- Departamento de Bioquímica y Biología Molecular, Facultad de Farmacia, Universidad de Sevilla, and Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío/CSIC, Seville, Spain
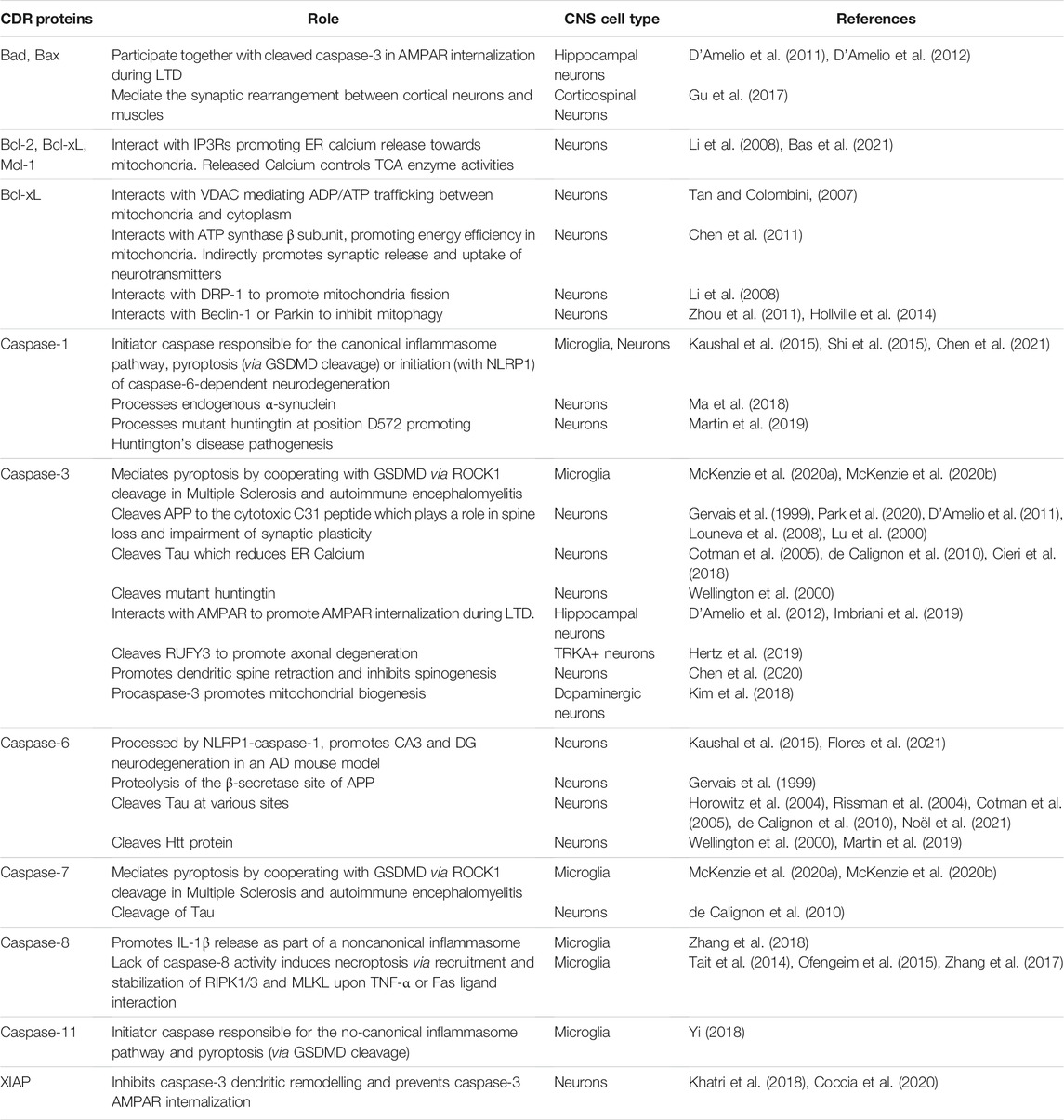
Cell death related (CDR) proteins are a diverse group of proteins whose original function was ascribed to apoptotic cell death signaling. Recently, descriptions of non-apoptotic functions for CDR proteins have increased. In this minireview, we comment on recent studies of CDR proteins outside the field of apoptosis in the CNS, encompassing areas such as the inflammasome and non-apoptotic cell death, cytoskeleton reorganization, synaptic plasticity, mitophagy, neurodegeneration and calcium signaling among others. Furthermore, we discuss the evolution of proteomic techniques used to predict caspase substrates that could potentially explain their non-apoptotic roles. Finally, we address new concepts in the field of non-apoptotic functions of CDR proteins that require further research such the effect of sexual dimorphism on non-apoptotic CDR protein function and the emergence of zymogen-specific caspase functions.
Introduction
CDR proteins include a variety of proteins (e.g. caspases, members of the Bcl-2 family, etc.) whose actions directly affect the outcome of the apoptotic cell death (i.e. pro-apoptotic vs. anti-apoptotic proteins). Considerable progress had been made in the field of apoptosis since its seminal discovery by the labs of Brenner, Sulston and Horvitz, whose findings were recognized with the Nobel Prize in Physiology or Medicine in 2002. Since then, knowledge of the individual proteins critical to the apoptotic process has increased considerably, and in fact widened to encompass new and exciting non-apoptotic functions. In this mini-review, we examine the non-apoptotic roles of caspases as well as other CDR proteins such as members of the Bcl-2 protein family and X-chromosome-linked inhibitor of apoptosis (XIAP). We will focus on particularly in the CNS, where these non-apoptotic roles play great relevance in the physiology of the different cell types forming it. We will briefly summarize these non-apoptotic functions [which have already been reviewed in depth in the CNS (Shen et al., 2018; Espinosa-Oliva et al., 2019; Rodríguez-Gómez et al., 2020)] to focus more on the most recent findings in this exciting field.
Diversity of Caspase Functions
Caspases are a family of cysteine proteases (Schauperl et al., 2015; Julien and Wells, 2017), traditionally known for their roles in apoptotic cell death during development and disease (Shen et al., 2018). However, since the late nineties, seminal studies have described various non-apoptotic roles for caspases, such as the differentiation of lens fiber cells (Ishizaki et al., 1998), and processes necessary for erythropoiesis (De Maria et al., 1999), erythroid terminal differentiation (Zermati et al., 2001) and maturation (Carlile et al., 2004). Since then, the list of distinct caspase functions has increased and diversified significantly, including within the CNS.
Inflammasome, Pyroptosis and Necroptosis
Some of the first non-apoptotic functions described for caspases were linked to inflammatory processes, and alternative cell death pathways, pyroptosis and necroptosis. The inflammasome is a multiprotein intracellular complex (Martinon et al., 2002), triggered by external stimuli that leads to the production and release of pro-inflammatory cytokines IL-1β and IL-18. The complex consists of a sensor protein such as NLRP1 or NLRP3, an adaptor protein named apoptosis-associated speck-like protein containing a caspase activation and recruitment domains (ASC), and caspase-1, caspase-11 (Schroder and Tschopp, 2010; Yi, 2018; Chen et al., 2021) or caspase-8 (Zhang et al., 2018) in microglia cells. There is a study that suggests that caspase-6, implicated in axonal degeneration and cognitive decline in AD (Simon et al., 2012; Geden et al., 2019), may play an additional role in inflammasome activation in neurons (Kaushal et al., 2015). Although the mechanism is not well elucidated, the authors showed that NLRP1-Caspase-1 activation of caspase-6 in neurons caused neurodegeneration in hippocampal regions CA3 and DG in an AD model (Kaushal et al., 2015), implicating this pathway as a therapeutic target (Flores et al., 2021).
