- 1Health Science Center, School of Biomedical Engineering, Shenzhen University, Shenzhen, China
- 2Marshall Laboratory of Biomedical Engineering, Shenzhen, China
- 3Beijing Ophthalmology and Visual Sciences Key Laboratory, Beijing Tongren Eye Center, Beijing Tongren Hospital, Beijing Institute of Ophthalmology, Capital Medical University, Beijing, China
- 4Ophthalmology and Visual Sciences Key Laboratory, Beijing, China
Meibomian gland dysfunction (MGD) is caused by abnormalities of the meibomian glands (MG) and is one of the causes of evaporative dry eye (DED). Precise MG segmentation is crucial for MGD-related DED diagnosis because the morphological parameters of MG are of importance. Deep learning has achieved state-of-the-art performance in medical image segmentation tasks, especially when training and test data come from the same distribution. But in practice, MG images can be acquired from different devices or hospitals. When testing image data from different distributions, deep learning models that have been trained on a specific distribution are prone to poor performance. Histogram specification (HS) has been reported as an effective method for contrast enhancement and improving model performance on images of different modalities. Additionally, contrast limited adaptive histogram equalization (CLAHE) will be used as a preprocessing method to enhance the contrast of MG images. In this study, we developed and evaluated the automatic segmentation method of the eyelid area and the MG area based on CNN and automatically calculated MG loss rate. This method is evaluated in the internal and external testing sets from two meibography devices. In addition, to assess whether HS and CLAHE improve segmentation results, we trained the network model using images from one device (internal testing set) and tested on images from another device (external testing set). High DSC (0.84 for MG region, 0.92 for eyelid region) for the internal test set was obtained, while for the external testing set, lower DSC (0.69–0.71 for MG region, 0.89–0.91 for eyelid region) was obtained. Also, HS and CLAHE were reported to have no statistical improvement in the segmentation results of MG in this experiment.
Introduction
Meibomian glands (MG) are special sebaceous gland located on the tarsal plate of eye (Erich et al., 2011). They produce meibum to prevent tear from over evaporation (Nichols et al., 2011), maintain the surface tension of the tear film, and trap tears between its oily edge and the eyeball. Healthy MG are elongated in shape, arrange in parallel and throughout the entire tarsal plate (Djalilian, 2018). Functional and/or structural problem of MG may cause meibomian gland dysfunction (MGD) (Driver and Lemp, 1996). MGD is a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion (Den et al., 2011; Nichols et al., 2011). MGD often leads to tear film alteration, ocular surface disease, and is the leading cause of evaporative dry eye (Turgut et al., 2018), which can seriously affect the patient’s life and increase the global public health and financial burden (McDonald et al., 2016).
Meibomian gland area loss is an important index to evaluate MGD (Arita et al., 2014). MGD can be directly observed using meibography, which is an optical imaging technique allowing visualizing MG morphology in vivo (Pult and Nichols, 2012). Recently, many non-contact infrared meibography methods were developed, making the MGD diagnosis process more patient-friendly and less time-consuming (Pult and Riede-Pult, 2012; Wong et al., 2019; Xiao et al., 2020; Hwang et al., 2021). These devices allow users to capture high-resolution images of meibomian glands in a short period of time, which can provide sufficient experimental material for MG analysis. Quantification of the area of meibomian glands loss is of importance when assessing MGD. To date, automatic methods based on image processing techniques have been developed for the automated assessment and classification of MGD (Arita et al., 2014; Celik et al., 2013; Koprowski et al., 2016; Koprowski et al., 2017; Liang et al., 2017; Llorens-Quintana et al., 2019; Pult and Riede-Pult, 2013; Xiao et al., 2021). In recent years, MGD automatic analysis methods based on convolutional neural network (CNN) have been developed rapidly. These works automatically segment the eyelid and MG, and calculate the loss rate and analyze the morphological parameters of MG (Wang et al., 2019). In order to promote the segmentation performance of MG, contrast limited adaptive histogram equalization (CLAHE) will be used as an image preprocessing step to enhance the contrast of MG images (Prabhu et al., 2020; Dai et al., 2021).
However, the MG images used in these works were acquired from a single device. The preset parameters of the method or the trained model are for the specific data domain used in the experiment. When testing these methods with images from other distribution domains (such as different image modalities, or different acquisition devices), a lower performance is usually obtained (Yan et al., 2019). Such problem is called “distribution shift” (Jo and Bengio, 2017). To reduce the performance gap, an effective method is to reduce the distribution domain distance of the data. Training a generative adversarial network (GAN) to generate fake images between two different domains is a common method to improve the generalization of the model to data from different domains (Perone et al., 2019; Yan et al., 2019); but it requires a large number of training samples. In some medical image processing tasks, histogram specification (HS) has been reported as an effective method for contrast enhancement and improving model performance on images of different modalities, such as between MRI and CT images (Naseem et al., 2021).
In this study, we focus on two open questions in the field of automated meibomian gland analysis:
1) In the CNN-based MG analysis method, does CLAHE improve the network performance?
2) Can HS improve the performance of models trained with data collected from a single device on cross-device data?
