
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 14 October 2021
Sec. Molecular and Cellular Oncology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.736927
This article is part of the Research Topic The Role of ncRNAs (non-coding RNAs) in Regulating Tumor Immune Microenvironment View all 32 articles
 Yating Xu1,2,3,4†
Yating Xu1,2,3,4† Xiao Yu1,2,3,4†
Xiao Yu1,2,3,4† Menggang Zhang1,2,3,4†
Menggang Zhang1,2,3,4† Qingyuan Zheng1,2,3,4
Qingyuan Zheng1,2,3,4 Zongzong Sun5
Zongzong Sun5 Yuting He1,2,3,4*
Yuting He1,2,3,4* Wenzhi Guo1,2,3,4*
Wenzhi Guo1,2,3,4*Long non-coding RNAs (lncRNAs) are RNAs with a length of no less than 200 nucleotides that are not translated into proteins. Accumulating evidence indicates that lncRNAs are pivotal regulators of biological processes in several diseases, particularly in several malignant tumors. Long intergenic non-protein coding RNA 1116 (LINC01116) is a lncRNA, whose aberrant expression is correlated with a variety of cancers, including lung cancer, gastric cancer, colorectal cancer, glioma, and osteosarcoma. LINC01116 plays a crucial role in facilitating cell proliferation, invasion, migration, and apoptosis. In addition, numerous studies have recently suggested that LINC01116 has emerged as a novel biomarker for prognosis and therapy in malignant tumors. Consequently, we summarize the clinical significance of LINC01116 associated with biological processes in various tumors and provide a hopeful orientation to guide clinical treatment of various cancers in future studies.
Cancer is one of the leading causes of death (Seow et al., 2020; Wang et al., 2020d; Buneviciene et al., 2021; Eloranta et al., 2021) worldwide and threatens human health and social happiness. Despite the advancements (Blessin et al., 2020) in clinical diagnosis and treatment of malignancies (Jung et al., 2020; Grady et al., 2021; Hussain et al., 2021), most patients still have a poor prognosis, and the overall survival (OS) rate remains low. Due to the lack of early diagnostic biomarkers, most cancers progress to the terminal stage. Therefore, it is urgent to understand the underlying molecular mechanisms in cancer, which is crucial for finding effective early diagnostic biomarkers and therapeutic methods.
Owing to the development of multiple RNA detection techniques (Shu et al., 2021; Toden et al., 2021; Wang et al., 2021; Zhong et al., 2021a), most RNAs have been found to lack the capacity to encode proteins. Although these RNAs do not directly translate into proteins (Gupta et al., 2020), driving evidence clarifies that they play an essential role in biological functions (Chen et al., 2020b; Jantrapirom et al., 2021; Jin et al., 2021; Lai et al., 2021; Zhang et al., 2021b). Such is the case of long non-coding RNAs (lncRNAs), which are no less than 200 nucleotides and participate in the initiation and progression of various diseases, especially cancers (Chen et al., 2020a; Huang et al., 2021; Kotani et al., 2021; Luo et al., 2021; Teng et al., 2021). Abnormal expression of these lncRNAs is involved in a variety of biological processes in tumors via regulation of gene expression that affects tumor size, metastasis, pathological stage, and prognosis in patients (Yin et al., 2018; Shuai et al., 2020; Li et al., 2021a; Zheng et al., 2021; Zhong et al., 2021b). Moreover, studies have confirmed that lncRNAs serve as competitive endogenous RNAs (ceRNAs) and could sponge microRNAs to regulate the expression of messenger RNA, which provides a promising direction for exploring the complicated molecular mechanisms of malignancies.
Long intergenic non-protein coding RNA 1116 (LINC01116), located in the 2q31.1 region, is currently reported to be an extraordinary regulator of proliferation, migration, and invasion of cancer cells (Meng et al., 2020; Cui et al., 2021; Lou et al., 2021; Ren et al., 2021; Zhang et al., 2021a). High expression of LINC01116 was identified in malignant tumors; LINC01116 might participate in tumorigenesis. For instance, previous studies have demonstrated that hepatocellular carcinoma (HCC) patients with LINC01116 overexpression generally have a dismal survival time (Jiang et al., 2019). Further studies verified that LINC01116 promoted oral squamous cell carcinoma (OSCC) proliferation, migration, and invasion (Chen et al., 2019). In contrast, knockdown of LINC01116 positively inhibited the proliferation of prostate cancer cells. Additionally, numerous reports have shown that LINC01116 functions as a major regulator of lung cancer (LC), gastric cancer (GC), colorectal cancer (CRC), glioma, osteosarcoma, glioma, head and neck squamous cell carcinoma (HNSC), epithelial ovarian cancer (EOC), and breast cancer (BC).
In this review, we highlight the latest studies concerning LINC01116, its abnormal expression related to clinical characteristics, and its influence on multiple biological functions of cancers. The present review could guide the further discovery of prospective and creative therapeutic targets.
Numerous studies have elucidated the significance of LINC01116 in malignancy. Therefore, we review the specific process in Table 1, which shows how LINC01116 expression exerts its impact on a variety of cancers.
Lung cancer is predominant worldwide and its incidence rate is the highest in men and the second in women (Bade and Dela Cruz, 2020; Chen et al., 2021; Ma et al., 2021; Yuan et al., 2021). Zeng et al. (2020) showed that LINC01116 was overexpressed in LC tumor tissues compared to normal adjacent tissues. LINC01116 expression is high in LC patients, and they generally have more unsatisfactory outcomes than the others. Thus, previous reports have suggested that LINC01116 is an independent prognostic factor in LC. Furthermore, the expression of LINC01116 was shown to be high in patients with advanced tumor stages. For instance, recent studies showed that a considerable number of patients with low expression of LINC01116 were generally diagnosed with TNM I rather than TNM I/III. These results indicate that LINC01116 can be considered as a latent regulator that participates in the progression of metastasis and invasiveness in LC (Shang et al., 2021). Additionally, silencing of LINC01116 reverses this effect. Eventually, LINC01116 plays crucial roles in tumor processes and could provide an orientation for being diagnostic and prognostic markers of LC, but its actual situation of clinical application still requires massive clinical and basic research.
Gastric cancer is the 4th most prevalent malignant tumor. Due to the lack of early diagnostic markers, patients are commonly diagnosed at terminal stages, with tumors that have metastasized to proximal or even remote regions in the body (Lin et al., 2019; Arnold et al., 2020; Ascherman et al., 2021; Puliga et al., 2021). Su et al. (2019) discovered that LINC01116 and CASC11 were upregulated in GC tissues, compared with cancer-adjacent tissues, and were positively correlated with clinical stages. In addition, the overexpression of LINC01116 and CASC11 was found to collectively increase the migration and invasion of GC cells. In contrast, low expression of LINC01116 was found to be associated with suppressed metastasis and invasiveness in GC patients. Moreover, CASC11 silencing alleviated the overexpression of LINC01116. Additionally, Chen et al. (2020c) demonstrated that patients with abundant expression of LINC01116 in GC cells generally have shorter survival time than those with low expression levels, and that the inhibition of LINC01116 expression could impede the proliferation of GC cells. These findings provide a novel direction for the diagnosis and treatment of GC.
