- 1Department of General Surgery, Pancreatic Disease Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Research Institute of Pancreatic Diseases, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of General Surgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background: For pancreatic ductal adenocarcinoma (PDAC) patients, chemotherapy failure is the major reason for postoperative recurrence and poor outcomes. Establishment of novel biomarkers and models for predicting chemotherapeutic efficacy may provide survival benefits by tailoring treatments.
Methods: Univariate cox regression analysis was employed to identify EMT-related genes with prognostic potential for DFS. These genes were subsequently submitted to LASSO regression analysis and multivariate cox regression analysis to identify an optimal gene signature in TCGA training cohort. The predictive accuracy was assessed by Kaplan–Meier (K-M), receiver operating characteristic (ROC) and calibration curves and was validated in PACA-CA cohort and our local cohort. Pathway enrichment and function annotation analyses were conducted to illuminate the biological implication of this risk signature.
Results: LASSO and multivariate Cox regression analyses selected an 8-gene signature comprised DLX2, FGF9, IL6R, ITGB6, MYC, LGR5, S100A2, and TNFSF12. The signature had the capability to classify PDAC patients with different DFS, both in the training and validation cohorts. It provided improved DFS prediction compared with clinical indicators. This signature was associated with several cancer-related pathways. In addition, the signature could also predict the response to immune-checkpoint inhibitors (ICIs)-based immunotherapy.
Conclusion: We established a novel EMT-related gene signature that was capable of predicting therapeutic response to adjuvant chemotherapy and immunotherapy. This signature might facilitate individualized treatment and appropriate management of PDAC patients.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly malignant and devastating disease with a 5 years survival rate not exceeding 10% and its incidence increases about 1% per year in the United States (Siegel et al., 2021). The dismal outcome of this malignancy is primarily due to a frequently late diagnosis, mostly at the metastatic and unresectable stage, and the notorious chemoresistance (Kamisawa et al., 2016). Surgery combined with adjuvant chemotherapy is the established therapy option for resectable PDAC patients (Mizrahi et al., 2020). Unfortunately, early postoperative recurrence in most patients caused by the inherent resistance to adjuvant chemotherapy limits the dramatic improvement of patient survival (Kleeff et al., 2016). Currently, adjuvant chemotherapy is administrated empirically, and individual survival benefit of this approach is still questionable. In PDAC patients, the clinical benefit response rates to regimens of chemotherapy are extremely low (Han et al., 2021). Non-responding patients are likely to suffer a variety of adverse events including asthenia and nausea (Phua et al., 2018). These intractable issues have motivated a number of groups to identify robust biomarkers that can predict therapeutic response to chemotherapy in PDAC patients (Kyrochristos et al., 2018).
As precision medicine has shown promising signs, a priori prediction of treatment response may facilitate individual management and maximize survival benefit of PDAC patients (Tu et al., 2016). Multiple studies have reported that a treatment-related decrease in serum CA19-9 can predict response to treatment (Xu et al., 2018; Aoki et al., 2019; Perri et al., 2020, 2021). Pre-clinical and clinical evidence demonstrates that patients with specific PDAC subtypes response differently to available treatments (Collisson et al., 2011; Aung et al., 2018). Several genes participating in drug uptake and metabolism have emerged as powerful predictors of drug sensitivity (Bird et al., 2017; Raffenne et al., 2019; Okamura et al., 2020). Recently, with the adventure of high throughput sequencing and bioinformatic technology, more and more gene expression signatures have been identified to evaluate drug sensitivity in PDAC (Kaissis et al., 2019; Clayton et al., 2020; Piquemal et al., 2020; Nicolle et al., 2021; Nishiwada et al., 2021).
Epithelial to mesenchymal transition (EMT) program is related to phenotypic conversion of epithelial cells into more aggressive mesenchymal-like cells and suppression of EMT results in enhanced gemcitabine sensitivity in PDAC mice (Zheng et al., 2015). Compelling evidence has proved a strong association between EMT-related gene expression and therapeutic resistance (Shibue and Weinberg, 2017). For instance, Byres et al. constructed a 76 gene signature based on EMT-related genes with satisfactory accuracy in predicting clinical response to EGFR and PI3K inhibitors for patients with non-small-cell lung carcinoma (Byers et al., 2013). These studies suggest EMT represents an under-explored source of credible biomarkers that could be used to predict drug response.
We purposed to establish a model for predicting response to adjuvant chemotherapy based on EMT-related genes in PDAC. We measured the association between EMT-related genes and disease-free survival (DFS), and established an 8-gene signature with excellent predictive performance in both training and validation datasets. Functionally, this signature is closely related to several pathways involved in drug response. Interestingly, we found that this signature also had potential to predict response to immune-checkpoint inhibitors (ICIs). These findings may facilitate personalized treatment and may potentially exempt patients from heavy finical burden and unnecessary adverse effects of overtreatment.
Materials and Methods
PDAC Cohorts
Two public PDAC cohorts were included in this study. Among them, TCGA cohort was used as the training set, while PACA-CA cohort was used for external validation. Processed RNA-sequencing data and corresponding clinical data of TCGA cohort were downloaded from TCGA hub at UCSC Xena1. In the cases of PACA-CA cohort, normalized RNA-sequencing data and clinical information were retrieved and downloaded from the International Cancer Genome Consortium (ICGC)2 database. In each cohort, the following criteria were used to exclude unqualified samples: (a) follow-up time < 1 month; (b) lack of survival and therapeutic data; (c) histopathological type is not PDAC. After a careful review, 99 samples in TCGA cohort and 105 samples in PACA-CA cohort were included in this study. All patients received adjuvant chemotherapy in both cohorts, and detail of chemotherapeutic drugs was only available in TCGA cohort. Patients whose response to chemotherapy is “clinical progressive disease” or “stable disease” were defined as chemotherapy-resistant, while patients whose response to chemotherapy is “complete response” or “partial response” were defined as chemotherapy-sensitive. Given the medium size of the cohorts we used, we additionally verified the EMT signature in our own cohort (Ruijin cohort). 48 PDAC frozen samples were collected as previously reported (Feng et al., 2020).
Construction of the EMT-Related Gene Signature for DFS Prediction
A total of 1,184 EMT-related genes were obtained from a previous article (Cai et al., 2020). In the TCGA training cohort, EMT-related genes that were significantly associated with DFS were screened using univariate cox regression analysis (P < 0.01). Subsequently, LASSO regression combined with multivariate cox regression analyses were used to determine the optimal risk model. The risk score was calculated as follows: Risk score = (coefficient 1 ∗ expression value of gene 1) + (coefficient 2 ∗ expression value of gene 2) + . + (coefficient X ∗ expression value of gene X).
Predictive Performance of the EMT-Related Gene Signature
Patients in each cohort were classified into low- and high-risk groups based on the medium value of risk scores. Kaplan–Meier (K-M) survival curves were employed to evaluate the DFS differences between low- and high-risk groups. Calibration plots comparing the predicted and observed survival probabilities were performed to assess the predictive accuracy. Receiver operating characteristic (ROC) curves were utilized to compare the efficiency of the signature with that of clinical predictors for DFS prediction. In addition, univariate and multivariate cox regression analyses were utilized to verify the independent prognostic role of the signature.
Functional Annotation and Pathway Enrichment
Aiming to clarify the biological function of the EMT signature, we conducted Pearson correlation analysis to identify genes whose expression levels were significantly (P < 0.05) correlated with risk scores in TCGA training cohort. Top 1,000 positively and negatively correlated genes were, respectively, submitted to Gene Ontology (GO) analysis and The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis on DAVID online website (Huang et al., 2007).
