- 1Department of Thoracic Surgery West China Hospital, Sichuan University, Chengdu, China
- 2West China Biomedical Big Data Center, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Periodical Press, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
- 4Nursing Key Laboratory of Sichuan Province, Chengdu, China
- 5Chinese Evidence-Based Medicine Center, West China Hospital, Sichuan University, Chengdu, China
Pharmaceutical therapies are essential for esophageal cancer (EC). For the advanced EC, the neoadjuvant therapy regimen, including chemotherapy plus radiotherapy and/or immunotherapy, is effective to achieve clinical benefit, even pathological complete response. For the unresectable, recurrent, and metastatic EC, the pharmaceutical therapy is the limited effective regimen to alleviate the disease and prolong the progression-free survival and overall survival. In this review, we focus on the pharmaceutical applications in EC treatment including cytotoxic agents, molecular targeted antibodies, and immune checkpoint inhibitors (ICIs). The chemotherapy regimen is based on cytotoxic agents such as platinum-based complexes, fluorinated pyrimidines and taxenes. Although the cytotoxic agents have been developed in past decades, the standard chemotherapy regimen is still the cisplatin and 5-FU or paclitaxel because the derived drugs have no significant advantages of overcoming the shortcomings of side effects and drug resistance. The targeted molecular therapy is an essential supplement for chemotherapy; however, there are only a few targeted therapies available in clinical practice. Trastuzumab and ramucirumab are the only two molecular therapy drugs which are approved by the US Food and Drug Administration to treat advanced and/or metastatic EC. Although the targeted therapy usually achieves effective benefits in the early stage therapy of EC, the patients will always develop drug resistance during treatment. ICIs have had a significant impact on routine clinical practice in cancer treatment. The anti-programmed cell death-1 monoclonal antibodies pembrolizumab and nivolumab, as the ICIs, are recommended for advanced EC by several clinical trials. However, the significant issues of pharmaceutical treatment are still the dose-limiting side effects and primary or secondary drug resistance. These defects of pharmaceutical therapy restrain the clinical application and diminish the effectiveness of treatment.
Introduction
Esophageal cancer (EC) is a global health challenge. It ranks the seventh most common cancer incidence and the sixth most common cause of cancer-related death worldwide (Li et al., 2019; Zhang et al., 2020). The histologic types of EC are mostly composed of esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), which have distinct epidemiology and biology. ESCC are highly prevalent in the East, East Africa and South America and account for 90% of EC. EAC is more common in Western developed countries than that in developing countries (Smyth et al., 2017; Chen et al., 2019).
The treatment regimen for EC is dependent on the general status of patient and the tumor stage, mainly the TNM stage (Lordick et al., 2016). Patients with early stage tumors should be treated with endoscopic or surgical resection, whereas local advanced tumors should be treated with systemic treatment regimen. Patients that are not suitable for surgical treatment should be treated with systemic regimens including definitive chemoradiotherapy, targeted therapy, immunotherapy, and palliative treatment (Smyth et al., 2017).
Most patients seek medical attention because of a period of progressive dysphagia (Lagergren et al., 2017), therefore, radical surgery is possible in only 15–20% of all cases (Zhao et al., 2020). Moreover, for resectable locally advanced EC, the overall prognosis is poor with surgery alone (Gebski et al., 2007). For the advanced or metastasis EC, although combination therapy has prolonged overall survival, the current median survival time remains almost 1 year (Shah et al., 2017; Ho and Smyth, 2020).
Although the overall survival of EC has improved in the past decades because of medicine development (Ho and Smyth, 2020), the overall 5-year survival rate is still 20% (Zeng et al., 2015; Njei et al., 2016). The main causes of treatment failure are recurrence and metastasis concurrent with treatment resistance. In this review, we focus on the most common pharmaceutical applications for EC and its drug resistance including chemotherapy, molecular targeted therapy, and immunotherapy.
Chemotherapy
For EAC, the FLOT regimen including docetaxel, oxaliplatin, leucovorin, and fluorouracil is recognized as the standard perioperative chemotherapy regimen. The regimen including cisplatin and fluorouracil is the alternative treatment while the FLOT regimen should not be implemented (Shah et al., 2020). For ESCC, the cisplatin plus fluorouracil regimen is the standard chemotherapy (Chen et al., 2019). It can be seen that the platinum containing cytotoxic agents are still the standard regimen of chemotherapy for EC.
Platinum Complexes
Cisplatin or cis-dichloro-diammine-platinum (II) is the representative drug of platinum complex. It was first used to a cancer patient in 1971 (Lebwohl and Canetta, 1998). Since then, platinum-based cytotoxic agents are widely used in tumor treatment (Shimizu et al., 2020). However, the mechanisms that determine sensitivity and resistance to platinum drugs remain elusive (De Vries et al., 2020).
The antitumor activities of cisplatin are associated with the formation of certain kinetically stable cisplatin–DNA adducts and many platinum-based drugs have been designed and synthesized based on the classical structure-activity relationships (Yang and Wang, 1996; Wheate et al., 2010). The general platinum-based complexes (II) structure has been summarized as [PtA2X2] and platinum-based complexes (IV) structure has been summarized as [PtA2X2Y2]. In these chemical structures, the Pt means metal platinum, the A2 mean two monodentate or one bidentate amine ligand, the X2 mean two monodentate or one bidentate anionic ligand and the Y means hydroxo, chloro, or carboxylato (Galanski, 2006).
The formation of intra-strand crosslink adducts leads to an impairment of DNA expression and stability (Yang and Wang, 1996; Heringova et al., 2009). These lesions could cause mutations and have detrimental effects on replication and transcription, which are thought to be the crucial antitumor activity of platinum-based drugs and ultimately lead to cellular apoptosis (Bai L. et al., 2017). Furthermore, cisplatin is known to interfere with cellular RNA processing by binding to RNA and assisting the antitumor activity of the drug (Chapman and Derose, 2010).
The platinum-based drug not only impairs cancerous cells but also the normal cells (Kelland, 2007). The toxicity of platinum-based drugs is directly associated with the aquated of the leaving groups (Galluzzi et al., 2012) and nephrotoxicity, neurotoxicity, ototoxicity, and myelosuppression are the most common side effects (Piccart et al., 2001). In order to diminish the toxicity and side effects of cisplatin, pharmacologists are dedicated to discovering new leaving groups such as carboplatin, oxaliplatin, nedaplatin, lobaplatin, and heptaplatin (Wheate et al., 2010; Dilruba and Kalayda, 2016; Bai L. et al., 2017). However, the toxicity and side effects are still the causes of dosage range limiting in clinic, such as myelosuppression of carboplatin, neurotoxicity and gastrointestinal toxicity of oxaliplatin (Hanada et al., 2008; Zhong et al., 2020), none of the newly platinum-based analogs have a more comprehensive effect than the cisplatin (Najjar et al., 2017). Because these new platinum-based drugs are derived from a basic cisplatin structure, the defects of cisplatin are inherited. Therefore, cisplatin is still the most widely used platinum drug in clinic, and there are only three agents including cisplatin, carboplatin, and oxaliplatin which have become globally used. Another three agents including nedaplatin, lobaplatin, and heptaplatin have received approval in regional areas (Wheate et al., 2010; Dilruba and Kalayda, 2016; Bai L. et al., 2017).
Resistance in Platinum-Based Drugs
Beyond the side effects of platinum-based drugs diminishing the effectiveness of clinical practice, the resistance of them, including intrinsic or acquired resistance, also limits the clinical application. Furthermore, the severe side effects of cisplatin restrict the dosage intake and the dose delivered to patients can be sub-lethal to tumors, which means that it could develop resistance in further treatment.
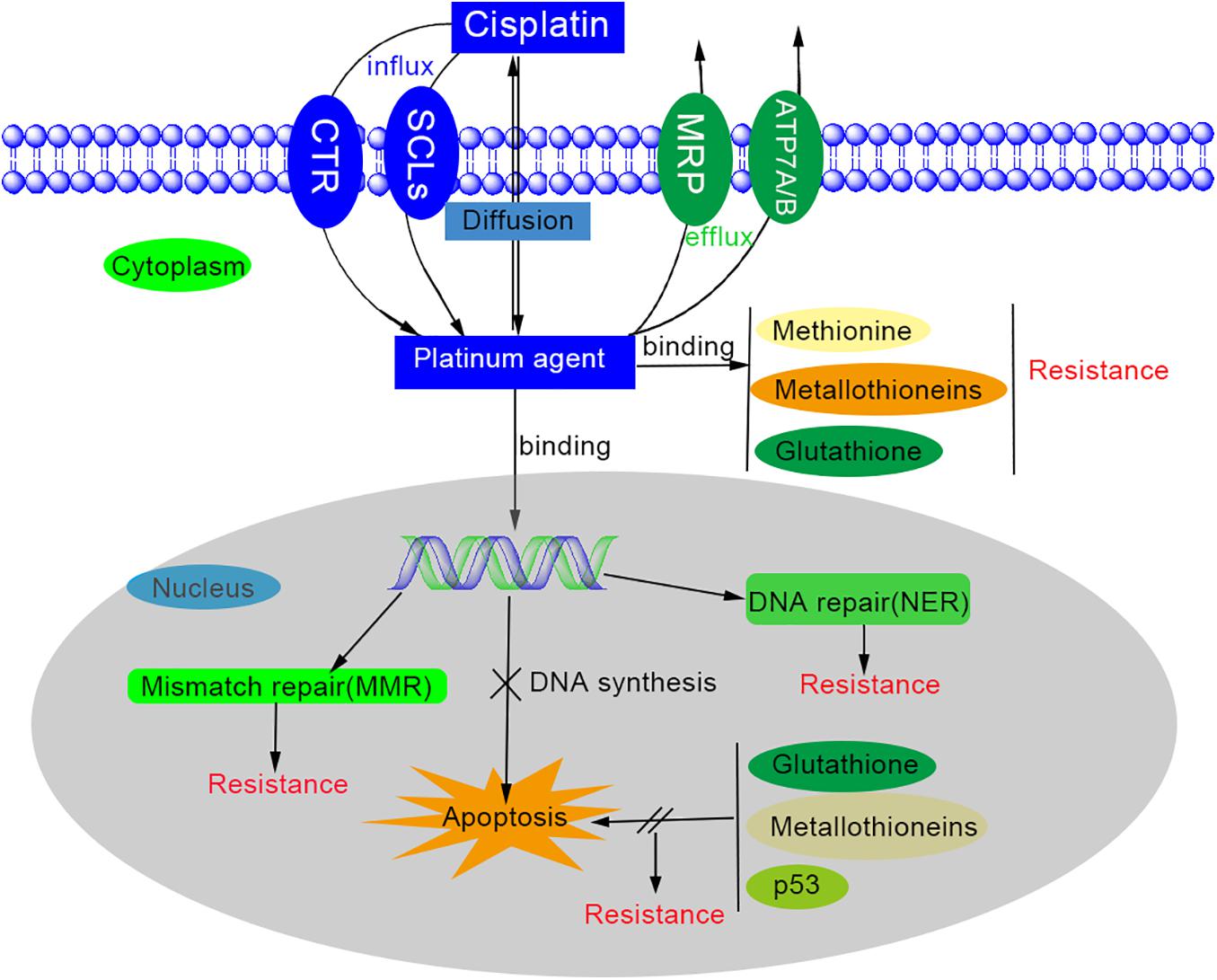
However, the underlying mechanisms are still far from elucidated. The main mechanisms of platinum-based drug resistance are possibly associated with changed cellular platinum accumulation, increased detoxification system, increased DNA repair, decreased apoptosis, and autophagy (Figure 1) (Kehe and Szinicz, 2005; Wheate et al., 2010; Zhou et al., 2020).
First, to accumulate the platinum antitumor agents inside the cells is the necessary process for cytotoxicity, so platinum resistance would generate while the platinum agent influx decreased and/or efflux increased. The way that platinum enters the cell is thought to be passive diffusion and through gated channels (Gately and Howell, 1993; Puckett et al., 2010). There are multiple transporters involved in platinum influx/efflux (Zhou et al., 2020), such as solute carrier superfamily (SLCs) of membrane transporters (Perland and Fredriksson, 2017), copper transporter 1/2(CTR1/2) (Holzer and Howell, 2006), copper-transporting ATPases (ATP 7A/7B) (Gupta and Lutsenko, 2009), multidrug resistance protein subfamily (MPR) (Yaneff et al., 2019) etc. The organic cation transporters and copper transporter are related to the influx, while ATP 7A/7B and MPR2 are involved in the isolation and efflux of platinum agents (Zhou et al., 2020). However, the mechanism of uptake of platinum-based drugs is not elucidated (Hall et al., 2008). Secondly, platinum agents can be deactivated by binding to detoxification components such as glutathione (GSH), methionine, metallothioneins, and other cysteine-rich proteins. This binding depletes cytoplasmic antioxidant reserves and results in the oxidative stress in cells. On the other hand, while the cytoplasmic nucleophiles level is elevated, the available reactive cisplatin would be diminished and thus contribute to cisplatin resistance (Dilruba and Kalayda, 2016; Zhou et al., 2020). Thirdly, the DNA repair process is significantly increased in platinum resistance cells (Wynne et al., 2007). Although platinum-based agents can induce cytotoxicity by forming the platinum-DNA adducts, the DNA lesion could be repaired by the DNA repair process (Zhou et al., 2020). One of these DNA repair processes is nucleotide excision repair (NER) system, which can remove most intra-strand crosslinks through reconstituted genetic integrity by excising damaged nucleotides and synthesizing DNA (Roos and Kaina, 2013). The expression level of excision repair cross-complementing (ERCC) members and breast cancer susceptibility genes (BRCAs) also have significant influence on platinum resistance (Dann et al., 2012; Foulkes and Shuen, 2013; Muggia and Safra, 2014). Fourthly, the dysfunction of apoptosis may be one of the causes of platinum drug resistance. The apoptosis would be activated while the DNA repair fails or excessive DNA lesions occurs after platinum agents and mitochondria will generate surplus reactive oxygen species (ROS) to kill the cells. However, this reaction may be neutralized by glutathione and metallothioneins. The platinum-resistant cells usually have a higher threshold to trigger apoptosis due to the defection of mitochondrial signaling and the overexpression of anti-apoptotic proteins. Many factors contribute to the regulation of apoptosis, including the signal pathways (such as MAPK/ERK, PI3k/AKT, NF-kB, Nrf2, p53), the tumor microenvironment (TME) (including hypoxia-inducible factor, HIF), cancer-associated fibroblasts (CAFs), and epigenetic regulation (Ramadoss et al., 2017; Zhou et al., 2020). Last but not least, autophagy was observed to be increased in platinum-resistant cells after platinum-based drug treatment (Wang Z. et al., 2019). Autophagy is a self-digestion process and essential for nutrient regulation, intracellular quality control and homeostasis (Mizushima and Klionsky, 2007). If persistent or excessive autophagy is carried out, it will trigger cell death. When autophagy activity is inhibited by autophagy inhibitors, interference of regulatory elements, or non-coding RNAs, it has been proven to diminish platinum resistance (Zhou et al., 2020).
However, the mechanisms of platinum resistance are far from elucidated and the dose-liming side effects and cytotoxicity still hinder clinical application. Therefore, the chemotherapy is mostly concurrent with two to three cytotoxic agents to reduce dose-limiting side effects and toxicity of platinum complexes. The most common concurrent cytotoxic agents in EC are fluorinated pyrimidines (5-fluorouracil) and taxanes (paclitaxel or docetaxel).
Fluorinated Pyrimidines
5-Fluorouracil (5-FU) is the representative drug of fluorinated pyrimidine, the chemical formula of which is C4H3FN2O2. It was first synthesized as the antitumor agent in 1957 by modified a pyrimidine chemical uracil, in which the hydrogen at the carbon-5 position of the pyrimidine ring was replaced by a fluorine atom (Alvarez et al., 2012).
