
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 23 March 2021
Sec. Molecular and Cellular Pathology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.619565
This article is part of the Research Topic Exosomes in Brain Health and Disease View all 18 articles
Exosomes are natural cells-derived vesicles, which are at the forefront toward clinical success for various diseases, including cerebral ischemia. Exosomes mediate cell-to-cell communication in different brain cells during both physiological and pathological conditions. Exosomes are an extensively studied type of extracellular vesicle, which are considered to be the best alternative for stem cell–based therapy. They can be secreted by various cell types and have unique biological properties. Even though native exosomes have potential for ischemic stroke therapy, some undesirable features prevent their success in clinical applications, including a short half-life, poor targeting property, low concentration at the target site, rapid clearance from the lesion region, and inefficient payload. In this review, we highlight exosome trafficking and cellular uptake and survey the latest discoveries in the context of exosome research as the best fit for brain targeting owing to its natural brain-homing abilities. Furthermore, we overview the methods by which researchers have bioengineered exosomes (BioEng-Exo) for stroke therapy. Finally, we summarize studies in which exosomes were bioengineered by a third party for stroke recovery. This review provides up-to-date knowledge about the versatile nature of exosomes with a special focus on BioEng-Exo for ischemic stroke. Standard exosome bioengineering techniques are mandatory for the future and will lead exosomes toward clinical success for stroke therapy.
Stroke is a leading cause of death and neurological disability, and it has no effective treatment up to now (Li et al., 2020a; McCann and Lawrence, 2020). Approximately 6.7 million people die due to stroke each year (Cunningham et al., 2020), and 87% of these deaths are due to ischemic stroke (Dabrowska et al., 2019). Pathophysiological responses after stroke are complex, and presently, no better choice is available for stroke treatment except tissue plasminogen activator (Otero-Ortega et al., 2019; Pan et al., 2020), the only useful drug within 4–6 h of clearly defined symptom onset (Malone et al., 2019; Wang J. et al., 2020). However, due to its narrow therapeutic window, less than 5% of patients benefit from it (Wang et al., 2018). Even though there is some progress in stroke treatment, current therapeutic concepts are limited, and development of novel therapy for stroke survivors is critical.
Since the discovery that mesenchymal stem cells (MSCs) could produce new neurons (Sanchez-Ramos et al., 2000), much improvement has been done in MSC research (Kruminis-Kaszkiel et al., 2020). MSCs are multipotent, self-renewing exogenous cell populations present in adults as well as developing individuals and can differentiate into neuron as well glial lineages (Bai et al., 2020). However, with the advancement of this field, scientists have found that MSCs can have unpredictable consequences for target as well non-target organs (Wei et al., 2019). MSC implantation in the brain is invasive and can cause damage to healthy tissue, and they have poor survival in hypoxic and inflammatory conditions (Kim H.Y. et al., 2020). MSCs are big (15–40 μm), which makes them easily get trapped in small-diameter vessels, causing vascular occlusion and a decrease in cerebral blood flow (Bang and Kim, 2019). Hence, MSC delivery to the ischemic region is difficult, and this limits their clinical use for the treatment of ischemic stroke. While considering the therapeutic abilities of stem cells, researchers are shifting their interest toward stem cell–derived products, one of which is the exosome.
Exosomes are cell membrane–derived vesicles secreted by almost all body cells (Wang S. et al., 2020). The secretion process of exosomes is related to the mechanism of waste disposal. Wolfers et al. (2001) found that exosomes were an important source of intercellular communication in cancer. However, now it is confirmed by numerous studies that exosomes mediate communication by delivering DNA, RNA, lipids, and proteins in between cells and tissues (van Niel et al., 2018; Lin et al., 2020), playing an important role in controlling the biological expression of the recipient cells and leading to the activation of their signaling pathways (Cheng et al., 2019).
Exosomes are the best choice because of their natural characteristics, such as negligible toxicity, circulation stability, production and storage advantages, ability to encapsulate endogenous bioactive molecules, strong protection for cargo, and excellent transport efficacy to distant body cells, specifically for brains cells as they can pass through the blood–brain barrier (BBB) easily (Rufino-Ramos et al., 2017; Bang and Kim, 2019). Stem cell–derived exosomes’ beneficial effects were studied in animal models of stroke, traumatic brain injury, Alzheimer’s disease, status epilepticus–induced brain injury, multiple sclerosis, and spinal cord injury (Kodali et al., 2020). Exosomes are the most promising therapeutic avenues capable of addressing the need for regenerative and therapeutic treatment deficiencies for stroke patients (Webb et al., 2018; Gharbi et al., 2020). In addition, synthetic therapeutic agents may suffer from immunogenicity and toxicity, but the origin of exosomes is biological, that is why they are less likely to induce adverse effects (de Abreu et al., 2020).
Exosomes’ ability to cross the BBB makes them a suitable candidate for brain diseases as well as increasing interest in utilization of exosomes as drug delivery systems to the brain (Wood et al., 2011). The BBB is composed of brain macrovascular endothelial cells (BMECs), pericytes, astrocytes, and tight junctions, which allow selective transport of some compounds while inhibiting entry of toxic substances in between the blood and brain (Ballabh et al., 2004). The exosome BBB crossing mechanism remains unclear; however, research examining exosome trafficking through the BBB points toward absorption by BMECs via endocytosis, where they fuse to BMEC endosomes and are released to the brain (Chen et al., 2016; Lu et al., 2020). Interaction between exosomes and BMECs in vitro shows that, in healthy and stroke-like conditions, exosomes retain their BBB crossing ability (Chen et al., 2016). Exosomes crossed the BBB and were taken up by endothelial cells in a 3-D BBB static model (Jakubec et al., 2020). This finding of exosome BBB crossing has been confirmed by a number of studies (Grapp et al., 2013; Long et al., 2017; Yuan et al., 2017; Qu et al., 2018) however, biodistribution studies of exosomes reveal that, after intravenous administration of native exosomes to the body, they were rapidly cleaned from the target site and accumulated in organs of the reticuloendothelial system, such as the lungs, liver, and spleen, and very few exosomes were found at the target site (Lai et al., 2014; Wiklander et al., 2015; Tian et al., 2018; Lu and Huang, 2020). Hence, there is need to engineer exosome targeting characteristics before its use as a therapeutic agent for stroke therapy.
Exosome research is still in its infancy, particularly in central nervous system (CNS) diseases; therefore, a better understanding of exosomes is of formidable importance to optimally utilize them for therapeutic purposes. Even though the potential therapeutic abilities of exosomes are considered to be well-established fact now, several challenges still need to be addressed before its successful clinical translation. These include the processes by which they are formed and their function, exosome targeting and trafficking, internalization, dose optimization, route of administration, and strategies for modification of exosomes to steer their delivery toward their target sites of action. Here, first we overview the exosome process of biogenesis, composition, and uptake by cells. Next, we survey some of the latest discoveries that add unexpected new twists to our understanding of exosome function and potential for stroke therapy. Furthermore, we highlight exosomes’ versatile nature as being the best fit for brain targeting and review the methodologies of exosome engineering for brain targeting. Finally, we focus on and summarize the studies in which exosomes were bioengineered by a third party for stroke therapy and discuss how this field could advance. In this review, we use the term “exosomes” for some studies on behalf of extracellular vesicles (EVs) (Chivet et al., 2014; Liu et al., 2019; Tieu et al., 2020).
Exosomes were first reported by Trams et al. (1981), Xin et al. (2014); Witwer and Théry (2019). They were discovered in in vitro cell culture of sheep red blood cell supernatants with structural size ranging from 40 to 200 nm (Yu et al., 2020). Exosomes are the most important subtype of EVs, whereas the literature also contains other EV subtypes, including microvesicles, apoptotic bodies, blebs, exomeres, migrasomes, oncosomes, and argosomes classified by size, biogenesis, origin, content, and function (Ma et al., 2015; Xie et al., 2020) (Figure 1). Exosomes differ from other types of EVs in the context of biogenesis, diameter, and contents.
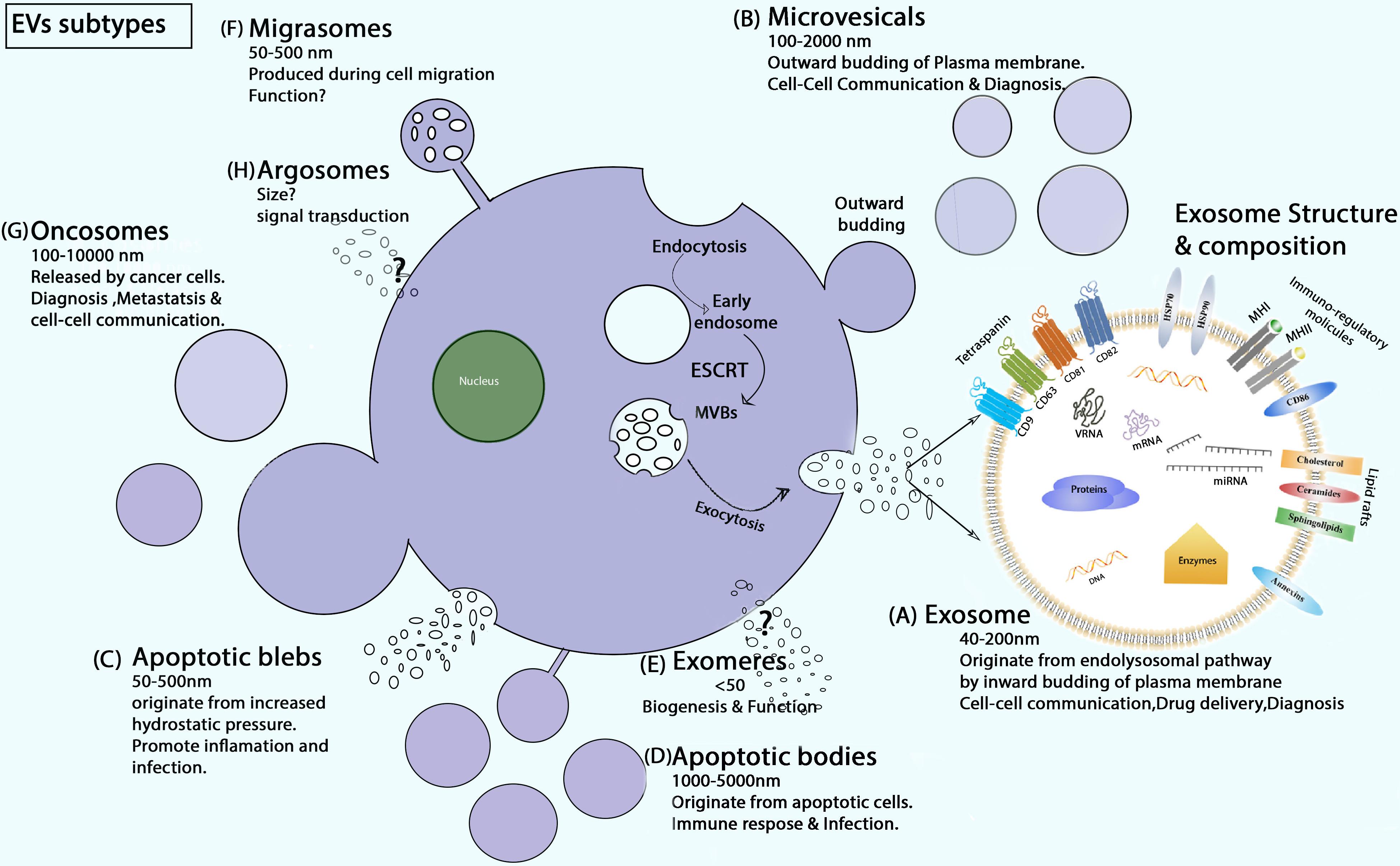
Figure 1. Schematic of EV biogenesis and subtypes. (A) Exosome secretion (ESCRT dependent) and composition. (B) MVs are formed during inflammatory and hypoxic conditions as buds off of the plasma membrane. (C) Apoptotic blebs are EVs that generate with increased cell contraction and hydrostatic pressure (Xie et al., 2020). (D) Apoptotic bodies are released only during programmed cell death (Jadli et al., 2020). (E) Exomeres are recently discovered EVs, and their biological function and biogenesis is yet unknown (Zhang et al., 2018; Xie et al., 2020). (F) Migrasomes are oval-shaped EVs produced during cell migration. (G) Oncosomes are membrane-derived large and small EVs released by cancer cells. They contain a unique signature of the tumor cells from which they are secreted (Chuo et al., 2018). (H) Argosomes are exosome-like vesicles, and their biogenesis starts from the basolateral membrane of Drosophila discoid cells and can take part directly in the transferring of molecules from producer to recipient cells (Borges et al., 2013).