Colorectal cancer is the frequent cancer globally and has high mortality and incidence rates (Wieszczy et al., 2020; Świerczyński et al., 2021; Zaborowski et al., 2021; Zhao et al., 2021). Bi et al. (2020) revealed that LINC01116 was highly expressed in CRC tissues compared to normal tissues, and patients with high expression of LINC01116 had a very poor prognosis. LINC01116 knockdown substantially prevented the migration, proliferation, and invasion of CRC cells and activated cell apoptosis. Emerging evidence showed that a large number of patients were commonly diagnosed at terminal stages with high expression of LINC01116. Liang et al. (2021) identified that LINC01116 facilitated the growth of CRC cells and tumorigenicity through the downregulation of TPM1 expression. Specifically, LINC01116 can bind with EZH2 to accelerate the methylation of TPM1, which blocks the transcription of TPM1. Additionally, low expression of LINC01116 considerably impeded the tumorigenicity and angiogenesis of CRC cells in nude mice. Despite LINC01116 could serve as a diagnostic biomarker for in CRC, the deficiency of clinical application needs to be further investigated. For instance, researches of LINC01116 participated in CRC are only tested in tissues, and diverse effects between LINC01116 and molecular target markers are supposed to be probed in blood and other body fluids.
Brain gliomas are the most common primary malignant tumors in the central nervous system, presenting with an increasing mortality rate. Several patients present with an OS time of less than 2 years (Mondal and Kulshreshtha, 2021; Petridis et al., 2021; Tanabe et al., 2021; Tu et al., 2021). Wang et al. (2020c) found that the upregulation of LINC01116 in glioma cells is related to poor prognosis. When LINC01116 is knocked, G1-G0 phase arrest in Ln229 and U87 cells might be induced. Therefore, the apoptosis of glioma could be significantly inhibited. Moreover, LINC01116 has been shown to stimulate IL-1β transcription to generate an army of cytokines, which are associated with tumorigenicity via DDX5. Wang et al. (2020c) showed that LINC01116 positively regulates MDM2 to repress the p53 pathway, which activates the development of glioma cells. Current research shows that LINC01116 can substantially promote the proliferation and invasion abilities of glioma cells (Cole et al., 1986). Ye et al. revealed that LINC01116 expression is higher in glioma tissues than in normal tissues, and it could elevate the capacity of the cell cycle and cell proliferation by regulating the expression of VEGFA. In summary, these findings further clarify the biological functions of LINC01116 in glioma tumorigenesis and provide a promising treatment target for glioma patients.
Osteosarcoma is a leading cause of cancer-related death among young adolescents. Osteosarcoma patients retain high levels of metastasis and recurrence, accounting for approximately 30%–40% of cases (Lu et al., 2020; Zheng et al., 2020; Li et al., 2021c; Liu et al., 2021b). Zhang et al. (2019) reported that LINC01116 might remarkably accelerate the proliferation, migration, and invasion of osteosarcoma cells, but substantially precludes cell apoptosis. These results corroborated that LINC01116 directly interacted with EZH2 to mediate PTEN and p53. Thus, EZH2 knockdown reverses the LINC01116 functional effect on osteosarcoma cells (Zhang et al., 2019). Zhang et al. (2018) silenced LINC01116, inhibiting the viability of osteosarcoma cells and promoting cell apoptosis. Moreover, high expression of LINC01116 in patients generally results in a poorer prognosis. These results revealed a crucial role for LINC01116 in osteosarcoma. However, further investigation is required before clinical application.
Wu et al. (2020) shed new light on the silencing of LINC01116, which was shown to inhibit the tumorigenicity of OSCC through the upregulation of miRNA-136. Likewise, LINC01116 knockdown was shown to decrease the migration and invasion of HNSC cells, probably via the epithelial-mesenchymal transition pathway (Xing et al., 2020). Yu et al. (2021) suggested that LINC01116 was an oncogene that accelerates the development of prostate adenocarcinoma (PRAD) cells. Fang et al. (2018) reported that the proliferation and migration of EOC cells was increased with the overexpression of LINC01116. Knockdown of LINC01116 could function as an essential suppressor to block the viability and cloning ability of BC cells (Hu et al., 2018). Consequently, LINC01116 is a promising prognostic and therapeutic target for OSCC, HNSC, PRAD, EOC, and BC.
To further assess the expression pattern of LINC01116 across pan-cancer, we used the GEPIA1 website to explore the expression level based on The Cancer Genome Atlas database (Tang et al., 2019). The results demonstrated that LINC01116 expression is upregulated in glioblastoma multiforme (GBM), head and neck squamous cell carcinoma (HNSC), lung squamous cell carcinoma (LUSC), pancreatic adenocarcinoma (PAAD), and skin cutaneous melanoma (SKCM). However, decreased expression of LINC01116 has been observed in kidney chromophobe (KICH), kidney renal papillary cell carcinoma (KIRP), testicular germ cell tumor (TGCT), and uterine corpus endometrial carcinoma (UCEC) (Figure 1A).
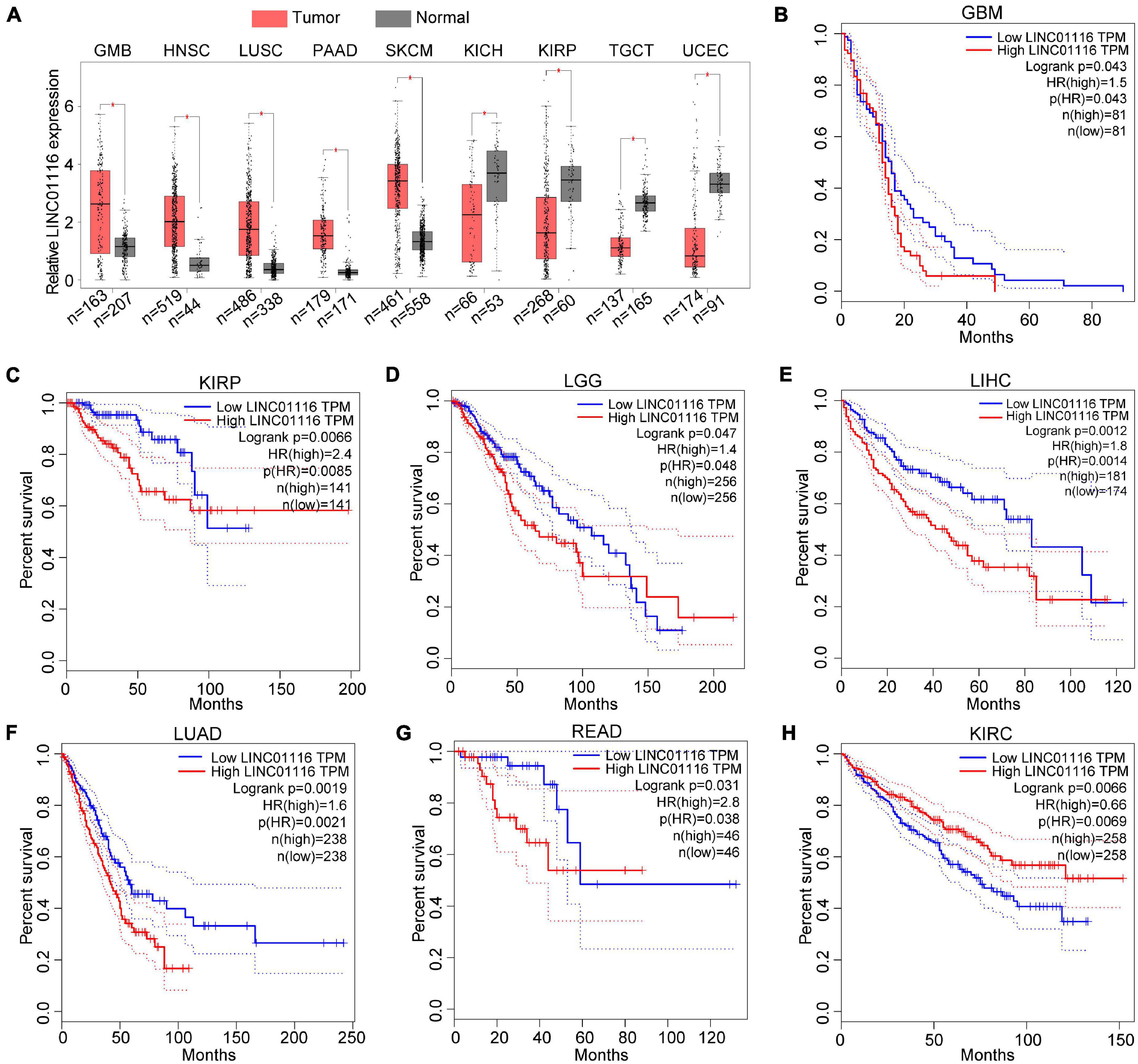
Figure 1. Expression and prognostic roles of LINC01116 in different cancer types. (A) Dysregulated expression of LINC01116 in GMB, HNSC, LUSC, PAAD, SKCM, KICH, KIRP, TGCT, and UCEC. (B–G) Patients with highly expressed LINC01116 had poor overall survival (OS) rate compared those with lowly expressed LINC01116 in GBM, KIRP, LGG, LIHC, LUAD, and READ. (H) Patients with decreased LINC01116 expression had poor overall survival (OS) rate compared those with increased LINC01116 expressed in KIRC.
