
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 03 May 2022
Sec. Epigenomics and Epigenetics
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.829826
This article is part of the Research Topic Genetic and Epigenetic Regulation of Insect Development, Reproduction, and Phenotypic Plasticity - Volume II View all 9 articles
Dendrolimus species (Lepidoptera, Lasiocampidae), are the most serious phytophagous pests of coniferous forests worldwide. Dendrolimus feed intensively on needles, leading to considerable economic loss and ecological damage. Notably, the outbreak of Dendrolimus is a somewhat periodic pattern, and those outbreaks cause rapid and large-scale destruction of pine forests, with those forests observed to look like “Fire without smoke”. Sex pheromones play an important role during insect mating and reproduction, and there has been extensive research into the pheromone of Dendrolimus. The pheromone components of several Dendrolimus have been identified, and functions of two most important pheromone recognition genes, pheromone-binding proteins (PBPs) and pheromone receptors (PRs), were clarified. The evolution of PBP gene sequences is in good agreement with the trends in structural changes of the sex pheromone components in several Dendrolimus species, and it is interesting that PRs of Dendrolimus spp. occupy a novel lineage of PRs tuned to Type I pheromones in Lepidoptera. We present the current state of research into the sex pheromone of these important forest pests and highlight the emerging topics, to clarify future urgent work into Dendrolimus.
Dendrolimus spp. (Lepidoptera, Lasiocampidae) are the most serious phytophagous pests of coniferous forests worldwide. More than 30 species in the genus Dendrolimus are identified around the world of which about 27 species are present in China (Hou, 1987). The most seriously damaging Dendrolimus in China consist of six species including Dendrolimus punctatus (Walker), D. superans (Butler), D. tabulaeformis Tsai et Liu, D. spectabilis Butler, D. houi lajonquière, and D. kikuchii Matsumura. Some Dendrolimus species cause damage multiple times a year, with young larvae of the filial generation in summer voraciously feeding on the pine trees. Their damage causes a considerable reduction in standing volume, annual increment, and resin productivity of the trees. In particular, the damaged forests are susceptible and vulnerable to subsequent diseases and insect pests, and even forest fire, all which lead directly to extensive economic loss and ecological disaster (Chen, 1990). Notably, the outbreaks of Dendrolimus are somewhat periodic (Zhang et al., 2002). Catastrophic outbreaks usually occur within a short period, and large-scale destruction of pine forests has been referred to as making those forests look like “fire without smoke” (Han et al., 2004). In addition, people and livestock can suffer from serious health problems because of the toxic/irritating hairs on the larvae and cocoons (Hou, 1987). However, controlling Dendrolimus with pesticides has impacted human health, the environment, the natural enemies of Dendrolimus, and biodiversity (Kong X. B. et al., 2007).
Concomitant with their threats to pine forests and ecosystems, great efforts have been devoted to developing sustainable control strategies for pine caterpillars. Generally, it is not easy to monitor and control forest pests due to the thickness of the forests and the presence of surrounding mountains, so pheromone detection and control are particularly important. As lepidopteran insects, sex pheromones were emitted by Dendrolimus females, and the males are highly sensitive to pheromones. For this reason, research into the pheromones of Dendrolimus has attracted considerable attention. This review focuses on the advances made in the identification and recognition of Dendrolimus spp. pheromones, as well as the further understanding needed to manage these forest pests more effectively.
Structure identification of pheromone compounds is the first step when researching insect pheromones. Such work in this field began in the 1930s. After years of research, Butenandt et al. (1959) isolated and identified the chemical structure of the first insect sex pheromone E8,Z10-16: OH, named “bombykol”, from more than 500,000 female silkworm moths. Since then, research into insect pheromone identification has developed rapidly. The application of gas chromatography (GC), gas chromatography–mass spectrometry (GC-MS), gas chromatography–electroantennogram (GC-EAD), high-pressure liquid chromatography (HPLC), other high-performance microanalyzers, and the improvement of separation and extraction technology of insect pheromones has reduced the number of insects needed for the identification of insect pheromones from hundreds of thousands of individuals to just hundreds or even dozens, expediting research in this area.
So far, the sex pheromones of seven Dendrolimus species have been elucidated, six of which consist of isomers of (5Z,7E)-dodecadien-1-ol (Z5,E7-12:OH), and/or the corresponding acetates, propionates, or aldehyde derivatives (Ando et al., 1982; Priesner et al., 1984; Kovalev et al., 1993; Klun et al., 2000; Kong et al., 2003; Kong X.-B. et al., 2007; Kong et al., 2011; Kong et al., 2012). The seventh species, D. houi, uses (5E,7Z)-dodecadien-1-ol (E5,Z7-12:OH) and the corresponding acetate and aldehyde as sex pheromone components (Kong X. B. et al., 2007). All the identified sex pheromone components in Dendrolimus spp. share a common chemical theme of being C12 5,7-dienes with alcohol, acetate, propionate, or aldehyde functional groups. The brief history of sex pheromone identification of Dendrolimus spp. is summarized as follows.
