
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 15 December 2021
Sec. Epigenomics and Epigenetics
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.774370
This article is part of the Research Topic RNA Modification and Non-Coding RNAs in Human Disease View all 22 articles
Osteoarthritis (OA) is a joint disease that is pervasive in life, and the incidence and mortality of OA are increasing, causing many adverse effects on people’s life. Therefore, it is very vital to identify new biomarkers and therapeutic targets in the clinical diagnosis and treatment of OA. ncRNA is a nonprotein-coding RNA that does not translate into proteins but participates in protein translation. At the RNA level, it can perform biological functions. Many studies have found that miRNA, lncRNA, and circRNA are closely related to the course of OA and play important regulatory roles in transcription, post-transcription, and post-translation, which can be used as biological targets for the prevention, diagnosis, and treatment of OA. In this review, we summarized and described the various roles of different types of miRNA, lncRNA, and circRNA in OA, the roles of different lncRNA/circRNA-miRNA-mRNA axis in OA, and the possible prospects of these ncRNAs in clinical application.
Osteoarthritis (OA) is a joint disease that is pervasive in life. It is largely caused by cartilaginous injury and affects the whole joint tissue (Pereira et al., 2015). Nearly half of people over 65 suffer from OA.(Sakalauskienė and Jauniškienė, 2010; Glyn-Jones et al., 2015). Globally, the incidence and mortality of OA are increasing (Bijlsma et al., 2011). Arthrodynia, swelling, and inability to move freely are the main symptoms of OA and cause many adverse effects on people’s lives. Several risk factors (Prieto-Alhambra et al., 2014), including age, sex, obesity, genetics, and joint damage, have been linked to OA progression (Felson et al., 2000; Vincent, 2019; Abramoff and Caldera, 2020). Articular cartilage degeneration and secondary osteogenesis are the main pathological manifestations of OA (Burr and Gallant, 2012). The long-term development of OA will not only affect people’s behaviors and activities but also cause depression, anxiety, and other negative emotions (Litwic et al., 2013). To provide more perfect, targeted treatment for patients with OA, the progression of OA needs to be studied. The specific pathogenesis of OA may be related to metalloproteinases (Mehana et al., 2019), cytokines (Boehme and Rolauffs, 2018), signaling pathways (Rigoglou and Papavassiliou, 2013), and noncoding RNA (ncRNA) (Sondag and Haqqi, 2016).
ncRNA is a nonprotein-coding RNA that does not translate into proteins but participates in protein translation. At the RNA level, it can perform biological functions (Wu et al., 2019). microRNA (miRNA), long ncRNA (lncRNA), circular RNA (circRNA), ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), small interfering RNA (siRNA), short hairpin RNA (shRNA) and Piwi-interactingRNA (piRNA) are the main ncRNAs(Chen et al., 2021). Studies have found that ncRNA is closely related to the occurrence of several diseases for the past few years (Esteller, 2011; Wang et al., 2019b). For example, promoter CpG methylation of two genes encoding members of the miR-200 family can easily lead to the occurrence and development of breast and colorectal cancer (Lim et al., 2013); miR-34b/c is a critical tumor suppressor. The methylation of miR-34b/c CpG island leads to the silence of miR-34b/c, thus increasing the incidence of tumors (Toyota et al., 2008); the decreased expression of miR-133 may induce myocardial hypertrophy by targeting the beta-1 adrenergic receptor pathway (Castaldi et al., 2014). Many studies have also found that miRNA, lncRNA, and circRNA are closely related to the course of OA, and play important regulatory roles in transcription, post-transcription, and post-translation (Li et al., 2019b; Zhang et al., 2021e). The interaction between lncRNA/circRNA, miRNA, and mRNA has attracted increasing attention. For example, lncRNA/circRNA can bind to miRNA, reduce the inhibitory effect of miRNA on mRNA, participate in regulating the progress of chondrocyte proliferation and apoptosis, extracellular matrix (ECM) degradation and inflammatory response in the progress of OA. Furthermore, lncRNA-p21 could induce chondrocyte apoptosis and slow the process of OA by binding to miR-451 and promoting the expression of downstream target gene mRNA (Tang et al., 2018a). This review describes the roles of miRNA, lncRNA, and circRNA in OA and the role of the lncRNA/circRNA–miRNA–mRNA axis in OA.
miRNA is a single-stranded RNA molecule with a length of about 20–24 nucleotides (Correia de Sousa et al., 2019). It belongs to one type of ncRNA and widely exists in eukaryotes to regulate the expression of other genes. miRNA regulates gene expression based on complete or incomplete pairing with mRNA. In most cases, the single-stranded miRNA in the complex is paired with the 3′UTR of the target mRNA in an incomplete complementary manner, blocking the translation of the gene and regulating gene expression. This process, called translation inhibition, is mainly found in animal cells. When the miRNA is completely complementary to the 3′UTR of the target mRNA, the mRNA in the complementary region would be specifically broken, eventually leading to gene silencing, and the process called post-transcriptional gene silencing, which will eventually lead to the degradation of target mRNA, mainly exists in plant cells (Liu et al., 2014a). The same gene can be regulated by multiple miRNAs, and multiple target genes can be regulated by the same miRNA (Iacona and Lutz, 2019). The formation and mechanism of miRNA are shown in Figure 1.
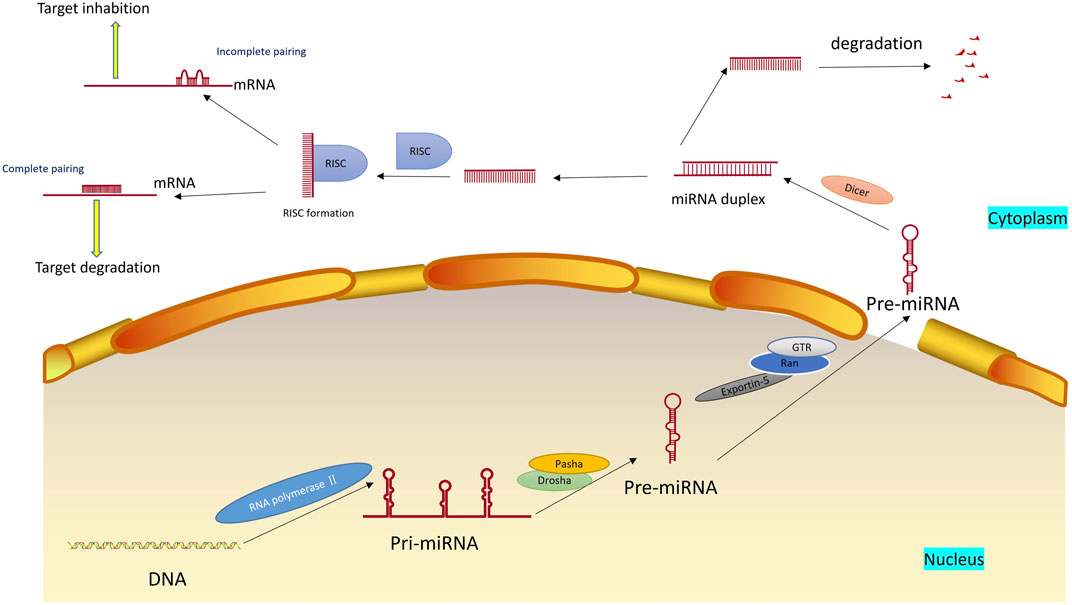
FIGURE 1. Formation and mechanism of miRNA. miRNAs are first transcripted into longer primary miRNAs in the nucleus and then processed into hairpin RNAs of 60–70 nucleotides in the nucleus by Drosha, Pasha et al. The precursor miRNAs are transported out of the cell nucleus with the help of the Ran-GTP-dependent nucleoplasmic/cell transporter Exprotin-5 and split into 21–25 nucleotide length double-stranded miRNAs in the cytoplasm by Dicer. Subsequently, the double helix is derotated by the action of the derotation enzyme, and one of the strands is integrated into the RNA-induced silencing complex (RISC), an asymmetric RISC assembly is formed, and the other chain is immediately degraded.
With the deepening of research, miRNAs have been discovered and studied increasingly, and they have become a potential target in disease prevention and treatment. miRNA has many functions roles in human diseases, such as regulating cell autophagy (Li et al., 2019h), epigenesis (Yao et al., 2019), glucose metabolism (Fu et al., 2015). Chen et al. (2018c) developed a computational model for disease association prediction to detect potential miRNA-disease associations accurately and efficiently. By studying three common human cancers (Zhang et al., 2021b), namely, colon cancer, esophagus cancer, and kidney cancer, many miRNAs were confirmed to be connected with the three kinds of cancer. In addition, many studies have proven that miRNA is related to the pathological processes of intervertebral disc degeneration (Shi et al., 2021b), muscle atrophy (Zhang et al., 2021a), and cardiovascular diseases (Liu et al., 2021a).
Currently, growing findings reveal that miRNA expression level changes exist in various tissues of patients with OA, leading to abnormal target gene expression. miRNA has many functions in OA, such as regulating cell autophagy and apoptosis (Yu et al., 2019b), inflammatory reaction (Sui et al., 2019), and cartilage degradation (Guo et al., 2020). Changes in miRNA expression levels in different tissues can be experimented with by gene sequencing. Gene sequencing is a new type of gene detection technology, which can analyze and determine the whole sequence of genes from blood or saliva to predict the possibility of suffering from various diseases and lock in individual diseased genes for early prevention and treatment. Zhou et al. (2020b) revealed 21 differentially expressed miRNAs in synovial tissues from OA patients compared with normal controls by gene sequencing technology. The expression levels of the first two DEmiRNAs(hsa-miR-17-5p and hsa-miR-20b-5p), which cover most of the DEmRNAs, were analyzed and found to be down-regulated in OA, which was also confirmed by qRT-PCR verification. Ntoumou et al. (2017) assessed differential miRNA expression by microarray analysis in the serum of patients with OA. Compared with the control group, 279 miRNAs were differentially expressed in OA. This study focused on analyzing and studying three differentially expressed miRNAs: hsa-miR-140-3p, hsa-miR-671-3p, and hsa-miR-33b-3p. We found that the expression of these three miRNAs was down-regulated in the serum of OA patients. Through serum microRNA array analysis and bioinformatics analysis, they determined that these three miRNAs were potential OA biomarkers involved in the metabolic processes of insulin and cholesterol. OA is a metabolic disease, and insulin resistance plays a vital role in metabolic syndrome. Therefore, the metabolic processes of insulin and cholesterol in the body are closely related to OA. In addition, based on RNA sequencing and miRNA analysis, Wu et al. (2021a) identified that miR-210-5p is highly enriched in the exosomes of OA sclerotic subchondral osteoblasts, triggering the expression of genes associated with catabolism in articular chondrocytes. Therefore, the abnormal up-regulation of miR-210-5p in exosomes could serve as a marker for OA. Notably, miRNA show obvious tissue specificity in different OA tissues. For example, the expression of miR-125b-5p in synovial fluid and chondrocytes is different in OA patients. Ge et al. (2017) found by PCR that miR-125b-5p in synovial fluid was significantly up-regulated in OA patients compared with normal subjects, promoting synovial cell apoptosis by targeting syvn1. Rasheed et al. (2019) treated chondrocytes with IL-1β to construct OA cell models and determined the expression of miR-125b-5p using Taqman analysis. They found that miR-125b-5p in chondrocytes was significantly down-regulated compared to healthy individuals and regulated inflammatory genes in OA chondrocytes by targeting TRAF6. Our appeal study found that expression levels of multiple miRNAs in the synovial membrane, cartilage, and subchondral bone were altered in OA patients compared to healthy individuals. In addition, even in the same tissue, if in different stages of development, the expression of miRNAs may be different. For example, in different stages of the knee joint cartilage of rats, Sun et al. (2011) used Solexa sequencing and RT-qPCR detection for the expression of miRNAs. They tested the miRNAs in the rat knee joint cartilage at the starting point, on Day 21 and Day 42, and found that the expression of miRNAs was different at each stage. Among them, 4 representative miRNAs were selected for further analysis. Compared with the initial stage, the expressions of aggrecan, colia1, and ColXa1 were up-regulated on day 21. The expression of ColXa1 was up-regulated on day 42, whereas those of aggrecan and colia1 were down-regulated. The expression of Sox9 showed minimal change during the three stages. Gabler et al. (2015) found that miRNA could control the differentiation of chondrocytes and regulate the occurrence of OA. During the development of human bone marrow mesenchymal stem cells (HMSCs), the expression of miRNA in different development stages is also different. By microarray analysis, the miR spectra of HMSCs in patients with OA at different development time points were measured. Among the 1,349 detected miRNAs, 553 were expressed in cartilage formation, they further performed miRNAs detection at 7, 14, 21, and 42 days after cartilage formation and found that their expression of miRNAs was also different. In summary, the expression of miRNAs in OA patients is different in different tissues and between different stages of development of the same tissue.
It is well known that many intracellular signaling pathways, such as nuclear factor-kappaB(NF-κB) and transforming growth factor β (TGF-β) played an vital roles in the pathogenesis of OA (Nishimura et al., 2020). In recent years, more studies discovered that miRNA can delay the pathological process of OA by promoting or inhibiting these pathways (Xu et al., 2016). NF-κB is an essential nuclear transcription factor in cells participating in the inflammatory and immune response of the body and apoptosis regulation (Lawrence, 2009). For example, as the 3′UTR of NF-κB contains the binding site of miR-143 and miR-124, when the DNA methylation degree of miR-143 and miR-124 promoters is reduced, the expression of miR-143 and miR-124 is up-regulated, and the transcription process is activated, thereby inhibiting the NF-κB signaling pathway, inhibiting apoptosis and delaying the progression of OA (Qiu et al., 2020). Similarly, When the expression levels of miR-34a and miR-181a were decreased, the expression of the BCL2 gene was increased, thereby limiting the term of NF-κ B translocation into the nucleus in OA Chondrocytes cultures and eventually reducing apoptosis and oxidative stressl (Cheleschi et al., 2019). The TGF-β signaling pathway is involved in many cellular processes in mature organisms and developing embryos, including cell growth, differentiation, apoptosis, dynamic cell balance, and other cellular functions. By promoting or inhibiting the TGF-β signaling pathway, we can regulate the cellular processes, thereby inducing or delaying the progression of OA (Shen et al., 2014). Hu et al. (2019b) established OA mouse models. QPCR and Western blot were used to compare the expression of miR-455-3p and PAK2 in the cartilage of healthy individuals and patients with OA, and the luciferase reporter gene was used to analyze the interaction between them. The results showed that miR-455-3p could inhibit the expression of pak 2, promote the TGF-β signaling pathway, and ultimately inhibit OA by directly targeting PAK2 3′UTR. In summary, various miRNAs are involved in regulating OA progression by handling a variety of intracellular signaling pathways. In addition, increasing evidence also emphasizes that changes in the expression of many miRNAs can also directly regulate the development of OA. The specific information of these miRNAs is listed in Table 1.
lncRNAs are ncRNAs with a length of more than 200 nucleotides that have little or no protein-coding potential, and account for more than 80% of total lncRNAs(Ponting et al., 2009). At first, lncRNA was considered the “noise” of genome transcription, with no biological function, and its mechanism of action was only in situ regulation, through recruitment and formation of chromatin modification complexes [such as IGF2RRNA antisense (AIR), XIST] to silence the transcription of neighboring genes. As more detection techniques were applied to RNA studies, such as microarray, RNA sequencing (RNA-seq), Northern blot, and real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) (Zhu et al., 2013), more biological functions of lncRNAs gradually being discovered. Recent studies have discovered several mechanisms of action of lncRNA, which can interact with proteins, DNA, and RNA to regulate many biological processes (Zhu et al., 2013). For example, lncRNA MALAT1 acts on miR-150-5P and AKT3 to regulate cell proliferation and apoptosis (Zhang et al., 2019g), thus participating in the growth and development of the body and the pathological process of diseases (Kopp and Mendell, 2018) (Figure 2).
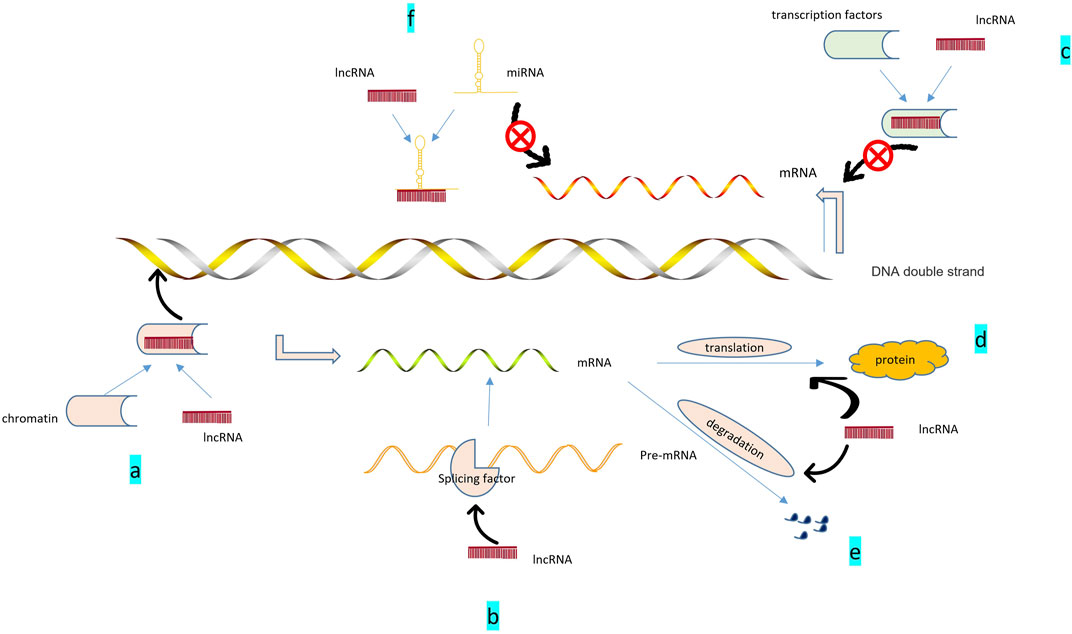
FIGURE 2. Role of lncRNAs: 1. Epigenetic regulation: (A). lncRNA recruits chromatin remodeling and modification complexes to specific sites; regulates DNA or RNA methylation status, chromosome structure; and promotes the expression of related genes.2. Transcriptional regulation: (B). lncRNA can help generate mature mRNA by promoting the binding of pre-mRNA to alternative splicing factors; (C). ncRNA binding with transcription factors can inhibit the activity of target genes and inhibit their gene expression. 3. Post-transcriptional regulation: (D). Participation in mRNA translation; (E). Involvement in mRNA degradation. 4. Regulation of miRNA: (F). ncRNA can act as sponges of miR-compete for miR and alleviate the inhibition of target genes.
lncRNA is closely related to cell growth, differentiation, and senescence. In addition, lncRNA has a special relationship with some human diseases, such as cardiovascular diseases (Huang, 2018), nervous system diseases (Zhang et al., 2019f), and immune-mediated diseases (Zhou et al., 2018b). In the recently updated database of lncRNA-related diseases, more than 200,000 lncRNAs have been recorded in their association with diseases (Bao et al., 2019).
lncRNA can regulate chondrocyte proliferation and apoptosis, inflammatory response, and extracellular matrix degradation, and promote the repair and stability of articular cartilage. Recent studies have shown an essential relationship between some changes or disorders of lncRNAs and the occurrence and development of OA. There are many studies to detect the expression of lncRNA in OA patients. Yang et al. (2021a) examined the lncRNA profiles of patients with OA and healthy individuals by RNA sequencing. They found that 25 lncRNAs are differentially expressed in patients with OA compared with the control group. Through microarray analysis, Xing et al. (2014) detected the expression of lncRNA in KOA cartilage and normal cartilage and further verified it by real-time polymerase chain reaction (RT-PCR). They found that the expression of 121 lncRNAs in KOA is different from normal cartilage: 73 up-regulated lncRNAs and 48 down-regulated lncRNAs. Among the up-regulated lncRNAs, HOTAIR is the most up-regulated. Pearson et al. (2016) separated OA chondrocytes through collagenase digestion and analyzed lncRNA expression through RNA sequencing (RNAseq) and qPCR. Finally, 983 lncRNAs were identified in OA chondrocytes. A total of 125 differentially expressed lncRNAs were identified after interleukin-1B (IL-1B) stimulation. Through microarray and qPCR analysis, Liu et al. (2014b) compared the expression of lncRNA in OA cartilage and normal cartilage, and found 152 differentially expressed lncRNAs in OA cartilage. Compared with normal cartilage, 82 increased lncRNAs and 70 decreased lncRNAs were in OA cartilage. Using mRNA and lncRNA microarray analysis, Zhang et al. (2020a) found that 990 lncRNAs were different in OA chondrocytes compared with the control group: 666 up-regulated, 324 down-regulated. In addition, 546 mRNAs had a different expression: 419 up-regulated, 127 down-regulated. Six lncRNAs (ENST00000606283.1, ENST00000436872.1, ENST00000488584.1, ENST00000603682.1, XR-245446.2, and ENST00000605586.1) were tested by qPCR. The results were consistent with the test results. In summary, through the detection of lncRNA expression levels in the chondrocytes of OA patients and healthy individuals, we can finally find that there are differences in the expression of a variety of lncRNAs. In addition to the lncRNAs of appeal, several lncRNAs are closely related to the progress of OA, as shown in Table 2.
The circRNA molecule is in a closed-loop structure and is not affected by RNA exonuclease. They are mainly in the cytoplasm or stored in exosomes. They are stable and not easily degradable, and widely exist in many eukaryotes. circRNAs are formed by reverse splicing through nonclassical splicing. One model believes that in the transcription of pre-RNA, due to the partial folding of RNA, the originally nonadjacent exons are pulled closer, and exon jumping occurs, resulting in the formation of circular RNA intermediates in the region to be crossed. Moreover, ring RNA molecules composed of exons are formed by lasso splicing. Another model suggests that the reverse complementary sequence located in the intron region leads to intron region pairing mediated reverse splicing, resulting in the formation of circular RNA molecules (Chen and Yang, 2015). To date, the biological functions of circRNAs that have been discovered mainly include interactions with miRNAs(Cao et al., 2019a), binding of regulatory proteins (Zang et al., 2020), transcription of regulatory genes (Zhang, 2020), and coding functions (Lei et al., 2020) (Figure 3). For example, circRNA.33186 increased MMP-13 expression by interacting with miR-127-5p to regulate cell proliferation and apoptosis (Zhou et al., 2019b).
