- 1Department of General Intensive Care Unit, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 2Translational Medicine Research Center, Medical Innovation Research Division and Fourth Medical Center of the Chinese PLA General Hospital, Beijing, China
Efferocytosis is the effective clearance of apoptotic cells by professional and non-professional phagocytes. The process is mechanically different from other forms of phagocytosis and involves the localization, binding, internalization, and degradation of apoptotic cells. Defective efferocytosis has been demonstrated to associate with the pathogenesis of various inflammatory disorders. In the current review, we summarize recent findings with regard to efferocytosis networks and discuss the relationship between efferocytosis and different immune cell populations, as well as describe how efferocytosis helps resolve inflammatory response and modulate immune balance. Our knowledge so far about efferocytosis suggests that it may be a useful target in the treatment of numerous inflammatory diseases.
Introduction
Cell turnover is usually achieved through apoptosis (Elmore, 2007) and other newly regulated cell death programs. Importantly, dying cells release molecular signals that direct phagocytes to sites of death and regulate immune response to maintain tissue homeostasis (Devitt and Marshall, 2011). This multi-step process is known as efferocytosis, which comes from the Latin word “effere”, meaning “take to the grave” (de Cathelineau and Henson, 2003). In the pathogenesis of efferocytosis, phagocytes such as macrophages, dendritic cells (DCs), monocytes, and epithelial cells destroy and recycle dead cells (Arandjelovic and Ravichandran, 2015). In fact, so-called “find me” signals recruit phagocytes, while “eat me” signals trigger uptake of apoptotic cells.
Accumulating evidence suggests that efferocytosis is vital for tissue repair, inflammation resolution, and immune system balance during homeostasis (Monks et al., 2005; Arandjelovic and Ravichandran, 2015; Sachet et al., 2017; Boada-Romero et al., 2020). Accordingly, impaired efferocytosis can lead to the accumulation of apoptotic cells in inflamed foci, subsequently resulting in cell necrosis, cytolysis, and the production of tissue-damaging intracellular components (Monks et al., 2005; Szondy et al., 2014; Arandjelovic and Ravichandran, 2015; Sachet et al., 2017). Moreover, abnormal efferocytosis may induce substantial inflammatory response and contribute to the development of various inflammatory disorders (McCubbrey and Curtis, 2013; Karaji and Sattentau, 2017; Abdolmaleki et al., 2018; Horst et al., 2019; Morioka et al., 2019; Doran et al., 2020; Yoshimura et al., 2020). Over recent years, the underlying mechanisms and regulatory pathways of efferocytosis in inflammatory and autoimmune diseases have been widely studied. This review summarizes the recent findings with regard to efferocytosis signals and their impacts on host immunity, which might be of importance to understand the pathophysiology of abnormal efferocytosis and inflammatory diseases.
Efferocytosis and Its Receptor Network
Apoptosis is a highly organized process that accelerates embryogenesis and maintains cell growth (Elmore, 2007). Generally, apoptosis is terminated by efferocytosis, which prevents the aggregation of cell corpses, inflammatory response, and secondary necrosis of other cells (Devitt and Marshall, 2011; Arandjelovic and Ravichandran, 2015). Efferocytosis is performed by professional phagocytes, including DCs and macrophages, and non-professional phagocytes, such as fibroblasts and epithelial cells, which recognize “find me” and “eat me” signals from apoptotic cells (Arandjelovic and Ravichandran, 2015). Specifically, efferocytosis involves four steps (Figure 1): 1) phagocyte recruitment regulated by “find me” signals, 2) identification of dead cells guided by “eat me” signals, 3) uptake of cellular corpses (Monks et al., 2005; Boada-Romero et al., 2020), 4) degradation of dying cells (Monks et al., 2005; Boada-Romero et al., 2020). Nevertheless, healthy cells can escape efferocytosis via “tolerate me” signals, also known as “keep me” or “don’t eat me” signals (Doran et al., 2020).
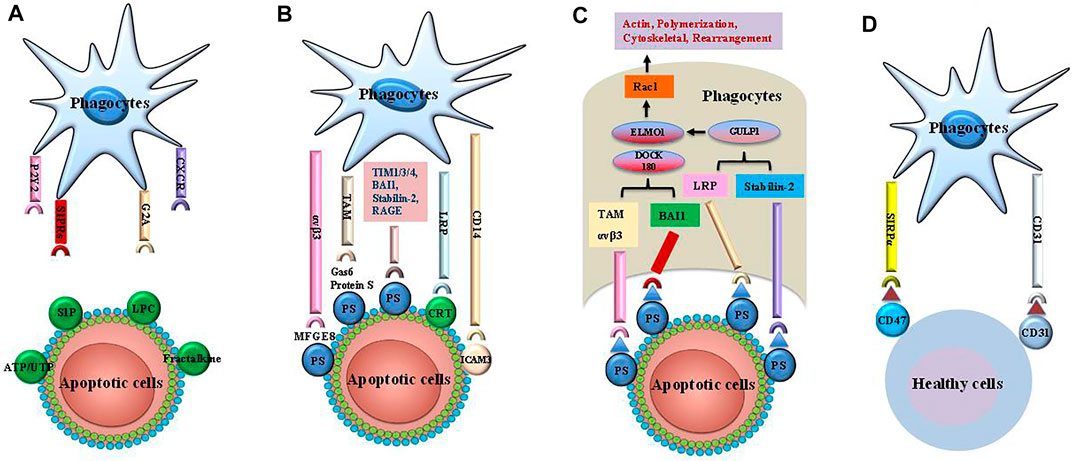
FIGURE 1. The steps of efferocytosis. Efferocytosis is a multi-steps process that involves several steps: finding apoptotic cells, binding apoptotic cells, internalizing and digestion of apoptotic cells. (A) The apoptotic cells release a series of “find me” signals including lysophosphatidylcholine sphingosine-1-phosphate (S1P), uridine diphosphate (UTP) and adenosine triphosphate (ATP), which attract phagocytes to region of apoptotic corpses. These signals are recognized by phagocytes using cognate receptors such as CXCR3, G-protein-coupled receptor (G2A), purinergic receptors (P2Y2), andsphingosine-1-phosphate receptor (S1PRs). (B) The “eat me” signals on the apoptotic cells are sensed by phagocytes, which ingest these dying cells via several receptors and bridging molecules. These crucial signals comprise brain-specific angiogenesis inhibitor 1 (BAI1), T cell immunoglobulin mucin receptor (TIM) 1, TIM3, TIM4, the receptor for advanced glycationend products (RAGE), stabilin-2, phosphatidylserine (PS)-specific bridging molecules, growth arrest specific 6 (Gas6), milk fat globule epidermal growth factor VIII (MFG-E8), and protein S. In addition, calreticulin (CRT) and intercellular adhesion molecule (ICAM) 3 act as the “eat me” signals via interaction with CD14 and low-density lipoprotein-related protein (LRP). (C) Engulfment of apoptotic cells are conducted by phagocytes by recruitment of ingestion receptors along with Rac pathways, the polymerization of actin and rearranging of cytoskeletal. Ingestion receptors can recruit the DOCK180/ELMO1 set [αvβ3, integrin, Tyro3-Ax1-MER proto-oncogene tyrosine kinase (MERTK) (TAM), stabilin-2, and LRP]. D. Healthy cells can resist efferocytosis and leave phagocytes unengulfed via “tolerate me” signals (e.g., CD47, CD31) on the cell surface. SIRPα on the surface of phagocytes can recognize CD47. Similarly, CD31 homodimerizes with CD31 on the phagocytes. Finally, mitochondrial fission and an increase in cytoplasmic calcium occurs in efferocytes. These cellular changes are critical for phagosome sealing. Phagosome fusion with lysosome leads to degradation of apoptotic cells via acid hydrolase activity. The processing of engulfed apoptotic corpses uses microtubule-associated protein light chain 3 (LC3)-dependent phagocytosis and exhibit anti-inflammatory activities.
“Find Me” Signals
“Find me” signals are released by apoptotic cells to distinguish them from healthy cells and to recruit phagocytes to sites of death (Ravichandran, 2010; Doran et al., 2020). These signals also act as danger-associated molecular patterns (DAMPs) and mediate the formation of various cytokines as well as chemokines for phagocyte activation (Karaji and Sattentau, 2017; Morioka et al., 2019). “Find me” signals consist of sphingosine-1-phosphate (S1P), lysophosphatidylcholine (LPC), C-X-C motif chemokine ligand 1 (CX3CL1), and nucleotides (Gude et al., 2008; Truman et al., 2008; Elliott et al., 2009; Chekeni et al., 2010; Garris et al., 2013). S1P is produced from sphingosine via sphingosine kinases and modulates phagocyte chemotaxis by engaging G-protein-coupled receptors (Gude et al., 2008; Garris et al., 2013), while LPC is generated by caspase-3 and phospholipase A2 (Ravichandran, 2010). Nucleotides such as uridine diphosphate and adenosine triphosphate (ATP) promote the interaction of phagocytes with purinergic receptors, thus favoring phagocytic clearance of apoptotic cells (Elliott et al., 2009). As a chemokine, CX3CL1 is released from apoptotic lymphocytes in a caspase- and Bcl-2-dependent manner. Macrophages are attracted to apoptotic sites via interaction with CX3CL1 and macrophage fractalkine receptor (a “find-me” signal) (Peter et al., 2008; Truman et al., 2008). However, the underlying molecular mechanisms are obscure.
“Eat Me” Signals
In the second step of efferocytosis, apoptotic cells bind directly to cell surface receptors (e.g., low-density lipoprotein receptor-related protein 1, T cell immunoglobulin mucin receptor (TIM) 1, TIM3, TIM4, adhesion G protein-coupled receptor B1, stabilin-1, and stabilin-2) (Gardai et al., 2005; Park et al., 2008a; Rodriguez-Manzanet et al., 2010; He et al., 2011; Mazaheri et al., 2014), in turn inducing pleiotropic effects through several bridging molecules such as protein S, milk fat globule epidermal growth factor 8 (MFG-E8), and vitamin K-dependent protein growth arrest specific 6 (Gas6) (Fadok et al., 1998a; Scott et al., 2001; Thorp et al., 2008; Hu et al., 2009; Qi et al., 2013; Nguyen et al., 2014; Soki et al., 2014; Toda et al., 2014; Nepal et al., 2019; Rymut et al., 2020). Similarly, transglutaminase 2 (TG2) acts as a coreceptor for integrin β3 and binds MFG-E8, inducing uptake of apoptotic cells via activating Rac 1. Conversely, integrin β3 cannot recognize the apoptotic cells in the absence of TG2 (Tóth et al., 2009; Sághy et al., 2019).
Phosphatidylserine (PS), which is found in the inner leaflet of living cells and is expressed externally via caspase signals during apoptosis (Park et al., 2008b; Segawa et al., 2014), appears to be a key factor in “eat me” signals. “Eat me” signals are recognized directly by PS binding receptors or indirectly by bridging mediators on phagocytes. Direct binding of PS to the receptor augments the formation of advanced glycation end products (RAGE) and macrophage efferocytosis (Thorp et al., 2008; Birge et al., 2016). MFG-E8 is capable of recognizing PS and being recognized by phagocyte (e.g., DCs, macrophages) surface receptors αVβ3 and αVβ5. Interaction with these receptors can lead to cytoskeletal rearrangements and then promote the uptake of apoptotic cells (Hanayama et al., 2004; Hu et al., 2009; Soki et al., 2014). In addition, bridging of the complement factor C1q with PS is recognized by scavenger receptor class F member 1 on endothelial and phagocytotic cells (Pulanco et al., 2017), while soluble CD93 interacting with PS and integrin αxβ2 on apoptotic cells mediates efferocytosis via an opsonin (Martin et al., 1996). These results suggest that interaction with PS can accelerate the engulfment of dying cells.
PS serves as the most characterized “eat-me” signal. PS can be recognized by several membrane receptors such as Stabilin-1, Stabilin-2, TIM4, RAGE, and BAI-1. It has been demonstrated that PS receptors play a key role in the recognition mechanism of dead cells (Park et al., 2008a; Rodriguez-Manzanet et al., 2010; Mazaheri et al., 2014; Nishi et al., 2014). For example, Stabilin-1 and -2 expressed by macrophages recognize PS on apoptotic cells and enhance the ingestion of apoptotic debris (Park et al., 2008b; Rantakari et al., 2016). This process is essential for the capture and elimination of PS-stimulated injured or aged erythrocytes. The CD300 family of type I transmembrane proteins can recognize PS and phosphatidylethanolamine during apoptosis (Voss et al., 2015). Thus, CD300f and CD300d deficiency can disrupt efferocytosis by macrophages (Tian et al., 2016). The scavenger receptors SR-A1, SR-B1, and CD36 recognize PS and promote efferocytosis by macrophages (Fadok et al., 1998b; Terpstra and van Berkel, 2000).
As we known, high mobility group box-1 protein (HMGB1) is a classical DAMP that can suppress RAGE/PS-mediated efferocytosis by binding to integrin αvβ3 in macrophages (Friggeri et l., 2010). Conversely, HMGB1-deficient macrophages effectively phagocytose apoptotic neutrophils and thymocytes (Wang et al., 2013), leading to translocation of HMGB1 into the cytoplasm and its secretion into the extracellular milieu (Banerjee et al., 2011). In addition, the Ras homolog family (Rho) of small GTPases, including Rho-associated coiled-coil-containing protein serine/threonine kinase, Rho A, CDC42, Rab5, and Rac, is critically involved in regulating the uptake of dying cells (Castellano et al., 2000; Erwig et al., 2006; Nakaya et al., 2006; Moon et al., 2010).