Exosome biogenesis occurs from the endo-lysosomal pathway, in particular, when inward budding of the plasma membrane happens. Biogenesis starts with the development of early endosomes (EEs), then intraluminal vesicles (ILVs) in intracellular multivesicular bodies (MVBs), transport of MVBs to the plasma layer, and union of MVBs with the plasma membrane. The first step in the formation of EEs is budding from the plasma membrane and the fusion of primary endocytic vesicles. The trans-Golgi network and endoplasmic reticulum could promote EE formation and content. In the conversion process of EEs to MVBs, Rab5 and its effector VPS34/p150 act as important regulators (Huotari and Helenius, 2011). The most important protein playing a role in ILV formation is the endosomal-sorting complex essential for transport (ESCRT) protein family. This protein family includes four complexes, ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III (Murphy et al., 2019; Kalluri and LeBleu, 2020). ILVs can also generate through the ESCRT-independent pathway. For the ESCRT-independent way of maturation, tetraspanin and CD63 are important factors. Neutral sphingomyelinase 2 enzyme is another important mediator of ESCRT-independent ILV maturation. This enzyme synthesizes the lipid ceramide that makes microdomains on the surface of MVBs, a process that is vital for ILV production (Murphy et al., 2019). Thus, MVB biogenesis can be ESCRT-dependent or -independent. In ESCRT-dependent biogenesis, MVBs take two paths, influenced by the protein ubiquitin checkpoint (Vietri et al., 2020). On the one hand, MVBs bind to lysosomes or autophagosomes in the cells and are degraded by the ubiquitinated cargo. On the other hand, MVBs are mediated by specific components of the actin, cortactin, microtubule skeleton, and Rab family (such as Rab27A and Rab27B), which are continuously transported and eventually stuck to the plasma membrane to secrete exosomes (Ostrowski et al., 2010; Villarroya-Beltri et al., 2016).
Through the biogenesis of exosomes, a series of molecular contents with cell biological activity selectively encapsulate into exosomes (Xu et al., 2018). The contents of exosomes can reflect the structure of donor cells (Valadi et al., 2007). Research indicates that exosomes are rich in many molecular substances, such as mRNA, miRNA, and other non-coding RNA (Vojtech et al., 2014; Xie et al., 2019); double-stranded DNA and mtDNA (Guescini et al., 2010; Sansone et al., 2017; Yang S. et al., 2017; Tsai et al., 2018); cholesterol, sphingolipids, and ceramides (Skotland et al., 2017); heat shock cognate proteins (HSP70, HSP90), adhesion molecules, and cell skeleton proteins (Muller, 2020); and receptors MHC-II, MHC-I, CD86 and tetraspanin proteins CD9, CD63, CD81, CD82, and enzymes (Thery et al., 2002; Pegtel and Gould, 2019). Once exosomes are grabbed by the recipient cell, they can release their exosomal cargo to the target cell performing different kinds of pathological and physiological functions.
Different uptake pathways involve exosome internalization by recipient cells. Exosome uptake routes are diverse and depend on both the donor and recipient cell type (Murphy et al., 2019). Researchers indicate that exosomes biologically resemble retroviruses as they share many properties. Both exosomes and retroviruses have comparable diameter of about 120 nm (Mathieu et al., 2019), are coated with a lipid membrane carrying genetic material originating from endosomal pathways (Margolis and Sadovsky, 2019), and trigger a specific reaction through their molecular contents in the recipient cells (Nolte-’t Hoen et al., 2016). The ability of exosomes to avoid a degradative pathway was revealed to resemble the route of human immunodeficiency virus (HIV) mechanism of cell uptake (Izquierdo-Useros et al., 2009). HIV causes infection by combining with the plasma membrane; likewise exosomes are taken up by recipient cells after combining with plasma membranes. However, the cells take up HIV virions through pathways, including multiple modes of macrophage proliferation, phagocytosis, and endocytosis (Melikyan, 2014). On the other hand, exosomes can also be taken up by different pathways, such as caveolin-mediated endocytosis, clathrin-mediated endocytosis, lipid raft-mediated endocytosis, macro-pinocytosis, and phagocytosis (Mulcahy et al., 2014; Murphy et al., 2019).
Even though exosomes have similarities to viruses in their uptake mechanism, there are some differences as well. In some cases, the uptake of exosomes is more complicated than viruses. For example, exosomes are abundant in macro-molecules rich in PS receptors, lectins, glycans, integrins, and other cell adhesion molecules. These allow exosomes to bind to and be taken up by nearby or faraway recipient cells via ligand-receptor interaction to release the substances they carry (van Dongen et al., 2016; Joshi et al., 2020). In summary, exosome uptake can be expressed in three ways: direct fusion with cell membranes, endocytosis, and ligand-receptor interaction. Other exosome surface proteins, such as CD9 and CD81, are included in cell–cell fusion, but whether they are included in the mediation of exosome–cell fusion lacks evidence, and further studies are needed to confirm.
Exosomes are nano-sized extracellular membrane vesicles that can work as a remedy for inflammation and improve functional and behavioral recovery in stroke models of rodents (Go et al., 2020).
Among various stem cell types, MSCs are vastly studied cells (Kabat et al., 2020). MSCs were believed to treat various disease conditions by differentiating into healthy cells and regaining functionality, but later on, researchers found that this was because of the paracrine effect of MSCs on the surrounding host cells (Murphy et al., 2019; Hur et al., 2020). The paracrine signals of MSCs are due to exosomes (Maacha et al., 2020). Nowadays, it is proven by multiple research groups that exosomes contain various types of bioactive molecules possessing the properties and contents of their origin cell (Riazifar et al., 2019; Hur et al., 2020). Stem cell exosomes have been isolated, characterized, evaluated, and designed for enhancing beneficial effects in brain injury and neurodegenerative disease (Upadhya and Shetty, 2019). MSCs exosomes (MSCs-Exo) mediate secretion of cell waste to extracellular fluid and transmit it in between producer and target/recipient brain cells (Glebov et al., 2015; Zhang Z.G. et al., 2019). Under preclinical and clinical research, stem cell–derived exosome-based approaches have been verified as a promising regenerative medical treatment for ischemic stroke (Table 1).
Exosomes’ brain disease–prevention characteristics are reported by a number of studies (Long et al., 2017; Zhang et al., 2017). Exosomes mainly protect ischemic brain by Li et al. (2020a) improving the microenvironment and mediating immune response, (McCann and Lawrence, 2020) inhibiting brain cell apoptosis and activating biogenesis (Cunningham et al., 2020), inducing vascular remolding and regeneration, and (Dabrowska et al., 2019) alleviating inflammation.
Exosome therapy decreases inflammation in the mouse brain and reduces infract volume and edema by reducing ROS, TNF-α, and NMDAR1 expression (Kalani et al., 2016). In a glutamate-induced nerve injury model, exosomes protected brain tissues by activating the release of cytokines and growth factors mainly by activating the phosphatidylinositol 3-kinase (PI3K)/Akt pathway (Wei et al., 2016). MSCs-Exo had a neuroprotective effect on hypoxic-ischemia injury in newborn mice, and this outcome was partly because of a decrease in neuron apoptosis and neuroinflammation. Within the injured brain, the miR-21a-5p was upregulated in neurons and microglia via uptake of MSCs-Exo (Xin et al., 2020). IV injection of exosomes improved the brain microenvironment by inducing vascular remodeling and promoting regeneration of damaged neurons in a stroke rat model (Han et al., 2018). MSCs-Exo enhanced motor function, learning, and memory abilities of rats after 7 days of middle cerebral artery occlusion (MCAO). Furthermore, an increase in production of anti-inflammatory cytokines and growth factors and a decrease in pro-inflammatory factors was found in both the hippocampus and cortex of the ischemic region as well as in OGD-treated microglia cells. Western blotting analysis results confirmed that CysLT2R expression and ERK1/2 phosphorylation were downregulated both in vivo and in vitro (Zhao et al., 2020). White blood cells, especially polymorphonuclear neutrophils, appear to be the key leukocyte population in the mediation of the neuroinflammatory response. Exosomes derived from hMSCs were studied to confer neurological recovery after focal cerebral ischemia in rodents by depleting polymorphonuclear neutrophils, especially monocytes and lymphocytes (Wang C. et al., 2020). Exosomes from MSCs were reported to reduce neuroinflammation after cortical injury in the aged brain of monkeys. Results show that recovery in exosome-treated aged monkeys was fast and efficient compared with aged control vehicle monkeys. Moreover, exosome treatment after injury is associated with greater densities of ramified, homeostatic microglia along with reduced pro-inflammatory microglial markers (Go et al., 2020). In general, exosomes’ potential therapeutic activities for stroke can be summarized as neuroprotection; reduced inflammation; immunomodulation; stimulation of new synapse formation; and activation of neurogenesis, astrogenesis, angiogenesis, and white matter remodeling (Figure 2).
