
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Cell Dev. Biol. , 30 August 2022
Sec. Signaling
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.1004376
This article is part of the Research Topic Regulation of Endoplasmic Reticulum and Mitochondria in Cellular Homeostasis View all 15 articles
Editorial on the Research Topic
Regulation of endoplasmic reticulum and mitochondria in cellular homeostasis
Endoplasmic reticulum (ER) is an interconnected single membrane-bound network and the largest organelle in the eukaryotic cell. The major function of ER includes the synthesis of proteins and lipids, protein folding and maturation, membrane biogenesis, xenobiotic detoxification, and cellular Ca2+ storage. Various mechanisms are conducted to help ER to complete these functions. ER-associated protein degradation (ERAD) mechanism is a conserved protein quality control pathway to erase misfolded, mistargeted and unassembled proteins to maintain ER homeostasis. ER stress, another critical mechanism to regulate ER homeostasis, is induced by the accumulation of misfolded proteins in the ER and triggers the unfolded protein response. Besides recovering cellular homeostasis to maintain cell survival, persistent or intense ER stress could also result in apoptosis, autophagic cell death, or ferroptosis. Altogether, targeting ER via modulating ERAD or ER stress would be beneficial for organism development and disease therapy.
Mitochondrion is a complex double-membraned cellular organelle that harbors their own genome. Mitochondrion acts as the energy-producing center in cells for producing ATP via the oxidative phosphorylation system. The mitochondrion also serves as a powerful center for the precursors of macromolecules, such as DNA, RNA, proteins, and lipids. Besides the well-known roles in cell metabolism and energy conversion, mitochondria also participate in redox homeostasis, Ca2+ homeostasis, stress response, metabolite transport, cell signaling, cell cycle distribution, differentiation, cell death, embryonic development, and aging. Emerging evidence has shown that mitochondrial malfunction is closely related to a broad spectrum of human diseases, including cancer and neurological disorder. Mitochondria execute these functions via various molecular and cellular mechanisms. For example, mitophagy, a tightly regulated biological process defined as autophagy-dependent selective degradation of damaged or excessive mitochondria, acts an important role in the quality and quantity control of mitochondria to maintain ultimate cellular homeostasis. Mitochondria-mediated intrinsic apoptosis, regulated by caspases, Bcl-2 family of proteins, Smac/DIABLO, death receptors, IAPs, Omi/HtrA2 and cytochrome c, is one of the major apoptotic pathways. In sum, modulating mitochondria-mediated cell apoptosis, mitochondrial dynamics, mitochondrial energy metabolism, and some other mitochondrial physiological processes would be critical for cells to rebuild homeostasis.
ER and mitochondria are all essential for maintaining cellular homeostasis. To fulfill the goal, an efficient and fast exchange of materials between the ER and mitochondria is required. In addition, there are physical interactions between ER and mitochondria at specific sites (named mitochondria-associated ER membrane, MAMs) in which the surfaces of the two organelles juxtapose at a constant distance, for several nm in length. The unique contact sites serve as the platform for communication between the two organelles and are critical for the regulation of cellular homeostasis, including Ca2+ homeostasis, proteostasis, and ER redox homeostasis. Different pathways and numerous mediators participate in the cross-talk between ER and mitochondria. For example, Mitofusin 2 (Mfn2), fission protein 1 homologue (Fis1) and B-cell receptor-associated protein 31 (BAP31) play a crucial role in mitochondrial fusion and fission (Barazzuol et al., 2021). Among them, BAP31, a polytopic integral membrane protein of ER, functions in apoptosis and participates in several kinds of diseases, including cancers (Li et al., 2022a). BAP31 interacting with Fis1 in MAMs generates an essential platform for the procaspase eight recruitment and transduces apoptotic signal from mitochondria to ER. BAP31 could be cleaved into proapoptotic p20BAP31 in case procaspase eight is recruited and activated to transmit ER calcium signals to mitochondria rapidly. Certainly, there are also some other proteins interacting in MAMs to bridge the crosstalk between ER and mitochondria.
In this Research Topic, we have published 14 papers. Excitedly, there are many novel findings regarding the mechanism and/or application associated with ER and mitochondria. 5-hydroxymethylfurfural, a furan-containing aldehyde widely presented in various sacchariferous foods, is shown to alleviate inflammatory lung injury by suppressing ER stress (Zhang et al.). In another study, a lipophilic glycoprotein named milk fat globule EGF factor 8 (MFG-E8) is reported to inhibit ER stress to maintain cellular homeostasis in pancreatic exocrine acinar cells during acute pancreatitis (Ren et al.). These studies convey a message that ER stress acts as an important mediator in the various physiological and pathological processes. In our previous studies, we also found that modulating ER stress could be beneficial for inhibiting cancer development (Huang et al., 2021; Li et al., 2022b). Since the existence of the ER-mitochondria interface, the stress from ER could also send a signal to mitochondria and even further receive feedback from mitochondria. In this Research Topic, Rajalekshmy Shyam etc. report that there was dilated ER and elevated expression of ER stress markers BIP and CHOP in mouse Slc4a11−/− corneal endothelial tissue, and concludes that mitochondrial ROS could induce ER stress (Shyam et al.). Furthermore, the Research Topic also collected several review articles deciphering the role of ER and mitochondria in many biological events.