Uptake and Degradation of Dying Cells
Phagocytes recognize and home to cell corpses, then internalize them via plasma membrane reorganization (Boada-Romero et al., 2020). The remodeling of actin leads to invagination and localized extravagation of the plasma membrane and phagosome formation. After cell engulfment, the resulting phagosome fuses with lysosomes to digest cell corpses (Boada-Romero et al., 2020; Doran et al., 2020). Lysosomes contain several lipases, nucleases, and proteases that digest the apoptotic cells to maintain homeostasis (Viaud et al., 2018). Microtubule-associated protein light chain 3 is involved in the canonical autophagy pathway, while unc-51-like kinase 1/2 complex plays a crucial role in the digestion of dying cells (Florey et al., 2011; Asare et al., 2020).
“Tolerate Me” Signals
Healthy cells express transmembrane molecules that down-regulate efferocytosis through “tolerate me” signals (Martinez, 2017). Binding of CD47 to the signal regulatory protein-α on the macrophage surface inhibits the actin cytoskeleton rearrangements required for phagocytosis (Willingham et al., 2012; Kojima et al., 2016; Cao et al., 2022). Phagocytic clearance is blocked by the sialoglycoprotein CD24, which binds to sialic acid-binding Ig like lectin 10 (Siglec-10) on macrophages (Barkal et al., 2019). CD31 on macrophages and healthy cells can inhibit phagocytosis (Brown et al., 2002).
Major histocompatibility complex (MHC) class I molecules are positively expressed on healthy cells and interact with the inhibitory receptor leukocyte immunoglobulin-like receptor subfamily B member 1, which contribute to blocking the engulfment of apoptotic cells and the expression of inflammatory molecules (Barkal et al., 2018). However, the release of lactoferrin glycoprotein from apoptotic cells acts as a “tolerate me” signal, in turn removing eosinophils and neutrophils from the sites of death (Bournazou et al., 2009; Bournazou et al., 2010). Recently, plasminogen activator inhibitor-1 has been shown to induce excessive accumulation of neutrophils and inflammatory response in tissues, leading to organ dysfunction. Therefore, it may be a novel “tolerate me” signal for apoptotic neutrophils (Park et al., 2008a).
Efferocytosis in Immune Cells and Underlying Regulatory Mechanisms
Efferocytosis and Macrophages
Macrophages play important roles in innate immunity and the restoration of tissue homeostasis, as they ingest infected apoptotic cells to kill bacteria and limit cell necrosis, while they drive adaptive immune response by degrading pathogen-associated antigens and presenting them to effector T cells (Martin et al., 2014; Blander, 2017).
Macrophages can exhibit two main phenotypes depending on the stimuli and microenvironment: a pro-inflammatory M1 phenotype, or a pro-resolving M2 (“alternatively activated”) phenotype (Angsana et al., 2016; Elliott et al., 2017). Efferocytosis shifts macrophages towards the M2 phenotype, which can reduce levels of pro-inflammatory cytokines [e.g., tumor necrosis factor alpha (TNF-α), CXCL-8, LBT4, interleukin (IL)-6] and enhance the release of anti-inflammatory mediators [IL-10 and transforming growth factor-beta (TGF-β)] as well as pro-resolving molecules (Fadok et al., 1998a; Dalli and Serhan, 2012; Angsana et al., 2016). The resolution of inflammation is regulated by the balance between pro-inflammatory cytokines and pro-resolving mediators, such as lipoxins and resolvins, which have been shown to augment efferocytosis of apoptotic cells by macrophages (Korns et al., 2011; Schif-Zuck et al., 2011). For instance, resolvin E4 effectively limits neutrophil infiltration and induces efferocytotic ingestion of apoptotic neutrophils and senescent red blood cells by macrophages. Likely, resolvin D1 improves efferocytosis in aging by limiting senescent cell-mediated MERTK cleavage (Schif-Zuck et al., 2011; Nishi et al., 2014). MERTK-binding bridging molecules contribute to efferocytosis-associated inflammatory resolution (Nishi et al., 2014; Toda et al., 2014), while the Tyro3-Ax1-MERTK (TAM) family of receptor tyrosine kinases in macrophages is involved in the recognition and clearance of dead cells, as well as the activation of anti-inflammatory signals (Qi et al., 2013; Toda et al., 2014; Bernsmeier et al., 2015). Furthermore, efferocytosis can promote non-inflammatory macrophage proliferation to help resolve tissue injury via inducing DNA-dependent protein kinase-mammalian target of rapamycin-rictor complex 2/Rictor pathway (Gerlach et al., 2021).
Efferocytosis is an anti-inflammatory process sensitive to cyclic adenosine 3,5′-monophosphate (cAMP). cAMP is a crucial intracellular molecule that affects phagocytosis and reprogramming of macrophages for inflammation resolution, while it can stimulate macrophage efferocytosis of apoptotic neutrophils by protein kinase A (PKA) signaling (Negreiros-Lima et al., 2020). In addition, the secreted protein endothelial locus-1 (DEL-1) has recently been found to inhibit leukocyte-endothelial adhesion and inflammation initiation, serving as a potent stimulator of efferocytosis (Kourtzelis et al., 2019). Moreover, DEL-1-mediated efferocytosis reprograms macrophages to adopt the pro-resolving phenotype, suggesting that DEL-1 facilitates homeostatic functions in the setting of inflammation (Kourtzelis et al., 2019).
Efferocytosis and Dendritic Cells
It is widely accepted that DCs are professional antigen-presenting cells that can activate T cells. Costimulatory molecules, including CD80, CD86, and MHC-II, are crucial for DC maturation and subsequent activation of native CD4+ T cells, and their deficiency contributes to T cell immunosuppression or tolerance (Blachere et al., 2005). DCs have been identified as key phagocytes in recognizing apoptotic cells and regulating adaptive immunity (Albert et al., 1998; Schaible et al., 2003; Blachere et al., 2005), while they efficiently mediate efferocytosis to suppress immune response to self-antigens. Moreover, phagocytosis of infected apoptotic cells by DCs releases high amounts of CD86 and CC-chemokine receptor type 7 and favors the production of prostaglandin E2 (PGE2), IL-10, and IL-1β (Pujol-Autonell et al., 2013; Dejani et al., 2018). In contrast, inhibiting the recognition of infected cells markedly prevent the maturation of DCs (de Aquino Penteado et al., 2017). Efferocytosis of sterile apoptotic cells only slightly affects the phenotype and immune properties of DCs (de Aquino Penteado et al., 2017), yet it acts via PGE2 to down-regulate host immunity to self-antigens (Pujol-Autonell et al., 2013). Of note, further studies should be performed to investigate the opposing impacts of efferocytosis on DC maturation.
Several studies have shown that efferocytosis is involved in the cross-presentation and activation of CD8+ and CD4+ T cells during viral infection and tumor growth (Yrlid and Wick, 2000; Larsson et al., 2002; Bosnjak et al., 2005; Desch et al., 2011; Tzelepis et al., 2015). DCs recognize and ingest infected apoptotic cells, thus increasing the immune response to invading organisms (Albert et al., 1998; Blachere et al., 2005). Efferocytosis of infected apoptotic cells improved the production of IL-6 and PGE2, and the expression of CCR7 and CD86, and migration on DCs. Following recognition of infected cells, the maturation and migration of DCs correlated with high expression of cyclooxygenase-2 (COX-2) and PGE2, and activation of pattern recognition receptors by bacterial components (Yrlid and Wick, 2000; Pujol-Autonell et al., 2013; Tzelepis et al., 2015; Dejani et al., 2018), which further promoted lymph node-directed migration and up-regulated a Th2-type immune response (Dejani et al., 2018). Similar results were observed for apoptotic cells infected with influenza virus, human immunodeficiency virus (HIV)-1, herpes simplex virus, Salmonella typhimurium, Mycobacterium tuberculosis, vaccinia virus, and human cytomegalovirus (Larsson et al., 2002; Bosnjak et al., 2005; Desch et al., 2011; Tzelepis et al., 2015). For example, HIV-1-infected dying monocytes were phagocytosed by DCs, resulting in antigen cross-presentation (MHC class I or II) and T cell activation (Larsson et al., 2002; Werfel and Cook, 2018). Collectively, the available data indicate that efferocytosis of infected apoptotic cells by DCs leads to antigen presentation and activation of effector T cells together with elevation of COX-2 and PGE2 levels.
Efferocytosis and Neutrophils
Neutrophils are short-lived cells that act as first responders of innate immunity and infiltrate into inflamed sites upon infection (Greenlee-Wacker, 2016). However, substantial neutrophil aggregation and secondary necrosis exacerbate inflammatory cascades, thereby leading to self-amplifying tissue injury and organ dysfunction (Greenlee-Wacker, 2016). Neutrophils can be inactivated via apoptosis, forming apoptotic cells that are removed by tissue-resident macrophages through an efferocytotic mechanism (Maimon et al., 2020; Sekheri et al., 2020). It was reported that culture of macrophages or monocytes with apoptotic neutrophils increased the levels of IL-10 and TGF-β (Kim et al., 2019), while their engulfment enhanced the release of specialized pro-resolving mediators, such as lipoxin B4 and resolvin 1/2, indicating the potential anti-inflammatory action of apoptotic neutrophils (Sun et al., 2015; Moges et al., 2018). Additionally, neutrophil efferocytosis guided by phagocytic signals was found to be regulated by urokinase receptor-associated procedures (Park et al., 2009).
There is increasing evidence that neutrophil function may affect efferocytotic activity in macrophages. Neutrophils exert a specific defense mechanism against microbes, known as neutrophil extracellular traps (NETs), which can capture and kill extracellular bacteria (Chen et al., 2021). NETs act through a unique form of non-apoptotic cell death, defined as NETosis, where molecules produced by neutrophils interfere with the recognition of dying cells (Bukong et al., 2018). For instance, HMGB1 serves as a DAMP that activates pro-inflammatory cascades [e.g., Toll-like-receptor (TLR), NLR family, pyrin domain-containing 3 (NLRP3)] and inhibits efferocytosis. In such process, HMGB1 binds to PS or RAGE receptors, thereby preventing the recognition of other PS-binding proteins (Friggeri et al., 2010; Banerjee et al., 2011; Wang et al., 2013).
Neutrophils modulate an efferocytotic “Trojan horse” way in certain infections. Bacteria such as Yersinia pestis and Chlamydia pneumonia can be phagocytosed by neutrophils, within which the bacteria multiply. Then neutrophils are engulfed by macrophages, promoting efferocytosis and increasing PS exposure (Jondle et al., 2018; Lim et al., 2020). Reportedly, phagocytosis of methicillin-resistant Staphylococcus aureus by neutrophils mediated the expression of the “tolerate me” signal CD47 and prevented macrophage efferocytosis (Willingham et al., 2012; Greenlee-Wacker et al., 2014). Failure to efferocytose dying and infected neutrophils resulted in neutrophil necrosis and the release of living bacteria (Lim et al., 2020). In line with these observations, Klebsiella pneumoniae escaped killing by macrophage-induced efferocytosis, as it prevented neutrophil pyroptosis and efferocytosis (Jondle et al., 2018).
Efferocytosis and Regulatory T Cells
Regulatory T cells (Tregs) interact with various innate and adaptive immune cells and exert potent immunosuppression by inhibiting T cell function, promoting macrophages to the M2 phenotype, releasing anti-inflammatory mediators, enhancing immune tolerance, and accelerating inflammation resolution (Josefowicz et al., 2012; Weirather et al., 2014). Tregs have been found to modulate macrophage efferocytosis in animal models of inflammatory states, including peritonitis, atherosclerosis, and acute lung injury (ALI) (Proto et al., 2018). IL-13 secretion by Tregs stimulates the production of IL-10 in macrophages, which in turn induces macrophage efferocytosis by initiating Rac1-associated actin accumulation in the phagosome and apoptotic cell internalization (Proto et al., 2018), thus enhancing the proliferative activity of Tregs. The disposal of apoptotic cells leads to the production of IL-10 and TGF-β in macrophages, further increasing the Treg population (Kleinclauss et al., 2006). Similarly, apoptotic cell infusion accelerates the expansion of Tregs (Cummings et al., 2016). CD103+ DCs also engulf apoptotic cells, stimulating the differentiation of Tregs and inducing intestinal epithelial cell apoptosis [106]. Consistent with these results, phagocytosis of non-infected cells by macrophages favors the generation of Tregs and the production of anti-inflammatory mediators including PGE2, platelet-activating factor, and TGF-β (Tiemessen et al., 2007; Proto et al., 2018).
Efferocytosis and Other Immune Cells
Ly6C+ monocytes were reported to induce efferocytosis via TLR ligation (Larson et al., 2016). For instance, TLR7 stimulated Ly6C+ monocytes to improve the cross-presentation of cell-associated antigen to CD8+ T cells, implicating a role for these monocytes in protective immunity (Arrode et al., 2000). Efferocytosis of infected apoptotic cells appeared to favor T helper (Th)17 immune response. Phagocytosis of Escherichia coli-infected cells by DCs facilitated the production of PGE2, IL-1β, and pro-Th17 cytokines, such as IL-6 and TGF-β (Torchinsky et al., 2009), while PGE2-EP4 signaling obviously inhibited Th17 cell differentiation and phosphorylation of signal transducer and activator of transcription (STAT) 3 (Monks et al., 2008). Strikingly, apoptotic epithelial cells were engulfed by residual viable epithelial cells into spacious efferosomes during post-lactation involution of the mouse mammary gland (Monks et al., 2005; Monks et al., 2008).
