
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cell Dev. Biol., 02 February 2021
Sec. Cell Death and Survival
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.627976
This article is part of the Research TopicDeciphering Phagocyte Functions across Different SpeciesView all 12 articles
The innate immune system is the primary defense response to limit invading pathogens for all invertebrate species. In insects, immune cells are central to both cellular and humoral immune responses, however few genetic resources exist beyond Drosophila to study immune cell function. Therefore, the development of innovative tools that can be widely applied to a variety of insect systems is of importance to advance the study of insect immunity. Here, we have adapted the use of clodronate liposomes (CLD) to deplete phagocytic immune cells in the vinegar fly, Drosophila melanogaster, and the yellow fever mosquito, Aedes aegypti. Through microscopy and molecular techniques, we validate the depletion of phagocytic cell populations in both insect species and demonstrate the integral role of phagocytes in combating bacterial pathogens. Together, these data demonstrate the wide utility of CLD in insect systems to advance the study of phagocyte function in insect innate immunity.
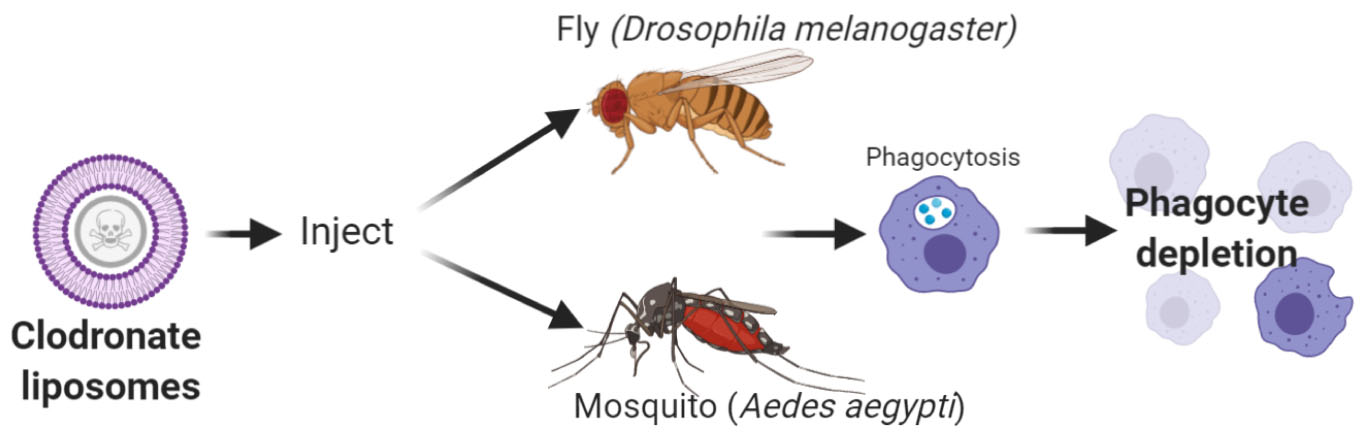
Graphical Abstract. Overview of phagocyte depletion experiments using clodronate liposomes to in Drosophila melanogaster or Aedes aegypti. Created with BioRender.com.
Insects rely on conserved cellular and humoral responses as the primary defense to invading pathogens. Immune cells, known as hemocytes, can directly participate in cellular responses such as phagocytosis and encapsulation (Lemaitre and Hoffmann, 2007; Hillyer and Strand, 2014), as well as mediate humoral signaling responses (Foley and O’Farrell, 2003; Wu et al., 2012) that limit pathogen survival. Studies in Drosophila have been aided by a wealth of genetic tools that include mutant and transgenic lines (Braun et al., 1997, 1998; Kurucz et al., 2003; Zettervall et al., 2004), as well as genetic techniques to ablate populations of plasmatocytes (Charroux and Royet, 2009; Defaye et al., 2009) that have significantly advanced our understanding of insect immune cells. However, the lack of genetic resources in non-model insect systems has severely limited studies of immune cell function. In mosquitoes, there has been a dependence on RNAi for reverse-genetic studies of hemocytes (Pinto et al., 2009; Ramirez et al., 2014; Smith et al., 2015, 2016), yet due to the absence of hemocyte markers and the systemic nature of gene-silencing, there have been significant limitations to address gene function in specific tissues or immune cell-types.
Evidence from vertebrate systems has demonstrated that chemical approaches can be utilized to target immune cells (Shek and Lukovich, 1982; Kagan and Hartmann, 1984; van Rooijen and Sanders, 1994), overcoming specific requirements for genetic tools to study immune cell function. Among these chemical approaches, clodronate liposomes (CLD) have shown the most promise and have been widely used in vertebrate systems to examine macrophage function (van Rooijen and Sanders, 1994; Lehenkari et al., 2002; van Rooijen and Hendrikx, 2010). Relying on the phagocytic properties of a subset of immune cells, CLD can be specifically delivered to macrophage populations, where after being phagocytosed they are degraded by the lysosome to promote apoptosis (van Rooijen and Sanders, 1994; van Rooijen and Hendrikx, 2010). Non-target cells lacking phagocytic abilities and lysosomal components are not affected by CLD treatment (van Rooijen and Sanders, 1994; van Rooijen and Hendrikx, 2010). This methodology has been widely applied in mammalian systems to understand autoimmune disease and macrophage contributions to infection biology (Jordan et al., 2003; Cockburn et al., 2010; Cha et al., 2015).
