
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 19 March 2021
Sec. Cell Growth and Division
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.654028
This article is part of the Research TopicQuality Control of Mammalian Oocyte Meiotic Maturation: Causes, Molecular Mechanisms and SolutionsView all 40 articles
Meiosis is the basis of sexual reproduction. In female mammals, meiosis of oocytes starts before birth and sustains at the dictyate stage of meiotic prophase I before gonadotropins-induced ovulation happens. Once meiosis gets started, the oocytes undergo the leptotene, zygotene, and pachytene stages, and then arrest at the dictyate stage. During each estrus cycle in mammals, or menstrual cycle in humans, a small portion of oocytes within preovulatory follicles may resume meiosis. It is crucial for females to supply high quality mature oocytes for sustaining fertility, which is generally achieved by fine-tuning oocyte meiotic arrest and resumption progression. Anything that disturbs the process may result in failure of oogenesis and seriously affect both the fertility and the health of females. Therefore, uncovering the regulatory network of oocyte meiosis progression illuminates not only how the foundations of mammalian reproduction are laid, but how mis-regulation of these steps result in infertility. In order to provide an overview of the recently uncovered cellular and molecular mechanism during oocyte maturation, especially epigenetic modification, the progress of the regulatory network of oocyte meiosis progression including meiosis arrest and meiosis resumption induced by gonadotropins is summarized. Then, advances in the epigenetic aspects, such as histone acetylation, phosphorylation, methylation, glycosylation, ubiquitination, and SUMOylation related to the quality of oocyte maturation are reviewed.
Uncovering the signals involved in controlling the resumption of oocyte meiosis is a major issue in female reproductive biology. The meiosis initiation and resumption of oocytes is different from sperm in at least three aspects. Female germ cells enter and undergo the first meiotic progression during embryonic development, and arrest at the diplotene stage of prophase I before birth. And, some of the arrested oocytes within fully grown follicles will resume meiosis after puberty in response to luteinizing hormones (LHs) during each estrous (animal) or menstrual cycle (human) (Mehlmann, 2005). Last, the cell division of oocytes is known as asymmetric cytokinesis. Interestingly, whenever fully grown oocytes are released from follicles and cultured in appropriate medium in vitro, spontaneous resumption happens as well (Pincus and Enzmann, 1935). Oocyte meiotic maturation is a complicated and vital process used to attain full competence required for the oocyte as well as early embryonic development. An oocyte arrested at meiotic prophase I contains a large nucleus covered by a nuclear envelope, which is known as the germinal vesicle (GV). With the arrival of LH surge, serial processes related to oocyte nuclear maturation, such as chromatin condensation and germinal vesicle breakdown (GVBD), occur in oocytes of fully grown follicles. After GVBD, oocytes enter the metaphase I (MI) stage (Moor et al., 1998). Later, after extrusion of the first polar body (PB1) containing a small portion of cytoplasm, an oocyte containing one set of chromosomes completes meiosis I. Very soon after that, the second meiosis starts and the oocyte (mature egg) arrests at metaphase II (MII) until fertilization. Actually, the oocyte accomplishes its meiosis progress only when fertilization happens.
In humans and animals, multiple factors including epigenetic molecules and different signaling pathways have been identified and proven to be pivotal for meiotic maturation. They not only regulate oocytes maturation, but also coordinate with each other to ensure good oocyte quality. This article aims to review the events and development around the quality control of mammalian oocyte meiotic maturation in nuclear and cytoplasm aspects, of which, the underlying molecular mechanisms are discussed to provide detailed information for better understanding of meiosis.
Before an oocyte is enclosed by ovarian granulosa cells to form primordial follicles, meiosis has been initiated and the cell has arrested at the diplotene stage of prophase I (Bowles et al., 2006; Bowles and Koopman, 2007). When females are sexually mature, a small portion of primordial follicles will be activated and start to grow gradually. Previous studies have indicated that molecules such as cyclic adenosine monophosphate (cAMP) within growing oocytes and the natriuretic peptide precursor type C (NPPC)/natriuretic peptide receptor 2 (NPR2) system in granulosa cells play essential roles in maintaining oocyte meiotic arrest during the long developmental journey. Later, oocytes in fully grown follicles in response to gonadotropins stimulation possess the capability to resume meiosis and ovulate in vivo.
In mammals, meiotic arrest is regulated by a high level of cAMP in the oocyte (Conti et al., 2002; Mehlmann, 2005). When oocytes are isolated from the antral follicles, the cAMP levels within the oocytes decrease and meiosis resumes spontaneously (Törnell et al., 1990). On the contrary, when they are cultured with the cAMP analog dibutyryl cAMP (dbcAMP) or cAMP phosphodiesterase (PDE) inhibitors such as isobutyl methyl xanthine (IBMX) and milrinone, the spontaneous meiotic maturation of mouse oocytes is prevented (Cho et al., 1974; Dekel et al., 1981; Schultz et al., 1983; Vivarelli et al., 1983; Eppig et al., 1985; Aktas et al., 1995). Therefore, a constantly higher level of cAMP becomes the priority for oocytes to sustain meiosis at the GV stage.
cAMP in oocytes plays a central role in the regulation of meiosis arrest (Zhang et al., 2009). Oocytes possess all of the necessary proteins including adenylyl cyclase (AC), Gs protein, and G protein-coupled receptor 3 (GPR3) for producing cAMP themselves. AC is responsible for specifically catalyzes ATP to form cAMP, and Gs protein, which stimulates AC3 activity in oocytes (Horner et al., 2003; Hinckley et al., 2005; Mehlmann, 2005). Mice oocytes lacking AC3 expression fail to maintain meiosis arrest (Horner et al., 2003). Similarly, blocking Gs function causes spontaneous resumption of meiosis in follicle-enclosed mouse oocytes (Mehlmann et al., 2002; Kalinowski et al., 2004). GPR3, which is located in the oocyte plasma membrane, is necessary to stimulate Gs activity and elevate the level of cAMP (Kalinowski et al., 2004). This is approved by the fact that oocytes undergo spontaneous meiotic resumption at an early antral stage in GPR3 KO mice and the phenomenon can be reversed by injection of GPR3 mRNA into the oocyte (Freudzon, 2005). The studies in pig oocytes are consistent with those in mice (Yang et al., 2012a). Although GPR3 is expressed in the human oocyte, it contributes nothing to premature ovarian failure, which is unlike the phenotype of GPR3 KO mice (Kovanci et al., 2008). While GPR and Gs are functional in generating intrinsic cAMP, PDE in mice oocytes is responsible for the degradation of cAMP (Sasseville et al., 2006). In a PDE3 knockout model, oocytes are permanently arrested at the GV stage and female mice are infertile (Vaccari et al., 2008). Specifically, inhibition of PDE3 elevates cAMP level and prevents oocyte spontaneous maturation simultaneously in cultured cumulus-oocyte-complexes (COCs) or denuded oocytes (DOs) (Kovanci et al., 2008). Simultaneously knockout of GPR3 and PDE3A result in oocyte maturation (Vaccari et al., 2008).
Meiosis inhibition is a process in which oocytes coordinate with granulosa cells to sustain a high level of cAMP. Cumulative data have proven that intrinsic cAMP produced by oocyte alone is not sufficient to maintain meiotic arrest. Instead, a sustained high level of cAMP in the oocyte depends on cGMP, which is produced in the surrounding granulosa cells, possibly by suppressing PDE3A activity (Zhang et al., 2010; Shuhaibar et al., 2015; Jaffe and Egbert, 2017). Generally, cGMP is produced from GTP by guanylyl cyclases in mural granulosa cells (MGCs) and cumulus granulosa cells (CGCs) and is transported to oocytes.
cGMP production in CGCs relies on the coordination of MGCs-secreted NPPC conjugating with its receptor, guanylyl cyclase NPR2 which is found on the membrane of CGCs. NPPC and NPR2 are both highly expressed in follicular granulosa cells (Zhang et al., 2010; Jaffe and Egbert, 2017). NPPC inhibits the spontaneous GVBD in COCs, but not in DOs in vitro. Besides, NPR2 mutant mice are infertile due to premature resumption of meiosis because of the shortage of cGMP production in CGCs, which results in oocyte fragmentation and poor embryo development (Geister et al., 2012; Tsuji et al., 2012). Consistently, applying NPPC in cultured COCs contributes to preventing spontaneous oocyte maturation by increasing the cGMP levels in the CGCs (Zhang et al., 2010). Together, these results suggest that cGMP produced in granulosa cells play a vital role in keeping the cAMP level high in the oocyte, and that maintaining oocyte meiotic arrest requires coordination between granulosa cells and an oocyte within a follicle.
How is the NPPC/NPR2 signaling pathway regulated in granulosa cells? One of the important actions of follicle stimulation hormones (FSHs) on MGCs and CGCs of antral follicles is to sustain high levels of NPPC/NPR2 in humans, rodents, and pig (Jankowski et al., 1997; Kawamura et al., 2011). Pregnant mare serum gonadotrophin (PMSG) that possesses primarily FSH activity induces the expression of NPPC and NPR2 mRNA in the ovary (Zhang and Xia, 2012). This is further approved by the fact that estrogen-promoted NPPC expression in granulosa cells can be enhanced by interaction with FSH (Lee et al., 2013). However, the oocytes within antral follicles did not show precocious resumption of meiosis after deletion of the estrogen receptor or Cyp19α1 (aromatase) (Krege et al., 1998; Dupont et al., 2000; Kiyama and Wada-Kiyama, 2015), possibly implying that there are other pathways mediating NPPC/NPR2 action. In line with this speculation, we have proved that the expression of the NPPC/NPR2 system in ovarian granulosa cells is up regulated by sex hormones, such as androgen and estrogen through respective hormone receptors (AR and ER) in physiological conditions, in polycystic ovary syndrome (PCOS) in mice ovaries, and in in vitro cultured granulosa cell lines (Liu et al., 2017; Reis and Honorato-Sampaio, 2018; Wang et al., 2018). Therefore, NPPC/NPR2 as a specific pathway potentially helps to explain the mechanism of the ovulatory disruption in PCOS (Reis and Honorato-Sampaio, 2018). In addition, Yang et al. (2019) proved that transforming growth factor β (TGF-β) could regulate the expression of NPPC in MGCs and oocyte maturation. In the presence of FSH, TGF-β further increased NPPC levels and inhibited the oocyte meiotic resumption of COCs (Yang et al., 2019). Interestingly, supplementary natriuretic peptide precursor type B (NPPB) and NPPC are effective at improving the developmental competence of oocytes recovered from small-sized antral follicles of porcine in vitro (Zhang W. et al., 2015; Zhang Y. et al., 2017).
Importantly, one of the important roles of LH surge is to downregulate the level of the NPPC/NPR2 system in MGCs and CGCs as well. The levels of NPPC/NPR2, as well as the activity of NPR2, are either completely decreased or inhibited in mouse and human ovaries after the activation of LH receptors, which occurs sufficiently earlier than GVBD. The underlined mechanism could be that LH significantly decreases AR and ER levels, and thus decreases NPPC/NPR2 levels and induces oocyte maturation (Liu et al., 2017; Wang et al., 2018; Yang et al., 2019). By suppressing the NPPC/NPR2 system, LH reduces cGMP level in CGCs as well as oocytes rapidly. Besides, the reduced cGMP level in oocytes releases PDE3A from the inhibitory state. As a result, cAMP is degraded and the maturation promoting factor (MPF) is activated, which induces the resumption of meiosis (Norris et al., 2009). However, it remains unclear how LH and FSH specifically regulate the expression of AR, ER, and TGF-β. The regulations of granulosa cells cooperate with oocytes to maintain oocyte meiotic arrest in mice, which are summarized in Figure 1.
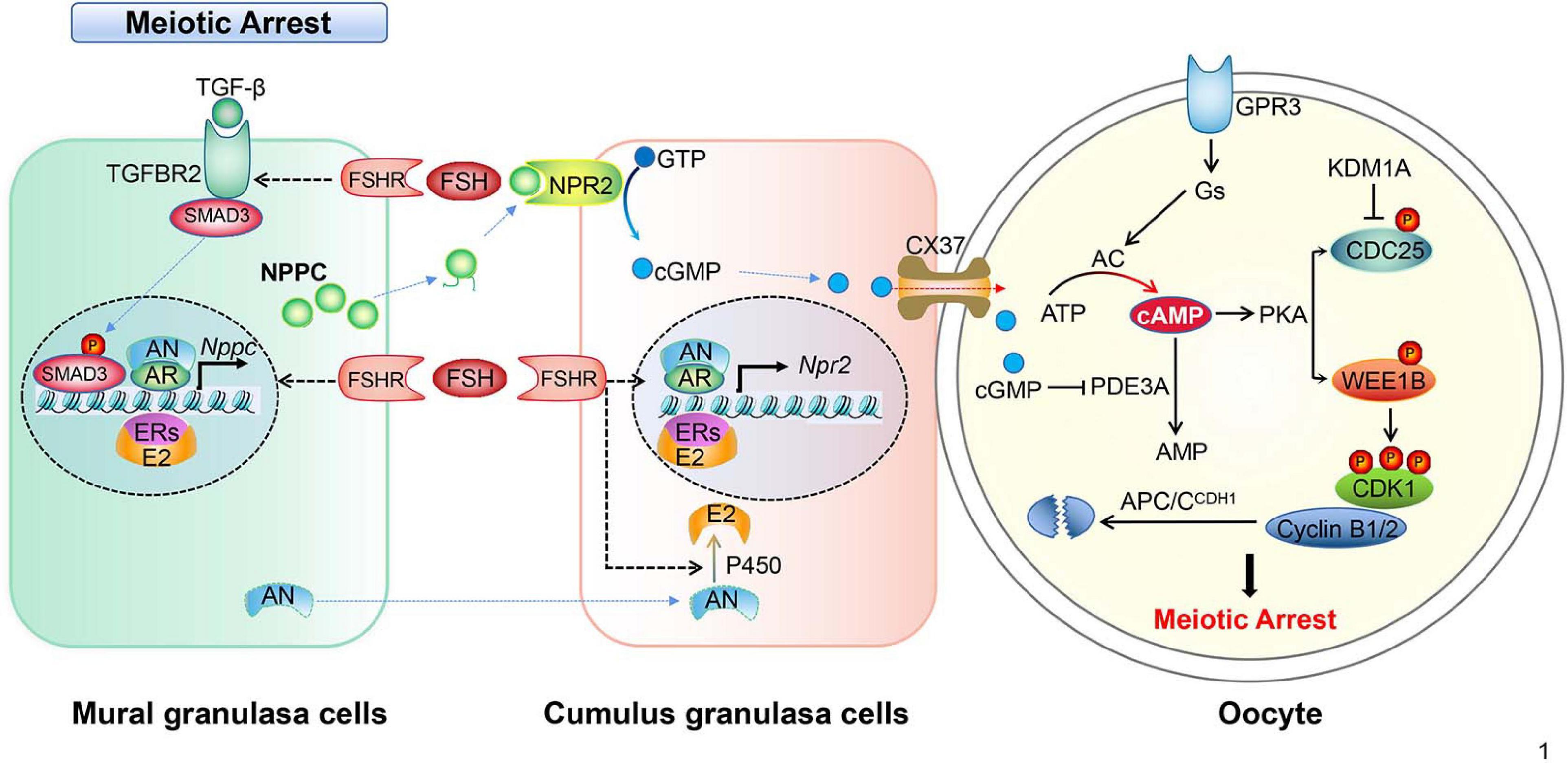
Figure 1. Schematic model depicting the mechanisms of meiotic arrest. Meiotic arrest in fully grown oocytes is required by the synthesis and maintenance of high levels of cAMP, the arrest state is maintained by the cooperation of granulosa cells and oocytes in the follicles. In mural granulosa cells, FSH binds its receptor (FSHR), collaborating with androgen/AR, estrogen/ER, and the TGF-β/TGFBR2 signal pathway to promote NPPC transcription and increase NPPC production. In cumulus granulosa cells, FSH binds FSHR, collaborating with androgen/AR and estrogen/ER to promote NPR2 transcription and increase NPR2 production. NPPC actives NPR2, GTP is converted into cGMP, then cGMP enters the oocyte through CX37 gap junctions. In oocytes, cGMP inhibits PDE3A activity, prevents the degradation of cAMP, cAMP activates protein kinase A (PKA) that in turn activates the WEE1B kinase and inhibits the CDC25B phosphatase leading to the inactivation of CDK1. In addition, CDC25B protein level is inhibited by histone lysine demethylases 1A (KDM1A). The constant degradation of cyclin B1/2 (cycB1/2) by APC/CDH1 prevents MPF activation in the arrested oocytes.
