- 1Department of Urology, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Shanghai Key Laboratory of Organ Transplantation, Shanghai, China
Intratumoral fibrosis is a histologic manifestation of fibrotic tumor stroma. The interaction between cancer cells and fibrotic stroma is intricate and reciprocal, involving dysregulations from multiple biological processes. Different components of tumor stroma are implicated via distinct manners. In the kidney, intratumoral fibrosis is frequently observed in renal cell carcinoma (RCC). However, the underlying mechanisms remain largely unclear. In this review, we recapitulate evidence demonstrating how fibrotic stroma interacts with cancer cells and mechanisms shared between RCC tumorigenesis and renal fibrogenesis, providing promising targets for future studies.
Introduction
Renal fibrosis is the common outcome of different chronic kidney diseases (CKDs), characterized by excessive accumulation of extracellular matrix (ECM) and disrupted renal microarchitecture (Hewitson, 2009). Formation of fibrosis involves numbers of cell subtypes, including epithelial, endothelial, and inflammatory cells with a purpose to trigger fibrosis and fibroblasts, pericytes that execute fibrosis (Lovisa et al., 2016). The intricate cross-talk between these cells has been brought to understanding but still remains largely controversial.
Renal cell carcinoma (RCC) is one of the most common malignancies. It accounts for 85% of kidney neoplasms (Cancer.Net, 2020), the global incidence of which was estimated to be 403,000 in 2018 worldwide (Bray et al., 2018). It is classified into mainly three subtypes, namely, clear cell RCC (ccRCC), papillary RCC (PRCC), and chromophobe RCC. The identification of von Hippel–Lindau (VHL) in ccRCC has furthered our understanding of the underlying mechanisms of RCC formation. Tumor suppressor VHL serves as a substrate recognition subunit of a ubiquitin ligase targeting hypoxia-inducible factor (HIF). Inactivation of VHL results in abnormal stabilization of HIF pathway, favoring atypical cell growth through promoting cell survival under hypoxia condition (Kaelin, 2008).
As a persistent tissue injury, cancer cells initiate a chronic wound healing response in tumors, namely, intratumoral fibrosis (ITF). ITF is the result of aberrant accumulation of collagen matrix produced by cancer-associated fibroblasts (CAFs) (Liu et al., 2019b). As a highly vascularized tumor, RCC is frequently found with ITF. Joung et al. (2018) reported that among 204 ccRCC cases, 167 (81.7%) showed ITF. Although the correlation between prognosis of ccRCC and ITF is not significant, ITF proves to be related to other poor prognostic factors in ccRCC including Fuhrman nuclear grade, intratumor necrosis, and lymphovascular invasion. It is safe to acknowledge the contribution of tumor cell microenvironment in tumorigenesis. A wide range of studies have been conducted to elucidate the underlying interactions between fibrosis and cancer. The microenvironment surrounding tumor cells serves as both powerful tumor suppressor and tumor promoter (Sternlicht et al., 1999). Fibroblasts, the dominant component of tumor stroma, were proved to induce oncogenic potential of adjacent epithelia (Bhowmick et al., 2004) and associated with early and advanced stages of tumor progression (Rønnov-Jessen et al., 1996; Giussani et al., 2015). Formation of mature tumor ECM is marked by high density of fibrillar collagens, especially type I collagen, and capable of resisting degradation and repetitive mechanical stress (Yamauchi et al., 2018). Cancer cells trigger the formation of tumor stroma and stiffening stroma benefits tumor growth in return, suggesting that the dynamics between stroma and cancer cells is mutual. Evidence in different organs sustains that instead of merely preceding or tailing cancer formation, fibrosis participates in the cancer formation and metastasis (Neesse et al., 2015; Saito et al., 2018; Tzouvelekis et al., 2019). However, the evidence describing the correlation between cancer and fibrotic stroma both clinically and mechanically in kidney is limited.
In this review, we introduce how fibrotic stroma interacts with tumor cells in different organs: (1) the interplay between fibrotic stroma and cancer cells via metabolic manners; (2) how signaling mediates features of fibronectin (FN) and enzymes regulating collagen exert a protumor effect; (3) robust reciprocal communications between cancer cells and CAFs mediated by secretory molecules; (4) demonstration of the pro-inflammatory feature of CAFs and the controversial involvement of ECM in tumor immunity. Next, we focus on demonstrating the potential role of different signaling pathways including mammalian target of rapamycin (mTOR), Wnt, and Notch and molecules including non-coding RNA (ncRNA), fumarate hydratase (FH), and other molecules, promoting both renal fibrosis and RCC, which hopefully may provide valid insights for future studies regarding the correlation between these two pathogeneses.
The Relationship Between Tumor and Stroma
Metabolic Interaction Between Cancer Cells and Stroma
Fibrotic stroma drives metabolic shifts in cancer cells, fostering multiple malignant features. After being activated, CAFs also shift to aerobic glycolysis (Vander Heiden et al., 2009). CAFs have been shown to promote glycolysis in ovarian cancer cells by inducing phosphorylation and activation of phosphoglucomutase 1, facilitating proliferation and metastasis (Curtis et al., 2019). Aspartate generated by CAFs is shown to promote tumor proliferation. In return, glutamate secreted by tumor cells contributes to maintaining redox homeostasis of CAFs through glutathione pathway (Bertero et al., 2019). Involvement of lactate and pyruvate in promoting the cell growth is also identified in different cancer cell types (Sanford-Crane et al., 2019). In addition to aberrant secretion from CAFs, alterations in ECM exert a certain influence on cancer cell metabolism. Increased collagen density in ECM was shown to be associated with decreased oxygen consumption and glucose metabolism in breast cancer cells (Morris et al., 2016). Degradation of hyaluronan promoted glucose uptake in several cancer cell lines. Induction of glycolysis by hyaluronidase accelerated cell migration (Sullivan et al., 2018).
The Protumor Effect of Fibronectin and Collagen
The components of tumor stroma contribute to various tumor hallmarks. Tumor-associated stroma rich in FN and type I collagen was proved to be associated with enhanced cancer progression (Li et al., 2003). As the adhesion protein, FN provides the basic scaffold for nascent collagen deposition by fibroblasts, which is crucial to regulate cell proliferation and migration (Sottile and Hocking, 2002). FN plays a significant role in directing signals, by binding to a wide range of growth factors including transforming growth factor-β (TGF-β) superfamily, fibroblast growth factor (FGF) family, insulin-like growth factor binding protein-5 (IGFBP-5), and IGFBP-3 via FN III12–14, a highly promiscuous GF binding domain (Martino and Hubbell, 2010). FN-rich ECM drives desmoplastic differentiation of normal fibroblasts (Amatangelo et al., 2005). In RCC, FN was shown to promote cell growth and migration in part via Src and TGF-β1 signaling in vitro, the mechanism of which was not clearly demonstrated (Ou et al., 2019).
As the most abundant ECM scaffolding protein in the stroma, collagen is significantly associated with the tensile strength (Kolácná et al., 2007). Type I collagen protected against tumor invasion, while increased collagen I expression was related to elevated incidence of metastasis (Ramaswamy et al., 2003). Lysyl oxidase (LOX) and LOX-like (LOXL) family members initiate collagen cross-linking by catalyzing the oxidative deamination of Lys and Hyl residues and are found elevated in different tumors (Erler et al., 2009). Levental et al. (2009) proved that LOX-mediated collagen cross-linking, final step of collagen biosynthesis, stiffened the matrix, thereby promoting focal adhesions and tumor progression. Cox et al. (2013) showed that cross-linking of collagen I enhanced metastatic growth and that LOX-mediated collagen cross-linking increased tumor cell proliferation and metastasis. In RCC, studies showed that procollagen-lysine, 2-oxoglutarate 5-dioxygenases1/2/3 (PLOD1/2/3), and LOXL2, both collagen-modifying enzymes, were related to high pathological grades; however, the underlying mechanisms are vaguely depicted (Hase et al., 2014; Xu et al., 2019). Lysyl hydroxylase 2 (LH2), which is responsible for the overhydroxylation of the collagen telopeptides (van der Slot et al., 2004), shifted the tumor stroma toward high-Hylald-derived collagen cross-links, low-Lysald-derived collagen cross-link state, increasing tumor stiffness, and enhanced tumor cell invasion and metastasis (Chen et al., 2015). This evidence suggests both that the quality and the quantity of collagen are related to tumor progression via different mechanisms.
Cancer-Associated Fibroblast and Cancer Cells
Among all the stromal cells, CAFs share most the intricate relationship with cancer cells. Under the unabated influence of a large array of stimuli, e.g., growth factors, cytokines, and chemokines, normal fibroblasts get activated into CAFs irreversibly. CAFs display promoted secretory phenotypes, ECM remodeling ability, and immunomodulatory functions, which regulate different cancer traits (Kalluri, 2016).
Stromal cell-derived factor 1 (SDF-1) secreted by CAFs was found to accelerate tumor growth directly and promote angiogenesis via recruiting endothelial progenitor cells (Orimo et al., 2005). In RCC, under hypoxic conditions, accumulation of HIF-1α upregulated chemokine receptor 4 (CXCR4), the receptor of SDF-1, leading to elevated metastatic ability (Pan et al., 2006). This evidence suggested that SDF-1/CXCR4 biological axis regulated organ-specific metastasis of RCC. As ECM-degrading proteases, matrix metalloproteinase (MMP)-1 and 3 produced by the CAFs contribute to tumor invasiveness (Lochter et al., 1997; Boire et al., 2005). A similar correlation was reported in RCC. Paracrine platelet-derived growth factor-CC (PDGF-CC) signaling pathway was reported to control breast cancer basal-like subtype (Roswall et al., 2018). The evidence of CAF secretion enhancing tumorigenesis is numerous and comprehensive. In breast cancer, cancer-derived osteopontin and WNT7A activated mesenchymal stem cells into CAFs and enhanced invasive features of CAFs, respectively, in a TGF-β-dependent manner (Weber et al., 2015; Avgustinova et al., 2016). ccRCC cells induced CAF-derived periostin expression, and elevated periostin promoted tumor cell itself and CAF proliferation, in return (Bakhtyar et al., 2013). Taken together, these evidences indicate a robust reciprocal relationship between cancer cells and CAFs. Particularly interesting is the physical force that CAFs exert on cancer cells promoting cancer invasion, via E-/N-cadherin adhesion (Labernadie et al., 2017).
