
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol., 18 March 2021
Sec. Molecular and Cellular Oncology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.655950
This article is part of the Research TopicEmerging Molecular Mechanisms of Cell Cycle Regulation in Cancer: Functions and Potential ApplicationsView all 23 articles
Objective: To screen lung adenocarcinoma (LUAC)-specific cell-cycle-related genes (CCRGs) and develop a prognostic signature for patients with LUAC.
Methods: The GSE68465, GSE42127, and GSE30219 data sets were downloaded from the GEO database. Single-sample gene set enrichment analysis was used to calculate the cell cycle enrichment of each sample in GSE68465 to identify CCRGs in LUAC. The differential CCRGs compared with LUAC data from The Cancer Genome Atlas were determined. The genetic data from GSE68465 were divided into an internal training group and a test group at a ratio of 1:1, and GSE42127 and GSE30219 were defined as external test groups. In addition, we combined LASSO (least absolute shrinkage and selection operator) and Cox regression analysis with the clinical information of the internal training group to construct a CCRG risk scoring model. Samples were divided into high- and low-risk groups according to the resulting risk values, and internal and external test sets were used to prove the validity of the signature. A nomogram evaluation model was used to predict prognosis. The CPTAC and HPA databases were chosen to verify the protein expression of CCRGs.
Results: We identified 10 LUAC-specific CCRGs (PKMYT1, ETF1, ECT2, BUB1B, RECQL4, TFRC, COCH, TUBB2B, PITX1, and CDC6) and constructed a model using the internal training group. Based on this model, LUAC patients were divided into high- and low-risk groups for further validation. Time-dependent receiver operating characteristic and Cox regression analyses suggested that the signature could precisely predict the prognosis of LUAC patients. Results obtained with CPTAC, HPA, and IHC supported significant dysregulation of these CCRGs in LUAC tissues.
Conclusion: This prognostic prediction signature based on CCRGs could help to evaluate the prognosis of LUAC patients. The 10 LUAC-specific CCRGs could be used as prognostic markers of LUAC.
Lung cancer (LC) remains one of the most common malignancies and is a major contributor to cancer-related deaths worldwide, accounting for 11.6% of cancers and 18.4% of deaths, respectively (Bray et al., 2018). In China, the burden of LC remains the highest of all cancers, with mortality and incidence rates 1.5 times those worldwide in 2017 (Liu et al., 2020). Non-small-cell LC (NSCLC) is the predominant type (approximately 85%) of LC; it includes lung adenocarcinoma (LUAC) and lung squamous cell carcinoma (LUSC), of which LUAC is the most prevalent type (Peng et al., 2017; Herbst et al., 2018). LUAC originates in the distal airway and has less correlation with chronic inflammation and smoking than LUSC (Peng et al., 2017).
Currently, anatomical surgical resection and mediastinal lymph node dissection are the most effective methods for treatment of early stage LUAC patients, and the main surgical procedures are lobectomy and sub-lobectomy (Lin et al., 2020; Yu et al., 2020). Advanced-stage LUAC covers a variety of disease manifestations and has an equally complex range of multimodal treatment options, including systemic and local therapies (chemotherapy, radiation therapy, etc.) for remote and local symptom control, respectively (Evison, 2020). With the application of biomarker-directed therapies targeting molecular changes (such as EGFR and BRAF V600E mutations, or ALK and ROS1 rearrangements), these therapies can prolong the survival of LC patients (Arbour and Riely, 2019). In the past few years, with the application of high-throughput sequencing (Illumina HiSeq, Illumina MiSeq, Ion PGMTM, etc.), increasing numbers of rare molecular changes in oncogenic drivers (including HER2, MET, and RET) have been identified (Yu et al., 2018). Specific tyrosine kinase inhibitors targeting these genomic changes have shown improved patient survival and satisfactory biological activity, mostly in phase III clinical trials, which has led the US Food and Drug Administration to accelerate the approval of some of these drugs (Lamberti et al., 2020). However, the 5 year survival rate has only increased by 5% in the past 20 years (Johnson et al., 2014).
Increasing numbers of studies show that the cell cycle is tightly bound to the growth and proliferation of LUAC cells, with certain genes potentially functioning as cycle regulators. For example, knockdown of GINS2 induced cell cycle arrest and apoptosis in A549 cells (Sun et al., 2021), and MITF could inhibit NSCLC progression by controlling the cell cycle (Hsiao et al., 2020). However, the results of such studies are difficult to translate into clinical practice. This is mainly because tumor occurrence and development are pathological processes driven by multiple genes and cannot adequately be explained by the abnormal expression of a single gene. Although changes in a certain gene may lead to differences in the prognosis of patients, the sensitivity and specificity of clinical tests for the gene in question are often not satisfactory. Thus, there is a need to develop more accurate methods for diagnosis and prediction of prognosis of LUAC patients.
The GSE68465 (Shedden et al., 2008), GSE42127 (Hight et al., 2020), and GSE30219 (Rousseaux et al., 2013) data sets (containing data from 443, 133, and 148 LUAC patients, respectively) were downloaded from the Gene Expression Omnibus database (Edgar et al., 2002). GSE68465 was defined as an internal group, and the other data sets were combined as an external group (test group). The batch effect was balanced using the “SVA” package in R (version 4.0.2) (Irizarry et al., 2003). When more than one probe was mapped to one gene ID, we took their average value for further analysis. In addition, mRNA expression profiles and corresponding clinical information of LUAC patients were obtained from The Cancer Genome Atlas (TCGA) database (Chang et al., 2015).
Single-sample gene set enrichment analysis (ssGSEA) was implemented using the “GSVA” R package (Barbie et al., 2009). The reference gene sets were from the MSigDB2 database (Liberzon et al., 2011): “KEGG_CELL_CYCLE” (Kanehisa, 2002) and “GO_CELL_CYCLE” (Mi et al., 2019). Spearman correlations were calculated between pairs of enrichment scores and each gene. Next, genes that met both of the following criteria were defined as LUAC-related CCRGs: absolute correlation > 0.3 and P < 0.01. The analysis of differentially expressed genes used the “limma” package. | Log2 fold change| > 1 and P < 0.05 were the criteria for determining differentially expressed genes. Finally, LUAC-specific CCRGs were screened out.
The samples from GSE68465 were divided into two groups at a ratio of 1:1 at random to form an internal training group and a test group. Univariate Cox regression analysis, LASSO regression, and multiple Cox regression analysis were used to investigate the prognostic value of CCRGs in predicting the overall survival (OS) of LUAC patients, and to construct a model. On the basis of the median value of the risk score, all samples in the training group were divided into low- and high-risk groups. Kaplan–Meier survival curves and time-dependent receiver operating characteristic curves for OS evaluation of the two groups were plotted to evaluate the accuracy of the signature in the internal training group. The results were further confirmed in the internal and external test groups. To demonstrate that the model represents an independent risk factor, the combination of the signature and clinical factors was further validated through univariate and multivariate Cox regression analysis. P < 0.05 was regarded as statistically significant.