A recent study in mosquitoes described the use of CLD to deplete phagocytic immune cell populations in Anopheles gambiae (Kwon and Smith, 2019), demonstrating for the first time that CLD can be utilized in an invertebrate. Based on the highly conserved phagocytic properties of immune cells, the use of CLD has significant potential as a tool to study invertebrate immune function, overcoming many of the technical hurdles for non-model insect species. To further examine its applicability to insect species, in this study we examine the use of CLD to similarly investigate phagocytic immune cell function in Drosophila melanogaster and Aedes aegypti. Through these studies, we demonstrate that CLD can effectively deplete phagocytic cell populations of both species, illustrating the broad application of the use of CLD to study innate immune cell function across insects.
Drosophila melanogaster fly stocks were maintained at 25°C on standard molasses-based fly medium (Archon Scientific). Previously described SRP-mCherry (w[1118]; P{w[ + mC] = srpHemo-3XmCherry}; stock #78358) and HeGal4-UAS-GFP (w[∗]; P{w[ + mC] = He-GAL4.Z}85, P{w[ + mC] = UAS-GFP.nls}8; stock #8700) transgenic lines (Zettervall et al., 2004; Gyoergy et al., 2018) which express fluorescent proteins under universal larval hemocyte markers were obtained from the Bloomington Stock Center.
Aedes aegypti (Liverpool strain) mosquitoes were reared at 27°C and 80% relative humidity with a 14:10 h light/dark period. Larvae were reared on a 50:50 diet of ground fish flakes (Tetramin, Tetra) and milk bone dog biscuits. Adults were maintained on a 10% sucrose solution. All experimental techniques were performed on cohorts of 4–6 days old adult female mosquitoes.
Adult flies (2–3 days old) and mosquitoes (3–5 days old) were intra-thoracically injected with 69 nl of control liposomes (LP) or CLD (Standard macrophage depletion kit, Encapsula NanoSciences LLC) using a Nanoject III injector (Drummond Scientific) as previously described (Kwon and Smith, 2019). To determine the ideal concentrations for each species to maximize CLD efficacy on phagocyte depletion while minimizing effects on survival, dilutions of commercially available stock solutions of LP (24.3 mM L-alpha-phosphatidylcholine, 10.9 mM cholesterol) and CLD (24.3 mM L-alpha-phosphatidylcholine, 10.9 mM cholesterol, 18.4 mM Clodronate [(Dichloro-phosphono-methyl)phosphonate) were prepared in 1X PBS (1 (stock), 1:2, 1:3, 1:4 (only Aedes), 1:5] and compared to 1× PBS serving as an injection control. Based on the resulting experiments, a 1:5 dilution was chosen for all subsequent experiments in Drosophila, while a 1:4 dilution of LP and CLD was used for experiments with Ae. aegypti.
To evaluate the efficacy of phagocyte depletion experiments, hemolymph perfusions were performed as previously (Smith et al., 2015; Kwon et al., 2017; Kwon and Smith, 2019) using anticoagulant buffer (vol/vol 60% Schneider’s insect medium, 10% fetal bovine serum and 30% citrate buffer, 98 mM NaOH, 186 mM NaCl, 1.7 mM EDTA, 41 mM citric acid, pH 4.5). Perfused hemolymph was placed onto a hemocytometer (Neubauer, C-Chip DHC-N01, INCYTO) where approximately 50 cells were counted per individual fly or approximately 200 cells per individual mosquito for both LP and CLD treated sub-groups. Hemocyte sub-populations were differentiated by morphology (size and shape) or fluorescence (red or green) in the Drosophila transgenic lines.
Drosophila samples were examined 48h post-injection, while Aedes were evaluated at both 24 and 48h post-injection. Additionally, to examine the effects of blood feeding, blood-fed mosquitoes were examined 24 h post blood-meal (48 h post-injection) after challenge with defibrinated sheep blood (Hemostat Laboratories) using an artificial membrane feeding system.
Cultures of Serratia marcesens and Staphylococcus aureus were grown overnight in LB at 37°C. For Drosophila experiments, bacterial cultures were centrifuged at 8,000 rpm for 5 min, washed twice with 1× PBS, and resuspended in 1× PBS at a concentration of OD600 = 0.1. Approximately 24 h after pre-treatment with LP or CLD, adult SRP-mCherry Drosophila (n = 20 per replicate) were injected with 23 nl (∼1 × 108 CFU/ml) of either bacterial suspensions (S. marcescens or S. aureus) using a Nanoject III injector as previously described (Troha and Buchon, 2019). Following challenge, flies were maintained at room temperature and survival was monitored every 24 h for 8 days.
For mosquito experiments, S. marcescens or S. aureus cultures were centrifuged at 8,000 rpm for 5 min, washed twice with 1× PBS and resuspended to a final concentration of OD600 = 0.4. OD. A 100× dilution of the bacterial cultures (∼4 × 106 CFU/ml) were injected (69 nl) into naïve adult mosquitoes (n = 30 per replicate) 48 h post-treatment with LP or CLD as previously (Kwon and Smith, 2019). The injection of 1× PBS was included as an additional control. The survival of mosquitoes following bacterial challenge was monitored every 24 h for 8 days to determine the effects of phagocyte depletion on mosquito survival.
Total RNA was isolated from pooled whole fly or mosquito samples using TRIzol (Thermo Fisher Scientific), of which 2 μg of total RNA was used a template for cDNA synthesis using the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific). To examine gene expression following phagocyte depletion, qRT-PCR was performed using PowerUp SYBR Green Master Mix (Thermo Fischer Scientific) on control- or clodronate-treated treated fly and mosquito samples.
