
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 26 July 2021
Sec. Stem Cell Research
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.682143
This article is part of the Research TopicMechanisms of Cellular Differentiation, Organ Development, and Novel Model SystemsView all 25 articles
 Diana Pereira
Diana Pereira Inês Sequeira*
Inês Sequeira*Epithelial tissues are the most rapidly dividing tissues in the body, holding a natural ability for renewal and regeneration. This ability is crucial for survival as epithelia are essential to provide the ultimate barrier against the external environment, protecting the underlying tissues. Tissue stem and progenitor cells are responsible for self-renewal and repair during homeostasis and following injury. Upon wounding, epithelial tissues undergo different phases of haemostasis, inflammation, proliferation and remodelling, often resulting in fibrosis and scarring. In this review, we explore the phenotypic differences between the skin, the oesophagus and the oral mucosa. We discuss the plasticity of these epithelial stem cells and contribution of different fibroblast subpopulations for tissue regeneration and wound healing. While these epithelial tissues share global mechanisms of stem cell behaviour for tissue renewal and regeneration, the oral mucosa is known for its outstanding healing potential with minimal scarring. We aim to provide an updated review of recent studies that combined cell therapy with bioengineering exporting the unique scarless properties of the oral mucosa to improve skin and oesophageal wound healing and to reduce fibrotic tissue formation. These advances open new avenues toward the ultimate goal of achieving scarless wound healing.
Epithelial tissues provide the body’s first line of protection from physical, chemical and biological damage. Mammalian epithelia vary in structure throughout the body according to their function and microenvironment. Skin is considered the largest organ of our body; however, it is not the only epithelium exposed to the external environment. The airways, digestive tract, as well as the urinary and reproductive systems, are all exposed to external stress and are lined by an epithelium, sharing some important structural and functional features.
In this review, we focus on three stratified squamous epithelial tissues – the skin, the oesophagus and the oral mucosa – and provide a comparative analysis of the architecture, cell composition and behaviour of these three different tissues during homeostasis and wound healing. We discuss the outstanding regenerative potential of the oral mucosa and how its scarless wound healing properties can be applied to the other tissues.
Adult epithelia harbour resident stem cells (SCs) responsible for homeostasis and tissue repair. The epithelial lining of the skin develops from the ectoderm, the oesophageal epithelium derives from the endoderm, while the oral epithelium derives both from ectoderm and endoderm (Wells and Melton, 1999; Fuchs, 2007; Que et al., 2007; Rothova et al., 2012). Skin, oesophagus and oral mucosa share global cellular architecture (Figure 1) and homeostasis, however several studies have highlighted different markers for their SCs and differentiated cells (Figure 2).
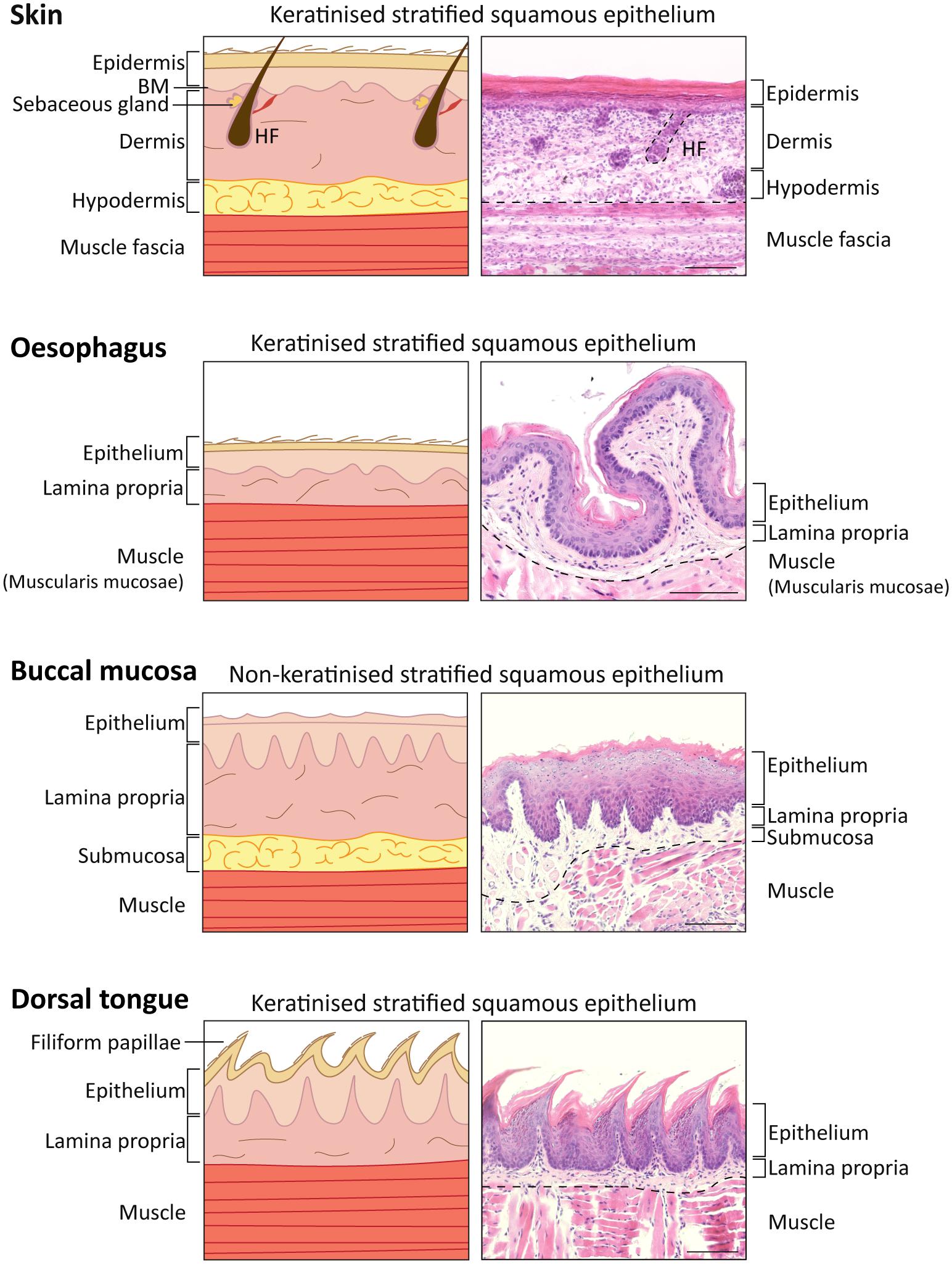
Figure 1. Comparison of skin, oesophagus, oral mucosal tissue structure in Mus musculus. Diagram (Left) and representative histological images (Right) of skin, oesophagus and oral mucosa keratinised (dorsal tongue) and non-keratinised (buccal mucosa) tissues identifying the different layers. 5 μm-sections collected from a 16-week-old C57BL/6 mouse stained with haematoxylin and eosin staining (H&E). Scale bar: 100 μm. HF, hair follicle.
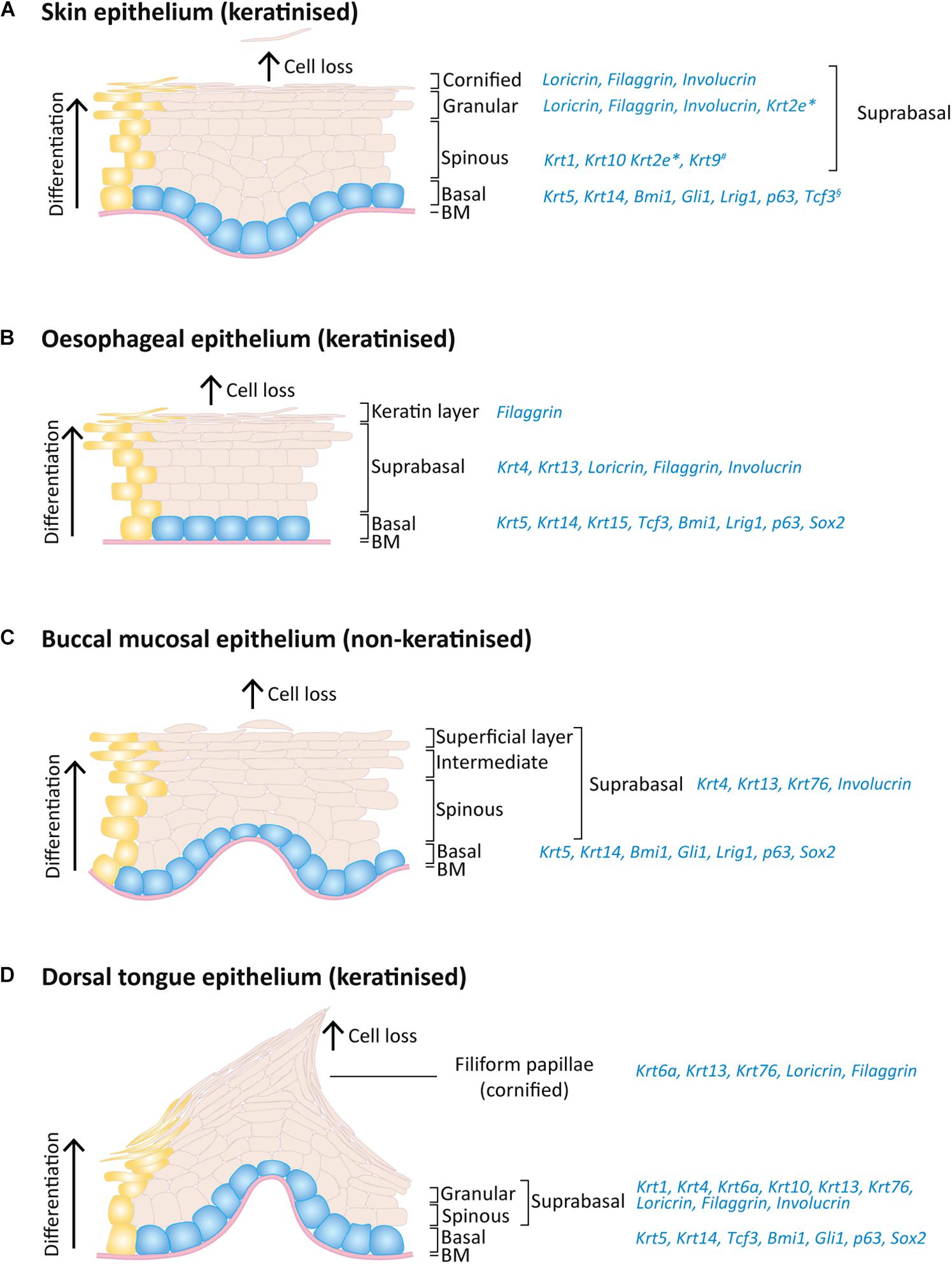
Figure 2. Expression pattern of keratins and others markers on the adult mouse skin, oesophagus and oral epithelia. Schematic of epithelial layers and respective expression markers for (A) skin, (B) oesophageal, (C) buccal mucosa, and (D) dorsal tongue epithelia. During normal epithelial homeostasis, epithelial cells proliferate on the basal layer (blue) and keratinocyte differentiation (yellow) is accompanied by an upward migration through the suprabasal layers, replacing dead cells that shed from the epithelium surface. *Expressed only on ear, sole and tail skin; #expressed only on sole and palm skin; §expressed only on paw skin.
The skin is comprised of three different layers: epidermis, dermis and hypodermis, and harbours additional appendages, such as hair follicles, nails, sweat and sebaceous glands (Watt, 2014). The interfollicular epidermis (IFE) is the outermost layer and is responsible not only for mechanical protection from the hostile environment, but also prevents from dehydration and invasion by microorganisms. The IFE is a multi-layered stratified squamous epithelium with four layers that have different degrees of differentiation: basal, spinous, granular and stratum corneum or cornified layer (Figures 1, 2A), and is composed of keratinocytes, Merkel cells, melanocytes, Langerhans cells and lymphocytes (Rushmer et al., 1966; Matoltsy, 1986; Odland, 1991; Holbrook, 1994; Joost et al., 2020). The IFE is separated from the underlying dermis by a basement membrane (Figure 1), an extracellular matrix (ECM) rich in type IV collagen and laminin (Timpl and Brown, 1996).
The dermis is the connective tissue layer that provides skin elasticity and tensile strength (Frantz et al., 2010) and it is mainly composed of fibroblasts, but also monocytes, macrophages, mast cells, lymphocytes, dermal adipocytes, as well as blood vessel- and sensory nerves-related cells (Lai-Cheong and McGrath, 2013). Using intra-vital imaging on normal mouse ear and paw skin showed that fibroblasts maintain a stable position, and that upon loss of neighbouring cells, the cell membranes extend to fill in the space in a Rac1-dependent process. This process is also conserved upon fibroblast loss in skin ageing (Marsh et al., 2018). However, it is the non-cellular component of the dermis – the ECM – that provides the scaffolding for the skin cellular constituents and that regulates the signalling required for tissue morphogenesis, differentiation and homeostasis (Sorrell and Caplan, 2004; Frantz et al., 2010).
The dermis can be separated into three spatially distinct layers with unique characteristics in development, regeneration and fibrosis: (1) the papillary layer, closest to the epidermis with a high cell density and loose connective tissue and expressing CD90+CD39+FAP+ in human; (2) the reticular layer, with lower cell density but rich in connective tissue and expressing FAP–CD90+ in human (and CD36+ for the lower reticular); and (3) the hypodermis which consists mainly of adipose tissue, loose connective tissue and is highly vascularised and rich in hormones and growth factors and expressing CD90+CD36+ in human (Figure 1; Harper and Grove, 1979; Azzarone and Macieira-Coelho, 1982; Schafer et al., 1985; Sorrell et al., 1996; Freinkel and Woodley, 2001; Sorrell and Caplan, 2004; Watt and Fujiwara, 2011; Driskell et al., 2013; Driskell and Watt, 2015; Sriram et al., 2015; Hiraoka et al., 2016; Philippeos et al., 2018; Korosec et al., 2019). Another fibroblast subpopulation associated with hair follicles lies in the dermal papilla and on the hair follicle dermal sheath, and belongs to the papillary lineage (Reynolds and Jahoda, 1991; Jahoda and Reynolds, 1996; Driskell et al., 2013; Joost et al., 2020). Several studies have highlighted the functional heterogeneity of fibroblasts with different healing potential (Driskell et al., 2013; Rinkevich et al., 2015; Mastrogiannaki et al., 2016; Jiang and Rinkevich, 2018; Jiang et al., 2018; Philippeos et al., 2018; Tabib et al., 2018; Correa-Gallegos et al., 2019; Guerrero-Juarez et al., 2019; Abbasi et al., 2020; Joost et al., 2020; Phan et al., 2021) as well as differences in the expression of collagen subtypes and proteoglycans (Meigel et al., 1977; Zimmermann et al., 1994; Sorrell et al., 1999; Sorrell and Caplan, 2004) and response to different signals originating from neoplastic epidermal SCs (Lichtenberger et al., 2016). Papillary fibroblasts are more proliferative than site-matched reticular fibroblasts, in both mouse and human skin (Harper and Grove, 1979; Azzarone and Macieira-Coelho, 1982; Schafer et al., 1985; Sorrell et al., 1996; Sorrell and Caplan, 2004) and more effectively support the formation of a multi-layered epithelium in two- and three-dimensional (3D) cultures (Higgins et al., 2017; Korosec and Lichtenberger, 2018; Philippeos et al., 2018). Reticular dermis is richer in fibrous connective tissue and, when in culture, reticular dermal fibroblasts contract collagen latices faster than papillary dermal fibroblasts (Schafer et al., 1985; Sorrell et al., 1996). According to lineage tracing and skin reconstitution assays in mice, reticular fibroblasts descending from PDGFRα+Dlk1+ progenitors are responsible for the first wave of dermal wound repair and produce the bulk of the ECM whereas papillary fibroblast lineage supports healthy skin regeneration and hair follicle development, namely through expression of the key transcription factor Lef1 (Driskell et al., 2013; Rognoni et al., 2016, 2018; Phan et al., 2020). More recently, the quiescence-associated factor hypermethylated in cancer 1 positive (Hic1+) progenitors, primarily distributed in the reticular dermis, was shown to robustly contribute to regenerate injured dermis and to populate neogenic hair follicles in adult mice (Abbasi et al., 2020). As for the hypodermis, the deepest layer of the mammalian skin that provides insulation and cushioning, is crucial for wound healing, re-epithelialisation and angiogenesis processes (Freinkel and Woodley, 2001; Rivera-Gonzalez et al., 2014; López et al., 2018; Zomer et al., 2020).
A recent study has identified an additional fibroblast subpopulation below the hypodermis called the fascia that contribute to skin scar formation (see section “The Outstanding Regenerative Potential of Oral Mucosa – Scarless Wound Healing”; Correa-Gallegos et al., 2019; Jiang et al., 2020a; Jiang and Rinkevich, 2021).
In continuity with the skin epithelium, the stratified oral mucosa provides an important barrier to the external challenges. The structure of the oral epithelium comprises a stratified squamous epithelium (keratinised or non-keratinised) and the underlying lamina propria, which is rich in connective tissue, fibroblasts, nerves, minor salivary glands and blood vessels (Jones and Klein, 2013; Hand and Frank, 2014; Figure 1).
The non-keratinised oral epithelia comprise basal, spinous, intermediate and superficial layers, while the keratinised oral epithelia resemble the skin epidermis and include basal, spinous, granular and cornified layers (example of keratinised vs. non-keratinised oral epithelia in Figures 1, 2; comparison between all mouse oral epithelia reviewed in Jones and Klein, 2013). Furthermore, the oral mucosa is subdivided in masticatory (hard palate and gingiva), specialised (dorsal tongue) and lining subtypes (soft palate, buccal mucosa, ventral tongue, intra-oral lips and alveolar mucosa) (Gartner, 1991; Jones and Klein, 2013), reflecting the different structures within the oral cavity. For instance, the cheek buccal mucosa and soft palate are covered by non-keratinised lining mucosa which confers flexibility (Figure 1). The hard palate and gingiva are characterised by a keratinised masticatory epithelium prepared for stresses caused by chewing food. The tongue presents two different phenotypes: the ventral surface displays a non-keratinised lining epithelium, and the dorsal surface is covered by a specialised keratinised epithelium (Figures 1, 2; Jones and Klein, 2013; Hand and Frank, 2014; Groeger and Meyle, 2019). The specialised epithelium of the dorsal tongue houses four types of lingual papillae, three gustatory papillae (fungiform, circumvallate and foliate) with taste buds for sensorial stimuli, and filiform papillae important to grip and process food (Mistretta and Kumari, 2017). Filiform papillae are found in large numbers through the dorsal tongue and present a spinous cone-shaped structure (Figures 1, 2D; Hume and Potten, 1976).
Oral (gingival) fibroblasts are known to resemble foetal skin regenerative potential, namely on their migratory capacity through production of MSF, a migration stimulating factor not present in adult skin (Irwin et al., 1994).
From a development perspective, dorsal skin and oral mucosal fibroblasts have different origins: while the non-cranial dorsal skin dermis has an Engrailed1-lineage-positive somitic origin, the oral mucosa lamina propria and cranial skin dermis originates from Wnt1-lineage-positive neural crest cells (Janebodin et al., 2011; Ishii et al., 2012; Rinkevich et al., 2015). This may be the basis for the intrinsic phenotypic differences between oral and skin fibroblasts in wound healing. For instance, CD90+CD26+ skin fibroblasts were linked to scarring in skin wound healing, however, in gingiva CD26+ fibroblasts are only residually present (Mah et al., 2017; Worthen et al., 2020). Oral mucosal fibroblasts are also primed with higher expression levels of hepatocyte growth factor and its most relevant isoform NK1, therefore more effectively resist to TGF-β1-driven myofibroblast differentiation when compared to dermal fibroblasts (Dally et al., 2017). Another crucial difference relies on the phenotypic activity of the matrix metalloproteinase (MMP) tissue inhibitors (TIMP), namely TIMP-1 and TIMP-2 production, which in the oral mucosa is reduced, therefore allowing for increased MMP-2 activity in the remodelling phase of oral wound healing (Stephens et al., 2001).
Importantly, the epithelial-stromal interaction is key determinant of the phenotypic dynamics of the epithelium in homeostasis and when challenged. The epithelium is affected by the underlying mesenchymal cells, as these produce keratinocyte growth factor and hepatocyte growth factor/scatter factor molecules, important for the regulation of epithelial growth and integrity (Grøn et al., 2002; Costea et al., 2003; McKeown et al., 2003; Shannon et al., 2006; Sa et al., 2019). Furthermore, the epithelial-stromal-immune cell crosstalk in gingival mucosa was recently described as determinant of inducing an immune response to environmental cues and in regulating mucosal immunity (Nowarski et al., 2017; Caetano et al., 2021; Williams et al., 2021).
The submucosal layer of the oral cavity can be compared to the hypodermis in skin, being composed of loose fatty or glandular connective tissue. The presence of a submucosal layer depends on the oral cavity region and is directly linked to the flexibility of the attachment of the oral mucosa to underlying structures. In regions of lining epithelium (such as the cheek buccal mucosa, lips and some hard palate regions) this layer separates the oral mucosa from the bone or muscle below (Figure 1), while regions of masticatory and specialised mucosa (such as gingiva and some hard palate regions) lack this layer (Squier and Kremer, 2001).
Compared to skin and oral mucosa, the oesophagus epithelium is relatively simpler. Given its physiological function of transferring food from the oral cavity to the stomach, this organ is extended from the upper to the lower oesophageal sphincters which are respectively overlapped by the pharyngoesophageal and gastroesophageal junctions. The sphincters open during swallowing and the oesophagus initiates the process of peristalsis to assure the unidirectional transport of the content to the stomach. The mouse oesophagus comprises a keratinised stratified squamous epithelium, differing from non-keratinised human oesophageal epithelium (Figure 1). There are a few other key aspects that differentiate the mouse and human oesophageal epithelia. In humans, the oesophageal epithelium is folded around structures called papillae, which separates the basal layer into either interpapillary or papillary basal layers; it is also characterised by the presence of submucosal glands. This contrasts with the simple epithelium found in mice, devoid of papillae and glands (Messier and Leblond, 1960; Seery, 2002; Doupé et al., 2012; Alcolea, 2017). Additionally, while in mice the oesophageal epithelium comprises a basal layer of proliferating cells (Goetsch, 1910; Messier and Leblond, 1960; Marques-Pereira and Leblond, 1965; Gavaghan, 1999), in humans, cycling cells extend to the 5th-6th suprabasal layers (Barbera et al., 2015).
The oesophagus mucosa is composed of two other layers: the lamina propria, which in this organ is a very thin layer of connective tissue supporting the epithelium, as well as a thin layer of longitudinally organised smooth muscle (Goetsch, 1910; Oezcelik and DeMeester, 2011). To add to this diversity, it is known that the human oesophagus is not only composed of squamous epithelium, but on the most distal area there is a 1-2cm transition to columnar epithelium, which is the same lining epithelium covering the stomach (Gavaghan, 1999). Furthermore, the muscularis mucosae thickness increases from the most proximal to the most distal part of the oesophagus (Goetsch, 1910; Oezcelik and DeMeester, 2011).