Likewise, we evaluated the effect of LINC01116 expression on the survival time of cancer patients. As shown in Figure 1B, when LINC01116 is highly expressed, patients with GBM generally have a shorter OS time than when the expression of LINC01116 is low. Similar results have been observed in KIRP, lower grade glioma (LGG), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), and rectum adenocarcinoma (READ) (Figures 1C–G). Conversely, patients with decreased LINC01116 expression had a lower OS rate in kidney renal clear cell carcinoma (KIRC) (Figure 1H). Collectively, LINC01116 could be a novel biomarker for the diagnosis and prognostic determination of different cancer types.
LINC01116 has effects on multiple functions of tumors via complicated molecular mechanisms. To further explore these underlying processes, we summarize the complex molecular mechanisms in Table 2 and elucidate the association between LINC01116 and the biological functions of various cancers (Figure 2).
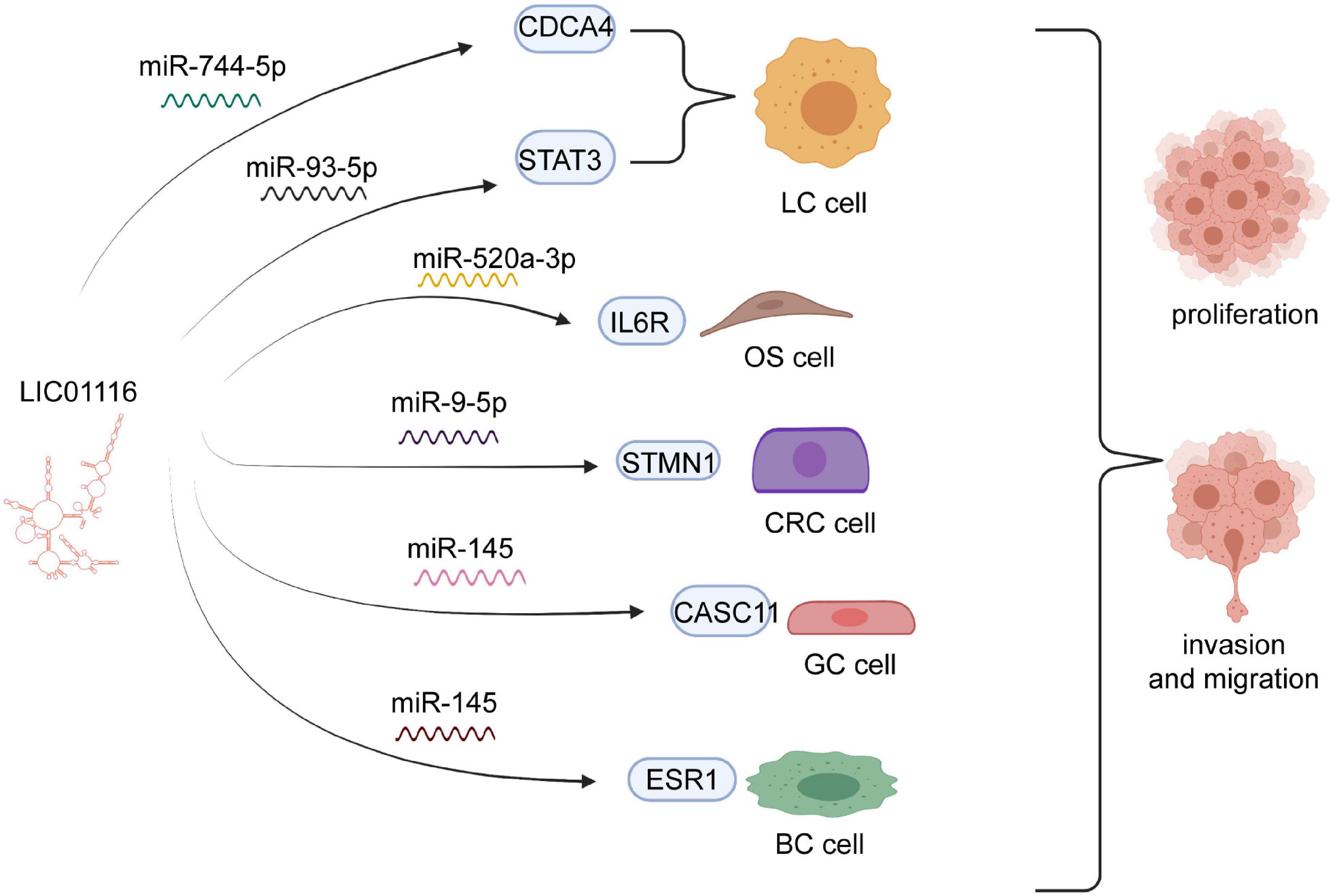
Figure 2. Diagram of the biological mechanisms involved in LINC01116 association with tumors. LINC01116 exerts an effect on the cell proliferation, invasion, and migration of multiple cancers, through sponging of miRNAs to mediate target regulators, such as miR-744-5p, miR-93-5p, miR-520a-3p, miR-9-5p, and miR-145.