Pheromone identification of Dendrolimus began in the 1970s. The sex pheromone of D. punctatus was the first one identified from the genus Dendrolimus. The insect hormone research group including three groups from the Institute of Zoology, Chinese Academy of Sciences; Jilin Institute of Applied Chemistry, Chinese Academy of Sciences; and the insect group of the Jiangxi forest pest control station, jointly identified the components of the pheromone of D. punctatus. The active components of the gonadal extract of D. punctatus were preliminarily obtained by extraction and separation; the compounds of the sex pheromone of D. punctatus were identified by GC-MS and characteristics MS peak analysis; the position of unsaturation of the aforementioned compounds were confirmed to be located at positions 5 and 7 by micro ozonolysis. Then, EAG (electroantennogram) reactions of single unsaturated dodecenol and its acetate to D. punctatus were analyzed, showing that the 5-position double bond could be a cis type, but the cis\trans structure of the 7-position double bond could not be determined. Various isomers (cis\trans, trans\cis, cis\cis, and trans\trans) of 5, 7-dodecadienol and its acetate were then synthesized, and the EAG activity of the antennae of male D. punctatus was tested. The results indicated that the cis-5-trans-7-isomers are the components of the pheromone of D. punctatus. Up to this point, the structure of the pheromone of D. punctatus had been analyzed step by step (Laboratory of Insect Pheromone, Institute of Zoology, Academia Sinica, Pine Caterpillar Moth Pheromone Section, Jilin Institute, of Applied Chemistry, Academia Sinica and Entomology Section, Forest Pest Control Experimental Station, Jiangxi, 1979). The active components of the pheromone gland extract of D. punctatus were identified as Z5,E7-12:OH, (5Z,7E)-5,7-dodecadien-1-yl acetate (Z5,E7-12:OAc), and (5Z,7E)-5,7-dodecadien-1-yl propionate (Z5,E7-12:OPr) (Laboratory of Insect Pheromone, Institute of Zoology, Academia Sinica, Pine Caterpillar Moth Pheromone Section, Jilin Institute, of Applied Chemistry, Academia Sinica and Entomology Section, Forest Pest Control Experimental Station, Jiangxi, 1979). This important progress demonstrated that researchers of insect pheromones in China were producing work of an international level at that time. Later, (5Z)-5-dodecen-1-yl acetate (Z5-12:OAc) and (5Z)-5-dodecen-1-ol (Z5-12:OH) were also found in the sex pheromone gland of D. punctatus, two compounds that, when added to the three-component sex pheromone blend, can increase the ease of trapping the D. punctatus moth (Zhao et al., 1993).
Subsequently, the sex pheromones of several other Dendrolimus species were identified. The sex pheromone components of D. spectabilis were identified as Z5,E7-12:OH by Japanese scholars (Ando et al., 1982), and Z5,E7-12:OAc and Z5,E7-12:OPr as minor pheromone components by Chinese scholars who have demonstrated that this strong moth lure is composed of these three components at a ratio of 100:3:25 (Kong et al., 2003). The sex pheromone components of D. pini were identified as Z5,E7-12:Ald (Priesner et al., 1984) but completed by Kovalev et al. (1993) who added Z5,E7-12:OH as a minor pheromone component (Kovalev et al., 1993). The sex pheromone components of D. superans were identified as Z5,E7-12:Ald and Z5,E7-12:OH at a ratio of 100:98 (Kong X.-B. et al., 2007), which clarified the active components and their isomers (Klun et al., 2000). In 2007, through pheromone gland extraction, GC-MS structure analysis (Figure 1), EAG activity tests, and field trapping verification, the pheromone components of D. houi were identified as E5,Z7-12:OH, E5,Z7-12:OAc, and E5,Z7-12:Ald, at a ratio of 100:39.7:5.6, and these chemicals are the first (E.Z)-isomer sex pheromones found in species of the genus Dendrolimus, the other species all being the (Z,E)-isomer (Kong X. B. et al., 2007). In 2011, the female sex pheromone of D. kikuchii was found to be Z5,E7-12:OAc, Z5,E7-12:OH, and Z5-12:OAc with an optimal attraction ratio of 100:20:25. In 2012, the sex pheromone components of D. tabulaeformis were identified as Z5,E7-12:OAc, Z5,E7-12:OH, and Z5,E7-12:OPr, with a ratio of 100:100:4.5 providing the optimal attraction (Kong et al., 2012).
