood, placenta, and amniotic fluid and may carry out their role in the foetal developmental process (Aagaard et al., 2014; Collado et al., 2016). Although some studies have defined the microbial composition of the fetal meconium has been defined in several studies (Moles et al., 2013; Stinson et al., 2019), others have linked premature birth with the decreased microbial diversity within the meconium microbiome (Ardissone et al., 2014). Interestingly some recent studies suggest a similarity within the microbial profile in between placenta, fetal meconium, and amniotic fluid (He et al., 2020; Senn et al., 2020). Younge et al. have confirmed maternal-fetalin utero-translocation of the gut microbiota using a mouse model (Younge et al., 2019). The preterm neonate meconium has been shown to have abundant microbes belonging to Lactobacillus, Staphylococcus, and Enterobacteriales (Madan et al., 2012). With low diversity and high inter-individual variability, the meconium microbiome is more abundant with genera Bacillus, Escherichia/Shigella, and Enterococcus, whereas Bacteroides and Bifidobacterium are less abundant concerning the fecal microbiome of infants (Moles et al., 2013; Bäckhed et al., 2015). The placenta is most abundantly colonized by phyla Proteobacteria and Bacteroidetes and various other phyla like Firmicutes, Fusobacteria, Tenericutes, and genera Mycoplasma and Ureaplasma (Brotman, 2011; Aagaard et al., 2014). At the time of birth, the babies born through standard delivery through the vagina are colonized mainly by the maternal vaginal microbial population, predominantly Lactobacillus and Prevotella (Dominguez-Bello et al., 2010). In contrast, cesarean section delivered babies are more exposed and colonized by Staphylococcus and Corynebacterium closer to the skin microbiome (Dominguez-Bello et al., 2010). The typical microbiome composition of a newborn is mainly dominated by Proteobacteria and Actinobacteria, where the former dominates immediately after birth and later towards the 4 months of age (Bäckhed et al., 2015; Dogra et al., 2015). During the 1st year of life, the gut of the new-born infant is predominantly inhabited by Bifidobacterium, Enterococcus, Escherichia/Shigella, Streptococcus, Bacteroides, and Rothia gut-microbial population closer to maternal microbiota in addition to Clostridium, Ruminococcus, Veilonella, Roseburia, Akkermansia, Alistipes, Eubacterium, Faecalibacterium, and Prevotella, species. Moreover, variation in the maternal microbiota has also been suggested as modulating factor of microbiome composition in offspring (Dotterud et al., 2015). The 1st 3 years of the lifetime are more crucial for establishing a healthy and stable structured microbiome. It has been reported that the process of the microbiome and neuronal development coincides in an intense and coordinated way within this critical time frame and is most vulnerable to disruption (Sharon et al., 2016; Jena et al., 2020; Acuña et al., 2021). A gut dysbiosis at such period has been reported to bring about many NDD/NPD like ADHD, ASD, SCZ, intellectual as well as learning disability, and behavioral problems (Borre et al., 2014; Chrobak et al., 2016; Bojović et al., 2020). Moreover, after this period, microbiota reconstitution does not normalize the behavioral phenotype or neurochemical disturbances during the critical developmental period (Sudo et al., 2004; Heijtz et al., 2011; Click or tap here to enter text; Clarke et al., 2013). Hence, maintaining a healthy and well-structured microbiota is essential during prenatal and postnatal periods up to a particular developmental phase.
Emerging studies provide evidence about the active role of microbiota during central nervous system development. It is now clear that gut microbes play an active role in the neurodevelopmental processes (Figure 2), including the establishment of the blood-brain barrier (BBB) (Parker et al., 2020), neurogenesis (Cerdó et al., 2020), maturation of microglia (Erny et al., 2015), and myelination (Hoban et al., 2016; Duncan and Watters, 2019). These processes are critical in shaping animal behavior and cognition. Various dietary components released from the gut are needed for the developing brain for neuronal cell maturity and proper functions (Prado and Dewey, 2014; Ratsika et al., 2021). Also, recent evidence describes that gut microbes can directly facilitate the development processes in the brain, which have long-lasting consequences in health (J. Lu and Claud, 2019).
The BBB establishes early in-utero, which constitutes capillary endothelial cells sealed by tight junction proteins, pericytes, and astrocytes, forming the restrictive barrier between the brain and systemic circulation. It also facilitates the exchange of molecules and nutrients for proper maintenance and functioning of the brain (Obermeier et al., 2013; Braniste et al., 2014). The presence of balanced gut microbiota and microbial-derived metabolites such as SCFAs are essential in regulating the formation and maintenance of intact BBB (Obermeier et al., 2013; Braniste et al., 2014; Michel and Prat, 2016). The permeability of BBB in developing sterile fetuses decreases towards adulthood (Mollgaard and Saunders, 1986). In germ-free (GF) mice, the permeability of BBB increases for macro-molecules due to reduced expression of basic junctional proteins occludin and claudin-5 in the brain endothelial layer (Braniste et al., 2014). Microbial colonization of the gut or administration of butyrate, a short-chain fatty acid (SCFA) produced by gut-microbial fermentation, has been shown to reduce the blood-brain permeability in GF mice (Braniste et al., 2014).
Neurogenesis refers to the development of new functional neurons through differentiation of the neural stem/progenitors’ cells (Gage, 2019; Cosacak et al., 2020). The functionality of neurogenesis and neuronal plasticity is critical for learning, memory, cognition, and stress response, especially in the hippocampus, as the cognitive center (Kempermann, 2019). A balanced gut-microbiota is involved directly or indirectly in maintaining the microenvironment to support the process of neuronal development (Sarubbo et al., 2022). A recent comparative study between GF and SPF mice outlines a range of gut microbial metabolites that can cross through the placenta into the foetal compartment, with the ability to induce and regulate the prenatal developmental process (Pessa-Morikawa et al., 2022). In addition, PG, a bacterial cell wall component, gets crossed through the placenta to reach the foetal brain, there it activates Toll-like receptor 2 (TLR2), triggering an increase in FOXG1 expression, a crucial transcription factor in regulating the development and neurogenesis, thereby inducing neuronal proliferation in the forebrain area (Kaul et al., 2012; Humann et al., 2016). A recent study provides direct evidence linking modulation of microbial function and associated cytokine by microbiota to influence the process of neurogenesis (Salvo et al., 2020).
Further, the gut-microbes may indirectly affect neuronal-plasticity by regulating neuronal migration and maturation in CNS may be via regulation of ephrin B and reelin pathway, where ephrin B plays a pivotal role in the maintenance of the gut epithelial barrier integrity and reelin, a membrane glycoprotein responsible for neuronal migration (Allam-Ndoul et al., 2020; Grandi et al., 2019; D’Arcangelo, 2014; White and Getsios, 2014; Sentürk et al., 2011; Hafner et al., 2005).
Accumulating evidence suggests that gut microbes can impact the neural stem cells’ fate by coordinating with intricate pathways of differentiation and survival through neurotrophins and neurotransmitters in different areas of the brain (Sharon et al., 2016). The process of synapse development and maturation is associated with neuronal maturation and plasticity. Experimental administration of neonatal prebiotic (BGOs) in comparison to other prebiotic in 22 days old rats has been reported to elevate hippocampal expression of synaptophysin and brain-derived neurotrophic factor (BDNF). Synaptophysin, a synaptic vesicle protein, which controls the synaptic vesicle endocytosis kinetics, and BDNF, a nerve growth factor secreted by neurons, which also act as a signaling molecule for neuronal survival, growth, maturation maintenance of various brain cell populations, as well as the establishment of neuronal circuitry through the formation of the synapse (S. Williams et al., 2016).
Serotonin, a neurotransmitter and signaling molecule, can also be synthesized and released by gut microbes into the gut lumen, known to promote adult neurogenesis (Alenina and Klempin 2015). In addition, gut microbes have been reported to have an essential involvement in serotonergic signaling pathways in the gut and multiple regions of the brain (O’Mahony et al., 2015). Several studies have also reported the role of gut microbiota in regulating adult neurogenesis. By labeling proliferating cells with bromo-deoxyuridine GF mice brain, an increase in the adult dorsal hippocampal neurogenesis was reported compared to conventionally grown mice, and even upon microbial colonization, the phenotypical condition couldn’t be reversed. This indicates that the absence of microbes induces a dysregulated increase in adult dorsal hippocampal neurogenesis, and the microbial signals during crucial early life developmental window act as a controlling force in regulating neurogenesis in the hippocampus (Ogbonnaya et al., 2015).
Furthermore, the usage of antibiotics, which has a negative impact on gut microbiota, was associated with decreased neurogenesis (Möhle et al., 2016). The molecular mechanism that regulates the process of adult neurogenesis via microbiota and its associated metabolites is not very clear. It is suggested that neuroinflammatory mechanisms mediate this process along with humoral and metabolic pathways (Liu et al., 2022). A number of recent studies provide evidence to support the role of neuroinflammatory mechanisms. Intestinal bacteria maintain the enteric nervous system in adult mice through Toll-like receptor 2-induced neurogenesis (Yarandi et al., 2020).
Additionally, a decrease in BDNF mRNA level in the whole hippocampus was reported, particularly with a significant decline in neuronal proliferation and survivability in vagotomized mice (O’Leary et al., 2018). Furthermore, microbiome also indirectly influences hippocampal neurogenesis via regulating the neuronal immune system. Induction of acute colonic inflammation via administrating dextran sodium sulfate in mice reported a dysbiotic gut microbial composition, the increased hippocampal expression level of pattern recognition receptor and T-helper 17 cell-associated cytokines and ionized calcium-binding adapter molecule 1, a microglial activation marker, concomitant to depreciated adult hippocampal neurogenesis with consequent behavioral deficits (Salvo et al., 2020).
Early life stress, like a lack of social interaction, can also alter the stability of the gut microbiome (Cong et al., 2015), along with reduced neurogenesis and IL-6 and IL-10 level in the hippocampus of socially isolated mice as compared to grouped controls (Dunphy-Doherty et al., 2018). A reduction in hippocampal neurogenesis is strongly associated with impaired learning, anxiety, depressive-like behaviors, neuroinflammation, which again have an explicit association with structural alterations gut microbiome (Liu et al., 2022).
A healthy/intact gut microbiome has been reported to modulate myelination (Hoban et al., 2016; Jena et al., 2020). Humans are born primarily with unmyelinated axons in the central nervous system (CNS) at the time of birth. Rapid myelination of maturing axons occurs within just a few years after childbirth by oligodendrocytes through the process of engagement and ensheathment (Lu et al., 2002; Williamson and Lyons, 2018), with a variable rate of myelination and myelin content over time (Benes, 1989), until early adulthood. (Lebel et al., 2012; Williamson and Lyons, 2018). Any aberration in this process may lead to long-lasting defects. Myelination has the most crucial role in cognitive function, and the scale of myelination has been linked with neuronal plasticity and function (Almeida and Lyons, 2017; Kaller et al., 2017). The gut microbiota regulates the critical process of myelination by regulating myelination-related gene expression in oligodendrocytes. Myelin deformities can have a detrimental impact on brain function and behavior (Gacias et al., 2016; Hoban et al., 2016; Ntranos and Casaccia, 2018). Most notably, the prefrontal cortex (PFC) area of the brain exhibits myelination at a later period, during the initial phase of an infantile life, which makes it more vulnerable to external influencing factors, like intestinal dysbiosis (Hoban et al., 2016). As in the case of GF mice, irregulated myelin formation in the PFC region has a harmful effect on social behavior (Gacias et al., 2016; Hoban et al., 2016). Furthermore, bacterial metabolites such as SCFAs have been demonstrated to have a beneficial impact over stress-induced behavioral difficulties, intestinal barrier dysfunctionalities, and in the regulation of the myelination process (Gacias et al., 2016; van de Wouw et al., 2018). SCFA butyrate, when orally administered, caused recovery of myelination impairments, intestinal physiology, and behavioral deficit in antibiotic-treated mice, indicating a pivotal role of gut microbiota in developing the microbiome-gut-brain (MGB) axis through regulation of myelination process in the PFC region (Keogh et al., 2021). Thus, the microbiota is crucial for myelination and maintenance of the plasticity of the myelin sheath.
The endocrine-neurocrine interaction between the hypothalamus, pituitary gland, and adrenal gland in response to stress is known as the hypothalamus-pituitary-adrenal axis, where the corticotropin-releasing factor (CRF) plays the central role in the stress response via initiating a course of events that leads to the release of glucocorticoids from the adrenal cortex by regulating the HPA-axis (S. M. Smith and Vale, 2006). In developing the HPA-axis, commensal microbiota also plays a significant role (Sudo, 2012). In GF mice, dopamine deficiency, particularly in the frontal cortex hippocampus and striatum, is one of the major causes of improper stress regulation and anxiety-like behavior (Zarrindast and Khakpai, 2015). The administration of probiotic formulation consisting of probiotic strains Lactobacillus helveticus and Bifidobacterium longum, the level of anxiety decreased substantially (Partrick et al., 2021). Also, the comparison between GF and specific-pathogen-free (SPF) mice revealed increased CRF mRNA level in the hypothalamus of the GF mice, indicating an enhanced stress response related to HPA-axis (Sudo et al., 2004).
Microglia are resident immune cells (macrophages) that belong to the glial system. They account for 10–15% of the whole total glial cell count in the CNS (Hugh Perry, 1998), thoroughly distributed across the brain and the spinal cord (Lawson et al., 1990). Unlike the neuronal cells, microglial cells that constitute the innate immune system of CNS are derived from a subset of primitive macrophages that originates from the progenitor cell of the yolk sac (Ginhoux et al., 2013). The functionalities of microglia include immune defense and maintenance of the CNS. The microglial cells consistently survey their local microenvironment to detect pathogenic invasion or tissue damage throughout the CNS, guarded by the BBB (Daneman, 2012). Microglia also regulate neuronal proliferation, differentiation, and formation of synaptic connections (Graeber, 2010; Hughes, 2012), that helps in the refurbishing of post-natal neural circuits via controlling synaptic truncation during postnatal development of the mice (Tremblay et al., 2010; Paolicelli et al., 2011). Microglia contributes to both innate and adaptive immune system defense in the CNS. Abnormal activation of microglia aberrantly induces inflammation, which has been observed in most brain-related pathologies. Emerging evidence also suggests microglia directly affect neuronal pathology and contributes to disease progression (Perry et al., 2010; Helmut et al., 2011; Kingwell, 2012). Recent research data have demonstrated that microbiota has a vital role in the development and maturation of microglia (Erny et al., 2015; Abdel-Haq et al., 2019). However, upon damage or depletion of microglial macrophages and the yolk progenitor cells the bone marrow-derived macrophages can replenish them by developing and differentiation with the help of microbial signals at an early point (Erny et al., 2015; J.; Han et al., 2019). Moreover, in GF mice, the microglial cells show a significantly altered developmental state, with morphological characteristics and gene expression profile of a developmental and maturation arrest condition. These GF mice-derived microglia usually displayed a limited response towards viral infections and microbe-associated molecular patterns (MAMP), which interestingly rescued by SCFA administration (Hanamsagar et al., 2017).
In response to eliminating invading microorganisms such as bacteria, viruses, and other pathogens, the innate immune system triggers an intracellular signaling pathway via activation of toll-like receptors (TLRs) for gene expression of interferons, cytokines, and other immunological mediators (El-Zayat et al., 2019). The molecules during pathogenic elimination can also contribute to the development of inflammation response and so-called inflammatory mediators. Inflammation can also be triggered in response to endogenous factors such as predisposition to genetic abnormalities, behavioral anomalies of immune cells (autoimmunity/allergy), as well as external factors such as trauma, heat and cold stress, environmental toxicants, pathogens, diseases, etc. (Chen et al., 2018). Among pro-inflammatory mediator IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-12, TNF-α, INF-γ and among anti-inflammatory mediator IL-4, IL-5, IL-10, TGF-β, C-reactive protein (CRP), and serum amyloid-A are considered as inflammation biomarkers (Brenner et al., 2014; Sands, 2015; Kany et al., 2019; Morris et al., 2020).
The gut microbes also have an essential role in the induction of inflammation. More specifically, the pathogenic/opportunistic pathogenic strains such as E. coli and B. fragilis from the Enterobacteriace family contribute to pathogenesis when outnumbered and eventually to inflammation (Hiippala et al., 2020). Lipopolysaccharides (LPS), a specific cell membrane component present mostly in Gram-negative bacteria like E. coli and B fragilis is endotoxic and, upon interaction with macrophages, cause the release of pro-inflammatory cytokine TNF-α, IL-6, IL-1, which lead to endocytic septic shock and often have fatal outcomes (Agarwal et al., 1995). The LPS from Bacteroides is also a potential stimulator of the innate immune system (Alexander and Rietschel, 2001). In systemic inflammation, there is a reported elevation in the expression of pro-inflammatory cytokines IL-6 and IL-8, which have been correlated with microbial taxa belonging to phylum Proteobacteria within total fecal microbiota (Biagi et al., 2010).
Moreover, the combination of different taxa of microbes can enhance the pathogenic effects that lead to inflammation. For example, co-infection of E. coli and B. fragilis can induce an increase in expression of TNF-α, keratinocytes-derived chemokine mRNA (KC mRNA), and proteins in peritoneal tissue and cause early peritonitis, abscess development, and death in the experimental mice model, which is not observed when infected singularly (J. M. Kim et al., 2000). Colonization of Chloristidium difficle has been reported to have an association with frequent occurrence of eczema, sensitization to allergy, and ectopic dermatitis (Lee et al., 2017).
In contrast to inflammation, inducing microbes, a fraction of gut microbiota can counteract the inflammation. These microbes can act either by directly inhibiting inflammation-inducing microbes, strengthening the gut epithelial/mucosal barrier or directly interacting with inflammation-inducing components of the immune system or all three ways simultaneously (Hakansson and Molin et al., 2011). Faecalibacterium prausnitzii from the family Ruminococcaceae has been reported to secrete anti-inflammatory metabolites that block the activation and secretion of NF-κB and IL-8 in Caco-2 cells (Sokol et al., 2008). Also, F. prausnitzii can induce an increase in the anti-inflammatory ratio of IL-10 and IL-12 (Alameddine et al., 2019). An endoscopic recurrence of Chron’s disease has been reported when there is a lower proportion of F. prausnitzii within the composition of the patient’s group (Sokol et al., 2008).
Moreover, oral administration of F. prausnitzii can reduce the disease severity induced by 2, 4, 6-trinitrobenzene sulfonic acid in the colitis mouse model (Y. Zhou et al., 2021). Lactobacillus and Bifidobacterium are well-known probiotics and are the most studied taxa having anti-inflammatory properties and are the most crucial part of the gut microbiota (Cristofori et al., 2021). Microbes belonging to taxa Lactobacillus and Bifidobacterium have been reported to suppress an undesired activation of the immune response as in case of autoimmunity and allergy, while capable of inducing an increase in nonspecific IgA antibody secretion in the intestine (Sjögren et al., 2009; Kandasamy et al., 2015). Gut dysbiosis can be the consequence of an increase in inflammation-inducing microbes in the gut. Both dysbiosis and inflammation have a negative effect on brain development and can result in NDDs (Figures 3, 4).
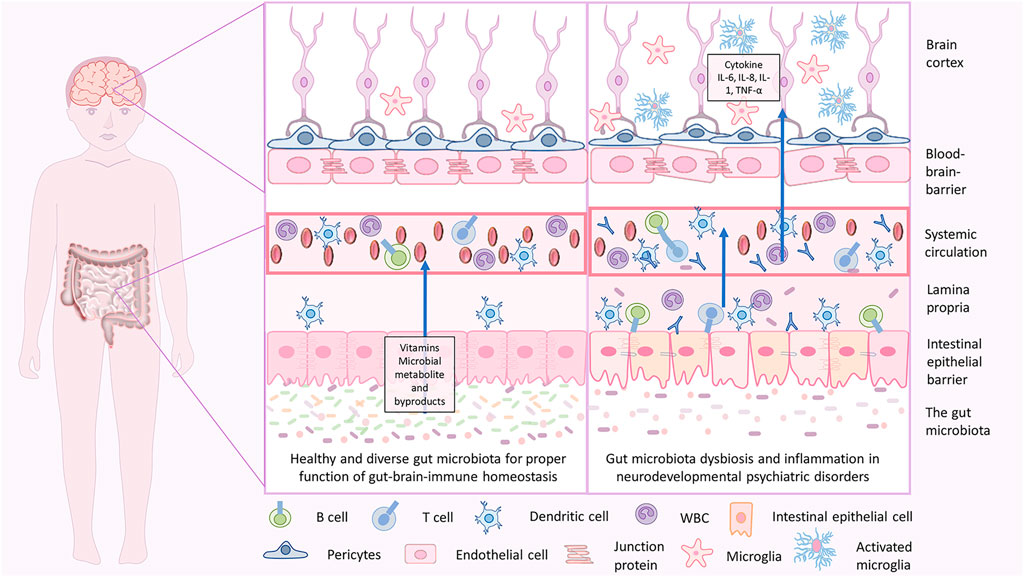
FIGURE 3. Gut-brain homeostasis and inflammatory mechanisms in neurodevelopmental psychiatric disorders due to gut brain dysbiosis. In infancy, the dysbiotic gut is characterized by less diverse microbiota with an abundance of pathogenic microbes, less beneficial microbes, and disrupted gut epithelial barrier with consequent GI tract-related disorders. When severe, the pathogenic microbes may cross through and enter the blood, which holds a massive immune response and releases inflammatory cytokines that lead to inflammation. Cytokine imbalance can induce microglial activation in the brain, which again causes neuroinflammation. This is associated with a disruption in brain development, and delay in neurodevelopmental subsequently may lead to NDD and NPDs.
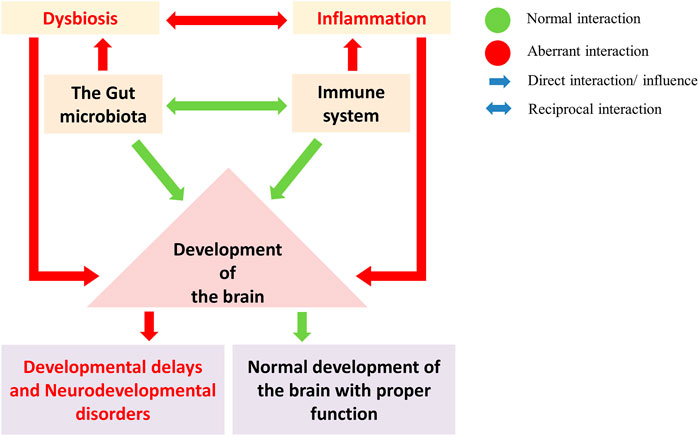
FIGURE 4. The gut microbes regulate the development and maturation of the immune system and play a crucial role in brain development (Green: normal interaction; Red: aberrant interaction). Gut dysbiosis and the immune system in brain development led to neurodevelopmental disorders. A gut dysbiosis alters the immune system homeostasis and leads to the activation of inflammatory response. Such aberration during brain development can disrupt the developmental pathways, leading to developmental delays and neurodevelopmental disorders.
Any alterations in the above neurodevelopmental process lead to a class of disorders affecting brain development and function that are collectively referred to as neurodevelopmental disorders, affecting 15% of the worldwide estimated population (Boyle et al., 2011). The gut microbiota has a significant role in neuronal development through a complex bidirectional communication, also known as the gut-brain axis. Alteration in the colonization of gut microbiota has a major impact on mammalian brain development that can influence the aberration in adult behavior by altering the expression pattern of several genetic events during the process (Heijtz et al., 2011). Also, the neurocrine disturbances (Clarke et al., 2013) and consequent behavioral phenotypes resulting upon disruption of the gut microbiota colonization during the developmental period cannot be reversed via reconstitution of microbiota at a later period (Sudo et al., 2004; Heijtz et al., 2011; Desbonnet et al., 2014). Inflammation-induced by dysregulation of the maternal immune system via maternal infection, maternal gut dysbiosis, or metabolic disorders during pregnancy has also been considered as modulating risk factors for abnormal brain development prenatally as well as neonatally, subsequently leading to a range of neurodevelopmental and neuropsychiatric disorders in the later period (Boulanger-Bertolus et al., 2018; Guma et al., 2021). Fetal exposure to maternal gut microbial products (propionic acid, LPS, or peptidoglycan), immune complexes, antibodies, and inflammation mediators can alter the normal neurodevelopmental process. Some of these specific factors are known to cross the placenta and the immature prenatal BBB and modulate several processes of brain development directly or by inducing neuroinflammation (Madore et al., 2016). Dysregulation of immune homeostasis and systemic inflammation share a direct connection with management complications of the gastrointestinal (GI) system (Chen et al., 2018), which eventually resulted in increased intestinal permeability (leaky gut) accompanied by various GI problems, and interestingly has a reciprocal correlation with gut microbial dysbiosis. Such GI complications have a reported association with various NDDs (Maguire and Maguire, 2019). After immediate birth among the first microbial colonizers, Bifidobacteria are the most important and dominant bacterial genera in the infant as well as adults’ gut commensals (Milani et al., 2017). Also, Bifidobacteria are considered among most potential probiotics and known to have anti-inflammatory, antimicrobial properties can utilize indigestible polysaccharides and can produce various B-group vitamins (O’Callaghan and van Sinderen, 2016). In addition, Bifidobacteria can reduce stress levels and help with depression by improving HPA axis stress response (Sudo et al., 2004), elevating the serotonergic precursor-tryptophan (Desbonnet et al., 2014), and having an anxiolytic effect (Bercik et al., 2011). Deficiency of intestinal Bifidobacteria has been linked with indigestion, vitamin B12 deficiency, dysregulated immune system, gut inflammation, depression, and anxiety-like behavior in individuals suffering from NDDs (Jolanta Wasilewska and Klukowski, 2015; Y.; Zhang et al., 2016). Moreover, in NDD patients, there is a significant decrease or complete absence of Bifidobacteria, while no such case was observed in any of the control subjects (Bojović et al., 2020). An association between the total SCFAs level and abundance level of Faecalibacterium, Ruminococcus, and Bifidobacterium was found (de Angelis et al., 2013), whereby R. champanellensis and F. prausnitzii are present in lower abundance in the patients, with several other strains from the genus Bifidobacterium. Desulfotomaculum guttoideum, Romboutsia ilealis, and Intestinibacter bartlettii are the microbial sp closely related to Clostridium clusters (Gerritsen et al., 2014) were commonly found in the NDD patients (Bojović et al., 2020). Moreover, microbes classified under genera Bifidobacterium and Ruminococcus cannot self-produce SCFA butyrate but can induce the production of SCFAs because of their ability to digest undigestible carbohydrates. They can induce butyrate production by providing substrates for butyrate-producing microbes in the colon area (Belenguer et al., 2006). SCFAs are important molecular intermediaries of the gut-brain axis, suggesting their involvement in developing autism-like symptoms (Rogers et al., 2016). Lower numbers of SCFA-producing bacteria, particularly the butyrate-producing bacteria in the affected individual’s gut, have been reported to exert a substantial impact on the etiopathology of NDDs (Bojović et al., 2020). One of the microbial species, namely Dialister invisus, is isolated from the oral cavity usually present in the gut microbiota of healthy humans (Downes et al., 2003), and a decrease in its abundance has been noticed in the NDD patient group (Bojović et al., 2020). This depicts that the variance in general commensal microbes in the NDD patients is significantly lower, with an abundance of potentially harmful bacteria than the healthy control group. Besides, people suffering from NDD commonly suffer from gut microbiota dysbiosis. However, there is a difference between the type of microbial richness and diversity within the gut microbiota and its association with different NDDs (Lacorte et al., 2019). Tables 1–3 provide a detailed summary of gut microbial abundance and altered cytokine levels in the case of NDDs.
