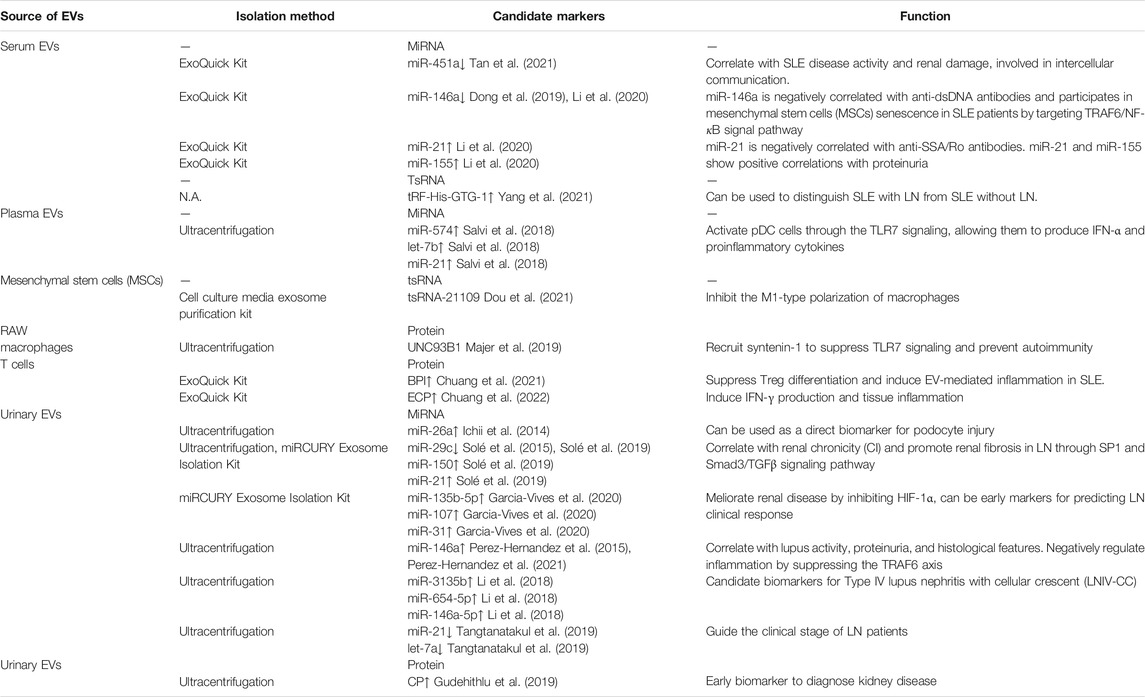
- Department of Dermatology, Huashan Hospital, Fudan University, Shanghai, China
Extracellular Vesicles (EVs) are small vesicles that can be actively secreted by most cell types into the extracellular environment. Evidence indicates that EVs can carry microRNAs (miRNAs), long non-coding RNAs (lncRNAs), tRNA-derived small RNAs (tsRNAs), proteins, and lipids to target cells or tissue organizations. Latest studies show that EVs play a vital role in the immune modulation and may contribute to the pathogenesis of autoimmune diseases. Systemic lupus erythematosus (SLE) is a common autoimmune disease characterized by abnormal T cell activation and sustained production of autoantibodies against self-antigens, resulting in inflammation and damage to multiple systems. Pathogenic mechanisms of SLE, however, are still not well understood. In this review, we summarize the latest research advances on the functions and mechanisms of EVs, and its role in the pathogenesis, diagnosis, and treatment of SLE.
1 Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease characterized by loss of tolerance and sustained production of autoantibodies against self-antigens that form immune complex deposits (Crispin et al., 2010). The prevalence of SLE varies from 30/100,000 to 50/100,000, and the disease is more common in women of childbearing age. SLE is hard to diagnose due to its complex pathogenesis and variable clinical symptoms. Most patients are diagnosed based on the 1997 American College of Rheumatology classification criteria, and the disease activity is assessed based on the SLE Disease Activity Index (SLEDAI). Nevertheless, it is not always effective. At present, a majority of scholars believe that it is the interaction of genetic susceptibility, environment, immunology, and hormone factors that lead to SLE, but the exact mechanism is not clear (Tsokos, 2011). Although non-steroidal anti-inflammatory drugs such as glucocorticoid (GCs), immunosuppressants, and biological agents are commonly used in the treatment of SLE, hurdles such as toxic side effects, the lack of target tissue, and non-response to treatment remain to be crossed (Tsokos, 2011).
First described as “platelet dust” by Peter Wolf in 1967, extracellular vesicles (EVs) are a collective term for phospholipids bilayer structures secreted by cells, which contain microRNAs (miRNAs), long non-coding RNAs (lncRNAs), tRNA-derived small RNAs (tsRNAs), proteins, lipids, and other substances (Raposo and Stoorvogel, 2013). The term “extracellular vesicles (EVs)” includes multiple types of vesicles. Specifically, there exist three main classified subtypes based on their biogenesis, size, and release mechanisms, namely microvesicles (100 nm-1 μm), apoptotic bodies (1–5 μm), and exosomes (30–100 nm in diameter) (Yanez-Mo et al., 2015). Microvesicles (MVs, also called microparticles) are larger than exosomes and pinch directly off from the outer cell membrane (Akers et al., 2013). Microvesicles formation is the result of molecular rearrangements of the plasma membrane regarding phospholipid and cytoskeletal protein composition as well as Ca2+ levels (Taylor and Bebawy, 2019). Apoptotic bodies are large structures, which are also produced by direct budding of the membrane and differ from exosomes and MVs as apoptotic bodies are formed only during programmed cell death. They are characterized by the presence of closely packed cellular organelles and fragments of nuleus (Saraste and Pulkki, 2000). Over the past few years, the cutting-edge knowledge about EV research provides insights into new tools for diagnosis, prognosis, and disease activity monitoring, novel therapeutic strategy, and innovative evaluation approaches for treatment effectiveness in SLE (Perez-Hernandez and Cortes, 2015; Perez-Hernandez et al., 2017; Wu et al., 2020; Zhang et al., 2020; Zhao et al., 2020).
As the smallest vesicles and probably the most prominently described class of EV, exosomes are ranging from 30–100 nm in diameter, and are released by almost all cell types, including stem cells, T and B lymphocytes, dendritic cells (DCs), macrophages, endothelial cells, neurons, adipocytes, and epithelial cells (Obregon et al., 2009; Mashouri et al., 2019; Rayamajhi et al., 2019). They can be found in a wide range of bodily fluids, such as blood, urine, saliva, breast milk, and in the supernatants of cultured cells after being released into the extracellular environment (Record et al., 2011; Matsumura et al., 2015). Exosome has a lipid bilayer membrane structure and contains bio-reactive macromolecules such as cell-specific proteins, lipids, and nucleic acids, which can protect the coating substances, targeting specific tissues and cells to perform their biological functions. Recently, evidence indicates that exosomes play important roles not only in physiological events, such as intracellular communication, immune modulation, and inflammation, but also in pathological conditions, including autoimmune and cardio-metabolic diseases, as well as development and metastasis of tumors (Shah et al., 2018; Stahl and Raposo, 2019). In this review, we summarize the recent progress of the potential role of exosomes in the pathogenesis, diagnosis, and treatment of SLE (Figure 1 and Table 1). However, there is always a heterogenous population of EVs regardless of the isolation method used. Additionally, none of the involved studies published to date can prove that the isolated fractions are exosomes only. In this context, we utilized the generic term “extracellular vesicles (EVs)” instead of “exosomes” throughout the rest of this survey. It is also in line with the recommended terminology from the international society for extracellular vesicles (ISEV) (Thery et al., 2018).
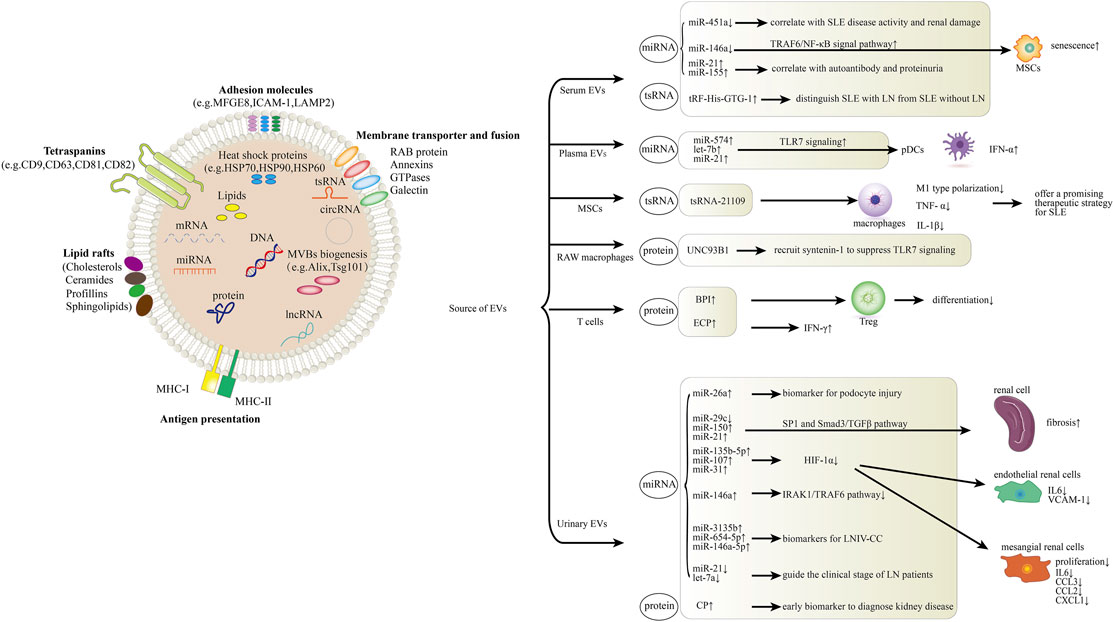
FIGURE 1. Role of EVs in systemic lupus erythematosus (SLE) and lupus nephritis (LN). The schematic diagram represents how EV components including miRNA, lncRNA, tsRNA and proteins are involved in the pathogenesis of SLE and LN. In serum, EV miR-451a is correlated with SLE disease activity and renal damage. MiR-146a could be internalized into mesenchymal stem cells (MSCs) via circulating EVs and participates in MSCs senescence in SLE patients by targeting TRAF6/NF-κB signal pathway. Serum EV miR-21 and miR-155 expression present correlations with autoantibodies and proteinuria. Levels of serum EV tRF-His-GTG-1 could be used to distinguish SLE with LN from SLE without LN. In plasma, EV miR-574, let-7b and miR-21 activate pDC cells through the TLR7 signaling. MSC-derived EV tsRNA-21109 inhibits the M1-type polarization of macrophages. UNC93B1 can be detected in RAW macrophage-derived EVs, it can recruit syntenin-1 to suppress TLR7 signaling and prevent autoimmunity. Overexpression of BPI in T cell-derived EVs suppresses Treg differentiation and induces EV-mediated inflammation in SLE. ECP overexpression in T cell-derived EVs induces IFN-γ production and tissue inflammation. MiR-26a from urinary EVs can be used as a direct biomarker for podocyte injury. Urinary EV miR-29c, miR-150 and miR-21 promote renal fibrosis through SP1 and Smad3/TGFβ signaling pathway. Urinary EV miR-135b-5p, miR-107 and miR-31 could meliorate renal disease by inhibiting HIF-1α. MiR-146a from urinary EVs negatively regulates inflammation by suppressing the TRAF6 axis. MiR-3135b, miR-654-5p and miR-146a-5p in urinary EVs are candidate biomarkers for Type IV lupus nephritis with cellular crescent (LNIV-CC). Urinary EV let-7a and miR-21 may guide the clinical staging of LN patients. CP, a protein from urinary EVs, could be an early biomarker to diagnose kidney disease.