Abnormal Efferocytosis and Inflammatory Disorders
Defects in efferocytosis has been demonstrated to result in substantial inflammatory responses that do not resolve, leading in turn to various pathologies (Morioka et al., 2019). Aberrant efferocytosis appears to be involved in several inflammatory and autoimmune disorders, including infection (Figure 2), ALI, asthma, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), diabetes, multiple sclerosis (MS), autoimmune lymphoproliferative syndrome (ALPS), and other inflammatory conditions (Figure 3 and Table 1) (McCubbrey and Curtis, 2013; Szondy et al., 2014; Karaji and Sattentau, 2017; Abdolmaleki et al., 2018; Horst et al., 2019; Doran et al., 2020; Yoshimura et al., 2020).
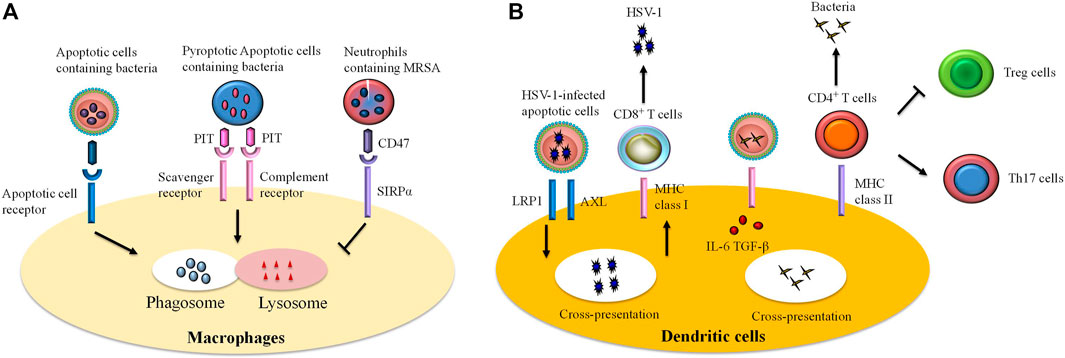
FIGURE 2. The role of efferocytosis by immune cells in infections. Efferocytosis participates in host defense against invading microbes. However, certain pathogens can escape efferocytosis and accelerate their spread. Efferocytosis is accomplished by professional and unprofessional phagocytes. Remarkably, macrophages and dendritic cells are professional phagocytes that are the most commonly studied of the efferocytes. (A) Ingestion of infected apoptotic cells by macrophages limits secondary necrosis of these cells and bacteria release, thereby improving bacteria clearance. In some cases, certain bacteria can trigger pyroptosis in infected cells. Efferocytotic receptors (i.e., scavenger receptor, complement receptor) are able to recognize pore-induced intracellular traps (PITs) and pytoptotic neutrophil containing bacteria. Subsequently, the process results in bacteria killing via infusion of phagosome to lysosome. However, some pathogens [e.g., methicillin-resistant Staphylococcus aureus (MRSA)] escape efferocytosis through “tolerate me” signal CD47 and signal regulatory protein α (SIRPα) on the infected cells. (B) Recognition and internalization of some viruses [such as herpes simplex virus type 1 (HSV-1)]-infected cells by dendritic cells interact with RAN-binding protein 9 (RANBP9), low-density lipoprotein receptor-related protein 1 (LRP1), and a protein complex comprising AXL. The cross presentation of viral antigen by dendritic cells on MHC class I molecules induces differentiation of CD8+ T cells against viruses. Similarly, dendritic cells swallow infected cells and thereby promote expansion of anti-bacteria effector T cells. In the infected cells, pathogen-associated molecular patterns (PAMPs) signal via Toll-like receptors (TLRs) and stimulate production of transforming growth factor-beta (TGF-β) and interleukin (IL)-6, thereby expanding the population of CD4+ T cells to T helper (Th)17 cells but inhibiting generation of regulatory T (Treg) cells.
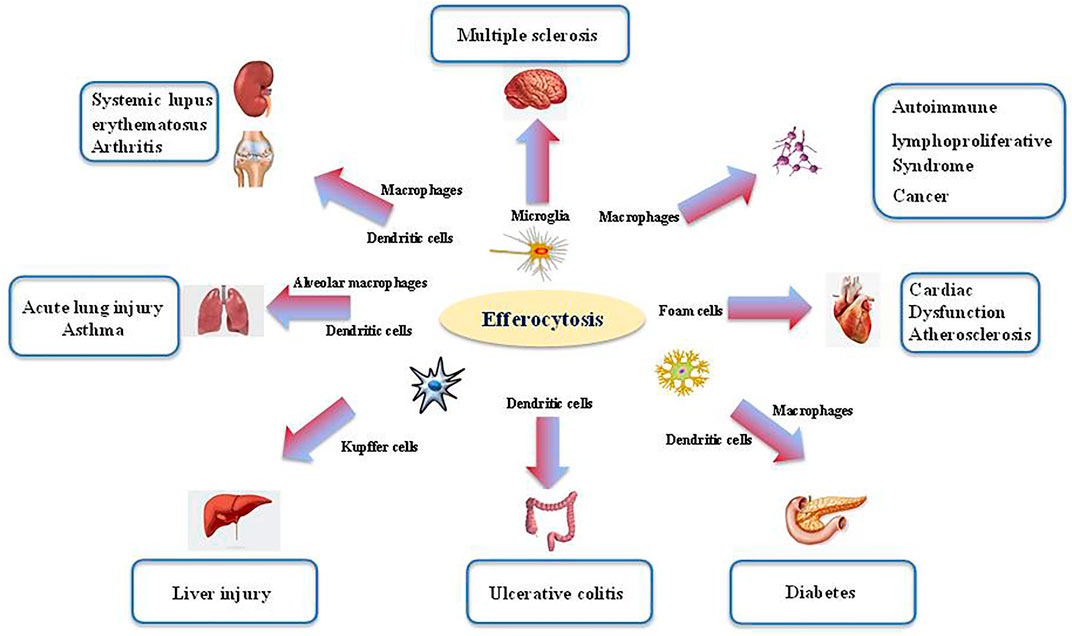
FIGURE 3. The role of efferocytosis in various inflammatory disorders. Efferocytosis is required for tissue homeostasis and organ development. The process is mainly orchestrated by several phagocytes (i.e., microglia, macrophage, dendritic cells). Aberrant efferocytosis has been associated with several inflammatory and autoimmune disorders, including infection, acute lung injury (ALI), asthma, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), diabetes, multiple sclerosis (MS), autoimmune lymphoproliferative syndrome (ALPS) and other inflammatory conditions.
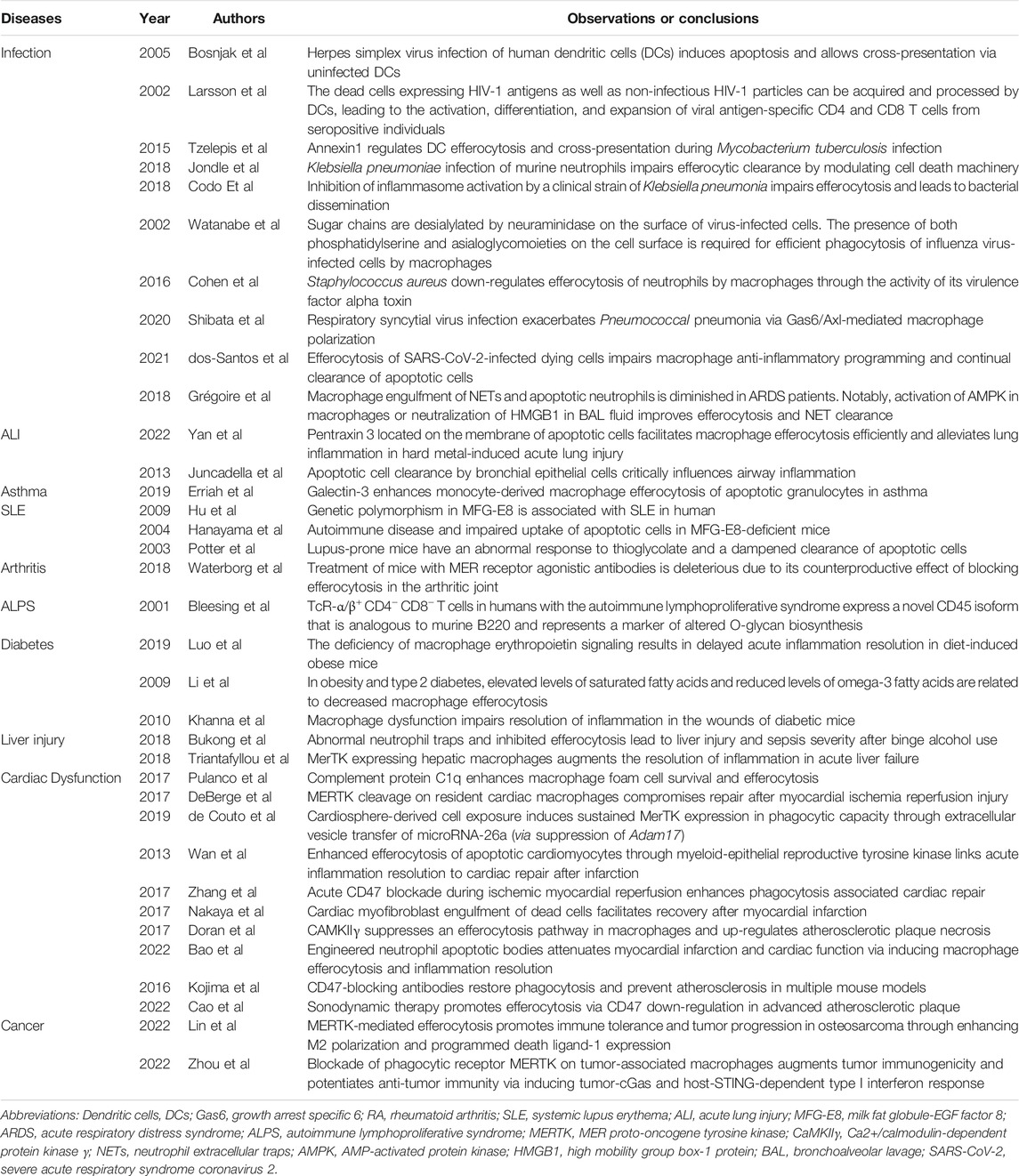
TABLE 1. Summary of studies concerning the significance of efferocytosis in various inflammatory diseases.
Infection
Pathogen-mediated cell death allows the host to limit pathogen multiplication and dissemination: the host’s defense cells engulf and eliminate bacteria through phagocytosis, while they eliminate apoptotic cells through efferocytosis (Figure 2) (Karaji and Sattentau, 2017). However, some pathogens may invade the phagocyte via efferocytosis and accelerate their own multiplication and spread by expressing the “tolerate me” signal CD47 on the surface of infected cells, which prevents the cells from being efferocytosed (Brown and Neher, 2012).
A recent study showed that low-virulence strains of Mycobacterium tuberculosis stimulated apoptosis, generating apoptotic cells that could be engulfed by phagocytes. Mycobacterium tuberculosis remained alive in the phagosome and lysosome-phagosome fusion, further promoting bacteria killing (Tzelepis et al., 2015). Stimulating efferocytosis actually exacerbated Mycobacterium tuberculosis infection in mice and blocked the engulfment of apoptotic Mycobacterium tuberculosis-infected macrophages (Andersson et al., 2020), and knocking out TIM4 in mice induced defective bacterial growth, suggesting that blockade of efferocytosis couldn’t neutralize bacteria (Divangahi et al., 2009; Nishi et al., 2014). Taken together, Mycobacterium tuberculosis infection drives cell necrosis, blocks the uptake of infected apoptotic cells by macrophages, and prevents efferocytosis, leading to bacterial spread (Tzelepis et al., 2015).
Klebsiella pneumoniae infection can inhibit apoptosis and trigger non-apoptotic programmed cell death mechanisms such as necroptosis (Jondle et al., 2018). At the same time, it can prevent the efferocytotic engulfment of neutrophils by macrophages in the lungs (Codo et al., 2018; Jondle et al., 2018). Klebsiella pneumoniae infection was noted to be associated with increased activity of PS transporter flippases and reduced PS externalization and caspase activity (Birge et al., 2016). Blockade of necroptosis restored the efferocytotic ingestion of Klebsiella pneumoniae-infected neutrophils (Jondle et al., 2018).
Macrophages phagocytose influenza A virus-infected HeLa cells in a phosphatidylserine-dependent manner during the process of cellular apoptosis. Moreover, engulfment of influenza A virus-treated cells resulted in suppression of virus growth. It was shown that influenza A virus-infected cells appeared to be susceptible to macrophage phagocytosis (Shiratsuchi et al., 2000; Watanabe et al., 2002; Lim et al., 2020). Efferocytotic engulfment of apoptotic HIV-1-infected cells by astrocytes in the brain increases resistance to infection and reduces viral spread (Larsson et al., 2002; Werfel and Cook, 2018). Recently, it was observed that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection could cause widespread cell apoptosis (Chan et al., 2020) and boost the release of chemokines and cytokines (Dutta et al., 2022). The “cytokine storm” was potent to impair macrophage function and impedes efferocytosis of apoptotic cells (dos-Santos et al., 2021). In contrast, the human papilloma virus suppresses efferocytosis, thus exacerbating infection (Bosnjak et al., 2005). Nevertheless, only a few studies on the effects of efferocytosis on viral spread have been reported.