Given the mounting publications delineating protumor effects of CAFs, it is reasonable to assume that increased fibrosis is positively associated with poor prognosis. However, signs of cancer cells progression being impeded by the tumor stroma have also been observed. In non-small cell lung carcinoma (NSCLC), a correlation of increased desmoplasia with longer survival was observed (Paulsson and Micke, 2014). Moreover, in pancreatic ductal adenocarcinoma cells, deletion of sonic hedgehog (SHH), an overexpressed soluble ligand driving formation of a fibroblast-rich desmoplastic stroma, results in more malignant features. The tumor-suppressing effect could be partially due to the unique capability of Hedgehog-driven stroma to restrain tumor angiogenesis (Rhim et al., 2014). Slit2 and Asporin, both secreted by stromal fibroblasts, were identified as tumor suppressor in breast cancer. Slit2, a ligand of Robo1 receptor, was found to restrain tumorigenesis via blocking PI3K/AKT/β-catenin pathway (Chang et al., 2012). High expression of Asporin, an inhibitor of TGF-β1, was significantly associated with less aggressive tumors (Maris et al., 2015). However, the exact subtype of stromal fibroblasts responsible for expressing Slit2 and Asporin remains to be determined. More studies are required to demonstrate the tangled functions of CAFs.
Fibrotic Stroma and Cancer Immunity
As another essential component of tumor stroma, immune cells receive heated attention following the success of novel immunotherapies targeting adaptive immune system. Chemokine ligand 12 (CXCL12) solely produced by CAFs was shown to negatively regulate T-cell accumulation. By targeting it, a promising synergistic effect with anti-PD-L1 immunotherapy was observed in pancreatic cancer (Feig et al., 2013). On the other hand, the innate immune system is of great significance as well, given its dynamic reciprocity between fibrosis and inflammation (Alexander and Cukierman, 2016).
CAFs regulate hallmark features of tumor by mediating tumor-promoting inflammation. A large array of cytokines and chemokines are related to CAFs and exert pro-inflammatory effects (Servais and Erez, 2013; Acerbi et al., 2015). Pro-inflammatory gene signature has been identified in CAFs in different organs, and the underlying mechanisms are becoming understood. CAFs were shown to promote tumor growth and macrophage recruitment with participation of nuclear factor-kappaB (NF-κB) signaling pathway (Erez et al., 2010). More evidence suggests that CAFs induce Th2 and Th17 inflammation response in a thymic stromal lymphopoietin (TSLP)-dependent manner and TLR, nucleotide oligomerization binding domain 2 signaling, respectively (Su et al., 2010; De Monte et al., 2011). In contrast to various cross-talks between immune cells and CAFs, fibrotic ECM serves as a barrier against immune cells. Matrix areas packed with aligned fiber and collagen hindered migration of T cells, blocking them from approaching cancer cells (Salmon et al., 2012; Hartmann et al., 2014; Chen et al., 2018). However, under the assistance of a novel computational imaging technology, Carstens et al. (2017) discovered no positive correlation between T-cell accumulation and collagen-I, α-SMA fibroblasts. Further investigation is required to determine the specific contribution of each component of ECM and to explore corresponding therapeutic treatments.
Cancer cells trigger the alterations in stroma. A reciprocal relationship is identified in all four sections, especially a notably beneficial interaction between CAFs and cancer cells. To various degrees, most components of fibrotic stroma including infiltrating lymphocytes are implicated. Secretory molecules play a vital part in the communications between fibrotic stroma and cancer cells owing to their capability of recruiting and activating the target cells. Tumor immunity and metabolism both contribute greatly to RCC tumorigenesis. Evidence revealed how metabolic alterations in RCC affected tumor immune microenvironment (Xiao and Meierhofer, 2019). However, no effort has been made to determine whether fibrotic stroma re-shapes the tumor immunity in RCC as well. Crossing these two fields yields a promising direction for future exploration.
Mechanisms Shared Between Renal Cell Carcinoma Tumorigenesis and Renal Fibrogenesis
Wnt Signaling
Initiated by Wnt ligands binding to the extracellular domain of frizzled (Fzd) receptor and co-receptors, low-density lipoprotein receptor-related proteins 5 and 6 (LRP5 and LRP6), the canonical Wnt signaling depends on the intracellular molecular β-catenin to exert its influence on multiple biologic processes (Clevers and Nusse, 2012). Wnt ligands embrace 19 different members; and 16 of them, except Wnt3a, Wnt8a, and Wnt10b, are upregulated in the unilateral ureteral obstruction (UUO) model (He et al., 2009). Zhou et al. (2017) reported that blocking the WNT secretion in renal tubular cells reduced β-catenin activation and inhibited myofibroblast activation in vivo, whereas blocking in fibroblasts showed little effect, suggesting that Wnt/β-catenin signaling displays its functions in the tubular epithelium in the renal fibrotic diseases. WNT1 has been reported to be related to both RCC and renal fibrosis. Not only high WNT1 was associated with more advanced stage, increased size, and overall survival, but it also promoted renal fibroblast proliferation in vitro (Kruck et al., 2013; Maarouf et al., 2016). However, few research on WNT1 has been conducted to explore the interaction between the two major diseases so far. Moreover, WNT2, WNT3A, and WNT4 were shown to induce fibroblast proliferation and myofibroblast differentiation in vitro, respectively (DiRocco et al., 2013; Zhou et al., 2017). WNT10A expression induced RCC cell proliferation and aggressiveness, while WNT7A displayed tumor suppression function in vitro (Hsu et al., 2012; Kondratov et al., 2012). Abnormal accumulation of β-catenin was related to both renal fibrogenesis and RCC carcinogenesis (Kruck et al., 2013; Maarouf et al., 2015). On the other hand, the expression of Fzd7 and mRNA expression of Fzd5 and 8 were shown to be upregulated in RCC and contributed to cell proliferation (Janssens et al., 2004; Xu et al., 2016), while no Fzd genes were repressed after obstructive injury, suggesting an underlying correlation to explore (He et al., 2009). Extensive studies have determined the functions of different components of Wnt signaling in renal fibrosis and RCC, whereas the interaction between these two fields has been rarely explained.
Mammalian Target of Rapamycin Signaling
The mTOR is a component of two distinct complexes, mTOR complex 1 (mTORC1) and mTORC2. As an evolutionarily conserved serine–threonine kinase, mTOR regulates cell growth, proliferation, autophagy, and metabolism (Ma et al., 2018). AMP-activated protein kinase (AMPK) and PI3K-AKT-dependent pathways converge on tuberous sclerosis complex (TSC), which subsequently activates mTORC1 by releasing Rheb, a Ras family GTPase. The well-described downstream factors of mTORC1 include p70S6K and 4EBP, which favor cell growth and proliferation via enhancing proteins and nucleotide synthesis (Fantus et al., 2016). Mechanically, mTORC1 is better characterized in both kidney malignancy and fibrosis. Chen et al. (2012) determined the interstitial macrophages and myofibroblasts as the main cell subtypes with persistent activation of mTORC1 signaling. Decreased levels of profibrotic cytokines, including TGF-β1, VEGF, glomerular connective tissue growth factor, and monocyte chemoattractant protein-1, were observed in models treated with rapamycin in vivo (Lloberas et al., 2006; Yang et al., 2007; Liu et al., 2014). Rapamycin was proved to reduce tubulointerstitial fibrosis in the UUO model and block TGF-β1-induced loss of E-cadherin expression, suggesting that mTOR signaling also contributed to the transdifferentiation from tubular epithelial cells to α-SMA-positive myofibroblasts (Wu et al., 2006). In addition to mTORC1, the engagement of mTORC2 in renal fibrogenesis was also recognized. Li et al. (2015) reported that Rictor/mTORC2 signaling induced TGF-β1-promoted fibroblast activation independent of mTORC1 signaling, indicating that both mTORC signaling was involved in the fibroblast response to TGF-β1.
As an intermediate regulator, a wide range of molecules contribute to RCC different malignant phenotypes via mTOR signaling pathway, including pyruvate kinase M2 (PKM2) (Dey et al., 2019), enoyl-CoA hydratase short-chain 1 (ECHS1) (Qu et al., 2020), nucleobindin-2 (NUCB-2) (Tao et al., 2020), miR-100 (Liu et al., 2020b), and maternal and embryonic leucine zipper kinase (MELK) (Zhang et al., 2019). Mutations in upstream factors of mTOR signaling pathway were also involved. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) mutation correlated with high-grade, advanced ccRCCs with enhanced ability of invasion (Kondo et al., 2001). In addition to the well-established TSC-mTOR signaling, Brugarolas et al. (2003) revealed an mTOR-independent pathway, possibly associated with chromatin remodeling. Intriguingly, epithelial–mesenchymal transition (EMT) was induced though mTOR pathway in two diseases (Wu et al., 2006; Tao et al., 2020).
Currently, use of mTOR inhibitor is mainly restricted to patients with advanced RCC and refractory to anti-VEGF therapy. Temsirolimus and everolimus both targeting mTORC1 were put into clinical use (Hudes et al., 2007; Motzer et al., 2008). In order to avoid activation of phosphatidylinositol 3-kinase (PI3K)/AKT initiated by sole inhibition of mTORC1, novel mTOR ATP-competitive blocker AZD-2014 targeting mTORC1/2 was developed and showed superior potency to restrain RCC cell growth both in vivo and in vitro as compared with mTORC1 inhibitor (Zheng et al., 2015). Interestingly, AZD-2014 activated cancer cells autophagy, which could prolong cancer cells survival. Co-administration of autophagy inhibitor 3-MA enhanced AZD-2014 growth arrest effect. However, in the randomized Phase II study, AZD-2014 failed to surpass everolimus in progression-free survival and overall survival in patients with VEGF-refractory metastatic ccRCC (Powles et al., 2016). Autophagy was also found to be activated in CAFs and foster tumor progression via modulating secretory factors including IL-6 and IL-8 in head and neck cancer (New et al., 2017). Focusing on the combined therapy of deactivation of autophagy in both cancer cells and tumor stroma and developing novel mTORC1/2 dual inhibitor could forward mTOR inhibitors to overcome resistance and display better efficacy in clinical trials.
