- 1Department of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Genetics, Faculty of Biological Sciences, Tarbiat Modares University, Tehran, Iran
- 3Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Skull Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
X-inactive–specific transcript (XIST) is one of the firstly discovered long non-coding RNAs with prominent roles in the process of X inactivation. Moreover, this transcript contributes in the carcinogenic process in different tissues. In addition to interacting with chromatin modifying molecules, XIST can be served as a molecular sponge for miRNAs to modulate expression of miRNA targets. Most of the studies have indicated an oncogenic role for XIST. However, in prostate cancer, a single study has indicated a tumor suppressor role for this lncRNA. Similar result has been reported for XIST in oral squamous cell carcinoma. In hepatocellular carcinoma, breast cancer, ovarian cancer, osteosarcoma, and renal cell carcinoma, different studies have reported inconsistent results. In the present manuscript, we review function of XIST in the carcinogenesis.
Introduction
X-inactive–specific transcript (XIST) RNA is among the firstly discovered long non-coding RNAs (lncRNAs) in humans (Brown et al., 1992). The gene coding this lncRNA has at least eight exons and spans an area of about 17 kb on the X chromosome, in a region containing the X inactivation center (Brown et al., 1992). XIST RNA is primarily localized in the nucleus to a location not discriminable from the X inactivation-associated Barr body (Brown et al., 1992). The first important function attributed to XIST has been related to the process of X inactivation during which XIST induces gene silencing through recruitment of several chromatin modifying molecules (Loda and Heard, 2019). The indispensable role of Xist in X inactivation has been proved by targeted mutagenesis and transgenic experiments in mice showing skewing of this process following deletion of the Xist gene (Penny et al., 1996; Marahrens et al., 1997). Several molecules have been identified to interact with XIST to contribute in chromosome-wide gene silencing. SPEN, RBM15, WTAP, hnRNP K, and LBR are among molecules that participate in this process through interplay with XIST (Chu et al., 2015; McHugh et al., 2015). In addition, XIST has a prominent role in the carcinogenic processes. Several in vitro, in vivo, and clinical investigations have verified this aspect of XIST functions. In the present manuscript, we review function of XIST in the carcinogenesis.
Cell Line Studies
Breast Cancer
Functional impact of XIST in the breast carcinogenesis has been assessed in a number of in vitro studies. Liu et al. (2020) have reported down-regulation of XIST and UBAP1 in breast cancer cells. Forced up-regulation of XIST has attenuated proliferation, migration and invasion of these cells, and accelerated cell apoptosis. From a mechanistical point of view, XIST can interact with miR-362-5p and miR-362-5p to exert its effects. UBAP1 has been identified as miR-362-5p target, thus XIST modulates expression this protein via sponging miR-362-5p (Liu et al., 2020). Li et al. (2020d) have demonstrated down-regulation of XIST in triple negative breast cancer cells. Up-regulation of XIST has blocked cell proliferation and epithelial mesenchymal transition (EMT) while inducing apoptosis in these cell lines. miR-454 has been identified as a target of XIST in these cells (Li et al., 2020d). On the other hand, Zong et al. (2020) XIST has reported up-regulation of XIST in breast cancer cells, parallel with down-regulation of miR-125b-5p and up-regulation of NLRC5. XIST silencing has remarkably suppressed cell proliferation, migration, and invasion aptitudes of breast cancer cells. XIST has been shown to sponge miR-125b-5p and subsequently influence NLRC5 expression (Zong et al., 2020). Moreover, expression of XIST has been reported to be higher in doxorubicin-resistant breast cancer cells compared with parental cells. Furthermore, XIST up-regulation enhances cell proliferation and prohibited apoptosis of doxorubicin-treated breast cancer cells through enhancing expression of ANLN. XIST functions as a sponge for miR-200c-3p, which regulates expression of ANLN (Zhang et al., 2020). Figure 1 depicts different roles of XIST in the breast carcinogenesis.
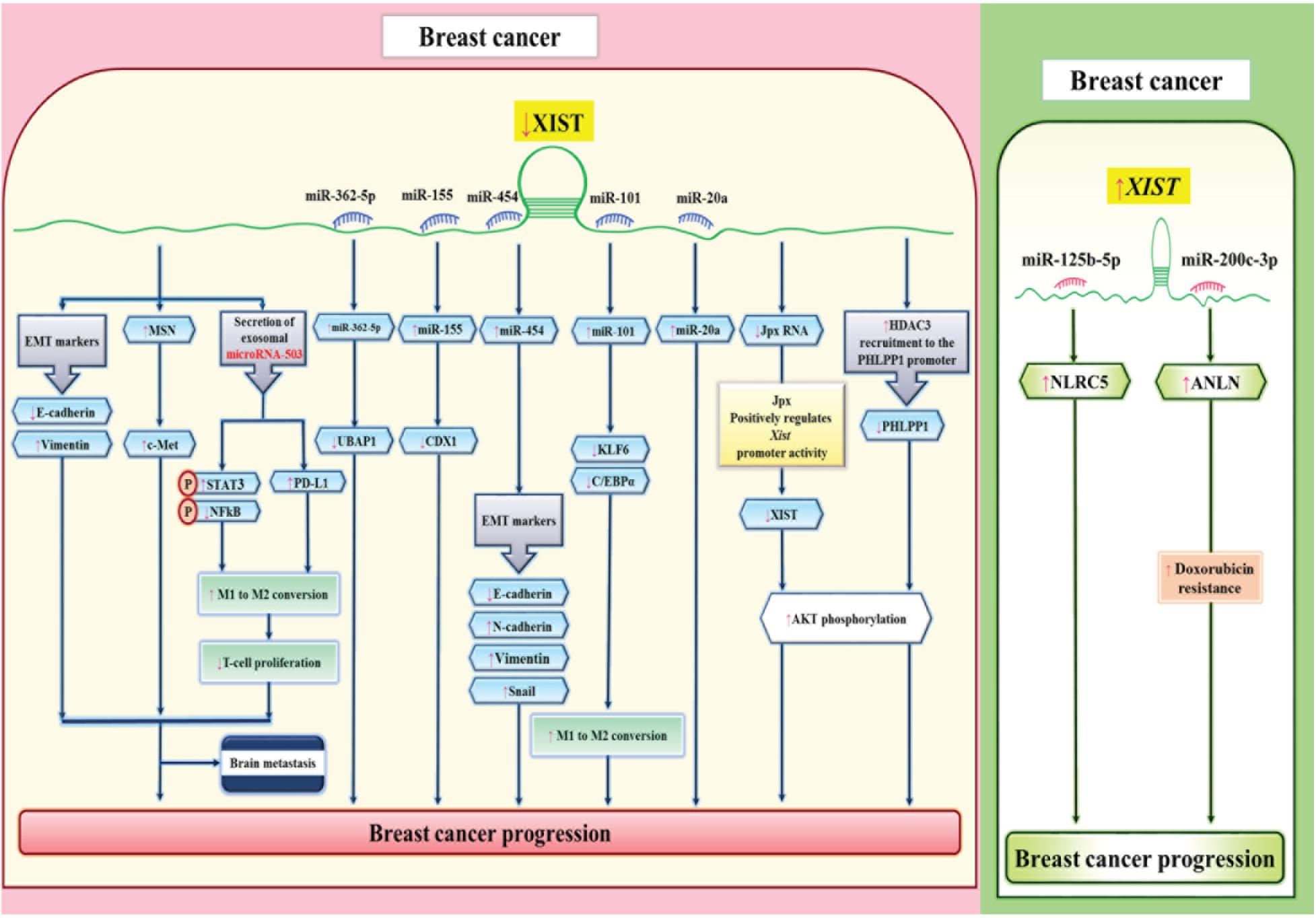
Figure 1. Different studies have shown the tumor suppressor role of XIST in breast cancer through sponging miRNAs (Li et al., 2020d; Liu et al., 2020) and regulating expression of epithelial-mesenchymal transition (EMT) markers (right panel). On the other hand, other studies have reported oncogenic roles for XIST (Zong et al., 2020) (left panel).
Gastric Cancer
In gastric cancer, XIST has been shown to exert oncogenic effects. Zheng et al. (2020) have demonstrated over-expression of XIST and down-regulation of miR-337 in these cells. XIST silencing has simultaneously suppressed proliferation, invasion, and migration of gastric cancer cells. Mechanistically, XIST increases expression of JAK2 through sponging miR-337 (Zheng et al., 2020). Consistently, over-expression of XIST in gastric cancer cells has been accompanied by up-regulation of PXN while down-regulation of miR-132. Furthermore, both XIST silencing and miR-132 over-expression could inhibit gastric cancer cell proliferation and migration (Li et al., 2020a). In this kind of cancer, XIST has also been shown to promote cell cycle progression at G1/S phase and block cell apoptosis through repressing miR-497 expression and up-regulating MACC1 levels (Ma et al., 2017). XIST can also sponge miR-185 to influence expression of TGF-β1 in gastric cancer cells (Zhang et al., 2018). Figure 2 depicts the role of XIST in gastric carcinogenesis.
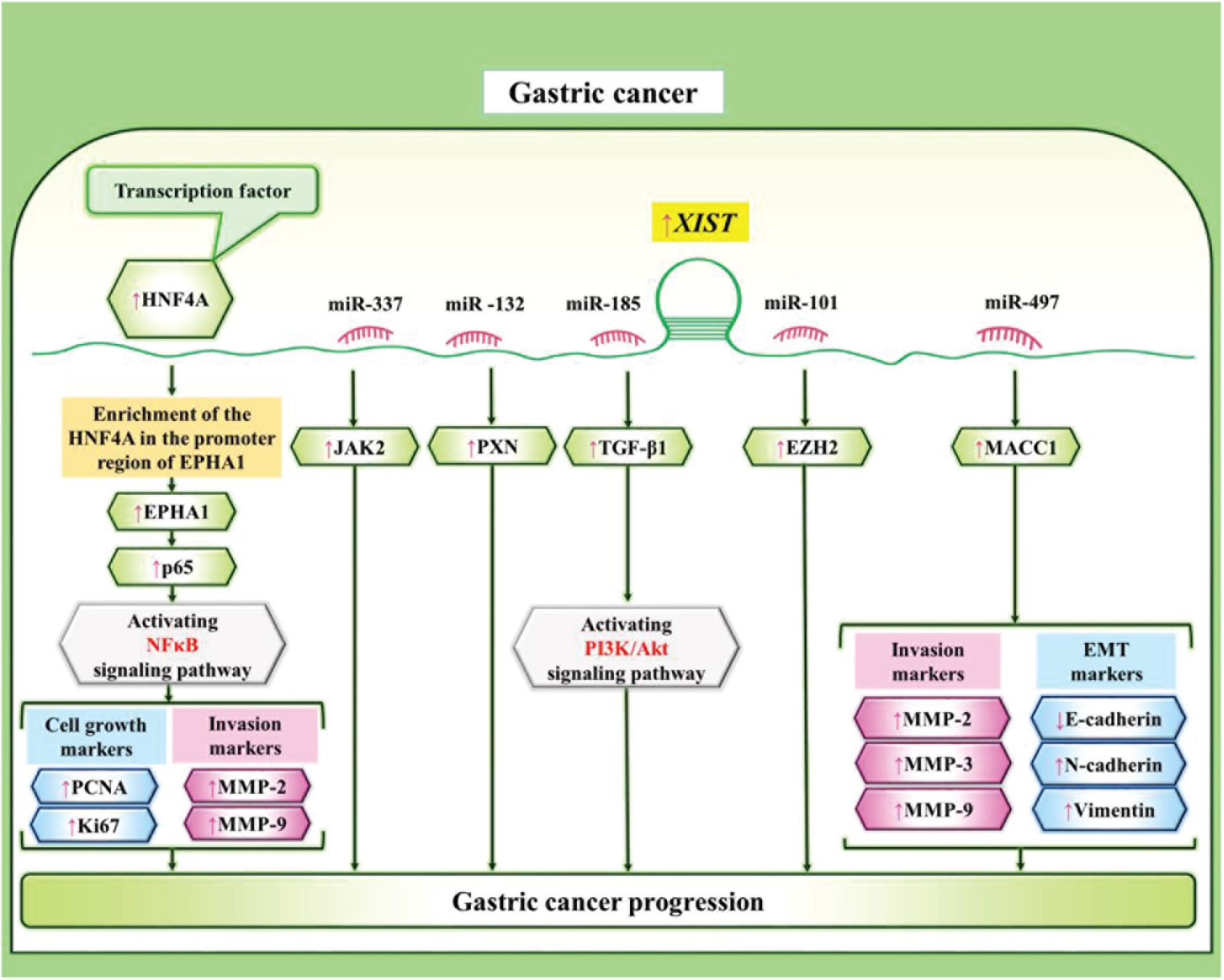
Figure 2. XIST can affect gastric carcinogenesis through sponging a number of miRNAs, thus regulating NF-κB and PI3K/AKT pathways (Chen et al., 2016; Lu et al., 2017; Ma et al., 2017; Zhang et al., 2018; Li et al., 2020a; Zheng et al., 2020).