Fully grown oocytes in early antral and preovulatory follicles have the capability to resume meiosis before LH surge (Holt et al., 2010). According to the hypothalamus-pituitary-ovary axis feedback theory, an LH surge in response to a peak estrogen surge initiates oocytes meiosis in vivo through positive feedback regulation. LH surge produces rapid changes in MGCs via intracellular pathways and extracellular paracrine loops. In brief, LH binds to the LH receptor (LHR) located in the membrane of theca cells and the MGCs of a follicle. As a result, the activated LHR induces serial affairs in follicular granulosa cells and oocytes. It reduces the cAMP level within the oocyte through downregulating the NPPC/NPR2 system and shutting down gap junctions between the oocyte and CGCs (Egbert et al., 2014; Shuhaibar et al., 2016). Also, it upregulates the activity of the epidermal-like growth factors (EGF) network in MGCs/CGCs (Conti et al., 2012; Jaffe and Egbert, 2017). In the oocyte, reduced cAMP levels activates the activity of MPF, which in turn phosphorylates proteins including APC and initiates GVBD and chromosome segregation (Adhikari and Liu, 2014).
The mechanism of how high levels of cAMP are necessary to prevent meiotic maturation in oocytes is more or less fully understood. It is clear that cAMP exerts its role by activating protein kinase A (PKA). PKA balances the activities of WEE1B/MYT1 kinase and CDC25 phosphatase, and thus regulates the activity of cyclin-dependent kinase 1 (CDK1). Briefly, the CDK1 and cyclin B complex, namely MPF, is essential for oocytes meiotic maturation (Jaffe and Egbert, 2017). The ability of CDK1 to phosphorylate target proteins at specific serine and threonine residues depends on its activity and binding with the cyclin B (Jones, 2004; Jaffe and Egbert, 2017). It has been found that activated CDK1 triggers CXXC-finger protein 1 (CXXC1, also known as CFP1) phosphorylation and degradation following meiotic resumption. The degradation of CFP1 ensures the absence of the SET domain containing 1 (SETD1)-CXXC1 complex from chromatin, thereby facilitating chromosome condensation during oocyte maturation. Besides, CFP1 coordinates histone H3 lysine-4 trimethylation and meiotic cell cycle progression in mouse oocytes (Sha et al., 2018). Therefore, one of the key points to initiate oocyte meiosis depends on when to activate CDK1. In arrested oocytes, a sustained high level of cAMP activates PKA, which in turn activates WEE1. WEE1 inactivates while CDC25 activates CDK1 through phosphorylates or dephosphorylates the Thr14 and Tyr15 residues of CDK1, respectively (Chen et al., 2001; Adhikari et al., 2016; Jaffe and Egbert, 2017). Thus, the activity of MPF is indirectly controlled by the level of cAMP (Jones, 2004; Han et al., 2005; Han and Conti, 2006; Kovo et al., 2006). Interestingly, epigenetic molecules, such as histone lysine demethylases KDM1A (also known as LSD1), are involved in regulating the expression of CDC25B to maintain meiotic arrest. Conditional deletion of LSD1 in growing oocytes results in precocious resumption of meiosis and spindle and chromosomal abnormalities (Kim et al., 2015).
Synthesis and accumulation of cyclin B1 and its interaction with CDK1 have long been considered prerequisites for oocyte MPF activation as well. As part of the MPF, cyclin B1 must be constantly degraded by a multi-subunit ubiquitin E3 ligase named the anaphase promoting complex (APC) to maintain meiosis arrest (Jaffe and Egbert, 2017). During this time, the role of cadherin 1 (CDH1) is important because it is an activator of the APC (Reis et al., 2006). Before GVBD happens, cyclin B1 translocation from the cytoplasm into the nucleus is required (Marangos and Carroll, 2004; Holt et al., 2010; Jaffe and Egbert, 2017). Interestingly, cyclin B1-null oocytes resumed and finished meiosis I but are then arrested at the meiosis interphase when cyclin B2 is available, indicating that cyclin B2 compensates for the shortage of cyclin B1 in oocyte meiosis I (Holt et al., 2010; Li et al., 2019).
Gap junction provides a direct communication channel between cells which allow molecules smaller than 1,000 Da be transferred to the adjacent cells (Nicholson and Bruzzone, 1997; Simon and Goodenough, 1998; Arroyo et al., 2020). In mice, as many as 20 connexins (Cxs) participate in forming the channels of the gap junction. Inside a follicle, cGMP produced in CGCs diffuses into oocytes through Cx43 and Cx37 GJs and thus elevates oocyte cGMP level (Solc et al., 2010). Importantly, the closure of GJs between MGCs and CGCs and between CGCs and oocytes are targets of LH signaling (Anderson and Albertini, 1976; Gilula et al., 1978). For instance, LH inhibits Cx43 translation and breaks down GJs to prevent cAMP and cGMP diffusion into the oocyte, which results in PKA inactivation and triggers the initiation of oocyte maturation (Kalma et al., 2004; Edry et al., 2006; Sela-Abramovich et al., 2006).
Of all connexins, Cx43 and Cx37 are the most studied ones in the follicle and may possess equal importance to folliculogenesis. In mice, Cx43 is mainly expressed in gap junctions between GCs and is regulated by extracellular signal regulated kinase-1 and -2 (ERK1/ERK2) signals in response to LH surge in vivo (Su et al., 2002; Sela-Abramovich et al., 2005; Dekel, 2009). However, PKCε-mediated mitogen-activated protein kinase (MAPK)-dependent signals might contribute to Cx43 phosphorylation in CGCs during FSH-induced oocyte meiotic resumption in vitro (Cai et al., 2018). Ovaries lacking Cx43 do not proceed beyond the primary follicle stage. Also, Cx37, which is mainly expressed between the oocyte and CGCs, is essential to oocyte growth and survival, which in turn is necessary to maintain proper MGC function (Li et al., 2007; Gershon et al., 2008). In Cx37-knockout mice, folliculogenesis is arrested at the early antral stage and this disruption results in sterility because mutant oocytes grow slowly and cannot survive (Carabatsos et al., 2000). To examine the roles that Cx37 and Cx43 play in oogenesis, a transgenic mouse model, in which Cx37 specifically replaced Cx43 in growing oocytes, was made. The generations of Cx43 transgene mice driven by zona pellucida 3 (ZP3) crossed with Cx37-null mice are fertile due to the restoration of oocyte–granulosa cell coupling, oocyte growth, and oocyte maturation (Li et al., 2007). Thus, despite their different properties, Cx43 may be physiologically equivalent to Cx37 in coupling oocytes with granulosa cells. Both of them are indispensable in the regulation of oocyte maturation.
EGF-related proteins are a set of proteins that respond to the LH signal and promote oocyte maturation. Different epidermal-like growth factors, such as amphiregulin (AREG), epiregulin (EREG), and beta-cellulin (BTC) are expressed in MGCs and CGCs in autocrine and paracrine manners through respective EGF receptors (EGFRs) (Tsafriri et al., 2005; Conti et al., 2006; Hsieh et al., 2007). The activation of EGFR is required for oocyte meiotic resumption and cumulus cell expansion (Fan et al., 2009; Reizel et al., 2010). Studies using inhibitors and gene deletion mouse models have identified that EGFs mediate LH action through EGFR (Park et al., 2004; Ashkenazi et al., 2005; Hsieh et al., 2007). For instance, the process of oocyte maturation, cumulus expansion, and ovulation stimulated by LH are either delayed in AREG or blocked in EGFR-deficient mice (Hsieh et al., 2007). Furthermore, in granulosa cell-specific EDFR deleted mice, oocytes cannot resume meiosis (Hsieh et al., 2011).
How does EGF signaling regulate meiosis resumption response to LH surge? When the LH surge arrives, LH decreases NPPC/NPR2 expression levels, thereby blocking cGMP synthesis, and stimulates MGCs to secrete EGFs to activate EGFR signaling in cumulus cells, and activates phosphodiesterase 5 (PDE5) (Egbert et al., 2016; Wang et al., 2019). The activation of PDE5 suppresses the production of NPPC and closes the gap junction communication between granulosa cells (Gershon et al., 2008; Kawamura et al., 2011; Liu et al., 2014), resulting in the decrease of cGMP levels and the reduction of oocyte cAMP levels. Then, cumulus expansion and oocyte maturation starts (Wang et al., 2019; Arroyo et al., 2020). Even though the expression of EGFR and the direct effects of EGF on oocytes has been reported (Das et al., 1991; Gall et al., 2004; Vigneron et al., 2004), how LH regulates EGF in detailed molecular mechanisms remain unclear. Recently, Wang et al. (2019) found that LH surge-induced histone deacetylase 3 (HDAC3) downregulation in GCs is essential for oocyte maturation. HDAC3 in GCs is a negative regulator of EGF expression before the LH surge. HDAC3 in GCs is recruited by transcription factors, such as FOXO1, to the AREG promoter to suppress the expression of AREG. With the LH surge, the HDAC3 level decreases while histone H3K14 acetylation increases, which enables transcription factor SP1 binding to the AREG promoter to initiate AREG transcription. Moreover, granulosa cell-specific knockout of HDAC3 in vivo or inhibition of HDAC3 activity in vitro increases the proportion of the oocyte maturation independent of LH (Wang et al., 2019). Unfortunately, the mechanism of HDAC3 downregulation after the LH surge remains unclear.
In addition, calcium signaling is involved in gonadotropin-induced oocyte maturation in many species (Veldhuis, 1987; Su et al., 1999; Zhang et al., 2007, 2009; Conti et al., 2012). It was reported that EGFR signaling activates phospholipase Cγ (Chattopadhyay et al., 1999), which may increase calcium levels (Wang et al., 2013). Moreover, the elevated calcium of cumulus granulosa cells inactivates NPR2, further decreasing the binding affinity of NPR2 for NPPC. As a result, cGMP levels and meiotic resumption decreases (Hao et al., 2016). The regulations of granulosa cells cooperate with oocytes to resume meiosis induced by LH surge in mice, which are summarized in Figure 2.
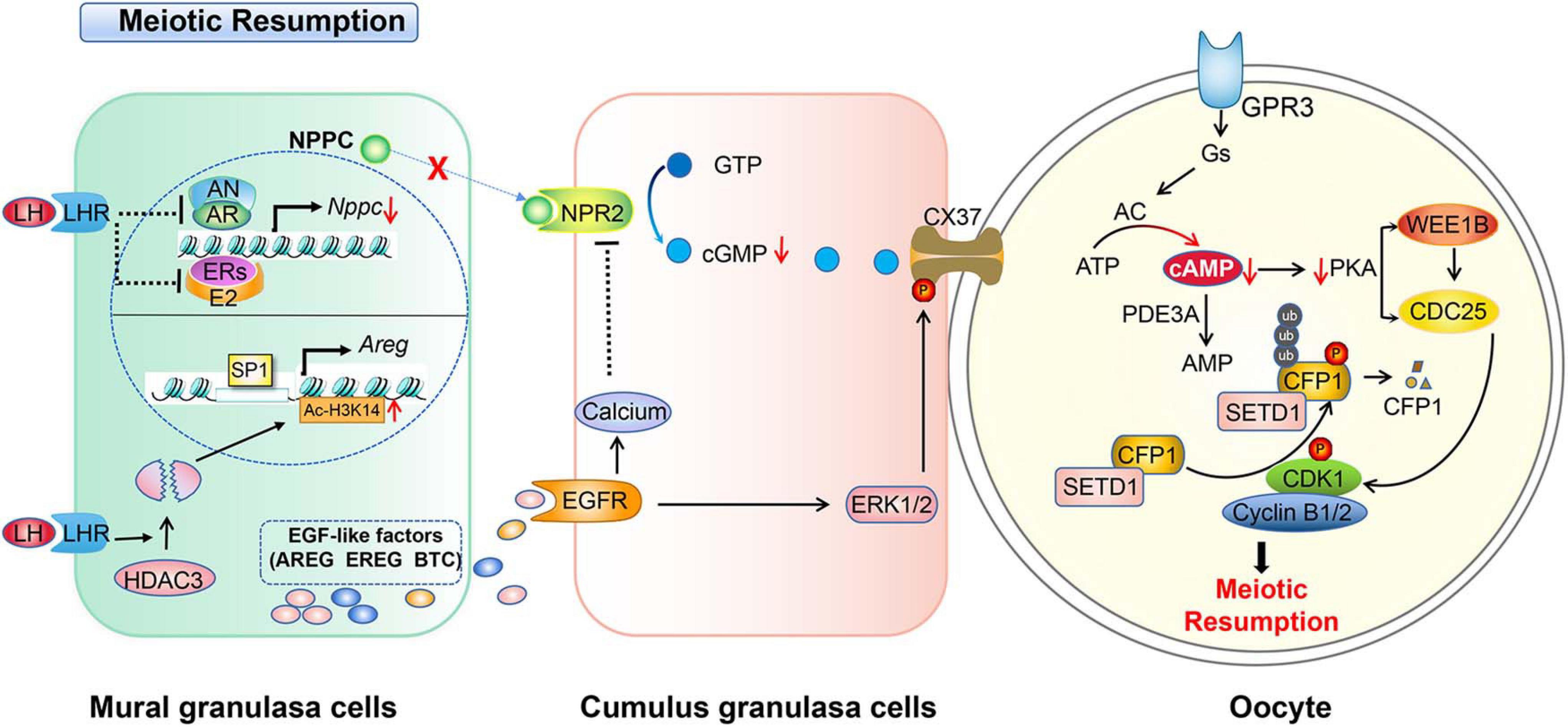
Figure 2. Schematic model depicting the mechanisms of LH-induced meiotic resumption. A preovulatory surge of LH binds its receptor (LHR) and induces a series of events in granulosa cells. In mural granulosa cells, on the one hand, LH/LHR inhibits AR and ER to reduce NPPC transcription and decrease NPPC production, on the other hand, it induces the degradation of histone deacetylase 3 (HDAC3) to decrease the Ac-H3K14 level which enables transcription factor SP1 binding to the AREG promoter to initiate AREG transcription, then increases the EGF level. The production of EGFs activates EGFR signaling and elevates the level of calcium in cumulus granulosa cells to further inactivate NPR2. LH-LHR also causes closure of gap junctions in the follicle and prevents cGMP delivery to oocytes. This in turn increases cAMP degradation by PDE3A. Low levels of cAMP and PKA can no longer activate WEE1B and inactivate CDC25B, and CDK1 becomes dephosphorylated and catalytically active. In addition, activated CDK1 triggers CXXC-finger protein 1 (CXXC1, also known as CFP1) phosphorylation and degradation following the meiotic resumption. The degradation of CFP1 ensures the absence of the SET domain containing 1 (SETD1)-CXXC1 complex from the chromatin, thereby facilitating chromosome condensation during oocyte maturation.