Proliferation of cancer cells generally occurs rapidly, which markedly affects the prognosis of patients (Liu et al., 2019; Zhang et al., 2020). A recent study validated that miR-744-5p might be a novel target of LINC01116 in lung (LAD). miR-744-5p was known as the suppressor to involving in malignant tumors and negatively through regulating CDCA4. Knockdown of miR-744-5p could promote tumor cell proliferation and migration in LAD. LINC01116 could mediate the expression of CDCA4 by competitively binding the sites of CDCA4 with miR-744-5p, which markedly increased cell growth in LAD (Ren et al., 2021)Numerous experiments have shown that LINC01116 is overexpressed in small cell lung carcinoma (SCLC) and that it could upregulate STAT3 to boost SCLC cell invasion and migration. However, high expression of miR-93-5p suppresses the effect of LINC01116 overexpression. Thus, LINC01116 is likely to modulate miR-93-5p, which contributes to the expression levels of STAT3 (Figure 3). However, high expression of miR-93-5p and LINC01116 did not exert an influence on mutual expression, implying that miR-93-5p might have other targets (Xiang et al., 2017). In osteosarcoma cells, LINC01116 was shown to accelerate cell proliferation by targeting miR-520a-3p and upregulating IL6R (Zhang et al., 2018). Similarly, Liu et al. showed that LINC01116 interacts with CASC11, which regulates the invasion and migration of GC cells (Figure 4). Accumulating evidence suggests that miR-145 might bridge the interaction between LINC01116 and CASC11 (Su et al., 2019).
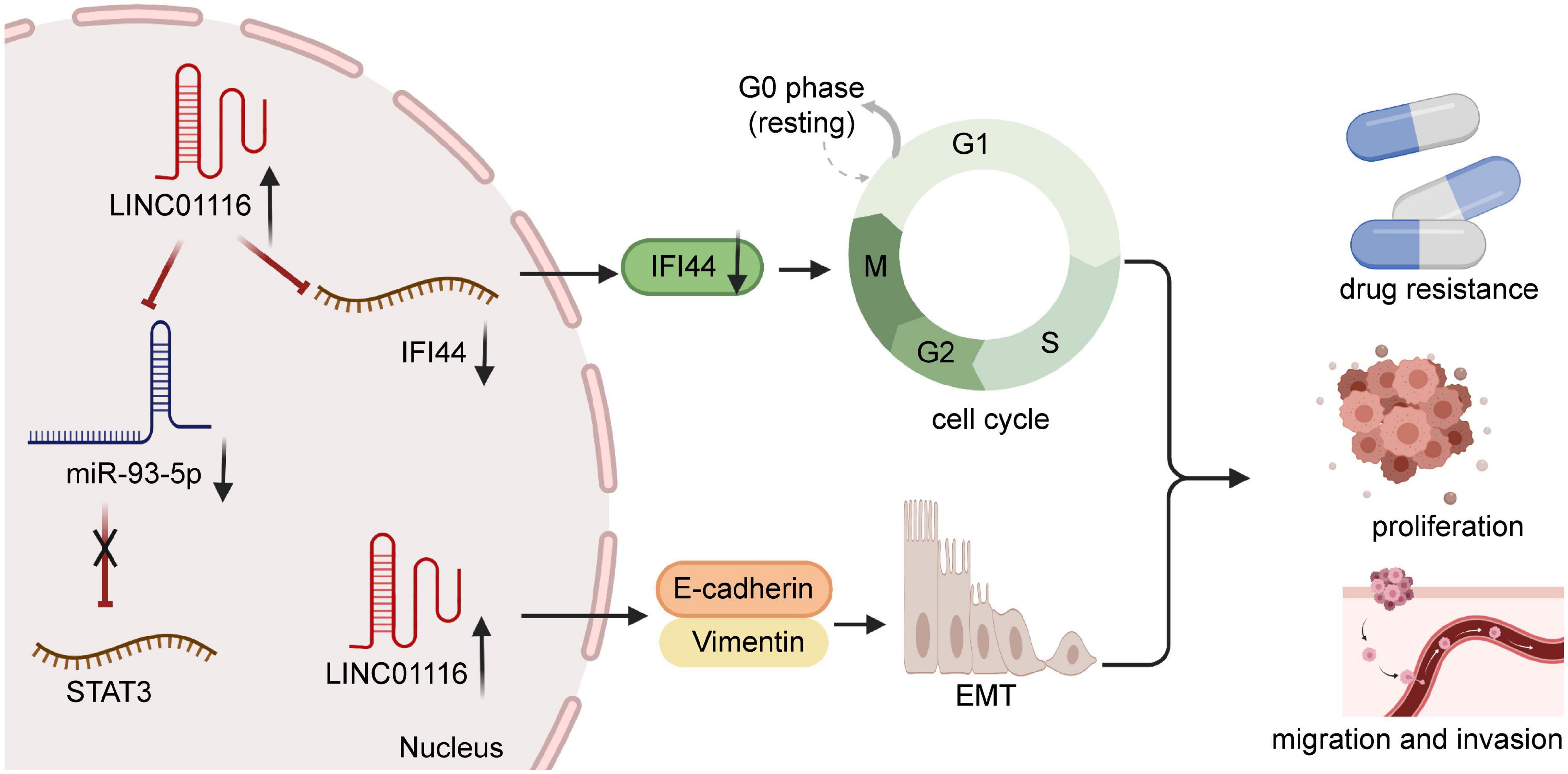
Figure 3. The overexpression of LINC00261 in non-small cell lung cancer accelerates tumor progression and chemoresistance.
In nasopharyngeal carcinoma (NPC) cells, LINC01116 was shown to induce the translation of MYC and enhance the expression of MYC protein, which plays an essential role in proliferation. Likewise, when MYC remains highly expressed, the effect of LINC01116 deletion can be recovered to some degree (Tran et al., 2016). In CRC cells, LINC01116 negatively correlates with miR-9-5p regulation, promoting the proliferation, invasion, and migration of cancer cells. In contrast, miR-9-5p rescues the function of LINC01116. miR-9-5p tends to bind STMN1 to preclude its expression. LINC01116 partly regulates STMN1 to target miR-9-5p (Bi et al., 2020; Figure 4). Liang et al. reported that LINC01116 enhanced the CRC cell proliferation, invasion and migration through interacting with EZH2 to potentiate methylation in the TPM1 promoter region to suppress the transcription of TPM1 (Figure 4). In addition, a previous study confirmed that LINC01116 was a regulator of ESR1 related to the proliferation of BC cells by sponging miR-145. In brief, LINC01116 actively stimulates the development of cell proliferation in many cancers by prompting miRNAs to mediate the expression of several proteins.
Chemotherapy is one of the primary treatment methods for several malignant tumors. However, the phenomenon of chemoresistance has increased, which has led to a serious dilemma in clinical treatment (Fatma et al., 2020; Jiang et al., 2020). Thus, there has been a great deal of research focusing on the molecular mechanisms of chemoresistance. Previous evidence has indicated that LINC01116 contributes to chemoresistance in some cancers (Li et al., 2021b), namely gefitinib and cisplatin resistance. Wang et al. (2020a) corroborated that LINC01116 contributed positively to the development of cisplatin resistance in LUAD, which depends on the EMT process (Figure 3). In contrast, LINC01116 silencing increases cisplatin sensitivity by mediating apoptosis and cell cycle distribution. Recent experiments have shed new light on the LINC01116-increasing effect of gefitinib resistance by regulating cell cycle through mediating IFI44 expression (Wang et al., 2020a; Figure 3). Furthermore, when LINC01116 is upregulated, the sensitivity of A549 cells to cisplatin is low. Conversely, silencing of LINC01116 generally leads to the inhibition of cisplatin resistance in A549 cells, via the stimulation of apoptosis and cell cycle arrest at the G0/G1 phase (Wang et al., 2020b). In summary, an abundance of chemoresistance mechanisms remain unclear. Thus, further studies are necessary.
