- 1Center for Physiology, Pathophysiology and Biophysics, Institute for Physiology and Pathophysiology, Paracelsus Medical University, Salzburg, Austria
- 2Institute for Physiology and Pathophysiology, Paracelsus Medical University, Nuremberg, Germany
- 3Gastein Research Institute, Paracelsus Medical University, Salzburg, Austria
- 4Ludwig Boltzmann Institute for Arthritis und Rehabilitation, Salzburg, Austria
- 5Kathmandu University School of Medical Sciences, Dhulikhel, Nepal
- 6Department of Biosciences, University of Salzburg, Salzburg, Austria
The volumes of a cell [cell volume (CV)] and its organelles are adjusted by osmoregulatory processes. During pinocytosis, extracellular fluid volume equivalent to its CV is incorporated within an hour and membrane area equivalent to the cell’s surface within 30 min. Since neither fluid uptake nor membrane consumption leads to swelling or shrinkage, cells must be equipped with potent volume regulatory mechanisms. Normally, cells respond to outwardly or inwardly directed osmotic gradients by a volume decrease and increase, respectively, i.e., they shrink or swell but then try to recover their CV. However, when a cell death (CD) pathway is triggered, CV persistently decreases in isotonic conditions in apoptosis and it increases in necrosis. One type of CD associated with cell swelling is due to a dysfunctional pinocytosis. Methuosis, a non-apoptotic CD phenotype, occurs when cells accumulate too much fluid by macropinocytosis. In contrast to functional pinocytosis, in methuosis, macropinosomes neither recycle nor fuse with lysosomes but with each other to form giant vacuoles, which finally cause rupture of the plasma membrane (PM). Understanding methuosis longs for the understanding of the ionic mechanisms of cell volume regulation (CVR) and vesicular volume regulation (VVR). In nascent macropinosomes, ion channels and transporters are derived from the PM. Along trafficking from the PM to the perinuclear area, the equipment of channels and transporters of the vesicle membrane changes by retrieval, addition, and recycling from and back to the PM, causing profound changes in vesicular ion concentrations, acidification, and—most importantly—shrinkage of the macropinosome, which is indispensable for its proper targeting and cargo processing. In this review, we discuss ion and water transport mechanisms with respect to CVR and VVR and with special emphasis on pinocytosis and methuosis. We describe various aspects of the complex mutual interplay between extracellular and intracellular ions and ion gradients, the PM and vesicular membrane, phosphoinositides, monomeric G proteins and their targets, as well as the submembranous cytoskeleton. Our aim is to highlight important cellular mechanisms, components, and processes that may lead to methuotic CD upon their derangement.
Introduction
Individual cells have the same need entire organisms have: They have to drink. At the cellular level, water drinking is known as pinocytosis and the fluid-containing organelle is the pinosome. Fluid uptake, intracellular distribution, and processing require precise spatial and temporal coordination of membrane and cytoskeletal proteins. Macropinocytosis is an actin-driven process, where a cup-like structure emerges from the cell surface, which engulfs extracellular fluid and forms a vesicle (Swanson and Watts, 1995; Swanson, 2008; Kerr and Teasdale, 2009; Lim and Gleeson, 2011; Hinze and Boucrot, 2018; Doodnauth et al., 2019; King and Kay, 2019; Swanson and King, 2019). The vesicle membrane contains ion channels and transporters that are used for the flux of water, osmolytes, and nutrients, and it is decorated by distinct phospholipids and proteins that are required for intracellular sorting and transport of the organelle (Bohdanowicz and Grinstein, 2013; Levin et al., 2015; Freeman and Grinstein, 2018; Williamson and Donaldson, 2019; Stow et al., 2020) (Table 1). Macropinocytosis serves seemingly unrelated cellular functions, such as nutrition acquisition to satisfy cellular energy demands (Recouvreux and Commisso, 2017; Palm, 2019; Lin et al., 2020), immune surveillance leading to antigen presentation to lymphocytes (Lanzavecchia, 1996; Von Delwig et al., 2006; Liu and Roche, 2015; Canton, 2018), intracellular replication of pathogenic bacteria (Bloomfield and Kay, 2016; Di Russo Case et al., 2016), and CD by drinking too much fluid, i.e., methuosis (Maltese and Overmeyer, 2014, 2015). Careful examination of these functions showed similarities in the initial steps of fluid uptake and differences in the final processing steps, such as fusion or not fusion with lysosomes.
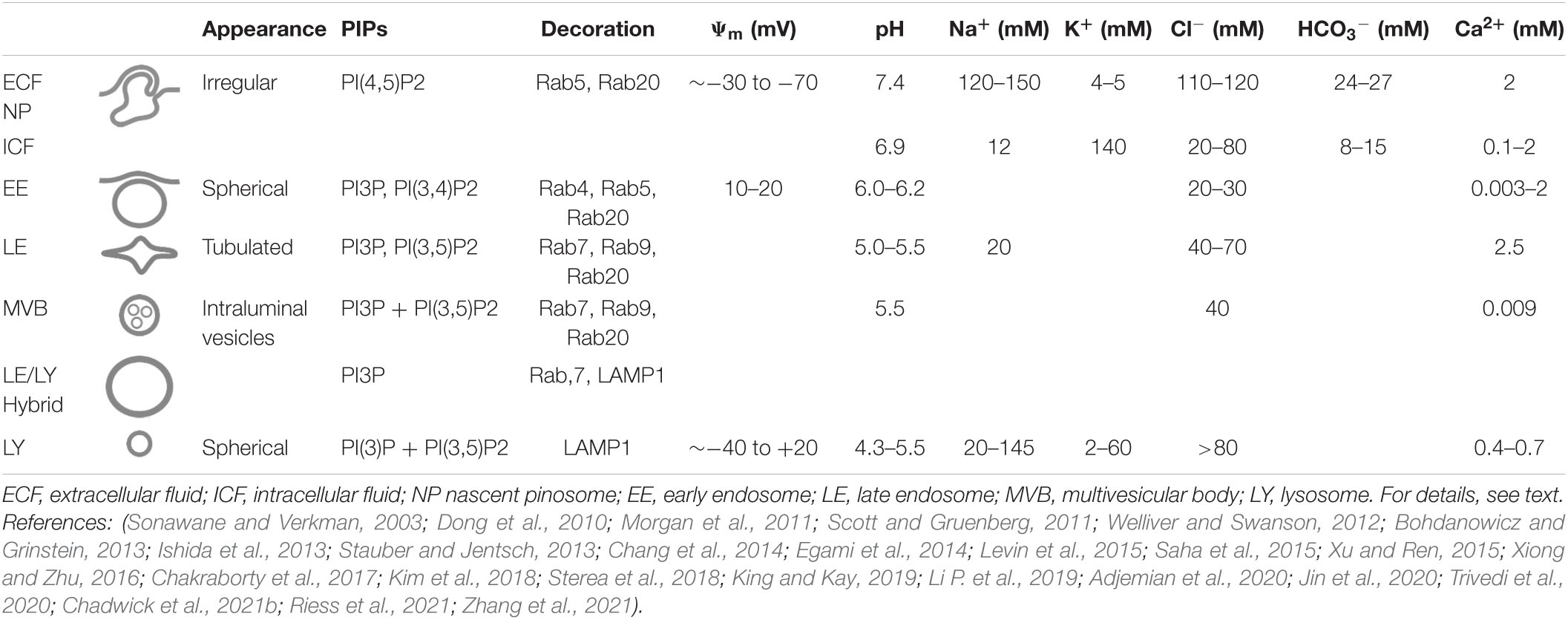
Table 1. Some characteristics and ion concentrations of macropinocytosis and the endolysosomal pathway.
The prototypic experiments examining pinocytosis were done on macrophages and malignant cells by Lewis in the 1930s (Lewis, 1936, 1937). Then, Lewis was among the few researchers using time-lapse microscopy to study the dynamics of living cells. Lewis introduced the term “pinocytosis” to describe the fact that ruffle formation is associated with vesicle formation and uptake of extracellular fluid (Lewis, 1936, 1937). In line with Lewis’s statement “Pinocytosis is easily seen in motion pictures” (Lewis, 1937), we use video microscopy to visualize the dynamics of pinocytosis (Figure 1 and Supplementary Videos 1, 2). In the words of Lewis, Supplementary Video 1 shows that vesicles “taken in vary greatly in size,” “several fuse… to form larger ones,” “move centrally,” and “finally reach… the neighborhood of… the nucleus” (Lewis, 1937). Lewis also suggested that proteins in the vesicles are “split by the digestive enzymes into simpler products which can be utilized or can diffuse out of the cell.” He also related the “disappearance” of the vesicles “with the completion of the digestion of their contents” as the vesicles “slowly shrink in size and disappear, leaving a small granule…” (Lewis, 1937). Lewis’s experiments also showed that the cells do not increase in volume, although “they may take in several times their volume of fluid” and he “assumed that the fluid diffuses out of the cells when the globules disappear” (Lewis, 1937). These experiments demonstrate that volume regulatory processes at the cellular and organelle levels are of paramount importance to maintain CV while cells incorporate large volumes of extracellular fluid and digest macromolecules present in the fluid. The fact that the fluid taken up by macropinocytosis outweighs its elimination by recycling vesicles (Swanson, 1989; Choy et al., 2018) highlights that VVR and CVR are inevitably linked to each other. This becomes evident from the massive cell swelling seen upon inhibition of water channels in pinocytosing cells (De Baey and Lanzavecchia, 2000). Furthermore, considering that the total volume of the endolysosomal compartment can make up a substantial part of the total CV (Choy et al., 2018) makes evident that the exchange of osmotically active solutes between the endolysosomal compartment and the cytosol will strictly affect the volumes of either part.
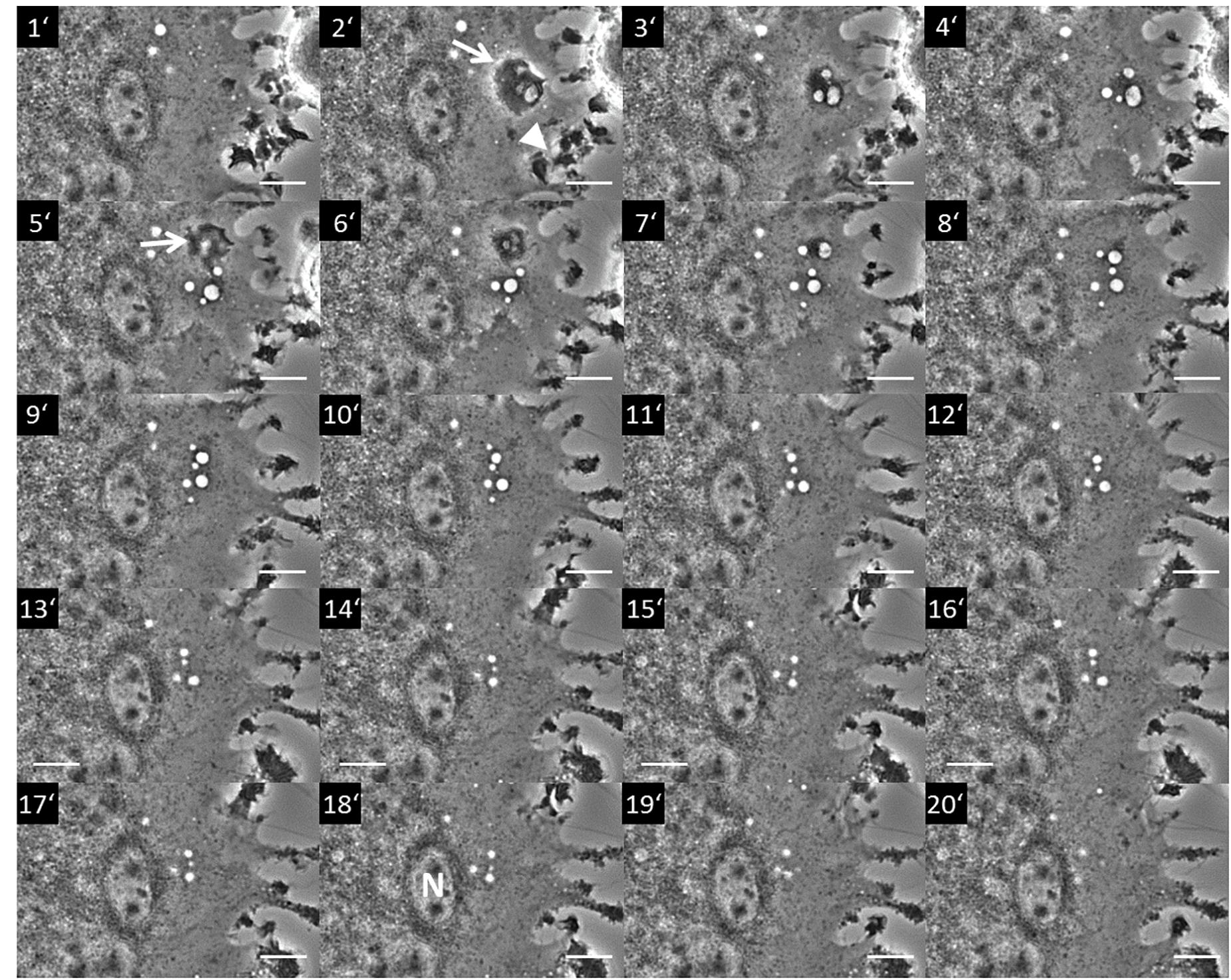
Figure 1. Macropinocytosis occurring in a section of a multinucleated giant cell from a rat non-parenchymal hepatic cell line putatively representing immortalized monocytes (Kupffer cells). The image series shows a section of a giant cell where pinocytosis occurs. The process starts with the formation of a membrane ruffle at minute 2 (2′; arrow) from which an array of vesicles (pinosomes) originates (3′–4′). Further ruffling can be seen at the cell periphery (2′; arrowhead). A second ruffle is forming at minute 5 (5′; arrow) from which additional pinosomes derive (5′–7′). The whole pinosome array subsequently moves toward the center area of the giant cell (8′–18′) locating to the vicinity of a nucleus (N in 18′). Terminally, the vesicles start to disappear in the perinuclear (pericentral) cytosol (20′). Scale bar = 10 μm. The whole live cell imaging sequence can be seen in Supplementary Video 1.
Given the importance of CVR, a central question is: What determines the size of a vesicle? That is, which transporters and ion channels in the PM and vesicle membrane are activated, incorporated, and terminated at which spatial and temporal check points? How does the macropinosomal solute composition and the vesicular membrane properties change after having gulped a lot of extracellular fluid during maturation along the endolysosomal pathway, and what are the determinants of this change? And finally: How are these cellular and subcellular volume regulatory mechanisms altered in methuosis?
To understand subcellular volume regulation, findings related to CVR provide clues to identify factors maintaining the set points of the vesicle.
As ion channels and transporters in the PM are central in CVR, they also contribute to VVR (Freeman and Grinstein, 2018; Freeman et al., 2020; Chadwick et al., 2021b). Thus, Lewis’s statement in the 1930s that “The factors involved in the diffusion of the fluid out of the cell are as mysterious as most of the other processes which take place” (Lewis, 1937) is now transforming to hypotheses trying to explain CVR and VVR in macropinocytosis at the molecular level and by facts generated by electrophysiological, molecular–biological, and imaging studies. Notably, Freeman and Grinstein (2018); Freeman et al. (2020), and Chadwick et al. (2021b) demonstrated that contributions from ion transporters are essential for normal shrinkage in macropinosome maturation.
This review focuses on the complex mutual interplay between extracellular and intracellular ions and ion gradients, the PM and vesicular membrane, phosphoinositides, monomeric G proteins and their targets, as well as the submembranous cytoskeleton in pinocytosis, with special emphasis on its connection to CVR and VVR. It aims at highlighting important cellular mechanisms and components that govern these processes and that may lead to methuotic cell death upon their derangement.
Figure 2 schematically shows key steps of vesicle/vacuole formation and processing during normal macropinocytosis and in methuosis.
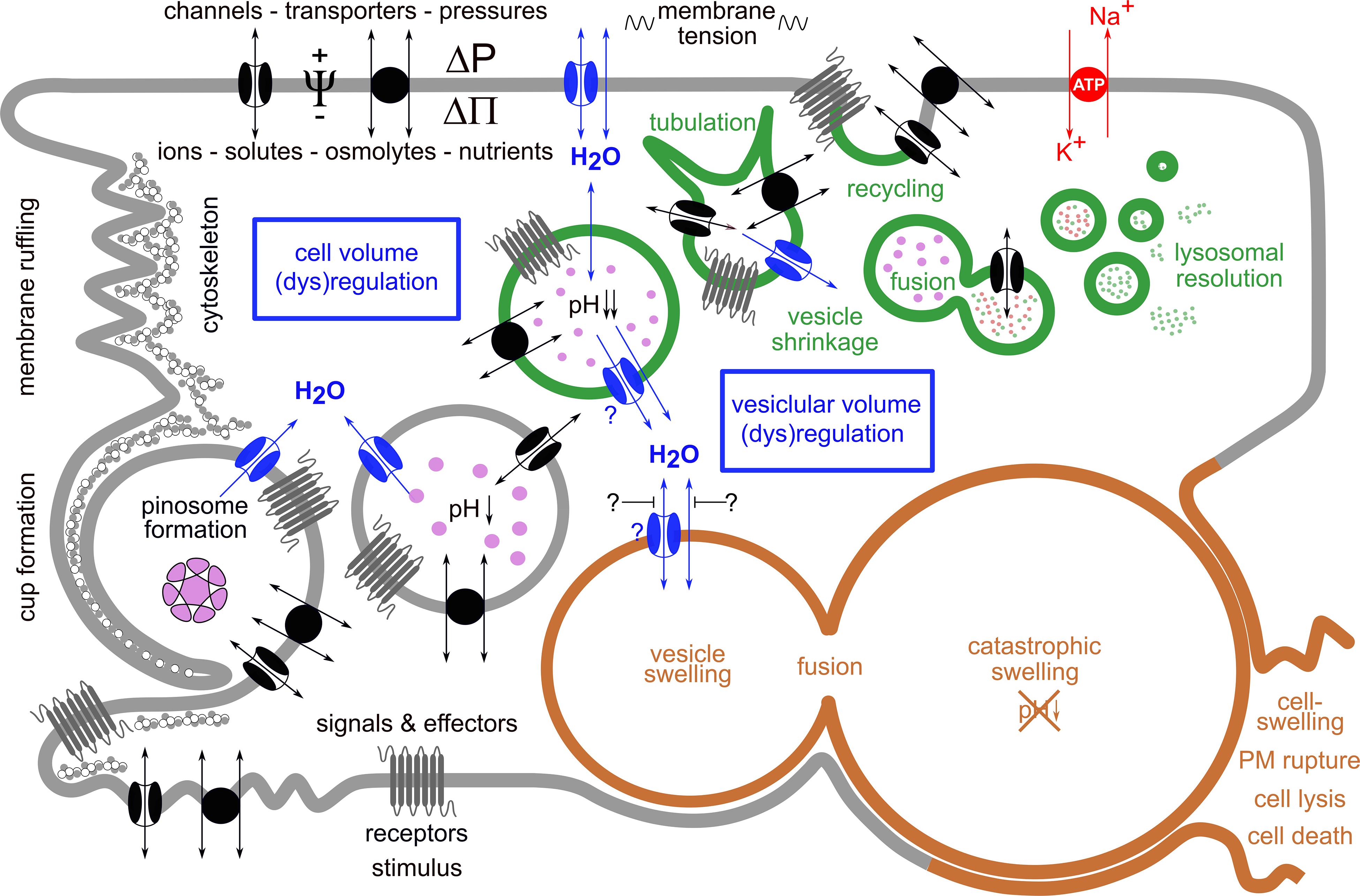
Figure 2. Simplified scheme of key processes in vesicle/vacuole formation and processing during normal macropinocytosis and in methuosis. Macropinocytosis is an actin-driven process that is triggered by various stimuli. It starts with ruffling of the plasma membrane (PM) and formation of a pinocytotic cup, which engulfs extracellular fluid and forms a pinosome by membrane fusion at the tip of a lamellipodium-like structure. Its membrane contains ion channels and transporters, receptors, among other various PM constituents, e.g., phospholipids. The nascent pinosome unselectively engulfs extracellular fluid, along with its ions, nutrients, and metabolites and eventually also toxins or drugs. It may also enclose particulate matter-like exosomes, micro- or nanoparticles, or pathogens such as bacteria and viruses (pink enclosed structure). Under normal conditions (green vesicles), pinosomes move centripetally, become more and more acidic, shrink along their route, and become tubulated, a process necessary for proper sorting and recycling of the vesicles and their cargo. This requires also its decoration with distinct phospholipids and proteins (not shown). Reusable membrane proteins may become inserted again into the PM when vesicles fuse with it. This also recycles incorporated membrane back to the PM and relieves its tension. Vesicles designated for delivery of their contents to lysosomes—for further processing, digestion, or destruction—fuse with them and finally resolve. The resulting products may be further used, e.g., for the cell’s nutrition. To ensure these processes, cell volume regulation and vesicular volume regulation must work hand in hand. This becomes evident from the fact that during pinocytosis, an extracellular fluid volume and membrane area equivalent to the cell’s volume and to the cell’s surface are incorporated within 1 h and 30 min, respectively. The volume regulatory mechanisms involve movement of ions and osmolytes across the PM by means of specific ion channels and transporters. The driving forces for these fluxes are determined by the electrochemical gradients. Water flux is driven mainly by the osmotic (ΔΠ) but eventually also by the hydrostatic (ΔP) pressure differences. The water permeability of the PM is greatly enhanced by water channels (aquaporins, blue). All of these movements are primarily driven by the activity of the Na+/K+-ATPase (red transporter in the PM), an ion pump that moves Na+ ions out of and K+ ions into the cell, while hydrolyzing ATP as energy source. This process sets up the required ionic and osmotic gradients and determines the electrical potential difference Ψ across the PM. The mechanisms for vesicular volume regulation follow the same rules and are in principle identical to those in cell volume regulation, while utilizing their individual set of transporters and channels. In methuosis, a lethal process of aberrant pinocytosis where cells “drink themselves to death,” these processes are severely disturbed (brown vesicles). Fluid uptake by macropinocytosis is enhanced, and instead of shrinking, the vesicles swell, do not acidify, remain non-functional, and do not fuse with lysosomes, but instead they with each other. This leads to the formation of giant vacuoles, catastrophic cell swelling, and consequently to rupture of the PM, cell lysis, and death.
Macropinocytosis
Macropinocytosis is a form of clathrin-independent endocytosis. Ruffling of cholesterol-rich membrane microdomains leads to the unselective incorporation of large volumes of extracellular fluid in macropinosomes with a diameter of 0.2 up to 5.0 μm (Levin et al., 2015; Donaldson, 2019). Macropinocytosis may occur constitutively or be induced by growth factors, chemokines, microbial products, viruses (Freeman et al., 2014; Marques et al., 2017; Canton, 2018; Doodnauth et al., 2019; Tejeda-Munoz et al., 2019), crosslinking of cell surface molecules (Imamura et al., 2011), knockdown of genes (Choi et al., 2017; Fomin et al., 2018; Fomin, 2019; Su et al., 2021), and constitutive expression (Kasahara et al., 2007) or mutations of signal transduction molecules (Yoo et al., 2020). Some cells, like innate immune cells and Ras-transformed cancer cells, are able to perform both forms of macropinocytosis (Amyere et al., 2000; Stow et al., 2020). Importantly, this process ensures that cells incorporate everything animals ingest and digest, including nutrients as well as toxic substances and metabolites released by neighboring cells, exosomes, microparticles, and pathogens, such as bacteria and viruses (Suda et al., 2007; Mercer and Helenius, 2009, 2012; Mercer et al., 2010; Commisso et al., 2013; Bloomfield and Kay, 2016; Jiang et al., 2017; Commisso, 2019). Furthermore, macropinocytosis is involved in cell migration (Wen et al., 2016; Swanson and King, 2019).
Remarkably, within 1 h, a volume equivalent of the entire cytoplasm is incorporated by macropinocytosis, and within 30 min, macropinosome formation requires the entire cellular PM surface (Steinman et al., 1976; Cullen and Steinberg, 2018; Freeman and Grinstein, 2018). Against the compelling background of conserved CV and cell surface, sorting mechanisms distinguishing between recycling and digestion routes are of eminent importance. Membrane recycling is not only of importance for maintaining the cell surface but also for supplying the PM with receptors and transporters, such as neonatal Fc-receptor (Toh et al., 2019), bone morphogenetic protein receptor (Kim et al., 2019), PDGF β-receptor (Schmees et al., 2012), EGF receptor (Chiasson-Mackenzie et al., 2018; Freeman et al., 2020), and other plasmalemmal components such as integrins (Buckley et al., 2016; Freeman et al., 2020) as well as small GTPases, which fuel macropinocytosis (Cullen and Steinberg, 2018). Furthermore, as the intercellular volume is usually small, changes in extracellular ion composition may greatly affect ion gradients, which drive nutrient transporters, e.g., Na+-dependent glucose or amino acid uptake (Ganapathy et al., 2008; Wright et al., 2011; Broer, 2014). This exceptional well-balanced system is keeping CV and cell surface during macropinocytic flow reasonably constant, but puts the cell at risk to damage either when CVR fails or when it accumulates metabolic waste products as seen in lysosomal storage diseases (Platt et al., 2012, 2018; Rappaport et al., 2016). Excessive fluid uptake in in vitro conditions leading to a distinct form of CD has been recently recognized and named methuosis (Maltese and Overmeyer, 2014, 2015).
Ruffle and Cup Formation
Ruffle and cup formation is an actin-driven process, which is closely related to the formation of phagocytic cups and pseudopods (Lim and Gleeson, 2011; Freeman and Grinstein, 2014; Buckley and King, 2017; Williamson and Donaldson, 2019). Actin cytoskeleton rearrangement depends on phospholipids, lipid kinases and phosphatases, small GTPases, actin-modulating proteins, ion channels, and proton (H+) pumps (Welliver and Swanson, 2012). Among the numerous small GTPases, the Ras and Rho family member, Rac1, is critical in the formation of ruffles and macropinocytic cups (Egami et al., 2014; Donaldson, 2019). For small GTPases, the switch from an inactive guanosine diphosphate (GDP)-bound form to an active guanosine triphosphate (GTP)-bound form is facilitated by GEFs, which promote GDP dissociation. The inactivation of the small GTPases is mediated by GTPase-activating proteins (GAPs), which enhance GTP hydrolysis (Cherfils and Zeghouf, 2013). Critically, the activation of the GTPase cycle—activation, inactivation, removal from the membrane—is transient. Oscillations of Ca2+i may drive parallel oscillatory association and dissociation of the Ras-GAP, Rasal (Ras-GTPase–activating-like protein), to and from the PM. Only when bound to the PM, Rasal is active and can inactivate Ras. Thus, Ras is repetitively activated and inactivated (Walker et al., 2004). In macropinocytosis, Ras activity is terminated by RasGAP, which is recruited to the cup as it closes (Veltman et al., 2016; Buckley et al., 2020). When GTPases are persistently activated, e.g., when RasGAPs are inactive like in neurofibromatosis (Bloomfield et al., 2015; Ghoshal et al., 2019) or when Ras is constitutively active like in oncogenic H-Ras mutants, macropinosome formation and maturation deviate from the normal physiological pathway. The Ras-related G protein Rap1 is a negative regulator of Ras (Zwartkruis and Bos, 1999; Nussinov et al., 2020). Rap1 is found in early and late endocytic vesicles and lysosomes (Pizon et al., 1994), and its overexpression negatively regulates macropinocytosis (Seastone et al., 1999). In Dictyostelium discoideum, hyperosmotic stress activates Rap1 and decreases endocytic activity due to cellular acidification (Pintsch et al., 2001), and it promotes the formation of giant vacuoles of pinocytotic origin (Yuan and Chia, 2001).
Notably, in human intestinal cells, hypotonicity increases the activity of the H-Ras–Raf1–Erk signaling pathway (Van Der Wijk et al., 1998). The clustering of Ras proteins in distinct microdomains at the PM (lipid rafts) influences Ras structure, orientation, and Ras-isoform accessibility and thus the activation states of its effectors. Accordingly, GTP-bound H-Ras may remain in a locked state and as such not able to associate with its downstream effectors (Jang et al., 2016; Nussinov et al., 2018). Activation occurs once the lipid-anchored GTPases Ras1 and H-Ras are shifted out of the lipid rafts. This happens upon thinning of the PM in combination with changes of its curvature induced by cell swelling (Cohen, 2018).
Active Rac and Ras are located at the cup wall. Rac1 activation is associated with ruffle formation, and Rac1 inactivation precedes cup closure (Buckley and King, 2017). In Dictyostelium discoideum, the multidomain protein, RGBARG (RCC1, RhoGEF, BAR, and RasGAP-containing protein), orchestrates Ras and Rac activity in a small membrane patch, where RGBARG is localized at the protruding rim region (Buckley et al., 2020). Oncogenic H-Ras promotes the translocation of the vacuolar H+-ATPase (v-ATPase) from intracellular vesicles to the PM. The accumulation of v-ATPase is necessary for the cholesterol-dependent association of Rac1 with the PM, which is a prerequisite for the stimulation of membrane ruffling and macropinocytosis. Knockdown of the v-ATPase or its inhibition suppresses macropinocytosis, while addition of cholesterol to these cells restores both Rac1 membrane localization and macropinocytosis (Ramirez et al., 2019). In addition, Ras binds and activates phosphatidylinositol 3-kinases (PI3Ks), which have a Ras-binding domain (Gupta et al., 2007; Castellano and Downward, 2011; Castellano et al., 2013). Activation of PI3Ks phosphorylates phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to phosphatidylinositol (3,4,5)-trisphosphate [PI(3,4,5)P3] and promotes its enrichment in the PM (Rupper et al., 2001; Nussinov et al., 2020). In EGF-stimulated A431 cells, PI(4,5)P2 increases in the ruffles-forming macropinocytic cups, reaches its maximum just before macropinosome closure, and then rapidly falls as the cup closes. In contrast, PI(3,4,5)P3 increases locally at the site of macropinosome formation and peaks at the time of closure (Araki et al., 2007). The extension of PI(3,4,5)P3 patches, which can reach a diameter of several micrometers, reflects the balanced activity of PI3Ks and the lipid phosphatase, phosphatase and tensin homolog (PTEN), which dephosphorylates PI(3,4,5)P3 back to PI(4,5)P2. The kinetics of PI(4,5)P2 and PI(3,4,5)P3 are mechanistically linked to actin-remodeling during macropinocytosis (Araki et al., 1996, 2007; Worby and Dixon, 2014; Swanson and Yoshida, 2019; Nussinov et al., 2020) and to the regulation of many ion channels and transporters relevant to pinocytosis as well as CVR (Araki et al., 1996; Lang et al., 1998; Ritter et al., 2001; Furst et al., 2002; Jakab et al., 2002; Okada, 2004; Lang, 2007; Suh and Hille, 2008; Hoffmann et al., 2009; Abu Jawdeh et al., 2011; Lang and Hoffmann, 2012; Balla, 2013; Hansen, 2015; Hille et al., 2015; Kunzelmann, 2015; Jentsch, 2016; Pasantes-Morales, 2016; De Los Heros et al., 2018; Delpire and Gagnon, 2018; Konig et al., 2019; Okada et al., 2019; Centeio et al., 2020; Larsen and Hoffmann, 2020; Model et al., 2020). For cup closure, the progressive dephosphorylation of PI(3,4,5)P3 seems to be important. Among its dephosphorylation products, PI(3)P has been shown to directly activate the Ca2+-activated K+-channel, KCa3.1, at ruffles, which is necessary for closure (Maekawa et al., 2014). The significance of Ras activation, which peaks shortly after cup closure (Welliver and Swanson, 2012; Egami et al., 2014), as well as of the antagonistic behavior of PI3Ks and PTEN in the initiation and termination of cup formation is nicely documented by the observations that injection of Ras in cells induces ruffle formation, that inhibitors of PI3K inhibit cup closure in macrophages, and that deletion of PTEN in prostate cancer cells enhances macropinocytosis (Bohdanowicz and Grinstein, 2013; Levin et al., 2015; Kim et al., 2018; King and Kay, 2019).
Phosphatidylinositol (3,4,5)-trisphosphate enrichment promotes the translocation of the serine/threonine protein kinase Akt/PKB via binding to the pleckstrin homology (PH) domain and kinase activation by target of rapamycin complex 2 (TORC2) and 3-phosphoinositide-dependent protein kinase-1 (PDK1) (Yoshida et al., 2018; Kay et al., 2019). Among the downstream targets of Akt is the tuberous sclerosis complex 2 (TSC2), which is—together with TSC1—located on the lysosomal membrane, from which it subsequently dissociates to act as a GAP for the Ras-related small GTPase, Rheb, which in turn activates mammalian target of rapamycin complex 1 (mTORC1). In Dictyostelium discoideum downstream of PI(3,4,5)P3, the homologs of mammalian Akt, PkbA, and of the related glucocorticoid-regulated kinase (SGK), PkbR1, as well as their activating protein kinases, TORC2 and PdkA, increase the size of the macropinocytic patch and macropinosome (Kay et al., 2019; Williams et al., 2019). Combined inhibition of mTORC1/mTORC2 induces massive catastrophic macropinocytosis, i.e., methuosis, in cancer cells (Srivastava et al., 2019). Akt and TORC2 target SGK1, which regulates a plentitude of cell functions, including ion channels and transporters (Lang et al., 2018) and endomembrane trafficking (Zhu et al., 2015). SGK transcription is stimulated by cell shrinkage via p38-kinase and inhibited by cell swelling due to transcriptional stop (Waldegger et al., 1997, 2000; Lang et al., 2006, 2018). Furthermore, the isoform Akt3 controls actin-dependent macropinocytosis in macrophages by suppressing the expression of with no lysine kinase 2 (WNK2) and the activity of SGK1, while increased activity of SGK1 leads to stimulation of Cdc42-mediated macropinocytosis of lipoprotein (Ding et al., 2017). However, in macrophages stimulated by macrophage colony-stimulating factor (M-CSF), the Akt pathway is induced by this growth factor but not required for macropinosome formation (Yoshida et al., 2015).
In myoblasts, an acute decrease of PM tension leads to phospholipase D2 activation, production of phosphatidic acid, F-actin and development of PI(4,5)P2-enriched membrane ruffling, and macropinocytosis without an increase in PI(3,4,5)P3 (Loh et al., 2019).
