
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol. , 25 April 2022
Sec. Molecular and Cellular Reproduction
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.850052
This article is part of the Research Topic The Role of Calcium Signaling in Gametogenesis and Early Embryogenesis View all 8 articles
 Haixia Chen1†
Haixia Chen1† Peng Li2†
Peng Li2† Xiaoling Du2
Xiaoling Du2 Yiding Zhao3
Yiding Zhao3 Lingling Wang2
Lingling Wang2 Ye Tian1
Ye Tian1 Xueru Song1
Xueru Song1 Ling Shuai3*
Ling Shuai3* Xiaohong Bai1*
Xiaohong Bai1* Lingyi Chen2*
Lingyi Chen2*The SEPTIN12 gene has been associated with male infertility. Male Septin12+/− chimera mice were infertile, supporting the prevailing view that SEPTIN12 haploinsufficiency causes male infertility. In this study, we identified a heterozygous mutation on SEPTIN12, c.72C>A (p.Cys24Ter) in the male partner of a patient couple, who had a previous fertilization failure (FF) after intracytoplasmic sperm injection (ICSI) and became pregnant after ICSI together with artificial oocyte activation (AOA). To investigate the role of SEPTIN12 in FF and oocyte activation, we constructed Septin12 knockout mice. Surprisingly, Septin12−/− male mice, but not Septin12+/− male mice, are infertile, and have reduced sperm counts and abnormal sperm morphology. Importantly, AOA treatment enhances the 2-cell embryo rate of ICSI embryos injected with Septin12−/− sperm, indicating that FF caused by male Septin12 deficiency is overcome by AOA. Mechanistically, loss of PLCζ around the acrosome might be the reason for FF of Septin12−/− sperm. Taken together, our data indicated that homozygous knockout of Septin12, but not Septin12 haploinsufficiency, leads to male infertility and FF.
Infertility is a worldwide health problem affecting about 15% reproductive-aged couples (Agarwal et al., 2015; Yeste et al., 2016). Assisted reproduction techniques (ARTs), especially intracytoplasmic sperm injection (ICSI), have allowed severe infertility couples to conceive. The procedure of ICSI is to inject a sperm into the oocyte cytoplasm, thus bypassing several key steps during fertilization, such as acrosome reaction, membrane fusion, and penetration into the oocyte. Even though the average fertilization rate in ICSI is around 70%, total fertilization failure still occurs in 1–3% of ICSI cycles (Van Steirteghem et al., 1993; Flaherty et al., 1998; Mahutte and Arici, 2003; Esfandiari et al., 2005). It has been revealed that more than 80% of unfertilized oocytes after ICSI are arrested at the metaphase II (MII), likely due to oocyte activation failure (OAF) (Flaherty S. P. et al., 1995; Flaherty S. et al., 1995; Flaherty et al., 1998). Thus, artificial oocyte activation (AOA), which triggers calcium oscillation by mechanical, electrical, or chemical stimuli, is applied to treat fertilization failure (FF) after ICSI. ICSI together with AOA (ICSI-AOA) improves reproductive outcomes in patients with previous FF (Sfontouris et al., 2015). Yet, not all patients benefit from AOA. Particularly, patients with oocyte-related activation deficiency show a less beneficial response to AOA treatment (Nasr-Esfahani et al., 2008; Vanden Meerschaut et al., 2012; Ferrer-Buitrago et al., 2018).
Obviously, identification of genetic defects responsible for FF allows us to accurately predict reproductive outcomes of AOA. PLCζ has been identified as a sperm oocyte-activating factor (Saleh et al., 2020). Injection of PLCζ mRNA into mouse oocytes activates calcium oscillation. Conversely, depletion of PLCζ in sperm extracts reduces their ability to induce calcium oscillation in oocytes (Cox et al., 2002; Saunders et al., 2002). The absence, abnormal localization, and genetic mutations of PLCζ in sperms have been identified in patients with low or total FF after ICSI (Yoon et al., 2008; Nomikos et al., 2011; Yelumalai et al., 2015; Escoffier et al., 2016; Nazarian et al., 2019; Torra-Massana et al., 2019; Dai et al., 2020; Mu et al., 2020; Yan et al., 2020; Yuan et al., 2020). It is highly possible that AOA treatment may efficiently improve reproductive outcomes for these patients. However, PLCζ defects are detected in only a subset of patients with low or total FF after ICSI, suggesting that additional genes involved in FF remain to be discovered.
SEPTIN12 belongs to the SEPTIN family which are GTP-binding proteins with unique filament forming capabilities. It is expressed specifically in testis, and located in the neck and annulus regions of mature sperm (Shen et al., 2017). SEPTIN12 forms complexes with other SEPTINs, 1, 2, 10 and 11 at the sperm neck, and 1, 4, 6 and 7 at the annulus, and is essential for the assembly of the connecting pieces and the annulus (Toure et al., 2011; Shen et al., 2017; Shen et al., 2020). It was first identified as a down-regulated gene in the testicular tissue of infertile men (Lin et al., 2006). Various mutations of SEPTIN12 have been identified in infertile males, implying SEPTIN12 as a male infertility gene (Kuo et al., 2012; Lin et al., 2012; Miyamoto et al., 2012; Geng et al., 2019; Rafaee et al., 2020). Moreover, it has been reported that male Septin12+/− chimera mice are infertile, indicating that haploinsufficiency of Septin12 may lead to male infertility (Lin et al., 2009).
In this study, a couple with previous total FF after ICSI became pregnant after ICSI-AOA. A heterozygous mutation on SEPTIN12, c.72C>A; p.Cys24Ter, was identified in the male patient through whole exome sequencing. To study the function and mechanism of SEPTIN12 in oocyte activation, Septin12 knockout (KO) mice were generated. In contrast to previously reported infertile male Septin12+/− chimera mice (Lin et al., 2009), our male Septin12+/− mice are fertile. Only Septin12−/− male mice, with reduced sperm count and defective sperms, are infertile. The 2-cell embryo rate of ICSI embryos injected with Septin12−/− sperm increases from about 15 to 40% after AOA treatment. We further demonstrated that the acrosomal distribution of PLCζ is diminished in Septin12−/− spermatozoa, providing a possible explanation for why Septin12−/− sperm fails to activate oocyte after ICSI. In summary, complete loss of Septin12, but not haploinsufficiency of Septin12, causes FF and infertility. ICSI-AOA may overcome the FF caused by male Septin12 deficiency.