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
Reverse transcription and qRT-PCR were performed as previously reported (Feng et al., 2020). The mRNA primer sequences are displayed in Supplementary Table 1.
Statistical Analysis
The statistical analysis and graphical work were done in the R environment (version 3.5.2). cox regression analyses were conducted by the “survival” package. K-M survival curves with log-rank tests were produced by the “survminer” package. LASSO regression analysis was done by the “glmnet” package. The ROC curves were plotted by the “survivalROC” package. Boxplots were depicted by the “ggpubr” package. Forest plot was derived from the “forestplot” package. Calibration curves were generated from the “rms” package. A two-sided log-rank P < 0.05 was considered significant.
Results
Construction of the EMT-Related Gene Signature
With the selection criteria of p < 0.01, a total of 35 credibly prognostic EMT-related genes were identified through the univariate cox regression analysis in the TCGA training cohort. The LASSO regression algorithm was subsequently applied, and 16 candidate genes with most powerful predictive features were screened (Figures 1A,B). Then, multivariate cox regression analysis was performed on the 16 genes to avoid overfitting, and it finally determined an optimal 8-gene signature for DFS prediction (Figure 1C). Based on the expression levels and corresponding coefficients of these eight genes, we constructed a risk-score formula: Risk score = (0.30407 × expression value of DLX2) − (0.24245 × expression value of FGF9) − (0.40586 × expression value of IL6R) + (0.214597 × expression value of ITGB6) − (0.15683 × expression value of LGR5) + (0.638384 × expression value of MYC) − (0.12315 × expression value of S100A2) − (0.44785 × expression value of TNFSF12). K-M analysis illustrated that these eight individual genes adequately captured the DFS differences between low- and high-expression groups in the TCGA cohort (Supplementary Figure 1). In addition, the risk scores of chemotherapy-resistant patients were significantly higher than those of chemotherapy-sensitive patients, indicating the hazardous role of the signature (Figure 1D).
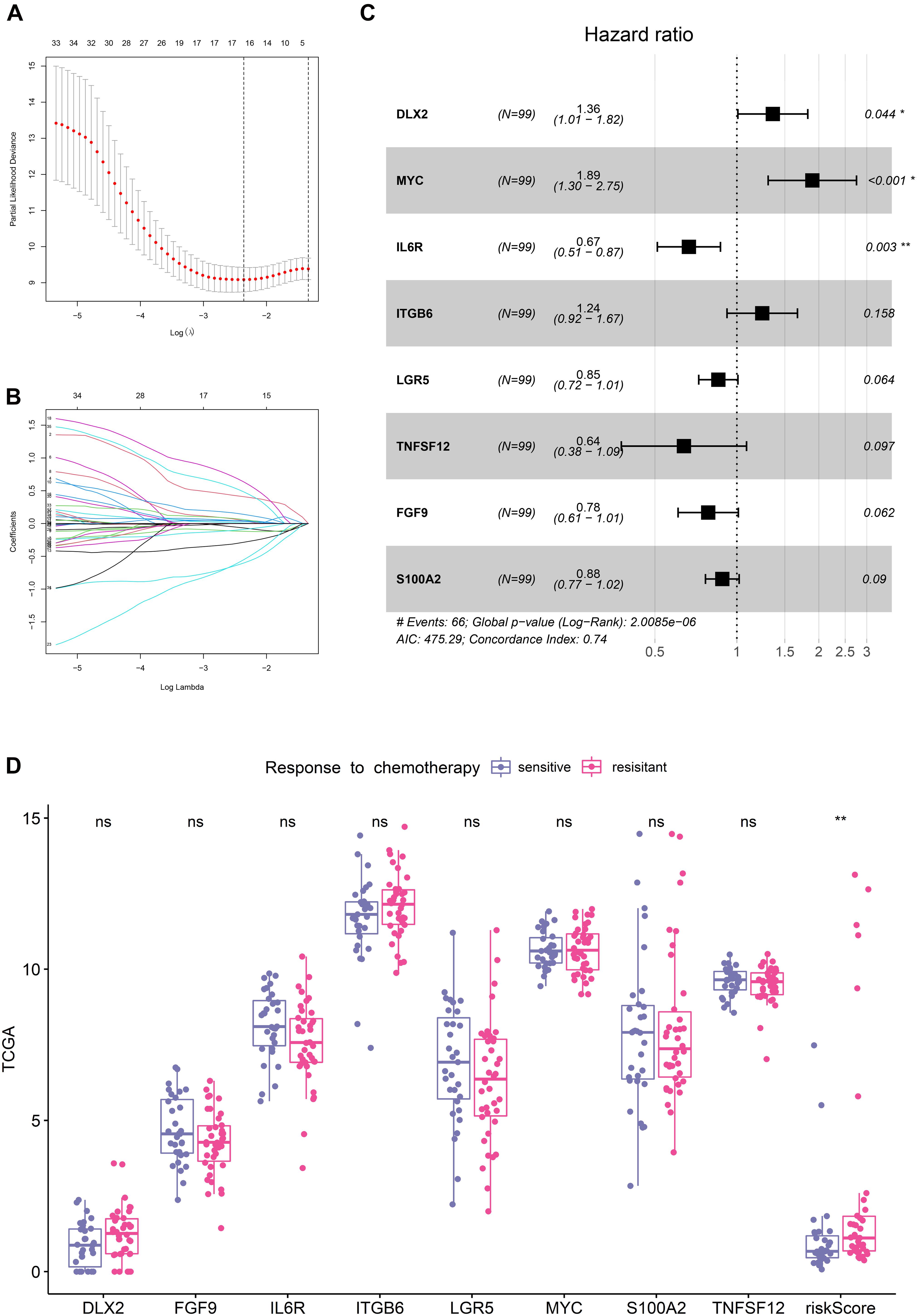
Figure 1. Establishment of the EMT-related gene signature in TCGA training cohort. (A) Cross-validation for tuning parameter (lambda) screening in the LASSO regression model. (B) LASSO coefficient profiles of 35 prognostic EMT-related genes. (C) Forest plot of the eight EMT-related genes. (D) Distribution of the eight genes and risk scores in patients stratified by the chemotherapy sensitivity.
Predictive Performance of the EMT-Related Gene Signature in TCGA Training Cohort
The distribution of the risk scores and survival status were shown in Figure 2A. The results suggested that patients in high-risk group had remarkably decreased DFS time.
