- 1Department of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Medical Genetics, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
- 3School of Advancement, Centennial College, Ashtonbee Campus, Toronto, ON, Canada
- 4Wake Forest Institute for Regenerative Medicine, School of Medicine, Wake Forest University, Winston-Salem, NC, United States
- 5Department of Anatomical Sciences, Faculty of Medicine, Biranjd University of Medical Sciences, Birjand, Iran
- 6Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
The insulin-like growth factors (IGFs) are polypeptides with similar sequences with insulin. These factors regulate cell growth, development, maturation, and aging via different processes including the interplay with MAPK, Akt, and PI3K. IGF signaling participates in the pathogenesis of neoplasia, insulin resistance, diabetes mellitus, polycystic ovarian syndrome, cerebral ischemic injury, fatty liver disease, and several other conditions. Recent investigations have demonstrated the interplay between non-coding RNAs and IGF signaling. This interplay has fundamental roles in the development of the mentioned disorders. We designed the current study to search the available data about the role of IGF-associated non-coding RNAs in the evolution of neoplasia and other conditions. As novel therapeutic strategies have been designed for modification of IGF signaling, identification of the impact of non-coding RNAs in this pathway is necessary for the prediction of response to these modalities.
Background
The insulin-like growth factors (IGFs) are involved in growth and developmental processes and are evolutionarily conserved among several species (Rosenzweig, 2020). The functions of IGFs are mediated through two receptor tyrosine kinases and receptors for IGF1 and insulin. Besides, several IGF binding proteins selectively inhibit IGF1 or IGF2. IGF1 receptors have been shown to be up-regulated in tumors, thus participating in the tumorigenesis, resistance to therapies, and facilitation of metastasis in various cancer kinds (Rosenzweig, 2020). IGF1 receptors are known inducers of the Akt and mitogen-activated protein kinase (MAPK) (Pollak, 2008). Besides, IGF signaling is involved in the pathogenesis of insulin resistance and other disorders (Rosenzweig, 2020). The contribution of IGF in the pathogenesis of a wide assortment of human disorders including neoplasia and other disorders is explained by its influence on energy metabolism and cell growth (Pollak, 2008). IGF1 acts downstream of the growth hormone and through activation of MAPK and PI3K pathways and anabolism, it promotes growth and maturation of almost all tissues. Therefore, it is also involved in the aging process (Wrigley et al., 2017). Figure 1 depicts an overview of Insulin-like growth factor (IGF) signal transduction and two downstream signaling pathways: PI3K/AKT and MAPK/ERK. The IGF signaling network is composed of three receptor tyrosine kinases (IGF1R, IGF2R, and INSR), three ligands (insulin, IGF1, and IGF2), and six serum insulin-like growth factor binding proteins (IGFBP). Figure 1 shows the IGF signal transduction and its downstream effectors.
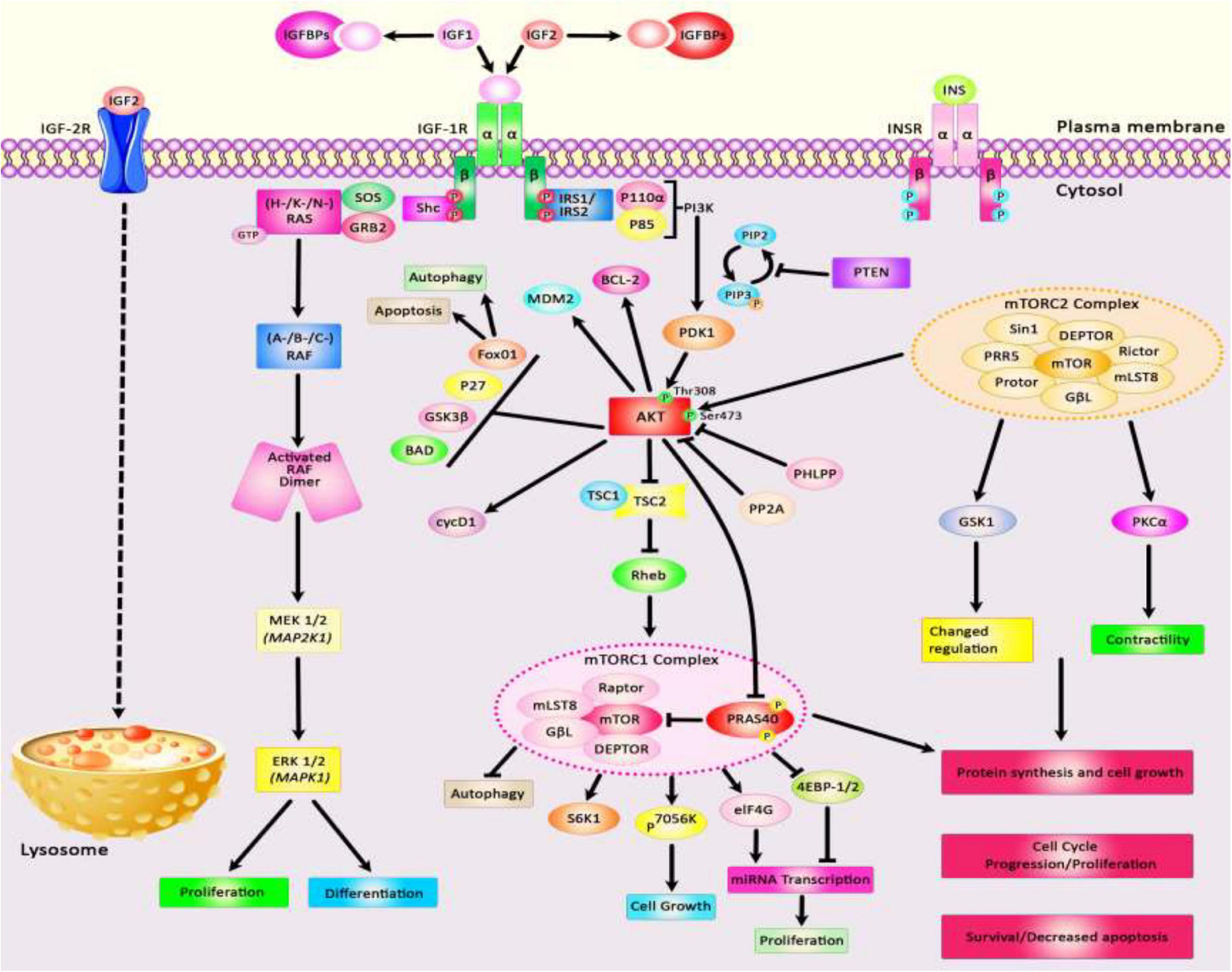
Figure 1. A schematic diagram of the insulin-like growth factor (IGF) signal transduction and main downstream effects. Both IGF-1 and IGF-2 could bind with plasma membrane IGF-1R and IGF-1/Insulin hybrid receptor leading to autophosphorylation of this target receptor in the intracellular β-subunits and thus triggering the catalytic function of the IGF-1R, while insulin binds only to the Insulin-R. IGFBPs could regulate the bioavailability of both IGF-1 and IGF-2 signaling cascades. The bioavailability of IGF-2 could also be regulated via binding to the IGF-2R which results in receptor-triggered internalization and endosomal degradation of IGF-2 in the lysosomes. Phosphorylated β-subunits could in turn create docking sites for the adaptor proteins IRS-1/2, and Shc that modulate the activation of two signaling pathways: PI3K/AKT and MAPK/ERK. Activation of the PI3K and AKT pathway leads to modulation of a variety of cell signaling cascades, such as regulation of TSC1/2 to suppress mTORC1 complex and modulation of 4EB-P1 and S6K1/2 phosphorylation, promoting cell survival via activation or suppression of major effectors like the Foxo transcription factors, BCL-2, BAD, and P27, upregulation of transformation of glucose to glycogen through suppression of GSK-3β, increasing protein synthesis, as well as suppressing apoptosis and autophagy. Activation of AKT family of kinases via PDK1 and mTORC2 leads to the phosphorylation at Thr308 and Ser473, respectively. Besides, docking of Grb2 to the phosphorylated IGF-1R β subunits could trigger the Ras/Raf/MEK/ERK axis. Shc binding to activated IGF-1R leads to stimulation of the MAPK/ERK cascade which regulates a kinase signaling pathway and eventually results in promoting cellular proliferation via enhancing transcription factors activities including ELK1.
Recent investigations have verified the influence of regulatory non-coding RNAs on IGF signaling (Chen B. et al., 2019). Most investigations in this regard have focused on long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) (Chen B. et al., 2019). LncRNAs are transcripts with sizes of more than 200 nucleotides which are principally produced by RNA polymerase II. These transcripts have various functions in the modulation of genomic structure, chromatin configuration, mRNA stability, alternative splicing, and enhancement or inhibition of transcription. The other types of regulatory non-coding RNAs, i.e., miRNAs mainly influence gene expression at the post-transcriptional phase via binding with the 3′ UTR of their specific targets. Both classes of non-coding RNAs participate in the pathogenesis of human diseases. We designed the current study to search the available data about the role of IGF-associated non-coding RNAs in the evolution of neoplasia and other conditions.
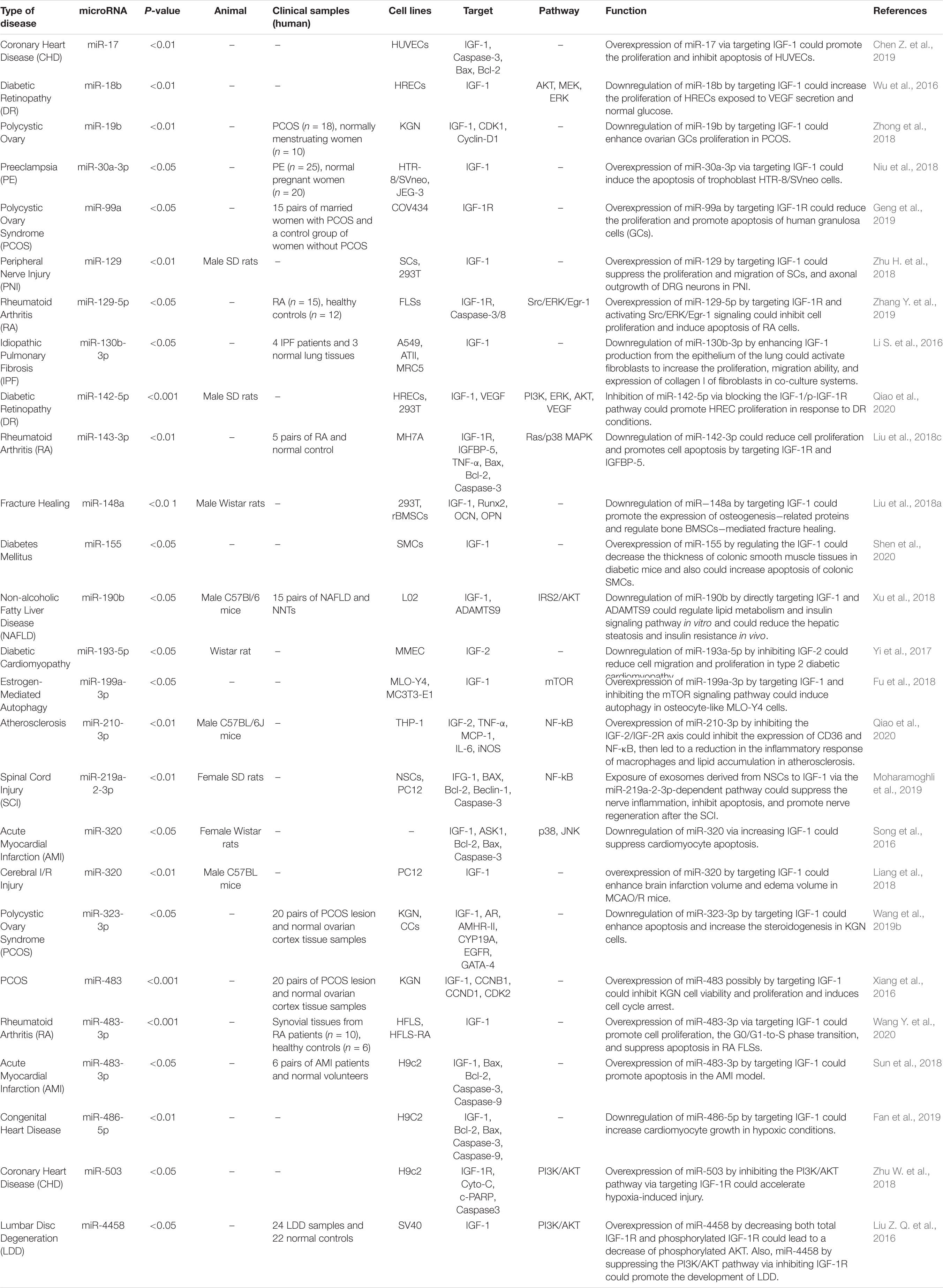
IGF-Associated miRNAs in Human Disorders
Several IGF-associated miRNAs have been dysregulated in neoplastic conditions. For instance, experiments in ovarian cancer cells have shown that miR−19a−3p suppresses the levels of IGF binding protein−3 (IGFBP−3), thus promoting the growth and migration of these cells. Notably, the expression of this miRNA can be modulated by NF−κB (Bai et al., 2019). Shastri et al. have demonstrated the inhibitory effects of the miR-29 family on IGF-1. Members of the miR-30 family can inhibit both IGF-1 and IGF-1R. Notably, calorie restriction has resulted in the over-expression of miR-29 and miR-30 in the normal liver and the liver being metastasized by breast cancer cells, indicating a possible role for dietary modifications in the management of liver metastases (Shastri et al., 2020). In nasopharyngeal squamous cell carcinoma cells, miR-30a inhibitor could reverse IGF-I-associated epithelial-mesenchymal transition (EMT). The IGF-1R/Src/miR-30a/E-cadherin axis has been identified as an important pathway in the regulation of EMT in these cells (Wang et al., 2016). miR-99a is another miRNA that can inhibit proliferation, migration, and invasion of breast carcinoma via suppression of IGF-1R (Xia et al., 2016). Being up-regulated in hepatocellular carcinoma cells, miR-155 can increase expression of IGF-II and IGF-1R, while decreasing IGFBP-3 expression. Through these pathways, miR-155 can increase proliferation, migration, and clonogenicity of hepatocellular carcinoma cells (El Tayebi et al., 2015). In the same type of cancer, miR-342-3p can inhibit cell proliferation through the suppression of the IGF-1R-associated Warburg effect (Liu et al., 2018d). In colorectal cancer cells, the oncogenic protein IGF2BP2 has a functional interaction with miR-195 through which it regulates RAF1 expression and participates in the carcinogenic process (Ye S. et al., 2016). Meanwhile, miR-197 can inhibit the expression of IGFBP3 through binding with its 3′-UTR, hence enhancing cell migratory potential and invasion of colorectal cancer cells (Zhou et al., 2018). Supplementary Table 1 reviews the results of studies that displayed the role of IGF-associated miRNAs in the neoplastic conditions.
The interaction between IGF-related proteins and miRNAs has been also assessed in non-neoplastic conditions. For instance, IGF-1 is targeted by miR-17. This miRNA has been over-expressed in ox-LDL treated human umbilical vascular endothelial cells (HUVECs) in association with down-regulation of IGF-1. Up-regulation of miR-17 has enhanced cell viability and suppressed the apoptosis of ox-LDL exposed cells. Such effects have been accompanied by down-regulation of Bax and Caspase3 expressions, while up-regulation of Bcl-2, suggesting a role for miR-17 as a biomarker for coronary heart disease (Chen Z. et al., 2019). Expression of miR-30a-3p has been elevated in the placenta samples of women with preeclampsia. This miRNA has been shown to regulate the expression of IGF-1, therefore influencing the invasive capacity and apoptosis of trophoblasts (Niu et al., 2018). Over-expression of miR-129 has suppressed proliferation and migration of Schwan cells and axonal outgrowth of dorsal root ganglion neurons through modulation of several targets including IGF-1 (Zhu H. et al., 2018). Yang et al. have reported up-regulation of miR-143-3p in synovial tissues of patients with rheumatoid arthritis compared with those affected with osteoarthritis. Down-regulation of miR-143-3p has inhibited cell proliferation, enhanced apoptosis, and reduced production of inflammatory cytokines. miR-143-3p has been shown to target IGF1R and IGFBP5 and regulate the Ras/p38 MAPK axis (Liu et al., 2018c). In colonic smooth muscle cells, miR-155 has been shown to down-regulate IGF-1 levels. Up-regulation of miR-155 has increased apoptosis of these cells and reduced the thickness of the related tissue in the diabetic mice, suggesting the role of this miRNA in the aggravation of colonic dysmotility (Shen et al., 2020). Figure 2 illustrates the IGF signaling cascade modulating by dysregulated miRNAs in various human diseases as well as cancers. Table 1 reviews the role of IGF-associated miRNAs in non-neoplastic conditions.
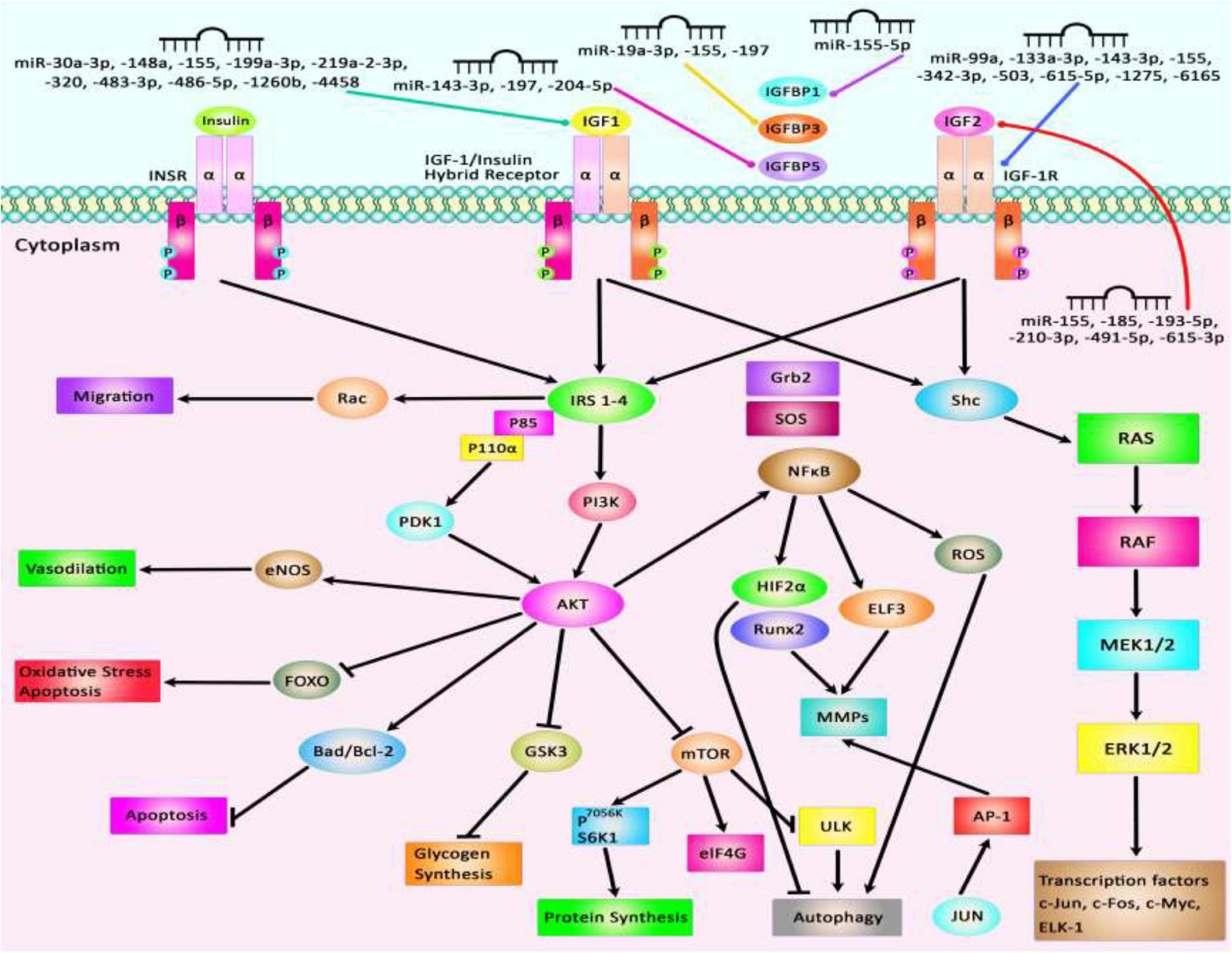
Figure 2. Several proteins in the IGF signaling pathway such as IGF1, IGF2, IGF-1R, and IGFBPs are regulated by miRNAs. Through modulation of these proteins, miRNAs can affect several cellular processes such as apoptosis, autophagy, protein synthesis, response to oxidative stress, and cell migration.
