- 1School of Life Science and Technology, ShanghaiTech University, Shanghai, China
- 2Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China
- 3NHC Key Lab of Reproduction Regulation, Shanghai Institute for Biomedical and Pharmaceutical Technologies, Reproduction and Development Institution, Fudan University, Shanghai, China
- 4Shanghai Institute for Advanced Immunochemical Studies, ShanghaiTech University, Shanghai, China
In the male reproductive tract, the epididymis is an essential organ for sperm maturation, in which sperm cells acquire mobility and the ability to fertilize oocytes while being stored in a protective microenvironment. Epididymal function involves a specialized luminal microenvironment established by the epithelial cells of epididymal mucosa. Low-calcium concentration is a unique feature of this epididymal luminal microenvironment, its relevance and regulation are, however, incompletely understood. In the rat epididymis, the vitamin D-related calcium-dependent TRPV6-TMEM16A channel-coupler has been shown to be involved in fluid transport, and, in a spatially complementary manner, vitamin K2-related γ-glutamyl carboxylase (GGCX)-dependent carboxylation of matrix Gla protein (MGP) plays an essential role in promoting calcium-dependent protein aggregation. An SNP in the human GGCX gene has been associated with asthenozoospermia. In addition, bioinformatic analysis also suggests the involvement of a vitamin B6-axis in calcium-dependent MGP-mediated protein aggregation. These findings suggest that vitamins interact with calcium homeostasis in the epididymis to ensure proper sperm maturation and male fertility. This review article discusses the regulation mechanisms of calcium homeostasis in the epididymis, and the potential role of vitamin interactions on epididymal calcium homeostasis, especially the role of matrix calcium in the epididymal lumen as a cofactor for the carboxylated MGP-mediated scavenging function.
General Introduction
The production of viable and functionally competent spermatozoa is a prerequisite for male fertility. This is achieved through normal spermatogenesis in the testis and maturation of spermatozoa in the epididymis. Spermatozoa are non-fertilising when released from the testis but become functionally competent during epididymal transit. The fertilizing competence of spermatozoa is conferred by their interaction with the epididymal luminal microenvironment, which is formed by the epithelial cellular activities and retained behind the blood-epididymis barrier (Dacheux and Dacheux, 2014; Dube and Cyr, 2012; Robaire and Hinton, 2015; Zhou et al., 2018). Spermatozoa therefore mature and are protected in a special physiologically and immunologically privileged epididymal microenvironment, in which they undergo a series of tightly-controlled sequential maturational processes in the precisely segmented luminal compartments (Cornwall, 2009; Gervasi and Visconti, 2017; Gregory and Cyr, 2014; Hermo, 2002; Mital et al., 2011; Pleuger et al., 2020; Robaire and Hinton, 2015; Voisin et al., 2019; Wong P. Y. D. et al., 2002; Zhou et al., 2018). Through these maturation processes, spermatozoa acquire the motility to swim within the female tract and to undergo a three-stage modification process that enables oocyte fertilization (Aitken, 2016; Breitbart, 2002; Reid et al., 2011; Stival et al., 2016), including capacitation and hyperactivation (Aitken and Nixon, 2013; Gervasi and Visconti, 2016), as well as the acrosome reaction (Brucker and Lipford, 1995) (Figure 1A). The minimal time for spermatozoa to transit through the entire epididymis usually takes 1–16 days, depending on the species. In humans, the average transit time is approximately 1–2 days (Amann and Howards, 1980), and spermatozoa can be stored in the cauda epididymidis for several days and even months (Robaire and Hinton, 2015). Although fertilization occurs within the female genital tract, the functional ability for fertilization is acquired by spermatozoa when they transit through the highly convoluted epididymal tubule, where spermatozoa mature but remain in a dormant stage (Figure 1B). Hence, the epididymis plays a vital role for sperm maturation and male reproduction.
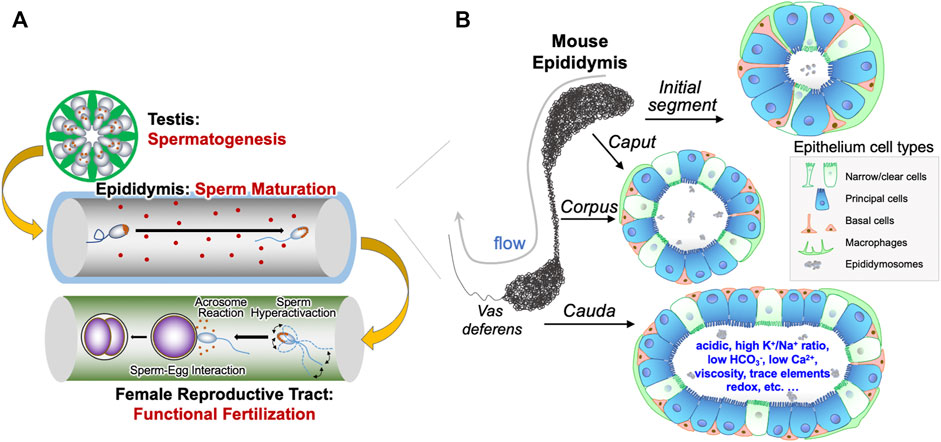
FIGURE 1. Epididymal epithelial cells and luminal microenvironment are important for male reproduction. (A) Graphic illustration of the generation of spermatozoa in the testis through spermatogenesis, the maturation of spermatozoa in the epididymis, and the spermatozoa functions, including sperm hyperactivation, acrosome reaction and sperm-egg interaction, in the female reproductive tract required for fertilization. (B) The different cell types in the various regions of epididymis, including basal cells, clear cells, narrow cells, and principal cells, as well as immunological cells from systemic circulation. These different cell types work in a concerted manner to form a unique luminal microenvironment that promotes maturation of sperm during its epididymal transit and ensures male fertility.
Epididymal function relies on a highly specialized epididymal luminal microenvironment, which is formed and maintained by the well synchronized cellular activities of the epithelial cells lining the epididymal mucosa. Owing to the complex functions and compartmentalisation of the epididymis, multiple causes of epididymal dysfunction resulting in male fertility disorders, and even the health of offspring, are conceivable (Chen et al., 2016a; Sharma et al., 2016; Chan et al., 2020). Defects in essential factors in the epididymis, which may originate from the testicles (Gatti et al., 1999; Shen et al., 2013; Kim et al., 2015; Koch et al., 2015; Zi et al., 2015; Liu et al., 2016), including proteins (Cornwall, 2009; Dacheux and Dacheux, 2014; Robaire and Hinton, 2015; Gervasi and Visconti, 2017), lipids (Haidl and Opper, 1997; Ouvrier et al., 2009; Saez et al., 2011; Bjorkgren et al., 2015), and non-coding RNAs (Belleannee, 2015; Chen et al., 2016a; Chen et al., 2016b; Sharma et al., 2016; Chu et al., 2017; Sharma et al., 2018), or sperm maturation deficits, impaired motility, and production of anti-sperm antibodies (Barak et al., 2000; Hamada et al., 2012), can all result in epididymal dysfunction associated male fertility deficits. Clinical practice relies on semen analysis to classify male infertility as asthenozoospermia, oligoasthenozoospermia, oligoteratozoospermia or the phenotypes associated with primitive biochemical parameters (WHO, 2021). Although male infertility is common, affecting approximately 15% of couples of child-bearing age worldwide (Zegers-Hochschild et al., 2009; Joffe, 2010; Esteves, 2013; Winters and Walsh, 2014; Tuttelmann et al., 2018; Agarwal et al., 2021), more than 50% of cases are idiopathic, i.e., of unknown cause, which limits our ability for developing targeted therapies. In some clinics before the intervention of antibiotics, epididymal deficits are estimated to be involved in 50–80% of male infertile patients (Silvert and Stanisic, 1980). Hence, an improved understanding of epididymal function permitting sperm maturation holds the promise of improved diagnosis and treatment of male infertility.
Calcium Homeostasis and Male Reproduction
While male fertility research is predominantly focused on spermatogenesis and the biology of spermatozoa following their release from the epididymis, accumulating evidence points to epididymal function being critical for sperm activity and male fertility (Silvert and Stanisic, 1980; Chen et al., 2016b; Sharma et al., 2016; Sharma et al., 2018; Tuttelmann et al., 2018; Conine et al., 2019; Sharma, 2019; Kiyozumi et al., 2020). One unique feature of the epididymal luminal environment is its acidity and low calcium (Ca2+) concentration (Levine and Marsh, 1971; Levine and Kelly, 1978; Au and Wong, 1980; Jenkins et al., 1980; Hong et al., 1984; Breton et al., 1996; Clulow and Jones, 2004; Da Silva et al., 2007; Weissgerber et al., 2011). Ca2+ homeostasis is essential for male reproduction (Karsenty, 2011; Oury et al., 2011; Laurentino et al., 2012; Miyata et al., 2015), and Ca2+ dysregulation is associated with male infertility (Okunade et al., 2004; Prasad et al., 2004; Brandenburger et al., 2011; Weissgerber et al., 2011), although the mechanisms regulating Ca2+ homeostasis remain largely unknown. In principle, Ca2+ homeostasis in male reproduction organs can be regulated through inter-organ and intra-organ mechanisms, in a manner of endocrine (Karsenty, 2011; Oury et al., 2011; Oury et al., 2013), paracrine (Gao da et al., 2016; Ma et al., 2019), or lumicrine (Laurentino et al., 2012; Kiyozumi et al., 2020), and so might affect epididymal function directly or indirectly (Lewis and Aitken, 2001; Ecroyd et al., 2004a; Miyata et al., 2015).
Ca2+ homeostasis requires a balance of Ca2+ efflux and influx to maintain intracellular and extracellular Ca2+ concentrations within optimal ranges in individual compartments of biological systems. The proteins and mechanisms underlying Ca2+ homeostasis are tightly regulated. Importantly, Ca2+ servers as an extracellular first messenger and intracellular second messenger in numerous physiological functions (Peng et al., 2003; Hoenderop et al., 2005; Breitwieser, 2008; Bagur and Hajnoczky, 2017). In the human body, Ca2+ is stored in bones and teeth, mainly in the form of hydroxyapatite and Ca2+ phosphate. In blood plasma, circulating throughout the body, [Ca2+] is controlled approximately 2.5 mM; whereas in the epididymis the Ca2+ concentration ranges from approximately 1.3 mM at the initial segment down to 0.25 mM in the cauda (Jenkins et al., 1980; Turner, 1991; Clulow et al., 1994; Turner, 2002; Carlson et al., 2003; Carlson et al., 2007; Ma et al., 2019). Understanding the mechanisms for Ca2+ regulation in the epididymis will provide insights into sperm physiology and associated functions of sperm fertilization, as well as male reproductive health.
As in other systems, Ca2+ homeostasis in the epididymis is strictly regulated by a network of cell-cell interactions and signaling pathways. The consequences of dysregulated Ca2+ levels and Ca2+-regulated proteins in the epididymis associated with male infertility are considered as interrupting this network (Okunade et al., 2004; Schuh et al., 2004; Weissgerber et al., 2011; Correia et al., 2013). In this review, we will focus on the Ca2+ homeostatic regulation mechanisms in the epididymis and discuss their physiological roles in regulating the luminal fluid microenvironment for sperm maturation. Specifically, the potential role of Ca2+ as a cofactor for matrix Gla protein (MGP)-mediated scavenging of extracellular metabolites in the epididymal microenvironment will be discussed, and the potential role of vitamins in this regard will be explored.
Vitamins and Male Reproduction
Whereas the essential roles of vitamins in general biology are well recognized, their specific roles in male reproductive health are remained incompletely understood. It has been reported that specific vitamin supplements can increase sperm quality in rats (Dawson et al., 1992; Wong W. Y. et al., 2002; Paradiso Galatioto et al., 2008; Blomberg Jensen et al., 2011), demonstrating that vitamins contribute to male fertility. Functional studies have revealed that vitamins A and B12 are involved in spermatogenesis (Watanabe et al., 2007; Raverdeau et al., 2012; Boucheron-Houston et al., 2013), whereas the antioxidant properties of vitamins C and E are believed to protect sperm DNA (Dawson et al., 1992; Greco et al., 2005). Vitamin B12 deficiency during maternal pregnancy or during growth of male rats was found to cause irreversible damage to the development of germ cells in embryos and affect the maturation of spermatozoa (Watanabe et al., 2003; Watanabe et al., 2007).
It is known that vitamins interact with Ca2+ homeostasis pathways. For example, vitamin B6 deficiency in rats was found to alter intracellular Ca2+-homeostasis in enterocytes, potentially via Ca2+ channel modulation without affecting net Ca2+ transport (Lal and Dakshinamurti, 1993; Matyaszczyk et al., 1993). Moreover, vitamin C can enhance intestinal Ca2+ absorption (Morcos et al., 1976), potentially by modulating epithelial transcellular and/or paracellular transport pathways. Vitamin C deficiency is related to secondary hyperparathyroidism in renal disease (Richter et al., 2008), and parathyroid hormone is an important endocrine signaling pathway for Ca2+-homeostasis (Lee and Partridge, 2009; Andrukhova et al., 2016). It is well-known that vitamin D plays an indispensable role in Ca2+ homeostasis regulation, possibly through the steroid sex hormone-related pathways and calcium-sensing receptor (Blomberg Jensen et al., 2010; Blomberg Jensen et al., 2011; Blomberg Jensen, 2014; Boisen et al., 2021). Our research on Ca2+ reabsorption in the rat epididymis pointed to the activity of transient receptor potential vanilloid channel 6 (TRPV6)- and transmembrane protein 16A (TMEM16A, also known as anoctamin-1)-associated activities in Ca2+- and fluid-homeostasis (Gao da et al., 2016), a pathway that has also been reported to be regulated by vitamin D (Walters et al., 2006) and that might be of fundamental importance for vital events (Rock et al., 2008; Benedetto et al., 2019), in addition to male fertility (Weissgerber et al., 2011; Weissgerber et al., 2012; Boisen et al., 2021). Vitamins D and E are lipid-soluble antioxidants and known to have protective effects on sperm quality and DNA integrity in rats challenged with oxidative stress (Greco et al., 2005; Momeni and Eskandari, 2012). While vitamin E administration alone in men of infertile couples did not improve sperm parameters upon conventional sperm analysis (Matorras et al., 2020), it did so when used in combination with selenium (Keskes-Ammar et al., 2003). Regarding vitamin K, the endocrine function of the vitamin K-dependent Ca2+-binding protein osteocalcin in male reproduction has been reported (Karsenty, 2011; Oury et al., 2011; Oury et al., 2013; Patti et al., 2013). Using a rat model of warfarin-induced vitamin K2-deficiency, we also confirmed the role of vitamin K-dependent MGP carboxylation in Ca2+-homeostasis, sperm maturation and male fertility (Ma et al., 2019). In this review, we will also discuss the potential role of vitamin interactions on epididymal calcium homeostasis, especially in relation to carboxylated MGP-mediated function.
Epididymal Epithelial Cells and Luminal Microenvironment Are Key Players of Male Reproduction
The mammalian epididymis is a single, long, and highly convoluted tubule that connects the efferent ducts from the testis to the vas deferens. Based on morphology and regional gene expression, the epididymis is divided into four main regions, namely the initial segment and the caput, corpus and cauda epididymidis (Figure 1B). The epididymal tubule is lined with an epithelium composed of a pseudo-stratified layer of specialized epithelial cells, including principal cells, narrow cells, clear cells, basal cells and immunological cells (Da Silva et al., 2011; Robaire and Hinton, 2015; Breton et al., 2019; Rinaldi et al., 2020). These epithelial cells have cell-to-cell contact with spermatozoa, create and maintain the epididymal luminal microenvironment, including secreting maturation-promoting factors and conveying the environmental factors to the spermatozoa, as well as removing potentially harmful metabolites from the lumen, and thus play a critical role in regulation of male fertility and even the health of offspring (Robaire and Hinton, 2015; Chen et al., 2016a; Chen et al., 2016b; Sharma et al., 2016; Gervasi and Visconti, 2017; Conine et al., 2018; Sharma et al., 2018; Sharma, 2019). Understanding the essential ingredients and optimal composition of the epididymal microenvironment will improve our understanding of sperm maturation and of sperm function in the female genital tract to required to fertilize an oocyte successfully.
Epididymal Epithelial Cells and Cell-Cell Crosstalk
Spermatozoa depend on the proper function of various types of epididymal epithelial cells during their transit (Battistone et al., 2020; Breton et al., 2019; Cheung et al., 2005; Leung et al., 2004; Ma et al., 2019; Robaire and Hinton, 2015; Wong P. Y. D. et al., 2002). The epithelial cells form a barrier that separates the luminal cavity from the bloodstream, and also transport nutrients and metabolites across it. Therefore, both secretory and resorptive epithelial machinery acts to create a special milieu in the lumen of the epididymis that is required for sperm maturation, while keeping them quiescent, preventing premature activation (Carr and Acott, 1984; Robaire and Hinton, 2015; Zhou et al., 2004). Epididymal function involves a network of complex regulatory mechanisms that are not yet fully understood. Using state-of-the-art tools, such as single-cell omics and systems biology analyses, recent studies have revealed the complexity of cell biology in epididymal function (de Lima et al., 2021; Rinaldi et al., 2020; Shi et al., 2021). A diagram of a basic model for the epithelial function, regulation of luminal microenvironment, and cell-cell interactions in the epididymis is presented in Figure 2. It has reported that the basal cells in the epididymis participate an active role in regulating the principal cell-mediated fluid secretion into the lumen using the prostaglandin-E2 (PGE2) signaling axis from the basolateral side (Leung et al., 2004; Cheung et al., 2005). In addition to this paradigm that basal cells solely play their role in the basolateral side, our research has also revealed that basal cells also have an important role from the luminal side, by sending out the antenna-like body projections to sense luminal hormonal factors, in particular angiotensin II (Shum et al., 2008). These basal cells express angiotensin type-2 receptor (AGTR2) and communicate their findings using the nitric oxide (NO) and soluble guanylyl cyclase signaling axis to the adjacent clear cells, which express high levels of proton pump vacuolar-ATPase (V-ATPase) and thereby modulate the luminal acidification (Breton and Brown, 2013), an essential process for sperm maturation and viability (Robaire and Hinton, 2015). The cell-cell interactions between clear cells and principal cells, between principal cells, as well as between macrophages and epithelial cells have also been described (Battistone et al., 2019; Breton et al., 2019; Battistone et al., 2020).