Colorectal Cancer
Expression of XIST has been increased in colon cancer cells. Mechanistically, XIST sponges miR-34a and increases expression of WNT1. XIST also affects expression of β-catenin, cyclinD1, c-Myc, and MMP-7 in colon cancer cells (Sun et al., 2018). Moreover, expression of this lncRNA has been up-regulated 5-Flurouracil-resitant colon cancer cells. XIST silencing has inverted resistance phenotype in these cells. XIST has been shown to promote expression of thymidylatesynthase, an enzyme which is targeted by 5-Flurouracil (Xiao et al., 2017). Another study in colon cancer cells has demonstrated over-expression of XIST and FOXK1, while down-regulation of miR-497-5p. This study has also confirmed the significance of XIST/miR-497-5p/FOXK1 in the pathogenesis of colon cancer (Wang et al., 2020b). Figure 3 depicts the downstream targets of XIST in colon cancer cells.
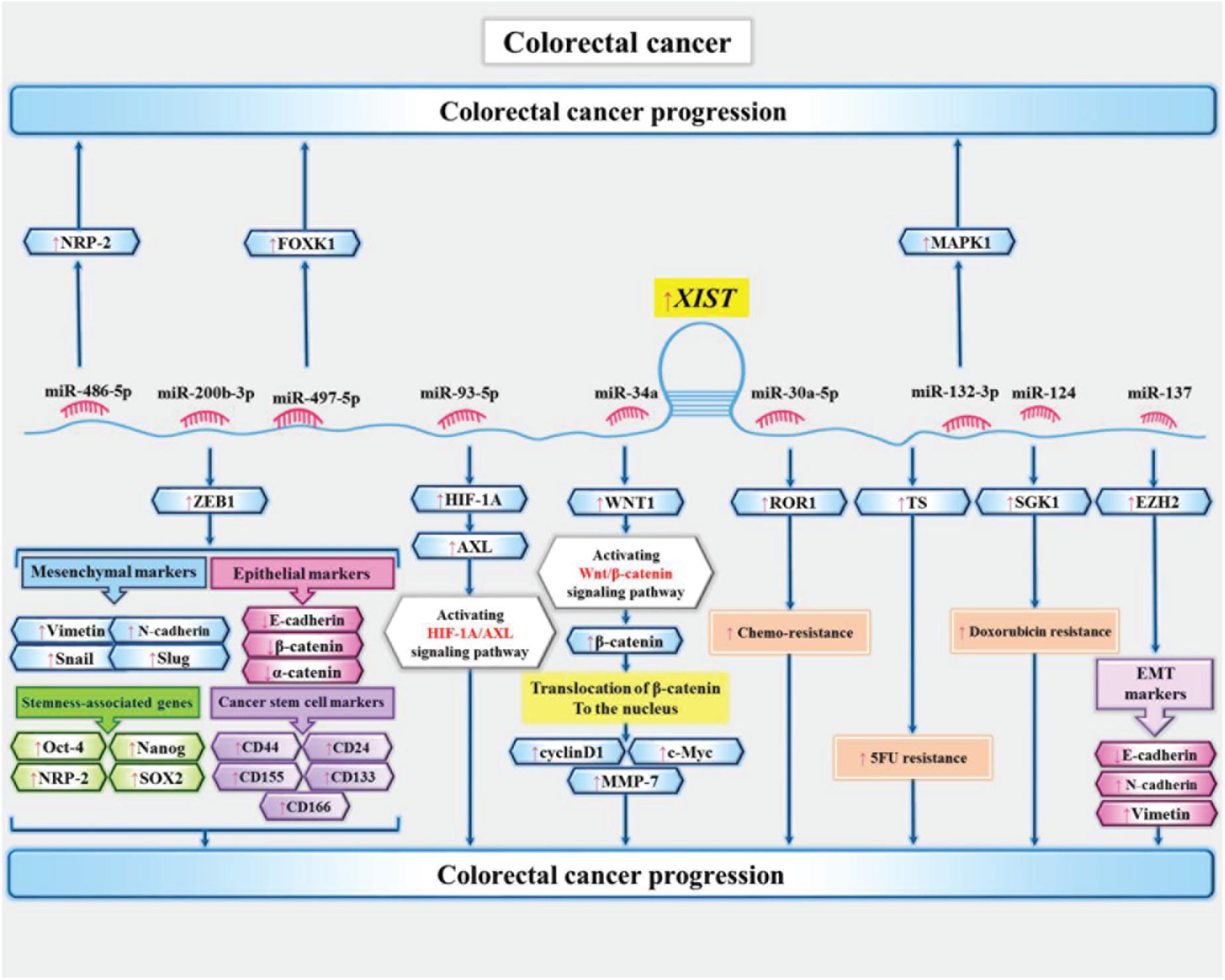
Figure 3. Downstream targets of XIST in colon cancer cells (Chen et al., 2017; Song et al., 2017; Xiao et al., 2017; Liu et al., 2020; Sun et al., 2018; Liu et al., 2019; Wang et al., 2020b). XIST enhances proliferation and epithelial–mesenchymal transition of colon cancer cells via sequestering miR-486-5p and increasing expression neuropilin-2 (Liu et al., 2019). It can also sequester miR-137 and subsequently increase expression of EZH2 to enhance metastatic ability of colon cancer cells (Liu et al., 2018).
Pancreatic Cancer
XIST has also been up-regulated in prostate cancer cell lines where it enhances their proliferation, migration and invasion, and suppresses cell their apoptosis. These effects are exerted through sponging miR-34a-5p (Sun et al., 2018). In these cells, XIST has also interactions with miR-137 through which it regulates expression of Notch1 (Liu et al., 2020). miR-141-3p is another miRNA which has been shown to be sponged by XIST in pancreatic cancer cells. XIST enhances expression of TGF-β2 through interacting with this miRNA (Sun and Zhang, 2019). Figure 4 depicts the interaction between XIST and miRNAs as well as their targets in pancreatic cancer cells.
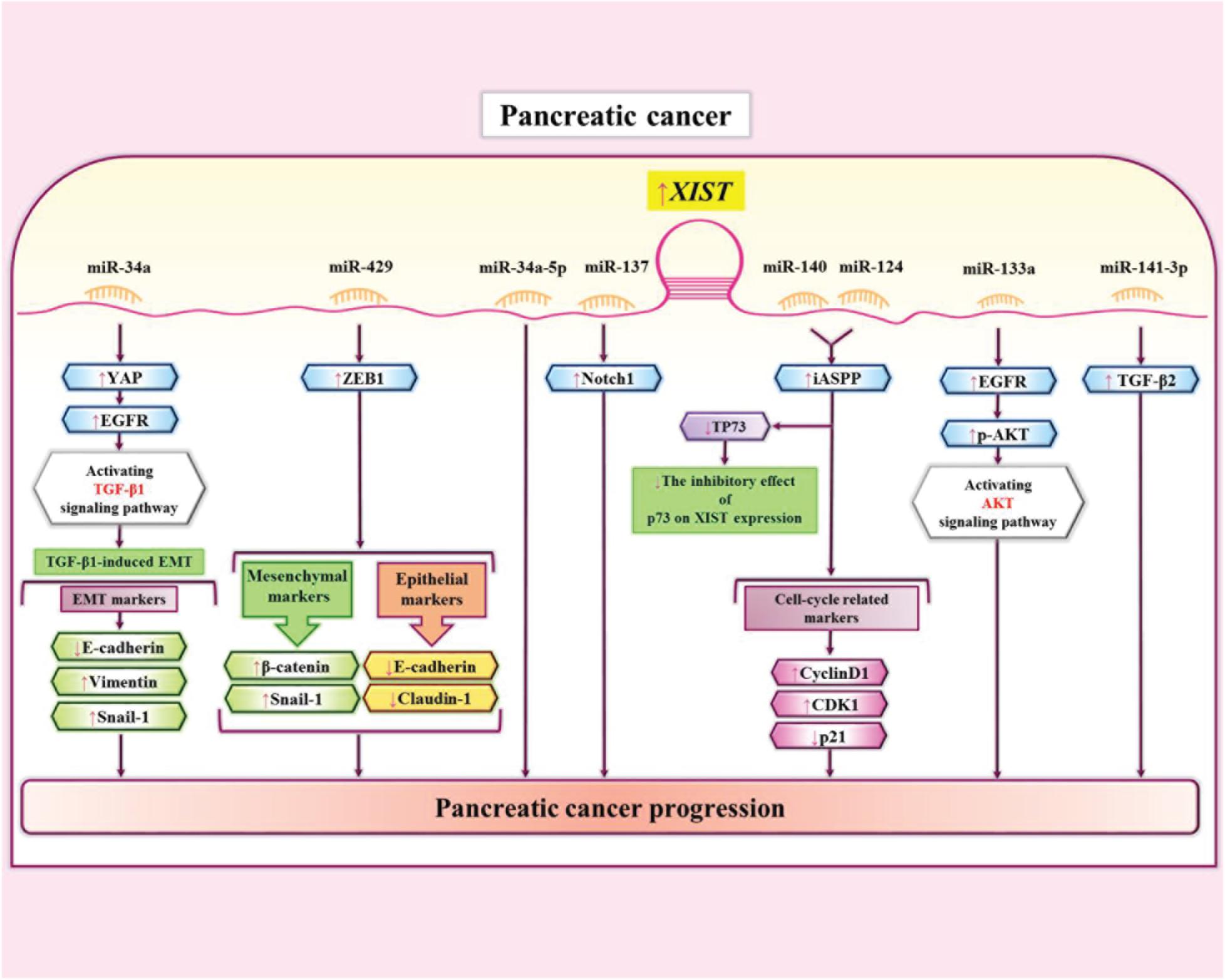
Figure 4. The interaction between XIST and miRNAs as well as their targets in pancreatic cancer cells (Liang et al., 2017; Wei et al., 2017; Sun et al., 2018; Shen et al., 2019; Sun and Zhang, 2019; Liu et al., 2020; Zou et al., 2020). XIST interaction with miR-140 and miR-124 increases expression of iASPP and promotes growth of pancreatic cancer cells (Liang et al., 2017). XIST also enhances expression of TGF-β2 through sequestering miR-141-3p. This interaction enhances invasiveness of pancreatic cancer cells (Sun and Zhang, 2019).
