
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 15 October 2021
Sec. Molecular and Cellular Oncology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.753706
This article is part of the Research Topic Non-coding RNAs in Gastrointestinal and Gynecological Cancers: New Insights into the Mechanisms of Cancer Therapeutic Resistance View all 22 articles
Long non-coding RNA (lncRNA) DANCR (also known as ANCR)—differentiation antagonizing non-protein coding RNA, was first reported in 2012 to suppress differentiation of epithelial cells. Emerging evidence demonstrates that DANCR is a cancer-associated lncRNA abnormally expressed in many cancers (e.g., lung cancer, gastric cancer, breast cancer, hepatocellular carcinoma). Increasing studies suggest that the dysregulation of DANCR plays critical roles in cancer cell proliferation, apoptosis, migration, invasion, and chemoresistance in vitro and tumor growth and metastasis in vivo. Mechanistic analyses show that DANCR can serve as miRNA sponges, stabilize mRNAs, and interact with proteins. Recent research reveals that DANCR can be detected in many body fluids such as serum, plasma, and exosomes, providing a quick and convenient method for cancer monitor. Thus DANCR can be used as a promising diagnostic and prognostic biomarker and therapeutic target for various types of cancer. This review focuses on the role and mechanism of DANCR in cancer progression with an emphasis on the clinical significance of DANCR in human cancers.
LncRNAs are non-coding RNAs (ncRNAs) consisting of longer than 200 nucleotides in length without initiation codon and termination codon. They were initially considered as “junk” transcriptional products without biological functions, thus attracting limited attention among scientists (Anastasiadou et al., 2018). However, in recent decades, large-scale genome-wide sequencing analysis has shown the tissue specificity and essential functions of non-coding RNAs in diverse biological processes (Fico et al., 2019). They can modulate gene expression at epigenetic, transcription, and post-transcription levels (Ma et al., 2019).
LncRNAs also play essential roles in human cancers. The previous studies have indicated that many lncRNAs are dysregulated in cancers (Iyer et al., 2015; Yan et al., 2015). Aberrant expression of lncRNAs is associated with tumor growth (Zhou et al., 2020), metastasis (Liao et al., 2021), angiogenesis (Jin et al., 2020), chemotherapy resistance (Ferretti and León, 2021), and metabolism (Xu et al., 2021). Therefore, lncRNAs are regarded as important regulators of the pathological processes in cancer cells.
Differentiation antagonizing non-protein coding RNA (DANCR or ANCR), located on human chromosome 4q12, was first reported in 2012 to suppress differentiation of epithelial cells, and then proved to promote the stemness features of hepatocellular carcinoma cells (Kretz et al., 2012; Yuan et al., 2016). Subsequently, in recent decades, many studies have been carried out to understand the function of DANCR in cancer. DANCR is aberrantly expressed in various kinds of cancers (Figure 1). The dysregulation of DANCR expression is closely associated with biological functions (Figure 2) and clinical pathological factors (Figure 3). In this review, we summarized the current evidence regarding the function and underlying mechanism of DANCR in numerous cancers. It is suggested that DANCR serve as a tumor promoter or suppressor, representing a promising cancer biomarker or therapeutic target in various cancers.
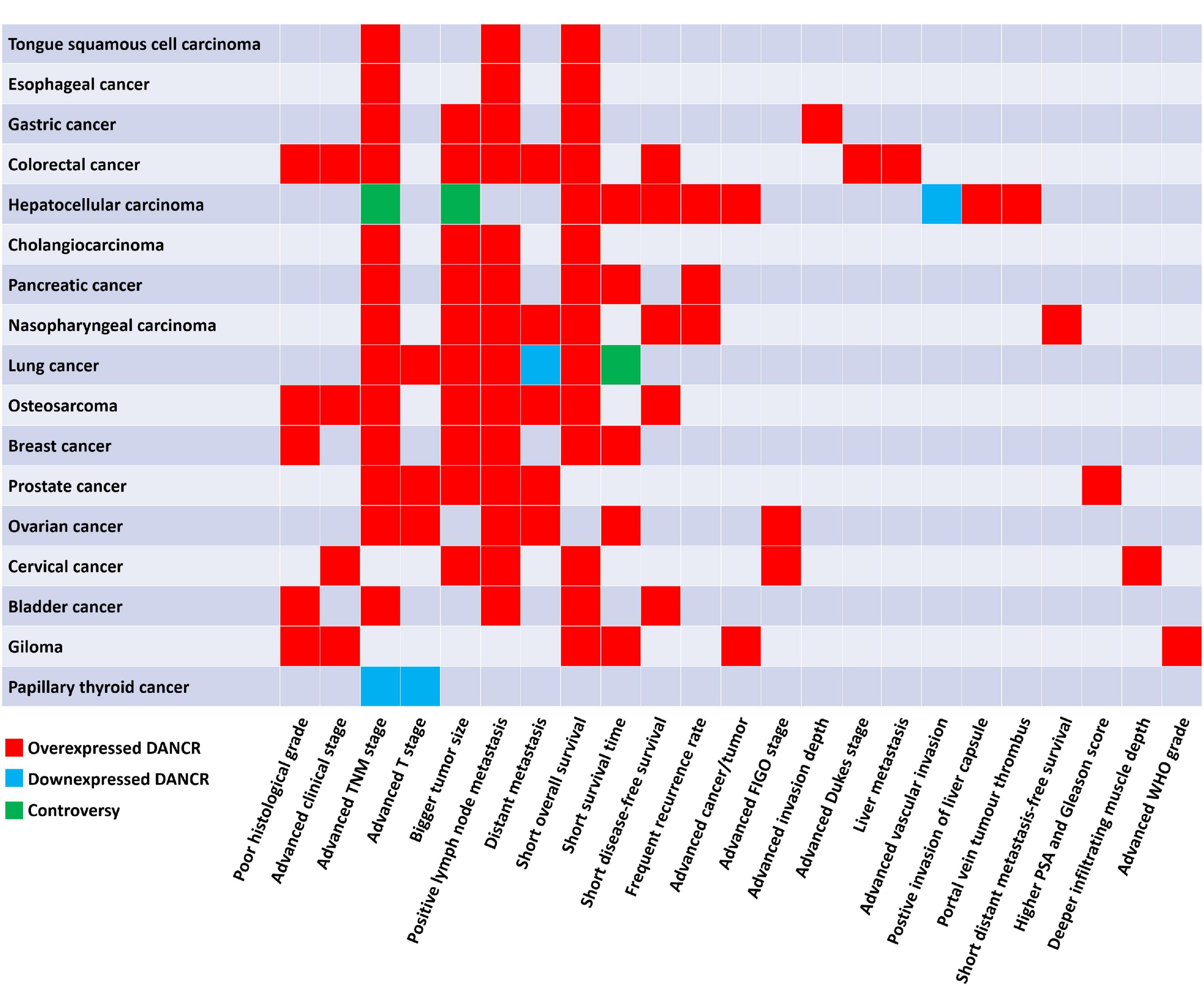
Figure 3. The correlation of DANCR expression level with clinicopathological factors of patients with cancers.
The downstream regulatory mechanisms for the biological roles of DANCR in cancers are complicated (Figure 4). The competing endogenous RNA (ceRNA) network, initially proposed in 2011, is one popular regulatory mechanism of DANCR in cancers (Table 1; Salmena et al., 2011). As we know, miRNAs could regulate gene expression at the post-transcriptional level by interacting with the 3′UTR region of mRNA via base-pairing (Fabian et al., 2010). Meanwhile, the miRNA recognition elements (MRE) of ceRNA could compete with relevant miRNAs to bind to mRNAs and regulate their expression (Salmena et al., 2011). Up to now, DANCR has been reported to bind to ∼ 50 miRNAs (Table 1).
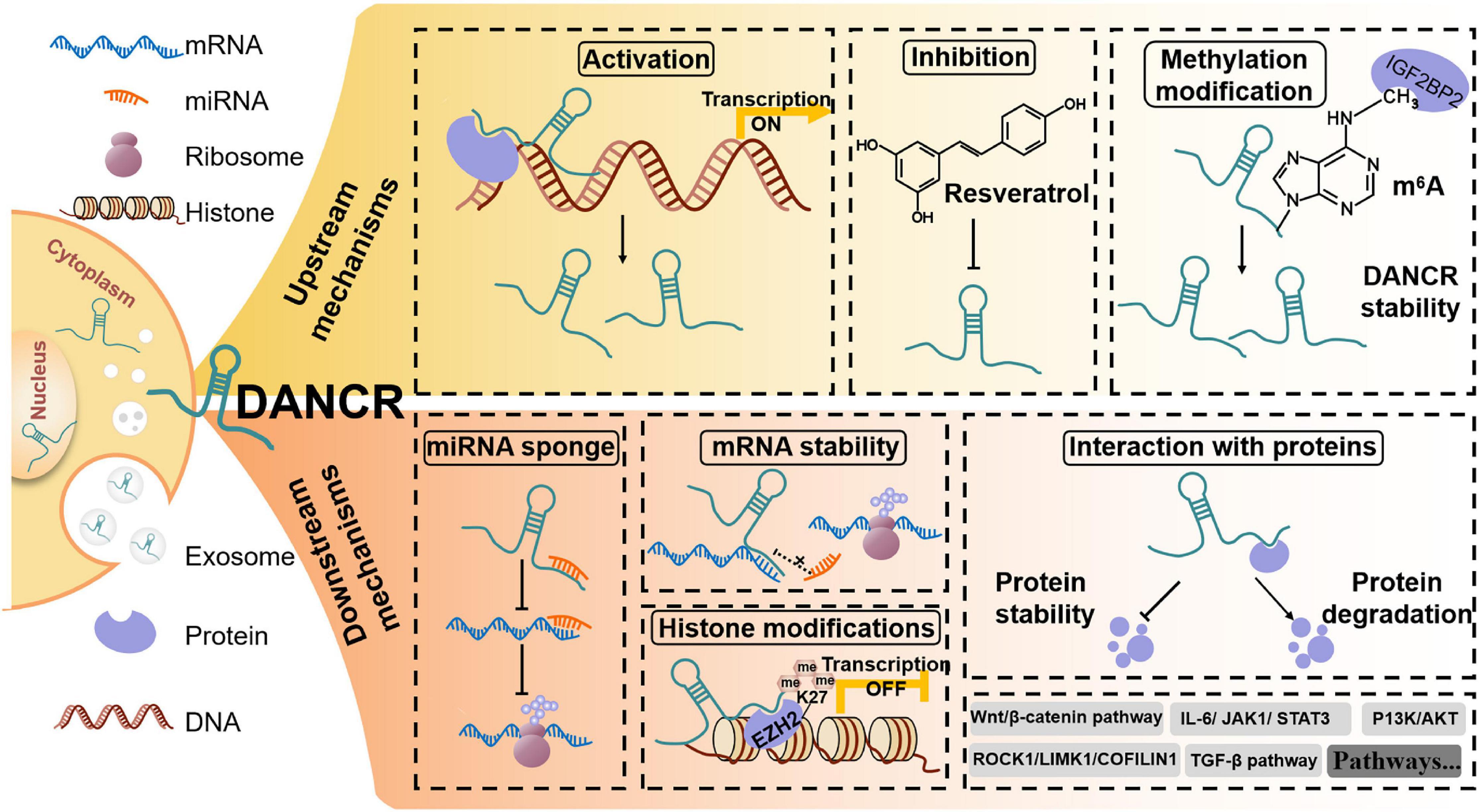
Figure 4. The upstream and downstream regulatory mechanisms for DANCR in cancer. For upstream mechanisms, factors like proteins or drugs can regulate the expression of DANCR. Meanwhile, methylation modification can also regulate DANCR expression. For downstream mechanisms, DANCR can act to sponge miRNAs, stabilize mRNAs and interact with proteins, finally regulating the activation of many signaling pathways.
