- Department of General Surgery, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
Cell death is a fundamental feature of multicellular organisms’ development and a key driver of degenerative diseases. Ferroptosis is a new regulatory cell death mediated by iron-dependent lipid peroxidation, which is different from apoptosis and necrosis in morphology, pathophysiology and mechanism. Recent studies have found that ferroptosis is involved in the development of many diseases including hepatocellular carcinoma (HCC). As further research progresses, specific mechanisms of ferroptosis in HCC are being revealed. In this review, we summarize these recent advances about the treatment of drug-resistance in HCC and the latest ferroptosis-related treatment for HCC.
Introduction
HCC is an invasive cancer prevalent worldwide, with a mortality rate ranked second among all the cancers, which was just behind lung cancer and colon cancer (Bray et al., 2018). The 5-years survival rate of HCC patients is less than 10%, and the average life expectancy is only 6 months for those patients who were not eligible for surgery. And the existing treatments, including radiofrequency therapy, radiotherapy therapy, and chemotherapy, do not significantly improve the prognosis of HCC patients. Currently, in terms of HCC chemotherapy, the US Food and Drug Administration (FDA) has approved a variety of small molecule multi-kinase inhibitors, such as sorafenib, for the treatment of advanced HCC (Boland and WU, 2018). However, the therapeutic effect of most patients is still limited due to the frequent drug resistance of those inhibitors. Therefore, different modulation strategies and administration routes have been proposed to enhance the antitumor activity of these agents.
Dixon identified an iron-dependent form of cell death in 2012 and defined this modality as ferroptosis. It is now considered that ferroptosis is triggered by both exogenous and endogenous pathways, either by inhibition of cell membrane transporters (cystine/glutamate transporter system) or by activation of iron transporters, serum transferrin, and lactoferrin. Endogenous pathways are activated by blocking intracellular antioxidant enzymes such as glutathione peroxidase 4 (GPX4) (Tang and KROEMER, 2020). Unlike other known modes of cell death, such as apoptosis, necrosis, and autophagy, ferroptosis has unique morphological, biochemical, and genetic characteristics, such as mitochondrial atrophy, increased membrane density, iron, and ROS accumulation.
Recent studies have found that ferroptosis is involved in the proliferation, invasion, and migration of HCC cells, and is also closely related to drug-resistance in HCC, of which the specific mechanism is being gradually revealed.
Regulation of Ferroptosis in HCC
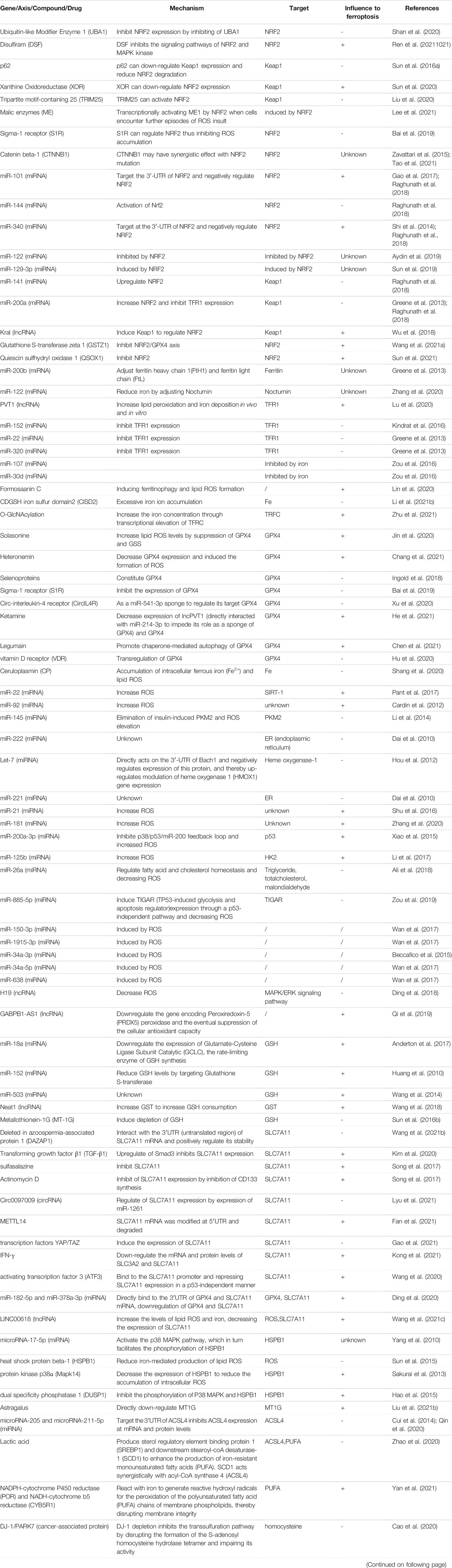
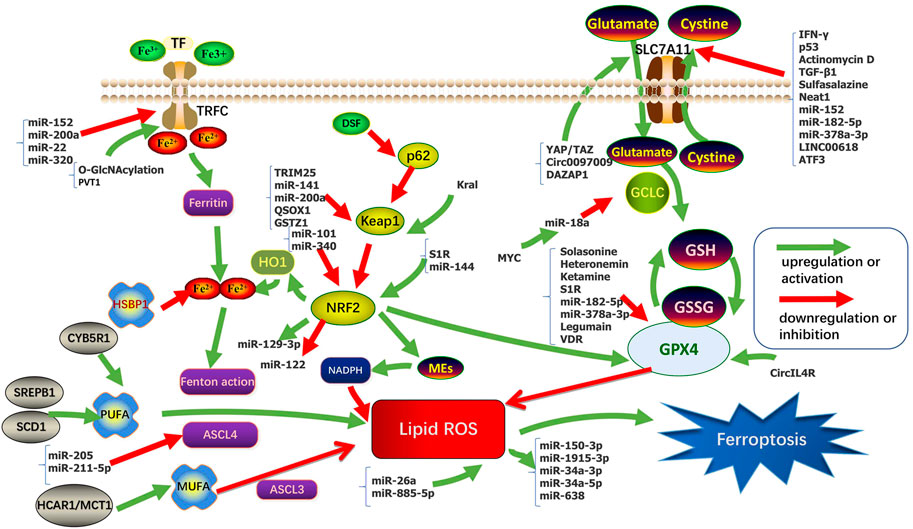
Sensitivity to ferroptosis is closely related to many biological processes, such as (anti-)oxidant metabolism, iron metabolism, lipid metabolism, energy metabolism, and regulation of non-coding RNAs (ncRNAs). NcRNAs participate in the regulation of tumorigenesis via various biological processes such as chromatin modification, alternative splicing, competition with endogenous RNAs, and interaction with proteins. Intervention in these key links may regulate the sensitivity of HCC cells to ferroptosis. The regulation of ferroptosis found in HCC in recent years was sorted out in Table 1 and Figure 1.
(Anti-)Oxidant Metabolism
(Anti-)oxidant Metabolism plays an important role in ferroptosis. Glutathione (GSH) metabolism and anti-oxidant capacity regulate sensitivity to ferroptosis. GSH is a tripeptide antioxidant that acts as a cofactor of Se-dependent GPX4 to reduce lipid hydroperoxides (Yant et al., 2003; Lu, 2009). Inhibition of cystine required for GSH synthesis eventually leads to depletion of intracellular GSH levels (Dixon et al., 2012; Dixon and STOCKWELL, 2014). GPX4 converts GSH between the reduced and oxidized states and converts lipid hydroperoxides to lipid alcohols. This process prevents the formation of Fe2+ dependent toxic lipid ROS (Labunskyy et al., 2014; Forcina and DIXON, 2019). GPX4 is the only reported enzyme that can directly reduce complex phospholipid peroxides and is the downstream target gene of NRF2 (Nuclear factor E2-related factor 2) (Forcina and DIXON, 2019; Friedmann Angeli et al., 2019). Erastin, a classical ferroptosis-inducing drug, depletes GSH and indirectly inactivates GPX4, leading to accumulation of toxic lipid ROS and subsequent lipid peroxidation (Dixon et al., 2012; Dixon and STOCKWELL, 2014), ultimately leading to ferroptosis.
At present, most studies on NRF2 in HCC involve the p62-Keap1 (Kelch-like ECH-associated protein 1)-NRF2 axis. The p62-Keap1-NRF2 signaling pathway is involved in the process of cell avoiding ferroptosis. NRF2 is a key regulator of the antioxidant response, including the expression of the Cystine/glutamate exchange system (system XC−) (Hassannia et al., 2019). Inhibition or knockdown of NRF2 enhances erastin- or sorafenib-induced ferroptosis in HCC in vitro and in vivo (Hassannia et al., 2019). The System XC− consists of solute carrier family 7 member 11 (SLC7A11, xCT) and solute carrier family 3 member 2 (SLC3A2, 4F2hc) by disulfide bonded, which import the extracellular oxidized form of cysteine and cystine, in exchange for intracellular glutamate. SLC7A11 indirectly inactivates GPX4 by reducing cysteine uptake, thereby limiting GSH synthesis, increasing lipid ROS, and ultimately leading to ferroptosis (Sato et al., 1999; Cao and DIXON, 2016). NRF2 has antioxidant elements and is regulated by Keap1. Its gene transcription is partially under the control of ROS. Sun et al. (2016a) found p62 expression prevents NRF2 degradation by Keap1 inactivation and enhances the subsequent nuclear accumulation of NRF2. They also demonstrate that NRF2-mediated anti-ferroptosis activity depends on the induction of NADPH (Reduced Nicotinamide Adenine Dinucleotide Phosphate) quinone oxidoreductase 1 (NQO1), heme oxygenase-1(HO-1), and ferritin heavy chain-1 (FTH1).
