
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 12 September 2022
Sec. Epigenomics and Epigenetics
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.1012193
This article is part of the Research TopicProtein Modifications in Epigenetic Dysfunctional Diseases: Mechanisms and Potential Therapeutic StrategiesView all 15 articles
Regenerating islet-derived 4 (REG4) gene was discovered by high-throughput sequencing of ulcerative colitis cDNA libraries. REG4 is involved in infection and inflammation by enhancing macrophage polarization to M2, via activation of epidermal growth factor receptor (EGFR)/Akt/cAMP-responsive element binding and the killing inflammatory Escherichia coli, and closely linked to tumorigenesis. Its expression was transcriptionally activated by caudal type homeobox 2, GATA binding protein 6, GLI family zinc finger 1, SRY-box transcription factor 9, CD44 intracytoplasmic domain, activating transcription factor 2, and specificity protein 1, and translationally activated by miR-24. REG4 can interact with transmembrane CD44, G protein-coupled receptor 37, mannan and heparin on cancer cells. Its overexpression was observed in gastric, colorectal, pancreatic, gallbladder, ovarian and urothelial cancers, and is closely linked to their aggressive behaviors and a poor prognosis. Additionally, REG4 expression and recombinant REG4 aggravated such cellular phenotypes as tumorigenesis, proliferation, anti-apoptosis, chemoradioresistance, migration, invasion, peritoneal dissemination, tumor growth, and cancer stemness via EGFR/Akt/activator protein-1 and Akt/glycogen synthase kinase three β/β-catenin/transcription factor 4 pathways. Sorted REG4-positive deep crypt secretory cells promote organoid formation of single Lgr5 (+) colon stem cells by Notch inhibition and Wnt activation. Histologically, REG4 protein is specifically expressed in neuroendocrine tumors and signet ring cell carcinomas of the gastrointestinal tract, pancreas, ovary, and lung. It might support the histogenesis of gastric intestinal–metaplasia–globoid dysplasia–signet ring cell carcinoma. In this review, we summarized the structure, biological functions, and effects of REG4 on inflammation and cancer. We conclude that REG4 may be employed as a biomarker of tumorigenesis, subsequent progression and poor prognosis of cancer, and may be a useful target for gene therapy.
In 1984, Yonemura et al. (Yonemura et al., 1984) discovered regenerating islet-derived (REG) proteins during the regeneration of pancreatic islets. The REG family belongs to the calcium-dependent lectin (C-type lectin) gene superfamily, which encodes four multi-functional and secreted small proteins. REG proteins serve as anti-apoptotic factors, acute phase reactants, lectins, and growth factors for neural cells, pancreatic β cells, and epithelial cells in the digestive system. To date, researchers have identified human REG I (Iα and Iβ), REG III (III and HIP/PAP), and REG4, which encode homologous 158–175aa proteins. REG genes are located on chromosomes 2p12 (HIP/PAP, REG Iα, REG Iβ, and REG III) and 1q12-q21 (REG4) (Nata et al., 2004).
REG4 was discovered by high-throughput sequencing of a cDNA library from an ulcerative colitis (UC) sample in 2001 (Hartupee et al., 2001). The REG4 gene has 10 exons and encodes four types of variants by alternative splicing, which contain different open reading frames. Its longest cDNA has an open reading frame of 477 bp and encodes an 18-kDa peptide of 158aa. The REG4 protein is composed of a 22aa signal peptide and calcium-dependent lectin domain, within which are an N-glycosylation site and two carbohydrate binding sites (Figure 1) (Zhang et al., 2021). In the rat, REG4 mRNA was detected in the brain cortex, stomach, pancreas, spleen, small intestine, colon, kidney, and urinary bladder, but not in the sciatic nerve, thymus, liver, cerebellum, suprarenal gland, heart, soleus muscle, lung, and esophagus by reverse transcriptase–PCR. Using western blot, REG4 protein expression was detectable in the pancreas, stomach, small intestine, colon, spleen, brain cortex, kidney, and urinary bladder, but not in the sciatic nerve, liver, thymus, cerebellum, suprarenal gland, soleus muscle, heart, esophagus, and lung. Immunohistochemically, positive REG4 staining was detected throughout the gastric mucosa and was mainly distributed in the basal portion of intestinal crypts. Pancreatic acinar cells appeared positive for REG4, but not pancreatic islet β cells. REG4 was expressed in large spleen cells with a large nucleus in the red pulp, but not the white pulp. Immunoreactivity for REG4 was found in large neurons of the brain cortex, and in glomerular and urinary bladder epithelial cells, but rarely in renal tubular cells (Azman et al., 2011). REG4 immunoreactivity was significantly higher in the ovary than the uterus. The expression of REG4 was strongly detectable in oocytes and granulosa cells of ovarian follicles, interstitial cells and corpus luteum, while only weak expression was found in the glandular and luminal epithelium of the rat endometrium (Du and Yao, 2013). The tissue-specific expression of REG4 is closely linked to its biological function in different organs.
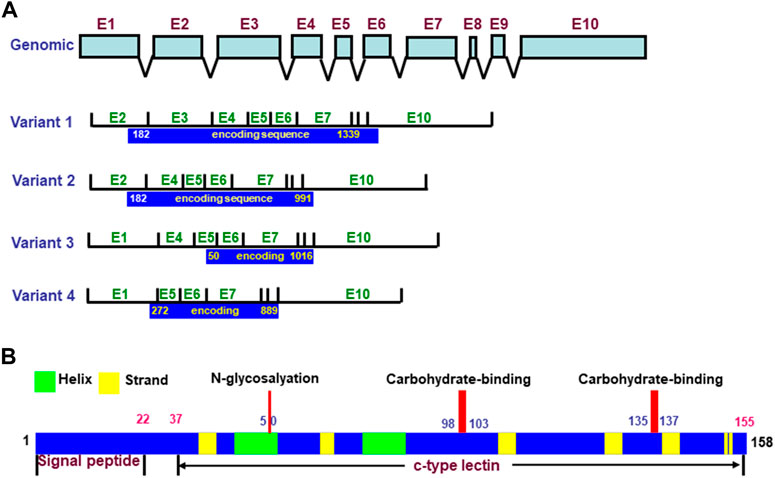
FIGURE 1. Structures of REG4 gene and protein. The REG4 gene has 10 exons and is alternatively spliced into four variants with different open reading frames (A). The encoding protein of REG4 variant one produces a 158aa protein with signal peptide and c-type lectin domain (B).
In mammalian cells, caudal type homeobox 2 (CDX2) was found to induce REG4 expression by binding to consensus CDX2-binding elements upstream of the REG4 gene, supported by the positive relationship between CDX2 and REG4 expression in gastric cancer cells and tissues (Naito et al., 2012; Chai et al., 2021). MicroRNA (miR)-363 suppressed the translation of GATA binding protein 6 (GATA6), which functioned as a transcriptional factor to induce REG4 and leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) expression essential for the growth of colon cancer cells under adherent conditions (Kawasaki et al., 2015). The key transcriptional factor in the Hedgehog signaling pathway, GLI family zinc finger 1 (GLI1) bound to REG4 promoter regions (GATCATCCA) for its transcription and translation in pancreatic cancer cells, supported by the synergic expression of REG4 and GLI1 (Wang et al., 2011). Additionally, SRY-box transcription factor 9 (SOX9) knockdown upregulated REG4 protein expression in gastric cancer cells; a positive correlation of REG4 expression with SOX9 expression in gastric cancer was noted (Zhang et al., 2018). The activating transcription factor 2 (ATF2) targeted the REG4 promoter to induce REG4 expression during enteritis (Xiao et al., 2019). Duan et al. (Duan et al., 2014) found that the tumor suppressor, miR-24, translationally restrained the progression of gastric cancer by down-regulating REG4. In short, REG4 expression was transcriptionally activated by CDX2, GATA6, GLI1, ATF2, and SOX9, and translationally activated by miR-24. However, further miRNAs may be discovered in future that are associated with the translational regulation of REG4.