Overexpression of IGF-associated miRNAs namely miR-30a-3p, miR-155, miR-199a-3p, and miR-486-5p has important roles in different conditions such as preeclampsia, hepatocellular carcinoma, estrogen-mediated autophagy, and congenital heart disease (El Tayebi et al., 2015; Fu et al., 2018; Niu et al., 2018; Fan et al., 2019). Besides, dysregulation of miR-210-3p, miR-491-5p, and miR-615-3p contributes to the pathogenesis of atherosclerosis, colorectal carcinoma, and non-small lung cancer through modulation of IGF2 expression level (Liu j. et al., 2019; Lu et al., 2019; Qiao et al., 2020). Besides, aberrant expressions of miR-204-5p, miR-197, and miR-155-5p participate in the pathogenesis of papillary thyroid carcinoma, colorectal cancer, and non-small lung cancer through affecting expressions of IGFBP5, IGFBP3, and IGFBP1, respectively (Ling et al., 2015; Liu L. et al., 2015; Zheng et al., 2018). miR-99a, miR-503, and miR-1275 contribute to the pathogenesis of polycystic ovary syndrome, coronary heart disease, and hepatocellular carcinoma by affecting IGF-1R levels (Fawzy et al., 2015; Zhu W. et al., 2018; Geng et al., 2019). Figure 2 summarizes the role of a number of IGF-associated miRNAs in human disorders including cancers.
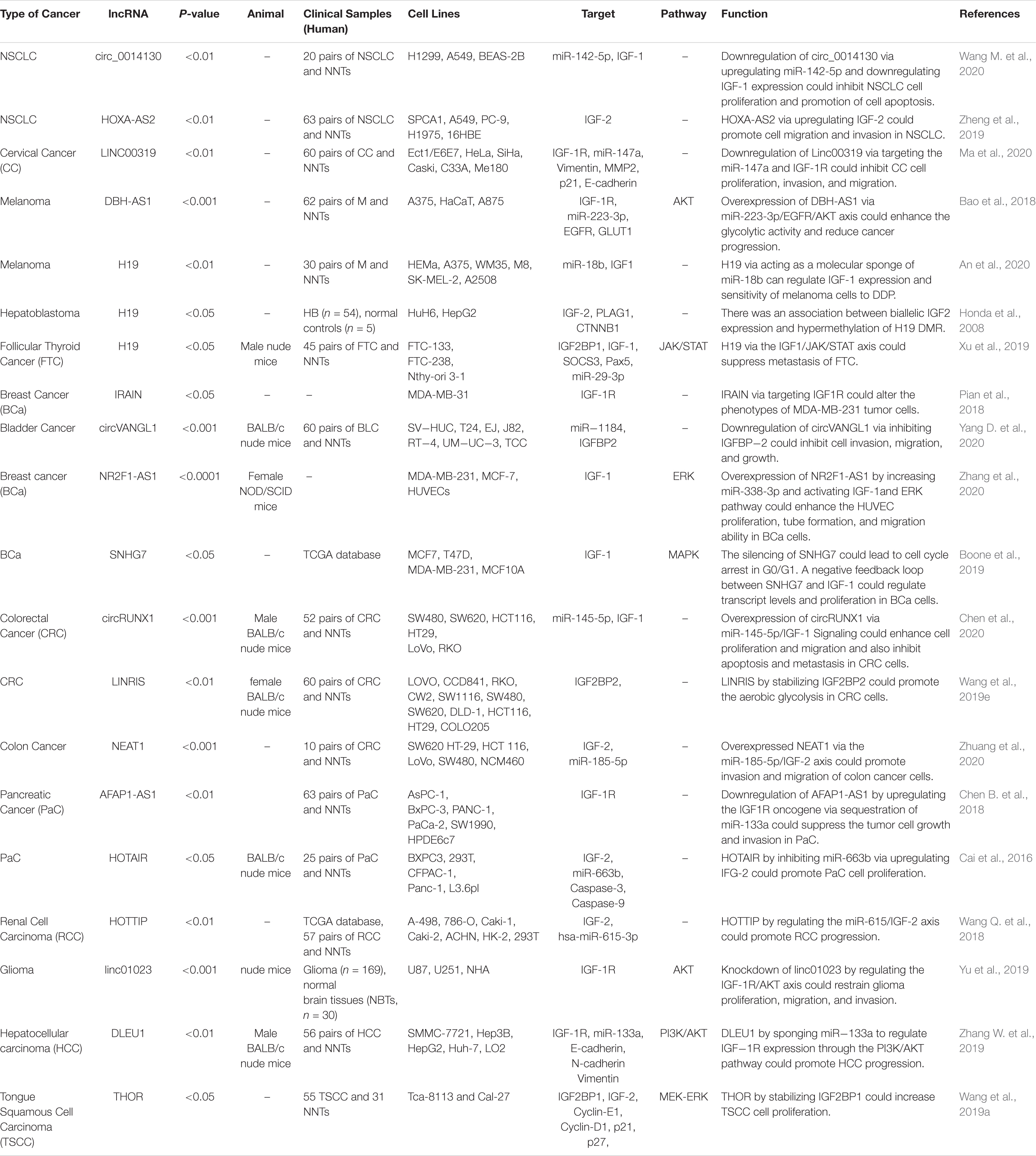
IGF-Associated lncRNAs in Human Disorders
Several lncRNAs have functional links with IGF-related proteins. Wang et al. have demonstrated over-expression of circ_0014130 in Non-Small Cell Lung Carcinoma tissues and cells. Down-regulation of this cirCRNA has suppressed cell proliferation and enhanced cell apoptosis in these cells. Circ_0014130 has functional interactions with miR-142-5p and IGF-1. Small interfering RNA-mediated circ_0014130 silencing has enhanced IGF-1 levels through up-regulation of miR-142-5p (Wang M. et al., 2020). Another study in this kind of cancer has shown up-regulation of HOXA-AS2 in the tumor samples. HOXA-AS2 silencing has decreased the expression of IGF2. Therefore, HOXA-AS2 promotes the migratory and invasive capacities of lung cancer cells by enhancing IGF2 expression (Zheng et al., 2019). In cervical cancer cells, the expression of linc00319 has been increased. Linc00319 silencing has suppressed cell proliferation, invasion, and migration of cervical cancer cells. This lncRNA interacts with miR-147a to modulate the expression of IGF1R (Ma et al., 2020). DBH-AS1 is another oncogenic lncRNA in hepatocellular carcinoma. Up-regulation of this lncRNA has been associated with the down-regulation of miR-138. DBH-AS1 knockdown and miR-138 up-regulation have decreased cell viability, repressed colony formation, and increased cell apoptosis. DBH-AS1 enhanced tumor growth and activated FAK/Src/ERK axis by modulating the expression of miR-138 (Bao et al., 2018). H19 is another up-regulated lncRNA in melanoma. H19 silencing has increased the sensitivity of melanoma cells to cisplatin, suppressed colony formation, and enhanced apoptosis of cisplatin-resistant melanoma cells. This lncRNA regulates IGF-1 expression through modulation of miR-18b expression (An et al., 2020). Honda et al. have assessed the methylation pattern of the H19 differentially methylated region (DMR), loss of heterozygosity, and allelic expression of IGF2 in hepatoblastoma. They reported associations between biallelic IGF2 expression and hypermethylation of H19 DMR. On the other hand, the monoallelic expression of IGF2 has been correlated with normal methylation of this region. They also reported over-expression of IGF2 and predominance of the embryonic P3 transcript in most hepatoblastoma with retention of imprinting (Honda et al., 2008). Table 2 summarizes the role of IGF-associated lncRNAs in cancers.
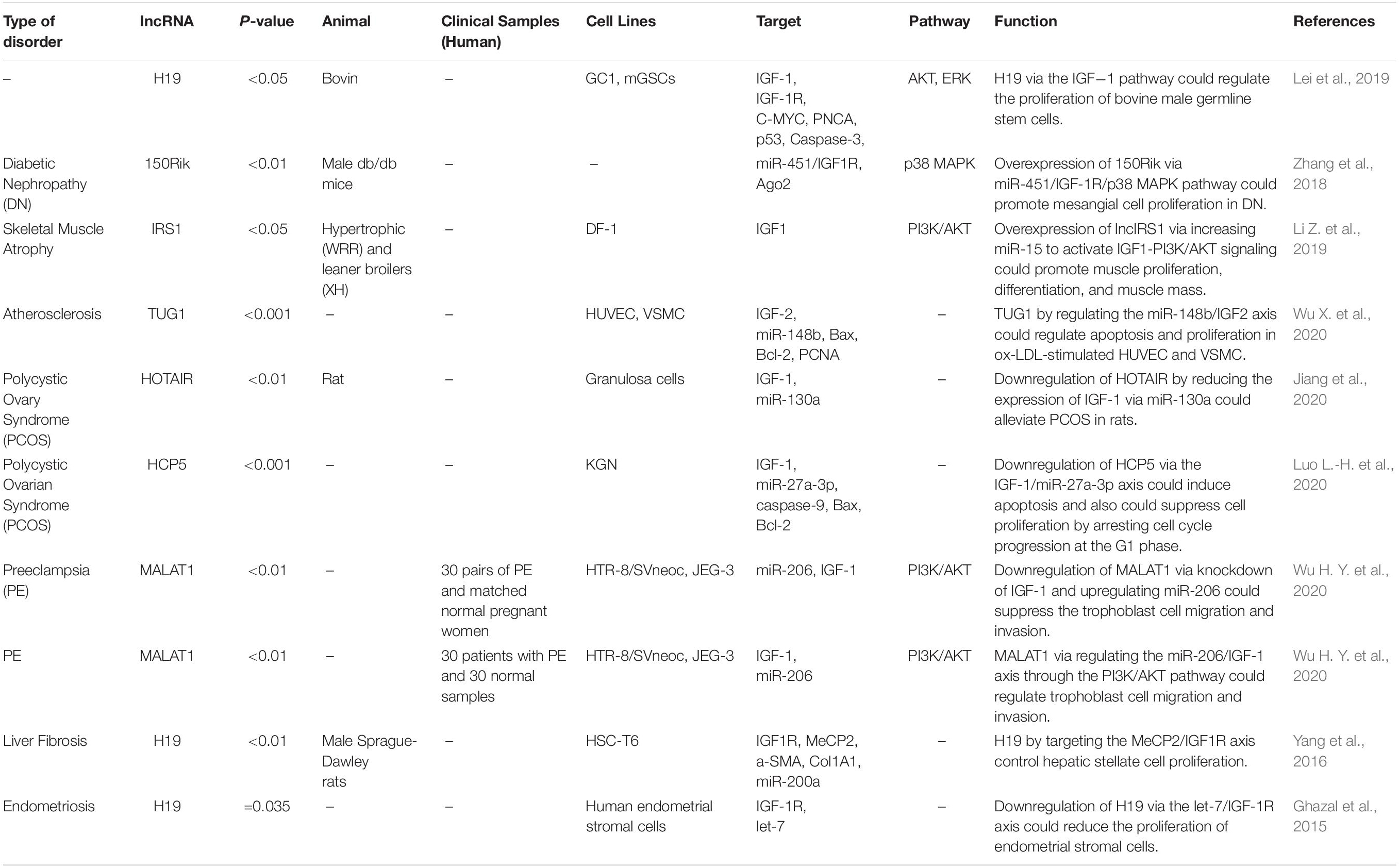
Via regulation of the IGF−1 signaling pathway, H19 can modulate proliferation and apoptosis of male germline stem cells. H19 silencing has reduced the cell quantities in the seminiferous tubule (Lei et al., 2019). Expression of the lncRNA 150Rik has been enhanced in renal tissue of animal models of diabetic nephropathy and in mesangial cells cultured in hyperglycemic media. This lncRNA regulates mesangial cell proliferation through interacting with miR-451, thus regulating the IGF1R/p38MAPK axis (Zhang et al., 2018). LncIRS1 has been shown to act as a molecular sponge for miR−15a, miR−15b−5p, and miR−15c−5p to modulate the expression of IRS1 a downstream target of the IGF1-R. Up-regulation of lncIRS1 has enhanced IRS1 expression and increased phosphorylation of AKT as an important element in the IGF-1 pathway. LncIRS1 can also regulate the expression of atrophy−associated genes and affect muscle atrophy (Li Z. et al., 2019). TUG1 is an up-regulated lncRNA in ox-LDL-exposed vascular smooth muscle cell (VSMC) and HUVEC. Its silencing has suppressed proliferation and enhanced apoptosis in ox-LDL-exposed VSMC but has exerted opposite effects in HUVEC. miR-148b has been identified as a target of TUG1 in these cells. In turn, miR-148b has been shown to target IGF2. Therefore, TUG1 enhances IGF2 levels by sequestering miR-148b (Wu X. et al., 2020). HCP5 is a lncRNA that is involved in the pathogenesis of polycystic ovarian syndrome (PCOS). Down-regulation of this lncRNA inhibits cell proliferation via inducing cell cycle arrest at the G1 phase and stimulating the mitochondrial apoptotic route. miR-27a-3p has been recognized as a direct target of HCP5. This miRNA can bind with IGF-1. Therefore, HCP5 can be involved in the development of PCOS via modulating the miR-27a-3p/IGF-1 axis (Luo L.-H. et al., 2020). Figure 3 represents the dysregulation of various types of lncRNAs which have a remarkable role in negatively modulating IGF1, IGF2, IGFBP2, and IGF-1R through the IGF signaling pathway in different human cancers. Table 3 summarizes the information about the role of IGF-associated lncRNAs in non-neoplastic conditions.
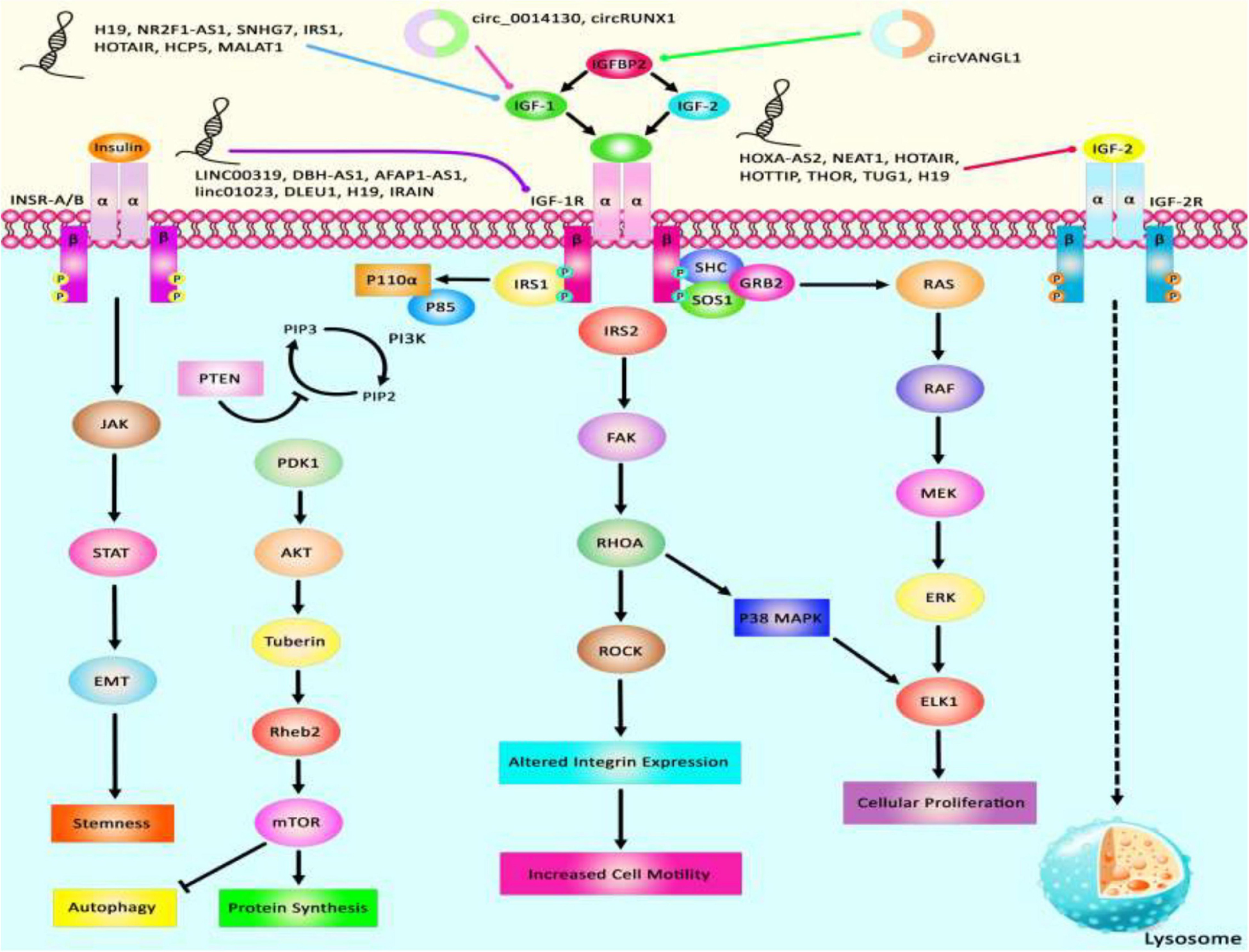
Figure 3. A schematic summary of lncRNAs that target IGF signaling cascade. IGF1, IGF2, IGFBP2, and IGF-1R are among proteins that are regulated by lncRNAs. Abnormal levels of lncRNAs can affect the carcinogenesis process by influencing autophagy, cell proliferation, protein synthesis, and stemness.