The female was 41-year-old and had been diagnosed with primary infertility for 3 years; BMI: 21.8 kg/m2; basal endocrine levels are normal; chromosome phenotype was 46XX. The male was 41 and diagnosed with oligospermia; BMI: 23.5 kg/m2, chromosome phenotype was 46XY; No Y-chromosome micro-deletions were detected; DNA fragment index (DFI) was 20.13%; high DNA stain-ability (HDS) was 18.38%.
For human ICSI procedure, cumulus stripping was performed 2 h after oocyte retrieval to examine oocyte maturation. Denudation of cumulus cells was performed by the exposure of oocytes to HYASETM (Vitrolife, Sweden) for a maximum of 60 s. Denudation of cumulus cells was performed by the use of glass tube slightly. The oocytes were washed three times in G-MOPS-Plus (Vitrolife, Sweden) after denudation. Only Metaphase II (MII) oocytes were inseminated by ICSI using the partner’s spermatozoa.
Human ICSI-AOA was performed 4–6 h later after oocytes retrieval. Once ICSI finished, oocytes were incubated in culture medium for 10 min and then transfer to culture medium containing 10 μM calcium ionophore A23187 (Sigma, United States) for 10 min at 37°C and 6.0% CO2. The oocytes were then washed twice in culture medium and finally placed in culture medium (G-1 Plus, Vitrolife Sweden) in the incubator at 37°C and 6.0% CO2.
Mouse ICSI was performed as previously described with slight modification (Li et al., 2012). Briefly, mature oocytes at MII stage were collected from 8-week-old CD-1 female mice by hormone administration. Spermatids minced from the epididymis of C57 sex-matured male mice were decapitated, and prepared in M2 medium (Sigma, M7167) for the subsequent injection. For injection, sperm head suspension and oocytes were manipulated in M2 medium covered with mineral oil (Sigma, M8410). Each sperm head was injected into one oocyte with an 8-μm injection needle using a Piezo-drill equipment (Prime Tech, PMAS-C7150). Reconstructed embryos were further cultured in KSOM-AA medium (Millipore, MR-020P-5F) at 37°C, 5% CO2. For artificial oocyte activation, the reconstructed embryos were activated by 10 mM SrCl2 (Sigma, 255521) in calcium-free CZB medium (Shuai et al., 2014) supplemented with 5 μg/ml of cytochalasin B (MCE, HY-16928) at 37°C, 5% CO2 for 6 h. The activated embryos were transferred to KSOM-AA medium at 37°C, 5% CO2 for further culture. The development efficiency of embryos was calculated every day until blastocyst stage.
Fertilization was scored 16–18 h after injection and insemination. Normal fertilization was when two pronuclei appear, abnormal fertilization when less or more than two pronuclei appear. The embryos were evaluated 24 h later (42 h after insemination or injection) by laboratory staff member. Embryos were cultured in the Vitrolife series of culture medium drops covered with mineral oil. Embryo transfer was performed on day 3 after injection. Luteal phase support with progesterone was administered from the day following oocyte retrieval until the day of the pregnancy test.
Whole exome sequencing was performed by AEGICARE (Shenzhen, China). Briefly, genomic DNA was isolated from peripheral blood samples using DNA extraction kit. Exon sequences were enriched and subjected to DNA sequencing on Illumina HiSeq 2000. Sequencing depth reached 123×. Copy number variation was analyzed with the Weaver (AEGICARE, Shenzhen, China). The reference genome GRCh37 was used for sequence alignment. The sequencing data is available in sequence read archive (SRA, accession number PRJNA753965).
The mutation site in the exon 2 of SEPTIN12 was verified by PCR and Sanger sequencing. The DNA fragment was amplified with primers 5′-gcagctcctggaagc-3′ and 5′-ggctatgaagatggggttt-3′.
The Septin12 KO mice were constructed by Cyagen (Guangzhou, China). The sgRNAs targeting Septin12 and Cas9 mRNA were co-injected into mouse zygotes to generate targeted KO offspring. The genotype was determined by PCR with the following primers. Forward primer: 5′- tcagagtaacccctctgagcc-3′; reverse primer 1 (R1): 5′-atttaatttcagccctcctgtgag-3′; reverse primer 2 (R2): 5′-gctctatacctaacgtcctgtgg-3′. Septin12−/− mice were obtained by mating between Septin12+/− mice.
The testis and epididymis were dissected immediately after euthanasia, fixed in 4% paraformaldehyde (PFA) for up to 24 h, dehydrated from 100% ethanol to 70% ethanol, and embedded in paraffin. The embedded tissues were cut into 5 μm sections, and mounted on glass slides. The sliced sections were deparaffinized, rehydrated, and stained with hematoxylin solution (Biosharp, BL702A) and 0.5% eosin (Solarbio, 15086-94-9) for histological examination.
For immunofluorescence assay of tissue sections, deparaffinizated sections described above were washed in phosphate-buffer saline (PBS) for 5 min. These sections were boiled in sodium citrate buffer for antigen retrieval for 15 min, and blocked in 5% BSA for 45 min. For immunofluorescence of spermatozoa, slides were treated with 3-Aminopropyl (APES, Sigma, 919-30-2) in advance. 50 μl of sperms at 1 × 106/ml was added onto the APES treated slides. Slides were fixed in 4% PFA, and blocked in 5% BSA supplemented with 0.3% Triton X-100 for 30 min. Slides were incubated with primary antibody, anti-SEPTIN12 (1:100, Abnova, H00124404-M), or anti-PLCζ (1:100, Abcam, ab124446), overnight at 4°C, and then detected by Alexa Fluor 488-AffiniPure Goat Anti-Mouse IgG (Jackson, 115-545-003) or Alexa Fluor 488-AffiniPure Goat Anti-Rabbit IgG (111-545-003). Hoechst 33342 was used for nucleus staining. Images were captured by Zeiss microscope with CCD.
For acrosome staining of tissue sections, after blocking in 5% BSA, slides were incubated with Alexa Fluor 488-lectin-PNA (20 μg/ml, Invitrogen, L21409) for 30 min, and then washed by PBS for three times. For acrosome staining of spermatozoa, slides were fixed by 4% PFA, permeabilized with 1% Triton X-100 for 5 min, incubated with 20 μg/ml Alexa Fluor 488-lectin-PNA at 37°C for 30 min, and then washed by PBS.
The cauda epididymis was dissected from adult mice. Sperms were extruded from the cauda epididymis and incubated for 15 min at 37°C in Human Tubal Fluid (HTF) medium. Hemocytometer was used for counting. For mouse motility analysis, a microscope (OLYMPUS, CX41) with the CASA system (Beijing Suijia) was used. At least 200 tracks were measured for each group.