Inflammasome activation may progress to a regulated cell death process termed pyroptosis, during which caspase-1 cleaves gasdermin D (GSDMD) provoking plasma membrane pore formation that causes cell lysis (Shi et al., 2015). Recently, studies have linked apoptotic cell death pathways with pyroptosis in the CNS, specifically in human primary microglia (McKenzie et al., 2020a). McKenzie et al. illustrated that active caspase-3/7 may mediate pyroptosis via ROCK1, alongside the well described caspase-1-GSDMD pathway, in microglia in multiple sclerosis and its animal model experimental autoimmune encephalomyelitis. Of interest, in human microglia, caspase-3/7 activation during pyroptosis required inflammasome activation, and treatment with VX-765 (caspase-1 inhibitor) reduced cleaved caspase-3 under pyroptotic but not apoptotic stimulus. This contribution supports the idea of convergence of different cell death pathways in the CNS and provides a novel therapeutic target for pyroptotic mediated cell death (McKenzie et al., 2020b).
Necroptosis in microglia is a regulated cell death mechanism initiated by the interaction of receptor-interacting protein kinase (RIPK)-1 and RIPK3, mixed lineage kinase domain-like protein (MKLK) and caspase-8 (Tait et al., 2014; Ofengeim et al., 2015). This interaction occurs upon ligand binding to death receptors such as TNFR or FAS. Under these conditions, activation of caspase-8 promotes apoptosis and actively inhibits necroptosis by direct cleavage of RIPK1 and RIPK3. By contrast, in the absence of caspase-8 following genetic ablation, pharmacological inhibition, or as a result of certain viral infections, RIPK1 and RIPK3 are stabilized and recruit mixed-lineage kinase domain-like protein (MLKL) into complex IIb, also known as the necrosome, which initiates necroptosis (Zhang et al., 2017).
Caspases and Aberrant Proteostasis
There are common pathological features shared by various neurodegenerative diseases such as Huntington’s disease, Alzheimer’s disease (AD), Parkinson’s disease, and frontotemporal lobar degeneration. One is a chronic inflammatory response and another is an aberrant proteostasis which leads to aggregation and deposition of intracellular and/or extracellular protein inclusions (Lee et al., 2011). Many of these neurodegeneration-linked aggregation-prone proteins such as amyloid precursor protein (APP) (Gervais et al., 1999; Park et al., 2020), tau (Cotman et al., 2005; de Calignon et al., 2010), α-synuclein (Ma et al., 2018), or huntingtin (Wellington et al., 2000; Martin et al., 2019) can be processed by caspases, generating toxic fragments that aggregate, thereby linking caspase activity with the etiopathogenesis of these diseases. Recent studies have described new characteristics of these caspase-generated toxic fragments. One example in mouse primary cortical neurons is a caspase-3 generated tau fragment, that aggregates and forms neurofibrillary tangles and induces a reduction of Ca2+ levels in the endoplasmic reticulum (ER) and enhancement of ER-mitochondria communication (Cieri et al., 2018). The exact relationship between caspase-3-cleaved tau and the enhancement of the contact sites between ER and mitochondria, and Ca2+ levels is unknown. However, the authors suggest that this could be an important pathological event in tau-related neurodegenerative disease. Similarly, the ability to impact neuronal ER-mitochondria communication, has been reported for α-synuclein (Calì et al., 2012), and β-amyloid (Aβ) oligomers (Calvo-Rodriguez et al., 2019). While the role for caspase-3 in facilitating nucleation-dependent tau filament formation is well described, the importance of caspase-6 in this process is not so evident. Early studies described caspase-6 as capable of processing tau (Horowitz et al., 2004; Rissman et al., 2004). However, in hippocampal and cortical neurons tau fragments generated by caspase-6 are insufficient per se to induce tau pathogenesis, as shown recently using a transgenic knock-in mouse model expressing full-length human tau together with human Casp6 (Noël et al., 2021).
Altered functional connectivity and synaptic degeneration is the best pathological correlate of the cognitive decline seen in AD that occurs at the early stages of disease (Terry et al., 1991). The role of caspase-3 in this process, based on its ability to cleave APP and release cytotoxic fragments, has been highlighted several times (D’Amelio et al., 2011; Louneva et al., 2008; Lu et al., 2000; Banwait et al., 2008). However, the intricate relationship amongst caspase-cleaved APP fragments, caspase activation and synaptic loss has not been directly shown. Recent experiments in hippocampal neuronal organotypic slice cultures from APP knock-in mice that are resistant to cleavage by caspase-3 prevented Aβ-dependent caspase activation and release of the putatively cytotoxic C31 peptide, which plays an essential role in Aβ-induced dendritic spine loss and impairment in synaptic plasticity (Park et al., 2020).
Synaptic plasticity in the context of learning and memory as well as neurodegeneration and neuronal injury can also be affected by caspase activity. To date, involvement of pro-apoptotic proteins (Li et al., 2010; Jiao and Li, 2011) such as caspase-3, Bad, and Bax have been shown to regulate internalization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) subunits in hippocampal synapses, a process necessary for long-term depression (LTD) (D’Amelio et al., 2012). Additionally, a recent Parkinson’s disease study reported that in striatal medium spiny neurons present in corticostriatal slices from PINK1 knockout (KO) mice exhibit impaired LTD, which was restored with low doses of caspase-3 activator α-(Trichloromethyl)-4-pyridineethanol. Interestingly when LTD was induced in PINK1 KO mice, reduced activity of caspase-3 compared to wild type was observed (Imbriani et al., 2019). It is likely the PINK1 deficiency impacts the fine modulation of caspase cascade which in turn may perturb long-term synaptic plasticity machinery, leading to synaptic dysfunction. Peripheral nerve injury downregulates caspase-3 expression in neurons present in the anterior cingulate cortex, where its interaction with AMPAR regulates LTD. The absence of LTD, due to downregulation of caspase-3, enhances synaptic transmission in somatosensory pathways and contributes to peripheral hypersensitivity. Intriguingly, caspase-3 directly regulates LTD via AMPAR subunit cleavage and subsequent internalization (Wang et al., 2020).
Caspase-Mediated Reorganization of the Cytoskeleton
Caspases are involved in the reorganization of the cytoskeleton of neurons, either for the correct wiring of the olfactory sensory neurons axons (Ohsawa et al., 2010) or by eliminating the dendrites, dendritic spines or axons (Cusack et al., 2013; Kaushal et al., 2015). Axonal or dendritic pruning allows for selective degeneration of excessive, misguided, or unnecessary neuronal extensions without degeneration of the entire cell, and occurs not only during brain development but also for plasticity in the mature brain (Saxena and Caroni, 2007). Importantly, failure of this process has been associated with neurodevelopmental (Riccomagno and Kolodkin, 2015; Thomas et al., 2016), and neurodegenerative disorders (Burke and O'Malley, 2013; Stokin et al., 2005; Vickers et al., 2009). Controlled axonal pruning is similar in many ways to the active self-destruction of cells during apoptosis (Geden et al., 2019). Hertz et al. found in Tropomyosin receptor kinase A positive neurons that dephosphorylation and cleavage of RUFY3, an adaptor protein for small GTPases (Kitagishi and Matsuda, 2013), which acts downstream of or in parallel to caspase 3 activation, is required for TRKA+ sensory axon degeneration upon trophic deprivation. Importantly, depletion of RUFY3 protects from axonal degeneration even in presence of active caspase-3 (Hertz et al., 2019). This protein could be a possible new checkpoint, allowing neurons to locally control caspase-driven degeneration. It is noteworthy that RUFY3 expression was increased in the olfactory bulbs of AD patients compared with healthy individuals across early and advanced AD (Zelaya et al., 2015).
Caspase-3 can control dendritic spine density and architecture (Mukherjee and Williams, 2017). Under stress conditions caused by oligomycin A in primary cultured hippocampal neurons, mitochondrial F1Fo ATP synthase dysfunction induces non-apoptotic caspase-3 activation in dendrites concurrent with dendritic spine retraction and spinogenesis suppression. Moreover, the inhibition of caspase activation using a pan-caspase inhibitor protected against dendritic spine damage. This finding illustrates the contribution of non-apoptotic caspase activity to dendritic spine turnover (Chen et al., 2020).