LncRNAs that regulate the expression of IGF1, IGF2, IGF-1R, and IGFBPs can participate in the pathogenesis of human disorders. H19, NR2F1-AS1, and SNHG7 participate in the development of melanoma and breast cancer through modulation of IGF1 (Boone et al., 2019; An et al., 2020; Zhang et al., 2020). NEAT1, THOR, and HOTTIP via targeting IGF2 affect carcinogenic processes in colorectal cancer, tongue squamous cell carcinoma, and renal cell carcinoma (Wang Q. et al., 2018; Yang et al., 2019; Zhuang et al., 2020). Additionally, downregulation of circVANGL1 through suppressing the expression level of IGFBP2 could attenuate breast cancer cell invasion, migration, and proliferation (Yang D. et al., 2020). Also, IRAIN, Linc00319, and DLEU1 through negatively regulating IGF-1R could cause breast cancer, cervical cancer, and hepatocellular carcinoma (Pian et al., 2018; Zhang W. et al., 2019; Ma et al., 2020). Figure 3 summarizes the role of these IGF-associated lncRNAs in human disorders.
Diagnostic/Prognostic Values of IGF-Associated miRNAs/lncRNAs in Cancers
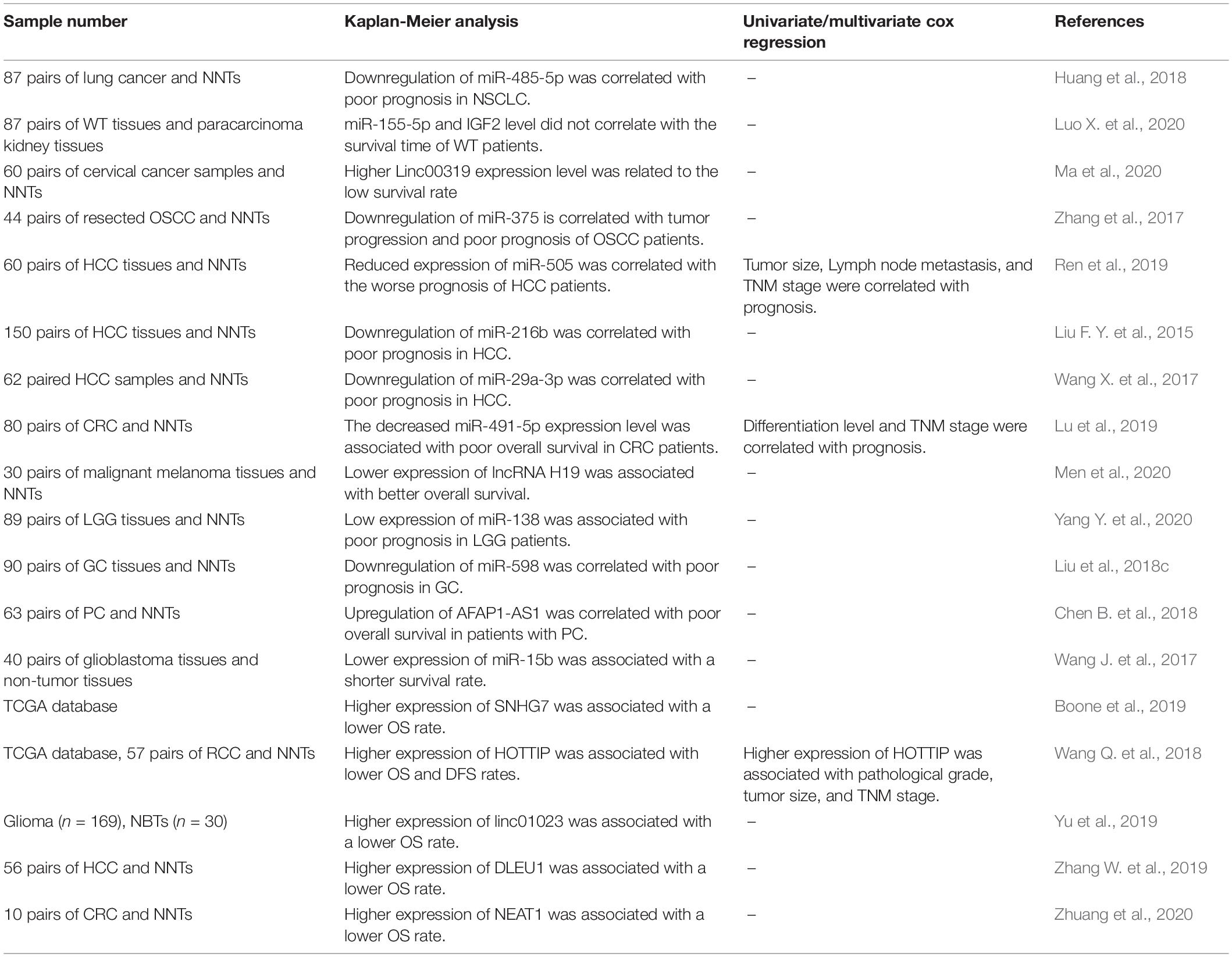
A number of miRNAs and lncRNAs which are functionally linked with IGF signaling have potential applications as diagnostic/prognostic markers in cancers. Zhuang et al. have demonstrated high accuracy of NEAT1 levels in distinguishing colon cancer tissues from normal ones (area under the receiver operating curve = 0.89) (Zhuang et al., 2020). Expression levels of the IGF-associated miRNAs miR-485-5p and miR-155-5p have been associated with the survival of patients with lung cancer and Wilms tumor, respectively (Huang et al., 2018; Luo X. et al., 2020). Also, Linc00319, H19, AFAP1-AS1, SNHG7, HOTTIP, linc01023, DLEU1, and NEAT1 have been identified as prognostic markers in diverse kinds of cancer (Table 4).
Importance of IGF-Associated Pathways in Response to Chemotherapy
IGF-associated molecules have been involved in the resistance of cancer cells to chemotherapeutic agents. In some cases, miRNAs or lncRNAs have been identified as molecules that mediate this phenotype. For instance, H19 silencing has enhanced the sensitivity of cancer cells to cisplatin and increased apoptosis of cisplatin-resistant melanoma cells through modulation of IGF1 expression (An et al., 2020). In a number of ovarian cancer cell lines, IGF-2 expression has been higher in Taxol-resistant cells compared with chemosensitive cell lines. Transient IGF2 silencing has enhanced Taxol sensitivity in these cells. However, IGF1R blocking did not affect the chemosensitivity of these cells. These results have supported the role of IGF-2 as a possible therapeutic target in drug-resistant ovarian cancer (Brouwer-Visser et al., 2014). IGF-1 has been shown to confer resistance to docetaxel in prostate cancer cells. IGF-I treatment has reduced expression of miR-143 expression, while enhanced expression IGF-1R and IRS1, direct targets of this miRNA. Up-regulation of miR-143 has stopped IGF-I-associated resistance to docetaxel, reduced expressions of IGF-I, IRS1, and VEGF in these cells (Niu et al., 2017). Table 5 reviews the importance of IGF-related pathways in response to chemotherapy.
IGF Signaling Pathway in Tumorigenesis and Progression of Chemotherapeutic Drug Resistance Providing the New Concepts in Cancer Therapy
One of the major impediments to current cancer remedy endeavors is the induction of drug resistance by tumors. Despite recent improvements in diagnostic methods and surgical interventions, many aggressive tumors have a poor response to adjuvant or neoadjuvant chemotherapy and radiation. The IGF signaling axis has been detected to have a pivotal role in the progression and development of a variety of tumors (Denduluri et al., 2015). The IGF-1R is involved in various human cancers, such as ovarian, breast, pancreatic, glioma, hepatocellular, lymphoma, and non-small lung cancers. In some cases, its anti-apoptotic attributes strengthen cancerous cells to resist the cytotoxic characteristics of chemotherapeutic agents or radiotherapy (Beauchamp et al., 2009; Dool et al., 2011; Awasthi et al., 2012; Zhou, 2015). Zhou et al. demonstrated that the IGF-1R kinase inhibitor nVp-ADW742 combined with temozolomide could trigger inhibition of P38, GSK3β, and AKT phosphorylation along with a considerable reduction in the intracellular expression levels of Bcl-2, P38, and GSK3β, thereby resulting in promoting response to chemotherapeutic drug temozolomide in medulloblastoma to a large extent (Zhou et al., 2011). Also, Vewinger et al. have illustrated that the IGF signaling pathway has an important role in HGNET-BCOR brain tumor since IGF-1R could be a significant target to improve the sensitivity of vinca alkaloids, vinblastine, doxorubicin, ceritinib, and actinomycin D as efficient drugs in patients affected with this kind of brain tumor. As a consequence, utilizing the off-target IGF1R suppressor ceritinib may pave the way for the remedy of tumor cells driven by IGF1R and IGF2 (Vewinger et al., 2019). In another study, Valerie et al. have indicated that the activity of histone deacetylase inhibitors (HDACi) has reduced in Ewing sarcoma patients. Drug combinations of temozolomide with the dual ALK and IGF-1R inhibitor, AZD3463 could suppress AKT and STAT3 to promote the cytotoxic impacts of temozolomide, and thereby decreasing cell proliferation and enhancing apoptosis via cleavage of PARP and caspase-3 indicating that AKT and STAT3 activation could be modulated by ALK and IGF-1R signaling pathway (Sampson et al., 2015). Additionally, Refolo et al. have figured out that the combined treatment with regorafenib, vitamin K1, and two IGF-1R tyrosine kinase inhibitors GSK1838705A or OSI-906 could strengthen antitumor effects of the target drug, improving their actions and decreasing their toxicity to a large extent. Therefore, both IGF1-R inhibitors could enhance the pro-apoptotic and antiproliferative impacts of regorafenib and VK1 in hepatocellular carcinoma downregulating both MAPK and PI3K/AKT signaling pathways (Refolo et al., 2017). Supplementary Table 2 summarizes the results of various studies that indicate utilizing IGF-1R drug inhibitors with the aim of suppressing the anti-apoptotic properties of IGFR which cause cancerous cells to resist the cytotoxicproperties of chemotherapeutic drugs or radiotherapy.
Epigenetic Regulation of IGF-I, IGF-II, IGF-1R, and IGFBPS of IGF Axis in a Variety of Human Cancers
Accumulating evidence indicates that dysregulation of epigenetic systems has an important role in cancer pathogenesis resulting in overexpression of altered target genes as well as malignant cellular transformation. Since the IGF axis could contribute to cancer progression and invasion, it is now widely accepted that aberrant methylation of IGFBP7, IGFBP-4, IGFBP-3, IGF-1R, IGF-1, and IGF-II promoters could be a potential factor in various common human cancers (Qian et al., 2011; Sato et al., 2011; Bolomsky et al., 2015; Ye P. et al., 2016). Beeghly et.al have demonstrated that differential promoter P2 and P3 methylation patterns of the IGF-II gene could be remarkably related to promoting the risk of disease progression in epithelial ovarian cancer, especially hypermethylation of P2 could be associated with unpleasant symptoms of this serious disease (Beeghly et al., 2007). Additionally, another research indicated that epigenetic alterations in the IGF signaling pathway could play an effective role in the emergence of hepatocellular carcinoma. Therefore, considerable demethylation and upregulation of IGFBP3 via employing 5-Aza-2′- deoxycytidine and trichostatin A therapy results in attenuating cell proliferation and decreasing colony formation in HCC cells (Han et al., 2015). Chang et al. have illustrated that hypermethylation of the IGFBP-3 promoter which dramatically suppressed the expression level of this target gene could be substantially related to poor prognosis among Non-Small Cell Lung Carcinoma patients. Therefore, utilizing demethylation agents to upregulate the expression of IGFBP-3 could pave the way for providing a pivotal remedial procedure for these patients (Chang et al., 2002). Besides, Dar et al. have discovered that epigenetic silencing of IGFBP3 via hypermethylation of its promoter in human melanoma cells. Upregulation of IGFBP3 through applying 5AZA treatment resulting in inhibiting cancer cell survival, triggering tumor cell death, decrease colony formation and invasion, inducing expression of the pro-apoptotic genes containing PUMA, p21, and BAX as well as caspase 3 cleavage and downregulating phosphorylation of AKT (Dar et al., 2010). Furthermore, Schayek et al. have indicated that hypermethylation of AR promoter in metastatic prostate cancer cells results in downregulation of IGF1R expression levels which indicates the fact that the IGF1R gene has been detected s a downstream target for AR action. Employing 5-Aza treatment could trigger demethylation of AR promoter and as a consequence the expression level of IGF1R could increase significantly which may consider as a promising therapy in human prostate cancer (Schayek et al., 2010). An overview of promoter methylation and epigenetic modulation of various genes relevant to the IGF signaling pathway in different human cancers is represented in Supplementary Table 3.
Applying Remedial Crispr and siRNA State-Of-The-Art Genome Editing Systems to Manipulate the IGF Signaling Pathway in Various Human Cancers
It is now accepted that gene silencing via CRISPR-Cas9 and small interfering RNA (siRNA) is becoming an inevitable gene-editing tool in biological research, especially to repair genetic defects via editing or knock out various genes related to the IGF signaling pathway. Via applying a CRISPR/Cas9 or siRNA genome editing tool, it could be possible to knock out or edit ectopic expression of various genes related to IGF signaling cascade through which we could be able to improve response to chemotherapeutic agents as well as attenuating tumor cell survival, proliferation, invasion, angiogenesis, and metastasis of different kinds to a large extent (Singh et al., 2008; Brouwer-Visser et al., 2014; Hussmann et al., 2017; Strub et al., 2018). Liu et al. have detected that knockdown of IGF2BP1 expression level through applying a CRISPR/Cas9 genome editing system could play a crucial role in repressing the expression levels of IGF2, Gli1, CD44, and Myc in skin SCC cells through which tumor cell proliferation and survival were suppressed considerably. Likewise, via utilizing siRNA-mediated knockout of IGF2BP1-bound lncRNA THOR, skin SCC cell growth could be suppressed dramatically (Liu et al., 2018e). In addition, another research demonstrated that silencing IGF1R expression through employing a CRISPR/Cas9 genome editing system leads to functional endpoint mechanism for TKI resistance in a targetable direction MET-amplification, and thereby resulting in improving response to treatment via suppressing resistance to Erlotinib in Non-Small Cell Lung Carcinoma cells and inhibiting epithelial-mesenchymal transition in tumor cells (Hussmann et al., 2017). Besides, Strub et al. have demonstrated that via applying a CRISPR–Cas9 screen targeting chromatin regulators the histone deacetylase SIRT6 haploinsufficiency could play an effective role in upregulating IGFBP2 expression level through promoting chromatin availability, H3K56 acetylation at the IGFBP2 locus, and overexpression of IGF-1R function as well as downstream AKT signaling cascade. Additionally, elevating the IGFBP2 expression could lead to attenuate sensitivity to MAPK signaling inhibitors, and thereby increasing BRAFV600E melanoma cell survival via triggering IGF-1R/AKT signaling pathway. Thus, incorporating a clinically suitable IGF-1Ri with BRAFi could pave the way for promoting the sensitivity of SIRT6 haploinsufficient melanoma cells (Strub et al., 2018). Besides, another research indicated that POU2F3 can be expressed particularly in variant SCLC cancers that have the insufficient expression of neuroendocrine markers and markers of a chemosensory lineage. They applied domain-focused CRISPR screening as a suitable procedure to identify POU2F3 as a significant transcription factor in a subset of SCLC cells and to display other important associations in POU2F3-expressing SCLC lines, containing the lineage TFs SOX9 and ASCL2 and IGF1R. Besides, this strategy shed light on the fact that upregulation of IGFBP5 through employing lentivirus in POU2F3high SCLC lines could suppress tumor cell growth remarkably (Wu et al., 2018). Baade Rø et al. have illustrated that there are an extreme intricacy and interaction between the chemokine and cytokine network triggering migration. They have detected the positive relevance among the degree of cytokine-induced migration and phosphorylation of PAK. PAK phosphorylation was considerably elevated when tumor cells were triggered by combinations of SDF-1a, IGF-1, and HGF which could play an effective role in promoting myeloma cell migration to the large extent. Therefore, via utilizing small interfering RNA, the expression of PAK was downregulated leading to attenuating cytokine-driven migration (Rø et al., 2013). Another study detected that silencing expression of IGFBP-6 or IGF-I or IGF-II through applying siRNA mechanism as well as knockdown IGF-1R activity on fibroblasts could lead to altering fibroblast mobilization, attenuating tumor invasion and TME remodeling through the IGFs/IGF-1R axis in breast epithelial cells which can be considered as a helpful tool for pivotal therapeutic of breast cancer related to dysregulation of IGF signaling pathway (De Vincenzo et al., 2019). Additionally, Brouwer-Visser et.al indicated that suppressing the expression level of IGF2 in ovarian cancer cells via employing RNA interference technology could elevate paclitaxel sensitivity and could restore sensitivity to both microtubule-stabilizing and destabilizing agents (Brouwer-Visser et al., 2014). A summary of clinical researches with the aim of editing or knocking down aberrant expression of different target genes relevant to IGF signaling pathway in various human cancers via employing CRISPR/Cas9 and siRNA gene-editing tools are demonstrated in Tables 6, 7, respectively.