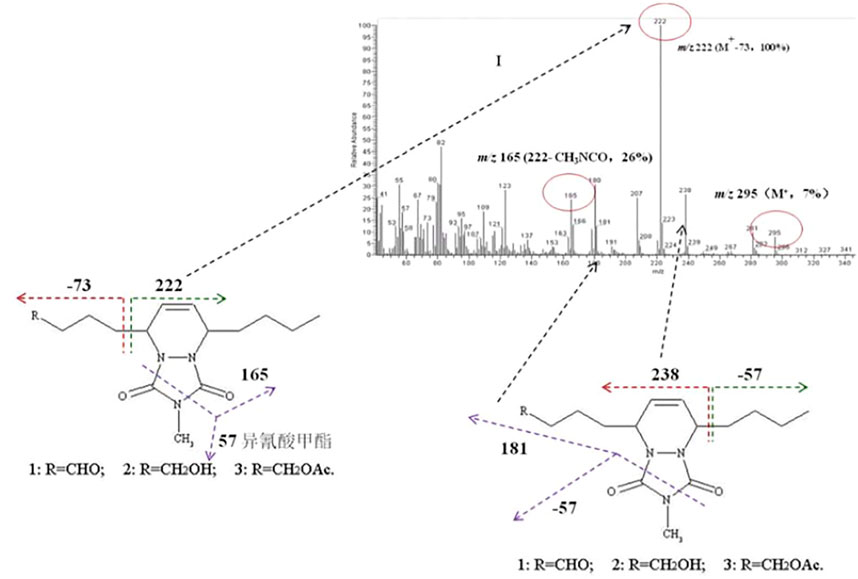
FIGURE 1. Mass spectrometry output of the derivatives of the sex components of (5E,7Z)-dodecadien-1-ol and 4-methyl-1,2,4-triazoline-3,5-dione, together with the cleavage law of characteristic fragmentations.
The relationships between sex pheromone components and Dendrolimus species are interesting. The sex pheromone of D. pini and D. superans belongs to the first sex pheromone type, which uses Z5,E7-12:Ald as major component without any ester components involved. D. punctatus, D. tabulaeformis, D. spectabilis, and D. kikuchii belong to the second sex pheromone type containing Z5,E7-12:OAc, Z5,E7-12:OH, and Z5,E7-12:OPr, but no aldehyde components. It is interesting that D. punctatus, D. tabulaeformis, and D. spectabilis all contain the same pheromone components but with different ratios. Finally, the sex pheromone components of D. houi are composed of E5,Z7-dodecadienol, and its aldehyde and acetate derivatives, representing the third sex pheromone type in Dendrolimus spp. (Figure 2). Based on the content changes of major sex pheromone components of different Dendrolimus species, it is evident that they follow certain rules: first, Z5,E7-12:Ald is the main pheromone component of D. pini and accounts for half of the total pheromones of D. superans; on the contrary, its potential as a sex pheromone inhibitor in D. punctatus, D. spectabilis, and D. kikuchii gradually strengthened (Figure 2, directed in red dotted triangle). Second, the ratio of Z5,E7-12:OH, which is a oxidation product of Z5,E7-12:Ald, in the pheromone mixtures of several Dendrolimus were directed in blue diamond. It is the most important sex pheromone component of D. spectabilis, D. punctatus, and D. tabulaeformis, but minor component of D. pini, D. superans, and D. kikuchii (Figure 2). At Last, Z5,E7-12:OAc, which is a acetate ester of Z5,E7-12:OH, is major sex pheromone component of D. punctatus, D. tabulaeformis, and D. kikuchii, minor pheromone component of D. spectabilis, antagonist of D. superans, and strong antagonist D. pini (Figure 2, directed in pink dotted triangle).
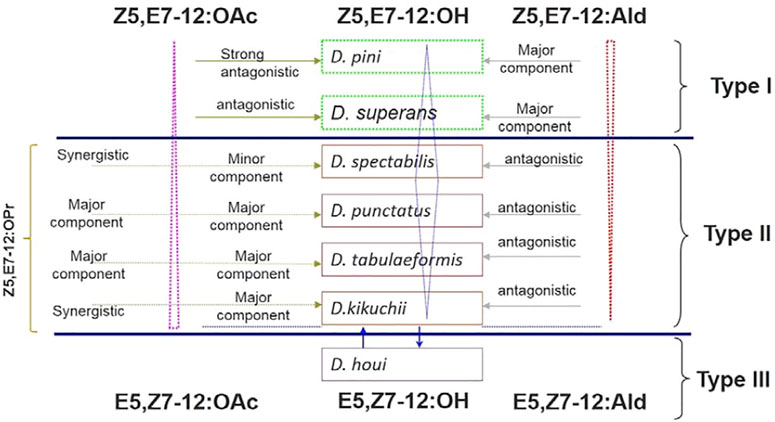
FIGURE 2. Relationships of sex pheromone components in Dendrolimus spp. Dotted pink triangle, blue diamond, and dotted red triangle directed the ratio and function of three important sex pheromone components, Z5,E7-12:OAc, Z5,E7-12:OH, and Z5,E7-12:Ald, respectively.
Along with the identification of sex pheromones, synthesis research in vitro and in vivo was also undertaken. The biosynthesis pathway of the sex pheromone in D. punctatus was gradually clarified (Liénard et al., 2010), which is affected by the pheromone biosynthesis activating neuropeptide (PBAN) produced in the brain and subesophageal ganglia complex (Zhao et al., 2002). Meanwhile, the in vitro chemical synthesis of the Dendrolimus sex pheromone components was developed and applied in field population monitoring (Laboratory of Insect Pheromone, Institute of Zoology, Academia Sinica, Pine Caterpillar Moth Pheromone Section, Jilin Institute, of Applied Chemistry, Academia Sinica and Entomology Section, Forest Pest Control Experimental Station, Jiangxi; Meng and Wang, 1983; Khrimian et al., 2002). Insect pheromone research is a subject closely aligned with practical application. Most of the pheromones or attractants identified and synthesized are from major agricultural or forestry pests, which play an important role in integrated pest management.