In morphology, ferroptosis mainly occurred in cells with reduced mitochondrial size, increased bilayer membrane density, and decreased or disappeared mitochondrial crest (Dixon et al., 2012; Yang and STOCKWELL, 2008; Yagoda et al., 2007). Mitochondria are the main source of ROS. Excessive ROS can cause significant oxidative stress and lead to cell and tissue damage (Czaja et al., 2013). Gao et al. (2019)showed that ROS derived from mitochondria are involved in cysteine deprivation induced ferroptosis. Li et al. (2021) found depletes cysteine can enhance sorafenib-induced ferroptosis and lipid ROS production, and increase oxidative stress and mitochondrial ROS accumulation. And they point out that sorafenib exerts its anti-HCC function partly by targeting the mitochondrial function. Huang et al. (2021a) found the use of ZZW-115 (Nuclear protein 1 inhibitor) induced ferroptosis and subsequent mitochondrial morphological changes, including the disintegration of mitochondrial network and severe mitochondrial metabolic disorders, which were compatible with the process of ferroptosis, and this process can be complementary to TFAM (a core mitochondrial transcription factor) (Zhao, 2019).
Iron Metabolism
Iron is a redox-active metal that can participate in the formation of free radicals and the propagation of lipid peroxidation. Elevated iron levels increase susceptibility to ferroptosis. Iron overload or excessive activity of heme oxygenase 1 (HMOX1) increases the labile iron pool (LIP) that cause ferroptosis. Excessive iron increases ROS through Fenton reaction (through reaction with hydrogen peroxide (H2O2), ferrous iron (Fe2+) is oxidized into trivalent iron (Fe3+), forming highly active hydroxyl radical) (Hassannia et al., 2019), ROS is reversely neutralized by iron (Arefieva et al., 2021). Iron metabolism mainly involves the interaction between transferrin (TF) and its receptor (TFR), the input of iron through divalent metal transporter 1 (DMT1), the storage of iron as ferritin and iron-sulfur clusters (ISC), and the output of iron through iron transporter (FPN) (Abeyawardhane and LUCAS, 2019; Wang et al., 2019a).
The protection of the p62-Keap1-NRF2 signaling pathway on ferroptosis in HCC cells also involves the regulation of Fe homeostasis. An early study showed an increase in TFR1 and a decrease in ferritin (FTL and FTH1) expression in ferroptosis sensitive cells compared with iron-resistant cells (Yang and STOCKWELL, 2008). Sun et al. (2016a) showed that it was FTH1, not FTL or TFR1, that was regulated by NRF2 in ferroptosis. FTH1 inhibited ferroptosis by storing and transporting Fe2+ in HCC cells. In addition, excess iron in the liver may play a role in carcinogenesis by promoting tumor growth and altering the immune system (Kowdley, 2004). It is important to note that induction of ferroptosis in the liver may have different roles in tumorigenesis and cancer therapy.
Lipid Metabolism
Ferroptosis is iron-dependent regulatory necrosis induced by lipid peroxidation that occurs in cell membranes, a peroxidation reaction by polyunsaturated fatty acids catalyzed by the synthesis of acyl-CoA synthetase long-chain family member 4 (ACSL4) (Doll et al., 2017; Conrad and PRATT, 2019). Some polyunsaturated fatty acids (PUFAs) such as phosphatidylethanolamine (PE) and phosphatidylcholine (PC) are responsible for inducing ferroptosis by lipid peroxidation. Since de novo synthesis of PUFAs is strictly limited in mammals, various PUFAs are produced by the PUFAs biosynthesis pathway through the uptake of essential fatty acids from the blood and lymphatic fluid by cells. Free polyunsaturated fatty acids can be incorporated into cell membranes by various enzymes, such as ACLS4 and LPCAT3 (lysophosphatidylcholine acyltransferase 3), and lipid peroxidation can be induced by enzyme-induced and non-enzyme-induced mechanisms, resulting in ferroptosis (Lin et al., 2021). In this regard, knockdown of ACLS4, which preferably converts arachidonoyl (AA) to acylated AA, or loss of LPCAT3, which catalyzes the insertion of acylated AA into PLs (phospholipids), and make cells resistant to ferroptosis (Dixon et al., 2015; Yuan et al., 2016; Doll et al., 2017; Kagan et al., 2017). Magtanong et al. (2019) found that acyl-CoA synthetase long-chain family member 3 (ACSL3) converts monounsaturated fatty acids (MUFAs) into its acyl-CoA ester for incorporation into membrane phospholipids, thereby protecting cancer cells from ferroptosis. However, the levels of fatty acids (include MUFAs and PUFAs) in human serum are much higher than those in classical media containing fetal bovine serum (FBS), so how cells maintain the level of free fatty acid pools in cells is important to determine whether cells experience ferroptosis (Kamphorst et al., 2013; Magtanong et al., 2019).
Energy Metabolism
Cellular energy metabolism is directly related to ferroptosis because it regulates antioxidant defense by mediating the synthesis of biological macromolecules and biological reductants such as NADPH (Zheng and CONRAD, 2020). Tumor cells typically exhibit upregulated glycolysis and PPP (pentose phosphate pathway) activity, which not only reduces ROS production by inhibiting mitochondrial respiration but also replenishes NADPH supply, thereby helps maintaining redox homeostasis to ensure cell survival. In energy metabolism, previous studies have reported that Cytochrome P450 oxidoreductase (POR) is a key mediator of ferroptosis, which promotes ferroptosis through the peroxidation of saturated phospholipids in cell membranes (Zou et al., 2020). Glucose 6-phosphate dehydrogenase (G6PD) is a key enzyme in PPP and plays a key role in NADPH production (Yang et al., 2019). G6PD may negatively regulate ferroptosis in HCC by regulating POR (Cao et al., 2021). Lu et al. (2018) pointed out that G6PD induces epithelial-mesenchymal transition (EMT) by activating the Signal Transducers and Activators of Transcription 3(STAT3) pathway, thereby promoting migration and invasion of HCC. Therefore, it can be concluded that disruption of tumor energy metabolism pathway not only changes the sensitivity of mutant tumor cells to ferroptosis, but also reduces their antioxidant defense ability to promote ferroptosis, and even affects tumor migration and invasion.
Regulation of Ferroptosis by Non-Coding RNAs
According to length and shapes, ncRNAs are divided into various types including microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), long ncRNAs (lncRNAs), circular RNAs (circRNAs), transfer RNAs (tRNAs), and ribosomal RNAs (rRNAs) (Wang et al., 2019b; Alzhrani et al., 2020). MiRNAs exhibit functions by binding to the 3′-untranslated regions of target mRNAs and suppressing their expression (Majidinia et al., 2020). MiRNA can regulate ferroptosis and control cancer progression by regulating GSH, iron levels, NRF2, and ROS. LncRNAs mainly act as the regulatory factors of transcription factors in the nucleus or as miRNAs of sponges in the cytoplasm to regulate ferroptosis (Wu et al., 2020). However, there were few studies on the relationship between ferroptosis and circRNA, tRNA, rRNA, piRNA, snRNA, and snoRNA. Studies have reported that the tRNA mutations in HCC leads to decreased expression of selenoproteins, except for GPX4 and GPX1 (glutathione peroxidase 1), and introduces some weak changes in ferroptosis (Kipp et al., 2013; Becker et al., 2014; De Spirt et al., 2016). The regulation of ferroptosis found in HCC about ncRNAs in recent years was sorted out in Table 1 and Figure 1. Wider and deeper studies are needed to explore the function of ncRNAs in ferroptosis.
Treatment of Ferroptosis in HCC
Ferroptosis Associated With Chemotherapy Resistance in HCC
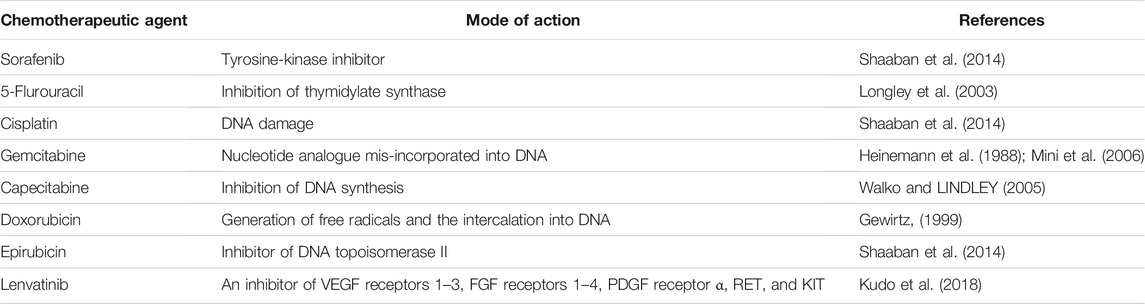
Although the treatments have become more diversified in recent years, the average life expectancy of HCC was lagged far behind those of other cancers. The result of systemic chemotherapy has been particularly disappointing, not only because of the chemotherapeutic resistance of HCC, but also the severe results of major side effects, making the treatment of advanced HCC depends on the degree of underlying liver dysfunction, the burden of malignancy, and the patient’s general profile or expectations. Treatment options for advanced HCC are limited comparing to early HCC. In this context, several therapeutic agents have been developed over the past 50 years to provide better responses and improve the average life expectancy in patients with HCC. Some common chemotherapeutic agents in HCC are summarized in Table 2. However, In two randomized clinical trials of advanced HCC patients in stage III, Sorafenib, which is a commonly used chemotherapy drug, only increased overall survival by 2.8 and 2.3 months compared to the placebo, suggested limited effect to drug-resistant HCC in advanced HCC (Llovet et al., 2008; Cheng et al., 2009). Therefore, overcome the resistance of sorafenib and find more effective new drugs has become an urgency for advanced HCC patients and postoperative adjuvant chemotherapy patients. Different regulatory strategies and delivery routes have been proposed to enhance the antitumor activity of these drugs (Kodama et al., 2008; Hung et al., 2012; Li et al., 2013; Song et al., 2013). Although some ferroptosis inducers, for example, Erastin, are very effective in killing cancer cells in vitro, their pharmacokinetic properties, such as solubility and metabolic stability, are not suitable for the usage in vivo (Yang et al., 2014). It is now believed that sorafenib can induce a new type of regulated cell death-ferroptosis (Louandre et al., 2013), distinct from apoptosis, necrosis, and autophagy (Dixon et al., 2012), not only sorafenib, Guo et al. (2018) killed a variety of tumor cells with cisplatin, which can simultaneously cause apoptosis and ferroptosis. Wu et al. (2018) found that some ncRNAs affect the sensitivity of 5-Fu-resistant cells by regulating some key steps of ferroptosis.