Non-Apoptotic Functions of Other CDR Proteins
CNS non-apoptotic functions have been described for CDR proteins such as XIAP and Bcl-2 family. In some cases, CDR proteins modulate caspase activity directly, affecting non-apoptotic caspase functions. For instance, regulation of XIAP degradation via ubiquitination may be responsible for some of the non-apoptotic roles of caspase-3. Specifically, XIAP inhibits the ability of caspase-3 to shape dendrites through cleavage of microtubules, a process that, unregulated, has been observed in various autism spectrum disorders (Khatri et al., 2018). Furthermore, in hippocampal neurons XIAP modulates caspase-3 mediated AMPAR internalization in synapses in NMDA receptor-dependent LTD (Coccia et al., 2020). The pro-apoptotic proteins Bax and Bak trigger non-apoptotic caspase activity required for synaptic rearrangement between corticospinal neurons and muscles in mice during early development (Gu et al., 2017).
Non-caspase CDR proteins also contribute to non-apoptotic CNS processes independently of their regulation of caspase activity. One example is the control of mitochondrial Ca2+ homeostasis through direct interaction of BCL-2 family members with Ca2+ transporters (such as IP3Rs) present at mitochondria-ER contact points. Bcl-2, Bcl-xL and Mcl-1 interact with IP3Rs (Nougarede et al., 2018). The outcome of this interaction on the permeability of IP3Rs is not yet clear. For instance, in the case of Bcl-2 homolog Nrh/BCL2L10, its interaction with I3PRs negatively regulates ER-Ca2+ release (Nougarede et al., 2018). In the case of Bcl-xL, it’s a matter of debate whether it’s interaction with IP3Rs affects IP3R permeability (topic reviewed in (Popgeorgiev and Gillet, 2021)). It has been reported that Bcl-xL interaction with IP3Rs can promote calcium release and regulate mitochondrial metabolism, due to the requirement of calcium by several enzymes of the tri-carboxylic acid cycle and the electronic transport chain, as well as the ADP/ATP translocator and ATP synthase in neurons (Bas et al., 2021). However a recent study has shown that Bcl-xL inhibits IP3R-mediated Ca2+ release, contributing to the anti-apoptotic response against Ca2+-driven apoptosis induced by drugs such as Staurosporine (Rosa et al., 2021). Bcl-xL can indirectly regulate cellular energy levels by modulating ADP/ATP trafficking between mitochondria and the cytoplasm, through its interaction with voltage-dependent anion channels (VDAC) (Tan and Colombini, 2007). Furthermore in neurons, Bcl-xL directly interacts with the ATP synthase β subunit (Chen et al., 2011), stabilizing the membrane potential across the inner mitochondrial membrane and increasing overall energetic efficiency. The interaction between Bcl-xL and the ATP synthase β-subunit is blocked upon Bcl-xL phosphorylation by the cyclin B1-Cdk1 complex, a process that is dysregulated in AD neurons (Veas-Pérez de Tudela et al., 2015). One of the consequences of an increase in neuronal ATP is the promotion of synaptic release and uptake of neurotransmitters. Interestingly, Bcl-xL itself promotes endocytic vesicle retrieval in hippocampal neurons through protein–protein interaction with components of the clathrin complex (Li et al., 2013). Bcl-xL affects mitochondrial dynamics, in particular mitochondria fission, by interacting with dynamin-related protein 1 in cultured hippocampal neurons (Li et al., 2008). Additionally, Bcl-xL inhibits mitophagy either via its inhibitory interaction with Beclin-1 [reviewed in Zhou et al. (2011)] or by blocking Parkin translocation to depolarized mitochondria (Hollville et al., 2014).
Proteomic Search for Non-Apoptotic Caspase Substrates in the CNS
New non-apoptotic roles are constantly emerging for the CDR proteins. The involvement of caspase enzyme activity in such a wide range of key biological signaling processes has led to a strong interest in the identification of caspase substrates. Key to the detection of caspase substrates has been the resolution of the caspase tetrapeptide substrate recognition motif, which specifies the preferred amino acid sequence recognized by specific caspases. Although the motifs have in common an aspartate (D) at position P1 (Talanian et al., 1997), the amino acids at the other positions vary for most caspases, and are merely a predictor of caspase cleavage site. For example, only 1% of known caspase-3/7 substrates contain the full DEVD* substrate recognition motif (Julien and Wells, 2017). Additionally, it should also be noted that many substrates are targets for multiple caspases, caspase-3 being the most promiscuous of the family (Benkova et al., 2009). Bioinformatic tools have been developed to identify potential caspase substrates using algorithms that predict substrate recognition motifs within a protein’s amino acid sequence such as PEPS (Lohmüller et al., 2003), GraBCas (Backes et al., 2005), and CasCleave, with the latter taking into account protein structural information and solvent accessibility (Song et al., 2010).
Experimentally, the hunt for caspase substrates at the proteomic level in native cells involves incubating a recombinant caspase with cell lysate, isolating the subsequently cleaved proteins with newly exposed N-termini, and digesting and identifying the peptide fragments by mass spectrometry (Gevaert et al., 2003; Mahrus et al., 2008). A variation of this method provides more certainty of the responsible caspase by neutralizing proteases in the cell lysate prior to the addition of exogenous purified caspase (Julien et al., 2016). These methods have resulted in the identification of hundreds of native substrates for many caspases (Benkova et al., 2009) under apoptotic (Dix et al., 2008; Mahrus et al., 2008) and inflammatory conditions (Agard et al., 2010) in Jurkat T lymphocytes or THP-1 monocyte cell lines (Araya et al., 2021). The search for CNS-relevant or CNS specific caspase substrates has been less well studied. Recently, once such study carried out in isolated neuronal synaptosomes from C57 mice identified approximately 70 substrates, 48 previously unidentified, of caspases 3 and 6 (Victor et al., 2018), signaling the importance of caspase activity in synaptic plasticity. Similarly, in a model of auditory brainstem development, extracellular vesicles were isolated from the brainstems of embryonic chicks, and subjected to nanoLC-MS/MS, leading to the identification of caspase-3 substrates (288/5,653 unique proteins), of which 193 were novel (Weghorst et al., 2020). Based on a functional enrichment analysis of the novel caspase-3 substrates found, the authors suggest a non-apoptotic role of caspase-3 in axon guidance and maturation of auditory nuclei. These proteomic studies highlight the utility of non-biased approaches to caspase substrate mining.
We have included a table summarizing the different non-apoptotic roles of the different CDRs discussed in this manuscript (Table 1) as well as a schematic representation for some of those non-apoptotic roles of CDR proteins in CNS (Figure 1).
