
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol., 07 October 2021
Sec. Molecular and Cellular Pathology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.716302
This article is part of the Research TopicNovel Insights into Biofilms in Infective EndocarditisView all 4 articles
Infective endocarditis is a challenging disease with a high mortality and morbidity rate. Antibiotic prophylaxis is currently recommended in high-risk infective endocarditis patients. However, the use of antibiotics faces the challenge of a low efficacy and contributes further to the emerging infection rate by antibiotic-resistant strains, emphasizing the need for new therapeutic strategies. Platelets are essential in the initial phase of infective endocarditis, acting as first-line immune responders. During the first phase of disease, bacteria can interact with platelets and counteract platelet antimicrobial activities. Mechanistic in vitro and animal studies on the effect of aspirin on bacteria-platelet interactions and the prevention of vegetation development showed promising results. However, data from clinical studies on the outcome of infective endocarditis patients who were receiving medically indicated aspirin therapy remain controversial. Therefore, the benefit of antiplatelet agents in infective endocarditis prevention has been questioned. Besides aspirin, it has been discovered that the platelet P2Y12 receptor antagonist ticagrelor has antibacterial properties in addition to its potent antiplatelet activity. Furthermore, a recent study in mice and a case report remarkably indicated the ability of this drug to eradicate Staphylococcus aureus bacteremia. This review will focus on current knowledge on antibacterial activity of ticagrelor, compared to aspirin, pointing out main unanswered questions. The goal is to provide food for thought as to whether a prior ticagrelor therapy might be beneficial for the prevention of infective endocarditis.
Infective endocarditis (IE) is a life-threatening infectious disease, affecting the heart valves, or (bio-) prosthetic valve implants (Holland et al., 2016). The disease has been associated with a one-year mortality rate of around 30–40% (Liesenborghs et al., 2020). Gram-positive bacteria are the main instigators of IE, with Staphylococcus aureus (S. aureus) being the most prominent and virulent one (Werdan et al., 2014; Tong et al., 2015; Cahill and Prendergast, 2016; Holland et al., 2016; Liesenborghs et al., 2018; Habib et al., 2019). IE is characterized by the formation of a vegetation on the heart valve surface, consisting of bacteria, platelets, fibrin, and leukocytes (Moreillon and Que, 2004; Que and Moreillon, 2011; Liesenborghs et al., 2019). Disease initiation depends on the overall ability of bacteria to be cleared from the blood stream, to adhere to damaged or inflamed endothelium, and to bypass the host defense (Bayer et al., 1997).
Antibiotic prophylaxis is currently recommended in patients at high risk to develop IE (Wilson et al., 2007; Que and Moreillon, 2011). However, this further contributes to a new pandemic of antibiotic-resistant bacterial strains, emphasizing the need for additional strategies to prevent IE development (Que and Moreillon, 2011).
Data from in vitro and in vivo preclinical studies indicated reduced vegetation growth when the antiplatelet agent aspirin was used as prophylactic or adjunct therapy (Nicolau et al., 1995, 1999; Kupferwasser et al., 1999a, b, 2003; Veloso et al., 2015a, b). Several prospective and retrospective clinical studies have evaluated the ability of aspirin to prevent embolic events in IE patients and improve outcome. However, the results of these studies are controversial (Chan et al., 2003, 2008; Anavekar et al., 2007; Eisen et al., 2009; Pepin et al., 2009; Snygg-Martin et al., 2011; Habib et al., 2013) and the clinical usefulness of antiplatelet approaches in IE has been questioned.
We will describe hereafter recent advances on the potential benefits of the platelet P2Y12 receptor inhibitor ticagrelor in IE in regard to data that have previously been obtained with aspirin.
Besides their primary role in thrombosis and hemostasis, it is now well established that platelets also act as first-line immune responders following pathogen invasion (Holinstat, 2017). Platelets express Toll-like receptors (TLRs), enabling them to recognize pathogen-associated molecular patterns (PAMPs). They can target and fight pathogens via the release of antimicrobial peptides from platelet α-granules, including defensins, thrombocidins, and kinocidins (Yeaman, 2010; Wong et al., 2013). Furthermore, they can communicate with, and modulate the function of other immune cells through the release of immunomodulating mediators (Wong et al., 2013; Gaertner et al., 2017; Surewaard et al., 2018). Platelets have been reported to be essential in transporting bacteria to the hepatic Kupffer cells via a “touch-and-go” mechanism, mediating macrophage-induced clearance of bacteria from the blood stream (Wong et al., 2013).
Because inhibiting platelets may prevent them to exert their antimicrobial activity, we need to better understand why and how targeting platelets could be beneficial in bacterial infectious diseases such as IE. The initial phase of IE development involves the interplay between platelets and bacteria (Figure 1; Hamzeh-Cognasse et al., 2015; Liesenborghs et al., 2018, 2019), which strongly suggests an essential role for platelets in early stages of IE. Vegetations could form either via indirect or direct interaction of bacteria with platelets (Hamzeh-Cognasse et al., 2015; Hannachi et al., 2020; Figure 1).