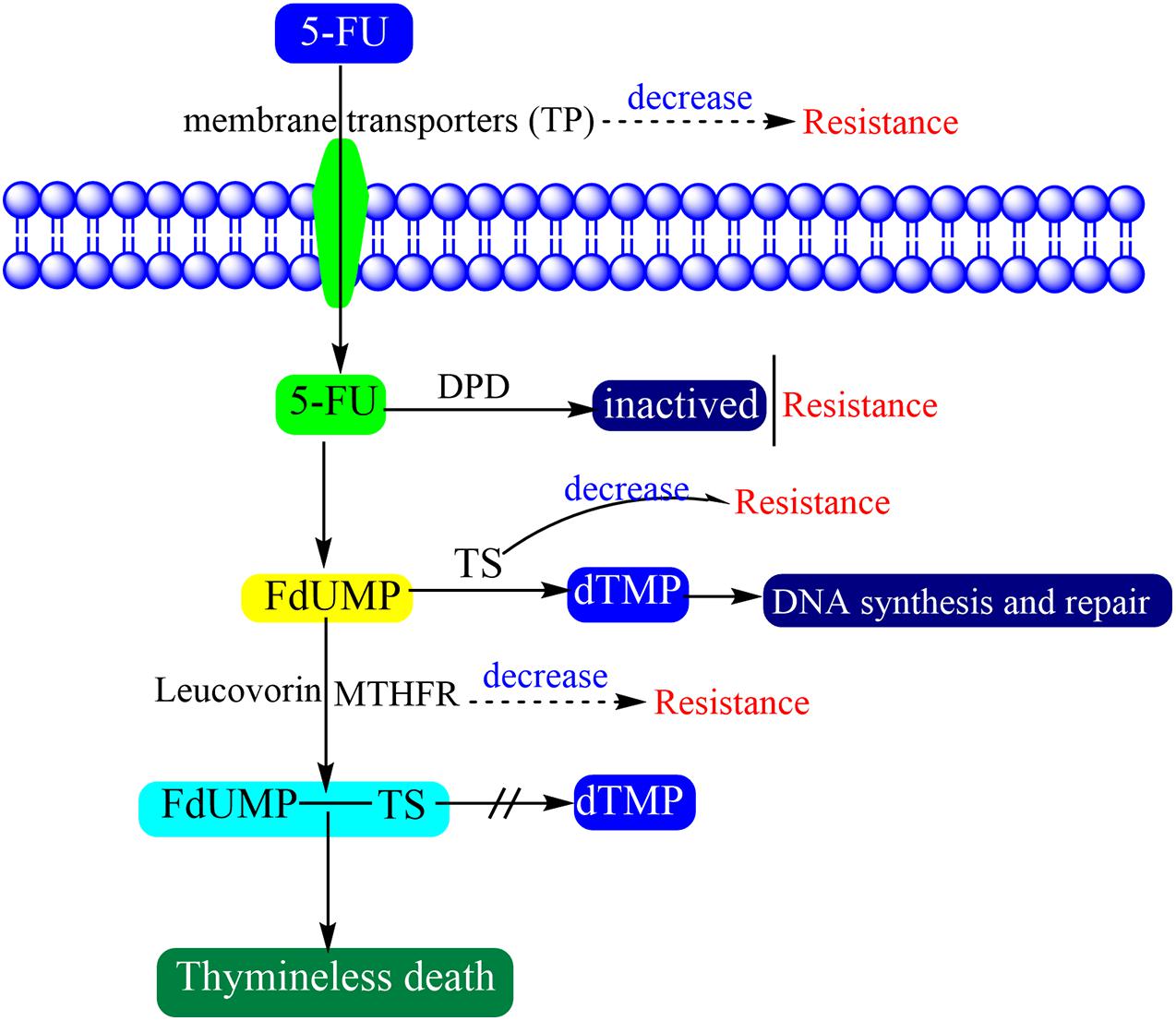
The biological activities of 5-FU including antitumor activity and systemic toxicities remain only partly characterized. A primary theory is the lethal synthesis in which a biological metabolism can transform a relatively non-toxic metabolite into a more toxic form (Weckbecker, 1991). 5-FU interferes with DNA synthesis and mRNA translation by being misincorporated into RNA and DNA in place of uracil or thymine, that lead to cytotoxicity and cell death (Noordhuis et al., 2004; An et al., 2007). While 5-FU enters the human body, most of it is inactivated by dihydropyrimidine dehydrogenase (DPD), and further converted to fluorodeoxyuridine monophosphate (FdUMP). The FdUMP could form a stable complex with thymidylate synthase (TS) in the presence of methylene tetrahydrofolate reductase (MTHFR) and then inhibits the production of deoxythymidine monophosphate (dTMP). While thymidylate synthase is inhibited, the cancer cells which reliant on the de novo thymidylate pathway undergo thymineless death (Houghton et al., 1997). Because dTMP is essential for DNA repair and replication, its depletion therefore causes cytotoxicity (Zhang et al., 2008). In clinical application, the combination of the folate analog Leucovorin and 5-FU can promote the clinical efficacy because it can promote thymidylate synthase ternary complex formation (Wolmark et al., 1999).
Resistance in Fluorinated Pyrimidines
There are many defects of 5-FU, including systemic toxicities because of non-specific cytotoxicity for tumor cell, loss of efficiency due to poor distribution to tumor sites, and severely limited efficacy because of drug resistance (Alvarez et al., 2012). The serious systemic toxicities of 5-FU are commonly seen in gastrointestinal and hematopoietic effects (Gmeiner, 2020).
There are multiple factors that may be responsible for 5-FU resistance (Figure 2) (Zhang et al., 2008). Antitumor drug resistance usually concentrates on alteration of drug influx and efflux, enhancement of drug deactivation, and mutation of the drug target (Longley and Johnston, 2005). The factors which affect the drug transition would affect the activation of 5-FU. 5-FU and other nucleic acid dugs present cytotoxicity only when pass through the cell membranes. However, the water-soluble character of 5-FU makes it so that it cannot pass through cell membranes by diffusion. Therefore, the specific nucleic acid membrane transporters are needed to help cells to absorb these drugs (Kong et al., 2004). Thymidine phosphatase (TP) is the main form of pyrimidine nucleoside phosphatase in humans, which helps cells to survive, promotes angiogenesis and inhibits apoptosis (Toi et al., 2005). When tumors present high levels of TP expression, they show more sensitivity to 5-FU (Soong et al., 2008).
Some enzymes are involved in the conversion and activation of 5-FU. DPD is an initial and rate-limiting factor in the catabolism of uracil and thymine which mediates the conversion of 5-FU to dihydrofluorouracil (DHFU) (Heggie et al., 1987; Zhang et al., 2008). The 5-FU resistance will generate while DPD activity increases in cancer patients, because the 5-FU will be converted to non-pharmacologically active metabolites before activation (Lu et al., 1993; Reti et al., 2010). TS is a key enzyme that catalyzes the conversion of dUMP to dTMP and is extremely important for DNA synthesis and repair. The TS loss will hamper cell proliferation and result in cell death (Costi et al., 2005). TS is overexpressed in most tumors and its high expression is an important factor of 5-FU resistance (Longley et al., 2003). MTHFR participate the conversion of 5-FU to a stable ternary complex which results in TS inhibition. The decrease in MTHFR activity finally inhibits the formation and stabilization of the ternary complex. Therefore, patients with a mutant genotype associated with decreased MTHFR activity are more sensitive to 5-FU (Lurje et al., 2008).
Autophagy and many signaling pathways also affect antitumor activity of 5-FU. Previous study has showed that inhibiting autophagy activity could enhance antitumor activity of 5-FU in colorectal cancers (Sasaki et al., 2012). Many signaling pathways involved 5-FU resistance, including Hippo/YAP, Wnt/β-catenin, Notch signaling pathway, Hedgehog, NF-kB signaling pathway, and so on (Xie et al., 2020).
To overcome the shortcomings of 5-FU, many fluorinated pyrimidines have been synthesized and are under biological evaluation. However, the DPD-inhibiting oral fluoropyrimidines such as eniluracil and 5-chloro-2,4-dihydroxypyridine (CDHP) have failed to improve outcomes for patients with metastatic colorectal cancer (Schmoll, 2003). The DPD inhibitors had combined with orally bioavailable fluorinated pyrimidine such as capecitabine or tegafur to verify the similar effect to continuous intra-venous infusion of 5-FU and did not prove molecules advantageous to continuous intra-venous infusion (Kobayakawa and Kojima, 2011; Aguado et al., 2014). Furthermore, more oral fluoropyrimidine such as S1 (tegafur/gimeracil/oteracil) are in progress phase I clinical trials for tumors, including EC (Ajani et al., 2020; Hosoda et al., 2020). However, up to date, the 5-FU is still the widest used fluorinated pyrimidine for cancer treatment. Many adjuvant drugs to promote activity of 5-FU, such as Non-steroidal anti-inflammatory drugs (NSAIDs), chloroquine (CQ), disulfiram, vitamin D analogs (VDAs), and Ca2+-activated G protein-coupled calcium-sensitive receptor (CaSR) (Xie et al., 2020). However, these agents are far from application in clinical practice.
Taxanes (Paclitaxel or Docetaxel)
Taxanes are naturally occurring compounds and belongs to genus of Taxus. Paclitaxel (PTX) is a key member of taxane family and is a semisynthetic plant alkaloid that restabilizes the microtubule cytoskeleton against depolymerization (Tan et al., 2006). PTX has been widely used to treat a number of cancers and has been considered as a successful antitumor agent since it was first approved for the treatment of ovarian cancer in 1992 (Zhu and Chen, 2019). Docetaxel (DTX) is another widely used taxane for antitumor therapy, which was first found in 1990 (Sohail et al., 2018). PTX is a tricyclic diterpenoid compound and has a chemical formula of C47H51NO41.
Microtubules are the key members of the cytoskeleton, which play a notable role in various biological processes including maintenance of cell shape, molecular signaling pathways, conducing to the transportation of cell organelles, and production of the mitotic spindle to ensure the progression of the cell cycle (Jordan and Wilson, 2004; Magnani et al., 2009). PTX has a different antitumor action compared with other common tubulin-binding antitumor drugs which exert antitumor activity through impeding the assembly of tubulin into microtubules. It has a distinctive antitumor function which enhances the assembly of tubulin into microtubules and the production of non-functional microtubules (Weaver, 2014; Lee and Tan, 2018). In the physiological condition, there is a balance in the process of entering and eliminating tubulin proteins from microtubules. Upon effects of PTX or DTX, this balance is interrupted and the microtubules receive a stabilized form (Yvon et al., 1999). This results in cell cycle arrest, inhibition of mitosis, inhibiting growth and proliferation of cancer cells and apoptotic cell death (Weaver, 2014; Ashrafizadeh et al., 2020b).
Resistance in Taxanes
Although the taxanes are highly active cytotoxic antitumor drugs, the serious adverse drug reactions and emergence of drug resistance still affect the clinical application.
The effects of antitumor activity could enhance in relation to drug dose increasing, however, the dose increasing not only promotes the drug’s effectiveness but also increases cytotoxicity (Ashrafizadeh et al., 2020a). The common toxicities of the drugs manifests in hair loss, hypersensitivity reactions, hematological toxicity (principally neutropenia), arthralgia, myalgias, and peripheral neuropathy (Markman, 2003). Fortunately, with appropriate management, both PTX and DTX generate easily treatable side effects and it would be a rare patient who is unable to proceed with taxane treatment due to unacceptable toxicity (Markman, 2003; Ashrafizadeh et al., 2020b).
A lot of molecular pathways and mechanisms are involved in the taxane resistance of cancer cells. Among the molecular mechanisms, autophagy is the most widely known mechanism related to PTX resistance. A study showed that the inhibition of autophagy can enhance antitumor activity of PTX. The self-digestion mechanism can stimulate cancer cells resistance into PTX chemotherapy. In this progress, non-coding RNAs is the most common molecular pathway that dually mediate or inhibit PTX resistance (Datta et al., 2019; Yang et al., 2020). Integrin subunit α2 (ITGA2) is an oncogene factor which promotes metastasis and growth ability of cancer cells and it is thought to reduce sensitivity of cancer cells of PTX through activation of Akt/FoxO1 signaling pathway (Ma et al., 2020; Qin et al., 2020). Furthermore, high mobility group box 1 (HMGB1) can activate c-Myc oncogene to induce PTX resistance (Jung et al., 2020; Lei et al., 2020).
To overcome the drug resistance and dose-limiting side effects, many studies have been proceeding. Many plant derived natural compound such as nobiletin (Feng et al., 2020), quercetin (Li et al., 2018), and resveratrol (Ozturk et al., 2019) have been used to inhibit PTX resistance and increase its antitumor activity in cancer cells. Irinotecan is a topoisomerase I inhibitor and proven to have benefits of survival and quality of life in 5-FU refractory colorectal cancer (Liu et al., 2020). Previous studies also considered it as a second- or third-line chemotherapy for EC which were refractory to platinum plus fluoropyrimidine chemotherapy (Wang et al., 2016).
For both EAC and ESCC, either adjuvant or neoadjuvant chemotherapy was proven to be superior to surgery alone in overall survival (Ando et al., 2003; Ychou et al., 2011). For the pathological node positive EC which received neoadjuvant chemotherapy or chemoradiotherapy, adjuvant chemotherapy showed a survival benefit compared to those that did not receive chemotherapy after esophagectomy (Samson et al., 2018). Platinum plus fluoropyrimidines (FP) or taxanes are an essential chemotherapy regimen for EC. Moreover, radiotherapy with FP regiment followed by surgery also showed superior overall survival compared with surgery alone (Tepper et al., 2008). Taxanes are also frequently used in chemotherapy for EC as a second-line chemotherapy for recurrent and metastasis EC. Many clinical trials indicate that paclitaxel and docetaxel are effective for both ESCC and EAC in extending median survival time (Muro et al., 2004; Ford et al., 2014). However, chemotherapy agents are exhibiting considerable cytotoxic effects including normal cells and the dose-limiting side effects restrict the chemotherapy’s effectiveness. Furthermore, the emergence of drug resistance is still the challenge for clinicians and drug developers and the effective approach to eliminate drug resistance and side effects has not been proven (Xie et al., 2020).
To overcome these limited therapeutic options and promote overall survival and quality of life, the personalized targeted therapies are designed based on the molecular characterization of EC (Maeda and Ando, 2019).
Molecular Targeted Therapy
Despite the EC being mostly categorized into ESCC and EAC, the treatment options are largely similar both in the different histological types (Lagergren et al., 2017). However, the cellular and molecular data suggest that different histological types represent different genomic characterization. ESCCs closely resemble head and neck cancers while EACs more resemble gastric cancers (Cancer Genome Atlas Research Network, Analysis Working Group, Asan University, BC Cancer Agency, Brigham and Women’s Hospital, Broad Institute, et al., 2017).
The molecular characterization of EAC is divided into four etiological/genetic subtypes based on gastric adenocarcinoma molecular characterization classification (Cancer Genome Atlas Research Network, 2014): (1) EBV-associated tumors; (2) Microsatellite instability (MSI) tumors commonly with PIK3CA, EGFR and human epidermal growth factor receptor 2 (HER2) mutations; (3) Genomically stable tumors, (4) Chromosomally instability (CIN) tumors with TP53 mutations as well as RTK/RAS, VEGFR, and p110 amplifications (Barsouk et al., 2019). In these genomic subtypes, the MSI-high and EBV subtypes have shown great responsiveness to immune checkpoint inhibitors (ICIs), such as pembrolizumab (Kim et al., 2018; Le et al., 2018; Shitara et al., 2018). Only the transtuzumab have positive treatment responses in the CIN subtype and the other targeted therapies such as the cMET inhibitor, FGFR inhibitor, PARP inhibitor have failed (Shah et al., 2016; Bang et al., 2017; Van Cutsem et al., 2017). Although the EGFR tyrosine kinase inhibitors gefitinib did not meet significant overall survival benefits compared with a placebo in unselected EC patients, partial patients achieved disease control and benefits in progression-free survival (Dutton et al., 2014; Petty et al., 2017).