Engulfment of Trypanosoma cruzi-infected apoptotic T cells by macrophages accelerated parasite expansion and promoted the production of TGF-β and PGE2, thereby improving virus infectivity (Decote-Ricardo et al., 2017). Efferocytosis is inhibited in sepsis through unknown mechanisms, and blockade of apoptotic cell efferocytosis by phagocytes exacerbates sepsis by increasing levels of sepsis-associated histones and DAMPs that impair apoptotic cell ingestion (Bukong et al., 2018). The extracellular cold-inducible RNA-binding protein (cCIRP), a DAMP that can initiate inflammatory response, has been recently identified. cCIRP-primed NETs prevented efferocytosis in a mouse sepsis model by reducing the levels of integrins αvβ3/αvβ5 in macrophages, indicating that targeting cCIRP via the efferocytotic pathway may be a new therapeutic approach against septic challenge (Cohen et al., 2016).
Acute Lung Injury
ALI is characterized by sustained inflammatory response, disruption of the endothelial-epithelial barrier, alveolar injury, and pulmonary edema, and its pathogenesis has been associated with impaired efferocytosis in a mouse model (McCubbrey and Curtis, 2013). Alveolar macrophages help maintain lung homeostasis during ALI by rapidly removing apoptotic neutrophils through efferocytosis and exerting antimicrobial activity. Moreover, M1-type macrophages induce the expression of STAT6 and relieve ALI by triggering the expression of Gas6, an efferocytotic ligand (Shibata et al., 2020). A recent study indicated that pentraxin 3 located on the membrane of apoptotic cells facilitated macrophage efferocytosis efficiently and alleviated lung inflammation in hard metal-induced acute lung injury (Yan et al., 2022).
NETs are critical for immobilizing and preventing pathogen invasion by releasing pro-inflammatory cytokines and proteases (Jorgensen et al., 2016). However, the increased formation of NETs along with their incomplete efferocytotic uptake may exacerbate inflammation in the development of ALI (Grégoire et al., 2018). Interestingly, restoration of AMP-activated protein kinase activity by metformin or blockade of HMGB1 in bronchoalveolar lavage fluid promoted NETs efferocytosis, and it might provide a potential therapeutic target for attenuating persistent lung inflammation in ALI (Fox et al., 2010).
Asthma
Asthma is characterized by hyper-responsiveness and exaggerated inflammatory cell infiltration in the airways. Airway allergens can induce the production of inflammatory cytokines by tissue-resident mast cells and facilitate eosinophil migration to the airways (McCubbrey and Curtis, 2013). Defective efferocytotic ingestion of apoptotic cells by airway macrophages has been associated with the pathogenesis of asthma: the excessive accumulation of dying cells stimulates sustained inflammation and secondary necrosis in the lungs (Juncadella et al., 2013). Hence, restoring efferocytosis may be a promising therapeutic approach to eliminate inflammation in asthma. For instance, the release of galectin-3 by macrophages significantly augmented phagocytosis, chemotaxis, and cell activation. However, galectin-3 levels in the sputum of asthma patients were low, thus impairing efferocytosis and allowing sustained airway inflammation (Erriah et al., 2019). Galectin-3 stimulates efferocytotic engulfment of apoptotic cells by airway macrophages in asthma, suggesting that elevated galectin-3 levels might be a way to rescue efferocytosis in asthma (Erriah et al., 2019).
Systemic Lupus Erythematosus and Arthritis
SLE is a common autoimmune disease with various clinical symptoms manifesting in the lung, heart, kidney, joint, skin, and nervous system, and its pathogenesis has recently been associated with abnormal efferocytosis (Abdolmaleki et al., 2018). The complement factor C1q binds to apoptotic cells via IgM and LPC signals and its deficiency may contribute to the development of SLE. Impairment of C1q in mice lacking the efferocytotic bridging molecule MFG-E8 markedly reduced the uptake of apoptotic cells and necrosis in response to autoantibodies and cellular compartments (Hanayama et al., 2004; Hu et al., 2009; Pulanco et al., 2017). By allowing the aggregation of unengulfed dying cells, C1q deficiency contributes to the development of SLE-associated glomerulonephritis. Indeed, genetic targeting of the complement C1q subcomponent subunit A increases the number of apoptotic cells and favors the generation of autoantibodies and glomerulonephritis (Potter et al., 2003).
RA is a chronic inflammatory and progressive joint disorder manifested by the production of serum autoantibodies against rheumatoid factor, complement protein C3, and citrullinated peptides (Abdolmaleki et al., 2018). Given that DNA is degraded by dnase II in lysosomes, dnase II deficiencies have been associated with the pathogenesis of RA and polyarthritis, which are lysosomal storage diseases (Martin et al., 2014). Aggregated DNA in macrophage lysosomes of dnase II-deficient mice activated innate immune response, but other undigested cellular constituents in the lysosomes stimulated production of TNF-α and interferon-β (Waterborg et al., 2018).
These results clearly support that aberrant efferocytosis can disrupt self-tolerance and contribute to the development of several autoimmune disorders. However, further studies are needed to explore the underlying mechanisms.
Autoimmune Lymphoproliferative Syndrome
ALPS is characterized by increased numbers of CD4−CD8− T cells, high levels of circulating IL-10 and Fas ligand (FasL), and hypergammaglobulinemia (Abdolmaleki et al., 2018). The Fas/FasL pathway is crucial for cell apoptosis and its mutation has been observed in a mouse model of ALPS. The TNF receptor family is involved in apoptosis and helps limit the accumulation of self-reactive T and B lymphocytes. Therefore, impaired apoptosis stimulates the immune system due to FasL mutation and defective signaling (Bleesing et al., 2001; Hao et al., 2008), while the Fas/FasL cascade may act as a “find me” signal during efferocytosis (Cullen et al., 2013).
Multiple Sclerosis
MS is a chronic degenerative disease of the central nervous system characterized by axonal injury, demyelination, oligodendroglial cell death, persistent inflammation (Abdolmaleki et al., 2018), and excitotoxicity and activation of metabotropic (P2Y) as well as ionotropic (P2X) receptors and ATP via the glutamate pathway [94]. P2Y and P2X have been found to recognize “find me” and “eat me” signals in MS, implicating dysregulated efferocytosis in the disease (North, 2002; Locovei et al., 2007). The link between efferocytosis and pathogens further needs to be clarified.
Diabetes
Pancreatic B cell destruction leads to hyperglycemia and insulin deficiency, and it has been closely related to the pathogenesis of diabetes, especially the type I form. Moreover, insufficient removal of apoptotic B cells results in aggregation of dying cells, which in turn mediates the release of autoantigens and the activation of inflammatory signals. Studies in mouse models had linked abnormal efferocytosis with diabetes (Morioka et al., 2019; Doran et al., 2020).
Abnormal efferocytosis in obesity, which is a key factor in type II diabetes, also contributes to defective erythropoietin (EPO) signaling. Specifically, S1P produced in apoptotic cells can bind to the cognate receptor on macrophages, promoting efferocytosis via the EPO-EPO receptor-peroxisome proliferator-activated receptor-γ signal (Luo et al., 2016; Luo et al., 2019).
A study in mice showed that low-density lipoprotein receptor deficiency resulted in lesional efferocytosis and to larger necrotic cores than those in healthy animals (Li et al., 2009). Incomplete efferocytosis can slow wound healing and allow persistent inflammation due to apoptotic cell accumulation at the wound site (Khanna et al., 2010), which are the most common complications in diabetes. Of note, much remains to be clarified concerning how abnormal efferocytosis contributes to diabetes.
Ulcerative Colitis
Ulcerative colitis (UC) is a chronic form of inflammatory bowel disease, characterized by the accumulation of uncleared apoptotic cells in inflammed tissue. Recent studies have shown that enhanced apoptosis or abnormal efferocytosis contribute to the pathogenesis of UC (Abdolmaleki et al., 2018; Morioka et al., 2019; Doran et al., 2020). Bacterial host recognition is critical to the pathophysiology of UC, as lipopolysaccharide (LPS), the main component of the bacterial cell wall, can bind to TLR4 and activate NF-κB-associated inflammatory cascades (Török et al., 2004). Other inflammatory complexes, such as bacterial permeability-increasing and LPS-binding proteins, are also recognized by CD14 in UC patients (Baird et al., 2016), while interaction of CD14 with intercellular adhesion molecule 3 favors efferocytosis by promoting the recognition and engulfment of dying cells (Weiss, 2003). Engulfment of apoptotic corpses by DCs and epithelial cells controlled the disease in a mouse model (Dejani et al., 2018), confirming that targeting efferocytosis might help reduce gut inflammation.
Liver Injury
MERTK, a TAM receptor, acts as a key bridging molecule during efferocytosis. TAM receptor-deficient mice were more prone to autoimmune hepatitis-like diseases, while increased MERTK-expressing macrophages that infiltrated into necrotic sites were been observed in patients with acute liver injury (Nguyen et al., 2014; Toda et al., 2014; Bernsmeier et al., 2015; Horst et al., 2019; Rymut et al., 2020). MERTK deficiency in a mouse model of acute liver injury increased numbers of myeloperoxidase (MPO)+ neutrophils and reduced numbers of liver macrophages, indicating the critical role of MERTK in the clearance of dying neutrophils (Triantafyllou et al., 2018). In addition, giving the mice with acute liver injury a secretory leukocyte protease inhibitor reduced the number of MPO+TUNEL+ neutrophils, suggesting a therapeutic approach against acute hepatic damage (Triantafyllou et al., 2018). Patients with decompensated cirrhosis and acute-on-chronic hepatic dysfunction showed an increased number of MERTK-expressing macrophages and monocytes, which linked to reduced levels of the pro-inflammatory mediators IL-6 and TNF-α (Bernsmeier et al., 2015). This clinical finding suggests that administering MERTK inhibitors can enhance the production of these pro-inflammatory cytokines in MERTK+ monocytes (Bernsmeier et al., 2015). Thus, expanding the population of MERTK-expressing myeloid cells may promote the removal of necrotic components and thereby serve as a treatment against acute liver injury.
Similar to MERTK, Gas6 is a bridging molecule that is highly expressed in liver macrophages and can be stimulated with carbon tetrachloride (CCI4). Gas6 deficiency reduces numbers of infiltrating monocytes and levels of pro-inflammatory mediators, limiting hepatocyte expansion and Kupffer cell proliferation (Nepal et al., 2019). Moreover, Gas6/Ax1 signaling in CCI4-induced hepatic injury blocks NLRP3 inflammasome activity and autophagy, which is important to homeostasis maintenance (Shibata et al., 2020). Incomplete neutrophil efferocytosis following excessive alcohol consumption in a mouse model exacerbated sepsis-associated liver injury, revealing a potential therapeutic target against liver injury (Bukong et al., 2018).
Cardiac Dysfunction
Efferocytosis has been reported to relate to the process of myocardial repair (Yoshimura et al., 2020). Deleting MERTK from macrophages in mice with reperfusion injury impaired cardiac function, increased infarct size, and reduced cardiac wound debridement (DeBerge et al., 2017). Conversely, delivery of MERTK and C1q to macrophages via extracellular vesicles enhanced efferocytosis and cardioprotection in mice after myocardial infarction (Wan et al., 2013; de Couto et al., 2019). Likewise, MFG-E8 deficiency leads to cardiac inflammation, necrosis, and cardiac dysfunction (Soki et al., 2014), whereas MFG-E8 administration has the opposite effects (Zhang et al., 2017). Cardiac myofibroblasts producing MEF-E8 can efficiently recognize dead cardiac cells and thereby promote recovery from myocardial infarction (Nakaya et al., 2017). Interestingly, a recent study showed that engineered neutrophil apoptotic bodies attenuated myocardial infarction and cardiac function via inducing macrophage efferocytosis and inflammation resolution (Bao et al., 2021).
Inflammatory signaling in apoptotic cells at lesion sites results in overexpression of the “tolerate me” signal CD47, promoting resistance to internalization and thereby compromising efferocytosis (Willingham et al., 2012). Treatment with a neutralizing anti-CD47 antibody during ischemic myocardial reperfusion accelerated the removal of apoptotic myocytes by phagocytes, promoted the resolution of cardiac inflammation, preserved cardiac function, and reduced infarct size, implying that targeting CD47 might protect against myocardial reperfusion (Kojima et al., 2016; Cao et al., 2022). Mesenchymal stem cells in a rat model were reported to improve cardiac function by promoting M2 macrophage-mediated efferocytosis of apoptotic neutrophils (Doran et al., 2017).
These results illustrate the close association between efferocytosis and inflammatory resolution in cardiac disorders. Notably, they demonstrate the potential for exploiting efferocytosis to attenuate myocardial ischemia-reperfusion injury and control immune response.
Cancer
Tumor-associated macrophages act as a type of phagocyte involved in efferocytosis. These macrophages are M2-polarized and promotes the production of anti-inflammatory mediators and regulatory T cells, suppressing effector T cells. Subsequently, removal of dying cells by macrophages inhibits inflammatory responses and provides tumor cells a microenvironment to escape from immunological surveillance (Soki et al., 2014). It has been demonstrated that efferocytosis not only facilitates the proliferation, invasion, metastasis, and angiogenesis of tumor cells, but also affects the drug resistance to anti-cancer treatments (Birge et al., 2016; Barkal et al., 2018; Barkal et al., 2019; Tajbakhsh et al., 2021). Recently, Lin et al. reported that MERTK-mediated efferocytosis promotes immune tolerance and tumor progression in osteosarcoma through enhancing M2 polarization and programmed death ligand-1 expression (Lin et al., 2022). Furthermore, an excellent study revealed that blockade of phagocytic receptor MERTK on tumor-associated macrophages augmented tumor immunogenicity and potentiated anti-tumor immunity via inducing tumor-cGas and host-STING-dependent type I interferon response (Zhou et al., 2020). Thus, efferocytosis-targeted therapy may represent a potential approach for treating cancers.