In addition to binding to miRNAs, DANCR could also interact with mRNAs or proteins. For example, DANCR was verified to interact with 3′UTR of CTNNB1 mRNA in hepatocellular carcinoma, thus competitively blocking miRNA site and reversing miRNA-mediated CTNNB1 suppression (Yuan et al., 2016). Similarly, in sorafenib-resistant HCC, DANCR could bind with PSMD10 mRNA to stabilize its expression via blocking the miRNA binding site (Liu et al., 2020a). In the case of binding to proteins, it is interesting that DANCR could regulate both protein stability (Wen et al., 2020) and protein degradation (Xie et al., 2020).
Another function of DANCR is epigenetic regulation. EZH2, a histone methyltransferase of polycomb repressive complex 2 (PRC2), is a common binding protein for DANCR. By interacting with EZH2, DANCR could epigenetically silence target gene transcription. In cholangiocarcinoma, DANCR-EZH2 interaction promoted FBP1 silence by regulating histone methylation of FBP1 promoter and then exacerbating tumorigenesis (Wang N. et al., 2019).
What’s more, DANCR expression is regulated at both epigenetic and transcriptional levels. It was suggested that in gastric cancer cells, DANCR could be activated by SALL4, which could bind to the promoter of DANCR (Pan et al., 2018). In addition, DANCR has been considered as a drug target. In nasopharyngeal carcinoma, resveratrol was verified to suppress cancer progression via DANCR/PTEN pathway (Zhang J. et al., 2020). Moreover, IGF2BP2 protein has been shown to regulate DANCR stability by m6A modification (Hu et al., 2020). In conclusion, the upstream and downstream mechanisms of DANCR are multifaceted and play vital roles in human cancers.
It has been verified that DANCR is aberrantly expressed in many cancers. Notably, DANCR is detected in some body fluids, including serum, plasma, and even exosomes (Table 2). This indicates that DANCR may help diagnosis and prognosis in many cancers by non-invasive methods with low cost and real-time monitoring.
Circulating DANCR expression is also positively associated with tissue DANCR level. For example, after HCC surgery treatment, plasma DANCR level decreased dramatically, which indicates that circulating DANCR may drive from tissues (Ma et al., 2016). As expected, the release of RNAs to circulation may reveal disease-associated information of tissue or activation of intercellular signaling pathways (Misawa et al., 2017). Thus, circulating DANCR may have a diagnostic capability. It was reported by Ma et al. (2016) that plasma DANCR had a better diagnostic value than AFP in HCC. As illustrated in Table 2, plasma DANCR (AUC = 0.868) showed a better effect in distinguishing HCC from healthy volunteers (HV), patients with CHB (chronic hepatitis B), and cirrhosis than AFP (AUC = 0.744). Recently, it has been confirmed that DANCR can be transported through exosomes, but the detailed mechanism is unclear. In addition, droplet digital PCR (ddPCR) has been used to detect exosomal DANCR to monitor HCV-HCC recurrence after curative surgical resection, showing a higher AUC (0.88) than qRT-PCR detection (0.831) (Wang S.C. et al., 2020).
These encouraging researches indicate that circulating DANCR may be a novel non−invasive biomarker for cancer diagnosis. However, the lncRNA-based liquid biopsy is still at its early stage. For further application, there is still a long way to go. For instance, standardized sample preparation and reliable endogenous controls need to be considered.
Tongue squamous cell carcinoma (TSCC) is one of the most common oral cavity malignancies, known for its aggressive biological behavior (Boldrup et al., 2017). Although significant progress has been made in treating TSCC, long-term survival does not improve significantly (Hu et al., 2017). Therefore, it would be beneficial to find promising biomarkers for TSCC detection and treatment.
DANCR expression was upregulated in TSCC cells. In vitro studies showed that DANCR could enhance TSCC cell proliferation, migration, and invasion. The molecular mechanism study suggested that DANCR act as a ceRNA to sponge miR-135a-5p and upregulate Kruppel-like Factor 8 (KLF8) expression. Similarly, knockdown of DANCR inhibited tumor growth and KLF8 expression in vivo, suggesting that DANCR may play an essential role via miR-135a-5p/KLF8 axis (Zheng et al., 2019). So DANCR may be a diagnostic biomarker and new therapeutic target in TSCC.
Esophageal cancer (EC) is one of the most frequent cancers in the digestive system and ranks the seventh leading cause of death in cancers (Siegel et al., 2021). Though the management and treatment of EC patients have improved, there is still no effective treatment, and 5-year post-esophagectomy survival rates are still poor (Hou et al., 2017; Huang and Yu, 2018). In addition, due to the deficiency of early clinical symptoms, it tends to be diagnosed in advanced stages (Encinas de la Iglesia et al., 2016).
Esophageal squamous cell carcinoma (ESCC) is one histological subtype of EC (Le Bras et al., 2016). Shi et al. (2018) monitored the expression of DANCR in ESCC, and they found that ESCC cell lines and tissues expressed a higher level of DANCR compared with that in adjacent normal counterparts. Its aberrant expression was implicated in accelerating cell proliferation, migration, invasion, and inhibiting apoptosis. Subsequently, Zhang C. et al. (2019) investigated the role of DANCR in ESCC. They found that DANCR sponged miR-33a-5p and upregulated zinc-finger-enhancer binding protein 1 (ZEB1) expression. Recently, Bi et al. (2020) discovered that DANCR was a direct downstream target of a tumor suppressor gene ZNF750. The mutations or deletions of ZNF750 were significantly associated with upregulated DANCR and poor prognosis, especially tumor metastasis in ESCC patients. Their further investigations verified that DANCR could competitively bind with miR-4707-3p and serve as a ceRNA to regulate FOXC2 expression in ESCC, which provides a novel DANCR/miR-4707-3p/FOXC2 pathway regulated by ZNF750 in ESCC. In summary, DANCR is associated with ESCC progression, and it may act as a novel prognostic and therapeutic target for ESCC.
Gastric cancer (GC) is a complex and heterogeneous disease, regarded as the fourth leading cause of cancer-related death (Serra et al., 2019). Traditional clinical indicators such as CEA, CA199, and CA724 are faced with shortcomings in diagnostic sensitivity and specificity. Therefore, there is an urgent need to find a potential marker for GC with higher diagnostic sensitivity and specificity (Cai et al., 2019).
Recent studies have revealed that DANCR expression was upregulated in GC tissues (Hao et al., 2017) and cell lines (Pan et al., 2018) compared to adjacent normal ones. High DANCR expression promoted cell proliferation, migration, and invasion in GC cells. Its expression was positively associated with tumor size, lymph node metastasis, invasion depth, and TNM stage of GC patients (Pan et al., 2018), suggesting that DANCR may correlate to the malignant progression of GC. For further function and mechanism analysis of DANCR in GC, Pan et al. (2018) proved that DANCR was regulated by SALL4 (sal-like protein 4), previously shown as a critical transcription factor to regulate the stemness of GC cells. Then DANCR accelerated GC progression via Wnt/β-catenin pathway (Zhang et al., 2014; Pan et al., 2018). A study by Xie et al. (2020) indicated another mechanism showing that DANCR inhibited FOXO1 expression by promoting its ubiquitination. As a result, M1 macrophage polarization was inhibited, which promoted GC cell invasion and metastasis.
Chemotherapy is the primary therapy for GC patients with unresectable tumors, and cisplatin (DDP) is used as the first-line drug (Zhu et al., 2019). However, DDP resistance is a major obstacle for DDP application. Thus, exploring underlying mechanisms of DDP-resistance development in GC remains an urgent issue (Guo Q. et al., 2019). Xu et al. (2019) found that DANCR was upregulated in DDP-resistant GC cells. Further study showed that the expression of multidrug resistance (MDR) related genes, MDR1 and MRP1, were induced by DANCR in GC cells. Their studies suggest that DANCR is associated with MDR development and may be a potential therapeutic target for GC with MDR.
Colorectal cancer (CRC) is one of the most commonly diagnosed malignant neoplasms among men (Siegel et al., 2019, 2021). Thanks to the improvements in screening tests and treatment, CRC incidence and mortality rates have declined for several years in developed countries (Siegel et al., 2017). However, in some low-income and middle-income regions, CRC incidence and mortality rates are still rising rapidly. Consequently, improvement in treatment options and accessibility is still necessary for economically backward areas (Arnold et al., 2017).
Liu et al. (2015) demonstrated that CRC tissues expressed a higher level of DANCR than adjacent normal ones. In addition, high DANCR expression was associated with worse overall survival and disease-free survival, and further study showed that DANCR might be an independent prognostic factor for CRC.
A recent study by Wang et al. (2018b) showed that miR-577 shared the same binding site for HSP27 (heat shock protein 27) with DANCR. They verified DANCR could enhance CRC cell proliferation and metastasis by acting as a miRNA sponge to promote HSP27 expression. Moreover, in in vivo study, the elevation of DANCR promoted tumor growth and liver metastasis of CRC. Another mechanism study by Lian et al. (2020) identified that DANCR could bind with lysine acetyltransferase 6A (KAT6A) and then triggered H3K23 acetyltransferase activity to promote CRC development. Intriguingly, DANCR could also promote the expression of an oncogenic lncRNA MALAT1 via enhancing its RNA stability, which suppressed doxorubicin-induced apoptosis in CRC cells (Xiong et al., 2021). In summary, these studies provided a potential therapeutic target for molecular treatment in CRC.
DANCR was also overexpressed in the serum of CRC patients. Serum DANCR level was positively associated with the TNM stage. Surprisingly, serum DANCR showed a better diagnostic ability than CEA and CA199 and a better performance in distinguishing CRC and colorectal polyps. Moreover, the combination of DANCR with CEA and CA199 showed better sensitivity than the single or double combination (Shen et al., 2020; Table 1), suggesting that DANCR has the potential to be a promising biomarker for CRC patients.
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer and ranks the sixth most common neoplasm and the third leading cause of cancer-related death (Forner et al., 2018). Sorafenib is an anti-angiogenic multi-kinase inhibitor for advanced hepatocellular carcinoma. However, the outcomes after therapy are not encouraging (Llovet et al., 2008). So, it is urgent to find other available opinions for treatment. As shown in recent studies, non-coding RNAs are considered promising biomarkers for diagnosis and treatment (Duan et al., 2019).
DANCR was upregulated in HCC cell lines, tissues, plasma and exosomes (Ma et al., 2016; Wang S.C. et al., 2020; Wen et al., 2020). It was implicated that DANCR played an essential role in HCC progression. For example, a high level of DANCR was associated with stemness features (Yuan et al., 2016), metastasis (Wen et al., 2020), and chemotherapeutic drug resistance in HCC (Liu et al., 2020a). In addition, the ROC (receiver operating characteristic) analysis showed that plasma DANCR and exosomal DANCR exhibited good discriminatory ability, suggesting it could be a promising diagnostic marker (Ma et al., 2016; Wang S.C. et al., 2020; Table 2).
