- 1National Institute of Biomedical Genomics, Kalyani, India
- 2Regional Centre for Biotechnology, Faridabad, India
RNA-binding proteins (RBPs) play a significant role in multiple cellular processes with their deregulations strongly associated with cancer. However, there are not adequate evidences regarding global alteration and functions of RBPs in pancreatic cancer, interrogated in a systematic manner. In this study, we have prepared an exhaustive list of RBPs from multiple sources, downloaded gene expression microarray data from a total of 241 pancreatic tumors and 124 normal pancreatic tissues, performed a meta-analysis, and obtained differentially expressed RBPs (DE-RBPs) using the Limma package of R Bioconductor. The results were validated in microarray datasets and the Cancer Genome Atlas (TCGA) RNA sequencing dataset for pancreatic adenocarcinoma (PAAD). Pathway enrichment analysis was performed using DE-RBPs, and we also constructed the protein–protein interaction (PPI) network to detect key modules and hub-RBPs. Coding and noncoding targets for top altered and hub RBPs were identified, and altered pathways modulated by these targets were also investigated. Our meta-analysis identified 45 upregulated and 15 downregulated RBPs as differentially expressed in pancreatic cancer, and pathway enrichment analysis demonstrated their important contribution in tumor development. As a result of PPI network analysis, 26 hub RBPs were detected and coding and noncoding targets for all these RBPs were categorized. Functional exploration characterized the pathways related to epithelial-to-mesenchymal transition (EMT), cell migration, and metastasis to emerge as major pathways interfered by the targets of these RBPs. Our study identified a unique meta-signature of 26 hub-RBPs to primarily modulate pancreatic tumor cell migration and metastasis in pancreatic cancer. IGF2BP3, ISG20, NIP7, PRDX1, RCC2, RUVBL1, SNRPD1, PAIP2B, and SIDT2 were found to play the most prominent role in the regulation of EMT in the process. The findings not only contribute to understand the biology of RBPs in pancreatic cancer but also to evaluate their candidature as possible therapeutic targets.
Background
Pancreatic cancer is one of the most morbid cancer types worldwide and is mostly diagnosed at a very advanced stage with a 5-year survival rate of 8.2% (Gordon-Dseagu et al., 2018). Recent studies have identified several mutations to be predominant in different pancreatic cancer subtypes, and about 80% of these mutations are found to be sporadic (Feldmann et al., 2007; McGuigan et al., 2018). Pancreatic cancer is a heterogeneous disease with aberrant gene expression patterns like any other cancer. Therefore, in order to understand the biology of the disease, altered regulatory events need to be portrayed at the level of gene to RNA to protein to signaling pathways of the cell.
After the expression of the genes, the roadway to the functionality of the transcripts is highly regulated at the posttranscriptional and translational level. RNA-binding proteins (RBPs) are an integral part of this regulatory machinery participating in all the cellular processes. RBPs exert multi-functionality in directing the fate of the transcripts such as splicing, stability, translocation, translation, and decay (Neelamraju et al., 2015). A transcript interacts with different RBPs at different stages of life, and its fate and functionality is decided accordingly. Canonical RBPs bind to the RNAs in a sequence-specific manner with the help of RNA-binding domains (RBDs) and form dynamic ribonucleoprotein (RNP) complexes to execute the regulatory function. RBPs can bind at the coding region of the mRNA, but it is more prevalent in the regulatory elements of the RNA such as the 5′ and 3′ untranslated regions (UTRs) and in the alternative UTRs of mRNA isoforms during RNA processing (Wurth and Gebauer, 2015). There are also evidences of RBP interaction with the regulatory non-coding RNAs such as lncRNAs and miRNAs to form more complex regulatory networks. Sometimes few unconventional RBPs act as “mRNA clothes,” to expose or hide specific coding and non-coding parts of an mRNA in order to drive its activity (Singh et al., 2015; Hentze et al., 2018). Being such a pivotal player of the regulatory mechanism, the level of RBPs in a cell should be precisely regulated. So, any alterations in the expression and alteration in function due to mutation in the binding site of RBPs can break the homeostasis and lead the cell toward oncogenesis and several other diseases. There is numerous evidence of modulation of normal repertoire of RBP in a cell in several cancer types (Kechavarzi and Janga, 2014). For example, overexpression and promoter mutation of TERT are prevalent in several types of cancer such as melanoma, cholangiocarcinoma, lung squamous cell carcinoma, and adrenocortical carcinoma (Colebatch et al., 2019). ELAVL1 is a well-reported oncogenic RBP that is found to induce tumorigenesis by facilitating the translation of several growth factors and proto-oncogenes (Abdelmohsen and Gorospe, 2010; Wang et al., 2013a). Reappearance of oncofetal proteins of the IGF2BP family is a major posttranscriptional driver of oncogenesis in many aggressive cancer types (Bell et al., 2013). The RNA-binding protein FXR1 also has diverse roles in cancer progression by means of cell proliferation and viability (Jin et al., 2016; Fan et al., 2017). Ribosomal proteins control tumor suppression via activation of p53, and deletion (43% of tumors) and mutation in genes encoding ribosomal proteins such as RPL22 are evidenced in several cancer types such as acute lymphoblastic leukemia (Sloan et al., 2013; Pelletier et al., 2018; Lessard et al., 2019). There are evidences of some onco-ribosomes as well like RPL10-R98S mutation in T-cell leukemia mimicking the oncogenic JAK-STAT pathway (Sulima et al., 2017; Girardi et al., 2018).
Recent studies have corroborated that RBPs are highly predominant and ubiquitously expressed than any other regulatory elements throughout the cell (Wang et al., 2018). But as opposed to other cancer types, dysregulation of RBPs in the case of pancreatic cancer is not much worked out. With the recent advancement of knowledge about the multifaceted role of RBPs in cancer progression and keeping in mind the aggressive nature of pancreatic cancer, the disparity of RBPs in pancreatic cancer development needs to be explored. Here, at the beginning, we have performed a meta-analysis to identify the differentially expressed RBPs in pancreatic cancer and validated them in a separate validation dataset as well as in the Human Protein Atlas (Uhlén et al., 2015). This was followed by the identification of “hub” RBPs, finding out their coding and noncoding targets, and exploration of biological processes supposed to be altered by the deregulation of both the RBPs and their targets. Our discovery of RBPs getting involved in important oncogenic activities not only contributes to the existing knowledge but also opens up immense therapeutic possibilities.
Methods
Enumeration of Human RBPs
A comprehensive list of 1,542 manually curated RBPs by Gerstberger et al. (2014) was the fundamental source of RBP in our entire list of RBPs. Additionally, we downloaded a list of RBPs from the RBPDB database under the category “Homo sapiens” (Cook et al., 2011). Moreover, an extensive search from literature resulted in a number of RBPs (Galante et al., 2009; Kechavarzi and Janga, 2014; Neelamraju et al., 2018). We also considered RBPs from high-throughput studies involving cross-linking, mass spectrometry, etc., and a common set of proteins from these studies yielded few additional RBPs (Baltz et al., 2012; Castello et al., 2012). We finally could catalogue an exhaustive list of 1,623 RBPs (Supplementary Table S1). We obtained the Entrez IDs of these RBPs using the “org.Hs.eg.db” package of Bioconductor, and missing Entrez Ids from the package were added to the list by manual search.
Selection of Microarray Datasets
The PDAC microarray gene expression datasets used in this study for meta-analysis were obtained from Gene Expression Omnibus (GEO) (Barrett et al., 2013). We used the keywords “pancreatic cancer”, “pancreatic adenocarcinoma,” and “pancreatic ductal adenocarcinoma” and selected only the datasets that contained information on pancreatic tumor and normal tissues of human patients. We selected five datasets for meta-analysis of the discovery cohort containing information of 241 pancreatic tumor tissues and 124 normal pancreatic tissues (Badea et al., 2008; Park et al., 2014; Janky et al., 2016; Yang et al., 2016; Chhatriya et al., 2020). Two additional datasets were chosen for meta-analysis in the validation cohort with 60 pancreatic tumor samples and 35 normal pancreatic samples (Ellsworth et al., 2013; Klett et al., 2018). Detailed information on these datasets is provided in Table 1. For validation of the RBPs in the EMT-induced pancreatic cancer cell line, a microarray gene expression dataset (GSE23952) was chosen from GEO. The dataset provides the gene expression profile of TGF-beta–induced epithelial–mesenchymal transition (EMT) in the pancreatic cancer cell line (Panc-1) compared to the normal Panc-1 cell line (Maupin et al., 2010).
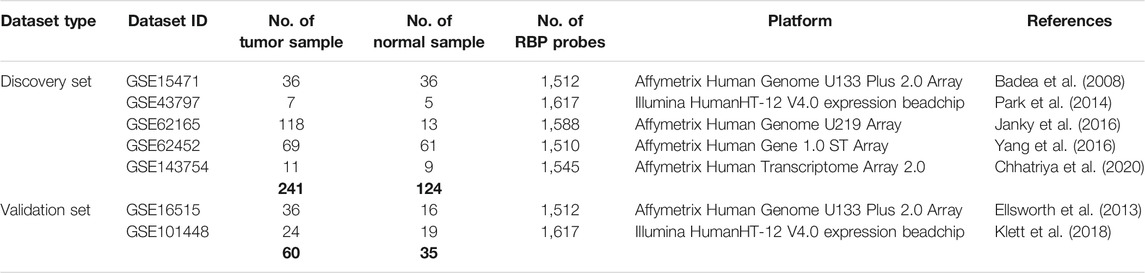
TABLE 1. Mircoarray dataset information from GEO used for meta-analysis. The top five datasets are used for meta-analysis of the discovery cohort, and last two datasets are used for meta-analysis of the validation cohort.
Meta-Analysis
Series matrix files of the datasets were downloaded from GEO, and information for only 1,623 RBPs was extracted from Log2-transformed processed data. Each dataset having the number of RBP probes is mentioned in Table 1. All the gene and probe IDs were converted to their corresponding Entrez IDs, and expression values of the same Entrez ID were aggregated with their mean value. All five microarray datasets of the discovery cohort were integrated and merged, and the corresponding combined expression set was made using the “Biobase” package of R Bioconductor. To remove batch effects among the datasets, batch correction was performed using “ComBat” function of the R Bioconductor package “sva.” Normalized data were used for the calculation of differential expression of genes in pancreatic tumor tissue compared to normal pancreatic tissue as controls. Differential expression analysis was performed using the “Limma” package of R Bioconductor (Ritchie et al., 2015). The Benjamini–Hochberg correction method was used minimize the false discovery rate (FDR). Genes with adjusted p-value below 0.05 and fold change (log2) cutoff of 0.5 were considered as differentially expressed genes.
