
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol. , 27 January 2022
Sec. Stem Cell Research
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.832354
This article is part of the Research Topic Ovarian Cancer Stem Cells and Drug Resistance View all 4 articles
Background: Ovarian cancer (OV) is one of the common malignant tumors and has a poor prognosis. Chromobox (CBX) family proteins are critical components of epigenetic regulation complexes that repress target genes transcriptionally via chromatin modification. Some studies have investigated the function specifications among several CBXs members in multiple cancer types, however, little is known about the functions and prognostic roles of distinct CBXs family proteins in ovarian cancer.
Methods: In this study, several bioinformatics databases and in vitro experiments were used to analyze the expression profiles, prognostic values, and therapeutic potential of the CBXs family (CBX1-8) in ovarian cancer.
Results: It was found that higher expression of CBX3/8 and lower expression of CBX1/6/7 were detected in OV tissues. CBX2/4/5/8 were significantly correlated with individual cancer stages of OV. The expression of CBX1/2/3 were all significantly associated with worse overall survival (OS) and progression-free survival (PFS) for OV patients, whereas the expression of other five CBXs members showed either irrelevant (CBX5 and CBX8) or inconsistent (CBX4, CBX6, and CBX7) results for both OS and PFS in OV. These results showed that only CBX3 had consistent results in expression and prognosis. Further cell experiments also showed that CBX3 promoted the proliferation of ovarian cancer cells. CBX3 was highly expressed in chemoresistant OV tissues. These results indicated that CBX3 was the most likely prognostic indicator and new therapeutic target in OV. Furthermore, gene enrichment analysis suggests that the CBXs family was primarily involved in mast cell activation and mast cell mediated immunity. Individual CBXs members were associated with varying degrees of the infiltration of immune cells, especially B cells. Finally, a high genetic alteration rate of CBXs family (39%) was observed in OV. The low methylation status of CBX3/8 in OV may be associated with their high expression levels.
Conclusions: Taken together, these findings exhibited the pivotal value of CBXs family members (especially CBX3) in the prognosis and chemoresistance of ovarian cancer. Our results may provide new insight to explore new prognostic biomarkers and therapeutic targets for ovarian cancer.
Ovarian cancer is the most frequent cause of death in patients with gynecological malignancy, with five-year survival rates of less than 45% (Webb and Jordan, 2017; Liu et al., 2019; Siegel et al., 2021). Because of its asymptomatic development and the lack of reliable diagnostic markers, patients with ovarian cancer are frequently diagnosed at an advanced stage (Scarlett and Conejo-Garcia, 2012). The current standardized therapy strategy for patients with ovarian cancer is optimal cytoreductive surgery and platinum-based chemotherapy (Gupta et al., 2019; Moufarrij et al., 2019). Despite ovarian cancers usually responding well to platinum-based first-line chemotherapy, the majority of patients relapses frequently and develop chemoresistance with poor prognosis (Lim and Ledger, 2016). Although many years of researches there is still a lack of an early diagnosis method enabling early detection and suitable for screening. Hence, there is an urgent need to find new diagnostic biomarkers and new therapeutic targets to improve the diagnostic and treatment efficacy of ovarian cancer. Therefore, in this study, we are aimed to explore novel biomarkers with diagnostic, prognostic potential as well as on the finding for the new targeted therapy.
Emerging evidence showed that aberration of epigenetic regulation played a critical role in the regulation and progression of ovarian cancer (Chen et al., 2011; Natanzon et al., 2018). Polycomb group (PcG) complexes, as a kind of epigenetic regulatory complexes, have been detected to be dysregulated in various cancers and participate in the tumorigenesis and progression of these cancers (Casa and Gabellini, 2012; Chan and Morey, 2019). The chromobox (CBX) proteins are the crucial components of PcG that are involved in the regulation of several critical biologic processes such as self-renewal of cancer stem cells and cell differentiation (Klauke et al., 2013; Gil and O’Loghlen, 2014). To date, eight members of the CBXs proteins have been identified and were further subdivided into two groups (Siegel et al., 2021): heterochromatin protein 1 (HP1) group including CBX1, CBX3, and CBX5, it consists of an N-terminal chromodomain and a C-terminal chromodomain and is necessary for DNA repair, gene silencing, and telomere function (Kwon and Workman, 2008; Morey et al., 2012). Webb and Jordan (2017) Polycomb (Pc) group including CBX2, CBX4, CBX6, CBX7, and CBX8, contains only a conserved N-terminal chromodomain and could regulate transcription of target genes through interaction with PcG complex (Wotton and Merrill, 2007; Di Croce and Helin, 2013). Different CBXs members were correlated with chromatin different parts and regulated specific transcription of target genes (Zhen et al., 2016; Zeng et al., 2018).
Existing evidence have reported abnormal expressions and prognostic values of several CBXs members in some cancer types such as hepatocellular carcinoma (HCC) and osteosarcoma (Wang et al., 2013; Ma et al., 2019). However, the roles of distinct CBX family members in the development and progression of ovarian cancer still remained incompletely understood. Based on the rapid development of second-generation sequencing technology and the establishment of a large number of databases, a comprehensive study of the CBXs family in ovarian cancer is the benefit to discover new biomarkers with diagnostic and prognostic potential and therapeutic targets for this deadly disease. In the present study, we analyzed the expression of CBXs members and their relations with clinical parameters in OV. Cell experiments were conducted to explore the role of CBX3 in the proliferation of ovarian cancer cells. Then, the correlation between CBX3 and OV chemoresistance was explored. In addition, the predicted functions and pathways that were associated with CBXs family and their 246 frequently altered neighbor genes were also been investigated. Finally, we further analyzed the relationship between CBXs family and prognosis/immune infiltration/methylation in ovarian cancer. Our findings suggested that several CBXs family members might be considered potential biomarkers for prognosis and therapeutic targets in ovarian cancer.
The ONCOMINE database is a publicly accessible online cancer microarray database, it usually analyzes DNA or RNA sequences to promote discovery from the gene-wide expression analyses (Rhodes et al., 2004). In this work, we used this database to explore the differential mRNA expression of eight CBXs members between various cancer types and their corresponding normal tissues. The student’s t test was applied to compare the difference mRNA expression. Cut-off of p-value was defined as 0.05, the fold change was set up at 2 and the gene rank was defined as 10%. The databases used in this study were summarized in Supplementary Table S1.
The GEPIA2 (Gene Expression Profiling Interactive Analysis 2) database is a newly developed online tool that includes thousands of tumor samples and normal samples data from the TCGA (Tang et al., 2019). In the present study, this database was used to explore the expression levels of different CBXs members in ovarian cancer tissues and normal tissues.
The Human Protein Atlas is an online database that contains immunohistochemistry-based expression data for various cancer types. It aims to help researchers discover the expression patterns of specific proteins expressed in specific cancer types and thus identify clinically useful biomarkers (Asplund et al., 2012; Yan et al., 2019). In this analysis, we compared the protein expression levels of different CBXs members between ovarian serous cystadenocarcinoma tissues and normal ovarian stromal tissues by immunohistochemistry image.
UALCAN is an interactive web resource based on TCGA datasets. it can analyze mRNA expression of specific genes between tumor tissues and normal tissues and the association of its expression with several clinicopathological characteristics such as tumor grade and individual cancer stages (Chandrashekar et al., 2017). In this work, we choose the “individual cancer stages” model to analyze the relations between the mRNA expression of different CBXs members and clinicopathological parameters in ovarian cancer. The student’s t test was applied and p < 0.05 was considered statistically significant.
The Kaplan–Meier Plotter is a web tool that information about the association of specific gene expression with the survival of patients with ovarian cancer, breast cancer, lung cancer, gastric cancer, and liver cancer could be easily accessed (Deng et al., 2019; Li et al., 2020a). In this research, we used this database to investigate the association between the expression of CBXs members and overall survival (OS) and progression-free survival (PFS) for OV patients. A p-value less than 0.05 was considered statistically significant.
cBioPortal is a comprehensive online resource with visual and multidimensional cancer genomics and clinical data (Gao et al., 2013). In this work, the genetic alterations and mRNA expression of the CBXs family were analyzed. We obtained the mRNA expression z scores (RNA Seq V2 RSEM) using a z score threshold of ±0.7.
WebGestalt a comprehensive and flexible online resource that can conduct interactive web-based analysis (Liao et al., 2019). In this study, we applied this database to analyze Gene ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway.
Cytoscape was applied to conduct functional integration on 246 frequently altered neighbor genes of the CBXs family screened from the cBioPortal database (Supplementary Table S2). The nodes’ size represented the degree values between these interacting proteins. The larger the circles, the higher the degree.
The TIMER2.0 is an open service that can assess immune cell infiltration and their clinical significance for 32 cancer types (Li et al., 2020b). In this work, we used the “Gene module” tool to generate scatterplots to analyze the association between the expression of the CBXs members and the infiltration of six immune cells including B cells, CD8 + T cells, CD4 + T cells, macrophages, neutrophils and dendritic cells in ovarian cancer.
DiseaseMeth2.0 is an accessible online resource that provides information on DNA methylation status in different kinds of human diseases, especially cancers (Lv et al., 2012; Xiong et al., 2017). In this analysis, we used this web to investigate the methylation status of the CBXs members between ovarian cancer tissues and normal tissues. p < 0.05 was considered as significant.
The human ovarian cancer cell lines SKOV3 and HO8910 were purchased from CRC/PUMC (Cell Resource Center, IBMS, CAMS/PUMC) and CCTCC (China Center for Type Culture Collection), respectively. These two cell lines were both cultured in 1640 RPMI medium supplemented with 10% fetal bovine serum.