Exosome pros for the treatment of stroke is studied enormously (as discussed). An ongoing clinical trial (NCT03384433) aims to investigate exosome safety and potential for ischemic stroke. The main aim of this trial is a safety check, i.e., to check adverse effects within 12 months of exosome therapy, and the secondary goal is to find out the efficacy of improvement in modified ranking score, i.e., measure the degree of disability in stroke patients after 12 months. Results of the study are yet to be announced. The pitfalls of exosome applications are that it can induce neurodegenerative disorder, autoimmunity, viral infections, or the spread of cancer, as they can transmit in between cells and deliver their contents. Cancer cell–derived exosomes were immune-suppressive and had low expression levels of tumor suppressor miRNA-15a, oncogenic protein, and cytokines, and it increased cancer progression (Roccaro et al., 2013; Wen et al., 2016). Besides this, the contents and function of exosomes may depend on donor cells or on metabolic activities of receipt cells, which makes the process of exosome therapy problematic (Wiklander et al., 2015). Exosome proapoptotic and pathological communications were studied in ischemic heart injury in obesity/diabetes mellitus. Diabetic serum exosome injection to the non-diabetic heart caused poorer cardiac function recovery, larger infract size, and increased apoptosis of cardiomyocytes. It was confirmed through various analysis that miRNA-130b-3p was responsible for the cardiotoxicity, and its direct downstream targets were AMPKα1/α2, Birc6, and Ucp3 (Gan et al., 2020). Microglia-derived exosomes were found to mediate neuroinflammation (Kumar et al., 2017) and Alzheimer’s disease propagation (Saeedi et al., 2019). Moreover, preclinical data show that optimum dose is an important indicator for neurological outcomes after stroke (Moniche et al., 2017); hence, exosome time and dose-dependent studies are of utmost importance for better and safe clinical outcomes. Exosomes’ high dose was found to be detrimental for neurons although low doses show neuroprotective effects (Venugopal et al., 2017). Another study demonstrates that a low dose of exosomes was productive in ischemic conditions although, during administration of high doses, exosomes were mostly detected in the lungs or liver (Bian et al., 2014; Moon et al., 2019; Williams et al., 2020). A dose-dependent study of exosomes for stroke therapy reveals that low doses (50–100 μg) had comparatively better outcomes. At least 50 μg exosomes were necessary for subcortical stroke recovery in rats and cell proliferation in OGD conditions although in vivo analysis of low dosage groups showed better functional recovery compared with high dose–treated animals (Otero-Ortega et al., 2020). An exosome high dose can be detrimental and can produce negative effects; therefore, optimum effective dosage studies are mandatory.
Furthermore, it is believed that exosomes function because of miRNAs although it should be kept in mind that some miRNAs are involved in cancer pathogenesis (Van Roosbroeck and Calin, 2017). In addition, off-target effects, short half-life, tracking procedures, and clinical-level large scale production are some basic limitations of exosomes to be addressed. Subsequently, scientists are engineering native exosomes to overcome the natural limitations that we discuss in another section.
Recently, various invasive and newly developed non-invasive methodologies based on overcoming the impeding action of the impermeable BBB and targeting the required disease sites of the brain have been explored. Researchers have put all their efforts toward finding therapeutic agents that can effectively target the brain. Traditional therapeutic agents do not effectively penetrate to the brain because they have to pass through four main barriers, including the BBB, blood brain tumor barrier, blood-cerebrospinal fluid barrier, and efflux protein (Khan et al., 2018). Exosomes have recently been investigated in many studies as a suitable alternative for the shortcomings of traditional agents due to their biological compatibility and particularly small size (Arrighetti et al., 2019) for brain targeting (Lapchak et al., 2018; Qu et al., 2018). Experimental studies provide new insight that exosomes and their cargo play important roles in nerve regeneration, synaptic function, plasticity, immune response, and exosome-mediated intercellular communication, contributing to brain reconstruction (Qing et al., 2018; Liu et al., 2019; Branscome et al., 2020). Exosomes’ BBB crossing ability opens new getaways to the CNS, targeting the treatment of various neurodegenerative disorders, such as stroke, Alzheimer’s disease, tumors, and autoimmune diseases (Abbott et al., 2006; Andreone et al., 2015). Luciferase-carrying exosomes can cross the BMEC monolayer under stroke mimicking inflamed condition, while not under the healthy normal conditions. Furthermore, the results show that exosomes were internalized by BMECs via endocytosis (Chen et al., 2016; Salunkhe et al., 2020). This study reveals the capability of exosomes in brain targeting by exploring their endocytic uptake via their interaction with BBB cells. Blood exosomes’ brain targeting ability was investigated in vivo in nude mice by injecting DiD-labeled blood exosomes through IV administration. By taking near-infrared fluorescence images at different times, it was found that exosomes were accumulated specifically in the brain between 1 and 10 h after injection, whereas the fluorescence intensity was at its peak about 4–8 h after administration of exosomes (Qu et al., 2018). MSCs-Exo were labeled with gold nanoparticles as a labeling agent to check exosome migration and brain-homing abilities. Neuroimaging results show that exosomes exactly targeted and accumulated in pathologically relevant murine model brain regions up to 96 h while in healthy controls up to 24 h (Perets et al., 2019). Exosomes can be used to deliver dopamine or drugs to the brain, and brain distribution can be increased (Yang et al., 2015; Qu et al., 2018). Exosomes are specifically internalized by microglia cells through the macro-pinocytosis pathway in a mix brain cells culture (Fitzner et al., 2011). Hence, exosomes have natural brain-homing abilities, making them suitable for brain disorders.
Considering the natural therapeutic abilities of exosomes for clinical translation yet the rapid clearance of exosomes from the human brain, researchers are focusing on bioengineering of exosomes to increase concentration on target sites, circulation time, and half-life in the body. At least two conditions should be met while engineering exosomes for brain targeting: (I) BioEng-Exo must not interact with the physiological barriers, which could limit their diffusion except the plasma membrane of the target cell, and (II) exosomes should be engineered to preferably target cell types relevant to disease pathogenesis, e.g., rabies virus glycoprotein (RVG) is used to engineer exosomes specifically for CNS disease. RVG is a 29-amino-acid peptide that specifically binds to neural cells’ nicotinic acetylcholine receptor (Ferrantelli et al., 2020). T7 peptides, that target the cellular transferrin receptor in the brain has been successfully used for targeting glioblastoma in the brain (Kim G. et al., 2020). In general, exosome engineering can be done by two basic methodologies: by either engineering exosome-producing cells or direct exosome modification (Figure 3).
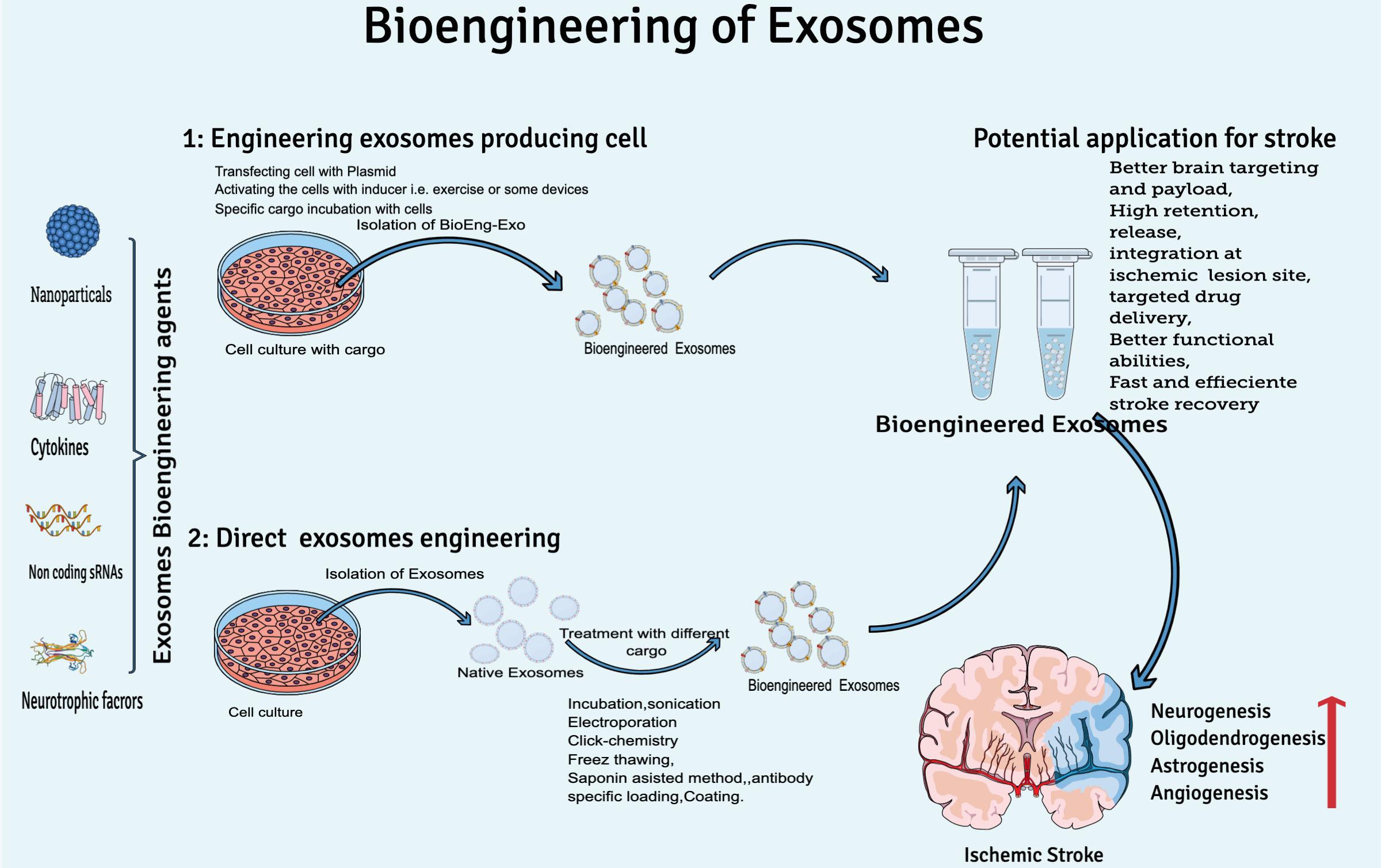
Figure 3. Overview of exosome bioengineering for better ischemic stroke therapy. Two basic approaches used for exosomes engineering are (1) engineering exosome-producing cells (transfecting, activating, or incubation of cargo with cells) and (2) direct exosome engineering (by loading or decorating specific cargo to exosomes after isolation).
During bioengineering of exosome-producing cells, mainly two approaches are practiced by scientists. Either the exosome-producing cell is incubated with cargo (a specific drug or other desired solution) or by genetic transfection of the parent cell (manipulation of the cell by plasmid-containing miRNA, siRNA, or pDNA) (Tanziela et al., 2020). Production of BioEng-Exo in this way is challenging because it is very time-consuming, and sometimes the required physical and chemical conditions are not favorable for viable cells. That is why scientists mostly prefer post exosome modification techniques.
Direct exosome bioengineering is done after isolating and cleaning exosomes from a cell’s ordinary liquid, such as culture medium, serum, and other cell debris. Postproduction exosome bioengineering is a feasible method because exosomes are non-living structure, so it is easy to apply the condition of choice, and high yield can be loaded onto exosomes compared with the cell-based modification approach. Direct exosome engineering can be done by incubation, sonication, electroporation, antibody-specific loading, the freeze-and-thaw method, and the saponin assist method (Tanziela et al., 2020). Through these modification techniques, researchers either modify the surface or contents of exosomes.
Despite recent progress unlocking exosomes’ secrets, advancements in surface modification techniques are mandatory for future clinical translation. Through surface manipulation strategies, exosome circulation, targeting, and stay time can be increased, and it can be done by both active (sonication, electroporation, etc.) or passive (incubation, diffusion etc.) techniques.