DANCR could act as sponges for multiple miRNAs including miR-216a-5p (Wang J. et al., 2019), miR-27a-3p (Guo D. et al., 2019), miR-125b-5p (Yang et al., 2020), miR-140-3p (Wen et al., 2020) and miR-222-3p (Wang X. et al., 2020) in HCC. DANCR could also stabilize PSMD10 (Liu et al., 2020a) and CTNNB1 (Yuan et al., 2016) mRNAs by binding to their 3′UTR region to prevent its degradation by miRNAs. Wen et al. (2020) found that DANCR could not only improve HNRNPA1 expression via DANCR/miR-140-3p/HNRNPA1 axis but also bind to HNRNPA1 protein and inhibit its degradation. However, DANCR was also considered as a tumor suppressor due to the suppression of Wnt/β-catenin signaling pathway in HCC (Song et al., 2020).
In conclusion, DANCR is a crucial factor in HCC progression and may serve as a new marker for HCC diagnosis and treatment.
Cholangiocarcinoma (CCA) is a rare but aggressive biliary epithelial tumor (Goyal et al., 2021). Surgery is the best therapeutic option; however, only a minority (35%) of CCA patients are diagnosed early, whereas most of them are diagnosed late due to the “silent” clinical character (Rizvi et al., 2018). Therefore, there is an urgent need for early diagnosis and new treatment methods to help improve CCA survival outcomes.
DANCR was overexpressed in CCA tissues compared with adjacent normal tissues. Its expression level was associated with tumor size, TNM stage, and lymph node metastasis. Moreover, CCA patients with higher levels of DANCR had a lower survival rate. Knockdown of DANCR impeded cell proliferation, migration, invasion, EMT process, angiogenesis, and enhanced apoptosis in vitro. miR-345-5p was predicted and verified as a target miRNA of DANCR, which regulated the expression of Twist1 (Twist-related protein 1) in CCA cells (Zhu et al., 2020). Wang N. et al. (2019) showed that DANCR inhibited FBP1 expression epigenetically via interacting with EZH2 and subsequently promoted CCA. In conclusion, DANCR may be a potential biomarker and therapeutic target for CCA.
Pancreatic cancer is the most life-threatening cancer with the lowest 5-year survival rate (10%) (Siegel et al., 2021). What is worse, early detection for pancreatic cancer is lacking, and the available treatment options are limited (Moore and Donahue, 2019).
DANCR showed higher expression in pancreatic cancer cell lines and tissues. Upregulated DANCR expression was associated with poor prognosis and short overall survival time in patients with pancreatic cancer. Knockdown of DANCR suppressed pancreatic cancer cell proliferation, migration, invasion in vitro, and tumor growth in vivo (Luo et al., 2019; Yao et al., 2019). Mechanistic studies showed that DANCR could be methylated at the N6 position of adenosine and then be stabilized by the m6A reader protein IGF2BP2 (Hu et al., 2020). In addition, DANCR sponged several miRNAs to regulate the expression of target mRNAs in pancreatic cancer cells (Luo et al., 2019; Yao et al., 2019; Tang et al., 2020). Interestingly, Liu et al. (2020b) found that DANCR could downregulate MLL3 expression to influence pancreatic cancer progression only at a late stage rather than an early stage.
Hence, DANCR was associated with pancreatic cancer development and regarded as a promising target for pancreatic cancer prognosis and treatment.
Nasopharyngeal carcinoma (NPC) is not a common cancer type but notable for its distinctive geographical distribution pattern. Most cases occur in the east and southeast parts of Asia (Chua et al., 2016). NPC patients at an early stage or with locoregional advanced disease could be well treated benefiting from the radiotherapy. However, distant metastasis is the primary cause of treatment failure (Yin et al., 2017).
DANCR was upregulated in NPC and promoted NPC cell proliferation, migration, invasion, and inhibited apoptosis in vitro. In addition, DANCR knockdown inhibited NPC tumor growth in vivo (Hao et al., 2019; Wen et al., 2018). Ma X. et al. (2018) found that DANCR could promote NPC proliferation and radiation resistance via DANCR/PTEN pathway. Interestingly, another research by Zhang J. et al. (2020) found that resveratrol could downregulate the expression of DANCR through this pathway. It validated DANCR as a promising target in NPC therapy.
Hypoxia is known as a target for cancer treatment, including NPC, and intratumoral hypoxia could lead to HIF-1α (hypoxia inducible factor-1α) overexpression (Semenza, 2003; Shan et al., 2018). Wen et al. (2018) found that DANCR overexpression was associated with lymph node metastasis and indicated a poor prognosis in NPC. Further study revealed that DANCR could stabilize HIF-1α mRNA through interacting with NF90/NF45 complex. These data suggest that DANCR might be a potential biomarker and therapeutic target of NPC.
Lung cancer is the leading cause of cancer-related deaths worldwide, both in men and women. It is reported that nearly a quarter of cancer deaths are related to lung cancer (Siegel et al., 2021). Non-small cell lung cancer (NSCLC) accounts for most types of lung cancer, and lung adenocarcinoma is the most common type of NSCLC. Despite improvements in early detection and standard treatment, NSCLC is often diagnosed at an advanced stage with a poor prognosis (Herbst et al., 2008). Understanding the molecular mechanism of NSCLC may bring better treatment for lung cancer.
Upregulated DANCR expression was detected in NSCLC tissue specimens and cell lines compared with normal counterparts. High level of DANCR was associated with larger tumor size, advanced TNM stage, and lymph node metastasis. Bai et al. (2019) found DANCR could activate EMT and act as a ceRNA to competitively bind to miR-138. And then, Sox4, a vital regulator involving tumor growth and metastasis, was regulated. In addition to miR-138, many other miRNAs have been verified as sponge targets for DANCR (Lu Q.C. et al., 2018; Wang and Jiang, 2018; Zhen et al., 2018; Chen Y.R. et al., 2020; Yu et al., 2020; Huang et al., 2021). Another study by Guo L. et al. (2019) showed that DANCR facilitated carcinogenesis by epigenetically silencing p21 expression via binding to EZH2. Thus, DANCR might be a key regulator of lung cancer progression and used as a promising biomarker.
Due to PSA testing and advances in early detection and treatment, the death rate for prostate cancer (PCa) dropped by 51% in the past two decades. However, PCa remains the second leading cause of cancer-related deaths in men, and the high rate of overdiagnosis is widely debated. Androgen deprivation therapy (ADT) is the principal treatment for advanced PCa (Magnan et al., 2015; Siegel et al., 2021). However, the majority of PCa will develop into castration-resistant prostate cancer (CRPC) inevitably over time (Nuhn et al., 2019). Compared with ADT, initial treatment with chemotherapy could improve the survival rate (Kahn et al., 2014; Litwin and Tan, 2017). However, the acquisition of chemoresistance inevitably develops, which is a major reason for therapy failure (Domanska et al., 2012).
Strong evidence has been presented about the oncogenic roles of DANCR in PCa. DANCR was upregulated in PCa tissues and cell lines (Jia et al., 2016; Zhao et al., 2019). Lu Y. et al. (2018) found that DANCR expression was induced by MYC, a common oncogene, which resulted in the reduction of p21, a protein required for cell cycle progression. Enzalutamide, a kind of AR (androgen receptor) inhibitor, was used to treat PCa. However, in some cases, it caused side effects such as PCa metastasis (Lin et al., 2013). DANCR knockdown limited the enzalutamide-induced metastasis. Mechanically, DNACR could inhibit TIMP2/3 expression by binding to EZH2 (Jia et al., 2016). Their studies suggested that DANCR might be a potential target for PCa.
Chemotherapy with Taxol (paclitaxel and its semisynthetic analog docetaxel) is commonly used for CRPC. However, there are obstacles to drug resistance (Gan et al., 2009; Jiang and Huang, 2010). Ma et al. (2019) and Zhao et al. (2019) found that DANCR could function as a sponge to miR-135a and miR-34a-5p, and eventually triggered the resistance to paclitaxel and docetaxel, respectively. Meanwhile, DANCR knockdown could promote the sensitivity of PCa cells to these drugs. Thus, DANCR provides a promising target to improve the effectiveness of chemotherapy for PCa.
Ovarian cancer (OC) is typically diagnosed at an advanced stage and has no effective screening strategy (Matulonis et al., 2016). Searching available biomarkers and developing appropriate targeted therapies are in need (Cortez et al., 2018).
DANCR was detected upregulated in ovarian cancer tissues and cell lines (Lin et al., 2019). Vascular endothelial growth factor (VEGF) is the master regulator of vessel formation, resulting in the growth and metastasis of tumors (Chen et al., 2018). Lin et al. (2019) suggested that DANCR can facilitate angiogenesis by regulating the DANCR/miR-145/VEGF axis in a manner of ceRNA. Additionally, DANCR showed a cancer-promoting property by negatively regulating UPF1. Enhanced UPF1 in OC cells was able to partly reverse the promotion of cell proliferation and migration by DANCR (Pei et al., 2019). Thus, DANCR might serve as a potential therapeutic target for ovarian cancer treatment.
Infection of specific types of human papillomavirus (HPV) is the primary biological etiology for cervical cancers (CC). Prophylactic vaccination for HPV provides the most effective method of primary prevention against HPV-related diseases (Kessler, 2017). However, the prognosis of advanced patients with cervical cancer is still poor (Siegel et al., 2021).
Liang et al. (2019) found that the expression of DANCR was aberrantly increased in cervical cancer tissues and cell lines and its high expression was associated with bigger tumor size, advanced FIGO stage, and poorer prognosis. Further functional analysis showed that DANCR could regulate ROCK1 expression by competitively binding to miR-335-5p and promote CC progression. Interestingly, another study by Ta et al. (2020) observed the diagnostic value of DANCR in distinguishing different types of CC. Their further investigation indicated that DANCR downregulated HIF-1α expression and inhibited the growth of HPV-negative CC under hypoxic conditions.
From Cancer statistics 2021, breast cancer (BC) has become the most common type of malignancy among women worldwide, responsible for 15% of deaths in women (Siegel et al., 2021). TNBC (triple-negative breast cancer), with a poor prognosis, is a subtype of breast cancer that does not express ER, PR, and HER2 (Wang X. et al., 2019). Though endocrine and HER2-targeted therapy have made great progress in recent years, targeted therapies for TNBC remain unsatisfactory (Costa et al., 2018). Therefore, it is necessary to identify new molecular targets for breast cancer therapy.
DANCR was significantly upregulated in TNBC tissues and cell lines compared with normal ones (Tang et al., 2018). Sha et al. (2017) found that DANCR expression was increased in TNBC tissues compared with that in adjacent normal tissues. Patients with higher DANCR expression tended to have worse TNM stage and poorer overall survival (OS). Tao et al. (2019) also found that DANCR knockdown significantly suppressed cancer cell proliferation and invasion, while the opposite phenomena were observed when DANCR was overexpressed. Inhibition of DANCR expression also impaired the growth of breast tumors in vivo.
The molecular mechanism analysis showed that DANCR played oncogenic roles by targeting miR-216a-5p as a ceRNA in MDA-MB-231 cells (TNBC cell line) (Tao et al., 2019). In addition, DANCR knockdown was associated with reduced expression of CD44, ABCG2, and ALDH1 in TNBC cells (Sha et al., 2017). Similarly, Tang et al. (2018) showed that DANCR could activate PI3K/AKT signaling through the activation of serine phosphorylation of RXRA by binding of GSK3β and RXRA, which promotes breast cancer progression. Zhang K.J. et al. (2020) verified that DANCR regulated EMT and cancer stemness in BC cells by binding to EZH2, which suppressed SOCS3 (suppressor of cytokine signaling 3) expression. Thus, DANCR was validated as an oncogene in breast cancer, and targeting DANCR may have therapeutic value in BC.