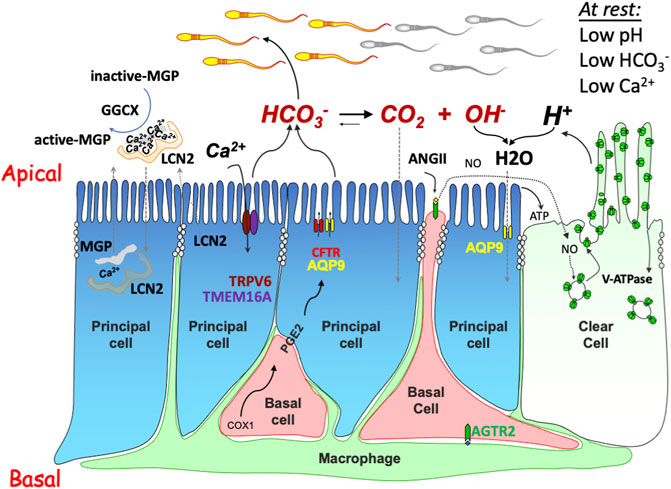
FIGURE 2. Schematic drawing showing the intercellular communication model regulating the luminal microenvironment in the epididymis. The model includes several physiological processes: 1) anion secretion (e.g., HCO3−) via apically located CFTR (and/or other anion channels, such as TMEM16A) provides an ionic gradient which drives fluid transport through water channels, e.g., aquaporin 9 (AQP9); 2) proton-pump V-ATPase acidifies the luminal content and promotes HCO3− hydrolysis for water and CO2 absorption, an essential acidification process required for maintaining sperm in a quiescent state and to prevent premature activation. The acidification process is mediated by either constitutively by recycling of the V-ATPases which are abundantly expressed in clear cells, or by the stimulation of luminal factors, such as ATP generated by principal cells or nitric oxide (NO) from the basal cells upon stimulation by the angiotensin receptor type 2 (AGTR2); and 3) Ca2+ reabsorption through either the epithelial Ca2+ channel TRPV6 and Ca2+-dependent Cl−-channel TMEM16A electrical coupler or via a process facilitated by GGCX-mediated carboxylation-dependent activation of MGP for Ca2+ chelation and simultaneous protein aggregation, such as lipocalin 2 (LCN2).
The epididymal epithelium is known to be active in secretion and absorption via the sophisticated exocytotic and endocytotic cellular machinery, predominantly in the principal cells and clear cells (Leung et al., 2004; Cheung et al., 2005; Robaire and Hinton, 2015; Gao da et al., 2016; Ma et al., 2019). These are the major cell types covering most of the epididymal luminal surface and thus mainly responsible for the controlling luminal ion compositions, luminal organic molecules, and liberating extracellular vesicles (EVs), also known as epididymosomes. These membraned-bound EVs serve as the key extracellular medium for two-way cell-to-cell communication between the nurtured spermatozoa and the nurturing epididymal cells (Sullivan et al., 2005; Sullivan et al., 2007; Sharma et al., 2016; Conine et al., 2018; Sharma et al., 2018; Leahy et al., 2020).
Other cell types including the basal cells and the immunological cells like dendritic cells can occasionally make contact to the luminal environment in a regional-dependent manner (Shum et al., 2008; Da Silva et al., 2011; Shum et al., 2014). Their contribution to the luminal environment is thought to be indirect and cell-cell interactions are the underlying indispensable mechanisms (Shum et al., 2008; Da Silva et al., 2011; Shum et al., 2014; Battistone et al., 2020). It is reasonable to hypothesize that the luminal microenvironment in epididymis critically affects sperm function, and thus male fertility and even transgenerational health of offspring (Chen et al., 2016a; Chen et al., 2016b; Sharma et al., 2016; Conine et al., 2018; Sharma, 2019; Chan et al., 2020).
The Composition of Epididymal Luminal Fluid
The epididymal luminal fluid, similar to other bodily extracellular fluids, comprises cations and anions, with organics constituents of proteins and other macromolecules and small molecules, glycans and lipids (Pastor-Soler et al., 2005; Cornwall, 2009; Shum et al., 2011; Robaire and Hinton, 2015; Tecle and Gagneux, 2015; Gervasi and Visconti, 2017; Zhou et al., 2018; Cornwall et al., 2019). The ionic composition together with the organic matter determines the fluid volume, pH and osmolarity. Before entering the epididymal tubule, immature spermatozoa in testicular fluid enter the efferent ducts (Wong and Yeung, 1978; Wong P. Y. D. et al., 2002), where the majority (>95%) of the fluid is reabsorbed (Hess et al., 1997; Clulow et al., 1998; Wong P. Y. D. et al., 2002; Turner, 2002), and replaced with epididymal transcellular fluid, the fluid within epithelial-lined space. Upon transit from the head down to the tail of epididymis, addition >90% of epididymal transcellular fluid is reabsorbed (Yamamoto et al., 1993; Wong P. Y. D. et al., 2002; Turner, 2002).
The Epididymal Luminal Fluid Is a Matrix With Regional Specificity
Epididymal fluid is a gel-like and viscous dynamic phase compartment, which can be characterized as a giant membraneless organelle (Cornwall, 2009; Shum et al., 2011; Robaire and Hinton, 2015; Tecle and Gagneux, 2015; Gervasi and Visconti, 2017; Zhou et al., 2018; Cornwall et al., 2019). The intraluminal fluidic contents are compartmentalized distinctly according to anatomical regions of the epididymal tubule, as revealed by a glance at the region-specific patterns of different types of epithelial cells and their absorption and secretory machinery, as well as the changes of surface contents on the surface of epididymal spermatozoa (Turner et al., 2003; Ecroyd et al., 2004b; Johnston et al., 2005; Pastor-Soler et al., 2005; Yudin et al., 2005; Finger et al., 2006; Jelinsky et al., 2007; Turner et al., 2007; Shum et al., 2011; Tollner et al., 2012; Shum et al., 2014; Robaire and Hinton, 2015; Zhou et al., 2018). The mechanisms underlying the formation and maintenance of epididymal fluid compartmentalization are not fully understood yet. However, liquid-liquid phase separation, a widespread mechanism for cells to handle membraneless matrices (Hyman et al., 2014; Shin and Brangwynne, 2017) suggest that this process could play a role in regulation of epididymal luminal microenvironment.
Water and Acid-Base Balance
The concentrations of all the epididymal fluid components reflect their dilution in water, whose volume in turn is adjusted as a function of the bathed ion concentrations; thus water transport is important for the optimal microenvironment in each compartment of the male excurrent duct, ensuring sperm maturation. And thus, water transport also influences Ca2+ homeostasis in the epididymal lumen, despite the regulation mechanism and the physiological implications still largely remain to explore. Proteins known to be relevant for water transport and electrolyte balance in epididymis, including cystic fibrosis transmembrane conductance regulator (CFTR) (Wong, 1998; Ruan et al., 2012), aquaporins (Stevens et al., 2000; Pastor-Soler et al., 2001; Cheung et al., 2003; Pastor-Soler et al., 2005; Da Silva et al., 2006), and the Na+-K+-pump and associated transporters (Breton et al., 1998; Bagnis et al., 2001; Wong P. Y. D. et al., 2002; Kujala et al., 2007; Zuo et al., 2011), have important role in maintaining epididymal function and male fertility (Wagenfeld et al., 2002; Xu et al., 2003; Yeung et al., 2004; Pruneda et al., 2007; Krausz and Riera-Escamilla, 2018; Xu et al., 2018).
Water is the medium of numerous hydrolysis processes in biological systems and carbon dioxide and its hydrolyzed derivative bicarbonate are not only the metabolic products but also an acid-base balancers in the body. In epididymis, bicarbonate-related acid-base balance is an essential process for acidification of luminal fluid, which is critical for maintaining spermatozoa in a quiescent state (Au and Wong, 1980; Carr and Acott, 1984; Breton et al., 1996; Newcombe et al., 2000; Pastor-Soler et al., 2005; Shum et al., 2011; Zuo et al., 2011). Hence, multitude of proteins must act in concert to establish and maintain optimal epididymal volume, electrolyte balance, and pH. The orchestration of these multiple proteins and their functions involves a complex network of cell-cell crosstalk involving autocrine, paracrine and lumicrine signaling (Kim et al., 2015; Robaire and Hinton, 2015; Gao da et al., 2016; Battistone et al., 2019; Ma et al., 2019; Kiyozumi et al., 2020). Figure 2 shows a basic model for the epithelial cell functions involved in epididymal fluid regulation, including water and ion transport, acid-base balance, and calcium homeostasis.
Inorganic Ions in the Epididymis
Table 1 summarises the concentrations of most inorganic ions contained in epididymal fluid, based on data published in the literature (Levine and Marsh, 1971, 1975; Levine and Kelly, 1978; Hinton and Setchell, 1980; Jenkins et al., 1980; Turner et al., 1984; Caflisch and DuBose, 1990; Hinton, 1990; Caflisch, 1992; Cooper et al., 1992; Clulow et al., 1994; Stoltenberg et al., 1996; Clulow et al., 1998; Sorensen et al., 1999; Newcombe et al., 2000). The high K+ ion concentration, up to approximately 55 mM in the distal cauda region (approximately ten-fold higher than in serum), and the low concentration of Ca2+ (down to 0.25 mM in the cauda region, approximately ten-fold lower than in serum) are notable. These special properties indicate that the microenvironment for sperm maturation is uniquely formed and maintained as spermatozoa transit the epididymal tubule.
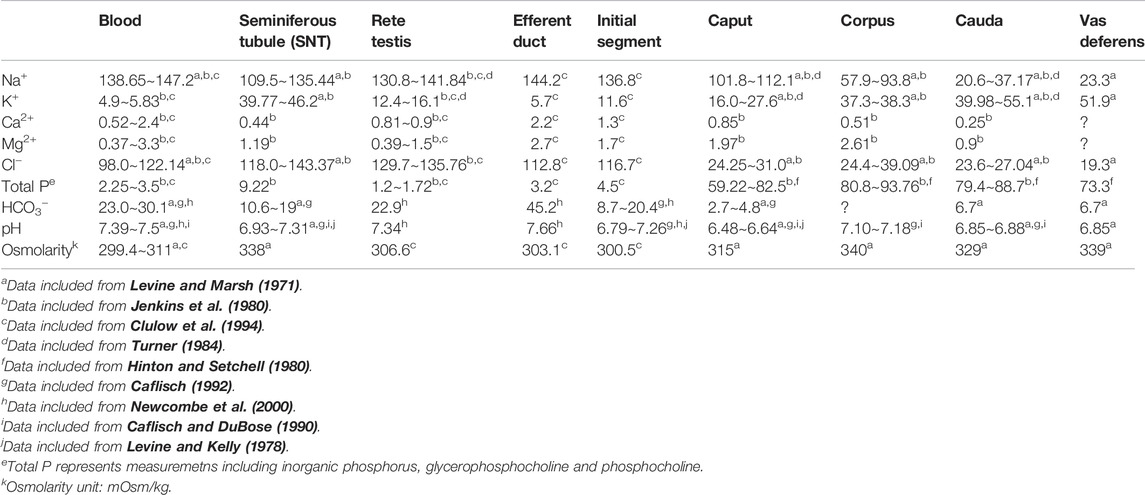
TABLE 1. Concentrations of inorganic elements (mM) and pH in blood plasma and intraluminal fluids from the excurrent duct of rats.
Calcium Homeostasis in the Epididymal Luminal Matrix
The Epididymis Maintains a Low Luminal Ca2+ Microenvironment
After spermatozoa are released from the testis, they migrate passively towards the excurrent duct and enter the epididymal tubule where they mature along a spatio-temporally changing milieu. As mentioned earlier, >90% of the initial epididymal fluid is reabsorbed by the epididymal epithelium during its transit toward the cauda. Therefore, it is suggestive that the low concentration of Ca2+ in the cauda depend on active Ca2+ reabsorption machinery in the epididymis. Consistent with the critical role of a low Ca2+ concentration in the epididymal fluid, dysregulation of epididymal Ca2+ homeostasis is one cause of male infertility (Prasad et al., 2004; Schuh et al., 2004; Brandenburger et al., 2011; Karsenty, 2011; Oury et al., 2011; Weissgerber et al., 2011; Laurentino et al., 2012; Oury et al., 2013; Miyata et al., 2015).
In general, epithelial absorption can occur through two main pathways for ion transport across epithelial cells: one is the paracellular pathway, in which electrochemical gradients passively drive ions through tight junctions and the paracellular space; the other is the transcellular pathway, which involves several steps including active apical absorption and intracellular trafficking as well as basolateral secretion (Hoenderop et al., 2005; Frizzell and Hanrahan, 2012; Garcia-Castillo et al., 2017). Because the transepithelial Ca2+ gradient does not support passive Ca2+ absorption through the paracellular pathway, transcellular Ca2+ absorption is expected the main pathway of Ca2+ absorption in the epididymis.
The transcellular transport of Ca2+ across epithelia is a multistep process (Hoenderop et al., 2005), beginning with passive entry of Ca2+ through the Ca2+ channels in the apical membranes followed by diffusion through cytosol facilitated by binding to intracellular Ca2+-binding proteins, e.g. calbindin-D28k in the kidney and calbindin-D9K in the intestine (Hoenderop et al., 2005), and eventually extrusion across the basolateral membranes by energy-requiring Na+/Ca2+ exchangers, plasma membrane Ca2+-ATPases (PMCAs), which operate against the electrochemical gradient for Ca2+ (Okunade et al., 2004; Hoenderop et al., 2005; Brandenburger et al., 2011; Patel et al., 2012). From a stoichiometric point of view, initial step of passive entry through apical Ca2+ channels is believed to be likely the rate-limiting step of the transepithelial Ca2+ (Nijenhuis et al., 2005). In epithelial tissues, only two highly Ca2+-selective ion channels act as apical channels for Ca2+ (re)absorption: Ca2+-selective ion channels TRPV5 and TRPV6 (Peng et al., 1999; Peng et al., 2000). Our previous study found that only TRPV6 was expressed in the apical pole of the rat epididymal epithelium of rats in a regional-dependent manner (Shum et al., 2006). Studies from other research groups using genetic deletion or mutant mouse models of the epithelial calcium channel TRPV6 have confirmed that apical Ca2+ influx through TRPV6 is important for the prevention of abnormal Ca2+ accumulation in the epididymis (Weissgerber et al., 2011; Weissgerber et al., 2012). In these studies, it was shown that after the ablation of TRPV6 channel function, the Ca2+ concentration in the distal cauda epididymidis was increased nearly ten-fold, sperm motility decreased, and fertilizing ability in mice was reduced. These studies showed that the epididymal Ca2+ homeostasis is critical for male fertility and sperm maturation.
Other mechanisms of epididymal Ca2+ homeostasis are also critical for male fertility and post-epididymal sperm functions. For example, in the plasma membrane Ca2+-pump 4 (PMCA4) knockout mice, sperm lacked motility and could not move forward in the female reproductive tract, resulting in male infertility (Okunade et al., 2004; Brandenburger et al., 2011). PMCA4a protein may be transported via epididymosomes from the epithelial cells to the sperm tail in the epididymis (Brandenburger et al., 2011), a process regulated by luminal constituents. Another observation pointing to the importance of Ca2+ homeostasis for male fertility is that loss of the Ca2+-dependent phosphatase calcineurin leads to infertility in male mice, owing to decreased sperm motility caused by an inflexible midpiece of spermatozoa (Miyata et al., 2015). Moreover, the functionality for sperm capacitation, which is triggered by Ca2+ influx in spermatozoa with subsequent activation of the cAMP-PKA signaling pathway and protein tyrosine phosphorylation, is formed in the epididymis and sensitive to extracellular Ca2+ (Luconi et al., 1996; Lewis and Aitken, 2001; Ecroyd et al., 2005; Gervasi and Visconti, 2016; Puga Molina et al., 2017; Takei et al., 2021). Another Ca2+-binding protein regucalcin, is abundant in epididymal luminal fluid, but any role in Ca2+ homeostasis remains to be demonstrated (Laurentino et al., 2012; Correia et al., 2013). Taken together, these findings point to the complexity and importance of Ca2+ homeostasis in the lumen of epididymis.
Regulation of Low Ca2+ Concentrations in the Epididymis and Its Molecular Mechanisms and Physiological Implications
It has been shown that defects in transepithelial Ca2+ absorption, including by defects of the apically located TRPV6 Ca2+ channels or the basolateral PMCA4 Ca2+ exclusion pump, result in Ca2+ accumulation in the luminal compartment of the distal epididymal tubule. This in turn impairs epididymal-dependent spermatozoa fertilization activities and impaired fertility without affecting spermatogenesis (Okunade et al., 2004; Schuh et al., 2004; Weissgerber et al., 2011; Weissgerber et al., 2012; Patel et al., 2013).
Regulating Epididymal Ca2+ Homeostasis by the TRPV6-TMEM16A Coupler
In further characterization of the mechanisms regulating Ca2+ absorption in the epididymis, our research has demonstrated that the TRPV6-associated extracellular pH-sensitive Ca2+ conductance functions in an electrically coupled manner with the intracellular Ca2+-sensitive chloride channel TMEM16A in isolated single principal cells of the distal part of the rat epididymis (Gao da et al., 2016) (see Figure 2). The interplay between TRPV6 and TMEM16A does not only regulate Ca2+ resorption but also Ca2+-dependent anionic current, a driving force of fluid secretion (Wong P. Y. D. et al., 2002). Our research also found that TRPV6-like Ca2+ conductivity is regulated by negative membrane potential and is sensitive to extracellular pH and divalent ions (Ca2+ and Mg2+), all of these physiological features are uniquely formed and maintained in the epididymis. Therefore, the coupling of TRPV6 with TMEM16A makes this coupler a key player of epididymal secretion. TMEM16A has been described to mediate epithelial mucin secretion, including in response to inflammation (Huang et al., 2012; Lin et al., 2015; Zhang et al., 2015; Benedetto et al., 2019). Our research has shown that TRPV6 co-localizes with TMEM16A in the apical membrane of the rat epididymal epithelium, as well as in some epididymosomes of the very proximal and the very distal rat epididymis (Gao da et al., 2016). These findings raise the possibility of TRPV6-TMEM16A contributing to the secretion of epididymosomes from the epididymal cells, an epididymal cell-to-cell communication mechanism that is essential for sperm maturation as well as transgenerational paternal epigenetic information transfer (Sullivan et al., 2005; Lotvall et al., 2014; Belleannee, 2015; Chen et al., 2016a; Chen et al., 2016b; Sharma et al., 2016; Tkach and Thery, 2016; Sharma, 2019; Chan et al., 2020).