Despite the ESCC and EAC being similar to the responsiveness of chemotherapeutic agents, ESCC is significantly distinct from EAC at the genomic level (Ilson and Van Hillegersberg, 2018). However, the differentiated epigenetic alterations of growth advantage in ESCC are still not elucidated (Kang et al., 2015). The ESCC genomic characterization are divided into three molecular subtypes by The Cancer Genome Atlas Research Network: (1) the alteration in NrF2 pathway, which regulates adaptation to oxidative stressors including some carcinogens and some chemotherapy agents; (2) higher rates of mutation of NOTCH1 or ZNF750, more frequent inactivating alterations of KDM6A and KDM2D, CDK6 amplification, and inactivation of PTEN or PIK3R1; (3) No evidence for genetic deregulation of the cell cycle and only a few of TP53 mutations (Cancer Genome Atlas Research Network, Analysis Working Group, Asan University, BC Cancer Agency, Brigham and Women’s Hospital, Broad Institute, et al., 2017). Unfortunately, only a few cancer drivers are targetable and the druggability of a target is still a research question. The most approved targeted drugs in clinic are directed against kinases and some of them have been used to treat ESCC (Kang et al., 2015).
Many oncogenes and tumor suppressor genes have been revealed as promising targets for targeted therapy and immunotherapy. These agents usually inhibit the vascular endothelial growth factor (VEGF) signaling or receptor tyrosine kinases (RTK), such as the EGFR, erb-b2 receptor tyrosine kinase 2 (ERBB2 or HER2), and MET (N-methyl-N0-nitroso-guanidine human osteosarcoma transforming gene) (Figure 3) (Ilson and Van Hillegersberg, 2018).
EGFR Inhibition
It is estimated that about 15–20% of advanced gastric and gastroesophageal junction (GEJ) cancers have overexpression or amplification of HER2 (Van Cutsem et al., 2015) and 12–41% in ESCC cases (Gonzaga et al., 2012; Zhan et al., 2012).
Cetuximab, as an anti-EGFR monoclonal chimeric antibody, is proven useful for head and neck cancers and colorectal cancer with the wild-type RAS gene. A phase 2 clinical study showed that cetuximab can be safely combined with standard chemotherapy and may increase the overall response rate, median progression-free survival, and median overall survival in advanced ESCC (Lorenzen et al., 2009). Moreover, another phase 2 clinical trial showed benefits of the addition of cetuximab to standard preoperative chemoradiotherapy in locally advanced ESCC (Brenner et al., 2019). However, it did not show additional benefit compared to chemotherapy alone for advanced gastric cancer (Lordick et al., 2013).
Gefitinib is EGFR tyrosine kinase inhibitor and a phase 3 trial did not show overall survival benefits for previous treated advanced EC including squamous-cell cancers (Dutton et al., 2014).
Lapatinib is a dual EGFR and HER2 tyrosine kinase domain. Some studies showed that the lapatinib combined with chemotherapy were efficacious for advanced ESCC (Reynolds et al., 2017; Guo et al., 2018). However, these studies are just in laboratory and phase 1 studies and it is far from being verified in patients.
Nimotuzumab is a recombinant humanized monoclonal antibody. It inhibits the EGFR-dependent intracellular signaling pathway via binding to the extracellular domain of EGFR (Hirano and Kato, 2019). Many phase 2 clinical trials and retrospective studies had showed that in combination of nimotuzumab to chemotherapy or chemoradiotherapy could achieve effective benefits for advanced and metastatic ESCC (Saumell et al., 2017; Jing et al., 2019). Pertuzumab is a humanized monoclonal HER2-targeted antibody which binds to a different epitope on the HER2 receptor protein than trastuzumab (Shitara et al., 2020). However, a phase 3 trial (JACOB) showed that adding pertuzumab to trastuzumab and chemotherapy did not significantly improve overall survival in patients with previously untreated HER2-positive metastatic gastric or GEJ cancer (Shitara et al., 2020).
Panitumumab as an anti-EGFR antibody did not promote survival for first-line chemotherapy for gastroesophageal adenocarcinomas in a phase 3 trial (REAL3) (Waddell et al., 2013). Moreover, it did improve survival and substantially increased toxicity for non-resectable, advanced, or metastatic ESCC in a phase 3 trial (POWER) (Moehler et al., 2020).
Trastuzumab is a recombinant humanized monoclonal antibody directed against HER2. In a phase 3 trial (ToG A), It was approved for the first-line treatment combined with chemotherapy for the metastatic, gastric, or GEJ cancers which have HER2 overexpression (Bang et al., 2010). However, many patients were still progressing under this regimen (Li and Li, 2016) and there were no clinical benefits for further trastuzumab utilization while the disease progressed (Makiyama et al., 2018). Trastuzumab emtansine (T-DM1) is a microtubule inhibitor, which conjugate monoclonal antibody agent trastuzumab and cytotoxic agent emtansine (DM1). However, the clinical trial showed that it was not superior to taxane as the second-line therapy for the previous treatment HER2-positive locally advanced or metastatic gastric or GEJ adenocarcinoma (Thuss-Patience et al., 2017).
VEGF Inhibition
VEGFR inhibitors are largely used in gastrointestinal cancer including gastric and GEJ adenocarcinomas (Rajabi and Mousa, 2017).
Bevacizumab is a monoclonal antibody targeting VEGF-A which is mediated by two tyrosine kinase receptors, VEGFR-1 and VEGFR-2. Although it is an efficient treatment for several malignancies in combination with chemotherapy including colorectal cancer and lung cancer, it was not proven superior in overall survival time compared to chemotherapy for advanced gastric or GEJ adenocaecinoma (AVAGAST) (Ohtsu et al., 2011).
Nintedanib is a multikinase inhibitor that potently inhibits VEGFR1-3, FGFR1-3, and PDGFRa/b. A phase 2 trial showed that nintedanib did not have significant benefits in disease progression of first-line chemotherapy or metastatic esophagogastric adenocarcinoma compared with VEGFR2 inhibition alone and it has no value for the further development of EC (Won et al., 2019).
Ramucirumab is a fully humanized monoclonal antibody and is designed to inhibit VEGFR-2 via ligand binding which prevents VEGF ligands binding to VEGFR-2 epitopes (Spratlin et al., 2010). A double-blind, randomized phase 3 trial (RAINBOW) showed that there were significant benefits in ramucirumab plus paclitaxel group than compared to the paclitaxel group including the overall survival, progression-free survival, and therapy response rate (Wilke et al., 2014). For the patients who underwent first-line treatment with transtuzumab, paclitaxel plus ramucirumab is recommended as a second-line therapy regimen regardless of HER2 expression (Wilke et al., 2014). There are many other VEFG inhibitors in the clinical trial with no exciting results for gastric or GEJ adenocarcinoma, such as apatinib (Li et al., 2016), regorafenib (Pavlakis et al., 2016), lapatinib (Hecht et al., 2016) et al.
MET Inhibition
MET is a proto-oncogene which encodes a receptor tyrosine kinase c-MET. Activation of c-MET is ordinarily indispensable to cell function such as morphogenesis, scattering and motility, cell proliferation, and protection from apoptosis (Mo and Liu, 2017). Rilotumumab is an inhibitor of the MET ligand hepatocyte growth factor. However, a phase 3 trial of chemotherapy plus rilotumumab as the first-line therapy for the advanced MET-positive gastric or GEJ cancer did not increase the overall survival time (Catenacci et al., 2017). Moreover, another phase 3 trial of the MET inhibitor onartuzumab which plus chemotherapy for HER2-negative and MET-positive advanced gastroesophageal adenocarcinoma also did not show significantly improved clinical benefits compared with chemotherapy (Shah et al., 2017).
Despite recent advances in genomic drivers of EC, there are only few targeted therapies available in clinical practice. Trastuzumab, ramucirumab, and pembrolizumab are the only three molecular therapy agents approved by the US Food and Drug Administration (FDA) for treatment of advanced and/or metastatic ECs (Fatehi Hassanabad et al., 2020).
Resistance in Targeted Therapy
Although targeted therapy is better tolerated than traditional chemotherapy, it does produce toxicity based on several main mechanisms. The common side effects include hypertension, rash, diarrhea, hypothyroidism, proteinuria, depigmentation, and hepatotoxicity. Either antibodies or small molecular kinase inhibitors, the same target may have similar side effects. VEGFR kinase inhibitors usually cause hypertension and EGFR antibodies and kinase inhibitors usually cause rash (Liu and Kurzrock, 2014; Kang et al., 2015).
The intratumoral, intermetastatic, intrametastatic, or interpatient heterogeneities are related to various combinations of drivers and pathways. These heterogeneities may explain how the different patients have different treatment responses and even resistance under the same treatment and the favorable response patients initially could develop resistance over time (Kang et al., 2015). It is considered that the carcinogenesis is a successive new mutation which is driven by natural selection. Correspondingly, radiotherapy and targeted therapy may be the artificial selection which has a potent source to alter clonal dynamics. Therefore, the antitumor therapy may induce drug resistance (Greaves and Maley, 2012). In fact, targeted therapy is associated with a high rate of resistance at the beginning of the targeted therapy used in clinic (Kang et al., 2015). Patients will always develop resistance during this treatment (Merz et al., 2020).
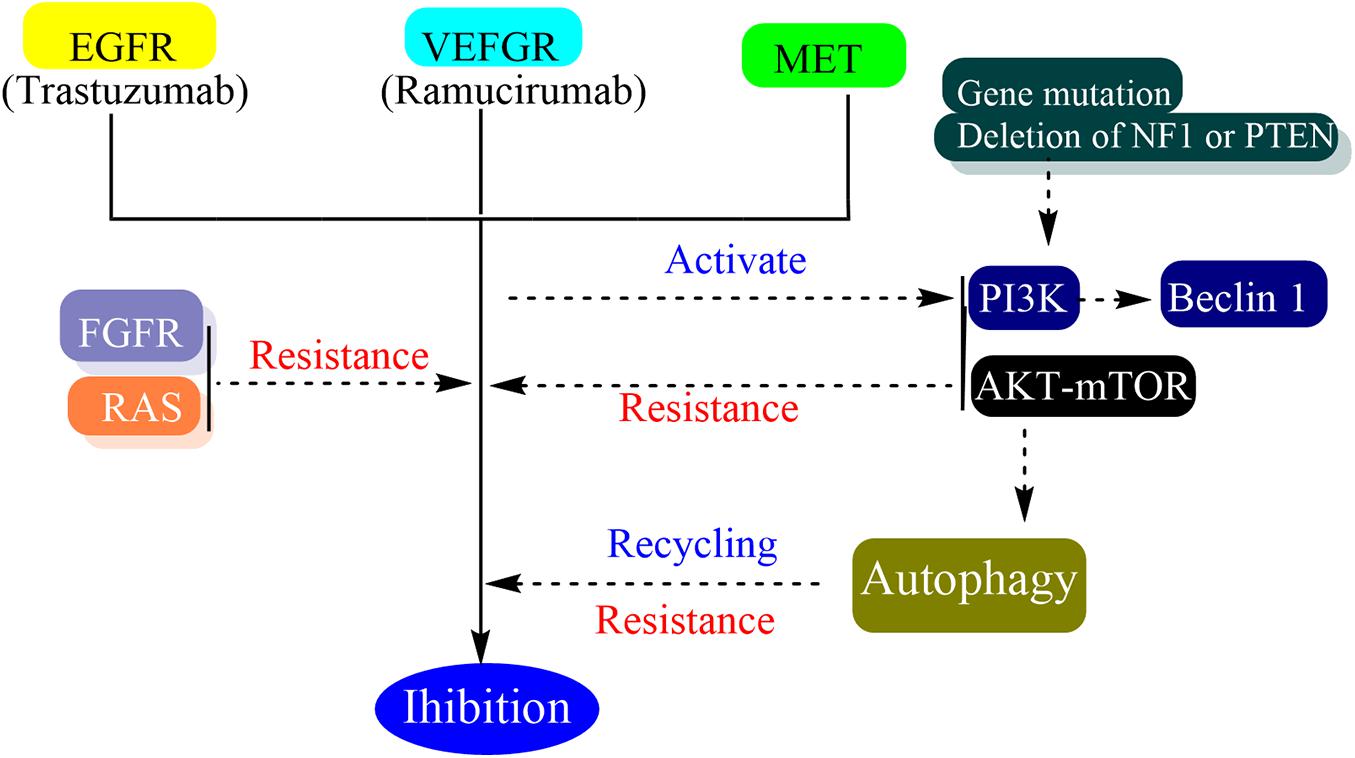
Autophagy can contribute to drug resistance because autophagy contributes to cell homeostasis by eliminating damaged organelles and its activation can mitigate metabolic, oxidative, and endoplasmic reticulum stresses (Das et al., 2012). In the process of targeted therapy resistance, autophagy has been shown to play an important role in many different cancer types (Mizushima, 2018). Autophagy is involved in the recycling of some receptors which finally reduce the efficacy of targeted therapy and the cells which are deficient in autophagy are more sensitive to target therapies (Janser et al., 2019). The targeted therapy can trigger autophagy by several mechanisms such as activation of Beclin 1 through class III PI3K, induction of oxidative and endoplasmic reticulum stress, and alteration of AKT-mTOR pathway (Vera-Ramirez et al., 2018). In vitro, trastuzumab has been shown to induce autophagy and the basal autophagy of cell line which present intrinsic or acquired resistance to trastuzumab are increased (Chen et al., 2016). Although autophagy inhibitors showed the effect of reverting the resistance and increasing drug affects in vitro and vivo, the different targeted therapy blocking different pathways in several cancer types would activate a similar response to promote drug resistance (Mele et al., 2020).
Besides autophagy, many other resistance mechanisms have been identified including alteration of the drug target, alterations in upstream and downstream effectors resulting in pathway reactivation and bypass mechanisms (Groenendijk and Bernards, 2014). The activation of the PI3K/AKT/mTOR pathway is one of the bypass pathways to targeted therapy resistance. The increased PI3K signaling can be due to loss of function through gene mutations or deletion of NF1 or PTEN, or the activation of a wide range RTKs, EGFR, FGFR1, PGFR-β etc. (Zuo et al., 2018). Moreover, the interaction between tumor cells and microenvironment is also an important factor in drug resistance (Ahmed and Haass, 2018; Mourah et al., 2020).
After a certain period of trastuzumab treatment, almost all patients will develop drug resistance even if the primary resistance is very rare in patients in HER2 overexpression. The common mechanism of trastuzumab resistance is that the selection of HER2 will not amplify clones (Piro et al., 2018; Cavaliere et al., 2019). There are many mechanisms of primary or acquired resistance to anti-HER2 therapies, including impaired drug binding to HER2, constitutive activation of signaling pathways parallel or downstream of HER2, metabolic reprogramming or reduced immune system activation. However, only a few of them have been validated in clinical series (Vernieri et al., 2019).
Fibroblast growth factor receptor (FGFR) pathway activation was considered to be one of the mechanisms related to various targeted therapeutic resistance including EGFR TKIs (Luo et al., 2018). The FGF family has four tyrosine kinase receptors and is involved in cellular growth and tumor angiogenesis (Mossahebi-Mohammadi et al., 2020). FGF9 has a unique affinity to FGFR3 and the overexpression of FGFR3 may be responsible for transtuzumab resistance (Hecht et al., 1995; Touat et al., 2015). Bemarituzumab is a humanized monoclonal antibody specific to the human FGFR2b receptor. It demonstrated monotherapy clinical activity in the overexpression of FDFR2b late-line gastric cancer. However, there is no phase 3 trial to verify its effectiveness (Catenacci et al., 2019). Dovitinib is a selective FGFR3 inhibitor. While the trastuzumab-resistant murine models were treated with it, the tumor burdens were more reduced and the overall survival rates were longer than the control group (Piro et al., 2016). Pemigatinib is a selective, potent, oral inhibitor of FGFR1, 2, and 3. A phase 2 trial which intended to assess the safety and activity of pemigatinib in HER2 trastuzumab-resistant gastric or GEJ cancer showed that trastuzumab-resistance may benefit from the utilization of pemigatinib (Merz et al., 2020).