In parallel to the lipid and protein phosphorylation cascades, the cortical actin filaments are reorganized. This is controlled by PI3K and PLC, Rac, Cdc42, Arf6, Rab5, and Pak (Swanson, 2008). In the cup region, polymerized actin is seen as a ring-like structure (Hinze and Boucrot, 2018). Active Ras and PIP(3,4,5)P3 coincidentally form patches in macropinosomes, which are surrounded by a ring of the Scar/WAVE complex, an activator of the Arp2/3 complex. Arp2/3 drives actin polymerization by filament branching, leading to the formation of dendritic F-actin, which populates the wall of the cup and forms rings of protrusive actin under the PM and the circular ruffles (Swanson, 2008; Saarikangas et al., 2010; Pollard, 2016; Buckley and King, 2017; Hinze and Boucrot, 2018; Kay et al., 2019; Williamson and Donaldson, 2019). PI(3,4,5)P3 also recruits myosin proteins to macropinocytic cups (Chen et al., 2012). The synthesis of PI(3,4,5)P3 from PI(4,5)P2 occurs simultaneously with the recruitment of Rab5 to the PM in COS-7 cells expressing oncogenic H-RasG12V (Porat-Shliom et al., 2008). Rab5 promotes macropinosome sealing and scission downstream of ruffling. To this end, Rab5-containing vesicles are recruited to circular ruffles of the PM, which requires soluble N-ethylmaleimide-sensitive-factor attachment receptor (SNARE)-dependent endomembrane fusion. This is paralleled by the disappearance of PI(4,5)P2 and accompanies macropinosome closure. The removal of PI(4,5)P2 is dependent on Rab5 through its recruitment of the inositol 5-phosphatase Inpp5b/OCRL and via APPL1, an adaptor protein that regulates vesicle trafficking and endosomal signaling (Maxson et al., 2021). Rab5 and RN-tre, which is a Rab5-specific GAP as well as a Rab5 effector, are also recruited to the PM. RN-tre interacts with actin as well as actinin-4, which contributes to actin bundling (Lanzetti et al., 2004). The Rab5 cycle is active on nascent macropinosomes and stabilizes the macropinosomes. Rab5 activity is increased on macropinosome tubules (Feliciano et al., 2011). In BHK-21 cells, H-RasG12V has been shown to separately activate Rab5 and Rac1 via distinct Ras signal transduction pathways. While Rab5 activation stimulates pinocytosis, Rac1 stimulation causes membrane ruffling but does not contribute to the stimulation of pinocytosis (Li et al., 1997).
Intracellular Trafficking of Macropinosomes
In Dictyostelium, the nascent vesicles lose their actin coat within 1 min after pinching off and internalization (Lee and Knecht, 2002). In rat basophilic leukemia (RBL) cells, “pinosomes… ignite a burst of actin polymerization when they are pinched off from the plasma membrane” and “then move into the cytosol at the tips of short-lived actin ‘comet tails,’” which fade within 2 min after their appearance. These brief bursts of actin polymerization are thought to help move the vesicles into the cytosol (Merrifield et al., 1999). They also require recruitment of annexin-2 to nascent macropinosome membranes as an essential prerequisite for actin polymerization-dependent vesicle locomotion (Merrifield et al., 2001).
Organelle shrinkage concentrates the to-be-digested material and recycles membrane back to the PM. Macropinosomes show two routes of structural adaptations to maximize the organelle surface area and to minimize its volume: tubulation and shrinkage (Freeman and Grinstein, 2018; King and Kay, 2019; Chadwick et al., 2021b). Macropinosomes retrieve v-ATPase from fusion with other vesicles and mature toward acidic organelles that contain hydrolytic enzymes, such as proteases, nucleases, and lipases, which are required for the degeneration of macromolecules, as well as transporters facilitating the efflux of cholesterol, cystine (the disulfide form of cysteine, which is generated during protein degradation), amino acids, cobalamin, and inorganic ions (Neuhaus et al., 2002; Buckley and King, 2017; Trivedi et al., 2020).
Mobilization and maturation of macropinosomes depend on the decoration of the vesicle membrane with distinct small GTPases (Egami et al., 2014; Egami, 2016). Transient activation of ADP-ribosylation factor 6 (Arf6), a member of the Ras superfamily, by the exchange factor, EFA6, promotes PM protrusion, formation of macropinosomes, and recycling of the vesicle membrane back to the PM (Brown et al., 2001). Arf6 colocalizes and activates phosphatidylinositol 4-phosphate 5-kinase (PIP 5-kinase), which phosphorylates phosphatidylinositol 4-phosphate PI(4)P to PI(4,5)P2 (Egami et al., 2014). Subsequently, PI(4,5)P2 and Cdc42-GTP coordinate the activation of Wiskott–Aldrich syndrome protein (WASP), which promotes the actin-nucleating and actin filament-branching activity of Arp2/3 (Higgs and Pollard, 2000). The recruitment of Arf6 and its exchange factor, ARF nucleotide binding-site opener (ARNO), from cytosol to endosomal membranes relies on v-ATPase-dependent intra-endosomal acidification, thus regulating the protein-degradative pathway (Hurtado-Lorenzo et al., 2006). Persistent activation of Arf6, as seen in the GTP hydrolysis-resistant mutant Arf6Q67L or by overexpression of the PIP 5-kinase, results in the accumulation of macropinosomes. Interestingly, PI(4,5)P2-enriched macropinosomes in apposition to each other fuse with one another and give rise to large vacuoles. Furthermore, entrapped PM proteins in these vacuoles are not recycled (Brown et al., 2001). The GTPase septin is involved in endosome fusion. Whereas septin downregulation decreases macropinosome fusion events as well as lysosomal delivery, septin overexpression increases delivery to lysosomes (Dolat and Spiliotis, 2016).
Early macropinosomes harbor Rab5 (Feliciano et al., 2011; Maxson et al., 2021), which in turn recruits the class III PI3K Vps34, which catalyzes the reaction from PI to PI(3)P (Christoforidis et al., 1999; Kerr and Teasdale, 2009). In macropinosomes routed toward lysosomes, Rab5 is replaced by Rab7 (Racoosin and Swanson, 1993; Kerr et al., 2006; Langemeyer et al., 2018; Morishita et al., 2019). In an analogy to an electrical safety-breaker, Del Conte-Zerial et al. (2008) compare the replacement of Rab5 by Rab7 as a “cut-out switch.” This model predicts that Rab5 drives Rab7 activation above a distinct threshold. Above the threshold, Rab7 shows a self-sustained activity and suppresses Rab5 activity via a negative feedback. In this model, crossing the Rab7 activation threshold excludes reactivation of Rab5 and activation of a different trafficking pathway. Thus, the Rab5 to Rab7 switch ensures a unidirectional route of cargo-loaded macropinosomes toward lysosomes. Using Förster/fluorescence resonance energy transfer (FRET) imaging, Morishita et al. (2019) describe that active Rab5 facilitates Rab7 activation until Rab7 sustains its own activity and inactivates Rab5. Furthermore, recruitment of amyotrophic lateral sclerosis 2 (ALS2) to the macropinosome coincides with Rab5 activation, and ALS2 detachment is associated with Rab5 inactivation (Morishita et al., 2019). In earlier studies using Texas red-labeled dextran macropinosomes, Racoosin and Swanson showed that Rab7-positive macropinosomes fuse with tubular lysosomes (Racoosin and Swanson, 1993). The fate of waste-containing vesicles is not known.
Dysfunction or inhibition of Vps34 or PIKfyve, which phosphorylates PI(3)P to PI(3,5)P2, leads to the formation of massive and progressively exacerbating cytoplasmic vacuolization due to loss of PI(3,5)P2. This requires active v-ATPase activity and a functional Rab5a cycle. Interestingly, the formation of the enlarged vacuoles does not require their acidification (Compton et al., 2016; Saveanu and Lotersztajn, 2016), pointing to an osmotic function of the v-ATPase. In melanoma cells, oncogenic class I PI3K elicits a hyperactive influx of macropinosomes, which is counteracted by Rab7A (Alonso-Curbelo et al., 2015). Furthermore, by stimulating RIN1, which is a Rab5 GEF, activation of H-Ras or H-RasG12V also mediates homotypic fusion of early endosomes, thus leading to endosome enlargement (Roberts et al., 2000; Tall et al., 2001).
Microtubules are associated with peripheral actin/myosin-enriched lamellae, and they are the scaffold for the unidirectional transport of macropinosomes. Critically, inhibitors of microtubule assembly, and the dynein inhibitor ciliobrevin D, decrease fluid uptake, indicating that the microtubules are involved in an early step of macropinocytosis (Williamson and Donaldson, 2019).
Cell Volume Regulation
In general, cells respond to alterations of the osmotic equilibrium with a change of their volume due to water movement into or out of the cell. While an increase in intracellular or a decrease in extracellular osmolarity leads to cell swelling, a decrease in intracellular or an increase in extracellular osmolarity leads to cell shrinkage. Physiologically, such changes arise when cells invade anisotonic extracellular environments, e.g., the renal medulla, or following changes in intracellular osmolyte concentrations, such as during uptake or release of ions or nutrients, but also from metabolic changes, such as formation or degradation of macromolecules, e.g., proteins or glycogen. Likewise, perturbations of the double Donnan equilibrium, established by the so-called pump–leak balance (Okada, 2004; Kay, 2017; Kay and Blaustein, 2019), such as changes in intracellular pH (pHi) or inhibition of the Na+/K+-ATPase by cardiac glycosides or low temperature (Russo et al., 2015), will lead to alterations of CV (for review, see Lang et al., 1998; Ritter et al., 2001; Furst et al., 2002; Jakab et al., 2002; Okada, 2004, 2020; Lang, 2007; Hoffmann et al., 2009; Kunzelmann, 2015; Jentsch, 2016; Pasantes-Morales, 2016; De Los Heros et al., 2018; Delpire and Gagnon, 2018; Okada et al., 2019; Centeio et al., 2020; Larsen and Hoffmann, 2020; Model et al., 2020).
The kinetics and degree of the actual volume changes critically depend on the water permeability of the PM, which is intrinsically low but greatly enhanced by aquaporins (AQPs) (Day et al., 2014; Kitchen et al., 2015) and also by the efficiency as well as the time of onset of CVR mechanisms. If the regulatory mechanisms [i.e., regulatory volume decrease (RVD) and regulatory volume increase (RVI); see below] have high transport efficiency and/or will start to work quickly, the degree of swelling or shrinkage will deviate from a perfect osmometer-like behavior.
When the actual CV deviates from the given set point, regulatory mechanisms are spurred, aiming at readjusting the original volume. Thus, upon swelling, cells initiate a process termed RVD (cells shrink back toward their original volume), while cell shrinkage is counteracted by RVI (cells swell back toward their original volume). The mechanisms driving the regulatory water fluxes during RVD and RVI are complex and involve rapid release or uptake of ions, amino acids, sugars, or alcohols across the cell membrane but also metabolic changes such as the formation or degradation of macromolecules to pack or unpack abundant osmotically active solutes.
Acute CVR relies on distinct sets of ion channels and transporters. The Na+/K+-ATPase actively pumps K+ into and Na+ out of the cell. K+ permeating through K+ channels generates a negative PM potential ΨPM and thus creates the driving force for the cellular exit of anions such as Cl– and HCO3–. Canonically, during RVD, volume-sensitive K+ and anion channels, KCl cotransport, or parallel activation of K+/H+ exchange and Cl–/HCO3– exchange is activated, while during RVI, Na+/H+ exchangers (NHEs) and Na+/K+/2Cl– cotransporters (NKCCs) in parallel to Cl–/HCO3– exchange or Na+ channels are activated to release or take up ions and osmotically obliged water through AQPs. Thus, RVD is mainly accomplished via cellular exit of K+, Cl–, and HCO3–, whereas RVI is achieved by uptake of Na+ and Cl–. Frequently, RVD mechanisms are inhibited during RVI and vice versa.
Furthermore, shrunken cells can accumulate organic osmolytes such as amino acids, myoinositol, betaine, and taurine either by synthesis or by Na+-coupled uptake of sorbitol, glycerophosphorylcholine, and monomeric amino acids. These osmolytes are then released upon cell swelling. Though inhibition of the Na+/K+-ATPase leads to cell swelling at least in some cells (Alvarez-Leefmans et al., 1992), interestingly, in certain cell types, CVR can also be performed by formation and exocytosis of vesicles even when the Na+/K+-ATPase is inhibited (Russo et al., 2015).
Cell volume also greatly affects macromolecular crowding, i.e., the concentration of macromolecules (mainly proteins and nucleic acids) within the cell and hence their biological activities, which in turn has widespread consequences for cellular functions, including CVR itself (Model et al., 2020). Macromolecular crowding also induces liquid–liquid phase separation as part of the cellular osmosensing system (Ishihara et al., 2021; Watanabe et al., 2021).
As mentioned above, in myoblasts, an acute decrease of PM tension induces macropinocytosis. This was established by hypotonic swelling (stretching the PM and inducing RVD) of the cells followed by returning to isosmotic conditions (inducing cell shrinkage and PM relaxation) (Loh et al., 2019).
Both PIPs and IPs are involved in CVR. As shown in Figure 3, PI(4,5)P2 is metabolized to Ins(1,4,5)P3 and further to the various inositol (poly)phosphates (Hatch and York, 2010). Prominently, Ins(1,4,5)P3 binds to its receptor on internal Ca2+ stores like the endoplasmic reticulum (ER) and triggers the release of Ca2+ into the cytosol (Berridge, 2009). Given the plentitude of cellular functions governed by Ca2+i and the numerous components of CVR dependent on it, Ins(1,4,5)P3 is crucial to it. Notably, the levels of IPs are altered in Ras-transformed cells (Fleischman et al., 1986; Maly et al., 1995; Ritter et al., 1997a). Besides, other inositol (poly)phosphates are activated or inhibited by anisotonicity and/or changes in CV (Fleischman et al., 1986; Lang et al., 1998; Jakab et al., 2002; Pesesse et al., 2004; Hoffmann et al., 2009; Wundenberg and Mayr, 2012; Lee et al., 2020).
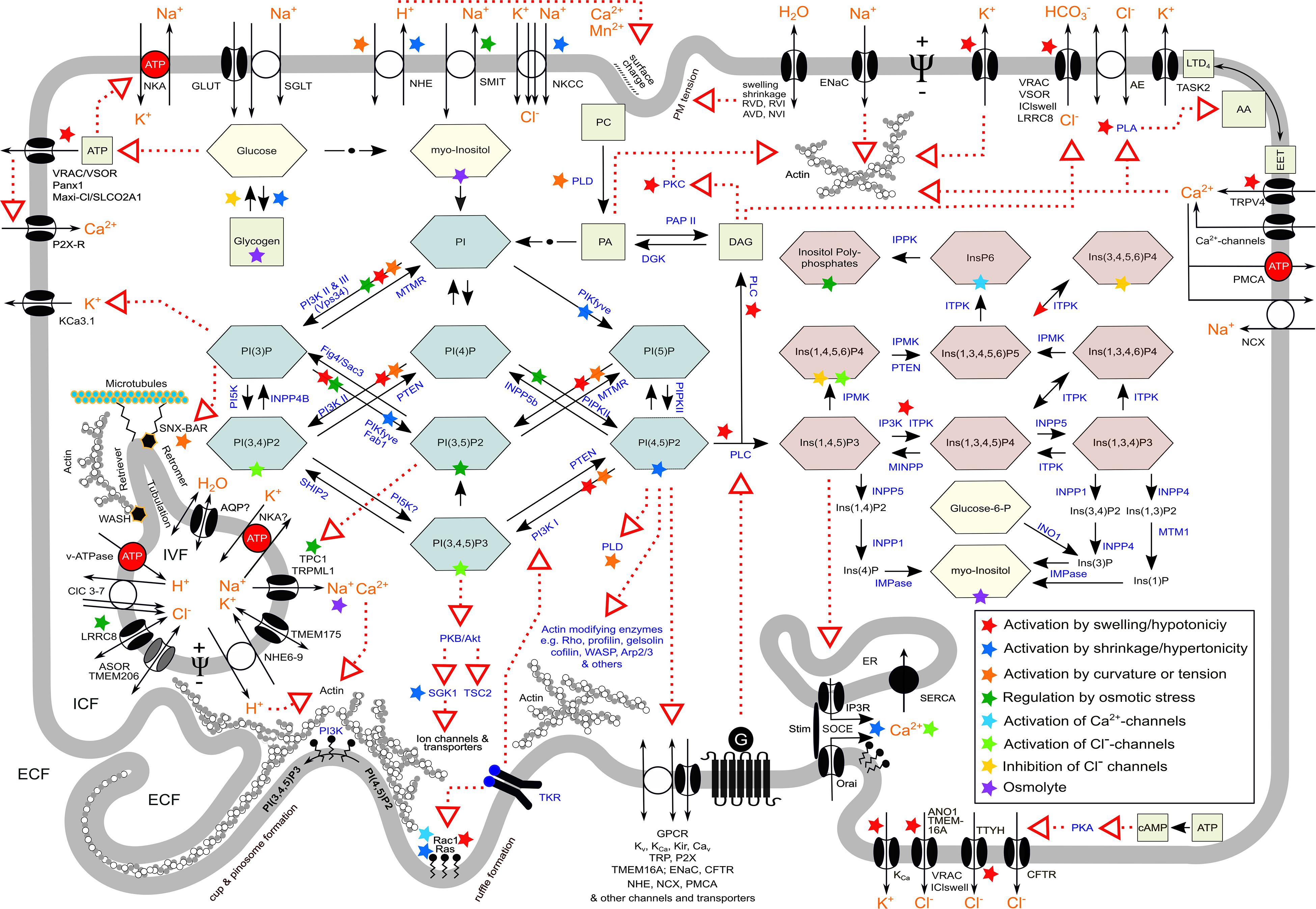
Figure 3. Synopsis of various aspects in macropinocytosis and ion transport in cell volume regulation and vesicular volume regulation, which are also relevant in methuosis. Red dotted arrows indicate action/s on target/s; black arrows indicate metabolic conversion; green combs, phosphoinositides; red combs, inositolphosphates; yellow combs, sugars; green squares, important metabolites; orange letters, ion(s); blue letters, enzymes; ECF, extracellular fluid; ICF, intracellular fluid; IVF, intravesicular fluid; G, heterotrimeric G protein; TKR, tyrosine kinase receptor; NKA, Na+/K+-ATPase; SOCE, store-operated Ca2+ entry; Ψ, transmembrane electrical potential difference. For the meaning of colored asterisks, see insert in the right lower part. For details, nomenclature, and further abbreviations, see text and list of abbreviations.
In programmed CD, cell shrinkage is characteristic (though not universal) of apoptosis and in its initial phase accomplished by RVD-like cellular exit of ions, termed apoptotic volume decrease (AVD). In contrast, cell swelling is characteristic of necrosis and ischemic cell death/onkosis (derived from the Greek word óγκoς, i.e., tumor/swelling Von Recklinghausen, 1910; Majno and Joris, 1995; Weerasinghe and Buja, 2012), termed necrotic volume increase (NVI) (Okada et al., 2001; Orlov and Hamet, 2004; Lang and Hoffmann, 2012, 2013a,b; Orlov et al., 2013; Bortner and Cidlowski, 2014, 2020; Model, 2014; Okada, 2020), and related modes of CD such as secondary necrosis, pyroptosis, or ferroptosis (Zong and Thompson, 2006; D’Arcy, 2019; Nirmala and Lopus, 2020; Riegman et al., 2020). In methousis, not only is cell shrinkage absent but cells actually are swollen (Overmeyer et al., 2008), such as seen in cells expressing an activated form of the H-RasG12V oncoprotein (see below) (Maltese and Overmeyer, 2014; Alonso-Curbelo et al., 2015). This may be related to altered ion transport in cells expressing the H-Ras oncogene.
The set point of a given cell for regulation of its volume is not a fixed constant but may change intrinsically to adjust the CV to altered functional needs. For instance, proliferating cells have to gain volume prior to mitosis, and hence, the set point for volume regulation varies in a cell cycle-dependent manner (Lang et al., 1992a, 2007; Doroshenko et al., 2001; Chen et al., 2002; Klausen et al., 2007; Pedersen et al., 2013). Expression of H-RasG12V in NIH 3T3 fibroblasts leads to an upshift of the set point for CVR, i.e., the cells swell (Lang et al., 1992a). This is related to alterations of cellular metabolism, ion transport, and structural remodeling. Such cells proliferate independently of growth factors and have altered PI metabolism (Fleischman et al., 1986; Maly et al., 1995), stimulated Ca2+ influx (Maly et al., 1995; Ritter et al., 1997b), and increased intracellular pH (pHi) due to activation of NHE (Maly et al., 1989; Ritter et al., 1997b) and Na+, K+, 2C1– cotransport (NKCC1) (Lang et al., 1992a). Furthermore, mitogens, like serum or bradykinin, cause pulsatile release of Ca2+ from internal stores and activation of a store-operated calcium entry (SOCE), which leads to oscillations of ΨPM via Ca2+-activated K+ channels, NHE activation, and actin depolymerization (Lang et al., 1992b; Dartsch et al., 1994b, 1995; Ritter et al., 1997b). In NIH 3T3 fibroblasts that do not express the H-Ras oncogene, bradykinin causes a single transient hyperpolarization, is without effect on actin stress fibers, and leads to cell shrinkage, unless actin is depolymerized (Lang et al., 1992b) (for review, see Ritter and Woll, 1996). The H-Ras-induced ΨPM oscillations are stimulated by hypertonic cell shrinkage (Ritter et al., 1993) presumably via fostering physical apposition and hence interaction of STIM and Orai, which accomplish SOCE (Lang et al., 2018). In line with that, SOCE has been shown to be inhibited by cell swelling (Liu et al., 2010). Furthermore, SOCE is stimulated by hypertonicity via NFAT5 (TonEBP) (Sahu et al., 2017) and SGK1 (Lang et al., 2018). Oncogenic K-RasG13D has been shown to suppress SOCE by altered expression of STIM1 in colorectal cancer cells (Huang and Rane, 1993; Pierro et al., 2018). Interestingly, fibroblastic L cells also display spontaneous oscillations of the cell membrane potential driven by oscillations of Ca2+i and concomitant K+ channel activation. These oscillations are modulated by low- and high-density lipoproteins in parallel with Ca2+-dependent stimulation of their pinocytosis (Tsuchiya et al., 1981; Rane, 1991; Huang and Rane, 1993). Oncogenic H-Ras also activates volume-regulated anion channels (VRACs) (Schneider et al., 2008). An inverse relationship between CV and cell number has been described for T24H-Ras (H-RasG12V) bearing Rat1 and M1 fibroblasts (Kunzschughart et al., 1995).
Water, Ions, and Their Channels and Transporters in Pinocytosis
Extracellular Ionic Composition, Osmolarity, and Extracellular pH
The ionic composition of the extracellular fluid affects pinocytosis by modulating the surface charge of the cell membrane and the cell membrane potential. Electrostatic interactions with the [normally negative (Cevc, 1990)] outer surface charge of a cell and/or the presence of positive charges in macromolecules play an important role in the induction of pinocytosis. In Amoeba proteus, monovalent cations induce pinocytosis in the order Cs+>K+>Na+>Li+, while divalent cations are less effective. Critically, Ca2+ and Mn2+ reduce the sensitivity of monovalent cations but are themselves without effects on pinocytosis (Stockem, 1966; Josefsson, 1968; Josefsson et al., 1975). Furthermore, the initial binding of pinocytosis inducers to the outer cell surface promotes displacement of surface-associated Ca2+ ions and induces changes in various membrane parameters, such as increased membrane conductance and decreased ΨPM along with an increase in plasmalemma hydration. Although pinocytosis in the amoeba can be induced by a great number of different solutes, all of them characteristically possess net positive charges that interact with negative surface charges. Hence, increasing extracellular Na+ displaces most of the exchangeable surface-associated Ca2+ and, therefore, induces pinocytosis. In the yolk sac, cationic macromolecules lead to pinocytosis (Lloyd, 1990). In human corneal epithelial cells, fibroblasts, and human umbilical vein endothelial cells (HUVECs), the uptake of an antibody–drug conjugate by macropinocytosis is facilitated by the presence of positive charges or hydrophobic residues on the surface of the macromolecule (Zhao et al., 2018). In contrast, in mammalian macrophages, anionic molecules are better inducers of pinocytosis than neutral or cationic ones (Cohn and Parks, 1967). The uptake of positively charged nanoparticles in colon carcinoma Caco-2 cells is reduced by inhibition of macropinocytosis with 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) and by cholesterol depletion of the PM, whereas these inhibitors have no effect on negatively charged systems (Bannunah et al., 2014). Modulation of the charge for intracellular delivery carriers aims at increasing the efficiency of macropinocytosis of cellular entry for therapeutic nucleic acids to tumor cells (Desai et al., 2019).
Anisotonicity modulates endocytosis in a diversity of cell types. In rat Kupffer cells, hypoosmotic and hyperosmotic conditions have been shown to stimulate and inhibit, respectively, phagocytosis (Warskulat et al., 1996), and in microglial BV-2 cells, preconditioning with hypotonic or hypertonic medium attenuates microsphere uptake (Harl et al., 2013). Hypertonic medium has been shown to disrupt the interaction of caveolae with endosomes. Increased phosphorylation due to phosphatase inhibition induces removal of caveolae from the PM. In the presence of hypertonic medium, this is followed by their redistribution to the center of the cell close to the microtubule-organizing center (Parton et al., 1994).
Only a few studies have addressed the action of anisotonicity on pinocytosis. In Dictyostelium, hyperosmotic conditions lead to a decrease in endocytic activity that can be attributed to cellular acidification (Pintsch et al., 2001), and it can foster the formation of giant vacuoles of pinocytotic origin, which appear to have a function in cellular osmoregulation (see above) (Yuan and Chia, 2001). In murine bone marrow-derived macrophages, hypotonic cell swelling stimulates phagocytosis and pinocytosis, both of which appear to require ClC-3 chloride channels. In this study, the ion channels have on the one hand been suggested to act as volume-activated anion channels in the PM and, on the other hand, shown to support acidification the endosomal compartment (Yan et al., 2014). Hypertonicity inhibits pinocytosis in rat hepatocytes (Synnes et al., 1999). In mouse L929 fibroblasts, cellular uptake of horseradish peroxidase is inhibited in hypertonic sucrose medium, which is reversed to stimulated uptake in the presence 10% PEG 1000 in the medium (Okada and Rechsteiner, 1982; Hughey et al., 2007). Similarly, in murine embryonic fibroblasts, cellular uptake of native proteins via macropinocytosis in hypertonic conditions is stimulated by alkali metal ions (Na+, Rb+, K+, Li+) but not by hypertonicity created by addition of sugars or sugar alcohols. This finding points to the contribution of surface charge in macropinocytosis. Also, hypotonic stress has been demonstrated to greatly enhance receptor-independent retroviral transduction efficiency in NIH 3T3 fibroblasts via stimulated intensive endocytosis (Lee and Peng, 2009).
An interesting concept explaining how extracellular pH elicits such effects is that protonation of the cell surface produces local charge asymmetries across the cell membrane, which induce inward bendings of the lipid bilayer, thus favoring vesicle formation and uptake of macromolecules (Ben-Dov and Korenstein, 2012).
In dendritic cells and bone marrow-derived macrophages, extracellular acidosis improves uptake and presentation of antigens by stimulation of macropinocytosis (Vermeulen et al., 2004; Martínez et al., 2007). This effect is related to acid-sensing ion channels (ASICs) and can be inhibited by their blocker amiloride (Kong et al., 2013). As macropinocytosis depends on phospholipase C (Amyere et al., 2002; Yoshida et al., 2015), its activation by proton-sensing G protein-coupled receptors such as ovarian cancer G protein-coupled receptor 1 (OGR1/GPR68), G protein-coupled receptor 4 (GPR4), T-cell death-associated gene 8 (TDAG8/GPR65), and GPR132/G2A (Seuwen et al., 2006; Alexander et al., 2017; Insel et al., 2020) may also explain the observed stimulatory effect of extracellular acidosis on pinocytosis. Notably, in HEK293 cells expressing mutated wild-type OGR1 or mutated OGR1L74P, receptor internalization, Ca2+i mobilization, and morphological changes observed upon activation of OGR1 by H+ or Ni2+ are severely compromised. This missense mutation of the H+ ion-sensing receptor has been found to cause familial amelogenesis imperfecta (Sato et al., 2020). Experiments with HEK293 cells transfected with active OGR1 receptor or a mutant lacking five histidine residues (H5Phe-OGR1) unraveled that receptor activation by H+ stimulates NHE- and v-ATPase activity only in OGR1- but not in H5Phe-OGR1-transfected cells. Furthermore, the known OGR1 inhibitors Zn2+ and Cu2+ reduce the stimulatory effect (Mohebbi et al., 2012). Given the importance of NHE1 and the v-ATPase for pinocytosis, it is tempting to speculate that OGR1 is a regulator thereof.
By contrast, in pancreatic acinar cells, low extracellular pH selectively impairs apical endocytosis. This is seen in mice lacking cystic fibrosis transmembrane conductance regulator (CFTR), which normally couples endocytosis at the apical PM to HCO3– secretion into the ductal lumen by normally rendering it alkaline. The acidic luminal fluid and impaired endocytosis due to lack of CFTR can be restored by alkalizing it in vitro (Freedman et al., 2001).
Ion Fluxes Drive Gel–Sol Transitions of the Cortical Actin Rim
Reorganization of the submembranous cytoskeleton is an essential step in pinocytosis. The cortical actin network is a major determinant of cell stiffness, and a correlation between stiffness of the actin network and the activity of endocytosis has been demonstrated (Planade et al., 2019). Extracellular K+ and Na+ antagonistically modulate the gel-to-sol transition of the cortical actin cytoskeleton beneath the PM (for review, see Oberleithner et al., 2009; Callies et al., 2011; Oberleithner and De Wardener, 2011; Warnock et al., 2014). Elevations of extracellular Na+ and K+ concentration stiffen and soften, respectively, the submembranous actin cytoskeleton of endothelial cells within minutes (Oberleithner et al., 2009). The Na+-dependent stiffening is mediated by an aldosterone-induced upregulation and activation of epithelial Na+ channels (ENaCs) and, presumably, by downregulation of the endothelial nitric oxide synthase (eNOS) activity. Conversely, inhibition of ENaCs upregulates nitric oxide [NO; formerly termed endothelium-derived relaxing factor (EDRF)] production. Depolarizing ΨPM by increasing extracellular K+ concentration, by blocking K+ channels with Ba2+, and by decreasing extracellular Cl– concentration decreases the mechanical stiffness of endothelial cells (Callies et al., 2011). Thus, modulation of the sol–gel transition of the actin cytoskeleton is thought to be due to the G-actin-dependent activation of eNOS (Fels et al., 2010).
It has to be mentioned that hypotonic swelling of endothelial cells was shown to cause stiffening of the PM due to an increase in cellular hydrostatic pressure rather than to disruption of the submembranous actin network. In this study, a change in membrane tension was not observed upon osmotic swelling, and depolymerization of F-actin did not abrogate swelling-induced stiffening of the PM (Ayee et al., 2018).
Cell Volume, Nitric Oxide, and the Cytoskeleton May Act Together in Regulating Endocytosis
In endothelial cells, a reciprocal regulatory relationship between eNOS and caveolin-1 (Cav-1) has been found, whereby Cav-1 and eNOS regulate the function of each other. On the one hand, Cav-1 stabilizes eNOS expression and regulates its activity, and eNOS-derived NO promotes caveolae-mediated endocytosis of albumin and insulin; on the other hand, a sustained NO production and persistent S-nitrosylation of Cav-1 lead to its ubiquitination and degradation (Chen et al., 2018).
Besides eNOS, also an association of inducible NO synthase (iNOS) with the submembranous actin cytoskeleton and intracytoplasmic vesicles in lipopolysaccharide (LPS)- and interferon gamma (INFγ)-stimulated macrophages and other cells was shown (Webb et al., 2001; Su et al., 2005).
Nitric oxide is a regulator of endocytosis, phagocytosis, and vesicle trafficking (Weinberg, 1998; Chen et al., 2018). For example, NO downregulates endocytosis in rat liver endothelial cells (Martinez et al., 1996) but promotes caveolae-mediated endocytosis as mentioned above (Chen et al., 2018). Macrophages stimulated by the thymic peptide thyαl display enhanced NO levels as well as increased pinocytosis (Shrivastava et al., 2004), as do amphotericin B-stimulated microglial cells (Kawabe et al., 2017). Conversely, mouse peritoneal macrophages stimulated with activin A display both reduced NO and pinocytosis (Zhou et al., 2009). Also, macropinocytosis of drugs may promote enhancement of iNOS expression, as exemplified for the chemotherapeutic nab-paclitaxel, which synergizes to this end with INFγ (Cullis et al., 2017).
Recently, it has been shown that NO regulates endocytic vesicle budding by S-nitrosylation of dynamin—a GTPase that regulates vesicle budding from the PM—and increases its enzymatic activity in response to NO (Wang et al., 2006). Dynamin is bound by NOS trafficking inducer (NOSTRIN), an eNOS-interacting adaptor protein, which forms a complex with Cav-1 and eNOS, and colocalizes with WASP and actin to promote its polymerization. It thus regulates caveolar endocytosis and eNOS internalization (Schilling et al., 2006; Su, 2014).
Nitric oxide/EDRF is also released in response to changes in extracellular osmolarity, which also greatly affect the cortical actin organization. This is also relevant in apoptosis, where NO metabolism, actin organization, and CV-regulatory ion transport are linked together (Bortner, 2005).
Nitric oxide release in response to hypertonicity occurs in vessels and endothelial cells (Steenbergen and Bohlen, 1993; Vacca et al., 1996; Zani and Bohlen, 2005). Hypertonicity downregulates eNOS in human aortic endothelial cells (HAECs), an effect that is mediated by activation of AQP1 and NHE-1, and which involves PKCβ-mediated intracellular signaling (Madonna et al., 2010). In insulin-treated HAECs, hypertonicity, established by high glucose or mannitol, downregulates the PI3K/Akt/mTOR/eNOS pathway and impairs their ability to respond to insulin. This may contribute to insulin resistance. The mechanism involves AQP1 and the transcription factor Ton/EBP (NFAT5) for osmosensing, and the effect can be reversed by silencing the transcription of these proteins (Madonna et al., 2020).
Hypotonic swelling has been shown to promote the release of NO and reactive nitrogen oxide species in various cells (Kimura et al., 2000; Haussinger and Schliess, 2005; Takeda-Nakazawa et al., 2007; Kruczek et al., 2009; Gonano et al., 2014) and to augment LPS-triggered iNOS expression in RAW 264.7 macrophages (Warskulat et al., 1998). Recently, it has been shown that in HUVECs, VRAC/SWELL1/LRRC8A mediates endothelial cell alignment via stretch-dependent Akt-eNOS signaling and formation of a signaling complex made up by Grb2, Cav-1, and eNOS (Alghanem et al., 2021).