This study aims at developing and validating an MG segmentation method based on CNN. Whether HS and CLAHE as preprocessing methods can effectively improve the segmentation performance of the CNN model was also investigated in this study. MG images captured from different devices were divided into different testing sets based on different preprocessing methods, and then segmented by CNN model to calculate MG loss rate. All the results will be compared with the ground-truth by the clinicians.
Related works
Histogram specification (HS) enhances the brightness and contrast of the input image and transforms the input image into an image with a similar shape to the template histogram. HS has the advantage of simplicity and low computational cost (Xiao et al., 2018). HS is very common in the preprocessing stage of medical images. HS is used to match the histogram of the input image to the template image to initialize and avoid gradient explosion before using CNN to segment the kidney based on CT images (da Cruz et al., 2020). Naseem et al. (2021) based on HS to enhance images of different modalities. They enhance low-contrast CT images based on MRI images based on the second-order distribution.
The raw MG image often has low contrast. In other word, the pixel intensity of glands and background have little difference, as a result, it is difficult for the observer to separate the glands from background, especially when the image is blur. CLAHE is applied for the meibography image enhancement. CLAHE enhances an image by limiting the height of the local region histogram, such as a 64 pixels neighborhood block, thereby overcome the global uneven illumination and noise amplification problem. CLAHE is often used as a preprocessing method to enhance the performance of MG segmentation (Prabhu et al., 2020; Dai et al., 2021).
Materials
This study involves 287 subjects (age: 56.1 ± 17.2 years old, 83 men and 204 women) collected by Beijing Tongren Hospital. The purpose and possible consequences of the study were explained to all involved subjects. Exclusion criteria included 1) ocular allergies, 2) history of ocular surgery, 3) history of ocular trauma, 4) other eye diseases, 5) long term or frequent contact lens use and 6) Any other eye or systemic disease known to affect the tear film. Excluded images include: 1) Images included something other than the eyelids and their surrounding tissueswere, 2) Images were not sufficiently clear for automatic analysis were excluded. 3) Patients’ eyes exhibited excessive meibomian lipid secretion. The study was approved by the Ethical Committee of the Beijing Tongren Hospital and was conducted in accordance with the tenets of the Declaration of Helsinki. A total of 1,074 images were collected by professional clinicians, including 888 MG images collected from Keratograph 5M (K5M; OCULUS Optikgeräte GmbH, Wetzlar, Germany) and 186 from KANGHUA DED-1L (KH). Note that
The ground-truth annotation was completed by a senior clinician using imageLabeler application in MATLAB (9.5.0.944444, R2018b, Java 1.8.0_152-b16). For labeling the eyelid region, the upper edge is defined at the opening of the gland, the lower edge is defined at the edge of proximal tarsal plate, and the horizontal borders is defined at the top and bottom borders intersected (Wang et al., 2019). Value 1 and 2 are used to denote the eyelid region and the MG region, respectively. Examples are shown in Figure 1.
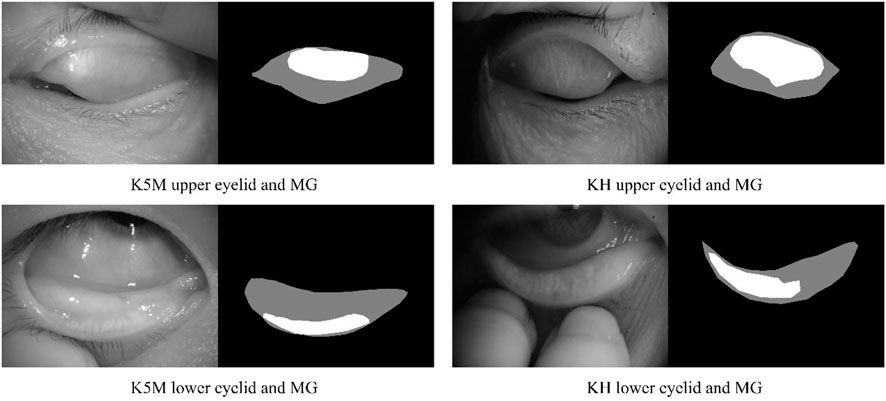
FIGURE 1. Examples of upper and lower eyelids and MG, and ground-truth for segmentation mask. Black, gray, and white pixel represent the background, eyelid region and MG region, respectively.
Methods
Image preprocessing
Images
MG segmentation network
To segment the eyelid region and MG region, we constructed a compact CNN based on U-Net architecture (Ronneberger et al., 2015). The segmentation network contains an encoder and a decoder, as shown in Figure. Specifically, we use ResNet-34 and remove its fully connection layers as the backbone of our network (He et al., 2016). Residual blocks in ResNet-34 use shortcuts to extract features more efficiently, transfer gradients and prevent gradients vanishing in deep layers. A residual block is consisted of convolution layers, batch normalization layers and rectified linear unit (ReLU), as shown in Figure 2.