Another Calcium Reabsorption Mechanism in Addition to TRPV6-TMEM16A Coupler in the Epididymis
It is known that the epididymal luminal fluid is acidic and under resting physiological conditions, it maintains a pH range of about 6.4–6.8 in the corpus and cauda epididymidis of rodents (see Table 1). Our research has shown that in single cauda epithelial cells isolated from rat epididymis, the TRPV6-related conductance is inhibited at acidic pH (for example, pH 6.4) and activated at alkaline pH (for example, 7.4) (Gao da et al., 2016). In addition, the TRPV6 activity is also sensitive to the resting membrane potential, and its conductance is inwardly rectifying at negative membrane potentials and is negligible at positive potentials (Bodding, 2005; Hoenderop et al., 2005). Whereas the resting membrane potential of epididymal epithelial cells has been determined to range between –6 mV and –39 mV, with an average value of approximately –22 mV (Cheung et al., 1976; Gao da et al., 2016). Our research has shown that in these resting potential ranges, the Ca2+-permeable current in single cauda epithelial cells at extracellular pH 6.4, or even at pH 7.4 is indeed undetectable or negligible, respectively (Gao da et al., 2016). We speculate that under physiological conditions of acidic epididymal lumen, Ca2+ influx through TRPV6 channel alone is silent or at least negligible, while the electrical coupling between TRPV6 and TMEM16A promotes Ca2+ cation influx by accompanying Cl− anion influx. However, the restricted localization of the extracellular pH- and Ca2+-dependent TRPV6-TMEM16A electrical coupler in the proximal and distal ends of the epididymis as well as in the epididymosomes not associated with extensive Ca2+ reabsorption could imply the presence of another calcium reabsorption mechanism or that TRPV6 and its coupler TMEM16A have a function other than Ca2+ reabsorption. Consistent with our speculation, a study of roosters found that those with epididymal stones had raised epididymal expression of TRPV6, although the connection between the two observations is still unclear (Oliveira et al., 2012).
Vitamin K2-Dependent Matrix Gla Protein-Mediated Ca2+-Homeostatic Regulation and Its Role in Epididymal Luminal Microenvironment and Male Fertility
To address whether in epididymidal regions where TRPV6-TMEM16A is absent, another Ca2+ reabsorption mechanism is active, our research has found vitamin K-dependent γ-glutamyl carboxylase (γ-carboxylase or GGCX)-mediated carboxylation of the substrate MGP to play a role in Ca2+ homeostasis in the epididymis (Ma et al., 2019). Consistent with our hypothesis, we found that GGCX-MGP expression was enriched in the epididymal regions with minimal TRPV6-TMEM16A coupler expression. Using a rat model of warfarin-induced vitamin K2 deficiency, we found that vitamin K2-dependent GGCX and its γ-carboxylation substrate MGP were essential for epididymal Ca2+ homeostasis, sperm maturation and thereby male fertility. Warfarin-induced vitamin K2-deficiency in rats leads to disruption of GGCX-mediated carboxylation of MGP, which leads to accumulation of stress granules and Ca2+ in the epididymal epithelium and lumen, and ultimately reduces male fertility (Ma et al., 2019).
Insights Into the Role of Carboxylation-Dependent Calcium Homeostasis in the Epididymis on Male Reproduction and Offspring Health
Vitamin K is a known key modulator of Ca2+ homeostasis. By facilitating GGCX-mediated γ-carboxylation of matrix proteins, including MGP, it maintains bone health and prevents vascular ectopic calcification (Weber, 2001; Wallin et al., 2008; Boraldi et al., 2013). MGP is a 14-kDa (103 amino acids) secretory protein containing five Ca2+-binding γ-carboxyglutamic acid (Gla) residues. It was first described as an extrahepatic matrix protein in the extracellular matrix of bone and cartilage and later detected in a wide variety of other tissues such as lung, heart, kidney and arterial vessel walls (Price et al., 1983; Fraser and Price, 1988; Hale et al., 1988). MGP is synthesized locally, at least in the vascular tissues, where it counterbalances ectopic mineral deposition (Murshed et al., 2004). The essential role of MGP has been revealed in mice lacking MGP, which die within 6–8 weeks of birth from large blood vessels ruptures, owing to massive vascular calcification (Luo et al., 1997). Humans with mutations in both alleles of the MGP gene develop Keutel syndrome, an autosomal recessive disorder characterized by abnormal calcification of soft tissues and female miscarriages (Munroe et al., 1999; Khosroshahi et al., 2014). The vitamin K antagonist, warfarin through inhibition of the vitamin K epoxide reductase complex 1 (VKORC1) to prevent carboxylation and activation of MGP. The treatment of normal mice with warfarin has been associated with a rapid calcification of elastic lamellae in arteries and heart valves that is reminiscent of the MGP-null phenotype in mice (Price et al., 1998). The importance of vitamin K status for MGP has also been confirmed in healthy people and in patients with long-term warfarin treatment (Rennenberg et al., 2010; Cranenburg et al., 2012). Importantly, the understanding of vitamin K-dependent mechanisms for local calcification regulation may also provide information about luminal calcium homeostasis in the epididymis.
Like other Gla proteins, MGP is activated by GGCX, which is the only gamma-carboxylation enzyme in the cell and which has no relevant homology to any known enzyme families (Tie et al., 2016). The Ca2+ binding function of MGP requires two post-translational modifications: γ-glutamate carboxylation and serine phosphorylation (Schurgers et al., 2007; Schurgers et al., 2013; Houben et al., 2016). The exact mechanism through which serine phosphorylation in MGP facilitates Ca2+ binding is unclear, and may involve indirect mechanisms such as promoting extracellular secretion of MGP (Wajih et al., 2004). In contrast, γ-glutamyl carboxylation is precisely related to increased Ca2+ binding affinity of MGP (Hackeng et al., 2001; Schurgers et al., 2005).
Our research has showed that MGP expression is high in the epididymis when compared with other organs containing epithelial-lining such as kidney and liver (Ma et al., 2019). In addition, GGCX and MGP co-localized in the vesicular structures on the luminal surface of epithelial and sperm membranes, potentially indicating that carboxylation takes place in the epididymal luminal microenvironment. These results may dicates that the vitamin K-dependent carboxylation and the MGP-associated prevention of Ca2+-deposition can reciprocally regulate epididymal luminal secretions. Since the expression of GGCX and MGP and their co-localization on vesicular granules, sperm surface, and in the cytoplasmic droplets progressively increases from the proximal epididymis distally, our study suggests that both proteins likely arose from the epididymosomes derived from epididymal epithelial cells. In view of the important role of epididymosomes in the male reproduction and the health of offspring (Sullivan et al., 2005; Chen et al., 2016a; Chen et al., 2016b; Sharma et al., 2016; Conine et al., 2018, 2019; Sharma et al., 2018), whereas GGCX-dependent carboxylation of MGP plays a vital role in life events (e.g., early embryo development) (Schurgers et al., 2013; Khosroshahi et al., 2014), the understanding the role of carboxylated-MGP dependent luminal calcium homeostasis in epididymis may provide insights into male reproduction and the health of offspring.
Ca2+ Behaves as a Cofactor of the Matrix Gla Protein-Promoted Scavenging Function of Ca2+-Precipitable Aggregates
Biphasic Ca2+-Dependent Chelation Properties of Matrix Gla Protein
In one of our previous studies (Ma et al., 2019), western blotting with anti-MGP antibody on rat epididymis and kidney always detected a major band at approximately 32-kDa that did not correspond to the expected ∼12-kDa molecular weight of MGP (Figure 3). Other publications also noted this band and interpreted it as a non-specific band due to the excessive protein lysis sample (Lomashvili et al., 2011). However, the intensity of this ∼32-kDa band was strikingly decreased when the tissue protein extracts were preincubated with the MGP peptide (Figure 3A). In addition, the intensity of the 32-kDa band and a few weaker bands at higher molecular weight became intensified when the divalent ion chelator EDTA was added to the protein extracts (Figure 3B), suggesting that these bands may represent Ca2+-dependent protein aggregates. Supporting this hypothesis, the 32-kDa band intensity from an epididymal cells line changed in a biphasic Ca2+-dependent manner. When the Ca2+ was added to the protein extract at sub-millimolar concentrations, the intensity of the Ca2+-bound MGP-positive protein complex increased. Maximum intensity of the MGP-positive protein complex was observed after addition of 0.1 mM Ca2+, suggesting that at this concentration protein aggregation is favored. When the Ca2+-concentration was further increased to the millimolar range, the band intensity was reduced, presumably because millimolar Ca2+ does not favor formation of MGP-containing protein aggregates (Figure 3C). This Ca2+-dependent, biphasic protein aggregation matches the Ca2+ levels that have been reported for the epididymal luminal microenvironment and range from ∼0.25 to ∼0.9 mM (Jenkins et al., 1980; Turner, 1991; Clulow et al., 1994; Turner, 2002). These results suggest that MGP-containing protein aggregates form in the presence of sub-millimolar Ca2+, but not at millimolar Ca2+ concentrations.
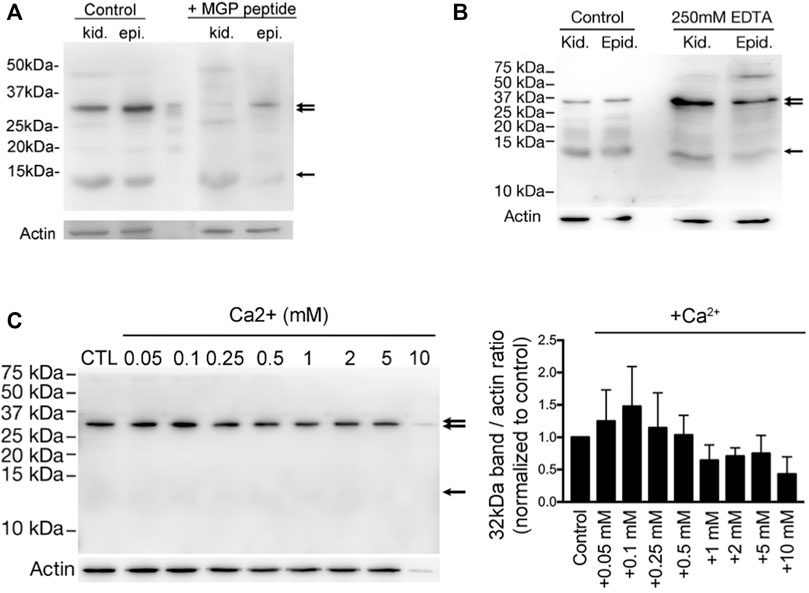
FIGURE 3. Vitamin K-dependent MGP-mediated calcium-promoted aggregation of a protein complex with a prominent band of ∼32-kDa. (A) Western blot detection of anti-MGP in total homogenates of kidney (kid.) and epididymis (epi.) from WT adult rats. A band at ∼12-KDa (arrow) corresponding to the expected molecular size of MGP, and another major band at around ∼32-kDa (double arrow) were detected. Both bands were almost abolished by the preincubation with a ten-fold excess of the MGP immunizing peptide (+MGP peptide). (B) The intensity of ∼32-kDa bands (double arrow) were significantly enriched in the low-Ca2+ condition by addition of 250 mM EDTA to the protein lysates, whereas the ∼12-kDa bands remained unchanged (arrow). Some bands at higher molecular sizes became obvious under the low-Ca2+ condition. (C) The same anti-MGP antibody was used to detect the intensity changes of ∼32-kDa band in DC2 cell protein lysates under various Ca2+ concentrations and the bar graph on the right shows the intensity of the band normalized to control (no additional Ca2+). This suggests that MGP-mediated protein-aggregation is dependent on sub-millimolar amount of Ca2+, whereas excessive Ca2+ (>0.25 mM) inhibits protein-aggregation. (Originally published in iScience (Ma et al., 2019), with permission to reproduce from iScience).
Unique Properties of Matrix Gla Protein for Ca2+ Chelation Facilitate Its Scavenging Function in the Epididymal Lumen
The formation of the MGP-binding protein complex can be interpreted from the biochemical nature of the MGP protein itself (Figure 4). The inactive form of uncarboxylated MGP (ucMGP) protein has an isoelectric point of about 8.8, so that in the epididymal microenvironment of around pH 6.4–6.8 (Levine and Marsh, 1971; Da Silva et al., 2007), the five γ-glutamate Glu residues of ucMGP endow the protein with five positive charges and thus hydrophilic. When these Glu sites are carboxylated by GGCX, the protein becomes its active Gla form of carboxylated MGP (cMGP), in which the positive charges are neutralized, so cMPG is electrically neutral and hydrophobic. However, the carboxylated sites have strong Ca2+ binding ability, which permit cMGP to bind five Ca2+ ions (Hackeng et al., 2001; Huang et al., 2003; Schurgers et al., 2005; Boraldi et al., 2013), and thus carrying ten positive charges, greatly enhancing its solubility. Therefore, in the acidic epididymal lumen, inactive ucMGP is soluble but lacks Ca2+ binding ability, whereas the active cMGP is insoluble but highly adherent to Ca2+, which confers the aggregating ability. In other words, Ca2+ ions could promote Ca2+-MGP scavenging function. Ca2+-MGP chelator function could be a perfect scavenger for the aggregation of either organic or inorganic extracellular matter or both, and regulated by the changing luminal microenvironment, including nutrients supply, acid-base balance and luminal Ca2+ levels, as well as other constituents in epididymal fluid.
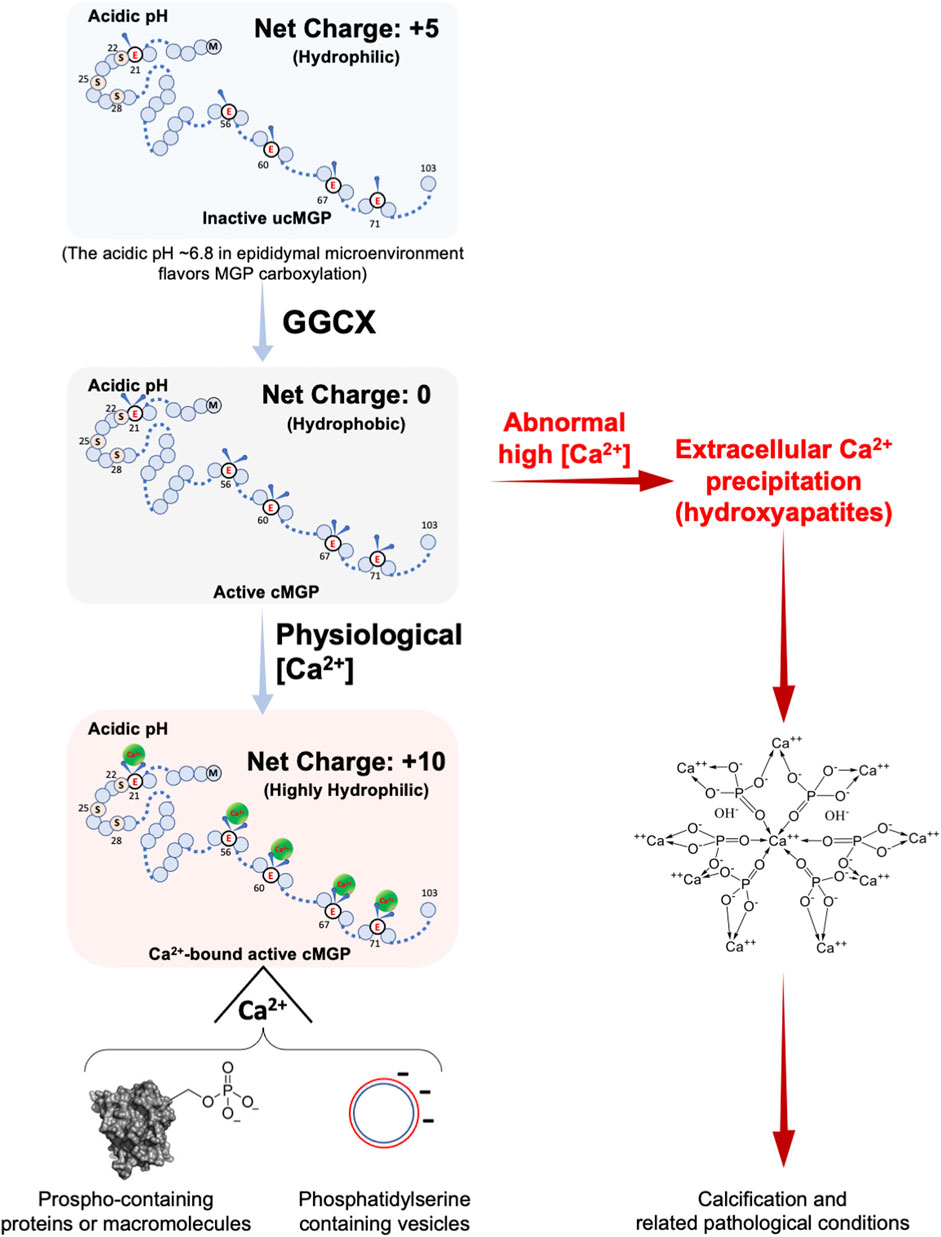
FIGURE 4. Calcium-dependent chelation property of MGP with or without carboxylation. MGP is a protein composed of 103 amino acids and can be post-translationally modified by carboxylation and phosphorylation. Uncarboxylated MGP (ucMGP) is an inactive form with an isoelectric point of around 9 (8.8 for rat and 9.6 for human MGP), which means that in an acidic environment, the ucMGP has a net positive charge of five, despite its five γ-glutamate residues, and thus it is hydrophilic. When the five γ-glutamate residues are carboxylated to γ-carboxylglutamates by GGCX enzymatic activity, the carboxylated MGP (cMGP) becomes active but is electrically neutral and thus hydrophobic. Intriguingly, the five carboxylated γ-glutamate residues of cMGP bear five negative charges, which favors the chelation of five Ca2+ ions and form a highly hydrophilic ten-positively-charged cMGP-Ca2+ compound, facilitating chelation of negative-moiety-bearing molecules under normal physiological conditions, particularly aggregation of organic or inorganic phosphates, or the membrane-embedded phosphatidylserine such as membranes of extracellular vesicles. Dysregulation of the Ca2+-binding property of cMGP results in ectopic Ca2+ precipitation and abnormal Ca2+ content, which causes cMGP to bind with hydroxylapatites and thereby precipitation nucleation, a precursor status of promoting matrix calcification and related pathological conditions.
The epididymal luminal microenvironment has a complex composition and contains components that can interact with MGP other than Ca2+, such as Mg2+ and phosphorus-containing electrolytes (see Table 1). EDTA chelates divalent cations and its affinity for Mg2+ is higher than for Ca2+. The increased intensity of the MGP-positive protein aggregate bands after the addition of exogeneous EDTA to protein lysates (see Figure 3B) can be explained by preferential chelation of Mg2+-ions and promotion of MGP interaction with Ca2+ and subsequent protein aggregate formation (Ma et al., 2019). This hypothesis is consistent with the finding that Mg2+ and Ca2+ competitively bind to MGP (Roy and Nishimoto, 2002). This also indicates that under the low Ca2+ concentrations of the epididymal lumen, activated MGP preferentially binds to Ca2+ and other organic matter, as illustrated in Figure 4. In the presence of high Ca2+, MGP has an affinity for inorganic phosphorous compounds (Roy and Nishimoto, 2002), but this would not occur in the epididymal lumen–the microenvironment for the maturation of spermatozoa whose membrane surfaces are enriched with phosphorates in lipids and proteins (Hamamah and Gatti, 1998; Jenkins et al., 1980). Under conditions with abnormally high Ca2+ (see Figure 4), such as impaired GGCX activity after warfarin treatment, Ca2+ precipitated granules result (Ma et al., 2019) and other pathological conditions may occur (Tan et al., 2012). A related phenomenon may be the clinical observations that patients undergoing continuous hemodialysis have a higher incidence of epididymal stones (Guvel et al., 2004; Bozzini et al., 2013), although the correlation and mechanism between these two phenomena are still unknown.