As one the key factors that heavily determines the quality of an oocyte, the cytoplasmic maturation of oocytes is critically important, which includes the synthesis, activation, and degradation of maternal mRNA as well as orderly arrangement of organelles (Schellander et al., 2007; Su et al., 2007; Walser and Lipshitz, 2011; Yu et al., 2016a), thereby affecting fertilization and embryonic development (Sirard, 2001; Chen et al., 2013; Li and Albertini, 2013; Coticchio et al., 2015; Pan et al., 2020; Sun et al., 2020; Xu et al., 2020).
The expression and degradation of maternal mRNA is developmental stage dependent. Along with the growth of activated follicles, the transcription of genes necessary for oocyte growth and meiosis resumption accumulate and are stored in the cytoplasm. With the initiation of meiotic resumption, not only does the transcription in oocytes cease because of staining agglutination, but the maternal mRNAs stored in oocytes are degraded and gradually consumed. As to protein synthesis, although a large amount of maternal mRNA exists in fully grown oocytes at the GV stage, they are translationally dormant in mice until meiotic maturation (Piccioni et al., 2005; Chen et al., 2011, 2013). The freshly translated proteins after oocytes resume meiosis play important roles in meiotic spindle assembly, MII arrest maintenance, and mRNA clearance during maternal zygotic transition (MZT) (Walser and Lipshitz, 2011). Generally, novel mRNA synthesis is initiated in the late stage of fertilized eggs (Piccioni et al., 2005).
How could the transcriptions of mRNA in growing oocytes remain stable before GVBD? Accumulative data show that there are stringent mRNA stabilizing mechanisms within GV-stage oocytes. For instance, cytoplasmic polyadenylation of the 3′-untranslated region (3′-UTR) is closely related to mRNA stability and mRNA translational activation, which plays an important role in oocyte maturation (Yang et al., 2017). Actually, the degree of polyadenylation of mRNA affects oocyte translation activation as well. Cytoplasmic polyadenylation is a key process that serves to unmask particular mRNAs and enables them to be translated (Richter, 2007; Ivshina et al., 2014). In its simplest form, masked mRNA refers to dormant transcripts in the oocyte that are to be translated during completion of meiotic divisions or in early embryos.
The degradation of maternal mRNA is controlled strictly in oocytes undergoing meiotic resumption and in early embryos. In mouse oocytes, transcriptional destruction, especially the transcripts of oxidative phosphorylation, energy production, and protein synthesis during the transition from GV to MII, is selective rather than promiscuous. It is stated that the selective degradation of the transcription of maternal mRNAs is a prerequisite for the activation of the zygotic genome (Su et al., 2007; Yu et al., 2016a,b). Particularly, regulation of maternal mRNA translation and degradation mainly occurs in maturing oocytes rather than in fertilized eggs, but these mechanisms are essential for the oocyte and zygote to build up competence to accomplish MZT.
The starting point of the MZT is oocyte activation from meiotic arrest rather than fertilization (Sha et al., 2019). About 90% of the maternal mRNA is degraded at the two-cell stage of early mice embryos (Schellander et al., 2007). In the major pathway of mRNA degradation, shortening of the poly(A) tail, or deadenylation, is the first and rate-limiting step (Walser and Lipshitz, 2011). Poly(A) tail shortening reduces the binding of poly(A) binding proteins (PABPs) and slows down translation (Ozturk and Uysal, 2017). In this aspect, prepared RNA-binding proteins (RBPs) in fully grown oocytes are important for sustaining genome stability, stabilizing and/or degrading mRNAs, or even for protein synthesis and degradation. For instance, meiosis arrest female 1 (MARF1) is an essential regulator of important oogenic processes leading to female fertility and the development of healthy offspring by suppressing levels of specific transcripts (Su et al., 2012a,b). More information about oocyte-specific RBPs regulating genome stability and mRNA stability needs to be uncovered.
The cytoplasmic maturation of oocytes also includes the maturation of various organelles, especially cortical granules, mitochondria, the endoplasmic reticulum (ER), and cytoskeleton. The time dependent distribution and structure of these organelles are indispensable for the respective functions.
Cortical granules are membranous organelles derived from Golgi complexes, which are found in the cortex of unfertilized oocytes (Liu et al., 2003) and play important roles during the fertilization process (Liu, 2011). Following fertilization, cortical granules undergo exocytosis to release their contents into the perivitelline space, which result in the blocking of polyspermy by modifying the oocytes’ extracellular matrices, such as the zona pellucida in mammals (Coticchio et al., 2015). Besides, mitochondria are the key to ATP energy supply in oocytes. Impaired oocyte quality, including meiosis chromosome separation, maturation, and fertilization failure, correlates with both abnormal mitochondrial rearrangement and low ATP level (Blerkom, 2004; Dumollard et al., 2006). In addition, ER is responsible for the storage and release of free Ca2+ in the cytoplasm in oocytes, which is important for calcium reaction at fertilization (Bootman et al., 2002; FitzHarris et al., 2007; Machaca, 2007). Since the cytoskeleton is mainly composed of microtubules and filaments and the recombination of spindles is strictly controlled by the microfilament network (Verlhac et al., 2000), anything that disrupts either microtubules or microfilament causes failure of chromosome movement and separation, in which case, the oocyte is arrested at the metaphase stage.
Multiple posttranslational modifications exist in developing oocytes, including acetylation, phosphorylation, methylation, glycosylation, ubiquitination, and SUMOylation of various proteins (Allfrey et al., 1964; Gutierrez and Hnilica, 1967; Huletsky et al., 1985; Kim J. H. et al., 2003; Sarmento et al., 2004; Nathan et al., 2006; Koprinarova and Russev, 2008; Rada-Iglesias et al., 2009; Xu et al., 2009), implying that epigenetic modification plays different but important roles during the oocyte maturation process under varying temporal and environmental conditions (Li, 2002; Richardson, 2002). As follows, the changes and regulation as well as functions of histone modifications during meiotic maturation of mammalian oocytes, with particular emphasis on histone acetylation and methylation are summarized.
Lysine acetylation of histones is generally controlled by histone acetyl transferases (HATs) and histone deacetylases (HDACs) (Gallinari et al., 2007). Acetylation of H3/4 leads to open chromatin configuration, enhances transcriptional activity, and thereby promotes transcription factor binding to DNA (Liu et al., 2011). Contrarily, deacetylation is associated with transcriptional inactivation. The key sites of histones for acetylation include at least four conserved lysines (K) in histone H4 (K5, K8, K12, and K16) and two conserved lysines (K) in H3 (K9 and K14) (Bjerling et al., 2002; de Ruijter et al., 2003). In general, all lysine residues are acetylated in fully grown GV oocytes, including H4K5ac, H4K8ac, H4K12ac, H4K16ac, H3K9ac, and H3K14ac, except for H4K8ac, which is deacetylated in condensed chromosomes and is maintained until the MII stage (Kim J. M. et al., 2003; Akiyama et al., 2004; Kageyama et al., 2007). In mammals, as many as 18 HDACs are identified and divided into four classes based on their homology with yeast proteins (Bolden et al., 2006). In which, class I HDACs are nuclear-localized, including HDAC 1, 2, 3, and 8 (de Ruijter et al., 2003; Ropero and Esteller, 2007). Class II is divided into IIa (HDAC 4, 5, 7, and 9) and IIb (HDAC 6 and 10), both of which can shuttle between the nucleus and cytoplasm (de Ruijter et al., 2003). Class III includes seven sirtuins (SIRT1-7), which are homologous with the yeast SIRT2 family proteins and require NAD+ as a cofactor to function (Sengupta and Seto, 2004). HDAC11 is the only member of class IV, which is homologous to both classes I and II (Gao et al., 2002). The respective actions of these proteins in oocytes are reviewed in the following.
Class I HDACs are important in oocyte development and maturation. HDAC1 and HDAC2 share high amino acid homology and work together in almost all repressive transcriptional complexes (Grozinger and Schreiber, 2002). HDAC1 and HDAC2 are located in the nucleus throughout oocyte growth (Ma et al., 2012). HDAC1 in the nucleus decreases gradually during the growth of oocytes and co-localizes with chromosomes following meiosis resumption. In contrast, HDAC2 in the nucleus increases between 5 and 12 days post-partum, and is relatively stable during the growing period of mice oocytes. After GVBD, HDAC2 in an oocyte is uniformly dispersed throughout the cytoplasm (Ma and Schultz, 2008; Ma et al., 2012). Germ-line deletion of either HDAC1 or HDAC2 will cause mouse embryo lethality (Montgomery et al., 2007; Leboeuf et al., 2010). However, conditional knockout HDAC1 by ZP3-Cre has no obvious impact on fertility and oocyte maturation. Although, the deletion of HDAC2 by ZP3-Cre did result in reduced fertility, but the follicular development was normal. Further, deletion of both HDAC1 and HDAC2, however, results in infertility due to oocyte development arrest at the secondary follicle stage (Ma et al., 2012). The low level acetylation of H4K16 is essential for the function of centromeres. Interestingly, the deletion of maternal HDAC2 caused high level acetylation of H4K16 and resulted in disorder in chromosome segregation and kinetochore function during MII in oocytes (Ma and Schultz, 2013). In summary, HDAC1/2 regulate oocyte growth with their compensatory function, and HDAC2 could be more critical than HDAC1 for oogenesis.
HDAC3 is expressed in the nucleus of GV oocytes and disperses in the cytoplasm of oocytes after meiotic resumption. The signal of HDAC3 accumulates on the meiotic spindle region from pre-metaphase I to MII. Knockdown of HDAC3 in oocytes results in spindle/chromosome organization failure, with severely impaired kinetochore-microtubule attachments. In addition, overexpression of HDAC3 modulates the acetylation status of α-tubulin in mouse oocytes (Li et al., 2017). HDAC3 also has functions in promoting meiotic apparatus assembly in aging mouse oocytes. Overexpression of HDAC3 in old oocytes not only partially prevents spindle/chromosome disorganization, but significantly lowers the incidence of aneuploidy (He et al., 2019). HDAC3 also plays important roles in GCs. Conditional knockout of HDAC3 in MGCs in vivo or inhibition of HDAC3 activity in vitro promotes the maturation of oocytes independent of LH (Wang et al., 2019). The above results indicate that HDAC3 in both granulosa cells and oocytes plays important regulatory roles in oocyte maturation.
HDAC8 could be important for oocyte maturation according to its distribution in growing oocytes. It is widely distributed in the cytoplasm of mouse oocytes at the GV stage. After GVBD, it starts to accumulate around the chromosomes, and shows a spindle pole-like localization pattern in both MI and MII. Inhibition of HDAC8 in fully grown oocytes causes spindle defects and chromosome misalignment during oocyte meiotic maturation, accompanied by impaired kinetochore-microtubule attachments (Zhang K. et al., 2017). Conditional deletion of HDAC8 by Vasa-Cre results in subfertile females, which is independent of chromosome segregation errors during meiosis (Vijay Pratap et al., 2019). On the whole, HDAC8 is important for oocyte development and maturation, but the mechanisms of its action on oocytes needs further study.
The function of class IIa HDACs in oocyte maturation has not been well studied. According to existing reports, the expression of HDAC4 is maintained at a high level in fully grown oocytes until the MII stage, and then dramatically decreased after fertilization, it may play specific roles during mouse oocyte maturation (Kageyama et al., 2006).
In class IIb, HDAC6 has been studied extensively, while HDAC10 has hardly been reported. HDAC6 localizes in the cytoplasm of mouse GV oocytes. Overexpression of HDAC6 results in GV oocytes and pronuclear zygotes which results in altered nuclear structure and causes compaction of the chromatin (Verdel et al., 2003). In addition, inhibition of HDAC6 in GV oocytes prevents PB1 extrusion later because of disrupted maturational progression and meiotic apparatus assembly (Zhou et al., 2017; Sui et al., 2020a). However, HDAC6 KO mice are viable and fertile and presented no major observable phenotype (Zhang et al., 2008). Despite that, the TuA-treated group presented significant changes in the expression of HDAC subfamily genes such as HDAC6, 10, and 11 and sirtuin 2, 5, 6, and 7 by RNA-sequencing, which may indicate that TuA is a multifunctional inhibitor which targets both HDAC and sirtuin activity rather than being a HDAC6-specific inhibitor in mouse oocytes (Choi et al., 2019).
Sirtuins are generally important for oocyte development. SIRT1, SIRT2, SIRT3, and SIRT6 are beneficial for improving the competence of oocytes grown or matured in vitro in humans and animals (Tatone et al., 2018). SIRT4, SIRT5, and SIRT7 have seldom been studied so far. Activation of SIRT1 by resveratrol in vitro improves oocyte quality and embryo development in mice, pigs, and cows (Liu et al., 2013; Takeo et al., 2014; Wang et al., 2014; Itami et al., 2015; Li et al., 2016; Khan et al., 2017). SIRT1 relates to mitochondria biosynthesis and degradation in oocytes because resveratrol supplementation improves the mitochondrial function and the developmental capability of the oocytes (Sato et al., 2014). In contrast, specifically inhibition of SIRT1 results in increased ROS production and abnormal MII plates in mouse oocytes (Di Emidio et al., 2014). Similarly, inhibition of SIRT2 during in vitro oocyte maturation or knockout of SIRT2 blocks the progression of oocyte development after GVBD (Riepsamen et al., 2015). SIRT2 knockdown also affects spindle organization and chromosome alignment during meiosis (Zhang L. et al., 2014). Besides, SIRT3 regulates the ROS level in oocytes. Overexpression of SIRT3 reduces the spindle defects and chromosome misalignment in oocytes (Zhang L. et al., 2015). Last, SIRT6 is important in regulating meiotic progression as well. Depleted SIRT6 results in disruption of spindle morphology and chromosome alignment in oocytes (Han et al., 2015).
The expression of HDAC11 in oocytes decreases from the GV to MII stage. Inhibition of HDAC11 by JB3-22 significantly interrupted mouse oocyte meiosis progress, possibly because of abnormal spindle organization and misaligned chromosomes, impaired kinetochore-microtubule attachment, and spindle assembly checkpoint function (Sui et al., 2020b).
The function of HDACs during oocyte maturation are summarized in Table 1.
Histone acetyl transferases, including MYST, GCNA5/PCAF, and p300/CREB-binding protein (CBP), regulate the acetylation of histones as well (Gu et al., 2010).
MYST is an acronym of its four founding members, including human MOZ (monocytic leukemia zinc finger protein), yeast Ybf2 (renamed Sas3, for something about silencing 3), yeast Sas2, and mammalian TIP60 (HIV Tat-interacting 60 kDa protein) (Carrozza et al., 2003; Yang, 2004; Thomas and Voss, 2007). Importantly, K (lysine) acetyltransferase 8 (KAT8) is a highly conserved MYST family member who is specifically responsible for H4K16 acetylation and is important for mouse oocyte development, by regulating reactive oxygen species levels (Thomas et al., 2007; Gupta et al., 2013; Yin et al., 2017). The expression of KAT8 increases dramatically between 14 days and full-grown GV-stage oocytes, followed by a sharp decrease in GVBD-MI-stage oocytes. The protein is mainly located in the nucleus throughout the growth phase, but upon GVBD, the staining intensity decreases and the signal becomes uniformly dispersed throughout the oocyte. Oocyte KAT8 deletion results in female infertility with defects in follicle development and increased oocyte apoptosis (Yin et al., 2017).