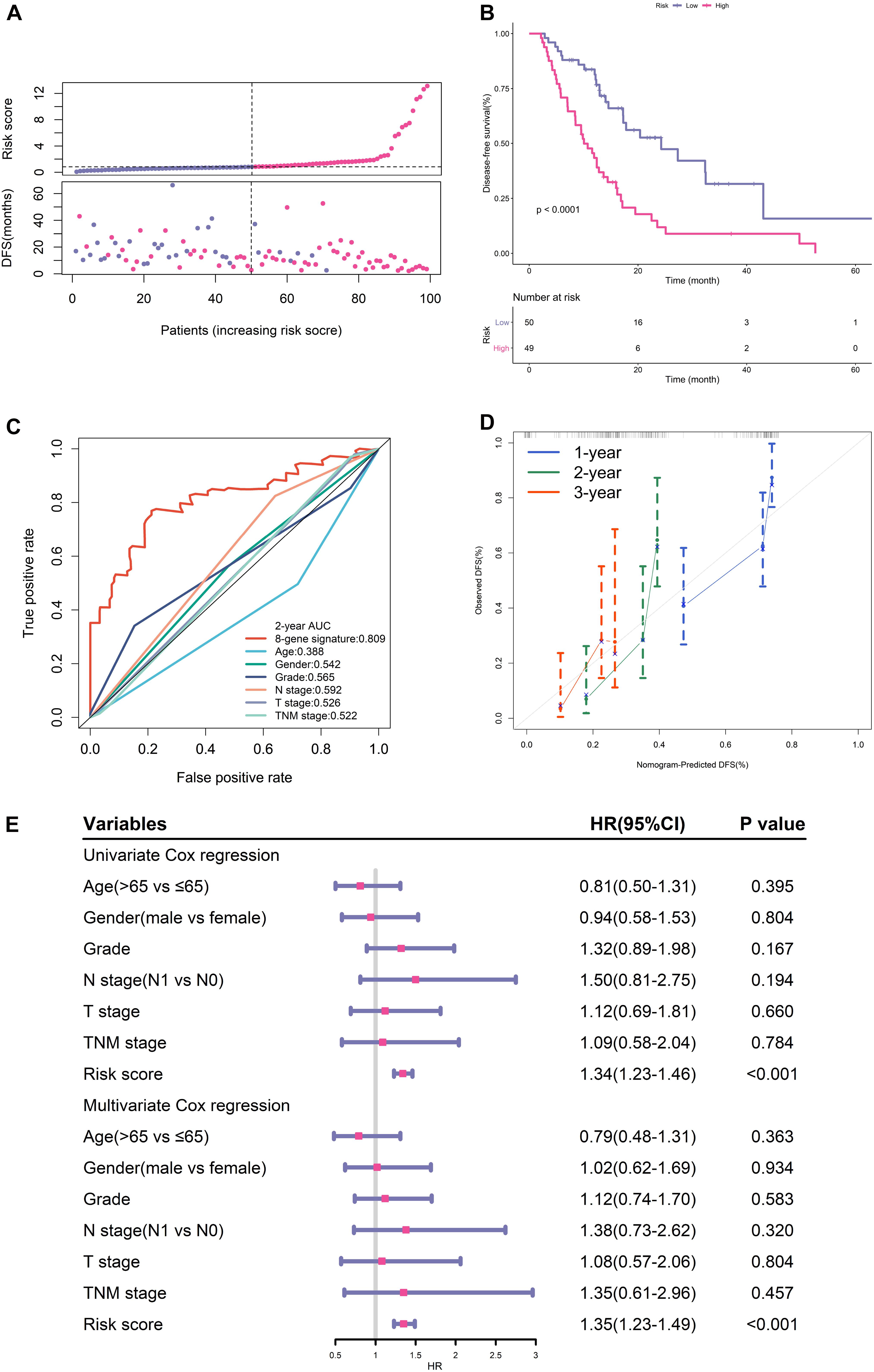
Figure 2. Prognostic performance of gene signature in TCGA training cohort. (A) From top to bottom are the risk score distribution and survival status distribution. (B) K-M survival curve for risk score. (C) ROC curve of the risk signature and clinical predictors. (D) Calibration curves for risk score. (E) Univariate and multivariate cox regression analyses of clinical parameters and gene signature for DFS.
K-M analysis illustrated that patients in the low-risk group had longer DFS (Figure 2B). ROC analysis demonstrated that this signature had high accuracy as the area under the curve (AUC) value was 0.809. What’s more, the AUC value of this signature was high than that of clinical predictors including histological grade and TNM stage (Figure 2C).
The calibration curves proved the good agreement between predicted DFS and observed DFS (Figure 2D). In addition, both univariate and multivariate cox regression analyses certified that the proposed EMT signature was an independent risk factor for DFS (Figure 2E).
Predictive Performance of the EMT-Related Gene Signature in Two Validation Cohorts
We next verified the predictive accuracy of this signature in another public PDAC cohort (PACA-CA) and our own cohort (Ruijin). Figures 3A,B showed the distribution of the risk scores and survival status in these two cohorts. We observed that patients with a high-risk score had markedly increased recurrence rates. K-M survival curves estimated significantly decreased DFS of high-risk patients in both PACA-CA and Ruijin cohorts (Figures 3C,D). ROC curves demonstrated that this signature outperformed clinical indicators in predicting DFS (Figures 3E,F).
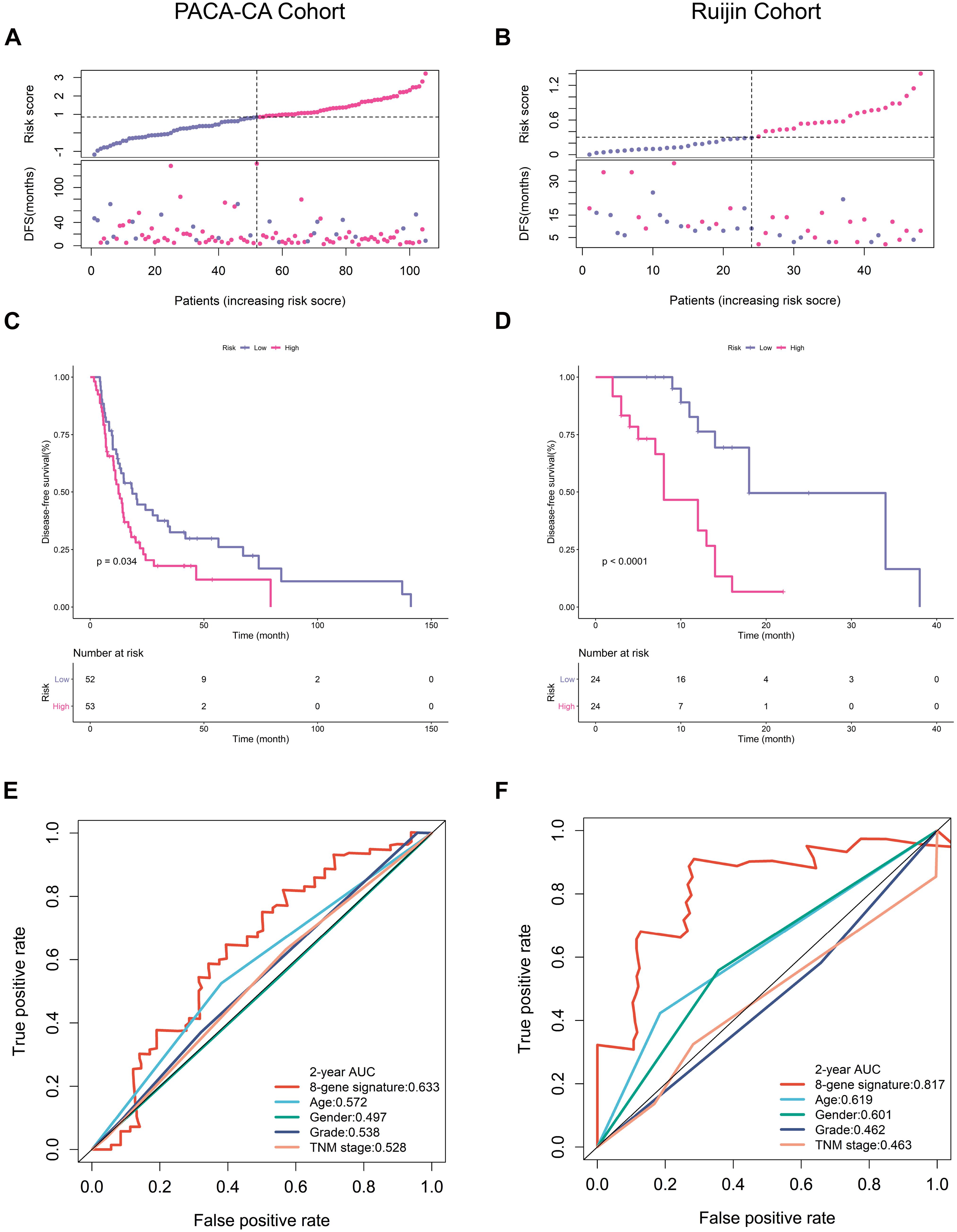
Figure 3. Prognostic validation in PACA-CA and Ruijin cohorts. (A,B) Distribution of risk score and survival status in PACA-CA and Ruijin cohorts, respectively. (C,D) K-M survival curves estimating DFS difference in two cohorts. (E,F) ROC curves of risk signature and clinical indicators in two cohorts.