Validation
Microarray datasets GSE16515 and GSE101448 were used for validation of the DE-RBPs from the discovery cohort. Meta-analysis of these two datasets was performed as described previously, and differentially expressed RBPs below the adjusted p-value cutoff 0.05 were considered for validation. For validation, TCGA pancreatic adenocarcinoma (PAAD) RNA-sequencing data were also used. Differentially expressed genes from RNA-sequencing were downloaded from the GEPIA and used for validation with adjusted p-value cutoff of <0.01. The computation of differential gene expression from the microarray dataset of EMT-induced Panc-1 cell line (GSE23952) was performed using GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/). DEGs with p-value below 0.05 were considered as significant. The TNMplot (https://www.tnmplot.com) database was used to compare gene expression of selected RBPs in normal, tumor, and metastatic tissues (Bartha and Győrffy, 2021). Gene chip data of the pancreas from the database were taken into account for the validation. We have also used The Human Protein Atlas to obtain tissue specific expression pattern of specific RBPs. For example, for IGF2BP3, normal pancreatic image was accessed using: https://www.proteinatlas.org/ENSG00000136231-IGF2BP3/tissue/pancreas, while pancreatic cancer image was accessed using: https://www.proteinatlas.org/ENSG00000136231-IGF2BP3/pathology/pancreatic+cancer.
Pathway Over-Representation Analysis
To explore the potential biological functions and molecular mechanisms modulated by the DE-RBPs and their coding target RNAs, we used Gene Ontology (GO) enrichment which comprises three major domains: biological process (BP), molecular function (MF), and cellular component (CC) (Ashburner et al., 2000). GO over-representation analysis was performed using the clusterProfiler package of R (version 4.0.3) with the significance threshold of p-value <0.05 (Yu et al., 2012). Kyoto Encyclopedia of Genes and Genomes (KEGG) is an integrated data resource for systematic analysis of gene functions (Kanehisa and Goto, 2000). KEGG pathway assignment was performed by Enrichr (https://maayanlab.cloud/Enrichr/), a web-based server for gene enrichment analysis (Kuleshov et al., 2016). Pathway enrichment for lncRNA targets of the RBPs was performed employing the LncSEA database (http://bio.liclab.net/LncSEA/index.php), a powerful platform for lncRNA enrichment analysis functions (Chen et al., 2021). DE-RBPs and their coding target RNAs were separated according to their upregulated and downregulated expression pattern in PDAC, and pathway analysis was performed separately for upregulated and downregulated groups in each category.
Protein–Protein Interaction Network Construction and Module Screening
Validated 60 differentially expressed RBPs were mapped to the STRING database (https://string-db.org/), and the protein—protein interaction network was built with the genes of the combined score ≥0.4 (medium confidence score) (Szklarczyk et al., 2017). Then, Cytoscape software (v3.8.2) was used to visualize the interaction network (Shannon et al., 2003). The significant gene module of the network was detected by the Molecular Complex Detection (MCODE) plugin of Cytoscape with the following criteria: degree cutoff = 2, node score cutoff = 0.2, k-core = 2, and maximum depth = 100 truncation standard to comprehend the network (Bader and Hogue, 2003). Furthermore, to screen out the hub genes of the network with the highest degree of connectivity, the cytoHubba plugin of Cytoscape was used, and the top five genes from each algorithm of the analysis were together considered hub genes (Chin et al., 2014).
Identification of RNA Targets for Selected RBPs
Targets of the RBPs, mainly those conventional RBPs that were being experimented for a long time with respect to their RNA-binding property, were derived primarily by extensive search in literature. Our next approach was to explore databases such as RNAInter (http://www.rna-society.org/rnainter/) and RNAct (https://rnact.crg.eu/), which contains information of RNAs and their interacting proteins. RNAInter (RNA Interactome Database) includes information on RNA-protein interactions based on strong and weak (high-throughput) experimental evidence and prediction algorithms (Lin et al., 2020). But, all the selected RBPs did not have experimentally validated targets and thus we also considered their targets derived from the computational prediction method. As, RNAInter does not have information for all the RBPs, we relied on the RNAct database for predicted target RNAs of the concerned RBPs (Lang et al., 2019). We also looked for target RNAs in the CLIP-sequencing database, CLIPdb (http://lulab.life.tsinghua.edu.cn/postar3/RBP.html) for protein-RNA interactions (Yang et al., 2015). Additionally, we had explored the CircInteractome database (https://circinteractome.irp.nia.nih.gov/) to investigate the interaction of RBPs with circular RNA (Dudekula et al., 2016).
Results
Meta-Analysis Identified Differentially Expressed RBPs in Pancreatic Cancer
Importance of the dysregulation of RBPs in cancer and the ambiguity of oncogenic RBPs as reported in different studies led us to perform a meta-analysis of multiple microarray datasets. The overall objective was to find out differentially expressed RBPs in pancreatic cancer resulting out of gene expression data from 241 tumor tissues and 124 normal pancreatic tissue samples. We initiated the process by analyzing the expression of 1,623 RBPs in these five different microarray datasets as the “discovery cohort” (Table 1), and our meta-analysis yielded a set of 49 upregulated and 21 downregulated RBPs (adj. p-value < 0.05, |log2FC| > 0.5). Detailed information on differential expression of these RBPs is summarized in Supplementary Table S2.
Validation of Meta-Analysis Result
In order to have more confidence in our findings, we further wanted to validate the differentially expressed RBPs from the discovery set separately in a validation meta-analysis set of the two microarray datasets (adj. p-value <0.05) (60 tumor tissues and 35 normal pancreatic tissue samples) and TCGA RNA-sequencing data of pancreatic ductal adenocarcinoma (PAAD) from GEPIA (adj. p-value <0.01) (179 tumor tissues and 171 normal pancreatic tissue samples) (Supplementary Figure S1). Finally, 45 upregulated and 15 downregulated RBPs in PAAD got validated following such stringent conditions (Supplementary Table S3). While the volcano plot demonstrates the distribution of validated upregulated and downregulated RBPs in Figure 1A, the heatmap portrays the expression profiles of 60 differentially expressed RBPs (45 upregulated and 15 downregulated) in the validation microarray dataset (Figure 1B). Hence, we report 60 RBPs having significantly altered expression in pancreatic cancer. Interestingly, we observed that there are reports of mutations and copy number variation in PAIP2B, PRDX1, RNASE1, BARD1, UHMK1, and RPL3L genes associated with pancreatic cancer (Bartsch et al., 2005; Barton, 2016; Tang et al., 2017; Wang et al., 2017; Alimirzaie et al., 2018; Grant et al., 2018). The finding further strengthens the importance of RBPs in pancreatic cancer.
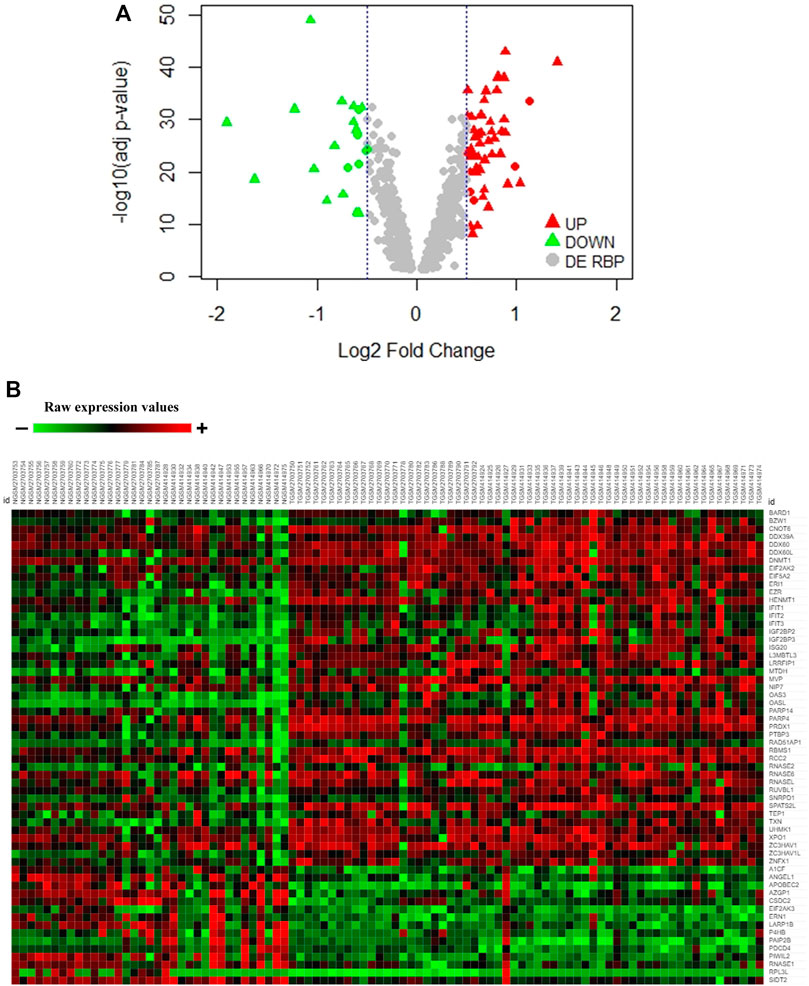
FIGURE 1. Expression pattern of DE-RBPs in pancreatic cancer. (A) Volcano plot showing significant DE-RBPs in the discovery dataset where red and green triangles denote validated upregulated and downregulated RBPs, respectively. (B) Heatmap of 60 validated DE-RBP expression in the tumor and normal tissue samples of the microarray validation dataset. Red indicates high expression and green indicates low expression.
Next, to have an idea about the most altered RBPs, we applied a fold change cutoff of |log2FC| > 1.0 to the validated DE-RBPs identifying IGF2BP3 and DDX60 as the most upregulated and PAIP2B, AZGP1, PDCD4, SIDT2, and RNASE1 as the most downregulated DE-RBPs in PDAC. We thought that an excellent way to validate the expression of these RBPs would be to check whether these proteins were also altered in PDAC patients. Therefore, the expression of those RBPs at the protein level in pancreatic tumor tissues and normal pancreatic tissues were further investigated using immunohistochemical data from the Human Protein Atlas database. While the results for the top altered RBPs are shown in Figure 2, we found similar changes for other RBPs too. The similarity of the expression pattern of these RBPs in these patient-derived tissues to that of our transcriptome meta-analysis results further validated our finding.
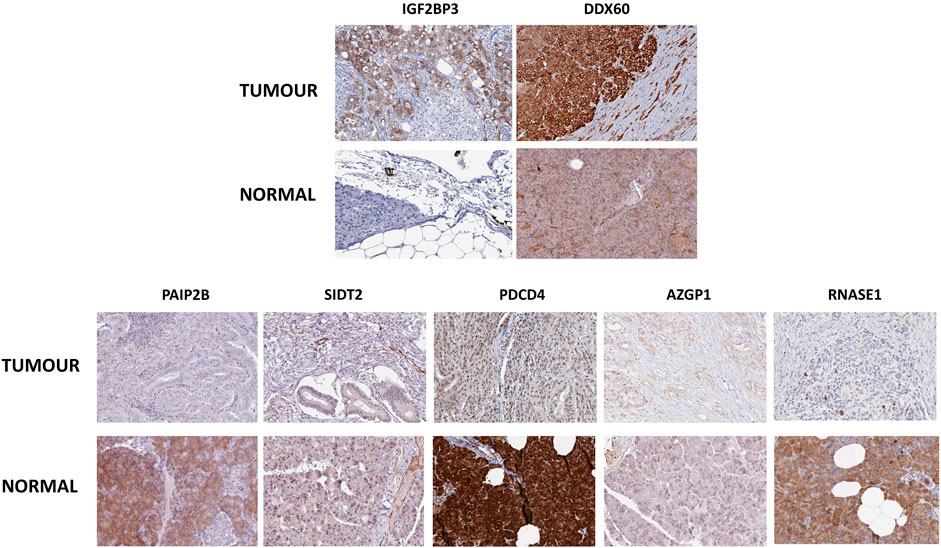
FIGURE 2. Representative Immunohistochemistry images showing expression of top-altered DE-RBPs in respective pancreatic tissues from the Human Protein Atlas Database. Upper panel shows the images of top overexpressed DE-RBPs and the lower panel shows images of most underexpressed DE-RBPs.