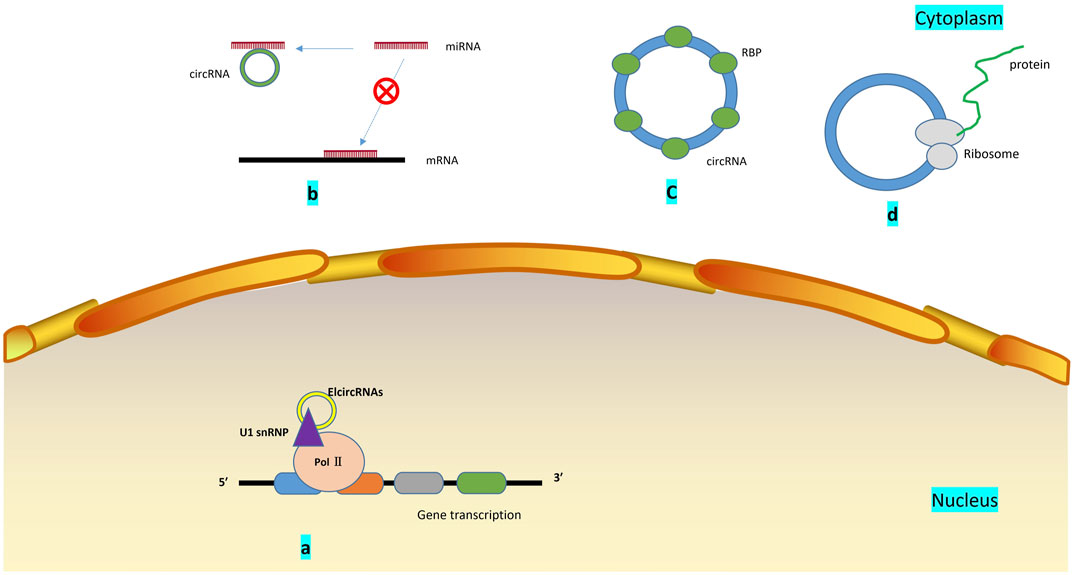
FIGURE 3. Biological functions of circRNA: (A). Regulation of gene transcription: Elcircrna can interact with small nuclear ribonucleoproteins and bind to RNA polymerase II; (B). miRNA sponge: circRNA contains miRNA binding sites, which can block miRNA binding to mRNA and promote or inhibit the expression of related genes by sponging miRNA; (C). circRNAs bind to mRNA regulatory binding protein, which influences the stability of mRNA, and may change the splicing pattern of circRNA; (D). By being translated by ribosomes and encoding polypeptides, several circRNAs can play a role in regulating and controlling human physiological processes.
Bipartite Network Projection allocates resources according to the known associations between different miRNAs and diseases, entirely using the similarity information of miRNA and diseases to predict various conditions accurately (Chen et al., 2018b). KATZ Measure is a graph-based calculation method, which converts the calculation of the similarity between lncRNA and diseases into the problem of similarity calculation between nodes in heterogeneous networks to predict the correlation between lncRNA and conditions. The integration of the two can recognize the association of circRNA with the disease (Chen, 2015). Through Bipartite Network Projection and KATZ Measure (Zhao et al., 2019a), many circRNAs related to diseases have been discovered, and circRNAs are involved in the diagnosis and treatment of atherosclerosis (Zhang et al., 2018a), cancer (Li et al., 2020f), cardiac hyperplasia (Li et al., 2020e), and other diseases. There are many experimental studies related to circRNA and diseases, and the main research types are cell experiments or animal experiments. Through these experiments, we have found multiple action mechanisms of circRNA on various conditions. For example, circRNA_100367 acts as a signaling molecule that regulates esophageal squamous cell carcinoma through the Wnt3 signaling pathway (Liu et al., 2019b); circRNA_0016624 regulates gene-based expression of interest in osteoporosis patients via sponge miR-98 (Yang et al., 2020d); circRNA_100395 mitigates the progression of breast cancer by directly targeting MAPK6 (Yu et al., 2020).
In addition, several circRNAs participate in the development of OA and the OA of the abnormal expression in various tissues. For example, Xiao et al. (2019a) used illumina sequencing platform to detect circRNA expression in patients with mild and severe KOA. In this paper, 197 differentially expressed circRNAs were identified. Among them, the up-regulation amplitude of Hg38_circ_0007474 is the largest, and the down-regulation amplitude of hg38_circ_0000118 is the largest. Further analysis of the three circRNAs selected from hsa_circ_0045714, hsa_circ_0005567, and hsa_circ_0002485 found that all three circRNAs can inhibit the function of the corresponding miRNA by serving as a sponge for miRNAs and indirectly promote its downstream process, thereby participating in the development of OA. Wang et al. (2019h) used microarray analysis to screen for circRNA expression in healthy and KOA articular cartilage. They found 1,380 circRNAs differentially expressed in the articular cartilage of knee joints of healthy individuals and patients with OA. Meanwhile, constructing a circRNA-miRNA network verified the ten most likely target genes related to circRNA. It was finally discovered that hsa_circ RNA_003231 might be involved in the occurrence and progression of OA. Zhou et al. (2018e) established OA models in interleukin-1β (IL1β)-treated mouse articular chondrocytes (MACs) to study the expression and function of circRNAs in OA using new sequencing methods and bioinformatic analysis. Compared with the control group, 255 circRNAs were differentially expressed in MACs treated with IL-1 β: 119 up-regulated, 136 down-regulated. Mmu-circRNA-30365 and Mmu-circRNA36866 were two substantially different circRNAs, and their specific expression changes in patients with OA and normal individuals were verified by QRT-PCR. Liu et al. (2016) analyzed circRNA expression between OA and normal cartilage samples by hierarchical clustering analysis and found that compared with normal cartilage, 71 circRNAs were differentially expressed (16 were increased, and 55 were decreased) in OA cartilage. In this study, we focused on the research of circRNA-CER. We found that this circRNA could compete with MMP13 for miR-136 and participate in the degradation of the extracellular matrix of chondrocytes. The above examples fully prove that the expression levels of circRNA in OA patients and healthy individuals are different, and these differentially expressed circRNA has a special relationship with the progression of OA.
Several studies have reported the functions and mechanisms of several circRNAs in OA, but relevant studies are few. Zhou et al. (2018d) established rat OA models, predicted the function of circRNA_ATP9b in rat knee chondrocytes through bioinformatic analysis, and finally found that circRNA_ATP9b regulated the degradation of extracellular matrix through sponge miR-138-5p, thereby controlling the progression of OA. Moreover, circRNA_ATP9b expression was increased, and miR-138-5p expression was down-regulated in IL-1β-induced chondrocytes. circRNA_ATP9b regulated the expression of related genes by targeting miR-138-5p. Li et al. (2017a) analyzed the dual-luciferase reporter genes and found that the transcriptional activity of miR-193b can be inhibited by overexpression of hsa_circ_0045714. Overexpression of hsa_circ_0045714 can also up-regulate the expression of insulin-like growth factor 1 receptor (IGF1R) because IGF1R is a crucial target gene of miR-193b. It is associated with cell proliferation and apoptosis. Further studies on the progression of circRNA in OA are presented in Table 3.
Studies have shown that lncRNA–miRNA–mRNA axis plays a vital control effect in the progression of several diseases, such as cardiovascular disease and cancer (He et al., 2018; Wang et al., 2019c). The mechanisms of interaction of lncRNAs, miRNAs, and mRNAs in various diseases are as follows: 1) The structure of most lncRNAs is similar to mRNAs, and miRNAs binding to mRNAs can reduce the expression of lncRNAs. lncRNA and miRNA compete to bind the 3′-UTR of target gene mRNA, thereby indirectly inhibiting the interaction between miRNA and mRNA. For example, in Alzheimer’s disease, the post-transcriptional regulation of BACE1 involves miR-485-5p, and the specific antisense transcription of BACE1 forms lncRNA-BacE1-As, which compete with lncRNA-Bace1-As to bind to the binding sites of related mRNAs (Faghihi et al., 2010). 2) lncRNAs sponge miRNAs as competitive endogenous RNAs (ceRNAs). lncRNA molecules contain miRNA binding sites, which can bind to miRNA, inhibit the interaction between miRNA and mRNA, improve the expression level of related mRNA, and regulate the expression of target genes. For example, Zhang et al. (2020l) constructed a complete mRNA-LncRNA-miRNA ceRNA regulatory network; lncRNAs ENST00000326237.3, ENST00000399702.5, and ENST00000463727.1 were found to regulate related genes through competitive binding of the same miRNA has-miR-1260a. Kong et al. (2019) demonstrated that lncRNA—CDC6 can further regulate CDC6 expression through direct uptake of miR-215 as a ceRNA. Luan et al.(Luan and Wang, 2018) found that in cervical cancer, XLOC_006390 may act as ceRNA and bind with miR-331-3p and miR-338-3p, thus regulating the expression of genes related to cervical cancer. 3) miRNAs mediate the degradation of lncRNAs. For example, miRNA-150 is the target gene for lncRNA CASC11 in human plasma, and increased concentrations of miRNA-150 decrease the activity of lncRNA CASC11(Luo et al., 2019b). 4) lncRNAs act as miRNAs precursors. For example, Tao et al. (2017) found that miR-869a and miR-160c could be clipped from lncRNAs npc83 and npc521. However, in OA, lncRNA mainly binds to miRNA as a competitive endogenous RNA (ceRNA), inhibiting its target genes’ expression and regulating OA’s progression by regulating cell proliferation, apoptosis, autophagy and extracellular matrix (ECM) degradation (Figure 4).
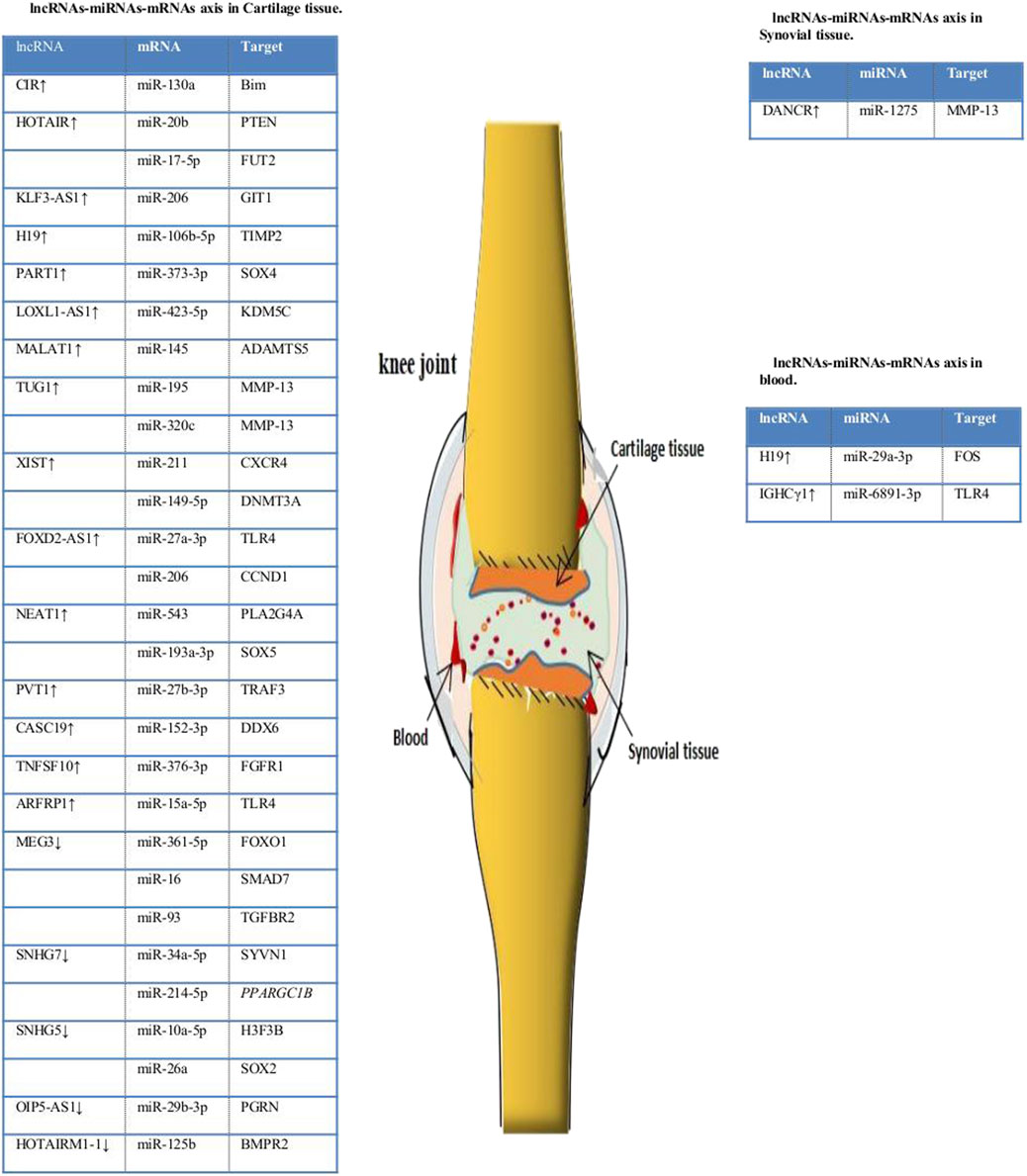
FIGURE 4. lncRNA–miRNA–mRNA axis in OA. lncRNA can combine with miRNA to promote the expression of related target genes. PTEN = phosphatase and tensin homolog; FUT2 = fucosyltransferase 2; Timp2 = tissue inhibitor of metalloproteinase 2; KDM5C = lysine demethylase 5C; DNMT3A = DNA methyltransferase 3A; TLR4 = toll-like receptor 4; CCND1 = cyclin D1; KLF4 = Krüppel-like factor 4; SYVN1 = synoviolin 1; PPARGC1B = PPARG coactivator 1 beta; H3F3B = H3 histone family 3B; PGRN = progranulin; DNM3OS = dynamin 3 opposite strand; BMPR2 = bone morphogenetic protein receptor 2.
There are many examples where lncRNA functions as a binding of ceRNA to miRNA in OA. For example, Zhang et al. (2020m) took IL -1β-induced OA chondrocytes as the research object to study the molecular mechanism of LINC00511 in regulating OA. The study found that the expression of LINC00511 was up-regulated, and the lncRNA could be used as a sponge of miR-150-5p and combined with 3′-UTR of transcription factor inhibit the proliferation of chondrocytes, promote apoptosis and degradation of ECM, and finally regulate OA. Liu et al. (2018) established an OA chondrocyte model induced by IL -1β and an OA mouse model caused by collagenase. The experiments were performed in vivo and in vitro at two levels, and the cell state was examined by the CCK-8 method and flow cytometry. Studies have found that KLF3-AS1, as a ceRNA interacting with miR-206, promotes the expression of GIT1 and then promotes the proliferation of chondrocytes and inhibits apoptosis, ultimately alleviating the progression of OA. Likewise, Tian et al. (2020) studied the relationship between SNHG7, miR-34a-5p, and SYVN1 in human chondrocytes. It has been found that in OA tissues, SNHG7 is down-regulated, and SNHG7 can regulate SYVN1 by sponging miR-34a-5p, thereby promoting cell proliferation and inhibiting apoptosis and autophagy. In addition, studies have found that lncRNA XIST is up-regulated in OA articular cartilage. Like a sponge, XIST regulates the target proteins miR-211, miR-17-5p, miR-149-5p, and miR-27b-3p, thereby promoting the proliferation and apoptosis of chondrocytes and finally inducing OA (Li et al., 2018b; Zhu et al., 2021). These results suggest that lncRNAs can act as miRNA sponges in the interaction of lncRNAs, miRNAs, and mRNA in OA.
Currently, research on the mechanism of interactions between circRNAs, miRNAs, and mRNAs is growing (Peng et al., 2020). circRNAs and miRNAs are closely related to the expression of disease-related mRNAs, and interactions between circRNAs, miRNAs, and mRNAs may be involved in the pathological mechanism of OA (Figure 5). At present, research on the interaction mechanism of circRNAs, miRNAs, and mRNAs is not comprehensive. Relevant research has three main types: 1) circRNAs interact with miRNAs. miRNA interacts with mRNA to inhibit mRNA expression. circRNA molecules contain miRNA binding sites, which can sponge miRNA and release miRNA’s inhibitory effect on target genes. For example, Hansen et al. (2013) found that CiRS-7 could sponge miR-7, inhibit the binding of miR-7 and its target genes, and indirectly promote the expression of related mRNA. Other research suggests that hsa_circ_101237, like a sponge for miRNA490-3p, promotes the expression of its target gene MAPK1. In patients with lung cancer, hsa_circ_101237 expression is up-regulated, thereby promoting the proliferation, differentiation, and migration of lung cancer cells (Zhang et al., 2020o). 2) circRNA can regulate the splicing of pre-mRNA, thus affecting the production of protein. 3) circRNA can pair with targeted mRNA directly through local bases. As the circRNA molecule is rich in miRNA binding sites, the circ RNA molecule functions as a miRNA sponge in cells so that the inhibition effect of the miRNA on target genes can be released, and the expression level of the target genes is increased. Therefore, in OA, the interaction mechanism of circRNA, miRNA, and mRNA is mainly circRNA sponging miRNA (Kulcheski et al., 2016). Many circRNA expressions in OA have been changed, and OA is regulated by adsorbing a specific miRNA. For example, hsa_circ_0005567 is down-regulated in OA patients and, by competitively binding to miR-495, terminates Atg14 expression and eventually induces human chondrocyte apoptosis (Zhang et al., 2020f); hsa_circ_0032131 is up-regulated in the human body, and knocking out hsa_circ_0032131 inactivates the STAT3 signaling pathway by sponging miR-502-5p, thereby relieving symptoms of OA in the body (Xu and Ma, 2021); circPSM3 is up-regulated in OA chondrocytes, and its low expression promotes chondrogenesis and OA development. circPSM3 can inhibit OA chondrogenesis by sponging miRNA-296-5p (Ni et al., 2020a). All these results prove the mechanism of circRNA sponge miRNA in osteoarthritis.
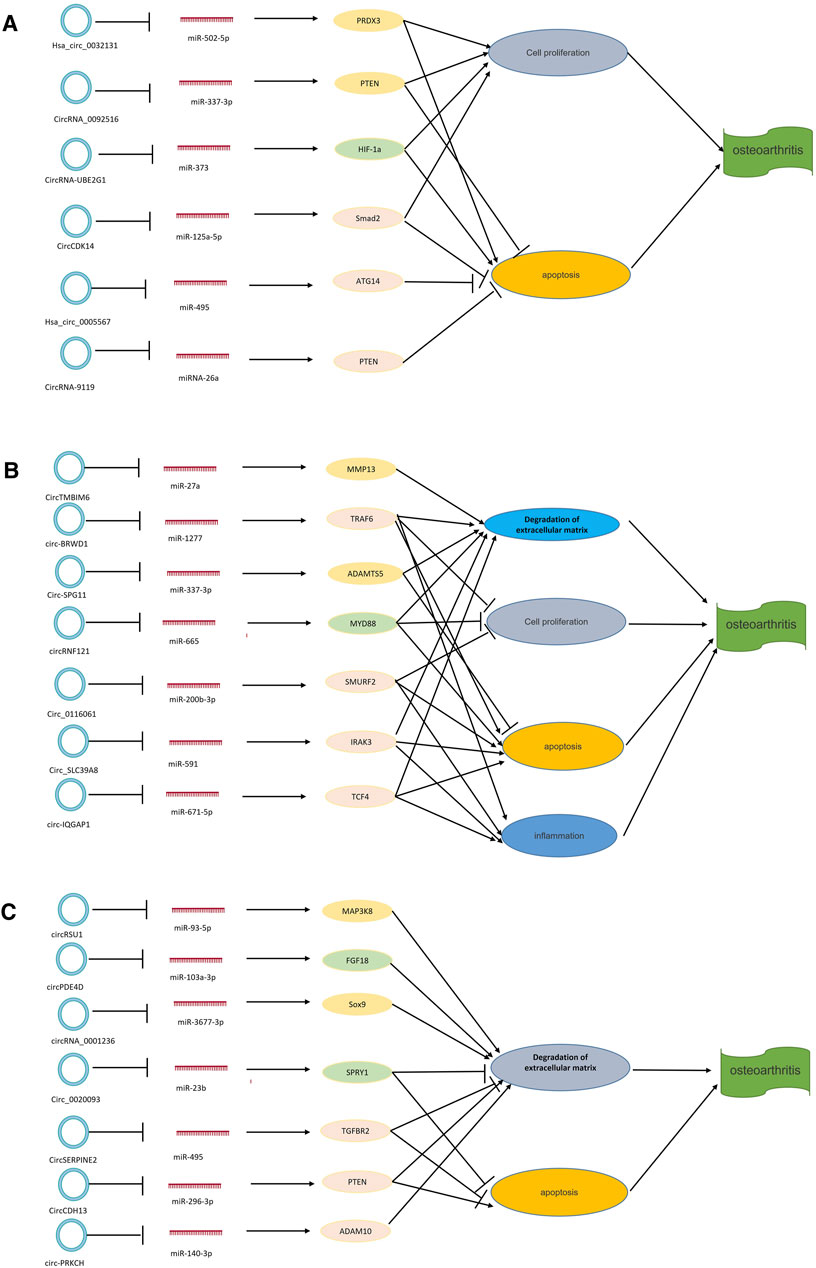
FIGURE 5. circRNA–miRNA–mRNA axis in OA. circRNAs can combine with miRNAs to promote the expression of related target genes. (A) circRNAs that play a role in cell proliferation and apoptosis. (B) circRNAs that play a role in degradation of the extracellular matrix and apoptosis. (C) circRNAs that play a role in degradation of the extracellular matrix, cell proliferation, apoptosis, and inflammation. NAMPT = nicotinamide phosphoribosyltransferase; MMP13 = matrix metalloproteinase.
