
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol. , 17 January 2022
Sec. Molecular and Cellular Pathology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.777989
 Yumeng Huang1†
Yumeng Huang1† Qian Ji2†
Qian Ji2† Yanyan Zhu1†
Yanyan Zhu1† Shengqiao Fu2†
Shengqiao Fu2† Shuangwei Chen3
Shuangwei Chen3 Liangmei Chu2
Liangmei Chu2 Yongfei Ren3
Yongfei Ren3 Yue Wang3
Yue Wang3 Xuan Lei1
Xuan Lei1 Jia Gu1
Jia Gu1 Ningzheng Tai1*
Ningzheng Tai1* Dadong Liu4*
Dadong Liu4*Excessive neutrophil extracellular trap (NET) formation is an important contributor to sepsis-induced acute lung injury (ALI). Recent reports indicate that platelets can induce neutrophil extracellular trap formation. However, the specific mechanism remains unclear. Tph1 gene, which encodes the rate-limiting enzyme for peripheral 5-hydroxytryptophan (5-HT) synthesis, was knocked out in mice to simulate peripheral 5-HT deficiency. Cecal ligation and puncture (CLP) surgery was performed to induce sepsis. We found that peripheral 5-HT deficiency reduced NET formation in lung tissues, alleviated sepsis-induced lung inflammatory injury, and reduced the mortality rate of CLP mice. In addition, peripheral 5-HT deficiency was shown to reduce the accumulation of platelets and NETs in the lung of septic mice. We found that platelets from wild-type (WT), but not Tph1 knockout (Tph1−/−), mice promote lipopolysaccharide (LPS)-induced NET formation. Exogenous 5-HT intervention increased LPS-induced NET formation when Tph1−/− platelets were co-cultured with WT neutrophils. Therefore, our study uncovers a mechanism by which peripheral 5-HT aggravated sepsis-induced ALI by promoting NET formation in the lung of septic mice.
Sepsis is a life-threatening organ dysfunction caused by the host’s unbalanced response to infection and continues to be a major cause of death resulting from infection (Fleischmann et al., 2016; Rhodes et al., 2017; Napolitano 2018; Xie et al., 2020). The lungs are usually the earliest organ suffering in sepsis (Costa et al., 2006; Wang et al., 2019). Sepsis-induced lung injury is one of the key factors that affect the prognosis of patients with sepsis (Park et al., 2019).
Neutrophils are the most abundant innate immune cells in human blood and constitute the first line of human immunity (Mantovani et al., 2011; Wang and Chen, 2018; Abrams et al., 2019). In the past years, it was believed that neutrophils kill bacteria through phagocytosis and degranulation (Kolaczkowska and Kubes, 2013). In 2004, a new mechanism of neutrophil bactericidal function was discovered by Brinkmann et al. (2004), namely, neutrophil extracellular traps (NETs). Subsequently, NETs were considered to be a protective mechanism through capturing and eradicating pathogens (Jenne et al., 2013a; Amulic et al., 2017; Knackstedt et al., 2019; Drury et al., 2021). With the continuous research on NETs, some investigators indicated that excessive NET formation was an important cause of sepsis-induced organ dysfunction and death (Sonego et al., 2016; Ravindran et al., 2019; Tan et al., 2020). Sepsis-induced acute lung injury (ALI) can also be accentuated by NETs (Jhelum et al., 2018; Wang et al., 2020; Yaqinuddin and Kashir, 2020). However, the mechanism still remains unclear.
Serotonin, also named 5-hydroxytryptophan (5-HT), acts as a neurotransmitter in the central nervous system and enteric nervous system (McLean et al., 2007; Brommage et al., 2015). In peripheral blood, 5-HT is synthesized by tryptophan hydroxylase 1 (TPH1) in enterochromaffin cells and mainly stored in dense granules of platelets (Mohammad-Zadeh et al., 2008). Accumulating studies have depicted the critical role of peripheral 5-HT in inflammatory response (Duerschmied et al., 2013; Li et al., 2016; Zhang et al., 2017). However, whether peripheral 5-HT involves in lung NET formation in sepsis remains unclear.
Our data reveal that peripheral 5-HT deficiency can protect against sepsis-induced lung injury and improve the survival rate of septic mice by inhibiting the formation of NETs in lung tissues. This is fundamental to the development of measures to protect organs from injury and dysfunction in sepsis.
Lipopolysaccharide (LPS), 5-HT, fetal bovine serum (FBS), bovine serum albumin (BSA), and Tyrode’s solution were obtained from Sigma-Aldrich (MO, United States). ELISA kits for tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) were obtained from Qiaoyi (Shanghai, China). HBSS and RPMI 1640 medium were obtained from Life Technologies (CA, United States). APC-Cy7-labeled anti-LY6G antibody and FITC-labeled anti-CD41 antibody were obtained from BD (NJ, United States). APC-labeled anti-CD62p antibody (p-selectin) was obtained from BioLegend (San Diego, United States). Rabbit anti-mouse citrullinated histone H3 (CitH3) was obtained from Abcam (Cambridge, United Kingdom). Rabbit anti-mouse Ly6G, rabbit anti-mouse CD41, and related fluorescent secondary antibodies were obtained from Cell Signaling Technology (MA, United States).
Wild-type (WT) and Tph1 knockout (Tph1−/−) C57BL/6 male mice (6–8 weeks, body weight 20 ± 2 g) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, Jiangsu, China) and raised in the Experimental Animal Center of Jiangsu University (Zhenjiang, Jiangsu, China). All mice were randomly divided into four groups, including the WT and Tph1−/− sham groups that received sham surgery as well as the WT and Tph1−/− cecal ligation and puncture (CLP) groups that underwent CLP surgery. During CLP surgery, the mice were anesthetized with sevoflurane inhalation, the abdomen was disinfected with alcohol, and a 1-cm midline abdominal incision was made to expose the cecum of the mice. The end 1/3 of the cecum was ligated with 3–0 nylon thread, the end cecum was punctured with a 21-gauge needle, and an appropriate amount of intestinal content spilled out from the perforation. After the treatment, the cecum was reset, and the abdominal incision of the mice was sutured. Sham surgery requires only the cecum to be properly turned over and exposed, and then, the cecum is reset and the abdominal incision is sutured layer by layer. For survival analysis, in the sham groups (WT and Tph1−/−), each had six mice, while in CLP groups (WT and Tph1−/−), each had 25 mice. For other experiments, there were four mice in each group. The experimental protocol on animal protection and welfare was approved by the Council on Animal Care and Use at Jiangsu University.