Functional Enrichment of DE-RBPs
Next, we wanted to find out what effects these altered RBPs could bring about in pancreatic cancer development and progression. To assess that, the first step would be to perform GO term enrichment and KEGG pathway analysis of DE-RBPs depicting their role in distinct biological functions and pathways. The enriched biological process (BP) section of GO displayed the immunomodulatory activity of the upregulated DE-RBPs, and the molecular function (MF) division showed that these RBPs are mainly associated with double-stranded RNA-binding, nuclease activity, translation regulation, and mRNA UTR binding (Figure 3A). On the other hand, downregulated DE-RBPs mainly exhibit phosphodiester bond hydrolysis and mRNA editing function in the BP category and stand for nuclease activity and transmembrane transport in the MF category (Figure 3B). Additionally, KEGG pathway analysis also indicated the involvement of upregulated RBPs in response to viruses such as those causing hepatitis C, influenza, and measles and in the NOD-like receptor signaling pathway which is known as a master regulator of inflammation and cancer (Saxena and Yeretssian, 2014) (Figure 3C). KEGG pathways linked to downregulated RBPs illustrate their role in protein processing, autophagy, mitophagy, and apoptosis (Figure 3D). To summarize, all the over-represented pathways either directly or indirectly substantiate the important contribution of the DE-RBPs in the pathophysiology of PDAC. The complete list of altered pathways is presented in Supplementary Table S4.
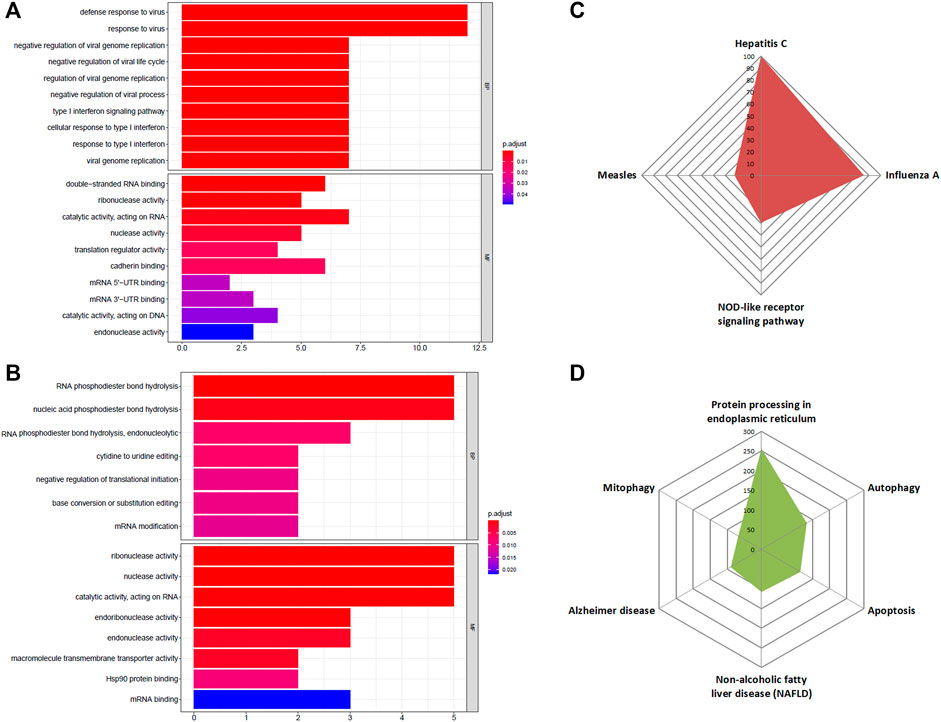
FIGURE 3. GO and KEGG enrichment analysis of DE-RBPs. (A) Barplot shows enriched GO terms of upregulated RBPs, (B) barplot shows enriched GO terms of downregulated RBPs, (C) radar plot represents the KEGG pathway analysis result of upregulated RBPs, (D) radar plot represents the KEGG pathway analysis result of downregulated RBPs. BP: biological processes, MF: molecular function.
PPI Network Construction and Identification of Significant Gene Modules
Leading-edge research emphasizes higher-order interactions between RBPs to enact the combinatorial regulation of specific mRNAs and coordinate complex cellular processes (Sternburg and Karginov, 2020). To have a comprehensive insight into those intricate regulatory networks between DE-RBPs, a PPI network was formed using the STRING database and visualized with Cytoscape Software_v3.8.2. The network resulted in 44 nodes and 87 edges where blue indicated upregulated RBPs and yellow indicated downregulated RBPs (Figure 4A). The MCODE plugin was used to find the highly interconnected regions in the main network, and two significant gene cluster modules were obtained (Figures 4B,C). To recognize the important hubs or nodes in the interactome network, the cytoHubba plugin was used where hub genes were ranked by 12 topological algorithms. We used the top five genes calculated from each algorithm, and totally 20 genes were identified as the key hub genes of the network among which EIF2AK2, RNASEL, ISG20, IFIT1, and OASL were found to overlap in at least five methods (Supplementary Figure S2). Hence, these 20 hub genes were used in subsequent analyses with 18 genes being upregulated and 2 genes being downregulated.
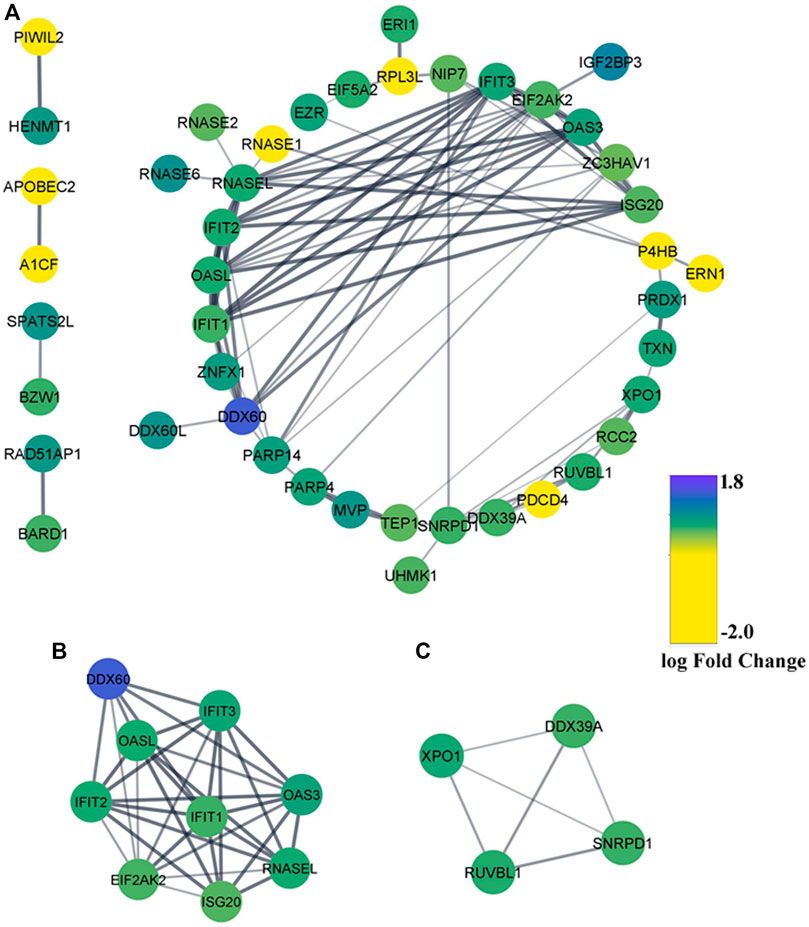
FIGURE 4. Protein–protein interaction (PPI) network of the differentially expressed RBPs. (A) PPI network of DE-RBPs constructed using the STRING database. RBPs without any interaction with another DE-RBP are removed. Blue denotes upregulated DE-RBPs and yellow denotes downregulated DE-RBPs. (B,C) Two MCODE modules from the PPI network.
Identification of Coding and Noncoding Targets of Selected RBPs
Overall physiological functions of RBPs are governed by the type of RNA they bind with and also by the subsequent fate of those RNAs as determined by the RBPs themselves and other associated factors. MRNAs are undoubtedly the most characterized targets of RBPs, while recent discoveries of various types of noncoding RNAs and their interaction with RBPs also carry crucial functional significance. Hub genes along with the top altered DE-RBPs (|log2FC| > 1.0) (DDX60 and IGF2BP3: upregulated, PAIP2B, AZGP1, PDCD4, SIDT2, and RNASE1: downregulated) (Table 2) were taken forward for target RNA identification. The targets of these 26 RBPs; identified from literature, strong and weak experimental evidences, and bioinformatic prediction were divided into coding and noncoding categories. Noncoding RNAs were again subdivided into two categories based on their length. Long noncoding RNA groups consisted of lncRNAs and lincRNAs, pseudo-gene RNAs, circular RNAs, and few processed transcripts whereas miRNAs, snoRNAs, and scaRNAs constituted the group of small noncoding RNAs. Only the coding and long noncoding transcript targets that were differentially expressed in PDAC (GEPIA) were selected. However, the expression of small noncoding RNA targets could not be validated due to poor availability of data. A total of 203 coding targets were identified for 26 RBPs (Supplementary Table S5). We were able to identify 418 long noncoding target RNA information for 20 RBPs and small noncoding RNA information could be retrieved for 7 RBPs only (Supplementary Table S6).
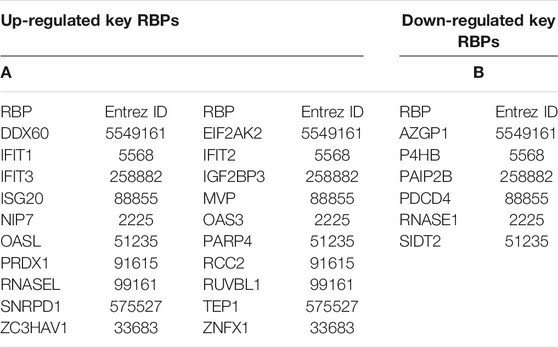
TABLE 2. Information of the DE-RBPs used for target RNA identification. Hub RBPs along with the top-altered RBPs are included in this group. These set of RBPs are presented in the table as (A) upregulated RBPs and B) downregulated RBPs.