Both the oral and the oesophageal epithelia are devoid of appendages. Although they belong to the gastrointestinal tract, they share the same stratified epithelium architecture as the skin rather than the single layer of cells that line the stomach, the small intestine and the large intestine, important for greater absorption capacity (Goetsch, 1910; Gordon, 1994).
Keratins are intermediate filament proteins of epithelial cells providing mechanical integrity and structure to the epithelia and act as a scaffold that enables cells to resist stress and damage, which is essential for normal tissue function (Coulombe et al., 1991; Moll et al., 2008). Changes in keratin synthesis leads to alterations in cell movement or cell differentiation and, consequently, their function (Vassar et al., 1991; Singh and Gupta, 1994). Mutations that impair keratin assembly have been identified in a range of human skin or multifactorial disorders, such as epidermolysis bullosa, typically leading to loss of epithelial integrity, abnormal differentiation and affecting epithelial regeneration (Lane, 1994; Quinlan et al., 1994; Knöbel et al., 2015; Herrmann and Aebi, 2016; Bardhan et al., 2020).
While different epithelia exhibit different patterns of keratin expression (Franke et al., 1981) the keratin patterns are similar between the same anatomic regions of different species. During epithelial homeostasis, epithelial cells migrate from the basal into the suprabasal layer and progressively loose their proliferative potential and begin to synthesise a set of structural proteins (Candi et al., 2005). The switch in the keratin expression from proliferating basal cells to differentiated suprabasal cells indicates a change in the cell cytoskeleton organisation, influencing their functional properties.
In all skin, oesophageal and oral stratified squamous epithelia, the basal dividing cells produce keratin 5 (Krt5) and Krt14 (Squier and Kremer, 2001; Rosekrans et al., 2015; Gonzales and Fuchs, 2017). Krt15 is additionally expressed in the oesophageal basal cells (Rosekrans et al., 2015; Giroux et al., 2017). As cells leave the basal layer and start differentiating, the keratin expression suffers a transition to other keratins and differences arise between types of epithelia. For instance, mouse skin epidermal suprabasal cells switch to expressing Krt10 and Krt1 on interfollicular epidermis and Krt2e on the ears, soles and tail (Fuchs and Green, 1980; Candi et al., 2005; Fischer et al., 2016; Figure 2A). Interestingly, the epidermis of palms and soles, which are the thickest epidermis withstanding the highest degree of mechanical stress the body is exposed to, also express Krt9 in suprabasal layers to provide additional mechanical reinforcement (Knapp et al., 1986; Moll et al., 1987; Candi et al., 2005; Fu et al., 2014).
The heterogeneity of oral epithelia is reflected in its suprabasal keratin expression. The non-keratinised lining mucosa shares the expression of Krt4 and Krt13 (Dale et al., 1990), whereas the palatal and gingival masticatory epithelia are keratinised and share the expression of Krt1, Krt2p (now called Krt76) and Krt10 with skin (Dale et al., 1990; Collin et al., 1992). The gingiva itself is composed of a heterogeneous combination of keratin expression varying between the gingival epithelium mentioned above, the sulcular epithelium (expressing Krt4 and Krt13) and junctional epithelium (expressing Krt8, Krt13, Krt16, Krt18 and Krt19) (Dale et al., 1990; Groeger and Meyle, 2019). Regarding the specialised epithelium of the dorsal tongue, a heterogeneous pattern is also found: Krt4 and Krt13 are expressed in the interpapillary zone and anterior papillae, Krt1 and Krt6a are expressed in the anterior papillae and Krt1 and Krt10 are locally expressed in the posterior papillae (Dale et al., 1990; Howard et al., 2014; Nishiguchi et al., 2016). A recent study has also shown the expression of Krt76 in the palate, buccal mucosa and dorsal tongue suprabasal layers, including filiform papillae (Figures 2C,D; Sequeira et al., 2018).
With more similarities with oral than with skin epithelia, the oesophageal epithelium expresses Krt4 and Krt13 on the suprabasal layers (Figure 2B; Treuting et al., 2012; Rosekrans et al., 2015; Zhang et al., 2017). The mouse oesophagus contains an acellular layer of keratin on the top of the squamous epithelium, similar to the skin, however this keratin layer is absent in the human oesophagus (Treuting et al., 2012).
In addition to keratins, important transcription factors are also expressed in the basal layer of different stratified epithelia: Lef/Tcf-family transcription factor Tcf3 was found in paw skin, dorsal tongue and oesophagus (Howard et al., 2014); Bmi1, Lrig1 and p63 are enriched as well in all these epithelial basal layers (Figure 2; Que et al., 2007; Senoo et al., 2007; Choy et al., 2012; Jones and Klein, 2013; Zhang et al., 2017; Byrd et al., 2019; Jones et al., 2019; Piedrafita et al., 2020); Gli1+ cells are present in oral mucosa and skin epithelial basal layer while Sox2 is in oesophageal and oral epithelia, including tongue taste bud cells (Que et al., 2007; Jones and Klein, 2013; Zhang et al., 2017; Jones et al., 2019; Ohmoto et al., 2020; Figure 2).
The proteins filaggrin, involucrin, and loricrin are also expressed in the suprabasal layers of these epithelia, being key differentiation proteins involved in the thickening of the cornified cell envelope (Figure 2; Mehrel et al., 1990; Squier and Kremer, 2001; Howard et al., 2014; Nishiguchi et al., 2016; Quiroz et al., 2020). Considering the lack a cornified layer in non-keratinised epithelia, keratinocytes retain their nucleus and despite presenting membrane-coating granules, the accumulation and aggregation of cytokeratins with formation of bundles of filaments seen in keratinised epithelia is much less pronounced (Squier, 1977).
Interestingly, keratin expression programmes can change when epithelial cells are exposed to a different environment. Epithelial cells respond to extrinsic signals and change their identity when placed in a different microenvironment, as observed when oesophageal, thymic or cornea epithelial are placed on skin microenvironment (Ferraris et al., 2000; Bonfanti et al., 2010; Bejar et al., 2021). For instance, when oesophageal epithelial cells are grafted into skin, the suprabasal layers loose Krt4 expression as it transforms into a skin identity (Bejar et al., 2021). The mechanisms regulating this identity change remain to be elucidated.
Tissues such as the squamous epithelia of the epidermis, oral cavity and oesophagus hold the natural capacity of self-renewal, with resident adult SCs actively replacing dying cells to accomplish homeostasis. The skin epidermis is by far the most studied epithelium, and this reflects the depth of the knowledge on SC behaviour and differentiation. In adult skin, different epithelia maintain homeostasis by their own pool of SC niches that are found in the basal layer of the IFE, as well as in the sweat glands, touch domes and hair follicle (Fuchs and Green, 1980; Cotsarelis et al., 1990; Blanpain et al., 2004; Morris et al., 2004; Ito et al., 2005; Legué and Nicolas, 2005; Clayton et al., 2007; Jaks et al., 2008; Jensen et al., 2009; Snippert et al., 2010b; Legué et al., 2012; Lu et al., 2012; Sequeira and Nicolas, 2012; Doucet et al., 2013; Page et al., 2013; Schepeler et al., 2014; Sada et al., 2016; Donati et al., 2017; Mesler et al., 2017; Yang et al., 2017).
There has been a great effort to understand the organisation and fate of stem cells in the basal layer that maintain tissue homeostasis. The first proposed model was the SC-transient amplifying cell hierarchy of the epidermal proliferative unit (EPU) (Potten, 1974; Figure 3A). The EPU model defends that each stack of cornified cells is maintained by a single slow-cycling SC basally located within the basal layer. The SC divides asymmetrically to generate another SC and a daughter transient amplifying cell, organised in 3D columns. The transient amplifying cells show high proliferative potential, undergo a fixed number of divisions prior upward migration and differentiation (Potten, 1974; Mackenzie, 1997). This model predicts clone size to rise into a plateau and then remain stable, although this was ruled out by lineage-tracing experiments (Clayton et al., 2007).
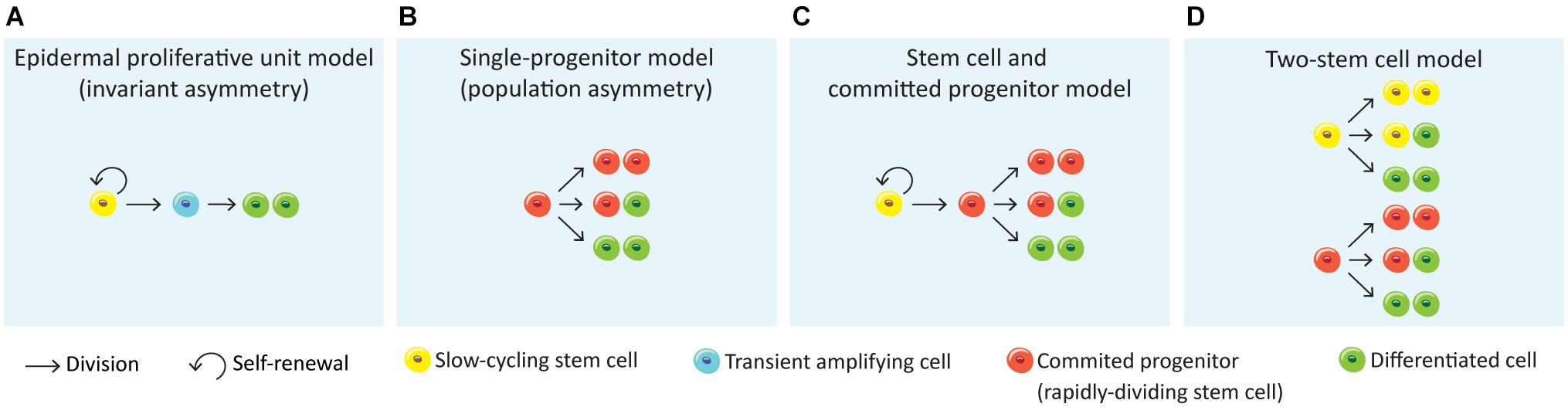
Figure 3. Models for epithelial self-renewal. (A) Epidermal proliferative unit or invariant asymmetry model (Potten, 1974) suggests that epithelial renewal relies on quiescent slow-cycling SCs that generate transient amplifying actively cells, which in turn generate non-dividing, differentiated cells. (B) A second model defends that the epithelial renewal is achieved long-term by a single population of actively cycling and through stochastic fate of committed progenitor cells that directly generate differentiated cells (single-progenitor model) (Clayton et al., 2007; Doupé et al., 2010). (C) The stem cell and committed progenitor model aroused from the observation of a fast-cycling stem cell population (committed progenitor) within the basal layer that is generated by slow-cycling stem cells. These progenitors eventually produce differentiated cells, however due to its short lifespan their contribution to wound healing is limited (Mascré et al., 2012; Sánchez-Danés et al., 2016). (D) The two stem cell model suggests the co-existence of two stem cell populations independent from each other, with different division rates (Joost et al., 2016; Rompolas et al., 2016; Sada et al., 2016; Aragona et al., 2020; Piedrafita et al., 2020).
More recently, another study proposed the “population asymmetry” or “single-progenitor” model (Clayton et al., 2007), where the epidermal maintenance is achieved long-term through stochastic fate of a single committed keratinocyte progenitor in the basal layer from a pool of relatively fast cycling undifferentiated Krt5+Krt14+ epidermal SCs (Figure 3B). This pool is maintained by an autocrine mechanism of Wnt signalling (Lim et al., 2013). According to this model, SCs divide to generate one basal cell that attaches to the basement membrane and one committed progenitor cell which will be prone to leave the basal layer to enter an upward differentiation process. The progenitor population will continuously divide wile committed cells leave the basal layer and differentiate. This model suggests that the progenitor population randomly undergoes either asymmetrical or symmetrical divisions, the latter giving two progenitors or two differentiated cells (Clayton et al., 2007; Doupé et al., 2010; Lim et al., 2013; Rompolas et al., 2016; Figure 3B). Lineage-tracing experiments have shown that following this stochastic choice between symmetric or asymmetric SC division, the mean clone size progressively increases with time (Clayton et al., 2007).
An alternative to this model is the “Stem cell – committed progenitor model” (Figure 3C) that proposes a hierarchy of slow-cycling SCs that will give rise to active SCs (progenitors) which will then follow symmetric of asymmetric divisions to self-renew or to generate the differentiated cells (Mascré et al., 2012; Sánchez-Danés et al., 2016; Figure 3C).
Finally, a fourth model proposes the existence of two SC populations that differ in their proliferative dynamics, their gene-expression profile and their ability to repair the epidermis after injury (Figure 3D; Rompolas et al., 2016; Sada et al., 2016; Piedrafita et al., 2020). Some studies have already demonstrated heterogeneity within the mouse IFE basal cells. Joost and colleagues found two basal subpopulations in mouse dorsal IFE basal I and basal II, differing in the additional expression of Avpi1, Krt16, Thbs1, and the transcription factor Bhlhe40 by IFE basal I (Joost et al., 2016). Furthermore, the IFE progenitors found in different regions of the body were slow-cycling cells able to both self-renew and give rise to intermediate progenitors with a shorter lifespan and greater tendency to differentiation (Mascré et al., 2012; Sada et al., 2016; Sánchez-Danés et al., 2016; Piedrafita et al., 2020). The different observations on IFE basal cell populations in relation to the anatomical position were proposed to be dependent on hair follicle density in those regions. Both the distance to the hair follicles and its cycling status were shown to influence clonal progression reflecting fast- and slow-cycling progenitors (Roy et al., 2016; Gonzales and Fuchs, 2017). This two-stem cell model (Figure 3D) was very recently reinforced by Aragona and colleagues through the study of cellular and molecular mechanisms underlying stretch-mediated expansion in vivo (Aragona et al., 2020). The authors show that stretching induces changes in the renewal activity of a subset of epidermal SCs, which is crucial for expansion, while a second progenitor subpopulation committed to differentiation is preserved. These events were shown to be more consistently governed by the two-stem cell model when compared to the single-progenitor model (Figures 3B,D). Interestingly, a recent single-cell RNA-sequencing analysis of human neonatal foreskin discovered four basal SC populations with differential spatial distribution on the rete ridges of the epidermis, agreeing with a model of multiple SC pools that differ in their proliferation capacity (Wang et al., 2020). Future lineage-tracing, single-cell and microscopic analysis will be needed to further elucidate the basal layer cellular heterogeneity as well as novel markers and regulators.
The hair follicle has separate pools of long-term SCs [CD34+ (Blanpain et al., 2004), Gata6+ (Donati et al., 2017), Lgr5+ (Jaks et al., 2008), Lgr6+ (Snippert et al., 2010a), Lrig1+ (Jensen et al., 2009)] that are responsible for the homeostasis and the cycling regeneration of the hair follicle; and some of these subpopulations can contribute to the IFE for wounding regeneration, although they do not contribute to normal homeostasis maintenance of the IFE (Ito et al., 2005; Legué et al., 2012; Mesa et al., 2015; Liakath-Ali et al., 2018; Dekoninck and Blanpain, 2019; Abbasi et al., 2020).
IFE and oesophageal epithelia appear to share common homeostasis mechanisms (Piedrafita et al., 2020). As for the IFE, oesophageal homeostasis mechanisms of cell behaviour remain controversial. Some studies postulated that a hierarchy of stem and transient amplifying cells maintains homeostasis. Croagh and colleagues reported the existence of three basal cell subpopulations, according to the expression profiles of α6integrin and transferrin receptor (CD71): one α6briCD71dim is a putative oesophageal SC population, the α6briCD71bri represents the transient amplifying cell population and the third population α6dim which is a population of early differentiating cells (Croagh et al., 2007). In agreement to the postulated heterogeneity of basal cells, DeWard and colleagues used a combination of cell-surface markers and labelled proliferating basal epithelial cells in vivo to infer cell-cycle profiles and proliferation kinetics. Differences on the expression of α6 integrin (Itgα6, also known as CD49f) and β4 integrin (Itgβ4, CD104) in Sox2+ basal cells, combined with CD73 and Krt14, Krt13and Krt4 revealed three different basal subpopulations: Itgα6/Itgβ4HighCD73+ is a SC population, the faster dividing Itgα6/Itgβ4HighCD73– is a transient-amplifying population and Itgα6/Itgβ4Low represented the more differentiated basal cell population (DeWard et al., 2014). However, more studies argue that proliferation of a single progenitor population is confined to the basal layer in contact to the basement membrane and as progenitors are committed to differentiation, they withdraw from the cell cycle and migrate from this layer toward the epithelial surface. The fate of a dividing cell is randomly assigned, however the probabilities are balanced, so equal proportions of progenitor and differentiated cells are generated to maintain cellular homeostasis (Piedrafita et al., 2020). How this balance is maintained is not yet clear (Jankowski, 1993; Doupé et al., 2010, 2012; Alcolea et al., 2014; Frede et al., 2016). Recently, Giroux and colleagues defended the existence of a long-lived Krt15+ population with stem/progenitor cell characteristics through in vivo lineage-tracing and pointed against the single-progenitor model (Giroux et al., 2017).
All these paradigms around the proposed models of skin and oesophageal epithelia cell dynamics prompted Piedrafita and colleagues to conduct an in-depth study of nine lineage-tracing datasets in both oesophagus and various skin regions (paw, ear, back, tail scale and tail interscale) (Doupé et al., 2010, 2012; Mascré et al., 2012; Lim et al., 2013; Füllgrabe et al., 2015; Sada et al., 2016; Sánchez-Danés et al., 2016; Giroux et al., 2017; Murai et al., 2018), defending that divergent hypothesis result from distinct datasets analysis through distinct interpreting and suitable procedures, lacking alternative hypotheses tests. The authors used cell-cycle properties from the H2B-GFP dilution data to fit lineage-tracing results by maximum likelihood parameter inference. The results show that all these datasets are in unison with the single-progenitor model (Figure 3B), with the exception of the tail inter-scale region of the skin, defending that skin and oesophageal epithelia homeostasis is equally controlled by this model of basal cell behaviour (Piedrafita et al., 2020).
Besides intrinsic ability for division, the factors that drive basal cells to make the decision to proliferate or differentiate were not yet disclosed. For instance, upon skin wounding different SC populations were shown to contribute to different compartments and change their behaviour in order to increase proliferation over differentiation until complete wound closure, only then reverting to homeostasis (Jaks et al., 2008; Lim et al., 2013; Roshan et al., 2016; Donati et al., 2017). This highlights their plasticity when challenged. More recently the concepts of local fate coordination and epidermal cell competition were brought into discussion as key players of epithelial cell dynamics (Lei and Chuong, 2018; Mesa et al., 2018; Murai et al., 2018; Piedrafita et al., 2020). SC self-renewal was shown to be driven by differentiation of neighbouring cells, supporting the concept of local fate coordination, needed to achieve a precise balance of SC activity (Mesa et al., 2018). Upon differentiation, the space left is occupied by one of the directly neighbouring progenitors which competes with the others for filling the space. Cell competition is the process of elimination of less fit cells that regulates tissue homeostasis and defence against mutant populations which ultimately could evolve to tumours (Murai et al., 2018). Cell competition has been found in different tissues, such as skin, oral mucosa, intestine and oesophagus, and it is often associated with differential gene expression between competing cells (Klein et al., 2010; Snippert et al., 2010b; Klein and Simons, 2011; Alcolea et al., 2014; Lynch et al., 2017; Martincorena et al., 2018; Corominas-Murtra et al., 2020). Clone growth is restricted by the limited size of the proliferating compartment; therefore, since the epithelial progenitors reside in a continuous sheet with no barriers, the mutant clones can expand and collide with other surrounding progenitors. When these encounter similar competitive cells, the fate of the mutant clones reverts to a homeostatic behaviour (Hall et al., 2018; Martincorena et al., 2018; Colom et al., 2020). Both the skin and oesophageal local fate coordination and competition events were shown to be compatible with the single-progenitor model, regulating epithelial cell dynamics governed by stochastic, but, also biased progenitor fates (Piedrafita et al., 2020).
The oral epithelia SCs remain largely uncharacterised and the attribution of the EPU model of homeostasis was often assumed from studies performed in other epithelia, mainly skin (Alonso and Fuchs, 2003; Dabelsteen and Mackenzie, 2006; Thomson, 2020). More recent studies have been exploring different regions of the oral cavity and pointing to which model of epithelial homeostasis suits best with the results. Some studies have defended the EPU model for mouse tongue SC patterns (Luo et al., 2009; Tanaka et al., 2013; Tang et al., 2013). The specialised epithelium of the tongue was demonstrated to house two different SC niches, one in the basal layer where long-term progenitors characterised as Krt14+Krt5+Trp63+Sox2Low maintain the physiology of filiform and fungiform papillae, circumvallate papilla and soft palate, and the other is located outside the taste buds and is a Krt14+Krt5+Trp63+Sox2+ population of bipotential progenitor cells which give rise to both taste pore keratinocytes and receptor cells of the taste buds (Okubo et al., 2009). Jones and colleagues presented a study using lineage-tracing, label retention and single-cell RNA-sequencing that argues against the EPU model, stating that both the dorsal tongue and buccal mucosa epithelia are maintained by the single-progenitor model of homeostasis (Figure 3B). Additionally, the oral epithelial progenitor cells responded to epithelial damage by amending their daughter cell fates (Jones et al., 2019). Label-retaining cells were not found in tongue and oropharynx epithelia, however they observed that the hard palate displays a heterogeneous pattern of proliferation. The palate rugae junctional zone was proposed to hold a reserve SC niche, as these present characteristics of quiescence, self-renewal by symmetric cell divisions, Lrig1 expression, and activation after injury (Byrd et al., 2019). Furthermore, Lrig1 plays a critical role in regulating the oral epithelial SCs of the hard palate: upon decrease of Lrig1 expression, cells exit their quiescence mode, inducing proliferation in response to stress and injury (Byrd et al., 2019). Another study points to a Wnt-responsive long-lived SC population in the hard and soft palates basally located, responsible for homeostasis and response to injury. However, the soft palate showed a more robust and faster re-epithelialisation (Yuan et al., 2019).
Overall, the oral cavity is composed of a variety of types of epithelia with different lineage origins (Rothova et al., 2012) that serve distinct functions. More studies are needed to unveil the mechanisms underlying normal physiology of these tissues.
The main differences between epithelia of the skin, oesophagus and oral cavity, are their function, external microenvironment and differentiation markers. Their SCs are also estimated to divide at different rates: proliferating cells on the oesophagus and the oral mucosa divide on average every 2.4 days, while on the epidermis on average between 3.5 and 6 days, depending on the body region (Jones et al., 2019; Piedrafita et al., 2020). Furthermore, tissue expansion during growth or in adulthood (for example, ventral skin during pregnancy) also regulates SC division rate and global behaviour. This further supports the notion that SC behaviour is regulated by a combination of molecular and mechanical cues that regulate tissue microenvironment and cell behaviour (Vining and Mooney, 2017; Li et al., 2018; Shyer et al., 2018; Aragona et al., 2020; Biggs et al., 2020; Mcginn et al., 2021). Importantly, one of the crucial components of the microenvironment surrounding epithelial progenitor cells are fibroblasts. It has been shown that different fibroblast subpopulations which carry regionally intrinsic signals, determine the behaviour of adult epithelial cells, namely in skin and oral mucosa (Locke et al., 2008; Rinkevich et al., 2015; Yang et al., 2017; Abbasi et al., 2020). For instance, in the gingiva structure, the gingival and the junctional epithelia are phenotypically distinct. This is in part due to heterogeneous resident fibroblasts that provide different support to the epithelial growth and differentiation (Locke et al., 2008). These interactions between epithelial and subepithelial tissues hold a key role in tissue repair (McKeown et al., 2003).