Evidence suggests that dysregulated inflammation may be at the root of deficits that occur in numerous neurodevelopmental disorders, including schizophrenia and autism spectrum disorder (Ransohoff et al., 2015). Correlation between inflammation and NPDs in children has been gaining interest increasingly due to the harmful effects of inflammation on neuronal plasticity, neurogenesis, and overall neuronal development (Garay and McAllister 2010; Nona M.; Jiang, 2016). Inflammatory molecules affect brain development via interacting with MHC class-I molecule, glial cells, monoamine metabolism pathway, and HPA axis (Nona M. Jiang, 2016). In addition, immune cells, including B-cell, T cells, and macrophages, play a crucial role in the processes of brain development and are responsible for the onset of NPD (Shogo and Toshihide, 2018). Besides B-1a cell, one of the B-cell subtypes prevalently localizes to the neonatal brain in response to choroid plexus, reportedly involved in oligodendrocyte maturation in the developing brain (Shogo and Toshihide, 2018). However, in normal physiological conditions, B-1a cells preferentially localize in the intra-peritoneal cavity and participate in the neuro-immune defense system against any pathogenicity through the innate immune capability to secrete IgM antibodies, along with suppressing neuroinflammation, hence maintaining the neuroimmune homeostasis of the developing brain (Baumgarth, 2011; Kurnellas et al., 2015). Likewise, T-cells producing interferon-γ (IFN-γ) and IL-4 have been reported to regulate neuronal connectivity, development of cognitive, social behavior, and learning-related memory, respectively (Derecki et al., 2010; Filiano et al., 2016). The complete absence of T-cells in nude mice shows significant impaired neurogenesis and brain dysfunctionality (Ziv et al., 2006). Additionally, M2 macrophages subtypes are known to secrete neurotrophic factors such as IL-4, IGF-1, and upon induction BDNF, which promotes hippocampal neurogenesis, neuronal survival, and enhancement of spatial memory (Rőszer, 2015; Qi et al., 2017). IGF-1 and IL-4 are also produced by microglia and T cells, respectively (Derecki et al., 2010; Ueno et al., 2013). Also, perivascular macrophages support BBB development by providing pericytes progenitor cells (Daneman et al., 2010; Yamamoto et al., 2017).
Throughout the pregnancy, the maternal immune system and a fetal-placental immune response protect both mother and fetus in coordination with each other via systematic expression of various immune receptors that recognize diverse extra/intracellular pathogen and related products. And with pathogenic encounters, effectively produce a moderated inflammatory response, downstream signaling pathways related to pathogen clearance along with the secretion of antimicrobial peptides, and Indoleamine 2,3-dioxygenase (IDO) that are directly involved with pathogen clearance at the uterine-placental interface (Hoo et al., 2020). Thus, the immune system is likely to be present in an activated state rather than in the modulated state. And any case of immune alteration during this period can result in an increased susceptibility towards microbial and parasitic infections (Mor and Cardenas, 2010). This may trigger the fetal inflammatory response syndrome (FIRS) and results in an increased cytokine level of IL-1, IL-6, IL-8, and TNF-α, which has reported an increased risk of fetal developmental abnormalities (Gotsch et al., 2007). Moreover, human studies have also demonstrated a significant correlation between MIA, FIRs and the development of neurodevelopmental psychiatric disorders such as ASD, schizophrenia, neurosensorial deficits, ADHD, and psychosis in early as well as in the later period of adulthood (Knuesel et al., 2014; Brown and Conway, 2019; Choudhury and Lennox, 2021).
Further, research in the experimental mouse model has characterized high pro-inflammatory cytokine levels in the blood and amniotic fluid upon immune stimulation during pregnancy (Mor and Cardenas, 2010). It may likely contribute to the immunopathology of NDDs in their offspring. Certain inflammatory and immune-related conditions specific to particular NDD conditions, associated with inflammation induced by MIA, FIRS induction, nvolved in the development of NDD in offspring (Gotsch et al., 2007; Knuesel et al., 2014; Jung et al., 2020). Tables 1–3 provides a detailed summary of elevation and downfall in cytokine level during the inflammatory conditions in NDDs. The association of well-known neurodevelopmental-psychiatric disorders with inflammation and gut microbiome are discussed below.
Children with ASDs show a range of communication problems like lack of interest in social involvement, trouble expressing their feelings, and mostly avoiding physical contact, with trouble in speaking tendency (NIH, 2016). In addition, abnormality restrictive and repetitive behaviors, like aligning toys, flapping hands, swinging their body, or revolving in circles, have been the hallmarks of ASD (S. H. Kim and Lord, 2010). Around 80% of the children with ASD suffer from GI symptoms that include dyspepsia, abdominal pain, and constipation. The main reason for these severe GI problems could be the disruption of the gut microflora (Jolanta Wasilewska and Klukowski, 2015). In a recent study, restrictive food preference-based diet has also been linked with dysbiosis and decreased gut microbial diversity in children with ASD (Yap et al., 2021). Whilst the gut microbiome can regulate dietary preference, food choices as well as eating-related behavior, and a healthy gut microbiome is associated with healthy food choices (Alcock et al., 2014; de Wouters d’Oplinter et al., 2021; Koponen et al., 2021). Moreover, during pregnancy, dysbiosis of the maternal gut effectively alters gut-microbial diversity and immunity in the offspring, bringing an early onset of NDD (Nyangahu et al., 2018; Di Gesù et al., 2021). Additionally, altered immunity and inflammation also cause dysbiosis (Zeng et al., 2017).
Increasing evidence supports an association between gut microbiota dysbiosis and ASD (Xu et al., 2019). Through culture methods, aggressive forms of Candida species have been identified in the stool samples of 57% of children suffering from ASD compared to healthy controls (Iovene et al., 2017). Investigation of correlation between microbiome and ASD revealed that Clostridium histolyticum, a pathogenic anaerobic bacterium (Clostridium clusters I and II), was highly abundant in the fecal microbiota of ASD individuals. Several research groups have also detected an overgrowth of Clostridium sp. in the microbiota of children with autism (Finegold et al., 2002; Parracho et al., 2005; de Angelis et al., 2013). Erysipelatoclostridium ramosum was isolated from the fecal specimens of autistic children (Finegold et al., 2002). Clostridium tetani produce a neurotoxin p-cresol and other toxic metabolites (phenols and indole derivatives) that cause anxiety-like behavior (Bolte, 1998; Hsiao et al., 2013). Clostridium bartlettii is known to synthesize trans-3-indole acrylic acid (IAA), and by glycine conjugation, it can get converted into Indolyl-3-acryloyl glycine (IAG), which is a speculative ASD urinary diagnostic marker (Shattock and Whiteley, 2002; Bull et al., 2003). Overgrowth of these kinds of microbes has been associated with ASD pathology. Abnormality in sulfur metabolisms, such as the decline of serum sulfur in blood with a higher urinary excretion, decreased trans-sulfuration, and reduction in methylation capacity, in addition to chronic oxidative stress (Finegold et al., 2012b), have been detected in children with autism. This may be due to an increase in another sulfate-reducing organism, D. guttoideum, similar to Desulfovibrio (Finegold et al., 2012a; Tomova et al., 2015). In addition to Chloristidium cluster bacteria, Sutterella, associated with gut-immune homeostasis, and GI symptoms, has been found closely related with the intestinal epithelial layer among autistic children while absent in controls (B. L. Williams et al., 2012). Moreover, a particular relation between microbes Actinomycetaceae, Allisonella, Barnesiella, Coprobacter, Fusobacterium, Olsenella in ASD individuals and constipation have been observed. In contrast, there was an increase in Holdemanella in the case of ASD without associated constipation (Lacorte et al., 2019). The microbiome of ASD individuals represents a phylum level ratiometric increase in Firmicutes/Bacteroidetes due to a significant increase in Firmicutes and a reduction in the abundance of Bacteroidetes (Finegold et al., 2010; Kang et al., 2013). A lower abundance of Eubacteriaceae, Erysipelotrichaceae, Faecalibacterium, Peptostreptococcaceae, Ruminococcaceae, and Streptococcaeceae, has been reported at a taxa level (de Angelis et al., 2013). At the family level, a considerably higher abundance of Bifidobacteriaceae, Lactobacillaceae, Enterobacteriaceae, and Veillonellaceae have been observed (Bezawada et al., 2020). At the genus level, there was a significant increase in Bifidobacterium, Bacteroides, Bacillus, Biophila, Faecalibacterium, Lactobacillus, Lachnospira, Lactococcus, Lachnobacterium, Megamonas, Megasphera, Mitsuokella Oscillospira, Parabacteroides, Sutterella (Lacorte et al., 2019), Collinsella, Corynebacterium, and Dorea (Strati et al., 2017). A lower abundance of genera Streptococcus, Veillonella, Escherichia (Zhang et al., 2018), Alistipes, Dialister, Parabacteroides (Strati et al., 2017) were observed in autistic children. Additionally, Yap et al. reported a specific decline in Romboutsia timonensis abundance, especially in the gut microbiota of ASD children (Yap et al., 2021). Furthermore, SCFAs are crucial for maintaining BBB integrity and essential during the process of brain development (Silva et al., 2020), with proper maintenance of the functional intestinal epithelial barrier (Peng et al., 2009). SCFAs like butyrate, propionate and acetate solely or in combination with others can stimulate the tight junctions (TJ) formation (Ohata et al., 2005; Peng et al., 2007, 2009). Especially butyrate regulates the formation of BBB (Nankova et al., 2014). Further, butyrate and propionate exert a strong epigenetic effect over-regulation of the gene expression profile of neurotransmitter systems, neuronal cell adhesion molecules, inflammation, mitochondrial function, oxidative stress, and lipid metabolism. All these have been reportedly associated with the development of ASD (Nankova et al., 2014). Lower SCFAs levels may disrupt epithelial barrier function in the intestine, and BBB thus increases the permeability as reported in ASD patients (Fiorentino et al., 2016). Decreased SCFAs levels in the gut can be related to the absence of potential SCFA-producing bacteria in ASD patient microbiota (Bojović et al., 2020). Assisting all these reports, lower Bifidobacterium sp. levels were found in children with autism (Wang et al., 2011).
Cytokines regulate immune response and are interestingly involved in neuronal development synaptic functions, differentiation, migration, proliferation, and behavioral impairments (Deverman and Patterson, 2009; Mousa and Bakhiet, 2013). Neuropoietic cytokines such as IL-6, IL-1β, and TNF-α have been reported to exert direct influence on the cortical neuron, dendrite development, neural activity, long-term potentiation, neurite outgrowth, regulation of synaptic plasticity in the hippocampus, overall neurodevelopment as well as behavior (Deverman and Patterson, 2009; Mousa and Bakhiet, 2013). Dysregulation in the cytokine levels creates disturbances in immune homeostasis, which potentially contribute to communication and behavioral impairment at the neuronal level in early childhood and, upon becoming severe, can give rise to NDDs like ASD (Goines and Ashwood, 2013; H. K.; Hughes et al., 2018). In children with ASD increased number of monocytes and plasma concentrations of IL-8, TNF-α, IL1β are observed, which indicate an abnormal inflammation condition (Rodríguez et al., 2017). Increased plasma concentration of IL-8 has been reported as a result of increased IL-17 released by activated Th17 cells in response to epithelial and endothelial infections (Suzuki et al., 2011). Immune dysfunction in ASD patients like abnormal T helper cell profile (Ashwood and Wakefield, 2006), increased concentration of complement factors (Corbett et al., 2007), pro-inflammatory interleukins (IL-1β, IL-6, IL-12p40) (Ashwood et al., 2011), TNF-α, and a decreased level of Transforming growth factor β (TGF-β) have been reported to cause acute inflammation analogous to ASD condition. Abnormal cytokine levels have been associated with poor health and communication, impaired social interaction, poor cognition/memory, and behavioral and neuronal dysfunction in ASD (Ashwood et al., 2011; Tye et al., 2019). Further, more studies conducted in children with ASD have revealed an elevated plasma level of IL-1β, IL-6, IL-17, IL-12p40, and IL-12p70, including both Th1 and Th2 cytokines, with down-regulation of IL-2 (Molloy et al., 2006; Jácome et al., 2016; Eftekharian et al., 2018). There also has been a report suggesting a sex-specific correlation of cytokine expression profile. According to which the mRNA expression level of TNF-α has been linked with expression of TGF-β, INF-γ, IL-17, and IL-6 in the case of males, whereas it's not the case with the females (Eftekharian et al., 2018). Besides, reports also show a difference in cytokine expression profile as per the disease severity in children with ASD. At a mildly severe condition, there is an elevation in plasma level of IL-12p40. In contrast, patients with moderate disease severity have higher plasma levels of TNF-α, which explains the correlation of disease severity with increased plasma concentration of TNF-α (Jácome et al., 2016; Xie et al., 2017).
In addition to all these inflammatory factors that contribute to ASD pathology, dysregulation in the maternal immune system during pregnancy has also been implicated in the development of ASD (Bilbo et al., 2018). Fetal brain reactive antibody, autoimmunity, or IgG antibody from mother can cross the placental barrier, thus entering into the fetal compartment where they can interfere with the developmental process by recognizing self-proteins. Also, in the fetus brain, the BBB is not fully formed or functional (Croen et al., 2008; Braunschweig and van de Water, 2012; Fiorentino et al., 2016). Therefore, the probability of immune-complexes and inflammatory mediators in action can reach the brain stem by crossing the BBB, contributing to microglial activation and neuronal inflammation eventually (Morgan et al., 2010; Fiorentino et al., 2016). Neuroinflammation within the CNS is also associated with severe disease conditions in ASD (Dipasquale et al., 2017; Eissa et al., 2020). Animal and human studies have suggested an elevated IL-6 expression in the autistic brain, causing anatomical aberration. Such overproduction of IL-6 can alter synapse formation and neurotransmission along with distorted patterns and distribution of dendritic spines (Wei et al., 2011, 2012, 2013). Moreover, the ASD animal model studies showed systemic inflammation with up-regulated IL-β, IL-6, IL-18, IL-33, IL-17, and TNF-α along with microglia activation and neuroinflammation principally contributing to the pathological mechanisms of ASD (Prata et al., 2017).
Attention-deficit/hyperactivity disorder is commonly characterized by persistent inattention and hyperactivity-impulsivity onset during childhood. Genetic factors (Faraone et al., 2005), streptococcal infection (Leslie et al., 2008), and environmental factors (Mulligan et al., 2013; Guney et al., 2015) have a close association with ADHD. However, dopamine (DA) deficiency and an altered level of norepinephrine (NE), serotonin, GABA has also been proposed for ADHD (Blum et al., 2008; Wilens and Spencer, 2010), including a decline in cortisol level, indicating disruption of the HPA-axis as well (Fortier et al., 2013). The microbiota has an important role in the development of the HPA-axis, and individuals with ADHD experience an imbalance in their microbiota and a reduced microbial diversity and composition, which resulted in lower levels of cortisol to cause improper management of the HPA-axis stress response (Huo et al., 2017; Checa-Ros et al., 2021). Children with ADHD unusually have a higher load of the family Bacteroidaceae, Neisseriaceae differing from a higher level of Prevotellaceae, Catabacteriaceae, and Porphiromonadaceae in the control group. An increased microbial load of family Neisseriaceae and Bacteroidaceae has been reported causing a significant decline in the gut microbial diversity in ADHD (Lacorte et al., 2019). At a family level, the ADHD-specific fecal microbiome represents a substantial rise in the richness of Moraxellaceae, Peptostreptococcaceae, Peptococcaceae, Xanthomonadaceae, and a firm decline in Alcaligenaceae (Jiang et al., 2018). While, at a genus level, Dialister, Faecalibacterium, Lachnoclostridium, and Sutterella were the major representatives that brought the differences among children with and without ADHD, respectively (Prehn-Kristensen et al., 2018). Besides, a certain group of gut microbiota can synthesize neuroactive monoamine molecules (dopamine, noradrenaline, serotonin, GABA) and its precursors (phenylalanine, tyrosine, tryptophan) that are involved in ADHD pathomechanism. These microbes also induce intestinal epithelial cells to synthesize neuroactive compounds and indirectly control the neurotransmission system to have a crucial impact on brain functioning and behavior (Strandwitz, 2018; Y.; Chen et al., 2021; Huang and Wu, 2021). In a study including 28 participants, functional magnetic resonance imaging (fMRI) revealed a nominal increase in the genus Bifidobacterium in ADHD (Hiergeist et al., 2020). This increase has a significant association with enhanced bacterial gene functionality that encodes the enzyme cyclohexadienyl dehydratase (Aarts et al., 2017), an enzyme involved in the phenylalanine (a dopamine precursor) synthesis pathway (Lou, 1994). Phenylalanine can cross the BBB and regulate dopamine synthesis positively or negatively by inhibiting tyrosine hydroxylase during dopamine synthesis (Lou, 1994). Dopamine deficiency can cause decreased neural responses to reward anticipation, which is one of the hallmarks of ADHD (Aarts et al., 2017). On the other hand, diminished Bifidobacterium population during early childhood has been correlated with a greater risk of developing ADHD (Pärtty et al., 2015). Bacillus macerans can synthesize dopamine, as it possesses an enzyme called cyclomaltodextrin glucosyltransferase or CTGase, a key enzyme to produce dopamine (Clarke et al., 2013). Deficiency and decrease in abundance of such microbes in gut microbiota may contribute to stress, depression, and anxiety-like behavior that has been witnessed in ADHD (Clapp et al., 2017). In individuals with ADHD, altered serotonin levels have been linked with abnormal cognitive function and various other processes during neuronal development (Blum et al., 2008). Studies have demonstrated that gut microbes regulate the serotonin (5-HT) levels in the colon and blood, while the GI tract contains the majority of total serotonin present in the body either as 5-hydroxytryptamine or 5-HT. Microbes in the colon produce certain metabolites that stimulate the chromaffin cells to increase Tph levels and 5-HT biosynthesis (Huang and Wu, 2021). The serotonergic system resolves brains involvement in stress, anxiety, and depression (Graeff et al., 1996). Specific gut bacteria can produce important inhibitory neurotransmitters gamma-aminobutyric acid (GABA). Moreover, Bifidobacterium and Lactobacillus can produce GABA, while Streptococcus sp. and Enterococcus sp. which produce serotonin, are less abundant in the fecal microbiota of ASD and ADHD patients (Bundgaard-Nielsen et al., 2020). Another study showed an increase in Alistipes and Oscillibacter and a significant decrease in Bacteroidales in depressed individuals (Frémont et al., 2013; Naseribafrouei et al., 2014). Bacteroidetes has been associated with obesity previously, which is again associated with depression and mild inflammatory conditions (Chen et al., 2018). In addition, the genus Oscillibacter produces a metabolic end-product valeric acid, which is structurally similar to gamma-Amino Butyric acid (GABA). As a structural homolog of GABA, it can also bind to GABA receptors (Katano et al., 2012), facilitating increased inhibitory neurotransmission. This signifies the correlation of intra-microbiome coordination with depression and anxiety-like behavior in NDDs such as ADHD.
ADHD is reported to be immune-associated with an acute inflammatory response that emerges during childhood. Immune-associated genetic factors and heritability have been the most common cause of ADHD, estimated at around 70–78% (Franke et al., 2012). Most potentially genetic polymorphism of cytokine genes IL-6 and tumor necrosis factor-alpha (TNF-α) has been found in ADHD patients (Drtilkova et al., 2008). Moreover, cytokines play a significant role in tryptophan metabolism and dopaminergic pathways in the brain (Miller et al., 2013). Therefore, in the case of ADHD, an alteration in proportional expression of pro-inflammatory and anti-inflammatory cytokine can alter the required level of monoamine neurotransmitter (Dunn et al., 2019). Administration of IL-6, IL-2, IL-1β caused altered neurotransmission in the rodent model due to increased norepinephrine and decreased dopamine level, resembling the ADHD condition (Zalcman et al., 1994; Anisman et al., 1996). Also, in ADHD patients, there is an increased expression of innate pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α) and a reduction in expression of anti-inflammatory cytokines like IL-4, IL-2, and INF-γ. These pro and anti-inflammatory cytokine alterations can induce microglial activation and neuroinflammation (Oades et al., 2010; Anand et al., 2017). The activated microglia release more pro-inflammatory cytokines and other associated factors, contributing to chronic neuroinflammation (Harry and Kraft, 2008). During the prenatal period, the peripheral and neuroinflammation alone or in combination may interfere with the maturation of the prefrontal cortex and neurotransmitter system, such as the dopaminergic system, which increases the risk of developing ADHD in offspring. Dopamine deficiency in CNS is one of the well-reported pathogenesis hallmarks of ADHD (Blum et al., 2008; Aarts et al., 2017). Moreover, allergic diseases that are considered high inflammatory disorders are also reported to be associated with ADHD (Wang et al., 2011; Miyazaki et al., 2017).
SCZ is a chronic, heterogeneous neurodevelopmental psychiatric disorder with multifactorial involvement of genetic and epigenetic disturbances, gut microbiome alteration, immune system dyshomeostasis, and environmental influence correlated with hallucinations, delusional thoughts, perturbing mental awareness, and social interaction (Van Os and Kapur 2009). The disorder commonly involves continuous or intermittent occurrences of paranoia (Owen et al., 2016), with positive, negative, and cognitive psychotic symptoms. The positive symptoms include hallucinations, unsystematic speech patterns, and cataleptic behavior based on the excessiveness of normal function. In contrast, the negative symptoms involve a reduction in normal physiological processes, such as lack of emotions or interest, poor speech, aimlessness, and cognitive impairments, which comprise poor retention of verbal information and confusion (Munawar et al., 2021). SCZ is thought to begin in utero and is associated with prenatal famine, poor pregnancy, fetal growth conditions, emergency C-section delivery, and low birth weight (King et al., 2010). The functionalities of bidirectional gut-brain communication are related to emotional and cognitive centers such as the central nervous system, peripheral nervous system, enteric nervous system, and enteroendocrine system. Dysregulation might hold implications over the occurrence and etiology of SCZ (Grenham et al., 2011; Rogers et al., 2016).
As in ASD, Schizophrenic individuals suffer high rates of GI problems like gastroenteritis, colitis, irritable bowel syndrome (IBS) (Garakani et al., 2003; Shah et al., 2012; Yeh et al., 2021) and are mostly at high risk for emergence of hyperglycemia/diabetes, obesity, hypertension, and cardiovascular disease. Such metabolic disorders are strongly associated with microbiota dysbiosis, including neuropsychiatric disorders such as SCZ (de Hert et al., 2009; Ventriglio et al., 2015). Furthermore, the comorbidities of metabolic disorders in SCZ and enrichment of the specific gut microbes may disrupt brain white matter (Spangaro et al., 2018), causing the manifestation of negative symptoms and cognitive functional anomalies (Socała et al., 2021). Alteration in the gut microbiota is associated with a decreased species diversity within the microbiota in SCZ (Patrono et al., 2021).
There is an increase in the abundance of oropharyngeal microbial species like Bifidobacterium dentium, Lactobacillus oris, Veillonella atypica, Dialister invisus, Veillonella dispar, and Streptococcus salivarius in schizophrenic individuals than in healthy controls (Zhu et al., 2020; Castro-Nallar et al., 2015). Whereby the increased abundance of genera Streptococcus and Veillonella have positive cross-correlation, which also indicates a close association between the intestinal and the buccal microbiota in SCZ (Zhu et al., 2020). The increased translocation of oral microbe can be linked with disrupted the mucosal barrier, giving rise to leaky gut, pathological intestinal conditions, and decreased immune surveillance towards foreign microbes in SCZ patients (Zhu et al., 2020). Schizophrenic patients harbor a unique combination of facultative anaerobes, including Lactobacillus fermentum, Alkaliphilus oremlandii, Enterococcus faecium, and Cronobacter sakazakii/turicensis, that are usually not present in a healthy gut (Zhu et al., 2020).
An elevated phylum level of Proteobacteria characterizes the schizophrenic microbiome. Particularly, with a substantial rise in genera Methanobrevibacter, Clostridium, Collinsella, Succinivibrio, Klebsiella, Megasphaera, with a decrease in Coprococcus, Blautia, and Roseburia, which also has been associated with disturbed metabolic pathways like fatty acid, vitamin B6 metabolism in SCZ (Shen et al., 2018). Contrarily a decline in phylum Proteobacteria, family Rumimococcaceae, genus Haemophilus, Sutterella, and Clostridium have been reportedly associated with disease progression. In contrast, increased Anaerococcus and Bacteroids has also been reported in such schizophrenic conditions. The relative decrease in Rumimococcaceae is directly related to negative symptoms, and increased Bacteroids have been reported in association with the increase in the symptoms of depression in SCZ patients (Nguyen et al., 2018). Moreover, in the early stages of the disease, an increase in Lactobacillaceae (Schwarz et al., 2018), and at a later stage, bacterial taxa Lachnospiraceae and Veillonellaceae have been linked with the disease severity in SCZ (Zheng et al., 2019). Higher Clostridiales, Lactobacillales, Bacteroidales suggest an overlap between altered microbiome with those identified in SCZ and ASD (de Angelis et al., 2013; Shen et al., 2018).