2 Biological Characteristics of EVs
2.1 The Biogenesis of EVs
The formation of EVs involves a variety of proteins and transport complexes, and the fusion of primary endocytic vesicles should be the first step of the early endosomes (EEs) formation. Then, two pathways are shown by the EEs. One is that EEs become recycling endosomes, returning to plasma membrane, and the other way is converting into “late endosomes” (LEs)/multivesicular bodies (MVBs) via inward budding of the membrane under the endosomal sorting complex required for transport (ESCRT)-dependent or ESCRT-independent mechanism. Afterwards, LEs are fused with cell membranes, released into the extracellular space under the control of Ras-related proteins in barin (Rab) GTPases and soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), which are called EVs (Thery et al., 2002b; Simons and Raposo, 2009; Hessvik and Llorente, 2018).
2.2 Isolation and Extraction of EVs
EVs play an essential role in mediating cell communications and participate in the pathological process of multiple diseases. How to extract EVs efficiently with high purity, high recovery, and low cost has become the key to further downstream cell function research. EV samples contain a large number of vesicles or proteins that have similar volume, density or surface charge to EVs, which can interfere with the result of the experiment. A variety of methods have been developed in this regard (Ramirez et al., 2018). Among them, ultracentrifugation is the most widely used protocol and has also evolved as the gold standard for EV separation. There are, however, still some inevitable downsides such as high instrument cost and long extraction time. Moreover, factors (e.g., multiple cleaning, high sample viscosity, etc.) are likely to shape the downstream analyses negatively in an uncertain manner (Momen-Heravi et al., 2012a; Momen-Heravi et al., 2012b). Size exclusion chromatography is a scheme using EV purification columns to separate EVs. It does not require the use of expensive centrifuges, and the obtained EVs have high purity. However, compared to ultracentrifugation, it is more challenging to handle large samples due to the limitations of the purification columns (Koh et al., 2018; Monguio-Tortajada et al., 2019). Faced with bodily fluids and other large-volume samples, ultrafiltration can be perceived as a solution. The principle of ultrafiltration is the same as membrane separation, and it takes less time, but impurities such as other vesicles and proteins tend to block the pores and reduce the extraction efficiency (Cheruvanky et al., 2007; Konoshenko et al., 2018). The above-mentioned traditional extraction methods have multiple drawbacks including low purity, low recovery rate, and low efficiency. Nowadays, a mounting number of new extraction methods are discovered. Lewis et al. (2018) utilized static electricity to adsorb EVs around the positive electrode, which largely improves the purity and specificity of the EVs (Lewis et al., 2018). Wu et al. developed a sonic-based separation method that can directly isolate EVs from whole blood, greatly reducing the time required to extract EVs (Wu M. et al., 2017). These new technologies bring new opportunities for the diagnosis and treatment of diseases in the future.
2.3 Function of EVs
EVs were originally considered to be vesicles employed to expel excess transferrin receptor vesicles (Pan and Johnstone, 1983). With the development of the research, various functions of EVs were gradually revealed to the public. The vesicle structure of EVs can protect its internal transported substances from the interference of soluble substances such as proteases in vivo. At present, it is generally believed that EVs serve as carriers and play a big part in mediating information exchange between cells by transporting microRNAs (miRNAs), long non-coding RNAs (lncRNAs), tRNA-derived small RNAs (tsRNAs), proteins, lipids, and other substances (Mathivanan et al., 2012; Barile and Vassalli, 2017; Mathieu et al., 2019). These substances may be involved in the pathogenesis of different diseases. EVs of nasal epithelial cells in patients with chronic rhinosinusitis with nasal polyps contain differentially expressed proteins, which are mainly involved in epithelial remodeling through p53 and other pathways, leading to sinus mucosal remodeling (Zhou et al., 2020). EVs can carry β-Amyloid, prion, and α-synuclein, thus spread disease-causing proteins in the brain, which may be involved in Alzheimer’s disease progression (Nath et al., 2012; Arellano-Anaya et al., 2015; Lööv et al., 2016). Evidence shows that EV-associated miRNAs and lncRNAs play essential roles in the pathogenesis of osteoarthritis (OA), including OA diagnosis, pathogenesis, and treatment (Maehara et al., 2021; Miao et al., 2021). In addition, EVs are involved in many physiological processes, such as intracellular communication, signal transduction, transport of genetic materials, and modulation of immune response (Natasha et al., 2014). Evidence in previous studies indicates that EVs are also involved in the progression of diseases, including cancers, neurodegenerative diseases, and autoimmune diseases (Anderson et al., 2010), such as rheumatoid arthritis (RA), Sjogren’s syndrome (SS), and SLE. In this review, we summarize the latest progress and recent advances in EV research, therapeutic potential, and mechanism of EVs in the pathogenesis of SLE, as well as their clinical implications.
3 Role of EVs in Immune Function
It was not until 1996 that B cells were found to induce T cell responses by secreting EVs with major histocompatibility complex (MHC) class II, which indicated the relationship between EVs and immune regulation (Raposo et al., 1996). After that, EVs from other immunocytes, such as T cells, natural killer (NK) cells, and dendritic cells (DCs), have also been proven to mediate either immune stimulation or immune modulation (Gutierrez-Vazquez et al., 2013; Zhang et al., 2014; Jong et al., 2017; Reis et al., 2018).
3.1 EVs and Innate Immune Cells
Immune cell-derived EVs are involved in the regulation of the innate immune responses. EVs released by neutrophils, macrophages, NK cells, and DCs act on the innate immune system as pro-inflammatory mediators via paracrine messengers (Yanez-Mo et al., 2015).
3.1.1 Neutrophils
Neutrophils are the most abundant leukocyte population in peripheral blood and are the first line of host defense against a wide range of infectious pathogens (Mayadas et al., 2014). In addition to regulating macrophage activation (Gasser and Schifferli, 2004), neutrophil-derived EVs have inhibitory effects on monocyte-derived DCs (Eken et al., 2008). These EVs modify the morphology of monocyte derived DCs (MoDCs) by inhibiting the formation of dendrites, downregulate their phagocytic activity and maturation, and inhibite the cytokine release of MoDCs, resulting in an attenuated capacity to stimulate T cell proliferation (Eken et al., 2008). Other studies identified several neutrophil-derived EV associated molecules which can influence DC and T cell function potentially, such as annexin A1 (Dalli et al., 2008; Gavins and Hickey, 2012) and arginase-1 (Leliefeld et al., 2015; Shen et al., 2017). What’s more, it was found that several proteases in neutrophil-derived EVs such as myeloperoxidase (MPO), elastase, cathepsin G and proteinase 3 may influence adaptive immunity (Hess et al., 1999; Gasser et al., 2003; Dalli et al., 2013; Timar et al., 2013; Slater et al., 2017).
3.1.2 Macrophages
Another type of innate immune cells which is a rich source of EVs is macrophages (Wang et al., 2020). Macrophages are important phagocytic cells distributed in essentially all tissues, where they respond to a complex variety of regulatory signals to coordinate immune functions involved in tissue development, homeostasis, metabolism, and repair (Wynn and Vannella, 2016). EVs secreted by bacterially infected macrophages have a pro-inflammatory effect, which can induce the maturation of DCs and activate CD4+ and CD8+ T cells (Giri and Schorey, 2008; Ramachandra et al., 2010). Besides, these macrophage-derived EVs promote the release of multiple pro-inflammatory cytokines and chemokines (Singh et al., 2012). Furthermore, several studies characterized EVs content and their effects on uninfected macrophages which revealed that EVs released from infected macrophages holds a vital role in immune surveillance (Bhatnagar et al., 2007; Bhatnagar and Schorey, 2007).