Additional mutations in receptor tyrosine kinases, RAS, and PI3K pathways appear to the mechanism of both intrinsic and acquired resistance to HER2 inhibitor. For patients with these co-alterations, there is a lower benefit from trastuzumab treatment and it has a shorter progression-free survival (Janjigian et al., 2018). The combination with anti-angiogenesis trastuzumab and anti-EGFR ramucirumab has a good experience in durations of response in esophagogastric cancer. It is considered that the resistance inducing by angiogenesis and HER2 signaling may be reversed with the inhibition of the angiogenesis pathway. However, the bevacizumab combined with cetuximab or panitumumab received disappointing results in the CAIRO-2 and PACCE studies (Hecht et al., 2009; Tol et al., 2009). Furthermore, the combination with HER2 inhibitor and VEGFR inhibitor were associated with higher rates of side effects, even a detrimental effect on survival.
In a short, although molecular-targeted therapy is widely used in other solid tumors such as breast, leukemia, colorectal, lung, and ovarian cancers (Lee et al., 2018), it has limited benefit in EC treatment because of intrinsic resistance or acquired resistance.
Immunotherapy
Immunotherapy has entered a new era in cancer treatment (Dong et al., 2018; Peinemann et al., 2019; Zhao and Huang, 2020; Zhu et al., 2020). A lot of immune modulatory strategies have been developed in the past few years, including ICIs, adoptive cellular therapy, cancer vaccines, oncolytic viruses, and so on. Among these, ICIs have greatly impacted on routine clinical practice which has achieved unprecedented results in many tumors (Dong et al., 2018; Ribas and Wolchok, 2018).
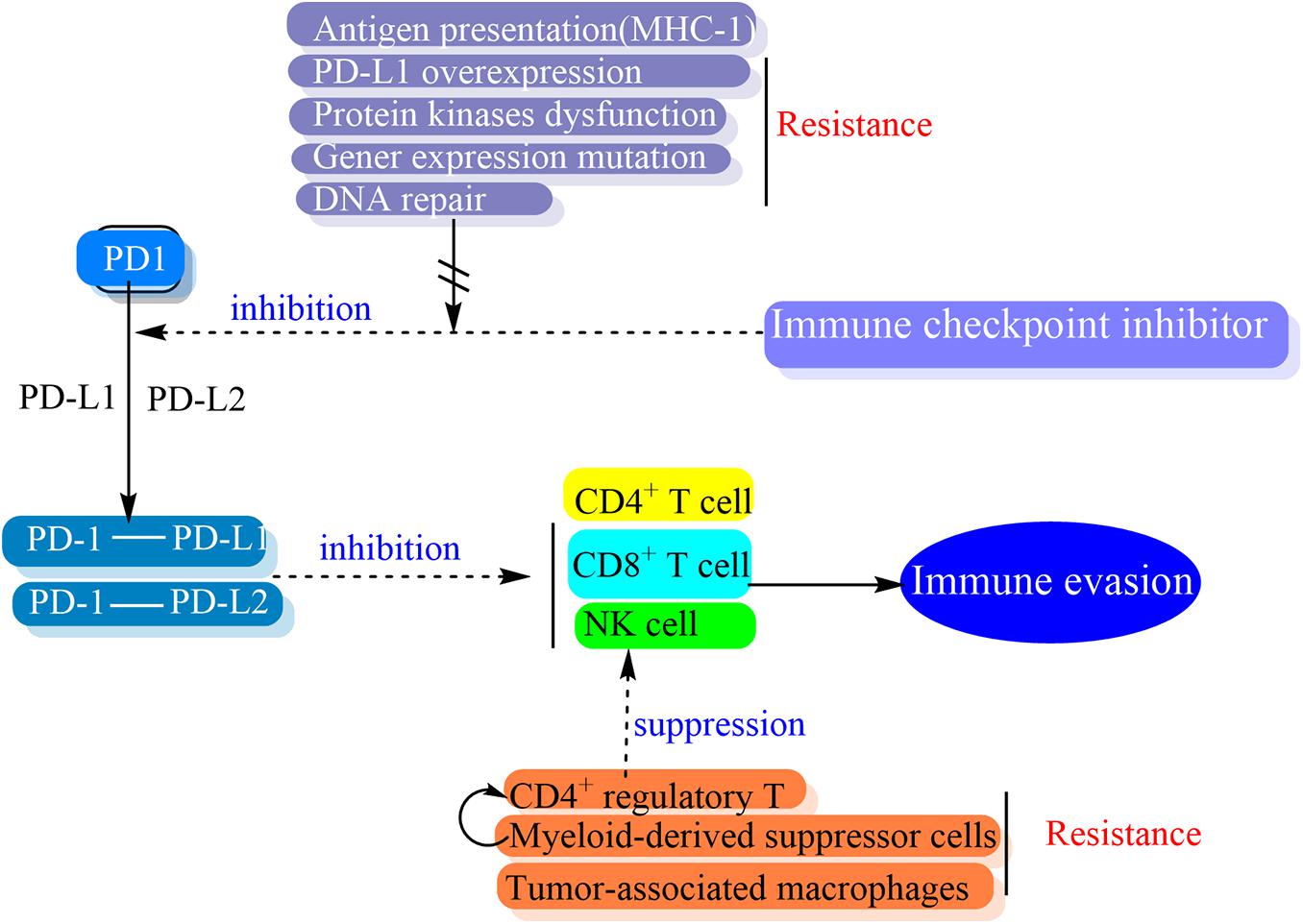
Programmed cell death-1 (PD-1) was first identified in 1992 and also called CD279 (Ishida et al., 1992). PD-1 is a 55-kDa transmembrane protein with 288 amino acids and a member of the B7-CD28 family of cell surface receptors including CTLA-4, CD28, BTLA, and ICOS, which is expressed on activated T cells, B cells, NKT cells, monocytes and macrophages (Sharpe and Freeman, 2002; Francisco et al., 2010). While PD-1 is binding to its ligands, the active immune cells are inhibited. The PD-L1 (CD279 and B7-H1) and PD-L2 (CD273 and B7-DC) are the two recognized ligands of PD-1, which belong to the B7 family (Sanmamed and Chen, 2014). Although PD-L1 and PD-L2 is different from the expression pattern, both of them bind to the same receptor and inhibit immune response, therefore they do not have any different roles (Momtaz and Postow, 2014). PD-L1 is constitutively expressed on the surface of immune cells, professional antigen-presenting cells (APCs) that are mainly defined by expressing MHC class 2, such as macrophages, B cells and dendritic cells (DCs), and costimulatory molecules to CD4+ T cells. PD-L1 is also expressed on the surface of non-professional APCs which present antigen to cytotoxic CD8+ T cells (Francisco et al., 2010).
The PD-1/PD-L1 pathway has a role in promoting mechanisms of tumor immune evasion. This is the theoretical basis of the development of monoclonal antibodies targeting either PD-1 or PD-L1. The block of the binding of PD-1 and its ligands has the potential to make tumor cells exposure to the immune regulatory activity of effector T cells and resume an effective antitumor immune response (Figure 4) (Sharpe and Freeman, 2002; Francisco et al., 2010; Pardoll, 2012).
The efficiency of the PD-1/PD-L1 inhibitors is associated with the PD-L1 expression and/or tumor mutation burden (TMB) in tumor cells (Lawrence et al., 2013). For EC, about 14.5–82.8% patients have tumors with PD-L1 expression and high TMB (Hong and Ding, 2019). The pembrolizumab and nivolumab are the recommended anti-PD-1 monoclonal antibodies based on several clinical trials for EC either ESCC or EAC (Hong and Ding, 2019).
Pembrolizumab is a fully humanized IgG4-k monoclonal antibody which is potent and highly selective against PD-1 (Wolchok, 2015). In many clinical trials, pembrolizumab demonstrated manageable toxicity and durable antitumor activity in patients with previous standard therapy failed, PD-L1–positive advanced, recurrent, and metastatic EC (Doi et al., 2018; Fuchs et al., 2018; Kojima et al., 2019; Shah et al., 2019). Most of the patient in these trials who achieved overall survival superiority had an ESCC and PD-L1 combined positive score (CPS) of more than 10 (Kojima et al., 2019). Based on these clinical trials, pembrolizumab as a second-line therapy has been approved by FDA for advanced ESCC patients with PD-L1 CPS ≥ 10 (Yamamoto and Kato, 2020). Moreover, many clinical trials had combined pembrolizumab and trastuzumab (HER2 inhibitor) plus standard first-line chemotherapy for HER2-positive advanced and metastatic gastroesophageal cancer and the results were safe and tolerable (Bang et al., 2010; Tabernero et al., 2019; Janjigian et al., 2020). The benefits were observed when adding trastuzumab and pertuzumab to neochemoradiotherapy for resectable HER2-positive EAC (Stroes et al., 2020).
Nivolumab is a high-affinity, humanized IgG4 monoclonal PD-1 antibody (Wolchok, 2015). Many clinical trials showed that nivolumab had benefits in improving the overall survival of metastatic renal cell carcinoma (Peinemann et al., 2019). A phase 2 trial (ATTRACTION-01) showed that nivolumab had significant efficacy and safety for metastatic or recurrent ESCC (Kudo et al., 2017). The phase 3 trial (ATTRACTION-3) showed superiority of nivolumab for the second-line treatment of all ESCC patients regardless of PD-L1 expression (Kato et al., 2019; Kojima et al., 2019). Thus, nivolumab has approved by the FDA as a monotherapy for advanced ESCC patients after prior standard chemotherapy regardless of PD-L1 expression (Yamamoto and Kato, 2020).
There are many clinical trials in progress for other anti-PD-1 antibodies, such as tislelizumab (Xu et al., 2020), atezolizumab (Bando et al., 2020), durvalumab (Mamdani et al., 2019), and so on. However, most of them are under phase 1/2 clinical trials and far from clinical application.
In short, for metastatic or recurrent ESCC patients, ICIs such as pembrolizumab and nivolumab are standard treatment for second-line chemotherapy (Kato et al., 2019; Kojima et al., 2019). However, there is little evidence for the efficacy and safety of ICIs for metastatic or recurrent EAC patients (Yamamoto and Kato, 2020).
Resistance in Immunotherapy
There are still many questions and important challenges to conquer in ICIs application (Akin Telli et al., 2020). Firstly, although the immunotherapy has desirable antitumor effects, the inhibition of immune checkpoints may also result in loss of peripheral tolerance and subsequently arouse immune activation on non-tumor cells, which finally causes unintended tissue damage and represents multisystem organ dysfunction. Almost every organ system could manifest as tissue damage, in which the dermatologic, gastrointestinal, endocrine, and pulmonary systems are the most commonly affected (Hryniewicki et al., 2018). In previous studies, the most common immune-related adverse events (irAEs) of PD-1 inhibitors (pembrolizumab and nivolumab) were fatigue, pruritus, rash, and diarrhea. Furthermore, most of them (more than 95%) are low grade adverse effects. Secondly, compared with molecular targeted drugs, the ICI drug is more prone to the occurrence of drug resistance (Lim and Rizos, 2018). The drug resistance of ICIs are also divided into intrinsic and acquired resistance (Sharma et al., 2017). Previous studies showed that most tumors rarely exceed 40% remission rates and most of them were partial response (Sharma and Allison, 2015). Furthermore, the resistance to anti-PD-1/PD-L1 therapy was up to 70% of patients and the primary resistance was up to 60% (O’donnell et al., 2017; Nowicki et al., 2018). In the ATTRACTION-3 trial, the treatment responses between nivolumab and chemotherapy groups for ECs were similar (19% vs. 22%) and the responses to immunotherapy needed more time to be apparent compared with chemotherapy. However, the durability of the response in the nivolumab group was longer than that in chemotherapy groups. Furthermore, the proportion of patients with progressive disease were higher in the nivolumab group than that in chemotherapy group. Based on this, we could think that the immunotherapy drug nivolumab may be as beneficial as chemotherapy, but the intrinsic resistance and acquired resistance remain major obstacles to immunotherapy of EC (Kato et al., 2019).
The mechanism of ICIs resistance has not been fully elucidated. One cause of it may be related to the heterogeneity of the tumor process and the essence of it is the tumor immune escape. Generally, the drug resistance of ICIs is considered to involve in the interaction of internal and external factors between tumor cells and immune cells within the tumor and the interaction may present a dynamic multilevel change process (Wang and Wu, 2020). The tumor-cell related factors of resistance are considered as intrinsic mechanisms while the immune-cell and micro-environment related factors refer to extrinsic mechanisms of resistance (Pitt et al., 2016). A variety of intrinsic and extrinsic factors are involved in tumor cell evasion from immune activation at the genetic, enzymatic, and cellular levels, such as the regulation of TME, intracellular protein mutations, oncogene signal transduction pathways, epigenetic changes, etc. (Wang and Wu, 2020). Whether intrinsic or extrinsic factors, the mechanisms of resistance are concentrated on antigen presentation, cytotoxic T-cell activation and trafficking, as well as the stimulation of the immune-inhibitory axis (Haibe et al., 2020).
There are many cancer cell alteration processes related to intrinsic resistance. One of them is the alteration of antigen presentation which affects the immune recognition. The surface of tumor cells often present loss or down-regulation of the major histocompatibility complex class I molecules (MHC-1), and this is suggested to be a mechanism of immune escape of the tumor (Baba et al., 2007). Every tumor type has a different escape mechanism (Yoo et al., 2019) and various tumors demonstrate deregulated expression of MHC-1 (Garrido et al., 2017). The frequency of MHC-1 loss with concurrent PD-L1 expression was approximately 12.2% in ESCC (Ito et al., 2016). The alteration of MHC-1 transcription and expression will impact on anti-PD-1/PD-L1 therapies (García-Aranda and Redondo, 2019). The cell signaling also affects immune response. The abnormal expression or dysregulation of protein kinases is involved in different hallmarks of cancer including survival, motility, metabolism, angiogenesis, proliferation, resistance to standard therapies, and immunotherapies and escape of antitumor immune responses (Gross et al., 2015; Garcia-Aranda and Redondo, 2017). PD-L1 overexpression can be a major hinderance for anti-PD-L1 therapy via overexpression tumor specific T-cells and apart from its immunosuppressive function (García-Aranda and Redondo, 2019). Besides the role of protein kinases on the expression of both PD-1 and its ligands, the dysregulation of protein kinase pathways is also a main cause of apoptosis resistance against immune response (Garcia-Aranda et al., 2018). The absence of tumor neoantigen expression and presentation leads to a lack of recognition by T-cells and results in the inability of tumors to respond to PD-1/PD-L1 inhibitor therapy (Bai J. et al., 2017), moreover, other alterations such as apoptosis suppression or DNA repair promoted are also associated with treatment resistance (Mansoori et al., 2017). The anti-apoptotic activity of altered protein kinases is tightly related to tumor resistance against most therapeutic treatments including immunotherapies (Garcia-Aranda and Redondo, 2017). Either the alterations of receptor kinases in their activity, abundance, cellular distribution and/or regulation can affect the functioning of signal transduction routes and result in the constitutive activation of downstream kinases like PI3K/AKT, MAPK, EGFR, or JAK/STAT (Garcia-Aranda and Redondo, 2019). Furthermore, the alterations also affect different transcription factors which command cell survival and trigger PD-L1 expression (Bai J. et al., 2017).
There are many gene expression mutations which affect immune response (Trujillo et al., 2019). Loss of B2M and defective IFN-γ signaling are tightly associated with T cell-resistant phenotype and tumor-intrinsic determinants of resistance to immunotherapies. The B2M is the subunit necessary for antigen presentation by MHC-1 and the tumor resistant anti-CTLA-4 or anti-PD-1 therapy no longer express B2M (Sade-Feldman et al., 2017). Defective IFN-γ signaling, such as through inactivating mutations in Janus kinases (JAK1 or JAK2) or in the interferon-gamma receptor 1 (IFNGR1), has also been suggested to relate to resistance of anti-PD-1 therapy (Sucker et al., 2017). WNT signaling has not only been involved in tumor progress but also in the ability of tumor cells escaping from different types of cellular stresses, including drug treatments and host immune response (Martin-Orozco et al., 2019). The tumor cell-intrinsic activation of the Wnt/β-catenin pathway mediate T cell exclusion from the tumor microenvironment and it lead to primary resistance to ICI therapy. Up-regulation of β-catenin by cancer cells could cause tumor recurrence and result in secondary resistance to immunotherapy (Spranger et al., 2015).