To validate phagocyte depletion in Drosophila, primers directed at either GFP or mCherry were examined in their respective transgenic lines using RpL32 as an internal control (Supplementary Table 1) using the following cycling conditions: 95°C for 10 min, 40 cycles with 95°C for 15 s and 65°C for 60 s. Similarly, phagocyte depletion was evaluated in Aedes using primers directed at the granulocyte-enriched genes, nimrod, and eater, with rpS17 as an internal control (Supplementary Table 1). qRT-PCR was performed for 40 cycles using the following cycling conditions: 98°C for 10s, 60°C for 10s and 72°C for 30 s. For both fly and mosquito samples, relative expression was evaluated using a comparative CT (2–ΔΔCt) method (Livak and Schmittgen, 2001).
To determine the applicability of using CLD to deplete phagocytic cell populations in other insect species (Kwon and Smith, 2019), we first examined the use of CLD in Drosophila melanogaster. Following the injection of either LP (empty) or CLD at different dilutions (1:2 or 1:5 in 1× PBS, Figure 1A), adult Drosophila (SRP-mCherry) were monitored over an 8-day period to examine the potential effects of liposome treatment on fly survival (Figure 1B). When compared to PBS-injected controls, LP treatment had no effect on survival [Mantel-Cox; PBS: LP (1:2), P = 0.0811; PBS: LP (1:5), P = 0.0551] (Figure 1B). In addition, no differences in Drosophila survival were seen between LP and CLD treatments [Mantel-Cox; LP (1:2):CLD (1:2), P = 0.5506; LP (1:5):CLD (1:5), P = 0.6947] (Figure 1B). Using the 1:5 dilutions of LP and CLD, we then evaluated the efficacy of phagocyte depletion by perfusing flies two days post-injection (Figure 1A). Taking advantage of transgenic stocks that express fluorescent proteins in phagocytic plasmatocyte populations (Zettervall et al., 2004; Gyoergy et al., 2018), we demonstrate that CLD treatment significantly reduces the percentage of mCherry+ (Figure 1C) and EGFP+ (Supplementary Figure 1) plasmatocytes in Drosophila adults. We further validated these depletion experiments in the SRP-mCherry line using qRT-PCR, demonstrating a significant reduction in mCherry expression 24 h after CLD treatment (Figure 1D). Similar qRT-PCR experiments with the HemeseGal4-UAS-GFP line did not display differences in GFP expression when evaluated 24 h post-treatment or at 48 h post-treatment to allow at additional incubation time (Supplementary Figure 1). Given the reduction of EGFP+ immune cells following clodronate treatment (Supplementary Figure 1), the lack of change to GFP expression levels may be due to GFP expression in other tissues beyond plasmatocyte populations as previously noted (Zettervall et al., 2004). Together, these data suggest that CLD are able to effectively deplete Drosophila phagocyte populations.
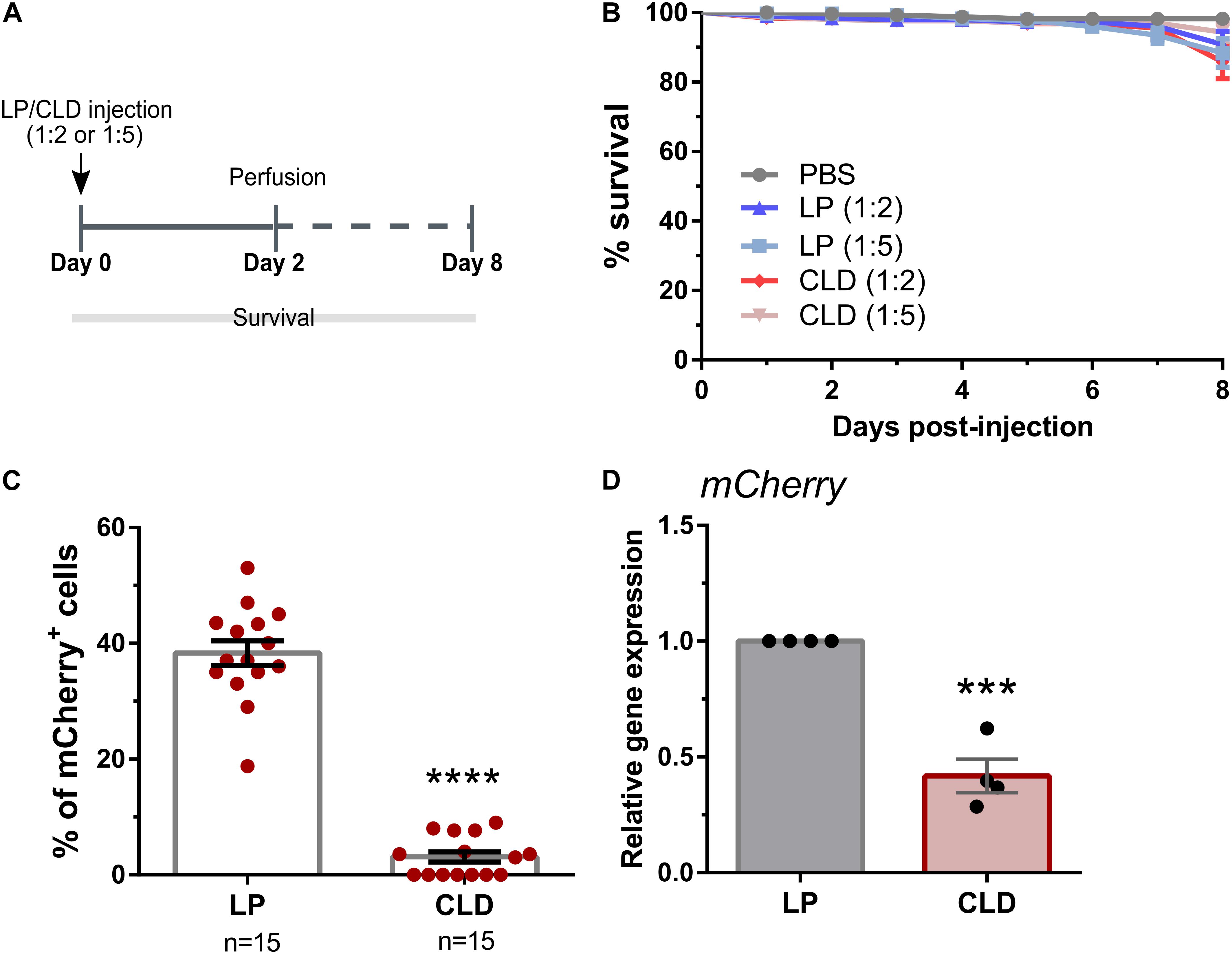