Moreover, the increase in diversity of the microbes in the blood can be related to the indefinite overall increase of microbial load in SCZ (Olde Loohuis et al., 2018). The blood microbiota is thought to emerge from the gut and the buccal cavity (Potgieter et al., 2015; Spadoni et al., 2015). Schizophrenic patients suffering from GI inflammation and IBS have been reported to have elevated levels of anti-Saccharomyces cerevisiae antibodies (ASCA), used for diagnosis of Crohn’s disease (Desplat-Jégo et al., 2007; Rodrigues-Amorim et al., 2018). Zhu and co-workers reported 11 bacterial species (Table. 3) that were significantly enriched and have been particularly associated with symptom severity and poor cognitive performance in SCZ (Zhu et al., 2020). Again, the frequency of Clostridium difficile infection has been highly related to the schizophrenic patient population (Argou-Cardozo and Zeidán-Chuliá, 2018). Zheng et al. reported an increased abundance of Acidaminococcus, Akkermansia, Alistipes, Citrobacter, Dialister, Veillonella in SCZ (Zheng et al., 2019). In addition, a metagenomic study in SCZ patients revealed a strong association of 12 increased taxonomic abundant groups including Deltaproteobacteria, Actinobacteria, Sphingomonadales, Actinomycetales, Sphingomonadaceae, Megasphaera, Eggerthella and, Megasphaera elsdeniis, Clostridium perfringens, Akkermansia, muciniphila, Lactobacillus gasseri, and Bifidobacterium adolescentis, along with 7 decreased taxonomic groups including Rhodocyclales, Enterococcaceae, Rikenellaceae, Alcaligenaceae, Rhodocyclaceae, Leuconostocaceae, and Enterococcus in comparison to age and sex-matched healthy controls (Xu et al., 2020).
A decrease in diversity of family Erysipelotrichaceae and genus Allobaculum, along with a dysbiosis, has been reported in the metabotropic glutamate receptor 5 (mGlu5) knockout mice model of SCZ (Gubert et al., 2020). Further, a significantly low level of Bifidobacterium, Lactobacillus, E. coli, and a higher level of Clostridium coccoides were reported in fecal samples of SCZ individuals compared to healthy subjects (Cuomo et al., 2018). However, treatment with either olanzapine or risperidone was reported to alter the level of Akkermansia, Sutterella, and Lachnospiraceae with a considerable increase in Bifidobacterium, E. coli and a decrease in fecal Clostridium coccoides and Lactobacillus compared to untreated (Flowers et al., 2017; Yuan et al., 2018). Studies have shown that prenatal and inherent microbial infections in neonates can increase the risk of developing SCZ (Babulas et al., 2006; Brown and Derkits, 2010). Infection by Helicobacter pylori is associated with nutrient malabsorption during childhood and induces disruption in biochemical homeostasis, which may play an essential role in the pathogenesis of SCZ (Yilmaz et al., 2008; Vitale et al., 2011). Toxoplasma gondii infection during pregnancy is positively associated with SCZ in offspring (Brown et al., 2005). Moreover, Streptococcus vestibularis has been considered a microbial biomarker of SCZ that can induce deficits in social behaviors, alteration in neurotransmitter levels (Zhu et al., 2020), hyperactivity, cognitive impairments associated with SCZ (Maes et al., 2019).
SCZ is associated with the aberration of various physiological pathways involving dopamine, glutamate, and γ-aminobutyric acid (GABA) signaling (Rowland et al., 2016; Hoftman et al., 2018; Kesby et al., 2018), synthesis of SCFAs like butyrate, acetate, propionate, and isovaleric acid, tryptophan metabolism, and synthesis of several neurotransmitters (glutamate, GABA, and nitric oxide) (Zheng et al., 2019). Alteration of which are associated with symptoms, such as depression, anxiety, and pathophysiology of SCZ (Maas et al., 1993; Möhler, 2012; Erhardt et al., 2017), which again can be related to the alteration of gut microbial diversity in SCZ. Experimental, fecal transfer from SCZ patients into the GF mice gut induces SCZ-related behaviors resembling glutamatergic hypo-functionality related SCZ mice model behavior in receiver mice with observably distorted glutamate, glutamine, and GABA ratio in the hippocampus region of the brain (Zheng et al., 2019).
Aberration in glutamatergic signaling system-related genes is closely associated development of SCZ. Meanwhile, Glutamate synthase (GOGAT), validated as SCZ gut marker, an increase in expression and activity in SCZ patients is co-related with a characteristic decrease in gut microbial diversity and an altered IgA related mucosal immunity as compared to healthy controls (Gubert et al., 2020; Xu et al., 2020).
Tryptophan metabolism is one of the essential pathways for the maintenance and homeostasis of gut microbiota and disturbances have been reported to contribute to the pathophysiology of SCZ (Erhardt et al., 2017; Plitman et al., 2017). Tryptophan is metabolized chiefly through the kynurenine pathway, and schizophrenic individuals are reported to have high serum kynurenine metabolites and low serum tryptophan levels (Zhu et al., 2020; Muneer, 2020). A study involving fecal microbiota transplantation from drug-free SCZ patients into SPF mice reported an alteration of the kynurenine metabolic pathway and schizophrenic behavior in receiver mice. This alteration in tryptophan and kynurenine pathway metabolite level is directly associated with the gut-microbiota alteration and enrichment of schizophrenic specific microbes (Zhu et al., 2020). The translocation of microbes along with microbial components like LPS into the bloodstream due to gut dysbiosis as well as increased gut epithelial barrier integrity may be inducing systemic inflammation, that consequently leads to neuroinflammation, neurological impairment, and apoptosis, leading to immune-mediated development of SCZ (Fasano, 2012; Alhasson et al., 2017; Yuan et al., 2019). In addition, SCFAs have a direct role in anxiety and behavioral changes such as antisocial, repetitive, ritualistic behaviors associated with neuropsychiatric disorders like obsessive-compulsive disorder hyperactivity in SCZ (MacFabe, 2012). Furthermore, the reported elevation in microbial translocation marker sCD14 has been correlated with a reduction in butyrate-producing species Roseburia and Coprococcus with increased inflammation and CNS infections (Machiels et al., 2014). SCFAs also indirectly regulate DNA repair mechanisms by reducing the excessive activity of histone deacetylases (HDACs) in schizophrenic conditions, and reduced levels can negatively affect the diseased condition (Oleskin and Shenderov, 2016), suggesting an additional indirect role of SCFA in SCZ.
SCZ affects approximately 1% of the worldwide population (Kahn et al., 2015). The complex interaction between genetic and environmental risk factors manipulates the immune system to cause inflammation, disruption of neuroimmune homeostasis, chronic neuroinflammation, and neurodevelopmental disturbances in SCZ (Comer et al., 2020). The risk factors include genetic anomaly, MIA, maternal infection, gut dysbiosis, early-life stress, and exposure to pollution, resulting in the dysfunctional BBB and microglial function that leads to neuroinflammation that gives rise to the onset and progression of SCZ (Comer et al., 2020). The characteristic brain damage in SCZ starts in the early periods of life manifests later in the lifetime (Altamura et al., 2013). The disease symptoms usually appear in late adolescence and early adulthood (Na et al., 2014).
Genome-wide association studies have revealed genetic polymorphism of immune-related genes in SCZ etiopathophysiology. For example, genetic polymorphisms of C4 complement factor, major histocompatibility complex (MHC) locus on chromosome 6 (Shi et al., 2009; Sekar et al., 2016), interleukin-1β, IL-6, the soluble IL-6 receptor (sIL6R), and IL-10 are associated with increased risk of SCZ (Xu and He, 2010; Gao et al., 2014; Shibuya et al., 2014; Hudson and Miller, 2018). In contrast, polymorphism of IL-2, IL-4, tumor necrosis factor-α (TNF-α), or transforming growth factor- β1 (TGF-β1), are not associated with increased risk of SCZ (Qin et al., 2013; Hudson and Miller, 2018). In addition, CD19 and CD20 genes associated with B-cell the onset of SCZ (Ripke et al., 2014). Genetic aberration in oligodendrocyte and myelination-associated genes also have been detected in schizophrenic patients (Katsel et al., 2005). Moreover, a significant rise of CD5+ B-cell is reported blood of schizophrenic individuals (Printz et al., 1999). This suggests a crucial contribution of B-cell functionalities in immunity, oligodendrogenesis, and myelination during brain development. A B-cell dysfunctionality was reported in pathophysiology and increased vulnerability in SCZ (Steiner et al., 2010; Birnbaum and Weinberger, 2017).
However, a higher blood level of acute-phase reactant CRP is negatively correlated with a decreased risk of SCZ (Prins et al., 2016; Ligthart et al., 2018), and higher soluble IL-2 receptors (sIL-2R) levels indicate an increased risk of schizophrenic events (Gaughran et al., 1998). Increased expression of complement protein C4A involved in microglia-mediated synaptic pruning has been reported to reduce connectivity/sociability, which contributes directly to pathophysiology in SCZ (Stevens et al., 2007; Schafer et al., 2012; Comer et al., 2020). In addition, variation in structural alleles of C4 poses an increased risk for autoimmunity and sex-specific vulnerability in SCZ in males (Kamitaki et al., 2020). A blood spot study on 892 newborns revealed increased C4A complement protein levels; those later developed SCZ (Cooper et al., 2017). CUB and sushi multiple domains 1 (CSMD1), expressed at the time of early perinatal development, which regulates C4 protein expression and a genetic disruption or pathway dysregulation may lead to deficits in average cognitive aptitude and management ability in SCZ (Kraus et al., 2006; Athanasiu et al., 2017).
Any deviation in expression of cytokines involving elevation in interferon regulatory factor 3 (IRF3) (crucial transcription factor during viral infection) (X. Li et al., 2015), IFN-γ (regulates viral multiplication) (Paul-Samojedny et al., 2011), pro-inflammatory IL-1α (Katila et al., 1999), IL-1β (Sasayama et al., 2011), IL-6 (Kalmady et al., 2014; Frydecka et al., 2015) and anti-inflammatory IL-10 (Gao et al., 2014), along with elevated mRNA levels of CRP, IL-6, IL-1β, TNF-β, and TGF-β, have been reported in SCZ individuals (Kroken et al., 2019). Immune receptors like MHC receptors that are involved in immune surveillance and Toll-like receptors (TLRs) that engaged in cognition of microbe-derived molecular signals by innate immune cells and microglia, early brain development (Mallard, 2012; C. Y.; Chen et al., 2019), synaptic plasticity and neurogenesis (Barak et al., 2014) have been found altered (Kang et al., 2013; García-Bueno et al., 2016; MacDowell et al., 2017) in either the blood or post-mortem brain tissue of SCZ individuals. A disruption in gene disrupted-in-schizophrenia- 1 (DISC1) was discovered in a Scottish family affected with SCZ (St Clair et al., 1990) and later worldwide (Chubb et al., 2008). Such genotypes disrupt the immune system network, thereby inducing inflammation (Brandon et al., 2009), suggesting a dysfunctional immune-related gene and inflammation co-ordinately contributes to the pathophysiology of SCZ (Trépanier et al., 2016; van Kesteren et al., 2017). MIA during pregnancy has reportedly caused an increase in mesencephalic dopaminergic neurons in the foetal brain, which is associated with excessive dopaminergic signaling in the midbrain area in SCZ (Winter et al., 2009). Besides, in utero MIA precondition offspring exhibit, a triggered HPA-axis stress response when exposed to cannabinoids in adolescence and exhibit SCZ associated behavior in a sex-specific manner with a remarkable decline in Bifidobacterium longum gut abundance (Katz-Barber et al., 2020). Exposure to viral or bacterial pathogens in-utero also has been reported to enhance the risk of developing SCZ. Maternal infection with Toxoplasma gondii, influenza, rubella, and Borna disease virus intensely increased the risk factor for SCZ (Brown et al., 2000; Brown et al., 2005). Moreover, autoimmune disorders (Benros et al., 2011), prenatal infections, and childhood exposure to different viruses (Brown et al., 2000; Pearce, 2001; Brown et al., 2004; Buka et al., 2008), Toxoplasma gondii infection (Brown et al., 2005) respiratory infections (Sørensen et al., 2009), genital or reproductive tract infections, (Babulas et al., 2006), and other infections (Gattaz et al., 2004; Dalman et al., 2008), have been reported to induce, behavioral and cognitive dysfunction and overall increased risk of development of SCZ in the offspring (Fineberg and Ellman, 2013; Na et al., 2014).
Unmedicated SCZ patients have a specific cytokine expression pattern with an abrupted type 1 IFN-γ, IL-2, sIL-2R, and a complimentary increase in type 2 cytokine pattern IL-6 and IL-10, indicating an imbalanced type 1 and type 2 immune responses in SCZ (Müller and Schwarz, 2006). An increased IL-8 level during the prenatal period is also associated with decreased brain cortex volume and increased ventricular volume in the schizophrenic progeny (Ellman et al., 2010). In addition, genetic risk for elevated expression of the immune marker IL-1β has been linked to brain volume loss (Meisenzahl et al., 2001). A recent study in SCZ patients has revealed increased serum cytokine levels of IL-6, IL-8, and IL-10, substantially associated with the cortical volume in the orbital frontal cortex, cingulate cortex, and frontotemporal gyrus (Wu et al., 2019). Increased level of IL-6 is associated with resurfacing SCZ associated factors preconditioned before birth (D. Zhou et al., 1993). Also, IL-6 has a strong influence on the decreased survivability of serotonergic neurons in the fetal brain (Jarskog et al., 1997), and IL-1β has been reported to induce the differentiation of mesencephalic progenitor cells into dopaminergic neurons in the animal model (Kabiersch et al., 1998; Potter et al., 1999). This is relatable to the potential impact of cytokines on the establishment of neurotransmitter systems in schizophrenic conditions. Further, higher blood levels of IL-6 in early life have been associated with an increased risk of experiencing psychotic episodes with manifestations of fulminant psychotic disorder at age 18 (Khandaker et al., 2014). IL-6 and IL-17 may bring on the poly (I: C), an immune stimulant in the MIA model, which affects the fetal brain (Choi et al., 2016; S. E. P.; Smith et al., 2007). Studies show associations of higher CRP levels with elevated serum IL-6 with a significantly altered cognition in chronic and first-episode SCZ, along with poor verbal and learning memory, poor management skills, and disrupted psychomotor speed (Miller et al., 2013; Misiak et al., 2018). Also, CRP levels have a significant positive correlation with BMI. In contrast, a negative correlation with HDL levels in psychosis periods (Carrizo et al., 2008) and higher CRP levels are associated with increased waist circumference in males compared to sex as well as age-matched patients (Fawzi et al., 2011). In addition, higher IL-6 and hs-CRP levels were notably positively correlated with BMI only in females (C. W. Kelly et al., 2019). Psychotic patients with an increased CRP level are most likely to have metabolic syndrome (Vuksan-Ćusa et al., 2010; Boyer et al., 2013). A meta-analysis revealed that increased inflammatory marker CRP, IL-8, and IL-10 in maternal blood during pregnancy, associated with higher risks, along with an early sign and symptoms in SCZ, suggesting prenatal inflammation alter the brain development process in the fetus, with increased susceptibility to SCZ (Zhang et al., 2018). CRP has essentially been correlated with negative symptoms and psychopathology, whereas IL-18 has been positively associated with total and general psychopathology in SCZ (B. Miller and Essali, 2019). In clinically high-risk patients, there has been a reported increase in CRP, IL-6, IL-4, IL-12, and a decrease in IL-1β, IL-10 (anti-inflammatory cytokine) (Perkins et al., 2015; Delaney et al., 2019; Park and Miller, 2020). A higher concentration of IL-12/IL-23p40 was detected in particulars who started to experience psychosis (Föcking et al., 2016).
In Patients with the first episode of psychosis (FEP), considerably higher levels of IFN-γ and IL-12 were inversely correlated with whole-brain grey matter (Lesh et al., 2018). Also, IL-12 is the only inflammation marker associated with the transition into psychosis in clinically high risk (CHR) individuals (Goldsmith et al., 2016). However, unlike CHR individuals, IL-1β and IL-10 are elevated in individuals with FEP than controls (Goldsmith et al., 2016). An increased concentration of IL-6 and decline of IL-17 in serum has been revealed in individuals at ultra-high risk (UHR) for psychosis, which explains the association that increased IL-17 levels can improve disease condition (Zeni-Graiff et al., 2016). And the expression of TNF-α has also been found to worsen the negative symptoms (Goldsmith et al., 2019). Collectively individuals with FEP and chronic psychosis has been found elevated cytokine levels, including IFN-γ, IL-1 receptor antagonist (IL-1RA), IL-1β, IL-6, IL-8, IL-10, IL-12, sIL-2R, TGF-β, and TNF-α (Goldsmith et al., 2016). However, among all IL-6 and TNF-α, levels were considerably higher in FEP individuals relative to healthy controls (Fraguas et al., 2019). Unlike FEP individuals, the IFN-γ level was lower in chronic outpatients (Goldsmith et al., 2016). Moreover, a meta-analysis study revealed an elevation in IL-1b, sIL-2R, IL-6, and TNF-α in FEP is independent of antipsychotic treatment (Upthegrove et al., 2014). Further inflammation has also been correlated with suicide. As in psychotic patients with mood disorders, increased serum levels of IL-1β and IL-6 have been detected in blood and autopsy brain samples from suicidality compared with both SCZ non-suicidal patients and healthy controls (Black and Miller, 2015). SCZ patients who committed suicide had an extreme rise in microglial density inside the dorsolateral prefrontal cortex, anterior cingulate cortex, mediodorsal thalamus, along with a course of hippocampal microgliosis (Steiner et al., 2008), which explains an extreme and hazardous level of neuroinflammation can induce suicidality in SCZ.
Furthermore, air pollution has also been reported to induce inflammation associated with SCZ pathogenesis (Horsdal et al., 2019). Interestingly, immune genes, most importantly microglia expressed genes, play a central role in the immune system’s interaction with pollution (Peters et al., 2006; Genc et al., 2012). Exposure to traffic-related air pollution (TRAP) revealed an elevated pro-inflammatory cytokine, IL-6, IL-1ß, CD14, and TNF-α in children, when compared to children living in more eco-friendly areas (Calderón-Garcidueñas et al., 2008, 2015; Gruzieva et al., 2017). Additionally, polluted air evoked an identical inflammatory cytokine profile in a healthy young population, with a characteristic increase of IL-6 and an increased frequency of inflammatory cells and related microparticles, which may indicate endothelial injury (Pope et al., 2016). Moreover, in animal models, TRAP exposure showed an elevated expression of IL-1α, IL-6, and TLR4 in the brain (Bos et al., 2012). TRAP specifically influences the microglial TLR4 signaling in a MyD88-dependent pathway (Woodward et al., 2017). In male children, this interactive inflammatory signaling is subsequently followed by circumstantial and auditory cue fear conditioning and anxiety along with behavioral deficits (Bolton et al., 2012, 2013, 2017). With such a condition, prolonged brings harmful consequences like altered synaptic plasticity (Gruol, 2015) altered brain development and function, featured on MIA models of SCZ (Girgis et al., 2014).
Treatment of NPDs with the drug has been challenging due to the involvement of diverse etiopathology, where drug resistance is posing a threat to the treatment due to improper counseling during treatment, post-zygotic genetic changes (Rylaarsdam and Guemez-Gamboa, 2019), unpredictable immune response, differential hormonal response, sex-specific (Jacquemont et al., 2014; Desachy et al., 2015) and the dose-dependent response of the drug (McCracken, 2005; Srisawasdi et al., 2017). The interference of drug in metabolic pathways also contribute to unforeseen development of metabolic disorders, and lastly, the drug addiction and behavioral issues in response to minimal treatment time and disease severity ensure difficulties towards drug-associated treatment (Scahill et al., 2016; Srisawasdi et al., 2017; Libowitz and Nurmi, 2021). Current treatment strategies for NDDs include psychotherapy, anti-inflammatory drugs, and antipsychotic drugs with significant side effects.
Meta-analysis studies revealed that nonsteroidal anti-inflammatory drugs hold considerable impact on schizophrenic total, positive and negative symptoms (Sommer et al., 2012). However, this effect is only in subjects with short-term disease or first episode manifestation (Nitta et al., 2013). Moreover, individuals with short-term (0–2 years) disease duration significantly benefited from the same treatment combined with celecoxib. In contrast, patients with longer disease duration and chronic inflammatory diseases did not differ from the placebo control. Anti-inflammatory treatment involving IFN-γ also comes with side effects, including a high risk of unexpected immune responses. Although long-term anti-inflammatory treatment in chronic SCZ has a considerable positive impact this also has been associated with higher inflammation and poorer response to antipsychotics (Grüber et al., 2014; Fond et al., 2020; Hong and Bang, 2020). Exposure to psychological stressors causes alteration and fluctuation in basal cortisol levels (Bradley and Dinan, 2010), correlated with fluctuating psychological symptoms. Antipsychotic medication directly affects cortisol secretion (Cohrs et al., 2006; Cherian et al., 2019). Celecoxib (cyclooxygenase-2 (COX-2) inhibitor) treatment along with risperidone has been significantly more efficient than the patients receiving risperidone alone in SCZ and had a positive effect on cognition (Müller et al., 2006). However, this therapeutic effect is only restricted until the first years of the schizophrenic disease progression (Müller et al., 2010). Risperidone (a second-generation atypical antipsychotic) treatment causes glucose dyshomeostasis and endocrine dysregulation in autistic children and young adults, bringing leptin and insulin resistance in a dose and treatment duration-dependent manner. Moreover, in children and teenagers suffering ASD, termination of medication is associated with aggressive behavior; thus, a long-life medication is suggested (McCracken, 2005; Srisawasdi et al., 2017). Individuals receiving a high dose of risperidone treatment for the long term are at increased risk of developing type 2 diabetes mellitus (Zuo et al., 2013).
Further, administration of olanzapine and aripiprazole for a short duration directly impacts tissue function enhances peripheral insulin resistance, independent of weight gain in healthy people (Teff et al., 2013). A second-generation antipsychotic treatment including amisulpride, clozapine, olanzapine, quetiapine, risperidone has been demonstrated to be associated with increased appetite, and gain in weight may be through interacting with dopamine receptors (Esen-Danaci et al., 2008). Besides, antipsychotic drugs administered to children and adolescents come with a risk of dyslipidemia and expression of HDL-C, and triglycerides are the main lipid biomarkers for cardiometabolic syndrome in SCZ (de Hert et al., 2011). PUFAs such as omega-3 and omega-6 protect the neuronal cells from oxidative damage inflammation, regulate neurogenesis and preserve overall neuronal function (Hashimoto et al., 2014; S. W.; Kim et al., 2016), therefore involved in many mental health conditions and related diseases such as SCZ (Amminger et al., 2015). In SCZ, patients have considerably low levels of omega-3 in the cell membranes (Hoen et al., 2013). Additionally, antipsychotic treatments have been reported to decrease the PUFAs levels (S. W. Kim et al., 2016). Antipsychotics can also potentially intervene in the metabolic process and biomarker levels, including the metabolites from the kynurenic pathway (Condray et al., 2011; Myint et al., 2011).
Twenty-four weeks of treatment with risperidone resulted in a significantly lower abundance of fecal E. coli, Lactobacillus spp. Bifidobacterium spp. when compared with healthy controls, suggesting that risperidone treatment causes a distinctive amendment in fecal microbiota, indicating metabolic changes induced by antipsychotic medication (Yuan et al., 2018). As discussed previously, microbial species have been substantially associated with disease severity, poor cognitive performance, and diagnosis. And antipsychotic treatment can influence the gut microbiota but cannot completely reconstruct the dysbiotic microbiota related to SCZ (Zhu et al., 2020). Current microbiome therapies have shown promising results in managing NDDs and metabolic disorders. Therefore, manipulating gut microbes with enrichment of beneficial microbes to create a balance and other microbiome therapy may have duplex therapeutic potential for neurodevelopmental and metabolic disorders.
NDDs can be readily linked with gut microbial dysbiosis and inflammatory state, which affect the development of learning, language, cognitive, motor, and behavioral skills with lifelong consequences (Jeste, 2015). Early identification of infants at risk for NDDs is a prerequisite for taking therapeutic measures hypothesized in (Figure 5). There is an imbalance in Bifidobacteria sp. Lactobacillus sp. and a deficiency of potential SCFA producing microbes in the gut microbiota of individuals suffering from NDD (Bojović et al., 2020). Treatment with the probiotic strain of Bifidobacteria sp. and Lactobacillus sp. and probiotic bacterial species to normalize the dysbiosis associated with inflammation, neuropsychological distress, GI, and behavioral problems. Moreover, treatment with B. fragilis has been reported to reduce the intestinal permeability, normalize the organization of the gut microbiota, and ease disease symptoms in the ASD mice model (Glibert et al., 2013; Hsiao et al., 2013). Administration of probiotics, Lactobacillus rhamnosus and Lactobacillus reuteri, have improved barrier integrity and function by regulation of the tight junction proteins expression and thereby reducing the microbial translocation in-vitro or in animal models (Ulluwishewa et al., 2011; Dicksved et al., 2012). Lactobacillus and Bifidobacteria were proven to decrease anxiety symptoms considerably (Rao et al., 2009). Supplementation of the probiotic formulation containing Lactobacillus, Bifidobacteria, and Streptococci has been reported to normalize the Bacteroidetes/Firmicutes ratio along with imbalanced abundance Desulfovibrio sp. and Bifidobacterium sp. in the fecal samples of autistic children, as with that of healthy controls (Tomova et al., 2015). In addition to probiotic therapy, fecal microbiota transplantation (FMT) and microbiota transfer therapy (MTT) have been increasingly considered promising therapies in treating NDDs. FMT has been reported to treat IBD and IBS successfully, resulting in a typical gut microbiota composition in patients, improving constipation symptoms (Aroniadis and Brandt, 2013; Rossen et al., 2015). Microbiota transfer therapy (MTT) is a kind of modified FMT. The patient goes through antibiotic treatment for 14 days and then cleans the bowel before administrating a high initial dose of systematized human gut microbiota (SHGM) for up to 7–8 weeks. MTT in a clinical trial has been reported to improve GI symptoms such as indigestion, abdominal pain, constipation, and diarrhea and symptoms related to ASD and normalized any alteration in the gut microbiota in ASD patients (D. W. Kang et al., 2017, 2019). Thus, there is increasing interest in using FMT and MTT as a therapeutic intervention for treating children with NDDs, following all the concerning safety issues (Gaonkar, 2021).