As for the cellular signaling pathway, recombinant human REG4 (rhREG4) treatment resulted in anti-apoptosis of colorectal cancer cells with the overexpression of B cell lymphoma-extra large (Bcl-xL), B cell lymphoma 2 (Bcl-2), survivin, and matrix metalloproteinase-7 (MMP-7), and the phosphorylation of epidermal growth factor receptor (EGFR) at Tyr992 and Tyr1068, and Akt at Thr308 and Ser473. It also strengthened the transcriptional activity of activator protein-1 (AP-1) by interaction with JunB, JunD, and FosB (Bishnupuri et al., 2006b). rhREG4 treatment also protected normal intestinal crypt cells from irradiation-induced apoptosis by enhancing the expression of Bcl-2, Bcl-xL, and survivin, in agreement with data from human colorectal cancer cells (Bishnupuri et al., 2010). rhREG4 treatment promoted G2 progression for the mitogenesis of colorectal cancer cells by Akt/glycogen synthase kinase three β (GSK3β)/β-catenin/transcription factor 4 (TCF-4) signaling (Bishnupuri et al., 2014). Meanwhile, REG4 might protect acinar cells against necrosis in experimental pancreatitis by enhancing the expression of Bcl-2 and Bcl-xL via activation of the EGFR/Akt pathway (Hu et al., 2011). Li et al. (Li et al., 2011) found anti-tumor effects of proteoglycan from Phellinus linteus on colorectal cancer cells via inactivation of the REG4/EGFR/Akt pathway. Taken together, these findings suggested that REG4 is a potent activator of the EGFR/Akt pathway for the proliferation and anti-apoptosis of colorectal cancer cells.
In addition, Ho et al. (2010) demonstrated that REG4 bound to mannan and heparin in the absence of calcium. In addition, REG4 was found to interact with CD44 to activate its regulated intramembrane proteolysis. This resulted in the γ-secretase–mediated cleavage and release of the CD44 intracytoplasmic domain (CD44ICD) that functions as a transcriptional activator of D-type cyclins involving in cell proliferation, and Kruppel-like factor 4 and SRY-box transcription factor 2 (SOX2) expression involved in the pluripotency of cancer stem cells (Figure 2) (Bishnupuri et al., 2022). A significant correlation between REG4 and CD44 or CD44ICD supported the above-mentioned hypothesis (Sninsky et al., 2021). Liu et al. (2013) found that REG4 down-regulation also resulted in the hypoexpression of p21 and p27, which negatively regulated cyclin D1 and blocked the G1/S transition of prostate cancer cells. Wang et al. (2016) showed that transforming growth factor (TGF)-α stimulated specificity protein 1 (SP1) to transcriptionally promote REG4 expression, while G protein-coupled receptor 37 (GPR37) complexed with REG4, which mediated EGFR signal transduction by REG4 and promoted peritoneal metastasis of gastric cancer cells. Therefore, TGF-α/EGFR/SP1 was responsible for the transcriptional activation of REG4 in a positive feedback loop.
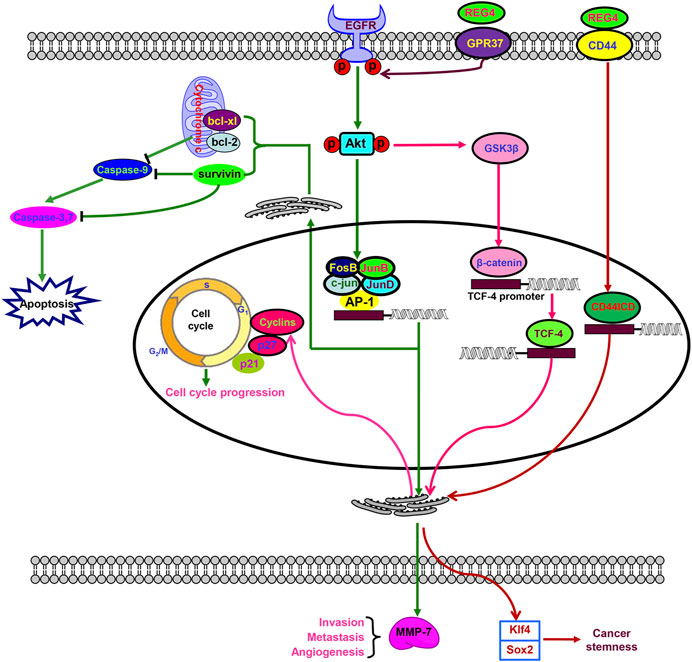
FIGURE 2. Biological functions of REG4. REG4 indirectly interacts with epidermal growth factor receptor (EGFR) to phosphorylate and activate Akt, which induces activator protein-1 (AP-1)–mediated transcriptional initiation by an AP-1/c-Jun/JunA/JunB complex and glycogen synthase kinase three β (GSK3β)–β-catenin–transcription factor 4 (TCF-4) signaling. Additionally, REG4 can bind to G protein-coupled receptor 37 (GPR37) to activate EGFR. REG4 interacts with CD44 to activate regulated transmembrane proteolysis of CD44 resulting in γ-secretase–mediated cleavage and release of the CD44 intracytoplasmic domain (CD44ICD). TCF-4 and CD44ICD serve as transcriptional activators that up-regulate the expression of cyclins for cell-cycle progression. CD44ICD-mediated Kruppel-like factor 4 (Klf4) and SRY-box transcription factor 2 (SOX2) expression is involved in cancer stemness. Activator protein-1–induced overexpression of B cell lymphoma-extra large (Bcl-xL), B cell lymphoma 2 (Bcl-2), survivin, and matrix metalloproteinase-7 (MMP7) plays an important role in apoptosis, angiogenesis, invasion, and metastasis.
REG4 gene was screened from UC samples (Hartupee et al., 2001). REG4 mRNA was found to be up-regulated in Crohn’s disease and UC samples (Takasawa et al., 2018). Nanakin et al. (2007) found that REG4 mRNA was strongly expressed in inflammatory epithelium, and dysplastic and cancerous lesions, and positively correlated with the expression of basic fibroblast growth factor (bFGF) and hepatocyte growth factor (HGF) mRNA expression in UC. In pediatric patients with intestinal failure, serum REG4 was positively correlated with serum interleukin (IL)-6 and tumor necrosis factor (TNF)-α, and REG4 protein was increased and highly expressed toward the luminal face of inflamed intestine. In intestinal conditional REG4 knockout mice, REG4 abrogation altered the colonic bacterial composition, and weakened bacterial adhesion to the colonic mucosa, finally ameliorating dextran sodium sulfate-induced colitis (Xiao et al., 2019). Further study indicated that REG4 stimulated complement-mediated attack complexes to eliminate intestinal dominant Escherichia coli in order to maintain homeostasis. These results supported the protective effects of REG4 on the epithelia of UC, possibly by being anti-inflammatory and anti-infection (Qi et al., 2020).
Hu et al. (Hu et al., 2011) also found that REG4 expression was significantly up-regulated during acute pancreatitis. The REG4 secreted by pancreatic cancer cells promoted macrophage polarization to M2 via EGFR/Akt/cAMP-responsive element binding activation, finally promoting tumor growth and distant metastasis (Ma et al., 2016). Li et al. (2021) found that rhREG4 attenuated the severity of rat osteoarthritis by facilitating the proliferation of articular chondrocytes. In a rat model of acutely-injured liver, treatment with recombinant interleukin 22 (IL-22) lentivirus reduced serum total bilirubin, alanine and aspartate transaminases, and enhanced REG4 expression, suggesting that REG4 might be involved in the protective effects of IL-22 on hepatic injury (Zhang et al., 2015).