3.1.3 Natural Killer Cells
Natural killer (NK) cells are innate lymphoid cells with potent cytolytic function toward viral invasion and prevent survival or spread of tumor cells (Morvan and Lanier, 2016). NK cells have multiple activating receptors (e.g., NKG2D) and inhibitory receptors (e.g., killer-cell immunoglobulin-like receptors, KIRs), and the balance between these signals determines whether or not NK cells are activated (Fernandez-Messina et al., 2012; Sivori et al., 2019). NK cells are found to secrete EVs in a constitutive way and independent of their activation status (Lugini et al., 2012). Several studies reported that NK cell-derived EVs show cytotoxic activity against tumor cells (Fais, 2013; Zhu et al., 2017) and activate immune cells (Lugini et al., 2012).
3.1.4 Dendritic Cells
As the sentinel antigen-presenting cells (APCs) of the immune system, dendritic cells (DCs) function as the link between innate and adaptive immunity, leading to either antigen-specific immunity initiation or tolerance (Steinman, 2012). Like DCs, EVs secreted by DCs were found to possess functional MHC-peptide complexes, costimulatory molecules, and other components that interact with immune cells (Thery et al., 1999; Thery et al., 2001; Thery et al., 2002a). EVs secreted by mature DCs contain class II MHC complexes and costimulatory molecules, which can directly interact with T cells to activate the immune system (Segura et al., 2005). On the other hand, EVs secreted by immature DCs can regulate the immune response, but do not function in direct T cell activation (Quah and O'Neill, 2005). In addition, studies have shown that DC-derived EVs can be absorbed by epithelial cells and promote the release of inflammatory mediators (MCP-1, IL-8, TNFα, RANTES) secreted by epithelial cells, suggesting that EVs promote immune-inflammatory response (Obregon et al., 2009).
3.2 EVs and Adaptive Immune Cells
The adaptive immune cells mainly include T and B lymphocytes.
3.2.1 T Cells
DC-T cell interaction results in T cell activation. The interaction is transmitted from T cells to DCs via the transfer of EV-DNA, making DCs more resistant to infections (Torralba et al., 2018). EVs derived from activated CD3+ T cell together with IL-2 can modulate the activity of immune cells, including other T cells (Wahlgren et al., 2012). In addition, depending on their activation status, CD4+ T cells regulate the release of distinct vesicle subpopulations with various abilities to activate other untouched T cells (van der Vlist et al., 2012). T cell tolerance is shown due to EVs secreted by CD8+ suppressor T cells (Bryniarski et al., 2013). The protein expression profile of T cell EVs change substantially after different stimuli (activation vs apoptosis induction). Induction of apoptosis causes T cells to release more apoptotic bodies than exosomes, while activated T cells release exosomes and microvesicles both in lower amounts (Tucher et al., 2018). Studies have shown that Treg-derived EVs express immunomodulatory molecules (CD25, CD73, CTLA4), which have immunosuppressive effects and can regulate effector T cell proliferation and cytokine secretion to regulate immune response (Agarwal et al., 2014).
3.2.2 B Cells
EVs derived from B cells exert a predominant role in antigen presentation and immunoregulation. B cell-derived EVs can induce antigen-specific MHC II-restricted T cell responses, suggesting antigen presentation capacities just like B cells (Raposo et al., 1996). Different types of antigens, carried by B cell-derived EVs, may dictate different types of immune responses (Hood, 2017). Recently, it has been suggested that B cell-derived EVs may have immunoregulatory functions which are independent of their ability to present antigen (Zhang et al., 2019). In addition, the role of different lymphocytes subsets (CD4+ T cells, CD8+ T cells, and NK cells) and DCs in CTL immune response to antigen presented on B-cell derived EVs has been described, demonstrating an complex interplay of cooperating lymphocytes for EV immunogenicity (Saunderson and McLellan, 2017).
4 Role of EVs in SLE and LN
4.1 EVs, SLE
EVs were found to be increased (Pereira et al., 2006; Sellam et al., 2009; Lee et al., 2016; Lopez et al., 2020) or decreased (Nielsen et al., 2011) in SLE patients compared to healthy controls. Proteins, mRNAs, miRNAs, lncRNAs, tsRNAs and other noncoding RNAs have been shown to be associated with EVs (Mathivanan et al., 2012; Barile and Vassalli, 2017; Mathieu et al., 2019). Recent studies have revealed that the ncRNAs play dominant roles in the pathogenesis of SLE (Long et al., 2018; Xie and Xu, 2018; Zhao et al., 2018; Chen et al., 2019; Liu et al., 2021). In this sense, miRNAs, lncRNAs, tsRNAs and proteins in SLE EVs might serve as biomarkers for disease diagnosis and therapeutic targets (Tan et al., 2016; Ortega et al., 2020).
4.1.1 EVs, miRNA, SLE
MiRNA is a type of single-stranded non-coding RNA with a length of about 19–24 nucleotides, which can regulate the expression of many genes in vivo and participate in the pathogenesis of many diseases. Abnormal expression of circulating miRNAs in SLE patients have been found, and some of these miRNAs are related to clinical parameters (Carlsen et al., 2013; Ishibe et al., 2018). Circulating miRNAs are extracellularly secreted miRNAs circulating in the peripheral blood, which are either encapsulated by extracellular vesicles such as exosomes and microvesicles or bound to molecules such as the Argonaute protein or HDL cholesterol (Arroyo et al., 2011; Vickers et al., 2011). Tan et al. (2021) have reported that compared with healthy controls, serum EV miR-451a was decreased in SLE patients, which correlated with SLE disease activity and renal damage (Tan et al., 2021). Moreover, they found that EV shuttled miR-451a was involved in intercellular communication (Tan et al., 2021). Li et al. (2020) demonstrated that compared with healthy controls, serum EV miR-21 and miR-155 of SLE patients were up-regulated, whereas the expression of miR-146a was down-regulated (Li et al., 2020). Additionally, the expression of miR-21 and miR-146a were negatively correlated with anti-SSA/Ro antibodies and anti-dsDNA antibodies, respectively (Li et al., 2020). What’s more, both EV miR-21 and miR-155 expression presented positive correlations with proteinuria. These findings indicated that the expression levels of EV miR-21 and miR-155 might serve as potential biomarkers for the diagnosis of SLE. The aforementioned studies, however, have some limitations yet to be addressed. The mechanism underlying the reported dysregulation of the EV-associated miRNAs expression and the cell origin of the EVs remain unclear in the studies, which are performed based on relatively limited samples.
With the continual advances in this thread, the mechanism of EV-associated miRNAs in SLE pathogenesis has been revealed gradually. It is shown that miR-574, let-7b and miR-21 in plasma EVs can activate plasmacytoid DCs (pDCs) through the TLR7 pathway, enabling them to continuously produce IFN-α and proinflammatory cytokines, which may contribute to the pathogenesis of SLE (Salvi et al., 2018). Another study suggests that miR-146a could be internalized into mesenchymal stem cells (MSCs) via circulating EVs and participates in MSCs senescence in SLE patients by targeting TRAF6/NF-κB signal pathway (Dong et al., 2019).
4.1.2 EVs, lncRNA, SLE
LncRNA is another regulatory noncoding RNA longer than 200 nucleotides, capable of modulating many biological functions more specifically than miRNA. Aberrant circulating lncRNA expressions are found in SLE patients as well. Wu et al. (2017), Wu et al. (2019) found that plasma levels of GAS5, lnc7074 and lnc-DC were significantly reduced, whereas levels of linc0597, linc0640 and lnc5150 were elevated in SLE patients compared with those of healthy controls (Wu G.-C. et al., 2017; Wu et al., 2019). However, due to the complexity of its role, there is no literature regarding EV-associated lncRNA’s role in the pathogenesis of SLE. Thus, this promising research line is worthwhile to be investigated.
4.1.3 EVs, tsRNA, SLE
Transfer RNAs (tRNAs) are a group of classic ncRNAs with a well-defined role in protein translation (Schulman and Abelson, 1988). tRNA-derived small RNAs (tsRNAs) are cleaved from precursor or mature tRNAs with a length of 18–40 nt and can be broadly classified into two main groups: tRNA halves and tRNA-derived fragments (tRFs) (Anderson and Ivanov, 2014). tRFs have been implicated to participate in diverse physiological processes and involved in many diseases through the protein synthesis by regulating mRNA expression (Zhu et al., 2018; Kim et al., 2020). In a recent study, Xu et al. (2020) found that tRNAs and tsRNAs were significantly differentially expressed in the PBMCs of SLE patients, compared with those of healthy donors, and the targeted genes of the differentially expressed tsRNAs were enriched in the signaling pathway involved in primary immunodeficiency, T cell receptor and Th cell differentiation, suggesting that tRNAs and tsRNAs play important roles in the pathogenesis of SLE (Xu et al., 2020). More precisely, Geng et al. (2021) showed that tRF-3009 was substantially over-expressed in CD4+ T cells of SLE patients than those of healthy donors. What’s more, tRF-3009 may be involved in SLE pathogenesis by modulation of IFN-α-induced CD4+ T cell oxidative phosphorylation (Geng et al., 2021). As for the EV, Dou et al. (2021) revealed that mesenchymal stem cell (MSC)-derived EV tsRNA-21109 inhibited the M1-type polarization of macrophages, offering a promising therapeutic strategy for SLE (Dou et al., 2021). Nowadays, Yang et al. (2021) found that tRF-His-GTG-1 was significantly upregulated both in serum of SLE without LN, and in serum EVs of SLE with LN compared with healthy controls, suggesting that it could be employed as a noninvasive biomarker for diagnosis and prediction of nephritis in SLE (Yang et al., 2021). Nonetheless, the exact mechanism underlying tsRNA-21109 and tRF-His-GTG-1 mediated SLE development remains yet to be elucidated.