However, it is a pity that we just received limited papers about the mechanisms of ER and mitochondria regulating cellular homeostasis, the contribution of modulating these two organelles to embryo and disease development, and novel methods to assess the crosstalk between ER and mitochondria. In addition, we consider that it would be wonderful to investigate the crosstalk between ER and mitochondria in future research using editing techniques, such as CRISPR-Cas9 and DdCBE. It would also be interesting to study the molecular mechanism between ER and mitochondria interplay and novel cell death modes, such as ferroptosis, NETosis, pyroptosis, and cuproptosis. Expectedly, it would have great significance for regulating the ER and mitochondria to maintain cellular homeostasis to prevent the occurrence of disease (Figure 1).
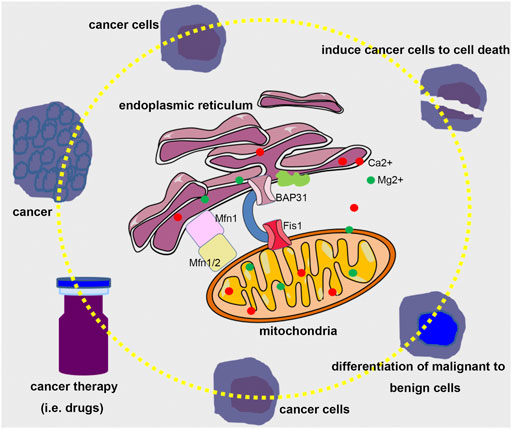
FIGURE 1. A bold assumption for modulating the crosstalk between ER and mitochondria in cancer therapy. Considering the significant role of ER and mitochondria in determining cells to restore cellular homeostasis or induce programmed cell death, it would be possible that tuning the interplay between ER and mitochondria using proper therapeutic means could guide cancer cells to death or differentiate malignant to benign cells.
YH drafted the manuscript, JJ revised the draft, QZ and JS made substantial contributions to the work through in-depth discussion. All the authors proposed the Research Topic theme, made a direct and intellectual contribution to the work, and approved the final version for publication.
This study was funded by the National Natural Science Foundation of China (No. 81502582). Funding was also provided by the Fundamental Research Funds for the Central Universities (N182004002), Natural Science Foundation of Liaoning Province (2021-MS-104, 2022-YGJC-39), and Fundamental Scientific Research Fund of Liaoning Provincial Education Department (LJKQZ2021002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Barazzuol, L., Giamogante, F., and Cali, T. (2021). Mitochondria associated membranes (MAMs): Architecture and physiopathological role. Cell Calcium 94, 102343. doi:10.1016/j.ceca.2020.102343
Huang, Y., Yuan, K., Tang, M., Yue, J., Bao, L., Wu, S., et al. (2021). Melatonin inhibiting the survival of human gastric cancer cells under ER stress involving autophagy and Ras-Raf-MAPK signalling. J. Cell. Mol. Med. 25 (3), 1480–1492. doi:10.1111/jcmm.16237
Li, T., Hao, Z., Tang, Z., Li, C., Cheng, L., Wang, T., et al. (2022a). BAP31 regulates wnt signaling to modulate cell migration in lung cancer. Front. Oncol. 12, 859195. doi:10.3389/fonc.2022.859195
Keywords: endoplasmic reticulum, mitochondria, homeostasis, redox, cell death, aging
Citation: Huang Y, Ji J, Zhao Q and Song J (2022) Editorial: Regulation of endoplasmic reticulum and mitochondria in cellular homeostasis. Front. Cell Dev. Biol. 10:1004376. doi: 10.3389/fcell.2022.1004376
Received: 27 July 2022; Accepted: 01 August 2022;
Published: 30 August 2022.
Edited and reviewed by:
Ana Cuenda, Spanish National Research Council (CSIC), SpainCopyright © 2022 Huang, Ji, Zhao and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongye Huang, aHVhbmd5b25neWU4OEAxNjMuY29t; Jianguang Ji, amlhbmd1YW5nLmppQG1lZC5sdS5zZQ==; Qi Zhao, emhhb3FpQGxudS5lZHUuY24=; Jun Song, c29uZ2p1bkBuZWF1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.