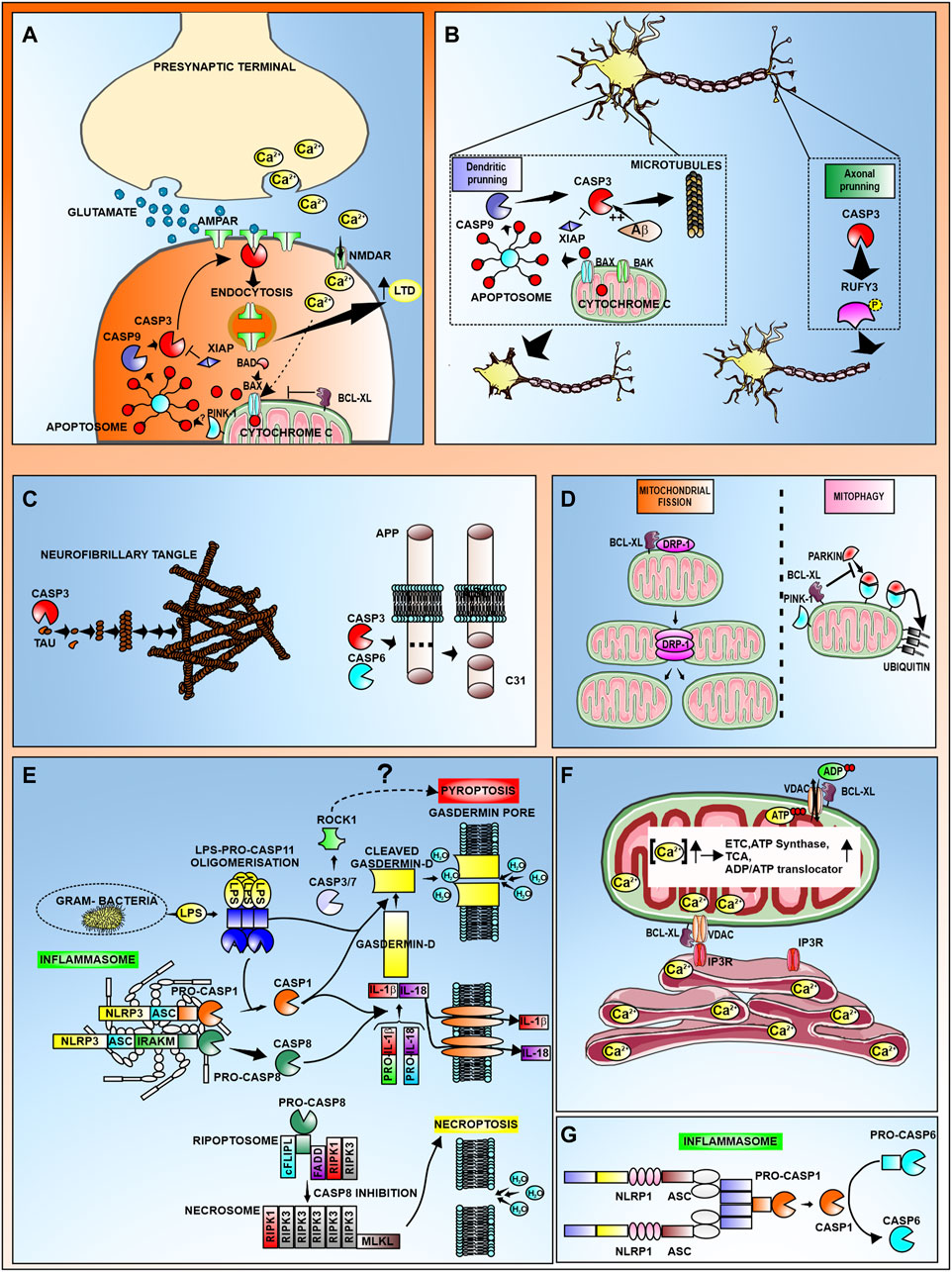
FIGURE 1. Schematic representation the non-apoptotic roles of CDR proteins in CNS. (A) Induction of Long term depression (LTD) requires active caspase-3 mediated internalization of AMPAR subunits. Calcium released at the presynaptic terminal is transported through NMDAR, promoting cytochrome c release from mitochondria. Cytochrome c can also be released via Bad/Bax translocation. Subsequent formation of the apoptosome leads to activation of caspase-3 at the postsynaptic terminal, where the active form binds to AMPAR, promoting its endocytosis. Internalization of AMPAR results in increased LTD. This process can be antagonized by the proteins XIAP and Bcl-xL. (B) Caspase-3 mediates dendritic and axonal pruning via cytoskeletal destabilization. During dendritic pruning, caspase-3 cleaves microtubules leading to a reduction in dendritic spines. In AD, Aβ promotes caspase-3 activation. Active caspase-3 cleaves dephosphorylated RUFY3 to promote axonal pruning (C) Among the neuronal substrates of caspases are aggregation-prone proteins such as Tau and APP. (D) Bcl-xL promotes mitochondrial fission by interacting with DRP-1, and inhibits mitophagy by preventing the Parkin-PINK1 ubiquitination of mitochondrial proteins. (E) Representation of canonical (caspase-1) and non-canonical (caspase-11 or capase-8) inflammasome activation that leads to the processing and release of IL-1β and IL-18 and the processing of Gasdermin D leading to pyroptosis. It has been suggested that active caspase-3/7 can also mediate pyroptosis through cleavage of ROCK-1. The complex formed by mixed lineage kinase domain like pseudokinase (MLKL) together with Receptor-interacting protein kinase (RIPK)-1 and RIPK3, can promote necroptosis in the absence of active caspase-8. (F) Bcl-xL promotes Calcium trafficking from the ER to mitochondria through interaction with IP3R and VDAC. The resulting increase in mitochondrial calcium concentration activates several proteins in the electron transport chain (ETC), tricarboxylic acid cycle (TCA), as well as ATP synthase and the ADP/ATP translocator which altogether leads to the increase in ATP production. Also, Bcl-xL modulates ADP/ATP trafficking from the cytosol to the mitochondria through its interaction with VDAC. (G) Activation of caspase-6 mediated by the NLRP1-caspase-1 inflammasome in neurons has been implicated in hippocampal AD neurodegeneration.
Sexual Dimorphism in CDR Protein Function
In the CNS, as well as in the periphery, there is increasing evidence of a gender-dependent natural bias in various disorders. In AD and Parkinson’s disease, the ratio of affected males to females and the severity of the disease depending on gender, differs significantly (Ullah et al., 2019). In ischemic stroke, females suffer worse cognitive outcomes than males (Selvamani and Sohrabji, 2017; Raval et al., 2019). An example of this dichotomy can be found in stroke, where mechanisms underlying ischemic cell death are caspase-independent in males and caspase-dependent in females (Siegel et al., 2011). The explanation for this difference in cell death mechanisms remains elusive although some studies have found differences in miRNAs expressed in each gender upon injury that could be linked with the exertion of one or other cell death mechanism. In an ischemic mouse model, the authors found an increase in the levels of miR-23a that targets XIAP in females, promoting caspase-dependent cell death mechanisms in this gender (Siegel et al., 2011). In a separate study, high levels of miR363-3p, that targets caspase-3, were found in the serum of adult female rats with small infarct volume compared to other groups with greater stroke-associated impairment (Selvamani and Sohrabji, 2017). Could sexual dimorphism affecting cell death mechanisms also affect non-apoptotic caspase roles? There are some indications that support this hypothesis. For instance, in a glaucoma model, treatment with estradiol inhibited the ability of caspase-3 to process tau, decreasing the formation of neurofibrillary tangles (Means et al., 2021). In a separate study, an increase in the expression of inflammasome components (including caspase-1) in aged female rats compared to aged-match males was linked to their decrease in estrogen levels (Raval et al., 2019). Taken together, these results suggest that the inclusion of both genders in future studies of non-apoptotic roles for caspase and other CDR proteins should be taken into consideration.
Discussion
A combination of traditional techniques in biochemistry, molecular biology and microscopy with other multi-omic and bioinformatic approaches have made possible the discovery of new non-apoptotic roles for caspase and other CDR proteins. Many of these new non apoptotic roles are based on well-established mechanisms, like for instance caspase’s enzymatic activity, that depending on the substrate will play either an apoptotic or non-apoptotic role. Interestingly, a new study highlighting a non-apoptotic function of caspases independently of their enzymatic activity has recently emerged in the CNS. Studies performed by Kim and colleagues have described a novel role for procaspase-3 in promoting mitochondrial biogenesis in dopaminergic neurons through induction of the synthesis of the transcription factors Tfam and Nrf-1, necessary for mitochondrial biogenesis (Kim et al., 2018). This highlights the possibility of new functions for other caspases independently of their protease activity. These roles could also be affected by a potential gender dependent natural bias and should be taken into consideration in future studies for non-apoptotic roles for CDR proteins.