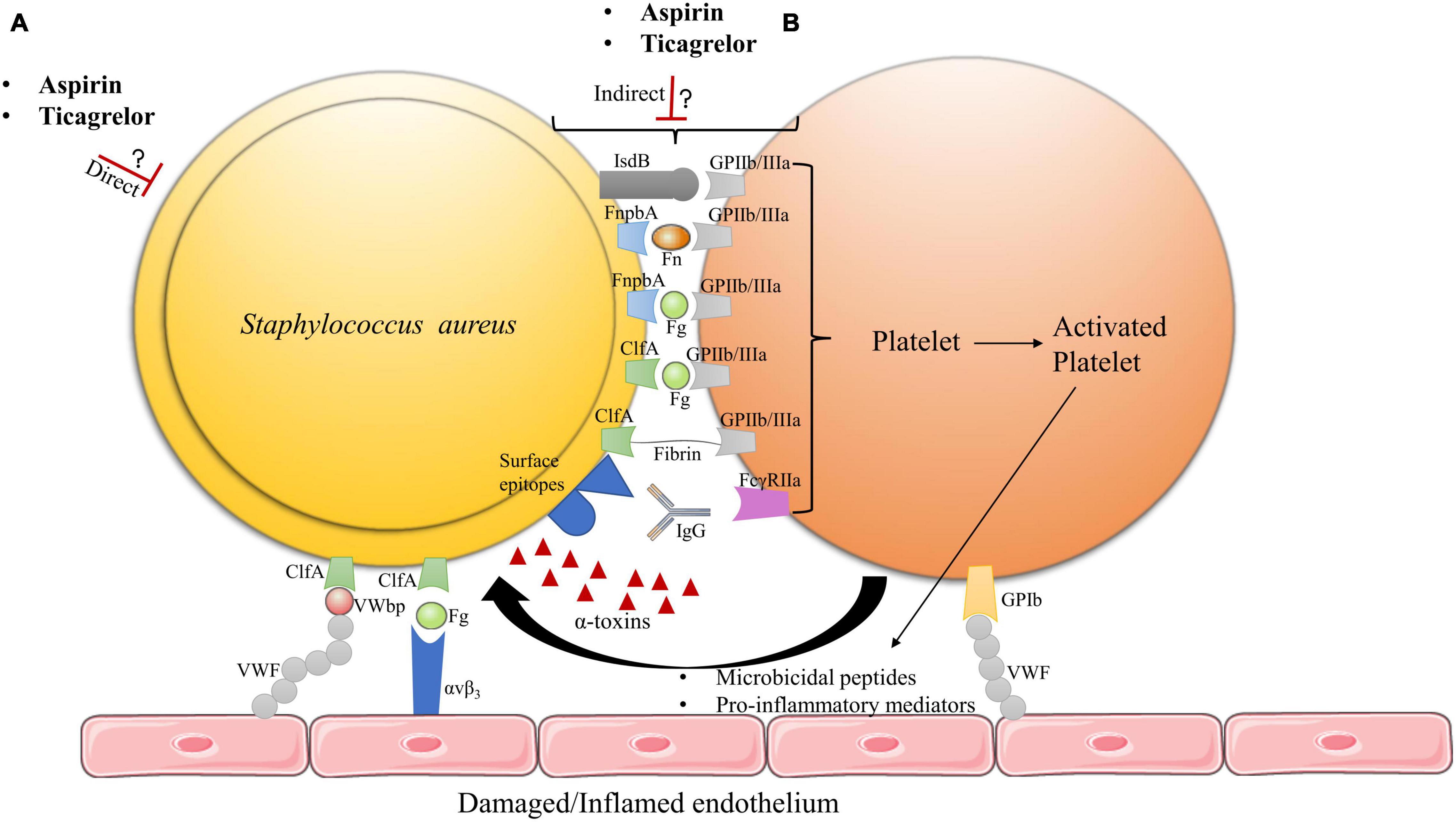
Figure 1. Hypothetical model of aspirin and ticagrelor action on S. aureus IE vegetation. (A) The antiplatelet drugs may exert direct effects on bacteria. (B) Ticagrelor and/or aspirin may inhibit bacteria–platelet interactions by acting on either platelet activation or bacteria toxins, which could also alter the adhesion of these hetero-aggregates on damaged or inflamed endothelia. The exact mechanisms of action of aspirin and ticagrelor on vegetation formation remain to be determined, as well as potential added value of ticagrelor compared to aspirin. FnbpA, fibronectin binding protein A; ClfA, clumping factor A; IsdB, iron regulated surface determinant B; Fn, fibronectin; Fg, fibrinogen; IgG, immunoglobulins; VWF, von Willebrand factor; VWbp, von Willebrand factor binding protein (Claes et al., 2017, 2018; Liesenborghs et al., 2018).