When synthetic insect pheromones are used in the field, it is necessary to select an appropriate carrier in order to achieve a stable release of the semiochemicals. The most widely used carriers are made of polyethylene plastic pipe, sleeve rubber, plastic film, microcapsules, etc. The release rates of Dendrolimus sex pheromone components on different carriers were evaluated (Li et al., 2015). The sulfur-free composite rubber carrier has good anti-isomerization ability and slow-release performance (Gao et al., 2001). The primary application of sex pheromones in pest control is population monitoring due to their high sensitivity, specificity, simplicity of operation, and low cost. The sex pheromone of Dendrolimus spp. has been used in monitoring since the 1970s. Population monitoring in low-density forest areas can directly ascertain the size of an insect population in the field (Wen et al., 2000), but different formula should be used for high density Dendrolimus populations (Du et al., 2016).
The pheromone recognition system of moths is very developed (Vogt and Riddiford, 1981). Dendrolimus are moth insect, and study on their pheromone recognition mechanisms can provide theoretical guidance for the development of efficient pest behavior regulation technology. The sex pheromone recognition system of pine moths is well developed, and study of their pheromone recognition mechanisms can provide theoretical guidance for the development of efficient population regulation technology.
At least six gene families involve in the detection of volatile and pheromone in insects, including three receptor families and two binding protein families (Odorant binding protein, OBP, and chemosensory protein, CSP), and the sensory neuron membrane proteins (SNMPs) (Su et al., 2009). The three receptor families are expressed in insect olfactory sensory neurons, including odorant receptors (OR) (Touhara and Vosshall, 2009), ionotropic receptors (IR) (Benton et al., 2009), and gustatory receptors (GR) (Agnihotri et al., 2016). Olfaction-related genes exhibit large sequence diversity, and identification of large repertoires of olfactory genes by purely homology-based methods is inefficient. Transcriptome researches greatly assisted the pheromone recognition related genes identification and analysis of Dendrolimus species.
Using transcriptome from different developmental stages and tissues, a complete sequence and expression profile of olfactory recognition related genes in D. punctatus was constructed (Zhang et al., 2017). Identification and comparison of olfactory recognition genes of D. kikuchii and D. houi indicated that odorant binding proteins (OBPs) and olfactory receptors (ORs) are the molecular basis of olfactory recognition differences between species (Zhang et al., 2014b). In order to solve the difficulty in identifying the pheromone receptor of D. punctatus, the antennae transcriptome of different mating stages of this species were compared and analyzed, and the candidate genes of sex pheromone receptor were found, which provided a solid foundation for the subsequent functional analysis of sex pheromone and the discovery of the new origin of pheromone receptor genes (Zhang et al., 2018).
Among the large number of chemosensory genes in insects (Vosshall and Stocker, 2007; Zhang et al., 2017; Zhang et al., 2018), there are two types of specific pheromone recognition genes: 1) pheromone binding proteins (PBPs) (Große-Wilde et al., 2006; Guo et al., 2012), which selectively bind and transport pheromone molecules to the dendrites of olfactory sensilla; 2) pheromone receptors (PRs) (Krieger et al., 2004; Leary et al., 2012; Zhang et al., 2015), which transform the chemical signals into specific neural electric signals. Therefore, the functional analysis of PBPs and PRs are the key to elucidating the pheromone molecular recognition mechanism of Dendrolimus spp.
Insect sex pheromones generally consist of 2–3 components at a particular ratio. Research into the function of PBPs obtained from D. kikuchii showed that the lower the proportion of a certain pheromone component, the higher the binding efficiency of PBPs to this component (Zhang et al., 2016). The functions of the PBP of D. tabulaeformis demonstrated the characteristics of inverse proportion recognition (Zhang et al., 2014c). Inverse proportion recognition might be an important way to recognize the minor sex pheromone components in Dendrolimus species. The chemical structure of sex pheromones changes gradually in several important Dendrolimus species, as described in Section 2. Phylogenetic analysis shows that the evolution of the PBP genes sequences associates well with the structural changes of pheromone components (Figure 3), indicating that the olfactory recognition genes and sex pheromone structures of Dendrolimus species are coevolutionary (Zhang S.-F. et al., 2014).
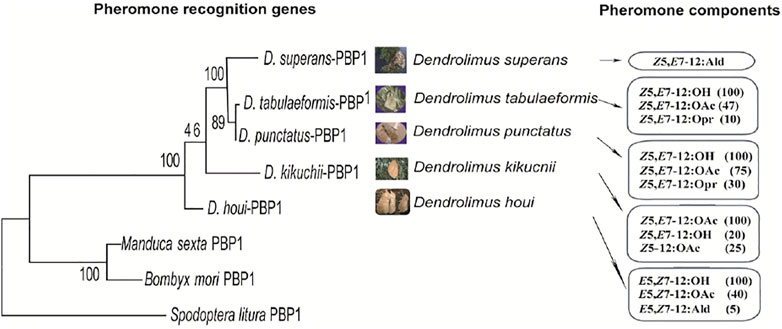
FIGURE 3. Comparison analysis of phylogenetic relationship of PBP1 and pheromone compound structures evolving in five Dendrolimus species. The PBP1 maximum likelihood tree was based on amino acid sequences, bootstrap values from 10,000 replications.