Bladder Cancer
In bladder cancer cells, XIST serves as a molecular sponge for miR-200c through which it enhances colony formation, self-renewal capacity and EMT in cancer stem cells -like cells (Xu et al., 2018). Another study has indicated parallel over-expressions of XIST and androgen receptor (AR) in bladder cancer cells. Mechanistically, XIST increases AR expression though sponging miR-124 (Xiong et al., 2017). Moreover, XIST can promote proliferation and metastatic ability of bladder cancer cells via modulating miR-139-5p expression and subsequent regulation of Wnt/β-catenin signaling pathway (Hu et al., 2017). Figure 5 depicts the interactions between XIST and miRNAs in bladder cancer cells.
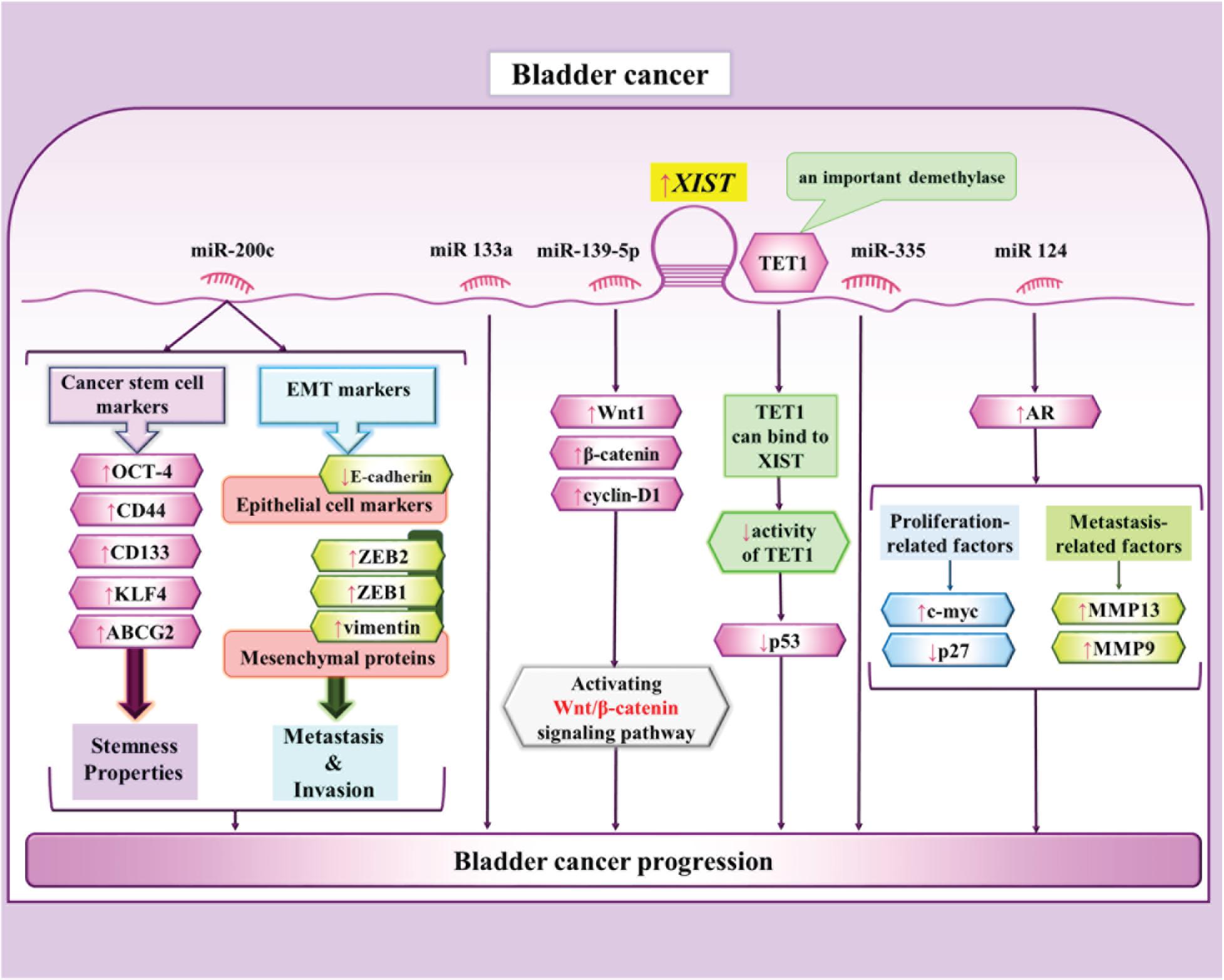
Figure 5. The interactions between XIST and miRNAs in bladder cancercells (Hu et al., 2017, 2019; Xiong et al., 2017; Xu et al., 2018; Zhou et al., 2019b). XIST influences progression of bladder cancer through suppressing p53 via binding to TET1 (Hu et al., 2019). Another route of participation of XIST in tumor growth and metastasis is exerted through regulation of miR-139-5p and Wnt/ β-catenin pathway (Hu et al., 2017).
Glioma
In glioma cells, XIST can modulate metabolism of glucose. XIST silencing has suppressed viability, migration, invasiveness, hypo-responsiveness to apoptotic stimuli, and glucose metabolism in glioblastoma. Mechanistically, XIST functions as a molecular sponge for miR-126 to subsequently regulate IRS1/PI3K/Akt pathway (Cheng et al., 2020). Another study in glioblastoma has shown the role of Steroid receptor coactivator-1 (SRC-1) in the regulation of XIST at posttranscriptional level. In fact, the impact of SRC-1 in enhancement of stemness features in glioblastoma is mediated through XIST. SRC-1 enhances expression of Kruppel-like factor 4 (KLF4) via the XIST/miR-152 axis (Gong et al., 2020). Moreover, miR-204-5p has been identified as another target of XIST in glioma cells. Interaction between XIST and miR-204-5p regulates expression of Bcl-2 (Shen et al., 2020). Figure 6 shows the interactions between XIST and miRNAs in glioma cells.
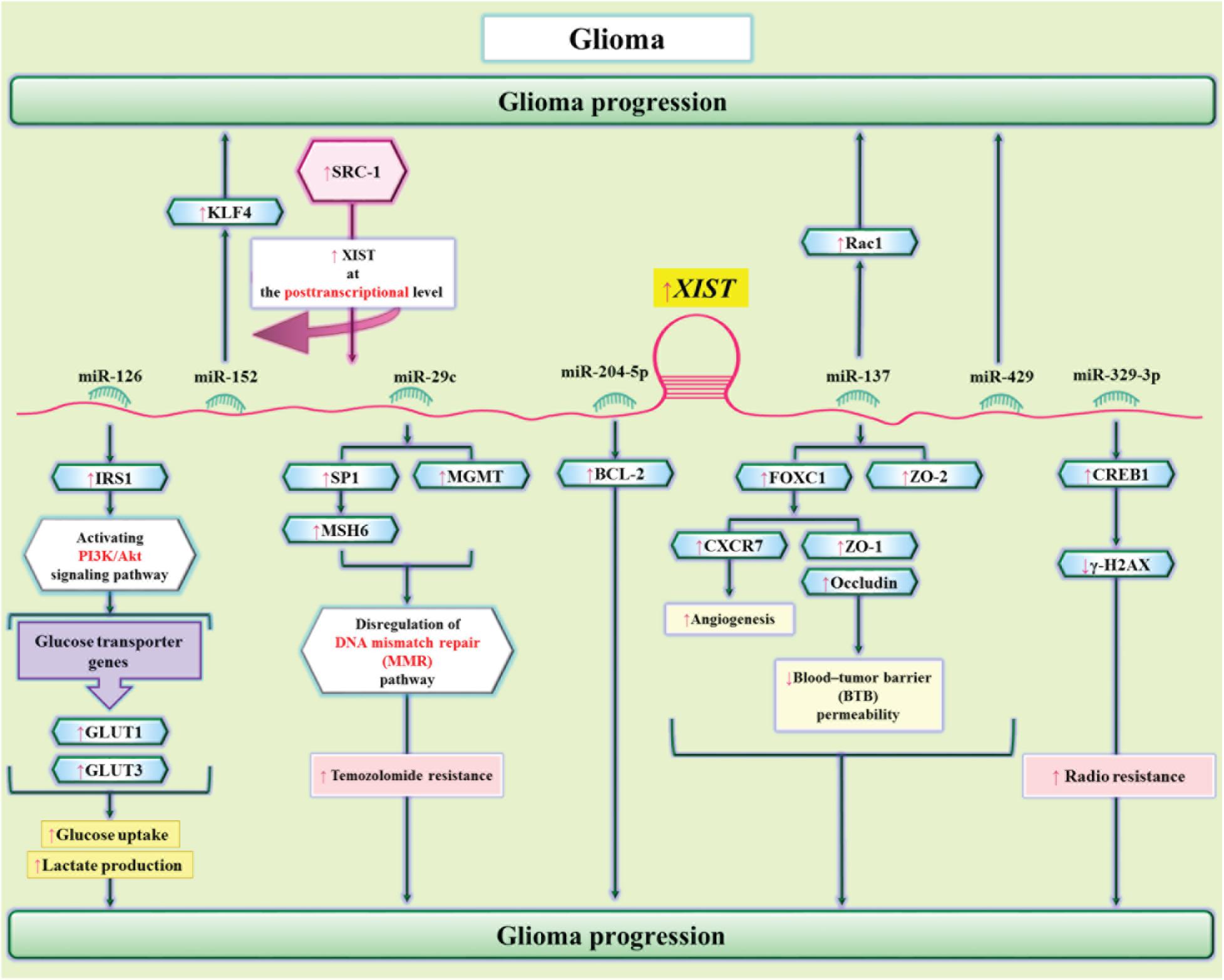
Figure 6. The interactions between XIST and miRNAs in glioma cells. XIST enhances glioma tumorigenic capacity and angiogenesis through sequestering miR-429 (Cheng et al., 2017). In addition, through sequestering miR-126, XIST regulates cell proliferation and glucose metabolism of glioma cells via influencing IRS1/PI3K/Akt axis (Cheng et al., 2020). The sponging effect of XIST on miR-29c modulates resistance of glioma cell to Temozolomide via DNA mismatch repair pathway (Du et al., 2017).