Subgroup Analyses of the EMT-Related Gene Signature
With the purpose to investigate the stability of this signature, we conducted subgroup analyses. As a small percentage of patients in PACA-CA cohort did not have clinical information regarding histological grade, we thus selected TCGA and Ruijin cohorts for further analyses. K-M curves showed that our signature had high-efficiency to distinguish patients with different DFS in every subgroup divided by age (Figure 4A), gender (Figure 4B), and histological grade (Figure 4C).
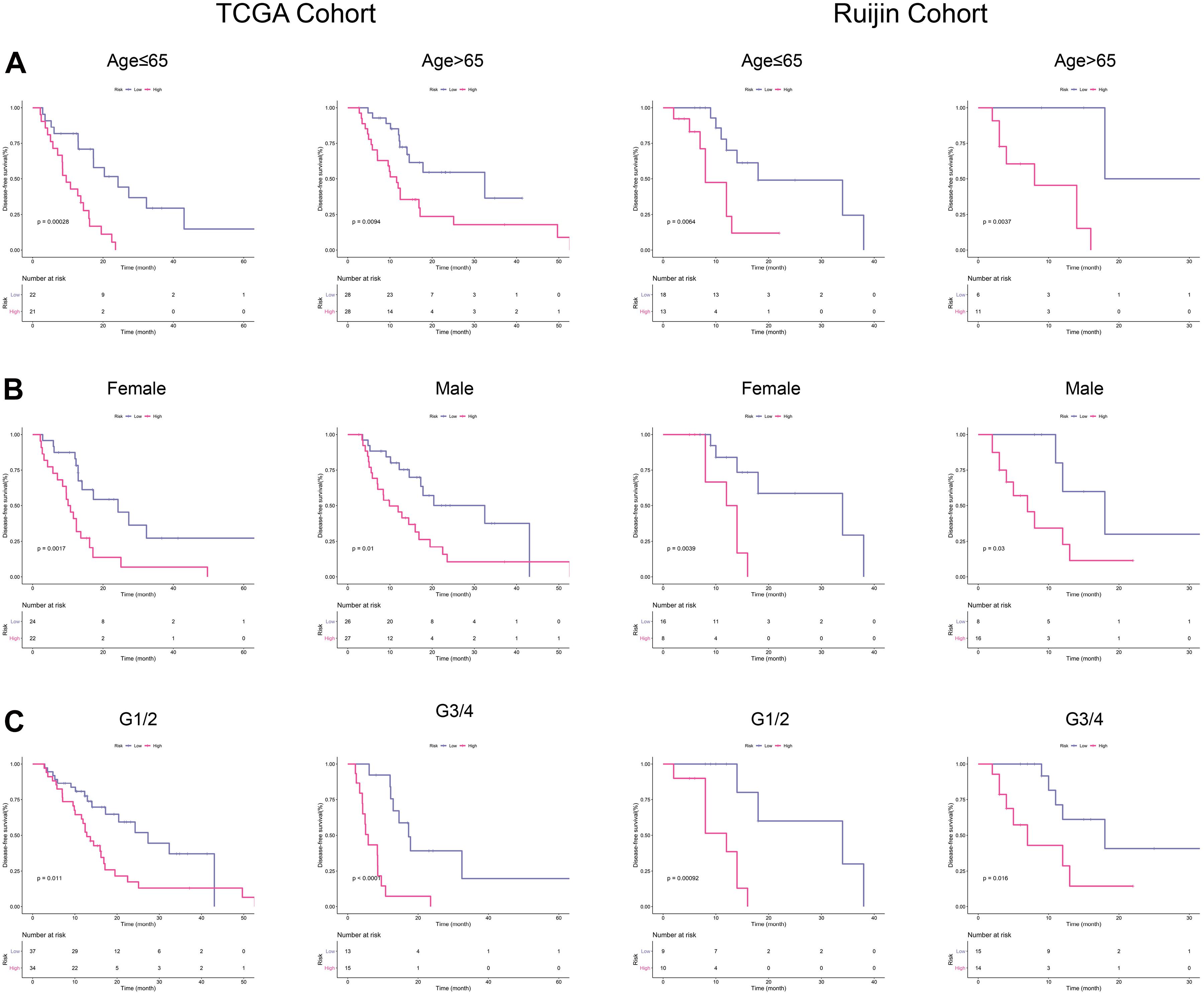
Figure 4. Subgroup analyses in TCGA and Ruijin cohorts. (A) K-M curves for the risk signature in patients stratified by age. (B) Survival difference in high- and low-risk patients stratified by gender. (C) K–M curves evaluating the DFS between low- and high-risk patients stratified by histological grade.
Relationship Between Risk Score and Response to Chemotherapeutic Regimes
Figure 5A illustrated that PDAC patients with a low-risk score had higher response rates to adjuvant chemotherapy than patients with a high-risk score in TCGA training cohort (61 vs. 32%, p < 0.001). Currently, adjuvant chemotherapy in PDAC is based on few regimes. Gemcitabine remains the most effective monotherapy and is often applied to patient who are ineligible for more aggressive treatments (Oba et al., 2020; Turpin et al., 2020). As for patients in good status, the polychemotherapy regimen including fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) is preferentially recommended in the adjuvant settings (Marabelle et al., 2020). Among samples receiving FOLFIRINOX chemotherapy, we found that patients in low-risk group had a longer DFS, although the difference was not statistically significant probably due to the limited sample size (Figure 5B). For samples receiving gemcitabine monotherapy, patients with a low-risk score had a significantly longer DFS (Figure 5C).