Oocytes were prepared as described in mouse ICSI procedure. Mature oocytes were treated with 0.3 mg/ml hyaluronidase for 5 min at 37°C, and then washed twice with M2 medium. Oocytes were incubated in M2 drops supplemented with 5 μM Fluo-4 AM (Invitrogen, F14217) at 37°C for 30 min, and then washed twice with M2 medium. Oocytes were incubated in M2 drops at 37°C for 15 min to remove excess dye. At the same time, sperms were isolated from the epididymis as described in mouse ICSI procedure. After ICSI, embryos were transferred into a KSOM drop covered by mineral oil, and imaged with live cell imaging microscope (Leica, AF7000) every 30 s and continuously for 1 h.
All data are represented as means ± SD. Statistical differences were analyzed through Student’s t test. p-values were considered significant at *p < 0.05; **p < 0.01; ***p < 0.001.
A couple, who had a failed ICSI attempt in another hospital, came to our reproductive medicine center for fertility treatment. The female was diagnosed with primary infertility for 3 years, and the male was diagnosed with oligospermia (Table 1). In the previous failed cycle, twenty MII oocytes were retrieved and then inseminated by ICSI. None of the twenty MII oocytes were fertilized. In our center, thirty-seven oocytes were obtained, among which thirty oocytes were at the MII stage. Considering the history of complete FF, thirty MII oocytes were randomly divided into two groups, and inseminated by conventional ICSI and ICSI-AOA respectively. In the ICSI group, only 1 out of 17 oocytes had two pronuclei, and this fertilized oocyte failed to develop to Day 5. In contrast, in the ICSI-AOA group, 12 out of 13 oocytes were fertilized, and 8 out of these 12 embryos developed to the 8-cell stage (Table 2). After two 8-cell embryos were transferred in uterus, the female patient was successfully pregnant and gave birth to a child.
To identify the genetic defect responsible for the OAF in the couple, whole exome sequencing was performed. No pathogenic copy number variation was detected in the exomes of the couple. We then looked for mutations on infertility genes. Four heterozygous mutations in SEPTIN12, FSIP2, CFAP69, and CEP19, in the male patient, and two heterozygous mutations in MSH5 and MCM8, in the female patient, were identified (Table 3). Among these mutated infertility genes, mutations of FSIP2, CFAP69, CEP19, MSH5 or MCM8 are recessive for spermatogenic failure or premature ovarian insufficiency (Guo et al., 2017; Dong et al., 2018; Martinez et al., 2018; Yildiz Bolukbasi et al., 2018; He et al., 2019; Liu et al., 2019; Zhang et al., 2020). Thus, the heterozygous mutations of these five genes are unlikely to be the causative mutation for the OAF phenotype. In contrast, given that male Septin12+/− chimera mice are infertile (Lin et al., 2009), haploinsufficiency of Septin12 was believed to cause male infertility. Therefore, we further investigated the role of SEPTIN12 in OAF after ICSI.
We first validated the heterozygous variant on SEPTIN12, c.72C>A, identified in the male patient, by PCR and Sanger sequencing (Figure 1A). This variant changes the cysteine codon (TGC) at position 24 to a stop codon (TGA) (p.Cys24Ter), leading to the deletion of SEPTIN12.
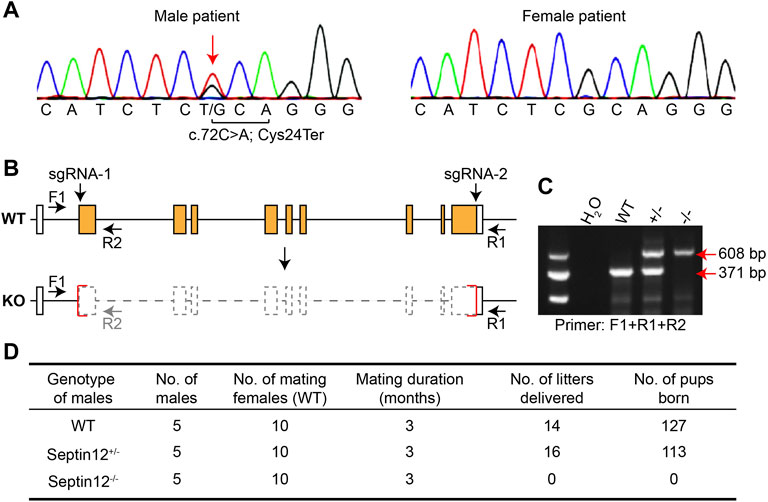
FIGURE 1. Male infertility of Septin12−/− mice. (A) Sequencing chromatograph showing the c.72C>A mutation on SEPTIN12 in the male patient. (B) Schematic illustration of the strategy for knocking out the Septin12 gene by CRISPR/Cas9. Genotyping primers F1, R1, and R2 are marked by arrows. (C) Genotyping of WT, Septin12+/−, and Septin12−/− mice by PCR. A 371 bp band is amplified from the WT allele with primers F1 and R1, while a 608 bp band is amplified from the KO allele with primers F1 and R2. (D) Male fertility of WT, Septin12+/−, and Septin12−/− mice.
To investigate the role of SEPTIN12 in OAF, we established Septin12 knockout mice using CRISPR/Cas9, and the genotypes of mice were determined by PCR (Figures 2B,C). Nearly the whole open reading frame of Septin12 is deleted in the KO allele (Figure 2B), thus mimicking the deletion of SEPTIN12 in the male patient. To our surprise, male Septin12+/− mice are fertile. However, no pups were born through the mating between Septin12−/− male and WT female mice, indicating male infertility of Septin12−/− mice (Figure 2D). These data are seemly conflicted with the infertility in the male patient with the heterozygous c.72C>A SEPTIN12 mutation and male Septin12+/− chimeric mice (Lin et al., 2009). We further discuss this issue in the later sections.