Lung Cancer
XIST has also been shown to be over-expressed in lung cancer cell lines promoting their proliferation ability through sponging miR-140. XIST silencing has repressed proliferation and enhanced apoptosis of lung cancer cells. Besides, inhibitor of apoptosis-stimulating protein of p53 (iASPP) has a prominent role in mediation of this effect (Tang et al., 2017). Expression of XIST expression has also been up-regulated in cisplatin-resistant lung cancer cells compared with the original cells. Up-regulation of this lncRNA has enhanced resistance to cisplatin through blocking apoptosis and increasing proliferation ability. These effects are mediated through sponging let-7i and regulating expression of BAG-1 (Sun et al., 2017). Figure 7 shows the interactions between XIST and miRNAs in lung cancer cells.
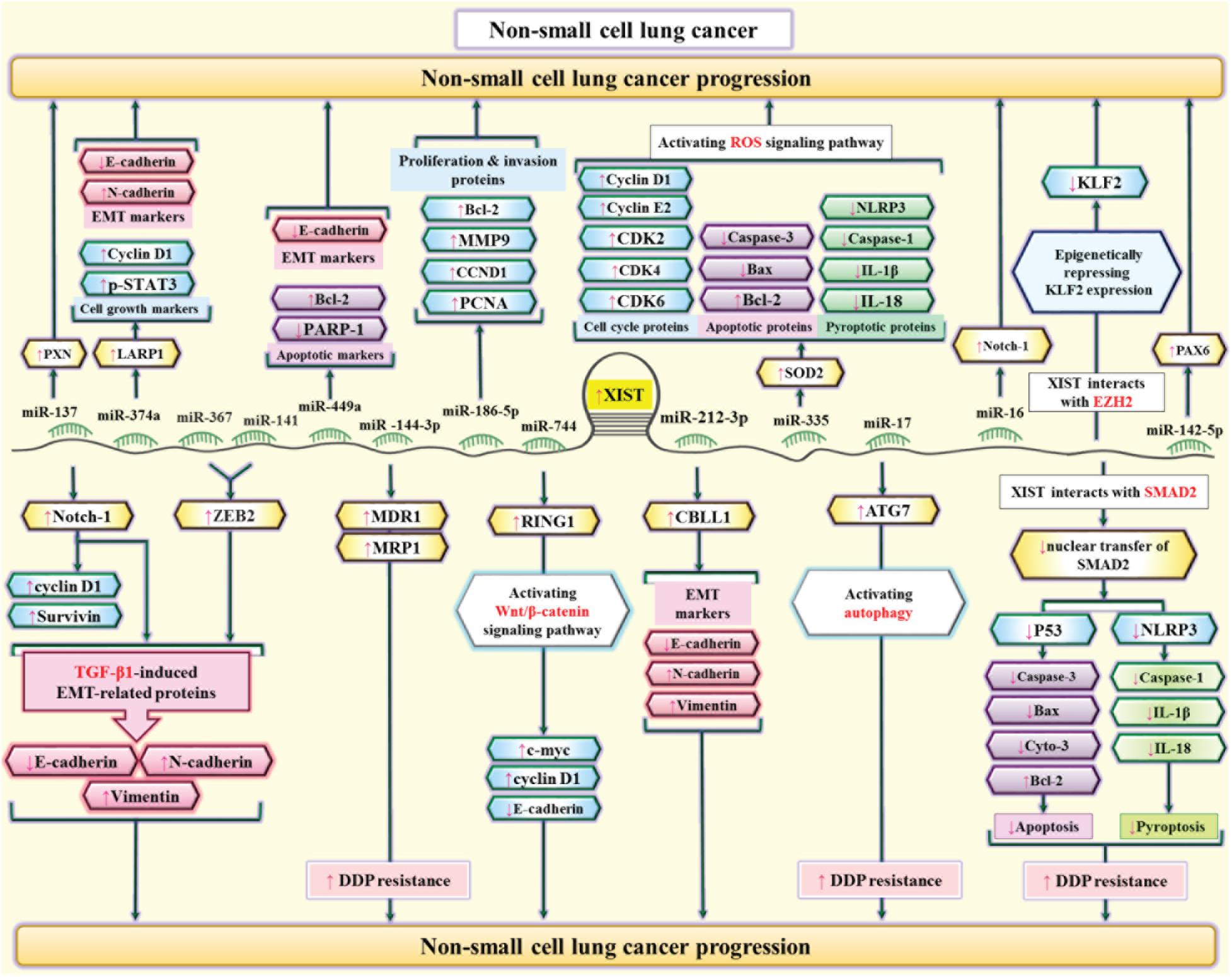
Figure 7. The interactions between XIST and miRNAs in lung cancer cells. XIST has an oncogenic role in lung cancer through different mechanisms including epigenetically silencing of KLF2 expression (Fang et al., 2016). Moreover, it can enhance viability and invasiveness of lung cancer cells through regulation of miR-137/PXN axis (Jiang et al., 2018). XIST can also enhance TGF-β-associated epithelial-mesenchymal transition through regulation of miR-367/141-ZEB2 (Li et al., 2018).
In addition to these types of malignancies, functional studies have verified the impact of XIST in the pathogenesis of almost all kinds of neoplasms. Supplementary Table 1 summarizes the results of in vitro studies.
Animal Studies
In line with in vitro studies, abnormal expression of XIST affects tumorigenesis in animal models of cancer. Almost all studies have indicated that up-regulation of XIST enhances tumorigenic ability of cancer cells, while its silencing has the opposite effects (Table 1). However, XIST has a tumor suppressor role in animal models of oral squamous cell carcinoma and renal cell carcinoma. Most notably, animal studies in hepatocellular carcinoma, breast cancer, ovarian cancer and osteosarcoma have indicated inconsistent results regarding the role of XIST (Table 1).
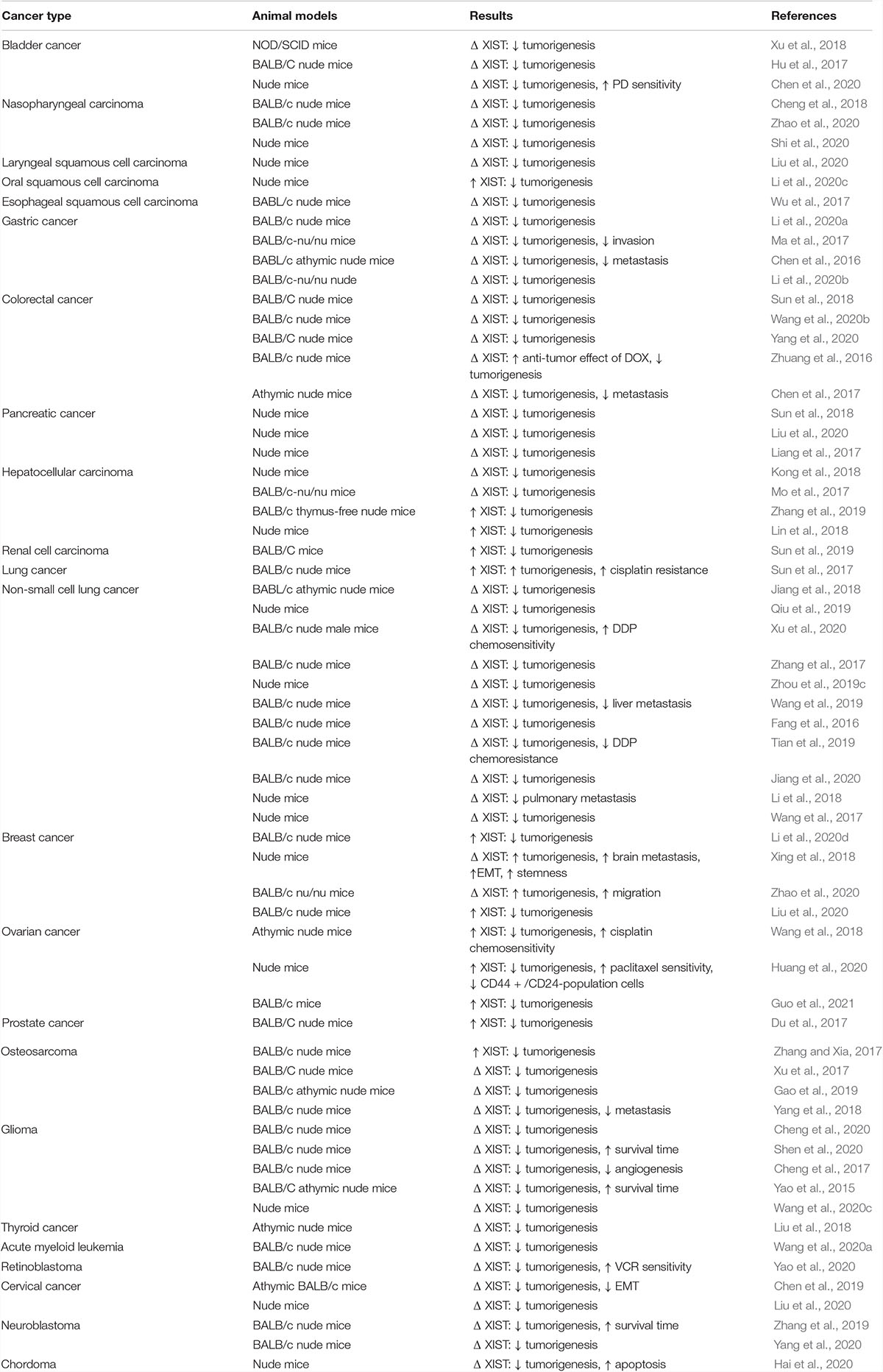
Table 1. Outcomes of studies which evaluated function of XIST in animal models (Δ: knock down or deletion).
