
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol., 20 January 2022
Sec. Molecular and Cellular Oncology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.783527
This article is part of the Research TopicMolecular Mechanisms and Targeted Therapies for Colorectal CancerView all 15 articles
 Cong Liu1
Cong Liu1 Dimitri Papukashvili1
Dimitri Papukashvili1 Yu Dong2
Yu Dong2 Xingyun Wang1
Xingyun Wang1 Xing Hu1
Xing Hu1 Nuo Yang1
Nuo Yang1 Jie Cai1
Jie Cai1 Fengfei Xie1
Fengfei Xie1 Nino Rcheulishvili1
Nino Rcheulishvili1 Peng George Wang1*
Peng George Wang1*mRNA vaccines have become a promising alternative to conventional cancer immunotherapy approaches. However, its application on colorectal cancer (CRC) remains poorly understood. We herein identified potential antigens for designing an effective mRNA vaccine, further to build an immune landscape for the accurate selection of patients for mRNA vaccine therapy. Raw transcriptome data from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases were retrieved. Consensus clustering algorithm was applied to divide the CRC samples into four immune subtypes. Immunogenomics analysis was further integrated to characterize the immune microenvironment of each immune subtype. Six tumor antigens were found to be associated with poor prognosis and infiltration of antigen-presenting cells (APCs) in CRC patients. Furthermore, each of the immune subtypes showed differential cellular and molecular features. The IS2 and IS4 exhibited significantly improved survival and higher immune cell infiltration compared with IS1 and IS3. Immune checkpoint molecules and human leukocyte antigen also showed significant differential expression in four immune subtypes. Moreover, we performed graph structure learning-based dimensionality reduction to visualize the immune landscape of CRC. Our results revealed a complex immune landscape that may provide directions for mRNA vaccine treatment of CRC and define appropriate vaccination patients.
Colorectal cancer (CRC) is the third most frequently diagnosed tumor, with the second highest mortality rate worldwide (Sung et al., 2021). Currently, effective treatments for CRC mainly include surgical resection, chemotherapy, and radiotherapy (van der Stok et al., 2017). However, morbidity and mortality remain high as approximately 80% of CRC patients show recurrence during the first 3 years (Augestad et al., 2017). Thus, new therapeutic strategies are needed to enhance the survival rate of patients with CRC.
To date, cancer immunotherapies, such as immune checkpoint blockade (ICB) and chimeric antigen receptor T (CAR-T) cell therapy have gained tremendous success (Hargadon et al., 2018; Pantin and Battiwalla, 2020). Messenger RNA (mRNA) vaccine has recently represented a promising alternative to conventional immunotherapy for anti-cancer treatments. In vitro transcribed (IVT) mRNA-based gene therapy was initially considered to be an unsuitable approach due to its high immunogenicity and instability. In recent years, along with the development of mRNA synthesis, chemical modification of mRNA and advancing the technology of delivery systems have greatly improved mRNA stability and translation efficacy. IVT mRNA can be designed to express proteins transiently by ensuring that its structure resembles the natural mRNA (Sahin et al., 2014; Kowalzik et al., 2021). Moreover, immunogenicity has gradually become controllable that is of great application prospect in tumor immunotherapy and other biological treatments (Kallen et al., 2013). Compared with the currently available drugs, the mRNA-based approach has a number of advantages: 1) mRNA is produced and purified in vitro, without any need of the complex process of protein drug and viral vector preparation; 2) IVT mRNA production process is highly versatile. The production of different target proteins is feasible, hence, time-saving for drug development and efficiency improvement; 3) mRNA only needs to enter the cytoplasm to be translated into protein without entering the nucleus, so no gene insertion and integration take place that improves the safety of the drug (De Keersmaecker et al., 2020); 4) The half-life can be changed by modulating the sequence and the delivery vector (Pardi et al., 2018). mRNA encoding tumor antigens transported via lipid nanoparticle enters into the antigen presenting cells (APCs) and is expressed into the targeted antigen. The expressed tumor antigens can be presented on the surface of APCs by major histocompatibility complexes (MHC) to evoke an anti-tumor response (Miao et al., 2021). Clinical trials have demonstrated that although the protein expression of mRNA is transient, it is effective for tumor immunotherapy applications. In the clinical trials, mRNA vaccines have been applied to treat solid tumors, including non-small cell lung cancers, melanoma, prostate cancer, and glioblastoma (Sebastian et al., 2014; Kubler et al., 2015; Papachristofilou et al., 2019; Batich et al., 2020). mRNA cancer vaccines are combined with ICB to further improve antitumor efficacy (Miao et al., 2021). A great deal of clinical trials has been started and consistently verified the feasibility and suitability of mRNA vaccines for cancer treatment.
Due to the high heterogeneity of CRC at the genetic and molecular level, which affects the efficacy of immunotherapy, there is no mRNA vaccine developed against CRC to date. The effectiveness of mRNA vaccines critically depends on making the antigen(s) of interest available to professional APCs.
The present study aims to predict the potential tumor antigen of CRC for designing an mRNA vaccine. Furthermore, the anti-CRC mRNA cancer vaccine is designed and the immune subtype for identifying suitable CRC patients for vaccination is also explored. Each of the four immune subtypes was correlated with different molecular, cellular features, and clinical outcomes. Our results might provide the theoretical basis of developing an mRNA cancer vaccine and facilitate the selection of optimal CRC patients for vaccination.
The RNA sequencing (RNA-seq) gene expression data of 612 CRC samples and their clinicopathologic information were downloaded from TCGA database (https://gdc-portal.nci.nih.gov/) as our discovery cohort. RNA-seq data of 598 CRC samples (GSE39582) and the clinical information were downloaded from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). An independent cohort GSE39582 was used for further validation. Building on TCGA datasets, gene mutation information was obtained via secondary databases like cBioportal (http://www.cbioportal.org/). To discover unbiased immune subtypes, we identified 1,989 IRGs in five different categories including single-cell RNA-seq data, the gene of co-stimulatory and co-inhibitory molecules, the gene of cytokine and cytokine receptors, genes involved in antigen processing and presentation, and other immune-related genes (Chen et al., 2019; Li et al., 2019).
The cBioPortal (http://www.cbioportal.org/) was used to analyze and visualize cancer genomics data sets including copy number variation and mutation (Cerami et al., 2012). The genetic alterations of potential tumor antigens against tumors were obtained from cBioPortal based on 612 CRC samples in TCGA.
The online database Gene Expression Profiling Interactive Analysis (GEPIA2) (http://gepia2.cancer-pku.cn/) which is based on samples from the TCGA and the GTEx (Genotype-Tissue Expression) databases was used for gene expression analysis to further clarify the relationship between tumor antigen gene expression and CRC prognosis (Tang et al., 2017). ANOVA was used for differential expression analysis to compare the tumor to paired normal samples. The chromosomal distribution of over- or under-expressed genes were plotted in differential genes with |Log2FC| > 1 and q-value < 0.01. Kaplan–Meier estimates of OS and RFS were performed to evaluate the prognostic value of identified tumor antigens.
To assess the correlation between the infiltration level of APCs and the expression of the identified tumor antigens TIMER database was used. (https://cistrome.shinyapps.io/timer/). TIMER includes 10,897 samples across 32 cancer types from TCGA to estimate the abundance of immune infiltrates. Prognostic gene expression data was used to calculate the abundance of six immune cell types (B cells, CD4+T cells, CD8+T cells, neutrophils, macrophages, and dendritic cells) in CRC patients using the TIMER (Li et al., 2017).