Interestingly, DANCR has also been suggested as a tumor suppressor in BC. Li et al. (2017c) revealed that DANCR expression was downregulated in BC cells as well as tumor tissues. It was verified that DANCR mediated EZH2 degradation and attenuated EMT and metastasis in BC. Another study from the same lab also showed that DANCR could inhibit TGF-β-induced EMT progress and BC metastasis by downregulating RUNX2 expression (Li et al., 2017b; Figure 5).
Bladder cancer (BCa) is among the top ten most common cancer types and the second most common genitourinary malignancy globally, with approximately 550,000 new cases annually (Bhanvadia, 2018; Richters et al., 2019). Diagnosis often occurs too late, particularly in women, due to their misinterpret of hematuria (Grayson, 2017). Thus, it is essential to find potential biomarkers to monitor tumorigenesis, development, and progression in BCa.
Zhan et al. (2018) showed that DANCR was aberrantly upregulated in BCa tissues compared to adjacent normal tissues. Moreover, increased DANCR expression was positively related to higher histological grade and advanced TNM stage. DANCR played as a miRNA sponge to positively regulate the expression of MSI2 (musashi RNA binding protein 2) via sponging miR-149 and subsequently promoted the malignant phenotypes of BCa cells.
Chen et al. (2019) also found that DANCR was significantly upregulated in bladder cancer with lymph node (LN) metastasis. Aberrant expression of DANCR in BCa was associated with LN metastasis and poor prognosis. DANCR guided leucine-rich pentatricopeptide repeat containing (LRPPRC) to stabilize target mRNAs, including IL-11, PLAU, and CCND1. Further investigations indicated that DANCR/LRPPRC/IL-11/STAT3 signaling pathway played an important role in BCa metastasis. These studies suggested that DANCR might be a valuable target for clinical intervention in LN-metastatic BCa.
Renal cell carcinoma (RCC) is one of the most common cancers in the urological system, originating from the renal tubular epithelial system. Surgical resection is an available choice for patients with localized RCC. However, due to the lack of sensitivity to radiotherapy and chemotherapy in RCC, targeted therapy is necessary for individuals without conditions for surgery (Lu et al., 2020).
Jin et al. (2017) found that DANCR was down-regulated in RCC tissues compared to adjacent normal tissues. Overexpression of DANCR caused the suppression of RCC cell proliferation, migration and invasion, and the induction of cell apoptosis. miR-3646 and miR-634 were predicted as the downstream targets of DANCR. However, biological validation studies are needed to confirm.
Papillary thyroid cancer (PTC) is the most prevalent form of thyroid cancer with a rapidly increasing incidence without a concomitant rise in mortality (Jegerlehner et al., 2017; Abdullah et al., 2019). Though the mean survival rate after 10 years is higher than 90%, disease recurrence cannot be ignored (Li et al., 2017a). An efficient and accurate diagnosis of PTC is still needed (Zhang H. et al., 2018).
Zhang K. et al. (2019) found that DANCR expression was lower in PTC tissues compared to that in normal thyroid tissues. Its expression was negatively associated with the clinical stage. ROC curve and AUC suggested the clinical diagnosis value of DANCR in PTC, which indicated DANCR might be a potential marker for PTC diagnosis. However, few mechanism studies about DANCR in PTC have been conducted.
Glioma is the most frequent and lethal central nervous system (CNS) tumor occurring both in children and adolescents (Sturm et al., 2017). DANCR expression was significantly higher in glioma cells than in normal human astrocytes (Wang W. et al., 2019). DANCR served as a ceRNA to modulate tumorigenesis, growth and metastasis by sponging miR-33a-5p (Yang et al., 2018), miR-634 (Xu et al., 2018), miR-216a (Wang W. et al., 2019) and miR-135a-5p (Feng et al., 2020) via regulating miR-33a-5p axis, miR-634/RAB1A axis, miR-216a/LGR5 axis, and miR-135a-5p/BMI1 axis, respectively. Li and Zhou (2018) also verified that high expression of DANCR might be a poor prognostic factor in glioma patients. Moreover, Ma Y. et al. (2018) found that DANCR promoted cisplatin resistance via activating AXL/PI3K/Akt/NF-κB signaling pathway through competitively binding with miRNAs, including miR-33a-5p, miR-33b-5p, miR-1-3p, miR-206, and miR-613 in glioma. These studies suggested that DANCR would be a potential biomarker for predicting cisplatin sensitivity and a therapeutic target for enhancing cisplatin efficacy in glioma.
Osteosarcoma is one of the most common primary solid malignancies of bone and mainly occurs in adolescence. Though the cure rate for conventional treatment of osteosarcoma is close to 70%, once osteosarcoma has spread to distant organs such as lungs, the survival rates are disappointing (Ritter and Bielack, 2010; Fan et al., 2020). Understanding the molecular mechanisms of osteosarcoma might provide new chances for early diagnosis and targets for therapy.
Increased expression of DANCR could be detected in osteosarcoma tissues and cell lines. Enhanced DANCR was found to have a positive correlation with poor prognostic outcomes (Jiang et al., 2017). DANCR suppression could restrain osteosarcoma progression by inhibiting autophagy (Pan et al., 2020). In recent studies, DANCR was found to function as a ceRNA to promote osteosarcoma progression by sponging miR-33a-5p (Jiang et al., 2017), miR-216a-5p (Pan et al., 2020), miR-149 (Zhang W. et al., 2020), miR-335-5p, and miR-1972 (Wang et al., 2018c). The interaction between DANCR and EZH2 was also found in osteosarcoma, which led to the inhibition of p21 and p27 expression. In conclusion, these studies indicated that DANCR could be utilized as a potential therapeutic target for the treatment of osteosarcoma.
Not only in cancer, DANCR could also participate in various biological processes and other diseases. Many research indicated that DANCR might play important role in the differentiation of mesenchymal stem cells (MSCs). Zhang J. et al. (2018) found DANCR was downregulated in human bone marrow-derived MSCs (BD-MSCs) during osteogenic differentiation. Their further investigation revealed DANCR could inhibit proliferation and osteogenic differentiation through p38 MAPK pathway. Similarly, Weng et al. (2021) observed the same function of DANCR in osteogenic differentiation and proposed another mechanism hypothesis. They considered that DANCR might suppress osteogenic differentiation via miR-1301-3p/PROX1 axis (Weng et al., 2021). With a similar situation to BD-MSCs, osteogenic differentiation capacity in periodontal ligament stem cells could also be inhibited by DANCR (Wang Z. et al., 2020). Apart from that, DANCR suppressed vascular smooth muscle cells transforming into osteoblast-like cells, thus attenuating arterial calcification (Zhang X. et al., 2020). Moreover, odontoblast differentiation in human dental pulp cells (Chen et al., 2016; Chen L. et al., 2020) and chondrogenic differentiation in human synovium-derived stem cells (Zhang et al., 2017) was inhibited and promoted by DANCR, respectively. To sum up, our increasing knowledge of DANCR indicated that targeting DANCR may be a novel therapeutic method in many diseases.
Dysregulation of lncRNAs is involved in regulating diverse malignant behaviors of cancer cells, leading to cancer progression and metastasis. It indicates that developing new diagnostic methods and therapeutic options targeting lncRNAs may be a new answer to the fight against cancers. LncRNA-DANCR, a booming researching topic in recent years, has been demonstrated to regulate many cellular functions such as proliferation, apoptosis, EMT, and CSC in various human cancers. The mechanism by which DANCR promotes tumor development is extremely complicated, including serving as a ceRNA for miRNAs, interacting with mRNAs or proteins, activating signaling pathways, and regulating epigenetic modulations. This review depicts a comprehensive picture of the biological roles of DANCR and its underlying mechanisms in cancer.
In most cancers, DANCR was upregulated and acted as an oncogene. However, a minority of studies reported that DANCR functioned as a potent tumor suppressor. Even in the same cancer type, the role of DANCR contradicted with each other (Li et al., 2017c; Zhang X.H. et al., 2020). This may be due to the heterogeneity in different cell lines, clinical sample selection, and experimental design (Table 3). Moreover, some of the confusing results in these studies may be explained by dynamic cancer progression. As in pancreatic cancer, MLL3 was only downregulated by DANCR at an advanced stage (Liu et al., 2020b). In addition, the study of gene expression always focuses on bulk analysis, only showing the information of the dominant cellular subset (Kanzaki and Pietras, 2020). Fortunately, emerging single-cell sequencing can reveal the uniqueness of individual cell and provide individualized therapy for patients (Ding et al., 2020). As shown in Figure 4, the downstream mechanisms are complicated. So when focusing on different targets of DANCR, we may come to different conclusions. These conjectures indicate that more studies on DANCR are needed to further clarify its role in specific cancer types and under distinct conditions.
DANCR is considered as a powerful biomarker not only in discriminating cancer patients from healthy people or patients with benign diseases but also in helping to predict the prognosis for cancer patients. Moreover, the combination of DANCR and other traditional biomarkers may enhance diagnostic efficiency. For cancer treatment, DANCR may be a promising target due to its essential role in cancers. Though DANCR was a promising biomarker and therapeutic target in cancer, more comprehensive and systematic clinical studies and more extensive sample tests are needed to further explore these complex issues. Further studies in DANCR mechanistic investigations and clinical application are still needed. Only after the mechanisms of DANCR in specific cancers have been elucidated can it likely be used for therapeutic purposes.
JY, XiZ, and XF conceived the project and supervised the writing. MW and JG wrote the draft of the review. XuZ and XF provided funds and assisted with preparation of the manuscript. All authors are involved in the revision and approved the final version of the manuscript.
This work was supported by the National Natural Science Foundation of China (81972310), the Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province (2019GSZDSYS01, 2019GSZDSYS02), the Six Talent Peaks Project in Jiangsu Province (2019-YY-188), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Postgraduate Research Innovation Program of Jiangsu Province (KYCX21-3405), and the Major Science and Technology Projects of Changzhou Municipal Committee of Health and Family Planning (ZD201917).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Some elements of Figure 1 were modified from Servier Medical Art (http://smart.servier.com/). We would like to acknowledge them for providing free academic licenses (https://creativecommons.org/licenses/by/3.0/).
Abdullah, M. I., Junit, S. M., Ng, K. L., Jayapalan, J. J., Karikalan, B., and Hashim, O. H. (2019). Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int. J. Med. Sci. 16, 450–460. doi: 10.7150/ijms.29935
Anastasiadou, E., Jacob, L. S., and Slack, F. J. (2018). Non-coding RNA networks in cancer. Nat. Rev. Cancer 18, 5–18. doi: 10.1038/nrc.2017.99
Arnold, M., Sierra, M. S., Laversanne, M., Soerjomataram, I., Jemal, A., and Bray, F. (2017). Global patterns and trends in colorectal cancer incidence and mortality. Gut 66, 683–691. doi: 10.1136/gutjnl-2015-310912
Bahreini, F., Saidijam, M., Mousivand, Z., Najafi, R., and Afshar, S. (2021). Assessment of lncRNA DANCR, miR-145-5p and NRAS axis as biomarkers for the diagnosis of colorectal cancer. Mol. Biol. Rep. 48, 3541–3547. doi: 10.1007/s11033-021-06373-2
Bai, Y., Zhang, G., Chu, H., Li, P., and Li, J. (2019). The positive feedback loop of lncRNA DANCR/miR-138/Sox4 facilitates malignancy in non-small cell lung cancer. Am. J. Cancer Res. 9, 270–284.