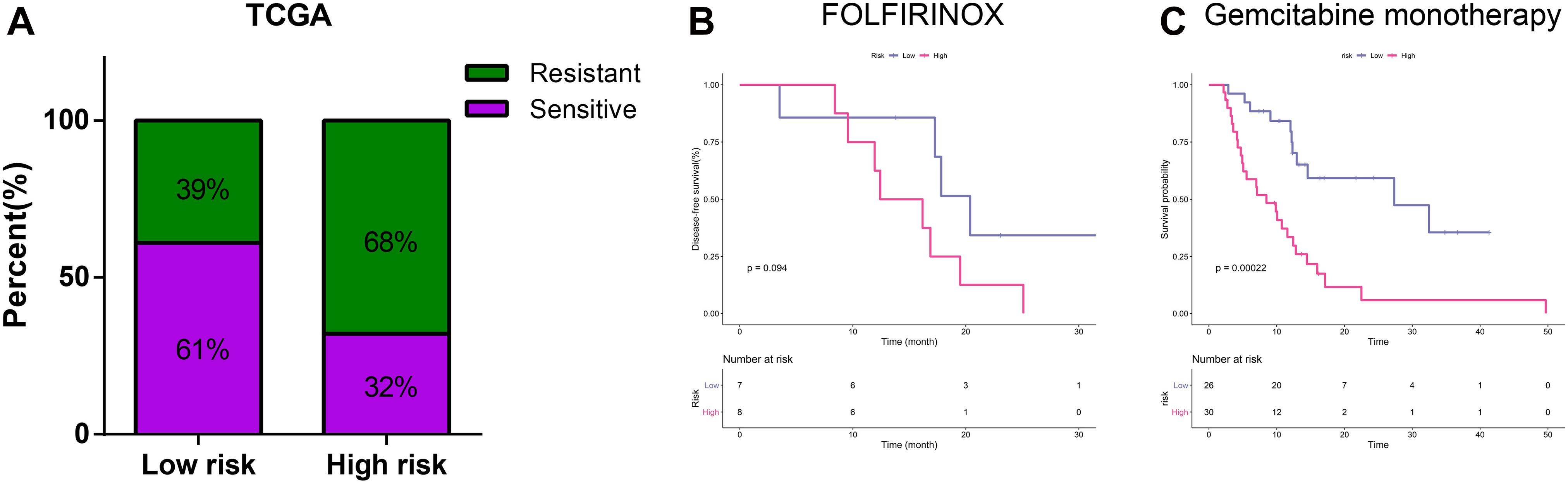
Figure 5. Relationship between risk score and chemotherapeutic regimes. (A) Relationship of risk score and chemotherapy sensitivity. (B) K-M curves for the risk signature in patients receiving FOLFIRINOX. (C) K-M curves for the risk signature in patients receiving gemcitabine monotherapy.
Annotated Functions and Enriched Pathways Associated With the EMT-Related Gene Signature
As illustrated in Figures 6A,B, positively correlated genes with risk scores were mainly involved in pathways associated with response to treatment, such as DNA repair, DNA replication, cell cycle and mismatch repair. Genes negatively correlated with risk scores were closely associated with several immunological pathways like adaptive immune response, T cell costimulation, chemotaxis and chemokine signaling pathways (Figures 6C,D).
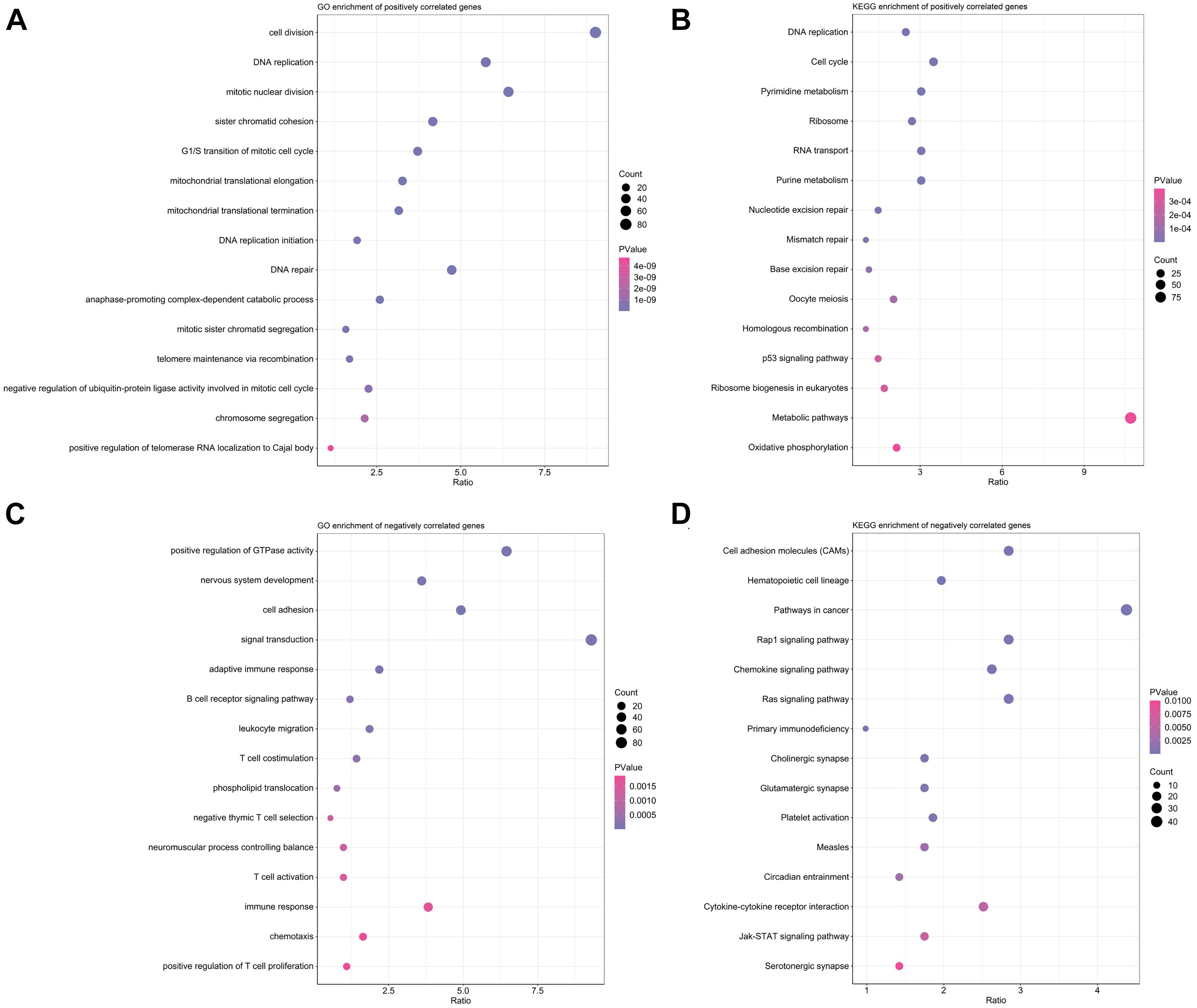
Figure 6. Function annotation and pathway enrichment analyses in TCGA training cohort. (A,B) Top 15 enriched biological processes in GO analysis (A) and pathways in KEGG analysis (B) for genes positively correlated with risk scores. (C,D) Top 15 enriched biological processes in GO analysis (C) and pathways in KEGG analysis (D) for genes positively correlated with risk scores.
Relationship Between Risk Scores and Expression Levels of Immune Check Points
Above findings suggested that risk scores were inversely correlated with T cell co-stimulation and immune response, so we wonder whether this signature could also predict response to ICIs. Recently, ICIs-based immunotherapy has drastically increased patient survival in certain cancers, but it is ineffective in the vast majority of patients with PDAC (Leinwand and Miller, 2020), biomarkers predicting response to ICIs thus are important for personalized oncology. As shown in Figures 7A–F, we observed that risk scores were negatively correlated with several common immune checkpoints, including CD28, CTLA4, PD1, TIGIT, TIM3, and VISTA. These findings indicated that PDAC patients who were not predicted to be sensitive to chemotherapy by our signature might be unsuitable for ICIs-based immunotherapy.