Target RNAs Drive Key Oncogenic Pathways
As mentioned before, the function of an RBP depends on the target RNAs it interacts with and hence, the pathways or biological processes modulated by these targets largely determine the role of the RBP in general; both in normal physiological conditions as well as in the diseased state. Therefore, it is imperative to explore the functions of both the coding and noncoding targets identified for our selected RBPs. Upregulated coding RNA targets underwent GO term enrichment and KEGG pathway analysis. The biological process (BP) segment of GO depicted the role of the target RNAs mainly in the regulation of epithelial cell proliferation and migration and mesenchymal development which indicated their involvement toward epithelial-to-mesenchymal transition (EMT). The regulation of protein kinase and serine/threonine kinase emerges as the top biological process mediated by the RBP targets, which is also a salient regulator of EMT. Positive regulation of pri-miRNA transcription by the RBP targets is also a significant biological process which denotes the direct and indirect involvement of the RBPs in miRNA biogenesis along with their targets (Figure5A). The GO-enriched terms of the cellular component exhibited the association of the target RNAs with transcription regulator complex, focal adhesion, cell-substrate junction, ribonucleoprotein granule, and cytoplasmic stress granules (Figure 5A). In the molecular function (MF) category, coding targets are mainly enriched in DNA-binding transcription activator activity, transcription cofactor binding, mRNA, double-stranded RNA, and UTR binding (Figure 5A). As the number of downregulated coding RNA targets is very less, we did not get any significant enriched GO terms for those. The KEGG pathway analysis of upregulated coding targets delineated their role in several signaling pathways such as the TGF-beta signaling, AGE-RAGE signaling, MAPK signaling, PI3K-Akt signaling, sphingolipid signaling, relaxin signaling, Ras signaling, HIF-1 signaling, ErbB signaling, C-type lectin receptor signaling, and VEGF signaling pathway. Additionally, they are also indicated to be involved in adherens junction, regulation of actin cytoskeleton, and Th17 cell differentiation (Figure 5B). Downregulated KEGG pathways due to downregulated coding genes involve nicotinate and nicotinamide metabolism, aldosterone-regulated sodium absorption, tight junction, and endocytosis (Figure 5C). In brief, we observed the alteration of very important pathways known to be involved in cancer development and progression, through the modulation of coding targets by RBPs. The list of all altered pathways is shown in Supplementary Table S7.
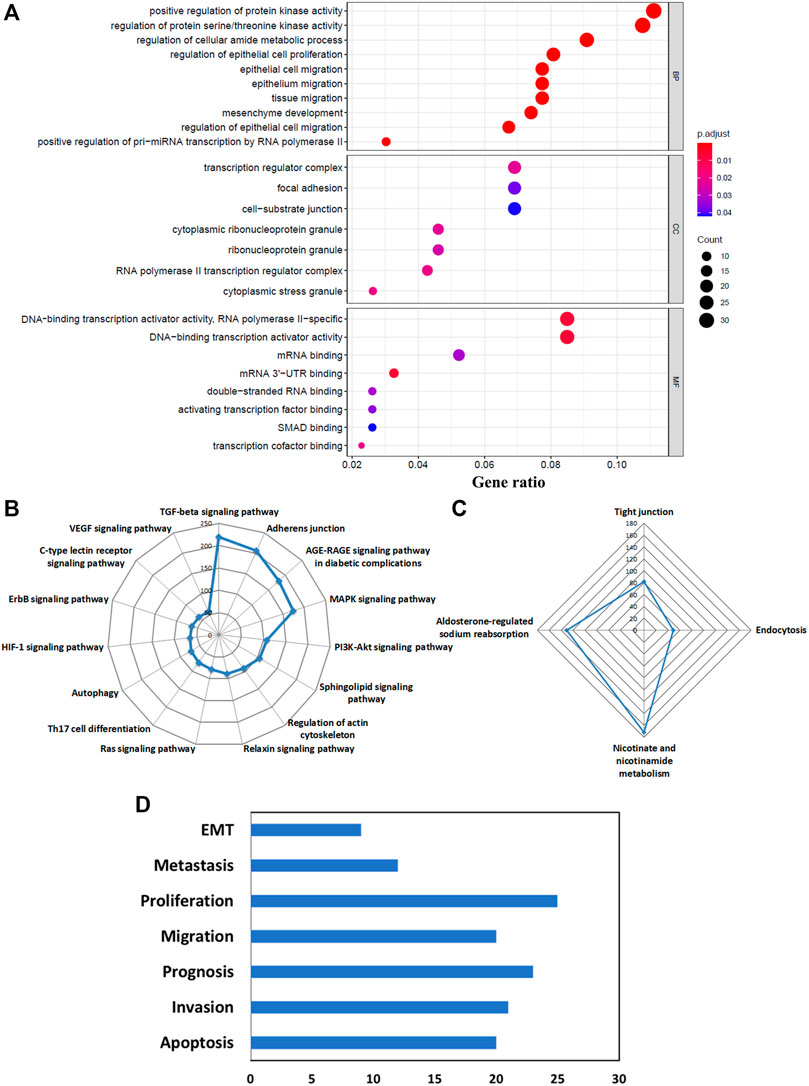
FIGURE 5. Biological functions and pathways of the predicted target RNAs of DE-RBPs. (A) Bubble plot shows the enriched GO terms of upregulated coding targets: biological processes (BP), Cellular Component (CC), and molecular functions (MF). (B) Radar plot shows KEGG pathway analysis of upregulated coding targets. (C) Radar plot is shows KEGG pathway analysis of downregulated coding targets. (D) Altered pathways represent cancer hallmarks for predicted lncRNA targets from the LncSEA database.
Like coding targets, we also wanted to find out the downstream pathway modulation by the long noncoding RNA targets of RBPs. The long noncoding Set Enrichment Analysis (LncSEA) webtool was used for this purpose and to our surprise, pathways leading to cancer cell migration, metastasis, and EMT were the most important ones (Figure 5D).
Epithelial-to-Mesenchymal Transition Emerged as the Most Prevalent Biological Process From RBP Targets
As already described in the previous segment, epithelial-to-mesenchymal transition (EMT) turned out to be the most distinct pathway modulated by the predicted targets of the selected RBPs. EMT is a process by which epithelial cells lose their cell polarity and cell–cell adhesion and attain migratory and invasive properties becoming the cell type of mesenchymal characteristics, and the process is considered a crucial step for tumor metastasis. We were curious to know exactly what were the RBPs which play a central role in this process and what were their targets. In order to do so, four EMT-related GO-enriched terms of the biological process (BP) category were selected from upregulated coding target–driven pathways, as shown in Figure 6 and a cnetplot was used to visualize the responsible target genes. The next obvious step was to go back and identify the RBPs targeting those RNAs and actually driving the process. Interestingly, we found that these were the target genes of all 26 RBPs used in the analysis. The result tells us that there is a clear indication that deregulated RBPs primarily promote EMT and thereby facilitate metastasis in pancreatic cancer.
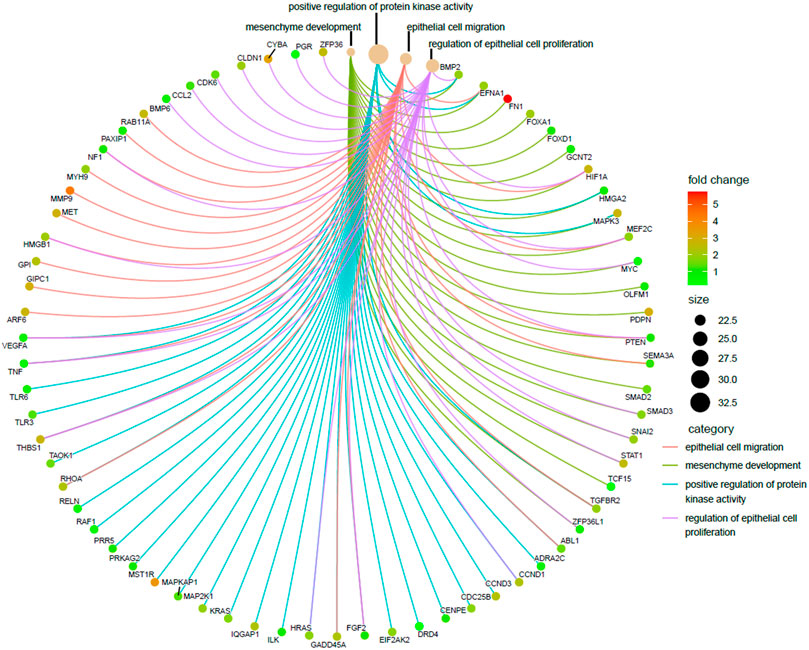
FIGURE 6. Visualization of enriched EMT-related GO terms of upregulated coding targets. Cnetplot showing four EMT-related enriched GO terms of the biological process (BP) category derived from upregulated coding targets along with the associated targets. Red represents the upregulated targets and green represents the downregulated targets.
Considering the fact that the finding is quite significant with respect to pancreatic carcinogenesis, we went on further to validate the results. There have been several experiments where the effect of any particular EMT-promoting factor has been tested on cancer cell lines by monitoring gene expression status at both epithelial and mesenchymal states. We found that one dataset (GSE23952) where TGF-β was used to induce EMT in Panc-1 pancreatic cancer cells, and gene expression profiling was performed before and after induction. We understand that the treatment by TGF-β cannot be the only method to induce EMT and might also not completely mimic the actual scenario prevailing in PDAC patients. Still, we moved forward and used this dataset to validate the expression of our selected RBPs over there, keeping in mind that the TGF-β–induced EMT model is the most widely used method, and the dataset is the best available option at present. We could validate the expression of nine RBPs in that dataset (Figure 7A), thereby supporting their role in EMT and indicating that TGF-β could also play a possible role here.
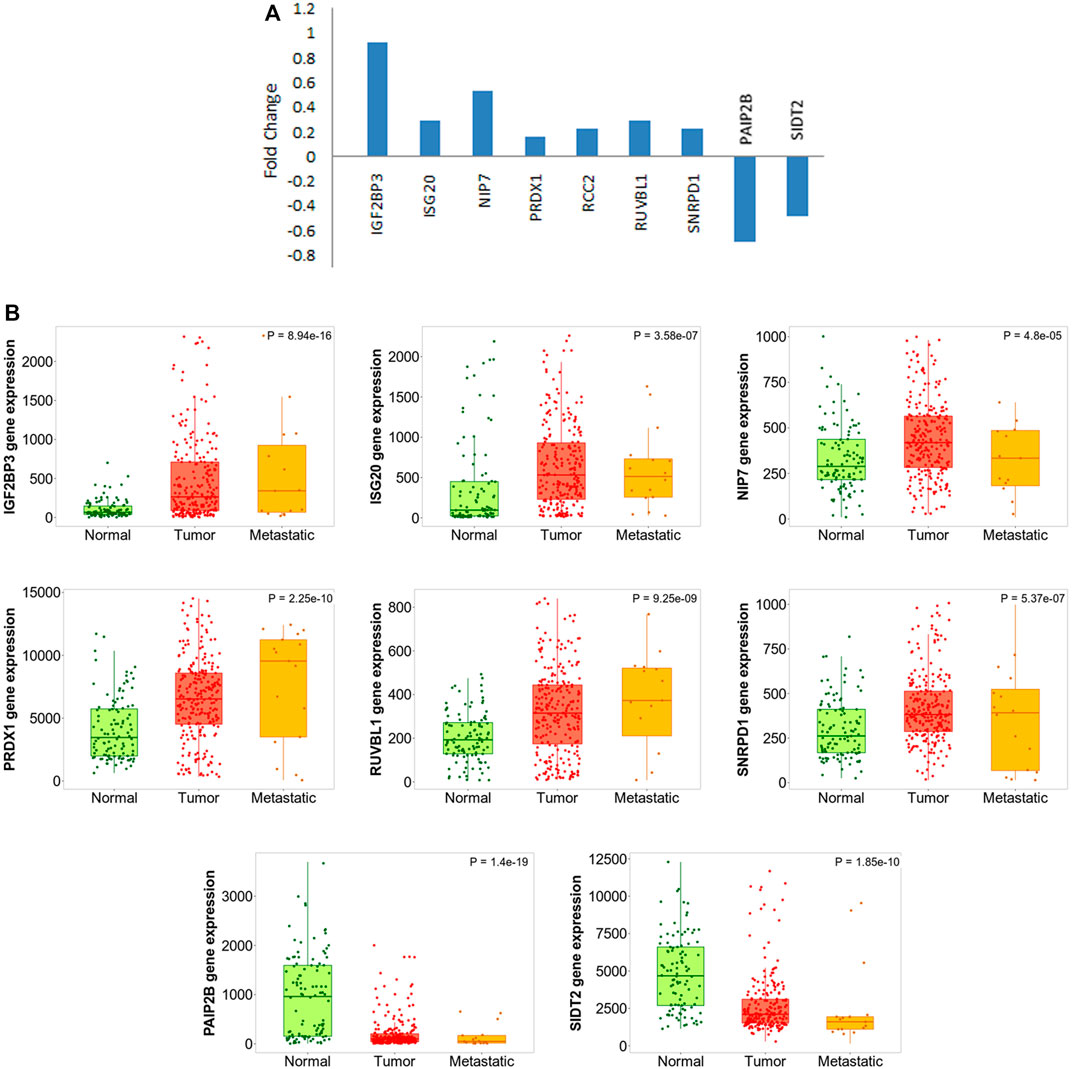
FIGURE 7. DE-RBP expression deregulated in EMT and metastasis. (A) Barplot shows the expression pattern (log2 fold change) of DE-RBPs to be differentially expressed in TGF-β–induced EMT in the Panc-1 cell line, as compared to uninduced Panc-1 cells, as obtained from the database GSE23952. (B) Boxplot comparing the expression of the abovementioned DE-RBPs in normal, tumor, and metastatic tissue samples as computed using the TNMplot database.