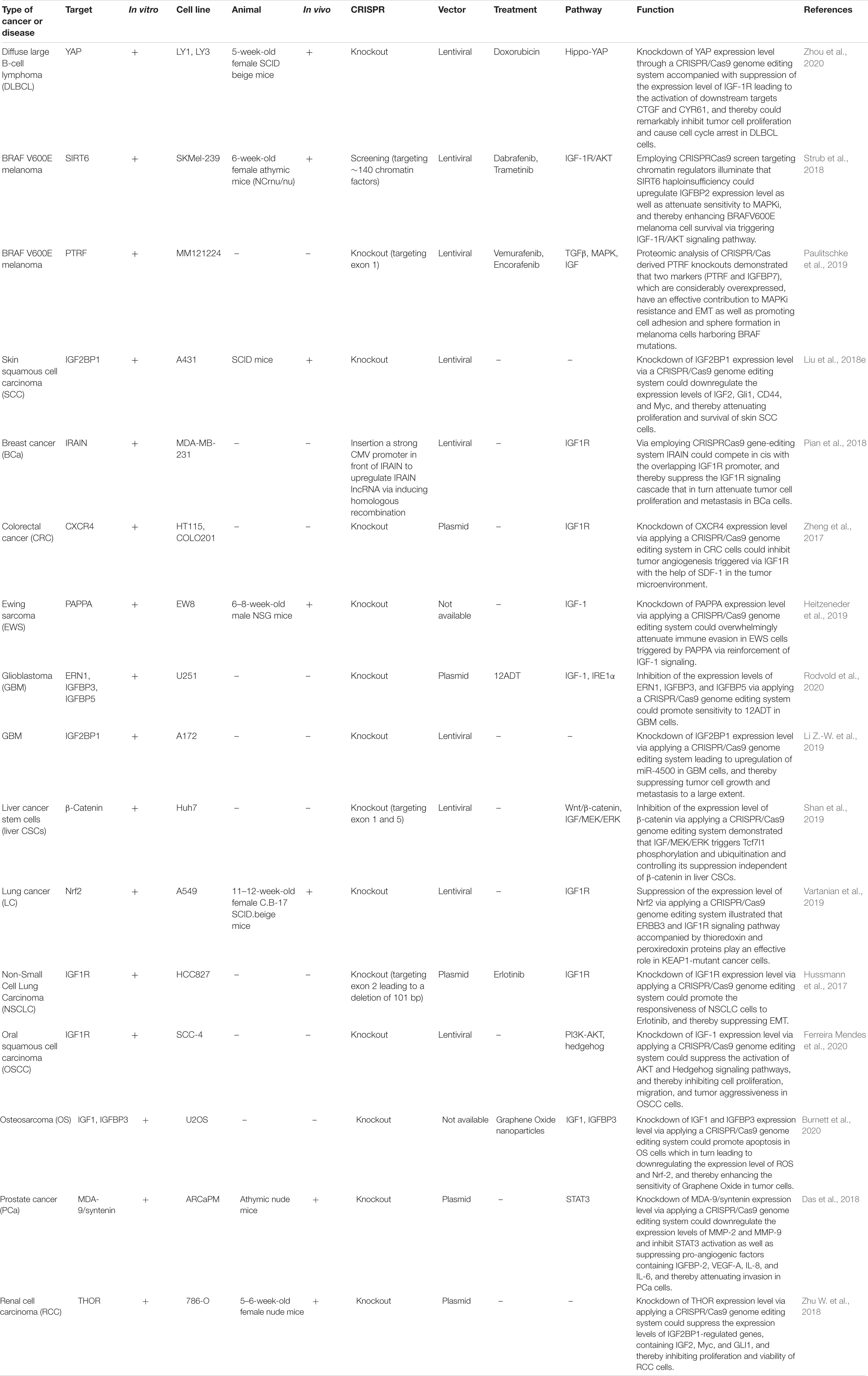
Table 6. Pre-clinical studies employing the CRISPR/Cas9 system with the aim of editing or knocking down various target genes related to the IGF signaling pathway in different human cancers.
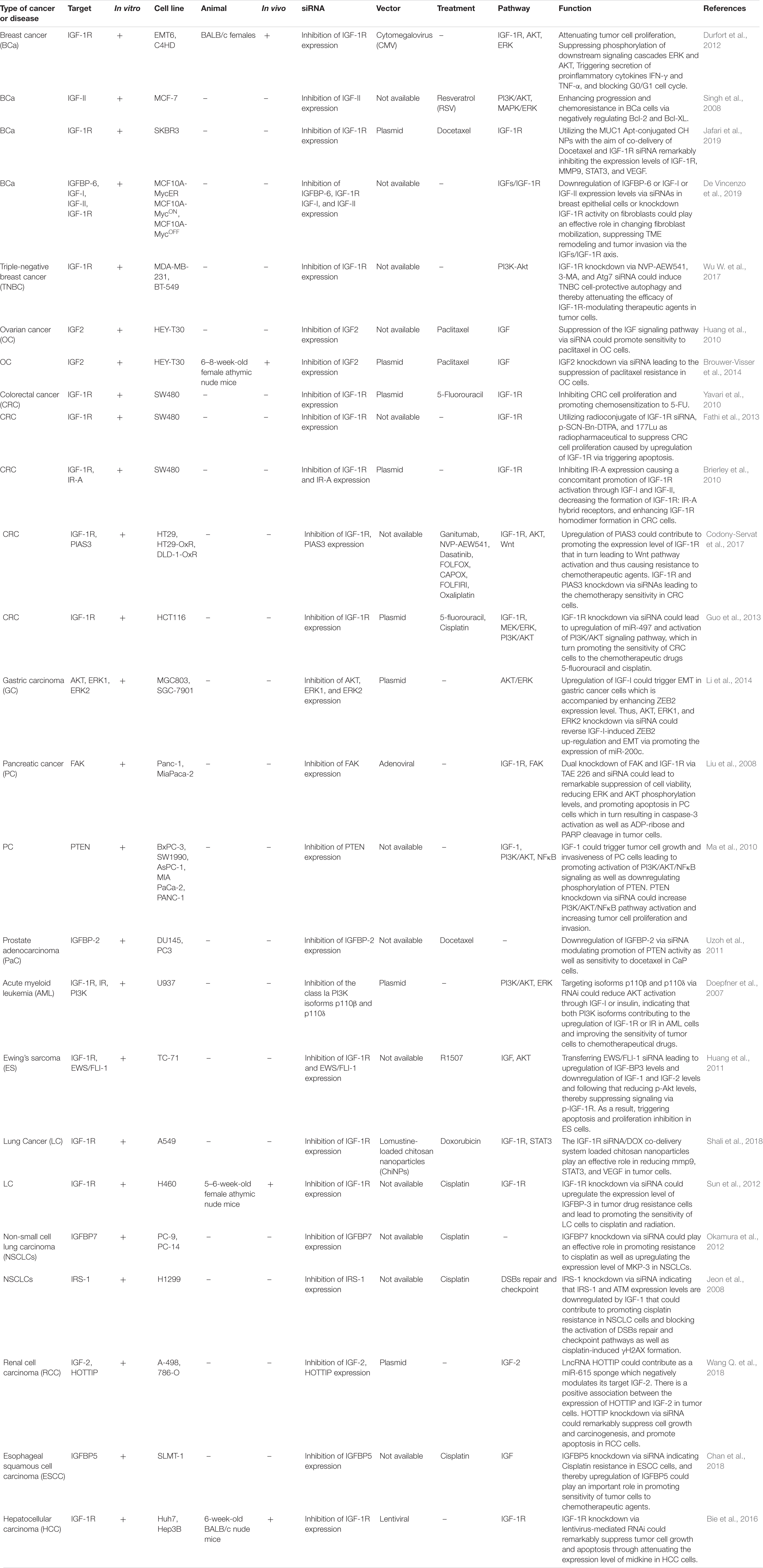
Table 7. Pre-clinical researches applying the siRNA silencing mechanism to edit or knockdown aberrant expression of target genes relevant to the IGF signaling pathway in various human cancers.
Discussion
IGFs and the related signal transduction networks partake in the pathogenesis of cancers, diabetes complications, atherosclerosis, PCOS, and other disorders. Meanwhile, these signaling pathways are regulated by hundreds of miRNAs and lncRNAs. Several members of IGF signaling including IGF-I, IGF-II, IGF-1R, and IGFBP-3 are targets of regulation by miRNAs and lncRNAs. Therefore, understanding the complex interplay between these factors is a necessary step in the design of appropriate therapeutic options for these conditions. The importance of this task has been further underscored by the availability of several IGF-modifying modalities including receptor-specific antibodies, inhibitors of receptor kinases, and activators of AMP-activated protein kinases (Pollak, 2008). In addition to these types of therapeutics, a number of alternative medicines act by affecting the expression of IGF-related non-coding RNAs. For instance, bufothionine induces gastric cancer cell apoptosis via up-regulating miR-133a-3p which sponges IGF1R and regulates PI3K/Akt associated production of reactive oxygen species (Hu Z. H. et al., 2020).
The data presented above indicate that most of the IGF-associated lncRNAs exert their roles via modulation of miRNAs. Examples of lncRNA/miRNA interactions in the IGF-related pathways are circ_0014130/miR-142-5p, Linc00319/miR-147a, TUG1/miR-148b, H19/miR-18b, HCP5/miR-27a-3p and DBH-AS1/miR-138. The association between lncRNAs/miRNAs and the IGF system has importance in regenerative medicine as well. IGF1R signaling has been shown to partake in the preservation of stem cell features and improvement of efficiency of stem cell therapy, as IGF1R-expressing stem cells exhibit strong pluripotent or multipotent features (Teng et al., 2018). Therefore, the lncRNA/miRNA-mediated regulation of IGF1R signaling might offer putative modalities for maintaining stem cell features and enhancing the effects of these therapeutics in clinical settings.
IGF-related miRNAs and lncRNAs can be used as potential markers for forecasting the prognosis of cancer. Moreover, expression levels of these transcripts can be used as diagnostic markers for neoplastic conditions. The importance of IGF signaling in the modulation of response of melanoma, ovarian cancer, breast cancer, pancreatic cancer, prostate cancer, colorectal cancer, and several other cancers to chemotherapeutic agents has been validated. Some lncRNAs and miRNAs such as H19, LUCAT1, miR-143, miR-497, and miR-223 are involved in this process. However, the role of other transcripts should be assessed in the upcoming researches. Based on the role of IGF-related miRNAs and lncRNAs in the modulation of response o chemotherapeutic agents, these transcripts are putative targets for the improvement of the response of cancer cells to these agents.
Besides, promoter methylation of IGF-1R, IGF-1, IGF-II, and especially IGFBP-3 in various regions could be associated with cancer prognosis (Supplementary Table 3). Methylation patterns of these promoters are important for the regulation of their expression and could have pivotal clinical implications in various cancers. Re-expression of IGFBP-3 will be really helpful in curing the majority of aggressive tumors and can solve the problem of intratumoral heterogeneity.
Furthermore, employing CRISPR-Cas9 or siRNAs gene editing tools with the aim of knockdown of ectopic expression of target genes including IGF1R, IGF1, IGF2, IGFBP3, and IGFBP-6 can play an important role in attenuating the tumorigenesis characteristics as well as improving response to treatment in various human cancer cells. Utilizing this effective method will pave the way for future clinical advancement.
Conclusion
The advent of novel genome editing modalities and clarification of the role of epigenetic factors including both genomic marks and non-coding RNAs have raised the possibility of management of human cancers particularly neoplastic disorders with novel therapeutics. Meanwhile, concomitant assessment of expression profile and genomic marks of IGF-related genes using high throughput methods would facilitate appropriate stratification of patients with regards to possible response to each therapeutic option. Further investigations are needed to appraise the clinical application of novel therapeutic modalities that target IGF signaling and related lncRNAs.
Availability of Data and Materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Author Contributions
MT and SG-F supervised the study, wrote the draft, and edited the submission. HS, AA, and MM performed the data collection, designed the tables and figures. All of the authors are contributed equally and fully aware of submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.634512/full#supplementary-material
Supplementary Table 1 | IGF-associated miRNAs in cancers (NNTs: nearby normal tissues).
Supplementary Table 2 | Role of different drug inhibitors in suppressing the IGF-1R activity and attenuating tumorigenesis as well as drug resistance in various human cancer cells and promoting response to treatment.
Supplementary Table 3 | Epigenetic regulation of different genes associated with the IGF signaling pathway in human cancers.
Abbreviations
IGFs, insulin-like growth factors; MAPK, mitogen-activated protein kinase; IGFBP, insulin-like growth factor binding proteins; lncRNAs, long non-coding RNAs; miRNAs, microRNAs; IGFBP-3, IGF binding protein-3; EMT, epithelial-mesenchymal transition; NNTs, nearby normal tissues; GBM, Glioblastoma; OC, ovarian cancer; OS, osteosarcoma; HCC, hepatocellular carcinoma; BCa, breast cancer; NPC, nasopharyngeal carcinoma; GC, gastric cancer; NSCLC, non-small cell lung carcinoma; LGG, low-grade gliomas; WT, wilms tumor; RB, retinoblastoma; OSCC, oral squamous cell carcinoma; CRC, colorectal cancer; RCC, renal cell carcinoma; ULM, uterine leiomyoma; PTC, papillary thyroid carcinoma; EC, endometrial carcinoma; M, melanoma; SCCHN, squamous cell carcinoma of head & neck; HUVECs, human umbilical vascular endothelial cells; CHD, coronary heart disease; DR, diabetic retinopathy; PE, preeclampsia; PCOS, polycystic ovary syndrome; PNI, peripheral nerve injury; RA, rheumatoid arthritis; IPF, idiopathic pulmonary fibrosis; NAFLD, non-alcoholic fatty liver disease; SCI, spinal cord injury; AMI, acute myocardial infarction; LDD, lumbar disc degeneration; DMR, differentially methylated region; PaC, pancreatic cancer; TSCC, tongue squamous cell carcinoma; VSMC, vascular smooth muscle cell; TNBC, triple-negative breast cancer; PaC, prostate adenocarcinoma; ACC, adrenocortical carcinoma; GIST, gastrointestinal stromal tumor; HDACi, histone deacetylase inhibitors; NICTH, non-islet cell tumor hypoglycemia; HB, hepatoblastoma; LA, lung adenocarcinoma; MM, multiple myeloma; EOC, epithelial ovarian cancer; DLBCL, diffuse large b-cell lymphoma; EWS, ewing sarcoma; liver CSCs, liver cancer stem cells.
References
Adachi, Y., Ohashi, H., Imsumran, A., Yamamoto, H., Matsunaga, Y., Taniguchi, H., et al. (2014). The effect of IGF-I receptor blockade for human esophageal squamous cell carcinoma and adenocarcinoma. Tumor Biol. 35, 973–985. doi: 10.1007/s13277-013-1131-2
Afshar, S., Najafi, R., Sedighi Pashaki, A., Sharifi, M., Nikzad, S., Gholami, M. H., et al. (2018). MiR-185 enhances radiosensitivity of colorectal cancer cells by targeting IGF1R and IGF2. Biomed. Pharmacother. 106, 763–769. doi: 10.1016/j.biopha.2018.07.002
Alyoussef, A. (2020). The therapeutic effects of blocking IGF-R1 on mice model of skin cancer. J. Dermatol. Treat. [Epub ahead of print].
An, L. F., Huang, J. W., Han, X., and Wang, J. (2020). Downregulation of lncRNA H19 sensitizes melanoma cells to cisplatin by regulating the miR-18b/IGF1 axis. Anticancer Drugs 31, 473–482. doi: 10.1097/cad.0000000000000888
Awasthi, N., Zhang, C., Ruan, W., Schwarz, M. A., and Schwarz, R. E. (2012). BMS-754807, a small-molecule inhibitor of insulin-like growth factor-1 receptor/insulin receptor, enhances gemcitabine response in pancreatic cancer. Mol. Cancer Ther. 11, 2644–2653. doi: 10.1158/1535-7163.mct-12-0447
Bai, R., Cui, Z., Ma, Y., Wu, Y., Wang, N., Huang, L., et al. (2019). The NF-kappaB-modulated miR-19a-3p enhances malignancy of human ovarian cancer cells through inhibition of IGFBP-3 expression. Mol. Carcinog. 58, 2254–2265. doi: 10.1002/mc.23113
Bao, J., Chen, X., Hou, Y., Kang, G., Li, Q., and Xu, Y. (2018). LncRNA DBH-AS1 facilitates the tumorigenesis of hepatocellular carcinoma by targeting miR-138 via FAK/Src/ERK pathway. Biomed. Pharmacother. 107, 824–833. doi: 10.1016/j.biopha.2018.08.079
Beauchamp, M.-C., Knafo, A., Yasmeen, A., Carboni, J. M., Gottardis, M. M., Pollak, M. N., et al. (2009). BMS-536924 sensitizes human epithelial ovarian cancer cells to the PARP inhibitor, 3-aminobenzamide. Gynecol. Oncology 115, 193–198. doi: 10.1016/j.ygyno.2009.07.009
Beck, O., Paret, C., Russo, A., Burhenne, J., Fresnais, M., Steimel, K., et al. (2020). Safety and activity of the combination of ceritinib and dasatinib in osteosarcoma. Cancers 12:793. doi: 10.3390/cancers12040793
Beeghly, A., Katsaros, D., Wiley, A., de la Longrais, I. R., Prescott, A., Chen, H., et al. (2007). IGF-II promoter methylation and ovarian cancer prognosis. J. Cancer Res. Clin. Oncol. 133, 713–723. doi: 10.1007/s00432-007-0211-3
Bie, C. Q., Liu, X. Y., Cao, M. R., Huang, Q. Y., Tang, H. J., Wang, M., et al. (2016). Lentivirus-mediated RNAi knockdown of insulin-like growth factor-1 receptor inhibits the growth and invasion of hepatocellular carcinoma via down-regulating midkine expression. Oncotarget 7:79305. doi: 10.18632/oncotarget.13027
Biernacka, K., Uzoh, C., Zeng, L., Persad, R., Bahl, A., Gillatt, D., et al. (2013). Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocr Relat. Cancer 20, 741–751. doi: 10.1530/erc-13-0077
Bolomsky, A., Hose, D., Schreder, M., Seckinger, A., Lipp, S., Klein, B., et al. (2015). Insulin like growth factor binding protein 7 (IGFBP7) expression is linked to poor prognosis but may protect from bone disease in multiple myeloma. J. Hematol. Oncol. 8:10. doi: 10.1186/s13045-014-0105-1
Boone, D. N., Warburton, A., Som, S., and Lee, A. V. (2019). A negative feedback loop between Insulin-like Growth Factor signaling and the lncRNA SNHG7 tightly regulates transcript levels and proliferation. bioRxiv [Preprint]. doi: 10.1101/709352
Brierley, G., Macaulay, S., Forbes, B., Wallace, J., Cosgrove, L., and Macaulay, V. (2010). Silencing of the insulin receptor isoform A favors formation of type 1 insulin-like growth factor receptor (IGF-1R) homodimers and enhances ligand-induced IGF-1R activation and viability of human colon carcinoma cells. Endocrinology 151, 1418–1427. doi: 10.1210/en.2009-1006
Brouwer-Visser, J., Lee, J., McCullagh, K., Cossio, M. J., Wang, Y., and Huang, G. S. (2014). Insulin-like growth factor 2 silencing restores taxol sensitivity in drug resistant ovarian cancer. PLoS One 9:e100165. doi: 10.1371/journal.pone.0100165
Burnett, M., Abuetabh, Y., Wronski, A., Shen, F., Persad, S., Leng, R., et al. (2020). Graphene oxide nanoparticles induce apoptosis in wild-type and CRISPR/Cas9-IGF/IGFBP3 knocked-out osteosarcoma cells. J. Cancer 11:5007. doi: 10.7150/jca.46464
Cai, H., An, Y., Chen, X., Sun, D., Chen, T., Peng, Y., et al. (2016). Epigenetic inhibition of miR-663b by long non-coding RNA HOTAIR promotes pancreatic cancer cell proliferation via up-regulation of insulin-like growth factor 2. Oncotarget 7:86857. doi: 10.18632/oncotarget.13490
Camblin, A. J., Pace, E. A., Adams, S., Curley, M. D., Rimkunas, V., Nie, L., et al. (2018). Dual inhibition of IGF-1R and ErbB3 enhances the activity of gemcitabine and nab-paclitaxel in preclinical models of pancreatic cancer. Clin. Cancer Res. 24, 2873–2885. doi: 10.1158/1078-0432.ccr-17-2262
Carrasco-Garcia, E., Martinez-Lacaci, I., Mayor-López, L., Tristante, E., Carballo-Santana, M., García-Morales, P., et al. (2018). PDGFR and IGF-1R inhibitors induce a G2/M arrest and subsequent cell death in human glioblastoma cell lines. Cells 7:131. doi: 10.3390/cells7090131
Chan, D., Zhou, Y., Chui, C. H., Lam, K. H., Law, S., and Chan, A. S.-C. (2018). Expression of insulin-like growth factor binding protein-5 (IGFBP5) reverses cisplatin-resistance in esophageal carcinoma. Cells 7:143. doi: 10.3390/cells7100143
Chang, Y. S., Wang, L., Liu, D., Mao, L., Hong, W. K., Khuri, F. R., et al. (2002). Correlation between insulin-like growth factor-binding protein-3 promoter methylation and prognosis of patients with stage I non-small cell lung cancer. Clin. Cancer Res. 8, 3669–3675.