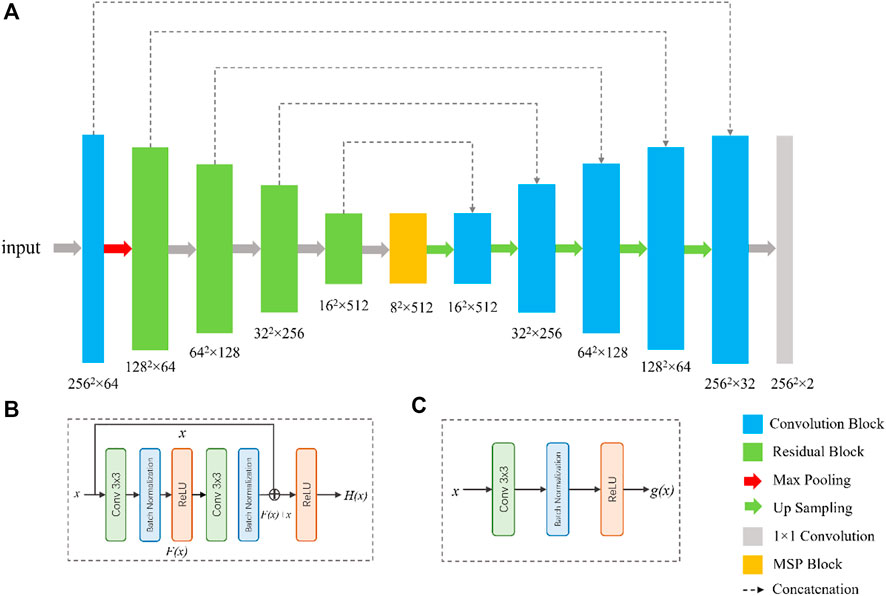
FIGURE 2. Network architecture for the segmentation of tear meniscus. The network (A) is a U-Net-like architecture with a modified ResNet-34 as encoder. The bottom of the network is multi-scale perception block (MSP). (B) and (C) are the illustrations of the residual block and convolution block, respectively.
Inspired by CE-Net (Zaiwang et al., 2019), we add a multi-scale perception block (MSP) between encoder and decoder, as shown in Figure 3. In order to save calculation cost, MSP realizes multi-scale convolution operation through dilated convolution, as shown in Figure 3. Convolution kernels of different sizes have different receptive fields, allowing more features to be captured. In this study, MSP contains 3 × 3, 5 × 5, and 7 × 7 convolution layers, which are realized by 3 × 3 dilatation convolution with dilated rate 1, 2, and 3, respectively. Finally, the feature graph is integrated by 1 × 1 convolution. After each convolutional layer, ReLU is added as activation function to increase the nonlinearity of the network.
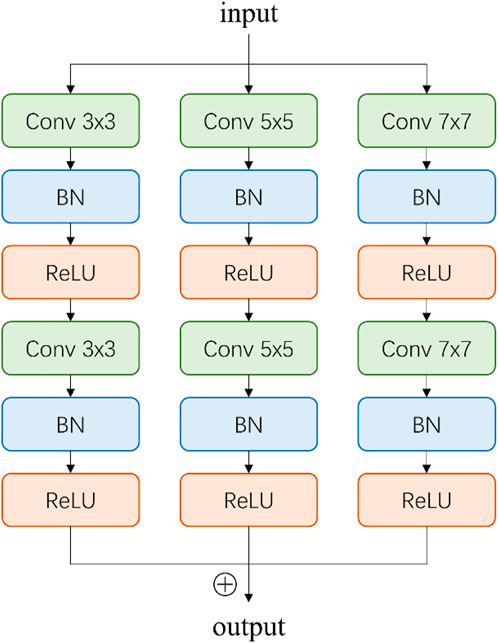
FIGURE 3. Illustration of multi-scale perception block (MSP). The 3 × 3, 5 × 5, and 7 × 7 convolution layers are realized by 3 × 3 dilatation convolution with dilated rate 1, 2, and 3, respectively.
In the decoder, each step consists of a bilinear interpolation operation, which is responsible for upscaling the feature map resolution by four times, followed by two convolutional blocks (Figure 2) and a concatenation with the corresponding feature maps from the encoder. At the final layer, a 1 × 1 padded convolutional layer was used to map the multi-channel feature maps to three classes that are belonged to eyelid region, MG region, and background region. The output of network was converted to a grayscale segmentation map, in which value 0, 1, and 2 are responding to background pixel, eyelid pixel, and MG region pixel, respectively.
MG loss rate calculation
MG loss rate is the ratio of the area of meibomian gland loss to the total eyelid area. Therefore, the MG loss rate is a positive real number in the range [0,1]. The loss rate can be expressed by Eq. 1.
MG loss rate is a parameter directly related to the severity of MGD and is the most used in clinic. When the meibomian gland atrophied by 33%, the severity of MGD increased by one grade, which is known as meiboscore (Arita et al., 2014; Arita et al., 2008).
Results
Two models were trained with the training set, including: 1) the model was trained with the k5m generation graph; 2) K5M images enhanced by CLAHE were used for training. Each training image and its corresponding mask were resized to 256 × 256 and augmented by gamma transformation, rotation, blur, noise addition, and image flip. The data augmentation increased the training images by 12 times. Training and evaluating of the proposed models were performed on a computer with an Nvidia GeForce GTX 3090 GPU. The deep learning models were implemented based on PyTorch (version 1.1.0) package in Python. All models were trained using the Adam optimizer (α = 0.9, β = 0.999), with an initial learning rate of 0.0003 and decays 0.8 times every five epochs. The batch size is set to 32 and the maximum epoch number of 50. L1 regulation was applied to prevent over-fitting (Hawkins, 2004). The loss function used in the experiment is Dice loss function (Jadon, 2020), which is represented by Eq. 2.