The improvement of research technology generates numerous possibilities for the study of lncRNAs. Substantial research has shown that lncRNAs are relevant to the biological process of tumor advancement as essential regulators of gene expression (Ramnarine et al., 2019; Olivero et al., 2020; Katsushima et al., 2021), and the dysregulation of LINC01116 has been identified as the promoter of the occurrence and progression of a variety of tumors. Plentiful expression of LINC01116 can be found in various cancers, such as lung cancer (Liu et al., 2021a; Mu et al., 2021; Zeng et al., 2021), gastric cancer, colorectal cancer, glioma, and osteosarcoma. When LINC01116 is highly expressed in multiple cancers, the survival time of these patients tends to be shorter. In several experiments, LINC01116 was found to be an independent prognostic factor in malignant tumors. Furthermore, accumulating evidence has revealed that LINC01116 can be regarded as a ceRNA that mediates gene expression by sponging miRNA, which plays an indispensable role in the proliferation, invasion, metastasis, chemoresistance, and apoptosis of tumors. For instance, LINC01116 functions as a regulator to positively promote the expression of STMN1 by interacting with miR-9-5p. In conclusion, we demonstrated that LINC01116 expression is linked to cancer, and LINC01116 has the potential of being a promising target in clinical tumor treatments. However, there are several dilemmas of LINC0116 applying to clinical treatment still need to be solved. Firstly, the molecular structure and functional information on LINC0116 remain uncharted. Without detailed understanding on the structure and functions of LINC0116, boosting LINC0116 -based therapy exists difficulty. Additionally, lncRNAs are considered to be weakly conserved across different species, the conversion from animal models to human clinical application might emerge block. Thus, it is necessary to further research to explore numerous mechanisms of LINC01116 associated with tumor biological processes.
YX, MZ, and XY drafted the manuscript. QZ and ZS drew the mechanism diagrams. YH and WG conceived of the study and guided the analysis. YH and YX edited and reviewed the manuscript. All authors read and approved the final manuscript.
This work was supported by the Youth Talent Lifting Project of Henan Province (2021HYTP059), Key Scientific Research Project of Henan Higher Education Institutions of China (21A320026), the Leading Talents of Zhongyuan Science and Technology Innovation (214200510027), the Henan Provincial Medical Science and Technology Research Plan (SBGJ2018002), the Science and Technology Innovation Talents in Henan Universities (19HASTIT003), and the Outstanding Foreign Scientist Studio in Henan Province (GZS2020004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arnold, M., Park, J. Y., Camargo, M. C., Lunet, N., Forman, D., and Soerjomataram, I. (2020). Is gastric cancer becoming a rare disease? a global assessment of predicted incidence trends to 2035. Gut 69, 823–829. doi: 10.1136/gutjnl-2019-320234
Ascherman, B., Oh, A., and Hur, C. (2021). International cost-effectiveness analysis evaluating endoscopic screening for gastric cancer for populations with low and high risk. Gastric Cancer 24, 878–887. doi: 10.1007/s10120-021-01162-z
Bade, B. C., and Dela Cruz, C. S. (2020). Lung cancer 2020: epidemiology, etiology, and prevention. Clin. Chest. Med. 41, 1–24. doi: 10.1016/j.ccm.2019.10.001
Bi, C., Cui, H., Fan, H., and Li, L. (2020). LncRNA LINC01116 promotes the development of colorectal cancer by targeting miR-9-5p/STMN1. Onco Targets Ther. 13, 10547–10558. doi: 10.2147/ott.S253532
Blessin, N. C., Spriestersbach, P., Li, W., Mandelkow, T., Dum, D., Simon, R., et al. (2020). Prevalence of CD8(+) cytotoxic lymphocytes in human neoplasms. Cell Oncol. (Dordr) 43, 421–430. doi: 10.1007/s13402-020-00496-497
Buneviciene, I., Mekary, R. A., Smith, T. R., Onnela, J. P., and Bunevicius, A. (2021). Can mHealth interventions improve quality of life of cancer patients? a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 157:103123. doi: 10.1016/j.critrevonc.2020.103123
Chen, J., Sun, Y., Ou, Z., Yeh, S., Huang, C. P., You, B., et al. (2020b). Androgen receptor-regulated circFNTA activates KRAS signaling to promote bladder cancer invasion. EMBO Rep. 21:e48467. doi: 10.15252/embr.201948467
Chen, C., Luo, Y., He, W., Zhao, Y., Kong, Y., Liu, H., et al. (2020a). Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Invest. 130, 404–421. doi: 10.1172/jci130892
Chen, J., Yuan, Z. H., Hou, X. H., Shi, M. H., and Jiang, R. (2020c). LINC01116 promotes the proliferation and inhibits the apoptosis of gastric cancer cells. Eur. Rev. Med. Pharmacol. Sci. 24, 1807–1814. doi: 10.26355/eurrev_202002_20358
Chen, X., Hao, B., Li, D., Reiter, R. J., Bai, Y., Abay, B., et al. (2021). Melatonin inhibits lung cancer development by reversing the Warburg effect via stimulating the SIRT3/PDH axis. J. Pineal. Res. doi: 10.1111/jpi.12755 Online ahead of print.
Chen, Z., Tao, Q., Qiao, B., and Zhang, L. (2019). Silencing of LINC01116 suppresses the development of oral squamous cell carcinoma by up-regulating microRNA-136 to inhibit FN1. Cancer Manag. Res. 11, 6043–6059. doi: 10.2147/cmar.S197583
Cole, E. H., Sweet, J., and Levy, G. A. (1986). Expression of macrophage procoagulant activity in murine systemic lupus erythematosus. J. Clin. Invest. 78, 887–893. doi: 10.1172/jci112676
Cui, L., Chen, S., Wang, D., and Yang, Q. (2021). LINC01116 promotes proliferation and migration of endometrial stromal cells by targeting FOXP1 via sponging miR-9-5p in endometriosis. J. Cell Mol. Med. 25, 2000–2012. doi: 10.1111/jcmm.16039
Eloranta, S., Smedby, K. E., Dickman, P. W., and Andersson, T. M. (2021). Cancer survival statistics for patients and healthcare professionals - a tutorial of real-world data analysis. J. Intern. Med. 289, 12–28. doi: 10.1111/joim.13139
Fang, Y. N., Huang, Z. L., Li, H., Tan, W. B., Zhang, Q. G., Wang, L., et al. (2018). LINC01116 promotes the progression of epithelial ovarian cancer via regulating cell apoptosis. Eur. Rev. Med. Pharmacol. Sci. 22, 5127–5133. doi: 10.26355/eurrev_201808_15707
Fatma, H., Maurya, S. K., and Siddique, H. R. (2020). Epigenetic modifications of c-MYC: role in cancer cell reprogramming, progression and chemoresistance. Semin. Cancer Biol. doi: 10.1016/j.semcancer.2020.11.008 Online ahead of print.