Mammalian epithelia are prepared to respond to assaults to the normal tissue homeostasis, including physical, chemical and biological stress that often result in wounding. Skin wound healing response has been extensively studied giving cues to what may also be happening in the process of wound healing in other tissues.
Wound healing response begins right after injury and comprises a series of coordinated events that make part of a highly dynamic process. Although there are variations among different species, the mammalian wound healing follows a general pattern organised in four main phases: haemostasis, inflammation, proliferation and remodelling. As a very tightly regulated mechanism, minor changes could lead to impaired healing (Gurtner et al., 2008; Shaw and Martin, 2009).
The first phase, haemostasis, is triggered by damaged blood vessels leading to bleeding. At first, blood vessels constrict to stop blood flow, platelets are activated and aggregate in order to seal the ruptured blood vessel wall. Consequently, a fibrin clot is formed to keep the platelets and blood cells in the wound site. The clot holds a role as an initial matrix scaffold rich in growth factors that will recruit cells for further wound healing stages (Etulain, 2018). Platelets were also shown to produce a positive effect on mouse skin wound healing by enhancing the angiogenic potential of mesenchymal SCs (Levoux et al., 2021). Upon activation, platelets release respiration-competent mitochondria that are internalised by recipient mesenchymal SCs, where it stimulates their metabolism to produce increased levels of certain metabolites. Particularly citrate, which works as the main fuel for de novo fatty acid synthesis that in turn increase secretion of pro-angiogenic factors by mesenchymal SCs (Levoux et al., 2021). The inflammatory phase of wounding response starts with the recruitment of immune cells that travel to the injury site in order to remove pathogenic microbes. Following the platelets, neutrophils and monocytes, which differentiate into macrophages, are recruited. These have been shown to also participate in later phases of wound healing, contributing largely to cytokines and growth factors secretion, which activates and recruits other cells important for the wound healing process (Park and Barbul, 2004). The proliferation phase of wound healing comprises the rebuild of the wound site where new tissue is generated. In skin, it starts from 2 to 10 days after injury and can last for up to 3 weeks. This phase is characterised by abundant formation of a highly vascularised granulation tissue through deposition of ECM by fibroblasts (mainly composed of type III collagen), replacing the fibrin matrix (Rognoni et al., 2018). Keratinocytes and endothelial cells are recruited and activated in the wound site, actively promoting re-epithelialisation and neovascularization. Fibroblasts in the wound bed will transition to an activated state, myofibroblasts, which will not only contribute for ECM deposition but also to allow wound closure through contraction (Hinz, 2007; Velnar et al., 2009; Darby et al., 2014; Rognoni et al., 2018; DesJardins-Park et al., 2019). Importantly, in mice the presence of a thin muscular layer, the panniculus carnosus, promotes skin contraction and union of the wound edges; while human skin is devoid of this muscular layer (Zomer and Trentin, 2018).
The last and longest phase of wound healing is the remodelling phase which starts around week 3 and can last for up to more than 1 year. During this phase the type III collagen is actively remodelled to type I collagen by fibroblasts, macrophages and endothelial cells, which secrete MMPs (Martins et al., 2013). This rearrangement of collagen fibres allows the new skin area to become stronger and reduces scar thickness over time; however, the tensile strength of the wound area can only reach 80% compared to unwounded tissue (Gurtner et al., 2008; Xue and Jackson, 2015; Marshall et al., 2018; DesJardins-Park et al., 2019). Recent findings highlighted the role of two fibroblast-expressing transcription factors in wound healing impairment and scarring of the skin: the cyclin-dependent kinase inhibitor p21 and the gap junction alpha-1 protein Connexin43 (Jiang et al., 2020b; Wan et al., 2021).
Wound healing in the oral cavity has a different timeline from the skin. Epithelial cells start migrating and proliferating 24h post wounding and, for wound areas up to 5mm, a complete re-epithelialisation is reached by day 2 to 3 in oral mucosa, while in skin it would take up to 7 days (Szpaderska et al., 2003; Chen et al., 2010; Larjava, 2012; Glim et al., 2013; Iglesias-Bartolome et al., 2018). Inflammation peaks at days 2 to 3 as well and is resolved by day 6 (Bodner et al., 1993; Szpaderska et al., 2003; Iglesias-Bartolome et al., 2018). The further proliferation phase takes place very early from day 2 to 7, being followed by the remodelling of collagen (Bodner et al., 1993; Nikoloudaki et al., 2020).
The cellular and molecular mechanisms underlying oesophageal wound healing have recently attracted attention. Despite comparisons with gastric healing, the similarities to the epidermis have also prompted studies to disclose possible critical players in oesophageal response to wounding (Baatar et al., 2002a, b; Chai et al., 2007; Tarnawski and Ahluwalia, 2012; Jönsson et al., 2016; Tabola et al., 2016; Cai et al., 2018; Komaki et al., 2019; Boudaka et al., 2020).
The regeneration of a skin wound will lead to fine scar formation in superficial injuries. However, there are more complex outcomes for scarring including widespread scars, atrophic scars, scar contractures, hypertrophic scars, and keloid scars (Karppinen et al., 2019). Hypertrophic and keloid scars are pathological outcomes that come with devastating consequences for patients, such as pain and itching. Hypertrophic scars are lifted, erythematous, pruritic lesions confined to the wound boundaries while keloids are benign fibroproliferative dermal scars, growing beyond the wound margins (Bayat et al., 2003; Brown et al., 2008; Karppinen et al., 2019). Given their quasi-neoplastic tendencies, it has been argued that keloids should be classified as a pathological disease rather than a scar (Ud-Din and Bayat, 2020). Besides minor traumatic wounds and acne, other cases can arise from clinical surgeries, chemical and thermal burns or in consequence of allergic reactions. Self-harm scarring and combat wounds also a matter of concern (Mitchell et al., 2019; Johnson et al., 2020). The traumatic wounds in the hostility of war context come with exposure of bone, ligaments and tendons, as well as contamination, and the limited available resources in conflict zones’ hospitals impede the treatment of these wounds (Johnson et al., 2020).
On the one hand, skin scars carry long-term psychosocial effects, including anxiety and avoiding social interaction. This behaviour will interfere with future work life and relationships. In some contexts, scars result from traumatising events and bury a psychological meaning (Brown et al., 2008; Gibson et al., 2018; Mitchell et al., 2019). On the other hand, while visible skin scarring implies a social burden, oral and oesophagus scarring result in difficulties swallowing food and weight loss (Campos et al., 2020).
The fibrous tissues formed upon oesophageal injury are named oesophageal strictures and are mainly a consequence of various benign and malignant disorders. Some other causes include radiation therapy and caustic ingestions. Peptic strictures are caused by gastroesophageal reflux disease when stomach acid damages the oesophagus epithelium over time (Yamasaki et al., 2016). Stricture formation may result from extended endoscopic mucosal resection and submucosal dissection, two techniques used for treatment of superficial gastrointestinal neoplasia, gastric cancer and superficial Barrett’s oesophagus (Yang et al., 2019; Huang et al., 2020). The oesophageal stricture may be persistent or recurrent despite application of several therapies. These can cause complications such as solid and liquid dysphagia, regurgitations or aspiration, abdominal and chest pain as well as obstruction of the oesophagus (Ferguson, 2005).
Compared to the skin and oesophagus, the oral mucosa has an exceptional regenerative ability, being much less prone to scar formation. Despite owning this scar-free healing capacity, there are some particular cases of scar formation. The mucosal trauma applied by oral and perioral piercings may in some rare cases cause complications. Moreover, the oral mucosa may form a keloid or hypertrophic scar as a consequence of medication or of systematic disease (Escudero-Castaño et al., 2008). Additional scar formation may be a consequence of the cleft lip, palate and gum reconstruction, as well as removal of benign and malignant oral tumours (Goodacre and Swan, 2008; Chang et al., 2012; Fierz et al., 2013; Botticelli et al., 2019). Some diseases are also associated with oral mucosal fibrosis, including submucous fibrosis, pemphigus vulgaris and cicatricial pemphigoid, lichen planus, epidermolysis bullosa and proliferative verrucous leukoplakia (Evans, 2017). These can lead to failure in normal growth and restricted oral aperture (Wright, 2010). The molecular mechanisms underpinning these changes in oral wound healing are a subject of ongoing research.
The only adult tissue with the potential to heal with minimal scar formation is the oral mucosa. This capacity is comparable to foetal skin scarless healing, occurring during the first and second trimesters of pregnancy (Rowlatt, 1979; Colwell et al., 2005; Karppinen et al., 2019). Several studies have evidenced that oral mucosa heals faster than skin (Szpaderska et al., 2003; Mak et al., 2009; Chen et al., 2010; Iglesias-Bartolome et al., 2018). Studies exploring the mechanisms of oral repair have allowed to point key differences responsible for the superior outcome when compared to skin (Figure 4). The main differences are:
(1) Environment: the oral mucosa comes in contact with a very different environment compared to skin. The external factors such as saliva and the oral microbiota have been shown to play a role in oral wound healing (Hutson et al., 1979; Bodner et al., 1993; Su et al., 2018). The oral microbiota was shown to affect wound repair through secretion of lipopolysaccharides which maintains oral mesenchymal SCs homeostasis via miRNA-21/Sp1/telomerase reverse transcriptase pathway (Su et al., 2018). Bacteria may accelerate wound healing with beneficial effects in the immune response, granulation tissue and collagen formation (Jones et al., 2004). The positive role of saliva in wound repair has been explained by it being composed of growth factors such as the epidermal growth factor and peptides as histatins with antimicrobial function, responsible for enhanced oral keratinocyte and fibroblast migration. Therefore, saliva modulates oral and eventually skin wound healing mediating the inflammatory response (Figure 4; Zelles et al., 1995; Oudhoff et al., 2008; Boink et al., 2016; Neves et al., 2019).
(2) Inflammation: the inflammatory response in oral wounds was shown to be reduced and to be concluded earlier than in skin wounds (Mak et al., 2009). In fact, there is much evidence linking excessive fibrosis with a strong inflammatory response to injury (Shaw et al., 2010; Wang et al., 2015). The number of immune cells such as neutrophils, macrophages and T cells in oral wound response is reduced when compared to skin, and linked with reduced levels of inflammatory cytokines [as interleukin (IL)-23, IL-24, IL-6, IL-8, tumour necrosis factor alpha (TNF-α)] and pro-fibrotic cytokines [transforming growth factor β1 (TGF-β1)], leading to decreased recruitment of inflammatory cells, and elevated anti-fibrotic cytokine TGF-β3 (Szpaderska et al., 2003; Schrementi et al., 2008; Chen et al., 2010; Glim et al., 2013). The reduced inflammation observed in the oral tissues during wound healing is a reflection of a tissue with the right tools to respond more efficiently. The local oral defences are constantly stimulated by the commensal microbiota and mastication, which trigger cellular crosstalk essential for homeostasis maintenance (Moutsopoulos and Konkel, 2018; Caetano et al., 2021; Williams et al., 2021). Another key immunosuppressive population in the mouth is Foxp3+ regulatory T cells (Park et al., 2018). Inflammatory response in the oral mucosa can be significantly amplified in cases of chemotherapy treatment or as a consequence of systemic conditions involving autoimmune responses, as of lichen planus, leading to increased probability of fibrotic tissue formation (Roopashree et al., 2010; Park et al., 2018; Basile et al., 2019). In fact the local oral tissue immunity can affect and be affected by extra-oral diseases (Moutsopoulos and Konkel, 2018; Kitamoto et al., 2020).
(3) Angiogenesis: reduced angiogenesis could be expected to impair healing, though some studies have proven that inhibition of the angiogenic response in oral wounds is linked to reduced scar formation (Szpaderska et al., 2003; Wilgus et al., 2008). Angiogenesis can directly affect scar formation through oedema, apoptosis and transition of recruited pericytes to an activated fibroblast phenotype (Dulmovits and Herman, 2012; Johnson and Di Pietro, 2013; DiPietro, 2016). Angiogenesis and the inflammatory response act together as inflammatory cells release pro-angiogenic molecules (vascular endothelial growth factor (VEGF) and CXC chemokines) to promote capillary growth, which in turn will support the inflammatory response (Lucas et al., 2010; DiPietro, 2016).
(4) Keratinocyte proliferation: the oral epithelia present faster re-epithelialisation (Szpaderska et al., 2003; Chen et al., 2010; Glim et al., 2014). Oral keratinocytes present higher proliferative potential and are less differentiated than skin keratinocytes therefore contributing with a greater regenerative potential (Glim et al., 2014; Turabelidze et al., 2014; Iglesias-Bartolome et al., 2018).
(5) Fibroblasts: the major players of the proliferative phase of wound healing are fibroblasts that are responsible for collagen deposition and wound contraction, being critical players in the process of scarring. Several studies have investigated how different fibroblast lineages contribute to oral and to skin wound healing (Rinkevich et al., 2015; Gölz et al., 2016; Jiang et al., 2020a). Apart from the Engrailed1-lineage-positive fibroblast subpopulation, the study from Rinkevich and colleagues reports a Wnt1-lineage-positive population in the oral dermis tightly linked to the non-fibrotic healing that characterises the oral mucosa. A reciprocal transplantation of these oral mucosal- and skin-derived fibroblast populations performed in mice revealed that these mimic the response of the tissue of origin. Thus, the grafting of Wnt1-lineage-positive oral fibroblasts in skin resulted in decreased scar tissue formation while skin fibroblasts contributed for a scar-like tissue formation in the oral wound site, proving that the oral fibroblast lineage is determinant for the scarless healing of the oral mucosa (Rinkevich et al., 2015). Comparison between dermal and gingival fibroblasts showed that the latter have increased in vitro proliferation, migration and efficiency in remodelling connective tissue (Chaussain et al., 2002; Boink et al., 2016; Isaac et al., 2018), however contradictory results were reported in regard to the contraction capacity of oral fibroblasts (Lygoe et al., 2007; Mak et al., 2009). Recent studies in mice revealed the contribution of subcutaneous fascia fibroblasts to large deep skin wound healing through deposition of matrix and further contraction into a more exuberant scar matrix architecture (Correa-Gallegos et al., 2019). This is mediated by migration and swarming to the surface involving N-cadherin-mediated cell-cell adhesion. Further experiments with an ex vivo explant technique termed scar-like tissue in a dish (SCAD) using oral mucosa (without fascia) showed that swarming is absent and N-cadherin is minimally expressed, agreeing with its typical scarless healing phenotype (Jiang et al., 2020a).
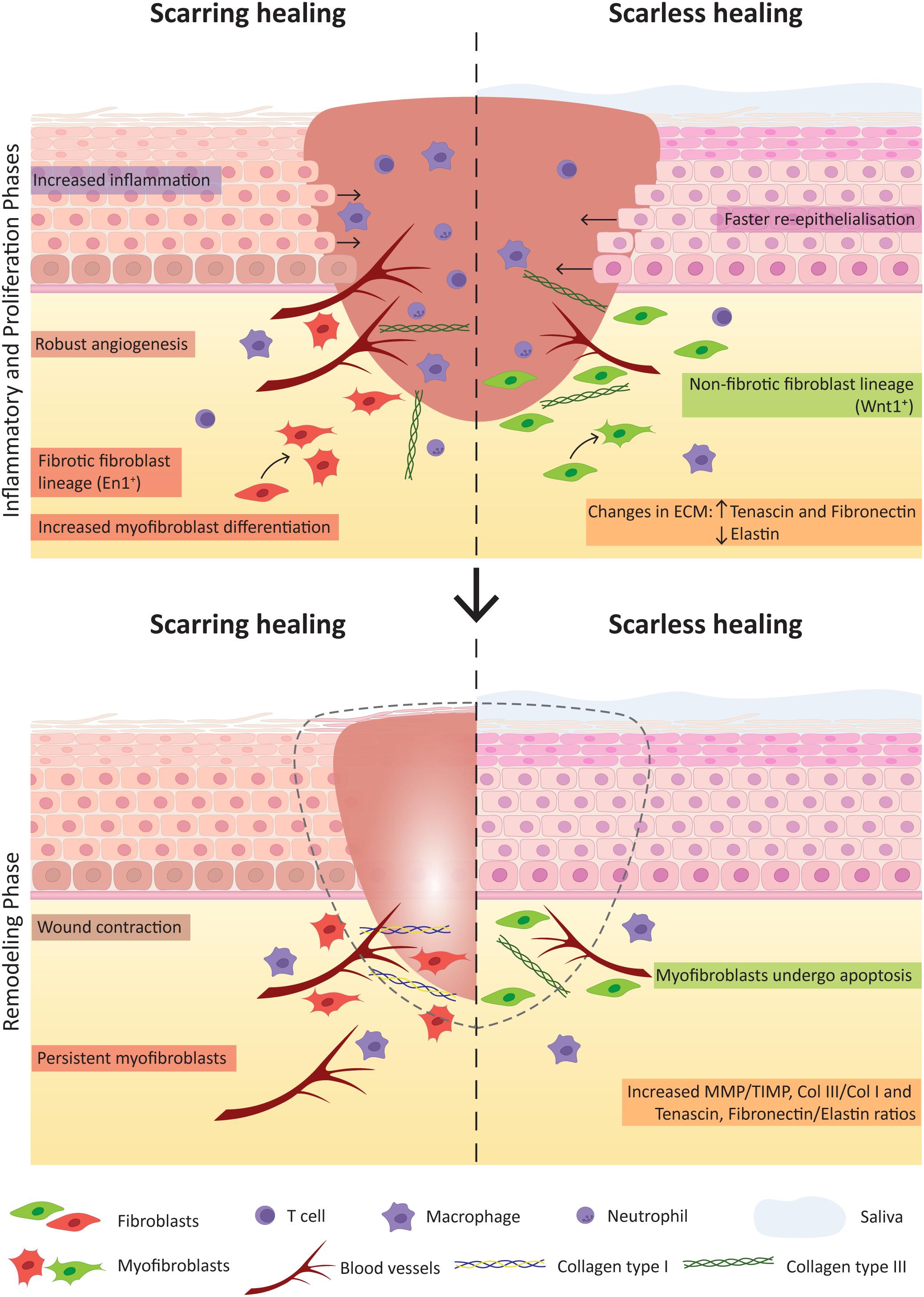
Figure 4. Key factors contributing for scarring and scarless wound healing. The comparison of the inflammatory and proliferation phases (Top) and the remodelling phase (Bottom) of wound healing highlight crucial factors that notably contribute to the distinct healing outcome of skin (with scar formation) and oral mucosa (scarless).
Several studies explored the differential response of oral and dermal fibroblasts to TGFβ1, a cytokine known to mediate fibroblast to myofibroblast differentiation and up-regulating the α-smooth muscle actin (αSMA) in these cells. Oral fibroblasts were shown not only to express higher basal levels of αSMA but also have higher number of αSMA-positive myofibroblasts in oral mucosal wounds (Lygoe et al., 2007; Mak et al., 2009). However, these were shown to resist more to TGFβ1-controlled myofibroblast differentiation, together with decreased levels of TGFβ1 in oral wounds, and supporting its non-scarring phenotype (Meran et al., 2007). This can be regulated by the increased expression of the hepatocyte growth factor (Dally et al., 2017).
(6) ECM: compared to cutaneous wounds, the ECM composition of oral wounds diverges and is a key determinant for the scarless phenotype. Oral wounds showed increased expression of hyaluronic acid, tenascin and fibronectin and decreased expression of elastin (Glim et al., 2013, 2014. MMP mediate ECM remodelling and are regulated by MMP tissue inhibitors. The balance between these two molecules was shown to be important for the final healing outcome. In oral wounds the ratio between MMP and MMP tissue inhibitors is high, namely the levels of MMP 2 and 3 (Stephens et al., 2001; Glim et al., 2013). Also, the collagen III to collagen I ratio is increased in oral wounds (Glim et al., 2013; Figure 4). The pro-fibrotic matricellular protein periostin was recently shown to be involved in ECM synthesis regulation in gingival wound healing, while in skin it appears as a mediator of myofibroblast differentiation through β1 integrin-focal adhesion kinase (FAK) signalling (Nikoloudaki et al., 2020). Another study related the activation of autophagic pathways with an increase in myofibroblast differentiation and noted heterogeneity within the oral cavity, namely between buccal mucosa and gingiva. The gingival tissue showed no autophagic process upon wound repair therefore leading to less myofibroblast differentiation when comparing to buccal mucosal tissue (Vescarelli et al., 2017). It would be interesting to deepen our knowledge on the different wound healing responses associated with different tissues of the oral cavity. Overall, the surrounding environment is capable of eliciting various responses that contribute for the scarless potential of oral mucosa, nevertheless, also inside the cells molecular differences can be pointed between skin and oral mucosa.
(7) Molecular cues: transcriptomic analysis have uncovered the molecular differences between skin and oral mucosal wound healing (Chen et al., 2010; Turabelidze et al., 2014; Iglesias-Bartolome et al., 2018). Healthy oral mucosa is primed with transcriptional networks readily prepared to respond to wounding, suggesting that the oral epithelia is equipped with a specially prepared intrinsic genetic response, particularly for cellular growth and proliferation and inflammatory response (Turabelidze et al., 2014; Iglesias-Bartolome et al., 2018). Importantly, the discovery of key players in transcriptional networks directly working for a scarless healing is of major importance. For instance, the Sox2 and Pitx1 transcription factors were shown to be the master regulators of the oral mucosal wound healing response (Iglesias-Bartolome et al., 2018). However, the intrinsic features playing to scarless healing are not restricted to the protein coding genes; microRNAs were differentially expressed between skin and oral wound healing, highlighting that genetic and epigenetic response of oral mucosa through growth factor production, SC levels and cellular proliferation capacity gives this epithelium its superior final repair (Simões et al., 2019).
To conclude, the ability of the oral mucosa to heal without scarring cannot be attributed to a single feature but to key extrinsic and intrinsic factors present in all stages of the wound healing process, which are crucial to the final improved outcome.
Improving wound healing in skin is an unmet need. Chronic skin wounds have devastating consequences for patients and treating chronic wounds costs the UK National Health Service £5 billion per annum (Guest et al., 2015). Development of more efficient wound treatments is urgently needed to increase the quality of life of patients and to effectively reduce healthcare costs.
Reconstruction of skin or oral mucosal tissues using tissue-engineering methods resembles wound healing processes. It requires active SCs, epithelial proliferation, epithelial and fibroblast cell migration and ECM production, all processes coordinated to regenerate the new 3D tissue with similar properties and functions.