S. aureus –bacteria interactions are mainly mediated through binding of the GPIIb/IIIa platelet receptor (integrin aIIbb3) (Hamzeh-Cognasse et al., 2015; Liesenborghs et al., 2018, 2020). Direct interaction of S. aureus with platelet GPIIb/IIIa can occur via the bacterial iron regulated surface determinant B (IsdB) receptor (Liesenborghs et al., 2018). Indirect interaction of S. aureus to platelets occurs via several surface membrane motifs referred to as microbial surface components recognizing adhesive matrix molecules (MSCRAMM’s), which comprise fibronectin binding protein A (FnbpA) and clumping factor A (ClfA) (Kerrigan et al., 2008; Cox et al., 2011; Liesenborghs et al., 2018). During IE development, ClfA would be essential for early valve colonization whereas FnbpA would be required for persistent infection and further disease progression (Kerrigan et al., 2008; Liesenborghs et al., 2018). MSCRAMM’s can bind plasma proteins, which enables bridging of S. aureus to platelets, mainly via GPIIb/IIIa (Pawar et al., 2004; Kerrigan et al., 2008; Liesenborghs et al., 2018). While ClfA binds with a high affinity to soluble and immobilized fibrinogen (Fg) as well as fibrin, FnbpA binds to both Fg and Fibronectin (Fn), but, with a higher affinity for Fn (Wann et al., 2000; Claes et al., 2018; Liesenborghs et al., 2018). In addition to GPIIb/IIIa platelet receptor activation, bridging of S. aureus via immunoglobulins (IgG) to the FcγRIIa platelet receptor is required to induce full aggregation of platelets (Liesenborghs et al., 2018). MSCRAMM’s are also essential in mediating bacterial adhesion to the heart valve surface. Such adhesion is achieved via direct binding of S. aureus to endothelial exposed von Willebrand factor (VWF) through the bacteria-secreted von Willebrand factor binding protein (VWbp) and bridging of S. aureus to the αvβ3 endothelial integrin via Fg. Furthermore, bacteria can use platelets to adhere to the endothelium despite the high shear stress that is encountered at heart valves (Claes et al., 2017, 2018; Liesenborghs et al., 2018, 2019). Based on these mechanisms, it is possible that inhibiting the aspirin-sensitive or the P2Y12 receptor ADP-dependent amplification pathways of platelet activation, downstream of GPIIb/IIIa, could impact platelet–bacteria interactions and eventually, IE development.
Bacteria like S. aureus are capable of developing several virulence mechanisms to counteract platelet antimicrobial activity (Hamzeh-Cognasse et al., 2015; Liesenborghs et al., 2020). These mechanisms may allow bacterial survival in the bloodstream and contribute to the development of endovascular infectious diseases such as IE. Of interest is the secreted S. aureus α-toxin (Cox et al., 2011; Hamzeh-Cognasse et al., 2015). This pore-forming protein, encoded by the Hla gene, interacts with several eukaryotic cell types, including myeloid cells, platelets, and endothelial cells via its disintegrin and metalloproteinase 10 (ADAM10) receptor (Powers et al., 2015; Surewaard et al., 2018). At sub-cytolytic concentration, α-toxin binding to ADAM10 induces proteolysis of VE-cadherin, causing activation of the endothelium (Powers et al., 2012). On platelets, the sub-cytolytic concentration of α-toxin induces ADAM10-mediated cleavage of the platelet GPVI receptor, which hampers platelet adhesion and aggregation. In contrast, at cytolytic concentration, α-toxin causes aberrant platelet activation and aggregation (Bhakdi et al., 1988; Surewaard et al., 2018). Accordingly, observations by Bhakdi et al. (1988) and Bayer et al. (1997) indicate that α-toxin can promote the formation of IE thrombi. α-toxin also enables bacteria to evade platelet antimicrobial activity and cause activation of pro-inflammatory pathways (Powers et al., 2015; Surewaard et al., 2018). Recently, Sun et al. (2021) reported α-toxin to induce the release of endogenous platelet sialidase, resulting in desialysation of platelet glycoproteins and β-galactose exposure. This process accelerates platelet clearance by the hepatic Ashwell-Morell receptor (AMR), which is responsible for S. aureus bacteremia-associated thrombocytopenia (Sørensen et al., 2009; Surewaard et al., 2018; Sun et al., 2021).
Thus, preventing α-toxin from inhibiting platelet antimicrobial activity could also be considered as part of the antiplatelet approach against IE.
In vitro studies focusing on the effect of aspirin on platelet–S. aureus interactions found that its main metabolite salicylic acid (SAL) regulates the expression of S. aureus genes encoding for virulence factors (Kupferwasser et al., 1999a). SAL has been linked to an overexpression of the sigma factor B operon, resulting in the repression of staphylococcal accessory regulator A (Sar A) and accessory gene regulator (Agr). By repressing Sar A and Agr, SAL can diminish the expression of MSCRAMM’s and α-toxin secretion (Kupferwasser et al., 2003; Gordon et al., 2013). The reduced expression of virulence factors could result in slowing down vegetation growth by decreasing platelet–bacteria interactions, thereby enhancing the antimicrobial activity of platelets. More particularly, the inhibition of α-toxin secretion could delay α-toxin enhanced platelet clearance via the hepatic AMR pathway, preserving platelet function (Surewaard et al., 2018; Sun et al., 2021).
Several studies have been performed in different animal models of IE in order to analyze the effect of aspirin on vegetation growth (Table 1). Kupferwasser et al. (1999b) described a significant reduction in bacterial density and vegetation weight using a prophylactic therapy of 8 mg/kg aspirin in a rabbit model of S. aureus IE (SAIE). Furthermore, this study indicated that pre-treatment of S. aureus with SAL reduced the ability of bacteria to adhere to vegetations (fibrin-platelet surface) (Kupferwasser et al., 1999b). Another study in rabbits showed a key role for both Sar A and the stress response gene sigB in mediating the antistaphylococcal effects of SAL in vivo (Kupferwasser et al., 2003). In contrast, studies by Nicolau et al. (1999) and Veloso et al. (2015b) described no reduction of vegetation weight when preventively using 10 and 8 mg/kg of aspirin as a monotherapy in a rabbit and rat model of IE, respectively. While Nicolau et al. (1999) reported this effect to be related to the low sample size of the study, Veloso et al. (2015b) stated a possible effect of bolus injection of bacteria in previous models, which induced transient bacteremia, thus negating the effect of preventive antiplatelet therapy. However, Veloso et al. (2015b) could observe a significant decrease in vegetation weight when using aspirin in combination with ticlopidine, another antiplatelet drug belonging to the thienopyridine class of platelet P2Y12 receptor inhibitors. Finally, a combination of aspirin with vancomycin was described to significantly decrease vegetation weight and bacterial density, emphasizing its potential as an adjuvant therapeutic agent (Nicolau et al., 1995; Veloso et al., 2015b).