While osmotic shrinkage in general is associated with an increase in actin polymerization, cell swelling leads to its depolymerization, though both with exceptions (Hoffmann et al., 2009).
Hypertonic shrinkage fosters the translocation of the actin-binding protein cortactin to the cortical actin net, where it interacts with the Arp2/3 complex, WASP, dynamin, and myosin light-chain kinase (for review, see Pedersen et al., 2001; Hoffmann et al., 2009). Cortactin stabilizes microfilament assembly at the cell periphery, is recruited to PM ruffles, and participates in macropinocytosis (Mettlen et al., 2006). In human-induced pluripotent stem cells, hyperosmolarity, created by high glucose or mannitol, upregulates AQP1 and induces cytoskeletal remodeling with increased ratios of F-actin to G-actin, effects that could be by reversed siRNA-mediated inhibition of AQP1 expression (Madonna et al., 2014).
Cell swelling can lead to reorganization of intermediate filaments (Dartsch et al., 1994a; Li J. et al., 2019). In Cos-7 fibroblast-like cells, hypotonicity causes a rapid calcium/calpain-dependent cleavage of the intermediate filament vimentin, whereby hypotonicity leads to the generation of IP3 by hydrolysis of PI(4,5)P2 and Ca2+ release from the ER (Pan et al., 2019). Vimentin in turn is involved in the regulation of vesicular trafficking (Margiotta and Bucci, 2016) and was also shown to be modulated by NO (Sripathi et al., 2012).
Na+/H+ Exchangers and Pinosome Formation
A distinctive hallmark to distinguish clathrin-dependent endocytosis from clathrin-independent macropinocytosis is the sensitivity of the latter to inhibitors of NHEs such as amiloride, EIPA, or HOE-694 (West et al., 1989; Koivusalo et al., 2010; Canton, 2018; Lin et al., 2020). Besides NHEs, amiloride also directly inhibits ion channels of the epithelial sodium channel/degenerin family, such as ENaC and ASIC1 (Kellenberger and Schild, 2002; Qadri et al., 2010) and growth factor receptor tyrosine kinase activity (Davis and Czech, 1985). Moreover, amiloride is a weak permeant base that can accumulate in acidic vesicles, thus, eventually dissipating the H+-gradient driving cation exchange, and thereby inhibiting pinocytosis (Dubinsky and Frizzell, 1983; Bakker-Grunwald et al., 1986). Of note, EIPA does not inhibit a newly described form of CD, which is induced by fenretinide, which is similar to methuosis characterized by hyperstimulated macropinocytosis and massive vacuolization (Brack et al., 2020).
Na+/H+ exchangers, which are members of the solute carrier (SLC) 9 family, are electroneutral transporters that exchange Na+ for H+ across membranes (Pedersen and Counillon, 2019). The NHE1 isoform is expressed almost ubiquitously and regulates cellular and organelle pH, motility, phagocytosis, proliferation, as well as cell survival and CD (Orlowski and Grinstein, 2011; Pedersen and Counillon, 2019). Cell shrinkage is a strong stimulator of NHE1 activity and serves for RVI (see above) (Ritter et al., 2001; Baumgartner et al., 2004; Orlowski and Grinstein, 2011; Pedersen and Counillon, 2019). NHE1 is activated by a drop in pHi, a broad diversity of PM receptors, such as tyrosine kinase receptors, G-protein-coupled receptors or integrin receptors, and it is regulated by second messengers, asymmetric membrane tensions, and phosphoinositides, such as PI(4,5)P2 (Orlowski and Grinstein, 2011; Pang et al., 2012; Pedersen and Counillon, 2019). In proximal tubular cells, NHE1 is activated by PI(4,5)P2 and inhibited by PI(3,4,5)P3 (Abu Jawdeh et al., 2011; Pang et al., 2012). NHE1-borne changes in pHi may also be confined to distinct subcellular domains or compartments. Such subcellular compartments are the lammelipodia formed during cell spreading and migration, pseudopodia in phagocytosis, ruffles and the cup wall formation in macropinocytosis. In all of these processes, NHE1 is also involved in cytoskeletal rearrangement (Schwab and Stock, 2014; Pedersen and Counillon, 2019). NHE1 is connected to actin via ezrin/radixin/moesin (ERM) family of actin-binding proteins. Migrating cells undergo cell polarity-dependent subcellular volume changes. Repetitive cycles of protrusion at the leading edge (lamellipodium) are followed by retraction of the cell’s rear, i.e., the trailing edge. Waves of local water movement across the PM locally increase and decrease CV during the protrusion and retraction of the lamellipodium, respectively. Hence, cell migration requires an alternating cycle of subcellular RVI at the leading edge and RVD at the trailing edge of the cell. Most of the ion transporters known to participate in CVR are also involved in the regulation of cell migration. As part of the RVI transporters, NHE1 is confined to the lamellipodium, where it also serves for local extracellular acidification (Lang et al., 1998; Schneider et al., 2000; Loitto et al., 2002, 2009; Jakab and Ritter, 2006; Hoffmann et al., 2009; Schwab et al., 2012; Stock et al., 2013). This is also given in breast cancer cell invadopodia, where NHE1 is allosterically regulated by NaV1.5 Na+ channels (Brisson et al., 2012, 2013). In T47D human breast cancer cells, NHE1 colocalizes with Akt and ERK in prolactin-induced ruffles (Pedraz-Cuesta et al., 2016).
Although inhibition of macropinocytosis by NHE inhibitors is a fingerprint feature of macropinocytosis, the molecular mechanisms how NHEs contribute to macropinocytosis are barely known. Interestingly, activation of the small GTPases, Rac1/Cdc42, and consequently actin polymerization and, thus, ruffle formation are sensitive to submembranous pH (pHsm). Submembranous acidification due to inhibition of NHE1 by amiloride prevents Rac1/Cdc42 activation and suppresses actin polymerization (Koivusalo et al., 2010). Moreover, NHE1 inhibition favors the accumulation of cytosolic H+, which neutralizes the negative charges on the inner leaflet of the PM. Thus the interaction of the negatively charged headgroups of phosphoinositides and the polybasic motifs in Rac and Cdc42 as well as the SCAR/WAVE complex and WASP is hampered. This also explains the inhibitory effect of NHE1 blockers on macropinocytosis (Marques et al., 2017). Therefore, amiloride might not directly inhibit macropinocytosis but cause submembranous accumulation of metabolically generated H+ in thin lamellipodium-like membrane extensions by inhibition of H+ efflux. Given the small volume of these structures, only a few H+ will change pHsm and, consequently, the state of actin polymerization. However, in HeLa cells, oncogenic Ras-induced macropinocytosis does not require a decrease in pHsm (Ramirez et al., 2019).
Notably, in mice bearing xenograft tumors derived from oncogenic K-Ras bearing MIA PaCa-2 cells, intratumoral macropinocytosis and tumor growth are suppressed by treatment of the animals with EIPA, which is, however, effective only in tumors with high but not low macropinocytic activity (Commisso et al., 2013; Recouvreux and Commisso, 2017).
The ionophoric NHE monensin induces vesicular swelling and the formation of giant multivesicular bodies (MVBs) (Stein and Sussman, 1986; Savina et al., 2003) and release of exosomes, which can be inhibited by the NHE inhibitor dimethyl amiloride (DMA) (Chalmin et al., 2010; Pironti et al., 2015). Moreover, deletion of the Saccharomyces cerevisiae NHE, Nhx1, disrupts the fusogenicity of the MVB in a manner dependent on pH and monovalent cation gradients (Karim and Brett, 2018).
The Ion Composition in Macropinosomes and Endolysosomal Vesicles
After pinching off, the nascent macropinosome has entrapped extracellular fluid that equals the extracellular fluid in the intimate vicinity of its formation site. Traveling along the endolysosomal pathway, its ionic composition is modified to render the vesicular volume, shape, and fluid suitable for processing its cargo, as shown in Table 1 (Scott and Gruenberg, 2011; Freeman and Grinstein, 2018; Chadwick et al., 2021b).
Water Permeability, Aquaporins, and Osmolarity
The permeabilities of the PM and vesicular membranes for water play crucial roles in pinocytosis. The importance of PM AQPs for macropinocytosis is seen in dendritic cells where they are needed as essential elements of a CV control mechanism necessary to concentrate the macrosolutes and present antigens (De Baey and Lanzavecchia, 2000; Hara-Chikuma et al., 2011; Kitchen et al., 2015).
Macropinosomes shrink along their cellular route by moving ions and osmotically obliged water out of its lumen into the cytosol (Freeman and Grinstein, 2018; Chadwick et al., 2021b). Despite the dramatic decrease in pinosome volume, the water permeation pathway is not characterized yet. Studies on osmotic water permeability (Pf) indicate that Pf values higher than 0.01 cm/s indicate water flux through channels (Verkman et al., 1996), Pf values in the range from 0.003 to 0.005 cm/s designate membranes lacking water channels, and Pf values between 0.0001 and 0.005 cm/s characterize pure phospholipid bilayers (Fettiplace and Haydon, 1980; Echevarria and Verkman, 1992; Olbrich et al., 2000). These values could be used to evaluate the presence or absence of water channels in endosomes.
Clathrin-coated vesicles from bovine brain that lack water channels have a low Pf of ∼0.001 cm/s and retain this value after stripping off the coat. Vesicles prepared from bovine renal cortex and inner medulla revealed two populations: one containing water channels with high (0.02 cm/s) and one lacking them with low values compared to brain-derived vesicles (Verkman et al., 1989). Vasopressin-induced endosomes of rat kidney papilla and toad bladder endocytic vesicles have Pf values of 0.03 and >0.1 cm/s, respectively, whereas isolated toad bladder granules have a Pf value as low as 0.0005 cm/s (Verkman and Masur, 1988; Verkman et al., 1988; Shi and Verkman, 1989; Verkman, 1989).
Only a few studies have investigated Pf of pinosomes and lysosomes. In J774 macrophages, the Pf of the PM is in the range of ∼0.004–0.009 cm/s (Fischbarg et al., 1989; Ye et al., 1989; Echevarria and Verkman, 1992). The water permeability of the macropinosome membrane of J774.A1 cells increases non-linearly from ∼0.001 cm/s 3 min after formation to ∼0.005 cm/s after 25 min of formation (Chaurra-Arboleda, 2009). The lysosomal Pf of CHO-K1 cells is in the same range (Chaurra et al., 2011). In J774.A1 cells, Pf shows pH dependency. It decreases from ∼0.007 to 0.0009 cm/s following lysosomal alkalinization with NH4Cl (pHlys 6.5–6.8) and to 0.0001 cm/s after inhibition of the v-ATPase (pHlys ∼7.0). These values are similar to those in early macropinosomes, which have a pH of ∼6.7 (Chaurra-Arboleda, 2009). Taken together, the low Pf values of J774 macrophages indicate the absence of AQPs in the endolysosomal compartment.
Despite these specific observations, the role of AQPs in the endolysosomal compartment is poorly investigated, while their importance in volume regulation of secretory vesicles is well documented (Cho and Jena, 2006; Sugiya et al., 2008; Jena, 2020). In toad urinary bladder endosomes, AQP-TB has been shown to be present (Siner et al., 1996), and incorporation of PM-derived AQP2 into endosomes is well known for AVP-stimulated renal collecting duct principal cells (Shi and Verkman, 1989; Verkman, 2005). In astrocytes, AQP4-laden vesicles may fuse with the PM upon cell swelling (Potokar et al., 2013; Vardjan et al., 2015). AQP6 was shown to be present in intracellular vesicles of acid-secreting intercalated cells of the renal collecting duct where it colocalizes with the H+-ATPase and serves also as an anion channel (Yasui et al., 1999; King et al., 2004). Lack of AQP11 causes defective endosomal pH regulation, as seen in mice devoid of AQP11. This is accompanied by the appearance of huge vacuoles in the renal proximal tubules and polycystic kidneys (Ishibashi et al., 2009).
An example for the function of organellar AQPs in VVR and CVR is evident in Trypanosoma cruzi, as briefly described below.
Alternatively to AQPs, water permeation through transporters and ion channels has been described for glucose transporters (Loike et al., 1993; Fischbarg and Vera, 1995), NKCC1, KCC, SGLT1, and CFTR (Hamann et al., 2010; Zeuthen, 2010; Zeuthen and Macaulay, 2012; Huang et al., 2017). Eventually, these transporters or ion channels could be used in pinosomes for water efflux as well.
By whatever way, as upon hypertonic cell shrinkage also vesicles rapidly shrink, it can be deduced that the intrinsic water permeability is sufficiently high to allow for timely vesicular volume changes during resolution of macropinosomes (Freeman and Grinstein, 2018). Using fluorescence enhancement of Lucifer yellow dextran by deuterated water, Li et al. (2020) have recently demonstrated that lysosomes rapidly swell in response to a hypoosmotic challenge, indicating that there is substantial water influx into the lumen of lysosomes soon after water penetration across the PM, again indicating that the intrinsic water permeability of these organelles is high.
Vesicular pH Regulation and Ions in Pinosome Maturation
The pH of the ingested fluid of nascent pinosomes resembles that of the extracellular fluid, which is under physiological conditions (7.4). During trafficking, vesicular pH (pHves) gradually decreases to the value prevailing in lysosomes, i.e., 4.5–5.0. pHves regulates enzyme activities and enables oxidation reactions, the release of internalized receptors from their ligands and their recycling back to the PM, movement and assembly of organellar surface coat proteins, vesicle maturation, as well as membrane fusion processes (Demaurex, 2002; Ohgaki et al., 2011).
Vacuolar ATPase
The acidification is primarily achieved by insertion of the v-ATPase. The v-ATPase is an evolutionarily highly conserved primary active H+ transporter. As already outlined, it can be found both in the PM and in various intracellular organelles. Structurally, it comprises a multiprotein complex that forms two distinct domains, i.e., the pore-forming transmembrane VO domain and the cytosolic V1 domain. The latter hydrolyzes ATP to drive H+ movement. This creates the electrochemical H+ gradient across the membrane, thereby driving secondary active transport processes, which act together for proper adjustment of pHves and vesicular ion and osmolyte composition. In particular, parallel to H+ pumping, ion channels and transporters move Cl–, H+, and other cations to establish an electrical shut aiming for electroneutrality of the net charge transfer. Otherwise, the acidification would be limited by the transmembrane potential Ψves, which is set up by the v-ATPase itself (Demaurex, 2002; Steinberg et al., 2010; Koivusalo et al., 2011; Xiong and Zhu, 2016; Freeman and Grinstein, 2018; Jentsch and Pusch, 2018; Sterea et al., 2018; Li P. et al., 2019; Freeman et al., 2020; Chadwick et al., 2021a). pHves is stabilized by the buffering capacity of the vesicle content (∼60 mM/pH at pH 4.5–5) (Weisz, 2003; Steinberg et al., 2010).
In addition, the v-ATPase also serves as a protein interaction hub. For the proper sorting and targeting of the vesicles, small GTPases are recruited to their membranes in an acidification-dependent manner. The v-ATPase itself is able to associate with some of them and with other regulatory proteins. Thus, the v-ATPase seems not only to serve for creating the acidic pHves but also to sense it and to transmit this information to its cytoplasmic domain, thus enabling trafficking molecules to bind and perform their targeting functions. The v-ATPase is also involved in signaling pathways for the regulation of macropinocytosis, as outlined throughout this review. Its dysfunction may play critical roles in various diseases including diabetes, cancer, neurodegeneration, osteopetrosis, skin disorders, or renal tubular acidosis and other pathologies. Furthermore, it is also essential for viral entry into cells (for reviews on v-ATPase, see Nishi and Forgac, 2002; Platt et al., 2012; Maxson and Grinstein, 2014; Rappaport et al., 2016; Kissing et al., 2018; Futai et al., 2019; Collins and Forgac, 2020; Song Q. et al., 2020; Vasanthakumar and Rubinstein, 2020; Chadwick et al., 2021a, b; Eaton et al., 2021).
Cl– Ions
Within 1 min after internalization, the luminal Cl– concentration in endosomes/macropinosomes drops from ∼120–150 mM to ∼20 mM (Sonawane et al., 2002). This decrease is insensitive to Cl– channel inhibition and can be attributed to Cl– expulsion by an interior negative Donnan potential (Ohshima and Ohki, 1985; Sonawane and Verkman, 2003; Hryciw et al., 2012). During maturation, the vesicular Cl– concentration increases again up to ∼130 mM in lysosomes (Saha et al., 2015). The Cl– accumulation of late endosomes can be suppressed by inhibition of the v-ATPase and restored by the K+ ionophore valinomycin. Also, replacement of Cl– by gluconate and Cl– channel inhibition slow endosomal acidification. Thus, Cl– is an important counter ion accompanying endosomal acidification (Sonawane and Verkman, 2003) (for review, see Faundez and Hartzell, 2004; Stauber and Jentsch, 2013). The accumulation of lysosomal Cl– appears to be important for the adjustment of the lysosomal volume and the activity of proteases. Reduced lysosomal Cl– concentrations may lead to lysosomal storage diseases, e.g., Gaucher’s disease (OMIM entries 230800, 230900, 231000) or Nieman–Pick’s disease (OMIN entries 257200, 607616, 257220, 607625) (Cigić and Pain, 1999; Platt et al., 2012, 2018; Stauber and Jentsch, 2013; Rappaport et al., 2016; Chakraborty et al., 2017; Astaburuaga et al., 2019).
The Cl– channels and transporters expressed in intracellular organelles include the ClC family members ClC-3 through 7, chloride intracellular channels (CLICs), CFTR, AQP6, transmembrane proteins (TMEM)16C–G/anoctamin (ANO) 3–7, bestrophin-1, Golgi pH regulator (GPHR) (reviewed in Stauber et al., 2012; Stauber and Jentsch, 2013), Tweety homolog 1 (Ttyh1) proteins (Wiernasz et al., 2014), LRRC8 (Li et al., 2020), and TMEM206 (Osei-Owusu et al., 2021).
The ClC-3 to 7 transporters are confined to distinct endolysosomal compartments with partially overlapping appearance (Jentsch and Pusch, 2018). They are electrogenic outwardly rectifying 2Cl–/H+ exchangers working in parallel with the v-ATPase. As per exchange cycle, two Cl– enter and one H+ leaves the vesicle, three negative charges accumulate in the vesicle. To maintain electroneutrality, three H+ are pumped in, one of which leaves the vesicle again via the 2Cl–/H+ exchanger, thus leading to a net uptake of two H+ (Guzman et al., 2013; Jentsch and Pusch, 2018). This proposed mechanism allows a more efficient H+ and Cl– accumulation, as it generates a more inside-negative Ψves than an ohmic Cl– conductance could do. Yet, this mechanism may be restricted to ClC-5 (Zifarelli, 2015). However, Ψves is generally thought to be negative (i.e., positive in the lumen) due to the rheogenic v-ATPase. The reason for this discrepancy is still elusive (Weinert et al., 2010) (reviewed in Jentsch, 2007; Stauber et al., 2012; Stauber and Jentsch, 2013; Jentsch and Pusch, 2018). Dysfunctional ClC exchangers may lead to a broad variety of symptoms and disorders including neurodegeneration and other neuropathies, proteinuria and kidney stones, osteopetrosis, albinism, and lysosomal storage diseases (Jentsch and Pusch, 2018; Nicoli et al., 2019; Schwappach, 2020; Bose et al., 2021). Overexpression of ClC-3 or gain-of-function mutations of ClC-6 or ClC-7/Ostm1 leads to swelling of late endosomes and lysosomes (Li et al., 2002; Nicoli et al., 2019; Polovitskaya et al., 2020). In B-cell non-Hodgkin lymphoma cells, the PIKFyfe inhibitor apilimod leads to CD by formation of giant vacuoles and disruption of endolysosomal function, an effect that requires functional ClC-7/Ostm1 transporters (Gayle et al., 2017). In Hela and NIH3T3 cells, a short natural ClC3 splice variant (Clc3s) has been shown to lead to the formation of large vacuoles (Wu et al., 2016), and in Chinese hamster ovary CHO-K1 or human hepatoma Huh-7 cells, the volume of such vesicles is governed by their Cl– concentration (Li et al., 2002).
Endothelial cells lacking CLIC4 display defective endothelial cell tubulogenesis and impaired acidification of large intracellular vesicles, while lysosomes are unaffected (Ulmasov et al., 2009).
Previous work showed that cell swelling leads to alkalinization of acidic cellular vesicles, regardless whether the cells are swollen by hypotonicity, isoosmotically with high-K+ solutions, inhibition of K+ channels, or concentrative uptake of solutes such as amino acids. At least in liver cells, swelling-induced alkalinization occurs rather in the pre-lysosomal than lysosomal compartments. The increased pHves affects proteolysis, trafficking of cell membrane proteins, and antigen presentation (Busch et al., 1994, 1996, 1997; Völkl et al., 1994). The mechanisms leading to swelling-induced rise of pHves are still elusive. Interestingly, overexpression of a naturally occurring C-terminally truncated splice variant of mouse bestrophin-3, Best3V2, leads to, besides swelling, alkalinization of lysosomes (Wu et al., 2016). Manipulation of the intracellular Cl– concentration leads to alterations of lysosomal volume due to the high ClC-3-endowed Cl– permeability. Accordingly, a decrease in intracellular Cl– concentration during RVD following cell swelling could alter pHves. In H-Ras oncogene-expressing fibroblasts, which have a higher CV (see above), the swelling-induced vesicular alkalinization is less pronounced compared to cells not expressing the oncogene. Hence, in H-Ras-expressing cells, any effect of the swelling-induced vesicle alkalinization on cell function may be altered (Busch et al., 1997). Notably, murine and human fibroblasts expressing oncogenic K-Ras also display significant alkalinization of lysosomes (Jiang et al., 1990).
In several cell types, the fusion of late endosomes to lysosomes is prevented by isotonic K+ buffers as a consequence of an increased permeability of cells to K+ and concomitant cell swelling. This inhibition is selective for late endosomes, since other endosome fusion events, such as homotypic fusion of early or late endosomes or fusion of recycling endosomes with the PM, are not affected. Cell swelling is regarded to be causative for this effect (Ward et al., 1990). As cell swelling and lack of fusion of endosomes to lysosomes are also hallmarks of methuosis (see below and Figure 2), it is tempting to speculate that cell swelling in methuosis might not only be a passive consequence of swollen vacuoles but also be a cause of it.
A link between CVR and vacuolar pH regulation is also established by the proteins MLC1 and GliaLCAM, which are defective in megalencephalic leukoencephalopathy with subcortical cysts. This rare congenital disease is characterized by macrocephaly, ataxia, seizures, degeneration of motor functions, and cognitive decline, morphologically by chronic white matter edema and subcortical cysts, and on the ultrastructural level by intra-myelinic vacuole formation and enlarged intracellular vacuoles (Van Der Knaap et al., 2012). The protein MLC1 is involved in astrocytic CVR. It may be a volume-sensitive ion channel itself, but it is also part of a macromolecular complex composed of the Na+/K+-ATPase, Kir4.1 K+-channels, AQP4, syntrophin, and caveolin-1, as well as volume-sensitive TRPV4 cation channels, which mediate cellular Ca2+ influx upon cell swelling. In addition, MLC1 also influences CLC-2 chloride channels as well as the volume-sensitive anion channel(s)/current(s) (VRACs) and therefore also RVD. It has been shown that knockdown of LRRC8 annihilates the potentiating effect of MLC1 on VRAC currents via modulation of the phosphorylation state of the channel subunit LRRC8C (Elorza-Vidal et al., 2018). GlialCAM serves as an escort protein for MLC1 and CLC-2, and it is necessary for its proper activation by cell swelling. MLC1 is expressed in early and recycling endosomes, which they use to travel to the PM during hyposmotic stress (Capdevila-Nortes et al., 2013). Importantly, MLC1 also interacts with the v-ATPase, and it is involved in regulating early endosomal pH (Lanciotti et al., 2012). Defective MLC1 may result in impaired recycling and retention of TRPV4 channels in the cytoplasmic perinuclear area and thus disturbed swelling-induced cellular Ca2+ influx (Lanciotti et al., 2012) such as seen in monocyte-derived macrophages from MLC patients (Ridder et al., 2011; Petrini et al., 2013; Brignone et al., 2014, 2015; Elorza-Vidal et al., 2018). Furthermore, CLC-2 knockout mice exhibit myelin vacuolization, which is thought to arise from dysregulation of extracellular ion concentrations (Stauber et al., 2012). Moreover, ClC-2 channels are functionally regulated by SGKs by inhibiting the ubiquitin ligase Nedd4-2, which in turn results in reduced clearance of ClC-2 protein from the PM (Palmada et al., 2004). As outlined above, SGK1 is involved in the regulation of macropinocytosis, and hence, both ClC-2 and SGKs may be tight together in the regulation of macropinocytosis.
The acid-activated outwardly rectifying chloride channel/current (ASOR; also termed proton-activated, outwardly rectifying anion current, PAC, PAORAC; or ICl,H) could be a candidate to serve as electrical shut for endolysosomal acidification. This anion channel or its core component is made up by TMEM206 proteins, which form a trimeric channel that is architecturally related to ENaCs/degenerin channels and ASICs (Ullrich et al., 2019; Ruan et al., 2020; Deng et al., 2021). TMEM206 has been shown to interact with Akt (Zhao et al., 2019) and to contribute to acid-induced CD (Osei-Owusu et al., 2020). The ASOR current is characterized by activation at pH values < 5.0, strong outward rectification, activation at positive transmembrane potentials, and sensitivity to typical Cl– channel blockers (Sato-Numata et al., 2013, 2017; Kittl et al., 2019, 2020; Ullrich et al., 2019). In contrast to CLCs, which are inhibited by extracellular/luminal acidic pH (Scheel et al., 2005; Jentsch, 2007; Jentsch and Pusch, 2018), ASOR is activated by it. Accordingly, endosomal ASOR and CLCs could regulate Ψves and vesicular ion concentrations. TMEM206 is mainly localized in the PM, but variable cytoplasmic labeling has been documented (Ullrich et al., 2019). Recently, it has been shown that TMEM206 indeed traffics from the PM to endosomes. Its deletion annihilates the endosomal Cl– conductance, raises the luminal Cl– concentration, lowers pHves, and increases transferrin receptor-mediated endocytosis. Moreover, its overexpression generates a large endosomal Cl– current with properties resembling the endogenous conductance and reduces endosomal acidification as well as transferrin uptake. Thus, endosomal TMEM206 appears to function as a sensor for low pH and may prevent hyperacidification by releasing Cl– from the lumen (Osei-Owusu et al., 2021). Clearly, the role of TMEM206/ASOR/PAORAC in the regulation for Ψves and pHves warrants further investigations. Likewise, its role in VVR needs to be investigated.
LRRC8A-E/SWELL1 proteins are essential components of the volume-regulated outwardly rectifying Cl– current (VRAC, VSOR, VSOAC, IClswell, IClvol) elicited in most cells during cell swelling (Qiu et al., 2014; Voss et al., 2014; Jentsch, 2016; Jentsch et al., 2016; Okada, 2020; Bertelli et al., 2021), and they are involved in a broad variety of cellular functions (Chen et al., 2019). LRRC8A-E/SWELL1 also regulate the PI3K-Akt, Erk1/2, mTOR signaling cascade (Kumar et al., 2020; Alghanem et al., 2021). Recently, Li et al. (2020) demonstrated the expression of LRRC8 family members on lysosomal membranes and their ability to build functional lysosomal VRACs (Lyso-VRACs). Patch clamp investigation of these vacuoles revealed that Lyso-VRACs are activated by ionic strength and permeable for anions, like Cl–, NO3–, HCO3–, acetate, aspartate, or glutamate. They are inhibited by known VRAC blockers. Early endosome membranes lack Lyso-VRACs. Hypoosmotic swelling promotes the formation of large cytoplasmic vacuoles containing markers for late endosomes and lysosomes with a diameter of >2 μm, which require functional Lyso-VRACs. Loss of VRAC channels enhances necrotic CD triggered by sustained hypoosmotic, hypoxic, and hypothermic stress. Furthermore, the authors demonstrated that endolysosome-derived giant vacuoles are subject to exocytosis. This releases PM tension and simultaneously reduces CV. Thus, by acting as water store-and-release compartments, the swelling-induced giant vesicles have been compared to the “cell’s ‘bladder,’ sequestering intracellular excess water through vacuolation and then expelling the potentially toxic level of water through exocytosis, which also relieves the plasma membrane tension stress” (Li et al., 2020).
AQP6 is activated by low pH and acts as Cl– channel in acid-secreting α-intercalated cells in renal collecting ducts. There it is found in endosomes where it colocalizes along with ClC-5 and the v-ATPase. However, ClC-5 is inhibited at acidic pH, whereas the anion conductance of AQP6 is turned on. Hence, ClC-5 may rather operate when acidification of the vesicles starts, whereas AQP6 may take over this role as their pH drops. Furthermore, AQP6, though its water permeability is low, may mediate vesicle swelling and membrane fusion during exocytosis or other cellular processes (Yasui et al., 1999; Hazama et al., 2002; Kitchen et al., 2015).
HCO3– Ions
The role of HCO3– in pinocytosis has not met much attention yet. Carbonic anhydrase (CA) catalyzes the dissociation of HCO3– to generate H+ of CO2. CA isoforms are present at the PM, in the cytosol, and in lysosomes (Rikihisa, 1985; Reibring et al., 2014). There they associate with numerous acid–base transporters such as anion exchangers 1-3 (AE1-3), sodium bicarbonate cotransporters NBCe1 and NBCn1, and NHE1 (Becker et al., 2014). Recently, Sedlyarov et al. (2018) found that electroneutral NBCn1 (NBC3, SLC4A7) is essential for phagosome acidification in macrophages. NBCn1 resides in the PM, and lack of it leads to cytoplasmic acidification, which in turn hampers phagosome maturation and impairs bacterial killing. If membrane-associated CA is internalized during the formation of the pinosomes, it will catalyze the dissociation of HCO3– to generate CO2, which in turn will rapidly equilibrate across the vesicular membrane. Notably, in global CVR, VRAC may actually work more effectively in driving RVD by passing HCO3– than Cl–, as the former can be virtually unlimitedly replenished from CO2 in the presence of CA, whereas Cl– can only exit the cell as long as the ΨPM is more negative than the equilibrium potential for this ion (Völkl and Lang, 1988; Ritter et al., 1991). In line with these considerations, the VRAC channels made up of LRRC8A with B through E subunits are permeable to HCO3– (Gaitan-Penas et al., 2016). Hence, Lyso-VRAC may provide a vesicular HCO3–-conductive pathway as well.
Na+ Ions
Among the internalized ion transporters, the Na+/K+-ATPase may limit endosomal acidification. Its rheogenic Na+ transport contributes to the Ψvesicle of endosomes, thereby opposing H+ transport. This is, however, only evident in early but not in late endosomes (Fuchs et al., 1989). In line with this, it has been shown in Swiss 3T3 fibroblasts that inhibition of the Na+/K+-ATPase by ouabain strongly enhances the acidification of early but not late endosomes or lysosomes. Ouabain has also been shown to produce stronger endosomal acidification and parallel Cl– accumulation in transferrin-labeled early and recycling endosomes of J774 cells (Sonawane and Verkman, 2003). Ouabain exerts its effect by acting on the interior side of the vesicles rather than on the PM (Zen et al., 1992). In contrast, in early rat liver endosomes, the Na+/K+-ATPase does not regulate acidification (Anbari et al., 1994).
Whether internalized PM retrieved NHE1 contributes to pinosomal acidification remains to be determined. According to the prevailing Na+ gradient between the lumen and the cytosol, NHEs should serve to acidify nascent macropinosomes. NHE1 does, however, not play a direct role in phagosome acidification due to the rapidly dissipating Na+ gradient between the phagosome and the cytosol and the absence of the Na+/K+-ATPase to maintain such a gradient. Instead, phagosomal acidification is established by the v-ATPase (Hackam et al., 1997, 1999). By analogy, this also may hold true for macropinosomes.
The human orthologs of yeast NHX1 are the endosomal Na+(K+)/H+ exchangers NHE-6 and NHE-9. NHE-6 is present in early and NHE-9 in late recycling vesicles. NHE-6 is also inserted into the PM upon vesicular recycling. NHE-6 and NHE-9 bind to the adaptor protein for activated PKC, receptor for activated C kinase (RACK1), which interacts with metabolic enzymes, kinase receptors, and ion transporters and contributes to the maintenance of pHves by regulating the distribution of the transporters between endosomes and the PM. They are involved in clathrin-dependent endocytosis by alkalinizing early endocytic vesicles. Endosomes of NHE-6-deficient neurons appear to be strongly acidic. Such a hyperacidification occurs upon hypoxia-induced mobilization of NHE-6 to the PM (Lucien et al., 2017). In mouse, loss of NHE-6 causes endolysosomal storage disease with accumulation of gangliosides and unesterified cholesterol in late endosomes and lysosomes of neurons of selective brain regions. In humans, mutations of NHE-6, NHE-7, and NHE-9 cause neurological syndromes (Ilie et al., 2014, 2016; Kondapalli et al., 2014; Schwede et al., 2014). NHE-5 may contribute to organellar pH regulation and regulate cell surface expression of the receptor tyrosine kinase MET and the EGF receptor (Fan et al., 2016). NHE-7 is found in the trans-Golgi network and in endosomes and also interacts with RACK1. Interestingly, NHE-7 mediates an acidification of intracellular vesicles that adds to that set up by the v-ATPase and that accelerates endocytosis (Milosavljevic et al., 2014). NHE-8 is found in the mid- to trans-Golgi compartment and MVBs. It regulates late endosomal morphology and function. In HeLa-M cells, NHE-8 silencing results in perturbation of MVB protein sorting, disrupted endosomal protein trafficking, and perinuclear clustering of endosomes and lysosomes (Lawrence et al., 2010). In the kidney, it also localizes to the apical PM and regions of coated pits. NHA-2 appears to localize to multiple compartments. It is expressed in endosomes of pancreatic β cells and synaptic-like microvesicles and participates in clathrin-dependent but not -independent endocytosis (Fuster and Alexander, 2014). In lysosomes, NHEs are absent (Nakamura et al., 2005) (for review, see Donowitz et al., 2013; Pedersen and Counillon, 2019).
Na+/H+ exchangers could fulfill a dual role in pHves regulation. In endosomes, NHEs face the acidic organellar interior and the high K+ concentration of the cytosol and act as H+/K+ exchangers to alkalinize vesicles. Once recycled through the PM, they face the high Na+ concentration within the nascent vesicle. As Na+ is then following its gradient into the cytosol, H+ is transported into the vesicular lumen, thus acidifying it (Pedersen and Counillon, 2019).