Non-coding RNA
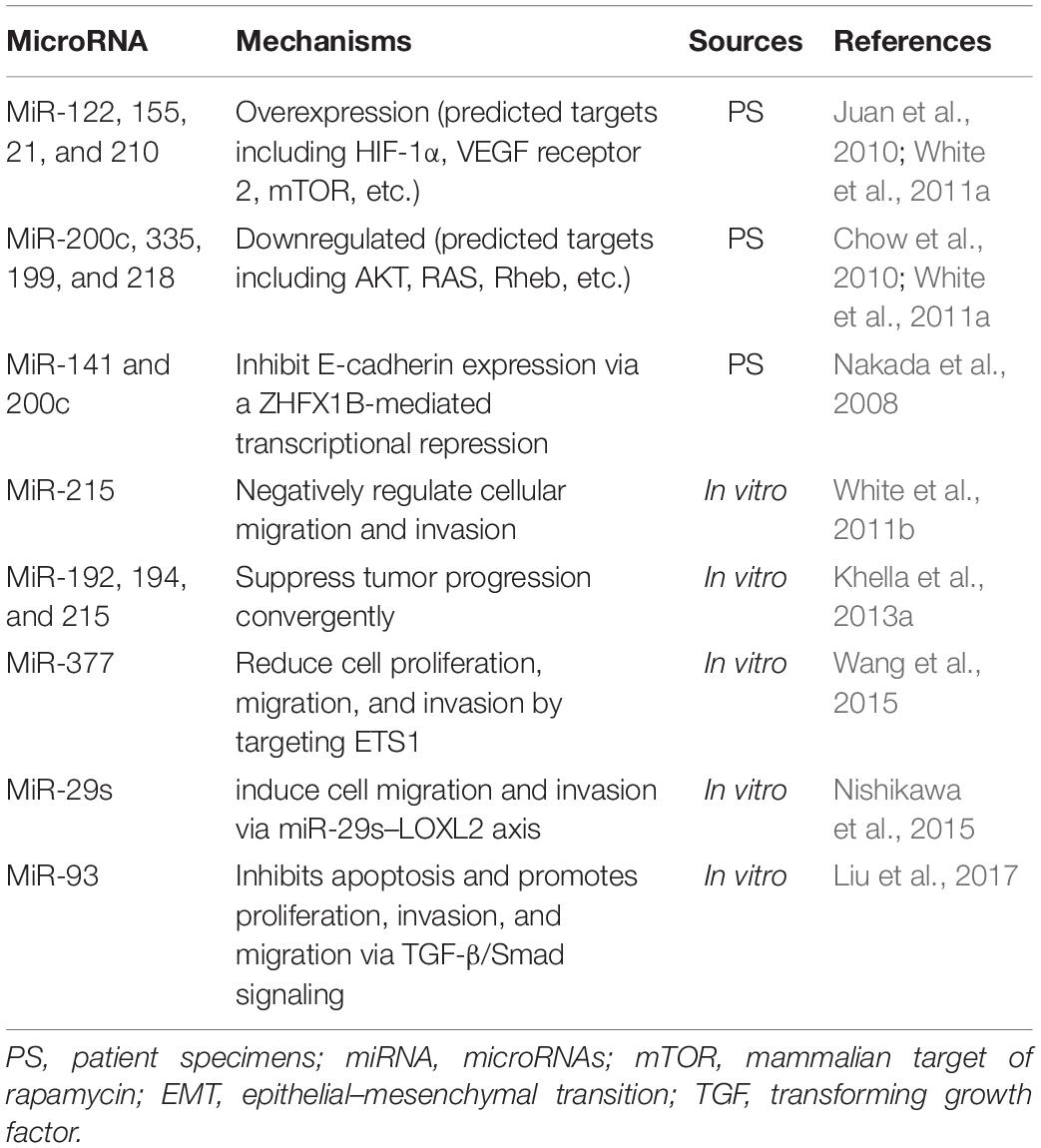
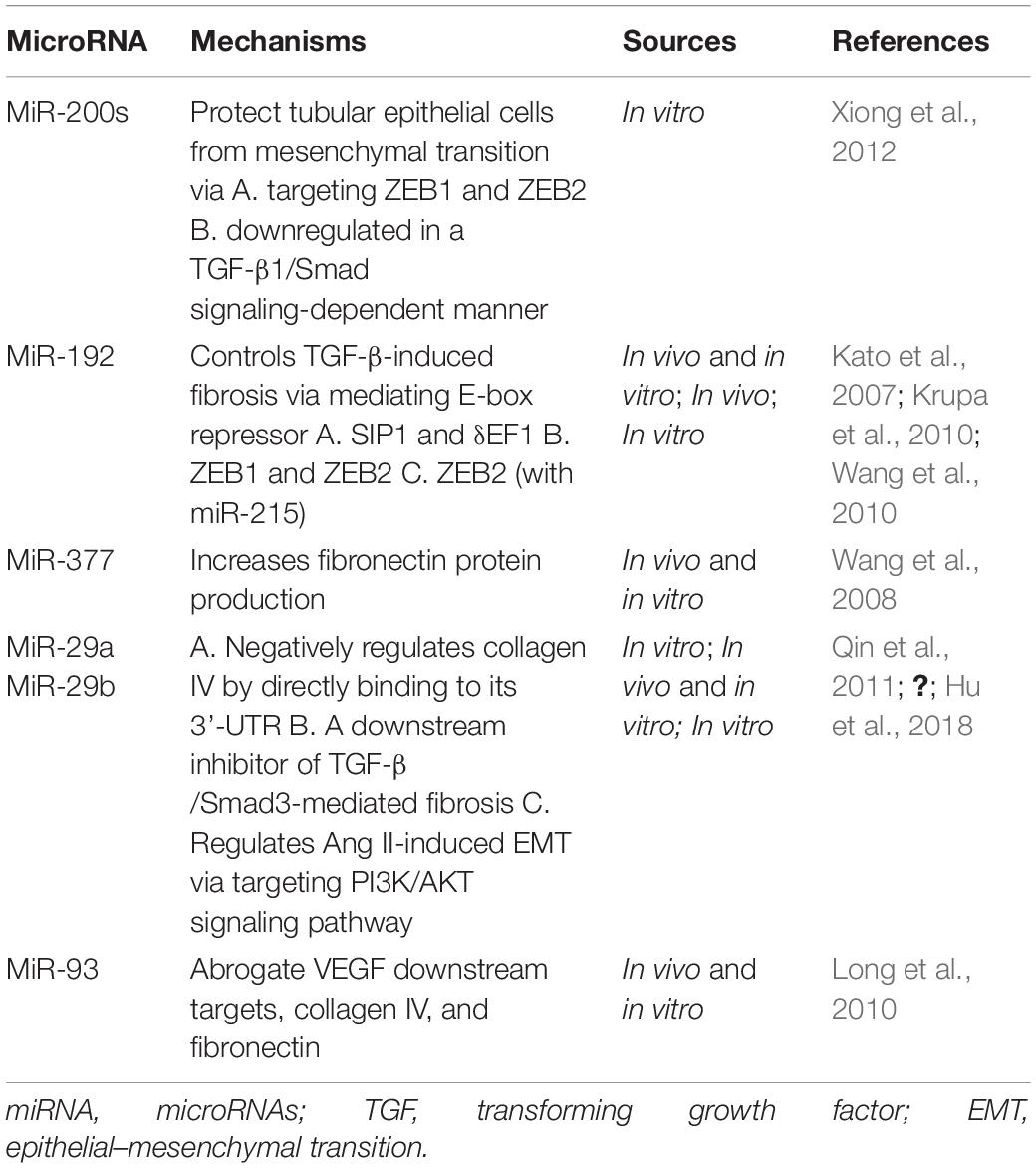
NcRNAs is divided into two classes mainly by their length: small (<200 nucleotides) and long (>200 nucleotides) ncRNAs. MicroRNAs (miRNAs) are included in the small ncRNAs, along with small interfering RNAs and small nucleolar RNAs (Mattick and Makunin, 2006). MiRNAs are short ncRNAs that modulate various physiological and pathological processes by negatively regulating the expression of their target genes via blockade of protein translation or by inducing mRNA degradation (Ambros, 2004). The studies of miRNAs profiling shed some light on the role of miRNAs in RCC tumorigenesis, while the underlying mechanism has not been well-demonstrated. A fraction of studies merely predicted the target genes of dysregulated miRNA using different analysis approaches without verifying it experimentally. Some of these genes are related to RCC tumorigenesis, remaining to be promising directions (Table 1). On the other hand, efforts have been made to delineate how miRNAs contribute to fibrosis, mostly in diabetic nephropathy (DN). Multiple studies we detected overlap on the E-box repressor such as δEF1, Smad-interacting protein 1 (SIP1), zinc finger E-box binding homeobox 1 (ZEB1), and ZEB2, indicating its significant role as a mediator in TGF-induced fibrosis (Table 2).
Nakada et al. (2008) reported that downregulation of miR-141 and miR-200c in ccRCC suppressed CDH1/E-cadherin transcription via upregulation of ZEB2, also known as ZFHX1B. MiR-200a and miR-141 were shown to abrogate EMT of tubular epithelial cells by targeting ZEB1 and ZEB2, revealing an anti-fibrotic effect of the miR-200 family (Jung et al., 2009). This evidence demonstrated that miR-200 family was involved in mediating the transcriptional repressor of E-cadherin and induction of EMT, leading to RCC and renal fibrosis separately. Additionally, several studies identified engagement of multiple miRNAs including miR-382 (Kriegel et al., 2010), miR-23a (Xu et al., 2018), and miR-133b and 199b (Sun et al., 2018) in renal fibrogenesis or tumorigenesis via EMT, suggesting EMT as a promising target to bridge two diseases.
Both classes are well-established to exert a certain influence on various biological processes, and the interactions among them are coming into the view (Yoon et al., 2014). Long ncRNAs (lncRNAs) regulated miRNA function by acting as miRNA sponges and inhibiting their binding to target mRNAs (Paraskevopoulou and Hatzigeorgiou, 2016). Fibrogenic effects of lncRNAs were observed in several CKD models. Liu et al. (2020a) showed that metastasis-associated lung adenocarcinoma transcript 1 (MALAT1)/miR-145/focal adhesion kinase (FAK) pathway was implicated in TGF-β1-induced renal fibrosis in obstructive nephropathy. MALAT1 regulated high glucose (HG)-induced EMT and fibrosis by functioning as a sponge RNA for miR-145, resulting in derepressing the expression of target gene ZEB2 (Liu et al., 2019a). Multiple publications revealed how MALAT1 contributed to RCC tumorigenesis (Xiao et al., 2015; Zhang et al., 2015). Hirata et al. (2015) reported that MALAT1 promoted RCC progression via Ezh2, the potential binding protein of MALAT1, and interacting with miR-205, which led to blockage of EMT via E-cadherin recovery and β-catenin downregulation. Additionally, various lncRNAs have been confirmed to facilitate processes such as cell migration, metastasis, invasion, proliferation, and apoptosis verified in different cell lines (Moghaddas Sani et al., 2018).