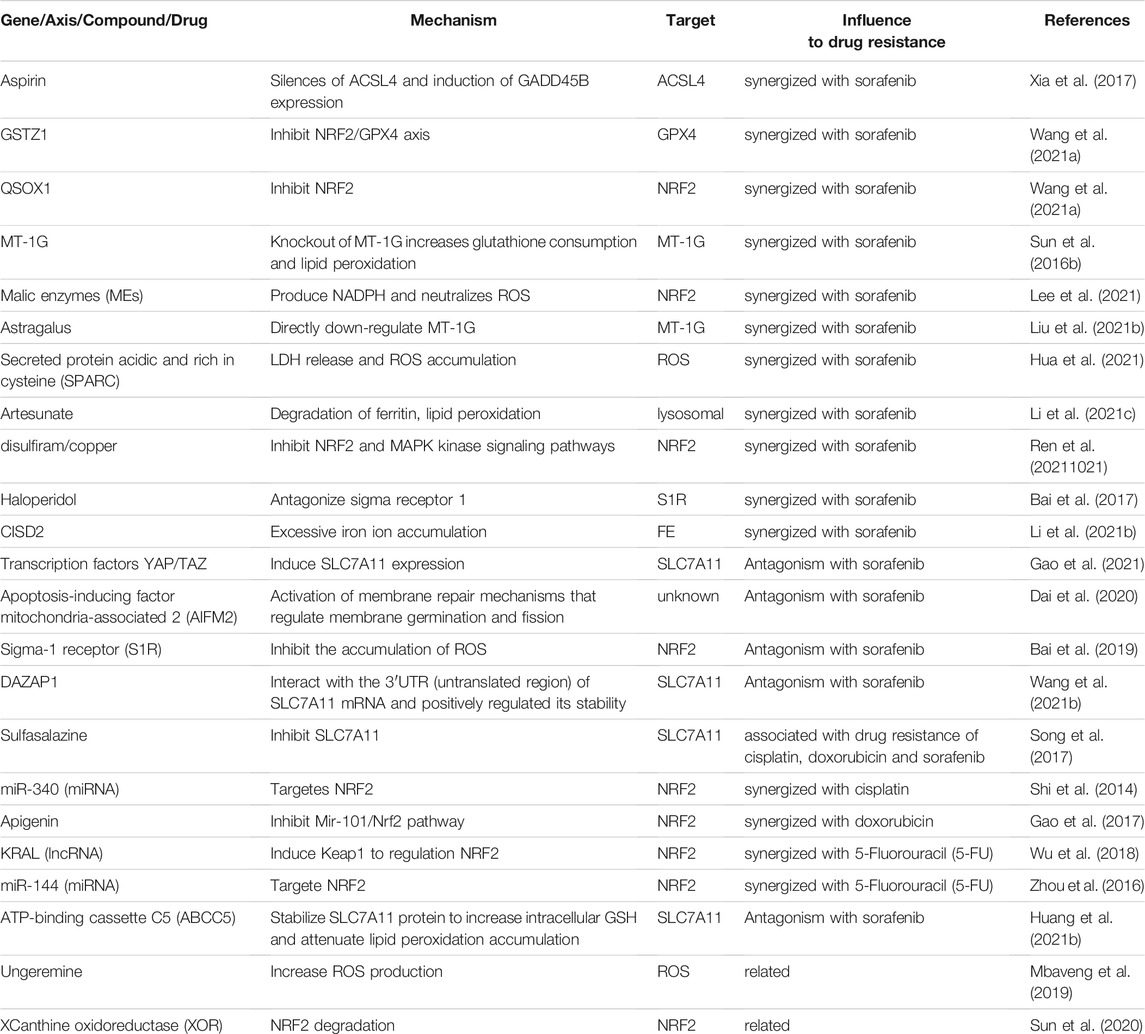
In recent years, the adjustment of HCC-related chemotherapy resistance is shown in Table 3.
Ferroptosis Associated With Radiotherapy Tolerance in HCC
Radiation therapy is an important non-surgical treatment for cancer, but the clinical problems such as low efficacy and severe side effects remained unsolved. Gene therapy can synergistically increase the effect of radiation therapy through its antitumor mechanisms, which may reduce the dose. Radiotherapy induces ferroptosis by down-regulation of SLC7A11 and up-regulation of ACSL4, resulting in GSH production, increasing lipid synthesis, and subsequent oxidative damage (Lang et al., 2019; Lei et al., 2020). Studies have found that collectrin (CLTRN), as a target of radiation, is regulated by NRF1 (nuclear respiratory factor 1)/RAN (RAS oncogene family)/DLD (dihydrolipoamide dehydrogenase) protein complex and enhances the radiosensitivity of HCC cells through ferroptosis (Yuan et al., 2021). A combination of gene therapy and radiation therapy is one way forward, allowing the radiation doses to be reduced and the side effects to be reduced. It is worth considering whether the application of iron death inhibitors to non-tumor cells can increase their radiation tolerance to reduce the adverse effects of radiotherapy.
Ferroptosis Associated With Emerging Therapies in HCC
The use of nano drugs to induce ferroptosis will become a new anticancer strategy (Shen et al., 2018). More and more anticancer nano drugs have been approved by FDA, and the development of drugs with higher efficacy and safety will become an emerging road for future cancer treatment (Bobo et al., 2016). Tang et al. (2019) synthesized manganese-doped mesoporous silica nanoparticles (MMSNs) from manganese and silica. This reaction resulted in the inactivation of GPX4 and the increase of intracellular lipid peroxides through the consumption of intracellular GSH induced by the degradation of MMSNs. Ou et al. (2017) used natural omega-3 fatty acid docosahexaenoic acid (LDL-DHA) reconstructed into Low-density lipoprotein nanoparticles to selectively kill HCC cells. LDL-DHA induces ferroptosis by increasing tissue lipid hydroperoxide levels and inhibition of GPX4 expression. Tian et al. (2022) reported a novel cascade copper-based metal-organic framework (MOF) therapeutic nanocatalyst using HKUST-1 (a kind of metal organic framework) combining meloxicam (Mel), a cyclooxygenase-2 (COX-2) inhibitor, and sorafenib (Sol). Down-regulation of COX-2 induces PINK1/Parkin-mediated mitochondrial autophagy, chemodynamic Therapy (CDT) -mediated cytotoxic ROS, accumulated lipid peroxides (LPO) and Sol through inhibition system XC−, the three interacted to activate ferroptosis and increase the sensitivity of HCC cells to chemotherapy. Liu et al. (2021a) constructed mil-101 (Fe) nanoparticles (NPs) loaded with sorafenib and iRGD (iRGD peptide (amino acid sequence: CRGDK/RGPD/EC) [MIL-101 (Fe) @ SOR], which co-administration significantly promoted the development of ferroptosis. Ma et al. (2017) enhanced the sensitivity of cancer cells to cisplatin by loading cisplatin prodrug onto iron oxide nanoparticles to increase ROS production. Du et al. (2021) designed an exosome with three parts, including surface functionalization of CD47, membrane loading of ferroptosis inducer Er (Erastin), and core of photosensitizer RB (Rose Bengal), and demonstrated potent antitumor therapeutic effects with surprisingly low toxicity.
Discussion
In this review, we summarize recent advances in potential regulators of ferroptosis in HCC and look into the ways ferroptosis can be used to create new therapies in the future. We demonstrate multiple advances in the drug resistance assessments in HCC treatment, the use of multiple genes or compounds to sensitize sorafenib, and the treatment of ferroptosis in HCC in some emerging areas, Nanoparticles such as MMSNs and LDL-DHA prepared in the tumor microenvironment and engineered exosomes with ferroptosis inducers are utilized to induce ferroptosis to bring better prognosis for patients.
The combination of ferroptosis with other therapies, such as immunotherapies, is also promising. Recently, it has been reported that anti-PD-L1 (programmed cell death-Ligand 1) immune checkpoint blockade can induce cancer cell ferroptosis responses by down-regulating SLC7A11 expression in cancer cells as a result of IFN-γ (Interferon γ) secreted by CD8+ T cells (Wang et al., 2019c). Therefore, we believe that therapeutic expansion in ferroptosis may realize effective treatment for patients with advanced HCC.
There are still some issues to be resolved: Although lipid peroxidation is an important factor affecting ferroptosis, what is the actual mechanism of ferroptosis downstream of phospholipid peroxidation? There are many mechanisms of ferroptosis, and many metabolic factors affect the death of tumor cells, the formation of drug resistance, and the avoidance of immune-induced metastasis. It is still unknown that which metabolic factor plays a more important decisive role. In vivo pharmacokinetics of some ferroptosis inducers are still not suitable for in vivo usage, especially how ferroptosis drugs work in liver-specific biotransformation in the treatment of HCC. The fatty acid pool of cells affects the progress of ferroptosis in cells, how to use the change of fatty acid in the blood to determine the progress of ferroptosis in cells? and how to create a fatty acid microenvironment that is conducive to killing tumor cells in the liver?