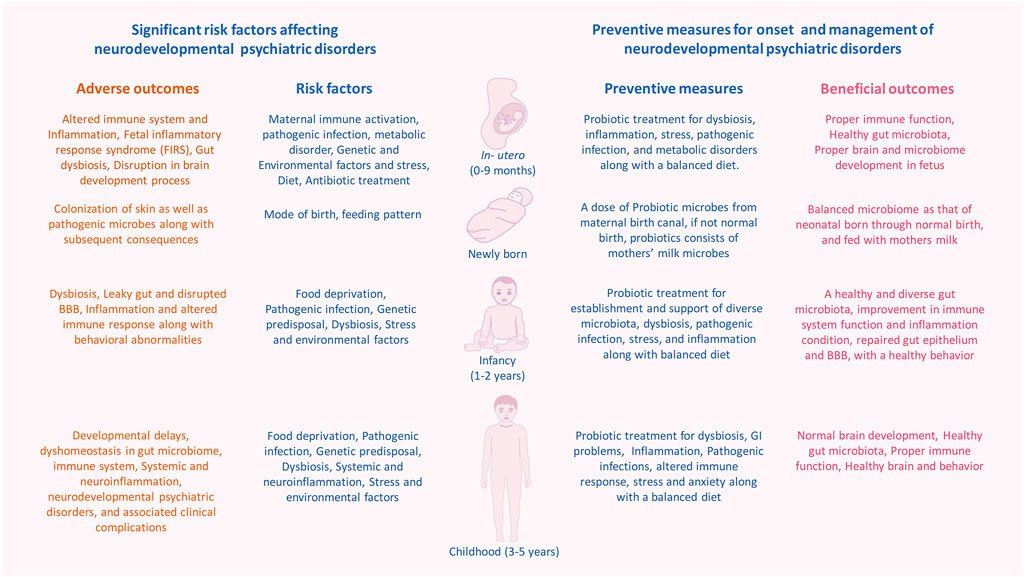
FIGURE 5. Probiotic therapy to overcome adverse effects NDDs at developmental time points. Probiotic treatment at specific time points such as in-utero, after immediate birth, infancy and childhood may help and support the establishment of stable and healthy gut microbiota, prevent the dysregulation in the developmental process, and play the role of a crucial preventive measure for the onset of neurodevelopmental and psychotic disorders via neutralizing the effects of inducing factors.
There is a bidirectional communication network between the CNS and the microbiome ecosystem that we possess (Carabotti et al., 2015). To understand this relationship in a more elaborative way still much has to be discovered. Disturbances in the simultaneous coordinated process of neuronal as well as gut-microbiome development due to overdoses of antibiotics in infants can lead to an inflammatory state at this critical phase of brain development (Neuman et al., 2018). Compositional alterations in the gut microbiome can result in systemic inflammation and neuroinflammation (Belkaid and Hand, 2014). Moreover, the microbiome also plays an essential role in the process of microglial maturation and can modulate glial activation in CNS, which also has been considered as regulating factor of neuroinflammation in the CNS (Abdel-Haq et al., 2019). All these events during the developmental process put up a foundation for the onset of NDDs like ASD, ADHD, SCZ, etc. Investigating intricate pathways for a brief illustration of the microbiome-gut-brain-immune axis in developing NDDs, disease onset, and progression will be beneficial towards the discovery of clinically relevant targeted biotherapies to combat the continuous rise in worldwide NDDs. The future perspective holds the exciting potential of sophisticated microbiome-based therapeutics to prevent the emergence and treatment of NDDs (Figure 5). Further studies are required to elucidate specific molecular signaling pathways associated with processes neuronal development, related disease, diagnosis, and to develop individualized microbiome therapeutics.
SD has conceptualized and prepared the original draft, YS and MK have validated, reviewed, edited and supervised the manuscript. All authors have approval for the publication of a final manuscript.
This work was funded by institute of advanced study in science and technology (IASST)and Department of science and technology (DST)-funded ST/SC community development project(SEED/TITE/2019/103) at IASST.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aagaard, K., Ma, J., Antony, K. M., Ganu, R., Petrosino, J., and Versalovic, J. (2014). The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 6 (237). doi:10.1126/scitranslmed.3008599
Aarts, E., Ederveen, T. H. A., Naaijen, J., Zwiers, M. P., Boekhorst, J., Timmerman, H. M., et al. (2017). Gut Microbiome in ADHD and its Relation to Neural Reward Anticipation. PLoS ONE 12 (9), e0183509. doi:10.1371/journal.pone.0183509
Abdel-Haq, R., Schlachetzki, J. C. M., Glass, C. K., and Mazmanian, S. K. (2019). Microbiome-microglia Connections via the Gut-Brain axis. J. Exp. Med. 216 (1), 41–59. doi:10.1084/jem.20180794
Acuña, I., Cerdó, T., Ruiz, A., Torres-Espínola, F. J., López-Moreno, A., Aguilera, M., et al. (2021). Infant Gut Microbiota Associated with fine Motor Skills. Nutrients 13 (5), 1673. doi:10.3390/nu13051673
Agarwal, S., Piesco, N. P., Johns, L. P., and Riccelli, A. E. (1995). Differential Expression of IL-1β, TNF-α, IL-6, and IL-8 in Human Monocytes in Response to Lipopolysaccharides from Different Microbes. J. Dent Res. 74 (4), 1057–1065. doi:10.1177/00220345950740040501
Alameddine, J., Godefroy, E., Papargyris, L., Sarrabayrouse, G., Tabiasco, J., Bridonneau, C., et al. (2019). Faecalibacterium Prausnitzii Skews Human DC to Prime IL10-Producing T Cells through TLR2/6/JNK Signaling and IL-10, IL-27, CD39, and Ido-1 Induction. Front. Immunol. 10 (2). doi:10.3389/fimmu.2019.00143
Alcock, J., Maley, C. C., and Aktipis, C. A. (2014). Is Eating Behavior Manipulated by the Gastrointestinal Microbiota? Evolutionary Pressures and Potential Mechanisms. Bioessays 36 (10), 940–949. doi:10.1002/bies.201400071
Alenina, N., and Klempin, F. (2015). The Role of Serotonin in Adult Hippocampal Neurogenesis. Behavioural Brain Research. Elsevier B.V.. doi:10.1016/j.bbr.2014.07.038
Alexander, C., and Rietschel, E. T. (2001). Bacterial Lipopolysaccharides and Innate Immunity. J. Endotoxin Res. 7 (3), 167–202. doi:10.1179/096805101101532675
Alhasson, F., Das, S., Seth, R., Dattaroy, D., Chandrashekaran, V., Ryan, C. N., et al. (2017). Altered Gut Microbiome in a Mouse Model of Gulf War Illness Causes Neuroinflammation and Intestinal Injury via Leaky Gut and TLR4 Activation. PLoS ONE 12 (3), e0172914. doi:10.1371/journal.pone.0172914
Allam-Ndoul, B., Castonguay-Paradis, S., and Veilleux, A. (2020). Gut Microbiota and Intestinal Trans-epithelial Permeability. Ijms 21 (17), 6402. doi:10.3390/ijms21176402
Almeida, R. G., and Lyons, D. A. (2017). On Myelinated Axon Plasticity and Neuronal Circuit Formation and Function. J. Neurosci. 37 (42), 10023–10034. doi:10.1523/JNEUROSCI.3185-16.2017
Altamura, A. C., Pozzoli, S., Fiorentini, A., and Dell'Osso, B. (2013). Neurodevelopment and Inflammatory Patterns in Schizophrenia in Relation to Pathophysiology. Prog. Neuro-Psychopharmacology Biol. Psychiatry 42, 63–70. doi:10.1016/j.pnpbp.2012.08.015
Amminger, G. P., Schäfer, M. R., Schlögelhofer, M., Klier, C. M., and McGorry, P. D. (2015). Longer-term Outcome in the Prevention of Psychotic Disorders by the Vienna omega-3 Study. Nat. Commun. 6. doi:10.1038/ncomms8934
Anand, D., Colpo, G. D., Zeni, G., Zeni, C. P., and Teixeira, A. L. (2017). Attention-deficit/hyperactivity Disorder and Inflammation: What Does Current Knowledge Tell US? A Systematic Review. Front. Psychiatry 8 (11). doi:10.3389/fpsyt.2017.00228
Anisman, H., Kokkinidis, L., and Merali, Z. (1996). Interleukin-2 Decreases Accumbal Dopamine Efflux and Responding for Rewarding Lateral Hypothalamic Stimulation. Brain Res. 731 (1–2), 1–11. doi:10.1016/0006-8993(96)00460-X
Ardissone, A. N., De La Cruz, D. M., Davis-Richardson, A. G., Rechcigl, K. T., Li, N., Drew, J. C., et al. (2014). Meconium Microbiome Analysis Identifies Bacteria Correlated with Premature Birth. PLoS ONE 9 (3). doi:10.1371/journal.pone.0090784
Argou-Cardozo, I., and Zeidán-Chuliá, F. (2018). Clostridium Bacteria and Autism Spectrum Conditions: A Systematic Review and Hypothetical Contribution of Environmental Glyphosate Levels. Med. Sci. 6 (2). doi:10.3390/medsci6020029
Aroniadis, O. C., and Brandt, L. J. (2013). Fecal Microbiota Transplantation: Past, Present and Future. Curr. Opin. Gastroenterol. 29 (1). doi:10.1097/MOG.0b013e32835a4b3e
Ashwood, P., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Pessah, I., and van de Water, J. (2011). Elevated Plasma Cytokines in Autism Spectrum Disorders Provide Evidence of Immune Dysfunction and Are Associated with Impaired Behavioral Outcome. Brain Behav. Immun. 25 (1). doi:10.1016/j.bbi.2010.08.003
Ashwood, P., and Wakefield, A. J. (2006). Immune Activation of Peripheral Blood and Mucosal CD3+ Lymphocyte Cytokine Profiles in Children with Autism and Gastrointestinal Symptoms. J. Neuroimmunology 173 (1–2). doi:10.1016/j.jneuroim.2005.12.007
Athanasiu, L., Giddaluru, S., Fernandes, C., Christoforou, A., Reinvang, I., Lundervold, A. J., et al. (2017). A Genetic Association Study of CSMD1 and CSMD2 with Cognitive Function. Brain Behav. Immun. 61. doi:10.1016/j.bbi.2016.11.026
Babulas, V., Factor-Litvak, P., Goetz, R., Schaefer, C. A., and Brown, A. S. (2006). Prenatal Exposure to Maternal Genital and Reproductive Infections and Adult Schizophrenia. Am. J. Psychiatry 163 (5). doi:10.1176/ajp.2006.163.5.927
Bäckhed, F., Roswall, J., Peng, Y., Feng, Q., Jia, H., Kovatcheva-Datchary, P., et al. (2015). Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host and Microbe 17 (5), 690–703. doi:10.1016/j.chom.2015.04.004
Barak, B., Feldman, N., and Okun, E. (2014). Toll-like Receptors as Developmental Tools that Regulate Neurogenesis during Development: an Update. Front. Neurosci. 8. doi:10.3389/fnins.2014.00272
Baumgarth, N. (2011). The Double Life of a B-1 Cell: Self-Reactivity Selects for Protective Effector Functions. Nat. Rev. Immunol. 11, 34–46. doi:10.1038/nri2901
Belenguer, A., Duncan, S. H., Calder, A. G., Holtrop, G., Louis, P., Lobley, G. E., et al. (2006). Two Routes of Metabolic Cross-Feeding between Bifidobacterium Adolescentis and Butyrate-Producing Anaerobes from the Human Gut. Appl. Environ. Microbiol. 72 (5). doi:10.1128/AEM.72.5.3593-3599.2006
Belkaid, Y., and Hand, T. W. (2014). Role of the Microbiota in Immunity and Inflammation. Cell 157 (1). doi:10.1016/j.cell.2014.03.011
Benes, F. M. (1989). Myelination of Cortical-Hippocampal Relays during Late Adolescence. Schizophrenia Bull. 15 (4). doi:10.1093/schbul/15.4.585
Benros, M. E., Nielsen, P. R., Nordentoft, M., Eaton, W. W., Dalton, S. O., and Mortensen, P. B. (2011). Autoimmune Diseases and Severe Infections as Risk Factors for Schizophrenia: A 30-year Population-Based Register Study. Am. J. Psychiatry 168 (12). doi:10.1176/appi.ajp.2011.11030516
Bercik, P., Denou, E., Collins, J., Jackson, W., Lu, J., Jury, J., et al. (2011). The Intestinal Microbiota Affect central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 141 (2). doi:10.1053/j.gastro.2011.04.052
Bezawada, N., Phang, T. H., Hold, G. L., and Hansen, R. (2020). Autism Spectrum Disorder and the Gut Microbiota in Children: A Systematic Review. Ann. Nutr. Metab. 76 (1). doi:10.1159/000505363
Biagi, E., Nylund, L., Candela, M., Ostan, R., Bucci, L., Pini, E., et al. (2010). Through Ageing, and beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 5 (5). doi:10.1371/journal.pone.0010667
Biasucci, G., Rubini, M., Riboni, S., Morelli, L., Bessi, E., and Retetangos, C. (2010). Mode of Delivery Affects the Bacterial Community in the Newborn Gut. Early Hum. Dev. 86 (1). doi:10.1016/j.earlhumdev.2010.01.004
Bilbo, S. D., Block, C. L., Bolton, J. L., Hanamsagar, R., and Tran, P. K. (2018). Beyond Infection - Maternal Immune Activation by Environmental Factors, Microglial Development, and Relevance for Autism Spectrum Disorders. Exp. Neurol. 299. doi:10.1016/j.expneurol.2017.07.002
Birnbaum, R., and Weinberger, D. R. (2017). Genetic Insights into the Neurodevelopmental Origins of Schizophrenia. Nat. Rev. Neurosci. 18 (12), 727–740. doi:10.1038/nrn.2017.125
Black, C., and Miller, B. J. (2015). Meta-analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal versus Nonsuicidal Patients. Biol. Psychiatry 78 (1). doi:10.1016/j.biopsych.2014.10.014
Blum, K., Chen, A. L. C., Braverman, E. R., Comings, D. E., Chen, T. J. H., Arcuri, V., et al. (2008). Attention-deficit-hyperactivity Disorder and Reward Deficiency Syndrome. Neuropsychiatr. Dis. Treat. 4 (5). doi:10.2147/ndt.s2627
Bojović, K., Ignjatović, Ð. d. i., Soković Bajić, S., Vojnović Milutinović, D., Tomić, M., Golić, N., et al. (2020). Gut Microbiota Dysbiosis Associated with Altered Production of Short Chain Fatty Acids in Children with Neurodevelopmental Disorders. Front. Cell Infect. Microbiol. 10. doi:10.3389/fcimb.2020.00223
Bolte, E. R. (1998). Autism and clostridium Tetani. Med. Hypotheses 51 (2). doi:10.1016/S0306-9877(98)90107-4
Bolton, J. L., Huff, N. C., Smith, S. H., Mason, S. N., Foster, W. M., Auten, R. L., et al. (2013). Maternal Stress and Effects of Prenatal Air Pollution on Offspring Mental Health Outcomes in Mice. Environ. Health Perspect. 121 (9). doi:10.1289/ehp.1306560
Bolton, J. L., Marinero, S., Hassanzadeh, T., Natesan, D., Le, D., Belliveau, C., et al. (2017). Gestational Exposure to Air Pollution Alters Cortical Volume, Microglial Morphology, and Microglia-Neuron Interactions in a Sex-specific Manner. Front. Synaptic Neurosci. 9 (5). doi:10.3389/fnsyn.2017.00010
Bolton, J. L., Smith, S. H., Huff, N. C., Gilmour, M. I., Foster, W. M., Auten, R. L., et al. (2012). Prenatal Air Pollution Exposure Induces Neuroinflammation and Predisposes Offspring to Weight Gain in Adulthood in a Sex-specific Manner. FASEB J. 26 (11). doi:10.1096/fj.12-210989
Borre, Y. E., Keeffe, G. W. O., Clarke, G., Stanton, C., Dinan, T. G., and Cryan, J. F. (2014). Microbiota and Neurodevelopmental Windows: Implications for Brain Disorders. Trends Mol. Med. 20 (9), 509–518. doi:10.1016/j.molmed.2014.05.002
Bos, I., de Boever, P., Emmerechts, J., Buekers, J., Vanoirbeek, J., Meeusen, R., et al. (2012). Changed Gene Expression in Brains of Mice Exposed to Traffic in a Highway Tunnel. Inhalation Toxicol. 24 (10). doi:10.3109/08958378.2012.714004
Boulanger-Bertolus, J., Pancaro, C., and Mashour, G. A. (2018). Increasing Role of Maternal Immune Activation in Neurodevelopmental Disorders. Front. Behav. Neurosci. 12. doi:10.3389/fnbeh.2018.00230
Boyer, L., Richieri, R., Dassa, D., Boucekine, M., Fernandez, J., Vaillant, F., et al. (2013). Association of Metabolic Syndrome and Inflammation with Neurocognition in Patients with Schizophrenia. Psychiatry Res. 210 (2). doi:10.1016/j.psychres.2013.06.020
Boyle, C. A., Boulet, S., Schieve, L. A., Cohen, R. A., Blumberg, S. J., Yeargin-Allsopp, M., et al. (2011). Trends in the Prevalence of Developmental Disabilities in US Children, 1997-2008. Pediatrics 127 (6), 1034–1042. doi:10.1542/peds.2010-2989
Bradley, A. J., and Dinan, T. G. (2010). A Systematic Review of Hypothalamic-Pituitary-Adrenal axis Function in Schizophrenia: Implications for Mortality. J. Psychopharmacol. (Oxford, England) 24 (4). doi:10.1177/1359786810385491
Brandon, N. J., Millar, J. K., Korth, C., Sive, H., Singh, K. K., and Sawa, A. (2009). Understanding the Role of DISC1 in Psychiatric Disease and during normal Development. J. Neurosci. 29 (41). doi:10.1523/JNEUROSCI.3355-09.2009
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Tóth, M., et al. (2014). The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Translational Med. 6 (263). doi:10.1126/scitranslmed.3009759
Braunschweig, D., and van de Water, J. (2012). Maternal Autoantibodies in Autism. Arch. Neurol. 69 (6). doi:10.1001/archneurol.2011.2506
Brenner, D. R., Scherer, D., Muir, K., Schildkraut, J., Boffetta, P., Spitz, M. R., et al. (2014). A Review of the Application of Inflammatory Biomarkers in Epidemiologic Cancer Research. Cancer Epidemiol. Biomarkers Prev. 23 (9). doi:10.1158/1055-9965.EPI-14-0064
Brotman, R. M. (2011). Vaginal Microbiome and Sexually Transmitted Infections: An Epidemiologic Perspective. J. Clin. Invest. 121 (12). doi:10.1172/JCI57172
Brown, A. S., Begg, M. D., Gravenstein, S., Schaefer, C. A., Wyatt, R. J., Bresnahan, M., et al. (2004). Serologic Evidence of Prenatal Influenza in the Etiology of Schizophrenia. Arch. Gen. Psychiatry 61 (8). doi:10.1001/archpsyc.61.8.774
Brown, A. S., Cohen, P., Greenwald, S., and Susser, E. (2000). Nonaffective Psychosis after Prenatal Exposure to Rubella. Am. J. Psychiatry 157 (3). doi:10.1176/appi.ajp.157.3.438
Brown, A. S., and Conway, F. (2019). Maternal Immune Activation and Related Factors in the Risk of Offspring Psychiatric Disorders. Front. Psychiatry 10 (5). doi:10.3389/fpsyt.2019.00430
Brown, A. S., and Derkits, E. J. (2010). Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 167 (3). doi:10.1176/appi.ajp.2009.09030361
Brown, A. S., Schaefer, C. A., Quesenberry, C. P., Liu, L., Babulas, V. P., and Susser, E. S. (2005). Maternal Exposure to Toxoplasmosis and Risk of Schizophrenia in Adult Offspring. Am. J. Psychiatry 162 (4). doi:10.1176/appi.ajp.162.4.767
Buka, S. L., Cannon, T. D., Torrey, E. F., and Yolken, R. H. (2008). Maternal Exposure to Herpes Simplex Virus and Risk of Psychosis Among Adult Offspring. Biol. Psychiatry 63 (8). doi:10.1016/j.biopsych.2007.09.022
Bull, G., Shattock, P., Whiteley, P., Anderson, R., Groundwater, P. W., Lough, J. W., et al. (2003). Indolyl-3-acryloylglycine (IAG) Is a Putative Diagnostic Urinary Marker for Autism Spectrum Disorders. Med. Sci. Monitor 9 (10).
Bull-Larsen, S., and Hasan, M. (2019). The Potential Influence of the Bacterial Microbiome on the Development and Progression of Adhd. Nutrients.11, 2805. doi:10.3390/nu11112805
Bundgaard-Nielsen, C., Knudsen, J., Leutscher, P. D. C., Lauritsen, M. B., Nyegaard, M., Hagstrøm, S., et al. (2020). Gut Microbiota Profiles of Autism Spectrum Disorder and Attention Deficit/hyperactivity Disorder: A Systematic Literature Review. Gut Microbes 11 (5). doi:10.1080/19490976.2020.1748258
Calderón-Garcidueñas, L., Franco-Lira, M., D’Angiulli, A., Rodríguez-Díaz, J., Blaurock-Busch, E., Busch, Y., et al. (2015). Mexico City normal Weight Children Exposed to High Concentrations of Ambient PM2.5 Show High Blood Leptin and Endothelin-1, Vitamin D Deficiency, and Food Reward Hormone Dysregulation versus Low Pollution Controls. Relevance for Obesity and Alzheimer Disease. Environ. Res. 140, 579–592. doi:10.1016/j.envres.2015.05.012
Calderón-Garcidueñas, L., Solt, A. C., Henríquez-Roldán, C., Torres-Jardón, R., Nuse, B., Herritt, L., et al. (2008). Long-term Air Pollution Exposure Is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood-Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid β-42 and α-synuclein in Children and Young Adults. Toxicologic Pathol. 36 (2), 289–310. doi:10.1177/0192623307313011
Carabotti, M., Scirocco, A., Antonietta, M., and Severi, C. (2015). The Gut-Brain axis: Interactions between Enteric Microbiota , central and Enteric Nervous Systems. Ann. Gastroenterol. 28, 203–209.