For transient transfections, CBX3 siRNAs or control siRNA (stB0002817A-1-5, Ribobio) were transfected into ovarian cancer cell lines using the Lipofectamine 3,000 reagent (Invitrogen) according to the manufacturer’s instruction. This siRNA of CBX3 product contained three different siRNA sequences.
These processes were performed as previously described (Li et al., 2020c). The primer sequences of CBX3 used in this study were F: 5′ TGGCCTCCAACAAAACTACA 3′; R: 5′ TCCCATTCACTACACGTCGA 3′. The GAPDH mRNA expression was applied as an internal reference for quantification.
This process was performed as previously described (Li et al., 2020c). Briefly, the BCA protein assay kit (Thermo Fisher Scientific) was used to detect the concentration of protein. Then, 40 ug proteins were loaded and separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and blocked with 5% milk for about 1 hour. Next, membranes were incubated at 4°C overnight with the following primary antibodies: CBX3 (ab217999, Abcam) and GAPDH (sc-47724, Santa Cruz Biotechnology). The protein band intensities were evaluated by the Image Lab software (Bio-Rad, CA, USA). The GAPDH protein expression level was used as an internal control for normalization.
After indicated treatment, SKOV3 and HO8910 cells were seeded and cultured in 96-well plates at a density of 3,000 cells/well. The cell proliferation ability was evaluated using the Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions.
SPSS, version 18.0 (Chicago, United States) was used for the statistical analysis. The data were expressed as mean ± SD. The differences were determined by the student’s t test for two groups and the one-way analysis of variance (ANOVA) for multiple groups. Statistical significance was set at p < 0.05.
First, we analyzed mRNA expression and protein expression of different CBXs family to explore their potential prognostic and therapeutic value in ovarian cancer patients. As shown in Supplementary Figure S1 and Supplementary Table S3, mRNA expression of CBXs members in various cancer types was explored by using the ONCOMINE database. Significant down-regulation of CBX1 and CBX7 was found in ovarian cancer (OV) tissues compared to normal tissues, while the expression levels of CBX3 were significantly up-regulated in OV tissues. In Bonome Ovarian dataset (Bonome et al., 2008), 2.56-fold decrease in CBX1 expression was shown in OV tissues (p = 3.91E-12). Similarly, the result from three datasets including Bonome Ovarian dataset, TCGA dataset, Yoshihara Ovarian dataset (Yoshihara et al., 2009) showed that there were 2.937-fold (p = 5.43E-12), 3.739-fold (p = 4.50E-6), and 5.422-fold (p = 7.48E-10) decrease in CBX7 mRNA expression in OV tissues compared to normal tissues, respectively. However, in the TCGA dataset, CBX3 overexpression was shown in OV tissues with a fold change of 2.064 (p = 1.40E-7). Furthermore, the mRNA expression levels of CBXs family were further explored by the GEPIA2 whose resources were different from ONCOMINE database. As shown in Figure 1A, the result supported that the mRNA expression of CBX7 was decreased in OV tissues compared with normal tissues. Besides, significant down-regulation of CBX6 and up-regulation of CBX8 were further obtained in OV tissues compared to normal tissues (Figure 1A). Then, we compared the relative expression of distinct CBXs members in OV by GEPIA2, and the result showed that CBX3 had the highest relative expression levels among all eight CBXs family members (Figure 1B).
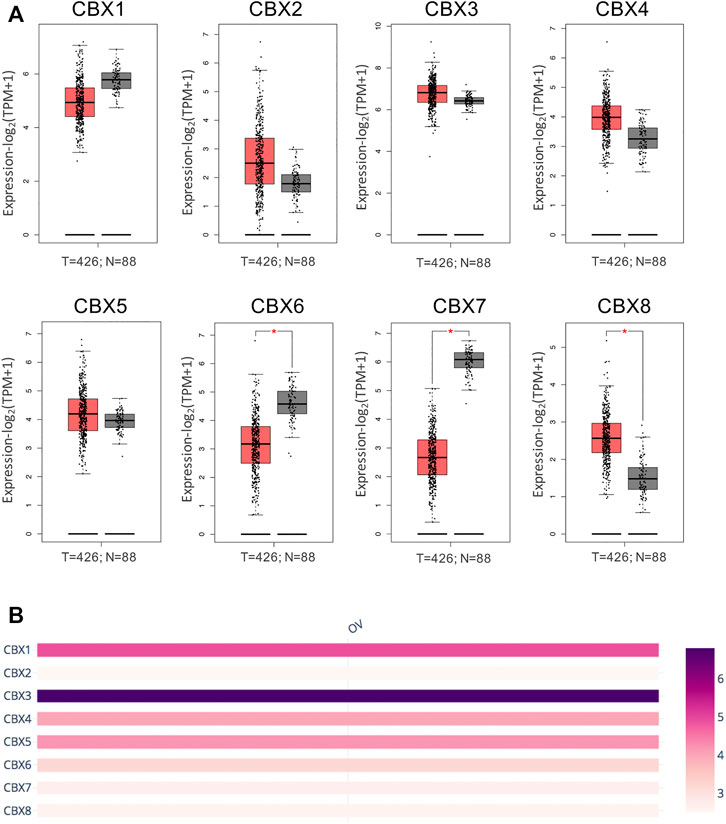
FIGURE 1. mRNA expression levels of the CBXs family members in OV tissues and normal ovarian tissues. (A) The mRNA expression profiles were obtained from the GEPIA2 databases. T represented ovarian cancer tissues; N represented normal ovarian tissues. *p < 0.05. (B) The relative expression of the CBXs family members in OV.
After a comprehensive analysis of the CBXs mRNA expression pattern in OV, we tried to use the Human Protein Atlas to investigate the protein expression levels of CBXs family in OV. As shown in Figure 2, the protein expression levels of CBX1/6/7 were decreased in OV tissues compared with normal ovarian tissues (high vs. medium; low vs. not detected; medium vs. low, respectively) (Figures 2A, F,G). Meanwhile, higher protein expression levels of CBX3/8 were found in OV tissues compared to normal tissues (medium vs. high; not detected vs. medium, respectively) (Figures 2C,H). These findings were consistent with our previous findings on the mRNA expression of CBXs family. Moreover, CBX2/4 had not detected expression in normal ovarian tissues and low expression in OV tissues (Figures 2B,D), while CBX5 had medium expression in normal tissues and low expression in OV tissues (Figure 2E).
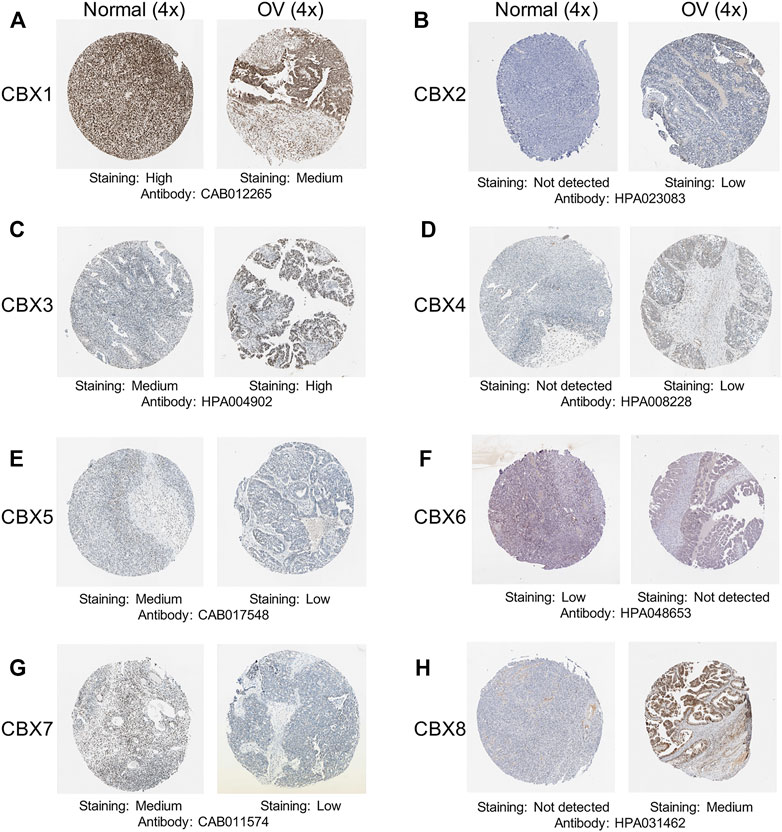
FIGURE 2. Representative immunohistochemistry images of the CBXs family members in OV tissues and normal ovarian tissues. (A–H) The protein expression profiles of CBX1-8 were collected from the Human Protein Atlas database.
Next, we explored the relationship between the mRNA expression of eight CBXs members and the individual cancer stages of OV by using the UALCAN database. The results showed that the mRNA expression levels of CBX2/4/5/8 were significantly correlated with individual cancer stages of OV, and patients who had higher cancer stages tended to express lower mRNA expression levels of CBXs. The lowest mRNA expression levels of CBX2/4/5/8 were detected in cancer stage 4 (Figures 3B,D,E,H). Moreover, the mRNA expression levels of CBX1/3/6 had a trend to lower expression in more advanced cancer stages, although that was not statistically significant (Figures 3A,C,F,G). That may be due to the small sample size and other reasons.