Bhanvadia, S. K. (2018). Bladder cancer survivorship. Curr. Urol. Rep. 19:111. doi: 10.1007/s11934-018-0860-6
Bi, Y., Guo, S., Xu, X., Kong, P., Cui, H., Yan, T., et al. (2020). Decreased ZNF750 promotes angiogenesis in a paracrine manner via activating DANCR/miR-4707-3p/FOXC2 axis in esophageal squamous cell carcinoma. Cell Death Dis. 11:296. doi: 10.1038/s41419-020-2492-2
Boldrup, L., Gu, X., Coates, P. J., Norberg-Spaak, L., Fahraeus, R., Laurell, G., et al. (2017). Gene expression changes in tumor free tongue tissue adjacent to tongue squamous cell carcinoma. Oncotarget 8, 19389–19402. doi: 10.18632/oncotarget.14288
Cai, C., Zhang, H., Zhu, Y., Zheng, P., Xu, Y., Sun, J., et al. (2019). Serum exosomal long noncoding RNA pcsk2-2:1 as a potential novel diagnostic biomarker for gastric cancer. Onco Targets Ther. 12, 10035–10041. doi: 10.2147/ott.S229033
Cao, L., Jin, H., Zheng, Y., Mao, Y., Fu, Z., Li, X., et al. (2019). DANCR-mediated microRNA-665 regulates proliferation and metastasis of cervical cancer through the ERK/SMAD pathway. Cancer Sci. 110, 913–925. doi: 10.1111/cas.13921
Chen, L., Song, Z., Huang, S., Wang, R., Qin, W., Guo, J., et al. (2016). lncRNA DANCR suppresses odontoblast-like differentiation of human dental pulp cells by inhibiting wnt/beta-catenin pathway. Cell Tissue Res. 364, 309–318. doi: 10.1007/s00441-015-2333-2
Chen, L., Song, Z., Wu, J., Huang, Q., Shen, Z., Wei, X., et al. (2020). LncRNA DANCR sponges miR-216a to inhibit odontoblast differentiation through upregulating c-Cbl. Exp. Cell Res. 387:111751. doi: 10.1016/j.yexcr.2019.111751
Chen, Y. R., Wu, Y. S., Wang, W. S., Zhang, J. S., and Wu, Q. G. (2020). Upregulation of lncRNA DANCR functions as an oncogenic role in non-small lung cancer by regulating miR-214-5p/CIZ1 axis. Eur. Rev. Med. Pharmacol. Sci. 24, 2539–2547. doi: 10.26355/eurrev_202003_20521
Chen, Y., Zhang, L., Liu, W. X., and Wang, K. (2018). VEGF and SEMA4D have synergistic effects on the promotion of angiogenesis in epithelial ovarian cancer. Cell. Mol. Biol. Lett. 23:2. doi: 10.1186/s11658-017-0058-9
Chen, Z., Chen, X., Xie, R., Huang, M., Dong, W., Han, J., et al. (2019). DANCR promotes metastasis and proliferation in bladder cancer cells by enhancing IL-11-STAT3 signaling and CCND1 expression. Mol. Ther. 27, 326–341. doi: 10.1016/j.ymthe.2018.12.015
Cheng, C., Dong, Y., Ru, X., Xia, Y., and Ji, Y. (2020). LncRNA ANCR promotes glioma cells invasion, migration, proliferation and inhibits apoptosis via interacting with EZH2 and repressing PTEN expression. Cancer Gene Ther. 28, 1025–1034. doi: 10.1038/s41417-020-00263-8
Chua, M. L. K., Wee, J. T. S., Hui, E. P., and Chan, A. T. C. (2016). Nasopharyngeal carcinoma. Lancet 387, 1012–1024. doi: 10.1016/s0140-6736(15)00055-0
Cortez, A. J., Tudrej, P., Kujawa, K. A., and Lisowska, K. M. (2018). Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 81, 17–38. doi: 10.1007/s00280-017-3501-8
Costa, R. L. B., Han, H. S., and Gradishar, W. J. (2018). Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res. Treat. 169, 397–406. doi: 10.1007/s10549-018-4697-y
Cui, P. H., Li, Z. Y., Li, D. H., Han, S. Y., and Zhang, Y. J. (2020). SP1-induced lncRNA DANCR contributes to proliferation and invasion of ovarian cancer. Kaohsiung J. Med. Sci. 37, 371–378. doi: 10.1002/kjm2.12316
Deng, H., Zhu, B., Dong, Z., Jiang, H., Zhao, X., and Wu, S. (2021). miR-214-5p targeted by LncRNA DANCR mediates TGF-β signaling pathway to accelerate proliferation, migration and inhibit apoptosis of prostate cancer cells. Am. J. Transl. Res. 13, 2224–2240.
Ding, S., Chen, X., and Shen, K. (2020). Single-cell RNA sequencing in breast cancer: understanding tumor heterogeneity and paving roads to individualized therapy. Cancer Commun. 40, 329–344. doi: 10.1002/cac2.12078
Domanska, U. M., Timmer-Bosscha, H., Nagengast, W. B., Oude Munnink, T. H., Kruizinga, R. C., Ananias, H. J., et al. (2012). CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia 14, 709–718. doi: 10.1593/neo.12324
Duan, J., Wu, Y., Liu, J., Zhang, J., Fu, Z., Feng, T., et al. (2019). Genetic biomarkers for hepatocellular carcinoma in the era of precision medicine. J. Hepatocell. Carcinoma 6, 151–166. doi: 10.2147/jhc.S224849
Encinas de la Iglesia, J., Corral de la Calle, M. A., Fernandez Perez, G. C., Ruano Perez, R., and Alvarez Delgado, A. (2016). Esophageal cancer: anatomic particularities, staging, and imaging techniques. Radiologia 58, 352–365. doi: 10.1016/j.rx.2016.06.004
Fabian, M. R., Sonenberg, N., and Filipowicz, W. (2010). Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379. doi: 10.1146/annurev-biochem-060308-103103
Fan, T. M., Roberts, R. D., and Lizardo, M. M. (2020). Understanding and modeling metastasis biology to improve therapeutic strategies for combating osteosarcoma progression. Front. Oncol. 10:13. doi: 10.3389/fonc.2020.00013
Feng, L., Lin, T., Che, H., and Wang, X. (2020). Long noncoding RNA DANCR knockdown inhibits proliferation, migration and invasion of glioma by regulating miR-135a-5p/BMI1. Cancer Cell Int. 20:53. doi: 10.1186/s12935-020-1123-4
Ferretti, V. A., and León, I. E. (2021). Long non-coding RNAs in cisplatin resistance in osteosarcoma. Curr. Treat. Options Oncol. 22:41. doi: 10.1007/s11864-021-00839-y
Fico, A., Fiorenzano, A., Pascale, E., Patriarca, E. J., and Minchiotti, G. (2019). Long non-coding RNA in stem cell pluripotency and lineage commitment: functions and evolutionary conservation. Cell. Mol. Life Sciences 76, 1459–1471. doi: 10.1007/s00018-018-3000-z
Forner, A., Reig, M., and Bruix, J. (2018). Hepatocellular carcinoma. Lancet 391, 1301–1314. doi: 10.1016/s0140-6736(18)30010-2
Gan, L., Chen, S., Wang, Y., Watahiki, A., Bohrer, L., Sun, Z., et al. (2009). Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res. 69, 8386–8394. doi: 10.1158/0008-5472.Can-09-1504
Goyal, L., Kongpetch, S., Crolley, V. E., and Bridgewater, J. (2021). Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat. Rev. 95:102170. doi: 10.1016/j.ctrv.2021.102170
Guo, D., Li, Y., Chen, Y., Zhang, D., Wang, X., Lu, G., et al. (2019). DANCR promotes HCC progression and regulates EMT by sponging miR-27a-3p via ROCK1/LIMK1/COFILIN1 pathway. Cell Prolif. 52:e12628. doi: 10.1111/cpr.12628
Guo, L., Gu, J., Hou, S., Liu, D., Zhou, M., Hua, T., et al. (2019). Long non-coding RNA DANCR promotes the progression of non-small-cell lung cancer by inhibiting p21 expression. Onco Targets Ther. 12, 135–146. doi: 10.2147/OTT.S186607
Guo, Q., Jing, F. J., Xu, W., Li, X., Li, X., Sun, J. L., et al. (2019). Ubenimex induces autophagy inhibition and EMT suppression to overcome cisplatin resistance in GC cells by perturbing the CD13/EMP3/PI3K/AKT/NF-kappaB axis. Aging 12, 80–105. doi: 10.18632/aging.102598
Hao, Y. P., Qiu, J. H., Zhang, D. B., and Yu, C. G. (2017). Long non-coding RNA DANCR, a prognostic indicator, promotes cell growth and tumorigenicity in gastric cancer. Tumour Biol. 39:1010428317699798. doi: 10.1177/1010428317699798
Hao, Y., Zhao, H., Jin, X., He, P., Zhang, J., Dong, Q., et al. (2019). Long noncoding RNA DANCR promotes nasopharyngeal carcinoma cell proliferation and migration. Mol. Med. Rep. 19, 2883–2889. doi: 10.3892/mmr.2019.9906
Herbst, R. S., Heymach, J. V., and Lippman, S. M. (2008). Lung cancer. N. Engl. J. Med. 359, 1367–1380. doi: 10.1056/NEJMra0802714
Hou, X., Wen, J., Ren, Z., and Zhang, G. (2017). Non-coding RNAs: new biomarkers and therapeutic targets for esophageal cancer. Oncotarget 8, 43571–43578. doi: 10.18632/oncotarget.16721
Hu, C., Han, Y., Zhu, G., Li, G., and Wu, X. (2021). Krüppel-like factor 5-induced overexpression of long non-coding RNA DANCR promotes the progression of cervical cancer via repressing microRNA-145-3p to target ZEB1. Cell Cycle 20, 1441–1454. doi: 10.1080/15384101.2021.1941625
Hu, H., Wang, Y., Li, Z., Zhu, Y., Zhang, W., Wang, D., et al. (2017). Overexpression of suppressor of zest 12 is associated with cervical node metastasis and unfavorable prognosis in tongue squamous cell carcinoma. Cancer Cell Int. 17:26. doi: 10.1186/s12935-017-0395-9
Hu, X., Peng, W. X., Zhou, H., Jiang, J., Zhou, X., Huang, D., et al. (2020). IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. 27, 1782–1794. doi: 10.1038/s41418-019-0461-z
Huang, F. L., and Yu, S. J. (2018). Esophageal cancer: risk factors, genetic association, and treatment. Asian J. Surg. 41, 210–215. doi: 10.1016/j.asjsur.2016.10.005
Huang, P., Qi, B., Yao, H., Zhang, L., Li, Y., and Li, Q. (2020). Knockdown of DANCR suppressed the biological behaviors of ovarian cancer cells treated with transforming growth factor-β (TGF-β) by sponging MiR-214. Med. Sci. Monit. 26:e922760. doi: 10.12659/msm.922760
Huang, Y. F., Zhang, Y., and Fu, X. (2021). Long non-coding RNA DANCR promoted non-small cell lung cancer cells metastasis via modulating of miR-1225-3p/ErbB2 signal. Eur. Rev. Med. Pharmacol. Sci. 25, 758–769. doi: 10.26355/eurrev_202101_24637
Iyer, M. K., Niknafs, Y. S., Malik, R., Singhal, U., Sahu, A., Hosono, Y., et al. (2015). The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 47, 199–208. doi: 10.1038/ng.3192
Jegerlehner, S., Bulliard, J. L., Aujesky, D., Rodondi, N., Germann, S., Konzelmann, I., et al. (2017). Overdiagnosis and overtreatment of thyroid cancer: a population-based temporal trend study. PLoS One 12:e0179387. doi: 10.1371/journal.pone.0179387
Jia, H., Liang, K., Liu, G., Zhang, Z., Shi, Y., Liang, H., et al. (2020). lncRNA DANCR promotes proliferation and metastasis of breast cancer cells through sponging miR-4319 and upregulating VAPB. Cancer Biother. Radiopharm. doi: 10.1089/cbr.2020.3675 [Epub ahead of print].