Scientists have coated exosomes with polyethylene glycol, which is a hydrophilic polymer, and increased the circulation time of nanoparticles (Suk et al., 2016). The PEGylation technique increases the circulation time of exosomes and reduces non-specific interaction with cells while improving interaction with EGFR-expressing cells. The PEGylation approach was employed by incubating the polyethylene glycol with exosomes 1:1 for 2 h at 40°C to enhance Neuro2A cell–derived exosome specificity and circulation time (Kooijmans et al., 2016). Exosomes targeted specifically toward the brain were achieved by engineering the exosome-producing cells to express an exosomal protein, lysosome-associated membrane protein 2 (Lamp2b), attached to the neuron-specific RVG peptide (Alvarez-Erviti et al., 2011). The glycosylation motif was introduced at various positions of the Lamp2b protein, which prevented the exosome-targeting peptide from degradation as well as stabilized targeted delivery of therapeutic exosomes (Hung and Leonard, 2015). Special membrane-penetrable agents can be stacked to exosomes by incubating the small compound with exosomes under specific conditions (25–37°C). Exosomes were decorated with a brain-targeting peptide (low-density lipoprotein) by the incubation approach for directing the exosome payload toward the glioma site in the brain. The peptide contained an ApoA-1 mimic sequence that enabled its linkage to exosomes by simple incubation (Ye et al., 2018). Click chemistry, originally introduced in 1999, is a reliable, simple, fast, and highly efficient technique for bioconjugation of small and macro molecules to the exosome surface (Hein et al., 2008; Smyth et al., 2014). The targeting ability of exosomes can be improved by surface functionalization. The peptide c(RGDyK), having high affinity to integrin αVβ3 in reactive cerebral vascular endothelial cells, after ischemia especially, was conjugated to the exosome surface through click chemistry reactions. Tail-vain injection of cRGD-Exo specifically targeted the lesion region of a mouse model of cerebral ischemia as well as entered the neuron, microglia, and astrocytes (Tian et al., 2018). Another group conjugated a neuropilin-1–targeting peptide to exosomes through click chemistry for glioma therapy (Jia et al., 2018). To cross the BBB, a gold nanoparticle surface was modified with brain-targeting exosomes. The unique brain-targeting ability of exosomes enabled the gold nanoparticles to cross the BBB while their binding to brain cells was examined under laminar flow conditions (Perets et al., 2019). GFP-CD63–labeled exosomes from the primary neuron are taken up by neurons preferentially, whereas those from a neuroblastoma cell line bind equally to astrocytes (Chivet et al., 2014). All the abovementioned studies confer exosomes with great potential to be polished and developed as effective, safe, and precise agents for CNS neurodegenerative disorders.
Desired cargo can be loaded onto exosomes by pre- or post-isolation modification methods. The most simple and straightforward technique for exosome cargo loading is incubation by which the desired cargo is incubated with exosome-producing cells or with exosomes. Exosomes are promising candidates for targeted delivery of various material to specific cells because of the lipid bilayer membrane decorated with multiple ligands and receptors that can interact with target molecules (Tang et al., 2020). Exosomes were loaded with catalase or quercetin by the incubation technique for neuroprotection in vivo and in vitro (Haney et al., 2015; Qi et al., 2020). Proteins were loaded onto exosomes by an optically reversible protein–protein interaction method, which significantly increased loaded protein levels in recipient cells (Yim et al., 2016). Another group manipulated exosome cargo by loading them with macromolecules, such as proteins and ribonucleoprotein, for cellular delivery (Zhang X. et al., 2020). MiR-210 loaded exosomes were produced by the incubation technique for targeted delivery of miR-210 to the ischemic stroke lesion (Zhang H. et al., 2019). Exosomes were produced through genetic modification for cerebral protection against deep hypothermic circulatory arrest. MSC culture was transfected with pre-miR-214 containing lentivirus vectors, which significantly increased the miR-214 expression in the extracted exosomes (Shi et al., 2020). Exosomes loaded with curcumin strongly suppressed the pro-inflammatory cytokines and cellular apoptosis in the stroke lesion area and activated microglia cells (Tian et al., 2018). Moreover, physical treatment techniques, such as sonication, electroporation, extrusion, surfactant treatment, and dialysis, are employed for loading of exosomes with specific cargo for brain targeting (Alvarez-Erviti et al., 2011; Haney et al., 2015; Fu et al., 2020).
Besides the stated exosome bioengineering methods and examples for the brain, some of the latest research on exosome engineering techniques for brain targeting (specifically for ischemic stroke therapy, Table 2) are discussed in the next sections, in which exosomes have been bioengineered by special agents for ischemic stroke therapy (Figure 3).
Advanced studies have demonstrated that exosomes Possess equivalent therapeutic potential of derived stem cells for ischemic stroke treatment (Zhang Z.G. et al., 2019). However, the most serious drawback of using exosomes is the poor targeting of the ischemic lesion in the brain. Magnetic nanovesicle (MNVs) derived from iron oxide nanoparticle-harbored MSCs increased the targeting of the ischemic region in the brain with the help of an external magnetic field by magnetic navigation. Magnetic navigation increased the exosomes’ ability to target the ischemic lesion by 5.1 times. Moreover, the MNVs considerably decreased the infract volume and improved motor function as well as promoted an anti-inflammatory response, angiogenesis, and anti-apoptosis in the ischemic brain lesion (Kim H.Y. et al., 2020). The exosomes are able to cross the BBB, but their migration and brain-homing abilities were yet to be discovered. A research group developed a method for longitudinal and quantitative in vivo neuroimaging of exosomes while combining exosomes with gold nanoparticles. Exosomes were tracked in different brain conditions, such as ischemic stroke, Alzheimer’s disease, etc. The results show that exosomes accumulated only in the diseased brain and were gradually cleared from the healthy brain, and the special protein structure of exosomes was important for their precise and extended accumulation in the brain (Perets et al., 2019). Glucose-coated gold nanoparticles were used in a mouse model of brain ischemia for non-invasive neuroimaging and tracking of exosomes, which helped to find the optimal administration route and size parameter (Betzer et al., 2017). Treatment and diagnosis for many neurological diseases are hindered by the inability of theranostic agents to cross the BBB (Moura et al., 2019). Following the concept of the nature biotechnology group, who developed exosomes as gene therapy vehicles for specific brain targeting (Alvarez-Erviti et al., 2011), exosome-coated gold nanoparticles were shown to induce targeted delivery to brain cells by increasing the permeability of nanoparticle to cross the BBB (Khongkow et al., 2019).
Cytokines are soluble glycoproteins that play an important role in the pathophysiology of stroke. The loss in between pro-inflammatory and anti-inflammatory cytokines occurs after stroke and affects infarct size and functional outcome (Doll et al., 2104; Klimiec-Moskal et al., 2020). A study compared the role of exosomes derived from interferon gamma (IFN-γ)-stimulating stem cells and control exosomes for treating ischemic stroke and found that IFN-γ preconditioning did not affect the secretion, but significantly altered the functional abilities of exosomes. Moreover, IFN-γ exosomes increased cell proliferation and cell survival as well as decreased cell apoptosis in vitro while exerting therapeutic effects in vivo in an ischemic rat model (Zhang G. et al., 2020). IFN-γ-exosomes have been used for treating neurodegenerative disorders, i.e., multiple sclerosis. IFN-γ-stimulated exosomes reduced demyelination and decreased neuroinflammation in a mouse model (Riazifar et al., 2019). The effect of tumor necrosis factor alpha and interleukin-1β cytokines was evaluated on the release and molecular composition of astrocyte-derived exosomes, and results confirm that TNFα- and IL-1β-treated astrocyte-derived exosomes were rich in miR-125a-5p and miR-16-5p that target proteins involved in neurotrophin signaling. In addition, they observed that cytokine-treated exosomes decrease neuronal NTKR3 and Bcl2 expression (Chaudhuri et al., 2018). Inflammatory cytokine IL-1β that regulates the brain’s injury inflammatory response was injected into brain; a striatal injection of IL-1β promoted an influx of Ly6b+ leukocytes to the lesion site as well as increasing circulating exosome levels in the plasma of mice compared with controls. IL-1β also induced the release of astrocyte-derived exosomes that rapidly crossed the BBB (Dickens et al., 2017). LPS/IFNγ-treated microglia-derived exosomes reduced brain tumor and promoted brain homeostasis recovery (Grimaldi et al., 2019). IL-4-polarized BV2-exosomes promoted angiogenesis in an MCAO model of ischemic stroke (Tian et al., 2019). IL-1-primed MSC-secreted conditioned medium was assessed to promote recovery after stroke. IL-1α-primed MSC-derived conditioned medium treatment led to ∼30% reduction in lesion volume and improved behavioral outcomes and neurological score in a mouse model of stroke (Cunningham et al., 2020). In another study, IL-4 and lipopolysaccharide polarized microglia BV2 cells were investigated for pro-angiogenesis effects. IL-4-polarized cells increased the tube formation of endothelial cells by secreting exosomes, and the miRNA-26a profile was higher compared with the LPS-polarized group (Tian et al., 2019). IL-6, a proinflammatory cytokine that can promote the prosurvival signaling pathway, preconditioned in neural stem cells to reduce ischemic injury in a mouse model of stroke (Sakata et al., 2012).
The field of exosome research is still in its infancy, particularly in studies of CNS diseases. The endothelial cells of brain capillaries form extremely tight junctions creating the BBB, which restricts the entry of all small molecules that are insoluble in lipids (90–98%) to the brain (Yang J. et al., 2017; Blanchard et al., 2020; Su et al., 2020). Yang et al. used exosomes for the delivery of circular RNA to the ischemic region of stroke. They specifically targeted neuronal cells by expressing RVG peptide on exosome membranes and used this RVG-Exo as a cargo delivery system for Circ-SCMHI RNA. RVG-circSCMH1-EVs improved neuronal plasticity by binding to MeCP2 and increased its downstream gene expression (Mobp, Igfbp3, Fxyd1, and Prodh), which maintains brain function. IV injection of RVG-CircSCMH1-Exo improved motor recovery, digit movement, and functional recovery in both rodents and non-human primate models. They suggested that RVG-CircSCMH1-Exo could have wider therapeutic potential window compared with current therapies as they could be administered 24 h after stroke onset (Yang L. et al., 2020).
MiR-124 is well known for its proneuronal role in both the developing and mature brain. Exosomes loaded with miR-124 promote cortical neural progenitors to obtain neuronal identity and confer recovery after ischemia by robust cortical neurogenesis (Yang J. et al., 2017). SiRNA was delivered by harnessing exosomes as shuttle servers. IV injection of exosomes delivered siRNA to neurons, microglia, and oligodendrocytes in the mouse brain. Exosome-mediated delivery of siRNA knocked downed the BACE1 gene, and the inhibition of BACE1 significantly decreased β-amyloid levels in the brain of wild-type mice (Alvarez-Erviti et al., 2011). Consecutively, another group used exosomes as an siRNA carrier assuming it as a possible key step toward siRNA clinical application (van den Boorn et al., 2011). MiRNA-210 holds great potential to improve angiogenesis for brain tissue repair after ischemia. Upregulation of miRNA-210 improved functional recovery after stroke. MiRNA-210 was delivered to the ischemic lesion by conjugating exosomes with c(RGDyK) peptide and then loading with miRNA-210. RGD-Exo miR-210 increased the miR-210 level at the ischemic site as well as upregulated integrinβ3, vascular endothelial growth factor, and CD34, thus increasing the animal survival rate (Zhang H. et al., 2019). Exosomes were utilized for HMGB1-siRNA delivery for treatment of ischemic stroke. Results indicate that exosomes combined with HMGB1-siRNA decreased HMGB1, TNF-α, apoptosis, and infract volume, showing potential for recovery of ischemic stroke (Kim et al., 2019). All these studies indicate that exosomes loaded with small RNAs can enormously alter its abilities for ischemic stroke treatment.