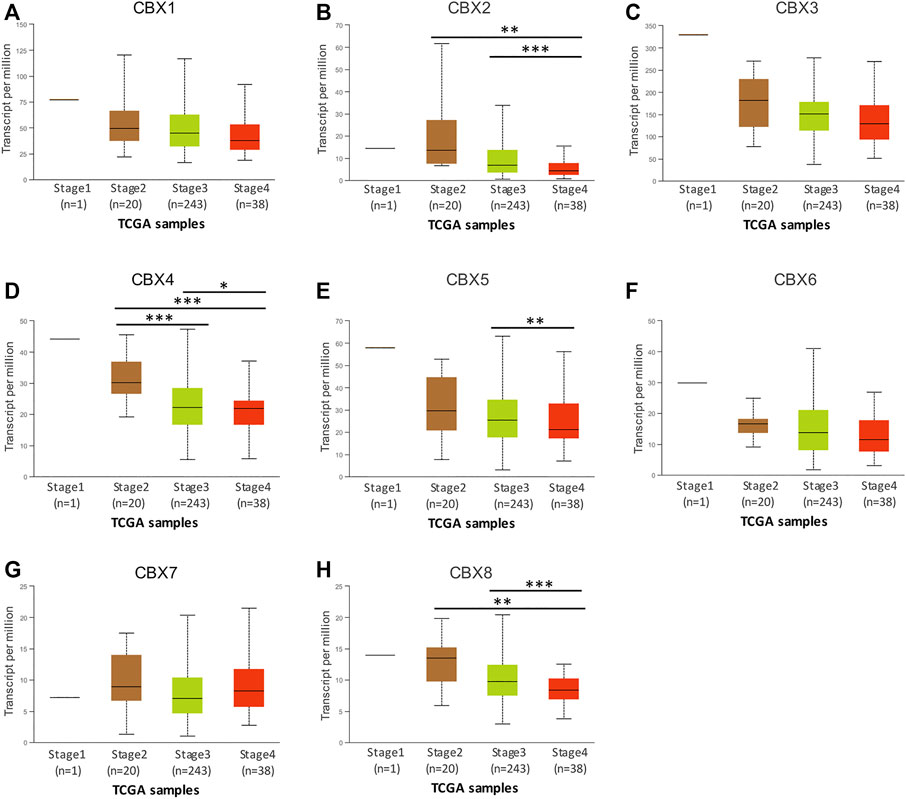
FIGURE 3. The relationship between the mRNA expression levels of different CBXs members and individual cancer stages in patients with OV. (A–H) The relationship information of CBX1-8 was evaluated from the UALCAN database. *p < 0.05, **p < 0.01, ***p < 0.001.
Further, we applied the Kaplan–Meier plotter database to investigate the prognostic value of mRNA expression of all eight CBXs members in patients with OV. As shown in Figure 4, higher mRNA expression levels of CBX1 (OS: HR = 1.38 (1.20–1.59), p < 0.001; PFS: HR = 1.31 (1.14–1.5), p = 0.00012), CBX2 (OS: HR = 1.35 (1.1–1.66), p = 0.0045; PFS: HR = 1.44 (1.16–1.79), p = 0.001) and CBX3 (OS: HR = 1.25 (1.09–1.44), p = 0.0019; PFS: HR = 1.19 (1.05–1.35), p = 0.0069) were all significantly associated with shorter overall survival (OS) and progression-free survival (PFS) for OV patients (Figure 4).
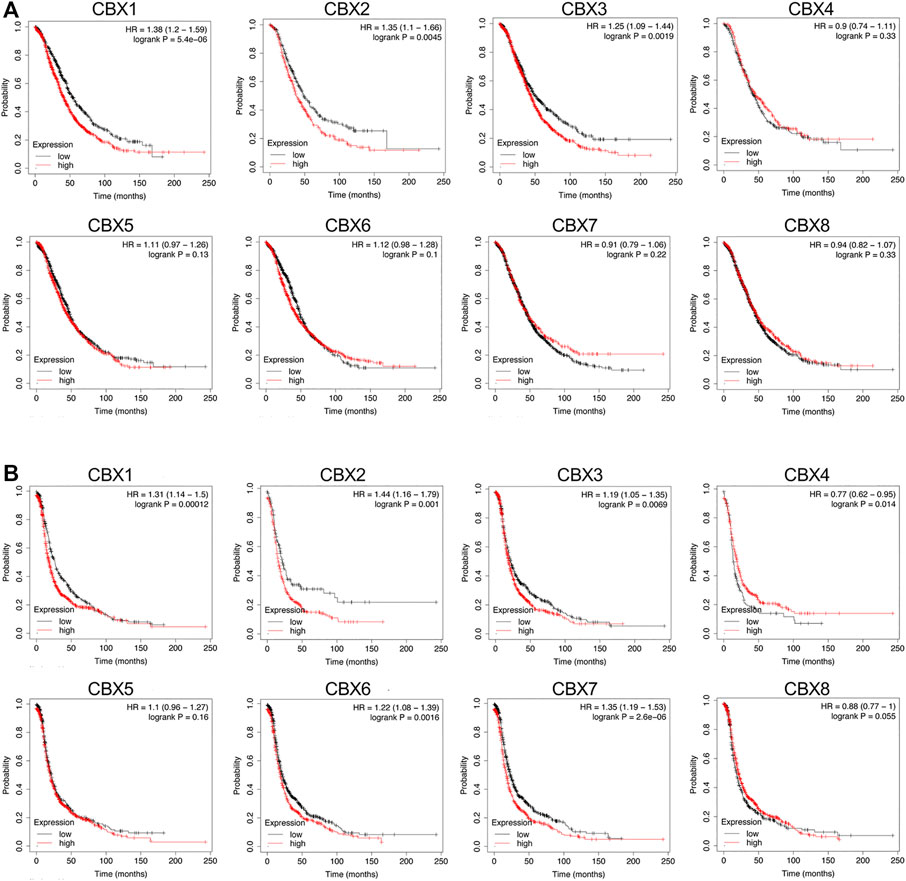
FIGURE 4. Prognostic value of the mRNA expression levels of the CBXs family members in patients with OV (the Kaplan-Meier Plotter). (A) The overall survival (OS) curve of CBX1-8 in patients with OV. (B) The progression-free survival (PFS) curve of CBX1-8 in patients with OV.
In contrast to the survival analysis results for CBX1/2/3 in OV patients, survival analysis focusing on the other five CBXs family members showed either irrelevant or inconsistent results for both OS and PFS in OV patients. Higher mRNA expression of CBX4 was not related to OS [HR = 0.9 (0.74–1.11), p = 0.33] but was related to favorable PFS [HR = 0.77 (0.62–0.95), p = 0.014] of OV patients. In addition, increased expression of CBX6 [HR = 1.12 (0.98–1.28), p = 0.1] and CBX7 [HR = 0.91 (0.79–1.06), p = 0.22] was not related to OS of OV patients, while increased expression of CBX6 [HR = 1.22 (1.08–1.39), p = 0.0016] and CBX7 [HR = 1.35 (1.19–1.53), p < 0.001] was significantly correlated with poor PFS of OV patients. Finally, the mRNA expression of CBX5 [OS: HR = 1.11 (0.97–1.26], p = 0.13; [PFS: HR = 1.1 (0.96–1.27), p = 0.16] and CBX8 [OS: HR = 0.94 (0.82–1.07), p = 0.33; PFS: HR = 0.88 (0.77–1), p = 0.055] did not show any relation to the prognosis in OV patients (Figure 4).
Based on the above expression and prognosis results of CBX family members, we found that only CBX3 was overexpressed in ovarian cancer tissues and closely related to the poor prognosis (OS and PFS) of patients with OV at the same time, indicating that CBX3 is the most likely potential diagnostic indicator and new therapeutic target for patients with ovarian cancer. Thus, we further investigated the function of CBX3 in ovarian cancer cells. CBX3 was reported to play an important role in cell growth in some cancer types, however, it is role in OV is still unclear. To investigate the impact of CBX3 on the proliferation of ovarian cancer cells, we conducted CCK8 assay to analyze the growth of ovarian cancer cells which were transfected with CBX3 siRNA (siCBX3) or siRNA control (siCtrl). The knockdown efficiency was evaluated and the results showed that compared with siCtrl group of two ovarian cancer cell lines including SKOV3 and HO8910, the expression of CBX3 was decreased in siCBX3 group in both protein and mRNA levels (Figures 5A,B). Next, we conducted CCK8 assay and the results showed that compared with siCtrl group, the growth of ovarian cancer cells was inhibited after transfected with siCBX3 (Figures 5C,D), suggesting that CBX3 was involved in the proliferation of ovarian cancer cells.