Author Contributions
Conceptualization, BB-K, EK, and MB; writing—review and editing BB-K, RM-G, AF-C, MG-C, EK, and MB; artwork, MB; supervision, project administration and funding acquisition MB. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by the Spanish Ministerio de Ciencia, Innovación y Universidades/FEDER/UE PID 2019-107948RA-I00, FEDER I + D + i-USE US-1265062. MAB. was funded by the Spanish Ministerio de Economia y Competitividad (Programa Ramón y Cajal: RYC-2017-21804). BB-K, was funded by a predoctoral fellowship granted by Spanish Ministerio de Ciencia e Innovación (PRE 2020-095781). AF-C is funded by Fondo Social Europeo (FSE) within Marco del Sistema Nacional de Garantía Juvenil y del Programa Operativo de Empleo Juvenil 2014–2020.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; APP, amyloid precursor protein; CDR, Cell Death Related; GSDMD, Gasdermin D; LTD, long term depression; RIPK, Receptor interacting protein kinase; VDAC, voltage-dependent anion channels.
References
Agard, N. J., Maltby, D., and Wells, J. A. (2010). Inflammatory Stimuli Regulate Caspase Substrate Profiles. Mol. Cell Proteomics 9 (5), 880–893. doi:10.1074/mcp.m900528-mcp200
Araya, L. E., Soni, I. V., Hardy, J. A., and Julien, O. (2021). Deorphanizing Caspase-3 and Caspase-9 Substrates in and Out of Apoptosis with Deep Substrate Profiling. ACS Chem. Biol. 16 (11), 2280–2296. doi:10.1021/acschembio.1c00456
Backes, C., Kuentzer, J., Lenhof, H.-P., Comtesse, N., and Meese, E. (2005). GraBCas: a Bioinformatics Tool for Score-Based Prediction of Caspase- and Granzyme B-Cleavage Sites in Protein Sequences. Nucleic Acids Res. 33 (Web Server issue), W208–W213. doi:10.1093/nar/gki433
Banwait, S., Galvan, V., Zhang, J., Gorostiza, O. F., Ataie, M., Huang, W., et al. (2008). C-terminal Cleavage of the Amyloid-β Protein Precursor at Asp664: A Switch Associated with Alzheimer's Disease. Jad 13 (1), 1–16. doi:10.3233/jad-2008-13101
Bas, J., Nguyen, T., and Gillet, G. (2021). Involvement of Bcl-xL in Neuronal Function and Development. Int. J. Mol. Sci. 22 (6), 3202. doi:10.3390/ijms22063202
Benkova, B., Lozanov, V., Ivanov, I. P., and Mitev, V. (2009). Evaluation of Recombinant Caspase Specificity by Competitive Substrates. Anal. Biochem. 394 (1), 68–74. doi:10.1016/j.ab.2009.07.012
Burke, R. E., and O'Malley, K. (2013). Axon Degeneration in Parkinson's Disease. Exp. Neurol. 246, 72–83. doi:10.1016/j.expneurol.2012.01.011
Calì, T., Ottolini, D., Negro, A., and Brini, M. (2012). α-Synuclein Controls Mitochondrial Calcium Homeostasis by Enhancing Endoplasmic Reticulum-Mitochondria Interactions. J. Biol. Chem. 287 (22), 17914–17929. doi:10.1074/jbc.m111.302794
Calvo-Rodriguez, M., Hernando-Perez, E., Nuñez, L., and Villalobos, C. (2019). Amyloid β Oligomers Increase ER-Mitochondria Ca2+ Cross Talk in Young Hippocampal Neurons and Exacerbate Aging-Induced Intracellular Ca2+ Remodeling. Front. Cel. Neurosci. 13, 22. doi:10.3389/fncel.2019.00022
Carlile, G. W., Smith, D. H., and Wiedmann, M. (2004). Caspase-3 Has a Nonapoptotic Function in Erythroid Maturation. Blood 103 (11), 4310–4316. doi:10.1182/blood-2003-09-3362
Chen, H., Tian, J., Guo, L., and Du, H. (2020). Caspase Inhibition Rescues F1Fo ATP Synthase Dysfunction-Mediated Dendritic Spine Elimination. Sci. Rep. 10 (1), 17589. doi:10.1038/s41598-020-74613-9
Chen, Y.-b., Aon, M. A., Hsu, Y.-T., Soane, L., Teng, X., McCaffery, J. M., et al. (2011). Bcl-xL Regulates Mitochondrial Energetics by Stabilizing the Inner Membrane Potential. J. Cel Biol 195 (2), 263–276. doi:10.1083/jcb.201108059
Chen, Y., Gong, K., Guo, L., Zhang, B., Chen, S., Li, Z., et al. (2021). Downregulation of Phosphoglycerate Mutase 5 Improves Microglial Inflammasome Activation after Traumatic Brain Injury. Cell Death Discov. 7 (1), 290. doi:10.1038/s41420-021-00686-8
Cieri, D., Vicario, M., Vallese, F., D'Orsi, B., Berto, P., Grinzato, A., et al. (2018). Tau Localises within Mitochondrial Sub-compartments and its Caspase Cleavage Affects ER-Mitochondria Interactions and Cellular Ca2+ Handling. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1864 (10), 3247–3256. doi:10.1016/j.bbadis.2018.07.011
Coccia, E., Planells-Ferrer, L., Badillos-Rodríguez, R., Pascual, M., Segura, M. F., Fernández-Hernández, R., et al. (2020). SIVA-1 Regulates Apoptosis and Synaptic Function by Modulating XIAP Interaction with the Death Receptor Antagonist FAIM-L. Cell Death Dis 11 (2), 82. doi:10.1038/s41419-020-2282-x
Cotman, C. W., Poon, W. W., Rissman, R. A., and Blurton-Jones, M. (2005). The Role of Caspase Cleavage of Tau in Alzheimer Disease Neuropathology. J. Neuropathol. Exp. Neurol. 64 (2), 104–112. doi:10.1093/jnen/64.2.104
Cusack, C. L., Swahari, V., Hampton Henley, W., Michael Ramsey, J., and Deshmukh, M. (2013). Distinct Pathways Mediate Axon Degeneration during Apoptosis and Axon-specific Pruning. Nat. Commun. 4, 1876. doi:10.1038/ncomms2910
D'Amelio, M., Sheng, M., and Cecconi, F. (2012). Caspase-3 in the central Nervous System: beyond Apoptosis. Trends Neurosci. 35 (11), 700–709. doi:10.1016/j.tins.2012.06.004
D'Amelio, M., Cavallucci, V., Middei, S., Marchetti, C., Pacioni, S., Ferri, A., et al. (2011). Caspase-3 Triggers Early Synaptic Dysfunction in a Mouse Model of Alzheimer's Disease. Nat. Neurosci. 14 (1), 69–76. doi:10.1038/nn.2709
de Calignon, A., Fox, L. M., Pitstick, R., Carlson, G. A., Bacskai, B. J., Spires-Jones, T. L., et al. (2010). Caspase Activation Precedes and Leads to Tangles. Nature 464 (7292), 1201–1204. doi:10.1038/nature08890
De Maria, R., Zeuner, A., Eramo, A., Domenichelli, C., Bonci, D., Grignani, F., et al. (1999). Negative Regulation of Erythropoiesis by Caspase-Mediated Cleavage of GATA-1. Nature 401 (6752), 489–493. doi:10.1038/46809
Dix, M. M., Simon, G. M., and Cravatt, B. F. (2008). Global Mapping of the Topography and Magnitude of Proteolytic Events in Apoptosis. Cell 134 (4), 679–691. doi:10.1016/j.cell.2008.06.038