Based on the expression of 1,989 IRGs, the 201 IRGs impacting prognosis of CRC patients by univariate Cox regression analysis was performed in subsequent analysis. Consensus clustering analysis was used to identify the four immune subtypes by using the R package “Consensus Cluster Plus” with parameters defined as a maximum of nine clusters. To assess the robustness of the identified immune subtypes, we further conducted the same algorithm in the validation cohort (GEO). In-group-proportion statistic was calculated to validate the similarity of immune subtypes across the discovery and validation cohorts.
The correlation between immune subtypes and clinicopathological characteristics was evaluated by the Chi-square test. The prognostic value of the immune subtypes with OS as the endpoint was analyzed by the log-rank test and multivariable Cox proportional hazards regression model. The ANOVA algorithm was performed to assess the correlation between immune subtypes and different immune-related molecular and cellular characteristics. Finally, we employed the ssGSEA algorithm via R packages “GSVA” to comprehensively calculate the enrichment score of CRC samples on each immune-related term.
Considering the dynamic nature of the immune system, the dimensionality reduction analysis using the graph learning-based method was performed to reveal the intrinsic structure and visualize the distribution of individual patients (Trapnell et al., 2014). The discriminative dimensionality reduction with trees (DDRTree) was performed for dimension reduction (Qiu et al., 2017). Finally, the immune landscape was visualized with the function plot cell trajectory (Monocle) with the color corresponding to the immune subtype identified above (Qi et al., 2017; Wang and Mao, 2019).
All statistical analyses and data visualization were performed in R version 4.0.5, and GraphPad Prism 8.0. p < 0.05 was considered statistically significant.
To identify potential tumor antigens of CRC, 2658 over-expressed genes that could potentially encode tumor-associated antigens (TAA) were detected by screening for aberrantly expressed genes (Figure 1A). In addition, 14,751 mutated genes encoding TAA were filtered by analyzing fraction of genome altered and tumor mutational count in CRC patients (Figures 1B,C). Mutational analysis showed that the adenomatous polyposis coli (APC), tumor suppressor P53 (TP53), KRAS proto-oncogene GTPase (KARS), titin (TTN), spectrin repeat containing nuclear envelope protein 1 (SYNE1), mucin 16 (MUC16), and low-density lipoprotein receptor-related protein 1B (LRP1B) were the most frequently mutated genes in the fraction genome alteration and tumor mutational count group (Figures 1D,E). Collectively, we identified 2052 over-expressed and mutated genes.
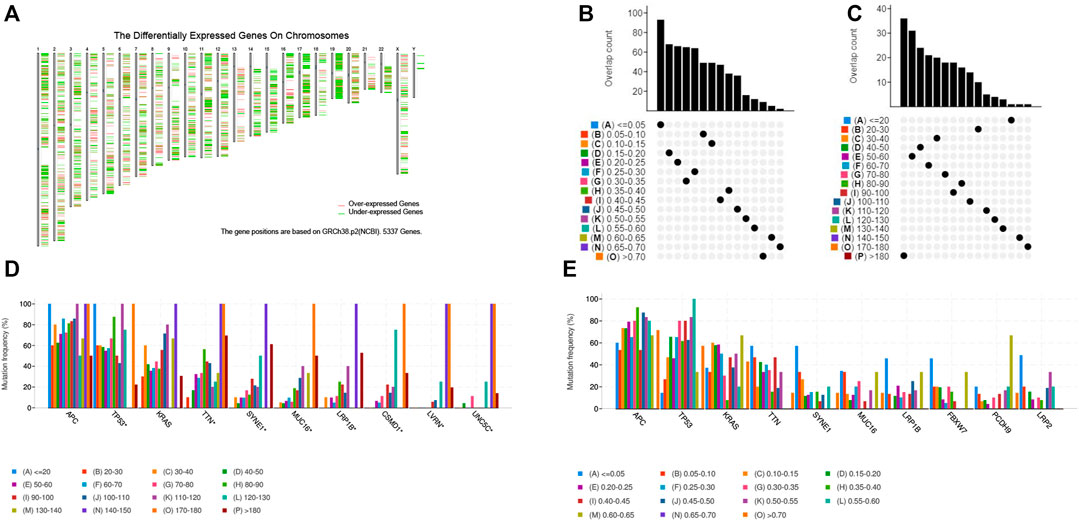
FIGURE 1. Identification of potential tumor-associated antigens of CRC. (A) Chromosomal distribution of up-regulated and down-regulated genes in CRC. (B) Overlapping samples in altered genome fraction group. (C) Overlapping samples in mutation count group. (D) Highest frequency genes in mutation count group. (E) Highest frequency genes in altered genome fraction group.
The prognosis-associated tumor antigens were selected from the above-mentioned genes as promising candidates for designing the mRNA vaccine. Fifty-one genes were strongly correlated with the overall survival (OS) of CRC. Only six genes were closely related to the disease-free survival (RFS) (Supplementary Figure S1). The survival analysis revealed that elevated expression of thrombospondin 2 (THBS2), follistatin like 3 (FSTL3), troponin T1 (TNNT1), biglycan (BGN), collagen triple helix repeat containing 1 (CTHRC1), and NADPH oxidase 4 (NOX4) were associated with poor OS in CRC (Figures 2A–F). In addition, we found a shorter RFS time in patients with higher expression levels of THBS2, FSTL3, TNNT1, BGN, CTHRC1, and NOX4 (Supplementary Figure S2). The effectiveness of mRNA vaccines strongly relies on the availability of the target antigen(s) to professional APCs, especially dendritic cells (DCs), macrophages, and B cells. Therefore, we investigated whether the expression of THBS2, FSTL3, TNNT1, BGN, CTHRC1, and NOX4 was correlated with immune cell infiltration levels in CRC via the Tumor Immune Estimation Resource (TIMER) database. The results demonstrated that elevated expression of THBS2, FSTL3, BGN, CTHRC1, and NOX4 was exhibited in increased infiltration of macrophages, DCs and B cells in CRC, although it was insignificant in B cells. Additionally, TNNT1 expression has the same tendency of increased infiltration of macrophages, DCs, and B cells (Figures 3A–F). Taken together, we designed mRNA encoding the antigen of interest flanked by 5′ and 3′ untranslated regions (UTRs). As shown in Figure 4, six tumor antigens (THBS2, FSTL3, TNNT1, BGN, CTHRC1, and NOX4) were identified as potential therapeutic candidates for the anti-CRC mRNA vaccine that can be processed and presented by APCs to activate a robust immune response.
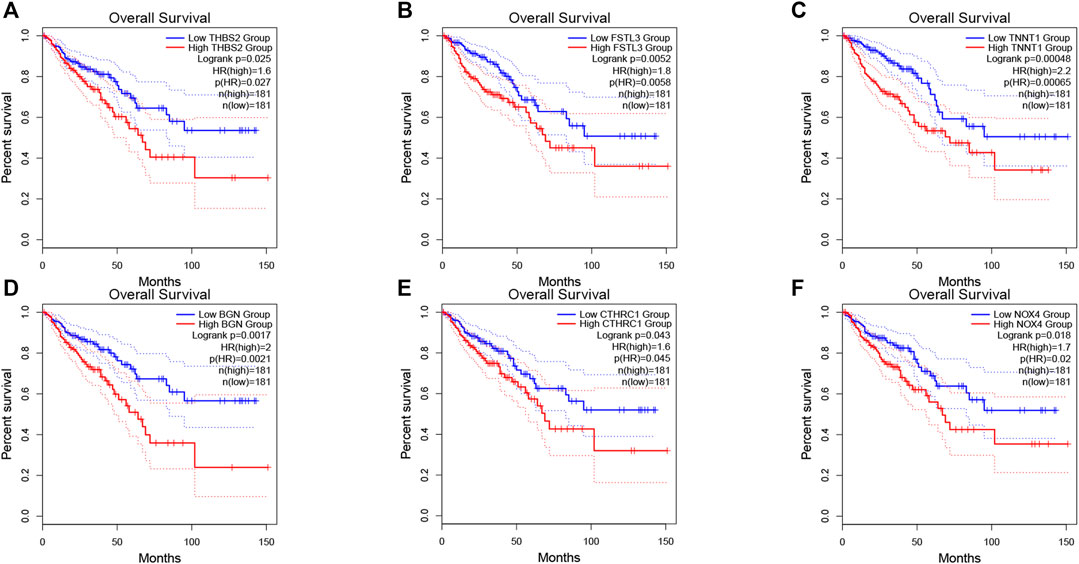
FIGURE 2. Correlation of tumor antigens with CRC prognosis. (A–F) The association of THBS2 (A), FSTL3 (B), TNNT1 (C), BGN (D), CTHRC1 (E), and NOX4 (F) expression levels with the OS of CRC patients. The red line represents the samples with highly expressed genes and the blue line indicates the samples with lowly expressed genes.