Jia, J., Li, F., Tang, X. S., Xu, S., Gao, Y., Shi, Q., et al. (2016). Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget 7, 37868–37881. doi: 10.18632/oncotarget.9350
Jiang, J., and Huang, H. (2010). Targeting the androgen receptor by taxol in castration-resistant prostate cancer. Mol. Cell. Pharmacol. 2, 1–5.
Jiang, N., Wang, X., Xie, X., Liao, Y., Liu, N., Liu, J., et al. (2017). lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 405, 46–55. doi: 10.1016/j.canlet.2017.06.009
Jin, K.-T., Yao, J.-Y., Fang, X.-L., Di, H., and Ma, Y.-Y. (2020). Roles of lncRNAs in cancer: focusing on angiogenesis. Life Sci. 252:117647. doi: 10.1016/j.lfs.2020.117647
Jin, L., Fu, H., Quan, J., Pan, X., He, T., Hu, J., et al. (2017). Overexpression of long non-coding RNA differentiation antagonizing non-protein coding RNA inhibits the proliferation, migration and invasion and promotes apoptosis of renal cell carcinoma. Mol. Med. Rep. 16, 4463–4468. doi: 10.3892/mmr.2017.7135
Kahn, B., Collazo, J., and Kyprianou, N. (2014). Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int. J. Biol. Sci. 10, 588–595. doi: 10.7150/ijbs.8671
Kanzaki, R., and Pietras, K. (2020). Heterogeneity of cancer-associated fibroblasts: opportunities for precision medicine. Cancer Sci. 111, 2708–2717. doi: 10.1111/cas.14537
Kessler, T. A. (2017). Cervical cancer: prevention and early detection. Semin. Oncol. Nurs. 33, 172–183. doi: 10.1016/j.soncn.2017.02.005
Kretz, M., Webster, D. E., Flockhart, R. J., Lee, C. S., Zehnder, A., Lopez-Pajares, V., et al. (2012). Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 26, 338–343. doi: 10.1101/gad.182121.111
Le Bras, G. F., Farooq, M. H., Falk, G. W., and Andl, C. D. (2016). Esophageal cancer: the latest on chemoprevention and state of the art therapies. Pharmacol. Res. 113(Pt A), 236–244. doi: 10.1016/j.phrs.2016.08.021
Li, J., and Zhou, L. (2018). Overexpression of lncRNA DANCR positively affects progression of glioma via activating Wnt/beta-catenin signaling. Biomed. Pharmacother. 102, 602–607. doi: 10.1016/j.biopha.2018.03.116
Li, Q., Jiang, Y., Zhong, G., Lu, Y., Song, T., Zhang, Y., et al. (2020). Long noncoding RNA DANCR regulates cell proliferation by stabilizing SOX2 mRNA in nasopharyngeal carcinoma. Am. J. Pathol. 190, 2343–2354. doi: 10.1016/j.ajpath.2020.09.005
Li, Z., Hou, P., Fan, D., Dong, M., Ma, M., Li, H., et al. (2017c). The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 24, 59–71. doi: 10.1038/cdd.2016.95
Li, Z., Dong, M., Fan, D., Hou, P., Li, H., Liu, L., et al. (2017b). LncRNA ANCR down-regulation promotes TGF-beta-induced EMT and metastasis in breast cancer. Oncotarget 8, 67329–67343. doi: 10.18632/oncotarget.18622
Li, Q., Li, H., Zhang, L., Zhang, C., Yan, W., and Wang, C. (2017a). Identification of novel long non-coding RNA biomarkers for prognosis prediction of papillary thyroid cancer. Oncotarget 8, 46136–46144. doi: 10.18632/oncotarget.17556
Lian, J., Zhang, H., Wei, F., Li, Q., Lu, Y., Yu, B., et al. (2020). Long non-coding RNA DANCR promotes colorectal tumor growth by binding to lysine acetyltransferase 6A. Cell. Signal. 67:109502. doi: 10.1016/j.cellsig.2019.109502
Liang, H., Zhang, C., Guan, H., Liu, J., and Cui, Y. (2019). LncRNA DANCR promotes cervical cancer progression by upregulating ROCK1 via sponging miR-335-5p. J. Cell. Physiol. 234, 7266–7278. doi: 10.1002/jcp.27484
Liao, Z., Nie, H., Wang, Y., Luo, J., Zhou, J., and Ou, C. (2021). The emerging landscape of long non-coding RNAs in colorectal cancer metastasis. Front. Oncol. 11:641343. doi: 10.3389/fonc.2021.641343
Lin, T. H., Izumi, K., Lee, S. O., Lin, W. J., Yeh, S., and Chang, C. (2013). Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis. 4:e764. doi: 10.1038/cddis.2013.270
Lin, X., Yang, F., Qi, X., Li, Q., Wang, D., Yi, T., et al. (2019). LncRNA DANCR promotes tumor growth and angiogenesis in ovarian cancer through direct targeting of miR-145. Mol. Carcinog. 58, 2286–2296. doi: 10.1002/mc.23117
Litwin, M. S., and Tan, H. J. (2017). The diagnosis and treatment of prostate cancer: a review. JAMA 317, 2532–2542. doi: 10.1001/jama.2017.7248
Liu, B., Zhao, H., Zhang, L., and Shi, X. (2019). Silencing of long-non-coding RNA ANCR suppresses the migration and invasion of osteosarcoma cells by activating the p38MAPK signalling pathway. BMC Cancer 19:1112. doi: 10.1186/s12885-019-6335-4
Liu, Y., Chen, L., Yuan, H., Guo, S., and Wu, G. (2020a). LncRNA DANCR promotes sorafenib resistance via activation of IL-6/STAT3 signaling in hepatocellular carcinoma cells. Onco Targets Ther. 13, 1145–1157. doi: 10.2147/ott.S229957
Liu, Y., Li, J., Yue, B., Liang, L., Zhang, S., and Chen, Y. (2020b). Long non-coding RNA DANCR regulate MLL3 and thereby it determines the progression of pancreatic cancer. J. BUON 25, 1954–1959.
Liu, Y., Zhang, M., Liang, L., Li, J., and Chen, Y. X. (2015). Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 8, 11480–11484.
Llovet, J. M., Ricci, S., Mazzaferro, V., Hilgard, P., Gane, E., Blanc, J.-F., et al. (2008). Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390. doi: 10.1056/NEJMoa0708857
Lu, J., Chen, Z., Zhao, H., Dong, H., Zhu, L., Zhang, Y., et al. (2020). ABAT and ALDH6A1, regulated by transcription factor HNF4A, suppress tumorigenic capability in clear cell renal cell carcinoma. J. Transl. Med. 18:101. doi: 10.1186/s12967-020-02268-1
Lu, Q. C., Rui, Z. H., Guo, Z. L., Xie, W., Shan, S., and Ren, T. (2018). LncRNA-DANCR contributes to lung adenocarcinoma progression by sponging miR-496 to modulate mTOR expression. J. Cell. Mol. Med. 22, 1527–1537. doi: 10.1111/jcmm.13420
Lu, W., Huang, Z., Wang, J., and Liu, H. (2021). Long non-coding RNA DANCR accelerates colorectal cancer progression via regulating the miR-185-5p/HMGA2 axis. J. Biochem. mvab011. doi: 10.1093/jb/mvab011 [Epub ahead of print].