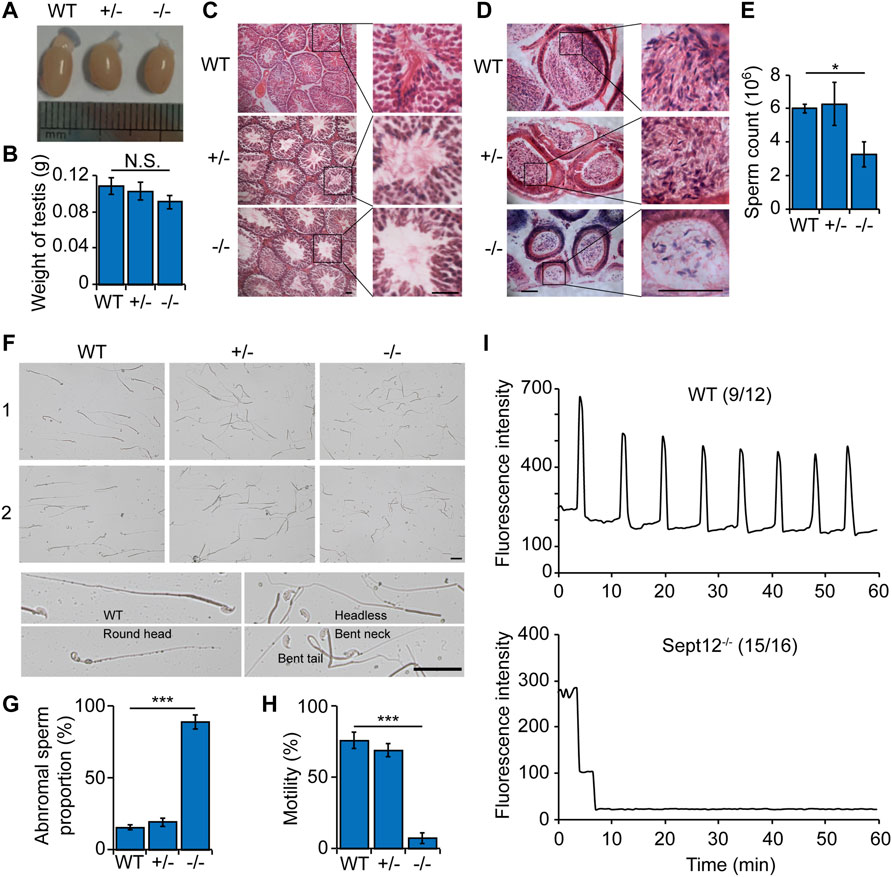
FIGURE 2. Abnormal sperm morphology and motility in Septin12−/− male mice. (A) The morphology of the testis from WT, Septin12+/−, and Septin12−/− mice. (B) Testis weight of WT, Septin12+/−, and Septin12−/− mice (n = 5 for each genotype). NS stands for not statistically significant. (C,D) Hematoxylin and eosin staining of testis (C) and epididymis (D) sections from WT, Septin12+/−, and Septin12−/− mice. Scale bars, 100 μm. (E–H) Sperm count (E), morphology (F,G), and motility (H) of WT, Septin12+/−, and Septin12−/− mice (n = 5 for each genotype). Sperms were isolated from the epididymis. The data are represented as means ± SD, ***p < 0.001. (F) Scale bars, 10 μm. (I) Ca2+ oscillation profiles of ICSI embryos with sperms from WT mice (left panel) or Sept12−/− mice (right panel). The numbers in parentheses denote the fraction of embryos presented in the plots. The numerator is the number of ICSI embryos represented in the plot from two independent experiments, and the denominator is the total number of ICSI embryos from two independent experiments.
Analysis of reproductive organs revealed no obvious difference in testis morphology and weight in WT, Septin12+/− and Septin12−/− males (Figures 2A,B). Histological analysis of tissue sections showed that there are less spermatozoa in Septin12−/− testis and epididymis, compared with their WT and Septin12+/− counterparts (Figures 2C,D). Consistently, the sperm count in Septin12−/− males is about half of those in WT and Septin12+/− male mice (Figure 2E). Moreover, a large fraction of Septin12−/− spermatozoa exhibit abnormal morphologies, such as round spermatid, headless, bent neck, and tail defects (Figures 2F,G), and lack mobility (Figure 2H). These data suggested that defects in spermatozoa, rather than abnormal reproductive organs, leads to male infertility of Septin12−/− mice.
Given the clinical result that ICSI-AOA allowed the patient couple with male SEPTIN12 deficiency to conceive, we next tested whether Septin12−/− sperms are defective in oocyte activation after ICSI, and whether the FF after ICSI with Septin12−/− sperm can be overcome by AOA. ICSI experiments were performed using sperms from WT, Septin12+/−, and Septin12−/− mouse, and WT mouse oocytes. Oocytes injected with WT and Septin12+/− sperms developed to the 2-cell stage with high efficiencies, 89.8 and 69.0%, respectively. In contrast, only 16.3 and 13.0% of oocytes injected with Septin12−/− sperms developed to 2-cell embryos, in two independent ICSI experiments (Table 4). These data indicated that male Septin12 deficiency causes FF after ICSI. AOA treatment significantly enhanced the 2-cell embryo rate in the ICSI group with Septin12−/− sperm, from ∼15 to ∼40%, while the 2-cell embryo rate in the ICSI group with Septin12+/− sperm was only slightly increased by AOA (Table 4). These data validated that AOA treatment indeed overcomes the FF after ICSI caused by male Septin12 deficiency. We further demonstrated that calcium oscillation is not properly triggered in ICSI embryos with Septin12−/− sperm (Figure 2I), indicating that failure in initiating calcium oscillation contributes to the FF after ICSI with Septin12−/− sperms.
ICSI embryos with Septin12+/− sperm have lower developmental rates to various embryo stages, compared with ICSI embryos with WT sperm (Table 4). It implies that haploinsufficiency of Septin12 might affect the quality of sperms. The slightly compromised quality of Septin12+/− sperms might be manifested in sub-optimal conditions, such as ICSI and in vitro embryo development, but not in in vivo fertilization and embryogenesis. It is obvious that the quality of Septin12−/− sperms are more severely impaired. Even with AOA treatment, only 2 out of total 73 embryos injected with Septin12−/− sperms developed to the blastocyst stage (Table 4), suggesting that defects in Septin12−/− sperms affect not only oocyte activation, but also the developmental potential of zygotes beyond the 2-cell stage.
We then addressed why Septin12−/− sperm fails to activate oocyte after ICSI. Since AOA treatment activates calcium oscillation and rescues the FF after ICSI due to male Septin12 deficiency, it is very likely that Septin12−/− sperms are defective in triggering calcium oscillation upon injected into an oocyte. Sperm-specific PLCζ plays an essential role in inducing calcium oscillation (Cox et al., 2002; Saunders et al., 2002). Thus, immunofluorescence experiments were performed and revealed that the expression of PLCζ at the acrosomal and post-acrosomal regions is diminished in Septin12−/− spermatozoa isolated from cauda epididymis (Figure 3A), which might account for the FF after ICSI with Septin12−/− spermatozoa.