Other studies have found interactions between circRNA, miRNA, and mRNA. Shen et al. (2020a) established a rabbit model of OA and studied the role and mechanism of circCDK14 in OA by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and other methods. miR-125a-5p is a downstream target protein of circCDK14, while Smad2 is an mRNA target protein of circCDK14. The mechanism of action of circCDK14 in OA is to down-regulate the expression of Smad2 through the sponge action of miR-125a-5p, resulting in dysfunction of the TGF-β signaling pathway. Chen et al. (2020a) studied the expression and action mechanism of circRNA-9119 in OA patients using bioinformatics prediction and double luciferase reporter gene detection. They found that the expression of circRNA-9119 was down-regulated to provide a sponge effect on miR-26a. At the same time, miR-26a targeted the 3' -UTR of PTEN to promote cell proliferation and inhibit apoptosis. Their results all demonstrated the mechanism of the interaction between circRNAs, miRNAs, and mRNAs in OA.
At present, the incidence of OA is very high, and its pathogenesis is still unclear. Studying the specific pathological process and molecular pathway of OA is of great clinical significance (Duan et al., 2020). First, ncRNA can be used to diagnose OA. The expression of many ncRNAs between patients with OA and normal individuals have remarkable differences, which can be seen in humans and animals. For example, Huang et al. (2019c) showed that miRNA-204 and miRNA-211 are decreased in OA, resulting in Runx2 accumulation in multiple types of joint cells and elevated OA markers, and leading to total joint degeneration. Second, several ncRNAs are associated with the prognosis of OA. Rousseau et al. (2020) took the miRNAs in the serum of female patients with KOA as the research objects. He first made a preliminary screening of the research objects through next-generation sequencing and then further analyzed the research objects through RT-QPCR. He found that miR-146A-5p is up-regulated in patients with mild OA, and the prognosis of OA caused by the up-regulation of miRNA is relatively good. In addition, the increase of miR-186-5p in an individual means that the individual might have the imaging changes of OA in the past 4 years, which could be prevented in advance to avoid the occurrence of OA as much as possible. Finally, several ncRNAs can be used for the treatment of OA. Several new drugs can be developed to promote or inhibit several ncRNAs, or change the pathway of action of ncRNA to treat OA. For example, miR-93 is down-regulated in mice with OA and lipopolysaccharide-treated chondrocytes, and acts directly on TLR4 to exert biological effects. miR-93 regulates OA by inhibiting the TLR4/NF-κB pathway, lipopolysaccharide-induced inflammation, and apoptosis. In patients with OA and down-regulation of miR-93, corresponding drugs can be developed to promote its up-regulation and inhibit the aggravation of OA (Ding et al., 2019). These studies indicate that ncRNA has great potential for clinical use in OA. At present, most of the tissue comes from cartilage and is found in the knee joint, and the chondrocytes are cultured to construct the OA cell model. Further research is needed, and more clinical trials must be explored to find biomarkers associated with OA while developing the immense potential of ncRNA.
In recent years, ncRNAs have become one of the most widely studied fields in the development of OA. However, the studies on the regulation of miRNA, lncRNA, and circRNA in diseases and their use as indicators for diagnosis or treatment of OA are still in the early stages, and the mechanism of action ofOA, which may involve multiple signaling pathways, is still unclear. This study reviews theinteractions between lncRNA/circRNA and miRNA in OA. Through high-throughput sequencingtechnologies such as microarray analysis and RNA sequencing, the findings reveal that a large number of miRNA, lncRNA, and circRNA are dysregulated in patients with OA, and the clinical trials related to ncRNA and OA are summarized. The present research progress of ncRNA in the prevention, diagnosis, and treatment of OA is illustrated, which provides a basis for the treatment of OA by ncRNA in the future.
X-AZ and X-QW: conceptualization, project administration, and funding acquisition. HK, X-AZ, and X-QW: writing—review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the Innovative Talents Support Program for Universities of Liaoning Province, No. WR2019024; Shanghai Frontiers Science Research Base of Exercise and Metabolic Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abouheif, M. M., Nakasa, T., Shibuya, H., Niimoto, T., Kongcharoensombat, W., and Ochi, M. (2010). Silencing microRNA-34a Inhibits Chondrocyte Apoptosis in a Rat Osteoarthritis Model In Vitro. Rheumatology 49 (11), 2054–2060. doi:10.1093/rheumatology/keq247
Abramoff, B., and Caldera, F. E. (2020). Osteoarthritis. Med. Clin. North America 104 (2), 293–311. doi:10.1016/j.mcna.2019.10.007
An, Y., Wan, G., Tao, J., Cui, M., Zhou, Q., and Hou, W. (2020). Down-regulation of microRNA-203a Suppresses IL-1β-induced Inflammation and Cartilage Degradation in Human Chondrocytes through Smad3 Signaling. Biosci. Rep. 40 (3), BSR20192723. doi:10.1042/BSR20192723
Bai, Y., Chen, K., Zhan, J., and Wu, M. (2020a). miR-122/SIRT1 axis Regulates Chondrocyte Extracellular Matrix Degradation in Osteoarthritis. Biosci. Rep. 40 (6), BSR20191908. doi:10.1042/BSR20191908
Bai, Z. M., Kang, M. M., Zhou, X. F., and Wang, D. (2020b). CircTMBIM6 Promotes Osteoarthritis-Induced Chondrocyte Extracellular Matrix Degradation via miR-27a/MMP13 axis. Eur. Rev. Med. Pharmacol. Sci. 24 (15), 7927–7936. doi:10.26355/eurrev_202008_22475
Bao, Z., Yang, Z., Huang, Z., Zhou, Y., Cui, Q., and Dong, D. (2019). LncRNADisease 2.0: an Updated Database of Long Non-coding RNA-Associated Diseases. Nucleic Acids Res. 47 (D1), D1034–D1037. doi:10.1093/nar/gky905
Bijlsma, J. W., Berenbaum, F., and Lafeber, F. P. (2011). Osteoarthritis: an Update with Relevance for Clinical Practice. The Lancet 377 (9783), 2115–2126. doi:10.1016/S0140-6736(11)60243-2
Boehme, K. A., and Rolauffs, B. (2018). Onset and Progression of Human Osteoarthritis-Can Growth Factors, Inflammatory Cytokines, or Differential miRNA Expression Concomitantly Induce Proliferation, ECM Degradation, and Inflammation in Articular Cartilage? Int. J. Mol. Sci. 19 (8), 2282. doi:10.3390/ijms19082282
Budd, E., de Andrés, M. C., Sanchez-Elsner, T., and Oreffo, R. O. C. (2017). MiR-146b Is Down-Regulated during the Chondrogenic Differentiation of Human Bone Marrow Derived Skeletal Stem Cells and Up-Regulated in Osteoarthritis. Sci. Rep. 7, 46704. doi:10.1038/srep46704
Burr, D. B., and Gallant, M. A. (2012). Bone Remodelling in Osteoarthritis. Nat. Rev. Rheumatol. 8 (11), 665–673. doi:10.1038/nrrheum.2012.130
Cao, J., Han, X., Qi, X., Jin, X., and Li, X. (2018a). miR-204-5p I-nhibits the O-ccurrence and D-evelopment of O-steoarthritis by T-argeting Runx2. Int. J. Mol. Med. 42 (5), 2560–2568. doi:10.3892/ijmm.2018.3811
Cao, L., Wang, Y., Wang, Q., and Huang, J. (2018b). LncRNA FOXD2-AS1 Regulates Chondrocyte Proliferation in Osteoarthritis by Acting as a Sponge of miR-206 to Modulate CCND1 Expression. Biomed. Pharmacother. 106, 1220–1226. doi:10.1016/j.biopha.2018.07.048
Cao, M., Zhang, L., Wang, J.-H., Zeng, H., Peng, Y., Zou, J., et al. (2019a). Identifying circRNA-Associated-ceRNA Networks in Retinal Neovascularization in Mice. Int. J. Med. Sci. 16 (10), 1356–1365. doi:10.7150/ijms.35149
Cao, X., Duan, Z., Yan, Z., Li, Y., Li, L., Sun, J., et al. (2019b). miR-195 Contributes to Human Osteoarthritis via Targeting PTHrP. J. Bone Miner Metab. 37 (4), 711–721. doi:10.1007/s00774-018-0973-5
Cao, Z., Liu, W., Qu, X., Bi, H., Sun, X., Yu, Q., et al. (2020). miR-296-5p Inhibits IL-1β-induced Apoptosis and Cartilage Degradation in Human Chondrocytes by Directly Targeting TGF-β1/CTGF/p38MAPK Pathway. Cell Cycle 19 (12), 1443–1453. doi:10.1080/15384101.2020.1750813
Castaldi, A., Zaglia, T., Di Mauro, V., Carullo, P., Viggiani, G., Borile, G., et al. (2014). MicroRNA-133 Modulates the β 1 -Adrenergic Receptor Transduction Cascade. Circ. Res. 115 (2), 273–283. doi:10.1161/CIRCRESAHA.115.303252
Chang, T., Xie, J., Li, H., Li, D., Liu, P., and Hu, Y. (2016). MicroRNA-30a Promotes Extracellular Matrix Degradation in Articular Cartilageviadownregulation of Sox9. Cell Prolif. 49 (2), 207–218. doi:10.1111/cpr.12246
Chang, Z. k., Meng, F. g., Zhang, Z. q., Mao, G. p., Huang, Z. y., Liao, W. m., et al. (2018). MicroRNA‐193b‐3p Regulates Matrix Metalloproteinase 19 Expression in Interleukin‐1β‐induced Human Chondrocytes. J. Cel. Biochem. 119 (6), 4775–4782. doi:10.1002/jcb.26669
Cheleschi, S., Tenti, S., Mondanelli, N., Corallo, C., Barbarino, M., Giannotti, S., et al. (2019). MicroRNA-34a and MicroRNA-181a Mediate Visfatin-Induced Apoptosis and Oxidative Stress via NF-Κb Pathway in Human Osteoarthritic Chondrocytes. Cells 8 (8), 874. doi:10.3390/cells8080874
Chen, C., and Xu, Y. (2021). Long Noncoding RNA LINC00671 Exacerbates Osteoarthritis by Promoting ONECUT2-Mediated Smurf2 Expression and Extracellular Matrix Degradation. Int. immunopharmacology 90, 106846. doi:10.1016/j.intimp.2020.106846
Chen, C., Yin, P., Hu, S., Sun, X., and Li, B. (2020a). Circular RNA-9119 Protects IL-1β-treated Chondrocytes from Apoptosis in an Osteoarthritis Cell Model by Intercepting the microRNA-26a/PTEN axis. Life Sci. 256, 117924. doi:10.1016/j.lfs.2020.117924
Chen, G., Liu, T., Yu, B., Wang, B., and Peng, Q. (2020b). CircRNA-UBE2G1 Regulates LPS-Induced Osteoarthritis through miR-373/HIF-1a axis. Cell Cycle 19 (13), 1696–1705. doi:10.1080/15384101.2020.1772545
Chen, H., Yang, J., and Tan, Z. (2019a). Upregulation of microRNA‐9‐5p Inhibits Apoptosis of Chondrocytes through Downregulating Tnc in Mice with Osteoarthritis Following Tibial Plateau Fracture. J. Cel Physiol 234 (12), 23326–23336. doi:10.1002/jcp.28900
Chen, J., and Wu, X. (2019). MicroRNA-103 Contributes to Osteoarthritis Development by Targeting Sox6. Biomed. Pharmacother. 118, 109186. doi:10.1016/j.biopha.2019.109186
Chen, K., Fang, H., and Xu, N. (2020c). LncRNA LOXL1-AS1 Is Transcriptionally Activated by JUND and Contributes to Osteoarthritis Progression via Targeting the miR-423-5p/KDM5C axis. Life Sci. 258, 118095. doi:10.1016/j.lfs.2020.118095
Chen, K., Zhu, H., Zheng, M.-Q., and Dong, Q.-R. (2019b). LncRNA MEG3 Inhibits the Degradation of the Extracellular Matrix of Chondrocytes in Osteoarthritis via Targeting miR-93/TGFBR2 Axis. Cartilage 2019, 194760351985575. doi:10.1177/1947603519855759
Chen, L.-L., and Yang, L. (2015). Regulation of circRNA Biogenesis. RNA Biol. 12 (4), 381–388. doi:10.1080/15476286.2015.1020271
Chen, L., Li, Q., Wang, J., Jin, S., Zheng, H., Lin, J., et al. (2017). MiR-29b-3p Promotes Chondrocyte Apoptosis and Facilitates the Occurrence and Development of Osteoarthritis by Targeting PGRN. J. Cel. Mol. Med. 21 (12), 3347–3359. doi:10.1111/jcmm.13237
Chen, Q., Wu, S., Wu, Y., Chen, L., and Pang, Q. (2018a). MiR-149 Suppresses the Inflammatory Response of Chondrocytes in Osteoarthritis by Down-Regulating the Activation of TAK1/NF-Κb. Biomed. Pharmacother. 101, 763–768. doi:10.1016/j.biopha.2018.02.133
Chen, R., Ye, B., Xie, H., Huang, Y., Wu, Z., Wu, H., et al. (2020d). miR-129-3p Alleviates Chondrocyte Apoptosis in Knee Joint Fracture-Induced Osteoarthritis through CPEB1. J. Orthop. Surg. Res. 15 (1), 552. doi:10.1186/s13018-020-02070-1
Chen, S., and Li, B. (2020). MiR-128-3p Post-Transcriptionally Inhibits WISP1 to Suppress Apoptosis and Inflammation in Human Articular Chondrocytes via the PI3K/AKT/NF-κB Signaling Pathway. Cel Transpl. 29, 096368972093913. doi:10.1177/0963689720939131
Chen, X., Guo, D.-Y., Yin, T.-L., and Yang, J. (2021). Non-Coding RNAs Regulate Placental Trophoblast Function and Participate in Recurrent Abortion. Front. Pharmacol. 12, 646521. doi:10.3389/fphar.2021.646521
Chen, X. (2015). KATZLDA: KATZ Measure for the lncRNA-Disease Association Prediction. Sci. Rep. 5, 16840. doi:10.1038/srep16840
Chen, X., Shi, Y., Xue, P., Ma, X., Li, J., and Zhang, J. (2020e). Mesenchymal Stem Cell-Derived Exosomal microRNA-136-5p Inhibits Chondrocyte Degeneration in Traumatic Osteoarthritis by Targeting ELF3. Arthritis Res. Ther. 22 (1), 256. doi:10.1186/s13075-020-02325-6
Chen, X., Xie, D., Wang, L., Zhao, Q., You, Z.-H., and Liu, H. (2018b). BNPMDA: Bipartite Network Projection for MiRNA-Disease Association Prediction. Bioinformatics (Oxford, England) 34 (18), 3178–3186. doi:10.1093/bioinformatics/bty333
Chen, X., Zhou, Z., and Zhao, Y. (2018c). ELLPMDA: Ensemble Learning and Link Prediction for miRNA-Disease Association Prediction. RNA Biol. 15 (6), 1–12. doi:10.1080/15476286.2018.1460016
Chen, Y., Zhang, L., Li, E., Zhang, G., Hou, Y., Yuan, W., et al. (2020f). Long-chain Non-coding RNA HOTAIR Promotes the Progression of Osteoarthritis via Sponging miR-20b/PTEN axis. Life Sci. 253, 117685. doi:10.1016/j.lfs.2020.117685
Cheng, F., Hu, H., Sun, K., Yan, F., and Geng, Y. (2020). miR-455-3p Enhances Chondrocytes Apoptosis and Inflammation by Targeting COL2A1 in the In Vitro Osteoarthritis Model. Biosci. Biotechnol. Biochem. 84 (4), 695–702. doi:10.1080/09168451.2019.1690974
Chu, P., Wang, Q., Wang, Z., and Gao, C. (2019). Long Non-coding RNA Highly Up-Regulated in Liver Cancer Protects Tumor Necrosis Factor-Alpha-Induced Inflammatory Injury by Down-Regulation of microRNA-101 in ATDC5 Cells. Int. immunopharmacology 72, 148–158. doi:10.1016/j.intimp.2019.04.004
Correia de Sousa, M., Gjorgjieva, M., Dolicka, D., Sobolewski, C., and Foti, M. (2019). Deciphering miRNAs' Action through miRNA Editing. Int. J. Mol. Sci. 20 (24), 6249. doi:10.3390/ijms20246249
Cui, X., Wang, S., Cai, H., Lin, Y., Zheng, X., Zhang, B., et al. (2016). Overexpression of microRNA-634 Suppresses Survival and Matrix Synthesis of Human Osteoarthritis Chondrocytes by Targeting PIK3R1. Sci. Rep. 6, 23117. doi:10.1038/srep23117
Dai, L., Zhang, X., Hu, X., Liu, Q., Man, Z., Huang, H., et al. (2015). Silencing of miR-101 Prevents Cartilage Degradation by Regulating Extracellular Matrix-Related Genes in a Rat Model of Osteoarthritis. Mol. Ther. 23 (8), 1331–1340. doi:10.1038/mt.2015.61
Dai, Y., Liu, S., Xie, X., Ding, M., Zhou, Q., and Zhou, X. (2019). MicroRNA-31 P-romotes C-hondrocyte P-roliferation by T-argeting C-X-C M-otif C-hemokine L-igand 12. Mol. Med. Rep. 19 (3), 2231–2237. doi:10.3892/mmr.2019.9859
Ding, L.-B., Li, Y., Liu, G.-Y., Li, T.-H., Li, F., Guan, J., et al. (2020). Long Non-coding RNA PVT1, a Molecular Sponge of miR-26b, Is Involved in the Progression of Hyperglycemia-Induced Collagen Degradation in Human Chondrocytes by Targeting CTGF/TGF-β Signal Ways. Innate Immun. 26 (3), 204–214. doi:10.1177/1753425919881778
Ding, Y., Wang, L., Zhao, Q., Wu, Z., and Kong, L. (2019). MicroRNA-93 I-nhibits C-hondrocyte A-poptosis and I-nflammation in O-steoarthritis by T-argeting the TLR4/NF-κB S-ignaling P-athway. Int. J. Mol. Med. 43 (2), 779–790. doi:10.3892/ijmm.2018.4033
Dou, P., He, Y., Yu, B., and Duan, J. (2020). Downregulation of microRNA-29b by DNMT3B Decelerates Chondrocyte Apoptosis and the Progression of Osteoarthritis via PTHLH/CDK4/RUNX2 axis. Aging 13 (5), 7676–7690. doi:10.18632/aging.103778
Duan, L., Duan, D., Wei, W., Sun, Z., Xu, H., Guo, L., et al. (2019a). MiR-19b-3p Attenuates IL-1β Induced Extracellular Matrix Degradation and Inflammatory Injury in Chondrocytes by Targeting GRK6. Mol. Cel Biochem 459 (1-2), 205–214. doi:10.1007/s11010-019-03563-2
Duan, L., Liang, Y., Xu, X., Wang, J., Li, X., Sun, D., et al. (2020). Noncoding RNAs in Subchondral Bone Osteoclast Function and Their Therapeutic Potential for Osteoarthritis. Arthritis Res. Ther. 22 (1), 279. doi:10.1186/s13075-020-02374-x
Duan, Z.-X., Huang, P., Tu, C., Liu, Q., Li, S.-Q., Long, Z.-L., et al. (2019b). MicroRNA-15a-5p Regulates the Development of Osteoarthritis by Targeting PTHrP in Chondrocytes. Biomed. Research International 2019, 1–11. doi:10.1155/2019/3904923
Esteller, M. (2011). Non-coding RNAs in Human Disease. Nat. Rev. Genet. 12 (12), 861–874. doi:10.1038/nrg3074
Faghihi, M. A., Zhang, M., Huang, J., Modarresi, F., Van der Brug, M. P., Nalls, M. A., et al. (2010). Evidence for Natural Antisense Transcript-Mediated Inhibition of microRNA Function. Genome Biol. 11 (5), R56. doi:10.1186/gb-2010-11-5-r56
Fan, X., Yuan, J., Xie, J., Pan, Z., Yao, X., Sun, X., et al. (2018). Long Non-protein Coding RNA DANCR Functions as a Competing Endogenous RNA to Regulate Osteoarthritis Progression via miR-577/SphK2 axis. Biochem. biophysical Res. Commun. 500 (3), 658–664. doi:10.1016/j.bbrc.2018.04.130
Fan, Z., Liu, Y., Shi, Z., Deng, K., Zhang, H., Li, Q., et al. (2020). MiR‐155 Promotes Interleukin‐1β‐induced Chondrocyte Apoptosis and Catabolic Activity by Targeting PIK3R1‐mediated PI3K/Akt Pathway. J. Cel Mol Med 24 (15), 8441–8451. doi:10.1111/jcmm.15388
Fang, P., Zhang, L. X., Hu, Y., Zhang, L., and Zhou, L. W. (2019). Long Non-coding RNA DANCR Induces Chondrogenesis by Regulating the miR-1275/MMP-13 axis in Synovial Fluid-Derived Mesenchymal Stem Cells. Eur. Rev. Med. Pharmacol. Sci. 23 (23), 10459–10469. doi:10.26355/eurrev_201912_19685
Felson, D. T., Lawrence, R. C., Dieppe, P. A., Hirsch, R., Helmick, C. G., Jordan, J. M., et al. (2000). Osteoarthritis: New Insights. Part 1: the Disease and its Risk Factors. Ann. Intern. Med. 133 (8), 635–646. doi:10.7326/0003-4819-133-8-200010170-00016
Feng, M., Jing, L., Cheng, J., An, S., Huang, J., and Yan, Q. (2021). Circ_0020093 Ameliorates IL-1β-induced Apoptosis and Extracellular Matrix Degradation of Human Chondrocytes by Upregulating SPRY1 via Targeting miR-23b. Mol. Cel Biochem 476 (10), 3623–3633. doi:10.1007/s11010-021-04186-2