In comparison with general odorant receptors (ORs), the sequences of PRs in moths are relatively conserved and tend to be clustered into the same branch (also called the PR branch) on the evolutionary tree. However, we found that none of the ORs of Dendrolimus clustered into the traditional PRs branch, indicating the PRs of Dendrolimus spp. have unique sequence characteristics. We cannot find PR candidates of Dendrolimus spp. by phylogenetic analysis of ORs. To solve this problem, a new method to identify the PRs gene of Dendrolimus spp. was developed, by combining the mating behavior process with gene expression patterns (Zhang et al., 2018). The ORs correspond with identified mating behaviors, and specifically expressed in the antennae of male D. punctatus, were selected as PR candidates. Next, six candidate PRs and Orco were cloned from D. punctatus and each of the six genes was expressed with an Orco gene found in Xenopus oocytes—this gene was physiologically tested with pheromone compounds and analogs of Dendrolimus spp. using two-electrode voltage-clamp recording methods. Finally, PR45 and PR46 were successfully identified as two sex pheromone receptors.
Phylogenetic tree analysis showed that D. punctatus PRs were located in a more distinct clade than other lepidopteran PRs, and motif analysis of PRs showed differences between Dendrolimus spp. and other tested moth species (Shen et al., 2020). Therefore, our work found a novel lineage of PRs tuned to Type I pheromones in Lepidoptera (Shen et al., 2020) (Figure 4). Genome and mitochondrial phylogenies both indicate that Dendrolimus spp. (Lasiocampidae) is close to Bombyx mori (Bombycoidea) (Qin et al., 2019; Zhang et al., 2020), but D. punctatus PRs appear to diverge more than expected. Maybe the reproductive systems of insects are under larger selection pressure, and these driven accelerating divergence of PR genes in Lepidoptera. Evolution of moths reproductive system is still a mystery, and more work on genetic divergence of close related species are need in the future (Groot et al., 2016).
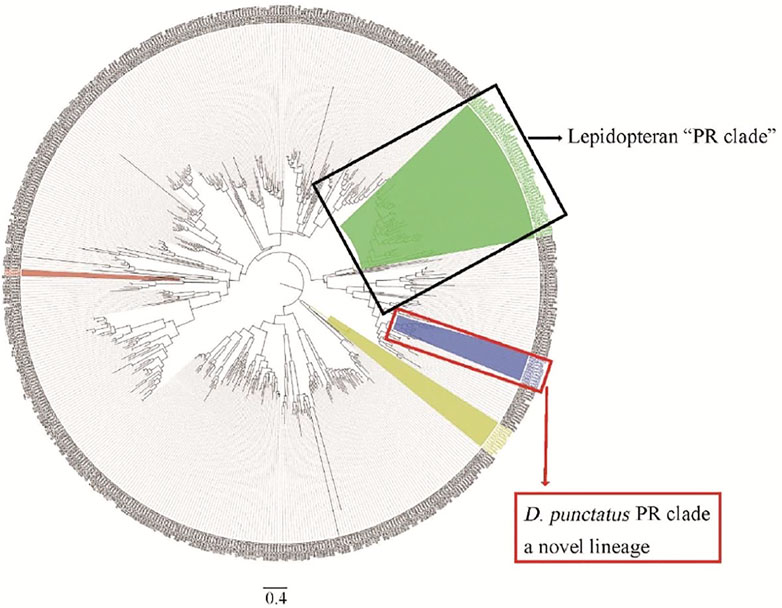
FIGURE 4. Phylogenetic analysis of ORs from Dendrolimus spp. and other lepidopteran insects, which indicates a new lineage of PRs. Dpun: D. punctatus, Bmor: Bombyx mori, Hvir: Heliothis virescens, Harm: Helicoverpa armigera, Hass: Helicoverpa assulta, Msex: Manduca sexta, Slit: Spodoptera litura, Ofur: Ostrinia furnacalis, Esem: Eriocrania semipurpurella, and Epos: Epiphyas postvittana. The clade of traditional PR clade is highlighted in green, conserved Orco in yellow, and D. punctatus in blue. Part of the picture comes from Shen et al. (2020).
As Dendrolimus species use same or very similar chemicals as sex pheromones, it is unclear that how PRs recognize and discriminate pheromones in closely related Dendrolimus species, and this deserves to be further studied.