Author Contributions
SZ reviewed articles, collected data, and wrote the main manuscript text. WZ conceived and designed this study. CY and GX made the chart and figure. XZ, YF, and CP critically analyzed the data and gave valuable advice. KY, JZ, and YM critically revised it for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Jiangsu Natural Science Foundation (SBK2019021253).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abeyawardhane, D. L., and Lucas, H. R. (2019). Iron Redox Chemistry and Implications in the Parkinson's Disease Brain [J]. Oxid Med. Cel Longev 2019, 4609702. doi:10.1155/2019/4609702
Ali, O., Darwish, H. A., Eldeib, K. M., and Abdel Azim, S. A. (2018). miR-26a Potentially Contributes to the Regulation of Fatty Acid and Sterol Metabolism In Vitro Human HepG2 Cell Model of Nonalcoholic Fatty Liver Disease. Oxid Med. Cel Longev 2018, 8515343. doi:10.1155/2018/8515343
Alzhrani, R., Alsaab, H. O., Petrovici, A., Bhise, K., Vanamala, K., Sau, S., et al. (2020). Improving the Therapeutic Efficiency of Noncoding RNAs in Cancers Using Targeted Drug Delivery Systems. Drug Discov. Today 25 (4), 718–730. doi:10.1016/j.drudis.2019.11.006
Anderton, B., Camarda, R., Balakrishnan, S., Balakrishnan, A., Kohnz, R. A., Lim, L., et al. (2017). MYC ‐driven Inhibition of the Glutamate‐cysteine Ligase Promotes Glutathione Depletion in Liver Cancer. EMBO Rep. 18 (4), 569–585. doi:10.15252/embr.201643068
Arefieva, O. D., Vasilyeva, M. S., Zemnukhova, L. A., and Timochkina, A. S. (2021). Heterogeneous Photo-Fenton Oxidation of Lignin of rice Husk Alkaline Hydrolysates Using Fe-Impregnated Silica Catalysts. Environ. Tech. 42 (14), 2220–2228. doi:10.1080/09593330.2019.1697376
Aydin, Y., Kurt, R., Song, K., Lin, D., Osman, H., Youngquist, B., et al. (2019). Hepatic Stress Response in HCV Infection Promotes STAT3-Mediated Inhibition of HNF4A-miR-122 Feedback Loop in Liver Fibrosis and Cancer Progression. Cancers (Basel) 11 (10). doi:10.3390/cancers11101407
Bai, T., Lei, P., Zhou, H., Liang, R., Zhu, R., Wang, W., et al. (2019). Sigma‐1 Receptor Protects against Ferroptosis in Hepatocellular Carcinoma Cells. J. Cel Mol Med 23 (11), 7349–7359. doi:10.1111/jcmm.14594
Bai, T., Wang, S., Zhao, Y., Zhu, R., Wang, W., and Sun, Y. (2017). Haloperidol, a Sigma Receptor 1 Antagonist, Promotes Ferroptosis in Hepatocellular Carcinoma Cells. Biochem. Biophysical Res. Commun. 491 (4), 919–925. doi:10.1016/j.bbrc.2017.07.136
Beccafico, S., Morozzi, G., Marchetti, M. C., Riccardi, C., Sidoni, A., Donato, R., et al. (2015). Artesunate Induces ROS- and P38 MAPK-Mediated Apoptosis and Counteracts Tumor Growthin Vivoin Embryonal Rhabdomyosarcoma Cells. Carcin 36 (9), 1071–1083. doi:10.1093/carcin/bgv098
Becker, N.-P., Martitz, J., Renko, K., Stoedter, M., Hybsier, S., Cramer, T., et al. (2014). Hypoxia Reduces and Redirects Selenoprotein Biosynthesis. Metallomics 6 (5), 1079–1086. doi:10.1039/c4mt00004h
Bobo, D., Robinson, K. J., Islam, J., Thurecht, K. J., and Corrie, S. R. (2016). Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 33 (10), 2373–2387. doi:10.1007/s11095-016-1958-5
Boland, P., and Wu, J. (2018). Systemic Therapy for Hepatocellular Carcinoma: beyond Sorafenib. Chin. Clin. Oncol. 7 (5), 50. doi:10.21037/cco.2018.10.10
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clinicians 68 (6), 394–424. doi:10.3322/caac.21492
Cao, F., Luo, A., and Yang, C. (2021). G6PD Inhibits Ferroptosis in Hepatocellular Carcinoma by Targeting Cytochrome P450 Oxidoreductase. Cell Signal. 87, 110098. doi:10.1016/j.cellsig.2021.110098
Cao, J., Chen, X., Jiang, L., Lu, B., Yuan, M., Zhu, D., et al. (2020). DJ-1 Suppresses Ferroptosis through Preserving the Activity of S-Adenosyl Homocysteine Hydrolase. Nat. Commun. 11 (1), 1251. doi:10.1038/s41467-020-15109-y
Cao, J. Y., and Dixon, S. J. (2016). Mechanisms of Ferroptosis. Cell Mol Life Sci 73 (11-12), 2195–2209. doi:10.1007/s00018-016-2194-1
Cardin, R., Romilda, C., Piciocchi, M., Marika, P., Sinigaglia, A., Alessandro, S., et al. (2012). Oxidative DNA Damage Correlates with Cell Immortalization and Mir-92 Expression in Hepatocellular Carcinoma. BMC Cancer 12, 177. doi:10.1186/1471-2407-12-177
Chang, W. T., Bow, Y. D., Fu, P. J., Li, C. Y., Wu, C. Y., Chang, Y. H., et al. (2021). A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways. Oxid Med. Cel Longev 2021, 7689045. doi:10.1155/2021/7689045
Chen, C. a., Wang, D., Yu, Y., Zhao, T., Min, N., Wu, Y., et al. (2021). Legumain Promotes Tubular Ferroptosis by Facilitating Chaperone-Mediated Autophagy of GPX4 in AKI. Cell Death Dis 12 (1), 65. doi:10.1038/s41419-020-03362-4
Cheng, A.-L., Kang, Y.-K., Chen, Z., Tsao, C.-J., Qin, S., Kim, J. S., et al. (2009). Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: a Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 10 (1), 25–34. doi:10.1016/s1470-2045(08)70285-7
Conrad, M., and Pratt, D. A. (2019). The Chemical Basis of Ferroptosis. Nat. Chem. Biol. 15 (12), 1137–1147. doi:10.1038/s41589-019-0408-1
Cui, M., Xiao, Z., Sun, B., Wang, Y., Zheng, M., Ye, L., et al. (2014). Involvement of Cholesterol in Hepatitis B Virus X Protein-Induced Abnormal Lipid Metabolism of Hepatoma Cells via Up-Regulating miR-205-Targeted ACSL4. Biochem. Biophysical Res. Commun. 445 (3), 651–655. doi:10.1016/j.bbrc.2014.02.068
Czaja, M. J., Ding, W.-X., Donohue, T. M., Friedman, S. L., Kim, J.-S., Komatsu, M., et al. (2013). Functions of Autophagy in normal and Diseased Liver. Autophagy 9 (8), 1131–1158. doi:10.4161/auto.25063
Dai, E., Zhang, W., Cong, D., Kang, R., Wang, J., and Tang, D. (2020). AIFM2 Blocks Ferroptosis Independent of Ubiquinol Metabolism. Biochem. Biophysical Res. Commun. 523 (4), 966–971. doi:10.1016/j.bbrc.2020.01.066
Dai, R., Li, J., Liu, Y., Yan, D., Chen, S., Duan, C., et al. (2010). miR-221/222 Suppression Protects against Endoplasmic Reticulum Stress-Induced Apoptosis via p27(Kip1)- and MEK/ERK-mediated Cell Cycle Regulation. Biol. Chem. 391 (7), 791–801. doi:10.1515/BC.2010.072
De Spirt, S., Eckers, A., Wehrend, C., Micoogullari, M., Sies, H., Stahl, W., et al. (2016). Interplay between the Chalcone Cardamonin and Selenium in the Biosynthesis of Nrf2-Regulated Antioxidant Enzymes in Intestinal Caco-2 Cells. Free Radic. Biol. Med. 91, 164–171. doi:10.1016/j.freeradbiomed.2015.12.011
Ding, C., Ding, X., Zheng, J., Wang, B., Li, Y., Xiang, H., et al. (2020). miR-182-5p and miR-378a-3p Regulate Ferroptosis in I/R-induced Renal Injury. Cel Death Dis 11 (10), 929. doi:10.1038/s41419-020-03135-z
Ding, K., Liao, Y., Gong, D., Zhao, X., and Ji, W. (2018). Effect of Long Non-coding RNA H19 on Oxidative Stress and Chemotherapy Resistance of CD133+ Cancer Stem Cells via the MAPK/ERK Signaling Pathway in Hepatocellular Carcinoma. Biochem. Biophysical Res. Commun. 502 (2), 194–201. doi:10.1016/j.bbrc.2018.05.143
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: An Iron-dependent Form of Nonapoptotic Cell Death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Dixon, S. J., and Stockwell, B. R. (2014). The Role of Iron and Reactive Oxygen Species in Cell Death. Nat. Chem. Biol. 10 (1), 9–17. doi:10.1038/nchembio.1416