At the mRNA level, REG4 gene was significantly up-regulated in gastric cancer compared with normal mucosa (Tao et al., 2011), while our group showed higher REG4 expression in intestinal metaplasia than in gastritis and gastric cancer (Zheng et al., 2010). REG4 mRNA was found to positively correlate with the wall penetration (Miyagawa et al., 2008), depth of invasion, and clinicopathological stages (Ying et al., 2013) of gastric cancer. These results indicate that REG4 mRNA expression might reflect gastric carcinogenesis and subsequent progression.
In the stomach, foveolar epithelium was negative for REG4, whereas goblet and neuroendocrine cells of intestinal metaplasia were positive for REG4 (Oue et al., 2005). Meanwhile, REG4 expression was significantly associated with both the intestinal mucin phenotype (mucin 2 [MUC2] and CDX2) and neuroendocrine differentiation of gastric cancer (Oue et al., 2005; Yamagishi et al., 2009). Zheng et al. (2010) reported that REG4 immunostaining was gradually decreased from intestinal metaplasia, adenoma, cancer to gastritis, positively correlated with mucin 5AC (MUC-5AC) and MUC-2 expression, and was most frequently expressed in signet ring cell carcinoma (SRCCs). The expression of REG4 was found to be significantly correlated with advanced T stage, N stage, M stage, TNM stage, frequent peritoneal recurrence and dissemination, diffuse-type carcinoma, and dedifferentiation of gastric cancer (Yamagishi et al., 2009; Tao et al., 2011; Moon et al., 2012). REG4 was detected in peritoneal lavage fluids of gastric cancer patients as well (Miyagawa et al., 2008; Kuniyasu et al., 2009; Yamagishi et al., 2009). The serum REG4 level was higher in patients with gastric cancer than in healthy individuals, in advanced than early gastric cancer patients, and in pre-surgical than post-surgical gastric cancer patients respectively (Mitani et al., 2007; Miyagawa et al., 2008; Kobayashi et al., 2010; Zheng et al., 2010). As for prognosis, REG4 immunoexpression was considered as an independent prognostic factor for both worse peritoneal recurrence-free and overall survival (Miyagawa et al., 2008; Tao et al., 2011; Moon et al., 2012). These data demonstrated that REG4 expression is involved in the histogenesis of gastric SRCC, neuroendocrine differentiation, and gastric carcinogenesis, and is closely linked to aggressive behaviors and adverse prognosis in gastric cancers.
With regard to drug resistance, Ying et al. (2013) found that up-regulation of REG4 mRNA was closely linked to the intrinsic drug resistance of gastric cancer cells to fluorouracil (5-FU) or its combination therapy. All 14 REG4-positive patients with gastric cancer showed no change or disease progression when treated with a combination of low-dose 5-FU and cisplatin (Mitani et al., 2007). In gastric cancer cells, REG4 enhanced the resistance of gastric cancer cells to 5-FU through the mitogen-activated protein kinase/extracellular-signal-regulated kinase/Bim pathway (Jin et al., 2017). REG4 antibody significantly inhibited proliferation and chemosensitivity of gastric cancer cells to 5-FU (Zhang et al., 2019) and REG4 silencing caused the loss of stemness properties (Zhou et al., 2013). It was suggested that REG4 overexpression predicted chemoresistance in gastric cancer cells, possibly by promoting proliferation and stemness.
As for molecular mechanisms, REG4 expression facilitated invasion and migration of gastric cancer cells by up-regulating SOX9 expression, in contrast to REG4 knockdown (Zhang et al., 2018). Another report described how REG4 promoted proliferation, tumor growth, and migration of gastric cancer cells through the protein kinase B pathway (Huang et al., 2014a). Katsuno et al. (2012) found that coexpression of aldehyde dehydrogenase one and REG4 was involved in the tumorigenesis of diffuse-type gastric carcinoma, which was blocked by TGF-β. Kuniyasu et al. (2009) found that REG4 overexpression increased levels of Bcl-xL, Bcl-2, survivin, phosphorylated Akt, and EGFR, and decreased nitric oxide-induced apoptosis in gastric cancer cells, in contrast to REG4 silencing. In mice models of gastric cancer, REG4 expression enhanced peritoneal metastasis, weakened apoptosis, and shortened survival time (Miyagawa et al., 2008; Kuniyasu et al., 2009). These results demonstrated that REG4 aggravated the proliferation, anti-apoptosis, tumor growth, and peritoneal metastasis of gastric cancer cells.
At the genetic level, Lu et al. (2013) found that a single nucleotide polymorphism in REG4 might be a genetic marker for the progression of colorectal cancer. In colorectal tissues, REG4 mRNA–positive cells are mostly enteroendocrine and goblet cells. Adenomatous and cancer cells positive for REG4 mRNA exhibited enterocyte-like, mucus-secreting, or undifferentiated features (Violette et al., 2003), in agreement with observations in the stomach (Oue et al., 2005; Yamagishi et al., 2009; Zheng et al., 2010). REG4 mRNA was found to be highly expressed in all adenoma samples, with or without concurrent colorectal carcinoma, compared to normal mucosa samples (Zhang et al., 2003a; Zhang et al., 2003b). Statistically, REG4 mRNA was more expressed in colorectal cancers (especially mucinous carcinomas) than in normal colorectal mucosa (Violette et al., 2003). Combining these results, we conclude that up-regulated REG4 mRNA expression is markedly observed in colorectal adenoma and adenocarcinoma.
At the protein level, REG4 expression was observed in both the middle and outer parts of crypts and superficial epithelium, especially goblets (Granlund et al., 2011). Statistically, REG4 expression was significantly lower in colorectal cancer than in normal mucosa or adenomas, and inversely correlated with poor differentiation, venous invasion, low expression of MUC2 and EGFR phosphorylated at Tyr1068 (Li et al., 2010). REG4 expression was less frequently observed in colorectal cancer than in adjacent non-neoplastic mucosa, in well- and moderately-differentiated adenomas than in mucinous carcinoma (Zheng et al., 2011), and in the cancers of the right colon than in the left colon and rectum, respectively (Kang et al., 2021). Further study showed that REG4 expression was associated with lymph node metastasis, distant metastasis, metastatic recurrence in the liver, advanced TNM stage, histologic grade, and MMP-7 expression in colorectal cancer (Oue et al., 2007; Zhu et al., 2015). Oue et al. (2007) found that the preoperative serum REG4 concentration was not elevated in patients with colorectal cancer at stages 0–III, but was significantly elevated in those at stage IV. Additionally, REG4 expression, as an independent predictor, was significantly linked to a worse prognosis in patients with colorectal cancer (Oue et al., 2007; Numata et al., 2011; He et al., 2014). However, Kaprio et al. (2014) reported that REG4 expression was an independent marker of a lower risk of death for patients with non-mucinous colorectal cancer, 65 years and younger, within 5 years. These findings demonstrated that aberrant REG4 expression is involved in the colorectal adenoma–adenocarcinoma sequence, and can be used to indicate the aggressive behaviors and prognosis of colorectal cancers.
A body of evidence has shown that REG4 was markedly related to chemoresistance, migration, and invasion of cancer cells. Violette et al. (Zhang et al., 2003b) discovered that REG4 protein was strongly expressed in drug-resistant rectal cancer cells, but expressed weakly in drug-sensitive rectal cancer cells. REG4 expression was found to correlate with γ-radiation sensitivity in rectal cancer patients receiving radiotherapy (Kobunai et al., 2011). In radiochemotherapy (RCT)-sensitive colorectal cancer cells, REG4 expression was down-regulated, while it was increased in radiochemoresistant cells (Gao et al., 2021). REG4–overexpressing cells had a high survival rate and showed few DNA breaks after irradiation (He et al., 2014). rhREG4 significantly induced resistance to ionizing radiation in colon adenocarcinoma cells by promoting anti-apoptotic Bcl-xL and Bcl-2 expression (Numata et al., 2011). Additionally, rhREG4 stimulated cell growth in a paracrine manner. Notably, REG4 promoted migration and invasion of colorectal cancer cells via its carbohydrate-recognition domain in both autocrine and paracrine manners, which was significantly decreased by anti-REG4 antibody (Guo et al., 2010; Rafa et al., 2010). Nanakin et al. (2007) found that REG4 expression was stimulated by TNFα, epidermal growth factor (EGF), bFGF, and HGF in colon cancer cells, and then promoted cell proliferation and resistance to H2O2-induced apoptosis. These data indicated that REG4 might be identified as a potential marker for RCT resistance.