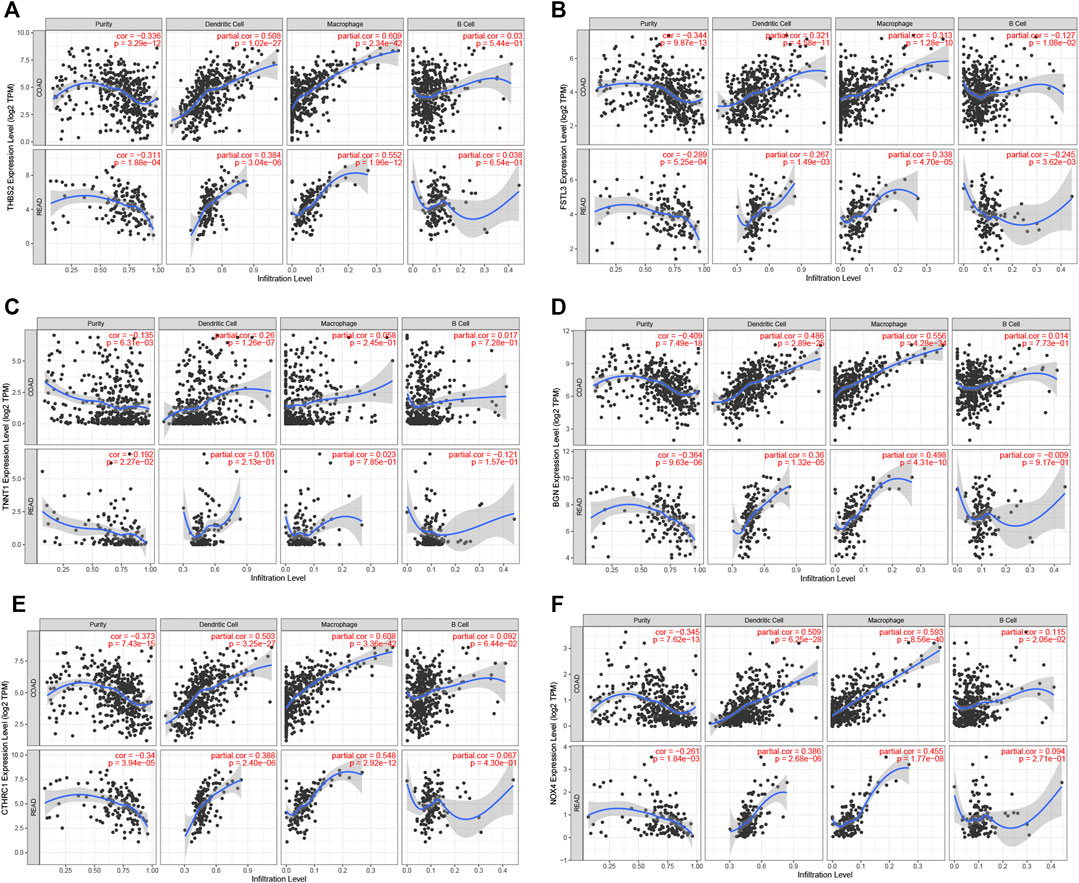
FIGURE 3. Correlation of tumor antigens expression with antigen-presenting cells level in CRC. (A–F) Correlation between THBS2 (A), FSTL3 (B), TNNT1 (C), BGN (D), CTHRC1 (E), and NOX4 (F) expression and the level of infiltrating immune cells dendritic cells, macrophages, and B cells in CRC.
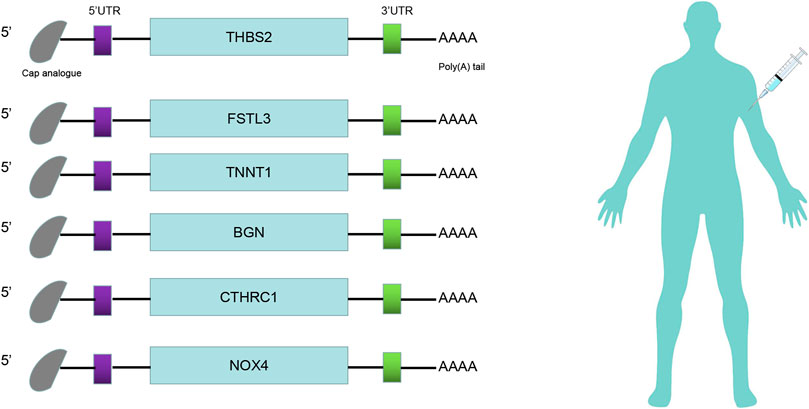
FIGURE 4. mRNA-vaccine design. Structure of the anti-CRC mRNA vaccine. The 5′-cap analog, 5′- and 3′-untranslated regions (UTRs), and poly(A) tail were optimized for stability and translational efficiency.
Immunophenotyping can be used to reflect the immune status of the tumor microenvironment, thus, allow the selection of patients for mRNA vaccine treatment. Therefore, consensus clustering was performed in 568 CRC samples based on the immune-related genes (IRGs). The results were visualized using a cumulative distribution function (CDF) plot and a delta area plot, in which k represents the number of immune subtypes. Four robust immune subtypes (IS1-IS4) were identified and the IRGs appeared to be stably clustered when K = 4 (Figures 5A–C). Significantly prognostic impact of the immune subtypes in TCGA cohort was observed. Immune subtypes IS2 and IS4 were associated with a better prognosis for OS. Conversely, patients in IS1 and IS3 displayed the poorer survival probability in the TCGA cohort (Figure 5D). Consistent with our results obtained from the TCGA cohort, each of the immune subtypes was related to prognosis in the GEO cohort as well (Figure 5E). Among all immune subtypes, IS2 and IS4 were associated with the lower stage (Figures 5F,G) compared with IS1 and IS3. Taken together, immunophenotyping could be a robust prognostic indicator and its accuracy is superior to conventional stage of the tumor in CRC.