Neutrophils were isolated from WT mice bone marrow by using density gradient centrifugation as previously described (Boxio et al., 2004; Szatmary et al., 2017). WT mice were sacrificed. Bone marrow was extracted from the femurs and tibias of the mice. Marrow cells were harvested from the bone marrow with a 70-µm cell strainer. Then, the cells were pelleted in a centrifuge and resuspended in HBSS. Following erythrocyte lysis, the neutrophil-containing solution was placed onto a discontinuous Percoll gradient solution (Percoll solution diluted to 78%, 69%, and 52% in HBSS), and the gradient was centrifuged at 1,500g at room temperature for 30 min. Neutrophils were collected from a band between the 78% and 69% layers. Finally, the neutrophils were resuspended in RPMI 1640 with 1% heat-inactivated FBS.
Platelets were isolated from WT and Tph1−/− mice peripheral blood as previously described (Liu et al., 2013). Mice peripheral blood was collected into a vacuum-anticoagulated tube containing trisodium citrate, and Tyrode’s solution was added. Platelet-rich plasma were isolated by centrifuging at 180×g at room temperature for 10 min, and platelets were isolated by centrifuging at 1,250×g at room temperature for 10 min. Then, platelets were washed and resuspended in Tyrode’s solution for at least 1 h at 37°C before use.
The purity (>97%) of cells was detected and adjusted by flow cytometry and an APC-Cy7-labeled anti-LY6G antibody (neutrophil) and FITC-labeled anti-CD41 antibody (platelet). The cell concentration was maintained at 1 × 106/ ml at every experiment. The activity of platelets was detected by flow cytometry and an APC-labeled anti-CD62p antibody.
WT neutrophils were randomly divided into three groups, including the WT neutrophil group, WT platelet and WT neutrophil co-culture group, and Tph1−/− platelet and WT neutrophil co-culture group. In each group, cells were randomly divided into four groups, with each containing 4 × 105 cells, including a control group, which received no intervention; an LPS group, which received LPS (1 μg/ ml) stimulation; a 5-HT group, which received 5-HT (100 μM) intervention; and an LPS + 5-HT group, which received 5-HT (100 μM) intervention and LPS (1 μg/ ml) stimulation. Cells were incubated in a 5% CO2 incubator at 37°C and 95% humidity. Twelve hours later, cells were harvested for subsequent experiments.
Immunofluorescence was used to detect the NET formation in the lung of mice and in vitro cultured cells. Lung tissues were sequentially fixed by 4% paraformaldehyde, embedded in paraffin, sectioned, and permeabilized with 0.05% Triton X-100. Cells were harvested, fixed, and permeabilized. Then, both lung and cell specimens were stained with primary antibodies (1:200), including citH3, Ly6G, and/or CD41, overnight at 4°C. Fluorescent secondary antibodies (1:200) were added and incubated at room temperature for 1 h in the dark. DAPI (1 μm/ ml) was incubated in the dark for 15 min. Finally, NET formation was detected by using an inverted phase-contrast microscope (×400 magnification). NETs expression was calculated based on CitH3 staining.
Lung tissue specimens (approximately 0.4 g) were harvested 12 h after surgery and fixed in 10% formalin. Then, the fixed specimens were sequentially embedded in paraffin, sectioned, and stained with hematoxylin/eosin (HE). A light microscope (×400 magnification) was applied to examine alveolar structure, cellular edema, and granulocyte infiltration in lung specimens.
Mice were sacrificed, and endotracheal intubation was performed 12 h after surgery. Bronchoalveolar lavage fluid (BALF) was collected from the endotracheal tube by lavage with PBS without Ca2+ and Mg2+. ELISA kits were used to detect the level of TNF-α and IL-6.
A total of 62 mice were randomly divided into four groups: WT sham group (n = 6), WT CLP group (n = 25), Tph1−/− sham group (n = 6), and Tph1−/− CLP group (n = 25). All mice were raised in the same environment, with no restrictions on their food or water intake. The mice were monitored every 6 h for 72 h.
Statistical analyses were performed with GraphPad Prism version 9.0 (United States). One-way analysis of variance was used for comparison between multiple groups, t-test was used for comparison between two groups, and Dunnett’s test was used for post-hoc analysis comparison. All data are presented as mean ± SD. Survival rate analysis was performed using the Kaplan–Meier method. A p value <0.05 was considered to be statistically significant.
Peripheral 5-HT, a monoamine neurotransmitter that is mainly synthesized by TPH1, can promote the inflammatory response by activating immune cells and increasing inflammatory cytokine release (Duerschmied et al., 2013; Li et al., 2016; Zhang et al., 2017). TPH1 is a rate-limiting enzyme for the synthesis of peripheral 5-HT (Zhang et al., 2020). In order to explore the role of peripheral 5-HT in sepsis, Tph1−/− mice were constructed and CLP was performed to induce sepsis. As shown in Supplementary Figure S1, the 5-HT concentration was markedly reduced in platelets of Tph1−/− mice, which confirmed the functional deficiency of TPH1.
The effect of peripheral 5-HT on the survival rate of septic mice was examined postoperatively. Results showed that peripheral 5-HT deficiency significantly increased the survival of septic mice (Figure 1A). Further study showed that in the Tph1−/− CLP mice, alveolar structure injury was less severe and the extent of alveolar wall granulocyte infiltration was reduced (Figures 1B, C). Meanwhile, we found that Tph1−/− CLP mice had a minor increase in BALF TNF-α and IL-6. The differences were statistically significant when compared to WT CLP mice (Figures 1D, E). These results suggested that peripheral 5-HT deficiency improves the survival rate of septic mice and alleviates lung inflammatory injury.