In an animal model, REG4 mRNA was elevated in the intestine of APCmin/+ mice carrying APC mutation at codon 850 for transcription stop before a spontaneous second mutation of APC. Adenomas from 14-week-old APCmin/+ mice showed significantly up-regulated expression of Bcl-2 and REG4 (Bishnupuri et al., 2006a). Sorted REG4-positive deep crypt secretory (DCS) cells facilitated organoid formation of single Lgr5 (+) stem cells, and DCS cells overwhelmingly originated from Lgr5 (+) stem cells by both Notch inactivation and Wnt activation (Sasaki et al., 2016). In an organoid model, mutant KRAS-induced REG4 promoted colorectal cancer stemness via a Wnt/β-catenin pathway (Hwang et al., 2020). This was also evidenced by the positive correlation of cancer stem markers with REG4 in intestinal tumors from APCmin+/KrasG12D LA2 mice. These results indicated that REG4 might be involved in the colorectal adenoma–adenocarcinoma sequence by the regulation of local stem cells.
In pancreatic tumors, REG4 mRNA expression was significantly higher in intestinal-type rather than in gastric-type intraductal papillary mucinous neoplasms and normal pancreatic ductal epithelium. REG4 expression was higher in borderline lesions and carcinoma than in adenoma, and in colloid carcinoma than that in tubular carcinoma, respectively, and positively correlated with CDX2 expression (Nakata et al., 2009). A high serum REG4 level could be used to discriminate chronic pancreatitis and pancreatic ductal adenocarcinoma, as well as predict worse survival (Takehara et al., 2006; Takayama et al., 2010; Saukkonen et al., 2018). The pancreatic cancer patients with a higher serum REG4 concentration had an unfavorable response to RCT and frequently experienced local recurrence after surgery (Eguchi et al., 2009). The knockdown of REG4 or an anti-REG4 antibody both attenuated the cell viability of pancreatic cancer cells, while rhREG4 exposure showed the opposite effect in a dose-dependent manner (Eguchi et al., 2009). He et al. (2012) found that REG4 promoted not only tumor growth but also invasion of pancreatic cancer cells by up-regulating MMP-7 and MMP-9. REG4–overexpressing pancreatic cancer cells were resistant to gemcitabine and γ-radiation (Eguchi et al., 2009). These data suggested that REG4 overexpression contributed to pancreatic carcinogenesis and subsequent progression by promoting proliferation, invasion, and RCT resistance.
REG4 mRNA expression was significantly higher in gallbladder adenocarcinoma than peritumoral normal tissues, adenoma, and cholecystitis (Yang et al., 2016), in line with findings by Tamura et al. (Tamura et al., 2009). Immunohistochemically, REG4 was negative in all normal gallbladders and cholelithiasis, but positive in 50% of intestinal metaplasia with adenomyomatosis, and positive in 56% of gallbladder carcinomas (Tamura et al., 2009). REG4 expression was positively correlated with dedifferentiation, local invasiveness, and lymph node metastasis of gallbladder cancer. REG4 expression was independently associated with a poor prognosis in patients with advanced gallbladder cancer (Yang et al., 2016). A high serum REG4 level was evident preoperatively in four (33%) of 12 patients with gallbladder cancer, but not in benign diseases, and was postoperatively reduced (Tamura et al., 2009). These findings indicated that REG4 is involved in the carcinogenesis and subsequent progression of gallbladder adenocarcinoma.
In ovarian carcinogenesis, REG4 mRNA and protein levels were higher in ovarian tumors than in normal ovaries, in mucinous carcinomas than in serous carcinomas, and in well- and moderately-differentiated carcinomas than in poorly-differentiated carcinomas, respectively (Chen et al., 2015), in line with another report (Xiang et al., 2022). REG4 protein expression was significantly higher in ovarian mucinous borderline tumors and mucinous carcinomas than in mucinous cystadenomas, and was more closely associated with ovarian borderline, intestinal-type, mucinous tumors rather than in endocervical-like type tumors. A significant positive correlation existed between CDX2 and REG4 expression in primary ovarian mucinous tumors (Huang et al., 2014b). High REG4 mRNA expression was inversely associated with inferior overall, progression-free and post-progression survival in patients with ovarian cancer receiving platinum chemotherapy (Xiang et al., 2022). As an independent factor, the expression of REG4 was an overall or relapse-free poor prognostic factor for patients with ovarian cancer (Chen et al., 2015). In ovarian cancer cells, REG4 expression or 5-FU chemoresistance was enhanced by CDX2 transfection, in contrast to CDX2 knockdown (Koh et al., 2019). Either REG4 overexpression or rhREG4 treatment promoted proliferation, G2/S progression, anti-apoptosis, migration, invasion, and cisplatin and paclitaxel resistance in ovarian cancer cells (Chen et al., 2015; Xiang et al., 2022). Taken together, REG4 overexpression might play an important role in ovarian carcinogenesis and subsequent aggressiveness.
In renal clear cell carcinoma, the immunoexpression of REG4 was not detectable due to a lack of neuroendocrine and intestinal differentiation (Hayashi et al., 2009). In prostate cancer, 14 (14%) of 98 cases had tissues positive for REG4 staining, which was associated with MUC2 and chromogranin A expression. The expression of REG4 was a significant prognostic factor and independent predictor of the relapse-free survival of patients with prostate cancer. In prostate cancer, the serum REG4 concentration was significantly higher in patients with prostate cancer than in control individuals (Ohara et al., 2008). The overexpression of REG4 was also observed in hormone refractory xenografts and refractory metastatic prostate cancer (Gu et al., 2005). rhREG4 treatment enhanced EGFR phosphorylation in prostate cancer cells (Ohara et al., 2008). Taking these findings together, we speculated that REG4 was involved in prostate carcinogenesis and subsequent progression, especially in those patients showing intestinal mucin and neuroendocrine differentiation.
REG4 is physiologically found in selected enteroendocrine cells (EEC). REG4-positive gastric EECs were associated with serotonin, gastrin, somatostatin, and pancreatic polypeptide (Sentani et al., 2010). REG4 was differentially expressed in ECCs of the small intestine and colon (Heiskala and Andersson, 2013). REG4 showed a cellular co-distribution with serotonin, substance P or chromogranin A in the gastrointestinal tract. Subpopulations of REG4-positive cells overlapped with EECs containing GLP-1, GLP-2, peptide YY, secretin, and ghrelin, relying on the anatomical sites of the tissues. Therefore, REG4 is thought to be involved in intestinal and neuroendocrine differentiation. Oue et al. (2005) found that insulin-secreting β cells of the pancreas were positive for REG4. Of 21 gastric SRCCs, 16 colorectal SRCCs, 10 breast SRCCs, and 47 lung SRCCs, all gastric and colorectal SRCCs showed REG4 immunopositivity, but the others did not indicate that REG4 might be a biomarker specific for gastrointestinal SRCCs (Sentani et al., 2008).