In another attempt to further validate our finding, we wanted to check what is the pattern of the expression of these RBPs during metastatic progression of the disease in actual patient data. If an RBP is either a promoter or inhibitor of EMT, we can expect that it is progressively up or downregulated expression from the normal pancreatic tissue to primary tumor to metastatic lesion. We used a web-platform TNMplot which is an integrated database using transcriptome-level datasets to compare gene expression in normal, tumor, and metastatic tissues. Expression values of these EMT-related RBPs were checked. Progressive nature of the expression of the RBPs which already got validated in TGF-β–mediated EMT induction study further validated their importance in EMT (Figure 7B). Additionally, the expression of remaining 19 RBPs was also tested using TNMplot, and 12 out of 18 RBPs were progressively altered, strengthening our finding that these RBPs play a significant role in EMT in pancreatic cancer (Supplementary Figure S3). Expression information on RCC2 and ZNFX1 were not found in the dataset.
Lastly, in order to further verify the expression status of altered RBPs in pancreatic cancer cell lines and tissue samples, we explored the published literature and used The Protein Atlas data. There were multiple studies where actually the expression of these specific RBPs was estimated in several pancreatic cancer cell lines or patient tissues using qPCR and/or Western blotting or immunohistochemical analysis; convincingly supporting our results (Supplementary Table S8) (Fernández-Salas et al., 2000; Kong et al., 2010; Bhatti et al., 2011; Taniuchi et al., 2014a; Cai et al., 2015; Taniuchi et al., 2015; Schultz et al., 2017; Tang et al., 2017; Yu et al., 2017; Nguyen et al., 2019; Cui et al., 2020; Konishi et al., 2021). Immunohistochemistry results for IGF2BP3, PAIP2B, and SIDT2 have already been shown in Figure 2, and we additionally present the results for the rest of the five RBPs in pancreatic cancer tissues as shown in Supplementary Figure S4, which strongly validate their expression in patient samples signifying their importance in the disease.
Discussion
The prime objective of this study was to identify the deregulated RBPs and investigate the modulation of gene expression by them in pancreatic cancer. As RBPs play an instrumental role in regulating normal cellular processes, the altered expression of RBP emerged as a major player in the development and progression of cancer. Apart from candidate RBP studies and their involvement in pancreatic cancer, there are very few reports of universal RBPome studies derived from TCGA RNA-sequencing data in pancreatic cancer which have discrepancies between the results (Glass et al., 2020; Wen et al., 2020). Disparity among the outcomes of these studies led us to investigate the RBPs which are universally altered in pancreatic cancer. The best unbiased approach we thought of was to access all available relevant gene expression microarray datasets and process them together to find out the expression information for the RBPs and perform the analysis.
The meta-analysis offers statistical methods to integrate results from multiple comparable studies with the aim of extrapolating the number of observations and statistical power and provide a precise estimate of the effects of an intervention (Fagard et al., 1996). Thus, we conducted a meta-analysis of five published transcriptomic microarray datasets for the discovery cohort, and the differentially expressed RBPs were validated by another meta-analysis of two more transcriptomic array datasets and DE-RBPs from TCGA RNA-sequencing data. Eventually, we identified 45 overexpressed and 15 underexpressed DE-RBPs that were invariably claimed to be differentially expressed in pancreatic cancer (Figure 1). Minute observations to the set of altered RBPs reflected the enrichment of some protein families such as IGF2BP, APOBEC, and OAS along with the assembly of DEAD-box helicases, RNAse, translation initiator, vault proteins, and some interferon-induced proteins. There are also some unconventional RBPs with no functional validation of their RNA-binding activity such as SPATS2L, TXN, UHMK1, ANGEL1, and P4HB. RBPs associated with innate immune response and immunomodulation are immensely represented in DE-RBPs, as also shown by the pathway enrichment. IFIT, OAS, and ZC3HAV family proteins are distinct interferon-stimulated genes (ISGs) (Justesen et al., 2000; Brice et al., 2019; Pidugu et al., 2019) whereas LRRFIP1 and PDCD4 are involved in the regulation of type I interferon response (Yang et al., 2010; Kroczynska et al., 2012). Another interesting feature is the downregulation of negative regulators of the typical translational machinery (EIF2AK3 and PDCD4) to combat the high energy demanding process of general translation (Supplementary Table S3). Altered expression of translation mediators indicate toward “translation acrobatics,” that is, exploiting alternate modes of translation initiation by cancer cells (Sriram et al., 2018). For example, the non‐canonical translation initiation factor EIF2AK2 is found to be upregulated, and the upregulated EIF5 determines the frequency of the leaky scanning mode of alternative translation and is reported as a novel oncogenic protein (Wang et al., 2013b). DDX60 was revealed to be the most overexpressed RBP in pancreatic adenocarcinoma. DDX60 is an ATP-dependent RNA helicase which induces type I IFN (IFN-α/β), and IFN- β is reported to influence oncogenic Ras transformation (Miyashita et al., 2011; Tsai et al., 2011). But there is no report of DDX60 involvement in pancreatic cancer, in spite of its consistent upregulation. IGF2BP3 is another important overexpressed KH-domain–containing RBP which is already reported to promote invasiveness and metastatic properties of the PDAC cells (Taniuchi et al., 2014b). Most downregulated RBPs include PAIP2B, SIDT2, PDCD4, AZGP1, and RNASE1, among which we report for the first-time involvement of PAIP2B, SIDT2, PDCD4, and RNASE1 in pancreatic cancer (Figure 2).
Some DE-RBPs exhibit protein–protein interaction among themselves, and the interaction network regulates several cellular processes. We also tried to find out the hub RBPs while searching for the RBPs having more connectivity and thus more regulatory capacity (Figure 4). RBPs act as the principal mediators of posttranscriptional regulation of gene expression by directly targeting various types of RNAs. Many experimental methods have been employed to identify the potential RNA partners of a particular RBP. Deep-sequencing approaches are the most convenient method nowadays to uncover the RBP-RNA interactome, but strong bioinformatic prediction methods can also help us identify targets of novel RBPs, which can serve as the basis for further prospective studies. However, we have tried to look for the targets of a definite RBP in every possible way to estimate the functions and pathways governed by the target RNAs of altered RBPs. Evidence from pathway enrichment intuitively manifested the connection between downstream target RNAs and EMT, a major event in metastasis (Figure 5). Being a highly dynamic process, metastatic cascade is also known to be promoted by the differentiation of tumor-infiltrating immune cells. Immune evasion of the cancer cells at the primary site of the tumor remotely prepares their pre-metastatic niche (Kitamura et al., 2015). Given the prevalence of immune response–related terms in RBP annotation results, as mentioned in Figure 3, it is worth justifying the potential role of target RNAs in pancreatic cancer metastasis. Apart from the direct involvement of target RNAs in promoting metastasis or EMT, concerned RBPs also act as direct or indirect regulators of EMT. We validated the expression of RBPs in a gene expression dataset of the TGF-β–induced EMT cell line of pancreatic cancer (Figure 7). There we came across nine RBPs differentially expressed in an identical pattern of our DE-RBPs. As the mode of regulation by RBP is a complex process, the expression level of target RNAs is not always linear with the expression of that particular RBP. Hence, the direction of altered expression is not always predictable using this approach. Moreover, TGF- β is not the sole inducer of EMT, and in the tumor microenvironment, there are other paracrine signaling pathways such as WNT pathway, NOTCH signaling, and mitogenic growth factor such as EGF and FGF-mediated activation pathways acting in combination to induce an EMT program (Dongre and Weinberg, 2019). Each pathway triggers EMT by modulating different sets of RBPs. As we have chosen a cell-line based model of EMT induced by a particular signaling pathway, the system primarily lacks other signaling modules and cross-talk with infiltrating immune cells which are known to be major player of EMT. Thus, the RBPs we found altered in TGF-β–induced EMT might be specific for the process. However, it does not mean that other RBPs do not have any contribution in EMT as they could always be part of other signaling pathways promoting EMT. The TNMplot dataset lastly verified the relatedness of the selected RBPs with EMT by comparing between normal, tumor, and metastatic tissue samples (Figure 7). We felt that we have tried to validate our findings by multiple means. Even, at the end, we had curated the published reports of experimental observations of other multiple groups where they had evaluated the expression of these RBPs in pancreatic cancer cell lines and patient samples (Supplementary Table S8). Still, detailed experimental interrogation of the functional role of individual RBPs in cell lines or animal models could have the final say on establishing their mechanism of action during the regulation of EMT.
Therefore, our study provides a comprehensive view of the dysregulated RBPs in pancreatic cancer and substantial insights into the molecular mechanisms of how RBPs interact among themselves and with their target RNAs, modulating one of the important hallmarks in pancreatic cancer, the epithelial-to-mesenchymal transition. “RBP”-eutics is a currently emerging field where RBPs are gaining attention as a promising target for cancer therapeutics using diverse approaches such as small-molecule inhibitors and oligonucleotide-based strategy (Mohibi et al., 2019). Thus, considering the importance of our identified RBPs in pancreatic cancer, their candidature as a suitable drug target will be worth investigating.
.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
MM carried out the formal analysis and curated data while SG conceptualized, designed, and supervised the study. Both MM and SG drafted the manuscript, prepared and approved the final version, and revised it critically for important intellectual content.