Dixon, S. J., Winter, G. E., Musavi, L. S., Lee, E. D., Snijder, B., Rebsamen, M., et al. (2015). Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 10 (7), 1604–1609. doi:10.1021/acschembio.5b00245
Doll, S., Proneth, B., Tyurina, Y. Y., Panzilius, E., Kobayashi, S., Ingold, I., et al. (2017). ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 13 (1), 91–98. doi:10.1038/nchembio.2239
Du, J., Wan, Z., Wang, C., Lu, F., Wei, M., Wang, D., et al. (2021). Designer Exosomes for Targeted and Efficient Ferroptosis Induction in Cancer via Chemo-Photodynamic Therapy. Theranostics 11 (17), 8185–8196. doi:10.7150/thno.59121
Fan, Z., Yang, G., Zhang, W., Liu, Q., Liu, G., Liu, P., et al. (2021). Hypoxia Blocks Ferroptosis of Hepatocellular Carcinoma via Suppression of METTL14 Triggered YTHDF2‐dependent Silencing of SLC7A11. J. Cell. Mol. Medi 25 (21), 10197–10212. doi:10.1111/jcmm.16957
Forcina, G. C., and Dixon, S. J. (2019). GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 19 (18), e1800311. doi:10.1002/pmic.201800311
Friedmann Angeli, J. P., Krysko, D. V., and Conrad, M. (2019). Ferroptosis at the Crossroads of Cancer-Acquired Drug Resistance and Immune Evasion. Nat. Rev. Cancer 19 (7), 405–414. doi:10.1038/s41568-019-0149-1
Gao, A.-M., Zhang, X.-Y., and Ke, Z.-P. (2017). Apigenin Sensitizes BEL-7402/ADM Cells to Doxorubicin through Inhibiting miR-101/Nrf2 Pathway. Oncotarget 8 (47), 82085–82091. doi:10.18632/oncotarget.18294
Gao, M., Yi, J., Zhu, J., Minikes, A. M., Monian, P., Thompson, C. B., et al. (2019). Role of Mitochondria in Ferroptosis. Mol. Cel 73 (2), 354–363. doi:10.1016/j.molcel.2018.10.042
Gao, R., Kalathur, R. K. R., Coto-Llerena, M., Ercan, C., Buechel, D., Shuang, S., et al. (2021). YAP/TAZ and ATF4 Drive Resistance to Sorafenib in Hepatocellular Carcinoma by Preventing Ferroptosis. EMBO Mol. Med. 13 (12), e14351. doi:10.15252/emmm.202114351
Gewirtz, D. (1999). A Critical Evaluation of the Mechanisms of Action Proposed for the Antitumor Effects of the Anthracycline Antibiotics Adriamycin and Daunorubicin. Biochem. Pharmacol. 57 (7), 727–741. doi:10.1016/s0006-2952(98)00307-4
Greene, C. M., Varley, R. B., and Lawless, M. W. (2013). MicroRNAs and Liver Cancer Associated with Iron Overload: Therapeutic Targets Unravelled. Wjg 19 (32), 5212–5226. doi:10.3748/wjg.v19.i32.5212
Guo, J., Xu, B., Han, Q., Zhou, H., Xia, Y., Gong, C., et al. (2018). Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res. Treat. 50 (2), 445–460. doi:10.4143/crt.2016.572
Hao, P.-P., Li, H., Lee, M.-J., Wang, Y.-P., Kim, J.-H., Yu, G.-R., et al. (2015). Disruption of a Regulatory Loop between DUSP1 and P53 Contributes to Hepatocellular Carcinoma Development and Progression. J. Hepatol. 62 (6), 1278–1286. doi:10.1016/j.jhep.2014.12.033
Hassannia, B., Vandenabeele, P., and Vanden Berghe, T. (2019). Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 35 (6), 830–849. doi:10.1016/j.ccell.2019.04.002
He, G.-N., Bao, N.-R., Wang, S., XI, M., Zhang, T.-H., and Chen, F.-S. (2021). Ketamine Induces Ferroptosis of Liver Cancer Cells by Targeting lncRNA PVT1/miR-214-3p/GPX4. Dddt 15, 3965–3978. doi:10.2147/dddt.s332847
Heinemann, V., Hertel, L. W., Grindey, G. B., and Plunkett, W. (1988). Comparison of the Cellular Pharmacokinetics and Toxicity of 2',2'-difluorodeoxycytidine and 1-Beta-D-Arabinofuranosylcytosine. Cancer Res. 48 (14), 4024–4031.
Hou, W., Tian, Q., Steuerwald, N. M., Schrum, L. W., and Bonkovsky, H. L. (2012). The Let-7 microRNA Enhances Heme Oxygenase-1 by Suppressing Bach1 and Attenuates Oxidant Injury in Human Hepatocytes. Biochim. Biophys. Acta 1819 (11-12), 1113–1122. doi:10.1016/j.bbagrm.2012.06.001
Hu, Z., Zhang, H., Yi, B., Yang, S., Liu, J., Hu, J., et al. (2020). VDR Activation Attenuate Cisplatin Induced AKI by Inhibiting Ferroptosis. Cel Death Dis 11 (1), 73. doi:10.1038/s41419-020-2256-z
Hua, H. W., Jiang, H. S., Jia, L., Jia, Y. P., Yao, Y. L., Chen, Y. W., et al. (2021). SPARC Regulates Ferroptosis Induced by Sorafenib in Human Hepatocellular Carcinoma [J]. Cancer Biomark 32 (4), 425–433. doi:10.3233/CBM-200101
Huang, C., Santofimia-Castaño, P., Liu, X., Xia, Y., Peng, L., Gotorbe, C., et al. (2021). NUPR1 Inhibitor ZZW-115 Induces Ferroptosis in a Mitochondria-dependent Manner. Cell Death Discov. 7 (1), 269. doi:10.1038/s41420-021-00662-2
Huang, J., Wang, Y., Guo, Y., and Sun, S. (2010). Down-regulated microRNA-152 Induces Aberrant DNA Methylation in Hepatitis B Virus-Related Hepatocellular Carcinoma by Targeting DNA Methyltransferase 1. Hepatology 52 (1), 60–70. doi:10.1002/hep.23660
Huang, W., Chen, K., Lu, Y., Zhang, D., Cheng, Y., Li, L., et al. (2021). ABCC5 Facilitates the Acquired Resistance of Sorafenib through the Inhibition of SLC7A11-Induced Ferroptosis in Hepatocellular Carcinoma. Neoplasia 23 (12), 1227–1239. doi:10.1016/j.neo.2021.11.002
Hung, C.-S., Lin, S.-F., Liu, H.-H., Kuo, L.-J., Li, L.-T., Su, H.-Y., et al. (2012). Survivin-mediated Therapeutic Efficacy of Gemcitabine through Glucose-Regulated Protein 78 in Hepatocellular Carcinoma. Ann. Surg. Oncol. 19 (8), 2744–2752. doi:10.1245/s10434-011-2188-z
Ingold, I., Berndt, C., Schmitt, S., Doll, S., Poschmann, G., Buday, K., et al. (2018). Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 172 (3), 409–422. doi:10.1016/j.cell.2017.11.048
Jin, M., Shi, C., Li, T., Wu, Y., Hu, C., and Huang, G. (2020). Solasonine Promotes Ferroptosis of Hepatoma Carcinoma Cells via Glutathione Peroxidase 4-induced Destruction of the Glutathione Redox System. Biomed. Pharmacother. 129, 110282. doi:10.1016/j.biopha.2020.110282
Kagan, V. E., Mao, G., Qu, F., Angeli, J. P. F., Doll, S., Croix, C. S., et al. (2017). Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 13 (1), 81–90. doi:10.1038/nchembio.2238
Kamphorst, J. J., Cross, J. R., Fan, J., De Stanchina, E., Mathew, R., White, E. P., et al. (2013). Hypoxic and Ras-Transformed Cells Support Growth by Scavenging Unsaturated Fatty Acids from Lysophospholipids. Proc. Natl. Acad. Sci. 110 (22), 8882–8887. doi:10.1073/pnas.1307237110
Kim, D. H., Kim, W. D., Kim, S. K., Moon, D. H., and Lee, S. J. (2020). TGF-β1-mediated Repression of SLC7A11 Drives Vulnerability to GPX4 Inhibition in Hepatocellular Carcinoma Cells. Cel Death Dis 11 (5), 406. doi:10.1038/s41419-020-2618-6
Kindrat, I., Tryndyak, V., De Conti, A., Shpyleva, S., Mudalige, T. K., Kobets, T., et al. (2016). MicroRNA-152-mediated Dysregulation of Hepatic Transferrin Receptor 1 in Liver Carcinogenesis. Oncotarget 7 (2), 1276–1287. doi:10.18632/oncotarget.6004
Kipp, A. P., Frombach, J., Deubel, S., and Brigelius-Flohé, R. (2013). Selenoprotein W as Biomarker for the Efficacy of Selenium Compounds to Act as Source for Selenoprotein Biosynthesis. Methods Enzymol. 527, 87–112. doi:10.1016/b978-0-12-405882-8.00005-2
Kodama, Y., Fumoto, S., Nishi, J., Nakashima, M., Sasaki, H., Nakamura, J., et al. (2008). Absorption and Distribution Characteristics of 5-Fluorouracil (5-FU) after an Application to the Liver Surface in Rats in Order to Reduce Systemic Side Effects. Biol. Pharm. Bull. 31 (5), 1049–1052. doi:10.1248/bpb.31.1049
Kong, R., Wang, N., Han, W., Bao, W., and Lu, J. (2021). Ifnγ‐mediated Repression of System Xc − Drives Vulnerability to Induced Ferroptosis in Hepatocellular Carcinoma Cells. J. Leukoc. Biol. 110 (2), 301–314. doi:10.1002/jlb.3ma1220-815rrr