K+ Ions
As mentioned above, extracellular and intracellular K+ greatly affects endocytosis and vesicle trafficking. After pinching off, the pinosomes may retain the channel equipment of the PM, and they may contribute to the initial changes in the ionic composition of the intra-pinosomal fluid. Given the normally high K+ conductance of the PM and the prevailing electrochemical driving force for K+, this is expected to be in favor of setting up a negative Ψves in the nascent pinosome, which in turn would initially drive its early ionic movements.
Following endosome formation, the intraluminal K+ concentration changes from ∼5 mM to values in lysosomes ranging from 2 to 60 mM in a manner depending on pHlysosome (Steinberg et al., 2010; Sterea et al., 2018). In maturing endosomes, the accumulation of K+ has been shown to be dependent on cholesterol, as its depletion impairs this process (Charlton et al., 2019).
The K+ channel KCa3.1 is activated via PI(3)P and a putative regulatory subunit that is required for Ca2+ gating. In addition, KCa3.1 interacts with the phosphatase myotubularin R6 (MTMR6), which dephosphorylates PI(3)P, thereby inactivating the ion channel (Srivastava et al., 2005, 2006). Using coelomocytes of Caenorhabditis elegans, Maekawa et al. (2014) showed that the sequential dephosphorylation of phosphoinositides [in the order PI(3,4,5)P3, PI(3,4)P2, PI(3)P, PI] by phosphoinositide phosphatases, which are activated after ruffle formation, is related to the activation of KCa3.1. Before induction of macropinocytosis with EGF, KCa3.1 is enriched in “intracellular punctate structures” but not in the PM. Following EGF treatment, KCa3.1 is recruited to F-actin-positive membrane ruffles. Interestingly, exposure of coelomocytes to TRAM-34, a selective inhibitor of KCa3.1 (Panyi et al., 2006), or using a mutant KCa3.1, which is not activated by PI(3)P, impairs cellular dextran uptake. TRAM-34 treatment does not inhibit ruffle formation or actin polymerization. Interestingly, supplementation by sucrose to cells treated with TRAM-34 restores macropinocytosis, indicating that KCa3.1 contributes to macropinocytosis at least partially by regulating local osmolarity via a decrease of the intracellular K+ concentration. The authors suggest that KCa3.1 is involved in the closure of ruffles and that amiloride and TRAM-34 might be used to dissect macropinosome formation pharmacologically—amiloride inhibits the formation and TRAM-34 the closure of the ruffle (Maekawa et al., 2014).
Endosomal and lysosomal K+ concentration and Ψvesicle are also regulated by the selective K+ channel TMEM175, which in turn regulates organellar functions including fusions with each other. Via this channel, vesicular K+ concentration and Ψvesicle are hence also influenced by the cytosolic K+ concentration. The “resting” Ψlysosome is, however, also largely determined by the vesicular H+ permeability. It is predicted to be in the range of −18 mV at pH 5.0 and +12 mV at pH 4.5 when assuming a cytosolic K+ concentration of 140 mM (Cang et al., 2014, 2015).
Furthermore, large-conductance Ca2+-activated K+ channels (BK channels) are found in lysosomes. They are physically and functionally coupled to TRPML1 channels and facilitate release of Ca2+ ions, which activate further BK channels. This hyperpolarizes Ψlysosome and thus further facilitates Ca2+ release. BK overexpression has been shown to rescue abnormal lysosomal storage in cells from patients with the lysosomal storage disease Niemann–Pick’s disease (Cao et al., 2015).
Ca2+ Ions
Ca2+ ions are indispensable regulators of pinocytosis and endolysosomal functions. They induce macropinocytosis via F-actin depolymerization (Kabayama et al., 2009) and establish compensating exocytosis of large endosomes in parallel to ongoing macropinocytosis, thereby preventing cellular volume overload (Falcone et al., 2006). Furthermore, they regulate lysosomal fusion events and condensation of the luminal content (Xu and Ren, 2015).
Intravesicular H+ and Ca2+ concentrations are interdependently tied together. In 3T3 Swiss fibroblasts, after uptake of 2 mM extracellular Ca2+, the endosomal concentration rapidly drops to ∼30 μM within 3 min and further on to ∼3 μM after 20 min. This is proportionally paralleled by a drop of pHvesicle from 7.4 to 7.0 and 5.7 at the same time points. The loss of vesicular Ca2+ can be prevented if the acidification is inhibited by blocking the v-ATPase. By reducing the external Ca2+ to 200 μM, the acidification is completely suppressed. Hence, the acidification can occur only when the initial Ca2+ concentration in the endosomes is high (Gerasimenko et al., 1998; Petersen et al., 2020).
Lysosomal Ca2+ homeostasis involves a putative Ca2+/H+ exchanger and Ca2+ pumps. The relationship between luminal Ca2+ and H+ is suggested to be as follows: the lower the pHlysosome, the higher the Ca2+ concentration (Morgan et al., 2011). In macrophages, the lysosomal Ca2+ concentration is ∼500 μM and dependent on extracellular and cytosolic Ca2+ concentration as well as on pHlysosome. Alkalizing pHlumen reversibly decreases Ca2+ by shifting it into the cytosol. However, in contrast to fibroblasts, alterations of extracellular or lysosomal Ca2+ do not alter pHlysosome in macrophages (Christensen et al., 2002; Sterea et al., 2018). This model has been challenged by the finding that lysosomal stores are rather refilled pH-independently with Ca2+ via the ER in an Ins(1,4,5)P3 receptor-dependent process and via involvement of an unknown Ca2+ transport mechanism to move Ca2+ into the lysosome (Garrity et al., 2016).
The Ca2+ permeability of the endolysosomal membranes is set up by several cation channels. Among them are the transient receptor potential channel family members TPRML1, 2, and 3 (mucolipins), two-pore channels TPC1, 2, and 3, and voltage-gated Ca2+ channels (Cheng et al., 2010; Abe and Puertollano, 2011; Grimm et al., 2012; Xiong and Zhu, 2016; Clement et al., 2020; Freeman et al., 2020; Jin et al., 2020).
TRP channel of mucolipin subfamilies mainly localize to lysosomes and endosomes but are also found in the PM. The channels are activated by PI(3,5)P2. TRPML1 is inhibited by PI(4,5)P2, which is abundantly present in the PM. Hence, if residing there, it is likely to be functionally suppressed (Sun et al., 2015; Venkatachalam et al., 2015).
TRPML1 is encoded by the Mcoln1 gene and expressed in late endosomes and lysosomes. It is responsible for the enlarged vacuole formation and the lysosomal storage disease mucolipidosis IV if mutated to loss of function (OMIM entry #252650) (Lloyd-Evans et al., 2008; Grimm et al., 2012). The channel is permeable to Ca2+, Na+, K+, Fe2+, and Mg2+, and activated by PI(3,5)P2, but inhibited by PI(4,5)P2 and sphingomyelin. TRPML1 acts as a lysosomal pH regulator as it senses the pHlysosome and initiates the release of H+. Being activated at low pH, it allows for Ca2+ influx (Li et al., 2017). Accordingly, lysosomal acidification is hindered when PI(3,5)P3 generation is suppressed by inhibition or lack of PIKfyve and rescued by overexpression of TRPML1 or raising lysosomal Ca2+. However, the vacuole formation observed in PIKfyve-deficient cells is not rescued by Ca2+ or overexpressed TRPML1 (Cheng et al., 2010; Isobe et al., 2019).
PIKfyve converts PI(3)P into PI(3,5)P2 in the endocytic pathway and the enzyme Fig4 dephosphorylates it back to educt. In yeast, hyperosmotic stress leads to the rapid transient synthesis of PI(3,5)P2 via activation of PI(3)P kinase (Dove et al., 1997; Duex et al., 2006) and the TRPML1 ortholog Yvc1. Lack of PI(3,5)P2 synthesis leads to severe swelling of the endolysosomal compartment due to concentrating K+ up to ∼85 mM. This effect is relieved by inactivating mutations of the vacuolar monovalent cation/H+-antiporter Vnx1 or v-ATPase or by activating mutations of Yvc1. This identifies PI(3,5)P3 and the ion transporters regulated by it as crucial osmoregulators (Dove et al., 1997; Wilson et al., 2018). In contrast to yeast, hyperosmotic stress decreases and hypoosmotic treatment enhances PI(3,5)P2 production in monkey Cos-7 cells (Dove et al., 1997). PI(3,5)P2 regulates vacuole size in part via TRPML1 channels (Krishna et al., 2016). Hypertonicity also activates PIKfyve and its upstream regulator hVac14 in differentiated 3T3-L1 adipocytes, but not in their undifferentiated precursor cells (Sbrissa and Shisheva, 2005). In dendritic cells, TRPML1 releases lysosomal Ca2+ upon bacterial sensing, which activates the actin-based motor protein myosin II for directional migration. In addition, it induces the activation of the transcription factor EB (TFEB), which translocates to the nucleus to maintain TRPML1 expression. This TRPML1–TFEB axis results from the downregulation of macropinocytosis after bacterial sensing (Bretou et al., 2017). Moreover, TRPML1 is a lysosomal sensor of reactive oxygen species (ROS) (Zhang et al., 2016), which are known, on the one hand, to be generated during cell swelling (Gorg et al., 2013) and, on the other hand, to activate VRACs (Lambert, 2003; Varela et al., 2004; Browe and Baumgarten, 2006; Lambert et al., 2015). Such a link between ROS, endosomal function, and CVR may be mediated by ClC-3-dependent endosomal ROS production and isosmotic activation of VRAC (Matsuda et al., 2010).
Activating H-Ras mutations—known to drive macropinocytosis—elevates TRPML1 expression. In H-Ras-driven cancer cells, the channel is needed to restore PM cholesterol, which gets internalized during endocytosis. Inhibition or lack of TRPML1 causes false localization of cholesterol from the PM to endolysosomes and loss of oncogenic H-Ras from the cell surface (Jung and Venkatachalam, 2019). Hence, cells expressing oncogenic H-Ras are vulnerable to inhibition of TRPML1 (Jung and Venkatachalam, 2019; Jung et al., 2019).
TRPML2 is an osmo/mechanosensitive endolysosomal channel conductive to Ca2+, Na+, K+, and Fe2+ and also activated by PI(3,5)P2. It is involved in Arf6-regulated recycling pathway and in endolysosomal membrane trafficking by means of its sensitivity to hypotonicity. In late endosomes, TRPML2 is activated by free cytosolic ADP-ribose and eventually by nicotinic acid adenine dinucleotide phosphate (NAADP) (Dong et al., 2010; Viet et al., 2019). Mutations in TRPML2 may induce CD due to Ca2+ overload (Sun et al., 2015; Sterea et al., 2018; Chen et al., 2020a).
TRPML3 localizes to both early endosomes and endolysosomes, is Ca2+ permeable, activated by PI(3,5)P2, and inhibited by PI(4,5)P2, Na+, as well as acidic pH (Dong et al., 2010).
The two-pore channels (TPCs) are voltage-gated cation channels that function as homodimers. TPC1 localizes predominantly to the proximal endosomal system, while TPC2 is found mainly on late endosomes and lysosomes, where they act as Na+ and Ca2+ release channels. They are activated by PI(3,5)P2 (rather than NAADP) (Wang et al., 2012; Cang et al., 2013; She et al., 2019; Jin et al., 2020) and play important roles for vesicular fusion and endosomal trafficking, autophagy, nutrient sensing, protein processing, and macropinocytic virus entry (Wang et al., 2012; Bellono and Oancea, 2014; Sakurai et al., 2015; Xiong and Zhu, 2016; Castonguay et al., 2017). Inhibition or loss of TPC channels critically reduces cellular entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Ou et al., 2020) and Ebola virus (Sakurai et al., 2015; Petersen et al., 2020). TPC1 activity is high at alkaline pHvesicle and open over a wide voltage range but low at acidic pHvesicle. Also, low intracellular ATP concentration increases TPC1 opening. This leads to Na+ efflux and thus facilitates the v-ATPase and luminal acidification (Cang et al., 2014). TPC2 channels are important in organelle fusion and pH regulation. They mediate the rapid decrease of vesicular Ca2+ after uptake (Petersen et al., 2020). TPC2 inhibition or loss suppresses virus uptake (Sakurai et al., 2015; Petersen et al., 2020), increases the risk to develop non-alcoholic steatohepatitis (NASH) and fatty liver disease (NAFLD); Grimm et al., 2014, p. 707]. TPC2 is also involved in pigmentation (Bellono and Oancea, 2014; Lin et al., 2015).
Lysosomes seem also to possess voltage-gated calcium channel, which might serve to release Ca2+ upon depolarization of Ψvesicle and which are required for lysosomal fusion with endosomes and autophagosomes (Tian et al., 2015).
Vesicular Volume Regulation
Macropinosome shrinkage or swelling is accomplished by osmotically driven exchange of water between the vesicle lumen and the cytosol (VVR) (Freeman and Grinstein, 2018; Chen et al., 2020b; King and Smythe, 2020; Chadwick et al., 2021b), in adjunction with global CVR (see above). The direction of ion flux—efflux or influx—is determined by the transmembrane ion gradients, the levels of ΨPM and Ψvesicle, and the reversal potentials of the respective ions. The composition of the fluid in a nascent macropinosome initially equals the fluid in the intimate extracellular vicinity of its emergence and may be identical to the extracellular fluid. This creates a transmembrane gradient across the membrane of the macropinosome and the cytosol similar to that across the PM. Macropinosome shrinkage is therefore largely mediated by Na+ flux across the vesicle membrane. Recent studies demonstrated that Na+ efflux down its gradient from the pinosome to the cytosol is controlled by the non-selective TRPML cation channels and TPC Na+ channels (Laplante et al., 2002; Freeman et al., 2020; Chadwick et al., 2021a, b; Saric and Freeman, 2021).
In addition to Na+, also Cl– permeates through the macropinosomal membrane (Freeman and Grinstein, 2018). As described above, vesicular LRRC8 channels/LysoVRACs may play a role for the flux of anions across the vesicular membrane, and their importance in VVR has been recognized (Li et al., 2020; Osei-Owusu et al., 2021). A role for TMEM206/ASOR/PAORAC seems feasible, and an eventual functional coupling of vesicular VSOR and ASOR channels/currents, as described to occur in the PM (Nobles et al., 2004; Lambert and Oberwinkler, 2005; Kittl et al., 2019, 2020), awaits future investigation.
The efflux of Na+ and Cl– is coupled to cotransporters such as the electroneutral cation–Cl– cotransporters (SLC12A family members; carrying K+), NHEs (SLC9A family members; carrying H+), and anion exchangers (AE; SLC4A family members; carrying HCO3–) (Freeman and Grinstein, 2018). Moreover, HCO3– forms water and CO2, which exits the macropinosome as described above. The net result of these ion fluxes leads to osmotically driven vesicular water exit and shrinkage in parallel to H+ ion accumulation, thus generating small acidic late endosomes, in which the to-be-digested substances are highly concentrated (for review, see Freeman and Grinstein, 2018; Chadwick et al., 2021b).
Accordingly, macropinosome shrinkage is prevented if Na+ or Cl– is replaced by a non-permeant cation or anion. Importantly, the osmotically driven vesicle shrinkage is necessary for the vesiculation, tubulation, and scission processes, as it confers—by creating membrane wrinkles—the appropriate curvature necessary to recruit and stabilize the protein complexes required for proper sorting and recycling of the vesicles and their cargo, e.g., anchor sites for microtubule-associated motors, branched actin generation, or cargo recognition proteins. The latter are composed of the sorting nexins (SNXs), which contain a Bin-amphiphysin-Rvs (BAR) domain. BAR domains are able to electrostatically interact with phospholipids of appropriately bent concave membrane stretches. Also, proteins of the ESCRT complexes take advantage of the slack membrane to induce inwardly budding intraluminal vesicles (ILVs) and further on MVBs. Thus, the vesicular shrinkage creates organelles with high surface-to-volume ratios, relieves the hydrostatic tension of their membranes, and is hence critical for their resolution (for review, see Freeman and Grinstein, 2018; Chen et al., 2020b; Saric and Freeman, 2020, 2021; Chadwick et al., 2021a, b).
As pointed out above, the role of and function of AQPs in VVR is still elusive. A clue about their usage may come from observations in T. cruzi epimastigotes. They respond to cell swelling with trafficking and fusion of acidocalcisomes, which contain osmolytes and the AQP, TcAQP1, to the so-called bladder of the contractile vacuole complex, thereby loading their content to it. This enables the bladder to take up and store excess cellular water, whereby it swells by the aid of TcAQP1. Subsequently, the water is expelled to the extracellular environment, thus driving RVD (reviewed in Docampo et al., 2011, 2013). The basic mechanistic principle of this mechanism—soak up, store, and release excess intracellular water to/from intracellular organelles—is somehow recapitulated in Cos1 cells in response to a hypotonicity as described above (Li et al., 2020). It will be interesting to find out whether this principle of linking CVR and VVR is a more general one and if it might function in other cells as well.
Methuosis
Regulated CD in response to perturbation of the extracellular or intracellular milieu is an essential hallmark of multicellular organisms and is mediated mainly by apoptotic and necrotic phenotypes (Galluzzi et al., 2018). Methuosis is a non-apoptotic CD phenotype characterized by large and lucent vacuoles, which are limited by a single membrane as well as by cell swelling and, finally, rupture of the PM (Maltese and Overmeyer, 2014). Currently, methuosis is seen as a dysfunctional pinocytosis. Although Lewis first described macropinocytosis already in 1937 (Lewis, 1937), the fatal consequences for the cell by “drinking too much” have been described by Overmeyer et al. (2008) several decades later. While macropinocytosis is associated with vesicle shrinkage during processing of the vesicle content, methuosis reflects the abnormal growth of pinosomes. Despite that cell vacuolization is a common feature in many diseases, such as the lysosomal storage disease Gaucher’s disease or in microglia in Alzheimer’s disease, it is still uncertain whether methuosis plays a critical role in the etiology of these diseases (Kruth et al., 2005; Rappaport et al., 2016). There is also evidence that methuosis may be an eventual CD modality outcome of cell senescence (Adjemian et al., 2020).
H-Ras is required for macropinocytosis, and oncogenic mutants of a Ras allele are associated with methuosis. Chi et al. (1999) tested the assumption that the persistent activation of an oncogenic mutant of a Ras allele induces CD on those malignant tumors, which in general do not show an expression of Ras alleles. They found that transfection of oncogenic H-Ras (RasG12V) in human malignant glioma cells and human gastric cancer cell lines causes CD associated with intense vacuolization (Chi et al., 1999). These authors concluded that H-Ras expression triggers a type-2 CD, meaning that, in this condition, autophagy leads to CD. Overmeyer et al. (2008) replicated, confirmed, and extended these findings, but they excluded a type-2 form of CD. As in the study of Chi et al. (1999), they used a human glioblastoma cell line and induced the expression of Ras. Within a few days, Ras-expressing cells revealed lucent cytoplasmic vacuoles in phase-contrast microscopy, which did contain neither cell organelles nor cytoplasmic components when analyzed using electron microscopy. Furthermore, electron microscopy revealed that the vacuoles are limited by a single and not by a double membrane, as would be distinctive for autophagy. Fluid-phase traces, like Lucifer yellow or dextran-Alexa Fluor 488, accumulate in large vacuoles, whereas transferrin-Alexa Fluor 594 labels small endosomes, which contain—in contrast to pinosomes—transferrin receptors. Furthermore, the small endosomes are decorated with clathrin, whereas large vacuoles do not contain a clathrin coat. In contrast to functional pinocytosis, large vacuoles are not acidic. Finally, the cells detach from the surface and swell, and the PM ruptures. Because of the similarities with pinocytosis, but the fatal consequences of fluid uptake, the authors suggest that this is a dysfunctional pinocytosis, which leads to a distinct form of CD. Accordingly, Overmeyer et al. (2008) named this form of CD methuosis, derived from the Greek word methuo, meaning “to drink to intoxication.”
In normal macropinocytosis, nascent macropinosomes dynamically engage with cytoskeletal elements and either are tagged for recycling or finally fuse with lysosomes. With methuosis, however, vesicles fuse with each other to form large vacuoles, leading to the presumption that methuosis is a dysfunctional pinocytosis (Maltese and Overmeyer, 2014). In molecular–biological terms, methuosis shows its relationship to macropinocytosis by its dependence on H-Ras. Despite having the late endosome markers, LAMP1 and Rab7, vacuoles are not acidic, as demonstrated by their failure to accumulate acridine orange and LysoTracker. Interestingly, inhibition of v-ATPase by bafilomycin A1 also inhibits vacuolization (Maltese and Overmeyer, 2014). An important aspect in macropinocytosis is shrinkage of the vesicles via osmoregulatory processes (see above). Ras and phosphoinositides are required for the activation of a variety of ion channels, including those involved in vesicle shrinkage and CVR (see above) (Ritter and Woll, 1996). Assuming that formation of large vacuoles is not only due to fusion of pinosomes but also related to organelle swelling, an imbalance in the signaling cascade from Ras to ion channels could play a key role in methuosis. In line with the crucial role of NHE1 in macropinocytosis is the observation that methuosis induced by an ursolic acid-derived small molecule, compound 17, has been shown to be prevented by EIPA (Sun et al., 2017). As outlined above, H-RasG12V-expressing cells experience to shift of CVR toward higher volumes and also the intracellular vesicles have more alkaline pH. Inducers of methuosis and formation of extremely enlarged vacuoles are given in Table 2.

Table 2. Inducers of methuosis, cell death associated with aberrant vacuolization, and formation of strongly enlarged vacuoles.
Figure 3 highlights major cellular aspects of the interrelation of (macro)pinocytosis, CVR, and VVR as discussed in this review.
Perspectives
Understanding the mechanisms of pinocytosis and how it exerts its cell protective effects is critical to appreciate its diversity of physiological functions. It is also necessary to understand its dysfunctions and how they might lead to disease as well as to unravel how influencing pinocytosis may translate to potential applications for medical and other use.
For instance, designing of compounds like proteolytically stable peptidomimetics that are taken up by macropinocytosis, but which are able to escape from endosomes, could be used for intracellular biomolecular targeting (Yoo et al., 2020). Likewise, development of versatile drug carriers with a high loading capacity, such as nanoparticles, which are optimized for specific binding to cell surface receptors, and which are favoring both macropinocytic uptake and intracellular release by degradation of their shells, may open new ways for, e.g., cancer therapy. This principle was proven for hyaluronic acid-modified polymeric biodegradable mesoporous silica nanoparticles (Palanikumar et al., 2018).
Cancer cells frequently depend on autophagy to support their metabolic and energetic demands. However, inhibition of autophagy leads to compensatory stimulation of macropinocytosis to ensure cellular nutrient supply. This switch depends on the transcription factor NRF2, which is recruited to promoter regions of macropinocytosis-related genes. Recently, it has been shown that dual inhibition of autophagy and macropinocytosis is a successful strategy in treating mice with pancreatic ductal adenocarcinomas. Advancing this strategy to clinical applications may be promising for cancer therapy (Staff, 2021; Su et al., 2021).
Cell death by methuosis should meet special attention. The formation of huge cellular vacuoles, which eventually leads to cell death, is a frequently observed phenomenon in numerous pathologies and known for decades (Cameron, 1952; Henics and Wheatley, 1999). However, in many studies, the origin and mechanisms of the development of such cellular vacuoles were not or could not be determined. The term methuosis was coined in 2008 (Overmeyer et al., 2008). Hence, it will be necessary to carefully scrutinize the available body of literature for work describing methuosis-like phenomena and to eventually reevaluate it for better understanding the underlying mechanisms of deadly vacuolization and related types of cell death (Chi et al., 1999).
Recent studies indicate that volume regulation in cells and cell organelles is governed by the same biophysical principles and by the overlapping use of ion channels and transporters in distinct cellular compartments (Saric and Freeman, 2020, 2021; Chadwick et al., 2021a, b). That is, it starts with the asymmetric distribution of ions and materializes in the well-orchestrated uptake and processing of engulfed material in specialized vesicles (Chadwick et al., 2021a).
However, the contribution of inorganic ions may go beyond volume regulation. Inflammation and tissue injury are associated with a local extracellular increase in Ca2+ and enhancement of macropinocytosis in antigen-presenting cells (Canton, 2018). Presumably, the extracellular Ca2+ concentration is sensed by the calcium-sensing receptor (CaSR), which is expressed in myeloid cells (Canton, 2018). Accordingly, change in the extracellular ion composition may be critical in antigen processing and presentation via major histocompatibility complexes (MHCs) as well as recognition of non-self-antigens or modified endogenous substances by pattern recognition receptors (PRRs) (Canton, 2018). Consequently, failure in proper pinocytosis due to the formation of large vesicles could impair degradation of organic compounds or its delivery to PRRs or MHCs, which may have detrimental consequences in the immune response. The studies on CVR and VVR, pinocytotic activity, and delivery of compounds to MHCs or PRRs exemplify the power of ion gradients to understand physiology and pathophysiology of pinocytosis and methuosis, respectively. Despite Ca2+, other inorganic ions may have comparable dual effects on volume regulation and delivering of ligands to MHCs or PRRs. Thus, identification of ion channels and transporters required for establishing ion gradients could lead to a more detailed understanding of the complex interaction between distinct inorganic ions and cellular responses to tissue injuries or inflammation.
Beyond that, targeting specific ion transport mechanisms or their regulators will help to combat cellular entry of pathogens such as viruses or bacteria. For example, inhibition of PIKfyve, TPCs, NHEs, or the v-ATPase could help prevent cellular infection by Ebola virus, SARS-CoV-2, and other viruses (Mercer and Helenius, 2009; Sakurai et al., 2015; Kang et al., 2020; Mercer et al., 2020; Petersen et al., 2020; Spix et al., 2020; Eaton et al., 2021).
The number of compounds and materials that can induce or inhibit methuosis in various cell types and organisms is growing, and the fields of their use are just emerging. It will be necessary to unravel not only their specific modes of actions but also their harmful effects, as they might be used for a broad variety of applications, e.g., for eco-friendly biological pest control in agriculture to overcome multidrug resistance of parasites (Bogner et al., 2017; Rajasekharan and Lee, 2020; Rajasekharan et al., 2020). Importantly, they may emerge as new treatment options for various diseases, e.g., cancer (Song S. et al., 2020; Xiao et al., 2021), ALS, or dementia (Fomin et al., 2018; Fomin, 2019).
Author Contributions
HK conceptualized the manuscript. HK, MR, and NB wrote and finalized the manuscript. NB captured and arranged the images displayed in Figure 1 and the accompanying Supplementary Video 1. MR prepared Tables 1, 2, and Figures 2, 3. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.651982/full#supplementary-material
Supplementary Video 1 | Pinocytosis occurring in a multinucleated giant cell from a rat non-parenchymal hepatic cell line putatively representing immortalized monocytes (Kupffer cells). For explanations, see Figure 1 and text.
Supplementary Video 2 | Close-up of Supplementary Video 1.
Abbreviations
AA, arachidonic acid; Akt1, AKT serine/threonine kinase 1; ALS2, amyotrophic lateral sclerosis 2; ANO, anoctamin; APPL1, adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1; AQP, aquaporin; Arf6, ADP-ribosylation factor 6; ARNO, ARF nucleotide binding-site opener; Arp2/3, actin-related protein 2/3; ASIC, acid-sensing ion channel; ALS2, alsin Rho guanine nucleotide exchange factor; ASOR, acid-activated outwardly rectifying chloride channel; ATP, adenosine triphosphate; AVD, apoptotic volume decrease; AVP, arginine vasopressin; BK channel, big-conductance Ca2+-activated K+ channel; CA, carbonic anhydrase; Ca2+i, intracellular Ca2+ concentration; CaMK, Ca2+/calmodulin-dependent kinase; Cav, voltage-dependent calcium channel; Cav-1, caveolin-1; CD, cell death; CK, casein kinase; Cdc42, cell division control protein 42 homolog; CFTR, cystic fibrosis transmembrane conductance regulator; ClC, voltage-dependent chloride channel/exchanger; CLIC, chloride intracellular channel; 3Cpro, 3C protease of human hepatitis A virus; CV, cell volume; CVR, cell volume regulation; EFA6, ARF6 guanine nucleotide exchange factor; ECF, extracellular fluid; EDRF, endothelium-derived relaxing factor; EGF, epidermal growth factor; EGFR, epdermal growth factor receptor; EIPA, 5-(N-ethyl-N-isopropyl)-amiloride; ENaC, epithelial Na+ channel; EET, 5 ′ -6 ′ -epoxyeicosatrienoic acid; ERK, extracellular signal-regulated kinase; Fig4, factor-induced gene 4; FRET, Förster/fluorescence resonance energy transfer; GAP, GTPase activating protein; GB, glioblastoma; GBM, glioblastoma multiforme; GEF, guanine nucleotide exchange factor; GluCL, glutamate-gated chloride channel; GLUT, glucose transporter; GPCR, see GPR; GPHR, Golgi pH regulator; GPR, G protein-coupled receptor; Grb2, growth factor receptor-bound protein 2; HLEK, human limbal keratinocytes; HGF, hepatocyte growth factor; hTCEpi, human limbal-derived epithelial cell line; HUVEC, human umbilical vein endothelial cell; ICF, intracellular fluid; IClswell, swelling-activated Cl– current; IGF-1, insulin like growth factor 1; IPMK, inositol polyphosphate multikinase; INO1, inositol-3-phosphate synthase; IMPase, inositol-1(or4)-monophosphatase; Ins(1,4,5)P3 or IP3, inositol 1,4,5-trisphosphate; IP, inositolphosphate; IP3K, inositol-trisphosphate 3-kinase; IP3R, IP3 receptor; IPP, inositol-1,4 biphosphate 1-phosphatase; INFγ, interferon gamma; INPP5, inositol-polyphosphate 5-phosphatase; INPase, inositol-phosphate phosphatase; INPP, inositol-1,4-bisphosphate 1-phosphatase; INPP5, inositol polyphosphate 5 phosphatase; IPPK, inositol-pentakisphosphate 2-kinase; ITPK, inositol 1,3,4-trisphosphate 5/6 kinase, acts also as inositol polyphosphate phosphatase; Kv, voltage-gated potassium channel; LPS, lipopolysaccharide; LTD4, leukotriene D4; mAB, monoclonal antibody; MAPK, mitogen-activated protein kinase; M-CSF, macrophage colony-stimulating factor; MHC, major histocompatibility complex; MINPP, multiple inositol-polyphosphate phosphatase; MIPP, 3-(2-methyl-1H indol-3-yl)-1-(4-pyridinyl)-2-propen-1-one; miR, microRNA; MKK4, mitogen-activated protein kinase kinase 4; MLC1, megalencephalic leukoencephalopathy with subcortical cysts 1; MOMIPP, 3-(5-methoxy, 2-methyl-1H-indol-3-yl)-1-(4-pyridinyl)-2-propen-1-one; MTM1, myotubularin 1; MTMR, myotubularin-related protein; MTMR6, myotubularin R6; mTOR, mammalian target of rapamycin; MVB, multi-vesicular body; NAADP, nicotinic acid adenine dinucleotide phosphate; NCX, Na+/Ca2+-exchanger; NFAT5, nuclear factor of activated T cells 5; NGF, nerve growth factor; NOSTRIN, nitric oxide synthase trafficking inducer; NRF2, nuclear factor erythroid 2-related factor 2; NVI, necrotic volume increase; OCR, Lowe oculocerebrorenal syndrome protein; Orai, named after the keepers of the gates of heaven in Greek mythology, synonym: CRACM, calcium release-activated calcium channel protein; OMIM, Online Mendelian Inheritance in Man®; Ostm1, osteoclastogenesis-associated transmembrane protein; p53, tumor protein P53 or cellular tumor antigen p53; Pak, p21-activated kinase; Panx1, pannexin 1; PDGF, platelet-derived growth factor; PDK1, 3-phosphoinositide-dependent protein kinase 1; PEG, polyethyleneglycol; Pf, osmotic water permeability; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6 biphosphatase 3; PH, pleckstrin homology; pHi, intracellular pH; PI, phosphoinositide or phosphatidylinositol; PI(3)P, phosphatidylinositol 3-phosphate; PI(3,4,5)P3, phosphatidylinositol (3,4,5)-trisphosphate; PI(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PI3K, phosphatidylinositol 3 kinase; PIKfyve, phosphoinositide kinase FYVE-type zinc finger containing; PKB, protein kinase B; PLA2, phospholipase A2; PLC, phospholipase C; PM, plasma membrane; PS, phosphatidylserine; PRR, pattern recognition receptor; PTEN, phosphatase and tensin homolog; P2X-R, ATP-gated P2X receptor cation channel; RA, retinoic acid; Rab, Ras-related in brain; Rac, Ras-related C3 botulinum toxin substrate; RACK1, receptor for activated C kinase 1; Raf1, rapidly accelerated fibrosarcoma 1; Rap1, Ras-proximate-1/Ras-related protein 1; Ras, rat sarcoma; Rasal, Ras-GTPase–activating-like protein; RGBARG, RCC1-RhoGEF-BAR-and-RasGAP-containing protein; Rheb, Ras homolog enriched in brain; Rho, Ras homologous; RIN1, Ras and Rab interactor 1; RN-tre, Related to the N-terminus of tre; ROS, reactive oxygen species; RVD, regulatory volume decrease; RVI, regulatory volume increase; SGK, serum- and glucocorticoid-inducible protein kinase; SLC, solute carrier; SMIT, Na+/myo-inositol transporter; SOCE, store-operated calcium entry; STIM1, stromal interaction molecule 1; TFEB, transcription factor EB; TMEM, transmembrane protein; TonEBP, tonicity responsive enhancer (TonE) binding protein; TORC, target of rapamycin complex; TPC, two-pore channel; TRP, transient receptor potential; TRPML, TRP channel of mucolipin subfamily; TRPM7, Transient receptor potential cation channel subfamily M member 7; TSC, tuberous sclerosis complex; Ttyh, Tweety homolog; v-ATPase, vacuolar H+-ATPase; Vps34, vacuolar protein sorting 34; VSOR, volume-sensitive outwardly rectifying Cl– channel/current; VRAC, volume-regulated anion channel/current; VVR, vesicular volume regulation; WASP, Wiskott–Aldrich syndrome protein; WNK, with no lysine kinase
References
Abe, K., and Puertollano, R. (2011). Role of TRP channels in the regulation of the endosomal pathway. Physiology 26, 14–22. doi: 10.1152/physiol.00048.2010
Abu Jawdeh, B. G., Khan, S., Deschenes, I., Hoshi, M., Goel, M., Lock, J. T., et al. (2011). Phosphoinositide binding differentially regulates NHE1 Na+/H+ exchanger-dependent proximal tubule cell survival. J. Biol. Chem. 286, 42435–42445. doi: 10.1074/jbc.m110.212845
Adjemian, S., Oltean, T., Martens, S., Wiernicki, B., Goossens, V., Vanden Berghe, T., et al. (2020). Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis. 11:1003.