The DNA damage responses also affect the treatment benefits. Many studies suggest that DNA repair plays an important role in driving sensitivity and response to ICIs (Mouw et al., 2017). Many reports have manifested that a specific DNA damage exposure or a specific DNA repair pathway deficiency is related to ICI response. Loss of normal DNA repair fidelity may lead to an increase in mutational burden and ICI response of tumors (Rizvi et al., 2015).
The expression of different checkpoints to prevent T-cell activation leads to secondary resistance. Single antagonistic PD1/PDL1 pathway has limited function in improving immune cells and is prone to drug resistance (De Sousa Linhares et al., 2018). Besides the PD-1, a lot of high expression of immune inhibitory checkpoints are related to T-cell function including T cells immune globulin mucin-3(TIM-3), CTLA-4, Lymphocyte activation gene 3(LAG-3), B and T lymphocytes attenuation factor (BTLA), T-cell immune globulin and ITIM structure domain proteins (TIGIT) etc., and these checkpoints also affect the efficacy of PD-1/PD-L1 antibody (Curdy et al., 2019).
Alteration of T-cell activation would lead to extrinsic resistance (Shitara and Nishikawa, 2018). CD4+ regulatory T (Treg) cells is one of the most common immune-suppressive cells in tumors and a highly immune-suppressive subset of CD4+ T cells that maintain immune homeostasis. It can suppress antitumor immunity and promote cancer progression (Togashi and Nishikawa, 2017). In vitro, PD-1 upregulation by Treg cells increases the suppression of the CD8+ T cell response (Park et al., 2015). PD-1 expression modulates the activation threshold of T cells and maintains the balance between regulatory and effector T cells (Zhang et al., 2016).
Myeloid-derived suppressor cells (MDSC) partly mediated profound immunosuppression in a number of patients which do not respond to immunotherapy. The heterogeneous population of immature myeloid cells can significantly inhibit anti-tumor activities of T and NK cells and stimulate Treg cells, which leads to tumor progression (Weber et al., 2018). Activated MDSC express high levels of PD-L1 that interacts with PD-1 on T cells and causes their exhaustion (Dieterich et al., 2017). MDSC has been shown to drive the differentiation of CD4+ T cells into immunosuppressive Treg cells and reduce the anti-tumor activity of effector T cells (Pan et al., 2010).
Tumor-associated macrophages (TAM) represents immune suppressor cells in the solid tumors that restrict anti-tumor immune reaction induced by CD8+ T cells (Cassetta and Kitamura, 2018). While the TAM is abundant in a tumor microenvironment, TAM is proposed as one of the important therapeutic targets to promote the efficacy of immunotherapies utilizing checkpoint antagonists (Mantovani et al., 2017).
Pro-inflammatory cytokines such as Interferon-α, Interleukin-2, TNF-α, TGF-β et al. can contribute to cancer immunotherapy, acting on every phase of the cancer immunity cycle (Chen and Mellman, 2017). The cellular immunotherapies can be optimized by the incorporation of cytokine genes (Chmielewski and Abken, 2015). Therefore, cytokines can elevate antigen priming, increase the number of effector immune cells in the TME and promote their cytolytic activity. The ability to expand and reactivate effector NK and T lymphocytes, promote tumor infiltration, and persist in the TME make the cytokines important molecules to overcome resistance (Berraondo et al., 2019).
Although many mechanisms are assumed to be associated with immunotherapy resistance, it is difficult to get a universal biomarker for resisting drug resistance (Haibe et al., 2020; Wang and Wu, 2020). Similarly, the specific mechanisms of immunotherapy resistance in EC are also far from elucidated because the immunotherapy agents have not been widely used in clinical practice. Until now, only pembrolizumab and nivolumab are approved for second-line therapy for advanced/metastatic EC by the FDA. Sun et al. (2020) had reported that an advanced ESCC patient who received nivolumab therapy developed hyperprogressive disease shortly. They analyzed that the mechanism of hyperprogressive disease may be associated with PI3K/AKT signaling pathway, because the PI3K/Akt signaling pathway was activated in tumor progression patient (Sun et al., 2020). Wang et al. (2019) had evaluated the expression of LAG-3, CTLA-4, and the density of CD8+ tumor-infiltrating lymphocyte in resected ESCC. The results showed that positive LAG-3 expression was significantly correlated with positive CTLA-4 expression and poor prognosis in ESCC (Wang W. et al., 2019). The positive expression of LAG-3 and CTLA-4 may prevent T-cell activation and lead to secondary resistance. Zheng et al. (2020) reported that CD39 was overexpressed on NK cells from EC patients and the frequency of CD39+ NK cells correlated with the poor prognosis. Moreover, IL-6 can induce CD39 expression and CD39+ NK cells presented an exhausted phenotype (Zheng et al., 2020). Because the dysfunction of NK cells could lead to immune escape, the activated IL-6 may increase CD39 expression and the NK cells exhaustion could cause immunotherapy resistance. However, these phenomena should not elucidate the mechanisms of immunotherapy resistance for EC and the studies of resisting the immunotherapy resistance are still ongoing.
In Summary
The pharmaceutical therapies are essential for EC. For the advanced EC, the neoadjuvant therapy including chemotherapy plus radiotherapy and/or immunotherapy is effective to achieve clinical benefit and even pathological complete response. For the recurrent, unresectable, and metastatic EC, the pharmaceutical therapy is a limited effective regimen to alleviate the disease and prolong the PFS and OS.
The regimen of platinum complexes plus fluorinated pyrimidines or taxanes is still the first-line therapy for EC. Although the targeted therapies are effective on other solid tumors such as non-small cell lung cancer, especially adenocarcinoma, the molecular targeted therapy for EC is not as effective as expected and only approved as a second-line therapy by the FDA. Furthermore, the targeted therapeutic effect in EAC is more effective compared with that in ESCC. Immunotherapy has entered a new era in cancer treatment. The ICIs are proven to provide a significant benefit in many malignant tumors compared to traditional therapy and will become the mainstream pharmaceutical therapy for the unresectable and progressive or metastatic EC. Compared to EAC, the ESCC receive more effective benefits from ICIs.
However, the significant issues of pharmaceutical treatment are still the dose-limiting side effects and primary or secondary drug resistance. Despite the fact that the mechanisms of drug resistance have achieved many profits, the dose-limiting side effects and drug resistance still restrain the clinical application and diminish the effectiveness of pharmaceutical treatment.
Author Contributions
CM and XZ contributed to the conceptual idea, reviewed, analyzed the literature, and wrote the manuscript. XX and SL assisted in compiling background material. YuY and CZ edited the manuscript and provided the suggestions for the revision of the manuscript. YZ and YoY provided the conceptual idea, analyzed the literature, and reviewed and edited the manuscript. All the authors contributed to the article and approved the submitted version.
Funding
This study was funded by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18010), and did not receive any commercial interest support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aguado, C., Garcia-Paredes, B., Sotelo, M. J., Sastre, J., and Diaz-Rubio, E. (2014). Should capecitabine replace 5-fluorouracil in the first-line treatment of metastatic colorectal cancer? World J. Gastroenterol. 20, 6092–6101. doi: 10.3748/wjg.v20.i20.6092
Ahmed, F., and Haass, N. K. (2018). Microenvironment-driven dynamic heterogeneity and phenotypic plasticity as a mechanism of melanoma therapy resistance. Front. Oncol. 8:173. doi: 10.3389/fonc.2018.00173
Ajani, J. A., Javle, M., Eng, C., Fogelman, D., Smith, J., Anderson, B., et al. (2020). Phase I study of DFP-11207, a novel oral fluoropyrimidine with reasonable AUC and low Cmax and improved tolerability, in patients with solid tumors. Invest. New Drugs 38, 1763–1773. doi: 10.1007/s10637-020-00939-w
Akin Telli, T., Bregni, G., Camera, S., Deleporte, A., Hendlisz, A., and Sclafani, F. (2020). PD-1 and PD-L1 inhibitors in oesophago-gastric cancers. Cancer Lett. 469, 142–150. doi: 10.1016/j.canlet.2019.10.036
Alvarez, P., Marchal, J. A., Boulaiz, H., Carrillo, E., Velez, C., Rodriguez-Serrano, F., et al. (2012). 5-Fluorouracil derivatives: a patent review. Expert Opin. Ther. Pat. 22, 107–123. doi: 10.1517/13543776.2012.661413
An, Q., Robins, P., Lindahl, T., and Barnes, D. E. (2007). 5-Fluorouracil incorporated into DNA is excised by the Smug1 DNA glycosylase to reduce drug cytotoxicity. Cancer Res. 67, 940–945. doi: 10.1158/0008-5472.can-06-2960
Ando, N., Iizuka, T., Ide, H., Ishida, K., Shinoda, M., Nishimaki, T., et al. (2003). Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study–JCOG9204. J. Clin. Oncol. 21, 4592–4596. doi: 10.1200/jco.2003.12.095
Ashrafizadeh, M., Ahmadi, Z., Mohammadinejad, R., Farkhondeh, T., and Samarghandian, S. (2020a). Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr. Mol. Med. 20, 116–133. doi: 10.2174/1566524019666191016150757
Ashrafizadeh, M., Zarrabi, A., Hashemi, F., Moghadam, E. R., Hashemi, F., Entezari, M., et al. (2020b). Curcumin in cancer therapy: a novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 256:117984. doi: 10.1016/j.lfs.2020.117984
Baba, T., Hanagiri, T., Ichiki, Y., Kuroda, K., Shigematsu, Y., Mizukami, M., et al. (2007). Lack and restoration of sensitivity of lung cancer cells to cellular attack with special reference to expression of human leukocyte antigen class I and/or major histocompatibility complex class I chain related molecules A/B. Cancer Sci. 98, 1795–1802. doi: 10.1111/j.1349-7006.2007.00586.x
Bai, J., Gao, Z., Li, X., Dong, L., Han, W., and Nie, J. (2017). Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget 8, 110693–110707. doi: 10.18632/oncotarget.22690
Bai, L., Gao, C., Liu, Q., Yu, C., Zhang, Z., Cai, L., et al. (2017). Research progress in modern structure of platinum complexes. Eur. J. Med. Chem. 140, 349–382. doi: 10.1016/j.ejmech.2017.09.034
Bando, H., Kotani, D., Tsushima, T., Hara, H., Kadowaki, S., Kato, K., et al. (2020). TENERGY: multicenter phase II study of Atezolizumab monotherapy following definitive Chemoradiotherapy with 5-FU plus Cisplatin in patients with unresectable locally advanced esophageal squamous cell carcinoma. BMC Cancer 20:336. doi: 10.1186/s12885-020-06716-5
Bang, Y. J., Van Cutsem, E., Feyereislova, A., Chung, H. C., Shen, L., Sawaki, A., et al. (2010). Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697. doi: 10.1016/s0140-6736(10)61121-x
Bang, Y. J., Xu, R. H., Chin, K., Lee, K. W., Park, S. H., Rha, S. Y., et al. (2017). Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1637–1651. doi: 10.1016/s1470-2045(17)30682-4
Barsouk, A., Rawla, P., Hadjinicolaou, A. V., Aluru, J. S., and Barsouk, A. (2019). Targeted therapies and immunotherapies in the treatment of esophageal cancers. Med. Sci. 7:100. doi: 10.3390/medsci7100100
Berraondo, P., Sanmamed, M. F., Ochoa, M. C., Etxeberria, I., Aznar, M. A., Perez-Gracia, J. L., et al. (2019). Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15.
Brenner, B., Purim, O., Gordon, N., Goshen-Lago, T., Idelevich, E., Kashtan, H., et al. (2019). The addition of cetuximab to preoperative chemoradiotherapy for locally advanced esophageal squamous cell carcinoma is associated with high rate of long term survival: mature results from a prospective phase Ib/II trial. Radiother. Oncol. 134, 74–80. doi: 10.1016/j.radonc.2019.01.013
Cancer Genome Atlas Research Network (2014). Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209. doi: 10.1038/nature13480
Cancer Genome Atlas Research Network, Analysis Working Group, Asan University, BC Cancer Agency, Brigham and Women’s Hospital, Broad Institute, et al. (2017). Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175. doi: 10.1038/nature20805
Cassetta, L., and Kitamura, T. (2018). Targeting tumor-associated macrophages as a potential strategy to enhance the response to immune checkpoint inhibitors. Front. Cell. Dev. Biol. 6:38. doi: 10.3389/fcell.2018.00038
Catenacci, D. V., Tesfaye, A., Tejani, M., Cheung, E., Eisenberg, P., Scott, A. J., et al. (2019). Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT Phase III study design. Future Oncol. 15, 2073–2082. doi: 10.2217/fon-2019-0141
Catenacci, D. V. T., Tebbutt, N. C., Davidenko, I., Murad, A. M., Al-Batran, S. E., Ilson, D. H., et al. (2017). Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1467–1482. doi: 10.1016/s1470-2045(17)30566-1
Cavaliere, A., Merz, V., Casalino, S., Zecchetto, C., Simionato, F., Salt, H. L., et al. (2019). Novel biomarkers for prediction of response to preoperative systemic therapies in gastric cancer. J. Gastric Cancer 19, 375–392. doi: 10.5230/jgc.2019.19.e39
Chapman, E. G., and Derose, V. J. (2010). Enzymatic processing of platinated RNAs. J. Am. Chem. Soc. 132, 1946–1952. doi: 10.1021/ja908419j
Chen, D. S., and Mellman, I. (2017). Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330. doi: 10.1038/nature21349
Chen, S., Zhu, X., Qiao, H., Ye, M., Lai, X., Yu, S., et al. (2016). Protective autophagy promotes the resistance of HER2-positive breast cancer cells to lapatinib. Tumour Biol. 37, 2321–2331. doi: 10.1007/s13277-015-3800-9
Chen, Y., Ye, J., Zhu, Z., Zhao, W., Zhou, J., Wu, C., et al. (2019). Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: a randomized, multicenter, phase III clinical trial. J. Clin. Oncol. 37, 1695–1703. doi: 10.1200/jco.18.02122
Chmielewski, M., and Abken, H. (2015). TRUCKs: the fourth generation of CARs. Expert Opin. Biol. Ther. 15, 1145–1154. doi: 10.1517/14712598.2015.1046430
Costi, M. P., Ferrari, S., Venturelli, A., Calo, S., Tondi, D., and Barlocco, D. (2005). Thymidylate synthase structure, function and implication in drug discovery. Curr. Med. Chem. 12, 2241–2258. doi: 10.2174/0929867054864868
Curdy, N., Lanvin, O., Laurent, C., Fournie, J. J., and Franchini, D. M. (2019). Regulatory mechanisms of inhibitory immune checkpoint receptors expression. Trends Cell Biol. 29, 777–790. doi: 10.1016/j.tcb.2019.07.002
Dann, R. B., Deloia, J. A., Timms, K. M., Zorn, K. K., Potter, J., Flake, D. D., et al. (2012). BRCA1/2 mutations and expression: response to platinum chemotherapy in patients with advanced stage epithelial ovarian cancer. Gynecol. Oncol. 125, 677–682. doi: 10.1016/j.ygyno.2012.03.006
Das, G., Shravage, B. V., and Baehrecke, E. H. (2012). Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb. Perspect. Biol. 4:a008813. doi: 10.1101/cshperspect.a008813
Datta, S., Choudhury, D., Das, A., Mukherjee, D. D., Dasgupta, M., Bandopadhyay, S., et al. (2019). Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A549 cells via ROS mediated modulation of beta-catenin pathway. Apoptosis 24, 414–433. doi: 10.1007/s10495-019-01526-y
De Sousa Linhares, A., Leitner, J., Grabmeier-Pfistershammer, K., and Steinberger, P. (2018). Not all immune checkpoints are created equal. Front. Immunol. 9:1909. doi: 10.3389/fimmu.2018.01909
De Vries, G., Rosas-Plaza, X., Van Vugt, M., Gietema, J. A., and De Jong, S. (2020). Testicular cancer: determinants of cisplatin sensitivity and novel therapeutic opportunities. Cancer Treat. Rev. 88:102054. doi: 10.1016/j.ctrv.2020.102054
Dieterich, L. C., Ikenberg, K., Cetintas, T., Kapaklikaya, K., Hutmacher, C., and Detmar, M. (2017). Tumor-associated lymphatic vessels upregulate PDL1 to inhibit T-cell activation. Front. Immunol. 8:66. doi: 10.3389/fimmu.2017.00066
Dilruba, S., and Kalayda, G. V. (2016). Platinum-based drugs: past, present and future. Cancer Chemother. Pharmacol. 77, 1103–1124. doi: 10.1007/s00280-016-2976-z
Doi, T., Piha-Paul, S. A., Jalal, S. I., Saraf, S., Lunceford, J., Koshiji, M., et al. (2018). Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J. Clin. Oncol. 36, 61–67. doi: 10.1200/jco.2017.74.9846
Dong, J., Li, B., Zhou, Q., and Huang, D. (2018). Advances in evidence-based medicine for immunotherapy of non-small cell lung cancer. J. Evid. Based Med. 11, 278–287. doi: 10.1111/jebm.12322
Dutton, S. J., Ferry, D. R., Blazeby, J. M., Abbas, H., Dahle-Smith, A., Mansoor, W., et al. (2014). Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 15, 894–904. doi: 10.1016/s1470-2045(14)70024-5
Fatehi Hassanabad, A., Chehade, R., Breadner, D., and Raphael, J. (2020). Esophageal carcinoma: towards targeted therapies. Cell Oncol. 43, 195–209. doi: 10.1007/s13402-019-00488-2
Feng, S., Zhou, H., Wu, D., Zheng, D., Qu, B., Liu, R., et al. (2020). Nobiletin and its derivatives overcome multidrug resistance (MDR) in cancer: total synthesis and discovery of potent MDR reversal agents. Acta Pharm. Sin. B 10, 327–343. doi: 10.1016/j.apsb.2019.07.007
Ford, H. E., Marshall, A., Bridgewater, J. A., Janowitz, T., Coxon, F. Y., Wadsley, J., et al. (2014). Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 15, 78–86. doi: 10.1016/s1470-2045(13)70549-7
Foulkes, W. D., and Shuen, A. Y. (2013). In brief: BRCA1 and BRCA2. J. Pathol. 230, 347–349. doi: 10.1002/path.4205
Francisco, L. M., Sage, P. T., and Sharpe, A. H. (2010). The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236, 219–242. doi: 10.1111/j.1600-065x.2010.00923.x
Fuchs, C. S., Doi, T., Jang, R. W., Muro, K., Satoh, T., Machado, M., et al. (2018). Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4:e180013. doi: 10.1001/jamaoncol.2018.0013
Galanski, M. (2006). Recent developments in the field of anticancer platinum complexes. Recent Pat. Anticancer Drug Discov. 1, 285–295. doi: 10.2174/157489206777442287
Galluzzi, L., Senovilla, L., Vitale, I., Michels, J., Martins, I., Kepp, O., et al. (2012). Molecular mechanisms of cisplatin resistance. Oncogene 31, 1869–1883.