Funding
The study has been supported by Intramural funding from the National Institute of Biomedical Genomics. MM is a Senior Research Fellow getting fellowship from the University Grants Commission, Ministry of Education, Government of India.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Bishnupriya Chhatriya for critical technical suggestions during the meta-analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.713852/full#supplementary-material
Supplementary Figure S1 | Flowchart for analyzing DE-RBPs in pancreatic cancer. Discovery cohort includes meta-analysis of five microarray datasets, and DE-RBPs from the analysis were validated in a meta-analysis to two microarray datasets and TCGA RNA-sequencing data.
Supplementary Figure S2 | Highest degree connectivity network of the top five hub genes for each topological algorithm. Top 5 hub genes for each 12 topological algorithms are identified from the cytoHubba plugin used in Cytoscape. Interaction networks are based on their highest degree of connectivity. Each network denotes each algorithm used: (A) Betweenness, (B) BottleNeck, (C) Closeness, (D) Clustering coefficient, (E) Degree, (F) Density of Maximum Neighborhood Component (DMNC), (G) EcCenticity, (H) Edge Percolated Component (EPC), (I) Maximal Clique Centrality (MCC), (J) Maximum Neighborhood Component (MNC), (K) Radiality, and (L) Stress.
Supplementary Figure S3 | Boxplot showing the role of hub RBPs and top-altered RBPs in metastasis. Boxplot compares the expression of the hub RBPs and top-altered RBPs in normal, tumor, and metastatic tissue samples from the TNMplot database. Expression of the remaining six RBPs is shown in Figure 7A.
Supplementary Figure S4 | Immunohistochemistry images showing the expression of EMT-specific RBPs in respective pancreatic tissues from the Human Protein Atlas database. Comparison of the expression of tumor and normal tissue samples has been shown. The database does not have any IHC staining data corresponding to ISG20.
Supplementary Table S1 | List of enumerated RBPs. The list includes the names of 1623 RBPs along with their Entrez IDs.
Supplementary Table S2 | Differentially expressed RBPs in pancreatic cancer from the meta- analysis of the discovery cohort. The table includes analyzed data of the RBPs in pancreatic tumor tissues compared to normal pancreatic tissues identified from the meta-analysis of the five microarray datasets.
Supplementary Table S3 | Validated differentially expressed RBPs in pancreatic cancer. DE- RBPs from the discovery cohort are validated in a meta-analysis of the two microarray datasets and TCGA RNA-sequencing data. The validated DE-RBPs which include 45 upregulated and 15 downregulated RBPs are listed in the table.
Supplementary Table S4 | GO term enrichment and KEGG Pathway analysis results of DE- RBPs. Result of GO enrichment for upregulated RBPs is shown in sheet 1 named as “UP RBP GO” and result for downregulated RBPs shown in sheet 2 named as “DOWN RBP GO.”Sheet 3 named as “KEGG” includes the result of KEGG pathway analysis for upregulated and downregulated RBPs.
Supplementary Table S5 | Interacting coding RNA partners of the selected DE-RBPs.
Supplementary Table S6 | Interacting noncoding RNA partners of the selected DE-RBPs. Noncoding target RNAs are divided into two categories depending on their size. First sheet of the file consists of the “Long Noncoding RNA” targets of the RBPs. This category includes the name of RBPs, name of interacting RNA, and biotype of the target transcripts such as lncRNAs and lincRNAs, pseudo-gene RNAs, circular RNAs, and few processed transcripts. Second sheet of the file consists of the “Small Noncoding RNA” targets of the RBPs. This category includes the name of RBPs, name of interacting RNA, and biotype of the target transcripts such as miRNAs, snoRNAs, and scaRNA.
Supplementary Table S7 | GO term enrichment and KEGG pathway analysis results of coding targets of selected RBPs. Result of GO enrichment for upregulated coding targets are shown in sheet 1 named as “UP TARGET GO.” KEGG pathway analysis results of upregulated coding targets are shown in sheet 2 named as “UP TARGET KEGG”, and results of downregulated coding targets are shown in sheet 3 named as “DOWN TARGET KEGG.”
Supplementary Table S8 | List of published reports of experimental validation verifying the expression status of altered RBPs in pancreatic cancer cell lines and patient samples.
References
Abdelmohsen, K., and Gorospe, M. (2010). Posttranscriptional Regulation of Cancer Traits by HuR. WIREs RNA 1 (2), 214–229. Epub 2011/09/22. doi:10.1002/wrna.4
Alimirzaie, S., Mohamadkhani, A., Masoudi, S., Sellars, E., Boffetta, P., Malekzadeh, R., et al. (2018). Mutations in Known and Novel Cancer Susceptibility Genes in Young Patients with Pancreatic Cancer. Arch. Iran Med. 21 (6), 228–233. Epub 2018/06/27.
Ashburner, M., Ball, C. A., Blake, J. A., Botstein, D., Butler, H., Cherry, J. M., et al. (2000). Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 25 (1), 25–29. Epub 2000/05/10. doi:10.1038/75556
Badea, L., Herlea, V., Dima, S. O., Dumitrascu, T., and Popescu, I. (2008). Combined Gene Expression Analysis of Whole-Tissue and Microdissected Pancreatic Ductal Adenocarcinoma Identifies Genes Specifically Overexpressed in Tumor Epithelia. Hepatogastroenterology 55 (88), 2016–2027. Epub 2009/03/06.
Bader, G. D., and Hogue, C. W. (2003). An Automated Method for Finding Molecular Complexes in Large Protein Interaction Networks. BMC bioinformatics 4, 2. Epub 2003/01/15. doi:10.1186/1471-2105-4-2
Baltz, A. G., Munschauer, M., Schwanhäusser, B., Vasile, A., Murakawa, Y., Schueler, M., et al. (2012). The mRNA-Bound Proteome and its Global Occupancy Profile on Protein-Coding Transcripts. Mol. Cel. 46 (5), 674–690. Epub 2012/06/12. doi:10.1016/j.molcel.2012.05.021
Barrett, T., Wilhite, S. E., Ledoux, P., Evangelista, C., Kim, I. F., Tomashevsky, M., et al. (2013). NCBI GEO: Archive for Functional Genomics Data Sets-Uupdate. Nucleic Acids Res. 41, D991–D995. Epub 2012/11/30. doi:10.1093/nar/gks1193
Bartha, Á., and Győrffy, B. (2021). TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 22 (5), 1. Epub 2021/04/04. doi:10.3390/ijms22052622
Barton, M. K. (2016). Germline Mutations in Pancreatic Cancer Become Better Defined. CA: a Cancer J. clinicians 66 (2), 93–94. Epub 2016/01/13. doi:10.3322/caac.21300
Bartsch, D. K., Fendrich, V., Slater, E. P., Sina-Frey, M., Rieder, H., Greenhalf, W., et al. (2005). RNASEL Germline Variants Are Associated with Pancreatic Cancer. Int. J. Cancer 117 (5), 718–722. Epub 2005/06/28. doi:10.1002/ijc.21254
Bell, J. L., Wächter, K., Mühleck, B., Pazaitis, N., Köhn, M., Lederer, M., et al. (2013). Insulin-like Growth Factor 2 mRNA-Binding Proteins (IGF2BPs): post-transcriptional Drivers of Cancer Progression? Cell. Mol. Life Sci. 70 (15), 2657–2675. Epub 2012/10/17. doi:10.1007/s00018-012-1186-z
Bhatti, I., Lee, A., James, V., Hall, R. I., Lund, J. N., Tufarelli, C., et al. (2011). Knockdown of microRNA-21 Inhibits Proliferation and Increases Cell Death by Targeting Programmed Cell Death 4 (PDCD4) in Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Surg. 15 (1), 199–208. Epub 2010/11/23. doi:10.1007/s11605-010-1381-x
Brice, D. C., Figgins, E., Yu, F., and Diamond, G. (2019). Type I Interferon and Interferon‐stimulated Gene Expression in Oral Epithelial Cells. Mol. Oral Microbiol. 34 (6), 245–253. Epub 2019/09/15. doi:10.1111/omi.12270
Cai, C.-Y., Zhai, L.-L., Wu, Y., and Tang, Z.-G. (2015). Expression and Clinical Value of Peroxiredoxin-1 in Patients with Pancreatic Cancer. Eur. J. Surg. Oncol. (Ejso) 41 (2), 228–235. Epub 2014/12/02. doi:10.1016/j.ejso.2014.11.037
Castello, A., Fischer, B., Eichelbaum, K., Horos, R., Beckmann, B. M., Strein, C., et al. (2012). Insights into RNA Biology from an Atlas of Mammalian mRNA-Binding Proteins. Cell 149 (6), 1393–1406. Epub 2012/06/05. doi:10.1016/j.cell.2012.04.031
Chen, J., Zhang, J., Gao, Y., Li, Y., Feng, C., Song, C., et al. (2021). LncSEA: a Platform for Long Non-coding RNA Related Sets and Enrichment Analysis. Nucleic Acids Res. 49 (D1), D969–D980. Epub 2020/10/13. doi:10.1093/nar/gkaa806
Chhatriya, B., Mukherjee, M., Ray, S., Saha, B., Lahiri, S., Halder, S., et al. (2020). Transcriptome Analysis Identifies Putative Multi-Gene Signature Distinguishing Benign and Malignant Pancreatic Head Mass. J. Transl Med. 18 (1), 420. Epub 2020/11/09. doi:10.1186/s12967-020-02597-1
Chin, C. H., Chen, S. H., Wu, H. H., Ho, C. W., Ko, M. T., and Lin, C. Y. (2014). cytoHubba: Identifying Hub Objects and Sub-networks from Complex Interactome. BMC Syst. Biol. 8 Suppl 4 (Suppl. 4), S11. Epub 2014/12/19. doi:10.1186/1752-0509-8-S4-S11
Colebatch, A. J., Dobrovic, A., and Cooper, W. A. (2019). TERT Gene: its Function and Dysregulation in Cancer. J. Clin. Pathol. 72 (4), 281–284. Epub 2019/01/31. doi:10.1136/jclinpath-2018-205653
Cook, K. B., Kazan, H., Zuberi, K., Morris, Q., and Hughes, T. R. (2011). RBPDB: a Database of RNA-Binding Specificities. Nucleic Acids Res. 39, D301–D308. Epub 2010/11/03. doi:10.1093/nar/gkq1069