Figure 1. Use of clodronate liposomes to deplete Drosophila plasmatocytes. (A) Overview of clodronate liposome experiments in Drosophila (SRP-mCherry). Control (LP)- or clodronate liposomes (CLD) were diluted at 1:2 or 1:5 in 1X PBS and intrathoracically injected into adult female flies. Survival was then monitored over an eight-day period (B). Error bars represent the mean ± SEM of three independent replicates. In each replicate, 20 female adult flies were used for each experimental condition. LP treatment had no effect on survival [Mantel-Cox; PBS: LP (1:2), P = 0.0811; PBS: LP (1:5), P = 0.0551], nor were there differences between LP and CLD treatments [Mantel-Cox; LP (1:2):CLD (1:2), P = 0.5506; LP (1:5):CLD (1:5), P = 0.6947]. Following perfusion two-days post-injection, the percentage of mCherry+ hemocytes were evaluated in LP- and CLD-treated flies (1:5 dilution) (C). Data represent the pooled mean ± SEM of three independent experiments and were analyzed by a Mann–Whitney test to determine significance. To further validate phagocyte depletion, mCherry expression was examined in whole flies by qRT-PCR (D). Relative mCherry transcripts were significantly reduced following CLD-treatment. Data represent the pooled mean ± SEM of four independent experiments and were analyzed using an unpaired t test to determine significance. n = number of individual flies examined. Asterisks denote significance (***P < 0.001, ****P < 0.0001).
Similar experiments were also performed in the yellow fever mosquito, Aedes aegypti, to evaluate the use of CLD for phagocyte depletion (Figure 2A). Concentrations of either LP or CLD at 1:2, 1:4, or 1:5 dilutions were examined, with none of the concentration having measurable impacts on adult mosquito survival (Figure 2B). Both the 1:4 and 1:5 dilutions were able to significantly reduce the percentage of granulocytes at 24- or 48-h post-treatment (Supplementary Figure 2), although phagocyte depletion was more effective at 48 h and with the 1:4 dilution (Supplementary Figure 2). Moreover, CLD treatment was able to effectively reduce phagocyte populations in mosquitoes under both naïve (Figure 2C) and blood-fed conditions (Figure 2D). These morphological observations were further validated using qRT-PCR on eater and nimrod, two transcripts associated with hemocyte phagocytic function (Kocks et al., 2005; Kurucz et al., 2007; Kwon and Smith, 2019). For both eater and nimrod, clodronate treatment significantly reduced the relative transcript abundance in naïve and blood-fed mosquitoes (Figure 2E). Together, these data suggest that CLD can effectively be used to study Ae. aegypti phagocyte function.
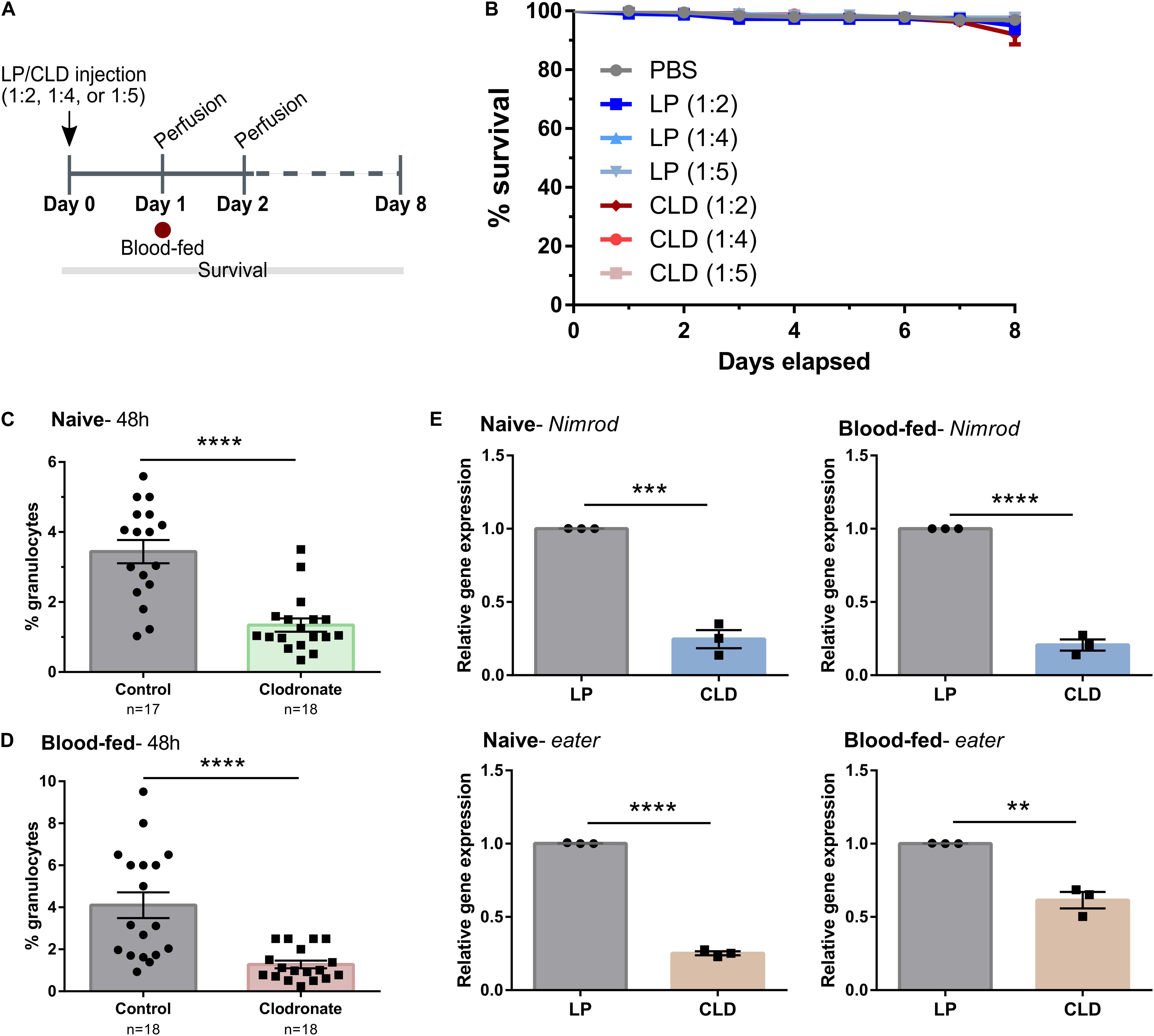