Garcia-Aranda, M., Perez-Ruiz, E., and Redondo, M. (2018). Bcl-2 inhibition to overcome resistance to chemo- and immunotherapy. Int. J. Mol. Sci. 19:3950. doi: 10.3390/ijms19123950
Garcia-Aranda, M., and Redondo, M. (2017). Protein kinase targets in breast cancer. Int. J. Mol. Sci. 18:2543. doi: 10.3390/ijms18122543
Garcia-Aranda, M., and Redondo, M. (2019). Targeting receptor kinases in colorectal cancer. Cancers 11:433. doi: 10.3390/cancers11040433
García-Aranda, M., and Redondo, M. (2019). Targeting protein kinases to enhance the response to anti-PD-1/PD-L1 immunotherapy. Int. J. Mol. Sci. 20:2296. doi: 10.3390/ijms20092296
Garrido, F., Ruiz-Cabello, F., and Aptsiauri, N. (2017). Rejection versus escape: the tumor MHC dilemma. Cancer Immunol. Immunother. 66, 259–271. doi: 10.1007/s00262-016-1947-x
Gately, D. P., and Howell, S. B. (1993). Cellular accumulation of the anticancer agent cisplatin: a review. Br. J. Cancer 67, 1171–1176. doi: 10.1038/bjc.1993.221
Gebski, V., Burmeister, B., Smithers, B. M., Foo, K., Zalcberg, J., Simes, J., et al. (2007). Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 8, 226–234. doi: 10.1016/s1470-2045(07)70039-6
Gmeiner, W. H. (2020). Chemistry of fluorinated pyrimidines in the era of personalized medicine. Molecules 25:3438. doi: 10.3390/molecules25153438
Gonzaga, I. M., Soares-Lima, S. C., De Santos, P. T., Blanco, T. C., De Reis, B. S., Quintella, D. C., et al. (2012). Alterations in epidermal growth factor receptors 1 and 2 in esophageal squamous cell carcinomas. BMC Cancer 12:569. doi: 10.1186/1471-2407-12-569
Groenendijk, F. H., and Bernards, R. (2014). Drug resistance to targeted therapies: deja vu all over again. Mol. Oncol. 8, 1067–1083. doi: 10.1016/j.molonc.2014.05.004
Gross, S., Rahal, R., Stransky, N., Lengauer, C., and Hoeflich, K. P. (2015). Targeting cancer with kinase inhibitors. J. Clin. Invest. 125, 1780–1789.
Guo, X. F., Li, S. S., Zhu, X. F., Dou, Q. H., and Liu, D. (2018). Lapatinib in combination with paclitaxel plays synergistic antitumor effects on esophageal squamous cancer. Cancer Chemother. Pharmacol. 82, 383–394. doi: 10.1007/s00280-018-3627-3
Gupta, A., and Lutsenko, S. (2009). Human copper transporters: mechanism, role in human diseases and therapeutic potential. Future Med. Chem. 1, 1125–1142. doi: 10.4155/fmc.09.84
Haibe, Y., El Husseini, Z., El Sayed, R., and Shamseddine, A. (2020). Resisting resistance to immune checkpoint therapy: a systematic review. Int. J. Mol. Sci. 21:6176. doi: 10.3390/ijms21176176
Hall, M. D., Okabe, M., Shen, D. W., Liang, X. J., and Gottesman, M. M. (2008). The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu. Rev. Pharmacol. Toxicol. 48, 495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426
Hanada, K., Asano, K., Nishimura, T., Chimata, T., Matsuo, Y., Tsuchiya, M., et al. (2008). Use of a toxicity factor to explain differences in nephrotoxicity and myelosuppression among the platinum antitumour derivatives cisplatin, carboplatin and nedaplatin in rats. J. Pharm. Pharmacol. 60, 317–322. doi: 10.1211/jpp.60.3.0006
Hecht, D., Zimmerman, N., Bedford, M., Avivi, A., and Yayon, A. (1995). Identification of fibroblast growth factor 9 (FGF9) as a high affinity, heparin dependent ligand for FGF receptors 3 and 2 but not for FGF receptors 1 and 4. Growth Factors 12, 223–233. doi: 10.3109/08977199509036882
Hecht, J. R., Bang, Y. J., Qin, S. K., Chung, H. C., Xu, J. M., Park, J. O., et al. (2016). Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J. Clin. Oncol. 34, 443–451. doi: 10.1200/jco.2015.62.6598
Hecht, J. R., Mitchell, E., Chidiac, T., Scroggin, C., Hagenstad, C., Spigel, D., et al. (2009). A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J. Clin. Oncol. 27, 672–680. doi: 10.1200/jco.2008.19.8135
Heggie, G. D., Sommadossi, J. P., Cross, D. S., Huster, W. J., and Diasio, R. B. (1987). Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 47, 2203–2206.
Heringova, P., Kasparkova, J., and Brabec, V. (2009). DNA adducts of antitumor cisplatin preclude telomeric sequences from forming G quadruplexes. J. Biol. Inorg. Chem. 14, 959–968. doi: 10.1007/s00775-009-0508-6
Hirano, H., and Kato, K. (2019). Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn. J. Clin. Oncol. 49, 412–420. doi: 10.1093/jjco/hyz034
Ho, A. L. K., and Smyth, E. C. (2020). A global perspective on oesophageal cancer: two diseases in one. Lancet Gastroenterol. Hepatol. 5, 521–522. doi: 10.1016/s2468-1253(20)30047-9
Holzer, A. K., and Howell, S. B. (2006). The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res. 66, 10944–10952. doi: 10.1158/0008-5472.can-06-1710
Hong, Y., and Ding, Z. Y. (2019). PD-1 inhibitors in the advanced esophageal cancer. Front. Pharmacol. 10:1418. doi: 10.3389/fphar.2019.01418
Hosoda, K., Azuma, M., Katada, C., Ishido, K., Niihara, M., Ushiku, H., et al. (2020). A phase I study of docetaxel/oxaliplatin/S-1 (DOS) combination neoadjuvant chemotherapy for patients with locally advanced adenocarcinoma of the esophagogastric junction. Int. J. Clin. Oncol. 25, 1090–1097. doi: 10.1007/s10147-020-01638-5
Houghton, J. A., Harwood, F. G., and Tillman, D. M. (1997). Thymineless death in colon carcinoma cells is mediated via fas signaling. Proc. Natl. Acad. Sci. U.S.A. 94, 8144–8149. doi: 10.1073/pnas.94.15.8144
Hryniewicki, A. T., Wang, C., Shatsky, R. A., and Coyne, C. J. (2018). Management of immune checkpoint inhibitor toxicities: a review and clinical guideline for emergency physicians. J. Emerg. Med. 55, 489–502. doi: 10.1016/j.jemermed.2018.07.005
Ilson, D. H., and Van Hillegersberg, R. (2018). Management of patients with adenocarcinoma or squamous cancer of the esophagus. Gastroenterology 154, 437–451. doi: 10.1053/j.gastro.2017.09.048
Ishida, Y., Agata, Y., Shibahara, K., and Honjo, T. (1992). Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x
Ito, S., Okano, S., Morita, M., Saeki, H., Tsutsumi, S., Tsukihara, H., et al. (2016). Expression of PD-L1 and HLA class I in esophageal squamous cell carcinoma: prognostic factors for patient outcome. Ann. Surg. Oncol. 23, 508–515. doi: 10.1245/s10434-016-5376-z
Janjigian, Y. Y., Maron, S. B., Chatila, W. K., Millang, B., Chavan, S. S., Alterman, C., et al. (2020). First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 21, 821–831. doi: 10.1016/s1470-2045(20)30169-8
Janjigian, Y. Y., Sanchez-Vega, F., Jonsson, P., Chatila, W. K., Hechtman, J. F., Ku, G. Y., et al. (2018). Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 8, 49–58.
Janser, F. A., Tschan, M. P., and Langer, R. (2019). The role of autophagy in HER2-targeted therapy. Swiss. Med. Wkly. 149:w20138.
Jing, W., Yan, W., Liu, Y., Li, J., Yu, J., and Zhu, H. (2019). Slight advantages of nimotuzumab versus cetuximab plus concurrent chemoradiotherapy in locally advanced esophageal squamous cell carcinoma. Cancer Biol. Ther. 20, 1121–1126. doi: 10.1080/15384047.2019.1598760
Jordan, M. A., and Wilson, L. (2004). Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4, 253–265. doi: 10.1038/nrc1317
Jung, J. H., Lee, H. J., Kim, J. H., Sim, D. Y., Im, E., Kim, S., et al. (2020). Colocalization of MID1IP1 and c-Myc is critically involved in liver cancer growth via regulation of ribosomal protein L5 and L11 and CNOT2. Cells 9:985. doi: 10.3390/cells9040985
Kang, X., Chen, K., Li, Y., Li, J., D’amico, T. A., and Chen, X. (2015). Personalized targeted therapy for esophageal squamous cell carcinoma. World J. Gastroenterol. 21, 7648–7658. doi: 10.3748/wjg.v21.i25.7648
Kato, K., Cho, B. C., Takahashi, M., Okada, M., Lin, C. Y., Chin, K., et al. (2019). Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20, 1506–1517. doi: 10.1016/s1470-2045(19)30626-6
Kehe, K., and Szinicz, L. (2005). Medical aspects of sulphur mustard poisoning. Toxicology 214, 198–209. doi: 10.1016/j.tox.2005.06.014
Kelland, L. (2007). The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7, 573–584. doi: 10.1038/nrc2167
Kim, S. T., Cristescu, R., Bass, A. J., Kim, K. M., Odegaard, J. I., Kim, K., et al. (2018). Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 24, 1449–1458. doi: 10.1038/s41591-018-0101-z
Kobayakawa, M., and Kojima, Y. (2011). Tegafur/gimeracil/oteracil (S-1) approved for the treatment of advanced gastric cancer in adults when given in combination with cisplatin: a review comparing it with other fluoropyrimidine-based therapies. Onco Targets Ther. 4, 193–201. doi: 10.2147/ott.s19059
Kojima, T., Muro, K., Francois, E., Hsu, C.-H., Moriwaki, T., Kim, S.-B., et al. (2019). Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase III KEYNOTE-181 study. J. Clin. Oncol. 37:2. doi: 10.1200/jco.2019.37.4_suppl.2
Kong, W., Engel, K., and Wang, J. (2004). Mammalian nucleoside transporters. Curr. Drug Metab. 5, 63–84. doi: 10.2174/1389200043489162
Kudo, T., Hamamoto, Y., Kato, K., Ura, T., Kojima, T., Tsushima, T., et al. (2017). Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 18, 631–639. doi: 10.1016/s1470-2045(17)30181-x
Lagergren, J., Smyth, E., Cunningham, D., and Lagergren, P. (2017). Oesophageal cancer. Lancet 390, 2383–2396.
Lawrence, M. S., Stojanov, P., Polak, P., Kryukov, G. V., Cibulskis, K., Sivachenko, A., et al. (2013). Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218.
Le, D. T., Kavan, P., Kim, T. W., Burge, M. E., Van Cutsem, E., Hara, H., et al. (2018). KEYNOTE-164: pembrolizumab for patients with advanced microsatellite instability high (MSI-H) colorectal cancer. J. Clin. Oncol. 36, 3514–3514. doi: 10.1200/jco.2018.36.15_suppl.3514
Lebwohl, D., and Canetta, R. (1998). Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur. J. Cancer 34, 1522–1534. doi: 10.1016/s0959-8049(98)00224-x
Lee, M. X., and Tan, D. S. (2018). Weekly versus 3-weekly paclitaxel in combination with carboplatin in advanced ovarian cancer: which is the optimal adjuvant chemotherapy regimen? J. Gynecol. Oncol. 29:e96.
Lee, Y. T., Tan, Y. J., and Oon, C. E. (2018). Molecular targeted therapy: treating cancer with specificity. Eur. J. Pharmacol. 834, 188–196. doi: 10.1016/j.ejphar.2018.07.034
Lei, X., Hu, X., Zhang, T., Zhang, J., Wu, C., Hong, W., et al. (2020). HMGB1 release promotes paclitaxel resistance in castration-resistant prostate cancer cells via activating c-Myc expression. Cell. Signal. 72:109631. doi: 10.1016/j.cellsig.2020.109631
Li, J., Qin, S., Xu, J., Xiong, J., Wu, C., Bai, Y., et al. (2016). Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34, 1448–1454. doi: 10.1200/jco.2015.63.5995
Li, K., and Li, J. (2016). Current molecular targeted therapy in advanced gastric cancer: a comprehensive review of therapeutic mechanism, clinical trials, and practical application. Gastroenterol. Res. Pract. 2016:4105615.