Fu, Q., Li, L., Wang, B., Wu, J., Li, H., Han, Y., et al. (2021). CircADAMTS6/miR‐431‐5p axis Regulate Interleukin‐1β Induced Chondrocyte Apoptosis. J. Gene Med. 23 (2), e3304. doi:10.1002/jgm.3304
Fu, X., Dong, B., Tian, Y., Lefebvre, P., Meng, Z., Wang, X., et al. (2015). MicroRNA-26a Regulates Insulin Sensitivity and Metabolism of Glucose and Lipids. J. Clin. Invest. 125 (6), 2497–2509. doi:10.1172/JCI75438
Gabler, J., Ruetze, M., Kynast, K. L., Grossner, T., Diederichs, S., and Richter, W. (2015). Stage-Specific miRs in Chondrocyte Maturation: Differentiation-dependent and Hypertrophy-Related miR Clusters and the miR-181 Family. Tissue Eng. A 21 (23-24), 2840–2851. doi:10.1089/ten.TEA.2015.0352
Gao, G. C., Cheng, X. G., Wei, Q. Q., Chen, W. C., and Huang, W. Z. (2019a). Long Noncoding RNA MALAT‐1 Inhibits Apoptosis and Matrix Metabolism Disorder in Interleukin‐1β‐induced Inflammation in Articular Chondrocytes via the JNK Signaling Pathway. J. Cel Biochem 120 (10), 17167–17179. doi:10.1002/jcb.28977
Gao, S. T., Yu, Y. M., Wan, L. P., Liu, Z. M., and Lin, J. X. (2020). LncRNA GAS5 Induces Chondrocyte Apoptosis by Down-Regulating miR-137. Eur. Rev. Med. Pharmacol. Sci. 24 (21), 10984–10991. doi:10.26355/eurrev_202011_23582
Gao, Y., Zhao, H., and Li, Y. (2019b). LncRNA MCM3AP-AS1 Regulates miR-142-3p/HMGB1 to Promote LPS-Induced Chondrocyte Apoptosis. BMC Musculoskelet. Disord. 20 (1), 605. doi:10.1186/s12891-019-2967-4
Ge, F.-X., Li, H., and Yin, X. (2017). Upregulation of microRNA-125b-5p Is Involved in the Pathogenesis of Osteoarthritis by Downregulating SYVN1. Oncol. Rep. 37 (4), 2490–2496. doi:10.3892/or.2017.5475
Glyn-Jones, S., Palmer, A. J. R., Agricola, R., Price, A. J., Vincent, T. L., Weinans, H., et al. (2015). Osteoarthritis. The Lancet 386 (9991), 376–387. doi:10.1016/S0140-6736(14)60802-3
Guo, Y., Tian, L., Du, X., and Deng, Z. (2020). MiR-203 Regulates Estrogen Receptor α and Cartilage Degradation in IL-1β-stimulated Chondrocytes. J. Bone Miner Metab. 38 (3), 346–356. doi:10.1007/s00774-019-01062-4
Guo, Z., Wang, H., Zhao, F., Liu, M., Wang, F., Kang, M., et al. (2021). Exosomal Circ-BRWD1 Contributes to Osteoarthritis Development through the Modulation of miR-1277/TRAF6 axis. Arthritis Res. Ther. 23 (1), 159. doi:10.1186/s13075-021-02541-8
Han, H., and Liu, L. (2021). Long Noncoding RNA TUG1 Regulates Degradation of Chondrocyte Extracellular Matrix via miR-320c/MMP-13 axis in Osteoarthritis. Open Life Sci. 16 (1), 384–394. doi:10.1515/biol-2021-0037
Hansen, T. B., Jensen, T. I., Clausen, B. H., Bramsen, J. B., Finsen, B., Damgaard, C. K., et al. (2013). Natural RNA Circles Function as Efficient microRNA Sponges. Nature 495 (7441), 384–388. doi:10.1038/nature11993
He, B., and Jiang, D. (2020). HOTAIR‐induced Apoptosis Is Mediated by Sponging miR‐130a‐3p to Repress Chondrocyte Autophagy in Knee Osteoarthritis. Cell Biol Int 44 (2), 524–535. doi:10.1002/cbin.11253
He, J.-H., Han, Z.-P., Zou, M.-X., Wang, L., Lv, Y. B., Zhou, J. B., et al. (2018). Analyzing the LncRNA, miRNA, and mRNA Regulatory Network in Prostate Cancer with Bioinformatics Software. J. Comput. Biol. 25 (2), 146–157. doi:10.1089/cmb.2016.0093
He, J., Wang, L., Ding, Y., Liu, H., and Zou, G. (2021a). lncRNA FER1L4 Is Dysregulated in Osteoarthritis and Regulates IL-6 Expression in Human Chondrocyte Cells. Sci. Rep. 11 (1), 13032. doi:10.1038/s41598-021-92474-8
He, J., Zhang, J., and Wang, D. (2017). Down-regulation of microRNA-216b Inhibits IL-1β-induced Chondrocyte Injury by Up-Regulation of Smad3. Biosci. Rep. 37 (2), BSR20160588. doi:10.1042/BSR20160588
He, X., and Deng, L. (2021). Potential of miR-25-3p in protection of Chondrocytes: Emphasis on Osteoarthritis. Folia Histochem. Cytobiol. 59 (1), 30–39. doi:10.5603/FHC.a2021.0004
He, X., Gao, K., Lu, S., and Wu, R. (2021b). LncRNA HOTTIP Leads to Osteoarthritis Progression via Regulating miR-663a/Fyn-Related Kinase axis. BMC Musculoskelet. Disord. 22 (1), 67. doi:10.1186/s12891-020-03861-7
Hu, G., Zhao, X., Wang, C., Geng, Y., Zhao, J., Xu, J., et al. (2017). MicroRNA-145 Attenuates TNF-α-Driven Cartilage Matrix Degradation in Osteoarthritis via Direct Suppression of MKK4. Cell Death Dis 8 (10), e3140. doi:10.1038/cddis.2017.522
Hu, J., Wang, Z., Pan, Y., Ma, J., Miao, X., Qi, X., et al. (2018a). MiR-26a and miR-26b Mediate Osteoarthritis Progression by Targeting FUT4 via NF-Κb Signaling Pathway. Int. J. Biochem. Cel Biol. 94, 79–88. doi:10.1016/j.biocel.2017.12.003
Hu, J., Wang, Z., Shan, Y., Pan, Y., Ma, J., and Jia, L. (2018b). Long Non-coding RNA HOTAIR Promotes Osteoarthritis Progression via miR-17-5p/FUT2/β-Catenin axis. Cel Death Dis 9 (7), 711. doi:10.1038/s41419-018-0746-z
Hu, S., Mao, G., Zhang, Z., Wu, P., Wen, X., Liao, W., et al. (2019a). MicroRNA-320c Inhibits Development of Osteoarthritis through Downregulation of Canonical Wnt Signaling Pathway. Life Sci. 228, 242–250. doi:10.1016/j.lfs.2019.05.011
Hu, S., Zhao, X., Mao, G., Zhang, Z., Wen, X., Zhang, C., et al. (2019b). MicroRNA-455-3p Promotes TGF-β Signaling and Inhibits Osteoarthritis Development by Directly Targeting PAK2. Exp. Mol. Med. 51 (10), 1–13. doi:10.1038/s12276-019-0322-3
Hu, Y., Zhu, H., Bu, L., and He, D. (2019c). Expression Profile of Circular RNA S in TMJ Osteoarthritis Synovial Tissues and Potential Functions of Hsa_circ_0000448 with Specific Back-Spliced junction. Am. J. Transl Res. 11 (9), 5357–5374.
Huang, B., Yu, H., Li, Y., Zhang, W., and Liu, X. (2019a). Upregulation of Long Noncoding TNFSF10 Contributes to Osteoarthritis Progression through the miR‐376‐3p/FGFR1 axis. J. Cel Biochem 120 (12), 19610–19620. doi:10.1002/jcb.29267
Huang, J., Liu, L., Yang, J., Ding, J., and Xu, X. (2019b). lncRNA DILC Is Downregulated in Osteoarthritis and Regulates IL‐6 Expression in Chondrocytes. J. Cel Biochem 120 (9), 16019–16024. doi:10.1002/jcb.28880
Huang, J., Zhao, L., Fan, Y., Liao, L., Ma, P. X., Xiao, G., et al. (2019c). The microRNAs miR-204 and miR-211 Maintain Joint Homeostasis and Protect against Osteoarthritis Progression. Nat. Commun. 10 (1), 2876. doi:10.1038/s41467-019-10753-5
Huang, T., Wang, J., Zhou, Y., Zhao, Y., Hang, D., and Cao, Y. (2019d). LncRNA CASC2 Is Up-Regulated in Osteoarthritis and Participates in the Regulation of IL-17 Expression and Chondrocyte Proliferation and Apoptosis. Biosci. Rep. 39 (5), BSR20182454. doi:10.1042/BSR20182454
Huang, Y. (2018). The Novel Regulatory Role of lncRNA-miRNA-mRNA axis in Cardiovascular Diseases. J. Cel Mol Med 22 (12), 5768–5775. doi:10.1111/jcmm.13866
Huang, Z., Ma, W., Xiao, J., Dai, X., and Ling, W. (2021). CircRNA_0092516 Regulates Chondrocyte Proliferation and Apoptosis in Osteoarthritis through the miR-337-3p/PTEN axis. J. Biochem. 169 (4), 467–475. doi:10.1093/jb/mvaa119
Huang, Z., Zhang, N., Ma, W., Dai, X., and Liu, J. (2017). MiR-337-3p Promotes Chondrocytes Proliferation and Inhibits Apoptosis by Regulating PTEN/AKT axis in Osteoarthritis. Biomed. Pharmacother. 95, 1194–1200. doi:10.1016/j.biopha.2017.09.016
Hwang, H. S., Park, S. J., Lee, M. H., and Kim, H. A. (2017). MicroRNA-365 Regulates IL-1β-induced Catabolic Factor Expression by Targeting HIF-2α in Primary Chondrocytes. Sci. Rep. 7 (1), 17889. doi:10.1038/s41598-017-18059-6
Iacona, J. R., and Lutz, C. S. (2019). miR‐146a‐5p: Expression, Regulation, and Functions in Cancer. WIREs RNA 10 (4), e1533. doi:10.1002/wrna.1533
Ji, Q., Qiao, X., Liu, Y., and Wang, D. (2021). Expression of Long-Chain Noncoding RNA GAS5 in Osteoarthritis and its Effect on Apoptosis and Autophagy of Osteoarthritis Chondrocytes. Histol. Histopathol 36 (4), 475–484. doi:10.14670/HH-18-312
Ji, Y., Fang, Q. Y., Wang, S. N., Zhang, Z. W., Hou, Z. J., Li, J. N., et al. (2020). Lnc-RNA BLACAT1 Regulates Differentiation of Bone Marrow Stromal Stem Cells by Targeting miR-142-5p in Osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 24 (6), 2893–2901. doi:10.26355/eurrev_202003_20653
Jiang, H., Pang, H., Wu, P., Cao, Z., Li, Z., and Yang, X. (2020a). LncRNA SNHG5 Promotes Chondrocyte Proliferation and Inhibits Apoptosis in Osteoarthritis by Regulating miR-10a-5p/H3F3B axis. Connect. Tissue Res. 2020, 1–10. doi:10.1080/03008207.2020.1825701
Jiang, L., Sun, X., and Kong, H. (2020b). microRNA-9 Might Be a Novel Protective Factor for Osteoarthritis Patients. Hereditas 157 (1), 15. doi:10.1186/s41065-020-00128-y
Jiang, M., Liu, J., Luo, T., Chen, Q., Lu, M., and Meng, D. (2019). LncRNA PACER Is Down-Regulated in Osteoarthritis and Regulates Chondrocyte Apoptosis and lncRNA HOTAIR Expression. Biosci. Rep. 39 (6), BSR20190404. doi:10.1042/BSR20190404
Jin, Z., Ren, J., and Qi, S. (2020). Human Bone Mesenchymal Stem Cells-Derived Exosomes Overexpressing microRNA-26a-5p Alleviate Osteoarthritis via Down-Regulation of PTGS2. Int. immunopharmacology 78, 105946. doi:10.1016/j.intimp.2019.105946
Kang, L., Yang, C., Song, Y., Liu, W., Wang, K., Li, S., et al. (2016a). MicroRNA-23a-3p Promotes the Development of Osteoarthritis by Directly Targeting SMAD3 in Chondrocytes. Biochem. biophysical Res. Commun. 478 (1), 467–473. doi:10.1016/j.bbrc.2016.06.071
Kang, Y., Song, J., Kim, D., Ahn, C., Park, S., Chun, C.-H., et al. (2016b). PCGEM1 Stimulates Proliferation of Osteoarthritic Synoviocytes by Acting as a Sponge for miR-770. J. Orthop. Res. 34 (3), 412–418. doi:10.1002/jor.23046
Karlsen, T. A., de Souza, G. A., Ødegaard, B., Engebretsen, L., and Brinchmann, J. E. (2016). microRNA-140 Inhibits Inflammation and Stimulates Chondrogenesis in a Model of Interleukin 1β-Induced Osteoarthritis. Mol. Ther. - Nucleic Acids 5 (10), e373. doi:10.1038/mtna.2016.64
Karlsen, T. A., Jakobsen, R. B., Mikkelsen, T. S., and Brinchmann, J. E. (2014). microRNA-140 Targets RALA and Regulates Chondrogenic Differentiation of Human Mesenchymal Stem Cells by Translational Enhancement of SOX9 and ACAN. Stem Cell Dev. 23 (3), 290–304. doi:10.1089/scd.2013.0209
Ko, J.-Y., Lee, M. S., Lian, W.-S., Weng, W.-T., Sun, Y.-C., Chen, Y.-S., et al. (2017). MicroRNA-29a Counteracts Synovitis in Knee Osteoarthritis Pathogenesis by Targeting VEGF. Sci. Rep. 7 (1), 3584. doi:10.1038/s41598-017-03616-w
Kong, X., Duan, Y., Sang, Y., Li, Y., Zhang, H., Liang, Y., et al. (2019). LncRNA-CDC6 Promotes Breast Cancer Progression and Function as ceRNA to Target CDC6 by Sponging microRNA‐215. J. Cel Physiol 234 (6), 9105–9117. doi:10.1002/jcp.27587
Kopp, F., and Mendell, J. T. (2018). Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 172 (3), 393–407. doi:10.1016/j.cell.2018.01.011
Kostopoulou, F., Malizos, K. N., Papathanasiou, I., and Tsezou, A. (2015). MicroRNA-33a Regulates Cholesterol Synthesis and Cholesterol Efflux-Related Genes in Osteoarthritic Chondrocytes. Arthritis Res. Ther. 17, 42. doi:10.1186/s13075-015-0556-y
Kulcheski, F. R., Christoff, A. P., and Margis, R. (2016). Circular RNAs Are miRNA Sponges and Can Be Used as a New Class of Biomarker. J. Biotechnol. 238, 42–51. doi:10.1016/j.jbiotec.2016.09.011
Lawrence, T. (2009). The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harbor Perspect. Biol. 1 (6), a001651. doi:10.1101/cshperspect.a001651
Lei, J., Fu, Y., Zhuang, Y., Zhang, K., and Lu, D. (2019). miR‐382‐3p Suppressed IL‐1β Induced Inflammatory Response of Chondrocytes via the TLR4/MyD88/NF‐κB Signaling Pathway by Directly Targeting CX43. J. Cel Physiol 234 (12), 23160–23168. doi:10.1002/jcp.28882
Lei, M., Zheng, G., Ning, Q., Zheng, J., and Dong, D. (2020). Translation and Functional Roles of Circular RNAs in Human Cancer. Mol. Cancer 19 (1), 30. doi:10.1186/s12943-020-1135-7
Li, B.-F., Zhang, Y., Xiao, J., Wang, F., Li, M., Guo, X.-Z., et al. (2017a). Hsa_circ_0045714 Regulates Chondrocyte Proliferation, Apoptosis and Extracellular Matrix Synthesis by Promoting the Expression of miR-193b Target Gene IGF1R. Hum. Cel. 30 (4), 311–318. doi:10.1007/s13577-017-0177-7
Li, C., Hu, Q., Chen, Z., Shen, B., Yang, J., Kang, P., et al. (2018a). MicroRNA-140 Suppresses Human Chondrocytes Hypertrophy by Targeting SMAD1 and Controlling the Bone Morphogenetic Protein Pathway in Osteoarthritis. Am. J. Med. Sci. 355 (5), 477–487. doi:10.1016/j.amjms.2018.01.004
Li, D., Sun, Y., Wan, Y., Wu, X., and Yang, W. (2020a). LncRNA NEAT1 Promotes Proliferation of Chondrocytes via Down‐regulation of miR‐16‐5p in Osteoarthritis. J. Gene Med. 22 (9), e3203. doi:10.1002/jgm.3203
Li, F., Yao, J., Hao, Q., and Duan, Z. (2019a). miRNA-103 Promotes Chondrocyte Apoptosis by Down-Regulation of Sphingosine Kinase-1 and Ameliorates PI3K/AKT Pathway in Osteoarthritis. Biosci. Rep. 39 (10), BSR20191255. doi:10.1042/BSR20191255
Li, H.-Z., Xu, X.-H., Lin, N., Wang, D.-W., Lin, Y.-M., Su, Z.-Z., et al. (2020b). Overexpression of miR-10a-5p Facilitates the Progression of Osteoarthritis. Aging 12 (7), 5948–5976. doi:10.18632/aging.102989
Li, H., Li, Z., Pi, Y., Chen, Y., Mei, L., Luo, Y., et al. (2020c). MicroRNA-375 Exacerbates Knee Osteoarthritis through Repressing Chondrocyte Autophagy by Targeting ATG2B. Aging 12 (8), 7248–7261. doi:10.18632/aging.103073
Li, H., Xie, S., Li, H., Zhang, R., and Zhang, H. (2020d). LncRNA MALAT1 Mediates Proliferation of LPS Treated-Articular Chondrocytes by Targeting the miR-146a-PI3K/Akt/mTOR axis. Life Sci. 254, 116801. doi:10.1016/j.lfs.2019.116801
Li, H., Xu, J.-D., Fang, X.-H., Zhu, J.-N., Yang, J., Pan, R., et al. (2020e). Circular RNA circRNA_000203 Aggravates Cardiac Hypertrophy via Suppressing miR-26b-5p and miR-140-3p Binding to Gata4. Cardiovasc. Res. 116 (7), 1323–1334. doi:10.1093/cvr/cvz215
Li, H., Yang, H. H., Sun, Z. G., Tang, H. B., and Min, J. K. (2019b). Whole-transcriptome Sequencing of Knee Joint Cartilage from Osteoarthritis Patients. Bone Jt. Res. 8 (7), 290–303. doi:10.1302/2046-3758.87.BJR-2018-0297.R1
Li, J., Huang, J., Dai, L., Yu, D., Chen, Q., Zhang, X., et al. (2012). miR-146a, an IL-1β Responsive miRNA, Induces Vascular Endothelial Growth Factor and Chondrocyte Apoptosis by Targeting Smad4. Arthritis Res. Ther. 14 (2), R75. doi:10.1186/ar3798
Li, L., Lv, G., Wang, B., and Kuang, L. (2018b). The Role of lncRNA XIST/miR-211 axis in Modulating the Proliferation and Apoptosis of Osteoarthritis Chondrocytes through CXCR4 and MAPK Signaling. Biochem. biophysical Res. Commun. 503 (4), 2555–2562. doi:10.1016/j.bbrc.2018.07.015
Li, L., Yang, C., Liu, X., Yang, S., Ye, S., Jia, J., et al. (2015). Elevated Expression of microRNA-30b in Osteoarthritis and its Role in ERG Regulation of Chondrocyte. Biomed. Pharmacother. 76, 94–99. doi:10.1016/j.biopha.2015.10.014
Li, Q., Zhang, Z., Guo, S., Tang, G., Lu, W., and Qi, X. (2019c). LncRNA ANCR Is Positively Correlated with Transforming Growth Factor‐β1 in Patients with Osteoarthritis. J. Cel Biochem 120 (9), 14226–14232. doi:10.1002/jcb.28881
Li, W., Zhao, S., Yang, H., Zhang, C., Kang, Q., Deng, J., et al. (2019d). Potential Novel Prediction of TMJ-OA: MiR-140-5p Regulates Inflammation through Smad/TGF-β Signaling. Front. Pharmacol. 10, 15. doi:10.3389/fphar.2019.00015
Li, X., Huang, T. L., Zhang, G. D., Jiang, J. T., and Guo, P. Y. (2019e). LncRNA ANRIL Impacts the Progress of Osteoarthritis via Regulating Proliferation and Apoptosis of Osteoarthritis Synoviocytes. Eur. Rev. Med. Pharmacol. Sci. 23 (22), 9729–9737. doi:10.26355/eurrev_201911_19535
Li, X., Ding, J., Wang, X., Cheng, Z., and Zhu, Q. (2020f). NUDT21 Regulates circRNA Cyclization and ceRNA Crosstalk in Hepatocellular Carcinoma. Oncogene 39 (4), 891–904. doi:10.1038/s41388-019-1030-0
Li, X., Yu, M., Chen, L., Sun, T., Wang, H., Zhao, L., et al. (2019f). RETRACTED: LncRNA PMS2L2 Protects ATDC5 Chondrocytes against Lipopolysaccharide-Induced Inflammatory Injury by Sponging miR-203. Life Sci. 217, 283–292. doi:10.1016/j.lfs.2018.12.020
Li, Y.-F., Li, S.-H., Liu, Y., and Luo, Y.-T. (2017b). Long Noncoding RNA CIR Promotes Chondrocyte Extracellular Matrix Degradation in Osteoarthritis by Acting as a Sponge for Mir-27b. Cell Physiol Biochem 43 (2), 602–610. doi:10.1159/000480532
Li, Y., Li, S., Luo, Y., Liu, Y., and Yu, N. (2017c). LncRNA PVT1 Regulates Chondrocyte Apoptosis in Osteoarthritis by Acting as a Sponge for miR-488-3p. DNA Cel. Biol. 36 (7), 571–580. doi:10.1089/dna.2017.3678
Li, Y., Li, Z., Li, C., Zeng, Y., and Liu, Y. (2019g). Long Noncoding RNA TM1P3 Is Involved in Osteoarthritis by Mediating Chondrocyte Extracellular Matrix Degradation. J. Cel Biochem 120 (8), 12702–12712. doi:10.1002/jcb.28539
Li, Y., Zhou, D., Ren, Y., Zhang, Z., Guo, X., Ma, M., et al. (2019h). Mir223 Restrains Autophagy and Promotes CNS Inflammation by Targeting ATG16L1. Autophagy 15 (3), 478–492. doi:10.1080/15548627.2018.1522467
Li, Z., Cheng, J., and Liu, J. (2020g). Baicalin Protects Human OA Chondrocytes against IL-1β-Induced Apoptosis and ECM Degradation by Activating Autophagy via MiR-766-3p/AIFM1 Axis. Drug Des. Devel Ther. 14, 2645–2655. doi:10.2147/DDDT.S255823
Li, Z., Meng, D., Li, G., Xu, J., Tian, K., and Li, Y. (2016). Overexpression of microRNA-210 Promotes Chondrocyte Proliferation and Extracellular Matrix Deposition by Targeting HIF-3α in Osteoarthritis. Mol. Med. Rep. 13 (3), 2769–2776. doi:10.3892/mmr.2016.4878
Li, Z., Yuan, B., Pei, Z., Zhang, K., Ding, Z., Zhu, S., et al. (2019i). Circ_0136474 and MMP‐13 Suppressed Cell Proliferation by Competitive Binding to miR‐127‐5p in Osteoarthritis. J. Cel Mol Med 23 (10), 6554–6564. doi:10.1111/jcmm.14400
Lian, W.-S., Ko, J.-Y., Wu, R.-W., Sun, Y.-C., Chen, Y.-S., Wu, S.-L., et al. (2018). MicroRNA-128a Represses Chondrocyte Autophagy and Exacerbates Knee Osteoarthritis by Disrupting Atg12. Cel Death Dis 9 (9), 919. doi:10.1038/s41419-018-0994-y