Cui, X.-H., Hu, S.-Y., Zhu, C.-F., and Qin, X.-H. (2020). Expression and Prognostic Analyses of the Insulin-like Growth Factor 2 mRNA Binding Protein Family in Human Pancreatic Cancer. BMC cancer 20 (1), 1160. Epub 2020/11/29. doi:10.1186/s12885-020-07590-x
Dongre, A., and Weinberg, R. A. (2019). New Insights into the Mechanisms of Epithelial-Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cel Biol. 20 (2), 69–84. Epub 2018/11/22. doi:10.1038/s41580-018-0080-4
Dudekula, D. B., Panda, A. C., Grammatikakis, I., De, S., Abdelmohsen, K., and Gorospe, M. (2016). CircInteractome: A Web Tool for Exploring Circular RNAs and Their Interacting Proteins and microRNAs. RNA Biol. 13 (1), 34–42. Epub 2015/12/17. doi:10.1080/15476286.2015.1128065
Ellsworth, K. A., Eckloff, B. W., Li, L., Moon, I., Fridley, B. L., Jenkins, G. D., et al. (2013). Contribution of FKBP5 Genetic Variation to Gemcitabine Treatment and Survival in Pancreatic Adenocarcinoma. PloS one 8 (8), e70216. Epub 2013/08/13. doi:10.1371/journal.pone.0070216
Fagard, R. H., Staessen, J. A., and Thijs, L. (1996). Advantages and Disadvantages of the Meta-Analysis Approach. J. Hypertens. Suppl. 14 (2), S9–S12. Epub 1996/09/01. doi:10.1097/00004872-199609002-00004
Fan, Y., Yue, J., Xiao, M., Han-Zhang, H., Wang, Y. V., Ma, C., et al. (2017). FXR1 Regulates Transcription and Is Required for Growth of Human Cancer Cells with TP53/FXR2 Homozygous Deletion. eLife 6. doi:10.7554/eLife.26129
Feldmann, G., Beaty, R., Hruban, R. H., and Maitra, A. (2007). Molecular Genetics of Pancreatic Intraepithelial Neoplasia. J. Hepatobiliary Pancreat. Surg. 14 (3), 224–232. Epub 2007/05/24. doi:10.1007/s00534-006-1166-5
Fernández-Salas, E., Peracaula, R., Frazier, M. L., and de Llorens, R. (2000). Ribonucleases Expressed by Human Pancreatic Adenocarcinoma Cell Lines. Eur. J. Biochem. 267 (5), 1484–1494. Epub 2000/02/26. doi:10.1046/j.1432-1327.2000.01148.x
Galante, P. A. F., Sandhu, D., de Sousa Abreu, R., Gradassi, M., Slager, N., Vogel, C., et al. (2009). A Comprehensive In Silico Expression Analysis of RNA Binding Proteins in normal and Tumor Tissue; Identification of Potential Players in Tumor Formation. RNA Biol. 6 (4), 426–433. Epub 2009/05/22. doi:10.4161/rna.6.4.8841
Gerstberger, S., Hafner, M., and Tuschl, T. (2014). A Census of Human RNA-Binding Proteins. Nat. Rev. Genet. 15 (12), 829–845. Epub 2014/11/05. doi:10.1038/nrg3813
Girardi, T., Vereecke, S., Sulima, S. O., Khan, Y., Fancello, L., Briggs, J. W., et al. (2018). The T-Cell Leukemia-Associated Ribosomal RPL10 R98S Mutation Enhances JAK-STAT Signaling. Leukemia 32 (3), 809–819. Epub 2017/07/27. doi:10.1038/leu.2017.225
Glass, M., Michl, P., and Hüttelmaier, A. S. (2020). RNA Binding Proteins as Drivers and Therapeutic Target Candidates in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 21 (11). Epub 2020/06/18. doi:10.3390/ijms21114190
Gordon-Dseagu, V. L., Devesa, S. S., Goggins, M., and Stolzenberg-Solomon, R. (2018). Pancreatic Cancer Incidence Trends: Evidence from the Surveillance, Epidemiology and End Results (SEER) Population-Based Data. Int. J. Epidemiol. 47 (2), 427–439. Epub 2017/11/18. doi:10.1093/ije/dyx232
Grant, R. C., Denroche, R. E., Borgida, A., Virtanen, C., Cook, N., Smith, A. L., et al. (2018). Exome-Wide Association Study of Pancreatic Cancer Risk. Gastroenterology 154 (3), 719–722. Epub 2017/10/28. doi:10.1053/j.gastro.2017.10.015
Hentze, M. W., Castello, A., Schwarzl, T., and Preiss, T. (2018). A Brave New World of RNA-Binding Proteins. Nat. Rev. Mol. Cel Biol. 19 (5), 327–341. Epub 2018/01/18. doi:10.1038/nrm.2017.130
Janky, R. s., Binda, M. M., Allemeersch, J., Van den Broeck, A., Govaere, O., Swinnen, J. V., et al. (2016). Prognostic Relevance of Molecular Subtypes and Master Regulators in Pancreatic Ductal Adenocarcinoma. BMC cancer 16, 632. Epub 2016/08/16. doi:10.1186/s12885-016-2540-6
Jin, X., Zhai, B., Fang, T., Guo, X., and Xu, L. (2016). FXR1 Is Elevated in Colorectal Cancer and Acts as an Oncogene. Tumor Biol. 37 (2), 2683–2690. Epub 2015/09/26. doi:10.1007/s13277-015-4068-9
Justesen, J., Hartmann, R., and Kjeldgaard, N. O. (2000). Gene Structure and Function of the 2′-5′-oligoadenylate Synthetase Family. Cmls, Cel. Mol. Life Sci. 57 (11), 1593–1612. Epub 2000/11/25. doi:10.1007/pl00000644
Kanehisa, M., and Goto, S. (2000). KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28 (1), 27–30. Epub 1999/12/11. doi:10.1093/nar/28.1.27
Kechavarzi, B., and Janga, S. (2014). Dissecting the Expression Landscape of RNA-Binding Proteins in Human Cancers. Genome Biol. 15 (1), R14. Epub 2014/01/15. doi:10.1186/gb-2014-15-1-r14
Kitamura, T., Qian, B.-Z., and Pollard, J. W. (2015). Immune Cell Promotion of Metastasis. Nat. Rev. Immunol. 15 (2), 73–86. Epub 2015/01/24. doi:10.1038/nri3789
Klett, H., Fuellgraf, H., Levit-Zerdoun, E., Hussung, S., Kowar, S., Küsters, S., et al. (2018). Identification and Validation of a Diagnostic and Prognostic Multi-Gene Biomarker Panel for Pancreatic Ductal Adenocarcinoma. Front. Genet. 9, 108. Epub 2018/04/21. doi:10.3389/fgene.2018.00108
Kong, B., Michalski, C. W., Hong, X., Valkovskaya, N., Rieder, S., Abiatari, I., et al. (2010). AZGP1 Is a Tumor Suppressor in Pancreatic Cancer Inducing Mesenchymal-To-Epithelial Transdifferentiation by Inhibiting TGF-β-Mediated ERK Signaling. Oncogene 29 (37), 5146–5158. Epub 2010/06/29. doi:10.1038/onc.2010.258
Konishi, H., Kashima, S., Goto, T., Ando, K., Sakatani, A., Tanaka, H., et al. (2021). The Identification of RNA-Binding Proteins Functionally Associated with Tumor Progression in Gastrointestinal Cancer. Cancers (Basel) 13 (13). Epub 2021/07/03. doi:10.3390/cancers13133165
Kroczynska, B., Sharma, B., Eklund, E. A., Fish, E. N., and Platanias, L. C. (2012). Regulatory Effects of Programmed Cell Death 4 (PDCD4) Protein in Interferon (IFN)-stimulated Gene Expression and Generation of Type I IFN Responses. Mol. Cel Biol. 32 (14), 2809–2822. Epub 2012/05/16. doi:10.1128/mcb.00310-12
Kuleshov, M. V., Jones, M. R., Rouillard, A. D., Fernandez, N. F., Duan, Q., Wang, Z., et al. (2016). Enrichr: a Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 44 (W1), W90–W97. Epub 2016/05/05. doi:10.1093/nar/gkw377
Lang, B., Armaos, A., and Tartaglia, G. G. (2019). RNAct: Protein-RNA Interaction Predictions for Model Organisms with Supporting Experimental Data. Nucleic Acids Res. 47 (D1), D601–D606. Epub 2018/11/18. doi:10.1093/nar/gky967
Lessard, F., Brakier-Gingras, L., and Ferbeyre, G. (2019). Ribosomal Proteins Control Tumor Suppressor Pathways in Response to Nucleolar Stress. Bioessays 41 (3), e1800183. Epub 2019/02/02. doi:10.1002/bies.201800183
Lin, Y., Liu, T., Cui, T., Wang, Z., Zhang, Y., Tan, P., et al. (2020). RNAInter in 2020: RNA Interactome Repository with Increased Coverage and Annotation. Nucleic Acids Res. 48 (D1), D189–D197. Epub 2020/01/08. doi:10.1093/nar/gkz804
Maupin, K. A., Sinha, A., Eugster, E., Miller, J., Ross, J., Paulino, V., et al. (2010). Glycogene Expression Alterations Associated with Pancreatic Cancer Epithelial-Mesenchymal Transition in Complementary Model Systems. PloS one 5 (9), e13002. Epub 2010/10/05. doi:10.1371/journal.pone.0013002
McGuigan, A., Kelly, P., Turkington, R. C., Jones, C., Coleman, H. G., and McCain, R. S. (2018). Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. Wjg 24 (43), 4846–4861. Epub 2018/11/30. doi:10.3748/wjg.v24.i43.4846
Miyashita, M., Oshiumi, H., Matsumoto, M., and Seya, T. (2011). DDX60, a DEXD/H Box Helicase, Is a Novel Antiviral Factor Promoting RIG-I-like Receptor-Mediated Signaling. Mol. Cel Biol. 31 (18), 3802–3819. Epub 2011/07/28. doi:10.1128/mcb.01368-10
Mohibi, S., Chen, X., and Zhang, J. (2019). Cancer the'RBP'eutics-RNA-binding Proteins as Therapeutic Targets for Cancer. Pharmacol. Ther. 203, 107390. Epub 2019/07/16. doi:10.1016/j.pharmthera.2019.07.001
Neelamraju, Y., Gonzalez-Perez, A., Bhat-Nakshatri, P., Nakshatri, H., and Janga, S. C. (2018). Mutational Landscape of RNA-Binding Proteins in Human Cancers. RNA Biol. 15 (1), 115–129. Epub 2017/10/13. doi:10.1080/15476286.2017.1391436
Neelamraju, Y., Hashemikhabir, S., and Janga, S. C. (2015). The Human RBPome: from Genes and Proteins to Human Disease. J. Proteomics 127 (Pt), 61–70. Epub 2015/05/20. doi:10.1016/j.jprot.2015.04.031
Nguyen, T. A., Bieging-Rolett, K. T., Putoczki, T. L., Wicks, I. P., Attardi, L. D., and Pang, K. C. (2019). SIDT2 RNA Transporter Promotes Lung and Gastrointestinal Tumor Development. iScience 20, 14–24. Epub 2019/09/24. doi:10.1016/j.isci.2019.09.009
Park, M., Kim, M., Hwang, D., Park, M., Kim, W. K., Kim, S. K., et al. (2014). Characterization of Gene Expression and Activated Signaling Pathways in Solid-Pseudopapillary Neoplasm of Pancreas. Mod. Pathol. 27 (4), 580–593. Epub 2013/09/28. doi:10.1038/modpathol.2013.154
Pelletier, J., Thomas, G., and Volarević, S. (2018). Ribosome Biogenesis in Cancer: New Players and Therapeutic Avenues. Nat. Rev. Cancer 18 (1), 51–63. Epub 2017/12/02. doi:10.1038/nrc.2017.104