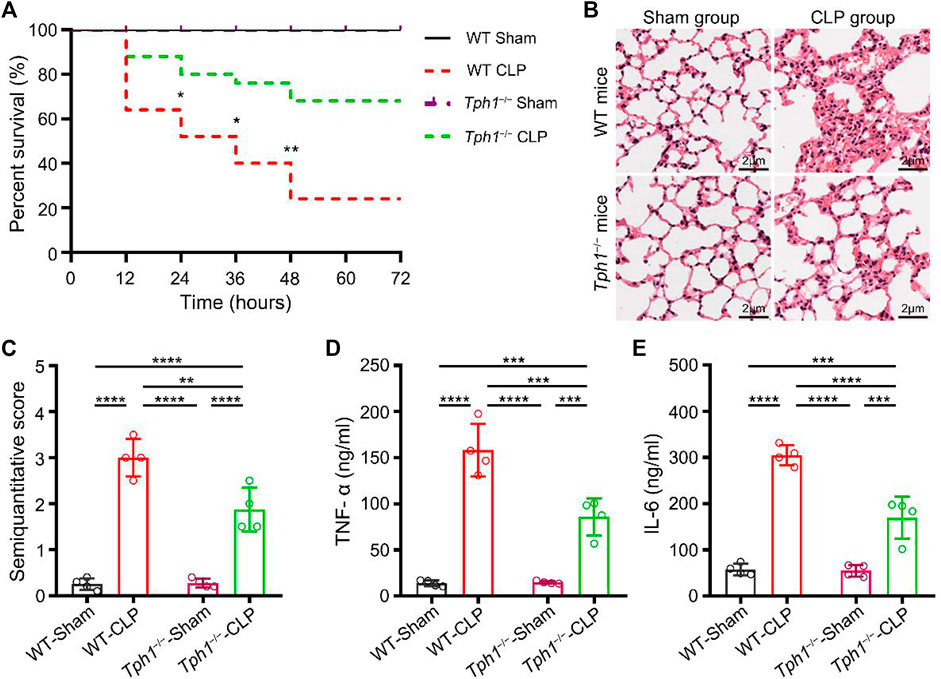
FIGURE 1. Peripheral 5-hydroxytryptophan (5-HT) deficiency improves the survival rate of septic mice and alleviates sepsis-induced acute lung injury. (A) The survival rate of mice. No deaths occurred in the wild-type (WT) and Tph1 knockout (Tph1−/−) sham groups. Most of the mice challenged with CLP died between 12 and 24 h postoperatively. The survival rate of WT mice was substantially low (only 52.00% at 24 h). In contrast, the survival rate of Tph1−/− mice was significantly increased (80.00% at 24 h). Moreover, at 72 h postoperatively, peripheral 5-HT deficiency dramatically increased the survival of septic mice from 24.00% to 68.00% (p < 0.01). For WT and Tph1−/− sham groups: n = 6; for WT and Tph1−/− cecal ligation and puncture (CLP) groups: n = 25. (B) Histopathological changes. Lung specimens from the sham mice showed normal architectures and lesser granulocyte infiltration, while lung specimens from WT CLP mice showed severe alveolar structure destruction, hyperemia, thickening of alveolar walls, and extensive granulocyte infiltration. On the contrary, lung specimens from the Tph1−/− CLP mice group showed less severe alveolar structure destruction and mild alveolar wall granulocyte infiltration. (C) Semiquantitative score of lung histological injury. Semiquantitative scores in WT CLP mice were significantly increased compared to sham groups. In contrast, Tph1−/− CLP mice showed lower semiquantitative scores. (D) Tumor necrosis factor alpha (TNF-α) concentrations. TNF-α concentrations in bronchoalveolar lavage fluid (BALF) of WT CLP mice were significantly increased compared to sham groups. In contrast, Tph1−/− CLP mice showed lower TNF-α concentrations. (E) Interleukin 6 (IL-6) concentrations. IL-6 concentrations in BALF of WT CLP mice were significantly increased compared to sham groups. In contrast, Tph1−/− CLP mice showed lower IL-6 concentrations. For each group: n = 4 (B–E). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Uncontrolled NET formation is reported to be a major contributor to sepsis-induced ALI and acute respiratory distress syndrome (ARDS) (Jhelum et al., 2018; Wang et al., 2020; Yaqinuddin and Kashir, 2020). Lefrancais et al. (2018) showed that inhibiting NET formation could reduce lung injury and improve mice survival. Lung specimens were collected, and immunofluorescence studies were performed to determine whether peripheral 5-HT deficiency affected NET formation. Results showed that lung tissues from sham mice had no NET formation. However, lung tissues from WT CLP mice had significantly enhanced NET formation, as indicated by the staining of CitH3. Interestingly, we observed that in the lung tissues of Tph1−/− CLP mice, NET formation was significantly reduced (Figure 2). This result indicates that peripheral 5-HT deficiency can reduce NET formation in the lung of septic mice.
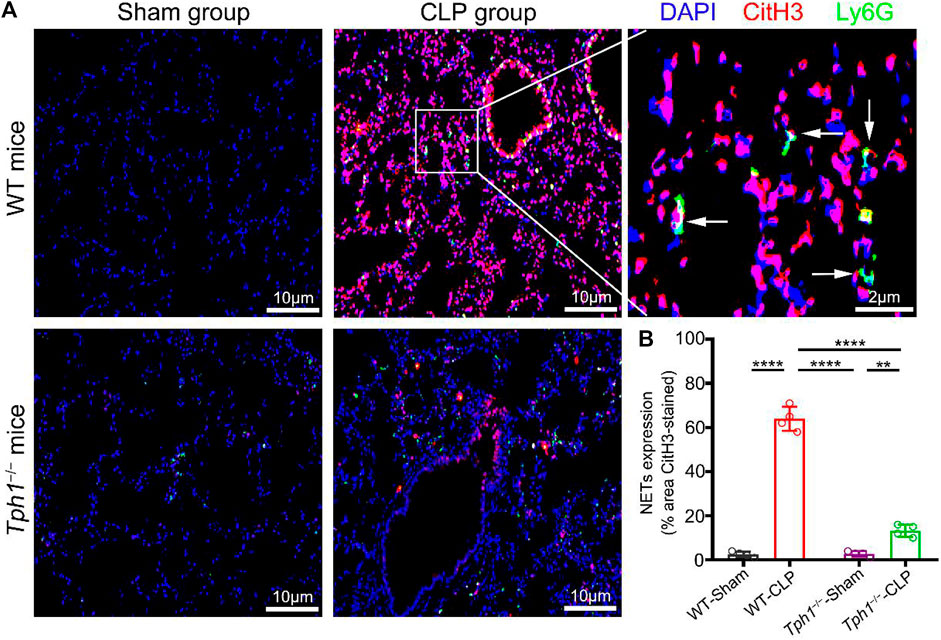
FIGURE 2. Peripheral 5-HT deficiency inhibits neutrophil extracellular trap (NET) formation in the lung of septic mice. Both WT and Tph1−/− mice (male, 6–8 weeks) were selected and randomly divided into sham and CLP groups. The mice were sacrificed 12 h after surgery, and lung specimens were collected and co-stained with Ly6G, CitH3, and DAPI fluorescent antibodies. The accumulation of NETs was detected by immunofluorescence microscopy. (A) Representative images of NET formation in the lung of septic mice. (B) Analysis of NET expression in mice lung specimens. For each group: n = 4. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Peripheral 5-HT is mainly stored in dense granules of platelets and released into the plasma during platelet activation (Mohammad-Zadeh et al., 2008). In order to investigate whether platelet-derived 5-HT is associated with NET formation, lung specimens were collected and co-stained with anti-CitH3 (labeled NETs) and anti-CD41 (labeled platelets). Results showed that the lungs from sham mice did not show NET formation, while the lungs from WT CLP mice showed a mass of the accumulation of platelets and NETs. Interestingly, the lungs from Tph1−/− CLP mice showed a significant decrease of the accumulation of platelets and NETs (Figure 3). We conclude that peripheral 5-HT deficiency can reduce the accumulation of platelets and NETs in the lung of septic mice.