Neurotrophic factor are studied as a neuroprotectant in neurovegetative disorders (Lindholm and Saarma, 2010; Houlton et al., 2019). They control neural stem cell differentiation, which is responsible for the recovery of neural cell function and vessel damage due to ischemic stroke (Abe, 2000). NTFs are emerging as a viable repair therapy in stroke, and they are recognized for their multifaceted neuroprotective role after ischemia (Ramos-Cejudo et al., 2015). However, NTF clinical application is yet not applicable because of the lack of an efficient systemic delivery approach to the ischemic region. Engineered exosomes were used for the delivery of nerve growth factor (NGF) to the ischemic cortex of mouse brain. HEK293 cells were incubated with NGF-Exo different quantities for 4 h, and the mRNA and NGF proteins were checked through qPCR, which showed significant increases in both mRNA and NGF protein expression levels, suggesting that engineered exosomes could successfully deliver NGF mRNA and protein to target cells in vitro. A photothrombotic ischemia model was used, and Dil-labeled exosomes were injected through the tail vein 24 h postischemia. Results show that a remarkable Dil-positive ischemic region was detected in both RVG and NGF-EXO compared with the control exosome group as well as the Dil signal in the ischemic region overlapped with the markers of neuron, astrocyte, and microglia, suggesting that exosomes were taken up by these cells (Yang J. et al., 2020). Brain-derived neurotrophic factor (BDNF) has been investigated enormously for conferring neuroprotection and anti-inflammatory properties. BDNF was encapsulated inside naïve exosomes and delivered to the brain against brain inflammation through IV administration to mice (Donoso-Quezada et al., 2020). MiR-206 knockdown exosomes attenuated early brain injury by upregulation of BDNF levels after exosome treatment (Zhao et al., 2019), which gives us an idea that bioengineering exosomes with BDNF could have a positive outcome for brain disorders, including stroke. However, the collaboration of both exosomes and NTFs has not been done for stroke therapy in depth; indeed, it is a worthwhile field to explore in the future.
Exosomes are secreted by almost all cell types and contain lipids, proteins, and nucleic acids of the origin cells. They are at the forefront of clinical success in many research areas. In the past few years, the effectiveness of exosomes has been enormously studied as being the best fit for brain targeting because of their native abilities to cross the biological barriers into the brain. However, there is evidence that exosomes get cleaned up from the brain quite quickly, and in a short time period, very low amounts of exosomes can be seen in the brain. Therefore, scientists have focused on bioengineering exosomes to increase their circulation half-life, increase their stay at the disease site, direct exosomes to target cells, and use them for targeted delivery of therapeutic molecules or for regenerative medicine. Scientists have BioEng-Exo with lots of agents (as discussed in the review) for targeted stroke therapy, but still, this field is in its infancy, and lot yet needs to be done. In preclinical studies, promising results are obtained, but the effect is still unknown in humans. Many studies are been carried out, but mostly in vitro, in which the culture condition may affect the exosomes’ biochemical and biophysical features; therefore, future studies are needed to better understand the physiological effects of these engineered exosomes on human health. Moreover, the bioactive molecules loaded in exosomes are extremely small and low in quantity; therefore, there is need of efficient Exo-specific recognition tools in recipient cells. Immune tolerance, potency, and toxicity of these exosomes need to be explored in in vivo studies. Moreover, further work is needed on stabilization, and optimization of these BioEng-Exo techniques as well as isolation, high scalable production, purity, and stability of relevant exosomes is mandatory for future potential clinical success.
HK conceived, designed, and wrote the manuscript. J-JP and YL provided critical revision and helped in the analysis of manuscript. G-YY and ZZ contributed to the discussion of ideas, helped in the correction, and proofread the manuscript. All the authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This contribution was financially supported by grants from the National Key R&D Program of China (#2016YFC1300602, G-YY), National Natural Science Foundation of China (NSFC) (81771251, G-YY and 81771244 and 81974179, ZZ), Shanghai Education Commission Research and Innovation Program (2019-01-07-00-02-E00064, G-YY), and K. C. Wong Education Foundation (G-YY).
BioEng-Exo, bioengineered exosomes; BBB, blood–brain barrier; BDNF, brain derived neurotrophic factor; BMECs, brain microvascular endothelial cells; CNS, central nervous system; EEs, early endosomes; EVs, extracellular vesicles; ESCRT, endosomal-sorting complex essential for transport; HIV, human immunodeficiency virus; ILVs, intraluminal vesicles; IFN- γ, interferon gamma; IL, interleukin; IONP, iron oxide nanoparticle; Lamp2b, lysosome-associated membrane protein 2; MSCs, mesenchymal stem cells; MVs, microvesicles; MVBs, multivesicular bodies; MCAO, middle cerebral artery occlusion; MSCs-Exo, MSCs exosomes; MNVs, magnetic nanovesicle; NTFs, neurotrophic factors; NGF, nerve growth factor; RVG, rabies virus glycoprotein.
Abbott, N. J., Ronnback, L., and Hansson, E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53. doi: 10.1038/nrn1824
Abe, K. (2000). Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J. Cereb. Blood Flow. Metab. 20, 1393–1408. doi: 10.1097/00004647-200010000-00001
Alvarez-Erviti, L., Seow, Y., Yin, H., Betts, C., Lakhal, S., and Wood, M. J. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345. doi: 10.1038/nbt.1807
Andreone, B. J., Lacoste, B., and Gu, C. (2015). Neuronal and vascular interactions. Annu. Rev. Neurosci. 38, 25–46. doi: 10.1146/annurev-neuro-071714-033835
Arrighetti, N., Corbo, C., Evangelopoulos, M., Pasto, A., Zuco, V., and Tasciotti, E. (2019). Exosome-like nanovectors for drug delivery in cancer. Curr. Med. Chem. 26, 6132–6148. doi: 10.2174/0929867325666180831150259
Bai, W.-F., Zhang, Y., Xu, W., Li, W., Li, M., Yuan, F., et al. (2020). Isolation and characterization of neural progenitor cells from bone marrow in cell replacement therapy of brain injury. Front. Cell. Neurosci. 14:49. doi: 10.3389/fncel.2020.00049
Ballabh, P., Braun, A., and Nedergaard, M. (2004). The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1–13. doi: 10.1016/j.nbd.2003.12.016
Bang, O. Y., and Kim, E. H. (2019). Mesenchymal stem cell-derived extracellular vesicle therapy for stroke: challenges and progress. Front. Neurol. 10:211.
Betzer, O., Perets, N., and Angel, A. (2017). In Vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 11, 10883–10893. doi: 10.1021/acsnano.7b04495
Bian, S., Zhang, L., Duan, L., Wang, X., Min, Y., and Yu, H. (2014). Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. 92, 387–397. doi: 10.1007/s00109-013-1110-5
Blanchard, J. W., Bula, M., Davila-Velderrain, J., Akay, L. A., Zhu, L., Frank, A., et al. (2020). Reconstruction of the human blood–brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat. Med. 26, 952–963. doi: 10.1038/s41591-020-0886-4
Borges, F. T., Reis, L. A., and Schor, N. (2013). Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Brazil. J. Med. Biol. Res. 46, 824–830. doi: 10.1590/1414-431x20132964
Branscome, H., Paul, S., Yin, D. Z., El-Hage, N., Agbottah, E. T., Zadeh, M. A., et al. (2020). Use of stem cell extracellular vesicles as a “Holistic” approach to CNS repair. Front. Cell. Dev. Biol. 8:455. doi: 10.3389/fcell.2020.00455
Chaudhuri, A. D., Dastgheyb, R. M., Yoo, S. W., Trout, A., Talbot, C. C. Jr., Hao, H., et al. (2018). TNFalpha and IL-1beta modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 9:363. doi: 10.1038/s41419-018-0369-4
Chen, C. C., Liu, L., Ma, F., Wong, C. W., Guo, X. E., Chacko, J. V., et al. (2016). Elucidation of exosome migration across the blood-brain barrier model In Vitro. Cell. Mol. Bioeng. 9, 509–529. doi: 10.1007/s12195-016-0458-3
Cheng, W. C., Liao, T. T., Lin, C. C., Yuan, L. T. E., Lan, H. Y., Lin, H. H., et al. (2019). RAB27B-activated secretion of stem-like tumor exosomes delivers the biomarker microRNA-146a-5p, which promotes tumorigenesis and associates with an immunosuppressive tumor microenvironment in colorectal cancer. Int. J. Cancer 145, 2209–2224. doi: 10.1002/ijc.32338
Chivet, M., Javalet, C., Laulagnier, K., Blot, B., Hemming, F. J., and Sadoul, R. (2014). Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J. Extracell. Vesicles 3, 24722–24722. doi: 10.3402/jev.v3.24722
Chuo, S. T., Chien, J. C., and Lai, C. P. (2018). Imaging extracellular vesicles: current and emerging methods. J. Biomed. Sci. 25:91. doi: 10.1186/s12929-018-0494-5
Cunningham, C. J., Wong, R., Barrington, J., Tamburrano, S., Pinteaux, E., and Allan, S. M. (2020). Systemic conditioned medium treatment from interleukin-1 primed mesenchymal stem cells promotes recovery after stroke. Stem Cell Res. Ther. 11:32. doi: 10.1186/s13287-020-1560-y
Dabrowska, S., Andrzejewska, A., Lukomska, B., and Janowski, M. (2019). Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J. Neuroinflam. 16:178. doi: 10.1186/s12974-019-1571-8
de Abreu, R. C., Fernandes, H., da Costa Martins, P. A., Sahoo, S., Emanueli, C., and Ferreira, L. (2020). Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 17, 685–697. doi: 10.1038/s41569-020-0389-5
Dickens, A. M., Tovar, Y. R. L. B., Yoo, S. W., Trout, A. L., Bae, M., Kanmogne, M., et al. (2017). Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Sign. 10:473. doi: 10.1126/scisignal.aai7696
Doll, D. N., Barr, T. L., and Simpkins, J. W. (2104). Cytokines their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis. 5, 294–306.