Pidugu, V. K., Pidugu, H. B., Wu, M.-M., Liu, C.-J., and Lee, T.-C. (2019). Emerging Functions of Human IFIT Proteins in Cancer. Front. Mol. Biosci. 6, 148. Epub 2020/01/11. doi:10.3389/fmolb.2019.00148
Ritchie, M. E., Phipson, B., Wu, D., Hu, Y., Law, C. W., Shi, W., et al. (2015). Limma powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 43 (7), e47. Epub 2015/01/22. doi:10.1093/nar/gkv007
Saxena, M., and Yeretssian, G. (2014). NOD-like Receptors: Master Regulators of Inflammation and Cancer. Front. Immunol. 5, 327. Epub 2014/07/30. doi:10.3389/fimmu.2014.00327
Schultz, M. A., Diaz, A. M., Smite, S., Lay, A. R., DeCant, B., McKinney, R., et al. (2017). Thioredoxin System-Mediated Regulation of Mutant Kras Associated Pancreatic Neoplasia and Cancer. Oncotarget 8 (54), 92667–92681. Epub 2017/12/02. doi:10.18632/oncotarget.21539
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 13 (11), 2498–2504. Epub 2003/11/05. doi:10.1101/gr.1239303
Singh, G., Pratt, G., Yeo, G. W., and Moore, M. J. (2015). The Clothes Make the mRNA: Past and Present Trends in mRNP Fashion. Annu. Rev. Biochem. 84, 325–354. Epub 2015/03/19. doi:10.1146/annurev-biochem-080111-092106
Sloan, K. E., Bohnsack, M. T., and Watkins, N. J. (2013). The 5S RNP Couples P53 Homeostasis to Ribosome Biogenesis and Nucleolar Stress. Cel Rep. 5 (1), 237–247. Epub 2013/10/15. doi:10.1016/j.celrep.2013.08.049
Sriram, A., Bohlen, J., and Teleman, A. A. (2018). Translation Acrobatics: How Cancer Cells Exploit Alternate Modes of Translational Initiation. EMBO Rep. 19 (10). Epub 2018/09/19. doi:10.15252/embr.201845947
Sternburg, E. L., and Karginov, F. V. (2020). Global Approaches in Studying RNA-Binding Protein Interaction Networks. Trends Biochemical Sciences 45 (7), 593–603. Epub 2020/06/13. doi:10.1016/j.tibs.2020.03.005
Sulima, S. O., Hofman, I. J. F., De Keersmaecker, K., and Dinman, J. D. (2017). How Ribosomes Translate Cancer. Cancer Discov. 7 (10), 1069–1087. Epub 2017/09/20. doi:10.1158/2159-8290.cd-17-0550
Szklarczyk, D., Morris, J. H., Cook, H., Kuhn, M., Wyder, S., Simonovic, M., et al. (2017). The STRING Database in 2017: Quality-Controlled Protein-Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res. 45 (D1), D362–D368. Epub 2016/12/08. doi:10.1093/nar/gkw937
Tang, H., Wei, P., Chang, P., Li, Y., Yan, D., Liu, C., et al. (2017). Genetic Polymorphisms Associated with Pancreatic Cancer Survival: a Genome-wide Association Study. Int. J. Cancer 141 (4), 678–686. Epub 2017/05/05. doi:10.1002/ijc.30762
Taniuchi, K., Furihata, M., Hanazaki, K., Iwasaki, S., Tanaka, K., Shimizu, T., et al. (2015). Peroxiredoxin 1 Promotes Pancreatic Cancer Cell Invasion by Modulating P38 MAPK Activity. Pancreas 44 (2), 331–340. Epub 2014/11/27. doi:10.1097/mpa.0000000000000270
Taniuchi, K., Furihata, M., Hanazaki, K., Saito, M., and Saibara, T. (2014). IGF2BP3-mediated Translation in Cell Protrusions Promotes Cell Invasiveness and Metastasis of Pancreatic Cancer. Oncotarget 5 (16), 6832–6845. Epub 2014/09/13. doi:10.18632/oncotarget.2257
Taniuchi, K., Furihata, M., Iwasaki, S., Tanaka, K., Shimizu, T., Saito, M., et al. (2014). RUVBL1 Directly Binds Actin Filaments and Induces Formation of Cell Protrusions to Promote Pancreatic Cancer Cell Invasion. Int. J. Oncol. 44 (6), 1945–1954. Epub 2014/04/15. doi:10.3892/ijo.2014.2380
Tsai, Y.-C., Pestka, S., Wang, L.-H., Runnels, L. W., Wan, S., Lyu, Y. L., et al. (2011). Interferon-β Signaling Contributes to Ras Transformation. PloS one 6 (8), e24291. Epub 2011/09/08. doi:10.1371/journal.pone.0024291
Uhlén, M., Fagerberg, L., Hallström, B. M., Lindskog, C., Oksvold, P., Mardinoglu, A., et al. (2015). Tissue-Based Map of the Human Proteome. Science 347, 1260419. doi:10.1126/science.1260419
Wang, F.-w., Guan, X.-y., and Xie, D. (2013). Roles of Eukaryotic Initiation Factor 5A2 in Human Cancer. Int. J. Biol. Sci. 9 (10), 1013–1020. Epub 2013/11/20. doi:10.7150/ijbs.7191
Wang, J., Dumartin, L., Mafficini, A., Ulug, P., Sangaralingam, A., Alamiry, N. A., et al. (2017). Splice Variants as Novel Targets in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 7 (1), 2980. Epub 2017/06/09. doi:10.1038/s41598-017-03354-z
Wang, J., Guo, Y., Chu, H., Guan, Y., Bi, J., and Wang, B. (2013). Multiple Functions of the RNA-Binding Protein HuR in Cancer Progression, Treatment Responses and Prognosis. Ijms 14 (5), 10015–10041. Epub 2013/05/15. doi:10.3390/ijms140510015
Wang, Z.-L., Li, B., Luo, Y.-X., Lin, Q., Liu, S.-R., Zhang, X.-Q., et al. (2018). Comprehensive Genomic Characterization of RNA-Binding Proteins across Human Cancers. Cel Rep. 22 (1), 286–298. Epub 2018/01/04. doi:10.1016/j.celrep.2017.12.035
Wen, X., Shao, Z., Chen, S., Wang, W., Wang, Y., Jiang, J., et al. (2020). Construction of an RNA-Binding Protein-Related Prognostic Model for Pancreatic Adenocarcinoma Based on TCGA and GTEx Databases. Front. Genet. 11, 610350. Epub 2021/02/16. doi:10.3389/fgene.2020.610350
Wurth, L., and Gebauer, F. (2015). RNA-binding Proteins, Multifaceted Translational Regulators in Cancer. Biochim. Biophys. Acta (Bba) - Gene Regul. Mech. 1849 (7), 881–886. Epub 2014/10/16. doi:10.1016/j.bbagrm.2014.10.001
Yang, P., An, H., Liu, X., Wen, M., Zheng, Y., Rui, Y., et al. (2010). The Cytosolic Nucleic Acid Sensor LRRFIP1 Mediates the Production of Type I Interferon via a β-catenin-dependent Pathway. Nat. Immunol. 11 (6), 487–494. Epub 2010/05/11. doi:10.1038/ni.1876
Yang, S., He, P., Wang, J., Schetter, A., Tang, W., Funamizu, N., et al. (2016). A Novel MIF Signaling Pathway Drives the Malignant Character of Pancreatic Cancer by Targeting NR3C2. Cancer Res. 76 (13), 3838–3850. Epub 2016/05/20. doi:10.1158/0008-5472.can-15-2841
Yang, Y.-C. T., Di, C., Hu, B., Zhou, M., Liu, Y., Song, N., et al. (2015). CLIPdb: a CLIP-Seq Database for Protein-RNA Interactions. BMC genomics 16, 51. Epub 2015/02/06. doi:10.1186/s12864-015-1273-2
Yu, G., Jia, B., Cheng, Y., Zhou, L., Qian, B., Liu, Z., et al. (2017). MicroRNA-429 Sensitizes Pancreatic Cancer Cells to Gemcitabine through Regulation of PDCD4. Am. J. Transl Res. 9 (11), 5048–5055. Epub 2017/12/09.
Yu, G., Wang, L.-G., Han, Y., and He, Q.-Y. (2012). clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. OMICS: A J. Integr. Biol. 16 (5), 284–287. Epub 2012/03/30. doi:10.1089/omi.2011.0118
Glossary
RBP RNA-binding protein
TERT telomerase reverse transcriptase
ELAVL1 ELAV-like RNA-binding protein 1
FXR1 FMR1 autosomal homolog 1
JAK-STAT Janus kinase- signal transducer and activator of transcription
TCGA The Cancer Genome Atlas
GEPIA gene expression profiling interactive analysis
DDX60 DEAD-box helicase 60
IFN Interferon Interferon
IGF2BP Insulin-like growth factor 2 mRNA-binding protein
PDAC Pancreatic ductal adenocarcinoma
PAIP2B Polyadenylate-binding protein–interacting protein 2B
SIDT2 SID1 transmembrane family member 2
PDCD4 Programmed cell death 4
AZGP1 Alpha-2-glycoprotein 1
RNASE1 Ribonuclease A family member 1
NOD Nucleotide-binding oligomerization domain
ISG20 Interferon-stimulated exonuclease gene 20
MAPK Mitogen-activated protein kinase
AGE Advanced glycation end products
RAGE Receptor of AGE
PI3K-Akt phosphatidylinositol 3-kinase - AKT serine/threonine kinase
HIF Hypoxia-inducible factor
VEGF Vascular endothelial growth factor
Th17 T-helper cell 17
RCC2 Regulator of chromosome condensation 2
APOBEC Apolipoprotein B MRNA editing enzyme catalytic subunit
OAS Oligoadenylate synthetase
SPATS2L Spermatogenesis-associated serine rich 2–like
TXN Thioredoxin
UHMK1 U2AF homology motif kinase 1
ANGEL1 Angel homolog 1
P4HB Prolyl 4-hydroxylase subunit beta
IFIT Interferon-inducedpProtein With tetratricopeptide repeats
LRRFIP1 LRR-binding FLII-interacting protein 1
EIF2AK Eukaryotic translation initiation factor 2 alpha kinase
EIF5 Eukaryotic translation initiation factor 5
IFN Interferon Interferon
TGF- β Tumor growth factor-beta
lncRNA Long noncoding RNA
WNT Wingless-type
EGF Epidermal growth factor
FGF Fibroblast growth factor
SnoRNA Small nucleolar RNA
ScaRNA small Cajal body-specific RNA
IHC Immunohistochemistry
Keywords: RNA-binding protein, pancreatic cancer, meta-analysis, epithelial-to-mesenchymal transition, target RNAs
Citation: Mukherjee M and Goswami S (2021) Identification of Key Deregulated RNA-Binding Proteins in Pancreatic Cancer by Meta-Analysis and Prediction of Their Role as Modulators of Oncogenesis. Front. Cell Dev. Biol. 9:713852. doi: 10.3389/fcell.2021.713852
Received: 24 May 2021; Accepted: 04 November 2021;
Published: 29 November 2021.
Edited by:
Sridhar Muthusami, Karpagam Academy of Higher Education, IndiaReviewed by:
Eduardo Castañeda Saucedo, Autonomous University of Guerrero, MexicoYi Pan, China Pharmaceutical University, China
Dinesh Babu Somasundaram, University of Oklahoma Health Sciences Center, United States
Copyright © 2021 Mukherjee and Goswami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Srikanta Goswami, c2cxQG5pYm1nLmFjLmlu
 Moumita Mukherjee
Moumita Mukherjee Srikanta Goswami
Srikanta Goswami