In the esophagus, REG4 staining was not detected in squamous cell carcinoma (SCC) and small cell carcinomas, whereas REG4 staining was found in four of 10 (40%) adenocarcinoma samples. The serum REG4 level was significantly higher in patients with SCC than in control participants, and correlated with the control participants’ age (Oue et al., 2011). In the lung, REG4 was highly expressed in KRAS-mutant adenocarcinoma with thyroid transcription factor-1 (TTF-1) hypoexpression. Silencing significantly REG4 reduced proliferation and tumor growth, and arrested the cell cycle by regulating E2F targets and the G2/M checkpoint (Sun et al., 2019). In glioma, REG4 expression was significantly higher in tumor than normal brain tissues. REG4 immunoreactivity was significantly associated with advanced pathological grade and a low Karnofsky performance score, and short survival as an independent prognostic factor (Wang et al., 2012). Sasahira et al. (2008) found that REG4 was expressed in salivary duct epithelia and acinus myoepithelia, but not in squamous epithelia. REG4 expression was found in 41% (17/41) of adenoid cystic carcinomas (ACCs), but not in SCCs, and was associated with lymph node involvement and a poor prognosis in ACC. These findings indicated that REG4 expression was not detectable in SCC, but in glioma, and ACC.
In conclusion, altered REG4 expression is involved in infection and inflammation, and in gastric, colorectal, pancreatic, gallbladder, ovarian and urothelial cancers, where it is closely linked to aggressive behaviors and a poor prognosis. REG4 expression and recombinant REG4 aggravated tumorigenesis, proliferation, anti-apoptosis, chemoradioresistance, migration, invasion, peritoneal dissemination, tumor growth, and cancer stemness by EGFR/Akt/AP-1 and Akt/GSK3β/β-catenin/TCF-4 pathways. Histologically, REG4 protein is highly expressed in neuroendocrine tumors and SRCCs. In accordance with recent findings about REG4, we believe that REG4 should be used as a biomarker for SRCC and neuroendocrine tumors, and contributes to the histogenesis of gastric intestinal–metaplasia–globoid dysplasia–SRCC. Aberrant REG4 expression should be employed to predict the tumorigenesis, aggressive behaviors and poor prognosis of malignancies, and the lavage REG4 level should be determined to guard against peritoneal dissemination.
HZ and HX were mainly responsible for literature review and manuscript writing. HZ and C-YZ completed the construction pictures. HZ designed the ideas of this paper and modified the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by: Award for Liaoning Distinguished Professor, Natural Science Foundation of Hebei Province (21377772D) and National Natural Scientific Foundation of China (81672700).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JY declared a shared parent affiliation with the author CZ to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Azman, J., Starcevic Klasan, G., Ivanac, D., Picard, A., Jurisic-Erzen, D., Nikolic, M., et al. (2011). Reg IV protein and mRNA expression in different rat organs. Acta Histochem. 113 (8), 793–797. doi:10.1016/j.acthis.2010.11.008
Bishnupuri, K. S., Luo, Q., Korzenik, J. R., Henderson, J. O., Houchen, C. W., Anant, S., et al. (2006a). Dysregulation of Reg gene expression occurs early in gastrointestinal tumorigenesis and regulates anti-apoptotic genes. Cancer Biol. Ther. 5 (12), 1714–1720. doi:10.4161/cbt.5.12.3469
Bishnupuri, K. S., Luo, Q., Murmu, N., Houchen, C. W., Anant, S., and Dieckgraefe, B. K. (2006b). Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology 130 (1), 137–149. doi:10.1053/j.gastro.2005.10.001
Bishnupuri, K. S., Luo, Q., Sainathan, S. K., Kikuchi, K., Sureban, S. M., Sabarinathan, M., et al. (2010). Reg IV regulates normal intestinal and colorectal cancer cell susceptibility to radiation-induced apoptosis. Gastroenterology 138 (2), 616–626. doi:10.1053/j.gastro.2009.10.050
Bishnupuri, K. S., Sainathan, S. K., Bishnupuri, K., Leahy, D. R., Luo, Q., Anant, S., et al. (2014). Reg4-induced mitogenesis involves Akt-GSK3β-β-Catenin-TCF-4 signaling in human colorectal cancer. Mol. Carcinog. 53 (101), E169–E180. doi:10.1002/mc.22088
Bishnupuri, K. S., Sainathan, S. K., Ciorba, M. A., Houchen, C. W., and Dieckgraefe, B. K. (2022). Reg4 interacts with CD44 to regulate proliferation and stemness of colorectal and pancreatic cancer cells. Mol. Cancer Res. 20 (3), 387–399. doi:10.1158/1541-7786.MCR-21-0224
Chai, D., Du, H., Li, K., Zhang, X., Li, X., Zhao, X., et al. (2021). CDX2 and Reg IV expression and correlation in gastric cancer. BMC Gastroenterol. 21 (1), 92. doi:10.1186/s12876-021-01678-9
Chen, S., Gou, W. F., Zhao, S., Niu, Z. F., Zhao, Y., Takano, Y., et al. (2015). The role of the REG4 gene and its encoding product in ovarian epithelial carcinoma. BMC Cancer 15, 471. doi:10.1186/s12885-015-1435-2
Du, F., and Yao, Z. W. (2013). The expression patterns of Reg IV gene in normal rat reproduction system. J. Exp. Zool. A Ecol. Genet. Physiol. 319 (1), 32–38. doi:10.1002/jez.1771
Duan, Y., Hu, L., Liu, B., Yu, B., Li, J., Yan, M., et al. (2014). Tumor suppressor miR-24 restrains gastric cancer progression by downregulating RegIV. Mol. Cancer 13, 127. doi:10.1186/1476-4598-13-127
Eguchi, H., Ishikawa, O., Ohigashi, H., Takahashi, H., Yano, M., Nishiyama, K., et al. (2009). Serum REG4 level is a predictive biomarker for the response to preoperative chemoradiotherapy in patients with pancreatic cancer. Pancreas 38 (7), 791–798. doi:10.1097/MPA.0b013e3181ac5337
Gao, L., Wu, X., Zhang, L., Dai, Y., Zhu, Z., Zhi, Y., et al. (2021). REG4 is a Potential biomarker for radiochemotherapy sensitivity in colorectal cancer. Onco. Targets. Ther. 14, 1605–1611. doi:10.2147/OTT.S296031
Granlund, A. V., Beisvag, V., Torp, S. H., Flatberg, A., Kleveland, P. M., Ostvik, A. E., et al. (2011). Activation of REG family proteins in colitis. Scand. J. Gastroenterol. 46 (11), 1316–1323. doi:10.3109/00365521.2011.605463
Gu, Z., Rubin, M. A., Yang, Y., Deprimo, S. E., Zhao, H., Horvath, S., et al. (2005). Reg IV: A promising marker of hormone refractory metastatic prostate cancer. Clin. Cancer Res. 11 (6), 2237–2243. doi:10.1158/1078-0432.CCR-04-0356
Guo, Y., Xu, J., Li, N., Gao, F., and Huang, P. (2010). RegIV potentiates colorectal carcinoma cell migration and invasion via its CRD domain. Cancer Genet. cytogenet. 199 (1), 38–44. doi:10.1016/j.cancergencyto.2010.01.011
Hartupee, J. C., Zhang, H., Bonaldo, M. F., Soares, M. B., and Dieckgraefe, B. K. (2001). Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochim. Biophys. Acta 1518 (3), 287–293. doi:10.1016/s0167-4781(00)00284-0
Hayashi, T., Matsubara, A., Ohara, S., Mita, K., Hasegawa, Y., Usui, T., et al. (2009). Immunohistochemical analysis of Reg IV in urogenital organs: Frequent expression of Reg IV in prostate cancer and potential utility as serum tumor marker. Oncol. Rep. 21 (1), 95–100.