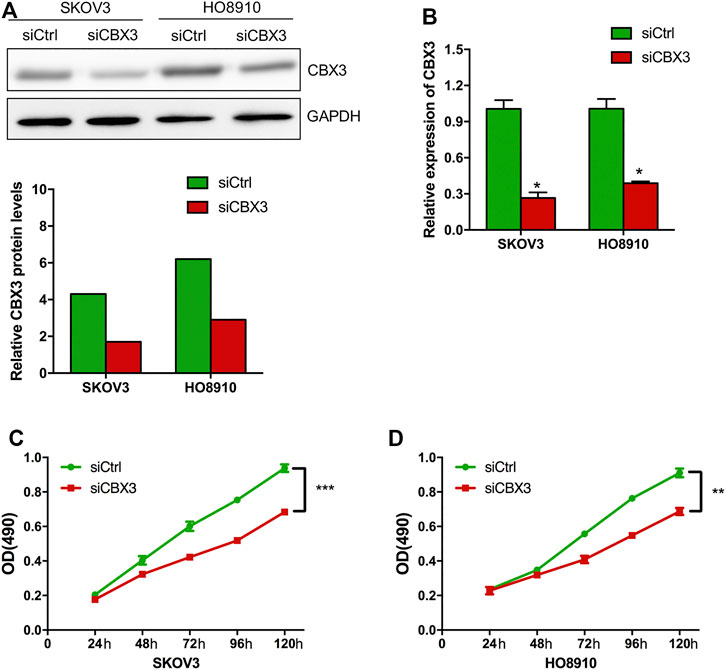
FIGURE 5. CBX3 knockdown inhibited the proliferation of ovarian cancer cells. (A) The knockdown efficiency of CBX3 in SKOV3 and HO8910 was evaluated by Western Blot. (B) The knockdown efficiency of CBX3 in SKOV3 and HO8910 was assessed by qRT-PCR. (C–D) CCK-8 assay was conducted to evaluate the effect of siCBX3 on the proliferation of SKOV3 and HO8910 cell lines. **p < 0.01, ***p < 0.001.
Furthermore, we explored the effect of CBX3 on the treatment outcomes of ovarian cancer patients. The expression level of CBX3 was checked in two microarray datasets related to platinum chemotherapy including GSE15709 and GSE1926. The results from these two datasets both presented that treatment with the platinum drugs upregulated the expression level of CBX3 expression in ovarian cancer tissues or cells (Figure 6). These results indicated that CBX3 may influence the chemotherapy responses of ovarian cancer patients.
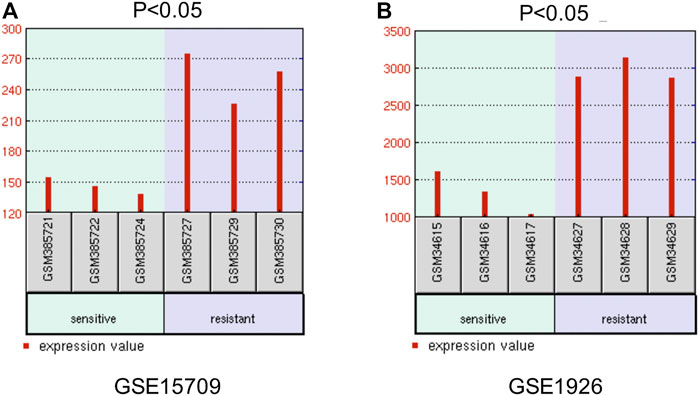
FIGURE 6. The effect of CBX3 on the treatment outcomes of ovarian cancer patients. (A–B) GSE15709 and GSE1926 are two datasets related to platinum-based chemotherapy and are used to explore the impacts of CBX3 expression levels on ovarian cancer therapy.
Next, we explored the genetic alterations of individual CBXs family members by using the temporary TCGA dataset. As shown in Figure 7A, all eight CBXs family members were altered in OV patients, with 4, 9, 12, 14, 5, 4, 2.2, and 13% alteration rates, respectively. CBX4/8/3 ranked the highest three genes with the genetic alteration. In the 182 sequenced OV patients/samples, the genetic alteration was detected in 71 cases and the total alteration rate was 39% (Figure 7A). Then, we measured the correlation between each CBXs member at the transcriptional level by using the GEPIA2, and Pearson’s correction was included. A remarkably positive correlation between CBX2 and CBX8, CBX1 and CBX5 were observed (Figure 7B).
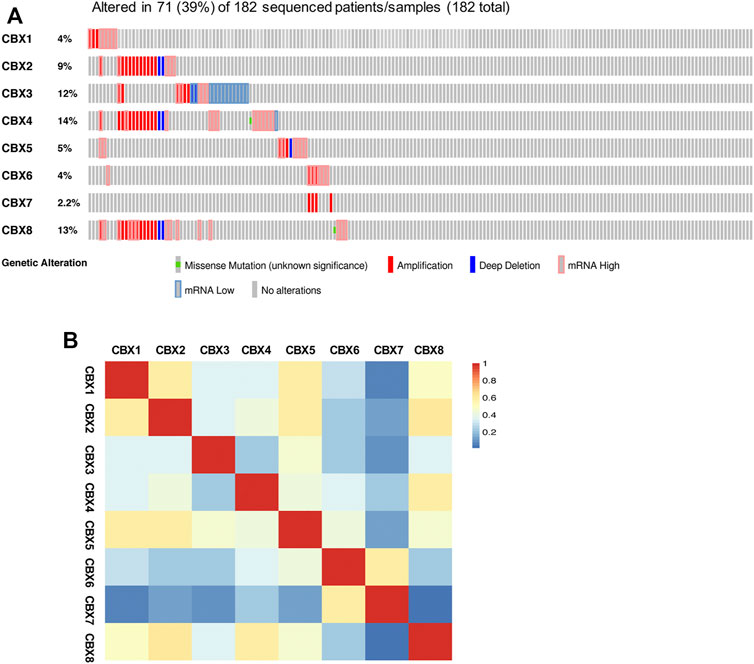
FIGURE 7. Genetic alterations and correlation analysis of distinct CBXs members in OV. (A) Summary of the alteration rates for CBX1-8 in OV (cBioPortal). (B) Correlation between eight CBXs family members in OV (GEPIA2).
We further investigated the function of the CBXs Family in OV patients. Since lots of proteins synergistically performed their functions by forming protein-protein complexes, it is essential to discover protein-protein interaction (PPI) patterns for better understanding the function of the CBXs family. We analyzed 246 frequently altered neighbor genes that were most relevant with CBXs members in OV patients by the cBioPortal and constructed an integrated network using the Cytoscape (Figure 7A, Supplementary Table S2). The results showed that PTPRC, ITGAM, CCR5, C3AR1, and CCR2 were primarily related to the function of the CBXs family in OV (Figure 8A). In addition, the biological functions of CBXs members and their 246 co-expressed genes were analyzed by GO annotation and KEGG pathway analysis using the WebGestalt (Figures 8B,C). Biological processes including biological regulation, response to stimulus, and multicellular organismal process were significantly regulated by the CBXs family. Cellular components such as membrane, endomembrane system and vesicle were significantly associated with the function of CBXs family. Besides, the CBXs family mainly affected the molecular functions including protein binding, ion binding, and molecular transducer activity (Figure 8B). As shown in Figure 8C, in terms of KEGG pathway analysis, 11 pathways were related to the function of CBXs family in OV, and the top three most relevant pathways were mast cell activity, mast cell-mediated immunity, and response to a chemokine (Figure 8C).
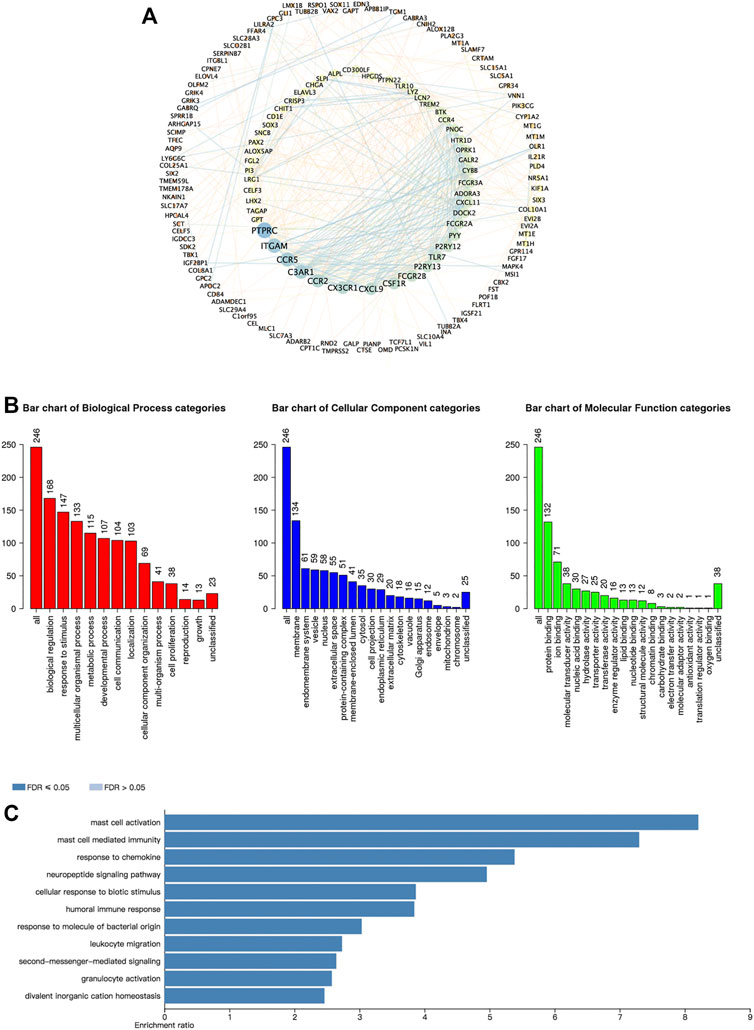
FIGURE 8. Predicted functions and pathways of the CBXs family members and their 246 co-expressed molecules in OV. (A) PPI network of the CBXs family interaction partners was constructed (cBioPortal and Cytoscape). The colors of nodes and edges: high values to dark colors; low values to bright colors. (B) Biological process, cellular components, and molecular functions of the CBXs family interaction partners was predicted by GO functional enrichment analysis (WebGestalt). (C) KEGG pathway analysis on the CBXs family interaction partners was shown (WebGestalt).