Espinosa-Oliva, A. M., García-Revilla, J., Alonso-Bellido, I. M., and Burguillos, M. A. (2019). Brainiac Caspases: Beyond the Wall of Apoptosis. Front. Cel. Neurosci. 13, 500. doi:10.3389/fncel.2019.00500
Flores, J., Noël, A., Fillion, M. L., and LeBlanc, A. C. (2021). Therapeutic Potential of Nlrp1 Inflammasome, Caspase-1, or Caspase-6 against Alzheimer Disease Cognitive Impairment. Cell Death Differ. doi:10.1038/s41418-021-00881-1
Geden, M. J., Romero, S. E., and Deshmukh, M. (2019). Apoptosis versus Axon Pruning: Molecular Intersection of Two Distinct Pathways for Axon Degeneration. Neurosci. Res. 139, 3–8. doi:10.1016/j.neures.2018.11.007
Gervais, F. G., Xu, D., Robertson, G. S., Vaillancourt, J. P., Zhu, Y., Huang, J., et al. (1999). Involvement of Caspases in Proteolytic Cleavage of Alzheimer's Amyloid-β Precursor Protein and Amyloidogenic Aβ Peptide Formation. Cell 97 (3), 395–406. doi:10.1016/s0092-8674(00)80748-5
Gevaert, K., Goethals, M., Martens, L., Van Damme, J., Staes, A., Thomas, G. R., et al. (2003). Exploring Proteomes and Analyzing Protein Processing by Mass Spectrometric Identification of Sorted N-Terminal Peptides. Nat. Biotechnol. 21 (5), 566–569. doi:10.1038/nbt810
Gu, Z., Serradj, N., Ueno, M., Liang, M., Li, J., Baccei, M. L., et al. (2017). Skilled Movements Require Non-apoptotic Bax/Bak Pathway-Mediated Corticospinal Circuit Reorganization. Neuron 94 (3), 626–641. e4. doi:10.1016/j.neuron.2017.04.019
Hertz, N. T., Adams, E. L., Weber, R. A., Shen, R. J., O’Rourke, M. K., Simon, D. J., et al. (2019). Neuronally Enriched RUFY3 Is Required for Caspase-Mediated Axon Degeneration. Neuron 103 (3), 412–422. e4. doi:10.1016/j.neuron.2019.05.030
Hollville, E., Carroll, R. G., Cullen, S. P., and Martin, S. J. (2014). Bcl-2 Family Proteins Participate in Mitochondrial Quality Control by Regulating Parkin/PINK1-dependent Mitophagy. Mol. Cel 55 (3), 451–466. doi:10.1016/j.molcel.2014.06.001
Horowitz, P. M., Patterson, K. R., Guillozet-Bongaarts, A. L., Reynolds, M. R., Carroll, C. A., Weintraub, S. T., et al. (2004). Early N-Terminal Changes and Caspase-6 Cleavage of Tau in Alzheimer's Disease. J. Neurosci. 24 (36), 7895–7902. doi:10.1523/jneurosci.1988-04.2004
Imbriani, P., Tassone, A., Meringolo, M., Ponterio, G., Madeo, G., Pisani, A., et al. (2019). Loss of Non-apoptotic Role of Caspase-3 in the PINK1 Mouse Model of Parkinson's Disease. Int. J. Mol. Sci. 20 (14), 3407. doi:10.3390/ijms20143407
Ishizaki, Y., Jacobson, M. D., and Raff, M. C. (1998). A Role for Caspases in Lens Fiber Differentiation. J. Cel Biol 140 (1), 153–158. doi:10.1083/jcb.140.1.153
Jiao, S., and Li, Z. (2011). Nonapoptotic Function of BAD and BAX in Long-Term Depression of Synaptic Transmission. Neuron 70 (4), 758–772. doi:10.1016/j.neuron.2011.04.004
Julien, O., and Wells, J. A. (2017). Caspases and Their Substrates. Cel Death Differ 24 (8), 1380–1389. doi:10.1038/cdd.2017.44
Julien, O., Zhuang, M., Wiita, A. P., O’Donoghue, A. J., Knudsen, G. M., Craik, C. S., et al. (2016). Quantitative MS-based Enzymology of Caspases Reveals Distinct Protein Substrate Specificities, Hierarchies, and Cellular Roles. Proc. Natl. Acad. Sci. USA 113 (14), E2001–E2010. doi:10.1073/pnas.1524900113
Kaushal, V., Dye, R., Pakavathkumar, P., Foveau, B., Flores, J., Hyman, B., et al. (2015). Neuronal NLRP1 Inflammasome Activation of Caspase-1 Coordinately Regulates Inflammatory Interleukin-1-Beta Production and Axonal Degeneration-Associated Caspase-6 Activation. Cel Death Differ 22 (10), 1676–1686. doi:10.1038/cdd.2015.16
Khatri, N., Gilbert, J. P., Huo, Y., Sharaflari, R., Nee, M., Qiao, H., et al. (2018). The Autism Protein Ube3A/E6AP Remodels Neuronal Dendritic Arborization via Caspase-dependent Microtubule Destabilization. J. Neurosci. 38 (2), 363–378. doi:10.1523/jneurosci.1511-17.2017
Kim, J. S., Ha, J. Y., Yang, S. j., and Son, J. H. (2018). A Novel Non‐Apoptotic Role of Procaspase‐3 in the Regulation of Mitochondrial Biogenesis Activators. J. Cel. Biochem. 119 (1), 347–357. doi:10.1002/jcb.26186
Kitagishi, Y., and Matsuda, S. (2013). RUFY, Rab and Rap Family Proteins Involved in a Regulation of Cell Polarity and Membrane Trafficking. Ijms 14 (3), 6487–6498. doi:10.3390/ijms14036487
Lee, S.-J., Lim, H.-S., Masliah, E., and Lee, H.-J. (2011). Protein Aggregate Spreading in Neurodegenerative Diseases: Problems and Perspectives. Neurosci. Res. 70 (4), 339–348. doi:10.1016/j.neures.2011.05.008
Li, H., Alavian, K. N., Lazrove, E., Mehta, N., Jones, A., Zhang, P., et al. (2013). A Bcl-xL-Drp1 Complex Regulates Synaptic Vesicle Membrane Dynamics during Endocytosis. Nat. Cel Biol 15 (7), 773–785. doi:10.1038/ncb2791
Li, H., Chen, Y., Jones, A. F., Sanger, R. H., Collis, L. P., Flannery, R., et al. (2008). Bcl-xL Induces Drp1-dependent Synapse Formation in Cultured Hippocampal Neurons. Proc. Natl. Acad. Sci. 105 (6), 2169–2174. doi:10.1073/pnas.0711647105
Li, Z., Jo, J., Jia, J.-M., Lo, S.-C., Whitcomb, D. J., Jiao, S., et al. (2010). Caspase-3 Activation via Mitochondria Is Required for Long-Term Depression and AMPA Receptor Internalization. Cell 141 (5), 859–871. doi:10.1016/j.cell.2010.03.053
Lohmüller, T., Wenzler, D., Hagemann, S., Kiess, W., Peters, C., Dandekar, T., et al. (2003). Toward Computer-Based Cleavage Site Prediction of Cysteine Endopeptidases. Biol. Chem. 384 (6), 899–909. doi:10.1515/BC.2003.101
Louneva, N., Cohen, J. W., Han, L.-Y., Talbot, K., Wilson, R. S., Bennett, D. A., et al. (2008). Caspase-3 Is Enriched in Postsynaptic Densities and Increased in Alzheimer's Disease. Am. J. Pathol. 173 (5), 1488–1495. doi:10.2353/ajpath.2008.080434
Lu, D. C., Rabizadeh, S., Chandra, S., Shayya, R. F., Ellerby, L. M., Ye, X., et al. (2000). A Second Cytotoxic Proteolytic Peptide Derived from Amyloid β-protein Precursor. Nat. Med. 6 (4), 397–404. doi:10.1038/74656