Li, S., Zhao, Q., Wang, B., Yuan, S., Wang, X., and Li, K. (2018). Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother. Res. 32, 1530–1536. doi: 10.1002/ptr.6081
Lim, S. Y., and Rizos, H. (2018). Immune cell profiling in the age of immune checkpoint inhibitors: implications for biomarker discovery and understanding of resistance mechanisms. Mamm. Genome 29, 866–878. doi: 10.1007/s00335-018-9757-4
Li, C. Y., Zhang, W. W., Xiang, J. L., Wang, X. H., Li, J., and Wang, J. L. (2019). Identification of microRNAs as novel biomarkers for esophageal squamous cell carcinoma: a study based on the cancer genome Atlas (TCGA) and bioinformatics. Chin. Med. J. (Engl.) 132, 2213–2222. doi: 10.1097/CM9.0000000000000427
Liu, M., Jia, Q., Wang, X., Sun, C., Yang, J., Chen, Y., et al. (2020). Clinical efficacy of irinotecan plus raltitrexed chemotherapy in refractory esophageal squamous cell cancer. Anticancer. Drugs 31, 403–410. doi: 10.1097/cad.0000000000000891
Liu, S., and Kurzrock, R. (2014). Toxicity of targeted therapy: implications for response and impact of genetic polymorphisms. Cancer Treat. Rev. 40, 883–891. doi: 10.1016/j.ctrv.2014.05.003
Longley, D. B., Harkin, D. P., and Johnston, P. G. (2003). 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 3, 330–338. doi: 10.1038/nrc1074
Longley, D. B., and Johnston, P. G. (2005). Molecular mechanisms of drug resistance. J. Pathol. 205, 275–292.
Lordick, F., Kang, Y. K., Chung, H. C., Salman, P., Oh, S. C., Bodoky, G., et al. (2013). Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 490–499. doi: 10.1016/s1470-2045(13)70102-5
Lordick, F., Mariette, C., Haustermans, K., Obermannova, R., Arnold, D., and Committee, E. G. (2016). Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27, v50–v57.
Lorenzen, S., Schuster, T., Porschen, R., Al-Batran, S. E., Hofheinz, R., Thuss-Patience, P., et al. (2009). Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the arbeitsgemeinschaft internistische onkologie. Ann. Oncol. 20, 1667–1673.
Lu, Z., Zhang, R., and Diasio, R. B. (1993). Dihydropyrimidine dehydrogenase activity in human peripheral blood mononuclear cells and liver: population characteristics, newly identified deficient patients, and clinical implication in 5-fluorouracil chemotherapy. Cancer Res. 53, 5433–5438.
Luo, H., Quan, J., Xiao, H., Luo, J., Zhang, Q., Pi, G., et al. (2018). FGFR inhibitor AZD4547 can enhance sensitivity of esophageal squamous cell carcinoma cells with epithelialmesenchymal transition to gefitinib. Oncol. Rep. 39, 2270–2278.
Lurje, G., Zhang, W., Yang, D., Groshen, S., Hendifar, A. E., Husain, H., et al. (2008). Thymidylate synthase haplotype is associated with tumor recurrence in stage II and stage III colon cancer. Pharmacogenet. Genomics 18, 161–168. doi: 10.1097/fpc.0b013e3282f4aea6
Ma, L., Sun, Y., Li, D., Li, H., Jin, X., and Ren, D. (2020). Overexpressed ITGA2 contributes to paclitaxel resistance by ovarian cancer cells through the activation of the AKT/FoxO1 pathway. Aging 12, 5336–5351. doi: 10.18632/aging.102954
Maeda, O., and Ando, Y. (2019). Recent progress of chemotherapy and biomarkers for gastroesophageal cancer. World J. Gastrointest. Oncol. 11, 518–526. doi: 10.4251/wjgo.v11.i7.518
Magnani, M., Maccari, G., Andreu, J. M., Diaz, J. F., and Botta, M. (2009). Possible binding site for paclitaxel at microtubule pores. FEBS J. 276, 2701–2712. doi: 10.1111/j.1742-4658.2009.06994.x
Makiyama, A., Sagara, K., Kawada, J., Kashiwada, T., Hosokawa, A., Horie, Y., et al. (2018). A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT). J. Clin. Oncol. 36, 4011–4011. doi: 10.1200/jco.2018.36.15_suppl.4011
Mamdani, H., Schneider, B. J., Abushahin, L. I., Birdas, T. J., Kesler, K., Burney, H., et al. (2019). Safety and efficacy of durvalumab following multimodality therapy for locally advanced esophageal and GEJ adenocarcinoma: results from Big Ten Cancer Research Consortium study. J. Clin. Oncol. 37, 4058–4058. doi: 10.1200/jco.2019.37.15_suppl.4058
Mansoori, B., Mohammadi, A., Davudian, S., Shirjang, S., and Baradaran, B. (2017). The different mechanisms of cancer drug resistance: a brief review. Adv. Pharm. Bull. 7, 339–348. doi: 10.15171/apb.2017.041
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L., and Allavena, P. (2017). Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416. doi: 10.1038/nrclinonc.2016.217
Markman, M. (2003). Management of toxicities associated with the administration of taxanes. Expert Opin. Drug Saf. 2, 141–146. doi: 10.1517/14740338.2.2.141
Martin-Orozco, E., Sanchez-Fernandez, A., Ortiz-Parra, I., and Ayala-San Nicolas, M. (2019). WNT signaling in tumors: the way to evade drugs and immunity. Front. Immunol. 10:2854. doi: 10.3389/fimmu.2019.02854
Mele, L., Del Vecchio, V., Liccardo, D., Prisco, C., Schwerdtfeger, M., Robinson, N., et al. (2020). The role of autophagy in resistance to targeted therapies. Cancer Treat. Rev. 88:102043.
Merz, V., Zecchetto, C., Simionato, F., Cavaliere, A., Casalino, S., Pavarana, M., et al. (2020). A phase II trial of the FGFR inhibitor pemigatinib in patients with metastatic esophageal-gastric junction/gastric cancer trastuzumab resistant: the FiGhTeR trial. Ther. Adv. Med. Oncol. 12:1758835920937889.
Mizushima, N. (2018). A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 20, 521–527. doi: 10.1038/s41556-018-0092-5
Mizushima, N., and Klionsky, D. J. (2007). Protein turnover via autophagy: implications for metabolism. Annu. Rev. Nutr. 27, 19–40. doi: 10.1146/annurev.nutr.27.061406.093749
Mo, H. N., and Liu, P. (2017). Targeting MET in cancer therapy. Chronic Dis. Transl. Med. 3, 148–153. doi: 10.1016/j.cdtm.2017.06.002
Moehler, M., Maderer, A., Thuss-Patience, P. C., Brenner, B., Meiler, J., Ettrich, T. J., et al. (2020). Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER). Ann. Oncol. 31, 228–235. doi: 10.1016/j.annonc.2019.10.018
Momtaz, P., and Postow, M. A. (2014). Immunologic checkpoints in cancer therapy: focus on the programmed death-1 (PD-1) receptor pathway. Pharmgenomics Pers. Med. 7, 357–365. doi: 10.2147/pgpm.s53163
Mossahebi-Mohammadi, M., Quan, M., Zhang, J. S., and Li, X. (2020). FGF signaling pathway: a key regulator of stem cell pluripotency. Front. Cell. Dev. Biol. 8:79. doi: 10.3389/fcell.2020.00079
Mourah, S., Louveau, B., and Dumaz, N. (2020). Mechanisms of resistance and predictive biomarkers of response to targeted therapies and immunotherapies in metastatic melanoma. Curr. Opin. Oncol. 32, 91–97. doi: 10.1097/cco.0000000000000603
Mouw, K. W., Goldberg, M. S., Konstantinopoulos, P. A., and D’andrea, A. D. (2017). DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 7, 675–693. doi: 10.1158/2159-8290.cd-17-0226
Muggia, F., and Safra, T. (2014). ‘BRCAness’ and its implications for platinum action in gynecologic cancer. Anticancer. Res. 34, 551–556.
Muro, K., Hamaguchi, T., Ohtsu, A., Boku, N., Chin, K., Hyodo, I., et al. (2004). A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann. Oncol. 15, 955–959. doi: 10.1093/annonc/mdh231
Najjar, A., Rajabi, N., and Karaman, R. (2017). Recent approaches to platinum(IV) prodrugs: a variety of strategies for enhanced delivery and efficacy. Curr. Pharm. Des. 23, 2366–2376.
Njei, B., Mccarty, T. R., and Birk, J. W. (2016). Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J. Gastroenterol. Hepatol. 31, 1141–1146. doi: 10.1111/jgh.13289
Noordhuis, P., Holwerda, U., Van Der Wilt, C. L., Van Groeningen, C. J., Smid, K., Meijer, S., et al. (2004). 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 15, 1025–1032. doi: 10.1093/annonc/mdh264
Nowicki, T. S., Hu-Lieskovan, S., and Ribas, A. (2018). Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J. 24, 47–53. doi: 10.1097/ppo.0000000000000303
O’donnell, J. S., Long, G. V., Scolyer, R. A., Teng, M. W., and Smyth, M. J. (2017). Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 52, 71–81. doi: 10.1016/j.ctrv.2016.11.007
Ohtsu, A., Shah, M. A., Van Cutsem, E., Rha, S. Y., Sawaki, A., Park, S. R., et al. (2011). Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 29, 3968–3976. doi: 10.1200/jco.2011.36.2236
Ozturk, Y., Gunaydin, C., Yalcin, F., Naziroglu, M., and Braidy, N. (2019). Resveratrol enhances apoptotic and oxidant effects of paclitaxel through TRPM2 channel activation in DBTRG glioblastoma cells. Oxid. Med. Cell Longev. 2019:4619865.
Pan, P. Y., Ma, G., Weber, K. J., Ozao-Choy, J., Wang, G., Yin, B., et al. (2010). Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 70, 99–108. doi: 10.1158/0008-5472.can-09-1882
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264. doi: 10.1038/nrc3239
Park, H. J., Park, J. S., Jeong, Y. H., Son, J., Ban, Y. H., Lee, B. H., et al. (2015). PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J. Immunol. 194, 5801–5811. doi: 10.4049/jimmunol.1401936
Pavlakis, N., Sjoquist, K. M., Martin, A. J., Tsobanis, E., Yip, S., Kang, Y. K., et al. (2016). Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II trial. J. Clin. Oncol. 34, 2728–2735.
Peinemann, F., Unverzagt, S., Hadjinicolaou, A. V., and Moldenhauer, I. (2019). Immunotherapy for metastatic renal cell carcinoma: a systematic review. J. Evid. Based Med. 12, 253–262. doi: 10.1111/jebm.12362
Perland, E., and Fredriksson, R. (2017). Classification systems of secondary active transporters. Trends Pharmacol. Sci. 38, 305–315. doi: 10.1016/j.tips.2016.11.008
Petty, R. D., Dahle-Smith, A., Stevenson, D. A. J., Osborne, A., Massie, D., Clark, C., et al. (2017). Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J. Clin. Oncol. 35, 2279–2287. doi: 10.1200/jco.2016.70.3934
Piccart, M. J., Lamb, H., and Vermorken, J. B. (2001). Current and future potential roles of the platinum drugs in the treatment of ovarian cancer. Ann. Oncol. 12, 1195–1203. doi: 10.1023/a:1012259625746
Piro, G., Carbone, C., Cataldo, I., Di Nicolantonio, F., Giacopuzzi, S., Aprile, G., et al. (2016). An FGFR3 autocrine loop sustains acquired resistance to trastuzumab in gastric cancer patients. Clin. Cancer Res. 22, 6164–6175. doi: 10.1158/1078-0432.ccr-16-0178
Piro, G., Carbone, C., Santoro, R., Tortora, G., and Melisi, D. (2018). Predictive biomarkers for the treatment of resectable esophageal and esophago-gastric junction adenocarcinoma: from hypothesis generation to clinical validation. Expert Rev. Mol. Diagn. 18, 357–370. doi: 10.1080/14737159.2018.1454312
Pitt, J. M., Vétizou, M., Daillère, R., Roberti, M. P., Yamazaki, T., Routy, B., et al. (2016). Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44, 1255–1269. doi: 10.1016/j.immuni.2016.06.001
Puckett, C. A., Ernst, R. J., and Barton, J. K. (2010). Exploring the cellular accumulation of metal complexes. Dalton Trans. 39, 1159–1170. doi: 10.1039/b922209j
Qin, A., Liu, Q., and Wang, J. (2020). Ropivacaine inhibits proliferation, invasion, migration and promotes apoptosis of papillary thyroid cancer cells via regulating ITGA2 expression. Drug Dev. Res. 81, 700–707. doi: 10.1002/ddr.21671
Rajabi, M., and Mousa, S. A. (2017). The role of angiogenesis in cancer treatment. Biomedicines 5:34. doi: 10.3390/biomedicines5020034
Ramadoss, S., Sen, S., Ramachandran, I., Roy, S., Chaudhuri, G., and Farias-Eisner, R. (2017). Lysine-specific demethylase KDM3A regulates ovarian cancer stemness and chemoresistance. Oncogene 36, 1537–1545. doi: 10.1038/onc.2016.320
Reti, A., Pap, E., Adleff, V., Jeney, A., Kralovanszky, J., and Budai, B. (2010). Enhanced 5-fluorouracil cytotoxicity in high cyclooxygenase-2 expressing colorectal cancer cells and xenografts induced by non-steroidal anti-inflammatory drugs via downregulation of dihydropyrimidine dehydrogenase. Cancer Chemother. Pharmacol. 66, 219–227. doi: 10.1007/s00280-009-1149-8
Reynolds, K. L., Bedard, P. L., Lee, S. H., Lin, C. C., Tabernero, J., Alsina, M., et al. (2017). A phase I open-label dose-escalation study of the anti-HER3 monoclonal antibody LJM716 in patients with advanced squamous cell carcinoma of the esophagus or head and neck and HER2-overexpressing breast or gastric cancer. BMC Cancer 17:646. doi: 10.1186/s12885-017-3641-6
Ribas, A., and Wolchok, J. D. (2018). Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355.
Rizvi, N. A., Hellmann, M. D., Snyder, A., Kvistborg, P., Makarov, V., Havel, J. J., et al. (2015). Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128.
Roos, W. P., and Kaina, B. (2013). DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 332, 237–248. doi: 10.1016/j.canlet.2012.01.007
Sade-Feldman, M., Jiao, Y. J., Chen, J. H., Rooney, M. S., Barzily-Rokni, M., Eliane, J. P., et al. (2017). Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8:1136.
Samson, P., Puri, V., Lockhart, A. C., Robinson, C., Broderick, S., Patterson, G. A., et al. (2018). Adjuvant chemotherapy for patients with pathologic node-positive esophageal cancer after induction chemotherapy is associated with improved survival. J. Thorac. Cardiovasc. Surg. 156, 1725–1735. doi: 10.1016/j.jtcvs.2018.05.100
Sanmamed, M. F., and Chen, L. (2014). Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 20, 256–261. doi: 10.1097/ppo.0000000000000061
Sasaki, K., Tsuno, N. H., Sunami, E., Kawai, K., Hongo, K., Hiyoshi, M., et al. (2012). Resistance of colon cancer to 5-fluorouracil may be overcome by combination with chloroquine, an in vivo study. Anticancer Drugs 23, 675–682. doi: 10.1097/cad.0b013e328353f8c7
Saumell, Y., Sanchez, L., González, S., Ortiz, R., Medina, E., Galán, Y., et al. (2017). Overall survival of patients with locally advanced or metastatic esophageal squamous cell carcinoma treated with nimotuzumab in the real world. Advances in Therapy 34, 2638–2647. doi: 10.1007/s12325-017-0631-7
Schmoll, H. J. (2003). Dihydropyrimidine dehydrogenase inhibition as a strategy for the oral administration of 5-fluorouracil: utility in the treatment of advanced colorectal cancer. Anticancer. Drugs 14, 695–702. doi: 10.1097/00001813-200310000-00003
Shah, M. A., Bang, Y. J., Lordick, F., Alsina, M., Chen, M., Hack, S. P., et al. (2017). Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-Negative, MET-positive gastroesophageal adenocarcinoma: the METGastric Randomized Clinical Trial. JAMA Oncol 3, 620–627.