Conclusion and Perspectives
Phagocytes rapidly remove apoptotic cells via efferocytosis to ensure tissue repair and organ development. Efferocytosis involves the recognition of “find me” and “eat me” signals, followed by phagosome-lysosome fusion and digestion of apoptotic corpses. Phagocytes selectively recognize and ingest apoptotic cells because normal cells display “tolerate me” signals. However, it is unclear whether the “tolerate me” and “eat me” signals are cell-specific and whether they exert different impacts on phagocytic cells in certain circumstances.
In this review, we focus on the potential role of efferocytosis in the regulation of immune response and homeostasis, and the effect of aberrant efferocytosis on the pathogenesis of inflammatory disorders. Macrophage efferocytosis of apoptotic cells stimulates the differentiation of macrophages into a pro-resolving phenotype by enhancing the production of pro-resolving mediators and angiogenic growth factors and by reducing the levels of pro-inflammatory cytokines. In this way, efferocytosis prevents an excessive inflammatory response and favors tissue repair. On the one hand, efferocytosis regulates immune response, such as by eliminating invading pathogens; on the other hand, some pathogens can “hijack” efferocytosis to infect phagocytes, where they multiply and spread in a protected manner via a “Trojan horse” mechanism.
The importance of efferocytosis is reflected in the fact that defects in the process contribute to various inflammatory disorders. Understanding more about what regulates efferocytosis and developing ways to activate it may be useful therapeutic approaches against inflammatory diseases. Most investigations of efferocytosis have been performed in cellular and animal studies, with little development being made in the clinical context. Further research is critically needed to explore the impact of regulating efferocytosis on susceptibility to inflammatory pathologies and the safety of this approach in clinical settings. Additionally, it is possible that there is a dynamic balance of efferocytosis involved in inflammation and immunity or tissue repair. Accordingly, studies are warranted to precisely evaluate the function of efferocytosis and immune responses in inflammatory disorders Weirather et al., 2014.
Author Contributions
YG and MH conducted the literature review and drafted the manuscript. Y-mY conceptualized and supervised the project, and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81,974,293, 81,873,946, 82,072,201, 81,730,057) and the Military Medical Innovation Program of Chinese PLA (18CXZ026), the Natural Science Foundation of Zhejiang Province (LY20H150011), and the Zhejiang Province Medicine and Health Science and Technology Program (2021KY710).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdolmaleki, F., Farahani, N., Gheibi Hayat, S. M., Pirro, M., Bianconi, V., Barreto, G. E., et al. (2018). The Role of Efferocytosis in Autoimmune Diseases. Front. Immunol. 9, 1645. doi:10.3389/fimmu.2018.01645
Albert, M. L., Sauter, B., and Bhardwaj, N. (1998). Dendritic Cells Acquire Antigen from Apoptotic Cells and Induce Class I-Restricted CTLs. Nature 392, 86–89. doi:10.1038/32183
Andersson, A.-M., Larsson, M., Stendahl, O., and Blomgran, R. (2020). Efferocytosis of Apoptotic Neutrophils Enhances Control of Mycobacterium tuberculosis in HIV-Coinfected Macrophages in a Myeloperoxidase-dependent Manner. J. Innate Immun. 12, 235–247. doi:10.1159/000500861
Angsana, J., Chen, J., Liu, L., Haller, C. A., and Chaikof, E. L. (2016). Efferocytosis as a Regulator of Macrophage Chemokine Receptor Expression and Polarization. Eur. J. Immunol. 46, 1592–1599. doi:10.1002/eji.201546262
Arandjelovic, S., and Ravichandran, K. S. (2015). Phagocytosis of Apoptotic Cells in Homeostasis. Nat. Immunol. 16, 907–917. doi:10.1038/ni.3253
Arrode, G., Boccaccio, C., Lulé, J., Allart, S., Moinard, N., Abastado, J.-P., et al. (2000). Incoming Human Cytomegalovirus Pp65 (UL83) Contained in Apoptotic Infected Fibroblasts Is Cross-Presented to CD8 + T Cells by Dendritic Cells. J. Virol. 74, 10018–10024. doi:10.1128/jvi.74.21.10018-10024.2000
Asare, P. F., Roscioli, E., Hurtado, P. R., Tran, H. B., Mah, C. Y., and Hodge, S. (2020). LC3-associated Phagocytosis (LAP): a Potentially Influential Mediator of Efferocytosis-Related Tumor Progression and Aggressiveness. Front. Oncol. 10, 1298. doi:10.3389/fonc.2020.01298
Baird, A. C., Mallon, D., Radford-Smith, G., Boyer, J., Piche, T., Prescott, S. L., et al. (2016). Dysregulation of Innate Immunity in Ulcerative Colitis Patients Who Fail Anti-tumor Necrosis Factor Therapy. World J. Gastroenterol. 22, 9104–9116. doi:10.3748/wjg.v22.i41.9104
Banerjee, S., de Freitas, A., Friggeri, A., Zmijewski, J. W., Liu, G., and Abraham, E. (2011). Intracellular HMGB1 Negatively Regulates Efferocytosis. J. Immunol. 187, 4686–4694. doi:10.4049/jimmunol.1101500
Bao, L., Dou, G., Tian, R., Lv, Y., Ding, F., Liu, S., et al. (2022). Engineered Neutrophil Apoptotic Bodies Ameliorate Myocardial Infarction by Promoting Macrophage Efferocytosis and Inflammation Resolution. Bioactive Mater. 9, 183–197. doi:10.1016/j.bioactmat.2021.08.008
Barkal, A. A., Brewer, R. E., Markovic, M., Kowarsky, M., Barkal, S. A., Zaro, B. W., et al. (2019). CD24 Signalling through Macrophage Siglec-10 Is a Target for Cancer Immunotherapy. Nature 572, 392–396. doi:10.1038/s41586-019-1456-0
Barkal, A. A., Weiskopf, K., Kao, K. S., Gordon, S. R., Rosental, B., Yiu, Y. Y., et al. (2018). Engagement of MHC Class I by the Inhibitory Receptor LILRB1 Suppresses Macrophages and Is a Target of Cancer Immunotherapy. Nat. Immunol. 19, 76–84. doi:10.1038/s41590-017-0004-z
Bernsmeier, C., Pop, O. T., Singanayagam, A., Triantafyllou, E., Patel, V. C., Weston, C. J., et al. (2015). Patients with Acute-On-Chronic Liver Failure Have Increased Numbers of Regulatory Immune Cells Expressing the Receptor Tyrosine Kinase MERTK. Gastroenterology 148, 603–615. e14. doi:10.1053/j.gastro.2014.11.045
Birge, R. B., Boeltz, S., Kumar, S., Carlson, J., Wanderley, J., Calianese, D., et al. (2016). Phosphatidylserine Is a Global Immunosuppressive Signal in Efferocytosis, Infectious Disease, and Cancer. Cell Death Differ 23, 962–978. doi:10.1038/cdd.2016.11
Blachère, N. E., Darnell, R. B., and Albert, M. L. (2005). Apoptotic Cells Deliver Processed Antigen to Dendritic Cells for Cross-Presentation. Plos Biol. 3, e185. doi:10.1371/journal.pbio.0030185
Blander, J. M. (2017). The many Ways Tissue Phagocytes Respond to Dying Cells. Immunol. Rev. 277, 158–173. doi:10.1111/imr.12537
Bleesing, J. J. H., Brown, M. R., Dale, J. K., Straus, S. E., Lenardo, M. J., Puck, J. M., et al. (2001). TcR-α/β+ CD4−CD8− T Cells in Humans with the Autoimmune Lymphoproliferative Syndrome Express a Novel CD45 Isoform that Is Analogous to Murine B220 and Represents a Marker of Altered O-Glycan Biosynthesis. Clin. Immunol. 100, 314–324. doi:10.1006/clim.2001.5069
Boada-Romero, E., Martinez, J., Heckmann, B. L., and Green, D. R. (2020). The Clearance of Dead Cells by Efferocytosis. Nat. Rev. Mol. Cel. Biol. 21, 398–414. doi:10.1038/s41580-020-0232-1
Bosnjak, L., Miranda-Saksena, M., Koelle, D. M., Boadle, R. A., Jones, C. A., Cunningham, A. L., et al. (2005). Herpes Simplex Virus Infection of Human Dendritic Cells Induces Apoptosis and Allows Cross-Presentation via Uninfected Dendritic Cells. J. Immunol. 174, 2220–2227. doi:10.4049/jimmunol.174.4.2220
Bournazou, I., Pound, J. D., Duffin, R., Bournazos, S., Melville, L. A., Brown, S. B., et al. (2009). Apoptotic Human Cells Inhibit Migration of Granulocytes via Release of Lactoferrin. J. Clin. Invest. 119, 20–32. doi:10.1172/JCI36226
Bournazou, I., Mackenzie, K. J., Duffin, R., Rossi, A. G., and Gregory, C. D. (2010). Inhibition of Eosinophil Migration by Lactoferrin. Immunol. Cel. Biol. 88, 220–223. doi:10.1038/icb.2009.86
Brown, G. C., and Neher, J. J. (2012). Eaten Alive! Cell Death by Primary Phagocytosis: 'phagoptosis'. Trends Biochem. Sci. 37, 325–332. doi:10.1016/j.tibs.2012.05.002
Brown, S., Heinisch, I., Ross, E., Shaw, K., Buckley, C. D., and Savill, J. (2002). Apoptosis Disables CD31-Mediated Cell Detachment from Phagocytes Promoting Binding and Engulfment. Nature 418, 200–203. doi:10.1038/nature00811
Bukong, T. N., Cho, Y., Iracheta-Vellve, A., Saha, B., Lowe, P., Adejumo, A., et al. (2018). Abnormal Neutrophil Traps and Impaired Efferocytosis Contribute to Liver Injury and Sepsis Severity after Binge Alcohol Use. J. Hepatol. 69, 1145–1154. doi:10.1016/j.jhep.2018.07.005
Cao, Y., Yao, J., Gao, W., Cao, Z., Diabakte, K., Wang, L., et al. (2022). Sonodynamic Therapy Promotes Efferocytosis via CD47 Down-Regulation in Advanced Atherosclerotic Plaque. Int. Heart J. 63, 131–140. doi:10.1536/ihj.21-233
Castellano, F., Montcourrier, P., and Chavrier, P. (2000). Membrane Recruitment of Rac1 Triggers Phagocytosis. J. Cel. Sci. 113, 2955–2961. PMID: 10934035. doi:10.1242/jcs.113.17.2955
Chan, J. F.-W., Zhang, A. J., Yuan, S., Poon, V. K.-M., Chan, C. C.-S., Lee, A. C.-Y., et al. (2020). Simulation of the Clinical and Pathological Manifestations of Coronavirus Disease 2019 (COVID-19) in a golden Syrian Hamster Model: Implications for Disease Pathogenesis and Transmissibility. Clin. Infect. Dis. 71, 2428–2446. doi:10.1093/cid/ciaa325
Chekeni, F. B., Elliott, M. R., Sandilos, J. K., Walk, S. F., Kinchen, J. M., Lazarowski, E. R., et al. (2010). Pannexin 1 Channels Mediate 'find-Me' Signal Release and Membrane Permeability during Apoptosis. Nature 467, 863–867. doi:10.1038/nature09413
Chen, K., Murao, A., Arif, A., Takizawa, S., Jin, H., Jiang, J., et al. (2021). Inhibition of Efferocytosis by Extracellular CIRP-Induced Neutrophil Extracellular Traps. J. Immunol. 206, 797–806. doi:10.4049/jimmunol.2000091
Codo, A. C., Saraiva, A. C., dos Santos, L. L., Visconde, M. F., Gales, A. C., Zamboni, D. S., et al. (2018). Inhibition of Inflammasome Activation by a Clinical Strain of Klebsiella pneumoniae Impairs Efferocytosis and Leads to Bacterial Dissemination. Cell Death Dis 9, 1182. doi:10.1038/s41419-018-1214-5
Cohen, T. S., Jones-Nelson, O., Hotz, M., Cheng, L., Miller, L. S., Suzich, J., et al. (2016). S. aureus Blocks Efferocytosis of Neutrophils by Macrophages through the Activity of its Virulence Factor Alpha Toxin. Sci. Rep. 6, 35466. doi:10.1038/srep35466