4.1.4 EVs, Protein, SLE
Proteins in EVs are also involved in the pathogenesis of SLE. Majer et al. (2019) found that UNC93B1 can be detected in RAW macrophage-derived EVs, it can limit TLR7 signaling and prevent TLR7-dependent autoimmunity in mice (Majer et al., 2019). Moreover, UNC93B1 mutation can enhance the TLR7 signaling pathway, leading to the development of autoimmune diseases (Majer et al., 2019). More recently, Chuang et al. (2021) proved that bactericidal/permeability-increasing protein (BPI) is a negative regulator of Treg differentiation (Chuang et al., 2021). They identified the overexpression of BPI in T cells and T cell-derived EVs contributed to autoimmune responses through both intrinsic (inhibition of Treg population) and extrinsic (induction of inflammatory EVs) pathways, which might be a biomarker and a pathogenic factor for SLE (Chuang et al., 2021). And just this month, they reported another EV-associated protein, Eosinophil Cationic Protein (ECP, also named RNase 3), which was overexpressed in SLE T cell-derived EVs (Chuang et al., 2022). What’s more, ECP overexpression in T cells resulted in an increase of inflammatory responses and T-cell activation. Notably, ECP-containing EVs from T cells led to tissue inflammation of the recipient mice. These results suggest that ECP-overexpressing T cells or ECP-containing EVs may play an important role in SLE pathogenesis (Chuang et al., 2022). Nevertheless, except for the small sample size, it would be challenging yet essential to dig into the fundamental mechanisms of BPI/ECP-induced inflammation via EVs in the future.
4.2 EVs, LN
Lupus nephritis (LN) is one of the most devastating manifestations of SLE, and a primary cause of morbidity and mortality of SLE (Hahn et al., 2012). At present, renal biopsy is still the gold standard for diagnosis and evaluation of residual nephron function. Renal puncture, however, presents many perilous complications and can only reflect the state of a small part of the renal tissue. Therefore, pursuing a non-invasive and sensitive diagnostic method is urgently needed (Ortega et al., 2010; Morell et al., 2021). Over the past few years, changes in urinary miRNAs have been reported in LN patients, and its expression may be relevant to disease activity (So et al., 2021).
4.2.1 EVs, miRNA, LN
Urinary EV-associated miRNAs are promising novel markers for the diagnosis and prognosis of disease, and also clinical outcomes. Lv et al. (2013) showed that high levels of miRNA were confined to urinary EVs in patients with a diversity of chronic diseases (Lv et al., 2013). Urinary EV in lupus nephritis was first described in 2014, Ichii et al. (2014) first found that in patients with lupus nephritis, the expression level of miR-26a in urinary EVs was significantly higher than that in the control group, and miR-26a expression was related to podocyte injury, suggesting that miR-26a can be used as a direct biomarker for podocyte injury in autoimmune glomerulonephritis (Ichii et al., 2014). Solé et al. (2015) showed reduced expression level of miR-29c in LN patients compared with healthy controls, and its level in urinary EVs was negatively correlated with the histological chronicity index and glomerular sclerosis, indicating that miR-29c level could be used as a novel non-invasive marker for predicting histological fibrosis of LN (Solé et al., 2015). As the research proceeds, the mechanism of urinary EV-associated miRNAs in LN pathogenesis has been gradually revealed. Recently, their team revealed that miR-21 and miR-150 were substantially up-regulated while miR-29c was down-regulated in the urinary EVs of LN patients, and their expression was strongly correlated with renal chronicity. They also demonstrated that these miRNAs promoted renal fibrosis through SP1 and Smad3/TGFβ signaling pathway (Solé et al., 2019). And a more recent study by their team found that the overexpression of urinary EV miR-135b-5p, miR-107, and miR-31 could meliorate renal disease by inhibiting HIF-1α, suggesting their potential to become early markers for predicting clinical response in LN (Garcia-Vives et al., 2020). Perez-Hernandez et al. (2015) confirmed that urinary miRNAs were contained mainly in EVs, and they reported an impressive increase in miRNA-146a in urinary EVs in patients with active lupus nephropathy, implying that miRNA-146a in EVs may be able to distinguish SLE patients with active LN from control group or SLE patients in absence of LN (Perez-Hernandez et al., 2015). Recently, their group identified a protective role that urinary EV miR-146a played in LN progression through negative regulation of inflammation by suppressing the TRAF6 axis (Perez-Hernandez et al., 2021). Furthermore, it has been evidenced that Type IV lupus nephritis with cellular crescent (LNIV-CC) has a unique urinary EV-associated miRNA expression profile, and urinary EV miR-3135b, miR-654-5p and miR-146a-5p are candidate biomarkers for LNIV-CC (Li et al., 2018). In addition, another study discovered that compared with inactive disease, let-7a and miR-21 in urine EVs were significantly down-regulated in LN patients with active disease. Interestingly, their expression increased after the entire course of treatment, indicating that urinary EV-related miRNA, let-7a and miR-21, may be leveraged to guide the clinical stage of LN patients (Tangtanatakul et al., 2019). The above findings indicate that miRNAs in urinary EVs may have great potential to serve as biomarkers in LN diagnosis and monitoring.
4.2.2 EVs, Protein, LN
Proteins in urine EVs are also involved in the pathogenesis of LN. Gudehithlu et al. (2019) found that urine EV ceruloplasmin (CP) was increased in LN patients. What’s more, in biopsied cases, CP was strongly localized to kidney tubules, suggesting that the CP found in urine EVs came from the kidney. Moreover, in mouse models, urine EV CP were observed to increase prior to proteinuria, indicating it could be an early biomarker to diagnose kidney disease (Gudehithlu et al., 2019).
These findings strongly suggest the potential role of urinary EVs as a non-invasive biomarker in the diagnosis and treatment of LN. Multiple limitations of the present studies, however, need to be acknowledged and are summarized as follows. Firstly, studies involving a reasonably larger patient cohort are necessary for further analyzing and validating these findings, especially for diagnosis. Secondly, most of the research works described above are cross-sectional studies. The longitudinal studies such as during disease flare or before and after treatment are also needed to further extend these findings. Thirdly, the thorough comparative analysis between the reported urinary EV-associated miRNAs/protein and the existing inflammatory and clinical markers of disease is missing and thus worthwhile to be performed in the further. Fourthly, the differentially expressed urinary EV-associated miRNAs/protein is delivered from various kidney cell types. Further study to characterize specific cell types that contribute to the dysregulation of miRNAs/protein in urine EVs is thus demanded. What’s more, the present studies lack functional experiments at molecular or cellular level to verify the association between miRNAs/protein and LN. All these imperfections are likely due to the low recovery ration, low yield and purity of EV extraction as well as the immature technology of EV transfection and infection.
5 Conclusion
In recent years, EVs have emerged as an important endogenous “nanovehicles” for carrying and transferring molecular mediators such as nucleic acids, proteins, and bioactive lipids for intercellular communications and signal transduction. Besides, it is well established that EVs are involved in a multitude of physiological and pathological processes, such as immune response, antigen presentation, cell differentiation, cell migration, and tumor invasion. A rapidly expanding body of evidence indicates that the presence of EV-specific patterns and their cargo play crucial physiological and pathological roles in SLE. In this context, miRNAs, tsRNAs, and proteins transported into serum/plasma/urinary EVs are correlated with glomerular damage, SLE disease activity, clinical stage and response, proteinuria as well as the severity of renal fibrosis in lupus nephritis. In this article, we provide the up-to-date survey of relevant literature evidencing that EVs have great potential in SLE disease diagnosis, prediction, prognosis, and targeted treatment (Figure 1 and Table 1). However, the underlying pathophysiological mechanisms of EVs in SLE pathogenesis and their functionality as therapeutic agents or targets are not fully understood. Future investigations into the exact mechanisms of EVs in SLE will undoubtedly bring new breakthroughs for SLE disease diagnosis and therapies.