The CBXs family were reported to participate in the tumor progression and the several immune cells infiltration in various cancer types, thus affecting patient prognosis and therapy (Chen et al., 2019; Zhou et al., 2021). In this study, we applied TIMER2.0 to explore the correlation between the individual CBXs family members and the immune infiltration of ovarian cancer. As shown in Figures 9A,B,H, CBX1/2/8 were all negatively correlated with B cells, macrophages, and dendritic cells, CBX2 was also negatively correlated with CD8 + T cells. CBX3 was negatively correlated with B cells (Figure 9C). CBX4/5 were negatively correlated with B cells and positively correlated with CD4 + T cells, CBX5 was also negatively correlated with dendritic cells (Figures 9D,E). CBX6 was negatively correlated with B cells and dendritic cells and positively correlated with CD8 + T cells and CD4 + T cells (Figure 9F). In addition, there were positive correlations between CBX7 and the infiltration of five immune cells including CD8 + T cells, CD4 + T cells, macrophages, neutrophils, and dendritic cells (Figure 9G, Supplementary Figure S2).
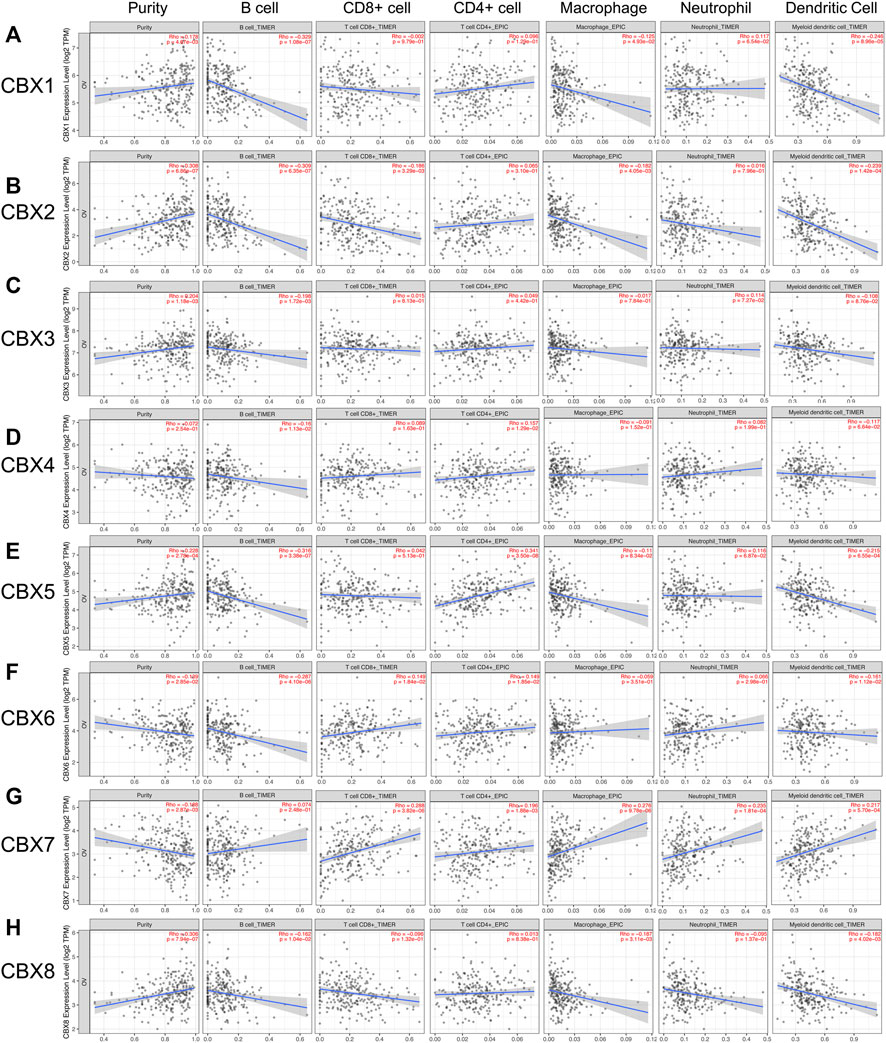
FIGURE 9. The correlations between differentially expressed CBXs family members and immune cell infiltration. (A–H) The effects of CBX1-8 on the immune cell infiltration were analyzed by TIMER2.0. Six immune cells were analyzed including B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils, and dendritic cells.
Increasing reports have indicated that DNA methylation is negatively associated with gene expression in cancers (Győrffy et al., 2016). Thus, in the present study, we tried to analyze whether the methylation status was correlated with the expression of CBXs members. We obtained the methylation expression data of CBXs family by using the DiseaseMeth. As shown in Supplementary Figure S3, the methylation expression levels of CBX2, CBX3, CBX4, CBX5, and CBX8 were significantly decreased in OV tissues compared to normal tissues. Our previous results on mRNA expression of CBXs members indicated that the upregulated expression of CBX3 and CBX8 in OV patients, these aberrant expression levels of CBX3/8 might be due to their low methylation levels in OV tissues. For the rest of CBXs members, in addition to methylation, there may be other factors affecting their expression levels, such as gene mutation, fusion, epigenetic modification, and so on.
Numerous studies reported that except for tumor genetics, abnormal epigenetic regulation has also been depicted to play a critical role in the development of ovarian cancer (Matei and Nephew, 2020). The CBXs family members, as critical components of epigenetic regulation complexes, are reported to take part in the development and progression of various cancer types, including ovarian cancer (Li et al., 2014a; Li et al., 2020c). Some researchers found that high expression of CBX1 was significantly related to an unfavorable progression and was correlated with chemoresistance in breast cancer (Liang et al., 2017). In hepatocellular carcinoma (HCC), CBX2 was found to promote HCC cells proliferation by decreasing YAP phosphorylation (Mao et al., 2019). High expression of CBX3 was observed in several cancers such as HCC and glioma and could accelerate cell proliferation (Zhao et al., 2019; Zhong et al., 2019). Hu et al. reported that knockdown of CBX4 could inhibit cell growth and migration in lung cancer through regulating the BMI-1 pathway (Hu et al., 2020). High expression of CBX5 was detected in gastric cancer and can promote cell migration and invasion (Guo et al., 2018). Zheng et al. reported that a higher expression level of CBX6 was associated with a worse prognosis in patients with HCC (Zheng et al., 2017). In our previous studies, CBX7 was proved to make TWIST-1 transcriptionally nonfunctional during mesenchymal-epithelial transition (MET) process in ovarian cancer, and a subclassification of ovarian cancers based on both CBX7 and TWIST-1 expression was then described which could predict clinical outcomes and patient prognosis in OV (Li et al., 2020c). Finally, CBX8 was found to promote invasion and migration in several cancer types including glioblastoma, lung cancer, and breast cancer (Jia et al., 2020). Despite a few members of the CBXs family having been discovered to play important roles in tumors, distinct roles of the CBXs family in ovarian cancer remained to be illuminated. In this work, the expression, gene alteration, prognostic values, immune infiltration, and methylation of different CBXs members in ovarian cancer were analyzed.
For the first time, the mRNA expression levels of eight CBXs family members were analyzed and summarized in ovarian cancer tissues compared with normal ovarian tissues by using both the ONCOMINE database and the GEPIA2 database. Higher mRNA expression levels of CBX3/8 and lower expression levels of CBX1/6/7 were observed in OV tissues compared to normal tissues. These mRNA expression data were consistent with the subsequent protein data of CBXs family. In addition, a further novel finding was that despite the mRNA expression levels of CBX2/4/5 were not statistically different between OV tissues and normal tissues, their mRNA expression levels in ovarian cancer tissues were significantly correlated with individual cancer stages. Ovarian cancer patients who had higher cancer stages tended to express lower mRNA expression levels of these CBXs. Besides, the mRNA expression levels of CBX1/3/6 showed a significant difference in OV tissues compared to normal tissues, however, their expression levels only had a trend to lower expression in more advanced cancer stages but without statistical significance. That may be due to the small sample size and other reasons.
Nowadays, the prognostic value of several CBXs members in cancers has been reported. CBX1 has been detected to have high protein expression in HCC which indicated that HCC patients with high expression of CBX1 had worse clinical outcomes (Yang et al., 2018). Elevated protein expression of CBX3 was involved in unfavorable prognosis in prostate cancer, and it also was an independent prognostic marker (Slezak et al., 2013). In lung cancer, CBX3 mRNA expression was increased and was associated with a shorter survival time (Alam et al., 2018). However, their prognostic values in ovarian cancer have not been fully illuminated. In this analysis, we explored the prognostic roles of eight CBXs family members in OV patients by using the Kaplan–Meier plotter. The results showed that CBX1, CBX2, and CBX3 were all associated with worse OS and PFS in OV patients. Nevertheless, the prognostic value of the other five CBXs family members for predicting OS and PFS in ovarian cancer patients was either irrelevant (CBX5 and CBX8) or inconsistent (CBX4, CBX6, and CBX7). These results suggested that more research was needed to further investigate their prognostic significance. According to these findings of the expression and prognosis of CBXs, we found that only CBX3 had consistent results in expression and prognosis. Thus, further cell experiments were conducted to explore the function of CBX3 in OV, and the results showed that CBX3 could promote ovarian cancer cell proliferation and impact the treatment outcomes of OV patients. These findings suggested that CBX3 may be the potential diagnostic indicator and new therapeutic target in ovarian cancer.