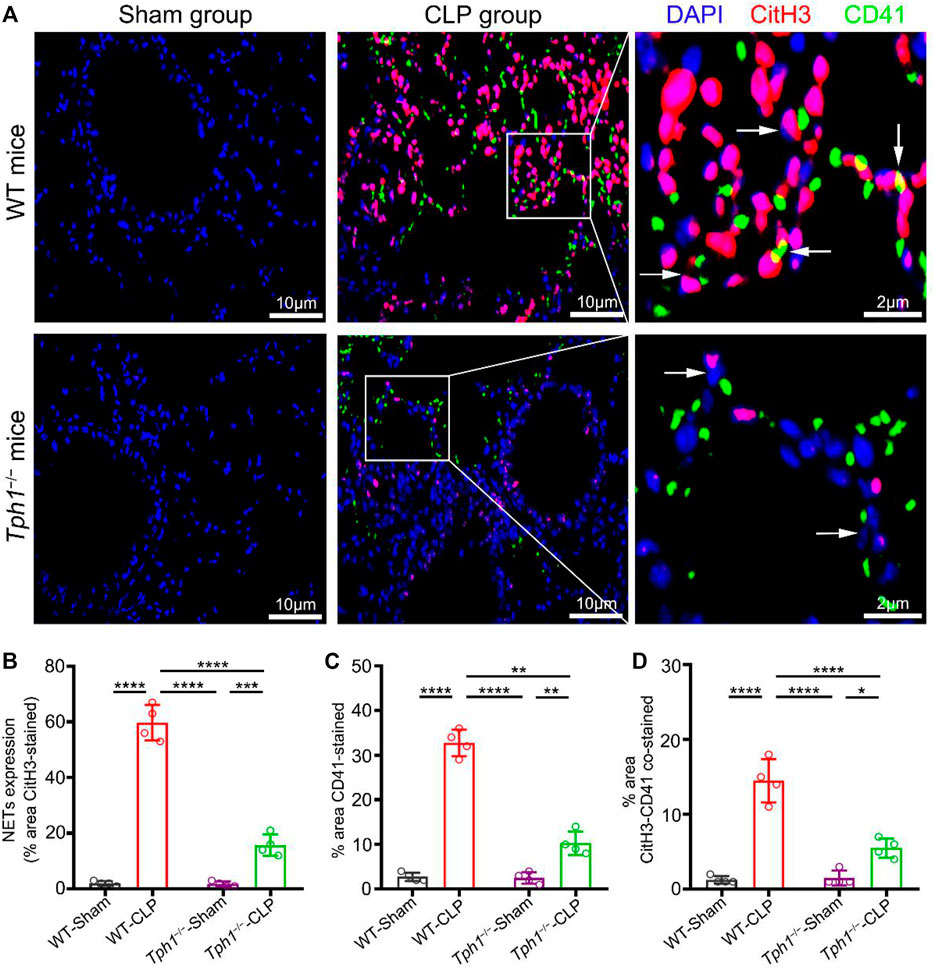
FIGURE 3. Peripheral 5-HT deficiency inhibits the accumulation of platelets with NETs in the lung of septic mice. Both WT and Tph1−/− mice (male, 6–8 weeks) were selected and randomly divided into sham and CLP groups. The mice were sacrificed 12 h after surgery, and lung specimens were collected and co-stained with CD41, CitH3, and DAPI fluorescent antibodies. The accumulation of NETs was detected by immunofluorescence microscopy. Lung specimens from sham mice showed little accumulation of platelets and NETs. Lung specimens from WT CLP mice group showed a large number of accumulation of platelets and NETs, while lung specimens from Tph1−/− CLP mice showed a significant decrease of the accumulation of platelets and NETs. (A) Representative images of the accumulation of platelets and NETs in the lung of septic mice. (B) Analysis of NET expression in mice lung specimens. (C) Analysis of the percentage of CD41-stained lung specimens in mice. (D) Analysis of the percentage of CitH3–CD41-stained lung specimens in mice. For each group: n = 4. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Although accumulating evidences reveal the role of platelets in NET formation, its mechanisms is unknown. To address this issue, neutrophils were isolated from WT mice and stimulated by LPS with or without exogenous 5-HT in vitro. NET generation was detected by staining with anti-CitH3 and DAPI. We noticed that LPS stimulation induced NET formation, while exogenous 5-HT intervention did not increase the LPS-induced NET formation in WT neutrophils cultured alone (Figures 4A, D). Recent studies showed that in bacterial sepsis, LPS induces non-classical activation of platelets (Clark et al., 2007; Carestia et al., 2016; Martinod and Deppermann, 2021). Consistent with the above studies, our mean fluorescence intensity (MFI) result showed that P-selectin (also called CD62p—a platelet activation marker) (van Velzen et al., 2012) was highly expressed in LPS-stimulated platelets. Furthermore, the MFI of P-selectin in WT platelets was higher than that in Tph1−/− platelets (Supplementary Figure S2). To further investigate whether platelet autocrine 5-HT accounted for NET formation, LPS was used to activate platelets. Platelets were isolated from WT or Tph1−/− mice and co-incubated with the WT neutrophils. Interestingly, results showed that WT platelets promoted LPS-induced NET formation (Figures 4B, E), while Tph1−/− platelets did not show a promotive effect (Figures 4C, F). However, exogenous 5-HT intervention remedied the deficiency of Tph1−/− platelets and promoted LPS-induced NET formation (Figures 4C, F). In light of the results shown above, we conclude that activated platelets promote NET formation through autocrine 5-HT signaling.