Author Contributions
LX and CZ analyzed the literature and wrote the manuscript. HQ, XL, XC, FL and LW assisted with constructing figures and polishing the language. LX, JX and XZ revised the manuscript. All authors listed have made substantial, direct, and intellectual contributions to the work, and approved the article for publication.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81903216 and 81872526) and from Shanghai Science and Technology Committee (21YF1404500).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, A., Fanelli, G., Letizia, M., Tung, S. L., Boardman, D., Lechler, R., et al. (2014). Regulatory T Cell-Derived Exosomes: Possible Therapeutic and Diagnostic Tools in Transplantation. Front. Immunol. 5, 555. doi:10.3389/fimmu.2014.00555
Akers, J. C., Gonda, D., Kim, R., Carter, B. S., and Chen, C. C. (2013). Biogenesis of Extracellular Vesicles (EV): Exosomes, Microvesicles, Retrovirus-Like Vesicles, and Apoptotic Bodies. J. Neurooncol. 113 (1), 1–11. doi:10.1007/s11060-013-1084-8
Anderson, H. C., Mulhall, D., and Garimella, R. (2010). Role of Extracellular Membrane Vesicles in the Pathogenesis of Various Diseases, Including Cancer, Renal Diseases, Atherosclerosis, and Arthritis. Lab. Invest. 90 (11), 1549–1557. doi:10.1038/labinvest.2010.152
Anderson, P., and Ivanov, P. (2014). tRNA Fragments in Human Health and Disease. FEBS Lett. 588 (23), 4297–4304. doi:10.1016/j.febslet.2014.09.001
Arellano-Anaya, Z. E., Huor, A., Leblanc, P., Lehmann, S., Provansal, M., Raposo, G., et al. (2015). Prion Strains are Differentially Released through the Exosomal Pathway. Cell. Mol. Life Sci. 72 (6), 1185–1196. doi:10.1007/s00018-014-1735-8
Arroyo, J. D., Chevillet, J. R., Kroh, E. M., Ruf, I. K., Pritchard, C. C., Gibson, D. F., et al. (2011). Argonaute2 Complexes Carry a Population of Circulating microRNAs Independent of Vesicles in Human Plasma. Proc. Natl. Acad. Sci. 108 (12), 5003–5008. doi:10.1073/pnas.1019055108
Barile, L., and Vassalli, G. (2017). Exosomes: Therapy Delivery Tools and Biomarkers of Diseases. Pharmacol. Ther. 174, 63–78. doi:10.1016/j.pharmthera.2017.02.020
Bhatnagar, S., and Schorey, J. S. (2007). Exosomes Released from Infected Macrophages Contain Mycobacterium A Glycopeptidolipids and Are Proinflammatory. J. Biol. Chem. 282 (35), 25779–25789. doi:10.1074/jbc.M702277200
Bhatnagar, S., Shinagawa, K., Castellino, F. J., and Schorey, J. S. (2007). Exosomes Released from Macrophages Infected with Intracellular Pathogens Stimulate a Proinflammatory Response In Vitro and In Vivo. Blood 110 (9), 3234–3244. doi:10.1182/blood-2007-03-079152
Bryniarski, K., Ptak, W., Jayakumar, A., Püllmann, K., Caplan, M. J., Chairoungdua, A., et al. (2013). Antigen-Specific, Antibody-Coated, Exosome-Like Nanovesicles Deliver Suppressor T-Cell microRNA-150 to Effector T Cells to Inhibit Contact Sensitivity. J. Allergy Clin. Immunol. 132 (1), 170–181. doi:10.1016/j.jaci.2013.04.048
Carlsen, A. L., Schetter, A. J., Nielsen, C. T., Lood, C., Knudsen, S., Voss, A., et al. (2013). Circulating microRNA Expression Profiles Associated with Systemic Lupus Erythematosus. Arthritis Rheum. 65 (5), 1324–1334. doi:10.1002/art.37890
Chen, S., Wang, Y., Qin, H., Lin, J., Xie, L., Chen, S., et al. (2019). Downregulation of miR-633 Activated AKT/mTOR Pathway by Targeting AKT1 in Lupus CD4+ T Cells. Lupus 28 (4), 510–519. doi:10.1177/0961203319829853
Cheruvanky, A., Zhou, H., Pisitkun, T., Kopp, J. B., Knepper, M. A., Yuen, P. S. T., et al. (2007). Rapid Isolation of Urinary Exosomal Biomarkers Using a Nanomembrane Ultrafiltration Concentrator. Am. J. Physiology-Renal Physiol. 292 (5), F1657–F1661. doi:10.1152/ajprenal.00434.2006
Chuang, H.-C., Chen, M.-H., Chen, Y.-M., Yang, H.-Y., Ciou, Y.-R., Hsueh, C.-H., et al. (2021). BPI Overexpression Suppresses Treg Differentiation and Induces Exosome-Mediated Inflammation in Systemic Lupus Erythematosus. Theranostics 11 (20), 9953–9966. doi:10.7150/thno.63743
Chuang, H. C., Chen, M. H., Chen, Y. M., Ciou, Y. R., Hsueh, C. H., Tsai, C. Y., et al. (2022). Induction of Interferon-γ and Tissue Inflammation by Overexpression of Eosinophil Cationic Protein in T Cells and Exosomes. Arthritis Rheumatol. 74 (1), 92–104. doi:10.1002/art.41920
Crispín, J. C., Liossis, S.-N. C., Kis-Toth, K., Lieberman, L. A., Kyttaris, V. C., Juang, Y.-T., et al. (2010). Pathogenesis of Human Systemic Lupus Erythematosus: Recent Advances. Trends Mol. Med. 16 (2), 47–57. doi:10.1016/j.molmed.2009.12.005
Dalli, J., Montero-Melendez, T., Norling, L. V., Yin, X., Hinds, C., Haskard, D., et al. (2013). Heterogeneity in Neutrophil Microparticles Reveals Distinct Proteome and Functional Properties. Mol. Cell Proteomics 12 (8), 2205–2219. doi:10.1074/mcp.M113.028589
Dalli, J., Norling, L. V., Renshaw, D., Cooper, D., Leung, K.-Y., and Perretti, M. (2008). Annexin 1 Mediates the Rapid Anti-Inflammatory Effects of Neutrophil-Derived Microparticles. Blood 112 (6), 2512–2519. doi:10.1182/blood-2008-02-140533
Dong, C., Zhou, Q., Fu, T., Zhao, R., Yang, J., Kong, X., et al. (2019). Circulating Exosomes Derived-miR-146a from Systemic Lupus Erythematosus Patients Regulates Senescence of Mesenchymal Stem Cells. Biomed. Res. Int. 2019, 6071308. doi:10.1155/2019/6071308
Dou, R., Zhang, X., Xu, X., Wang, P., and Yan, B. (2021). Mesenchymal Stem Cell Exosomal tsRNA-21109 Alleviate Systemic Lupus Erythematosus by Inhibiting Macrophage M1 Polarization. Mol. Immunol. 139, 106–114. doi:10.1016/j.molimm.2021.08.015
Eken, C., Gasser, O., Zenhaeusern, G., Oehri, I., Hess, C., and Schifferli, J. A. (2008). Polymorphonuclear Neutrophil-Derived Ectosomes Interfere with the Maturation of Monocyte-Derived Dendritic Cells. J. Immunol. 180 (2), 817–824. doi:10.4049/jimmunol.180.2.817
Fais, S. (2013). NK Cell-Released Exosomes: Natural Nanobullets against Tumors. Oncoimmunology 2 (1), e22337. doi:10.4161/onci.22337
Fernández-Messina, L., Reyburn, H. T., and Valés-Gómez, M. (2012). Human NKG2D-Ligands: Cell Biology Strategies to Ensure Immune Recognition. Front. Immun. 3, 299. doi:10.3389/fimmu.2012.00299
Garcia-Vives, E., Solé, C., Moliné, T., Vidal, M., Agraz, I., Ordi-Ros, J., et al. (2020). The Urinary Exosomal miRNA Expression Profile Is Predictive of Clinical Response in Lupus Nephritis. Int. J. Mol. Sci. 21 (4), 1372. doi:10.3390/ijms21041372
Gasser, O., Hess, C., Miot, S., Deon, C., Sanchez, J.-C., and Schifferli, J. U. A. (2003). Characterisation and Properties of Ectosomes Released by Human Polymorphonuclear Neutrophils. Exp. Cel Res. 285 (2), 243–257. doi:10.1016/s0014-4827(03)00055-7
Gasser, O., and Schifferli, J. A. (2004). Activated Polymorphonuclear Neutrophils Disseminate Anti-Inflammatory Microparticles by Ectocytosis. Blood 104 (8), 2543–2548. doi:10.1182/blood-2004-01-0361
Gavins, F. N. E., and Hickey, M. J. (2012). Annexin A1 and the Regulation of Innate and Adaptive Immunity. Front. Immun. 3, 354. doi:10.3389/fimmu.2012.00354
Geng, G., Wang, H., Xin, W., Liu, Z., Chen, J., Danting, Z., et al. (2021). tRNA Derived Fragment (tRF)-3009 Participates in Modulation of IFN-α-Induced CD4+ T Cell Oxidative Phosphorylation in Lupus Patients. J. Transl Med. 19 (1), 305. doi:10.1186/s12967-021-02967-3
Giri, P. K., and Schorey, J. S. (2008). Exosomes Derived from M. Bovis BCG Infected Macrophages Activate Antigen-Specific CD4+ and CD8+ T Cells In Vitro and In Vivo. PLoS One 3 (6), e2461. doi:10.1371/journal.pone.0002461
Gudehithlu, K. P., Hart, P., Joshi, A., Garcia-Gomez, I., Cimbaluk, D. J., Dunea, G., et al. (2019). Urine Exosomal Ceruloplasmin: A Potential Early Biomarker of Underlying Kidney Disease. Clin. Exp. Nephrol. 23 (8), 1013–1021. doi:10.1007/s10157-019-01734-5
Gutiérrez-Vázquez, C., Villarroya-Beltri, C., Mittelbrunn, M., and Sánchez-Madrid, F. (2013). Transfer of Extracellular Vesicles during Immune Cell-Cell Interactions. Immunol. Rev. 251 (1), 125–142. doi:10.1111/imr.12013
Hahn, B. H., McMahon, M. A., Wilkinson, A., Wallace, W. D., Daikh, D. I., Fitzgerald, J. D., et al. (2012). American College of Rheumatology Guidelines for Screening, Treatment, and Management of Lupus Nephritis. Arthritis Care Res. 64 (6), 797–808. doi:10.1002/acr.21664
Hess, C., Sadallah, S., Hefti, A., Landmann, R., and Schifferli, J. A. (1999). Ectosomes Released by Human Neutrophils Are Specialized Functional Units. J. Immunol. 163 (8), 4564–4573.