New targets and functions of miRNAs are being determined at a tremendous rate; however, our understanding of miRNAs fails to go further correspondingly. The overlap of target gene prediction using different algorithms is far from satisfactory, and subsequent experimental validations are inadequate. Moreover, one target gene could be controlled by multiple miRNAs, and vice versa (Khella et al., 2013b). Studies focusing on convergent and divergent effects of certain miRNAs would be more eloquent to elucidate how such network regulates its target gene. On the grounds that lncRNAs display its function partly through regulating miRNAs, it is paramount to launch studies delineating the network interactions between the two different classes of ncRNAs, hopefully reaching a more comprehensive understanding.
Notch Signaling
The Notch signaling pathway is an evolutionarily conserved signaling pathway, composed of four Notch receptors (Notch 1–4) and five ligands [delta-like ligand (DLL)-1, DLL-3, DLL-4, Jagged-1, and Jagged-2] (Artavanis-Tsakonas et al., 1999). Its role in renal malignancy and fibrosis was demonstrated separately. Huang et al. (2018) recognized that Jagged1 and Notch2 contributed to kidney fibrosis development by regulating mitochondrial transcription factor A (Tfam) expression and metabolic reprogramming. Notch-induced kidney fibrosis was related to metabolic dysregulations and could be restored by peroxisomal proliferator-g coactivator-1a (PGC-1a). The downstream target of Notch1 signaling, Hes1, was capable of regulating PGC-1a directly (Han et al., 2017). Both publications revealed aberrant metabolism disturbed by activated Notch signaling, resulting in a profibrotic feature.
High-level expression of Notch signaling positively correlates with tumor size, nuclear grade, and TNM stage and risk of metastasis in T1 stage ccRCC (Wu et al., 2011; Ai et al., 2012). JAGGED1 and 2 were confirmed to be associated with loss of CpG methylation of H3K4me1-associated enhancer regions and gene amplification, respectively, indicating that the activation of Notch signaling pathway could result from both genetic and epigenetic alterations (Bhagat et al., 2017). Activated Notch signaling was identified in renal cancer stem cells by both transcriptional profiling and single-cell sequencing (Fendler et al., 2020). Xiao et al. (2017) showed that overexpression of Notch1 exerted an upregulatory impact on chemotaxis of RCC cancer stem cells via SDF-1/CXCR4 axis.
Fumarate Hydratase
In terms of FH, extensive researches have been carried out to demonstrate how such mutations of metabolic enzymes engage in hereditary cancer syndromes. FH inactivation was proved to predispose individuals to hereditary leiomyomatosis and renal cell cancer (HLRCC) (Kim and Kaelin, 2006). In addition to Krebs cycle, further studies spotted significant changes in the urea cycle and determined cytosolic metabolic pathways in FH-associated oncogenesis (Adam et al., 2013). Both in vivo and in vitro evidence supported that accumulation of fumarate caused stabilization of HIF-1α (Pollard et al., 2005). However, based on a much more thorough study, a distinct mechanism of Fh1-dependent, the murine homolog of FH, cyst formation was proposed. Adam et al. (2011) provided solid evidence asserting the absence of Hif/Phd pathway and introducing nuclear factor-like 2 (NRF2) dysregulation as an oncogenic pathway involved in FH-associated disease. Interestingly, FH inactivation also engages in renal fibrosis. Reduction of FH caused the accumulation of fumarate, leading to fibrosis in DN in Goto–Kakizaki (GK) rats. Increased levels of HIF-1α and TGF-β1 were detected, suggesting candidate mechanisms accounting for such fibrosis (Miura et al., 2019). Although these studies on FH inactivation leading to renal carcinoma and fibrosis were conducted separately, it still provided insights to bridge our understanding of two major diseases.
Other Molecules
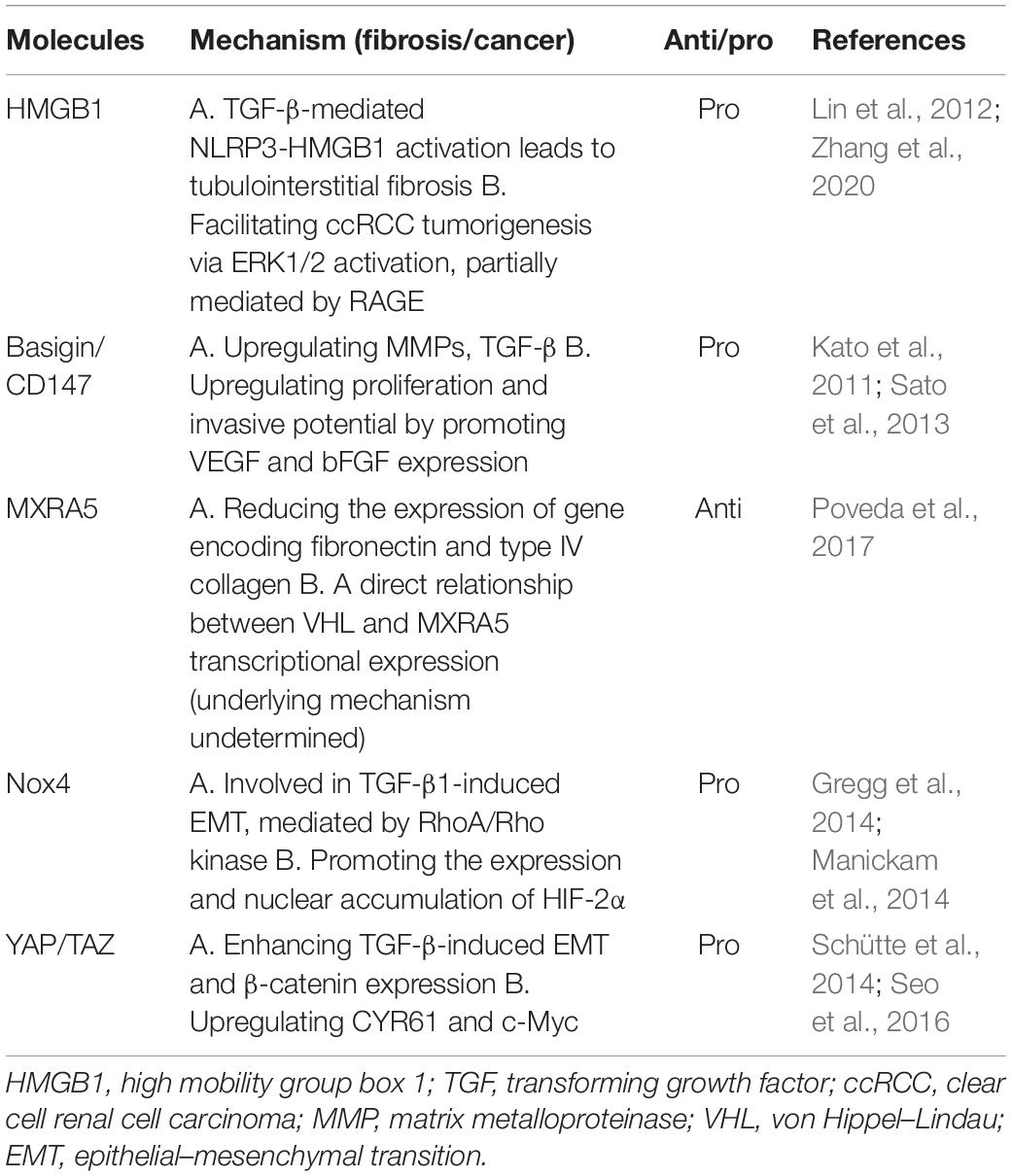
Apart from the major signaling pathways and molecules that we mentioned above, several scattered individual studies also come into our view. YAP/TAZ is associated with the mechanical traits of the cell microenvironment, while not as well-described as the pathways we mentioned before. We detected two proteins regarding ECM remodeling, Basigin and MXRA5, suggesting a more comprehensive engagement of ECM in RCC tumorigenesis. High mobility group box 1 (HMGB1) is a nuclear protein that acts as a co-factor for gene transcription. As the major NADPH isoform in kidney, Nox4 contributes to redox processes by mainly producing H2O2. These publications receive less attention but still broaden our view and provide interesting insights to better demonstrate how RCC and fibrosis may interact with each other (Table 3).
All these signaling pathways and molecules are related to renal fibrogenesis and tumorigenesis, some components of which are directly involved in both pathologies. Future experiments focusing on these directions may be of importance to unveil how fibrotic stroma facilitates RCC aggressiveness. EMT is a canonical process that shifts the cancer cells into a mesenchymal phenotype, hence being a driver of the metastasis. TGF-β1 secreted by CAFs led to EMT of urinary bladder cancer cells through lncRNA-ZEB2NAT (Zhuang et al., 2015). This study revealed that tumor stroma could prompt cancer development via inducing EMT of cancer cells. As we demonstrated above, EMT and its secretory mediator TGF-β1 have been identified in two renal diseases repeatedly; however, in RCC, there has been no study conducted to determine whether EMT caused by tumor stroma is capable of facilitating RCC tumorigenesis. It remains to be a promising direction to explore ncRNA and different signaling pathways as demonstrated, which may further our understanding by elaborating the underlying mechanisms.
Conclusion
The contribution of tumor stroma to cancer cell is widely acknowledged. We provide evidence in different organs depicting how reciprocal interactions between cancer cells and fibrotic stroma function, few of which are regarding RCC. On the grounds that ITF was shown to correlate with several indicators of poor prognosis of ccRCC, it is logical to broaden our view of RCC by investigating the contribution of fibrotic stroma and delineating concerned mechanisms. We show that such reciprocal interactions are joint efforts from different dysregulations, including various components of excessive ECM, aberrant metabolisms, activation of CAFs, and tumor immunity (shown in Figure 1). Next, we recapitulate mechanisms shared between RCC progression, metastases, and formation of renal fibrosis. mTOR, Notch, Wnt signaling pathways, and ncRNA widely participate in RCC tumorigenesis and renal fibrogenesis via different manners. Additionally, secretory molecules and process of EMT are widely implicated and may be promising targets. The majority of publications we detected regarding the interactions between fibrotic stroma and cancer cells are based on experiments conducted in the lung, breast, pancreas, etc., suggesting an absence of deserved attention on the kidney. Hopefully, the evidence we collect may provide promising targets for future experiments.