Donoso-Quezada, J., Ayala-Mar, S., and Gonzalez-Valdez, J. (2020). State-of-the-art exosome loading and functionalization techniques for enhanced therapeutics: a review. Crit. Rev. Biotechnol. 40, 804–820. doi: 10.1080/07388551.2020.1785385
Ferrantelli, F., Chiozzini, C., Leone, P., Manfredi, F., and Federico, M. (2020). Engineered extracellular vesicles/exosomes as a new tool against neurodegenerative diseases. Pharmaceutics 12:529. doi: 10.3390/pharmaceutics12060529
Fitzner, D., Schnaars, M., van Rossum, D., Krishnamoorthy, G., Dibaj, P., Bakhti, M., et al. (2011). Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell. Sci. 124, 447–458. doi: 10.1242/jcs.074088
Fu, S. Y., Wang, Y., Xia, X. H., and Zheng, J. L. C. (2020). Exosome engineering: current progress in cargo loading and targeted delivery. Nanoimpact 20:100261. doi: 10.1016/j.impact.2020.100261
Gan, L., Xie, D. N., Liu, J., Lau, W. N., Christopher, T. A., Lopez, B., et al. (2020). Small extracellular microvesicles mediated pathological communications between dysfunctional adipocytes and cardiomyocytes as a novel mechanism exacerbating ischemia/reperfusion injury in diabetic mice. Circulation 141, 968–983. doi: 10.1161/Circulationaha.119.042640
Gharbi, T., Zhang, Z., and Yang, G. Y. (2020). The function of astrocyte mediated extracellular vesicles in central nervous system diseases. Front. Cell Dev. Biol. 8:568889. doi: 10.3389/fcell.2020.568889
Glebov, K., Lochner, M., Jabs, R., Lau, T., Merkel, O., Schloss, P., et al. (2015). Serotonin stimulates secretion of exosomes from microglia cells. Glia 63, 626–634. doi: 10.1002/glia.22772
Go, V., Bowley, B. G. E., Pessina, M. A., Zhang, Z. G., Chopp, M., Finklestein, S. P., et al. (2020). Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience 42, 1–17. doi: 10.1007/s11357-019-00115-w
Grapp, M., Wrede, A., Schweizer, M., Huwel, S., Galla, H. J., Snaidero, N., et al. (2013). Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat. Commun. 4:2123. doi: 10.1038/ncomms3123
Grimaldi, A., Serpe, C., Chece, G., Nigro, V., Sarra, A., Ruzicka, B., et al. (2019). Microglia-derived microvesicles affect microglia phenotype in glioma. Front. Cell. Neurosci. 13:41. doi: 10.3389/fncel.2019.00041
Guescini, M., Guidolin, D., Vallorani, L., Casadei, L., Gioacchini, A. M., Tibollo, P., et al. (2010). C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp. Cell. Res. 316, 1977–1984. doi: 10.1016/j.yexcr.2010.04.006
Han, Y., Seyfried, D., Meng, Y., Yang, D., Schultz, L., Chopp, M., et al. (2018). Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J. Neurosurg. 131, 290–300. doi: 10.3171/2018.2.JNS171475
Haney, M. J., Klyachko, N. L., Zhaoa, Y. L., Gupta, R., Plotnikova, E. G., He, Z. J., et al. (2015). Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 207, 18–30. doi: 10.1016/j.jconrel.2015.03.033
Hein, C. D., Liu, X. M., and Wang, D. (2008). Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 25, 2216–2230. doi: 10.1007/s11095-008-9616-1
Houlton, J., Abumaria, N., Hinkley, S. F. R., and Clarkson, A. N. (2019). Therapeutic potential of neurotrophins for repair after brain injury: a helping hand from biomaterials. Front. Neurosci. 13:790. doi: 10.3389/fnins.2019.00790
Hung, M. E., and Leonard, J. N. (2015). Stabilization of exosome-targeting peptides via engineered glycosylation. J. Biol. Chem. 290, 8166–8172. doi: 10.1074/jbc.M114.621383
Huotari, J., and Helenius, A. (2011). Endosome maturation. EMBO J. 30, 3481–3500. doi: 10.1038/emboj.2011.286
Hur, Y. H., Cerione, R. A., and Antonyak, M. A. (2020). Extracellular vesicles and their roles in stem cell biology. Stem Cells 38, 469–476. doi: 10.1002/stem.3140
Izquierdo-Useros, N., Naranjo-Gomez, M., Archer, J., Hatch, S. C., Erkizia, I., Blanco, J., et al. (2009). Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 113, 2732–2741. doi: 10.1182/blood-2008-05-158642
Jadli, A. S., Ballasy, N., Edalat, P., and Patel, V. B. (2020). Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol. Cell. Biochem. 467, 77–94. doi: 10.1007/s11010-020-03703-z
Jakubec, M., Maple-Grodem, J., Akbari, S., Nesse, S., Halskau, O., and Mork-Jansson, A. E. (2020). Plasma-derived exosome-like vesicles are enriched in lyso-phospholipids and pass the blood-brain barrier. PLoS One 15:e0232442. doi: 10.1371/journal.pone.0232442
Jia, G., Han, Y., An, Y., Ding, Y., He, C., Wang, X., et al. (2018). NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 178, 302–316. doi: 10.1016/j.biomaterials.2018.06.029
Joshi, B. S., de Beer, M. A., Giepmans, B. N. G., and Zuhorn, I. S. (2020). Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano 14, 4444–4455. doi: 10.1021/acsnano.9b10033
Kabat, M., Bobkov, I., Kumar, S., and Grumet, M. (2020). Trends in mesenchymal stem cell clinical trials 2004-2018: is efficacy optimal in a narrow dose range? Stem Cells Transl. Med. 9, 17–27. doi: 10.1002/sctm.19-0202
Kalani, A., Chaturvedi, P., Kamat, P. K., Maldonado, C., Bauer, P., Joshua, I. G., et al. (2016). Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell. Biol. 79, 360–369. doi: 10.1016/j.biocel.2016.09.002
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367:6478. doi: 10.1126/science.aau6977
Khan, A. R., Yang, X., Fu, M., and Zhai, G. (2018). Recent progress of drug nanoformulations targeting to brain. J. Control Release 291, 37–64. doi: 10.1016/j.jconrel.2018.10.004
Khongkow, M., Yata, T., Boonrungsiman, S., Ruktanonchai, U. R., Graham, D., and Namdee, K. (2019). Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci. Rep. 9:8278. doi: 10.1038/s41598-019-44569-6
Kim, G., Kim, M., Lee, Y., Byun, J. W., Hwang, D. W., and Lee, M. (2020). Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control. Release 317, 273–281. doi: 10.1016/j.jconrel.2019.11.009
Kim, H. Y., Kim, T. J., Kang, L., Kim, Y. J., Kang, M. K., Kim, J., et al. (2020). Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials 243:119942. doi: 10.1016/j.biomaterials.2020.119942
Kim, M., Kim, G., Hwang, D. W., and Lee, M. (2019). Delivery of high mobility group box-1 siRNA using brain-targeting exosomes for ischemic stroke therapy. J. Biomed. Nanotechnol. 15, 2401–2412. doi: 10.1166/jbn.2019.2866
Klimiec-Moskal, E., Piechota, M., Pera, J., Weglarczyk, K., Slowik, A., Siedlar, M., et al. (2020). The specific ex vivo released cytokine profile is associated with ischemic stroke outcome and improves its prediction. J. Neuroinflammation 17:7. doi: 10.1186/s12974-019-1691-1
Kodali, M., Castro, O. W., Kim, D. K., Thomas, A., Shuai, B., Attaluri, S., et al. (2020). Intranasally administered human MSC derived extracellular vesicles pervasively incorporate into neurons and microglia in both intact and status epilepticus injured forebrain. Int. J. Mol. Sci. 21:181. doi: 10.3390/ijms21010181
Kooijmans, S. A. A., Fliervoet, L. A. L., van der Meel, R., Fens, M. H. A. M., Heijnen, H. F. G., Henegouwen, P. M. P. V. E., et al. (2016). PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 224, 77–85. doi: 10.1016/j.jconrel.2016.01.009
Kruminis-Kaszkiel, E., Osowski, A., Bejer-Olenska, E., Dziekonski, M., and Wojtkiewicz, J. (2020). Differentiation of human mesenchymal stem cells from whartons jelly towards neural stem cells using a feasible and repeatable protocol. Cells 9:739. doi: 10.3390/cells9030739
Kumar, A., Stoica, B. A., Loane, D. J., Yang, M., Abulwerdi, G., Khan, N., et al. (2017). Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflam. 14:47. doi: 10.1186/s12974-017-0819-4
Lai, C. P., Mardini, O., Ericsson, M., Prabhakar, S., Maguire, C. A., Chen, J. W., et al. (2014). Dynamic biodistribution of extracellular vesicles in Vivo using a multimodal imaging reporter. Acs Nano 8, 483–494. doi: 10.1021/nn404945r
Lapchak, P. A., Boitano, P. D., de Couto, G., and Marban, E. (2018). Intravenous xenogeneic human cardiosphere-derived cell extracellular vesicles (exosomes) improves behavioral function in small-clot embolized rabbits. Exp. Neurol. 307, 109–117. doi: 10.1016/j.expneurol.2018.06.007
Li, L., Scott, C. A., and Rothwell, P. M. (2020a). Trends in stroke incidence in high-income countries in the 21st century: population-based study and systematic review. Stroke 51, 1372–1380. doi: 10.1161/STROKEAHA.119.028484
Li, L., Zhang, Y., Mu, J., Chen, J., Zhang, C., Cao, H., et al. (2020b). Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 20, 4298–4305. doi: 10.1021/acs.nanolett.0c00929
Lin, Y., Zhang, C., Xiang, P., Shen, J., Sun, W., and Yu, H. (2020). Exosomes derived from HeLa cells break down vascular integrity by triggering endoplasmic reticulum stress in endothelial cells. J. Extracell. Vesicles 9:1722385. doi: 10.1080/20013078.2020.1722385
Lindholm, P., and Saarma, M. (2010). Novel CDNF/MANF family of neurotrophic factors. Dev. Neurobiol. 70, 360–371. doi: 10.1002/dneu.20760
Liu, W., Bai, X., Zhang, A., Huang, J., Xu, S., and Zhang, J. (2019). Role of exosomes in central nervous system diseases. Front. Mol. Neurosci. 12:240. doi: 10.3389/fnmol.2019.00240
Long, Q., Upadhya, D., Hattiangady, B., Kim, D. K., An, S. Y., Shuai, B., et al. (2017). Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. U S A. 114, E3536–E3545. doi: 10.1073/pnas.1703920114
Lu, M., and Huang, Y. (2020). Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 242:119925. doi: 10.1016/j.biomaterials.2020.119925
Lu, Y., Chen, L., Li, L., and Cao, Y. (2020). Exosomes derived from brain metastatic breast cancer cells destroy the blood-brain barrier by carrying lncRNA GS1-600G8.5. Biomed. Res. Int. 2020:7461727. doi: 10.1155/2020/7461727
Ma, L., Li, Y., Peng, J., Wu, D., Zhao, X., Cui, Y., et al. (2015). Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell. Res. 25, 24–38. doi: 10.1038/cr.2014.135
Maacha, S., Sidahmed, H., Jacob, S., Gentilcore, G., Calzone, R., Grivel, J. C., et al. (2020). Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020:4356359. doi: 10.1155/2020/4356359