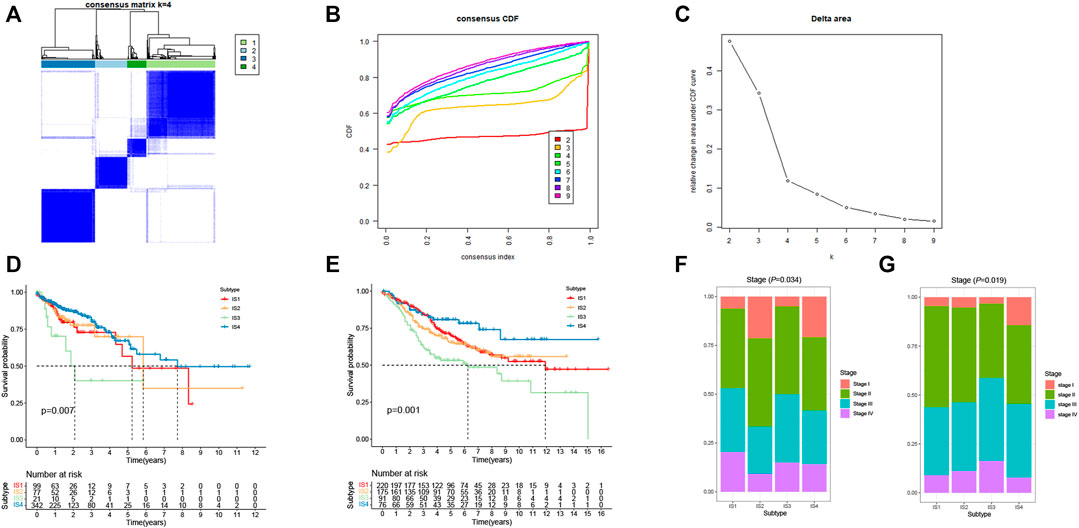
FIGURE 5. Identification of immune subtypes of CRC. (A) Consensus clustering matrix of CRC samples for k = 4. (B) Cumulative distribution function (CDF) curve of the consistency score for different immune subtype numbers (k = 2–9). (C) Delta area plot showing the relative change in area under the CDF curve from k = 2 to k = 9, which assisted in determining the optimal immune subtype numbers. (D) Kaplan-Meier survival curves of CRC immune subtypes in TCGA cohort. (E) Kaplan-Meier survival curves of CRC immune subtypes in GEO cohort. (F) Stages of CRC patients among the IS1-IS4 immune subtypes in TCGA cohort. (G) Stages of CRC patients among the IS1-IS4 immune subtypes in GEO cohort.
High tumor mutational burden (TMB) can increase the tumor immunogenicity, allow the immune system to recognize and attack the cancer cells by T cell-mediated antitumor immunity. Therefore, TMB for each patient can be detected and calculated using the VarScan method in the TCGA cohort. Subtypes IS2 and IS4 had significantly higher TMB than subtypes IS1 and IS3 (Figure 6A). Furthermore, somatic mutation profiling revealed a high frequency of mutations in APC (78%), TP53 (59%), TTN (50%), KRAS (43%), SYNE1 (29%), and MUC16 (28%) across immune subtype (Figure 6B). These findings suggest that the immune subtype is a promising tool to predict TMB in CRC patients and clinical response to mRNA vaccine. Therefore, patients belong to IS2 and IS4 may respond positively to the mRNA vaccine.
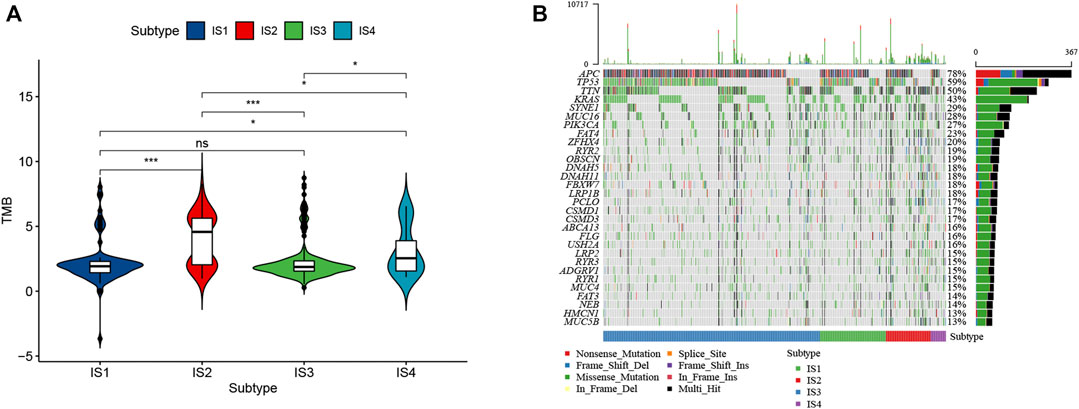
FIGURE 6. Correlation of immune subtypes with TMB in CRC. (A) The TMB of CRC samples from four immune subtypes. (B) The alteration landscape of CRC across four immune subtypes.
Immune checkpoint molecules and human leukocyte antigen (HLA) are essential for immune function and have diverse clinical implications in cancer immunotherapy. Therefore, we next analyzed the relationship between the CRC immune subtypes and the expression level of immune checkpoint molecule genes and HLA. We found that 30 immune checkpoint-related genes were significantly differentially expressed among the immune subtypes. For example, TNFSF4, TNFRSF9, TNFRSF4, TNFRSF18, LAG3, JAK2, PTPRC, IL12B, IFNG, ICOS, HAVCR2, CTLA4, CD8A, CD86, CD80, CD40, CD28, and CD274 were significantly downregulated in IS3 tumors, while TNFSF9, TNFSF4 TNFRSF9, TNFRSF4, TNFRSF18, PTPRC, CTLA4, CD8A, CD86, CD80, CD40, CD28, and CD274 were strongly elevated in IS2 tumors (Figures 7A,B). Next, we assessed the expression level of HLA genes in the four immune subtypes and determined that the expression levels were significantly elevated in IS2 and IS4 than in IS1 and IS3 (Figures 7C,D). HLA plays important role in the activation of anti-tumor immunity through interactions with T cell receptors. Thus, the response of patients with IS2 and IS4 on mRNA vaccine treatment might be more promising.