Liang, Q., Asila, A., Deng, Y., Liao, J., Liu, Z., and Fang, R. (2021). Osteopontin‐induced lncRNA HOTAIR Expression Is Involved in Osteoarthritis by Regulating Cell Proliferation. BMC Geriatr. 21 (1), 57. doi:10.1186/s12877-020-01993-y
Liang, Y., Duan, L., Xiong, J., Zhu, W., Liu, Q., Wang, D., et al. (2016). E2 Regulates MMP-13 via Targeting miR-140 in IL-1β-induced Extracellular Matrix Degradation in Human Chondrocytes. Arthritis Res. Ther. 18 (1), 105. doi:10.1186/s13075-016-0997-y
Liang, Z.-J., Zhuang, H., Wang, G.-X., Li, Z., Zhang, H.-T., Yu, T.-Q., et al. (2012). MiRNA-140 Is a Negative Feedback Regulator of MMP-13 in IL-1β-stimulated Human Articular Chondrocyte C28/I2 Cells. Inflamm. Res. 61 (5), 503–509. doi:10.1007/s00011-012-0438-6
Liao, H.-X., Zhang, Z.-H., Chen, H.-L., Huang, Y.-M., Liu, Z.-L., and Huang, J. (2021). CircHYBID Regulates Hyaluronan Metabolism in Chondrocytes via Hsa-miR-29b-3p/TGF-Β1 axis. Mol. Med. 27 (1), 56. doi:10.1186/s10020-021-00319-x
Lim, Y., Wright, J. A., Attema, J. L., Gregory, P. A., Bert, A. G., Smith, E., et al. (2013). Epigenetic Modulation of the miR-200 Family Is Associated with Transition to a Breast Cancer Stem Cell-like State. J. Cel. Sci. 126 (Pt 10), 2256–2266. doi:10.1242/jcs.122275
Lin, Z., Tian, X. Y., Huang, X. X., He, L. L., and Xu, F. (2019). microRNA‐186 Inhibition of PI3K-AKT Pathway via SPP1 Inhibits Chondrocyte Apoptosis in Mice with Osteoarthritis. J. Cel Physiol 234 (5), 6042–6053. doi:10.1002/jcp.27225
Litwic, A., Edwards, M. H., Dennison, E. M., and Cooper, C. (2013). Epidemiology and burden of Osteoarthritis. Br. Med. Bull. 105, 185–199. doi:10.1093/bmb/lds038
Liu, B., Li, J., and Cairns, M. J. (2014a). Identifying miRNAs, Targets and Functions. Brief. Bioinformatics 15 (1), 1–19. doi:10.1093/bib/bbs075
Liu, C., Ren, S., Zhao, S., and Wang, Y. (2019a). LncRNA MALAT1/MiR-145 Adjusts IL-1β-Induced Chondrocytes Viability and Cartilage Matrix Degradation by Regulating ADAMTS5 in Human Osteoarthritis. Yonsei Med. J. 60 (11), 1081–1092. doi:10.3349/ymj.2019.60.11.1081
Liu, F., Liu, X., Yang, Y., Sun, Z., Deng, S., Jiang, Z., et al. (2020a). NEAT1/miR‐193a‐3p/SOX5 axis Regulates Cartilage Matrix Degradation in Human Osteoarthritis. Cel Biol Int 44 (4), 947–957. doi:10.1002/cbin.11291
Liu, H., and Luo, J. (2019). miR-211-5p Contributes to Chondrocyte Differentiation by Suppressing Fibulin-4 Expression to Play a Role in Osteoarthritis. J. Biochem. 166 (6), 495–502. doi:10.1093/jb/mvz065
Liu, J., Liu, Y., Wang, F., and Liang, M. (2021a). miR-204: Molecular Regulation and Role in Cardiovascular and Renal Diseases. Hypertension 78 (2), 270–281. doi:10.1161/HYPERTENSIONAHA.121.14536
Liu, J., Xue, N., Guo, Y., Niu, K., Gao, L., Zhang, S., et al. (2019b). CircRNA_100367 Regulated the Radiation Sensitivity of Esophageal Squamous Cell Carcinomas through miR-217/Wnt3 Pathway. Aging 11 (24), 12412–12427. doi:10.18632/aging.102580
Liu, Q., Zhang, X., Dai, L., Hu, X., Zhu, J., Li, L., et al. (2014b). Long Noncoding RNA Related to Cartilage Injury Promotes Chondrocyte Extracellular Matrix Degradation in Osteoarthritis. Arthritis Rheumatol. 66 (4), 969–978. doi:10.1002/art.38309
Liu, Q., Zhang, X., Hu, X., Dai, L., Fu, X., Zhang, J., et al. (2016). Circular RNA Related to the Chondrocyte ECM Regulates MMP13 Expression by Functioning as a MiR-136 'Sponge' in Human Cartilage Degradation. Sci. Rep. 6, 22572. doi:10.1038/srep22572
Liu, W., Zha, Z., and Wang, H. (2019c). Upregulation of microRNA‐27a Inhibits Synovial Angiogenesis and Chondrocyte Apoptosis in Knee Osteoarthritis Rats through the Inhibition of PLK2. J. Cel Physiol 234 (12), 22972–22984. doi:10.1002/jcp.28858
Liu, X. C., Xu, L., Cai, Y. L., Zheng, Z. Y., Dai, E. N., and Sun, S. (2020b). MiR‐1207‐5p/CX3CR1 axis Regulates the Progression of Osteoarthritis via the Modulation of the Activity of NF‐κB Pathway. Int. J. Rheum. Dis. 23 (8), 1057–1065. doi:10.1111/1756-185X.13898
Liu, X., Liu, L., Zhang, H., Shao, Y., Chen, Z., Feng, X., et al. (2019d). MiR-146b Accelerates Osteoarthritis Progression by Targeting Alpha-2-Macroglobulin. Aging 11 (16), 6014–6028. doi:10.18632/aging.102160
Liu, Y., Li, Q., Gao, Z., Lei, F., and Gao, X. (2021b). Circ-SPG11 Knockdown Hampers IL-1β-induced Osteoarthritis Progression via Targeting miR-337-3p/ADAMTS5. J. Orthop. Surg. Res. 16 (1), 392. doi:10.1186/s13018-021-02526-y
Liu, Y., Lin, L., Zou, R., Wen, C., Wang, Z., and Lin, F. (2018). MSC-derived Exosomes Promote Proliferation and Inhibit Apoptosis of Chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in Osteoarthritis. Cell Cycle 17 (21-22), 2411–2422. doi:10.1080/15384101.2018.1526603
Liu, Y., Liu, K., Tang, C., Shi, Z., Jing, K., and Zheng, J. (2020c). Long Non-coding RNA XIST Contributes to Osteoarthritis Progression via miR-149-5p/DNMT3A axis. Biomed. Pharmacother. 128, 110349. doi:10.1016/j.biopha.2020.110349
Liu, Z., Chen, S., Yang, Y., Lu, S., Zhao, X., Hu, B., et al. (2019e). MicroRNA-671-3p R-egulates the D-evelopment of K-nee O-steoarthritis by T-argeting TRAF3 in C-hondrocytes. Mol. Med. Rep. 20 (3), 2843–2850. doi:10.3892/mmr.2019.10488
Long, H., Li, Q., Xiao, Z., and Yang, B. (2021). LncRNA MIR22HG Promotes Osteoarthritis Progression via Regulating miR-9-3p/ADAMTS5 Pathway. Bioengineered 12 (1), 3148–3158. doi:10.1080/21655979.2021.1945362
Lu, C., Li, Z., Hu, S., Cai, Y., and Peng, K. (2019). LncRNA PART‐1 Targets TGFBR2/Smad3 to Regulate Cell Viability and Apoptosis of Chondrocytes via Acting as miR‐590‐3p Sponge in Osteoarthritis. J. Cel Mol Med 23 (12), 8196–8205. doi:10.1111/jcmm.14690
Lü, G., Li, L., Wang, B., and Kuang, L. (2020). LINC00623/miR-101/HRAS axis Modulates IL-1β-mediated ECM Degradation, Apoptosis and Senescence of Osteoarthritis Chondrocytes. Aging 12 (4), 3218–3237. doi:10.18632/aging.102801
Lu, J., Ji, M.-L., Zhang, X.-J., Shi, P.-L., Wu, H., Wang, C., et al. (2017). MicroRNA-218-5p as a Potential Target for the Treatment of Human Osteoarthritis. Mol. Ther. 25 (12), 2676–2688. doi:10.1016/j.ymthe.2017.08.009
Lu, M., and Zhou, E. (2020). Long Noncoding RNA LINC00662‐miR‐15b‐5p Mediated GPR120 Dysregulation Contributes to Osteoarthritis. Pathol. Int. 70 (3), 155–165. doi:10.1111/pin.12875
Lu, X., Yu, Y., Yin, F., Yang, C., Li, B., Lin, J., et al. (2020). Knockdown of PVT1 Inhibits IL-1β-induced Injury in Chondrocytes by Regulating miR-27b-3p/TRAF3 axis. Int. immunopharmacology 79, 106052. doi:10.1016/j.intimp.2019.106052
Lu, Z., Luo, M., and Huang, Y. (2018). lncRNA‐CIR Regulates Cell Apoptosis of Chondrocytes in Osteoarthritis. J. Cel Biochem 120, 7229–7237. doi:10.1002/jcb.27997
Luan, X., and Wang, Y. (2018). LncRNA XLOC_006390 Facilitates Cervical Cancer Tumorigenesis and Metastasis as a ceRNA Against miR-331-3p and miR-338-3p. J. Gynecol. Oncol. 29 (6), e95. doi:10.3802/jgo.2018.29.e95
Luo, C., Liang, J. S., Gong, J., Zhang, H. L., Feng, Z. J., Yang, H. T., et al. (2019a). The Function of microRNA-34a in Osteoarthritis. Bratisl Lek Listy 120 (5), 386–391. doi:10.4149/BLL_2019_063
Luo, H., Xu, C., Le, W., Ge, B., and Wang, T. (2019b). lncRNA CASC11 Promotes Cancer Cell Proliferation in Bladder Cancer through miRNA‐150. J. Cel Biochem 120 (8), 13487–13493. doi:10.1002/jcb.28622
Ma, F., Li, G., Yu, Y., Xu, J., and Wu, X. (2019a). MiR-33b-3p Promotes Chondrocyte Proliferation and Inhibits Chondrocyte Apoptosis and Cartilage ECM Degradation by Targeting DNMT3A in Osteoarthritis. Biochem. biophysical Res. Commun. 519 (2), 430–437. doi:10.1016/j.bbrc.2019.09.022
Ma, H. R., Mu, W. B., Zhang, K. Y., Zhou, H. K., Jiang, R. D., and Cao, L. (2020). CircVCAN Regulates the Proliferation and Apoptosis of Osteoarthritis Chondrocyte through NF-Κb Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 24 (12), 6517–6525. doi:10.26355/eurrev_202006_21635
Ma, Y., Wu, Y., Chen, J., Huang, K., Ji, B., Chen, Z., et al. (2019b). miR-10a-5p Promotes Chondrocyte Apoptosis in Osteoarthritis by Targeting HOXA1. Mol. Ther. - Nucleic Acids 14, 398–409. doi:10.1016/j.omtn.2018.12.012
Makki, M. S., and Haqqi, T. M. (2015). miR-139 Modulates MCPIP1/IL-6 Expression and Induces Apoptosis in Human OA Chondrocytes. Exp. Mol. Med. 47, e189. doi:10.1038/emm.2015.66
Mao, D., Wu, M., Wei, J., Zhou, X., Yang, L., and Chen, F. (2021a). MicroRNA‐101a‐3p Could Be Involved in the Pathogenesis of Temporomandibular Joint Osteoarthritis by Mediating UBE2D1 and FZD4. J. Oral Pathol. Med. 50 (2), 236–243. doi:10.1111/jop.13131
Mao, G., Hu, S., Zhang, Z., Wu, P., Zhao, X., Lin, R., et al. (2018a). Exosomal miR-95-5p Regulates Chondrogenesis and Cartilage Degradation via Histone Deacetylase 2/8. J. Cel Mol Med 22 (11), 5354–5366. doi:10.1111/jcmm.13808
Mao, G., Kang, Y., Lin, R., Hu, S., Zhang, Z., Li, H., et al. (2019a). Long Non-coding RNA HOTTIP Promotes CCL3 Expression and Induces Cartilage Degradation by Sponging miR-455-3p. Front. Cel Dev. Biol. 7, 161. doi:10.3389/fcell.2019.00161
Mao, G., Wu, P., Zhang, Z., Zhang, Z., Liao, W., Li, Y., et al. (2017a). MicroRNA-92a-3p Regulates Aggrecanase-1 and Aggrecanase-2 Expression in Chondrogenesis and IL-1β-Induced Catabolism in Human Articular Chondrocytes. Cel Physiol Biochem 44 (1), 38–52. doi:10.1159/000484579
Mao, G., Xu, Y., Long, D., Sun, H., Li, H., Xin, R., et al. (2021b). Exosome-transported circRNA_0001236 Enhances Chondrogenesis and Suppress Cartilage Degradation via the miR-3677-3p/Sox9 axis. Stem Cel Res Ther 12 (1), 389. doi:10.1186/s13287-021-02431-5
Mao, G., Zhang, Z., Hu, S., Zhang, Z., Chang, Z., Huang, Z., et al. (2018b). Exosomes Derived from miR-92a-3p-Overexpressing Human Mesenchymal Stem Cells Enhance Chondrogenesis and Suppress Cartilage Degradation via Targeting WNT5A. Stem Cel Res Ther 9 (1), 247. doi:10.1186/s13287-018-1004-0
Mao, G., Zhang, Z., Huang, Z., Chen, W., Huang, G., Meng, F., et al. (2017b). MicroRNA-92a-3p Regulates the Expression of Cartilage-specific Genes by Directly Targeting Histone Deacetylase 2 in Chondrogenesis and Degradation. Osteoarthritis and cartilage 25 (4), 521–532. doi:10.1016/j.joca.2016.11.006
Mao, T., He, C., Wu, H., Yang, B., and Li, X. (2019b). Silencing lncRNA HOTAIR Declines Synovial Inflammation and Synoviocyte Proliferation and Promotes Synoviocyte Apoptosis in Osteoarthritis Rats by Inhibiting Wnt/β-Catenin Signaling Pathway. Cell Cycle 18 (22), 3189–3205. doi:10.1080/15384101.2019.1671716
Matsukawa, T., Sakai, T., Yonezawa, T., Hiraiwa, H., Hamada, T., Nakashima, M., et al. (2013). MicroRNA-125b Regulates the Expression of Aggrecanase-1 (ADAMTS-4) in Human Osteoarthritic Chondrocytes. Arthritis Res. Ther. 15 (1), R28. doi:10.1186/ar4164
Mayer, U., Benditz, A., and Grässel, S. (2017). miR-29b Regulates Expression of Collagens I and III in Chondrogenically Differentiating BMSC in an Osteoarthritic Environment. Sci. Rep. 7 (1), 13297. doi:10.1038/s41598-017-13567-x
Mehana, E.-S. E., Khafaga, A. F., and El-Blehi, S. S. (2019). The Role of Matrix Metalloproteinases in Osteoarthritis Pathogenesis: An Updated Review. Life Sci. 234, 116786. doi:10.1016/j.lfs.2019.116786
Mei, X., Tong, J., Zhu, W., and Zhu, Y. (2019). lncRNA-NR024118 O-verexpression R-everses LPS-induced I-nflammatory I-njury and A-poptosis via NF-κB/Nrf2 S-ignaling in ATDC5 C-hondrocytes. Mol. Med. Rep. 20 (4), 3867–3873. doi:10.3892/mmr.2019.10639
Meng, F., Li, Z., Zhang, Z., Yang, Z., Kang, Y., Zhao, X., et al. (2018). MicroRNA-193b-3p Regulates Chondrogenesis and Chondrocyte Metabolism by Targeting HDAC3. Theranostics 8 (10), 2862–2883. doi:10.7150/thno.23547
Meng, F., Zhang, Z., Chen, W., Huang, G., He, A., Hou, C., et al. (2016). MicroRNA-320 Regulates Matrix Metalloproteinase-13 Expression in Chondrogenesis and Interleukin-1β-Induced Chondrocyte Responses. Osteoarthritis and cartilage 24 (5), 932–941. doi:10.1016/j.joca.2015.12.012
Miao, G., Zang, X., Hou, H., Sun, H., Wang, L., Zhang, T., et al. (2019). Bax Targeted by miR-29a Regulates Chondrocyte Apoptosis in Osteoarthritis. Biomed. Research International 2019, 1–9. doi:10.1155/2019/1434538
Miyaki, S., Sato, T., Inoue, A., Otsuki, S., Ito, Y., Yokoyama, S., et al. (2010). MicroRNA-140 Plays Dual Roles in Both Cartilage Development and Homeostasis. Genes Dev. 24 (11), 1173–1185. doi:10.1101/gad.1915510
Moulin, D., Salone, V., Koufany, M., Clément, T., Behm-Ansmant, I., Branlant, C., et al. (2017). MicroRNA-29b Contributes to Collagens Imbalance in Human Osteoarthritic and Dedifferentiated Articular Chondrocytes. Biomed. Research International 2017, 1–12. doi:10.1155/2017/9792512
Ni, J. L., Dang, X. Q., and Shi, Z. B. (2020a). CircPSM3 Inhibits the Proliferation and Differentiation of OA Chondrocytes by Targeting miRNA-296-5p. Eur. Rev. Med. Pharmacol. Sci. 24 (7), 3467–3475. doi:10.26355/eurrev_202004_20805
Ni, S., Xu, C., Zhuang, C., Zhao, G., Li, C., Wang, Y., et al. (2020b). LncRNA LUADT1 Regulates miR‐34a/SIRT1 to Participate in Chondrocyte Apoptosis. J. Cel Biochem 122, 1003–1008. doi:10.1002/jcb.29637
Ni, W., Jiang, C., Wu, Y., Zhang, H., Wang, L., Yik, J. H. N., et al. (2021). CircSLC7A2 Protects against Osteoarthritis through Inhibition of the miR‐4498/TIMP3 axis. Cell Prolif 54 (6), e13047. doi:10.1111/cpr.13047
Ni, Z., Shang, X., Tang, G., and Niu, L. (2018). Expression of miR-206 in Human Knee Articular Chondrocytes and Effects of miR-206 on Proliferation and Apoptosis of Articular Chondrocytes. Am. J. Med. Sci. 355 (3), 240–246. doi:10.1016/j.amjms.2017.11.003
Nishimura, R., Hata, K., Takahata, Y., Murakami, T., Nakamura, E., Ohkawa, M., et al. (2020). Role of Signal Transduction Pathways and Transcription Factors in Cartilage and Joint Diseases. Int. J. Mol. Sci. 21 (4), 1340. doi:10.3390/ijms21041340
Ntoumou, E., Tzetis, M., Braoudaki, M., Lambrou, G., Poulou, M., Malizos, K., et al. (2017). Serum microRNA Array Analysis Identifies miR-140-3p, miR-33b-3p and miR-671-3p as Potential Osteoarthritis Biomarkers Involved in Metabolic Processes. Clin. Epigenet 9, 127. doi:10.1186/s13148-017-0428-1
Pan, H., Dai, H., Wang, L., Lin, S., Tao, Y., Zheng, Y., et al. (2020). MicroRNA-410-3p Modulates Chondrocyte Apoptosis and Inflammation by Targeting High Mobility Group Box 1 (HMGB1) in an Osteoarthritis Mouse Model. BMC Musculoskelet. Disord. 21 (1), 486. doi:10.1186/s12891-020-03489-7
Park, S. J., Cheon, E. J., and Kim, H. A. (2013). MicroRNA-558 Regulates the Expression of Cyclooxygenase-2 and IL-1β-induced Catabolic Effects in Human Articular Chondrocytes. Osteoarthritis and cartilage 21 (7), 981–989. doi:10.1016/j.joca.2013.04.012
Pearson, M. J., Philp, A. M., Heward, J. A., Roux, B. T., Walsh, D. A., Davis, E. T., et al. (2016). Long Intergenic Noncoding RNAs Mediate the Human Chondrocyte Inflammatory Response and Are Differentially Expressed in Osteoarthritis Cartilage. Arthritis Rheumatol. 68 (4), 845–856. doi:10.1002/art.39520
Peng, L., Deng, M., Ma, Y., Hu, W., and Liang, F. (2021). miR-520c-3p Regulates IL-1β-stimulated Human Chondrocyte Apoptosis and Cartilage Degradation by Targeting GAS2. J. Orthop. Surg. Res. 16 (1), 347. doi:10.1186/s13018-021-02466-7
Peng, P., Zhang, B., Huang, J., Xing, C., Liu, W., Sun, C., et al. (2020). Identification of a circRNA-miRNA-mRNA Network to Explore the Effects of circRNAs on Pathogenesis and Treatment of Spinal Cord Injury. Life Sci. 257, 118039. doi:10.1016/j.lfs.2020.118039
Ponting, C. P., Oliver, P. L., and Reik, W. (2009). Evolution and Functions of Long Noncoding RNAs. Cell 136 (14), 629–641. doi:10.1016/j.cell.2009.02.006
Prieto-Alhambra, D., Judge, A., Javaid, M. K., Cooper, C., Diez-Perez, A., and Arden, N. K. (2014). Incidence and Risk Factors for Clinically Diagnosed Knee, Hip and Hand Osteoarthritis: Influences of Age, Gender and Osteoarthritis Affecting Other Joints. Ann. Rheum. Dis. 73 (9), 1659–1664. doi:10.1136/annrheumdis-2013-203355
Qin, G. H., Yang, W. C., Yao, J. N., Zhao, Y., and Wu, X. J. (2021). LncRNA OIP5-AS1 Affects the Biological Behaviors of Chondrocytes of Patients with Osteoarthritis by Regulating Micro-30a-5p. Eur. Rev. Med. Pharmacol. Sci. 25 (3), 1215–1224. doi:10.26355/eurrev_202102_24825
Qiu, B., Xu, X., Yi, P., and Hao, Y. (2020). Curcumin Reinforces MSC‐derived Exosomes in Attenuating Osteoarthritis via Modulating the miR‐124/NF‐kB and miR‐143/ROCK1/TLR9 Signalling Pathways. J. Cel Mol Med 24 (18), 10855–10865. doi:10.1111/jcmm.15714
Qiu, W.-J., Xu, M.-Z., Zhu, X.-D., and Ji, Y.-H. (2019). MicroRNA-27a Alleviates IL-1β-induced Inflammatory Response and Articular Cartilage Degradation via TLR4/NF-Κb Signaling Pathway in Articular Chondrocytes. Int. immunopharmacology 76, 105839. doi:10.1016/j.intimp.2019.105839
Rasheed, Z., Rasheed, N., Abdulmonem, W. A., and Khan, M. I. (2019). Author Correction: MicroRNA-125b-5p Regulates IL-1β Induced Inflammatory Genes via Targeting TRAF6-Mediated MAPKs and NF-Κb Signaling in Human Osteoarthritic Chondrocytes. Sci. Rep. 9 (1), 14729. doi:10.1038/s41598-019-50844-3
Ren, T., Wei, P., Song, Q., Ye, Z., Wang, Y., and Huang, L. (2020). MiR-140-3p Ameliorates the Progression of Osteoarthritis via Targeting CXCR4. Biol. Pharm. Bull. 43 (5), 810–816. doi:10.1248/bpb.b19-00959
Rigoglou, S., and Papavassiliou, A. G. (2013). The NF-Κb Signalling Pathway in Osteoarthritis. Int. J. Biochem. Cel Biol. 45 (11), 2580–2584. doi:10.1016/j.biocel.2013.08.018
Rousseau, J.-C., Millet, M., Croset, M., Sornay-Rendu, E., Borel, O., and Chapurlat, R. (2020). Association of Circulating microRNAs with Prevalent and Incident Knee Osteoarthritis in Women: the OFELY Study. Arthritis Res. Ther. 22 (1), 2. doi:10.1186/s13075-019-2086-5
Sakalauskienė, G., and Jauniškienė, D. (2010). Osteoarthritis: Etiology, Epidemiology, Impact on the Individual and Society and the Main Principles of Management. Medicina (Kaunas) 46 (11), 790–797.