To predict 1, 3, and 5 years OS, we established a nomogram and plotted its calibration curve based on all independent prognostic factors determined by multivariate analysis. Cox regression analysis was conducted with the R software to observe the relationship between the predicted probabilities and the actual occurrence rates.
With the R package “ConsensusClusterPlus,” the gene expression matrix contained in the model was used to identify molecular subtypes in LUAC. Then, survival rate analysis was performed and displayed for single subtypes.
Data from the Clinical Proteomic Tumor Analysis Consortium (CTPAC) (Rudnick et al., 2016) and the Human Protein Atlas (HPA) (Pontén et al., 2008) were chosen to verify the protein expression of LUAC-specific CCRGs in tumor tissues and normal tissues, and to determine whether the expression differences were consistent with the previous mRNA results from TCGA. Differences were considered notable if P < 0.05.
After obtaining the consent of 3 LUAC patients, tissue sections were obtained from the pathology department of our hospital. After blocking with endogenous peroxide and protein, the sections were then incubated with diluted specific anti-ECT2 or anti-BUB1B at 4°C overnight. The next day, the sections were incubated with the secondary antibody at 37°C for 1 h. The sections were stained with 3,3-diaminobenzidine solution for 3 min and counterstained with hematoxylin. The slices are finally observed and photographed under a microscope.
The workflow of the study is shown in Figure 1. On the basis of the mRNA data and clinical features from GSE68465, we conducted ssGSEA and used the CCRG sets as a reference, identifying 1,029 genes as LUAC-related CCRGs. Then, univariate Cox analysis was performed to screen out CCRGs that were significantly related to survival, resulting in 801 genes (Supplementary Table 1). By comparison with mRNA expression in the TCGA LUAC data set, we screened out 148 differentially expressed CCRGs (Supplementary Table 2) as LUAC-specific CCRGs (Figure 2A).
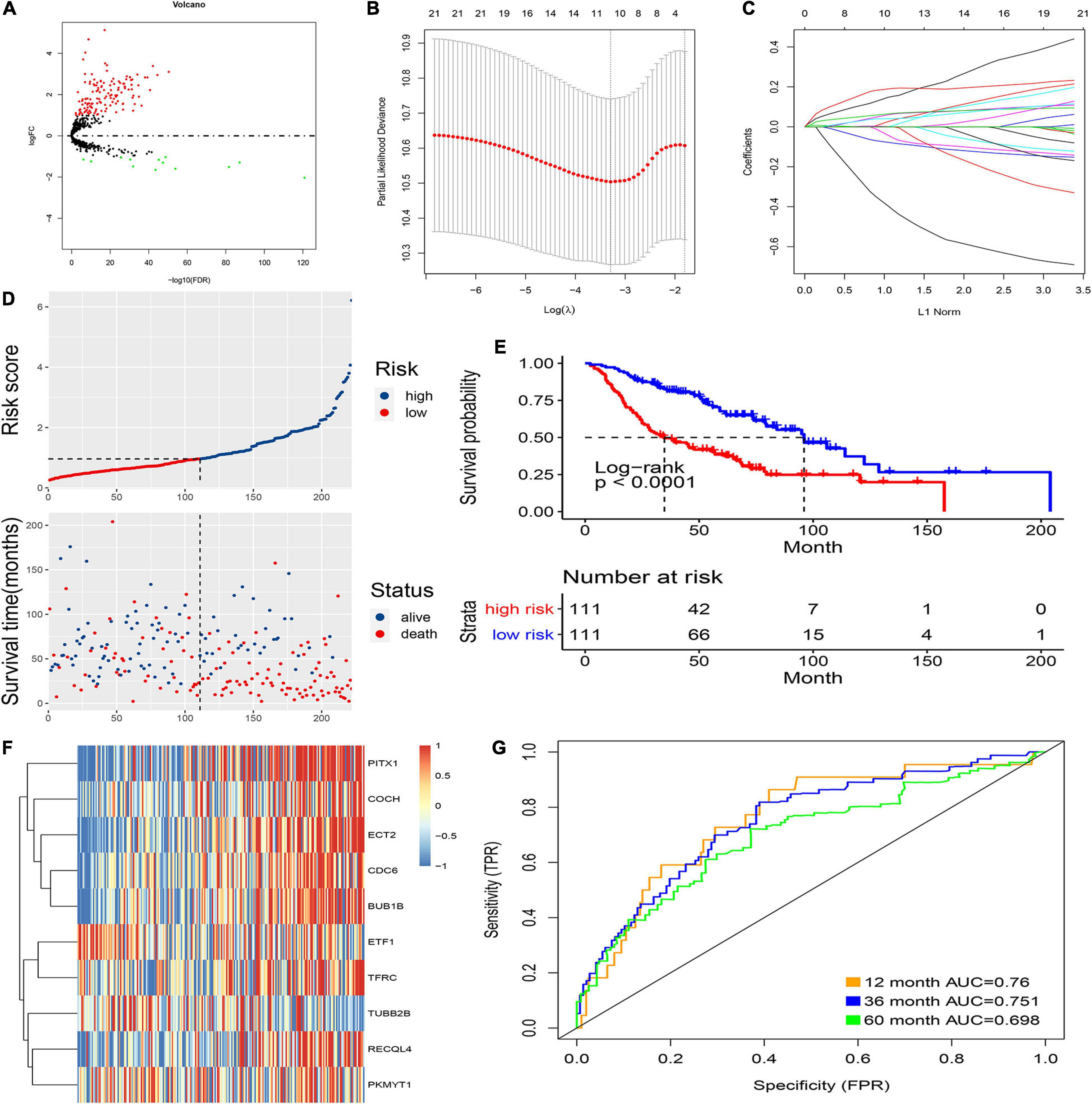
Figure 2. Prognostic model of the training cohort and risk signature with the 10 CCRGs. (A) LUAC-specific CCRGs were screened out. (B) The association between deviance and log(λ). (C) The association between coefficients of genes and log(λ). (D) Risk score of the high and low groups. (E) Survival analysis of the high and low groups. (F) Heatmap of the expression of 10 CCRGs. (G) The AUC of the ROC.