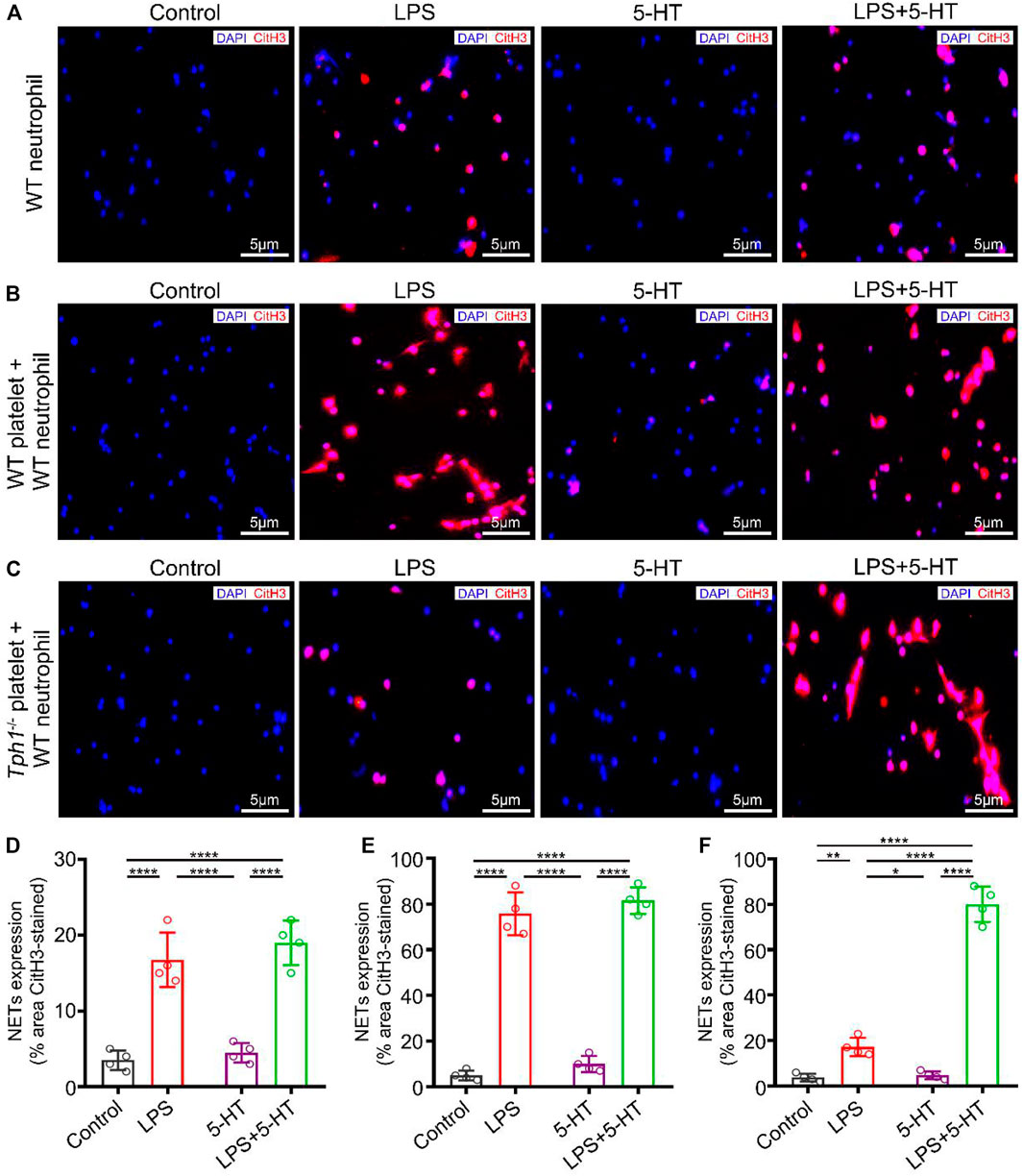
FIGURE 4. Activated platelets promote NET formation through autocrine 5-HT. Neutrophils were isolated from WT mice. Platelets were isolated from WT and Tph1−/− mice. The WT neutrophils were divided into WT neutrophil culture alone, WT neutrophil co-culture with WT platelet, and WT neutrophil co-culture with Tph1−/− platelet groups. In each group, cells were randomly divided into control, lipopolysaccharide (LPS), 5-HT, and 5-HT + LPS subgroups. After 12 h of cultivation, cells were fixed and co-stained with CitH3 fluorescent antibodies and DAPI. The accumulation of NETs was detected by immunofluorescence microscopy. Representative images of NET formation are presented in this figure. (A) Representative images of NET formation in WT neutrophils cultured alone. (B) Representative images of NET formation in WT neutrophils co-cultured with WT platelets. (C) Representative images of NET formation in WT neutrophils co-cultured with Tph1−/− platelets. (D) Analysis of NET expression in WT neutrophils. (E) Analysis of NET expression in WT neutrophils co-cultured with WT platelets. (F) Analysis of NET expression in WT neutrophils co-cultured with Tph1−/− platelets. For each group: n = 4. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
NETs are extracellular strands of decondensed DNA that are decorated with histones and neutrophil granule proteins. Since the discovery of NETs, numerous studies on NETs have been published. Among these findings, one widely accepted fact is that NETs may function as a double-edged sword (Kaplan and Radic, 2012). First of all, NET formation plays a central role in antimicrobial immunity. It constitutes an effective antimicrobial defense by neutralizing and killing pathogens at the infected site (Brinkmann et al., 2004; Papayannopoulos, 2018). On the other hand, NETs and their components may amplify the inflammatory process and promote organ damage, especially in non-infected organs of sepsis (Folco et al., 2018; Denning et al., 2019). In addition, detection of circulating NET components (including myeloperoxidase-DNA and citrullinated histone H3) can be used to assess organ impairment and predict 28-day mortality rate in septic patients (Li et al., 2011; Maruchi et al., 2018; Nomura et al., 2019). Consistent with previous reports, our results also revealed that the NET formation in lung tissue is associated with lung inflammatory injury and high mortality of CLP mice. Therefore, further studies were performed to explore the specific mechanism of sepsis-induced NET formation in the lungs.
The 5-HT receptor is classically recognized as a neurotransmitter (Okaty et al., 2019). Recently, the pro-inflammatory effect of platelet-derived 5-HT has attracted extensive attention (Duerschmied et al., 2013; Wu et al., 2019). Cloutier et al.,(2012) found that inhibiting the uptake of 5-HT in platelets could reduce joint effusion during arthritis. In recent years, studies have shown that the severity of pneumonia is also related to the tryptophan/serotonin pathway (Meier et al., 2017). Also, 5-HT can increase the exudation of neutrophils during ALI, which is believed to be related to the recruitment of neutrophils in innate immunity (Duerschmied et al., 2013). Our in vivo studies confirmed that peripheral 5-HT deficiency protects the lungs of mice from sepsis-induced lung injury and at the same time reduces NET formation in the lung tissues. However, in further in vitro experiments, we found that exogenous addition of 5-HT could not directly affect the NET formation induced by LPS. Therefore, we further explored its potential mechanisms.