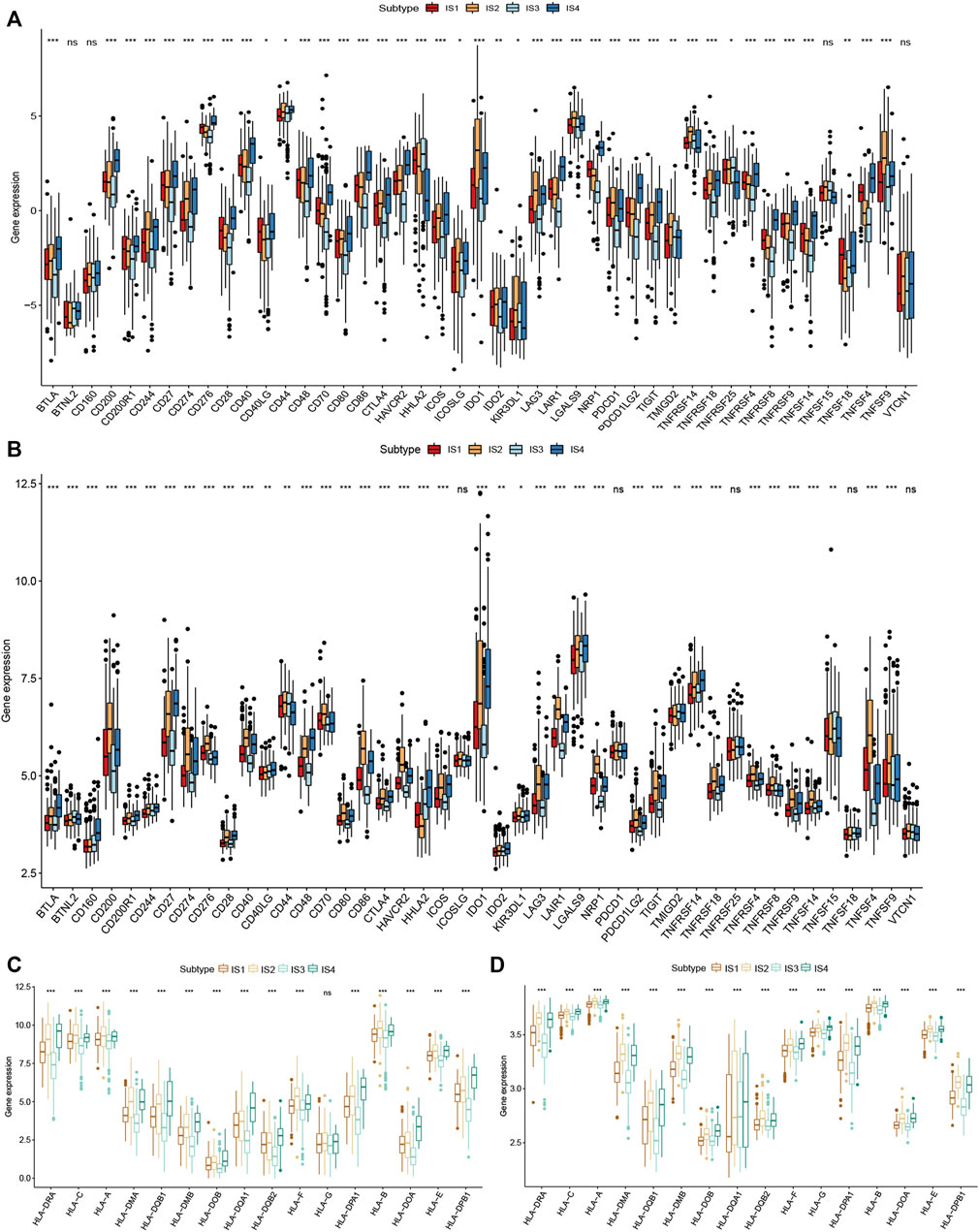
FIGURE 7. Correlation of immune subtypes with immune checkpoint molecules and HLA genes expression in CRC. (A) The expression of immune checkpoint molecules among CRC immune subtypes in TCGA cohort. (B) The expression of immune checkpoint molecules among CRC immune subtypes in GEO cohort. (C) The expression of HLA genes among the CRC immune subtypes in TCGA cohort. (D) The expression of HLA genes among the CRC immune subtypes in GEO cohort. *p < 0.05, **p < 0.01, and ***p < 0.001.
The tumor immune microenvironment (TIME) plays a crucial role in CRC progression, thus influences the effectiveness of the mRNA vaccine. We assessed the relationship between the immune subtypes and 29 immune-related molecular features in the TCGA and GEO cohorts by the single-sample gene set enrichment analysis (ssGSEA) algorithm (Figures 8A,B). The results showed that IS2 and IS4 have significantly higher levels of B cells, CD+8 T cells, Th1 cells, and macrophages compared with IS1 and IS3. Moreover, we calculated stromal and immune scores to quantify the abundances of infiltrating stromal and immune cells for each sample in TCGA and GEO. These results also demonstrated that IS2 and IS4 have higher Immune Score than IS1 and IS3 (Figure 8C). Thus, IS2 and IS4 are immune-hot phenotypes, while IS1 and IS3 are immune-cold phenotypes. Similar results were also validated in patients in the GEO cohort (Figure 8D).
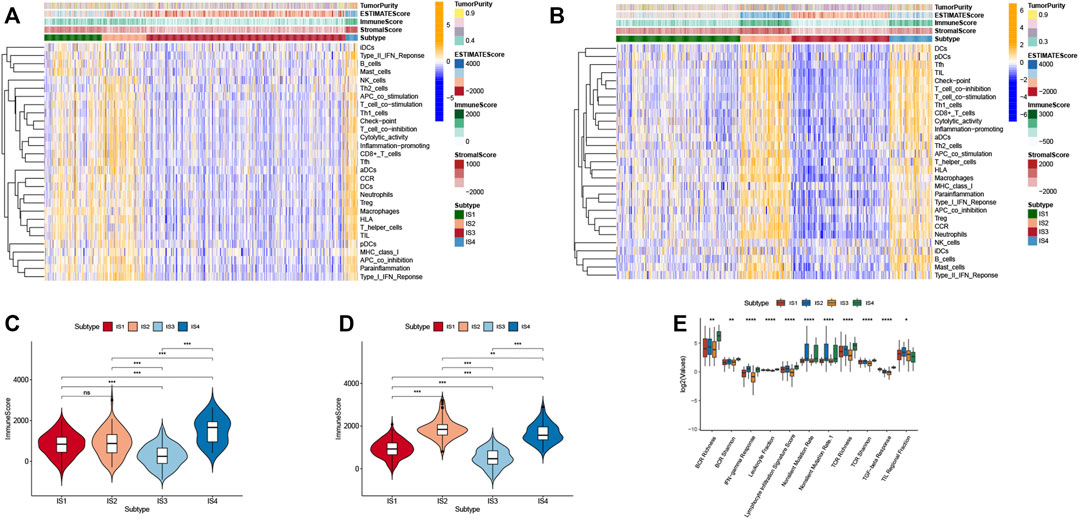
FIGURE 8. Cellular and molecular features of immune subtypes. (A) The enrichment levels of 29 immune-related gene sets among CRC immune subtypes in TCGA cohort. (B) The difference in Immune Scores between four immune subtypes in TCGA cohort. (C) The enrichment levels of 29 immune-related gene sets among CRC immune subtypes in GEO cohort. (D) The difference in Immune Scores between four immune subtypes in GEO cohort. (E) The enrichment levels of 11 immune-related gene sets among CRC immune subtypes.
To better understand the association of CRC immune subtypes with six pan-cancer immunotypes we extracted 11 immune-related molecular features from a previous study and demonstrated that the expression of 11 molecular signatures was significantly different among the four immune subtypes (Figure 8E). Antigen-specific T cell receptor (TCR) and B cell receptor (BCR) repertoires play a significant role in the immune system for the recognition of malignant cells. We observed that IS2 and IS4 had higher scores in TCR richness and BCR richness. Higher TCR richness may enhance anticancer immunity. Therefore, immunotype can be used to reflect CRC immune status and to select suitable patients for mRNA vaccine treatment.
The immune landscape is increasingly recognized to be important in cancer development, progression, and response to therapy. Subsequently, we sought to visualize the immune landscape by the graph learning-based dimensionality reduction technique. We observed that the immune-hot subtype IS2 and immune-cold subtype IS3 were distributed at the opposite end of the horizontal axis in the immune landscape (Figure 9A). The horizontal axis in the immune landscape may represent the overall immune infiltration. As shown in Figure 9B, the horizontal axis was associated with various immune cells, of which activated CD8+ T cells, activated CD4+ T cells, and type 1 T helper cells showed the positive correlation.