He, H. L., Lee, Y. E., Shiue, Y. L., Lee, S. W., Lin, L. C., Chen, T. J., et al. (2014). Overexpression of REG4 confers an independent negative prognosticator in rectal cancers receiving concurrent chemoradiotherapy. J. Surg. Oncol. 110 (8), 1002–1010. doi:10.1002/jso.23764
He, X. J., Jiang, X. T., Ma, Y. Y., Xia, Y. J., Wang, H. J., Guan, T. P., et al. (2012). REG4 contributes to the invasiveness of pancreatic cancer by upregulating MMP-7 and MMP-9. Cancer Sci. 103 (12), 2082–2091. doi:10.1111/cas.12018
Heiskala, K., and Andersson, L. C. (2013). Reg IV is differently expressed in enteroendocrine cells of human small intestine and colon. Regul. Pept. 183, 27–34. doi:10.1016/j.regpep.2013.03.007
Ho, M. R., Lou, Y. C., Wei, S. Y., Luo, S. C., Lin, W. C., Lyu, P. C., et al. (2010). Human RegIV protein adopts a typical C-type lectin fold but binds mannan with two calcium-independent sites. J. Mol. Biol. 402 (4), 682–695. doi:10.1016/j.jmb.2010.07.061
Hu, G., Shen, J., Cheng, L., Guo, C., Xu, X., Wang, F., et al. (2011). Reg4 protects against acinar cell necrosis in experimental pancreatitis. Gut 60 (6), 820–828. doi:10.1136/gut.2010.215178
Huang, J., Yang, Y., Yang, J., and Li, X. (2014a). Regenerating gene family member 4 promotes growth and migration of gastric cancer through protein kinase B pathway. Int. J. Clin. Exp. Med. 7 (9), 3037–3044.
Huang, Q., Chen, X., Lu, W., Lai, M., and Lu, B. (2014b). Expression of REG4 in ovarian mucinous tumors. Appl. Immunohistochem. Mol. Morphol. 22 (4), 295–301. doi:10.1097/PAI.0b013e3182936d8e
Hwang, J. H., Yoon, J., Cho, Y. H., Cha, P. H., Park, J. C., and Choi, K. Y. (2020). A mutant KRAS-induced factor REG4 promotes cancer stem cell properties via Wnt/β-catenin signaling. Int. J. Cancer 146 (10), 2877–2890. doi:10.1002/ijc.32728
Jin, J., Lv, H., Wu, J., Li, D., Chen, K., Zhang, F., et al. (2017). Regenerating family member 4 (Reg4) enhances 5-Fluorouracil resistance of gastric cancer through activating MAPK/Erk/Bim signaling pathway. Med. Sci. Monit. 23, 3715–3721. doi:10.12659/msm.903134
Kang, G., Oh, I., Pyo, J., Kang, D., and Son, B. (2021). Clinicopathological significance and prognostic implications of REG4 immunohistochemical expression in colorectal cancer. Med. Kaunas. 57 (9), 938. doi:10.3390/medicina57090938
Kaprio, T., Hagström, J., Mustonen, H., Koskensalo, S., Andersson, L. C., and Haglund, C. (2014). REG4 independently predicts better prognosis in non-mucinous colorectal cancer. PLoS One 9 (10), e109600. doi:10.1371/journal.pone.0109600
Katsuno, Y., Ehata, S., Yashiro, M., Yanagihara, K., Hirakawa, K., and Miyazono, K. (2012). Coordinated expression of REG4 and aldehyde dehydrogenase 1 regulating tumourigenic capacity of diffuse-type gastric carcinoma-initiating cells is inhibited by TGF-β. J. Pathol. 228 (3), 391–404. doi:10.1002/path.4020
Kawasaki, Y., Matsumura, K., Miyamoto, M., Tsuji, S., Okuno, M., Suda, S., et al. (2015). REG4 is a transcriptional target of GATA6 and is essential for colorectal tumorigenesis. Sci. Rep. 5, 14291. doi:10.1038/srep14291
Kobayashi, Y., Niwa, Y., Tajika, M., Kawai, H., Kondo, S., Hara, K., et al. (2010). Serum tumor antigen REG4 as a useful diagnostic biomarker in gastric cancer. Hepatogastroenterology. 57 (104), 1631–1634.
Kobunai, T., Watanabe, T., and Fukusato, T. (2011). REG4, NEIL2, and BIRC5 gene expression correlates with gamma-radiation sensitivity in patients with rectal cancer receiving radiotherapy. Anticancer Res. 31 (12), 4147–4153.
Koh, I., Nosaka, S., Sekine, M., Sugimoto, J., Hirata, E., and Kudo, Y. (2019). Regulation of REG4 expression and prediction of 5-Fluorouracil sensitivity by CDX2 in ovarian mucinous carcinoma. Cancer Genomics Proteomics 16 (6), 481–490. doi:10.21873/cgp.20151
Kuniyasu, H., Oue, N., Sasahira, T., Yi, L., Moriwaka, Y., Shimomoto, T., et al. (2009). Reg IV enhances peritoneal metastasis in gastric carcinomas. Cell Prolif. 42 (1), 110–121. doi:10.1111/j.1365-2184.2008.00577.x
Li, X. H., Zheng, Y., Zheng, H. C., Takahashi, H., Yang, X. H., Masuda, S., et al. (2010). REG IV overexpression in an early stage of colorectal carcinogenesis: An immunohistochemical study. Histol. Histopathol. 25 (4), 473–484. doi:10.14670/HH-25.473
Li, X. J., Zhu, F., Li, B., Zhang, D., and Liang, C. W. (2021). Recombinant human regenerating gene 4 attenuates the severity of osteoarthritis by promoting the proliferation of articular chondrocyte in an animal model. Curr. Mol. Pharmacol. 15, 693–699. doi:10.2174/1874467214666210901163144
Li, Y. G., Ji, D. F., Zhong, S., Zhu, J. X., Chen, S., and Hu, G. Y. (2011). Anti-tumor effects of proteoglycan from Phellinus linteus by immunomodulating and inhibiting Reg IV/EGFR/Akt signaling pathway in colorectal carcinoma. Int. J. Biol. Macromol. 48 (3), 511–517. doi:10.1016/j.ijbiomac.2011.01.014
Liu, C. M., Hsieh, C. L., He, Y. C., Lo, S. J., Liang, J. A., Hsieh, T. F., et al. (2013). In vivo targeting of ADAM9 gene expression using lentivirus-delivered shRNA suppresses prostate cancer growth by regulating REG4 dependent cell cycle progression. PLoS One 8 (1), e53795. doi:10.1371/journal.pone.0053795
Lu, S., Bevier, M., Huhn, S., Sainz, J., Lascorz, J., Pardini, B., et al. (2013). Genetic variants in C-type lectin genes are associated with colorectal cancer susceptibility and clinical outcome. Int. J. Cancer 133 (10), 2325–2333. doi:10.1002/ijc.28251
Ma, X., Wu, D., Zhou, S., Wan, F., Liu, H., Xu, X., et al. (2016). The pancreatic cancer secreted REG4 promotes macrophage polarization to M2 through EGFR/AKT/CREB pathway. Oncol. Rep. 35 (1), 189–196. doi:10.3892/or.2015.4357
Mitani, Y., Oue, N., Matsumura, S., Yoshida, K., Noguchi, T., Ito, M., et al. (2007). Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5-fluorouracil-based chemotherapy. Oncogene 26 (30), 4383–4393. doi:10.1038/sj.onc.1210215
Miyagawa, K., Sakakura, C., Nakashima, S., Yoshikawa, T., Fukuda, K., Kin, S., et al. (2008). Overexpression of RegIV in peritoneal dissemination of gastric cancer and its potential as A novel marker for the detection of peritoneal micrometastasis. Anticancer Res. 28 (2B), 1169–1179.