In bovine, the levels of MYST4 mRNA in both GV and MII oocytes are high. MYST4 protein accumulates in the nucleus of GV oocytes. It concentrates in the vicinity of the meiotic spindle rather than on chromosomes in the MI stage (Champagne et al., 1999; McGraw et al., 2007).
Histone acetyltransferases p300 and the CBP subfamily are constitutively expressed in the GCs of growing and ovulatory follicles in a gonadotrophin-independent manner. ED-rich tail (CITED) protein CITED4 formed an endogenous protein complex with CBP and transcription factors CCAAT/enhancer binding protein C/EBP/b, which docked on the promoters of LH and ERK1/2 target genes. Both CITED4 expression and CBP acetyltransferase activity were indispensable for ovulation-related molecular and histological events. Moreover, the dynamic histone acetylation changes (histone H2B-Lys5 and H3-Lys9) in GCs were regulated by LH, CBP, and HDACs during ovulation (Zhang Y. L. et al., 2014). In addition to the above two subfamilies, the constant expression of HAT1 and GCN5 mRNA was also detected during bovine oocyte maturation (McGraw et al., 2003).
Taken together, HATs have essential roles in mouse follicle development and oocyte maturation, and the potential functions of HATs in oocytes maturation needs more exploration.
Histone methylation correlates with chromatins activity. For instance, H3K4 methylation is associated with the activation of chromatins and occurs mainly in the promoter regions of active genes, while the methylations of either H3K9 or H3K27 relates to gene inactivation (Werner and Ruthenburg, 2011). In developing mouse oocytes, the level of H3K4me3 are elevated during the transition of chromatin configuration from the non-surrounded nucleolus (NSN) to surrounded nucleolus (SN) type (Yu et al., 2017), the latter of which have better developmental competence after fertilization (Ma et al., 2013; Zhang et al., 2019). H3K4me and H3K4me2 levels elevate, but H3K4me3 level decreases after GVBD and reaches its lowest point in anaphase I (Sha et al., 2018).
Histone methylation modification is regulated by histone lysine methyl transferases (KMTs) and histone lysine demethylases (KDMs) via modifying lysine residues and the number of methyl groups (Sha et al., 2020). There are six known histone H3 methyltransferases, including SET domain containing 1A/B (SETD1A/B), lysine (K) methyltransferase 2A/B (KMT2A/B), and KMT3/4 in mammals (Shilatifard, 2012).
The SETD1/COMPASS histone methyltransferase complex is the primary enzyme that methylates histone H3K4 by recognizing its basic subunit, CXXC1 (Roguev et al., 2001; Lee and Skalnik, 2005; Brown et al., 2017). The expression of SETD1A and SETD2B persists from oocyte to blastocyst. SETD1A is first required at the epiblast stage, whereas SETD1B becomes essential after gastrulation (Bledau et al., 2014). In GDF9-Cre-driven SETD1B deficient mice, the number of follicles decreases gradually with time, the ovulated MII oocytes exhibit meiotic spindle abnormalities. And the oocytes as well as zygotes display perturbed cytoplasmic organelles and aggregated lipid droplets (Brici et al., 2017). SETD1-CXXC1 conjugation regulates H3K4me3 in mice oocytes (Yu et al., 2017). A stabilized CXXC1 in fully grown GV oocytes resulted in decreased GVBD and PB1 emission rates as well as spindle assembly defects in mice (Sha et al., 2018).
KMT2B activates gene expression by regulating H3K4me3 (Glaser et al., 2006). Loss of KMT2B in mouse oocytes induced by GDF9-Cre resulted in abnormal meiosis maturation, anovulation, oocyte death, and female sterility, in which H3K4 level decreased and gene expression was abnormal (Andreu-Vieyra et al., 2010; Hanna et al., 2018). KDMs consist of KDM1 and the KDM2-KDM7 subfamily, which contain a Jumonji C (JmjC) domain (Xhabija and Kidder, 2019). KDM1A is expressed in the oocyte nucleus, which specifically catalyzes the demethylation of H3K4me1 and H3K4me2. KDM1A-null oocytes display defects in maintaining prophase I arrest and undergo precocious GVBD. Most KDM1A-null oocytes undergo apoptosis before the completion of meiotic maturation (Kim et al., 2015). KDM1B is highly expressed in growing oocytes and the level persists through later stages of oogenesis, but it is hardly detectible in oocytes of primordial and primary follicles in mice. The deletion of KDM1B in mice does not affect embryo development, animal survival, or oocyte growth. However, oocytes from KDM1B-deleted females show high levels of H3K4 methylation and fail-to-deposit DNA methylation marks at four out of seven imprinted genes. Early embryos derived from these oocytes show biallelic expression or suppression of the affected genes and died before mid-gestation (Ciccone et al., 2009).
KDM4A and KDM4B are located in human oocytes, granulosa cells, theca cells, and luteal cells in reproductive-aged women (Krieg et al., 2018). Deletion of KDM4A during oogenesis had no significant impact on ovulation since KDM4A–/– and wild-type females ovulated similar numbers of fertilizable oocytes. MII oocytes from KDM4A–/– females were euploid with no evidence of major chromosomal breakages or aneuploidies. KDM4A is the major demethylase functional in MII oocytes and is required to maintain the genomic stability of pre-implantation embryos (Sankar et al., 2020).
The KDM5 family consists of KDM5A to KDM5D (Xhabija and Kidder, 2019). During knockout of KDM5A, the mice are viable and fertile (Klose et al., 2007), whereas knockout of KDM5B resulted in early embryonic lethality (Catchpole et al., 2011), suggesting that KDM5B is the major functional KDM family member in vivo.
Protein phosphorylation occurs most often on serine, threonine, or tyrosine residues and competently regulates cell cycle stage-related affairs in a variety of different signal transduction pathways (Schatten and Sun, 2014). For example, histone H3 phosphorylation at Ser10 and Ser28 affects chromatin condensation of either mitosis or meiosis (Bradbury et al., 1973; Bradbury, 1992; Wei et al., 1998, 1999; Goto et al., 1999; Houben et al., 2005; Swain et al., 2007). Aurora B phosphorylates histone H3 at Ser28 in mitotic cells and Ser10 and Ser28 in meiosis cell. ZM447439 (an inhibitor of the Aurora kinase family) treatment prevented Aurora B activity and significantly decreased the phosphorylation levels of both H3/Ser10 and H3/Ser28 in mouse oocytes, resulting in chromosome misalignment (Jeliìnkovaì and Kubelka, 2006). In addition, protein phosphatase 1 (PP1) dephosphorylates H3 at Ser10 in budding yeast and nematodes. Inhibition of PP1/PP2a induces rapid chromosome condensation with hyperphosphorylated histone H3 (Bui et al., 2004; Swain et al., 2007). Taken together, the balance of Aurora B kinase and PP1 activities regulate the meiotic phosphorylation of histone H3 in mammalian oocytes.
The ubiquitination/deubiquitination system is important for the degradation of proteins, cell cycle progression, and transcriptional regulation (Bassermann et al., 2014), which is also important for oocyte maturation (Dekel, 2005; Susor et al., 2010; Mtango et al., 2012). Importantly, APC initiates the metaphase to anaphase transition by inducing the degradation of cyclin B and securin (Jones, 2011). Also, protein ubiquitin (Ub) E3 ligases trigger specific protein degradation and thus plays an important role in the process of both the meiotic and mitotic cell cycle (Huo et al., 2004).
Interestingly, cullin ring-finger ubiquitin ligase 4 (CRL4) is one of E3 ligase members who exert multiple functions in the maintenance of oocyte survival and meiotic cell cycle progression (Jones, 2011). DCAF13, a CRL4 adaptor, stimulates the meiotic resumption-coupled activation of protein synthesis in oocytes. Deletion of DCAF13 in oocytes resulted in not only decreased CDK1 activity and impaired meiotic cell cycle progression as well as chromosome condensation defects, but also polyubiquitination and degradation of PTEN (Zhang et al., 2020).
In addition, protein-ubiquitination mediated CCNB1 and securin degradation is essential for the metaphase to anterograde transition during oocyte meiotic maturation (Herbert et al., 2003; Marangos and Carroll, 2008).
Ubiquitin C-terminal hydrolases (UCHs) are a deubiquitin enzyme that catalyzes the hydrolysis of peptides, isopeptides, or UB portions (Kim J. H. et al., 2003; Wilkinson, 2009). UCHs present in oocytes in many species. UCHs have a complimentary distribution in porcine, bovine, and murine oocytes. UCHL1, one of the most abundant proteins in mammalian oocytes, accumulates in the oocyte cortex. UCHL3 is associated with oocyte spindle (Mtango et al., 2012). Inhibiting UCH activity causes excessively large PB1, distorts the meiotic spindle, and disrupts other spindle attributes, such as chromosome alignment (Mtango et al., 2012). In vitro, inhibition of UCHL3 reduces the expansion of cumulus cells (Mtango et al., 2014).
In follicular granulosa cells, the ubiquitin-proteasome system (UPS) was involved in regulating the deposition of the extracellular matrix of cumulus and steroidal formation during the expansion of cumulus cells, implying that this system may be pivotal for follicle development (Nagyova et al., 2012).
In the female reproductive system, a large number of proteins, including FSH, LH, GDF9, BMP15, and AMH are glycosylated (Saito et al., 2008; Bousfield and Harvey, 2019). Protein glycosylation is one of the most frequent post-translational modifications (PTMs), which affects many things, such as protein folding, distribution, stability, and activity. There are two main types of glycosylation in cells, N-linked and O-linked glycosylation (Ohtsubo and Marth, 2006). Defects in the process of protein glycosylation leads to many clinical diseases.
Protein N-glycosylation in oocytes is crucial for female fertility. For example, DPAGT1 is an enzyme involved in the process of protein N-glycosylation. DPAGT1 missense mutation causes subfertility in females due to defective follicular development and less ovulation (Li et al., 2020). Also, due to the decreased glycosylation of ZP proteins, the mutant oocytes have a thin and fragile ZP layer and have poor developmental ability after in vitro fertilization. Furthermore, the first meiotic division is accelerated in such mutant oocytes. Importantly, the phenotypes of conditional knockout of DPAGT1 in infertile mouse oocytes is consistent with those in humans (Li et al., 2020).
Protein O-glycosylation plays a small role in oocyte maturation. In vitro, O-glycosylation is elevated in bovine COCs exposed to glucosamine (Sutton-McDowall et al., 2006). Glucosamine treatment during in vitro maturation does not affect the meiotic maturation of cow, pig, or mouse oocytes, but blastocyst development was severely inhibited (Schelbach et al., 2010). This suggests that protein O-glycosylation does not affect oocyte maturation, but it affects the quality of oocytes.
SUMOylation and de-SUMOylation modification refers to the reversible addition and removal of SUMO (small ubiquitin-related modifier) polypeptides on lysine residues (Schatten and Sun, 2014). SUMO proteins, such as SUMO1, SUMO2, SUMO3, and UBE2I, are expressed in and are required for oocyte maturation in events like oocyte meiotic resumption and spindle formation (Ihara et al., 2008; Wang et al., 2010; Feitosa et al., 2018; Rodriguez et al., 2019).
The localization of SUMO1, SUMO2, and SUMO3 in oocytes depends on the developmental stage of the oocytes. In immature oocytes, SUMO1 localizes to the nuclear membrane while SUMO2/3 are within the nucleoplasm. During oocyte meiosis, SUMO1 localizes to the spindle poles and around the chromosomes whereas SUMO2/3 locate near the centromeres (Yuan et al., 2014). UBE2I primarily expresses in the nucleoplasm of mouse growing oocytes at least from postnatal day 13 to GV-stage fully grown oocytes. UBE2I is downregulated following meiotic resumption (Ihara et al., 2008).
The importance of SUMOylation on oocytes maturation could be highlighted by the following facts. After endogenous SUMO1 or UBC9 activities in oocytes were either inhibited or silenced, the percentage of GVBD and PB1 extrusion was significantly reduced, together with abnormal spindle organization, chromosome misalignment, segregation defects, and aneuploidy in matured oocytes (Yuan et al., 2014). Similarly, inhibition of UBE2I for GV-stage mouse oocytes disrupts meiotic maturation and causes defects in spindle organization (Yuan et al., 2014), while overexpression of UBE2I in meiotic incompetent oocytes stimulates gene transcription in vitro (Ihara et al., 2008). Deletion of UBE21 by GDF9-Cre results in complex infertility phenotypes, including defects appearing at multiple critical oocyte transition points, such as unstable ovarian reserves, impaired communication with granulosa cells, and defective resumption of meiosis and meiotic progression (Rodriguez et al., 2019).
Other proteins that affect oocyte maturation are also involved in the process of SUMOylation. For instances, overexpression of the SUMO-specific isopeptidase, sentrin/SUMO-specific protease 2 (SENP2), leads to defects in MII spindle organization by changing the localization of SUMO-modified proteins in oocytes (Wang et al., 2010). Septin, a conserved GTP-binding protein that is modified by SUMO1, is also required for chromosome congression in mouse oocytes (Zhu et al., 2010). And the spindle-assembly checkpoint protein Bub1-related kinase, or MAD3/Bub1b (BUBR1), may be SUMOylated by SUMO1 and is necessary for homologous chromosome alignment as well (Wei et al., 2010; Yang et al., 2012b).
Oocyte maturation is a complex process involving multiple steps and is regulated by many molecules and signaling pathways. In recent years, due to the rapid development and popularization of technologies like the genetic modification of animal models, molecular biology, and biochemistry, researchers have gained a better understanding of oocyte GV arrest and meiosis I resumption. The major cellular and molecular affairs, especially the epigenetic modification events related to oocyte maturation in response to hormone induction, and the major advances in this field, are highlighted in this review.
Since the development of an oocyte depends not only on the oocyte itself, but on mutual communication and physical contact with follicular granulosa cells, it is important to focus more on epigenetic changes within oocytes, ovarian granulosa cells in response to hormones, and other extracellular molecules induction. Besides, applying microscopes with high resolution and a high-throughput analysis technique, such as mono-cellular based sequencing and omics techniques, should be emphasized to present clearer 3D or even time-dependent 4D representations of critical affairs that happen during oogenesis. Finding more specific oocyte-expressed proteins, such as RBPs and oocyte-derived paracrine molecules, may contribute to uncover the mysterious mechanisms of oocyte meiosis as well. Further, integration of analysis of sequencing data, comparing the data collected from different breeds, and verifying the function of each individual molecule in vitro and in vivo simultaneously based on multiple animal models are also plausible.
MH and TZ collected the information and wrote the manuscript. CW and YY revised the manuscript. All authors read and approved the final manuscript.
This study was supported by grants from the National Key Research & Developmental Program of China (2018YFC1003701 and 2018YFC1003801); the National Basic Research Program of China (2013CB945501); the Institution of Higher Education Projects of Building First-Class Discipline Construction in Ningxia Region (Biology) (NXYLXK2017B05); the National Natural Science Foundation of China (31872792, 32071132, and 32070839); and the Project of the State Key Laboratory of Agrobiotechnology (2015SKLAB4-1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors wish to thank each member of Xia Lab for their valuable discussion.