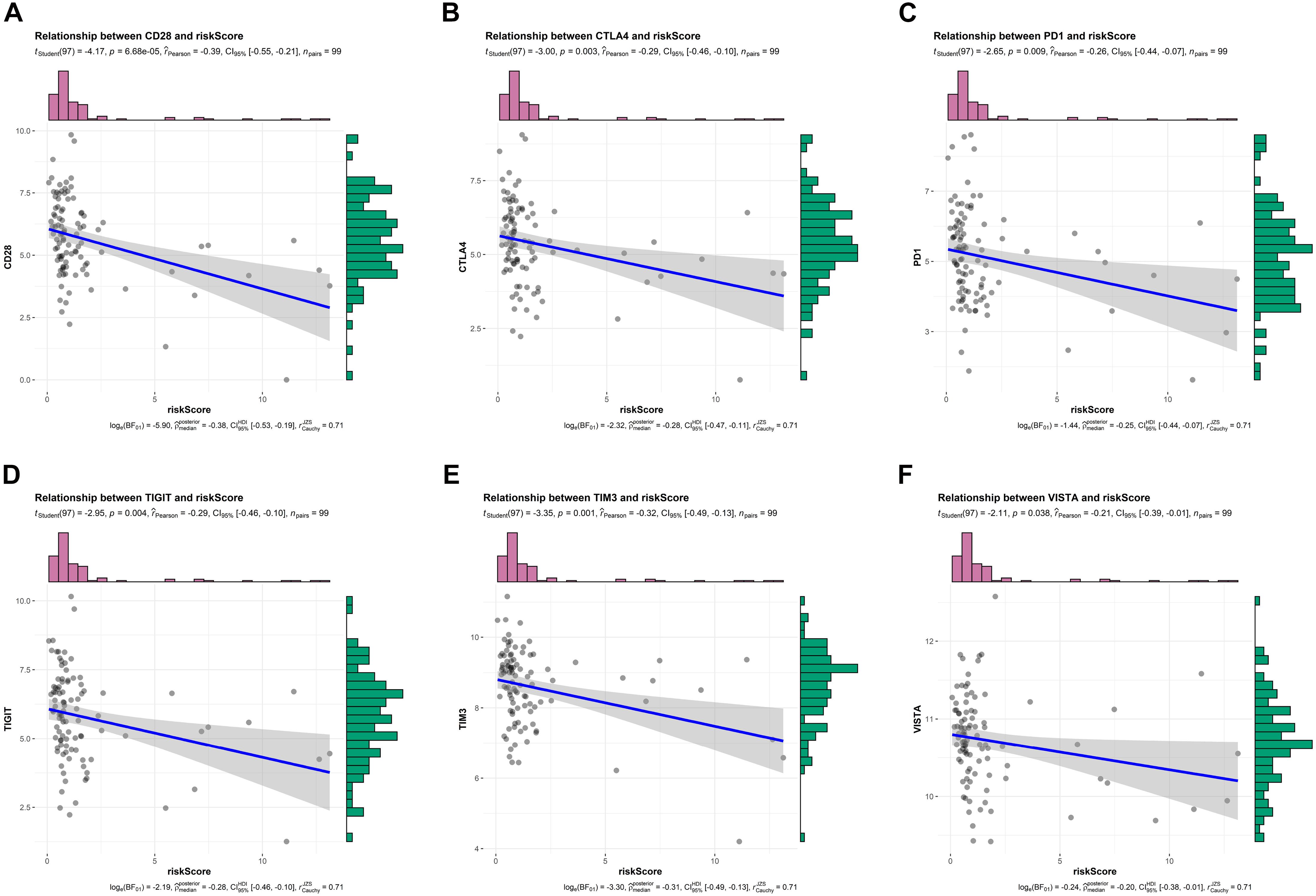
Figure 7. Relationship between risk scores and expression levels of immune checkpoints in TCGA training cohort. (A–F) Relationship between risk scores and CD28, CTLA4, PD1, TIGIT, TIM3, and VISTA, respectively.
Discussion
PDAC is a very devastating disease with extremely poor outcomes. As we all know, chemotherapy failure is one of the major problems to cure this disease and improve patient survival. PDAC features a notable intra- (Yachida and Iacobuzio-Donahue, 2013) and inter-tumoral (Cancer Genome Atlas Research Network, 2017) heterogeneity that drives chemoresistance. As cancer treatment has entered into the area of precision medicine, personalized therapy is a very attractive and laudable strategy. Determining the most effective drug to treat each patient with well balance between potential side events and expected survival benefits is definitely helpful to achieve the most favorable outcome. However, compared with other cancers, personalized treatments that translate the increased understanding of tumor molecular profiles into the clinical management are in their relative infancy for PDAC (Santofimia-Castaño and Iovanna, 2021).
Molecular characterization and subtyping of PDAC is providing a unique insight into predictive biomarkers for individualized treatments. The transcriptomic data has been a practical tool for PDAC subtyping and multiple stratification systems have been proposed to date by analyzing the transcriptional networks (Collisson et al., 2019). In addition, combining transcriptomic data with genomic sequencing, mutational landscape, immune infiltrate or genetic alteration can identify additional subtypes with clinical relevance (Bailey et al., 2016; Connor et al., 2017; Brunton et al., 2020; Rashid et al., 2020). More importantly than predicting patient prognosis and disease aggressiveness, recent studies find that transcriptomic data is also good at predicting chemotherapy sensitivity for PDAC (Deng et al., 2020; Nicolle et al., 2021; Nishiwada et al., 2021).
In this study, we initially analyzed the prognostic potential of EMT-related genes in predicting DFS through univariate cox regression analysis. Subsequently LASSO regression analysis and multivariate cox regression analysis identified an 8-gene signature for predicting response to adjuvant chemotherapy. K-M survival curves, ROC curves and calibration curves collectively proved the moderate accuracy of the signature in predicting DFS. Functional analysis indicated that this signature was closely related to several cancer-related pathways. Subgroup analysis demonstrated the cross-clinicopathology stability. Intriguingly, except from chemotherapy, the signature also had great potential to predict response to ICIs. In other words, patients with a high-risk score predicted by our signature were very likely to be insensitive to neither chemotherapy nor ICIs-based immunotherapy. In this way, high-risk patients might be exempted from unnecessary drug toxicity and heavy finical burden.
The EMT program plays an indispensable role in therapeutic resistance in cancers. Mechanically, it inhibits multiple apoptotic signaling pathways, enhances drug efflux, and gives rise to cancer stem cells. These all contribute to cancer cells’ increased resistance to anti-cancer drugs. In addition, EMT also upregulates several pathways that allow cancer cells to stave off the lethal effects of cytotoxic T cells, thus enhances resistance to immunotherapy (Huang et al., 2007). Transcriptional prognostic signatures based on EMT-related genes have been extensively reported recently (Cao et al., 2020; Zhang et al., 2020; Zhong et al., 2020; Cheng et al., 2021), but are rare in PDAC. Our study firstly constructed a robust response prediction model based on the eight EMT-related genes. We validated this signature in two public cohorts including American and Canadian populations, and our local cohort of Asian population, which enhanced the reliability and clinical applicability of this signature.
Despite explicit validation and considerable clinical relevance, this work is still based on retrospective data and has many limitations. Firstly, the cohorts used in this study are relatively small probably due to the low curative resection rates for PDAC patients.