Hessvik, N. P., and Llorente, A. (2018). Current Knowledge on Exosome Biogenesis and Release. Cel. Mol. Life Sci. 75 (2), 193–208. doi:10.1007/s00018-017-2595-9
Hood, J. L. (2017). The Association of Exosomes with Lymph Nodes. Semin. Cel Develop. Biol. 67, 29–38. doi:10.1016/j.semcdb.2016.12.002
Ichii, O., Otsuka-Kanazawa, S., Horino, T., Kimura, J., Nakamura, T., Matsumoto, M., et al. (2014). Decreased miR-26a Expression Correlates with the Progression of Podocyte Injury in Autoimmune Glomerulonephritis. PLoS One 9 (10), e110383. doi:10.1371/journal.pone.0110383
Ishibe, Y., Kusaoi, M., Murayama, G., Nemoto, T., Kon, T., Ogasawara, M., et al. (2018). Changes in the Expression of Circulating microRNAs in Systemic Lupus Erythematosus Patient Blood Plasma after Passing through a Plasma Adsorption Membrane. Ther. Apher. Dial. 22 (3), 278–289. doi:10.1111/1744-9987.12695
Jong, A. Y., Wu, C.-H., Li, J., Sun, J., Fabbri, M., Wayne, A. S., et al. (2017). Large-Scale Isolation and Cytotoxicity of Extracellular Vesicles Derived from Activated Human Natural Killer Cells. J. Extracellular Vesicles 6 (1), 1294368. doi:10.1080/20013078.2017.1294368
Kim, H. K., Yeom, J.-H., and Kay, M. A. (2020). Transfer RNA-Derived Small RNAs: Another Layer of Gene Regulation and Novel Targets for Disease Therapeutics. Mol. Ther. 28 (11), 2340–2357. doi:10.1016/j.ymthe.2020.09.013
Koh, Y. Q., Almughlliq, F. B., Vaswani, K., Peiris, H. N., and Mitchell, M. D. (2018). Exosome Enrichment by Ultracentrifugation and Size Exclusion Chromatography. Front. Biosci. 23, 865–874. doi:10.2741/4621
Konoshenko, M. Y., Lekchnov, E. A., Vlassov, A. V., and Laktionov, P. P. (2018). Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed. Res. Int. 2018, 8545347. doi:10.1155/2018/8545347
Lee, J. Y., Park, J. K., Lee, E. Y., Lee, E. B., and Song, Y. W. (2016). Circulating Exosomes from Patients with Systemic Lupus Erythematosus Induce a Proinflammatory Immune Response. Arthritis Res. Ther. 18 (1), 264. doi:10.1186/s13075-016-1159-y
Leliefeld, P. H. C., Koenderman, L., and Pillay, J. (2015). How Neutrophils Shape Adaptive Immune Responses. Front. Immunol. 6, 471. doi:10.3389/fimmu.2015.00471
Lewis, J. M., Vyas, A. D., Qiu, Y., Messer, K. S., White, R., and Heller, M. J. (2018). Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano 12 (4), 3311–3320. doi:10.1021/acsnano.7b08199
Li, W., Liu, S., Chen, Y., Weng, R., Zhang, K., He, X., et al. (2020). Circulating Exosomal microRNAs as Biomarkers of Systemic Lupus Erythematosus. Clinics (Sao Paulo) 75, e1528. doi:10.6061/clinics/2020/e1528
Li, Y., Xu, X., Tang, X., Bian, X., Shen, B., Zhao, H., et al. (2018). MicroRNA Expression Profile of Urinary Exosomes in Type IV Lupus Nephritis Complicated by Cellular Crescent. J. Biol. Res-thessaloniki 25, 16. doi:10.1186/s40709-018-0088-0
Liu, X., Lin, J., Wu, H., Wang, Y., Xie, L., Wu, J., et al. (2021). A Novel Long Noncoding RNA lincRNA00892 Activates CD4(+) T Cells in Systemic Lupus Erythematosus by Regulating CD40L. Front. Pharmacol. 12, 733902. doi:10.3389/fphar.2021.733902
Long, H., Wang, X., Chen, Y., Wang, L., Zhao, M., and Lu, Q. (2018). Dysregulation of microRNAs in Autoimmune Diseases: Pathogenesis, Biomarkers and Potential Therapeutic Targets. Cancer Lett. 428, 90–103. doi:10.1016/j.canlet.2018.04.016
Lööv, C., Scherzer, C. R., Hyman, B. T., Breakefield, X. O., and Ingelsson, M. (2016). α-Synuclein in Extracellular Vesicles: Functional Implications and Diagnostic Opportunities. Cell Mol Neurobiol 36 (3), 437–448. doi:10.1007/s10571-015-0317-0
López, P., Rodríguez-Carrio, J., Caminal-Montero, L., and Suárez, A. (2020). Relationship between T-Cell Exosomes and Cellular Subsets in SLE According to Type I IFN-Signaling. Front. Med. 7, 604098. doi:10.3389/fmed.2020.604098
Lugini, L., Cecchetti, S., Huber, V., Luciani, F., Macchia, G., Spadaro, F., et al. (2012). Immune Surveillance Properties of Human NK Cell-Derived Exosomes. J. Immunol. 189 (6), 2833–2842. doi:10.4049/jimmunol.1101988
Lv, L.-L., Cao, Y., Liu, D., Xu, M., Liu, H., Tang, R.-N., et al. (2013). Isolation and Quantification of microRNAs from Urinary Exosomes/microvesicles for Biomarker Discovery. Int. J. Biol. Sci. 9 (10), 1021–1031. doi:10.7150/ijbs.6100
Maehara, M., Toyoda, E., Takahashi, T., Watanabe, M., and Sato, M. (2021). Potential of Exosomes for Diagnosis and Treatment of Joint Disease: Towards a Point-of-Care Therapy for Osteoarthritis of the Knee. Int. J. Mol. Sci. 22 (5), 2666. doi:10.3390/ijms22052666
Majer, O., Liu, B., Kreuk, L. S. M., Krogan, N., and Barton, G. M. (2019). UNC93B1 Recruits Syntenin-1 to Dampen TLR7 Signalling and Prevent Autoimmunity. Nature 575 (7782), 366–370. doi:10.1038/s41586-019-1612-6
Mashouri, L., Yousefi, H., Aref, A. R., Ahadi, A. m., Molaei, F., and Alahari, S. K. (2019). Exosomes: Composition, Biogenesis, and Mechanisms in Cancer Metastasis and Drug Resistance. Mol. Cancer 18 (1), 75. doi:10.1186/s12943-019-0991-5
Mathieu, M., Martin-Jaular, L., Lavieu, G., and Théry, C. (2019). Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-To-Cell Communication. Nat. Cel Biol 21 (1), 9–17. doi:10.1038/s41556-018-0250-9
Mathivanan, S., Fahner, C. J., Reid, G. E., and Simpson, R. J. (2012). ExoCarta 2012: Database of Exosomal Proteins, RNA and Lipids. Nucleic Acids Res. 40 (Database issue), D1241–D1244. doi:10.1093/nar/gkr828
Matsumura, T., Sugimachi, K., Iinuma, H., Takahashi, Y., Kurashige, J., Sawada, G., et al. (2015). Exosomal microRNA in Serum Is a Novel Biomarker of Recurrence in Human Colorectal Cancer. Br. J. Cancer 113 (2), 275–281. doi:10.1038/bjc.2015.201
Mayadas, T. N., Cullere, X., and Lowell, C. A. (2014). The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 9, 181–218. doi:10.1146/annurev-pathol-020712-164023
Miao, C., Zhou, W., Wang, X., and Fang, J. (2021). The Research Progress of Exosomes in Osteoarthritis, with Particular Emphasis on the Mediating Roles of miRNAs and lncRNAs. Front. Pharmacol. 12, 685623. doi:10.3389/fphar.2021.685623
Momen-Heravi, F., Balaj, L., Alian, S., Tigges, J., Toxavidis, V., Ericsson, M., et al. (2012a). Alternative Methods for Characterization of Extracellular Vesicles. Front. Physio. 3, 354. doi:10.3389/fphys.2012.00354
Momen-Heravi, F., Balaj, L., Alian, S., Trachtenberg, A. J., Hochberg, F. H., Skog, J., et al. (2012b). Impact of Biofluid Viscosity on Size and Sedimentation Efficiency of the Isolated Microvesicles. Front. Physio. 3, 162. doi:10.3389/fphys.2012.00162
Monguió-Tortajada, M., Gálvez-Montón, C., Bayes-Genis, A., Roura, S., and Borràs, F. E. (2019). Extracellular Vesicle Isolation Methods: Rising Impact of Size-Exclusion Chromatography. Cel. Mol. Life Sci. 76 (12), 2369–2382. doi:10.1007/s00018-019-03071-y
Morell, M., Pérez-Cózar, F., and Marañón, C. (2021). Immune-Related Urine Biomarkers for the Diagnosis of Lupus Nephritis. Int. J. Mol. Sci. 22 (13), 7143. doi:10.3390/ijms22137143
Morvan, M. G., and Lanier, L. L. (2016). NK Cells and Cancer: You Can Teach Innate Cells New Tricks. Nat. Rev. Cancer 16 (1), 7–19. doi:10.1038/nrc.2015.5
Natasha G, G., Gundogan, B., Tan, A., Farhatnia, Y., Wu, W., Rajadas, J., et al. (2014). Exosomes as Immunotheranostic Nanoparticles. Clin. Ther. 36 (6), 820–829. doi:10.1016/j.clinthera.2014.04.019
Nath, S., Agholme, L., Kurudenkandy, F. R., Granseth, B., Marcusson, J., and Hallbeck, M. (2012). Spreading of Neurodegenerative Pathology via Neuron-To-Neuron Transmission of -Amyloid. J. Neurosci. 32 (26), 8767–8777. doi:10.1523/jneurosci.0615-12.2012
Nielsen, C. T., Østergaard, O., Johnsen, C., Jacobsen, S., and Heegaard, N. H. H. (2011). Distinct Features of Circulating Microparticles and Their Relationship to Clinical Manifestations in Systemic Lupus Erythematosus. Arthritis Rheum. 63 (10), 3067–3077. doi:10.1002/art.30499