Adhikari, D., Busayavalasa, K., Zhang, J., Hu, M., Risal, S., Bayazit, M. B., et al. (2016). Inhibitory phosphorylation of Cdk1 mediates prolonged prophase I arrest in female germ cells and is essential for female reproductive lifespan. Cell Res. 26, 1212–1225. doi: 10.1038/cr.2016.119
Adhikari, D., and Liu, K. (2014). The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol. Cell. Endocrinol. 382, 480–487. doi: 10.1016/j.mce.2013.07.027
Akiyama, T., Kim, J. M., Nagata, M., and Aoki, F. (2004). Regulation of histone acetylation during meiotic maturation in mouse oocytes. Mol. Reprod. Dev. 69, 222–227. doi: 10.1002/mrd.20121
Aktas, H., Wheeler, M. B., Rosenkrans, C. F. Jr., First, N. L., and Leibfried-Rutledge, M. L. (1995). Maintenance of bovine oocytes in prophase of meiosis I by high [cAMP]i. J. Reprod. Fertil. 105, 227–235. doi: 10.1530/jrf.0.1050227
Allfrey, V. G., Faulkner, R., and Mirsky, A. E. (1964). Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl. Acad. Sci. U.S.A. 51, 786–794. doi: 10.1073/pnas.51.5.786
Anderson, E., and Albertini, D. F. (1976). Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J. Cell Biol. 71, 680–686. doi: 10.1083/jcb.71.2.680
Andreu-Vieyra, C. V., Chen, R., Agno, J. E., Glaser, S., Anastassiadis, K., Stewart, A. F., et al. (2010). MLL2 is required in oocytes for bulk histone 3 lysine 4 trimethylation and transcriptional silencing. PLoS Biol. 8:e1000453. doi: 10.1371/journal.pbio.1000453
Arroyo, A., Kim, B., and Yeh, J. (2020). Luteinizing hormone action in human oocyte maturation and quality: signaling pathways, regulation, and clinical impact. Reprod. Sci. 27, 1223–1252. doi: 10.1007/s43032-019-00137-x
Ashkenazi, H., Cao, X., Motola, S., Popliker, M., Conti, M., and Tsafriri, A. (2005). Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 146, 77–84. doi: 10.1210/en.2004-0588
Bassermann, F., Eichner, R., and Pagano, M. (2014). The ubiquitin proteasome system — Implications for cell cycle control and the targeted treatment of cancer. Biochim. Biophys. Acta 1843, 150–162. doi: 10.1016/j.bbamcr.2013.02.028
Bhaskara, S., Chyla, B. J., Amann, J. M., Knutson, S. K., Cortez, D., Sun, Z.-W., et al. (2008). Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol. Cell 30, 61–72. doi: 10.1016/j.molcel.2008.02.030
Bjerling, P., Silverstein, R. A., Thon, G., Caudy, A., Grewal, S., and Ekwall, K. (2002). Functional divergence between histone deacetylases in fission Yeast by distinct cellular localization and in vivo specificity. Mol. Cell Biol. 22, 2170–2181. doi: 10.1128/mcb.22.7.2170-2181.2002
Bledau, A. S., Schmidt, K., Neumann, K., Hill, U., Ciotta, G., Gupta, A., et al. (2014). The H3K4 methyltransferase Setd1a is first required at the epiblast stage, whereas Setd1b becomes essential after gastrulation. Development 141, 1022–1035. doi: 10.1242/dev.098152
Blerkom, J. V. (2004). Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction 128, 269–280. doi: 10.1530/rep.1.00240
Bobrowska, A., Paganetti, P., Matthias, P., and Bates, G. P. (2011). Hdac6 knock-out increases tubulin acetylation but does not modify disease progression in the R6/2 mouse model of huntington’s disease. PLoS One 6:e20696. doi: 10.1371/journal.pone.0020696
Bolden, J. E., Peart, M. J., and Johnstone, R. W. (2006). Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5, 769–784. doi: 10.1038/nrd2133
Bootman, M. D., Berridge, M. J., and Roderick, H. L. (2002). Calcium signalling: more messengers, more channels, more complexity. Curr. Biol. 12, R563–R565. doi: 10.1016/S0960-9822(02)01055-2
Bousfield, G. R., and Harvey, D. J. (2019). Follicle-stimulating hormone glycobiology. Endocrinology 160, 1515–1535. doi: 10.1210/en.2019-00001
Bowles, J., Knight, D., Smith, C., Wilhelm, D., Richman, J., Mamiya, S., et al. (2006). Retinoid signaling determines germ cell fate in mice. Science 312, 596–600. doi: 10.1126/science.1125691
Bowles, J., and Koopman, P. (2007). Retinoic acid, meiosis and germ cell fate in mammals. Development 134, 3401–3411. doi: 10.1242/dev.001107
Bradbury, E. M. (1992). Reversible histone modifications and the chromosome cell cycle. Bioessays 14, 9–16. doi: 10.1002/bies.950140103
Bradbury, E. M., Inglis, R. J., Matthews, H. R., and Sarner, N. (1973). Phosphorylation of very-lysine-rich histone in physarum polycephalum. porrelation with chromosome condensation. Eur. J. Biochem. 33, 131–139. doi: 10.1111/j.1432-1033.1973.tb02664.x
Brici, D., Zhang, Q., Reinhardt, S., Dahl, A., Hartmann, H., Schmidt, K., et al. (2017). Setd1b, encoding a histone 3 lysine 4 methyltransferase, is a maternal effect gene required for the oogenic gene expression program. Development 144, 2606–2617. doi: 10.1242/dev.143347
Brown, D. A., Di Cerbo, V., Feldmann, A., Ahn, J., Ito, S., Blackledge, N. P., et al. (2017). The SET1 complex selects actively transcribed target genes via multivalent interaction with CpG island chromatin. Cell Rep. 20, 2313–2327. doi: 10.1016/j.celrep.2017.08.030
Bui, H. T., Yamaoka, E., and Miyano, T. (2004). Involvement of Histone H3 (Ser10) phosphorylation in chromosome condensation without Cdc2 kinase and mitogen-activated protein kinase activation in pig oocytes. Biol. Reprod. 70, 1843–1851. doi: 10.1095/biolreprod.103.026070
Cai, H., Liu, B., Yang, T., Yang, Y., Xu, J., Wei, Z., et al. (2018). Involvement of PKCε in FSH-induced connexin43 phosphorylation and oocyte maturation in mouse. Biol. Open 7:bio034678. doi: 10.1242/bio.034678
Carabatsos, M. J., Sellitto, C., Goodenough, D. A., and Albertini, D. F. (2000). Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev. Biol. 226, 167–179. doi: 10.1006/dbio.2000.9863
Carrozza, M. J., Utley, R. T., Workman, J. L., and Côté, J. (2003). The diverse functions of histone acetyltransferase complexes. Trends Genet. 19, 321–329. doi: 10.1016/s0168-9525(03)00115-x
Catchpole, S., Spencer-Dene, B., Hall, D., Santangelo, S., Rosewell, I., Guenatri, M., et al. (2011). PLU-1/JARID1B/KDM5B is required for embryonic survival and contributes to cell proliferation in the mammary gland and in ER+ breast cancer cells. Int. J. Oncol. 38, 1267–1277. doi: 10.3892/ijo.2011.956
Champagne, N., Bertos, N. R., Pelletier, N., Wang, A. H., Vezmar, M., Yang, Y., et al. (1999). Identification of a human histone acetyltransferase related to monocytic leukemia zinc finger protein. J. Biol. Chem. 274, 28528–28536. doi: 10.1074/jbc.274.40.28528
Chattopadhyay, A., Vecchi, M., Ji, Q., Mernaugh, R., and Carpenter, G. (1999). The role of individual SH2 domains in mediating association of phospholipase C-gamma1 with the activated EGF receptor. J. Biol. Chem. 274, 26091–26097. doi: 10.1074/jbc.274.37.26091
Cheng, F., Lienlaf, M., Perez-Villarroel, P., Wang, H.-W., Lee, C., Woan, K., et al. (2014). Divergent roles of histone deacetylase 6 (HDAC6) and histone deacetylase 11 (HDAC11) on the transcriptional regulation of IL10 in antigen presenting cells. Mol. Immunol. 60, 44–53. doi: 10.1016/j.molimm.2014.02.019
Chen, J., Melton, C., Suh, N., Oh, J. S., Horner, K., Xie, F., et al. (2011). Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 25, 755–766. doi: 10.1101/gad.2028911
Chen, J., Torcia, S., Xie, F., Lin, C. J., Cakmak, H., Franciosi, F., et al. (2013). Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat. Cell Biol. 15, 1415–1423. doi: 10.1038/ncb2873
Chen, M. S., Hurov, J., White, L. S., Woodford-Thomas, T., and Piwnica-Worms, H. (2001). Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol. Cell. Biol. 21, 3853–3861. doi: 10.1128/mcb.21.12.3853-3861.2001
Cho, W. K., Stern, S., and Biggers, J. D. (1974). Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J. Exp. Zool. 187, 383–386. doi: 10.1002/jez.1401870307
Choi, Y. J., Kang, M. H., Hong, K., and Kim, J. H. (2019). Tubastatin A inhibits HDAC and Sirtuin activity rather than being a HDAC6-specific inhibitor in mouse oocytes. Aging 11, 1759–1777. doi: 10.18632/aging.101867
Grozinger, C. M., and Schreiber, S. L. (2002). Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem. Biol. 9, 3–16. doi: 10.1016/s1074-5521(02)00092-3
Ciccone, D. N., Su, H., Hevi, S., Gay, F., Lei, H., Bajko, J., et al. (2009). KDM1B is a histone H3K4 demethylase required to establish maternal genomic imprints. Nature 461, 415–418. doi: 10.1038/nature08315
Conti, M., Andersen, C. B., Richard, F., Mehats, C., Chun, S. Y., Horner, K., et al. (2002). Role of cyclic nucleotide signaling in oocyte maturation. Mol. Cell Endocrinol. 187, 153–159. doi: 10.1016/s0303-7207(01)00686-4
Conti, M., Hsieh, M., Musa Zamah, A., and Oh, J. S. (2012). Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell Endocrinol. 356, 65–73. doi: 10.1016/j.mce.2011.11.002
Conti, M., Hsieh, M., Park, J. Y., and Su, Y. Q. (2006). Role of the epidermal growth factor network in ovarian follicles. Mol. Endocrinol. 20, 715–723. doi: 10.1210/me.2005-0185
Coticchio, G., Dal Canto, M., Mignini Renzini, M., Guglielmo, M. C., Brambillasca, F., Turchi, D., et al. (2015). Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 21, 427–454. doi: 10.1093/humupd/dmv011
Das, K., Stout, L. E., Hensleigh, H. C., Tagatz, G. E., Phipps, W. R., and Leung, B. S. (1991). Direct positive effect of epidermal growth factor on the cytoplasmic maturation of mouse and human oocytes. Fertil. Steril. 55, 1000–1004. doi: 10.1016/s0015-0282(16)54313-1
de Ruijter, A. J., van Gennip, A. H., Caron, H. N., Kemp, S., and van Kuilenburg, A. B. (2003). Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 370, 737–749. doi: 10.1042/bj20021321
Dekel, N. (2005). Cellular, biochemical and molecular mechanisms regulating oocyte maturation. Mol. Cell Endocrinol. 234, 19–25. doi: 10.1016/j.mce.2004.09.010
Dekel, N. (2009). Master regulators of female fertility. N. Engl. J. Med. 361, 718–719. doi: 10.1056/NEJMcibr0904558
Dekel, N., Lawrence, T. S., Gilula, N. B., and Beers, W. H. (1981). Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev. Biol. 86, 356–362. doi: 10.1016/0012-1606(81)90193-7
Di Emidio, G., Falone, S., Vitti, M., D’Alessandro, A. M., Vento, M., Di Pietro, C., et al. (2014). SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum. Reprod. 29, 2006–2017. doi: 10.1093/humrep/deu160
Dumollard, R., Duchen, M., and Sardet, C. (2006). Calcium signals and mitochondria at fertilisation. Semin. Cell Dev. Biol. 17, 314–323. doi: 10.1016/j.semcdb.2006.02.009
Dupont, S., Krust, A., Gansmuller, A., Dierich, A., Chambon, P., and Mark, M. (2000). Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127, 4277–4291.