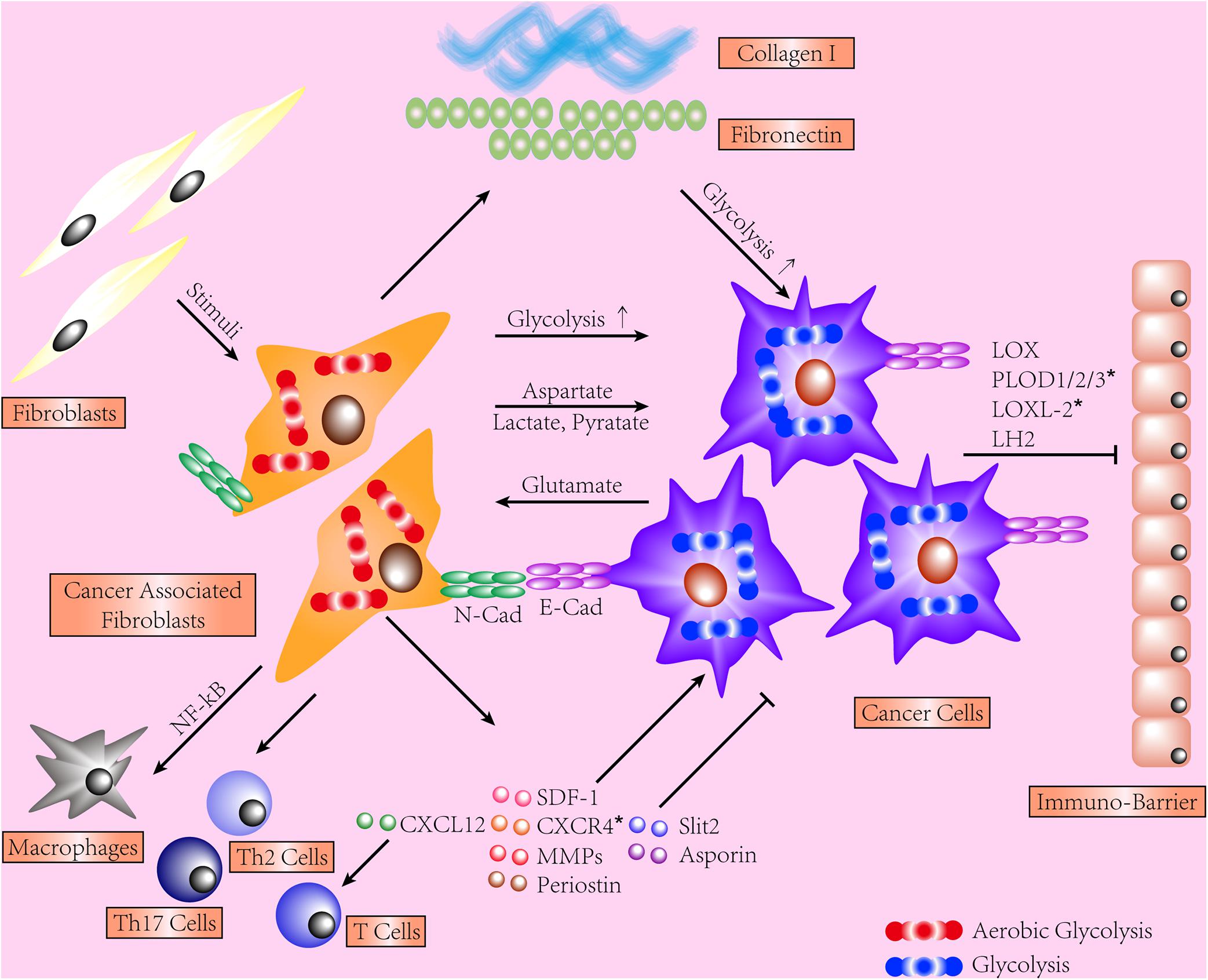
Figure 1. This schematic shows the interactions between stroma and cancer cells. Cancer-associated fibroblasts (CAFs) display an enhanced secretory phenotype, regulating immune cells and cancer cells and producing excessive extracellular matrix (ECM). Both CAFs and cancer cells shift to glycolysis and share a dynamic exchange of metabolites. The force transmission is mediated by E-cadherin/N-cadherin. Fibrotic ECM induces CAF activation, facilitates tumor invasion, and hinders T-cell migration. Type I collagen exerts a quantity-dependent pro- or anti-tumor effect on cancer cells. *Represents the data collected from renal cell carcinoma (RCC) models.
Author Contributions
CH and TZ conceived and designed the study and coordinated this work. CH, YZ, and XW collected, reviewed the literature, and composed the draft. CH, YZ, XW, and TZ organized the manuscript. All authors helped with data interpretation and manuscript editing.
Funding
This work was supported by the grants to CH from Shanghai Municipal Science and Technology Commission (Shanghai Sailing Program, 19YF1406600) and the Natural Science Foundation of China (81900682).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Acerbi, I., Cassereau, L., Dean, I., Shi, Q., Au, A., Park, C., et al. (2015). Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. (Camb) 7, 1120–1134. doi: 10.1039/c5ib00040h
Adam, J., Hatipoglu, E., O’Flaherty, L., Ternette, N., Sahgal, N., Lockstone, H., et al. (2011). Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20, 524–537. doi: 10.1016/j.ccr.2011.09.006
Adam, J., Yang, M., Bauerschmidt, C., Kitagawa, M., O’Flaherty, L., Maheswaran, P., et al. (2013). A role for cytosolic fumarate hydratase in urea cycle metabolism and renal neoplasia. Cell Rep. 3, 1440–1448. doi: 10.1016/j.celrep.2013.04.006
Ai, Q., Ma, X., Huang, Q., Liu, S., Shi, T., Zhang, C., et al. (2012). High-level expression of Notch1 increased the risk of metastasis in T1 stage clear cell renal cell carcinoma. PLoS One 7:e35022. doi: 10.1371/journal.pone.0035022
Alexander, J., and Cukierman, E. (2016). Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions. Curr. Opin. Cell Biol. 42, 80–93. doi: 10.1016/j.ceb.2016.05.002
Amatangelo, M. D., Bassi, D. E., Klein-Szanto, A. J., and Cukierman, E. (2005). Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am. J. Pathol. 167, 475–488. doi: 10.1016/s0002-9440(10)62991-4
Artavanis-Tsakonas, S., Rand, M. D., and Lake, R. J. (1999). Notch signaling: cell fate control and signal integration in development. Science 284, 770–776. doi: 10.1126/science.284.5415.770
Avgustinova, A., Iravani, M., Robertson, D., Fearns, A., Gao, Q., Klingbeil, P., et al. (2016). Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat. Commun. 7:10305. doi: 10.1038/ncomms10305
Bakhtyar, N., Wong, N., Kapoor, A., Cutz, J. C., Hill, B., Ghert, M., et al. (2013). Clear cell renal cell carcinoma induces fibroblast-mediated production of stromal periostin. Eur. J. Cancer 49, 3537–3546. doi: 10.1016/j.ejca.2013.06.032
Bertero, T., Oldham, W. M., Grasset, E. M., Bourget, I., Boulter, E., Pisano, S., et al. (2019). Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab. 29, 124–140.e10. doi: 10.1016/j.cmet.2018.09.012
Bhagat, T. D., Zou, Y., Huang, S., Park, J., Palmer, M. B., Hu, C., et al. (2017). Notch pathway is activated via genetic and epigenetic alterations and is a therapeutic target in clear cell renal cancer. J. Biol. Chem. 292, 837–846. doi: 10.1074/jbc.M116.745208
Bhowmick, N. A., Chytil, A., Plieth, D., Gorska, A. E., Dumont, N., Shappell, S., et al. (2004). TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851. doi: 10.1126/science.1090922
Boire, A., Covic, L., Agarwal, A., Jacques, S., Sherifi, S., and Kuliopulos, A. (2005). PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120, 303–313. doi: 10.1016/j.cell.2004.12.018
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Brugarolas, J. B., Vazquez, F., Reddy, A., Sellers, W. R., and Kaelin, W. G. Jr. (2003). TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4, 147–158. doi: 10.1016/s1535-6108(03)00187-9
Cancer.Net (2020). Kidney Cancer: Introduction. Available online at: https://www.cancer.net/cancer-types/kidney-cancer/introduction (accessed October 19, 2020).