A large number of studies have been exploring SC therapies to improve skin regeneration. A major breakthrough recently published has used autologous transgenic skin epithelial cultures to regenerate an entire, fully functional epidermis from a patient with an epidermolysis bullosa disease caused by a mutation in laminin 332 usually expressed in skin’s basement membrane (Hirsch et al., 2017). Using retrovirus bearing healthy copies of the needed gene, LAMB3, epithelial cells from the patient were corrected, expanded in culture and grafted back to the patient. By combining cell and gene therapy, this clinical study demonstrated a life-saving regeneration of virtually the entire epidermis. This study inspires the use of other tissues for skin regeneration. Oral mucosal cells present advantages over skin cells in therapeutic applications due to their unique scarless properties and are an easy source to harvest reducing time for surgical procedures and accelerating patient’s recovery time (Izumi et al., 2015; Chapple, 2020). However, the direct use of mucosal grafts comes with various disadvantages associated with availability of sufficient amount of donor tissue as well as other graft-associated problems, such as donor site morbidity, recipient site, pain and risk of infection (Llames et al., 2014). To overcome these problems, the clinical use of tissue-engineered oral mucosa (TEOM) is the most adopted method (Figure 5).
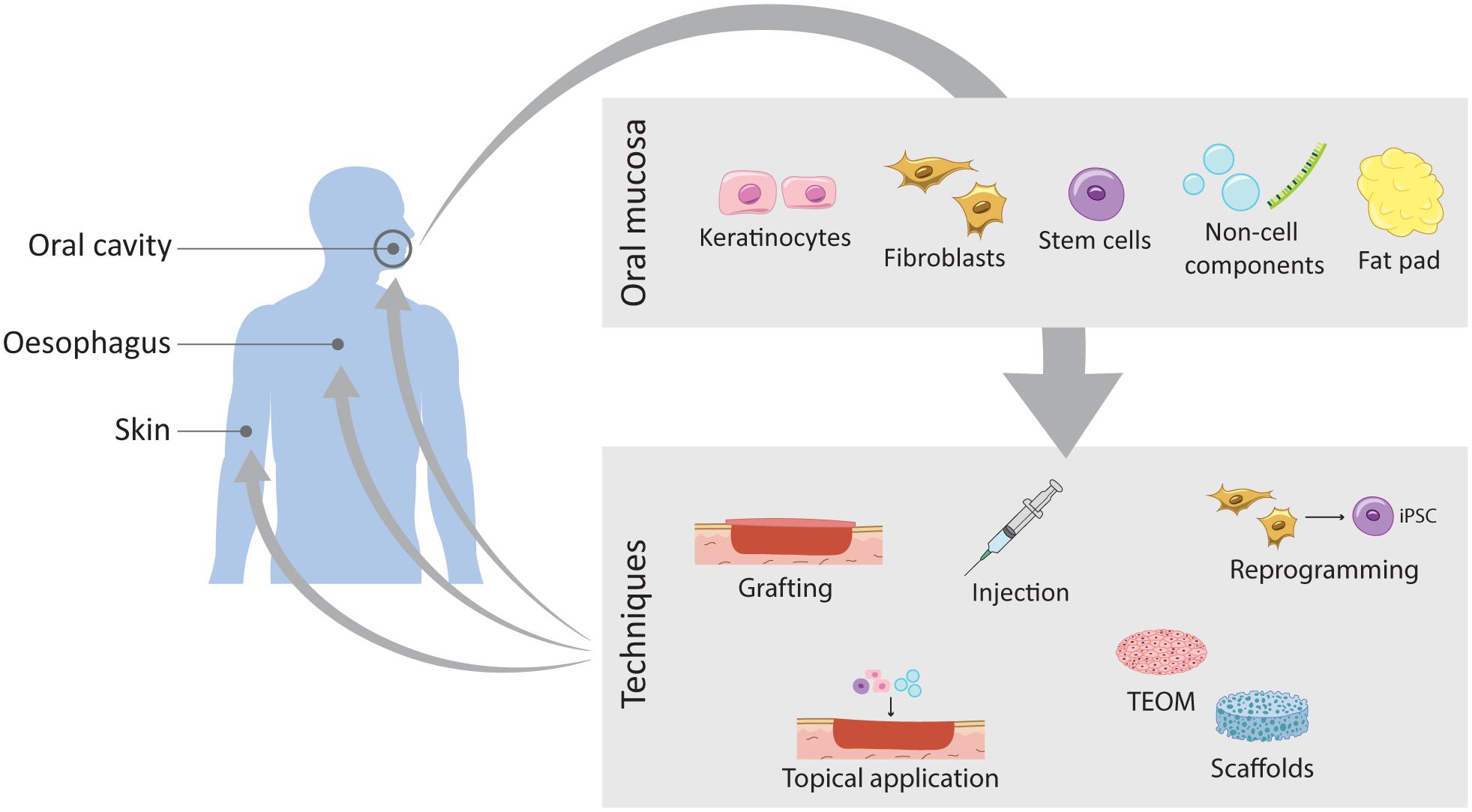
Figure 5. Schematic representation of the current work on wound healing improvement using oral mucosa. The oral mucosa represents a valuable source of different components that translates into different routes of exploration and expansion of its unique healing potential. Through different techniques these components can be applied to different tissues such as the oral mucosa itself, the skin and the oesophagus.
TEOMs are based on a scaffold matrix that provides structural support for the cells to seed, or as a scaffold used to deliver drugs or growth factors directly into the injured tissue, upon transplantation. The key factors are the optimal choice of the scaffold and the cells to seed. Collagen scaffolds are the golden standard, but advances in tissue engineering are proposing other synthetic scaffolds such as biodegradable hydrogels, as well as decellularised dermis (Figure 5). TEOM is a potential technique to reconstruct the oral cavity after tumour excision or after injury, and to repair congenital defects, such as cleft palate. Furthermore, it is a great model for in vitro testing of oral care products efficiency and safety, for evaluating cigarette smoke effects and to analyse cellular and molecular mechanisms of infection in the oral cavity (Chen et al., 2020; Wang et al., 2020; Zhong et al., 2020; Huang et al., 2021).
The TEOM explores the outstanding regenerative potential of the oral mucosal to reconstruct the oral cavity itself or in other tissues of the body. The following subchapters cover pre-clinical and clinical studies on the use of the oral mucosal tissue to improve the healing outcome of other intra- and extra-oral tissues (Tables 1, 2).
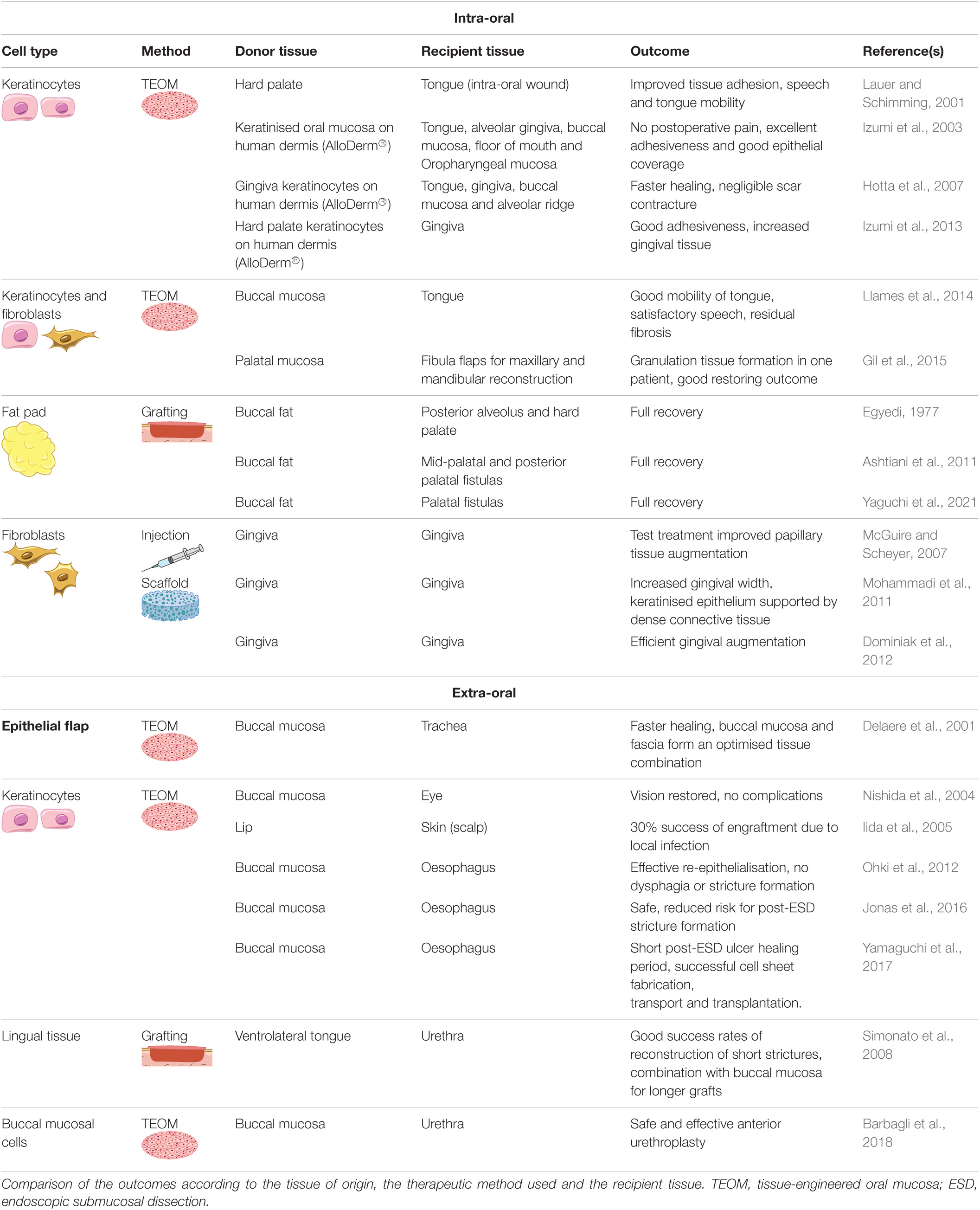
Table 1. Clinical application of cellular therapy using human oral mucosa cells to regenerate oral tissues or other recipient tissues.
The human clinical application of oral mucosal scarless potential and exceptional properties for repair is still scarce, however the number of case reports and pilot studies has been growing (Figure 5 and Table 1). TEOM produced ex vivo from autologous keratinocytes from the hard palate or gingiva were successfully used for reconstruction of intra-oral lining tissues and periodontal plastic surgeries (Lauer and Schimming, 2001; Izumi et al., 2003; Hotta et al., 2007), while full-thickness TEOM combined with fibula flap allowed for the lining reconstruction of maxilla and mandible (Gil et al., 2015). Other cases of congenital anomalies such as hemifacial microsomia, ankyloglossia (tongue-tie) and cleft palate were treated with TEOM yielding satisfactory outcomes (Llames et al., 2014; Hixon et al., 2019). The use of TEOM to repair mucogingival defects demonstrated its capacity to integrate and vascularise (Izumi et al., 2013), however this technique still needs to be improved to avoid postoperative wound shrinkage.
The buccal fat pad flap is reported to be a reliable and effective flap with clinical application in reconstruction of oral defects due to its high vascularity, reducing tissue hypoxia and improving graft survival. This has been used to treat oroantral fistula, congenital defects such as the cleft palate, osteonecrosis of the jawbone and defects induced by removal of tumours or cysts (Egyedi, 1977; Ashtiani et al., 2011; Kim et al., 2017; Yaguchi et al., 2021).
The clinical use of oral-derived SCs is still limited. Oral SCs have been derived from dental pulp, periodontal ligament, exfoliated deciduous teeth, apical papilla, dental follicle, gingiva, oral mucosa, salivary glands and alveolar bone (Kanwal et al., 2017; Bryja et al., 2019; Sanz et al., 2019). The work with oral SCs for hard and soft tissue regeneration within the oral cavity has focused on the use of oral SCs for reconstructing periodontal, bone, dentin and pulp tissues (Seo et al., 2004; Feng et al., 2010; Giuliani et al., 2013; Shiehzadeh et al., 2014; Surendran and Sivamurthy, 2015; Chen et al., 2016; Kanwal et al., 2017). Human gingival and mouse palatal epithelial cells were used to develop teeth in combination with mouse embryonic tooth mesenchyme following transplantation into renal capsules (Nakagawa et al., 2009; Volponi et al., 2013). The combination of human oral epithelial cells and dental pulp SCs using a matrigel as scaffold allowed the 3D construction of an epithelial invagination model, an important feature of early tooth development (Xiao and Tsutsui, 2012). Furthermore, human salivary gland-derived SCs were used to restore saliva production after radiation of salivary glands, opening doors for the treatment of hyposalivation resulting from head and neck cancer radiotherapy (Pringle et al., 2016).
Several clinical studies explored the potential of using oral mucosal fibroblasts for gingival tissue augmentation. Autologous gingival fibroblasts seeded in different scaffolds improved keratinised tissue formation (Mohammadi et al., 2011; Dominiak et al., 2012). Additionally, the injection of autologous fibroblasts harvested from keratinised tissue from the maxillary tuberosity in interdental papillary recession defects improved the papillary tissue augmentation (McGuire and Scheyer, 2007; Table 1).
In addition to clinical studies, in vitro and in vivo research is progressing with more viable alternatives (Table 2). A large size ex vivo fabricated oral mucosal equivalent was successfully achieved using higher cell seeding density of oral keratinocytes and a thinner AlloDerm scaffold, reaching a final 15cm2 size, which can be applied in the reconstruction of significant soft tissue defects (Kato et al., 2015). Cryopreservation of abundant lip mucosa tissues harvested upon cleft lip repair proved to be a useful approach to biobank oral keratinocytes for TEOMs. The 4- to 6-month cryopreservation did not affect the characteristics of the TEOM when compared with the equivalents engineered from fresh lip and palate (Xiong et al., 2010). Furthermore, pre-vascularised oral mucosal cell sheets grafted into deep wounds in the buccal region of rats healed more rapidly and without fibrosis (Lee et al., 2017).
Using cell surface coating through layer-by-layer assembled ECM films succeeded in creating the 3D oral mucosal equivalents composed of epithelium, lamina propria and blood capillaries, recreating the tissue cellular heterogeneity (Nishiyama et al., 2019). Furthermore, the in vitro incorporation of oral mucosa and bone components in a composite scaffold model that mimics the natural structure of alveolar bone with an overlying oral mucosa was achieved and is a possible future application in cleft palate repair (Almela et al., 2016; Hixon et al., 2019).
Oral mucosal fibroblasts were recently reprogrammed into induced pluripotent SCs (Miyoshi et al., 2010) and were shown to be efficient feeders for induced pluripotent SCs expansion (Yu et al., 2016; Figure 5). This represents a promising tool for ex vivo cell expansion.
Recent technologies have been exploring the use of cell-free therapies for oral maxillofacial regeneration, particularly the use of extracellular vesicles (Figure 5; reviewed in Lv et al., 2019; Shi et al., 2020). This technology overcomes the shortcoming of cells and instead explores the cell paracrine effects that can activate endogenous repair pathways (Jiang et al., 2017; Zheng et al., 2018; Ren, 2019). As in TEOMs, it remains important to consider the origin and the differentiation status of the secreting cells to guarantee not only improved outcome but also safe clinical applications.
It is well known that adult skin wounds are frequently accompanied by scar formation that can become fibrotic, while oral mucosal wounds heal in an accelerated fashion, displaying minimal scar formation (see section “Wound Repair Mechanisms in Skin, Oesophagus, and Oral Epithelia”). While surgical reconstruction of the oral cavity with skin grafts has been easily and routinely accomplished for a long time, particularly after tumour resection, the clinical application of oral mucosa grafts into skin has been less explored (Schramm and Myers, 1980; Schramm et al., 1983; de Bree et al., 2008). However, exploring the scarless potential of the oral mucosa offers an exciting strategy to accelerate skin wound healing and to improve the quality of life for patients with chronic wounds. There have been promising studies around this topic, but few clinical studies. Iida and colleagues used TEOMs based on an acellular allogeneic dermal matrix grafted into scalp skin with extensive deep skin burn showing 30% of the graft efficacy (Iida et al., 2005; Table 1).
In vitro studies have demonstrated the potential of oral mucosal fibroblasts, to improve wound healing after biostimulation with low-level laser therapy (Basso et al., 2012). A recent study by Kong and colleagues engineered a biomimetic gel inspired by the characteristics of oral mucosal wound healing. The hydrogel was loaded with epidermal growth factor, basic fibroblast growth factor, lysozyme and hyaluronic acid, as these were observed to be highly expressed in the oral mucosal wound healing when comparing to skin (Kong et al., 2019). The authors were able to simulate the oral mucosal trauma microenvironment through controlled release of these molecules resorting to microspheres and chitosan thermo-sensitive gels. The use of this biomimetic hydrogel in skin wounds of rats resulted in rapid wound healing and reduced scar formation (Kong et al., 2019).
As for pre-clinical studies, topical grafting of human oral keratinocytes onto skin wounds in nude mice improved regeneration of skin wounds, with increased production of keratinocyte growth factor and cytokines IL-6, and IL-1α (Kim et al., 2013).
SCs from human exfoliated deciduous teeth and oral mucosa were shown to improve skin wound healing in mice when injected around the wound or topically applied onto the wound bed highlighting the plasticity of different SC types that could be used to regenerate skin (Nishino et al., 2011; Kuperman et al., 2020). Other studies have explored the potential of oral mucosal engineered cell sheets to apply on skin excisional and burn wounds. All reported results indicated the plasticity of the cell sheets in adapting to the skin wounds, the contribution to accelerated healing and limited scar formation (Roh et al., 2017; Lee et al., 2018, 2019).
The implementation of recent knowledge in the prevention of oesophageal stricture after endoscopic submucosal dissection has attracted increasing attention. Autologous oral mucosal keratinocytes were endoscopically implanted after oesophageal resection in a porcine model, resulting in accelerated re-epithelialisation and wound healing (Sakurai et al., 2007). On a “bench to bedside” approach, tissue-engineered autologous oral epithelial cell sheets and full-thickness substitutes were endoscopically transplanted into an oesophageal ulcer immediately after a large endoscopic submucosal dissection, showing excellent results with no cases of dysphagia or stricture formation (Ohki et al., 2006, 2012, 2015; Arakelian et al., 2018; Ohki and Yamamoto, 2020). The first clinical trial using cell sheet technology for oesophageal reconstruction in Europe used cell sheets from autologous oral epithelial cells, transplanted right after endoscopic submucosal dissection of Barrett’s neoplasms, resulting in decreased risk and extent of strictures (Jonas et al., 2016). On a canine model, the replacement of the full-circumference and full-thickness intrathoracic oesophagus was achieved using autologous oral keratinocytes and fibroblasts seeded on amniotic membrane, sheeted on polyglycolic acid filled with smooth muscle tissue (Nakase et al., 2008), a technique later also achieved without animal-derived materials that could compromise future human clinical trials (Takagi et al., 2010). On a porcine model, an in vitro engineered oesophageal substitute was obtained with an acellular small intestinal submucosa scaffold seeded with autologous skeletal myoblasts, covered with a human amniotic membrane and seeded with autologous oral epithelial cells (Poghosyan et al., 2013).
Other non-cellular-based approaches have also emerged which explore the benefits of oral microRNA and exosomes for skin and oesophageal wound repair (Shi et al., 2017; Chen et al., 2019; Sjöqvist et al., 2019; Mi et al., 2020).
Finally, an interesting and challenging study tested the safety and feasibility of transplanting engineered autologous oral mucosal cell sheets in patients who had undergone extensive endoscopic submucosal dissection for oesophageal squamous cell carcinoma removal. The challenge of this study was the use on non-autologous cell sheets produced 1200km away from the patients that were transported by air for 7h before transplantation (Yamaguchi et al., 2017). Such studies are of major relevance when thinking about future tissue-engineering therapies reaching remote hospitals with no tissue engineering facilities, or hospitals in conflict zones.
The use of oral mucosa to improve wound healing in other tissues has taken its first steps with successful applications in the clinical field, such as in urethral reconstruction and stricture repair through grafting, with recent research focused on improving the TEOM for better outcomes (Simonato et al., 2008; Barbagli and Lazzeri, 2015; Horiguchi, 2017; Barbagli et al., 2018; Simsek et al., 2018; Chapple, 2020; Yudintceva et al., 2020; Zhang et al., 2021). The advantages of using buccal mucosa instead of other donor sites, such as skin, are the fact that the buccal mucosa is a non-keratinised squamous epithelium that lacks hair (Figure 1) and has low associated morbidity and short harvest time.
Tissue-engineered cell sheets of autologous oral epithelial cells alone, or combined with other tissues have been used for ocular diseases treatment, including eyelid and corneal reconstructions (Nishida et al., 2004; Yoshizawa et al., 2004; Oliva et al., 2020; Sasaki et al., 2020). Using an in vivo rabbit model of total limbal SC deficiency, dental pulp SCs were used in a tissue-engineered cell sheet for ocular surface reconstruction (Gomes et al., 2010) highlighting the plasticity of these cells. Furthermore, oral mucosal fibroblasts containing neural crest-origin cells showed plasticity in differentiating into mesenchymal and neural cell lineages, being proposed as an autologous cell source for establishing human corneal epithelial cell sheets suitable for corneal regeneration (Higa et al., 2017). Other applications of the oral mucosa comprise the treatment of tracheal defects (Delaere et al., 2001; Li et al., 2019) and prevention of intrauterine adhesions caused by endometrial damage (Kuramoto et al., 2015).
While in full-thickness skin transplant into the oral cavity (to reconstruct tongue or buccal mucosa), the recipient organ keeps characteristics of the donor tissue (Vural and Suen, 2000; Sebastian et al., 2008; Amin et al., 2011; Aslam-Pervez et al., 2018), there is mounting evidence that grafted epithelial cells can adopt the phenotype of the recipient tissue, stressing the plasticity of these cells and the importance of the underlying connective tissue and fibroblasts to determine epithelial cells phenotype. Recent animal work has successfully grafted oesophageal tissue into skin demonstrating that when exposed to the adult skin dermis, oesophageal epithelial cells transition to a skin identity following a cell fate conversion process (Bejar et al., 2021). This highlights how the stromal cells influence the final epithelial phenotype in a homeostatic tissue. However, this is a topic of much controversy and variability of results over the years, especially when considering clinical and in vivo applications (Billingham and Silvers, 1967; Mackenzie and Hill, 1984; Luca et al., 1990; Katou et al., 2003). Whether the epithelium is transplanted alone or with subepithelial tissue as full thickness flaps, or applied as single-cell suspensions of fully differentiated cells or stem cells alone might contribute for the observed heterogenous responses. Additionally, the majority of these events lack a more in depth study of the molecular mechanisms driving the final outcomes. Nonetheless, we can’t discard the intrinsic identity and programmes that epithelial and fibroblastic cells carry themselves, which differ between oral mucosa and skin (Turabelidze et al., 2014; Rinkevich et al., 2015; Iglesias-Bartolome et al., 2018). This heterogeneity could as well explain the predisposition of a tissue to resemble the origin characteristics or to be more influenced by the recipient. Regardless, the studies presented in this final chapter highlight a very promising venue for using these intrinsic cellular properties into other tissue wounds, mainly through improved in vitro tissue engineering.
Techniques to improve skin wound healing are currently under development and are an unmet need, particularly following large burns and war injuries, where treatment is still primarily performed by split-thickness skin grafting and accompanied by problems associated with limited donor tissue, pain and scarring (Singh et al., 2015; Connolly et al., 2016).