Human Studies
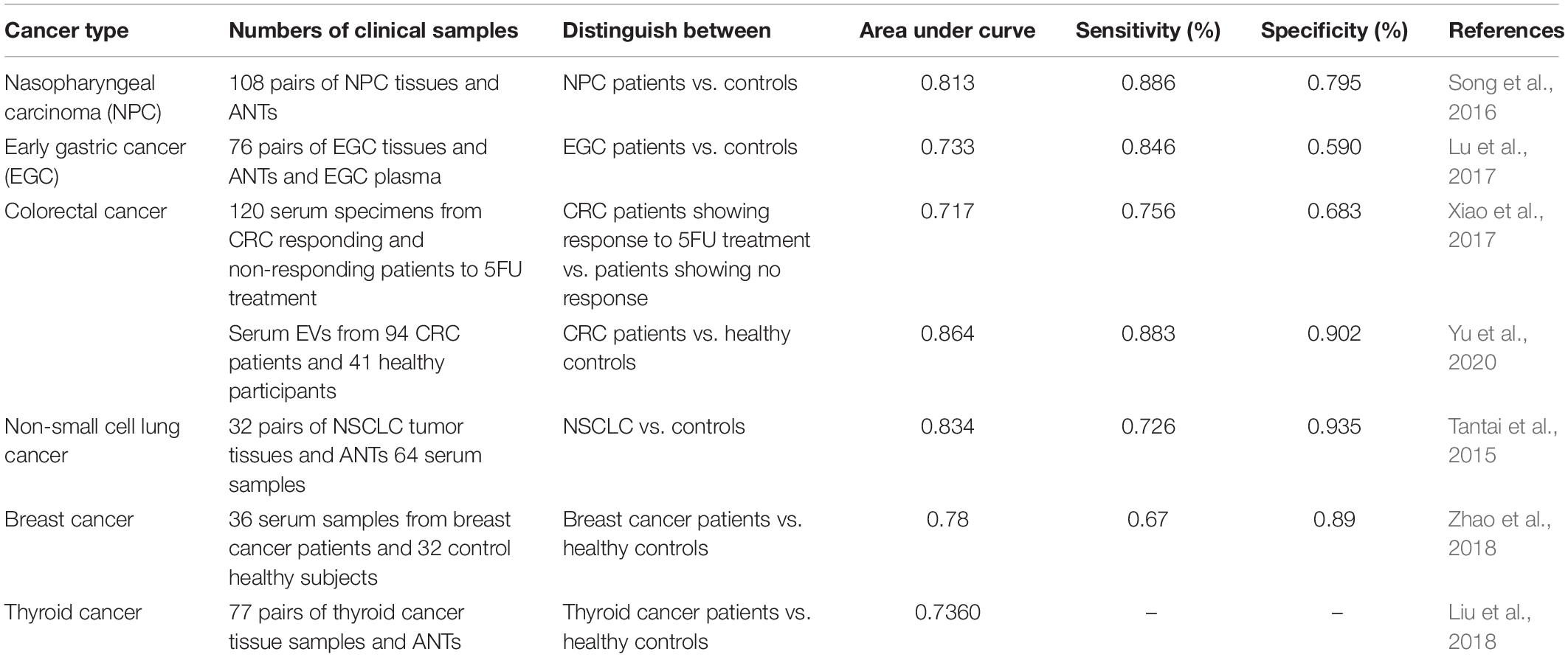
Experiments in clinical samples obtained from patients have shown that expression of XIST is principally increased in tumoral samples compared with nearby non-cancerous samples (Supplementary Table 2). However, in oral squamous cell carcinoma, its expression has been decreased (Li et al., 2020c). In hepatocellular carcinoma, most of studies have indicated its down-regulation (Chang et al., 2017; Lin et al., 2018; Zhang et al., 2019). However, few studies have reported opposite results (Mo et al., 2017; Kong et al., 2018). Similarly, different studies in breast cancer, ovarian cancer, osteosarcoma and renal cell carcinoma(Supplementary Table 2) have reported inconsistent results. Moreover, expression levels of XIST have been correlated with patients’ survival in different kinds of cancers including bladder cancer, esophageal squamous cell carcinoma, nasopharyngeal carcinoma, lung cancer, gastric cancer, colorectal cancer and breast cancer.
In nasopharyngeal carcinoma, XIST expression levels could differentiate tumoral tissues from nearby non-cancerous samples with diagnostic power of 0.813 (Song et al., 2016). In colorectal cancer, up-regulation of XIST in extracellular vesicles isolated from serum samples had an appropriate diagnostic value [Area under curve (AUC) = 0.86, sensitivity = 0.88 and specificity = 0.90]. Most notably, over-expression of XIST in serum extracellular vesicles has been associated with survival rates (Yu et al., 2020). Expression levels of XIST have also been shown to be appropriate markers for follow-up of patients with lung cancer, since they have been reduced following surgical removal of tumors. Receiver operating characteristic curves have demonstrated the ability of XIST expression levels in separation between the patients and healthy controls with an AUC value of 0.834. In addition, combination of expression levels of XIST and HIF1A-AS1 in serum samples has enhanced the diagnostic power (Tantai et al., 2015). Finally, serum levels of XIST could separate breast cancer patients from healthy controls with AUC value of 0.78 (Zhao et al., 2018). Table 2 summarizes the outcomes of studies which evaluated this aspect of XIST application in clinical settings.
Discussion
Although XIST has been primarily identified as a transcript whichregulates X inactivation, subsequent studies have show thatthislncRNA has several regulatory roles beyond thisphysiologicalprocess. In addition to interacting with chromatin modifyingmolecules, XIST can be served as a molecularsponge for miRNAs to modulate expression of miRNA targets. miR-362-5p/UBAP1, miR-125b-5p/NLRC5, miR-200c-3p/ANLN, miR-337/JAK2, miR-132/PXN, miR-497/MACC1, miR-185/TGF-β1, miR-497-5p/FOXK1, miR-141-5p/TGF-β2, miR-152/KLF4, and let-7i/BAG-1 are among molecular cascades downstream of XIST which are involved in the carcinogenesis process.
XIST can modulate resistance to chemotherapeutic agents in a number of cancers including breast and lung cancers (Sun et al., 2017; Zhang et al., 2020). Thus, modulation of its expression might beregarded as a strategy for combatting chemoresistance of cancercells. However, tissue-specific effects of XIST in conferring resistance to chemotherapeutic agents should be considered. Most of the above-mentioned studies have indicated an oncogenic role for XIST. However, in prostate cancer, a single study has indicated a tumor suppressor role for this lncRNA (Du et al., 2017). Similar result has been reported for XIST in oral squamous cell carcinoma (Li et al., 2020c). In hepatocellular carcinoma, breast cancer, ovarian cancer, osteosarcoma and renal cell carcinoma, different studies have reported inconsistent results (Supplementary Table 2). Most notably, animal studies in hepatocellular carcinoma, breast cancer, ovarian cancer and osteosarcoma have indicated inconsistent results regarding the role of XIST. Although these discrepancies might be due to possible tissue-specific roles for XIST or differences in cell lines (particularly passage number) and animal models, future studies with larger sample sizes from different ethnic groups are needed to solve these discrepancies.
XIST has both diagnostic and prognostic values in different cancers, albeit the prognostic value of this lncRNA has been more validated. Both tissue and serum levels of XIST can be used to distinguish disease status, yet the latter source is superior regarding the non-invasive route of access. The best diagnostic power values have been reported in CRC, NSCLC, and nasopharyngeal carcinoma, respectively. However, all of these studies lack validation in independent samples. So, future studies should assess this aspect of XIST application in larger cohorts of patients.
In brief, XIST has been shown to affect carcinogenic process possibly in a tissue-specific manner. Therefore, therapeutic strategies targeting this lncRNA should consider this point to design a personalized regimen for treatment of cancer.
Author Contributions
MT and SG-F wrote the draft and revised it. SD, MF, and SM collected the data and designed the tables and figures. All the authors approved submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.690522/full#supplementary-material
References
Brown, C. J., Hendrich, B. D., Rupert, J. L., Lafrenière, R. G., Xing, Y., Lawrence, J., et al. (1992). The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71, 527–542. doi: 10.1016/0092-8674(92)90520-M
Chang, S., Chen, B., Wang, X., Wu, K., and Sun, Y. (2017). Long non-coding RNA XIST regulates PTEN expression by sponging miR-181a and promotes hepatocellular carcinoma progression. BMC Cancer 17:248. doi: 10.1186/s12885-017-3216-6
Chen, D., Chen, T., Guo, Y., Wang, C., Dong, L., and Lu, C. (2020). Platycodin D (PD) regulates LncRNA-XIST/miR-335 axis to slow down bladder cancer progression in vitro and in vivo. Exper. Cell Res. 396:112281. doi: 10.1016/j.yexcr.2020.112281
Chen, D.-L., Chen, L.-Z., Lu, Y.-X., Zhang, D.-S., Zeng, Z.-L., Pan, Z.-Z., et al. (2017). Long noncoding RNA XIST expedites metastasis and modulates epithelial–mesenchymal transition in colorectal cancer. Cell Death Dis. 8:e3011. doi: 10.1038/cddis.2017.421
Chen, D.-L., Ju, H.-Q., Lu, Y.-X., Chen, L.-Z., Zeng, Z.-L., Zhang, D.-S., et al. (2016). Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J. Exper. Clin. Cancer Res. 35:142. doi: 10.1186/s13046-016-0420-1
Chen, X., Xiong, D., Ye, L., Wang, K., Huang, L., Mei, S., et al. (2019). Up-regulated lncRNA XIST contributes to progression of cervical cancer via regulating miR-140-5p and ORC1. Cancer Cell Intern. 19, 1–19. doi: 10.1186/s12935-019-0744-y
Chen, Z., Hu, X., Wu, Y., Cong, L., He, X., Lu, J., et al. (2019). Long non-coding RNA XIST promotes the development of esophageal cancer by sponging miR-494 to regulate CDK6 expression. Biomed. Pharmacother. 109, 2228–2236. doi: 10.1016/j.biopha.2018.11.049
Cheng, Q., Xu, X., Jiang, H., Xu, L., and Li, Q. (2018). Knockdown of long non-coding RNA XIST suppresses nasopharyngeal carcinoma progression by activating miR-491-5p. J. Cell. Biochem. 119, 3936–3944. doi: 10.1002/jcb.26535
Cheng, Y., Chang, Q., Zheng, B., Xu, J., Li, H., and Wang, R. (2019). LncRNA XIST promotes the epithelial to mesenchymal transition of retinoblastoma via sponging miR-101. Eur. J. Pharmacol. 843, 210–216. doi: 10.1016/j.ejphar.2018.11.028
Cheng, Z., Li, Z., Ma, K., Li, X., Tian, N., Duan, J., et al. (2017). Long non-coding RNA XIST promotes glioma tumorigenicity and angiogenesis by acting as a molecular sponge of miR-429. J. Cancer 8:4106. doi: 10.7150/jca.21024
Cheng, Z., Luo, C., and Guo, Z. (2020). LncRNA-XIST/microRNA-126 sponge mediates cell proliferation and glucose metabolism through the IRS1/PI3K/Akt pathway in glioma. J. Cell. Biochem. 121, 2170–2183. doi: 10.1002/jcb.29440
Chu, C., Zhang, Q. C., da Rocha, S. T., Flynn, R. A., Bharadwaj, M., Calabrese, J. M., et al. (2015). Systematic discovery of Xist RNA binding proteins. Cell 161, 404–416. doi: 10.1016/j.cell.2015.03.025
Du, P., Zhao, H., Peng, R., Liu, Q., Yuan, J., Peng, G., et al. (2017). LncRNA-XIST interacts with miR-29c to modulate the chemoresistance of glioma cell to TMZ through DNA mismatch repair pathway. Biosci. Rep. 37:BSR20170696. doi: 10.1042/BSR20170696
Du, Y., Weng, X.-D., Wang, L., Liu, X.-H., Zhu, H.-C., Guo, J., et al. (2017). LncRNA XIST acts as a tumor suppressor in prostate cancer through sponging miR-23a to modulate RKIP expression. Oncotarget 8:94358. doi: 10.18632/oncotarget.21719
Du, Y.-L., Liang, Y., Cao, Y., Liu, L., Li, J., and Shi, G.-Q. (2020). LncRNA XIST promotes migration and invasion of papillary thyroid cancer cell by modulating MiR-101-3p/CLDN1 axis. Biochem. Genet. 59, 437–452. doi: 10.1007/s10528-020-09985-8
Fang, H., Yang, L., Fan, Y., Mo, C., Luo, L., Liang, D., et al. (2020). Upregulation of tissue long noncoding RNA X inactive specific transcript predicts poor postoperative survival in patients with non-small cell lung cancer. Medicine 99, e21789. doi: 10.1097/MD.0000000000021789
Fang, J., Sun, C.-C., and Gong, C. (2016). Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem. Biophys. Res. Commun. 478, 811–817. doi: 10.1016/j.bbrc.2016.08.030
Gao, W., Gao, J., Chen, L., Ren, Y., and Ma, J. (2019). Targeting XIST induced apoptosis of human osteosarcoma cells by activation of NF-kB/PUMA signal. Bioengineered 10, 261–270. doi: 10.1080/21655979.2019.1631104
Gong, M., Wang, X., Mu, L., Wang, Y., Pan, J., Yuan, X., et al. (2020). SRC-1 enhances the stemness of glioblastoma by activating lncRNA XIST/ miR-152/ KLF4 pathway. Cancer Sci. 112, 604–618. doi: 10.1111/cas.14685