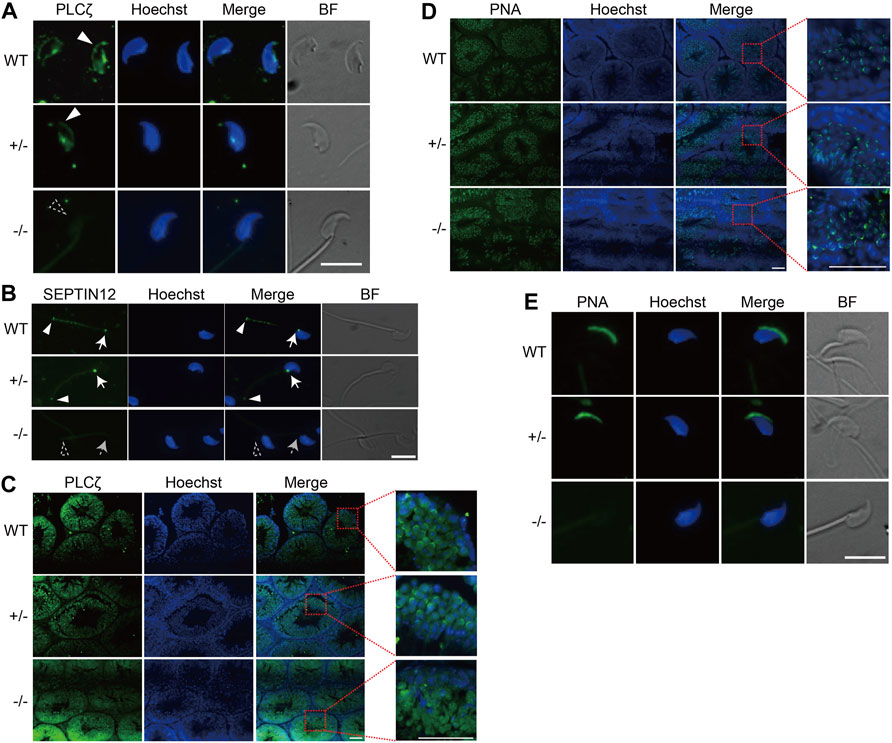
FIGURE 3. Disturbed distribution of PLCζ and acrosome malformation in Septin12−/− spermatozoa. (A,B) Immunofluorescence staining of PLCζ (A) and SEPTIN12 (B) in spermatozoa isolated from the epididymis of WT, Septin12+/−, and Septin12−/− mice. Scale bars: 10 μm. (A) The PLCζ signal at the acrosome region is marked by solid white triangle, and the dashed triangle shows the lack of PLCζ signal at the acrosome region of Septin12−/− spermatozoa. (B) The signals of SEPTIN12 at the neck and annulus are indicated by solid white arrows and triangles, respectively. Dashed arrow and triangle point to the neck and annulus without SEPTIN12 signal. (C,D) Immunofluorescence staining of PLCζ (C) and acrosome (D) in testis sections of WT, Septin12+/−, and Septin12−/− mice. Enlarge images are shown in the right side. Scale bars: 50 μm. (D) The acrosome was stained by Alexa Fluor 488-lectin-PNA. (E) Immunofluorescence staining of acrosome in spermatozoa isolated from the epididymis of WT, Septin12+/−, and Septin12−/− mice. Scale bar: 10 μm.
How does SEPTIN12, localized at the neck and annulus of spermatozoa (Figure 3B), affect the expression and distribution of PLCζ in the head of spermatozoa? It has been shown that during human spermiogenesis, SEPTIN12 is first concentrated around the acrosome, and translocated to the neck and annulus regions in spermatozoa (Lin et al., 2011), implying that SEPTIN12 might regulate the acrosomal distribution of PLCζ at the early steps of spermiogenesis. Indeed, immunofluorescent staining of testicular tissue sections revealed that PLCζ is enriched in the acrosome region of WT and Septin12+/− spermatids. In contrast, the acrosomal enrichment of PLCζ is diminished in Septin12−/− spermatids (Figure 3C). The acrosome formation appears to be normal in Septin12−/− spermatids (Figure 3D), suggesting that the disturbed distribution of PLCζ in Septin12−/− spermatids is not due to abnormal acrosome formation. Surprisingly, no acrosome signal in Septin12−/− spermatozoa isolated from cauda epididymis was detected by peanut agglutinin (PNA) staining (Figure 3E), reflecting no acrosome or lack of β-D-galactosylation on acrosomal membrane proteins. Given that the acrosome is formed normally in Septin12−/− testis, it is more likely that β-D-galactosylation of acrosomal membrane proteins is removed during sperm transit in the epididymis. Taken together, Septin12 deficiency affects the acrosomal enrichment of PLCζ at the late cap phase and the early acrosome phase of spermiogenesis, and the formation of acrosome at the maturation phase.
AOA treatment improves reproductive outcomes in some patients, but not all, with previous FF after ICSI. Thus, identification of genetic defects responsible for FF which may be overcome by AOA is important for selective application of ICSI-AOA in patients with high beneficial potential. In this study, we showed that Septin12−/− male mice, but not Septin12+/− male mice, are infertile. Importantly, the 2-cell embryo rate of ICSI embryos with sperms from Septin12−/− mice was increased by AOA treatment (Table 4). These data suggest an essential role of SEPTIN12 in oocyte activation and male infertility.
The male infertility in Septin12−/− mice seems to be conflicted with the infertile phenotype in the male patient with a heterozygous c.72C>A SEPTIN12 mutation. Compound mutation might account for the infertility in the male patient. Consistently, in addition to the SEPTIN12 mutation, heterozygous mutations were identified in three other infertility genes in the male patient (Table 3). Further studies are necessary to address whether compound mutations of SEPTIN12 and other infertility genes lead to infertility. Nevertheless, we cannot rule out the possibility that heterozygosity for the null SEPTIN12 mutation leads to male infertility in the human, but not in the mouse, due to species difference.
Our study also clarified the role of SEPTIN12 in mouse spermatogenesis. Previous studies showed that male Septin12+/− chimeric mice are infertile (Lin et al., 2009). However, our male Septin12+/− mice are fertile, whereas male Septin12−/− mice are sterile. The main difference is that their Septin12+/− chimeric mice were generated by blastocyst injection of Septin12+/− embryonic stem cells (ESCs), while our Septin12+/− founder mice were established by CRISPR/Cas9 mediated gene editing in the zygote. The quality of injected Septin12+/− ESCs might affect the experimental result. For example, these ESCs might harbor a mutation in addition to the knockout of Septin12, thus impairing the development and maturation of sperms. The genetic background difference might also contribute to the conflicted results. The Septin12+/− ESCs were derived from the 129Sv mouse (Lin et al., 2009), while our Septin12 knockout mice are in the C57BL/6 background. With current data, it is convincing that male Septin12+/− C57BL/6 mice are fertile.