Shao, J., Ding, Z., Peng, J., Zhou, R., Li, L., Qian, Q., et al. (2020). MiR-146a-5p Promotes IL-1β-induced Chondrocyte Apoptosis through the TRAF6-Mediated NF-kB Pathway. Inflamm. Res. 69 (6), 619–630. doi:10.1007/s00011-020-01346-w
Shen, H., Wang, Y., Shi, W., Sun, G., Hong, L., and Zhang, Y. (2018). LncRNA SNHG5/miR-26a/SOX2 Signal axis Enhances Proliferation of Chondrocyte in Osteoarthritis. Acta Biochim. Biophys. Sinica 50 (2), 191–198. doi:10.1093/abbs/gmx141
Shen, J., Li, S., and Chen, D. (2014). TGF-β Signaling and the Development of Osteoarthritis. Bone Res. 2, 14002. doi:10.1038/boneres.2014.2
Shen, P., Yang, Y., Liu, G., Chen, W., Chen, J., Wang, Q., et al. (2020a). CircCDK14 Protects against Osteoarthritis by Sponging miR-125a-5p and Promoting the Expression of Smad2. Theranostics 10 (20), 9113–9131. doi:10.7150/thno.45993
Shen, S., Wu, Y., Chen, J., Xie, Z., Huang, K., Wang, G., et al. (2019). CircSERPINE2 Protects against Osteoarthritis by Targeting miR-1271 and ETS-Related Gene. Ann. Rheum. Dis. 78 (6), 826–836. doi:10.1136/annrheumdis-2018-214786
Shen, S., Yang, Y., Shen, P., Ma, J., Fang, B., Wang, Q., et al. (2021). circPDE4B Prevents Articular Cartilage Degeneration and Promotes Repair by Acting as a Scaffold for RIC8A and MID1. Ann. Rheum. Dis. 80 (9), 1209–1219. doi:10.1136/annrheumdis-2021-219969
Shen, X.-F., Cheng, Y., Dong, Q.-R., and Zheng, M.-Q. (2020b). MicroRNA-675-3p Regulates IL-1β-stimulated Human Chondrocyte Apoptosis and Cartilage Degradation by Targeting GNG5. Biochem. biophysical Res. Commun. 527 (2), 458–465. doi:10.1016/j.bbrc.2020.04.044
Shi, F.-L., and Ren, L.-X. (2020). Up-regulated miR-374a-3p Relieves Lipopolysaccharides Induced Injury in CHON-001 Cells via Regulating Wingless-type MMTV Integration Site Family Member 5B. Mol. Cell. probes 51, 101541. doi:10.1016/j.mcp.2020.101541
Shi, J., Cao, F., Chang, Y., Xin, C., Jiang, X., Xu, J., et al. (2021a). Long Non-coding RNA MCM3AP-AS1 Protects Chondrocytes ATDC5 and CHON-001 from IL-1β-induced Inflammation via Regulating miR-138-5p/SIRT1. Bioengineered 12 (1), 1445–1456. doi:10.1080/21655979.2021.1905247
Shi, J., Guo, K., Su, S., Li, J., and Li, C. (2018). miR-486-5p I-s U-pregulated in O-steoarthritis and I-nhibits C-hondrocyte P-roliferation and M-igration by S-uppressing SMAD2. Mol. Med. Rep. 18 (1), 502–508. doi:10.3892/mmr.2018.8931
Shi, J., Wang, S., He, Q., Liu, K., Zhao, W., Xie, Q., et al. (2021b). TNF‐α Induces Up‐regulation of MicroRNA‐27a via the P38 Signalling Pathway, Which Inhibits Intervertebral Disc Degeneration by Targeting FSTL1. J. Cel Mol Med 25 (15), 7146–7156. doi:10.1111/jcmm.16745
Shu, Y., Long, J., Guo, W., and Ye, W. (2019). MicroRNA-195-5p I-nhibitor P-revents the D-evelopment of O-steoarthritis by T-argeting REGγ. Mol. Med. Rep. 19 (6), 4561–4568. doi:10.3892/mmr.2019.10124
Si, H.-b., Zeng, Y., Zhou, Z.-k., Pei, F.-x., Lu, Y.-r., Cheng, J.-q., et al. (2016). Expression of miRNA-140 in Chondrocytes and Synovial Fluid of Knee Joints in Patients with Osteoarthritis. Chin. Med. Sci. J. 31 (4), 207–212. doi:10.1016/s1001-9294(17)30002-0
Si, Z., Zhou, S., Shen, Z., Luan, F., and Yan, J. (2021). lncRNA HAND2-AS1 Is Downregulated in Osteoarthritis and Regulates IL-6 Expression in Chondrocytes. J. Orthop. Surg. Res. 16 (1), 68. doi:10.1186/s13018-021-02216-9
Skrzypa, M., Szala, D., Gablo, N., Czech, J., Pajak, J., Kopanska, M., et al. (2019). miRNA-146a-5p Is Upregulated in Serum and Cartilage Samples of Patients with Osteoarthritis. Pol. Przegl Chir 91 (3), 1–5. doi:10.5604/01.3001.0013.0135
Sondag, G. R., and Haqqi, T. M. (2016). The Role of MicroRNAs and Their Targets in Osteoarthritis. Curr. Rheumatol. Rep. 18 (8), 56. doi:10.1007/s11926-016-0604-x
Song, J., Ahn, C., Chun, C.-H., and Jin, E.-J. (2014). A Long Non-coding RNA, GAS5, Plays a Critical Role in the Regulation of miR-21 during Osteoarthritis. J. Orthop. Res. 32 (12), 1628–1635. doi:10.1002/jor.22718
Song, J., Jin, E.-H., Kim, D., Kim, K. Y., Chun, C.-H., and Jin, E.-J. (2015). MicroRNA-222 Regulates MMP-13 via Targeting HDAC-4 during Osteoarthritis Pathogenesis. BBA Clin. 3, 79–89. doi:10.1016/j.bbacli.2014.11.009
Song, J., Kim, D., Lee, C. H., Lee, M. S., Chun, C.-H., and Jin, E.-J. (2013). MicroRNA-488 Regulates Zinc Transporter SLC39A8/ZIP8 during Pathogenesis of Osteoarthritis. J. Biomed. Sci. 20, 31. doi:10.1186/1423-0127-20-31
Steck, E., Boeuf, S., Gabler, J., Werth, N., Schnatzer, P., Diederichs, S., et al. (2012). Regulation of H19 and its Encoded microRNA-675 in Osteoarthritis and under Anabolic and Catabolic In Vitro Conditions. J. Mol. Med. 90 (10), 1185–1195. doi:10.1007/s00109-012-0895-y
Su, W., Xie, W., Shang, Q., and Su, B. (2015). The Long Noncoding RNA MEG3 Is Downregulated and Inversely Associated with VEGF Levels in Osteoarthritis. Biomed. Research International 2015, 1–5. doi:10.1155/2015/356893
Sui, C., Liu, D., Que, Y., Xu, S., and Hu, Y. (2021). Knockdown of Hsa_circ_0037658 Inhibits the Progression of Osteoarthritis via Inducing Autophagy. Hum. Cel. 34 (1), 76–85. doi:10.1007/s13577-020-00440-9
Sui, C., Zhang, L., and Hu, Y. (2019). MicroRNA-let-7a I-nhibition I-nhibits LPS-induced I-nflammatory I-njury of C-hondrocytes by T-argeting IL6R. Mol. Med. Rep. 20 (3), 2633–2640. doi:10.3892/mmr.2019.10493
Sun, J. L., Yan, J. F., Yu, S. B., Zhao, J., Lin, Q. Q., and Jiao, K. (2020). MicroRNA-29b Promotes Subchondral Bone Loss in TMJ Osteoarthritis. J. Dent Res. 99 (13), 1469–1477. doi:10.1177/0022034520937617
Sun, J., Zhong, N., Li, Q., Min, Z., Zhao, W., Sun, Q., et al. (2011). MicroRNAs of Rat Articular Cartilage at Different Developmental Stages Identified by Solexa Sequencing. Osteoarthritis and cartilage 19 (10), 1237–1245. doi:10.1016/j.joca.2011.07.002
Sun, T., Li, X., Song, H., Gao, F., Zhou, G., Li, X., et al. (2017). MiR-146a Aggravates LPS-Induced Inflammatory Injury by Targeting CXCR4 in the Articular Chondrocytes. Cel Physiol Biochem 44 (4), 1282–1294. doi:10.1159/000485488
Tan, F., Wang, D., and Yuan, Z. (2020). The Fibroblast-like Synoviocyte Derived Exosomal Long Non-coding RNA H19 Alleviates Osteoarthritis Progression through the miR-106b-5p/TIMP2 Axis. Inflammation 43 (4), 1498–1509. doi:10.1007/s10753-020-01227-8
Tang, L., Ding, J., Zhou, G., and Liu, Z. (2018a). LncRNA-p21 P-romotes C-hondrocyte A-poptosis in O-steoarthritis by A-cting as a S-ponge for miR-451. Mol. Med. Rep. 18 (6), 5295–5301. doi:10.3892/mmr.2018.9506
Tang, L. P., Ding, J. B., Liu, Z. H., and Zhou, G. J. (2018b). LncRNA TUG1 Promotes Osteoarthritis-Induced Degradation of Chondrocyte Extracellular Matrix via miR-195/MMP-13 axis. Eur. Rev. Med. Pharmacol. Sci. 22 (24), 8574–8581. doi:10.26355/eurrev_201812_16620
Tao, H., Cheng, L., and Yang, R. (2020). Downregulation of miR-34a Promotes Proliferation and Inhibits Apoptosis of Rat Osteoarthritic Cartilage Cells by Activating PI3K/Akt Pathway. Clin. Interv. Aging 15, 373–385. doi:10.2147/CIA.S241855
Tao, S.-C., Huang, J.-Y., Gao, Y., Li, Z.-X., Wei, Z.-Y., Dawes, H., et al. (2021). Small Extracellular Vesicles in Combination with Sleep-Related circRNA3503: A Targeted Therapeutic Agent with Injectable Thermosensitive Hydrogel to Prevent Osteoarthritis. Bioactive Mater. 6 (12), 4455–4469. doi:10.1016/j.bioactmat.2021.04.031
Tao, S.-C., Yuan, T., Zhang, Y.-L., Yin, W.-J., Guo, S.-C., and Zhang, C.-Q. (2017). Exosomes Derived from miR-140-5p-Overexpressing Human Synovial Mesenchymal Stem Cells Enhance Cartilage Tissue Regeneration and Prevent Osteoarthritis of the Knee in a Rat Model. Theranostics 7 (1), 180–195. doi:10.7150/thno.17133
Tardif, G., Hum, D., Pelletier, J.-P., Duval, N., and Martel-Pelletier, J. (2009). Regulation of the IGFBP-5 and MMP-13 Genes by the microRNAs miR-140 and miR-27a in Human Osteoarthritic Chondrocytes. BMC Musculoskelet. Disord. 10, 148. doi:10.1186/1471-2474-10-148
Tardif, G., Pelletier, J.-P., Fahmi, H., Hum, D., Zhang, Y., Kapoor, M., et al. (2013). NFAT3 and TGF-Β/smad3 Regulate the Expression of miR-140 in Osteoarthritis. Arthritis Res. Ther. 15 (6), R197. doi:10.1186/ar4387
Tian, F., Wang, J., Zhang, Z., and Yang, J. (2020). LncRNA SNHG7/miR-34a-5p/SYVN1 axis Plays a Vital Role in Proliferation, Apoptosis and Autophagy in Osteoarthritis. Biol. Res. 53 (1), 9. doi:10.1186/s40659-020-00275-6
Tian, L., Su, Z., Ma, X., Wang, F., and Guo, Y. (2019). Inhibition of miR-203 Ameliorates Osteoarthritis Cartilage Degradation in the Postmenopausal Rat Model: Involvement of Estrogen Receptor α. Hum. Gene Ther. Clin. Develop. 30 (4), 160–168. doi:10.1089/humc.2019.101
Tian, Y., Guo, R., Shi, B., Chen, L., Yang, L., and Fu, Q. (2016). MicroRNA-30a Promotes Chondrogenic Differentiation of Mesenchymal Stem Cells through Inhibiting Delta-like 4 Expression. Life Sci. 148, 220–228. doi:10.1016/j.lfs.2016.02.031
Toyota, M., Suzuki, H., Sasaki, Y., Maruyama, R., Imai, K., Shinomura, Y., et al. (2008). Epigenetic Silencing of microRNA-34b/c and B-Cell Translocation Gene 4 Is Associated with CpG Island Methylation in Colorectal Cancer. Cancer Res. 68 (11), 4123–4132. doi:10.1158/0008-5472.CAN-08-0325
Tu, Y., Ma, T., Wen, T., Yang, T., Xue, L., Cai, M., et al. (2020). MicroRNA-377-3p Alleviates IL-1β-caused Chondrocyte Apoptosis and Cartilage Degradation in Osteoarthritis in Part by Downregulating ITGA6. Biochem. biophysical Res. Commun. 523 (1), 46–53. doi:10.1016/j.bbrc.2019.11.186
Vincent, T. L. (2019). Mechanoflammation in Osteoarthritis Pathogenesis. Semin. Arthritis Rheum. 49 (3S), S36–S38. doi:10.1016/j.semarthrit.2019.09.018
Wan, D., Qu, Y., Ai, S., and Cheng, L. (2020). miR-152 Attenuates Apoptosis in Chondrocytes and Degeneration of Cartilages in Osteoarthritis Rats via TCF-4 Pathway. Dose-Response 18 (4), 155932582094691. doi:10.1177/1559325820946918
Wang, A., Hu, N., Zhang, Y., Chen, Y., Su, C., Lv, Y., et al. (2019a). MEG3 Promotes Proliferation and Inhibits Apoptosis in Osteoarthritis Chondrocytes by miR-361-5p/FOXO1 axis. BMC Med. Genomics 12 (1), 201. doi:10.1186/s12920-019-0649-6
Wang, C.-L., Peng, J.-P., and Chen, X.-D. (2018a). LncRNA-CIR Promotes Articular Cartilage Degeneration in Osteoarthritis by Regulating Autophagy. Biochem. biophysical Res. Commun. 505 (3), 692–698. doi:10.1016/j.bbrc.2018.09.163
Wang, C., Li, N., Liu, Q., Su, L., Wang, S., Chen, Y., et al. (2021a). The Role of circRNA Derived from RUNX2 in the Serum of Osteoarthritis and its Clinical Value. J. Clin. Lab. Anal. 35 (7), e23858. doi:10.1002/jcla.23858
Wang, G.-D., Zhao, X.-W., Zhang, Y.-G., Kong, Y., Niu, S.-S., Ma, L.-F., et al. (2017a). Effects of miR-145 on the Inhibition of Chondrocyte Proliferation and Fibrosis by Targeting TNFRSF11B in Human Osteoarthritis. Mol. Med. Rep. 15 (1), 75–80. doi:10.3892/mmr.2016.5981
Wang, G.-L., Wu, Y.-B., Liu, J.-T., and Li, C.-Y. (2016a). Upregulation of miR-98 Inhibits Apoptosis in Cartilage Cells in Osteoarthritis. Genet. Test. Mol. biomarkers 20 (11), 645–653. doi:10.1089/gtmb.2016.0011
Wang, H., Zhang, H., Sun, Q., Wang, Y., Yang, J., Yang, J., et al. (2017b). Intra-articular Delivery of Antago-miR-483-5p Inhibits Osteoarthritis by Modulating Matrilin 3 and Tissue Inhibitor of Metalloproteinase 2. Mol. Ther. 25 (3), 715–727. doi:10.1016/j.ymthe.2016.12.020
Wang, J., Chen, L., Jin, S., Lin, J., Zheng, H., Zhang, H., et al. (2017c). Altered Expression of microRNA-98 in IL-1β-induced Cartilage Degradation and its Role in Chondrocyte Apoptosis. Mol. Med. Rep. 16 (3), 3208–3216. doi:10.3892/mmr.2017.7028
Wang, J., Chen, L., Jin, S., Lin, J., Zheng, H., Zhang, H., et al. (2016b). MiR-98 Promotes Chondrocyte Apoptosis by Decreasing Bcl-2 Expression in a Rat Model of Osteoarthritis. Acta Biochim. Biophys. Sin 48 (10), 923–929. doi:10.1093/abbs/gmw084
Wang, J., Fang, L., Ye, L., Ma, S., Huang, H., Lan, X., et al. (2020a). miR-137 Targets the Inhibition of TCF4 to Reverse the Progression of Osteoarthritis through the AMPK/NF-κB Signaling Pathway. Biosci. Rep. 40 (6), BSR20200466. doi:10.1042/BSR20200466
Wang, J., Zhu, S., Meng, N., He, Y., Lu, R., and Yan, G.-R. (2019b). ncRNA-Encoded Peptides or Proteins and Cancer. Mol. Ther. 27 (10), 1718–1725. doi:10.1016/j.ymthe.2019.09.001
Wang, L., Cho, K. B., Li, Y., Tao, G., Xie, Z., and Guo, B. (2019c). Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int. J. Mol. Sci. 20 (22), 5758. doi:10.3390/ijms20225758
Wang, Q., Wang, W., Zhang, F., Deng, Y., and Long, Z. (2017d). NEAT1/miR‐181c Regulates Osteopontin (OPN)‐Mediated Synoviocyte Proliferation in Osteoarthritis. J. Cel. Biochem. 118 (11), 3775–3784. doi:10.1002/jcb.26025
Wang, T., Liu, Y., Wang, Y., Huang, X., Zhao, W., and Zhao, Z. (2019d). Long Non-coding RNA XIST Promotes Extracellular Matrix Degradation by Functioning as a Competing Endogenous RNA of miR-1277-5p in Osteoarthritis. Int. J. Mol. Med. 44 (2), 630–642. doi:10.3892/ijmm.2019.4240
Wang, W.-T., Huang, Z.-P., Sui, S., Liu, J.-H., Yu, D.-M., and Wang, W.-B. (2020b). microRNA-1236 Promotes Chondrocyte Apoptosis in Osteoarthritis via Direct Suppression of PIK3R3. Life Sci. 253, 117694. doi:10.1016/j.lfs.2020.117694
Wang, W. F., Liu, S. Y., Qi, Z. F., Lv, Z. H., Ding, H. R., and Zhou, W. J. (2020c). MiR-145 Targeting BNIP3 Reduces Apoptosis of Chondrocytes in Osteoarthritis through Notch Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 24 (16), 8263–8272. doi:10.26355/eurrev_202008_22622
Wang, X.-B., Zhao, F.-C., Yi, L.-H., Tang, J.-L., Zhu, Z.-Y., Pang, Y., et al. (2019e). MicroRNA-21-5p as a Novel Therapeutic Target for Osteoarthritis. Rheumatology (Oxford) 58, 1485–1497. doi:10.1093/rheumatology/kez102
Wang, X., Guo, Y., Wang, C., Yu, H., Yu, X., and Yu, H. (2016c). MicroRNA-142-3p Inhibits Chondrocyte Apoptosis and Inflammation in Osteoarthritis by Targeting HMGB1. Inflammation 39 (5), 1718–1728. doi:10.1007/s10753-016-0406-3
Wang, Y., Cao, L., Wang, Q., Huang, J., and Xu, S. (2019f). LncRNA FOXD2-AS1 Induces Chondrocyte Proliferation through Sponging miR-27a-3p in Osteoarthritis. Artif. Cell nanomedicine, Biotechnol. 47 (1), 1241–1247. doi:10.1080/21691401.2019.1596940
Wang, Y., and Kong, D. (2018). Retracted : MicroRNA‐136 Promotes Lipopolysaccharide‐induced ATDC5 Cell Injury and Inflammatory Cytokine Expression by Targeting Myeloid Cell Leukemia 1. J. Cel Biochem 119 (11), 9316–9326. doi:10.1002/jcb.27208
Wang, Y., Shen, S., Li, Z., Li, W., and Weng, X. (2020d). MIR-140-5p Affects Chondrocyte Proliferation, Apoptosis, and Inflammation by Targeting HMGB1 in Osteoarthritis. Inflamm. Res. 69 (1), 63–73. doi:10.1007/s00011-019-01294-0
Wang, Y., Wu, C., Yang, Y., Ren, Z., Lammi, M. J., and Guo, X. (2019g). Preliminary Exploration of Hsa_circ_0032131 Levels in Peripheral Blood as a Potential Diagnostic Biomarker of Osteoarthritis. Genet. Test. Mol. biomarkers 23 (10), 717–721. doi:10.1089/gtmb.2019.0036
Wang, Y., Wu, C., Zhang, F., Zhang, Y., Ren, Z., Lammi, M. J., et al. (2019h). Screening for Differentially Expressed Circular RNAs in the Cartilage of Osteoarthritis Patients for Their Diagnostic Value. Genet. Test. Mol. biomarkers 23 (10), 706–716. doi:10.1089/gtmb.2019.0108
Wang, Z., Hu, J., Pan, Y., Shan, Y., Jiang, L., Qi, X., et al. (2018b). miR-140-5p/miR-149 Affects Chondrocyte Proliferation, Apoptosis, and Autophagy by Targeting FUT1 in Osteoarthritis. Inflammation 41 (3), 959–971. doi:10.1007/s10753-018-0750-6