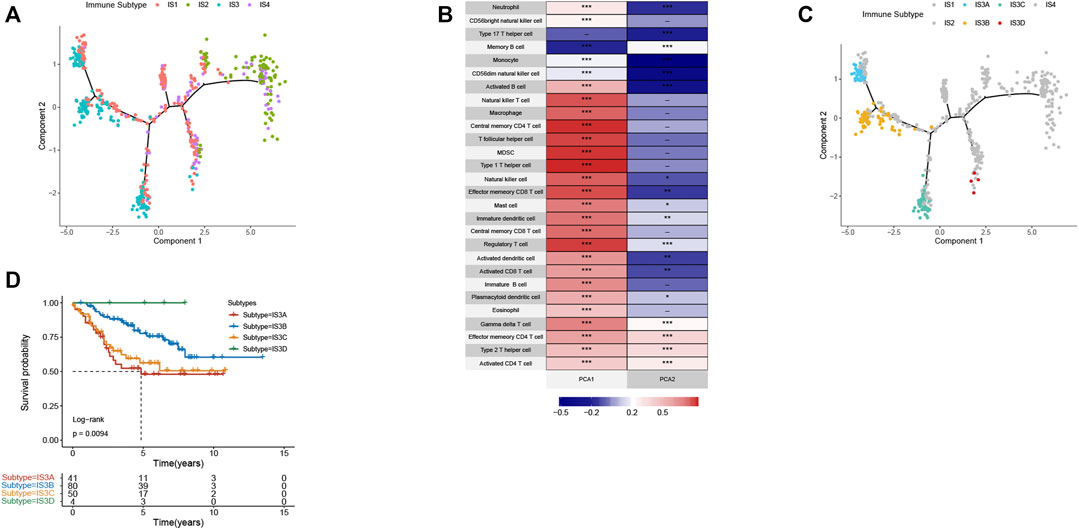
FIGURE 9. The immune landscape of CRC. (A) The immune landscape of CRC. Each point represents a patient with colors corresponding to the immune subtype defined previously. (B) Correlation between PCA1/2 and immune cell signatures. (C) Immune landscape of the subsets of CRC IS3 based on their location in the immune landscape. (D) Different subtypes in IS3 associated with different prognoses.
The immune landscape further revealed significant intra-cluster heterogeneity within each subtype. IS3 could be further divided into four subgroups based on the distribution location in the immune landscape (Figure 9C). The further prognostic analysis demonstrated that IS3D has the best prognosis among IS3A, IS3B, and IS3C (Figure 9D). Collectively, the immune landscape based on immune subtypes can precisely recognize immune components of each CRC patient as well as predict their prognoses that are beneficial for selecting personalized therapeutics for mRNA vaccine.
Therapy of CRC has been improved over the years with advanced surgical and chemotherapeutic procedures but challenges in terms of efficiency and adverse effects still need to be accomplished. Especially late-stage CRC patients still have a relatively poor prognosis (Hammond et al., 2016; Russo et al., 2019). mRNA vaccines have become a promising platform for cancer treatment. Nevertheless, tumor heterogeneity is one of the biggest setbacks in implementing mRNA vaccine-based therapy. In this study, the abnormal gene expression patterns and mutational landscape of CRC were constructed and a range of targeted antigens, including THBS2, FSTL3, TNNT1, BGN, CTHRC1, and NOX4 were identified as promising mRNA vaccine candidates. The overexpression of these antigens was associated with poor prognosis and high infiltration of APCs in CRC. Thus, these antigens play important roles in CRC development and progression. According to the abovementioned, the sequence of tumor antigens can be modified and cloned into appropriate plasmids for synthesizing mRNA. IVT mRNA will be encapsulated with lipid nanoparticles. Optimized mRNA encoding CRC antigens will be injected into CRC patients to induce an immune response against tumor cells to eradicate the tumor. mRNA vaccines encoding these tumor antigens (THBS2, FSTL3, TNNT1, BGN, CTHRC1, and NOX4) induce a cell-mediated immune response and humoral immune response that are beneficial for efficient clearance of cancer cells. Although further clinical evaluation of the candidates is required, their potential for anti-CRC mRNA vaccine development is consolidated by previous studies. For instance, THBS2 is a biomarker for clinical stages and a strong prognostic indicator in CRC. Increased THBS2 expression was significantly associated with clinical stages (TNM). Furthermore, THBS2 has been reported to regulate apoptosis, cell proliferation, and adhesion (Wang et al., 2016; Tian et al., 2018). FSTL3 is an oncogene and may be involved in CRC progression via the promotion of bone morphogenetic protein (BMP) signaling pathway (Karagiannis et al., 2013). Similarly, TNNT1 expression was closely correlated with the clinical stage, facilitated the proliferation, migration, and invasion of CRC cells (Chen Y. et al., 2020). BGN has been reported to be implicated in CRC liver metastasis (Ma et al., 2018). BGN activity influences CRC progression via its participation in other pathways (Chen D. et al., 2020). Previous studies have reported that knockout of BGN can inhibit the proliferation and migration of CRC cells (Xing et al., 2015a). In addition, BGN can promote angiogenesis through upregulating vascular endothelial growth factor (VEGF) expression in CRC patients (Xing et al., 2015b; Suhovskih et al., 2015). Moreover, BGN can also serve as a prognostic biomarker in CRC patients (You et al., 2015). CTHRC1 promotes human CRC cell proliferation and invasiveness by activating Wnt/planar cell polarity (PCP) signaling pathway (Yang et al., 2015). Upregulated CTHRC1 predicts poor prognosis and promotes epithelial-mesenchymal transition in CRC (Ni et al., 2018). NOX4 plays an important role in CRC progression and metastasis (Shen et al., 2020). High NOX4 expression was significantly correlated with clinicopathologic classifications and poor survival of CRC patients. NOX4 promoted CRC cells proliferation, migration, and invasion by regulating relevant pathway (Lin et al., 2017). To our knowledge, this is the first study that screened the CRC antigens for the development of mRNA vaccine for cancer.
Given that tumor heterogeneity restricts the widespread application of mRNA vaccine in CRC patients, a comprehensive understanding of the immune landscape of CRC is therefore essential for the design of efficient immunotherapy strategies. Four reproducible immune subtypes of CRC were identified based on IRGs expression profile to provide new insights for designing advanced mRNA vaccine strategies for CRC treatment. We observed that each of the four immune subtypes was correlated with distinct clinical characteristics, and accordingly revealed different patterns in molecular and cellular features. IS2 and IS4 tumors displayed a better prognosis than other subtypes in TCGA and GEO datasets. TMB is a critical intrinsic factor that affects tumor response to immunotherapy. High TMB can be used as an independent biomarker for the selection of patients for mRNA vaccine therapy (Havel et al., 2019). Patients with IS2 and IS4 tumors showed higher TMB, which may have a greater response to mRNA vaccine treatment. Moreover, we found that the IS2 and IS4 tumors had higher expression of HLA and immune checkpoint molecules and showed a more prominent Th1/IFNγ gene signature. HLA expression contributes to the activation of anti-tumor immunity through interactions with T cell receptors. Moreover, the complex immune landscape of CRC revealed substantial heterogeneity between individual patients and the same immune subtype, which provided complementary information for personalized mRNA-based cancer vaccines.
TIME plays a vital role for mRNA vaccine to elicit tumor immune response, it is critical to further understand diverse immune cell components of different immune subtypes. We found that each of the four immune subtypes was related to distinct cellular and molecular features. Among all immune subtypes, IS2 and IS4 have high immune scores compared with IS1 and IS3, which indicated that IS2 and IS4 are immunologically “hot” tumors while IS1 and IS3 are immunologically “cold” tumors. High immune scores subtype (IS2 and IS4) showed better survival outcomes. Previous studies proposed the “hot” tumor displayed higher tumor sensitivity to immunotherapy (Galon et al., 2006; Galon and Bruni, 2019). These findings further showed that patients in the immune-cold subtype have the lowest degree of B cells and CD4+T cells infiltration, while the immune-hot subtype exhibited high levels of infiltrating immune cells. IS2 and IS4 displayed abundant tumor-infiltrating lymphocytes (TILs), cytotoxic T cells, natural killer cells, and macrophages, the high level of TILs recognize ample tumor antigens with their diverse receptors. IS2 and IS4 had higher scores in TCR richness and BCR richness. Higher TCR richness may enhance anticancer immunity (Thorsson et al., 2018). Moreover, IS2 and IS4 exhibited higher inflammation-promoting, IFN-γ response, and TGF-β response gene expression compared with IS1 and IS3. Thus, patients with IS2 and IS4 tumors are more suitable for mRNA vaccine therapy, IS1 and IS3 may respond to mRNA vaccine combined with ICB therapy. However, our study was limited because it was retrospective, and identified mRNA vaccine antigens of the current investigation demand validation by prospective studies. The findings should be interpreted with this limitation in mind. Additionally, we will attempt to validate the accuracy of the identified six antigens in our following work.