To explore the influence of HS and CLAHE on model performance, k5m images and KH images were divided into different testing sets, which were: 1)
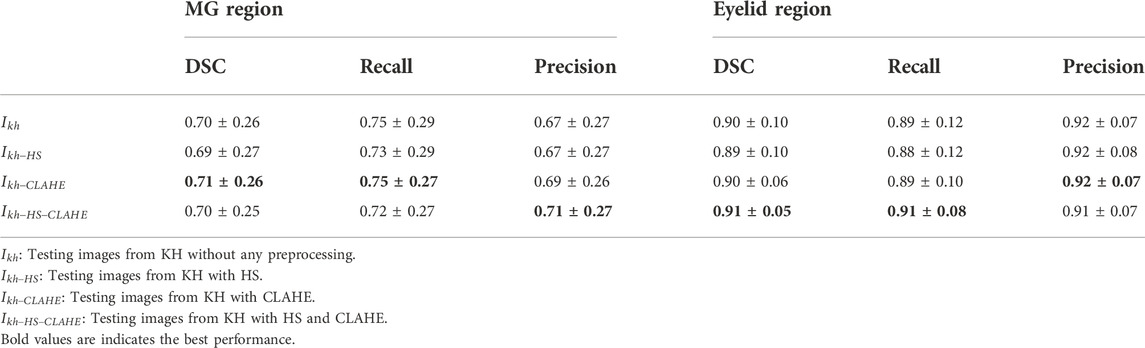
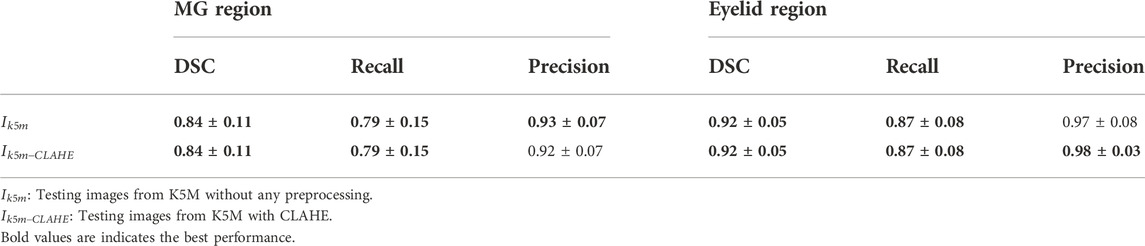
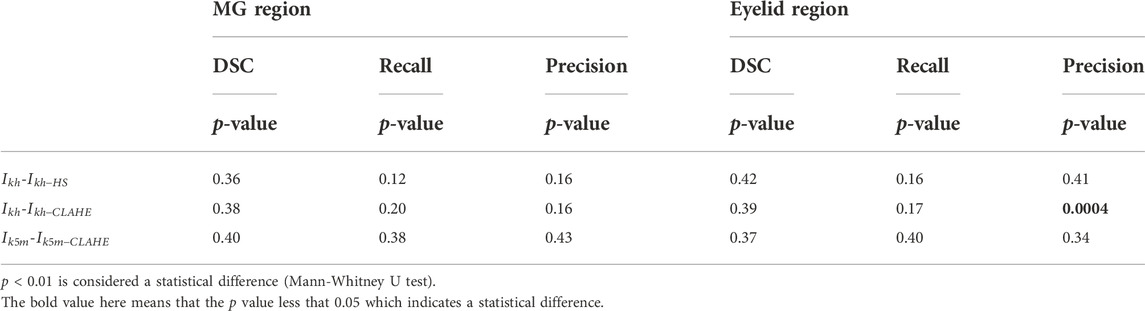
The performance of the MG segmentation models was assessed by dice similarity coefficient (DSC), recall, and precision. These results were reported in Table 1 and Table 2. The results in Table 1 show that all images have close results. The maximum value of each indicator is marked in bold. Table 3 shows the comparison between segmentation results of different preprocessing methods on two image set. Note that there is no statistical difference in all different testing sets, except that the precision between the
Figure 4 shows some visualization of MG segmentation results. Most of the images in both the internal testing set and the external testing set have segmentation results with high DSC score, and the network predicted segmentation masks and ground-truth masks have high similarity and coincidence. But some images in the external testing set have very low segmentation results, as shown in Figure 5, both the eyelid region and the MG region are under-segmented, with rough edges and anomalous masks. These under-segmented images have dissimilar features when compared with the predicted results on average level.