Insights Into the Mechanism Linking the GGCX arginine325 to glutamine325 (R325Q) rs699664 Polymorphism With the Increased Infertility Risk in Men
As illustrated in Figure 5A, GGCX requires the reduced vitamin KH2 (VKH2) as an obligatory cofactor and its carboxylating activity. Vitamin K generally undergoes a pathway called the vitamin K cycle, in which vitamin K is reduced to VKH2 by vitamin K epoxide reductase (VKOR or VKORC1); VKH2 is then re-oxidized during γ-glutamyl carboxylation by GGCX to vitamin K epoxide (VKO), which is subsequently converted to VKH2 by VKORC1 to complete the VK cycle (Stafford, 2005; Rishavy and Berkner, 2012; Tie and Stafford, 2016).
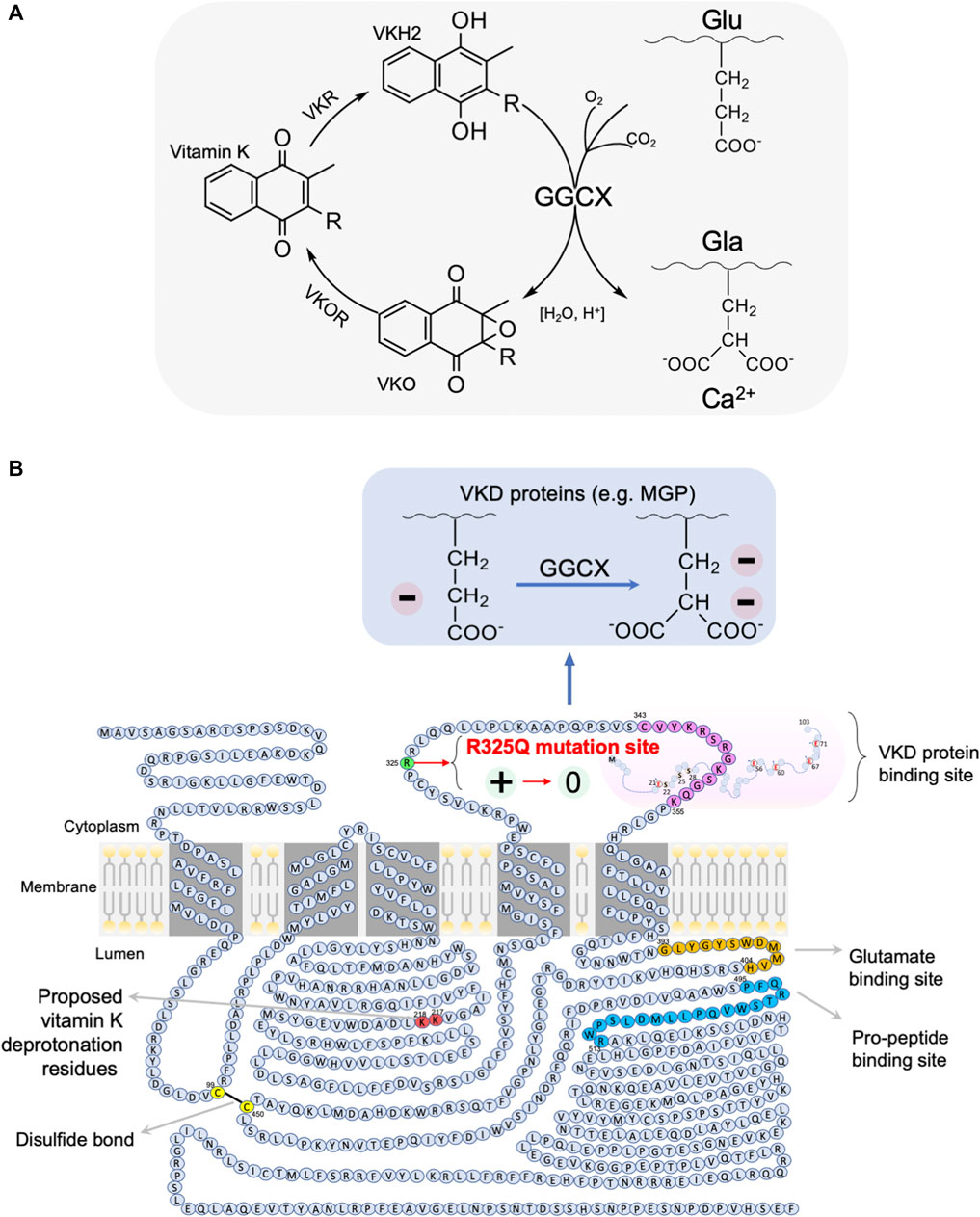
FIGURE 5. A mechanistic model for the association of the GGCX arginine325 to glutamine325 (R325Q) SNP with asthenozoospermia infertility risk in men. (A) Vitamin K cycle: during vitamin K dependent (VKD) carboxylation, the carboxylase GGCX mediates the pH-dependent conversion of glutamate (Glu) residues to Ca2+-binding gamma-carboxyglutamate (Gla) residues of the vitamin K-dependent substrates (e.g., MGP) or their pro-peptides, with CO2, O2 and reduced vitamin K as the co-substrates. The carboxylation reaction also oxidises the reduced vitamin K-H2 to vitamin K epoxide (VKO), which is then reduced back to vitamin K-H2 by vitamin K epoxide reductase (VKOR). The cycle involves redox- and pH-sensitive carboxylation and oxygenation processes (Rishavy and Berkner, 2012). (B) GGCX is a membrane protein with a size of ∼87.5 kDa, which has two intracellular loops and two luminal loops of amino acid sequences, in addition to its cytoplasmic N-terminus and a long luminal C-terminus. The binding sites for glutamate and pro-peptide are located on the long C-terminal tail, which can form a disulfide bond with the first luminal loop of amino acids. It has been hypothesized that all five Glu residues of MGP are carboxylated in one round in sequential order by GGCX, presumably due to MGP stabilisation by the singly positively charged arginine325 of GGCX (Rishavy and Berkner, 2012). In our proposed model, the rs699664 SNP-mediated change of arginine325 to a neutral glutamine325 moiety in the GGCX leads to the loss of GGCX binding stability to MGP for carboxylation, thus resulting in decreased carboxylation of VKD proteins, and thereby to increased calcium mineralization and Ca2+-mediated proliferation of stress granules, and eventually in a disordered epididymal luminal microenvironment, causing impaired sperm maturation and male infertility. Graphic illustration is adapted from (Tie et al., 2016) with modifications. For more details, see the main text.
GGCX is a membrane protein with a size of approximately 87.5 kDa and has five transmembrane domains, with its N-terminus facing the cytoplasmic side and the C-terminus facing the luminal side, in addition to two intracellular loops and two luminal loops, as illustrated in Figure 5B (Tie et al., 2000; Tie et al., 2003; Rishavy and Berkner, 2012; Tie and Stafford, 2016). It is believed that the second intracellular loop contains the binding sequence for the inactive form of MGP (Pudota et al., 2001). On the luminal side, a disulfide bond is formed at position of Cys-99 and Cys-450, which is the essential site for the epoxidation and carboxylation of GGCX (Pudota et al., 2000). This disulfide bond is on the same side as the sequence residues for vitamin K deprotonation (Rishavy et al., 2006) and as the glutamate and pro-peptide binding sites (Mutucumarana et al., 2000; Mutucumarana et al., 2003). On the basis of the nature of this redox-sensitive binding, as well as the epoxidation and deprotonation nature of GGCX, the carboxylation and the other reactions in the vitamin-K cycle are all affected by redox status and acid-base balance (Rishavy and Berkner, 2012). Both of these two physiological factors are known to be essential for sperm maturation and male fertility (Au and Wong, 1980; Carr and Acott, 1984; Breton et al., 1996; Newcombe et al., 2000; Vernet et al., 2004; Pastor-Soler et al., 2005; Aitken and Curry, 2011; Shum et al., 2011; Robaire and Hinton, 2015; Aitken, 2016). It is suspected that the GGCX activity and redox- and pH-sensitive processes in the epididymis reciprocally influence each other.
In our previous study, we identified a single nucleotide polymorphism (SNP), rs699664, in the GGCX gene of infertile men with asthenozoospermia (Ma et al., 2019). This SNP replaces an arginine325 with glutamine325 (Arg325Gln) in the GGCX protein (Kinoshita et al., 2007; Rieder et al., 2007). The same SNP (Arg325Gln) has been reported to be associated with bone mineral density in aged females, and increased GGCX activity for MGP and bone matrix protein osteocalcin (Kinoshita et al., 2007). The SNP is located in the same second intracellular loop of GGCX, in close proximity to where the binding site of vitamin K-dependent proteins (i.e., MGP) is, as illustrated in Figure 5B. It has been reported that all the five Glu residues of MGP are converted into active Gla in one round of stepwise action of GGCX (Rishavy and Berkner, 2012). This process enables MGP to bind Ca2+ ions, Ca2+ crystals and bone morphogenetic proteins (Nadeem et al., 2004; Schurgers et al., 2013).
While further investigation is required, we speculate that the positively charged arginine325 residue of GGCX is responsible for stabilizing the negatively charged Glu residue of MGP during the stepwise carboxylation process, which is promoted in the acidic conditions of the epididymal lumen. Such charge-charge interaction stability is lost as a result of the SNP, in which the charged arginine325 residue is replaced with the neutral glutamine325, thereby changing the binding preference of MGP. Accordingly, the charge-charge interaction stability in the wild-type protein ensures sufficient time for complete carboxylation of each residue in the substrates and avoids their incomplete activation (Rishavy and Berkner, 2012). Supporting this interpretation is the finding that the rs699664 SNP in GGCX results in higher carboxylase enzymatic activity in aged females, and was associated with osteoporosis (Kinoshita et al., 2007). We speculate that the rs699664 SNP has similar effects in the epididymis of asthenozoospermic infertile males, i.e., increases early release of incompletely carboxylated substrate, resulting in under-carboxylated MGP, impaired Ca2+-binding, disturbed Ca2+-homeostasis, and ultimately to impaired sperm maturation and to male infertility.
A Potential Interplay Between Vitamins in Ca2+ Homeostasis in the Epididymis
An interplay of multiple vitamins may be required for optimal Ca2+ homeostasis, including fat- or water-soluble vitamins, as illustrated in Figure 6A. Vitamins B and C are water soluble, whereas other vitamins including vitamins A, D, E and K are all fat-soluble compounds. It has been reported that the systemic action of vitamin D in Ca2+ homeostasis can be antagonized by vitamin A, resulting in higher incidents of osteoporosis in man (Johansson and Melhus, 2001). A synergistic interaction between vitamins D and K in bone and cardiovascular health has been proposed, in which vitamin D promotes the production of vitamin K-dependent proteins, including osteocalcin and MGP (Oury et al., 2013; van Ballegooijen et al., 2017). Consistent with this notion, our studies showed that both the vitamin D-related TRPV6-TMEP16A and the vitamin K-dependent GGCX-mediated carboxylation of MGP pathways participate in epididymal Ca2+ homeostasis in a spatial complementary manner (Gao da et al., 2016; Ma et al., 2019). To elucidate further the role of vitamin K-dependent Ca2+-bound carboxylation-dependent MGP in the epididymal Ca2+, we performed a bioinformatic analysis on our published proteome results (Ma et al., 2019), and we found that the vitamin B6-related signaling was also involved in vitamin K-related Ca2+-dependent MGP-aggregation, as demonstrated in Figure 6B. These results suggest an interplay of multiple vitamin pathways in regulating epididymal Ca2+ homeostasis.
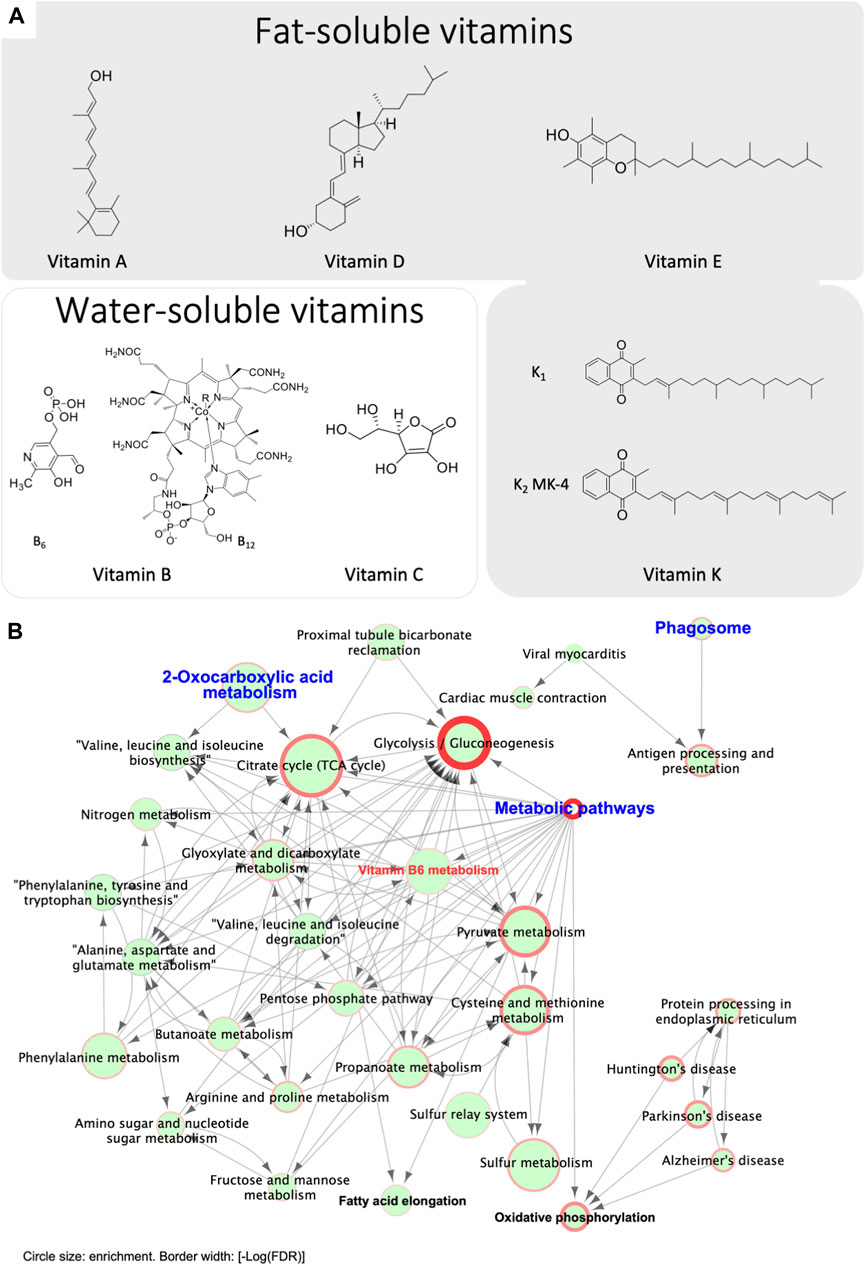
FIGURE 6. Bioinformatic analysis reveals potential involvement of vitamin B6 pathways in regulating epididymal Ca2+ homeostasis and Ca2+-dependent MGP-aggregation. (A) Chemical structures of the key members of various fat-soluble and water-soluble vitamins. (B) Bioinformatic analysis reveals a potential involvement of vitamin B6 in epididymal Ca2+ homeostasis. Presented data were originally published in iScience (Ma et al., 2019) and reanalyzed. Briefly, proteomic analysis the in-gel digested MGP-positive 32-kDa band was performed with LC-MS/MS analysis (n = 3 rats). The MGP-positive 32-kDa band in whole epididymis protein lysates underwent proteomic analysis followed by GO and KEGG-based bioinformatic analysis and identified 301 genes present in all 3 lysates. 2-oxocarboxylic acid metabolism is one of the primary enriched gene clusters, consistent with MGP carboxylation. Other key enriched clusters include phagosome and its downstream pathway of antigen processing and presentation, proximal tubule bicarbonate reclamation, and metabolic pathways. The cluster of metabolic pathways mainly contains sub-clusters for catabolic pathways, including glycolysis and gluconeogenesis, TCA cycle and pyruvate metabolism, in addition to a vitamin-B6 metabolic pathway, suggesting the involvement of vitamin-B6 in the metabolism of Ca2+-MGP aggregates. The end products of most of these pathways are substrates for oxidative phosphorylation and fatty acid elongation. These enriched pathways are in consistent with the notion of that chelation activity of carboxylated Ca2+-bound MGP contributes to the scavenging of extracellular metabolites, and Ca2+ serves as a cofactor in this process. Circle size indicates enrichment degree of each pathway, and border width represents the value of statistical significance of the enriched gene clusters [-Log (False Discovery Rate, FDR)].
On the basis of the pathway network analysis in Figure 6B, we found that 2-oxocarboxylic acid metabolism as one of the primary enriched gene clusters, consistent with the association of a carboxylation-dependent function of Ca2+-bound MGP. In addition, we found that phagosome and antigen processing and presentation as the other key enriched pathways, which were separated from the main primary cluster of metabolic pathways. This may suggest that bound MGP-dependent Ca2+-chelation participates in immunological function in the epididymis, which is essential for sperm function (Stammler et al., 2015; Fijak et al., 2018; Voisin et al., 2019). The downstream of metabolic pathways identified in the network analysis are mainly the catabolic pathways, including glycolysis and gluconeogenesis, TCA cycle and pyruvate metabolism. Interestingly, a vitamin B6 metabolic pathway is also one of the identified downstream metabolic pathways, suggesting the involvement of B6 in the metabolism of Ca2+-MGP aggregates. The metabolite products of the identified pathways are substrates for oxidative phosphorylation and fatty acid elongation. These results suggested that Ca2+-bound MGP aggregates are involved in lipid generation, together with the carbohydrate-related catabolism. In view of the maturing spermatozoa in the epididymal lumen, and the various vitamin-dependent pathways in Ca2+-homeostatic regulation, we hypothesized that there is an interplay of different vitamins in the regulation of Ca2+ homeostasis in the epididymis, and that the biphasic chelation activity of carboxylated MGP contributes to scavenging of extracellular metabolites, whereas Ca2+ serves as the cofactor in this process.