Ahlstedt, J., Förnvik, K., Zolfaghari, S., Kwak, D., Hammarström, L. G. J., Ernfors, P., et al. (2018). Evaluating vacquinol-1 in rats carrying glioblastoma models RG2 and NS1. Oncotarget 9, 8391–8399. doi: 10.18632/oncotarget.23842
Alexander, S. P., Kelly, E., Marrion, N. V., Peters, J. A., Faccenda, E., Harding, S. D., et al. (2017). THE concise guide to pharmacology 2017/18: overview. Br. J. Pharmacol. 174(Suppl. 1), S1–S16.
Alghanem, A. F., Abello, J., Maurer, J. M., Kumar, A., Ta, C. M., Gunasekar, S. K., et al. (2021). The SWELL1-LRRC8 complex regulates endothelial AKT-eNOS signaling and vascular function. eLife 10:e61313.
Alonso-Curbelo, D., Osterloh, L., Canon, E., Calvo, T. G., Martinez-Herranz, R., Karras, P., et al. (2015). RAB7 counteracts PI3K-driven macropinocytosis activated at early stages of melanoma development. Oncotarget 6, 11848–11862. doi: 10.18632/oncotarget.4055
Alvarez-Leefmans, F. J., Gamino, S. M., and Reuss, L. (1992). Cell volume changes upon sodium pump inhibition in Helix aspersa neurones. J. Physiol. 458, 603–619. doi: 10.1113/jphysiol.1992.sp019436
Amyere, M., Mettlen, M., Van Der Smissen, P., Platek, A., Payrastre, B., Veithen, A., et al. (2002). Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int. J. Med. Microbiol. 291, 487–494. doi: 10.1078/1438-4221-00157
Amyere, M., Payrastre, B., Krause, U., Van Der Smissen, P., Veithen, A., and Courtoy, P. J. (2000). Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol. Biol. Cell. 11, 3453–3467. doi: 10.1091/mbc.11.10.3453
Anbari, M., Root, K. V., and Van Dyke, R. W. (1994). Role of Na,K-ATPase in regulating acidification of early rat liver endocytic vesicles. Hepatology 19, 1034–1043. doi: 10.1016/0270-9139(94)90306-9
Araki, N., Egami, Y., Watanabe, Y., and Hatae, T. (2007). Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp. Cell. Res. 313, 1496–1507. doi: 10.1016/j.yexcr.2007.02.012
Araki, N., Johnson, M. T., and Swanson, J. A. (1996). A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135, 1249–1260. doi: 10.1083/jcb.135.5.1249
Astaburuaga, R., Quintanar Haro, O. D., Stauber, T., and Relogio, A. (2019). A mathematical model of lysosomal ion homeostasis points to differential effects of Cl(-) transport in Ca(2+) dynamics. Cells 8:1263. doi: 10.3390/cells8101263
Ayee, M. A. A., Lemaster, E., Teng, T., Lee, J., and Levitan, I. (2018). Hypotonic challenge of endothelial cells increases membrane stiffness with no effect on tether force. Biophys. J. 114, 929–938. doi: 10.1016/j.bpj.2017.12.032
Bakker-Grunwald, T., Keller, F., and Trissl, D. (1986). Effects of amiloride on Na+ content and pinocytosis in Entamoeba histolytica. Biochim. Biophys. Acta Biomembr. 854, 265–269. doi: 10.1016/0005-2736(86)90119-7
Balla, T. (2013). Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137. doi: 10.1152/physrev.00028.2012
Bannunah, A. M., Vllasaliu, D., Lord, J., and Stolnik, S. (2014). Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: effect of size and surface charge. Molecular Pharmaceutics 11, 4363–4373. doi: 10.1021/mp500439c
Baumgartner, M., Patel, H., and Barber, D. L. (2004). Na(+)/H(+) exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am. J. Physiol. Cell Physiol. 287, C844–C850.
Becker, H. M., Klier, M., and Deitmer, J. W. (2014). “Carbonic anhydrases and their interplay with acid/base-coupled membrane transporters,” in Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications, eds S. C. Frost and R. Mckenna (Dordrecht: Springer), 105–134. doi: 10.1007/978-94-007-7359-2_7
Bellono, N. W., and Oancea, E. V. (2014). Ion transport in pigmentation. Arch. Biochem. Biophys. 563, 35–41. doi: 10.1016/j.abb.2014.06.020
Ben-Dov, N., and Korenstein, R. (2012). Enhancement of cell membrane invaginations, vesiculation and uptake of macromolecules by protonation of the cell surface. PLoS One 7:e35204. doi: 10.1371/journal.pone.0035204
Berridge, M. J. (2009). Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 1793, 933–940. doi: 10.1016/j.bbamcr.2008.10.005
Bertelli, S., Remigante, A., Zuccolini, P., Barbieri, R., Ferrera, L., Picco, C., et al. (2021). Mechanisms of activation of LRRC8 volume regulated anion channels. Cell Physiol. Biochem. 55, 41–56. doi: 10.33594/000000329
Bhanot, H., Young, A. M., Overmeyer, J. H., and Maltese, W. A. (2010). Induction of nonapoptotic cell death by activated Ras requires inverse regulation of Rac1 and Arf6. Mol. Cancer Res. 8, 1358–1374. doi: 10.1158/1541-7786.mcr-10-0090
Bloomfield, G., and Kay, R. R. (2016). Uses and abuses of macropinocytosis. J. Cell Sci. 129, 2697–2705.
Bloomfield, G., Traynor, D., Sander, S. P., Veltman, D. M., Pachebat, J. A., and Kay, R. R. (2015). Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. eLife 4:e04940.
Bogner, C. W., Kamdem, R. S., Sichtermann, G., Matthaus, C., Holscher, D., Popp, J., et al. (2017). Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microb. Biotechnol. 10, 175–188. doi: 10.1111/1751-7915.12467
Bohdanowicz, M., and Grinstein, S. (2013). Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol. Rev. 93, 69–106. doi: 10.1152/physrev.00002.2012
Bortner, C. D. (2005). Apoptotic volume decrease and nitric oxide. Toxicology 208, 213–221. doi: 10.1016/j.tox.2004.11.024
Bortner, C. D., and Cidlowski, J. A. (2014). Ion channels and apoptosis in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20130104. doi: 10.1098/rstb.2013.0104
Bortner, C. D., and Cidlowski, J. A. (2020). Ions, the movement of water and the apoptotic volume decrease. Front. Cell Dev. Biol. 8:611211. doi: 10.3389/fcell.2020.611211
Bose, S., He, H., and Stauber, T. (2021). Neurodegeneration upon dysfunction of endosomal/lysosomal CLC chloride transporters. Front. Cell Dev. Biol. 9:639231. doi: 10.3389/fcell.2021.639231
Brack, E., Wachtel, M., Wolf, A., Kaech, A., Ziegler, U., and Schafer, B. W. (2020). Fenretinide induces a new form of dynamin-dependent cell death in pediatric sarcoma. Cell Death Differ. 27, 2500–2516. doi: 10.1038/s41418-020-0518-z
Brel, V., Annereau, J. P., Vispe, S., Kruczynski, A., Bailly, C., and Guilbaud, N. (2011). Cytotoxicity and cell death mechanisms induced by the polyamine-vectorized anti-cancer drug F14512 targeting topoisomerase II. Biochem. Pharmacol. 82, 1843–1852. doi: 10.1016/j.bcp.2011.08.028
Bretou, M., Saez, P. J., Sanseau, D., Maurin, M., Lankar, D., Chabaud, M., et al. (2017). Lysosome signaling controls the migration of dendritic cells. Sci. Immunol. 2:eaak9573. doi: 10.1126/sciimmunol.aak9573
Brignone, M. S., Lanciotti, A., Camerini, S., De Nuccio, C., Petrucci, T. C., Visentin, S., et al. (2015). MLC1 protein: a likely link between leukodystrophies and brain channelopathies. Front. Cell. Neurosci. 9:66. doi: 10.3389/fncel.2015.00106
Brignone, M. S., Lanciotti, A., Visentin, S., De Nuccio, C., Molinari, P., Camerini, S., et al. (2014). Megalencephalic leukoencephalopathy with subcortical cysts protein-1 modulates endosomal pH and protein trafficking in astrocytes: relevance to MLC disease pathogenesis. Neurobiol. Dis. 66, 1–18. doi: 10.1016/j.nbd.2014.02.003
Brisson, L., Driffort, V., Benoist, L., Poet, M., Counillon, L., Antelmi, E., et al. (2013). NaV1.5 Na(+) channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J. Cell Sci. 126, 4835–4842.
Brisson, L., Reshkin, S. J., Gore, J., and Roger, S. (2012). pH regulators in invadosomal functioning: proton delivery for matrix tasting. Eur. J. Cell Biol. 91, 847–860. doi: 10.1016/j.ejcb.2012.04.004
Broer, S. (2014). The SLC38 family of sodium-amino acid co-transporters. Pflugers Arch. 466, 155–172. doi: 10.1007/s00424-013-1393-y
Browe, D. M., and Baumgarten, C. M. (2006). EGFR kinase regulates volume-sensitive chloride current elicited by integrin stretch via PI-3K and NADPH oxidase in ventricular myocytes. J. Gen. Physiol. 127, 237–251. doi: 10.1085/jgp.200509366
Brown, F. D., Rozelle, A. L., Yin, H. L., Balla, T., and Donaldson, J. G. (2001). Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154, 1007–1017. doi: 10.1083/jcb.200103107
Buckley, C. M., Gopaldass, N., Bosmani, C., Johnston, S. A., Soldati, T., Insall, R. H., et al. (2016). WASH drives early recycling from macropinosomes and phagosomes to maintain surface phagocytic receptors. Proc. Natl. Acad. Sci. U.S.A. 113, E5906–E5915.
Buckley, C. M., and King, J. S. (2017). Drinking problems: mechanisms of macropinosome formation and maturation. FEBS J. 284, 3778–3790. doi: 10.1111/febs.14115
Buckley, C. M., Pots, H., Gueho, A., Vines, J. H., Munn, C. J., Phillips, B. A., et al. (2020). Coordinated Ras and Rac activity shapes macropinocytic cups and enables phagocytosis of geometrically diverse bacteria. Curr. Biol. 30, 2912.e5–2926.e5.
Busch, G., Völkl, H., Haller, T., Ritter, M., Siemen, D., Moest, J., et al. (1997). Vesicular pH is sensitive to changes in cell volume. Cell. Physiol. Biochem. 7, 25–34. doi: 10.1159/000154849
Busch, G. L., Lang, H. J., and Lang, F. (1996). Studies on the mechanism of swelling-induced lysosomal alkalinization in vascular smooth muscle cells. Pflügers Arch. 431, 690–696. doi: 10.1007/BF02253831
Busch, G. L., Schreiber, R., Dartsch, P. C., Völkl, H., Vom Dahl, S., Häussinger, D., et al. (1994). Involvement of microtubules in the link between cell volume and pH of acidic cellular compartments in rat and human hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 91, 9165–9169. doi: 10.1073/pnas.91.19.9165
Callies, C., Fels, J., Liashkovich, I., Kliche, K., Jeggle, P., Kusche-Vihrog, K., et al. (2011). Membrane potential depolarization decreases the stiffness of vascular endothelial cells. J. Cell. Sci. 124, 1936–1942. doi: 10.1242/jcs.084657
Cang, C., Aranda, K., Seo, Y. J., Gasnier, B., and Ren, D. (2015). TMEM175 is an organelle K(+) channel regulating lysosomal function. Cell 162, 1101–1112. doi: 10.1016/j.cell.2015.08.002
Cang, C., Bekele, B., and Ren, D. (2014). The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol. 10, 463–469. doi: 10.1038/nchembio.1522
Cang, C., Zhou, Y., Navarro, B., Seo, Y. J., Aranda, K., Shi, L., et al. (2013). mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 152, 778–790. doi: 10.1016/j.cell.2013.01.023
Canton, J. (2018). Macropinocytosis: new insights into its underappreciated role in innate immune cell surveillance. Front. Immunol. 9:2286. doi: 10.3389/fimmu.2018.02286
Cao, Q., Zhong, X. Z., Zou, Y., Zhang, Z., Toro, L., and Dong, X. P. (2015). BK channels alleviate lysosomal storage diseases by providing positive feedback regulation of lysosomal Ca2+ release. Dev. Cell. 33, 427–441. doi: 10.1016/j.devcel.2015.04.010
Capdevila-Nortes, X., Lopez-Hernandez, T., Apaja, P. M., Lopez De Heredia, M., Sirisi, S., Callejo, G., et al. (2013). Insights into MLC pathogenesis: glialCAM is an MLC1 chaperone required for proper activation of volume-regulated anion currents. Hum. Mol. Genet. 22, 4405–4416. doi: 10.1093/hmg/ddt290
Castellano, E., and Downward, J. (2011). “Role of RAS in the regulation of PI 3-kinase,” in Phosphoinositide 3-kinase in Health and Disease, eds C. Rommel, B. Vanhaesebroeck, and P. K. Vogt (Berlin: Springer), 143–169. doi: 10.1007/82_2010_56
Castellano, E., Sheridan, C., Thin, M. Z., Nye, E., Spencer-Dene, B., Diefenbacher, M. E., et al. (2013). Requirement for interaction of PI3-kinase p110alpha with RAS in lung tumor maintenance. Cancer Cell 24, 617–630. doi: 10.1016/j.ccr.2013.09.012
Castonguay, J., Orth, J. H. C., Muller, T., Sleman, F., Grimm, C., Wahl-Schott, C., et al. (2017). The two-pore channel TPC1 is required for efficient protein processing through early and recycling endosomes. Sci. Rep. 7:10038.
Centeio, R., Ousingsawat, J., Schreiber, R., and Kunzelmann, K. (2020). Ca2+ dependence of volume-regulated VRAC/LRRC8 and TMEM16A Cl– channels. Front. Cell Dev. Biol. 8:596879. doi: 10.3389/fcell.2020.596879
Chadwick, S. R., Grinstein, S., and Freeman, S. A. (2021a). From the inside out: ion fluxes at the centre of endocytic traffic. Curr. Opin. Cell Biol. 71, 77–86. doi: 10.1016/j.ceb.2021.02.006
Chadwick, S. R., Wu, J., and Freeman, S. A. (2021b). Solute transport controls membrane tension and organellar volume. Cell Physiol. Biochem. 55, 1–24. doi: 10.33594/000000318
Chakraborty, K., Leung, K., and Krishnan, Y. (2017). High lumenal chloride in the lysosome is critical for lysosome function. eLife 6:e28862.
Chalmin, F., Ladoire, S., Mignot, G., Vincent, J., Bruchard, M., Remy-Martin, J. P., et al. (2010). Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 120, 457–471.
Chang, H. Y., Huang, T. C., Chen, N. N., Huang, H. C., and Juan, H. F. (2014). Combination therapy targeting ectopic ATP synthase and 26S proteasome induces ER stress in breast cancer cells. Cell Death Dis. 5:e1540. doi: 10.1038/cddis.2014.504
Charlton, F. W., Hover, S., Fuller, J., Hewson, R., Fontana, J., Barr, J. N., et al. (2019). Cellular cholesterol abundance regulates potassium accumulation within endosomes and is an important determinant in bunyavirus entry. J. Biol. Chem. 294, 7335–7347. doi: 10.1074/jbc.ra119.007618
Chaurra, A., Gutzman, B. M., Taylor, E., Ackroyd, P. C., and Christensen, K. A. (2011). Lucifer Yellow as a live cell fluorescent probe for imaging water transport in subcellular organelles. Appl. Spectrosc. 65, 20–25. doi: 10.1366/10-06095
Chaurra-Arboleda, A. M. C. (2009). Development of A Fluorescent Probe for Determination of Water Transport in Subcellular Organelles. Clemson: Clemson University.
Chen, C. C., Krogsaeter, E., Butz, E. S., Li, Y., Puertollano, R., Wahl-Schott, C., et al. (2020a). TRPML2 is an osmo/mechanosensitive cation channel in endolysosomal organelles. Sci. Adv. 6:eabb5064. doi: 10.1126/sciadv.abb5064
Chen, C. C., Krogsaeter, E., and Grimm, C. (2020b). Two-pore and TRP cation channels in endolysosomal osmo-/mechanosensation and volume regulation. Biochim. Biophys. Acta Mol. Cell. Res. 1868:118921. doi: 10.1016/j.bbamcr.2020.118921
Chen, C. L., Wang, Y., Sesaki, H., and Iijima, M. (2012). Myosin I links PIP3 signaling to remodeling of the actin cytoskeleton in chemotaxis. Sci. Signal. 5:ra10. doi: 10.1126/scisignal.2002446
Chen, L., Konig, B., Liu, T., Pervaiz, S., Razzaque, Y. S., and Stauber, T. (2019). More than just a pressure relief valve: physiological roles of volume-regulated LRRC8 anion channels. Biol. Chem. 400, 1481–1496. doi: 10.1515/hsz-2019-0189
Chen, L., Wang, L., Zhu, L., Nie, S., Zhang, J., Zhong, P., et al. (2002). Cell cycle-dependent expression of volume-activated chloride currents in nasopharyngeal carcinoma cells. Am. J. Physiol. Cell. Physiol. 283, C1313–C1323.
Chen, Z., Zimnicka, A. M., Jiang, Y., Sharma, T., Chen, S., Lazarov, O., et al. (2018). Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol. Biol. Cell. 29, 1190–1202. doi: 10.1091/mbc.e17-01-0049
Cheng, X., Shen, D., Samie, M., and Xu, H. (2010). Mucolipins: intracellular TRPML1-3 channels. FEBS Lett. 584, 2013–2021. doi: 10.1016/j.febslet.2009.12.056
Cherfils, J., and Zeghouf, M. (2013). Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309. doi: 10.1152/physrev.00003.2012
Chi, S., Kitanaka, C., Noguchi, K., Mochizuki, T., Nagashima, Y., Shirouzu, M., et al. (1999). Oncogenic Ras triggers cell suicide through the activation of a caspase-independent cell death program in human cancer cells. Oncogene 18, 2281–2290. doi: 10.1038/sj.onc.1202538
Chiasson-Mackenzie, C., Morris, Z. S., Liu, C. H., Bradford, W. B., Koorman, T., and Mcclatchey, A. I. (2018). Merlin/ERM proteins regulate growth factor-induced macropinocytosis and receptor recycling by organizing the plasma membrane:cytoskeleton interface. Genes Dev. 32, 1201–1214. doi: 10.1101/gad.317354.118
Cho, H., Geno, E., Patoor, M., Reid, A., Mcdonald, R., Hild, M., et al. (2018). Indolyl-pyridinyl-propenone-induced methuosis through the inhibition of PIKFYVE. ACS Omega 3, 6097–6103. doi: 10.1021/acsomega.8b00202
Cho, S.-J., and Jena, B. P. (2006). “Secretory vesicle swelling by atomic force microscopy,” in Cell Imaging Techniques: Methods and Protocols, eds D. J. Taatjes and B. T. Mossman (Totowa, NJ: Humana Press), 317–330. doi: 10.1007/978-1-59259-993-6_16
Choi, J., Kim, H., Bae, Y. K., and Cheong, H. (2017). REP1 modulates autophagy and macropinocytosis to enhance cancer cell survival. Int. J. Mol. Sci. 18:1866. doi: 10.3390/ijms18091866
Choy, C. H., Saffi, G., Gray, M. A., Wallace, C., Dayam, R. M., Ou, Z. A., et al. (2018). Lysosome enlargement during inhibition of the lipid kinase PIKfyve proceeds through lysosome coalescence. J. Cell Sci. 131:jcs213587.
Christensen, K. A., Myers, J. T., and Swanson, J. A. (2002). pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 115, 599–607. doi: 10.1242/jcs.115.3.599
Christoforidis, S., Miaczynska, M., Ashman, K., Wilm, M., Zhao, L., Yip, S. C., et al. (1999). Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1, 249–252. doi: 10.1038/12075
Cigić, B., and Pain, R. H. (1999). Location of the binding site for chloride ion activation of cathepsin C. Eur. J. Biochem. 264, 944–951. doi: 10.1046/j.1432-1327.1999.00697.x
Cingolani, F., Simbari, F., Abad, J. L., Casasampere, M., Fabrias, G., Futerman, A. H., et al. (2017). Jaspine B induces nonapoptotic cell death in gastric cancer cells independently of its inhibition of ceramide synthase. J. Lipid Res. 58, 1500–1513. doi: 10.1194/jlr.m072611
Clement, D., Goodridge, J. P., Grimm, C., Patel, S., and Malmberg, K. J. (2020). TRP channels as interior designers: remodeling the endolysosomal compartment in natural killer cells. Front. Immunol. 11:753. doi: 10.3389/fimmu.2020.00753
Cohen, B. E. (2018). Membrane thickness as a key factor contributing to the activation of osmosensors and essential ras signaling pathways. Front. Cell Dev. Biol. 6:76. doi: 10.3389/fcell.2018.00076
Cohn, Z. A., and Parks, E. (1967). The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J. Exp. Med. 125, 213–232. doi: 10.1084/jem.125.2.213
Colin, M., Delporte, C., Janky, R., Lechon, A. S., Renard, G., Van Antwerpen, P., et al. (2019). Dysregulation of macropinocytosis processes in glioblastomas may be exploited to increase intracellular anti-cancer drug levels: the example of temozolomide. Cancers 11:411. doi: 10.3390/cancers11030411
Collins, M. P., and Forgac, M. (2020). Regulation and function of V-ATPases in physiology and disease. Biochim. Biophys. Acta Biomembr. 1862:183341. doi: 10.1016/j.bbamem.2020.183341
Commisso, C. (2019). The pervasiveness of macropinocytosis in oncological malignancies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180153. doi: 10.1098/rstb.2018.0153
Commisso, C., Davidson, S. M., Soydaner-Azeloglu, R. G., Parker, S. J., Kamphorst, J. J., Hackett, S., et al. (2013). Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637. doi: 10.1038/nature12138
Compton, L. M., Ikonomov, O. C., Sbrissa, D., Garg, P., and Shisheva, A. (2016). Active vacuolar H+ ATPase and functional cycle of Rab5 are required for the vacuolation defect triggered by PtdIns(3,5)P2 loss under PIKfyve or Vps34 deficiency. Am. J. Physiol. Cell Physiol. 311, C366–C377.
Cullen, P. J., and Steinberg, F. (2018). To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696. doi: 10.1038/s41580-018-0053-7
Cullis, J., Siolas, D., Avanzi, A., Barui, S., Maitra, A., and Bar-Sagi, D. (2017). Macropinocytosis of Nab-paclitaxel drives macrophage activation in pancreatic cancer. Cancer Immunol. Res. 5, 182–190. doi: 10.1158/2326-6066.cir-16-0125
D’Amore, C., Moro, E., Borgo, C., Itami, K., Hirota, T., Pinna, L. A., et al. (2020). “Janus” efficacy of CX-5011: CK2 inhibition and methuosis induction by independent mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 1867:118807. doi: 10.1016/j.bbamcr.2020.118807
D’Arcy, M. S. (2019). Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 43, 582–592. doi: 10.1002/cbin.11137
Dartsch, P. C., Kolb, H. A., Beckmann, M., and Lang, F. (1994a). Morphological alterations and cytoskeletal reorganization in opossum kidney (OK) cells during osmotic swelling and volume regulation. Histochemistry 102, 69–75. doi: 10.1007/bf00271051
Dartsch, P. C., Ritter, M., Haussinger, D., and Lang, F. (1994b). Cytoskeletal reorganization in NIH 3T3 fibroblasts expressing the ras oncogene. Eur. J. Cell. Biol. 63, 316–325.
Dartsch, P. C., Ritter, M., Gschwentner, M., Lang, H. J., and Lang, F. (1995). Effects of calcium channel blockers on NIH 3T3 fibroblasts expressing the Ha-ras oncogene. Eur. J. Cell Biol. 67, 372–378.
Davis, R. J., and Czech, M. P. (1985). Amiloride directly inhibits growth factor receptor tyrosine kinase activity. J. Biol. Chem. 260, 2543–2551. doi: 10.1016/s0021-9258(18)89586-2
Day, R. E., Kitchen, P., Owen, D. S., Bland, C., Marshall, L., Conner, A. C., et al. (2014). Human aquaporins: regulators of transcellular water flow. Biochim. Biophys. Acta 1840, 1492–1506. doi: 10.1016/j.bbagen.2013.09.033
De Baey, A., and Lanzavecchia, A. (2000). The role of aquaporins in dendritic cell macropinocytosis. J. Exp. Med. 191, 743–748. doi: 10.1084/jem.191.4.743
De Los Heros, P., Pacheco-Alvarez, D., and Gamba, G. (2018). “Chapter seven - role of WNK kinases in the modulation of cell volume,” in Current Topics in Membranes, eds I. Levitane, E. Delpire, and H. Rasgado-Flores (London: Academic Press), 207–235. doi: 10.1016/bs.ctm.2018.08.002
Del Conte-Zerial, P., Brusch, L., Rink, J. C., Collinet, C., Kalaidzidis, Y., Zerial, M., et al. (2008). Membrane identity and GTPase cascades regulated by toggle and cut-out switches. Mol. Syst. Biol. 4:206. doi: 10.1038/msb.2008.45
Delpire, E., and Gagnon, K. B. (2018). Water homeostasis and cell volume maintenance and regulation. Curr. Top. Membr. 81, 3–52. doi: 10.1016/bs.ctm.2018.08.001
Demaurex, N. (2002). pH Homeostasis of cellular organelles. News Physiol. Sci. 17, 1–5. doi: 10.1152/physiologyonline.2002.17.1.1
Dendo, K., Yugawa, T., Nakahara, T., Ohno, S. I., Goshima, N., Arakawa, H., et al. (2018). Induction of non-apoptotic programmed cell death by oncogenic RAS in human epithelial cells and its suppression by MYC overexpression. Carcinogenesis 39, 202–213. doi: 10.1093/carcin/bgx124
Deng, Z., Zhao, Y., Feng, J., Zhang, J., Zhao, H., Rau, M. J., et al. (2021). Cryo-EM structure of a proton-activated chloride channel TMEM206. Sci. Adv. 7:eabe5983. doi: 10.1126/sciadv.abe5983
Desai, A. S., Hunter, M. R., and Kapustin, A. N. (2019). Using macropinocytosis for intracellular delivery of therapeutic nucleic acids to tumour cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180156. doi: 10.1098/rstb.2018.0156
Di Russo Case, E. D., Smith, J. A., Ficht, T. A., Samuel, J. E., and De Figueiredo, P. (2016). Space: a final frontier for vacuolar pathogens. Traffic 17, 461–474. doi: 10.1111/tra.12382
Ding, L., Zhang, L., Kim, M., Byzova, T., and Podrez, E. (2017). Akt3 kinase suppresses pinocytosis of low-density lipoprotein by macrophages via a novel WNK/SGK1/Cdc42 protein pathway. J. Biol. Chem. 292, 9283–9293. doi: 10.1074/jbc.m116.773739
Docampo, R., Jimenez, V., King-Keller, S., Li, Z. H., and Moreno, S. N. (2011). The role of acidocalcisomes in the stress response of Trypanosoma cruzi. Adv. Parasitol. 75, 307–324. doi: 10.1016/b978-0-12-385863-4.00014-9
Docampo, R., Jimenez, V., Lander, N., Li, Z. H., and Niyogi, S. (2013). New insights into roles of acidocalcisomes and contractile vacuole complex in osmoregulation in protists. Int. Rev. Cell. Mol. Biol. 305, 69–113. doi: 10.1016/b978-0-12-407695-2.00002-0
Dolat, L., and Spiliotis, E. T. (2016). Septins promote macropinosome maturation and traffic to the lysosome by facilitating membrane fusion. J. Cell. Biol. 214, 517–527. doi: 10.1083/jcb.201603030
Donaldson, J. G. (2019). Macropinosome formation, maturation and membrane recycling: lessons from clathrin-independent endosomal membrane systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180148. doi: 10.1098/rstb.2018.0148
Dong, X. P., Wang, X., and Xu, H. (2010). TRP channels of intracellular membranes. J. Neurochem. 113, 313–328. doi: 10.1111/j.1471-4159.2010.06626.x
Donowitz, M., Ming Tse, C., and Fuster, D. (2013). SLC9/NHE gene family, a plasma membrane and organellar family of Na(+)/H(+) exchangers. Mol. Aspects Med. 34, 236–251. doi: 10.1016/j.mam.2012.05.001
Doodnauth, S. A., Grinstein, S., and Maxson, M. E. (2019). Constitutive and stimulated macropinocytosis in macrophages: roles in immunity and in the pathogenesis of atherosclerosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180147. doi: 10.1098/rstb.2018.0147
Doroshenko, P., Sabanov, V., and Doroshenko, N. (2001). Cell cycle-related changes in regulatory volume decrease and volume-sensitive chloride conductance in mouse fibroblasts. J. Cell Physiol. 187, 65–72. doi: 10.1002/1097-4652(200104)187:1<65::aid-jcp1052>3.0.co;2-a
Dove, S. K., Cooke, F. T., Douglas, M. R., Sayers, L. G., Parker, P. J., and Michell, R. H. (1997). Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390, 187–192. doi: 10.1038/36613
Dubinsky, W. P. Jr., and Frizzell, R. A. (1983). A novel effect of amiloride on H+-dependent Na+ transport. Am. J. Physiol. 245, C157–C159.
Duex, J. E., Nau, J. J., Kauffman, E. J., and Weisman, L. S. (2006). Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot Cell 5, 723–731. doi: 10.1128/ec.5.4.723-731.2006
Eaton, A. F., Merkulova, M., and Brown, D. (2021). The H(+)-ATPase (V-ATPase): from proton pump to signaling complex in health and disease. Am. J. Physiol. Cell Physiol. 320, C392–C414.
Echevarria, M., and Verkman, A. S. (1992). Optical measurement of osmotic water transport in cultured cells. Role of glucose transporters. J. Gen. Physiol. 99, 573–589. doi: 10.1085/jgp.99.4.573
Egami, Y. (2016). Molecular imaging analysis of Rab GTPases in the regulation of phagocytosis and macropinocytosis. Anat. Sci. Int. 91, 35–42. doi: 10.1007/s12565-015-0313-y
Egami, Y., Taguchi, T., Maekawa, M., Arai, H., and Araki, N. (2014). Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation. Front. Physiol. 5:374. doi: 10.3389/fphys.2014.00374
Elorza-Vidal, X., Sirisi, S., Gaitán-Peñas, H., Pérez-Rius, C., Alonso-Gardón, M., Armand-Ugón, M., et al. (2018). GlialCAM/MLC1 modulates LRRC8/VRAC currents in an indirect manner: implications for megalencephalic leukoencephalopathy. Neurobiol. Dis. 119, 88–99. doi: 10.1016/j.nbd.2018.07.031
Falcone, S., Cocucci, E., Podini, P., Kirchhausen, T., Clementi, E., and Meldolesi, J. (2006). Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events. J. Cell Sci. 119, 4758–4769. doi: 10.1242/jcs.03238
Fan, S. H., Numata, Y., and Numata, M. (2016). Endosomal Na+/H+ exchanger NHE5 influences MET recycling and cell migration. Mol. Biol. Cell. 27, 702–715. doi: 10.1091/mbc.e15-04-0257
Faundez, V., and Hartzell, H. C. (2004). Intracellular chloride channels: determinants of function in the endosomal pathway. Sci. STKE 2004:re8. doi: 10.1126/stke.2332004re8
Feliciano, W. D., Yoshida, S., Straight, S. W., and Swanson, J. A. (2011). Coordination of the Rab5 cycle on macropinosomes. Traffic 12, 1911–1922. doi: 10.1111/j.1600-0854.2011.01280.x
Fels, J., Callies, C., Kusche-Vihrog, K., and Oberleithner, H. (2010). Nitric oxide release follows endothelial nanomechanics and not vice versa. Pflugers Arch. 460, 915–923. doi: 10.1007/s00424-010-0871-8
Fettiplace, R., and Haydon, D. A. (1980). Water permeability of lipid membranes. Physiol. Rev. 60, 510–550. doi: 10.1152/physrev.1980.60.2.510
Fischbarg, J., Kuang, K. Y., Hirsch, J., Lecuona, S., Rogozinski, L., Silverstein, S. C., et al. (1989). Evidence that the glucose transporter serves as a water channel in J774 macrophages. Proc. Natl. Acad. Sci. U.S.A. 86, 8397–8401. doi: 10.1073/pnas.86.21.8397
Fischbarg, J., and Vera, J. C. (1995). Multifunctional transporter models: lessons from the transport of water, sugars, and ring compounds by GLUTs. Am. J. Physiol. 268, C1077–C1089.
Fleischman, L. F., Chahwala, S. B., and Cantley, L. (1986). ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science 231, 407–410. doi: 10.1126/science.3001936
Fomin, V. (2019). Novel Functions of C9ORF72, A Gene Involved in ALS/FTD. New York, NY: Columbia University.
Fomin, V., Richard, P., Hoque, M., Li, C., Gu, Z., Fissore-O’leary, M., et al. (2018). The C9ORF72 gene, implicated in amyotrophic lateral sclerosis and frontotemporal dementia, encodes a protein that functions in control of endothelin and glutamate signaling. Mol. Cell Biol. 38:e00155-18.
Freedman, S. D., Kern, H. F., and Scheele, G. A. (2001). Pancreatic acinar cell dysfunction in CFTR(-/-) mice is associated with impairments in luminal pH and endocytosis. Gastroenterology 121, 950–957. doi: 10.1053/gast.2001.27992
Freeman, M. C., Peek, C. T., Becker, M. M., Smith, E. C., and Denison, M. R. (2014). Coronaviruses induce entry-independent, continuous macropinocytosis. mBio 5:e01340-14.