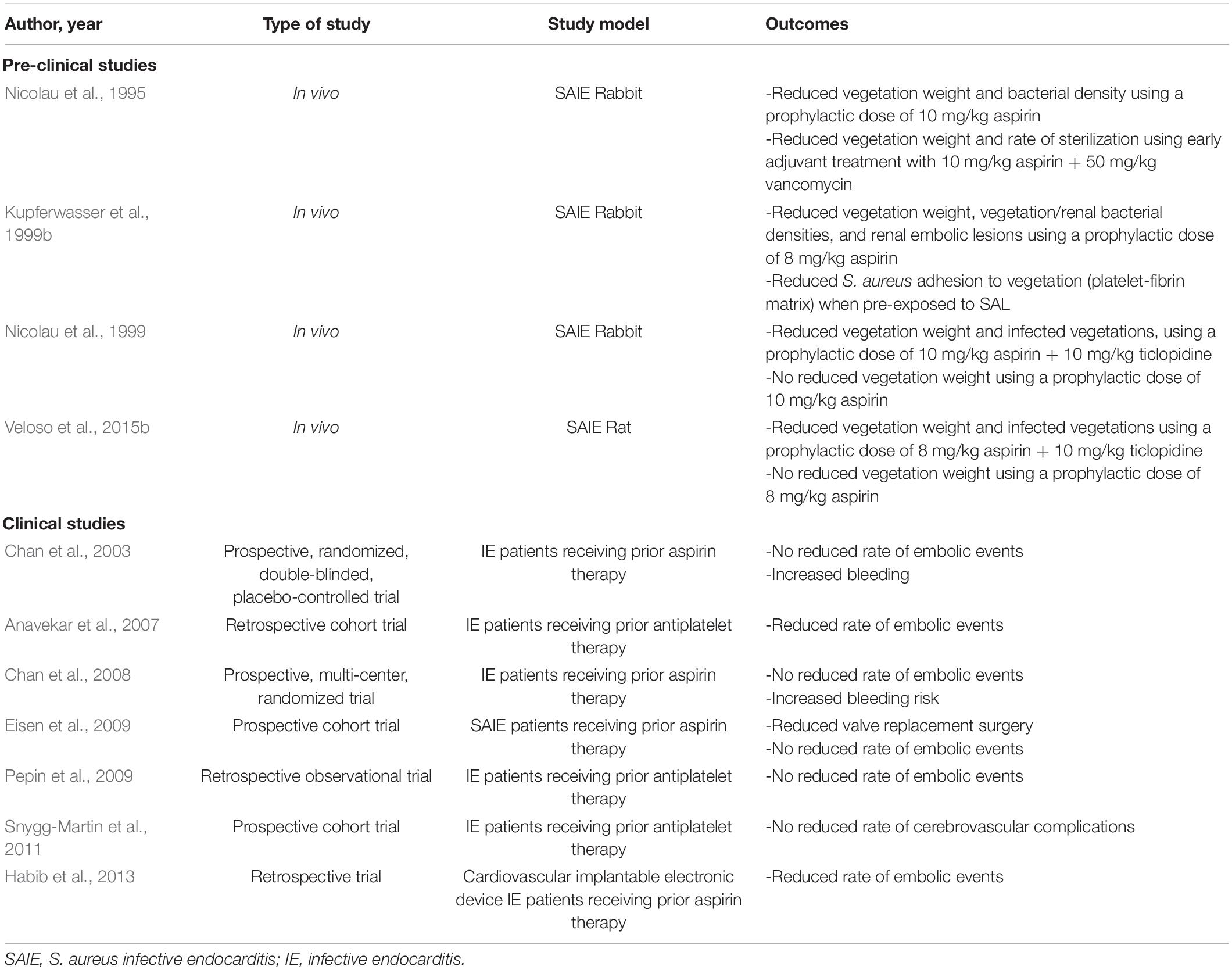
Table 1. Overview of pre-clinical and clinical studies on the use of antiplatelet therapy in the prevention of infective endocarditis.
Clinical studies focusing on prior aspirin therapy in patients at high risk of IE described variable outcomes in relation to the prevention of embolic events (Table 1). Chan et al. (2003, 2008) reported no benefit of aspirin in reducing the risk of embolic events in IE patients, however increased bleeding was observed. This was further confirmed by Snygg-Martin et al. (2011), Eisen et al. (2009), and Pepin et al. (2009), showing no reduction of cerebrovascular complications or embolic events in patients on previously established antiplatelet therapy (mostly aspirin) (Trauer et al., 2017). In contrast, Anavekar et al. (2007) and Habib et al. (2013) described prior aspirin therapy, to reduce vegetation formation and embolic events. Despite promising mechanistic in vitro and animal studies, clinical studies showed controversial results. Thus, there is currently no evidence for any benefits of antiplatelet drugs such as aspirin in improving IE patient outcome. Nevertheless, many of these clinical studies had a low sample size which made it difficult to obtain sufficient statistical power. Furthermore, there is a large heterogeneity in patient age, comorbidities, the duration and dose of antiplatelet therapy prior to IE development or after, and bacterial strains involved in disease development.