Carstens, J. L., Correa de Sampaio, P., Yang, D., Barua, S., Wang, H., Rao, A., et al. (2017). Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 8:15095. doi: 10.1038/ncomms15095
Chang, P. H., Hwang-Verslues, W. W., Chang, Y. C., Chen, C. C., Hsiao, M., Jeng, Y. M., et al. (2012). Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/β-catenin pathway. Cancer Res. 72, 4652–4661. doi: 10.1158/0008-5472.Can-12-0877
Chen, G., Chen, H., Wang, C., Peng, Y., Sun, L., Liu, H., et al. (2012). Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One 7:e33626. doi: 10.1371/journal.pone.0033626
Chen, J., Weihs, D., and Vermolen, F. J. (2018). A model for cell migration in non-isotropic fibrin networks with an application to pancreatic tumor islets. Biomech. Model Mechanobiol. 17, 367–386. doi: 10.1007/s10237-017-0966-7
Chen, Y., Terajima, M., Yang, Y., Sun, L., Ahn, Y. H., Pankova, D., et al. (2015). Lysyl hydroxylase 2 induces a collagen cross-link switch in tumor stroma. J. Clin. Invest. 125, 1147–1162. doi: 10.1172/jci74725
Chow, T. F., Youssef, Y. M., Lianidou, E., Romaschin, A. D., Honey, R. J., Stewart, R., et al. (2010). Differential expression profiling of microRNAs and their potential involvement in renal cell carcinoma pathogenesis. Clin. Biochem. 43, 150–158. doi: 10.1016/j.clinbiochem.2009.07.020
Clevers, H., and Nusse, R. (2012). Wnt/β-catenin signaling and disease. Cell 149, 1192–1205. doi: 10.1016/j.cell.2012.05.012
Cox, T. R., Bird, D., Baker, A. M., Barker, H. E., Ho, M. W., Lang, G., et al. (2013). LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 73, 1721–1732. doi: 10.1158/0008-5472.Can-12-2233
Curtis, M., Kenny, H. A., Ashcroft, B., Mukherjee, A., Johnson, A., Zhang, Y., et al. (2019). Fibroblasts mobilize tumor cell glycogen to promote proliferation and metastasis. Cell Metab. 29, 141–155.e9. doi: 10.1016/j.cmet.2018.08.007
De Monte, L., Reni, M., Tassi, E., Clavenna, D., Papa, I., Recalde, H., et al. (2011). Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 208, 469–478. doi: 10.1084/jem.20101876
Dey, P., Son, J. Y., Kundu, A., Kim, K. S., Lee, Y., Yoon, K., et al. (2019). Knockdown of pyruvate kinase M2 inhibits cell proliferation, metabolism, and migration in renal cell carcinoma. Int. J. Mol. Sci. 20:5622. doi: 10.3390/ijms20225622
DiRocco, D. P., Kobayashi, A., Taketo, M. M., McMahon, A. P., and Humphreys, B. D. (2013). Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J. Am. Soc. Nephrol. 24, 1399–1412. doi: 10.1681/asn.2012050512
Erez, N., Truitt, M., Olson, P., Arron, S. T., and Hanahan, D. (2010). Cancer-associated fibroblasts are activated in incipient Neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17, 135–147. doi: 10.1016/j.ccr.2009.12.041
Erler, J. T., Bennewith, K. L., Cox, T. R., Lang, G., Bird, D., Koong, A., et al. (2009). Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44. doi: 10.1016/j.ccr.2008.11.012
Fantus, D., Rogers, N. M., Grahammer, F., Huber, T. B., and Thomson, A. W. (2016). Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat. Rev. Nephrol. 12, 587–609. doi: 10.1038/nrneph.2016.108
Feig, C., Jones, J. O., Kraman, M., Wells, R. J., Deonarine, A., Chan, D. S., et al. (2013). Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 20212–20217. doi: 10.1073/pnas.1320318110
Fendler, A., Bauer, D., Busch, J., Jung, K., Wulf-Goldenberg, A., Kunz, S., et al. (2020). Inhibiting WNT and NOTCH in renal cancer stem cells and the implications for human patients. Nat. Commun. 11:929. doi: 10.1038/s41467-020-14700-7
Giussani, M., Merlino, G., Cappelletti, V., Tagliabue, E., and Daidone, M. G. (2015). Tumor-extracellular matrix interactions: identification of tools associated with breast cancer progression. Semin. Cancer Biol. 35, 3–10. doi: 10.1016/j.semcancer.2015.09.012
Gregg, J. L., Turner, R. M. II, Chang, G., Joshi, D., Zhan, Y., Chen, L., et al. (2014). NADPH oxidase NOX4 supports renal tumorigenesis by promoting the expression and nuclear accumulation of HIF2α. Cancer Res. 74, 3501–3511. doi: 10.1158/0008-5472.Can-13-2979
Han, S. H., Wu, M. Y., Nam, B. Y., Park, J. T., Yoo, T. H., Kang, S. W., et al. (2017). PGC-1α protects from notch-induced kidney fibrosis development. J. Am. Soc. Nephrol. 28, 3312–3322. doi: 10.1681/asn.2017020130
Hartmann, N., Giese, N. A., Giese, T., Poschke, I., Offringa, R., Werner, J., et al. (2014). Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin. Cancer Res. 20, 3422–3433. doi: 10.1158/1078-0432.Ccr-13-2972
Hase, H., Jingushi, K., Ueda, Y., Kitae, K., Egawa, H., Ohshio, I., et al. (2014). LOXL2 status correlates with tumor stage and regulates integrin levels to promote tumor progression in ccRCC. Mol. Cancer Res. 12, 1807–1817. doi: 10.1158/1541-7786.Mcr-14-0233
He, W., Dai, C., Li, Y., Zeng, G., Monga, S. P., and Liu, Y. (2009). Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776. doi: 10.1681/asn.2008060566
Hewitson, T. D. (2009). Renal tubulointerstitial fibrosis: common but never simple. Am. J. Physiol. Renal. Physiol. 296, F1239–F1244. doi: 10.1152/ajprenal.90521.2008
Hirata, H., Hinoda, Y., Shahryari, V., Deng, G., Nakajima, K., Tabatabai, Z. L., et al. (2015). Long Noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res. 75, 1322–1331. doi: 10.1158/0008-5472.Can-14-2931
Hsu, R. J., Ho, J. Y., Cha, T. L., Yu, D. S., Wu, C. L., Huang, W. P., et al. (2012). WNT10A plays an oncogenic role in renal cell carcinoma by activating WNT/β-catenin pathway. PLoS One 7:e47649. doi: 10.1371/journal.pone.0047649
Hu, H., Hu, S., Xu, S., Gao, Y., Zeng, F., and Shui, H. (2018). miR-29b regulates Ang II-induced EMT of rat renal tubular epithelial cells via targeting PI3K/AKT signaling pathway. Int. J. Mol. Med. 42, 453–460. doi: 10.3892/ijmm.2018.3579
Huang, S., Park, J., Qiu, C., Chung, K. W., Li, S. Y., Sirin, Y., et al. (2018). Jagged1/Notch2 controls kidney fibrosis via Tfam-mediated metabolic reprogramming. PLoS Biol. 16:e2005233. doi: 10.1371/journal.pbio.2005233
Hudes, G., Carducci, M., Tomczak, P., Dutcher, J., Figlin, R., Kapoor, A., et al. (2007). Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 356, 2271–2281. doi: 10.1056/NEJMoa066838
Janssens, N., Andries, L., Janicot, M., Perera, T., and Bakker, A. (2004). Alteration of frizzled expression in renal cell carcinoma. Tumour. Biol. 25, 161–171. doi: 10.1159/000081098
Joung, J. W., Oh, H. K., Lee, S. J., Kim, Y. A., and Jung, H. J. (2018). Significance of intratumoral fibrosis in clear cell renal cell carcinoma. J. Pathol. Transl. Med. 52, 323–330. doi: 10.4132/jptm.2018.07.21
Juan, D., Alexe, G., Antes, T., Liu, H., Madabhushi, A., Delisi, C., et al. (2010). Identification of a microRNA panel for clear-cell kidney cancer. Urology 75, 835–841. doi: 10.1016/j.urology.2009.10.033
Jung, M., Mollenkopf, H. J., Grimm, C., Wagner, I., Albrecht, M., Waller, T., et al. (2009). MicroRNA profiling of clear cell renal cell cancer identifies a robust signature to define renal malignancy. J. Cell Mol. Med. 13, 3918–3928. doi: 10.1111/j.1582-4934.2009.00705.x
Kaelin, W. G. Jr. (2008). The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873. doi: 10.1038/nrc2502
Kalluri, R. (2016). The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598. doi: 10.1038/nrc.2016.73
Kato, M., Zhang, J., Wang, M., Lanting, L., Yuan, H., Rossi, J. J., et al. (2007). MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc. Natl. Acad. Sci. U.S.A. 104, 3432–3437. doi: 10.1073/pnas.0611192104
Kato, N., Kosugi, T., Sato, W., Ishimoto, T., Kojima, H., Sato, Y., et al. (2011). Basigin/CD147 promotes renal fibrosis after unilateral ureteral obstruction. Am. J. Pathol. 178, 572–579. doi: 10.1016/j.ajpath.2010.10.009
Khella, H. W., Bakhet, M., Allo, G., Jewett, M. A., Girgis, A. H., Latif, A., et al. (2013a). miR-192, miR-194 and miR-215: a convergent microRNA network suppressing tumor progression in renal cell carcinoma. Carcinogenesis 34, 2231–2239. doi: 10.1093/carcin/bgt184
Khella, H. W., Bakhet, M., Lichner, Z., Romaschin, A. D., Jewett, M. A., and Yousef, G. M. (2013b). MicroRNAs in kidney disease: an emerging understanding. Am. J. Kidney Dis. 61, 798–808. doi: 10.1053/j.ajkd.2012.09.018
Kim, W. Y., and Kaelin, W. G. Jr. (2006). Molecular pathways in renal cell carcinoma–rationale for targeted treatment. Semin. Oncol. 33, 588–595. doi: 10.1053/j.seminoncol.2006.06.001
Kolácná, L., Bakesová, J., Varga, F., Kostáková, E., Plánka, L., Necas, A., et al. (2007). Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol. Res. 56 Suppl 1, S51–S60.
Kondo, K., Yao, M., Kobayashi, K., Ota, S., Yoshida, M., Kaneko, S., et al. (2001). PTEN/MMAC1/TEP1 mutations in human primary renal-cell carcinomas and renal carcinoma cell lines. Int. J. Cancer 91, 219–224. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1034<3.0.co;2-s
Kondratov, A. G., Kvasha, S. M., Stoliar, L. A., Romanenko, A. M., Zgonnyk, Y. M., Gordiyuk, V. V., et al. (2012). Alterations of the WNT7A gene in clear cell renal cell carcinomas. PLoS One 7:e47012. doi: 10.1371/journal.pone.0047012
Kriegel, A. J., Fang, Y., Liu, Y., Tian, Z., Mladinov, D., Matus, I. R., et al. (2010). MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor beta 1: a novel role of miR-382. Nucleic Acids Res. 38, 8338–8347. doi: 10.1093/nar/gkq718
Kruck, S., Eyrich, C., Scharpf, M., Sievert, K. D., Fend, F., Stenzl, A., et al. (2013). Impact of an altered Wnt1/β-catenin expression on clinicopathology and prognosis in clear cell renal cell carcinoma. Int. J. Mol. Sci. 14, 10944–10957. doi: 10.3390/ijms140610944
Krupa, A., Jenkins, R., Luo, D. D., Lewis, A., Phillips, A., and Fraser, D. (2010). Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J. Am. Soc. Nephrol. 21, 438–447. doi: 10.1681/asn.2009050530
Labernadie, A., Kato, T., Brugués, A., Serra-Picamal, X., Derzsi, S., Arwert, E., et al. (2017). A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19, 224–237. doi: 10.1038/ncb3478
Levental, K. R., Yu, H., Kass, L., Lakins, J. N., Egeblad, M., Erler, J. T., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906. doi: 10.1016/j.cell.2009.10.027
Li, G., Satyamoorthy, K., Meier, F., Berking, C., Bogenrieder, T., and Herlyn, M. (2003). Function and regulation of melanoma-stromal fibroblast interactions: when seeds meet soil. Oncogene 22, 3162–3171. doi: 10.1038/sj.onc.1206455
Li, J., Ren, J., Liu, X., Jiang, L., He, W., Yuan, W., et al. (2015). Rictor/mTORC2 signaling mediates TGFβ1-induced fibroblast activation and kidney fibrosis. Kidney Int. 88, 515–527. doi: 10.1038/ki.2015.119
Lin, L., Zhong, K., Sun, Z., Wu, G., and Ding, G. (2012). Receptor for advanced glycation end products (RAGE) partially mediates HMGB1-ERKs activation in clear cell renal cell carcinoma. J. Cancer Res. Clin. Oncol. 138, 11–22. doi: 10.1007/s00432-011-1067-0
Liu, B., Qiang, L., Wang, G. D., Duan, Q., and Liu, J. (2019a). LncRNA MALAT1 facilities high glucose induced endothelial to mesenchymal transition and fibrosis via targeting miR-145/ZEB2 axis. Eur. Rev. Med. Pharmacol. Sci. 23, 3478–3486. doi: 10.26355/eurrev_201904_17713
Liu, C. F., Liu, H., Fang, Y., Jiang, S. H., Zhu, J. M., and Ding, X. Q. (2014). Rapamycin reduces renal hypoxia, interstitial inflammation and fibrosis in a rat model of unilateral ureteral obstruction. Clin. Invest. Med. 37:E142. doi: 10.25011/cim.v37i3.21381
Liu, L. J., Yu, J. J., and Xu, X. L. (2017). MicroRNA-93 inhibits apoptosis and promotes proliferation, invasion and migration of renal cell carcinoma ACHN cells via the TGF-β/Smad signaling pathway by targeting RUNX3. Am. J. Transl. Res. 9, 3499–3513.