Remarkable progress has been made in the identification and recognition of the sex pheromone of Dendrolimus spp., which provides a basis for further theoretical and practical research. First, the pheromone components of several main species of Dendrolimus have been identified, and the sex pheromone components of different Dendrolimus species are gradually changed. Second, inverse proportion recognition may be an important way for PBPs to recognize the trace sex pheromone component of Dendrolimus spp. Third, the evolution of the sequences of their PBP genes is in good agreement with the trends in structural changes of the sex pheromone components of the major Dendrolimus species, which reveals that the olfactory recognition genes and pheromone structures of Dendrolimus evolved synergistically. Fourth, PRs of Dendrolimus spp. occupy a novel lineage of PRs tuned to Type I pheromones in Lepidoptera. However, more work on pheromone recognition and discrimination of Dendrolimus are needed. For example, how closely related Dendrolimus species recognize their own sex pheromones accurately (not similar pheromones from closely related speces) and direct mating with homospecies? Does PBPs and PRs sequence mutations or different subsequent neural single integration process responsible for pheromone discrimination among closely related species? This is not only important for Dendrolimus but is likely to reveal some of the secrets still hidden in many other sister insect species.
SZ drafted the manuscript and generated the reference list. XK and ZZ revised the manuscript.
This work was supported by the Fundamental Research Funds of CAF (CAFYBB2020QC001) and the National Natural Science Foundation of China (31670657, 31470654).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agnihotri, A. R., Roy, A. A., and Joshi, R. S. (2016). Gustatory Receptors in Lepidoptera: Chemosensation and beyond. Insect Mol. Biol. 25, 519–529. doi:10.1111/imb.12246
Ando, T., Vu, M. H., Yoshida, S., Takahashi, N., Tatsuki, S., Katagiri, K., et al. (1982). (5Z, 7E)-5,7-Dodecadien-1-Ol: Female Sex Pheromone of the Pine Moth Dendrolimus Spectabilis Butler. Agric. Biol. Chem. 46, 709–715. doi:10.1080/00021369.1982.1086511710.1271/bbb1961.46.709
Benton, R., Vannice, K. S., Gomez-Diaz, C., and Vosshall, L. B. (2009). Variant Ionotropic Glutamate Receptors as Chemosensory Receptors in Drosophila. Cell 136, 149–162. doi:10.1016/j.cell.2008.12.001
Butenandt, A., Beckmann, R., Stamm, D., and Hecker, E. (1959). Über den sexual-lockstoff des seidenspinners Bombyx mori. Z. Naturforsch. 14, 283.
Chen, C. (1990). “Species, Geographic Distributions, and Biological Characteristics of pine Caterpillars in China,” in Integrated Management of pine Caterpillas in China (Beijing: China Forestry Publishing House), 5–18.
Du, K., Gao, C., Liu, W., Zhang, L., Lu, P., and Luo, Y. (2016). The Correlation Analysis Among Sex Pheromone Trap Catch, Leaf Loss Rate and Population Density of Chinese-pine Caterpillar Dendrolimus tabulaeformis. J. Plant Prot. 43, 966–971. doi:10.13802/j.cnki.zwbhxb.2016.06.012
Gao, W., Zhao, C., and Shi, Z. (2001). A Study on Stability of Dendrolimus punctatus Sex Pheromone in Different Types of Baits. Acta Entomologica Sinica 44, 213–220.
Groot, A. T., Dekker, T., and Heckel, D. G. (2016). The Genetic Basis of Pheromone Evolution in Moths. Annu. Rev. Entomol. 61, 99–117. doi:10.1146/annurev-ento-010715-023638
Grosse-Wilde, E., Svatoš, A., and Krieger, J. (2006). A Pheromone-Binding Protein Mediates the Bombykol-Induced Activation of a Pheromone Receptor In Vitro. Chem. Senses 31, 547–555. doi:10.1093/chemse/bjj059
Guo, H., Huang, L.-Q., Pelosi, P., and Wang, C.-Z. (2012). Three Pheromone-Binding Proteins Help Segregation between Two Helicoverpa Species Utilizing the Same Pheromone Components. Insect Biochem. Mol. Biol. 42, 708–716. doi:10.1016/j.ibmb.2012.06.004
Han, R., He, Z., and Ge, F. (2004). Factor Influencing the Population Dynamics of pine Caterpillars. Entomolocial Knowledge 41, 504–511.
Hou, T. (1987). “Introduction to pine Caterpillars,” in The pine Caterpillar in China. Editor T. Hou (Beijing: Science Press), 1–26.