Freeman, S. A., and Grinstein, S. (2014). Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol. Rev. 262, 193–215. doi: 10.1111/imr.12212
Freeman, S. A., and Grinstein, S. (2018). Resolution of macropinosomes, phagosomes and autolysosomes: osmotically driven shrinkage enables tubulation and vesiculation. Traffic 19, 965–974. doi: 10.1111/tra.12614
Freeman, S. A., Uderhardt, S., Saric, A., Collins, R. F., Buckley, C. M., Mylvaganam, S., et al. (2020). Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance. Science 367, 301–305. doi: 10.1126/science.aaw9544
Fuchs, R., Schmid, S., and Mellman, I. (1989). A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc. Natl. Acad. Sci. U.S.A. 86, 539–543. doi: 10.1073/pnas.86.2.539
Furst, J., Gschwentner, M., Ritter, M., Botta, G., Jakab, M., Mayer, M., et al. (2002). Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. Pflugers Arch. 444, 1–25. doi: 10.1007/s00424-002-0805-1
Fuster, D. G., and Alexander, R. T. (2014). Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 466, 61–76. doi: 10.1007/s00424-013-1408-8
Futai, M., Sun-Wada, G. H., Wada, Y., Matsumoto, N., and Nakanishi-Matsui, M. (2019). Vacuolar-type ATPase: a proton pump to lysosomal trafficking. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 95, 261–277. doi: 10.2183/pjab.95.018
Gaitan-Penas, H., Gradogna, A., Laparra-Cuervo, L., Solsona, C., Fernandez-Duenas, V., Barrallo-Gimeno, A., et al. (2016). Investigation of LRRC8-mediated volume-regulated anion currents in Xenopus oocytes. Biophys. J. 111, 1429–1443. doi: 10.1016/j.bpj.2016.08.030
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Agostinis, P., et al. (2018). Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541.
Ganapathy, V., Thangaraju, M., Gopal, E., Martin, P. M., Itagaki, S., Miyauchi, S., et al. (2008). Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 10, 193–199.
Gao, X., Ruan, X., Ji, H., Peng, L., Qiu, Y., Yang, D., et al. (2020). Maduramicin triggers methuosis-like cell death in primary chicken myocardial cells. Toxicol. Lett. 333, 105–114. doi: 10.1016/j.toxlet.2020.07.025
Garrity, A. G., Wang, W., Collier, C. M., Levey, S. A., Gao, Q., and Xu, H. (2016). The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. eLife 5:e15887.
Gayle, S., Landrette, S., Beeharry, N., Conrad, C., Hernandez, M., Beckett, P., et al. (2017). Identification of apilimod as a first-in-class PIKfyve kinase inhibitor for treatment of B-cell non-Hodgkin lymphoma. Blood 129, 1768–1778. doi: 10.1182/blood-2016-09-736892
Gerasimenko, J. V., Tepikin, A. V., Petersen, O. H., and Gerasimenko, O. V. (1998). Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr. Biol. 8, 1335–1338. doi: 10.1016/s0960-9822(07)00565-9
Ghoshal, P., Singla, B., Lin, H., Cherian-Shaw, M., Tritz, R., Padgett, C. A., et al. (2019). Loss of GTPase activating protein neurofibromin stimulates paracrine cell communication via macropinocytosis. Redox Biol. 27:101224. doi: 10.1016/j.redox.2019.101224
Gonano, L. A., Morell, M., Burgos, J. I., Dulce, R. A., De Giusti, V. C., Aiello, E. A., et al. (2014). Hypotonic swelling promotes nitric oxide release in cardiac ventricular myocytes: impact on swelling-induced negative inotropic effect. Cardiovasc. Res. 104, 456–466. doi: 10.1093/cvr/cvu230
Gong, X., Sun, R., Gao, Z., Han, W., Liu, Y., Zhao, L., et al. (2018). Tubeimoside 1 acts as a chemotherapeutic synergist via stimulating macropinocytosis. Front. Pharmacol. 9:1044. doi: 10.3389/fphar.2018.01044
Gorg, B., Schliess, F., and Haussinger, D. (2013). Osmotic and oxidative/nitrosative stress in ammonia toxicity and hepatic encephalopathy. Arch. Biochem. Biophys. 536, 158–163. doi: 10.1016/j.abb.2013.03.010
Grimm, C., Hassan, S., Wahl-Schott, C., and Biel, M. (2012). Role of TRPML and two-pore channels in endolysosomal cation homeostasis. J. Pharmacol. Exp. Ther. 342, 236–244. doi: 10.1124/jpet.112.192880
Grimm, C., Holdt, L. M., Chen, C. C., Hassan, S., Muller, C., Jors, S., et al. (2014). High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat. Commun. 5, 4699. doi: 10.1038/ncomms5699
Gupta, S., Ramjaun, A. R., Haiko, P., Wang, Y., Warne, P. H., Nicke, B., et al. (2007). Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell 129, 957–968. doi: 10.1016/j.cell.2007.03.051
Guzman, R. E., Grieschat, M., Fahlke, C., and Alekov, A. K. (2013). ClC-3 is an intracellular chloride/proton exchanger with large voltage-dependent nonlinear capacitance. ACS Chem. Neurosci. 4, 994–1003. doi: 10.1021/cn400032z
Hackam, D. J., Rotstein, O. D., and Grinstein, S. (1999). “Phagosomal acidification mechanisms and functional significance,” in Phagocytosis: The Host, ed. S. Gordon (Mumbai: JAI), 299–319. doi: 10.1016/s1874-5172(99)80037-6
Hackam, D. J., Rotstein, O. D., Zhang, W. J., Demaurex, N., Woodside, M., Tsai, O., et al. (1997). Regulation of phagosomal acidification. Differential targeting of Na+/H+ exchangers, Na+/K+-ATPases, and vacuolar-type H+-atpases. J. Biol. Chem. 272, 29810–29820.
Hamann, S., Herrera-Perez, J. J., Zeuthen, T., and Alvarez-Leefmans, F. J. (2010). Cotransport of water by the Na+-K+-2Cl(-) cotransporter NKCC1 in mammalian epithelial cells. J. Physiol. 588, 4089–4101. doi: 10.1113/jphysiol.2010.194738
Hansen, S. B. (2015). Lipid agonism: the PIP2 paradigm of ligand-gated ion channels. Biochim. Biophys. Acta 1851, 620–628. doi: 10.1016/j.bbalip.2015.01.011
Hara-Chikuma, M., Sugiyama, Y., Kabashima, K., Sohara, E., Uchida, S., Sasaki, S., et al. (2011). Involvement of aquaporin-7 in the cutaneous primary immune response through modulation of antigen uptake and migration in dendritic cells. FASEB J. 26, 211–218. doi: 10.1096/fj.11-186627
Harl, B., Schmolzer, J., Jakab, M., Ritter, M., and Kerschbaum, H. H. (2013). Chloride channel blockers suppress formation of engulfment pseudopodia in microglial cells. Cell Physiol. Biochem. 31, 319–337. doi: 10.1159/000343370
Haussinger, D., and Schliess, F. (2005). Astrocyte swelling and protein tyrosine nitration in hepatic encephalopathy. Neurochem. Int. 47, 64–70. doi: 10.1016/j.neuint.2005.04.008
Hazama, A., Kozono, D., Guggino, W. B., Agre, P., and Yasui, M. (2002). Ion permeation of AQP6 water channel protein. Single channel recordings after Hg2+ activation. J. Biol. Chem. 277, 29224–29230. doi: 10.1074/jbc.m204258200
Henics, T., and Wheatley, D. N. (1999). Cytoplasmic vacuolation, adaptation and cell death: a view on new perspectives and features. Biol. Cell 91, 485–498. doi: 10.1016/s0248-4900(00)88205-2
Higgs, H. N., and Pollard, T. D. (2000). Activation by Cdc42 and PIP(2) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell. Biol. 150, 1311–1320. doi: 10.1083/jcb.150.6.1311
Hille, B., Dickson, E. J., Kruse, M., Vivas, O., and Suh, B. C. (2015). Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851, 844–856. doi: 10.1016/j.bbalip.2014.09.010
Hinze, C., and Boucrot, E. (2018). Local actin polymerization during endocytic carrier formation. Biochem. Soc. Trans. 46, 565–576. doi: 10.1042/bst20170355
Hoffmann, E. K., Lambert, I. H., and Pedersen, S. F. (2009). Physiology of cell volume regulation in vertebrates. Physiol. Rev. 89, 193–277. doi: 10.1152/physrev.00037.2007
Hryciw, D. H., Jenkin, K. A., Simcocks, A. C., Grinfeld, E., Mcainch, A. J., and Poronnik, P. (2012). The interaction between megalin and ClC-5 is scaffolded by the Na+–H+ exchanger regulatory factor 2 (NHERF2) in proximal tubule cells. Int. J. Biochem. Cell Biol. 44, 815–823. doi: 10.1016/j.biocel.2012.02.007
Huang, B., Wang, H., and Yang, B. (2017). “Water transport mediated by other membrane proteins,” in Aquaporins, ed. B. Yang (Dordrecht: Springer), 251–261. doi: 10.1007/978-94-024-1057-0_17
Huang, W., Sun, X., Li, Y., He, Z., Li, L., Deng, Z., et al. (2018). Discovery and identification of small molecules as methuosis inducers with in vivo antitumor activities. J. Med. Chem. 61, 5424–5434. doi: 10.1021/acs.jmedchem.8b00753
Huang, Y., and Rane, S. G. (1993). Single channel study of a Ca(2+)-activated K+ current associated with ras-induced cell transformation. J. Physiol. 461, 601–618. doi: 10.1113/jphysiol.1993.sp019531
Hughey, J. J., Wikswo, J. P., and Seale, K. T. (2007). “Intra-microfluidic pinocytic loading of human T cells,” in Proceedings of the 2007 IEEE/Nih Life Science Systems and Applications Workshop (Piscataway, NJ: IEEE), 132–135.
Hurtado-Lorenzo, A., Skinner, M., El Annan, J., Futai, M., Sun-Wada, G. H., Bourgoin, S., et al. (2006). V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 8, 124–136. doi: 10.1038/ncb1348
Ikonomov, O. C., Altankov, G., Sbrissa, D., and Shisheva, A. (2018). PIKfyve inhibitor cytotoxicity requires AKT suppression and excessive cytoplasmic vacuolation. Toxicol. Appl. Pharmacol. 356, 151–158. doi: 10.1016/j.taap.2018.08.001
Ilie, A., Gao, A. Y., Reid, J., Boucher, A., Mcewan, C., Barriere, H., et al. (2016). A Christianson syndrome-linked deletion mutation ((287)ES(288)) in SLC9A6 disrupts recycling endosomal function and elicits neurodegeneration and cell death. Mol. Neurodegener. 11:63.
Ilie, A., Weinstein, E., Boucher, A., Mckinney, R. A., and Orlowski, J. (2014). Impaired posttranslational processing and trafficking of an endosomal Na+/H+ exchanger NHE6 mutant (Delta(370)WST(372)) associated with X-linked intellectual disability and autism. Neurochem. Int. 73, 192–203. doi: 10.1016/j.neuint.2013.09.020
Imamura, J., Suzuki, Y., Gonda, K., Roy, C. N., Gatanaga, H., Ohuchi, N., et al. (2011). Single particle tracking confirms that multivalent Tat protein transduction domain-induced heparan sulfate proteoglycan cross-linkage activates Rac1 for internalization. J. Biol. Chem. 286, 10581–10592. doi: 10.1074/jbc.m110.187450
Insel, P. A., Sriram, K., Salmeron, C., and Wiley, S. Z. (2020). Proton-sensing G protein-coupled receptors: detectors of tumor acidosis and candidate drug targets. Future Med. Chem. 12, 523–532. doi: 10.4155/fmc-2019-0357
Ishibashi, K., Koike, S., Kondo, S., Hara, S., and Tanaka, Y. (2009). The role of a group III AQP, AQP11 in intracellular organelle homeostasis. J. Med. Investig. 56, 312–317. doi: 10.2152/jmi.56.312
Ishida, Y., Nayak, S., Mindell, J. A., and Grabe, M. (2013). A model of lysosomal pH regulation. J. Gen. Physiol. 141, 705–720. doi: 10.1085/jgp.201210930
Ishihara, S., Ichijo, H., and Watanabe, K. (2021). A novel lens for cell volume regulation: liquid–liquid phase separation. Cell Physiol. Biochem. 55, 135–160. doi: 10.33594/000000357
Isobe, Y., Nigorikawa, K., Tsurumi, G., Takemasu, S., Takasuga, S., Kofuji, S., et al. (2019). PIKfyve accelerates phagosome acidification through activation of TRPML1 while arrests aberrant vacuolation independent of the Ca2+ channel. J. Biochem. 165, 75–84. doi: 10.1093/jb/mvy084
Jakab, M., Furst, J., Gschwentner, M., Botta, G., Garavaglia, M. L., Bazzini, C., et al. (2002). Mechanisms sensing and modulating signals arising from cell swelling. Cell Physiol. Biochem. 12, 235–258. doi: 10.1159/000067895
Jakab, M., and Ritter, M. (2006). Cell volume regulatory ion transport in the regulation of cell migration. Contrib. Nephrol. 152, 161–180. doi: 10.1159/000096322
Jang, H., Banerjee, A., Chavan, T. S., Lu, S., Zhang, J., Gaponenko, V., et al. (2016). The higher level of complexity of K-Ras4B activation at the membrane. FASEB J. 30, 1643–1655. doi: 10.1096/fj.15-279091
Jefferies, H. B., Cooke, F. T., Jat, P., Boucheron, C., Koizumi, T., Hayakawa, M., et al. (2008). A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 9, 164–170. doi: 10.1038/sj.embor.7401155
Jena, B. P. (2020). “Aquaporin regulation: lessons from secretory vesicles,” in Aquaporin Regulation, ed. G. Litwack (New York, NY: Academic Press), 147–162. doi: 10.1016/bs.vh.2019.08.007
Jentsch, T. J. (2007). Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J. Physiol. 578, 633–640. doi: 10.1113/jphysiol.2006.124719
Jentsch, T. J. (2016). VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat. Rev. Mol. Cell Biol. 17, 293–307. doi: 10.1038/nrm.2016.29
Jentsch, T. J., Lutter, D., Planells-Cases, R., Ullrich, F., and Voss, F. K. (2016). VRAC: molecular identification as LRRC8 heteromers with differential functions. Pflügers Arch. Eur. J. Physiol. 468, 385–393. doi: 10.1007/s00424-015-1766-5
Jentsch, T. J., and Pusch, M. (2018). CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol. Rev. 98, 1493–1590. doi: 10.1152/physrev.00047.2017
Jiang, J., Kao, C. Y., and Papoutsakis, E. T. (2017). How do megakaryocytic microparticles target and deliver cargo to alter the fate of hematopoietic stem cells? J. Control. Release 247, 1–18. doi: 10.1016/j.jconrel.2016.12.021
Jiang, L. W., Maher, V. M., Mccormick, J. J., and Schindler, M. (1990). Alkalinization of the lysosomes is correlated with ras transformation of murine and human fibroblasts. J. Biol. Chem. 265, 4775–4777. doi: 10.1016/s0021-9258(19)34037-2
Jin, X., Zhang, Y., Alharbi, A., Hanbashi, A., Alhoshani, A., and Parrington, J. (2020). Targeting two-pore channels: current progress and future challenges. Trends Pharmacol. Sci. 41, 582–594. doi: 10.1016/j.tips.2020.06.002
John, S., Sivakumar, K. C., and Mishra, R. (2017). Bacoside a induces tumor cell death in human glioblastoma cell lines through catastrophic macropinocytosis. Front. Mol. Neurosci. 10:171. doi: 10.3389/fnmol.2017.00171
Josefsson, J. O. (1968). Induction and inhibition of pinocytosis in Amoeba proteus. Acta Physiol. Scand. 73, 481–490. doi: 10.1111/j.1365-201x.1968.tb10887.x
Josefsson, J.-O., Holmer, N.-G., and Hansson, S. E. (1975). Membrane potential and conductance during piocytosis induced in amoeba proteus with alkali metal lons. Acta Physiol. Scand. 94, 278–288. doi: 10.1111/j.1748-1716.1975.tb05887.x
Jung, J., Cho, K. J., Naji, A. K., Clemons, K. N., Wong, C. O., Villanueva, M., et al. (2019). HRAS-driven cancer cells are vulnerable to TRPML1 inhibition. EMBO Rep. 20:e46685.
Jung, J., and Venkatachalam, K. (2019). TRPML1 and RAS-driven cancers - exploring a link with great therapeutic potential. Channels 13, 374–381. doi: 10.1080/19336950.2019.1666457
Kabayama, H., Nakamura, T., Takeuchi, M., Iwasaki, H., Taniguchi, M., Tokushige, N., et al. (2009). Ca2+ induces macropinocytosis via F-actin depolymerization during growth cone collapse. Mol. Cell. Neurosci. 40, 27–38. doi: 10.1016/j.mcn.2008.08.009
Kang, Y. L., Chou, Y. Y., Rothlauf, P. W., Liu, Z., Soh, T. K., Cureton, D., et al. (2020). Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2. Proc. Natl. Acad. Sci. U.S.A. 117, 20803–20813. doi: 10.1073/pnas.2007837117
Karim, M. A., and Brett, C. L. (2018). The Na(+)(K(+))/H(+) exchanger Nhx1 controls multivesicular body-vacuolar lysosome fusion. Mol. Biol. Cell 29, 317–325. doi: 10.1091/mbc.e17-08-0496
Kasahara, K., Nakayama, Y., Sato, I., Ikeda, K., Hoshino, M., Endo, T., et al. (2007). Role of Src-family kinases in formation and trafficking of macropinosomes. J. Cell. Physiol. 211, 220–232. doi: 10.1002/jcp.20931
Kaul, A., Overmeyer, J. H., and Maltese, W. A. (2007). Activated Ras induces cytoplasmic vacuolation and non-apoptotic death in glioblastoma cells via novel effector pathways. Cell Signal. 19, 1034–1043. doi: 10.1016/j.cellsig.2006.11.010
Kawabe, K., Takano, K., Moriyama, M., and Nakamura, Y. (2017). Amphotericin B Increases Transglutaminase 2 Expression Associated with Upregulation of Endocytotic Activity in Mouse Microglial Cell Line BV-2. Neurochem. Res. 42, 1488–1495. doi: 10.1007/s11064-017-2205-0
Kay, A. R. (2017). How cells can control their size by pumping ions. Front. Cell Dev. Biol. 5:41. doi: 10.3389/fcell.2017.00041
Kay, A. R., and Blaustein, M. P. (2019). Evolution of our understanding of cell volume regulation by the pump-leak mechanism. J. Gen. Physiol. 151, 407–416. doi: 10.1085/jgp.201812274
Kay, R. R., Williams, T. D., Manton, J. D., Traynor, D., and Paschke, P. (2019). Living on soup: macropinocytic feeding in amoebae. Int. J. Dev. Biol. 63, 473–483. doi: 10.1387/ijdb.190220rk
Kellenberger, S., and Schild, L. (2002). Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82, 735–767. doi: 10.1152/physrev.00007.2002
Kerr, M. C., Lindsay, M. R., Luetterforst, R., Hamilton, N., Simpson, F., Parton, R. G., et al. (2006). Visualisation of macropinosome maturation by the recruitment of sorting nexins. J. Cell Sci. 119, 3967–3980. doi: 10.1242/jcs.03167
Kerr, M. C., and Teasdale, R. D. (2009). Defining macropinocytosis. Traffic 10, 364–371. doi: 10.1111/j.1600-0854.2009.00878.x
Kim, N., Kim, S., Nahm, M., Kopke, D., Kim, J., Cho, E., et al. (2019). BMP-dependent synaptic development requires Abi-Abl-Rac signaling of BMP receptor macropinocytosis. Nat. Commun. 10:684.
Kim, S. M., Nguyen, T. T., Ravi, A., Kubiniok, P., Finicle, B. T., Jayashankar, V., et al. (2018). PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov. 8, 866–883. doi: 10.1158/2159-8290.cd-17-1215
Kimura, C., Koyama, T., Oike, M., and Ito, Y. (2000). Hypotonic stress-induced NO production in endothelium depends on endogenous ATP. Biochem. Biophys. Res. Commun. 274, 736–740. doi: 10.1006/bbrc.2000.3205
King, J. S., and Kay, R. R. (2019). The origins and evolution of macropinocytosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180158. doi: 10.1098/rstb.2018.0158
King, J. S., and Smythe, E. (2020). Water loss regulates cell and vesicle volume. Science 367, 246–247. doi: 10.1126/science.aba3623
King, L. S., Kozono, D., and Agre, P. (2004). From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 5, 687–698. doi: 10.1038/nrm1469
Kissing, S., Saftig, P., and Haas, A. (2018). Vacuolar ATPase in phago(lyso)some biology. Int. J. Med. Microbiol. 308, 58–67. doi: 10.1016/j.ijmm.2017.08.007
Kitchen, P., Day, R. E., Salman, M. M., Conner, M. T., Bill, R. M., and Conner, A. C. (2015). Beyond water homeostasis: diverse functional roles of mammalian aquaporins. Biochim. Biophys. Acta 1850, 2410–2421. doi: 10.1016/j.bbagen.2015.08.023
Kittl, M., Helm, K., Beyreis, M., Mayr, C., Gaisberger, M., Winklmayr, M., et al. (2019). Acid- and volume-sensitive chloride currents in microglial cells. Int. J. Mol. Sci. 20:3475. doi: 10.3390/ijms20143475
Kittl, M., Winklmayr, M., Helm, K., Lettner, J., Gaisberger, M., Ritter, M., et al. (2020). Acid- and volume-sensitive chloride currents in human chondrocytes. Front. Cell Dev. Biol. 8:583131. doi: 10.3389/fcell.2020.583131
Klausen, T. K., Bergdahl, A., Hougaard, C., Christophersen, P., Pedersen, S. F., and Hoffmann, E. K. (2007). Cell cycle-dependent activity of the volume- and Ca2+-activated anion currents in Ehrlich lettre ascites cells. J. Cell Physiol. 210, 831–842. doi: 10.1002/jcp.20918
Koivusalo, M., Steinberg, B. E., Mason, D., and Grinstein, S. (2011). In situ measurement of the electrical potential across the lysosomal membrane using FRET. Traffic 12, 972–982. doi: 10.1111/j.1600-0854.2011.01215.x
Koivusalo, M., Welch, C., Hayashi, H., Scott, C. C., Kim, M., Alexander, T., et al. (2010). Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 188, 547–563. doi: 10.1083/jcb.200908086
Kondapalli, K. C., Prasad, H., and Rao, R. (2014). An inside job: how endosomal Na(+)/H(+) exchangers link to autism and neurological disease. Front. Cell Neurosci. 8:172. doi: 10.3389/fncel.2014.00172
Kong, X., Tang, X., Du, W., Tong, J., Yan, Y., Zheng, F., et al. (2013). Extracellular acidosis modulates the endocytosis and maturation of macrophages. Cell Immunol. 281, 44–50. doi: 10.1016/j.cellimm.2012.12.009
Konig, B., Hao, Y., Schwartz, S., Plested, A. J., and Stauber, T. (2019). A FRET sensor of C-terminal movement reveals VRAC activation by plasma membrane DAG signaling rather than ionic strength. eLife 8:e45421.
Krishna, S., Palm, W., Lee, Y., Yang, W., Bandyopadhyay, U., Xu, H., et al. (2016). PIKfyve regulates vacuole maturation and nutrient recovery following engulfment. Dev. Cell 38, 536–547. doi: 10.1016/j.devcel.2016.08.001
Kruczek, C., Gorg, B., Keitel, V., Pirev, E., Kroncke, K. D., Schliess, F., et al. (2009). Hypoosmotic swelling affects zinc homeostasis in cultured rat astrocytes. Glia 57, 79–92. doi: 10.1002/glia.20737
Kruth, H. S., Jones, N. L., Huang, W., Zhao, B., Ishii, I., Chang, J., et al. (2005). Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J. Biol. Chem. 280, 2352–2360. doi: 10.1074/jbc.m407167200
Kumar, A., Xie, L., Ta, C. M., Hinton, A. O., Gunasekar, S. K., Minerath, R. A., et al. (2020). SWELL1 regulates skeletal muscle cell size, intracellular signaling, adiposity and glucose metabolism. eLife 9:e58941.
Kunzelmann, K. (2015). TMEM16, LRRC8A, bestrophin: chloride channels controlled by Ca(2+) and cell volume. Trends Biochem. Sci. 40, 535–543. doi: 10.1016/j.tibs.2015.07.005
Kunzschughart, L., Simm, A., and Muellerklieser, W. (1995). Oncogene-associated transformation of rodent fibroblasts is accompanied by large morphologic and metabolic alterations. Oncol. Rep. 2, 651–661. doi: 10.3892/or.2.4.651
Labudda, M., Rozanska, E., Prabucka, B., Muszynska, E., Marecka, D., Kozak, M., et al. (2020). Activity profiling of barley vacuolar processing enzymes provides new insights into the plant and cyst nematode interaction. Mol. Plant Pathol. 21, 38–52. doi: 10.1111/mpp.12878
Lambert, I. H. (2003). Reactive oxygen species regulate swelling-induced taurine efflux in NIH3T3 mouse fibroblasts. J. Membr. Biol. 192, 19–32. doi: 10.1007/s00232-002-1061-1
Lambert, I. H., Kristensen, D., Holm, J. B., and Mortensen, O. H. (2015). Physiological role of taurine–from organism to organelle. Acta Physiol. 213, 191–212. doi: 10.1111/apha.12365
Lambert, S., and Oberwinkler, J. (2005). Characterization of a proton-activated, outwardly rectifying anion channel. J. Physiol. 567, 191–213. doi: 10.1113/jphysiol.2005.089888
Lanciotti, A., Brignone, M. S., Molinari, P., Visentin, S., De Nuccio, C., Macchia, G., et al. (2012). Megalencephalic leukoencephalopathy with subcortical cysts protein 1 functionally cooperates with the TRPV4 cation channel to activate the response of astrocytes to osmotic stress: dysregulation by pathological mutations. Hum. Mol. Genet. 21, 2166–2180. doi: 10.1093/hmg/dds032
Lang, F. (2007). Mechanisms and significance of cell volume regulation. J. Am. Coll. Nutr. 26, 613S–623S.
Lang, F., Böhmer, C., Palmada, M., Seebohm, G., Strutz-Seebohm, N., and Vallon, V. (2006). (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 86, 1151–1178. doi: 10.1152/physrev.00050.2005
Lang, F., Busch, G. L., Ritter, M., Volkl, H., Waldegger, S., Gulbins, E., et al. (1998). Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78, 247–306. doi: 10.1152/physrev.1998.78.1.247
Lang, F., Föller, M., Lang, K., Lang, P., Ritter, M., Vereninov, A., et al. (2007). “Cell volume regulatory ion channels in cell proliferation and cell death,” in Osmosensing and Osmosignaling, eds D. Häussinger and H. Sies (London: Academic Press), 209–225. doi: 10.1016/s0076-6879(07)28011-5
Lang, F., and Hoffmann, E. K. (2012). “Role of ion transport in control of apoptotic cell death,” in Comprehensive Physiology, ed. Y. S. Prakash (Hoboken, NJ: Wiley), 2037–2061.
Lang, F., and Hoffmann, E. K. (2013a). CrossTalk proposal: cell volume changes are an essential step in the cell death machinery. J. Physiol. 591, 6119–6121. doi: 10.1113/jphysiol.2013.258632
Lang, F., and Hoffmann, E. K. (2013b). Rebuttal from florian lang and else K. Hoffmann. J. Physiol. 591:6127. doi: 10.1113/jphysiol.2013.265231
Lang, F., Ritter, M., Woll, E., Weiss, H., Haussinger, D., Hoflacher, J., et al. (1992a). Altered cell volume regulation in ras oncogene expressing NIH fibroblasts. Pflugers Arch. 420, 424–427. doi: 10.1007/bf00374615
Lang, F., Stournaras, C., Zacharopoulou, N., Voelkl, J., and Alesutan, I. (2018). Serum- and glucocorticoid-inducible kinase 1 and the response to cell stress. Cell Stress 3, 1–8. doi: 10.15698/cst2019.01.170
Lang, F., Woll, E., Waldegger, S., Friedrich, F., Ritter, M., Pinggera, G., et al. (1992b). Cell membrane potential oscillations induced by kinins in fibroblasts expressing the Ha-ras oncogene. Agents Actions Suppl. 38(Pt 2), 73–80.
Langemeyer, L., Fröhlich, F., and Ungermann, C. (2018). Rab GTPase function in endosome and lysosome biogenesis. Trends Cell Biol. 28, 957–970. doi: 10.1016/j.tcb.2018.06.007
Lanzavecchia, A. (1996). Mechanisms of antigen uptake for presentation. Curr. Opin. Immunol. 8, 348–354. doi: 10.1016/s0952-7915(96)80124-5
Lanzetti, L., Palamidessi, A., Areces, L., Scita, G., and Di Fiore, P. P. (2004). Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature 429, 309–314. doi: 10.1038/nature02542
Laplante, J. M., Falardeau, J., Sun, M., Kanazirska, M., Brown, E. M., Slaugenhaupt, S. A., et al. (2002). Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV, a disorder affecting the lysosomal pathway. FEBS Lett. 532, 183–187. doi: 10.1016/s0014-5793(02)03670-0
Larsen, E. H., and Hoffmann, E. K. (2020). “Volume regulation in epithelia,” in Basic Epithelial Ion Transport Principles and Function, eds K. L. Hamilton and D. C. Devor (Cham: Springer), 395–460.
Lawrence, S. P., Bright, N. A., Luzio, J. P., and Bowers, K. (2010). The sodium/proton exchanger NHE8 regulates late endosomal morphology and function. Mol. Biol. Cell 21, 3540–3551. doi: 10.1091/mbc.e09-12-1053
Lee, E., and Knecht, D. A. (2002). Visualization of actin dynamics during macropinocytosis and exocytosis. Traffic 3, 186–192. doi: 10.1034/j.1600-0854.2002.030304.x
Lee, S., Kim, M. G., Ahn, H., and Kim, S. (2020). Inositol pyrophosphates: signaling molecules with pleiotropic actions in mammals. Molecules 25:2208. doi: 10.3390/molecules25092208
Lee, Y. H., and Peng, C. A. (2009). Effect of hypotonic stress on retroviral transduction. Biochem. Biophys. Res. Commun. 390, 1367–1371. doi: 10.1016/j.bbrc.2009.10.161
Lertsuwan, J., Lertsuwan, K., Sawasdichai, A., Tasnawijitwong, N., Lee, K. Y., Kitchen, P., et al. (2018). CX-4945 induces methuosis in cholangiocarcinoma cell lines by a CK2-independent mechanism. Cancers 10:283. doi: 10.3390/cancers10090283
Levin, R., Grinstein, S., and Schlam, D. (2015). Phosphoinositides in phagocytosis and macropinocytosis. Biochim. Biophys. Acta 1851, 805–823. doi: 10.1016/j.bbalip.2014.09.005
Lewis, W. H. (1936). Pinocytosis: Drinking by Cells. Baltimore, MD: The Johns Hopkins Medical Institutions.
Li, C., Macdonald, J. I., Hryciw, T., and Meakin, S. O. (2010). Nerve growth factor activation of the TrkA receptor induces cell death, by macropinocytosis, in medulloblastoma Daoy cells. J. Neurochem. 112, 882–899. doi: 10.1111/j.1471-4159.2009.06507.x
Li, G., D’souza-Schorey, C., Barbieri, M. A., Cooper, J. A., and Stahl, P. D. (1997). Uncoupling of membrane ruffling and pinocytosis during Ras signal transduction. J. Biol. Chem. 272, 10337–10340. doi: 10.1074/jbc.272.16.10337
Li, J., Gao, W., Zhang, Y., Cheng, F., Eriksson, J. E., Etienne-Manneville, S., et al. (2019). Engagement of vimentin intermediate filaments in hypotonic stress. J. Cell Biochem. 120, 13168–13176. doi: 10.1002/jcb.28591
Li, M., Zhang, W. K., Benvin, N. M., Zhou, X., Su, D., Li, H., et al. (2017). Structural basis of dual Ca(2+)/pH regulation of the endolysosomal TRPML1 channel. Nat. Struct. Mol. Biol. 24, 205–213. doi: 10.1038/nsmb.3362
Li, P., Gu, M., and Xu, H. (2019). Lysosomal ion channels as decoders of cellular signals. Trends Biochem. Sci. 44, 110–124. doi: 10.1016/j.tibs.2018.10.006
Li, P., Hu, M., Wang, C., Feng, X., Zhao, Z., Yang, Y., et al. (2020). LRRC8 family proteins within lysosomes regulate cellular osmoregulation and enhance cell survival to multiple physiological stresses. Proc. Natl. Acad. Sci. U.S.A. 117, 29155–29165. doi: 10.1073/pnas.2016539117
Li, X., Wang, T., Zhao, Z., and Weinman, S. A. (2002). The ClC-3 chloride channel promotes acidification of lysosomes in CHO-K1 and Huh-7 cells. Am. J. Physiol. Cell Physiol. 282, C1483–C1491.
Li, Z., Mbah, N. E., Overmeyer, J. H., Sarver, J. G., George, S., Trabbic, C. J., et al. (2019). The JNK signaling pathway plays a key role in methuosis (non-apoptotic cell death) induced by MOMIPP in glioblastoma. BMC Cancer 19:77. doi: 10.1186/s12885-019-5288-y
Lim, J. P., and Gleeson, P. A. (2011). Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol. Cell Biol. 89, 836–843. doi: 10.1038/icb.2011.20
Lin, J., Shi, S. S., Zhang, J. Q., Zhang, Y. J., Zhang, L., Liu, Y., et al. (2016). Giant cellular vacuoles induced by rare earth oxide nanoparticles are abnormally enlarged Endo/lysosomes and promote mTOR-dependent TFEB nucleus translocation. Small 12, 5759–5768. doi: 10.1002/smll.201601903
Lin, P. H., Duann, P., Komazaki, S., Park, K. H., Li, H., Sun, M., et al. (2015). Lysosomal two-pore channel subtype 2 (TPC2) regulates skeletal muscle autophagic signaling. J. Biol. Chem. 290, 3377–3389. doi: 10.1074/jbc.m114.608471
Lin, X. P., Mintern, J. D., and Gleeson, P. A. (2020). Macropinocytosis in different cell types: similarities and differences. Membranes 10:177. doi: 10.3390/membranes10080177
Liu, J., Ying, M., Wu, B., and Fu, C. (2020). Ethanol extract of the infructescence of Platycarya strobilacea Sieb. et Zucc. Induces methuosis of human nasopharyngeal carcinoma cells. Evid. Based Complement. Alternat. Med. 2020:2760979.
Liu, X., Ong, H. L., Pani, B., Johnson, K., Swaim, W. B., Singh, B., et al. (2010). Effect of cell swelling on ER/PM junctional interactions and channel assembly involved in SOCE. Cell Calcium 47, 491–499. doi: 10.1016/j.ceca.2010.04.002
Liu, X., Wang, S., Zheng, H., Liu, Q., Shen, T., Wang, X., et al. (2020). Epimedokoreanin c, a prenylated flavonoid isolated from epimedium koreanum, induces non-apoptotic cell death with the characteristics of methuosis in lung cancer cells. Res. Square [Epub ahead of print].