Figure 2. Application of clodronate liposomes to deplete Ae. aegypti phagocytic immune cells. (A) Overview of clodronate liposome experiments in mosquitoes. Control (LP)- or clodronate liposomes (CLD) were diluted at 1:2, 1:4, or 1:5 in 1X PBS and intrathoracically injected into adult female mosquitoes. Survival was then monitored over an eight-day period (B). Error bars represent the mean ± SEM of two independent replicates. In each replicate, 30 adult female adult mosquitoes were used for each experimental condition. LP treatment had no effect on survival [Mantel-Cox; PBS: LP (1:2), P = 0.4464; PBS: LP (1:4), P = 0.7120; PBS: LP (1:5), P = 0.7978] and there were no differences in survival between LP and CLD treatments [Mantel-Cox; LP (1:2):CLD (1:2), P = 0.9425; LP (1:4):CLD (1:4), P = 0.7992; LP (1:5):CLD (1:5), P = 0.8361]. To evaluate phagocyte depletion, the percentage of granulocytes were examined by light microscopy two-days (48 h) post-injection (1:4 dilution) under either naïve (C) or blood-fed conditions (D). Data represent the pooled mean ± SEM of three independent experiments that were analyzed by a Mann–Whitney test to determine significance. To further validate phagocyte depletion, molecular marker of phagocytic cells, nimrod and eater, were examined in whole mosquitoes by qRT-PCR (E). Relative nimrod and eater transcripts were significantly reduced following CLD-treatment under both naïve and blood-fed conditions. Data represent the pooled mean ± SEM of three independent experiments that were analyzed using an unpaired t test to determine significance. n = number of individual mosquitoes examined. Asterisks denote significance (**P < 0.01, ***P < 0.001, ****P < 0.0001).
To determine the effects of phagocyte depletion on immune function and host survival, we challenged adult flies and mosquitoes with bacteria after treatment with LP or CLD (Figure 3). Drosophila displayed significantly reduced survival following phagocyte depletion when challenged with S. marcescens and S. aureus (Figure 3A) similar to previous reports in which plasmatocytes were depleted through genetic experiments (Charroux and Royet, 2009; Defaye et al., 2009). However, these effects were considered more moderate when compared to the strong phenotypes resulting from similar experiments in Ae. aegypti, where the survival of CLD-treated mosquitoes was severely reduced upon challenge of either S. marcescens or S. aureus (Figure 3B). Similar to previous work in the mosquito Anopheles gambiae (Kwon and Smith, 2019), S. marcescens challenge caused significant pathogenicity in control- and clodronate-treated Ae. aegypti, although phagocyte depletion led to significant mortality within 3 days post-challenge (Figure 3B). S. aureus challenge also led to severe mortality in the phagocyte-depleted background with little effect in control mosquitoes (Figure 3B). In agreement with previous studies implicating phagocytic immune cells in mediating insect responses to bacterial challenge (Kocks et al., 2005; Kurucz et al., 2007; Hashimoto et al., 2009; Kwon and Smith, 2019), these results provide further support that CLD can serve as a valuable tool to study cellular immune function and phagocyte contributions to innate immune responses to pathogens across a variety of insect systems.