Khrimian, A., Klun, J. A., Hijji, Y., Baranchikov, Y. N., Pet'ko, V. M., Mastro, V. C., et al. (2002). Syntheses of (Z,E)-5,7-dodecadienol and (E,Z)-10,12-hexadecadienol, Lepidoptera Pheromone Components, via Zinc Reduction of Enyne Precursors. Test of Pheromone Efficacy against the Siberian Moth. J. Agric. Food Chem. 50, 6366–6370. doi:10.1021/jf020472s
Klun, J. A., Baranchikov, Y. N., Mastro, V. C., Hijji, Y., Nicholson, J., Ragenovich, I., et al. (2000). A Sex Attractant for the Siberian Moth Dendrolimus superans Sibiricus (Lepidoptera: Lasiocampidae). J. Entomol. Sci. 35, 158–166. doi:10.18474/0749-8004-35.2.158
Kong, X.-B., Liu, K.-W., Wang, H.-B., Zhang, S.-F., and Zhang, Z. (2012). Identification and Behavioral Evaluation of Sex Pheromone Components of the Chinese pine Caterpillar Moth, Dendrolimus tabulaeformis. PLoS One 7, e33381. doi:10.1371/journal.pone.0033381
Kong, X.-B., Sun, X.-L., Wang, H.-B., Zhang, Z., Zhao, C.-H., and Booij, K. C. J. H. (2011). Identification of Components of the Female Sex Pheromone of the Simao pine Caterpillar Moth, Dendrolimus kikuchii Matsumura. J. Chem. Ecol. 37, 412–419. doi:10.1007/s10886-011-9932-5
Kong, X.-B., Zhao, C.-H., and Wang, R. (2007a). Sex Pheromone of the Larch Caterpillar Moth, Dendrolimus superans, from Northeastern China. Entomol. Exper Applic 124, 37–44. doi:10.1111/j.1570-7458.2007.00562.x
Kong, X. B., Zhang, Z., Zhao, C. H., and Wang, H. B. (2007b). Female Sex Pheromone of the Yunnan pine Caterpillar Moth Dendrolimus houi: First (E,Z)-Isomers in Pheromone Components of Dendrolimus Spp. J. Chem. Ecol. 33, 1316–1327. doi:10.1007/s10886-007-9313-2
Kong, X. B., Zhao, C. H., Sun, Y., Feng, S., and Wu, H. (2003). Identification of Minor Components of the pine Caterpillar Moth, Dendrolimus Spectabilis Sex Pheromone: Components, Electrophysiological Activity and Field Effects. Acta Entomologica Sinica 46, 131–137.
Kovalev, B. G., Bolgar', T. S., Zubov, P. A., Zharkov, D. G., Golosova, M., Nesterov, E. A., et al. (1993). Identification of Additional Components of the Sex Pheromone of Dendrolimus Pini. Chem. Nat. Compd. 29, 135–136. doi:10.1007/bf00631042
Krieger, J., Grosse-Wilde, E., Gohl, T., Dewer, Y. M. E., Raming, K., and Breer, H. (2004). Genes Encoding Candidate Pheromone Receptors in a Moth ( Heliothis virescens ). Proc. Natl. Acad. Sci. U.S.A. 101, 11845–11850. doi:10.1073/pnas.0403052101
Laboratory of Insect Pheromone, Institute of Zoology, Academia Sinica; Pine Caterpillar Moth Pheromone Section, Jilin Institute, of Applied Chemistry, Academia Sinica and Entomology Section, Forest Pest Control Experimental Station, Jiangxi (1979). Isolation, Identification and Synthesis of EAG-Active Components of the Pine-caterpillar Moth Sex Pheromone. Sci. Bull. 21, 1004–1008.
Leary, G. P., Allen, J. E., Bunger, P. L., Luginbill, J. B., Linn, C. E., Macallister, I. E., et al. (2012). Single Mutation to a Sex Pheromone Receptor Provides Adaptive Specificity between Closely Related Moth Species. Proc. Natl. Acad. Sci. U.S.A. 109, 14081–14086. doi:10.1073/pnas.1204661109
Li, X., Kong, X., Zhang, S., Wang, H., Zhang, Z., and Yang, M. (2015). Researches on the Release Rates of Four Types of Insect Semiochemicals from Four Dispenser Types. Scientia Silvae Sinicae 51, 63–70. doi:10.11707/j.1001-7488.20151208
Liénard, M. A., Lassance, J.-M., Wang, H.-L., Zhao, C.-H., Piškur, J., Johansson, T., et al. (2010). Elucidation of the Sex-Pheromone Biosynthesis Producing 5,7-dodecadienes in Dendrolimus punctatus (Lepidoptera: Lasiocampidae) Reveals Δ11- and Δ9-desaturases with Unusual Catalytic Properties. Insect Biochem. Mol. Biol. 40, 440–452. doi:10.1016/j.ibmb.2010.04.003
Meng, X., and Wang, H. (1983). Synthesis and Field experiment of Sex Attractent for pine Caterpillar Dendrolimus tabulaeformis. Scientia Silvae Sinicae 19, 137–140.
Priesner, E., Bogenschütz, H., Albert, R., Reed, D. W., and Chisholm, M. D. (1984). Identification and Field Evaluation of a Sex Pheromone of the European pine Moth. Z. Naturforsch. 39, 1192–1195. doi:10.1515/znc-1984-11-1236
Qin, J., Li, J., Gao, Q., Wilson, J.-J., and Zhang, A.-B. (2019). Mitochondrial Phylogeny and Comparative Mitogenomics of Closely Related pine Moth Pests (Lepidoptera:Dendrolimus). PeerJ 7, e7317. doi:10.7717/peerj.7317
Shen, S., Cao, S., Zhang, Z., Kong, X., Liu, F., Wang, G., et al. (2020). Evolution of Sex Pheromone Receptors in Dendrolimus punctatus Walker (Lepidoptera: Lasiocampidae) Is Divergent from Other Moth Species. Insect Biochem. Mol. Biol. 122, 103375. doi:10.1016/j.ibmb.2020.103375
Su, C.-Y., Menuz, K., and Carlson, J. R. (2009). Olfactory Perception: Receptors, Cells, and Circuits. Cell 139, 45–59. doi:10.1016/j.cell.2009.09.015
Touhara, K., and Vosshall, L. B. (2009). Sensing Odorants and Pheromones with Chemosensory Receptors. Annu. Rev. Physiol. 71 (1), 307–332. doi:10.1146/annurev.physiol.010908.163209
Vogt, R. G., and Riddiford, L. M. (1981). Pheromone Binding and Inactivation by Moth Antennae. Nature 293 (5828), 161–163. doi:10.1038/293161a0
Vosshall, L. B., and Stocker, R. F. (2007). Molecular Architecture of Smell and Taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533. doi:10.1146/annurev.neuro.30.051606.094306
Wen, Z., Wang, S., and Ren, Y. (2000). Prediction of Occurrence Period and Monitoring of Dendrolimus punctatus by Pheromone Attraction. For. Sci. Tech. 5, 17–19.