Grady, W. M., Yu, M., and Markowitz, S. D. (2021). Epigenetic alterations in the gastrointestinal tract: current and emerging use for biomarkers of cancer. Gastroenterology 160, 690–709. doi: 10.1053/j.gastro.2020.09.058
Gupta, S. C., Awasthee, N., Rai, V., Chava, S., Gunda, V., and Challagundla, K. B. (2020). Long non-coding RNAs and nuclear factor-κB crosstalk in cancer and other human diseases. Biochim. Biophys. Acta Rev. Cancer 1873:188316. doi: 10.1016/j.bbcan.2019.188316
Hu, H. B., Chen, Q., and Ding, S. Q. (2018). LncRNA LINC01116 competes with miR-145 for the regulation of ESR1 expression in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 22, 1987–1993. doi: 10.26355/eurrev_201804_14726
Huang, X., Pan, L., Zuo, Z., Li, M., Zeng, L., Li, R., et al. (2021). LINC00842 inactivates transcription co-regulator PGC-1α to promote pancreatic cancer malignancy through metabolic remodelling. Nat. Commun. 12:3830. doi: 10.1038/s41467-021-23904-23904
Hussain, S., Tulsyan, S., Dar, S. A., Sisodiya, S., Abiha, U., Kumar, R., et al. (2021). Role of epigenetics in carcinogenesis: recent advancements in anticancer therapy. Semin. Cancer Biol. doi: 10.1016/j.semcancer.2021.06.023 Online ahead of print.
Jantrapirom, S., Koonrungsesomboon, N., Yoshida, H., Candeias, M. M., Pruksakorn, D., and Lo Piccolo, L. (2021). Long noncoding RNA-dependent methylation of nonhistone proteins. Wiley Interdiscip. Rev. RNA. doi: 10.1002/wrna.1661 Online ahead of print.
Jiang, H., Shi, X., Ye, G., Xu, Y., Xu, J., Lu, J., et al. (2019). Up-regulated long non-coding RNA DUXAP8 promotes cell growth through repressing Krüppel-like factor 2 expression in human hepatocellular carcinoma. Onco. Targets Ther. 12, 7429–7436. doi: 10.2147/ott.S214336
Jiang, W., Xia, J., Xie, S., Zou, R., Pan, S., Wang, Z. W., et al. (2020). Long non-coding RNAs as a determinant of cancer drug resistance: towards the overcoming of chemoresistance via modulation of lncRNAs. Drug Resist. Update 50:100683. doi: 10.1016/j.drup.2020.100683
Jin, F., Li, J., Zhang, Y. B., Liu, X., Cai, M., Liu, M., et al. (2021). A functional motif of long noncoding RNA Nron against osteoporosis. Nat. Commun. 12:3319. doi: 10.1038/s41467-021-23642-23647
Jung, G., Hernández-Illán, E., Moreira, L., Balaguer, F., and Goel, A. (2020). Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 17, 111–130. doi: 10.1038/s41575-019-0230-y
Katsushima, K., Lee, B., Kunhiraman, H., Zhong, C., Murad, R., Yin, J., et al. (2021). The long noncoding RNA lnc-HLX-2-7 is oncogenic in Group 3 medulloblastomas. Neuro. Oncol. 23, 572–585. doi: 10.1093/neuonc/noaa235
Kotani, A., Ito, M., and Kudo, K. (2021). Non-coding RNAs and lipids mediate the function of extracellular vesicles in cancer cross-talk. Semin. Cancer Biol. 74, 121–133. doi: 10.1016/j.semcancer.2021.04.017
Lai, C., Liu, L., Liu, Q., Wang, K., Cheng, S., Zhao, L., et al. (2021). Long noncoding RNA AVAN promotes antiviral innate immunity by interacting with TRIM25 and enhancing the transcription of FOXO3a. Cell Death Differ. doi: 10.1038/s41418-021-00791-792 Online ahead of print.
Li, M., Yu, X., Zheng, Q., Zhang, Q., He, Y., and Guo, W. (2021a). Promising role of long non-coding RNA PCAT6 in malignancies. Biomed. Pharmacother. 137:111402. doi: 10.1016/j.biopha.2021.111402
Li, Z., Li, X., Xu, D., Chen, X., Li, S., Zhang, L., et al. (2021c). An update on the roles of circular RNAs in osteosarcoma. Cell Prolif. 54:e12936. doi: 10.1111/cpr.12936
Li, R., Ruan, Q., Zheng, J., Zhang, B., and Yang, H. (2021b). LINC01116 promotes doxorubicin resistance in osteosarcoma by epigenetically silencing miR-424-5p and inducing epithelial-mesenchymal transition. Front. Pharmacol. 12:632206. doi: 10.3389/fphar.2021.632206
Liang, W., Wu, J., and Qiu, X. (2021). LINC01116 facilitates colorectal cancer cell proliferation and angiogenesis through targeting EZH2-regulated TPM1. J. Transl. Med. 19:45. doi: 10.1186/s12967-021-02707-2707
Lin, C., He, H., Liu, H., Li, R., Chen, Y., Qi, Y., et al. (2019). Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 68, 1764–1773. doi: 10.1136/gutjnl-2018-316324
Liu, Y., Qiao, Z., Gao, J., Wu, F., Sun, B., Lian, M., et al. (2021b). Hydroxyapatite-Bovine serum albumin-paclitaxel nanoparticles for locoregional treatment of osteosarcoma. Adv. Healthc. Mater. 10:e2000573. doi: 10.1002/adhm.202000573
Liu, W., Liang, F., Yang, G., and Xian, L. (2021a). LncRNA LINC01116 sponges miR-93-5p to promote cell invasion and migration in small cell lung cancer. BMC Pulm. Med. 21:50. doi: 10.1186/s12890-020-01369-1363
Liu, X., Chen, Y., Li, Y., Petersen, R. B., and Huang, K. (2019). Targeting mitosis exit: a brake for cancer cell proliferation. Biochim. Biophys. Acta Rev. Cancer 1871, 179–191. doi: 10.1016/j.bbcan.2018.12.007
Lou, J., Wang, P., Chang, K., Wang, G., Geng, X., Wu, Y., et al. (2021). Knocking down LINC01116 can inhibit the regulation of TGF-β through miR-774-5p axis and inhibit the occurrence and development of glioma. Am. J. Transl. Res. 13, 5702–5719.
Lu, K. H., Lu, E. W., Lin, C. W., Yang, J. S., and Yang, S. F. (2020). New insights into molecular and cellular mechanisms of zoledronate in human osteosarcoma. Pharmacol. Ther. 214:107611. doi: 10.1016/j.pharmthera.2020.107611
Luo, Y., Zheng, S., Wu, Q., Wu, J., Zhou, R., Wang, C., et al. (2021). Long noncoding RNA (lncRNA) EIF3J-DT induces chemoresistance of gastric cancer via autophagy activation. Autophagy doi: 10.1080/15548627.2021.1901204 Online ahead of print.