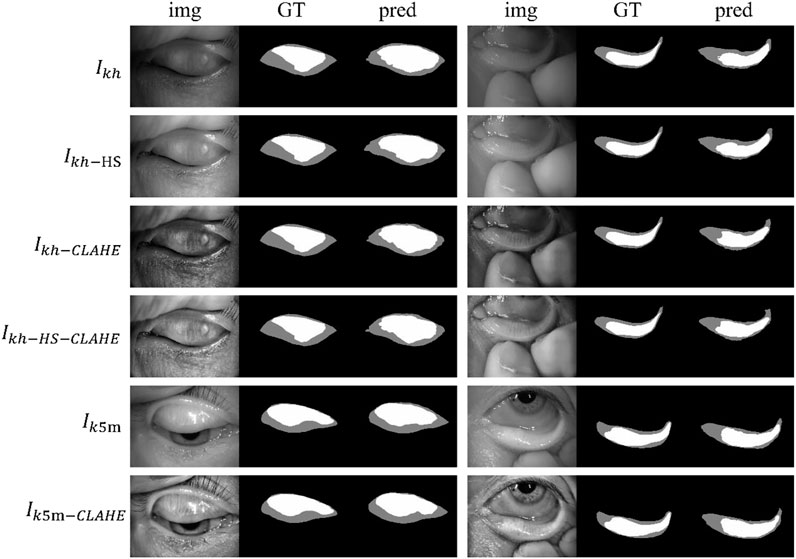
FIGURE 4. Examples of segmentation results for external and internal testing images. Img, GT, and pred represent input images, ground-truth masks, and predicted segmentation masks, respectively.
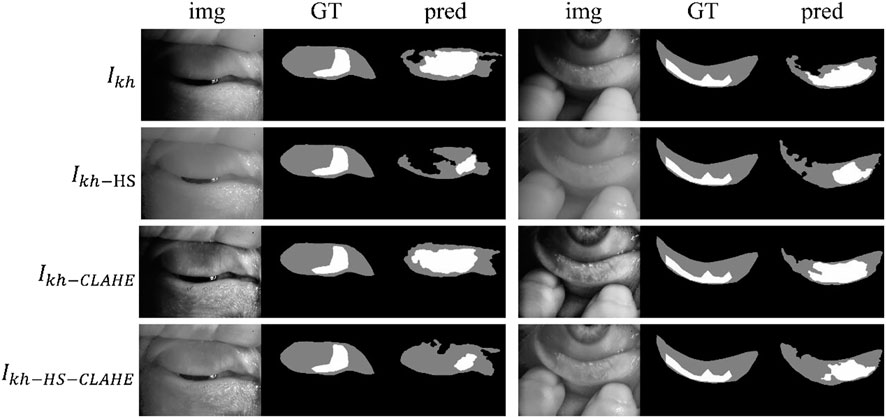
FIGURE 5. Some under-segmented examples in the external testing set due to the distribution gap. Img, GT, and pred represent input images, ground-truth masks, and predicted segmentation masks, respectively.
Figure 6 and Figure 7 show the direct comparison results between the MG loss rate and GT for the external and internal testing sets. Most of the predicted MG loss rates are distributed near the ideal line (predicted MG loss rate equals to ground-truth MG loss rate), which indicates that the MG loss rates calculated according to the predicted segmentation results of the network are relatively close to the ground-truth. Root Mean Squared Error (RMSE) in Table 4 was used to quantify and compare the MG loss rate with the ground-truth. Testing set
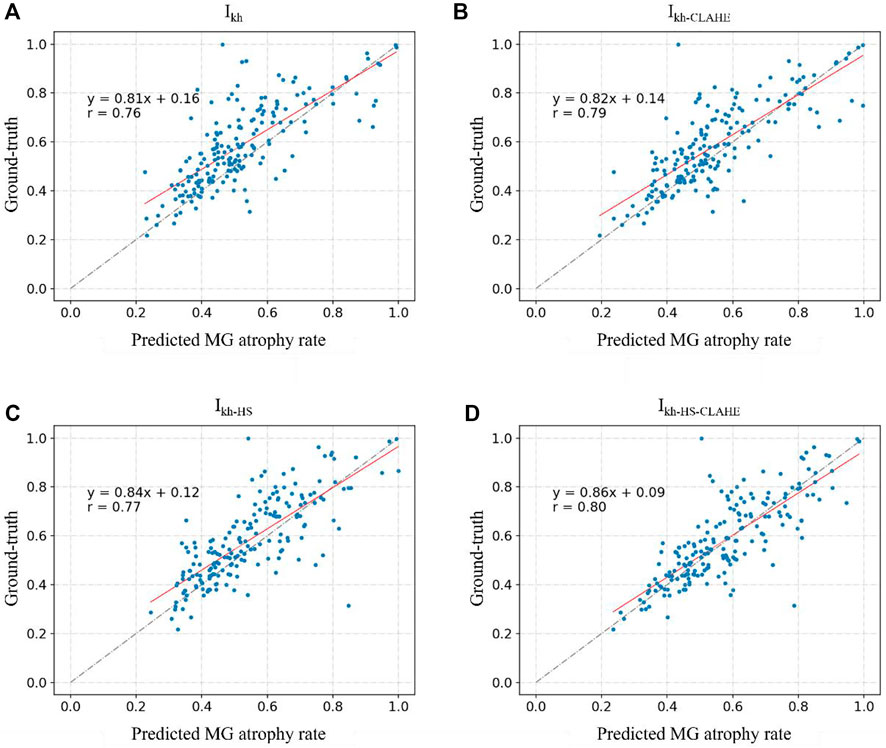
FIGURE 6. (A–D) represent the direct comparison of the ground truth and predicted MG atrophy rates of the unprocessed image set Ikh, image set Ikh-CLAHE processed with CLAHE, image set Ikh-HS processed with HS, and image set Ikh-HS-CLAHE processed with HS and CLAHE, respectively.