Cullen, S. P., Henry, C. M., Kearney, C. J., Logue, S. E., Feoktistova, M., Tynan, G. A., et al. (2013). Fas/CD95-Induced Chemokines Can Serve as "Find-Me" Signals for Apoptotic Cells. Mol. Cel 49, 1034–1048. doi:10.1016/j.molcel.2013.01.025
Cummings, R. J., Barbet, G., Bongers, G., Hartmann, B. M., Gettler, K., Muniz, L., et al. (2016). Different Tissue Phagocytes Sample Apoptotic Cells to Direct Distinct Homeostasis Programs. Nature 539, 565–569. doi:10.1038/nature20138
Dalli, J., and Serhan, C. N. (2012). Specific Lipid Mediator Signatures of Human Phagocytes: Microparticles Stimulate Macrophage Efferocytosis and Pro-resolving Mediators. Blood 120, e60–e72. doi:10.1182/blood-2012-04-423525
de Aquino Penteado, L., Dejani, N. N., Verdan, F. F., Orlando, A. B., Niño, V. E., Dias, F. D. N., et al. (2017). Distinctive Role of Efferocytosis in Dendritic Cell Maturation and Migration in Sterile or Infectious Conditions. Immunology 151, 304–313. doi:10.1111/imm.12731
de Couto, G., Jaghatspanyan, E., DeBerge, M., Liu, W., Luther, K., Wang, Y., et al. (2019). Mechanism of Enhanced MerTK-dependent Macrophage Efferocytosis by Extracellular Vesicles. Arteriosclerosis, Thromb. Vasc. Biol. 39, 2082–2096. doi:10.1161/ATVBAHA.119.313115
DeBerge, M., Yeap, X. Y., Dehn, S., Zhang, S., Grigoryeva, L., Misener, S., et al. (2017). MerTK Cleavage on Resident Cardiac Macrophages Compromises Repair after Myocardial Ischemia Reperfusion Injury. Circ. Res. 121, 930–940. doi:10.1161/CIRCRESAHA.117.311327
deCathelineau, A. M., and Henson, P. M. (2003). The Final Step in Programmed Cell Death: Phagocytes Carry Apoptotic Cells to the Grave. Essays Biochem. 39, 105–117. doi:10.1042/bse0390105
Decote-Ricardo, D., Nunes, M. P., Morrot, A., and Freire-de-Lima, C. G. (2017). Implication of Apoptosis for the Pathogenesis of Trypanosoma Cruzi Infection. Front. Immunol. 8, 518. doi:10.3389/fimmu.2017.00518
Dejani, N. N., Orlando, A. B., Niño, V. E., Penteado, L. d. A., Verdan, F. F., Bazzano, J. M. R., et al. (2018). Intestinal Host Defense Outcome Is Dictated by PGE2 Production during Efferocytosis of Infected Cells. Proc. Natl. Acad. Sci. USA 115, E8469–E8478. doi:10.1073/pnas.1722016115
Desch, A. N., Randolph, G. J., Murphy, K., Gautier, E. L., Kedl, R. M., Lahoud, M. H., et al. (2011). CD103+ Pulmonary Dendritic Cells Preferentially Acquire and Present Apoptotic Cell-Associated Antigen. J. Exp. Med. 208, 1789–1797. doi:10.1084/jem.20110538
Devitt, A., and Marshall, L. J. (2011). The Innate Immune System and the Clearance of Apoptotic Cells. J. Leukoc. Biol. 90, 447–457. doi:10.1189/jlb.0211095
Divangahi, M., Chen, M., Gan, H., Desjardins, D., Hickman, T. T., Lee, D. M., et al. (2009). Mycobacterium tuberculosis Evades Macrophage Defenses by Inhibiting Plasma Membrane Repair. Nat. Immunol. 10, 899–906. doi:10.1038/ni.1758
Doran, A. C., Ozcan, L., Cai, B., Zheng, Z., Fredman, G., Rymond, C. C., et al. (2017). CAMKIIγ Suppresses an Efferocytosis Pathway in Macrophages and Promotes Atherosclerotic Plaque Necrosis. J. Clin. Invest. 127, 4075–4089. doi:10.1172/JCI94735
Doran, A. C., Yurdagul, A., and Tabas, I. (2020). Efferocytosis in Health and Disease. Nat. Rev. Immunol. 20, 254–267. doi:10.1038/s41577-019-0240-6
dos-Santos, D., Salina, A. C., Rodrigues, T. S., Rocha, M. F., Freitas-Filho, E. G., Alzamora-Terrel, D. L., et al. (2021). Efferocytosis of SARS-CoV-2-Infected Dying Cells Impairs Macrophage Anti-inflammatory Programming and Continual Clearance of Apoptotic Cells. MedRxiv. doi:10.1101/2021.02.18.21251504
Dutta, S., Mukherjee, A., and Nongthomba, U. (2022). Before the "cytokine Storm": Boosting Efferocytosis as an Effective Strategy against SARS-CoV-2 Infection and Associated Complications. Cytokine Growth Factor. Rev. online. doi:10.1016/j.cytogfr.2022.01.002
Elliott, M. R., Chekeni, F. B., Trampont, P. C., Lazarowski, E. R., Kadl, A., Walk, S. F., et al. (2009). Nucleotides Released by Apoptotic Cells Act as a Find-Me Signal to Promote Phagocytic Clearance. Nature 461, 282–286. doi:10.1038/nature08296
Elliott, M. R., Koster, K. M., and Murphy, P. S. (2017). Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J. Immunol. 198, 1387–1394. doi:10.4049/jimmunol.1601520
Elmore, S. (2007). Apoptosis: a Review of Programmed Cell Death. Toxicol. Pathol. 35, 495–516. doi:10.1080/01926230701320337
Erriah, M., Pabreja, K., Fricker, M., Baines, K. J., Donnelly, L. E., Bylund, J., et al. (2019). Galectin-3 Enhances Monocyte-Derived Macrophage Efferocytosis of Apoptotic Granulocytes in Asthma. Respir. Res. 20, 1. doi:10.1186/s12931-018-0967-9
Erwig, L.-P., Mcphilips, K. A., Wynes, M. W., Ivetic, A., Ridley, A. J., and Henson, P. M. (2006). Differential Regulation of Phagosome Maturation in Macrophages and Dendritic Cells Mediated by Rho GTPases and Ezrin-Radixin-Moesin (ERM) Proteins. Proc. Natl. Acad. Sci. 103, 12825–12830. doi:10.1073/pnas.0605331103
Fadok, V. A., Warner, M. L., Bratton, D. L., and Henson, P. M. (1998a). CD36 Is Required for Phagocytosis of Apoptotic Cells by Human Macrophages that Use Either a Phosphatidylserine Receptor or the Vitronectin Receptor (Alpha V Beta 3). J. Immunol. 161, 6250–6257. http://www.jimmunol.org/content/161/11/6250
Fadok, V. A., Bratton, D. L., Konowal, A., Freed, P. W., Westcott, J. Y., and Henson, P. M. (1998b). Macrophages that Have Ingested Apoptotic Cells In Vitro Inhibit Proinflammatory Cytokine Production through Autocrine/paracrine Mechanisms Involving TGF-Beta, PGE2, and PAF. J. Clin. Invest. 101, 890–898. doi:10.1172/JCI1112
Florey, O., Kim, S. E., Sandoval, C. P., Haynes, C. M., and Overholtzer, M. (2011). Autophagy Machinery Mediates Macroendocytic Processing and Entotic Cell Death by Targeting Single Membranes. Nat. Cel. Biol. 13, 1335–1343. doi:10.1038/ncb2363
Fox, S., Leitch, A. E., Duffin, R., Haslett, C., and Rossi, A. G. (2010). Neutrophil Apoptosis: Relevance to the Innate Immune Response and Inflammatory Disease. J. Innate Immun. 2, 216–227. doi:10.1159/000284367
Friggeri, A., Yang, Y., Banerjee, S., Park, Y.-J., Liu, G., and Abraham, E. (2010). HMGB1 Inhibits Macrophage Activity in Efferocytosis through Binding to the αvβ3-integrin. Am. J. Physiology-Cell Physiol. 299, C1267–C1276. doi:10.1152/ajpcell.00152.2010
Gardai, S. J., McPhillips, K. A., Frasch, S. C., Janssen, W. J., Starefeldt, A., Murphy-Ullrich, J. E., et al. (2005). Cell-surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through Trans-activation of LRP on the Phagocyte. Cell 123, 321–334. doi:10.1016/j.cell.2005.08.032
Garris, C. S., Wu, L., Acharya, S., Arac, A., Blaho, V. A., Huang, Y., et al. (2013). Defective Sphingosine 1-phosphate Receptor 1 (S1P1) Phosphorylation Exacerbates TH17-Mediated Autoimmune Neuroinflammation. Nat. Immunol. 14, 1166–1172. doi:10.1038/ni.2730
Gerlach, B. D., Ampomah, P. B., Yurdagul, A., Liu, C., Lauring, M. C., Wang, X., et al. (2021). Efferocytosis Induces Macrophage Proliferation to Help Resolve Tissue Injury. Cel Metab. 33, 2445–2463. e8. doi:10.1016/j.cmet.2021.10.015
Greenlee-Wacker, M. C. (2016). Clearance of Apoptotic Neutrophils and Resolution of Inflammation. Immunol. Rev. 273, 357–370. doi:10.1111/imr.12453
Greenlee-Wacker, M. C., Rigby, K. M., Kobayashi, S. D., Porter, A. R., DeLeo, F. R., and Nauseef, W. M. (2014). Phagocytosis of Staphylococcus aureus by Human Neutrophils Prevents Macrophage Efferocytosis and Induces Programmed Necrosis. J. Immunol. 192, 4709–4717. doi:10.4049/jimmunol.1302692
Grégoire, M., Uhel, F., Lesouhaitier, M., Gacouin, A., Guirriec, M., Mourcin, F., et al. (2018). Impaired Efferocytosis and Neutrophil Extracellular Trap Clearance by Macrophages in ARDS. Eur. Respir. J. 52, 1702590. doi:10.1183/13993003.02590-2017
Gude, D. R., Alvarez, S. E., Paugh, S. W., Mitra, P., Yu, J., Griffiths, R., et al. (2008). Apoptosis Induces Expression of Sphingosine Kinase 1 to Release Sphingosine-1-Phosphate as a "Come-And-Get-Me" Signal. FASEB J. 22, 2629–2638. doi:10.1096/fj.08-107169
Hanayama, R., Tanaka, M., Miyasaka, K., Aozasa, K., Koike, M., Uchiyama, Y., et al. (2004). Autoimmune Disease and Impaired Uptake of Apoptotic Cells in MFG-E8-Deficient Mice. Science 304, 1147–1150. doi:10.1126/science.1094359
Hao, Z., Duncan, G. S., Seagal, J., Su, Y.-W., Hong, C., Haight, J., et al. (2008). Fas Receptor Expression in Germinal-center B Cells Is Essential for T and B Lymphocyte Homeostasis. Immunity 29, 615–627. doi:10.1016/j.immuni.2008.07.016
He, M., Kubo, H., Morimoto, K., Fujino, N., Suzuki, T., Takahasi, T., et al. (2011). Receptor for Advanced Glycation End Products Binds to Phosphatidylserine and Assists in the Clearance of Apoptotic Cells. EMBO Rep. 12, 358–364. doi:10.1038/embor.2011.28
Horst, A. K., Tiegs, G., and Diehl, L. (2019). Contribution of Macrophage Efferocytosis to Liver Homeostasis and Disease. Front. Immunol. 10, 2670. doi:10.3389/fimmu.2019.02670
Hu, C., Wu, C., Tsai, H., Chang, S., Tsai, W., and Hsu, P. (2009). Genetic Polymorphism in Milk Fat Globule-EGF Factor 8 (MFG-E8) Is Associated with Systemic Lupus Erythematosus in Human. Lupus 18, 676–681. doi:10.1177/0961203309103027
Jondle, C. N., Gupta, K., Mishra, B. B., and Sharma, J. (2018). Klebsiella pneumoniae Infection of Murine Neutrophils Impairs Their Efferocytic Clearance by Modulating Cell Death Machinery. Plos Pathog. 14, e1007338. doi:10.1371/journal.ppat.1007338
Jorgensen, I., Zhang, Y., Krantz, B. A., and Miao, E. A. (2016). Pyroptosis Triggers Pore-Induced Intracellular Traps (PITs) that Capture Bacteria and lead to Their Clearance by Efferocytosis. J. Exp. Med. 213, 2113–2128. doi:10.1084/jem.20151613
Josefowicz, S. Z., Lu, L.-F., and Rudensky, A. Y. (2012). Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 30, 531–564. doi:10.1146/annurev.immunol.25.022106.141623
Juncadella, I. J., Kadl, A., Sharma, A. K., Shim, Y. M., Hochreiter-Hufford, A., Borish, L., et al. (2013). Apoptotic Cell Clearance by Bronchial Epithelial Cells Critically Influences Airway Inflammation. Nature 493, 547–551. doi:10.1038/nature11714
Karaji, N., and Sattentau, Q. J. (2017). Efferocytosis of Pathogen-Infected Cells. Front. Immunol. 8, 1863. doi:10.3389/fimmu.2017.01863
Khanna, S., Biswas, S., Shang, Y., Collard, E., Azad, A., Kauh, C., et al. (2010). Macrophage Dysfunction Impairs Resolution of Inflammation in the Wounds of Diabetic Mice. PLoS One 5, e9539. doi:10.1371/journal.pone.0009539