Ma, Y., Liu, Y., Teng, L., Luo, E., Liu, D., Zhou, F., et al. (2021). Zi shen decoction inhibits growth and metastasis of lung cancer via regulating the AKT/GSK-3β/β-Catenin pathway. Oxid. Med. Cell Longev. 2021:6685282. doi: 10.1155/2021/6685282
Meng, L., Xing, Z., Guo, Z., and Liu, Z. (2020). LINC01106 post-transcriptionally regulates ELK3 and HOXD8 to promote bladder cancer progression. Cell Death Dis. 11:1063. doi: 10.1038/s41419-020-03236-3239
Mondal, I., and Kulshreshtha, R. (2021). Potential of microRNA based diagnostics and therapeutics in glioma: a patent review. Expert Opin. Ther. Pat. 31, 91–106. doi: 10.1080/13543776.2021.1837775
Mu, L., Ding, K., Tu, R., and Yang, W. (2021). Identification of 4 immune cells and a 5-lncRNA risk signature with prognosis for early-stage lung adenocarcinoma. J. Transl. Med. 19:127. doi: 10.1186/s12967-021-02800-x
Olivero, C. E., Martínez-Terroba, E., Zimmer, J., Liao, C., Tesfaye, E., Hooshdaran, N., et al. (2020). p53 activates the long noncoding RNA Pvt1b to inhibit Myc and suppress tumorigenesis. Mol. Cell 77, 761–774.e8. doi: 10.1016/j.molcel.2019.12.014
Petridis, P. D., Horenstein, C., Pereira, B., Wu, P., Samanamud, J., Marie, T., et al. (2021). BOLD asynchrony elucidates tumor burden in IDH-Mutated gliomas. Neuro Oncol. doi: 10.1093/neuonc/noab154 Online ahead of print.
Puliga, E., Corso, S., Pietrantonio, F., and Giordano, S. (2021). Microsatellite instability in Gastric cancer: between lights and shadows. Cancer Treat. Rev. 95:102175. doi: 10.1016/j.ctrv.2021.102175
Ramnarine, V. R., Kobelev, M., Gibb, E. A., Nouri, M., Lin, D., Wang, Y., et al. (2019). The evolution of long noncoding RNA acceptance in prostate cancer initiation, progression, and its clinical utility in disease management. Eur. Urol. 76, 546–559. doi: 10.1016/j.eururo.2019.07.040
Ren, P., Chang, L., Hong, X., Xing, L., and Zhang, H. (2021). Long non-coding RNA LINC01116 is activated by EGR1 and facilitates lung adenocarcinoma oncogenicity via targeting miR-744-5p/CDCA4 axis. Cancer Cell Int. 21:292. doi: 10.1186/s12935-021-01994-w
Seow, H., Tanuseputro, P., Barbera, L., Earle, C., Guthrie, D., Isenberg, S., et al. (2020). Development and validation of a prognostic survival model with patient-reported outcomes for patients with cancer. JAMA Netw. Open 3:e201768. doi: 10.1001/jamanetworkopen.2020.1768
Shang, B., Li, Z., Li, M., Jiang, S., Feng, Z., Cao, Z., et al. (2021). Silencing LINC01116 suppresses the development of lung adenocarcinoma via the AKT signaling pathway. Thorac. Cancer 12, 2093–2103. doi: 10.1111/1759-7714.14042
Shu, B., Lin, L., Wu, B., Huang, E., Wang, Y., Li, Z., et al. (2021). A pocket-sized device automates multiplexed point-of-care RNA testing for rapid screening of infectious pathogens. Biosens. Bioelectron. 181:113145. doi: 10.1016/j.bios.2021.113145
Shuai, Y., Ma, Z., Liu, W., Yu, T., Yan, C., Jiang, H., et al. (2020). TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2. Mol. Cancer 19:6. doi: 10.1186/s12943-019-1104-1101
Su, X., Zhang, J., Luo, X., Yang, W., Liu, Y., Liu, Y., et al. (2019). LncRNA LINC01116 promotes cancer cell proliferation, migration and invasion in gastric cancer by positively interacting with lncRNA CASC11. Onco Targets Ther. 12, 8117–8123. doi: 10.2147/ott.S208133
Świerczyński, M., Szymaszkiewicz, A., Fichna, J., and Zielińska, M. (2021). New insights into molecular pathways in colorectal cancer: adiponectin, interleukin-6 and opioid signaling. Biochim. Biophys. Acta Rev. Cancer 1875:188460. doi: 10.1016/j.bbcan.2020.188460
Tanabe, R., Miyazono, K., Todo, T., Saito, N., Iwata, C., Komuro, A., et al. (2021). PRRX1 induced by BMP signaling decreases tumorigenesis by epigenetically regulating glioma-initiating cell properties via DNA methyltransferase 3A. Mol. Oncol. doi: 10.1002/1878-0261.13051 Online ahead of print.
Tang, Z., Kang, B., Li, C., Chen, T., and Zhang, Z. (2019). GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–W560. doi: 10.1093/nar/gkz430
Teng, L., Feng, Y. C., Guo, S. T., Wang, P. L., Qi, T. F., Yue, Y. M., et al. (2021). The pan-cancer lncRNA PLANE regulates an alternative splicing program to promote cancer pathogenesis. Nat. Commun. 12:3734. doi: 10.1038/s41467-021-24099-24094
Toden, S., Zumwalt, T. J., and Goel, A. (2021). Non-coding RNAs and potential therapeutic targeting in cancer. Biochim. Biophys. Acta Rev. Cancer 1875:188491. doi: 10.1016/j.bbcan.2020.188491
Tran, N. T., Su, H., Khodadadi-Jamayran, A., Lin, S., Zhang, L., Zhou, D., et al. (2016). The AS-RBM15 lncRNA enhances RBM15 protein translation during megakaryocyte differentiation. EMBO Rep. 17, 887–900. doi: 10.15252/embr.201541970
Tu, J., Fang, Y., Han, D., Tan, X., Jiang, H., Gong, X., et al. (2021). Activation of nuclear factor-κB in the angiogenesis of glioma: insights into the associated molecular mechanisms and targeted therapies. Cell Prolif. 54:e12929. doi: 10.1111/cpr.12929
Wang, W., Hu, S., Gu, Y., Yan, Y., Stovall, D. B., Li, D., et al. (2020d). Human MYC G-quadruplex: from discovery to a cancer therapeutic target. Biochim. Biophys. Acta Rev. Cancer 1874:188410. doi: 10.1016/j.bbcan.2020.188410
Wang, T., Cao, L., Dong, X., Wu, F., De, W., Huang, L., et al. (2020c). LINC01116 promotes tumor proliferation and neutrophil recruitment via DDX5-mediated regulation of IL-1β in glioma cell. Cell Death Dis. 11:302. doi: 10.1038/s41419-020-2506-2500
Wang, H., Lu, B., Ren, S., Wu, F., Wang, X., Yan, C., et al. (2020a). Long noncoding RNA LINC01116 contributes to gefitinib resistance in non-small cell lung cancer through regulating IFI44. Mol. Ther. Nucleic Acids 19, 218–227. doi: 10.1016/j.omtn.2019.10.039