Carrizo, E., Fernández, V., Quintero, J., Connell, L., Rodríguez, Z., Mosquera, M., et al. (2008). Coagulation and Inflammation Markers during Atypical or Typical Antipsychotic Treatment in Schizophrenia Patients and Drug-free First-Degree Relatives. Schizophrenia Res. 103 (1–3). doi:10.1016/j.schres.2008.03.004
Castro-Nallar, E., Bendall, M. L., Pérez-Losada, M., Sabuncyan, S., Severance, E. G., Dickerson, F. B., et al. (2015). Composition, Taxonomy and Functional Diversity of the Oropharynx Microbiome in Individuals with Schizophrenia and Controls. PeerJ 2015 (8). doi:10.7717/peerj.1140
Cerdó, T., Diéguez, E., and Campoy, C. (2020). Impact of Gut Microbiota on Neurogenesis and Neurological Diseases during Infancy. Curr. Opin. Pharmacol. 50. doi:10.1016/j.coph.2019.11.006
Checa-Ros, A., Jeréz-Calero, A., Molina-Carballo, A., Campoy, C., and Muñoz-Hoyos, A. (2021). Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients 13 (1). doi:10.3390/nu13010249
Chen, C. Y., Shih, Y. C., Hung, Y. F., and Hsueh, Y. P. (2019). Beyond Defense: Regulation of Neuronal Morphogenesis and Brain Functions via Toll-like Receptors. J. Biomed. Sci. 26 (1). doi:10.1186/s12929-019-0584-z
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., et al. (2018). Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 9 (6). doi:10.18632/oncotarget.23208
Chen, Y., Xu, J., and Chen, Y. (2021). Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 13 (6). doi:10.3390/nu13062099
Cherian, K., Schatzberg, A. F., and Keller, J. (2019). HPA axis in Psychotic Major Depression and Schizophrenia Spectrum Disorders: Cortisol, Clinical Symptomatology, and Cognition. Schizophrenia Res. 213. doi:10.1016/j.schres.2019.07.003
Choi, G. B., Yim, Y. S., Wong, H., Kim, S., Kim, H., Kim, S. v., et al. (2016). The Maternal Interleukin-17a Pathway in Mice Promotes Autism-like Phenotypes in Offspring. Science 351 (6276). doi:10.1126/science.aad0314
Choudhury, Z., and Lennox, B. (2021). Maternal Immune Activation and Schizophrenia–Evidence for an Immune Priming Disorder. Front. Psychiatry 12. doi:10.3389/fpsyt.2021.585742
Chrobak, A. A., Nowakowski, J., and Dudek, D. (2016). Interactions between the Gut Microbiome and the central Nervous System and Their Role in Schizophrenia, Bipolar Disorder and Depression. Arch. Psychiatry Psychotherapy 18 (2). doi:10.12740/APP/62962
Chubb, J. E., Bradshaw, N. J., Soares, D. C., Porteous, D. J., and Millar, J. K. (2008). The DISC Locus in Psychiatric Illness. Mol. Psychiatry 13 (1). doi:10.1038/sj.mp.4002106
Clapp, M., Aurora, N., Herrera, L., Bhatia, M., Wilen, E., and Wakefield, S. (2017). Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 7 (4). doi:10.4081/cp.2017.987
Clarke, G., Grenham, S., Scully, P., Fitzgerald, P., Moloney, R. D., Shanahan, F., et al. (2013). The Microbiome-Gut-Brain axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-dependent Manner. Mol. Psychiatry 18, 666–673. doi:10.1038/mp.2012.77
Di Gesù, C. M., Matz, L. M., and Buffington, S. A. (2021). Diet-Induced Dysbiosis of the Maternal Gut Microbiome in Early Life Programming of Neurodevelopmental Disorders. Neuroscience Research. Elsevier Ireland Ltd. doi:10.1016/j.neures.2021.05.003
Cohrs, S., Röher, C., Jordan, W., Meier, A., Huether, G., Wuttke, W., et al. (2006). The Atypical Antipsychotics Olanzapine and Quetiapine, but Not Haloperidol, Reduce ACTH and Cortisol Secretion in Healthy Subjects. Psychopharmacology 185 (1). doi:10.1007/s00213-005-0279-x
Collado, M. C., Rautava, S., Aakko, J., Isolauri, E., and Salminen, S. (2016). Human Gut Colonisation May Be Initiated In Utero by Distinct Microbial Communities in the Placenta and Amniotic Fluid. Scientific Rep. 6. doi:10.1038/SREP23129
Comer, A. L., Carrier, M., Tremblay, M. È., and Cruz-Martín, A. (2020). The Inflamed Brain in Schizophrenia: The Convergence of Genetic and Environmental Risk Factors that Lead to Uncontrolled Neuroinflammation. Front. Cell Neurosci. 14. doi:10.3389/fncel.2020.00274
Condray, R., Dougherty, G. G., Keshavan, M. S., Reddy, R. D., Haas, G. L., Montrose, D. M., et al. (2011). 3-Hydroxykynurenine and Clinical Symptoms in First-Episode Neuroleptic-Naive Patients with Schizophrenia. Int. J. Neuropsychopharmacol. 14 (6). doi:10.1017/S1461145710001689
Cong, X., Henderson, W. A., Graf, J., and McGrath, J. M. (2015). Early Life Experience and Gut Microbiome: The Brain-Gut-Microbiota Signaling System. Adv. Neonatal. Care 15 (5), 314–E2. doi:10.1097/ANC.0000000000000191
Cooper, J. D., Ozcan, S., Gardner, R. M., Rustogi, N., Wicks, S., van Rees, G. F., et al. (2017). Schizophrenia-risk and Urban Birth Are Associated with Proteomic Changes in Neonatal Dried Blood Spots. Translational Psychiatry 7 (12). doi:10.1038/s41398-017-0027-0
Corbett, B. A., Kantor, A. B., Schulman, H., Walker, W. L., Lit, L., Ashwood, P., et al. (2007). A Proteomic Study of Serum from Children with Autism Showing Differential Expression of Apolipoproteins and Complement Proteins. Mol. Psychiatry 12 (3). doi:10.1038/sj.mp.4001943
Cosacak, M., Bhattarai, P., and Kizil, C. (2020). “Alzheimer’s Disease, Neural Stem Cells and Neurogenesis: Cellular Phase at Single-Cell Level,” in Neural Regeneration Research (Mumbai: Wolters Kluwer Medknow Publications). doi:10.4103/1673-5374.268896
Cowen, P., Harrison, P., and Burns, T. (2012). Shorter Oxford Textbook of Psychiatry. Oxford University Press. doi:10.1093/med/9780199605613.001.0001
Cristofori, F., Dargenio, V. N., Dargenio, C., Miniello, V. L., Barone, M., and Francavilla, R. (2021). Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 12, 578386. doi:10.3389/fimmu.2021.578386
Croen, L. A., Braunschweig, D., Haapanen, L., Yoshida, C. K., Fireman, B., Grether, J. K., et al. (2008). Maternal Mid-pregnancy Autoantibodies to Fetal Brain Protein: The Early Markers for Autism Study. Biol. Psychiatry 64 (7). doi:10.1016/j.biopsych.2008.05.006
Cuomo, A., Maina, G., Rosso, G., Crescenzi, B. B., Bolognesi, S., Muro, A. D., et al. (2018). The Microbiome: A New Target for Research and Treatment of Schizophrenia and its Resistant Presentations? A Systematic Literature Search and Review. Front. Pharmacol. 9 (10). doi:10.3389/fphar.2018.01040
Dalman, C., Allebeck, P., Gunnell, D., Harrison, G., Kristensson, K., Lewis, G., et al. (2008). Infections in the CNS during Childhood and the Risk of Subsequent Psychotic Illness: A Cohort Study of More Than One Million Swedish Subjects. Am. J. Psychiatry 165 (1). doi:10.1176/appi.ajp.2007.07050740
Daneman, R. (2012). The Blood-Brain Barrier in Health and Disease. Ann. Neurol. 72 (5). doi:10.1002/ana.23648
Daneman, R., Zhou, L., Kebede, A., and Barres, B. (2010). Pericytes Are Required for Blood–Brain Barrier Integrity during Embryogenesis. Nature 468, 562–566. doi:10.1038/nature09513
D’Arcangelo, G. (2014). Reelin in the Years: Controlling Neuronal Migration and Maturation in the Mammalian Brain. Adv. Neurosci. 2014. doi:10.1155/2014/597395
de Agüero, M. G., Ganal-Vonarburg, S. C., Fuhrer, T., Rupp, S., Uchimura, Y., Li, H., et al. (2016). The Maternal Microbiota Drives Early Postnatal Innate Immune Development. Science 351 (6279), 1296–1302. doi:10.1126/science.aad2571
de Angelis, M., Piccolo, M., Vannini, L., Siragusa, S., de Giacomo, A., Serrazzanetti, D. I., et al. (2013). Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 8 (10). doi:10.1371/journal.pone.0076993
de Hert, M., Dobbelaere, M., Sheridan, E. M., Cohen, D., and Correll, C. U. (2011). Metabolic and Endocrine Adverse Effects of Second-Generation Antipsychotics in Children and Adolescents: A Systematic Review of Randomized, Placebo Controlled Trials and Guidelines for Clinical Practice. Eur. Psychiatry 26 (3). doi:10.1016/j.eurpsy.2010.09.011
de Hert, M., Schreurs, V., Vancampfort, D., and van Winkel, R. (2009). Metabolic Syndrome in People with Schizophrenia: A Review. World Psychiatry 8 (1). doi:10.1002/j.2051-5545.2009.tb00199.x
de Wouters d’Oplinter, A., Rastelli, M., Van Hul, M., Delzenne, N. M., Cani, P. D., and Everard, A. (2021). Gut Microbes Participate in Food Preference Alterations during Obesity. Gut microbes 13 (1), 1959242. doi:10.1080/19490976.2021.1959242
Delaney, S., Fallon, B., Alaedini, A., Yolken, R., Indart, A., Feng, T., et al. (2019). Inflammatory Biomarkers in Psychosis and Clinical High Risk Populations. Schizophrenia Res. 206. doi:10.1016/j.schres.2018.10.017
Derecki, N. C., Cardani, A. N., Yang, C. H., Quinnies, K. M., Crihfield, A., Lynch, K. R., et al. (2010). Regulation of Learning and Memory by Meningeal Immunity: a Key Role for IL-4. J. Exp. Med. 207 (5), 1067–1080. doi:10.1084/jem.20091419
Desachy, G., Croen, L. A., Torres, A. R., Kharrazi, M., Delorenze, G. N., Windham, G. C., et al. (2015). Increased Female Autosomal burden of Rare Copy Number Variants in Human Populations and in Autism Families. Mol. Psychiatry 20 (2). doi:10.1038/mp.2014.179
Desbonnet, L., Clarke, G., Shanahan, F., Dinan, T. G., and Cryan, J. F. (2014). Microbiota Is Essential for Social Development in the Mouse. Mol. Psychiatry 19 (2), 146–148. doi:10.1038/mp.2013.65
Desplat-Jégo, S., Johanet, C., Escande, A., Goetz, J., Fabien, N., Olsson, N., et al. (2007). Update in Anti-Saccharomyces cerevisiae Antibodies, Anti-nuclear Associated Anti-neutrophil Antibodies and Antibodies to Exocrine Pancreas Detected by Indirect Immunofluorescence as Biomarkers in Chronic Inflammatory Bowel Diseases: Results of a Multicenter Study. World J. Gastroenterol. 13 (16). doi:10.3748/wjg.v13.i16.2312
Deverman, B. E., and Patterson, P. H. (2009). Cytokines and CNS Development. Neuron 64 (1). doi:10.1016/j.neuron.2009.09.002
Di Gesù, C. M., Matz, L. M., and Buffington, S. A. (2021). Diet-induced Dysbiosis of the Maternal Gut Microbiome in Early Life Programming of Neurodevelopmental Disorders. Neurosci. Res. 168. doi:10.1016/j.neures.2021.05.003
Dicksved, J., Schreiber, O., Willing, B., Petersson, J., Rang, S., Phillipson, M., et al. (2012). Lactobacillus Reuteri Maintains a Functional Mucosal Barrier during DSS Treatment Despite Mucus Layer Dysfunction. PLoS ONE 7 (9). doi:10.1371/journal.pone.0046399
Dipasquale, V., Cutrupi, M. C., Colavita, L., Manti, S., Cuppari, C., and Salpietro, C. (2017). Neuroinflammation in Autism Spectrum Disorders: Role of High Mobility Group Box 1 Protein. Int. J. Mol. Cell Med. 6 (3). doi:10.22088/acadpub.BUMS.6.3.148
Dogra, S., Sakwinska, O., Soh, S. E., Ngom-Bru, C., Brück, W. M., Berger, B., et al. (2015). Rate of Establishing the Gut Microbiota in Infancy Has Consequences for Future Health. Gut Microbes 6 (5). doi:10.1080/19490976.2015.1078051
Dominguez-Bello, M. G., Costello, E. K., Contreras, M., Magris, M., Hidalgo, G., Fierer, N., et al. (2010). Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. United States America 107 (26). doi:10.1073/pnas.1002601107
Dotterud, C. K., Avershina, E., Sekelja, M., Simpson, M. R., Rudi, K., Storrø, O., et al. (2015). Does Maternal Perinatal Probiotic Supplementation Alter the Intestinal Microbiota of Mother and Child? J. Pediatr. Gastroenterol. Nutr. 61 (2). doi:10.1097/MPG.0000000000000781
Downes, J., Munson, M., and Wade, W. G. (2003). Dialister Invisus Sp. nov., Isolated from the Human Oral Cavity. Int. J. Syst. Evol. Microbiol. 53 (6). doi:10.1099/ijs.0.02640-0
Drtilkova, I., Sery, O., Theiner, P., Uhrova, A., Zackova, M., Balastikova, B., et al. (2008). Clinical and Molecular-Genetic Markers of ADHD in Children. Neuroendocrinology Lett. 29 (3).
Duncan, I. D., and Watters, J. J. (2019). “Remyelination and the Gut−brain axis,” in Proceedings of the National Academy of Sciences of the United States of America (Washington, DC: National Academy of Science). doi:10.1073/pnas.1918897116
Dunn, G. A., Nigg, J. T., and Sullivan, E. L. (2019). Neuroinflammation as a Risk Factor for Attention Deficit Hyperactivity Disorder. Pharmacol. Biochem. Behav. 182. doi:10.1016/j.pbb.2019.05.005
Dunphy-Doherty, F., O'Mahony, S. M., Peterson, V. L., O'Sullivan, O., Crispie, F., Cotter, P. D., et al. (2018). Post-weaning Social Isolation of Rats Leads to Long-Term Disruption of the Gut Microbiota-Immune-Brain axis. Brain Behav. Immun. 68, 261–273. doi:10.1016/j.bbi.2017.10.024
Eftekharian, M. M., Ghafouri-Fard, S., Noroozi, R., Omrani, M. D., Arsang-jang, S., Ganji, M., et al. (2018). Cytokine Profile in Autistic Patients. Cytokine 108. doi:10.1016/j.cyto.2018.03.034
Eissa, N., Sadeq, A., Sasse, A., and Sadek, B. (2020). Role of Neuroinflammation in Autism Spectrum Disorder and the Emergence of Brain Histaminergic System. Lessons Also for BPSD? Front. Pharmacol. 11. doi:10.3389/fphar.2020.00886
El-Zayat, S. R., Sibaii, H., and Mannaa, F. A. (2019). Toll-like Receptors Activation, Signaling, and Targeting: an Overview. Bull. Natl. Res. Centre 43 (1). doi:10.1186/s42269-019-0227-2
Ellman, L. M., Deicken, R. F., Vinogradov, S., Kremen, W. S., Poole, J. H., Kern, D. M., et al. (2010). Structural Brain Alterations in Schizophrenia Following Fetal Exposure to the Inflammatory Cytokine Interleukin-8. Schizophrenia Res. 121 (1–3). doi:10.1016/j.schres.2010.05.014
Erhardt, S., Schwieler, L., Imbeault, S., and Engberg, G. (2017). The Kynurenine Pathway in Schizophrenia and Bipolar Disorder. Neuropharmacology 112. doi:10.1016/j.neuropharm.2016.05.020
Ernesto Martínez-González, A., and Andreo-Martínez, P. (2019). The Role of Gut Microbiota in Gastrointestinal Symptoms of Children with ASD. Medicina 55 (8), 408. doi:10.3390/medicina55080408
Erny, D., De Angelis, A. L. H., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 18 (7), 965–977. doi:10.1038/nn.4030
Esen-Danaci, A., Sarandöl, A., Taneli, F., Yurtsever, F., and Özlen, N. (2008). Effects of Second Generation Antipsychotics on Leptin and Ghrelin. Prog. Neuro-Psychopharmacology Biol. Psychiatry 32 (6). doi:10.1016/j.pnpbp.2008.03.015
Faraone, S. v., Perlis, R. H., Doyle, A. E., Smoller, J. W., Goralnick, J. J., Holmgren, M. A., et al. (2005). Molecular Genetics of Attention-Deficit/hyperactivity Disorder. Biol. Psychiatry 57 (11). doi:10.1016/j.biopsych.2004.11.024
Fasano, A. (2012). Zonulin, Regulation of Tight Junctions, and Autoimmune Diseases. Ann. N Y Acad. Sci. 1258 (1). doi:10.1111/j.1749-6632.2012.06538.x
Fawzi, M. H., Fawzi, M. M., Fawzi, M. M., and Said, N. S. (2011). C-reactive Protein Serum Level in Drug-free Male Egyptian Patients with Schizophrenia. Psychiatry Res. 190 (1). doi:10.1016/j.psychres.2011.05.010
Filiano, A., Xu, Y., Tustison, N., and Marsh, R. (2016). Unexpected Role of Interferon-γ in Regulating Neuronal Connectivity and Social Behaviour. Nature 535, 425–429. doi:10.1038/nature18626
Fineberg, A. M., and Ellman, L. M. (2013). Inflammatory Cytokines and Neurological and Neurocognitive Alterations in the Course of Schizophrenia. Biol. Psychiatry 73 (10). doi:10.1016/j.biopsych.2013.01.001
Finegold, S. M., Dowd, S. E., Gontcharova, V., Liu, C., Henley, K. E., Wolcott, R. D., et al. (2010). Pyrosequencing Study of Fecal Microflora of Autistic and Control Children. Anaerobe 16 (4). doi:10.1016/j.anaerobe.2010.06.008
Finegold, S. M., Downes, J., and Summanen, P. H. (2012a). Microbiology of Regressive Autism. Anaerobe 18 (2). doi:10.1016/j.anaerobe.2011.12.018
Finegold, S. M., Downes, J., and Summanen, P. H. (2012b). Pathogenesis and Toxins Microbiology of Regressive Autism. Anaerobe 18. doi:10.1016/j.anaerobe.2011.12.018
Finegold, S. M., Molitoris, D., Song, Y., Liu, C., Vaisanen, M. L., Bolte, E., et al. (2002). Gastrointestinal Microflora Studies in Late-Onset Autism. Clin. Infect. Dis. 35. doi:10.1086/341914
Fiorentino, M., Sapone, A., Senger, S., Camhi, S. S., Kadzielski, S. M., Buie, T. M., et al. (2016). Blood-brain Barrier and Intestinal Epithelial Barrier Alterations in Autism Spectrum Disorders. Mol. Autism 7 (1). doi:10.1186/s13229-016-0110-z
Flowers, S. A., Evans, S. J., Ward, K. M., McInnis, M. G., and Ellingrod, V. L. (2017). Interaction between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort. Pharmacotherapy 37 (3). doi:10.1002/phar.1890
Föcking, M., Dicker, P., Lopez, L. M., Cannon, M., Schäfer, M. R., McGorry, P. D., et al. (2016). Differential Expression of the Inflammation Marker IL12p40 in the At-Risk Mental State for Psychosis: A Predictor of Transition to Psychotic Disorder? BMC Psychiatry 16 (1). doi:10.1186/s12888-016-1039-7
Fond, G., Lançon, C., Korchia, T., Auquier, P., and Boyer, L. (2020). The Role of Inflammation in the Treatment of Schizophrenia. Front. Psychiatry 11. doi:10.3389/fpsyt.2020.00160
Fortier, M. È., Sengupta, S. M., Grizenko, N., Choudhry, Z., Thakur, G., and Joober, R. (2013). Genetic Evidence for the Association of the Hypothalamic-Pituitary-Adrenal (HPA) axis with ADHD and Methylphenidate Treatment Response. NeuroMolecular Med. 15 (1). doi:10.1007/s12017-012-8202-1
Fraguas, D., Díaz-Caneja, C. M., Ayora, M., Hernández-Álvarez, F., Rodríguez-Quiroga, A., Recio, S., et al. (2019). Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-Analysis. Schizophrenia Bull. 45 (4). doi:10.1093/schbul/sby125
Frémont, M., Coomans, D., Massart, S., and de Meirleir, K. (2013). High-throughput 16S rRNA Gene Sequencing Reveals Alterations of Intestinal Microbiota in Myalgic Encephalomyelitis/chronic Fatigue Syndrome Patients. Anaerobe 22. doi:10.1016/j.anaerobe.2013.06.002
Franke, B., Faraone, S. V., Asherson, P., Buitelaar, J., Bau, C. H. D., Ramos-Quiroga, J. A., et al. (2012). The Genetics of Attention Deficit/Hyperactivity Disorder in Adults, a Review. Molecular Psychiatry. doi:10.1038/mp.2011.138
Frydecka, D., Misiak, B., Pawlak-Adamska, E., Karabon, L., Tomkiewicz, A., Sedlaczek, P., et al. (2015). Interleukin-6: the Missing Element of the Neurocognitive Deterioration in Schizophrenia? the Focus on Genetic Underpinnings, Cognitive Impairment and Clinical Manifestation. Eur. Arch. Psychiatry Clin. Neurosci. 265 (6). doi:10.1007/s00406-014-0533-5
Gacias, M., Gaspari, S., Santos, P. M. G., Tamburini, S., Andrade, M., Zhang, F., et al. (2016). Microbiota-driven Transcriptional Changes in Prefrontal Cortex Override Genetic Differences in Social Behavior. ELife 5. doi:10.7554/eLife.13442
Gage, F. H. (2019). Adult Neurogenesis in Mammals Neurogenesis in Adulthood Has Implications for Sense of Self, Memory, and Disease. Am. Assoc. Adv. Sci. 364, 827–828. doi:10.1126/science.aav6885
Gao, L., Li, Z., Chang, S., and Wang, J. (2014). Association of Interleukin-10 Polymorphisms with Schizophrenia: A Meta-Analysis. PLoS ONE 9 (3). doi:10.1371/journal.pone.0090407
Gaonkar, P. (2021). “Safety and Potential Risks with Fecal Microbiota Transplantation,” in Contemporary Topics in Patient Safety - Volume 1 [Working Title] (London: IntechOpen). doi:10.5772/intechopen.95907
Garakani, A., Win, T., Virk, S., Gupta, S., Kaplan, D., and Masand, P. S. (2003). Comorbidity of Irritable Bowel Syndrome in Psychiatric Patients: a Review. Am. J. Ther. 10 (1). doi:10.1097/00045391-200301000-00014
Garay, P. A., and McAllister, A. K. (2010). Novel Roles for Immune Molecules in Neural Development: Implications for Neurodevelopmental Disorders. Front. Synaptic Neurosci. 1 (9). doi:10.3389/fnsyn.2010.00136
García-Bueno, B., Gassó, P., MacDowell, K. S., Callado, L. F., Mas, S., Bernardo, M., et al. (2016). Evidence of Activation of the Toll-like Receptor-4 Proinflammatory Pathway in Patients with Schizophrenia. J. Psychiatry Neurosci. 41 (3). doi:10.1503/jpn.150195
Gattaz, W. F., Abrahão, A. L., and Foccacia, R. (2004). Childhood Meningitis, Brain Maturation and the Risk of Psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 254 (1). doi:10.1007/s00406-004-0431-3
Gaughran, F., O’Neill, E., Cole, M., Collins, K., Daly, R. J., and Shanahan, F. (1998). Increased Soluble Interleukin 2 Receptor Levels in Schizophrenia. Schizophrenia Res. 29 (3). doi:10.1016/S0920-9964(97)00099-6
Gaughran, F., O’Neill, E., Sham, P., Daly, R. J., and Shanahan, F. (2002). Soluble Interleukin 2 Receptor Levels in Families of People with Schizophrenia. Schizophrenia Res. 56 (3). doi:10.1016/S0920-9964(01)00275-4
Genc, S., Zadeoglulari, Z., Fuss, S. H., and Genc, K. (2012). The Adverse Effects of Air Pollution on the Nervous System. J. Toxicol. 2012. doi:10.1155/2012/782462
Gensollen, T., Iyer, S. S., Kasper, D. L., and Blumberg, R. S. (2016). How Colonization by Microbiota in Early Life Shapes the Immune System. Science 352 (6285). doi:10.1126/science.aad9378
Gerritsen, J., Fuentes, S., Grievink, W., van Niftrik, L., Tindall, B. J., Timmerman, H. M., et al. (2014). Characterization of Romboutsia Ilealis Gen. nov., Sp. nov., Isolated from the Gastro-Intestinal Tract of a Rat, and Proposal for the Reclassification of Five Closely Related Members of the Genus Clostridium into the Genera Romboutsia Gen. nov., Intestinibacter Gen. nov., Terrisporobacter Gen. Nov. And Asaccharospora Gen. Nov. Int. J. Syst. Evol. Microbiol. 64 (5). doi:10.1099/ijs.0.059543-0
Ginhoux, F., Lim, S., Hoeffel, G., Low, D., and Huber, T. (2013). Origin and Differentiation of Microglia. Front. Cell Neurosci. 7 (3). doi:10.3389/fncel.2013.00045
Girgis, R. R., Kumar, S. S., and Brown, A. S. (2014). The Cytokine Model of Schizophrenia: Emerging Therapeutic Strategies. Biol. Psychiatry 75 (4). doi:10.1016/j.biopsych.2013.12.002
Glibert, J. A., Krajmalnik-Brown, R., Porazinska, D. L., Weiss, S. J., and Knight, R. (2013). Towards Effective Probiotics for Autism and Other Mental Disorders? Cell 155 (7). doi:10.1016/j.cell.2013.11.035
Goines, P. E., and Ashwood, P. (2013). Cytokine Dysregulation in Autism Spectrum Disorders (ASD): Possible Role of the Environment. Neurotoxicology and Teratology 36. doi:10.1016/j.ntt.2012.07.006
Goldsmith, D. R., Haroon, E., Miller, A. H., Addington, J., Bearden, C., Cadenhead, K., et al. (2019). Association of Baseline Inflammatory Markers and the Development of Negative Symptoms in Individuals at Clinical High Risk for Psychosis. Brain Behav. Immun. 76. doi:10.1016/j.bbi.2018.11.315
Goldsmith, D. R., Rapaport, M. H., and Miller, B. J. (2016). A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons between Schizophrenia, Bipolar Disorder and Depression. Mol. Psychiatry 21 (12). doi:10.1038/mp.2016.3
Gotsch, F., Romero, R., Kusanovic, J. P., Mazaki-Tovi, S., Pineles, B. L., Erez, O., et al. (2007). The Fetal Inflammatory Response Syndrome. Clin. Obstet. Gynecol. 50 (3). doi:10.1097/GRF.0b013e31811ebef6
Graeff, F. G., Guimarães, F. S., de Andrade, T. G. C. S., and Deakin, J. F. W. (1996). Role of 5-HT in Stress, Anxiety, and Depression. Pharmacol. Biochem. Behav. 54 (1). doi:10.1016/0091-3057(95)02135-3
Grandi, A., Zini, I., Palese, S., Giorgio, C., Tognolini, M., Marchesani, F., et al. (2019). Targeting the Eph/ephrin System as Anti-inflammatory Strategy in IBD. Front. Pharmacol. 10 (JUN). doi:10.3389/fphar.2019.00691
Grenham, S., Clarke, G., Cryan, J. F., and Dinan, T. G. (2011). Brain-gut-microbe Communication in Health and Disease. Front. Physiol. 2. doi:10.3389/fphys.2011.00094
Grüber, L., Bunse, T., Weidinger, E., Reichard, H., and Müller, N. (2014). Adjunctive Recombinant Human Interferon Gamma-1b for Treatment-Resistant Schizophrenia in 2 Patients. J. Clin. Psychiatry 75 (11). doi:10.4088/JCP.14l09005
Gruol, D. L. (2015). IL-6 Regulation of Synaptic Function in the CNS. Neuropharmacology 96. doi:10.1016/j.neuropharm.2014.10.023
Gruzieva, O., Merid, S. K., Gref, A., Gajulapuri, A., Lemonnier, N., Ballereau, S., et al. (2017). Exposure to Traffic-Related Air Pollution and Serum Inflammatory Cytokines in Children. Environ. Health Perspect. 125 (6). doi:10.1289/EHP460
Gubert, C., Kong, G., Uzungil, V., Zeleznikow-Johnston, A. M., Burrows, E. L., Renoir, T., et al. (2020). Microbiome Profiling Reveals Gut Dysbiosis in the Metabotropic Glutamate Receptor 5 Knockout Mouse Model of Schizophrenia. Front. Cel. Dev. Biol. 8, 1233. doi:10.3389/fcell.2020.582320