Kim, G. T., Hahn, K. W., Sohn, K. Y., Yoon, S. Y., and Kim, J. W. (2019). PLAG Enhances Macrophage Mobility for Efferocytosis of Apoptotic Neutrophils via Membrane Redistribution of P2Y2. FEBS. J. 286, 5016–5029. doi:10.1111/febs.15135
Kleinclauss, F., Perruche, S., Masson, E., de Carvalho Bittencourt, M., Biichle, S., Remy-Martin, J.-P., et al. (2006). Intravenous Apoptotic Spleen Cell Infusion Induces a TGF-β-dependent Regulatory T-Cell Expansion. Cel Death Differ 13, 41–52. doi:10.1038/sj.cdd.4401699
Kojima, Y., Volkmer, J.-P., McKenna, K., Civelek, M., Lusis, A. J., Miller, C. L., et al. (2016). CD47-blocking Antibodies Restore Phagocytosis and Prevent Atherosclerosis. Nature 536, 86–90. doi:10.1038/nature18935
Korns, D., Frasch, S. C., Fernandez-Boyanapalli, R., Henson, P. M., and Bratton, D. L. (2011). Modulation of Macrophage Efferocytosis in Inflammation. Front. Immun. 2, 57. doi:10.3389/fimmu.2011.00057
Kourtzelis, I., Li, X., Mitroulis, I., Grosser, D., Kajikawa, T., Wang, B., et al. (2019). DEL-1 Promotes Macrophage Efferocytosis and Clearance of Inflammation. Nat. Immunol. 20, 40–49. doi:10.1038/s41590-018-0249-1
Larson, S. R., Atif, S. M., Gibbings, S. L., Thomas, S. M., Prabagar, M. G., Danhorn, T., et al. (2016). Ly6C+ Monocyte Efferocytosis and Cross-Presentation of Cell-Associated Antigens. Cel Death Differ 23, 997–1003. doi:10.1038/cdd.2016.24
Larsson, M., Fonteneau, J.-F., Lirvall, M., Haslett, P., Lifson, J. D., Bhardwaj, N., et al. (2002). Activation of HIV-1 Specific CD4 and CD8 T Cells by Human Dendritic Cells: Roles for Cross-Presentation and Non-infectious HIV-1 Virus. AIDS 16, 1319–1329. doi:10.1097/00002030-200207050-00003
Li, S., Sun, Y., Liang, C.-P., Thorp, E. B., Han, S., Jehle, A. W., et al. (2009). Defective Phagocytosis of Apoptotic Cells by Macrophages in Atherosclerotic Lesions of Ob/ob Mice and Reversal by a Fish Oil Diet. Circ. Res. 105, 1072–1082. doi:10.1161/CIRCRESAHA.109.199570
Lim, K., Kim, T.-h., Trzeciak, A., Amitrano, A. M., Reilly, E. C., Prizant, H., et al. (2020). In Situ neutrophil Efferocytosis Shapes T Cell Immunity to Influenza Infection. Nat. Immunol. 21, 1046–1057. doi:10.1038/s41590-020-0746-x
Lin, J., Xu, A., Jin, J., Zhang, M., Lou, J., Qian, C., et al. (2022). MerTK-mediated Efferocytosis Promotes Immune Tolerance and Tumor Progression in Osteosarcoma through Enhancing M2 Polarization and PD-L1 Expression. Oncoimmunology 11, 2024941. doi:10.1080/2162402X.2021.2024941
Locovei, S., Scemes, E., Qiu, F., Spray, D. C., and Dahl, G. (2007). Pannexin1 Is Part of the Pore Forming Unit of the P2X7receptor Death Complex. FEBS. Lett. 581, 483–488. doi:10.1016/j.febslet.2006.12.056
Luo, B., Gan, W., Liu, Z., Shen, Z., Wang, J., Shi, R., et al. (2016). Erythropoeitin Signaling in Macrophages Promotes Dying Cell Clearance and Immune Tolerance. Immunity 44, 287–302. doi:10.1016/j.immuni.2016.01.002
Luo, B., Wang, Z., Zhang, Z., Shen, Z., and Zhang, Z. (2019). The Deficiency of Macrophage Erythropoietin Signaling Contributes to Delayed Acute Inflammation Resolution in Diet-Induced Obese Mice. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1865, 339–349. doi:10.1016/j.bbadis.2018.10.005
Maimon, N., Zamir, Z. Z., Kalkar, P., Zeytuni-Timor, O., Schif-Zuck, S., Larisch, S., et al. (2020). The Pro-apoptotic ARTS Protein Induces Neutrophil Apoptosis, Efferocytosis, and Macrophage Reprogramming to Promote Resolution of Inflammation. Apoptosis 25, 558–573. doi:10.1007/s10495-020-01615-3
Martin, C. J., Peters, K. N., and Behar, S. M. (2014). Macrophages Clean up: Efferocytosis and Microbial Control. Curr. Opin. Microbiol. 17, 17–23. doi:10.1016/j.mib.2013.10.007
Martin, S. J., Finucane, D. M., Amarante-Mendes, G. P., O'Brien, G. A., and Green, D. R. (1996). Phosphatidylserine Externalization during CD95-Induced Apoptosis of Cells and Cytoplasts Requires ICE/CED-3 Protease Activity. J. Biol. Chem. 271, 28753–28756. doi:10.1074/jbc.271.46.28753
Martinez, J. (2015). Prix Fixe: Efferocytosis as a Four-Course Meal. Curr. Top. Microbiol. Immunol. 403, 1–36. doi:10.1007/82_2015_467
Mazaheri, F., Breus, O., Durdu, S., Haas, P., Wittbrodt, J., Gilmour, D., et al. (2014). Distinct Roles for Bai1 and TIM-4 in the Engulfment of Dying Neurons by Microglia. Nat. Commun. 5, 4046. doi:10.1038/ncomms5046
McCubbrey, A. L., and Curtis, J. L. (2013). Efferocytosis and Lung Disease. Chest 143, 1750–1757. doi:10.1378/chest.12-2413
Moges, R., De Lamache, D. D., Sajedy, S., Renaux, B. S., Hollenberg, M. D., Muench, G., et al. (2018). Anti-Inflammatory Benefits of Antibiotics: Tylvalosin Induces Apoptosis of Porcine Neutrophils and Macrophages, Promotes Efferocytosis, and Inhibits Pro-inflammatory CXCL-8, IL1α, and LTB4 Production, while Inducing the Release of Pro-resolving Lipoxin A4 and Resolvin D1. Front. Vet. Sci. 5, 57. doi:10.3389/fvets.2018.00057
Monks, J., Rosner, D., Jon Geske, F., Lehman, L., Hanson, L., Neville, M. C., et al. (2005). Epithelial Cells as Phagocytes: Apoptotic Epithelial Cells Are Engulfed by Mammary Alveolar Epithelial Cells and Repress Inflammatory Mediator Release. Cel Death Differ 12, 107–114. doi:10.1038/sj.cdd.4401517
Monks, J., Smith-Steinhart, C., Kruk, E. R., Fadok, V. A., and Henson, P. M. (2008). Epithelial Cells Remove Apoptotic Epithelial Cells during Post-Lactation Involution of the Mouse Mammary Gland1. Biol. Reprod. 78, 586–594. doi:10.1095/biolreprod.107.065045
Moon, C., Lee, Y.-J., Park, H.-J., Chong, Y. H., and Kang, J. L. (2010). N-acetylcysteine Inhibits RhoA and Promotes Apoptotic Cell Clearance during Intense Lung Inflammation. Am. J. Respir. Crit. Care Med. 181, 374–387. doi:10.1164/rccm.200907-1061OC
Morioka, S., Maueröder, C., and Ravichandran, K. S. (2019). Living on the Edge: Efferocytosis at the Interface of Homeostasis and Pathology. Immunity 50, 1149–1162. doi:10.1016/j.immuni.2019.04.018
Nakaya, M., Tanaka, M., Okabe, Y., Hanayama, R., and Nagata, S. (2006). Opposite Effects of Rho Family GTPases on Engulfment of Apoptotic Cells by Macrophages. J. Biol. Chem. 281, 8836–8842. doi:10.1074/jbc.M510972200
Nakaya, M., Watari, K., Tajima, M., Nakaya, T., Matsuda, S., Ohara, H., et al. (2016). Cardiac Myofibroblast Engulfment of Dead Cells Facilitates Recovery after Myocardial Infarction. J. Clin. Invest. 127, 383–401. doi:10.1172/JCI83822
Negreiros-Lima, G. L., Lima, K. M., Moreira, I. Z., Jardim, B. L. O., Vago, J. P., Galvão, I., et al. (2020). Cyclic AMP Regulates Key Features of Macrophages via PKA: Recruitment, Reprogramming and Efferocytosis. Cells 9, 128. doi:10.3390/cells9010128
Nepal, S., Tiruppathi, C., Tsukasaki, Y., Farahany, J., Mittal, M., Rehman, J., et al. (2019). STAT6 Induces Expression of Gas6 in Macrophages to clear Apoptotic Neutrophils and Resolve Inflammation. Proc. Natl. Acad. Sci. USA 116, 16513–16518. doi:10.1073/pnas.1821601116
Nguyen, K.-Q. N., Tsou, W.-I., Calarese, D. A., Kimani, S. G., Singh, S., Hsieh, S., et al. (2014). Overexpression of MERTK Receptor Tyrosine Kinase in Epithelial Cancer Cells Drives Efferocytosis in a Gain-Of-Function Capacity. J. Biol. Chem. 289, 25737–25749. doi:10.1074/jbc.M114.570838
Nishi, C., Toda, S., Segawa, K., and Nagata, S. (2014). Tim4- and MerTK-Mediated Engulfment of Apoptotic Cells by Mouse Resident Peritoneal Macrophages. Mol. Cel. Biol. 34, 1512–1520. doi:10.1128/MCB.01394-13
North, R. A. (2002). Molecular Physiology of P2X Receptors. Physiol. Rev. 82, 1013–1067. doi:10.1152/physrev.00015.2002
Park, S.-Y., Jung, M.-Y., Kim, H.-J., Lee, S.-J., Kim, S.-Y., Lee, B.-H., et al. (2008a). Rapid Cell Corpse Clearance by Stabilin-2, a Membrane Phosphatidylserine Receptor. Cel Death Differ 15, 192–201. doi:10.1038/sj.cdd.4402242
Park, Y.-J., Liu, G., Lorne, E. F., Zhao, X., Wang, J., Tsuruta, Y., et al. (2008b). PAI-1 Inhibits Neutrophil Efferocytosis. Proc. Natl. Acad. Sci. 105, 11784–11789. doi:10.1073/pnas.0801394105
Park, Y.-J., Liu, G., Tsuruta, Y., Lorne, E., and Abraham, E. (2009). Participation of the Urokinase Receptor in Neutrophil Efferocytosis. Blood 114, 860–870. doi:10.1182/blood-2008-12-193524
Peter, C., Waibel, M., Radu, C. G., Yang, L. V., Witte, O. N., Schulze-Osthoff, K., et al. (2008). Migration to Apoptotic "Find-Me" Signals Is Mediated via the Phagocyte Receptor G2A. J. Biol. Chem. 283, 5296–5305. doi:10.1074/jbc.M706586200
Potter, P. K., Cortes-Hernandez, J., Quartier, P., Botto, M., and Walport, M. J. (2003). Lupus-prone Mice Have an Abnormal Response to Thioglycolate and an Impaired Clearance of Apoptotic Cells. J. Immunol. 170, 3223–3232. doi:10.4049/jimmunol.170.6.3223
Proto, J. D., Doran, A. C., Gusarova, G., Yurdagul, A., Sozen, E., Subramanian, M., et al. (2018). Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 49, 666–677. doi:10.1016/j.immuni.2018.07.015
Pujol-Autonell, I., Ampudia, R.-M., Planas, R., Marin-Gallen, S., Carrascal, J., Sanchez, A., et al. (2013). Efferocytosis Promotes Suppressive Effects on Dendritic Cells through Prostaglandin E2 Production in the Context of Autoimmunity. PLoS One 8, e63296. doi:10.1371/journal.pone.0063296
Pulanco, M. C., Cosman, J., Ho, M.-M., Huynh, J., Fing, K., Turcu, J., et al. (2017). Complement Protein C1q Enhances Macrophage Foam Cell Survival and Efferocytosis. J. Immunol. 198, 472–480. doi:10.4049/jimmunol.1601445
Qi, N., Liu, P., Zhang, Y., Wu, H., Chen, Y., and Han, D. (2013). Development of a Spontaneous Liver Disease Resembling Autoimmune Hepatitis in Mice Lacking Tyro3, Axl and Mer Receptor Tyrosine Kinases. PLoS One 8, e66604. doi:10.1371/journal.pone.0066604
Rantakari, P., Patten, D. A., Valtonen, J., Karikoski, M., Gerke, H., Dawes, H., et al. (2016). Stabilin-1 Expression Defines a Subset of Macrophages that Mediate Tissue Homeostasis and Prevent Fibrosis in Chronic Liver Injury. Proc. Natl. Acad. Sci. USA 113, 9298–9303. doi:10.1073/pnas.1604780113
Ravichandran, K. S. (2010). Find-me and Eat-Me Signals in Apoptotic Cell Clearance: Progress and Conundrums. J. Exp. Med. 207, 1807–1817. doi:10.1084/jem.20101157
Rodriguez-Manzanet, R., Sanjuan, M. A., Wu, H. Y., Quintana, F. J., Xiao, S., Anderson, A. C., et al. (2010). T and B Cell Hyperactivity and Autoimmunity Associated with Niche-specific Defects in Apoptotic Body Clearance in TIM-4-Deficient Mice. Proc. Natl. Acad. Sci. 107, 8706–8711. doi:10.1073/pnas.0910359107