Obregon, C., Rothen-Rutishauser, B., Gerber, P., Gehr, P., and Nicod, L. P. (2009). Active Uptake of Dendritic Cell-Derived Exovesicles by Epithelial Cells Induces the Release of Inflammatory Mediators through a TNF-α-Mediated Pathway. Am. J. Pathol. 175 (2), 696–705. doi:10.2353/ajpath.2009.080716
Ortega, A., Martinez-Arroyo, O., Forner, M. J., and Cortes, R. (2020). Exosomes as Drug Delivery Systems: Endogenous Nanovehicles for Treatment of Systemic Lupus Erythematosus. Pharmaceutics 13 (1), 3. doi:10.3390/pharmaceutics13010003
Ortega, L., Schultz, D., Lenz, O., Pardo, V., and Contreras, G. (2010). Review: Lupus Nephritis: Pathologic Features, Epidemiology and a Guide to Therapeutic Decisions. Lupus 19 (5), 557–574. doi:10.1177/0961203309358187
Pan, B.-T., and Johnstone, R. M. (1983). Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes In Vitro: Selective Externalization of the Receptor. Cell 33 (3), 967–978. doi:10.1016/0092-8674(83)90040-5
Pereira, J., Alfaro, G., Goycoolea, M., Quiroga, T., Ocqueteau, M., Massardo, L., et al. (2006). Circulating Platelet-Derived Microparticles in Systemic Lupus Erythematosus. Association with Increased Thrombin Generation and Procoagulant State. Thromb. Haemost. 95 (1), 94–99. doi:10.1160/TH05-05-0310
Perez-Hernandez, J., and Cortes, R. (2015). Extracellular Vesicles as Biomarkers of Systemic Lupus Erythematosus. Dis. Markers 2015, 613536. doi:10.1155/2015/613536
Perez-Hernandez, J., Forner, M. J., Pinto, C., Chaves, F. J., Cortes, R., and Redon, J. (2015). Increased Urinary Exosomal MicroRNAs in Patients with Systemic Lupus Erythematosus. PLoS One 10 (9), e0138618. doi:10.1371/journal.pone.0138618
Perez-Hernandez, J., Martinez-Arroyo, O., Ortega, A., Galera, M., Solis-Salguero, M. A., Chaves, F. J., et al. (2021). Urinary Exosomal miR-146a as a Marker of Albuminuria, Activity Changes and Disease Flares in Lupus Nephritis. J. Nephrol. 34 (4), 1157–1167. doi:10.1007/s40620-020-00832-y
Perez-Hernandez, J., Redon, J., and Cortes, R. (2017). Extracellular Vesicles as Therapeutic Agents in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 18 (4), 717. doi:10.3390/ijms18040717
Quah, B. J. C., and O'Neill, H. C. (2005). The Immunogenicity of Dendritic Cell-Derived Exosomes. Blood Cell Mol. Dis. 35 (2), 94–110. doi:10.1016/j.bcmd.2005.05.002
Ramachandra, L., Qu, Y., Wang, Y., Lewis, C. J., Cobb, B. A., Takatsu, K., et al. (2010). Mycobacterium T Synergizes with ATP to Induce Release of Microvesicles and Exosomes Containing Major Histocompatibility Complex Class II Molecules Capable of Antigen Presentation. Infect. Immun. 78 (12), 5116–5125. doi:10.1128/IAI.01089-09
Ramirez, M. I., Amorim, M. G., Gadelha, C., Milic, I., Welsh, J. A., Freitas, V. M., et al. (2018). Technical Challenges of Working with Extracellular Vesicles. Nanoscale 10 (3), 881–906. doi:10.1039/c7nr08360b
Raposo, G., Nijman, H. W., Stoorvogel, W., Liejendekker, R., Harding, C. V., Melief, C. J., et al. (1996). B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 183 (3), 1161–1172. doi:10.1084/jem.183.3.1161
Raposo, G., and Stoorvogel, W. (2013). Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cel Biol 200 (4), 373–383. doi:10.1083/jcb.201211138
Rayamajhi, S., Nguyen, T. D. T., Marasini, R., and Aryal, S. (2019). Macrophage-derived Exosome-Mimetic Hybrid Vesicles for Tumor Targeted Drug Delivery. Acta Biomater. 94, 482–494. doi:10.1016/j.actbio.2019.05.054
Record, M., Subra, C., Silvente-Poirot, S., and Poirot, M. (2011). Exosomes as Intercellular Signalosomes and Pharmacological Effectors. Biochem. Pharmacol. 81 (10), 1171–1182. doi:10.1016/j.bcp.2011.02.011
Reis, M., Mavin, E., Nicholson, L., Green, K., Dickinson, A. M., and Wang, X.-N. (2018). Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front. Immunol. 9, 2538. doi:10.3389/fimmu.2018.02538
Salvi, V., Gianello, V., Busatto, S., Bergese, P., Andreoli, L., D’Oro, U., et al. (2018). Exosome-Delivered microRNAs Promote IFN-α Secretion by Human Plasmacytoid DCs via TLR7. JCI Insight 3 (10), e98204. doi:10.1172/jci.insight.98204
Saraste, A., and Pulkki, K. (2000). Morphologic and Biochemical Hallmarks of Apoptosis. Cardiovasc. Res. 45 (3), 528–537. doi:10.1016/s0008-6363(99)00384-3
Saunderson, S. C., and McLellan, A. D. (2017). Role of Lymphocyte Subsets in the Immune Response to Primary B Cell-Derived Exosomes. J. Immunol. 199 (7), 2225–2235. doi:10.4049/jimmunol.1601537
Schulman, L. H., and Abelson, J. (1988). Recent Excitement in Understanding Transfer RNA Identity. Science 240 (4859), 1591–1592. doi:10.1126/science.2454505
Segura, E., Amigorena, S., and Théry, C. (2005). Mature Dendritic Cells Secrete Exosomes with strong Ability to Induce Antigen-specific Effector Immune Responses. Blood Cell Mol. Dis. 35 (2), 89–93. doi:10.1016/j.bcmd.2005.05.003
Sellam, J., Proulle, V., Jüngel, A., Ittah, M., Miceli Richard, C., Gottenberg, J.-E., et al. (2009). Increased Levels of Circulating Microparticles in Primary Sjögren's Syndrome, Systemic Lupus Erythematosus and Rheumatoid Arthritis and Relation with Disease Activity. Arthritis Res. Ther. 11 (5), R156. doi:10.1186/ar2833
Shah, R., Patel, T., and Freedman, J. E. (2018). Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 379 (10), 958–966. doi:10.1056/NEJMra1704286
Shen, G., Krienke, S., Schiller, P., Niessen, A., Neu, S., Eckstein, V., et al. (2017). Microvesicles Released by Apoptotic Human Neutrophils Suppress Proliferation and IL-2/IL-2 Receptor Expression of Resting T Helper Cells. Eur. J. Immunol. 47 (5), 900–910. doi:10.1002/eji.201546203
Simons, M., and Raposo, G. (2009). Exosomes - Vesicular Carriers for Intercellular Communication. Curr. Opin. Cel Biol. 21 (4), 575–581. doi:10.1016/j.ceb.2009.03.007
Singh, P. P., Smith, V. L., Karakousis, P. C., and Schorey, J. S. (2012). Exosomes Isolated from Mycobacteria-Infected Mice or Cultured Macrophages Can Recruit and Activate Immune Cells In Vitro and In Vivo. J. Immunol. 189 (2), 777–785. doi:10.4049/jimmunol.1103638
Sivori, S., Vacca, P., Del Zotto, G., Munari, E., Mingari, M. C., and Moretta, L. (2019). Human NK Cells: Surface Receptors, Inhibitory Checkpoints, and Translational Applications. Cell Mol Immunol 16 (5), 430–441. doi:10.1038/s41423-019-0206-4
Slater, T. W., Finkielsztein, A., Mascarenhas, L. A., Mehl, L. C., Butin-Israeli, V., and Sumagin, R. (2017). Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa to Inhibit Epithelial Wound Healing. J. Immunol. 198 (7), 2886–2897. doi:10.4049/jimmunol.1601810
So, B. Y. F., Yap, D. Y. H., and Chan, T. M. (2021). MicroRNAs in Lupus Nephritis-Role in Disease Pathogenesis and Clinical Applications. Int. J. Mol. Sci. 22 (19), 10737. doi:10.3390/ijms221910737
Solé, C., Cortés-Hernández, J., Felip, M. L., Vidal, M., and Ordi-Ros, J. (2015). miR-29c in Urinary Exosomes as Predictor of Early Renal Fibrosis in Lupus Nephritis. Nephrol. Dial. Transpl. 30 (9), 1488–1496. doi:10.1093/ndt/gfv128
Solé, C., Moliné, T., Vidal, M., Ordi-Ros, J., and Cortés-Hernández, J. (2019). An Exosomal Urinary miRNA Signature for Early Diagnosis of Renal Fibrosis in Lupus Nephritis. Cells 8 (8), 773. doi:10.3390/cells8080773
Stahl, P. D., and Raposo, G. (2019). Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 34 (3), 169–177. doi:10.1152/physiol.00045.2018
Steinman, R. M. (2012). Decisions about Dendritic Cells: Past, Present, and Future. Annu. Rev. Immunol. 30, 1–22. doi:10.1146/annurev-immunol-100311-102839
Tan, L., Wu, H., Liu, Y., Zhao, M., Li, D., and Lu, Q. (2016). Recent Advances of Exosomes in Immune Modulation and Autoimmune Diseases. Autoimmunity 49 (6), 357–365. doi:10.1080/08916934.2016.1191477
Tan, L., Zhao, M., Wu, H., Zhang, Y., Tong, X., Gao, L., et al. (2021). Downregulated Serum Exosomal miR-451a Expression Correlates with Renal Damage and its Intercellular Communication Role in Systemic Lupus Erythematosus. Front. Immunol. 12, 630112. doi:10.3389/fimmu.2021.630112