Guma, E., Bordignon, P. do. C., Devenyi, G. A., Gallino, D., Anastassiadis, C., Cvetkovska, V., et al. (2021). Early or Late Gestational Exposure to Maternal Immune Activation Alters Neurodevelopmental Trajectories in Mice: An Integrated Neuroimaging, Behavioral, and Transcriptional Study. Biol. Psychiatry 90. doi:10.1016/j.biopsych.2021.03.017
Guney, E., Cetin, F. H., and Iseri, E. (2015). “The Role of Environmental Factors in Etiology of Attention- Deficit Hyperactivity Disorder,” in ADHD - New Directions in Diagnosis and Treatment (London: IntechOpen). doi:10.5772/61025
Hakansson, A., and Molin, G. (2011). Gut Microbiota and Inflammation. Nutrients MDPI AG. doi:10.3390/nu3060637
Hafner, C., Meyer, S., Langmann, T., Schmitz, G., Bataille, F., Hagen, I., et al. (2005). Ephrin-B2 Is Differentially Expressed in the Intestinal Epithelium in Crohn’s Disease and Contributes to Accelerated Epithelial Wound Healing In Vitro. World J. Gastroenterol. 11 (26). doi:10.3748/wjg.v11.i26.4024
Han, J., Zhu, K., Zhang, X. M., and Harris, R. A. (2019). Enforced Microglial Depletion and Repopulation as a Promising Strategy for the Treatment of Neurological Disorders. GLIA 67 (2). doi:10.1002/glia.23529
Han, V. X., Patel, S., Jones, H. F., and Dale, R. C. (2021). Maternal Immune Activation and Neuroinflammation in Human Neurodevelopmental Disorders. Nat. Rev. Neurol. 17 (9). doi:10.1038/s41582-021-00530-8
Hanamsagar, R., Alter, M. D., Block, C. S., Sullivan, H., Bolton, J. L., and Bilbo, S. D. (2017). Generation of a Microglial Developmental Index in Mice and in Humans Reveals a Sex Difference in Maturation and Immune Reactivity. GLIA 65 (9), 1504–1520. doi:10.1002/glia.23176
Harry, G. J., and Kraft, A. D. (2008). Neuroinflammation and Microglia: Considerations and Approaches for Neurotoxicity Assessment. Expert Opin. Drug Metab. Toxicol. 4 (10). doi:10.1517/17425255.4.10.1265
Hashimoto, M., Maekawa, M., Katakura, M., Hamazaki, K., and Matsuoka, Y. (2014). Possibility of Polyunsaturated Fatty Acids for the Prevention and Treatment of Neuropsychiatric Illnesses. J. Pharmacol. Sci. 124 (3). doi:10.1254/jphs.13R14CP
He, Q., Kwok, L. Y., Xi, X., Zhong, Z., Ma, T., Xu, H., et al. (2020). The Meconium Microbiota Shares More Features with the Amniotic Fluid Microbiota Than the Maternal Fecal and Vaginal Microbiota. Gut Microbes 12 (1). doi:10.1080/19490976.2020.1794266
Heijtz, R. D., Wang, S., Anuar, F., Qian, Y., Björkholm, B., Samuelsson, A., et al. (2011). Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. United States America 108 (7). doi:10.1073/pnas.1010529108
Helmut, K., Hanisch, U. K., Noda, M., and Verkhratsky, A. (2011). Physiology of Microglia. Physiol. Rev. 91 (2). doi:10.1152/physrev.00011.2010
Hiergeist, A., Gessner, J., and Gessner, A. (2020). Current Limitations for the Assessment of the Role of the Gut Microbiome for Attention Deficit Hyperactivity Disorder (ADHD). Front. Psychiatry 11. doi:10.3389/fpsyt.2020.00623
Hiippala, K., Kainulainen, V., Suutarinen, M., Heini, T., Bowers, J. R., Jasso-Selles, D., et al. (2020). Isolation of Anti-inflammatory and Epithelium Reinforcing bacteroides and parabacteroides Spp. From a Healthy Fecal Donor. Nutrients 12 (4). doi:10.3390/nu12040935
Hoban, A. E., Stilling, R. M., Ryan, F. J., Shanahan, F., Dinan, T. G., Claesson, M. J., et al. (2016). Regulation of Prefrontal Cortex Myelination by the Microbiota. Translational Psychiatry 6. doi:10.1038/tp.2016.42
Hoen, W. P., Lijmer, J. G., Duran, M., Wanders, R. J. A., van Beveren, N. J. M., and de Haan, L. (2013). Red Blood Cell Polyunsaturated Fatty Acids Measured in Red Blood Cells and Schizophrenia: A Meta-Analysis. Psychiatry Res. 207, 1–12. doi:10.1016/j.psychres.2012.09.041
Hoftman, G. D., Dienel, S. J., Bazmi, H. H., Zhang, Y., Chen, K., and Lewis, D. A. (2018). Altered Gradients of Glutamate and Gamma-Aminobutyric Acid Transcripts in the Cortical Visuospatial Working Memory Network in Schizophrenia. Biol. Psychiatry 83 (8). doi:10.1016/j.biopsych.2017.11.029
Hong, J., and Bang, M. (2020). Anti-inflammatory Strategies for Schizophrenia: A Review of Evidence for Therapeutic Applications and Drug Repurposing. Clin. Psychopharmacol. Neurosci. 18 (1). doi:10.9758/CPN.2020.18.1.10
Hong, S., Beja-Glasser, V. F., Nfonoyim, B. M., Frouin, A., Li, S., Ramakrishnan, S., et al. (2016). Complement and Microglia Mediate Early Synapse Loss in Alzheimer Mouse Models. Science 352 (6286). doi:10.1126/science.aad8373
Hoo, R., Nakimuli, A., and Vento-Tormo, R. (2020). Innate Immune Mechanisms to Protect against Infection at the Human Decidual-Placental Interface. Front. Immunol. 11. doi:10.3389/fimmu.2020.02070
Horsdal, H. T., Agerbo, E., McGrath, J. J., Vilhjálmsson, B. J., Antonsen, S., Closter, A. M., et al. (2019). Association of Childhood Exposure to Nitrogen Dioxide and Polygenic Risk Score for Schizophrenia with the Risk of Developing Schizophrenia. JAMA Netw. Open 2 (11). doi:10.1001/jamanetworkopen.2019.14401
Hsiao, E. Y., Mcbride, S. W., Hsien, S., Sharon, G., Hyde, E. R., Mccue, T., et al. (2013). The Microbiota Modulates Gut Physiology and Behavioral Abnormalities Associated with Autism. Cell 155 (7). doi:10.1016/j.cell.2013.11.024
Hu, J., Nomura, Y., Bashir, A., Fernandez-Hernandez, H., Itzkowitz, S., Pei, Z., et al. (2013). Diversified Microbiota of Meconium Is Affected by Maternal Diabetes Status. PLoS ONE 8 (11). doi:10.1371/journal.pone.0078257
Huang, F., and Wu, X. (2021). Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cel Dev. Biol. 9. doi:10.3389/fcell.2021.649103
Hudson, Z. D., and Miller, B. J. (2018). Meta-analysis of Cytokine and Chemokine Genes in Schizophrenia. Clin. Schizophrenia Relat. Psychoses 12 (3). doi:10.3371/CSRP.HUMI.070516
Hugh Perry, V. (1998). A Revised View of the Central Nervous System Microenvironment and Major Histocompatibility Complex Class II Antigen Presentation. Journal of Neuroimmunology. doi:10.1016/S0165-5728(98)00145-3
Hughes, H. K., Mills Ko, E., Rose, D., and Ashwood, P. (2018). Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front. Cell Neurosci. 12. doi:10.3389/fncel.2018.00405
Humann, J., Mann, B., Gao, G., Moresco, P., Ramahi, J., Loh, L. N., et al. (2016). Bacterial Peptidoglycan Transverses the Placenta to Induce Fetal Neuroproliferation and Aberrant Postnatal Behavior. Cell Host and Microbe 19 (3). doi:10.1016/j.chom.2016.02.009
Huo, R., Zeng, B., Zeng, L., Cheng, K., Li, B., Luo, Y., et al. (2017). Microbiota Modulate Anxiety-like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal axis. Front. Cell Infect. Microbiol. 7 (11). doi:10.3389/fcimb.2017.00489
Iovene, M. R., Bombace, F., Maresca, R., Sapone, A., Iardino, P., Picardi, A., et al. (2017). Intestinal Dysbiosis and Yeast Isolation in Stool of Subjects with Autism Spectrum Disorders. Mycopathologia 182 (3–4). doi:10.1007/s11046-016-0068-6
Jácome, M. C. I., Chacòn, L. M. M., Cuesta, H. V., Rizo, C. M., Santiesteban, M. W., Hernandez, L. R., et al. (2016). Peripheral Inflammatory Markers Contributing to Comorbidities in Autism. Behav. Sci. 6 (4). doi:10.3390/bs6040029
Jacquemont, S., Coe, B. P., Hersch, M., Duyzend, M. H., Krumm, N., Bergmann, S., et al. (2014). A Higher Mutational burden in Females Supports a “Female Protective Model” in Neurodevelopmental Disorders. Am. J. Hum. Genet. 94 (3). doi:10.1016/j.ajhg.2014.02.001
Jarskog, L. F., Xiao, H., Wilkie, M. B., Lauder, J. M., and Gilmore, J. H. (1997). Cytokine Regulation of Embryonic Rat Dopamine and Serotonin Neuronal Survival In Vitro. Int. J. Dev. Neurosci. 15 (6). doi:10.1016/S0736-5748(97)00029-4
Jena, A., Montoya, C. A., Mullaney, J. A., Dilger, R. N., Young, W., McNabb, W. C., et al. (2020). Gut-Brain Axis in the Early Postnatal Years of Life: A Developmental Perspective. Front. Integr. Neurosci. 14. doi:10.3389/fnint.2020.00044
Jeste, S. S. (2015). Neurodevelopmental Behavioral and Cognitive Disorders. CONTINUUM Lifelong Learn. Neurol. 21 (3). doi:10.1212/01.CON.0000466661.89908.3c
Jiang, H. Y., Zhou, Y. Y., Zhou, G. L., Li, Y. C., Yuan, J., Li, X. H., et al. (2018). Gut Microbiota Profiles in Treatment-Naïve Children with Attention Deficit Hyperactivity Disorder. Behav. Brain Res. 347. doi:10.1016/j.bbr.2018.03.036
Jiang, Nona. M. (2016). The Impact of Systemic Inflammation on Neurodevelopment. Physiol. BehaviorThe 176 (1), 100–106. doi:10.1016/j.molmed.2018.06.008
Jolanta Wasilewska, J., and Klukowski, M. (2015). Gastrointestinal Symptoms and Autism Spectrum Disorder: Links and Risks – a Possible New Overlap Syndrome. Pediatr. Health Med. Ther. 153. doi:10.2147/phmt.s85717
Jung, E., Romero, R., Yeo, L., Diaz-Primera, R., Marin-Concha, J., Para, R., et al. (2020). The Fetal Inflammatory Response Syndrome: the Origins of a Concept, Pathophysiology, Diagnosis, and Obstetrical Implications. Semin. Fetal Neonatal Med. 25 (4). doi:10.1016/j.siny.2020.101146
Kabiersch, A., Furukawa, H., Rey, A. D., and Besedovsky, H. O. (1998). Administration of Interleukin-1 at Birth Affects Dopaminergic Neurons in Adult Mice. Ann. N Y Acad. Sci. 840. doi:10.1111/j.1749-6632.1998.tb09556.x
Kahn, R. S., Sommer, I. E., Murray, R. M., Meyer-Lindenberg, A., Weinberger, D. R., Cannon, T. D., et al. (2015). Schizophrenia. Nat. Rev. Dis. Primers 1. doi:10.1038/nrdp.2015.67
Kaller, M. S., Lazari, A., Blanco-Duque, C., Sampaio-Baptista, C., and Johansen-Berg, H. (2017). Myelin Plasticity and Behaviour — Connecting the Dots. Curr. Opin. Neurobiol. 47. doi:10.1016/j.conb.2017.09.014
Kalmady, S. V., Venkatasubramanian, G., Shivakumar, V., Gautham, S., Subramaniam, A., Jose, D. A., et al. (2014). Relationship between Interleukin-6 Gene Polymorphism and Hippocampal Volume in Antipsychotic-Naïve Schizophrenia: Evidence for Differential Susceptibility? PLoS ONE 9 (5). doi:10.1371/journal.pone.0096021
Kamitaki, N., Sekar, A., Handsaker, R. E., de Rivera, H., Tooley, K., Morris, D. L., et al. (2020). Complement Genes Contribute Sex-Biased Vulnerability in Diverse Disorders. Nature 582 (7813). doi:10.1038/s41586-020-2277-x
Kandasamy, S., Chattha, K. S., Vlasova, A. N., Rajashekara, G., and Saif, L. J. (2015). Lactobacilli and Bifidobacteria Enhance Mucosal B Cell Responses and Differentially Modulate Systemic Antibody Responses to an Oral Human Rotavirus Vaccine in a Neonatal Gnotobiotic Pig Disease Model. Gut Microbes 5 (5). doi:10.4161/19490976.2014.969972
Kang Dw, D. W., Park, J. G., Ilhan, Z. E., Wallstrom, G., LaBaer, J., Adams, J. B., et al. (2013). Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 8 (7). doi:10.1371/journal.pone.0068322
Kang, D. W., Adams, J. B., Coleman, D. M., Pollard, E. L., Maldonado, J., McDonough-Means, S., et al. (2019). Long-term Benefit of Microbiota Transfer Therapy on Autism Symptoms and Gut Microbiota. Scientific Rep. 9 (1). doi:10.1038/s41598-019-42183-0
Kang, D. W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., et al. (2017). Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 5 (1). doi:10.1186/s40168-016-0225-7
Kang Ws, W. S., Park, J. K., Lee, S. M., Kim, S. K., Park, H. J., and Kim, J. W. (2013). Association between Genetic Polymorphisms of Toll-like Receptor 2 (TLR2) and Schizophrenia in the Korean Population. Gene 526 (2). doi:10.1016/j.gene.2013.04.058
Kany, S., Vollrath, J. T., and Relja, B. (2019). Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 20 (23). doi:10.3390/ijms20236008
Kapoor, R. (2002). Neurological, Psychiatric, and Developmental Disorders: Meeting the challenge in the Developing World. Trans. R. Soc. Trop. Med. Hyg. 96 (3). doi:10.1016/s0035-9203(02)90116-1
Katano, Y., Fujinami, S., Kawakoshi, A., Nakazawa, H., Oji, S., Iino, T., et al. (2012). Complete Genome Sequence of Oscillibacter Valericigenes Sjm18-20 T (=NBRC 101213 T). Stand. Genomic Sci. 6 (3). doi:10.4056/sigs.2826118
Katila, H., Hänninen, K., and Hurme, M. (1999). Polymorphisms of the Interleukin-1 Gene Complex in Schizophrenia. Mol. Psychiatry 4 (2). doi:10.1038/sj.mp.4000483
Katsel, P., Davis, K. L., and Haroutunian, V. (2005). Variations in Myelin and Oligodendrocyte-Related Gene Expression across Multiple Brain Regions in Schizophrenia: a Gene Ontology Study. Schizophrenia Res. 79 (2-3), 157–173. doi:10.1016/j.schres.2005.06.007
Katz-Barber, M. W., Hollins, S. L., Cuskelly, A., Leong, A. J., Dunn, A., Harms, L., et al. (2020). Investigating the Gut-Brain axis in a Neurodevelopmental Rodent Model of Schizophrenia. Brain Behav. Immunity-Health 3, 100048. doi:10.1016/j.bbih.2020.100048
Kaul, D., Habbel, P., Derkow, K., Krüger, C., Franzoni, E., Wulczyn, F. G., et al. (2012). Expression of Toll-like Receptors in the Developing Brain. PLoS ONE 7 (5), e37767. doi:10.1371/journal.pone.0037767
Kelly, C. W., McEvoy, J. P., and Miller, B. J. (2019). Total and Differential white Blood Cell Counts, Inflammatory Markers, Adipokines, and Incident Metabolic Syndrome in Phase 1 of the Clinical Antipsychotic Trials of Intervention Effectiveness Study. Schizophrenia Res. 209. doi:10.1016/j.schres.2019.04.021
Kelly, J. R., Minuto, C., Cryan, J. F., Clarke, G., and Dinan, T. G. (2021). The Role of the Gut Microbiome in the Development of Schizophrenia. Schizophrenia Res. 234. doi:10.1016/j.schres.2020.02.010
Kempermann, G. (2019). Environmental Enrichment, New Neurons and the Neurobiology of Individuality. Nat. Rev. Neurosci. 20 (4). doi:10.1038/s41583-019-0120-x
Keogh, C. E., Kim, D. H. J., Pusceddu, M. M., Knotts, T. A., Rabasa, G., Sladek, J. A., et al. (2021). Myelin as a Regulator of Development of the Microbiota-Gut-Brain axis. Brain Behav. Immun. 91, 437–450. doi:10.1016/j.bbi.2020.11.001
Kesby, J. P., Eyles, D. W., McGrath, J. J., and Scott, J. G. (2018). Dopamine, Psychosis and Schizophrenia: The Widening gap between Basic and Clinical Neuroscience. Translational Psychiatry 8 (1). doi:10.1038/s41398-017-0071-9
Khandaker, G. M., Pearson, R. M., Zammit, S., Lewis, G., and Jones, P. B. (2014). Association of Serum Interleukin 6 and C-Reactive Protein in Childhood with Depression and Psychosis in Young Adult Life a Population-Based Longitudinal Study. JAMA Psychiatry 71 (10). doi:10.1001/jamapsychiatry.2014.1332
Kim, J. M., Kim, Y. J., and Cho, Y. J. (2000). Synergy of bacteroides Fragilis and escherichia Coli in the Induction of KC Gene Expression in Mouse Peritoneal Tissues. Scand. J. Infect. Dis. 32 (6). doi:10.1080/003655400459568
Kim, S. H., and Lord, C. (2010). Restricted and Repetitive Behaviors in Toddlers and Preschoolers with Autism Spectrum Disorders Based on the Autism Diagnostic Observation Schedule (ADOS). Autism Res. 3 (4). doi:10.1002/aur.142
Kim, S. W., Jhon, M., Kim, J. M., Smesny, S., Rice, S., Berk, M., et al. (2016). Relationship between Erythrocyte Fatty Acid Composition and Psychopathology in the Vienna omega-3 Study. PLoS ONE 11 (3). doi:10.1371/journal.pone.0151417
King, S., St-Hilaire, A., and Heidkamp, D. (2010). Prenatal Factors in Schizophrenia. Curr. Dir. Psychol. Sci. 19 (4). doi:10.1177/0963721410378360
Kingwell, K. (2012). Neurodegenerative Disease: Microglia in Early Disease Stages. Nat. Rev. Neurol. 8 (9). doi:10.1038/nrneurol.2012.172
Klimkeit, E., Rinehart, N., May, T., and Bradshaw, J. (2016). Neurodevelopmental Disorders. Amsterdam: International Encyclopedia of Public Health, 223–230. doi:10.1016/B978-0-12-803678-5.00299-X
Knuesel, I., Chicha, L., Britschgi, M., Schobel, S. A., Bodmer, M., Hellings, J. A., et al. (2014). Maternal Immune Activation and Abnormal Brain Development across CNS Disorders. Nat. Rev. Neurol. 10 (11). doi:10.1038/nrneurol.2014.187
Koenig, J. E., Spor, A., Scalfone, N., Fricker, A. D., Stombaugh, J., Knight, R., et al. (2011). Succession of Microbial Consortia in the Developing Infant Gut Microbiome. Proc. Natl. Acad. Sci. United States America 108 (1). doi:10.1073/pnas.1000081107
Koponen, K. K., Salosensaari, A., Ruuskanen, M. O., Havulinna, A. S., Männistö, S., Jousilahti, P., et al. (2021). Associations of Healthy Food Choices with Gut Microbiota Profiles. Am. J. Clin. Nutr. 114. doi:10.1093/ajcn/nqab077
Kraus, D. M., Elliott, G. S., Chute, H., Horan, T., Pfenninger, K. H., Sanford, S. D., et al. (2006). CSMD1 Is a Novel Multiple Domain Complement-Regulatory Protein Highly Expressed in the Central Nervous System and Epithelial Tissues. J. Immunol. 176 (7). doi:10.4049/jimmunol.176.7.4419
Kroken, R. A., Sommer, I. E., Steen, V. M., Dieset, I., and Johnsen, E. (2019). Constructing the Immune Signature of Schizophrenia for Clinical Use and Research; an Integrative Review Translating Descriptives into Diagnostics. Front. Psychiatry 10 (1). doi:10.3389/fpsyt.2018.00753
Kurnellas, M. P., Ghosn, E. E. B., Schartner, J. M., Baker, J., Rothbard, J. J., Negrin, R. S., et al. (2015). Amyloid Fibrils Activate B-1a Lymphocytes to Ameliorate Inflammatory Brain Disease. Proc. Natl. Acad. Sci. 112 (49), 15016–15023. doi:10.1073/pnas.1521206112
Lacorte, E., Gervasi, G., Bacigalupo, I., Vanacore, N., Raucci, U., and Parisi, P. (2019). A Systematic Review of the Microbiome in Children with Neurodevelopmental Disorders. Front. Neurol. 10. doi:10.3389/fneur.2019.00727
Lawson, L. J., Perry, V. H., Dri, P., and Gordon, S. (1990). Heterogeneity in the Distribution and Morphology of Microglia in the normal Adult Mouse Brain. Neuroscience 39 (1). doi:10.1016/0306-4522(90)90229-W
Lebel, C., Gee, M., Camicioli, R., Wieler, M., Martin, W., and Beaulieu, C. (2012). Diffusion Tensor Imaging of white Matter Tract Evolution over the Lifespan. NeuroImage 60 (1). doi:10.1016/j.neuroimage.2011.11.094
Lee, S. H., Gong, Y. N., and Ryoo, E. (2017). Clostridium difficile Colonization And/or Infection during Infancy and the Risk of Childhood Allergic Diseases. Korean J. Pediatr. 60 (5). doi:10.3345/kjp.2017.60.5.145
Lesh, T. A., Careaga, M., Rose, D. R., McAllister, A. K., van de Water, J., Carter, C. S., et al. (2018). Cytokine Alterations in First-Episode Schizophrenia and Bipolar Disorder: Relationships to Brain Structure and Symptoms. J. Neuroinflammation 15 (1). doi:10.1186/s12974-018-1197-2
Leslie, D. L., Kozma, L., Martin, A., Landeros, A., Katsovich, L., King, R. A., et al. (2008). Neuropsychiatric Disorders Associated with Streptococcal Infection: A Case-Control Study Among Privately Insured Children. J. Am. Acad. Child Adolesc. Psychiatry 47 (10). doi:10.1097/CHI.0b013e3181825a3d
Li, W., Chen, M., Feng, X., Song, M., Shao, M., Yang, Y., et al. (2021). Maternal Immune Activation Alters Adult Behavior, Intestinal Integrity, Gut Microbiota and the Gut Inflammation. Brain Behav. 11 (5). doi:10.1002/brb3.2133
Li, X., Zhang, W., Lencz, T., Darvasi, A., Alkelai, A., Lerer, B., et al. (2015). Common Variants of IRF3 Conferring Risk of Schizophrenia. J. Psychiatr. Res. 64. doi:10.1016/j.jpsychires.2015.03.008
Libowitz, M. R., and Nurmi, E. L. (2021). The Burden of Antipsychotic-Induced Weight Gain and Metabolic Syndrome in Children. Front. Psychiatry 12. doi:10.3389/fpsyt.2021.623681
Ligthart, S., Vaez, A., Võsa, U., Stathopoulou, M. G., de Vries, P. S., Prins, B. P., et al. (2018). Genome Analyses of >200,000 Individuals Identify 58 Loci for Chronic Inflammation and Highlight Pathways that Link Inflammation and Complex Disorders. Am. J. Hum. Genet. 103 (5). doi:10.1016/j.ajhg.2018.09.009
Liu, C., Yang, S. Y., Wang, L., and Zhou, F. (2022). The Gut Microbiome: Implications for Neurogenesis and Neurological Diseases. Neural Regen. Res. 17, 53–58. doi:10.4103/1673-5374.315227
Lou, H. (1994). Dopamine Precursors and Brain Function in Phenylalanine Hydroxylase Deficiency. Acta Pædiatrica 83. doi:10.1111/j.1651-2227.1994.tb13461.x
Lu, J., and Claud, E. C. (2019). Connection between Gut Microbiome and Brain Development in Preterm Infants. Developmental Psychobiology 61 (5). doi:10.1002/dev.21806
Lu, Q. R., Sun, T., Zhu, Z., Ma, N., Garcia, M., Stiles, C. D., et al. (2002). Common Developmental Requirement for Olig Function Indicates a Motor Neuron/oligodendrocyte Connection. Cell 109 (1). doi:10.1016/S0092-8674(02)00678-5
Maas, J. W., Contreras, S. A., Miller, A. L., Bennan, N., Bowden, C. L., Javors, M. A., et al. (1993). Studies of Catecholamine Metabolism in Schizophrenia/psychosis-I. Neuropsychopharmacology 8 (2). doi:10.1038/npp.1993.11
MacDowell, K. S., Pinacho, R., Leza, J. C., Costa, J., Ramos, B., and García-Bueno, B. (2017). Differential Regulation of the TLR4 Signalling Pathway in post-mortem Prefrontal Cortex and Cerebellum in Chronic Schizophrenia: Relationship with SP Transcription Factors. Prog. Neuro-Psychopharmacology Biol. Psychiatry 79. doi:10.1016/j.pnpbp.2017.08.005
MacFabe, D. F. (2012). Short-chain Fatty Acid Fermentation Products of the Gut Microbiome: Implications in Autism Spectrum Disorders. Microb. Ecol. Health Dis. 23 (0). doi:10.3402/mehd.v23i0.19260
Machiels, K., Joossens, M., Sabino, J., de Preter, V., Arijs, I., Eeckhaut, V., et al. (2014). A Decrease of the Butyrate-Producing Species Roseburia Hominis and Faecalibacterium Prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 63 (8). doi:10.1136/gutjnl-2013-304833
Madan, J. C., Salari, R. C., Saxena, D., Davidson, L., O’Toole, G. A., Moore, J. H., et al. (2012). Gut Microbial Colonisation in Premature Neonates Predicts Neonatal Sepsis. Arch. Dis. Child. Fetal Neonatal Edition 97 (6). doi:10.1136/fetalneonatal-2011-301373
Madore, C., Leyrolle, Q., Lacabanne, C., Benmamar-Badel, A., Joffre, C., Nadjar, A., et al. (2016). Neuroinflammation in Autism: Plausible Role of Maternal Inflammation, Dietary Omega 3, and Microbiota. Neural Plasticity 2016, 3597209. doi:10.1155/2016/3597209
Maes, M., Kanchanatawan, B., Sirivichayakul, S., and Carvalho, A. F. (2019). In Schizophrenia, Increased Plasma IgM/IgA Responses to Gut Commensal Bacteria Are Associated with Negative Symptoms, Neurocognitive Impairments, and the Deficit Phenotype. Neurotoxicity Res. 35 (3). doi:10.1007/s12640-018-9987-y
Maguire, M., and Maguire, G. (2019). Gut Dysbiosis, Leaky Gut, and Intestinal Epithelial Proliferation in Neurological Disorders: Towards the Development of a New Therapeutic Using Amino Acids, Prebiotics, Probiotics, and Postbiotics. Rev. Neurosciences 30 (2). doi:10.1515/revneuro-2018-0024
Mallard, C. (2012). Innate Immune Regulation by Toll-like Receptors in the Brain. ISRN Neurol. 2012. doi:10.5402/2012/701950
Marín, O. (2016). Developmental Timing and Critical Windows for the Treatment of Psychiatric Disorders. Nat. Med. 22 (11). doi:10.1038/nm.4225
Martínez-Gras, I., García-Sánchez, F., Guaza, C., Rodríguez-Jiménez, R., Andrés-Esteban, E., Palomo, T., et al. (2012). Altered Immune Function in Unaffected First-Degree Biological Relatives of Schizophrenia Patients. Psychiatry Res. 200 (2–3). doi:10.1016/j.psychres.2012.05.036
McCracken (2005). Risperidone Treatment of Autistic Disorder: Longer-Term Benefits and Blinded Discontinuation after 6 Months. Am. J. Psychiatry 162 (7). doi:10.1176/appi.ajp.162.7.1361
Meisenzahl, E. M., Rujescu, D., Kirner, A., Giegling, I., Kathmann, N., Leinsinger, G., et al. (2001). Association of an Interleukin-1β Genetic Polymorphism with Altered Brain Structure in Patients with Schizophrenia. Am. J. Psychiatry 158 (8). doi:10.1176/appi.ajp.158.8.1316
Michel, L., and Prat, A. (2016). One More Role for the Gut: Microbiota and Blood Brain Barrier. Ann. Translational Med. 4 (1). doi:10.3978/j.issn.2305-5839.2015.10.16
Milani, C., Duranti, S., Bottacini, F., Casey, E., Turroni, F., Mahony, J., et al. (2017). The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 81 (4). doi:10.1128/mmbr.00036-17
Miller Ah, A. H., Haroon, E., Raison, C. L., and Felger, J. C. (2013). Cytokine Targets in the Brain: Impact on Neurotransmitters and Neurocircuits. Depress. Anxiety 30 (4). doi:10.1002/da.22084
Miller B, B., Mellor, A., and Buckley, P. F. (2013). Interleukin-6 and Cognition in Nonaffective Psychosis. Schizophrenia Bull. 39.