Liu, Z., and Roche, P. A. (2015). Macropinocytosis in phagocytes: regulation of MHC class-II-restricted antigen presentation in dendritic cells. Front. Physiol. 6:1. doi: 10.3389/fphys.2015.00001
Lloyd, J. B. (1990). Cell physiology of the rat visceral yolk sac: a study of pinocytosis and lysosome function. Teratology 41, 383–393. doi: 10.1002/tera.1420410404
Lloyd-Evans, E., Morgan, A. J., He, X., Smith, D. A., Elliot-Smith, E., Sillence, D. J., et al. (2008). Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 14:1247. doi: 10.1038/nm.1876
Loh, J., Chuang, M. C., Lin, S. S., Joseph, J., Su, Y. A., Hsieh, T. L., et al. (2019). An acute decrease in plasma membrane tension induces macropinocytosis via PLD2 activation. J. Cell. Sci. 132:jcs232579.
Loike, J. D., Cao, L., Kuang, K., Vera, J. C., Silverstein, S. C., and Fischbarg, J. (1993). Role of facilitative glucose transporters in diffusional water permeability through J774 cells. J. Gen. Physiol. 102, 897–906. doi: 10.1085/jgp.102.5.897
Loitto, V. M., Forslund, T., Sundqvist, T., Magnusson, K. E., and Gustafsson, M. (2002). Neutrophil leukocyte motility requires directed water influx. J. Leukoc. Biol. 71, 212–222.
Loitto, V. M., Karlsson, T., and Magnusson, K. E. (2009). Water flux in cell motility: expanding the mechanisms of membrane protrusion. Cell Motil. Cytoskeleton. 66, 237–247. doi: 10.1002/cm.20357
Lucien, F., Pelletier, P. P., Lavoie, R. R., Lacroix, J. M., Roy, S., Parent, J. L., et al. (2017). Hypoxia-induced mobilization of NHE6 to the plasma membrane triggers endosome hyperacidification and chemoresistance. Nat. Commun. 8:15884.
Madonna, R., Geng, Y. J., Shelat, H., Ferdinandy, P., and De Caterina, R. (2014). High glucose-induced hyperosmolarity impacts proliferation, cytoskeleton remodeling and migration of human induced pluripotent stem cells via aquaporin-1. Biochim. Biophys. Acta 1842, 2266–2275. doi: 10.1016/j.bbadis.2014.07.030
Madonna, R., Montebello, E., Lazzerini, G., Zurro, M., and De Caterina, R. (2010). NA+/H+ exchanger 1- and aquaporin-1-dependent hyperosmolarity changes decrease nitric oxide production and induce VCAM-1 expression in endothelial cells exposed to high glucose. Int. J. Immunopathol. Pharmacol. 23, 755–765. doi: 10.1177/039463201002300309
Madonna, R., Pieragostino, D., Rossi, C., Confalone, P., Cicalini, I., Minnucci, I., et al. (2020). Simulated hyperglycemia impairs insulin signaling in endothelial cells through a hyperosmolar mechanism. Vascul. Pharmacol. 130:106678. doi: 10.1016/j.vph.2020.106678
Maekawa, M., Terasaka, S., Mochizuki, Y., Kawai, K., Ikeda, Y., Araki, N., et al. (2014). Sequential breakdown of 3-phosphorylated phosphoinositides is essential for the completion of macropinocytosis. Proc. Natl. Acad. Sci. U.S.A. 111, E978–E987.
Majno, G., and Joris, I. (1995). Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 146, 3–15.
Maltese, W. A., and Overmeyer, J. H. (2014). Methuosis: nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments. Am. J. Pathol. 184, 1630–1642.
Maltese, W. A., and Overmeyer, J. H. (2015). Non-apoptotic cell death associated with perturbations of macropinocytosis. Front. Physiol. 6:38. doi: 10.3389/fphys.2015.00038
Maly, K., Kindler, E., Tinhofer, I., and Grunicke, H. H. (1995). Activation of Ca2+ influx by transforming Ha-ras. Cell Calcium 18, 120–134. doi: 10.1016/0143-4160(95)90003-9
Maly, K., Uberall, F., Loferer, H., Doppler, W., Oberhuber, H., Groner, B., et al. (1989). Ha-ras activates the Na+/H+ antiporter by a protein kinase C-independent mechanism. J. Biol. Chem. 264, 11839–11842. doi: 10.1016/s0021-9258(18)80142-9
Manara, M. C., Terracciano, M., Mancarella, C., Sciandra, M., Guerzoni, C., Pasello, M., et al. (2016). CD99 triggering induces methuosis of Ewing sarcoma cells through IGF-1R/RAS/Rac1 signaling. Oncotarget 7, 79925–79942. doi: 10.18632/oncotarget.13160
Margiotta, A., and Bucci, C. (2016). Role of intermediate filaments in vesicular traffic. Cells 5:20. doi: 10.3390/cells5020020
Marques, P. E., Grinstein, S., and Freeman, S. A. (2017). SnapShot:macropinocytosis. Cell 169, 766.e1–766.e1.
Martínez, D., Vermeulen, M., Von Euw, E., Sabatté, J., Maggíni, J., Ceballos, A., et al. (2007). Extracellular acidosis triggers the maturation of human dendritic cells and the production of IL-12. J. Immunol. 179, 1950–1959. doi: 10.4049/jimmunol.179.3.1950
Martinez, I., Sveinbjornsson, B., and Smedsrod, B. (1996). Nitric oxide down-regulates endocytosis in rat liver endothelial cells. Biochem. Biophys. Res. Commun. 222, 688–693. doi: 10.1006/bbrc.1996.0805
Matsuda, J. J., Filali, M. S., Moreland, J. G., Miller, F. J., and Lamb, F. S. (2010). Activation of swelling-activated chloride current by tumor necrosis factor-alpha requires ClC-3-dependent endosomal reactive oxygen production. J. Biol. Chem. 285, 22864–22873. doi: 10.1074/jbc.m109.099838
Maxson, M. E., and Grinstein, S. (2014). The vacuolar-type H(+)-ATPase at a glance - more than a proton pump. J. Cell. Sci. 127, 4987–4993. doi: 10.1242/jcs.158550
Maxson, M. E., Sarantis, H., Volchuk, A., Brumell, J. H., and Grinstein, S. (2021). Rab5 regulates macropinocytosis by recruiting the inositol 5-phosphatases OCRL/Inpp5b that hydrolyze PtdIns(4,5)P2. J. Cell. Sci. [Epub ahead of print].
Mercer, J., and Helenius, A. (2009). Virus entry by macropinocytosis. Nat. Cell Biol. 11, 510–520. doi: 10.1038/ncb0509-510
Mercer, J., and Helenius, A. (2012). Gulping rather than sipping: macropinocytosis as a way of virus entry. Curr. Opin. Microbiol. 15, 490–499. doi: 10.1016/j.mib.2012.05.016
Mercer, J., Lee, J. E., Saphire, E. O., and Freeman, S. A. (2020). SnapShot: enveloped virus entry. Cell 182, 786.e1–786.e1.
Mercer, J., Schelhaas, M., and Helenius, A. (2010). Virus entry by endocytosis. Annu. Rev. Biochem. 79, 803–833. doi: 10.1146/annurev-biochem-060208-104626
Merrifield, C. J., Moss, S. E., Ballestrem, C., Imhof, B. A., Giese, G., Wunderlich, I., et al. (1999). Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat. Cell Biol. 1, 72–74. doi: 10.1038/9048
Merrifield, C. J., Rescher, U., Almers, W., Proust, J., Gerke, V., Sechi, A. S., et al. (2001). Annexin 2 has an essential role in actin-based macropinocytic rocketing. Curr. Biol. 11, 1136–1141. doi: 10.1016/s0960-9822(01)00321-9
Mettlen, M., Platek, A., Van Der Smissen, P., Carpentier, S., Amyere, M., Lanzetti, L., et al. (2006). Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic 7, 589–603. doi: 10.1111/j.1600-0854.2006.00412.x
Milosavljevic, N., Monet, M., Lena, I., Brau, F., Lacas-Gervais, S., Feliciangeli, S., et al. (2014). The intracellular Na(+)/H(+) exchanger NHE7 effects a Na(+)-coupled, but not K(+)-coupled proton-loading mechanism in endocytosis. Cell Rep. 7, 689–696. doi: 10.1016/j.celrep.2014.03.054
Minna, E., Romeo, P., De Cecco, L., Dugo, M., Cassinelli, G., Pilotti, S., et al. (2014). miR-199a-3p displays tumor suppressor functions in papillary thyroid carcinoma. Oncotarget 5, 2513–2528. doi: 10.18632/oncotarget.1830
Model, M. A. (2014). Possible causes of apoptotic volume decrease: an attempt at quantitative review. Am. J. Physiol. Cell Physiol. 306, C417–C424.
Model, M. A., Hollembeak, J. E., and Kurokawa, M. (2020). Macromolecular crowding: a hidden link between cell volume and everything else. Cell Physiol. Biochem. 55, 25–40. doi: 10.33594/000000319
Mohebbi, N., Benabbas, C., Vidal, S., Daryadel, A., Bourgeois, S., Velic, A., et al. (2012). The proton-activated G protein coupled receptor OGR1 acutely regulates the activity of epithelial proton transport proteins. Cell Physiol. Biochem. 29, 313–324. doi: 10.1159/000338486
Morgan, A. J., Platt, F. M., Lloyd-Evans, E., and Galione, A. (2011). Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 439, 349–374. doi: 10.1042/bj20110949
Morishita, S., Wada, N., Fukuda, M., and Nakamura, T. (2019). Rab5 activation on macropinosomes requires ALS2, and subsequent Rab5 inactivation through ALS2 detachment requires active Rab7. FEBS Lett. 593, 230–241.
Nakamura, N., Tanaka, S., Teko, Y., Mitsui, K., and Kanazawa, H. (2005). Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. 280, 1561–1572. doi: 10.1074/jbc.m410041200
Nara, A., Aki, T., Funakoshi, T., and Uemura, K. (2010). Methamphetamine induces macropinocytosis in differentiated SH-SY5Y human neuroblastoma cells. Brain Res. 1352, 1–10. doi: 10.1016/j.brainres.2010.07.043
Neuhaus, E. M., Almers, W., and Soldati, T. (2002). Morphology and dynamics of the endocytic pathway in Dictyostelium discoideum. Mol. Biol. Cell. 13, 1390–1407. doi: 10.1091/mbc.01-08-0392
Nicoli, E.-R., Weston, M. R., Hackbarth, M., Becerril, A., Larson, A., Zein, W. M., et al. (2019). Lysosomal storage and albinism due to effects of a de novo CLCN7 variant on lysosomal acidification. Am. J. Hum. Genet. 104, 1127–1138.
Nirmala, J. G., and Lopus, M. (2020). Cell death mechanisms in eukaryotes. Cell. Biol. Toxicol. 36, 145–164. doi: 10.1007/s10565-019-09496-2
Nishi, T., and Forgac, M. (2002). The vacuolar (H+)-ATPases–nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 3, 94–103. doi: 10.1038/nrm729
Nobles, M., Higgins, C. F., and Sardini, A. (2004). Extracellular acidification elicits a chloride current that shares characteristics with ICl(swell). Am. J. Physiol. Cell Physiol. 287, C1426–C1435.
Nussinov, R., Tsai, C.-J., and Jang, H. (2018). Oncogenic Ras isoforms signaling specificity at the membrane. Cancer Res. 78, 593–602. doi: 10.1158/0008-5472.can-17-2727
Nussinov, R., Tsai, C. J., and Jang, H. (2020). Ras assemblies and signaling at the membrane. Curr. Opin. Struct. Biol. 62, 140–148. doi: 10.1016/j.sbi.2020.01.009
Oberleithner, H., Callies, C., Kusche-Vihrog, K., Schillers, H., Shahin, V., Riethmuller, C., et al. (2009). Potassium softens vascular endothelium and increases nitric oxide release. Proc. Natl. Acad. Sci. U.S.A. 106, 2829–2834. doi: 10.1073/pnas.0813069106
Oberleithner, H., and De Wardener, H. E. (2011). Sodium: a wolf in sheep’s clothing. Blood Purif. 31, 82–85. doi: 10.1159/000321842
Ohgaki, R., Van, I. S. C., Matsushita, M., Hoekstra, D., and Kanazawa, H. (2011). Organellar Na+/H+ exchangers: novel players in organelle pH regulation and their emerging functions. Biochemistry 50, 443–450. doi: 10.1021/bi101082e
Ohshima, H., and Ohki, S. (1985). Donnan potential and surface potential of a charged membrane. Biophys. J. 47, 673–678. doi: 10.1016/s0006-3495(85)83963-1
Okada, C. Y., and Rechsteiner, M. (1982). Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell 29, 33–41. doi: 10.1016/0092-8674(82)90087-3
Okada, Y. (2004). Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem. Biophys. 41, 233–258. doi: 10.1385/cbb:41:2:233
Okada, Y. (2020). Cell death induction and protection by activation of ubiquitously expressed anion/cation channels. Part 1: roles of VSOR/VRAC in cell volume regulation, release of double-edged signals and apoptotic/necrotic cell death. Front. Cell Dev. Biol. 8:614040. doi: 10.3389/fcell.2020.614040
Okada, Y., Maeno, E., Shimizu, T., Dezaki, K., Wang, J., and Morishima, S. (2001). Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 532, 3–16. doi: 10.1111/j.1469-7793.2001.0003g.x
Okada, Y., Okada, T., Sato-Numata, K., Islam, M. R., Ando-Akatsuka, Y., Numata, T., et al. (2019). Cell volume-activated and volume-correlated anion channels in mammalian cells: their biophysical, molecular, and pharmacological properties. Pharmacol. Rev. 71, 49–88. doi: 10.1124/pr.118.015917
Olbrich, K., Rawicz, W., Needham, D., and Evans, E. (2000). Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophys. J. 79, 321–327. doi: 10.1016/s0006-3495(00)76294-1
Oppong, F., Li, Z., Fakhrabadi, E. A., Raorane, T., Giri, P. M., Liberatore, M. W., et al. (2020). Investigating the potential to deliver and maintain plasma and brain levels of a novel practically insoluble methuosis inducing anticancer agent 5-methoxy MOMIPP through an injectable in situ forming thermoresponsive hydrogel formulation. J. Pharm. Sci. 109, 2719–2728. doi: 10.1016/j.xphs.2020.05.014
Orlov, S. N., and Hamet, P. (2004). Apoptosis vs. oncosis: role of cell volume and intracellular monovalent cations. Adv. Exp. Med. Biol. 559, 219–233. doi: 10.1007/0-387-23752-6_21
Orlov, S. N., Model, M. A., and Grygorczyk, R. (2013). CrossTalk opposing view: the triggering and progression of the cell death machinery can occur without cell volume perturbations. J. Physiol. 591, 6123–6125. doi: 10.1113/jphysiol.2013.258624
Orlowski, J., and Grinstein, S. (2011). “Na+/H+ exchangers,” in Comprehensive Physiology, ed. Y. S. Prakash (Hoboken, NJ: Wiley), 2083–2100.
Osei-Owusu, J., Yang, J., Del Carmen Vitery, M., Tian, M., and Qiu, Z. (2020). PAC proton-activated chloride channel contributes to acid-induced cell death in primary rat cortical neurons. Channels 14, 53–58. doi: 10.1080/19336950.2020.1730019
Osei-Owusu, J., Yang, J., Leung, K. H., Ruan, Z., Lu, W., Krishnan, Y., et al. (2021). Proton-activated chloride channel PAC regulates endosomal acidification and transferrin receptor-mediated endocytosis. Cell. Rep. 34:108683. doi: 10.1016/j.celrep.2020.108683
Ou, X., Liu, Y., Lei, X., Li, P., Mi, D., Ren, L., et al. (2020). Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 11:1620.
Overmeyer, J. H., Kaul, A., Johnson, E. E., and Maltese, W. A. (2008). Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol. Cancer Res. 6, 965–977. doi: 10.1158/1541-7786.mcr-07-2036
Overmeyer, J. H., Young, A. M., Bhanot, H., and Maltese, W. A. (2011). A chalcone-related small molecule that induces methuosis, a novel form of non-apoptotic cell death, in glioblastoma cells. Mol. Cancer 10:69. doi: 10.1186/1476-4598-10-69
Palanikumar, L., Kim, J., Oh, J. Y., Choi, H., Park, M. H., Kim, C., et al. (2018). Hyaluronic acid-modified polymeric gatekeepers on biodegradable mesoporous silica nanoparticles for targeted cancer therapy. ACS Biomater. Sci. Eng. 4, 1716–1722. doi: 10.1021/acsbiomaterials.8b00218
Palm, W. (2019). Metabolic functions of macropinocytosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180285. doi: 10.1098/rstb.2018.0285
Palmada, M., Dieter, M., Boehmer, C., Waldegger, S., and Lang, F. (2004). Serum and glucocorticoid inducible kinases functionally regulate ClC-2 channels. Biochem. Biophys. Res. Commun. 321, 1001–1006. doi: 10.1016/j.bbrc.2004.07.064
Pan, L., Zhang, P., Hu, F., Yan, R., He, M., Li, W., et al. (2019). Hypotonic stress induces fast, reversible degradation of the vimentin cytoskeleton via intracellular calcium release. Adv. Sci. 6:1900865. doi: 10.1002/advs.201900865
Pang, V., Counillon, L., Lagadic-Gossmann, D., Poet, M., Lacroix, J., Sergent, O., et al. (2012). On the role of the difference in surface tensions involved in the allosteric regulation of NHE-1 induced by low to mild osmotic pressure, membrane tension and lipid asymmetry. Cell Biochem. Biophys. 63, 47–57. doi: 10.1007/s12013-012-9340-7
Panyi, G., Possani, L. D., Rodriguez De La Vega, R. C., Gaspar, R., and Varga, Z. (2006). K+ channel blockers: novel tools to inhibit T cell activation leading to specific immunosuppression. Curr. Pharm. Des. 12, 2199–2220. doi: 10.2174/138161206777585120
Park, J. K., Peng, H., Katsnelson, J., Yang, W., Kaplan, N., Dong, Y., et al. (2016). MicroRNAs-103/107 coordinately regulate macropinocytosis and autophagy. J. Cell Biol. 215, 667–685. doi: 10.1083/jcb.201604032
Parton, R. G., Joggerst, B., and Simons, K. (1994). Regulated internalization of caveolae. J. Cell Biol. 127, 1199–1215. doi: 10.1083/jcb.127.5.1199
Pasantes-Morales, H. (2016). Channels and volume changes in the life and death of the cell. Mol. Pharmacol. 90, 358–370. doi: 10.1124/mol.116.104158
Pedersen, S. F., and Counillon, L. (2019). The SLC9A-C mammalian Na(+)/H(+) exchanger family: molecules. Mech. Physiol. Physiol. Rev. 99, 2015–2113. doi: 10.1152/physrev.00028.2018
Pedersen, S. F., Hoffmann, E. K., and Mills, J. W. (2001). The cytoskeleton and cell volume regulation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130, 385–399.
Pedersen, S. F., Hoffmann, E. K., and Novak, I. (2013). Cell volume regulation in epithelial physiology and cancer. Front. Physiol. 4:233. doi: 10.3389/fphys.2013.00233
Pedraz-Cuesta, E., Fredsted, J., Jensen, H. H., Bornebusch, A., Nejsum, L. N., Kragelund, B. B., et al. (2016). Prolactin signaling stimulates invasion via Na(+)/H(+) exchanger NHE1 in T47D human breast cancer cells. Mol. Endocrinol. 30, 693–708. doi: 10.1210/me.2015-1299
Pesesse, X., Choi, K., Zhang, T., and Shears, S. B. (2004). Signaling by higher inositol polyphosphates. Synthesis of bisdiphosphoinositol tetrakisphosphate (“InsP8”) is selectively activated by hyperosmotic stress. J. Biol. Chem. 279, 43378–43381.
Petersen, O. H., Gerasimenko, J. V., and Gerasimenko, O. V. (2020). Endocytic uptake of SARS-CoV-2: the critical roles of pH, Ca2+, and NAADP. Function 1:zqaa003.
Petrini, S., Minnone, G., Coccetti, M., Frank, C., Aiello, C., Cutarelli, A., et al. (2013). Monocytes and macrophages as biomarkers for the diagnosis of megalencephalic leukoencephalopathy with subcortical cysts. Mol. Cell Neurosci. 56, 307–321. doi: 10.1016/j.mcn.2013.07.001
Pierro, C., Zhang, X., Kankeu, C., Trebak, M., Bootman, M. D., and Roderick, H. L. (2018). Oncogenic KRAS suppresses store-operated Ca(2+) entry and ICRAC through ERK pathway-dependent remodelling of STIM expression in colorectal cancer cell lines. Cell Calcium 72, 70–80. doi: 10.1016/j.ceca.2018.03.002
Pintsch, T., Satre, M., Klein, G., Martin, J. B., and Schuster, S. C. (2001). Cytosolic acidification as a signal mediating hyperosmotic stress responses in Dictyostelium discoideum. BMC Cell Biol. 2:9. doi: 10.1186/1471-2121-2-9
Pironti, G., Strachan, R. T., Abraham, D., Mon-Wei Yu, S., Chen, M., Chen, W., et al. (2015). Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131, 2120–2130. doi: 10.1161/circulationaha.115.015687
Pizon, V., Desjardins, M., Bucci, C., Parton, R. G., and Zerial, M. (1994). Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J. Cell Sci. 107(Pt 6), 1661–1670. doi: 10.1242/jcs.107.6.1661
Planade, J., Belbahri, R., Boiero Sanders, M., Guillotin, A., Du Roure, O., Michelot, A., et al. (2019). Mechanical stiffness of reconstituted actin patches correlates tightly with endocytosis efficiency. PLoS Biol. 17:e3000500. doi: 10.1371/journal.pbio.3000500
Platt, F. M., Boland, B., and Van Der Spoel, A. C. (2012). The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J. Cell Biol. 199, 723–734. doi: 10.1083/jcb.201208152
Platt, F. M., D’azzo, A., Davidson, B. L., Neufeld, E. F., and Tifft, C. J. (2018). Lysosomal storage diseases. Nat. Rev. Dis Primers 4:27.
Pollard, T. D. (2016). Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol. 8:a018226.
Polovitskaya, M. M., Barbini, C., Martinelli, D., Harms, F. L., Cole, F. S., Calligari, P., et al. (2020). A recurrent gain-of-function mutation in CLCN6, encoding the ClC-6 Cl(-)/H(+)-exchanger, causes early-onset neurodegeneration. Am. J. Hum. Genet. 107, 1062–1077. doi: 10.1016/j.ajhg.2020.11.004
Porat-Shliom, N., Kloog, Y., and Donaldson, J. G. (2008). A unique platform for H-Ras signaling involving clathrin-independent endocytosis. Mol. Biol. Cell. 19, 765–775. doi: 10.1091/mbc.e07-08-0841
Potokar, M., Stenovec, M., Jorgacevski, J., Holen, T., Kreft, M., Ottersen, O. P., et al. (2013). Regulation of AQP4 surface expression via vesicle mobility in astrocytes. Glia 61, 917–928. doi: 10.1002/glia.22485
Qadri, Y. J., Song, Y., Fuller, C. M., and Benos, D. J. (2010). Amiloride docking to acid-sensing ion channel-1. J. Biol. Chem. 285, 9627–9635. doi: 10.1074/jbc.m109.082735
Qiu, Z., Dubin, A. E., Mathur, J., Tu, B., Reddy, K., Miraglia, L. J., et al. (2014). SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157, 447–458. doi: 10.1016/j.cell.2014.03.024
Racoosin, E. L., and Swanson, J. A. (1993). Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 121, 1011–1020. doi: 10.1083/jcb.121.5.1011
Rajasekharan, S. K., Kim, S., Kim, J. C., and Lee, J. (2020). Nematicidal activity of 5-iodoindole against root-knot nematodes. Pestic Biochem. Physiol. 163, 76–83. doi: 10.1016/j.pestbp.2019.10.012
Rajasekharan, S. K., and Lee, J. (2020). Hydropic anthelmintics against parasitic nematodes. PLoS Pathog. 16:e1008202. doi: 10.1371/journal.ppat.1008202
Rajasekharan, S. K., Lee, J., Ravichandran, V., Kim, J., Park, J. G., and Lee, J. (2019). Nematicidal and insecticidal activities of halogenated indoles. Sci. Rep. 9:2010. doi: 10.1038/s41598-019-38561-3
Rajasekharan, S. K., Lee, J. H., Ravichandran, V., and Lee, J. (2017). Assessments of iodoindoles and abamectin as inducers of methuosis in pinewood nematode, Bursaphelenchus xylophilus. Sci. Rep. 7:6803.
Ramirez, C., Hauser, A. D., Vucic, E. A., and Bar-Sagi, D. (2019). Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis. Nature 576, 477–481. doi: 10.1038/s41586-019-1831-x
Rane, S. G. (1991). A Ca2(+)-activated K+ current in ras-transformed fibroblasts is absent from nontransformed cells. Am. J. Physiol. 260, C104–C112.
Rappaport, J., Manthe, R. L., Solomon, M., Garnacho, C., and Muro, S. (2016). A comparative study on the alterations of endocytic pathways in multiple lysosomal storage disorders. Mol. Pharm. 13, 357–368. doi: 10.1021/acs.molpharmaceut.5b00542
Recouvreux, M. V., and Commisso, C. (2017). Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front. Endocrinol. 8:261. doi: 10.3389/fendo.2017.00261
Reibring, C. G., El Shahawy, M., Hallberg, K., Kannius-Janson, M., Nilsson, J., Parkkila, S., et al. (2014). Expression patterns and subcellular localization of carbonic anhydrases are developmentally regulated during tooth formation. PLoS One 9:e96007. doi: 10.1371/journal.pone.0096007
Reyes-Reyes, E. M., Salipur, F. R., Shams, M., Forsthoefel, M. K., and Bates, P. J. (2015). Mechanistic studies of anticancer aptamer AS1411 reveal a novel role for nucleolin in regulating Rac1 activation. Mol. Oncol. 9, 1392–1405. doi: 10.1016/j.molonc.2015.03.012
Ridder, M. C., Boor, I., Lodder, J. C., Postma, N. L., Capdevila-Nortes, X., Duarri, A., et al. (2011). Megalencephalic leucoencephalopathy with cysts: defect in chloride currents and cell volume regulation. Brain 134, 3342–3354. doi: 10.1093/brain/awr255
Riegman, M., Sagie, L., Galed, C., Levin, T., Steinberg, N., Dixon, S. J., et al. (2020). Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell. Biol. 22, 1042–1048. doi: 10.1038/s41556-020-0565-1
Riess, C., Koczan, D., Schneider, B., Linke, C., Del Moral, K., Classen, C. F., et al. (2021). Cyclin-dependent kinase inhibitors exert distinct effects on patient-derived 2D and 3D glioblastoma cell culture models. Cell Death Discov. 7:54.
Rikihisa, Y. (1985). Ultrastructural localization of carbonic anhydrase in lysosomes. Anat. Rec. 211, 1–8. doi: 10.1002/ar.1092110102
Ritter, M., Dartsch, P., Waldegger, S., Haller, T., Zwierzina, H., Lang, H. J., et al. (1997a). Effects of bradykinin on NIH 3T3 fibroblasts pretreated with lithium. Mimicking events of Ha-ras oncogene expression. Biochim. Biophys. Acta 1358, 23–30. doi: 10.1016/s0167-4889(97)00046-3
Ritter, M., Fuerst, J., Woll, E., Chwatal, S., Gschwentner, M., Lang, F., et al. (2001). Na(+)/H(+)exchangers: linking osmotic dysequilibrium to modified cell function. Cell Physiol. Biochem. 11, 1–18. doi: 10.1159/000047787
Ritter, M., Woll, E., Haller, T., Dartsch, P. C., Zwierzina, H., and Lang, F. (1997b). Activation of Na+/H(+)-exchanger by transforming Ha-ras requires stimulated cellular calcium influx and is associated with rearrangement of the actin cytoskeleton. Eur. J. Cell. Biol. 72, 222–228.
Ritter, M., Paulmichl, M., and Lang, F. (1991). Further characterization of volume regulatory decrease in cultured renal epitheloid (MDCK) cells. Pflügers Arch. 418, 35–39. doi: 10.1007/bf00370449
Ritter, M., and Woll, E. (1996). Modification of cellular ion transport by the ha-ras oncogene: steps towards malignant transformation. Cell Physiol. Biochem. 6, 245–270. doi: 10.1159/000154827
Ritter, M., Woll, E., Waldegger, S., Haussinger, D., Lang, H. J., Scholz, W., et al. (1993). Cell shrinkage stimulates bradykinin-induced cell membrane potential oscillations in NIH 3T3 fibroblasts expressing the ras-oncogene. Pflugers Arch. 423, 221–224. doi: 10.1007/bf00374398
Roberts, R. L., Barbieri, M. A., Ullrich, J., and Stahl, P. D. (2000). Dynamics of rab5 activation in endocytosis and phagocytosis. J. Leukoc. Biol. 68, 627–632.
Robinson, M. W., Overmeyer, J. H., Young, A. M., Erhardt, P. W., and Maltese, W. A. (2012). Synthesis and evaluation of indole-based chalcones as inducers of methuosis, a novel type of nonapoptotic cell death. J. Med. Chem. 55, 1940–1956. doi: 10.1021/jm201006x
Ruan, Z., Osei-Owusu, J., Du, J., Qiu, Z., and Lu, W. (2020). Structures and pH-sensing mechanism of the proton-activated chloride channel. Nature 588, 350–354. doi: 10.1038/s41586-020-2875-7
Rupper, A., Lee, K., Knecht, D., and Cardelli, J. (2001). Sequential activities of phosphoinositide 3-kinase, PKB/Aakt, and Rab7 during macropinosome formation in Dictyostelium. Mol. Biol. Cell. 12, 2813–2824. doi: 10.1091/mbc.12.9.2813
Russo, M. A., Morgante, E., Russo, A., Van Rossum, G. D., and Tafani, M. (2015). Ouabain-induced cytoplasmic vesicles and their role in cell volume maintenance. Biomed. Res. Int. 2015:487256.
Saarikangas, J., Zhao, H., and Lappalainen, P. (2010). Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 90, 259–289. doi: 10.1152/physrev.00036.2009
Saha, S., Prakash, V., Halder, S., Chakraborty, K., and Krishnan, Y. (2015). A pH-independent DNA nanodevice for quantifying chloride transport in organelles of living cells. Nat. Nanotechnol. 10, 645–651. doi: 10.1038/nnano.2015.130
Sahu, I., Pelzl, L., Sukkar, B., Fakhri, H., Al-Maghout, T., Cao, H., et al. (2017). NFAT5-sensitive Orai1 expression and store-operated Ca(2+) entry in megakaryocytes. FASEB J. 31, 3439–3448. doi: 10.1096/fj.201601211r
Sakurai, Y., Kolokoltsov, A. A., Chen, C. C., Tidwell, M. W., Bauta, W. E., Klugbauer, N., et al. (2015). Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 347, 995–998. doi: 10.1126/science.1258758
Sander, P., Mostafa, H., Soboh, A., Schneider, J. M., Pala, A., Baron, A. K., et al. (2017). Vacquinol-1 inducible cell death in glioblastoma multiforme is counter regulated by TRPM7 activity induced by exogenous ATP. Oncotarget 8, 35124–35137. doi: 10.18632/oncotarget.16703
Sano, O., Kazetani, K., Funata, M., Fukuda, Y., Matsui, J., and Iwata, H. (2016). Vacuolin-1 inhibits autophagy by impairing lysosomal maturation via PIKfyve inhibition. FEBS Lett. 590, 1576–1585. doi: 10.1002/1873-3468.12195
Saric, A., and Freeman, S. A. (2020). Endomembrane tension and trafficking. Front. Cell Dev. Biol. 8:611326. doi: 10.3389/fcell.2020.611326
Saric, A., and Freeman, S. A. (2021). Solutes as controllers of endomembrane dynamics. Nat. Rev. Mol. Cell Biol. 22, 237–238. doi: 10.1038/s41580-021-00334-0
Sarkar Bhattacharya, S., Thirusangu, P., Jin, L., Roy, D., Jung, D., Xiao, Y., et al. (2019). PFKFB3 inhibition reprograms malignant pleural mesothelioma to nutrient stress-induced macropinocytosis and ER stress as independent binary adaptive responses. Cell Death Dis. 10:725.
Sato, K., Mogi, C., Mighell, A. J., and Okajima, F. (2020). A missense mutation of Leu74Pro of OGR1 found in familial amelogenesis imperfecta actually causes the loss of the pH-sensing mechanism. Biochem. Biophys. Res. Commun. 526, 920–926. doi: 10.1016/j.bbrc.2020.04.005
Sato-Numata, K., Numata, T., Inoue, R., Sabirov, R. Z., and Okada, Y. (2017). Distinct contributions of LRRC8A and its paralogs to the VSOR anion channel from those of the ASOR anion channel. Channels 11, 167–172. doi: 10.1080/19336950.2016.1230574
Sato-Numata, K., Numata, T., Okada, T., and Okada, Y. (2013). Acid-sensitive outwardly rectifying (ASOR) anion channels in human epithelial cells are highly sensitive to temperature and independent of ClC-3. Pflugers Arch. 465, 1535–1543. doi: 10.1007/s00424-013-1296-y
Saveanu, L., and Lotersztajn, S. (2016). New pieces in the complex puzzle of aberrant vacuolation. Focus on “Active vacuolar H+ ATPase and functional cycle of Rab5 are required for the vacuolation defect triggered by PtdIns(3,5)P2 loss under PIKfyve or Vps34 deficiency”. Am. J. Physiol. Cell Physiol. 311, C363–C365.
Savina, A., Furlán, M., Vidal, M., and Colombo, M. I. (2003). Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 278, 20083–20090. doi: 10.1074/jbc.m301642200
Sbrissa, D., Ikonomov, O. C., Filios, C., Delvecchio, K., and Shisheva, A. (2012). Functional dissociation between PIKfyve-synthesized PtdIns5P and PtdIns (3, 5) P2 by means of the PIKfyve inhibitor YM201636. Am. J. Physiol. Cell Physiol. 303, C436–C446.