Wang, Z., Zhou, N., Wang, W., Yu, Y., Xia, L., and Li, N. (2021b). HDAC2 Interacts with microRNA-503-5p to Regulate SGK1 in Osteoarthritis. Arthritis Res. Ther. 23 (1), 78. doi:10.1186/s13075-020-02373-y
Wei, M., Xie, Q., Zhu, J., Wang, T., Zhang, F., Cheng, Y., et al. (2016). MicroRNA-33 Suppresses CCL2 Expression in Chondrocytes. Biosci. Rep. 36 (3), BSR20160068. doi:10.1042/BSR20160068
Wei, W., He, S., Wang, Z., Dong, J., Xiang, D., Li, Y., et al. (2019). LINC01534 Promotes the Aberrant Metabolic Dysfunction and Inflammation in IL-1β-Simulated Osteoarthritic Chondrocytes by Targeting miR-140-5p. Cartilage 2019, 194760351988878. doi:10.1177/1947603519888787
Wen, X., Li, H., Sun, H., Zeng, A., Lin, R., Zhao, J., et al. (2020). MiR-455-3p Reduces Apoptosis and Alleviates Degeneration of Chondrocyte through Regulating PI3K/AKT Pathway. Life Sci. 253, 117718. doi:10.1016/j.lfs.2020.117718
Woods, S., Barter, M. J., Elliott, H. R., McGillivray, C. M., Birch, M. A., Clark, I. M., et al. (2019). miR-324-5p Is up Regulated in End-Stage Osteoarthritis and Regulates Indian Hedgehog Signalling by Differing Mechanisms in Human and Mouse. Matrix Biol. 77, 87–100. doi:10.1016/j.matbio.2018.08.009
Wu, D.-P., Zhang, J.-L., Wang, J.-Y., Cui, M.-X., Jia, J.-L., Liu, X.-H., et al. (2017a). MiR-1246 Promotes LPS-Induced Inflammatory Injury in Chondrogenic Cells ATDC5 by Targeting HNF4γ. Cel Physiol Biochem 43 (5), 2010–2021. doi:10.1159/000484162
Wu, J., Zou, M., Ping, A., Deng, Z., and Cai, L. (2018a). MicroRNA-449a Upregulation Promotes Chondrocyte Extracellular Matrix Degradation in Osteoarthritis. Biomed. Pharmacother. 105, 940–946. doi:10.1016/j.biopha.2018.06.074
Wu, Q., Yuan, Z. H., Ma, X. B., and Tang, X. H. (2020). Low Expression of CircRNA HIPK3 Promotes Osteoarthritis Chondrocyte Apoptosis by Serving as a Sponge of miR-124 to Regulate SOX8. Eur. Rev. Med. Pharmacol. Sci. 24 (15), 7937–7945. doi:10.26355/eurrev_202008_22476
Wu, X.-F., Zhou, Z.-H., and Zou, J. (2017b). MicroRNA-181 Inhibits Proliferation and Promotes Apoptosis of Chondrocytes in Osteoarthritis by Targeting PTEN. Biochem. Cel Biol. 95 (3), 437–444. doi:10.1139/bcb-2016-0078
Wu, X., Crawford, R., Xiao, Y., Mao, X., and Prasadam, I. (2021a). Osteoarthritic Subchondral Bone Release Exosomes that Promote Cartilage Degeneration. Cells 10 (2), 251. doi:10.3390/cells10020251
Wu, Y. H., Liu, W., Zhang, L., Liu, X. Y., Wang, Y., Xue, B., et al. (2018b). Retracted : Effects of microRNA‐24 Targeting C‐myc on Apoptosis, Proliferation, and Cytokine Expressions in Chondrocytes of Rats with Osteoarthritis via MAPK Signaling Pathway. J. Cel. Biochem. 119 (10), 7944–7958. doi:10.1002/jcb.26514
Wu, Y., Hong, Z., Xu, W., Chen, J., Wang, Q., Chen, J., et al. (2021b). Circular RNA circPDE4D Protects against Osteoarthritis by Binding to miR-103a-3p and Regulating FGF18. Mol. Ther. 29 (1), 308–323. doi:10.1016/j.ymthe.2020.09.002
Wu, Y., Lu, X., Shen, B., and Zeng, Y. (2019). The Therapeutic Potential and Role of miRNA, lncRNA, and circRNA in Osteoarthritis. Curr. Gene Ther. 19 (4), 255–263. doi:10.2174/1566523219666190716092203
Wu, Y., Zhang, Y., Zhang, Y., and Wang, J.-J. (2017c). CircRNA Hsa_circ_0005105 Upregulates NAMPT Expression and Promotes Chondrocyte Extracellular Matrix Degradation by Sponging miR-26a. Cel Biol Int 41 (12), 1283–1289. doi:10.1002/cbin.10761
Xi, P., Zhang, C. l., Wu, S. y., Liu, L., Li, W. j., and Li, Y. m. (2021). CircRNA circ‐IQGAP1 Knockdown Alleviates Interleukin‐1β‐Induced Osteoarthritis Progression via Targeting miR‐671‐5p/TCF4. Orthop. Surg. 13 (3), 1036–1046. doi:10.1111/os.12923
Xiao, K., Xia, Z., Feng, B., Bian, Y., Fan, Y., Li, Z., et al. (2019a). Circular RNA Expression Profile of Knee Condyle in Osteoarthritis by Illumina HiSeq Platform. J. Cel Biochem 120 (10), 17500–17511. doi:10.1002/jcb.29014
Xiao, P., Zhu, X., Sun, J., Zhang, Y., Qiu, W., Li, J., et al. (2021). LncRNA NEAT1 Regulates Chondrocyte Proliferation and Apoptosis via Targeting miR-543/PLA2G4A axis. Hum. Cel. 34 (1), 60–75. doi:10.1007/s13577-020-00433-8
Xiao, Y., Bao, Y., Tang, L., and Wang, L. (2019b). LncRNA MIR4435-2HG Is Downregulated in Osteoarthritis and Regulates Chondrocyte Cell Proliferation and Apoptosis. J. Orthop. Surg. Res. 14 (1), 247. doi:10.1186/s13018-019-1278-7
Xiao, Y., Yan, X., Yang, Y., and Ma, X. (2019c). Downregulation of Long Noncoding RNA HOTAIRM1 Variant 1 Contributes to Osteoarthritis via Regulating miR-125b/BMPR2 axis and Activating JNK/MAPK/ERK Pathway. Biomed. Pharmacother. 109, 1569–1577. doi:10.1016/j.biopha.2018.10.181
Xing, D., Liang, J.-q., Li, Y., Lu, J., Jia, H.-b., Xu, L.-y., et al. (2014). Identification of Long Noncoding RNA Associated with Osteoarthritis in Humans. Orthopaedic Surg. 6 (4), 288–293. doi:10.1111/os.12147
Xu, B., Li, Y.-y., Ma, J., and Pei, F.-x. (2016). Roles of microRNA and Signaling Pathway in Osteoarthritis Pathogenesis. J. Zhejiang Univ. Sci. B 17 (3), 200–208. doi:10.1631/jzus.B1500267
Xu, J., and Ma, X. (2021). Hsa_circ_0032131 Knockdown Inhibits Osteoarthritis Progression via the miR-502-5p/PRDX3 axis. Aging 13 (11), 15100–15113. doi:10.18632/aging.203073
Xu, J., Pei, Y., Lu, J., Liang, X., Li, Y., Wang, J., et al. (2021). LncRNA SNHG7 Alleviates IL-1β-induced Osteoarthritis by Inhibiting miR-214-5p-Mediated PPARGC1B Signaling Pathways. Int. immunopharmacology 90, 107150. doi:10.1016/j.intimp.2020.107150
Xu, J., and Xu, Y. (2017). The lncRNA MEG3 Downregulation Leads to Osteoarthritis Progression via miR-16/SMAD7 axis. Cell Biosci 7, 69. doi:10.1186/s13578-017-0195-x
Xu, K., Meng, Z., Xian, X.-M., Deng, M.-H., Meng, Q.-G., Fang, W., et al. (2020). LncRNA PVT1 Induces Chondrocyte Apoptosis through Upregulation of TNF-α in Synoviocytes by Sponging miR-211-3p. Mol. Cell. probes 52, 101560. doi:10.1016/j.mcp.2020.101560
Xue, H., Yu, P., Wang, W. Z., Niu, Y. Y., and Li, X. (2020). The Reduced lncRNA NKILA Inhibited Proliferation and Promoted Apoptosis of Chondrocytes via miR-145/sp1/nf-Κb Signaling in Human Osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 24 (2), 535–548. doi:10.26355/eurrev_202001_20030
Xue, H., Tu, Y., Ma, T., Wen, T., Yang, T., Xue, L., et al. (2019). miR-93-5p Attenuates IL-1β-induced Chondrocyte Apoptosis and Cartilage Degradation in Osteoarthritis Partially by Targeting TCF4. Bone 123, 129–136. doi:10.1016/j.bone.2019.03.035
Yan, S., Wang, M., Zhao, J., Zhang, H., Zhou, C., Jin, L., et al. (2016). MicroRNA-34a Affects Chondrocyte Apoptosis and Proliferation by Targeting the SIRT1/p53 Signaling Pathway during the Pathogenesis of Osteoarthritis. Int. J. Mol. Med. 38 (1), 201–209. doi:10.3892/ijmm.2016.2618
Yang, B., Ni, J., Long, H., Huang, J., Yang, C., and Huang, X. (2018a). IL‐1β‐induced miR‐34a Up‐regulation Inhibits Cyr61 to Modulate Osteoarthritis Chondrocyte Proliferation through ADAMTS‐4. J. Cel. Biochem. 119 (10), 7959–7970. doi:10.1002/jcb.26600
Yang, B., Xu, L., and Wang, S. (2020a). Regulation of lncRNA-H19/miR-140-5p in Cartilage Matrix Degradation and Calcification in Osteoarthritis. Ann. Palliat. Med. 9 (4), 1896–1904. doi:10.21037/apm-20-929
Yang, D. W., Zhang, X., Qian, G. B., Jiang, M. J., Wang, P., and Wang, K. Z. (2019a). Downregulation of Long Noncoding RNA LOC101928134 Inhibits the Synovial Hyperplasia and Cartilage Destruction of Osteoarthritis Rats through the Activation of the Janus Kinase/signal Transducers and Activators of Transcription Signaling Pathway by Upregulating IFNA1. J. Cel Physiol 234 (7), 10523–10534. doi:10.1002/jcp.27730
Yang, F., Huang, R., Ma, H., Zhao, X., and Wang, G. (2020b). miRNA-411 Regulates Chondrocyte Autophagy in Osteoarthritis by Targeting Hypoxia-Inducible Factor 1 Alpha (HIF-1α). Med. Sci. Monit. 26, e921155. doi:10.12659/MSM.921155
Yang, G., Tang, K., Qiao, L., Li, Y., and Sun, S. (2021a). Identification of Critical Genes and lncRNAs in Osteolysis after Total Hip Arthroplasty and Osteoarthritis by RNA Sequencing. Biomed. Research International 2021, 1–13. doi:10.1155/2021/6681925
Yang, H., Wu, D., Li, H., Chen, N., and Shang, Y. (2018b). Downregulation of microRNA-448 Inhibits IL-1β-induced Cartilage Degradation in Human Chondrocytes via Upregulation of Matrilin-3. Cell Mol Biol Lett 23, 7. doi:10.1186/s11658-018-0072-6
Yang, Q., Yao, Y., Zhao, D., Zou, H., Lai, C., Xiang, G., et al. (2021b). LncRNA H19 Secreted by Umbilical Cord Blood Mesenchymal Stem Cells through microRNA-29a-3p/FOS axis for central Sensitization of Pain in Advanced Osteoarthritis. Am. J. Transl Res. 13 (3), 1245–1256.
Yang, Q., Zhou, Y., Cai, P., Fu, W., Wang, J., Wei, Q., et al. (2019b). Downregulation of microRNA-23b-3p Alleviates IL-1β-induced Injury in Chondrogenic CHON-001 Cells. Drug Des. Devel Ther. 13, 2503–2512. doi:10.2147/DDDT.S211051
Yang, Y., Shen, P., Yao, T., Ma, J., Chen, Z., Zhu, J., et al. (2021c). Novel Role of circRSU1 in the Progression of Osteoarthritis by Adjusting Oxidative Stress. Theranostics 11 (4), 1877–1900. doi:10.7150/thno.53307
Yang, Y., Xing, D., Wang, Y., Jia, H., Li, B., and Li, J. J. (2020c). A Long Non-coding RNA, HOTAIR, Promotes Cartilage Degradation in Osteoarthritis by Inhibiting WIF-1 Expression and Activating Wnt Pathway. BMC Mol. Cel Biol 21 (1), 53. doi:10.1186/s12860-020-00299-6
Yang, Y., Yujiao, W., Fang, W., Linhui, Y., Ziqi, G., Zhichen, W., et al. (2020d). The Roles of miRNA, lncRNA and circRNA in the Development of Osteoporosis. Biol. Res. 53 (1), 40. doi:10.1186/s40659-020-00309-z
Yang, Z., Tang, Y., Lu, H., Shi, B., Ye, Y., Xu, G., et al. (2018c). Retracted : Long Non‐coding RNA Reprogramming (lncRNA‐ROR) Regulates Cell Apoptosis and Autophagy in Chondrocytes. J. Cel. Biochem. 119 (10), 8432–8440. doi:10.1002/jcb.27057
Yao, Q., Chen, Y., and Zhou, X. (2019). The Roles of microRNAs in Epigenetic Regulation. Curr. Opin. Chem. Biol. 51, 11–17. doi:10.1016/j.cbpa.2019.01.024
Ye, D., Jian, W., Feng, J., and Liao, X. (2018). Role of Long Noncoding RNA ZFAS1 in Proliferation, Apoptosis and Migration of Chondrocytes in Osteoarthritis. Biomed. Pharmacother. 104, 825–831. doi:10.1016/j.biopha.2018.04.124
Ying, H., Wang, Y., Gao, Z., and Zhang, Q. (2019). Long Non-coding RNA Activated by Transforming Growth Factor Beta Alleviates Lipopolysaccharide-Induced Inflammatory Injury via Regulating microRNA-223 in ATDC5 Cells. Int. immunopharmacology 69, 313–320. doi:10.1016/j.intimp.2019.01.056
You, D., Yang, C., Huang, J., Gong, H., Yan, M., and Ni, J. (2019). Long Non-coding RNA MEG3 Inhibits Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells by Epigenetically Inhibiting TRIB2 via Methyltransferase EZH2. Cell Signal. 63, 109379. doi:10.1016/j.cellsig.2019.109379
Yu, C., Shi, D., Li, Z., Wan, G., and Shi, X. (2019a). Retracted : Long Noncoding RNA CHRF Exacerbates IL‐6‐induced Inflammatory Damages by Downregulating microRNA‐146a in ATDC5 Cells. J. Cel Physiol 234 (12), 21851–21859. doi:10.1002/jcp.28749
Yu, C., and Wang, Y. (2018). RETRACTED: MicroRNA-19a Promotes Cell Viability and Migration of Chondrocytes via Up-Regulating SOX9 through NF-Κb Pathway. Biomed. Pharmacother. 98, 746–753. doi:10.1016/j.biopha.2017.11.132
Yu, J., Qin, Y., and Zhou, N. (2021). Knockdown of Circ_SLC39A8 Protects against the Progression of Osteoarthritis by Regulating miR-591/IRAK3 axis. J. Orthop. Surg. Res. 16 (1), 170. doi:10.1186/s13018-021-02323-7
Yu, Q., Zhao, B., He, Q., Zhang, Y., and Peng, X. B. (2019b). microRNA‐206 Is Required for Osteoarthritis Development through its Effect on Apoptosis and Autophagy of Articular Chondrocytes via Modulating the Phosphoinositide 3‐kinase/protein Kinase B‐mTOR Pathway by Targeting Insulin‐like Growth Factor‐1. J. Cel Biochem 120 (4), 5287–5303. doi:10.1002/jcb.27803
Yu, X. P., Liu, C. G., Qiu, F., Xu, Y. Q., Xing, F., Yin, J. Q., et al. (2020). CircRNA_100395 Protects Breast Carcinoma Deterioration by Targeting MAPK6. Eur. Rev. Med. Pharmacol. Sci. 24 (23), 12216–12223. doi:10.26355/eurrev_202012_24012
Zang, J., Lu, D., and Xu, A. (2020). The Interaction of circRNAs and RNA Binding Proteins: An Important Part of circRNA Maintenance and Function. J. Neurosci. Res. 98 (1), 87–97. doi:10.1002/jnr.24356
Zhai, X., Meng, R., Li, H., Li, J., Jing, L., Qin, L., et al. (2017). miR-181a Modulates Chondrocyte Apoptosis by Targeting Glycerol-3-Phosphate Dehydrogenase 1-Like Protein (GPD1L) in Osteoarthritis. Med. Sci. Monit. 23, 1224–1231. doi:10.12659/msm.899228
Zhang, B., Sun, M., Wang, J., Ma, C., Hao, T., Liu, G., et al. (2019a). MiR-671 Ameliorates the Progression of Osteoarthritis In Vitro and In Vivo. Pathol. - Res. Pract. 215 (7), 152423. doi:10.1016/j.prp.2019.04.015
Zhang, C.-Y., Yang, C.-Q., Chen, Q., Liu, J., Zhang, G., Dong, C., et al. (2021a). miR-194-Loaded Gelatin Nanospheres Target MEF2C to Suppress Muscle Atrophy in a Mechanical Unloading Model. Mol. Pharmaceutics 18 (8), 2959–2973. doi:10.1021/acs.molpharmaceut.1c00121
Zhang, C., Wang, P., Jiang, P., Lv, Y., Dong, C., Dai, X., et al. (2016a). Upregulation of lncRNA HOTAIR Contributes to IL-1β-induced MMP Overexpression and Chondrocytes Apoptosis in Temporomandibular Joint Osteoarthritis. Gene 586 (2), 248–253. doi:10.1016/j.gene.2016.04.016
Zhang, C., Zhang, Z., Chang, Z., Mao, G., Hu, S., Zeng, A., et al. (2019b). miR‐193b‐5p Regulates Chondrocytes Metabolism by Directly Targeting Histone Deacetylase 7 in Interleukin‐1β‐induced Osteoarthritis. J. Cel Biochem 120 (8), 12775–12784. doi:10.1002/jcb.28545
Zhang, D., Cao, X., Li, J., and Zhao, G. (2015). MiR-210 Inhibits NF-Κb Signaling Pathway by Targeting DR6 in Osteoarthritis. Sci. Rep. 5, 12775. doi:10.1038/srep12775
Zhang, D., Wang, K., Wei, W., Liu, Y., and Liu, S. (2021b). Multifunctional Plasmonic Core-Satellites Nanoprobe for Cancer Diagnosis and Therapy Based on a Cascade Reaction Induced by MicroRNA. Anal. Chem. 93 (27), 9521–9530. doi:10.1021/acs.analchem.1c01539
Zhang, F. e., Lammi, M. J., Tan, S., Meng, P., Wu, C., and Guo, X. (2020a). Cell Cycle-Related lncRNAs and mRNAs in Osteoarthritis Chondrocytes in a Northwest Chinese Han Population. Medicine 99 (24), e19905. doi:10.1097/MD.0000000000019905
Zhang, F. Q., Wang, Z., Zhang, H., Liu, L., Luo, X. L., and Liu, W. W. (2019c). MiR-27a Alleviates Osteoarthritis in Rabbits via Inhibiting Inflammation. Eur. Rev. Med. Pharmacol. Sci. 23 (3 Suppl. l), 89–95. doi:10.26355/eurrev_201908_18634
Zhang, F., Zhang, R., Zhang, X., Wu, Y., Li, X., Zhang, S., et al. (2018a). Comprehensive Analysis of circRNA Expression Pattern and circRNA-miRNA-mRNA Network in the Pathogenesis of Atherosclerosis in Rabbits. Aging 10 (9), 2266–2283. doi:10.18632/aging.101541
Zhang, G., Sun, Y., Wang, Y., Liu, R., Bao, Y., and Li, Q. (2016b). MiR-502-5p Inhibits IL-1β-induced Chondrocyte Injury by Targeting TRAF2. Cell Immunol. 302, 50–57. doi:10.1016/j.cellimm.2016.01.007
Zhang, G., Wu, Y., Xu, D., and Yan, X. (2016c). Long Noncoding RNA UFC1 Promotes Proliferation of Chondrocyte in Osteoarthritis by Acting as a Sponge for miR-34a. DNA Cel. Biol. 35 (11), 691–695. doi:10.1089/dna.2016.3397
Zhang, G., Zhang, Q., Zhu, J., Tang, J., and Nie, M. (2020b). LncRNA ARFRP1 Knockdown Inhibits LPS-Induced the Injury of Chondrocytes by Regulation of NF-Κb Pathway through Modulating miR-15a-5p/TLR4 axis. Life Sci. 261, 118429. doi:10.1016/j.lfs.2020.118429