Ma, L., Yang, C., Zhang, X., Li, Y., Wang, S., Zheng, L., et al. (2018). C-Terminal Truncation Exacerbates the Aggregation and Cytotoxicity of α-Synuclein: A Vicious Cycle in Parkinson's Disease. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1864 (12), 3714–3725. doi:10.1016/j.bbadis.2018.10.003
Mahrus, S., Trinidad, J. C., Barkan, D. T., Sali, A., Burlingame, A. L., and Wells, J. A. (2008). Global Sequencing of Proteolytic Cleavage Sites in Apoptosis by Specific Labeling of Protein N Termini. Cell 134 (5), 866–876. doi:10.1016/j.cell.2008.08.012
Martin, D. D. O., Schmidt, M. E., Nguyen, Y. T., Lazic, N., and Hayden, M. R. (2019). Identification of a Novel Caspase Cleavage Site in Huntingtin that Regulates Mutant Huntingtin Clearance. FASEB j. 33 (3), 3190–3197. doi:10.1096/fj.201701510rrr
Martinon, F., Burns, K., and Tschopp, J. (2002). The Inflammasome. Mol. Cel 10 (2), 417–426. doi:10.1016/s1097-2765(02)00599-3
McKenzie, B. A., Dixit, V. M., and Power, C. (2020a). Fiery Cell Death: Pyroptosis in the Central Nervous System. Trends Neurosciences 43 (1), 55–73. doi:10.1016/j.tins.2019.11.005
McKenzie, B. A., Fernandes, J. P., Doan, M. A. L., Schmitt, L. M., Branton, W. G., and Power, C. (2020b). Activation of the Executioner Caspases-3 and -7 Promotes Microglial Pyroptosis in Models of Multiple Sclerosis. J. Neuroinflammation 17 (1), 253. doi:10.1186/s12974-020-01902-5
Means, J. C., Lopez, A. A., and Koulen, P. (2021). Estrogen Protects Optic Nerve Head Astrocytes against Oxidative Stress by Preventing Caspase-3 Activation, Tau Dephosphorylation at Ser422 and the Formation of Tau Protein Aggregates. Cell Mol Neurobiol 41 (3), 449–458. doi:10.1007/s10571-020-00859-6
Mukherjee, A., and Williams, D. W. (2017). More Alive Than Dead: Non-apoptotic Roles for Caspases in Neuronal Development, Plasticity and Disease. Cel Death Differ 24 (8), 1411–1421. doi:10.1038/cdd.2017.64
Noël, A., Foveau, B., and LeBlanc, A. C. (2021). Caspase-6-cleaved Tau Fails to Induce Tau Hyperphosphorylation and Aggregation, Neurodegeneration, Glial Inflammation, and Cognitive Deficits. Cel Death Dis 12 (3), 227. doi:10.1038/s41419-021-03506-0
Nougarede, A., Popgeorgiev, N., Kassem, L., Omarjee, S., Borel, S., Mikaelian, I., et al. (2018). Breast Cancer Targeting through Inhibition of the Endoplasmic Reticulum-Based Apoptosis Regulator Nrh/BCL2L10. Cancer Res. 78 (6), 1404–1417. doi:10.1158/0008-5472.can-17-0846
Ofengeim, D., Ito, Y., Najafov, A., Zhang, Y., Shan, B., DeWitt, J. P., et al. (2015). Activation of Necroptosis in Multiple Sclerosis. Cel Rep. 10 (11), 1836–1849. doi:10.1016/j.celrep.2015.02.051
Ohsawa, S., Hamada, S., Kuida, K., Yoshida, H., Igaki, T., and Miura, M. (2010). Maturation of the Olfactory Sensory Neurons by Apaf-1/caspase-9-Mediated Caspase Activity. Proc. Natl. Acad. Sci. 107 (30), 13366–13371. doi:10.1073/pnas.0910488107
Park, G., Nhan, H. S., Tyan, S.-H., Kawakatsu, Y., Zhang, C., Navarro, M., et al. (2020). Caspase Activation and Caspase-Mediated Cleavage of APP Is Associated with Amyloid β-Protein-Induced Synapse Loss in Alzheimer's Disease. Cel Rep. 31 (13), 107839. doi:10.1016/j.celrep.2020.107839
Popgeorgiev, N., and Gillet, G. (2021). Bcl-xL and IP3R Interaction: Intimate Relationship with an Uncertain Outcome. Cell Calcium 101, 102504. doi:10.1016/j.ceca.2021.102504
Raval, A. P., Martinez, C. C., Mejias, N. H., and de Rivero Vaccari, J. P. (2019). Sexual Dimorphism in Inflammasome-Containing Extracellular Vesicles and the Regulation of Innate Immunity in the Brain of Reproductive Senescent Females. Neurochem. Int. 127, 29–37. doi:10.1016/j.neuint.2018.11.018
Riccomagno, M. M., and Kolodkin, A. L. (2015). Sculpting Neural Circuits by Axon and Dendrite Pruning. Annu. Rev. Cel Dev. Biol. 31, 779–805. doi:10.1146/annurev-cellbio-100913-013038
Rissman, R. A., Poon, W. W., Blurton-Jones, M., Oddo, S., Torp, R., Vitek, M. P., et al. (2004). Caspase-cleavage of Tau Is an Early Event in Alzheimer Disease Tangle Pathology. J. Clin. Invest. 114 (1), 121–130. doi:10.1172/jci200420640
Rodríguez-Gómez, J. A., Kavanagh, E., Engskog-Vlachos, P., Engskog, M. K. R., Herrera, A. J., Espinosa-Oliva, A. M., et al. (2020). Microglia: Agents of the CNS Pro-inflammatory Response. Cells 9 (7), 1717. doi:10.3390/cells9071717
Rosa, N., Ivanova, H., Wagner, L. E., Kale, J., La Rovere, R., Welkenhuyzen, K., et al. (2021). Bcl-xL Acts as an Inhibitor of IP 3 R Channels, Thereby Antagonizing Ca2+-Driven Apoptosis. Cel Death Differ. doi:10.1038/s41418-021-00894-w
Saxena, S., and Caroni, P. (2007). Mechanisms of Axon Degeneration: from Development to Disease. Prog. Neurobiol. 83 (3), 174–191. doi:10.1016/j.pneurobio.2007.07.007
Schauperl, M., Fuchs, J. E., Waldner, B. J., Huber, R. G., Kramer, C., and Liedl, K. R. (2015). Characterizing Protease Specificity: How Many Substrates Do We Need. PLoS One 10 (11), e0142658. doi:10.1371/journal.pone.0142658
Schroder, K., and Tschopp, J. (2010). The Inflammasomes. Cell 140 (6), 821–832. doi:10.1016/j.cell.2010.01.040
Selvamani, A., and Sohrabji, F. (2017). Mir363-3p Improves Ischemic Stroke Outcomes in Female but Not Male Rats. Neurochem. Int. 107, 168–181. doi:10.1016/j.neuint.2016.10.008
Shen, X., Venero, J. L., Joseph, B., and Burguillos, M. A. (2018). Caspases Orchestrate Microglia Instrumental Functions. Prog. Neurobiol. 171, 50–71. doi:10.1016/j.pneurobio.2018.09.007
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., et al. (2015). Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 526 (7575), 660–665. doi:10.1038/nature15514
Siegel, C., Li, J., Liu, F., Benashski, S. E., and McCullough, L. D. (2011). miR-23a Regulation of X-Linked Inhibitor of Apoptosis (XIAP) Contributes to Sex Differences in the Response to Cerebral Ischemia. Proc. Natl. Acad. Sci. 108 (28), 11662–11667. doi:10.1073/pnas.1102635108