It is widely known that genetic alteration is a general phenomenon in various tumors including ovarian cancer and plays a critical role in several cell biological processes such as cell growth, cell apoptosis, and cell cycle (Sanchez-Vega et al., 2018). All eight CBXs family members were detected to be altered in OV patients, and the total genetic alteration rate of CBXs family was 39%. The results above comprehensively suggested that genetic alterations of the CBXs family probably play a pivotal role in ovarian cancer. Moreover, methylation is also associated with the expression levels of genes and takes part in tumor development (Győrffy et al., 2016). There were several clues that existed revealing the role of methylation of CBXs in cancers. For example, CBX1, CBX3, and CBX5 were found to be act as the methyl readers which were involved in the interpretation of H3K9me3 marks induced by H3K9 methyltransferases (van Wijnen et al., 2021). Moreover, differentially expressed CBXs were found to have a strong association with the SUMOylation of DNA methylation proteins in colorectal cancer (Li et al., 2020d). In our study, we showed that higher expression levels of CBX3/8 in OV tissues might be due to their low methylation levels. For the rest of CBXs members, in addition to methylation, there may be other factors affecting their expression levels, such as genetic alterations that we mentioned above.
Next, the molecular biological functions of CBXs members and their 246 co-expressed genes were analyzed. Protein-protein network interactions exhibited PTPRC, ITGAM, and CCR5 were most related to the modulation and function of the differentially expressed CBXs family members in ovarian cancer. Biological processes such as mast cell activation, mast cell-mediated immunity were remarkably regulated by the CBXs family members in ovarian cancer. Mast cells are a kind of immune cells that could secrete diverse active compounds to take part in the immune response (Komi and Redegeld, 2020). Giuseppe et al. reported that mast cell density was increased in gastric cancer and exerted a protumorigenic role through the release of angiogenic and lymphangiogenic factors (Sammarco et al., 2019). Mast cell infiltration was found to participate in poor response to neoadjuvant chemotherapy in inflammatory breast cancer (Reddy et al., 2019). Despite mounting research showing that mast cells were consistently infiltrating tumors, the consequences of their presence as tumor suppressors or tumor drivers still remain unclear (Aponte-López et al., 2018). Particularly, in ovarian cancer, their roles and functions were still under discussion. Our results suggested that CBXs family may play a critical role in mast cell infiltration in ovarian cancer.
Mounting evidence supported that immune cell infiltration was significantly associated with tumor progression and recurrence and is considered as a pivotal determinant of clinical outcome and immune therapy response (Li et al., 2016). Lele et al. showed that tumor-infiltrating immune cells were correlated with clinical features in colorectal cancer and could act as a prognostic marker (Ye et al., 2019). Liu et al. found that profiling of immune infiltration plays an important role in the prediction of prognosis in ovarian cancer, and it could also be considered in therapeutic modulation (Liu et al., 2020). Moreover, epigenetic regulation of innate immune response is an emerging field these years. There were evidence showing that in macrophages, CBX2 can bind to and recruit Jmjd3 to the Ifnb promoter, resulting in the demethylation of H3K27me3, thereby increasing the transcription of IFN-β, an essential factor involved in the antiviral innate immunity (Sun et al., 2019). In addition, another published paper also demonstrated that CBX7 knockdown leads to apoptosis of CD4+ T cells via hyper demethylation of FasL gene promoter and increased expression of FasL (Li et al., 2014b). In this work, we found a remarkable correlation between the expression of individual CBXs family members and the infiltration of six immune cells, thus suggesting that CBXs members may reflect the immune status of ovarian cancer. Particularly, we found that almost all CBXs members except CBX7 were significate associated with B cell infiltration in ovarian cancer. B cell, as a critical immune cell, harbored various functions in the immune response. Increasing studies supported that tumor-infiltrating B lymphocytes could suppress tumor progression by secreting antibodies, promoting T cell response, and killing tumor cells directly (Wang et al., 2019). Several studies reported that B cell infiltration was related to poorer survival in patients with ovarian cancer (Dong et al., 2006; Yang et al., 2013). Furthermore, in the present study, we found that CBX7 was positive correlations with five of six immune cells infiltration including CD8 + T cells, CD4 + T cells, macrophages, neutrophils, and dendritic cells, suggesting that CBX7 may play a critical role in affecting the immune status of ovarian cancer.
There were several limitations in this work. Firstly, most of the data used for analysis in this study were retrieved from online services, further cell-based studies and clinical experiments were needed to confirm our results and to investigate the clinical application of the CBXs family in ovarian cancer. Besides, we lacked the research on the detailed mechanisms of individual CBXs members in ovarian cancer. Further studies were required to explore the specific mechanism between each CBXs member and ovarian cancer.
In conclusion, we first comprehensively analyzed the expression, prognostic values, gene alteration, immune infiltration, and methylation of different CBXs family members in ovarian cancer. Higher expression of CBX3/8 and lower expression of CBX1/6/7 were detected in OV tissues. CBX1/2/3 were all associated with worse OS and PFS in OV patients. CBX3 promoted ovarian cancer cell proliferation and impacts the treatment outcomes of OV patients. Besides, a high genetic alteration rate of CBXs family (39%) was observed in OV. The low methylation status of CBX3/8 may be associated with their high expression levels in OV. Moreover, individual CBXs members were associated with varying degrees of the infiltration of immune cells, especially B cells. These findings exhibited the pivotal value of the CBXs family in the prognosis and therapy of ovarian cancer. Our results may provide new insight to explore new prognostic biomarkers and therapeutic targets for ovarian cancer.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Conception and design: KH and JL. Writing, review, and/or revision of the manuscript: JL and LY. Administrative, technical, or material support: ZX and YY. All authors approved final version of manuscript.
This study is supported by grants from National Natural Science Foundation of China (82103300, 82102743), Outstanding Postdoctoral Innovative Talents Foundation (2021RC2022), Youth Science Foundation of Xiangya Hospital (2020Q07).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2022.832354/full#supplementary-material
Supplementary Figure S1 | Transcriptional expression levels of the CBXs family members in different cancer types (ONCOMINE). Red cell represented gene overexpression; blue cell represented decreased expression. The numbers in each cell represented the evidential frequencies. The deeper the color, the higher the significance.
Supplementary Figure S2 | The heat map for the correlations between differentially expressed CBXs family members and immune cell infiltration.
Supplementary Figure S3 | The methylation levels of distinct CBXs family in OV. (A–H) The methylation values of CBX1-8 were collected from the DiseaseMeth database.
Alam, H., Li, N., Dhar, S. S., Wu, S. J., Lv, J., Chen, K., et al. (2018). HP1γ Promotes Lung Adenocarcinoma by Downregulating the Transcription-Repressive Regulators NCOR2 and ZBTB7A. Cancer Res. 78 (14), 3834–3848. PubMed PMID: 29764865. doi:10.1158/0008-5472.can-17-3571