In summary, our findings depicted the immune landscape of CRC and eventually identified four immune subtypes. THBS2, FSTL3, TNNT1, BGN, CTHRC1, and NOX4 are the potential targets of the CRC mRNA vaccine. Patients with IS2 and IS4 are suitable candidates for vaccination. In addition, we design an anti-CRC mRNA vaccine. Hence, this study may guide a personalized approach for mRNA vaccine development against CRC.
Publicly available datasets were analyzed in this study. This data can be found here: TCGA database (https://gdc-portal.nci.nih.gov/), GEO database (https://www.ncbi.nlm.nih.gov/geo/), cBioPortal (http://www.cbioportal.org/), GEPIA2 (http://gepia2.cancer-pku.cn/), and TIMER database https://cistrome.shinyapps.io/timer/.
CL and PW designed the study. CL performed the bioinformatic analyses and wrote the manuscript. YD, XW, XH, NY, JC, NR, and FX participated in data collection. DP and PW revised the manuscript. All authors reviewed and approved the final manuscript.
This work is supported by grant from Department of Education of Guangdong Province Research Project (2020KZDZX1181).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.783527/full#supplementary-material
Augestad, K. M., Merok, M. A., and Ignatovic, D. (2017). Tailored Treatment of Colorectal Cancer: Surgical, Molecular, and Genetic Considerations. Clin. Med. Insights Oncol. 11, 117955491769076. doi:10.1177/1179554917690766
Batich, K. A., Mitchell, D. A., Healy, P., Herndon, J. E., Sampson, J. H., and Sampson, J. H. (2020). Once, Twice, Three Times a Finding: Reproducibility of Dendritic Cell Vaccine Trials Targeting Cytomegalovirus in Glioblastoma. Clin. Cancer Res. 26 (20), 5297–5303. doi:10.1158/1078-0432.CCR-20-1082
Cerami, E., Gao, J., Dogrusoz, U., Gross, B. E., Sumer, S. O., Aksoy, B. A., et al. (2012). The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov. 2 (5), 401–404. doi:10.1158/2159-8290.CD-12-0095
Chen, D., Qin, Y., Dai, M., Li, L., Liu, H., Zhou, Y., et al. (2020). BGN and COL11A1 Regulatory Network Analysis in Colorectal Cancer (CRC) Reveals that BGN Influences CRC Cell Biological Functions and Interacts with miR-6828-5p. Cmar Vol. 12, 13051–13069. doi:10.2147/CMAR.S277261
Chen, Y.-P., Wang, Y.-Q., Lv, J.-W., Li, Y.-Q., Chua, M. L. K., Le, Q.-T., et al. (2019). Identification and Validation of Novel Microenvironment-Based Immune Molecular Subgroups of Head and Neck Squamous Cell Carcinoma: Implications for Immunotherapy. Ann. Oncol. 30 (1), 68–75. doi:10.1093/annonc/mdy470
Chen, Y., Wang, J., Wang, D., Kang, T., Du, J., Yan, Z., et al. (2020). TNNT1, Negatively Regulated by miR‐873, Promotes the Progression of Colorectal Cancer. J. Gene Med. 22 (2), e3152. doi:10.1002/jgm.3152
De Keersmaecker, B., Claerhout, S., Carrasco, J., Bar, I., Corthals, J., Wilgenhof, S., et al. (2020). TriMix and Tumor Antigen mRNA Electroporated Dendritic Cell Vaccination Plus Ipilimumab: Link between T-Cell Activation and Clinical Responses in Advanced Melanoma. J. Immunother. Cancer 8 (1), e000329. doi:10.1136/jitc-2019-000329
Galon, J., and Bruni, D. (2019). Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 18 (3), 197–218. doi:10.1038/s41573-018-0007-y
Galon, J., Costes, A., Sanchez-Cabo, F., Kirilovsky, A., Mlecnik, B., Lagorce-Pagès, C., et al. (2006). Type, Density, and Location of Immune Cells within Human Colorectal Tumors Predict Clinical Outcome. Science 313 (5795), 1960–1964. doi:10.1126/science.1129139
Hammond, W. A., Swaika, A., and Mody, K. (2016). Pharmacologic Resistance in Colorectal Cancer: a Review. Ther. Adv. Med. Oncol. 8 (1), 57–84. doi:10.1177/1758834015614530
Hargadon, K. M., Johnson, C. E., and Williams, C. J. (2018). Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacology 62, 29–39. doi:10.1016/j.intimp.2018.06.001
Havel, J. J., Chowell, D., and Chan, T. A. (2019). The Evolving Landscape of Biomarkers for Checkpoint Inhibitor Immunotherapy. Nat. Rev. Cancer 19 (3), 133–150. doi:10.1038/s41568-019-0116-x
Kallen, K.-J., Heidenreich, R., Schnee, M., Petsch, B., Schlake, T., Thess, A., et al. (2013). A Novel, Disruptive Vaccination Technology. Hum. Vaccin. Immunother. 9 (10), 2263–2276. doi:10.4161/hv.25181
Karagiannis, G. S., Berk, A., Dimitromanolakis, A., and Diamandis, E. P. (2013). Enrichment Map Profiling of the Cancer Invasion Front Suggests Regulation of Colorectal Cancer Progression by the Bone Morphogenetic Protein Antagonist, Gremlin-1. Mol. Oncol. 7 (4), 826–839. doi:10.1016/j.molonc.2013.04.002
Kowalzik, F., Schreiner, D., Jensen, C., Teschner, D., Gehring, S., and Zepp, F. (2021). mRNA-Based Vaccines. Vaccines 9 (4), 390. doi:10.3390/vaccines9040390
Kübler, H., Scheel, B., Gnad-Vogt, U., Miller, K., Schultze-Seemann, W., Vom Dorp, F., et al. (2015). Self-adjuvanted mRNA Vaccination in Advanced Prostate Cancer Patients: a First-In-Man Phase I/IIa Study. J. Immunotherapy Cancer 3, 26. doi:10.1186/s40425-015-0068-y
Li, B., Cui, Y., Nambiar, D. K., Sunwoo, J. B., and Li, R. (2019). The Immune Subtypes and Landscape of Squamous Cell Carcinoma. Clin. Cancer Res. 25 (12), 3537. doi:10.1158/1078-0432.CCR-18-4085
Li, T., Fan, J., Wang, B., Traugh, N., Chen, Q., Liu, J. S., et al. (2017). TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 77 (21), e108–e110. doi:10.1158/0008-5472.CAN-17-0307
Lin, X.-L., Yang, L., Fu, S.-W., Lin, W.-F., Gao, Y.-J., Chen, H.-Y., et al. (2017). Overexpression of NOX4 Predicts Poor Prognosis and Promotes Tumor Progression in Human Colorectal Cancer. Oncotarget 8 (20), 33586–33600. doi:10.18632/oncotarget.16829
Ma, Y.-S., Huang, T., Zhong, X.-M., Zhang, H.-W., Cong, X.-L., Xu, H., et al. (2018). Proteogenomic Characterization and Comprehensive Integrative Genomic Analysis of Human Colorectal Cancer Liver Metastasis. Mol. Cancer 17 (1), 139. doi:10.1186/s12943-018-0890-1