Predictive efficiency needs to be verified in more prospective studies and larger cohorts. Second, owing to the limited sample size, some subgroup analyses cannot be implemented. For instance, 89 of 99 samples in TCGA cohorts are at stage II, subgroup analysis on tumor stage is thus meaningless. Third, detailed chemotherapy regimens are largely unknown in PACA-CA cohorts and incomplete in TCGA cohorts. Fourth, more in vivo and in vitro experiments are needed to elucidate biological function of eight genes in PDAC progression.
In conclusion, we proposed an EMT-related gene signature with satisfactory performance in predicting response to adjuvant chemotherapy. Functionally, it was associated with cell cycle, DNA repair and DNA replication. The signature outperformed clinical indicators in predictive chemotherapy sensitivity. After all, this signature was based on the retrospective cohorts and needed to be further validated in more prospective cohorts.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Ruijin Hospital affiliated with Shanghai Jiao Tong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZF and CP designed the study and wrote the manuscript. ZF, KL, and JL participated in data analysis, discussion, and language editing. YW reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81672325 and 81802316).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the National Natural Science Foundation of China for the grant funding. We also acknowledge the contributions from UCSC, TCGA, and ICGC databases.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.665161/full#supplementary-material
Abbreviations
AUC, area under the curve; DFS, disease-free survival; EMT, epithelial to mesenchymal transition; GOm Gene Ontology; ICGC, International Cancer Genome Consortium; ICIs, Immune-checkpoint inhibitors; KEGG, Kyoto Encyclopedia of Genes and Genomes (KEGG); K-M, Kaplan-Meier; PDAC, pancreatic ductal adenocarcinoma; QRT-PCR, quantitative real time polymerase chain reaction; ROC, receiver operating characteristic; TCGA, The Cancer Genome Atlas; TNM, tumor, node, metastasis.
Footnotes
References
Aoki, S., Motoi, F., Murakami, Y., Sho, M., Satoi, S., and Honda, G, et al. (2019). Decreased serum carbohydrate antigen 19-9 levels after neoadjuvant therapy predict a better prognosis for patients with pancreatic adenocarcinoma: a multicenter case-control study of 240 patients. BMC Cancer 19:252. doi: 10.1186/s12885-019-5460-4
Aung, KL., Fischer, SE., Denroche, RE., Jang, GH., Dodd, A., and Creighton, S, et al. (2018). Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin. Cancer Res. 24, 1344–1354. doi: 10.1158/1078-0432.Ccr-17-2994
Bailey, P., Chang, DK., Nones, K., Johns, AL., Patch, AM., and Gingras, MC, et al. (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52. doi: 10.1038/nature16965
Bird, NT., Elmasry, M., Jones, R., Psarelli, E., Dodd, J., and Malik, H, et al. (2017). Immunohistochemical hENT1 expression as a prognostic biomarker in patients with resected pancreatic ductal adenocarcinoma undergoing adjuvant gemcitabine-based chemotherapy. Br. J. Surg. 104, 328–336. doi: 10.1002/bjs.10482
Brunton, H., Caligiuri, G., Cunningham, R., Upstill-Goddard, R., Bailey, UM., and Garner, IM, et al. (2020). HNF4A and GATA6 loss reveals therapeutically actionable subtypes in pancreatic cancer. Cell Rep. 31:107625. doi: 10.1016/j.celrep.2020.107625
Byers, LA., Diao, L., Wang, J., Saintigny, P., Girard, L., and Peyton, M, et al. (2013). An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 19, 279–290. doi: 10.1158/1078-0432.Ccr-12-1558
Cai, L., Hu, C., Yu, S., Liu, L., Zhao, J., and Zhao, Y, et al. (2020). Identification of EMT-related gene signatures to predict the prognosis of patients with endometrial cancer. Front. Genet. 11:582274. doi: 10.3389/fgene.2020.582274
Cancer Genome Atlas Research Network (2017). Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 32, 185–203.e13. doi: 10.1016/j.ccell.2017.07.007
Cao, R., Yuan, L., Ma, B., Wang, G., Qiu, W., and Tian, Y. (2020). An EMT-related gene signature for the prognosis of human bladder cancer. J. Cell Mol. Med. 24, 605–617. doi: 10.1111/jcmm.14767
Cheng, YB., Li, S., Zhang, YP., Zhu, ZH., and Zhao, N. (2021). An epithelial-mesenchymal transition-related long non-coding RNA signature to predict overall survival and immune microenvironment in kidney renal clear cell carcinoma. Bioengineered. 12, 555–564. doi: 10.1080/21655979.2021.1880718
Clayton, EA., Pujol, TA., McDonald, JF., and Qiu, P. (2020). Leveraging TCGA gene expression data to build predictive models for cancer drug response. BMC Bioinformatics 21:364. doi: 10.1186/s12859-020-03690-4
Collisson, EA., Bailey, P., Chang, DK., and Biankin, AV. (2019). Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 16, 207–220. doi: 10.1038/s41575-019-0109-y
Collisson, EA., Sadanandam, A., Olson, P., Gibb, WJ., Truitt, M., and Gu, S, et al. (2011). Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 17, 500–503. doi: 10.1038/nm.2344
Connor, AA., Denroche, RE., Jang, GH., Timms, L., Kalimuthu, SN., and Selander, I, et al. (2017). Association of distinct mutational signatures with correlates of increased immune activity in pancreatic ductal adenocarcinoma. JAMA Oncol. 3, 774–783. doi: 10.1001/jamaoncol.2016.3916
Deng, Z., Li, X., Shi, Y., Lu, Y., Yao, W., and Wang, J. (2020). A novel autophagy-related incrnas signature for prognostic prediction and clinical value in patients with pancreatic cancer. Front. Cell Dev. Biol. 8:606817. doi: 10.3389/fcell.2020.606817
Feng, Z., Shi, M., Li, K., Ma, Y., Jiang, L., and Chen, H, et al. (2020). Development and validation of a cancer stem cell-related signature for prognostic prediction in pancreatic ductal adenocarcinoma. J. Transl. Med. 18:360. doi: 10.1186/s12967-020-02527-1
Han, B., Kim, BJ., Kim, HS., Choi, DR., Shim, BY., and Lee, KH, et al. (2021). A phase II study of gemcitabine, erlotinib and S-1 in patients with advanced pancreatic cancer. J. Cancer 12, 912–917. doi: 10.7150/jca.50514
Huang, DW., Sherman, BT., Tan, Q., Kir, J., Liu, D., and Bryant, D, et al. (2007). DAVID bioinformatics resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 35, W169–W175. doi: 10.1093/nar/gkm415
Kaissis, G., Ziegelmayer, S., Lohöfer, F., Steiger, K., Algül, H., and Muckenhuber, A, et al. (2019). A machine learning algorithm predicts molecular subtypes in pancreatic ductal adenocarcinoma with differential response to gemcitabine-based versus FOLFIRINOX chemotherapy. PLoS One 14:e0218642. doi: 10.1371/journal.pone.0218642