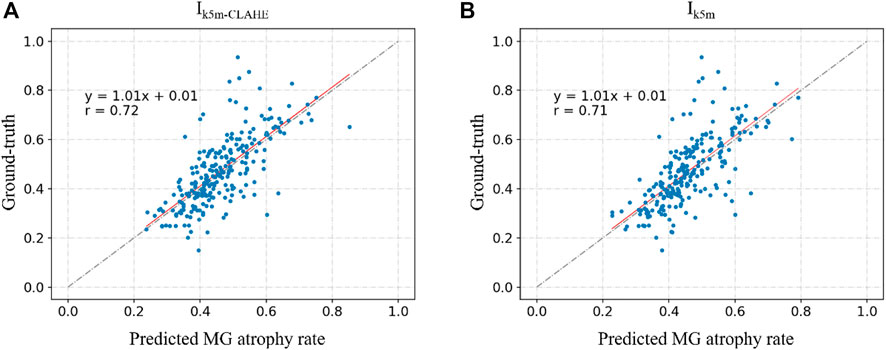
FIGURE 7. (A) and (B) represent the direct comparison of the ground real and predicted MG atrophy rates of the unprocessed image set Ik5m, image set I5m-CLAHE processed with CLAHE, respectively.
Discussion
Meibomian gland morphology analysis is an important index to evaluate MGD. However, the current manual grading methods have the problems of heavy workload, low efficiency and large error, which is not conducive to the standardized diagnosis and treatment of MGD and dry eye. In this study, we developed and evaluated the automatic segmentation method of the eyelid area and the MG area based on CNN and automatically calculated MG loss rate. This method is evaluated in the internal and external testing sets from the two meibography devices. In addition, we also tested that the pre-processing method of HS and CLAHE as MG images has not improved significantly.
In this experiment, the segmentation results of the external testing set (
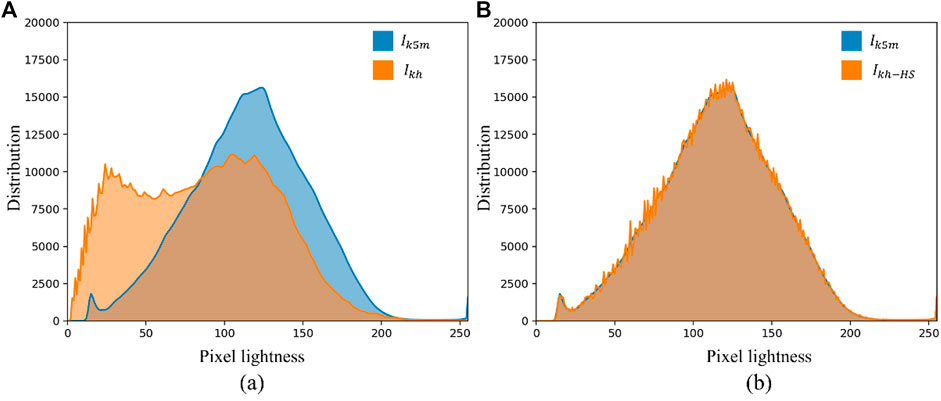
FIGURE 8. Histogram comparison of internal and external test sets. (A) Shows the histograms of
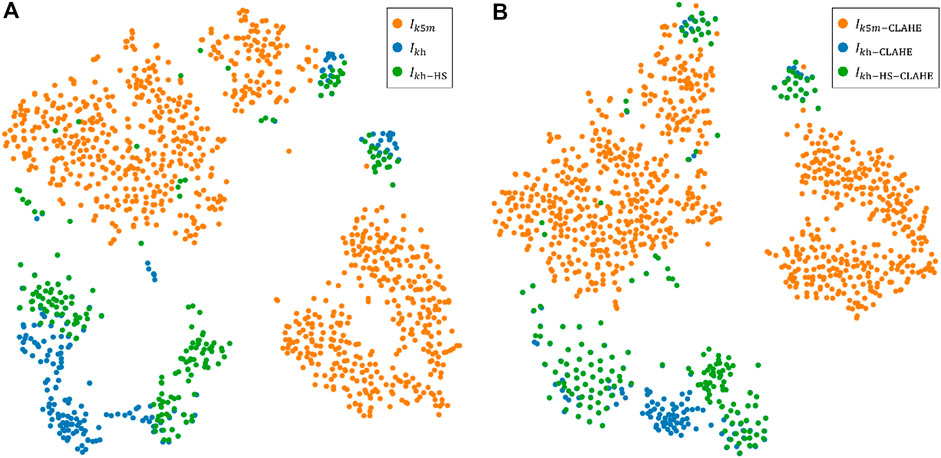
FIGURE 9. Execution of t-SNE algorithm for images from two different devices. Colors represent data from different devices. (A) A visualization of the t-SNE 2D non-linear embedding projection for the images without CLAHE. (B) A visualization of the t-SNE 2D non-linear embedding projection for the images with CLAHE for contrast enhancement.