In contrast, the relatively more recent antiplatelet drug ticagrelor, a reversible platelet P2Y12 receptor inhibitor, has become subject of discussion. In a sub-study of the large, randomized PLATO clinical trial, ticagrelor therapy was associated with a lower risk of death related to infection as compared to the thienopyridine clopidogrel. In addition, the small XANTHIPPE clinical study showed improved lung function in pneumonia patients treated with ticagrelor (Wallentin et al., 2009; Sexton et al., 2018; Lupu et al., 2020). The study by Lancellotti et al. (2019a) demonstrated bactericidal activity of ticagrelor and its main metabolite against Gram-positive bacteria such as methicillin-susceptible S. aureus (MSSA) and E. faecalis, as well as Gram-positive resistant strains, including methicillin-resistant S. epidermidis (MRSE), methicillin-resistant S. aureus (MRSA), and Vancomycin-resistant Enterococcus (VRE). Importantly, these effects were not observed with the active metabolite of prasugrel, another thienopyridine P2Y12 inhibitor (Wallentin et al., 2009; Lancellotti et al., 2019a).
The reported in vitro antibacterial concentration (Minimum inhibitory concentration value) of ticagrelor against MSSA and MRSA was around ten times higher than the recommended antiplatelet dosage (Lancellotti et al., 2019a). However, the use of a mouse model implanted with an S. aureus-infected subcutaneous disc supported the antibacterial effect of ticagrelor at antiplatelet dosage, as shown by a significant decrease of S. aureus biofilm growth on implants and dissemination of bacteria to surrounding tissues (Lancellotti et al., 2019a). Although systemically, bactericidal concentrations are not reached in vivo, bactericidal activity at the infection site could still be achieved at antiplatelet dosage through an unknown mechanism, hypothesized to be platelet related (Lancellotti et al., 2019a). Recently, a preclinical and in vitro study has been performed focusing on the role of ticagrelor in eradicating S. aureus bacteremia and preserving the ability of platelets to kill bacteria (Sun et al., 2021; Ulloa et al., 2021). Ulloa et al. (2021) described successful use of ticagrelor as an adjuvant therapy to antibiotics in a case report of a male patient with MSSA bacteremia and thrombocytopenia. The patient received antibiotic treatment but remained bacteremic. On day five, ticagrelor was administered which resulted in a decreased, non-detectable bacterial blood count and an increase in platelet count to a low-normal range (Ulloa et al., 2021). Furthermore, the case report result was supported by an in vitro study, showing that ticagrelor could prevent α-toxin-induced inhibition of platelet antibacterial activity (Sun et al., 2021; Ulloa et al., 2021). Indeed, in vitro, platelet pre-treatment with ticagrelor improved S. aureus killing (Sun et al., 2021; Ulloa et al., 2021). However, the mechanism of such an effect remains unclear.
To date, no studies have investigated the potential effect of prior ticagrelor therapy in preventing IE development.
The use of antiplatelet drugs as an adjunct therapy to prevent vegetation growth, embolic events, or to improve the outcome in high-risk cardiovascular patients with IE has been and should still be a matter of great interest. While the mode of action and possible benefits of aspirin in the prevention of IE progression have been widely investigated, the more recent antiplatelet drug ticagrelor deserves attention. Several hypotheses have been proposed regarding its antibacterial properties. Lancellotti et al. (2019b) reported a possible role of platelets for ticagrelor transport to the site of infection, allowing a local antibacterial effect. This hypothesis is based on the reversible binding properties of ticagrelor to the P2Y12 receptor, and on studies indicating that platelets are recruited to the site of infection, similar to immune cells (Lancellotti et al., 2019b). Heying et al. (2019) proposed that the bactericidal properties of ticagrelor could resemble the aspirin effect, modulating the expression of S. aureus virulence factors with a decrease in the expression of MSCRAMM’s and toxins. The potential inhibitory effect of ticagrelor on bacteria-platelet interactions was further supported by an in vitro study reporting the highest inhibitory effect of bacteria-induced platelet aggregation by ticagrelor as compared to aspirin, aspirin plus ticagrelor, or tirofiban (Hannachi et al., 2020). Very recently, two studies described an inhibitory effect of ticagrelor on α-toxin mediated platelet clearance by the hepatic AMR pathways, thereby preserving the antibacterial activity of platelets (Sun et al., 2021; Ulloa et al., 2021). In addition, antiplatelet drugs could also inhibit the immune and inflammatory role of platelets (Tiwari et al., 2020). While aspirin inhibits the release of inflammatory mediators by platelets such as leukotrienes, ticagrelor blocks the formation of platelet-leukocyte aggregates (Tiwari et al., 2020), which could also play a role during the process of infection, as proposed by Sexton et al. (2018).