First, the internal group (GSE68465) was randomly divided into two groups, which were used as an internal training group and a test group. Then, LASSO regression was used, and cross-validation was performed in the internal training group (Figure 2B). The preliminary signature included 21 CCRGs: ETF1, TFRC, RRM2, MYBL2, CDC6, RECQL4, BUB1B, ECT2, PITX1, TRIP13, RPL39L, PKMYT1, CKS1B, KIF23, MCM10, TUBB2B, COCH, DTL, CENPE, BLM, and DHRS2 (Figure 2C). Then, multivariate Cox regression was performed to build prognostic signatures on the basis of these CCRGs. Finally, a signature of 10 CCRGs was selected, and risk scores were calculated as follows: risk score = (−0.655 ∗ exp of ETF1) + (0.106 ∗ exp of TFRC) + (0.122 ∗ exp of CDC6) + (0.099 ∗ exp of RECQL4) + (0.178 ∗ exp of BUB1B) + (0.22 ∗ exp of ECT2) + (0.094 ∗ exp of PITX1) + (−0.212 ∗ exp of PKMYT1) + (−0.119 ∗ exp of TUBB2B) + (0.042 ∗ exp of COCH). After calculating the risk scores of individual patients, 1.0145 was chosen as the cutoff value to distinguish the high- and low-risk groups (Figure 2D). The survival analysis showed striking differences between the two groups (Figure 2E). The mRNA expression of the 10 LUAC-specific CCRGs in each sample is shown in Figure 2F. The accuracy was evaluated by the area under the curve (AUC) of the ROC curve; as shown in Figure 2G, the AUC values were 0.76 at 1 year, 0.751 at 3 years, and 0.698 at 5 years.
The internal and external test groups were used to verify the accuracy of the model. Risk score analysis, survival analysis, and ROC analysis were performed repeatedly for each group (Figures 3A–D). The model could distinguish the high-risk group from the low-risk group efficiently. Survival analysis proved that the critical value remained valid. The AUC of the ROC curve in different groups further proved the robustness of the signature. The AUC values at 1, 3, and 5 years were 0.611, 0.665, and 0.679, respectively, for the internal test group; 0.664, 0.711, and 0.688 for the entire internal group; 0.768, 0.735, and 0.753 for the external validation group; and, finally, 0.674, 0.686, and 0.675 for all samples.
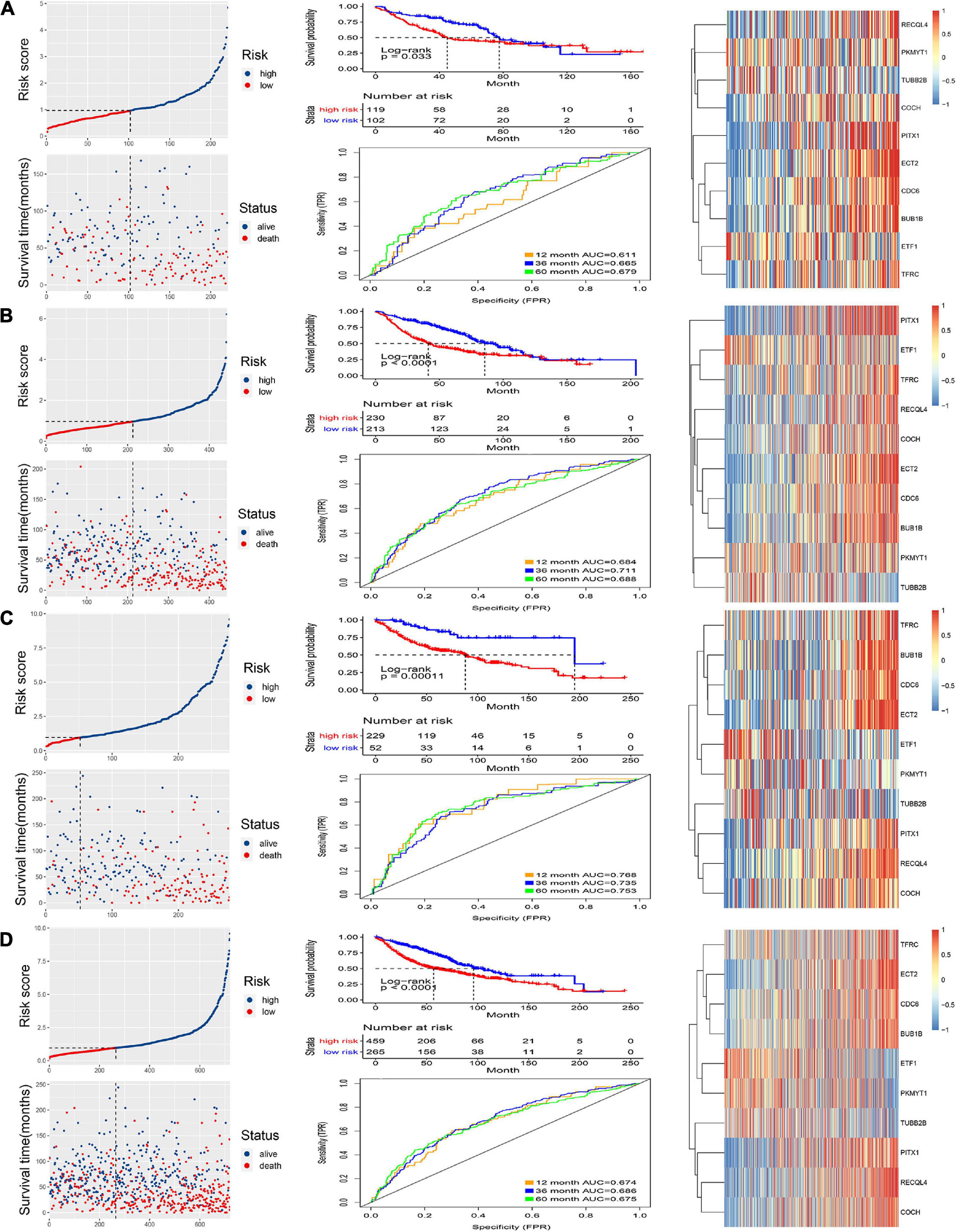
Figure 3. Validation of the signature. Risk score and survival analysis of the high and low groups, heatmap of the expression of 10 CCRGs, and the AUC of the ROC in (A) internal test group, (B) internal group, (C) external test group and (D) all samples.
In order to analyze the efficiency of the signature in different situations, patients were divided into different groups on the basis of different clinical characteristics (age, gender, lymph node metastasis, whether they received adjuvant radiotherapy, and whether they relapsed). Figures 4A–E shows the results of ROC and survival analysis for each group under different conditions; the OS rate of the high-risk group was significantly lower than that of the low-risk group. These results showed that the signature was highly efficient and stable in different situations. In addition, we used univariate and multivariate Cox regression in GSE68465, GSE42127, and GSE30219 to analyze the prognostic value of the risk score in specimens with different clinicopathological factors (Figures 5A–C).