CLAHE can effectively enhance the visual effect of MG images, which is beneficial for doctors to read and measure MG images manually. However, CLAHE does not significantly improve the segmentation results of neural networks, because CLAHE also fails to close the distribution gap between the distributions of
Conclusion
In this study, we developed and validated a CNN-based automatic MG analysis method based on MG images acquired by two different devices, K5M and KH. Predictions of MG loss rates were in high agreement with the gold standard obtained by physicians. We also found that in this study HS and CLAHE do not significantly improve the performance of CNN for segmenting MG, which may indicate that in future deep learning-based MG analysis tasks, no additional computational cost is required for image preprocessing phase.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Beijing Tongren Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YoZ and YJ developed the plot and designed the experiments, which were executed by YiZ and AL; XD and LT did the discussion and wrote the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arita, R., Itoh, K., Inoue, K., and Amano, S. (2008). Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 115, 911–915. doi:10.1016/j.ophtha.2007.06.031
Arita, R., Suehiro, J., Haraguchi, T., Shirakawa, R., Tokoro, H., and Amano, S. (2014). Objective image analysis of the meibomian gland area. Br. J. Ophthalmol. 98, 746–755. doi:10.1136/bjophthalmol-2012-303014
Celik, T., Lee, H. K., Petznick, A., and Tong, L. (2013). Bioimage informatics approach to automated meibomian gland analysis in infrared images of meibography. J. Optometry 6, 194–204. doi:10.1016/j.optom.2013.09.001
da Cruz, L. B., Araújo, J. D. L., Ferreira, J. L., Diniz, J. O. B., Silva, A. C., de Almeida, J. D. S., et al. (2020). Kidney segmentation from computed tomography images using deep neural network. Comput. Biol. Med. 123, 103906. doi:10.1016/j.compbiomed.2020.103906
Dai, Q., Liu, X., Lin, X., Fu, Y., Chen, C., Yu, X., et al. (2021). A novel meibomian gland morphology analytic system based on a convolutional neural network. IEEE Access 9, 23083–23094. doi:10.1109/ACCESS.2021.3056234
Den, S., Danielnelson, J., Mcculley, J. P., Shimazaki, J., Craig, J., Benitez-Del-Castillo, J. M., et al. (2011). The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Invest. Ophthalmol. Vis. Sci. 52, 1930–1937. doi:10.1167/iovs.10-6997b
Djalilian, A. R. (2018). “Ocular surface disease,” in Update in the diagnosis and management of meibomian gland dysfunction (Berlin, Germany: Springer), 17–29. doi:10.1007/978-3-319-15823–5
Driver, P. J., and Lemp, M. A. (1996). Meibomian gland dysfunction. Surv. Ophthalmol. 40, 343–367. doi:10.1016/s0039-6257(96)80064-6
Erich, K., Nadja, K., Thomas, M., Hiroto, O., and Sullivan, D. A. (2011). The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest. Ophthalmol. Vis. Sci. 52, 1938–1978. doi:10.1167/iovs.10-6997c
Hawkins, D. M. (2004). The problem of overfitting. J. Chem. Inf. Comput. Sci. 44, 1–12. doi:10.1021/ci0342472
He, K., Zhang, X., Ren, S., and Sun, J. (2016). “Deep residual learning for image recognition,” in Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27-30 June 2016, 770–778.
Hwang, H. S., Mikula, E., Xie, Y., Brown, D. J., and Jester, J. V. (2021). A novel transillumination meibography device for in vivo imaging of mouse meibomian glands. Ocul. Surf. 19, 201–209. doi:10.1016/j.jtos.2020.08.012
Jadon, S., 2020. A survey of loss functions for semantic segmentation. arXiv preprint arXiv:2006.14822.
Jo, J., and Bengio, Y., 2017. Measuring the tendency of cnns to learn surface statistical regularities. arXiv preprint arXiv:1711.11561.
Koprowski, R., Tian, L., and Olczyk, P. (2017). A clinical utility assessment of the automatic measurement method of the quality of Meibomian glands. Biomed. Eng. Online 16, 82. doi:10.1186/s12938-017-0373-4
Koprowski, R., Wilczyński, S., Olczyk, P., Nowińska, A., Węglarz, B., and Wylęgała, E. (2016). A quantitative method for assessing the quality of meibomian glands. Comput. Biol. Med. 75, 130–138. doi:10.1016/j.compbiomed.2016.06.001
Liang, F., Xu, Y., Li, W., Ning, X., Liu, X., and Liu, A. (2017). Recognition algorithm based on improved FCM and rough sets for meibomian gland morphology. Appl. Sci. 7, 192. doi:10.3390/app7020192
Llorens-Quintana, C., Rico-del-Viejo, L., Syga, P., Madrid-Costa, D., and Iskander, D. R. (2019). A novel automated approach for infrared-based assessment of meibomian gland morphology. Trans. Vis. Sci. Tech. 8, 17. doi:10.1167/tvst.8.4.17