It is known that vitamin K is a key modulator of Ca2+ homeostasis in maintaining bone health and for preventing vascular ectopic calcification (Weber, 2001; Wallin et al., 2008; Schurgers et al., 2013), which may also influence male fertility systemically (Karsenty, 2011; Oury et al., 2011; Oury et al., 2013). Since the epididymis is indispensable for sperm maturation, and sperm maturation requires precise regulation Ca2+ signaling (Turner, 2002; Weissgerber et al., 2011; Weissgerber et al., 2012; Dacheux and Dacheux, 2014; Robaire and Hinton, 2015), the elucidation of Ca2+ homeostasis mechanisms in the epididymis should provide insights into the causes of epididymal-dependent sperm dysfunction and male infertility.
While further investigations are required for a full picture of the interplay between the vitamin signaling pathways, all pathways are connected to Ca2+ homeostasis, particularly extracellular Ca2+ homeostasis in epididymal fluid. Therefore, low Ca2+ is a key factor in regulation of sperm maturation and in male reproductive health. Taken together, the evidence presented here indicates that dysregulated Ca2+ homeostasis due to defective expression of Ca2+-binding proteins, abnormal Ca2+ signaling, or different kinds of vitamin deficiency in the male genital tract may negatively affect male reproductive health.
Future Perspectives and Conclusion
Accumulating evidence has indicated the important role of epididymal function in male fertility outcomes, and this function depends to a large degree on the congenial luminal microenvironment established by the epithelial cells lining within the excurrent duct. Important aspects of the luminal microenvironment include Ca2+homeostasis of acidity, both of which are strictly regulated by a network of cell-cell interactions and signaling pathways, as depicted in Figure 7. We have provided evidence for the potential role of luminal Ca2+ in functioning as a cofactor for the GGCX-dependent carboxylation of MGP, which in turn acts as a scavenger for extracellular metabolites. In addition, we discussed potential interactions of vitamins in these pathways, which are essential for sperm maturation and male reproductive health. This raises the possibility that these multi-vitamin-dependent Ca2+ homeostatic-dependent pathways could be leveraged for novel interventions aimed at treating and preventing sperm dysfunctions and male reproductive defecits.
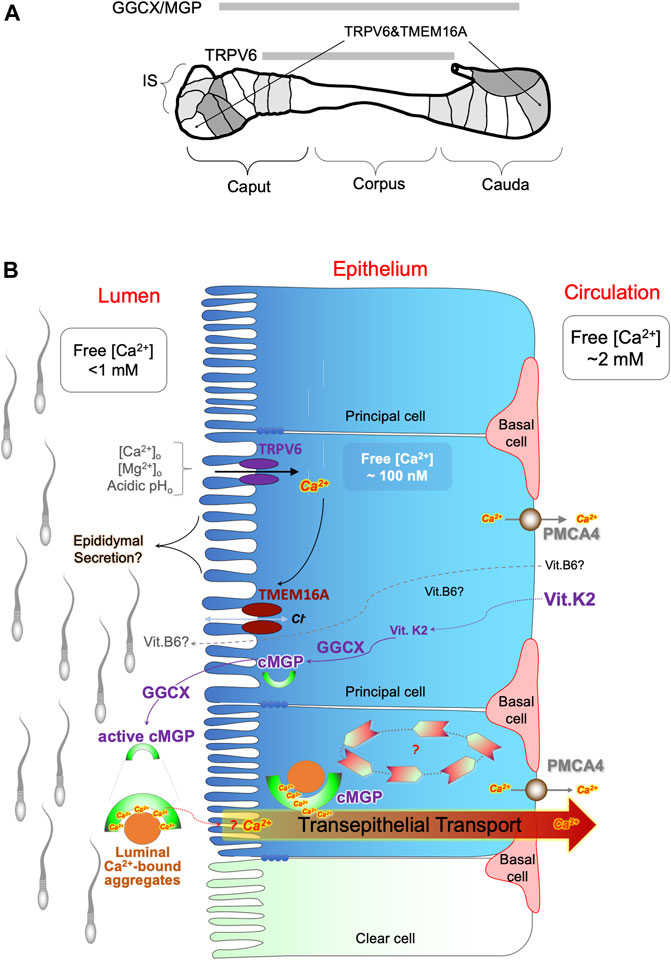
FIGURE 7. Potential involvement of multiple vitamin pathways in regulation of epididymal Ca2+ homeostasis. (A) Schematic illustration for the regional expression patterns of the key players regulating Ca2+ homeostasis in the rat epididymis, including epithelial Ca2+ channel TRPV6, Ca2+-dependent chloride channel TMEM16A, γ-carboxylase GGCX and its substrate MGP. IS: initial segment; caput, corpus and cauda indicate head, body and tail region of the epididymis. Spermatozoa mainly mature in the IS and caput epididymidis regions and are stored in the whereas cauda epididymidal region in a dormant stage. (B) Schematic diagram of the hypothetical model of Ca2+ homeostatic regulation in the epididymal luminal microenvironment during sperm maturation. It involves vitamin D-associated electrical coupling of TRPV6 and TMEM16A channels and vitamin K2-dependent GGCX carboxylation of MGP for luminal Ca2+ modulation and protein aggregation. The TRPV6-TMEM16A electrical coupler is involved in fluid transport in a manner of sensitive to extracellular Ca2+ and pH. The GGCX-dependent MGP carboxylation plays a role in a spatially complementary manner in the epididymis to promote Ca2+-facilitated protein aggregation, thereby maintaining Ca2+-homeostasis and pathological calcifications. The potential role of vitamin-B6 in regulating the epididymal luminal microenvironment is also depicted in the proposed model. Ca2+-bound MGP-containing protein aggregates in the epididymal lumen act as scavengers in which Ca2+ is an essential cofactor for the chelation of Ca2+-precipitable metabolites in the extracellular matrix, such as epididymal secretory proteins and shedded remnants from spermatozoa.
Author Contributions
WS conceived of the presented idea and took the lead in writing the manuscript. BZ provided critical feedback and helped the writing of the manuscript. ASC, XZ, SMS, ZYZ, and LYG contributed to the graphics and result analysis. All authors discussed the presented idea and contributed to the final manuscript.
Funding
This work is supported by NNSFC grants (31871166; 82071704), Science and Technology Commission of Shanghai Municipality grant (19140903400), and the startup of ShanghaiTech University to WWS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, A., Baskaran, S., Parekh, N., Cho, C.-L., Henkel, R., Vij, S., et al. (2021). Male Infertility. The Lancet 397, 319–333. doi:10.1016/s0140-6736(20)32667-2
Aitken, R. J., and Curry, B. J. (2011). Redox Regulation of Human Sperm Function: from the Physiological Control of Sperm Capacitation to the Etiology of Infertility and DNA Damage in the Germ Line. Antioxid. Redox Signaling 14, 367–381. doi:10.1089/ars.2010.3186
Aitken, R. J., and Nixon, B. (2013). Sperm Capacitation: a Distant Landscape Glimpsed but Unexplored. Mol. Hum. Reprod. 19, 785–793. doi:10.1093/molehr/gat067
Aitken, R. J. (2016). Oxidative Stress and the Etiology of Male Infertility. J. Assist Reprod. Genet. 33, 1691–1692. doi:10.1007/s10815-016-0791-4
Amann, R. P., and Howards, S. S. (1980). Daily Spermatozoal Production and Epididymal Spermatozoal Reserves of the Human Male. J. Urol. 124, 211–215. doi:10.1016/s0022-5347(17)55377-x
Andrukhova, O., Streicher, C., Zeitz, U., and Erben, R. G. (2016). Fgf23 and Parathyroid Hormone Signaling Interact in Kidney and Bone. Mol. Cell Endocrinol. 436, 224–239. doi:10.1016/j.mce.2016.07.035
Au, C. L., and Wong, P. Y. D. (1980). Luminal Acidification by the Perfused Rat Cauda Epididymidis. J. Physiol. 309, 419–427. doi:10.1113/jphysiol.1980.sp013517
Bagnis, C., Marsolais, M., Biemesderfer, D., Laprade, R., and Breton, S. (2001). Na+/H+-exchange Activity and Immunolocalization of NHE3 in Rat Epididymis. Am. J. Physiology-Renal Physiol. 280, F426–F436. doi:10.1152/ajprenal.2001.280.3.f426
Bagur, R., and Hajnóczky, G. (2017). Intracellular Ca 2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 66, 780–788. doi:10.1016/j.molcel.2017.05.028
Barak, S., and Baker, H. W. G. (2000). Clinical Management of Male Infertility. South Dartmouth (MA).
Battistone, M. A., Mendelsohn, A. C., Spallanzani, R. G., Brown, D., Nair, A. V., and Breton, S. (2020). Region-specific Transcriptomic and Functional Signatures of Mononuclear Phagocytes in the Epididymis. Mol. Hum. Reprod. 26, 14–29. doi:10.1093/molehr/gaz059
Battistone, M. A., Merkulova, M., Park, Y. J., Peralta, M. A., Gombar, F., Brown, D., et al. (2019). Unravelling Purinergic Regulation in the Epididymis: Activation of V‐ATPase‐dependent Acidification by Luminal ATP and Adenosine. J. Physiol. 597, 1957–1973. doi:10.1113/jp277565
Belleannée, C. (2015). Extracellular microRNAs from the Epididymis as Potential Mediators of Cell-To-Cell Communication. Asian J. Androl. 17, 730–736. doi:10.4103/1008-682X.155532
Benedetto, R., Cabrita, I., Schreiber, R., and Kunzelmann, K. (2019). TMEM16A Is Indispensable for Basal Mucus Secretion in Airways and Intestine. FASEB j. 33, 4502–4512. doi:10.1096/fj.201801333rrr
Björkgren, I., Gylling, H., Turunen, H., Huhtaniemi, I., Strauss, L., Poutanen, M., et al. (2015). Imbalanced Lipid Homeostasis in the Conditional Dicer1 Knockout Mouse Epididymis Causes Instability of the Sperm Membrane. FASEB j. 29, 433–442. doi:10.1096/fj.14-259382
Blomberg Jensen, M., Bjerrum, P. J., Jessen, T. E., Nielsen, J. E., Joensen, U. N., Olesen, I. A., et al. (2011). Vitamin D Is Positively Associated with Sperm Motility and Increases Intracellular Calcium in Human Spermatozoa. Hum. Reprod. 26, 1307–1317. doi:10.1093/humrep/der059
Blomberg Jensen, M., Nielsen, J. E., Jorgensen, A., Rajpert-De Meyts, E., Kristensen, D. M., Jorgensen, N., et al. (2010). Vitamin D Receptor and Vitamin D Metabolizing Enzymes Are Expressed in the Human Male Reproductive Tract. Hum. Reprod. 25, 1303–1311. doi:10.1093/humrep/deq024
Bödding, M. (2005). Voltage-dependent Changes of TRPV6-Mediated Ca2+ Currents. J. Biol. Chem. 280, 7022–7029. doi:10.1074/jbc.m410184200
Boisen, I. M., Nielsen, J. E., Verlinden, L., Lorenzen, M., Holt, R., Pinborg, A., et al. (2021). Calcium Transport in Male Reproduction Is Possibly Influenced by Vitamin D and CaSR. J. Endocrinol. 251, 207–222. doi:10.1530/joe-20-0321
Boraldi, F., Garcia-Fernandez, M., Paolinelli-Devincenzi, C., Annovi, G., Schurgers, L., Vermeer, C., et al. (2013). Ectopic Calcification in β-thalassemia Patients Is Associated with Increased Oxidative Stress and Lower MGP Carboxylation. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1832, 2077–2084. doi:10.1016/j.bbadis.2013.07.017
Boucheron-Houston, C., Canterel-Thouennon, L., Lee, T.-L., Baxendale, V., Nagrani, S., Chan, W.-Y., et al. (2013). Long-term Vitamin A Deficiency Induces Alteration of Adult Mouse Spermatogenesis and Spermatogonial Differentiation: Direct Effect on Spermatogonial Gene Expression and Indirect Effects via Somatic Cells. J. Nutr. Biochem. 24, 1123–1135. doi:10.1016/j.jnutbio.2012.08.013
Bozzini, G., Lunelli, L., Berlingheri, M., Groppali, E., and Carmignani, L. (2013). Epididymis Microlithiasis and Semen Abnormalities in Young Adult Kidney Transplant Recipients. Andrologia 45, 357–360. doi:10.1111/and.12036
Brandenburger, T., Strehler, E. E., Filoteo, A. G., Caride, A. J., Aumüller, G., Post, H., et al. (2011). Switch of PMCA4 Splice Variants in Bovine Epididymis Results in Altered Isoform Expression during Functional Sperm Maturation. J. Biol. Chem. 286, 7938–7946. doi:10.1074/jbc.m110.142836
Breitbart, H. (2002). Intracellular Calcium Regulation in Sperm Capacitation and Acrosomal Reaction. Mol. Cell Endocrinol. 187, 139–144. doi:10.1016/s0303-7207(01)00704-3
Breitwieser, G. E. (2008). Extracellular Calcium as an Integrator of Tissue Function. Int. J. Biochem. Cell Biol. 40, 1467–1480. doi:10.1016/j.biocel.2008.01.019
Breton, S., Nair, A. V., and Battistone, M. A. (2019). Epithelial Dynamics in the Epididymis: Role in the Maturation, protection, and Storage of Spermatozoa. Andrology 7, 631–643. doi:10.1111/andr.12632
Breton, S., and Brown, D. (2013). Regulation of Luminal Acidification by the V-ATPase. Physiology 28, 318–329. doi:10.1152/physiol.00007.2013
Breton, S., Hammar, K., Smith, P. J. S., and Brown, D. (1998). Proton Secretion in the Male Reproductive Tract: Involvement of Cl−-independent HCO 3 − Transport. Am. J. Physiology-Cell Physiol. 275, C1134–C1142. doi:10.1152/ajpcell.1998.275.4.c1134
Breton, S., Smith, P. J. S., Lui, B., and Brown, D. (1996). Acidification of the Male Reproductive Tract by a Proton pumping(H+)-ATPase. Nat. Med. 2, 470–472. doi:10.1038/nm0496-470
Brucker, C., and Lipford, G. B. (1995). The Human Sperm Acrosome Reaction: Physiology and Regulatory Mechanisms. An Update. Hum. Reprod. Update 1, 51–62. doi:10.1093/humupd/1.1.51
Caflisch, C. R. (1992). Acidification of Testicular and Epididymal Fluids in the Rat after Surgically-Induced Varicocele. Int. J. Androl. 15, 238–245. doi:10.1111/j.1365-2605.1992.tb01344.x
Caflisch, C. R., and DuBose, T. D. (1990). Direct Evaluation of Acidification by Rat Testis and Epididymis: Role of Carbonic Anhydrase. Am. J. Physiology-Endocrinology Metab. 258, E143–E150. doi:10.1152/ajpendo.1990.258.1.e143
Carlson, A. E., Hille, B., and Babcock, D. F. (2007). External Ca2+ Acts Upstream of Adenylyl Cyclase SACY in the Bicarbonate Signaled Activation of Sperm Motility. Developmental Biol. 312, 183–192. doi:10.1016/j.ydbio.2007.09.017
Carlson, A. E., Westenbroek, R. E., Quill, T., Ren, D., Clapham, D. E., Hille, B., et al. (2003). CatSper1 Required for Evoked Ca2+ Entry and Control of Flagellar Function in Sperm. Proc. Natl. Acad. Sci. 100, 14864–14868. doi:10.1073/pnas.2536658100
Carr, D. W., and Acott, T. S. (1984). Inhibition of Bovine Spermatozoa by Caudal Epididymal Fluid: I. Studies of a Sperm Motility Quiescence Factor 1. Biol. Reprod. 30, 913–925. doi:10.1095/biolreprod30.4.913
Chan, J. C., Morgan, C. P., Adrian Leu, N., Shetty, A., Cisse, Y. M., Nugent, B. M., et al. (2020). Reproductive Tract Extracellular Vesicles Are Sufficient to Transmit Intergenerational Stress and Program Neurodevelopment. Nat. Commun. 11, 1499. doi:10.1038/s41467-020-15305-w
Chen, Q., Yan, M., Cao, Z., Li, X., Zhang, Y., Shi, J., et al. (2016a). Sperm tsRNAs Contribute to Intergenerational Inheritance of an Acquired Metabolic Disorder. Science 351, 397–400. doi:10.1126/science.aad7977
Chen, Q., Yan, W., and Duan, E. (2016b). Epigenetic Inheritance of Acquired Traits through Sperm RNAs and Sperm RNA Modifications. Nat. Rev. Genet. 17, 733–743. doi:10.1038/nrg.2016.106
Cheung, K.-h., Leung, G. P. H., Leung, M. C. T., Shum, W. W. C., Zhou, W.-l., and Wong, P. Y. D. (2005). Cell-cell Interaction Underlies Formation of Fluid in the Male Reproductive Tract of the Rat. J. Gen. Physiol. 125, 443–454. doi:10.1085/jgp.200409205
Cheung, K. H., Leung, C. T., Leung, G. P. H., and Wong, P. Y. D. (2003). Synergistic Effects of Cystic Fibrosis Transmembrane Conductance Regulator and Aquaporin-9 in the Rat Epididymis1. Biol. Reprod. 68, 1505–1510. doi:10.1095/biolreprod.102.010017
Cheung, Y. M., Hwang, J. C., and Wong, P. Y. (1976). Epithelial Membrane Potentials of the Epididymis in Rats [proceedings]. J. Physiol. 263, 280P.
Chu, C., Yu, L., Wu, B., Ma, L., Gou, L.-T., He, M., et al. (2017). A Sequence of 28S rRNA-Derived Small RNAs Is Enriched in Mature Sperm and Various Somatic Tissues and Possibly Associates with Inflammation. J. Mol. Cell Biol 9, 256–259. doi:10.1093/jmcb/mjx016
Clulow, J., Jones, R. C., Hansen, L. A., and Man, S. Y. (1998). Fluid and Electrolyte Reabsorption in the Ductuli Efferentes Testis. J. Reprod. Fertil. Suppl. 53, 1–14.