Guo, T., Yuan, D., Zhang, W., Zhu, D., Xiao, A., Mao, G., et al. (2021). Upregulation of long noncoding RNA XIST has anticancer effects on ovarian cancer through sponging miR-106a. Hum. Cell 34, 579–587. doi: 10.1007/s13577-020-00469-w
Hai, B., Pan, X., Du, C., Mao, T., Jia, F., Liu, Y., et al. (2020). LncRNA XIST promotes growth of human chordoma cells by regulating miR-124-3p/iASPP pathway. Oncotargets Therapy 13:4755. doi: 10.2147/OTT.S252195
Han, Q., Li, L., Liang, H., Li, Y., Xie, J., and Wang, Z. (2017). Downregulation of lncRNA X inactive specific transcript (XIST) suppresses cell proliferation and enhances radiosensitivity by upregulating mir-29c in nasopharyngeal carcinoma cells. Med. Sci. Monit. 23:4798. doi: 10.12659/MSM.905370
Hu, B., Shi, G., Li, Q., Li, W., and Zhou, H. (2019). Long noncoding RNA XIST participates in bladder cancer by downregulating p53 via binding to TET1. J. Cell. Biochem. 120, 6330–6338. doi: 10.1002/jcb.27920
Hu, C., Liu, S., Han, M., Wang, Y., and Xu, C. (2018). Knockdown of lncRNA XIST inhibits retinoblastomaprogression by modulating the miR-124/STAT3 axis. Biomed. Pharmacother. 107547–554. doi: 10.1016/j.biopha.2018.08.020
Hu, Y., Deng, C., Zhang, H., Zhang, J., Peng, B., and Hu, C. (2017). Long non-coding RNA XIST promotes cell growth and metastasis through regulating miR-139-5p mediated Wnt/β-catenin signaling pathway in bladder cancer. Oncotarget 8:94554. doi: 10.18632/oncotarget.21791
Huang, R., Zhu, L., and Zhang, Y. (2020). XIST lost induces ovarian cancer stem cells to acquire taxol resistance via a KMT2C-dependent way. Cancer Cell Intern. 20, 1–11. doi: 10.1186/s12935-020-01500-8
Huang, Y.-S., Chang, C.-C., Lee, S.-S., Jou, Y.-S., and Shih, H.-M. (2016). Xist reduction in breast cancer upregulates AKT phosphorylation via HDAC3-mediated repression of PHLPP1 expression. Oncotarget 7:43256. doi: 10.18632/oncotarget.9673
Jiang, H., Zhang, H., Hu, X., and Li, W. (2018). Knockdown of long non-coding RNA XIST inhibits cell viability and invasion by regulating miR-137/PXN axis in non-small cell lung cancer. Intern. J. Biol. Macromol. 111, 623–631. doi: 10.1016/j.ijbiomac.2018.01.022
Jiang, Q., Xing, W., Cheng, J., and Yu, Y. (2020). Knockdown of lncRNA XIST suppresses cell tumorigenicity in human non-small cell lung cancer by regulating miR-142-5p/PAX6 Axis. Oncotargets Therapy 13:4919. doi: 10.2147/OTT.S238808
Kobayashi, R., Miyagawa, R., Yamashita, H., Morikawa, T., Okuma, K., Fukayama, M., et al. (2016). Increased expression of long non-coding RNA XIST predicts favorable prognosis of cervical squamous cell carcinoma subsequent to definitive chemoradiation therapy. Oncol. Lett. 12, 3066–3074. doi: 10.3892/ol.2016.5054
Kong, Q., Zhang, S., Liang, C., Zhang, Y., Kong, Q., Chen, S., et al. (2018). LncRNA XIST functions as a molecular sponge of miR-194-5p to regulate MAPK1 expression in hepatocellular carcinoma cell. J. Cell. Biochem. 119, 4458–4468. doi: 10.1002/jcb.26540
Li, C., Wan, L., Liu, Z., Xu, G., Wang, S., Su, Z., et al. (2018). Long non-coding RNA XIST promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 418, 185–195. doi: 10.1016/j.canlet.2018.01.036
Li, G., Wu, Y., Li, Y., and Li, J. (2017). High expression of long non-coding RNA XIST in osteosarcoma is associated with cell proliferation and poor prognosis. Eur. Rev. Med. Pharmacol. Sci. 21, 2829–2834.
Li, H., Cui, J., Xu, B., He, S., Yang, H., and Liu, L. (2019). Long non-coding RNA XIST serves an oncogenic role in osteosarcoma by sponging miR-137. Exper. Therap. Med. 17, 730–738. doi: 10.3892/etm.2018.7032
Li, J., and He, D. (2019). Long noncoding RNA Xist predicts the presence of lymph node metastases in human oesophageal squamous cell carcinoma. Br. J. Biomed. Sci. 76, 147–149. doi: 10.1080/09674845.2019.1594489
Li, P., Wang, L., Li, P., Hu, F., Cao, Y., Tang, D., et al. (2020a). Silencing lncRNA XIST exhibits antiproliferative and proapoptotic effects on gastric cancer cells by up-regulating microRNA-132 and down-regulating PXN. Aging 12:635. doi: 10.18632/aging.103635
Li, P., Wang, L., Li, P., Hu, F., Cao, Y., Tang, D., et al. (2020b). Silencing of long non-coding RNA XIST represses gastric cancer progression through blocking NFκB pathway via inhibiting HNF4A-mediated transcription of EPHA1. Cancer Gene Therapy 28, 307–320. doi: 10.1038/s41417-020-00220-5
Li, Q., Sun, Q., and Zhu, B. (2020c). LncRNA XIST inhibits the progression of oral squamous cell carcinoma via sponging miR-455-3p/BTG2 Axis. Oncotargets Therapy 13:11211. doi: 10.2147/OTT.S267937
Li, X., Hou, L., Yin, L., and Zhao, S. (2020d). LncRNA XIST interacts with miR-454 to inhibit cells proliferation, epithelial mesenchymal transition and induces apoptosis in triple-negative breast cancer. J. Biosci. 45, 1–11. doi: 10.1007/s12038-020-9999-7
Liang, S., Gong, X., Zhang, G., Huang, G., Lu, Y., and Li, Y. (2017). The lncRNA XIST interacts with miR-140/miR-124/iASPP axis to promote pancreatic carcinoma growth. Oncotarget 8:113701. doi: 10.18632/oncotarget.22555
Lin, X.-Q., Huang, Z.-M., Chen, X., Wu, F., and Wu, W. (2018). XIST induced by JPX suppresses hepatocellular carcinoma by sponging miR-155-5p. Yonsei Med. J. 59:816. doi: 10.3349/ymj.2018.59.7.816
Liu, A., Liu, L., and Lu, H. (2019). LncRNA XIST facilitates proliferation and epithelial–mesenchymal transition of colorectal cancer cells through targeting miR-486-5p and promoting neuropilin-2. J. Cell. Physiol. 234, 13747–13761. doi: 10.1002/jcp.28054
Liu, B., Luo, C., Lin, H., Ji, X., Zhang, E., and Li, X. (2020). Long noncoding RNA XIST acts as a ceRNA of miR-362-5p to suppress breast cancer progression. Cancer Biother. Radiopharm. doi: 10.1089/cbr.2019.3481 [Epub ahead of print].
Liu, C., Lu, Z., Liu, H., Zhuang, S., and Guo, P. (2020). LncRNA XIST promotes the progression of laryngeal squamous cell carcinoma via sponging miR-125b-5p to modulate TRIB2. Biosci. Rep. 40:BSR20193172. doi: 10.1042/BSR20193172
Liu, H., Deng, H., Zhao, Y., Li, C., and Liang, Y. (2018). LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J. Exper. Clin. Cancer Res. 37, 1–12. doi: 10.1186/s13046-018-0950-9
Liu, J., Yao, L., Zhang, M., Jiang, J., Yang, M., and Wang, Y. (2019). Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging 11:7830. doi: 10.18632/aging.102291
Liu, P., Pan, Y., Wang, D., and You, D. (2020). Long non-coding RNA XIST promotes cell proliferation of pancreatic cancer through miR-137 and Notch1 pathway. Eur. Rev. Med. Pharmacol. Sci. 24, 12161–12170.
Liu, X., Cui, L., and Hua, D. (2018). Long noncoding RNA XIST regulates miR-137-EZH2 Axis to promote tumor metastasis in colorectal cancer. Oncol. Res. Featur. Preclinic. Clin. Cancer Therap. 27, 99–106. doi: 10.3727/096504018X15195193936573
Liu, X., Xie, S., Zhang, J., and Kang, Y. (2020). Long noncoding RNA XIST contributes to cervical cancer development through targeting miR-889-3p/SIX1 axis. Cancer Biother. Radiopharm. 35, 640–649. doi: 10.1089/cbr.2019.3318
Loda, A., and Heard, E. (2019). Xist RNA in action: past, present, and future. PLoS Genet. 15:e1008333. doi: 10.1371/journal.pgen.1008333
Lu, Q., Yu, T., Ou, X., Cao, D., Xie, T., and Chen, X. (2017). Potential lncRNA diagnostic biomarkers for early gastric cancer. Mol. Med. Rep. 16, 9545–9552. doi: 10.3892/mmr.2017.7770
Lv, G.-Y., Miao, J., and Zhang, X.-L. (2017). Long noncoding RNA XIST promotes osteosarcoma progression by targeting Ras-related protein RAP2B via miR-320b. Oncol. Res. 26, 837–846. doi: 10.3727/096504017X14920318811721
Lyu, X., Ma, Y., Wu, F., Wang, L., and Wang, L. (2019). LncRNA NKILA inhibits retinoblastoma by downregulating lncRNA XIST. Curr. Eye Res. 44, 975–979. doi: 10.1080/02713683.2019.1606253
Ma, L., Zhou, Y., Luo, X., Gao, H., Deng, X., and Jiang, Y. (2017). Long non-coding RNA XIST promotes cell growth and invasion through regulating miR-497/MACC1 axis in gastric cancer. Oncotarget 8:4125. doi: 10.18632/oncotarget.13670
Ma, W., Wang, H., Jing, W., Zhou, F., Chang, L., Hong, Z., et al. (2017). Downregulation of long non-coding RNAs JPX and XIST is associated with the prognosis of hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 41, 163–170. doi: 10.1016/j.clinre.2016.09.002
Marahrens, Y., Panning, B., Dausman, J., Strauss, W., and Jaenisch, R. (1997). Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 11, 156–166. doi: 10.1101/gad.11.2.156
McHugh, C. A., Chen, C.-K., Chow, A., Surka, C. F., Tran, C., McDonel, P., et al. (2015). The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521, 232–236. doi: 10.1038/nature14443
Mo, Y., Lu, Y., Wang, P., Huang, S., He, L., Li, D., et al. (2017). Long non-coding RNA XIST promotes cell growth by regulating miR-139-5p/PDK1/AKT axis in hepatocellular carcinoma. Tumor Biol. 39:1010428317690999. doi: 10.1177/1010428317690999
Pan, B., Lin, X., Zhang, L., Hong, W., and Zhang, Y. (2019). Long noncoding RNA X-inactive specific transcript promotes malignant melanoma progression and oxaliplatin resistance. Melanoma Res. 29, 254–262. doi: 10.1097/CMR.0000000000000560
Penny, G. D., Kay, G. F., Sheardown, S. A., Rastan, S., and Brockdorff, N. (1996). Requirement for Xist in X chromosome inactivation. Nature 379, 131–137. doi: 10.1038/379131a0
Qiu, H., Yang, K., Yu, H., and Liu, M. (2019). Downregulation of long non-coding RNA XIST inhibits cell proliferation, migration, invasion and EMT by regulating miR-212-3p/CBLL1 axis in non-small cell lung cancer cells. Eur. Rev. Med. Pharmacol. Sci. 23, 8391–8402.