Mounting evidence over the last two decades has demonstrated a remarkable plasticity in adult epithelial cell fate while in in different niches, leaving behind the concept of strict SC fates (Bonfanti et al., 2010; Blanpain and Fuchs, 2014; Chacón-Martínez et al., 2018; Yuan et al., 2019). While epithelial cells and fibroblasts reciprocal grafting have dissected their contribution to wound healing, the plasticity of these cells in the new microenvironment and their cellular behaviour need further investigation. There is an exciting future for collaborative efforts to understand the heterogeneity and plasticity between these tissues and yet their common features and the mechanisms behind the cell fate conversion, in order to improve the use of heterotypic transplants for future therapeutic strategies.
In this review, we elucidate the intrinsic properties that prime the oral mucosa with scarless wound healing response. Lessons should be learned from the oral mucosa to apply on other tissues, exploring these unique properties in future innovative therapies combining cell therapy with bioengineering to prevent pathologies associated with impaired wound healing and scar formation in both skin and oesophagus.
DP and IS: conceptualisation, writing, editing, and figures. Both authors contributed to the article and approved the submitted version.
IS was funded by a Barts Charity Lectureship (MGU045) and Royal Society (RGS\R2\202291). DP was funded by INOV Contacto (AICEP, Portugal) and Fundação para a Ciência e a Tecnologia (2020.08715.BD).
IS is a consultant for L’Oréal Research and Innovation.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge Beate Lichtenberger and Oliver Culley for comments on the manuscript.
Abbasi, S., Sinha, S., Labit, E., Rosin, N. L., Yoon, G., Rahmani, W., et al. (2020). Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell 27, 396–412.e6. doi: 10.1016/j.stem.2020.07.008
Alcolea, M. P. (2017). “Oesophageal stem cells and cancer,” in Stem Cell Microenvironments and Beyond, ed. A. Birbrair (Cham: Springer), 187–206. doi: 10.1007/978-3-319-69194-7_10
Alcolea, M. P., Greulich, P., Wabik, A., Frede, J., Simons, B. D., and Jones, P. H. (2014). Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat. Cell Biol. 16, 612–619. doi: 10.1038/ncb2963
Almela, T., Brook, I. M., and Moharamzadeh, K. (2016). Development of three-dimensional tissue engineered bone-oral mucosal composite models. J. Mater. Sci. Mater. Med. 27:4. doi: 10.1007/s10856-016-5676-7
Alonso, L., and Fuchs, E. (2003). Stem cells of the skin epithelium. Proc. Natl. Acad. Sci. U.S.A. 100(Suppl. 1), 11830–11835. doi: 10.1073/pnas.1734203100
Amin, A. A., Sakkary, M. A., Khalil, A. A., Rifaat, M. A., and Zayed, S. B. (2011). The submental flap for oral cavity reconstruction: extended indications and technical refinements. Head Neck Oncol. 3, 1–7. doi: 10.1186/1758-3284-3-51
Aragona, M., Sifrim, A., Malfait, M., Song, Y., Van Herck, J., Dekoninck, S., et al. (2020). Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 584, 268–273. doi: 10.1038/s41586-020-2555-7
Arakelian, L., Kanai, N., Dua, K., Durand, M., Cattan, P., and Ohki, T. (2018). Esophageal tissue engineering: from bench to bedside. Ann. N. Y. Acad. Sci. 1434, 156–163. doi: 10.1111/nyas.13951
Ashtiani, A. K., Bohluli, B., Motamedi, M. H. K., Fatemi, M. J., and Moharamnejad, N. (2011). Effectiveness of buccal fat in closing residual midpalatal and posterior palatal fistulas in patients previously treated for clefts. Int. J. Oral Maxillofac. Surg. 69, e416–e419. doi: 10.1016/j.joms.2011.02.010
Aslam-Pervez, N., Caldroney, S. J., Isaiah, A., and Lubek, J. E. (2018). A retrospective volume matched analysis of the submental artery island pedicled flap as compared to the forearm free flap: is it a good alternative choice for the reconstruction of defects of the oral cavity and oropharynx? J. Oral Maxillofac. Surg. 76, 656–663. doi: 10.1016/j.joms.2017.08.003
Azzarone, B., and Macieira-Coelho, A. (1982). Heterogeneity of the kinetics of proliferation within the human skin fibroblastic cell populations. J. Cell Sci. 57, 177–187.
Baatar, D., Jones, M. K., Tsugawa, K., Pai, R., Moon, W. S., Koh, G. Y., et al. (2002a). Esophageal ulceration triggers expression of hypoxia-inducible factor-1α and activates vascular endothelial growth factor gene: implications for angiogenesis and ulcer healing. Am. J. Pathol. 161, 1449–1457. doi: 10.1016/S0002-9440(10)64420-3
Baatar, D., Kawanaka, H., Szabo, I. L., Pai, R., Jones, M. K., Kitano, S., et al. (2002b). Esophageal ulceration activates keratinocyte growth factor and its receptor in rats: implications for ulcer healing. Gastroenterology 122, 458–468. doi: 10.1053/gast.2002.31004
Barbagli, G., Akbarov, I., Heidenreich, A., Zugor, V., Olianas, R., Aragona, M., et al. (2018). Anterior urethroplasty using a new tissue engineered oral mucosa graft: surgical techniques and outcomes. J. Urol. 200, 448–456. doi: 10.1016/j.juro.2018.02.3102
Barbagli, G., and Lazzeri, M. (2015). Clinical experience with urethral reconstruction using tissue-engineered oral mucosa: a quiet revolution. Eur. Urol. 68, 917–918. doi: 10.1016/j.eururo.2015.05.043
Barbera, M., Pietro, M. D., Walker, E., Brierley, C., MacRae, S., Simons, B. D., et al. (2015). The human squamous oesophagus has widespread capacity for clonal expansion from cells at diverse stages of differentiation. Gut 64, 11–19. doi: 10.1136/gutjnl-2013-306171
Bardhan, A., Bruckner-Tuderman, L., Chapple, I. L. C., Fine, J. D., Harper, N., Has, C., et al. (2020). Epidermolysis bullosa. Nat. Rev. Dis. Primers 6:1. doi: 10.1038/s41572-020-0210-0
Basile, D., Di Nardo, P., Corvaja, C., Garattini, S. K., Pelizzari, G., Lisanti, C., et al. (2019). Mucosal injury during anti-cancer treatment: from pathobiology to bedside. Cancers 11, 1–22. doi: 10.3390/cancers11060857
Basso, F. G., Pansani, T. N., Turrioni, A. P. S., Bagnato, V. S., Hebling, J., and Costa, C. A. S. (2012). In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. Int. J. Dent. 2012:719452. doi: 10.1155/2012/719452
Bayat, A., McGrouther, D. A., and Ferguson, M. W. J. (2003). Skin scarring: clinical review. BMJ 326, 88–92.
Bejar, M. T., Jimenez-gomez, P., Moutsopoulos, I., Colom, B., and Han, S. (2021). Defining the transcriptional signature of esophageal-to-skin lineage conversion. bioRxiv [Preprint] doi: 10.1101/2021.02.19.431899
Biggs, L. C., Kim, C. S., Miroshnikova, Y. A., and Wickström, S. A. (2020). Mechanical forces in the skin: roles in tissue architecture, stability, and function. J. Invest. Dermatol. 140, 284–290. doi: 10.1016/j.jid.2019.06.137
Billingham, R. E., and Silvers, W. K. (1967). Studies on the conservation of epidermal specificities of skin and certain mucosas in adult mammals. J. Exp. Med. 125, 429–446. doi: 10.1084/jem.125.3.429
Blanpain, C., and Fuchs, E. (2014). Plasticity of epithelial stem cells in tissue regeneration. Science 344:1242281. doi: 10.1126/science.1242281
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L., and Fuchs, E. (2004). Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648. doi: 10.1016/j.cell.2004.08.012
Bodner, L., Dayan, D., Pinto, Y., and Hammel, I. (1993). Characteristics of palatal wound healing in desalivated rats. Arch. Oral Biol. 38, 17–21. doi: 10.1016/0003-9969(93)90149-G
Boink, M. A., van den Broek, L. J., Roffel, S., Nazmi, K., Bolscher, J. G. M., Gefen, A., et al. (2016). Different wound healing properties of dermis, adipose, and gingiva mesenchymal stromal cells. Wound Repair Regen. 24, 100–109. doi: 10.1111/wrr.12380
Bonfanti, P., Claudinot, S., Amici, A. W., Farley, A., Blackburn, C. C., and Barrandon, Y. (2010). Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature 466, 978–982. doi: 10.1038/nature09269
Botticelli, S., Küseler, A., Mølsted, K., Ovsenik, M., Nørholt, S. E., Dalstra, M., et al. (2019). Palatal morphology in unilateral cleft lip and palate patients: association with infant cleft dimensions and timing of hard palate repair. Orthod. Craniofac. Res. 22, 270–280. doi: 10.1111/ocr.12318
Boudaka, A., Saito, C. T., and Tominaga, M. (2020). Deletion of TRPV4 enhances in vitro wound healing of murine esophageal keratinocytes. Sci. Rep. 10:11349. doi: 10.1038/s41598-020-68269-8
Brown, B. C., McKenna, S. P., Siddhi, K., McGrouther, D. A., and Bayat, A. (2008). The hidden cost of skin scars: quality of life after skin scarring. J. Plast. Reconstr. Aesthetic Surg. 61, 1049–1058. doi: 10.1016/j.bjps.2008.03.020
Bryja, A., Stefañska, K., Budna-Tukan, J., Kempisty, B., and Dyszkiewicz-Konwiñska, M. (2019). Oral derived stem cells potential – current status. CPQ Dent. 1:4.
Byrd, K. M., Piehl, N. C., Patel, J. H., Huh, W. J., Sequeira, I., Lough, K. J., et al. (2019). Heterogeneity within stratified epithelial stem cell populations maintains the oral mucosa in response to physiological stress. Cell Stem Cell 25, 814–829.e6. doi: 10.1016/j.stem.2019.11.005
Caetano, A. J., Yianni, V., Volponi, A., Booth, V., D’Agostino, E. M., and Sharpe, P. (2021). Defining human mesenchymal and epithelial heterogeneity in response to oral inflammatory disease. ELife 10:e62810. doi: 10.7554/eLife.62810
Cai, H., Li, Y., Wang, R., and Cui, Y. (2018). The effects of NF-K B signal pathway on the process of anastomotic stricture after the radical resection of esophageal carcinoma. Eur. J. Inflamm. 16, 1–8. doi: 10.1177/2058739218777593
Campos, J., Tanny, S. P. T., Kuyruk, S., Sekaran, P., Hawley, A., Brooks, J. A., et al. (2020). The burden of esophageal dilatations following repair of esophageal atresia. J. Pediatr. Surg. 55, 2329–2334. doi: 10.1016/j.jpedsurg.2020.02.018
Candi, E., Schmidt, R., and Melino, G. (2005). The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340. doi: 10.1038/nrm1619
Chacón-Martínez, C. A., Koester, J., and Wickström, S. A. (2018). Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development 145:15. doi: 10.1242/dev.165399
Chai, J., Norng, M., Tarnawski, A. S., and Chow, J. (2007). A critical role of serum response factor in myofibroblast differentiation during experimental oesophageal ulcer healing in rats. Gut 56, 621–630. doi: 10.1136/gut.2006.106674
Chang, C. S., Wong, A., Rohde, C. H., Ascherman, J. A., and Wu, J. K. (2012). Management of lip hemangiomas: minimizing peri-oral scars. J. Plast. Reconstr. Aesthet. Surg. 65, 163–168. doi: 10.1016/j.bjps.2011.08.033
Chapple, C. (2020). Tissue engineering of the urethra: where are we in 2019? World J. Urol. 38, 2101–2105. doi: 10.1007/s00345-019-02826-3
Chaussain, C., Septier, D., Bonnefoix, M., Lecolle, S., Lebreton-Decoster, C., Coulomb, B., et al. (2002). Human dermal and gingival fibroblasts in a three-dimensional culture: a comparative study on matrix remodeling. Clin. Oral Investig. 6, 39–50. doi: 10.1007/s00784-001-0143-2
Chen, F. M., Gao, L. N., Tian, B. M., Zhang, X. Y., Zhang, Y. J., Dong, G. Y., et al. (2016). Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res. Ther. 7, 1–11. doi: 10.1186/s13287-016-0288-1
Chen, L., Arbieva, Z. H., Guo, S., Marucha, P. T., Mustoe, T. A., and DiPietro, L. A. (2010). Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics 11:47. doi: 10.1186/1471-2164-11-47
Chen, L., Simões, A., Chen, Z., Zhao, Y., Wu, X., Dai, Y., et al. (2019). Overexpression of the oral mucosa-specific microRNA-31 promotes skin wound closure. Int. J. Mol. Sci. 20:3679. doi: 10.3390/ijms20153679
Chen, L., Zhao, J., Peng, J., Li, X., Deng, X., Geng, Z., et al. (2020). Detection of 2019-NCoV in Saliva and Characterization of Oral Symptoms in COVID-19 Patients. Available online at: https://ssrn.com/abstract=3556665 (accessed February 27, 2021).
Choy, B., Bandla, S., Xia, Y., Tan, D., Pennathur, A., Luketich, J. D., et al. (2012). Clinicopathologic characteristics of high expression of Bmi-1 in esophageal adenocarcinoma and squamous cell carcinoma. BMC Gastroenterol. 12:146. doi: 10.1186/1471-230X-12-146
Clayton, E., Doupé, D. P., Klein, A. M., Winton, D. J., Simons, B. D., and Jones, P. H. (2007). A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189. doi: 10.1038/nature05574
Collin, C., Ouhayoun, J. P., Grund, C., and Franke, W. W. (1992). Suprabasal marker proteins distinguishing keratinizing squamous epithelia: cytokeratin 2 polypeptides of oral masticatory epithelium and epidermis are different. Differentiation 51, 137–148. doi: 10.1111/j.1432-0436.1992.tb00690.x
Colom, B., Alcolea, M. P., Piedrafita, G., Hall, M. W. J., Wabik, A., Dentro, S. C., et al. (2020). Spatial competition shapes the dynamic mutational landscape of normal esophageal epithelium. Nat. Genet. 52, 604–614. doi: 10.1038/s41588-020-0624-3
Colwell, A. S., Longaker, M. T., and Lorenz, H. P. (2005). “Mammalian fetal organ regeneration,” in Regenerative Medicine I, Vol. 93, ed. I. V. Yannas (Berlin: Springer), 83–100. doi: 10.1007/b99972
Connolly, M., Ibrahim, Z. R., and Johnson, O. N. (2016). changing paradigms in lower extremity reconstruction in war-related injuries. Mil. Med. Res. 3, 1–6. doi: 10.1186/S40779-016-0080-7
Corominas-Murtra, B., Scheele, C. L. G. J., Kishi, K., Ellenbroek, S. I. J., Simons, B. D., Van Rheenen, J., et al. (2020). Stem cell lineage survival as a noisy competition for niche access. Proc. Natl. Acad. Sci. U.S.A. 117, 16969–16975. doi: 10.1073/pnas.1921205117
Correa-Gallegos, D., Jiang, D., Christ, S., Ramesh, P., Ye, H., Wannemacher, J., et al. (2019). Patch repair of deep wounds by mobilized fascia. Nature 576, 287–292. doi: 10.1038/s41586-019-1794-y
Costea, D. E., Loro, L. L., Dimba, E. A. O., Vintermyr, O. K., and Johannessen, A. C. (2003). Crucial effects of fibroblasts and keratinocyte growth factor on morphogenesis of reconstituted human oral epithelium. J. Invest. Dermatol. 121, 1479–1486. doi: 10.1111/j.1523-1747.2003.12616.x
Cotsarelis, G., Sun, T. T., and Lavker, R. M. (1990). Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337. doi: 10.1016/0092-8674(90)90696-C
Coulombe, P. A., Hutton, M. E., Vassar, R., and Fuchs, E. (1991). A function for keratins and a common thread among different types of epidermolysis bullosa simplex diseases. J. Cell Biol. 115, 1661–1674. doi: 10.1083/jcb.115.6.1661
Croagh, D., Phillips, W. A., Redvers, R., Thomas, R. J. S., and Kaur, P. (2007). Identification of candidate murine esophageal stem cells using a combination of cell kinetic studies and cell surface markers. Stem Cells 25, 313–318. doi: 10.1634/stemcells.2006-0421
Dabelsteen, S., and Mackenzie, I. C. (2006). The stem cell concept in oral mucosa and in cancer. Nor. Tannlegeforen. Tid. 116, 6–11.
Dale, B. A., Salonen, J., and Jones, A. H. (1990). New approaches and concepts in the study of differentiation of oral epithelia. Crit. Rev. Oral Biol. Med. 1, 167–190. doi: 10.1177/10454411900010030201
Dally, J., Khan, J. S., Voisey, A., Charalambous, C., John, H. L., Woods, E. L., et al. (2017). Hepatocyte growth factor mediates enhanced wound healing responses and resistance to transforming growth factor-B 1-driven myo?broblast differentiation in oral mucosal fibroblasts. Int. J. Mol. Sci. 18, 1–15. doi: 10.3390/ijms18091843
Darby, I. A., Laverdet, B., Bonté, F., and Desmoulière, A. (2014). Clinical, cosmetic and investigational dermatology dovepress fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 4, 301–311. doi: 10.2147/CCID.S50046
de Bree, R., Rinaldo, A., Genden, E. M., Suárez, C., Rodrigo, J. P., Fagan, J. J., et al. (2008). Modern reconstruction techniques for oral and pharyngeal defects after tumor resection. Eur. Arch. Otorhinolaryngol. 265, 1–9. doi: 10.1007/s00405-007-0413-y
Dekoninck, S., and Blanpain, C. (2019). Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 21, 18–24. doi: 10.1038/s41556-018-0237-6
Delaere, P. R., Hermans, R., Hardillo, J., and Van Den Hof, B. (2001). Prefabrication of composite tissue for improved tracheal reconstruction. Ann. Otol. Rhinol. Laryngol. 110, 849–860. doi: 10.1177/000348940111000909
DesJardins-Park, H. E., Mascharak, S., Chinta, M. S., Wan, D. C., and Longaker, M. T. (2019). The spectrum of scarring in craniofacial wound repair. Front. Physiol. 10:322. doi: 10.3389/fphys.2019.00322
DeWard, A. D., Cramer, J., and Lagasse, E. (2014). Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 9, 701–711. doi: 10.1016/j.celrep.2014.09.027
DiPietro, L. A. (2016). Angiogenesis and wound repair: when enough is enough. J. Leukoc. Biol. 100, 979–984. doi: 10.1189/jlb.4mr0316-102r
Dominiak, M., Łysiak-Drwal, K., Saczko, J., Kunert-Keil, C., and Gedrange, T. (2012). The clinical efficacy of primary culture of human fibroblasts in gingival augmentation procedures-a preliminary report. Ann. Anat. 194, 502–507. doi: 10.1016/j.aanat.2012.03.011
Donati, G., Rognoni, E., Hiratsuka, T., Liakath-Ali, K., Hoste, E., Kar, G., et al. (2017). Wounding induces dedifferentiation of epidermal gata6 + cells and acquisition of stem cell properties. Nat. Cell Biol. 19, 603–613. doi: 10.1038/ncb3532
Doucet, Y. S., Woo, S. H., Ruiz, M. E., and Owens, D. M. (2013). The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Rep. 3, 1759–1765. doi: 10.1016/j.celrep.2013.04.026
Doupé, D. P., Alcolea, M. P., Roshan, A., Zhang, G., Klein, A. M., Simons, B. D., et al. (2012). A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science 337, 1091–1093. doi: 10.1126/science.1218835
Doupé, D. P., Klein, A. M., Simons, B. D., and Jones, P. H. (2010). The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell 18, 317–323. doi: 10.1016/j.devcel.2009.12.016
Driskell, R. R., and Watt, F. M. (2015). Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 25, 92–99. doi: 10.1016/j.tcb.2014.10.001
Driskell, R. R., Lichtenberger, B. M., Hoste, E., Kretzschmar, K., Simons, B. D., Charalambous, M., et al. (2013). Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281. doi: 10.1038/nature12783
Dulmovits, B. M., and Herman, I. M. (2012). Microvascular remodeling and wound healing: a role for pericytes. Int. J. Biochem. Cell Biol. 44, 1800–1812. doi: 10.1016/j.biocel.2012.06.031
Egyedi, P. (1977). Utilization of the buccal fat pad for closure of oro-antral and/or oro-nasal communications. J. Maxillofac. Surg. 5, 241–244. doi: 10.1016/S0301-0503(77)80117-3
Escudero-Castaño, N., Perea-García, M. A., Campo-Trapero, J., Sánchez, C., and Bascones-Martínez, A. (2008). Oral and perioral piercing complications. Open Dent. J. 2, 133–136. doi: 10.2174/1874210600802010133
Etulain, J. (2018). Platelets in wound healing and regenerative medicine. Platelets 29, 556–568. doi: 10.1080/09537104.2018.1430357
Evans, E. W. (2017). Treating scars on the oral mucosa. Facial Plast. Surg. Clin. North. Am. 25, 89–97. doi: 10.1016/j.fsc.2016.08.008
Feng, F., Akiyama, K., Liu, Y., Yamaza, T., Wang, T. M., Chen, J. H., et al. (2010). Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 16, 20–28. doi: 10.1111/j.1601-0825.2009.01593.x
Ferguson, D. D. (2005). Evaluation and management of benign esophageal strictures. Dis. Esophagus 18, 359–364. doi: 10.1111/j.1442-2050.2005.00516.x
Ferraris, C., Chevalier, G., Favier, B., Jahoda, C. A. B., and Dhouailly, D. (2000). Adult corneal epithelium basal cells possess the capacity to activate epidermal, pilosebaceous and sweat gland genetic programs in response to embryonic dermal stimuli. Development 127, 5487–5495.
Fierz, J., Hallermann, W., and Mericske-Stern, R. (2013). Patients with oral tumors. Part 1: prosthetic rehabilitation following tumor resection. Schweiz. Monatsschr. Zahnmed. 123, 91–105. doi: 10.7892/boris.40796
Fischer, H., Langbein, L., Reichelt, J., Buchberger, M., Tschachler, E., and Eckhart, L. (2016). Keratins K2 and K10 are essential for the epidermal integrity of plantar skin. J. Dermatol. Sci. 81, 10–16. doi: 10.1016/j.jdermsci.2015.10.008
Franke, W. W., Schiller, D. L., Moll, R., Winter, S., Schmid, E., Engelbrecht, I., et al. (1981). Diversity of cytokeratins. differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J. Mol. Biol. 153, 933–959. doi: 10.1016/0022-2836(81)90460-5
Frantz, C., Stewart, K., and Weaver, V. (2010). The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200. doi: 10.1242/jcs.023820
Frede, J., Greulich, P., Nagy, T., Simons, B. D., and Jones, P. H. (2016). A single dividing cell population with imbalanced fate drives oesophageal tumour growth. Nat. Cell Biol. 18, 967–978. doi: 10.1038/ncb3400
Freinkel, R. K., and Woodley, D. T. (eds) (2001). The Biology of the Skin. Boca Raton, FL: CRC Press.