Septin12−/− mouse spermatozoa display multiple defective phenotypes, including round spermatid, headless, bent neck, tail defects, and reduced mobility. These phenotypes are directly associated with the function of SEPTIN12 in the neck and annulus regions of spermatozoa. ICSI should be able to treat the male infertility caused by these defects. In addition, Septin12−/− mouse spermatozoa have other defects not directly related to the neck and annulus regions, such as abnormal PLCζ distribution and acrosome formation. These defects reflect the function of SEPTIN12 out of the neck and annulus regions. During spermiogenesis, SEPTIN12 migrates from the acrosome to the neck and the annulus (Lin et al., 2011). Its presence in the acrosomal region appears to be essential for the acrosomal recruitment and enrichment of PLCζ during the late cap and the early acrosome phases, as well as the acrosome formation at the maturation phase. The acrosome defect should be overcome by ICSI, while the abnormal PLCζ distribution might lead to OAF, and thus require the treatment of ICSI-AOA. ICSI-AOA leads to high fertilization and pregnancy rate for globozoospermia patients (Tavalaee et al., 2018; Modarres et al., 2019). However, it is notable that even with ICSI-AOA, low blastocyst rates, 10.6 and 0–4.1%, were achieved for embryos injected with Septin12+/− and Septin12−/− sperms, respectively (Table 4). It implies that Septin12 deficiency may cause additional defect(s) in sperms, which compromise the in vitro developmental potential of zygotes, and are not rescued by ICSI-AOA. Nuclear defects have been observed in sperms isolated from Septin12+/− chimeric mice (Lin et al., 2011). Consistently, DNA fragment index (DFI) and high DNA stain-ability (HDS) of the male patient were 20.13 and 18.38%, respectively, indicating DNA defects in the spermatozoa. Further investigations are required to characterize these additional defects in Septin12−/− sperms.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA753965.
The studies involving human participants were reviewed and approved by The Ethics Committee of Tianjin Medical University General Hospital. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Nankai Animal Care and Use Committee.
HC, PL, XD, YZ, LW, YT and XS performed experiments, HC and PL analyzed the data and contributed to the paper writing, LS, XB and LC designed the experiments and wrote the paper.
LC was supported by the National Key R & D Program of China (Grant Nos. 2021YFA1101002 and 2018YFA0107002), the National Natural Science Foundation of China (Grant No. 31871485), the Natural Science Foundation of Tianjin (Grant No. 18JCJQJC48400), the 111 Project Grant (B08011), and the Fundamental Research Funds for the Central Universities. HC was supported by the National Natural Science Foundation of China (Grant No. 82001617). YT was supported by the National Natural Science Foundation of China (Grant No. 82171625).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agarwal, A., Mulgund, A., Hamada, A., and Chyatte, M. R. (2015). A Unique View on Male Infertility Around the globe. Reprod. Biol. Endocrinol. 13, 37. doi:10.1186/s12958-015-0032-1
Cox, L., Larman, M., Saunders, C., Hashimoto, K., Swann, K., and Lai, F. (2002). Sperm Phospholipase Czeta from Humans and Cynomolgus Monkeys Triggers Ca2+ Oscillations, Activation and Development of Mouse Oocytes. Reproduction 124 (5), 611–623. doi:10.1530/rep.0.1240611
Dai, J., Dai, C., Guo, J., Zheng, W., Zhang, T., Li, Y., et al. (2020). Novel Homozygous Variations in PLCZ1 lead to Poor or Failed Fertilization Characterized by Abnormal Localization Patterns of PLCζ in Sperm. Clin. Genet. 97 (2), 347–351. doi:10.1111/cge.13636
Dong, F. N., Amiri-Yekta, A., Martinez, G., Saut, A., Tek, J., Stouvenel, L., et al. (2018). Absence of CFAP69 Causes Male Infertility Due to Multiple Morphological Abnormalities of the Flagella in Human and Mouse. Am. J. Hum. Genet. 102 (4), 636–648. doi:10.1016/j.ajhg.2018.03.007
Escoffier, J., Lee, H. C., Yassine, S., Zouari, R., Martinez, G., Karaouzène, T., et al. (2016). Homozygous Mutation of PLCZ1 Leads to Defective Human Oocyte Activation and Infertility that Is Not Rescued by the WW-Binding Protein PAWP. Hum. Mol. Genet. 25 (5), 878–891. doi:10.1093/hmg/ddv617
Esfandiari, N., Javed, M. H., Gotlieb, L., and Casper, R. F. (2005). Complete Failed Fertilization after Intracytoplasmic Sperm Injection-Aanalysis of 10 years' Data. Int. J. Fertil. Womens Med. 50 (4), 187–192.