Malone, K., Amu, S., Moore, A. C., and Waeber, C. (2019). The immune system and stroke: from current targets to future therapy. Immunol. Cell. Biol. 97, 5–16. doi: 10.1111/imcb.12191
Margolis, L., and Sadovsky, Y. (2019). The biology of extracellular vesicles: the known unknowns. PLoS Biol. 17:e3000363. doi: 10.1371/journal.pbio.3000363
Mathieu, M., Martin-Jaular, L., Lavieu, G., and Thery, C. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell. Biol. 21, 9–17. doi: 10.1038/s41556-018-0250-9
McCann, S. K., and Lawrence, C. B. (2020). Comorbidity and age in the modelling of stroke: are we still failing to consider the characteristics of stroke patients? BMJ Open Sci. 4:e100013. doi: 10.1136/bmjos-2019-100013
Melikyan, G. B. (2014). HIV entry: a game of hide-and-fuse? Curr. Opin. Virol. 4, 1–7. doi: 10.1016/j.coviro.2013.09.004
Moniche, F., Rosado-de-Castro, P. H., Escudero, I., Zapata, E., Laviana, F. J. D., Mendez-Otero, R., et al. (2017). Increasing dose of autologous bone marrow mononuclear cells transplantation is related to stroke outcome: results from a pooled analysis of two clinical trials. Stem Cells Int. 2016:8657173. doi: 10.1155/2017/7946930
Moon, G. J., Sung, J. H., Kim, D. H., Kim, E. H., Cho, Y. H., Son, J. P., et al. (2019). Application of mesenchymal stem cell-derived extracellular vesicles for stroke: biodistribution and MicroRNA study. Transl. Stroke Res. 10, 509–521. doi: 10.1007/s12975-018-0668-1
Moore, T. L., Bowley, B. G. E., Pessina, M. A., Calderazzo, S. M., Medalla, M., Go, V., et al. (2019). Mesenchymal derived exosomes enhance recovery of motor function in a monkey model of cortical injury. Restor. Neurol. Neurosci. 37, 347–362. doi: 10.3233/RNN-190910
Moura, R. P., Sousa, F., Almeida, A., Pinto, S., and Sarmento, B. (2019). Theranostic biomaterials for regulation of the blood–brain barrier. Theranostic Bionanomat. 2019, 303–319. doi: 10.1016/b978-0-12-815341-3.00013-4
Mulcahy, L. A., Pink, R. C., and Carter, D. R. (2014). Routes and mechanisms of extracellular vesicle uptake. J. Extracell Vesicles 3:24641. doi: 10.3402/jev.v3.24641
Muller, U. (2020). Exosome-mediated protection of auditory hair cells from ototoxic insults. J. Clin. Invest. 130, 2206–2208. doi: 10.1172/JCI135710
Murphy, D. E., de Jong, O. G., Brouwer, M., Wood, M. J., Lavieu, G., Schiffelers, R. M., et al. (2019). Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp. Mol. Med. 51, 1–12. doi: 10.1038/s12276-019-0223-5
Nolte-’t Hoen, E., Cremer, T., Gallo, R. C., and Margolis, L. B. (2016). Extracellular vesicles and viruses: are they close relatives? Proc. Natl. Acad. Sci. U S A. 113, 9155–9161. doi: 10.1073/pnas.1605146113
Ostrowski, M., Carmo, N. B., Krumeich, S., Fanget, I., Raposo, G., Savina, A., et al. (2010). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell. Biol. 12, 19–30. doi: 10.1038/ncb2000
Otero-Ortega, L., Laso-García, F., Frutos, M. C. G., Diekhorst, L., Martínez-Arroyo, A., Alonso-López, E., et al. (2020). Low dose of extracellular vesicles identified that promote recovery after ischemic stroke. Stem Cell Res. Ther. 11:70. doi: 10.1186/s13287-020-01601-1
Otero-Ortega, L., Laso-García, F., Frutos, M. G.-D., Fuentes, B., Diekhorst, L., Díez-Tejedor, E., et al. (2019). Role of exosomes as a treatment and potential biomarker for stroke. Transl. Stroke Res. 10, 241–249. doi: 10.1007/s12975-018-0654-7
Pan, J., Qu, M., Li, Y., Wang, L., Zhang, L., Wang, Y., et al. (2020). MicroRNA-126-3p/-5p overexpression attenuates blood-brain barrier disruption in a mouse model of middle cerebral artery occlusion. Stroke 51, 619–627. doi: 10.1161/STROKEAHA.119.027531
Pegtel, D. M., and Gould, S. J. (2019). Exosomes. Annu. Rev. Biochem. 88, 487–514. doi: 10.1146/annurev-biochem-013118-111902
Perets, N., Betzer, O., Shapira, R., Brenstein, S., Angel, A., Sadan, T., et al. (2019). Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 19, 3422–3431. doi: 10.1021/acs.nanolett.8b04148
Qi, Y., Guo, L., Jiang, Y., Shi, Y., Sui, H., and Zhao, L. (2020). Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 27, 745–755. doi: 10.1080/10717544.2020.1762262
Qing, L. M., Chen, H. W., Tang, J. Y., and Jia, X. F. (2018). Exosomes and their MicroRNA cargo: new players in peripheral nerve regeneration. Neurorehabil Neural Repair 32, 765–776. doi: 10.1177/1545968318798955
Qu, M. K., Lin, Q., Huang, L. Y., Fu, Y., Wang, L. Y., He, S. S., et al. (2018). Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 287, 156–166. doi: 10.1016/j.jconrel.2018.08.035
Ramos-Cejudo, J., Gutierrez-Fernandez, M., Otero-Ortega, L., Rodriguez-Frutos, B., Fuentes, B., Vallejo-Cremades, M. T., et al. (2015). Brain-derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke 46:221. doi: 10.1161/Strokeaha.114.006692
Riazifar, M., Mohammadi, M. R., Pone, E. J., Yeri, A., Lässer, C., Segaliny, A. I., et al. (2019). Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano 13, 6670–6688. doi: 10.1021/acsnano.9b01004
Roccaro, A. M., Sacco, A., Maiso, P., Azab, A. K., Tai, Y. T., Reagan, M., et al. (2013). BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Invest. 123, 1542–1555. doi: 10.1172/JCI66517
Rufino-Ramos, D., Albuquerque, P. R., Carmona, V., Perfeito, R., Nobre, R. J., and Pereira de Almeida, L. (2017). Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J. Control Release 262, 247–258. doi: 10.1016/j.jconrel.2017.07.001
Saeedi, S., Israel, S., Nagy, C., and Turecki, G. (2019). The emerging role of exosomes in mental disorders. Transl. Psychiatry 9:122. doi: 10.1038/s41398-019-0459-9
Sakata, H., Narasimhan, P., Niizuma, K., Maier, C. M., Wakai, T., and Chan, P. H. (2012). Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain 135, 3298–3310. doi: 10.1093/brain/aws259
Salunkhe, S., Dheeraj, Basak, M., Chitkara, D., and Mittal, A. (2020). Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J. Control. Release 326, 599–614. doi: 10.1016/j.jconrel.2020.07.042
Sanchez-Ramos, J., Song, S., Cardozo-Pelaez, F., Hazzi, C., Stedeford, T., Willing, A., et al. (2000). Adult bone marrow stromal cells differentiate into neural cells in vitro.”. Exp. Neurol. 164, 247–256. doi: 10.1006/exnr.2000.7389
Sansone, P., Savini, C., Kurelac, I., Chang, Q., Amato, L. B., Strillacci, A., et al. (2017). Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. U S A. 114, E9066–E9075. doi: 10.1073/pnas.1704862114
Shi, J., Jiang, X., Gao, S., Zhu, Y., Liu, J., Gu, T., et al. (2020). Gene-modified exosomes protect the brain against prolonged deep hypothermic circulatory arrest. Ann. Thorac Surg. 111, 576–585. doi: 10.1016/j.athoracsur.2020.05.075
Skotland, T., Sandvig, K., and Llorente, A. (2017). Lipids in exosomes: current knowledge and the way forward. Prog. Lipid Res. 66, 30–41. doi: 10.1016/j.plipres.2017.03.001
Smyth, T., Petrova, K., Persaud, I., Redzic, J. S., Gruner, M. W., Smith-Jones, P., et al. (2014). Surface functionalization of exosomes using click chemistry. Bioconjugate Chem. 25, 1777–1784. doi: 10.1021/bc500291r
Song, Y., Li, Z., He, T., Qu, M., Jiang, L., Li, W., et al. (2019). M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 9, 2910–2923. doi: 10.7150/thno.30879
Su, Y. L., Kuo, L. W., Hsu, C. H., Chiang, C. S., Lu, Y. J., Chang, S. J., et al. (2020). Rabies virus glycoprotein-amplified hierarchical targeted hybrids capable of magneto-electric penetration delivery to orthotopic brain tumor. J. Control. Release 321, 159–173. doi: 10.1016/j.jconrel.2020.02.018
Suk, J. S., Xu, Q. G., Kim, N., Hanes, J., and Ensign, L. M. (2016). PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 99, 28–51. doi: 10.1016/j.addr.2015.09.012
Tang, T. T., Wang, B., Wu, M., Li, Z. L., Feng, Y., Cao, J. Y., et al. (2020). Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci. Adv. 6:eaaz0748. doi: 10.1126/sciadv.aaz0748
Tanziela, T., Shaikh, S., Jiang, H., Lu, Z., and Wang, X. (2020). Efficient encapsulation of biocompatible nanoparticles in exosomes for cancer theranostics. Nano Today 35:100964. doi: 10.1016/j.nantod.2020.100964
Thery, C., Zitvogel, L., and Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579. doi: 10.1038/nri855
Tian, T., Zhang, H. X., He, C. P., Fan, S., Zhu, Y. L., Qi, C., et al. (2018). Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150, 137–149. doi: 10.1016/j.biomaterials.2017.10.012
Tian, Y., Zhu, P., Liu, S., Jin, Z., Li, D., Zhao, H., et al. (2019). IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv. Clin. Exp. Med. 28, 421–430. doi: 10.17219/acem/91826
Tieu, A., Lalu, M. M., Slobodian, M., Gnyra, C., Fergusson, D. A., Montroy, J., et al. (2020). An analysis of mesenchymal stem cell-derived extracellular vesicles for preclinical use. ACS Nano 14, 9728–9743. doi: 10.1021/acsnano.0c01363
Trams, E. G., Lauter, C. J., and Salem, N. Jr., and Heine, U. (1981). Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta. 645:63–70. doi: 10.1016/0005-2736(81)90512-5
Tsai, M. J., Hsu, Y. L., and Kuo, P. L. (2018). Circulating extracellular vesicles in human disease. N. Engl. J. Med. 379, 2179–2180. doi: 10.1056/NEJMc1813170
Upadhya, D., and Shetty, A. K. (2019). Extracellular vesicles as therapeutics for brain injury and disease. Curr. Pharmaceutical. Design 25, 3500–3505. doi: 10.2174/1381612825666191014164950
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., and Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell. Biol. 9, 654–659. doi: 10.1038/ncb1596
van den Boorn, J. G., Schlee, M., Coch, C., and Hartmann, G. (2011). SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 29, 325–326. doi: 10.1038/nbt.1830
van Dongen, H. M., Masoumi, N., Witwer, K. W., and Pegtel, D. M. (2016). Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol. Mol. Biol. Rev. 80, 369–386. doi: 10.1128/MMBR.00063-15