Antibiotic prophylaxis is currently recommended to prevent IE development in high-risk patients. However, the use of antibiotics faces the challenge of a low efficacy due to the steadily increasing infection rate by resistant bacteria strains, which is further enhanced by using antibiotics. This review suggests that the antiplatelet drug ticagrelor combined with antibiotics may play a role in the prevention of SAIE. Indeed, this drug was recently described to have antibacterial properties in addition to its potent antiplatelet activity. Moreover, a recent study in mice and a case report remarkably indicated the ability of ticagrelor to eradicate S. aureus bacteremia. Therefore, further investigations should be performed in order to evaluate whether prior ticagrelor therapy could be beneficial for the prevention of IE or other endovascular infectious diseases. This new strategy could contribute to a decrease in antibiotic resistance and a significant reduction in disease-associated mortality.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
CO is Research Director at the National Fund for Scientific Research, Belgium, and obtained a grant from FNRS-F.R.S. (T.0190.20 – PDR).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anavekar, N. S., Tleyjeh, I. M., Anavekar, N. S., Mirzoyev, Z., Steckelberg, J. M., Haddad, C., et al. (2007). Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin. Infect. Dis. 44, 1180–1186. doi: 10.1086/513197
Bayer, A. S., Ramos, M. D., Menzies, B. E., Yeaman, M. R., Shen, A. J., and Cheung, A. L. (1997). Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect. Immun. 65, 4652–4660. doi: 10.1128/iai.65.11.4652-4660.1997
Bhakdi, S., Muhly, M., Mannhardt, U., Hugo, F., Klapettek, K., Mueller-Eckhardt, C., et al. (1988). Staphylococcal α toxin promotes blood coagulation via attack on human platelets. J. Exp. Med. 168, 527–542. doi: 10.1084/jem.168.2.527
Cahill, T. J., and Prendergast, B. D. (2016). Infective endocarditis. Lancet 387, 882–893. doi: 10.1016/S0140-6736(15)00067-7
Chan, K. L., Dumesnil, J. G., Cujec, B., Sanfilippo, A. J., Jue, J., Turek, M. A., et al. (2003). A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J. Am. Coll. Cardiol. 42, 775–780. doi: 10.1016/S0735-1097(03)00829-5
Chan, K. L., Tam, J., Dumesnil, J. G., Cujec, B., Sanfilippo, A. J., Jue, J., et al. (2008). Effect of long-term aspirin use on embolic events in infective endocarditis. Clin. Infect. Dis. 46, 37–41. doi: 10.1086/524021
Claes, J., Ditkowski, B., Liesenborghs, L., Veloso, T. R., Entenza, J. M., Moreillon, P., et al. (2018). Assessment of the dual role of clumping factor A in S. Aureus adhesion to endothelium in absence and presence of plasma. Thromb. Haemost. 118, 1230–1241. doi: 10.1055/s-0038-1660435
Claes, J., Liesenborghs, L., Peetermans, M., Veloso, T. R., Missiakas, D., Schneewind, O., et al. (2017). Clumping factor A, von Willebrand factor-binding protein and von Willebrand factor anchor Staphylococcus aureus to the vessel wall. J. Thromb. Haemost. 15, 1009–1019. doi: 10.1111/jth.13653
Cox, D., Kerrigan, S. W., and Watson, S. P. (2011). Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J. Thromb. Haemost. 9, 1097–1107. doi: 10.1111/j.1538-7836.2011.04264.x
Eisen, D. P., Corey, G. R., McBryde, E. S., Fowler, V. G., Miro, J. M., Cabell, C. H., et al. (2009). Reduced valve replacement surgery and complication rate in Staphylococcus aureus endocarditis patients receiving acetyl-salicylic acid. J. Infect. 58, 332–338. doi: 10.1016/j.jinf.2009.03.006
Gaertner, F., Ahmad, Z., Rosenberger, G., Fan, S., Nicolai, L., Busch, B., et al. (2017). Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell 171, 1368.e–1382.e. doi: 10.1016/j.cell.2017.11.001
Gordon, C. P., Williams, P., and Chan, W. C. (2013). Attenuating Staphylococcus aureus virulence gene regulation: a medicinal chemistry perspective. J. Med. Chem. 56, 1389–1404. doi: 10.1021/jm3014635
Habib, A., Irfan, M., Baddour, L. M., Le, K. Y., Anavekar, N. S., Lohse, C. M., et al. (2013). Impact of prior aspirin therapy on clinical manifestations of cardiovascular implantable electronic device infections. Europace 15, 227–235. doi: 10.1093/europace/eus292
Habib, G., Lancellotti, P., Erba, P. A., Sadeghpour, A., Meshaal, M., Sambola, A., et al. (2019). The ESC-EORP EURO-ENDO (European Infective Endocarditis) registry. Eur. Heart J. Qual. Care Clin. Outcomes 5, 202–207. doi: 10.1093/ehjqcco/qcz018
Hamzeh-Cognasse, H., Damien, P., Chabert, A., Pozzetto, B., Cognasse, F., and Garraud, O. (2015). Platelets and infections-Complex interactions with bacteria. Front. Immunol. 6:82. doi: 10.3389/fimmu.2015.00082