Lu, Y., Hu, Z., Mangala, L. S., Stine, Z. E., Hu, X., Jiang, D., et al. (2018). MYC targeted long noncoding RNA DANCR promotes cancer in part by reducing p21 levels. Cancer Res. 78, 64–74. doi: 10.1158/0008-5472.CAN-17-0815
Luo, Y., Wang, Q., Teng, L., Zhang, J., Song, J., Bo, W., et al. (2019). LncRNA DANCR promotes proliferation and metastasis in pancreatic cancer by regulating miRNA-33b. FEBS Open Bio 10, 18–27. doi: 10.1002/2211-5463.12732
Ma, X., Wang, X., Yang, C., Wang, Z., Han, B., Wu, L., et al. (2016). DANCR acts as a diagnostic biomarker and promotes tumor growth and metastasis in hepatocellular carcinoma. Anticancer Res. 36, 6389–6398. doi: 10.21873/anticanres.11236
Ma, X., Yuan, Y., Lu, J., Li, M., Yu, Y., Liu, J., et al. (2021). Long noncoding RNA ANCR promotes migration, invasion, EMT progress and stemness of nasopharyngeal carcinoma cells via the miR-4731-5p/NMT1 axis. Pathol. Res. Pract. 224:153540. doi: 10.1016/j.prp.2021.153540
Ma, X., Zhou, J., Liu, J., Wu, G., Yu, Y., Zhu, H., et al. (2018). LncRNA ANCR promotes proliferation and radiation resistance of nasopharyngeal carcinoma by inhibiting PTEN expression. Onco Targets Ther. 11, 8399–8408. doi: 10.2147/ott.S182573
Ma, Y., Fan, B., Ren, Z., Liu, B., and Wang, Y. (2019). Long noncoding RNA DANCR contributes to docetaxel resistance in prostate cancer through targeting the miR-34a-5p/JAG1 pathway. Onco Targets Ther. 12, 5485–5497. doi: 10.2147/OTT.S197009
Ma, Y., Zhou, G., Li, M., Hu, D., Zhang, L., Liu, P., et al. (2018). Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-kappaB signaling pathway. Neurochem. Int. 118, 233–241. doi: 10.1016/j.neuint.2018.03.011
Magnan, S., Zarychanski, R., Pilote, L., Bernier, L., Shemilt, M., Vigneault, E., et al. (2015). Intermittent vs continuous androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. JAMA Oncol. 1, 1261–1269. doi: 10.1001/jamaoncol.2015.2895
Malakootian, M., Mirzadeh Azad, F., Fouani, Y., Taheri Bajgan, E., Saberi, H., and Mowla, S. J. (2018). Anti-differentiation non-coding RNA, ANCR, is differentially expressed in different types of brain tumors. J. Neuro Oncol. 138, 261–270. doi: 10.1007/s11060-018-2809-5
Matulonis, U. A., Sood, A. K., Fallowfield, L., Howitt, B. E., Sehouli, J., and Karlan, B. Y. (2016). Ovarian cancer. Nat. Rev. Dis. Primers 2:16061. doi: 10.1038/nrdp.2016.61
Misawa, A., Takayama, K. I., and Inoue, S. (2017). Long non-coding RNAs and prostate cancer. Cancer Sci. 108, 2107–2114. doi: 10.1111/cas.13352
Nuhn, P., De Bono, J. S., Fizazi, K., Freedland, S. J., Grilli, M., Kantoff, P. W., et al. (2019). Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur. Urol. 75, 88–99. doi: 10.1016/j.eururo.2018.03.028
Pan, L., Liang, W., Gu, J., Zang, X., Huang, Z., Shi, H., et al. (2018). Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget 9, 1915–1930. doi: 10.18632/oncotarget.23019
Pan, Z., Wu, C., Li, Y., Li, H., An, Y., Wang, G., et al. (2020). LncRNA DANCR silence inhibits SOX5-medicated progression and autophagy in osteosarcoma via regulating miR-216a-5p. Biomed. Pharmacother. 122:109707. doi: 10.1016/j.biopha.2019.109707
Pei, C. L., Fei, K. L., Yuan, X. Y., and Gong, X. J. (2019). LncRNA DANCR aggravates the progression of ovarian cancer by downregulating UPF1. Eur. Rev. Med. Pharmacol. Sci. 23, 10657–10663. doi: 10.26355/eurrev_201912_19763
Ping, Q., Shi, Y., Yang, M., Li, H., Zhong, Y., Li, J., et al. (2021). LncRNA DANCR regulates lymphatic metastasis of bladder cancer via the miR-335/VEGF-C axis. Transl. Androl. Urol. 10, 1743–1753. doi: 10.21037/tau-21-226
Richters, A., Aben, K. K. H., and Kiemeney, L. (2019). The global burden of urinary bladder cancer: an update. World J. Urol. 38, 1895–1904. doi: 10.1007/s00345-019-02984-4
Ritter, J., and Bielack, S. S. (2010). Osteosarcoma. Ann. Oncol. 21(Suppl. 7), vii320–vii325. doi: 10.1093/annonc/mdq276
Rizvi, S., Khan, S. A., Hallemeier, C. L., Kelley, R. K., and Gores, G. J. (2018). Cholangiocarcinoma – evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 15, 95–111. doi: 10.1038/nrclinonc.2017.157
Salmena, L., Poliseno, L., Tay, Y., Kats, L., and Pandolfi, P. P. (2011). A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146, 353–358. doi: 10.1016/j.cell.2011.07.014
Semenza, G. L. (2003). Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732. doi: 10.1038/nrc1187
Serra, O., Galan, M., Ginesta, M. M., Calvo, M., Sala, N., and Salazar, R. (2019). Comparison and applicability of molecular classifications for gastric cancer. Cancer Treat. Rev. 77, 29–34. doi: 10.1016/j.ctrv.2019.05.005
Sha, S., Yuan, D., Liu, Y., Han, B., and Zhong, N. (2017). Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol. Open 6, 1310–1316. doi: 10.1242/bio.023135
Shan, Y., You, B., Shi, S., Shi, W., Zhang, Z., Zhang, Q., et al. (2018). Hypoxia-induced matrix metalloproteinase-13 expression in exosomes from nasopharyngeal carcinoma enhances metastases. Cell Death Dis. 9:382. doi: 10.1038/s41419-018-0425-0
Shen, X., Xue, Y., Cong, H., Wang, X., Fan, Z., Cui, X., et al. (2020). Circulating lncRNA DANCR as a potential auxillary biomarker for the diagnosis and prognostic prediction of colorectal cancer. Biosci. Rep. 40:BSR20191481. doi: 10.1042/bsr20191481
Shi, H., Li, K., Feng, J., Liu, G., Feng, Y., and Zhang, X. (2020). LncRNA-DANCR interferes with miR-125b-5p/HK2 axis to desensitize colon cancer cells to cisplatin vis activating anaerobic glycolysis. Front. Oncol. 10:1034. doi: 10.3389/fonc.2020.01034
Shi, H., Shi, J., Zhang, Y., Guan, C., Zhu, J., Wang, F., et al. (2018). Long non-coding RNA DANCR promotes cell proliferation, migration, invasion and resistance to apoptosis in esophageal cancer. J. Thorac. Dis. 10, 2573–2582. doi: 10.21037/jtd.2018.04.109
Siegel, R. L., Miller, K. D., Fedewa, S. A., Ahnen, D. J., Meester, R. G. S., Barzi, A., et al. (2017). Colorectal cancer statistics, 2017. CA Cancer J. Clin. 67, 177–193. doi: 10.3322/caac.21395
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2021). Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33. doi: 10.3322/caac.21654
Siegel, R. L., Miller, K. D., and Jemal, A. (2019). Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34. doi: 10.3322/caac.21551
Song, X. Z., Xu, X. J., Ren, X. N., Ruan, X. X., Wang, Y. L., and Yao, T. T. (2020). LncRNA ANCR suppresses the progression of hepatocellular carcinoma through the inhibition of Wnt/β-catenin signaling pathway. Onco Targets Ther. 13, 8907–8917. doi: 10.2147/ott.S260556
Sturm, D., Pfister, S. M., and Jones, D. T. W. (2017). Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J. Clin. Oncol. 35, 2370–2377. doi: 10.1200/jco.2017.73.0242
Sun, W., Zu, S., Shao, G., Wang, W., and Gong, F. (2021). Long non-coding DANCR targets miR-185-5p to upregulate LIM and SH3 protein 1 promoting prostate cancer via the FAK/PI3K/AKT/GSK3β/snail pathway. J. Gene Med. 23:e3344. doi: 10.1002/jgm.3344
Sun, Y., Cao, B., and Zhou, J. (2020). Roles of DANCR/microRNA-518a-3p/MDMA ceRNA network in the growth and malignant behaviors of colon cancer cells. BMC Cancer 20:434. doi: 10.1186/s12885-020-06856-8
Ta, W., Zhang, Y., Zhang, S., and Sun, P. (2020). LncRNA ANCR downregulates hypoxiainducible factor 1alpha and inhibits the growth of HPV negative cervical squamous cell carcinoma under hypoxic conditions. Mol. Med. Rep. 21, 413–419. doi: 10.3892/mmr.2019.10792
Tang, J., Zhong, G., Zhang, H., Yu, B., Wei, F., Luo, L., et al. (2018). LncRNA DANCR upregulates PI3K/AKT signaling through activating serine phosphorylation of RXRA. Cell Death Dis. 9:1167. doi: 10.1038/s41419-018-1220-7
Tang, Y., Cao, G., Zhao, G., Wang, C., and Qin, Q. (2020). LncRNA differentiation antagonizing non-protein coding RNA promotes proliferation and invasion through regulating miR-135a/NLRP37 axis in pancreatic cancer. Invest. New Drugs 38, 714–721. doi: 10.1007/s10637-019-00798-0
Tao, W., Wang, C., Zhu, B., Zhang, G., and Pang, D. (2019). LncRNA DANCR contributes to tumor progression via targetting miR-216a-5p in breast cancer: lncRNA DANCR contributes to tumor progression. Biosci. Rep. 39:BSR20181618. doi: 10.1042/BSR20181618
Tian, W., Lei, N., Guo, R., Yuan, Z., and Chang, L. (2020). Long non-coding RNA DANCR promotes cervical cancer growth via activation of the Wnt/beta-catenin signaling pathway. Cancer Cell Int. 20:61. doi: 10.1186/s12935-020-1139-9
Wang, J., Pu, J., Zhang, Y., Yao, T., Luo, Z., Li, W., et al. (2019). DANCR contributed to hepatocellular carcinoma malignancy via sponging miR-216a-5p and modulating KLF12. J. Cell. Physiol. 234, 9408–9416. doi: 10.1002/jcp.27625
Wang, N., Zhang, C., Wang, W., Liu, J., Yu, Y., Li, Y., et al. (2019). Long noncoding RNA DANCR regulates proliferation and migration by epigenetically silencing FBP1 in tumorigenesis of cholangiocarcinoma. Cell Death Dis. 10:585. doi: 10.1038/s41419-019-1810-z
Wang, S., and Jiang, M. (2018). The long non-coding RNA-DANCR exerts oncogenic functions in non-small cell lung cancer via miR-758-3p. Biomed. Pharmacother. 103, 94–100. doi: 10.1016/j.biopha.2018.03.053
Wang, Y., Lu, Z., Wang, N., Feng, J., Zhang, J., Luan, L., et al. (2018b). Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp. Mol. Med. 50:57. doi: 10.1038/s12276-018-0082-5
Wang, Y., Zeng, X., Wang, N., Zhao, W., Zhang, X., Teng, S., et al. (2018c). Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma. Mol. Cancer 17:89. doi: 10.1186/s12943-018-0837-6
Wang, S., Lan, F., and Xia, Y. (2018a). lncRA ANCR inhibits non-small cell lung cancer cell migration and invasion by inactivating TGF-beta pathway. Med. Sci. Monit. 24, 6002–6009. doi: 10.12659/msm.911492
Wang, S. C., Li, C. Y., Chang, W. T., Cheng, W. C., Yen, C. H., Tu, W. Y., et al. (2020). Exosome-derived differentiation antagonizing non-protein coding RNA with risk of hepatitis C virus-related hepatocellular carcinoma recurrence. Liver Int. 41, 956–968. doi: 10.1111/liv.14772
Wang, W., Li, Y., Ma, Q., Yan, H., and Su, W. (2019). Differentiation antagonizing non-protein coding RNA modulates the proliferation, migration, and angiogenesis of glioma cells by targeting the miR-216a/LGR5 axis and the PI3K/AKT signaling pathway. Onco Targets Ther. 12, 2439–2449. doi: 10.2147/ott.S196851
Wang, X., Cheng, M. L., Gong, Y., Ma, W. J., Li, B., and Jiang, Y. Z. (2020). LncRNA DANCR promotes ATG7 expression to accelerate hepatocellular carcinoma cell proliferation and autophagy by sponging miR-222-3p. Eur. Rev. Med. Pharmacol. Sci. 24, 8778–8787. doi: 10.26355/eurrev_202009_22816
Wang, X., Qi, Y., Kong, X., Zhai, J., Li, Y., Song, Y., et al. (2019). Immunological therapy: a novel thriving area for triple-negative breast cancer treatment. Cancer Lett. 442, 409–428. doi: 10.1016/j.canlet.2018.10.042
Wang, Z., Huang, Y., and Tan, L. (2020). Downregulation of lncRNA DANCR promotes osteogenic differentiation of periodontal ligament stem cells. BMC Dev. Biol. 20:2. doi: 10.1186/s12861-019-0206-8
Wen, X., Liu, X., Mao, Y. P., Yang, X. J., Wang, Y. Q., Zhang, P. P., et al. (2018). Long non-coding RNA DANCR stabilizes HIF-1alpha and promotes metastasis by interacting with NF90/NF45 complex in nasopharyngeal carcinoma. Theranostics 8, 5676–5689. doi: 10.7150/thno.28538
Wen, Z., Lian, L., Ding, H., Hu, Y., Xiao, Z., Xiong, K., et al. (2020). LncRNA ANCR promotes hepatocellular carcinoma metastasis through upregulating HNRNPA1 expression. RNA Biol. 17, 381–394. doi: 10.1080/15476286.2019.1708547
Weng, W., Di, S., Xing, S., Sun, Z., Shen, Z., Dou, X., et al. (2021). Long non-coding RNA DANCR modulates osteogenic differentiation by regulating the miR-1301-3p/PROX1 axis. Mol. Cell. Biochem. 476, 2503–2512. doi: 10.1007/s11010-021-04074-9
Wu, G., Zhou, H., Li, D., Zhi, Y., Liu, Y., Li, J., et al. (2020). LncRNA DANCR upregulation induced by TUFT1 promotes malignant progression in triple negative breast cancer via miR-874-3p-SOX2 axis. Exp. Cell Res. 396:112331. doi: 10.1016/j.yexcr.2020.112331
Xie, C., Guo, Y., and Lou, S. (2020). LncRNA ANCR promotes invasion and migration of gastric cancer by regulating FoxO1 expression to inhibit macrophage M1 polarization. Dig. Dis. Sci. 65, 2863–2872. doi: 10.1007/s10620-019-06019-1
Xiong, M., Wu, M., Dan, P., Huang, W., Chen, Z., Ke, H., et al. (2021). LncRNA DANCR represses Doxorubicin-induced apoptosis through stabilizing MALAT1 expression in colorectal cancer cells. Cell Death Dis. 12:24. doi: 10.1038/s41419-020-03318-8
Xu, D., Yu, J., Gao, G., Lu, G., Zhang, Y., and Ma, P. (2018). LncRNA DANCR functions as a competing endogenous RNA to regulate RAB1A expression by sponging miR-634 in glioma. Biosci. Rep. 38:BSR20171664. doi: 10.1042/BSR20171664
Xu, Y. D., Shang, J., Li, M., and Zhang, Y. Y. (2019). LncRNA DANCR accelerates the development of multidrug resistance of gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 23, 2794–2802. doi: 10.26355/eurrev_201904_17554
Xu, Y., Qiu, M., Shen, M., Dong, S., Ye, G., Shi, X., et al. (2021). The emerging regulatory roles of long non-coding RNAs implicated in cancer metabolism. Mol. Ther. 29, 2209–2218. doi: 10.1016/j.ymthe.2021.03.017
Yan, X., Hu, Z., Feng, Y., Hu, X., Yuan, J., Zhao, S. D., et al. (2015). Comprehensive genomic characterization of long non-coding RNAs across human cancers. Cancer Cell 28, 529–540. doi: 10.1016/j.ccell.2015.09.006
Yang, J. X., Sun, Y., Gao, L., Meng, Q., and Yang, B. Y. (2018). Long non-coding RNA DANCR facilitates glioma malignancy by sponging miR-33a-5p. Neoplasma 65, 790–798. doi: 10.4149/neo_2018_170724N498
Yang, L., Jiang, M. N., Liu, Y., Wu, C. Q., and Liu, H. (2020). Crosstalk between lncRNA DANCR and miR-125b-5p in HCC cell progression. Tumori 300891620977010. doi: 10.1177/0300891620977010 [Epub ahead of print].