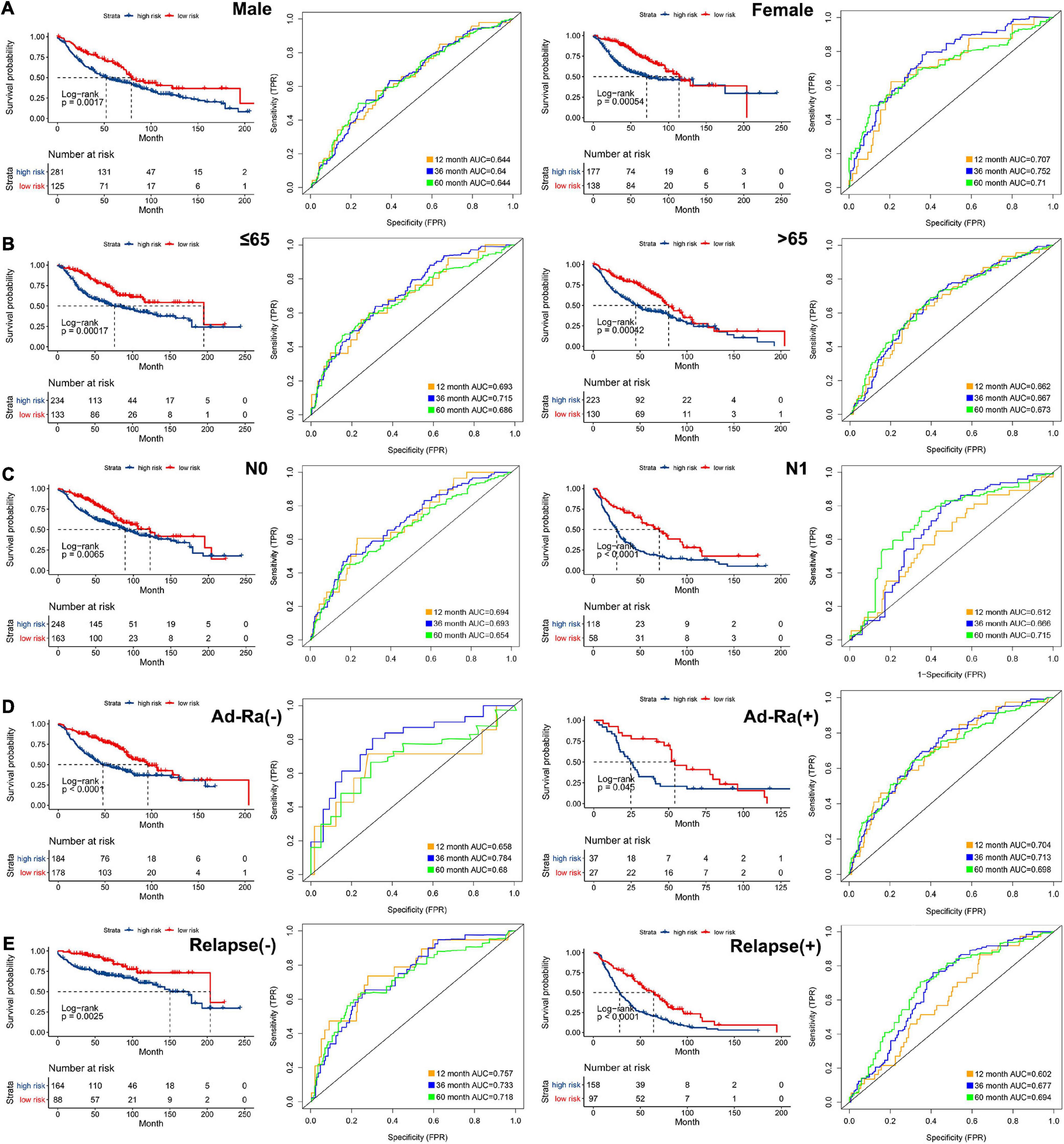
Figure 4. The AUC of the ROC that was computed by the signature under diverse situations. Survival analysis of the high and low groups and the AUC of the ROC in (A) gender, (B) age, (C) status of node metastasis, (D) whether they received adjuvant radiotherapy, and (E) whether relapsed.
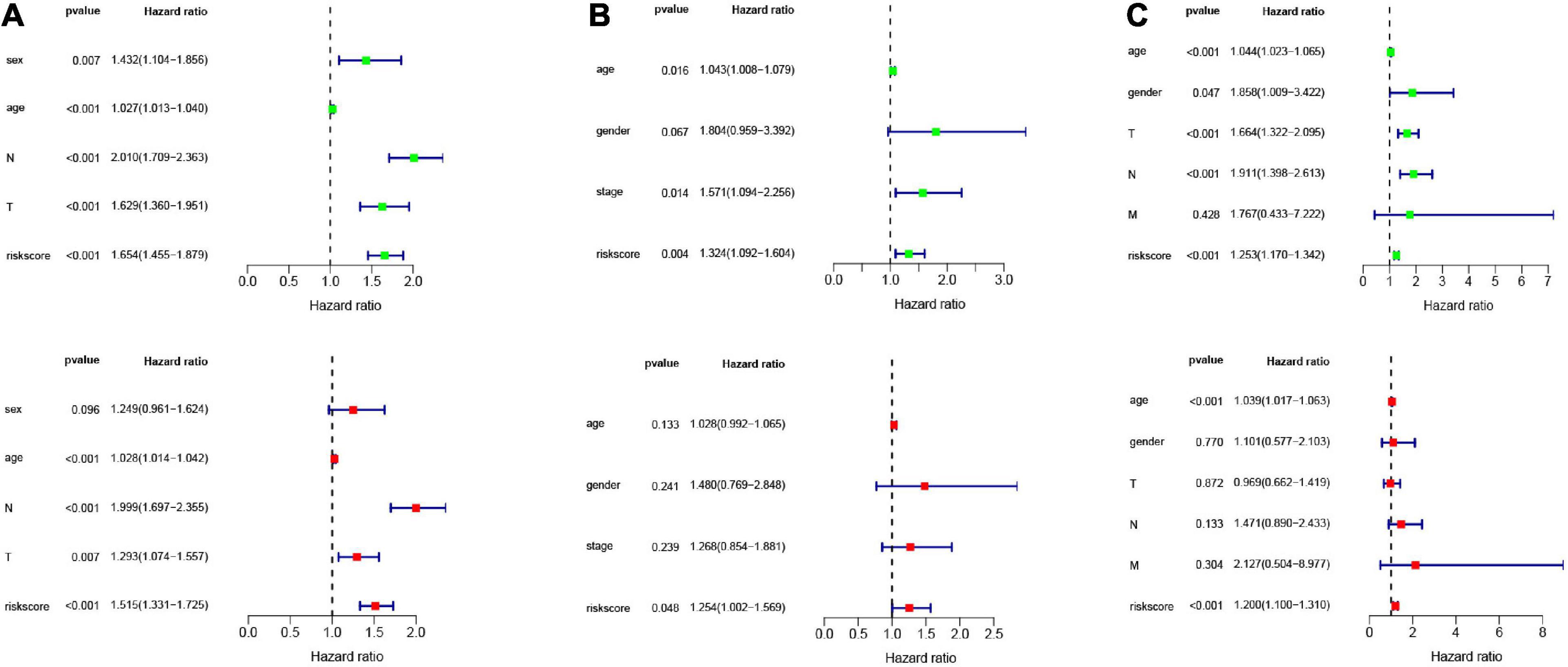
Figure 5. Univariate and multivariate Cox analysis of the signature combined clinical features. Univariate and multivariate Cox analysis in (A) GSE68465 (B) GSE42127 (C) GSE30219.