Chang, Y. S., Wang, L., Suh, Y.-A., Mao, L., Karpen, S. J., Khuri, F. R., et al. (2004). Mechanisms underlying lack of insulin-like growth factor-binding protein-3 expression in non-small-cell lung cancer. Oncogene 23, 6569–6580. doi: 10.1038/sj.onc.1207882
Chen, B., Li, J., Chi, D., Sahnoune, I., Calin, S., Girnita, L., et al. (2019). Non-Coding RNAs in IGF-1R signaling regulation: the underlying pathophysiological link between diabetes and cancer. Cells 8:1638. doi: 10.3390/cells8121638
Chen, B., Li, Q., Zhou, Y., Wang, X., Zhang, Q., Wang, Y., et al. (2018). The long coding RNA AFAP1-AS1 promotes tumor cell growth and invasion in pancreatic cancer through upregulating the IGF1R oncogene via sequestration of miR-133a. Cell Cycle 17, 1949–1966. doi: 10.1080/15384101.2018.1496741
Chen, J., Deng, T., Li, X., and Cai, W. (2019). MiR-193b inhibits the growth and metastasis of renal cell carcinoma by targeting IGF1R. Artif. Cells Nanomed. Biotechnol. 47, 2058–2064. doi: 10.1080/21691401.2019.1620251
Chen, P. H., Cheng, C. H., Shih, C. M., Ho, K. H., Lin, C. W., Lee, C. C., et al. (2016). The Inhibition of microRNA-128 on IGF-1-activating mTOR signaling involves in temozolomide-induced glioma cell apoptotic death. PLoS One 11:e0167096. doi: 10.1371/journal.pone.0167096
Chen, Z., Pan, X., Sheng, Z., Yan, G., Chen, L., and Ma, G. (2019). miR-17 regulates the proliferation and apoptosis of endothelial cells in coronary heart disease via targeting insulin-like-growth factor 1. Pathol. Res. Pract. 215:152512. doi: 10.1016/j.prp.2019.152512
Chen, Z., Yang, H., Nie, Y., and Xing, Y. (2018). miR-145 regulates the proliferation and apoptosis of Y79 human retinoblastoma cells by targeting IGF-1R. Int. J. Clin. Exp. Pathol. 11, 4331–4338.
Chen, Z. L., Li, X. N., Ye, C. X., Chen, H. Y., and Wang, Z. J. (2020). Elevated levels of circRUNX1 in colorectal cancer promote cell growth and metastasis via miR-145-5p/IGF1 Signalling. Onco Targets Ther. 13, 4035–4048. doi: 10.2147/ott.s254133
Cho, Y.-L., Hur, S.-M., Kim, J.-Y., Kim, J.-H., Lee, D.-K., Choe, J., et al. (2015). Specific activation of insulin-like growth factor-1 receptor by ginsenoside Rg5 promotes angiogenesis and vasorelaxation. J. Biol. Chem. 290, 467–477. doi: 10.1074/jbc.m114.603142
Chu, S., Gu, J., Feng, L., Liu, J., Zhang, M., Jia, X., et al. (2014). Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharmacol. 19, 317–326. doi: 10.1016/j.intimp.2014.01.018
Codony-Servat, J., Cuatrecasas, M., Asensio, E., Montironi, C., Martínez-Cardús, A., Marín-Aguilera, M., et al. (2017). Nuclear IGF-1R predicts chemotherapy and targeted therapy resistance in metastatic colorectal cancer. Br. J. Cancer 117, 1777–1786. doi: 10.1038/bjc.2017.279
Cornelissen, B., McLarty, K., Kersemans, V., and Reilly, R. M. (2008). The level of insulin growth factor-1 receptor expression is directly correlated with the tumor uptake of 111In-IGF-1 (E3R) in vivo and the clonogenic survival of breast cancer cells exposed in vitro to trastuzumab (Herceptin). Nuclear Med. Biol. 35, 645–653. doi: 10.1016/j.nucmedbio.2008.05.010
Cortes-Sempere, M., De Miguel, M., Pernia, O., Rodriguez, C., de Castro Carpeno, J., Nistal, M., et al. (2013). IGFBP-3 methylation-derived deficiency mediates the resistance to cisplatin through the activation of the IGFIR/Akt pathway in non-small cell lung cancer. Oncogene 32, 1274–1283. doi: 10.1038/onc.2012.146
Cunningham, M. P., Thomas, H., Marks, C., Green, M., Fan, Z., and Modjtahedi, H. (2008). Co-targeting the EGFR and IGF-1R with anti-EGFR monoclonal antibody ICR62 and the IGF-1R tyrosine kinase inhibitor NVP-AEW541 in colorectal cancer cells. Int. J. Oncol. 33, 1107–1113.
Dang, X., Li, X., Wang, L., Sun, X., and Tian, X. (2017). MicroRNA-3941 targets IGF-1 to regulate cell proliferation and migration of breast cancer cells. Int. J. Clin. Exp. Pathol. 10, 7650–7660.
Dar, A. A., Majid, S., Nosrati, M., De Semir, D., Federman, S., and Kashani-Sabet, M. (2010). Functional modulation of IGF-binding protein-3 expression in melanoma. J. Investig. Dermatol. 130, 2071–2079. doi: 10.1038/jid.2010.70
Das, F., Dey, N., Bera, A., Kasinath, B. S., Ghosh-Choudhury, N., and Choudhury, G. G. (2016). MicroRNA-214 reduces insulin-like growth factor-1 (IGF-1) receptor expression and downstream mTORC1 signaling in renal carcinoma cells. J. Biol. Chem. 291, 14662–14676. doi: 10.1074/jbc.m115.694331
Das, S. K., Pradhan, A. K., Bhoopathi, P., Talukdar, S., Shen, X.-N., Sarkar, D., et al. (2018). The MDA-9/Syntenin/IGF1R/STAT3 axis directs prostate cancer invasion. Cancer Res. 78, 2852–2863. doi: 10.1158/0008-5472.can-17-2992
De Caceres, I. I., Cortes-Sempere, M., Moratilla, C., Machado-Pinilla, R., Rodriguez-Fanjul, V., Manguan-Garcia, C., et al. (2010). IGFBP-3 hypermethylation-derived deficiency mediates cisplatin resistance in non-small-cell lung cancer. Oncogene 29, 1681–1690. doi: 10.1038/onc.2009.454
De Martino, M. C., van Koetsveld, P. M., Feelders, R. A., de Herder, W. W., Dogan, F., Janssen, J., et al. (2019). IGF and mTOR pathway expression and in vitro effects of linsitinib and mTOR inhibitors in adrenocortical cancer. Endocrine 64, 673–684. doi: 10.1007/s12020-019-01869-1
De Vincenzo, A., Belli, S., Franco, P., Telesca, M., Iaccarino, I., Botti, G., et al. (2019). Paracrine recruitment and activation of fibroblasts by c-Myc expressing breast epithelial cells through the IGFs/IGF-1R axis. Int. J. Cancer 145, 2827–2839. doi: 10.1002/ijc.32613
Denduluri, S. K., Idowu, O., Wang, Z., Liao, Z., Yan, Z., Mohammed, M. K., et al. (2015). Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2, 13–25. doi: 10.1016/j.gendis.2014.10.004
Ding, L., Wang, L., and Guo, F. (2017). microRNA188 acts as a tumour suppressor in glioma by directly targeting the IGF2BP2 gene. Mol. Med. Rep. 16, 7124–7130. doi: 10.3892/mmr.2017.7433
Doepfner, K., Spertini, O., and Arcaro, A. (2007). Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia 21, 1921–1930. doi: 10.1038/sj.leu.2404813
Dool, C. J., Mashhedi, H., Zakikhani, M., David, S., Zhao, Y., Birman, E., et al. (2011). IGF1/insulin receptor kinase inhibition by BMS-536924 is better tolerated than alloxan-induced hypoinsulinemia and more effective than metformin in the treatment of experimental insulin-responsive breast cancer. Endocrine Relat. Cancer 18:699. doi: 10.1530/erc-11-0136
Durfort, T., Tkach, M., Meschaninova, M. I., Rivas, M. A., Elizalde, P. V., Venyaminova, A. G., et al. (2012). Small interfering RNA targeted to IGF-1R delays tumor growth and induces proinflammatory cytokines in a mouse breast cancer model. PLoS One 7:e29213. doi: 10.1371/journal.pone.0029213
Eckstein, N., Servan, K., Hildebrandt, B., Politz, A., von Jonquieres, G., Wolf-Kummeth, S., et al. (2009). Hyperactivation of the insulin-like growth factor receptor I signaling pathway is an essential event for cisplatin resistance of ovarian cancer cells. Cancer Res. 69, 2996–3003. doi: 10.1158/0008-5472.can-08-3153
Economou, M. A., Andersson, S., Vasilcanu, D., All-Ericsson, C., Menu, E., Girnita, A., et al. (2008). Oral picropodophyllin (PPP) is well tolerated in vivo and inhibits IGF-1R expression and growth of uveal melanoma. Investig. Ophthalmol. Vis. Sci. 49, 2337–2342. doi: 10.1167/iovs.07-0819
El Tayebi, H. M., Waly, A. A., Assal, R. A., Hosny, K. A., Esmat, G., and Abdelaziz, A. I. (2015). Transcriptional activation of the IGF-II/IGF-1R axis and inhibition of IGFBP-3 by miR-155 in hepatocellular carcinoma. Oncol. Lett. 10, 3206–3212. doi: 10.3892/ol.2015.3725
Fabbi, P., Spallarossa, P., Garibaldi, S., Barisione, C., Mura, M., Altieri, P., et al. (2015). Doxorubicin impairs the insulin-like growth factor-1 system and causes insulin-like growth factor-1 resistance in cardiomyocytes. PLoS One 10:e0124643. doi: 10.1371/journal.pone.0124643
Fan, J., Shi, S., Qiu, Y., Zheng, Z., and Yu, L. (2019). MicroRNA-486-5p down-regulation protects cardiomyocytes against hypoxia-induced cell injury by targeting IGF-1. Int. J. Clin. Exp. Pathol. 12, 2544–2551.
Fathi, M., Taghikhani, M., Ghannadi-Maragheh, M., and Yavari, K. (2013). Demonstration of dose dependent cytotoxic activity in SW480 colon cancer cells by 177Lu-labeled siRNA targeting IGF-1R. Nuclear Med. Biol. 40, 529–536. doi: 10.1016/j.nucmedbio.2012.05.001
Fawzy, I. O., Hamza, M. T., Hosny, K. A., Esmat, G., El Tayebi, H. M., and Abdelaziz, A. I. (2015). miR-1275: a single microRNA that targets the three IGF2-mRNA-binding proteins hindering tumor growth in hepatocellular carcinoma. FEBS Lett. 589, 2257–2265. doi: 10.1016/j.febslet.2015.06.038
Fei, H.-D., Yuan, Q., Mao, L., Chen, F.-L., Cui, Z.-H., Tao, S., et al. (2017). Assessment of GSK1904529A as a promising anti-osteosarcoma agent. Oncotarget 8:49646. doi: 10.18632/oncotarget.17911
Ferreira Mendes, J. M., de Faro Valverde, L., Torres Andion Vidal, M., Paredes, B. D., Coelho, P., Allahdadi, K. J., et al. (2020). Effects of IGF-1 on proliferation, angiogenesis, tumor stem cell populations and activation of AKT and hedgehog pathways in oral squamous cell carcinoma. Int. J. Mol. Sci. 21:6487. doi: 10.3390/ijms21186487
Fowler, C. A., Perks, C. M., Newcomb, P. V., Savage, P. B., Farndon, J. R., and Holly, J. M. (2000). Insulin-like growth factor binding protein-3 (IGFBP-3) potentiates paclitaxel-induced apoptosis in human breast cancer cells. Int. J. Cancer 88, 448–453. doi: 10.1002/1097-0215(20001101)88:3<448::aid-ijc18>3.0.co;2-v
Franks, S. E., Jones, R. A., Briah, R., Murray, P., and Moorehead, R. A. (2016). BMS-754807 is cytotoxic to non-small cell lung cancer cells and enhances the effects of platinum chemotherapeutics in the human lung cancer cell line A549. BMC Res. Notes 9:134. doi: 10.1186/s13104-016-1919-4
Fu, J., Hao, L., Tian, Y., Liu, Y., Gu, Y., and Wu, J. (2018). miR-199a-3p is involved in estrogen-mediated autophagy through the IGF-1/mTOR pathway in osteocyte-like MLO-Y4 cells. J. Cell. Physiol. 233, 2292–2303. doi: 10.1002/jcp.26101
Gable, K. L., Maddux, B. A., Penaranda, C., Zavodovskaya, M., Campbell, M. J., Lobo, M., et al. (2006). Diarylureas are small-molecule inhibitors of insulin-like growth factor I receptor signaling and breast cancer cell growth. Mol. Cancer Ther. 5, 1079–1086. doi: 10.1158/1535-7163.mct-05-0397
Gebeshuber, C. A., and Martinez, J. (2013). miR-100 suppresses IGF2 and inhibits breast tumorigenesis by interfering with proliferation and survival signaling. Oncogene 32, 3306–3310. doi: 10.1038/onc.2012.372
Geng, Y., Sui, C., Xun, Y., Lai, Q., and Jin, L. (2019). MiRNA-99a can regulate proliferation and apoptosis of human granulosa cells via targeting IGF-1R in polycystic ovary syndrome. J. Assist. Reprod. Genet. 36, 211–221. doi: 10.1007/s10815-018-1335-x
George, B., George, S. K., Shi, W., Haque, A., Shi, P., Eskandari, G., et al. (2019). Dual inhibition of IGF-1R and ALK as an effective strategy to eradicate NPM-ALK+ T-cell lymphoma. J. Hematol. Oncol. 12:80.
Ghazal, S., McKinnon, B., Zhou, J., Mueller, M., Men, Y., Yang, L., et al. (2015). H19 lnc RNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol. Med. 7, 996–1003. doi: 10.15252/emmm.201505245
Gigek, C. O., Leal, M. F., Lisboa, L. C. F., Silva, P. N. O., Chen, E. S., Lima, E. M., et al. (2010). Insulin-like growth factor binding protein-3 gene methylation and protein expression in gastric adenocarcinoma. Growth Hormone IGF Res. 20, 234–238. doi: 10.1016/j.ghir.2010.02.005
Guan, J., Zhou, Y., Mao, F., Lin, Y., Shen, S., Zhang, Y., et al. (2018). MicroRNA320a suppresses tumor cell growth and invasion of human breast cancer by targeting insulinlike growth factor 1 receptor. Oncol. Rep. 40, 849–858.
Guo, S. T., Jiang, C. C., Wang, G. P., Li, Y. P., Wang, C. Y., Guo, X. Y., et al. (2013). MicroRNA-497 targets insulin-like growth factor 1 receptor and has a tumour suppressive role in human colorectal cancer. Oncogene 32, 1910–1920. doi: 10.1038/onc.2012.214
Han, J.-J., Xue, D.-W., Han, Q.-R., Liang, X.-H., Xie, L., Li, S., et al. (2015). Induction of apoptosis by IGFBP3 overexpression in hepatocellular carcinoma cells. Asian Pac. J. Cancer Prevent. 15, 10085–10089. doi: 10.7314/apjcp.2014.15.23.10085
Hanafusa, T., Shinji, T., Shiraha, H., Nouso, K., Iwasaki, Y., Yumoto, E., et al. (2005). Functional promoter upstream p53 regulatory sequence of IGFBP3 that is silenced by tumor specific methylation. BMC Cancer 5:9. doi: 10.1186/1471-2407-5-9
Hanafusa, T., Yumoto, Y., Nouso, K., Nakatsukasa, H., Onishi, T., Fujikawa, T., et al. (2002). Reduced expression of insulin-like growth factor binding protein-3 and its promoter hypermethylation in human hepatocellular carcinoma. Cancer Lett. 176, 149–158. doi: 10.1016/s0304-3835(01)00736-4
Hassanlou, M., Soltani, B. M., Medlej, A., Kay, M., and Mowla, S. J. (2020). Hsa-miR-6165 downregulates insulin-like growth factor-1 receptor (IGF-1R) expression and enhances apoptosis in SW480 cells. Biol. Chem. 401, 477–485. doi: 10.1515/hsz-2018-0421
He, Y., Zhang, J., Zheng, J., Du, W., Xiao, H., Liu, W., et al. (2010). The insulin-like growth factor-1 receptor kinase inhibitor, NVP-ADW742, suppresses survival and resistance to chemotherapy in acute myeloid leukemia cells. Oncol. Res. Featuring Preclin. Clin. Cancer Ther. 19, 35–43. doi: 10.3727/096504010x12828372551821
Heitzeneder, S., Sotillo, E., Shern, J. F., Sindiri, S., Xu, P., Jones, R., et al. (2019). Pregnancy-associated plasma protein-A (PAPP-A) in Ewing sarcoma: role in tumor growth and immune evasion. JNCI J. Natl. Cancer Instit. 111, 970–982. doi: 10.1093/jnci/djy209
Hendrickson, A. E. W., Haluska, P., Schneider, P. A., Loegering, D. A., Peterson, K. L., Attar, R., et al. (2009). Expression of insulin receptor isoform A and insulin-like growth factor-1 receptor in human acute myelogenous leukemia: effect of the dual-receptor inhibitor BMS-536924 in vitro. Cancer Res. 69, 7635–7643. doi: 10.1158/0008-5472.can-09-0511
Honda, S., Arai, Y., Haruta, M., Sasaki, F., Ohira, M., Yamaoka, H., et al. (2008). Loss of imprinting of IGF2 correlates with hypermethylation of the H19 differentially methylated region in hepatoblastoma. Br. J. Cancer 99, 1891–1899. doi: 10.1038/sj.bjc.6604754
Hou, X., Huang, F., Macedo, L. F., Harrington, S. C., Reeves, K. A., Greer, A., et al. (2011). Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res. 71, 7597–7607. doi: 10.1158/0008-5472.can-11-1080
Hu, G.-F., Wang, C., Hu, G.-X., Wu, G., Zhang, C., Zhu, W., et al. (2020). AZD3463, an IGF-1R inhibitor, suppresses breast cancer metastasis to bone via modulation of the PI3K-Akt pathway. Ann. Transl. Med. 8:336. doi: 10.21037/atm.2020.02.110
Hu, Y., Yang, Z., Bao, D., Ni, J. S., and Lou, J. (2019). miR-455-5p suppresses hepatocellular carcinoma cell growth and invasion via IGF-1R/AKT/GLUT1 pathway by targeting IGF-1R. Pathol. Res. Pract. 215:152674. doi: 10.1016/j.prp.2019.152674
Hu, Z. H., Wang, G. J., Li, R. X., Zhu, T. Y., Wang, Z. Y., Ding, H. X., et al. (2020). Upregulation of miR-133a-3p enhances Bufothionine-induced gastric cancer cell death by modulating IGF1R/PI3K/Akt signal pathway mediated ER stress. Life Sci. 259:118180. doi: 10.1016/j.lfs.2020.118180
Huang, F., Chang, H., Greer, A., Hillerman, S., Reeves, K. A., Hurlburt, W., et al. (2015). IRS2 copy number gain, KRAS and BRAF mutation status as predictive biomarkers for response to the IGF-1R/IR inhibitor BMS-754807 in colorectal cancer cell lines. Mol. Cancer Ther. 14, 620–630. doi: 10.1158/1535-7163.mct-14-0794-t
Huang, G. S., Brouwer-Visser, J., Ramirez, M. J., Kim, C. H., Hebert, T. M., Lin, J., et al. (2010). Insulin-like growth factor 2 expression modulates Taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin. Cancer Res. 16, 2999–3010. doi: 10.1158/1078-0432.ccr-09-3233
Huang, H. J., Angelo, L. S., Rodon, J., Sun, M., Kuenkele, K.-P., Parsons, H. A., et al. (2011). R1507, an anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, and EWS/FLI-1 siRNA in Ewing’s sarcoma: convergence at the IGF/IGFR/Akt axis. PLoS One 6:e26060. doi: 10.1371/journal.pone.0026060
Huang, R. S., Zheng, Y. L., Li, C., Ding, C., Xu, C., and Zhao, J. (2018). MicroRNA-485-5p suppresses growth and metastasis in non-small cell lung cancer cells by targeting IGF2BP2. Life Sci. 199, 104–111. doi: 10.1016/j.lfs.2018.03.005
Hussmann, D., Madsen, A. T., Jakobsen, K. R., Luo, Y., Sorensen, B. S., and Nielsen, A. L. (2017). IGF1R depletion facilitates MET-amplification as mechanism of acquired resistance to erlotinib in HCC827 NSCLC cells. Oncotarget 8:33300. doi: 10.18632/oncotarget.16350
Ireland, L., Santos, A., Campbell, F., Figueiredo, C., Hammond, D., Ellies, L. G., et al. (2018). Blockade of insulin-like growth factors increases efficacy of paclitaxel in metastatic breast cancer. Oncogene 37, 2022–2036. doi: 10.1038/s41388-017-0115-x
Jafari, R., Zolbanin, N. M., Majidi, J., Atyabi, F., Yousefi, M., Jadidi-Niaragh, F., et al. (2019). Anti-Mucin1 Aptamer-conjugated Chitosan nanoparticles for targeted co-delivery of Docetaxel and IGF-1R siRNA to SKBR3 metastatic breast cancer cells. Iranian Biomed. J. 23:21.