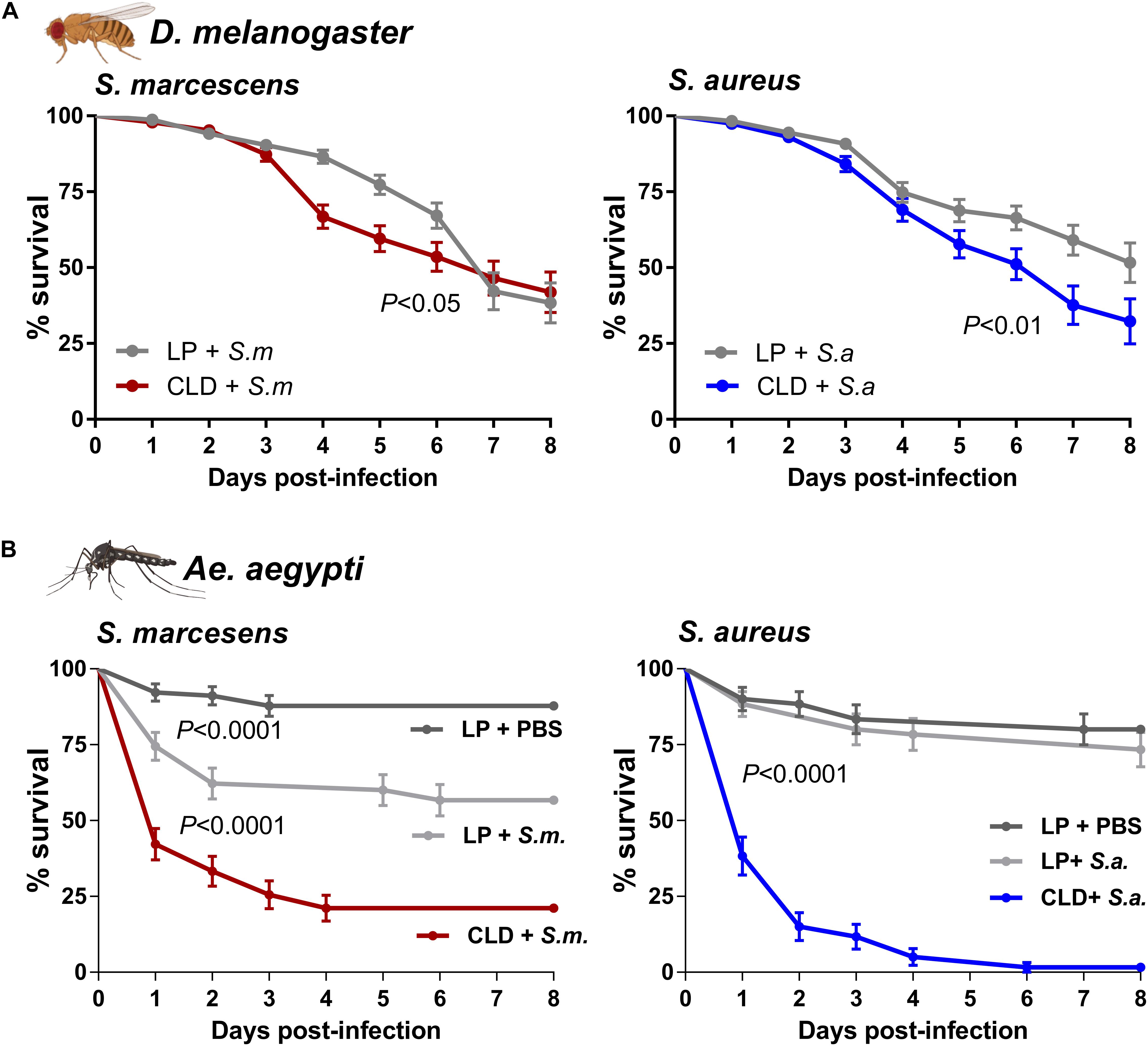
Figure 3. The depletion of phagocytic immune cells influences survival after bacterial challenge. Bacterial challenge assays were performed in flies (A) or mosquitoes (B) following treatment with control (LP)- or clodronate liposomes (CLD). Survivorship was monitored in every day over an 8-day period to evaluate the effects of S. marcescens or S. aureus challenge. For mosquito challenge experiments (B), an additional control was added in which LP-treated mosquitoes were challenged with the injection of sterile PBS. Error bars represent the mean ± SEM of three independent replicates for Drosophila (20 per replicate) and Ae. aegypti (30 per replicate). Data were analyzed using a log-rank (Mantel-Cox) test using GraphPad Prism 6.0. Fly and mosquito images were created with BioRender.com.
Insects have developed a robust innate immune system for defense against a variety of microorganisms that are the result of developments in diverse ecological systems and environments, as well as the hematophagous behaviors that expose many insect species to bacterial, viral, fungal, and parasitic pathogens. With evidence of immune memory (Pham et al., 2007; Rodrigues et al., 2010; Cooper and Eleftherianos, 2017) and the conservation of immune signaling pathways with mammalian systems (Buchon et al., 2014; Hillyer, 2016), the study of insect immunity offers several advantages for comparative immunology. Moreover, insects have integral roles in the transmission of disease that influence agriculture or that are of veterinary or medical importance. While Drosophila has served as an excellent model for insect systems, it is not representative of the diversity in insect systems where studies of non-model insects have been limited by the lack of genetic tools.
Herein, we expand on previous reports in An. gambiae (Kwon and Smith, 2019) to describe the use of CLD in D. melanogaster and Ae. aegypti to deplete phagocytic immune cells. Widely used in mammalian systems to deplete macrophage populations function (van Rooijen and Sanders, 1994; Lehenkari et al., 2002; van Rooijen and Hendrikx, 2010), our results provide further evidence that CLD can also be utilized in a variety of insect systems and is supported by conserved, functional similarities between insect and mammalian phagocytes (Browne et al., 2013).