Fu, D. J., Thomson, C., Lunny, D. P., Dopping-Hepenstal, P. J., McGrath, J. A., Smith, F. J. D., et al. (2014). Keratin 9 is required for the structural integrity and terminal differentiation of the palmoplantar epidermis. J. Invest. Dermatol. 134, 754–763. doi: 10.1038/jid.2013.356
Fuchs, E. (2007). Scratching the surface of skin development. Nature 445, 834–842. doi: 10.1038/nature05659
Fuchs, E., and Green, H. (1980). Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19, 1033–1042. doi: 10.1016/0092-8674(80)90094-X
Füllgrabe, A., Joost, S., Are, A., Jacob, T., Sivan, U., Haegebarth, A., et al. (2015). Dynamics of Lgr6+ progenitor cells in the hair follicle, sebaceous gland, and interfollicular epidermis. Stem Cell Rep. 5, 843–855. doi: 10.1016/j.stemcr.2015.09.013
Gavaghan, M. (1999). Anatomy and physiology of the esophagus. AORN J. 69, 370–386. doi: 10.1016/s0001-2092(07)68909-1
Gibson, J. A. G., Ackling, E., Bisson, J. I, Dobbs, T. D., and Whitaker, I. S. (2018). The association of affective disorders and facial scarring: systematic review and meta-analysis. J. Affect. Disord. 239, 1–10. doi: 10.1016/j.jad.2018.06.013
Gil, S. R., Pagés, C. M., Díez, E. G., Llames, S., Fuertes, A. F., and Vilagran, J. L. (2015). Tissue-engineered oral mucosa grafts for intraoral lining reconstruction of the maxilla and mandible with a fibula flap. J. Oral Maxillofac. Surg. 73, 195.e1–195.e16. doi: 10.1016/j.joms.2014.09.001
Giroux, V., Lento, A. A., Islam, M., Pitarresi, J. R., Kharbanda, A., Hamilton, K. E., et al. (2017). Long-lived keratin 15+ esophageal progenitor cells contribute to homeostasis and regeneration. J. Clin. Invest. 127, 2378–2391. doi: 10.1172/JCI88941
Giuliani, A., Manescu, A., Langer, M., Rustichelli, F., Desiderio, V., Paino, F., et al. (2013). Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cells Transl. Med. 2, 316–324. doi: 10.5966/sctm.2012-0136
Glim, J. E., Everts, V., Niessen, F. B., Ulrich, M. M., and Beelen, R. H. J. (2014). Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Arch. Oral Biol. 50, 1048–1055. doi: 10.1016/j.archoralbio.2014.05.019
Glim, J. E., van Egmond, M., Niessen, F. B., Everts, V., and Beelen, R. H. J. (2013). Detrimental dermal wound healing: what can we learn from the oral mucosa? Wound Repair Regen. 21, 648–660. doi: 10.1111/wrr.12072
Goetsch, E. (1910). The structure of the mammalian oesophagus. Am. J. Anat. 10, 1–40. doi: 10.1002/aja.1000100102
Gölz, L., Vestewig, E., Blankart, M., Kraus, D., Appel, T., Frede, S., et al. (2016). Differences in human gingival and dermal fibroblasts may contribute to oral-induced tolerance against nickel. J. Allergy Clin. Immunol. 138, 1202–1205.e3. doi: 10.1016/j.jaci.2016.03.036
Gomes, J. ÁP., Monteiro, B. G., Melo, G. B., Smith, R. L., Silva, M. C. P., Lizier, N. F., et al. (2010). Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Investig. Ophthalmol. Vis. Sci. 51, 1408–1414. doi: 10.1167/iovs.09-4029
Gonzales, K. A. U., and Fuchs, E. (2017). Skin and its regenerative powers: an alliance between stem cells and their niche. Dev. Cell 43, 387–401. doi: 10.1016/j.devcel.2017.10.001
Goodacre, T., and Swan, M. C. (2008). Cleft lip and palate: current management. J. Paediatr. Child Health 18, 283–292. doi: 10.1016/j.paed.2008.03.008
Gordon, J. I. (1994). Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr. Opin. Cell Biol. 6, 795–803. doi: 10.1016/0955-0674(94)90047-7
Groeger, S., and Meyle, J. (2019). Oral mucosal epithelial cells. Front. Immunol. 10:208. doi: 10.3389/fimmu.2019.00208
Grøn, B., Stoltze, K., Andersson, A., and Dabelsteen, E. (2002). Oral fibroblasts produce more HGF and KGF than skin fibroblasts in response to co-culture with keratinocytes. APMIS 110, 892–898. doi: 10.1034/j.1600-0463.2002.1101208.x
Guerrero-Juarez, C. F., Dedhia, P. H., Jin, S., Ruiz-Vega, R., Ma, D., Liu, Y., et al. (2019). Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat. Commun. 10:650. doi: 10.1038/s41467-018-08247-x
Guest, J. F., Ayoub, N., McIlwraith, T., Uchegbu, I., Gerrish, A., Weidlich, D., et al. (2015). Health economic burden that wounds impose on the national health service in the UK. BMJ Open 5:e009283. doi: 10.1136/bmjopen-2015-009283
Gurtner, G. C., Werner, S., Barrandon, Y., and Longaker, M. T. (2008). Wound repair and regeneration. Nature 453, 314–321. doi: 10.1038/nature07039
Hall, M. W. J., Jones, P. H., and Hall, B. A. (2018). Relating evolutionary selection and mutant clonal dynamics in normal epithelia. bioRxiv [Preprint] doi: 10.1101/480756
Hand, A. R., and Frank, M. E. (2014). Fundamentals of Oral Histology and Physiology. Hoboken, NJ: John Wiley & Sons, Inc.
Harper, R. A., and Grove, G. (1979). Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science 204, 526–527. doi: 10.1126/science.432659
Herrmann, H., and Aebi, U. (2016). Intermediate filaments: structure and assembly. Cold Spring Harb. Perspect. Biol. 8:a018242. doi: 10.1101/cshperspect.a018242
Higa, K., Satake, Y., and Shimazaki, J. (2017). The characterization of human oral mucosal fibroblasts and their use as feeder cells in cultivated epithelial sheets. Future Sci. OA 3:FSO243. doi: 10.4155/fsoa-2017-0074
Higgins, C. A., Roger, M. F., Hill, R. P., Ali-Khan, A. S., Garlick, J. A., Christiano, A. M., et al. (2017). Multifaceted role of hair follicle dermal cells in bioengineered skins. Br. J. Dermatol. 176, 1259–1269. doi: 10.1111/bjd.15087
Hinz, B. (2007). Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 127, 526–537. doi: 10.1038/sj.jid.5700613
Hiraoka, C., Toki, F., Shiraishi, K., Sayama, K., Nishimura, E. K., Miura, H., et al. (2016). Two clonal types of human skin fibroblasts with different potentials for proliferation and tissue remodeling ability. J. Dermatol. Sci. 82, 84–94. doi: 10.1016/j.jdermsci.2016.01.009
Hirsch, T., Rothoeft, T., Teig, N., Bauer, J. W., Pellegrini, G., De Rosa, L., et al. (2017). Regeneration of the entire human epidermis using transgenic stem cells. Nature 551, 327–332. doi: 10.1038/nature24487
Hixon, K. R., Lin, A. Y., and Sell, S. A. (2019). “Scaffolds for cleft lip and cleft palate reconstruction,” in Handbook of Tissue Engineering Scaffolds, Vol. 1, eds M. Mozafari, F. Sefat, and A. Atala (Sawston: Woodhead Publishing), 421–435. doi: 10.1016/B978-0-08-102563-5.00020-4
Holbrook, K. (1994). “Ultrastructure of the epidermis,” in The Keratinocyte Handbook, eds I. M. Leigh, E. B. Lane, and F. M. Watt (Cambridge: Cambridge University Press), 3–39.
Horiguchi, A. (2017). Substitution urethroplasty using oral mucosa graft for male anterior urethral stricture disease: current topics and reviews. Int. J. Urol. 24, 493–503. doi: 10.1111/iju.13356
Hotta, T., Yokoo, S., Terashi, H., and Komori, T. (2007). Clinical and histopathological analysis of healing process of intraoral reconstruction with ex vivo produced oral mucosa equivalent. Kobe J. Med. Sci. 53, 1–14.
Howard, J. M., Nuguid, J. M., Ngole, D., and Nguyen, H. (2014). Tcf3 expression marks both stem and progenitor cells in multiple epithelia. Development 141, 3143–3152. doi: 10.1242/dev.106989
Huang, N., Perez, P., Kato, T., Mikami, Y., Okuda, K., Gilmore, R. C., et al. (2021). SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 27, 892–903.
Huang, Z., Wei, W., and Cheng, F. (2020). Endoscopic radial incision method for two strictures of the esophagus after endoscopic submucosal dissection: a case report. World J. Surg. Oncol. 18, 1–6. doi: 10.1186/s12957-020-01812-z
Hume, W. J., and Potten, C. S. (1976). The ordered columnar structure of mouse filiform papillae. J. Cell Sci. 22, 149–160.
Hutson, J. M., Niall, M., Evans, D., and Fowler, R. (1979). Effect of salivary glands on wound contraction in mice. Nature 279, 793–795. doi: 10.1038/279793a0
Iglesias-Bartolome, R., Uchiyama, A., Molinolo, A. A., Abusleme, L., Brooks, S. R., Callejas-Valera, J. L., et al. (2018). Transcriptional signature primes human oral mucosa for rapid wound healing. Sci. Transl. Med. 10:451. doi: 10.1126/scitranslmed.aap8798
Iida, T., Takami, Y., Yamaguchi, R., Shimazaki, S., and Harii, K. (2005). Development of a tissue-engineered human oral mucosa equivalent based on an acellular allogeneic dermal matrix: a preliminary report of clinical application to burn wounds. Scand. J. Plast. Reconstr. Surg. Hand Surg. 39, 138–146. doi: 10.1080/0284431051006376
Irwin, C. R., Picardo, M., Ellis, I., Sloan, P., Grey, A. M., McGurk, M., et al. (1994). Inter- and Intra-site heterogeneity in the expression of fetal-like phenotypic characteristics by gingival fibroblasts: potential significance for wound healing. J. Cell Sci. 107, 1333–1346.
Isaac, J., Nassif, A., Asselin, A., Taïhi, I., Fohrer-Ting, H., Klein, C., et al. (2018). Involvement of neural crest and paraxial mesoderm in oral mucosal development and healing. Biomaterials 172, 41–53. doi: 10.1016/j.biomaterials.2018.04.036
Ishii, M., Arias, A. C., Liu, L., Chen, Y., Bronner, M. E., and Maxson, R. E. (2012). A stable cranial neural crest cell line from mouse. Stem Cells Dev. 21, 3069–3080. doi: 10.1089/scd.2012.0155
Ito, M., Liu, Y., Yang, Z., Nguyen, J., Liang, F., Morris, R. J., et al. (2005). Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11, 1351–1354. doi: 10.1038/nm1328
Izumi, K., Feinberg, S. E., Iida, A., and Yoshizawa, M. (2003). Intraoral grafting of an Ex vivo produced oral mucosa equivalent: a preliminary report. Int. J. Oral Maxillofac. Surg. 32, 188–197. doi: 10.1054/ijom.2002.0365
Izumi, K., Kato, H., and Feinberg, S. E. (2015). “Tissue engineered oral mucosa,” in Stem Cell Biology and Tissue Engineering in Dental Sciences, eds A. Vishwakarma, P. Sharpe, S. Shi, and M. Ramalingam (Amsterdam: Elsevier Inc.), 721–731. doi: 10.1016/B978-0-12-397157-9.00077-1
Izumi, K., Neiva, R. F., and Feinberg, S. E. (2013). Intraoral grafting of tissue-engineered human oral mucosa. Int. J. Oral Maxillofac. Implants 28, e295–e303. doi: 10.11607/jomi.te11
Jahoda, C. A. B., and Reynolds, A. J. (1996). Dermal-epidermal interactions. Adult follicle-derived cell populations and hair growth. Dermatol. Clin. 14, 573–583. doi: 10.1016/s0733-8635(05)70385-5
Jaks, V., Barker, N., Kasper, M., Van Es, J. H., Snippert, H. J., Clevers, H., et al. (2008). Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 40, 1291–1299. doi: 10.1038/ng.239
Janebodin, K., Horst, O. V., Ieronimakis, N., Balasundaram, G., Reesukumal, K., Pratumvinit, B., et al. (2011). Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One 6:e27526. doi: 10.1371/journal.pone.0027526
Jankowski, J. (1993). Gene expression in Barrett’s mucosa: acute and chronic adaptive responses in the oesophagus. Gut 32, 1649–1650.
Jensen, K. B., Collins, C. A., Nascimento, E., Tan, D. W., Frye, M., Itami, S., et al. (2009). Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4, 427–439. doi: 10.1016/j.stem.2009.04.014
Jiang, D., and Rinkevich, Y. (2018). Defining skin fibroblastic cell types beyond CD90. Front. Cell Dev. Biol. 6:133. doi: 10.3389/fcell.2018.00133
Jiang, D., and Rinkevich, Y. (2021). Distinct fibroblasts in scars and regeneration. Curr. Opin. Genet. Dev. 70, 7–14. doi: 10.1016/j.gde.2021.04.005
Jiang, D., Christ, S., Correa-Gallegos, D., Ramesh, P., Gopal, S. K., Wannemacher, J., et al. (2020a). Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 11:5653. doi: 10.1038/s41467-020-19425-1
Jiang, D., Correa-Gallegos, D., Christ, S., Stefanska, A., Liu, J., Ramesh, P., et al. (2018). Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat. Cell Biol. 20, 422–431. doi: 10.1038/s41556-018-0073-8
Jiang, D., de Vries, J. C., Muschhammer, J., Schatz, S., Ye, H., Hein, T., et al. (2020b). Local and transient inhibition of P21 expression ameliorates age-related delayed wound healing. Wound Repair Regen. 28, 49–60. doi: 10.1111/wrr.12763
Jiang, N., Xiang, L., He, L., Yang, G., Zheng, J., Wang, C., et al. (2017). Exosomes mediate epithelium-mesenchyme crosstalk in organ development. ACS Nano 11, 7736–7746. doi: 10.1021/acsnano.7b01087
Johnson, A., and Di Pietro, L. A. (2013). Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J. 27, 3893–3901. doi: 10.1096/fj.12-214189
Johnson, O. N., Nelson, M., Estabrooke, I., Sopko, N., and Swanson, E. W. (2020). Successful treatment of war zone traumatic lower extremity wound with exposed tendons using an autologous homologous skin construct. Cureus 12, 1–7. doi: 10.7759/cureus.7952
Jonas, E., Sjöqvist, S., Elbe, P., Kanai, N., Enger, J., Haas, S. L., et al. (2016). Transplantation of tissue-engineered cell sheets for stricture prevention after endoscopic submucosal dissection of the oesophagus. United Eur. Gastroenterol. J. 4, 741–753. doi: 10.1177/2050640616631205
Jones, K. B., and Klein, O. D. (2013). Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. Int. J. Oral Sci. 5, 121–129. doi: 10.1038/ijos.2013.46
Jones, K. B., Furukawa, S., Marangoni, P., Ma, H., Pinkard, H., D’Urso, R., et al. (2019). Quantitative clonal analysis and single-cell transcriptomics reveal division kinetics, hierarchy, and fate of oral epithelial progenitor cells. Cell Stem Cell 24, 183–192.e8. doi: 10.1016/j.stem.2018.10.015
Jones, S. G., Edwards, R., and Thomas, D. W. (2004). Inflammation and wound healing: the role of bacteria in the immuno-regulation of wound healing. Int. J. Low Extrem. Wounds 3, 201–208. doi: 10.1177/1534734604271810
Jönsson, L., Blom, M. D., Friberg, L., Gatzinsky, V., Holmquist, O., Jennische, E., et al. (2016). Macrophage phenotype is associated with the regenerative response in experimental replacement of the porcine esophagus. Artif. Organs 40, 950–958. doi: 10.1111/aor.12652
Joost, S., Annusver, K., Jacob, T., Sun, X., Dalessandri, T., Sivan, U., et al. (2020). The molecular anatomy of mouse skin during hair growth and rest. Cell Stem Cell 26, 441–457.e7. doi: 10.1016/j.stem.2020.01.012
Joost, S., Zeisel, A., Jacob, T., Sun, X., La Manno, G., Lönnerberg, P., et al. (2016). Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst. 3, 221–237.e9. doi: 10.1016/j.cels.2016.08.010
Kanwal, A., D’souza, J., Muthiah, L., and Srividya, S. (2017). The current status of stem cell regeneration in intra oral applications—a systematic review. Open J. Stomatol. 7, 197–224. doi: 10.4236/ojst.2017.74015
Karppinen, S. M., Heljasvaara, R., Gullberg, D., Tasanen, K., and Pihlajaniemi, T. (2019). Toward understanding scarless skin wound healing and pathological scarring. F1000Res. 8:787. doi: 10.12688/f1000research.18293.1
Kato, H., Marcelo, C. L., Washington, J. B., Bingham, E. L., and Feinberg, S. E. (2015). Fabrication of large size ex vivo-produced oral mucosal equivalents for clinical application. Tissue Eng. Part C Methods 21, 872–880. doi: 10.1089/ten.tec.2014.0600
Katou, F., Shirai, N., Kamakura, S., Tagami, H., Nagura, H., and Motegi, K. (2003). Differential expression of cornified cell envelope precursors in normal skin, intraorally transplanted skin and normal oral mucosa. Br. J. Dermatol. 148, 898–905. doi: 10.1046/j.1365-2133.2003.05288.x
Kim, H. S., Kim, N. H., Kim, J., and Cha, I. H. (2013). Inducing re-epithelialization in skin wound through cultured oral mucosal keratinocytes. J. Korean Assoc. Oral Maxillofac. Surg. 39:63. doi: 10.5125/jkaoms.2013.39.2.63
Kim, M., Han, W., and Kim, S. (2017). The use of the buccal fat pad flap for oral reconstruction. Maxillofac. Plast. Reconstr. Surg. 39, 1–9. doi: 10.1186/s40902-017-0105-5
Kitamoto, S., Nagao-Kitamoto, H., Jiao, Y., Gillilland, M. G., Hayashi, A., Imai, J., et al. (2020). The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 182, 447–462.e14. doi: 10.1016/j.cell.2020.05.048
Klein, A. M., and Simons, B. D. (2011). Universal patterns of stem cell fate in cycling adult tissues. Development 138, 3103–3111. doi: 10.1242/dev.060103
Klein, A. M., Brash, D. E., Jones, P. H., and Simons, B. D. (2010). Stochastic fate of P53-mutant epidermal progenitor cells is tilted toward proliferation by UV B during preneoplasia. Proc. Natl. Acad. Sci. U.S.A. 107, 270–275. doi: 10.1073/pnas.0909738107
Knapp, A. C., Franke, W. W., Heid, H., Hatzfeld, M., Jorcano, J. L., and Moll, R. (1986). Cytokeratin no. 9, an epidermal type i keratin characteristic of a special program of keratinocyte differentiation displaying body site specificity. J. Cell Biol. 103, 657–667. doi: 10.1083/jcb.103.2.657
Knöbel, M., O’Toole, E. A., and Smith, F. J. D. (2015). Keratins and skin disease. Cell Tissue Res. 360, 583–589. doi: 10.1007/s00441-014-2105-4
Komaki, Y., Kanmura, S., Sasaki, F., Maeda, H., Oda, K., Arima, S., et al. (2019). Hepatocyte growth factor facilitates esophageal mucosal repair and inhibits the submucosal fibrosis in a rat model of esophageal ulcer. Digestion 99, 227–238. doi: 10.1159/000491876
Kong, X., Fu, J., Shao, K., Wang, L., Lan, X., and Shi, J. (2019). Biomimetic hydrogel for rapid and scar-free healing of skin wounds inspired by the healing process of oral mucosa. Acta Biomater. 100, 255–269. doi: 10.1016/j.actbio.2019.10.011
Korosec, A., and Lichtenberger, B. M. (2018). “12 in vitro models to study hair follicle generation,” in Skin Tissue Models, eds A. Marques, R. Reis, R. Pirraco, and M. Cerqueira (Cambridge, MA: Academic Press), 279–301.
Korosec, A., Frech, S., Gesslbauer, B., Vierhapper, M., Radtke, C., Petzelbauer, P., et al. (2019). Lineage identity and location within the dermis determine the function of papillary and reticular fibroblasts in human skin. J. Invest. Dermatol. 139, 342–351. doi: 10.1016/j.jid.2018.07.033
Kuperman, S., Efraty, R., Arie, I., Rahmanov, A., Gavrielov, M. R., Noff, M., et al. (2020). Examination of the therapeutic potential of mouse oral mucosa stem cells in a wound-healing diabetic mice model. Int. J. Environ. Res. Public Health 17, 1–10. doi: 10.3390/ijerph17134854
Kuramoto, G., Takagi, S., Ishitani, K., Shimizu, T., Okano, T., and Matsui, H. (2015). Preventive effect of oral mucosal epithelial cell sheets on intrauterine adhesions. Hum. Reprod. 30, 406–416. doi: 10.1093/humrep/deu326
Lai-Cheong, J. E., and McGrath, J. A. (2013). Structure and function of skin, hair and nails. Medcine 41, 317–320. doi: 10.1016/j.mpmed.2013.04.017
Lane, E. B. (1994). Keratin diseases. Curr. Opin. Genet. Dev. 4, 412–418. doi: 10.1016/0959-437X(94)90030-2
Larjava, H. (ed.) (2012). Oral Wound Healing: Cell Biology and Clinical Management. West Sussex: John Wiley & Sons, Inc.