Moon, J. H., Fujiwara, Y., Nakamura, Y., Okada, K., Hanada, H., Sakakura, C., et al. (2012). REGIV as a potential biomarker for peritoneal dissemination in gastric adenocarcinoma. J. Surg. Oncol. 105 (2), 189–194. doi:10.1002/jso.22021
Naito, Y., Oue, N., Hinoi, T., Sakamoto, N., Sentani, K., Ohdan, H., et al. (2012). Reg IV is a direct target of intestinal transcriptional factor CDX2 in gastric cancer. PLoS One 7 (11), e47545. doi:10.1371/journal.pone.0047545
Nakata, K., Nagai, E., Ohuchida, K., Aishima, S., Hayashi, A., Miyasaka, Y., et al. (2009). REG4 is associated with carcinogenesis in the 'intestinal' pathway of intraductal papillary mucinous neoplasms. Mod. Pathol. 22 (3), 460–468. doi:10.1038/modpathol.2008.205
Nanakin, A., Fukui, H., Fujii, S., Sekikawa, A., Kanda, N., Hisatsune, H., et al. (2007). Expression of the REG IV gene in ulcerative colitis. Lab. Invest. 87 (3), 304–314. doi:10.1038/labinvest.3700507
Nata, K., Liu, Y., Xu, L., Ikeda, T., Akiyama, T., Noguchi, N., et al. (2004). Molecular cloning, expression and chromosomal localization of a novel human REG family gene, REG III. Gene 340 (1), 161–170. doi:10.1016/j.gene.2004.06.010
Numata, M., Oshima, T., Yoshihara, K., Watanabe, T., Tsuchida, K., Tamagawa, H., et al. (2011). Relationship between RegIV gene expression to outcomes in colorectal cancer. J. Surg. Oncol. 104 (2), 205–209. doi:10.1002/jso.21906
Ohara, S., Oue, N., Matsubara, A., Mita, K., Hasegawa, Y., Hayashi, T., et al. (2008). Reg IV is an independent prognostic factor for relapse in patients with clinically localized prostate cancer. Cancer Sci. 99 (8), 1570–1577. doi:10.1111/j.1349-7006.2008.00846.x
Oue, N., Kuniyasu, H., Noguchi, T., Sentani, K., Ito, M., Tanaka, S., et al. (2007). Serum concentration of reg IV in patients with colorectal cancer: Overexpression and high serum levels of reg IV are associated with liver metastasis. Oncology 72 (5-6), 371–380. doi:10.1159/000113147
Oue, N., Mitani, Y., Aung, P. P., Sakakura, C., Takeshima, Y., Kaneko, M., et al. (2005). Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J. Pathol. 207 (2), 185–198. doi:10.1002/path.1827
Oue, N., Noguchi, T., Anami, K., Sentani, K., Sakamoto, N., Uraoka, N., et al. (2011). Serum concentration and expression of Reg IV in patients with esophageal cancer: Age-related elevation of serum Reg IV concentration. Oncol. Lett. 2 (2), 235–239. doi:10.3892/ol.2011.239
Qi, H., Wei, J., Gao, Y., Yang, Y., Li, Y., Zhu, H., et al. (2020). Reg4 and complement factor D prevent the overgrowth of E. coli in the mouse gut. Commun. Biol. 3 (1), 483. doi:10.1038/s42003-020-01219-2
Rafa, L., Dessein, A. F., Devisme, L., Buob, D., Truant, S., Porchet, N., et al. (2010). REG4 acts as a mitogenic, motility and pro-invasive factor for colon cancer cells. Int. J. Oncol. 36 (3), 689–698. doi:10.3892/ijo_00000544
Sasahira, T., Oue, N., Kirita, T., Luo, Y., Bhawal, U. K., Fujii, K., et al. (2008). Reg IV expression is associated with cell growth and prognosis of adenoid cystic carcinoma in the salivary gland. Histopathology 53 (6), 667–675. doi:10.1111/j.1365-2559.2008.03188.x
Sasaki, N., Sachs, N., Wiebrands, K., Ellenbroek, S. I., Fumagalli, A., Lyubimova, A., et al. (2016). Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc. Natl. Acad. Sci. U. S. A. 113 (37), E5399–E5407. doi:10.1073/pnas.1607327113
Saukkonen, K., Hagström, J., Mustonen, H., Lehtinen, L., Carpen, O., Andersson, L. C., et al. (2018). Prognostic and diagnostic value of REG4 serum and tissue expression in pancreatic ductal adenocarcinoma. Tumour Biol. 40 (3), 1010428318761494. doi:10.1177/1010428318761494
Sentani, K., Oue, N., Noguchi, T., Sakamoto, N., Matsusaki, K., and Yasui, W. (2010). Immunostaining of gastric cancer with neuroendocrine differentiation: Reg IV-positive neuroendocrine cells are associated with gastrin, serotonin, pancreatic polypeptide and somatostatin. Pathol. Int. 60 (4), 291–297. doi:10.1111/j.1440-1827.2010.02519.x
Sentani, K., Oue, N., Tashiro, T., Sakamoto, N., Nishisaka, T., Fukuhara, T., et al. (2008). Immunohistochemical staining of Reg IV and claudin-18 is useful in the diagnosis of gastrointestinal signet ring cell carcinoma. Am. J. Surg. Pathol. 32 (8), 1182–1189. doi:10.1097/PAS.0b013e318163a8f8
Sninsky, J. A., Bishnupuri, K. S., González, I., Trikalinos, N. A., Chen, L., and Dieckgraefe, B. K. (2021). Reg4 and its downstream transcriptional activator CD44ICD in stage II and III colorectal cancer. Oncotarget 12 (4), 278–291. doi:10.18632/oncotarget.27896
Sun, S., Hu, Z., Huang, S., Ye, X., Wang, J., Chang, J., et al. (2019). REG4 is an indicator for KRAS mutant lung adenocarcinoma with TTF-1 low expression. J. Cancer Res. Clin. Oncol. 145 (9), 2273–2283. doi:10.1007/s00432-019-02988-y
Takasawa, S., Tsuchida, C., Sakuramoto-Tsuchida, S., Takeda, M., Itaya-Hironaka, A., Yamauchi, A., et al. (2018). Expression of human REG family genes in inflammatory bowel disease and their molecular mechanism. Immunol. Res. 66 (6), 800–805. doi:10.1007/s12026-019-9067-2
Takayama, R., Nakagawa, H., Sawaki, A., Mizuno, N., Kawai, H., Tajika, M., et al. (2010). Serum tumor antigen REG4 as a diagnostic biomarker in pancreatic ductal adenocarcinoma. J. Gastroenterol. 45 (1), 52–59. doi:10.1007/s00535-009-0114-y
Takehara, A., Eguchi, H., Ohigashi, H., Ishikawa, O., Kasugai, T., Hosokawa, M., et al. (2006). Novel tumor marker REG4 detected in serum of patients with resectable pancreatic cancer and feasibility for antibody therapy targeting REG4. Cancer Sci. 97 (11), 1191–1197. doi:10.1111/j.1349-7006.2006.00297.x
Tamura, H., Ohtsuka, M., Washiro, M., Kimura, F., Shimizu, H., Yoshidome, H., et al. (2009). Reg IV expression and clinicopathologic features of gallbladder carcinoma. Hum. Pathol. 40 (12), 1686–1692. doi:10.1016/j.humpath.2009.06.001
Tao, H. Q., He, X. J., Ma, Y. Y., Wang, H. J., Xia, Y. J., Ye, Z. Y., et al. (2011). Evaluation of REG4 for early diagnosis and prognosis of gastric cancer. Hum. Pathol. 42 (10), 1401–1409. doi:10.1016/j.humpath.2010.08.023
Violette, S., Festor, E., Pandrea-Vasile, I., Mitchell, V., Adida, C., Dussaulx, E., et al. (2003). Reg IV, a new member of the regenerating gene family, is overexpressed in colorectal carcinomas. Int. J. Cancer 103 (2), 185–193. doi:10.1002/ijc.10788