Zhang, J., Walker, W. B., and Wang, G. (2015). “Pheromone Reception in Moths,” in Progress in Molecular Biology and Translational Science. Editor G. Richard (New York: Academic Press), 109–128. doi:10.1016/bs.pmbts.2014.11.005
Zhang, S.-F., Liu, H.-H., Kong, X.-B., Wang, H.-B., Liu, F., and Zhang, Z. (2017). Identification and Expression Profiling of Chemosensory Genes in Dendrolimus punctatus Walker. Front. Physiol. 8, 471. doi:10.3389/fphys.2017.00471
Zhang, S.-F., Zhang, Z., Kong, X.-B., Wang, H.-B., and Liu, F. (2018). Dynamic Changes in Chemosensory Gene Expression during the Dendrolimus punctatus Mating Process. Front. Physiol. 8, 1127. doi:10.3389/fphys.2017.01127
Zhang, S.-F., Zhang, Z., Kong, X.-B., and Wang, H.-B. (2014a). Molecular Characterization and Phylogenetic Analysis of Three Odorant Binding Protein Gene Transcripts in Dendrolimus species (Lepidoptera: Lasiocampidae). Insect Sci. 21, 597–608. doi:10.1111/1744-7917.12074
Zhang, S., Kong, X., Ze, S., Wang, H., Lin, A., Liu, F., et al. (2016). Discrimination of Cis-Trans Sex Pheromone Components in Two Sympatric Lepidopteran Species. Insect Biochem. Mol. Biol. 73, 47–54. doi:10.1016/j.ibmb.2016.04.004
Zhang, S., Shen, S., Peng, J., Zhou, X., Kong, X., Ren, P., et al. (2020). Chromosome‐level Genome Assembly of an Important pine Defoliator, Dendrolimus punctatus (Lepidoptera; Lasiocampidae). Mol. Ecol. Resour. 20 (4), 1023–1037. doi:10.1111/1755-0998.13169
Zhang, S., Zhang, Z., Wang, H., and Kong, X. (2014b). Antennal Transcriptome Analysis and Comparison of Olfactory Genes in Two Sympatric Defoliators, Dendrolimus houi and Dendrolimus kikuchii (Lepidoptera: Lasiocampidae). Insect Biochem. Mol. Biol. 52, 69–81. doi:10.1016/j.ibmb.2014.06.006
Zhang, S., Zhang, Z., Wang, H., and Kong, X. (2014c). Molecular Characterization, Expression Pattern, and Ligand-Binding Property of Three Odorant Binding Protein Genes from Dendrolimus tabulaeformis. J. Chem. Ecol. 40, 396–406. doi:10.1007/s10886-014-0412-6
Zhang, Z., Li, D., and Cha, G. (2002). Time Series Analysis and Complex Dynamics of Mason Pine Caterpillar, Dendrolimus punctatus Walker (Lepidoptera Lasiocampidae). Acta Ecologica Sinica 22, 1061–1067.
Zhao, C. H., Li, Q., and Gao, W. (2002). Stimulation of Sex Pheromone Production by PBAN-like Substance in the Pine Caterpillar Moth, Dendrolimus punctatus (Lepidoptera: Lasiocampidae). Arch. Insect Biochem. Physiol. 49, 137–148. doi:10.1002/arch.10010
Keywords: forest pests, periodic outbreak, pheromone, PBP, PR
Citation: Zhang S, Kong X and Zhang Z (2022) Research Progress on the Dendrolimus spp. Pheromone: From Identification to Molecular Recognition. Front. Cell Dev. Biol. 10:829826. doi: 10.3389/fcell.2022.829826
Received: 06 December 2021; Accepted: 05 April 2022;
Published: 03 May 2022.
Edited by:
Emma Despland, Concordia University, CanadaReviewed by:
Guanghong Liang, Fujian Agriculture and Forestry University, ChinaCopyright © 2022 Zhang, Kong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sufang Zhang, emhhbmdzZkBjYWYuYWMuY24=; Xiangbo Kong, eGJrb25nQHNpbmEuY29t; Zhen Zhang, emhhbmd6aGVuQGNhZi5hYy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.