Jameson, M. J., Beckler, A. D., Taniguchi, L. E., Allak, A., VanWagner, L. B., Lee, N. G., et al. (2011). Activation of the insulin-like growth factor-1 receptor induces resistance to epidermal growth factor receptor antagonism in head and neck squamous carcinoma cells. Mol. Cancer Ther. 10, 2124–2134. doi: 10.1158/1535-7163.mct-11-0294
Jeon, J. H., Kim, S. K., Kim, H. J., Chang, J., Ahn, C. M., and Chang, Y. S. (2008). Insulin-like growth factor-1 attenuates cisplatin-induced gammaH2AX formation and DNA double-strand breaks repair pathway in non-small cell lung cancer. Cancer Lett. 272, 232–241. doi: 10.1016/j.canlet.2008.07.011
Jeon, S. H., Yoo, J. K., Kim, C. M., Lim, E. S., Lee, S. J., Lee, J. M., et al. (2018). The novel hsa-miR-12528 regulates tumourigenesis and metastasis through hypo-phosphorylation of AKT cascade by targeting IGF-1R in human lung cancer. Cell Death Dis. 9:493.
Jia, T., Ren, Y., Wang, F., Zhao, R., Qiao, B., Xing, L., et al. (2020). MiR-148a inhibits oral squamous cell carcinoma progression through ERK/MAPK pathway via targeting IGF-1R. Biosci. Rep. 40:BSR20182458.
Jiang, B., Xue, M., Xu, D., Song, J., and Zhu, S. (2020). Down-regulated lncRNA HOTAIR alleviates polycystic ovaries syndrome in rats by reducing expression of insulin-like growth factor 1 via microRNA-130a. J. Cell. Mol. Med. 24, 451–464. doi: 10.1111/jcmm.14753
Julovi, S. M., Martin, J. L., and Baxter, R. C. (2018). Nuclear insulin-like growth factor binding protein-3 as a biomarker in triple-negative breast cancer xenograft tumors: effect of targeted therapy and comparison with chemotherapy. Front. Endocrinol. 9:120. doi: 10.3389/fendo.2018.00120
Kajdaniuk, D., Marek, B., and Kos-Kudla, B. (2001). Influence of adjuvant chemotherapy with cyclophosphamide, methotrexate and 5-fluorouracil on plasma melatonin and chosen hormones in breast cancer premenopausal patients. J. Clin. Pharm. Ther. 26, 297–301.
Kawasaki, T., Nosho, K., Ohnishi, M., Suemoto, Y., Kirkner, G. J., Fuchs, C. S., et al. (2007). IGFBP3 promoter methylation in colorectal cancer: relationship with microsatellite instability, CpG island methylator phenotype, p53. Neoplasia 9, 1091–1098. doi: 10.1593/neo.07760
Kim, M. S., and Lee, D. Y. (2015). Insulin-like growth factor binding protein-3 enhances etoposide-induced cell growth inhibition by suppressing the NF-kappaB activity in gastric cancer cells. Mol. Cell Biochem. 403, 107–113.
Kim, S. T., Jang, H.-L., Lee, J., Park, S. H., Park, Y. S., Lim, H. Y., et al. (2015). Clinical significance of IGFBP-3 methylation in patients with early stage gastric cancer. Transl. Oncol. 8, 288–294. doi: 10.1016/j.tranon.2015.06.001
Kruger, D. T., Alexi, X., Opdam, M., Schuurman, K., Voorwerk, L., Sanders, J., et al. (2020). IGF-1R pathway activation as putative biomarker for linsitinib therapy to revert tamoxifen resistance in ER-positive breast cancer. Int. J. Cancer 146, 2348–2359. doi: 10.1002/ijc.32668
Küffer, S., Gutting, T., Belharazem, D., Sauer, C., Michel, M. S., Marx, A., et al. (2018). Insulin-like growth factor 2 expression in prostate cancer is regulated by promoter-specific methylation. Mol. Oncol. 12, 256–266. doi: 10.1002/1878-0261.12164
Kurio, N., Shimo, T., Fukazawa, T., Takaoka, M., Okui, T., Hassan, N. M. M., et al. (2011). Anti-tumor effect in human breast cancer by TAE226, a dual inhibitor for FAK and IGF-1R in vitro and in vivo. Exp. Cell Res. 317, 1134–1146. doi: 10.1016/j.yexcr.2011.02.008
Lamhamedi-Cherradi, S.-E., Menegaz, B. A., Ramamoorthy, V., Vishwamitra, D., Wang, Y., Maywald, R. L., et al. (2016). IGF-1R and mTOR blockade: novel resistance mechanisms and synergistic drug combinations for Ewing sarcoma. JNCI J. Natl. Cancer Instit. 108:djw182. doi: 10.1093/jnci/djw182
Lawson, E. A., Zhang, X., Crocker, J. T., Wang, W.-L., and Klibanski, A. (2009). Hypoglycemia from IGF2 overexpression associated with activation of fetal promoters and loss of imprinting in a metastatic hemangiopericytoma. J. Clin. Endocrinol. Metab. 94, 2226–2231. doi: 10.1210/jc.2009-0153
Lei, Q., Pan, Q., Li, N., Zhou, Z., Zhang, J., He, X., et al. (2019). H19 regulates the proliferation of bovine male germline stem cells via IGF-1 signaling pathway. J. Cell. Physiol. 234, 915–926. doi: 10.1002/jcp.26920
Li, B., Ge, L., Li, M., Wang, L., and Li, Z. (2016). miR-448 suppresses proliferation and invasion by regulating IGF1R in colorectal cancer cells. Am. J. Transl. Res. 8, 3013–3022.
Li, H., Xu, L., Li, C., Zhao, L., Ma, Y., Zheng, H., et al. (2014). Ubiquitin ligase Cbl-b represses IGF-I-induced epithelial mesenchymal transition via ZEB2 and microRNA-200c regulation in gastric cancer cells. Mol. Cancer 13:136. doi: 10.1186/1476-4598-13-136
Li, S., Geng, J., Xu, X., Huang, X., Leng, D., Jiang, D., et al. (2016). miR-130b-3p modulates epithelial-mesenchymal crosstalk in lung fibrosis by targeting IGF-1. PLoS One 11:e0150418. doi: 10.1371/journal.pone.0150418
Li, X., Li, Y., and Lu, H. (2017). miR-1193 suppresses proliferation and invasion of human breast cancer cells through directly targeting IGF2BP2. Oncol. Res. 25, 579–585. doi: 10.3727/97818823455816x14760504645779
Li, Y., Wang, K., Song, N., Hou, K., Che, X., Zhou, Y., et al. (2019). Activation of IGF-1R pathway and NPM-ALK G1269A mutation confer resistance to crizotinib treatment in NPM-ALK positive lymphoma. Investig. New Drugs 38, 599–609. doi: 10.1007/s10637-019-00802-7
Li, Z., Cai, B., Abdalla, B. A., Zhu, X., Zheng, M., Han, P., et al. (2019). LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J. Cachexia Sarcopenia Muscle 10, 391–410. doi: 10.1002/jcsm.12374
Li, Z.-W., Xue, M., Zhu, B.-X., Yue, C.-L., Chen, M., and Qin, H.-H. (2019). microRNA-4500 inhibits human glioma cell progression by targeting IGF2BP1. Biochem. Biophys. Res. Commun. 513, 800–806. doi: 10.1016/j.bbrc.2019.04.058
Liang, L., Wang, J., Yuan, Y., Zhang, Y., Liu, H., Wu, C., et al. (2018). MicRNA-320 facilitates the brain parenchyma injury via regulating IGF-1 during cerebral I/R injury in mice. Biomed. Pharmacother. 102, 86–93. doi: 10.1016/j.biopha.2018.03.036
Ling, J., Jiang, L., Zhang, C., Dai, J., Wu, Q., and Tan, J. (2015). Upregulation of miR-197 inhibits cell proliferation by directly targeting IGFBP5 in human uterine leiomyoma cells. In Vitro Cell Dev. Biol. Anim. 51, 835–842. doi: 10.1007/s11626-015-9887-x
Liu, F. Y., Zhou, S. J., Deng, Y. L., Zhang, Z. Y., Zhang, E. L., Wu, Z. B., et al. (2015a). MiR-216b is involved in pathogenesis and progression of hepatocellular carcinoma through HBx-miR-216b-IGF2BP2 signaling pathway. Cell Death Dis. 6:e1670. doi: 10.1038/cddis.2015.46
Liu, H., Su, H., Wang, X., and Hao, W. (2018a). MiR-148a regulates bone marrow mesenchymal stem cells-mediated fracture healing by targeting insulin-like growth factor 1. J. Cell. Biochem. [Epub ahead of print].
Liu, J., Jia, Y., Jia, L., Li, T., Yang, L., and Zhang, G. (2019). MicroRNA 615-3p Inhibits the Tumor Growth and Metastasis of NSCLC via Inhibiting IGF2. Oncol. Res. 27, 269–279. doi: 10.3727/096504018x15215019227688
Liu, L., Wang, J., Li, X., Ma, J., Shi, C., Zhu, H., et al. (2015b). MiR-204-5p suppresses cell proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma. Biochem. Biophys. Res. Commun. 457, 621–626. doi: 10.1016/j.bbrc.2015.01.037
Liu, M. D., Wu, H., Wang, S., Pang, P., Jin, S., Sun, C. F., et al. (2018b). MiR-1275 promotes cell migration, invasion and proliferation in squamous cell carcinoma of head and neck via up-regulating IGF-1R and CCR7. Gene 646, 1–7. doi: 10.1016/j.gene.2017.12.049
Liu, N., Yang, H., and Wang, H. (2018c). miR-598 acts as a tumor suppressor in human gastric cancer by targeting IGF-1R. Onco Targets Ther. 11, 2911–2923. doi: 10.2147/ott.s166597
Liu, P., Hu, Y., Ma, L., Du, M., Xia, L., and Hu, Z. (2015c). miR-425 inhibits melanoma metastasis through repression of PI3K-Akt pathway by targeting IGF-1. Biomed. Pharmacother. 75, 51–57. doi: 10.1016/j.biopha.2015.08.010
Liu, T.-J., LaFortune, T., Honda, T., Ohmori, O., Hatakeyama, S., Meyer, T., et al. (2007). Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol. Cancer Ther. 6, 1357–1367. doi: 10.1158/1535-7163.mct-06-0476
Liu, W., Bloom, D. A., Cance, W. G., Kurenova, E. V., Golubovskaya, V. M., and Hochwald, S. N. (2008). FAK and IGF-1R interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis 29, 1096–1107. doi: 10.1093/carcin/bgn026
Liu, W., Kang, L., Han, J., Wang, Y., Shen, C., Yan, Z., et al. (2018d). miR-342-3p suppresses hepatocellular carcinoma proliferation through inhibition of IGF-1R-mediated Warburg effect. Onco Targets Ther. 11, 1643–1653. doi: 10.2147/ott.s161586
Liu, X. Y., Tang, S. H., Wu, S. L., Luo, Y. H., Cao, M. R., Zhou, H. K., et al. (2015d). Epigenetic modulation of insulin-like growth factor-II overexpression by hepatitis B virus X protein in hepatocellular carcinoma. Am. J. Cancer Res. 5:956.
Liu, Y., Zhu, S. T., Wang, X., Deng, J., Li, W. H., Zhang, P., et al. (2016). MiR-100 inhibits osteosarcoma cell proliferation, migration, and invasion and enhances chemosensitivity by targeting IGFIR. Technol. Cancer Res. Treat. 15, N40–N48.
Liu, Z. Q., Fu, W. Q., Zhao, S., and Zhao, X. (2016). Regulation of insulin-like growth factor 1 receptor signaling by microRNA-4458 in the development of lumbar disc degeneration. Am. J. Transl. Res. 8, 2309–2316.
Liu, Z., He, F., OuYang, S., Li, Y., Ma, F., Chang, H., et al. (2019). miR-140-5p could suppress tumor proliferation and progression by targeting TGFBRI/SMAD2/3 and IGF-1R/AKT signaling pathways in Wilms’ tumor. BMC Cancer 19:405. doi: 10.1186/s12885-019-5609-1
Liu, Z., Wu, G., Lin, C., Guo, H., Xu, J., and Zhao, T. (2018e). IGF2BP1 over-expression in skin squamous cell carcinoma cells is essential for cell growth. Biochem. Biophys. Res. Commun. 501, 731–738. doi: 10.1016/j.bbrc.2018.05.057
Lu, L., Cai, M., Peng, M., Wang, F., and Zhai, X. (2019). miR-491-5p functions as a tumor suppressor by targeting IGF2 in colorectal cancer. Cancer Manag. Res. 11, 1805–1816. doi: 10.2147/cmar.s183085
Lu, L., Risch, E., Deng, Q., Biglia, N., Picardo, E., Katsaros, D., et al. (2013). An insulin-like growth factor-II intronic variant affects local DNA conformation and ovarian cancer survival. Carcinogenesis 34, 2024–2030. doi: 10.1093/carcin/bgt168
Luo, L.-H., Rao, L., Luo, L.-F., Chen, K., Ran, R.-Z., and Liu, X.-L. (2020). Long non-coding RNA NKILA inhibited angiogenesis of breast cancer through NF-κB/IL-6 signaling pathway. Microvasc. Res. 129:103968. doi: 10.1016/j.mvr.2019.103968
Luo, X., Dong, J., He, X., Shen, L., Long, C., Liu, F., et al. (2020). MiR-155-5p exerts tumor-suppressing functions in Wilms tumor by targeting IGF2 via the PI3K signaling pathway. Biomed. Pharmacother. 125:109880. doi: 10.1016/j.biopha.2020.109880
Ma, J., Sawai, H., Matsuo, Y., Ochi, N., Yasuda, A., Takahashi, H., et al. (2010). IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J. Surg. Res. 160, 90–101. doi: 10.1016/j.jss.2008.08.016
Ma, Z., Cai, Y., Zhang, L., Tian, C., and Lyu, L. (2020). LINC00319 promotes cervical cancer progression via targeting miR-147a/IGF1R pathway. Cancer Biother. Radiopharm. [Epub ahead of print].
Martinez-Quetglas, I., Pinyol, R., Dauch, D., Torrecilla, S., Tovar, V., Moeini, A., et al. (2016). IGF2 is up-regulated by epigenetic mechanisms in hepatocellular carcinomas and is an actionable oncogene product in experimental models. Gastroenterology 151, 1192–1205. doi: 10.1053/j.gastro.2016.09.001
Mata, R., Palladino, C., Nicolosi, M. L., Lo Presti, A. R., Malaguarnera, R., Ragusa, M., et al. (2016). IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget 7, 7683–7700. doi: 10.18632/oncotarget.6524
Men, Y., Ye, L., Risgaard, R. D., Promes, V., Zhao, X., Paukert, M., et al. (2020). Astroglial FMRP deficiency cell-autonomously up-regulates miR-128 and disrupts developmental astroglial mGluR5 signaling. Proc. Natl. Acad. Sci. U.S.A. 117, 25092–25103. doi: 10.1073/pnas.2014080117
Moharamoghli, M., Hassan-Zadeh, V., Dolatshahi, E., Alizadeh, Z., and Farazmand, A. (2019). The expression of GAS5, THRIL, and RMRP lncRNAs is increased in T cells of patients with rheumatoid arthritis. Clin. Rheumatol. 38, 3073–3080. doi: 10.1007/s10067-019-04694-z
Moritake, H., Saito, Y., Sawa, D., Sameshima, N., Yamada, A., Kinoshita, M., et al. (2019). TAE226, a dual inhibitor of focal adhesion kinase and insulin-like growth factor-I receptor, is effective for Ewing sarcoma. Cancer Med. 8, 7809–7821. doi: 10.1002/cam4.2647
Moser, C., Schachtschneider, P., Lang, S. A., Gaumann, A., Mori, A., Zimmermann, J., et al. (2008). Inhibition of insulin-like growth factor-I receptor (IGF-1R) using NVP-AEW541, a small molecule kinase inhibitor, reduces orthotopic pancreatic cancer growth and angiogenesis. Eur. J. Cancer 44, 1577–1586. doi: 10.1016/j.ejca.2008.04.003
Mukohara, T., Shimada, H., Ogasawara, N., Wanikawa, R., Shimomura, M., Nakatsura, T., et al. (2009). Sensitivity of breast cancer cell lines to the novel insulin-like growth factor-1 receptor (IGF-1R) inhibitor NVP-AEW541 is dependent on the level of IRS-1 expression. Cancer Lett. 282, 14–24. doi: 10.1016/j.canlet.2009.02.056
Niu, X. B., Fu, G. B., Wang, L., Ge, X., Liu, W. T., Wen, Y. Y., et al. (2017). Insulin-like growth factor-I induces chemoresistence to docetaxel by inhibiting miR-143 in human prostate cancer. Oncotarget 8, 107157–107166. doi: 10.18632/oncotarget.22362
Niu, Z.-R., Han, T., Sun, X.-L., Luan, L.-X., Gou, W.-L., and Zhu, X.-M. (2018). MicroRNA-30a-3p is overexpressed in the placentas of patients with preeclampsia and affects trophoblast invasion and apoptosis by its effects on IGF-1. Am. J. Obstetr. Gynecol. 218, 249.e1–249.e12.