Wang, F., Xu, L., Guo, C., Ke, A., Hu, G., Xu, X., et al. (2011). Identification of RegIV as a novel GLI1 target gene in human pancreatic cancer. PLoS One 6 (4), e18434. doi:10.1371/journal.pone.0018434
Wang, H., Hu, L., Zang, M., Zhang, B., Duan, Y., Fan, Z., et al. (2016). REG4 promotes peritoneal metastasis of gastric cancer through GPR37. Oncotarget 7 (19), 27874–27888. doi:10.18632/oncotarget.8442
Wang, Q., Deng, J., Yuan, J., Wang, L., Zhao, Z., He, S., et al. (2012). Oncogenic Reg IV is a novel prognostic marker for glioma patient survival. Diagn. Pathol. 7, 69. doi:10.1186/1746-1596-7-69
Xiang, L. W., Xue, H., Ha, M. W., Yu, D. Y., Xiao, L. J., and Zheng, H. (2022). The effects of REG4 expression on chemoresistance of ovarian cancer. J. Obstet. Gynaecol. Tokyo. 1995., 1–9. doi:10.1080/01443615.2022.2106834
Xiao, Y., Lu, Y., Wang, Y., Yan, W., and Cai, W. (2019). Deficiency in intestinal epithelial Reg4 ameliorates intestinal inflammation and alters the colonic bacterial composition. Mucosal Immunol. 12 (4), 919–929. doi:10.1038/s41385-019-0161-5
Yamagishi, H., Fukui, H., Sekikawa, A., Kono, T., Fujii, S., Ichikawa, K., et al. (2009). Expression profile of REG family proteins REG ialpha and REG IV in advanced gastric cancer: Comparison with mucin phenotype and prognostic markers. Mod. Pathol. 22 (7), 906–913. doi:10.1038/modpathol.2009.41
Yang, L., Lan, S., Liu, J., and Yang, Z. (2016). Expression of MK-1 and RegIV and its clinicopathological significances in the benign and malignant lesions of gallbladder. Diagn. Pathol. 6, 100. doi:10.1186/1746-1596-6-100
Ying, L. S., Yu, J. L., Lu, X. X., and Ling, Z. Q. (2013). Enhanced RegIV expression predicts the intrinsic 5-fluorouracil (5-FU) resistance in advanced gastric cancer. Dig. Dis. Sci. 58 (2), 414–422. doi:10.1007/s10620-012-2381-3
Yonemura, Y., Takashima, T., Miwa, K., Miyazaki, I., Yamamoto, H., and Okamoto, H. (1984). Amelioration of diabetes mellitus in partially depancreatized rats by poly(ADP-ribose) synthetase inhibitors. Evidence of islet B-cell regeneration. Diabetes 33 (4), 401–404. doi:10.2337/diab.33.4.401
Zhang, H. B., Luo, H. C., Xin, X. J., and Zeng, A. Z. (2015). Up-regulated Reg proteins induced by Interleukin-22 treatment ameliorate acute liver injury in rat model. Int. J. Clin. Exp. Med. 8 (1), 1253–1258.
Zhang, J., Zhu, Z., Miao, Z., Huang, X., Sun, Z., Xu, H., et al. (2021). The clinical significance and mechanisms of REG4 in human cancers. Front. Oncol. 10, 559230. doi:10.3389/fonc.2020.559230
Zhang, N., Chai, D., Du, H., Li, K., Xie, W., Li, X., et al. (2018). Expression of Reg IV and SOX9 and their correlation in human gastric cancer. BMC Cancer 18, 344. doi:10.1186/s12885-018-4285-x
Zhang, X. Q., Yu, L. T., Du, P., Yin, T. Q., Zhang, Z. Y., Xu, Y., et al. (2019). Single-chain antibody against Reg4 suppresses gastric cancer cell growth and enhances 5-FU-induced cell death in vitro. Anticancer. Agents Med. Chem. 19 (5), 610–619. doi:10.2174/1871520619666181122104720
Zhang, Y., Lai, M., Gu, X., Luo, M., and Shao, L. (2003). Reg IV, a differentially expressed gene in colorectal adenoma. Chin. Med. J. 116 (6), 918–922.
Zhang, Y., Lai, M., Lv, B., Gu, X., Wang, H., Zhu, Y., et al. (2003). Overexpression of Reg IV in colorectal adenoma. Cancer Lett. 200 (1), 69–76. doi:10.1016/s0304-3835(03)00460-9
Zheng, H. C., Sugawara, A., Okamoto, H., Takasawa, S., Takahashi, H., Masuda, S., et al. (2011). Expression profile of the REG gene family in colorectal carcinoma. J. Histochem. Cytochem. 59 (1), 106–115. doi:10.1369/jhc.2010.956961
Zheng, H. C., Xu, X. Y., Yu, M., Takahashi, H., Masuda, S., and Takano, Y. (2010). The role of Reg IV gene and its encoding product in gastric carcinogenesis. Hum. Pathol. 41 (1), 59–69. doi:10.1016/j.humpath.2009.06.013
Zhou, W., Sun, M., Wang, D. L., Wang, Y., Jin, F., Zhang, Y. Y., et al. (2013). Silencing of RegIV by shRNA causes the loss of stemness properties of cancer stem cells in MKN45 gastric cancer cells. Oncol. Rep. 30 (6), 2685–2690. doi:10.3892/or.2013.2745
Zhu, X., Han, Y., Yuan, C., Tu, W., Qiu, G., Lu, S., et al. (2015). Overexpression of Reg4, alone or combined with MMP-7 overexpression, is predictive of poor prognosis in colorectal cancer. Oncol. Rep. 33 (1), 320–328. doi:10.3892/or.2014.3559
ACCs adenoid cystic carcinomas
AP-1 activator protein-1
ATF2 activating transcription factor 2
Bcl-xl B cell lymphoma-extra large
Bcl-2 B cell lymphoma two
bFGF basic fibroblast growth factor
CDX2 caudal type homeobox two
CD44ICD CD44 intracytoplasmic domain
C-type lectin calcium-dependent lectin
DCS deep crypt secretory
EEC enteroendocrine cells
EGF epidermal growth factor
EGFR epidermal growth factor receptor
GATA6 GATA binding protein six
GLI1 GLI family zinc finger one;
GPR37 G protein-coupled receptor 37
GSK3β glycogen synthase kinase three β
HGF hepatocyte growth factor
IL interleukin
Klf4 Kruppel-like factor 4
Lgr5 leucine-rich repeat-containing G-protein coupled receptor five
miR MicroRNA
MMP-7 matrix metalloproteinase-7
MUC2 mucin two
RCT radiochemotherapy
REG regenerating islet-derived
REG4 Regenerating islet-derived four
rhREG4 recombinant human REG4
SCC squamous cell carcinoma
SOX2 SRY-box transcription factor 2
SOX9 SRY-box transcription factor 9
SP1 specificity protein one
SRCCs signet ring cell carcinomas
TCF-4 transcription factor 4
TGF transforming growth factor
TNF tumor necrosis factor
TTF-1 transcription factor-1;
UC ulcerative colitis
5-FU 5-fluorouracil
Keywords: cancer, REG4, tumor suppressor, tumor phenotype, transcriptional regulation
Citation: Zheng H-C, Xue H and Zhang C-Y (2022) REG4 promotes the proliferation and anti-apoptosis of cancer. Front. Cell Dev. Biol. 10:1012193. doi: 10.3389/fcell.2022.1012193
Received: 05 August 2022; Accepted: 26 August 2022;
Published: 12 September 2022.
Edited by:
Bin Liu, Jiangsu Ocean Universiity, ChinaCopyright © 2022 Zheng, Xue and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua-Chuan Zheng, emhlbmdfaHVhY2h1YW5AaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.