Hannachi, N., Ogé-Ganaye, E., Baudoin, J. P., Fontanini, A., Bernot, D., Habib, G., et al. (2020). Antiplatelet agents have a distinct efficacy on platelet aggregation induced by infectious bacteria. Front. Pharmacol. 11:863. doi: 10.3389/fphar.2020.00863
Heying, R., Vanassche, T., and Moreillon, P. (2019). Are antiplatelet agents beneficial in prevention of infective endocarditis? JAMA Cardiol. 4:1177. doi: 10.1001/jamacardio.2019.3130
Holinstat, M. (2017). Normal platelet function. Cancer Metastasis Rev. 36, 195–198. doi: 10.1007/s10555-017-9677-x
Holland, T. L., Baddour, L. M., Bayer, A. S., Hoen, B., Miro, J. M., and Fowler, V. G. (2016). Infective endocarditis. Nat. Rev. Dis. Prim. 2:16059. doi: 10.1038/nrdp.2016.59
Kerrigan, S. W., Clarke, N., Loughman, A., Meade, G., Foster, T. J., and Cox, D. (2008). Molecular basis for Staphylococcus aureus-mediated platelet aggregate formation under arterial shear in vitro. Arterioscler. Thromb. Vasc. Biol. 28, 335–340. doi: 10.1161/ATVBAHA.107.152058
Kupferwasser, L. I., Skurray, R. A., Brown, M. H., Firth, N., Yeaman, M. R., and Bayer, A. S. (1999a). Plasmid-mediated resistance to thrombin-induced platelet microbicidal protein in Staphylococci: role of the qacA locus. Antimicrob. Agents Chemother. 43, 2395–2399. doi: 10.1128/aac.43.10.2395
Kupferwasser, L. I., Yeaman, M. R., Nast, C. C., Kupferwasser, D., Xiong, Y.-Q., Palma, M., et al. (2003). Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J. Clin. Invest. 112, 222–233. doi: 10.1172/jci16876
Kupferwasser, L. I., Yeaman, M. R., Shapiro, S. M., Nast, C. C., Sullam, P. M., Filler, S. G., et al. (1999b). Acetylsalicylic acid reduces vegetation bacterial density, hematogenous bacterial dissemination, and frequency of embolic events in experimental Staphylococcus aureus endocarditis through antiplatelet and antibacterial effects. Circulation 99, 2791–2797. doi: 10.1161/01.CIR.99.21.2791
Lancellotti, P., Musumeci, L., Jacques, N., Servais, L., Goffin, E., Pirotte, B., et al. (2019a). Antibacterial activity of ticagrelor in conventional antiplatelet dosages against antibiotic-resistant gram-positive bacteria. JAMA Cardiol. 4, 596–599. doi: 10.1001/jamacardio.2019.1189
Lancellotti, P., Musumeci, L., and Oury, C. (2019b). Are antiplatelet agents beneficial in prevention of infective endocarditis?-Reply. JAMA Cardiol. 4, 1177–1178.
Liesenborghs, L., Meyers, S., Lox, M., Criel, M., Claes, J., Peetermans, M., et al. (2019). Staphylococcus aureus endocarditis: distinct mechanisms of bacterial adhesion to damaged and inflamed heart valves. Eur. Heart J. 40, 3248–3259. doi: 10.1093/eurheartj/ehz175
Liesenborghs, L., Meyers, S., Vanassche, T., and Verhamme, P. (2020). Coagulation: at the heart of infective endocarditis. J. Thromb. Haemost. 18, 995–1008. doi: 10.1111/jth.14736
Liesenborghs, L., Verhamme, P., and Vanassche, T. (2018). Staphylococcus aureus, master manipulator of the human hemostatic system. J. Thromb. Haemost. 16, 441–454. doi: 10.1111/jth.13928
Lupu, L., Shepshelovich, D., Banai, S., Hershkoviz, R., and Isakov, O. (2020). Effect of ticagrelor on reducing the risk of gram-positive infections in patients with acute coronary syndrome. Am. J. Cardiol. 130, 56–63. doi: 10.1016/j.amjcard.2020.06.016
Moreillon, P., and Que, Y. A. (2004). Infective endocarditis. Lancet 363, 139–149. doi: 10.1016/S0140-6736(03)15266-X
Nicolau, D. P., Marangos, M. N., Nightingale, C. H., and Quintiliani, R. (1995). Influence of aspirin on development and treatment of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 39, 1748–1751. doi: 10.1128/AAC.39.8.1748
Nicolau, D. P., Tessier, P. R., and Nightingale, C. H. (1999). Beneficial effect of combination antiplatelet therapy on the development of experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents 11, 159–161. doi: 10.1016/S0924-8579(98)00092-2
Pawar, P., Shin, P. K., Mousa, S. A., Ross, J. M., and Konstantopoulos, K. (2004). Fluid shear regulates the kinetics and receptor specificity of Staphylococcus aureus binding to activated platelets. J. Immunol. 173, 1258–1265. doi: 10.4049/jimmunol.173.2.1258
Pepin, J., Tremblay, V., Bechard, D., Rodier, F., Walker, C., Dufresne, D., et al. (2009). Chronic antiplatelet therapy and mortality among patients with infective endocarditis. Clin. Microbiol. Infect. 15, 193–199. doi: 10.1111/j.1469-0691.2008.02665.x
Powers, M. E., Becker, R. E. N., Sailer, A., Turner, J. R., and Bubeck Wardenburg, J. (2015). Synergistic action of Staphylococcus aureus α-toxin on platelets and myeloid lineage cells contributes to lethal sepsis. Cell Host Microbe 17, 775–787. doi: 10.1016/j.chom.2015.05.011
Powers, M. E., Kim, H. K., Wang, Y., and Wardenburg, J. B. (2012). ADAM10 mediates vascular injury induced by Staphylococcus aureus α-hemolysin. J. Infect. Dis. 206, 352–356. doi: 10.1093/infdis/jis192