Rymut, N., Heinz, J., Sadhu, S., Hosseini, Z., Riley, C. O., Marinello, M., et al. (2020). Resolvin D1 Promotes Efferocytosis in Aging by Limiting Senescent Cell-Induced MerTK Cleavage. FASEB J. 34, 597–609. doi:10.1096/fj.201902126R
Sachet, M., Liang, Y. Y., and Oehler, R. (2017). The Immune Response to Secondary Necrotic Cells. Apoptosis 22, 1189–1204. doi:10.1007/s10495-017-1413-z
Sághy, T., Köröskényi, K., Hegedűs, K., Antal, M., Bankó, C., Bacsó, Z., et al. (2019). Loss of Transglutaminase 2 Sensitizes for Diet-Induced Obesity-Related Inflammation and Insulin Resistance Due to Enhanced Macrophage C-Src Signaling. Cel Death Dis 10, 439. doi:10.1038/s41419-019-1677-z
Schaible, U. E., Winau, F., Sieling, P. A., Fischer, K., Collins, H. L., Hagens, K., et al. (2003). Apoptosis Facilitates Antigen Presentation to T Lymphocytes through MHC-I and CD1 in Tuberculosis. Nat. Med. 9, 1039–1046. doi:10.1038/nm906
Schif-Zuck, S., Gross, N., Assi, S., Rostoker, R., Serhan, C. N., and Ariel, A. (2011). Saturated-efferocytosis Generates Pro-resolving CD11blow Macrophages: Modulation by Resolvins and Glucocorticoids. Eur. J. Immunol. 41, 366–379. doi:10.1002/eji.201040801
Scott, R. S., McMahon, E. J., Pop, S. M., Reap, E. A., Caricchio, R., Cohen, P. L., et al. (2001). Phagocytosis and Clearance of Apoptotic Cells Is Mediated by MER. Nature 411, 207–211. doi:10.1038/35075603
Segawa, K., Kurata, S., Yanagihashi, Y., Brummelkamp, T. R., Matsuda, F., and Nagata, S. (2014). Caspase-mediated Cleavage of Phospholipid Flippase for Apoptotic Phosphatidylserine Exposure. Science 344, 1164–1168. doi:10.1126/science.1252809
Sekheri, M., El Kebir, D., Edner, N., and Filep, J. G. (2020). 15-Epi-LXA4and 17-Epi-RvD1 Restore TLR9-Mediated Impaired Neutrophil Phagocytosis and Accelerate Resolution of Lung Inflammation. Proc. Natl. Acad. Sci. USA 117, 7971–7980. doi:10.1073/pnas.1920193117
Shibata, T., Makino, A., Ogata, R., Nakamura, S., Ito, T., Nagata, K., et al. (2020). Respiratory Syncytial Virus Infection Exacerbates Pneumococcal Pneumonia via Gas6/Axl-Mediated Macrophage Polarization. J. Clin. Invest. 130, 3021–3037. doi:10.1172/JCI125505
Shiratsuchi, A., Kaido, M., Takizawa, T., and Nakanishi, Y. (2000). Phosphatidylserine-mediated Phagocytosis of Influenza A Virus-Infected Cells by Mouse Peritoneal Macrophages. J. Virol. 74, 9240–9244. doi:10.1128/jvi.74.19.9240-9244.2000
Soki, F. N., Koh, A. J., Jones, J. D., Kim, Y. W., Dai, J., Keller, E. T., et al. (2014). Polarization of Prostate Cancer-Associated Macrophages Is Induced by Milk Fat Globule-EGF Factor 8 (MFG-E8)-Mediated Efferocytosis. J. Biol. Chem. 289, 24560–24572. doi:10.1074/jbc.M114.571620
Sun, L., Zhou, H., Zhu, Z., Yan, Q., Wang, L., Liang, Q., et al. (2015). Ex Vivo and In Vitro Effect of Serum Amyloid a in the Induction of Macrophage M2 Markers and Efferocytosis of Apoptotic Neutrophils. J. Immunol. 194, 4891–4900. doi:10.4049/jimmunol.1402164
Szondy, Z., Garabuczi, Ã. v., Joós, G., Tsay, G. J., and Sarang, Z. (2014). Impaired Clearance of Apoptotic Cells in Chronic Inflammatory Diseases: Therapeutic Implications. Front. Immunol. 5, 354. doi:10.3389/fimmu.2014.00354
Tajbakhsh, A., Gheibi hayat, S. M., Movahedpour, A., Savardashtaki, A., Loveless, R., Barreto, G. E., et al. (2021). The Complex Roles of Efferocytosis in Cancer Development, Metastasis, and Treatment. Biomed. Pharmacother. 140, 111776. doi:10.1016/j.biopha.2021.111776
Terpstra, V., and van Berkel, T. J. C. (2000). Scavenger Receptors on Liver Kupffer Cells Mediate the In Vivo Uptake of Oxidatively Damaged Red Blood Cells in Mice. Blood 95, 2157–2163. PMID: 10706889. doi:10.1182/blood.v95.6.2157
Thorp, E., Cui, D., Schrijvers, D. M., Kuriakose, G., and Tabas, I. (2008). Mertk Receptor Mutation Reduces Efferocytosis Efficiency and Promotes Apoptotic Cell Accumulation and Plaque Necrosis in Atherosclerotic Lesions of Apoe −/− Mice. Atvb 28, 1421–1428. doi:10.1161/ATVBAHA.108.167197
Tian, L., Choi, S.-C., Lee, H.-N., Murakami, Y., Qi, C.-F., Sengottuvelu, M., et al. (2016). Enhanced Efferocytosis by Dendritic Cells Underlies Memory T-Cell Expansion and Susceptibility to Autoimmune Disease in CD300f-Deficient Mice. Cel Death Differ 23, 1086–1096. doi:10.1038/cdd.2015.161
Tiemessen, M. M., Jagger, A. L., Evans, H. G., van Herwijnen, M. J. C., John, S., and Taams, L. S. (2007). CD4+CD25+Foxp3+ Regulatory T Cells Induce Alternative Activation of Human Monocytes/macrophages. Proc. Natl. Acad. Sci. 104, 19446–19451. doi:10.1073/pnas.0706832104
Toda, S., Segawa, K., and Nagata, S. (2014). MerTK-mediated Engulfment of Pyrenocytes by central Macrophages in Erythroblastic Islands. Blood 123, 3963–3971. doi:10.1182/blood-2014-01-547976
Torchinsky, M. B., Garaude, J., Martin, A. P., and Blander, J. M. (2009). Innate Immune Recognition of Infected Apoptotic Cells Directs TH17 Cell Differentiation. Nature 458, 78–82. doi:10.1038/nature07781
Török, H.-P., Glas, J., Tonenchi, L., Mussack, T., and Folwaczny, C. (2004). Polymorphisms of the Lipopolysaccharide-Signaling Complex in Inflammatory Bowel Disease: Association of a Mutation in the Toll-like Receptor 4 Gene with Ulcerative Colitis. Clin. Immunol. 112, 85–91. doi:10.1016/j.clim.2004.03.002
Tóth, B., Garabuczi, É., Sarang, Z., Vereb, G., Vámosi, G., Aeschlimann, D., et al. (2009). Transglutaminase 2 Is Needed for the Formation of an Efficient Phagocyte portal in Macrophages Engulfing Apoptotic Cells. J. Immunol. 182, 2084–2092. doi:10.4049/jimmunol.0803444
Triantafyllou, E., Pop, O. T., Possamai, L. A., Wilhelm, A., Liaskou, E., Singanayagam, A., et al. (2018). MerTK Expressing Hepatic Macrophages Promote the Resolution of Inflammation in Acute Liver Failure. Gut 67, 333–347. doi:10.1136/gutjnl-2016-313615
Truman, L. A., Ford, C. A., Pasikowska, M., Pound, J. D., Wilkinson, S. J., Dumitriu, I. E., et al. (2008). CX3CL1/fractalkine Is Released from Apoptotic Lymphocytes to Stimulate Macrophage Chemotaxis. Blood 112, 5026–5036. doi:10.1182/blood-2008-06-162404
Tzelepis, F., Verway, M., Daoud, J., Gillard, J., Hassani-Ardakani, K., Dunn, J., et al. (2015). Annexin1 Regulates DC Efferocytosis and Cross-Presentation during Mycobacterium tuberculosis Infection. J. Clin. Invest. 125, 752–768. doi:10.1172/JCI77014
Viaud, M., Ivanov, S., Vujic, N., Duta-Mare, M., Aira, L.-E., Barouillet, T., et al. (2018). Lysosomal Cholesterol Hydrolysis Couples Efferocytosis to Anti-inflammatory Oxysterol Production. Circ. Res. 122, 1369–1384. doi:10.1161/CIRCRESAHA.117.312333
Voss, O. H., Tian, L., Murakami, Y., Coligan, J. E., and Krzewski, K. (2015). Emerging Role of CD300 Receptors in Regulating Myeloid Cell Efferocytosis. Mol. Cell Oncol. 2, e964625. doi:10.4161/23723548.2014.964625
Wan, E., Yeap, X. Y., Dehn, S., Terry, R., Novak, M., Zhang, S., et al. (2013). Enhanced Efferocytosis of Apoptotic Cardiomyocytes through Myeloid-Epithelial-Reproductive Tyrosine Kinase Links Acute Inflammation Resolution to Cardiac Repair after Infarction. Circ. Res. 113, 1004–1012. doi:10.1161/CIRCRESAHA.113.301198
Wang, X., Bu, H.-F., Zhong, W., Asai, A., Zhou, Z., and Tan, X.-D. (2013). MFG-E8 and HMGB1 Are Involved in the Mechanism Underlying Alcohol-Induced Impairment of Macrophage Efferocytosis. Mol. Med. 19, 170–182. doi:10.2119/molmed.2012.00260
Watanabe, Y., Shiratsuchi, A., Shimizu, K., Takizawa, T., and Nakanishi, Y. (2002). Role of Phosphatidylserine Exposure and Sugar Chain Desialylation at the Surface of Influenza Virus-Infected Cells in Efficient Phagocytosis by Macrophages. J. Biol. Chem. 277, 18222–18228. doi:10.1074/jbc.M201074200
Waterborg, C. E. J., Beermann, S., Broeren, M. G. A., Bennink, M. B., Koenders, M. I., van Lent, P. L. E. M., et al. (2018). Protective Role of the MER Tyrosine Kinase via Efferocytosis in Rheumatoid Arthritis Models. Front. Immunol. 9, 742. doi:10.3389/fimmu.2018.00742
Weirather, J., Hofmann, U. D. W., Beyersdorf, N., Ramos, G. C., Vogel, B., Frey, A., et al. (2014). Foxp3 + CD4 + T Cells Improve Healing after Myocardial Infarction by Modulating Monocyte/Macrophage Differentiation. Circ. Res. 115, 55–67. doi:10.1161/CIRCRESAHA.115.303895
Weiss, J. (2003). Bactericidal/permeability-increasing Protein (BPI) and Lipopolysaccharide-Binding Protein (LBP): Structure, Function and Regulation in Host Defence against Gram-Negative Bacteria. Biochem. Soc. Trans. 31, 785–790. doi:10.1042/bst0310785
Werfel, T. A., and Cook, R. S. (2018). Efferocytosis in the Tumor Microenvironment. Semin. Immunopathol. 40, 545–554. doi:10.1007/s00281-018-0698-5
Willingham, S. B., Volkmer, J. P., Gentles, A. J., Sahoo, D., Dalerba, P., Mitra, S. S., et al. (2012). The CD47-Signal Regulatory Protein Alpha (SIRPa) Interaction Is a Therapeutic Target for Human Solid Tumors. Proc. Natl. Acad. Sci. U S A. 109, 6662–6667. doi:10.1073/pnas.1121623109
Yan, W., Ma, D., Liu, Y., Sun, W., Cheng, D., Li, G., et al. (2022). PTX3 Alleviates Hard Metal-Induced Acute Lung Injury through Potentiating Efferocytosis. Ecotoxicology Environ. Saf. 230, 113139. doi:10.1016/j.ecoenv.2021.113139
Yoshimura, C., Nagasaka, A., Kurose, H., and Nakaya, M. (2020). Efferocytosis during Myocardial Infarction. J. Biochem. 168, 1–6. doi:10.1093/jb/mvaa051
Yrlid, U., and Wick, M. J. (2000). Salmonella-induced Apoptosis of Infected Macrophages Results in Presentation of a Bacteria-Encoded Antigen after Uptake by Bystander Dendritic Cells. J. Exp. Med. 191, 613–624. doi:10.1084/jem.191.4.613
Zhang, S., Yeap, X.-Y., DeBerge, M., Naresh, N. K., Wang, K., Jiang, Z., et al. (2017). Acute CD47 Blockade during Ischemic Myocardial Reperfusion Enhances Phagocytosis-Associated Cardiac Repair. JACC: Basic Translational Sci. 2, 386–397. doi:10.1016/j.jacbts.2017.03.013
Keywords: apoptosis, efferocytosis, inflammatory diseases, immune response, phagocytosis
Citation: Ge Y, Huang M and Yao Y-m (2022) Efferocytosis and Its Role in Inflammatory Disorders. Front. Cell Dev. Biol. 10:839248. doi: 10.3389/fcell.2022.839248
Received: 19 December 2021; Accepted: 10 February 2022;
Published: 25 February 2022.
Edited by:
Ivan Dzhagalov, National Yang-Ming University, TaiwanReviewed by:
Zsolt Sarang, University of Debrecen, HungaryAlvaro De Mingo Pulido, Moffitt Cancer Center, United States
Copyright © 2022 Ge, Huang and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-ming Yao, Y19mZkBzaW5hLmNvbQ==; Man Huang, aHVhbmdtYW5Aemp1LmVkdS5jbg==
 Yun Ge
Yun Ge Man Huang
Man Huang Yong-ming Yao
Yong-ming Yao