Wang, J., Gao, J., Chen, Q., Zou, W., Yang, F., Wei, C., et al. (2020b). LncRNA LINC01116 contributes to cisplatin resistance in lung adenocarcinoma. Onco Targets Ther. 13, 9333–9348. doi: 10.2147/ott.S244879
Wang, J., Yang, J., Li, D., and Li, J. (2021). Technologies for targeting DNA methylation modifications: basic mechanism and potential application in cancer. Biochim. Biophys. Acta Rev. Cancer 1875:188454. doi: 10.1016/j.bbcan.2020.188454
Wieszczy, P., Kaminski, M. F., Franczyk, R., Loberg, M., Kobiela, J., Rupinska, M., et al. (2020). Colorectal cancer incidence and mortality after removal of adenomas during screening colonoscopies. Gastroenterology 158, 875–883.e5. doi: 10.1053/j.gastro.2019.09.011
Wu, J., Chen, Z., Zhang, L., Cao, J., Li, X., Gong, Z., et al. (2020). Knockdown of LINC01116 inhibits cell migration and invasion in head and neck squamous cell carcinoma through epithelial-mesenchymal transition pathway. J. Cell. Biochem. 121, 867–875. doi: 10.1002/jcb.29331
Xiang, Y., Liao, X. H., Yu, C. X., Yao, A., Qin, H., Li, J. P., et al. (2017). MiR-93-5p inhibits the EMT of breast cancer cells via targeting MKL-1 and STAT3. Exp. Cell Res. 357, 135–144. doi: 10.1016/j.yexcr.2017.05.007
Xing, H., Sun, H., and Du, W. (2020). LINC01116 accelerates nasopharyngeal carcinoma progression based on its enhancement on MYC transcription activity. Cancer Med. 9, 269–277. doi: 10.1002/cam4.2624
Yin, D., Lu, X., Su, J., He, X., De, W., Yang, J., et al. (2018). Long noncoding RNA AFAP1-AS1 predicts a poor prognosis and regulates non-small cell lung cancer cell proliferation by epigenetically repressing p21 expression. Mol. Cancer 17:92. doi: 10.1186/s12943-018-0836-837
Yu, S., Yu, H., Zhang, Y., Liu, C., Zhang, W., and Zhang, Y. (2021). Long non-coding RNA LINC01116 acts as an oncogene in prostate cancer cells through regulation of miR-744-5p/UBE2L3 axis. Cancer Cell Int. 21:168. doi: 10.1186/s12935-021-01843-w
Yuan, J., Xing, H., Li, Y., Song, Y., Zhang, N., Xie, M., et al. (2021). EPB41 suppresses the Wnt/β-catenin signaling in non-small cell lung cancer by sponging ALDOC. Cancer Lett. 499, 255–264. doi: 10.1016/j.canlet.2020.11.024
Zaborowski, A. M., Abdile, A., Adamina, M., Aigner, F., D’allens, L., Allmer, C., et al. (2021). Characteristics of early-onset vs late-onset colorectal cancer: a review. JAMA Surg. 156, 865–874. doi: 10.1001/jamasurg.2021.2380
Zeng, L., Lyu, X., Yuan, J., Wang, W., Zhao, N., Liu, B., et al. (2020). Long non-coding RNA LINC01116 is overexpressed in lung adenocarcinoma and promotes tumor proliferation and metastasis. Am. J. Transl. Res. 12, 4302–4313.
Zeng, L., Lyu, X., Yuan, J., Wang, W., Zhao, N., Liu, B., et al. (2021). Erratum: long non-coding RNA LINC01116 is overexpressed in lung adenocarcinoma and promotes tumor proliferation and metastasis. Am. J. Transl. Res. 13, 3919–3920.
Zhang, B., Yu, L., Han, N., Hu, Z., Wang, S., Ding, L., et al. (2018). LINC01116 targets miR-520a-3p and affects IL6R to promote the proliferation and migration of osteosarcoma cells through the Jak-stat signaling pathway. Biomed. Pharmacother. 107, 270–282. doi: 10.1016/j.biopha.2018.07.119
Zhang, B., Yu, L., Han, N., Hu, Z., Wang, S., Ding, L., et al. (2021a). Corrigendum to “LINC01116 targets miR-520a-3p and affects IL6R to promote the proliferation and migration of osteosarcoma cells through the Jak-stat signaling pathway” [Biomed. Pharmacother. 107 (2018) 270-282]. Biomed. Pharmacother. 133:110893. doi: 10.1016/j.biopha.2020.110893
Zhang, F., Yang, Y., Chen, X., et al. (2021b). The long non-coding RNA βFaar regulates islet β-cell function and survival during obesity in mice. Nat. Commun. 12:3997. doi: 10.1038/s41467-021-24302-24306
Zhang, K., Zhang, M., Luo, Z., Wen, Z., and Yan, X. (2020). The dichotomous role of TGF-β in controlling liver cancer cell survival and proliferation. J. Genet Genomics 47, 497–512. doi: 10.1016/j.jgg.2020.09.005
Zhang, Z. F., Xu, H. H., Hu, W. H., Hu, T. Y., and Wang, X. B. (2019). LINC01116 promotes proliferation, invasion and migration of osteosarcoma cells by silencing p53 and EZH2. Eur. Rev. Med. Pharmacol. Sci. 23, 6813–6823. doi: 10.26355/eurrev_201908_18720
Zhao, Y., Wang, C., and Goel, A. (2021). Role of gut microbiota in epigenetic regulation of colorectal Cancer. Biochim. Biophys. Acta Rev. Cancer 1875:188490. doi: 10.1016/j.bbcan.2020.188490
Zheng, C., Tang, F., Min, L., Hornicek, F., Duan, Z., and Tu, C. (2020). PTEN in osteosarcoma: recent advances and the therapeutic potential. Biochim. Biophys. Acta Rev. Cancer 1874:188405. doi: 10.1016/j.bbcan.2020.188405
Zheng, Q., Zhang, Q., Yu, X., He, Y., and Guo, W. (2021). FENDRR: a pivotal, cancer-related, long non-coding RNA. Biomed. Pharmacother. 137:111390. doi: 10.1016/j.biopha.2021.111390
Zhong, G. X., Ye, C. L., Wei, H. X., Yang, L. Y., Wei, Q. X., Liu, Z. J., et al. (2021a). Ultrasensitive detection of RNA with single-base resolution by coupling electrochemical sensing strategy with chimeric DNA probe-aided ligase chain reaction. Anal. Chem. 93, 911–919. doi: 10.1021/acs.analchem.0c03563
Keywords: long non-coding RNA, LINC01116, human cancers, prognosis, chemoresistance
Citation: Xu Y, Yu X, Zhang M, Zheng Q, Sun Z, He Y and Guo W (2021) Promising Advances in LINC01116 Related to Cancer. Front. Cell Dev. Biol. 9:736927. doi: 10.3389/fcell.2021.736927
Received: 06 July 2021; Accepted: 24 September 2021;
Published: 14 October 2021.
Edited by:
Shiv K. Gupta, Mayo Clinic, United StatesReviewed by:
Luciana N. S. Andrade, Universidade de São Paulo, BrazilCopyright © 2021 Xu, Yu, Zhang, Zheng, Sun, He and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuting He, ZmNjaGV5dDFAenp1LmVkdS5jbg==; Wenzhi Guo, ZmNjZ3Vvd3pAenp1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.