Sbrissa, D., Naisan, G., Ikonomov, O. C., and Shisheva, A. (2018). Apilimod, a candidate anticancer therapeutic, arrests not only PtdIns(3,5)P2 but also PtdIns5P synthesis by PIKfyve and induces bafilomycin A1-reversible aberrant endomembrane dilation. PLoS One 13:e0204532. doi: 10.1371/journal.pone.0204532
Sbrissa, D., and Shisheva, A. (2005). Acquisition of unprecedented phosphatidylinositol 3,5-bisphosphate rise in hyperosmotically stressed 3T3-L1 adipocytes, mediated by ArPIKfyve-PIKfyve pathway. J. Biol. Chem. 280, 7883–7889. doi: 10.1074/jbc.m412729200
Scheel, O., Zdebik, A. A., Lourdel, S., and Jentsch, T. J. (2005). Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436, 424–427. doi: 10.1038/nature03860
Schilling, K., Opitz, N., Wiesenthal, A., Oess, S., Tikkanen, R., Muller-Esterl, W., et al. (2006). Translocation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol. Biol. Cell 17, 3870–3880. doi: 10.1091/mbc.e05-08-0709
Schmees, C., Villasenor, R., Zheng, W., Ma, H., Zerial, M., Heldin, C. H., et al. (2012). Macropinocytosis of the PDGF beta-receptor promotes fibroblast transformation by H-RasG12V. Mol. Biol. Cell 23, 2571–2582. doi: 10.1091/mbc.e11-04-0317
Schneider, L., Klausen, T. K., Stock, C., Mally, S., Christensen, S. T., Pedersen, S. F., et al. (2008). H-ras transformation sensitizes volume-activated anion channels and increases migratory activity of NIH3T3 fibroblasts. Pflugers Arch. 455, 1055–1062. doi: 10.1007/s00424-007-0367-3
Schneider, S. W., Pagel, P., Rotsch, C., Danker, T., Oberleithner, H., Radmacher, M., et al. (2000). Volume dynamics in migrating epithelial cells measured with atomic force microscopy. Pflugers Arch. 439, 297–303. doi: 10.1007/s004249900176
Schwab, A., Fabian, A., Hanley, P. J., and Stock, C. (2012). Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913. doi: 10.1152/physrev.00018.2011
Schwab, A., and Stock, C. (2014). Ion channels and transporters in tumour cell migration and invasion. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20130102. doi: 10.1098/rstb.2013.0102
Schwappach, B. (2020). Chloride accumulation in endosomes and lysosomes: facts checked in mice. EMBO J. 39:e104812.
Schwede, M., Garbett, K., Mirnics, K., Geschwind, D. H., and Morrow, E. M. (2014). Genes for endosomal NHE6 and NHE9 are misregulated in autism brains. Mol. Psychiatry 19, 277–279. doi: 10.1038/mp.2013.28
Scott, C. C., and Gruenberg, J. (2011). Ion flux and the function of endosomes and lysosomes: pH is just the start: the flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays 33, 103–110. doi: 10.1002/bies.201000108
Seastone, D. J., Zhang, L., Buczynski, G., Rebstein, P., Weeks, G., Spiegelman, G., et al. (1999). The small Mr Ras-like GTPase Rap1 and the phospholipase C pathway act to regulate phagocytosis in Dictyostelium discoideum. Mol. Biol. Cell. 10, 393–406. doi: 10.1091/mbc.10.2.393
Sedlyarov, V., Eichner, R., Girardi, E., Essletzbichler, P., Goldmann, U., Nunes-Hasler, P., et al. (2018). The bicarbonate transporter SLC4A7 plays a key role in macrophage phagosome acidification. Cell Host Microbe 23, 766.e5–774.e5.
Seuwen, K., Ludwig, M. G., and Wolf, R. M. (2006). Receptors for protons or lipid messengers or both? J. Recept. Signal. Transduct. Res. 26, 599–610. doi: 10.1080/10799890600932220
She, J., Zeng, W., Guo, J., Chen, Q., Bai, X. C., and Jiang, Y. (2019). Structural mechanisms of phospholipid activation of the human TPC2 channel. eLife 8:e45222.
Shi, L. B., and Verkman, A. S. (1989). Very high water permeability in vasopressin-induced endocytic vesicles from toad urinary bladder. J. Gen. Physiol. 94, 1101–1115. doi: 10.1085/jgp.94.6.1101
Shrivastava, P., Singh, S. M., and Singh, N. (2004). Activation of tumor-associated macrophages by thymosin alpha 1. Int. J. Immunopathol. Pharmacol. 17, 39–47. doi: 10.1177/039463200401700106
Shubin, A. V., Demidyuk, I. V., Lunina, N. A., Komissarov, A. A., Roschina, M. P., Leonova, O. G., et al. (2015). Protease 3C of hepatitis A virus induces vacuolization of lysosomal/endosomal organelles and caspase-independent cell death. BMC Cell. Biol. 16:4. doi: 10.1186/s12860-015-0050-z
Silva-Pavez, E., Villar, P., Trigo, C., Caamano, E., Niechi, I., Perez, P., et al. (2019). CK2 inhibition with silmitasertib promotes methuosis-like cell death associated to catastrophic massive vacuolization of colorectal cancer cells. Cell Death Dis. 10:73.
Siner, J., Paredes, A., Hosselet, C., Hammond, T., Strange, K., and Harris, H. W. (1996). Cloning of an aquaporin homologue present in water channel containing endosomes of toad urinary bladder. Am. J. Physiol. 270, C372–C381.
Sonawane, N., Thiagarajah, J. R., and Verkman, A. (2002). Chloride concentration in endosomes measured using a ratioable fluorescent Cl- indicator evidence for chloride accumulation during acidification. J. Biol. Chem. 277, 5506–5513. doi: 10.1074/jbc.m110818200
Sonawane, N. D., and Verkman, A. S. (2003). Determinants of [Cl-] in recycling and late endosomes and Golgi complex measured using fluorescent ligands. J. Cell Biol. 160, 1129–1138. doi: 10.1083/jcb.200211098
Song, Q., Meng, B., Xu, H., and Mao, Z. (2020). The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases. Transl. Neurodegener. 9:17.
Song, S., Zhang, Y., Ding, T., Ji, N., and Zhao, H. (2020). The dual role of macropinocytosis in cancers: promoting growth and inducing methuosis to participate in anticancer therapies as targets. Front. Oncol. 10:570108. doi: 10.3389/fonc.2020.570108
Spix, B., Chao, Y. K., Abrahamian, C., Chen, C. C., and Grimm, C. (2020). TRPML cation channels in inflammation and immunity. Front. Immunol. 11:225. doi: 10.3389/fimmu.2020.00225
Sripathi, S. R., He, W., Um, J. Y., Moser, T., Dehnbostel, S., Kindt, K., et al. (2012). Nitric oxide leads to cytoskeletal reorganization in the retinal pigment epithelium under oxidative stress. Adv. Biosci. Biotechnol. 3, 1167–1178. doi: 10.4236/abb.2012.38143
Srivastava, R. K., Li, C., Khan, J., Banerjee, N. S., Chow, L. T., and Athar, M. (2019). Combined mTORC1/mTORC2 inhibition blocks growth and induces catastrophic macropinocytosis in cancer cells. Proc. Natl. Acad. Sci. U.S.A. 116, 24583–24592. doi: 10.1073/pnas.1911393116
Srivastava, S., Choudhury, P., Li, Z., Liu, G., Nadkarni, V., Ko, K., et al. (2006). Phosphatidylinositol 3-phosphate indirectly activates KCa3.1 via 14 amino acids in the carboxy terminus of KCa3.1. Mol. Biol. Cell. 17, 146–154. doi: 10.1091/mbc.e05-08-0763
Srivastava, S., Li, Z., Lin, L., Liu, G., Ko, K., Coetzee, W. A., et al. (2005). The phosphatidylinositol 3-phosphate phosphatase myotubularin- related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3.1. Mol. Cell. Biol. 25, 3630–3638. doi: 10.1128/mcb.25.9.3630-3638.2005
Staff, C. D. E. (2021). Autophagy-deficient pancreatic cancer cells depend on macropinocytosis. Cancer Discov. [Epub ahead of print].
Stauber, T., and Jentsch, T. J. (2013). Chloride in vesicular trafficking and function. Annu. Rev. Physiol. 75, 453–477. doi: 10.1146/annurev-physiol-030212-183702
Stauber, T., Weinert, S., and Jentsch, T. J. (2012). Cell biology and physiology of CLC chloride channels and transporters. Compr. Physiol. 2, 1701– 1744.
Steenbergen, J. M., and Bohlen, H. G. (1993). Sodium hyperosmolarity of intestinal lymph causes arteriolar vasodilation in part mediated by EDRF. Am. J. Physiol. 265, H323–H328.
Stein, B. S., and Sussman, H. H. (1986). Demonstration of two distinct transferrin receptor recycling pathways and transferrin-independent receptor internalization in K562 cells. J. Biol. Chem. 261, 10319–10331. doi: 10.1016/s0021-9258(18)67527-1
Steinberg, B. E., Huynh, K. K., Brodovitch, A., Jabs, S., Stauber, T., Jentsch, T. J., et al. (2010). A cation counterflux supports lysosomal acidification. J. Cell. Biol. 189, 1171–1186. doi: 10.1083/jcb.200911083
Steinman, R. M., Brodie, S. E., and Cohn, Z. A. (1976). Membrane flow during pinocytosis. A stereologic analysis. J. Cell Biol. 68, 665–687. doi: 10.1083/jcb.68.3.665
Sterea, A. M., Almasi, S., and El Hiani, Y. (2018). The hidden potential of lysosomal ion channels: a new era of oncogenes. Cell Calcium 72, 91–103. doi: 10.1016/j.ceca.2018.02.006
Stock, C., Ludwig, F. T., Hanley, P. J., and Schwab, A. (2013). Roles of ion transport in control of cell motility. Compr. Physiol. 5, 59–119.
Stockem, W. (1966). Pinocytose und bewegung von amöben. Z. Zellforschung Mikroskopische Anatomie 74, 372–400. doi: 10.1007/bf00401263
Stow, J. L., Hung, Y., and Wall, A. A. (2020). Macropinocytosis: insights from immunology and cancer. Curr. Opin. Cell Biol. 65, 131–140. doi: 10.1016/j.ceb.2020.06.005
Su, H., Yang, F., Fu, R., Li, X., French, R., Mose, E., et al. (2021). Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell. 39, 678.e11–693.e11.
Su, Y. (2014). Regulation of endothelial nitric oxide synthase activity by protein-protein interaction. Curr. Pharm. Des. 20, 3514–3520. doi: 10.2174/13816128113196660752
Su, Y., Kondrikov, D., and Block, E. R. (2005). Cytoskeletal regulation of nitric oxide synthase. Cell Biochem. Biophys. 43, 439–449. doi: 10.1385/cbb:43:3:439
Suda, T., Gao, X., Stolz, D. B., and Liu, D. (2007). Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 14, 129–137. doi: 10.1038/sj.gt.3302865
Sugiya, H., Matsuki-Fukushima, M., and Hashimoto, S. (2008). Role of aquaporins and regulation of secretory vesicle volume in cell secretion. J. Cell Mol. Med. 12, 1486–1494. doi: 10.1111/j.1582-4934.2008.00239.x
Suh, B. C., and Hille, B. (2008). PIP2 is a necessary cofactor for ion channel function: how and why? Annu. Rev. Biophys. 37, 175–195. doi: 10.1146/annurev.biophys.37.032807.125859
Sun, L., Hua, Y., Vergarajauregui, S., Diab, H. I., and Puertollano, R. (2015). Novel role of TRPML2 in the regulation of the innate immune response. J. Immunol. 195, 4922–4932. doi: 10.4049/jimmunol.1500163
Sun, L., Li, B., Su, X., Chen, G., Li, Y., Yu, L., et al. (2017). An ursolic acid derived small molecule triggers cancer cell death through hyperstimulation of macropinocytosis. J. Med. Chem. 60, 6638–6648. doi: 10.1021/acs.jmedchem.7b00592
Swanson, J. A. (1989). Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J. Cell Sci. 94, 135–142. doi: 10.1242/jcs.94.1.135
Swanson, J. A. (2008). Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 9, 639–649. doi: 10.1038/nrm2447
Swanson, J. A., and King, J. S. (2019). The breadth of macropinocytosis research. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180146. doi: 10.1098/rstb.2018.0146
Swanson, J. A., and Yoshida, S. (2019). Macropinosomes as units of signal transduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180157. doi: 10.1098/rstb.2018.0157
Synnes, M., Prydz, K., Lovdal, T., Brech, A., and Berg, T. (1999). Fluid phase endocytosis and galactosyl receptor-mediated endocytosis employ different early endosomes. Biochim. Biophys. Acta 1421, 317–328. doi: 10.1016/s0005-2736(99)00134-0
Takeda-Nakazawa, H., Harada, N., Shen, J., Kubo, N., Zenner, H. P., and Yamashita, T. (2007). Hyposmotic stimulation-induced nitric oxide production in outer hair cells of the guinea pig cochlea. Hear. Res. 227, 59–70. doi: 10.1016/j.heares.2006.09.007
Tall, G. G., Barbieri, M. A., Stahl, P. D., and Horazdovsky, B. F. (2001). Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1, 73–82. doi: 10.1016/s1534-5807(01)00008-9
Tejeda-Munoz, N., Albrecht, L. V., Bui, M. H., and De Robertis, E. M. (2019). Wnt canonical pathway activates macropinocytosis and lysosomal degradation of extracellular proteins. Proc. Natl. Acad. Sci. U.S.A. 116, 10402–10411. doi: 10.1073/pnas.1903506116
Tian, X., Gala, U., Zhang, Y., Shang, W., Nagarkar Jaiswal, S., Di Ronza, A., et al. (2015). A voltage-gated calcium channel regulates lysosomal fusion with endosomes and autophagosomes and is required for neuronal homeostasis. PLoS Biol. 13:e1002103. doi: 10.1371/journal.pbio.1002103
Toh, W. H., Louber, J., Mahmoud, I. S., Chia, J., Bass, G. T., Dower, S. K., et al. (2019). FcRn mediates fast recycling of endocytosed albumin and IgG from early macropinosomes in primary macrophages. J. Cell. Sci. 133:jcs235416.
Trabbic, C. J., Dietsch, H. M., Alexander, E. M., Nagy, P. I., Robinson, M. W., Overmeyer, J. H., et al. (2014). Differential induction of cytoplasmic vacuolization and methuosis by novel 2-indolyl-substituted pyridinylpropenones. ACS Med. Chem. Lett. 5, 73–77. doi: 10.1021/ml4003925
Trabbic, C. J., George, S. M., Alexander, E. M., Du, S., Offenbacher, J. M., Crissman, E. J., et al. (2016). Synthesis and biological evaluation of isomeric methoxy substitutions on anti-cancer indolyl-pyridinyl-propenones: effects on potency and mode of activity. Eur. J. Med. Chem. 122, 79–91. doi: 10.1016/j.ejmech.2016.06.016
Trabbic, C. J., Overmeyer, J. H., Alexander, E. M., Crissman, E. J., Kvale, H. M., Smith, M. A., et al. (2015). Synthesis and biological evaluation of indolyl-pyridinyl-propenones having either methuosis or microtubule disruption activity. J. Med. Chem. 58, 2489–2512. doi: 10.1021/jm501997q
Trivedi, P. C., Bartlett, J. J., and Pulinilkunnil, T. (2020). Lysosomal biology and function: modern view of cellular debris bin. Cells 9:1131. doi: 10.3390/cells9051131
Tsuchiya, W., Okada, Y., Yano, J., Murai, A., Miyahara, T., and Tanaka, T. (1981). Membrane potential changes associated with pinocytosis of serum lipoproteins in L cells. Exp. Cell Res. 136, 271–278. doi: 10.1016/0014-4827(81)90005-7
Ullrich, F., Blin, S., Lazarow, K., Daubitz, T., Von Kries, J. P., and Jentsch, T. J. (2019). Identification of TMEM206 proteins as pore of PAORAC/ASOR acid-sensitive chloride channels. eLife 8:e49187.
Ulmasov, B., Bruno, J., Gordon, N., Hartnett, M. E., and Edwards, J. C. (2009). Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am. J. Pathol. 174, 1084–1096. doi: 10.2353/ajpath.2009.080625
Unni, A. M., Lockwood, W. W., Zejnullahu, K., Lee-Lin, S. Q., and Varmus, H. (2015). Evidence that synthetic lethality underlies the mutual exclusivity of oncogenic KRAS and EGFR mutations in lung adenocarcinoma. eLife 4: e06907.
Vacca, G., Papillo, B., Battaglia, A., Grossini, E., Mary, D. A., and Pelosi, G. (1996). The effects of hypertonic saline solution on coronary blood flow in anaesthetized pigs. J. Physiol. 491(Pt 3), 843–851. doi: 10.1113/jphysiol.1996.sp021261
Van Der Knaap, M. S., Boor, I., and Estévez, R. (2012). Megalencephalic leukoencephalopathy with subcortical cysts: chronic white matter oedema due to a defect in brain ion and water homoeostasis. Lancet Neurol. 11, 973–985. doi: 10.1016/s1474-4422(12)70192-8
Van Der Wijk, T., Dorrestijn, J., Narumiya, S., Maassen, J. A., De Jonge, H. R., and Tilly, B. C. (1998). Osmotic swelling-induced activation of the extracellular-signal-regulated protein kinases Erk-1 and Erk-2 in intestine 407 cells involves the Ras/Raf-signalling pathway. Biochem. J. 331(Pt 3), 863–869. doi: 10.1042/bj3310863
Vardjan, N., Verkhratsky, A., and Zorec, R. (2015). Pathologic potential of astrocytic vesicle traffic: new targets to treat neurologic diseases? Cell Transplant 24, 599–612. doi: 10.3727/096368915x687750
Varela, D., Simon, F., Riveros, A., Jørgensen, F., and Stutzin, A. (2004). NAD (P) H oxidase-derived H2O2 signals chloride channel activation in cell volume regulation and cell proliferation. J. Biol. Chem. 279, 13301–13304. doi: 10.1074/jbc.c400020200
Vasanthakumar, T., and Rubinstein, J. L. (2020). Structure and Roles of V-type ATPases. Trends Biochem. Sci. 45, 295–307. doi: 10.1016/j.tibs.2019.12.007
Veltman, D. M., Williams, T. D., Bloomfield, G., Chen, B. C., Betzig, E., Insall, R. H., et al. (2016). A plasma membrane template for macropinocytic cups. eLife 5:e20085.
Venkatachalam, K., Wong, C. O., and Zhu, M. X. (2015). The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium 58, 48–56. doi: 10.1016/j.ceca.2014.10.008
Verkman, A., Weyer, P., Brown, D., and Ausiello, D. (1989). Functional water channels are present in clathrin-coated vesicles from bovine kidney but not from brain. J. Biol. Chem. 264, 20608–20613. doi: 10.1016/s0021-9258(19)47106-8
Verkman, A. S. (1989). Mechanisms and regulation of water permeability in renal epithelia. Am. J. Physiol. 257, C837–C850.
Verkman, A. S. (2005). More than just water channels: unexpected cellular roles of aquaporins. J. Cell Sci. 118, 3225–3232. doi: 10.1242/jcs.02519
Verkman, A. S., Lencer, W. I., Brown, D., and Ausiello, D. A. (1988). Endosomes from kidney collecting tubule cells contain the vasopressin-sensitive water channel. Nature 333, 268–269. doi: 10.1038/333268a0
Verkman, A. S., and Masur, S. K. (1988). Very low osmotic water permeability and membrane fluidity in isolated toad bladder granules. J. Membr. Biol. 104, 241–251. doi: 10.1007/bf01872326
Verkman, A. S., Van Hoek, A. N., Ma, T., Frigeri, A., Skach, W. R., Mitra, A., et al. (1996). Water transport across mammalian cell membranes. Am. J. Physiol. 270, C12–C30.
Vermeulen, M., Giordano, M., Trevani, A. S., Sedlik, C., Gamberale, R., Fernández-Calotti, P., et al. (2004). Acidosis improves uptake of antigens and MHC class I-restricted presentation by dendritic cells. J. Immunol. 172, 3196–3204. doi: 10.4049/jimmunol.172.5.3196
Viet, K. K., Wagner, A., Schwickert, K., Hellwig, N., Brennich, M., Bader, N., et al. (2019). Structure of the human TRPML2 ion channel extracytosolic/lumenal domain. Structure 27, 1246.e5–1257.e5.
Völkl, H., Busch, G. L., Häussinger, D., and Lang, F. (1994). Alkalinization of acidic cellular compartments following cell swelling. FEBS Lett. 338, 27–30. doi: 10.1016/0014-5793(94)80110-x
Völkl, H., and Lang, F. (1988). Ionic requirement for regulatory cell volume decrease in renal straight proximal tubules. Pflügers Arch. 412, 1–6. doi: 10.1007/bf00583723
Von Delwig, A., Hilkens, C. M., Altmann, D. M., Holmdahl, R., Isaacs, J. D., Harding, C. V., et al. (2006). Inhibition of macropinocytosis blocks antigen presentation of type II collagen in vitro and in vivo in HLA-DR1 transgenic mice. Arthritis Res. Ther. 8:R93.
Voss, F. K., Ullrich, F., Munch, J., Lazarow, K., Lutter, D., Mah, N., et al. (2014). Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344, 634–638. doi: 10.1126/science.1252826
Waldegger, S., Barth, P., Raber, G., and Lang, F. (1997). Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc. Natl. Acad. Sci. U.S.A. 94, 4440–4445. doi: 10.1073/pnas.94.9.4440
Waldegger, S., Gabrysch, S., Barth, P., Fillon, S., and Lang, F. (2000). h-sgk serine-threonine protein kinase as transcriptional target of p38/MAP kinase pathway in HepG2 human hepatoma cells. Cell Physiol. Biochem. 10, 203–208. doi: 10.1159/000016351
Walker, S. A., Kupzig, S., Bouyoucef, D., Davies, L. C., Tsuboi, T., Bivona, T. G., et al. (2004). Identification of a Ras GTPase-activating protein regulated by receptor-mediated Ca2+ oscillations. EMBO J. 23, 1749–1760. doi: 10.1038/sj.emboj.7600197
Wang, G., Moniri, N. H., Ozawa, K., Stamler, J. S., and Daaka, Y. (2006). Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc. Natl. Acad. Sci. U.S.A. 103, 1295–1300. doi: 10.1073/pnas.0508354103
Wang, X., Zhang, X., Dong, X. P., Samie, M., Li, X., Cheng, X., et al. (2012). TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 151, 372–383. doi: 10.1016/j.cell.2012.08.036
Ward, D. M., Hackenyos, D. P., Davis-Kaplan, S., and Kaplan, J. (1990). Inhibition of late endosome-lysosome fusion: studies on the mechanism by which isotonic-K+ buffers alter intracellular ligand movement. J. Cell Physiol. 145, 522–530. doi: 10.1002/jcp.1041450319
Warnock, D. G., Kusche-Vihrog, K., Tarjus, A., Sheng, S., Oberleithner, H., Kleyman, T. R., et al. (2014). Blood pressure and amiloride-sensitive sodium channels in vascular and renal cells. Nat. Rev. Nephrol. 10, 146–157. doi: 10.1038/nrneph.2013.275
Warskulat, U., Schliess, F., and Haussinger, D. (1998). Compatible organic osmolytes and osmotic modulation of inducible nitric oxide synthetase in RAW 264.7 mouse macrophages. Biol. Chem. 379, 867–874. doi: 10.1515/bchm.1998.379.7.867
Warskulat, U., Zhang, F., and Haussinger, D. (1996). Modulation of phagocytosis by anisoosmolarity and betaine in rat liver macrophages (Kupffer cells) and RAW 264.7 mouse macrophages. FEBS Lett. 391, 287–292. doi: 10.1016/0014-5793(96)00753-3
Watanabe, K., Morishita, K., Zhou, X., Shiizaki, S., Uchiyama, Y., Koike, M., et al. (2021). Cells recognize osmotic stress through liquid-liquid phase separation lubricated with poly(ADP-ribose). Nat. Commun. 12:1353.
Webb, J. L., Harvey, M. W., Holden, D. W., and Evans, T. J. (2001). Macrophage nitric oxide synthase associates with cortical actin but is not recruited to phagosomes. Infect. Immun. 69, 6391–6400. doi: 10.1128/iai.69.10.6391-6400.2001
Weerasinghe, P., and Buja, L. M. (2012). Oncosis: an important non-apoptotic mode of cell death. Exp. Mol. Pathol. 93, 302–308. doi: 10.1016/j.yexmp.2012.09.018
Weinberg, J. B. (1998). Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol. Med. 4, 557–591. doi: 10.1007/bf03401758
Weinert, S., Jabs, S., Supanchart, C., Schweizer, M., Gimber, N., Richter, M., et al. (2010). Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl–accumulation. Science 328, 1401–1403. doi: 10.1126/science.1188072
Weisz, O. A. (2003). Acidification and protein traffic. Int. Rev. Cytol. 226, 259–319. doi: 10.1016/s0074-7696(03)01005-2
Welliver, T. P., and Swanson, J. A. (2012). A growth factor signaling cascade confined to circular ruffles in macrophages. Biol. Open 1, 754–760. doi: 10.1242/bio.20121784
Wen, M. H., Wang, J. Y., Chiu, Y. T., Wang, M. P., Lee, S. P., and Tai, C. Y. (2016). N-cadherin regulates cell migration through a Rab5-dependent temporal control of macropinocytosis. Traffic 17, 769–785. doi: 10.1111/tra.12402
West, M. A., Bretscher, M. S., and Watts, C. (1989). Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 109, 2731–2739. doi: 10.1083/jcb.109.6.2731
Wiernasz, E., Kaliszewska, A., Brutkowski, W., Bednarczyk, J., Gorniak, M., Kaza, B., et al. (2014). Ttyh1 protein is expressed in glia in vitro and shows elevated expression in activated astrocytes following status epilepticus. Neurochem. Res. 39, 2516–2526. doi: 10.1007/s11064-014-1455-3
Williams, T. D., Peak-Chew, S. Y., Paschke, P., and Kay, R. R. (2019). Akt and SGK protein kinases are required for efficient feeding by macropinocytosis. J. Cell Sci. 132:jcs224998.
Williamson, C. D., and Donaldson, J. G. (2019). Arf6, JIP3, and dynein shape and mediate macropinocytosis. Mol. Biol. Cell 30, 1477–1489. doi: 10.1091/mbc.e19-01-0022
Wilson, Z. N., Scott, A. L., Dowell, R. D., and Odorizzi, G. (2018). PI(3,5)P2 controls vacuole potassium transport to support cellular osmoregulation. Mol. Biol. Cell 29, 1718–1731. doi: 10.1091/mbc.e18-01-0015
Wright, E. M., Loo, D. D., and Hirayama, B. A. (2011). Biology of human sodium glucose transporters. Physiol. Rev. 91, 733–794. doi: 10.1152/physrev.00055.2009
Wu, L., Sun, Y., Ma, L., Zhu, J., Zhang, B., Pan, Q., et al. (2016). A C-terminally truncated mouse Best3 splice variant targets and alters the ion balance in lysosome-endosome hybrids and the endoplasmic reticulum. Sci. Rep. 6:27332.
Wundenberg, T., and Mayr, G. W. (2012). Synthesis and biological actions of diphosphoinositol phosphates (inositol pyrophosphates), regulators of cell homeostasis. Biol. Chem. 393, 979–998. doi: 10.1515/hsz-2012-0133
Xiao, F., Li, J., Huang, K., Li, X., Xiong, Y., Wu, M., et al. (2021). Macropinocytosis: mechanism and targeted therapy in cancers. Am. J. Cancer Res. 11, 14–30.
Xiong, J., and Zhu, M. X. (2016). Regulation of lysosomal ion homeostasis by channels and transporters. Sci. China Life Sci. 59, 777–791. doi: 10.1007/s11427-016-5090-x
Xu, H., and Ren, D. (2015). Lysosomal physiology. Annu. Rev. Physiol. 77, 57–80. doi: 10.1146/annurev-physiol-021014-071649
Yan, Y., Ding, Y., Ming, B., Du, W., Kong, X., Tian, L., et al. (2014). Increase in hypotonic stress-induced endocytic activity in macrophages via ClC-3. Mol. Cells 37, 418–425. doi: 10.14348/molcells.2014.0031
Yang, L., Song, L., Zhao, S., Ma, C., Wu, D., and Wu, Y. L. (2019). Isobavachalcone reveals novel characteristics of methuosis-like cell death in leukemia cells. Chem. Biol. Interact. 304, 131–138. doi: 10.1016/j.cbi.2019.03.011
Yasui, M., Hazama, A., Kwon, T. H., Nielsen, S., Guggino, W. B., and Agre, P. (1999). Rapid gating and anion permeability of an intracellular aquaporin. Nature 402, 184–187. doi: 10.1038/46045
Ye, R. G., Shi, L. B., Lencer, W. I., and Verkman, A. S. (1989). Functional colocalization of water channels and proton pumps in endosomes from kidney proximal tubule. J. Gen. Physiol. 93, 885–902. doi: 10.1085/jgp.93.5.885
Yoo, D. Y., Barros, S. A., Brown, G. C., Rabot, C., Bar-Sagi, D., and Arora, P. S. (2020). Macropinocytosis as a key determinant of peptidomimetic uptake in cancer cells. J. Am. Chem. Soc. 142, 14461–14471. doi: 10.1021/jacs.0c02109
Yoshida, S., Gaeta, I., Pacitto, R., Krienke, L., Alge, O., Gregorka, B., et al. (2015). Differential signaling during macropinocytosis in response to M-CSF and PMA in macrophages. Front. Physiol. 6:8. doi: 10.3389/fphys.2015.00008
Yoshida, S., Pacitto, R., Sesi, C., Kotula, L., and Swanson, J. A. (2018). Dorsal ruffles enhance activation of Akt by growth factors. J. Cell Sci. 131:jcs220517.
Yuan, A., and Chia, C. P. (2001). Giant vacuoles observed in Dictyostelium discoideum. Cell Biol. Int. 25, 147–155. doi: 10.1006/cbir.2000.0577
Zani, B. G., and Bohlen, H. G. (2005). Sodium channels are required during in vivo sodium chloride hyperosmolarity to stimulate increase in intestinal endothelial nitric oxide production. Am. J. Physiol. Heart Circ. Physiol. 288, H89–H95.
Zen, K., Biwersi, J., Periasamy, N., and Verkman, A. S. (1992). Second messengers regulate endosomal acidification in Swiss 3T3 fibroblasts. J. Cell Biol. 119, 99–110. doi: 10.1083/jcb.119.1.99
Zeuthen, T. (2010). Water-transporting proteins. J. Membr. Biol. 234, 57–73. doi: 10.1007/s00232-009-9216-y
Zeuthen, T., and Macaulay, N. (2012). Cotransport of water by Na(+)-K(+)-2Cl(-) cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J. Physiol. 590, 1139–1154. doi: 10.1113/jphysiol.2011.226316
Zhang, L., Qu, Z., Wu, J., Yao, S., Zhang, Q., Zhang, T., et al. (2021). SARs of a novel series of s-triazine compounds targeting vimentin to induce methuotic phenotype. Eur. J. Med. Chem. 214:113188. doi: 10.1016/j.ejmech.2021.113188
Zhang, X., Cheng, X., Yu, L., Yang, J., Calvo, R., Patnaik, S., et al. (2016). MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 7: 12109.
Zhao, H., Atkinson, J., Gulesserian, S., Zeng, Z., Nater, J., Ou, J., et al. (2018). Modulation of macropinocytosis-mediated internalization decreases ocular toxicity of antibody-drug conjugates. Cancer Res. 78, 2115–2126. doi: 10.1158/0008-5472.can-17-3202
Zhao, J., Zhu, D., Zhang, X., Zhang, Y., Zhou, J., and Dong, M. (2019). TMEM206 promotes the malignancy of colorectal cancer cells by interacting with AKT and extracellular signal-regulated kinase signaling pathways. J. Cell Physiol. 234, 10888–10898. doi: 10.1002/jcp.27751
Zheng, T., Gao, Y., Deng, X., Liu, H., Liu, J., Liu, R., et al. (2018). Comparisons between graphene oxide and graphdiyne oxide in physicochemistry biology and cytotoxicity. ACS Appl. Mater. Interfaces 10, 32946–32954. doi: 10.1021/acsami.8b06804
Zhou, J., Tai, G., Liu, H., Ge, J., Feng, Y., Chen, F., et al. (2009). Activin A down-regulates the phagocytosis of lipopolysaccharide-activated mouse peritoneal macrophages in vitro and in vivo. Cell Immunol. 255, 69–75. doi: 10.1016/j.cellimm.2008.11.001
Zhu, J. Y., Tu, W., Zeng, C., Mao, H. X., Du, Q. F., and Cai, H. B. (2017). [Mechanism of Platycarya strobilacea Sieb. et Zucc extract-induced methuosis in human nasopharyngeal carcinoma CNE1 and CNE2 cells]. Nan Fang Yi Ke Da Xue Xue Bao 37, 827–832.
Zhu, M., Wu, G., Li, Y. X., Stevens, J. K., Fan, C. X., Spang, A., et al. (2015). Serum- and glucocorticoid-inducible kinase-1 (SGK-1) plays a role in membrane trafficking in Caenorhabditis elegans. PLoS One 10:e0130778. doi: 10.1371/journal.pone.0130778
Zifarelli, G. (2015). A tale of two CLCs: biophysical insights toward understanding ClC-5 and ClC-7 function in endosomes and lysosomes. J. Physiol. 593, 4139–4150. doi: 10.1113/jp270604
Zong, W. X., and Thompson, C. B. (2006). Necrotic death as a cell fate. Genes Dev. 20, 1–15. doi: 10.1101/gad.1376506
Keywords: pinocytosis, macropinocytosis, endocytosis, intracellular vesicle, ion transport, cell volume regulation, cell death, methuosis
Citation: Ritter M, Bresgen N and Kerschbaum HH (2021) From Pinocytosis to Methuosis—Fluid Consumption as a Risk Factor for Cell Death. Front. Cell Dev. Biol. 9:651982. doi: 10.3389/fcell.2021.651982
Received: 11 January 2021; Accepted: 29 April 2021;
Published: 23 June 2021.
Edited by:
Baojun Zhang, Xi’an Jiaotong University, ChinaReviewed by:
Yong Liu, Xuzhou Medical University, ChinaLianjun Zhang, Center of Systems Medicine, Chinese Academy of Medical Sciences, Suzhou Institute of Systems Medicine, China
Copyright © 2021 Ritter, Bresgen and Kerschbaum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Markus Ritter, bWFya3VzLnJpdHRlckBwbXUuYWMuYXQ=; Hubert H. Kerschbaum, SHViZXJ0LktlcnNjaGJhdW1Ac2JnLmFjLmF0
 Markus Ritter
Markus Ritter Nikolaus Bresgen6
Nikolaus Bresgen6 Hubert H. Kerschbaum
Hubert H. Kerschbaum