Miller, B., and Essali, N. (2019). O10.5. Meta-Analysis of Cytokine Levels and Psychopathology in Schizophrenia. Schizophrenia Bull. 45 (2). doi:10.1093/schbul/sbz021.253
Misiak, B., Stańczykiewicz, B., Kotowicz, K., Rybakowski, J. K., Samochowiec, J., and Frydecka, D. (2018). Cytokines and C-Reactive Protein Alterations with Respect to Cognitive Impairment in Schizophrenia and Bipolar Disorder: A Systematic Review. Schizophrenia Res. 192. doi:10.1016/j.schres.2017.04.015
Miyazaki, C., Koyama, M., Ota, E., Swa, T., Mlunde, L. B., Amiya, R. M., et al. (2017). Allergic Diseases in Children with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. BMC Psychiatry 17 (1). doi:10.1186/s12888-017-1281-7
Möhle, L., Mattei, D., Heimesaat, M. M., Bereswill, S., Fischer, A., Alutis, M., et al. (2016). Ly6Chi Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cel Rep. 15 (9), 1945–1956. doi:10.1016/j.celrep.2016.04.074
Möhler, H. (2012). The GABA System in Anxiety and Depression and its Therapeutic Potential. Neuropharmacology 62 (1). doi:10.1016/j.neuropharm.2011.08.040
Moles, L., Gómez, M., Heilig, H., Bustos, G., Fuentes, S., de Vos, W., et al. (2013). Bacterial Diversity in Meconium of Preterm Neonates and Evolution of Their Fecal Microbiota during the First Month of Life. PLoS ONE 8 (6). doi:10.1371/journal.pone.0066986
Mollgaard, K., and Saunders, N. R. (1986). The Development of the Human Blood-Brain and Blood-CSF Barriers. Neuropathol. Appl. Neurobiol. 12 (4). doi:10.1111/j.1365-2990.1986.tb00146.x
Molloy, C. A., Morrow, A. L., Meinzen-Derr, J., Schleifer, K., Dienger, K., Manning-Courtney, P., et al. (2006). Elevated Cytokine Levels in Children with Autism Spectrum Disorder. J. Neuroimmunology 172 (1–2). doi:10.1016/j.jneuroim.2005.11.007
Mor, G., and Cardenas, I. (2010). The Immune System in Pregnancy: A Unique Complexity. Am. J. Reprod. Immunol. 63 (6). doi:10.1111/j.1600-0897.2010.00836.x
Morgan, J. T., Chana, G., Pardo, C. A., Achim, C., Semendeferi, K., Buckwalter, J., et al. (2010). Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol. Psychiatry 68 (4). doi:10.1016/j.biopsych.2010.05.024
Morris, P., Ali, K., Merritt, M., Pelletier, J., and Macedo, L. G. (2020). A Systematic Review of the Role of Inflammatory Biomarkers in Acute, Subacute and Chronic Non-specific Low Back Pain. BMC Musculoskelet. Disord. 21 (1). doi:10.1186/s12891-020-3154-3
Mousa, A., and Bakhiet, M. (2013). Role of Cytokine Signaling during Nervous System Development. Int. J. Mol. Sci. 14 (7). doi:10.3390/ijms140713931
Müller, N., Krause, D., Dehning, S., Musil, R., Schennach-Wolff, R., Obermeier, M., et al. (2010). Celecoxib Treatment in an Early Stage of Schizophrenia: Results of a Randomized, Double-Blind, Placebo-Controlled Trial of Celecoxib Augmentation of Amisulpride Treatment. Schizophrenia Res. 121 (1–3). doi:10.1016/j.schres.2010.04.015
Müller, N., Schwarz, M. J., Dehning, S., Douhe, A., Cerovecki, A., Goldstein-Müller, B., et al. (2006). The Cyclooxygenase-2 Inhibitor Celecoxib Has Therapeutic Effects in Major Depression: Results of a Double-Blind, Randomized, Placebo Controlled, Add-On Pilot Study to Reboxetine. Mol. Psychiatry 11 (7). doi:10.1038/sj.mp.4001805
Müller, N., and Schwarz, M. J. (2006). Neuroimmune-endocrine Crosstalk in Schizophrenia and Mood Disorders. Expert Rev. Neurotherapeutics 6 (7). doi:10.1586/14737175.6.7.1017
Mulligan, A., Anney, R., Butler, L., O’Regan, M., Richardson, T., Tulewicz, E. M., et al. (2013). Home Environment: Association with Hyperactivity/impulsivity in Children with ADHD and Their Non-ADHD Siblings. Child. Care Health Dev. 39 (2). doi:10.1111/j.1365-2214.2011.01345.x
Munawar, N., Ahsan, K., Muhammad, K., Ahmad, A., Anwar, M. A., Shah, I., et al. (2021). Hidden Role of Gut Microbiome Dysbiosis in Schizophrenia: Antipsychotics or Psychobiotics as Therapeutics? Int. J. Mol. Sci. 22 (14). doi:10.3390/ijms22147671
Muneer, A. (2020). Kynurenine Pathway of Tryptophan Metabolism in Neuropsychiatric Disorders: Pathophysiologic and Therapeutic Considerations. Clin. Psychopharmacol. Neurosci. 18 (4). doi:10.9758/CPN.2020.18.4.507
Myint, A. M., Schwarz, M. J., Verkerk, R., Mueller, H. H., Zach, J., Scharpé, S., et al. (2011). Reversal of Imbalance between Kynurenic Acid and 3-hydroxykynurenine by Antipsychotics in Medication-Naïve and Medication-free Schizophrenic Patients. Brain Behav. Immun. 25 (8). doi:10.1016/j.bbi.2011.05.005
Na, K. S., Jung, H. Y., and Kim, Y. K. (2014). The Role of Pro-inflammatory Cytokines in the Neuroinflammation and Neurogenesis of Schizophrenia. Prog. Neuro-Psychopharmacology Biol. Psychiatry 48. doi:10.1016/j.pnpbp.2012.10.022
Nankova, B. B., Agarwal, R., MacFabe, D. F., and la Gamma, E. F. (2014). Enteric Bacterial Metabolites Propionic and Butyric Acid Modulate Gene Expression, Including CREB-dependent Catecholaminergic Neurotransmission, in PC12 Cells - Possible Relevance to Autism Spectrum Disorders. PLoS ONE 9 (8). doi:10.1371/journal.pone.0103740
Naseribafrouei, A., Hestad, K., Avershina, E., Sekelja, M., Linløkken, A., Wilson, R., et al. (2014). Correlation between the Human Fecal Microbiota and Depression. Neurogastroenterology Motil. 26 (8). doi:10.1111/nmo.12378
Neuman, H., Forsythe, P., Uzan, A., Avni, O., and Koren, O. (2018). Antibiotics in Early Life: Dysbiosis and the Damage Done. FEMS Microbiol. Rev. 42 (4). doi:10.1093/femsre/fuy018
Nguyen, T. T., Kosciolek, T., Eyler, L. T., Knight, R., and Jeste, D. v. (2018). Overview and Systematic Review of Studies of Microbiome in Schizophrenia and Bipolar Disorder. J. Psychiatr. Res. 99. doi:10.1016/j.jpsychires.2018.01.013
NIH (2016). Autism Spectrum Disorder: Communication Problems in Children. Bethesda: Cdc: National Institute on Deafness and Other Communication Disorders (NIDCD).
Nitta, M., Kishimoto, T., Müller, N., Weiser, M., Davidson, M., Kane, J. M., et al. (2013). Adjunctive Use of Nonsteroidal Anti-inflammatory Drugs for Schizophrenia: A Meta-Analytic Investigation of Randomized Controlled Trials. Schizophrenia Bull. 39 (6). doi:10.1093/schbul/sbt070
Ntranos, A., and Casaccia, P. (2018). The Microbiome–Gut–Behavior Axis: Crosstalk between the Gut Microbiome and Oligodendrocytes Modulates Behavioral Responses. Neurotherapeutics 15 (1). doi:10.1007/s13311-017-0597-9
Nyangahu, D. D., Lennard, K. S., Brown, B. P., Darby, M. G., Wendoh, J. M., Havyarimana, E., et al. (2018). Disruption of Maternal Gut Microbiota during Gestation Alters Offspring Microbiota and Immunity. Microbiome 6, 124. doi:10.1186/s40168-018-0511-7
Oades, R. D., Myint, A. M., Dauvermann, M. R., Schimmelmann, B. G., and Schwarz, M. J. (2010). Attention-deficit Hyperactivity Disorder (ADHD) and Glial Integrity: An Exploration of Associations of Cytokines and Kynurenine Metabolites with Symptoms and Attention. Behav. Brain Functions 6. doi:10.1186/1744-9081-6-32
Oades, R. D. (2011). An Exploration of the Associations of Pregnancy and Perinatal Features with Cytokines and Tryptophan/Kynurenine Metabolism in Children with Attention-Deficit Hyperactivity Disorder (ADHD). ADHD Attention Deficit and Hyperactivity Disorders 3 (4), 301–318. doi:10.1007/s12402-011-0062-2
Obermeier, B., Daneman, R., and Ransohoff, R. M. (2013). Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 19 (12). doi:10.1038/nm.3407
O’Callaghan, A., and van Sinderen, D. (2016). Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 7 (6). doi:10.3389/fmicb.2016.00925
Ogbonnaya, E. S., Clarke, G., Shanahan, F., Dinan, T. G., Cryan, J. F., and O’Leary, O. F. (2015). Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol. Psychiatry 78 (4). doi:10.1016/j.biopsych.2014.12.023
Ohata, A., Usami, M., and Miyoshi, M. (2005). Short-chain Fatty Acids Alter Tight junction Permeability in Intestinal Monolayer Cells via Lipoxygenase Activation. Nutrition 21 (7–8). doi:10.1016/j.nut.2004.12.004
Olde Loohuis, L. M., Mangul, S., Ori, A. P. S., Jospin, G., Koslicki, D., Yang, H. T., et al. (2018). Transcriptome Analysis in Whole Blood Reveals Increased Microbial Diversity in Schizophrenia. Translational Psychiatry 8 (1). doi:10.1038/s41398-018-0107-9
O’Leary, O. F., Ogbonnaya, E. S., Felice, D., Levone, B. R., Conroy, L. C., Fitzgerald, P., et al. (2018). The Vagus Nerve Modulates BDNF Expression and Neurogenesis in the hippocampus. Eur. Neuropsychopharmacol. 28 (2), 307–316. doi:10.1016/j.euroneuro.2017.12.004
Oleskin, A. v., and Shenderov, B. A. (2016). Neuromodulatory Effects and Targets of the SCFAs and Gasotransmitters Produced by the Human Symbiotic Microbiota. Microb. Ecol. Health Dis. 27 (0). doi:10.3402/mehd.v27.30971
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., and Cryan, J. F. (2015). Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome axis. Behav. Brain Res. 277. doi:10.1016/j.bbr.2014.07.027
O’Shea, T. M., Joseph, R. M., Kuban, K. C. K., Allred, E. N., Ware, J., Coster, T., et al. (2014). Elevated Blood Levels of Inflammation-Related Proteins Are Associated with an Attention Problem at Age 24 Mo in Extremely Preterm Infants. Pediatr. Res. 75 (6). doi:10.1038/pr.2014.41
Owen, M. J., Sawa, A., and Mortensen, P. B. (2016). Schizophrenia. Lancet. (London: England) 388 (10039), 86–97. doi:10.1016/S0140-6736(15)01121-6
Paolicelli, R. C., Bolasco, G., Pagani, F., Maggi, L., Scianni, M., Panzanelli, P., et al. (2011). Synaptic Pruning by Microglia Is Necessary for normal Brain Development. Science 333 (6048), 1456–1458. doi:10.1126/science.1202529
Park, S., and Miller, B. J. (2020). Meta-analysis of Cytokine and C-Reactive Protein Levels in High-Risk Psychosis. Schizophrenia Res. 226. doi:10.1016/j.schres.2019.03.012
Parker, A., Fonseca, S., and Carding, S. R. (2020). Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 11 (2), 135–157. doi:10.1080/19490976.2019.1638722
Parracho, H. M. R. T., Bingham, M. O., Gibson, G. R., and McCartney, A. L. (2005). Differences between the Gut Microflora of Children with Autistic Spectrum Disorders and that of Healthy Children. J. Med. Microbiol. 54 (10). doi:10.1099/jmm.0.46101-0
Partrick, K. A., Rosenhauer, A. M., Auger, J., Arnold, A. R., Ronczkowski, N. M., Jackson, L. M., et al. (2021). Ingestion of Probiotic (Lactobacillus Helveticus and Bifidobacterium Longum) Alters Intestinal Microbial Structure and Behavioral Expression Following Social Defeat Stress. Scientific Rep. 11 (1). doi:10.1038/s41598-021-83284-z
Pärtty, A., Kalliomäki, M., Wacklin, P., Salminen, S., and Isolauri, E. (2015). A Possible Link between Early Probiotic Intervention and the Risk of Neuropsychiatric Disorders Later in Childhood: A Randomized Trial. Pediatr. Res. 77 (6). doi:10.1038/pr.2015.51
Patrono, E., Svoboda, J., and Stuchlík, A. (2021). Schizophrenia, the Gut Microbiota, and New Opportunities from Optogenetic Manipulations of the Gut-Brain axis. Behav. Brain Functions 17 (1). doi:10.1186/s12993-021-00180-2
Paul-Samojedny, M., Owczarek, A., Suchanek, R., Kowalczyk, M., Fila-Danilow, A., Borkowska, P., et al. (2011). Association Study of Interferon Gamma (IFN-γ) +874T/A Gene Polymorphism in Patients with Paranoid Schizophrenia. J. Mol. Neurosci. 43 (3). doi:10.1007/s12031-010-9442-x
Pearce, B. D. (2001). Schizophrenia and Viral Infection during Neurodevelopment: A Focus on Mechanisms. Mol. Psychiatry 6 (6). doi:10.1038/sj.mp.4000956
Peng, L., He, Z., Chen, W., Holzman, I. R., and Lin, J. (2007). Effects of Butyrate on Intestinal Barrier Function in a Caco-2 Cell Monolayer Model of Intestinal Barrier. Pediatr. Res. 61 (1). doi:10.1203/01.pdr.0000250014.92242.f3
Peng, L., Li, Z. R., Green, R. S., Holzman, I. R., and Lin, J. (2009). Butyrate Enhances the Intestinal Barrier by Facilitating Tight junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 139 (9). doi:10.3945/jn.109.104638
Perkins, D. O., Jeffries, C. D., Addington, J., Bearden, C. E., Cadenhead, K. S., Cannon, T. D., et al. (2015). Towards a Psychosis Risk Blood Diagnostic for Persons Experiencing High-Risk Symptoms: Preliminary Results from the NAPLS Project. Schizophrenia Bull. 41 (2). doi:10.1093/schbul/sbu099
Perry, V. H., Nicoll, J. A. R., and Holmes, C. (2010). Microglia in Neurodegenerative Disease. Nat. Rev. Neurol. 6 (4). doi:10.1038/nrneurol.2010.17
Pessa-Morikawa, T., Husso, A., Kärkkäinen, O., and Koistinen, V. M. (2022). Maternal Microbiota-Derived Metabolic Profile in Fetal Murine Intestine, Brain and Placenta. BMC Microbiol. 22, 46. doi:10.1186/s12866-022-02457-6
Peters, A., Veronesi, B., Calderón-Garcidueñas, L., Gehr, P., Chen, L. C., Geiser, M., et al. (2006). Translocation and Potential Neurological Effects of fine and Ultrafine Particles a Critical Update. Part. Fibre Toxicol. 3. doi:10.1186/1743-8977-3-13
Plitman, E., Iwata, Y., Caravaggio, F., Nakajima, S., Chung, J. K., Gerretsen, P., et al. (2017). Kynurenic Acid in Schizophrenia: A Systematic Review and Meta-Analysis. Schizophrenia Bull. 43 (4). doi:10.1093/schbul/sbw221
Pope, C. A., Bhatnagar, A., McCracken, J. P., Abplanalp, W., Conklin, D. J., and O’Toole, T. (2016). Exposure to Fine Particulate Air Pollution Is Associated with Endothelial Injury and Systemic Inflammation. Circ. Res. 119 (11). doi:10.1161/CIRCRESAHA.116.309279
Potgieter, M., Bester, J., Kell, D. B., and Pretorius, E. (2015). The Dormant Blood Microbiome in Chronic, Inflammatory Diseases. FEMS Microbiol. Rev. 39 (4). doi:10.1093/femsre/fuv013
Potter, E. D., Ling, Z. D., and Carvey, P. M. (1999). Cytokine-induced Conversion of Mesencephalic-Derived Progenitor Cells into Dopamine Neurons. Cel Tissue Res. 296 (2). doi:10.1007/s004410051285
Prado, E. L., and Dewey, K. G. (2014). Nutrition and Brain Development in Early Life. Nutr. Rev. 72 (4). doi:10.1111/nure.12102
Prata, J., Santos, S. G., Almeida, M. I., Coelho, R., and Barbosa, M. A. (2017). Bridging Autism Spectrum Disorders and Schizophrenia through Inflammation and Biomarkers - Pre-clinical and Clinical Investigations. J. Neuroinflammation 14 (1). doi:10.1186/s12974-017-0938-y
Prehn-Kristensen, A., Zimmermann, A., Tittmann, L., Lieb, W., Schreiber, S., Baving, L., et al. (2018). Reduced Microbiome Alpha Diversity in Young Patients with ADHD. PLoS ONE 13 (7). doi:10.1371/journal.pone.0200728
Prins, B. P., Abbasi, A., Wong, A., Vaez, A., Nolte, I., Franceschini, N., et al. (2016). Investigating the Causal Relationship of C-Reactive Protein with 32 Complex Somatic and Psychiatric Outcomes: A Large-Scale Cross-Consortium Mendelian Randomization Study. PLoS Med. 13 (6). doi:10.1371/journal.pmed.1001976
Printz, D. J., Strauss, D. H., Goetz, R., Sadiq, S., Malaspina, D., Krolewski, J., et al. (1999). Elevation of CD5+ B Lymphocytes in Schizophrenia. Biol. Psychiatry 46 (1), 110–118. doi:10.1016/S0006-3223(98)00307-2
Qi, F., Zuo, Z., Yang, J., Hu, S., Yang, Y., Yuan, Q., et al. (2017). Combined Effect of BCG Vaccination and Enriched Environment Promote Neurogenesis and Spatial Cognition via a Shift in Meningeal Macrophage M2 Polarization. J. Neuroinflammation 14, 32. doi:10.1186/s12974-017-0808-7
Qin, H., Zhang, L., Xu, G., and Pan, X. (2013). Lack of Association between TNFα Rs1800629 Polymorphism and Schizophrenia Risk: A Meta-Analysis. Psychiatry Res. 209 (3). doi:10.1016/j.psychres.2013.01.019
Ransohoff, R. M., Schafer, D., Vincent, A., Blachère, N. E., and Bar-Or, A. (2015). Neuroinflammation: Ways in Which the Immune System Affects the Brain. Neurotherapeutics 12 (4), 896–909. doi:10.1007/s13311-015-0385-3
Rao, A. V., Bested, A. C., Beaulne, T. M., Katzman, M. A., Iorio, C., Berardi, J. M., et al. (2009). A Randomized, Double-Blind, Placebo-Controlled Pilot Study of a Probiotic in Emotional Symptoms of Chronic Fatigue Syndrome. Gut Pathog. 1 (1). doi:10.1186/1757-4749-1-6
Rapaport, M. H., Torrey, E. F., McAllister, C. G., Nelson, D. L., Pickar, D., and Paul, S. M. (1993). Increased Serum Soluble Interleukin-2 Receptors in Schizophrenic Monozygotic Twins. Eur. Arch. Psychiatry Clin. Neurosci. 243 (1). doi:10.1007/BF02191517
Ratsika, A., Codagnone, M. C., O’mahony, S., Stanton, C., and Cryan, J. F. (2021). Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain axis. Nutrients 13 (2). doi:10.3390/nu13020423
Ripke, S., Neale, B. M., Corvin, A., Walters, J. T., Farh, K. H., Holmans, P. A., et al. (2014). Biological Insights from 108 Schizophrenia-Associated Genetic Loci. Nature 511 (7510), 421. doi:10.1038/nature13595
Rodrigues-Amorim, D., Rivera-Baltanás, T., Regueiro, B., Spuch, C., de las Heras, M. E., Vázquez-Noguerol Méndez, R., et al. (2018). The Role of the Gut Microbiota in Schizophrenia: Current and Future Perspectives. World J. Biol. Psychiatry 19 (8). doi:10.1080/15622975.2018.1433878
Rodríguez, N., Morer, A., González-Navarro, E. A., Serra-Pages, C., Boloc, D., Torres, T., et al. (2017). Inflammatory Dysregulation of Monocytes in Pediatric Patients with Obsessive-Compulsive Disorder. J. Neuroinflammation 14 (1). doi:10.1186/s12974-017-1042-z
Rogers, G. B., Keating, D. J., Young, R. L., Wong, M. L., Licinio, J., and Wesselingh, S. (2016). From Gut Dysbiosis to Altered Brain Function and Mental Illness: Mechanisms and Pathways. Mol. Psychiatry 21 (6). doi:10.1038/mp.2016.50
Rossen, N. G., MacDonald, J. K., de Vries, E. M., D’Haens, G. R., de Vos, W. M., Zoetendal, E. G., et al. (2015). Fecal Microbiota Transplantation as Novel Therapy in Gastroenterology: A Systematic Review. World J. Gastroenterol. 21 (17). doi:10.3748/wjg.v21.i17.5359
Rőszer, T. (2015). Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015. doi:10.1155/2015/816460
Rowland, L. M., Summerfelt, A., Wijtenburg, S. A., Du, X., Chiappelli, J. J., Krishna, N., et al. (2016). Frontal Glutamate and γ-aminobutyric Acid Levels and Their Associations with Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. JAMA Psychiatry 73 (2). doi:10.1001/jamapsychiatry.2015.2680
Rylaarsdam, L., and Guemez-Gamboa, A. (2019). Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell Neurosci. 13. doi:10.3389/fncel.2019.00385
Salvo, E., Stokes, P., Keogh, C. E., Brust-Mascher, I., Hennessey, C., Knotts, T. A., et al. (2020). A Murine Model of Pediatric Inflammatory Bowel Disease Causes Microbiota-Gut-Brain axis Deficits in Adulthood. Am. J. Physiology-Gastrointestinal Liver Physiol. 319 (3), G361–G374. doi:10.1152/ajpgi.00177.2020
Sands, B. E. (2015). Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology 149 (5). doi:10.1053/j.gastro.2015.07.003
Sarubbo, F., Cavallucci, V., and Pani, G. (2022). The Influence of Gut Microbiota on Neurogenesis: Evidence and Hopes. Cells 11 (3), 382. doi:10.3390/cells11030382
Sasayama, D., Hori, H., Teraishi, T., Hattori, K., Ota, M., Iijima, Y., et al. (2011). Possible Association between Interleukin-1beta Gene and Schizophrenia in a Japanese Population. Behav. Brain Functions 7. doi:10.1186/1744-9081-7-35
Scahill, L., Jeon, S., Boorin, S. J., McDougle, C. J., Aman, M. G., Dziura, J., et al. (2016). Weight Gain and Metabolic Consequences of Risperidone in Young Children with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 55 (5). doi:10.1016/j.jaac.2016.02.016
Schafer, D. P., Lehrman, E. K., Kautzman, A. G., Koyama, R., Mardinly, A. R., Yamasaki, R., et al. (2012). Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-dependent Manner. Neuron 74 (4). doi:10.1016/j.neuron.2012.03.026
Schwarz, E., Maukonen, J., Hyytiäinen, T., Kieseppä, T., Orešič, M., Sabunciyan, S., et al. (2018). Analysis of Microbiota in First Episode Psychosis Identifies Preliminary Associations with Symptom Severity and Treatment Response. Schizophrenia Res. 192. doi:10.1016/j.schres.2017.04.017
Sekar, A., Bialas, A. R., de Rivera, H., Davis, A., Hammond, T. R., Kamitaki, N., et al. (2016). Schizophrenia Risk from Complex Variation of Complement Component 4. Nature 530 (7589). doi:10.1038/nature16549
Senn, V., Bassler, D., Choudhury, R., Scholkmann, F., Righini-Grunder, F., Vuille-dit-Bile, R. N., et al. (2020). Microbial Colonization from the Fetus to Early Childhood—A Comprehensive Review. Front. Cell Infect. Microbiol. 10. doi:10.3389/fcimb.2020.573735
Sentürk, A., Pfennig, S., Weiss, A., Burk, K., and Acker-Palmer, A. (2011). Ephrin Bs Are Essential Components of the Reelin Pathway to Regulate Neuronal Migration. Nature 472 (7343). doi:10.1038/nature09874