Clulow, J., and Jones, R. C. (2004). Composition of Luminal Fluid Secreted by the Seminiferous Tubules and after Reabsorption by the Extratesticular Ducts of the Japanese Quail, Coturnix coturnix Japonica1. Biol. Reprod. 71, 1508–1516. doi:10.1095/biolreprod.104.031401
Clulow, J., Jones, R., and Hansen, L. (1994). Micropuncture and Cannulation Studies of Fluid Composition and Transport in the Ductuli Efferentes Testis of the Rat: Comparisons with the Homologous Metanephric Proximal Tubule. Exp. Physiol. 79, 915–928. doi:10.1113/expphysiol.1994.sp003817
Conine, C. C., Sun, F., Song, L., Rivera-Pérez, J. A., and Rando, O. J. (2019). MicroRNAs Absent in Caput Sperm Are Required for Normal Embryonic Development. Developmental Cell 50, 7–8. doi:10.1016/j.devcel.2019.06.007
Conine, C. C., Sun, F., Song, L., Rivera-Pérez, J. A., and Rando, O. J. (2018). Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice. Developmental Cell 46, 470–480. doi:10.1016/j.devcel.2018.06.024
Cooper, T. G., Raczek, S., Yeung, C. H., Schwab, E., Schulze, H., and Hertle, L. (1992). Composition of Fluids Obtained from Human Epididymal Cysts. Urol. Res. 20, 275–280. doi:10.1007/bf00300258
Cornwall, G. A. (2009). New Insights into Epididymal Biology and Function. Hum. Reprod. Update 15, 213–227. doi:10.1093/humupd/dmn055
Cornwall, G. A., Do, H. Q., Hewetson, A., Muthusubramanian, A., and Myers, C. (2019). The Epididymal Amyloid Matrix: Structure and Putative Functions. Andrology 7, 603–609. doi:10.1111/andr.12586
Correia, S., Oliveira, P. F., Guerreiro, P. M., Lopes, G., Alves, M. G., Canario, A. V. M., et al. (2013). Sperm Parameters and Epididymis Function in Transgenic Rats Overexpressing the Ca2+-Binding Protein Regucalcin: a Hidden Role for Ca2+ in Sperm Maturation? Mol. Hum. Reprod. 19, 581–589. doi:10.1093/molehr/gat030
Cranenburg, E. C. M., Schurgers, L. J., Uiterwijk, H. H., Beulens, J. W. J., Dalmeijer, G. W., Westerhuis, R., et al. (2012). Vitamin K Intake and Status Are Low in Hemodialysis Patients. Kidney Int. 82, 605–610. doi:10.1038/ki.2012.191
Da Silva, N., Cortez-Retamozo, V., Reinecker, H.-C., Wildgruber, M., Hill, E., Brown, D., et al. (2011). A Dense Network of Dendritic Cells Populates the Murine Epididymis. Reproduction 141, 653–663. doi:10.1530/rep-10-0493
Da Silva, N., Piétrement, C., Brown, D., and Breton, S. (2006). Segmental and Cellular Expression of Aquaporins in the Male Excurrent Duct. Biochim. Biophys. Acta (Bba) - Biomembranes 1758, 1025–1033. doi:10.1016/j.bbamem.2006.06.026
Da Silva, N., Shum, W. W. C., El-Annan, J., Păunescu, T. G., McKee, M., Smith, P. J. S., et al. (2007). Relocalization of the V-ATPase B2 Subunit to the Apical Membrane of Epididymal clear Cells of Mice Deficient in the B1 Subunit. Am. J. Physiology-Cell Physiol. 293, C199–C210. doi:10.1152/ajpcell.00596.2006
Dacheux, J.-L., and Dacheux, F. (2014). New Insights into Epididymal Function in Relation to Sperm Maturation. Reproduction 147, R27–R42. doi:10.1530/rep-13-0420
Dawson, E. B., Harris, W. A., Teter, M. C., and Powell, L. C. (1992). Effect of Ascorbic Acid Supplementation on the Sperm Quality of smokers**Supported by Hoffman-La Roche, Inc., Nutley, New Jersey.††Presented at the 47th Annual Meeting of the American Fertility Society, Orlando, Florida, October 21 to 24, 1991. Fertil. Sterility 58, 1034–1039. doi:10.1016/s0015-0282(16)55456-9
de Lima, A. O., Afonso, J., Edson, J., Marcellin, E., Palfreyman, R., Porto-Neto, L. R., et al. (2021). Network Analyses Predict Small RNAs that Might Modulate Gene Expression in the Testis and Epididymis of Bos indicus Bulls. Front. Genet. 12, 610116. doi:10.3389/fgene.2021.610116
Dubé, E., and Cyr, D. G. (2012). The Blood-Epididymis Barrier and Human Male Fertility. Adv. Exp. Med. Biol. 763, 218–236. doi:10.1007/978-1-4614-4711-5_11
Ecroyd, H., Asquith, K. L., Jones, R. C., and Aitken, R. J. (2004a). The Development of Signal Transduction Pathways during Epididymal Maturation Is Calcium Dependent. Developmental Biol. 268, 53–63. doi:10.1016/j.ydbio.2003.12.015
Ecroyd, H., Belghazi, M., Dacheux, J.-L., and Gatti, J.-L. (2005). The Epididymal Soluble Prion Protein Forms a High-Molecular-Mass Complex in Association with Hydrophobic Proteins. Biochem. J. 392, 211–219. doi:10.1042/bj20050459
Ecroyd, H., Sarradin, P., Dacheux, J.-L., and Gatti, J.-L. (2004b). Compartmentalization of Prion Isoforms within the Reproductive Tract of the Ram1. Biol. Reprod. 71, 993–1001. doi:10.1095/biolreprod.104.029801
Esteves, S. (2013). A Clinical Appraisal of the Genetic Basis in Unexplained Male Infertility. J. Hum. Reprod. Sci. 6, 176–182. doi:10.4103/0974-1208.121419
Fijak, M., Pilatz, A., Hedger, M. P., Nicolas, N., Bhushan, S., Michel, V., et al. (2018). Infectious, Inflammatory and 'autoimmune' Male Factor Infertility: How Do Rodent Models Inform Clinical Practice? Hum. Reprod. Update 24, 416–441. doi:10.1093/humupd/dmy009
Finger, J. N., Jelinsky, S. A., Bang, H. J., Wilson, E., Kopf, G. S., Turner, T. T., et al. (2006). The Rat Epididymal Transcriptome: Transcriptional Profiling of Segmental Gene Expression in the Rat Epididymis. J. Androl., 62. doi:10.1095/biolreprod.105.039719
Fraser, J. D., and Price, P. A. (1988). Lung, Heart, and Kidney Express High Levels of mRNA for the Vitamin K-dependent Matrix Gla Protein. Implications for the Possible Functions of Matrix Gla Protein and for the Tissue Distribution of the Gamma-Carboxylase. J. Biol. Chem. 263, 11033–11036. doi:10.1016/s0021-9258(18)37912-2
Frizzell, R. A., and Hanrahan, J. W. (2012). Physiology of Epithelial Chloride and Fluid Secretion. Cold Spring Harbor Perspect. Med. 2, a009563. doi:10.1101/cshperspect.a009563
Gao, D. Y., Zhang, B. L., Leung, M. C. T., Au, S. C. L., Wong, P. Y. D., and Shum, W. W. C. (2016). Coupling of TRPV6 and TMEM16A in Epithelial Principal Cells of the Rat Epididymis. J. Gen. Physiol. 148, 161–182. doi:10.1085/jgp.201611626
Garcia-Castillo, M. D., Chinnapen, D. J., and Lencer, W. I. (2017). Membrane Transport across Polarized Epithelia. Cold Spring Harb Perspect. Biol. 9. doi:10.1101/cshperspect.a027912
Gatti, J.-L., Druart, X., Guérin, Y., Dacheux, F., and Dacheux, J.-L. (1999). A 105- to 94-Kilodalton Protein in the Epididymal Fluids of Domestic Mammals Is Angiotensin I-Converting Enzyme (ACE); Evidence that Sperm Are the Source of This ACE1. Biol. Reprod. 60, 937–945. doi:10.1095/biolreprod60.4.937
Gervasi, M. G., and Visconti, P. E. (2016). Chang's Meaning of Capacitation: A Molecular Perspective. Mol. Reprod. Dev. 83, 860–874. doi:10.1002/mrd.22663
Gervasi, M. G., and Visconti, P. E. (2017). Molecular Changes and Signaling Events Occurring in Spermatozoa during Epididymal Maturation. Andrology 5, 204–218. doi:10.1111/andr.12320
Greco, E., Iacobelli, M., Rienzi, L., Ubaldi, F., Ferrero, S., and Tesarik, J. (2005). Reduction of the Incidence of Sperm DNA Fragmentation by Oral Antioxidant Treatment. J. Androl. 26, 349–353. doi:10.2164/jandrol.04146
Gregory, M., and Cyr, D. G. (2014). The Blood-Epididymis Barrier and Inflammation. Spermatogenesis 4, e979619. doi:10.4161/21565562.2014.979619
Guvel, S., Pourbagher, M. A., Torun, D., Egilmez, T., Pourbagher, A., and Ozkardes, H. (2004). Calcification of the Epididymis and the tunica Albuginea of the Corpora Cavernosa in Patients on Maintenance Hemodialysis. J. Androl. 25, 752–756. doi:10.1002/j.1939-4640.2004.tb02851.x
Hackeng, T. M., Rosing, J., Spronk, H. M., and Vermeer, C. (2001). Total Chemical Synthesis of Human Matrix Gla Protein. Protein Sci. 10, 864–870. doi:10.1110/ps.44701
Haidl, G., and Opper, C. (1997). Changes in Lipids and Membrane Anisotropy in Human Spermatozoa during Epididymal Maturation. Hum. Reprod. 12, 2720–2723. doi:10.1093/humrep/12.12.2720
Hale, J. E., Fraser, J. D., and Price, P. A. (1988). The Identification of Matrix Gla Protein in Cartilage. J. Biol. Chem. 263, 5820–5824. doi:10.1016/s0021-9258(18)60639-8
Hamada, A., Esteves, S. C., Nizza, M., and Agarwal, A. (2012). Unexplained Male Infertility: Diagnosis and Management. Int. Braz. J Urol. 38, 576–594. doi:10.1590/s1677-55382012000500002
Hamamah, S., and Gatti, J. L. (1998). Role of the Ionic Environment and Internal pH on Sperm Activity. Hum. Reprod. 13 (Suppl. 4), 20–30. doi:10.1093/humrep/13.suppl_4.20
Hermo, L. R. B. (2002). “Epididymis Cell Types and Their Function,” in The Epididymis: From Molecules to Clinical Practice. Editor H. B. Robaire (New York: Kluwer Academic/Plenum Publishers).
Hess, R. A., Bunick, D., Lee, K.-H., Bahr, J., Taylor, J. A., Korach, K. S., et al. (1997). A Role for Oestrogens in the Male Reproductive System. Nature 390, 509–512. doi:10.1038/37352
Hinton, B. T. (1990). The Testicular and Epididymal Luminal Amino Acid Microenvironment in the Rat. J. Androl. 11, 498–505.
Hinton, B. T., and Setchell, B. P. (1980). Concentrations of Glycerophosphocholine, Phosphocholine and Free Inorganic Phosphate in the Luminal Fluid of the Rat Testis and Epididymis. Reproduction 58, 401–406. doi:10.1530/jrf.0.0580401
Hoenderop, J. G. J., Nilius, B., and Bindels, R. J. M. (2005). Calcium Absorption across Epithelia. Physiol. Rev. 85, 373–422. doi:10.1152/physrev.00003.2004
Hong, C. Y., Chiang, B. N., and Turner, P. (1984). Calcium Ion Is the Key Regulator of Human Sperm Function. The Lancet 324, 1449–1451. doi:10.1016/s0140-6736(84)91634-9
Houben, E., Neradova, A., Schurgers, L., and Marc, V. (2016). The Influence of Phosphate, Calcium and Magnesium on Matrix Gla-Protein and Vascular Calcification. G Ital. Nefro 33 (6), gin/33.6.5.
Huang, F., Zhang, H., Wu, M., Yang, H., Kudo, M., Peters, C. J., et al. (2012). Calcium-activated Chloride Channel TMEM16A Modulates Mucin Secretion and Airway Smooth Muscle Contraction. Proc. Natl. Acad. Sci. 109, 16354–16359. doi:10.1073/pnas.1214596109
Huang, M., Rigby, A. C., Morelli, X., Grant, M. A., Huang, G., Furie, B., et al. (2003). Structural Basis of Membrane Binding by Gla Domains of Vitamin K-dependent Proteins. Nat. Struct. Mol. Biol. 10, 751–756. doi:10.1038/nsb971
Hyman, A. A., Weber, C. A., and Jülicher, F. (2014). Liquid-liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 30, 39–58. doi:10.1146/annurev-cellbio-100913-013325
Jelinsky, S. A., Turner, T. T., Bang, H. J., Finger, J. N., Solarz, M. K., Wilson, E., et al. (2007). The Rat Epididymal Transcriptome: Comparison of Segmental Gene Expression in the Rat and Mouse Epididymides1. Biol. Reprod. 76, 561–570. doi:10.1095/biolreprod.106.057323
Jenkins, A. D., Lechene, C. P., and Howards, S. S. (1980). Concentrations of Seven Elements in the Intraluminal Fluids of the Rat Seminiferous Tubules, Rete Testis, and Epididymis1. Biol. Reprod. 23, 981–987. doi:10.1095/biolreprod23.5.981
Jensen, M. B. (2014). Vitamin D and Male Reproduction. Nat. Rev. Endocrinol. 10, 175–186. doi:10.1038/nrendo.2013.262
Joffe, M. (2010). What Has Happened to Human Fertility? Hum. Reprod. 25, 295–307. doi:10.1093/humrep/dep390
Johansson, S., and Melhus, H. (2001). Vitamin A Antagonizes Calcium Response to Vitamin D in Man. J. Bone Miner Res. 16, 1899–1905. doi:10.1359/jbmr.2001.16.10.1899
Johnston, D. S., Jelinsky, S. A., Bang, H. J., DiCandeloro, P., Wilson, E., Kopf, G. S., et al. (2005). The Mouse Epididymal Transcriptome: Transcriptional Profiling of Segmental Gene Expression in the Epididymis1. Biol. Reprod. 73, 404–413. doi:10.1095/biolreprod.105.039719
Karsenty, G. (2011). Regulation of Male Fertility by Bone. Cold Spring Harbor Symposia Quantitative Biol. 76, 279–283. doi:10.1101/sqb.2011.76.010934
Keskes-Ammar, L., Feki-Chakroun, N., Rebai, T., Sahnoun, Z., Ghozzi, H., Hammami, S., et al. (2003). Sperm Oxidative Stress and the Effect of an Oral Vitamin E and Selenium Supplement on Semen Quality in Infertile Men. Arch. Androl. 49, 83–94. doi:10.1080/01485010390129269
Khosroshahi, H. E., Sahin, S. C., Akyuz, Y., and Ede, H. (2014). Long Term Follow‐up of Four Patients with Keutel Syndrome. Am. J. Med. Genet. 164, 2849–2856. doi:10.1002/ajmg.a.36699
Kim, B., Roy, J., Shum, W. W., Da Silva, N., and Breton, S. (2015). Role of Testicular Luminal Factors on Basal Cell Elongation and Proliferation in the Mouse Epididymis. Biol. Reprod. 92, 9. doi:10.1095/biolreprod.114.123943
Kinoshita, H., Nakagawa, K., Narusawa, K. i., Goseki-Sone, M., Fukushi-Irie, M., Mizoi, L., et al. (2007). A Functional Single Nucleotide Polymorphism in the Vitamin-K-dependent Gamma-Glutamyl Carboxylase Gene (Arg325Gln) Is Associated with Bone mineral Density in Elderly Japanese Women. Bone 40, 451–456. doi:10.1016/j.bone.2006.08.007
Kiyozumi, D., Noda, T., Yamaguchi, R., Tobita, T., Matsumura, T., Shimada, K., et al. (2020). NELL2-mediated Lumicrine Signaling through OVCH2 Is Required for Male Fertility. Science 368, 1132–1135. doi:10.1126/science.aay5134
Koch, S., Acebron, S. P., Herbst, J., Hatiboglu, G., and Niehrs, C. (2015). Post-transcriptional Wnt Signaling Governs Epididymal Sperm Maturation. Cell 163, 1225–1236. doi:10.1016/j.cell.2015.10.029
Krausz, C., and Riera-Escamilla, A. (2018). Genetics of Male Infertility. Nat. Rev. Urol. 15, 369–384. doi:10.1038/s41585-018-0003-3
Kujala, M., Hihnala, S., Tienari, J., Kaunisto, K., Hästbacka, J., Holmberg, C., et al. (2007). Expression of Ion Transport-Associated Proteins in Human Efferent and Epididymal Ducts. Reproduction 133, 775–784. doi:10.1530/rep.1.00964
Lal, K. J., and Dakshinamurti, K. (1993). Calcium Channels in Vitamin B6 Deficiency-Induced Hypertension. J. Hypertens. 11, 1357–1362. doi:10.1097/00004872-199312000-00006
Laurentino, S. S., Correia, S., Cavaco, J. E., Oliveira, P. F., Sousa, M. d., Barros, A., et al. (2012). Regucalcin, a Calcium-Binding Protein with a Role in Male Reproduction? Mol. Hum. Reprod. 18, 161–170. doi:10.1093/molehr/gar075
Leahy, T., Rickard, J. P., Pini, T., Gadella, B. M., and de Graaf, S. P. (2020). Quantitative Proteomic Analysis of Seminal Plasma, Sperm Membrane Proteins, and Seminal Extracellular Vesicles Suggests Vesicular Mechanisms Aid in the Removal and Addition of Proteins to the Ram Sperm Membrane. Proteomics 20, e1900289. doi:10.1002/pmic.201900289
Lee, M., and Partridge, N. C. (2009). Parathyroid Hormone Signaling in Bone and Kidney. Curr. Opin. Nephrol. Hypertens. 18, 298–302. doi:10.1097/mnh.0b013e32832c2264
Leung, G. P. H., Cheung, K. H., Leung, C. T., Tsang, M. W., and Wong, P. Y. D. (2004). Regulation of Epididymal Principal Cell Functions by Basal Cells: Role of Transient Receptor Potential (Trp) Proteins and Cyclooxygenase-1 (COX-1). Mol. Cell Endocrinol. 216, 5–13. doi:10.1016/j.mce.2003.10.077
Levine, N., and Kelly, H. (1978). Measurement of pH in the Rat Epididymis In Vivo. Reproduction 52, 333–335. doi:10.1530/jrf.0.0520333
Levine, N., and Marsh, D. J. (1971). Micropuncture Studies of the Electrochemical Aspects of Fluid and Electrolyte Transport in Individual Seminiferous Tubules, the Epididymis and the Vas Deferens in Rats. J. Physiol. 213, 557–570. doi:10.1113/jphysiol.1971.sp009400
Levine, N., and Marsh, D. J. (1975). Micropuncture Study of the Fluid Composition of `Sertoli Cell-Only' Seminiferous Tubules in Rats. Reproduction 43, 547–549. doi:10.1530/jrf.0.0430547
Lewis, B., and John Aitken, R. (2001). Impact of Epididymal Maturation on the Tyrosine Phosphorylation Patterns Exhibited by Rat Spermatozoa1. Biol. Reprod. 64, 1545–1556. doi:10.1095/biolreprod64.5.1545
Lin, J., Jiang, Y., Li, L., Liu, Y., Tang, H., and Jiang, D. (2015). TMEM16A Mediates the Hypersecretion of Mucus Induced by Interleukin-13. Exp. Cell Res. 334, 260–269. doi:10.1016/j.yexcr.2015.02.026
Liu, J., Shen, C., Fan, W., Chen, Y., Zhang, A., Feng, Y., et al. (2016). Low Levels of PRSS37 Protein in Sperm Are Associated with many Cases of Unexplained Male Infertility. Acta Biochim. Biophys. Sin 48, 1058–1065. doi:10.1093/abbs/gmw096
Lomashvili, K. A., Wang, X., Wallin, R., and O'Neill, W. C. (2011). Matrix Gla Protein Metabolism in Vascular Smooth Muscle and Role in Uremic Vascular Calcification. J. Biol. Chem. 286, 28715–28722. doi:10.1074/jbc.m111.251462
Lötvall, J., Hill, A. F., Hochberg, F., Buzás, E. I., Di Vizio, D., Gardiner, C., et al. (2014). Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: a Position Statement from the International Society for Extracellular Vesicles. J. Extracellular Vesicles 3, 26913. doi:10.3402/jev.v3.26913
Luconi, M., Krausz, C., Forti, G., and Baldi, E. (1996). Extracellular Calcium Negatively Modulates Tyrosine Phosphorylation and Tyrosine Kinase Activity during Capacitation of Human Spermatozoa1. Biol. Reprod. 55, 207–216. doi:10.1095/biolreprod55.1.207
Luo, G., Ducy, P., McKee, M. D., Pinero, G. J., Loyer, E., Behringer, R. R., et al. (1997). Spontaneous Calcification of Arteries and Cartilage in Mice Lacking Matrix GLA Protein. Nature 386, 78–81. doi:10.1038/386078a0
Ma, H., Zhang, B. L., Liu, B. Y., Shi, S., Gao, D. Y., Zhang, T. C., et al. (2019). Vitamin K2-dependent GGCX and MGP Are Required for Homeostatic Calcium Regulation of Sperm Maturation. iScience 14, 210–225. doi:10.1016/j.isci.2019.03.030
Matorras, R., Pérez-Sanz, J., Corcóstegui, B., Pérez-Ruiz, I., Malaina, I., Quevedo, S., et al. (2020). Effect of Vitamin E Administered to Men in Infertile Couples on Sperm and Assisted Reproduction Outcomes: a Double-Blind Randomized Study. F&S Rep. 1, 219–226. doi:10.1016/j.xfre.2020.09.006
Matyaszczyk, M., Karczmarewicz, E., Czarnowska, E., Reynolds, R. D., and Lorenc, R. S. (1993). Vitamin B-6 Deficiency Alters Rat Enterocyte Calcium Homeostasis but Not Duodenal Transport. J. Nutr. 123, 204–215. doi:10.1093/jn/123.2.204
Mital, P., Hinton, B. T., and Dufour, J. M. (2011). The Blood-Testis and Blood-Epididymis Barriers Are More Than Just Their Tight Junctions1. Biol. Reprod. 84, 851–858. doi:10.1095/biolreprod.110.087452
Miyata, H., Satouh, Y., Mashiko, D., Muto, M., Nozawa, K., Shiba, K., et al. (2015). Sperm Calcineurin Inhibition Prevents Mouse Fertility with Implications for Male Contraceptive. Science 350, 442–445. doi:10.1126/science.aad0836
Momeni, H. R., and Eskandari, N. (2012). Effect of Vitamin E on Sperm Parameters and DNA Integrity in Sodium Arsenite-Treated Rats. Iran J. Reprod. Med. 10, 249–256.