In order to integrate multiple predictors, show the relationship between the variables in the predictive model, we used the gender, age, relapse, T, M, N, and risk score to build a nomogram model (Figure 6A). The calibration curve was close to the ideal curve, indicating that the signature produced results consistent with the actual results (Figure 6B).
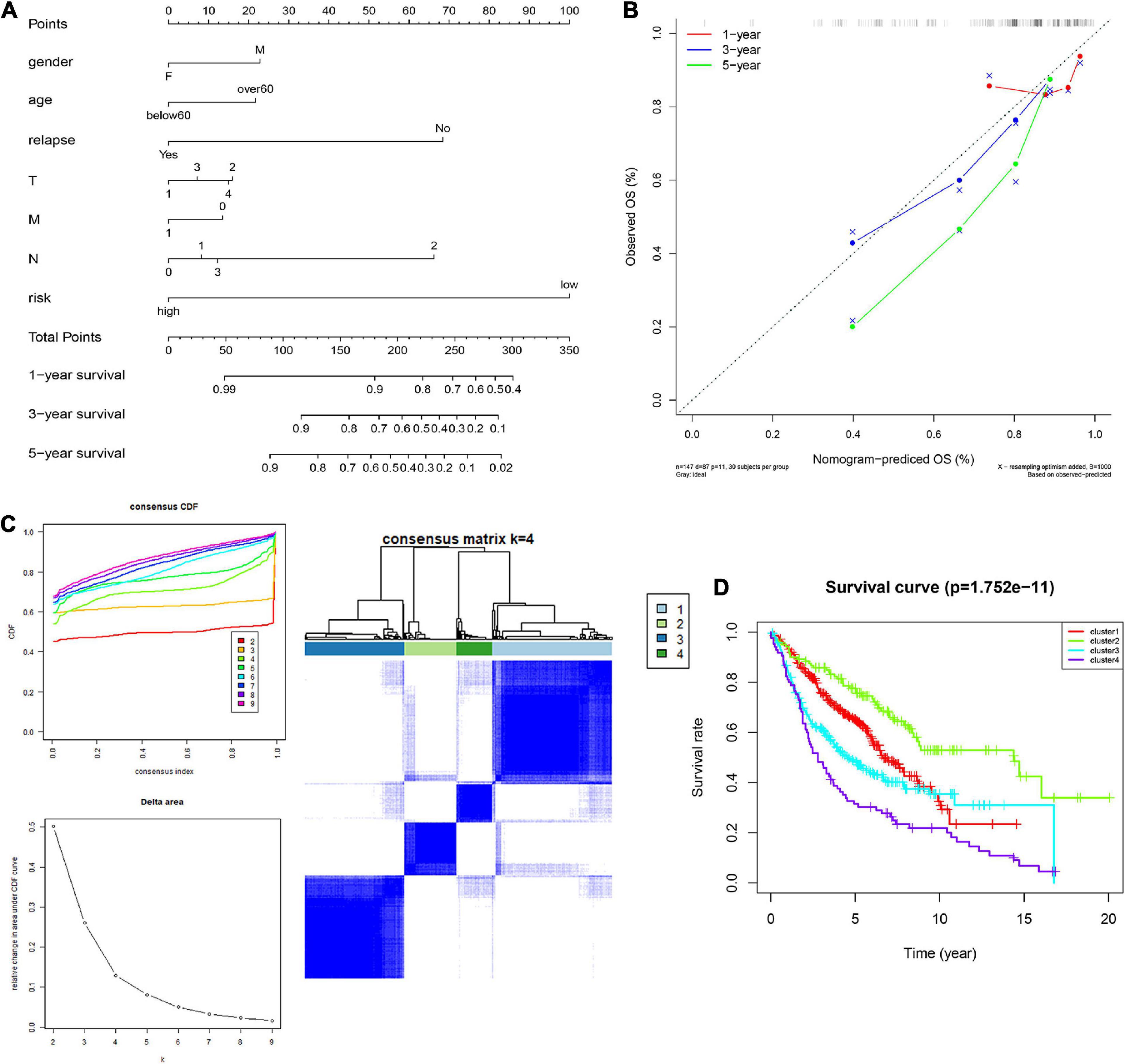
Figure 6. Construction and validation of the nomogram. (A) Details of the nomogram, (B) Calibration analysis based on the nomogram. (C) Molecular subgrouping based on 10 CCRGs: Elbow and gap plot for different numbers of subgroups; Consensus heatmap of the clusters. (D) Survival analysis of the four subgroups.
We further verified whether the 10 CCRGs could divide patients into different molecular subgroups. The subgroup effect was most significant with a k-value of 4. OS analysis of different molecular subtypes confirmed the prognostic significance of molecular subtype classification methods for clinical patients (Figures 6C,D). These findings suggest that the 10 CCRGs are potential LUAC biomarkers that could have a vital role in clinical treatment.
According to the CPTAC data, the protein expression of PKMYT1, ETF1, ECT2, BUB1B, and RECQL4 in tumor tissues was significantly increased, while the expression of TFRC was significantly reduced, and the expression of COCH and TUBB2B did not change significantly (PITX1 and CDC6 were not included) (Figure 7A). In the HPA data, compared with normal tissues, the expression of PKMYT1, ETF1, RECQL4, TUBB2B, and CDC6 in tumor tissues was remarkably upregulated, while the expression of TFRC and PITX1 was significantly downregulated (,ECT2, BUBB1B, and COCH were not included) (Figure 7B). In addition, through IHC, we found that ECT2 and BUBB1B are highly expressed in LUAC tissues (Figure 7C). The verification results basically coincided with the previous results.