Severance, E. G., Alaedini, A., Yang, S., Halling, M., Gressitt, K. L., Stallings, C. R., et al. (2012). Gastrointestinal Inflammation and Associated Immune Activation in Schizophrenia. Schizophrenia Res. 138 (1). doi:10.1016/j.schres.2012.02.025
Shah, E. D., Riddle, M. S., Chang, C., and Pimentel, M. (2012). Estimating the Contribution of Acute Gastroenteritis to the Overall Prevalence of Irritable Bowel Syndrome. J. Neurogastroenterology Motil. 18 (2). doi:10.5056/jnm.2012.18.2.200
Sharon, G., Sampson, T. R., Geschwind, D. H., and Mazmanian, S. K. (2016). The Central Nervous System and the Gut Microbiome. Cell 167 (4). doi:10.1016/j.cell.2016.10.027
Shattock, P., and Whiteley, P. (2002). Biochemical Aspects in Autism Spectrum Disorders: Updating the Opioid-Excess Theory and Presenting New Opportunities for Biomedical Intervention. Expert Opin. Ther. Targets 6 (2). doi:10.1517/14728222.6.2.175
Shen, Y., Xu, J., Li, Z., Huang, Y., Yuan, Y., Wang, J., et al. (2018). Analysis of Gut Microbiota Diversity and Auxiliary Diagnosis as a Biomarker in Patients with Schizophrenia: A Cross-Sectional Study. Schizophrenia Res. 197. doi:10.1016/j.schres.2018.01.002
Shi, J., Levinson, D. F., Duan, J., Sanders, A. R., Zheng, Y., Péer, I., et al. (2009). Common Variants on Chromosome 6p22.1 Are Associated with Schizophrenia. Nature 460 (7256). doi:10.1038/nature08192
Shibuya, M., Watanabe, Y., Nunokawa, A., Egawa, J., Kaneko, N., Igeta, H., et al. (2014). Interleukin 1 Beta Gene and Risk of Schizophrenia: Detailed Case-Control and Family-Based Studies and an Updated Meta-Analysis. Hum. Psychopharmacol. 29 (1). doi:10.1002/hup.2365
Shogo, T., and Toshihide, Y. (2018). The Role of Immune Cells in Brain Development and Neurodevelopmental Diseases. Int. Immunol. 30 (10), 437–444. doi:10.1093/intimm/dxy041
Silva, Y. P., Bernardi, A., and Frozza, R. L. (2020). The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 11. doi:10.3389/fendo.2020.00025
Sjögren, Y. M., Tomicic, S., Lundberg, A., Böttcher, M. F., Björkstén, B., Sverremark-Ekström, E., et al. (2009). Influence of Early Gut Microbiota on the Maturation of Childhood Mucosal and Systemic Immune Responses: Gut Microbiota and Immune Responses. Clin. Exp. Allergy 39 (12). doi:10.1111/j.1365-2222.2009.03326.x
Smith, S. E. P., Li, J., Garbett, K., Mirnics, K., and Patterson, P. H. (2007). Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. J. Neurosci. 27 (40). doi:10.1523/JNEUROSCI.2178-07.2007
Smith, S. M., and Vale, W. W. (2006). The Role of the Hypothalamic-Pituitary-Adrenal axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 8 (4). doi:10.31887/dcns.2006.8.4/ssmith
Socała, K., Doboszewska, U., Szopa, A., Serefko, A., Włodarczyk, M., Zielińska, A., et al. (2021). The Role of Microbiota-Gut-Brain axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 172. doi:10.1016/j.phrs.2021.105840
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermúdez-Humarán, L. G., Gratadoux, J. J., et al. (2008). Faecalibacterium Prausnitzii Is an Anti-inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. United States America 105 (43). doi:10.1073/pnas.0804812105
Sommer, I. E., de Witte, L., Begemann, M., and Kahn, R. S. (2012). Nonsteroidal Anti-inflammatory Drugs in Schizophrenia: Ready for Practice or a Good Start? A Meta-Analysis. J. Clin. Psychiatry 73 (4). doi:10.4088/JCP.10r06823
Sørensen, H. J., Mortensen, E. L., Reinisch, J. M., and Mednick, S. A. (2009). Association between Prenatal Exposure to Bacterial Infection and Risk of Schizophrenia. Schizophrenia Bull. 35 (3). doi:10.1093/schbul/sbn121
Sotgiu, S., Manca, S., Gagliano, A., Minutolo, A., Melis, M. C., Pisuttu, G., et al. (2020). Immune Regulation of Neurodevelopment at the Mother–Foetus Interface: the Case of Autism. Clin. Translational Immunol. 9 (11). doi:10.1002/cti2.1211
Spadoni, I., Zagato, E., Bertocchi, A., Paolinelli, R., Hot, E., di Sabatino, A., et al. (2015). A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 350 (6262). doi:10.1126/science.aad0135
Spangaro, M., Mazza, E., Poletti, S., Cavallaro, R., and Benedetti, F. (2018). Obesity Influences white Matter Integrity in Schizophrenia. Psychoneuroendocrinology 97. doi:10.1016/j.psyneuen.2018.07.017
Srisawasdi, P., Vanwong, N., Hongkaew, Y., Puangpetch, A., Vanavanan, S., Intachak, B., et al. (2017). Impact of Risperidone on Leptin and Insulin in Children and Adolescents with Autistic Spectrum Disorders. Clin. Biochem. 50 (12). doi:10.1016/j.clinbiochem.2017.02.003
St Clair, D., Blackwood, D., Muir, W., Walker, M., St Clair, D., Muir, W., et al. (1990). Association within a Family of a Balanced Autosomal Translocation with Major Mental Illness. The Lancet 336 (8706). doi:10.1016/0140-6736(90)91520-K
Steiner, J., Bielau, H., Brisch, R., Danos, P., Ullrich, O., Mawrin, C., et al. (2008). Immunological Aspects in the Neurobiology of Suicide: Elevated Microglial Density in Schizophrenia and Depression Is Associated with Suicide. J. Psychiatr. Res. 42 (2). doi:10.1016/j.jpsychires.2006.10.013
Steiner, J., Jacobs, R., Panteli, B., Brauner, M., Schiltz, K., Bahn, S., et al. (2010). Acute Schizophrenia Is Accompanied by Reduced T Cell and Increased B Cell Immunity. Eur. Arch. Psychiatry Clin. Neurosci. 260, 509–518. doi:10.1007/s00406-010-0098-x
Stevens, B., Allen, N. J., Vazquez, L. E., Howell, G. R., Christopherson, K. S., Nouri, N., et al. (2007). The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 131 (6), 1164–1178. doi:10.1016/j.cell.2007.10.036
Stinson, L. F., Boyce, M. C., Payne, M. S., and Keelan, J. A. (2019). The Not-so-sterile Womb: Evidence that the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 10 (JUN). doi:10.3389/fmicb.2019.01124
Strandwitz, P. (2018). Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 1693. doi:10.1016/j.brainres.2018.03.015
Strati, F., Cavalieri, D., Albanese, D., de Felice, C., Donati, C., Hayek, J., et al. (2017). New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 5 (1). doi:10.1186/s40168-017-0242-1
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X.-N., et al. (2004). Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 558, 263–275. doi:10.1113/jphysiol.2004.063388
Sudo, N. (2012). Role of Microbiome in Regulating the HPA axis and its Relevance to Allergy. Chem. Immunol. Allergy 98. doi:10.1159/00033651013
Suzuki, K., Matsuzaki, H., Iwata, K., Kameno, Y., Shimmura, C., Kawai, S., et al. (2011). Plasma Cytokine Profiles in Subjects with High-Functioning Autism Spectrum Disorders. PLoS ONE 6 (5). doi:10.1371/journal.pone.0020470
Teff, K. L., Rickels, M. R., Grudziak, J., Fuller, C., Nguyen, H. L., and Rickels, K. (2013). Antipsychotic-induced Insulin Resistance and Postprandial Hormonal Dysregulation Independent of Weight Gain or Psychiatric Disease. Diabetes 62 (9). doi:10.2337/db13-0430
Tomova, A., Husarova, V., Lakatosova, S., Bakos, J., Vlkova, B., Babinska, K., et al. (2015). Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 138. doi:10.1016/j.physbeh.2014.10.033
Tremblay, M. Ě., Lowery, R. L., and Majewska, A. K. (2010). Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol. 8 (11). doi:10.1371/journal.pbio.1000527
Trépanier, M. O., Hopperton, K. E., Mizrahi, R., Mechawar, N., and Bazinet, R. P. (2016). Postmortem Evidence of Cerebral Inflammation in Schizophrenia: A Systematic Review. Mol. Psychiatry 21 (8). doi:10.1038/mp.2016.90
Tye, C., Runicles, A., Whitehouse, A. J. O., and Alvares, G. A. (2019). Corrigendum: Characterizing the Interplay between Autism Spectrum Disorder and Comorbid Medical Conditions: An Integrative Review. Front. Psychiatry 10 (6). doi:10.3389/fpsyt.2019.00438
Ueno, M., Fujita, Y., Tanaka, T., Nakamura, Y., Kikuta, J., Ishii, M., et al. (2013). Layer V Cortical Neurons Require Microglial Support for Survival during Postnatal Development. Nat. Neurosci. 16, 543–551. doi:10.1038/nn.3358
Ulluwishewa, D., Anderson, R. C., McNabb, W. C., Moughan, P. J., Wells, J. M., and Roy, N. C. (2011). Regulation of Tight junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 141 (5). doi:10.3945/jn.110.135657
Upthegrove, R., Manzanares-Teson, N., and Barnes, N. M. (2014). Cytokine Function in Medication-Naive First Episode Psychosis: A Systematic Review and Meta-Analysis. Schizophrenia Res. 155 (1–3). doi:10.1016/j.schres.2014.03.005
van de Wouw, M., Boehme, M., Lyte, J. M., Wiley, N., Strain, C., O’Sullivan, O., et al. (2018). Short-chain Fatty Acids: Microbial Metabolites that Alleviate Stress-Induced Brain–Gut axis Alterations. J. Physiol. 596 (20). doi:10.1113/JP276431
van Kesteren, C. F. M. G., Gremmels, H., de Witte, L. D., Hol, E. M., van Gool, A. R., Falkai, P. G., et al. (2017). Immune Involvement in the Pathogenesis of Schizophrenia: A Meta-Analysis on Postmortem Brain Studies. Translational Psychiatry 7 (3). doi:10.1038/tp.2017.4
Van Os, J., and Kapur, S. (2009). Schizophrenia. Lancet. London: England 374 (9690), 635–645. doi:10.1016/S0140-6736(09)60995-8
Vangay, P., Ward, T., Gerber, J. S., and Knights, D. (2015). Antibiotics, Pediatric Dysbiosis, and Disease. Cell Host and Microbe 17 (5). doi:10.1016/j.chom.2015.04.006
Ventriglio, A., Gentile, A., Stella, E., and Bellomo, A. (2015). Metabolic Issues in Patients Affected by Schizophrenia: Clinical Characteristics and Medical Management. Front. Neurosci. 9 (9). doi:10.3389/fnins.2015.00297
Villagomez, A. N., Muñoz, F. M., Peterson, R. L., Colbert, A. M., Gladstone, M., MacDonald, B., et al. (2019). Neurodevelopmental Delay: Case Definition and Guidelines for Data Collection, Analysis, and Presentation of Immunization Safety Data. Vaccine 37 (52), 7623–7641. doi:10.1016/j.vaccine.2019.05.027
Vitale, G., Barbaro, F., Ianiro, G., Cesario, V., Gasbarrini, G., Franceschi, F., et al. (2011). Nutritional Aspects of Helicobacter pylori Infection. Minerva Gastroenterologica e Dietologica 57 (4).
Vuksan-Ćusa, B., Šagud, M., and Jakovljević, M. (2010). C-reactive Protein and Metabolic Syndrome in Patients with Bipolar Disorder Compared to Patients with Schizophrenia. Psychiatria Danubina 22 (2).
Wampach, L., Heintz-Buschart, A., Hogan, A., Muller, E. E. L., Narayanasamy, S., Laczny, C. C., et al. (2017). Colonization and Succession within the Human Gut Microbiome by Archaea, Bacteria, and Microeukaryotes during the First Year of Life. Front. Microbiol. 8 (MAY). doi:10.3389/fmicb.2017.00738
Wang, L., Christophersen, C. T., Sorich, M. J., Gerber, J. P., Angley, M. T., and Conlon, M. A. (2011). Low Relative Abundances of the Mucolytic Bacterium Akkermansia Muciniphila and Bifidobacterium Spp. In Feces of Children with Autism. Appl. Environ. Microbiol. 77 (18). doi:10.1128/AEM.05212-11
Wei, H., Alberts, I., and Li, X. (2013). Brain IL-6 and Autism. Neuroscience 252. doi:10.1016/j.neuroscience.2013.08.025
Wei, H., Chadman, K. K., McCloskey, D. P., Sheikh, A. M., Malik, M., Brown, W. T., et al. (2012). Brain IL-6 Elevation Causes Neuronal Circuitry Imbalances and Mediates Autism-like Behaviors. Biochim. Biophys. Acta - Mol. Basis Dis. 1822 (6). doi:10.1016/j.bbadis.2012.01.011
Wei, H., Zou, H., Sheikh, A. M., Malik, M., Dobkin, C., Brown, W. T., et al. (2011). IL-6 Is Increased in the Cerebellum of Autistic Brain and Alters Neural Cell Adhesion, Migration and Synaptic Formation. J. Neuroinflammation 8. doi:10.1186/1742-2094-8-52
White, B. E. P., and Getsios, S. (2014). Eph Receptor and Ephrin Function in Breast, Gut, and Skin Epithelia. Cell Adhes. Migration 8 (4). doi:10.4161/19336918.2014.970012
Wilens, T. E., and Spencer, T. J. (2010). Understanding Attention-Deficit/hyperactivity Disorder from Childhood to Adulthood. Postgrad. Med. 122 (5). doi:10.3810/pgm.2010.09.2206
Williams, B. L., Hornig, M., Parekh, T., and Ian Lipkin, W. (2012). Application of Novel PCR-Based Methods for Detection, Quantitation, and Phylogenetic Characterization of Sutterella Species in Intestinal Biopsy Samples from Children with Autism and Gastrointestinal Disturbances. MBio 3 (1). doi:10.1128/mBio.00261-11
Williams, S., Chen, L., Savignac, H. M., Tzortzis, G., Anthony, D. C., and Burnet, P. W. (2016). Neonatal Prebiotic (BGOS) Supplementation Increases the Levels of Synaptophysin, GluN2A-Subunits and BDNF Proteins in the Adult Rat hippocampus. Synapse 70 (3). doi:10.1002/syn.21880
Williamson, J. M., and Lyons, D. A. (2018). Myelin Dynamics throughout Life: An Ever-Changing Landscape? Front. Cell Neurosci. 12. doi:10.3389/fncel.2018.00424
Winter, C., Djodari-Irani, A., Sohr, R., Morgenstern, R., Feldon, J., Juckel, G., et al. (2009). Prenatal Immune Activation Leads to Multiple Changes in Basal Neurotransmitter Levels in the Adult Brain: Implications for Brain Disorders of Neurodevelopmental Origin Such as Schizophrenia. Int. J. Neuropsychopharmacol. 12 (4). doi:10.1017/S1461145708009206
Woodward, N. C., Levine, M. C., Haghani, A., Shirmohammadi, F., Saffari, A., Sioutas, C., et al. (2017). Toll-like Receptor 4 in Glial Inflammatory Responses to Air Pollution In Vitro and In Vivo. J. Neuroinflammation 14 (1). doi:10.1186/s12974-017-0858-x
Wu, D., Lv, P., Li, F., Zhang, W., Fu, G., Dai, J., et al. (2019). Association of Peripheral Cytokine Levels with Cerebral Structural Abnormalities in Schizophrenia. Brain Res. 1724. doi:10.1016/j.brainres.2019.146463
Xie, J., Huang, L., Li, X., Li, H., Zhou, Y., Zhu, H., et al. (2017). Immunological Cytokine Profiling Identifies TNF-α as a Key Molecule Dysregulated in Autistic Children. Oncotarget 8 (47). doi:10.18632/oncotarget.19326
Xu, M., and He, L. (2010). Convergent Evidence Shows a Positive Association of Interleukin-1 Gene Complex Locus with Susceptibility to Schizophrenia in the Caucasian Population. Schizophrenia Res. 120 (1–3). doi:10.1016/j.schres.2010.02.1031
Xu, M., Xu, X., Li, J., and Li, F. (2019). Association between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 10 (7). doi:10.3389/fpsyt.2019.00473
Xu, R., Wu, B., Liang, J., He, F., Gu, W., Li, K., et al. (2020). Altered Gut Microbiota and Mucosal Immunity in Patients with Schizophrenia. Brain Behav. Immun. 85, 120–127. doi:10.1016/j.bbi.2019.06.039
Yamamoto, S., Muramatsu, M., Azuma, E., Ikutani, M., Nagai, Y., Sagara, H., et al. (2017). A Subset of Cerebrovascular Pericytes Originates from Mature Macrophages in the Very Early Phase of Vascular Development in CNS. Sci. Rep. 7, 3855. doi:10.1038/s41598-017-03994-1
Yap, C. X., Henders, A. K., Alvares, G. A., Wood, D. L. A., Krause, L., Tyson, G. W., et al. (2021). Autism-related Dietary Preferences Mediate Autism-Gut Microbiome Associations. Cell 184 (24), 5916–5931. e17. doi:10.1016/j.cell.2021.10.015
Yap, I. K. S., and Martin, F. P. (2015). “Deciphering the Gut Microbial Contribution to the Etiology of Autism Development,” in Molecular and Integrative Toxicology (Berlin/Heidelberg: Springer Science+Business Media), 311–322. doi:10.1007/978-1-4471-6539-2_14
Yarandi, S. S., Kulkarni, S., Saha, M., Sylvia, K. E., Sears, C. L., and Pasricha, P. J. (2020). Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice. Gastroenterology 159 (1), 200–213. doi:10.1053/j.gastro.2020.03.050
Yeh, T. C., Bai, Y. M., Tsai, S. J., Chen, T. J., Liang, C. S., and Chen, M. H. (2021). Risks of Major Mental Disorders and Irritable Bowel Syndrome Among the Offspring of Parents with Irritable Bowel Syndrome: A Nationwide Study. Int. J. Environ. Res. Public Health 18 (9). doi:10.3390/ijerph18094679
Yilmaz, Y., Gul, C. B., Arabul, M., and Eren, M. A. (2008). Helicobacter pylori: A Role in Schizophrenia? Med. Sci. Monitor 14 (7).
Younge, N., McCann, J. R., Ballard, J., Plunkett, C., Akhtar, S., Araújo-Pérez, F., et al. (2019). Fetal Exposure to the Maternal Microbiota in Humans and Mice. JCI Insight 4 (19). doi:10.1172/jci.insight.127806
Yuan, X., Kang, Y., Zhuo, C., Huang, X. F., and Song, X. (2019). The Gut Microbiota Promotes the Pathogenesis of Schizophrenia via Multiple Pathways. Biochem. Biophysical Res. Commun. 512 (2). doi:10.1016/j.bbrc.2019.02.152
Yuan, X., Zhang, P., Wang, Y., Liu, Y., Li, X., Kumar, B. U., et al. (2018). Changes in Metabolism and Microbiota after 24-week Risperidone Treatment in Drug Naïve, normal Weight Patients with First Episode Schizophrenia. Schizophrenia Res. 201. doi:10.1016/j.schres.2018.05.017
Zalcman, S., Green-Johnson, J. M., Murray, L., Nance, D. M., Dyck, D., Anisman, H., et al. (1994). Cytokine-specific central Monoamine Alterations Induced by Interleukin-1, - 2 and - 6. Brain Res. 643 (1–2). doi:10.1016/0006-8993(94)90006-X
Zarrindast, M. R., and Khakpai, F. (2015). The Modulatory Role of Dopamine in Anxiety-like Behavior. Arch. Iranian Med. 18 (9), 0151809.
Zeng, M., Inohara, N., and Nuñez, G. (2017). Mechanisms of Inflammation-Driven Bacterial Dysbiosis in the Gut. Mucosal Immunol. 10, 18–26. doi:10.1038/mi.2016.75
Zeni-Graiff, M., Rizzo, L. B., Mansur, R. B., Maurya, P. K., Sethi, S., Cunha, G. R., et al. (2016). Peripheral Immuno-Inflammatory Abnormalities in Ultra-high Risk of Developing Psychosis. Schizophrenia Res. 176 (2–3). doi:10.1016/j.schres.2016.06.031
Zhang J, J., Luo, W., Huang, P., Peng, L., and Huang, Q. (2018). Maternal C-Reactive Protein and Cytokine Levels during Pregnancy and the Risk of Selected Neuropsychiatric Disorders in Offspring: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 105. doi:10.1016/j.jpsychires.2018.09.002
Zhang M, M., Ma, W., Zhang, J., He, Y., and Wang, J. (2018). Analysis of Gut Microbiota Profiles and Microbe-Disease Associations in Children with Autism Spectrum Disorders in China. Scientific Rep. 8 (1). doi:10.1038/s41598-018-32219-2
Zhang, Y., Hodgson, N. W., Trivedi, M. S., Abdolmaleky, H. M., Fournier, M., Cuenod, M., et al. (2016). Decreased Brain Levels of Vitamin B12 in Aging, Autism and Schizophrenia. PLoS ONE 11 (1). doi:10.1371/journal.pone.0146797
Zheng, P., Zeng, B., Liu, M., Chen, J., Pan, J., Han, Y., et al. (2019). The Gut Microbiome from Patients with Schizophrenia Modulates the Glutamate-Glutamine-GABA Cycle and Schizophrenia-Relevant Behaviors in Mice. Sci. Adv. 5 (2). doi:10.1126/sciadv.aau8317
Zhou, D., Kusnecov, A. W., Shurin, M. R., Depaoli, M., and Rabin, B. S. (1993). Exposure to Physical and Psychological Stressors Elevates Plasma Interleukin 6: Relationship to the Activation of Hypothalamic-Pituitary-Adrenal axis. Endocrinology 133 (6). doi:10.1210/endo.133.6.8243274
Zhou, Y., Xu, H., Xu, J., Guo, X., Zhao, H., Chen, Y., et al. (2021). F. Prausnitzii and its Supernatant Increase SCFAs-Producing Bacteria to Restore Gut Dysbiosis in TNBS-Induced Colitis. AMB Express 11 (1). doi:10.1186/s13568-021-01197-6
Zhu, F., Guo, R., Wang, W., Ju, Y., Wang, Q., Ma, Q., et al. (2020b). Transplantation of Microbiota from Drug-free Patients with Schizophrenia Causes Schizophrenia-like Abnormal Behaviors and Dysregulated Kynurenine Metabolism in Mice. Mol. Psychiatry 25, 2905–2918. doi:10.1038/s41380-019-0475-4
Zhu, F., Ju, Y., Wang, W., Wang, Q., Guo, R., Ma, Q., et al. (2020a). Metagenome-wide Association of Gut Microbiome Features for Schizophrenia. Nat. Commun. 11 (1). doi:10.1038/s41467-020-15457-9
Ziv, Y., Ron, N., Butovsky, O., Landa, G., Sudai, E., Greenberg, N., et al. (2006). Immune Cells Contribute to the Maintenance of Neurogenesis and Spatial Learning Abilities in Adulthood. Nat. Neurosci. 9, 268–275. doi:10.1038/nn1629
Keywords: gut microbiome, inflammation, autism spectrum disorder, attention-deficit/hyperactivity disorder, schizophrenia, neurodevelopmental psychiatric disorder, drug resistance, probiotics
Citation: Dash S, Syed YA and Khan MR (2022) Understanding the Role of the Gut Microbiome in Brain Development and Its Association With Neurodevelopmental Psychiatric Disorders. Front. Cell Dev. Biol. 10:880544. doi: 10.3389/fcell.2022.880544
Received: 21 February 2022; Accepted: 28 March 2022;
Published: 14 April 2022.
Edited by:
Chunliang Xu, The Chinese University of Hong Kong, ChinaReviewed by:
Liyang Ma, Albert Einstein College of Medicine, United StatesCopyright © 2022 Dash, Syed and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mojibur R. Khan, bW9qaWJ1ci5raGFuQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.