Ferrer-Buitrago, M., Dhaenens, L., Lu, Y., Bonte, D., Vanden Meerschaut, F., De Sutter, P., et al. (2018). Human Oocyte Calcium Analysis Predicts the Response to Assisted Oocyte Activation in Patients Experiencing Fertilization Failure after ICSI. Hum. Reprod. 33 (3), 416–425. doi:10.1093/humrep/dex376
Flaherty, S., Payne, D., Swann, N., and Matthews, C. (1995b). Assessment of Fertilization Failure and Abnormal Fertilization after Intracytoplasmic Sperm Injection (ICSI). Reprod. Fertil. Dev. 7 (2), 197–210. doi:10.1071/rd9950197
Flaherty, S. P., Dianna, P., Swann, N. J., and Matthews, C. D. (1995a). Aetiology of Failed and Abnormal Fertilization after Intracytoplasmic Sperm Injection1. Hum. Reprod. 10 (10), 2623–2629. doi:10.1093/oxfordjournals.humrep.a135757
Flaherty, S. P., Payne, D., and Matthews, C. D. (1998). Fertilization Failures and Abnormal Fertilization after Intracytoplasmic Sperm Injection. Hum. Reprod. 13 (Suppl. 1), 155–164. doi:10.1093/humrep/13.suppl_1.155
Geng, D., Yang, X., Zhang, H., Liu, X., Yu, Y., Jiang, Y., et al. (2019). Association of Single Nucleotide Polymorphism c.673C>A/p.Gln225Lys in SEPT12 Gene with Spermatogenesis Failure in Male Idiopathic Infertility in Northeast China. J. Int. Med. Res. 47 (2), 992–998. doi:10.1177/0300060518811770
Guo, T., Zhao, S., Zhao, S., Chen, M., Li, G., Jiao, X., et al. (2017). Mutations in MSH5 in Primary Ovarian Insufficiency. Hum. Mol. Genet. 26 (8), 1452–1457. doi:10.1093/hmg/ddx044
He, X., Li, W., Wu, H., Lv, M., Liu, W., Liu, C., et al. (2019). Novel Homozygous CFAP69 Mutations in Humans and Mice Cause Severe Asthenoteratospermia with Multiple Morphological Abnormalities of the Sperm Flagella. J. Med. Genet. 56 (2), 96–103. doi:10.1136/jmedgenet-2018-105486
Kuo, Y.-C., Lin, Y.-H., Chen, H.-I., Wang, Y.-Y., Chiou, Y.-W., Lin, H.-H., et al. (2012). SEPT12mutations Cause Male Infertility with Defective Sperm Annulus. Hum. Mutat. 33 (4), 710–719. doi:10.1002/humu.22028
Li, W., Shuai, L., Wan, H., Dong, M., Wang, M., Sang, L., et al. (2012). Androgenetic Haploid Embryonic Stem Cells Produce Live Transgenic Mice. Nature 490 (7420), 407–411. doi:10.1038/nature11435
Lin, Y.-H., Chou, C.-K., Hung, Y.-C., Yu, I.-S., Pan, H.-A., Lin, S.-W., et al. (2011). SEPT12 Deficiency Causes Sperm Nucleus Damage and Developmental Arrest of Preimplantation Embryos. Fertil. Sterility 95 (1), 363–365. doi:10.1016/j.fertnstert.2010.07.1064
Lin, Y.-H., Lin, Y.-M., Teng, Y.-N., Hsieh, T.-Y. T., Lin, Y.-S., and Kuo, P.-L. (2006). Identification of Ten Novel Genes Involved in Human Spermatogenesis by Microarray Analysis of Testicular Tissue. Fertil. Sterility 86 (6), 1650–1658. doi:10.1016/j.fertnstert.2006.04.039
Lin, Y.-H., Lin, Y.-M., Wang, Y.-Y., Yu, I.-S., Lin, Y.-W., Wang, Y.-H., et al. (2009). The Expression Level of Septin12 Is Critical for Spermiogenesis. Am. J. Pathol. 174 (5), 1857–1868. doi:10.2353/ajpath.2009.080955
Lin, Y.-H., Wang, Y.-Y., Chen, H.-I., Kuo, Y.-C., Chiou, Y.-W., Lin, H.-H., et al. (2012). SEPTIN12 Genetic Variants Confer Susceptibility to Teratozoospermia. PLoS One 7 (3), e34011. doi:10.1371/journal.pone.0034011
Liu, W., Wu, H., Wang, L., Yang, X., Liu, C., He, X., et al. (2019). Homozygous Loss-Of-Function Mutations in FSIP2 Cause Male Infertility with Asthenoteratospermia. J. Genet. Genomics 46 (1), 53–56. doi:10.1016/j.jgg.2018.09.006
Mahutte, N. G., and Arici, A. (2003). Failed Fertilization: Is it Predictable? Curr. Opin. Obstet. Gynecol. 15 (3), 211–218. doi:10.1097/00001703-200306000-00001
Martinez, G., Kherraf, Z.-E., Zouari, R., Fourati Ben Mustapha, S., Saut, A., Pernet-Gallay, K., et al. (2018). Whole-exome Sequencing Identifies Mutations in FSIP2 as a Recurrent Cause of Multiple Morphological Abnormalities of the Sperm Flagella. Hum. Reprod. 33 (10), 1973–1984. doi:10.1093/humrep/dey264
Miyamoto, T., Tsujimura, A., Miyagawa, Y., Koh, E., Namiki, M., Horikawa, M., et al. (2012). Single Nucleotide Polymorphisms in the SEPTIN12 Gene May Be Associated with Azoospermia by Meiotic Arrest in Japanese Men. J. Assist. Reprod. Genet. 29 (1), 47–51. doi:10.1007/s10815-011-9679-5
Modarres, P., Tavalaee, M., Ghaedi, K., and Nasr-Esfahani, M. H. (2019). An Overview of the Globozoospermia as A Multigenic Identified Syndrome. Int. J. Fertil. Steril 12 (4), 273–277. doi:10.22074/ijfs.2019.5561
Mu, J., Zhang, Z., Wu, L., Fu, J., Chen, B., Yan, Z., et al. (2020). The Identification of Novel Mutations in PLCZ1 Responsible for Human Fertilization Failure and a Therapeutic Intervention by Artificial Oocyte Activation. Mol. Hum. Reprod. 26 (2), 80–87. doi:10.1093/molehr/gaaa003
Nasr-Esfahani, M. H., Razavi, S., Javdan, Z., and Tavalaee, M. (2008). Artificial Oocyte Activation in Severe Teratozoospermia Undergoing Intracytoplasmic Sperm Injection. Fertil. Sterility 90 (6), 2231–2237. doi:10.1016/j.fertnstert.2007.10.047
Nazarian, H., Azad, N., Nazari, L., Piryaei, A., Heidari, M. H., Masteri-Farahani, R., et al. (2019). Effect of Artificial Oocyte Activation on Intra-cytoplasmic Sperm Injection Outcomes in Patients with Lower Percentage of Sperm Containing Phospholipase Cζ: A Randomized Clinical Trial. J. Reprod. Infertil 20 (1), 3–9.