Kowdley, K. V. (2004). Iron, Hemochromatosis, and Hepatocellular Carcinoma. Gastroenterology 127 (5 Suppl. 1), S79–S86. doi:10.1016/j.gastro.2004.09.019
Kudo, M., Finn, R. S., Qin, S., Han, K.-H., Ikeda, K., Piscaglia, F., et al. (2018). Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: a Randomised Phase 3 Non-inferiority Trial. The Lancet 391 (10126), 1163–1173. doi:10.1016/s0140-6736(18)30207-1
Labunskyy, V. M., Hatfield, D. L., and Gladyshev, V. N. (2014). Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 94 (3), 739–777. doi:10.1152/physrev.00039.2013
Lang, X., Green, M. D., Wang, W., Yu, J., Choi, J. E., Jiang, L., et al. (2019). Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 9 (12), 1673–1685. doi:10.1158/2159-8290.cd-19-0338
Lee, D., Zhang, M. S., Tsang, F. H. C., Bao, M. H. R., Xu, I. M. J., Lai, R. K. H., et al. (2021). Adaptive and Constitutive Activations of Malic Enzymes Confer Liver Cancer Multilayered Protection against Reactive Oxygen Species. Hepatology 74 (2), 776–796. doi:10.1002/hep.31761
Lei, G., Zhang, Y., Koppula, P., Liu, X., Zhang, J., Lin, S. H., et al. (2020). The Role of Ferroptosis in Ionizing Radiation-Induced Cell Death and Tumor Suppression. Cell Res 30 (2), 146–162. doi:10.1038/s41422-019-0263-3
Li, B., Wei, S., Yang, L., Peng, X., Ma, Y., Wu, B., et al. (2021). CISD2 Promotes Resistance to Sorafenib-Induced Ferroptosis by Regulating Autophagy in Hepatocellular Carcinoma. Front. Oncol. 11, 657723. doi:10.3389/fonc.2021.657723
Li, Q., Liu, X., Yin, Y., Zheng, J. T., Jiang, C. F., Wang, J., et al. (2014). Insulin Regulates Glucose Consumption and Lactate Production through Reactive Oxygen Species and Pyruvate Kinase M2. Oxid Med. Cel Longev 2014, 504953. doi:10.1155/2014/504953
Li, T., Dong, Z.-R., Guo, Z.-Y., Wang, C.-H., Tang, Z.-Y., Qu, S.-F., et al. (2013). Aspirin Enhances IFN-α-Induced Growth Inhibition and Apoptosis of Hepatocellular Carcinoma via JAK1/STAT1 Pathway. Cancer Gene Ther. 20 (6), 366–374. doi:10.1038/cgt.2013.29
Li, W., Hao, J., Zhang, L., Cheng, Z., Deng, X., and Shu, G. (2017). Astragalin Reduces Hexokinase 2 through Increasing miR-125b to Inhibit the Proliferation of Hepatocellular Carcinoma Cells In Vitro and In Vivo. J. Agric. Food Chem. 65 (29), 5961–5972. doi:10.1021/acs.jafc.7b02120
Li, Y., Xia, J., Shao, F., Zhou, Y., Yu, J., Wu, H., et al. (2021). Sorafenib Induces Mitochondrial Dysfunction and Exhibits Synergistic Effect with Cysteine Depletion by Promoting HCC Cells Ferroptosis. Biochem. Biophysical Res. Commun. 534, 877–884. doi:10.1016/j.bbrc.2020.10.083
Li, Z.-j., Dai, H.-q., Huang, X.-w., Feng, J., Deng, J.-h., Wang, Z.-x., et al. (2021). Artesunate Synergizes with Sorafenib to Induce Ferroptosis in Hepatocellular Carcinoma. Acta Pharmacol. Sin 42 (2), 301–310. doi:10.1038/s41401-020-0478-3
Lin, P. L., Tang, H. H., Wu, S. Y., Shaw, N. S., and Su, C. L. (2020). Saponin Formosanin C-Induced Ferritinophagy and Ferroptosis in Human Hepatocellular Carcinoma Cells. Antioxidants (Basel) 9 (8). doi:10.3390/antiox9080682
Lin, Z., Liu, J., Kang, R., Yang, M., and Tang, D. (2021). Lipid Metabolism in Ferroptosis [J]. Adv. Biol. 5 (8), e2100396. doi:10.1002/adbi.202100396
Liu, X., Zhu, X., Qi, X., Meng, X., and Xu, K. (2021). Co-Administration of iRGD with Sorafenib-Loaded Iron-Based Metal-Organic Framework as a Targeted Ferroptosis Agent for Liver Cancer Therapy. Ijn 16, 1037–1050. doi:10.2147/ijn.s292528
Liu, Y., Tao, S., Liao, L., Li, Y., Li, H., Li, Z., et al. (2020). TRIM25 Promotes the Cell Survival and Growth of Hepatocellular Carcinoma through Targeting Keap1-Nrf2 Pathway. Nat. Commun. 11 (1), 348. doi:10.1038/s41467-019-14190-2
Liu, Z., Ma, H., and Lai, Z. (2021). Revealing the Potential Mechanism of Astragalus Membranaceus Improving Prognosis of Hepatocellular Carcinoma by Combining Transcriptomics and Network Pharmacology. BMC Complement. Med. Ther. 21 (1), 263. doi:10.1186/s12906-021-03425-9
Llovet, J. M., Ricci, S., Mazzaferro, V., Hilgard, P., Gane, E., Blanc, J.-F., et al. (2008). Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 359 (4), 378–390. doi:10.1056/nejmoa0708857
Longley, D. B., Latif, T., Boyer, J., Allen, W. L., Maxwell, P. J., and Johnston, P. G. (2003). The Interaction of Thymidylate Synthase Expression with P53-Regulated Signaling Pathways in Tumor Cells. Semin. Oncol. 30 (3 Suppl. 6), 3–9. doi:10.1016/s0093-7754(03)00119-2
Louandre, C., Ezzoukhry, Z., Godin, C., Barbare, J.-C., Mazière, J.-C., Chauffert, B., et al. (2013). Iron-dependent Cell Death of Hepatocellular Carcinoma Cells Exposed to Sorafenib. Int. J. Cancer 133 (7), 1732–1742. doi:10.1002/ijc.28159
Lu, J., Xu, F., and Lu, H. (2020). LncRNA PVT1 Regulates Ferroptosis through miR-214-Mediated TFR1 and P53. Life Sci. 260, 118305. doi:10.1016/j.lfs.2020.118305
Lu, M., Lu, L., Dong, Q., Yu, G., Chen, J., Qin, L., et al. (2018). Elevated G6PD Expression Contributes to Migration and Invasion of Hepatocellular Carcinoma Cells by Inducing Epithelial-Mesenchymal Transition. Acta Biochim. Biophys. Sin (Shanghai) 50 (4), 370–380. doi:10.1093/abbs/gmy009
Lu, S. C. (2009). Regulation of Glutathione Synthesis. Mol. Aspects Med. 30 (1-2), 42–59. doi:10.1016/j.mam.2008.05.005
Lyu, N., Zeng, Y., Kong, Y., Chen, Q., Deng, H., Ou, S., et al. (2021). Ferroptosis Is Involved in the Progression of Hepatocellular Carcinoma through the circ0097009/miR-1261/SLC7A11 axis. Ann. Transl Med. 9 (8), 675. doi:10.21037/atm-21-997
Ma, P. a., Xiao, H., Yu, C., Liu, J., Cheng, Z., Song, H., et al. (2017). Enhanced Cisplatin Chemotherapy by Iron Oxide Nanocarrier-Mediated Generation of Highly Toxic Reactive Oxygen Species. Nano Lett. 17 (2), 928–937. doi:10.1021/acs.nanolett.6b04269
Magtanong, L., Ko, P.-J., To, M., Cao, J. Y., Forcina, G. C., Tarangelo, A., et al. (2019). Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cel Chem. Biol. 26 (3), 420–432. doi:10.1016/j.chembiol.2018.11.016
Majidinia, M., Karimian, A., Alemi, F., Yousefi, B., and Safa, A. (2020). Targeting miRNAs by Polyphenols: Novel Therapeutic Strategy for Aging. Biochem. Pharmacol. 173, 113688. doi:10.1016/j.bcp.2019.113688
Mbaveng, A. T., Bitchagno, G. T. M., Kuete, V., Tane, P., and Efferth, T. (2019). Cytotoxicity of Ungeremine towards Multi-Factorial Drug Resistant Cancer Cells and Induction of Apoptosis, Ferroptosis, Necroptosis and Autophagy. Phytomedicine 60, 152832. doi:10.1016/j.phymed.2019.152832
Mini, E., Nobili, S., Caciagli, B., Landini, I., and Mazzei, T. (2006). Cellular Pharmacology of Gemcitabine. Ann. Oncol. 17 (Suppl. 5), v7–v12. doi:10.1093/annonc/mdj941
Ou, W., Mulik, R. S., Anwar, A., Mcdonald, J. G., He, X., and Corbin, I. R. (2017). Low-density Lipoprotein Docosahexaenoic Acid Nanoparticles Induce Ferroptotic Cell Death in Hepatocellular Carcinoma. Free Radic. Biol. Med. 112, 597–607. doi:10.1016/j.freeradbiomed.2017.09.002
Pant, K., Yadav, A. K., Gupta, P., Islam, R., Saraya, A., and Venugopal, S. K. (2017). Butyrate Induces ROS-Mediated Apoptosis by Modulating miR-22/SIRT-1 Pathway in Hepatic Cancer Cells. Redox Biol. 12, 340–349. doi:10.1016/j.redox.2017.03.006