In our proof of principle experiments, we demonstrate through microscopy and qRT-PCR techniques that CLD can significantly reduce phagocytic plasmatocyte or granulocyte populations respectively in adult D. melanogaster and Ae. aegypti. While mutations (Braun et al., 1997, 1998) or other methods of genetic ablation (Charroux and Royet, 2009; Defaye et al., 2009) to study phagocyte function already exist in Drosophila, similar tools have not yet been developed in mosquitoes. Alternative methods to inhibit phagocyte function have been utilized in both Drosophila (Elrod-Erickson et al., 2000; Lamiable et al., 2016) and mosquitoes (Castillo et al., 2017) that rely on saturating the phagocytic machinery via the injection of polystyrene beads, yet may not fully impair phagocyte function. Therefore, we believe that the use of CLD provides a convenient method to study phagocyte function in non-model insects, as well as an alternative methodology for model systems such as Drosophila. Moreover, the ability to deplete phagocytic cell populations also enables the study of phagocyte contributions to insect-pathogen interactions. This is supported by recent experiments demonstrating phagocyte contributions to anti-Plasmodium immunity in An. gambiae (Kwon and Smith, 2019) and may be similarly utilized in the future to examine phagocyte function in the context of arbovirus infection in Ae. aegypti.
Additional experiments demonstrate the importance of phagocyte function for insect survival following bacterial challenge, wherein both flies and mosquitoes display reduced survival to gram (−) and gram (+) bacteria following phagocyte depletion similar to previous experiments (Charroux and Royet, 2009; Defaye et al., 2009; Kwon and Smith, 2019). Of interest, these survival phenotypes were much stronger in Ae. aegypti where few mosquitoes survived challenge with either S. marcescens or S. aureus, and may potentially represent differences in the cellular and humoral defenses to bacterial pathogens between mosquitoes and flies that warrant further study.
In summary, we believe that our experiments with CLD support their utility to deplete phagocytes in flies and mosquitoes, providing new or alternative methods to study the cellular and humoral contributions of phagocytes to the defense of invading pathogens. With the conserved utility of CLD in mammals and insects, as well as its ease of use, we believe that CLD can be a significant new resource for the study of invertebrate immunity.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
JR, JS, and HK performed the experiments and analyzed the results. RS conceived the experiments, analyzed data, and wrote the initial draft of the manuscript. All authors contributed to the editing and writing of the final manuscript.
This work was supported in part by R21AI149118 from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (to RS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.627976/full#supplementary-material
Braun, A., Hoffmann, J. A., and Meister, M. (1998). Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc. Natl. Acad. Sci. 95, 14337–14342. doi: 10.1073/pnas.95.24.14337
Braun, A., Lemaitre, B., Lanot, R., Zachary, D., and Meister, M. (1997). Drosophila immunity: Analysis of larval hemocytes by P-element-mediated enhancer trap. Genetics 147, 623–634. doi: 10.1093/genetics/147.2.623
Browne, N., Heelan, M., and Kavanagh, K. (2013). An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 4, 597–603. doi: 10.4161/viru.25906
Buchon, N., Silverman, N., and Cherry, S. (2014). Immunity in Drosophila melanogaster-from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 14, 796–810. doi: 10.1038/nri3763
Castillo, J. C., Beatriz, A., Ferreira, B., Trisnadi, N., and Barillas-Mury, C. (2017). Activation of mosquito complement antiplasmodial response requires cellular immunity. Sci. Immunol. 2:eaal1505. doi: 10.1126/sciimmunol.aal1505
Cha, S.-J., Park, K., Srinivasan, P., Schindler, C. W., van Rooijen, N., Stins, M., et al. (2015). CD68 acts as a major gateway for malaria sporozoite liver infection. J. Exp. Med. 212, 1391–1403. doi: 10.1084/jem.20110575
Charroux, B., and Royet, J. (2009). Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc. Natl. Acad. Sci. U. S. A. 106, 9797–9802. doi: 10.1073/pnas.0903971106
Cockburn, I. A., Chen, Y. C., Overstreet, M. G., Lees, J. R., van Rooijen, N., Farber, D. L., et al. (2010). Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog. 6, 1–13. doi: 10.1371/journal.ppat.1000877
Cooper, D., and Eleftherianos, I. (2017). Memory and specificity in the insect immune system: Current perspectives and future challenges. Front. Immunol. 8:539. doi: 10.3389/fimmu.2017.00539
Defaye, A., Evans, I., Crozatier, M., Wood, W., Lemaitre, B., and Leulier, F. (2009). Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J. Innate Immun. 1, 322–334. doi: 10.1159/000210264
Elrod-Erickson, M., Mishra, S., and Schneider, D. (2000). Interactions between the cellular and humoral immune responses in Drosophila. Curr. Biol. 10, 781–784. doi: 10.1016/s0960-9822(00)00569-8
Foley, E., and O’Farrell, P. H. (2003). Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in Drosophila. Genes Dev. 17, 115–125. doi: 10.1101/gad.1018503
Gyoergy, A., Roblek, M., Ratheesh, A., Valoskova, K., Belyaeva, V., Wachner, S., et al. (2018). Tools allowing independent visualization and genetic manipulation of Drosophila melanogaster macrophages and surrounding tissues. G3 Genes Genomes Genet. 8, 845–857. doi: 10.1534/g3.117.300452
Hashimoto, Y., Tabuchi, Y., Sakurai, K., Kutsuna, M., Kurokawa, K., Awasaki, T., et al. (2009). Identification of lipoteichoic acid as a ligand for draper in the phagocytosis of Staphylococcus aureus by Drosophila hemocytes. J. Immunol. 183, 7451–7460.