Lauer, G., and Schimming, R. (2001). Tissue-engineered mucosa graft for reconstruction of the intraoral lining after freeing of the tongue: a clinical and immunohistologic study. J. Oral Maxillofac. Surg. 59, 169–175. doi: 10.1053/joms.2001.20489
Lee, J., Kim, E. H., Shin, D., and Roh, J. L. (2017). Accelerated oral wound healing using a pre-vascularized mucosal cell sheet. Sci. Rep. 7:10667. doi: 10.1038/s41598-017-10991-x
Lee, J., Shin, D., and Roh, J. L. (2018). Use of a pre-vascularised oral mucosal cell sheet for promoting cutaneous burn wound healing. Theranostics 8, 5703–5712. doi: 10.7150/thno.28754
Lee, J., Shin, D., and Roh, J. L. (2019). Promotion of skin wound healing using prevascularized oral mucosal cell sheet. Head Neck 41, 774–779. doi: 10.1002/hed.25432
Legué, E., and Nicolas, J. F. (2005). Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development 132, 4143–4154. doi: 10.1242/dev.01975
Legué, E., Sequeira, I., and Nicolas, J. (2012). “Hair follicle stem cells,” in Stem Cells and Cancer Stem Cells, ed. M. Hayat (Dordrecht: Springer), 35–47. doi: 10.1007/978-94-007-2415-0
Lei, M., and Chuong, C. M. (2018). Epidermal darwinism and competitive equilibrium within the epidermis. Cell Stem Cell 23, 627–629. doi: 10.1016/j.stem.2018.10.019
Levoux, J., Prola, A., Lafuste, P., Gervais, M., Chevallier, N., Koumaiha, Z., et al. (2021). Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab. 33, 283–299.e9. doi: 10.1016/j.cmet.2020.12.006
Li, D., Yin, Z., Liu, Y., Feng, S., Liu, Y., Lu, F., et al. (2019). Regeneration of trachea graft with cartilage support, vascularization, and epithelization. Acta Biomater. 89, 206–216. doi: 10.1016/j.actbio.2019.03.003
Li, J., Wang, Z., Chu, Q., Jiang, K., Li, J., and Tang, N. (2018). The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Dev. Cell 44, 297–312.e5. doi: 10.1016/j.devcel.2018.01.008
Liakath-Ali, K., Mills, E. W., Sequeira, I., Lichtenberger, B. M., Pisco, A. O., Sipilä, K. H., et al. (2018). An evolutionarily conserved ribosome-rescue pathway maintains epidermal homeostasis. Nature 556, 367–380. doi: 10.1038/s41586-018-0032-3
Lichtenberger, B. M., Mastrogiannaki, M., and Watt, F. M. (2016). Epidermal β-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat. Commun. 7:10537. doi: 10.1038/ncomms10537
Lim, X., Tan, S. H., Koh, W. L. C., Chau, R. M. W., Yan, K. S., Kuo, C. J., et al. (2013). Interfollicular epidermal stem cells self-renew via autocrine wnt signaling. Science 342, 1226–1230. doi: 10.1126/science.1239730
Llames, S., Recuero, I., Romance, A., García, E., Peña, I., Del Valle, ÁF., et al. (2014). Tissue-engineered oral mucosa for mucosal reconstruction in a pediatric patient with hemifacial microsomia and ankyloglossia. Cleft Palate Craniofac. J. 51, 246–251. doi: 10.1597/12-245
Locke, M., Hyland, P. L., Irwin, C. R., and Mackenzie, I. C. (2008). Modulation of gingival epithelial phenotypes by interactions with regionally defined populations of fibroblasts. J. Periodontal Res. 43, 279–289. doi: 10.1111/j.1600-0765.2007.01028.x
López, J. F., Sarkanen, J. R., Huttala, O., Kaartinen, I. S., Kuokkanen, H. O., and Ylikomi, T. (2018). Adipose tissue extract shows potential for wound healing: in vitro proliferation and migration of cell types contributing to wound healing in the presence of adipose tissue preparation and platelet rich plasma. Cytotechnology 70, 1193–1204. doi: 10.1007/s10616-018-0211-y
Lu, C. P., Polak, L., Rocha, A. S., Pasolli, H. A., Chen, S. C., Sharma, N., et al. (2012). Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150, 136–150. doi: 10.1016/j.cell.2012.04.045
Luca, M., Albanese, E., Megna, M., Cancedda, R., Mangiante, P. E., Cadoni, A., et al. (1990). Evidence that human oral epithelium reconstituted in vitro and transplanted onto patients with defects in the oral mucosa retains properties of the original donor site. Transplantation 50, 454–459. doi: 10.1097/00007890-199009000-00019
Lucas, T., Waisman, A., Ranjan, R., Roes, J., Krieg, T., Müller, W., et al. (2010). Differential roles of macrophages in diverse phases of skin repair. J. Immunol. Res. 184, 3964–3977. doi: 10.4049/jimmunol.0903356
Luo, X., Okubo, T., Randell, S., and Hogan, B. L. M. (2009). Culture of endodermal stem/progenitor cells of the mouse tongue. In Vitro Cell. Dev. Biol. Anim. 45, 44–54. doi: 10.1007/s11626-008-9149-2
Lv, L., Sheng, C., and Zhou, Y. (2019). Extracellular vesicles as a novel therapeutic tool for cell-free regenerative medicine in oral rehabilitation. J. Oral Rehabil. 47, 29–54. doi: 10.1111/joor.12885
Lygoe, K. A., Wall, I., Stephens, P., and Lewis, M. P. (2007). Role of vitronectin and fibronectin receptors in oral mucosal and dermal myofibroblast differentiation. Biol. Cell 99, 601–614. doi: 10.1042/bc20070008
Lynch, M. D., Lynch, C. N. S., Craythorne, E., Liakath-Ali, K., Mallipeddi, R., Barker, J. N., et al. (2017). Spatial constraints govern competition of mutant clones in human epidermis. Nat. Commun. 8:1119. doi: 10.1038/s41467-017-00993-8
Mackenzie, I. C. (1997). Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J. Invest. Dermatol. 109, 377–383. doi: 10.1111/1523-1747.ep12336255
Mackenzie, I. C., and Hill, M. W. (1984). Connective tissue influences on patterns of epithelial architecture and keratinization in skin and oral mucosa of the adult mouse. Cell Tissue Res. 235, 551–559. doi: 10.1007/BF00226952
Mah, W., Jiang, G., Olver, D., Gallant-Behm, C., Wiebe, C., Hart, D. A., et al. (2017). Elevated CD26 expression by skin fibroblasts distinguishes a profibrotic phenotype involved in scar formation compared to gingival fibroblasts. Am. J. Pathol. 187, 1717–1735. doi: 10.1016/j.ajpath.2017.04.017
Mak, K., Manji, A., Gallant-Behm, C., Wiebe, C., Hart, D. A., Larjava, H., et al. (2009). Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red duroc pig model. J. Dermatol. Sci. 56, 168–180. doi: 10.1016/j.jdermsci.2009.09.005
Marques-Pereira, J. P., and Leblond, C. P. (1965). Mitosis and differentiation in the stratified squamous epithelium of the rat esophagus. Am. J. Anat. 117, 73–87. doi: 10.1002/aja.1001170106
Marsh, E., Gonzalez, D. G., Lathrop, E. A., Boucher, J., and Greco, V. (2018). Positional stability and membrane occupancy define skin fibroblast homeostasis in vivo. Cell 175, 1620–1633.e13. doi: 10.1016/j.cell.2018.10.013
Marshall, C. D., Hu, M. S., Leavitt, T., Barnes, L. A., Lorenz, H. P., and Longaker, M. T. (2018). Cutaneous scarring: basic science, current treatments, and future directions. Adv. Wound Care 7, 29–45. doi: 10.1089/wound.2016.0696
Martincorena, I., Fowler, J. C., Wabik, A., Lawson, A. R. J., Abascal, F., Hall, M. W. J., et al. (2018). Somatic mutant clones colonize the human esophagus with age. Science 362, 911–917. doi: 10.1126/science.aau3879
Martins, V. L., Caley, M., and O’Toole, E. A. (2013). Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 351, 255–268. doi: 10.1007/s00441-012-1410-z
Mascré, G., Dekoninck, S., Drogat, B., Youssef, K. K., Brohée, S., Sotiropoulou, P. A., et al. (2012). Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489, 257–262. doi: 10.1038/nature11393
Mastrogiannaki, M., Lichtenberger, B. M., Reimer, A., Collins, C. A., Driskell, R. R., and Watt, F. M. (2016). β-catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of the reticular dermis. J. Invest. Dermatol. 136, 1130–1142. doi: 10.1016/j.jid.2016.01.036
Matoltsy, A. G. (1986). “Structure and function of the mammalian epidermis,” in Biology of the Integument, eds J. Bereiter-Hahn, A. G. Matoltsy, and K. S. Richards (Berlin: Springer), 255–271. doi: 10.1007/978-3-662-00989-5_14
Mcginn, J., Hallou, A., Han, S., Krizic, K., Ulyanchenko, S., Iglesias-Bartolome, R., et al. (2021). A biomechanical switch regulates the transition towards homeostasis in esophageal epithelium. bioRxiv [Preprint] Available online at: https://www.biorxiv.org/content/10.1101/2021.02.03.428820v1 (accessed March 2, 2021)
McGuire, M. K., and Scheyer, E. T. (2007). A randomized, double-blind, placebo-controlled study to determine the safety and efficacy of cultured and expanded autologous fibroblast injections for the treatment of interdental papillary insufficiency associated with the papilla priming procedure. J. Periodontol. 78, 4–17. doi: 10.1902/jop.2007.060105
McKeown, S. T. W., Hyland, P. L., Locke, M., Mackenzie, I. C., and Irwin, C. R. (2003). Keratinocyte growth factor and scatter factor expression by regionally defined oral fibroblasts. Eur. J. Oral Sci. 111, 42–50. doi: 10.1034/j.1600-0722.2003.00002.x
Mehrel, T., Hohl, D., Rothnagel, J. A., Longley, M. A., Bundman, D., Cheng, C., et al. (1990). Identification of a major keratinocyte cell envelope protein, loricrin. Cell 61, 1103–1112. doi: 10.1016/0092-8674(90)90073-N
Meigel, W. N., Gay, S., and Weber, L. (1977). Dermal architecture and collagen type distribution. Arch. Dermatol. Res. 259, 1–10. doi: 10.1007/BF00562732
Meran, S., Thomas, D., Stephens, P., Martin, J., Bowen, T., Phillips, A., et al. (2007). Involvement of hyaluronan in regulation of fibroblast phenotype. J. Biol. Chem. 282, 25687–25697. doi: 10.1074/jbc.M700773200
Mesa, K. R., Kawaguchi, K., Cockburn, K., Gonzalez, D., Boucher, J., Xin, T., et al. (2018). Homeostatic epidermal stem cell self-renewal is driven by local differentiation. Cell Stem Cell 23, 677.e–686.e. doi: 10.1016/j.stem.2018.09.005
Mesa, K. R., Rompolas, P., and Greco, V. (2015). The dynamic duo: niche/stem cell interdependency. Stem Cell Rep. 4, 961–966. doi: 10.1016/j.stemcr.2015.05.001
Mesler, A. L., Veniaminova, N. A., Lull, M. V., and Wong, S. Y. (2017). Hair follicle terminal differentiation is orchestrated by distinct early and late matrix progenitors. Cell Rep. 19, 809–821. doi: 10.1016/j.celrep.2017.03.077
Messier, B., and Leblond, C. P. (1960). Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am. J. Anat. 106, 247–285. doi: 10.1002/aja.1001060305
Mi, B., Chen, L., Xiong, Y., Yan, C., Xue, H., Panayi, A. C., et al. (2020). Saliva exosomes-derived UBE2O MRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J. Nanobiotechnol. 18, 1–14. doi: 10.1186/s12951-020-00624-3
Mistretta, C. M., and Kumari, A. (2017). Tongue and taste organ biology and function: homeostasis maintained by hedgehog signaling. Annu. Rev. Physiol. 79, 335–356. doi: 10.1146/annurev-physiol-022516-034202
Mitchell, H., Abel, K. M., Dunlop, B. J., Walker, T., Ranote, S., Robinson, L., et al. (2019). Acceptability and feasibility pilot randomised controlled trial of medical skin camouflage for recovery of women prisoners with self-harm scarring (COVER): the study protocol. BMJ Open 9:e021891. doi: 10.1136/bmjopen-2018-021891
Miyoshi, K., Tsuji, D., Kudoh, K., Satomura, K., Muto, T., Itoh, K., et al. (2010). Generation of human induced pluripotent stem cells from oral mucosa. J. Biosci. Bioeng. 110, 345–350. doi: 10.1016/j.jbiosc.2010.03.004
Mohammadi, M., Mofid, R., and Shokrgozar, M. A. (2011). peri-implant soft tissue management through use of cultured gingival graft: a case report. Acta Med. Iran. 49, 319–324.
Moll, I., Heid, H., Franke, W. W., and Moll, R. (1987). Distribution of a special subset of keratinocytes characterized by the expression of cytokeratin 9 in adult and fetal human epidermis of various body sites. Differentiation 33, 254–265. doi: 10.1111/j.1432-0436.1987.tb01565.x
Moll, R., Divo, M., and Langbein, L. (2008). The human keratins: biology and pathology. Histochem. Cell Biol. 129, 705–733. doi: 10.1007/s00418-008-0435-6
Morris, R. J., Liu, Y., Marles, L., Yang, Z., Trempus, C., Li, S., et al. (2004). Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22, 411–417. doi: 10.1038/nbt950
Moutsopoulos, N. M., and Konkel, J. E. (2018). Tissue-specific immunity at the oral mucosal barrier. Trends Immunol. 39, 276–287. doi: 10.1016/j.it.2017.08.005
Murai, K., Skrupskelyte, G., Piedrafita, G., Hall, M., Kostiou, V., Ong, S. H., et al. (2018). Epidermal Tissue adapts to restrain progenitors carrying clonal P53 mutations. Cell Stem Cell 23, 687.e–699.e. doi: 10.1016/j.stem.2018.08.017
Nakagawa, E., Itoh, T., Yoshie, H., and Satokata, I. (2009). Odontogenic potential of post-natal oral mucosal epithelium. J. Dent. Res. 88, 219–223. doi: 10.1177/0022034509333198
Nakase, Y., Nakamura, T., Kin, S., Nakashima, S., Yoshikawa, T., Kuriu, Y., et al. (2008). Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J. Thorac. Cardiovasc. Surg. 136, 850–859. doi: 10.1016/j.jtcvs.2008.05.027
Neves, C. R., Buskermolen, J., Roffel, S., Waaijman, T., Thon, M., Veerman, E., et al. (2019). Human saliva stimulates skin and oral wound healing in vitro. J. Tissue Eng. Regen. Med. 13, 1079–1092. doi: 10.1002/term.2865
Nikoloudaki, G., Creber, K., and Hamilton, D. W. (2020). Wound healing and fibrosis: a contrasting role for periostin in skin and the oral mucosa. Am. J. Physiol. Cell Physiol. 318, C1065–C1077. doi: 10.1152/ajpcell.00035.2020
Nishida, K., Yamato, M., Hayashida, Y., Watanabe, K., Yamamoto, K., Adachi, E., et al. (2004). Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351, 1187–1196. doi: 10.1056/nejmoa040455
Nishiguchi, Y., Ohmoto, M., Koki, J., Enomoto, T., Kominami, R., Matsumoto, I., et al. (2016). Bcl11b/Ctip2 is required for development of lingual papillae in mice. Dev. Biol. 416, 98–110. doi: 10.1016/j.ydbio.2016.06.001
Nishino, Y., Yamada, Y., Ebisawa, K., Nakamura, S., Okabe, K., Umemura, E., et al. (2011). Stem cells from human exfoliated deciduous teeth (SHED) enhance wound healing and the possibility of novel cell therapy. Cytotherapy 13, 598–605. doi: 10.3109/14653249.2010.542462
Nishiyama, K., Akagi, T., Iwai, S., and Akashi, M. (2019). Construction of vascularized oral mucosa equivalents using a layer-by-layer cell coating technology. Tissue Eng. Part C Methods 25, 262–275. doi: 10.1089/ten.tec.2018.0337
Nowarski, R., Jackson, R., and Flavell, R. A. (2017). The stromal intervention: regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell 168, 362–375. doi: 10.1016/j.cell.2016.11.040
Odland, G. F. (1991). “Structure of the skin,” in Physiology, Biochemistry, and Molecular Biology of the Skin, ed. L. A. Goldsmith (Oxford: Oxford University Press), 3–62.
Oezcelik, A., and DeMeester, S. R. (2011). General anatomy of the esophagus. Thorac. Surg. Clin. 21, 289–297. doi: 10.1016/j.thorsurg.2011.01.003
Ohki, T., and Yamamoto, M. (2020). Esophageal regenerative therapy using cell sheet technology. Regen. Ther. 13, 8–17. doi: 10.1016/j.reth.2020.04.009
Ohki, T., Yamato, M., Murakami, D., Takagi, R., Yang, J., Namiki, H., et al. (2006). Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 55, 1704–1710. doi: 10.1136/gut.2005.088518
Ohki, T., Yamato, M., Ota, M., Takagi, R., Kondo, M., Kanai, N., et al. (2015). Application of regenerative medical technology using tissue-engineered cell sheets for endoscopic submucosal dissection of esophageal neoplasms. Dig. Endosc. 27, 182–188. doi: 10.1111/den.12354
Ohki, T., Yamato, M., Ota, M., Takagi, R., Murakami, D., Kondo, M., et al. (2012). Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 143, 582–588.e2. doi: 10.1053/j.gastro.2012.04.050
Ohmoto, M., Lei, W., Yamashita, J., Hirota, J., Jiang, P., and Matsumoto, I. (2020). SOX2 regulates homeostasis of taste bud cells and lingual epithelial cells in posterior tongue. Edited by Hiroaki Matsunami. PLoS One 15:e0240848. doi: 10.1371/journal.pone.0240848
Okubo, T., Clark, C., and Hogan, B. L. M. (2009). Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells 27, 442–450. doi: 10.1634/stemcells.2008-0611
Oliva, J., Bardag-Gorce, F., and Niihara, Y. (2020). Clinical trials of limbal stem cell deficiency treated with oral mucosal epithelial cells. Int. J. Mol. Sci. 21:411. doi: 10.3390/ijms21020411
Oudhoff, M. J., Bolscher, J. G. M., Nazmi, K., Kalay, H., Hof, W., Amerongen, A. V. N., et al. (2008). Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J. 22, 3805–3812. doi: 10.1096/fj.08-112003
Page, M. E., Lombard, P., Ng, F., Göttgens, B., and Jensen, K. B. (2013). The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13, 471–482. doi: 10.1016/j.stem.2013.07.010
Park, J. E., and Barbul, A. (2004). Understanding the role of immune regulation in wound healing. Am. J. Surg. 187, S11–S16. doi: 10.1016/S0002-9610(03)00296-4
Park, J. Y., Chung, H., Dipalma, D. T., Tai, X., and Park, J. H. (2018). Immune quiescence in the oral mucosa is maintained by a uniquely large population of highly activated Foxp3+ regulatory T cells article. Mucosal Immunol. 11, 1092–1102. doi: 10.1038/s41385-018-0027-2
Peramo, A., Marcelo, C. L., and Feinberg, S. E. (2012). Tissue engineering of lips and muco-cutaneous junctions: in vitro development of tissue engineered constructs of oral mucosa and skin for lip reconstruction. Tissue Eng. Part C Methods 18, 273–282. doi: 10.1089/ten.tec.2011.0406
Phan, Q. M., Fine, G. M., Salz, L., Herrera, G. G., Wildman, B., Driskell, I. M., et al. (2020). Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. Elife 9:e60066. doi: 10.7554/ELIFE.60066
Phan, Q. M., Sinha, S., Biernaskie, J., and Driskell, R. R. (2021). Single-cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Exp. Dermatol. 30, 92–101. doi: 10.1111/exd.14244
Philippeos, C., Telerman, S. B., Oulès, B., Pisco, A. O., Shaw, T. J., Elgueta, R., et al. (2018). Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J. Invest. Dermatol. 138, 811–825. doi: 10.1016/j.jid.2018.01.016
Piedrafita, G., Kostiou, V., Wabik, A., Colom, B., Fernandez-Antoran, D., Herms, A., et al. (2020). A single-progenitor model as the unifying paradigm of epidermal and esophageal epithelial maintenance in mice. Nat. Commun. 11:1429. doi: 10.1038/s41467-020-15258-0
Poghosyan, T., Gaujoux, S., Vanneaux, V., Bruneval, P., Domet, T., Lecourt, S., et al. (2013). In vitro development and characterization of a tissue-engineered conduit resembling esophageal wall using human and pig skeletal myoblast, oral epithelial cells, and biologic scaffolds. Tissue Eng. Part A 19, 2242–2252. doi: 10.1089/ten.tea.2012.0565
Potten, C. S. (1974). The epidermal proliferative unit: the possible role of the central basal cell. Cell Prolif. 7, 77–88. doi: 10.1111/j.1365-2184.1974.tb00401.x
Pringle, S., Maimets, M., van der Zwaag, M., Stokman, M. A., van Gosliga, D., Zwart, E., et al. (2016). Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 34, 640–652. doi: 10.1002/stem.2278
Qi, S., Yang, C., Zhu, M., and Chen, H. (2019). Effect of oral mucosal transplantation on the expression of EGF and VEGF−C during skin wound repair. Exp. Ther. Med. 18, 320–325. doi: 10.3892/etm.2019.7546
Que, J., Okubo, T., Goldenring, J. R., Nam, K. T., Kurotani, R., Morrisey, E. E., et al. (2007). Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134, 2521–2531. doi: 10.1242/dev.003855
Quinlan, R., Hutchison, C., and Lane, B. (1994). Intermediate filament proteins. Protein Profile 1, 779–911.