Mao, Q., Wang, L., Tsang, I. W., and Sun, Y. (2017). Principal Graph and Structure Learning Based on Reversed Graph Embedding. IEEE Trans. Pattern Anal. Mach. Intell. 39 (11), 2227–2241. doi:10.1109/TPAMI.2016.2635657
Miao, L., Zhang, Y., and Huang, L. (2021). mRNA Vaccine for Cancer Immunotherapy. Mol. Cancer 20 (1), 41. doi:10.1186/s12943-021-01335-5
Ni, S., Ren, F., Xu, M., Tan, C., Weng, W., Huang, Z., et al. (2018). CTHRC1 Overexpression Predicts Poor Survival and Enhances Epithelial-Mesenchymal Transition in Colorectal Cancer. Cancer Med. 7 (11), 5643–5654. doi:10.1002/cam4.1807
Pantin, J., and Battiwalla, M. (2020). Upsetting the Apple CAR‐T (Chimeric Antigen Receptor T‐cell Therapy) ‐ Sustainability Mandates USA Innovation. Br. J. Haematol. 190 (6), 851–853. doi:10.1111/bjh.16685
Papachristofilou, A., Hipp, M. M., Klinkhardt, U., Früh, M., Sebastian, M., Weiss, C., et al. (2019). Phase Ib Evaluation of a Self-Adjuvanted Protamine Formulated mRNA-Based Active Cancer Immunotherapy, BI1361849 (CV9202), Combined with Local Radiation Treatment in Patients with Stage IV Non-small Cell Lung Cancer. J. Immunotherapy Cancer 7 (1), 38. doi:10.1186/s40425-019-0520-5
Pardi, N., Hogan, M. J., Porter, F. W., and Weissman, D. (2018). mRNA Vaccines - a new era in Vaccinology. Nat. Rev. Drug Discov. 17 (4), 261–279. doi:10.1038/nrd.2017.243
Qiu, X., Mao, Q., Tang, Y., Wang, L., Chawla, R., Pliner, H. A., et al. (2017). Reversed Graph Embedding Resolves Complex Single-Cell Trajectories. Nat. Methods 14 (10), 979–982. doi:10.1038/nmeth.4402
Russo, M., Crisafulli, G., Sogari, A., Reilly, N. M., Arena, S., Lamba, S., et al. (2019). Adaptive Mutability of Colorectal Cancers in Response to Targeted Therapies. Science 366 (6472), 1473–1480. doi:10.1126/science.aav4474
Sahin, U., Karikó, K., and Türeci, Ö. (2014). mRNA-based Therapeutics - Developing a New Class of Drugs. Nat. Rev. Drug Discov. 13 (10), 759–780. doi:10.1038/nrd4278
Sebastian, M., Papachristofilou, A., Weiss, C., Früh, M., Cathomas, R., Hilbe, W., et al. (2014). Phase Ib Study Evaluating a Self-Adjuvanted mRNA Cancer Vaccine (RNActive) Combined with Local Radiation as Consolidation and Maintenance Treatment for Patients with Stage IV Non-small Cell Lung Cancer. BMC Cancer 14, 748. doi:10.1186/1471-2407-14-748
Shen, C.-J., Chang, K.-Y., Lin, B.-W., Lin, W.-T., Su, C.-M., Tsai, J.-P., et al. (2020). Oleic Acid-Induced NOX4 Is Dependent on ANGPTL4 Expression to Promote Human Colorectal Cancer Metastasis. Theranostics 10 (16), 7083–7099. doi:10.7150/thno.44744
Suhovskih, A. V., Aidagulova, S. V., Kashuba, V. I., and Grigorieva, E. V. (2015). Proteoglycans as Potential Microenvironmental Biomarkers for colon Cancer. Cell Tissue Res 361 (3), 833–844. doi:10.1007/s00441-015-2141-8
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tang, Z., Li, C., Kang, B., Gao, G., Li, C., and Zhang, Z. (2017). GEPIA: a Web Server for Cancer and normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res. 45 (W1), W98–W102. doi:10.1093/nar/gkx247
Thorsson, V., Gibbs, D. L., Brown, S. D., Wolf, D., Bortone, D. S., Ou Yang, T. H., et al. (2018). The Immune Landscape of Cancer. Immunity 48 (4), 812–e14. doi:10.1016/j.immuni.2018.03.023
Tian, Q., Liu, Y., Zhang, Y., Song, Z., Yang, J., Zhang, J., et al. (2018). THBS2 Is a Biomarker for AJCC Stages and a strong Prognostic Indicator in Colorectal Cancer. J. BUON 23 (5), 1331–1336.
Trapnell, C., Cacchiarelli, D., Grimsby, J., Pokharel, P., Li, S., Morse, M., et al. (2014). The Dynamics and Regulators of Cell Fate Decisions Are Revealed by Pseudotemporal Ordering of Single Cells. Nat. Biotechnol. 32 (4), 381–386. doi:10.1038/nbt.2859
van der Stok, E. P., Spaander, M. C. W., Grünhagen, D. J., Verhoef, C., and Kuipers, E. J. (2017). Surveillance after Curative Treatment for Colorectal Cancer. Nat. Rev. Clin. Oncol. 14 (5), 297–315. doi:10.1038/nrclinonc.2016.199
Wang, L., and Mao, Q. (2019). Probabilistic Dimensionality Reduction via Structure Learning. IEEE Trans. Pattern Anal. Mach. Intell. 41 (1), 205–219. doi:10.1109/TPAMI.2017.2785402
Wang, X., Zhang, L., Li, H., Sun, W., Zhang, H., and Lai, M. (2016). THBS2 Is a Potential Prognostic Biomarker in Colorectal Cancer. Sci. Rep. 6, 33366. doi:10.1038/srep33366
Xing, X., Gu, X., and Ma, T. (2015a). Knockdown of Biglycan Expression by RNA Interference Inhibits the Proliferation and Invasion of, and Induces Apoptosis in, the HCT116 colon Cancer Cell Line. Mol. Med. Rep. 12 (5), 7538–7544. doi:10.3892/mmr.2015.4383
Xing, X., Gu, X., Ma, T., and Ye, H. (2015b). Biglycan Up-Regulated Vascular Endothelial Growth Factor (VEGF) Expression and Promoted Angiogenesis in colon Cancer. Tumor Biol. 36 (3), 1773–1780. doi:10.1007/s13277-014-2779-y
Yang, X. M., You, H. Y., Li, Q., Ma, H., Wang, Y. H., Zhang, Y. L., et al. (2015). CTHRC1 Promotes Human Colorectal Cancer Cell Proliferation and Invasiveness by Activating Wnt/PCP Signaling. Int. J. Clin. Exp. Pathol. 8 (10), 12793–12801.
Keywords: mRNA vaccine, colorectal cancer, tumor immune microenvironment, tumor antigens, immune subtype, immune landscape
Citation: Liu C, Papukashvili D, Dong Y, Wang X, Hu X, Yang N, Cai J, Xie F, Rcheulishvili N and Wang PG (2022) Identification of Tumor Antigens and Design of mRNA Vaccine for Colorectal Cancer Based on the Immune Subtype. Front. Cell Dev. Biol. 9:783527. doi: 10.3389/fcell.2021.783527
Received: 26 September 2021; Accepted: 31 December 2021;
Published: 20 January 2022.
Edited by:
Shilpa S. Dhar, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Marcio Chaim Bajgelman, National Center for Research in Energy and Materials, BrazilCopyright © 2022 Liu, Papukashvili, Dong, Wang, Hu, Yang, Cai, Xie, Rcheulishvili and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng George Wang, d2FuZ3A2QHN1c3RlY2guZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.