Tangtanatakul, P., Klinchanhom, S., Sodsai, P., Sutichet, T., Promjeen, C., Avihingsanon, Y., et al. (2019). Down-regulation of Let-7a and miR-21 in Urine Exosomes from Lupus Nephritis Patients during Disease Flare. Asian Pac. J. Allergy Immunol. 37 (4), 189–197. doi:10.12932/ap-130318-0280
Taylor, J., and Bebawy, M. (2019). Proteins Regulating Microvesicle Biogenesis and Multidrug Resistance in Cancer. Proteomics 19 (1-2), e1800165. doi:10.1002/pmic.201800165
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell Vesicles 7 (1), 1535750. doi:10.1080/20013078.2018.1535750
Théry, C., Boussac, M., Véron, P., Ricciardi-Castagnoli, P., Raposo, G., Garin, J., et al. (2001). Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 166 (12), 7309–7318. doi:10.4049/jimmunol.166.12.7309
Théry, C., Duban, L., Segura, E., Véron, P., Lantz, O., and Amigorena, S. (2002a). Indirect Activation of Naïve CD4+ T Cells by Dendritic Cell-Derived Exosomes. Nat. Immunol. 3 (12), 1156–1162. doi:10.1038/ni854
Théry, C., Regnault, A., Garin, J., Wolfers, J., Zitvogel, L., Ricciardi-Castagnoli, P., et al. (1999). Molecular Characterization of Dendritic Cell-Derived Exosomes. Selective Accumulation of the Heat Shock Protein Hsc73. J. Cel Biol 147 (3), 599–610. doi:10.1083/jcb.147.3.599
Théry, C., Zitvogel, L., and Amigorena, S. (2002b). Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2 (8), 569–579. doi:10.1038/nri855
Timár, C. I., Lőrincz, Á. M., Csépányi-Kömi, R., Vályi-Nagy, A., Nagy, G., Buzás, E. I., et al. (2013). Antibacterial Effect of Microvesicles Released from Human Neutrophilic Granulocytes. Blood 121 (3), 510–518. doi:10.1182/blood-2012-05-431114
Torralba, D., Baixauli, F., Villarroya-Beltri, C., Fernández-Delgado, I., Latorre-Pellicer, A., Acín-Pérez, R., et al. (2018). Priming of Dendritic Cells by DNA-Containing Extracellular Vesicles from Activated T Cells through Antigen-Driven Contacts. Nat. Commun. 9 (1), 2658. doi:10.1038/s41467-018-05077-9
Tsokos, G. C. (2011). Systemic Lupus Erythematosus. N. Engl. J. Med. 365 (22), 2110–2121. doi:10.1056/NEJMra1100359
Tucher, C., Bode, K., Schiller, P., Classen, L., Birr, C., Souto-Carneiro, M. M., et al. (2018). Extracellular Vesicle Subtypes Released from Activated or Apoptotic T-Lymphocytes Carry a Specific and Stimulus-Dependent Protein Cargo. Front. Immunol. 9, 534. doi:10.3389/fimmu.2018.00534
van der Vlist, E. J., Arkesteijn, G. J. A., van de Lest, C. H. A., Stoorvogel, W., Nolte-'t Hoen, E. N. M., and Wauben, M. H. M. (2012). CD4(+) T Cell Activation Promotes the Differential Release of Distinct Populations of Nanosized Vesicles. J. Extracellular Vesicles 1, 18364. doi:10.3402/jev.v1i0.18364
Vickers, K. C., Palmisano, B. T., Shoucri, B. M., Shamburek, R. D., and Remaley, A. T. (2011). MicroRNAs Are Transported in Plasma and Delivered to Recipient Cells by High-Density Lipoproteins. Nat. Cel Biol 13 (4), 423–433. doi:10.1038/ncb2210
Wahlgren, J., Karlson, T. D. L., Glader, P., Telemo, E., and Valadi, H. (2012). Activated Human T Cells Secrete Exosomes that Participate in IL-2 Mediated Immune Response Signaling. PLoS One 7 (11), e49723. doi:10.1371/journal.pone.0049723
Wang, Y., Zhao, M., Liu, S., Guo, J., Lu, Y., Cheng, J., et al. (2020). Macrophage-Derived Extracellular Vesicles: Diverse Mediators of Pathology and Therapeutics in Multiple Diseases. Cell Death Dis 11 (10), 924. doi:10.1038/s41419-020-03127-z
Wu, G.-C., Hu, Y., Guan, S.-Y., Ye, D.-Q., and Pan, H.-F. (2019). Differential Plasma Expression Profiles of Long Non-Coding RNAs Reveal Potential Biomarkers for Systemic Lupus Erythematosus. Biomolecules 9 (6), 206. doi:10.3390/biom9060206
Wu, G.-C., Li, J., Leng, R.-X., Li, X.-P., Li, X.-M., Wang, D.-G., et al. (2017a). Identification of Long Non-coding RNAs GAS5, Linc0597 and Lnc-DC in Plasma as Novel Biomarkers for Systemic Lupus Erythematosus. Oncotarget 8 (14), 23650–23663. doi:10.18632/oncotarget.15569
Wu, M., Ouyang, Y., Wang, Z., Zhang, R., Huang, P.-H., Chen, C., et al. (2017b). Isolation of Exosomes from Whole Blood by Integrating Acoustics and Microfluidics. Proc. Natl. Acad. Sci. USA 114 (40), 10584–10589. doi:10.1073/pnas.1709210114
Wu, W.-C., Song, S.-J., Zhang, Y., and Li, X. (2020). Role of Extracellular Vesicles in Autoimmune Pathogenesis. Front. Immunol. 11, 579043. doi:10.3389/fimmu.2020.579043
Wynn, T. A., and Vannella, K. M. (2016). Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44 (3), 450–462. doi:10.1016/j.immuni.2016.02.015
Xie, L., and Xu, J. (2018). Role of MiR-98 and its Underlying Mechanisms in Systemic Lupus Erythematosus. J. Rheumatol. 45 (10), 1397–1405. doi:10.3899/jrheum.171290
Xu, H., Chen, W., Zheng, F., Tang, D., Dai, W., Huang, S., et al. (2020). The Potential Role of tRNAs and Small RNAs Derived from tRNAs in the Occurrence and Development of Systemic Lupus Erythematosus. Biochem. Biophysical Res. Commun. 527 (2), 561–567. doi:10.1016/j.bbrc.2020.04.114
Yáñez-Mó, M., Siljander, P. R.-M., Andreu, Z., Bedina Zavec, A., Borràs, F. E., Buzas, E. I., et al. (2015). Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracellular Vesicles 4, 27066. doi:10.3402/jev.v4.27066
Yang, P., Zhang, X., Chen, S., Tao, Y., Ning, M., Zhu, Y., et al. (2021). A Novel Serum tsRNA for Diagnosis and Prediction of Nephritis in SLE. Front. Immunol. 12, 735105. doi:10.3389/fimmu.2021.735105
Zhang, B., Yin, Y., Lai, R. C., and Lim, S. K. (2014). Immunotherapeutic Potential of Extracellular Vesicles. Front. Immunol. 5, 518. doi:10.3389/fimmu.2014.00518
Zhang, B., Zhao, M., and Lu, Q. (2020). Extracellular Vesicles in Rheumatoid Arthritis and Systemic Lupus Erythematosus: Functions and Applications. Front. Immunol. 11, 575712. doi:10.3389/fimmu.2020.575712
Zhang, F., Li, R., Yang, Y., Shi, C., Shen, Y., Lu, C., et al. (2019). Specific Decrease in B-Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8(+) T Cell Responses. Immunity 50 (3), 738–750. doi:10.1016/j.immuni.2019.01.010
Zhao, C.-N., Mao, Y.-M., Liu, L.-N., Li, X.-M., Wang, D.-G., and Pan, H.-F. (2018). Emerging Role of lncRNAs in Systemic Lupus Erythematosus. Biomed. Pharmacother. 106, 584–592. doi:10.1016/j.biopha.2018.06.175
Zhao, Y., Wei, W., and Liu, M.-L. (2020). Extracellular Vesicles and Lupus Nephritis - New Insights into Pathophysiology and Clinical Implications. J. Autoimmun. 115, 102540. doi:10.1016/j.jaut.2020.102540
Zhou, M., Tan, K. S., Guan, W.-J., Jiang, L.-J., Deng, J., Gao, W.-X., et al. (2020). Proteomics Profiling of Epithelium-Derived Exosomes from Nasal Polyps Revealed Signaling Functions Affecting Cellular Proliferation. Respir. Med. 162, 105871. doi:10.1016/j.rmed.2020.105871
Zhu, L., Kalimuthu, S., Gangadaran, P., Oh, J. M., Lee, H. W., Baek, S. H., et al. (2017). Exosomes Derived from Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 7 (10), 2732–2745. doi:10.7150/thno.18752
Keywords: exosome, systemic erythematosus lupus, lupus nephritis, intercellular communication, research progress, extracellular vesicle
Citation: Zheng C, Xie L, Qin H, Liu X, Chen X, Lv F, Wang L, Zhu X and Xu J (2022) The Role of Extracellular Vesicles in Systemic Lupus Erythematosus. Front. Cell Dev. Biol. 10:835566. doi: 10.3389/fcell.2022.835566
Received: 14 December 2021; Accepted: 07 February 2022;
Published: 02 March 2022.
Edited by:
Gal Bitan, University of California, Los Angeles, United StatesReviewed by:
Dwijendra K. Gupta, Jai Prakash Vishwavidyalaya, IndiaMuhammad Nawaz, University of Gothenburg, Sweden
Copyright © 2022 Zheng, Xie, Qin, Liu, Chen, Lv, Wang, Zhu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Xie, eGllbGluMTI2XzIwMDdAMTI2LmNvbQ==; Xiaohua Zhu, YWxwaGFiZXRpc3RAMTYzLmNvbQ==; Jinhua Xu, amluaHVheHVAZnVkYW4uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Chenghui Zheng
Chenghui Zheng Lin Xie
Lin Xie Haihong Qin
Haihong Qin Xiao Liu
Xiao Liu Xi Chen
Xi Chen Fan Lv
Fan Lv Li Wang
Li Wang Jinhua Xu
Jinhua Xu