Quiroz, F. G., Fiore, V. F., Levorse, J., Polak, L., Wong, E., Pasolli, H. A., et al. (2020). Liquid-liquid phase separation drives skin barrier formation. Science 367:6483. doi: 10.1126/science.aax9554
Ren, K. (2019). Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology 107, 271–284. doi: 10.1007/s10266-018-0395-9
Reynolds, A. J., and Jahoda, C. A. B. (1991). Inductive properties of hair follicle cells. Ann. N. Y. Acad. Sci. 641, 226–241. doi: 10.1111/j.1749-6632.1991.tb24390.x
Rinkevich, Y., Walmsley, G. G., Hu, M. S., Maan, Z. N., Newman, A. M., Drukker, M., et al. (2015). Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348:aaa2151. doi: 10.1126/science.aaa2151
Rivera-Gonzalez, G., Shook, B., and Horsley, V. (2014). Adipocytes in skin health and disease. Cold Spring Harb. Perspect. Med. 4:3. doi: 10.1101/cshperspect.a015271
Rognoni, E., Gomez, C., Pisco, A. O., Rawlins, E. L., Simons, B. D., Watt, F. M., et al. (2016). Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 143, 2522–2535. doi: 10.1242/dev.131797
Rognoni, E., Pisco, A. O., Hiratsuka, T., Sipilä, K. H., Belmonte, J. M., Mobasseri, S. A., et al. (2018). Fibroblast state switching orchestrates dermal maturation and wound healing. Mol. Syst. Biol. 14:8. doi: 10.15252/msb.20178174
Roh, J. L., Lee, J., Kim, E. H., and Shin, D. (2017). Plasticity of oral mucosal cell sheets for accelerated and scarless skin wound healing. Oral Oncol. 75, 81–88. doi: 10.1016/j.oraloncology.2017.10.024
Rompolas, P., Mesa, K. R., Kawaguchi, K., Park, S., Gonzalez, D., Brown, S., et al. (2016). Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science 352, 1471–1474. doi: 10.1126/science.aaf7012
Roopashree, M. R., Gondhalekar, R. V., Shashikanth, M. C., George, J., Thippeswamy, S. H., and Shukla, A. (2010). Pathogenesis of oral lichen planus – a review. J. Oral Pathol. Med. 39, 729–734. doi: 10.1111/j.1600-0714.2010.00946.x
Rosekrans, S. L., Baan, B., Muncan, V., and Van Den Brink, G. R. (2015). Esophageal development and epithelial homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G216–G228. doi: 10.1152/ajpgi.00088.2015
Roshan, A., Murai, K., Fowler, J., Simons, B. D., Nikolaidou-Neokosmidou, V., and Jones, P. H. (2016). Human keratinocytes have two interconvertible modes of proliferation. Nat. Cell Biol. 18, 145–156. doi: 10.1038/ncb3282
Rothova, M., Thompson, H., Lickert, H., and Tucker, A. S. (2012). Lineage tracing of the endoderm during oral development. Dev. Dyn. 241, 1183–1191. doi: 10.1002/dvdy.23804
Rowlatt, U. (1979). Intrauterine wound healing in a 20 week human fetus. Virchows Arch. A Pathol. Anat. Histopathol. 381, 353–361. doi: 10.1007/BF00432477
Roy, E., Neufeld, Z., Cerone, L., Wong, H. Y., Hodgson, S., Livet, J., et al. (2016). Bimodal behaviour of interfollicular epidermal progenitors regulated by hair follicle position and cycling. EMBO J. 35, 2658–2670. doi: 10.15252/embj.201693806
Rushmer, R. F., Buettner, K. J., Short, J. M., and Odland, G. F. (1966). The skin. Am. Assoc. Adv. Sci. 154, 343–348. doi: 10.1177/002205741508100313
Sa, G., Liu, Z., Ren, J., Wan, Q., Xiong, X., Yu, Z., et al. (2019). Keratinocyte growth factor (KGF) induces podosome formation via integrin-Erk1/2 signaling in human immortalized oral epithelial cells. Cell. Signal. 61, 39–47. doi: 10.1016/j.cellsig.2019.05.007
Sada, A., Jacob, F., Leung, E., Wang, S., White, B. S., and Tumbar, T. (2016). Defining the cellular lineage hierarchy in the interfollicular epidermis of adult skin. Nat. Cell Biol. 18, 619–631. doi: 10.1038/ncb3359.Defining
Sakurai, T., Miyazaki, S., Miyata, G., Satomi, S., and Hori, Y. (2007). Autologous buccal keratinocyte implantation for the prevention of stenosis after emr of the esophagus. Gastrointest. Endosc. 66, 167–173. doi: 10.1016/j.gie.2006.12.062
Sánchez-Danés, A., Hannezo, E., Larsimont, J. C., Liagre, M., Youssef, K. K., Simons, B. D., et al. (2016). Defining the clonal dynamics leading to mouse skin tumour initiation. Nature 536, 298–303. doi: 10.1038/nature19069
Sanz, M., Dahlin, C., Apatzidou, D., Artzi, Z., Bozic, D., Calciolari, E., et al. (2019). Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: consensus report of group 2 of the 15th european workshop on periodontology on bone regeneration. J. Clin. Periodontol. 46(Suppl. 21), 82–91. doi: 10.1111/jcpe.13123
Sasaki, K., Fujita, Y., Imai, Y., Oshima, J., Sasaki, M., Aihara, Y., et al. (2020). Total upper and lower eyelid reconstruction using periosteal flap canthoplasty combined with auricular cartilage and oral mucosa grafts. Eur. J. Plast. Surg. 43, 83–86. doi: 10.1007/s00238-019-01556-4
Schafer, I. A., Pandy, M., Ferguson, R., and Davis, B. R. (1985). Comparative observation of fibroblasts derived from the papillary and reticular dermis of infants and adults: growth kinetics, packing density at confluence and surface morphology. Mech. Ageing Dev. 31, 275–293. doi: 10.1016/0047-6374(85)90095-8
Schepeler, T., Page, M. E., and Jensen, K. B. (2014). Heterogeneity and plasticity of epidermal stem cells. Development 141, 2559–2567. doi: 10.1242/dev.104588
Schramm, V. L., and Myers, E. N. (1980). Skin grafts in oral cavity reconstruction. Arch. Otolaryngol. 106, 528–532. doi: 10.1001/archotol.1980.00790330008005
Schramm, V. L., Johnson, J. T., and Myers, E. N. (1983). Skin grafts and flaps in oral cavity reconstruction. Arch. Otolaryngol. 109, 175–177. doi: 10.1001/archotol.1983.00800170041011
Schrementi, M. E., Ferreira, A. M., Zender, C., and DiPietro, L. A. (2008). Site-specific production of TGF-β in oral mucosal and cutaneous wounds. Wound Repair Regen. 16, 80–86. doi: 10.1111/j.1524-475X.2007.00320.x
Sebastian, P., Thomas, S., Varghese, B. T., Iype, E. M., Balagopal, P. G., and Mathew, P. C. (2008). The submental island flap for reconstruction of intraoral defects in oral cancer patients. Oral Oncol. 44, 1014–1018. doi: 10.1016/j.oraloncology.2008.02.013
Senoo, M., Pinto, F., Crum, C. P., and McKeon, F. (2007). P63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129, 523–536. doi: 10.1016/j.cell.2007.02.045
Seo, B. M., Miura, M., Gronthos, S., Bartold, P. M., Batouli, S., Brahim, J., et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149–155. doi: 10.1016/S0140-6736(04)16627-0
Sequeira, I., and Nicolas, J. F. (2012). Redefining the structure of the hair follicle by 3D clonal analysis. Development 139, 3741–3751. doi: 10.1242/dev.081091
Sequeira, I., Neves, J. F., Carrero, D., Peng, Q., Palasz, N., Liakath-Ali, K., et al. (2018). Immunomodulatory role of keratin 76 in oral and gastric cancer. Nat. Commun. 9:3437. doi: 10.1038/s41467-018-05872-4
Shannon, D. B., McKeown, S. T. W., Lundy, F. T., and Irwin, C. R. (2006). Phenotypic differences between oral and skin fibroblasts in wound contraction and growth factor expression. Wound Repair Regen. 14, 172–178. doi: 10.1111/j.1743-6109.2006.00107.x
Shaw, T. J., and Martin, P. (2009). Wound repair at a glance. J. Cell Sci. 122, 3209–3213. doi: 10.1242/jcs.031187
Shaw, T. J., Kishi, K., and Mori, R. (2010). Wound-associated skin fibrosis: mechanisms and treatments based on modulating the inflammatory response. Endocr. Metab. Immune Disord. Drug Targets 10, 320–330. doi: 10.2174/1871530311006040320
Shi, Q., Huo, N., Wang, X., Yang, S., Wang, J., and Zhang, T. (2020). Exosomes from oral tissue stem cells: biological effects and applications. Cell Biosci. 10:108. doi: 10.1186/s13578-020-00471-7
Shi, Q., Qian, Z., Liu, D., Sun, J., Wang, X., Liu, H., et al. (2017). GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front. Physiol. 8:904. doi: 10.3389/fphys.2017.00904
Shiehzadeh, V., Aghmasheh, F., Shiehzadeh, F., Joulae, M., Kosarieh, E., and Shiehzadeh, F. (2014). Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: new method and three case reports. Indian J. Dent. Res. 25, 248–253. doi: 10.4103/0970-9290.135937
Shyer, A. E., Rodrigues, A. R., Schroeder, G. G., Kassianidou, E., and Harland, R. M. (2018). Initiate follicle pattern in the avian skin. Science 357, 811–815.
Simões, A., Chen, L., Chen, Z., Zhao, Y., Gao, S., Marucha, P. T., et al. (2019). Differential microRNA profile underlies the divergent healing responses in skin and oral mucosal wounds. Sci. Rep. 9:7160. doi: 10.1038/s41598-019-43682-w
Simonato, A., Gregori, A., Ambruosi, C., Venzano, F., Varca, V., Romagnoli, A., et al. (2008). Lingual mucosal graft urethroplasty for anterior urethral reconstruction. Eur. Urol. 54, 79–87. doi: 10.1016/j.eururo.2008.01.023
Simsek, A., Bullock, A. J., Roman, S., Chapple, C. R., and MacNeil, S. (2018). Developing improved tissue-engineered buccal mucosa grafts for urethral reconstruction. Can. Urol. Assoc. J. 12, E234–E242. doi: 10.5489/cuaj.4826
Singh, M., Nuutila, K., Kruse, C., Robson, M. C., Caterson, E., and Eriksson, E. (2015). Challenging the conventional therapy: emerging skin graft techniques for wound healing. Plast. Reconstr. Surg. 136, 524e–530e. doi: 10.1097/PRS.0000000000001634
Singh, S., and Gupta, P. D. (1994). Tampering with cytokeratin expression results in cell dysfunction. Epithelial Cell Biol. 3, 79–83.
Sjöqvist, S., Ishikawa, T., Shimura, D., Kasai, Y., Imafuku, A., Bou-Ghannam, S., et al. (2019). Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J. Extracell. Vesicles 8:1. doi: 10.1080/20013078.2019.1565264
Snippert, H. J., Haegebarth, A., Kasper, M., Jaks, V., Van Es, J. H., Barker, N., et al. (2010a). Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389. doi: 10.1126/science.1184733
Snippert, H. J., van der Flier, L. G., Sato, T., van Es, J. H., van den Born, M., Kroon-Veenboer, C., et al. (2010b). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144. doi: 10.1016/j.cell.2010.09.016
Sorrell, J. M., and Caplan, A. I. (2004). Fibroblast heterogeneity: more than skin deep. J. Cell Sci. 117(Pt 5), 667–675. doi: 10.1242/jcs.01005
Sorrell, J. M., Baber, M. A., and Caplan, A. I. (1996). Construction of a bilayered dermal equivalent containing human papillary and reticular dermal fibroblasts: use of fluorescent vital dyes. Tissue Eng. 2, 39–50. doi: 10.1089/ten.1996.2.39
Sorrell, J. M., Carrino, D. A., Baber, M. A., Assclineau, D., and Caplan, A. I. (1999). A monoclonal antibody which recognizes a glycosaminoglycan epitope in both dermatan sulfate and chondroitin sulfate proteoglycans of human skin. Histochem. J. 31, 549–558. doi: 10.1023/A:1003896124595
Squier, C. A. (1977). Membrane coating granules in nonkeratinizing oral epithelium. J. Ultrastruct. Res. 60, 212–220. doi: 10.1016/s0022-5320(77)80066-x
Squier, C. A., and Kremer, M. J. (2001). Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 52242, 7–15. doi: 10.1093/oxfordjournals.jncimonographs.a003443
Sriram, G., Bigliardi, P. L., and Bigliardi-Qi, M. (2015). Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur. J. Cell Biol. 94, 483–512. doi: 10.1016/j.ejcb.2015.08.001
Stephens, P., Davies, K. J., Occleston, N., Pleass, R. D., Kon, C., Daniels, J., et al. (2001). Skin and oral fibroblasts exhibit phenotypic differences in extracellular matrix reorganization and matrix metalloproteinase activity. Br. J. Dermatol. 144, 229–237. doi: 10.1046/j.1365-2133.2001.04006.x
Su, Y., Chen, C., Guo, L., Du, J., Li, X., and Liu, Y. (2018). Ecological balance of oral microbiota is required to maintain oral mesenchymal stem cell homeostasis. Stem Cells 36, 551–561. doi: 10.1002/stem.2762
Surendran, S., and Sivamurthy, G. (2015). Current applications and future prospects of stem cells in dentistry. Dent. Update 42, 556–561. doi: 10.12968/denu.2015.42.6.556
Szpaderska, A. M., Zuckerman, J. D., and DiPietro, L. A. (2003). Differential injury responses in oral mucosal and cutaneous wounds. J. Dent. Res. 82, 621–626. doi: 10.1177/154405910308200810
Tabib, T., Morse, C., Wang, T., Chen, W., and Lafyatis, R. (2018). SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J. Invest. Dermatol. 138, 802–810. doi: 10.1016/j.jid.2017.09.045
Tabola, R., Augoff, K., Lewandowski, A., Ziolkowski, P., Szelachowski, P., and Grabowski, K. (2016). Esophageal anastomosis how the granulation phase of wound healing improves the incidence of anastomotic leakage. Oncol. Lett. 12, 2038–2044. doi: 10.3892/ol.2016.4873
Takagi, R., Murakami, D., Kondo, M., Ohki, T., Sasaki, R., Mizutani, M., et al. (2010). Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest. Endosc. 72, 1253–1259. doi: 10.1016/j.gie.2010.08.007
Tanaka, T., Komai, Y., Tokuyama, Y., Yanai, H., Ohe, S., Okazaki, K., et al. (2013). Identification of stem cells that maintain and regenerate lingual keratinized epithelial cells. Nat. Cell Biol. 15, 511–518. doi: 10.1038/ncb2719
Tang, X. H., Scognamiglio, T., and Gudas, L. J. (2013). Basal stem cells contribute to squamous cell carcinomas in the oral cavity. Carcinogenesis 34, 1158–1164. doi: 10.1093/carcin/bgt021
Tarnawski, A. S., and Ahluwalia, A. (2012). Molecular mechanisms of epithelial regeneration and neovascularization during healing of gastric and esophageal ulcers. Curr. Med. Chem. 19, 16–27. doi: 10.2174/092986712803414088
Thomson, P. (2020). Back to the future: revisiting oral carcinogenesis, stem cells and epithelial cell proliferation. Fac. Dent. J. 11, 30–34. doi: 10.1308/rcsfdj.2020.30
Timpl, R., and Brown, J. C. (1996). Supramolecular assembly of basement membranes. Bioessays 18, 123–132.
Treuting, P. M., Dintzis, S. M., Liggitt, D., and Frevert, C. W. (eds) (2012). Comparative Anatomy and Histology: A Mouse and Human Atlas (Expert Consult). Cambridge, MA: Academic Press.
Turabelidze, A., Guo, S., Chung, A. Y., Chen, L., Dai, Y., Marucha, P. T., et al. (2014). Intrinsic differences between oral and skin keratinocytes. PLoS One 9:e101480. doi: 10.1371/journal.pone.0101480
Ud-Din, S., and Bayat, A. (2020). Keloid scarring or disease: unresolved quasi-neoplastic tendencies in the human skin. Wound Repair Regen. 28, 422–426. doi: 10.1111/wrr.12793
Vassar, R., Coulombe, P. A., Degenstein, L., Albers, K., and Fuchs, E. (1991). Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell 64, 365–380. doi: 10.1016/0092-8674(91)90645-F
Velnar, T., Bailey, T., and Smrkolj, V. (2009). The wound healing process: an overview of the cellular and molecular mechanisms. Int. J. Med. Res. 37, 1528–1542. doi: 10.1177/147323000903700531
Vescarelli, E., Pilloni, A., Dominici, F., Pontecorvi, P., Angeloni, A., Polimeni, A., et al. (2017). Autophagy activation is required for myofibroblast differentiation during healing of oral mucosa. J. Clin. Periodontol. 44, 1039–1050. doi: 10.1111/jcpe.12767
Vining, K. H., and Mooney, D. J. (2017). Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 18, 728–742. doi: 10.1038/nrm.2017.108
Volponi, A. A., Kawasaki, M., and Sharpe, P. T. (2013). Adult human gingival epithelial cells as a source for whole-tooth bioengineering. J. Dent. Res. 92, 329–334. doi: 10.1177/0022034513481041
Vural, E., and Suen, J. Y. (2000). The submental island flap in head and neck reconstruction. Head Neck 22, 572–578. doi: 10.1002/1097-0347(200009)22:6<572::AID-HED5<3.0.CO;2-K
Wan, L., Jiang, D., Correa-Gallegos, D., Ramesh, P., Zhao, J., Ye, H., et al. (2021). Connexin43 gap junction drives fascia mobilization and repair of deep skin wounds. Matrix Biol. 97, 58–71. doi: 10.1016/j.matbio.2021.01.005
Wang, B., Kovalchuk, A., Li, D., Rodriguez-Juarez, R., Ilnytskyy, Y., Kovalchuk, I., et al. (2020). In search of preventive strategies: novel high-CBD cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. PrePrints [Preprint] doi: 10.20944/preprints202004.0315.v1
Wang, H., Chen, Z., Li, X. J., Ma, L., and Tang, Y. L. (2015). Anti-inflammatory cytokine TSG-6 inhibits hypertrophic scar formation in a rabbit ear model. Eur. J. Pharmacol. 751, 42–49. doi: 10.1016/j.ejphar.2015.01.040
Wang, S., Drummond, M. L., Guerrero-Juarez, C. F., Tarapore, E., MacLean, A. L., Stabell, A. R., et al. (2020). Single cell transcriptomics of human epidermis identifies basal stem cell transition states. Nat. Commun. 11:4239. doi: 10.1038/s41467-020-18075-7
Watt, F. M. (2014). Mammalian skin cell biology: at the interface between laboratory and clinic. Science 346, 937–940. doi: 10.1126/science.1253734
Watt, F. M., and Fujiwara, H. (2011). Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 3:a005124. doi: 10.1101/cshperspect.a005124
Wells, J. M., and Melton, D. (1999). Vertebrate endoderm development. Annu. Rev. Cell Dev. Biol. 15, 393–410.
Wilgus, T. A., Ferreira, A. M., Oberyszyn, T. M., Bergdall, V. K., and DiPietro, L. A. (2008). Regulation of scar formation by vascular endothelial growth factor. Lab. Invest. 88, 579–590. doi: 10.1038/labinvest.2008.36
Williams, D. W., Greenwell-Wild, T., Brenchley, L., Dutzan, N., Overmiller, A., Sawaya, A. P., et al. (2021). Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell 184, 1–15. doi: 10.1016/j.cell.2021.05.013
Worthen, C. A., Cui, Y., Orringer, J. S., Johnson, T. M., Voorhees, J. J., and Fisher, G. J. (2020). CD26 identifies a subpopulation of fibroblasts that produce the majority of collagen during wound healing in human skin. J. Invest. Dermatol. 140, 2515.e–2524.e. doi: 10.1016/j.jid.2020.04.010
Wright, J. T. (2010). Oral manifestations in the epidermolysis bullosa spectrum. Dermatol. Clin. 28, 159–164. doi: 10.1016/j.det.2009.10.022
Xiao, L., and Tsutsui, T. (2012). Three-dimensional epithelial and mesenchymal cell co-cultures form early tooth epithelium invagination-like structures: expression patterns of relevant molecules. J. Cell. Biochem. 113, 1875–1885. doi: 10.1002/jcb.24056
Xiong, X., Jia, J., He, S., and Zhao, Y. (2010). Cryopreserved lip mucosa tissue derived keratinocytes can fabricate tissue engineered palatal mucosa equivalent. J. Biomed. Mater. Res. Part B Appl. Biomater. 94, 165–170. doi: 10.1002/jbm.b.31637
Xue, M., and Jackson, C. J. (2015). Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 4, 119–136. doi: 10.1089/wound.2013.0485
Yaguchi, K., Fujita, K., Noguchi, M., Nagai, F., and Yuzuriha, S. (2021). The palatal fistula closure using buccal fat graft after palatoplasty for cleft palate: two case reports. Cleft Palate Craniofac. J. doi: 10.1177/10556656211007000 [Epub ahead of print].
Yamaguchi, N., Isomoto, H., Kobayashi, S., Kanai, N., Kanetaka, K., Sakai, Y., et al. (2017). Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci. Rep. 7:17460. doi: 10.1038/s41598-017-17663-w
Yamasaki, Y., Ozawa, S., Oguma, J., Kazuno, A., and Ninomiya, Y. (2016). Long peptic strictures of the esophagus due to reflux esophagitis: a case report. Surg. Case. Rep. 2:64. doi: 10.1186/s40792-016-0190-1
Yang, D., Othman, M., and Draganov, P. V. (2019). Endoscopic mucosal resection vs endoscopic submucosal dissection for Barrett’s esophagus and colorectal neoplasia. Clin. Gastroenterol. Hepatol. 17, 1019–1028. doi: 10.1016/j.cgh.2018.09.030
Yang, H., Adam, R. C., Ge, Y., Hua, Z. L., and Fuchs, E. (2017). Epithelial-mesenchymal micro-niches govern stem cell lineage choices. Cell 169, 483–496.e13. doi: 10.1016/j.cell.2017.03.038
Yoshizawa, M., Feinberg, S. E., Marcelo, C. L., and Elner, V. M. (2004). Ex vivo produced human conjunctiva and oral mucosa equivalents grown in a serum-free culture system. J. Oral Maxillofac. Surg. 62, 980–988. doi: 10.1016/j.joms.2004.02.010
Yu, G., Okawa, H., Okita, K., Kamano, Y., Wang, F., Saeki, M., et al. (2016). Gingival fibroblasts as autologous feeders for induced pluripotent stem cells. J. Dent. Res. 95, 110–118. doi: 10.1177/0022034515611602
Yuan, X., Xu, Q., Zhang, X., Van Brunt, L. A., Ticha, P., and Helms, J. A. (2019). Wnt-responsive stem cell fates in the oral mucosa. IScience 21, 84–94. doi: 10.1016/j.isci.2019.10.016
Yudintceva, N. M., Nashchekina, Y. A., Shevtsov, M. A., Mikhailova, N. A., Vinogradova, T. I., Gorelova, A. A., et al. (2020). Application of tissue engineering construct seeded with buccal epithelium cells for replacement urethroplasty. Cell Tissue Biol. 14, 481–491. doi: 10.1134/S1990519X20060103
Zelles, T., Purushotham, K. R., Macauley, S. P., Oxford, G. E., and Humphreys-Beher, M. G. (1995). Concise review: saliva and growth factors: the fountain of youth resides in us all. J. Dent. Res. 74, 1826–1832. doi: 10.1177/00220345950740120301
Zhang, C., Zhang, Y., Feng, Z., Zhang, F., Liu, Z., Sun, X., et al. (2018). Therapeutic effect of dental pulp stem cell transplantation on a rat model of radioactivity-induced esophageal injury. Cell Death Dis. 9, 1–13. doi: 10.1038/s41419-018-0753-0
Zhang, Q., Tian, Y., Ding, T., and Femg, D. (2021). A preliminary study of constructing tissue engineered oral mucosa material and methods. Research Square [Preprint] doi: 10.21203/rs.3.rs-299660/v1
Zhang, Y., Jiang, M., Kim, E., Lin, S., Liu, K., Lan, X., et al. (2017). Development and stem cells of the esophagus. Semin. Cell Dev. Biol. 66, 25–35. doi: 10.1016/j.semcdb.2016.12.008
Zheng, G., Huang, R., Qiu, G., Ge, M., Wang, J., Shu, Q., et al. (2018). Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 374, 1–15. doi: 10.1007/s00441-018-2871-5
Zhong, M., Lin, B., Pathak, J. L., Gao, H., Young, A. J., Wang, X., et al. (2020). ACE2 and furin expressions in oral epithelial cells possibly facilitate COVID-19 infection via respiratory and fecal–oral routes. Front. Med. 7:580796. doi: 10.3389/fmed.2020.580796
Zimmermann, D. R., Dours-Zimmermann, M. T., Schubert, M., and Bruckner-Tuderman, L. (1994). Versican is expressed in the proliferating zone in the epidermis and in association with the elastic network of the dermis. J. Cell Biol. 124, 817–825. doi: 10.1083/jcb.124.5.817
Zomer, H. D., and Trentin, A. G. (2018). Skin wound healing in humans and mice: challenges in translational research. J. Dermatol. Sci. 90, 3–12. doi: 10.1016/j.jdermsci.2017.12.009
Keywords: oral mucosa, oesophagus, skin, homeostasis, wound repair, regenerative therapy, tissue engineering, regenerative medicine
Citation: Pereira D and Sequeira I (2021) A Scarless Healing Tale: Comparing Homeostasis and Wound Healing of Oral Mucosa With Skin and Oesophagus. Front. Cell Dev. Biol. 9:682143. doi: 10.3389/fcell.2021.682143
Received: 17 March 2021; Accepted: 24 June 2021;
Published: 26 July 2021.
Edited by:
Kai Kretzschmar, University Hospital Würzburg, GermanyReviewed by:
Ophir D. Klein, University of California, San Francisco, United StatesCopyright © 2021 Pereira and Sequeira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Inês Sequeira, aS5zZXF1ZWlyYUBxbXVsLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.