McDonald, M., Patel, D. A., Keith, M. S., and Snedecor, S. J. (2016). Economic and humanistic burden of dry eye disease in europe, north America, and asia: A systematic literature review. Ocul. Surf. 14, 144–167. doi:10.1016/j.jtos.2015.11.002
Naseem, R., Khan, Z. A., Satpute, N., Beghdadi, A., Cheikh, F. A., and Olivares, J. (2021). Cross-modality guided contrast enhancement for improved liver tumor image segmentation. IEEE Access 9, 118154–118167. doi:10.1109/ACCESS.2021.3107473
Nichols, K. K., Foulks, G. N., Bron, A. J., Glasgow, B. J., Dogru, M., Tsubota, K., et al. (2011). The international workshop on meibomian gland dysfunction: Executive summary. Invest. Ophthalmol. Vis. Sci. 52, 1922–1929. doi:10.1167/iovs.10-6997a
Perone, C. S., Ballester, P., Barros, R. C., and Cohen-Adad, J. (2019). Unsupervised domain adaptation for medical imaging segmentation with self-ensembling. NeuroImage 194, 1–11. doi:10.1016/j.neuroimage.2019.03.026
Prabhu, S. M., Chakiat, A., S, S., Vunnava, K. P., and Shetty, R. (2020). Deep learning segmentation and quantification of Meibomian glands. Biomed. Signal Process. Control 57, 101776. doi:10.1016/j.bspc.2019.101776
Pult, H., and Nichols, J. J. (2012). A review of meibography. Optom. Vis. Sci. 89, E760–E769. doi:10.1097/OPX.0b013e3182512ac1
Pult, H., and Riede-Pult, B. (2013). Comparison of subjective grading and objective assessment in meibography. Cont. Lens Anterior Eye 36, 22–27. doi:10.1016/j.clae.2012.10.074
Pult, H., and Riede-Pult, B. H. (2012). Non-contact meibography: Keep it simple but effective. Cont. Lens Anterior Eye 35, 77–80. doi:10.1016/j.clae.2011.08.003
Ronneberger, O., Fischer, P., and Brox, T. (2015). “U-net: Convolutional networks for biomedical image segmentation,” in International Conference on Medical Image Computing and Computer-Assisted Intervention, Berlin, Germany, 18 November 2015 (Springer), 234–241.
Turgut, B., Çatak, O., and Demir, T. (2018). Meibomian gland dysfunction: An overlooked eyelid disease. Adv. Ophthalmol. Vis. Syst. 8. doi:10.15406/aovs.2018.08.00295
Van der Maaten, L., and Hinton, G. (2008). Visualizing non-metric similarities in multiple maps. Mach. Learn. 9, 33–55. doi:10.1007/s10994-011-5273-4
Wang, J., Yeh, T. N., Chakraborty, R., Yu, S. X., and Lin, M. C. (2019). A deep learning approach for meibomian gland atrophy evaluation in meibography images. Trans. Vis. Sci. Tech. 8, 37. doi:10.1167/tvst.8.6.37
Wong, S., Srinivasan, S., Murphy, P. J., and Jones, L. (2019). Comparison of meibomian gland dropout using two infrared imaging devices. Cont. Lens Anterior Eye 42, 311–317. doi:10.1016/j.clae.2018.10.014
Xiao, B., Tang, H., Jiang, Y., Li, W., and Wang, G. (2018). Brightness and contrast controllable image enhancement based on histogram specification. Neurocomputing 275, 2798–2809. doi:10.1016/j.neucom.2017.11.057
Xiao, J., Adil, M. Y., Chen, X., Utheim, Ø. A., Ræder, S., Tønseth, K. A., et al. (2020). Functional and morphological evaluation of meibomian glands in the assessment of meibomian gland dysfunction subtype and severity. Am. J. Ophthalmol. 209, 160–167. doi:10.1016/j.ajo.2019.09.005
Xiao, P., Luo, Z., Deng, Y., Wang, G., and Yuan, J. (2021). An automated and multiparametric algorithm for objective analysis of meibography images. Quant. Imaging Med. Surg. 11, 1586–1599. doi:10.21037/qims-20-611
Yan, W., Wang, Y., Gu, S., Huang, L., Yan, F., Xia, L., et al. (2019). “The domain shift problem of medical image segmentation and vendor-adaptation by unet-GAN,” in Medical image computing and computer assisted intervention – MICCAI 2019, lecture notes in computer science. Editors D. Shen, T. Liu, T. M. Peters, L. H. Staib, C. Essert, S. Zhouet al. (Cham: Springer International Publishing), 623–631. doi:10.1007/978-3-030-32245-8_69
Zaiwang, G., Jun, C., Huazhu, F., Kang, Z., Huaying, H., Yitian, Z., et al. 2019, CE-Net: Context encoder network for 2D medical image segmentation. arXiv:1903.02740v1.
Keywords: Meibomian gland, segmentation, histogram, image, model performance
Citation: Deng X, Tian L, Zhang Y, Li A, Cai S, Zhou Y and Jie Y (2022) Is histogram manipulation always beneficial when trying to improve model performance across devices? Experiments using a Meibomian gland segmentation model. Front. Cell Dev. Biol. 10:1067914. doi: 10.3389/fcell.2022.1067914
Received: 12 October 2022; Accepted: 14 November 2022;
Published: 05 December 2022.
Edited by:
Weihua Yang, Jinan University, ChinaCopyright © 2022 Deng, Tian, Zhang, Li, Cai, Zhou and Jie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjin Zhou, eWp6aG91QHN6dS5lZHUuY24=; Ying Jie, amllX3lpbmdjbkBhbGl5dW4uY29t
†The first two authors, Deng and Tian, contributed equally to this work
 Xianyu Deng1,2†
Xianyu Deng1,2† Lei Tian
Lei Tian Yinghuai Zhang
Yinghuai Zhang Yongjin Zhou
Yongjin Zhou Ying Jie
Ying Jie