van Niel, G., D’Angelo, G., and Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 19, 213–228. doi: 10.1038/nrm.2017.125
Van Roosbroeck, K., and Calin, G. A. (2017). Cancer hallmarks and MicroRNAs: the therapeutic connection. Adv. Cancer Res. 135, 119–149. doi: 10.1016/bs.acr.2017.06.002
Venugopal, C., Shamir, C., Senthilkumar, S., Babu, J. V., Sonu, P. K., Nishtha, K. J., et al. (2017). Dosage and passage dependent neuroprotective effects of exosomes derived from rat bone marrow mesenchymal stem cells: an In Vitro analysis. Curr. Gene. Ther. 17, 379–390. doi: 10.2174/1566523218666180125091952
Vietri, M., Radulovic, M., and Stenmark, H. (2020). The many functions of ESCRTs. Nat. Rev. Mol. Cell. Biol. 21, 25–42. doi: 10.1038/s41580-019-0177-4
Villarroya-Beltri, C., Baixauli, F., Mittelbrunn, M., Fernandez-Delgado, I., Torralba, D., Moreno-Gonzalo, O., et al. (2016). ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 7:13588. doi: 10.1038/ncomms13588
Vojtech, L., Woo, S., Hughes, S., Levy, C., Ballweber, L., Sauteraud, R. P., et al. (2014). Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 42, 7290–7304. doi: 10.1093/nar/gku347
Wang, C., Borger, V., Sardari, M., Murke, F., Skuljec, J., Pul, R., et al. (2020). Mesenchymal stromal cell-derived small extracellular vesicles induce ischemic neuroprotection by modulating leukocytes and specifically neutrophils. Stroke 51, 1825–1834. doi: 10.1161/STROKEAHA.119.028012
Wang, F., Tang, H., Zhu, J., and Zhang, J. H. (2018). Transplanting mesenchymal stem cells for treatment of ischemic stroke. Cell Transplant 27, 1825–1834. doi: 10.1177/0963689718795424
Wang, J., Liu, H., Chen, S., Zhang, W., Chen, Y., and Yang, Y. (2020). Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell derived exosomes. Exp. Neurol. 330:113325. doi: 10.1016/j.expneurol.2020.113325
Wang, S., Khan, A., Huang, R., Ye, S., Di, K., Xiong, T., et al. (2020). Recent advances in single extracellular vesicle detection methods. Biosens Bioelec. 154:112056. doi: 10.1016/j.bios.2020.112056
Webb, R. L., Kaiser, E. E., Scoville, S. L., Thompson, T. A., Fatima, S., Pandya, C., et al. (2018). Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl. Stroke Res. 9, 530–539. doi: 10.1007/s12975-017-0599-2
Wei, J. J., Chen, Y. F., Xue, C. L., Ma, B. T., Shen, Y. M., Guan, J., et al. (2016). Protection of nerve injury with exosome extracted from mesenchymal stem cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 38, 33–36. doi: 10.3881/j.issn.1000-503X.2016.01.006
Wei, W., Wu, D., Duan, Y., Elkin, K. B., Chandra, A., Guan, L., et al. (2019). Neuroprotection by mesenchymal stem cell (MSC) administration is enhanced by local cooling infusion (LCI) in ischemia. Brain Res. 1724:146406. doi: 10.1016/j.brainres.2019.146406
Wen, S. W., Sceneay, J., Lima, L. G., Wong, C. S., Becker, M., Krumeich, S., et al. (2016). The biodistribution and immune suppressive effects of breast cancer-derived exosomes. Cancer Res. 76, 6816–6827. doi: 10.1158/0008-5472.CAN-16-0868
Wiklander, O. P. B., Nordin, J. Z., O’Loughlin, A., Gustafsson, Y., Corso, G., Mager, I., et al. (2015). Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4:26316. doi: 10.3402/jev.v4.26316
Williams, A. M., Wu, Z., Bhatti, U. F., Biesterveld, B. E., Kemp, M. T., Wakam, G. K., et al. (2020). Early single-dose exosome treatment improves neurologic outcomes in a 7-day swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 89, 388–396. doi: 10.1097/TA.0000000000002698
Witwer, K. W., and Théry, C. (2019). Extracellular vesicles or exosomes? on primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 8:1648167. doi: 10.1080/20013078.2019.1648167
Wolfers, J., Lozier, A., Raposo, G., Regnault, A., Thery, C., Masurier, C., et al. (2001). Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 7, 297–303. doi: 10.1038/85438
Wood, M. J., O’Loughlin, A. J., and Samira, L. (2011). Exosomes and the blood-brain barrier: implications for neurological diseases. Ther. Deliv. 2, 1095–1069. doi: 10.4155/tde.11.83
Xie, H., Di, K., Huang, R., Khan, A., Xia, Y., Xu, H., et al. (2020). Extracellular vesicles based electrochemical biosensors for detection of cancer cells:a review. Chinese Chem. Lett. 31, 1737–1745. doi: 10.1016/j.cclet.2020.02.049
Xie, Y., Dang, W., Zhang, S., Yue, W., Yang, L., Zhai, X., et al. (2019). The role of exosomal non-coding RNAs in cancer. Mol. Cancer 18:37. doi: 10.1186/s12943-019-0984-4
Xin, D., Li, T., Chu, X., Ke, H., Yu, Z., Cao, L., et al. (2020). Mesenchymal stromal cell-derived extracellular vesicles modulate microglia/macrophage polarization and protect the brain against hypoxia-ischemic injury in neonatal mice by targeting delivery of miR-21a-5p. Acta Biomater. 113, 597–613. doi: 10.1016/j.actbio.2020.06.037
Xin, H., Katakowski, M., Wang, F., Qian, J. Y., Liu, X. S., Ali, M. M., et al. (2017). MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 48, 747–753. doi: 10.1161/STROKEAHA.116.015204
Xin, H., Li, Y., and Chopp, M. (2014). Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 8:377. doi: 10.3389/fncel.2014.00377
Xin, H., Li, Y., Cui, Y., Yang, J. J., Zhang, Z. G., and Chopp, M. (2013a). Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 33, 1711–17145. doi: 10.1038/jcbfm.2013.152
Xin, H., Li, Y., Liu, Z., Wang, X., Shang, X., Cui, Y., et al. (2013b). MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31, 2737–2746. doi: 10.1002/stem.1409
Xu, R., Rai, A., Chen, M., Suwakulsiri, W., Greening, D. W., and Simpson, R. J. (2018). Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 15, 617–638. doi: 10.1038/s41571-018-0036-9
Yang, J., Wu, S., Hou, L., Zhu, D., Yin, S., Yang, G., et al. (2020). Therapeutic effects of simultaneous delivery of nerve growth factor mrna and protein via exosomes on cerebral ischemia. Mol. Ther. Nucleic Acids 21, 512–522. doi: 10.1016/j.omtn.2020.06.013
Yang, J., Zhang, X., Chen, X., Wang, L., and Yang, G. (2017). Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol. Ther. Nucleic Acids 7, 278–287. doi: 10.1016/j.omtn.2017.04.010
Yang, L., Han, B., Zhang, Z., Wang, S., Bai, Y., Zhang, Y., et al. (2020). Extracellular vesicle-mediated delivery of CircSCMH1 promotes functional recovery in rodent and non-human primate ischemic stroke models. Circulation 142, 556–574. doi: 10.1161/CIRCULATIONAHA.120.045765
Yang, S., Che, S. P., Kurywchak, P., Tavormina, J. L., Gansmo, L. B., Correa de Sampaio, P., et al. (2017). Detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer. Cancer Biol. Ther. 18, 158–165. doi: 10.1080/15384047.2017.1281499
Yang, T. Z., Martin, P., Fogarty, B., Brown, A., Schurman, K., Phipps, R., et al. (2015). Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in danio rerio. Pharmaceutical Res. 32, 2003–2014. doi: 10.1007/s11095-014-1593-y
Ye, Z., Zhang, T., He, W., Jin, H., Liu, C., Yang, Z., et al. (2018). Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl. Mater Interfaces 10, 12341–12350. doi: 10.1021/acsami.7b18135
Yim, N., Ryu, S. W., Choi, K., Lee, K. R., Lee, S., Choi, H., et al. (2016). Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 7:12277. doi: 10.1038/ncomms12277
Yu, Q., Zhao, Q., Wang, S., Zhao, S., Zhang, S., Yin, Y., et al. (2020). Development of a lateral flow aptamer assay strip for facile identification of theranostic exosomes isolated from human lung carcinoma cells. Anal. Biochem. 594:113591. doi: 10.1016/j.ab.2020.113591
Yuan, D. F., Zhao, Y. L., Banks, W. A., Bullock, K. M., Haney, M., Batrakova, E., et al. (2017). Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142, 1–12. doi: 10.1016/j.biomaterials.2017.07.011
Zhang, G., Zhu, Z., Wang, H., Yu, Y., Chen, W., Waqas, A., et al. (2020). Exosomes derived from human neural stem cells stimulated by interferon gamma improve therapeutic ability in ischemic stroke model. J. Adv. Res. 24, 435–445. doi: 10.1016/j.jare.2020.05.017
Zhang, H., Freitas, D., Kim, H. S., Fabijanic, K., Li, Z., Chen, H., et al. (2018). Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell. Biol. 20, 332–343. doi: 10.1038/s41556-018-0040-4
Zhang, H., Wu, J., Wu, J., Fan, Q., Zhou, J., Wu, J., et al. (2019). Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnol. 17:29. doi: 10.1186/s12951-019-0461-7
Zhang, X., Xu, Q., Zi, Z., Liu, Z., Wan, C., Crisman, L., et al. (2020). Programmable extracellular vesicles for macromolecule delivery and genome modifications. Dev. Cell. 55, 784–801. doi: 10.1016/j.devcel.2020.11.007
Zhang, Y., Chopp, M., Liu, X. S., Katakowski, M., Wang, X., Tian, X., et al. (2017). Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol. Neurobiol. 54, 2659–2673. doi: 10.1007/s12035-016-9851-0
Zhang, Z. G., Buller, B., and Chopp, M. (2019). Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 15, 193–203. doi: 10.1038/s41582-018-0126-4
Zhao, H., Li, Y., Chen, L., Shen, C., Xiao, Z., Xu, R., et al. (2019). HucMSCs-Derived miR-206-knockdown exosomes contribute to neuroprotection in subarachnoid hemorrhage induced early brain injury by targeting BDNF. Neuroscience 417, 11–23. doi: 10.1016/j.neuroscience.2019.07.051
Keywords: brain ischemia, extracellular vesicles, stroke, exosomes, bioengineered exosomes
Citation: Khan H, Pan J-J, Li Y, Zhang Z and Yang G-Y (2021) Native and Bioengineered Exosomes for Ischemic Stroke Therapy. Front. Cell Dev. Biol. 9:619565. doi: 10.3389/fcell.2021.619565
Received: 20 October 2020; Accepted: 15 February 2021;
Published: 23 March 2021.
Edited by:
Konstantin Glebov, University of Plymouth, United KingdomReviewed by:
Ellis Fok, The Chinese University of Hong Kong, ChinaCopyright © 2021 Khan, Pan, Li, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijun Zhang, emhhbmd6akBzanR1LmVkdS5jbg==; Guo-Yuan Yang, Z3l5YW5nQHNqdHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.