Ohshima-Hosoyama, S., Hosoyama, T., Nelon, L. D., and Keller, C. (2010). IGF-1 receptor inhibition by picropodophyllin in medulloblastoma. Biochem. Biophys. Res. Commun. 399, 727–732. doi: 10.1016/j.bbrc.2010.08.009
Okamura, J., Huang, Y., Moon, D., Brait, M., Chang, X., and Kim, M. S. (2012). Downregulation of insulin-like growth factor-binding protein 7 in cisplatin-resistant non-small cell lung cancer. Cancer Biol. Ther. 13, 148–155. doi: 10.4161/cbt.13.3.18695
Otani, H., Yamamoto, H., Takaoka, M., Sakaguchi, M., Soh, J., Jida, M., et al. (2015). TAE226, a bis-anilino pyrimidine compound, inhibits the EGFR-mutant kinase including T790M mutant to show anti-tumor effect on EGFR-mutant non-small cell lung cancer cells. PLoS One 10:e0129838. doi: 10.1371/journal.pone.0129838
Øy, G. F., Slipicevic, A., Davidson, B., Solberg Faye, R. M., Mælandsmo, G., and Flørenes, V. A. (2010). Biological effects induced by insulin-like growth factor binding protein 3 (IGFBP-3) in malignant melanoma. Int. J. Cancer 126, 350–361. doi: 10.1002/ijc.24727
Pan, Y.-H., Jiao, L., Lin, C.-Y., Lu, C.-H., Li, L., Chen, H.-Y., et al. (2018). Combined treatment with metformin and gefitinib overcomes primary resistance to EGFR-TKIs with EGFR mutation via targeting IGF-1R signaling pathway. Biol. Targets Ther. 12:75. doi: 10.2147/btt.s166867
Paulitschke, V., Eichhoff, O., Gerner, C., Paulitschke, P., Bileck, A., Mohr, T., et al. (2019). Proteomic identification of a marker signature for MAPK i resistance in melanoma. EMBO J. 38:e95874.
Pian, L., Wen, X., Kang, L., Li, Z., Nie, Y., Du, Z., et al. (2018). Targeting the IGF1R pathway in breast cancer using antisense lncRNA-mediated promoter cis competition. Mol. Ther. Nucleic Acids 12, 105–117. doi: 10.1016/j.omtn.2018.04.013
Pollak, M. (2008). Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 8, 915–928. doi: 10.1038/nrc2536
Premkumar, D. R., Jane, E. P., and Pollack, I. F. (2010). Co-administration of NVP-AEW541 and dasatinib induces mitochondrial-mediated apoptosis through Bax activation in malignant human glioma cell lines. Int. J. Oncol. 37, 633–643.
Qi, B., Zhang, R., Sun, R., Guo, M., Zhang, M., Wei, G., et al. (2019). IGF-1R inhibitor PQ401 inhibits osteosarcoma cell proliferation, migration and colony formation. Int. J. Clin. Exp. Pathol. 12:1589.
Qian, B., Katsaros, D., Lu, L., Canuto, E. M., Benedetto, C., Beeghly-Fadiel, A., et al. (2011). IGF-II promoter specific methylation and expression in epithelial ovarian cancer and their associations with disease characteristics. Oncol. Rep. 25, 203–213.
Qian, X., Yu, J., Yin, Y., He, J., Wang, L., Li, Q., et al. (2013). MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle 12, 1385–1394. doi: 10.4161/cc.24477
Qiao, X. R., Wang, L., Liu, M., Tian, Y., and Chen, T. (2020). MiR-210-3p attenuates lipid accumulation and inflammation in atherosclerosis by repressing IGF2. Biosci. Biotechnol. Biochem. 84, 321–329. doi: 10.1080/09168451.2019.1685370
Rahmoon, M. A., Youness, R. A., Gomaa, A. I., Hamza, M. T., Waked, I., El Tayebi, H. M., et al. (2017). MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Fact. 35, 76–87. doi: 10.1080/08977194.2017.1354859
Ramachandran, C., Khatib, Z., Petkarou, A., Fort, J., Fonseca, H. B., Melnick, S. J., et al. (2004). Tamoxifen modulation of etoposide cytotoxicity involves inhibition of protein kinase C activity and insulin-like growth factor II expression in brain tumor cells. J. Neurooncol. 67, 19–28. doi: 10.1023/b:neon.0000021738.77612.1b
Refolo, M. G., D’Alessandro, R., Lippolis, C., Carella, N., Cavallini, A., Messa, C., et al. (2017). IGF-1R tyrosine kinase inhibitors and Vitamin K1 enhance the antitumor effects of Regorafenib in HCC cell lines. Oncotarget 8:103465. doi: 10.18632/oncotarget.21403
Regel, I., Eichenmüller, M., Joppien, S., Liebl, J., Häberle, B., Müller-Höcker, J., et al. (2012). IGFBP3 impedes aggressive growth of pediatric liver cancer and is epigenetically silenced in vascular invasive and metastatic tumors. Mol. Cancer 11, 1–11.
Ren, L., Yao, Y., Wang, Y., and Wang, S. (2019). MiR-505 suppressed the growth of hepatocellular carcinoma cells via targeting IGF-1R. Biosci. Rep. 39:BSR20182442.
Rø, T. B., Holien, T., Fagerli, U.-M., Hov, H., Misund, K., Waage, A., et al. (2013). HGF and IGF-1 synergize with SDF-1α in promoting migration of myeloma cells by cooperative activation of p21-activated kinase. Exp. Hematol. 41, 646–655. doi: 10.1016/j.exphem.2013.03.002
Rodvold, J. J., Xian, S., Nussbacher, J., Tsui, B., Waller, T. C., Searles, S. C., et al. (2020). IRE1α and iGf signaling predict resistance to an endoplasmic reticulum stress-inducing drug in glioblastoma cells. Sci. Rep. 10, 1–12. doi: 10.1515/ersc-2018-0001
Rosenzweig, S. A. (2020). The continuing evolution of insulin-like growth factor signaling. F1000Research 9:F1000FacultyRev–205.
Russo, A., Paret, C., Alt, F., Burhenne, J., Fresnais, M., Wagner, W., et al. (2019). Ceritinib-Induced regression of an insulin-like growth factor-driven neuroepithelial brain tumor. Int. J. Mol. Sci. 20:4267. doi: 10.3390/ijms20174267
Sabbatini, P., Korenchuk, S., Rowand, J. L., Groy, A., Liu, Q., Leperi, D., et al. (2009). GSK1838705A inhibits the insulin-like growth factor-1 receptor and anaplastic lymphoma kinase and shows antitumor activity in experimental models of human cancers. Mol. Cancer Ther. 8, 2811–2820. doi: 10.1158/1535-7163.mct-09-0423
Sampson, V. B., Vetter, N. S., Kamara, D. F., Collier, A. B., Gresh, R. C., and Kolb, E. A. (2015). Vorinostat enhances cytotoxicity of SN-38 and temozolomide in ewing sarcoma cells and activates STAT3/AKT/MAPK pathways. PLoS One 10:e0142704. doi: 10.1371/journal.pone.0142704
Sato, H., Sakaeda, M., Ishii, J., Kashiwagi, K., Shimoyamada, H., Okudela, K., et al. (2011). Insulin-like growth factor binding protein-4 gene silencing in lung adenocarcinomas. Pathol. Int. 61, 19–27. doi: 10.1111/j.1440-1827.2010.02612.x
Schayek, H., Bentov, I., Sun, S., Plymate, S. R., and Werner, H. (2010). Progression to metastatic stage in a cellular model of prostate cancer is associated with methylation of the androgen receptor gene and transcriptional suppression of the insulin-like growth factor-I receptor gene. Exp. Cell Res. 316, 1479–1488. doi: 10.1016/j.yexcr.2010.03.007
Shali, H., Shabani, M., Pourgholi, F., Hajivalili, M., Aghebati-Maleki, L., Jadidi-Niaragh, F., et al. (2018). Co-delivery of insulin-like growth factor 1 receptor specific siRNA and doxorubicin using chitosan-based nanoparticles enhanced anticancer efficacy in A549 lung cancer cell line. Artif. Cells Nanomed. Biotechnol. 46, 293–302. doi: 10.1080/21691401.2017.1307212
Shan, J., Shen, J., Wu, M., Zhou, H., Feng, J., Yao, C., et al. (2019). Tcf7l1 acts as a suppressor for the self-renewal of liver cancer stem cells and is regulated by IGF/MEK/ERK signaling independent of β-catenin. Stem Cells 37, 1389–1400. doi: 10.1002/stem.3063
Shastri, A. A., Saleh, A., Savage, J. E., DeAngelis, T., Camphausen, K., and Simone, N. L. (2020). Dietary alterations modulate the microRNA 29/30 and IGF-1/AKT signaling axis in breast Cancer liver metastasis. Nutr. Metab. 17:23.
Shen, X., Zhao, Z., and Yang, B. (2020). MicroRNA-155 promotes apoptosis of colonic smooth muscle cells and aggravates colonic dysmotility by targeting IGF-1. Exp. Ther. Med. 19, 2725–2732.
Shi, X., and Teng, F. (2015). Down-regulated miR-28-5p in human hepatocellular carcinoma correlated with tumor proliferation and migration by targeting insulin-like growth factor-1 (IGF-1). Mol. Cell. Biochem. 408, 283–293. doi: 10.1007/s11010-015-2506-z
Simpson, A. D., Soo, Y. W. J., Rieunier, G., Aleksic, T., Ansorge, O., Jones, C., et al. (2020). Type 1 IGF receptor associates with adverse outcome and cellular radioresistance in paediatric high-grade glioma. Br. J. Cancer 122, 624–629. doi: 10.1038/s41416-019-0677-1
Sin, S. T., Li, Y., Liu, M., Ma, S., and Guan, X.-Y. (2019). TROP-2 exhibits tumor suppressive functions in cervical cancer by dual inhibition of IGF-1R and ALK signaling. Gynecol. Oncol. 152, 185–193. doi: 10.1016/j.ygyno.2018.10.039
Singh, S. K., Moretta, D., Almaguel, F., De Leon, M., and De Leon, D. D. (2008). Precursor IGF-II (proIGF-II) and mature IGF-II (mIGF-II) induce Bcl-2 and Bcl-XL expression through different signaling pathways in breast cancer cells. Growth Fact. 26:92. doi: 10.1080/08977190802057258
Song, C. L., Liu, B., Diao, H. Y., Shi, Y. F., Zhang, J. C., Li, Y. X., et al. (2016). Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget 7, 39740–39757. doi: 10.18632/oncotarget.9240
Song, R. X.-D., Chen, Y., Zhang, Z., Bao, Y., Yue, W., Wang, J.-P., et al. (2010). Estrogen utilization of IGF-1-R and EGF-R to signal in breast cancer cells. J. Steroid Biochem. Mol. Biol. 118, 219–230. doi: 10.1016/j.jsbmb.2009.09.018
Spiliotaki, M., Markomanolaki, H., Mela, M., Mavroudis, D., Georgoulias, V., and Agelaki, S. (2011). Targeting the insulin-like growth factor I receptor inhibits proliferation and VEGF production of non-small cell lung cancer cells and enhances paclitaxel-mediated anti-tumor effect. Lung Cancer 73, 158–165. doi: 10.1016/j.lungcan.2010.11.010
Strub, T., Ghiraldini, F. G., Carcamo, S., Li, M., Wroblewska, A., Singh, R., et al. (2018). SIRT6 haploinsufficiency induces BRAF V600E melanoma cell resistance to MAPK inhibitors via IGF signalling. Nat. Commun. 9, 1–13.
Sun, H., Cai, J., Xu, L., Liu, J., Chen, M., Zheng, M., et al. (2018). miR-483-3p regulates acute myocardial infarction by transcriptionally repressing insulin growth factor 1 expression. Mol. Med. Rep. 17, 4785–4790.
Sun, Y., Zheng, S., Torossian, A., Speirs, C. K., Schleicher, S., Giacalone, N. J., et al. (2012). Role of insulin-like growth factor-1 signaling pathway in cisplatin-resistant lung cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 82, e563–e572.
Tai, B.-J., Yao, M., Zheng, W.-J., Shen, Y.-C., Wang, L., Sun, J.-Y., et al. (2019). Alteration of oncogenic IGF-II gene methylation status associates with hepatocyte malignant transformation. Hepatob. Pancreatic Dis. Int. 18, 158–163. doi: 10.1016/j.hbpd.2019.01.003
Tan, X., Fan, S., Wu, W., and Zhang, Y. (2015). MicroRNA-26a inhibits osteosarcoma cell proliferation by targeting IGF-1. Bone Res. 3:15033.
Tang, S. H., Yang, D. H., Huang, W., Zhou, H. K., Lu, X. H., and Ye, G. (2006). Hypomethylated P4 promoter induces expression of the insulin-like growth factor-II gene in hepatocellular carcinoma in a Chinese population. Clin. Cancer Res. 12, 4171–4177. doi: 10.1158/1078-0432.ccr-05-2261
Teng, C.-F., Jeng, L.-B., and Shyu, W.-C. (2018). Role of insulin-like growth factor 1 receptor signaling in stem cell stemness and therapeutic efficacy. Cell Transpl. 27, 1313–1319. doi: 10.1177/0963689718779777
Tomizawa, M., Shinozaki, F., Motoyoshi, Y., Sugiyama, T., Yamamoto, S., and Sueishi, M. (2014). Picropodophyllin and sorafenib synergistically suppress the proliferation and motility of hepatocellular carcinoma cells. Oncol. Lett. 8, 2023–2026. doi: 10.3892/ol.2014.2484
Torng, P.-L., Lin, C.-W., Chan, M. W., Yang, H.-W., Huang, S.-C., and Lin, C.-T. (2009). Promoter methylation of IGFBP-3 and p53 expression in ovarian endometrioid carcinoma. Mol. Cancer 8:120. doi: 10.1186/1476-4598-8-120
Tu, C., Wang, F., and Wan, J. (2018). MicroRNA-381 inhibits cell proliferation and invasion in endometrial carcinoma by targeting the IGF-1R. Mol. Med. Rep. 17, 4090–4098.
Uzoh, C. C., Holly, J. M., Biernacka, K. M., Persad, R. A., Bahl, A., Gillatt, D., et al. (2011). Insulin-like growth factor-binding protein-2 promotes prostate cancer cell growth via IGF-dependent or -independent mechanisms and reduces the efficacy of docetaxel. Br. J. Cancer 104, 1587–1593. doi: 10.1038/bjc.2011.127
Vartanian, S., Lee, J., Klijn, C., Gnad, F., Bagniewska, M., Schaefer, G., et al. (2019). ERBB3 and IGF1R signaling are required for Nrf2-dependent growth in KEAP1-mutant lung cancer. Cancer Res. 79, 4828–4839. doi: 10.1158/0008-5472.can-18-2086
Vewinger, N., Huprich, S., Seidmann, L., Russo, A., Alt, F., Bender, H., et al. (2019). IGF1R is a potential new therapeutic target for HGNET-BCOR brain tumor patients. Int. J. Mol. Sci. 20:3027. doi: 10.3390/ijms20123027
Volkova, E., Robinson, B. A., Willis, J., Currie, M. J., and Dachs, G. U. (2014). Marginal effects of glucose, insulin and insulin-like growth factor on chemotherapy response in endothelial and colorectal cancer cells. Oncol. Lett. 7, 311–320. doi: 10.3892/ol.2013.1710
Von Mehren, M., George, S., Heinrich, M. C., Schuetze, S. M., Yap, J. T., Jain, Q. Y., et al. (2020). Linsitinib (OSI-906) for the treatment of adult and pediatric wild-type gastrointestinal stromal tumors, a SARC phase II study. Clin. Cancer Res. 26, 1837–1845. doi: 10.1158/1078-0432.ccr-19-1069
Wang, H., Yan, X., Ji, L. Y., Ji, X. T., Wang, P., Guo, S. W., et al. (2017). miR-139 Functions as An Antioncomir to Repress Glioma Progression Through Targeting IGF-1 R, AMY-1, and PGC-1beta. Technol. Cancer Res. Treat. 16, 497–511. doi: 10.1177/1533034616630866
Wang, J., Liu, H., Tian, L., Wang, F., Han, L., Zhang, W., et al. (2017). miR-15b inhibits the progression of glioblastoma cells through targeting insulin-like growth factor receptor 1. Horm. Cancer 8, 49–57. doi: 10.1007/s12672-016-0276-z
Wang, L., Luan, T., Zhou, S., Lin, J., Yang, Y., Liu, W., et al. (2019a). LncRNA HCP5 promotes triple negative breast cancer progression as a ceRNA to regulate BIRC3 by sponging miR-219a-5p. Cancer Med. 8, 4389–4403. doi: 10.1002/cam4.2335
Wang, M., Shi, J., Jiang, H., Xu, K., and Huang, Z. (2020). Circ_0014130 Participates in the Proliferation and Apoptosis of Nonsmall Cell Lung Cancer Cells via the miR-142-5p/IGF-1 Axis. Cancer Biother. Radiopharm. 35, 233–240. doi: 10.1089/cbr.2019.2965
Wang, Q., Wu, G., Zhang, Z., Tang, Q., Zheng, W., Chen, X., et al. (2018). Long non-coding RNA HOTTIP promotes renal cell carcinoma progression through the regulation of the miR-615/IGF-2 pathway. Int. J. Oncol. 53, 2278– 2288.
Wang, Q., Zhang, Y., Zhu, J., Zheng, H., Chen, S., Chen, L., et al. (2020). IGF-1R inhibition induces MEK phosphorylation to promote survival in colon carcinomas. Signal Transd. Target. Ther. 5, 1–11.
Wang, R., Li, H., Guo, X., Wang, Z., Liang, S., and Dang, C. I. G. F.-I. (2016). Induces epithelial-to-mesenchymal transition via the IGF-1R-Src-MicroRNA-30a-E-cadherin pathway in nasopharyngeal carcinoma cells. Oncol. Res. 24, 225–231. doi: 10.3727/096504016x14648701447931
Wang, T., Liu, Y., Lv, M., Xing, Q., Zhang, Z., He, X., et al. (2019b). miR-323-3p regulates the steroidogenesis and cell apoptosis in polycystic ovary syndrome (PCOS) by targeting IGF-1. Gene 683, 87–100. doi: 10.1016/j.gene.2018.10.006
Wang, W., Dong, M., Zhang, W., and Liu, T. (2019c). Long noncoding LUCAT1 promotes cisplatin resistance of non-small cell lung cancer by promoting IGF-2. Eur. Rev. Med. Pharmacol. Sci. 23, 5229–5234.