Que, Y. A., and Moreillon, P. (2011). Infective endocarditis. Nat. Rev. Cardiol. 8, 322–336. doi: 10.1038/nrcardio.2011.43
Sexton, T. R., Zhang, G., Macaulay, T. E., Callahan, L. A., Charnigo, R., Vsevolozhskaya, O. A., et al. (2018). Ticagrelor reduces thromboinflammatory markers in patients with pneumonia. JACC Basic Transl. Sci. 3, 435–449. doi: 10.1016/j.jacbts.2018.05.005
Snygg-Martin, U., Rasmussen, R. V., Hassager, C., Bruun, N. E., Andersson, R., and Olaison, L. (2011). The relationship between cerebrovascular complications and previously established use of antiplatelet therapy in left-sided infective endocarditis. Scand. J. Infect. Dis. 43, 899–904. doi: 10.3109/00365548.2011.603742
Sørensen, A. L., Rumjantseva, V., Nayeb-Hashemi, S., Clausen, H., Hartwig, J. H., Wandall, H. H., et al. (2009). Role of sialic acid for platelet life span: exposure of β-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood 114, 1645–1654. doi: 10.1182/blood-2009-01-199414
Sun, J., Uchiyama, S., Olson, J., Morodomi, Y., Cornax, I., Ando, N., et al. (2021). Repurposed drugs block toxin-driven platelet clearance by the hepatic Ashwell-Morell receptor to clear Staphylococcus aureus bacteremia. Sci. Transl. Med. 13:eabd6737. doi: 10.1126/scitranslmed.abd6737
Surewaard, B. G. J., Thanabalasuriar, A., Zeng, Z., Tkaczyk, C., Cohen, T. S., Bardoel, B. W., et al. (2018). α-Toxin Induces Platelet Aggregation and Liver Injury during Staphylococcus aureus Sepsis. Cell Host Microbe 24, 271.e–284.e. doi: 10.1016/j.chom.2018.06.017
Tiwari, N. R., Chaudhari, K. S., Sharma, R., Haas, K. P., and Sharma, V. R. (2020). Antiplatelet agents in sepsis-putting it all together: a call to action. Indian J. Crit. Care Med. 24, 483–484. doi: 10.5005/jp-journals-10071-23450
Tong, S. Y. C., Davis, J. S., Eichenberger, E., Holland, T. L., and Fowler, V. G. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. doi: 10.1128/CMR.00134-14
Trauer, J., Muhi, S., McBryde, E. S., Al Harbi, S. A., Arabi, Y. M., Boyle, A. J., et al. (2017). Quantifying the effects of prior acetyl-salicylic acid on sepsis-related deaths: an individual patient data meta-analysis using propensity matching. Crit. Care Med. 45, 1871–1879. doi: 10.1097/CCM.0000000000002654
Ulloa, E. R., Uchiyama, S., Gillespie, R., Nizet, V., and Sakoulas, G. (2021). Ticagrelor increases platelet-mediated Staphylococcus aureus killing resulting in clearance of bacteremia. J. Infect. Dis. 146:jiab146. doi: 10.1093/infdis/jiab146
Veloso, T. R., Oechslin, F., Que, Y. A., Moreillon, P., Entenza, J. M., and Mancini, S. (2015a). Aspirin plus ticlopidine prevented experimental endocarditis due to Enterococcus faecalis and Streptococcus gallolyticus. Pathog. Dis. 73:ftv060.
Veloso, T. R., Que, Y.-A., Chaouch, A., Giddey, M., Vouillamoz, J., Rousson, V., et al. (2015b). Prophylaxis of experimental endocarditis with antiplatelet and antithrombin agents: a role for long-term prevention of infective endocarditis in humans? J. Infect. Dis. 211, 72–79. doi: 10.1093/infdis/jiu426
Wallentin, L., Becker, R. C., Budaj, A., Cannon, C. P., Emanuelsson, H., Held, C., et al. (2009). Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057. doi: 10.1056/nejmoa0904327
Wann, E. R., Gurusiddappa, S., and Hook, M. (2000). The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 275, 13863–13871. doi: 10.1074/jbc.275.18.13863
Werdan, K., Dietz, S., Löffler, B., Niemann, S., Bushnaq, H., Silber, R. E., et al. (2014). Mechanisms of infective endocarditis: pathogen-host interaction and risk states. Nat. Rev. Cardiol. 11, 35–50. doi: 10.1038/nrcardio.2013.174
Wilson, W., Taubert, K. A., Gewitz, M., Lockhart, P. B., Baddour, L. M., Levison, M., et al. (2007). Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 116, 1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095
Wong, C. H. Y., Jenne, C. N., Petri, B., Chrobok, N. L., and Kubes, P. (2013). Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat. Immunol. 14, 785–792. doi: 10.1038/ni.2631
Keywords: infective endocarditis, antiplatelet drugs, ticagrelor, aspirin, biofilm, Staphylococcus aureus
Citation: Leeten K, Jacques N, Lancellotti P and Oury C (2021) Aspirin or Ticagrelor in Staphylococcus aureus Infective Endocarditis: Where Do We Stand? Front. Cell Dev. Biol. 9:716302. doi: 10.3389/fcell.2021.716302
Received: 28 May 2021; Accepted: 21 September 2021;
Published: 07 October 2021.
Edited by:
Marc F. Hoylaerts, KU Leuven, BelgiumReviewed by:
Ulrika Snygg-Martin, University of Gothenburg – Sahlgrenska University Hospital, SwedenCopyright © 2021 Leeten, Jacques, Lancellotti and Oury. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cécile Oury, Y2VjaWxlLm91cnlAdWxpZWdlLmJl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.