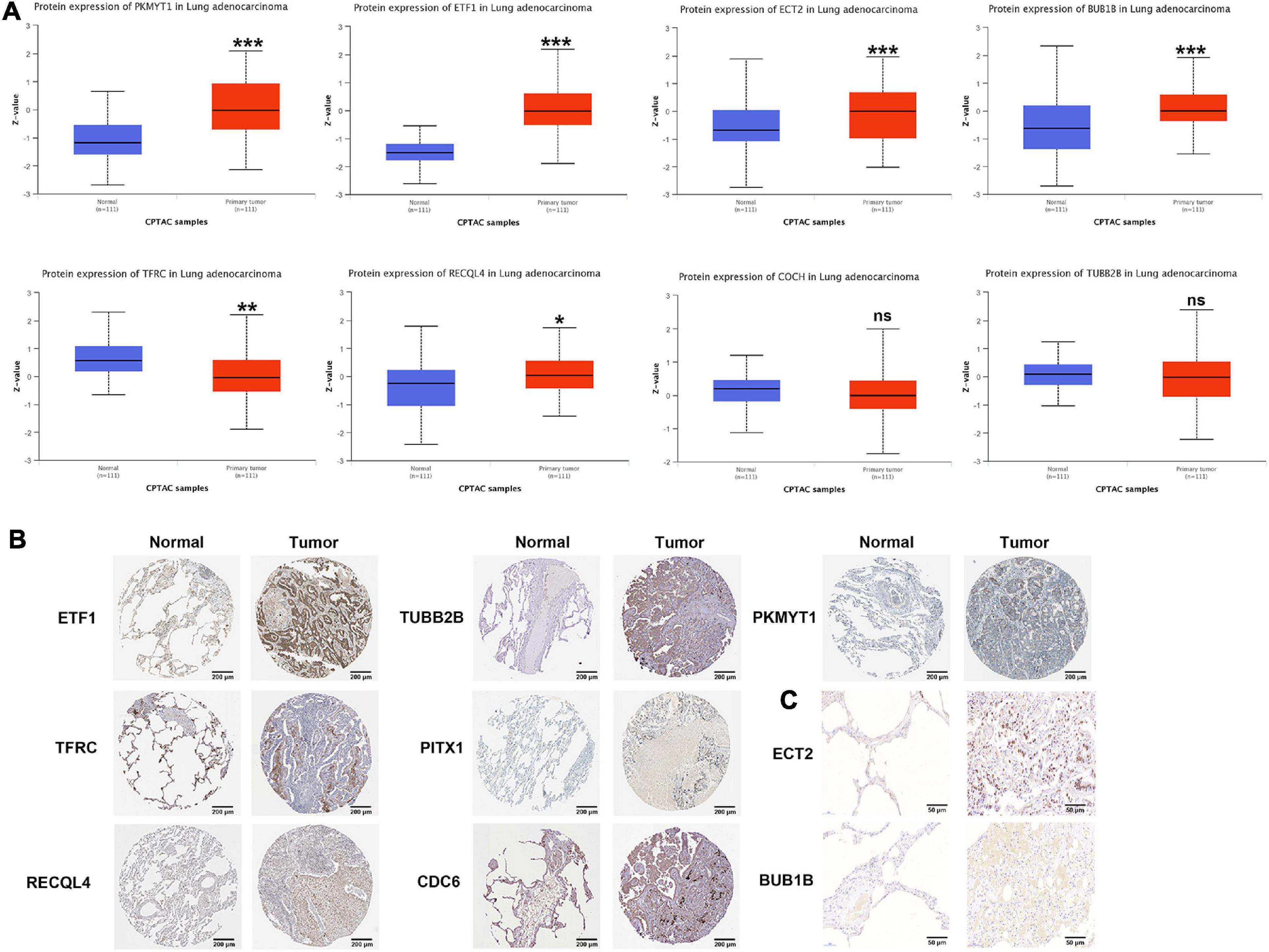
Figure 7. Protein level of CCRGs in LUAC tumor tissues and normal tissues. (A) CPTAC database. (B) HPA database. (C) IHC *P < 0.05, **P < 0.01, ***P < 0.001.
LC is the most common type of cancer and a major contributor to cancer-related deaths worldwide. It accounts for 11.6% of all cancers, and there were approximately 2.1 million new cases in 2018 (Bray et al., 2018). In addition to the prevalence of LC, the prognosis of most LC patients is very poor, with a 5 years predicted survival rate of about 17.8% (Zappa and Mousa, 2016). LUAC is the main type of LC. Therefore, there is an urgent need to find a more accurate way to diagnose LUAC patients and predict their prognosis.
Recent research has confirmed that genes changes can regulate the cell cycle in cancer. Precise regulation of the cell cycle is a basic requirement for eukaryotic cell homeostasis. The progression of the cell cycle consists of five consecutive phases: G0, G1, S, G2, and M (Schafer, 1998). A complex balance of different cyclin-dependent kinases (CDKs) and cyclins determines whether a cell enters the G1 phase of the cell cycle (Wood and Endicott, 2018). In addition, the G1 and G2 cell cycle checkpoints are considered to be an important part of regulating the cell cycle and are regulated by a variety of molecules (Afshari and Barrett, 1993). An extensive regulatory network composed of CCRGs is indispensable for the progression of the cell cycle. Understanding the expression levels of these factors and their combined regulatory modes is essential to predicting patient outcomes and prognosis. The development of drugs targeting one or more CCRGs may be the general trend of LUAC treatment in the future. At present, there is no research specifically analyzing which genes in CCRGs have an impact on the prognosis of LUAC patients. Therefore, it is of great significance to screen out these genes that play an important role in the progression of LUAC and the prognosis of patients.
In this work, we aimed to develop a prognostic signature related to the cell cycle. LUAC samples were divided into an internal training group, internal test group, and external test group. We used the internal training group to establish a prognostic model through Cox and LASSO regression analysis, and used the internal test group and external test group to verify the model. PKMYT1, ETF1, ECT2, BUB1B, RECQL4, TFRC, COCH, TUBB2B, PITX1, and CDC6 were screened out. We also studied some clinical features that may affect the survival of LUAC patients and constructed a nomogram to prove that the model can be better translated into clinical applications. Although new CCRGs are discovered every day, the use of genetic signatures can highlight the most vital markers for clinical applications.