In the lungs of septic mice, we found that platelets were accumulated with NETs, which suggested that platelets might have contributed to the NET formation in septic lungs. Platelets are tiny disk-shaped anucleate cell fragments derived from bone marrow megakaryocytes that play central roles in thrombosis and inflammation (Weiss, 1975; Jenne et al., 2013b; Deppermann and Kubes, 2018). Platelets contain three types of granules: α-granules, dense granules, and lysosomes. When platelets are activated, these granules can release a variety of secretions (Koupenova et al., 2018). Recent studies have shown that platelets are involved in NET formation (Clark et al., 2007). Meanwhile, platelet-promoted NET formation is associated with inflammation and thrombosis (Papayannopoulos, 2018; Mauler et al., 2019; Martinod and Deppermann, 2021). Similar, in our in vitro study, we also found that WT mice-derived platelets activated by LPS could significantly promote NET formation. However, the specific mechanism of platelet-promoted NET formation is still controversial.
Peripheral 5-HT is mainly stored in platelets and released after platelets are activated (Walther et al., 2003; Mammadova-Bach et al., 2018). The platelet autocrine 5-HT can further activate platelets (Li et al., 1997). In our study, we found that the activated Tph1−/− platelets (lacking 5-HT) did not increase NET formation, while the exogenous addition of 5-HT intervention can effectively reverse this phenomenon. These evidences suggest that platelet autocrine 5-HT plays a promoter role in LPS-induced NET formation. P-selectin can promote NET formation by mediating the binding of platelets to neutrophils (Pircher et al., 2019). However, it is still controversial whether P-selectin participated into the platelet-promoted NET formation (Maugeri et al., 2014; Etulain et al., 2015). In our study, we found that compared with Tph1−/− platelets, LPS could induce P-selectin expression in WT platelets more strongly. It indicates that platelet autocrine 5-HT-induced NET formation may involve P-selectin-mediated platelet and neutrophil interaction. Of course, we cannot completely ignore that other secretions may also have certain effects.
In conclusion, our findings indicate that platelet activation promotes NET formation in the lung tissue of septic mice through autocrine 5-HT signaling. Peripheral 5-HT deficiency protects the lung from sepsis-induced injury and improves the survival rate of septic mice by inhibiting the NET formation in lung tissues. This study is only conducted in animal and cell experiments and did not further explore the pathway through which activated platelet autocrine 5-HT acted on platelets to increase NET formation; further exploration and further clinical studies are warranted.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Council on Animal Care and Use at Jiangsu University.
YH, DL, and NT contributed to conception and design of the study. DL, YH, QJ, XL, JG, and YZ performed the statistical analysis. YH and DL wrote the first draft of the manuscript. QJ, SF, SC, YR, XL, YW, and DL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the sixth phase “Project 169” of Zhenjiang (DL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all of the mice who participated in this study for their contribution of specimens.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.777989/full#supplementary-material
Abrams, S. T., Morton, B., Alhamdi, Y., Alsabani, M., Lane, S., Welters, I. D., et al. (2019). A Novel Assay for Neutrophil Extracellular Trap Formation Independently Predicts Disseminated Intravascular Coagulation and Mortality in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 200 (7), 869–880. doi:10.1164/rccm.201811-2111OC
Amulic, B., Knackstedt, S. L., Abu Abed, U., Deigendesch, N., Harbort, C. J., Caffrey, B. E., et al. (2017). Cell-Cycle Proteins Control Production of Neutrophil Extracellular Traps. Develop. Cel 43 (4), 449–462. e5. doi:10.1016/j.devcel.2017.10.013
Boxio, R., Bossenmeyer-Pourié, C., Steinckwich, N., Dournon, C., and Nüße, O. (2004). Mouse Bone Marrow Contains Large Numbers of Functionally Competent Neutrophils. J. Leukoc. Biol. 75 (4), 604–611. doi:10.1189/jlb.0703340
Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, D. S., et al. (2004). Neutrophil Extracellular Traps Kill Bacteria. Science 303 (5663), 1532–1535. doi:10.1126/science.1092385
Brommage, R., Liu, J., Doree, D., Yu, W., Powell, D. R., and Melissa Yang, Q. (2015). Adult Tph2 Knockout Mice without Brain Serotonin Have Moderately Elevated Spine Trabecular Bone but Moderately Low Cortical Bone Thickness. Bonekey Rep. 4, 718. doi:10.1038/bonekey.2015.87
Carestia, A., Kaufman, T., Rivadeneyra, L., Landoni, V. I., Pozner, R. G., Negrotto, S., et al. (2016). Mediators and Molecular Pathways Involved in the Regulation of Neutrophil Extracellular Trap Formation Mediated by Activated Platelets. J. Leukoc. Biol. 99 (1), 153–162. doi:10.1189/jlb.3A0415-161R
Clark, S. R., Ma, A. C., Tavener, S. A., McDonald, B., Goodarzi, Z., Kelly, M. M., et al. (2007). Platelet TLR4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Septic Blood. Nat. Med. 13 (4), 463–469. doi:10.1038/nm1565
Cloutier, N., Paré, A., Farndale, R. W., Schumacher, H. R., Nigrovic, P. A., Lacroix, S., et al. (2012). Platelets Can Enhance Vascular Permeability. Blood 120 (6), 1334–1343. doi:10.1182/blood-2012-02-413047
Costa, E., Schettino, I., and Schettino, G. (2006). The Lung in Sepsis: Guilty or Innocent? Endocr. Metab. Immune Disord. Drug Targets 6 (2), 213–216. doi:10.2174/187153006777442413