Shah, M. A., Cho, J. Y., Tan, I. B., Tebbutt, N. C., Yen, C. J., Kang, A., et al. (2016). A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncologist 21, 1085–1090. doi: 10.1634/theoncologist.2016-0038
Shah, M. A., Kennedy, E. B., Catenacci, D. V., Deighton, D. C., Goodman, K. A., Malhotra, N. K., et al. (2020). Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol 38, 2677–2694. doi: 10.1200/jco.20.00866
Shah, M. A., Kojima, T., Hochhauser, D., Enzinger, P., Raimbourg, J., Hollebecque, A., et al. (2019). Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol 5, 546–550. doi: 10.1001/jamaoncol.2018.5441
Sharma, P., and Allison, J. P. (2015). The future of immune checkpoint therapy. Science 348, 56–61. doi: 10.1126/science.aaa8172
Sharma, P., Hu-Lieskovan, S., Wargo, J. A., and Ribas, A. (2017). Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723. doi: 10.1016/j.cell.2017.01.017
Sharpe, A. H., and Freeman, G. J. (2002). The B7-CD28 superfamily. Nat. Rev. Immunol. 2, 116–126. doi: 10.1038/nri727
Shimizu, T., Fujii, T., and Sakai, H. (2020). The relationship between actin cytoskeleton and membrane transporters in cisplatin resistance of cancer cells. Front. Cell. Dev. Biol. 8:597835. doi: 10.3389/fcell.2020.597835
Shitara, K., Hara, H., Yoshikawa, T., Fujitani, K., Nishina, T., Hosokawa, A., et al. (2020). Pertuzumab plus trastuzumab and chemotherapy for Japanese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subgroup analysis of the JACOB trial. Int. J. Clin. Oncol. 25, 301–311. doi: 10.1007/s10147-019-01558-z
Shitara, K., and Nishikawa, H. (2018). Regulatory T cells: a potential target in cancer immunotherapy. Ann. N. Y. Acad. Sci. 1417, 104–115. doi: 10.1111/nyas.13625
Shitara, K., Ozguroglu, M., Bang, Y. J., Di Bartolomeo, M., Mandala, M., Ryu, M. H., et al. (2018). Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392, 123–133.
Smyth, E. C., Lagergren, J., Fitzgerald, R. C., Lordick, F., Shah, M. A., Lagergren, P., et al. (2017). Oesophageal cancer. Nat. Rev. Dis. Primers 3:17048.
Sohail, M. F., Rehman, M., Sarwar, H. S., Naveed, S., Salman, O., Bukhari, N. I., et al. (2018). Advancements in the oral delivery of Docetaxel: challenges, current state-of-the-art and future trends. Int. J. Nanomedicine 13, 3145–3161. doi: 10.2147/ijn.s164518
Soong, R., Shah, N., Salto-Tellez, M., Tai, B. C., Soo, R. A., Han, H. C., et al. (2008). Prognostic significance of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase protein expression in colorectal cancer patients treated with or without 5-fluorouracil-based chemotherapy. Ann. Oncol. 19, 915–919. doi: 10.1093/annonc/mdm599
Spranger, S., Bao, R., and Gajewski, T. F. (2015). Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235. doi: 10.1038/nature14404
Spratlin, J. L., Cohen, R. B., Eadens, M., Gore, L., Camidge, D. R., Diab, S., et al. (2010). Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J. Clin. Oncol. 28, 780–787. doi: 10.1200/jco.2009.23.7537
Stroes, C. I., Schokker, S., Creemers, A., Molenaar, R. J., Hulshof, M., Van Der Woude, S. O., et al. (2020). Phase II feasibility and biomarker study of neoadjuvant trastuzumab and pertuzumab with chemoradiotherapy for resectable human epidermal growth factor receptor 2-positive esophageal adenocarcinoma: TRAP study. J. Clin. Oncol. 38, 462–471. doi: 10.1200/jco.19.01814
Sucker, A., Zhao, F., Pieper, N., Heeke, C., Maltaner, R., Stadtler, N., et al. (2017). Acquired IFNγ resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat. Commun. 8:15440.
Sun, D., Liu, D., Liu, Q., and Hou, H. (2020). Nivolumab induced hyperprogressive disease in advanced esophageal squamous cell carcinoma. Cancer Biol. Ther. 21, 1097–1104. doi: 10.1080/15384047.2020.1834319
Tabernero, J., Van Cutsem, E., Bang, Y.-J., Fuchs, C. S., Wyrwicz, L., Lee, K. W., et al. (2019). Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J. Clin. Oncol. 37, LBA4007–LBA4007.
Tan, T. H., Stevenson, B., and Yip, D. (2006). Docetaxel-induced nasal septal perforation. Intern. Med. J. 36, 471–472. doi: 10.1111/j.1445-5994.2006.01105.x
Tepper, J., Krasna, M. J., Niedzwiecki, D., Hollis, D., Reed, C. E., Goldberg, R., et al. (2008). Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 26, 1086–1092.
Thuss-Patience, P. C., Shah, M. A., Ohtsu, A., Van Cutsem, E., Ajani, J. A., Castro, H., et al. (2017). Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 18, 640–653.
Togashi, Y., and Nishikawa, H. (2017). Regulatory T cells: molecular and cellular basis for immunoregulation. Curr. Top. Microbiol. Immunol. 410, 3–27. doi: 10.1007/82_2017_58
Toi, M., Atiqur Rahman, M., Bando, H., and Chow, L. W. (2005). Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 6, 158–166. doi: 10.1016/s1470-2045(05)01766-3
Tol, J., Koopman, M., Cats, A., Rodenburg, C. J., Creemers, G. J., Schrama, J. G., et al. (2009). Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N. Engl. J. Med. 360, 563–572.
Touat, M., Ileana, E., Postel-Vinay, S., Andre, F., and Soria, J. C. (2015). Targeting FGFR signaling in cancer. Clin. Cancer Res. 21, 2684–2694. doi: 10.1158/1078-0432.ccr-14-2329
Trujillo, J. A., Luke, J. J., Zha, Y., Segal, J. P., Ritterhouse, L. L., Spranger, S., et al. (2019). Secondary resistance to immunotherapy associated with β-catenin pathway activation or PTEN loss in metastatic melanoma. J. Immunother. Cancer 7:295.
Van Cutsem, E., Bang, Y. J., Feng-Yi, F., Xu, J. M., Lee, K. W., Jiao, S. C., et al. (2015). HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18, 476–484. doi: 10.1007/s10120-014-0402-y
Van Cutsem, E., Bang, Y. J., Mansoor, W., Petty, R. D., Chao, Y., Cunningham, D., et al. (2017). A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann. Oncol. 28, 1316–1324. doi: 10.1093/annonc/mdx107
Vera-Ramirez, L., Vodnala, S. K., Nini, R., Hunter, K. W., and Green, J. E. (2018). Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat. Commun. 9:1944.
Vernieri, C., Milano, M., Brambilla, M., Mennitto, A., Maggi, C., Cona, M. S., et al. (2019). Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: current knowledge, new research directions and therapeutic perspectives. Crit. Rev. Oncol. Hematol. 139, 53–66. doi: 10.1016/j.critrevonc.2019.05.001
Waddell, T., Chau, I., Cunningham, D., Gonzalez, D., Okines, A. F., Okines, C., et al. (2013). Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 481–489. doi: 10.1016/s1470-2045(13)70096-2
Wang, K., Zhang, L., He, Z. H., Liu, Z. J., Zhang, L., Hu, N., et al. (2019). A population-based survey of gastroesophageal reflux disease in a region with high prevalence of esophageal cancer in China. Chin. Med. J. (Engl.) 132, 1516–1523. doi: 10.1097/CM9.0000000000000275
Wang, W., Chen, D., Zhao, Y., Zhao, T., Wen, J., Mao, Y., et al. (2019). Characterization of LAG-3, CTLA-4, and CD8(+) TIL density and their joint influence on the prognosis of patients with esophageal squamous cell carcinoma. Ann. Transl. Med. 7:776. doi: 10.21037/atm.2019.11.38
Wang, X., Wang, X., and Huang, J. (2016). Irinotecan plus fluorouracil-based regimen as second or third-line chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma. Thorac. Cancer 7, 246–250. doi: 10.1111/1759-7714.12323
Wang, Z., Liu, G., and Jiang, J. (2019). Profiling of apoptosis- and autophagy-associated molecules in human lung cancer A549 cells in response to cisplatin treatment using stable isotope labeling with amino acids in cell culture. Int. J. Oncol. 54, 1071–1085.
Wang, Z., and Wu, X. (2020). Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer Med. [Epub ahead of print].
Weaver, B. A. (2014). How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 25, 2677–2681. doi: 10.1091/mbc.e14-04-0916
Weber, R., Fleming, V., Hu, X., Nagibin, V., Groth, C., Altevogt, P., et al. (2018). Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front. Immunol. 9:1310. doi: 10.3389/fimmu.2018.01310
Weckbecker, G. (1991). Biochemical pharmacology and analysis of fluoropyrimidines alone and in combination with modulators. Pharmacol. Ther. 50, 367–424. doi: 10.1016/0163-7258(91)90051-m
Wheate, N. J., Walker, S., Craig, G. E., and Oun, R. (2010). The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 39, 8113–8127. doi: 10.1039/c0dt00292e
Wilke, H., Muro, K., Van Cutsem, E., Oh, S. C., Bodoky, G., Shimada, Y., et al. (2014). Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 15, 1224–1235. doi: 10.1016/s1470-2045(14)70420-6
Wolmark, N., Rockette, H., Mamounas, E., Jones, J., Wieand, S., Wickerham, D. L., et al. (1999). Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J. Clin. Oncol. 17, 3553–3559.
Won, E., Basunia, A., Chatila, W. K., Hechtman, J. F., Chou, J. F., Ku, G. Y., et al. (2019). Efficacy of combined VEGFR1-3, PDGFalpha/beta, and FGFR1-3 blockade using nintedanib for esophagogastric cancer. Clin. Cancer Res. 25, 3811–3817. doi: 10.1158/1078-0432.ccr-18-3789
Wynne, P., Newton, C., Ledermann, J. A., Olaitan, A., Mould, T. A., and Hartley, J. A. (2007). Enhanced repair of DNA interstrand crosslinking in ovarian cancer cells from patients following treatment with platinum-based chemotherapy. Br. J. Cancer 97, 927–933. doi: 10.1038/sj.bjc.6603973
Xie, P., Mo, J. L., Liu, J. H., Li, X., Tan, L. M., Zhang, W., et al. (2020). Pharmacogenomics of 5-fluorouracil in colorectal cancer: review and update. Cell Oncol. 43, 989–1001. doi: 10.1007/s13402-020-00529-1
Xu, J., Bai, Y., Xu, N., Li, E., Wang, B., Wang, J., et al. (2020). Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Clin. Cancer Res. 26, 4542–4550. doi: 10.1158/1078-0432.ccr-19-3561
Yamamoto, S., and Kato, K. (2020). Immuno-oncology for esophageal cancer. Future Oncol. 16, 2673–2681. doi: 10.2217/fon-2020-0545
Yaneff, A., Sahores, A., Gomez, N., Carozzo, A., Shayo, C., and Davio, C. (2019). MRP4/ABCC4 as a new therapeutic target: meta-analysis to determine cAMP binding sites as a tool for drug design. Curr. Med. Chem. 26, 1270–1307. doi: 10.2174/0929867325666171229133259
Yang, D., and Wang, A. H. (1996). Structural studies of interactions between anticancer platinum drugs and DNA. Prog. Biophys. Mol. Biol. 66, 81–111. doi: 10.1016/s0079-6107(96)00017-x
Yang, W., Gong, P., Yang, Y., Yang, C., Yang, B., and Ren, L. (2020). Circ-ABCB10 Contributes to Paclitaxel Resistance in Breast Cancer Through Let-7a-5p/DUSP7 Axis. Cancer Manag. Res. 12, 2327–2337. doi: 10.2147/cmar.s238513
Ychou, M., Boige, V., Pignon, J. P., Conroy, T., Bouche, O., Lebreton, G., et al. (2011). Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 29, 1715–1721. doi: 10.1200/jco.2010.33.0597
Yoo, S. H., Keam, B., Ock, C. Y., Kim, S., Han, B., Kim, J. W., et al. (2019). Prognostic value of the association between MHC class I downregulation and PD-L1 upregulation in head and neck squamous cell carcinoma patients. Sci. Rep. 9:7680.
Yvon, A. M., Wadsworth, P., and Jordan, M. A. (1999). Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell 10, 947–959. doi: 10.1091/mbc.10.4.947
Zeng, H., Zheng, R., Guo, Y., Zhang, S., Zou, X., Wang, N., et al. (2015). Cancer survival in China, 2003-2005: a population-based study. Int. J. Cancer 136, 1921–1930.
Zhan, N., Dong, W. G., Tang, Y. F., Wang, Z. S., and Xiong, C. L. (2012). Analysis of HER2 gene amplification and protein expression in esophageal squamous cell carcinoma. Med. Oncol. 29, 933–940. doi: 10.1007/s12032-011-9850-y
Zhang, B., Chikuma, S., Hori, S., Fagarasan, S., and Honjo, T. (2016). Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc. Natl. Acad. Sci. U.S.A. 113, 8490–8495. doi: 10.1073/pnas.1608873113
Zhang, C. J., Zhang, J. X., Wang, Z. Z., Li, P., Shang, J. W., and Guo, Y. J. (2020). Prognostic evaluation of human papillomavirus and p16 in esophageal squamous cell carcinoma. Chin. Med. J. (Engl.) 133, 751–752. doi: 10.1097/CM9.0000000000000662
Zhang, N., Yin, Y., Xu, S. J., and Chen, W. S. (2008). 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules 13, 1551–1569. doi: 10.3390/molecules13081551
Zhao, J., and Huang, J. (2020). Breast cancer immunology and immunotherapy: targeting the programmed cell death protein-1/programmed cell death protein ligand-1. Chin. Med. J. (Engl.) 133, 853–862. doi: 10.1097/CM9.0000000000000710
Zhao, Z., Zhang, Y., Wang, X., Geng, X., Zhu, L., and Li, M. (2020). Clinical response to chemoradiotherapy in esophageal carcinoma is associated with survival and benefit of consolidation chemotherapy. Cancer Med. 9, 5881–5888. doi: 10.1002/cam4.3273
Zheng, Y., Li, Y., Tang, B., Zhao, Q., Wang, D., Liu, Y., et al. (2020). IL-6-induced CD39 expression on tumor-infiltrating NK cells predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Immunol. Immunother. 69, 2371–2380. doi: 10.1007/s00262-020-02629-1
Zhong, Y., Jia, C., Zhang, X., Liao, X., Yang, B., Cong, Y., et al. (2020). Targeting drug delivery system for platinum(IV)-based antitumor complexes. Eur. J. Med. Chem. 194:112229. doi: 10.1016/j.ejmech.2020.112229
Zhou, J., Kang, Y., Chen, L., Wang, H., Liu, J., Zeng, S., et al. (2020). The drug-resistance mechanisms of five platinum-based antitumor agents. Front. Pharmacol. 11:343. doi: 10.3389/fphar.2020.00343
Zhu, H. H., Feng, Y., and Hu, X. S. (2020). Emerging immunotherapy targets in lung cancer. Chin. Med. J. (Engl.) 133, 2456–2465. doi: 10.1097/CM9.0000000000001082
Zhu, L., and Chen, L. (2019). Progress in research on paclitaxel and tumor immunotherapy. Cell Mol. Biol. Lett. 24:40.
Keywords: esophageal cancer, chemotherapy, molecular targeted therapy, immunotherapy, drug resistance
Citation: Mao C, Zeng X, Zhang C, Yang Y, Xiao X, Luan S, Zhang Y and Yuan Y (2021) Mechanisms of Pharmaceutical Therapy and Drug Resistance in Esophageal Cancer. Front. Cell Dev. Biol. 9:612451. doi: 10.3389/fcell.2021.612451
Received: 30 September 2020; Accepted: 04 January 2021;
Published: 11 February 2021.
Edited by:
Wei Zhao, Chengdu Medical College, ChinaReviewed by:
Xufeng Guo, Shanghai Jiao Tong University, ChinaLiang Dai, Beijing Cancer Hospital, China
Copyright © 2021 Mao, Zeng, Zhang, Yang, Xiao, Luan, Zhang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonggang Zhang, amVibV96aGFuZ0B5YWhvby5jb20=; Yong Yuan, eW9uZ3l1YW5Ac2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Chengyi Mao
Chengyi Mao Xiaoxi Zeng2†
Xiaoxi Zeng2† Siyuan Luan
Siyuan Luan Yonggang Zhang
Yonggang Zhang Yong Yuan
Yong Yuan