Simon, D. J., Weimer, R. M., McLaughlin, T., Kallop, D., Stanger, K., Yang, J., et al. (2012). A Caspase cascade Regulating Developmental Axon Degeneration. J. Neurosci. 32 (49), 17540–17553. doi:10.1523/jneurosci.3012-12.2012
Song, J., Tan, H., Shen, H., Mahmood, K., Boyd, S. E., Webb, G. I., et al. (2010). Cascleave: towards More Accurate Prediction of Caspase Substrate Cleavage Sites. Bioinformatics 26 (6), 752–760. doi:10.1093/bioinformatics/btq043
Stokin, G. B., Lillo, C., Falzone, T. L., Brusch, R. G., Rockenstein, E., Mount, S. L., et al. (2005). Axonopathy and Transport Deficits Early in the Pathogenesis of Alzheimer's Disease. Science 307 (5713), 1282–1288. doi:10.1126/science.1105681
Tait, S. W., Ichim, G., and Green, D. R. (2014). Die Another Way-NnonAapoptotic Mechanisms of Cell Death. J. Cel Sci 127 (Pt 10), 2135–2144. doi:10.1242/jcs.093575
Talanian, R. V., Quinlan, C., Trautz, S., Hackett, M. C., Mankovich, J. A., Banach, D., et al. (1997). Substrate Specificities of Caspase Family Proteases. J. Biol. Chem. 272 (15), 9677–9682. doi:10.1074/jbc.272.15.9677
Tan, W., and Colombini, M. (2007). VDAC Closure Increases Calcium Ion Flux. Biochim. Biophys. Acta 1768 (10), 2510–2515. doi:10.1016/j.bbamem.2007.06.002
Terry, R. D., Masliah, E., Salmon, D. P., Butters, N., DeTeresa, R., Hill, R., et al. (1991). Physical Basis of Cognitive Alterations in Alzheimer's Disease: Synapse Loss Is the Major Correlate of Cognitive Impairment. Ann. Neurol. 30 (4), 572–580. doi:10.1002/ana.410300410
Thomas, M. S. C., Davis, R., Karmiloff-Smith, A., Knowland, V. C. P., and Charman, T. (2016). The Over-pruning Hypothesis of Autism. Dev. Sci. 19 (2), 284–305. doi:10.1111/desc.12303
Ullah, M. F., Ahmad, A., Bhat, S. H., Abu-Duhier, F. M., Barreto, G. E., and Ashraf, G. M. (2019). Impact of Sex Differences and Gender Specificity on Behavioral Characteristics and Pathophysiology of Neurodegenerative Disorders. Neurosci. Biobehavioral Rev. 102, 95–105. doi:10.1016/j.neubiorev.2019.04.003
Veas-Pérez de Tudela, M., Delgado-Esteban, M., Maestre, C., Bobo-Jiménez, V., Jiménez-Blasco, D., Vecino, R., et al. (2015). Regulation of Bcl-xL-ATP Synthase Interaction by Mitochondrial Cyclin B1-cyclin-dependent Kinase-1 Determines Neuronal Survival. J. Neurosci. 35 (25), 9287–9301. doi:10.1523/jneurosci.4712-14.2015
Vickers, J. C., King, A. E., Woodhouse, A., Kirkcaldie, M. T., Staal, J. A., McCormack, G. H., et al. (2009). Axonopathy and Cytoskeletal Disruption in Degenerative Diseases of the central Nervous System. Brain Res. Bull. 80 (4-5), 217–223. doi:10.1016/j.brainresbull.2009.08.004
Victor, K. G., Heffron, D. S., Sokolowski, J. D., Majumder, U., Leblanc, A., and Mandell, J. W. (2018). Proteomic Identification of Synaptic Caspase Substrates. Synapse 72 (1). doi:10.1002/syn.22014
Wang, Y.-J., Liu, M.-G., Wang, J.-H., Cao, W., Wu, C., Wang, Z.-Y., et al. (2020). Restoration of Cingulate Long-Term Depression by Enhancing Non-apoptotic Caspase 3 Alleviates Peripheral Pain Hypersensitivity. Cel Rep. 33 (6), 108369. doi:10.1016/j.celrep.2020.108369
Weghorst, F., Mirzakhanyan, Y., Samimi, K., Dhillon, M., Barzik, M., Cunningham, L. L., et al. (2020). Caspase-3 Cleaves Extracellular Vesicle Proteins during Auditory Brainstem Development. Front. Cel. Neurosci. 14, 573345. doi:10.3389/fncel.2020.573345
Wellington, C. L., Singaraja, R., Ellerby, L., Savill, J., Roy, S., Leavitt, B., et al. (2000). Inhibiting Caspase Cleavage of Huntingtin Reduces Toxicity and Aggregate Formation in Neuronal and Nonneuronal Cells. J. Biol. Chem. 275 (26), 19831–19838. doi:10.1074/jbc.m001475200
Yi, Y. S. (2018). Regulatory Roles of the Caspase-11 Non-canonical Inflammasome in Inflammatory Diseases. Immune Netw. 18 (6), e41. doi:10.4110/in.2018.18.e41
Zelaya, M. V., Pérez-Valderrama, E., de Morentin, X. M., Tuñon, T., Ferrer, I., Luquin, M. R., et al. (2015). Olfactory Bulb Proteome Dynamics during the Progression of Sporadic Alzheimer's Disease: Identification of Common and Distinct Olfactory Targets across Alzheimer-Related Co-pathologies. Oncotarget 6 (37), 39437–39456. doi:10.18632/oncotarget.6254
Zermati, Y., Garrido, C., Amsellem, S., Fishelson, S., Bouscary, D., Valensi, F., et al. (2001). Caspase Activation Is Required for Terminal Erythroid Differentiation. J. Exp. Med. 193 (2), 247–254. doi:10.1084/jem.193.2.247
Zhang, C.-J., Jiang, M., Zhou, H., Liu, W., Wang, C., Kang, Z., et al. (2018). TLR-stimulated IRAKM Activates Caspase-8 Inflammasome in Microglia and Promotes Neuroinflammation. J. Clin. Invest. 128 (12), 5399–5412. doi:10.1172/jci121901
Zhang, Y., Su, S. S., Zhao, S., Yang, Z., Zhong, C.-Q., Chen, X., et al. (2017). RIP1 Autophosphorylation Is Promoted by Mitochondrial ROS and Is Essential for RIP3 Recruitment into Necrosome. Nat. Commun. 8, 14329. doi:10.1038/ncomms14329
Keywords: caspase, Bcl-2, Bcl-xL, non-apoptotic, CNS, sexual dymorphism, neurodegeneration
Citation: Bahatyrevich-Kharitonik B, Medina-Guzman R, Flores-Cortes A, García-Cruzado M, Kavanagh E and Burguillos MA (2022) Cell Death Related Proteins Beyond Apoptosis in the CNS. Front. Cell Dev. Biol. 9:825747. doi: 10.3389/fcell.2021.825747
Received: 30 November 2021; Accepted: 28 December 2021;
Published: 14 January 2022.
Edited by:
Zheng Li, National Institutes of Health (NIH), United StatesReviewed by:
Germain Gillet, Université Claude Bernard Lyon 1, FranceXiang-Yao Li, Zhejiang University, China
Copyright © 2022 Bahatyrevich-Kharitonik, Medina-Guzman, Flores-Cortes, García-Cruzado, Kavanagh and Burguillos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miguel Angel Burguillos, bWFidXJndWlsbG9zQHVzLmVz
 Bazhena Bahatyrevich-Kharitonik
Bazhena Bahatyrevich-Kharitonik Rafael Medina-Guzman
Rafael Medina-Guzman Alicia Flores-Cortes
Alicia Flores-Cortes Marta García-Cruzado
Marta García-Cruzado Edel Kavanagh
Edel Kavanagh Miguel Angel Burguillos
Miguel Angel Burguillos