Denning, N.-L., Aziz, M., Gurien, S. D., and Wang, P. (2019). DAMPs and NETs in Sepsis. Front. Immunol. 10, 2536. doi:10.3389/fimmu.2019.02536
Deppermann, C., and Kubes, P. (2018). Start a Fire, Kill the Bug: The Role of Platelets in Inflammation and Infection. Innate Immun. 24 (6), 335–348. doi:10.1177/1753425918789255
Drury, B., Hardisty, G., Gray, R. D., and Ho, G.-T. (2021). Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell Mol. Gastroenterol. Hepatol. 12, 321–333. doi:10.1016/j.jcmgh.2021.03.002
Duerschmied, D., Suidan, G. L., Demers, M., Herr, N., Carbo, C., Brill, A., et al. (2013). Platelet Serotonin Promotes the Recruitment of Neutrophils to Sites of Acute Inflammation in Mice. Blood 121 (6), 1008–1015. doi:10.1182/blood-2012-06-437392
Etulain, J., Martinod, K., Wong, S. L., Cifuni, S. M., Schattner, M., and Wagner, D. D. (2015). P-Selectin Promotes Neutrophil Extracellular Trap Formation in Mice. Blood 126 (2), 242–246. doi:10.1182/blood-2015-01-624023
Fleischmann, C., Scherag, A., Adhikari, N. K. J., Hartog, C. S., Tsaganos, T., Schlattmann, P., et al. (2016). Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 193 (3), 259–272. doi:10.1164/rccm.201504-0781OC
Folco, E. J., Mawson, T. L., Vromman, A., Bernardes-Souza, B., Franck, G., Persson, O., et al. (2018). Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production through Interleukin-1α and Cathepsin G. Arterioscler Thromb. Vasc. Biol. 38 (8), 1901–1912. doi:10.1161/ATVBAHA.118.311150
Jenne, C. N., Urrutia, R., and Kubes, P. (2013b). Platelets: Bridging Hemostasis, Inflammation, and Immunity. Int. Jnl. Lab. Hem. 35 (3), 254–261. doi:10.1111/ijlh.12084
Jenne, C. N., Wong, C. H. Y., Zemp, F. J., McDonald, B., Rahman, M. M., Forsyth, P. A., et al. (2013a). Neutrophils Recruited to Sites of Infection Protect from Virus Challenge by Releasing Neutrophil Extracellular Traps. Cell Host & Microbe 13 (2), 169–180. doi:10.1016/j.chom.2013.01.005
Jhelum, H., Sori, H., and Sehgal, D. (2018). A Novel Extracellular Vesicle-Associated Endodeoxyribonuclease Helps Streptococcus Pneumoniae Evade Neutrophil Extracellular Traps and Is Required for Full Virulence. Sci. Rep. 8 (1), 7985. doi:10.1038/s41598-018-25865-z
Kaplan, M. J., and Radic, M. (2012). Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J. Immunol. 189 (6), 2689–2695. doi:10.4049/jimmunol.1201719
Knackstedt, S. L., Georgiadou, A., Apel, F., Abu-Abed, U., Moxon, C. A., Cunnington, A. J., et al. (2019). Neutrophil Extracellular Traps Drive Inflammatory Pathogenesis in Malaria. Sci. Immunol. 4 (40), eaaw0336. doi:10.1126/sciimmunol.aaw0336
Kolaczkowska, E., and Kubes, P. (2013). Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 13 (3), 159–175. doi:10.1038/nri3399
Koupenova, M., Clancy, L., Corkrey, H. A., and Freedman, J. E. (2018). Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 122 (2), 337–351. doi:10.1161/CIRCRESAHA.117.310795
Lefrançais, E., Mallavia, B., Zhuo, H., Calfee, C. S., and Looney, M. R. (2018). Maladaptive Role of Neutrophil Extracellular Traps in Pathogen-Induced Lung Injury. JCI Insight 3 (3), e98178. doi:10.1172/jci.insight.98178
Li, N., Wallén, N. H., Ladjevardi, M., and Hjemdahl, P. (1997). Effects of Serotonin on Platelet Activation in Whole Blood. Blood Coagul. Fibrinolysis 8 (8), 517–524. doi:10.1097/00001721-199711000-00006
Li, Y., Hadden, C., Cooper, A., Ahmed, A., Wu, H., Lupashin, V. V., et al. (2016). Retracted Article: Sepsis-Induced Elevation in Plasma Serotonin Facilitates Endothelial Hyperpermeability. Sci. Rep. 6, 22747. doi:10.1038/srep22747
Li, Y., Liu, B., Fukudome, E. Y., Lu, J., Chong, W., Jin, G., et al. (2011). Identification of Citrullinated Histone H3 as a Potential Serum Protein Biomarker in a Lethal Model of Lipopolysaccharide-Induced Shock. Surgery 150 (3), 442–451. doi:10.1016/j.surg.2011.07.003
Liu, D., Liang, F., Wang, X., Cao, J., Qin, W., and Sun, B. (2013). Suppressive Effect of CORM-2 on LPS-Induced Platelet Activation by Glycoprotein Mediated HS1 Phosphorylation Interference. PLoS One 8 (12), e83112. doi:10.1371/journal.pone.0083112
Mammadova-Bach, E., Mauler, M., Braun, A., and Duerschmied, D. (2018). Autocrine and Paracrine Regulatory Functions of Platelet Serotonin. Platelets 29 (6), 541–548. doi:10.1080/09537104.2018.1478072
Mantovani, A., Cassatella, M. A., Costantini, C., and Jaillon, S. (2011). Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 11 (8), 519–531. doi:10.1038/nri3024
Martinod, K., and Deppermann, C. (2021). Immunothrombosis and Thromboinflammation in Host Defense and Disease. Platelets 32 (3), 314–324. doi:10.1080/09537104.2020.1817360
Maruchi, Y., Tsuda, M., Mori, H., Takenaka, N., Gocho, T., Huq, M. A., et al. (2018). Plasma Myeloperoxidase-Conjugated DNA Level Predicts Outcomes and Organ Dysfunction in Patients with Septic Shock. Crit. Care 22 (1), 176. doi:10.1186/s13054-018-2109-7
Maugeri, N., Campana, L., Gavina, M., Covino, C., De Metrio, M., Panciroli, C., et al. (2014). Activated Platelets Present High Mobility Group Box 1 to Neutrophils, Inducing Autophagy and Promoting the Extrusion of Neutrophil Extracellular Traps. J. Thromb. Haemost. 12 (12), 2074–2088. doi:10.1111/jth.12710
Mauler, M., Herr, N., Schoenichen, C., Witsch, T., Marchini, T., Härdtner, C., et al. (2019). Platelet Serotonin Aggravates Myocardial Ischemia/Reperfusion Injury via Neutrophil Degranulation. Circulation 139 (7), 918–931. doi:10.1161/CIRCULATIONAHA.118.033942
McLean, P. G., Borman, R. A., and Lee, K. (2007). 5-HT in the Enteric Nervous System: Gut Function and Neuropharmacology. Trends Neurosciences 30 (1), 9–13. doi:10.1016/j.tins.2006.11.002