Qi, W., Li, Z., Xia, L., Dai, J., Zhang, Q., Wu, C., et al. (2019). LncRNA GABPB1-AS1 and GABPB1 Regulate Oxidative Stress during Erastin-Induced Ferroptosis in HepG2 Hepatocellular Carcinoma Cells. Sci. Rep. 9 (1), 16185. doi:10.1038/s41598-019-52837-8
Qin, X., Zhang, J., Lin, Y., Sun, X.-m., Zhang, J.-n., and Cheng, Z.-q. (2020). Identification of MiR-211-5p as a Tumor Suppressor by Targeting ACSL4 in Hepatocellular Carcinoma. J. Transl Med. 18 (1), 326. doi:10.1186/s12967-020-02494-7
Raghunath, A., Sundarraj, K., Arfuso, F., Sethi, G., and Perumal, E. (2018). Dysregulation of Nrf2 in Hepatocellular Carcinoma: Role in Cancer Progression and Chemoresistance. Cancers (Basel) 10 (12). doi:10.3390/cancers10120481
Ren, X., Li, Y., Zhou, Y., Hu, W., Yang, C., Jing, Q., et al. (202110212). Overcoming the Compensatory Elevation of NRF2 Renders Hepatocellular Carcinoma Cells More Vulnerable to Disulfiram/copper-Induced Ferroptosis. Redox Biol. 46, 102122. doi:10.1016/j.redox.2021.102122
Sakurai, T., Kudo, M., Umemura, A., He, G., Elsharkawy, A. M., Seki, E., et al. (2013). p38α Inhibits Liver Fibrogenesis and Consequent Hepatocarcinogenesis by Curtailing Accumulation of Reactive Oxygen Species. Cancer Res. 73 (1), 215–224. doi:10.1158/0008-5472.can-12-1602
Sato, H., Tamba, M., Ishii, T., and Bannai, S. (1999). Cloning and Expression of a Plasma Membrane Cystine/Glutamate Exchange Transporter Composed of Two Distinct Proteins. J. Biol. Chem. 274 (17), 11455–11458. doi:10.1074/jbc.274.17.11455
Shaaban, S., Negm, A., Ibrahim, E. E., and Elrazak, A. A. (2014). Chemotherapeutic Agents for the Treatment of Hepatocellular Carcinoma: Efficacy and Mode of Action. Oncol. Rev. 8 (1), 246. doi:10.4081/oncol.2014.246
Shan, Y., Yang, G., Huang, H., Zhou, Y., Hu, X., Lu, Q., et al. (2020). Ubiquitin-Like Modifier Activating Enzyme 1 as a Novel Diagnostic and Prognostic Indicator that Correlates with Ferroptosis and the Malignant Phenotypes of Liver Cancer Cells. Front. Oncol. 10, 592413. doi:10.3389/fonc.2020.592413
Shang, Y., Luo, M., Yao, F., Wang, S., Yuan, Z., and Yang, Y. (2020). Ceruloplasmin Suppresses Ferroptosis by Regulating Iron Homeostasis in Hepatocellular Carcinoma Cells. Cell Signal 72, 109633. doi:10.1016/j.cellsig.2020.109633
Shen, Z., Song, J., Yung, B. C., Zhou, Z., Wu, A., and Chen, X. (2018). Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv. Mater. 30 (12), e1704007. doi:10.1002/adma.201704007
Shi, L., Chen, Z. G., Wu, L. L., Zheng, J. J., Yang, J. R., Chen, X. F., et al. (2014). miR-340 Reverses Cisplatin Resistance of Hepatocellular Carcinoma Cell Lines by Targeting Nrf2-dependent Antioxidant Pathway. Asian Pac. J. Cancer Prev. 15 (23), 10439–10444. doi:10.7314/apjcp.2014.15.23.10439
Shu, X. L., Fan, C. B., Long, B., Zhou, X., and Wang, Y. (2016). The Anti-cancer Effects of Cisplatin on Hepatic Cancer Are Associated with Modulation of miRNA-21 and miRNA-122 Expression. Eur. Rev. Med. Pharmacol. Sci. 20 (21), 4459–4465.
Song, D. S., Bae, S. H., Song, M. J., Lee, S. W., Kim, H. Y., Lee, Y. J., et al. (2013). Hepatic Arterial Infusion Chemotherapy in Hepatocellular Carcinoma with portal Vein Tumor Thrombosis. Wjg 19 (29), 4679–4688. doi:10.3748/wjg.v19.i29.4679
Song, Y., Jang, J., Shin, T.-H., Bae, S. M., Kim, J.-s., Kim, K. M., et al. (2017). Sulfasalazine Attenuates Evading Anticancer Response of CD133-Positive Hepatocellular Carcinoma Cells. J. Exp. Clin. Cancer Res. 36 (1), 38. doi:10.1186/s13046-017-0511-7
Sun, J., Zhou, C., Zhao, Y., Zhang, X., Chen, W., Zhou, Q., et al. (2021). Quiescin Sulfhydryl Oxidase 1 Promotes Sorafenib-Induced Ferroptosis in Hepatocellular Carcinoma by D Riving EGFR Endosomal Trafficking and Inhibiting NRF2 Activation [J]. Redox Biol. 41, 101942. doi:10.1016/j.redox.2021.101942
Sun, Q., Zhang, Z., Lu, Y., Liu, Q., Xu, X., Xu, J., et al. (2020). Loss of Xanthine Oxidoreductase Potentiates Propagation of Hepatocellular Carcinoma Stem Cells. Hepatology 71 (6), 2033–2049. doi:10.1002/hep.30978
Sun, W., Yi, Y., Xia, G., Zhao, Y., Yu, Y., Li, L., et al. (2019). Nrf2-miR-129-3p-mTOR Axis Controls an miRNA Regulatory Network Involved in HDACi-Induced Autophagy. Mol. Ther. 27 (5), 1039–1050. doi:10.1016/j.ymthe.2019.02.010
Sun, X., Niu, X., Chen, R., He, W., Chen, D., Kang, R., et al. (2016). Metallothionein‐1G Facilitates Sorafenib Resistance through Inhibition of Ferroptosis. Hepatology 64 (2), 488–500. doi:10.1002/hep.28574
Sun, X., Ou, Z., Chen, R., Niu, X., Chen, D., Kang, R., et al. (2016). Activation of the P62-Keap1-NRF2 Pathway Protects against Ferroptosis in Hepatocellular Carcinoma Cells. Hepatology 63 (1), 173–184. doi:10.1002/hep.28251
Sun, X., Ou, Z., Xie, M., Kang, R., Fan, Y., Niu, X., et al. (2015). HSPB1 as a Novel Regulator of Ferroptotic Cancer Cell Death. Oncogene 34 (45), 5617–5625. doi:10.1038/onc.2015.32
Tang, D., and Kroemer, G. (2020). Ferroptosis. Curr. Biol. 30 (21), R1292–R1297. doi:10.1016/j.cub.2020.09.068
Tang, H., Chen, D., Li, C., Zheng, C., Wu, X., Zhang, Y., et al. (2019). Dual GSH-Exhausting Sorafenib Loaded Manganese-Silica Nanodrugs for Inducing the Ferroptosis of Hepatocellular Carcinoma Cells. Int. J. Pharm. 572, 118782. doi:10.1016/j.ijpharm.2019.118782
Tao, J., Krutsenko, Y., Moghe, A., Singh, S., Poddar, M., Bell, A., et al. (2021). Nuclear Factor Erythroid 2-related Factor 2 and β‐Catenin Coactivation in Hepatocellular Cancer: Biological and Therapeutic Implications. Hepatology 74 (2), 741–759. doi:10.1002/hep.31730
Tian, H., Zhao, S., Nice, E. C., Huang, C., He, W., Zou, B., et al. (2022). A Cascaded Copper-Based Nanocatalyst by Modulating Glutathione and Cyclooxygenase-2 for Hepatocellular Carcinoma Therapy. J. Colloid Interf. Sci. 607 (Pt 2), 1516–1526. doi:10.1016/j.jcis.2021.09.049
Walko, C. M., and Lindley, C. (2005). Capecitabine: A Review. Clin. Ther. 27 (1), 23–44. doi:10.1016/j.clinthera.2005.01.005
Wan, Y., Cui, R., Gu, J., Zhang, X., Xiang, X., Liu, C., et al. (2017). Dentification of Four Oxidative Stress-Responsive MicroRNAs, miR-34a-5p, miR-1915-3p, miR-638, and M iR-150-3p, in Hepatocellular Carcinoma. Oxid Med. Cel Longev, 2017, 5189138. doi:10.1155/2017/5189138
Wang, D., Zhang, N., Ye, Y., Qian, J., Zhu, Y., and Wang, C. (2014). Role and Mechanisms of microRNA-503 in Drug Resistance Reversal in HepG2/ADM Human Hepatocellular Carcinoma Cells. Mol. Med. Rep. 10 (6), 3268–3274. doi:10.3892/mmr.2014.2591
Wang, F., Lv, H., Zhao, B., Zhou, L., Wang, S., Luo, J., et al. (2019). Iron and Leukemia: New Insights for Future Treatments. J. Exp. Clin. Cancer Res. 38 (1), 406. doi:10.1186/s13046-019-1397-3
Wang, J., Zhu, S., Meng, N., He, Y., Lu, R., and Yan, G.-R. (2019). ncRNA-Encoded Peptides or Proteins and Cancer. Mol. Ther. 27 (10), 1718–1725. doi:10.1016/j.ymthe.2019.09.001
Wang, L., Liu, Y., Du, T., Yang, H., Lei, L., Guo, M., et al. (2020). ATF3 Promotes Erastin-Induced Ferroptosis by Suppressing System Xc-. Cell Death Differ 27 (2), 662–675. doi:10.1038/s41418-019-0380-z
Wang, Q., Bin, C., Xue, Q., Gao, Q., Huang, A., Wang, K., et al. (2021). GSTZ1 Sensitizes Hepatocellular Carcinoma Cells to Sorafenib-Induced Ferroptosis via Inhibition of NRF2/GPX4 axis. Cel Death Dis 12 (5), 426. doi:10.1038/s41419-021-03718-4