Hillyer, J. F. (2016). Insect immunology and hematopoiesis. Dev. Comp. Immunol. 58, 102–118. doi: 10.1016/j.dci.2015.12.006
Hillyer, J. F., and Strand, M. R. (2014). Mosquito hemocyte-mediated immune responses. Curr. Opin. Insect Sci. 3, 14–21. doi: 10.1016/j.cois.2014.07.002
Jordan, M. B., Van Rooijen, N., Izui, S., Kappler, J., and Marrack, P. (2003). Liposomal clodronate as a novel agent for treating autoimmune hemolytic anemia in a mouse model. Blood 101, 594–601. doi: 10.1182/blood-2001-11-0061
Kagan, E., and Hartmann, D. P. (1984). [32] Elimination of macrophages with silica and asbestos. Methods Enzymol. 108, 325–335. doi: 10.1016/s0076-6879(84)08099-x
Kocks, C., Cho, J. H., Nehme, N., Ulvila, J., Pearson, A. M., Meister, M., et al. (2005). Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 123, 335–346. doi: 10.1016/j.cell.2005.08.034
Kurucz, É, Márkus, R., Zsámboki, J., Folkl-Medzihradszky, K., Darula, Z., Vilmos, P., et al. (2007). Nimrod, a putative phagocytosis receptor with EGF Repeats in Drosophila plasmatocytes. Curr. Biol. 17, 649–654. doi: 10.1016/j.cub.2007.02.041
Kurucz, E., Zettervall, C.-J., Sinka, R., Vilmos, P., Pivarcsi, A., Ekengren, S., et al. (2003). Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 100, 2622–2627. doi: 10.1073/pnas.0436940100
Kwon, H., Arends, B. R., and Smith, R. C. (2017). Late-phase immune responses limiting oocyst survival are independent of TEP1 function yet display strain specific differences in Anopheles gambiae. Parasit. Vectors 10:369.
Kwon, H., and Smith, R. C. (2019). Chemical depletion of phagocytic immune cells in Anopheles gambiae reveals dual roles of mosquito hemocytes in anti-Plasmodium immunity. Proc. Natl. Acad. Sci. 116, 14119–14128. doi: 10.1073/pnas.1900147116
Lamiable, O., Arnold, J., da Silva, de Faria, I. J., Proveti Olmo, R., Bergami, F., et al. (2016). Analysis of the contribution of hemocytes and autophagy to Drosophila antiviral immunity. J. Virol. 90, JVI.238–JVI.216.
Lehenkari, P. P., Kellinsalmi, M., Näpänkangas, J. P., Ylitalo, K. V., Mönkkönen, J., Rogers, M. J., et al. (2002). Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharmacol. 61, 1255–1262. doi: 10.1124/mol.61.5.1255
Lemaitre, B., and Hoffmann, J. (2007). The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743.
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Pham, L. N., Dionne, M. S., Shirasu-Hiza, M., and Schneider, D. S. (2007). A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 3:e26. doi: 10.1371/journal.ppat.0030026
Pinto, S. B., Lombardo, F., Koutsos, A. C., Waterhouse, R. M., McKay, K., An, C., et al. (2009). Discovery of Plasmodium modulators by genome-wide analysis of circulating hemocytes in Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 106, 21270–21275.
Ramirez, J. L., Garver, L. S., Brayner, F. A., Alves, L. C., Rodrigues, J., Molina-Cruz, A., et al. (2014). The role of hemocytes in Anopheles gambiae antiplasmodial immunity. J. Innate Immun. 6, 119–128. doi: 10.1159/000353765
Rodrigues, J., Brayner, F. A., Alves, L. C., Dixit, R., and Barillas-Mury, C. (2010). Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329, 1353–1355. doi: 10.1126/science.1190689
Shek, P. N., and Lukovich, S. (1982). The role of macrophages in promoting the antibody response mediated by liposome-associated protein antigens. Immunol. Lett. 5, 305–309. doi: 10.1016/0165-2478(82)90118-3
Smith, R. C., Barillas-Mury, C., and Jacobs-Lorena, M. (2015). Hemocyte differentiation mediates the mosquito late-phase immune response against Plasmodium in Anopheles gambiae. Proc. Natl. Acad. Sci. 112, E3412–E3420.
Smith, R. C., King, J. G., Tao, D., Tomescu, O., Brando, C., Thallinger, G. G., et al. (2016). Molecular profiling of phagocytic immune cells in Anopheles gambiae reveals integral roles for hemocytes in mosquito innate immunity. Mol. Cell. Proteomics 15, 3373–3387. doi: 10.1074/mcp.m116.060723
Troha, K., and Buchon, N. (2019). Methods for the study of innate immunity in Drosophila melanogaster. Wiley Interdiscip. Rev. Dev. Biol. 8, 1–25.
van Rooijen, N., and Hendrikx, E. (2010). Liposomes for specific depletion of macrophages from organs and tissues. Methods Mole. Biol. 605, 189–203. doi: 10.1007/978-1-60327-360-2_13
van Rooijen, N., and Sanders, A. (1994). Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93. doi: 10.1016/0022-1759(94)90012-4
Wu, S. C., Liao, C. W., Pan, R. L., and Juang, J. L. (2012). Infection-induced intestinal oxidative stress triggers organ-to-organ immunological communication in Drosophila. Cell Host Microbe 11, 410–417. doi: 10.1016/j.chom.2012.03.004
Keywords: phagocytosis, hemocytes, immune cells, phagocyte depletion, clodronate liposomes, Aedes (Ae.) aegypti, Drosophila melanogaster
Citation: Ramesh Kumar J, Smith JP, Kwon H and Smith RC (2021) Use of Clodronate Liposomes to Deplete Phagocytic Immune Cells in Drosophila melanogaster and Aedes aegypti. Front. Cell Dev. Biol. 9:627976. doi: 10.3389/fcell.2021.627976
Received: 10 November 2020; Accepted: 11 January 2021;
Published: 02 February 2021.
Edited by:
Marc S. Dionne, Imperial College London, United KingdomReviewed by:
Alysia Vrailas-Mortimer, Illinois State University, United StatesCopyright © 2021 Ramesh Kumar, Smith, Kwon and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryan C. Smith, c21pdGhyQGlhc3RhdGUuZWR1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.