In addition, we used the expression of these 10 CCRGs to classify LUAC patients into four different molecular subtypes. These four groups showed differences in prognosis, suggesting that these 10 genes have potential applications in LUAC diagnosis and treatment. The CPTAC and HPA databases were used to verify the differential expression of these genes. PKMYT1 is a membrane-associated kinase that can negatively regulate the G2/M transition of the cell cycle by phosphorylation and inactivation of CDK1 (Schmidt et al., 2017). In the cytoplasm, PKMYT1 can also promote the cytoplasmic separation of CDK1, thereby promoting the activation of mitosis-promoting factor and accelerating the cycle process (Lolli and Johnson, 2005). ETF1 is dysregulated in various types of cancer (Dubourg et al., 2002). ECT2 is a necessary link between the cell cycle machinery and Rho signaling pathways involved in the regulation of cell division, and its exchange function relies on its phosphorylation during the G2 and M phases (Tatsumoto et al., 1999). Expression of the BUB1B gene cannot be detected in G1 but reaches a peak in G2/M, and its absence can cause genome instability and the progression of LC (Myslinski et al., 2007). RecQL4 can protect chromosome stability by coordinating and regulating cell proliferation and cell cycle progression (Fang et al., 2018). TFRC accelerates cell proliferation and metastasis by upregulating AXIN2 in epithelial ovarian cancer (Huang et al., 2020). Methylation levels of COCH are elevated in the plasma of NSCLC patients with lymph node metastasis (Chen et al., 2020). Upregulation of TUBB2B may contribute to the development of neuroblastoma (Liu and Li, 2019). The p53 gene is the direct transcriptional target of PITX1 (Liu and Lobie, 2007). The synergistic effects of CDC6 and cyclin E induce DNA replication in resting cells when CDC6 and CDT1 are ectopically expressed (Borlado and Méndez, 2008). Our signature was constructed and verified in a comprehensive cohort and could be used in clinical practice with a large number of genes.
However, our research had some limitations. Our results based on data from TCGA, CPTAC, and HPA partially proved that 10 CCRGs are dysregulated in LUAC, suggesting that they may have vital roles in the occurrence and development of LUAC. Functional experiments are needed to further uncover the possible molecular regulatory mechanisms of these CCRGs.
The current study shows that the cell cycle pathway is mainly responsible for the occurrence and development of LUAC. A prognostic signature was constructed based on LUAC-specific CCRGs, which could be used for prognostic evaluation of LUAC patients. In addition, the specific CCRGs screened out could be used as new targets for the treatment of LUAC.
The datasets generated for this study can be found in the online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
YF: research design. WJ and JX: experiment implementation and drafting the manuscript. GL: literature search. ZL: help modify articles and collate references. CZ: review and revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant No. 81800279) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20180197).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.655950/full#supplementary-material
Supplementary Table 1 | Details of 801 CCRGs in LUAC.
Supplementary Table 2 | Details of 148 LUAC-specific CCRGs in LUAC.
CCRGs, cell-cycle-related genes; NSCLC, non-small-cell lung cancer; LC, lung cancer; LUAC, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; ssGSEA, single-sample gene set enrichment analysis; LASSO, least absolute shrinkage and selection operator; PKMYT1, protein kinase, membrane associated tyrosine/threonine 1; ETF1, eukaryotic translation termination factor 1; ECT2, epithelial cell transforming 2; BUB1B, BUB1 mitotic checkpoint serine/threonine kinase B; RECQL4, RecQ like helicase 4; TFRC, transferrin receptor; COCH, cochlin; TUBB2B, tubulin beta 2B class IIb; PITX1, paired like homeodomain 1; CDC6, cell division cycle 6.
Afshari, C. A., and Barrett, J. C. (1993). Cell cycle controls: potential targets for chemical carcinogens? Environ. Health Perspect. 101(Suppl. 5), 9–14. doi: 10.1289/ehp.93101s59
Arbour, K. C., and Riely, G. J. (2019). Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA 322, 764–774. doi: 10.1001/jama.2019.11058
Barbie, D. A., Tamayo, P., Boehm, J. S., Kim, S. Y., Moody, S. E., Dunn, I. F., et al. (2009). Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 462, 108–112. doi: 10.1038/nature08460
Borlado, L. R., and Méndez, J. (2008). CDC6: from DNA replication to cell cycle checkpoints and oncogenesis. Carcinogenesis 29, 237–243. doi: 10.1093/carcin/bgm268
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Chang, J. T., Lee, Y. M., and Huang, R. S. (2015). The impact of the cancer genome atlas on lung cancer. Transl. Res. 166, 568–585. doi: 10.1016/j.trsl.2015.08.001
Chen, Z., Xiong, S., Li, J., Ou, L., Li, C., Tao, J., et al. (2020). DNA methylation markers that correlate with occult lymph node metastases of non-small cell lung cancer and a preliminary prediction model. Transl. Lung Cancer Res. 9, 280–287. doi: 10.21037/tlcr.2020.03.13
Dubourg, C., Toutain, B., Hélias, C., Henry, C., Lessard, M., Le Gall, J. Y., et al. (2002). Evaluation of ETF1/eRF1, mapping to 5q31, as a candidate myeloid tumor suppressor gene. Cancer Genet. Cytogenet. 134, 33–37. doi: 10.1016/s0165-4608(01)00605-7
Edgar, R., Domrachev, M., and Lash, A. E. (2002). Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210. doi: 10.1093/nar/30.1.207
Evison, M. (2020). The current treatment landscape in the UK for stage III NSCLC. Br J Cancer 123(Suppl. 1), 3–9. doi: 10.1038/s41416-020-01069-z
Fang, H., Niu, K., Mo, D., Zhu, Y., Tan, Q., Wei, D., et al. (2018). RecQL4-Aurora B kinase axis is essential for cellular proliferation, cell cycle progression, and mitotic integrity. Oncogenesis 7:68. doi: 10.1038/s41389-018-0080-4
Herbst, R. S., Morgensztern, D., and Boshoff, C. (2018). The biology and management of non-small cell lung cancer. Nature 553, 446–454. doi: 10.1038/nature25183
Hight, S. K., Mootz, A., Kollipara, R. K., McMillan, E., Yenerall, P., Otaki, Y., et al. (2020). An in vivo functional genomics screen of nuclear receptors and their co-regulators identifies FOXA1 as an essential gene in lung tumorigenesis. Neoplasia 22, 294–310. doi: 10.1016/j.neo.2020.04.005
Hsiao, Y. J., Chang, W. H., Chen, H. Y., Hsu, Y. C., Chiu, S. C., Chiang, C. C., et al. (2020). MITF functions as a tumor suppressor in non-small cell lung cancer beyond the canonically oncogenic role. Aging (Albany N. Y.) 13, 646–674. doi: 10.18632/aging.202171
Huang, Y., Huang, J., Huang, Y., Gan, L., Long, L., Pu, A., et al. (2020). TFRC promotes epithelial ovarian cancer cell proliferation and metastasis via up-regulation of AXIN2 expression. Am. J. Cancer Res. 10, 131–147.