Yang, Z. Y., Yang, F., Zhang, Y. L., Liu, B., Wang, M., Hong, X., et al. (2017). LncRNA-ANCR down-regulation suppresses invasion and migration of colorectal cancer cells by regulating EZH2 expression. Cancer Biomark. 18, 95–104. doi: 10.3233/cbm-161715
Yao, Z., Chen, Q., Ni, Z., Zhou, L., Wang, Y., Yang, Y., et al. (2019). Long non-coding RNA differentiation antagonizing nonprotein coding RNA (DANCR) promotes proliferation and invasion of pancreatic cancer by sponging miR-214-5p to regulate E2F2 expression. Med. Sci. Monit. 25, 4544–4552. doi: 10.12659/MSM.916960
Yin, Z., Zhang, X., Wang, Y., Wang, P., and Yuan, Z. (2017). The combination of systemic therapy and locoregional radiotherapy prolongs survival in newly diagnosed metastatic nasopharyngeal carcinoma patients. Onco Targets Ther. 10, 5677–5683. doi: 10.2147/ott.S150035
Yu, J. E., Ju, J. A., Musacchio, N., Mathias, T. J., and Vitolo, M. I. (2020). Long noncoding RNA DANCR activates Wnt/β-catenin signaling through MiR-216a inhibition in non-small cell lung cancer. Biomolecules 10:1646. doi: 10.3390/biom10121646
Yuan, S. X., Wang, J., Yang, F., Tao, Q. F., Zhang, J., Wang, L. L., et al. (2016). Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology 63, 499–511. doi: 10.1002/hep.27893
Zhan, Y., Chen, Z., Li, Y., He, A., He, S., Gong, Y., et al. (2018). Long non-coding RNA DANCR promotes malignant phenotypes of bladder cancer cells by modulating the miR-149/MSI2 axis as a ceRNA. J. Exp. Clin. Cancer Res. 37:273. doi: 10.1186/s13046-018-0921-1
Zhang, C., Wang, L., Yang, J., Fu, Y., Li, H., Xie, L., et al. (2019). MicroRNA-33a-5p suppresses esophageal squamous cell carcinoma progression via regulation of lncRNA DANCR and ZEB1. Eur. J. Pharmacol. 861:172590. doi: 10.1016/j.ejphar.2019.172590
Zhang, F., and Peng, H. (2017). LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. J. Orthop. Surg. Res. 12:103. doi: 10.1186/s13018-017-0599-7
Zhang, H., Cai, Y., Zheng, L., Zhang, Z., Lin, X., and Jiang, N. (2018). Long noncoding RNA NEAT1 regulate papillary thyroid cancer progression by modulating miR-129-5p/KLK7 expression. J. Cell. Physiol. 233, 6638–6648. doi: 10.1002/jcp.26425
Zhang, J., Jin, X., Zhou, C., Zhao, H., He, P., Hao, Y., et al. (2020). Resveratrol suppresses human nasopharyngeal carcinoma cell growth via inhibiting differentiation antagonizing non-protein coding RNA (DANCR) expression. Med. Sci. Monit. 26:e923622. doi: 10.12659/msm.923622
Zhang, J., Tao, Z., and Wang, Y. (2018). Long non-coding RNA DANCR regulates the proliferation and osteogenic differentiation of human bone-derived marrow mesenchymal stem cells via the p38 MAPK pathway. Int. J. Mol. Med. 41, 213–219. doi: 10.3892/ijmm.2017.3215
Zhang, K., Lv, J., Peng, X., Liu, J., Li, C., Li, J., et al. (2019). Down-regulation of DANCR acts as a potential biomarker for papillary thyroid cancer diagnosis. Biosci. Rep. 39:BSR20181616. doi: 10.1042/BSR20181616
Zhang, K. J., Tan, X. L., and Guo, L. (2020). The long non-coding RNA DANCR regulates the inflammatory phenotype of breast cancer cells and promotes breast cancer progression via EZH2-dependent suppression of SOCS3 transcription. Mol. Oncol. 14, 309–328. doi: 10.1002/1878-0261.12622
Zhang, L., Xu, Z., Xu, X., Zhang, B., Wu, H., Wang, M., et al. (2014). SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene 33, 5491–5500. doi: 10.1038/onc.2013.495
Zhang, L., Yang, C., Chen, S., Wang, G., Shi, B., Tao, X., et al. (2017). Long noncoding RNA DANCR is a positive regulator of proliferation and chondrogenic differentiation in human synovium-derived stem cells. DNA Cell Biol. 36, 136–142. doi: 10.1089/dna.2016.3544
Zhang, N., and Jiang, W. (2019). Long noncoding RNA DANCR promotes HMGA2mediated invasion in lung adenocarcinoma cells. Oncol. Rep. 41, 1083–1090. doi: 10.3892/or.2018.6897
Zhang, W., Li, J. Z., Tai, Q. Y., Tang, J. J., Huang, Y. H., and Gao, S. B. (2020). LncRNA DANCR regulates osteosarcoma migration and invasion by targeting miR-149/MSI2 axis. Eur. Rev. Med. Pharmacol. Sci. 24, 6551–6560. doi: 10.26355/eurrev_202006_21639
Zhang, X., Chen, J., Meng, Q., Li, D., Hu, F. Z., Zhu, Y. Q., et al. (2020). The protective effects of long non-coding RNA-ANCR on arterial calcification. J. Bone Miner. Metab. 38, 421–431. doi: 10.1007/s00774-019-01076-y
Zhang, X., Yang, J., Bian, Z., Shi, D., and Cao, Z. (2019). Long noncoding RNA DANCR promotes nasopharyngeal carcinoma progression by interacting with STAT3, enhancing IL-6/JAK1/STAT3 signaling. Biomed. Pharmacother. 113:108713. doi: 10.1016/j.biopha.2019.108713
Zhang, X. H., Li, B. F., Ding, J., Shi, L., Ren, H. M., Liu, K., et al. (2020). LncRNA DANCR-miR-758-3p-PAX6 molecular network regulates apoptosis and autophagy of breast cancer cells. Cancer Manag. Res. 12, 4073–4084. doi: 10.2147/cmar.S254069
Zhao, H. F., Zhang, Z. C., Shi, B. K., and Jiang, X. Z. (2019). DANCR sponges miR-135a to regulate paclitaxel sensitivity in prostate cancer. Eur. Rev. Med. Pharmacol. Sci. 23, 6849–6857. doi: 10.26355/eurrev_201908_18724
Zhen, Q., Gao, L. N., Wang, R. F., Chu, W. W., Zhang, Y. X., Zhao, X. J., et al. (2018). LncRNA DANCR promotes lung cancer by sequestering miR-216a. Cancer Control 25:1073274818769849. doi: 10.1177/1073274818769849
Zheng, Y., Zheng, B., Meng, X., Yan, Y., He, J., and Liu, Y. (2019). LncRNA DANCR promotes the proliferation, migration, and invasion of tongue squamous cell carcinoma cells through miR-135a-5p/KLF8 axis. Cancer Cell Int. 19:302. doi: 10.1186/s12935-019-1016-6
Zhou, Y., Tian, B., Tang, J., Wu, J., Wang, H., Wu, Z., et al. (2020). SNHG7: a novel vital oncogenic lncRNA in human cancers. Biomed. Pharmacother. 124:109921. doi: 10.1016/j.biopha.2020.109921
Zhu, C. Y., Fan, C. R., Zhang, Y. L., Sun, Q. X., Yan, M. J., Wei, W., et al. (2020). LncRNA DANCR affected cell growth, EMT and angiogenesis by sponging miR-345-5p through modulating Twist1 in cholangiocarcinoma. Eur. Rev. Med. Pharmacol. Sci. 24, 2321–2334. doi: 10.26355/eurrev_202003_20498
Keywords: long non-coding RNA, DANCR, cancer, biomarker, diagnosis, therapy, target
Citation: Wang M, Gu J, Zhang X, Yang J, Zhang X and Fang X (2021) Long Non-coding RNA DANCR in Cancer: Roles, Mechanisms, and Implications. Front. Cell Dev. Biol. 9:753706. doi: 10.3389/fcell.2021.753706
Received: 05 August 2021; Accepted: 20 September 2021;
Published: 15 October 2021.
Edited by:
Feng Wang, Affiliated Hospital of Nantong University, ChinaCopyright © 2021 Wang, Gu, Zhang, Yang, Zhang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Yang, NDM2NTUzMDRAcXEuY29t; Xiaoxin Zhang, emhhbmd4aWFveGluMjAxNEAxNjMuY29t; Xinjian Fang, bHlnZnhqQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.