Wang, Q., Guo, Y., Wang, W., Liu, B., Yang, G., Xu, Z., et al. (2021). RNA Binding Protein DAZAP1 Promotes HCC Progression and Regulates Ferroptosis by Interacting with SLC7A11 mRNA. Exp. Cel Res. 399 (1), 112453. doi:10.1016/j.yexcr.2020.112453
Wang, S., Zhang, Q., Wang, Q., Shen, Q., Chen, X., Li, Z., et al. (2018). NEAT1 Paraspeckle Promotes Human Hepatocellular Carcinoma Progression by Strengthening IL-6/STAT3 Signaling. Oncoimmunology 7 (11), e1503913. doi:10.1080/2162402x.2018.1503913
Wang, W., Green, M., Choi, J. E., Gijón, M., Kennedy, P. D., Johnson, J. K., et al. (2019). CD8+ T Cells Regulate Tumour Ferroptosis during Cancer Immunotherapy. Nature 569 (7755), 270–274. doi:10.1038/s41586-019-1170-y
Wang, Z., Chen, X., Liu, N., Shi, Y., Liu, Y., Ouyang, L., et al. (2021). A Nuclear Long Non-coding RNA LINC00618 Accelerates Ferroptosis in a Manner Dependent upon Apoptosis. Mol. Ther. 29 (1), 263–274. doi:10.1016/j.ymthe.2020.09.024
Wu, L., Pan, C., Wei, X., Shi, Y., Zheng, J., Lin, X., et al. (2018). lncRNA KRAL Reverses 5-fluorouracil Resistance in Hepatocellular Carcinoma Cells by Acting as a ceRNA against miR-141. Cell Commun Signal 16 (1), 47. doi:10.1186/s12964-018-0260-z
Wu, Z.-y., Trenner, M., Boon, R. A., Spin, J. M., and Maegdefessel, L. (2020). Long Noncoding RNAs in Key Cellular Processes Involved in Aortic Aneurysms. Atherosclerosis 292, 112–118. doi:10.1016/j.atherosclerosis.2019.11.013
Xia, H., Lee, K. W., Chen, J., Kong, S. N., Sekar, K., Deivasigamani, A., et al. (2017). Simultaneous Silencing of ACSL4 and Induction of GADD45B in Hepatocellular Carcinoma Cells Amplifies the Synergistic Therapeutic Effect of Aspirin and Sorafenib. Cel Death Discov. 3, 17058. doi:10.1038/cddiscovery.2017.58
Xiao, Y., Yan, W., Lu, L., Wang, Y., Lu, W., Cao, Y., et al. (2015). p38/p53/miR-200a-3p Feedback Loop Promotes Oxidative Stress-Mediated Liver Cell Death. Cell Cycle 14 (10), 1548–1558. doi:10.1080/15384101.2015.1026491
Xu, Q., Zhou, L., Yang, G., Meng, F., Wan, Y., Wang, L., et al. (2020). CircIL4R Facilitates the Tumorigenesis and Inhibits Ferroptosis in Hepatocellular Carcinoma by Regulating the miR‐541‐3p/GPX4 axis. Cell Biol Int 44 (11), 2344–2356. doi:10.1002/cbin.11444
Yagoda, N., Von Rechenberg, M., Zaganjor, E., Bauer, A. J., Yang, W. S., Fridman, D. J., et al. (2007). RAS-RAF-MEK-dependent Oxidative Cell Death Involving Voltage-dependent Anion Channels. Nature 447 (7146), 864–868. doi:10.1038/nature05859
Yan, B., Ai, Y., Sun, Q., Ma, Y., Cao, Y., Wang, J., et al. (2021). Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol. Cel 81 (2), 355–369. doi:10.1016/j.molcel.2020.11.024
Yang, F., Yin, Y., Wang, F., Wang, Y., Zhang, L., Tang, Y., et al. (2010). miR-17-5p Promotes Migration of Human Hepatocellular Carcinoma Cells through the P38 Mitogen-Activated Protein Kinase-Heat Shock Protein 27 Pathway. Hepatology 51 (5), 1614–1623. doi:10.1002/hep.23566
Yang, H. C., Wu, Y. H., Yen, W. C., Liu, H. Y., Hwang, T. L., Stern, A., et al. (2019). The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer. Cells 8 (9). doi:10.3390/cells8091055
Yang, W. S., Sriramaratnam, R., Welsch, M. E., Shimada, K., Skouta, R., Viswanathan, V. S., et al. (2014). Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 156 (1-2), 317–331. doi:10.1016/j.cell.2013.12.010
Yang, W. S., and Stockwell, B. R. (2008). Synthetic Lethal Screening Identifies Compounds Activating Iron-dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 15 (3), 234–245. doi:10.1016/j.chembiol.2008.02.010
Yant, L. J., Ran, Q., Rao, L., Van Remmen, H., Shibatani, T., Belter, J. G., et al. (2003). The Selenoprotein GPX4 Is Essential for Mouse Development and Protects from Radiation and Oxidative Damage Insults. Free Radic. Biol. Med. 34 (4), 496–502. doi:10.1016/s0891-5849(02)01360-6
Yuan, H., Li, X., Zhang, X., Kang, R., and Tang, D. (2016). Identification of ACSL4 as a Biomarker and Contributor of Ferroptosis. Biochem. Biophysical Res. Commun. 478 (3), 1338–1343. doi:10.1016/j.bbrc.2016.08.124
Yuan, Y., Cao, W., Zhou, H., Qian, H., and Wang, H. (2021). CLTRN, Regulated by NRF1/RAN/DLD Protein Complex, Enhances Radiation Sensitivity of Hepatocellular Carcinoma Cells through Ferroptosis Pathway. Int. J. Radiat. Oncology*Biology*Physics 110 (3), 859–871. doi:10.1016/j.ijrobp.2020.12.062
Zavattari, P., Perra, A., Menegon, S., Kowalik, M. A., Petrelli, A., Angioni, M. M., et al. (2015). Nrf2, but Not β-catenin, Mutation Represents an Early Event in Rat Hepatocarcinogenesis. Hepatology 62 (3), 851–862. doi:10.1002/hep.27790
Zhang, X., Wang, L., Li, H., Zhang, L., Zheng, X., and Cheng, W. (2020). Crosstalk between Noncoding RNAs and Ferroptosis: New Dawn for Overcoming Cancer Progression. Cel Death Dis 11 (7), 580. doi:10.1038/s41419-020-02772-8
Zhao, L. (2019). Mitochondrial DNA Degradation: A Quality Control Measure for Mitochondrial Genome Maintenance and Stress Response. Enzymes 45, 311–341. doi:10.1016/bs.enz.2019.08.004
Zhao, Y., Li, M., Yao, X., Fei, Y., Lin, Z., Li, Z., et al. (2020). HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and its Therapeutic Implications. Cel Rep. 33 (10), 108487. doi:10.1016/j.celrep.2020.108487
Zheng, J., and Conrad, M. (2020). The Metabolic Underpinnings of Ferroptosis. Cel Metab. 32 (6), 920–937. doi:10.1016/j.cmet.2020.10.011
Zhou, S., Ye, W., Zhang, Y., Yu, D., Shao, Q., Liang, J., et al. (2016). miR-144 Reverses Chemoresistance of Hepatocellular Carcinoma Cell Lines by Targeting Nrf2-dependent Antioxidant Pathway. Am. J. Transl Res. 8 (7), 2992–3002.
Zhu, G., Murshed, A., Li, H., Ma, J., Zhen, N., Ding, M., et al. (2021). O-GlcNAcylation Enhances Sensitivity to RSL3-Induced Ferroptosis via the YAP/TFRC Pathway in Liver Cancer. Cel Death Discov. 7 (1), 83. doi:10.1038/s41420-021-00468-2
Zou, C., Zou, C., Cheng, W., Li, Q., Han, Z., Wang, X., et al. (2016). Heme Oxygenase-1 Retards Hepatocellular Carcinoma Progression through the microRNA Pathway. Oncol. Rep. 36 (5), 2715–2722. doi:10.3892/or.2016.5056
Zou, S., Rao, Y., and Chen, W. (2019). miR‐885‐5p Plays an Accomplice Role in Liver Cancer by Instigating TIGAR Expression via Targeting its Promoter. Biotechnol. Appl. Biochem. 66 (5), 763–771. doi:10.1002/bab.1767
Keywords: ferroptosis, drug resistance, treatment, hepatocellular carcinoma, regulatory cell death
Citation: Zhao S, Zheng W, Yu C, Xu G, Zhang X, Pan C, Feng Y, Yang K, Zhou J and Ma Y (2022) The Role of Ferroptosis in the Treatment and Drug Resistance of Hepatocellular Carcinoma. Front. Cell Dev. Biol. 10:845232. doi: 10.3389/fcell.2022.845232
Received: 05 January 2022; Accepted: 04 February 2022;
Published: 03 March 2022.
Edited by:
Lian Xiang Luo, Guangdong Medical University, ChinaReviewed by:
Liang He, University of California, San Francisco, United StatesXiang Wang, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH), United States
Yixuan Guo, The University of Utah, United States
Copyright © 2022 Zhao, Zheng, Yu, Xu, Zhang, Pan, Feng, Yang, Zhou and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kunxing Yang, MjQ3MjcwMTY1M0BxcS5jb20=; Jin Zhou, Z2VvcmdlbmptdUAxNjMuY29t; Yong Ma, eW1hMDkxN0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Siqi Zhao
Siqi Zhao Wubin Zheng†
Wubin Zheng† Chao Yu
Chao Yu Gaoxin Xu
Gaoxin Xu Jin Zhou
Jin Zhou Yong Ma
Yong Ma