Irizarry, R. A., Hobbs, B., Collin, F., Beazer-Barclay, Y. D., Antonellis, K. J., Scherf, U., et al. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264. doi: 10.1093/biostatistics/4.2.249
Johnson, D. H., Schiller, J. H., and Bunn, P. A. Jr. (2014). Recent clinical advances in lung cancer management. J. Clin. Oncol. 32, 973–982. doi: 10.1200/jco.2013.53.1228
Kanehisa, M. (2002). The KEGG database. Novartis Found. Symp. 247, 91–101; discussion 101-103, 119-128, 244-152.
Lamberti, G., Andrini, E., Sisi, M., Rizzo, A., Parisi, C., Di Federico, A., et al. (2020). Beyond EGFR, ALK and ROS1: current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit. Rev. Oncol. Hematol. 156:103119. doi: 10.1016/j.critrevonc.2020.103119
Liberzon, A., Subramanian, A., Pinchback, R., Thorvaldsdóttir, H., Tamayo, P., and Mesirov, J. P. (2011). Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740. doi: 10.1093/bioinformatics/btr260
Lin, S. F., Zheng, Y. Z., Li, X. Q., Xu, H. P., Wang, J. J., Wang, W., et al. (2020). Impact of treatment modality on long-term survival of stage IA small-cell lung cancer patients: a cohort study of the U.S. SEER database. Ann. Transl. Med. 8:1292. doi: 10.21037/atm-20-5525
Liu, D. X., and Lobie, P. E. (2007). Transcriptional activation of p53 by Pitx1. Cell Death Differ. 14, 1893–1907. doi: 10.1038/sj.cdd.4402209
Liu, J., and Li, Y. (2019). Upregulation of MAPK10, TUBB2B and RASL11B may contribute to the development of neuroblastoma. Mol. Med. Rep. 20, 3475–3486. doi: 10.3892/mmr.2019.10589
Liu, X., Zhou, M., Wang, F., Mubarik, S., Wang, Y., Meng, R., et al. (2020). Secular trend of cancer death and incidence in 29 cancer groups in China, 1990-2017: a joinpoint and age-period-cohort analysis. Cancer Manag. Res. 12, 6221–6238. doi: 10.2147/cmar.S247648
Lolli, G., and Johnson, L. N. (2005). CAK-cyclin-dependent activating kinase: a key kinase in cell cycle control and a target for drugs? Cell Cycle 4, 572–577.
Mi, H., Muruganujan, A., Ebert, D., Huang, X., and Thomas, P. D. (2019). PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 47, D419–D426. doi: 10.1093/nar/gky1038
Myslinski, E., Gérard, M.-A., Krol, A., and Carbon, P. (2007). Transcription of the human cell cycle regulated BUB1B gene requires hStaf/ZNF143. Nucleic Acids Res. 35, 3453–3464. doi: 10.1093/nar/gkm239
Peng, Z., Wang, J., Shan, B., Yuan, F., Li, B., Dong, Y., et al. (2017). Genome-wide analyses of long noncoding RNA expression profiles in lung adenocarcinoma. Sci. Rep. 7:15331. doi: 10.1038/s41598-017-15712-y
Pontén, F., Jirström, K., and Uhlen, M. (2008). The human protein atlas–a tool for pathology. J. Pathol. 216, 387–393. doi: 10.1002/path.2440
Rousseaux, S., Debernardi, A., Jacquiau, B., Vitte, A. L., Vesin, A., Nagy-Mignotte, H., et al. (2013). Ectopic activation of germline and placental genes identifies aggressive metastasis-prone lung cancers. Sci. Transl. Med. 5:186ra166. doi: 10.1126/scitranslmed.3005723
Rudnick, P. A., Markey, S. P., Roth, J., Mirokhin, Y., Yan, X., Tchekhovskoi, D. V., et al. (2016). A description of the clinical proteomic tumor analysis consortium (CPTAC) common data analysis pipeline. J. Proteome Res. 15, 1023–1032. doi: 10.1021/acs.jproteome.5b01091
Schafer, K. A. (1998). The cell cycle: a review. Vet. Pathol. 35, 461–478. doi: 10.1177/030098589803500601
Schmidt, M., Rohe, A., Platzer, C., Najjar, A., Erdmann, F., and Sippl, W. (2017). Regulation of G2/M transition by inhibition of WEE1 and PKMYT1 kinases. Molecules (Basel Switzerland) 22, 2045. doi: 10.3390/molecules22122045
Shedden, K., Taylor, J. M., Enkemann, S. A., Tsao, M. S., Yeatman, T. J., Gerald, W. L., et al. (2008). Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat. Med. 14, 822–827. doi: 10.1038/nm.1790
Sun, D., Zong, Y., Cheng, J., Li, Z., Xing, L., and Yu, J. (2021). GINS2 attenuates the development of lung cancer by inhibiting the STAT signaling pathway. J. Cancer 12, 99–110. doi: 10.7150/jca.46744
Tatsumoto, T., Xie, X., Blumenthal, R., Okamoto, I., and Miki, T. (1999). Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J. Cell Biol. 147, 921–928. doi: 10.1083/jcb.147.5.921
Wood, D. J., and Endicott, J. A. (2018). Structural insights into the functional diversity of the CDK-cyclin family. Open Biol. 8:180112. doi: 10.1098/rsob.180112
Yu, H. A., Planchard, D., and Lovly, C. M. (2018). Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am. Soc. Clin. Oncol. Educ. Book 38, 726–739. doi: 10.1200/edbk_201331
Yu, Y., Huang, R., Wang, P., Wang, S., Ling, X., Zhang, P., et al. (2020). Sublobectomy versus lobectomy for long-term survival outcomes of early-stage non-small cell lung cancer with a tumor size = 2 cm accompanied by visceral pleural invasion: a SEER population-based study. J. Thorac. Dis. 12, 592–604. doi: 10.21037/jtd.2019.12.121
Keywords: lung adenocarcinoma, cell cycle-related genes, prognostic signature, overall survival, GEO
Citation: Jiang W, Xu J, Liao Z, Li G, Zhang C and Feng Y (2021) Prognostic Signature for Lung Adenocarcinoma Patients Based on Cell-Cycle-Related Genes. Front. Cell Dev. Biol. 9:655950. doi: 10.3389/fcell.2021.655950
Received: 19 January 2021; Accepted: 02 March 2021;
Published: 18 March 2021.
Edited by:
Yueming Sun, Nanjing Medical University, ChinaReviewed by:
Hongcheng Zhu, Fudan University, ChinaCopyright © 2021 Jiang, Xu, Liao, Li, Zhang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengpeng Zhang, emNweGptMTk5NkAxNjMuY29t; Yu Feng, ZmVuZ3l1MUBzdWRhLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.