Shen, J., Hong, L., Yu, D., Cao, T., Zhou, Z., and He, S. (2019). LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Intern. J. Biochem. Cell Biol. 113, 17–26. doi: 10.1016/j.biocel.2019.05.021
Shen, J., Xiong, J., Shao, X., Cheng, H., Fang, X., Sun, Y., et al. (2020). Knockdown of the long noncoding RNA XIST suppresses glioma progression by upregulating miR-204-5p. J. Cancer 11:4550. doi: 10.7150/jca.45676
Shi, J., Tan, S., Song, L., Song, L., and Wang, Y. (2020). LncRNA XIST knockdown suppresses the malignancy of human nasopharyngeal carcinoma through XIST/miRNA-148a-3p/ADAM17 pathway in vitro and in vivo. Biomed. Pharmacother. 121:109620. doi: 10.1016/j.biopha.2019.109620
Song, H., He, P., Shao, T., Li, Y., Li, J., and Zhang, Y. (2017). Long non-coding RNA XIST functions as an oncogene in human colorectal cancer by targeting miR-132-3p. J. Buon. 22, 696–703.
Song, P., Ye, L.-F., Zhang, C., Peng, T., and Zhou, X.-H. (2016). Long non-coding RNA XIST exerts oncogenic functions in human nasopharyngeal carcinoma by targeting miR-34a-5p. Gene 592, 8–14. doi: 10.1016/j.gene.2016.07.055
Sun, J., and Zhang, Y. (2019). LncRNA XIST enhanced TGF-β2 expression by targeting miR-141-3p to promote pancreatic cancer cells invasion. Biosci. Rep. 39:BSR20190332. doi: 10.1042/BSR20190332
Sun, J., Pan, L.-M., Chen, L.-B., and Wang, Y. (2017). LncRNA XIST promotes human lung adenocarcinoma cells to cisplatin resistance via let-7i/BAG-1 axis. Cell Cycle 16, 2100–2107. doi: 10.1080/15384101.2017.1361071
Sun, K., Jia, Z., Duan, R., Yan, Z., Jin, Z., Yan, L., et al. (2019). Long non-coding RNA XIST regulates miR-106b-5p/P21 axis to suppress tumor progression in renal cell carcinoma. Biochem. Biophys. Res. Commun. 510, 416–420. doi: 10.1016/j.bbrc.2019.01.116
Sun, N., Zhang, G., and Liu, Y. (2018). Long non-coding RNA XIST sponges miR-34a to promotes colon cancer progression via Wnt/β-catenin signaling pathway. Gene 665, 141–148. doi: 10.1016/j.gene.2018.04.014
Sun, W., Zu, Y., Fu, X., and Deng, Y. (2017). Knockdown of lncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. Oncol. Rep. 38, 3347–3354. doi: 10.3892/or.2017.6056
Sun, X., Wei, B., Peng, Z.-H., Fu, Q.-L., Wang, C.-J., Zheng, J.-C., et al. (2019). Knockdown of lncRNA XIST suppresses osteosarcoma progression by inactivating AKT/mTOR signaling pathway by sponging miR-375-3p. Intern. J. Clin. Exper. Pathol. 12:1507.
Sun, Z., Zhang, B., and Cui, T. (2018). Long non-coding RNA XIST exerts oncogenic functions in pancreatic cancer via miR-34a-5p. Oncol. Rep. 39, 1591–1600. doi: 10.3892/or.2018.6245
Tang, Y., He, R., An, J., Deng, P., Huang, L., and Yang, W. (2017). lncRNA XIST interacts with miR-140 to modulate lung cancer growth by targeting iASPP. Oncol. Rep. 38, 941–948. doi: 10.3892/or.2017.5751
Tantai, J., Hu, D., Yang, Y., and Geng, J. (2015). Combined identification of long non-coding RNA XIST and HIF1A-AS1 in serum as an effective screening for non-small cell lung cancer. Intern. J. Clin. Exper. Pathol. 8:7887.
Tian, L.-J., Wu, Y.-P., Wang, D., Zhou, Z.-H., Xue, S.-B., Zhang, D.-Y., et al. (2019). Upregulation of long noncoding RNA (lncRNA) X-Inactive Specific Transcript (XIST) is associated with cisplatin resistance in non-small cell lung cancer (NSCLC) by downregulating MicroRNA-144-3p. Med. Sci. Monitor 25:8095. doi: 10.12659/MSM.916075
Wang, C., Li, L., Li, M., Wang, W., Liu, Y., and Wang, S. (2020a). Silencing long non-coding RNA XIST suppresses drug resistance in acute myeloid leukemia through down-regulation of MYC by elevating microRNA-29a expression. Mol. Med. 26, 1–11. doi: 10.1186/s10020-020-00229-4
Wang, C., Qi, S., Xie, C., Li, C., Wang, P., and Liu, D. (2018). Upregulation of long non-coding RNA XIST has anticancer effects on epithelial ovarian cancer cells through inverse downregulation of hsa-miR-214-3p. J. Gynecol. Oncol. 29:e99. doi: 10.3802/jgo.2018.29.e99
Wang, H., Shen, Q., Zhang, X., Yang, C., Cui, S., Sun, Y., et al. (2017). The long non-coding RNA XIST controls non-small cell lung cancer proliferation and invasion by modulating miR-186-5p. Cell. Physiol. Biochem. 41, 2221–2229. doi: 10.1159/000475637
Wang, J., Cai, H., Dai, Z., and Wang, G. (2019). Down-regulation of lncRNA XIST inhibits cell proliferation via regulating miR-744/RING1 axis in non-small cell lung cancer. Clin. Sci. 133, 1567–1579. doi: 10.1042/CS20190519
Wang, N., He, J.-X., Jia, G.-Z., Wang, K., Zhou, S., Wu, T., et al. (2020b). The lncRNA XIST promotes colorectal cancer cell growth through regulating the miR-497-5p/FOXK1 axis. Cancer Cell Intern. 20, 1–11. doi: 10.1186/s12935-020-01647-4
Wang, W., Shen, H., Cao, G., and Huang, J. (2019). Long non-coding RNA XIST predicts poor prognosis and promotes malignant phenotypes in osteosarcoma. Oncol. Lett. 17, 256–262. doi: 10.3892/ol.2018.9596
Wang, X., Zhang, G., Cheng, Z., Dai, L., Jia, L., Jing, X., et al. (2018). Knockdown of LncRNA-XIST suppresses proliferation and TGF-β1-induced EMT in NSCLC through the notch-1 pathway by regulation of miR-137. Genet. Test. Mol. Biomark. 22, 333–342. doi: 10.1089/gtmb.2018.0026
Wang, Y., Li, H., Chen, J., Kong, F., Mo, Z., Wang, J., et al. (2020c). Overexpression of XIST facilitates cell proliferation, invasion and suppresses cell apoptosis by reducing radio-sensitivity of glioma cells via miR-329-3p/CREB1 axis. Eur. Rev. Med. Pharmacol. Sci. 24, 3190–3203.
Wang, Y., Sun, D., Sheng, Y., Guo, H., Meng, F., and Song, T. (2020d). XIST promotes cell proliferation and invasion by regulating miR-140-5p and SOX4 in retinoblastoma. World J. Surg. Oncol. 18, 1–8. doi: 10.1186/s12957-020-01825-8
Wang, Z., Yuan, J., Li, L., Yang, Y., Xu, X., and Wang, Y. (2017). Long non-coding RNA XIST exerts oncogenic functions in human glioma by targeting miR-137. Am. J. Transl. Res. 9:1845.
Wei, W., Liu, Y., Lu, Y., Yang, B., and Tang, L. (2017). LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J. Cell. Biochem. 118, 3349–3358. doi: 10.1002/jcb.25988
Wen, J. F., Jiang, Y. Q., Li, C., Dai, X. K., Wu, T., and Yin, W. Z. (2020). LncRNA-XIST promotes the oxidative stress-induced migration, invasion, and epithelial-to-mesenchymal transition of osteosarcoma cancer cells through miR-153-SNAI1 axis. Cell Biol. Intern. 44, 1991–2001. doi: 10.1002/cbin.11405
Wu, X., Dinglin, X., Wang, X., Luo, W., Shen, Q., Li, Y., et al. (2017). Long noncoding RNA XIST promotes malignancies of esophageal squamous cell carcinoma via regulation of miR-101/EZH2. Oncotarget 8:76015. doi: 10.18632/oncotarget.18638
Xiao, D., Cui, X., and Wang, X. (2019). Long noncoding RNA XIST increases the aggressiveness of laryngeal squamous cell carcinoma by regulating miR-124-3p/EZH2. Exper. Cell Res. 381, 172–178. doi: 10.1016/j.yexcr.2019.04.034
Xiao, Y., Yurievich, U. A., and Yosypovych, S. V. (2017). Long noncoding RNA XIST is a prognostic factor in colorectal cancer and inhibits 5-fluorouracil-induced cell cytotoxicity through promoting thymidylate synthase expression. Oncotarget 8:83171. doi: 10.18632/oncotarget.20487
Xing, F., Liu, Y., Wu, S.-Y., Wu, K., Sharma, S., Mo, Y.-Y., et al. (2018). Loss of XIST in breast cancer activates MSN-c-Met and reprograms microglia via exosomal miRNA to promote brain metastasis. Cancer Res. 78, 4316–4330. doi: 10.1158/0008-5472.CAN-18-1102
Xiong, Y., Wang, L., Li, Y., Chen, M., He, W., and Qi, L. (2017). The long non-coding RNA XIST interacted with MiR-124 to modulate bladder cancer growth, invasion and migration by targeting androgen receptor (AR). Cell. Physiol. Biochem. 43, 405–418. doi: 10.1159/000480419
Xu, R., Zhu, X., Chen, F., Huang, C., Ai, K., Wu, H., et al. (2018). LncRNA XIST/miR-200c regulates the stemness properties and tumourigenicity of human bladder cancer stem cell-like cells. Cancer Cell Intern. 18, 1–10. doi: 10.1186/s12935-018-0540-0
Xu, T., Jiang, W., Fan, L., Gao, Q., and Li, G. (2017). Upregulation of long noncoding RNA Xist promotes proliferation of osteosarcoma by epigenetic silencing of P21. Oncotarget 8:101406. doi: 10.18632/oncotarget.20738
Xu, X., Zhou, X., Chen, Z., Gao, C., Zhao, L., and Cui, Y. (2020). Silencing of lncRNA XIST inhibits non-small cell lung cancer growth and promotes chemosensitivity to cisplatin. Aging 12:4711. doi: 10.18632/aging.102673
Xu, Y., Wang, J., and Wang, J. (2018). Long noncoding RNA XIST promotes proliferation and invasion by targeting miR-141 in papillary thyroid carcinoma. Oncotargets Therapy 11:5035. doi: 10.2147/OTT.S170439
Xu, Z., Xu, J., Lu, H., Lin, B., Cai, S., Guo, J., et al. (2017). LARP1 is regulated by the XIST/miR-374a axis and functions as an oncogene in non-small cell lung carcinoma. Oncol. Rep. 38, 3659–3667. doi: 10.3892/or.2017.6040
Yang, C., Wu, K., Wang, S., and Wei, G. (2018). Long non-coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR-195-5p. J. Cell. Biochem. 119, 5646–5656. doi: 10.1002/jcb.26743
Yang, H., Zhang, X., Zhao, Y., Sun, G., Zhang, J., Gao, Y., et al. (2020). Downregulation of lncRNA XIST represses tumor growth and boosts radiosensitivity of neuroblastoma via modulation of the miR-375/L1CAM Axis. Neurochem. Res. 45, 2679–2690. doi: 10.1007/s11064-020-03117-9
Yang, L. G., Cao, M. Z., Zhang, J., Li, X. Y., and Sun, Q. L. (2020). LncRNA XIST modulates HIF-1A/AXL signaling pathway by inhibiting miR-93-5p in colorectal cancer. Mol. Genet. Genom. Med. 8:e1112. doi: 10.1002/mgg3.1112
Yao, L., Yang, L., Song, H., Liu, T., and Yan, H. (2020). Silencing of lncRNA XIST suppresses proliferation and autophagy and enhances vincristine sensitivity in retinoblastoma cells by sponging miR-204-5p. Eur. Rev. Med. Pharmacol. Sci. 24, 3526–3537.