Morcos, S. R., El-Shobaki, F. A., El-Hawary, Z., and Saleh, N. (1976). Effect of Vitamin C and Carotene on the Absorption of Calcium from the Intestine. Z. Ernährungswiss 15, 387–390. doi:10.1007/bf02020506
Munroe, P. B., Olgunturk, R. O., Fryns, J.-P., Maldergem, L. V., Ziereisen, F., Yuksel, B., et al. (1999). Mutations in the Gene Encoding the Human Matrix Gla Protein Cause Keutel Syndrome. Nat. Genet. 21, 142–144. doi:10.1038/5102
Murshed, M., Schinke, T., McKee, M. D., and Karsenty, G. (2004). Extracellular Matrix Mineralization Is Regulated Locally; Different Roles of Two Gla-Containing Proteins. J. Cel. Biol. 165, 625–630. doi:10.1083/jcb.200402046
Mutucumarana, V. P., Acher, F., Straight, D. L., Jin, D.-Y., and Stafford, D. W. (2003). A Conserved Region of Human Vitamin K-dependent Carboxylase between Residues 393 and 404 Is Important for its Interaction with the Glutamate Substrate. J. Biol. Chem. 278, 46488–46493. doi:10.1074/jbc.m307707200
Mutucumarana, V. P., Stafford, D. W., Stanley, T. B., Jin, D.-Y., Solera, J., Brenner, B., et al. (2000). Expression and Characterization of the Naturally Occurring Mutation L394R in Human γ-Glutamyl Carboxylase. J. Biol. Chem. 275, 32572–32577. doi:10.1074/jbc.m006808200
Nadeem, W., Terete, B., Wei, X., Susan, M. H., and Reidar, W. (2004). Processing and Transport of Matrix Gla Protein (MGP) and Bone Morphogenetic Protein-2 (BMP-2) in Cultured Human Vascular Smooth Muscle Cell. J. Biol. Chem. 279 (41), 43052–43060. doi:10.1074/jbc.M40718020
Newcombe, N., Clulow, J., Man, S. Y., and Jones, R. C. (2000). pH and Bicarbonate in the Ductuli Efferentes Testis of the Rat. Int. J. Androl. 23, 46–50. doi:10.1046/j.1365-2605.2000.00204.x
Nijenhuis, T., Vallon, V., van der Kemp, A. W. C. M., Loffing, J., Hoenderop, J. G. J., and Bindels, R. J. M. (2005). Enhanced Passive Ca2+ Reabsorption and Reduced Mg2+ Channel Abundance Explains Thiazide-Induced Hypocalciuria and Hypomagnesemia. J. Clin. Invest. 115, 1651–1658. doi:10.1172/jci24134
Okunade, G. W., Miller, M. L., Pyne, G. J., Sutliff, R. L., O'Connor, K. T., Neumann, J. C., et al. (2004). Targeted Ablation of Plasma Membrane Ca2+-ATPase (PMCA) 1 and 4 Indicates a Major Housekeeping Function for PMCA1 and a Critical Role in Hyperactivated Sperm Motility and Male Fertility for PMCA4. J. Biol. Chem. 279, 33742–33750. doi:10.1074/jbc.m404628200
Oliveira, A. G., Aquino, D. J. Q., Mahecha, G. A. B., and Oliveira, C. A. (2012). Involvement of the Transepithelial Calcium Transport Disruption and the Formation of Epididymal Stones in Roosters. Reproduction 143, 835–844. doi:10.1530/rep-12-0034
Oury, F., Ferron, M., Huizhen, W., Confavreux, C., Xu, L., Lacombe, J., et al. (2013). Osteocalcin Regulates Murine and Human Fertility through a Pancreas-Bone-Testis axis. J. Clin. Invest. 123, 2421–2433. doi:10.1172/jci65952
Oury, F., Sumara, G., Sumara, O., Ferron, M., Chang, H., Smith, C. E., et al. (2011). Endocrine Regulation of Male Fertility by the Skeleton. Cell 144, 796–809. doi:10.1016/j.cell.2011.02.004
Ouvrier, A., Cadet, R., Vernet, P., Laillet, B., Chardigny, J.-M., Lobaccaro, J.-M. A., et al. (2009). LXR and ABCA1 Control Cholesterol Homeostasis in the Proximal Mouse Epididymis in a Cell-specific Manner. J. Lipid Res. 50, 1766–1775. doi:10.1194/jlr.m800657-jlr200
Paradiso Galatioto, G., Gravina, G. L., Angelozzi, G., Sacchetti, A., Innominato, P. F., Pace, G., et al. (2008). May Antioxidant Therapy Improve Sperm Parameters of Men with Persistent Oligospermia after Retrograde Embolization for Varicocele? World J. Urol. 26, 97–102. doi:10.1007/s00345-007-0218-z
Pastor-Soler, N., Bagnis, C., Sabolic, I., Tyszkowski, R., McKee, M., Van Hoek, A., et al. (2001). Aquaporin 9 Expression along the Male Reproductive Tract1. Biol. Reprod. 65, 384–393. doi:10.1095/biolreprod65.2.384
Pastor-Soler, N., Piétrement, C., and Breton, S. (2005). Role of Acid/base Transporters in the Male Reproductive Tract and Potential Consequences of Their Malfunction. Physiology 20, 417–428. doi:10.1152/physiol.00036.2005
Patel, R., Al-Dossary, A. A., Stabley, D. L., Barone, C., Galileo, D. S., Strehler, E. E., et al. (2013). Plasma Membrane Ca2+-ATPase 4 in Murine Epididymis: Secretion of Splice Variants in the Luminal Fluid and a Role in Sperm Maturation. Biol. Reprod. 89, 6. doi:10.1095/biolreprod.113.108712
Patel, R., Stabley, D., Strehler, E., and Martin-DeLeon, P. (2012). Expression of the Major Splice Variants of Pmca4 in the Murine Epididymis and Caudal Sperm: The Role of Pmca4a in Epididymal Maturation and Sperm Function. J. Androl. 33, 55.
Patti, A., Gennari, L., Merlotti, D., Dotta, F., and Nuti, R. (2013). Endocrine Actions of Osteocalcin. Int. J. Endocrinol. doi:10.1155/2013/846480
Peng, J.-B., Brown, E. M., and Hediger, M. A. (2003). Epithelial Ca2+ Entry Channels: Transcellular Ca2+ Transport and beyond. J. Physiol. 551, 729–740. doi:10.1113/jphysiol.2003.043349
Peng, J.-B., Chen, X.-Z., Berger, U. V., Vassilev, P. M., Brown, E. M., and Hediger, M. A. (2000). A Rat Kidney-specific Calcium Transporter in the Distal Nephron. J. Biol. Chem. 275, 28186–28194. doi:10.1074/jbc.m909686199
Peng, J.-B., Chen, X.-Z., Berger, U. V., Vassilev, P. M., Tsukaguchi, H., Brown, E. M., et al. (1999). Molecular Cloning and Characterization of a Channel-like Transporter Mediating Intestinal Calcium Absorption. J. Biol. Chem. 274, 22739–22746. doi:10.1074/jbc.274.32.22739
Pleuger, C., Silva, E. J. R., Pilatz, A., Bhushan, S., and Meinhardt, A. (2020). Differential Immune Response to Infection and Acute Inflammation along the Epididymis. Front. Immunol. 11, 599594. doi:10.3389/fimmu.2020.599594
Prasad, V., Okunade, G. W., Miller, M. L., and Shull, G. E. (2004). Phenotypes of SERCA and PMCA Knockout Mice. Biochem. Biophysical Res. Commun. 322, 1192–1203. doi:10.1016/j.bbrc.2004.07.156
Price, P. A., Faus, S. A., and Williamson, M. K. (1998). Warfarin Causes Rapid Calcification of the Elastic Lamellae in Rat Arteries and Heart Valves. Atvb 18, 1400–1407. doi:10.1161/01.atv.18.9.1400
Price, P. A., Urist, M. R., and Otawara, Y. (1983). Matrix Gla Protein, a New γ-carboxyglutamic Acid-Containing Protein Which Is Associated with the Organic Matrix of Bone. Biochem. Biophysical Res. Commun. 117, 765–771. doi:10.1016/0006-291x(83)91663-7
Pruneda, A., Yeung, C.-H., Bonet, S., Pinart, E., and Cooper, T. G. (2007). Concentrations of Carnitine, Glutamate and Myo-Inositol in Epididymal Fluid and Spermatozoa from Boars. Anim. Reprod. Sci. 97, 344–355. doi:10.1016/j.anireprosci.2006.01.013
Pudota, B. N., Hommema, E. L., Hallgren, K. W., McNally, B. A., Lee, S., and Berkner, K. L. (2001). Identification of Sequences within the γ-Carboxylase that Represent a Novel Contact Site with Vitamin K-dependent Proteins and that Are Required for Activity. J. Biol. Chem. 276, 46878–46886. doi:10.1074/jbc.m108696200
Pudota, B. N., Miyagi, M., Hallgren, K. W., West, K. A., Crabb, J. W., Misono, K. S., et al. (2000). Identification of the Vitamin K-dependent Carboxylase Active Site: Cys-99 and Cys-450 Are Required for Both Epoxidation and Carboxylation. Proc. Natl. Acad. Sci. 97, 13033–13038. doi:10.1073/pnas.97.24.13033
Puga Molina, L. C., Pinto, N. A., Torres Rodríguez, P., Romarowski, A., Vicens Sanchez, A., Visconti, P. E., et al. (2017). Essential Role of CFTR in PKA-dependent Phosphorylation, Alkalinization, and Hyperpolarization during Human Sperm Capacitation. J. Cell. Physiol. 232, 1404–1414. doi:10.1002/jcp.25634
Raverdeau, M., Gely-Pernot, A., Feret, B., Dennefeld, C., Benoit, G., Davidson, I., et al. (2012). Retinoic Acid Induces Sertoli Cell Paracrine Signals for Spermatogonia Differentiation but Cell Autonomously Drives Spermatocyte Meiosis. Proc. Natl. Acad. Sci. 109, 16582–16587. doi:10.1073/pnas.1214936109
Reid, A. T., Redgrove, K., Aitken, R. J., and Nixon, B. (2011). Cellular Mechanisms Regulating Sperm-Zona Pellucida Interaction. Asian J. Androl. 13, 88–96. doi:10.1038/aja.2010.74
Rennenberg, R. J. M. W., van Varik, B. J., Schurgers, L. J., Hamulyak, K., Ten Cate, H., Leiner, T., et al. (2010). Chronic Coumarin Treatment Is Associated with Increased Extracoronary Arterial Calcification in Humans. Blood 115, 5121–5123. doi:10.1182/blood-2010-01-264598
Richter, A., Kuhlmann, M. K., Seibert, E., Kotanko, P., Levin, N. W., and Handelman, G. J. (2008). Vitamin C Deficiency and Secondary Hyperparathyroidism in Chronic Haemodialysis Patients. Nephrol. Dial. Transplant. 23, 2058–2063. doi:10.1093/ndt/gfn084
Rieder, M. J., Reiner, A. P., and Rettie, A. E. (2007). γ-Glutamyl Carboxylase (GGCX) tagSNPs Have Limited Utility for Predicting Warfarin Maintenance Dose. J. Thromb. Haemost. 5, 2227–2234. doi:10.1111/j.1538-7836.2007.02744.x
Rinaldi, V. D., Donnard, E., Gellatly, K., Rasmussen, M., Kucukural, A., Yukselen, O., et al. (2020). An Atlas of Cell Types in the Mouse Epididymis and Vas Deferens. Elife 9. doi:10.7554/eLife.55474
Rishavy, M. A., and Berkner, K. L. (2012). Vitamin K Oxygenation, Glutamate Carboxylation, and Processivity: Defining the Three Critical Facets of Catalysis by the Vitamin K-dependent Carboxylase. Adv. Nutr. 3, 135–148. doi:10.3945/an.111.001719
Rishavy, M. A., Hallgren, K. W., Yakubenko, A. V., Shtofman, R. L., Runge, K. W., and Berkner, K. L. (2006). Brønsted Analysis Reveals Lys218 as the Carboxylase Active Site Base that Deprotonates Vitamin K Hydroquinone to Initiate Vitamin K-dependent Protein Carboxylation. Biochemistry 45, 13239–13248. doi:10.1021/bi0609523
Robaire, B., and Hinton, B. T. (2015). “The Epididymis,” in Knobil and Neill's Physiology of Reproduction. Editors T. M. Plant, and A. J. Zeleznik (Academic Press, Elsevier Inc.), 691–771. doi:10.1016/b978-0-12-397175-3.00017-x
Rock, J. R., Futtner, C. R., and Harfe, B. D. (2008). The Transmembrane Protein TMEM16A Is Required for normal Development of the Murine Trachea. Developmental Biol. 321, 141–149. doi:10.1016/j.ydbio.2008.06.009
Roy, M. E., and Nishimoto, S. K. (2002). Matrix Gla Protein Binding to Hydroxyapatite Is Dependent on the Ionic Environment: Calcium Enhances Binding Affinity but Phosphate and Magnesium Decrease Affinity. Bone 31, 296–302. doi:10.1016/s8756-3282(02)00821-9
Ruan, Y. C., Shum, W. W. C., Belleannée, C., Da Silva, N., and Breton, S. (2012). ATP Secretion in the Male Reproductive Tract: Essential Role of CFTR. J. Physiol. 590, 4209–4222. doi:10.1113/jphysiol.2012.230581
Saez, F., Ouvrier, A., and Drevet, J. R. (2011). Epididymis Cholesterol Homeostasis and Sperm Fertilizing Ability. Asian J. Androl. 13, 11–17. doi:10.1038/aja.2010.64
Schuh, K., Cartwright, E. J., Jankevics, E., Bundschu, K., Liebermann, J., Williams, J. C., et al. (2004). Plasma Membrane Ca2+ ATPase 4 Is Required for Sperm Motility and Male Fertility. J. Biol. Chem. 279, 28220–28226. doi:10.1074/jbc.m312599200
Schurgers, L. J., Spronk, H. M. H., Skepper, J. N., Hackeng, T. M., Shanahan, C. M., Vermeer, C., et al. (2007). Post-translational Modifications Regulate Matrix Gla Protein Function: Importance for Inhibition of Vascular Smooth Muscle Cell Calcification. J. Thromb. Haemost. 5, 2503–2511. doi:10.1111/j.1538-7836.2007.02758.x
Schurgers, L. J., Teunissen, K. J. F., Knapen, M. H. J., Kwaijtaal, M., van Diest, R., Appels, A., et al. (2005). Novel Conformation-specific Antibodies against Matrix γ-Carboxyglutamic Acid (Gla) Protein. Atvb 25, 1629–1633. doi:10.1161/01.atv.0000173313.46222.43
Schurgers, L. J., Uitto, J., and Reutelingsperger, C. P. (2013). Vitamin K-dependent Carboxylation of Matrix Gla-Protein: a Crucial Switch to Control Ectopic Mineralization. Trends Mol. Med. 19, 217–226. doi:10.1016/j.molmed.2012.12.008
Sharma, U., Conine, C. C., Shea, J. M., Boskovic, A., Derr, A. G., Bing, X. Y., et al. (2016). Biogenesis and Function of tRNA Fragments during Sperm Maturation and Fertilization in Mammals. Science 351, 391–396. doi:10.1126/science.aad6780
Sharma, U. (2019). Paternal Contributions to Offspring Health: Role of Sperm Small RNAs in Intergenerational Transmission of Epigenetic Information. Front. Cell Dev. Biol. 7, 215. doi:10.3389/fcell.2019.00215
Sharma, U., Sun, F., Conine, C. C., Reichholf, B., Kukreja, S., Herzog, V. A., et al. (2018). Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Developmental Cell 46, 481–494. doi:10.1016/j.devcel.2018.06.023
Shen, C., Kuang, Y., Liu, J., Feng, J., Chen, X., Wu, W., et al. (2013). Prss37 Is Required for Male Fertility in the Mouse. Biol. Reprod. 88, 123. doi:10.1095/biolreprod.112.107086
Shi, J., Fok, K. L., Dai, P., Qiao, F., Zhang, M., Liu, H., et al. (2021). Spatio-temporal Landscape of Mouse Epididymal Cells and Specific Mitochondria-Rich Segments Defined by Large-Scale Single-Cell RNA-Seq. Cell Discov 7, 34. doi:10.1038/s41421-021-00260-7
Shin, Y., and Brangwynne, C. P. (2017). Liquid Phase Condensation in Cell Physiology and Disease. Science 357. doi:10.1126/science.aaf4382
Shum, W. W., Smith, T. B., Cortez-Retamozo, V., Grigoryeva, L. S., Roy, J. W., Hill, E., et al. (2014). Epithelial Basal Cells Are Distinct from Dendritic Cells and Macrophages in the Mouse Epididymis. Biol. Reprod. 90, 90. doi:10.1095/biolreprod.113.116681
Shum, W. W. C., Da Silva, N., McKee, M., Smith, P. J. S., Brown, D., and Breton, S. (2008). Transepithelial Projections from Basal Cells Are Luminal Sensors in Pseudostratified Epithelia. Cell 135, 1108–1117. doi:10.1016/j.cell.2008.10.020
Shum, W. W., Leung, M. C. T., Cheng, G. F. Y., Au, S. C. L., and Wong, P. Y. (2006). Identification, Localization and Functional Study of Epithelial Ca2+ Channel TRPV6 in the Rat Epididymis. Proc. Physiol. Soc. 2, PC3.