Liu, P., Zhang, B., Chen, Z., He, Y., Du, Y., Liu, Y., et al. (2020a). m(6)A-induced lncRNA MALAT1 aggravates renal fibrogenesis in obstructive nephropathy through the miR-145/FAK pathway. Aging (Albany NY) 12, 5280–5299. doi: 10.18632/aging.102950
Liu, T., Zhou, L., Li, D., Andl, T., and Zhang, Y. (2019b). Cancer-associated fibroblasts build and secure the tumor microenvironment. Front. Cell Dev. Biol. 7:60. doi: 10.3389/fcell.2019.00060
Liu, X., Zhong, L., Li, P., and Zhao, P. (2020b). MicroRNA-100 enhances autophagy and suppresses migration and invasion of renal cell carcinoma cells via disruption of NOX4-dependent mTOR pathway. Clin. Transl. Sci. doi: 10.1111/cts.12798 [Epub ahead of print].
Lloberas, N., Cruzado, J. M., Franquesa, M., Herrero-Fresneda, I., Torras, J., Alperovich, G., et al. (2006). Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J. Am. Soc. Nephrol. 17, 1395–1404. doi: 10.1681/asn.2005050549
Lochter, A., Galosy, S., Muschler, J., Freedman, N., Werb, Z., and Bissell, M. J. (1997). Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J. Cell Biol. 139, 1861–1872. doi: 10.1083/jcb.139.7.1861
Long, J., Wang, Y., Wang, W., Chang, B. H., and Danesh, F. R. (2010). Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J. Biol. Chem. 285, 23457–23465. doi: 10.1074/jbc.M110.136168
Lovisa, S., Zeisberg, M., and Kalluri, R. (2016). Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis. Trends Endocrinol. Metab. 27, 681–695. doi: 10.1016/j.tem.2016.06.004
Ma, M. K. M., Yung, S., and Chan, T. M. (2018). mTOR inhibition and kidney diseases. Transplantation 102(2SSuppl. 1), S32–S40. doi: 10.1097/TP.0000000000001729
Maarouf, O. H., Aravamudhan, A., Rangarajan, D., Kusaba, T., Zhang, V., Welborn, J., et al. (2016). Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J. Am. Soc. Nephrol. 27, 781–790. doi: 10.1681/asn.2014121188
Maarouf, O. H., Ikeda, Y., and Humphreys, B. D. (2015). Wnt signaling in kidney tubulointerstitium during disease. Histol. Histopathol. 30, 163–171. doi: 10.14670/hh-30.163
Manickam, N., Patel, M., Griendling, K. K., Gorin, Y., and Barnes, J. L. (2014). RhoA/Rho kinase mediates TGF-β1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am. J. Physiol. Renal. Physiol. 307, F159–F171. doi: 10.1152/ajprenal.00546.2013
Maris, P., Blomme, A., Palacios, A. P., Costanza, B., Bellahcène, A., Bianchi, E., et al. (2015). Asporin is a fibroblast-derived TGF-β1 inhibitor and a tumor suppressor associated with good prognosis in breast cancer. PLoS Med. 12:e1001871. doi: 10.1371/journal.pmed.1001871
Martino, M. M., and Hubbell, J. A. (2010). The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 24, 4711–4721. doi: 10.1096/fj.09-151282
Mattick, J. S., and Makunin, I. V. (2006). Non-coding RNA. Hum. Mol. Genet. 15 Spec No 1, R17–R29. doi: 10.1093/hmg/ddl046
Miura, Y., Hayakawa, A., Kikuchi, S., Tsumoto, H., Umezawa, K., Chiba, Y., et al. (2019). Fumarate accumulation involved in renal diabetic fibrosis in Goto-Kakizaki rats. Arch. Biochem. Biophys. 678:108167. doi: 10.1016/j.abb.2019.108167
Moghaddas Sani, H., Hejazian, M., Hosseinian Khatibi, S. M., Ardalan, M., and Zununi Vahed, S. (2018). Long non-coding RNAs: an essential emerging field in kidney pathogenesis. Biomed. Pharmacother. 99, 755–765. doi: 10.1016/j.biopha.2018.01.122
Morris, B. A., Burkel, B., Ponik, S. M., Fan, J., Condeelis, J. S., Aguirre-Ghiso, J. A., et al. (2016). Collagen matrix density drives the metabolic shift in breast cancer cells. EBioMedicine 13, 146–156. doi: 10.1016/j.ebiom.2016.10.012
Motzer, R. J., Escudier, B., Oudard, S., Hutson, T. E., Porta, C., Bracarda, S., et al. (2008). Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456. doi: 10.1016/s0140-6736(08)61039-9
Nakada, C., Matsuura, K., Tsukamoto, Y., Tanigawa, M., Yoshimoto, T., Narimatsu, T., et al. (2008). Genome-wide microRNA expression profiling in renal cell carcinoma: significant down-regulation of miR-141 and miR-200c. J. Pathol. 216, 418–427. doi: 10.1002/path.2437
Neesse, A., Algül, H., Tuveson, D. A., and Gress, T. M. (2015). Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64, 1476–1484. doi: 10.1136/gutjnl-2015-309304
New, J., Arnold, L., Ananth, M., Alvi, S., Thornton, M., Werner, L., et al. (2017). Secretory autophagy in cancer-associated fibroblasts promotes head and neck cancer progression and offers a novel therapeutic target. Cancer Res. 77, 6679–6691. doi: 10.1158/0008-5472.can-17-1077
Nishikawa, R., Chiyomaru, T., Enokida, H., Inoguchi, S., Ishihara, T., Matsushita, R., et al. (2015). Tumour-suppressive microRNA-29s directly regulate LOXL2 expression and inhibit cancer cell migration and invasion in renal cell carcinoma. FEBS Lett. 589, 2136–2145. doi: 10.1016/j.febslet.2015.06.005
Orimo, A., Gupta, P. B., Sgroi, D. C., Arenzana-Seisdedos, F., Delaunay, T., Naeem, R., et al. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348. doi: 10.1016/j.cell.2005.02.034
Ou, Y. C., Li, J. R., Wang, J. D., Chang, C. Y., Wu, C. C., Chen, W. Y., et al. (2019). Fibronectin promotes cell growth and migration in human renal cell carcinoma cells. Int. J. Mol. Sci. 20:2792. doi: 10.3390/ijms20112792
Pan, J., Mestas, J., Burdick, M. D., Phillips, R. J., Thomas, G. V., Reckamp, K., et al. (2006). Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol. Cancer 5:56. doi: 10.1186/1476-4598-5-56
Paraskevopoulou, M. D., and Hatzigeorgiou, A. G. (2016). Analyzing MiRNA-LncRNA Interactions. Methods Mol. Biol. 1402, 271–286. doi: 10.1007/978-1-4939-3378-5_21
Paulsson, J., and Micke, P. (2014). Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin Cancer Biol. 25, 61–68. doi: 10.1016/j.semcancer.2014.02.006
Pollard, P. J., Brière, J. J., Alam, N. A., Barwell, J., Barclay, E., Wortham, N. C., et al. (2005). Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 14, 2231–2239. doi: 10.1093/hmg/ddi227
Poveda, J., Sanz, A. B., Fernandez-Fernandez, B., Carrasco, S., Ruiz-Ortega, M., Cannata-Ortiz, P., et al. (2017). MXRA5 is a TGF-β1-regulated human protein with anti-inflammatory and anti-fibrotic properties. J. Cell Mol. Med. 21, 154–164. doi: 10.1111/jcmm.12953
Powles, T., Wheater, M., Din, O., Geldart, T., Boleti, E., Stockdale, A., et al. (2016). A randomised phase 2 study of AZD2014 versus everolimus in patients with VEGF-refractory metastatic clear cell renal cancer. Eur. Urol. 69, 450–456. doi: 10.1016/j.eururo.2015.08.035
Qin, W., Chung, A. C., Huang, X. R., Meng, X. M., Hui, D. S., Yu, C. M., et al. (2011). TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J. Am. Soc. Nephrol. 22, 1462–1474. doi: 10.1681/asn.2010121308
Qu, Y. Y., Zhao, R., Zhang, H. L., Zhou, Q., Xu, F. J., Zhang, X., et al. (2020). Inactivation of the AMPK-GATA3-ECHS1 pathway induces fatty acid synthesis that promotes clear cell renal cell carcinoma growth. Cancer Res. 80, 319–333. doi: 10.1158/0008-5472.Can-19-1023
Ramaswamy, S., Ross, K. N., Lander, E. S., and Golub, T. R. (2003). A molecular signature of metastasis in primary solid tumors. Nat. Genet. 33, 49–54. doi: 10.1038/ng1060
Rhim, A. D., Oberstein, P. E., Thomas, D. H., Mirek, E. T., Palermo, C. F., Sastra, S. A., et al. (2014). Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747. doi: 10.1016/j.ccr.2014.04.021
Rønnov-Jessen, L., Petersen, O. W., and Bissell, M. J. (1996). Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol. Rev. 76, 69–125. doi: 10.1152/physrev.1996.76.1.69
Roswall, P., Bocci, M., Bartoschek, M., Li, H., Kristiansen, G., Jansson, S., et al. (2018). Microenvironmental control of breast cancer subtype elicited through paracrine platelet-derived growth factor-CC signaling. Nat. Med. 24, 463–473. doi: 10.1038/nm.4494
Saito, H., Fushida, S., Harada, S., Miyashita, T., Oyama, K., Yamaguchi, T., et al. (2018). Importance of human peritoneal mesothelial cells in the progression, fibrosis, and control of gastric cancer: inhibition of growth and fibrosis by tranilast. Gastric Cancer 21, 55–67. doi: 10.1007/s10120-017-0726-5
Salmon, H., Franciszkiewicz, K., Damotte, D., Dieu-Nosjean, M. C., Validire, P., Trautmann, A., et al. (2012). Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122, 899–910. doi: 10.1172/jci45817
Sanford-Crane, H., Abrego, J., and Sherman, M. H. (2019). Fibroblasts as modulators of local and systemic cancer metabolism. Cancers (Basel) 11:619. doi: 10.3390/cancers11050619
Sato, M., Nakai, Y., Nakata, W., Yoshida, T., Hatano, K., Kawashima, A., et al. (2013). EMMPRIN promotes angiogenesis, proliferation, invasion and resistance to sunitinib in renal cell carcinoma, and its level predicts patient outcome. PLoS One 8:e74313. doi: 10.1371/journal.pone.0074313