Aponte-López, A., Fuentes-Pananá, E. M., Cortes-Muñoz, D., and Muñoz-Cruz, S. (2018). Mast Cell, the Neglected Member of the Tumor Microenvironment: Role in Breast Cancer. J. Immunol. Res. 2018, 1–11. PubMed PMID: 29651440. doi:10.1155/2018/2584243
Asplund, A., Edqvist, P.-H. D., Schwenk, J. M., and Pontén, F. (2012). Antibodies for Profiling the Human Proteome-The Human Protein Atlas as a Resource for Cancer Research. Proteomics 12 (13), 2067–2077. PubMed PMID: 22623277. doi:10.1002/pmic.201100504
Bonome, T., Levine, D. A., Shih, J., Randonovich, M., Pise-Masison, C. A., Bogomolniy, F., et al. (2008). A Gene Signature Predicting for Survival in Suboptimally Debulked Patients with Ovarian Cancer. Cancer Res. 68 (13), 5478–5486. PubMed PMID: 18593951. doi:10.1158/0008-5472.can-07-6595
Casa, V., and Gabellini, D. (2012). A Repetitive Elements Perspective in Polycomb Epigenetics. Front. Gene 3, 199. PubMed PMID: 23060903. doi:10.3389/fgene.2012.00199
Chan, H. L., and Morey, L. (2019). Emerging Roles for Polycomb-Group Proteins in Stem Cells and Cancer. Trends Biochem. Sci. 44 (8), 688–700. PubMed PMID: 31085088. doi:10.1016/j.tibs.2019.04.005
Chandrashekar, D. S., Bashel, B., Balasubramanya, S. A. H., Creighton, C. J., Ponce-Rodriguez, I., Chakravarthi, B. V. S. K., et al. (2017). UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 19 (8), 649–658. PubMed PMID: 28732212. doi:10.1016/j.neo.2017.05.002
Chen, H., Hardy, T. M., and Tollefsbol, T. O. (2011). Epigenomics of Ovarian Cancer and its Chemoprevention. Front. Gene 2, 67. PubMed PMID: 22303362. doi:10.3389/fgene.2011.00067
Chen, Y., Lu, W., Jin, Z., Yu, J., and Shi, B. (2019). Carbenoxolone Ameliorates Hepatic Lipid Metabolism and Inflammation in Obese Mice Induced by High Fat Diet via Regulating the JAK2/STAT3 Signaling Pathway. Int. Immunopharmacology 74, 105498. PubMed PMID: 31261036. doi:10.1016/j.intimp.2019.03.011
Deng, J.-L., Xu, Y.-h., and Wang, G. (2019). Identification of Potential Crucial Genes and Key Pathways in Breast Cancer Using Bioinformatic Analysis. Front. Genet. 10, 695. PubMed PMID: 31428132. doi:10.3389/fgene.2019.00695
Di Croce, L., and Helin, K. (2013). Transcriptional Regulation by Polycomb Group Proteins. Nat. Struct. Mol. Biol. 20 (10), 1147–1155. PubMed PMID: 24096405. doi:10.1038/nsmb.2669
Dong, H. P., Elstrand, M. B., Holth, A., Silins, I., Berner, A., Trope, C. G., et al. (2006). NK- and B-Cell Infiltration Correlates with Worse Outcome in Metastatic Ovarian Carcinoma. Am. J. Clin. Pathol. 125 (3), 451–458. PubMed PMID: 16613351. doi:10.1309/15b66dqmfyym78cj
Gao, J., Aksoy, B. A., Dogrusoz, U., Dresdner, G., Gross, B., Sumer, S. O., et al. (2013). Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 6 (269), pl1. PubMed PMID: 23550210. doi:10.1126/scisignal.2004088
Gil, J., and O’Loghlen, A. (2014). PRC1 Complex Diversity: where Is it Taking Us? Trends Cel Biol. 24 (11), 632–641. PubMed PMID: 25065329. doi:10.1016/j.tcb.2014.06.005
Guo, J., Zhang, Z., Pan, L., and Zhou, Y. (2018). RETRACTED: Identification of miR-758-3p as Potential Modulator of CBX5 Expression in Gastric Cancer. Technol. Cancer Res. Treat. 17, 153303381881606. PubMed PMID: 30486755. doi:10.1177/1533033818816061
Gupta, S., Nag, S., Aggarwal, S., Rauthan, A., and Warrier, N. (2019). Maintenance Therapy for Recurrent Epithelial Ovarian Cancer: Current Therapies and Future Perspectives - a Review. J. Ovarian Res. 12 (1), 103. PubMed PMID: 31685032. doi:10.1186/s13048-019-0579-0
Győrffy, B., Bottai, G., Fleischer, T., Munkácsy, G., Budczies, J., Paladini, L., et al. (2016). Aberrant DNA Methylation Impacts Gene Expression and Prognosis in Breast Cancer Subtypes. Int. J. Cancer 138 (1), 87–97. PubMed PMID: 26174627. doi:10.1002/ijc.29684
Hu, C., Zhang, Q., Tang, Q., Zhou, H., Liu, W., Huang, J., et al. (2020). CBX4 Promotes the Proliferation and Metastasis via Regulating BMI‐1 in Lung Cancer. J. Cell. Mol. Medi 24 (1), 618–631. PubMed PMID: 31724308. doi:10.1111/jcmm.14771
Jia, Y., Wang, Y., Zhang, C., and Chen, M. Y. (2020). Upregulated CBX8 Promotes Cancer Metastasis via the WNK2/MMP2 Pathway. Mol. Ther. - Oncolytics 19, 188–196. PubMed PMID: 33251331. doi:10.1016/j.omto.2020.09.012
Klauke, K., Radulović, V., Broekhuis, M., Weersing, E., Zwart, E., Olthof, S., et al. (2013). Polycomb Cbx Family Members Mediate the Balance between Haematopoietic Stem Cell Self-Renewal and Differentiation. Nat. Cel Biol 15 (4), 353–362. PubMed PMID: 23502315. doi:10.1038/ncb2701
Komi, D. E. A., and Redegeld, F. A. (2020). Role of Mast Cells in Shaping the Tumor Microenvironment. Clinic Rev. Allerg Immunol. 58 (3), 313–325. PubMed PMID: 31256327. doi:10.1007/s12016-019-08753-w
Kwon, S. H., and Workman, J. L. (2008). The Heterochromatin Protein 1 (HP1) Family: Put Away a Bias toward HP1. Mol. Cell 26 (3), 217–227. Epub ahead of print.
Li, B., Severson, E., Pignon, J.-C., Zhao, H., Li, T., Novak, J., et al. (2016). Comprehensive Analyses of Tumor Immunity: Implications for Cancer Immunotherapy. Genome Biol. 17 (1), 174. PubMed PMID: 27549193. doi:10.1186/s13059-016-1028-7
Li, J., Alvero, A. B., Nuti, S., Tedja, R., Roberts, C. M., Pitruzzello, M., et al. (2020). CBX7 Binds the E-Box to Inhibit TWIST-1 Function and Inhibit Tumorigenicity and Metastatic Potential. Oncogene 39 (20), 3965–3979. PubMed PMID: 32205869. doi:10.1038/s41388-020-1269-5
Li, J., Hu, K., He, D., Zhou, L., Wang, Z., and Tao, Y. (2020). Prognostic Value of PLXND1 and TGF-β1 Coexpression and its Correlation with Immune Infiltrates in Hepatocellular Carcinoma. Front. Oncol. 10, 604131. PubMed PMID: 33489909. doi:10.3389/fonc.2020.604131
Li, J., Li, Y., Cao, Y., Yuan, M., Gao, Z., Guo, X., et al. (2014). Polycomb Chromobox (Cbx) 7 Modulates Activation-Induced CD4+ T Cell Apoptosis. Arch. Biochem. Biophys. 564, 184–188. PubMed PMID: 25449062. doi:10.1016/j.abb.2014.10.004
Li, J., Xu, Y., Long, X.-D., Wang, W., Jiao, H.-K., Mei, Z., et al. (2014). Cbx4 Governs HIF-1α to Potentiate Angiogenesis of Hepatocellular Carcinoma by its SUMO E3 Ligase Activity. Cancer Cell 25 (1), 118–131. PubMed PMID: 24434214. doi:10.1016/j.ccr.2013.12.008
Li, Q., Pan, Y., Cao, Z., and Zhao, S. (2020). Comprehensive Analysis of Prognostic Value and Immune Infiltration of Chromobox Family Members in Colorectal Cancer. Front. Oncol. 10, 582667. PubMed PMID: 33014884. doi:10.3389/fonc.2020.582667
Li, T., Fu, J., Zeng, Z., Cohen, D., Li, J., Chen, Q., et al. (2020). TIMER2.0 for Analysis of Tumor-Infiltrating Immune Cells. Nucleic Acids Res. 48 (W1), W509–W514. PubMed PMID: 32442275. doi:10.1093/nar/gkaa407
Liang, Y.-K., Lin, H.-Y., Chen, C.-F., and Zeng, D. (2017). Prognostic Values of Distinct CBX Family Members in Breast Cancer. Oncotarget 8 (54), 92375–92387. PubMed PMID: 29190923. doi:10.18632/oncotarget.21325
Liao, Y., Wang, J., Jaehnig, E. J., Shi, Z., and Zhang, B. (2019). WebGestalt 2019: Gene Set Analysis Toolkit with Revamped UIs and APIs. Nucleic Acids Res. 47 (W1), W199–W205. PubMed PMID: 31114916. doi:10.1093/nar/gkz401
Lim, H. J., and Ledger, W. (2016). Targeted Therapy in Ovarian Cancer. Womens Health (Lond Engl. 12 (3), 363–378. PubMed PMID: 27215391. doi:10.2217/whe.16.4
Liu, J., Meng, H., Li, S., Shen, Y., Wang, H., Shan, W., et al. (2019). Identification of Potential Biomarkers in Association with Progression and Prognosis in Epithelial Ovarian Cancer by Integrated Bioinformatics Analysis. Front. Genet. 10, 1031. PubMed PMID: 31708970. doi:10.3389/fgene.2019.01031
Liu, J., Tan, Z., He, J., Jin, T., Han, Y., Hu, L., et al. (2020). Identification of Three Molecular Subtypes Based on Immune Infiltration in Ovarian Cancer and its Prognostic Value. Biosci. Rep. 40 (10). PubMed PMID: 33043974. doi:10.1042/bsr20201431
Lv, J., Liu, H., Su, J., Wu, X., Liu, H., Li, B., et al. (2012). DiseaseMeth: a Human Disease Methylation Database. Nucleic Acids Res. 40 (Database issue), D1030–D1035. PubMed PMID: 22135302. doi:10.1093/nar/gkr1169
Ma, C., Nie, X. G., Wang, Y. L., Liu, X. H., Liang, X., Zhou, Q. L., et al. (2019). CBX3 Predicts an Unfavorable Prognosis and Promotes Tumorigenesis in Osteosarcoma. Mol. Med. Rep. 19 (5), 4205–4212. PubMed PMID: 30942427. doi:10.3892/mmr.2019.10104