Shum, W. W., Ruan, Y. C., Da Silva, N., and Breton, S. (2011). Establishment of Cell-Cell Crosstalk in the Epididymis: Control of Luminal Acidification. J. Androl. doi:10.2164/jandrol.111.012971
Silvert, M. A., and Stanisic, T. H. (1980). “Epididymectomy, Seminal Vesiculectomy and Hydrocelectomy for Epididymitis, Seminal Vesiculitis and Hydrocele,” in Surgery of the Male Reproductive Tract. Editors L. I. Lipshultz, J. N. Corriere, and E. S. E. Hafez (Kluwer Academic Publishers Group), 195–212. doi:10.1007/978-94-009-8849-1_17
Sorensen, M. B., Bergdahl, I. A., Hjollund, N. H., Bonde, J. P., Stoltenberg, M., and Ernst, E. (1999). Zinc, Magnesium and Calcium in Human Seminal Fluid: Relations to Other Semen Parameters and Fertility. Mol. Hum. Reprod. 5, 331–337. doi:10.1093/molehr/5.4.331
Stafford, D. W. (2005). The Vitamin K Cycle. J. Thromb. Haemost. 3, 1873–1878. doi:10.1111/j.1538-7836.2005.01419.x
Stammler, A., Hau, T., Bhushan, S., Meinhardt, A., Jonigk, D., Lippmann, T., et al. (2015). Epididymitis: Ascending Infection Restricted by Segmental Boundaries. Hum. Reprod. 30, 1557–1565. doi:10.1093/humrep/dev112
Stevens, A. L., Breton, S., Gustafson, C. E., Bouley, R., Nelson, R. D., Kohan, D. E., et al. (2000). Aquaporin 2 Is a Vasopressin-independent, Constitutive Apical Membrane Protein in Rat Vas Deferens. Am. J. Physiology-Cell Physiol. 278, C791–C802. doi:10.1152/ajpcell.2000.278.4.c791
Stival, C., Puga Molina, L. d. C., Paudel, B., Buffone, M. G., Visconti, P. E., and Krapf, D. (2016). Sperm Capacitation and Acrosome Reaction in Mammalian Sperm. Adv. Anat. Embryol. Cell Biol 220, 93–106. doi:10.1007/978-3-319-30567-7_5
Stoltenberg, M., Ernst, E., Andreasen, A., and Danscher, G. (1996). Histochemical Localization of Zinc Ions in the Epididymis of the Rat. Histochem. J. 28, 173–185. doi:10.1007/bf02331441
Sullivan, R., Frenette, G., and Girouard, J. (2007). Epididymosomes Are Involved in the Acquisition of New Sperm Proteins during Epididymal Transit. Asian J. Androl. 9, 483–491. doi:10.1111/j.1745-7262.2007.00281.x
Sullivan, R., Saez, F., Girouard, J., and Frenette, G. (2005). Role of Exosomes in Sperm Maturation during the Transit along the Male Reproductive Tract. Blood Cell Mol. Dis. 35, 1–10. doi:10.1016/j.bcmd.2005.03.005
Takei, G. L., Tourzani, D. A., Paudel, B., and Visconti, P. E. (2021). Activation of cAMP‐dependent Phosphorylation Pathways Is Independent of ROS Production during Mouse Sperm Capacitation. Mol. Reprod. Dev. 88, 544–557. doi:10.1002/mrd.23524
Tan, S., Özcan, M. F., Karaoğlanoğlu, M., Ipek, A., Özcan, A. S., and Arslan, H. (2012). Prevalence of Scrotal Calculi and Their Relationship with Pain. Diagn. Interv. Radiol. 18, 303–306. doi:10.4261/1305-3825.DIR.4808-11.1
Tecle, E., and Gagneux, P. (2015). Sugar‐coated Sperm: Unraveling the Functions of the Mammalian Sperm Glycocalyx. Mol. Reprod. Dev. 82, 635–650. doi:10.1002/mrd.22500
Tie, J.-K., Carneiro, J. D. A., Jin, D.-Y., Martinhago, C. D., Vermeer, C., and Stafford, D. W. (2016). Characterization of Vitamin K-dependent Carboxylase Mutations that Cause Bleeding and Nonbleeding Disorders. Blood 127, 1847–1855. doi:10.1182/blood-2015-10-677633
Tie, J.-K., Mutucumarana, V. P., Straight, D. L., Carrick, K. L., Pope, R. M., and Stafford, D. W. (2003). Determination of Disulfide Bond Assignment of Human Vitamin K-dependent γ-Glutamyl Carboxylase by Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry. J. Biol. Chem. 278, 45468–45475. doi:10.1074/jbc.m309164200
Tie, J.-K., and Stafford, D. W. (2016). Structural and Functional Insights into Enzymes of the Vitamin K Cycle. J. Thromb. Haemost. 14, 236–247. doi:10.1111/jth.13217
Tie, J., Wu, S.-M., Jin, D., Nicchitta, C. V., and Stafford, D. W. (2000). A Topological Study of the Human γ-glutamyl Carboxylase. Blood 96, 973–978. doi:10.1182/blood.v96.3.973.015k55_973_978
Tkach, M., and Théry, C. (2016). Communication by Extracellular Vesicles: Where We Are and where We Need to Go. Cell 164, 1226–1232. doi:10.1016/j.cell.2016.01.043
Tollner, T. L., Bevins, C. L., and Cherr, G. N. (2012). Multifunctional Glycoprotein DEFB126-A Curious story of Defensin-Clad Spermatozoa. Nat. Rev. Urol. 9, 365–375. doi:10.1038/nrurol.2012.109
Turner, T., Bomgardner, D., Jacobs, J., and Nguyen, Q. (2003). Association of Segmentation of the Epididymal Interstitium with Segmented Tubule Function in Rats and Mice. Reproduction 125, 871–878. doi:10.1530/rep.0.1250871
Turner, T. T., Johnston, D. S., Jelinsky, S. A., Tomsig, J. L., and Finger, J. N. (2007). Segment Boundaries of the Adult Rat Epididymis Limit Interstitial Signaling by Potential Paracrine Factors and Segments Lose Differential Gene Expression after Efferent Duct Ligation. Asian J. Androl. 9, 565–573. doi:10.1111/j.1745-7262.2007.00302.x
Turner, T. T., Jones, C. E., Howards, S. S., Ewing, L. L., Zegeye, B., and Gunsalus, G. L. (1984). On the Androgen Microenvironment of Maturing Spermatozoa*. Endocrinology 115, 1925–1932. doi:10.1210/endo-115-5-1925
Turner, T. T. (2002). “Necessity's Potion: Inorganic Ions and Small Organic Molecules in the Epididymal Lumen,” in The Epididymis: From Molecules to Clinical Practice. Editors B. H. Robaire, and B. T. (New York: Kluwer Academic/Plenum Publishers), 131–150. doi:10.1007/978-1-4615-0679-9_8
Turner, T. T. (1991). Spermatozoa Are Exposed to a Complex Microenvironment as They Traverse the Epididymis. Ann. NY Acad. Sci. 637, 364–383. doi:10.1111/j.1749-6632.1991.tb27323.x
Tüttelmann, F., Ruckert, C., and Röpke, A. (2018). Disorders of Spermatogenesis. Med. Genet. 30, 12–20. doi:10.1007/s11825-018-0181-7
van Ballegooijen, A. J., Pilz, S., Tomaschitz, A., Grubler, M. R., and Verheyen, N. (20172017). The Synergistic Interplay between Vitamins D and K for Bone and Cardiovascular Health: A Narrative Review. Int. J. Endocrinol. doi:10.1155/2017/7454376
Vernet, P., Aitken, R. J., and Drevet, J. R. (2004). Antioxidant Strategies in the Epididymis. Mol. Cell Endocrinol. 216, 31–39. doi:10.1016/j.mce.2003.10.069
Voisin, A., Saez, F., Drevet, J. R., and Guiton, R. (2019). The Epididymal Immune Balance: a Key to Preserving Male Fertility. Asian J. Androl. 21 (6), 531–539. doi:10.4103/aja.aja_11_19
Wagenfeld, A., Yeung, C. H., Lehnert, W., Nieschlag, E., and Cooper, T. G. (2002). Lack of Glutamate Transporter EAAC1 in the Epididymis of Infertile C-Ros Receptor Tyrosine-Kinase Deficient Mice. J. Androl. 23, 772–782.
Wajih, N., Borras, T., Xue, W., Hutson, S. M., and Wallin, R. (2004). Processing and Transport of Matrix γ-Carboxyglutamic Acid Protein and Bone Morphogenetic Protein-2 in Cultured Human Vascular Smooth Muscle Cells. J. Biol. Chem. 279, 43052–43060. doi:10.1074/jbc.m407180200
Wallin, R., Schurgers, L., and Wajih, N. (2008). Effects of the Blood Coagulation Vitamin K as an Inhibitor of Arterial Calcification. Thromb. Res. 122, 411–417. doi:10.1016/j.thromres.2007.12.005
Walters, J. R., Balesaria, S., Chavele, K.-M., Taylor, V., Berry, J. L., Khair, U., et al. (2006). Calcium Channel TRPV6 Expression in Human Duodenum: Different Relationships to the Vitamin D System and Aging in Men and Women. J. Bone Miner Res. 21, 1770–1777. doi:10.1359/jbmr.060721
Watanabe, T., Ebara, S., Kimura, S., Maeda, K., Watanabe, Y., Watanabe, H., et al. (2007). Maternal Vitamin B12deficiency Affects Spermatogenesis at the Embryonic and Immature Stages in Rats. Congenit. Anom. 47, 9–15. doi:10.1111/j.1741-4520.2006.00135.x
Watanabe, T., Ohkawa, K., Kasai, S., Ebara, S., Nakano, Y., and Watanabe, Y. (2003). The Effects of Dietary Vitamin B12 Deficiency on Sperm Maturation in Developing and Growing Male Rats. Congenit. Anom. (Kyoto) 43, 57–64. doi:10.1111/j.1399-0004.2003.tb02307.x
Weber, P. (2001). Vitamin K and Bone Health. Nutrition 17, 880–887. doi:10.1016/s0899-9007(01)00709-2
Weissgerber, P., Kriebs, U., Tsvilovskyy, V., Olausson, J., Kretz, O., Stoerger, C., et al. (2011). Male Fertility Depends on Ca²+ Absorption by TRPV6 in Epididymal Epithelia. Sci. Signal 4, ra27. doi:10.1126/scisignal.2001791
Weissgerber, P., Kriebs, U., Tsvilovskyy, V., Olausson, J., Kretz, O., Stoerger, C., et al. (2012). Excision of Trpv6 Gene Leads to Severe Defects in Epididymal Ca2+ Absorption and Male Fertility Much like Single D541A Pore Mutation. J. Biol. Chem. 287, 17930–17941. doi:10.1074/jbc.m111.328286
WHO (2021). WHO Laboratory Manual for the Examination and Processing of Human Semen. 6th edition. Geneva: World Health Organization.
Winters, B. R., and Walsh, T. J. (2014). The Epidemiology of Male Infertility. Urol. Clin. North America 41, 195–204. doi:10.1016/j.ucl.2013.08.006
Wong, P. (1998). CFTR Gene and Male Fertility. Mol. Hum. Reprod. 4, 107–110. doi:10.1093/molehr/4.2.107
Wong, P. Y. D., Gong, X. D., Leung, G. P. H., and Cheuk, B. L. Y. (2002a). “Formation of the Epididymal Fluid Microenvironment,” in The Epididymis: From Molecular to Clinical Practice A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. Editors B. Robaire, and B. T. Hinton (New York: Kluwer Academic/Plenum), 119–130. doi:10.1007/978-1-4615-0679-9_7
Wong, P. Y., and Yeung, C. H. (1978). Absorptive and Secretory Functions of the Perfused Rat Cauda Epididymidis. J. Physiol. 275, 13–26. doi:10.1113/jphysiol.1978.sp012174
Wong, W. Y., Merkus, H. M. W. M., Thomas, C. M. G., Menkveld, R., Zielhuis, G. A., and Steegers-Theunissen, R. P. M. (2002b). Effects of Folic Acid and Zinc Sulfate on Male Factor Subfertility: a Double-Blind, Randomized, Placebo-Controlled Trial. Fertil. Sterility 77, 491–498. doi:10.1016/s0015-0282(01)03229-0
Xu, B., Turner, S. D., and Hinton, B. T. (2018). Alteration of Transporter Activities in the Epididymides of Infertile Initial Segment-specific Pten Knockout Mice†. Biol. Reprod. 99, 536–545. doi:10.1093/biolre/ioy073
Xu, Y.-X., Wagenfeld, A., Yeung, C.-H., Lehnert, W., and Cooper, T. G. (2003). Expression and Location of Taurine Transporters and Channels in the Epididymis of Infertile C-Ros Receptor Tyrosine Kinase-Deficient and fertile Heterozygous Mice. Mol. Reprod. Dev. 64, 144–151. doi:10.1002/mrd.10250
Yamamoto, M., Tsuji, Y., and Miyake, K. (1993). Microassay of Sperm Concentration in the Rat Epididymis by Micropuncture Technique. Nagoya J. Med. Sci. 55, 71–75.
Yeung, C.-H., Anapolski, M., Setiawan, I., Lang, F., and Cooper, T. G. (2004). Effects of Putative Epididymal Osmolytes on Sperm Volume Regulation of Fertile and Infertile C-rosTransgenic Mice. J. Androl. 25, 216–223. doi:10.1002/j.1939-4640.2004.tb02781.x
Yudin, A. I., Generao, S. E., Tollner, T. L., Treece, C. A., Overstreet, J. W., and Cherr, G. N. (2005). Beta-Defensin 126 on the Cell Surface Protects Sperm from Immunorecognition and Binding of Anti-sperm Antibodies1. Biol. Reprod. 73, 1243–1252. doi:10.1095/biolreprod.105.042432
Zegers-Hochschild, F., Adamson, G. D., de Mouzon, J., Ishihara, O., Mansour, R., Nygren, K., et al. (20092009). International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary of ART Terminology, 2009∗. Fertil. Sterility 92, 1520–1524. doi:10.1016/j.fertnstert.2009.09.009
Zhang, Y., Wang, X., Wang, H., Jiao, J., Li, Y., Fan, E., et al. (2015). TMEM16A-Mediated Mucin Secretion in IL-13-Induced Nasal Epithelial Cells from Chronic Rhinosinusitis Patients. Allergy Asthma Immunol. Res. 7, 367–375. doi:10.4168/aair.2015.7.4.367
Zhou, C. X., Zhang, Y.-L., Xiao, L., Zheng, M., Leung, K. M., Chan, M. Y., et al. (2004). An Epididymis-specific β-defensin Is Important for the Initiation of Sperm Maturation. Nat. Cell Biol 6, 458–464. doi:10.1038/ncb1127
Zhou, W., De Iuliis, G. N., Dun, M. D., and Nixon, B. (2018). Characteristics of the Epididymal Luminal Environment Responsible for Sperm Maturation and Storage. Front. Endocrinol. 9, 59. doi:10.3389/fendo.2018.00059
Zi, Z., Zhang, Z., Li, Q., An, W., Zeng, L., Gao, D., et al. (2015). CCNYL1, but Not CCNY, Cooperates with CDK16 to Regulate Spermatogenesis in Mouse. Plos Genet. 11, e1005485. doi:10.1371/journal.pgen.1005485
Keywords: sperm maturation, epididymis, calcium homeostasis, luminal microenvironment, GGCX, matrix gla protein (MGP), Trpv6, Tmem16a
Citation: Shum W, Zhang BL, Cao AS, Zhou X, Shi SM, Zhang ZY, Gu LY and Shi S (2022) Calcium Homeostasis in the Epididymal Microenvironment: Is Extracellular Calcium a Cofactor for Matrix Gla Protein-Dependent Scavenging Regulated by Vitamins. Front. Cell Dev. Biol. 10:827940. doi: 10.3389/fcell.2022.827940
Received: 02 December 2021; Accepted: 19 January 2022;
Published: 17 February 2022.
Edited by:
Junaid Kashir, Alfaisal University, Saudi ArabiaReviewed by:
Bart Gadella, Utrecht University, NetherlandsMariana Weigel Muñoz, CONICET Instituto de Biología y Medicina Experimental (IBYME), Argentina
Copyright © 2022 Shum, Zhang, Cao, Zhou, Shi, Zhang, Gu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Winnie Shum, c2h1bXdAc2hhbmdoYWl0ZWNoLmVkdS5jbg==
 Winnie Shum
Winnie Shum Bao Li Zhang1,2,3
Bao Li Zhang1,2,3 Albert Shang Cao
Albert Shang Cao Ze Yang Zhang
Ze Yang Zhang Lou Yi Gu
Lou Yi Gu