Zhang, G., Zhou, Y., Su, M., Yang, X., and Zeng, B. (2020c). Inhibition of microRNA ‐27b‐3p Relieves Osteoarthritis Pain via Regulation of KDM4B ‐dependent DLX5. BioFactors 46 (5), 788–802. doi:10.1002/biof.1670
Zhang, H., Chen, C., Cui, Y., Li, Y., Wang, Z., Mao, X., et al. (2019d). lnc-SAMD14-4 Can Regulate Expression of the COL1A1 and COL1A2 in Human Chondrocytes. PeerJ 7, e7491. doi:10.7717/peerj.7491
Zhang, H., Li, J., Shao, W., and Shen, N. (2020d). LncRNA CTBP1-AS2 Is Upregulated in Osteoarthritis and Increases the Methylation of miR-130a Gene to Inhibit Chondrocyte Proliferation. Clin. Rheumatol. 39 (11), 3473–3478. doi:10.1007/s10067-020-05113-4
Zhang, H., Li, J., Shao, W., and Shen, N. (2020e). LncRNA SNHG9 Is Downregulated in Osteoarthritis and Inhibits Chondrocyte Apoptosis by Downregulating miR-34a through Methylation. BMC Musculoskelet. Disord. 21 (1), 511. doi:10.1186/s12891-020-03497-7
Zhang, J., Cheng, F., Rong, G., Tang, Z., and Gui, B. (2020f). Hsa_circ_0005567 Activates Autophagy and Suppresses IL-1β-Induced Chondrocyte Apoptosis by Regulating miR-495. Front. Mol. Biosci. 7, 216. doi:10.3389/fmolb.2020.00216
Zhang, L. L. (2020). CircRNA-PTPRA Promoted the Progression of Atherosclerosis through Sponging with miR-636 and Upregulating the Transcription Factor SP1. Eur. Rev. Med. Pharmacol. Sci. 24 (23), 12437–12449. doi:10.26355/eurrev_202012_24039
Zhang, L., Zhang, P., Sun, X., Zhou, L., and Zhao, J. (2018b). Long Non-coding RNA DANCR Regulates Proliferation and Apoptosis of Chondrocytes in Osteoarthritis via miR-216a-5p-JAK2-STAT3 axis. Biosci. Rep. 38 (6), BSR20181228. doi:10.1042/BSR20181228
Zhang, P., Gao, G., Zhou, Z., and He, X. (2021c). microRNA-130b Downregulation Potentiates Chondrogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Targeting SOX9. Braz. J. Med. Biol. Res. 54 (4), e10345. doi:10.1590/1414-431X202010345
Zhang, P., Sun, J., Liang, C., Gu, B., Xu, Y., Lu, H., et al. (2020g). lncRNA IGHCγ1 Acts as a ceRNA to Regulate Macrophage Inflammation via the miR-6891-3p/TLR4 Axis in Osteoarthritis. Mediators Inflamm. 2020, 1–11. doi:10.1155/2020/9743037
Zhang, Q., Qiao, X., and Xia, W. (2020h). CircSERPINE2 Weakens IL-1β-caused Apoptosis and Extracellular Matrix Degradation of Chondrocytes by Regulating miR-495/TGFBR2 axis. Biosci. Rep. 40 (11), BSR20201601. doi:10.1042/BSR20201601
Zhang, Q., Wang, Y., Zhang, M., and Ying, H. (2019e). Retracted : Green tea Polyphenols Attenuate LPS‐induced Inflammation through Upregulating microRNA‐9 in Murine Chondrogenic ATDC5 Cells. J. Cel Physiol 234 (12), 22604–22612. doi:10.1002/jcp.28826
Zhang, W., Cheng, P., Hu, W., Yin, W., Guo, F., Chen, A., et al. (2020i). Correction: Inhibition of microRNA-384-5p Alleviates Osteoarthritis through its Effects on Inhibiting Apoptosis of Cartilage Cells via the NF-Κb Signaling Pathway by Targeting SOX9. Cancer Gene Ther. 27 (10-11), 836–837. doi:10.1038/s41417-020-0202-y
Zhang, W., Cheng, P., Hu, W., Yin, W., Guo, F., Chen, A., et al. (2018c). Downregulated microRNA‐340‐5p Promotes Proliferation and Inhibits Apoptosis of Chondrocytes in Osteoarthritis Mice through Inhibiting the Extracellular Signal‐regulated Kinase Signaling Pathway by Negatively Targeting the FMOD Gene. J. Cel Physiol 234 (1), 927–939. doi:10.1002/jcp.26921
Zhang, W., Hsu, P., Zhong, B., Guo, S., Zhang, C., Wang, Y., et al. (2018d). MiR-34a Enhances Chondrocyte Apoptosis, Senescence and Facilitates Development of Osteoarthritis by Targeting DLL1 and Regulating PI3K/AKT Pathway. Cel Physiol Biochem 48 (3), 1304–1316. doi:10.1159/000492090
Zhang, W., Hu, C., Zhang, C., Luo, C., Zhong, B., and Yu, X. (2021d). MiRNA-132 Regulates the Development of Osteoarthritis in Correlation with the Modulation of PTEN/PI3K/AKT Signaling. BMC Geriatr. 21 (1), 175. doi:10.1186/s12877-021-02046-8
Zhang, W., Qi, L., Chen, R., He, J., Liu, Z., Wang, W., et al. (2021e). Circular RNAs in Osteoarthritis: Indispensable Regulators and Novel Strategies in Clinical Implications. Arthritis Res. Ther. 23 (1), 23. doi:10.1186/s13075-021-02420-2
Zhang, W., Zhang, C., Hu, C., Luo, C., Zhong, B., and Yu, X. (2020j). Circular RNA-CDR1as Acts as the Sponge of microRNA-641 to Promote Osteoarthritis Progression. J. Inflamm. 17, 8. doi:10.1186/s12950-020-0234-y
Zhang, W., Zhong, B., Zhang, C., Luo, C., and Zhan, Y. (2018e). miR-373 Regulates Inflammatory Cytokine-Mediated Chondrocyte Proliferation in Osteoarthritis by Targeting the P2X7 Receptor. FEBS open bio 8 (3), 325–331. doi:10.1002/2211-5463.12345
Zhang, X., Huang, C.-R., Pan, S., Pang, Y., Chen, Y.-S., Zha, G.-C., et al. (2020k). Long Non-coding RNA SNHG15 Is a Competing Endogenous RNA of miR-141-3p that Prevents Osteoarthritis Progression by Upregulating BCL2L13 Expression. Int. immunopharmacology 83, 106425. doi:10.1016/j.intimp.2020.106425
Zhang, X., Liang, H., Kourkoumelis, N., Wu, Z., Li, G., and Shang, X. (2020l). Comprehensive Analysis of lncRNA and miRNA Expression Profiles and ceRNA Network Construction in Osteoporosis. Calcif Tissue Int. 106 (4), 343–354. doi:10.1007/s00223-019-00643-9
Zhang, X., Wang, C., Zhao, J., Xu, J., Geng, Y., Dai, L., et al. (2017). miR-146a Facilitates Osteoarthritis by Regulating Cartilage Homeostasis via Targeting Camk2d and Ppp3r2. Cel Death Dis 8 (4), e2734. doi:10.1038/cddis.2017.146
Zhang, X., Zhu, X.-L., Ji, B.-Y., Cao, X., Yu, L.-J., Zhang, Y., et al. (2019f). LncRNA-1810034E14Rik Reduces Microglia Activation in Experimental Ischemic Stroke. J. Neuroinflammation 16 (1), 75. doi:10.1186/s12974-019-1464-x
Zhang, Y., Dong, Q., and Sun, X. (2020m). Positive Feedback Loop LINC00511/miR-150-5p/SP1 Modulates Chondrocyte Apoptosis and Proliferation in Osteoarthritis. DNA Cel. Biol. 39 (9), 1506–1512. doi:10.1089/dna.2020.5718
Zhang, Y., Jia, J., Yang, S., Liu, X., Ye, S., and Tian, H. (2014). MicroRNA-21 Controls the Development of Osteoarthritis by Targeting GDF-5 in Chondrocytes. Exp. Mol. Med. 46, e79. doi:10.1038/emm.2013.152
Zhang, Y., Ma, L., Wang, C., Wang, L., Guo, Y., and Wang, G. (2020n). Long Noncoding RNA LINC00461 Induced Osteoarthritis Progression by Inhibiting miR-30a-5p. Aging 12 (5), 4111–4123. doi:10.18632/aging.102839
Zhang, Y., Wang, F., Chen, G., He, R., and Yang, L. (2019g). LncRNA MALAT1 Promotes Osteoarthritis by Modulating miR-150-5p/AKT3 axis. Cel Biosci 9, 54. doi:10.1186/s13578-019-0302-2
Zhang, Z.-Y., Gao, X.-H., Ma, M.-Y., Zhao, C.-L., Zhang, Y.-L., and Guo, S.-S. (2020o). CircRNA_101237 Promotes NSCLC Progression via the miRNA-490-3p/MAPK1 axis. Sci. Rep. 10 (1), 9024. doi:10.1038/s41598-020-65920-2
Zhao, C., Wang, Y., Jin, H., and Yu, T. (2017). Knockdown of microRNA-203 Alleviates LPS-Induced Injury by Targeting MCL-1 in C28/I2 Chondrocytes. Exp. Cel. Res. 359 (1), 171–178. doi:10.1016/j.yexcr.2017.07.034
Zhao, G., and Gu, W. (2020). Effects of miR-146a-5p on Chondrocyte Interleukin-1β-Induced Inflammation and Apoptosis Involving Thioredoxin Interacting Protein Regulation. J. Int. Med. Res. 48 (11), 030006052096955. doi:10.1177/0300060520969550
Zhao, H., and Gong, N. (2019). miR-20a Regulates Inflammatory in Osteoarthritis by Targeting the IκBβ and Regulates NK-Κb Signaling Pathway Activation. Biochem. biophysical Res. Commun. 518 (4), 632–637. doi:10.1016/j.bbrc.2019.08.109
Zhao, J., Li, T., and Luo, W. (2021). Silencing of Circ-PRKCH Protects against Lipopolysaccharide (LPS)-evoked Chondrocyte Damage and Extracellular Matrix Loss by the miR-140-3p/ADAM10 axis. Gen. Physiol. Biophys. 40 (2), 89–101. doi:10.4149/gpb_2021001
Zhao, Q., Yang, Y., Ren, G., Ge, E., and Fan, C. (2019a). Integrating Bipartite Network Projection and KATZ Measure to Identify Novel CircRNA-Disease Associations. IEEE Trans.on Nanobioscience 18 (4), 578–584. doi:10.1109/TNB.2019.2922214
Zhao, X., Li, H., and Wang, L. (2019b). MicroRNA-107 Regulates Autophagy and Apoptosis of Osteoarthritis Chondrocytes by Targeting TRAF3. Int. immunopharmacology 71, 181–187. doi:10.1016/j.intimp.2019.03.005
Zhao, Y., Zhao, J., Guo, X., She, J., and Liu, Y. (2018). Long Non-coding RNA PVT1, a Molecular Sponge for miR-149, Contributes Aberrant Metabolic Dysfunction and Inflammation in IL-1β-simulated Osteoarthritic Chondrocytes. Biosci. Rep. 38 (5), BSR20180576. doi:10.1042/BSR20180576
Zhao, Z., Dai, X.-S., Wang, Z.-Y., Bao, Z.-Q., and Guan, J.-Z. (2019c). MicroRNA-26a Reduces Synovial Inflammation and Cartilage Injury in Osteoarthritis of Knee Joints through Impairing the NF-Κb Signaling Pathway. Biosci. Rep. 39 (4), BSR20182025. doi:10.1042/BSR20182025
Zheng, W., Hou, G., and Li, Y. (2021). Circ_0116061 Regulated the Proliferation, Apoptosis, and Inflammation of Osteoarthritis Chondrocytes through Regulating the miR-200b-3p/SMURF2 axis. J. Orthop. Surg. Res. 16 (1), 253. doi:10.1186/s13018-021-02391-9
Zheng, X., Zhao, F.-C., Pang, Y., Li, D.-Y., Yao, S.-C., Sun, S.-S., et al. (2017). Downregulation of miR-221-3p Contributes to IL-1β-induced Cartilage Degradation by Directly Targeting the SDF1/CXCR4 Signaling Pathway. J. Mol. Med. 95 (6), 615–627. doi:10.1007/s00109-017-1516-6
Zhi, L., Zhao, J., Zhao, H., Qing, Z., Liu, H., and Ma, J. (2020). Downregulation of LncRNA OIP5-AS1 Induced by IL-1β Aggravates Osteoarthritis via Regulating miR-29b-3p/PGRN. Cartilage 2020, 194760351990080. doi:10.1177/1947603519900801
Zhong, J.-H., Li, J., Liu, C.-F., Liu, N., Bian, R.-X., Zhao, S.-M., et al. (2017). Effects of microRNA-146a on the Proliferation and Apoptosis of Human Osteoarthritis Chondrocytes by Targeting TRAF6 through the NF-Κb Signalling Pathway. Biosci. Rep. 37 (2), BSR20160578. doi:10.1042/BSR20160578
Zhou, B., Li, H., and Shi, J. (2017). miR-27 Inhibits the NF-Κb Signaling Pathway by Targeting Leptin in Osteoarthritic Chondrocytes. Int. J. Mol. Med. 40 (2), 523–530. doi:10.3892/ijmm.2017.3021
Zhou, C., He, T., and Chen, L. (2021a). LncRNA CASC19 Accelerates Chondrocytes Apoptosis and Proinflammatory Cytokine Production to Exacerbate Osteoarthritis Development through Regulating the miR-152-3p/DDX6 axis. J. Orthop. Surg. Res. 16 (1), 399. doi:10.1186/s13018-021-02543-x
Zhou, J. L., Deng, S., Fang, H. S., Du, X. j., Peng, H., and Hu, Q. j. (2021b). Circular RNA circANKRD36 Regulates Casz1 by Targeting miR‐599 to Prevent Osteoarthritis Chondrocyte Apoptosis and Inflammation. J. Cel. Mol. Med. 25 (1), 120–131. doi:10.1111/jcmm.15884
Zhou, J. X., Tian, Z. G., Zhu, L. F., Wu, W. D., Zhou, S. L., Zhao, Y. T., et al. (2018a). MicroRNA-615-3p Promotes the Osteoarthritis Progression by Inhibiting Chondrogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Eur. Rev. Med. Pharmacol. Sci. 22 (19), 6212–6220. doi:10.26355/eurrev_201810_16027
Zhou, L., Gu, M., Ma, X., Wen, L., Zhang, B., Lin, Y., et al. (2021c). Long Non-coding RNA PCAT-1 Regulates Apoptosis of Chondrocytes in Osteoarthritis by Sponging miR-27b-3p. J. Bone Miner Metab. 39 (2), 139–147. doi:10.1007/s00774-020-01128-8
Zhou, M., Zhang, Z., Zhao, H., Bao, S., Cheng, L., and Sun, J. (2018b). An Immune-Related Six-lncRNA Signature to Improve Prognosis Prediction of Glioblastoma Multiforme. Mol. Neurobiol. 55 (5), 3684–3697. doi:10.1007/s12035-017-0572-9
Zhou, X., Jiang, L., Fan, G., Yang, H., Wu, L., Huang, Y., et al. (2019a). Role of the ciRS-7/miR-7 axis in the Regulation of Proliferation, Apoptosis and Inflammation of Chondrocytes Induced by IL-1β. Int. immunopharmacology 71, 233–240. doi:10.1016/j.intimp.2019.03.037
Zhou, X., Li, J., Zhou, Y., Yang, Z., Yang, H., Li, D., et al. (2020a). Down-regulated ciRS-7/up-Regulated miR-7 axis Aggravated Cartilage Degradation and Autophagy Defection by PI3K/AKT/mTOR Activation Mediated by IL-17A in Osteoarthritis. Aging 12 (20), 20163–20183. doi:10.18632/aging.103731
Zhou, X., Luo, D., Sun, H., Qi, Y., Xu, W., Jin, X., et al. (2018c). MiR‐132‐3p Regulates ADAMTS‐5 Expression and Promotes Chondrogenic Differentiation of Rat Mesenchymal Stem Cells. J. Cel. Biochem. 119 (3), 2579–2587. doi:10.1002/jcb.26421
Zhou, Y., Ming, J., Li, Y., Li, B., Deng, M., Ma, Y., et al. (2021d). Exosomes Derived from miR-126-3p-Overexpressing Synovial Fibroblasts Suppress Chondrocyte Inflammation and Cartilage Degradation in a Rat Model of Osteoarthritis. Cell Death Discov. 7 (1), 37. doi:10.1038/s41420-021-00418-y
Zhou, Y., Wang, Z., Chen, X., Zhang, J., Yang, L., Liu, S., et al. (2020b). Identification of Differentially Expressed miRNAs and mRNAs in Synovial of Osteoarthritis via RNA-Sequencing. BMC Med. Genet. 21 (1), 46. doi:10.1186/s12881-020-0978-5
Zhou, Z.-B., Du, D., Huang, G.-X., Chen, A., and Zhu, L. (2018d). Circular RNA Atp9b, a Competing Endogenous RNA, Regulates the Progression of Osteoarthritis by Targeting miR-138-5p. Gene 646, 203–209. doi:10.1016/j.gene.2017.12.064
Zhou, Z.-B., Huang, G.-X., Fu, Q., Han, B., Lu, J.-J., Chen, A.-M., et al. (2019b). circRNA.33186 Contributes to the Pathogenesis of Osteoarthritis by Sponging miR-127-5p. Mol. Ther. 27 (3), 531–541. doi:10.1016/j.ymthe.2019.01.006
Zhou, Z., Du, D., Chen, A., and Zhu, L. (2018e). Circular RNA Expression Profile of Articular Chondrocytes in an IL-1β-induced Mouse Model of Osteoarthritis. Gene 644, 20–26. doi:10.1016/j.gene.2017.12.020
Zhou, Z., Ma, J., Lu, J., Chen, A., and Zhu, L. (2021e). Circular RNA CircCDH13 Contributes to the Pathogenesis of Osteoarthritis via CircCDH13/miR‐296‐3p/PTEN axis. J. Cel Physiol 236 (5), 3521–3535. doi:10.1002/jcp.30091
Zhu, H., Hu, Y., Wang, C., Zhang, X., and He, D. (2020). CircGCN1L1 Promotes Synoviocyte Proliferation and Chondrocyte Apoptosis by Targeting miR-330-3p and TNF-α in TMJ Osteoarthritis. Cel Death Dis 11 (4), 284. doi:10.1038/s41419-020-2447-7
Zhu, J., Fu, H., Wu, Y., and Zheng, X. (2013). Function of lncRNAs and Approaches to lncRNA-Protein Interactions. Sci. China Life Sci. 56 (10), 876–885. doi:10.1007/s11427-013-4553-6
Zhu, J. K., He, T. D., Wei, Z. X., and Wang, Y. M. (2018). LncRNA FAS-AS1 Promotes the Degradation of Extracellular Matrix of Cartilage in Osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 22 (10), 2966–2972. doi:10.26355/eurrev_201805_15051
Zhu, Y. J., and Jiang, D. M. (2019). LncRNA PART1 Modulates Chondrocyte Proliferation, Apoptosis, and Extracellular Matrix Degradation in Osteoarthritis via Regulating miR-373-3p/SOX4 axis. Eur. Rev. Med. Pharmacol. Sci. 23 (19), 8175–8185. doi:10.26355/eurrev_201910_19124
Zhu, Y., Li, R., and Wen, L.-M. (2021). Long Non-coding RNA XIST Regulates Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells from Temporomandibular Joint via miR-27b-3p/ADAMTS-5 axis. Cytokine 137, 155352. doi:10.1016/j.cyto.2020.155352
Keywords: osteoarthritis, miRNA, lncRNA, circRNA, lncRNA/circRNA-miRNA-mRNA axis
Citation: Kong H, Sun M-L, Zhang X-A and Wang X-Q (2021) Crosstalk Among circRNA/lncRNA, miRNA, and mRNA in Osteoarthritis. Front. Cell Dev. Biol. 9:774370. doi: 10.3389/fcell.2021.774370
Received: 11 September 2021; Accepted: 29 November 2021;
Published: 15 December 2021.
Edited by:
Jing Zhang, Shanghai Jiao Tong University, ChinaReviewed by:
Lei Zhao, University of Wisconsin-Madison, United StatesCopyright © 2021 Kong, Sun, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-An Zhang, emhhbmd4YTI3MjVAMTYzLmNvbQ==; Xue-Qiang Wang, cWlhbmc4OTdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.