Mao, J., Tian, Y., Wang, C., Jiang, K., Li, R., Yao, Y., et al. (2019). CBX2 Regulates Proliferation and Apoptosis via the Phosphorylation of YAP in Hepatocellular Carcinoma. J. Cancer 10 (12), 2706–2719. PubMed PMID: 31258779. doi:10.7150/jca.31845
Matei, D., and Nephew, K. P. (2020). Epigenetic Attire in Ovarian Cancer: The Emperor's New Clothes. Cancer Res. 80 (18), 3775–3785. PubMed PMID: 32381656. doi:10.1158/0008-5472.can-19-3837
Morey, L., Pascual, G., Cozzuto, L., Roma, G., Wutz, A., Benitah, S. A., et al. (2012). Nonoverlapping Functions of the Polycomb Group Cbx Family of Proteins in Embryonic Stem Cells. Cell Stem Cell 10 (1), 47–62. PubMed PMID: 22226355. doi:10.1016/j.stem.2011.12.006
Moufarrij, S., Dandapani, M., Arthofer, E., Gomez, S., Srivastava, A., Lopez-Acevedo, M., et al. (2019). Epigenetic Therapy for Ovarian Cancer: Promise and Progress. Clin. Epigenet 11 (1), 7. PubMed PMID: 30646939. doi:10.1186/s13148-018-0602-0
Natanzon, Y., Goode, E. L., and Cunningham, J. M. (2018). Epigenetics in Ovarian Cancer. Semin. Cancer Biol. 51, 160–169. PubMed PMID: 28782606. doi:10.1016/j.semcancer.2017.08.003
Reddy, S. M., Reuben, A., Barua, S., Jiang, H., Zhang, S., Wang, L., et al. (2019). Poor Response to Neoadjuvant Chemotherapy Correlates with Mast Cell Infiltration in Inflammatory Breast Cancer. Cancer Immunol. Res. 7 (6), 1025–1035. PubMed PMID: 31043414. doi:10.1158/2326-6066.cir-18-0619
Rhodes, D. R., Yu, J., Shanker, K., Deshpande, N., Varambally, R., Ghosh, D., et al. (2004). ONCOMINE: a Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia 6 (1), 1–6. PubMed PMID: 15068665. doi:10.1016/s1476-5586(04)80047-2
Sammarco, G., Varricchi, G., Ferraro, V., Ammendola, M., De Fazio, M., Altomare, D. F., et al. (2019). Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer. Int. J. Mol. Sci. 20 (9), 2106. PubMed PMID: 31035644. doi:10.3390/ijms20092106
Sanchez-Vega, F., Mina, M., Armenia, J., Chatila, W. K., Luna, A., La, K. C., et al. (2018). Oncogenic Signaling Pathways in the Cancer Genome Atlas. Cell 173 (2), 321–337.e10. PubMed PMID: 29625050. doi:10.1016/j.cell.2018.03.035
Scarlett, U. K., and Conejo-Garcia, J. R. (2012). Modulating the Tumor Immune Microenvironment as an Ovarian Cancer Treatment Strategy. Expert Rev. Obstet. Gynecol. 7 (5), 413–419. PubMed PMID: 24039628. doi:10.1586/eog.12.41
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2021). Cancer Statistics, 2021. CA A. Cancer J. Clin. 71 (1), 7–33. PubMed PMID: 33433946. doi:10.3322/caac.21654
Slezak, J., Truong, M., Huang, W., and Jarrard, D. (2013). HP1γ Expression Is Elevated in Prostate Cancer and Is superior to Gleason Score as a Predictor of Biochemical Recurrence after Radical Prostatectomy. BMC Cancer 13, 148. PubMed PMID: 23522301. doi:10.1186/1471-2407-13-148
Sun, D., Cao, X., and Wang, C. (2019). Polycomb Chromobox Cbx2 Enhances Antiviral Innate Immunity by Promoting Jmjd3-Mediated Demethylation of H3K27 at the Ifnb Promoter. Protein Cell 10 (4), 285–294. PubMed PMID: 30357595. doi:10.1007/s13238-018-0581-0
Tang, Z., Kang, B., Li, C., Chen, T., and Zhang, Z. (2019). GEPIA2: an Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 47 (W1), W556–W560. PubMed PMID: 31114875. doi:10.1093/nar/gkz430
van Wijnen, A. J., Bagheri, L., Badreldin, A. A., Larson, A. N., Dudakovic, A., Thaler, R., et al. (2021). Biological Functions of Chromobox (CBX) Proteins in Stem Cell Self-Renewal, Lineage-Commitment, Cancer and Development. Bone 143, 115659. PubMed PMID: 32979540. doi:10.1016/j.bone.2020.115659
Wang, B., Tang, J., Liao, D., Wang, G., Zhang, M., Sang, Y., et al. (2013). Chromobox Homolog 4 Is Correlated with Prognosis and Tumor Cell Growth in Hepatocellular Carcinoma. Ann. Surg. Oncol. 20 (Suppl. 3), 684–692. PubMed PMID: 23943028. doi:10.1245/s10434-013-3171-7
Wang, S.-s., Liu, W., Ly, D., Xu, H., Qu, L., and Zhang, L. (2019). Tumor-infiltrating B Cells: Their Role and Application in Anti-tumor Immunity in Lung Cancer. Cell Mol Immunol 16 (1), 6–18. PubMed PMID: 29628498. doi:10.1038/s41423-018-0027-x
Webb, P. M., and Jordan, S. J. (2017). Epidemiology of Epithelial Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 41, 3–14. PubMed PMID: 27743768. doi:10.1016/j.bpobgyn.2016.08.006
Wotton, D., and Merrill, J. C. (2007). Pc2 and SUMOylation. Biochem. Soc. Trans. 35 (Pt 6), 1401–1404. PubMed PMID: 18031231. doi:10.1042/bst0351401
Xiong, Y., Wei, Y., Gu, Y., Zhang, S., Lyu, J., Zhang, B., et al. (2017). DiseaseMeth Version 2.0: a Major Expansion and Update of the Human Disease Methylation Database. Nucleic Acids Res. 45 (D1), D888–D895. PubMed PMID: 27899673. doi:10.1093/nar/gkw1123
Yan, Y., Xu, Z., Qian, L., Zeng, S., Zhou, Y., Chen, X., et al. (2019). Identification of CAV1 and DCN as Potential Predictive Biomarkers for Lung Adenocarcinoma. Am. J. Physiology-Lung Cell Mol. Physiol. 316 (4), L630–L643. PubMed PMID: 30604627. doi:10.1152/ajplung.00364.2018
Yang, C., Lee, H., Jove, V., Deng, J., Zhang, W., Liu, X., et al. (2013). Prognostic Significance of B-Cells and pSTAT3 in Patients with Ovarian Cancer. PLoS One 8 (1), e54029. PubMed PMID: 23326565. doi:10.1371/journal.pone.0054029
Yang, Y.-F., Pan, Y.-H., Tian, Q.-H., Wu, D.-C., and Su, S.-G. (2018). CBX1 Indicates Poor Outcomes and Exerts Oncogenic Activity in Hepatocellular Carcinoma. Translational Oncol. 11 (5), 1110–1118. PubMed PMID: 30031230. doi:10.1016/j.tranon.2018.07.002
Ye, L., Zhang, T., Kang, Z., Guo, G., Sun, Y., Lin, K., et al. (2019). Tumor-Infiltrating Immune Cells Act as a Marker for Prognosis in Colorectal Cancer. Front. Immunol. 10, 2368. PubMed PMID: 31681276. doi:10.3389/fimmu.2019.02368
Yoshihara, K., Tajima, A., Komata, D., Yamamoto, T., Kodama, S., Fujiwara, H., et al. (2009). Gene Expression Profiling of Advanced-Stage Serous Ovarian Cancers Distinguishes Novel Subclasses and implicatesZEB2in Tumor Progression and Prognosis. Cancer Sci. 100 (8), 1421–1428. PubMed PMID: 19486012. doi:10.1111/j.1349-7006.2009.01204.x
Zeng, J.-S., Zhang, Z.-D., Pei, L., Bai, Z.-Z., Yang, Y., Yang, H., et al. (2018). CBX4 Exhibits Oncogenic Activities in Breast Cancer via Notch1 Signaling. Int. J. Biochem. Cel Biol. 95, 1–8. PubMed PMID: 29229426. doi:10.1016/j.biocel.2017.12.006
Zhao, S.-P., Wang, F., Yang, M., Wang, X.-Y., Jin, C.-L., Ji, Q.-K., et al. (2019). CBX3 Promotes Glioma U87 Cell Proliferation and Predicts an Unfavorable Prognosis. J. Neurooncol. 145 (1), 35–48. PubMed PMID: 31502042. doi:10.1007/s11060-019-03286-w
Zhen, C. Y., Tatavosian, R., Huynh, T. N., Duc, H. N., Das, R., Kokotovic, M., et al. (2016). Live-cell Single-Molecule Tracking Reveals Co-recognition of H3K27me3 and DNA Targets Polycomb Cbx7-PRC1 to Chromatin. Elife 5, 5. PubMed PMID: 27723458. doi:10.7554/eLife.17667
Zheng, H., Jiang, W.-h., Tian, T., Tan, H.-s., Chen, Y., Qiao, G.-l., et al. (2017). CBX6 Overexpression Contributes to Tumor Progression and Is Predictive of a Poor Prognosis in Hepatocellular Carcinoma. Oncotarget 8 (12), 18872–18884. PubMed PMID: 28122351. doi:10.18632/oncotarget.14770
Zhong, X., Kan, A., Zhang, W., Zhou, J., Zhang, H., Chen, J., et al. (2019). CBX3/HP1γ Promotes Tumor Proliferation and Predicts Poor Survival in Hepatocellular Carcinoma. Aging 11 (15), 5483–5497. PubMed PMID: 31375643. doi:10.18632/aging.102132
Keywords: CBX family, ovarian cancer, expression profiles, prognosis, chemoresistance
Citation: Hu K, Yao L, Xu Z, Yan Y and Li J (2022) Prognostic Value and Therapeutic Potential of CBX Family Members in Ovarian Cancer. Front. Cell Dev. Biol. 10:832354. doi: 10.3389/fcell.2022.832354
Received: 09 December 2021; Accepted: 12 January 2022;
Published: 27 January 2022.
Edited by:
Dongling Zou, Chongqing University, ChinaReviewed by:
Xinyan Zhu, Tongji University, ChinaCopyright © 2022 Hu, Yao, Xu, Yan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juanni Li, bGlqdWFubmkyMDE0QGNzdS5lZHUuY24=; Yuanliang Yan, eWFueXVhbmxpYW5nQGNzdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.