Yao, Y., Ma, J., Xue, Y., Wang, P., Li, Z., Liu, J., et al. (2015). Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 359, 75–86. doi: 10.1016/j.canlet.2014.12.051
Yu, H., Xue, Y., Wang, P., Liu, X., Ma, J., Zheng, J., et al. (2017). Knockdown of long non-coding RNA XIST increases blood–tumor barrier permeability and inhibits glioma angiogenesis by targeting miR-137. Oncogenesis 6:e303. doi: 10.1038/oncsis.2017.7
Yu, J., Dong, W., and Liang, J. (2020). Extracellular vesicle-transported long non-coding RNA (LncRNA) X inactive-specific transcript (XIST) in Serum is a potential novel biomarker for colorectal cancer diagnosis. Med. Sci. Monitor 26:e0924448-41. doi: 10.12659/MSM.924448
Zhang, J., Cao, Z., Ding, X., Wei, X., Zhang, X., Hou, J., et al. (2017). The lncRNA XIST regulates the tumorigenicity of renal cell carcinoma cells via the miR-302c/SDC1 axis. Intern. J. Clin. Exper. Pathol. 10:7481.
Zhang, J., Li, W.-Y., Yang, Y., Yan, L.-Z., Zhang, S.-Y., He, J., et al. (2019). LncRNA XIST facilitates cell growth, migration and invasion via modulating H3 histone methylation of DKK1 in neuroblastoma. Cell Cycle 18, 1882–1892. doi: 10.1080/15384101.2019.1632134
Zhang, M., Wang, F., Xiang, Z., Huang, T., and Zhou, W. B. (2020). LncRNA XIST promotes chemoresistance of breast cancer cells to doxorubicin by sponging miR-200c-3p to upregulate ANLN. Clin. Exper. Pharmacol. Physiol. 47, 1464–1472. doi: 10.1111/1440-1681.13307
Zhang, Q., Chen, B., Liu, P., and Yang, J. (2018). XIST promotes gastric cancer (GC) progression through TGF-β1 via targeting miR-185. J. Cell. Biochem. 119, 2787–2796. doi: 10.1002/jcb.26447
Zhang, R., and Xia, T. (2017). Long non-coding RNA XIST regulates PDCD4 expression by interacting with miR-21-5p and inhibits osteosarcoma cell growth and metastasis. Intern. J. Oncol. 51, 1460–1470. doi: 10.3892/ijo.2017.4127
Zhang, R., Wang, Z., Yu, Q., Shen, J., He, W., Zhou, D., et al. (2019). Atractylenolide II reverses the influence of lncRNA XIST/miR-30a-5p/ROR1 axis on chemo-resistance of colorectal cancer cells. J. Cell. Mol. Med. 23, 3151–3165. doi: 10.1111/jcmm.14148
Zhang, X.-T., Pan, S.-X., Wang, A.-H., Kong, Q.-Y., Jiang, K.-T., and Yu, Z.-B. (2019). Long non-coding RNA (lncRNA) X-Inactive Specific Transcript (XIST) plays a critical role in predicting clinical prognosis and progression of colorectal cancer. Med. Sci. Monitor 25:6429. doi: 10.12659/MSM.915329
Zhang, Y., Zhu, Z., Huang, S., Zhao, Q., Huang, C., Tang, Y., et al. (2019). lncRNA XIST regulates proliferation and migration of hepatocellular carcinoma cells by acting as miR-497-5p molecular sponge and targeting PDCD4. Cancer Cell Intern. 19, 1–13. doi: 10.1186/s12935-019-0909-8
Zhang, Y.-L., Li, X.-B., Hou, Y.-X., Fang, N.-Z., You, J.-C., and Zhou, Q.-H. (2017). The lncRNA XIST exhibits oncogenic properties via regulation of miR-449a and Bcl-2 in human non-small cell lung cancer. Acta Pharmacol. Sinica 38, 371–381. doi: 10.1038/aps.2016.133
Zhao, C., Bai, X., and Hu, X. (2020). Knockdown of lncRNA XIST inhibits hypoxia-induced glycolysis, migration and invasion through regulating miR-381-3p/NEK5 axis in nasopharyngeal carcinoma. Eur. Rev. Med. Pharmacol. Sci. 24, 2505–2517.
Zhao, L., Zhao, Y., He, Y., Li, Q., and Mao, Y. (2018). The functional pathway analysis and clinical significance of miR-20a and its related lncRNAs in breast cancer. Cell. Signal. 51, 152–165. doi: 10.1016/j.cellsig.2018.08.004
Zhao, Y., Yu, Z., Ma, R., Zhang, Y., Zhao, L., Yan, Y., et al. (2020). LncRNA-Xist/miR-101-3p/KLF6-C/EBPα axis promotes TAMs polarization to regulate cancer cells proliferation and migration. Mol. Therapy Nucleic Acids 23, 536–551. doi: 10.1016/j.omtn.2020.12.005
Zheng, R., Lin, S., Guan, L., Yuan, H., Liu, K., Liu, C., et al. (2018). Long non-coding RNA XIST inhibited breast cancer cell growth, migration, and invasion via miR-155/CDX1 axis. Biochem. Biophys. Res. Commun. 498, 1002–1008. doi: 10.1016/j.bbrc.2018.03.104
Zheng, W., Li, J., Zhou, X., Cui, L., and Wang, Y. (2020). The lncRNA XIST promotes proliferation, migration and invasion of gastric cancer cells by targeting miR-337. Arab J. Gastroenterol. 21, 199–206. doi: 10.1016/j.ajg.2020.07.010
Zhou, K., Li, S., Du, G., Fan, Y., Wu, P., Sun, H., et al. (2019a). LncRNA XIST depletion prevents cancer progression in invasive pituitary neuroendocrine tumor by inhibiting bFGF via upregulation of microRNA-424-5p. Oncotargets Therapy 12:7095. doi: 10.2147/OTT.S208329
Zhou, K., Yang, J., Li, X., and Chen, W. (2019b). Long non-coding RNA XIST promotes cell proliferation and migration through targeting miR-133a in bladder cancer. Exper. Therap. Med. 18, 3475–3483. doi: 10.3892/etm.2019.7960
Zhou, X., Xu, X., Gao, C., and Cui, Y. (2019c). XIST promote the proliferation and migration of non-small cell lung cancer cells via sponging miR-16 and regulating CDK8 expression. Am. J. Transl. Res. 11:6196.
Zhu, H., Zheng, T., Yu, J., Zhou, L., and Wang, L. (2018). LncRNA XIST accelerates cervical cancer progression via upregulating Fus through competitively binding with miR-200a. Biomed. Pharmacother. 105, 789–797. doi: 10.1016/j.biopha.2018.05.053
Zhu, J., Zhang, R., Yang, D., Li, J., Yan, X., Jin, K., et al. (2018). Knockdown of long non-coding RNA XIST inhibited doxorubicin resistance in colorectal cancer by upregulation of miR-124 and downregulation of SGK1. Cell. Physiol. Biochem. 51, 113–128. doi: 10.1159/000495168
Zhuang, L., Yang, Y., Ma, X., Han, B., Wang, Z., Zhao, Q., et al. (2016). MicroRNA-92b promotes hepatocellular carcinoma progression by targeting Smad7 and is mediated by long non-coding RNA XIST. Cell Death Dis. 7:e2203. doi: 10.1038/cddis.2016.100
Zong, Y., Zhang, Y., Hou, D., Xu, J., Cui, F., Qin, Y., et al. (2020). The lncRNA XIST promotes the progression of breast cancer by sponging miR-125b-5p to modulate NLRC5. Am. J. Transl. Res. 12:3501.
Zou, L., Chen, F.-R., Xia, R.-P., Wang, H.-W., Xie, Z.-R., Xu, Y., et al. (2020). Long noncoding RNA XIST regulates the EGF receptor to promote TGF-β1-induced epithelial–mesenchymal transition in pancreatic cancer. Biochem. Cell Biol. 98, 267–276. doi: 10.1139/bcb-2018-0274
Keywords: lncRNA, X-inactive-specific transcript, expression, biomarker, cancer
Citation: Ghafouri-Fard S, Dashti S, Farsi M, Taheri M and Mousavinejad SA (2021) X-Inactive-Specific Transcript: Review of Its Functions in the Carcinogenesis. Front. Cell Dev. Biol. 9:690522. doi: 10.3389/fcell.2021.690522
Received: 03 April 2021; Accepted: 13 May 2021;
Published: 11 June 2021.
Edited by:
Simone Patergnani, University of Ferrara, ItalyReviewed by:
Lu Feng, Zhengzhou University, ChinaWilliam K. K. Wu, Chinese University of Hong Kong, China
Copyright © 2021 Ghafouri-Fard, Dashti, Farsi, Taheri and Mousavinejad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, bW9oYW1tYWRfODIzQHlhaG9vLmNvbQ==; Seyed Ali Mousavinejad, YWxpbW91c2F2aTY1QHlhaG9vLmNvbQ==
 Soudeh Ghafouri-Fard
Soudeh Ghafouri-Fard Sepideh Dashti1
Sepideh Dashti1 Molood Farsi
Molood Farsi Mohammad Taheri
Mohammad Taheri