Edry, I., Sela-Abramovich, S., and Dekel, N. (2006). Meiotic arrest of oocytes depends on cell-to-cell communication in the ovarian follicle. Mol. Cell Endocrinol. 252, 102–106. doi: 10.1016/j.mce.2006.03.009
Egbert, J. R., Shuhaibar, L. C., Edmund, A. B., Van Helden, D. A., Robinson, J. W., Uliasz, T. F., et al. (2014). Dephosphorylation and inactivation of NPR2 guanylyl cyclase in granulosa cells contributes to the LH-induced decrease in cGMP that causes resumption of meiosis in rat oocytes. Development 141, 3594–3604. doi: 10.1242/dev.112219
Egbert, J. R., Uliasz, T. F., Shuhaibar, L. C., Geerts, A., Wunder, F., Kleiman, R. J., et al. (2016). Luteinizing hormone causes phosphorylation and activation of the cGMP phosphodiesterase PDE5 in rat ovarian follicles, contributing, together with PDE1 activity, to the resumption of meiosis. Biol. Reprod. 94:110. doi: 10.1095/biolreprod.115.135897
Eppig, J. J., Ward-Bailey, P. F., and Coleman, D. L. (1985). Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biol. Reprod. 33, 1041–1049. doi: 10.1095/biolreprod33.5.1041
Fan, H. Y., Liu, Z., Shimada, M., Sterneck, E., Johnson, P. F., Hedrick, S. M., et al. (2009). MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324, 938–941. doi: 10.1126/science.1171396
Feitosa, W. B., Hwang, K., and Morris, P. L. (2018). Temporal and SUMO-specific SUMOylation contribute to the dynamics of Polo-like kinase 1 (PLK1) and spindle integrity during mouse oocyte meiosis. Dev. Biol. 434, 278–291. doi: 10.1016/j.ydbio.2017.12.011
FitzHarris, G., Marangos, P., and Carroll, J. (2007). Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev. Biol. 305, 133–144. doi: 10.1016/j.ydbio.2007.02.006
Freudzon, L. (2005). Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J. Cell Biol. 171, 255–265. doi: 10.1083/jcb.200506194
Gall, L., Chene, N., Dahirel, M., Ruffini, S., and Boulesteix, C. (2004). Expression of epidermal growth factor receptor in the goat cumulus-oocyte complex. Mol. Reprod. Dev. 67, 439–445. doi: 10.1002/mrd.20040
Gallinari, P., Di Marco, S., Jones, P., Pallaoro, M., and Steinkühler, C. (2007). HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 17, 195–211. doi: 10.1038/sj.cr.7310149
Gao, L., Cueto, M. A., Asselbergs, F., and Atadja, P. (2002). Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J. Biol. Chem. 277, 25748–25755. doi: 10.1074/jbc.M111871200
Geister, K. A., Brinkmeier, M. L., Hsieh, M., Faust, S. M., Karolyi, I. J., Perosky, J. E., et al. (2012). A novel loss-of-function mutation in Npr2 clarifies primary role in female reproduction and reveals a potential therapy for acromesomelic dysplasia, Maroteaux type. Hum. Mol. Genet. 22, 345–357. doi: 10.1093/hmg/dds432
Gershon, E., Plaks, V., and Dekel, N. (2008). Gap junctions in the ovary: expression, localization and function. Mol. Cell Endocrinol. 282, 18–25. doi: 10.1016/j.mce.2007.11.001
Gilula, N. B., Epstein, M. L., and Beers, W. H. (1978). Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J. Cell Biol. 78, 58–75. doi: 10.1083/jcb.78.1.58
Glaser, S., Schaft, J., Lubitz, S., Vintersten, K., van der Hoeven, F., Tufteland, K. R., et al. (2006). Multiple epigenetic maintenance factors implicated by the loss of Mll2 in mouse development. Development 133, 1423–1432. doi: 10.1242/dev.02302
Goto, H., Tomono, Y., Ajiro, K., Kosako, H., Fujita, M., Sakurai, M., et al. (1999). Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J. Biol. Chem. 274, 25543–25549. doi: 10.1074/jbc.274.36.25543
Gu, L., Wang, Q., and Sun, Q. Y. (2010). Histone modifications during mammalian oocyte maturation: dynamics, regulation and functions. Cell Cycle 9, 1942–1950. doi: 10.4161/cc.9.10.11599
Gupta, A., Hunt, C. R., Pandita, R. K., Pae, J., Komal, K., Singh, M., et al. (2013). T-cell-specific deletion of Mof blocks their differentiation and results in genomic instability in mice. Mutagenesis 28, 263–270. doi: 10.1093/mutage/ges080
Gutierrez, R. M., and Hnilica, L. S. (1967). Tissue specificity of histone phosphorylation. Science 157, 1324–1325. doi: 10.1126/science.157.3794.1324
Han, L., Ge, J., Zhang, L., Ma, R., Hou, X., Li, B., et al. (2015). Sirt6 depletion causes spindle defects and chromosome misalignment during meiosis of mouse oocyte. Sci. Rep. 5:15366. doi: 10.1038/srep15366
Han, S. J., Chen, R., Paronetto, M. P., and Conti, M. (2005). Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr. Biol. 15, 1670–1676. doi: 10.1016/j.cub.2005.07.056
Han, S. J., and Conti, M. (2006). New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle 5, 227–231. doi: 10.4161/cc.5.3.2395
Hanna, C. W., Taudt, A., Huang, J., Gahurova, L., Kranz, A., Andrews, S., et al. (2018). MLL2 conveys transcription-independent H3K4 trimethylation in oocytes. Nat. Struct. Mol. Biol. 25, 73–82. doi: 10.1038/s41594-017-0013-5
Hao, X., Wang, Y., Kong, N., Zhang, Y., Zhao, Y., Xia, G., et al. (2016). Epidermal growth factor-mobilized intracellular calcium of cumulus cells decreases natriuretic peptide receptor 2 affinity for natriuretic peptide type C and induces oocyte meiotic resumption in the mouse. Biol. Reprod. 95, 3401–3409. doi: 10.1095/biolreprod.116.140137
He, Y., Li, X., Gao, M., Liu, H., and Gu, L. (2019). Loss of HDAC3 contributes to meiotic defects in aged oocytes. Aging Cell 18:e13036. doi: 10.1111/acel.13036
Herbert, M., Levasseur, M., Homer, H., Yallop, K., Murdoch, A., and McDougall, A. (2003). Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B. Nat. Cell Biol. 5, 1023–1025. doi: 10.1038/ncb1062
Hinckley, M., Vaccari, S., Horner, K., Chen, R., and Conti, M. (2005). The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev. Biol. 287, 249–261. doi: 10.1016/j.ydbio.2005.08.019
Holt, J. E., Weaver, J., and Jones, K. T. (2010). Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development 137, 1297–1304. doi: 10.1242/dev.047555
Horner, K., Livera, G., Hinckley, M., Trinh, K., Storm, D., and Conti, M. (2003). Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev. Biol. 258, 385–396. doi: 10.1016/S0012-1606(03)00134-9
Houben, A., Demidov, D., Rutten, T., and Scheidtmann, K. H. (2005). Novel phosphorylation of histone H3 at threonine 11 that temporally correlates with condensation of mitotic and meiotic chromosomes in plant cells. Cytogenet. Genome Res. 109, 148–155. doi: 10.1159/000082394
Hsieh, M., Lee, D., Panigone, S., Horner, K., Chen, R., Theologis, A., et al. (2007). Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol. Cell Biol. 27, 1914–1924. doi: 10.1128/mcb.01919-06
Hsieh, M., Thao, K., and Conti, M. (2011). Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS One 6:e21574. doi: 10.1371/journal.pone.0021574
Huletsky, A., Niedergang, C., Fréchette, A., Aubin, R., Gaudreau, A., and Poirier, G. G. (1985). Sequential ADP-ribosylation pattern of nucleosomal histones. ADP-ribosylation of nucleosomal histones. Eur. J. Biochem. 146, 277–285. doi: 10.1111/j.1432-1033.1985.tb08650.x
Huo, L. J., Fan, H. Y., Liang, C. G., Yu, L. Z., Zhong, Z. S., Chen, D. Y., et al. (2004). Regulation of ubiquitin-proteasome pathway on pig oocyte meiotic maturation and fertilization. Biol. Reprod. 71, 853–862. doi: 10.1095/biolreprod.104.028134
Ihara, M., Stein, P., and Schultz, R. M. (2008). UBE2I (UBC9), a SUMO-conjugating enzyme, localizes to nuclear speckles and stimulates transcription in mouse oocytes. Biol. Reprod. 79, 906–913. doi: 10.1095/biolreprod.108.070474
Itami, N., Shirasuna, K., Kuwayama, T., and Iwata, H. (2015). Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology 83, 1360–1367. doi: 10.1016/j.theriogenology.2015.01.029
Ivshina, M., Lasko, P., and Richter, J. D. (2014). Cytoplasmic polyadenylation element binding proteins in development, health, and disease. Annu. Rev. Cell Dev. Biol. 30, 393–415. doi: 10.1146/annurev-cellbio-101011-155831
Schelbach, C. J., Kind, K. L., Lane, M., and Thompson, J. G. (2010). Mechanisms contributing to the reduced developmental competence of glucosamine-exposed mouse oocytes. Reprod. Fertil. Dev. 22, 771–779. doi: 10.1071/RD09193
Jaffe, L. A., and Egbert, J. R. (2017). Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle. Annu. Rev. Physiol. 79, 237–260. doi: 10.1146/annurev-physiol-022516-034102
Jankowski, M., Reis, A. M., Mukaddam-Daher, S., Dam, T. V., Farookhi, R., and Gutkowska, J. (1997). C-Type natriuretic peptide and the guanylyl cyclase receptors in the rat ovary are modulated by the estrous cycle. Biol. Reprod. 56, 59–66. doi: 10.1095/biolreprod56.1.59
Jeliìnkovaì, L., and Kubelka, M. (2006). Neither aurora B activity nor histone H3 phosphorylation is essential for chromosome condensation during meiotic maturation of porcine oocytes. Biol. Reprod. 74, 905–912. doi: 10.1095/biolreprod.105.047886
Jones, K. T. (2004). Turning it on and off: M-phase promoting factor during meiotic maturation and fertilization. Mol. Hum. Reprod. 10, 1–5. doi: 10.1093/molehr/gah009
Jones, K. T. (2011). Anaphase-promoting complex control in female mouse meiosis. Res. Probl. Cell Differ. 53, 343–363. doi: 10.1007/978-3-642-19065-0-15
Kageyama, S.-I., Liu, H., Kaneko, N., Ooga, M., Nagata, M., and Aoki, F. (2007). Alterations in epigenetic modifications during oocyte growth in mice. Reproduction 133, 85–94. doi: 10.1530/rep-06-0025
Kageyama, S.-I., Liu, H., Nagata, M., and Aoki, F. (2006). Stage specific expression of histone deacetylase 4 (HDAC4) during oogenesis and early preimplantation development in mice. J. Reprod. Dev. 52, 99–106. doi: 10.1262/jrd.17044
Kalinowski, R. R., Berlot, C. H., Jones, T. L., Ross, L. F., Jaffe, L. A., and Mehlmann, L. M. (2004). Maintenance of meiotic prophase arrest in vertebrate oocytes by a Gs protein-mediated pathway. Dev. Biol. 267, 1–13. doi: 10.1016/j.ydbio.2003.11.011
Kalma, Y., Granot, I., Galiani, D., Barash, A., and Dekel, N. (2004). Luteinizing hormone-induced connexin 43 down-regulation: inhibition of translation. Endocrinology 145, 1617–1624. doi: 10.1210/en.2003-1051
Kawamura, K., Cheng, Y., Kawamura, N., Takae, S., Okada, A., Kawagoe, Y., et al. (2011). Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum. Reprod. 26, 3094–3101. doi: 10.1093/humrep/der282
Khan, I., Kim, S. W., Lee, K. L., Song, S. H., Mesalam, A., Chowdhury, M. M. R., et al. (2017). Polydatin improves the developmental competence of bovine embryos in vitro via induction of sirtuin 1 (Sirt1). Reprod. Fertil. Dev. 29, 2011–2020. doi: 10.1071/RD16302
Kim, J. M., Liu, H., Tazaki, M., Nagata, M., and Aoki, F. (2003). Changes in histone acetylation during mouse oocyte meiosis. J. Cell Biol. 162, 37–46. doi: 10.1083/jcb.200303047
Kim, J., Singh, A. K., Takata, Y., Lin, K., Shen, J., Lu, Y., et al. (2015). LSD1 is essential for oocyte meiotic progression by regulating CDC25B expression in mice. Nat. Commun. 6:10116. doi: 10.1038/ncomms10116
Kim, J. H., Park, K. C., Chung, S. S., Bang, O., and Chung, C. H. (2003). Deubiquitinating enzymes as cellular regulators. J. Biochem. 134, 9–18. doi: 10.1093/jb/mvg107
Kiyama, R., and Wada-Kiyama, Y. (2015). Estrogenic endocrine disruptors: molecular mechanisms of action. Environ. Int. 83, 11–40. doi: 10.1016/j.envint.2015.05.012
Klose, R. J., Yan, Q., Tothova, Z., Yamane, K., Erdjument-Bromage, H., Tempst, P., et al. (2007). The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell 128, 889–900. doi: 10.1016/j.cell.2007.02.013
Koprinarova, M. A., and Russev, G. C. (2008). Dynamics of histone H4 acetylation during the cell cycle. Cell Cycle 7, 414–416. doi: 10.4161/cc.7.3.5314
Kovanci, E., Simpson, J. L., Amato, P., Rohozinski, J., Heard, M. J., Bishop, C. E., et al. (2008). Oocyte-specific G-protein-coupled receptor 3 (GPR3): no perturbations found in 82 women with premature ovarian failure (first report). Fertil. Steril. 90, 1269–1271. doi: 10.1016/j.fertnstert.2007.07.1373
Kovo, M., Kandli-Cohen, M., Ben-Haim, M., Galiani, D., Carr, D. W., and Dekel, N. (2006). An active protein kinase A (PKA) is involved in meiotic arrest of rat growing oocytes. Reproduction 132, 33–43. doi: 10.1530/rep.1.00824
Krege, J. H., Hodgin, J. B., Couse, J. F., Enmark, E., Warner, M., Mahler, J. F., et al. (1998). Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc. Natl. Acad. Sci. U.S.A. 95, 15677–15682. doi: 10.1073/pnas.95.26.15677
Krieg, A. J., Mullinax, S. R., Grimstad, F., Marquis, K., Constance, E., Hong, Y., et al. (2018). Histone demethylase KDM4A and KDM4B expression in granulosa cells from women undergoing in vitro fertilization. J. Assist. Reprod. Genet. 35, 993–1003. doi: 10.1007/s10815-018-1151-3
Leboeuf, M., Terrell, A., Trivedi, S., Sinha, S., Epstein, J. A., Olson, E. N., et al. (2010). Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev. Cell 19, 807–818. doi: 10.1016/j.devcel.2010.10.015
Lee, J.-H., and Skalnik, D. G. (2005). CpG-binding protein (CXXC Finger Protein 1) is a component of the mammalian Set1 Histone H3-Lys4 methyltransferase complex, the analogue of the Yeast Set1/COMPASS complex. J. Biol. Chem. 280, 41725–41731. doi: 10.1074/jbc.M508312200
Lee, K. B., Zhang, M., Sugiura, K., Wigglesworth, K., Uliasz, T., Jaffe, L. A., et al. (2013). Hormonal coordination of natriuretic peptide type C and natriuretic peptide receptor 3 expression in mouse granulosa cells. Biol. Reprod. 88:42. doi: 10.1095/biolreprod.112.104810
Li, E. (2002). Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 3, 662–673. doi: 10.1038/nrg887
Li, H., You, L., Tian, Y., Guo, J., Fang, X., Zhou, C., et al. (2020). DPAGT1-mediated protein N-Glycosylation is indispensable for oocyte and follicle development in mice. Adv. Sci. 7:2000531. doi: 10.1002/advs.202000531
Li, J., Qian, W. P., and Sun, Q. Y. (2019). Cyclins regulating oocyte meiotic cell cycle progression. Biol. Reprod. 101, 878–881. doi: 10.1093/biolre/ioz143
Li, R., and Albertini, D. F. (2013). The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 14, 141–152. doi: 10.1038/nrm3531
Li, T. Y., Colley, D., Barr, K. J., Yee, S.-P., and Kidder, G. M. (2007). Rescue of oogenesis in Cx37-null mutant mice by oocyte-specific replacement with Cx43. J. Cell Sci. 120, 4117–4125. doi: 10.1242/jcs.03488
Li, X., Liu, X., Gao, M., Han, L., Qiu, D., Wang, H., et al. (2017). HDAC3 promotes meiotic apparatus assembly in mouse oocytes by modulating tubulin acetylation. Development 144, 3789–3797. doi: 10.1242/dev.153353
Li, Y., Wang, J., Zhang, Z., Yi, J., He, C., Wang, F., et al. (2016). Resveratrol compares with melatonin in improving in vitro porcine oocyte maturation under heat stress. J. Anim. Sci. Biotechnol. 7:33. doi: 10.1186/s40104-016-0093-9
Liu, M. (2011). The biology and dynamics of mammalian cortical granules. Reprod. Biol. Endocrinol. 9:149. doi: 10.1186/1477-7827-9-149
Liu, M., Sims, D., Calarco, P., and Talbot, P. (2003). Biochemical heterogeneity, migration, and pre-fertilization release of mouse oocyte cortical granules. Reprod. Biol. Endocrinol. 1:77. doi: 10.1186/1477-7827-1-77
Liu, M., Yin, Y., Ye, X., Zeng, M., Zhao, Q., Keefe, D. L., et al. (2013). Resveratrol protects against age-associated infertility in mice. Hum. Reprod. 28, 707–717. doi: 10.1093/humrep/des437
Liu, W., Xin, Q., Wang, X., Wang, S., Wang, H., Zhang, W., et al. (2017). Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals. Cell Death Dis. 8:e2662. doi: 10.1038/cddis.2017.82
Liu, Y., Lu, C., Yang, Y., Fan, Y., Yang, R., Liu, C. F., et al. (2011). Influence of histone tails and H4 tail acetylations on nucleosome-nucleosome interactions. J. Mol. Biol. 414, 749–764. doi: 10.1016/j.jmb.2011.10.031
Lombard, D. B., Alt, F. W., Cheng, H. L., Bunkenborg, J., Streeper, R. S., Mostoslavsky, R., et al. (2007). Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27, 8807–8814. doi: 10.1128/MCB.01636-07
Ma, J. Y., Li, M., Luo, Y.-B., Song, S., Tian, D., Yang, J., et al. (2013). Maternal factors required for oocyte developmental competence in mice: transcriptome analysis of non-surrounded nucleolus (NSN) and surrounded nucleolus (SN) oocytes. Cell Cycle 12, 1928–1938. doi: 10.4161/cc.24991
Ma, P., Hua, P., Montgomery, R. L., Olson, E. N., and Schultz, R. M. (2012). Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. Proc. Natl. Acad. Sci. U.S.A. 109, 2704–2704. doi: 10.1073/pnas.1118403109
Ma, P., and Schultz, R. M. (2008). Histone deacetylase 1 (HDAC1) regulates histone acetylation, development, and gene expression in preimplantation mouse embryos. Dev. Biol. 319, 110–120. doi: 10.1016/j.ydbio.2008.04.011
Ma, P., and Schultz, R. M. (2013). Histone deacetylase 2 (HDAC2) regulates chromosome segregation and kinetochore function via H4K16 deacetylation during oocyte maturation in mouse. PLoS Genet. 9:e1003377. doi: 10.1371/journal.pgen.1003377
Machaca, K. (2007). Ca2+ signaling differentiation during oocyte maturation. J. Cell Physiol. 213, 331–340. doi: 10.1002/jcp.21194
Marangos, P., and Carroll, J. (2004). The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction 128, 153–162. doi: 10.1530/rep.1.00192
Marangos, P., and Carroll, J. (2008). Securin regulates entry into M-phase by modulating the stability of cyclin B. Nat. Cell Biol. 10, 445–451. doi: 10.1038/ncb1707
McGraw, S., Morin, G., Vigneault, C., Leclerc, P., and Sirard, M. A. (2007). Investigation of MYST4 histone acetyltransferase and its involvement in mammalian gametogenesis. BMC Dev. Biol. 7:123. doi: 10.1186/1471-213x-7-123
McGraw, S., Robert, C., Massicotte, L., and Sirard, M. A. (2003). Quantification of histone acetyltransferase and histone deacetylase transcripts during early bovine embryo development. Biol. Reprod. 68, 383–389. doi: 10.1095/biolreprod.102.005991
Mehlmann, L. M. (2005). Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 130, 791–799. doi: 10.1530/rep.1.00793
Mehlmann, L. M., Jones, T. L., and Jaffe, L. A. (2002). Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 297, 1343–1345. doi: 10.1126/science.1073978
Montgomery, R. L., Davis, C. A., Potthoff, M. J., Haberland, M., Fielitz, J., Qi, X., et al. (2007). Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 21, 1790–1802. doi: 10.1101/gad.1563807
Moor, R. M., Dai, Y., Lee, C., and Fulka, J. Jr. (1998). Oocyte maturation and embryonic failure. Hum. Reprod. Update 4, 223–236. doi: 10.1093/humupd/4.3.223
Mostoslavsky, R., Chua, K. F., Lombard, D. B., Pang, W. W., Fischer, M. R., Gellon, L., et al. (2006). Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329. doi: 10.1016/j.cell.2005.11.044
Mtango, N. R., Latham, K. E., and Sutovsky, P. (2014). “Deubiquitinating enzymes in oocyte maturation, fertilization and preimplantation embryo development,” in Posttranslational Protein Modifications in the Reproductive System. Advances in Experimental Medicine and Biology, Vol. 759, ed. P. Sutovsky (New York, NY: Springer).