Wang, X., Liu, S., Cao, L., Zhang, T., Yue, D., Wang, L., et al. (2017). miR-29a-3p suppresses cell proliferation and migration by downregulating IGF1R in hepatocellular carcinoma. Oncotarget 8, 86592–86603. doi: 10.18632/oncotarget.21246
Wang, Y., Hou, L., Yuan, X., Xu, N., Zhao, S., Yang, L., et al. (2020). miR-483-3p promotes cell proliferation and suppresses apoptosis in rheumatoid arthritis fibroblast-like synoviocytes by targeting IGF-1. Biomed. Pharmacother. 130:110519. doi: 10.1016/j.biopha.2020.110519
Wang, Y., Jia, L., Wang, B., Diao, S., Jia, R., and Shang, J. (2019d). MiR-495/IGF-1/AKT signaling as a novel axis is involved in the epithelial-to-mesenchymal transition of oral squamous cell carcinoma. J. Oral Maxillofac. Surg. 77, 1009–1021. doi: 10.1016/j.joms.2018.12.021
Wang, Y., Lu, J.-H., Wu, Q.-N., Jin, Y., Wang, D.-S., Chen, Y.-X., et al. (2019e). LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol. Cancer 18, 1–18.
Wang, Z. G., Fukazawa, T., Nishikawa, T., Watanabe, N., Sakurama, K., Motoki, T., et al. (2008). TAE226, a dual inhibitor for FAK and IGF-1R, has inhibitory effects on mTOR signaling in esophageal cancer cells. Oncol. Rep. 20, 1473–1477.
Wang, Z., Liu, G., Mao, J., Xie, M., Zhao, M., Guo, X., et al. (2019f). IGF-1R inhibition suppresses cell proliferation and increases radiosensitivity in nasopharyngeal carcinoma cells. Mediat. Inflamm. 2019:5497467.
Wang, Z., Zhao, Z., Yang, Y., Luo, M., Zhang, M., Wang, X., et al. (2018). MiR-99b-5p and miR-203a-3p function as tumor suppressors by targeting IGF-1R in gastric cancer. Sci. Rep. 8:10119.
Warshamana-Greene, G. S., Litz, J., Buchdunger, E., García-Echeverría, C., Hofmann, F., and Krystal, G. W. (2005). The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin. Cancer Res. 11, 1563–1571. doi: 10.1158/1078-0432.ccr-04-1544
Warshamana-Greene, G. S., Litz, J., Buchdunger, E., Hofmann, F., García-Echeverría, C., and Krystal, G. W. (2004). The insulin-like growth factor-I (IGF-I) receptor kinase inhibitor NVP-ADW742, in combination with STI571, delineates a spectrum of dependence of small cell lung cancer on IGF-I and stem cell factor signaling. Mol. Cancer Ther. 3, 527–536.
Watanabe, N., Takaoka, M., Sakurama, K., Tomono, Y., Hatakeyama, S., Ohmori, O., et al. (2008). Dual tyrosine kinase inhibitor for focal adhesion kinase and insulin-like growth factor-I receptor exhibits anticancer effect in esophageal adenocarcinoma in vitro and in vivo. Clin. Cancer Res. 14, 4631–4639. doi: 10.1158/1078-0432.ccr-07-4755
Wen, Y. Y., Liu, W. T., Sun, H. R., Ge, X., Shi, Z. M., Wang, M., et al. (2017). IGF-1-mediated PKM2/beta-catenin/miR-152 regulatory circuit in breast cancer. Sci. Rep. 7:15897.
Wrigley, S., Arafa, D., and Tropea, D. (2017). Insulin-like growth factor 1: at the crossroads of brain development and aging. Front. Cell. Neurosci. 11:14. doi: 10.3389/fncel.2017.00014
Wu, G., Liu, J., Wu, Z., Wu, X., and Yao, X. (2017). MicroRNA-184 inhibits cell proliferation and metastasis in human colorectal cancer by directly targeting IGF-1R. Oncol. Lett. 14, 3215–3222. doi: 10.3892/ol.2017.6499
Wu, H. Y., Wang, X. H., Liu, K., and Zhang, J. L. (2020). LncRNA MALAT1 regulates trophoblast cells migration and invasion via miR-206/IGF-1 axis. Cell Cycle 19, 39–52. doi: 10.1080/15384101.2019.1691787
Wu, J. H., Wang, Y. H., Wang, W., Shen, W., Sang, Y. Z., Liu, L., et al. (2016). MiR-18b suppresses high-glucose-induced proliferation in HRECs by targeting IGF-1/IGF1R signaling pathways. Int. J. Biochem. Cell. Biol. 73, 41–52. doi: 10.1016/j.biocel.2016.02.002
Wu, W., Chen, F., Cui, X., Yang, L., Chen, J., Zhao, J., et al. (2018). LncRNA NKILA suppresses TGF-β-induced epithelial–mesenchymal transition by blocking NF-κB signaling in breast cancer. Int. J. Cancer 143, 2213–2224. doi: 10.1002/ijc.31605
Wu, W., Ma, J., Shao, N., Shi, Y., Liu, R., Li, W., et al. (2017). Co-targeting IGF-1R and autophagy enhances the effects of cell growth suppression and apoptosis induced by the IGF-1R inhibitor NVP-AEW541 in triple-negative breast cancer cellsA. PLoS One 12:e0169229. doi: 10.1371/journal.pone.0169229
Wu, X., Wu, Q., Zhou, X., and Huang, J. (2019). SphK1 functions downstream of IGF-1 to modulate IGF-1-induced EMT, migration and paclitaxel resistance of A549 cells: a preliminary in vitro study. J. Cancer 10, 4264–4269. doi: 10.7150/jca.32646
Wu, X., Zheng, X., Cheng, J., Zhang, K., and Ma, C. (2020). LncRNA TUG1 regulates proliferation and apoptosis by regulating miR-148b/IGF2 axis in ox-LDL-stimulated VSMC and HUVEC. Life Sci. 243:117287. doi: 10.1016/j.lfs.2020.117287
Xia, M., Li, H., Wang, J. J., Zeng, H. J., and Wang, S. H. (2016). MiR-99a suppress proliferation, migration and invasion through regulating insulin-like growth factor 1 receptor in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 20, 1755–1763.
Xiang, Y., Song, Y., Li, Y., Zhao, D., Ma, L., and Tan, L. (2016). miR-483 is down-regulated in polycystic ovarian syndrome and inhibits KGN cell proliferation via targeting insulin-like growth factor 1 (IGF1). Med. Sci. Monit. 22, 3383–3393. doi: 10.12659/msm.897301
Xiao, J., He, C., Zhong, Y., Liu, L., Xiao, Y., and Liu, W. (2019). miR-223 regulates proliferation and apoptosis by targeting insulin-like growth factor 1 receptor (IGF1R) in osteosarcoma cells. Am. Sci. Publish. 9, 459–466. doi: 10.1166/mex.2019.1516
Xie, R., Wang, M., Zhou, W., Wang, D., Yuan, Y., Shi, H., et al. (2019). Long non-coding RNA (LncRNA) UFC1/miR-34a contributes to proliferation and migration in breast cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 25:7149. doi: 10.12659/msm.917562
Xu, G., Yuan, Y., Wang, M., Yang, J., Wang, H., Sun, Y., et al. (2019). LncRNA H19 suppresses metastasis of follicular thyroid carcinoma via the IGF1/JAK/STAT pathway. STAT Pathway [Epub ahead of print].
Xu, M., Zheng, X. M., Jiang, F., and Qiu, W. Q. (2018). MicroRNA-190b regulates lipid metabolism and insulin sensitivity by targeting IGF-1 and ADAMTS9 in non-alcoholic fatty liver disease. J. Cell. Biochem. 119, 5864–5874. doi: 10.1002/jcb.26776
Yang, D., Qian, H., Fang, Z., Xu, A., Zhao, S., Liu, B., et al. (2020). Silencing circular RNA VANGL1 inhibits progression of bladder cancer by regulating miR-1184/IGFBP2 axis. Cancer Med. 9, 700–710. doi: 10.1002/cam4.2650
Yang, H., Fu, G., Liu, F., Hu, C., Lin, J., Tan, Z., et al. (2019). LncRNA THOR promotes tongue squamous cell carcinomas by stabilizing IGF2BP1 downstream targets. Biochimie 165, 9–18. doi: 10.1016/j.biochi.2019.06.012
Yang, J.-J., Liu, L.-P., Tao, H., Hu, W., Shi, P., Deng, Z.-Y., et al. (2016). MeCP2 silencing of LncRNA H19 controls hepatic stellate cell proliferation by targeting IGF1R. Toxicology 359, 39–46. doi: 10.1016/j.tox.2016.06.016
Yang, Y., Liu, X., Cheng, L., Li, L., Wei, Z., Wang, Z., et al. (2020). Tumor suppressor microRNA-138 suppresses low-grade glioma development and metastasis via regulating IGF2BP2. Onco Targets Ther. 13, 2247–2260. doi: 10.2147/ott.s232795
Yao, Y., Song, T., Xiong, G., Wu, Z., Li, Q., Xia, H., et al. (2018). Combination of peripheral blood mononuclear cell miR-19b-5p, miR-221, miR-25-5p, and hypertension correlates with an increased heart failure risk in coronary heart disease patients. Anatolian J. Cardiol. 20:100.
Yavari, K., Taghikhani, M., Maragheh, M. G., Mesbah-Namin, S. A., and Babaei, M. H. (2010). Downregulation of IGF-1R expression by RNAi inhibits proliferation and enhances chemosensitization of human colon cancer cells. Int. J. Colorect. Dis. 25, 9–16. doi: 10.1007/s00384-009-0783-2
Ye, P., Qu, C.-F., and Hu, X.-L. (2016). Impact of IGF-1, IGF-1R, and IGFBP-3 promoter methylation on the risk and prognosis of esophageal carcinoma. Tumor Biol. 37, 6893–6904. doi: 10.1007/s13277-015-4489-5
Ye, S., Song, W., Xu, X., Zhao, X., and Yang, L. (2016). IGF2BP2 promotes colorectal cancer cell proliferation and survival through interfering with RAF-1 degradation by miR-195. FEBS Lett. 590, 1641–1650. doi: 10.1002/1873-3468.12205
Yi, F., Shang, Y., Li, B., Dai, S., Wu, W., Cheng, L., et al. (2017). MicroRNA-193-5p modulates angiogenesis through IGF2 in type 2 diabetic cardiomyopathy. Biochem. Biophys. Res. Commun. 491, 876–882. doi: 10.1016/j.bbrc.2017.07.108
Yin, D., Tamaki, N., Parent, A. D., and Zhang, J. H. (2005). Insulin-like growth factor-I decreased etoposide-induced apoptosis in glioma cells by increasing bcl-2 expression and decreasing CPP32 activity. Neurol. Res. 27, 27–35. doi: 10.1179/016164105x18151
Youness, R. A., El-Tayebi, H. M., Assal, R. A., Hosny, K., Esmat, G., and Abdelaziz, A. I. (2016). MicroRNA-486-5p enhances hepatocellular carcinoma tumor suppression through repression of IGF-1R and its downstream mTOR, STAT3 and c-Myc. Oncol. Lett. 12, 2567–2573. doi: 10.3892/ol.2016.4914
Yu, M., Yu, S., Gong, W., Chen, D., Guan, J., and Liu, Y. (2019). Knockdown of linc01023 restrains glioma proliferation, migration and invasion by regulating IGF-1R/AKT pathway. J. Cancer 10:2961. doi: 10.7150/jca.31004
Zeng, L., Jarrett, C., Brown, K., Gillespie, K. M., Holly, J. M., and Perks, C. M. (2013). Insulin-like growth factor binding protein-3 (IGFBP-3) plays a role in the anti-tumorigenic effects of 5-Aza-2′-deoxycytidine (AZA) in breast cancer cells. Exp. Cell Res. 319, 2282–2295. doi: 10.1016/j.yexcr.2013.06.011
Zhang, B., Li, Y., Hou, D., Shi, Q., Yang, S., and Li, Q. (2017). MicroRNA-375 Inhibits Growth and Enhances Radiosensitivity in Oral Squamous Cell Carcinoma by Targeting Insulin Like Growth Factor 1 Receptor. Cell Physiol. Biochem. 42, 2105–2117. doi: 10.1159/000479913
Zhang, Q., Li, T., Wang, Z., Kuang, X., Shao, N., and Lin, Y. (2020). lncRNA NR2F1-AS1 promotes breast cancer angiogenesis through activating IGF-1/IGF-1R/ERK pathway. J. Cell. Mol. Med. 24, 8236–8247. doi: 10.1111/jcmm.15499
Zhang, W., Liu, S., Liu, K., and Liu, Y. (2019). Long non-coding RNA deleted in lymphocytic leukaemia 1 promotes hepatocellular carcinoma progression by sponging miR-133a to regulate IGF-1R expression. J. Cell. Mol. Med. 23, 5154–5164. doi: 10.1111/jcmm.14384
Zhang, Y., Sun, Y., Peng, R., Liu, H., He, W., Zhang, L., et al. (2018). The long noncoding RNA 150Rik promotes mesangial cell proliferation via miR-451/IGF1R/p38 MAPK signaling in diabetic nephropathy. Cell Physiol. Biochem. 51, 1410–1428. doi: 10.1159/000495590
Zhang, Y., Yan, N., Wang, X., Chang, Y., and Wang, Y. (2019). MiR-129-5p regulates cell proliferation and apoptosis via IGF-1R/Src/ERK/Egr-1 pathway in RA-fibroblast-like synoviocytes. Biosci. Rep. 39:BSR20192009.
Zhao, F. Y., Han, J., Chen, X. W., Wang, J., Wang, X. D., Sun, J. G., et al. (2016). miR-223 enhances the sensitivity of non-small cell lung cancer cells to erlotinib by targeting the insulin-like growth factor-1 receptor. Int. J. Mol. Med. 38, 183–191. doi: 10.3892/ijmm.2016.2588
Zheng, F., Tang, Q., Zheng, X. H., Wu, J., Huang, H., Zhang, H., et al. (2018). Inactivation of Stat3 and crosstalk of miRNA155-5p and FOXO3a contribute to the induction of IGFBP1 expression by beta-elemene in human lung cancer. Exp. Mol. Med. 50:121.
Zheng, F., Wang, X., Zheng, W., and Zhao, J. (2019). Long noncoding RNA HOXA-AS2 promotes cell migration and invasion via upregulating IGF-2 in non-small cell lung cancer as an oncogene. Eur. Rev. Med. Pharmacol. Sci. 23, 4793–4799.
Zheng, F., Zhang, Z., Flamini, V., Jiang, W. G., and Cui, Y. (2017). The axis of CXCR4/SDF-1 plays a role in colon cancer cell adhesion through regulation of the AKT and IGF1R signalling pathways. Anticancer Res. 37, 4361–4369.
Zhong, Z., Li, F., Li, Y., Qin, S., Wen, C., Fu, Y., et al. (2018). Inhibition of microRNA-19b promotes ovarian granulosa cell proliferation by targeting IGF-1 in polycystic ovary syndrome. Mol. Med. Rep. 17, 4889–4898.
Zhou, F., Nie, L., Feng, D., Guo, S., and Luo, R. (2017). MicroRNA-379 acts as a tumor suppressor in non-small cell lung cancer by targeting the IGF1R-mediated AKT and ERK pathways. Oncol. Rep. 38, 1857–1866. doi: 10.3892/or.2017.5835
Zhou, H., Rao, J., Lin, J., Yin, B., Sheng, H., Lin, F., et al. (2011). The insulin-like growth factor-I receptor kinase inhibitor NVP-ADW742 sensitizes medulloblastoma to the effects of chemotherapy. Oncol. Rep. 25, 1565–1571.
Zhou, N., Sun, Z., Li, N., Ge, Y., Zhou, J., Han, Q., et al. (2018). miR197 promotes the invasion and migration of colorectal cancer by targeting insulinlike growth factorbinding protein 3. Oncol. Rep. 40, 2710–2721.
Zhou, Q. (2015). BMS-536924, an ATP-competitive IGF-1R/IR inhibitor, decreases viability and migration of temozolomide-resistant glioma cells in vitro and suppresses tumor growth in vivo. OncoTargets Ther. 8:689.
Zhou, X., Chen, N., Xu, H., Zhou, X., Wang, J., Fang, X., et al. (2020). Regulation of Hippo-YAP signaling by insulin-like growth factor-1 receptor in the tumorigenesis of diffuse large B-cell lymphoma. J. Hematol. Oncol. 13, 1–15.
Zhou, X., Shen, F., Ma, P., Hui, H., Pei, S., Chen, M., et al. (2015). GSK1838705A, an IGF-1R inhibitor, inhibits glioma cell proliferation and suppresses tumor growth in vivo. Mol. Med. Rep. 12, 5641–5646. doi: 10.3892/mmr.2015.4129
Zhou, X., Zhao, X., Li, X., Ping, G., Pei, S., Chen, M., et al. (2016). PQ401, an IGF-1R inhibitor, induces apoptosis and inhibits growth, proliferation and migration of glioma cells. J. Chemother. 28, 44–49. doi: 10.1179/1973947815y.0000000026
Zhou, Y., Li, S., Li, J., Wang, D., and Li, Q. (2017). Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol. Biochem. 42, 1431–1446. doi: 10.1159/000479207
Zhu, H., Xue, C., Yao, M., Wang, H., Zhang, P., Qian, T., et al. (2018). miR-129 controls axonal regeneration via regulating insulin-like growth factor-1 in peripheral nerve injury. Cell Death Dis. 9:720.
Zhu, W., Lei, J., Bai, X., Wang, R., Ye, Y., and Bao, J. (2018). MicroRNA-503 regulates hypoxia-induced cardiomyocytes apoptosis through PI3K/Akt pathway by targeting IGF-1R. Biochem. Biophys. Res. Commun. 506, 1026–1031. doi: 10.1016/j.bbrc.2018.10.160
Keywords: IGF, miRNA, lncRNA, expression, disorders
Citation: Ghafouri-Fard S, Abak A, Mohaqiq M, Shoorei H and Taheri M (2021) The Interplay Between Non-coding RNAs and Insulin-Like Growth Factor Signaling in the Pathogenesis of Neoplasia. Front. Cell Dev. Biol. 9:634512. doi: 10.3389/fcell.2021.634512
Received: 27 November 2020; Accepted: 02 February 2021;
Published: 09 March 2021.
Edited by:
Tatjana S. Kostic, University of Novi Sad, SerbiaReviewed by:
Haitao Wang, Southern Medical University, ChinaDeepak Chhangani, University of Florida, United States
Copyright © 2021 Ghafouri-Fard, Abak, Mohaqiq, Shoorei and Taheri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamed Shoorei, aC5zaG9vcmVpQGdtYWlsLmNvbQ==; Mohammad Taheri, bW9oYW1tYWRfODIzQHlhaG9vLmNvbQ==
 Soudeh Ghafouri-Fard1
Soudeh Ghafouri-Fard1 Atefe Abak
Atefe Abak Hamed Shoorei
Hamed Shoorei Mohammad Taheri
Mohammad Taheri