Schütte, U., Bisht, S., Heukamp, L. C., Kebschull, M., Florin, A., Haarmann, J., et al. (2014). Hippo signaling mediates proliferation, invasiveness, and metastatic potential of clear cell renal cell carcinoma. Transl. Oncol. 7, 309–321. doi: 10.1016/j.tranon.2014.02.005
Seo, E., Kim, W. Y., Hur, J., Kim, H., Nam, S. A., Choi, A., et al. (2016). The Hippo-Salvador signaling pathway regulates renal tubulointerstitial fibrosis. Sci. Rep. 6:31931. doi: 10.1038/srep31931
Servais, C., and Erez, N. (2013). From sentinel cells to inflammatory culprits: cancer-associated fibroblasts in tumour-related inflammation. J. Pathol. 229, 198–207. doi: 10.1002/path.4103
Sottile, J., and Hocking, D. C. (2002). Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell 13, 3546–3559. doi: 10.1091/mbc.e02-01-0048
Sternlicht, M. D., Lochter, A., Sympson, C. J., Huey, B., Rougier, J. P., Gray, J. W., et al. (1999). The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 98, 137–146. doi: 10.1016/s0092-8674(00)81009-0
Su, X., Ye, J., Hsueh, E. C., Zhang, Y., Hoft, D. F., and Peng, G. (2010). Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J. Immunol. 184, 1630–1641. doi: 10.4049/jimmunol.0902813
Sullivan, W. J., Mullen, P. J., Schmid, E. W., Flores, A., Momcilovic, M., Sharpley, M. S., et al. (2018). Extracellular matrix remodeling regulates glucose metabolism through TXNIP destabilization. Cell 175, 117–132.e21. doi: 10.1016/j.cell.2018.08.017
Sun, Z., Ma, Y., Chen, F., Wang, S., Chen, B., and Shi, J. (2018). miR-133b and miR-199b knockdown attenuate TGF-β1-induced epithelial to mesenchymal transition and renal fibrosis by targeting SIRT1 in diabetic nephropathy. Eur. J. Pharmacol. 837, 96–104. doi: 10.1016/j.ejphar.2018.08.022
Tao, R., Niu, W. B., Dou, P. H., Ni, S. B., Yu, Y. P., Cai, L. C., et al. (2020). Nucleobindin-2 enhances the epithelial-mesenchymal transition in renal cell carcinoma. Oncol. Lett. 19, 3653–3664. doi: 10.3892/ol.2020.11526
Tzouvelekis, A., Gomatou, G., Bouros, E., Trigidou, R., Tzilas, V., and Bouros, D. (2019). Common Pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest 156, 383–391. doi: 10.1016/j.chest.2019.04.114
van der Slot, A. J., Zuurmond, A. M., van den Bogaerdt, A. J., Ulrich, M. M., Middelkoop, E., Boers, W., et al. (2004). Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 23, 251–257. doi: 10.1016/j.matbio.2004.06.001
Vander Heiden, M. G., Cantley, L. C., and Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033. doi: 10.1126/science.1160809
Wang, B., Herman-Edelstein, M., Koh, P., Burns, W., Jandeleit-Dahm, K., Watson, A., et al. (2010). E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes 59, 1794–1802. doi: 10.2337/db09-1736
Wang, B., Komers, R., Carew, R., Winbanks, C. E., Xu, B., Herman-Edelstein, M., et al. (2012). Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 23, 252–265. doi: 10.1681/ASN.2011010055
Wang, Q., Wang, Y., Minto, A. W., Wang, J., Shi, Q., Li, X., et al. (2008). MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 22, 4126–4135. doi: 10.1096/fj.08-112326
Wang, R., Ma, Y., Yu, D., Zhao, J., and Ma, P. (2015). miR-377 functions as a tumor suppressor in human clear cell renal cell carcinoma by targeting ETS1. Biomed. Pharmacother. 70, 64–71. doi: 10.1016/j.biopha.2015.01.012
Weber, C. E., Kothari, A. N., Wai, P. Y., Li, N. Y., Driver, J., Zapf, M. A., et al. (2015). Osteopontin mediates an MZF1-TGF-β1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene 34, 4821–4833. doi: 10.1038/onc.2014.410
White, N. M., Bao, T. T., Grigull, J., Youssef, Y. M., Girgis, A., Diamandis, M., et al. (2011a). miRNA profiling for clear cell renal cell carcinoma: biomarker discovery and identification of potential controls and consequences of miRNA dysregulation. J. Urol. 186, 1077–1083. doi: 10.1016/j.juro.2011.04.110
White, N. M., Khella, H. W., Grigull, J., Adzovic, S., Youssef, Y. M., Honey, R. J., et al. (2011b). miRNA profiling in metastatic renal cell carcinoma reveals a tumour-suppressor effect for miR-215. Br. J. Cancer 105, 1741–1749. doi: 10.1038/bjc.2011.401
Wu, K., Xu, L., Zhang, L., Lin, Z., and Hou, J. (2011). High Jagged1 expression predicts poor outcome in clear cell renal cell carcinoma. Jpn. J. Clin. Oncol. 41, 411–416. doi: 10.1093/jjco/hyq205
Wu, M. J., Wen, M. C., Chiu, Y. T., Chiou, Y. Y., Shu, K. H., and Tang, M. J. (2006). Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 69, 2029–2036. doi: 10.1038/sj.ki.5000161
Xiao, H., Tang, K., Liu, P., Chen, K., Hu, J., Zeng, J., et al. (2015). LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell kidney carcinoma. Oncotarget 6, 38005–38015. doi: 10.18632/oncotarget.5357
Xiao, W., Gao, Z., Duan, Y., Yuan, W., and Ke, Y. (2017). Notch signaling plays a crucial role in cancer stem-like cells maintaining stemness and mediating chemotaxis in renal cell carcinoma. J. Exp. Clin. Cancer Res. 36:41. doi: 10.1186/s13046-017-0507-3
Xiao, Y., and Meierhofer, D. (2019). Glutathione metabolism in renal cell carcinoma progression and implications for therapies. Int. J. Mol. Sci. 20:3672. doi: 10.3390/ijms20153672
Xiong, M., Jiang, L., Zhou, Y., Qiu, W., Fang, L., Tan, R., et al. (2012). The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am. J. Physiol. Renal. Physiol. 302, F369–F379. doi: 10.1152/ajprenal.00268.2011
Xu, H., Sun, F., Li, X., and Sun, L. (2018). Down-regulation of miR-23a inhibits high glucose-induced EMT and renal fibrogenesis by up-regulation of SnoN. Hum. Cell 31, 22–32. doi: 10.1007/s13577-017-0180-z
Xu, R., Zeng, S., Xie, W., Sun, C., Chen, Y. L., Chen, M. J., et al. (2016). The expression and function of Frizzled-7 in human renal cell carcinoma. Clin. Transl. Oncol. 18, 269–276. doi: 10.1007/s12094-015-1362-3
Xu, W. H., Xu, Y., Wang, J., Tian, X., Wu, J., Wan, F. N., et al. (2019). Procollagen-lysine, 2-oxoglutarate 5-dioxygenases 1, 2, and 3 are potential prognostic indicators in patients with clear cell renal cell carcinoma. Aging (Albany NY) 11, 6503–6521. doi: 10.18632/aging.102206
Yamauchi, M., Barker, T. H., Gibbons, D. L., and Kurie, J. M. (2018). The fibrotic tumor stroma. J. Clin. Invest. 128, 16–25. doi: 10.1172/jci93554
Yang, Y., Wang, J., Qin, L., Shou, Z., Zhao, J., Wang, H., et al. (2007). Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am. J. Nephrol. 27, 495–502. doi: 10.1159/000106782
Yoon, J. H., Abdelmohsen, K., and Gorospe, M. (2014). Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 34, 9–14. doi: 10.1016/j.semcdb.2014.05.015
Zhang, H., Wei, P., Lv, W., Han, X., Yang, J., Qin, S., et al. (2019). MELK is upregulated in advanced clear cell renal cell carcinoma and promotes disease progression by phosphorylating PRAS40. Cell Transplant. 28(1_suppl), 37s–50s. doi: 10.1177/0963689719890860
Zhang, H. M., Yang, F. Q., Chen, S. J., Che, J., and Zheng, J. H. (2015). Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour. Biol. 36, 2947–2955. doi: 10.1007/s13277-014-2925-6
Zhang, K., Fan, C., Cai, D., Zhang, Y., Zuo, R., Zhu, L., et al. (2020). Contribution of TGF-beta-mediated NLRP3-HMGB1 activation to tubulointerstitial fibrosis in rat with angiotensin II-induced chronic kidney disease. Front. Cell Dev. Biol. 8:1. doi: 10.3389/fcell.2020.00001
Zheng, B., Mao, J. H., Qian, L., Zhu, H., Gu, D. H., Pan, X. D., et al. (2015). Pre-clinical evaluation of AZD-2014, a novel mTORC1/2 dual inhibitor, against renal cell carcinoma. Cancer Lett. 357, 468–475. doi: 10.1016/j.canlet.2014.11.012
Zhou, D., Fu, H., Zhang, L., Zhang, K., Min, Y., Xiao, L., et al. (2017). Tubule-Derived Wnts are required for fibroblast activation and kidney fibrosis. J. Am. Soc. Nephrol. 28, 2322–2336. doi: 10.1681/asn.2016080902
Keywords: cancer-associated fibroblast, intratumoral fibrosis, metabolism, microRNA, renal cell carcinoma
Citation: Hu C, Zhao Y, Wang X and Zhu T (2021) Intratumoral Fibrosis in Facilitating Renal Cancer Aggressiveness: Underlying Mechanisms and Promising Targets. Front. Cell Dev. Biol. 9:651620. doi: 10.3389/fcell.2021.651620
Received: 10 January 2021; Accepted: 05 February 2021;
Published: 11 March 2021.
Edited by:
Jin Qian, Stanford University, United StatesReviewed by:
Blake Kun Zhang, Duke University, United StatesYanting Liu, The First Affiliated Hospital of Xinxiang Medical University, China
Copyright © 2021 Hu, Zhao, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuanchuan Wang, d2FuZy54dWFuY2h1YW5AenMtaG9zcGl0YWwuc2guY24=; Tongyu Zhu, enNfdHl6aHVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Chao Hu
Chao Hu Yufeng Zhao1,2†
Yufeng Zhao1,2†