Mtango, N. R., Sutovsky, M., VandeVoort, C. A., Latham, K. E., and Sutovsky, P. (2012). Essential role of ubiquitin C-terminal hydrolases UCHL1 and UCHL3 in mammalian oocyte maturation. J. Cell Physiol. 227, 2022–2029. doi: 10.1002/jcp.22931
Nagyova, E., Scsukova, S., Nemcova, L., Mlynarcikova, A., Yi, Y. J., Sutovsky, M., et al. (2012). Inhibition of proteasomal proteolysis affects expression of extracellular matrix components and steroidogenesis in porcine oocyte-cumulus complexes. Domest. Anim. Endocrinol. 42, 50–62. doi: 10.1016/j.domaniend.2011.09.003
Nathan, D., Ingvarsdottir, K., Sterner, D. E., Bylebyl, G. R., Dokmanovic, M., Dorsey, J. A., et al. (2006). Histone sumoylation is a negative regulator in saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev. 20, 966–976. doi: 10.1101/gad.1404206
Nicholson, S. M., and Bruzzone, R. (1997). Gap junctions: getting the message through. Curr. Biol. 7, R340–R344. doi: 10.1016/s0960-9822(06)00169-2
Norris, R. P., Ratzan, W. J., Freudzon, M., Mehlmann, L. M., Krall, J., Movsesian, M. A., et al. (2009). Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136, 1869–1878. doi: 10.1242/dev.035238
Ohtsubo, K., and Marth, J. D. (2006). Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867. doi: 10.1016/j.cell.2006.08.019
Ozturk, S., and Uysal, F. (2017). Poly(A)-binding proteins are required for translational regulation in vertebrate oocytes and early embryos. Reprod. Fertil. Dev. 29, 1890–1901. doi: 10.1071/RD16283
Pan, Z. N., Pan, M. H., Sun, M. H., Li, X. H., Zhang, Y., and Sun, S. C. (2020). RAB7 GTPase regulates actin dynamics for DRP1-mediated mitochondria function and spindle migration in mouse oocyte meiosis. FASEB J. 34, 9615–9627. doi: 10.1096/fj.201903013R
Park, J. Y., Su, Y. Q., Ariga, M., Law, E., Jin, S. L., and Conti, M. (2004). EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303, 682–684. doi: 10.1126/science.1092463
Piccioni, F., Zappavigna, V., and Verrotti, A. C. (2005). Translational regulation during oogenesis and early development: the cap-poly(A) tail relationship. C. R. Biol. 328, 863–881. doi: 10.1016/j.crvi.2005.05.006
Pincus, G., and Enzmann, E. V. (1935). The comparative behavior of mammalian eggs in vivo and in vitro: I. the activation of ovarian eggs. J. Exp. Med. 62, 665–675. doi: 10.1084/jem.62.5.665
Rada-Iglesias, A., Enroth, S., Andersson, R., Wanders, A., Påhlman, L., Komorowski, J., et al. (2009). Histone H3 lysine 27 trimethylation in adult differentiated colon associated to cancer DNA hypermethylation. Epigenetics 4, 107–113. doi: 10.4161/epi.4.2.8038
Reis, A., Chang, H. Y., Levasseur, M., and Jones, K. T. (2006). APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat. Cell Biol. 8, 539–540. doi: 10.1038/ncb1406
Reis, A. M., and Honorato-Sampaio, K. (2018). C-type natriuretic peptide: a link between hyperandrogenism and anovulation in a mouse model of polycystic ovary syndrome. Clin. Sci. 132, 905–908. doi: 10.1042/CS20171491
Reizel, Y., Elbaz, J., and Dekel, N. (2010). Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Endocr. Rev. 31, 137–138. doi: 10.1210/edrv.31.1.9998
Richardson, B. C. (2002). Role of DNA methylation in the regulation of cell function: autoimmunity, aging and cancer. J. Nutr. 132, 2401s–2405s. doi: 10.1093/jn/132.8.2401S
Richter, J. D. (2007). CPEB: a life in translation. Trends Biochem. Sci. 32, 279–285. doi: 10.1016/j.tibs.2007.04.004
Riepsamen, A., Wu, L., Lau, L., Listijono, D., Ledger, W., Sinclair, D., et al. (2015). Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes. PLoS One 10:e0126194. doi: 10.1371/journal.pone.0126194
Rodriguez, A., Briley, S. M., Patton, B. K., Tripurani, S. K., Rajapakshe, K., Coarfa, C., et al. (2019). Loss of the E2 SUMO-conjugating enzyme Ube2i in oocytes during ovarian folliculogenesis causes infertility in mice. Development 146:dev176701. doi: 10.1242/dev.176701
Roguev, A., Schaft, D., Shevchenko, A., Pijnappel, W. W. M. P., Wilm, M., Aasland, R., et al. (2001). The Saccharomyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone 3 lysine 4. EMBO J. 20, 7137–7148. doi: 10.1093/emboj/20.24.7137
Ropero, S., and Esteller, M. (2007). The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 1, 19–25. doi: 10.1016/j.molonc.2007.01.001
Sela-Abramovich, S., Chorev, E., Galiani, D., and Dekel, N. (2005). Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146, 1236–1244. doi: 10.1210/en.2004-1006
Saito, S., Yano, K., Sharma, S., McMahon, H. E., and Shimasaki, S. (2008). Characterization of the post-translational modification of recombinant human BMP-15 mature protein. Protein Sci. 17, 362–370. doi: 10.1110/ps.073232608
Sankar, A., Lerdrup, M., Manaf, A., Johansen, J. V., Gonzalez, J. M., Borup, R., et al. (2020). KDM4A regulates the maternal-to-zygotic transition by protecting broad H3K4me3 domains from H3K9me3 invasion in oocytes. Nat. Cell Biol. 22, 380–388. doi: 10.1038/s41556-020-0494-z
Sarmento, O. F., Digilio, L. C., Wang, Y., Perlin, J., Herr, J. C., Allis, C. D., et al. (2004). Dynamic alterations of specific histone modifications during early murine development. J. Cell Sci. 117, 4449–4459. doi: 10.1242/jcs.01328
Sasseville, M., Côté, N., Guillemette, C., and Richard, F. J. (2006). New insight into the role of phosphodiesterase 3A in porcine oocyte maturation. BMC Dev. Biol. 6:47. doi: 10.1186/1471-213X-6-47
Sato, D., Itami, N., Tasaki, H., Takeo, S., Kuwayama, T., and Iwata, H. (2014). Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS One 9:e94488. doi: 10.1371/journal.pone.0094488
Schatten, H., and Sun, Q. Y. (2014). “Posttranslationally modified tubulins and other cytoskeletal proteins: their role in gametogenesis, oocyte maturation, fertilization and pre-implantation embryo development,” in Posttranslational Protein Modifications in the Reproductive System, Vol. 59, ed. P. Sutovsky (New York, NY: Springer New York), 57–87.
Schellander, K., Hoelker, M., and Tesfaye, D. (2007). Selective degradation of transcripts in mammalian oocytes and embryos. Theriogenology 68(Suppl. 1), S107–S115. doi: 10.1016/j.theriogenology.2007.05.054
Schultz, R. M., Montgomery, R. R., and Belanoff, J. R. (1983). Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev. Biol. 97, 264–273. doi: 10.1016/0012-1606(83)90085-4
Sela-Abramovich, S., Edry, I., Galiani, D., Nevo, N., and Dekel, N. (2006). Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 147, 2280–2286. doi: 10.1210/en.2005-1011
Sengupta, N., and Seto, E. (2004). Regulation of histone deacetylase activities. J. Cell. Biochem. 93, 57–67. doi: 10.1002/jcb.20179
Sha, Q. Q., Dai, X. X., Jiang, J. C., Yu, C., Jiang, Y., Liu, J., et al. (2018). CFP1 coordinates histone H3 lysine-4 trimethylation and meiotic cell cycle progression in mouse oocytes. Nat. Commun. 9:3477. doi: 10.1038/s41467-018-05930-x
Sha, Q. Q., Zhang, J., and Fan, H. Y. (2019). A story of birth and death: mRNA translation and clearance at the onset of maternal-to-zygotic transition in mammals. Biol. Reprod. 101, 579–590. doi: 10.1093/biolre/ioz012
Sha, Q. Q., Zhang, J., and Fan, H. Y. (2020). Function and regulation of histone H3 lysine-4 methylation during oocyte meiosis and maternal-to-zygotic transition. Front. Cell Dev. Biol. 8:597498. doi: 10.3389/fcell.2020.597498
Shilatifard, A. (2012). The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu. Rev. Biochem. 81, 65–95. doi: 10.1146/annurev-biochem-051710-134100
Shuhaibar, L. C., Egbert, J. R., Edmund, A. B., Uliasz, T. F., Dickey, D. M., Yee, S.-P., et al. (2016). Dephosphorylation of juxtamembrane serines and threonines of the NPR2 guanylyl cyclase is required for rapid resumption of oocyte meiosis in response to luteinizing hormone. Dev. Biol. 409, 194–201. doi: 10.1016/j.ydbio.2015.10.025
Shuhaibar, L. C., Egbert, J. R., Norris, R. P., Lampe, P. D., Nikolaev, V. O., Thunemann, M., et al. (2015). Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc. Natl. Acad. Sci. U.S.A. 112, 5527–5532. doi: 10.1073/pnas.1423598112
Simon, A. M., and Goodenough, D. A. (1998). Diverse functions of vertebrate gap junctions. Trends Cell Biol. 8, 477–483. doi: 10.1016/s0962-8924(98)01372-5
Sirard, M. A. (2001). Resumption of meiosis: mechanism involved in meiotic progression and its relation with developmental competence. Theriogenology 55, 1241–1254. doi: 10.1016/s0093-691x(01)00480-0
Solc, P., Schultz, R. M., and Motlik, J. (2010). Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol. Hum. Reprod. 16, 654–664. doi: 10.1093/molehr/gaq034
Su, Y.-Q., Sugiura, K., Sun, F., Pendola, J. K., Cox, G. A., Handel, M. A., et al. (2012a). MARF1 regulates essential oogenic processes in mice. Science 335, 1496–1499. doi: 10.1126/science.1214680
Su, Y.-Q., Sun, F., Handel, M. A., Schimenti, J. C., and Eppig, J. J. (2012b). Meiosis arrest female 1 (MARF1) has nuage-like function in mammalian oocytes. Proc. Natl. Acad. Sci. U.S.A. 109, 18653–18660. doi: 10.1073/pnas.1216904109
Su, Y. Q., Wigglesworth, K., Pendola, F. L., O’Brien, M. J., and Eppig, J. J. (2002). Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 143, 2221–2232. doi: 10.1210/endo.143.6.8845
Su, Y. Q., Xia, G. L., Grete Byskov, A., Fu, G. D., and Yang, C. R. (1999). Protein kinase C and intracellular calcium are involved in follicle-stimulating hormone-mediated meiotic resumption of cumulus cell-enclosed porcine oocytes in hypoxanthine-supplemented medium. Mol. Reprod. Dev. 53, 51–58. doi: 10.1002/(SICI)1098-2795(199905)53:1<51::AID-MRD6<3.0.CO;2-4
Su, Y. Q., Sugiura, K., Woo, Y., Wigglesworth, K., Kamdar, S., Affourtit, J., et al. (2007). Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev. Biol. 302, 104–117. doi: 10.1016/j.ydbio.2006.09.008
Sui, L., Huang, R., Yu, H., Zhang, S., and Li, Z. (2020a). Inhibition of HDAC6 by tubastatin A disrupts mouse oocyte meiosis via regulating histone modifications and mRNA expression. J. Cell. Physiol. 235, 7030–7042. doi: 10.1002/jcp.29599
Sui, L., Zhang, S., Huang, R., and Li, Z. (2020b). HDAC11 promotes meiotic apparatus assembly during mouse oocyte maturation via decreasing H4K16 and α-tubulin acetylation. Cell Cycle 19, 354–362. doi: 10.1080/15384101.2019.1711315
Sun, M. H., Li, X. H., Xu, Y., Xu, Y., Pan, Z. N., and Sun, S. C. (2020). Citrinin exposure disrupts organelle distribution and functions in mouse oocytes. Environ. Res. 185:109476. doi: 10.1016/j.envres.2020.109476
Susor, A., Liskova, L., Toralova, T., Pavlok, A., Pivonkova, K., Karabinova, P., et al. (2010). Role of ubiquitin C-terminal hydrolase-L1 in antipolyspermy defense of mammalian oocytes. Biol. Reprod. 82, 1151–1161. doi: 10.1095/biolreprod.109.081547
Sutton-McDowall, M. L., Mitchell, M., Cetica, P., Dalvit, G., Pantaleon, M., Lane, M., et al. (2006). Glucosamine supplementation during in vitro maturation inhibits subsequent embryo development: possible role of the hexosamine pathway as a regulator of developmental competence. Biol. Reprod. 74, 881–888. doi: 10.1095/biolreprod.105.048553
Swain, J. E., Ding, J., Brautigan, D. L., Villa-Moruzzi, E., and Smith, G. D. (2007). Proper chromatin condensation and maintenance of histone H3 phosphorylation during mouse oocyte meiosis requires protein phosphatase activity. Biol. Reprod. 76, 628–638. doi: 10.1095/biolreprod.106.055798
Takeo, S., Sato, D., Kimura, K., Monji, Y., Kuwayama, T., Kawahara-Miki, R., et al. (2014). Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J. Reprod. Dev. 60, 92–99. doi: 10.1262/jrd.2013-102