Meier, M. A., Ottiger, M., Vögeli, A., Steuer, C., Bernasconi, L., Thomann, R., et al. (2017). Activation of the Tryptophan/Serotonin Pathway Is Associated with Severity and Predicts Outcomes in Pneumonia: Results of a Long-Term Cohort Study. Clin. Chem. Lab. Med. 55 (7), 1060–1069. doi:10.1515/cclm-2016-0912
Mohammad-Zadeh, L. F., Moses, L., and Gwaltney-Brant, S. M. (2008). Serotonin: A Review. J. Vet. Pharmacol. Ther. 31 (3), 187–199. doi:10.1111/j.1365-2885.2008.00944.x
Napolitano, L. M. (2018). Sepsis 2018: Definitions and Guideline Changes. Surg. Infections 19 (2), 117–125. doi:10.1089/sur.2017.278
Nomura, K., Miyashita, T., Yamamoto, Y., Munesue, S., Harashima, A., Takayama, H., et al. (2019). Citrullinated Histone H3: Early Biomarker of Neutrophil Extracellular Traps in Septic Liver Damage. J. Surg. Res. 234, 132–138. doi:10.1016/j.jss.2018.08.014
Okaty, B. W., Commons, K. G., and Dymecki, S. M. (2019). Embracing Diversity in the 5-HT Neuronal System. Nat. Rev. Neurosci. 20 (7), 397–424. doi:10.1038/s41583-019-0151-3
Papayannopoulos, V. (2018). Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 18 (2), 134–147. doi:10.1038/nri.2017.105
Park, I., Kim, M., Choe, K., Song, E., Seo, H., Hwang, Y., et al. (2019). Neutrophils Disturb Pulmonary Microcirculation in Sepsis-Induced Acute Lung Injury. Eur. Respir. J. 53 (3), 1800786. doi:10.1183/13993003.00786-2018
Pircher, J., Engelmann, B., Massberg, S., and Schulz, C. (2019). Platelet-Neutrophil Crosstalk in Atherothrombosis. Thromb. Haemost. 119 (8), 1274–1282. doi:10.1055/s-0039-1692983
Ravindran, M., Khan, M. A., and Palaniyar, N. (2019). Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules 9 (8), 365. doi:10.3390/biom9080365
Rhodes, A., Evans, L. E., Alhazzani, W., Levy, M. M., Antonelli, M., Ferrer, R., et al. (2017). Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 43 (3), 304–377. doi:10.1007/s00134-017-4683-6
Sônego, F., Castanheira, F. V. E. S., Ferreira, R. G., Kanashiro, A., Leite, C. A. V. G., Nascimento, D. C., et al. (2016). Paradoxical Roles of the Neutrophil in Sepsis: Protective and Deleterious. Front. Immunol. 7, 155. doi:10.3389/fimmu.2016.00155
Szatmary, P., Liu, T., Abrams, S. T., Voronina, S., Wen, L., Chvanov, M., et al. (2017). Systemic Histone Release Disrupts Plasmalemma and Contributes to Necrosis in Acute Pancreatitis. Pancreatology 17 (6), 884–892. doi:10.1016/j.pan.2017.10.002
Tan, C., Aziz, M., and Wang, P. (2020). The Vitals of NETs. J. Leukoc. Biol. 110, 797–808. doi:10.1002/JLB.3RU0620-375R
van Velzen, J. F., Laros-van Gorkom, B. A. P., Pop, G. A. M., and van Heerde, W. L. (2012). Multicolor Flow Cytometry for Evaluation of Platelet Surface Antigens and Activation Markers. Thromb. Res. 130 (1), 92–98. doi:10.1016/j.thromres.2012.02.041
Walther, D. J., Peter, J.-U., Winter, S., Höltje, M., Paulmann, N., Grohmann, M., et al. (2003). Serotonylation of Small GTPases Is a Signal Transduction Pathway that Triggers Platelet α-Granule Release. Cell 115 (7), 851–862. doi:10.1016/s0092-8674(03)01014-6
Wang, J., Gong, S., Wang, F., Niu, M., Wei, G., He, Z., et al. (2019). Granisetron Protects Polymicrobial Sepsis-Induced Acute Lung Injury in Mice. Biochem. Biophysical Res. Commun. 508 (4), 1004–1010. doi:10.1016/j.bbrc.2018.12.031
Wang, X., and Chen, D. (2018). Purinergic Regulation of Neutrophil Function. Front. Immunol. 9, 399. doi:10.3389/fimmu.2018.00399
Wang, Y.-P., Guo, Y., Wen, P.-S., Zhao, Z.-Z., Xie, J., Yang, K., et al. (2020). Three Ingredients of Safflower Alleviate Acute Lung Injury and Inhibit NET Release Induced by Lipopolysaccharide. Mediators Inflamm. 2020, 1–12. doi:10.1155/2020/2720369
Weiss, H. J. (1975). Platelet Physiology and Abnormalities of Platelet Function. N. Engl. J. Med. 293 (12), 580–588. doi:10.1056/NEJM197509182931204
Wu, H., Denna, T. H., Storkersen, J. N., and Gerriets, V. A. (2019). Beyond a Neurotransmitter: The Role of Serotonin in Inflammation and Immunity. Pharmacol. Res. 140, 100–114. doi:10.1016/j.phrs.2018.06.015
Xie, J., Wang, H., Kang, Y., Zhou, L., Liu, Z., Qin, B., et al. (2020). The Epidemiology of Sepsis in Chinese ICUs: A National Cross-Sectional Survey. Crit. Care Med. 48 (3), e209–e218. doi:10.1097/CCM.0000000000004155
Yaqinuddin, A., and Kashir, J. (2020). Novel Therapeutic Targets for SARS-CoV-2-Induced Acute Lung Injury: Targeting a Potential IL-1β/Neutrophil Extracellular Traps Feedback Loop. Med. Hypotheses 143, 109906. doi:10.1016/j.mehy.2020.109906
Zhang, J., Bi, J., Liu, S., Pang, Q., Zhang, R., Wang, S., et al. (2017). 5-HT Drives Mortality in Sepsis Induced by Cecal Ligation and Puncture in Mice. Mediators Inflamm. 2017, 1–12. doi:10.1155/2017/6374283
Keywords: sepsis, acute lung injury (ALI), 5-hydroxytryptophan (5-HT), neutrophil extracellular traps (NETs), neutrophil, platelet
Citation: Huang Y, Ji Q, Zhu Y, Fu S, Chen S, Chu L, Ren Y, Wang Y, Lei X, Gu J, Tai N and Liu D (2022) Activated Platelets Autocrine 5-Hydroxytryptophan Aggravates Sepsis-Induced Acute Lung Injury by Promoting Neutrophils Extracellular Traps Formation. Front. Cell Dev. Biol. 9:777989. doi: 10.3389/fcell.2021.777989
Received: 16 September 2021; Accepted: 25 November 2021;
Published: 17 January 2022.
Edited by:
Jian Song, University Hospital Münster, GermanyCopyright © 2022 Huang, Ji, Zhu, Fu, Chen, Chu, Ren, Wang, Lei, Gu, Tai and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ningzheng Tai, NzEwMjA0OTE0QHFxLmNvbQ==; Dadong Liu, NTgzMDM3OTMxQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.