Kamisawa, T., Wood, LD., Itoi, T., and Takaori, K. (2016). Pancreatic cancer. Lancet 388, 73–85. doi: 10.1016/s0140-6736(16)00141-0
Kleeff, J., Korc, M., Apte, M., La Vecchia, C., Johnson, CD., and Biankin, AV, et al. (2016). Pancreatic cancer. Nat. Rev. Dis. Primers. 2:16022. doi: 10.1038/nrdp.2016.22
Kyrochristos, ID., Ziogas, DE., Glantzounis, GK., and Roukos, DH. (2018). Prediction of pancreatic cancer risk and therapeutic response with next-generation sequencing. Biomark. Med. 12, 5–8. doi: 10.2217/bmm-2017-0315
Leinwand, J., and Miller, G. (2020). Regulation and modulation of antitumor immunity in pancreatic cancer. Nat. Immunol. 21, 1152–1159. doi: 10.1038/s41590-020-0761-y
Marabelle, A., Le, DT., Ascierto, PA., Di Giacomo, AM., De Jesus-Acosta, A., and Delord, JP, et al. (2020). Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10. doi: 10.1200/jco.19.02105
Mizrahi, JD., Surana, R., Valle, JW., and Shroff, RT. (2020). Pancreatic cancer. Lancet 395, 2008–2020. doi: 10.1016/s0140-6736(20)30974-0
Nicolle, R., Gayet, O., Duconseil, P., Vanbrugghe, C., Roques, J., and Bigonnet, M, et al. (2021). A transcriptomic signature to predict adjuvant gemcitabine sensitivity in pancreatic adenocarcinoma. Ann. Oncol. 32, 250–260. doi: 10.1016/j.annonc.2020.10.601
Nishiwada, S., Sho, M., Cui, Y., Yamamura, K., Akahori, T., and Nakagawa, K, et al. (2021). A gene expression signature for predicting response to neoadjuvant chemoradiotherapy in pancreatic ductal adenocarcinoma. Int. J. Cancer 148, 769–779. doi: 10.1002/ijc.33284
Oba, A., Ho, F., Bao, QR., Al-Musawi, MH., Schulick, RD., and Del Chiaro, M. (2020). Neoadjuvant treatment in pancreatic cancer. Front. Oncol. 10:245. doi: 10.3389/fonc.2020.00245
Okamura, Y., Yasukawa, S., Narimatsu, H., Boku, N., Fukutomi, A., and Konishi, M, et al. (2020). Human equilibrative nucleoside transporter-1 expression is a predictor in patients with resected pancreatic cancer treated with adjuvant S-1 chemotherapy. Cancer Sci. 111, 548–560. doi: 10.1111/cas.14258
Perri, G., Prakash, L., Qiao, W., Varadhachary, GR., Wolff, R., and Fogelman, D, et al. (2020). Response and survival associated with first-line FOLFIRINOX vs Gemcitabine and nab-paclitaxel chemotherapy for localized pancreatic ductal adenocarcinoma. JAMA Surg. 155, 832–839. doi: 10.1001/jamasurg.2020.2286
Perri, G., Prakash, L., Wang, H., Bhosale, P., Varadhachary, GR., and Wolff, R, et al. (2021). Radiographic and serologic predictors of pathologic major response to preoperative therapy for pancreatic cancer. Ann. Surg. 273, 806–813. doi: 10.1097/sla.0000000000003442
Phua, LC., Goh, S., Tai, DWM., Leow, WQ., Alkaff, SMF., and Chan, CY, et al. (2018). Metabolomic prediction of treatment outcome in pancreatic ductal adenocarcinoma patients receiving gemcitabine. Cancer Chemother. Pharmacol. 81, 277–289. doi: 10.1007/s00280-017-3475-6
Piquemal, D., Noguier, F., Pierrat, F., Bruno, R., and Cros, J. (2020). Predictive values of blood-based rna signatures for the gemcitabine response in advanced pancreatic cancer. Cancers (Basel) 12:3204. doi: 10.3390/cancers12113204
Raffenne, J., Nicolle, R., Puleo, F., Le Corre, D., Boyez, C., and Marechal, R, et al. (2019). hENT1 testing in pancreatic ductal adenocarcinoma: are we ready? a multimodal evaluation of hENT1 status. Cancers (Basel) 11:1808. doi: 10.3390/cancers11111808
Rashid, NU., Peng, XL., Jin, C., Moffitt, RA., Volmar, KE., and Belt, BA, et al. (2020). Purity Independent Subtyping of Tumors (PurIST), a clinically robust, single-sample classifier for tumor subtyping in pancreatic cancer. Clin. Cancer Res. 26, 82–92. doi: 10.1158/1078-0432.Ccr-19-1467
Santofimia-Castaño, P., and Iovanna, J. (2021). Combating pancreatic cancer chemoresistance by triggering multiple cell death pathways. Pancreatology 21, 522–529. doi: 10.1016/j.pan.2021.01.010
Shibue, T., and Weinberg, RA. (2017). EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 14, 611–629. doi: 10.1038/nrclinonc.2017.44
Siegel, RL., Miller, KD., Fuchs, HE., and Jemal, A. (2021). Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33. doi: 10.3322/caac.21654
Tu, SM., Bilen, MA., and Tannir, NM. (2016). Personalised cancer care: promises and challenges of targeted therapy. J. R. Soc. Med. 109, 98–105. doi: 10.1177/0141076816631154
Turpin, A., El Amrani, M., Bachet, JB., Pietrasz, D., Schwarz, L., and Hammel, P. (2020). Adjuvant pancreatic cancer management: towards new perspectives in 2021. Cancers (Basel) 12:3866. doi: 10.3390/cancers12123866
Xu, HX., Li, S., Wu, CT., Qi, ZH., Wang, WQ., and Jin, W, et al. (2018). Postoperative serum CA19-9, CEA and CA125 predicts the response to adjuvant chemoradiotherapy following radical resection in pancreatic adenocarcinoma. Pancreatology 18, 671–677. doi: 10.1016/j.pan.2018.05.479
Yachida, S., and Iacobuzio-Donahue, CA. (2013). Evolution and dynamics of pancreatic cancer progression. Oncogene 32, 5253–5260. doi: 10.1038/onc.2013.29
Zhang, D., Zhou, S., and Liu, B. (2020). Identification and validation of an individualized EMT-related prognostic risk score formula in gastric adenocarcinoma patients. Biomed. Res. Int. 2020:7082408. doi: 10.1155/2020/7082408
Zheng, X., Carstens, JL., Kim, J., Scheible, M., Kaye, J., and Sugimoto, H, et al. (2015). Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530. doi: 10.1038/nature16064
Keywords: PDAC, EMT, adjuvant chemotherapy, sensitivity, disease-free survival, risk score, prognostic signature
Citation: Feng Z, Li K, Lou J, Wu Y and Peng C (2021) An EMT-Related Gene Signature for Predicting Response to Adjuvant Chemotherapy in Pancreatic Ductal Adenocarcinoma. Front. Cell Dev. Biol. 9:665161. doi: 10.3389/fcell.2021.665161
Received: 07 February 2021; Accepted: 13 April 2021;
Published: 30 April 2021.
Edited by:
Andrew Davis, Washington University in St. Louis, United StatesReviewed by:
Ali M. Ardekani, Avicenna Research Institute (ARI), IranTaiping Zhang, Peking Union Medical College Hospital (CAMS), China
Copyright © 2021 Feng, Li, Lou, Wu and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulian Wu, eXVsaWFud3VAemp1LmVkdS5jbg==; Chenghong Peng, Y2hocGVuZ0B5ZWFoLm5ldA==
 Zengyu Feng
Zengyu Feng Kexian Li
Kexian Li Jianyao Lou
Jianyao Lou Yulian Wu
Yulian Wu Chenghong Peng
Chenghong Peng