Nomikos, M., Elgmati, K., Theodoridou, M., Georgilis, A., Gonzalez-Garcia, J. R., Nounesis, G., et al. (2011). Novel Regulation of PLCζ Activity via its XY-Linker. Biochem. J. 438 (3), 427–432. doi:10.1042/BJ20110953
Rafaee, A., Mohseni Meybodi, A., Yaghmaei, P., Hosseini, S. H., and Sabbaghian, M. (2020). Single‐nucleotide Polymorphism c.474G>A in the SEPT12 Gene Is a Predisposing Factor in Male Infertility. Mol. Reprod. Dev. 87 (2), 251–259. doi:10.1002/mrd.23310
Saleh, A., Kashir, J., Thanassoulas, A., Safieh-Garabedian, B., Lai, F. A., and Nomikos, M. (2020). Essential Role of Sperm-specific PLC-Zeta in Egg Activation and Male Factor Infertility: An Update. Front. Cel Dev. Biol. 8, 28. doi:10.3389/fcell.2020.00028
Saunders, C. M., Larman, M. G., Parrington, J., Cox, L. J., Royse, J., Blayney, L. M., et al. (2002). PLCζ: a Sperm-specific Trigger of Ca2+ Oscillations in Eggs and Embryo Development. Development 129 (15), 3533–3544. doi:10.1242/dev.129.15.3533
Sfontouris, I. A., Nastri, C. O., Lima, M. L. S., Tahmasbpourmarzouni, E., Raine-Fenning, N., and Martins, W. P. (2015). Artificial Oocyte Activation to Improve Reproductive Outcomes in Women with Previous Fertilization Failure: a Systematic Review and Meta-Analysis of RCTs. Hum. Reprod. 30 (8), 1831–1841. doi:10.1093/humrep/dev136
Shen, Y.-R., Wang, H.-Y., Kuo, Y.-C., Shih, S.-C., Hsu, C.-H., Chen, Y.-R., et al. (2017). SEPT12 Phosphorylation Results in Loss of the Septin Ring/sperm Annulus, Defective Sperm Motility and Poor Male Fertility. Plos Genet. 13 (3), e1006631. doi:10.1371/journal.pgen.1006631
Shen, Y.-R., Wang, H.-Y., Tsai, Y.-C., Kuo, Y.-C., Wu, S.-R., Wang, C.-Y., et al. (2020). The SEPT12 Complex Is Required for the Establishment of a Functional Sperm Head-Tail junction. Mol. Hum. Reprod. 26 (6), 402–412. doi:10.1093/molehr/gaaa031
Shuai, L., Li, W., Wan, H., Zhao, X. Y., Wang, L., and Zhou, Q. (2014). Generation of Mammalian Offspring by Haploid Embryonic Stem Cells Microinjection. Curr. Protoc. Stem Cel Biol. 31, 1A 6 1–15. doi:10.1002/9780470151808.sc01a06s31
Tavalaee, M., Nomikos, M., Lai, F. A., and Nasr-Esfahani, M. H. (2018). Expression of Sperm PLCζ and Clinical Outcomes of ICSI-AOA in Men Affected by Globozoospermia Due to DPY19L2 Deletion. Reprod. BioMedicine Online 36 (3), 348–355. doi:10.1016/j.rbmo.2017.12.013
Torra-Massana, M., Cornet-Bartolomé, D., Barragán, M., Durban, M., Ferrer-Vaquer, A., Zambelli, F., et al. (2019). Novel Phospholipase C Zeta 1 Mutations Associated with Fertilization Failures after ICSI. Hum. Reprod. 34 (8), 1494–1504. doi:10.1093/humrep/dez094
Toure, A., Rode, B., Hunnicutt, G. R., Escalier, D., and Gacon, G. (2011). Septins at the Annulus of Mammalian Sperm. Biol. Chem. 392 (8-9), 799–803. doi:10.1515/BC.2011.074
Van Steirteghem, A. C., Nagy, Z., Joris, H., Liu, J., Staessen, C., Smitz, J., et al. (1993). High Fertilization and Implantation Rates after Intracytoplasmic Sperm Injection. Hum. Reprod. 8 (7), 1061–1066. doi:10.1093/oxfordjournals.humrep.a138192
Vanden Meerschaut, F., Nikiforaki, D., De Gheselle, S., Dullaerts, V., Van den Abbeel, E., Gerris, J., et al. (2012). Assisted Oocyte Activation Is Not Beneficial for All Patients with a Suspected Oocyte-Related Activation Deficiency. Hum. Reprod. 27 (7), 1977–1984. doi:10.1093/humrep/des097
Yan, Z., Fan, Y., Wang, F., Yan, Z., Li, M., Ouyang, J., et al. (2020). Novel Mutations in PLCZ1 Cause Male Infertility Due to Fertilization Failure or Poor Fertilization. Hum. Reprod. 35 (2), 472–481. doi:10.1093/humrep/dez282
Yelumalai, S., Yeste, M., Jones, C., Amdani, S. N., Kashir, J., Mounce, G., et al. (2015). Total Levels, Localization Patterns, and Proportions of Sperm Exhibiting Phospholipase C Zeta Are Significantly Correlated with Fertilization Rates after Intracytoplasmic Sperm Injection. Fertil. Sterility 104 (3), 561–568. e564. doi:10.1016/j.fertnstert.2015.05.018
Yeste, M., Jones, C., Amdani, S. N., Patel, S., and Coward, K. (2016). Oocyte Activation Deficiency: a Role for an Oocyte Contribution? Hum. Reprod. Update 22 (1), 23–47. doi:10.1093/humupd/dmv040
Yıldız Bölükbaşı, E., Mumtaz, S., Afzal, M., Woehlbier, U., Malik, S., and Tolun, A. (2018). Homozygous Mutation in CEP19, a Gene Mutated in Morbid Obesity, in Bardet-Biedl Syndrome with Predominant Postaxial Polydactyly. J. Med. Genet. 55 (3), 189–197. doi:10.1136/jmedgenet-2017-104758
Yoon, S.-Y., Jellerette, T., Salicioni, A. M., Lee, H. C., Yoo, M.-s., Coward, K., et al. (2008). Human Sperm Devoid of PLC, Zeta 1 Fail to Induce Ca2+ Release and Are Unable to Initiate the First Step of Embryo Development. J. Clin. Invest. 118 (11), 3671–3681. doi:10.1172/JCI36942
Yuan, P., Yang, C., Ren, Y., Yan, J., Nie, Y., Yan, L., et al. (2020). A Novel Homozygous Mutation of Phospholipase C Zeta Leading to Defective Human Oocyte Activation and Fertilization Failure. Hum. Reprod. 35 (4), 977–985. doi:10.1093/humrep/dez293
Keywords: SEPTIN12, oocyte activation, fertilization failure, calcium oscillation, male infertility
Citation: Chen H, Li P, Du X, Zhao Y, Wang L, Tian Y, Song X, Shuai L, Bai X and Chen L (2022) Homozygous Loss of Septin12, but not its Haploinsufficiency, Leads to Male Infertility and Fertilization Failure. Front. Cell Dev. Biol. 10:850052. doi: 10.3389/fcell.2022.850052
Received: 07 January 2022; Accepted: 06 April 2022;
Published: 25 April 2022.
Edited by:
Michail Nomikos, Qatar University, QatarReviewed by:
Mohammad Hossein Nasr-Esfahani, Royan Institute, IranCopyright © 2022 Chen, Li, Du, Zhao, Wang, Tian, Song, Shuai, Bai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingyi Chen, bGluZ3lpY2hlbkBuYW5rYWkuZWR1LmNu; Xiaohong Bai, YnhoampAMTYzLmNvbQ==; Ling Shuai, MDE1MTk5QG5hbmthaS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.