Tatone, C., Di Emidio, G., Barbonetti, A., Carta, G., Luciano, A. M., Falone, S., et al. (2018). Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Update 24, 267–289. doi: 10.1093/humupd/dmy003
Thomas, T., Loveland, K. L., and Voss, A. K. (2007). The genes coding for the MYST family histone acetyltransferases, Tip60 and Mof, are expressed at high levels during sperm development. Gene Expr. Patterns 7, 657–665. doi: 10.1016/j.modgep.2007.03.005
Thomas, T., and Voss, A. K. (2007). The diverse biological roles of MYST histone acetyltransferase family proteins. Cell Cycle 6, 696–704. doi: 10.4161/cc.6.6.4013
Törnell, J., Billig, H., and Hillensjö, T. (1990). Resumption of rat oocyte meiosis is paralleled by a decrease in guanosine 3’,5’-cyclic monophosphate (cGMP) and is inhibited by microinjection of cGMP. Acta Physiol. Scand. 139, 511–517. doi: 10.1111/j.1748-1716.1990.tb08953.x
Tsafriri, A., Cao, X., Ashkenazi, H., Motola, S., Popliker, M., and Pomerantz, S. H. (2005). Resumption of oocyte meiosis in mammals: on models, meiosis activating sterols, steroids and EGF-like factors. Mol. Cell Endocrinol. 234, 37–45. doi: 10.1016/j.mce.2004.09.009
Tsuji, T., Kiyosu, C., Akiyama, K., and Kunieda, T. (2012). CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol. Reprod. Dev. 79, 795–802. doi: 10.1002/mrd.22114
Vaccari, S., Horner, K., Mehlmann, L. M., and Conti, M. (2008). Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev. Biol. 316, 124–134. doi: 10.1016/j.ydbio.2008.01.018
Veldhuis, J. D. (1987). Mechanisms subserving hormone action in the ovary: role of calcium ions as assessed by steady state calcium exchange in cultured swine granulosa cells. Endocrinology 120, 445–449. doi: 10.1210/endo-120-2-445
Verdel, A., Seigneurin-Berny, D., Faure, A.-K., Eddahbi, M., Khochbin, S., and Nonchev, S. (2003). HDAC6-induced premature chromatin compaction in mouse oocytes and fertilised eggs. Zygote 11, 323–328. doi: 10.1017/S0967199403002387
Verlhac, M. H., Lefebvre, C., Guillaud, P., Rassinier, P., and Maro, B. (2000). Asymmetric division in mouse oocytes: with or without Mos. Curr. Biol. 10, 1303–1306. doi: 10.1016/S0960-9822(00)00753-3
Vigneron, C., Perreau, C., Dupont, J., Uzbekova, S., Prigent, C., and Mermillod, P. (2004). Several signaling pathways are involved in the control of cattle oocyte maturation. Mol. Reprod. Dev. 69, 466–474. doi: 10.1002/mrd.20173
Vijay Pratap, S., Wei-Ting, Y., Jennifer, L. G., and Francesca, E. D. (2019). Oocyte-specific deletion of Hdac8 in mice reveals stage-specific effects on fertility. Reproduction 157, 305–316. doi: 10.1530/REP-18-0560
Vivarelli, E., Conti, M., De Felici, M., and Siracusa, G. (1983). Meiotic resumption and intracellular cAMP levels in mouse oocytes treated with compounds which act on cAMP metabolism. Cell Differ. 12, 271–276. doi: 10.1016/0045-6039(83)90023-4
Walser, C. B., and Lipshitz, H. D. (2011). Transcript clearance during the maternal-to-zygotic transition. Curr. Opin. Genet. Dev. 21, 431–443. doi: 10.1016/j.gde.2011.03.003
Wang, F., Tian, X., Zhang, L., He, C., Ji, P., Li, Y., et al. (2014). Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 101, 577–586. doi: 10.1016/j.fertnstert.2013.10.041
Wang, H., Cai, H., Wang, X., Zhang, M., Liu, B., Chen, Z., et al. (2019). HDAC3 maintains oocyte meiosis arrest by repressing amphiregulin expression before the LH surge. Nat. Commun. 10:5719. doi: 10.1038/s41467-019-13671-8
Wang, X., Wang, H., Liu, W., Zhang, Z., Zhang, Y., Zhang, W., et al. (2018). High level of C-type natriuretic peptide induced by hyperandrogen-mediated anovulation in polycystic ovary syndrome mice. Clin. Sci. 132, 759–776. doi: 10.1042/cs20171394
Wang, Y., Kong, N., Li, N., Hao, X., Wei, K., Xiang, X., et al. (2013). Epidermal growth factor receptor signaling-dependent Calcium elevation in cumulus cells is required for NPR2 inhibition and meiotic resumption in mouse oocytes. Endocrinology 154, 3401–3409. doi: 10.1210/en.2013-1133
Wang, Z. B., Ou, X. H., Tong, J. S., Li, S., Wei, L., Ouyang, Y. C., et al. (2010). The SUMO pathway functions in mouse oocyte maturation. Cell Cycle 9, 2640–2646. doi: 10.4161/cc.9.13.12120
Wei, L., Liang, X. W., Zhang, Q. H., Li, M., Yuan, J., Li, S., et al. (2010). BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte. Cell Cycle 9, 1112–1121. doi: 10.4161/cc.9.6.10957
Wei, Y., Mizzen, C. A., Cook, R. G., Gorovsky, M. A., and Allis, C. D. (1998). Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc. Natl. Acad. Sci. U.S.A. 95, 7480–7484. doi: 10.1073/pnas.95.13.7480
Wei, Y., Yu, L., Bowen, J., Gorovsky, M. A., and Allis, C. D. (1999). Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell 97, 99–109. doi: 10.1016/s0092-8674(00)80718-7
Werner, M., and Ruthenburg, A. J. (2011). The United States of histone ubiquitylation and methylation. Mol. Cell 43, 5–7. doi: 10.1016/j.molcel.2011.06.015
Xhabija, B., and Kidder, B. L. (2019). KDM5B is a master regulator of the H3K4-methylome in stem cells, development and cancer. Semin. Cancer Biol. 57, 79–85. doi: 10.1016/j.semcancer.2018.11.001
Xu, D., Bai, J., Duan, Q., Costa, M., and Dai, W. (2009). Covalent modifications of histones during mitosis and meiosis. Cell Cycle 8, 3688–3694. doi: 10.4161/cc.8.22.9908
Xu, Y., Sun, M. H., Xu, Y., Ju, J. Q., Pan, M. H., Pan, Z. N., et al. (2020). Nonylphenol exposure affects mouse oocyte quality by inducing spindle defects and mitochondria dysfunction. Environ. Pollut. 266:114967. doi: 10.1016/j.envpol.2020.114967
Liu, X., Xie, F., Zamah, A. M., Cao, B., and Conti, M. (2014). Multiple pathways mediate luteinizing hormone regulation of cGMP signaling in the mouse ovarian follicle. Biol. Reprod. 91:9. doi: 10.1095/biolreprod.113.116814
Yang, C. R., Wei, Y., Qi, S. T., Lei, C., Zhang, Q. H., Ma, J. Y., et al. (2012a). The G protein coupled receptor 3 is involved in cAMP and cGMP signaling and maintenance of meiotic arrest in porcine oocytes. PLoS One 7:e38807. doi: 10.1371/journal.pone.0038807
Yang, F., Hu, L., Chen, C., Yu, J., O’Connell, C. B., Khodjakov, A., et al. (2012b). BubR1 is modified by sumoylation during mitotic progression. J. Biol. Chem. 287, 4875–4882. doi: 10.1074/jbc.M111.318261
Yang, J., Zhang, Y., Xu, X., Li, J., Yuan, F., Bo, S., et al. (2019). Transforming growth factor-β is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells. Cell Death Dis. 10:558. doi: 10.1038/s41419-019-1797-5
Yang, X. J. (2004). The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 32, 959–976. doi: 10.1093/nar/gkh252
Yang, Y., Yang, C. R., Han, S. J., Daldello, E. M., Cho, A., Martins, J. P. S., et al. (2017). Maternal mRNAs with distinct 3’ UTRs define the temporal pattern of Ccnb1 synthesis during mouse oocyte meiotic maturation. Genes Dev. 31, 1302–1307. doi: 10.1101/gad.296871.117
Yin, S., Jiang, X., Jiang, H., Gao, Q., Wang, F., Fan, S., et al. (2017). Histone acetyltransferase KAT8 is essential for mouse oocyte development by regulating reactive oxygen species levels. Development 144, 2165–2174. doi: 10.1242/dev.149518
Yu, C., Fan, X., Sha, Q. Q., Wang, H. H., Li, B. T., Dai, X. X., et al. (2017). CFP1 regulates histone H3K4 trimethylation and developmental potential in mouse oocytes. Cell Rep. 20, 1161–1172. doi: 10.1016/j.celrep.2017.07.011
Yu, C., Ji, S. Y., Dang, Y. J., Sha, Q. Q., Yuan, Y. F., Zhou, J. J., et al. (2016a). Oocyte-expressed yes-associated protein is a key activator of the early zygotic genome in mouse. Cell Res. 26, 275–287. doi: 10.1038/cr.2016.20
Yu, C., Ji, S. Y., Sha, Q. Q., Dang, Y., Zhou, J. J., Zhang, Y. L., et al. (2016b). BTG4 is a meiotic cell cycle–coupled maternal-zygotic-transition licensing factor in oocytes. Nat. Struct. Mol. Biol. 23, 387–394. doi: 10.1038/nsmb.3204
Yuan, Y. F., Zhai, R., Liu, X. M., Khan, H. A., Zhen, Y. H., and Huo, L. J. (2014). SUMO-1 plays crucial roles for spindle organization, chromosome congression, and chromosome segregation during mouse oocyte meiotic maturation. Mol. Reprod. Dev. 81, 712–724. doi: 10.1002/mrd.22339
Zhang, J., Zhang, Y. L., Zhao, L. W., Guo, J. X., Yu, J. L., Ji, S. Y., et al. (2019). Mammalian nucleolar protein DCAF13 is essential for ovarian follicle maintenance and oocyte growth by mediating rRNA processing. Cell Death Differ. 26, 1251–1266. doi: 10.1038/s41418-018-0203-7
Zhang, J., Zhang, Y. L., Zhao, L. W., Pi, S. B., Zhang, S. Y., Tong, C., et al. (2020). The CRL4-DCAF13 ubiquitin E3 ligase supports oocyte meiotic resumption by targeting PTEN degradation. Cell Mol. Life Sci. 77, 2181–2197. doi: 10.1007/s00018-019-03280-5
Zhang, K., Lu, Y., Jiang, C., Liu, W., Shu, J., Chen, X., et al. (2017). HDAC8 functions in spindle assembly during mouse oocyte meiosis. Oncotarget 8, 20092–20102. doi: 10.18632/oncotarget.15383
Zhang, L., Han, L., Ma, R., Hou, X., Yu, Y., Sun, S., et al. (2015). Sirt3 prevents maternal obesity-associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle 14, 2959–2968. doi: 10.1080/15384101.2015.1026517
Zhang, L., Hou, X., Ma, R., Moley, K., Schedl, T., and Wang, Q. (2014). Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J. 28, 1435–1445. doi: 10.1096/fj.13-244111
Zhang, M., Ouyang, H., and Xia, G. (2009). The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol. Hum. Reprod. 15, 399–409. doi: 10.1093/molehr/gap031
Zhang, M., Su, Y. Q., Sugiura, K., Xia, G., and Eppig, J. J. (2010). Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330, 366–369. doi: 10.1126/science.1193573
Zhang, M., and Xia, G. (2012). Hormonal control of mammalian oocyte meiosis at diplotene stage. Cell. Mol. Life Sci. 69, 1279–1288. doi: 10.1007/s00018-011-0867-3
Zhang, M., Xia, G., Zhou, B., and Wang, C. (2007). Gonadotropin-controlled mammal oocyte meiotic resumption. Front. Biosci. 12:282–296. doi: 10.2741/2064
Zhang, W., Yang, Y., Liu, W., Chen, Q., Wang, H., Wang, X., et al. (2015). Brain natriuretic peptide and C-Type natriuretic peptide maintain porcine oocyte meiotic arrest. J. Cell. Physiol. 230, 71–81. doi: 10.1002/jcp.24682
Zhang, Y. L., Xia, Y., Yu, C., Richards, J. S., Liu, J., and Fan, H. Y. (2014). CBP-CITED4 is required for luteinizing hormone-triggered target gene expression during ovulation. Mol. Hum. Reprod. 20, 850–860. doi: 10.1093/molehr/gau040
Zhang, Y., Kwon, S., Yamaguchi, T., Cubizolles, F., Rousseaux, S., Kneissel, M., et al. (2008). Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol. Cell. Biol. 28, 1688–1701. doi: 10.1128/mcb.01154-06
Zhang, Y., Wang, H., Liu, W., Yang, Y., Wang, X., Zhang, Z., et al. (2017). Natriuretic peptides improve the developmental competence of in vitro cultured porcine oocytes. Reprod. Biol. Endocrinol. 15:41. doi: 10.1186/s12958-017-0258-1
Zhou, D., Choi, Y. J., and Kim, J. H. (2017). Histone deacetylase 6 (HDAC6) is an essential factor for oocyte maturation and asymmetric division in mice. Sci. Rep. 7:8131. doi: 10.1038/s41598-017-08650-2
Keywords: ovary, oocyte, meiosis arrest, meiosis resumption, oocyte maturation
Citation: He M, Zhang T, Yang Y and Wang C (2021) Mechanisms of Oocyte Maturation and Related Epigenetic Regulation. Front. Cell Dev. Biol. 9:654028. doi: 10.3389/fcell.2021.654028
Received: 15 January 2021; Accepted: 25 February 2021;
Published: 19 March 2021.
Edited by:
Shao-Chen Sun, Nanjing Agricultural University, ChinaReviewed by:
Yuanwei Zhang, University of Science and Technology of China, ChinaCopyright © 2021 He, Zhang, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Wang, d2FuZ2NhbUAxMjYuY29t; d2FuZ2NhbUBjYXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.